Abstract
Background:
Physical activity can cause glucose fluctuations both during and after it is performed, leading to hurdles in optimal insulin dosing in people with type 1 diabetes (T1D). We conducted a pilot clinical trial assessing the safety and feasibility of a physical activity-informed mealtime insulin bolus advisor that adjusts the meal bolus according to previous physical activity, based on step count data collected through an off-the-shelf physical activity tracker.
Methods:
Fifteen adults with T1D, each using a continuous glucose monitor (CGM) and an insulin pump with carbohydrate counting, completed two randomized crossover daily visits. Participants performed a 30 to 45-minute brisk walk before lunch and lunchtime insulin boluses were calculated based on either their standard therapy (ST) or the physical activity-informed bolus method. Post-lunch glycemic excursions were assessed using CGM readings.
Results:
There was no significant difference between visits in the time spent in hypoglycemia in the post-lunch period (median [IQR] standard: 0 [0]% vs physical activity-informed: 0 [0]%, P = NS). Standard therapy bolus yielded a higher time spent in 70 to 180 mg/dL target range (mean ± standard: 77% ± 27% vs physical activity-informed: 59% ± 31%, P = .03) yet, it was associated with a steeper negative slope in the early postprandial phase (P = .032).
Conclusions:
Use of step count to adjust mealtime insulin following a walking bout has proved to be safe and feasible in a cohort of 15 T1D subjects. Physical activity-informed insulin dosing of meals eaten soon after a walking bout has a potential of mitigating physical activity related glucose reduction in the early postprandial phase.
Keywords: exercise, physical activity, treatment advisory systems, type 1 diabetes, walking, wearables
Introduction
Physical activity is recommended for all individuals and proven to decrease the risk of cardiovascular disease, cancer, hypertension, obesity, depression, and osteoporosis. 1 In addition to its general health benefits, physical activity is also associated with increased longevity 2 and with decreased risk of diabetes-related complications, such as retinopathy and neuropathy.3,4 Given the role of physical activity in improving health and preventing the aforementioned comorbidities, it is alarming that still more than 60% of the population with T1D remains sedentary. 5
Despite well-documented guidelines for physical activity related blood glucose (BG) management6,7 and advancements in technologies facilitating glycemic control, physical activity remains one of the main impediments to optimizing glycemic control in T1D.8,9 The current guidelines provide strategies based on the intensity and duration of the physical activity as well as the metabolic state (eg, glucose and insulin levels) of the patient at the time of a structured physical activity bout.6,7 The performance of these strategies is limited to the accuracy of the evaluation of these factors by the patient. The rising availability of off-the-shelf physical activity trackers offers a more objective alternative to self-evaluation. Recent studies show that the use of physical activity signals from these trackers are effective in detecting physical activity and improving glycemic control in closed-loop control systems.10-13
Beyond structured physical activity, even short episodes of walking accumulated throughout a day have a significant impact on glycemic control.14-18 Therefore, glycemic control in people with T1D may improve when all daily physical activity is considered in the design and adjustment of insulin therapy.
In this study, we adjust mealtime insulin treatment for physical activities beyond the routine of individuals. The motivation of our method is to facilitate physical activity related glucose management in a patient-specific manner and based on objectively measured physical activity. Here, we present results from a pilot study designed to assess the safety and feasibility of this method—using step count as a ubiquitously available physical activity indicator—to adjust mealtime insulin and thereby improve protection against hypoglycemia, following a walking physical activity bout. To do so, we compare the post-meal glycemic control performance of our method to the standard therapy in a randomized crossover clinical trial.
Study Design and Methods
Participants
Fifteen insulin pump users with T1D participated in a randomized, crossover study. The inclusion criteria were duration of diabetes of at least one year, using an insulin pump for at least six months, using defined parameters for calculating meal, and correction insulin boluses, and willingness to maintain a consistent physical activity regimen during the data collection period. Exclusion criteria were pregnancy, diabetic ketoacidosis or severe hypoglycemia in the 6 months prior to enrollment, use of non-insulin medications intended to lower glucose (eg, GLP-1 agonists, metformin), current use of a clearly defined method for insulin bolus adjustment to compensate for significant physical activity, inability to be physically active for more than 30 minutes per day, or current enrollment in another intervention clinical trial. The Institutional Review Board at the University of Virginia approved the study protocol and consent form. Informed consent was obtained from every participant.
