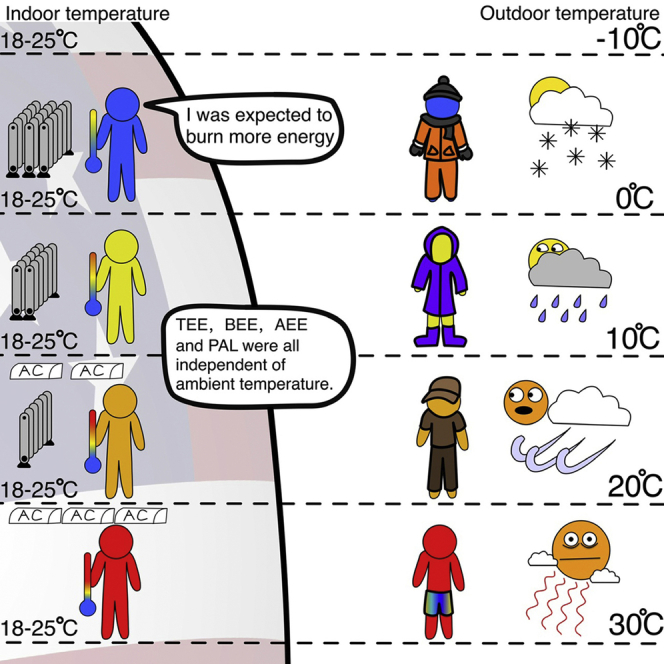
Summary
Lower ambient temperature (Ta) requires greater energy expenditure to sustain body temperature. However, effects of Ta on human energetics may be buffered by environmental modification and behavioral compensation. We used the IAEA DLW database for adults in the USA (n = 3213) to determine the effect of Ta (−10 to +30°C) on TEE, basal (BEE) and activity energy expenditure (AEE) and physical activity level (PAL). There were no significant relationships (p > 0.05) between maximum, minimum and average Ta and TEE, BEE, AEE and PAL. After adjustment for fat-free mass, fat mass and age, statistically significant (p < 0.01) relationships between TEE, BEE and Ta emerged in females but the effect sizes were not biologically meaningful. Temperatures inside buildings are regulated at 18–25°C independent of latitude. Hence, adults in the US modify their environments to keep TEE constant across a wide range of external ambient temperatures.
Subject areas: Human activity in medical context, Human Physiology, Human metabolism
Graphical abstract
Highlights
-
•
Human total, activity and basal energy expenditure was unrelated to outdoor temperature
-
•
Indoor temperature in the USA was independent of latitude and was 18–25°C
-
•
Human metabolism is independent of outdoor temperature because we buffer exposure
-
•
Keeping mice at 30°C does not create the best translational efficiency to humans
Human activity in medical context; Human Physiology; Human metabolism
Introduction
Obesity is a state of excess deposition of white adipose tissue (WAT) that has become a major global health issue (Blüher, 2019; Gregg and Shaw, 2017)because it leads to elevated risks for several chronic non-communicable diseases like type 2 diabetes, hypertension and cancer (Babu et al., 2018; Pi-Sunyer, 2019). Although obesity is caused by an imbalance between energy intake and expenditure (Hall et al., 2012), the causes of this imbalance remain unclear and disputed (Hall et al., 2022; Speakman and Hall, 2021). In addition to white adipocytes, whose primary role is to store triglycerides, Eutherian mammals also have other types of adipocytes that appear to serve different functions (Cypess, 2022). In particular, brown adipocytes are characterized by multilocular lipid droplets and large numbers of mitochondria that carry the uncoupling protein 1 (UCP1) on their inner membranes that can uncouple the passage of protons across the inner membrane from the synthesis of ATP, thereby releasing their chemiosmotic potential directly as heat (Cannon and Nedergaard, 2004). The third type of adipocytes, called beige or brite adipocytes (Ishibashi and Seale, 2010; Wu et al., 2012), seem to display both white and an attenuated brown phenotype under different circumstances (Rosenwald et al., 2013).
Brown adipose tissue, mainly comprising brown adipocytes, is abundant in small mammals and neonatal humans (Cannon and Nedergaard, 2004). Its presence in adult humans was controversial, but it was conclusively demonstrated around 10–15 years ago (Cypess et al., 2009; Nedergaard et al., 2007; Pfannenberg et al., 2010; Saito et al., 2009; van Marken Lichtenbelt et al., 2009). Interestingly the amounts of BAT in adult humans decline as we age (Cypess, 2022; Pfannenberg et al., 2010), coincident with a decline in whole-body metabolic rate (Pontzer et al., 2021) and an increase in adiposity (Schautz et al., 2012). Moreover, levels of BAT appear to be inversely related to WAT (Betz and Enerbäck, 2015; van Marken Lichtenbelt et al., 2009; Wang et al., 2015), suggesting that activation of BAT might be protective against obesity. But this inverse relation may also be because greater levels of WAT confer better cold resistance due to its insulative properties (Speakman, 2018) and hence lower the need for thermogenesis derived from BAT. Nevertheless, the potential to stimulate BAT, or force the conversion of beige adipocytes from their white to brown form, has received enormous attention, particularly in studies of mice (Li et al., 2022; Nedergaard and Cannon, 2014; Rosenwald et al., 2013; Wang et al., 2016). This has been stimulated in part by observations that transplanting BAT but not WAT in mice causes weight loss and can reverse diet-induced or genetic obesity (Liu et al., 2013, 2015; Stanford and Goodyear, 2013).
When animals are exposed to the cold, they increase their energy expenditure to balance the elevated heat loss (Scholander et al., 1950), and this heat requirement is supplied mainly by the BAT (Foster and Frydman, 1979). This effect is consistent with the fact that levels of BAT in humans detected by PET-CT increase during the temperate winter (Au-Yong et al., 2009; Saito et al., 2009) and at colder ambient temperatures (Cypess, 2022). If such increases are linked to defense against obesity, one would anticipate obesity would be less common in areas where it is colder. Yet within the mainland USA, such a relationship is not observed (Speakman and Heidari-Bakavoli, 2016). There are several potential reasons why this may pertain. Notably, humans may buffer themselves from environmental temperature changes by modulating their external insulation and controlling the environments where they spend most of their time. This would then decouple their metabolic responses from environmental exposures. Indeed, such decoupling may have encouraged humans into positive energy balance and be a root issue in the obesity epidemic (Dhurandhar and Keith, 2014; Johnson et al., 2011; Keith et al., 2006; McAllister et al., 2009).
Our understanding, however, is hindered by the fact that we have no information on how human total energy requirements vary as a function of ambient temperature for free-living humans. Measurements in the laboratory suggest humans conform to the normal ‘Scholander relation’ (Celi et al., 2010; Chen et al., 2013), but such individuals cannot react to the temperature any other way than physiologically. This raises the question whether free-living humans buffer such environmental changes entirely by behavioral changes or altering external insulation, leading to no effect of environmental temperature on metabolic rate. This would then explain the absence of an effect of ambient temperature on obesity. If true, switching on human BAT may be a viable treatment strategy. Alternatively, the buffering of ambient temperature impacts may be incomplete, but stimulated BAT in the cold is ineffective at regulating adiposity because it also stimulates food intake (Cypess, 2022) or compensations elsewhere in the energy budget (Careau et al., 2021). This would compromise the whole strategy of stimulating BAT to reduce WAT levels. The current paper provide an analysis of the total, basal and activity energy expenditure of 3213 adult humans measured across the USA in relation to ambient temperature, showing that indeed environmental buffering is complete across the range from −10 to 30°C.
