Abstract
Cryptorchidism-caused adult infertility is a common component of idiopathic reasons for male infertility. Retinoic acid (RA) has a vital effect on the spermatogenesis process. Here, we found that the expression of c-Kit, Stra8, and Sycp3 could be up-regulated via the activation of retinoic acid receptor α (RARα) after RA supplementation in neonatal cryptorchid infertile rats. We also demonstrated that the protein expression of PI3K, p-Akt/pan-Akt, and p-mTOR/mTOR was higher in cryptorchid than in normal testes, and could be suppressed with RA in vivo. After RA treatment in infertile cryptorchid testis in vivo, the levels of the autophagy proteins LC3 and Beclin1 increased and those of P62 decreased. Biotin tracer indicated that the permeability of blood-testis barrier (BTB) in cryptorchid rats decreased after RA administration. Additionally, after blocking the RARα with AR7 (an RARα antagonist) in testicle culture in vitro, we observed that compared with normal testes, the PI3K-Akt-mTOR signaling pathway and the autophagy pathway was increased and decreased, respectively, which were coincident with cryptorchisd testes in vivo. Additionally, the appropriate concentrations of RA treatment could depress the PI3K-Akt-mTOR signaling pathway and improve the autophagy pathway. The results confirmed that RA can rehabilitate BTB function and drive key protein levels in spermatogonial differentiation through depressing the PI3K-Akt-mTOR signaling pathway via RARα.
Keywords: Cryptorchidism, Male infertility, Retinoic acid, Spermatogenesis, Testis
Introduction
Infertility affects almost 70 million couples, with 50% caused by male reproduction problems.1 The majority of infertile men (>45%) are diagnosed with idiopathic reasons, including reproductive system diseases in children. Cryptorchidism is a representative condition.2 Male infertility may be caused by childhood reproductive diseases. This etiology of infertility often comes to the urologist's attention. Cryptorchid children are more likely to become infertile in adulthood, and about 10% of infertile men report a history of cryptorchidism or orchidopexy. Nearly 90% of men with untreated bilateral cryptorchidism will suffer azoospermia as seen with long-term follow up,3,4 this is a serious threat to human reproductive health. Finding a way to reestablish the spermatogenesis function of these cryptorchidism patients is extremely urgent.
It is known that the transformation of gonocytes into adult dark (AD) spermatogonia during the first year is crucial for male fertility.5 In undescended testes, testicular biopsy results indicate that the process of gonocyte transfer into AD spermatogonia is blocked.6,7 In addition, in cryptorchid testes, dysfunction of Sertoli and Leydig cells causes spermatogenic microenvironment disturbances and the initiation of meiosis. The detailed mechanism of spermatogenesis is complex. Researchers have suggested that retinoic acid (RA) deficiency could cause reproductive abnormalities and disorders of the spermatogenic process, which would indicate that RA is crucial for normal spermatogenesis.8,9 Additionally, RA is sufficient for inducing the differentiation of leptotene/zygotene spermatocytes from cultured mouse spermatogonial stem cells and is crucial in the initiation of meiosis by stimulating retinoic acid gene 8 (Stra8).10 In our previous study, we found that RA concentration is lower in rats with cryptorchidism. The spermatogenesis-blocking process in cryptorchidism is consistent with the spermatogenesis failure process in the RA-deletion model. Hence, we hypothesize that administration of exogenous RA to young rats with cryptorchidism during the spermatogenic critical period could ameliorate the spermatogenic damage process in adults.
RA binds retinoic acid receptors (RARs) to function in its biological role in testes. RARs is composed of three subtypes (α, β, and γ).11 RARα is indispensable for normal spermatogenesis. Mice with an RARα-/- null mutation exhibit defects in spermatogenesis and loss of proper germ cell organization, with a phenotype similar to RA or vitamin A deficient testes.12 In RARα knockout testes, the differentiation of spermatogonial stem cells is disrupted, and this phenotype is also presented in cryptorchidism.13 It was discovered in a recent pilot study that RA treatment could reprogram human dysfunctional sperm metabolism toward capacitation status, and that RA has the function to control the cell junctional physiology in Sertoli cells.14 Thus, the aim of this study is to verify whether the RARα signaling pathway could restart spermatogonial stem cell differentiation in cryptorchidism.
Autophagy is a self-degradative process, acting on various conditions of cellular stress, such as starvation, hyperthermia, and cytotoxic injuries to allow the cell to adapt to changing environments. Uncontrolled activation of autophagy can promote cell death.15 In the progress of spermatogenesis, autophagy has been shown to regulate germ cell maturation.16,17 It has been reported that autophagy is abnormal in sperm cells from patients with cryptorchid history.18 Regulating autophagy may be a suitable way to improve sperm quality in men with cryptorchidism.
Mammalian target of rapamycin (mTOR) is a conserved kinase that interacts with autophagy pathways to regulate different cellular processes, including spermatogonial development. Orderly spermatogonial differentiation is blocked when mTOR is inhibited with rapamycin in neonatal pups, and Kit and spermatogenesis and oogenesis specific basic helix-loop-helix (SOHLH) (two differentiation markers) genes are also degenerated.19 mTOR mediates the expression of c-Kit and Nanos2 for spermatogonial stem cell renewal. In an acute promyelocytic leukemia model, RA has been demonstrated to promote autophagy level and restore GOS2 (G0/G1 switch 2, a direct retinoic acid target gene) gene expression, which promotes autophagy.20 However, the mechanisms of correlation between RA and mTOR-mediated autophagy in the spermatogonium differentiation process in cryptorchidism-induced infertility have not been described.
In a previous study, we have demonstrated that the concentration of RA in cryptorchidism-induced infertility rat testes was decreased, and sperm quality could be partially improved with restoration of the blood-testis barrier after exogenous RA administration in cryptorchidism.21 Thus, the objective of this study was to investigate the mechanism by which RA improves spermatogenesis in cryptorchidism.
