Abstract
Background
Sarcopenia and myosteatosis have been associated to a poor prognosis of cirrhosis and to a higher incidence of hepatic encephalopathy (HE). The prognostic implications of visceral and subcutaneous adiposity are less known.
Aim
To evaluate the modifications of visceral and subcutaneous adipose tissue after TIPS and to investigate their relationships with the modification of muscle mass and with the incidence of post‐TIPS HE.
Patients and Methods
35 cirrhotic patients submitted to TIPS were retrospectively studied. The modification of skeletal muscle index (SMI), muscle attenuation (myosteatosis), subcutaneous adipose tissue index (SATI), visceral adipose tissue index (VATI), assessed by CT‐scan and plasma ammonia were evaluated before and after a mean follow‐up of 19 ± 15 months after TIPS. The number of episodes of overt HE was also recorded.
Results
During the follow‐up, the mean SMI and muscle attenuation increased significantly; SATI significantly increased while VATI significantly decreased, although not uniformly in all patients. By comparing the patients with or without improvement in their nutritional status after TIPS, MELD remained stable while the number of episodes of overt HE was significantly lower in the patients with improved SMI and in the patients with improved SATI. Finally, inverse correlation was observed between the variation of ammonia and SATI (r = −.40; P < .05).
Conclusion
In addition to muscle mass, adipose tissue is modified after TIPS. The improvement of subcutaneous adipose tissue as well as of sarcopenia and myosteatosis is associated to the amelioration of cognitive impairment independently of liver function. The correlation between adipose tissue and ammonia modification may suggest an active role of the adipose tissue in the inter‐organ ammonia trafficking.
Keywords: adipose tissue, ammonia, hepatic encephalopathy, sarcopenia, TIPS
Abbreviations
- CT
computed tomography
- HE
hepatic encephalopathy
- HU
Hounsfield unit
- MELD
model of end‐stage liver disease
- SATI
subcutaneous adipose tissue index
- SMI
skeletal muscle index
- TIPS
transjugular intrahepatic portosystemic shunt
- VATI
visceral adipose tissue index
Key points.
It has been showed that the TIPS placement, a radiological procedure performed to reduce portal hypertension secondary to cirrhosis, lead to an amelioration of both quantitative and qualitative muscle alterations.
In the present study, we show that TIPS lead also to modification in adipose tissue (subcutaneous adipose tissue increased and visceral adipose tissue decrease after TIPS).
The patients with an improved muscle mass and an improved subcutaneous adipose tissue had a lower number of episodes of hepatic encephalopathy after TIPS suggesting an active role of the adipose tissue in the inter‐organ ammonia trafficking.
1. INTRODUCTION
It is known for a long time, that patients with cirrhosis have an altered nutritional and metabolic status and that such alterations are clinically and prognostically important. 1 , 2 , 3 Most of the studies concentrated their attention on protein malnutrition and most recently to the relation between quantitative and qualitative muscle alterations and the clinical outcomes in cirrhosis. Several studies have shown that sarcopenia (low muscle mass) and myosteatosis 4 (pathological fat infiltration into skeletal muscle) are associated with a poor prognosis and with different complications 5 , 6 , 7 , 8 , 9 , 10 including hepatic encephalopathy (HE). 11 , 12 , 13 The relations between muscle mass and hepatic encephalopathy may be due to the capacity of muscle in ammonia handling by means of muscle glutamine synthetase activity.
For still incompletely known reason, both qualitative and quantitative muscle alterations ameliorate in most cirrhotic patients successfully treated with a transjugular intrahepatic portosystemic shunt (TIPS) 14 , 15 , 16 and such improvement was shown to be associated with an amelioration of the incidence of HE independent on the modification of liver function 17 during the follow‐up. The pathophysiological basis of these observations is not well defined and the improvement in muscle mass is not observable in all patients after TIPS.
Recently, methods to study the quantitative alterations of adipose tissue became available but the clinical implications of visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) in terms of survival and prevalence of complications in cirrhotic patients are still poorly understood. 18
The aim of the present study was to evaluate the modifications of both visceral and subcutaneous adipose tissue assessed by CT scan in 35 patients submitted to TIPS insertion and follow up in order to investigate their concurrence and relationships with the modification of muscle alterations and with the incidence of post‐TIPS hepatic encephalopathy.
2. PATIENTS AND METHODS
All consecutive cirrhotic patients submitted to TIPS from January 2017 to December 2020 in whom a CT scan was available before TIPS and during the follow‐up were retrospectively enrolled in this study. During this study period, 80 cirrhotic patients candidate to TIPS were evaluated; among them, 17 were not submitted to the procedure because of the exclusion criteria for TIPS placement (Figure 1). In our Centre (Unit of Gastroenterology of Policlinico Umberto I of Rome), exclusion criteria for TIPS placement are age >75 years, bilirubin levels >5 mg/dL, creatinine levels >3 mg/dL, a serious cardiac or pulmonary dysfunction, a Child‐Pugh's score >11 (except for patients who were candidates for early TIPS), a MELD score >18, the presence of portal vein thrombosis, a diagnosis of hepatocellular carcinoma, sepsis, spontaneous bacterial peritonitis. Present HE or previous spontaneous/recurrent HE is also a contraindication to TIPS; however, patients with only one episode of HE precipitated by variceal bleeding and ameliorated after the bleeding was controlled are not excluded.
