Abstract
This article is the work product of the Continuous Ketone Monitoring Consensus Panel, which was organized by Diabetes Technology Society and met virtually on April 20, 2021. The panel consisted of 20 US-based experts in the use of diabetes technology, representing adult endocrinology, pediatric endocrinology, advanced practice nursing, diabetes care and education, clinical chemistry, and bioengineering. The panelists were from universities, hospitals, freestanding research institutes, government, and private practice. Panelists reviewed the medical literature pertaining to ten topics: (1) physiology of ketone production, (2) measurement of ketones, (3) performance of the first continuous ketone monitor (CKM) reported to be used in human trials, (4) demographics and epidemiology of diabetic ketoacidosis (DKA), (5) atypical hyperketonemia, (6) prevention of DKA, (7) non-DKA states of fasting ketonemia and ketonuria, (8) potential integration of CKMs with pumps and automated insulin delivery systems to prevent DKA, (9) clinical trials of CKMs, and (10) the future of CKMs. The panelists summarized the medical literature for each of the ten topics in this report. They also developed 30 conclusions (amounting to three conclusions for each topic) about CKMs and voted unanimously to adopt the 30 conclusions. This report is intended to support the development of safe and effective continuous ketone monitoring and to apply this technology in ways that will benefit people with diabetes.
Keywords: acetoacetate, β-hydroxybutyrate, continuous, ketoacidosis, ketone
Introduction
Background
Spot ketone tests are used by some people with diabetes (PwD) to detect impending diabetic ketoacidosis (DKA) if ketone bodies are present in the blood, urine, or breath in greater than normal concentrations. DKA is a serious complication of type 1 diabetes (T1D) caused by an absence of circulating insulin. Ketones can also be present in a few cases of starvation or very low carbohydrate intake. Ketone testing has been used in the field of nutrition to monitor the effectiveness of very low carbohydrate diets and in managing a few rare diseases where ketogenic diets have been prescribed as a treatment. Various activities by people with T1D can lead to rapid production of ketone bodies, resulting in DKA within a few hours. However, few patients test themselves for ketones because of lack of (1) awareness of the importance of ketone testing, (2) convenience because they must carry a special type of testing strip or device, (3) knowledge of what to do with the information from ketone testing, (4) insurance coverage.
The Panel Meeting Process
In 2021 a major breakthrough in diabetes management was announced in an article by Alva et al. that described the first-in-human results of a pilot study of a continuous ketone monitor (CKM). 1 The electrochemical sensor used wired enzyme technology to measure β-hydroxybutyrate (BHB), which is the major pathologic analyte in DKA. This sensor delivered good accuracy and stability, both in vitro and in vivo, for 14 days with a single retrospective calibration. This CKM delivered a linear response over a clinically relevant range of BHB. A CKM could provide new and useful data for managing diabetes by automatically measuring interstitial fluid ketone concentrations (which were noted in that study to be similar to blood ketone concentrations), wirelessly transmitting the data to a repository, and alerting the CKM wearer of an elevated, risky ketone concentration. This is exactly how a continuous glucose monitor (CGM) operates when it sounds an alert for an out-of-range glucose concentration. The Alva article studied only 12 volunteers and much more work will be needed before this system can become an actual product cleared for use.
On April 20, 2021, Diabetes Technology Society (DTS), led by Dr. David Klonoff, convened the Continuous Ketone Monitoring Consensus Panel. The panel met virtually because of travel restrictions caused by COVID-19. The consensus panel consisted of 20 United States (US)-based experts in the use of diabetes technology. The purposes of the panel were to (1) provide insights to clinicians, researchers, regulators, and payers for understanding the technical and clinical performance of CKMs and (2) facilitate the use of this type of tool for monitoring ketone concentrations in people with T1D to prevent DKA.
Panelists included diabetes technology experts from adult endocrinology, pediatric endocrinology, advanced practice nursing, diabetes care and education, clinical chemistry, and bioengineering. The panelists were from universities, hospitals, freestanding research institutes, government, and private practice. Panelists reviewed the medical literature pertaining to ten sessions, each focused on a topic related to the use of ketone monitoring: (1) physiology of ketone production, (2) measurement of ketones, (3) performance of the first CKM reported to be used in human trials, (4) demographics and epidemiology of DKA, (5) atypical hyperketonemia, (6) prevention of DKA, (7) non-DKA states of fasting ketonemia and ketonuria, (8) potential integration of CKMs with pumps and automated insulin delivery (AID) systems to prevent DKA, (9) clinical trials of CKMs, and (10) the future of CKMs. Each of the ten sessions was co-chaired by two experts (presented in alphabetical order) and all panelists participated in every session.
The Panel Meeting Outcome
The panelists summarized the medical literature for each of the ten topics related to the measurement and use of CKM data. Recommendations for consensus conclusions about CKMs were proposed by panelists and then reviewed by the entire panel for favorability. The panel developed 30 conclusions about CKMs (amounting to three conclusions for each topic) and voted unanimously to accept all 30 of the conclusions. The 20 panelists and 4 meeting organizers from DTS all contributed to this report. The report is organized to contain, for each of the ten topics, three current status statements, a discussion of the role of CKM, and three conclusions.
Physiology of Ketone Production
Co-Chair: Suneil K. Koliwad, MD, PhD
University of California, San Francisco, San Francisco, California, USA
Co-Chair: Amisha Wallia, MD, MS
Northwestern University, Chicago, Illinois, USA
Current Status
Ketone bodies are alternative energy sources used in response to reduced glucose availability.
There are three ketone bodies, including acetoacetate, β-hydroxybutyrate, and acetone. Ketone bodies are formed when fatty acids are converted to acetoacetate, which is then reduced to β-hydroxybutyrate. Acetoacetate can also spontaneously decarboxylate to produce acetone.
If ketone bodies are produced more quickly than they can be metabolized, then the concentration of ketone bodies in the blood rises. Diabetic ketoacidosis refers to a state of severely elevated levels of circulating ketone bodies in people with diabetes, which is associated with extreme acidemia and is dangerous and potentially fatal.
Discussion
Like other mammals, humans have evolved with the ability to withstand brief periods of food unavailability and starvation by utilizing energy stored within adipose tissue. Ketogenesis is a metabolic process that helps provide energy substrates to tissues needing fuel during low carbohydrate states by liberating energy stored within adipose tissue.2,3 As a result of fasting or starvation, or in low insulin states, lipolysis occurs within the adipocyte, which generates free fatty acids (FFA). The liver converts these fatty acids into two 4-carbon short chain fatty acids known as ketone bodies. These ketone bodies, acetoacetate (AcAc) and BHB, can be utilized as a source of energy for the brain and other organs during states of carbohydrate deficiency. 4 Dietary ketogenic amino acids leucine and lysine may contribute to limited ketogenesis. 5 Additional ketone production in the gut has recently been demonstrated through the consumption of medium chain fats or through changes in the composition and function of the microbiome. 4 Ketosis is a state of reliance on ketone bodies as a fuel source as an alternative to glucose. Ketosis occurs physiologically during fasting, starvation, low carbohydrate intake, intense exercise, or pathologically because of a complete lack of insulin in inadequately treated diabetes. 6
Ketogenesis largely occurs within hepatocyte mitochondria. There, medium chain fatty acids and long chain fatty acids are converted into a pair of ketone bodies including, initially AcAc, which is then reduced to BHB, the predominant ketone body present in the blood and is available for ketolysis in peripheral tissues. Acetone is a third ketone body that is generated by the spontaneous decarboxylation of AcAc, but this substance is the least abundant ketone body. The structure of each of the three ketone bodies is given in Figure 1. AcAc and BHB can be considered as essential fuel sources, especially for the brain when glucose availability is low, but when they are present in large concentrations these acids can be associated with acidemia. 7 Ketogenesis is promoted by glucagon and suppressed by insulin. For example, an elevated glucagon/low insulin state can promote ketogenesis by increasing β-oxidation of FFA in the liver and lipolysis in adipocytes. On the other hand, insulin powerfully inhibits ketogenesis, mainly through suppression of lipolysis by adipocytes and reduction of the supply of FFA, which are the substrate for ketone body production.
Figure 1.
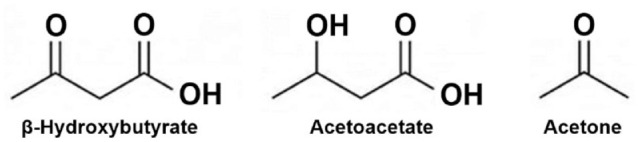
Structures of the three major ketone bodies.
In DKA, there is a lack of insulin activity and there are elevations in counterregulatory hormones, which combine to stimulate lipolysis in the adipose tissue and unchecked ketogenesis in the liver. Given the crucial role of insulin in regulating normal intermediary metabolism, an insufficient amount or total absence of insulin can lead to ketone bodies being produced more quickly than they can be metabolized. DKA is a state of severely elevated circulating ketone bodies. DKA is associated with extreme acidemia, which can be dangerous or fatal. Metabolic processes and hormone imbalances leading to ketogenesis are presented in Figure 2.
Figure 2.
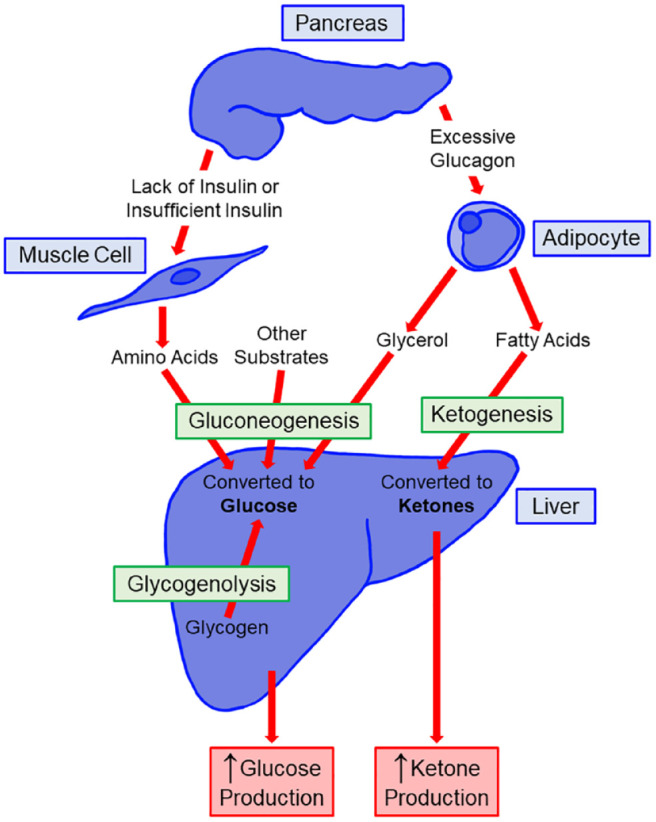
Metabolic processes and hormone imbalances resulting in ketogenesis.