Materials and methods
Animals and treatments
Female Sprague-Dawley rats (250-300 g) from the Experimental Animal Center of Chongqing Medical University (SCXK 2018-0003) were housed in the Animal Center of Chongqing Children's Hospital (SYXK 2017-0012). All of the animals had free access to food and tap water and were housed in a pathogen-free environment (12-h light/12-h dark; 25 ± 2 °C; humidity 55 ± 5%). The females were paired with males overnight and checked for a vaginal sperm plug in the morning, which was designated as embryonic day (ED) 0.5. The animal experiments were approved by the Experimental Animal Committee of Chongqing Medical University (license number: 2017-0001).
All of the pregnant female rats were randomly apportioned to three groups (n = 10, each): normal control, treated with flutamide only (Flu-only), and treated with Flu and RA (Flu + RA). The control rats were injected daily with 1 mL/kg/d corn oil by hypodermic needle at ED12.5 to ED19.5. The Flu-only group was injected daily with 25 mg/kg/d flutamide (with corn oil as the carrier) at ED 12.5−ED 20.5 to induce cryptorchidism in the male fetuses (the model). The Flu + RA group received the same treatment as the Flu group, but in addition the male offspring cubs were injected with all-trans RA (RA, R2625, Sigma) at 1 mg/kg from postnatal birth day (PND) 1 to PND 10. The incidence of cryptorchidism was detected at PND20. For investigating the effect of RA supplementation at different time periods on cryptorchidism, the RA was administered between PND 1–10, PND 20–30, and PND 50–60. Rats were euthanized by CO2 asphyxiation and cervical dislocation, and the testes were stored at −80 °C until use. All of the regents are listed in Table S1.
Pathway prediction with bioinformatics analysis
We investigated the potential key pathway in RA induction of the stem cell differentiation process. The gene expression profile data of GSE34279 was obtained from the Gene Expression Omnibus (GEO) database of the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/gds) for prediction. The data were described in mouse embryonic stem cells (mES), which were induced by RA. The differentially expressed genes (DEGs) were screened and obtained using the cutoffs of fold change > 2 and adj. P < 0.001. The first 200 DEGs (Table S2) were uploaded in DIVID6.7 website (https://david.ncifcrf.gov/) for gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment prediction analysis.
Western blot
The proteins of the testes were extracted with radioimmunoprecipitation assay (RIPA, Beyotime) buffer containing phenylmethanesulfonyl fluoride (PMSF), and centrifuged at 4 °C at 12,000 rpm for 20 min. Protein concentrations were measured using the bicinchoninic acid method (BCA, Beyotime). Total protein was diluted with 1/4 volume loading buffer and boiled 10 min to fully denature it before loading onto a 10% SDS-PAGE gel. Proteins were transferred to a polyvinylidene difluoride membrane (Millipore, USA). Tris-buffered saline Tween solution (TBST) with 5% non-fat milk was used to block the nonspecific protein binding at room temperature for 1 h. The primary antibodies were serially added, incubated at 4 °C overnight, and washed in TBST 3 × , 10 min each time; the corresponding secondary antibody (1:5000) was added for 1 h at room temperature. Finally, bands were detected with a chemiluminescent reaction (Millipore), and image collection and densitometry analysis were performed using Image Lab6.0.0 analysis software. The levels were relative to the reference β-actin.
Immunofluorescence analysis
Four-micron-thick sections obtained as described above were deparaffinized, rehydrated, and retrieved with antigen. Slides were dipped in 3% H2O2 (Keshi, China) for 20 min and 0.5% bovine serum albumin (ZSGB-BIO) for 1 h. Sections were incubated serially in the primary antibody overnight at 4 °C, and then the corresponding fluorescein-conjugated secondary antibody for 2 h at room temperature in the dark.
Nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) or Hoechst 33342 (Life Technologies) for 30 min. Images were processed using NIS Elements Basic Research software (Nikon, USA).
BTB integrity assay
The permeability of the BTB was assessed by a biotin tracer. Procedures were conducted as previously described.22 Briefly, PND 60 rats from different groups (n = 4, each group) were anesthetized and injected with 40 μL of 10 mg/mL EZ-Link Sulfo–NHS–LC-Biotin (Pierce Biotechnology, Rockford, IL, USA), which was dissolved in PBS containing 1 mM CaCl2, into the interstitium of cryptorchid testes in the Flu-only group and the Flu + RA group. The testes were dissected and removed after 30 min, and then frozen in liquid nitrogen. Next, 10-μm frozen sections were prepared and fixed with 4% paraformaldehyde (PFA) plus 5% bovine serum albumin for 30 min, and incubated with anti-streptavidin antibody (4 μg/mL in PBS, Alexa Fluor 488 streptavidin conjugate) for 1 h. Finally, slides were washed in PBS and mounted with DAPI. Images of the seminiferous tubules were acquired on a DM4000B microscope (Leica) at a 400 × magnification.
Testicle tissue cultures
To detect the effect of RA on the PI3K-Akt-mTOR pathway in vitro, testicle tissue cultures were performed as previously described.23 The brief process was conducted as follows: small pieces (cut into ~3 mm × 3 mm) of testis were isolated from 7-day-old normal male Sprague-Dawley rats (obtained from the Experimental Animal Center of Chongqing Medical University), and cultured in Dulbecco's Modified Eagle's Medium/F-12 (DMEM/F-12, Gibco, USA) mixed with 10% KnockOut Serum Replacement specific for tissue culture (KSR, Gibco, USA). Cultures were incubated in a humidified atmosphere of 95% air and 5% CO2 (vol/vol) at 34 °C. The testicular tissue was cultured 1 w to establish a steady state, and the medium was changed twice per week. To determine a suitable concentration of AR7 (an RARα antagonist) and RA, AR7 was added to the medium at 10, 20, and 50 μmol/L. Then, RA was added at 1, 10, or 50 μmol/L into the medium with the varying AR7 concentrations. Testicular tissue was collected after culturing with these media for 24, 48, or 72 h.
