Abstract
Somatic activating mutations in the epidermal growth factor receptor (EGFR) are one of the most common oncogenic drivers in cancers such as non-small-cell lung cancer (NSCLC), metastatic colorectal cancer, glioblastoma, head and neck cancer, pancreatic cancer, and breast cancer. Molecular-targeted agents against EGFR signaling pathways have shown robust clinical efficacy, but patients inevitably experience acquired resistance. Although immune checkpoint inhibitors (ICIs) targeting PD-1/PD-L1 have exhibited durable anti-tumor responses in a subset of patients across multiple cancer types, their efficacy is limited in cancers harboring activating gene alterations of EGFR. Increasing studies have demonstrated that upregulation of new B7/CD28 family members such as B7-H3, B7x and HHLA2, is associated with EGFR signaling and may contribute to resistance to EGFR-targeted therapies by creating an immunosuppressive tumor microenvironment (TME). In this review, we discuss the regulatory effect of EGFR signaling on the PD-1/PD-L1 pathway and new B7/CD28 family member pathways. Understanding these interactions may inform combination therapeutic strategies and potentially overcome the current challenge of resistance to EGFR-targeted therapies. We also summarize clinical data of anti-PD-1/PD-L1 therapies in EGFR-mutated cancers, as well as ongoing clinical trials of combination of EGFR-targeted therapies and anti-PD-1/PD-L1 immunotherapies.
Keywords: Combination therapies, EGFR, Immune checkpoint blockade, New B7/CD28 members, PD-1/PD-L1 pathway
Introduction
The epidermal growth factor receptor (EGFR, also known as ERBB1 or HER1) belongs to the ERBB family of cell-surface receptor tyrosine kinases (RTKs) that also includes HER2 (ERBB2), HER3 (ERBB3) and HER4 (ERBB4).1,2 EGFR ligands binding to EGFR triggers homodimerization or heterodimerization with other ERBB members and induces receptor phosphorylation and activation of downstream signaling cascades including the RAS/RAF/MEK/ERK/MAPK, PI3K/AKT/mTOR, and JAK/STAT signaling pathways, leading to cell proliferation, evasion of apoptosis, angiogenesis, and metastasis.1,3,4 EGFR is frequently expressed in epithelial tumors such as non-small cell lung cancer (NSCLC), breast, colorectal, pancreatic, and head and neck squamous cell carcinoma (HNSCC).3,5 Somatic EGFR-activating mutations, most commonly exon 19 deletions (EGFRΔ19) and L858R, account for 85% of all EGFR mutations,6, 7, 8 and promote oncogenesis, tumor growth, and progression.5,9 Molecular-targeted therapies, including tyrosine kinase inhibitors (TKIs) and monoclonal antibodies (mAbs), against EGFR signaling have been successfully developed and are now the first-line treatment for EGFR-mutant NSCLC, resulting in the improved survival of patients with cancers harboring these alterations.4,10,11 However, cancer patients who initially benefit from EGFR-targeted therapies inevitably develop acquired resistance. A better understanding of the complex mechanisms of resistance may provide an opportunity to develop new mechanism-based inhibitors and combination therapies to prevent or overcome therapeutic resistance in tumors.
The T cell-based immune system has evolved to recognize and destroy abnormal cells, such as pathogen-infected cells and cancer cells, through interaction of the T cell receptor (TCR) on T cells with peptide-major histocompatibility complexes (MHC) on target cells.12,13 The functional activity of antigen-specific T cells is to a very large extent regulated by a series of co-stimulatory receptors, and co-inhibitory receptors (also known as immune checkpoints), and their ligands, thus playing a critical role in maintaining self-tolerance and limiting tissue damages.13 Among all immune checkpoints, the B7-1/B7-2–CTLA-4 and PD-L1–PD-1 pathways of the B7/CD28 family have stood out because of their proven value as therapeutic targets in a large number of malignancies. The ability to successfully target checkpoint regulators has led to growing numbers of clinical trials with antibodies targeting the pathways of other B7/CD28 members. The current B7 family members and their receptors can be phylogenetically divided into three groups: group I consisting of B7-1/B7-2/CD28/CTLA-4 and B7h (ICOSL)/ICOS; group II containing PD-L1/PD-L2/PD-1; group III including B7-H3 (CD276), B7x (B7-H4/B7S1), and HHLA2 (B7y/B7H7/B7-H5)/TMIGD2 (IGPR-1/CD28H)/KIR3DL3.14, 15, 16, 17, 18, 19, 20 Compelling evidence indicates that B7 molecules not only provide crucial positive signals to stimulate and support T cell activation, but also offer negative signals that control and suppress T-cell responses.
Increasing numbers of studies have demonstrated that the upregulation of B7/CD28 family members, such as PD-L1, B7x and HHLA2, is associated with activating EGFR mutations,21, 22, 23, 24, 25, 26, 27, 28 which may contribute to resistance to EGFR-targeted therapies by creating an immunosuppressive tumor microenvironment (TME). In this review, we will discuss the regulatory effect of EGFR signaling on the B7/CD28 pathways, which may inform future combination therapeutic strategies and overcome the current challenge of EGFR-TKI resistance. We also summarize clinical data and ongoing clinical trials of combination of EGFR-targeted therapies with anti-PD-1/PD-L1 immunotherapies in EGFR-mutant cancers.
Crosstalk between the EGFR pathway and the PD-1/PD-L1 axis
The role of the EGFR signaling pathway on the PD-1/PD-L1 pathway
The PD-1/PD-L1 axis is a critical co-inhibitory immune pathway involved in the tumor immune evasion by counteracting T cell effector functions and promoting exhaustion. Aberrant PD-L1 expression is frequently observed in human cancers. PD-L1 binding to its receptor PD-1 on activated T cells alters the function of T cells by means of inhibiting T cell proliferation and survival, cytokine production, and other effector functions. Consistent with the role of PD-1/PD-L1 in suppressing T cell functions, antibody-based PD-1/PD-L1 inhibitors display striking responses in patients with diverse advanced cancers, which has led to clinical approval for the treatment of melanoma, NSCLC, renal cell carcinoma (RCC), Hodgkin's lymphoma, bladder cancer, HNSCC, Merkel-cell carcinoma, and microsatellite instable-high (MSI-H) or mismatch repair-deficient (dMMR) solid tumors.29,30 Despite considerable improvement in patient outcomes, durable responses to these therapies are observed only in a minority of patients. Indeed, PD-L1 expression on tumor cells and in TME has been closely associated with clinical responses,31, 32, 33, 34 highlighting the necessity of a better understanding of the mechanisms which control PD-L1 expression.
Expression of PD-L1 in tumor cells is determined by complex regulatory mechanisms, including but not limited to, aberrant oncogenic signaling, inflammatory signaling, posttranslational modulation, and genomic alterations.30 Compelling findings have demonstrated that aberrant oncogenic signaling may contribute to tumor outgrowth by driving PD-L1 expression in a tumor cell-intrinsic manner.30 The EGFR signaling pathway is one of the most important oncogenic pathways in NSCLC,35, 36, 37 metastatic colorectal cancer (CRC),38,39 glioblastoma,40, 41, 42 HNSCC,43 pancreatic cancer,44,45 breast cancer,46, 47, 48 and many other types of tumors. A series of preclinical and retrospective studies show that activating EGFR mutations as well as stimulation with the ligands can induce PD-L1 expression in bronchial epithelial cells,49 NSCLC,21,50 HNSCC,51 and breast cancer52 cells via downstream effector pathways. In line with this finding, the expression level of PD-L1 is significantly higher in NSCLC cell lines with mutant EGFR than in cells with wild type (WT) EGFR.49 Pharmacological inhibition of EGFR activity by EGFR-TKIs, e.g., gefitinib or erlotinib, decreases the constitutive expression of PD-L1 in NSCLC with mutant EGFR.21,49,50,53,54 In an EGFR-driven mouse model of lung cancer, microarray expression profiling of mice carrying EGFR mutations as compared to WT controls reveals elevated PD-L1 and PD-1 expression, thus leading to an immunosuppressive lung microenvironment.49
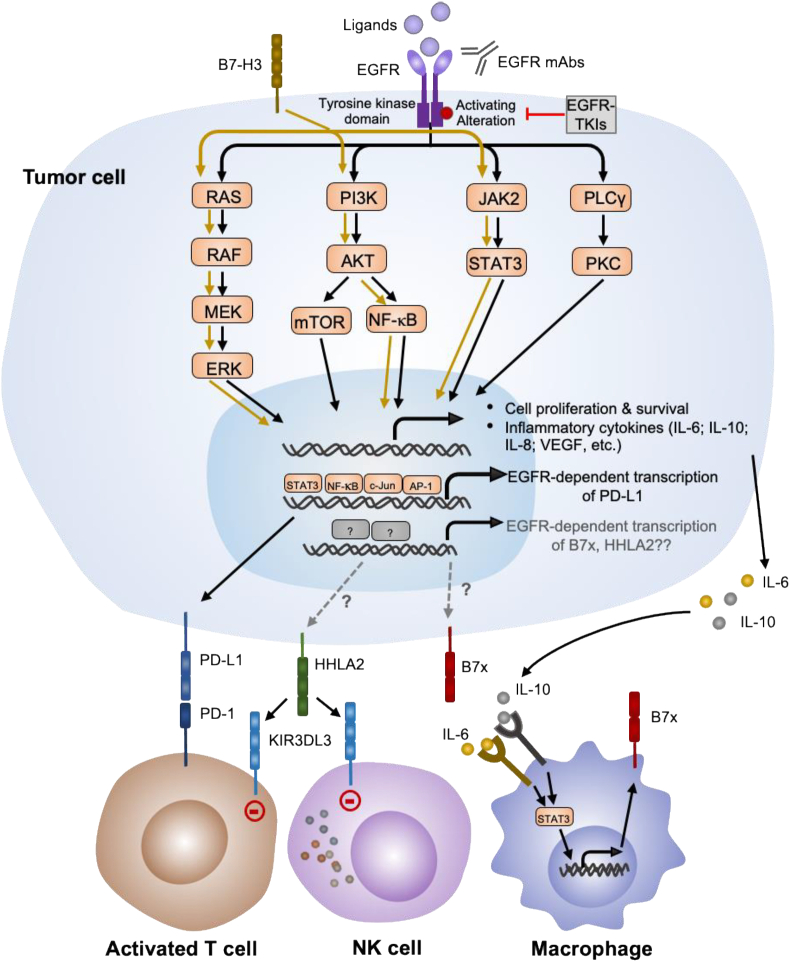
Mechanisms underlying the EGFR-medicated upregulation of PD-L1 are complicated. Upregulation of PD-L1 expression in EGFR mutant NSCLC cells can be blocked by the mTOR inhibitor rapamycin55 and by an ERK inhibitor,21 suggesting mTOR and ERK pathways may be responsible for the EGFR-mediated PD-L1 expression. In addition, several studies in HNSCC and NSCLC provide evidence for a model in which NF-κB, STAT3, and/or JAK2-STAT1 may also serve as mediators between EGFR signaling and PD-L1 expression50,51,54 (Fig. 1).