Conclusions
Ketone bodies are a type of fuel substrate produced by the liver upon oxidation of fatty acids that flux from adipose tissues where they are liberated by fasting or starvation, carbohydrate-deficient “ketogenic” diets, and in response to severe insulin deficiency as seen in diabetic ketoacidosis.
An elevated glucagon/low insulin state can promote ketogenesis by increasing β-oxidation of free fatty acids in the liver and lipolysis in adipocytes, whereas insulin inhibits ketogenesis, mainly through suppression of lipolysis by adipocytes and reduction of the supply of free fatty acids.
In diabetic ketoacidosis, an increase in counterregulatory hormones and lack of insulin can promote ketogenesis.
Measurement of Ketones
Co-Chair: James H. Nichols, PhD, DABCC, FAACC
Vanderbilt University Medical Center, Nashville, Tennessee, USA
Co-Chair: Mark R. Prausnitz, PhD
Georgia Institute of Technology, Atlanta, Georgia, USA
Current Status
Urine ketone tests based on nitroprusside are inexpensive and available as dipsticks for home use. They measure only acetoacetate, provide only qualitative results, and are subject to (1) medication interferences, by such agents as sulfhydryl drugs and captopril and (2) color discrimination errors when reading the test strip. Urinary acetoacetate assays, compared to blood β-hydroxybutyrate assays, may not reliably identify the onset and resolution of ketosis.
Meters that quantify β-hydroxybutyrate from capillary sampling are the preferred tests for the self-monitoring of ketones during illness and hyperglycemia. They have been associated with earlier detection of ketosis and may provide information required to prevent progression to diabetic ketoacidosis. Blood β-hydroxybutyrate tests are not subject to color discrimination like urine ketone dipsticks are.
Breath analyzers for ketones measure acetone, which is a breakdown product of acetoacetate. While convenient and less painful than fingerstick blood testing, these devices do not measure β-hydroxybutyrate which is the ketone body present in the greatest concentration during diabetic ketoacidosis.
Discussion
In healthy people plasma ketone concentrations usually range from 0–0.3 mM, but can increase to around 1 mM with prolonged exercise or fasting for 24 hours, and can increase to as high as 5–10 mM with prolonged fasting for 3 days or more.8-10 In PwD, plasma ketone concentrations of 0.6 mM or greater signify an increased risk of ketoacidosis, as presented in Table 1. 11
Table 1.
Risk of DKA According to Plasma Ketone Concentrations in People with Diabetes.
| Plasma ketone concentration | Risk of DKA |
|---|---|
| < 0.6 mmol/L | No particular increased risk |
| 0.6 to 1.5 mmol/L | Slightly increased risk |
| 1.6 to 2.9 mmol/L | Increased risk |
| ≥ 3 mmol/L | Very high risk |
Management of diabetes benefits from the measurement of ketones, especially for prevention of DKA. 12 There are three different types of ketone tests: (1) urine ketone tests that measure AcAc, (2) blood ketone tests that measure BHB, and (3) breath ketone tests that measure acetone. Products used to test for ketones in these three ways are presented in Table 2A to C.
Table 2.
Three Lists of Ketone Monitors That Are Currently Available on the Market in the United States and Whether They Are Cleared by the FDA. (A) A List of Blood Ketone Monitors. Table Modified from Zhang et al 19 (B) A List of Urine Ketone Monitors. (C) A List of Breath Ketone Monitors.
Fda regulatory status was determined for each device by searching the Fda’s 510(k) Premarket Notification database 20 for either the device name or company (applicant) name. Some of the products in our three tables were noted to be listed as cleared while others were not listed as cleared. If the product was listed as cleared on the Fda database, then the product identification number (known as the K number) was presented. No*, company website claims that the product is cleared, but as of July 23, 2021, the product was not on the Fda database of cleared devices. NI, no information; information could not be found about the manufacturers through searching their Amazon seller pages, the FDA’s 510(k) Premarket Notification database, or the Google database.
| A) Blood Ketone Monitors | |||
|---|---|---|---|
| Product | Manufacturer | Manufacturer location | Regulatory status—cleared by the FDA? |
| Bruno MD6 21 | Bruno MD | Boca Raton, FL, USA | No |
| CareSens N Plus Bluetooth Blood Diabetes Monitoring Kit 22 | i-SENS | Torrance, CA, USA | Yes - K083468 23 |
| CareTouch Blood Ketone Monitoring System 24 | CareTouch | Brooklyn, NY, USA | No |
| Femometer Blood Ketone Monitoring Kit 25 | Femometer | Wanchai, Hong Kong | No |
| FORA 6 Bluetooth Blood Ketone Meter and Glucose Monitor 26 | Taidoc Technology Corporation | New Taipei City, Taiwan | Yes - K161738 27 |
| FreeStyle Precision Pro 28 | Abbott Diabetes Care | Alameda, CA, USA | Yes - K132511 29 |
| GlucoMen LX Plus 30 | Menarini Diagnostics | Florence, Italy | No |
| GlucoRX HCT & Ketone Blood Glucose Monitoring System 31 | GlucoRx | Surrey, UK | No |
| KetoBM 32 | KetoBM | Sheridan, WY, USA | No |
| KetoCoach Blood Ketone Test Meter 33 | KetoCoach | Minneapolis, MN, USA | No* |
| Keto-Doc Advanced Ketone Blood Meter Testing Kit 34 | Ketodoc | Los Angeles, CA, USA | No |
| Keto-Mojo 35 | Taidoc Technology Corporation | New Taipei City, Taiwan | Yes - K161738 27 |
| KetoSens Blood Ketone Monitoring Kit 36 | i-SENS | Torrance, CA, USA | Yes - K201551 37 |
| KetoTrak Blood Ketone Monitoring System 38 | ACON Laboratories | San Diego, CA, USA | No |
| Kiss My Keto Ketone Blood Meter Kit 39 | Kiss My Keto | Los Angeles, CA, USA | No* |
| Nova Max Plus 40 | Nova Diabetes Care | Billerica, MA, USA | Yes - K091547 41 |
| Precision Xceed Pro 42 | Abbott Diabetes Care | Alameda, CA, USA | Yes - K080960 43 |
| Precision Xtra Blood Glucose & Ketone Monitoring 44 | Abbott Diabetes Care | Alameda, CA, USA | Yes - K060768 45 |
| B) Urine Ketone Monitors | |||
| Product | Manufacturer | Manufacturer location | Regulatory status—cleared by the FDA? |
| 310 Nutrition Ketone Test Strips 46 | 310 Nutrition | Las Vegas, NV, USA | No |
| Ascensia Ketostix Reagent Strips for Urinalysis 47 | Ascensia Diabetes Care | Basel, Switzerland | No |
| CVS Health Urinanalysis Ketone Test Strips 48 | CVS Health | Woonsocket, RI, USA | No |
| Easy@Home Ketone Tests Strips 49 | Easy@Home | NI | No |
| EZ Keto Ketone Testing Strips 50 | Easy KETO | NI | No |
| HealthyWiser UriTest Strips 51 | HealthyWiser | NI | No |
| INVBIO pH+Keto 2-in-1, Urinalysis Urine Test Strips 52 | Innovation Biotech Co., LTD | Beijing, China | No |
| JNW Direct Ketone Test Strips 53 | JNW Direct | NI | No |
| Just Fitter Ketone Urine Test Strips 54 | Just Fitter | Chicago, IL, USA | No |
| Keto Mojo Ketone Urine Test Strips 55 | Keto Mojo | Napa, CA | No |
| Kiss My Keto 56 | Kiss My Keto | Los Angeles, CA, USA | No |
| Med Lab Ketone Testing Kit Strips 57 | Medical Diagnostic Laboratories, L.L.C. | Hamilton Township, NJ, USA | No |
| Nurse Hatty 58 | Hatty, Inc. | NI | No |
| One Earth Health Ketone Strips 59 | One Earth Direct | NI | No |
| Perfect Keto Test Strips 60 | Perfect Keto | Austin, TX, USA | No |
| ReliOn Ketone Test Strips 62 | Walmart Inc. | Bentonville, AR, USA | No |
| Rock the Fork Keto Ketone Test Strips 63 | Rock the Fork | NI | No |
| Smack Fat Keto Strips 64 | smackfat | NI | No |
| Stript Health Urinanalysis Test Strips 65 | Stript Health | NI | No |
| Top Notch Nutrition Ketone Test Strips 66 | Top Notch Nutrition | Cheyenne, WY | No |
| TRUEplus Ketone Test Strips 67 | Trividia Health | Fort Lauderdale, FL, USA | No |
| VALI Ketone Test Strips 68 | VALI | NI | No |
| VitaPhoenix Ketone Strips 69 | VitaPhoenix | NI | No |
| Walgreens Ketone Test Strips 70 | Walgreen Company | Deerfield, IL, USA | No |
| Wondview Ketone Urine Reagent Strips 71 | Teco Diagnostics | Anaheim, CA, USA | No |
| Zenda Naturals Ketone Test Strips 72 | Zenda Naturals | NI | No |
| Zhou Keto Test Strips 73 | Nutraceutical Corporation | Park City, UT, USA | No |
| C) Breath Ketone Monitors | |||
| Product | Manufacturer | Manufacturer location | Regulatory status—cleared by the FDA? |
| Biosense Breath Ketone Monitor 74 | Readout Health | St. Louis, MO, USA | No |
| Coolker Ketone Breath Analyzer 75 | coolker | NI | No |
| GREENWON Breath Ketone Meter 76 | Shenzhen Hualixin Technologies Co., LTD | Guangdong, China | No |
| House of Keto Monitor 77 | House of Keto | New Territories, Hong Kong | No |
| Huainafajk Breath Ketone Analyzer 78 | Huainafajk | NI | No |
| KETONIX Professional Breath Ketone Analyzer 79 | Ketonix AB | Varberg, Hallands län, Sweden | No |
| KETOSCAN Lite Breath Ketone Meter 80 | SENTECH GMI Corp | Mapo-gu Seoul, South Korea | No |
| KETOSCAN Mini Breath Ketone Meter 81 | SENTECH GMI Corp | Mapo-gu Seoul, South Korea | No |
| KetoStat Breath Ketone Monitor 82 | Keto-Stat | NI | No |
| Keyto Breath Sensor 83 | Keyto Health | San Francisco, CA, USA | No |
| Lencool Ketone Breath Meter 84 | Lencool Limited | Castleblayney, Monaghan, Ireland | No |
| SXINEN Ketone Breath Meter 85 | SXINEN | NI | No |
Urine and blood ketone tests can report nitroprusside-based measurement of AcAc. This ketone reacts with nitroprusside to produce a purple-colored complex. 13 Urine ketone strips report AcAc concentrations qualitatively and usually report results as small (approximately < 20 mg/dL), moderate (approximately 30–40 mg/dL), and large (approximately > 80 mg/dL). 14 The nitroprusside reaction (also known as Legal’s test), however, cannot be used to measure BHB. 15 Early in DKA, the ratio of AcAc to BHB shifts to predominantly favor BHB, as shown in Figure 3. Because of the shift in ketone body concentrations during DKA to favor BHB, methods that measure AcAc may provide misleading clinical information and underestimate the amount of total body ketonemia. 16 In the diagnosis of DKA, nitroprusside-based methods should only be used as an adjunct to a clinical assessment and results of other tests (such as glucose and pH). Nitroprusside-based assays that measure only AcAc can be used to diagnose DKA but should not be used to monitor DKA treatment. When the acidemia of DKA resolves, BHB becomes oxidized to AcAc and BHB levels in the blood fall. However, this process causes a rise in urine ketone readings. The rise in AcAc levels lags behind the rise in BHB levels early in the course of DKA, and during the resolution of DKA when blood BHB levels are falling, AcAc levels paradoxically increase as this ketone is excreted in the urine. This increase in AcAc could falsely suggest that the DKA is worsening. Thus, measuring urinary AcAc, compared to measuring blood BHB, does not reliably identify the onset and resolution of ketoacidosis.