Results
We present here an analysis of measurements of TEE in 3213 adults (aged 18 to 101) living in the mainland USA, measured using the doubly-labelled water (DLW) method, drawn from the IAEA DLW database (Speakman et al., 2019). All original estimates of TEE were re-calculated using a standard equation which has been shown to perform best in validation studies (Speakman et al., 2021b). We split the data by sex: 2426 measurements of females and 787 measurements of males. In addition, for 185 of the males and 414 of the females, we also had measures of basal energy expenditure (BEE) from which we derived activity energy expenditure (AEE) and the physical activity level (PAL) – for calculation method, see STAR Methods. We matched the individual estimates of TEE, based on reported dates and locations for each measurement in the database, to the local ambient temperature and precipitation available from the National Centers for Environmental Information (NCEI) (Durre, 2018). This database contains averages of daily maximum, minimum, and average temperature (TMAX, TMIN, and TAVG) for the contiguous USA between January 1, 1951, and the present, based on gridded fields at a resolution of ½4 of a degree (0.041667°). This is equivalent to each grid being 4.6 km latitudinally and ranges from 3.0 km in the North to 4.2 km in the South longitudinally. Daily weather data were extracted from the database for the days of each DLW measurement (typically 14 to 21 days) for each subject and then averaged across these days. Approximately 80% of the temperature measurements were below the laboratory determined lower critical temperature (lower bound of the thermoneutral zone) of around 23°C (Brychta et al., 2019).
The relationships between total, basal, activity energy expenditure and ambient temperature
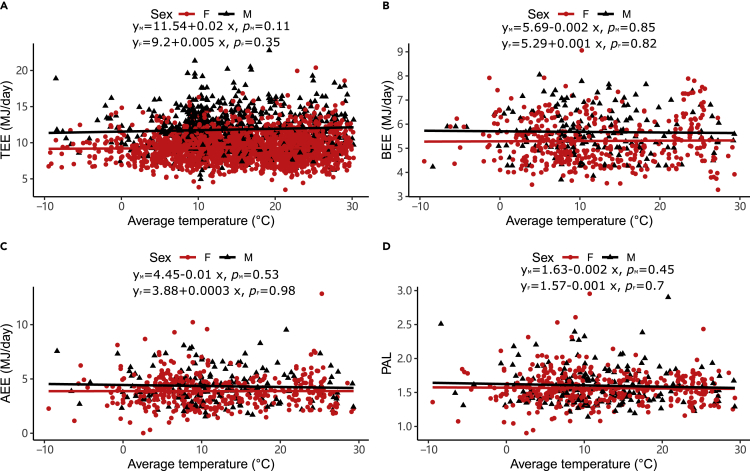
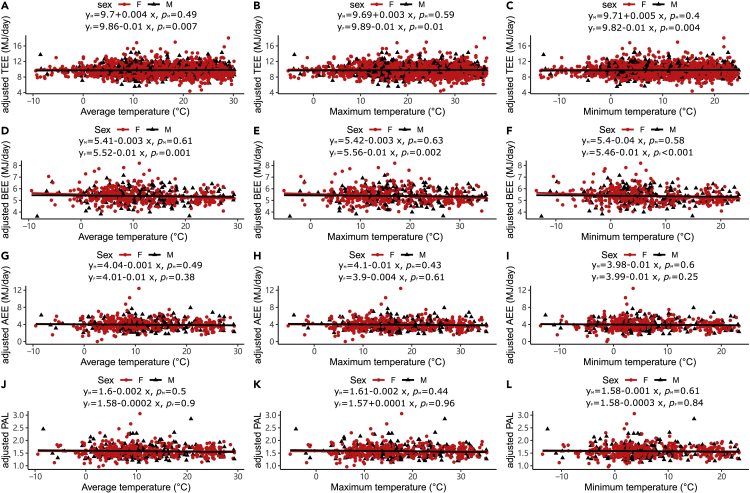
In both males and females, there were no significant relationships between the average temperature and the unadjusted TEE, BEE, AEE and PAL (Figure 1). There were no significant relationships between the two other measurement temperature and the unadjusted TEE, BEE, AEE and PAL (see Figure S1). There were no significant relationships between body mass, FFM, and FM and the three temperature measurements (see Figure S2). We adjusted the levels of TEE, BEE, AEE and PAL for body size and age using the residuals from general linear models with FFM, FM, age and age2 as predictor variables, as all these variables were significantly related to TEE, BEE and AEE (see Figure S3) (Pontzer et al., 2021). In males, none of the adjusted expenditure measures changed significantly with the three measures of ambient temperature (Figure 2). In females, there was no relationship between adjusted AEE and PAL and temperature measures (Figure 2). Still, there was a significant decline in adjusted TEE with increased ambient temperature (Figure 2A, r2 (TEE) TAVG = 0.3%, F (TEE) TAVG = 7.23, P (TEE) TAVG = 0.007; Figure 2B r2 (TEE) TMAX = 0.25%, F (TEE) TMAX = 5.99, P (TEE) TMAX = 0.01; Figure 2C r2 (TEE) TMIN = 0.33%, F (TEE) TMIN = 8.13, P (TEE) TMIN = 0.004). However, the variance explained by temperature was very low (<0.5%) and the gradient of decline (0.01 MJ/day, se (SE) = 0.003 MJ/day) was equivalent to an average reduction in adjusted TEE by only 0.1 MJ (1.03%) over 10°C (95%CI = 0.15 to 0.02 MJ/day). The effect size for temperature on TEE was far lower than the direct impact of temperature on energy expenditure observed in laboratory cold exposure studies. For example, RMR increases by about 25% coincident with a 3°C drop in ambient temperature below thermoneutrality27, an effect about 75× greater than reported here.
Figure 1.
Associations between unadjusted total, basal, activity energy expenditure, physical activity levels, and ambient temperature
(A) average temperature (oC) vs TEE (MJ/day).
(B) average temperature (oC) vs BEE (MJ/day).
(C) average temperature (oC) vs AEE (MJ/day).
(D) average temperature (oC) vs PAL. Each data point is a different individual. The black lines are the least-squares fitted regression lines for males, and the red lines are for females.
Figure 2.
Associations between adjusted total, basal, activity energy expenditure, physical activity levels (adjusted for fat-free mass, fat mass, age, and age2), and ambient temperature
(A) average (B) maximum and (C) minimum temperature (oC) vs adjusted TEE (MJ/day).
(D) average, (E) maximum, and (F) minimum temperature (oC) vs adjusted BEE (MJ/day).
(G) average, (H) maximum, and (I) minimum temperature (oC) vs adjusted AEE (MJ/day).
(J) average, (K) maximum, and (L) minimum temperature (oC) vs adjusted PAL. Each data point is a different individual. The black lines are the least-squares fitted regression lines for males, and the red lines are for females.
