Abstract
Objective
To report long‐term post hoc efficacy and safety data from 10 US study sites from an open‐label Phase 3 study of adjunctive cenobamate (NCT02535091).
Methods
Patients with uncontrolled focal seizures taking stable doses of 1–3 antiseizure medications (ASMs) were administered increasing daily doses of cenobamate (12.5, 25, 50, 100, 150, 200 mg/day) over 12 weeks at 2‐week intervals (target dose = 200 mg/day). Further increases to 400 mg/day by 50‐mg/day increments biweekly were allowed during the maintenance phase. Dose adjustments of cenobamate and concomitant ASMs were allowed. Data were assessed until the last clinic visit on or after September 1, 2019.
Results
Of 255 patients, 240 with focal aware motor, focal impaired awareness, or focal to bilateral tonic–clonic seizure data while on treatment were evaluated (median [maximum] exposure = 30.2 [43.0] months across the entire study). Median baseline seizure frequency/28 days was 2.8 (mean = 18.1). Of the 240 patients, 177 (73.8%) were continuing cenobamate treatment at data cutoff. The ≥50% responder rate for the total treatment duration was 71.7% (172/240). During titration, the ≥50% responder rates were 48.1% during Weeks 1–4 (12.5–25 mg/day cenobamate) and 61.7% during Weeks 5–8 (50–100 mg/day cenobamate). Among all patients who received a dose of cenobamate in the maintenance phase (n = 214), 13.1% (28/214) and 40.2% (86/214) achieved 100% and ≥90% seizure reduction during their entire maintenance treatment duration (median = 29.5 months). Among all patients, 87 (36.3%) had any consecutive ≥12‐month duration of 100% seizure reduction. Common treatment‐emergent adverse events among all 240 patients included fatigue (34.6%), dizziness (32.1%), and somnolence (29.6%).
Significance
This post hoc analysis of a subset of patients from the long‐term open‐label study showed high rates of sustained 100% and ≥90% seizure reduction, with many achieving response early during titration. These findings suggest durable seizure frequency reduction with cenobamate in adults with uncontrolled focal seizures.
Keywords: cenobamate, efficacy, focal epilepsy, long term, safety/tolerability
Key Points.
Of 240 patients in this analysis (median exposure = 30.2 months, range = 0.10–43.0), 177 (73.8%) were still taking cenobamate at data cutoff
During titration, ≥50% responder rates were 48.1% for Weeks 1–4 (12.5–25 mg/day cenobamate) and 61.7% for Weeks 5–8 (50–100 mg/day cenobamate)
During the entire maintenance phase (median = 29.5 months, population n = 214), 13.1% of patients had 100% seizure reduction, and 40.2% had ≥90% reduction
Eighty‐seven of 240 (36.3%) patients had 100% seizure reduction for ≥12 consecutive months during the study
Most common adverse events (fatigue, dizziness, somnolence) in this subset of patients were consistent with those in patients from the primary study
1. INTRODUCTION
Pharmacotherapy is the mainstay of therapy for epilepsy, and many patients require life‐long treatment with antiseizure medications (ASMs). 1 , 2 However, although 20 new ASMs have been introduced in the past 30 years, approximately 35%‐40% of newly diagnosed patients with epilepsy fail to achieve sustained seizure freedom (100% seizure reduction) despite use of multiple medications. 3 , 4 Failure to control seizures carries serious consequences, including increased mortality and morbidity compared with the general population. 5 , 6 , 7 , 8
Cenobamate (XCOPRI, SK Life Science) is an ASM approved by the US Food and Drug Administration (FDA) for treatment of adults with focal (partial onset) seizures. The safety, efficacy, and tolerability of cenobamate were demonstrated in two randomized, double‐blind, placebo‐controlled, multicenter studies (treatment lengths ≤ 18 weeks) in adults with uncontrolled focal seizures despite taking 1–3 ASMs (NCT01397968 [C013] 9 and NCT01866111 [C017] 9 , 10 ). In the 12‐week placebo‐controlled, randomized C013 study, cenobamate produced a median 55.6% reduction in seizure frequency, in contrast to a 21.5% reduction observed in placebo‐treated patients. 9 In the 18‐week, dose‐ranging C017 study, doses from 100 to 400 mg/day were shown to be efficacious, with median percent reduction of up to 55% in patients treated with 200 or 400 mg compared with a median percent reduction of 24% with placebo. 10 During the maintenance phases, 28.3% of patients in C013 and up to 21% of patients in C017 treated with cenobamate 200–400 mg/day achieved 100% seizure reduction (placebo‐adjusted treatment effect of ~20% in both studies). 9 , 10 The most common adverse events (AEs) in these studies were central nervous system (CNS)‐related. 9 , 10
Randomized, controlled, short‐term studies, with stringent protocols and inclusion criteria, provide a safe and standardized approach for regulatory authorities to evaluate the efficacy, safety, and tolerability of a new ASM. 11 Although short‐term trials are valuable, long‐term studies of efficacy, safety, and tolerability are needed to provide clinical insights about new ASMs. Long‐term data for cenobamate in a large, global, open‐label, Phase 3 safety study (NCT02535091 [C021]) with 1347 patients supported the safety and tolerability profile of cenobamate. 12 The C021 safety study, however, did not require reporting of seizure outcomes, because long‐term efficacy was not assessed in C021. Patients enrolled in C021 must have had uncontrolled seizures despite treatment with 1–3 ASMs. However, the study design had less stringent inclusion criteria for baseline seizure severity than the randomized clinical studies. 9 , 10 , 12 Therefore, the C021 study population as a whole had less severe focal epilepsy than the study populations in the short‐term clinical studies. Given the value of long‐term efficacy data, a protocol amendment permitted the post hoc collection of seizure data and AEs from patient diaries and clinic notes to assess seizure responses. This post hoc analysis examined the efficacy data from US study sites in the C021 study that had high‐quality long‐term seizure outcomes recorded.