Figure 1.
Schematic overview of crosstalk between the EGFR signaling and the B7/CD28 family. Pathways known to be involved in the EGFR-mediated signaling are depicted. Activation of EGFR by either ligand interaction or activating gene alteration leads to downstream signaling pathways that ultimately drive tumor proliferation, survival and induce pro-tumoral cytokines. EGFR signaling activation induces PD-L1 upregulation by activation of the PI3K/AKT, RAS/RAF/MEK/ERK or JAK/STAT3 pathways. These pathways involved in the EGFR signaling also mediate B7-H3 signaling, however, the crosstalk between these two pathways remains to be determined. B7x and HHLA2 are also associated with activating EGFR mutations through unclear mechanisms. IL-6 and IL-10 induced by EGFR activation may induce B7x expression in macrophages through JAK/STAT3 signaling, which represents a potential mechanism by which EGFR upregulates B7x expression. The upregulation of HHLA2 could result in the inhibition of T and NK cell response through interacting with its inhibitory receptor KIR3DL3. JAK, Janus kinase; NF-κB, nuclear factor kappa B; PI3K, phosphatidylinositol-4,5-Bisphosphate 3-kinase; PLC, phospholipase C; PD-1, programmed cell death protein-1; PD-Ll, programmed death ligand-1; STAT3, signal transducer and activator of transcription 3; HHLA2, human endogenous retrovirus-H long terminal repeat-associating protein 2; KIR3DL3, killer cell immunoglobulin-like receptor, three immunoglobulin domains and long cytoplasmic tail 3; NK cell, natural killer cell; B7x, B7 homolog x.
The effectiveness of PD-1/PD-L1 inhibitors in EGFR mutant NSCLC is currently controversial. Theoretically, increased PD-L1 expression in EGFR mutant and EGFR-TKI resistant NSCLC cell lines suggests that PD-1/PD-L1 blockade may be a promising approach in NSCLC with EGFR mutations. However, the overall clinical proof of principle currently could not fully support this therapeutic approach in EGFR-mutant NSCLC patients. Patients with NSCLC harboring EGFR mutations have poor clinical outcomes and are associated with low objective response rate (ORR) in response to treatment with PD-1/PD-L1 inhibitors.56, 57, 58 In a retrospective analysis of 58 patients treated with PD-1/PD-L1 inhibitors, relatively higher ORRs were observed in EGFR-WT patients (23.3%) versus EGFR-mutant patients (3.6%).56 Several meta-analyses exploring the efficacy of PD-1/PD-L1 inhibitors versus the chemotherapy docetaxel show that PD-1/PD-L1 inhibitors prolonged the overall survival (OS) of patients with EGFR-WT, but not EGFR-mutant advanced NSCLC.58, 59, 60, 61 In addition, one study not only analyzed the data from NSCLC, but also other types of cancer, and demonstrated that significantly prolonged progression-free survival (PFS) and OS were observed in patients with EGFR-WT cancers receiving PD-1 blockade but not in those with EGFR-mutated cancers.62 The underlying association between EGFR mutations and immune resistance remains largely unclear. We will review the potential mechanisms by which EGFR mutations impair response to PD-1/PD-L1 blockade and summarize the current clinical data of ICIs in EGFR-mutant cancers in the following section (Section Aberrant EGFR signaling regulates anti-tumor immunity and is associated with resistance to ICIs).
The PD-1/PD-L1 pathway modulates the efficacy of EGFR-TKIs
EGFR TKIs have been a first-line therapy in the treatment of NSCLC harboring EGFR sensitive mutations. Four FDA-approved EGFR TKIs are currently in clinical use, with response rates of ∼50–80%, including the first-generation non-covalent inhibitors erlotinib and gefitinib, the second-generation covalent inhibitor afatinib, and the more recently approved third-generation, WT-sparing, mutant EGFR-specific TKI osimertinib.63, 64, 65 Growing evidence suggests that inhibiting EGFR by EGFR-TKIs modulates the tumor immune microenvironment, including enhancing MHC I and MHC II expression, attenuating the suppressive function of regulatory T cells (Tregs), promoting the activity of cytotoxic T cells (CTLs) and reducing the apoptosis of T cells.21,66, 67, 68, 69 Although treatment with EGFR TKIs improves outcomes in patients with NSCLC, responses to these agents remain largely incomplete and temporary due to acquired resistance.
The association between PD-L1 expression status and clinical outcomes of EGFR-TKIs in patients with EGFR mutant advanced NSCLC has been explored. However, currently there is no consensus on the prognostic role of PD-L1 expression in EGFR mutant NSCLC treated with EGFR-TKIs. Two studies have reported that PD-L1 positive compared to PD-L1 negative EGFR-mutant NSCLC patients treated with EGFR-TKIs have a significantly longer PFS.70,71 Conversely, several other studies show that high tumor PD-L1 expression in EGFR-mutant NSCLC was associated with a shorter PFS.72,73 The inconsistent findings among the studies may be due to differences in prior treatment, detection approach, PD-L1 antibody clones, and scoring cutoffs. PD-L1 expression is dynamic during the time course of EGFR-TKI treatment. The expression of PD-L1 could be reduced by EGFR-TKIs,21,49,54 while it is upregulated in some patients who acquired resistance to EGFR-TKIs.56,74,75 The mechanisms underlying acquired resistance to EGFR-TKIs are complex, and more than 50% were mediated by T790M.76,77 The third-generation EGFR-TKI osimertinib significantly improves survival of T790M mutation positive patients, but not T790M mutation negative patients who developed resistance to EGFR-TKIs. Studies show PD-L1 expression was significantly higher in T790M negative patients compared to T790M positive patients.74 Collectively, PD-L1 upregulation might be correlated with EGFR-TKI resistance in a subset of patients, especially in T790M negative patients.
Crosstalk between the EGFR pathway and new B7 family pathways
The molecules B7-H3, B7x and HHLA2 in the third group of B7/CD28 family have been found to be overexpressed in various human malignancies.78,79 Cumulative studies reveal their aberrant expression is associated with poor prognoses and clinical outcomes in certain human cancers. Given their emerging roles in suppressing anti-tumor immune responses, there are gaining increasing interest as new therapeutic targets for the treatment of human diseases. In this section, we will review the advances of B7-H3, B7x and HHLA2 and their association with the EGFR pathway, thereby expanding the scope of treatment strategies for cancer patients with EGFR mutations and acquired EGFR-TKI resistance.
The correlation of B7-H3 with the EGFR signaling pathway
B7-H3 (also known as CD276) is a type I transmembrane protein that belongs to the B7 family. B7-H3 expression is not detectable on peripheral blood lymphocytes, but is induced on T cells, B cells and NK cells upon in vitro stimulation. B7-H3 was initially identified as a T cell co-stimulator in humans due to its ability to promote T-cell proliferation and interferon gamma (IFN-γ) secretion.80 However, increasing evidence suggests that it is a negative regulator.81, 82, 83, 84 In mice, B7-H3 inhibits T cell activation and effector cytokine production, so an anti-B7-H3 antagonistic mAb exacerbated experimental autoimmune encephalomyelitis (EAE) in vivo.85 In the context of graft-versus-host disease (GVHD), absent B7-H3 expression on allogeneic donor T cells leads to accelerated GVHD lethality associated with increased T-cell proliferation and inflammatory cytokines,84 demonstrating that B7-H3 provides a negative signal to T cells. In humans, B7-H3 expression is absent or low in normal tissues, however, more than 60% and up to 93% of patient tumor tissues display aberrant expression of B7-H3 in the vast majority of malignancies, and its expression is significantly linked to poor prognosis and decreased OS in patients with RCC,86 lung cancer,87 prostate cancer,88 CRC,89 gallbladder cancer,90 esophageal squamous cancer,91 osteosarcoma,92 and breast cancer,93 suggesting that B7-H3 is an attractive biomarker and high-value target for multiple cancer immunotherapy strategies.
