Abstract
The sediment–water interface of spiked‐sediment toxicity tests is a complex exposure system, where multiple uptake pathways exist for benthic organisms. The freely dissolved concentration (C free) in sediment porewater has been proposed as a relevant exposure metric to hydrophobic organic contaminants (HOCs) in this system. However, C free has rarely been measured in spiked‐sediment toxicity tests. We first developed a direct immersion solid‐phase microextraction method for measuring C free in overlying water and porewater in a sediment test using polydimethylsiloxane‐coated glass fibers, resulting in sensitive and repeatable in situ measurements of HOCs. Then, we measured C free and total dissolved concentrations (C diss) in the sediment test systems with the freshwater amphipod Hyalella azteca and thoroughly evaluated the temporal and spatial profiles of four HOCs (phenanthrene, pyrene, benzo[a]pyrene, and chlorpyrifos). Furthermore, we examined the relationship between the measured concentrations and the lethality of H. azteca. We found that the test system was far from an equilibrium state for all four chemicals tested, where C diss in overlying water changed over the test duration and a vertical C free gradient existed at the sediment–water interface. In porewater C diss was larger than C free by a factor of 170 to 220 for benzo[a]pyrene because of the strong binding to dissolved organic carbon. Comparison of the median lethal concentrations of chlorpyrifos in the sediment test and those in water‐only tests indicates that C free in porewater was the most representative indicator for toxicity of this chemical. The method and findings presented in the present study warrant further research on the chemical transport mechanisms and the actual exposure in sediment tests using different chemicals, sediments, and test species. Environ Toxicol Chem 2021;40:3148–3158. © 2021 The Authors. Environmental Toxicology and Chemistry published by Wiley Periodicals LLC on behalf of SETAC.
Keywords: Freely dissolved concentration, Spiked‐sediment toxicity test, Amphipod, Solid‐phase microextraction
INTRODUCTION
Spiked‐sediment toxicity tests are widely used methods for assessing the ecological risk of chemicals present in sediment. Tests are conducted by exposing benthic organisms to field sediment or artificial sediment spiked with a test chemical. This approach provides direct exposure‐effect relationships for a single organism and the test chemical and eliminates uncertainties caused by the presence of other species and contaminants (Nowell et al., 2016). Also, standardized test methods are available for several benthic organisms including amphipods, midges, polychaetes, and oligochaetes (ASTM International, 2019; Organisation for Economic Co‐operation and Development, 2004, 2007, 2010; US Environmental Protection Agency, 1994, 2000). Toxicity data of spiked‐sediment tests have been widely used to derive sediment quality guidelines (Nowell et al., 2016; Simpson et al., 2005).
Over the last decades, observed toxicity of hydrophobic organic contaminants (HOCs) in spiked‐sediment toxicity tests was usually linked to the nominal (C nom) or measured sediment (C sed) concentration, which resulted in a large variability in observed toxicities between test conditions, following differences in bioavailability (Di Toro et al., 1991). The freely dissolved concentration (C free) is generally considered a suitable metric for the bioavailable fraction of the contaminant in sediment (Lydy et al., 2014) and should normalize variabilities in chemical bioavailability between test conditions. It can consider aggregated exposure from water, sediment particles, and dissolved organic carbon (DOC) if the biota–sediment–water partition equilibrium is established (Di Toro et al., 1991). Toxicity as expressed with C free in spiked‐sediment toxicity tests can be linked to C free measured in environmental sediments for risk‐assessment purposes, emphasizing the importance of accurate quantification of C free in sediment toxicity tests.
The standard approach of quantifying C free of HOCs in spiked‐sediment toxicity tests is estimation based on the nominal or measured chemical concentration in the sediment and the chemical's organic carbon–water partitioning coefficient (K OC; Ankley et al., 1994; Di Toro et al., 1991; US Environmental Protection Agency, 2012). Alternatively, equilibrium passive sampling with solid‐phase microextraction (SPME) fibers was applied to measure C free using separated porewater or an aliquot of spiked sediment after toxicity testing (Ding et al., 2013; Harwood et al., 2013; Jonker et al., 2018; Xu et al., 2007; You et al., 2006). Surprisingly, however, direct immersion of SPME fibers in the sediment and overlying water within the sediment toxicity test system has not been applied yet, although it should provide an indisputable measurement of the free concentration at a particular sampling location. Direct immersion SPME is now an established method to measure porewater C free in contaminated soils and sediments (Mayer et al., 2000; Ter Laak et al., 2006).
While C free in sediment pores is increasingly accepted as a useful exposure metric, the actual sediment–water test systems are highly complex and offer multiple exposure pathways to benthic organisms. For example, a continuous or intermittent water exchange with uncontaminated water is often applied, which could cause a temporal change in the exposure concentration in sediment toxicity tests. Moreover, epibenthic organisms can mainly be present on the sediment surface or swimming in the overlying water instead of burying into the sediment and more often exposed to corresponding chemical concentrations (Chapman et al., 2002). Furthermore, previous studies indicated that the bound fraction significantly increases the uptake by organisms from what would be expected from the freely dissolved fraction alone (Lin et al., 2018), potentially resulting in a higher observed toxicity in the presence of DOC in the water (Fischer et al., 2016). Thus, in addition to C free in porewater, knowing the temporal and spatial variation of chemical concentrations in both free and bound states within the sediment toxicity test system should be beneficial.
The present study examined the distribution and state of HOCs in the sediment toxicity test system by measuring various types of concentrations including the total dissolved concentration (C diss = C free + concentration bound to DOC) and C free in porewater and overlying water. A particular emphasis was placed on the measurement of C free using direct immersion of SPME fibers. As a model sediment toxicity test system, a standardized semi‐flow‐through test was performed using formulated sediment spiked with HOCs. The HOCs considered in the present study were phenanthrene (Phe; log octanol–water partition coefficient [K OW] 4.4), pyrene (Pyr; log K OW 4.9), benzo[a]pyrene (BaP; log K OW 6.1), and chlorpyrifos (CPS; log K OW 5.1), covering a wide range of hydrophobicity. We first evaluated a direct immersion SPME method in terms of equilibrium time, repeatability, and sensitivity. Toxicity tests were then performed with the freshwater amphipod Hyalella azteca to discuss the relevance of each concentration type for toxicity.
MATERIALS AND METHODS
Study design
We performed four experimental runs with the semi‐flow‐through toxicity test system, each run with different purposes (Table 1). All experiments were conducted using formulated sediment spiked with HOCs. Moreover, in all runs, polydimethylsiloxane (PDMS)–coated fibers were added to the experimental systems to measure C free, and overlying water was sampled and filtered to measure C diss over time. In addition, in Run 1, we evaluated the attainment of PDMS fiber/water equilibrium for three polycyclic aromatic hydrocarbons (PAHs; i.e., Phe, Pyr, and BaP) with different hydrophobicity in porewater and overlying water by following the concentrations in PDMS over time. Next, we measured temporal and spatial concentration profiles of the three PAHs in sediment toxicity tests in more detail (Run 2). These two runs were performed without addition of amphipods and food. Finally, to relate various types of HOC concentrations to lethality of H. azteca, we performed sediment toxicity tests of BaP (Run 3) and CPS (Run 4), which were selected for toxicity tests as model chemicals with high hydrophobicity and high toxicity, respectively. Experimental setups are summarized in Table 1 and explained in more detail in the following sections.
Table 1.