Protocol
The study consisted of a free-living data collection period followed by two outpatient day-long supervised visits conducted at the University of Virginia Center for Diabetes Technology, following a randomized crossover design (clinicaltrials.gov: NCT03394352). During data collection period, records of CGM, insulin, meal, and physical activity were collected for more than 20 days under participants’ free-living conditions. CGM data were obtained via Dexcom G6 (Dexcom, San Diego, CA) and physical activity data were collected via Fitbit Charge 2 (Fitbit, San Francisco, CA) wristband. Subjects were instructed to enter any consumed carbohydrates into the bolus calculator of their pumps or the CGM mobile application.
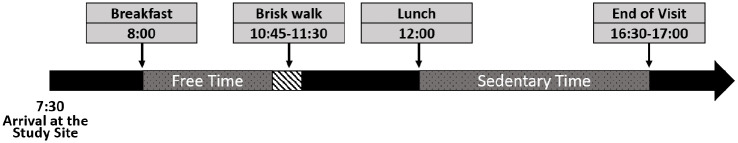
The 2 outpatient supervised visits had the same timeline (see Figure 1) and were separated by at least 2 days to allow wash-out. These visits started at 7:30 am upon the participants’ arrival at the study unit. After arrival, each participant’s basal insulin rate was set to their regular basal insulin profiles, and no temporary basal rates were used during the visits. Around 8 AM, a mixed-meal with 24 g of carbohydrates was provided for breakfast. In both visits, breakfast boluses were calculated according to the participants’ usual treatment parameters and with a target glucose of 110 mg/dl. Until 10:45 AM, participants were given free time with no constraints on their activities during their first visit. In their second visit, they were asked to repeat physical activity behavior similar to their previous visit. At 10:45 AM, the physical activity session started. Participants walked briskly at a steady pace on a track for an assigned time of either 30 or 45 minutes. This duration was determined for each participant in a manner to exceed their habitual accumulated physical activity by lunchtime. At noon, lunch was provided with a carbohydrate content matching each participant’s routine lunch. Participants’ habitual accumulated physical activity by lunchtime and routine lunch carbohydrate contents were derived from their data collected during the free-living data collection phase. Each participant had the same breakfast and lunch in both visits to control for the meal effect. Participants selected their own meal without any limitation on the composition except for the amount of the carbohydrates.
Figure 1.
Timeline of outpatient study visits.
In the control visit, insulin boluses for lunch were calculated according to the standard therapy (ST). In the experimental visit, these boluses were adjusted according to the physical activity informed bolus method. The adjustment was limited to 50% of the standard meal bolus. This was an empirically chosen safety layer for this pilot study. Following lunch, participants were asked to limit their physical activity to the minimum possible until the end of both study visits in order to observe the post-prandial glycemic control performance with minimal distortion. Hypoglycemia events were treated with carbohydrate administration at an amount determined by the study physician and were recorded at all times. Both study visits ended after the post-lunch glycemic excursions were completed, approximately 4.5 to 5 hours after lunch. At the end of each study visit, participants’ CGM, insulin pump, and physical activity tracker data were downloaded for the visit and were used in the analyses provided in this manuscript.
