Abstract
In a phase 2 trial of once‐weekly tirzepatide (1, 5, 10, or 15 mg), dulaglutide (1.5 mg), or placebo, the dual glucose‐dependent insulinotropic polypeptide and glucagon‐like peptide‐1 receptor agonist tirzepatide dose‐dependently reduced HbA1c and body weight in patients with type 2 diabetes. In this post hoc analysis, inflammation, endothelial dysfunction, and cellular stress biomarkers were measured at baseline, 4, 12, and 26 weeks to evaluate the additional effects of tirzepatide on cardiovascular risk factors. At 26 weeks, tirzepatide 10 and 15 mg decreased YKL‐40 (also known as chitinase‐3 like‐protein‐1), intercellular adhesion molecule 1 (ICAM‐1), leptin, and growth differentiation factor 15 levels versus baseline, and YKL‐40 and leptin levels versus placebo and dulaglutide. Tirzepatide 15 mg also decreased ICAM‐1 levels versus placebo and dulaglutide, and high‐sensitivity C‐reactive protein (hsCRP) levels versus baseline and placebo, but not dulaglutide. GlycA, interleukin 6, vascular cell adhesion molecule 1, and N‐terminal‐pro hormone B‐type natriuretic peptide levels were not significantly changed in any group. YKL‐40, hsCRP, and ICAM‐1 levels rapidly decreased within 4 weeks of treatment with tirzepatide 10 and 15 mg, whereas the decrease in leptin levels was more gradual and did not plateau by 26 weeks. In this hypothesis‐generating exploratory analysis, tirzepatide decreased several biomarkers that have been associated with cardiovascular risk.
Keywords: cardiovascular disease, GIP, GLP‐1, incretin therapy, type 2 diabetes
1. INTRODUCTION
Glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) are effective therapies for the treatment of type 2 diabetes. In addition to improving glycaemic control and reducing body weight, they have shown efficacy in reducing major adverse cardiovascular events (MACE). 1 While glucose lowering and weight loss probably contribute to reduced cardiovascular risk, they do not account for the full effect, 2 and additional mechanisms, including improvement in endothelial dysfunction and reduction of inflammation, may also contribute. 3 , 4
Dual agonism of glucose‐dependent insulinotropic polypeptide (GIP) and GLP‐1 receptors is a promising new approach currently in clinical development for the treatment of diabetes, obesity, and associated complications. 5 Human genetics has shown protection against coronary heart disease associated with a GLP1R variant, consistent with the effect of GLP‐1RAs on reducing MACE. 6 GIP has also shown anti‐inflammatory and vascular protective effects in preclinical studies. 7 However, in one study, short‐term infusion of GIP in humans was shown to increase monocyte chemoattractant protein‐1 (MCP‐1) and interleukin 6 (IL‐6) mRNA levels in adipose tissue, as well as circulating plasma concentrations of MCP‐1. 8 It is thus critically important to further understand the role of GIP in the context of dual GIP and GLP‐1 receptor agonism on key cardiovascular risk factors. 9
In a phase 2b study of patients with type 2 diabetes, the dual GIP and GLP‐1 RA tirzepatide showed significantly better glucose control and weight loss than the selective GLP‐1RA dulaglutide. 10 In this post hoc analysis, we report circulating levels of biomarkers of systemic inflammation, cytokines, adhesion molecules, the adipokine leptin, and the cardiomyocyte stress marker N‐terminal‐pro hormone B‐type natriuretic peptide (NT‐proBNP), to better understand the possible effects of tirzepatide on cardiovascular risk.
2. METHODS
2.1. Trial design
In this phase 2b, double‐blind (patient and investigator) study, participants (aged 18‐75 years) had type 2 diabetes for 6 months or longer (HbA1c 7.0%‐10.5%), which was inadequately controlled with diet and exercise alone or with stable metformin therapy, and a body mass index of 23‐50 kg/m2. 10 Overall, 318 participants were randomized (1:1:1:1:1:1) to once‐weekly subcutaneous 1 (N = 53), 5 (N = 55), 10 (N = 52), or 15 mg (N = 53) tirzepatide, 1.5 mg dulaglutide (N = 54), or placebo (N = 51), with 316 participants included in the modified intention‐to‐treat population. Participants were treated for 26 weeks after a 1‐week screening and 2‐week lead‐in period. The study (NCT03131687) was approved by the relevant ethics committees and conducted in accordance with the principles of the Declaration of Helsinki, Council of International Organizations of Medical Sciences International Ethical Guidelines, and Good Clinical Practice guidelines. All participants provided written informed consent prior to participation.