FIGURE 1.
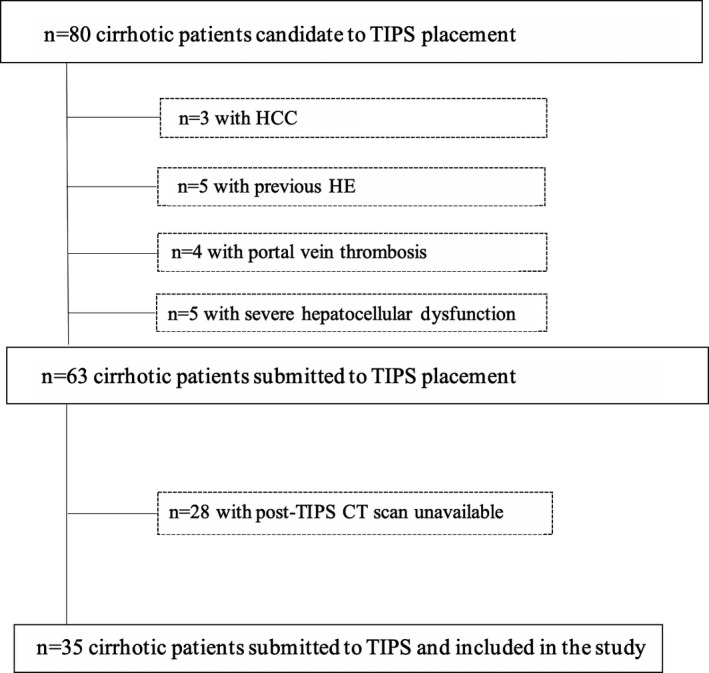
Patient flowchart
Moreover, a total of 28 patients submitted to TIPS were not included in the present analysis because of unavailability of the follow‐up CT scan (Figure 1). The characteristics of the 28 cirrhotic patients excluded (sex [M/F]: 21/7; age: 55.1 ± 7.4; aetiology [virus/alcohol/other]: 10/14/4; TIPS indication [variceal bleeding/refractory ascites]: 13/15; previous overt HE [yes/no]: 3/25; MELD score 11.4 ± 3.9; Child‐Pugh class [A/B/C]: 14/10/2; Child‐Pugh score: 7.9 ± 1.7; sodium [mEq/dL]: 134 ± 4; venous plasma ammonia [μg/dL]: 49.4 ± 19.5) were not statistically different from those of the 35 patients included.
Finally, 35 patients were included in the analysis. Data recovered from medical charts included gender, age, weight, height, cirrhosis aetiology, presence of ascites or hepatic encephalopathy, liver biochemistries including plasmatic ammonia levels. The clinical and laboratory data used to calculate MELD and Child–Pugh scores were obtained the day before the TIPS placement and at the moment of the second abdominal CT scan. The 'Sapienza' University of Rome Ethical Committee approved the collection of data and informed consent for their data analysis was obtained for each patient.
2.1. Skeletal muscle and adipose tissue assessment
All patients underwent a CT scan before TIPS for the evaluation of the vascular hepatic system. The CT scan was performed 3.2 ± 3.2 months before TIPS placement. The CT scan was then repeated during the follow‐up for the evaluation of the TIPS patency and the detection of hepatocellular carcinoma when clinically indicated. The follow‐up CT scan was chosen when the interval from the index scan was at least 6 months in all patients but one and the mean time interval was 19 ± 15 months. For the quantitative analysis of muscle mass and of subcutaneous and visceral adipose tissue, the transverse CT image at the L3 level was selected for each scan. All CT images were analysed by two trained observers (SG, LC) with SliceOmatic V4.2 software (Tomovision), which enables specific tissue demarcation by using previously reported Hounsfield unit (HU) thresholds. Skeletal muscle is identified and quantified by HU thresholds of −29 to +150. 19 With these specific HU thresholds, measurements of the muscle mass are not influenced by the presence of ascites. Subcutaneous adipose tissue (SAT) is defined as adipose tissue below the skin and above the parietal peritoneal lining and it is identified and quantified by HU thresholds of −190 to −30 while visceral adipose tissue (VAT) is defined as intraperitoneal adipose tissue identified and it is quantified by HU thresholds of −150 to −50.
Cross‐sectional areas (cm2) were automatically computed by summing tissue pixels and multiplying by pixel surface area. Muscle cross‐sectional, subcutaneous and visceral adipose tissue area were normalised for stature to obtain, respectively, the skeletal muscle index (SMI), the subcutaneous adipose tissue index (SATI) and visceral adipose tissue index (VATI) in cm2/m2.
The prevalence of sarcopenic patients was defined according to previously validated cut‐offs. 20
Because muscle attenuation indirectly measures fat infiltration in muscles, mean muscle attenuation in HU was reported for the entire muscle area from the same image used to calculate L3 SMI. To define the prevalence of myosteatosic patients we used cut‐off values that have been associated with mortality: <41 HU in patients with a BMI up to 24.9 and <33 in those with a BMI ≥25. 10 , 21 As there are not well‐validated cut‐offs for both the sexes to define the clinically significance of VAT or SAT in cirrhosis, the prevalence of patients with altered SAT or VAT cannot be defined and the absolute modifications of SATI and VATI before and after TIPS were compared.