2. MATERIALS AND METHODS
2.1. Study design and patients
C021 was a large, multicenter, open‐label safety study in patients with uncontrolled focal (partial) seizures. The study included a screening period of up to 21 days and an open‐label treatment period, consisting of a 12‐week titration phase, followed by an open‐label maintenance phase. The maintenance phase continued for the length of the study (total study duration was up to 43 months). Detailed patient eligibility criteria have been previously published. 12 Briefly, eligible patients were 18–70 years old, with a diagnosis of focal epilepsy by International League Against Epilepsy seizure classification criteria. Patients’ focal seizures must have been uncontrolled despite stable doses of 1–3 concomitant ASMs. 12 , 13 US sites that enrolled ≥11 patients who had recorded high‐quality seizure data were eligible to participate in this retrospective study.
For the post hoc analysis, patients must have had at least one focal aware motor, focal impaired awareness, or focal to bilateral tonic–clonic (FBTC) seizure per 13 weeks baseline prior to screening visit; data on focal aware motor, focal impaired awareness, or FBTC seizures while on treatment; consistent documentation of raw seizure data; and good‐quality data for ≥85% of the time spent in the study. Patients initiated cenobamate treatment at 12.5 mg/day for 2 weeks, followed by 25 mg/day for 2 weeks and 50 mg/day for 2 weeks. 12 Cenobamate dose was then increased by 50 mg/day at 2‐week intervals to the target dose of 200 mg/day. After reaching 200 mg/day, further increases up to 400 mg/day using biweekly increments of 50 mg/day were allowed during a maintenance phase. Reductions below 200 mg were allowed according to investigators’ clinical judgment (minimum allowed dose = 50 mg/day). Cenobamate monotherapy was not allowed. Patient visits occurred every 2 weeks for 16 weeks and then every 1–3 months.
The C021 study was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice guidelines. The post hoc analysis was approved by an independent ethics committee or institutional review board. Written informed consent was obtained from each patient in the C021 study (N = 1347) prior to study participation. No new patient consent was required for the post hoc analysis.
2.2. Assessments and analysis
Responder rates (≥50%, ≥75%, ≥90%, and 100% reduction in seizure frequency from baseline) were assessed during the entire treatment period, during the titration phase, and during the maintenance phase. The median percent reduction in seizure frequency was also examined during the maintenance phase by seizure subtype.
All patients meeting the post hoc inclusion criteria were included in the efficacy analyses. Data were assessed until the last clinic visit on or after September 1, 2019, depending on when sites delivered their data. For analysis during titration, the ≥50% responder rate was examined in 4‐week intervals versus baseline up to Week 12 using patients with available seizure data at each 4‐week interval as the denominator. All patients were included in this analysis and counted as a ≥50% responder if the data showed a ≥50% reduction from baseline seizure frequency during each 4‐week interval. For analysis during the maintenance phase, the maintenance population was defined as all patients meeting inclusion criteria who received a dose of cenobamate after the 12‐week titration phase. Duration of 100% seizure reduction was assessed at ≥3, ≥6, and ≥12 months. Assessments were performed moving backward from the patient's last clinical visit (ie, interval includes their last clinic visit) and during any consecutive ≥12‐month interval (ie, interval does not have to include the patient's last clinic visit). Patients could be assessed up to 15 days before the 12‐month duration, 10 days before the 6‐month duration, and 5 days before the 3‐month duration. Patients with any missing seizure frequency data could not be counted as having 100% seizure reduction. For ≥50%, ≥75%, and ≥90% responder rates, the seizure frequency was analyzed as observed with imputation for missing seizure data during the assessed interval. Descriptive statistics were used to summarize the data.
3. RESULTS
3.1. Patients
Among the 1347 patients enrolled in the C021 study, 1340 patients were treated with cenobamate as of July 2019. Retention at 12 and 24 months of treatment was 80% and 73%, respectively (Figure S1). Among the 1340 patients, 12 US sites (299 patients) were potentially eligible to participate in the post hoc study. Two sites were unable to participate, leaving 10 sites with 255 patients for the post hoc analysis. Of these 255 patients, 15 did not meet the post hoc inclusion criteria, leaving 240 patients who could be evaluated for efficacy. For these 240 patients, the first patient was enrolled on July 20, 2016, and the last patient was enrolled on January 19, 2018. Demographic (mean age = 41.8 years, 43.8% female) and disease characteristics of the 240 patients were similar to the remaining patient population from the primary study (Table 1). Median baseline seizure frequency per 28 days was 2.8 (mean = 18.1; Table 1). Most patients (88% [211/240]) were taking two or three ASMs prior to receiving the first dose of cenobamate.