Figure 3.
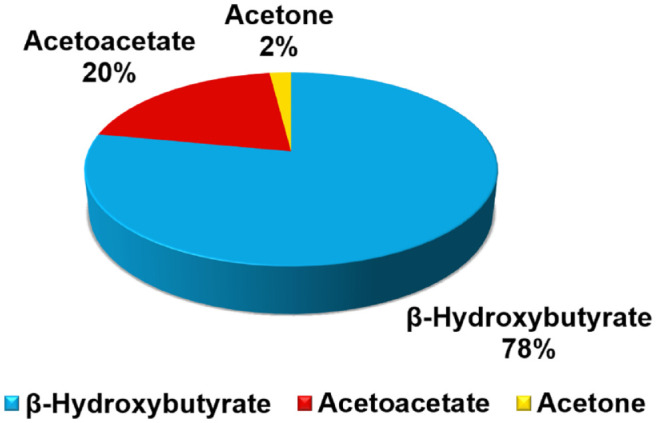
Relationship between the relative concentrations of the three ketone bodies in DKA. The ratio of BHB to AcAc shifts during DKA, from a usual ratio of 1:1, to instead favor BHB as shown in the figure. Figure modified from EKF Diagnostics. 86
Blood tests specific for BHB provide clinically useful information. Meters that quantify BHB ketones from capillary blood samples, compared to urine AcAc ketone tests, are preferred for self-monitoring of ketones in illness and hyperglycemia as well as earlier detection of ketosis that may prevent progression to DKA. 17 This may be especially useful for people with T1D using continuous subcutaneous insulin infusion (CSII) in their therapy because interruption of insulin delivery can result in rapid onset of DKA.
Breath analyzers measure acetone, which is a breakdown product of AcAc, and do not measure BHB. Although breath analyzers may be convenient and easy-to-use, breath ketone analyzers are new to the market. Their performance in self-testing and their value in clinical practice will need to be evaluated. 18
The importance of ketone monitoring, specifically of BHB, is underappreciated in current medical practice, in the clinic, hospital, and at home. In addition to lack of knowledge by patients and healthcare professionals, insurance coverage of ketone measurement may be limited.
Conclusions
Urine ketone tests based on nitroprusside may provide misleading clinical information, underestimating total body ketone concentrations early in diabetic ketoacidosis and overestimating total body ketone concentrations during resolution of diabetic ketoacidosis. They should only be used as an adjunct to diagnose diabetic ketoacidosis and not to monitor diabetic ketoacidosis treatment.
Blood β-hydroxybutyrate measurements provide more clinically useful information than methods that measure urine acetoacetate or acetone levels. β-hydroxybutyrate levels directly track with treatment progression, unlike urine nitroprusside tests.
Breath ketone analyzers are new to the market and their performance in self-testing and clinical practice has not been thoroughly evaluated.
Performance of the First Continuous Ketone Monitor Reported to be Used in Human Trials
Co-Chair: Ananda Basu, MBBS, MD, FRCP (UK)
University of Virginia, Charlottesville, Virginia, USA
Co-Chair: Kristin Castorino, DO, BC-ADM
Sansum Diabetes Research Institute, Santa Barbara, California, USA
Current Status
For the first time, continuous ketone monitoring technology has been shown in humans.
It has been feasible to perform continuous ketone monitoring in 12 adults over a 14-day wear period, using a single-calibration system, which measures β-hydroxybutyrate in interstitial fluid: with capillary ketone as reference.
A continuous ketone monitor has been shown to be stable over 14 days, with a linear response over the 0–8 mM range and with good accuracy.
Discussion
A pilot study of a continuous ketone monitor
Continuous ketone monitoring is a novel and evolving surveillance tool in the therapeutic portfolio that could potentially be useful for the management of T1D and other states that increase risks for ketosis, such as ketogenic diets, starvation, and sodium-glucose co-transporter-2 (SGLT2) inhibitor therapy. A recent publication by Alva et al. 1 provides important proof of concept of such a technique that could make this a clinical possibility in the near future.
In this pilot/feasibility study of a CKM sensor, an in vitro characterization of the sensor was followed by testing its in vivo performance in a cohort of 12 research participants on a low carbohydrate “ketogenic” diet. The CKM sensor is similar in size and structure to an existing glucose sensor, the Freestyle Libre®. Through the use of wired enzyme electrochemical technology, this sensor is designed to measure BHB in the interstitial fluid compartment when inserted subcutaneously using a method identical to that for placing the Freestyle Libre® system. Capillary ketone measurements using the Precision Xtra® ketone test strips were the reference method.
Study results
The in vitro study demonstrated satisfactory linear results across a range of BHB concentrations from 0 mM to 8 mM in phosphate buffer at 37°C (R 2 = 0.999) with an average CV (coefficient of variation) of 5%. The average response time to changing BHB concentrations was 228 seconds (~ 4 minutes). The operational stability of the sensor was 14 days at a BHB concentration of 8 mM with an average daily signal loss of 0.15%. There was interference of approximately 0.2 mM with ascorbic acid at a concentration of 2 mg/dL, which is equivalent to a maximum plasma concentration after ingesting 1 g of vitamin C. 87
The in vivo study was conducted in 12 adult volunteers (one with T1D and 11 not PwD) over the course of 14 days. All participants were on a ketogenic diet for the duration of the study. No participants were taking SGLT2 inhibitors. Each participant wore four sensors (three functional continuous ketone monitoring sensors and one non-functional background sensor) for 14 days. All sensor results were masked to the study participants. Each participant was required to perform eight daily fingerstick measurements using the Precision Xtra® ketone test strips. Six (five CKM and one non-functional) of the 48 sensors failed to collect any valid data because of device problems and were excluded from analyses.
The accuracy and bias results of the study with a single retrospective calibration are summarized in Table 3. Results showed that for BHB concentrations < 1.5 mM, the MAD (mean absolute difference) was 0.129 mM and 91.7% of the sensor results were within ± 0.3 mM of the reference. For BHB ≥ 1.5 mM the MARD (mean absolute relative difference) was 14.4%, 76% of the sensor results were within 20% and 89.7% of the CKM results within 30% of the reference.
Table 3.
Accuracy of the In Vivo Interstitial Fluid Ketone Sensor Results Compared to the Capillary Ketone Strip Reference. Table Reproduced from Alva et al. 1
| Concentration range | Percentage within | Mean MAD/MARD | SD/%CV | Number of paired data points | ||
|---|---|---|---|---|---|---|
| 0.225 mM/20% | 0.3 mM/30% | Between subject | Within subject | |||
| <1.5 mM | 83.4% | 91.7% | 0.129 mM | 0.051 mM | 0.029 mM | 2724 |
| ≥1.5 mM | 76.0% | 89.7% | 14.4% | 5.40% | 7.46% | 408 |
| Combined | 82.4% | 91.4% | – | – | – | 3132 |
Accuracy and bias results for ketone concentrations <1.5 mM are in mM. SD is the standard deviation and CV is the coefficient of variation.
Limitations and future directions
A limitation of this study was that it predominantly enrolled healthy participants who did not have diabetes. Furthermore, even though the reference BHB measurement ranged from 0 mM to 5.1 mM, 87% of the BHB values were below 1.5 mM (mild elevation at best) with a median value of 0.6 mM.
Several important and unanswered questions need to be further investigated prior to clinical use of the CKM. These include but are not limited to:
What is the lag time between plasma and interstitial fluid BHB both during rising and falling circulating BHB concentrations spanning the clinically relevant range (up to 10 mM)?
What is the accuracy of the CKM across the range of clinically relevant BHB levels?
How accurate is the CKM during rapidly changing circulating BHB concentrations?
What is the benefit of a CKM vs. intermittent ketone fingerstick monitoring in the diagnosis or treatment of a patient with ketoacidosis?
Can continuous ketone monitoring lower the incidence of cases and hospitalizations for DKA in high-risk patients?
What is the role of continuous ketone monitoring as an additional input to AID algorithms—possibly to detect under-insulinized states regardless of glucose levels (eg, pump/line failure, SGLT2 adjuvant therapy). 88
Is the CKM affected by interference with drugs other than ascorbic acid?
Conclusions
A continuous ketone monitor has been reported to measure β-hydroxybutyrate in vitro and in vivo in interstitial fluid with a linear response over the 0–8 mM range and with good accuracy.
Future studies of this sensor will be required to assess performance in intended patient populations across the clinical range of circulating ketone bodies. Addition of prospective factory calibration will support real time measurements.
For diagnosis and treatment of diabetic ketoacidosis, this sensor’s clinical role and usability for patients and healthcare professionals remain to be determined.
Demographics and Epidemiology of Diabetic Ketoacidosis
Co-Chair: Richard M. Bergenstal, MD
International Diabetes Center, Minneapolis, Minnesota, USA
Co-Chair: Priya Prahalad, MD, PhD
Stanford University, Stanford, California, USA
Current Status
Diabetic ketoacidosis occurs in ~30% of youth at diabetes onset, with greater frequency in those from minority ethnic groups, those on public insurance, or those with no insurance, and is the leading cause of death in children and adults with diabetes below the age of 58 years.
Healthcare expenditure in the United States for diabetic ketoacidosis was $5.1 billion in 2014.
Few people with diabetes have an illness management kit that includes a blood ketone monitor and clear guidance in insulin and fluid intake.