Adjusted BEE also decreased with the increase of ambient temperature for females (Figure 2D, r2 (BEE) TAVG = 2.69%, F (BEE) TAVG = 11.38, P (BEE) TAVG = 0.001; Figure 2E r2 (BEE) TMAX = 2.37%, F (BEE) TMAX = 10.01, P (BEE) TMAX = 0.002; Figure 2F r2 (BEE) TMIN = 3.16%, F (BEE) TMIN = 13.47, P (BEE) TMIN < 0.001). As with TEE, the explained variance was small and the gradient of decline (0.01 MJ/day, se = 0.004 MJ/day) was equivalent to an average reduction in adjusted BEE of just 0.1 MJ (1.86%) over 10° (95%CI = 0.19 to 0.05 MJ/day).
Associations between outdoor temperature (oC), indoor temperature (oC) and latitude (oN)
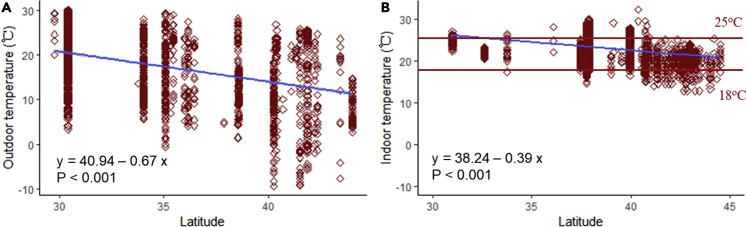
We explored the relationships between outdoor ambient temperature, indoor ambient temperature and latitude. We found significant negative relationships between latitude and both outdoor and indoor temperature (Figure 3A, Foutdoor = 490.89, p < 0.001; Figure 3B, Findoor = 1713.03, p < 0.001). However, the gradient of the effect of latitude on indoor temperatures was significantly shallower (Fgroup = 8.81, p = 0.003; Flatitude = 1989.87, p < 0.001, Fgroup x latitude = 134.87, p < 0.001) with the consequence that virtually all indoor temperatures were in the range 18–25°C (Figure 3B).
Figure 3.
Associations between (A): outdoor temperature (oC) and (B): indoor temperature (oC) and latitude (oN)
The outdoor temperature is the average temperature. For the indoor temperatures, the limits of 25°C and 18°C are shown in red.
Seasonal variations of ambient temperature and seasonal effects on energetics
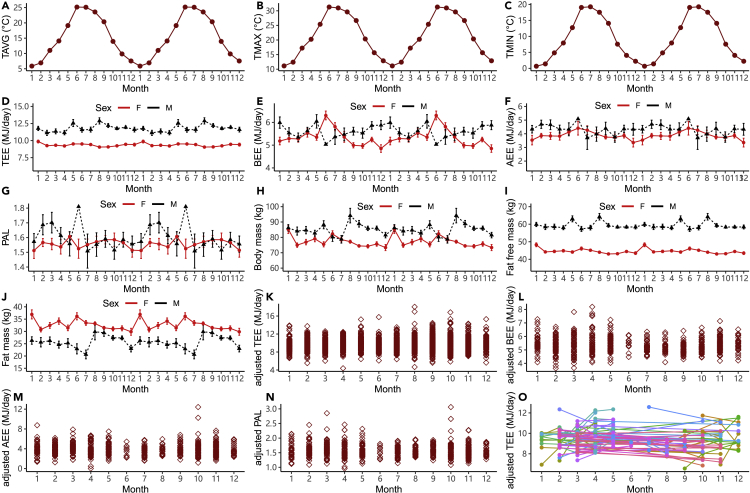
There was clear seasonal variation in ambient temperature (Figure 4). Month had a significant effect on the ambient temperature (Figures 4A and 4F (TAVG) month = 679.66 p < 0.001; Figures 4B and 4F (TMAX) month = 592.50, p < 0.001; Figures 4C and 4F (TMIN) month = 685.23, p < 0.001). The highest average level of TMAX was 31.30°C (se = 0.27) in June, while the lowest average level of TMIN was 0.66°C (se = 0.46) in January. We explored the seasonal effects on the unadjusted TEE, BEE, AEE, PAL, body mass (BM), fat free mass (FFM) and fat mass (FM) (Figure 4). Month had significant effects on all the traits for both males and females. TEE levels varied seasonally (Figure 4D, FM (TEE) month = 2.31, PM = 0.02; FF (TEE) month = 2.54, PF = 0.003). In males, the average TEE over time was 11.82 MJ/day (se = 0.09 MJ/day) with the highest level in August (12.88 MJ/day, se = 0.46 MJ/day) and lowest level in February (11.13 MJ/day, se = 0.38 MJ/day). The average level of TEE for females was 9.28 MJ/day (se = 0.04 MJ/day) which was significantly lower than males (F = 914.38, p < 0.001). The seasonal trend of TEE for females was different from that in males with the highest level in January (9.86 MJ/day, se = 0.21 MJ/day) and lowest level in August (9.05 MJ/day, se = 0.09 MJ/day). Month also had an effect on BM (Figure 4H, FM (BM) month = 2.31, p = 0.009; FF (BM) month = 4.02, p < 0.001), including clear seasonal trends in both FFM and FM (Figure 4I, FM (FFM) month = 2.31, p = 0.009; FF (FFM) month = 4.30, p < 0.001; Figure 4J, FM (FM) month = 2.37, p = 0.007; FF (FM) month = 3.29, p < 0.001).
Figure 4.
Seasonal variations of ambient temperature and seasonal effects for TEE, BEE, AEE, PAL, body mass, fat-free mass, fat mass, adjusted TEE, adjusted BEE, adjusted AEE, adjusted PAL (adjusted for fat-free mass, fat mass, age, and age2)
Plots A to J are shown as double plots to illuminate the seasonal trends, and all error bars are present as SE.
(A) average temperature (oC).
(B) maximum temperature (oC).
(C) minimum temperature (oC).
(D) Total energy expenditure (MJ/d).
(E) Basal energy expenditure (MJ/d).
(F) Activity energy expenditure (MJ/d).
(G) Physical activity level.
(H) Body mass (kg).
(I) fat-free mass (kg).
(J) fat mass (kg).
(K) adjusted TEE.
(L) adjusted BEE.
(M) adjusted AEE, and (N) adjusted PAL.
(O) repeated measures of adjusted TEE. Lines join repeat measures of the same individual in different months. Unadjusted and adjusted TEE, BEE, and AEE are in MJ/day, and each data point is a different individual.
Once we adjusted for the differences in fat-free, fat mass and age there was no seasonal cycle apparent in the adjusted TEE, AEE and PAL data (Figure 4K, r2 (TEE) month = 0.55%, F (TEE) month = 1.62, P (TEE) month = 0.09; Figure 4M, r2 (AEE) month = 1.52%, F (AEE) month = 0.82, P (AEE) month = 0.62; Figure 4N, r2 (PAL) month = 1.06%, F (PAL) month = 0.57, P (PAL) month = 0.85). However, there was a marginally significant seasonal effect on adjusted BEE (Figure 4L, r2 (BEE) month = 3.8%, F (BEE) month = 2.11, P (BEE) month = 0.02). We also had repeated measures data for 109 adults that were measured twice in different months. We found there was a between individual effect and also month/seasonal effect on adjusted TEE (Figure 4O, F month = 1.98, P month = 0.04; F ID = 2.91, P ID < 0.001). These repeated measurements were mostly older (postmenopausal women) from geographically diverse regions of the US.