Algorithm Description
The standard therapy bolus for pre-meal insulin dose calculation in T1D has three components: the amount required to compensate for the carbohydrates ingested in the meal, the amount required to correct for any current elevated glucose level at the time of the meal, and the insulin on board (IOB) that is the active insulin from previous injections. 19 The resulting bolus calculation formula is as follows:
where is the amount of ingested carbohydrates in the meal, is the carbohydrate-to-insulin ratio (g/U), is the target BG value, is the BG correction factor (mg/dL/U), is the self-monitored BG value or CGM reading at the time of the meal, and is the insulin on board from previous basal and correction insulin injections. The IOB component is an important element to account for the lasting impact on BG of previously administered insulin. 20 A similarity between the effect of physical activity and insulin is rooted in the fact that the glycemic impact of physical activity is also prolonged. 21 Therefore, we propose a physical activity informed insulin bolus method that augments the standard therapy bolus formula with a physical activity component inspired by the concept of IOB. This component corresponds to the amount of insulin required to compensate for estimated glycemic disturbances related to previously performed physical activity. The modulation relies on a wearable activity tracker based calculation of accumulated physical activity through a weighted sum of the historical steps taken within the previous 12 hours. The resulting metric, AOB, characterizes the accumulated physical activity that is still actively impacting glucose uptake. Once the is obtained at the time of the meal bolus, we calculate the physical activity informed boluses as follows:
The is the AOB calculated for the meal m consumed on day d. is the profile that captures the routine daily accumulated physical activity of a participant around a selected standard meal m such as breakfast, lunch or dinner. Note that serves as a reference for the regular accumulated physical activity of the patient at the selected mealtime for which the average treatment is designed. Deviations from the regular physical activity are expected to cause changes in the insulin needs and the physical activity informed bolus is designed to modulate the insulin dose accordingly. Our patient-specific bolus correction parameter, activity factor (AF), translates the anticipated glycemic change generated by the physical activity deviations into insulin units with a similar impact. The direction of the resulting bolus adjustment depends on whether is greater or smaller than . Its magnitude is a function of the amount of the deviation from the profile and the value of the AF. The optimized meal bolus parameters, CR and AF, used in physical activity informed bolus calculations are obtained for each participant following the steps provided in the supplementary document and detailed in Ozaslan et al. 22
In the calculation of the lunchtime bolus, optimized CRs were used in both outpatient visits while AF was used to correct for the previous physical activity only in the experimental visit.
Outcomes
The primary outcome measure was the time spent in hypoglycemia during the post-prandial window following lunch. Secondary outcomes were the time spent in target range (70-180 mg/dL), low blood glucose index (LBGI), and high blood glucose index (HBGI). The paired Wilcoxon rank test was used to statistically compare both the hypoglycemia counts and LBGI due to their non-Gaussian distributions. The paired t test was used to compare the percentage of time spent in the target range and the HBGI metrics. To assess the hypoglycemia mitigation potential of the two bolus methods, we also report the rate of glucose change in the first 2 hours in the post-prandial period. This time window corresponds to the phase that the rate of physical activity-induced glucose uptake is maximum. 21 For this assessment, we use linear mixed-effect analysis with covariates of categorical study visit variable and continuous lunchtime BG variable. In our models, we control for the hypoglycemia treatments administered from 1 hour prior to lunch until the end of the post-prandial window. Participant effect is accounted for as a random effect.
Results
Fifteen subjects (6 men and 9 women) completed the study. Participant demographic characteristics, expressed in means ± standard deviation, are presented in Table 1.
Table 1.
Demographic Characteristics of Study Participants.
| Mean ± SD | Minimum | Maximum | |
|---|---|---|---|
| Age (years) | 47.8 ± 10.6 | 23 | 60 |
| Height (cm) | 168.3 ± 10.1 | 154 | 181 |
| Weight (kg) | 77.7 ± 16.9 | 53.3 | 110 |
| BMI (kg/m2) | 27.5 ± 6.2 | 21.6 | 44.1 |
| HbA1c (%) | 7.2 ± 0.9 | 6.1 | 9.2 |
| Type 1 diabetes duration (years) | 26.7 ± 13.5 | 2 | 47 |
For four participants, CR optimization for lunch was not feasible (eg, lack of meal bolus between 11 AM and 2 PM on most days). Therefore, we used these participants’ original lunchtime CR during both study visits. For six participants, the optimization failed in finding an optimum AF within the optimization boundaries. In these cases, we used 2200 accumulated steps as an approximated average AF. In cases where CR optimization was feasible, it led to a 2% ± 18% change from the participant’s original CR with no statistically significant difference (original: 10.6 ± 2.7 g/U, optimized: 11 ± 3.8 g/U, P = .64). The average of optimum AFs, computed from the 9 patients that the optimization was feasible for, was 2926 ± 1267 accumulated steps/U.