TABLE 1.
Patient demographic and clinical characteristics
| Characteristic | All cenobamate, N = 240 | Patients from primary safety study population not included in post hoc analysis, N = 1100 a |
|---|---|---|
| Age at screening, years | ||
| Mean (SD) | 41.8 (14.63) | 39.3 (12.4) |
| Median | 40.5 | 38.0 |
| Minimum, maximum | 18, 70 | 18, 72 |
| Sex, n (%) | ||
| Male | 135 (56.3) | 538 (48.9) |
| Female | 105 (43.8) | 561 (51.1) |
| Race, n (%) | ||
| White | 203 (84.6) | 873 (79.4) |
| Black or African American | 21 (8.8) | 25 (2.3) |
| Hispanic | 12 (5.0) | 127 (11.5) |
| Asian | 4 (1.7) | 75 (6.8) |
| Seizure frequency at screening/28 days | ||
| Mean (SD) | 18.1 (64.17) | — |
| Median (minimum, maximum) | 2.8 (0.2, 562.3) | — |
| Concomitant ASMs at baseline in ≥10% of either group, n (%) | ||
| Lacosamide | 98 (40.8) | 199 (18.1) |
| Levetiracetam | 89 (37.1) | 397 (36.1) |
| Lamotrigine | 66 (27.5) | 344 (31.3) |
| Clobazam | 38 (15.8) | 121 (11.0) |
| Zonisamide | 39 (16.3) | 73 (6.6) |
| Carbamazepine | 24 (10.0) | 300 (27.3) |
| Valproate | 21 (8.8) | 366 (33.3) |
| Oxcarbazepine | 20 (8.3) | 128 (11.6) |
Abbreviation: ASM, antiseizure medication.
Primary safety study population as of July 2019 was N = 1340. Two‐hundred forty patients were included in the post hoc analysis, leaving 1100 patients who were not included.
3.2. Efficacy
The median duration of exposure for the 240 patients in the post hoc open‐label study was 30.2 months (range = 0.10–43 months). Of the 240 patients, 177 (73.8%) were still taking cenobamate as of the data cutoff visit on or after September 1, 2019 (median duration of exposure = 32.9 months [range = 22.1–43 months]). Among all 240 patients, the ≥50% responder rate for the total treatment duration was 71.7% (172/240). Concomitant ASMs did not appear to relate to response (data are presented in a companion paper by Rosenfeld et al. 2021). 14
3.2.1. Seizure reduction during titration
The ≥50% responder rates during the titration phase in 4‐week intervals with cenobamate doses up to 200 mg/day are shown in Figure 1. Among all patients with available seizure data, 48.1% (114/237) achieved a ≥50% seizure reduction during the first 4 weeks of titration with doses of 12.5–25 mg/day (Figure 1). Additional patients achieved ≥50% seizure reduction at each additional 4‐week interval during titration doses of 50–100 mg/day and 150–200 mg/day (Figure 1); hence, most who benefited responded early in the course of titration.
FIGURE 1.
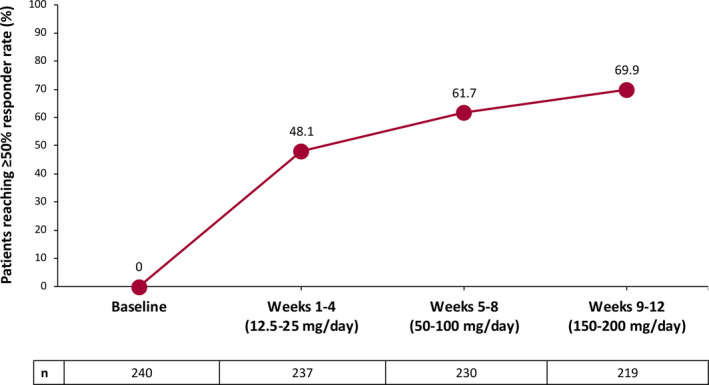
Responder rates of ≥50% during titration for all patients (n = 240)
3.2.2. Seizure reduction during maintenance treatment
Unlike the double‐blind studies with maintenance treatment durations of 6 and 12 weeks (Table S1), the median maintenance treatment duration for all patients in this post hoc analysis was 29.5 months. Among all patients who entered the maintenance phase (n = 214), the ≥50%, ≥75%, ≥90%, and 100% responder rates over the course of their entire maintenance phase duration were 75.7% (162/214), 57.5% (123/214), 40.2% (86/214), and 13.1% (28/214; Figure 2). Hence, 13.1% of all patients who entered the maintenance phase completely stopped having seizures for the entire duration they received maintenance treatment (median duration = 29.5 months, range = 0.8–40.2 months), and 40.2% achieved ≥90% seizure reduction during their entire duration of maintenance treatment. Among all 240 patients, 58.3% (140/240) were on doses greater than 200 mg/day at their last visit, not including visits related to taper and discontinuation (Table 2); 26.3% (63/240) were on 400 mg/day at their last visit.
FIGURE 2.