Discussion
The absence of a relationship between TEE and ambient temperature is probably because of two behavioral responses that humans display in response to changes in ambient temperature. The first is to heat or cool homes to a relatively narrow range of 18–25°C (Figure 3B). This buffers inhabitants from the temperature extremes outside, which in the present study varied from −10 to 30°C (Figure 3A). Interestingly despite the numerous claims that humans always live inside the thermoneutral zone (Fischer et al., 2018, 2019), these indoor temperatures are almost all lower than the laboratory observed lower critical temperature (lower bound of the thermoneutral zone) for lightly clothed humans of around 23°C (Brychta et al., 2019). This is probably because the lower critical point is by definition the lowest temperature at which the demands for thermoregulation are matched by the heat produced from basal metabolism (IUPS Thermal commission, 2003; Scholander et al., 1950). However, we spend very little of our time metabolizing at basal levels of energy expenditure (BEE). Most of the time, we expend energy at levels higher than BEE. On average, using the data from the present study, TEE was about 70% higher than BEE. If we lived perpetually inside the thermoneutral zone, we would have to continually dissipate this extra heat above BEE by either evaporation or becoming mildly hyperthermic to increase the driving gradient for heat loss. Probably largely to avoid these options, we habitually occupy an environment cooler than the lower critical temperature (Figure 3B). Our routine heat production can then be dissipated along the gradient from core temperature to environmental temperature without the need to elevate evaporative water loss or body temperature. Nevertheless, there was a shallow relationship between indoor temperature and latitude, which could be because there is some carryover between outdoor and indoor clothing. This would mean that the lower critical temperature was not fixed at 23°C (reflecting light clothing). Observations of what people wear indoors and outdoors at different latitudes and the thermal insulation of such clothing would be necessary to test this idea.
This observation has implications for the debate about the best temperature at which to house mice to provide the best translation to studies of humans ((Fischer et al., 2018, 2019; Ganeshan and Chawla, 2017; Gordon, 2017; Keijer et al., 2019a, b; Li and Speakman, 2022; Maloney et al., 2014; Speakman and Keijer, 2014). Although it is generally agreed that keeping mice at 20–22°C is too cold, the present data suggest that the widely promoted alternative of keeping them at 30°C because a) that is mouse thermoneutral, and b) humans always live at thermoneutral, is based on an error in the second statement. Moreover, the first statement is also incorrect for many mouse strains (Speakman and Keijer, 2014). Responses of mice at 30°C do indeed often differ from those reported at 20–22°C (Feldmann et al., 2009; Giles et al., 2016, 2017; Kokolus et al., 2013; Rudaya et al., 2005). However, whether these mice give us any better translational efficiency to humans is questionable since humans do not routinely live in equivalent thermal conditions.
The absence of an effect of temperature on TEE in the present data is probably, therefore, primarily driven by the fact that humans in the modern United States are largely buffered from external temperatures. Whether this pattern is also evident in populations that do not have access to air-conditioning and heating is interesting, but at the moment, we do not have a large enough sample of TEE data from such communities to test the idea. Studies of forager-horticulturalists (the Tsimane) suggest no effect of ambient temperature on RMR in a fully adjusted model (Gurven et al., 2016). However, the range of temperatures experienced by this population is relatively low (the average varies from 27°C in May to 32°C in September). The Yakut living in NE Siberia experience a much lower temperature range but use stoves to keep warm and have a difference in BMR between summer and winter of about 6% (Leonard et al., 2014). Studies of subjects living in Basel (Senn et al., 2018) showed that BMR was unrelated to the average outdoor temperature, which varied between 5°C and 30°C).
However, even humans living in the USA do not live their entire lives indoors. The second reason why TEE does not increase when it is colder is that humans have a unique capability to modulate their external insulation in response to ambient temperature conditions by adding and taking off outer clothing. Animals can also anticipate seasonal changes in ambient temperature and respond by molting into pelage that provides greater or less external insulation (Lindström et al., 1993; McNab, 2002; Schieltz and Murphy, 1997; Scholander et al., 1950; Speakman et al., 2021a). However, the speed and flexibility of this response are considerably slower than for humans, who can vary their clothing extremely rapidly in response to changing external demands. Hence, even when humans go outdoors in the cold, they likely do not significantly elevate their expenditure on thermoregulation. A final possibility explaining the absence of an impact of ambient temperature on TEE would be that significant demands of thermoregulation are somehow compensated in the energy budget. This could happen, for example, if lower levels of physical activity offset greater expenditure on thermoregulation. Since these both affect AEE, our data does not allow us to separate these component costs. The alternative that greater levels of thermoregulation at lower temperatures lead to suppressed basal metabolism (Careau et al., 2021) can be discounted as BEE was also independent of ambient temperature.
The effective absence of an impact of average ambient temperature between −10 and +30°C was unexpected in the light of previous observations that brown adipose tissue appears to be seasonally activated (Au-Yong et al., 2009; Johnson et al., 2011; Ouellet et al., 2011; Yoneshiro et al., 2016), suggesting some physiological acclimatization to lowered temperature and elevated thermoregulatory requirements. This discrepancy suggests that elevations of BAT activity may not translate to changes in TEE. There are several potential explanations for this disconnect. The effect of changes in BAT on TEE may be too small to be significant because the periods of exposure to cold sufficient to stimulate BAT are too short for that expenditure to be detectable within the 2–3 weeks study period of a DLW measurement. Alternatively, activation of BAT may only be observed in a subset of the population (Au-Yong et al., 2009; Ouellet et al., 2011; Yoneshiro et al., 2016), and the effects in these responders are swamped by the majority who do not respond.
The absence of an association between ambient temperature and TEE is consistent with the lack of a relationship between ambient temperature and obesity prevalence for the same population (Speakman and Heidari-Bakavoli, 2016). The absence of this relationship (also in the present individual-level data) could be because lower temperatures stimulate TEE, but this is offset by increases in food intake, or because the lower temperatures do not stimulate TEE because of the buffering effects discussed above. The current data support this latter interpretation. This is an important distinction because if elevated TEE due to thermoregulatory demands were offset by elevated food intake, then the strategy to increase TEE by, for example, switching on brown adipose tissue, or forcing the conversion of beige adipocytes from white to brown phenotypes, would be compromised. The absence of such a relationship leaves unexplained the strong relationship between ambient temperature and the prevalence of type 2 diabetes (Speakman and Heidari-Bakavoli, 2016). Potentially this is because stimulation of BAT in the cold is sufficient to affect glucose homeostasis but insufficient to affect energy budgets. Understanding the links of brown adipose tissue activity to free-living TEE is lacking and should be a key future goal.
In conclusion, the absence of a relationship between ambient temperature and human total energy expenditure suggests humans completely buffer themselves from the environmental impact of temperature probably by a combination of changing the thermal environment of their homes and office spaces and modulating external insulation (clothing) during periods spent outside these spaces.
Limitations of the study
Although we were able to connect 3213 measurements of TEE with external ambient temperature records, our study has several clear limitations. First, the temperature exposure was inferred from the general area where the people lived. We did not have actual temperature exposures for given individuals, which could in theory be measured by individuals carrying around temperature sensing devices while their expenditure was being measured. Moreover, we had no data on the clothing the individuals wore, both indoor and outdoor, which might indicate the insulation effects they were experiencing. Considerable individual variation in TEE (and AEE and BEE) at each temperature might reflect different actual exposures and clothing differences that we could not quantify. Second, we did not have individual measures of BAT activity regarding either the individual temperature exposure or the energy expenditure parameters. Connecting these observations together will provide much more insight into the links of BAT activity to total expenditure and its potential as a therapy for weight control. Third, while we did not observe substantial sex differences, the sample for females was much larger than for males, and hence the reason we were able to detect some minor effects in females but not males could have been a power issue. Finally, it would be interesting to contrast these data with measurements of energy demands of people living in environments where access to air-conditioning and heating is limited.