Discussion
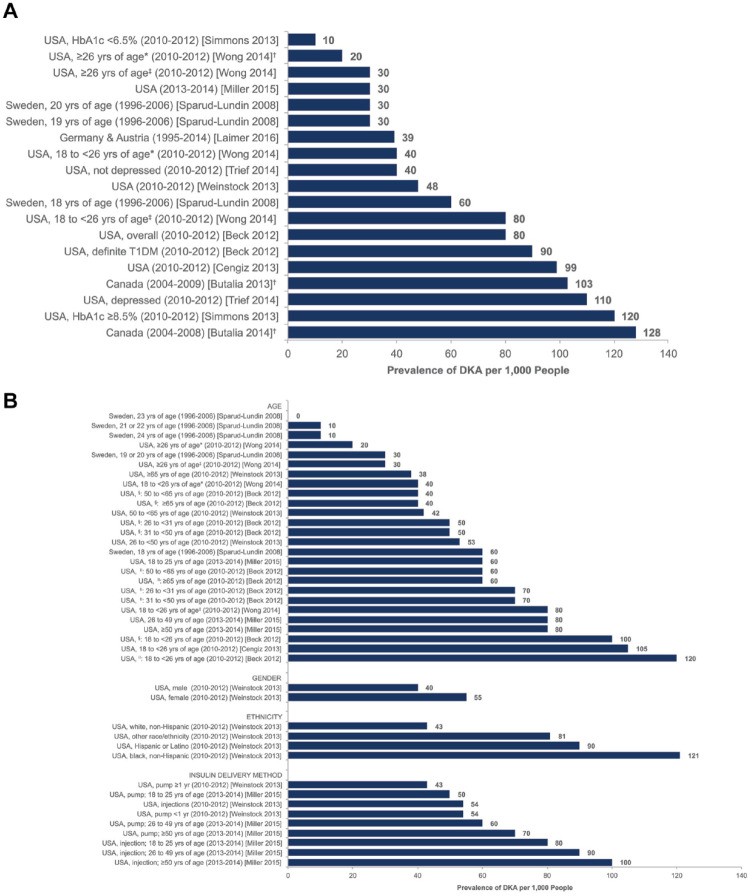
DKA is characterized by “D,” elevated blood glucose levels; “K,” the presence of high urinary or blood ketoacids; and “A,” a high anion gap metabolic acidosis. 10 It is the most common hyperglycemic emergency in PwD and is associated with an absolute or relative insulin deficiency. Based on data from the Centers for Disease Control and Prevention, the incidence of DKA in adults increased from 19.5 per 1,000 person-years in 2000–2009 to 30.2 per 1,000 person-years between 2009 and 2014. 89 In the US, the cost of a single DKA admission was $26,566 in 2014, which amounted to $5.1 billion in healthcare expenditure for the year. 90 Between 2006 and 2016, mean hospital charges for DKA in the US increased from $14,548 to $20,997, even as the length of stay decreased from 2.51 days to 2.28 days during the same period. 91 In adult cases of DKA, two-thirds occur in those with T1D while one-third occurs in those with type 2 diabetes (T2D).92,93 For those with new onset diabetes, the incidence of DKA in the US is 17.2–23.8%. 94 Worldwide, the leading risk factors for DKA in adults with established diabetes are infection and suboptimal engagement with treatment. 10 In the US, the leading risk factors for DKA in established PwD include suboptimal engagement with treatment, infection, adolescent age group, female gender, minority race or ethnicity status, multiple daily insulin injection (MDI) therapy, and use of SGLT2 inhibitors. 10 The prevalence of DKA in adults with T1D in North America (US and Canada) and parts of Europe (Sweden, Germany, and Austria) is presented in Figure 4. In children, DKA most commonly occurs at the time of a diabetes diagnosis and the incidence is between 13% and 80%, varying based on the prevalence of diabetes in the population.95-97 Children under the age of 5 years and those who have difficulty accessing medical care have the highest rates of DKA at diabetes onset.98-100 In children with established diabetes, risk factors for DKA are insulin omission, poor diabetes control, a prior episode of DKA, minority race or ethnicity status, psychosocial challenges, adolescence, limited access to medical care, and infections. 101 There has been a significant increase in DKA in children presenting with new onset of T1D during the COVID-19 pandemic period. 102
Figure 4.
Prevalence of DKA in adults with T1D by country: (a) Overall prevalence of DKA in adults with T1D, (b) Prevalence in specific subgroups of adults with T1D.
Abbreviation: HbA1c, glycosylated hemoglobin; yrs, years.
*CGM non-user.
†Calculated value based on data contained within publication. 94
‡CGM user.
§Overall study population.
||Definite T1D.
Figure reproduced from Fazeli Farsani et al. 94 under the Creative Commons Attribution 4.0 International License.
Fortunately, mortality rates for DKA are low. In developed nations, the mortality rate is less than 1% for children and adults, but in developing nations, the mortality rate increases to 3–13% in children89,93,103 and up to 30–40% in adults. 10 DKA is the leading cause of mortality in children with diabetes and in adults with diabetes over the age of 58 years. 104 In adults who are over the age of 60 years, mortality rates are higher with a more severe precipitating illness. 10
DKA is a relatively common, largely preventable, complication of diabetes. While the mortality rates are low in the developed world, preventing and recognizing DKA can potentially decrease health care spending for PwD worldwide.
Conclusions
In established cases of type 1 diabetes, diabetic ketoacidosis is often precipitated by behavioral and social factors.
Increased awareness among healthcare professionals and the general population of the signs and symptoms of diabetes can potentially decrease the incidence of diabetic ketoacidosis.
In people with established diabetes, suboptimal engagement with diabetes management and lack of awareness of this impending complication are associated with an increased incidence of diabetic ketoacidosis. Personalizing diabetes management to increase adherence to prescribed insulin therapy as well as monitor pertinent metabolic analytes, such as ketones, could decrease the incidence of diabetic ketoacidosis in those with established diabetes.
Atypical Hyperketonemia
Co-Chair: Jennifer L. Sherr, MD, PhD
Yale University, New Haven, Connecticut, USA
Co-Chair: Guillermo E. Umpierrez, MD, CDE, FACP, FACE
Emory University, Atlanta, Georgia, USA
Current Status
Alcoholic ketoacidosis presents with a high anion gap metabolic acidosis in the setting of hyperketonemia but without significant hyperglycemia (unlike in diabetic ketoacidosis).
An increased risk of diabetic ketoacidosis in type 1 diabetes has been observed with the use of sodium-glucose co-transporter-2 inhibitors. Applications to approve members of this drug class for treating type 1 diabetes have not received regulatory approval in the United States but have received such approval in Europe.
There is evidence of fetal harm in diabetic ketoacidosis during pregnancy, but data evaluating the association between elevated maternal ketone concentra-tions and low childhood intelligence quotient is conflicting.
Discussion
While DKA is the most commonly encountered form of ketoacidosis, it is critical to also understand situations where atypical hyperketonemia may arise. This phenomenon can be seen in a few situations including alcoholic ketoacidosis (AKA), SGLT2-associated DKA, and physiologic ketosis seen during pregnancy.
AKA is a clinical syndrome seen in chronic alcoholics who binge drink. For those who are not chronic drinkers, binge drinking rarely leads to AKA. The hallmark of AKA is presentation with a high anion gap metabolic acidosis in the setting of hyperketonemia without significant hyperglycemia. Resolution of ketoacidosis has been studied in those with DKA and AKA by Umpierrez and colleagues. They demonstrated that DKA presents with hyperglycemia that resolves with insulin therapy. 105 Furthermore, they found that people with DKA, compared to those with AKA, presented with lower venous pH levels and experienced faster resolution of ketosis. Insulin levels remained flat in those with AKA because no insulin deficiency is present, while elevated levels of counterregulatory hormones including glucagon, cortisol, growth hormone and epinephrine were similar in both types of ketoacidosis and normalized over time. 105 Thus, detection of AKA will primarily be through a history of chronic alcohol use in a setting of ketoacidosis without concomitant hyperglycemia at presentation.
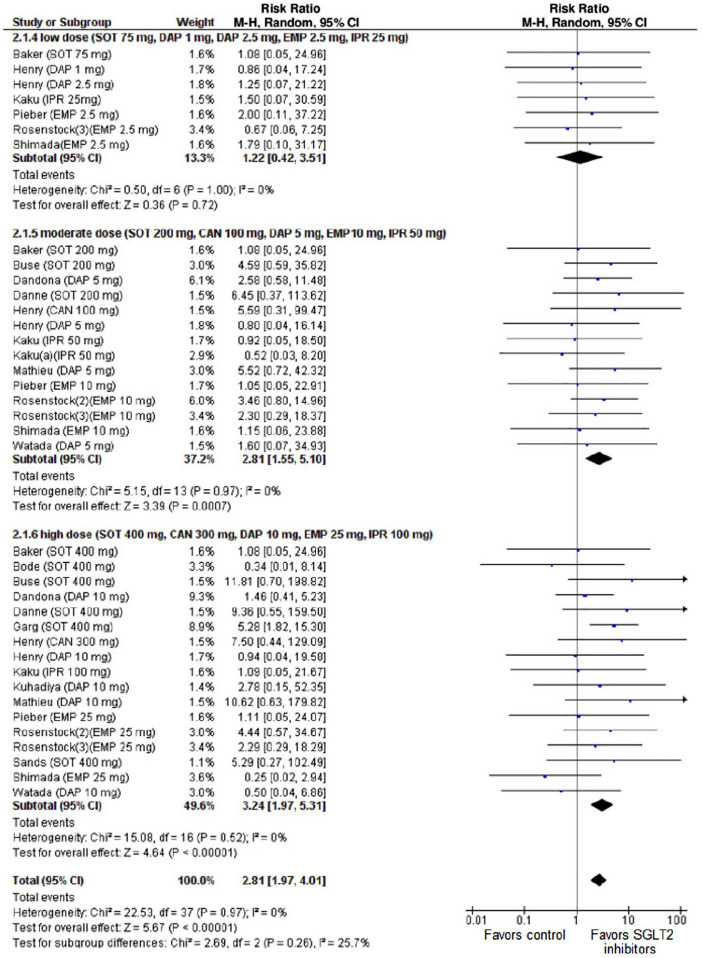
Since 2014, when the US Food and Drug Administration (FDA) released a warning regarding development of DKA with use of SGLT2 inhibitors, the body of literature on this topic has rapidly grown with 398 publications identified on PubMed as of July 19, 2021. 106 Early studies of canagliflozin identified this issue noting ketone-related adverse events in nearly 10% participants on high dose (300 mg) and 5% of participants on low dose (100 mg) therapy. 107 A consistent picture has emerged demonstrating that the risk of DKA is 3–5-fold higher with use of SGLT2 inhibitor therapy. Importantly, a metanalysis by Musso et al. 108 demonstrated a dose dependent relationship with the risk of DKA, presented in Figure 5.