2.2. TIPS placement, follow‐up and HE evaluation
All TIPS procedures were carried out by the same radiology team, using PTFE‐covered stents of 10 mm diameter as previously described. 22
The day before the procedure, a basal evaluation of HE, including an examination and grading of the patients' mental state and asterixis as well as the determination of venous blood ammonia were carried out as previously described. 23 None of the patients received any pharmacological treatment to prevent the occurrence of HE. After TIPS, the patients remained hospitalised for 1 week. The evaluation of the patients’ mental status was repeated before the discharge. The patients were then followed‐up once a week in the out‐patients department for the first month and then every 3 months and contacted by phone every month for the first 6 months. Thereafter, the patients were seen every 6 months until the execution of the follow‐up CT scan. Moreover, both the patients and their families were instructed about the importance of an immediate contact with the medical staff should any alteration in their mental state (lethargy, apathy, obvious personality changes, inappropriate behaviour or disorientation to time and space) occur between the scheduled visits. In this case, the HE evaluation was repeated to confirm and stage the degree of HE. A grade II HE or higher was considered an episode of overt HE. 23 All episodes, which occurred during the follow‐up, were recorded.
2.3. Statistical analysis
Data are expressed as mean ± SD, unless specified otherwise. Comparison between 2 groups was performed by chi‐square test, for discrete variables, and by unpaired Student's t‐test or Mann‐Whitney test, when necessary, for continuous variables. The comparisons between data recorded twice in the same patients (SMI, SATI, VATI, MELD score, etc) were performed by paired Student's t test and by Wilcoxon Signed‐Rank test when necessary. The relationship between variables were analysed by stepwise multiple regression analysis. The sample size was based on the data of a previous study 17 in which a sample size of 27 patients was sufficient to detect modification of muscle mass and its relationship with hepatic encephalopathy. The Number Cruncher Statistical System (NCSS) was used for all computations.
3. RESULTS
The demographic, clinical and biochemical characteristics of 35 patients included in this study are reported in Table 1. In one patient the TIPS placement was performed as an 'early TIPS' for the management of acute variceal bleeding.
TABLE 1.
Clinical and demographic characteristics of the 35 patients at study inclusion (before TIPS)
| Patients (n = 35) | |
|---|---|
| Age (mean ± SD) | 58.6 ± 6.3 |
| Sex (M/F) | 28/7 |
| Aetiology (virus/alcohol/other) | 12/16/7 |
| TIPS indication (bleeding/refractory ascites) | 16/19 |
| Child Pugh class (A/B/C) | 17/16/2 |
| Child Pugh Score | 7 ± 1.3 |
| MELD score | 11.9 ± 4 |
| Previous HE (no/yes) | 33/2 |
| Ascites (no/yes) | 9/26 |
| Previous variceal bleeding (no/yes) | 16/19 |
| Sarcopenia pre‐TIPS (no/yes) | 16/19 |
| SMI pre‐TIPS (cm2/m2) | 47.3 ± 10.7 |
| Muscle attenuation (HU) | 37.2 ± 8.6 |
| SATI pre‐TIPS (cm2/m2) | 51.8 ± 34.4 |
| VATI pre‐TIPS (cm2 m2) | 46 ± 21.5 |
| BMI (kg/m2) | 25.2 ± 0.7 |
| Body surface (m2) | 1.97 ± 0.6 |
| Albumin (g/dL) | 3.4 ± 0.61 |
| Bilirubin (mg/dL) | 1.36 ± 0.61 |
| INR | 1.31 ± 0.15 |
| Creatinine (mg/dL) | 0.9 ± 0.2 |
| Sodium (mEq/dL) | 136.5 ± 5 |
| Venous plasma ammonia (μg/dL) | 54 ± 23.19 |
Data are expressed as mean ±standard deviation, for the continue variables, and as number of patients for the discrete variables
Before TIPS, according to SMI cut‐off, sarcopenia was present in 19 patients (54%; CI 37%‐71%). After a mean follow‐up of 19 ± 15 months from TIPS placement, SMI significantly improved from 47.3 ± 10.7 a 49.7 ± 10.6 cm2/m2 (P = .04) with an average increase of 2.39 ± 6.7 cm2/m2 corresponding to an increase of 6.6%. At the end of the follow‐up, the increase in SMI was observed in 22 patients (63%) whereas in 13 patients (37%) the SMI remained unchanged or worsened.
Moreover, after TIPS, muscle attenuation significantly improved from 37.22 ± 8.63 to 40.9 ± 8.6 (P = .003) with an average increase of 12.5%, indicating a reduction of myosteatosis.
SATI was significantly increased from 51.8 ± 34.4 a 67.7 ± 39.3 cm2/m2 (P = .004) with an average increase of 15.9 ± 30.2 cm2/m2 corresponding to an increase of 74.4%. Twenty‐eight patients (80%) experienced an improvement of SATI and 7 (20%) did not.
VATI was significantly reduced from 45.9 ± 21.4 a 36.7 ± 18.2 cm2/m2 (P = .007) with an average decrease of 9.2 ± 19.1 cm2/m2 corresponding to a decrease of 10.7%. Twenty‐four patients (69%) experienced a reduction of VATI and 11 (31%) did not.