2.2. Biomarker analysis
Biomarkers were quantified in serum or EDTA plasma collected in the fasting state from patients at baseline, 4, 12, and 26 weeks and stored at −80°C until analysis. High‐sensitivity C‐reactive protein (hsCRP) (Roche, Indianapolis, IN); GlycA (LabCorp, Morrisville, NC); growth differentiation factor 15 (GDF‐15), YKL‐40 (also known as chitinase‐3 like‐protein‐1), MCP‐1, and leptin (R&D Systems, Minneapolis, MN); and IL‐6, intercellular adhesion molecule 1 (ICAM‐1), vascular cell adhesion molecule 1 (VCAM‐1), and NT‐proBNP (MesoScale Discovery, Rockville, MD), were measured by immunoassay. Additional biomarker descriptions, background information, and details of assay methods, are provided in Tables S1 and S2.
2.3. Statistical analysis
Analyses were performed on the modified intention‐to‐treat population, excluding data after study drug discontinuation or rescue drug initiation. Subjects included in analyses had non‐missing baseline values and at least one non‐missing postbaseline value of the response variable. Biomarker distribution was examined. Changes in biomarkers were analysed using mixed model with repeated measure for all biomarkers except GlycA, which was analysed using analysis of covariance because of data only being available at baseline and 26 weeks. Biomarker at baseline was included as a covariate in the models. Log‐transformation for models was used for biomarkers with a skewed distribution. To investigate the impact of changes in body weight on changes in hsCRP, YKL‐40, ICAM‐1, and leptin, linear regression analyses were conducted in each treatment group, as well as a combined tirzepatide 10 + 15 mg group. A linear model selected by a forward model selection analysis was conducted with biomarker change from baseline at 26 weeks as the response and weight loss at 26 weeks as the independent variable. The forward model selection analysis was run starting with the model with treatment, weight loss, and their interaction using samples from all treatments. Potential covariates for model selection were baseline body weight, age, sex, baseline HbA1c, and baseline biomarker levels. The best model was selected by the Akaike information criterion and this was used for each treatment separately to calculate the contribution of weight loss (type III sum of squares) to total variability (total sum of squares) in change from baseline of the biomarker. Spearman correlation coefficients were computed between all biomarkers within the tirzepatide‐15 mg and dulaglutide groups. A two‐sided P value of .05 was taken to indicate statistical significance.
3. RESULTS
Baseline demographics, clinical characteristics, and lipid profiles were published previously and were similar across treatment groups. 10 , 11 Baseline biomarker levels measured in this study were also similar across treatment groups (Table 1).
TABLE 1.