Protein-protein interaction (PPI) network
We investigated the interaction of all proteins in this study to validate our assumptions about the mechanism. All of the detected proteins in this study were uploaded to STRING11.0 (https://string-db.org/) with multiple proteins with official names. The central proteins between axes were considered critical proteins.
Statistical analysis
All of the experiments were replicated at least three times with different samples. Data were analyzed with SPSS13.0 (Chicago, USA) and are presented as mean ± standard error. All graphs were plotted using GraphPad Prism7.0 (San Diego, California, USA). Statistical analyses were determined using one-way analysis of variance (ANOVA) and then Tukey's post-hoc test. An ANOVA P-value < 0.05 was considered to be statistically significant.
Results
Spermatogenesis pivotal markers and RARα change associated with RA treatment
Spermatogonia differentiation and spermatocyte meiosis protein levels were determined by immunofluorescence and Western blot analysis. Compared with the normal control group, the levels of c-Kit, Sycp3, and Stra8 were significantly lower in the Flu-only group (Fig. 1A–C). However, these proteins were significantly higher in the Flu + RA group compared with the Flu-only group. The expression of MVH had no statistical differences in different groups (Fig. 1A, C). At PND 60, the protein expression levels of Stra8 and Sycp3 were higher when RA was administrated between PND 1-10 and PND 50-60. Nevertheless, those protein levels had no significant differences between the RA (50-60) group and the Flu-only group detected at PND 70 (Fig. S1). RARα was vital for Aal-A1 transformation and normal spermatogenesis, which were also blocked in cryptorchid animals. Quantitative analysis of RARα with Western blotting indicated that the level of RARα protein was lower in the Flu group. In contrast, the RARα level was higher after RA administration (Fig. 1D).
Figure 1.
RA may promote the expression of spermatogenesis markers via RARα in PND30 cryptorchidism-infertility testis. Representative images of the germ cell (MVH) and Aal-A1 transformation protein markers (c-Kit) (A), and meiosis initiation proteins (Stra8) (B) with immunofluorescence. Blue represents the cell nucleus, red represents corresponding protein. Quantitative analysis of spermatogenesis markers by Western blot (n = 5) (C). Quantitative analysis the protein expression of RARα in vivo with Western blot (n = 6) (D). The y-axis represents the target protein value relative to β-actin. Error bars represent mean ± standard error of estimate from three independent experiments. ∗ Compared with the normal control and Flu + RA groups; P < 0.05.
Potential signaling pathways associated with RA treatment in stem cell translation
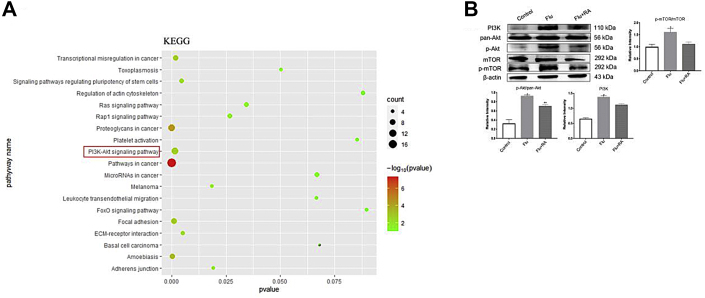
KEGG analysis with DAVID Bioinformatics Resources tools showed that top enrichment pathways were in cancers, and PI3K-Akt was the one of the most significantly changed pathways when mES cells were treated with RA for differentiation (Fig. 2A). The PI3K-Akt pathway plays an important role in regulating metabolism, cell growth, proliferation, and differentiation. mTOR plays an important role in the maintenance and differentiation of SSCs, regulating the redox balance and metabolic activity of Sertoli cells. mTOR could be regulated by the PI3K-Akt signaling pathway. Ten genes in the PI3K-Akt pathways were presented in Figure S2. Thus, we deduced that the PI3K-Akt-mTOR signaling pathway might play a pivotal role in the RA-mediated stem cell differentiation process.
Figure 2.
RA could depress the activation of PI3K-Akt-mTOR signaling pathway caused by cryptorchidism-infertility testis at PND60 in vivo. The KEGG pathway analysis of GSE34279 (A). PI3K-Akt signaling pathway is one of the most interrelated pathway (Red box). Quantitative analysis the protein expression of PI3K, pan-Akt, p-Akt, mTOR and p-mTOR in vivo with Western-blot (n = 5) (B). The y-axis represents the target protein value relative to β-actin. Error bars represent mean ± standard error of estimate from three independent experiments. ∗ Compared with the normal control and Flu + RA groups; P < 0.05.
Response of PI3K-Akt-mTOR pathway key markers after RA treatment
In the Flu-only group, the protein levels of PI3k, p-Akt/pan-Akt, and p-mTOR/mTOR were activated compared with the normal control group. These protein levels were significantly lower after administration with RA compared with the Flu-only group, and the p-Akt/pan-Akt level also significantly up-regulated compared with the normal control group (Fig. 2B).