B7-H3 promotes cancer cell survival, invasion, migration, metastasis and drug resistance in various types of cancer that is independent of its immune function by acting upstream from signal transduction pathways, such as PI3K/AKT, JAK2/STAT3, and Raf/MEK/ERK1/2, to induce anti-apoptotic and proliferative mechanisms.94, 95, 96, 97, 98 These pathways are also involved in EGFR-triggered signaling in lung adenocarcinoma cells (Fig. 1), thus the correlation between the two signaling pathways has been elucidated in several studies. B7-H3 knockout increases susceptibility of NSCLC cell lines harboring activating EGFR mutations to EGFR inhibitor gefitinib.25 Furthermore, B7-H3 ablation displayed significant synergistic effects with gefitinib in vitro.25 Additionally, high B7-H3 expression is associated with EGFR WT status (multivariable OR = 2.80, 95% CI = 1.38–5.84; P = 0.0042),99 hinting at the potential effectiveness of anti-B7-H3 therapy in EGFR WT lung adenocarcinoma. In pancreatic cancer, activation of B7-H3 by an agonist induced the expression of EGFR,24 suggesting that B7-H3 may be involved in regulating the EGFR signaling pathway in different cancer settings. However, the exact mechanism needs to be explored. Collectively, these studies reveal the correlation of B7-H3-induced signaling with the EGFR signaling pathway, and the translational potential of combined therapy targeting B7-H3 and EGFR in human cancers.
B7x is aberrantly expressed in EGFR mutated cancers
B7x (also known as B7S1, B7-H4, VTCN1) is a B7 ligand that was discovered by us and others simultaneously.14,100,101 B7x mRNA is widely distributed in mouse and human peripheral tissues, however, protein expression is restricted and can be induced on antigen presenting cells (APCs) after in vitro stimulation.14,102 Previous studies have found that aberrant B7x is expressed on a broad spectrum of cancers, including those of the stomach, kidney, ovary, lung, uterus, breast, prostate, and others.79 The binding of B7x to its unidentified receptor(s) inhibits TCR-mediated T cell proliferation by cell-cycle arrest and decreases IL-2 production,14,100,101 indicative of its role as a negative regulator of T cell responses. In addition to dampening effector T cell activity, B7x also promotes myeloid-derived suppressor cells103, 104, 105, 106 and macrophages,107,108 thus inducing an overall immunologically tolerant and immunosuppressive TME. Clinically, B7x expression is significantly associated with greater disease progression and poorer prognosis in patients with RCC,106,109 lung cancer,110,111 CRC,112 cholangiocarcinoma,113 glioma,108 pancreatic cancer,114 and others. Interestingly, the role of B7x in cancer seems to be conserved across mammalian species given that B7x expression is also associated with worse prognosis in canine bladder cancer.115 Blockade of B7x has demonstrated significant therapeutic efficacy across multiple murine models by reducing primary tumor growth and metastasis.116, 117, 118, 119 Collectively, these findings recognize that targeting B7x is a very attractive strategy for cancer immunotherapy.
Our previous study shows that, in tissue microarrays consisting of 392 resected NSCLC tumors, B7x was expressed in 69% of tumors and the co-expression of PD-L1 with B7x was infrequent.27 Although our univariate analysis revealed no significant difference between WT and mutated EGFR tumors in both the discovery (P = 0.06) and validation cohorts (P = 0.55),27 another independent study showed that the expression of B7x in the EGFR mutant group was significantly higher than those in the WT group (mutant vs. WT IHC score: 3.250 [0–7.000] vs. 5.000 [1.000–7.000]; P = 0.045).28 Moreover, nearly half of the PD-L1 negative patients expressed B7x, indicating that B7x may be a promising immune target for patients with EGFR mutant lung cancer independent of PD-L1 expression. The functional mechanisms of upregulation of B7x in EGFR mutant cancers have not yet been investigated. Previous findings show that EGFR-driven lung tumors inhibit antitumor immunity by activating the PD-1/PD-L1 pathway to suppress T-cell function and increase levels of proinflammatory cytokines including IL-6, IL-10, IL-8, and VEGF.49 Of note, IL-6, together with IL-10, can induce B7x expression in infiltrating myeloid cells through JAK/STAT3 signaling,108,120 which may represent a potential mechanism by which EGFR upregulates B7x expression in NSCLC (Fig. 1).
HHLA2 is associated with EGFR mutation in NSCLC
We recently identified HHLA2 (also known as B7-H7, B7-H5, B7y) as the newest member of the B7 family15 that shares 23%–33% similarity to other human B7 proteins, with the greatest phylogenetical similarity to B7-H3 and B7x. Notably, HHLA2 is the only B7 family member that is found in humans but not in mice. The HHLA2 protein is constitutively expressed on human monocytes/macrophages, and its expression on mature DCs and monocytes is modestly upregulated by inflammatory signals like lipopolysaccharides (LPS), IFN-γ, and poly I:C.15,16 HHLA2 protein is widely expressed in human cancers from the breast, lung, thyroid, melanoma, pancreas, ovary, liver, bladder, colon, prostate, kidney, and esophagus.17 Studies have shown that high expression of HHLA2 in tumors is correlated with a worse prognosis for lung cancer, osteosarcoma,121 gastric cancer,122 breast carcinoma,17 ccRCC,123 intrahepatic cholangiocarcinoma,124 bladder urothelial carcinoma125 and CRC,126 but is associated with a better prognosis for pancreatic ductal adenocarcinoma127 and glioma.128 This could be because HHLA2 can exert either a costimulatory or coinhibitory effect on T and NK cell activation. The costimulatory effect of HHLA2 is mediated through the CD28 family member transmembrane and immunoglobulin domain containing 2 (TMIGD2, also called CD28H or IGPR-1).16,17 Recently, we and other groups identified killer cell immunoglobulin-like receptor, three immunoglobulin domains and long cytoplasmic tail 3 (KIR3DL3) as an inhibitory receptor for HHLA2 on T and NK cells.19,20,129,130 Monoclonal antibodies targeting KIR3DL3 and HHLA2 effectively blocked both receptor–ligand interactions and inhibitory functions.19 HHLA2 expression is nonoverlapping with PD-L1 expression in NSCLC27 and intrahepatic cholangiocarcinoma,124 suggesting that HHLA2 mediates a mechanism of tumor immune evasion independent from the PD-1/PD-L1 pathway. Given its emerging roles in suppressing tumor immune responses with different immune evasion mechanisms from the PD-1/PD-L1 pathways, HHLA2 is attracting interest as a new therapeutic immune target.
We previously investigated the expression and clinical significance of HHLA2 in human lung cancer and found that HHLA2 was not detected in most of normal lung tissues but expressed in 66% of 679 NSCLC tumor tissues across different subtypes.27 EGFR-mutated NSCLC was significantly associated with higher tumor HHLA2 expression in both the discovery (EGFR vs. WT: 76% vs. 53%, P = 0.01) and validation cohorts (89% vs. 69%, P = 0.01).26 In line with our findings, another independent study also observed HHLA2 was prominently upregulated in lung adenocarcinoma with EGFR mutation.28 In the multivariate analysis, high tumor-infiltrating lymphocyte intensity and EGFR mutation status were independently associated with HHLA2 expression in lung adenocarcinoma.26 In addition, in the EGFR-mutated subgroup, patients with high HHLA2+ tumors trended toward poorer survival,26 suggesting HHLA2 may be a new therapeutic target for lung cancer, especially for patients with EGFR mutations. Interestingly, a notably negative correlation between WT EGFR and HHLA2 expression was demonstrated in a study including 47 NSCLC cancer tissues and 4 cell lines.131 EGFR silencing and EGFR-TKI treatment significantly increased HHLA2 mRNA and protein expression in NCI-H1299 cells.131 Collectively, these studies suggest that EGFR may participate in immune evasion through the regulation of HHLA2 expression in NSCLCs. Further investigation on the mechanism(s) regulating HHLA2 expression in EGFR-mutated cancers is clearly warranted.