During the experimental visit, physical activity related bolus reduction was on average 28% ± 15% of the total bolus. Participants’ glucose values at lunchtime were similar between study visits (control: 126 ± 41 mg/dL, experimental: 127 ± 35 mg/dL, P = .94). There was no significant difference between visits for the AOB at lunchtime (control: 5438 ± 1048 accumulated steps, experimental: 5258 ± 969 accumulated steps, P = .32). Nine hypoglycemia instances occurred within the previous hour of lunch (five in experimental, four in the control admission), and were treated with rescue carbohydrates. Hypoglycemia occurrence was rather rare in the post-lunch period in both visits; glucose levels below 70 mg/dL occurred in only 3 out of 30 observations (1 in the experimental, and 2 in the control visit) and treated with 25.7 ± 5.1 g of rescue carbohydrates. As a result, there was no statistically significant difference in the percentage of time spent in hypoglycemia between visits (median [IQR], control: 0 [0]%, experimental: 0 [0]% P = 1). The percentage of time spent in the target range was lower with physical activity informed boluses (control: 77% ± 27% experimental: 59% ± 31% P = .03). However, no significant difference was observed for LBGI (median [IQR], control: 0 [0.03], experimental: 0 [0.02], P = .62) or HBGI (control: 0.82 ± 0.8, experimental: 0.78 ± 0.6, P = .83) in the post-lunch period.
Overall, the afternoon physical activity, as measured by total number of steps, was significantly lower during the study visits compared to the participants’ routine physical activity extracted from the data collection period (control: 1095 ± 451 steps, experimental: 1288 ± 702 steps, data collection: 2555 ± 1618 steps, with P < .01 for both control vs data collection and experimental vs data collection afternoon physical activity). No significant difference was observed between the total number of steps that participants took in the afternoon portion of control vs experimental visits (P = .18).
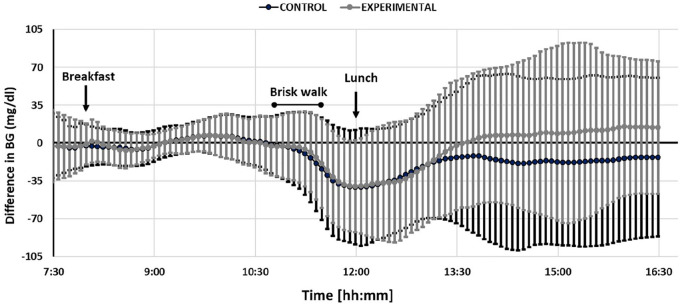
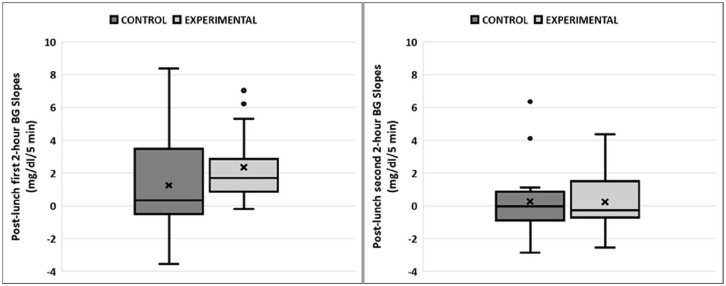
As indicated in Figure 2, on average, standard therapy bolus yielded a late post-prandial glucose below the morning average glucose while the physical activity informed bolus yielded a higher glucose than this reference; albeit statistically not significant (ΔCGMreference control: −16 ± 74 mg/dL, ΔCGMreference experimental: 10 ± 68 mg/dL, P = .09). The differences in the direction and magnitude of glucose change in the early and late phases of post-lunch glycemic excursion are evaluated through CGM slopes in linear mixed effects regression analyses with the results provided in Table 2. While study visit was a significant factor associated with the CGM trend in the first two hours following bolus injection (P = .032), no significant difference was observed in the CGM trend in the late post-lunch phase (between second and fourth hours after lunch) between visits (P = .71). CGM slopes for the first two-hour phase were negative in 40% of the observations in the control visit and this was reduced to 7% in the experimental visit (see Figure 3). Additional information and plots per participant are available in the Supplementary Material.
Figure 2.
Change in CGM in the control (dark grey) and the experimental (light grey) visits. The reference CGM value for each visit and participant is their average morning CGM calculated from CGM readings between 7:30 AM and 10:45 AM for the related visit. All values are then obtained by subtracting the subject and visit specific reference CGM values from the observed CGM readings from 7:30 AM until 16:30 PM during the study visit. The glucose values in the afternoon had a higher variation compared to the morning since glucose levels were managed by the study physician in the morning to bring them to a safe glucose range for physical activity. Furthermore, the range of meals taken for lunch are wider than the ones for breakfast.