Autophagy-related protein expression levels in vivo
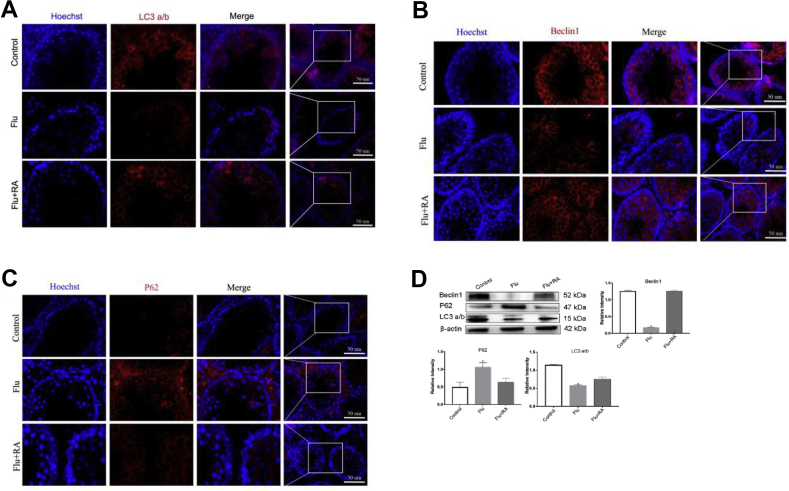
Autophagy is crucial to keep steady the processes of spermatogonial stem cell transformation and sustained spermatogenesis. This process is downstream of the PI3K-Akt-mTOR pathway. Hence, we examined autophagy level in testis. Immunofluorescence analysis indicated that the signals of LC3 a/b and Beclin 1 were lower in the Flu-only group, and increased to the normal standard after RA treatment. The expression of P62 (also known as SQSTM1, a substrate during autophagic degradation) was higher in the Flu group compared with the normal control and Flu + RA groups (Fig. 3A–C). As shown in Figure 3D, the expression of LC3 a/b and Beclin1 are markedly reduced in the Flu-only group compared with the control group. In addition, the level of P62 protein was higher in the Flu-only group when compared with the normal control and Flu + RA groups (Fig. 3D).
Figure 3.
RA associated with normalized levels of autophagy proteins in PND30 testes. Representative images of the autophagy proteins (white arrows) with immunofluorescence revealing the autophagy level increased after RA treatment in vivo of the testis. Blue represents the cell nucleus, red represents autophagy-relate protein level LC3 a/b (A), Beclin (B) and P62 (C) (n = 3). Western blot used for quantitative analysis of autophagy level in vivo(D) (n = 5). The y-axis represents the target protein value relative to β-actin. Error bars represent mean ± standard error of estimate from three independent experiments. ∗ Compared with the normal control and Flu + RA groups; P < 0.05.
BTB permeability analysis in PND60 rat testes
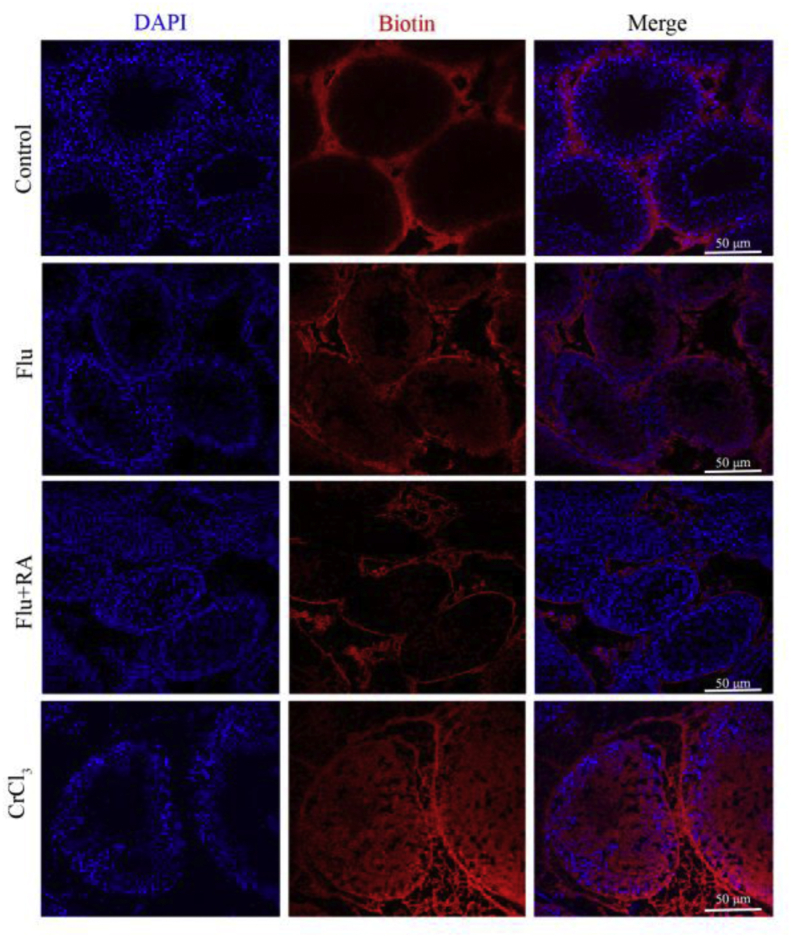
Because the integrity of BTB is a prerequisite of meiosis, we assessed BTB permeability in vivo using biotin as the tracer. In normal testes, the biotin signal was not detected in the seminiferous lumen. However, the biotin tracer permeated through the BTB into the tubule lumen for an obviously greater distance, indicating that BTB integrity was disrupted in the Flu-only group. As expected, the BTB function was improved after treatment with RA in cryptorchid testis, as there was less biotin signal in the tubules of the Flu + RA group compared with the Flu-only group (Fig. 4).
Figure 4.
Biotin tracer detects for BTB permeability in PND60 rats. Representative images depict the BTB permeability in different groups (n = 4). Red represents the signal of biotin tracer. Blue represents the cell nucleus. PND60 testis treated with CrCl3 group conducted as a positive control group.
Confirmation of AR7 (RARα antagonist) and RA concentration in testicular tissue culture system
We aimed to verify that expression levels of PI3K-Akt-mTOR pathway proteins change in RA-treated testis in vitro, as in a previous study.24 Therefore, we used three concentrations of AR7 (10, 20, and 50 μmol/L) and determined with the expression of Stra8 (a gene stimulated directly by RA) protein at 24, 48, or 72 h after intervention. As shown in Figure 5, the optimal condition is to use AR7 at 20 μmol/L for 48 h. Then, doses of RA at 1, 10, or 50 μmol/L were added into medium with AR7 at 20 μmol/L. Detection with Western blot at 24, 48, and 72 h for the level of Stra8 showed that this protein was most obviously improved with RA at 10 μmol/L for 48 h (Fig. 5B, D). Consequently, in the following experiments, the intervention conditions for AR7 and RA were selected as 20 μmol/L and 10 μmol/L, respectively, and the treatment time was 48 h (Fig. 5C, D).