Experimental overview of the present study
| Tested chemicals | Spiked concentrations (mg/kg‐dry) | Organisms | Data acquired | |||
|---|---|---|---|---|---|---|
| Common to all runsa | Time points for C diss,over (days) | Specific to particular run | ||||
| Run 1 | Phe, Pyr, BaP | 5 and 50 | No |
C sed, C free,pore, C free,over, C diss,pore, C diss,over DOC, pH, DO, temperature, and conductivity |
0, 1, 2, 5, 7, and 10 | –Time‐series concentrations in PDMS fiber (C PDMS) |
| Run 2 | Phe, Pyr, BaP | 5 and 50 | No | 0, 2, 4, 6, 8, and 9 |
–C diss,over in top and bottom layers of overlying water –C free at the sediment–water interface (i.e., C free,intf) –Concentrations in filtered (i.e., C diss,over) and unfiltered (i.e., C total,over) overlying water |
|
| Run 3 | BaP | 0 (control), 50, 100, 200, and 400 | Yes | 0, 1, 4, 6, 7, and 10 |
–Toxicity endpoints (lethality, dry wt, body length) –Body concentrationsb –Ammonia concentrations in overlying water |
|
| Run 4 | CPS | 0 (control), 0.01, 0.032, 0.1, 0.32, and 1 | Yes | 0, 1, 3, 7, and 10 | ||
C diss,pore was not measured in Run 1; C free,intf was measured instead of C free,over in Run 2; C free,over was not measured in Run 3; C total,over was measured instead of C diss,over only in Run 4.
Body concentrations were determined only for BaP in Run 3.
Phe = phenanthrene; Pyr = pyrene; BaP = benzo[a]pyrene; CPS = chlorpyrifos; C sed = concentration in spiked sediment; C free,pore = freely dissolved concentration in porewater; C free,over = freely dissolved concentration in overlying water; C free,intf = freely dissolved concentration at the sediment–water interface; C diss,pore = total dissolved concentration in porewater; C diss,over = total dissolved concentration in overlying water; C total,over = concentration in unfiltered overlying water; DOC = dissolved organic carbon; DO = dissolved oxygen; PDMS = polydimethylsiloxane.
Materials and chemicals
Benzo[a]pyrene (>97.0% purity, Chemical Abstracts Service [CAS] no. 50‐32‐8), CPS (>99.0% purity, CAS no. 2921‐88‐2), Phe (>99.0% purity, CAS no. 85‐01‐8), Pyr (>98.0% purity, CAS no. 129‐00‐0), acetone (>99.5% purity), acetonitrile (>99.8% purity), and kaolin (practical grade) were purchased from FujiFilm Wako Pure Chemicals. Chlorpyrifos‐d10 was purchased from Kanto Chemical. Quartz sand (0.2–0.8 mm particle ≥40%, loss on ignition at 900 °C ≤0.05%) was purchased from Merck. Peat was purchased form Midori, which was collected from Hokkaido, Japan. The elemental composition of the peat as determined by an elemental analyzer (Flash EA1112; Thermo Fisher Scientific) was as follows: N 1.5% w/w, C 37.6% w/w, H 4.4% w/w.
Test organisms
Hyalella azteca was obtained from a brood stock which has been maintained for more than 10 years at the National Institute for Environmental Studies, Japan. The stock culture was maintained in aquaria containing dechlorinated tap water and nylon mesh sheets (mesh size 500 μm) at 22 °C under a 16:8‐h of light: dark photoperiod, supplied with continuous aeration, and fed synthesized food (Halios; Feed One) twice a week. The dechlorinated tap water was confirmed to have an alkalinity of approximately 60 mg CaCO3/L, 35 mg Cl/L, and 0.20 mg Br/L, which met the criteria recommended by Ivey and Ingersoll (2016). A reference 96‐h water‐only test with cadmium chloride was performed according to Environment and Climate Change Canada (2017). The median lethal concentration (LC50) was 6.1 μg Cd/L (95% confidence interval [CI] 4.5–7.7, based on nominal concentrations), which fell within two standard deviations (SDs) of the laboratory's historical LC50 of 4.4 ± 1.4 μg Cd/L (mean ± SD), thus indicating acceptable sensitivity of the population.
Spiked‐sediment toxicity test
The tests were performed according to standardized protocols (Environment and Climate Change Canada, 2017; US Environmental Protection Agency, 2000) with slight modifications. Formulated sediment was prepared according to the Organisation for Economic Co‐operation and Development test guideline 218 (2004). Briefly, 140 g of ground peat moss powder (<250 μm) was suspended in 550 ml of deionized water for 2 days under stirring. The pH value was adjusted to 5.5 ± 0.5 and 6.0 ± 0.5 with calcium carbonate at the start and end of stirring, respectively. Defined volumes of acetonic stock solutions containing the test chemical(s) and an additional 50 ml of acetone were added to 100 g quartz sand in a 1‐L brown bottle. The bottle was gently shaken to coat the quartz with the acetone, and the solvent was evaporated to complete dryness under nitrogen. The rest of the quartz sand (75% w/w), peat moss (5% on the basis of dry wt), kaolinite (20% w/w), and dechlorinated tap water (achieving 30% water content w/w) were added to the bottle. The bottle was then shaken on a roller shaker at 6 to 8 °C (Runs 2 and 4) or room temperature (Runs 1 and 3) at 50 to 100 rpm for 4 to 14 days (varied between experiments; Supporting Information, Table S1). Two concentration levels (i.e., 5 and 50 mg/kg‐dry) were prepared for Runs 1 and 2, while the following 4 (Run 3) or 5 (Run 4) levels were prepared for toxicity experiments: 50, 100, 200, and 400 for Run 3 and 0.01, 0.032, 0.1, 0.32, and 1 mg/kg‐dry for Run 4 (Table 1). These concentrations in Runs 3 and 4 were selected based on the results of preliminary range‐finding tests (data not shown). The control sediment without spiking was also prepared in Runs 3 and 4.
Ninety grams (wet wt) of the prepared sediment (equivalent to 55 ml) and 220 ml of dechlorinated tap water were transferred to a 300‐ml tall beaker. Ten replicate beakers were prepared in Run 1, and two beakers each were sacrificed 1, 3, 5, 7, and 10 days after the beginning of water flow to follow the time course of the concentration in PDMS fiber. Four, eight, and eight replicate beakers were prepared in Runs 2, 3, and 4, respectively, and one, two, and two beakers were used to collect porewater by centrifugation at the end of tests (i.e., 11 days after the beginning of water flow). Approximately 10 g of the wet sediment were taken for the measurement of concentration in sediment and the total organic carbon (TOC) content. The beakers were placed below a semi‐flow‐through water renewal system modified from Zumwalt et al. (1994; Figure 1; Supporting Information, Figure S2), which dropped dechlorinated tap water at 880 ml/day to each beaker for a 24‐h pre‐equilibration period to flush out fine powders that were easily suspended in overlying water and that led to high water turbidity, following 440 ml/day during the 10‐day toxicity test. After the 24‐h pre‐equilibration (i.e., at day 0), 10 juvenile amphipods (7–9 days old) were added to each beaker in Runs 3 and 4. Over the test duration, water quality in overlying water was checked (i.e., dissolved oxygen, pH, conductivity, and temperature; Supporting Information, Tables S5 and S9), and amphipods were fed 1.75 ml of yeast–cerophyl–trout chow (Recenttec) and 3.15 mg of synthesized food (Halios) three times per week. Exposure beakers were kept at 22.5 °C under a 16:8‐h light: dark photoperiod. At the start and end of tests (i.e., days 0 and 10), the ammonia concentration in overlying water was measured to ensure that it was below the effective concentration for H. azteca (Environment and Climate Change Canada, 2017). At day 10, the survival of amphipods was judged by eye, and the missing were considered dead. Methods of measurement of growth and bioaccumulation are described in the Supporting Information.