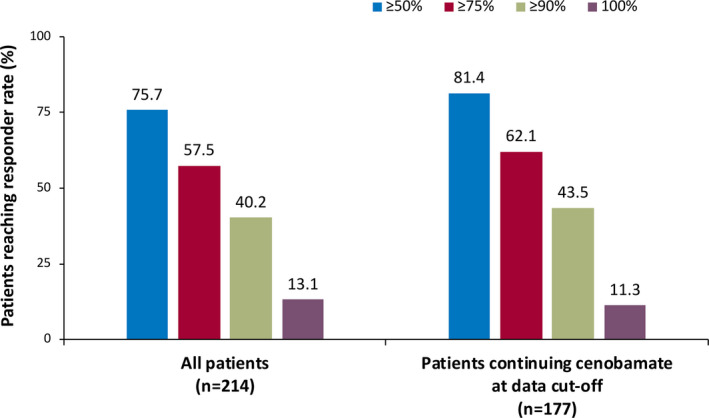
Responder rates during the entire maintenance phase among all patients (maintenance population, n = 214) and patients continuing cenobamate at data cutoff (n = 177). The median treatment duration for all patients in the maintenance population was 29.5 months. The median treatment duration for patients continuing cenobamate at data cutoff was 30.2 months
TABLE 2.
Dose at last visit among all patients, at data cutoff for patients continuing cenobamate, and at start of drug taper for patients discontinuing cenobamate
| All cenobamate, N = 240, n (%) a | Continuing cenobamate at data cutoff, N = 177, n (%) | Patients who discontinued cenobamate, N = 63, n (%) | |
|---|---|---|---|
| 12.5 mg/day | 2 (0.8) | 0 (0) | 2 (3.2) |
| 25 mg/day | 6 (2.5) | 0 (0) | 6 (9.5) |
| 50 mg/day | 11 (4.6) | 2 (1.1) | 9 (14.3) |
| 100 mg/day | 9 (3.8) | 6 (3.4) | 3 (4.8) |
| 150 mg/day | 17 (7.1) | 13 (7.3) | 4 (6.3) |
| 200 mg/day | 55 (22.9) | 41 (23.2) | 14 (22.2) |
| 250 mg/day | 23 (9.6) | 16 (9.0) | 7 (11.1) |
| 300 mg/day | 31 (12.9) | 28 (15.8) | 3 (4.8) |
| 350 mg/day | 23 (9.6) | 19 (10.7) | 4 (6.3) |
| 400 mg/day | 63 (26.3) | 52 (29.4) | 11 (17.5) |
For all cenobamate patients (N = 240), dose at the last visit did not include visits for drug taper/discontinuation.
Of the 177 patients taking cenobamate at data cutoff, the ≥50%, ≥75%, ≥90%, and 100% responder rates for the entire maintenance phase were 81.4% (144/177), 62.1% (110/177), 43.5% (77/177), and 11.3% (20/177), respectively (Figure 2). At data cutoff, 65.0% (115/177) of patients continuing cenobamate were on doses greater than 200 mg/day (Table 2); 29.4% (52/177) were on 400 mg/day at data cutoff.
Responder rates calculated over consecutive 6‐month intervals during the maintenance phase were higher during Months 27–33 as compared to Months 3–9 (Figure S2). The percentage of patients achieving 100% seizure reduction increased from 24.8% (53/214) to 43.7% (66/151). The percentage of patients achieving ≥90% seizure reduction increased from 35.0% (75/214) to 58.9% (89/151). The ≥75% responder rate increased from 54.2% (116/214) to 71.5% (108/151), and the ≥50% responder rate increased from 71.5% (153/214) to 83.4% (126/151). Among all patients, the median (minimum, maximum) dose at the beginning of the maintenance phase (starting at Week 13 [end of Month 3]) was 200 mg/day (50, 250 mg/day) and increased to 300 mg/day (50, 400 mg/day) at Month 33.
Efficacy, as shown by median percent seizure reduction during the entire study and responder rates during the maintenance phase (Figure 3A,B), was observed within each assessed focal seizure subtype (focal aware motor, focal impaired awareness, and FBTC). FBTC seizures were reduced by a median of 89.5%. Among patients with FBTC seizures who entered the maintenance phase, 35.4% (17/48) achieved 100% FBTC seizure reduction during their entire duration of maintenance treatment.
FIGURE 3.