Consortia
The IAEA database consortium: This group authorship contains the names of people whose data were contributed into the database by the analysis laboratory but they later could not be traced, or they did not respond to emails to assent inclusion among the authorship. The list also includes some researchers who did not assent inclusion because they felt their contribution was not sufficient to merit authorship Lene F. Andersen, Liam J. Anderson, Lenore Arab, Issad Baddou, Bedu—Addo, Stephane Blanc, Alberto Bonomi, Carlijn V.C. Bouten, Pascal Bovet, Stefan Branth, Niels C. De Bruin, Nancy F Butte, Lisa H. Colbert, Stephan G. Camps, Alice E. Dutman, Simon D. Eaton, Ulf Ekelund, Sonja Entringer, Cara Ebbeling, Sölve Elmståhl, Mikael Fogelholm, Terrence Forrester, Barry W. Fudge, Tamara Harris, Rik Heijligenberg, Annelies H Goris, Catherine Hambly, Marije B. Hoos, Hans U. Jorgensen, Annemiek M. Joosen, Kitty P. Kempen, Misaka Kimura, Watanee Kriengsinyos, Estelle V. Lambert, Christel L. Larsson, Nader Lessan, David S. Ludwig, Margaret McCloskey, Anine C. Medin, Gerwin A. Meijer, Eric Matsiko, Alida Melse-Boonstra, James C. Morehen, James P. Morton, Theresa A. Nicklas, Daphne L. Pannemans, Kirsi H. Pietiläinen, Renaat M. Philippaerts, Roberto A. Rabinovich, John J. Reilly, Elisabet M. Rothenberg, Albertine J. Schuit, Sabine Schulz, Anders M. Sjödin, Amy Subar, Minna Tanskanen, Ricardo Uauy, Giulio Valenti, Ludo M. Van Etten, Rita Van den Berg-Emons, Wim G. Van Gemert, Erica J. Velthuis-te Wierik, Wilhelmine W. Verboeket-van de Venne, Jeanine A. Verbunt, Jonathan C.K. Wells, George Wilson.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Raw and analyzed data | This paper | https://doubly-labelled-water-database.iaea.org/home and www.dlwdatabase.org |
| Software and algorithms | ||
| Minitab | This paper | V19. |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, John Roger Speakman.
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
This version of the database comprises 7,340 measurements of TEE using the DLW method. We selected from the database measurements of adults aged >18y, living in the USA, that also had a record of age, sex and date of measurement. In total, this resulted in 3254 measurements across both sexes. Estimates of TEE were recalculated using a standard equation shown to perform best in validation studies. These were then converted into energy expenditure using the Weir equation (Weir, 1949) with food quotients derived from the original studies. This included 815 measurements of males and 2439 measurements of females. The individual studies that contributed to the database were all individually proved by multiple review boards.
Method details
The DLW method is based on the differential elimination of isotopes of oxygen and hydrogen introduced into the body water2. The details of the practical implementation of the method and its theoretical basis have been previously published (Speakman, 1997).
For 185 of the males and 414 of the females, we also had measurements of basal metabolic rate (BMR). BMR measurements were derived either from hood calorimetry or minimal metabolic rate determined overnight during chamber calorimetry (strictly sleeping metabolic rates or SMR). We converted these BMR or SMR to estimates of basal energy expenditure (BEE). BMR and SMR are measured for relatively short periods lasting 30 minutes to an hour. BEE is a theoretical value for the energy expenditure that would pertain if this BMR/SMR measurement was sustained for 24h. We estimated several other traits for those individuals where we could estimate BEE. These were the activity energy expenditure (AEE), defined as AEE = (0.9∗TEE) - BEE, and the physical activity level or PAL, defined as PAL = TEE/BEE.
Additional characteristics of the subjects (BM, age, and sex) were measured using standard protocols. We estimated the FFM of individuals using the estimated total body water and assumed hydration constant for lean tissue of 0.732 (Wang et al., 1999) and then calculated FM by difference (FM = BM - FFM). This approach assumes no temperature effects on the hydration coefficient. The data span almost 35 years, with the first measurements in late 1981 and the latest measurements made in late 2017.
We obtained daily averages of ambient temperature for each county of the USA available from the National Centers for Environmental Information (NCEI)’s FTP site (ftp://ftp.ncdc.noaa.gov/pub/data/daily-grids/). This dataset contains area averages of daily maximum, minimum, and average temperature (TMAX, TMIN, and TAVG) for the contiguous USA between January 1, 1951, and the present (Durre, 2018). Data in the database are available for gridded fields covering the land area between 24°N and 49°N and between 67°W and 125°W at a resolution of 0.041667°. This means the grid point spacing is 4.6 km between latitudes and varies from 3.0 km in the North to 4.2 km in the South between longitudes. These data are compiled into averages for nine different types of area which includes countries. For each DLW measurement we used the known geographical location to identify the county where the person was measured and then extracted the daily averages for the duration of the measurement which was also provided from the IAEA DLW database. We then generated an average maximum, minimum and mean temperature exposure for each individual measurement.
The indoor temperature data used in this study were selected from existing databases: ASHRAE Thermal Comfort Database I (De Dear, 1998), ASHRAE Global Thermal Comfort Database II (Ličina et al., 2018) NREL RITS database (Booten et al., 2017), NYSERDA database (NYSERDA, 2019) and Indoor temperature - office work performance database (Porras-Salazar et al., 2021). We filtered those data mainly considering three aspects: datasets include indoor temperature or not; buildings located in the USA or not; buildings have location information (latitude/region/county) or not. We filtered 12,732 data points from the original databases. These data included 74 cities from different counties in the USA, with latitudes ranging from 31 to 44.5. The annual total energy consumption per capita is based on the latest data from the EIA for 2018 (https://majorenergy.com/whats-the-average-home-energy-consumption-in-your-state/). Using the same dataset, these data was compared to average temperature and days with a maximum temperature of less than 18 degrees in each state in the same year using the same dataset (ftp://ftp.ncdc.noaa.gov/pub/data/daily-grids/).
Quantification and statistical analysis
All results were presented as mean ± se (standard error). n = 3254 and n represents the number of human subjects. We first calculated the seasonal variations of ambient temperature using One–way ANOVA. Then we calculated seasonal and latitude effects for all the traits (TEE, BEE, AEE, PAL, BM, FM and FFM) using one-way ANOVA and linear regression models. In the subsequent analyses, we used the unadjusted TEE measures as dependent variables in linear regression models with ambient temperature as the predictors. All analyses were performed using Minitab v19. It is well established that TEE depends on body composition and subject age. Patterns of variation in unadjusted values with ambient temperature might then reflect biased population sampling concerning these traits. We used general linear modeling to adjust (logged) TEE using log FFM, log FM, and age as the predictor variables. For log TEE, Log BEE and Log AEE the predictors Age, Age2, log FFM and log FM were all significant. Following the above procedure, we then calculated the residuals to the fitted models and added them back to the mean logged TEE, BEE and AEE across all measurements. These values were then converted back to ‘adjusted TEE, BEE and AEE’ measures by taking the exponent of the derived values. We then sought relationships between the adjusted variables and ambient temperature using linear regression. Significance was defined as p-value less than 0.05.