Aberrant EGFR signaling regulates anti-tumor immunity and is associated with resistance to ICIs
ICIs, represented by anti-PD-1/PD-L1 immunotherapies, have showed superior outcomes over traditional chemotherapy in the treatment for multiple solid tumors. However, they failed to achieve similar efficacy in EGFR-mutant NSCLCs in multiple large-scale, randomized controlled clinical trials.31,132,133 A phase II clinical trial was designed to study the efficacy of the anti-PD-1 antibody pembrolizumab in patients with EGFR-mutant, PD-L1 positive, TKI treatment naïve, advanced NSCLC, but it was terminated early due to lack of efficacy after 11/25 planned patients were treated.134 This result was validated by a systemic review and meta-analysis studying patients with advanced NSCLC treated with checkpoint inhibitors.59 Therefore, it is not recommended to use single-agent immunotherapy for patients with stage IV NSCLC and any activating EGFR mutation based on the latest American Society of Clinical Oncology (ASCO) and Ontario Health (OH) guidelines.135
The exact mechanism behind the important clinical question of why EGFR-mutant NSCLC is treatment resistant to PD-1/PD-L1 inhibitors, is not yet clear, but it is considered to be associated with low tumor mutation burden (TMB), as well as an immunosuppressive TME in EGFR-signaling aberrant tumors.136 One study retrospectively examined 171 EGFR-mutant NSCLC patients who were treated with ICIs and concluded that patients with the EGFR L858R mutation tumors had similar OS compared with patients with EGFR WT tumors, whereas patients with EGFRΔ19 had inferior outcomes.137 This finding was independent of PD-L1 expression status. Another cohort of patients with sequencing data available showed that patients with the EGFRΔ19 mutations carried a lower TMB compared with patients with the EGFR L858R mutation. Of note, NSCLCs with high TMB are associated with a better response rate to ICIs in large scale clinical trials.138,139 Another study retrospectively examined 25 patients with EGFR-mutant NSCLC who received anti-PD-1 inhibitor nivolumab after disease progression on EGFR-TKI therapy, to evaluate immunotherapy efficacy and the TME. Patients with the negative EGFR T790M mutation were more likely to respond to nivolumab than those with the positive EGFR T790M mutation, possibly secondary to a higher PD-L1 expression level. However, the PFS, OS, PD-L1 expression level and tumor infiltrating CD8 T cell densities did not reach statistical significance, potentially due to the limited sample size (n = 25).140 An evaluation of 153 patients with advanced, EGFR-mutant NSCLC (with only the EGFRΔ19 mutation or EGFR L858R mutation included) showed that patients with high TMB (defined as > 4.85 mutations/Mb) had shorter time-to-treatment discontinuation (TTD) and shorter OS than patients with low/intermediate TMB (low: ≤ 2.83 mutations/Mb; intermediate: 2.83–4.85 mutations/Mb).141 Interestingly, rebiopsied tumor tissue post TKI treatment from patients who had disease progression during EGFR-TKI treatment, showed a higher percentage of high PD-L1 expression (14% → 28%; high expression defined as PD-L1 level ≥50%) and higher TMB (3.3 → 4.1 mutations/Mb). Importantly, in this cohort, patients with high PD-L1 expression had a longer median PFS than patients with low PD-L1 expression after treatment of anti-PD-1 immunotherapy.142 Together, the current evidence suggests that while EGFR-mutant NSCLC responds relatively poorly to ICIs compared with EGFR WT tumors, a selected subgroup of patients with EGFR-mutant NSCLC may potentially benefit from ICIs. Further studies are warranted to identify the group of patients with advanced EGFR-mutant lung cancers who might benefit from ICIs, such as those with high TMB or specific EGFR mutations (e.g., L858R).
Combination of EGFR-targeted therapies with ICIs
There is substantial interest to overcome the immunosuppressive effects in EGFR-mutant NSCLC, as patients who initially responded to EGFR-TKIs eventually developed treatment resistance and ICIs could potentially provide durable effects.143 Preclinical studies demonstrated that EGFRΔ19 mouse tumors treated with EGFR-TKI erlotinib were infiltrated with lower frequencies of Tregs and higher frequencies of cytotoxic CD8 T cells, suggesting a more inflamed TME, compared with EGFR-mutant controls without erlotinib treatment.144 Furthermore, a combination of erlotinib and anti-PD-1 therapy significantly inhibited EGFR-mutant mouse tumor growth compared with either therapy alone.144 Therefore, the joint usage of EGFR-targeted therapies could potentially increase the efficacy of PD-1 blockade immunotherapy in EGFR mutant lung cancers, leading to the increasing numbers of clinical trials evaluating combinations of EGFR-targeted therapies and PD-1/PD-L1 inhibitors (Table 1).
Table 1.
Selected clinical trials evaluating combinations ofEGFR-targetedtherapies andPD-1/PD-L1inhibitors.
| Disease | Phase | treatment regimen | Outcome | Clinicaltrials.gov identifier |
|---|---|---|---|---|
| Advanced NSCLC | I/II | Anti-EGFR: Erlotinib (group D) ICI: Nivolumab |
Unpublished | NCT02574078 |
| Stage IIIb/Iv NSCLC | I | Anti-EGFR: Erlotinib (arm E) ICI: Nivolumab |
ORR 15%, 24-week PFS 48%149 | NCT01454102 |
| EGFR positive, advanced NSCLC | I | Anti-EGFR: Erlotinib ICI: Atezolizumab |
ORR 75%, Median PFS 15.4 months, OS 32.7 months150 | NCT02013219 |
| EGFR positive, advanced lung cancer | I | Anti-EGFR: Osimertinib ICI: Durvalumab (MEDI4736) |
Terminated early due to increased incidence of interstitial lung disease146 | NCT02143466 |
| Advanced or metastatic HNSCC; NSCLC (multiple subtypes) |
I/II | Anti-EGFR: CIMAvax Vaccinea158 ICI: Nivolumab or Pembrolizumab |
Ongoing | NCT04298606 |
| Intermediate and high-risk local-regionally advanced HNSCC | I | Anti-EGFR: Cetuximab (arm 3) ICI: Nivolumab And radiation therapy (IMRT) |
Ongoing | NCT02764593 |
| Advanced colorectal cancer | II | Anti-EGFR: Cetuximab ICI: Sintilimab And Regorafenib |
Ongoing | NCT04745130 |
| Locally advanced HNSCC | I/II | Anti-EGFR: Cetuximab ICI: Durvalumab And radiation therapy (IMRT) |
Ongoing | NCT03051906 |
| Recurrent/metastatic HNSCC | II | Anti-EGFR: Cetuximab ICI: Pembrolizumab |
Ongoing | NCT03082534 |
| EGFR-driven advanced solid tumors | I/Ib | Anti-EGFR: BCA101b ICI: Pembrolizumab |
Ongoing | NCT04429542 |
| Recurrent or metastatic HNSCC (PD-L1 positive); Advanced or metastatic cutaneous SCC |
I/II | Anti-EGFR: ASP-1929c photoimmunotherapy ICI: Pembrolizumab or Cemiplimab |
Ongoing | NCT04305795 |
EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; HNSCC, head neck squamous cell carcinoma; SCC, squamous cell carcinoma.
CIMAvax Vaccine: CIMAvax-EGF vaccine is a therapeutic cancer vaccine, consisting of a chemical conjugate of the EGF with the P64 protein derived from the Meningitis B bacteria and Montanide ISA 51, as adjuvant.
BCA101: EGFR/TGFβ-trap bifunctional antibody.
ASP-1929: antibody drug conjugate of cetuximab and IRDye 700DX®, a phthalocyanine dye.
However, several early-phase clinical trials conducted to explore the safety and efficacy of combining EGFR-TKIs and anti-PD-1/PD-L1 immunotherapies were ceased early due to severe liver or pulmonary toxicities.145 Specifically, in one arm of the TATTON study (NCT02143466), combining osimertinib (a third generation EGFR TKI) and durvalumab (an anti-PD-L1 mAb) in EGFR-mutant lung cancer, 5/23 (22%) patients developed interstitial lung disease, which led to early discontinuation of the arm.146 The CAURAL trial (NCT02454933), an open label, phase III trial to compare osimertinib, with or without durvalumab, was terminated early as a similar regimen used in TATTON trial caused increased incidence of interstitial pneumonitis.147 In the phase I/II KEYNOTE-021 study (NCT02039674), the cohort that received pembrolizumab and gefitinib combination therapy showed high incidence (5/7 patients, 71.4%) of grade 3/4 liver toxicity.145 A large database study of 20,516 participants with NSCLC in the US FDA adverse event report system reported a much higher rate of interstitial pneumonitis (18/70, 25.7%) in patients who received both EGFR-TKIs and nivolumab, compared with 4.80% for total participants and 4.59% for patients received EGFR-TKIs only.148 These studies raised serious safety concerns regarding the combination of EGFR-TKIs and anti-PD-1/PD-L1 immunotherapies.
Meanwhile, several other studies reported tolerable toxicities when other combination regimens were used.145,149,150 In the KEYNOTE-021 study (NCT02039674), the combination of pembrolizumab and erlotinib was feasible with similar toxicities to monotherapy. The combination therapy of nivolumab and erlotinib in a phase I trial in advanced, EGFR-mutant NSCLC patients, showed that this regimen was tolerable with durable responses in a small portion of patients (3/20, 15%) (NCT01454102). One patient, who was EGFR-TKI naïve when treated with this regimen, achieved a complete response lasting more than 5 years at the time of this report.149 A phase Ib study combined the EGFR-TKI erlotinib with the anti-PD-L1 atezolizumab in 28 patients with locally advanced or metastatic NSCLC (NCT02013219).150 The safety profile was tolerable, similar to erlotinib monotherapy, and the final outcome data are not yet available. In summary, these reports, although with small patient samples, suggest that certain combinations of EGFR-TKIs and anti-PD-1/PD-L1 immunotherapies are feasible and can potentially provide durable response in some patients.
Besides EGFR-TKIs, cetuximab (a chimeric mouse-human IgG1 antibody) and panitumumab (a fully humanized IgG2 antibody) are two anti-EGFR mAbs whose major mechanisms of action are blocking ligand binding, promoting receptor internalization, and mediating antibody- and complement-mediated cytotoxicity (ADCC and CDC, respectively). Cetuximab has been approved for locally advanced and recurrent and/or metastatic HNSCCs and metastatic CRCs. Cetuximab treatment upregulates PD-1 expression in NK cells, and PD-1 blockade increases cetuximab-mediated ADCC against PD-L1hi HNSCC cells without EGFR amplification, indicating that the combination of anti-EGFR mAbs with ICIs could augment both innate and acquired anti-tumor immune responses against EGFR-expressing HNSCC.151 Currently, anti-PD-1/PD-L1 mAbs in combination with cetuximab are being evaluated in phase II clinical trials in recurrent and/or metastatic HNSCC (NCT03082534 and NCT03082534).