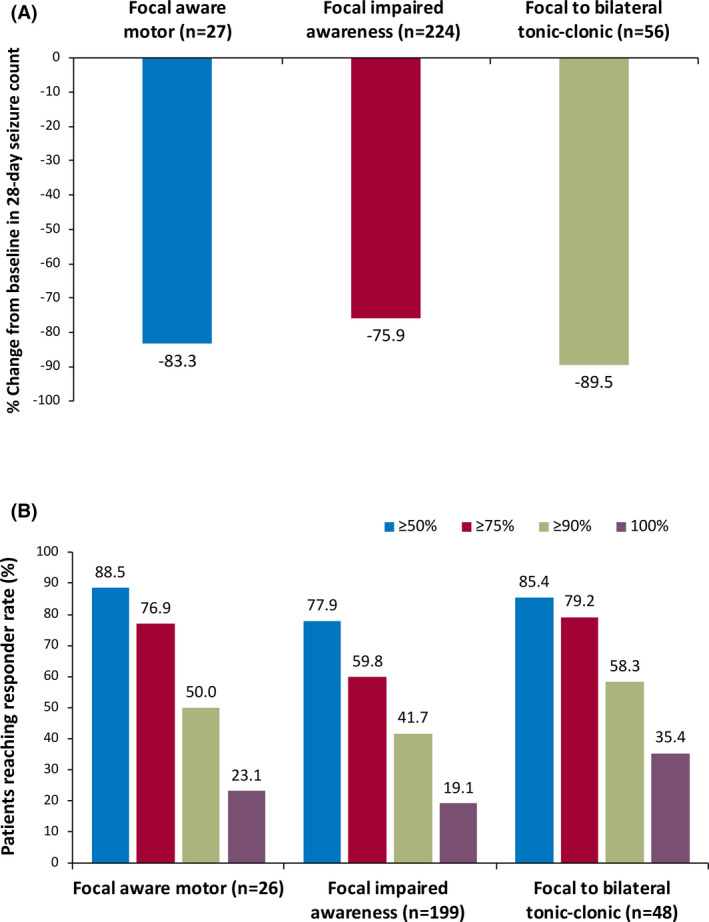
(A) Median percent reduction by focal seizure subtypes (n = 240) and (B) responder rates by focal seizure subtypes during the entire maintenance phase among all patients (maintenance population, n = 214). The median treatment duration for all patients in the maintenance population was 29.5 months
3.2.3. One hundred percent seizure reduction duration
For all 240 patients, 25.8% (62/240) experienced 100% seizure reduction for ≥12 months at their last visit (Figure 4A); mean duration of 100% seizure reduction for these 62 patients was 23.5 months (range = 11.6–40.1 months). Any consecutive ≥12‐month duration of 100% seizure reduction was observed in 36.3% (87/240) of all patients.
FIGURE 4.
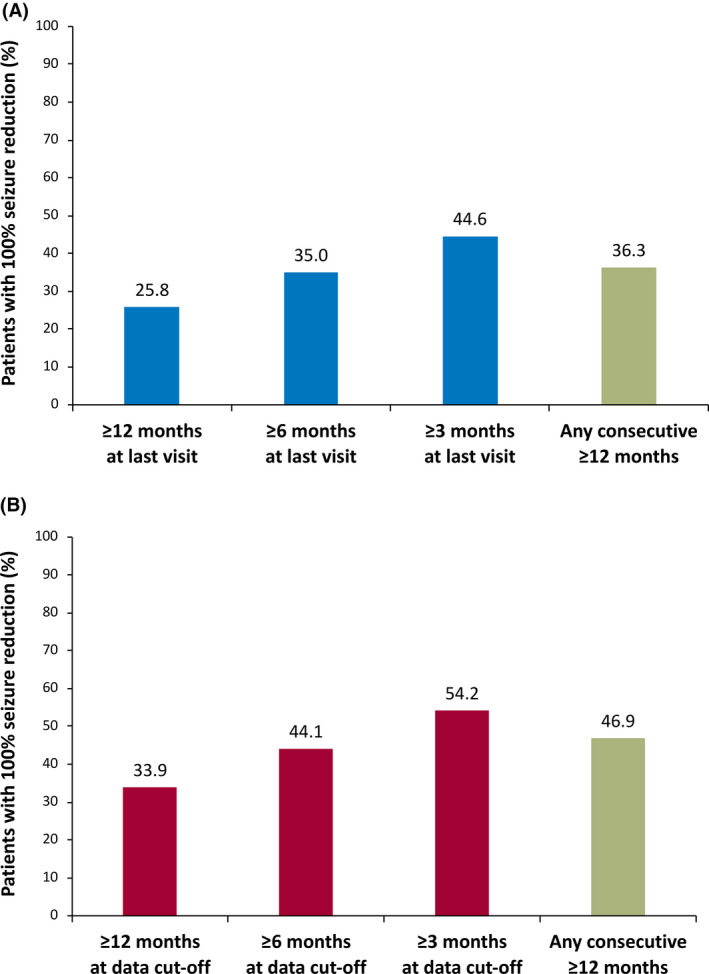
One hundred percent seizure reduction among (A) all patients (n = 240) and (B) patients continuing cenobamate at data cutoff (n = 177)
Of the 177 patients continuing cenobamate treatment at data cutoff, 33.9% (60/177) had 100% seizure reduction for ≥12 months at data cutoff (Figure 4B). For these 60 patients, the median dose of cenobamate was 208.3 mg/day (mean dose = 236 mg/day). Any consecutive ≥12‐month duration of 100% seizure reduction occurred in 46.9% (83/177) of patients continuing cenobamate.
3.3. Safety
The most common treatment‐emergent AEs were CNS‐related (fatigue [34.6%], dizziness [32.1%], and somnolence [29.6%]; Table 3). Serious treatment‐emergent AEs were reported in 50 of 240 patients (20.8%). Seizure was the only serious treatment‐emergent AE reported in ≥2% of all patients (n = 7, 2.9%). Three deaths, previously reported in the primary study population, 12 occurred (sudden death with no autopsy, traumatic intracranial hemorrhage after a fall, and respiratory failure in a patient with Angelman syndrome). Two of the deaths were considered unrelated to cenobamate treatment; the sudden death was considered remotely related to the study drug by the investigator. No cases of drug rash with eosinophilia and systemic symptoms (DRESS) were reported. Further details about treatment‐emergent AEs and concomitant ASMs are provided in a companion paper. 14
TABLE 3.