Table 2.
Regression Analysis Results on Post-Lunch CGM Slope Trends in the First 2 Hours (Left) and in the Second 2 Hours (Right) Following the Lunch Meals.
| Post-lunch [0 h, 2 h] | Value ± SE | P-value | Post-lunch [2 h, 4 h] | Value ± SE | P-value |
|---|---|---|---|---|---|
| Slope analysis | Slope analysis | ||||
| Intercept | 3.9 ± 1.49 | .04* | Intercept | 0.16 ± 1.48 | .917 |
| CGMlunchtime | −0.02 ± 0.01 | .032 | CGMlunchtime | −0.002 ± 0.01 | .853 |
| Experimental visit | 1.2 ± 0.5 | .032* | Experimental visit | 0.2 ± 0.52 | .712 |
The variable “Experimental Visit” in the regression models is categorical and the coefficients associated with it show marginal difference in the slopes in experimental compared to the control visit.
Figure 3.
Boxplot representation of post-lunch CGM slope trends in study visits separated for the observation in the first 2 hours (left) and in the second 2 hours (right) following the lunch meals.
Discussion
The goal of this pilot feasibility study was to assess whether step-count based physical activity information could be used to enhance hypoglycemia protection of a meal bolus following a brisk walking session. Negative CGM slopes in the early post-prandial phase support the need for formal incorporation of previous physical activity into insulin bolus calculations. While the physical activity-informed bolus method was able to successfully compensate for the decrease in glucose levels, results imply that it might be too conservative against the risk of hypoglycemia since it yielded an average glucose trend higher than the computed participant-specific reference glucose averages in the experimental arm of the study. In other words, results showed that meal treatment with the standard care following a walking physical activity session led to below the reference glucose levels, implying an increased risk for hypoglycemia, albeit not as much as anticipated. Despite full bolus administration in the control session, we observed a rather low rate of hypoglycemia and higher time in the target range compared to the experimental session. More specifically, only 3 instances of post-lunch hypoglycemia occurred during the trial. As such, participants indeed benefited from the walking exercise in terms of the time spent in target range. These findings suggest that additional factors should be considered while adjusting the insulin bolus for physical activity. Along this study, factors attenuating the activity-induced increase in the glucose uptake may include the high protein and fat content of the breakfast, which was designed for having minimum IOB at the time of the walking exercise. Additionally, the afternoon portion of the study incorporated minimum physical activity that was significantly lower than the participants’ routine afternoon physical activity upon which the treatment parameters were prescribed and optimized. These conditions, along with the unbolused rescue carbohydrates around lunch, likely led to higher glucose values than expected. Strategies to improve the glycemic outcomes with physical activity informed boluses, such as considering the meal composition, and anticipated physical activity levels in the following hours remain to be further investigated.
We also note three aspects of the approach taken in this paper that are worth further examination. First, physical activity on board is computed via a convolution with a reference signal (see the Supplementary Material). Different reference signal values may affect the outcomes of the bolus decisions. We hypothesize that, based on our mathematical intuition, if the reference signal approximates the decreasing nature of the effect of physical activity on blood glucose well enough, the corresponding adjustment decisions are valid for our purpose. Second, the literature on the prolonged glycemic effect of physical activity in individuals with T1D is limited. For instance, our reference study demonstrated a biphasic change in glucose uptake in adolescents after an exercise bout performed by adolescents late in the day. 21 However, it was shown in Davey et al 23 that when the exercise bout was at mid-day, the change in the glucose uptake was rather stable from one hour after the exercise until 11 hours. Similar studies in adults at differing times of the day and for different physical activity modalities could provide more accurate signals for our method. The third aspect is the fact that physical activity has a longer horizon of effect (12 hours) than the one for insulin injections (5 hours).21,23 This discrepancy translates to possible over-reduction of insulin dose for the performed physical activity as our AOB predictions anticipate further blood glucose decrease for hours after the insulin was used. Additionally, we observed the glycemic outcomes for up to five hours after the end of the physical activity session and about half of the total glycemic impact from the performed physical activity was anticipated to occur in the hours after our observation window, based on McMahon et al.’s study. 21 One possible way to reconcile this discrepancy is to make physical activity corrections to compensate only for the changes anticipated to occur within the duration of insulin action.