Acknowledgments
The DLW database, which can be found at https://www.dlwdatabase.org, is hosted by the International Atomic Energy Agency (IAEA) and generously supported by Taiyo Nippon Sanso and SERCON. We are grateful to the IAEA and these companies for their support. XYZ was supported by the Chinese Academy of Sciences (grant CAS 153E11KYSB20190045 to J.R.S.), and the database was also supported by the US National Science Foundation (grant BCS-1824466 to H.P.). The funders played no role in the content of this manuscript.
Author contributions
Analyzed the data and wrote the paper: XYZ, JRS. Commented on paper drafts and provided insights to analysis: YY, HS, MB, SKD, WEK, GP, SBR, SSU, WWW, HP, DAS, KRW. Compiled the database, JRS, AHL, HP, JR, DAS, HS, KRW, WWW, YY, AJM-A, CL, Contributed data to the database, PNA, EEB, MSB, GLC, JAC, SKD, LRD, MG, AEH, SMH, NJ, PK, WEK, RFK, WRL, CB, EPM, MLN, RMO, YPP, GP, RLP, SBR, ER, LMR, RMR, SBR, LBS, AMS, ES, SSU, EAvM, BMW, AJM-A, CL, AHL, HP, JR, HS, DAS, JRS, KRW, WWW, YY.
Declaration of interests
The authors declare no conflicts of interest.
Published: August 19, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.104682.
Contributor Information
Yosuke Yamada, Email: yyamada831@gmail.com.
Hiroyuki Sagayama, Email: sagayama.hiroyuki.ka@u.tsukuba.ac.jp.
Amy H. Luke, Email: aluke@luc.edu.
Jennifer Rood, Email: jennifer.rood@pbrc.edu.
Dale A. Schoeller, Email: dschoell@nutrisci.wisc.edu.
Klaas R. Westerterp, Email: k.westerterp@maastrichtuniversity.nl.
William W. Wong, Email: wwong@bcm.edu.
Herman Pontzer, Email: herman.pontzer@duke.edu.
John R. Speakman, Email: j.speakman@abdn.ac.uk.
the IAEA DLW database consortium:
Lene F. Andersen, Liam J. Anderson, Lenore Arab, Issad Baddou, Bedu Addo, Stephane Blanc, Alberto Bonomi, Carlijn V.C. Bouten, Pascal Bovet, Stefan Branth, Niels C. De Bruin, Nancy F. Butte, Lisa H. Colbert, Stephan G. Camps, Alice E. Dutman, Simon D. Eaton, Ulf Ekelund, Sonja Entringer, Cara Ebbeling, Sölve Elmståhl, Mikael Fogelholm, Terrence Forrester, Barry W. Fudge, Tamara Harris, Rik Heijligenberg, Annelies H. Goris, Catherine Hambly, Marije B. Hoos, Hans U. Jorgensen, Annemiek M. Joosen, Kitty P. Kempen, Misaka Kimura, Watanee Kriengsinyos, Estelle V. Lambert, Christel L. Larsson, Nader Lessan, David S. Ludwig, Margaret McCloskey, Anine C. Medin, Gerwin A. Meijer, Eric Matsiko, Alida Melse-Boonstra, James C. Morehen, James P. Morton, Theresa A. Nicklas, Daphne L. Pannemans, Kirsi H. Pietiläinen, Renaat M. Philippaerts, Roberto A. Rabinovich, John J. Reilly, Elisabet M. Rothenberg, Albertine J. Schuit, Sabine Schulz, Anders M. Sjödin, Amy Subar, Minna Tanskanen, Ricardo Uauy, Giulio Valenti, Ludo M. Van Etten, Rita Van den Berg-Emons, Wim G. Van Gemert, Erica J. Velthuis-te Wierik, Wilhelmine W. Verboeket-van de Venne, Jeanine A. Verbunt, Jonathan C.K. Wells, and George Wilson
Supplemental information
Data and code availability
Data
Data were extracted from the IAEA Doubly Labelled Water (DLW) Database (Speakman et al., 2019), version 3.4, compiled in April 2021. Data reported in this paper will be shared by the lead contact upon request.
Code
This paper does not report any original code.
Additional information
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Au-Yong I.T., Thorn N., Ganatra R., Perkins A.C., Symonds M.E. Brown adipose tissue and seasonal variation in humans. Diabetes. 2009;58:2583–2587. doi: 10.2337/db09-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu G.R., Murthy G.V.S., Ana Y., Patel P., Deepa R., Neelon S.E.B., Kinra S., Reddy K.S. Association of obesity with hypertension and type 2 diabetes mellitus in India: a meta-analysis of observational studies. World J. Diabetes. 2018;9:40–52. doi: 10.4239/wjd.v9.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz M.J., Enerbäck S. Human brown adipose tissue: what we have learned so far. Diabetes. 2015;64:2352–2360. doi: 10.2337/db15-0146. [DOI] [PubMed] [Google Scholar]
- Blüher M. Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019;15:288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- Booten C., Robertson J., Christensen D., Heaney M., Brown D., Norton P., Smith C. National Renewable Energy Lab (NREL); Golden, CO (United States): 2017. Residential Indoor Temperature Study. [Google Scholar]
- Brychta R.J., Huang S., Wang J., Leitner B.P., Hattenbach J.D., Bell S.L., Fletcher L.A., Perron Wood R., Idelson C.R., Duckworth C.J., et al. Quantification of the capacity for cold-induced thermogenesis in young men with and without obesity. J. Clin. Endocrinol. Metab. 2019;104:4865–4878. doi: 10.1210/jc.2019-00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Careau V., Halsey L.G., Pontzer H., Ainslie P.N., Andersen L.F., Anderson L.J., Arab L., Baddou I., Bedu-Addo K., Blaak E.E., et al. Energy compensation and adiposity in humans. Curr. Biol. 2021;31:4659–4666.e2. doi: 10.1016/j.cub.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celi F.S., Brychta R.J., Linderman J.D., Butler P.W., Alberobello A.T., Smith S., Courville A.B., Lai E.W., Costello R., Skarulis M.C., et al. Minimal changes in environmental temperature result in a significant increase in energy expenditure and changes in the hormonal homeostasis in healthy adults. Eur. J. Endocrinol. 2010;163:863–872. doi: 10.1530/EJE-10-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.Y., Brychta R.J., Linderman J.D., Smith S., Courville A., Dieckmann W., Herscovitch P., Millo C.M., Remaley A., Lee P., et al. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. J. Clin. Endocrinol. Metab. 2013;98:E1218–E1223. doi: 10.1210/jc.2012-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B., Kuo F.C., Palmer E.L., Tseng Y.-H., Doria A., et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess A.M. Reassessing human adipose tissue. N. Engl. J. Med. 2022;386:768–779. doi: 10.1056/NEJMra2032804. [DOI] [PubMed] [Google Scholar]
- De Dear R.J. A global database of thermal comfort field experiments. ASHRAE Trans. 1998;104:1141. [Google Scholar]
- Durre I. Public Beta Release; 2018. Daily Grids and Area Averages of Temperature and Precipitation for the Contiguous united states, 1951-present (nClimGrid-d and nClimDiv-d) [Google Scholar]