Figure 5.
Blocking RARα with AR7 for detecting the effect of RA on PI3K-Akt-mTOR pathway and autophagy with testicle culture in vitro. 20 μmol/L AR7 for 48h (A, C) and RA 10 μmol/L for 48h (B, D) are selected as proper intervention condition with the protein level change of Stra8 by Western blot in vitro. The y-axis represents the target protein value relative to β-actin in all histogram (B, D) The proteins level of PI3K, pan-Akt, p-Akt, mTOR, and p-mTOR are quantitative analysis with Western blot after corresponding AR7 and RA treatment (E). Quantitative analysis of autophagy level is evaluated by the expression of Beclin1, LC3 a/b and P62 with Western blot in vitro (F). The y-axis represents the target protein value relative to β-actin in all histogram. Error bars represent mean ± standard error of estimate from three independent experiments. ∗ Compared with the normal control and Flu + RA groups; P < 0.05.
PI3K-Akt-mTOR signaling pathway changes after AR7 or RA treatment
AR7 was used to determine whether RA regulated the PI3K-Akt-mTOR signaling pathway through RARα. As expected, the protein expression levels of key markers in the PI3K-Akt-mTOR pathway (PI3k, p-Akt/pan-Akt, and p-mTOR/mTOR) were significantly higher when compared with the normal control group and the AR7 + RA group. The phenomenon was similar in Flu-induced cryptorchid testes. After 10 μmol/L RA treatment for 48 h, the protein levels of PI3k, p-Akt/pan-Akt, and p-mTOR/mTOR had decreased and were close to those of the normal control group (Fig. 5E).
Changes in autophagy markers are associated with AR7 and RA intervention in vitro
To study whether the change of autophagy after RARα antagonist treatment was in accordance with cryptorchid testis, the level of autophagy was evaluated with LC3 a/b, Beclin1, and P62 by Western blot. After AR7 treatment, the protein levels of LC3 a/b and Beclin1 were decreased, and the P62 protein level was increased. In contrast, these protein levels approached normal when RARα was restored with RA treatment in vitro (Fig. 5F).
Key proteins in present study with PPI network validation
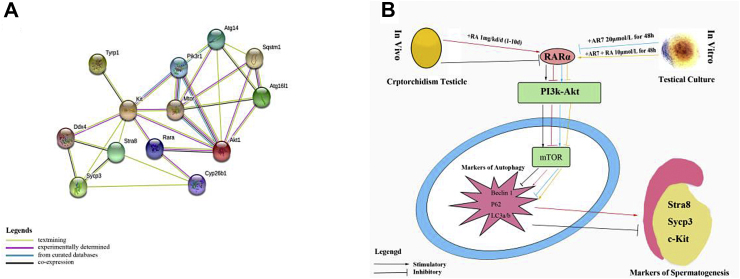
A PPI network was obtained with all proteins in this study. As shown in Figure 6A, RARα, mTOR, and c-Kit locate at the center in this network's axes. Thus, the PPI network supported our hypothesis retrospectively, demonstrating that RA may influence the expression of spermatogenic-related proteins through mTOR-related pathways via RARα in a cryptorchidism-damaged model of infertility.
Figure 6.
Test the hypothetical mechanism with PPI network. RARα, mTOR and c-Kit locate at axis center considered as crucial proteins in this PPI network constituting with all protein in this study (A). Schematic representation of the mechanism in this study (B). We show that spermatogenesis markers is lower in cryptorchidism-infertiltiy testis, and partial restoration after treatment with RA 1 mg/kg in neonatal rats in vivo. RA may increase the abnormality autophagy level in cryptorchidism-infertility testis through PI3K-Akt-mTOR pathway via RARα. This mechanism is also confirmation after blocking RARα and intervention with RA with testicle culture in vitro.
Discussion
It is well known that RA is, indeed, necessary for normal spermatogonial differentiation. However, the effect of RA on the processes of reproductive malformations and abnormal spermatogenesis still leaves many questions in our understanding. Thus, we provide the first evidence to introduce the importance of RA in spermatogonial differentiation in a cryptorchidism-induced animal model, which acted as a representative of reproductive-malformation-caused infertility. Our data suggest that RA may play a crucial role in regulating the autophagy level in cryptorchidism-related infertility through a suppressed PI3K-Akt-mTOR signaling pathway, via binding to RARα and restoring BTB function, finally partially reconstructing spermatogenesis.
In normal testis, RA is secreted by spermatogonia stem cells (SSCs) after birth, while cryptorchidism can lead to a deficient pool of stem cells.25,26 We also found that RA concentration was decreased in cryptorchid rat testes in a previous study.21 The response of spermatogonia to RA may depend on RAR binding to RXR to form an RAR/RXR heterodimer, which activates downstream target genes, including c-Kit, Sycp3, and Stra8.24 Therefore, RARα is necessary for the first wave of spermatogonia differentiation in SSCs. This process is basic for keeping spermatogenesis continuous.8,27 Combining this study with our previous study, we found the activation of RARα was inhibited, caused by RA intrinsic deficiency in cryptorchidism-infertile rats. In addition, we found that markers of spermatogonia differentiation and spermatocyte meiosis were significantly decreased in cryptorchid testes, and these marker levels were restored after RA supplementation. Consistent with our findings, administration with RA improved mouse spermatocytes’ induction efficiency in an RA/pup Sertoli cell-based in vitro model.25 One clinical trial showed oral RA improved sperm count in 19 infertile males.14 In future studies, we need to find the proper time period of RA treatment for cryptorchidism to give some reliable data for clinical practice.