Cardiovascular risk factor biomarkers at baseline and percentage change from baseline at 26 weeks
| Placebo N = 51 | Tirzepatide 1 mg N = 52 | Tirzepatide 5 mg N = 55 | Tirzepatide 10 mg N = 51 | Tirzepatide 15 mg N = 53 | Dulaglutide 1.5 mg N = 54 | ||
|---|---|---|---|---|---|---|---|
| hsCRP, mg/L | Baseline | 3.7 ± 0.6 | 2.9 ± 0.5 | 2.5 ± 0.3 | 3.7 ± 0.6 | 2.7 ± 0.4 | 3.1 ± 0.4 |
| % change from baseline | −1.7 (13.2) | −5.6 (11.8) | −13.2 (10.6) | −16.0 (10.9) | −36.2 (8.9)* , † | −18.2 (10.1) | |
| GlycA, μmol/L | Baseline | 468.2 ± 11.8 | 466.1 ± 11.7 | 437.7 ± 10.3 | 449.9 ± 11.1 | 463.8 ± 13.0 | 462.6 ± 11.0 |
| % change from baseline | 1.5 (2.0) | −0.9 (2.0) | −1.7 (1.8) | −0.3 (1.9) | −2.9 (2.2) | 0.9 (1.9) | |
| GDF‐15, pg/mL | Baseline | 1333 ± 82 | 1477 ± 102 | 1446 ± 96 | 1510 ± 120 | 1454 ± 123 | 1575 ± 109 |
| % change from baseline | 1.2 (6.1) | −11.6 (5.3)* | −9.4 (5.1) | −12.1 (5.2)* | −12.9 (5.8)* | −15.4 (4.9)* | |
| IL‐6, pg/mL | Baseline | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.5 ± 0.1 | 1.8 ± 0.2 | 1.7 ± 0.2 | 1.8 ± 0.1 |
| % change from baseline | 10.7 (7.6) | −1.8 (6.5) | −0.1 (6.3) | 0.5 (7.2) | −2.0 (7.3) | −2.5 (6.4) | |
| MCP‐1, pg/mL | Baseline | 376 ± 17 | 396 ± 18 | 378 ± 23 | 359 ± 22 | 406 ± 23 | 420 ± 25 |
| % change from baseline | 1.2 (3.6) | 3.5 (3.6) | 7.6 (3.5)* | −6.3 (3.3) ‡ | −5.7 (3.6) | 4.3 (3.6) | |
| YKL‐40, ng/mL | Baseline | 57.5 ± 5.8 | 66.5 ± 5.8 | 69.6 ± 7.1 | 63.6 ± 6.1 | 61.0 ± 8.3 | 66.3 ± 6.0 |
| % change from baseline | 10.8 (7.6) | −0.1 (6.8) | −8.8 (5.9) † | −26.1 (5.0)** , †† , ‡ | −30.8 (5.3)** , †† , ‡ | −6.2 (6.1) | |
| ICAM‐1, ng/mL | Baseline | 460 ± 100 | 449 ± 132 | 456 ± 142 | 467 ± 128 | 446 ± 130 | 481 ± 139 |
| % change from baseline | −1.6 (2.3) | −2.1 (2.3) | −4.5 (2.2)* | −7.2 (2.3)* , † | −11.2 (2.5)** , † , ‡ | −3.7 (2.2) | |
| VCAM‐1, ng/mL | Baseline | 629 ± 149 | 646 ± 156 | 655 ± 185 | 667 ± 233 | 628 ± 146 | 646 ± 144 |
| % change from baseline | −0.9 (2.2) | −1.2 (2.2) | −0.6 (2.1) | 1.3 (2.2) | 0.5 (2.4) | −4.2 (2.1) | |
| Leptin, ng/mL | Baseline | 19.7 ± 16.5 | 24.1 ± 21.2 | 22.6 ± 18.4 | 22.3 ± 16.8 | 27.7 ± 20.2 | 21.8 ± 15.6 |
| % change from baseline | 8.1 (7.0) | 8.6 (7.2) | 0.6 (6.8) | −28.2 (7.6)** , †† , ‡‡ | −34.1 (7.9)** , †† , ‡‡ | 10.7 (6.8) | |
| NT‐proBNP, pg/mL | Baseline | 171 ± 21 | 190 ± 31 | 183 ± 28 | 213 ± 35 | 251 ± 41 | 214 ± 32 |
| % change from baseline | −10.7 (10.6) | −0.7 (11.3) | −8.3 (10.1) | 2.4 (12.0) | −3.8 (12.0) | −6.6 (10.1) | |
Note: Data are presented as mean ± SD (ICAM‐1, VCAM‐1, leptin) or geometric mean ± SE (hsCRP, GlycA, GDF‐15, IL‐6, MCP‐1, YKL‐40, NT‐proBNP) at baseline and LSM (SE) percentage change from baseline at 26 weeks from ANCOVA (GlycA) or MMRM (all other biomarkers), mITT population. Log transformation was used for hsCRP, GDF‐15, IL‐6, MCP‐1, YKL‐40, and NT‐proBNP.