Summary of TEAEs
| All cenobamate, N = 240, n (%) | Continuing cenobamate at data cutoff, N = 177, n (%) | |
|---|---|---|
| Patients with ≥1 TEAE | 236 (98.3) | 175 (98.9) |
| Serious TEAEs | 50 (20.8) | 36 (20.3) |
| TEAEs in ≥10% of patients | ||
| Fatigue | 83 (34.6) | 67 (37.9) |
| Dizziness | 77 (32.1) | 59 (33.3) |
| Somnolence | 71 (29.6) | 53 (29.9) |
| Upper respiratory tract infection | 38 (15.8) | 34 (19.2) |
| Balance disorder | 37 (15.4) | 29 (16.4) |
| Headache | 36 (15.0) | 29 (16.4) |
| Nausea | 28 (11.7) | 24 (13.6) |
| Fall | 25 (10.4) | 18 (10.2) |
| Weight decreased | 24 (10.0) | 18 (10.2) |
Abbreviation: TEAE, treatment‐emergent adverse event.
4. DISCUSSION
This post hoc analysis of a subset of patients from the open‐label safety study demonstrates durable and sustained improvement in seizure control in adults treated with cenobamate. Moreover, high rates of sustained 100% seizure frequency reduction (≥12 months) were achieved, which is a striking response. Approximately 36% of all patients attained 100% seizure reduction for a ≥12‐month period. Robust seizure reduction was also noted for all assessed focal seizure types, including FBTC seizures, the seizure type most associated with morbidity and mortality. Retention was high for this cohort, with 73.8% remaining on drug throughout the analysis period (median = 2.7 years), further supporting long‐term, sustained efficacy and tolerability. Hence, this long‐term assessment provides useful insights about cenobamate and may prove helpful when treating patients in clinical practice.
Rates of seizure freedom for treatment durations over 12 months are not frequently reported for other ASMs, which, along with methodological differences, makes it difficult to compare these rates with other studies. In various open‐label, long‐term follow‐up extensions of clinical studies of newer adjunctive ASMs, sustained rates of 100% seizure reduction, when reported, are generally low (rates up to 13% for any 12‐month period and 5.6% for any 24‐month period). 15 , 16 , 17 , 18
The implications of seizure freedom, elimination of FBTC seizures, and marked reduction in seizure frequency should not be underestimated. Uncontrolled seizures, particularly tonic–clonic seizures, are associated with increased risk of death, including sudden unexpected death in epilepsy. 19 Patients with uncontrolled seizures are also subject to significant risks for injury. 7 By markedly reducing or abolishing the rate at which seizures occur, particularly tonic–clonic seizures, one can reasonably expect a reduction in risk of death and injury. This has been demonstrated to occur after successful epilepsy surgery, and it is likely that successful medical therapy reduces these risks as well. 20 Sustained improvements in seizure reduction among responders to drug therapy are likely to be associated with measurable improvements in other meaningful outcomes. Driving a motor vehicle, for instance, particularly in the United States, is a goal of many patients with epilepsy. Lengthy periods of 100% seizure reduction in this study, as indicated as of the data cutoff (median duration = ~2 years) for many patients (33.9%), may make this possible for some patients.
In this post hoc analysis of a subset of patients from the open‐label Phase 3 study, 40.2% of patients achieved sustained ≥90% reduction in seizure frequency during the maintenance treatment phase. Although long‐term ≥90% responder rates are not commonly reported in the literature, the high rate achieved with cenobamate may translate well into clinical practice, where patient factors such as intercurrent infection, adherence, and other seizure‐provoking factors may make 100% seizure control difficult to attain.
This study is also the first analysis to demonstrate efficacy with cenobamate using the FDA‐approved titration regimen. Using a start‐low, go‐slow cenobamate titration schedule, patients started to have seizure reduction within the first 4 weeks of titration at doses of 12.5–25 mg/day, with further reduction observed in the following 4‐week intervals through Week 12. Of note, steady‐state concentrations of cenobamate are attained after approximately 2 weeks of once‐daily dosing, 21 meaning that patients starting cenobamate likely only reached steady state by the end of the 2‐week, 12.5‐mg titration step. These data suggest that, despite cenobamate's 12‐week titration regimen, many patients respond during the first 4–8 weeks of titration, providing time to safely titrate patients to the desired maintenance dose. A separate analysis conducted by the FDA projected that the C021 titration regimen would be expected to produce half the maximum effect by Week 5 of titration and a percent seizure frequency reduction of ~40% at 7–8 weeks of titration (at a dose of 100 mg/day). In this retrospective analysis of a small subset of the C021 study patients, 61.7% of the included patients achieved an ≥50% reduction during Weeks 5–8 with doses of 50–100 mg/day. Based on the early efficacy results, patients may tolerate reduction in their concomitant ASMs during the titration schedule at low doses of cenobamate should a tolerability or interaction issue develop. Additional analyses of the C021 data, reported separately, 14 will help guide such dose adjustments for concomitant ASMs during cenobamate titration. Patients with less early seizure reduction may also respond over time, given that additional seizure reduction, including 100% and ≥90% seizure reduction, occurred with continued increases beyond the early doses. During the maintenance treatment phase, most patients’ doses ranged from 200 to 300 mg daily. However, some patients who displayed either improvement or 100% seizure reduction received as much as 400 mg daily.