In different periods of administration with RA in cryptorchid testes, we found that RA has little efficiency on spermatogenesis when supplemented during the post-pubescent period (PND 30) in cryptorchid infertile rats. There seems to be almost identical effects when RA supplementation occurs between PND 50-60 and the neonatal period. However, our follow-up results showed that this effect could not be further observed when detecting after 10 days (PND 70), although RA was administrated between PND 50 and PND 60. Other studies also mention that in neonatal testicular tubules, a pulsed concentration of RA leads to spermatogonia differentiation in a precise and repetitive manner, so that sperm could be produced continuously in adult testes.28,29 Additionally, we find that supplementing exogenous RA may only cause a transient improvement of spermatogenesis in adult cryptorchidism-infertility pathological states. This result is consistent with a recent study, in which exposure to Vitamin A also could lead to high RA concentration in adult testicle, but the dose was not sufficient to affect spermatogenesis.30 Another study reports that RA exposure in adult testes increased the expression CYP26A1 (an enzyme that degrades RA), and could not continuously stimulate spermatogenesis.31 Therefore, we deduced that exogenous RA supplementation may drive the entire abnormal undifferentiated germ cell population into a regular differentiation pathway in neonatal cryptorchid rats, but not in adult. The earlier the treatment for cryptorchidism, the better the curative effect. This is also agreed on by urologists.
However, how RA improves spermatogenesis is still unknown. The BTB is the key factor to guard the spermatogenesis process. Thus, after confirmation that the expression of RARα was improved in the RA treatment group, BTB functional analysis was performed. Biotin tracing analysis indicated that the permeability of the BTB was increased in Flu-induced cryptorchidism tests and could be ameliorated with RA. Hasegawa et al also found that if there was negative RARα expression in Sertoli cells, BTB formation would be disrupted.32 A recent study reported that blocking RA signaling not only presented with a loss of advanced germ cells but also increased the permeability of the BTB.11 In the present cryptorchidism-infertility model, the changes in spermatogonia differentiation and meiosis markers are improved with BTB permeability amelioration. With the cyclical changes of the BTB, the spermatogenesis process can continuously happen. We believe the role of RA in BTB integrity is related to the mTOR pathway.33 In cryptorchidic testes, we found p-mTOR was up-regulated. mTOR plays a crucial role in BTB integrity. Several reports have shown that mTORC1 promotes the restructuring of the BTB, leaving the BTB “leaky”34,35; whereas mTORC2 promotes BTB integrity, making the BTB “tighter”.36 The imbalance of mTORC1 and mTORC2 must lead to BTB disruption and spermatogenesis dysfunction. Thus, restoration of BTB function may become a goal in the treatment of cryptorchidism, although the detailed mechanism still needs to be investigated.
We aimed to find the signaling pathways by which RA improves spermatogenesis in cryptorchidism. The data of RA inducing the mouse embryonic stem cell differentiation process were analyzed. The KEGG pathway analysis results showed that the PI3K-Akt signaling pathway may act in a crucial role in RA intervention in mouse embryonic stem cell differentiation. Then, we found that the protein levels of PI3K, p-Akt, and p-mTOR were activated in cryptorchidism-induced infertile testes and in RARα antagonist treatment. RA effectively modified these key markers of the PI3K-Akt-mTOR pathway, so they approach the normal levels in vivo and in vitro. There is also evidence that RA could induce efficient translation of c-Kit mRNA along with the PI3K/Akt/mTOR pathway, promoting the spermatogonia-forming process.37 Similarly, in a liver ischemia and reperfusion injury study, RA bound to RARα, promoting autophagy through decreasing p-Akt expression.38 Jonathan et al reported that RA may induce KIT gene expression through activation of PI3K/Akt/mTOR pathway in testicular tissue.39 Other studies on nasopharyngeal carcinoma, acute promyelocytic leukemia, and neuroblastoma have all reported that RA plays an important role in autophagy by inhibiting PI3K/Akt/mTOR signaling pathway. Studies on peripheral blood B cells have shown that RA plays an immunomodulatory role by inducing and activating IgG production, increasing LC3A/B protein expression, decreasing P62 protein expression, and increasing autophagy levels.40, 41, 42 We hypothesized that the PI3K-Akt-mTOR pathway exists in several states in different models. Cryptorchidism is a disease state. Our results were consistent with the previous studies in other tissues, so we speculated that RA could play a role in balancing the level of autophagy in testicular tissue.
An mTOR-related autophagy level is not only known as being downstream of PI3K/Akt/mTOR pathway but also regulate the balance between spermatogonial stem cell self-renewal and differentiation.43 In this study, combined in vivo and in vitro results showed that the protein levels of Beclin1 and LC3 a/b were decreased. The level of P62 was increased in cryptorchidism-induced infertility or after RARα antagonist treatment. The protein levels of Beclin1 and LC3 a/b were higher, and P62 was lower after RA supplementation. In a spinal cord injury model, RA administration could prevent disruption of the blood−spinal barrier via the activation of autophagic flux and the inhibition of ER stress-induced cell apoptosis.44 While cultured samples without any other factors for more than one week could confirm the molecular mechanism in vitro, this may a limitation of present study. Based on these results, correct abnormal autophagy levels in testes may become the aim of drug treatment for treating cryptorchidism.
In summary, we find that RA could restore key protein levels of spermatogonial differentiation and cause recovery of the permeability of the BTB in cryptorchidism-induced infertility. This effect is partially attributed to the suppression of the PI3K/Akt/mTOR signaling pathway through RARα, thereby maintaining the changes of autophagy so they approach the normal standard. This study provides a novel insight into the treatment of adult infertility caused by childhood reproductive disease, especially cryptorchidism. However, advanced applications in cryptorchid children still need further clinical trial support, and the detailed mechanism of RA in autophagy also requires investigation with more methods and under different conditions.
Conflict of interests
The authors declare that they do not have any conflicts of interest.