Ipilimumab is an anti-CTLA-4 antibody that has been approved for treating several malignancies given its role in enhancing effector cells and depleting Tregs. Few studies have explored the feasibilities and potential benefit of combining EGFR-targeted therapies and ipilimumab. In a study of patients with stage III/IV HNSCC who were treated with cetuximab, CTLA-4+ Tregs were found to suppress cetuximab-mediated ADCC effects and correlate with poor clinical outcome. Ipilimumab could eliminate intratumoral Tregs and restore NK cell mediated ADCC in an ex vivo assay, suggesting that addition of ipilimumab could improve the clinical efficacy of cetuximab treatment.152 In another study of patients with EFGR-mutant NSCLC treated with first line EGFR-TKIs, CTLA-4+ immune shaped cell (ISC) counts were found to be borderline correlated with OS in such patients (P = 0.061).73 Additionally, the combination of ipilimumab plus EGFR-TKI erlotinib notably improved the PFS and OS of patients with EGFR-mutated advanced NSCLC in a phase I trial, although erlotinib plus ipilimumab caused excessive gastrointestinal toxicity resulting in early study closure.153 Overall, more convincing evidence is warranted to support the combination therapy of EGFR-targeted therapies and anti-CTLA-4 immunotherapy. A phase 1b clinical trial is ongoing to evaluate the safety and efficacy of combining third-generation EGFR-TKI osimertinib and ipilimumab in patients with EGFR-mutant NSCLC (NCT04141644).
More preclinical and clinical studies are warranted to explore the mechanism, safety, and efficacy of EGFR-TKIs and ICIs combination therapy. With the evidence of increased incidences of toxicities in some clinical trials, especially interstitial lung diseases, exceptional precautions should be taken when designing such trials.143,154, 155, 156
Conclusion and perspectives
EGFR signaling in cancer cells contributes to tumor progression by promoting cell growth, survival, and establishing an immunosuppressive TME. Molecular-targeted therapies against EGFR currently benefit a subset of cancer patients with activating EGFR mutations. The upregulation of PD-L1 expression in tumor cells following TKI therapy might contribute to an immunosuppressive TME by inhibiting T cell-mediated antitumor cytotoxicity. Thus, EGFR-targeted therapies in combination with PD-1/PD-L1 inhibitors are being evaluated in clinical trials as a valuable therapeutic approach. To optimize PD-1/PD-L1 inhibitors in patients with aberrant EFGR signaling, it is essential to develop novel therapeutic strategies targeting the immunosuppressive pathways caused by EGFR signaling. In addition to PD-L1, the new B7/CD28 members B7-H3, B7x, and HHLA2 are also correlated with EGFR signaling, although the underlying mechanisms remain elusive. Importantly, B7-H3,157 B7x27,120 and HHLA219,20,27 tend to have mutually exclusive expression from PD-L1 in cancer cells, suggesting that these new B7 members mediate mechanisms of tumor immune evasion that are independent from the PD-1/PD-L1 pathway. The roles of these new B7/CD28 pathways in mediating immune evasion in PD-L1-negative tumors should be explored to provide the rationale for an effective immunotherapy strategy in these tumors. Nevertheless, this may suggest that ICIs targeting these new B7 members could be an alternative option for PD-1/PD-L1 inhibitors in combination with EGFR-targeted therapies for treating patients with low or negative PD-L1 expression. Encouragingly, several inhibitors blocking these new B7 members have been under investigation in clinical trials and have shown very promising outcomes. Collectively, targeting EGFR signaling with molecular-targeted therapies in combination with cancer immunotherapies, particularly ICIs targeting new B7/CD28 members, could contribute to developing optimal cancer therapies, thus augmenting anti-tumor efficacy in the clinic.
Author contributions
Writing—original draft preparation, X.R. and Y.L.; writing—review and editing, X.R., Y.L., C.N. and X.Z.; supervision, X.Z. All authors have read and agreed to the published version of the manuscript.
Conflict of interests
The authors declare no conflict of interest.
Funding
Research in the Zang lab is supported by NIH R01CA175495 and R01DK100525, Department of Defense BC190403, Irma T. Hirschl/Monique Weill-Caulier Trust, and Cancer Research Institute.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Bazley L.A., Gullick W.J. The epidermal growth factor receptor family. Endocr Relat Cancer. 2005;12(Suppl 1):S17–S27. doi: 10.1677/erc.1.01032. [DOI] [PubMed] [Google Scholar]
- 2.Cataldo V.D., Gibbons D.L., Pérez-Soler R., Quintás-Cardama A. Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N Engl J Med. 2011;364(10):947–955. doi: 10.1056/NEJMct0807960. [DOI] [PubMed] [Google Scholar]
- 3.Arteaga C.L., Engelman J.A. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014;25(3):282–303. doi: 10.1016/j.ccr.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciardiello F., Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358(11):1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 5.Chong C.R., Jänne P.A. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med. 2013;19(11):1389–1400. doi: 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosell R., Moran T., Queralt C., et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 7.Sharma S.V., Bell D.W., Settleman J., Haber D.A. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 8.Shigematsu H., Gazdar A.F. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006;118(2):257–262. doi: 10.1002/ijc.21496. [DOI] [PubMed] [Google Scholar]
- 9.Bell D.W., Gore I., Okimoto R.A., et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet. 2005;37(12):1315–1316. doi: 10.1038/ng1671. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg S.B., Redman M.W., Lilenbaum R., et al. Randomized trial of afatinib plus cetuximab versus afatinib alone for first-line treatment of EGFR-mutant non-small-cell lung cancer: final results from SWOG S1403. J Clin Oncol. 2020;38(34):4076–4085. doi: 10.1200/JCO.20.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paez J.G., Jänne P.A., Lee J.C., et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 12.Bour-Jordan H., Esensten J.H., Martinez-Llordella M., Penaranda C., Stumpf M., Bluestone J.A. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/B7 family. Immunol Rev. 2011;241(1):180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zang X., Allison J.P. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13(18 Pt 1):5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 14.Zang X., Loke P., Kim J., Murphy K., Waitz R., Allison J.P. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A. 2003;100(18):10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao R., Chinai J.M., Buhl S., et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci U S A. 2013;110(24):9879–9884. doi: 10.1073/pnas.1303524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y., Yao S., Iliopoulou B.P., et al. B7-H5 costimulates human T cells via CD28H. Nat Commun. 2013;4:2043. doi: 10.1038/ncomms3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janakiram M., Chinai J.M., Fineberg S., et al. Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 protein. Clin Cancer Res. 2015;21(10):2359–2366. doi: 10.1158/1078-0432.CCR-14-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zang X. Cold Spring Harbor Asia Conference on Precision Cancer Biology: From Targeted to Immune Therapies, Suzhou, China. 18 to 22 September. 2017. New immune checkpoint pathways: HHLA2 and its receptors including TMIGD2. [Google Scholar]
- 19.Wei Y., Ren X., Galbo P.M., et al. KIR3DL3-HHLA2 is a human immunosuppressive pathway and a therapeutic target. Sci Immunol. 2021;6(61):eabf9792. doi: 10.1126/sciimmunol.abf9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatt R.S., Berjis A., Konge J.C., et al. KIR3DL3 is an inhibitory receptor for HHLA2 that mediates an alternative immunoinhibitory pathway to PD1. Cancer Immunol Res. 2021;9(2):156–169. doi: 10.1158/2326-6066.CIR-20-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen N., Fang W., Zhan J., et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol. 2015;10(6):910–923. doi: 10.1097/JTO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 22.Ji M., Liu Y., Li Q., et al. PD-1/PD-L1 pathway in non-small-cell lung cancer and its relation with EGFR mutation. J Transl Med. 2015;13:5. doi: 10.1186/s12967-014-0373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X., Lian Z., Wang S., Xing L., Yu J. Interactions between EGFR and PD-1/PD-L1 pathway: implications for treatment of NSCLC. Cancer Lett. 2018;418:1–9. doi: 10.1016/j.canlet.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Li D., Wang J., Zhou J., et al. B7-H3 combats apoptosis induced by chemotherapy by delivering signals to pancreatic cancer cells. Oncotarget. 2017;8(43):74856–74868. doi: 10.18632/oncotarget.20421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding M., Liao H., Zhou N., Yang Y., Guan S., Chen L. B7-H3-induced signaling in lung adenocarcinoma cell lines with divergent epidermal growth factor receptor mutation patterns. Biomed Res Int. 2020;2020:e8824805. doi: 10.1155/2020/8824805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng H., Janakiram M., Borczuk A., et al. HHLA2, a new immune checkpoint member of the B7 family, is widely expressed in human lung cancer and associated with EGFR mutational status. Clin Cancer Res. 2017;23(3):825–832. doi: 10.1158/1078-0432.CCR-15-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng H., Borczuk A., Janakiram M., et al. Wide expression and significance of alternative immune checkpoint molecules, B7x and HHLA2, in PD-L1-negative human lung cancers. Clin Cancer Res. 2018;24(8):1954–1964. doi: 10.1158/1078-0432.CCR-17-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y., Hu R., Li X., et al. B7-H4 and HHLA2, members of B7 family, are aberrantly expressed in EGFR mutated lung adenocarcinoma. Pathol Res Pract. 2020;216(10):153134. doi: 10.1016/j.prp.2020.153134. [DOI] [PubMed] [Google Scholar]