Figure 1.
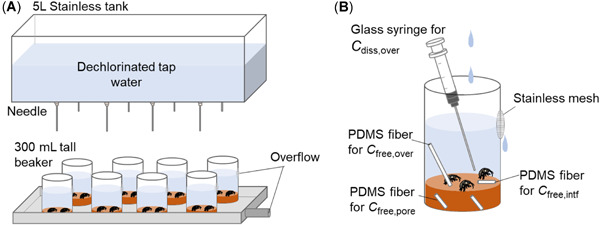
Semi flow‐through sediment toxicity test system with Hyalella azteca. (A) Overview of test system consisting of exposure beakers and a water storage tank equipped with eight needles. (B) Exposure beaker with a hole covered with a stainless‐steel mesh. Devices to measure various types of concentrations (C free,over, C free,pore, C free,intf, and C diss,over) are also shown. C diss,over = total dissolved concentration in overlying water; PDMS = polydimethylsiloxane; C free,over = freely dissolved concentration in overlying water; C free,pore = freely dissolved concentration in porewater; C free,intf = freely dissolved concentration at the sediment–water interface.
Concentration measurement
C free measurement using PDMS fiber
Values of C free,pore and C free,over were measured using a passive sampling method with PDMS‐coated glass fibers. In this method, the concentrations in the PDMS coating phase (C PDMS) were measured and converted to the corresponding freely dissolved concentrations via the equation C free,pore (or C free,over) = C PDMS/K PDMS/w, where K PDMS/w is the PDMS–water partition coefficient. The PDMS fibers (30 µm coating thickness; Polymicro Technologies) were cut into lengths of 3, 4, and 7 cm (PDMS volume of 0.39, 0.53, and 0.92 µl, respectively). Longer PDMS fibers (i.e., 7 cm) were used for C free,over measurement to increase the sensitivity. The fibers were rinsed twice for 15 minutes with excess ethyl acetate and methanol, dried under the fume hood, and stored in Milli‐Q water until use. Log K PDMS/w for Phe, Pyr, and BaP were taken from Muijs and Jonker (2009; 3.73, 4.28, and 5.22, respectively). Log K PDMS/w for CPS was measured (4.42 ± 0.01) by the method described in the Supporting Information. This value is close to that reported previously for a PDMS sheet–water partition coefficient (4.36; Stenzel et al., 2013).
At day −1 of the sediment toxicity tests (i.e., the day when all beakers received the spiked sediment, which is 1 day before amphipods were added to the beakers), 1 to 4 pieces per beaker of 3‐ or 4‐cm PDMS fibers were buried into the sediment (the numbers of fiber pieces are given in Supporting Information, Table S1). At day 0, two pieces of 3‐ or 7‐cm PDMS fibers were placed in the overlying water, standing on the sediment and leaning against the wall diagonally to the sediment (C free,over; Figure 1; Supporting Information, Table S1). At day 10, the PDMS fibers were retrieved from the overlying water and sediment, wiped with moist lint‐free tissues, and transferred to a 1.5‐ml vial, which received the extraction solvent (acetonitrile for Phe, Pyr, and BaP and n‐hexane for CPS; volume varied between experiments). Extracts for Phe, Pyr, and BaP were measured with high‐performance liquid chromatography (HPLC) and those for CPS with gas chromatography–mass spectroscopy (GC/MS). Conditions for HPLC and GC/MS are described in the Supporting Information.
C diss and C total measurement
Over the 10‐day sediment toxicity tests, up to 6 ml of overlying water were repeatedly sampled (Table 1) with a glass syringe and filtered with a glass fiber filter (GB‐140, pore size 0.4 μm; Advantec) to measure C diss,over. The filter was baked in advance in an oven at 550 °C for 1 h. Also, the unfiltered overlying water was collected in Run 2 to measure the total aqueous concentrations (C total = C diss + concentration sorbed to suspended particles). Water samples were not filtered for the C diss,over measurement of CPS in Run 4 because preliminary experiments showed that there was no difference between filtered and unfiltered concentrations for CPS and thus indicated negligible binding of CPS to suspended particles in overlying water (i.e., C total,over = C diss,over). The overlying water samples were taken from 1 to 2 cm above the sediment surface in all runs. In Run 2, water was also collected from just below the water surface for comparison. At day 10, porewater was collected by ultracentrifugation of wet sediment at 10 000 g for 30 min (CR21; Hitachi) followed by filtration with the glass fiber filter. Water samples containing Phe, Pyr, and BaP were diluted 1:1 in acetonitrile; filtered with a poly(tetrafluoroethylene) membrane (pore size 0.45 μm) if not filtered at the time of water sampling; and then analyzed with HPLC. For CPS, the water sample was transferred to a glass tube, liquid–liquid‐extracted with n‐hexane containing CPS‐d10 as a surrogate for GC/MS analysis, and concentrated under N2 gas if necessary. The filtered overlying water and porewater samples were used also for DOC determination with a TOC‐L analyzer (Shimadzu).
C sed measurement
Concentrations in spiked sediment (C sed) were measured using sediment samples collected at days −1 and 10 of the test, following freeze‐drying, addition of CPS‐d10 as surrogate (only for CPS), and extraction with a 1:1 mixture of acetone and n‐hexane in an ultrasonic bath for 15 min. The extracts were analyzed with HPLC for Phe, Pyr, and BaP and with GC/MS for CPS. The ratio of measured to nominal C sed (i.e., recovery ratio) at day −1 was 42 to 78% for PAHs in Run 2 (Supporting Information, Table S3) and 59 to 82% for CPS in Run 4 (Supporting Information, Table S7); however, it was decreased to 33 to 63% and 53 to 64% at day 10 in Runs 2 and 4, respectively. Also, TOC was measured using the freeze‐dried sediment samples with TOC‐L equipped with a solid sample module (SSM‐5000A; Shimadzu), which resulted in 1.8 ± 0.3% at day −1 and 1.4 ± 0.4% at day 10 in Run 3 (Supporting Information, Table S4) ans 2.0 ± 0.3% at day −1 and 1.9 ± 0.3% at day 10 in Run 4 (Supporting Information, Table S8).
Data analysis
All data analyses were performed with R software, Ver 4.0.5 (R Foundation for Statistical Computing, 2021). In situ K OC (liters per kilogram), the partition coefficient between sediment organic carbon and the freely dissolved phase in porewater was calculated as (C sed/TOC)/C free,pore, and K DOC (liters per kilogram) between DOC and the freely dissolved phase in porewater as [(C diss,pore − C free,pore)/DOC]/C free,pore. Lethal concentrations (e.g., LC50) were calculated based on the 2‐parameter log logistic equation (Equation 1) with a binomial error distribution using the drc R package (Ver 3.0‐1 [Ritz et al., 2015]).
| (1) |
In Equation 1, x represents the exposure concentration (i.e., C sed, C free,over, C free,pore, C diss,over, C diss,pore), b denotes the slope of the regression curve, and e is the LC50. For x, the arithmetic mean value of C diss,over over exposure duration was used because data were available at multiple time points. Similarly, the arithmetic mean value of C sed (for Run 4) at the start and end of exposure was used for x. Values for C sed (for Run 3), C free,over, C free,pore, and C diss,pore were only measured at the end of exposure; thus, these values were directly used for x.