Given the retrospective nature of this study, it is hard to establish how long after TIPS placement the improvement in SMI and SATI/VATI appears. By dividing the patients according to the availability of the second CT scan within 12 months (n = 18) or after 12 months (n = 17), it seems that the proportion of patients with the improvement in the body composition parameters was around 60%‐80% already in the first year of follow‐up.
Finally, the increase of BMI and body surface after TIPS was not statistically significant.
The patients were then divided into groups with or without modifications of each index (SMI, SATI and VATI); and compared for their demographic, clinical, biochemical characteristics at the moment of TIPS placement. The results of these comparisons are reported in Tables S1‐S3. There were no statistically significant differences in terms of sex, age, aetiology, stage of liver disease, biochemical parameters, indication to TIPS placement and previous overt HE episodes between each subgroup. Venous plasma ammonia levels before TIPS were also similar. However, in the 23 patients who experienced an increase of SMI values, the initial SMI value was significantly lower and the prevalence of ascites before TIPS placement was significantly higher (86%) than in the 13 patients without increase (54%) (P = .03) (Figure 2, panel A). The presence of ascites was also more frequent in patients with SATI increase compared to those without (82% vs 43%; P = .03) (Figure 2, panel B). At variance with SMI and SATI, VATI modifications were independent on the presence of ascites before TIPS. The only difference observed in the group of patients in whom VATI was reduced after TIPS was basal VATI, which resulted to be significantly higher in the 24 patients who experienced a decrease in VATI value after TIPS compared to the 11 patients who did not (51.5 ± 19 vs 33.9 ± 22.3; P = .02) (Table S3).
FIGURE 2.
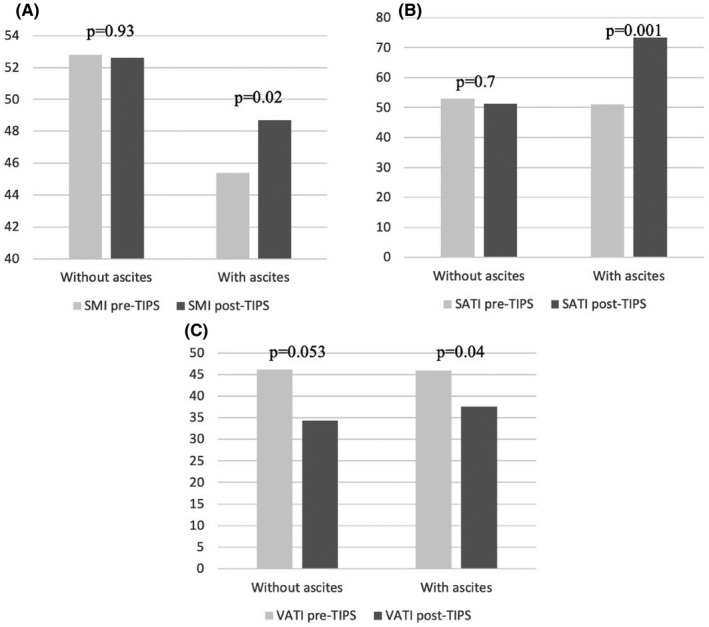
Comparison of the variations of SMI (Panel A), of SATI (Panel B) and of VATI (Panel C) before and after TIPS between the patients with or without ascites before TIPS placement
Thereafter, the basal value of SMI, SATI, VATI and muscle attenuation were correlated with each other to evaluate the reciprocal relationship and the reason why some patients experience the improvement of the nutritional status and other do not. Actually, no correlations were found between SMI, SATI and VATI before TIPS but an inverse correlation was observed between the basal values of muscle attenuation with both basal SATI and VATI (r = −.39, P < .01 and r = −.45, P < .005 respectively) (Figure 3, panel A and B).
FIGURE 3.
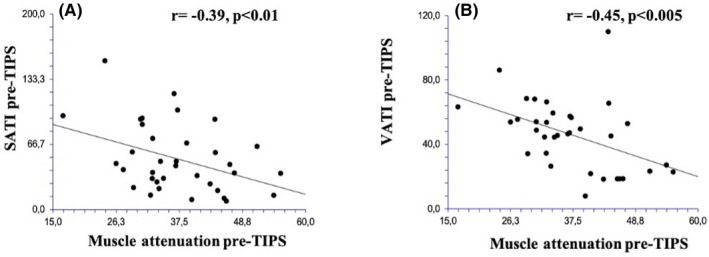
Correlation between muscle attenuation and subcutaneous adipose tissue index (SATI) before TIPS (Panel A) and correlation between muscle attenuation and visceral adipose tissue index (VATI) before TIPS in the 35 patients included in this study
The patients with or without the increase of each index considered (SMI, SATI and VATI) were followed up until the time of the second CT scan. At least one episode of overt HE was recorded in 25 patients. The number of episodes of post‐TIPS overt HE and of blood ammonia levels are reported in Table 2. In the group of patients with an improvement of SMI, the number of episodes of HE was lower than in those without the improvement (P = .01). In particular, the number of episodes of overt HE which occurred in the first month after TIPS was similar in the two groups while the number of episodes of overt HE occurring in the following months was significantly lower in the patients with the improvement of SMI, despite similar length of follow‐up. The same results were observed comparing the patients with and without the improvement of SATI. No differences were observed in the patients with and without reduction of VATI.