- 29.Sharma P., Hu-Lieskovan S., Wargo J.A., Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun C., Mezzadra R., Schumacher T.N. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48(3):434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borghaei H., Paz-Ares L., Horn L., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robert C., Long G.V., Brady B., et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg J.E., Hoffman-Censits J., Powles T., et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber J., Mandala M., Del Vecchio M., et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 35.Veale D., Ashcroft T., Marsh C., Gibson G.J., Harris A.L. Epidermal growth factor receptors in non-small cell lung cancer. Br J Cancer. 1987;55(5):513–516. doi: 10.1038/bjc.1987.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sridhar S.S., Seymour L., Shepherd F.A. Inhibitors of epidermal-growth-factor receptors: a review of clinical research with a focus on non-small-cell lung cancer. Lancet Oncol. 2003;4(7):397–406. doi: 10.1016/s1470-2045(03)01137-9. [DOI] [PubMed] [Google Scholar]
- 37.Meng X., Yu J.M. Detecting the epidermal growth factor receptors status in non-small cell lung cancer. Chin Med J (Engl) 2011;124(24):4324–4329. [PubMed] [Google Scholar]
- 38.Wu M., Rivkin A., Pham T. Panitumumab: human monoclonal antibody against epidermal growth factor receptors for the treatment of metastatic colorectal cancer. Clin Ther. 2008;30(1):14–30. doi: 10.1016/j.clinthera.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 39.Di Leo A., Linsalata M., Cavallini A., Messa C., Russo F. Sex steroid hormone receptors, epidermal growth factor receptor, and polyamines in human colorectal cancer. Dis Colon Rectum. 1992;35(4):305–309. doi: 10.1007/BF02048105. [DOI] [PubMed] [Google Scholar]
- 40.Thomas C.Y., Chouinard M., Cox M., et al. Spontaneous activation and signaling by overexpressed epidermal growth factor receptors in glioblastoma cells. Int J Cancer. 2003;104(1):19–27. doi: 10.1002/ijc.10880. [DOI] [PubMed] [Google Scholar]
- 41.Kwan K., Schneider J.R., Kobets A., Boockvar J.A. Targeting epidermal growth factor receptors in recurrent glioblastoma via a novel epithelial growth factor receptor-conjugated nanocell doxorubicin delivery system. Neurosurgery. 2018;82(3):N23–N24. doi: 10.1093/neuros/nyx594. [DOI] [PubMed] [Google Scholar]
- 42.Humphrey P.A., Wong A.J., Vogelstein B., et al. Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci U S A. 1990;87(11):4207–4211. doi: 10.1073/pnas.87.11.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ugurluer G., Ozsahin M. Early investigational drugs that target epidermal growth factor receptors for the treatment of head and neck cancer. Expert Opin Investig Drugs. 2014;23(12):1637–1654. doi: 10.1517/13543784.2014.951435. [DOI] [PubMed] [Google Scholar]
- 44.Yamanaka Y. The immunohistochemical expressions of epidermal growth factors, epidermal growth factor receptors and c-erbB-2 oncoprotein in human pancreatic cancer. Nihon Ika Daigaku Zasshi. 1992;59(1):51–61. doi: 10.1272/jnms1923.59.51. [DOI] [PubMed] [Google Scholar]
- 45.Aggarwal S., Gupta S., Gupta M.K., Murthy R.S., Vyas S.P. Possible role of epidermal growth factor receptors in the therapy of pancreatic cancer. Crit Rev Ther Drug Carrier Syst. 2011;28(4):293–356. doi: 10.1615/critrevtherdrugcarriersyst.v28.i4.10. [DOI] [PubMed] [Google Scholar]
- 46.Sainsbury R. Epidermal growth factor receptors and prognosis in breast cancer. Pathol Biol. 1990;38(8):771–772. [PubMed] [Google Scholar]
- 47.Pollak M.N. Epidermal-growth-factor receptors and breast cancer. Lancet. 1987;2(8558):562. doi: 10.1016/s0140-6736(87)92940-0. [DOI] [PubMed] [Google Scholar]
- 48.McIntyre E., Blackburn E., Brown P.J., Johnson C.G., Gullick W.J. The complete family of epidermal growth factor receptors and their ligands are co-ordinately expressed in breast cancer. Breast Cancer Res Treat. 2010;122(1):105–110. doi: 10.1007/s10549-009-0536-5. [DOI] [PubMed] [Google Scholar]
- 49.Akbay E.A., Koyama S., Carretero J., et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang N., Zeng Y., Du W., et al. The EGFR pathway is involved in the regulation of PD-L1 expression via the IL-6/JAK/STAT3 signaling pathway in EGFR-mutated non-small cell lung cancer. Int J Oncol. 2016;49(4):1360–1368. doi: 10.3892/ijo.2016.3632. [DOI] [PubMed] [Google Scholar]
- 51.Concha-Benavente F., Srivastava R.M., Trivedi S., et al. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNγ that induce PD-L1 expression in head and neck cancer. Cancer Res. 2016;76(5):1031–1043. doi: 10.1158/0008-5472.CAN-15-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li C.W., Lim S.O., Xia W., et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016;7:12632. doi: 10.1038/ncomms12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coelho M.A., de Carné Trécesson S., Rana S., et al. Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA. Immunity. 2017;47(6):1083–1099. doi: 10.1016/j.immuni.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin K., Cheng J., Yang T., Li Y., Zhu B. EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-κB. Biochem Biophys Res Commun. 2015;463(1–2):95–101. doi: 10.1016/j.bbrc.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 55.Lastwika K.J., Wilson W., 3rd, Li Q.K., et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76(2):227–238. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 56.Gainor J.F., Shaw A.T., Sequist L.V., et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong Z.Y., Zhang J.T., Liu S.Y., et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology. 2017;6(11):e1356145. doi: 10.1080/2162402X.2017.1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee C.K., Man J., Lord S., et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lungcancer-A meta-analysis. J Thorac Oncol. 2017;12(2):403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Lee C.K., Man J., Lord S., et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):210–216. doi: 10.1001/jamaoncol.2017.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khan M., Lin J., Liao G., et al. Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2018;97(33):e11936. doi: 10.1097/MD.0000000000011936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang C., Yu X., Wang W. A meta-analysis of efficacy and safety of antibodies targeting PD-1/PD-L1 in treatment of advanced nonsmall cell lung cancer. Medicine (Baltimore) 2016;95(52):e5539. doi: 10.1097/MD.0000000000005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun L., Zhang L., Yu J., et al. Clinical efficacy and safety of anti-PD-1/PD-L1 inhibitors for the treatment of advanced or metastatic cancer: a systematic review and meta-analysis. Sci Rep. 2020;10(1):2083. doi: 10.1038/s41598-020-58674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou C., Wu Y.L., Chen G., et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 64.Mok T.S., Wu Y.L., Ahn M.J., et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sequist L.V., Yang J.C., Yamamoto N., et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 66.Garrido G., Rabasa A., Sánchez B., et al. Induction of immunogenic apoptosis by blockade of epidermal growth factor receptor activation with a specific antibody. J Immunol. 2011;187(10):4954–4966. doi: 10.4049/jimmunol.1003477. [DOI] [PubMed] [Google Scholar]
- 67.Wang S., Zhang Y., Wang Y., et al. Amphiregulin confers regulatory T cell suppressive function and tumor invasion via the EGFR/GSK-3β/Foxp3 axis. J Biol Chem. 2016;291(40):21085–21095. doi: 10.1074/jbc.M116.717892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mascia F., Schloemann D.T., Cataisson C., et al. Cell autonomous or systemic EGFR blockade alters the immune-environment in squamous cell carcinomas. Int J Cancer. 2016;139(11):2593–2597. doi: 10.1002/ijc.30376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pollack B.P., Sapkota B., Cartee T.V. Epidermal growth factor receptor inhibition augments the expression of MHC class I and II genes. Clin Cancer Res. 2011;17(13):4400–4413. doi: 10.1158/1078-0432.CCR-10-3283. [DOI] [PubMed] [Google Scholar]