Funding
This study was financially supported by the National Natural Science Foundation of China (No. 81771566), the Science and Technology Research Program of Chongqing Municipal Education Commission, China (No. KJQN201900444), the High-level Medical Reserved Personnel Training Project of Chongqing, China (No. [2018]230), and the Chongqing Science and Technology Commission, China (No. cstc2018jcyjAX0193).
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2021.03.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Deshpande P.S., Gupta A.S. Causes and prevalence of factors causing infertility in a public health facility. J Hum Reprod Sci. 2019;12(4):287–293. doi: 10.4103/jhrs.JHRS_140_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeqiraj A., Beadini S., Beadini N., et al. Male infertility and sperm DNA fragmentation. Open Access Maced J Med Sci. 2018;6(8):1342–1345. doi: 10.3889/oamjms.2018.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hildorf S., Clasen-Linde E., Cortes D., Fossum M., Thorup J. Fertility potential is compromised in 20% to 25% of boys with nonsyndromic cryptorchidism despite orchiopexy within the first year of life. J Urol. 2020;203(4):832–840. doi: 10.1097/JU.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 4.Virtanen H.E., Toppari J. Cryptorchidism and fertility. Endocrinol Metab Clin North Am. 2015;44(4):751–760. doi: 10.1016/j.ecl.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Griswold M.D. Spermatogenesis: the commitment to meiosis. Physiol Rev. 2016;96(1):1–17. doi: 10.1152/physrev.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verkauskas G., Malcius D., Dasevicius D., Hadziselimovic F. Histopathology of unilateral cryptorchidism. Pediatr Dev Pathol. 2019;22(1):53–58. doi: 10.1177/1093526618789300. [DOI] [PubMed] [Google Scholar]
- 7.Rodprasert W., Virtanen H.E., Mäkelä J.A., Toppari J. Hypogonadism and cryptorchidism. Front Endocrinol. 2019;10:906. doi: 10.3389/fendo.2019.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y., Ma L., Hogarth C., Wei G., Griswold M.D., Tong M.H. Retinoid signaling controls spermatogonial differentiation by regulating expression of replication-dependent core histone genes. Development. 2016;143(9):1502–1511. doi: 10.1242/dev.135939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y., Reese D.H. Disruption of retinol (vitamin A) signaling by phthalate esters: SAR and mechanism studies. PLoS One. 2016;11(8):e0161167. doi: 10.1371/journal.pone.0161167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lord T., Oatley M.J., Oatley J.M. Testicular architecture is critical for mediation of retinoic acid responsiveness by undifferentiated spermatogonial subtypes in the mouse. Stem Cell Reports. 2018;10(2):538–552. doi: 10.1016/j.stemcr.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jauregui E.J., Mitchell D., Topping T., Hogarth C.A., Griswold M.D. Retinoic acid receptor signaling is necessary in steroidogenic cells for normal spermatogenesis and epididymal function. Development. 2018;145(13):dev160465. doi: 10.1242/dev.160465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung S.S., Sung W., Wang X., Wolgemuth D.J. Retinoic acid receptor alpha is required for synchronization of spermatogenic cycles and its absence results in progressive breakdown of the spermatogenic process. Dev Dyn. 2004;230(4):754–766. doi: 10.1002/dvdy.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogarth C.A., Griswold M.D. Retinoic acid regulation of male meiosis. Curr Opin Endocrinol Diabetes Obes. 2013;20(3):217–223. doi: 10.1097/MED.0b013e32836067cf. [DOI] [PubMed] [Google Scholar]
- 14.Amory J.K., Ostrowski K.A., Gannon J.R., et al. Isotretinoin administration improves sperm production in men with infertility from oligoasthenozoospermia: a pilot study. Andrology. 2017;5(6):1115–1123. doi: 10.1111/andr.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Y., He D., Yao Z., Klionsky D.J. The machinery of macroautophagy. Cell Res. 2014;24(1):24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang Y., Wang H., Jia P., et al. Autophagy regulates spermatid differentiation via degradation of PDLIM1. Autophagy. 2016;12(9):1575–1592. doi: 10.1080/15548627.2016.1192750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C., Wang H., Shang Y., et al. Autophagy is required for ectoplasmic specialization assembly in sertoli cells. Autophagy. 2016;12(5):814–832. doi: 10.1080/15548627.2016.1159377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yefimova M.G., Buschiazzo A., Burel A., et al. Autophagy is increased in cryptorchid testis resulting in abnormal spermatozoa. Asian J Androl. 2019;21(6):570–576. doi: 10.4103/aja.aja_12_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin J., Ni B., Tian Z.Q., Yang F., Liao W.G., Gao Y.Q. Regulatory effects of autophagy on spermatogenesis. Biol Reprod. 2017;96(3):525–530. doi: 10.1095/biolreprod.116.144063. [DOI] [PubMed] [Google Scholar]
- 20.Wu D., Shao K., Zhou Q., et al. Autophagy and ubiquitin-mediated proteolytic degradation of PML/Rarα fusion protein in matrine-induced differentiation sensitivity recovery of ATRA-resistant APL (NB4-LR1) cells: in vitro and in vivo studies. Cell Physiol Biochem. 2018;48(6):2286–2301. doi: 10.1159/000492646. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y., Zhang D., Hu D., et al. Retinoic acid: a potential therapeutic agent for cryptorchidism infertility based on investigation of flutamide-induced cryptorchid rats in vivo and in vitro. Reprod Toxicol. 2019;87:108–117. doi: 10.1016/j.reprotox.2019.05.063. [DOI] [PubMed] [Google Scholar]
- 22.Xu Y., Wu W., Fan Y., Jiang S., Jia X., Su W. MiR-142-3p inhibits TGF-β3-induced blood-testis barrier impairment by targeting lethal giant larvae homolog 2. Cell Physiol Biochem. 2018;46(1):253–268. doi: 10.1159/000488427. [DOI] [PubMed] [Google Scholar]