No new safety signals were detected with long‐term cenobamate treatment of up to 43 months in this subset of patients from the open‐label safety study. The AE profile during long‐term cenobamate treatment in this subset of patients was similar to the AE profile previously reported in the primary C021 study, 12 with most treatment‐emergent AEs CNS‐related (fatigue, dizziness, somnolence). No cases of DRESS were reported.
These findings must be interpreted in the context of the study limitations. First, the C021 study was open‐label and was not designed to assess efficacy. The retrospective analysis included a population selected from a subset of experienced clinical sites, which may reflect a selection bias, although this cohort generally resembled the remaining C021 sample and 88% of the patient cohort were receiving two or three ASMs before starting cenobamate. The open‐label study design allowed clinicians to make changes to cenobamate dose and concomitant ASMs, which better reflects clinical practice. Assessment of seizure frequency during the titration period using 4‐week intervals is a limitation, particularly as the C021 study had less stringent inclusion criteria for baseline seizure severity than the double‐blind clinical studies. However, the higher rates of ≥50% seizure reduction occurring later in titration, during doses of 50–200 mg/day, support the overall pattern of early efficacy. An early response to cenobamate was also noted in the Phase 2 clinical studies using 4‐week intervals, 9 , 10 which corresponds to our observation. Further examination of responses among patients in C021 by baseline seizure frequency is ongoing. Although signs of efficacy may occur early in titration, patients may need to reach doses of 200–300 mg/day, or as high as 400 mg/day, to achieve optimal response.
5. CONCLUSIONS
High rates of retention and sustained reduction in seizure frequency were observed in this post hoc analysis of a subset of patients from the open‐label Phase 3 study of cenobamate, extending the findings from the Phase 2 studies, with a significant proportion of patients experiencing ≥90% and 100% reduction in seizure frequency for prolonged periods. In addition, many patients responded relatively early during the 12‐week titration phase using the “start low, go slow” titration regimen. Most treatment‐emergent AEs were CNS‐related, similar to the primary study. Together, these findings suggest that cenobamate is safe and effective during long‐term treatment.
CONFLICT OF INTEREST
M.R.S.: Consultant/advisor: Medtronic, Neurelis; speaker: Eisai, International Medical Press, Medscape, NeurologyLive, Projects in Knowledge, UCB Pharma; research support: Cavion, Cerevel, Eisai, Engage, Medtronic, Neurelis, SK Life Science, Takeda, UCB Pharma, Xenon; royalty: Oxford University Press. B.A.‐K.: Research support: Otsuka, SK Life Science, UCB Pharma, Xenon. S.A.: Consultant/advisor: Eisai, SK Life Science; speaker: Eisai, Sunovion. P.B.: Research support: SK Life Science. V.B.: Research support: SK Life Science. P.K.: Consultant/advisor: Abbott, Aquestive, Arvelle, Eisai, Engage, Neurelis, SK Life Science, UCB Pharma; speaker: Aquestive, Eisai, Neurelis, Sunovion, UCB Pharma; research support: Eisai, Lundbeck; member, medical advisory board for Alliance‐Stratus, scientific advisory board for OB Pharma; CEO: PrevEp. G.L.K.: Consultant/advisor: Adamas, Eisai, Otsuka, Shire; research support: Biogen, SK Life Science, UCB Pharma, Upsher‐Smith. D.G.V.: Consultant/advisor: Otsuka, SK Life Science; speaker: Greenwich Biosciences, Neurelis, SK Life Science, UCB Pharma; research support: Biogen, Eisai, SK Life Science, UCB Pharma, Xenon. R.W.: Consultant/advisor: Brain Sentinel, Eisai, Engage, Greenwich Biosciences, Lundbeck, SK Life Science, Sunovion, UCB Pharma; speaker: Aquestive, Eisai, Greenwich Biosciences, LivaNova, Sunovion, UCB Pharma; research support: Aquestive, Biogen, Eisai, Engage, Greenwich Biosciences, Lundbeck, Pfizer, SK Life Science, Sunovion, UCB Pharma, Xenon, Zogenix. L.F., M.G.: Employees, SK Life Science. W.E.R.: Consultant/advisor: SK Life Science; speaker: Eisai, Greenwich Biosciences (GW Pharmaceuticals), SK Life Science, Sunovion, UCB Pharma; research support: Greenwich Biosciences, Marinus, Medtronic, Neurelis, Ovid, SK Life Science, Takeda, UCB Pharma, Upsher‐Smith. The authors confirm that they have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
PREVIOUS PRESENTATION
Presented at the American Epilepsy Society Virtual Annual Meeting, December 4–8, 2020, Poster 340, and encored at the American Academy of Neurology Virtual Annual Meeting 2021.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by SK Life Science. Medical writing and editorial assistance were provided by Sarah Mizne, PharmD, and Don Fallon, ELS, of MedVal Scientific Information Services, and were funded by SK Life Science. This article was prepared according to the International Society for Medical Publication Professionals’ Good Publication Practice for Communicating Company‐Sponsored Medical Research: GPP3.