RESULTS AND DISCUSSION
Equilibrium time for passive sampling with PDMS fibers
In Run 1, C PDMS of Phe, Pyr, and BaP was measured over time to examine the time to reach partition equilibrium between PDMS fiber and water. In all cases, C PDMS of Phe and Pyr reached the highest values within 5 days (Figure 2; Supporting Information, Figure S3). Partitioning of BaP required a longer time, although there was no statistically significant difference between C PDMS at days 7 and 10 for both porewater and overlying water. These results indicate that PDMS fiber/water partition equilibrium for these chemicals can be established during the 10‐day toxicity test. Because the time needed to reach equilibrium typically increases with increasing passive sampler/water partition coefficients (Doong et al., 2000), other HOCs with log K PDMS/w lower than that of BaP (5.22) are also expected to reach equilibrium in the experimental setting of the present study. Note that C PDMS of Phe and Pyr in Run 1 apparently decreased after 5 days. This result may be related to the decrease of the water phase concentrations that the fibers were exposed to (Supporting Information, Figure S4). Temporal courses of overlying water concentration will be discussed in the section Temporal change in concentrations using more extensive data from Runs 2 through 4.
Figure 2.
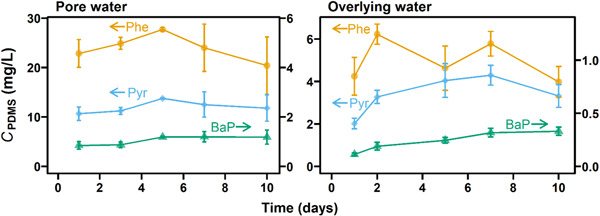
Concentration–time curves for phenanthrene (yellow circles), pyrene (blue diamonds), and benzo[a]pyrene (green triangles) in polydimethylsiloxane fiber (Run 1; nominal sediment concentration, 5 mg/kg‐dry). Error bars represent standard deviations (n = 4–6). Left and right panels represent the data for porewater and overlying water, respectively. Arrows indicate the axes that the data refer to. PDMS = polydimethylsiloxane; Phe = phenanthrene; Pyr = pyrene; BaP = benzo[a]pyrene.
Repeatability and sensitivity of C free measurements
In Runs 2 to 4, C free,pore, C free,over, and/or the freely dissolved concentration at the sediment–water interface (C free,intf) were measured using PDMS fiber (Figure 3). The beaker‐to‐beaker variation was negligible compared to the fiber‐to‐fiber variation in a single beaker; thus, all fiber pieces in the replicate beakers were considered replicate measurements. The C free measurement was variable in Run 2, with the coefficient of variation being 5 to 75% for Phe, Pyr, and BaP (n = 6). The repeatability largely improved in Runs 3 and 4 with 7 to 34% (BaP, n = 15–28) and 3 to 27% (CPS, n = 6–12). The reason for the improvement is unknown but could be related to improved handling of fibers by operators. In all cases, measured C free linearly increased with nominal spiked concentration in sediment. The lowest C free measured for CPS in the present study was as low as 0.001 μg/L, which is well below the level that causes toxic effects on aquatic organisms (Huang et al., 2020). High sensitivity is particularly important for insecticides such as CPS because the toxicity threshold value is often extremely low. The lowest C free values measured for Phe, Pyr, and BaP in the present study were 0.1, 0.2, and 0.01 μg/L, respectively, which corresponded to approximately 0.5, 4, and 2 μg/L in final acetonitrile solution injected to HPLC. Considering the peak intensities of these three chemicals in HPLC measurements, 100 times lower concentrations could also be quantified. We have not attempted measurement at such low concentrations because Phe, Pyr, and BaP cause toxicity to benthic organisms at much higher concentrations. All in all, the direct immersion SPME methods used in the present study have sufficient sensitivity to be applied for spiked‐sediment toxicity tests even for highly toxic chemicals like CPS.
Figure 3.
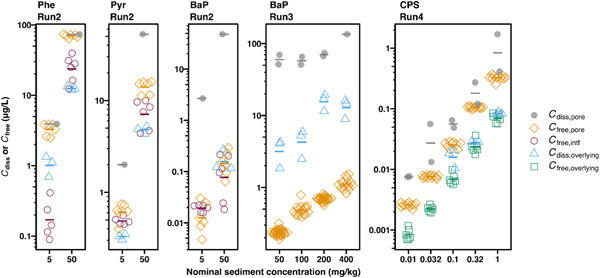
Measured C diss,pore, C diss,over, C free,pore, C free,intf, and C free,over of phenanthrene, pyrene, chlorpyrifos (CPS), and benzo[a]pyrene (BaP). Dots and bars represent measured values in each beaker or of polydimethylsiloxane fiber and the geometric means, respectively. Arithmetic means of C diss,over over 10 days are shown. Values of C diss,over of BaP at 5 mg/kg in Run 2 and of CPS at 0.01 and 0.032 mg/kg in Run 4 were under the detection limit. In Run 3 C free,over of BaP was not measured. Phe = phenanthrene; Pyr = pyrene; C diss = total dissolved concentration; C free = freely dissolved concentration; C diss,pore = total dissolved concentration in porewater; C free,pore = freely dissolved concentration in porewater; C free,intf = freely dissolved concentration at the sediment–water interface; C diss,overlying = total dissolved concentration in overlying water; C free,overlying = freely dissolved concentration in overlying water.
Temporal change in concentrations
In all Runs, C diss,over was measured over time (Figure 4; Supporting Information, Figures S4, S6, S9, and S15). Temporal changes in C diss,over were observed in all cases, showing the maximum concentration from days 0 to 2 and a decreasing trend after day 2. No clear time trend was observed for BaP because of the high variability in C diss,over. The increase of C diss,over from days 0 to 2 is likely due to the decrease in the exchange rate of overlying water from 880 to 440 ml/day at day 0. Thus, the outflow rate decreased by a factor of 2 at day 0, and so did the mass loss due to the outflow, which increased C diss,over. The successive concentration decrease after day 2 confirms that the continuous water renewal decreases C diss,over in the long run. Such temporal changes in C diss,over are expected in other sediment toxicity tests that apply water exchange of uncontaminated water to provide sufficient oxygen and remove excretes (e.g., ammonia; ASTM International, 2019; US Environmental Protection Agency, 2000).
Figure 4.
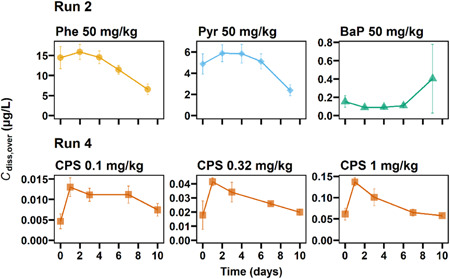
Time‐course changes of total dissolved concentrations of phenanthrene, pyrene, benzo[a]pyrene, and chlorpyrifos in overlying water in Runs 2 and 4. Error bars represent standard deviations (n = 3). Phe = phenanthrene; Pyr = pyrene; BaP = benzo[a]pyrene; CPS = chlorpyrifos; C diss,over = total dissolved concentration in overlying water.