Abbreviations: ANCOVA, analysis of covariance; GDF‐15, growth differentiation factor 15; hsCRP, high‐sensitivity C‐reactive protein; ICAM‐1, intercellular adhesion molecule 1; IL‐6, interleukin 6; LSM, least squares mean; MCP‐1, monocyte chemoattractant protein‐1; MMRM, mixed model with repeated measure; mITT, modified intention‐to‐treat; NT‐proBNP, N‐terminal‐pro hormone B‐type natriuretic peptide; SD, standard deviation; SE, standard error; VCAM‐1, vascular cell adhesion molecule 1; YKL‐40, also known as chitinase‐3 like‐protein‐1.
P < .05 versus baseline.
P < .001 versus baseline.
P < .05 versus placebo.
P < .001 versus placebo.
P < .05 versus dulaglutide 1.5 mg.
P < .001 versus dulaglutide 1.5 mg.
Table 1 and Figure S1 present percentage change from baseline at 26 weeks. Tirzepatide dose‐dependently decreased hsCRP from baseline levels, with statistical significance in the tirzepatide‐15 mg group (least squares mean [LSM] standard error [SE]: −36.2% [8.9]). Tirzepatide 15 mg also significantly reduced hsCRP versus placebo, but not versus dulaglutide. A trend of reduction in GlycA levels occurred in all tirzepatide dose groups, in contrast to increases in the placebo and dulaglutide groups. However, differences between groups were not statistically significant.
GDF‐15 levels decreased in all tirzepatide dose groups (1 mg: −11.6% [5.3], P < .05; 5 mg: −9.4% [5.1], P > .05; 10 mg: −12.1% [5.2], P < .05; 15 mg: −12.9% [5.8], P < .05), and the dulaglutide group (−15.4% [4.9], P < .05). The magnitudes of differences in GDF‐15 levels were not significant between any tirzepatide group and placebo or dulaglutide. IL‐6 levels were unchanged in all groups. MCP‐1 levels were increased from baseline in the 5‐mg group and decreased versus dulaglutide in the 10‐mg group, with no other significant differences.
Tirzepatide dose‐dependently decreased YKL‐40 levels with a significant change in the tirzepatide 10‐mg (−26.1% [5.0]) and 15‐mg (−30.8% [5.3]) groups (both P < .001). There was no significant change in the placebo (10.8% [7.6]) or dulaglutide (−6.2% [6.1]) groups. YKL‐40 levels were significantly decreased in the 5‐, 10‐, and 15‐mg tirzepatide dose groups versus placebo and the 10‐ and 15‐mg groups versus dulaglutide.
Tirzepatide dose‐dependently decreased ICAM‐1 levels, with significant decreases compared with baseline in the 5‐mg (−4.5% [2.2]), 10‐mg (−7.2% [2.3]), and 15‐mg (−11.2% [2.5]) groups. ICAM‐1 levels were also significantly lower in the tirzepatide 15‐mg group versus placebo and dulaglutide. VCAM‐1 levels did not change significantly in any group and there were no differences between groups in VCAM‐1 levels.
Leptin levels significantly decreased in the tirzepatide 10‐mg (−28.2% [7.6]) and 15‐mg (−34.1% [7.9]) groups compared with baseline and the placebo and dulaglutide groups.
There were no significant changes in NT‐proBNP levels in any group and no differences between groups.
Among the 10 biomarkers measured, four (hsCRP, YKL‐40, ICAM‐1, and leptin) were significantly reduced in the high‐dose tirzepatide treatment group compared with the placebo group. Changes over time in these biomarkers are presented in Figure 1. Dose‐dependent reductions in hsCRP, YKL‐40, and ICAM‐1 levels occurred within 4 weeks of tirzepatide treatment. By contrast, in the tirzepatide 10‐ and 15‐mg groups, leptin levels decreased throughout the study and did not plateau by 26 weeks.
FIGURE 1.