Sperling MR, Abou‐Khalil B, Aboumatar S, Bhatia P, Biton V, Klein P, et al. Efficacy of cenobamate for uncontrolled focal seizures: Post hoc analysis of a Phase 3, multicenter, open‐label study. Epilepsia. 2021;62:3005–3015. 10.1111/epi.17091
DATA AVAILABILITY STATEMENT
The data for the analyses described in this article are available by request from the corresponding author or SK Life Science, the company sponsoring the clinical development of cenobamate for the treatment of focal epilepsy.
REFERENCES
- 1. Perucca P, Scheffer IE, Kiley M. The management of epilepsy in children and adults. Med J Aust. 2018;208(5):226–33. [DOI] [PubMed] [Google Scholar]
- 2. Golyala A, Kwan P. Drug development for refractory epilepsy: the past 25 years and beyond. Seizure. 2017;44:147–56. [DOI] [PubMed] [Google Scholar]
- 3. Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30‐year longitudinal cohort study. JAMA Neurol. 2018;75(3):279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tian N, Boring M, Kobau R, Zack MM, Croft JB. Active epilepsy and seizure control in adults—United States, 2013 and 2015. MMWR Morb Mortal Wkly Rep. 2018;67(15):437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nevalainen O, Ansakorpi H, Simola M, Raitanen J, Isojarvi J, Artama M, et al. Epilepsy‐related clinical characteristics and mortality: a systematic review and meta‐analysis. Neurology. 2014;83(21):1968–77. [DOI] [PubMed] [Google Scholar]
- 6. Laxer KD, Trinka E, Hirsch LJ, Cendes F, Langfitt J, Delanty N, et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014;37:59–70. [DOI] [PubMed] [Google Scholar]
- 7. Lawn ND, Bamlet WR, Radhakrishnan K, O'Brien PC, So EL. Injuries due to seizures in persons with epilepsy: a population‐based study. Neurology. 2004;63(9):1565–70. [DOI] [PubMed] [Google Scholar]
- 8. Thurman DJ, Logroscino G, Beghi E, Hauser WA, Hesdorffer DC, Newton CR, et al. The burden of premature mortality of epilepsy in high‐income countries: a systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia. 2017;58(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chung SS, French JA, Kowalski J, Krauss GL, Lee SK, Maciejowski M, et al. Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology. 2020;94(22):e2311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krauss GL, Klein P, Brandt C, Lee SK, Milanov I, Milovanovic M, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double‐blind, randomised, placebo‐controlled, dose‐response trial. Lancet Neurol. 2020;19(1):38–48. [DOI] [PubMed] [Google Scholar]
- 11. Mohanraj R, Brodie MJ. Measuring the efficacy of antiepileptic drugs. Seizure. 2003;12(7):413–43. [DOI] [PubMed] [Google Scholar]
- 12. Sperling MR, Klein P, Aboumatar S, Gelfand M, Halford JJ, Krauss GL, et al. Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open‐label safety study. Epilepsia. 2020;61(6):1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenfeld WE, Abou‐Khalil B, Aboumatar S, Bhatia P, Biton V, Krauss GL, et al. Post‐hoc analysis of a Phase 3, multicenter, open‐label study of cenobamate for treatment of uncontrolled focal seizures: Effects of dose adjustments of concomitant antiseizure medications. Epilepsia. 2021. 10.1111/epi.17092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Toledo M, Whitesides J, Schiemann J, Johnson ME, Eckhardt K, McDonough B, et al. Safety, tolerability, and seizure control during long‐term treatment with adjunctive brivaracetam for partial‐onset seizures. Epilepsia. 2016;57(7):1139–51. [DOI] [PubMed] [Google Scholar]
- 16. Husain A, Chung S, Faught E, Isojarvi J, McShea C, Doty P. Long‐term safety and efficacy in patients with uncontrolled partial‐onset seizures treated with adjunctive lacosamide: results from a phase III open‐label extension trial. Epilepsia. 2012;53(3):521–8. [DOI] [PubMed] [Google Scholar]
- 17. Krauss GL, Perucca E, Kwan P, Ben‐Menachem E, Wang X‐F, Shih JJ, et al. Final safety, tolerability, and seizure outcomes in patients with focal epilepsy treated with adjunctive perampanel for up to 4 years in an open‐label extension of phase III randomized trials: study 307. Epilepsia. 2018;59(4):866–76. [DOI] [PubMed] [Google Scholar]
- 18. Inoue Y, Liao W, Wang X, Du X, Tennigkeit F, Sasamoto H, et al. Safety and efficacy of adjunctive lacosamide in Chinese and Japanese adults with epilepsy and focal seizures: a long‐term, open‐label extension of a randomized, controlled trial. Epilepsy Res. 2021;176:106705. [DOI] [PubMed] [Google Scholar]
- 19. Sveinsson O, Andersson T, Mattsson P, Carlsson S, Tomson T. Clinical risk factors in SUDEP: a nationwide population‐based case‐control study. Neurology. 2020;94(4):e419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sperling MR, Barshow S, Nei M, Asadi‐Pooya AA. A reappraisal of mortality after epilepsy surgery. Neurology. 2016;86(21):1938–44. [DOI] [PubMed] [Google Scholar]
- 21. XCOPRI® (cenobamate tablets), for oral use, CV [prescribing information]. Paramus, NJ: SK Life Science; 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data for the analyses described in this article are available by request from the corresponding author or SK Life Science, the company sponsoring the clinical development of cenobamate for the treatment of focal epilepsy.