We believe that the aforementioned issues can be addressed with future controlled studies that explore the glycemic response to unstructured physical activity in T1D. More data would support rigorous investigation on the mathematical modeling of the effect of physical activities combined with insulin intakes. Despite these limitations, it is noteworthy that in the first 2 hours of post-lunch phase, standard boluses yielded a higher tendency toward decreasing glucose compared to the physical activity informed boluses. These results are compatible with the current knowledge of an increased glucose uptake up to 2 hours following moderate-intensity physical activity.21,23
In conclusion, this pilot study presents early safety and feasibility data of a novel method to quantify physical activity via a ubiquitously available measurement, step count, and utilize this quantity to modulate prandial insulin boluses. This method can be adapted to different physical activity indicators, physical activity accumulation methods, as well as the changing baseline physical activity behavior of individuals through the parameters AOB and AF. Our results suggest that person-specific treatment adjustments through step-based quantifications of physical activity are safe, feasible, and have potential in mitigating physical activity-induced BG decrease in the early post-meal window in people with T1D. Nonetheless, further research and data are required to address the limitations mentioned in this manuscript such that a more comprehensive evaluation of our method’s performance in enhancing glucose control could be obtained.
Supplemental Material
Supplemental material, sj-pdf-1-dst-10.1177_1932296821997917 for Safety and Feasibility Evaluation of Step Count Informed Meal Boluses in Type 1 Diabetes: A Pilot Study by Basak Ozaslan, Sue A. Brown, Jennifer Pinnata, Charlotte L. Barnett, Kelly Carr, Christian A. Wakeman, Mary Clancy-Oliveri and Marc D. Breton in Journal of Diabetes Science and Technology
Acknowledgments
The authors thank Ke Wang for setting the database of physical activity data collection, Jacob A. Hellmann for his help in data downloads, Sandra Foster for her support in study coordination. B.O would like to thank Jonathan Hughes for his insightful comments, and proofreading of this article.
Footnotes
Abbreviations: AF, Activity Factor; AOB, Activity on Board; BG, Blood Glucose; CGM, Continuous Glucose Monitor; CHO, Carbohydrate; CR, Carbohydrate Ratio; CF, Correction Factor; HBGI, High Blood Glucose Index; IOB, Insulin on Board; IQR, Interquartile Range; LBGI, Low Blood Glucose Index; PA, Physical Activity; ST, Standard Therapy; STD, Standard Deviation; T1D, Type 1 Diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: B.O has no conflict of interest to disclose. MDB reports research support from Dexcom, Sanofi, and Tandem Diabetes Care; SAB reports research support from Tandem Diabetes Care, Dexcom, Roche Diagnostics, Insulet, and Tolerion outside the submitted work; MDB reports consulting fees and honoraria from Air Liquide, Dexcom, and Tandem Diabetes Care; MDB reports royalties from IP licenses in this field, managed by the University of Virginia. CW reports research support from Tandem Diabetes Care and Dexcom Inc., other from Dexcom, Inc., other from Tandem Diabetes Care, outside the submitted work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NIH NIDDK DP3DK106826.