TABLE 2.
Number of episodes of overt hepatic encephalopathy in the patients with or without improvement of SMI, SATI and VATI at the end of follow‐up
|
No SMI improvement (n = 13) |
SMI improvement (n = 22) |
P value |
No SATI increase (n = 7) |
SATI increase (n = 28) |
P value |
No VATI decrease (n = 11) |
VATI decrease (n = 24) |
P value |
|
|---|---|---|---|---|---|---|---|---|---|
|
n° episodes HE post‐TIPS |
2 (1‐3) | 1 (0‐1) | .01 | 3 (0‐3) | 1 (0‐1) | .04 | 2 (0‐3) | 1 (0‐2) | .22 |
| n° episodes HE first month post‐TIPS | 1 (0‐2) | 1 (0‐1) | .49 | 1 (0‐2) | 1 (0‐1) | .2 | 1 (0‐2) | 1 (0‐1) | .59 |
| n° episodes HE the months after | 1 (0‐2) | 0 (0‐0) | .004 | 1 (0‐2) | 0 (0‐0) | .01 | 0 (0‐2) | 0 (0‐1) | .14 |
| Venous plasma ammonia (μg/dL) | 82 (40‐134) | 42 (31‐76) | .02 | 58 (31‐112) | 44 (32‐104) | .93 | 79 (22‐134) | 44 (31‐65) | .12 |
| Follow‐up (months) | 11 (7‐31) | 13 (8‐22) | .7 | 18.5 (6‐56) | 10 (8‐22) | .64 | 11 (6‐27) | 11 (8‐22) | .76 |
The comparison between the groups of patients (no SMI improvement and SMI improvement; no SATI increase and SATI increase; no VATI decrease and VATI decrease) was performed by unpaired Student t test or Mann‐Whitney test, when necessary. Data are expressed as median and interquartile range (IQR).
No differences in survival were observed by dividing the patients according to the improvement or not of SMI, of SATI or of VATI.
Finally, the correlation between the variations of ammonia and SMI, muscle attenuation, SATI and VATI (i.e. the difference between the final and initial value collected at the time of the two muscle evaluations) before and after TIPS are reported in Figure 4. Inverse correlations were observed between delta ammonia and delta SMI (r = −.61; P < .005), delta ammonia and delta SATI (r = −.40; P < .05), delta ammonia and delta muscle attenuation (r = −.40; P < .05).
FIGURE 4.
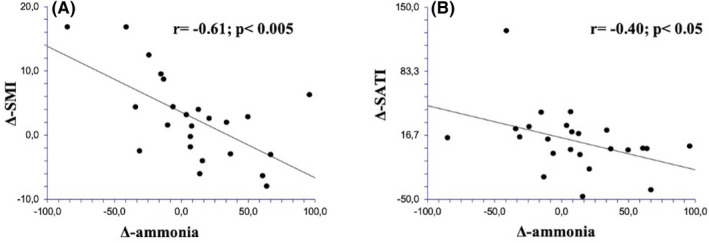
Correlation between the variations of ammonia and skeletal muscle index (SMI) (Panel A) and correlation between the variations of ammonia and subcutaneous adipose tissue index (SATI) (Panel B) before and after transjugular intrahepatic portosystemic shunt in the 35 patients included in this study
4. DISCUSSION
The present study investigates the modifications of body composition occurring after TIPS placement in cirrhotic patients. In addition to the modification of sarcopenia and myosteatosis, the paper describes the quantitative changes of both subcutaneous and visceral adipose tissue derivable by the CT scan evaluation, which is now considered a reliable tool for the quantitative estimation of both muscle and fat mass. This study shows that after TIPS there is an amelioration of nutritional status involving both muscle and fat tissue. After a mean follow‐up of 19 ± 15 months from TIPS placement, SMI and muscle attenuation, a measure of sarcopenia and myosteatosis, respectively, significantly improved, 14 , 15 , 16 , 17 subcutaneous adipose tissue index (SATI) increased, while visceral adipose tissue index (VATI) decreased. These results are in line with those of the study by Artru et al 24 that shows an increase of subcutaneous fat surface and a decrease of visceral fat surface at the CT scan performed at 1‐3 months and at 6 months after TIPS placement in a group of 179 cirrhotic patients. Notably, in our study, the changes in body composition were independent on liver function modifications, being the MELD stable or even worsened after TIPS.
After TIPS insertion, the modifications of body composition were observed in the majority of patients, although not in all. In the patients with an improvement of nutritional status, the modifications of muscle and fat mass after TIPS are associated with a reduction of the episode of post‐TIPS‐HE and to a reduction of plasma ammonia which is significantly correlated to both SMI and SATI modifications.