The continuous water renewal discharged test chemicals out of the test beaker, which was reflected by the reduction in C sed during tests. The reduction in C sed during tests was 26 ± 7% for Phe (Run 2), 24 ± 1% for Pyr (Run 2), 13 ± 10% for CPS (Run 4), and 23 ± 7% for BaP (Run 2).
Vertical concentration profile in sediment toxicity test system
In Run 1, we found that C free,pore > C free,over for all three chemicals tested. This indicates that there is a vertical concentration gradient in the sediment–water test system. More detailed depth‐related analysis was performed in Run 2, where we measured total (i.e., unfiltered) aqueous concentrations (C total) of Phe, Pyr, and BaP in the overlying water <1 cm below the water surface (“top“) and 1 to 2 cm above the sediment surface (“bottom“). Note that filtered (C diss) and unfiltered (C total) concentrations agreed well for Phe and Pyr, whereas there was a considerable difference for BaP because of the high hydrophobicity and resulting significant binding to suspended solids that are removed during filtration (Supporting Information, Figure S6). The top and bottom C total agreed well for all three chemicals (Figure 5), which indicates that the overlying water was well mixed over the entire test duration at least from 1 cm above the sediment surface to the water surface. In Run 2, PDMS fiber was placed on the sediment surface with the intention to measure C free right at the sediment–water interface (C free,intf). The measured C free,intf was 2.7 to 16 and 1.3 to 1.9 times lower than C free,pore for Phe and Pyr, respectively, whereas only a small difference (0.8–1.5 times) was measured for BaP (Figure 3). These results indicate that the extent of C free gradient depends on the chemical properties. Moderately hydrophobic chemicals (i.e., Phe, Pyr) would be depleted in the upper sediment layer because of lower sediment sorption and fast transport and dilution in overlying water by water renewal, resulting in a larger gap between C free,pore and C free,over compared to highly hydrophobic chemicals that are strongly bound to the sediment and less soluble in water. Depletion of the concentration in the top layer of sediment was also reported in a previous study that used more hydrophilic chemicals for spiked‐sediment tests (Dorn et al., 2021).
Figure 5.
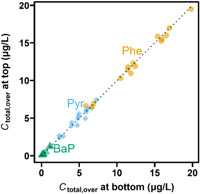
Relationship between total aqueous concentrations of phenanthrene (yellow circles), pyrene (blue diamonds), and benzo[a]pyrene (green triangles) at bottom and top of overlying water (Run 2; nominal sediment concentration, 50 mg/kg‐dry). Overlying water at the bottom and top was taken from approximately 1 cm above the sediment and from <1 cm below the water surface, respectively. The dotted line indicates a 1:1 ratio. C total,over = concentration in unfiltered overlying water; Phe = phenanthrene; Pyr = pyrene; BaP = benzo[a]pyrene.
Overlying versus porewater and total dissolved versus freely dissolved concentrations
While temporal and spatial concentration profiles of HOCs in the sediment test systems were chemical‐specific, the following general trend was observed: C diss,pore ≥ C free,pore > C free,intf > C diss,over ≥ C free,over (Figure 3). For all tested chemicals, C diss,pore showed the highest value of the measured types of concentrations and C free,pore the second highest, with the exception of BaP whose C diss,over showed the second highest. As discussed, this result indicates a nonequilibrium state between overlying water and porewater and the presence of a vertical concentration gradient in the system, partially caused by the semicontinuous refreshment of overlying water. The value of C free,pore was lower than that of C diss,pore by a factor of 1.1 ± 0.1 for Phe (Run 2), 3.5 ± 0.4 for Pyr (Run 2), 2.5 ± 0.8 for CPS (Run 4), 217 ± 112 for BaP (Run 2), and 165 ± 10 for BaP (Run 3). This difference between C diss,pore and C free,pore resulted from the significant binding of the HOCs to DOC in porewater and increased with increasing hydrophobicity, and hence K DOC, of the HOCs. The corresponding log K DOC values were 3.0 ± 0.4 for Phe (Run 2), 4.5 ± 0.0 for Pyr (Run 2), 4.0 ± 0.2 for CPS (Run 4), 6.4 ± 0.2 for BaP (Run 2), and 6.1 ± 0.0 for BaP (Run 3). These log K DOC were lower than the log K OC calculated from the respective runs (5.0 ± 0.1 for CPS in Run 4 and 7.0 ± 0.2 for BaP in Run 3). Compared with the significant difference between C diss,pore and C free,pore, the difference between C diss,over and C free,over was absent for CPS (a factor of 1.1 ± 0.2) because of a low level of DOC in the overlying water (i.e., 6 mg C/L on average of all runs, ∼15 times lower than that in porewater).
Concentrations representative for amphipod toxicity
The 10‐day spiked‐sediment toxicity tests resulted in >80% control survival of H. azteca, >0.1 mg dry weight per surviving amphipod, and a monotonically increasing concentration–response relationship. The calculated LC50 was 1.0 × 104 (95% CI 5.7 × 103−1.5 × 104) mg/kg of organic carbon (kg‐OC) and 2.6 (95% CI 2.2−3.0) mg/kg‐OC for BaP and CPS, respectively (Figure 6). The obtained LC50 value for CPS was comparable with those reported in previous studies with H. azteca, ranging from 1.8 to 4.4 mg/kg‐OC (Amweg & Weston, 2007; Hintzen et al., 2009; Weston & Amweg, 2007). To our knowledge, no LC50 value for BaP has been reported for H. azteca in a 10‐day spiked‐sediment toxicity assay. Growth inhibition and body concentration showed a monotonic concentration–response relationship for BaP (Supporting Information, Figures S12 and S13), whereas no significant effect on growth was observed for CPS (Supporting Information, Figure S18).
Figure 6.
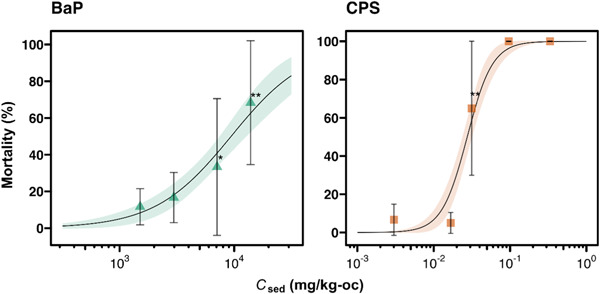
Concentration–response relationships for the effects of benzo[a]pyrene and chlorpyrifos on 10‐day amphipod mortality based on sediment concentration. Shaded areas are the 95% confidence interval of regression curves. Error bars represent standard deviations (n = 6). Asterisks indicate statistically significant difference in mortality from the control (*p < 0.05, **p < 0.01, Dunnett's test). BaP = benzo[a]pyrene; CPS = chlorpyrifos; C sed = concentration in spiked sediment.