- Dhurandhar E.J., Keith S.W. The aetiology of obesity beyond eating more and exercising less. Best Pract. Res. Clin. Gastroenterol. 2014;28:533–544. doi: 10.1016/j.bpg.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Feldmann H.M., Golozoubova V., Cannon B., Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Fischer A.W., Cannon B., Nedergaard J. Optimal housing temperatures for mice to mimic the thermal environment of humans: an experimental study. Mol. Metab. 2018;7:161–170. doi: 10.1016/j.molmet.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A.W., Cannon B., Nedergaard J. The answer to the question “What is the best housing temperature to translate mouse experiments to humans?” is: thermoneutrality. Mol. Metab. 2019;26:1–3. doi: 10.1016/j.molmet.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D.O., Frydman M.L. Tissue distribution of cold-induced thermogenesis in conscious warm-or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can. J. Physiol. Pharmacol. 1979;57:257–270. doi: 10.1139/y79-039. [DOI] [PubMed] [Google Scholar]
- Ganeshan K., Chawla A. Warming the mouse to model human diseases. Nat. Rev. Endocrinol. 2017;13:458–465. doi: 10.1038/nrendo.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles D.A., Moreno-Fernandez M.E., Stankiewicz T.E., Graspeuntner S., Cappelletti M., Wu D., Mukherjee R., Chan C.C., Lawson M.J., Klarquist J., et al. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat. Med. 2017;23:829–838. doi: 10.1038/nm.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles D.A., Ramkhelawon B., Donelan E.M., Stankiewicz T.E., Hutchison S.B., Mukherjee R., Cappelletti M., Karns R., Karp C.L., Moore K.J., et al. Modulation of ambient temperature promotes inflammation and initiates atherosclerosis in wild type C57BL/6 mice. Mol. Metab. 2016;5:1121–1130. doi: 10.1016/j.molmet.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C.J. The mouse thermoregulatory system: its impact on translating biomedical data to humans. Physiol. Behav. 2017;179:55–66. doi: 10.1016/j.physbeh.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg E.W., Shaw J.E. Global health effects of overweight and obesity. N. Engl. J. Med. 2017;377:80–81. doi: 10.1056/NEJMe1706095. [DOI] [PubMed] [Google Scholar]
- Gurven M.D., Trumble B.C., Stieglitz J., Yetish G., Cummings D., Blackwell A.D., Beheim B., Kaplan H.S., Pontzer H. High resting metabolic rate among Amazonian forager-horticulturalists experiencing high pathogen burden. Am. J. Phys. Anthropol. 2016;161:414–425. doi: 10.1002/ajpa.23040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall K.D., Farooqi I.S., Friedman J.M., Klein S., Loos R.J.F., Mangelsdorf D.J., O'Rahilly S., Ravussin E., Redman L.M., Ryan D.H., et al. The energy balance model of obesity: beyond calories in, calories out. Am. J. Clin. Nutr. 2022;115:1243–1254. doi: 10.1093/ajcn/nqac031. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall K.D., Heymsfield S.B., Kemnitz J.W., Klein S., Schoeller D.A., Speakman J.R. Energy balance and its components: implications for body weight regulation. Am. J. Clin. Nutr. 2012;95:989–994. doi: 10.3945/ajcn.112.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi J., Seale P. Beige can be slimming. Science. 2010;328:1113–1114. doi: 10.1126/science.1190816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUPS Thermal Commission Glossary of terms for thermal physiology. J. Therm. Biol. 2003;28:75–106. [Google Scholar]
- Johnson F., Mavrogianni A., Ucci M., Vidal-Puig A., Wardle J. Could increased time spent in a thermal comfort zone contribute to population increases in obesity? Obes. Rev. 2011;12:543–551. doi: 10.1111/j.1467-789X.2010.00851.x. [DOI] [PubMed] [Google Scholar]
- Keijer J., Li M., Speakman J.R. To best mimic human thermal conditions, mice should be housed slightly below thermoneutrality. Mol. Metab. 2019;26:4. doi: 10.1016/j.molmet.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijer J., Li M., Speakman J.R. What is the best housing temperature to translate mouse experiments to humans? Mol. Metab. 2019;25:168–176. doi: 10.1016/j.molmet.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith S.W., Redden D.T., Katzmarzyk P.T., Boggiano M.M., Hanlon E.C., Benca R.M., Ruden D., Pietrobelli A., Barger J.L., Fontaine K.R., et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int. J. Obes. 2006;30:1585–1594. doi: 10.1038/sj.ijo.0803326. [DOI] [PubMed] [Google Scholar]
- Kokolus K.M., Capitano M.L., Lee C.-T., Eng J.W.-L., Waight J.D., Hylander B.L., Sexton S., Hong C.-C., Gordon C.J., Abrams S.I. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc. Natl. Acad. Sci. USA. 2013;110:20176–20181. doi: 10.1073/pnas.1304291110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W., Levy S., Tarskaia L., Klimova T., Fedorova V., Baltakhinova M., Krivoshapkin V., Snodgrass J.J. Seasonal variation in basal metabolic rates among the Yakut (Sakha) of Northeastern Siberia. Am. J. Hum. Biol. 2014;26:437–445. doi: 10.1002/ajhb.22524. [DOI] [PubMed] [Google Scholar]
- Li M., Speakman J.R. Setting ambient temperature conditions to optimize translation of molecular work from the mouse to human: the “goldilocks solution”. Methods Mol. Biol. 2022;2448:235–250. doi: 10.1007/978-1-0716-2087-8_15. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang D., Ping X., Zhang Y., Zhang T., Wang L., Jin L., Zhao W., Guo M., Shen F., et al. Local hyperthermia therapy induces browning of white fat and treats obesity. Cell. 2022;185:949–966.e19. doi: 10.1016/j.cell.2022.02.004. [DOI] [PubMed] [Google Scholar]
- Ličina V.F., Cheung T., Zhanga H., Dear R., Parkinson T., Arens E., Chun C., Schiavon S., Luo M., Brager G., et al. Development of the ASHRAE global thermal comfort database II. Build. Environ. 2018;142:502–512. [Google Scholar]
- Lindström Å., Visser G.H., Daan S. The energetic cost of feather synthesis is proportional to basal metabolic rate. Physiol. Zool. 1993;66:490–510. doi: 10.1086/physzool.66.4.30163805. [DOI] [Google Scholar]
- Liu X., Wang S., You Y., Meng M., Zheng Z., Dong M., Lin J., Zhao Q., Zhang C., Yuan X., et al. Brown adipose tissue transplantation reverses obesity in Ob/Ob mice. Endocrinology. 2015;156:2461–2469. doi: 10.1210/en.2014-1598. [DOI] [PubMed] [Google Scholar]
- Liu X., Zheng Z., Zhu X., Meng M., Li L., Shen Y., Chi Q., Wang D., Zhang Z., Li C., et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 2013;23:851–854. doi: 10.1038/cr.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney S.K., Fuller A., Mitchell D., Gordon C., Overton J.M. Translating animal model research: does it matter that our rodents are cold? Physiology. 2014;29:413–420. doi: 10.1152/physiol.00029.2014. [DOI] [PubMed] [Google Scholar]
- McAllister E.J., Dhurandhar N.V., Keith S.W., Aronne L.J., Barger J., Baskin M., Benca R.M., Biggio J., Boggiano M.M., Eisenmann J.C., et al. Ten putative contributors to the obesity epidemic. Crit. Rev. Food Sci. Nutr. 2009;49:868–913. doi: 10.1080/10408390903372599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab B.K. Cornell University Press; 2002. The Physiological Ecology of Vertebrates: A View from Energetics. [Google Scholar]