The amelioration in nutritional parameters after TIPS has been repeatedly observed and described. 14 , 15 , 16 , 17 The mechanisms leading to the observed modifications and the reason why they occur in a portion of the patients are not completely understood. In our study, the modifications of muscular and adipose tissue parameters were more pronounced in the patients with ascites before TIPS (Figure 2) than in those without. In cirrhotic patients with ascites, an increase in basal energy expenditure (BEE) reversed by a large volume paracentesis has been described. 25 The reason is not completely understood: weight of ascites per se and a higher abdominal heat dispersion due to the presence of ascites may play a role. In any case, the presence of ascites, by increasing the energy expenditure may accelerate the appearance of protein‐energy malnutrition. Thus, the long‐term resolution of ascites induced by TIPS can contribute to the amelioration of body composition particularly in the patients with ascites before TIPS. In a study by Allard JP et al, 26 BEE was measured in 14 cirrhotic patients submitted to TIPS because of refractory ascites who showed a significant improvement in their nutritional parameters. Actually, BEE remained stable in these patients when expressed as unit of lean body mass. The possibility that the amelioration in body composition in the ascitic patients is due to a reduction in the BEE after TIPS needs therefore to be confirmed.
The degree of muscle and adipose tissue modifications after TIPS may also be influenced by the initial values of SMI, VATI and SATI. In the present study, the modifications of these parameters were more evident in the patients with the more pronounced muscular and adipose tissue alterations before TIPS (Supplementary Tables), confirming the observation of Tsien C et al study where a lower pre‐TIPS muscle area was an independent predictor of reversal of sarcopenia after TIPS. 15
As far as the adipose tissue modifications, TIPS placement may lead to favourable changes by modifying the serum levels of adipokines. Thomsen et al observed that adiponectin increased and retinol‐binding protein 4 decreased after a TIPS configuring an anabolic condition where the adipose tissue possessed residual capacity for energy storage. 27
In cirrhotic patients, sarcopenia has been associated with poor outcomes in terms of survival and incidence of complications 5 , 6 , 7 , 8 , 9 , 10 including infections and hepatic encephalopathy. 4 , 11 , 12 , 28 , 29 However, the data on the prognostic relevance of the alteration of adipose tissue and their influence on the clinical outcomes of cirrhotic patients are less known and a clear cut‐off for considering the modifications of these indices as clinically significant is lacking. Montano‐Loza et al described that in male patients with cirrhosis, high visceral adiposity (VATI ≥ 65 cm2/m2) was associated with a higher risk of hepatocellular carcinoma before liver transplant as well as with a higher rate of recurrence after liver transplant. 30 According to these data, a reduction of VATI observed in our study after TIPS may be considered as desirable but further studies are needed to establish the role of such component of fat mass in patients with cirrhosis. Low subcutaneous adiposity (SATI < 60 cm2/m2) was independently associated with mortality after adjusting for age, alcohol‐related cirrhosis, albumin and MELD score in female patients with cirrhosis. 31 Moreover, SATI resulted to be lower in patients with previous or ongoing episodes of clinical decompensation compared to compensated patients and a significant inverse correlation between SATI and hepatic venous pressure gradient was showed, suggesting a link between subcutaneous adipose tissue and portal hypertension. 32 Thus, again the increment in SATI observed in the present study may be considered as desirable.
The most relevant observation of the present study is on the effects of the modifications of body composition on the occurrence of HE after TIPS. The incidence and/or the recurrence of HE probably depends on several factors (liver function, portosystemic shunting, body composition, etc). What we are showing here is that, given such balance among different factors, the modification of one of them, muscle and fat, may have effect on hepatic encephalopathy.
Sarcopenia before TIPS is strongly correlated to post‐TIPS HE 13 and our results, by confirming what already observed on the TIPS induced modifications of sarcopenia and myosteatosis, 17 add data on a possible role of the adipose tissue. In fact, in the subgroup of patients who experienced an increase in SATI, a significantly lower number of episodes of HE after TIPS occurred during the follow‐up compared to those observed in the group without fat modification. In our series the modifications, adipose tissue paralleled those of muscle indices in the majority of patients and the modification of only one of the parameters occurs in a small proportion of patients. Therefore, it is very hard to discriminate the specific role of adipose tissue or muscle improvement observed after TIPS on post TIPS hepatic encephalopathy. The correlation between the differences of subcutaneous fat (delta‐SATI) and of ammonia observed in the present study before and after the TIPS may suggest the hypothesis of a role of subcutaneous adipose tissue on ammonia handling and on ammonia levels' reduction. As the correlation between the modification of SATI and ammonia was only moderate, this result must be interpreted with caution. However, the observation may have its physio‐pathological basis in an improvement of ammonia metabolisms by means of an increase activity of glutamine synthetase, which in conditions such as liver failure and portosystemic shunting may become the main mechanism for ammonia disposal. This metabolic adaptation has been well documented by studies based on ammonia balance through splanchnic area (liver) and peripheral tissue (legs) and attributed to the capacity of muscle tissue in ammonia handling by glutamine synthetase activity. 33 The exact fate of ammonia in adipose tissue is less well known, but there is the possibility that ammonia and glutamate conversion to glutamine by glutamine synthase may also occur in adipose tissue. By selective cannulation of the brachial artery and a subcutaneous abdominal vein and the determination of glutamine to glutamate ratio in adipose tissue biopsies Esbjornsson M. et al showed, in normal individuals during an exercise, a positive arterio‐subcutaneous abdominal venous plasma NH3 concentration differences, indicating a net uptake of NH3 from blood to adipose tissue 34 and opening a new perspective about the role of the adipose tissue on ammonia metabolism.