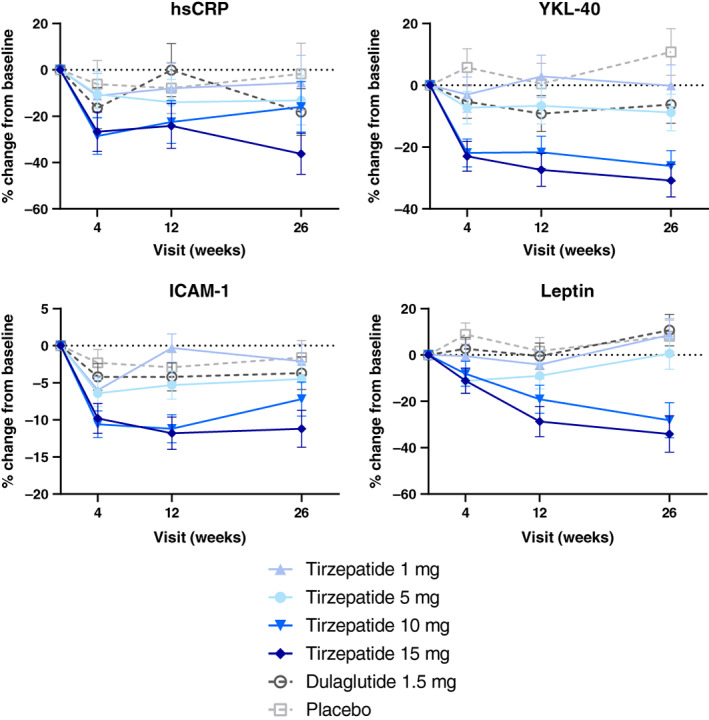
Percentage change from baseline over time in hsCRP, YKL‐40, ICAM‐1, and leptin. Data are presented as LSM (SE) percentage change from baseline over time from MMRM; mITT population. Log‐transformation was used for hsCRP and YKL‐40. hsCRP, high‐sensitivity C‐reactive protein; ICAM‐1, intercellular adhesion molecule 1; LSM, least squares mean; mITT, modified intention‐to‐treat; MMRM, mixed model with repeated measure; SE, standard error; YKL‐40, also known as chitinase‐3 like‐protein‐1
Heatmap plots of Spearman's correlation coefficients for changes from baseline at 26 weeks in the tirzepatide 15‐mg and dulaglutide groups are presented in Figure S2. Changes in ICAM‐1 showed significant correlations with changes in hsCRP, GlycA, and VCAM‐1 in both groups. Changes in YKL‐40 levels correlated with changes in VCAM‐1 and NT‐proBNP, but only in the dulaglutide group. Interestingly, changes in leptin levels did not correlate well with changes in other biomarkers, except for a significant correlation with changes in GDF‐15 only in the tirzepatide 15‐mg group.
Body weight change explained 23% and 20% of leptin variability in the dulaglutide and tirzepatide (10 + 15 mg combined) groups, respectively. The percentage of leptin variability explained by body weight change generally increased with increasing tirzepatide doses (1 mg, 6%; 5 mg, 11%; 10 mg, 18%; 15 mg, 18%). Change in body weight with tirzepatide (10 + 15 mg combined) also explained 4% of the variability in ICAM‐1 levels (vs. 1% with dulaglutide). Finally, body weight change with dulaglutide explained 13% of hsCRP and 21% of YKL‐40 variability compared with 0% and 6% with tirzepatide, respectively.
4. DISCUSSION
Patients with type 2 diabetes are at an increased risk of cardiovascular disease. Mechanisms related to inflammation and endothelial dysfunction have been implicated, among others. 12 In this post hoc analysis in patients with type 2 diabetes, we show that tirzepatide treatment reduces the circulating levels of several biomarkers associated with cardiovascular risk compared with both placebo and dulaglutide treatment.