In search of an exposure metric that represents H. azteca toxicity in varying conditions, the LC50 values based on four concentration types (i.e., C diss,pore, C free,pore, C diss,over, C free,over) were compared to those in 10‐ or 4‐day water‐only tests reported in the present study and the literature (Ding et al., 2012; Phipps et al., 1995; Tani et al., 2021; Supporting Information, Figure S14; Table 2). Note that water‐only toxicity is only available on the basis of C diss or C total, except that Ding et al. (2012) also inferred C free for CPS using their SPME method. The comparison showed that LC50 values for CPS based on overlying water concentrations in the present sediment tests, be it C diss,over or C free,over, were 3 to 10 times lower than LC50 values in water‐only tests. In contrast, the LC50 values from the sediment porewater concentrations, regardless of C diss,pore or C free,pore, and those from water‐only tests agree within a factor of 2.2. This result suggests that the porewater concentration is a more representative indicator than the overlying water for the toxicity of CPS in sediment tests with H. azteca. In particular, the C free,pore‐based LC50 for CPS from our sediment test was in excellent agreement with the C free‐based LC50 from the water‐only test by Ding et al. (2012; within a factor of 1.1). Data that allow such comparison are, however, limited, particularly C free data in water‐only tests. For BaP, for example, we were not able to find a C free‐based LC50 value for H. azteca in water‐only tests. Instead, a C free‐based bioconcentration factor (BCF) measured in a water‐only test is available (Schlechtriem et al., 2019), the value of which (3.5 ± 0.1 log units) fell between the C free,pore‐based and C diss,pore‐based BCF in the present study (4.4 ± 0.1 and 3.0 ± 0.5 log units, respectively; see Supporting Information, Table S6). This indicates that the bound fraction of chemicals might contribute to the bioaccumulation of BaP as well as the freely dissolved fraction. Measuring C free also in water‐only toxicity tests is highly recommended for highly hydrophobic compounds such as BaP to further evaluate C free as a unifying toxicity metric.
Table 2.
Comparison of median lethal concentration values based on different concentrations
| C diss (μg/L) | C free (μg/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| Test system | Duration | C sed (mg/kg‐OC) | C diss,pore | C diss,over a | C free,pore | C free,over | Reference | |
| BaP | Sediment–water | 10 days | 1.0 × 104 (5.7 × 103−1.5 × 104) | 101 (89−114) | 16 (11−21) | 0.9 (0.7−1.0) | NA | Present study |
| BaP | Water‐only | 96 h | — | 2.8 (2.1−3.5)b | NA | Present study | ||
| BaP | Water‐only | 96 h | — | 6.4 (4.7−9.4)b | NA | Tani et al. (2021) | ||
| CPS | Sediment–water | 10 days | 2.6 (2.2−3.0) | 0.053 (0.046−0.060) | 0.007 (0.006−0.008) | 0.019 (0.015−0.023) | 0.005 (0.004−0.006) | Present study |
| CPS | Water‐only | 10 days | — | 0.086 | NA | Phipps et al. (1995) | ||
| CPS | Water‐only | 10 days | — | 0.024 (0.017−0.026) | 0.021 (0.019−0.029) | Ding et al. (2012) | ||
The arithmetic mean of C diss,over over 10 days was used for median lethal concentration estimation.
Based on C total, not on C diss.
The median lethal concentration was calculated based on the measured concentrations. Values in parentheses represent 95% confidence intervals.
C sed = sediment concentration; OC = organic carbon; C diss = total dissolved concentration; C free = freely dissolved concentration; C diss,pore = total dissolved concentration in porewater; C diss,over = total dissolved concentration in overlying water; C free,pore = freely dissolved concentration in porewater; C free,over = freely dissolved concentration in overlying water; BaP = benzo[a]pyrene; CPS = chlorpyrifos; — = not applicable; NA = not available.
It may be worth reiterating that the C diss,over‐based LC50 values in the present study were calculated using the arithmetic mean of multiple measurements over 10‐day exposure. The individual measurements differed from the arithmetic mean by a factor of up to 4 for CPS and 40 for BaP, which were even larger than the differences between C diss,over and C diss,pore and between C diss,over and C free,over. As shown in the present study, instability of the overlying water concentration is unavoidable under the standard semi‐flow‐through condition because overlying water is repeatedly diluted with freshwater. Hence, observed toxicity cannot be linked to a steady‐state concentration in overlying water, which complicates the interpretation and extrapolation to other exposure scenarios. For direct comparison of water‐only and spiked‐sediment tests, both tests should be operated in more stable conditions (e.g., using passive dosing exposure [Fischer et al., 2016]). Porewater concentrations, in contrast, are expected to be more stable than overlying water concentrations.
Implications for sediment risk assessments
The method for the measurement of C free,pore and C free,over in the H. azteca sediment test presented in the present study may be adopted in the practice of spiked‐sediment toxicity testing of HOCs for improved effect interpretation on the basis of different exposure concentrations. The experimental observations emphasize the complexity of chemical exposure in sediment toxicity tests. Although protocols exist, there remain variations between test setups (e.g., static, manual, or automated water exchange; Hiki et al., 2021), leading to differences in chemical exposure. The application of immersed SPME fibers to measure C free in situ in porewater and overlying water may help to account for these differences, eventually increasing the comparability between toxicity data from different laboratories. Furthermore, for risk‐assessment purposes, effect concentrations on the basis of C free measurements can directly be compared to environmental C free measured in field sediments, which accounts for the chemical bioavailability both in the laboratory experiment and in the environment.
The observed gap between C free,pore and C free,over is important to consider when the toxicity of chemicals to organisms that are primarily present in the sediment, in the overlying water, or at the sediment–water interface is interpreted. Although porewater concentration was proved to be a more representative indicator for CPS sediment toxicity to H. azteca, it is known that this species can change its burrowing behavior depending on sediment type (e.g., sandy or silty; Doig & Liber, 2010) and some toxicants (e.g., ammonia; Whiteman et al., 1996), thereby being more exposed to overlying water. In this case, the application of equilibrium calculations or ex situ equilibrium SPME in sediment to estimate or measure C free in porewater will lead to overestimated exposure and hence underestimated chemical toxicity because C free in overlying water or at the sediment–water interface can be significantly lower, especially in test systems that apply water renewal. Our experimental results not only suggest that chemical distribution is influenced by water replacement but indicate a transport resistance at the sediment–water interface. Further investigation on the chemical transport using mechanistic modeling approaches may be useful, for which C free data as provided in the present study should be useful input.
It is a matter of ongoing debate what role the bound fraction of chemicals can play in the toxicity and bioaccumulation of HOCs to aquatic organisms (Fischer et al., 2016; Lin et al., 2018). Particularly, the large difference between freely dissolved and bound concentrations was found for very hydrophobic chemicals (e.g., BaP), and the role of the bound species in additional uptake and toxicity compared to the free species needs to be clarified for such chemicals. The relevance of the DOC‐bound species will depend on the chemical's sorptive affinity, the type of organic matter in the sediment, and the water exchange setup, which influences the DOC concentrations. Further experiments with more chemicals, in the presence of sediments with different properties, and with test species of different habitats and feeding strategies are needed to investigate the role of bound chemicals in uptake in sediment toxicity tests. Experimental C free and C diss determined by the methods described in the present study will serve as anchors to distinguish these different uptake routes in future experiments.
Supporting Information
The Supporting Information is available on the Wiley Online Library at https://doi.org/10.1002/etc.5199.
Author Contributions Statement
K. Hiki: conceptualization, methodology, investigation, data curation, formal analysis, writing–original draft, review and editing. F.C. Fischer: conceptualization, methodology, investigation, data curation, formal analysis, writing–original draft, review and editing. T. Nishimori: investigation, writing–review and editing. H. Watanabe: conceptualization, methodology, writing–review and editing. H. Yamamoto: writing–review and editing. S. Endo: conceptualization, methodology, investigation, data curation, formal analysis, writing–original draft, review and editing.
Supporting information
This article includes online‐only Supporting Information.
Supporting information.