4.1. Limitations of this study
This study has several limitations mainly related to its retrospective design. Data on the patients' body composition were obtained by reviewing the CT scans and a second scan was available only in 56% of them, when clinically indicated. Although the comparison of the basal characteristics of the patients included and excluded showed no differences the reason that led to the second CT scan could have introduced a selection bias.
Another consequence of the retrospective design of this study is that time since TIPS and second TC scan is not homogeneous and not allowing to study how long after TIPS placement the improvement in SMI and SATI/VATI appears.
Another note of caution in the interpretation of the results suggesting that the increase in adipose tissue decreases post‐TIPS HE derives by the fact that the improvement in muscle and fat parameters after TIPS occurred at the same times making it difficult to know if improvement of adipose tissue without improvement of skeletal muscle would also have a beneficial impact on HE. We considered inadequate analysing the patients with selective improvement in muscle or adipose parameters due to the limited sample size and to the possible interactions expected in subgroup analysis. This study can only suggest a contribution of adipose tissue to the observed phenomenon of the parallel amelioration of post‐TIPS encephalopathy and nutritional parameters.
4.2. Conclusion
In conclusion, after TIPS there is a significant amelioration of body composition involving both muscle and adipose tissue in the majority of patients. The effect is more evident in the patients with ascites before TIPS. In the patients with muscle and fat mass improvement, a significant lower number of episodes of HE after TIPS can be recorded in comparison with the patients not showing an improvement in nutritional status. Plasma ammonia is also reduced in the ameliorated patients and its modification is significantly correlated to the modification of subcutaneous fat, supporting the hypothesis of a significant contribution of subcutaneous adipose tissue to ammonia metabolism and disposal which may become very important in conditions such as liver failure and portosystemic shunting. These observations confirm the prognostic importance of nutritional status and body composition in cirrhotic patients and their relevance in the complications of advanced liver disease such as hepatic encephalopathy. The amelioration of nutritional status and body composition should be considered an important endpoint in the management of cirrhotic patients.
CONFLICT OF INTEREST
The authors declare that no conflict of interest exists concerning this paper.
AUTHOR CONTRIBUTIONS
Stefania Gioia: acquisition of data, analysis and interpretation of data; manuscript preparation. Lorenzo Ridola: final drafting of the manuscript. Ludovica Cristofaro: acquisition of data. Manuela Merli: study supervision. Jessica Faccioli: acquisition of data. Oliviero Riggio: study concept and design, analysis and interpretation of data; manuscript preparation; final drafting of the manuscript; study supervision. Silvia Nardelli: final drafting of the manuscript; study supervision.
Supporting information
Table S1‐S3
5. ACKNOWLEDGMENTS
Open Access Funding provided by Universita degli Studi di Roma La Sapienza within the CRUI‐CARE Agreement. [Correction added on 3 JUne 2022, after first online publication: CRUI funding statement has been added.]
Gioia S, Ridola L, Cristofaro L, et al. The improvement in body composition including subcutaneous and visceral fat reduces ammonia and hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Liver Int. 2021;41:2965–2973. doi: 10.1111/liv.15060
Handling Editor: Luca Valenti
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author [SG], upon reasonable request.
REFERENCES
- 1. Merli M, Riggio O, Dally L. Does malnutrition affect survival in cirrhosis? Hepatol. 1996;23:1041‐1046. [DOI] [PubMed] [Google Scholar]
- 2. Plauth M, Merli M, Kondrup J, et al. ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr. 1997;16(2):43‐55. [DOI] [PubMed] [Google Scholar]
- 3. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing. 2010;39:412‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhanji RA, Moctezuma‐Velazquez C, Duarte‐Rojo A, et al. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol Int. 2018;12:377‐386. [DOI] [PubMed] [Google Scholar]
- 5. Montano–Loza AJ, Meza–Junco J, Prado CMM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166‐173. [DOI] [PubMed] [Google Scholar]
- 6. van Vugt JLA, Buettner S, Alferink LJM, et al. Low skeletal muscle mass is associated with increased hospital costs in patients with cirrhosis listed for liver transplantation‐a retrospective study. Transpl Int. 2018;31:165‐174. [DOI] [PubMed] [Google Scholar]
- 7. Huisman EJ, Trip EJ, Siersema PD, et al. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol. 2011;23:982‐989. [DOI] [PubMed] [Google Scholar]
- 8. Tandon P, Ney M, Irwin I, et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209‐1216. [DOI] [PubMed] [Google Scholar]