- 70.Lin C., Chen X., Li M., et al. Programmed death-ligand 1 expression predicts tyrosine kinase inhibitor response and better prognosis in a cohort of patients with epidermal growth factor receptor mutation-positive lung adenocarcinoma. Clin Lung Cancer. 2015;16(5):e25–35. doi: 10.1016/j.cllc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 71.D'Incecco A., Andreozzi M., Ludovini V., et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112(1):95–102. doi: 10.1038/bjc.2014.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang Y., Fang W., Zhang Y., et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget. 2015;6(16):14209–14219. doi: 10.18632/oncotarget.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soo R.A., Kim H.R., Asuncion B.R., et al. Significance of immune checkpoint proteins in EGFR-mutant non-small cell lung cancer. Lung Cancer. 2017;105:17–22. doi: 10.1016/j.lungcan.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 74.Han J.J., Kim D.W., Koh J., et al. Change in PD-L1 expression after acquiring resistance to gefitinib in EGFR-mutant non-small-cell lung cancer. Clin Lung Cancer. 2016;17(4):263–270. doi: 10.1016/j.cllc.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 75.Hata A., Katakami N., Nanjo S., et al. Programmed death-ligand 1 expression and T790M status in EGFR-mutant non-small cell lung cancer. Lung Cancer. 2017;111:182–189. doi: 10.1016/j.lungcan.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 76.Yu H.A., Arcila M.E., Rekhtman N., et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pao W., Miller V.A., Politi K.A., et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ni L., Dong C. New B7 family checkpoints in human cancers. Mol Cancer Ther. 2017;16(7):1203–1211. doi: 10.1158/1535-7163.MCT-16-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Janakiram M., Shah U.A., Liu W., Zhao A., Schoenberg M.P., Zang X. The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3. Immunol Rev. 2017;276(1):26–39. doi: 10.1111/imr.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chapoval A.I., Ni J., Lau J.S., et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2(3):269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 81.Ueno T., Yeung M.Y., McGrath M., et al. Intact B7-H3 signaling promotes allograft prolongation through preferential suppression of Th1 effector responses. Eur J Immunol. 2012;42(9):2343–2353. doi: 10.1002/eji.201242501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steinberger P., Majdic O., Derdak S.V., et al. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol. 2004;172(4):2352–2359. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- 83.Suh W.K., Gajewska B.U., Okada H., et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4(9):899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 84.Veenstra R.G., Flynn R., Kreymborg K., et al. B7-H3 expression in donor T cells and host cells negatively regulates acute graft-versus-host disease lethality. Blood. 2015;125(21):3335–3346. doi: 10.1182/blood-2014-09-603357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prasad D.V., Nguyen T., Li Z., et al. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173(4):2500–2506. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 86.Crispen P.L., Sheinin Y., Roth T.J., et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14(16):5150–5157. doi: 10.1158/1078-0432.CCR-08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu S., Zhao X., Wu S., et al. Overexpression of B7-H3 correlates with aggressive clinicopathological characteristics in non-small cell lung cancer. Oncotarget. 2016;7(49):81750–81756. doi: 10.18632/oncotarget.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benzon B., Zhao S.G., Haffner M.C., et al. Correlation of B7-H3 with androgen receptor, immune pathways and poor outcome in prostate cancer: an expression-based analysis. Prostate Cancer Prostatic Dis. 2017;20(1):28–35. doi: 10.1038/pcan.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fan H., Zhu J.H., Yao X.Q. Prognostic significance of B7-H3 expression in patients with colorectal cancer: a meta-analysis. Pak J Med Sci. 2016;32(6):1568–1573. doi: 10.12669/pjms.326.11511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu C.L., Zang X.X., Huang H., et al. The expression of B7-H3 and B7-H4 in human gallbladder carcinoma and their clinical implications. Eur Rev Med Pharmacol Sci. 2016;20(21):4466–4473. [PubMed] [Google Scholar]
- 91.Song J., Shi W., Zhang Y., Sun M., Liang X., Zheng S. Epidermal growth factor receptor and B7-H3 expression in esophageal squamous tissues correlate to patient prognosis. Onco Targets Ther. 2016;9:6257–6263. doi: 10.2147/OTT.S111691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang L., Kang F.B., Sun N., et al. The tumor suppressor miR-124 inhibits cell proliferation and invasion by targeting B7-H3 in osteosarcoma. Tumour Biol. 2016;37(11):14939–14947. doi: 10.1007/s13277-016-5386-2. [DOI] [PubMed] [Google Scholar]
- 93.Bachawal S.V., Jensen K.C., Wilson K.E., Tian L., Lutz A.M., Willmann J.K. Breast cancer detection by B7-H3-targeted ultrasound molecular imaging. Cancer Res. 2015;75(12):2501–2509. doi: 10.1158/0008-5472.CAN-14-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li H., Huang C., Zhang Z., et al. MEK inhibitor augments antitumor activity of B7-H3-redirected bispecific antibody. Front Oncol. 2020;10:1527. doi: 10.3389/fonc.2020.01527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nunes-Xavier C.E., Karlsen K.F., Tekle C., et al. Decreased expression of B7-H3 reduces the glycolytic capacity and sensitizes breast cancer cells to AKT/mTOR inhibitors. Oncotarget. 2016;7(6):6891–6901. doi: 10.18632/oncotarget.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhong C., Tao B., Chen Y., et al. B7-H3 regulates glioma growth and cell invasion through a JAK2/STAT3/Slug-dependent signaling pathway. Onco Targets Ther. 2020;13:2215–2224. doi: 10.2147/OTT.S237841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Y., Guo G., Song J., et al. B7-H3 promotes the migration and invasion of human bladder cancer cells via the PI3K/Akt/STAT3 signaling pathway. J Cancer. 2017;8(5):816–824. doi: 10.7150/jca.17759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fan T.F., Deng W.W., Bu L.L., Wu T.F., Zhang W.F., Sun Z.J. B7-H3 regulates migration and invasion in salivary gland adenoid cystic carcinoma via the JAK2/STAT3 signaling pathway. Am J Transl Res. 2017;9(3):1369–1380. [PMC free article] [PubMed] [Google Scholar]
- 99.Inamura K., Yokouchi Y., Kobayashi M., et al. Tumor B7-H3 (CD276) expression and smoking history in relation to lung adenocarcinoma prognosis. Lung Cancer. 2017;103:44–51. doi: 10.1016/j.lungcan.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 100.Sica G.L., Choi I.H., Zhu G., et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18(6):849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 101.Prasad D.V., Richards S., Mai X.M., Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18(6):863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 102.Choi I.H., Zhu G., Sica G.L., et al. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol. 2003;171(9):4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 103.Abadi Y.M., Jeon H., Ohaegbulam K.C., et al. Host B7x promotes pulmonary metastasis of breast cancer. J Immunol. 2013;190(7):3806–3814. doi: 10.4049/jimmunol.1202439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jeon H., Ohaegbulam K.C., Abadi Y.M., Zang X. B7x and myeloid-derived suppressor cells in the tumor microenvironment: a tale of two cities. Oncoimmunology. 2013;2(7):e24744. doi: 10.4161/onci.24744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shan Z.G., Yan Z.B., Peng L.S., et al. Granulocyte-macrophage colony-stimulating factor-activated neutrophils express B7-H4 that correlates with gastric cancer progression and poor patient survival. J Immunol Res. 2021;2021:e6613247. doi: 10.1155/2021/6613247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li A., Zhang N., Zhao Z., Chen Y., Zhang L. Overexpression of B7-H4 promotes renal cell carcinoma progression by recruiting tumor-associated neutrophils via upregulation of CXCL8. Oncol Lett. 2020;20(2):1535–1544. doi: 10.3892/ol.2020.11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kryczek I., Zou L., Rodriguez P., et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203(4):871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yao Y., Ye H., Qi Z., et al. B7-H4(B7x)-mediated cross-talk between glioma-initiating cells and macrophages via the IL6/JAK/STAT3 pathway lead to poor prognosis in glioma patients. Clin Cancer Res. 2016;22(11):2778–2790. doi: 10.1158/1078-0432.CCR-15-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krambeck A.E., Thompson R.H., Dong H., et al. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006;103(27):10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tan Z., Shen W. Prognostic role of B7-H4 in patients with non-small cell lung cancer: a meta-analysis. Oncotarget. 2017;8(16):27137–27144. doi: 10.18632/oncotarget.15648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang X., Cai L., Zhang G., Shen Y., Huang J. B7-H4 promotes tumor growth and metastatic progression in lung cancer by impacting cell proliferation and survival. Oncotarget. 2017;8(12):18861–18871. doi: 10.18632/oncotarget.14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ding S., Lv X., Liu Z., et al. Overexpression of B7-H4 is associated with infiltrating immune cells and poor prognosis in metastatic colorectal cancer. Int Immunopharmacol. 2021;90:e107144. doi: 10.1016/j.intimp.2020.107144. [DOI] [PubMed] [Google Scholar]
- 113.Zhao X., Guo F., Li Z., et al. Aberrant expression of B7-H4 correlates with poor prognosis and suppresses tumor-infiltration of CD8+ T lymphocytes in human cholangiocarcinoma. Oncol Rep. 2016;36(1):419–427. doi: 10.3892/or.2016.4807. [DOI] [PubMed] [Google Scholar]