Acknowledgment
The present study was financially supported by the Environment Research and Technology Development Fund (JPMEERF20195002K) of the Environmental Restoration and Conservation Agency, Japan. K. Hiki was funded by the Japan Society for the Promotion of Science (JSPS; Research Fellowship 19J00074), and F. C. Fischer was also funded by the JSPS (Research Fellowship 20F20753). We are grateful to two anonymous reviewers for their valuable comments.
Contributor Information
Kyoshiro Hiki, Email: hiki.kyoshiro@nies.go.jp.
Satoshi Endo, Email: endo.satoshi@nies.go.jp.
Data Availability Statement
Data, associated metadata, and calculation tools are available from the corresponding authors (hiki.kyoshiro@nies.go.jp; endo.satoshi@nies.go.jp).
REFERENCES
- Amweg, E. L. , & Weston, D. P. (2007). Whole‐sediment toxicity identification evaluation tools for pyrethroid insecticides: I. Piperonyl butoxide addition. Environmental Toxicology and Chemistry, 26(11), 2389–2396. 10.1897/07-017R.1 [DOI] [PubMed] [Google Scholar]
- Ankley, G. T. , Call, D. J. , Cox, J. S. , Kahl, M. D. , Hoke, R. A. , & Kosian, P. A. (1994). Organic carbon partitioning as a basis for predicting the toxicity of chlorpyrifos in sediments. Environmental Toxicology and Chemistry, 13(4), 621–626. 10.1002/etc.5620130411 [DOI] [Google Scholar]
- ASTM International . (2019). Standard test method for measuring the toxicity of sediment‐associated contaminants with freshwater invertebrates. 05 (Reapproved 2010). E1706. 10.1520/E1706-05R10.1 [DOI]
- Chapman, P. M. , Wang, F. , Germano, J. D. , & Batley, G. (2002). Pore water testing and analysis: The good, the bad, and the ugly. Marine Pollution Bulletin, 44(5), 359–366. 10.1016/S0025-326X(01)00243-0 [DOI] [PubMed] [Google Scholar]
- Ding, Y. , Landrum, P. F. , You, J. , Harwood, A. D. , & Lydy, M. J. (2012). Use of solid phase microextraction to estimate toxicity: Relating fiber concentrations to toxicity—Part I. Environmental Toxicology and Chemistry, 31(9), 2159–2167. 10.1002/etc.1935 [DOI] [PubMed] [Google Scholar]
- Ding, Y. , Landrum, P. F. , You, J. , & Lydy, M. J. (2013). Assessing bioavailability and toxicity of permethrin and DDT in sediment using matrix solid phase microextraction. Ecotoxicology, 22(1), 109–117. 10.1007/s10646-012-1007-z [DOI] [PubMed] [Google Scholar]
- Di Toro, D. M. , Zarba, C. S. , Hansen, D. J. , Berry, W. J. , Swartz, R. C. , Cowan, C. E. , Pavlou, S. P. , Allen, H. E. , Thomas, N. A. , & Paquin, P. R. (1991). Technical basis for establishing sediment quality criteria for nonionic organic chemicals using equilibrium partitioning. Environmental Toxicology and Chemistry, 10(12), 1541–1583. 10.1002/etc.5620101203 [DOI] [Google Scholar]
- Doig, L. E. , & Liber, K. (2010). An assessment of Hyalella azteca burrowing activity under laboratory sediment toxicity testing conditions. Chemosphere, 81(2), 261–265. 10.1016/j.chemosphere.2010.05.054 [DOI] [PubMed] [Google Scholar]
- Doong, R. , Chang, S. , & Sun, Y. (2000). Solid‐phase microextraction for determining the distribution of sixteen US Environmental Protection Agency polycyclic aromatic hydrocarbons in water samples. Journal of Chromatography A, 879(2), 177–188. 10.1016/S0021-9673(00)00347-2 [DOI] [PubMed] [Google Scholar]
- Dorn, A. , Hammel, K. , Dalkmann, P. , Faber, D. , Hellpointner, E. , Lamshoeft, M. , Telscher, M. , Bruns, E. , Seidel, E. , & Hollert, H. (2021). What is the actual exposure of organic compounds on Chironomus riparius?—A novel methodology enabling the depth‐related analysis in sediment microcosms. Chemosphere, 279, Article 130424. 10.1016/j.chemosphere.2021.130424 [DOI] [PubMed] [Google Scholar]
- Environment and Climate Change Canada . (2017). Biological test method: Test for survival and growth in sediment using the freshwater amphipod Hyalella azteca.
- Fischer, F. , Böhm, L. , Höss, S. , Möhlenkamp, C. , Claus, E. , Düring, R. A. , & Schäfer, S. (2016). Passive dosing in chronic toxicity tests with the nematode Caenorhabditis elegans . Environmental Science & Technology, 50(17), 9708–9716. 10.1021/acs.est.6b02956 [DOI] [PubMed] [Google Scholar]
- Harwood, A. D. , Landrum, P. F. , & Lydy, M. J. (2013). Bioavailability‐based toxicity endpoints of bifenthrin for Hyalella azteca and Chironomus dilutus . Chemosphere, 90(3), 1117–1122. 10.1016/j.chemosphere.2012.09.017 [DOI] [PubMed] [Google Scholar]
- Hiki, K. , Watanabe, H. , & Yamamoto, H. (2021). Sources of variation in sediment toxicity of hydrophobic organic chemicals: Meta‐analysis of 10–14‐day spiked‐sediment tests with Hyalella azteca and Chironomus dilutus . Integrated environmental assessment and management, 17(5), 1003–1013. 10.1002/ieam.4413 [DOI] [PubMed] [Google Scholar]
- Hintzen, E. P. , Lydy, M. J. , & Belden, J. B. (2009). Occurrence and potential toxicity of pyrethroids and other insecticides in bed sediments of urban streams in central Texas. Environmental Pollution, 157(1), 110–116. 10.1016/j.envpol.2008.07.023 [DOI] [PubMed] [Google Scholar]
- Huang, X. , Cui, H. , & Duan, W. (2020). Ecotoxicity of chlorpyrifos to aquatic organisms: A review. Ecotoxicology and Environmental Safety, 200, Article 110731. 10.1016/j.ecoenv.2020.110731 [DOI] [PubMed] [Google Scholar]
- Ivey, C. D. , & Ingersoll, C. G. (2016). Influence of bromide on the performance of the amphipod Hyalella azteca in reconstituted waters. Environmental Toxicology and Chemistry, 35(10), 2425–2429. 10.1002/etc.3421 [DOI] [PubMed] [Google Scholar]
- Jonker, M. T. O. , Van Der Heijden, S. A. , Adelman, D. , Apell, J. N. , Burgess, R. M. , Choi, Y. , Fernandez, L. A. , Flavetta, G. M. , Ghosh, U. , Gschwend, P. M. , Hale, S. E. , Jalalizadeh, M. , Khairy, M. , Lampi, M. A. , Lao, W. , Lohmann, R. , Lydy, M. J. , Maruya, K. A. , Nutile, S. A. , … Wu, Y. (2018). Advancing the use of passive sampling in risk assessment and management of sediments contaminated with hydrophobic organic chemicals: Results of an international ex situ passive sampling interlaboratory comparison. Environmental Science & Technology, 52(6), 3574–3582. 10.1021/acs.est.7b05752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, H. , Xia, X. , Jiang, X. , Bi, S. , Wang, H. , Zhai, Y. , Wen, W. , & Guo, X. (2018). Bioavailability of pyrene associated with different types of protein compounds: Direct evidence for its uptake by Daphnia magna . Environmental Science & Technology, 52(17), 9851–9860. 10.1021/acs.est.8b03349 [DOI] [PubMed] [Google Scholar]
- Lydy, M. J. , Landrum, P. F. , Oen, A. M. , Allinson, M. , Smedes, F. , Harwood, A. D. , Li, H. , Maruya, K. A. , & Liu, J. (2014). Passive sampling methods for contaminated sediments: State of the science for organic contaminants. Integrated environmental assessment and management, 10(2), 167–178. 10.1002/ieam.1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, P. , Vaes, W. H. J. , Wijnker, F. , Legierse, K. C. H. M. , Kraaij, R. H. , Tolls, J. , & Hermens, J. L. M. (2000). Sensing dissolved sediment porewater concentrations of persistent and bioaccumulative pollutants using disposable solid‐phase microextraction fibers. Environmental Science & Technology, 34(24), 5177–5183. 10.1021/es001179g [DOI] [Google Scholar]
- Muijs, B. , & Jonker, M. T. O. (2009). Temperature‐dependent bioaccumulation of polycyclic aromatic hydrocarbons. Environmental Science & Technology, 43(12), 4517–4523. 10.1021/es803462y [DOI] [PubMed] [Google Scholar]
- Nowell, L. H. , Norman, J. E. , Ingersoll, C. G. , & Moran, P. W. (2016). Development and application of freshwater sediment‐toxicity benchmarks for currently used pesticides. Science of the Total Environment, 550, 835–850. 10.1016/j.scitotenv.2016.01.081 [DOI] [PubMed] [Google Scholar]
- Organisation for Economic Co‐operation and Development . (2004). Test No. 218: Sediment–water chironomid toxicity using spiked sediment. OECD Guidelines for the Testing of Chemicals. 10.1787/9789264070264-en [DOI]
- Organisation for Economic Co‐operation and Development . (2007). Test No. 225: Sediment–water Lumbriculus toxicity test using spiked sediment. OECD Guidelines for the Testing of Chemicals.