Figure 5.
Forest plot comparison of the dose dependent relationship between SGLT2 inhibitor use and the incidence of DKA. Compared with placebo, SGLT2 inhibitors were associated with an increased risk of DKA (RR 2.81, 95% CI: 1.97 to 4.01, P < .001). 38 comparisons were made of 7,396 participants; trial durations ranged from 1 to 52 weeks. Figure reproduced from Musso et al 108 under the Creative Commons Attribution 4.0 International License.
This risk of DKA has influenced the FDA’s decision to not approve these agents for people with T1D. In contrast, the European Medicines Agency has approved this therapy for people with T1D who have a BMI (body mass index) greater than 27 kg/m2. An international consensus panel on risk management of DKA in patients with T1D treated with SGLT2 inhibitors recommended “self-measurement of capillary blood ketones, specifically BHB as a matter of routine in assessing the metabolic state of patients with T1D treated with SGLT2 inhibitors.” 109 Protocols for rigorous ketone monitoring and treatment of ketosis may aid eventual integration of these therapies into the treatment regimen for people with T1D. 110
Finally, it is well known that ketosis occurs quite readily during normal pregnancy, especially in the morning while fasting. During pregnancy, levels of ketones are 2–3-fold higher following a fasting period and nearly 30% of pregnant women have urinary ketones when assessed. Ketone bodies cross the placenta, and this has been concerning because animal experiments have shown high levels of ketones are associated with suboptimal maternal and neonatal outcomes. Indeed, mouse studies have shown a tendency for macrosomia as well as decreased brain volume and increased heart volume in response to exposure to ketosis as a fetus. 111 Recognizing that low carbohydrate diets have recently become more popular, a question remains as to whether these mouse findings hold true in humans. To date, the picture has been unclear in studies conducted to explore the relationship between maternal ketones in women with diabetes during pregnancy and subsequent measures of childhood intelligence. In non-DKA-associated ketosis, there does not appear to be an impact on development in babies or intelligence quotient in children. However, further studies on ketosis in pregnancy are warranted.
With these atypical hyperketonemia scenarios, it is important to understand the unique pathophysiologic mechanisms leading to their development. While there may be little benefit to more regular ketone monitoring in AKA, the story with SGLT2 inhibitor use and pregnancy is quite different. Clinicians should be aware of assessing for AKA when individuals present with ketosis and a high anion gap metabolic acidosis that is not associated with hyperglycemia. Determination of alcohol use will also aid diagnosis of this condition. SGLT2 inhibitors are currently used as approved agents in the clinical care of T1D in countries where these agents have gained regulatory approval. They are also used off-label by some PwD in other countries, including the US. Real-world observation studies regarding the frequency and evolution of ketosis will provide insight into the natural history of DKA in these populations. The use of monitoring tools, like CKMs, may allow for earlier detection of a potentially precarious situation. Additionally, because pregnancy makes women more prone to developing ketosis, an opportunity to better understand how ketosis impacts the developing fetus could come from measuring ketone concentrations during pregnancy and following long-term outcomes in the children of these women. With the current popularity of lower carbohydrate diets, it is likely that even more women will experience ketosis during their pregnancies.
Conclusions
Access to β-hydroxybutyrate-based ketone monitoring, should be assured for all insulin-requiring people with diabetes.
Education regarding when and how to interpret ketone levels should be included at each follow up visit for diabetes as well as for women who are pregnant, those on very low carbohydrate diets, those at risk for alcoholic ketoacidosis, those using adjunctive sodium-glucose co-transporter-2 inhibitor therapy, and those with prolonged fasting periods.
Development of more refined tools, such as continuous ketone sensors, would help facilitate earlier detection of ketones, such as could occur with failed infusion sets or may aide with integration of sodium-glucose co-transporter-2 inhibitors as an adjunctive therapy for type 1 diabetes.
Prevention of Diabetic Ketoacidosis
Co-Chair: Lori M. Laffel, MD, MPH
Joslin Diabetes Center, Boston, Massachusetts, USA
Co-Chair: Jane Jeffrie Seley, DNP, MPH, MSN, GNP, BC-ADM, CDCES, CDTC, FADCES
Weill Cornell Medicine, New York, New York, USA
Current Status
β-hydroxybutyrate monitoring can reduce progression of hyperketonemia to diabetic ketoacidosis.
Patient populations at higher risk of diabetic ketoacidosis based on demographics include children, adolescents, seniors, and pregnant women.
Patient populations at higher risk based on diabetes regimen include use of insulin pumps, automated insulin delivery systems, multiple daily insulin injection therapy, sodium-glucose co-transporter-2 inhibitor therapy, pembrolizumab, ketogenic diets, and intermittent fasting.
Discussion
Diabetic ketoacidosis risk factors
Although the risk of DKA is very real for some PwD, they may lack the skills and knowledge needed to identify, treat, and hopefully prevent DKA. Empowering PwD who are at higher risk of DKA to be aware of their individual risk factors, recognize the signs and symptoms, and take immediate steps to mitigate the onset and progression of DKA is a key component of diabetes self-care education and support. While 20% of DKA cases occur at the time of T1D diagnosis and an additional 20% occur from precipitating factors, the majority of cases are due to mistakes in diabetes management. While DKA is generally more prevalent in those with T1D, there are times in a person’s lifespan when their risk of developing DKA is higher, especially during childhood, adolescence, and pregnancy. 95 PwD wearing insulin pumps or AID systems should be aware of their overall increased risk, especially if they have an infusion set malfunction. 112 Increased risk when following popular dietary practices such as intermittent fasting and ketogenic diets, and taking SGLT2 inhibitors should be discussed. The monoclonal antibody pembrolizumab, used for advanced cancer, binds to the programmed cell death 1 (PD-1) receptors on T-cells and activates the immune system to attack tumor cells, but in a handful of cases this drug has been reported to precipitate DKA. 9 Possible additional triggers also include omitting insulin (intentionally or in error), eating disorders, emotional stress, infections, surgery, and drugs such as corticosteroids, alcohol, and cocaine.
Sick-day guidelines
Basic principles of sick day management include monitoring glucose and checking ketone concentrations when the glucose level is above 250 mg/dL. Patients should be instructed to take supplemental insulin every 3–4 hours based on these results as well as to maintain hydration. In reality, many at-risk patients do not have ketone testing supplies available at home or elsewhere. A conversation about when and how to check ketones may not be addressed adequately enough or at all during clinician visits to motivate the patient to obtain supplies. An important question is whether we should recommend urine ketone or blood ketone testing. Urine tests measure AcAc and are subject to interference from medications and variability when reading the color change on the test strip. These assays may not reliably identify the onset and resolution of ketosis as rapidly as those that measure BHB concentrations in the blood, since AcAc and acetone concentrations can increase as BHB concentrations decrease during DKA treatment. Blood ketone meters that check BHB from capillary blood are preferred over urine testing for monitoring ketones during illness and hyperglycemia because they provide earlier detection of the presence of ketones and are not subject to misreading of the color change in urine ketone strips. Although the urine testing method is much more affordable, it may not provide the timely information needed to identify or prevent DKA. In a 2006 prospective, randomized study by Laffel et al. 13 comparing blood ketone testing vs urine ketone testing in children and young adults with T1D, the blood ketone group, compared to the urine ketone group, had 49% fewer events requiring emergency assessment, treatment, and hospitalization, as shown in Figure 6.
Figure 6.
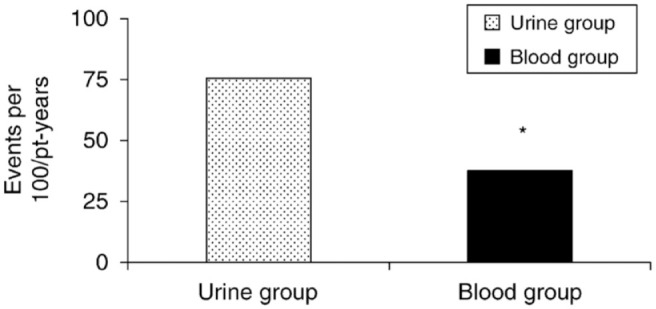
Incidence of acute events (emergency room use and hospitalizations) during 6-month follow-up in a prospective, randomized study by Laffel et al comparing patients in a urine ketone testing group vs. a blood ketone testing group. The blood ketone group, compared to the urine ketone group, had a significantly lower rate of acute complications: 38 per 100 patient-years (blood ketones) vs. 75 per 100 patient-years (urine ketones) (*P = .05). Compared with the urine ketone group, the blood ketone group had 49% fewer events requiring emergency assessment, treatment, and hospitalization. Figure reproduced from Laffel et al. 13
Challenges with sick-day management education
Patients may have difficulty retaining sick day management education since they are often not sick when they learn about it. Additionally, the notion of taking extra insulin when you do not feel like eating may seem counterintuitive to many. It is also true that hyperglycemia, compared to hypoglycemia, does not elicit the same level of concern, leading to further delays in responding. Clinicians should make a point of discussing sick-day management in detail at least annually and assess patient knowledge and availability of ketone testing supplies at every visit. Wearable continuous ketone monitoring technology is currently in development, and such devices might provide additional safety as an early warning system for our patients at high risk for DKA. Guidelines for treatment will be needed as to what to do with standalone continuous ketone information.
Conclusions
People with diabetes who are at risk for diabetic ketoacidosis should be made aware of their individual risk factors as well as key prevention and treatment strategies such as insulin, fluid replacement, and ketone testing.
Sick day management should be discussed in detail with people with diabetes at risk for diabetic ketoacidosis at least annually with reminders at subsequent visits.
Education should include the importance of ketone testing including when and how to perform the test, the differences between urine and blood/interstitial fluid testing for ketones, and the appropriate responses to ketone results.
Non-Diabetic Ketoacidosis States of Fasting Ketonemia and Ketonuria
Co-Chair: Kong Y. Chen, PhD, MSCI
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland, USA
Co-Chair: David Kerr, MBChB, DM, FRCPE, FRCP
Sansum Diabetes Research Institute, Santa Barbara, California, USA
Current Status
Measurement of ketones can be useful in settings of ketosis outside of diabetes.
Starvation ketosis occurs as daily calorie intake reduces below 500 kcal/day.
Measurement of ketones can be made with ketogenic diets.