- 23.Sato T., Katagiri K., Gohbara A., et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471(7339):504–507. doi: 10.1038/nature09850. [DOI] [PubMed] [Google Scholar]
- 24.Kasimanickam V.R., Kasimanickam R.K. Retinoic acid signaling biomarkers after treatment with retinoic acid and retinoic acid receptor alpha antagonist (Ro 41-5253) in canine testis: an in vitro organ culture study. Theriogenology. 2013;79(1):10–16. doi: 10.1016/j.theriogenology.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Wang S., Wang X., Ma L., et al. Retinoic acid is sufficient for the in vitro induction of mouse spermatocytes. Stem Cell Reports. 2016;7(1):80–94. doi: 10.1016/j.stemcr.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandran U., Indu S., Kumar A.T., et al. Expression of Cnnm1 and its association with stemness, cell cycle, and differentiation in spermatogenic cells in mouse testis. Biol Reprod. 2016;95(1):7. doi: 10.1095/biolreprod.115.130369. [DOI] [PubMed] [Google Scholar]
- 27.Bowles J., Knight D., Smith C., et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312(5773):596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 28.Vernet N., Dennefeld C., Rochette-Egly C., et al. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology. 2006;147(1):96–110. doi: 10.1210/en.2005-0953. [DOI] [PubMed] [Google Scholar]
- 29.Sugimoto R., Nabeshima Y., Yoshida S. Retinoic acid metabolism links the periodical differentiation of germ cells with the cycle of Sertoli cells in mouse seminiferous epithelium. Mech Dev. 2012;128(11–12):610–624. doi: 10.1016/j.mod.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Agrimson K.S., Onken J., Mitchell D., et al. Characterizing the spermatogonial response to retinoic acid during the onset of spermatogenesis and following synchronization in the neonatal mouse testis. Biol Reprod. 2016;95(4):81. doi: 10.1095/biolreprod.116.141770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X., Long X.Y., Xie Y.J., Zeng X., Chen X., Mo Z.C. The roles of retinoic acid in the differentiation of spermatogonia and spermatogenic disorders. Clin Chim Acta. 2019;497:54–60. doi: 10.1016/j.cca.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa K., Saga Y. Retinoic acid signaling in Sertoli cells regulates organization of the blood-testis barrier through cyclical changes in gene expression. Development. 2012;139(23):4347–4355. doi: 10.1242/dev.080119. [DOI] [PubMed] [Google Scholar]
- 33.Boyer A., Girard M., Thimmanahalli D.S., et al. mTOR regulates gap junction alpha-1 protein trafficking in Sertoli cells and is required for the maintenance of spermatogenesis in mice. Biol Reprod. 2016;95(1):13. doi: 10.1095/biolreprod.115.138016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S.Y.T., Yan M., Chen H., et al. mTORC1/rpS6 regulates blood-testis barrier dynamics and spermatogenetic function in the testis in vivo. Am J Physiol Endocrinol Metab. 2018;314(2):E174–E190. doi: 10.1152/ajpendo.00263.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan M., Li L., Mao B., et al. mTORC1/rpS6 signaling complex modifies BTB transport function: an in vivo study using the adjudin model. Am J Physiol Endocrinol Metab. 2019;317(1):E121–E138. doi: 10.1152/ajpendo.00553.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mok K.W., Mruk D.D., Lee W.M., Cheng C.Y. Rictor/mTORC2 regulates blood-testis barrier dynamics via its effects on gap junction communications and actin filament network. FASEB J. 2013;27(3):1137–1152. doi: 10.1096/fj.12-212977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minkina A., Lindeman R.E., Gearhart M.D., et al. Retinoic acid signaling is dispensable for somatic development and function in the mammalian ovary. Dev Biol. 2017;424(2):208–220. doi: 10.1016/j.ydbio.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong C., Pu L.Y., Fang M.M., Gu Z., Rao J.H., Wang X.H. Retinoic acid receptor α promotes autophagy to alleviate liver ischemia and reperfusion injury. World J Gastroenterol. 2015;21(43):12381–12391. doi: 10.3748/wjg.v21.i43.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Busada J.T., Chappell V.A., Niedenberger B.A., et al. Retinoic acid regulates Kit translation during spermatogonial differentiation in the mouse. Dev Biol. 2015;397(1):140–149. doi: 10.1016/j.ydbio.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y., Liu Q., Chen S., et al. APLNR is involved in ATRA-induced growth inhibition of nasopharyngeal carcinoma and may suppress EMT through PI3K-Akt-mTOR signaling. FASEB J. 2019;33(11):11959–11972. doi: 10.1096/fj.201802416RR. [DOI] [PubMed] [Google Scholar]
- 41.Humbert M., Federzoni E.A., Tschan M.P. Distinct TP73-DAPK2-ATG5 pathway involvement in ATO-mediated cell death versus ATRA-mediated autophagy responses in APL. J Leukoc Biol. 2017;102(6):1357–1370. doi: 10.1189/jlb.1A0317-132R. [DOI] [PubMed] [Google Scholar]
- 42.Eriksen A.B., Torgersen M.L., Holm K.L., et al. Retinoic acid-induced IgG production in TLR-activated human primary B cells involves ULK1-mediated autophagy. Autophagy. 2015;11(3):460–471. doi: 10.1080/15548627.2015.1009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serra N.D., Velte E.K., Niedenberger B.A., Kirsanov O., Geyer C.B. Cell-autonomous requirement for mammalian target of rapamycin (Mtor) in spermatogonial proliferation and differentiation in the mouse†. Biol Reprod. 2017;96(4):816–828. doi: 10.1093/biolre/iox022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Y., Zhang H., Zheng B., et al. Retinoic acid induced-autophagic flux inhibits ER-stress dependent apoptosis and prevents disruption of blood-spinal cord barrier after spinal cord injury. Int J Biol Sci. 2016;12(1):87–99. doi: 10.7150/ijbs.13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.