Tirzepatide dose‐dependently decreased hsCRP, YKL‐40, ICAM‐1, and leptin levels after 26 weeks of treatment. The dose‐ and time‐dependent reduction in leptin levels with tirzepatide was more gradual compared with rapid decreases in hsCRP, YKL‐40, and ICAM‐1 at 4 weeks, and did not plateau after 26 weeks. This pattern suggests an earlier direct effect of tirzepatide in suppressing inflammation and improving endothelial function, independent of weight loss. Leptin is a pro‐inflammatory adipokine mainly secreted from the adipose tissue, 13 and circulating leptin levels correlate with the degree of obesity. The decrease observed here is consistent with, although not completely explained by, the effect of tirzepatide on body weight. The rapid decline of the well‐established systemic inflammation marker hsCRP, 14 the pro‐inflammatory cytokine YKL‐40, 15 and the endothelial dysfunction marker ICAM‐1, 16 are consistent with the suppression of inflammatory responses in monocytes, macrophages, and adipocytes and the overall vascular protective effects of GIP and GLP‐1 reported in preclinical studies. 3 , 7 In epidemiological studies in humans, these inflammatory biomarkers are positively associated with atherosclerotic cardiovascular disease. High levels of hsCRP, YKL‐40, and ICAM‐1 are associated with an increased risk of MACE. Pharmacological treatments that reduce hsCRP, including statins and anti‐IL‐1β antibody, are associated with improved cardiovascular outcomes. 13 , 14 , 15 , 16 , 17 In the current 26‐week study, we also observed a trend for reduction in MCP‐1 levels with the 10 and 15 mg doses of tirzepatide, and no changes in IL‐6 levels, thus suggesting that the increase in MCP‐1 concentrations and adipose tissue MCP‐1 and IL‐6 mRNA levels described following a 240‐min infusion of synthetic human GIP (1‐42) is transitory. 8 The decrease of leptin, YKL‐40, ICAM‐1, and MCP‐1 levels following treatment with tirzepatide 10 and/or 15 mg compared with dulaglutide suggests improved anti‐inflammatory activity of tirzepatide and could translate into a favourable effect of this molecule in reducing cardiovascular risk.
This study is strengthened by comparison with both placebo and dulaglutide, a GLP‐1 RA with proven reduction in risk of MACE events, 1 the magnitude of which cannot be fully explained by its effects on glucose, weight, or systolic blood pressure, 2 suggesting that inflammatory, endothelial function, or other mechanisms may be involved. An inherent limitation of its post hoc design is that sample sizes were based on power calculations for primary study endpoints, participant flow through the trial, and stored sample availability, rather than prespecified analyses or a prepublished protocol. 10 The inclusion of 10 cardiovascular risk biomarkers allowed exploration of possible additional effects of tirzepatide treatment, but this study was intended to be hypothesis generating, rather than to provide definitive data on the effects of tirzepatide on cardiovascular outcomes.
In conclusion, in this 26‐week study in patients with type 2 diabetes, treatment with tirzepatide resulted in the reduction of several biomarkers of inflammation and endothelial dysfunction associated with cardiovascular risk. These findings will be explored further in larger studies, such as SURPASS‐4 (NCT03730662) and, particularly, SURPASS‐CVOT (NCT04255433). In the SURPASS‐4 study, the efficacy of tirzepatide versus insulin glargine in patients with type 2 diabetes and increased cardiovascular risk is being investigated, while in the SURPASS‐CVOT, the effect of tirzepatide versus dulaglutide on major cardiovascular events in patients with type 2 diabetes and established cardiovascular disease is being assessed.
CONFLICT OF INTEREST
J.M.W., Y.L., M.J.L., A.L.C., L.M.B., D.A.R., A.H., K.L.D., and G.R. are employees and shareholders of Eli Lilly and Company. G.C. is a contractor for Advanced Testing Laboratories and works with Eli Lilly and Company.
AUTHOR CONTRIBUTIONS
J.M.W., Y.L., D.A.R., A.H., K.L.D., and G.R. contributed to the study design. A.H. and G.R. provided medical oversight during the trial. J.M.W., G.R., A.L.C., and L.M.B. contributed to data analysis. Y.L. was responsible for the statistical analysis. All authors participated in interpretation of the data and critical review of the manuscript, had full access to all study data, and approved this manuscript to be submitted for publication.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14553.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGEMENTS
This paper is presented in remembrance of Jeffrey S. Riesmeyer. The authors thank the study participants and investigators for their contributions to the trial, and Andrea Hemmingway, PhD, for writing and editorial contributions. This study was sponsored by Eli Lilly and Company.