Discussion
For most clinicians with an interest in diabetes, ketone bodies are usually thought of as noxious, toxic, spillover agents causing DKA. 113 However, nutritional ketosis, characterized by lower ketone body concentrations than those seen in DKA, has been used for treating epilepsy in children, infantile spasms, glycogen storage diseases, and other rare metabolic diseases. 114 More recently, BHB therapy has been purported to have clinical benefits in a wide variety of conditions ranging from migraine, stroke, dementia, cancer, and heart failure, as well as enhancing exercise performance. From a cardio-metabolic perspective, there is also growing interest in using BHB for the management of severe obesity and its associated metabolic diseases/conditions such as dyslipidemia, insulin resistance, T2D, inflammation, and nonalcoholic fatty liver disease. 115
In healthy individuals, BHB levels are 0–0.3 mmol/L. With absolute starvation, this can rise to 5–10 mmol/L without severe acidosis due to adaptation by muscle and the kidneys.8,10 Nutritional ketosis or a ketogenic diet is generally characterized by raising blood ketone levels (especially BHB) above the normal baseline of 0.3 mmol/L through either prolonged fasting or adherence to low carbohydrate intakes (<30 g/d), which force the body to switch to metabolizing fats as fuels. An example of BHB concentrations of subjects in a very low carbohydrate diet program is presented in Figure 7. Initial weight loss from ketogenic diets can be rapid, with or without metabolic comorbidities such as T2D, which is appealing. The evidence of long-term weight loss from ketogenic diets is scarce, mostly because of the difficulty in maintaining a strict low carbohydrate diet over time and challenges related to obtaining repeated blood ketone measurements outside of laboratory settings. There is limited evidence that individuals with T2D achieving nutritional ketosis can have measurable clinical benefits for up to two years, although this requires regular professional support. 116 With sufficient accuracy and precision in the range typically targeted by nutritional ketosis (0–5 mmol/L), a CKM would potentially be helpful to provide data with high time-resolutions (once every 5–30 minutes) to help researchers understand the physiology of ketone metabolism, explore mechanisms of benefits from ketogenic diets, and identify early signs of risks all in free-living environments. It is highly likely that, even though the initial goal developing a CKM is for monitoring the risks of DKA, its potential target applications could eventually be widened to the fields of personalized nutrition, weight loss, exercise, behavioral modifications, and prediabetes monitoring.
Figure 7.
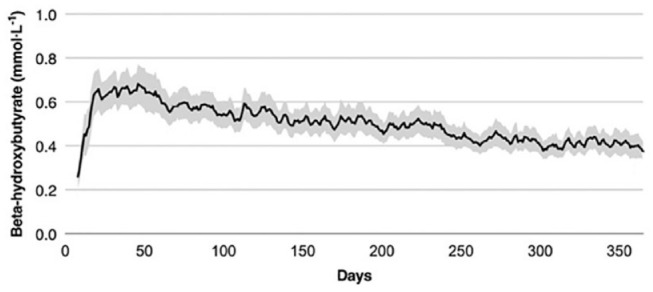
BHB concentrations of subjects in a very low carbohydrate diet program after one year. 349 subjects with T2D enrolled and after one year, 218 of them remained enrolled. Each day, participating subjects consumed 30 g of carbohydrates, 1.5 g/kg of protein, and fat ad lib for satiety. Mean BHB concentrations in the blood increased from baseline 0.18 mmol/L to 0.31 mmol/L at one year and remained elevated at 0.27 mmol/L at 2 years (BHB concentration after one year is not shown in the figure). 62% of subjects uploaded a BHB concentration above 0.5 mmol/L at least once. Figure modified from Hallberg et al 117 under the Creative Commons Attribution 4.0 International License.
Conclusions
Ketosis can be a result of prolonged fasting or low-carbohydrate diets.
Controlled mild ketosis by nutritional means may be beneficial for certain disease management such as epilepsy.
An accurate and precise continuous ketone monitor for measuring ketones levels would be a useful tool for current research in ketogenic diets and poten-tial future clinical applications beyond diabetic ketoacidosis.
Potential Integration of Continuous Ketone Monitors with Pumps and Automated Insulin Delivery Systems to Prevent DKA
Co-Chair: L. Kurt Midyett, MD
Midwest Pediatric Specialists, Overland Parks, Kansas, USA
Co-Chair: Joshua D. Miller, MD, MPH
Stony Brook University, Stony Brook, New York, USA
Current Status
A continuous ketone monitor integrated into an automated insulin delivery system could potentially provide useful information to improve the insulin dosing algorithm and lead to better outcomes, especially in the event of an infusion set blockage.
It remains to be determined whether a patient would use continuous ketone monitor data to inform manual insulin bolusing with their automated insulin delivery system (similar to the use of continuous glucose monitor data to inform manual correction bolus dosing with a hybrid automated insulin delivery system) or whether their continuous ketone monitor data would automatically become integrated into the dosing software of the automated insulin delivery system and if so then whether the patient could set a target range or target threshold for ketonemia.
Incorporation of continuous ketone monitoring into continuous subcutaneous insulin infusion (CSII) therapy must account for the impact on a patient’s quality of life in addition to any current burden of using a subcutaneous insulin infusion system.
Discussion
Despite advancements in technology, DKA remains a significant morbidity issue especially in children and young adults. One of the primary causes of DKA in these age groups is inadequate insulin delivery. 118 AID systems have the option to more dynamically adjust insulin dosing and even give bolus adjustments, which can help to minimize missed boluses that could possibly lead to DKA. An individual using an AID system can fail to receive insulin because of a problem with their insulin pump or infusion set. Missed bolus insulin doses or failure to receive basal insulin can result in DKA. A common cause of unintentionally missed insulin in patients with T1D using an AID system is pump infusion site malfunction. Studies have shown that BHB levels rise faster than glucose levels in patients that have an interruption in their insulin delivery by CSII.119,120 A PwD on an AID system might notice that their glucose levels are rising but would typically be unaware of the rapidly rising ketone concentrations. If the PwD used an AID system focused only on glucose levels, compared to focusing on both glucose and ketone concentrations, then treatment of ketosis might be delayed. These types of scenarios indicate that a CKM could potentially provide benefit to those patients on AID systems and help to prevent DKA by alerting the individual or their family members and allow them to intervene early enough to reverse the ketosis. Scenarios where addition of CKM data might enhance the value of using an AID system are presented in Table 4. 121 A PwD using open loop insulin pump control with a CGM and a CKM would be able to manually increase their basal or bolus insulin doses beyond their requirements for controlling glucose concentrations if they received an alert that they had an elevated ketone concentration. The extra insulin could be sufficient to prevent the development of DKA.
Table 4.
Scenarios Where Addition of CKM Data Might Enhance the Value of Using an AID System.
| 1 | Failure of insulin delivery by the AID |
| 2 | Inadequate bolus dosing |
| 3 | Recurrent DKA |
| 4 | Sick days |
| 5 | Stress |
| 6 | Reduced carbohydrate intake |
| 7 | Fasting |
| 8 | High intensity exercise |
| 9 | SGLT2 inhibitor therapy |
| 10 | Pregnancy |
| 11 | Heavy alcohol intake |
| 12 | Prolonged sedentary state |
| 13 | Social isolation |
However, successful integration of a continuous ketone monitoring device into an integrated insulin delivery system needs to consider the potential burden of this therapy on the PwD. Along with the possibility for transformative care, pump therapy includes a potential (and sometimes substantial) burden to the patient. Often underacknowledged challenges for PwD are the intricacies of device wearability in daily life with diabetes. Limited “real estate” for placing ketone and glucose sensors (if they cannot be combined into a single device) could possibly sway patients in their willingness to adopt CKMs into their daily pump life. Alarm fatigue is a real phenomenon. 122 PwD on pump therapy fall along a wide and diverse phenotypic spectrum. Adding an additional monitoring device onto an AID system requires a PwD to have a clear understanding of how a CKM should be integrated into the system to allow flexibility for adjusting and modifying the alert/alarm interface. This would be important to prevent the known issues with alarm fatigue.
The integration of CKMs into CSII therapies will require a different conceptualization from CGMs. Continuous glucose monitoring is a dynamic process and minute-to-minute changes in glucose levels are important to provide data to AID systems and their users. Ketone monitoring might not require that same degree of immediate data processing, but its output could potentially be reported to the user based on thresholds rather than rate of change, however the availability of a trend arrow in a CKM would be an attractive feature. A CKM might provide an option to simply work in the background and it could be programmed to alert only if a specific threshold of BHB has been crossed. This approach could provide adequate safety measures without overwhelming the user or their family with alerts or alarms.
An issue to consider with the possible addition of continuous ketone monitoring into an AID system is whether the CKM integration is intended for the patient or the pump. With a hybrid AID system, a PwD might be authorized to use information provided by a CKM to inform an occasional manual correction insulin bolus for an elevated ketone concentration similar to how CGM data can inform an occasional manual correction insulin bolus for an elevated glucose concentration. However, the software might be designed to automatically incorporate ketone data into its insulin dosing regimen, such that a manual correction bolus for an elevated ketone concentration might be a form of stacking and could result in hypoglycemia. With automatic control in response to the ketone concentration, it remains to be determined whether a PwD could set their own target range or target threshold for ketonemia.
Regarding the potential benefits of integrating CKM data into AID systems, it is currently unknown whether there is a need or benefit to utilize CKM data with AID systems to inform manual correction boluses for elevated ketone concentrations. Similarly, it is not known whether data from a CKM could facilitate automated adjustments to insulin delivery that could improve safety or effectiveness outcomes for the end user without overburdening them. Just as the development of continuous glucose monitoring sensors elevated the capabilities of inulin pumps to improve glycemic control, it is possible that continuous ketone monitoring sensors will further elevate these capabilities of current AID systems.
Conclusions
Successful integration of a continuous ketone monitor into an automated insulin delivery system, will require development of new treatment algorithms based on ketone data. Additionally, there will need to be agreement on how to apply continuous ketone monitor data with subcutaneous multiple daily insulin injection dosing to best avoid diabetes ketoacidosis and optimize glycemia.
The least burdensome form of integration of a continuous ketone monitor with a sensor augmented pump or an automated insulin delivery system would be to automatically process both continuous glucose monitor and continuous ketone monitor data together and integrate therapy based on both data streams. However, there could be an opportunity for manual insulin correction bolusing in response to elevated ketone concentrations.
Ketone-specific alarms for elevated ketone levels and trend arrow information (or rate of change) will be attractive features of a continuous ketone monitor integrated with a pump or an automated insulin delivery system.
Clinical Trials of Continuous Ketone Monitors
Co-Chair: Nestoras Mathioudakis, MD, MHS
Johns Hopkins University, Baltimore, Maryland, USA
Co-Chair: Francisco J. Pasquel, MD, MPH
Emory University, Atlanta, Georgia, USA
Current Status
Prospective studies are needed to determine the feasibility, acceptability, usability, efficacy, and safety of continuous ketone monitoring in patients at risk for diabetic ketoacidosis.
Further studies are also needed to evaluate the effectiveness of insulin dosing protocols in adults during sick days in the outpatient setting for managing elevated ketone concentrations.