- 9. DiMartini A, Cruz RJ, Dew MA, et al. Muscle mass predicts outcomes following liver transplantation. Liver Transpl. 2013;19:1172‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Montano‐Loza AJ, Angulo P, Meza‐Junco J, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7:126‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Merli M, Giusto M, Lucidi C, et al. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis. 2013;28(2):281‐284. [DOI] [PubMed] [Google Scholar]
- 12. Lucero C, Verna EC. The role of sarcopenia and frailty in hepatic encephalopathy management. Clin Liver Dis. 2015;19(3):507‐528. [DOI] [PubMed] [Google Scholar]
- 13. Nardelli S, Lattanzi B, Torrisi S, et al. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clin Gastroenterol Hepatol. 2017;15(6):934‐936. [DOI] [PubMed] [Google Scholar]
- 14. Dasarathy J, Alkhouri N, Dasarathy S. Changes in body composition after transjugular intrahepatic portosystemic stent in cirrhosis: a critical review of literature. Liver Int. 2011;31(9):1250‐1258. Review. [DOI] [PubMed] [Google Scholar]
- 15. Tsien C, Shah SN, McCullough AJ, et al. Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent. Eur J Gastroenterol Hepatol. 2013;25(1):85‐93. [DOI] [PubMed] [Google Scholar]
- 16. Jahangiri Y, Pathak P, Tomozawa Y, et al. Muscle gain after transjugular intrahepatic portosystemic shunt creation: time course and prognostic implications for survival in cirrhosis. J Vasc Interv Radiol. 2019;30:866‐872.e4. [DOI] [PubMed] [Google Scholar]
- 17. Gioia S, Merli M, Nardelli S, et al. The modification of quantity and quality of muscle mass improves the cognitive impairment after TIPS. Liver Int. 2019;39(5):871‐877. [DOI] [PubMed] [Google Scholar]
- 18. Ebadi M, Bhanji RA, Tandon P, Mazurak V, Baracos VE, Montano‐Loza AJ. Review article: prognostic significance of body composition abnormalities in patients with cirrhosis. Aliment Pharmacol Ther. 2020;52(4):600‐618. [DOI] [PubMed] [Google Scholar]
- 19. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115‐122. [DOI] [PubMed] [Google Scholar]
- 20. Carey EJ, Lai JC, Wang CW, et al. A multi‐center study to define sarcopenia in patients with end‐stage liver disease. Liver Transplant. 2017;23(5):625‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin L, Birdsell L, MacDonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539‐1547. [DOI] [PubMed] [Google Scholar]
- 22. Angeloni S, Merli M, Salvatori FM, et al. Polytetrafluorethylene‐covered stent‐graft for TIPS procedure: 1‐year patency and clinical results. Am J Gastroenterol. 2004;99:280‐285. [DOI] [PubMed] [Google Scholar]
- 23. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the study of liver diseases and the European Association for the study of the liver. Hepatol. 2014;60(2):715‐735. [DOI] [PubMed] [Google Scholar]
- 24. Artru F, Miquet X, Azahaf M, et al. Consequences of TIPSS placement on the body composition of patients with cirrhosis and severe portal hypertension: a large retrospective CT‐based surveillance. Aliment Pharmacol Ther. 2020;52(9):1516‐1526. [DOI] [PubMed] [Google Scholar]
- 25. Dolz C, Raurich JM, Ibanez J, et al. Ascites increases the resting energy expenditure in liver cirrhosis. Gastroenterol. 1991;100(3):738‐744. [DOI] [PubMed] [Google Scholar]
- 26. Allard JP, Chau J, Sandokji K, et al. Effects of ascites resolution after successful TIPS on nutrition in cirrhotic patients with refractory ascites. Am J Gastroenterol. 2001;96(8):2442‐2447. [DOI] [PubMed] [Google Scholar]
- 27. Thomsen KL, Sandahl TD, Holland‐Fischer P, et al. Changes in adipokines after transjugular intrahepatic porto‐systemic shunt indicate an anabolic shift in metabolism. Clin Nutr. 2012;31(6):940‐945. [DOI] [PubMed] [Google Scholar]
- 28. Hanai T, Shiraki M, Watanabe S, et al. Sarcopenia predicts minimal hepatic encephalopathy in patients with liver cirrhosis. Hepatol Res. 2017;47:1359‐1367. [DOI] [PubMed] [Google Scholar]
- 29. Nardelli S, Lattanzi B, Merli M, et al. Muscle alterations are associated with minimal and overt hepatic encephalopathy in patients with liver cirrhosis. Hepatol. 2019;70(5):1704‐1713. [DOI] [PubMed] [Google Scholar]
- 30. Montano‐Loza AJ, Mazurak VC, Ebadi M, et al. Visceral adiposity increases risk for hepatocellular carcinoma in male patients with cirrhosis and recurrence after liver transplant. Hepatol. 2018;67:914‐923. [DOI] [PubMed] [Google Scholar]
- 31. Ebadi M, Tandon P, Moctezuma‐Velazquez C, et al. Low subcutaneous adiposity associates with higher mortality in female patients with cirrhosis. J Hepatol. 2018;69:608‐616. [DOI] [PubMed] [Google Scholar]
- 32. Rodrigues SG, Brabandt B, Stirnimann G, et al. Adipopenia correlates with higher portal pressure in patients with cirrhosis. Liver Int. 2019;39:1672‐1681. [DOI] [PubMed] [Google Scholar]
- 33. Damink SWMO, Jalan R, Redhead DN, et al. Inter organ ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatol. 2002;37:1163‐1170. [DOI] [PubMed] [Google Scholar]
- 34. Esbjörnsson M, Bülow J, Norman B, et al. Adipose tissue extracts plasma ammonia after sprint exercise in women and men. J Appl Physiol. 2006;101(6):1576‐1580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [SG], upon reasonable request.