- Nedergaard J., Bengtsson T., Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- Nedergaard J., Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- NYSERDA New York State. New York State Residential Building Stock Assessment. https://data.ny.gov/d/3drn-bhzv
- Ouellet V., Routhier-Labadie A., Bellemare W., Lakhal-Chaieb L., Turcotte E., Carpentier A.C., Richard D. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J. Clin. Endocrinol. Metab. 2011;96:192–199. doi: 10.1210/jc.2010-0989. [DOI] [PubMed] [Google Scholar]
- Pfannenberg C., Werner M.K., Ripkens S., Stef I., Deckert A., Schmadl M., Reimold M., Häring H.U., Claussen C.D., Stefan N. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59:1789–1793. doi: 10.2337/db10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi-Sunyer X. Changes in body composition and metabolic disease risk. Eur. J. Clin. Nutr. 2019;73:231–235. doi: 10.1038/s41430-018-0320-x. [DOI] [PubMed] [Google Scholar]
- Pontzer H., Yamada Y., Sagayama H., Ainslie P.N., Andersen L.F., Anderson L.J., Arab L., Baddou I., Bedu-Addo K., Blaak E.E., et al. Daily energy expenditure through the human life course. Science. 2021;373:808–812. doi: 10.1126/science.abe5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras-Salazar J.A., Schiavon S., Wargocki P., Cheung T., Tham K.W. Dryad, Dataset; 2021. Indoor Temperature - Office Work Performance Database. [DOI] [Google Scholar]
- Rosenwald M., Perdikari A., Rülicke T., Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- Rudaya A.Y., Steiner A.A., Robbins J.R., Dragic A.S., Romanovsky A.A. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R1244–R1252. doi: 10.1152/ajpregu.00370.2005. [DOI] [PubMed] [Google Scholar]
- Saito M., Okamatsu-Ogura Y., Matsushita M., Watanabe K., Yoneshiro T., Nio-Kobayashi J., Iwanaga T., Miyagawa M., Kameya T., Nakada K., et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schautz B., Later W., Heller M., Peters A., Müller M.J., Bosy-Westphal A. Impact of age on leptin and adiponectin independent of adiposity. Br. J. Nutr. 2012;108:363–370. doi: 10.1017/S0007114511005605. [DOI] [PubMed] [Google Scholar]
- Schieltz P.C., Murphy M.E. The contribution of insulation changes to the energy cost of avian molt. Can. J. Zool. 1997;75:396–400. doi: 10.1139/z97-049. [DOI] [Google Scholar]
- Scholander P.F., Hock R., Walters V., Irving L. Adaptation to cold in arctic and tropical mammals and birds in relation to body temperature, insulation, and basal metabolic rate. Biol. Bull. 1950;99:259–271. doi: 10.2307/1538742. [DOI] [PubMed] [Google Scholar]
- Senn J.R., Maushart C.I., Gashi G., Michel R., Lalive d’Epinay M., Vogt R., Becker A.S., Müller J., Baláz M., Wolfrum C., et al. Outdoor temperature influences cold induced thermogenesis in humans. Front. Physiol. 2018;9:1184. doi: 10.3389/fphys.2018.01184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J.R. Springer Science & Business Media; 1997. Doubly Labelled Water: Theory and Practice. [Google Scholar]
- Speakman J.R. Obesity and thermoregulation. Handb. Clin. Neurol. 2018;156:431–443. doi: 10.1016/b978-0-444-63912-7.00026-6. [DOI] [PubMed] [Google Scholar]
- Speakman J.R., Chi Q., Ołdakowski Ł., Fu H., Fletcher Q.E., Hambly C., Togo J., Liu X., Piertney S.B., Wang X., et al. Surviving winter on the Qinghai-Tibetan Plateau: pikas suppress energy demands and exploit yak feces to survive winter. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2100707118. e2100707118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J.R., Hall K.D. Carbohydrates, insulin, and obesity. Science. 2021;372:577–578. doi: 10.1126/science.aav0448. [DOI] [PubMed] [Google Scholar]
- Speakman J.R., Heidari-Bakavoli S. Type 2 diabetes, but not obesity, prevalence is positively associated with ambient temperature. Sci. Rep. 2016;6:30409. doi: 10.1038/srep30409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J.R., Keijer J. Not so nuanced: Reply to the comments of Gaskill and Garner on ‘Not so hot: Optimal housing temperatures for mice to mimic the environment of humans. Mol. Metab. 2014;3:337. doi: 10.1016/j.molmet.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J., Pontzer H., Rood J., Sagayama H., Schoeller D., Westerterp K., Wong W., Yamada Y., Loechl C., Murphy-Alford A. The international atomic energy agency international doubly labelled water database: aims, scope and procedures. Ann. Nutr. Metab. 2019;75:114–118. doi: 10.1159/000503668. [DOI] [PubMed] [Google Scholar]
- Speakman J.R., Yamada Y., Sagayama H., Berman E.S., Ainslie P.N., Andersen L.F., Anderson L.J., Arab L., Baddou I., Bedu-Addo K., et al. A standard calculation methodology for human doubly labeled water studies. Cell Rep. Med. 2021;2:100203. doi: 10.1016/j.xcrm.2021.100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford K.I., Goodyear L.J. The therapeutic potential of brown adipose tissue. Hepatobiliary Surg. Nutr. 2013;2:286–287. doi: 10.3978/j.issn.2304-3881.2013.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M., Kemerink G.J., Bouvy N.D., Schrauwen P., Teule G.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Wang H., Liu L., Lin J.Z., Aprahamian T.R., Farmer S.R. Browning of white adipose tissue with roscovitine induces a distinct population of UCP1+ adipocytes. Cell Metab. 2016;24:835–847. doi: 10.1016/j.cmet.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhang M., Xu M., Gu W., Xi Y., Qi L., Li B., Wang W. Brown adipose tissue activation is inversely related to central obesity and metabolic parameters in adult human. PLoS One. 2015;10:e0123795. doi: 10.1371/journal.pone.0123795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Deurenberg P., Wang W., Pietrobelli A., Baumgartner R.N., Heymsfield S.B. Hydration of fat-free body mass: review and critique of a classic body-composition constant. Am. J. Clin. Nutr. 1999;69:833–841. doi: 10.1093/ajcn/69.5.833. [DOI] [PubMed] [Google Scholar]
- Weir J.B.d.V. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Boström P., Sparks L., Ye L., Choi J., Giang A.-H., Khandekar M., Virtanen K., Nuutila P., Schaart G., et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshiro T., Matsushita M., Nakae S., Kameya T., Sugie H., Tanaka S., Saito M. Brown adipose tissue is involved in the seasonal variation of cold-induced thermogenesis in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;310:R999–R1009. doi: 10.1152/ajpregu.00057.2015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data
Data were extracted from the IAEA Doubly Labelled Water (DLW) Database (Speakman et al., 2019), version 3.4, compiled in April 2021. Data reported in this paper will be shared by the lead contact upon request.
Code
This paper does not report any original code.
Additional information
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.