Clinical trials focused on outcomes will be needed to change payer policies. These trials will focus on both free-living outpatients and hospital inpatients and will include economic analyses.
Discussion
Based on the experiences gained with CGMs over the past two decades, there is now an opportunity to design clinical trials for CKMs following a similar development framework. Trials will be needed to determine the feasibility, acceptability, usability, efficacy, and safety of CKMs in diverse populations at risk for ketoacidosis. Endpoints of interest are presented in Table 5.
Table 5.
Endpoints of Interest in CKM Trials (Numbers 2–7 Would be Randomized Controlled Trials).
| Endpoints of interest in CKM trials | |
|---|---|
| 1 | Analytical accuracy (bias and precision) in four ketone concentrations ranges: highest range (DKA), mid-range (impending DKA), lower range (diet-induced ketosis), and lowest range (healthy controls) |
| 2 | Frequency of DKA episodes in high-risk individuals (T1D and insulin-treated T2D, use of sodium-glucose co-transporter-1 and 2 inhibitors, high-risk patients following ketogenic diets) |
| 3 | Reduction in events leading to emergency department visits or hospitalizations |
| 4 | In-hospital use during DKA resolution (length of stay, time to transition from intravenous to subcutaneous insulin, readmission rate) |
| 5 | Incremental benefit of CKM added to CGM in various clinical situations (but for statistically significant results they might require being powered with an impractically large n if the anticipated incremental benefit is small) |
| 6 | In-hospital resource use and economic analysis |
| 7 | Weight loss assisted by a CKM for a ketogenic diet |
| 8 | Patient-reported outcomes/satisfaction/user experience/user interface |
Table 6 presents four suggested phases for CKM investigation in clinical studies from proof-of-concept through feasibility and safety through efficacy and effectiveness through post-marketing surveillance. Confirmation of CKM sensor accuracy during situations associated with dynamically changing ketone levels to determine clinical reliability would be important. Such studies should be designed in coordination with the FDA to determine the need for an investigational device exemption (IDE) in the conduct of clinical studies in controlled settings considering brief withholding of insulin ( phase 1 ). Confirmation of accuracy during acute events in patients presenting with DKA to the hospital can further expand knowledge about the reliability of continuous ketone monitoring during significant ketonemia. Evaluating the use of CKMs both along, with, and in place of capillary or urine ketone testing for patients at risk for DKA ( phases 2–3 ) would be of great interest to the diabetes community. After confirmation of the accuracy of CKM sensors, a clearer definition of normal ketone levels may be possible in patients with and without diabetes to determine ideal reference ranges and meaningful trend alarms. Such advances would then likely be applicable to multiple settings and patient populations (ie, ketogenic diets, pregnancy, epilepsy, SGLT-2 inhibitor use). In addition, these studies may also facilitate the development of standardized reports (Ambulatory Glucose Profile equivalent), clinical target ketonemia ranges, and guidelines for sick-day management in adult patients at risk for ketoacidosis, which is an understudied clinical scenario. Post-market surveillance ( phase 4 ) including cost-effectiveness analyses will be needed to determine the populations most likely to benefit from the technology, from a medical and a resource utilization perspective. Showing clear benefit will be important for policy changes that ensure reimbursement for those with the highest need. 19
Table 6.
Suggested Research Phases for Clinical Trial Development of Continuous Ketone Monitoring.
| Phase 0 | Phase 1 | Phase 2 | Phase 3 | Phase 4 | |
|---|---|---|---|---|---|
| Goal | Proof-of-concept | Feasibility/safety | Efficacy | Efficacy/Effectiveness | Post-Marketing Surveillance |
| Question | Does CKM do what it is expected to do in healthy people? | Does CKM do what it is expected to do in patients at risk for DKA? | Can CKM be used in place of capillary ketone testing for patients at risk for DKA? | What else do we need to know? | |
| Population | Healthy participants on low carbohydrate diets | At-risk for DKA | |||
| Sample size | 12 | 24–36 | 25–100 | 100–300 | – |
| Reference testing | Capillary ketone (8× per day) | Serum β-hydroxybutyrate (clinical research unit- withhold insulin) | – | – | – |
| Comparator group | – | – | Capillary ketone (8× per day) during sick days | – | |
Three types of trial designs can be used to test the efficacy and effectiveness of CKMs. First are randomized controlled trials (RCTs) to assess efficacy, which will be useful in assessing most of the endpoints presented in Table 5. These can be conducted with blinded CKM sensors in the control group or with a crossover design. Second are pragmatic real-world trials, which compared to RCTs impose less control over the test subjects’ experience, to assess the effectiveness of the intervention. Third are real world observational studies, which are non-interventional and can be performed from a review of records, to assess the effectiveness of the intervention. 123
Conclusions
Endpoints of interest in assessing continuous ketone monitors can include measures of (1) analytical accuracy and (2) clinical performance defined both as efficacy and effectiveness.
Patient-reported outcomes and user experience/user interface are dimensions of performance that must be measured and maximized to enhance product uptake.
Determination of overall resource utilization compared to incremental clinical benefit will be critically important in determining how widely new continuous ketone monitor technology will be adopted.
The Future of Continuous Ketone Monitors
Co-Chair: David C. Klonoff, MD, FACP, FRCP (Edin), Fellow AIMBE
Mills-Peninsula Medical Center, San Mateo, California, USA
Co-Chair: Elias K. Spanakis, MD
University of Maryland, Baltimore, Maryland, USA
Current Status
Future studies will be needed to demonstrate that continuous ketone monitoring systems provide accurate information and that their use leads to better clinical outcomes associated with predicting and preventing metabolic decompensation in type 1 diabetes.
In the future, continuous ketone data will be used by patients, patient caregivers, and healthcare professionals, through real time wireless transmission to designated parties and through incorporation in the electronic health record.
In the future, if low carbohydrate diets become more widely prescribed for type 2 diabetes, obesity, seizures, dementia, and other diseases, then use of continuous ketone monitors will become part of those regimens.
Discussion
Future continuous ketone monitoring devices will be found beneficial in many different diabetes populations, who are at high risk for DKA. These populations include those with a history of multiple episodes of DKA, those with T1D and high hemoglobin A1c concentrations, and those with T2D on MDI therapy during metabolic stress. Pediatric patients and older individuals may especially benefit more from continuous ketone monitoring systems because these individuals require closer monitoring and are at higher risk of developing DKA. Future CKMs could be utilized as protective systems to prevent episodes of DKA in patients with CSII because these systems frequently experience system failures and stop delivering insulin. Common causes of occlusion of CSII systems, which can block insulin delivery and predispose to DKA, are presented in Table 7. 124 CKMs could be utilized in diabetes patients who require SGLT2 inhibitor therapy because of their cardiovascular and renal benefits, but who are not able to use them because of concerns with developing DKA. In addition to use by PwD, CKMs can be used by patients who experience non-diabetes related ketoacidosis (ie, alcoholic or low carbohydrate induced ketosis or in pregnant patients). In the inpatient setting, real-time continuous ketone monitoring may help both in categorizing cases of DKA based on their severity as well as managing these patients. Continuous ketone monitoring could potentially be utilized to monitor high-risk hospitalized patients intraoperatively or guide treatment decisions of DKA in the intensive care units.
Table 7.
Common Causes of Occlusion of Continuous Subcutaneous Insulin Infusion Systems. 125
| Insulin fibrils precipitating in the tubing or cannula |
| Kinking of the cannula |
| Kinked insulin pump tubing |
| Air bubbles in the tubing |
| Compression of the skin around the infusion site |
| Displacement of the insulin infusion set post-insertion |
| Incorrect insertion of the insulin infusion set |
Prior to developing future continuous ketone monitoring systems, industry should seek feedback not only from healthcare professionals but also from individual PwD, who will be using these systems and can provide guidance for how future CKMs should function. 125 Future continuous ketone monitoring system manufacturers should take into consideration the burden that these devices may cause in PwD. Many of these individuals wear multiple devices, such as CSII devices or CGMs, and adding another device like a CKM could perhaps be difficult.
Researchers will need to define the role of continuous ketone monitoring systems. These devices will need to be tested in order to ensure that they will be accurate and will not be subject to interference by common medications and substances. A challenge for CKM developers will be accurately calibrating a ketone sensor when a wearer not in DKA at the time might have an interstitial fluid ketone concentration of 0 mM. A measurement at 0 mM is effectively a blank and it is still necessary for a linear measurement to test another point where the concentration of analyte is not zero. All subsequent measurements can then subtract the 0-point measurement as background. This calibration process is different from calibrating a glucose sensor because a typical random glucose level has a lot of room both below and above. Data on how rapidly ketone levels are changing in blood is also needed so that ketone sensor lag can be quantified. Finally, clinical studies are needed that will show that CKM systems can lead to better clinical outcomes, a decreased incidence of DKA, and decreased hospitalizations and emergency department (ED) visits.
CKMs will need to be incorporated into the healthcare ecosystem. CKMs can be integrated with other hardware systems, such as continuous glucose monitoring and CSII devices in a common sensor platform, as well as with other sensor readouts in a multi-sensor software dashboard. This integration of hardware and software can lead to the development of automated sophisticated algorithms, which can initiate corrective actions to treat ketonemia. Embedding alarms is an important feature which can notify users of a need to prevent impending DKA and therefore ED visits and/or hospitalizations, but considerable efforts should be made to decrease the burden that frequent alarms may cause in PwD. The CKM data and the alarms should not only be available to patients but should also be transmitted wirelessly to their family members, their healthcare professional team, and the electronic health record to support more effective use of the data. These devices should be able to predict an impending increase in ketone levels and support the initiation of appropriate treatment.
As for the future adoption of CKMs, these devices will need to be financially affordable for PwD. After they are fully developed, healthcare professionals and patients will all require education about the proper use of CKMs and appropriate ways to respond to CKM data. As for any medical device, future CKMs should be built as ecologically friendly as possible to reduce medical waste, with consideration of sustainability throughout these products’ lifecycles. CKM manufacturers should be able to demonstrate that CKM data will be secure by utilizing secure-by-design engineering with sound cybersecurity systems that are “built in” rather than “bolted on.” Achieving all these accomplishments will ensure a bright future for CKMs as a key technology in the management of T1D and other metabolic disorders.
Conclusions
In the future, wearable continuous ketone monitoring sensors may become part of wearable multisensor systems capable of measuring multiple analytes for people with type 1 diabetes to detect impending diabetic ketoacidosis and prevent emergency department visits and hospitalizations for diabetic ketoacidosis.
Future continuous ketone monitoring systems should integrate with continuous glucose monitoring systems and support a dashboard to read continuous ketone monitor data along with other automatically collected continuous wearable sensor data.
Continuous ketone monitoring systems will need to be accurate, safe, effective, affordable, and cybersecure to be widely adopted.