- 114.Xu H., Chen X., Tao M., et al. B7-H3 and B7-H4 are independent predictors of a poor prognosis in patients with pancreatic cancer. Oncol Lett. 2016;11(3):1841–1846. doi: 10.3892/ol.2016.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chand D., Dhawan D., Sankin A., et al. Immune checkpoint B7x (B7-H4/B7S1/VTCN1) is over expressed in spontaneous canine bladder cancer: the first report and its Implications in a preclinical model. Bladder Cancer. 2019;5(1):63–71. doi: 10.3233/BLC-180204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jeon H., Vigdorovich V., Garrett-Thomson S.C., et al. Structure and cancer immunotherapy of the B7 family member B7x. Cell Rep. 2014;9(3):1089–1098. doi: 10.1016/j.celrep.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li J., Lee Y., Li Y., et al. Co-inhibitory molecule B7 superfamily member 1 expressed by tumor-infiltrating myeloid cells induces dysfunction of anti-tumor CD8(+) T cells. Immunity. 2018;48(4):773–786. doi: 10.1016/j.immuni.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 118.Song X., Zhou Z., Li H., et al. Pharmacologic suppression of B7-H4 glycosylation restores antitumor immunity in immune-cold breast cancers. Cancer Discov. 2020;10(12):1872–1893. doi: 10.1158/2159-8290.CD-20-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Podojil J.R., Glaser A.P., Baker D., et al. Antibody targeting of B7-H4 enhances the immune response in urothelial carcinoma. Oncoimmunology. 2020;9(1):e1744897. doi: 10.1080/2162402X.2020.1744897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schalper K.A., Carvajal-Hausdorf D., McLaughlin J., et al. Differential expression and significance of PD-L1, IDO-1, and B7-H4 in human lung cancer. Clin Cancer Res. 2017;23(2):370–378. doi: 10.1158/1078-0432.CCR-16-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Koirala P., Roth M.E., Gill J., et al. HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival. Sci Rep. 2016;6:31154. doi: 10.1038/srep31154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wei L., Tang L., Chang H., Huo S., Li Y. HHLA2 overexpression is a novel biomarker of malignant status and poor prognosis in gastric cancer. Hum Cell. 2020;33(1):116–122. doi: 10.1007/s13577-019-00280-2. [DOI] [PubMed] [Google Scholar]
- 123.Chen L., Zhu D., Feng J., et al. Overexpression of HHLA2 in human clear cell renal cell carcinoma is significantly associated with poor survival of the patients. Cancer Cell Int. 2019;19:101. doi: 10.1186/s12935-019-0813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jing C.Y., Fu Y.P., Yi Y., et al. HHLA2 in intrahepatic cholangiocarcinoma: an immune checkpoint with prognostic significance and wider expression compared with PD-L1. J Immunother Cancer. 2019;7(1):77. doi: 10.1186/s40425-019-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin G., Ye H., Wang J., Chen S., Chen X., Zhang C. Immune checkpoint human endogenous retrovirus-H long terminal repeat-associating protein 2 is upregulated and independently predicts unfavorable prognosis in bladder urothelial carcinoma. Nephron. 2019;141(4):256–264. doi: 10.1159/000495887. [DOI] [PubMed] [Google Scholar]
- 126.Zhu Z., Dong W. Overexpression of HHLA2, a member of the B7 family, is associated with worse survival in human colorectal carcinoma. Onco Targets Ther. 2018;11:1563–1570. doi: 10.2147/OTT.S160493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yan H., Qiu W., Koehne de Gonzalez A.K., et al. HHLA2 is a novel immune checkpoint protein in pancreatic ductal adenocarcinoma and predicts post-surgical survival. Cancer Lett. 2019;442:333–340. doi: 10.1016/j.canlet.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qi Y., Deng G., Xu P., et al. HHLA2 is a novel prognostic predictor and potential therapeutic target in malignant glioma. Oncol Rep. 2019;42(6):2309–2322. doi: 10.3892/or.2019.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Verschueren E., Husain B., Yuen K., et al. The immunoglobulin superfamily receptome defines cancer-relevant networks associated with clinical outcome. Cell. 2020;182(2):329–344. doi: 10.1016/j.cell.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 130.Wojtowicz W.M., Vielmetter J., Fernandes R.A., et al. A human IgSF cell-surface interactome reveals a complex network of protein-protein interactions. Cell. 2020;182(4):1027–1043. doi: 10.1016/j.cell.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dong Z., Zhang L., Xu W., Zhang G. EGFR may participate in immune evasion through regulation of B7-H5 expression in non-small cell lung carcinoma. Mol Med Rep. 2018;18(4):3769–3779. doi: 10.3892/mmr.2018.9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Herbst R.S., Baas P., Kim D.W., et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 133.Rittmeyer A., Barlesi F., Waterkamp D., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lisberg A., Cummings A., Goldman J.W., et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced NSCLC. J Thorac Oncol. 2018;13(8):1138–1145. doi: 10.1016/j.jtho.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hanna N.H., Robinson A.G., Temin S., et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. 2021;39(9):1040–1091. doi: 10.1200/JCO.20.03570. [DOI] [PubMed] [Google Scholar]
- 136.Kumagai S., Koyama S., Nishikawa H. Antitumour immunity regulated by aberrant ERBB family signalling. Nat Rev Cancer. 2021;21(3):181–197. doi: 10.1038/s41568-020-00322-0. [DOI] [PubMed] [Google Scholar]
- 137.Hastings K., Yu H.A., Wei W., et al. EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann Oncol. 2019;30(8):1311–1320. doi: 10.1093/annonc/mdz141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hellmann M.D., Ciuleanu T.E., Pluzanski A., et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rizvi H., Sanchez-Vega F., La K., et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Haratani K., Hayashi H., Tanaka T., et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol. 2017;28(7):1532–1539. doi: 10.1093/annonc/mdx183. [DOI] [PubMed] [Google Scholar]
- 141.Offin M., Rizvi H., Tenet M., et al. Tumor mutation burden and efficacy of EGFR-tyrosine kinase inhibitors in patients with EGFR-mutant lung cancers. Clin Cancer Res. 2019;25(3):1063–1069. doi: 10.1158/1078-0432.CCR-18-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Isomoto K., Haratani K., Hayashi H., et al. Impact of EGFR-TKI treatment on the tumor immune microenvironment in EGFR mutation-positive non-small cell lung cancer. Clin Cancer Res. 2020;26(8):2037–2046. doi: 10.1158/1078-0432.CCR-19-2027. [DOI] [PubMed] [Google Scholar]
- 143.Soo R.A., Lim S.M., Syn N.L., et al. Immune checkpoint inhibitors in epidermal growth factor receptor mutant non-small cell lung cancer: current controversies and future directions. Lung Cancer. 2018;115:12–20. doi: 10.1016/j.lungcan.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 144.Sugiyama E., Togashi Y., Takeuchi Y., et al. Blockade of EGFR improves responsiveness to PD-1 blockade in EGFR-mutated non-small cell lung cancer. Sci Immunol. 2020;5(43):eaav3937. doi: 10.1126/sciimmunol.aav3937. [DOI] [PubMed] [Google Scholar]
- 145.Yang J.C., Gadgeel S.M., Sequist L.V., et al. Pembrolizumab in combination with erlotinib or gefitinib as first-line therapy for advanced NSCLC with sensitizing EGFR mutation. J Thorac Oncol. 2019;14(3):553–559. doi: 10.1016/j.jtho.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 146.Oxnard G.R., Yang J.C., Yu H., et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol. 2020;31(4):507–516. doi: 10.1016/j.annonc.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 147.Yang J.C., Shepherd F.A., Kim D.W., et al. Osimertinib plus durvalumab versus osimertinib monotherapy in EGFR T790M-positive NSCLC following previous EGFR TKI therapy: CAURAL brief report. J Thorac Oncol. 2019;14(5):933–939. doi: 10.1016/j.jtho.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 148.Oshima Y., Tanimoto T., Yuji K., Tojo A. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol. 2018;4(8):1112–1115. doi: 10.1001/jamaoncol.2017.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gettinger S., Hellmann M.D., Chow L.Q.M., et al. Nivolumab plus erlotinib in patients with EGFR-mutant advanced NSCLC. J Thorac Oncol. 2018;13(9):1363–1372. doi: 10.1016/j.jtho.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 150.Rudin C., Cervantes A., Dowlati A., et al. Long-term safety and clinical activity results from a phase Ib study of erlotinib plus atezolizumab in advanced NSCLC. J Thorac Oncol. 2018;13(10):S407. [Google Scholar]
- 151.Concha-Benavente F., Kansy B., Moskovitz J., Moy J., Chandran U., Ferris R.L. PD-L1 mediates dysfunction in activated PD-1(+) NK cells in head and neck cancer patients. Cancer Immunol Res. 2018;6(12):1548–1560. doi: 10.1158/2326-6066.CIR-18-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jie H.B., Schuler P.J., Lee S.C., et al. CTLA-4(+) regulatory T cells increased in cetuximab-treated head and neck cancer patients suppress NK cell cytotoxicity and correlate with poor prognosis. Cancer Res. 2015;75(11):2200–2210. doi: 10.1158/0008-5472.CAN-14-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chalmers A.W., Patel S., Boucher K., et al. Phase I trial of targeted EGFR or ALK therapy with ipilimumab in metastatic NSCLC with long-term follow-up. Target Oncol. 2019;14(4):417–421. doi: 10.1007/s11523-019-00658-0. [DOI] [PubMed] [Google Scholar]
- 154.Wu L., Ke L., Zhang Z., Yu J., Meng X. Development of EGFR TKIs and options to manage resistance of third-generation EGFR TKI osimertinib: conventional ways and immune checkpoint inhibitors. Front Oncol. 2020;10:e602762. doi: 10.3389/fonc.2020.602762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ahn M.J., Sun J.M., Lee S.H., Ahn J.S., Park K. EGFR TKI combination with immunotherapy in non-small cell lung cancer. Expet Opin Drug Saf. 2017;16(4):465–469. doi: 10.1080/14740338.2017.1300656. [DOI] [PubMed] [Google Scholar]
- 156.Liang H., Liu X., Wang M. Immunotherapy combined with epidermal growth factor receptor-tyrosine kinase inhibitors in non-small-cell lung cancer treatment. Onco Targets Ther. 2018;11:6189–6196. doi: 10.2147/OTT.S178497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Altan M., Pelekanou V., Schalper K.A., et al. B7-H3 expression in NSCLC and its association with B7-H4, PD-L1 and tumor-infiltrating lymphocytes. Clin Cancer Res. 2017;23(17):5202–5209. doi: 10.1158/1078-0432.CCR-16-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Saavedra D., Crombet T. CIMAvax-EGF: a new therapeutic vaccine for advanced non-small cell lung cancer patients. Front Immunol. 2017;8:269. doi: 10.3389/fimmu.2017.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]