Wilson JM, Lin Y, Luo MJ, et al. The dual glucose‐dependent insulinotropic polypeptide and glucagon‐like peptide‐1 receptor agonist tirzepatide improves cardiovascular risk biomarkers in patients with type 2 diabetes: A post hoc analysis. Diabetes Obes Metab. 2022;24(1):148-153. doi:10.1111/dom.14553
Funding information Eli Lilly and Company
DATA AVAILABILITY STATEMENT
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
REFERENCES
- 1. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP‐1 receptor agonists in patients with type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776‐785. [DOI] [PubMed] [Google Scholar]
- 2. Konig M, Riddle MC, Colhoun HM, et al. Exploring potential mediators of the cardiovascular benefit of dulaglutide in type 2 diabetes patients in REWIND. Cardiovasc Diabetol. 2021;20(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gallego‐Colon E, Wojakowski W, Francuz T. Incretin drugs as modulators of atherosclerosis. Atherosclerosis. 2018;278:29‐38. [DOI] [PubMed] [Google Scholar]
- 4. Bray JJH, Foster‐Davies H, Salem A, et al. Glucagon‐like peptide‐1 receptor agonists improve biomarkers of inflammation and oxidative stress: a systematic review and meta‐analysis of randomised controlled trials. Diabetes Obes Metab. 2021;23(8):1806‐1822. [DOI] [PubMed] [Google Scholar]
- 5. Finan B, Müller TD, Clemmensen C, Perez‐Tilve D, DiMarchi RD, Tschöp MH. Reappraisal of GIP pharmacology for metabolic diseases. Trends Mol Med. 2016;22(5):359‐376. [DOI] [PubMed] [Google Scholar]
- 6. Scott RA, Freitag DF, Li L, et al. A genomic approach to therapeutic target validation identifies a glucose‐lowering GLP1R variant protective for coronary heart disease. Sci Transl Medicine. 2016;8(341):341ra76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mori Y, Matsui T, Hirano T, Yamagishi SI. GIP as a potential therapeutic target for atherosclerotic cardiovascular disease‐a systematic review. Int J Mol Sci. 2020;21(4):1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gögebakan Ö, Osterhoff MA, Schüler R, et al. GIP increases adipose tissue expression and blood levels of MCP‐1 in humans and links high energy diets to inflammation: a randomised trial. Diabetologia. 2015;58(8):1759‐1768. [DOI] [PubMed] [Google Scholar]
- 9. Heimbürger SM, Bergmann NC, Augustin R, Gasbjerg LS, Christensen MB, Knop FK. Glucose‐dependent insulinotropic polypeptide (GIP) and cardiovascular disease. Peptides. 2020;125:170174. [DOI] [PubMed] [Google Scholar]
- 10. Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP‐1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo‐controlled and active comparator‐controlled phase 2 trial. Lancet. 2018;392(10160):2180‐2193. [DOI] [PubMed] [Google Scholar]
- 11. Wilson JM, Nikooienejad A, Robins DA, et al. The dual glucose‐dependent insulinotropic peptide and glucagon‐like peptide‐1 receptor agonist, tirzepatide, improves lipoprotein biomarkers associated with insulin resistance and cardiovascular risk in patients with type 2 diabetes. Diabetes Obes Metab. 2020;12:2451‐2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Libby P. The changing landscape of atherosclerosis. Nature. 2021;592(7855):524‐533. [DOI] [PubMed] [Google Scholar]
- 13. Pérez‐Pérez A, Sánchez‐Jiménez F, Vilariño‐García T, Sánchez‐Margalet V. Role of leptin in inflammation and vice versa. Int J Mol Sci. 2020;21(16):5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Emerging Risk Factors Collaboration , Kaptoge S, Di Angelantonio E, et al. C‐reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta‐analysis. Lancet. 2010;375(9709):132‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao T, Su Z, Li Y, Zhang X, You Q. Chitinase‐3 like‐protein‐1 function and its role in diseases. Signal Transduct Target Ther. 2020;5(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Witkowska AM, Borawska MH. Soluble intercellular adhesion molecule‐1 (sICAM‐1): an overview. Euro Cytokine Netw. 2004;15(2):91‐98. [PubMed] [Google Scholar]
- 17. Ridker PM. Clinician's guide to reducing inflammation to reduce atherothrombotic risk: JACC review topic of the week. J Am Coll Cardiol. 2018;72(25):3320‐3331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.