ORCID iDs: Basak Ozaslan  https://orcid.org/0000-0002-8537-1892
https://orcid.org/0000-0002-8537-1892
Marc D. Breton  https://orcid.org/0000-0001-7645-2693
https://orcid.org/0000-0001-7645-2693
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. Can Med Assoc J. 2006;174(6):801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paffenbarger RS, Hyde R, Wing AL, Hsieh C. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314(10):605-613. [DOI] [PubMed] [Google Scholar]
- 3. Balducci S, Iacobellis G, Parisi L, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications 2006;20(4):216-223. [DOI] [PubMed] [Google Scholar]
- 4. Bohn B, Herbst A, Pfeifer M, et al. Impact of physical activity on glycemic control and prevalence of cardiovascular risk factors in adults with type 1 diabetes: a cross-sectional multicenter study of 18,028 patients. Diabetes Care. 2015;38(8):1536-1543. [DOI] [PubMed] [Google Scholar]
- 5. Brazeau A-S, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care. 2008;31(11):2108-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riddell MC, Gallen IW, Smart CE, et al. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017;5(5):377-390. [DOI] [PubMed] [Google Scholar]
- 7. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cinar A. Multivariable adaptive artificial pancreas system in type 1 diabetes. Curr Diab Rep. 2017;17(10):88. [DOI] [PubMed] [Google Scholar]
- 9. Riddell MC, Zaharieva DP, Yavelberg L, Cinar A, Jamnik VK. Exercise and the development of the artificial pancreas: one of the more difficult series of hurdles. J Diabetes Sci Technol. 2015;9(6):1217-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeBoer MD, Cherñavvsky DR, Topchyan K, Kovatchev BP, Francis GL, Breton MD. Heart rate informed artificial pancreas system enhances glycemic control during exercise in adolescents with T1D. Pediatr Diabetes. 2017;18(7):540-546. [DOI] [PubMed] [Google Scholar]
- 11. Breton MD, Brown SA, Karvetski CH, et al. Adding heart rate signal to a control-to-range artificial pancreas system improves the protection against hypoglycemia during exercise in type 1 diabetes. Diabetes Technol Ther. 2014;16(8):506-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turksoy K, Bayrak ES, Quinn L, Littlejohn E, Cinar A. Multivariable adaptive closed-loop control of an artificial pancreas without meal and activity announcement. Diabetes Technol Ther. 2013;15(5):386-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia-Tirado J, Brown SA, Laichuthai N, et al. Anticipation of historical exercise patterns by a novel artificial pancreas system reduces hypoglycemia during and after moderate-intensity physical activity in people with type 1 diabetes. Diabetes Technol Ther. Published online December 1, 2020. doi: 10.1089/dia.2020.0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dunstan DW, Kingwell BA, Larsen R, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35(5):976-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bailey DP, Locke CD. Breaking up prolonged sitting with light-intensity walking improves postprandial glycemia, but breaking up sitting with standing does not. J Sci Med Sport. 2015;18(3):294-298. [DOI] [PubMed] [Google Scholar]
- 16. Peddie MC, Bone JL, Rehrer NJ, Skeaff CM, Gray AR, Perry TL. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: a randomized crossover trial. Am J Clin Nutr. 2013;98(2):358-366. [DOI] [PubMed] [Google Scholar]
- 17. Manohar C, Levine JA, Nandy DK, et al. The effect of walking on postprandial glycemic excursion in patients with type 1 diabetes and healthy people. Diabetes Care. 2012;35(12):2493-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ozaslan B, Patek SD, Breton MD. Impact of daily physical activity as measured by commonly available wearables on meal time glucose control in type 1 diabetes. Diabetes Technol Ther. 2020;22(10):742-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cappon G, Marturano F, Vettoretti M, Facchinetti A, Sparacino G. In silico assessment of literature insulin bolus calculation methods accounting for glucose rate of change. J Diabetes Sci Technol. 2019;13(1):103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Swan KL, Dziura JD, Steil GM, et al. Effect of age of infusion site and type of rapid-acting analog on pharmacodynamic parameters of insulin boluses in youth with type 1 diabetes receiving insulin pump therapy. Diabetes Care. 2009;32(2):240-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McMahon SK, Ferreira LD, Ratnam N, et al. Glucose requirements to maintain euglycemia after moderate-intensity afternoon exercise in adolescents with type 1 diabetes are increased in a biphasic manner. J Clin Endocrinol Metab. 2007;92(3):963-968. [DOI] [PubMed] [Google Scholar]
- 22. Ozaslan B, Fabris C, Patek SD, Breton M. Automatically accounting for physical activity in insulin dosing for type 1 diabetes. Comput Methods Programs Biomed. Published online September 21, 2020. doi: 10.1016/j.cmpb.2020.105757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davey RJ, Howe W, Paramalingam N, et al. The effect of midday moderate-intensity exercise on postexercise hypoglycemia risk in individuals with type 1 diabetes. J Clin Endocrinol Metab. 2013;98(7):2908-2914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-dst-10.1177_1932296821997917 for Safety and Feasibility Evaluation of Step Count Informed Meal Boluses in Type 1 Diabetes: A Pilot Study by Basak Ozaslan, Sue A. Brown, Jennifer Pinnata, Charlotte L. Barnett, Kelly Carr, Christian A. Wakeman, Mary Clancy-Oliveri and Marc D. Breton in Journal of Diabetes Science and Technology