- Organisation for Economic Co‐operation and Development . (2010). Test No. 233: Sediment–water chironomid life‐cycle toxicity test using spiked water or spiked sediment. OECD Guidelines for the Testing of Chemicals.
- Phipps, G. L. , Mattson, V. R. , & Ankley, G. T. (1995). Relative sensitivity of three freshwater benthic macroinvertebrates to ten contaminants. Archives of Environmental Contamination and Toxicology, 28(3), 281–286. 10.1007/BF00213103 [DOI] [Google Scholar]
- R Foundation for Statistical Computing . (2021). R: A language and environment for statistical computing. https://www.r-project.org/
- Ritz, C. , Baty, F. , Streibig, J. C. , & Gerhard, D. (2015). Dose–response analysis using R. PLoS One, 10(12), Article e0146021. 10.1371/journal.pone.0146021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlechtriem, C. , Kampe, S. , Bruckert, H.‐J. , Bischof, I. , Ebersbach, I. , Kosfeld, V. , Kotthoff, M. , Schäfers, C. , & L'Haridon, J. (2019). Bioconcentration studies with the freshwater amphipod Hyalella azteca: Are the results predictive of bioconcentration in fish? Environmental Science and Pollution Research, 26(2), 1628–1641. 10.1007/s11356-018-3677-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, B. S. L. , Batley, G. E. , Chariton, A. A. , Stauber, J. L. , King, C. K. , Chapman, J. C. , Hyne, R. V. , Gale, S. A. , Roach, A. C. , & Maher, W. A. (2005). Handbook for sediment quality assessment quality assessment. CSIRO Energy Technology. http://hdl.handle.net/102.100.100/182300?index=1
- Stenzel, A. , Goss, K. U. , & Endo, S. (2013). Experimental determination of polyparameter linear free energy relationship (pp‐LFER) substance descriptors for pesticides and other contaminants: New measurements and recommendations. Environmental Science & Technology, 47(24), 14204–14214. 10.1021/es404150e [DOI] [PubMed] [Google Scholar]
- Tani, K. , Watanabe, H. , Noguchi, M. , Hiki, K. , Yamagishi, T. , Tatarazako, N. , & Yamamoto, H. (2021). Toxicity assessment of typical polycyclic aromatic hydrocarbons to Daphnia magna and Hyalella azteca in water‐only and sediment–water exposure systems. Science of the Total Environment, 784, Article 147156. 10.1016/j.scitotenv.2021.147156 [DOI] [PubMed] [Google Scholar]
- Ter Laak, T. L. , Agbo, S. O. , Barendregt, A. , & Hermens, J. L. M. (2006). Freely dissolved concentrations of PAHs in soil pore water: Measurements via solid‐phase extraction and consequences for soil tests. Environmental Science & Technology, 40(4), 1307–1313. 10.1021/es0514803 [DOI] [PubMed] [Google Scholar]
- US Environmental Protection Agency . (1994). Methods for assessing the toxicity of sediment associated contaminants with estuarine and marine amphipods. EPA‐600/R94‐025.
- US Environmental Protection Agency . (2000). Methods for measuring the toxicity and bioaccumulation of sediment‐associated contaminants with freshwater invertebrates. EPA/600/R‐99/064.
- US Environmental Protection Agency . (2012). Equilibrium partitioning sediment benchmarks (ESBs) for the protection of benthic organisms: Procedures for the determination of the freely dissolved interstitial water concentrations of nonionic organics. EPA/600/R‐02/012. https://cfpub.epa.gov/si/si_public_record_Report.cfm?Lab=NHEERL%26dirEntryID=237621
- Weston, D. P. , & Amweg, E. L. (2007). Whole‐sediment toxicity identification evaluation tools for pyrethroid insecticides: II. Esterase addition. Environmental Toxicology and Chemistry, 26(11), 2397–2404. 10.1897/07-018R.1 [DOI] [PubMed] [Google Scholar]
- Whiteman, F. W. , Ankley, G. T. , Kahl, M. D. , Rau, D. M. , & Balcer, M. D. (1996). Evaluation of interstitial water as a route of exposure for ammonia in sediment tests with benthic macroinvertebrates. Environmental Toxicology and Chemistry, 15(5), 794–801. [DOI] [Google Scholar]
- Xu, Y. , Spurlock, F. , Wang, Z. , & Gan, J. (2007). Comparison of five methods for measuring sediment toxicity of hydrophobic contaminants. Environmental Science & Technology, 41(24), 8394–8399. 10.1021/es071911c [DOI] [PubMed] [Google Scholar]
- You, J. , Landrum, P. F. , & Lydy, M. J. (2006). Comparison of chemical approaches for assessing bioavailability of sediment‐associated contaminants. Environmental Science & Technology, 40(20), 6348–6353. 10.1021/es060830y [DOI] [PubMed] [Google Scholar]
- Zumwalt, D. C. , Dwyer, F. J. , Greer, I. E. , & Ingersoll, C. G. (1994). A water‐renewal system that accurately delivers small volumes of water to exposure chambers. Environmental Toxicology and Chemistry, 13(8), 1311–1314. 10.1002/etc.5620130813 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article includes online‐only Supporting Information.
Supporting information.
Data Availability Statement
Data, associated metadata, and calculation tools are available from the corresponding authors (hiki.kyoshiro@nies.go.jp; endo.satoshi@nies.go.jp).