Conclusion
This consensus report for the use of CKMs was created to provide guidance to clinicians, researchers, regulators, and payers for understanding the technical and clinical performance of this novel type of sensor in the prevention of DKA in people with T1D and the management of metabolic conditions associated with ketogenesis. Through a consensus process, an expert panel voted on 30 conclusions. All 30 conclusions were agreed upon unanimously by the panel members. The consensus panel’s conclusions are all compiled in Table 8. The panel’s conclusions are intended to support a better understanding of (1) the physiology of ketones, (2) the pathophysiology of DKA, (3) the association of ketogenesis with insulin deficiency, starvation, and use of triggering ketogenic agents, and (4) the use of continuous automatic ketone monitoring to prevent and manage patients who develop the dangerous condition of DKA associated with severe ketonemia and less dangerous conditions associated with mild ketonemia. The authors hope that the conclusions in this report will contribute to development of better technologies and better treatments for PwD.
Table 8.
Thirty Continuous Ketone Monitoring Consensus Panel Conclusions.
| Physiology of ketone production | |
| 1 | Ketone bodies are a type of fuel substrate produced by the liver upon oxidation of fatty acids that flux from adipose tissues where they are liberated by fasting or starvation, carbohydrate-deficient “ketogenic” diets, and in response to severe insulin deficiency as seen in diabetic ketoacidosis. |
| 2 | An elevated glucagon/low insulin state can promote ketogenesis by increasing β-oxidation of free fatty acids in the liver and lipolysis in adipocytes, whereas insulin inhibits ketogenesis, mainly through suppression of lipolysis by adipocytes and reduction of the supply of free fatty acids. |
| 3 | In diabetic ketoacidosis, an increase in counter-regulatory hormones and lack of insulin can promote ketogenesis. |
| Measurement of ketones | |
| 4 | Urine ketone tests based on nitroprusside may provide misleading clinical information, underestimating total body ketone concentrations early in diabetic ketoacidosis and overestimating total body ketone concentrations during resolution of diabetic ketoacidosis. They should only be used as an adjunct to diagnose diabetic ketoacidosis and not to monitor diabetic ketoacidosis treatment. |
| 5 | Blood β-hydroxybutyrate measurements provide more clinically useful information than methods that measure urine acetoacetate or acetone levels. β-hydroxybutyrate levels directly track with treatment progression, unlike urine nitroprusside tests. |
| 6 | Breath ketone analyzers are new to the market and their performance in self-testing and clinical practice has not been thoroughly evaluated. |
| Performance of the first CKM reported to be used in human trials | |
| 7 | A continuous ketone monitor has been reported to measure β-hydroxybutyrate in vitro and in vivo in interstitial fluid with a linear response over the 0–8 mM range and with good accuracy. |
| 8 | Future studies of this sensor will be required to assess performance in intended patient populations across the clinical range of circulating ketone bodies. Addition of prospective factory calibration will support real time measurements. |
| 9 | For diagnosis and treatment of diabetic ketoacidosis, this sensor’s clinical role and usability for patients and healthcare professionals remain to be determined. |
| Demographics and epidemiology of DKA | |
| 10 | In established cases of type 1 diabetes, diabetic ketoacidosis is often precipitated by behavioral and social factors. |
| 11 | Increased awareness among healthcare professionals and the general population of the signs and symptoms of diabetes can potentially decrease the incidence of diabetic ketoacidosis. |
| 12 | In people with established diabetes, suboptimal engagement with diabetes management and lack of awareness of this impending complication are associated with an increased incidence of diabetic ketoacidosis. Personalizing diabetes management to increase adherence to prescribed insulin therapy as well as monitor pertinent metabolic analytes, such as ketones, could decrease the incidence of diabetic ketoacidosis in those with established diabetes. |
| Atypical Hyperketonemia | |
| 13 | Access to β-hydroxybutyrate-based ketone monitoring, should be assured for all insulin-requiring people with diabetes. |
| 14 | Education regarding when and how to interpret ketone levels should be included at each follow up visit for diabetes as well as for women who are pregnant, those on very low carbohydrate diets, those at risk for alcoholic ketoacidosis, those using adjunctive sodium-glucose co-transporter-2 inhibitor therapy, and those with prolonged fasting periods. |
| 15 | Development of more refined tools, such as continuous ketone sensors, would help facilitate earlier detection of ketones, such as could occur with failed infusion sets or may aide with integration of sodium-glucose co-transporter-2 inhibitors as an adjunctive therapy for type 1 diabetes. |
| Prevention of DKA | |
| 16 | People with diabetes who are at risk for diabetic ketoacidosis should be made aware of their individual risk factors as well as key prevention and treatment strategies such as insulin, fluid replacement, and ketone testing. |
| 17 | Sick day management should be discussed in detail with people with diabetes at risk for diabetic ketoacidosis at least annually with reminders at subsequent visits. |
| 18 | Education should include the importance of ketone testing including when and how to perform the test, the differences between urine and blood/interstitial fluid testing for ketones, and the appropriate responses to ketone results. |
| Non-DKA states of fasting ketonemia and ketonuria | |
| 19 | Ketosis can be a result of prolonged fasting or low-carbohydrate diets. |
| 20 | Controlled mild ketosis by nutritional means may be beneficial for certain disease management such as epilepsy. |
| 21 | An accurate and precise continuous ketone monitor for measuring ketones levels would be a useful tool for current research in ketogenic diets and potential future clinical applications beyond diabetic ketoacidosis. |
| Potential integration of CKMs with pumps and AID systems to prevent DKA | |
| 22 | Successful integration of a continuous ketone monitor into an automated insulin delivery system, will require development of new treatment algorithms based on ketone data. Additionally, there will need to be agreement on how to apply continuous ketone monitor data with subcutaneous multiple daily insulin injection dosing to best avoid diabetes ketoacidosis and optimize glycemia. |
| 23 | The least burdensome form of integration of a continuous ketone monitor with a sensor augmented pump or an automated insulin delivery system would be to automatically process both continuous glucose monitor and continuous ketone monitor data together and integrate therapy based on both data streams. However, there could be an opportunity for manual insulin correction bolusing in response to elevated ketone concentrations. |
| 24 | Ketone-specific alarms for elevated ketone levels and trend arrow information (or rate of change) will be attractive features of a continuous ketone monitor integrated with a pump or an automated insulin delivery system. |
| Clinical trials of CKMs | |
| 25 | Endpoints of interest in assessing continuous ketone monitors can include measures of (1) analytical accuracy and (2) clinical performance defined both as efficacy and effectiveness. |
| 26 | Patient-reported outcomes and user experience/user interface are dimensions of performance that must be measured and maximized to enhance product uptake. |
| 27 | Determination of overall resource utilization compared to incremental clinical benefit will be critically important in determining how widely new continuous ketone monitor technology will be adopted. |
| The future of CKMs | |
| 28 | In the future, wearable continuous ketone monitoring sensors may become part of wearable multisensor systems capable of measuring multiple analytes for people with type 1 diabetes to detect impending diabetic ketoacidosis and prevent emergency department visits and hospitalizations for diabetic ketoacidosis. |
| 29 | Future continuous ketone monitoring systems should integrate with continuous glucose monitoring systems and support a dashboard to read continuous ketone monitor data along with other automatically collected continuous wearable sensor data. |
| 30 | Continuous ketone monitoring systems will need to be accurate, safe, effective, affordable, and cybersecure to be widely adopted. |
Acknowledgments
We thank Annamarie Sucher-Jones for her expert editorial assistance.
Footnotes
Abbreviations: AcAc, acetoacetate; AID, automated insulin delivery; AKA, alcoholic ketoacidosis; BHB, β-hydroxybutyrate; CGM, continuous glucose monitor; CKM, continuous ketone monitor; CSII, continuous subcutaneous insulin infusion; CV, coefficient of variation; DKA, diabetic ketoacidosis; DTS, Diabetes Technology Society; ED, emergency department; FDA, US Food and Drug Administration; FFA, free fatty acids; IDE, investigational device exemption; MAD, mean absolute difference; MARD, mean absolute relative difference; PwD, people with diabetes; RCT, randomized controlled trial; SGLT2, sodium-glucose co-transporter-2; T1D, type 1 diabetes; T2D, type 2 diabetes; US, United States;
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KTN, NYX, JYZ, TS, AB, KYC, NM, JJS, GEU, and PP have nothing relevant to disclose. RMB reports research support, has consulted, or has served on a scientific advisory board for Abbott Diabetes Care, Ascensia, CeQur Corporation, Dexcom, Hygieia, Insulet, Johnson & Johnson, Lilly, Medtronic, Novo Nordisk, Onduo, Roche, Sanofi, and United Healthcare. He receives funding for technology research from NIH/NIDDK. All contracts are with his employer’s non-profit institute, and he receives no direct personal income from these activities. KC receives grant support and supplies provided to her institution from Dexcom, grant support provided to her institution from Abbott Labs, Abbott Diabetes Care, Medtronic, Insulet and Novo Nordisk. DK has received honoraria for participation in Advisory Boards related to Digital health for Sanofi and Novo Nordisk, is in receipt of research funding from Novo Nordisk, and share options from Glooko. SKK receives grant support from the UCSF Nutrition and Obesity Research Center (NIH P30 DK098722) and is an advisor to Yes Health, Suggestic, and Signos. LML reports grant support to her institution from NIH, JDRF, Helmsley Charitable Trust, Eli Lilly and Company, Insulet, Dexcom, and Boehringer Ingelheim. LML is a consultant for Janssen, Insulet, Eli Lilly and Company, Novo Nordisk, Boehringer Ingelheim, Medtronic, Dompe, and Provention. LKM is a consultant for Abbott Diabetes Care. JDM is an advisor and speaker for Medtronic Diabetes and has received a grant from Dexcom, Inc. JHN is an advisor for Dexcom and has received speaker honoraria from Abbott Laboratories. His institution has received research support from Abbott Laboratories and Roche Diagnostics. FJP is partially supported by the National Institutes of Health under award numbers 1K23GM128221-01A3 and P30DK111024-05S1. FJP reports receiving research support from Dexcom, Insulet, and Merck and consulting fees from Boehringer Ingelheim outside the submitted work. MRP has a financial interest in technologies to collect interstitial fluid using microneedles as a consultant, inventor, and company founder/shareholder. This conflict of interest is managed by Georgia Institute of Technology. JLS has had research support from the NIH, JDRF, and the Helmsley Charitable Trust and her institution has had research support from Medtronic and Insulet. JLS serves as a consultant to Cecelia Health, Lexicon, Lilly, Insulet, Medtronic, and Sanofi. JLS is also a member of the advisory board for Bigfoot Biomedical, Cecelia Health, Insulet, Medtronic, and the T1D Fund. EKS was partially supported by the VA MERIT award (#1I01CX001825) from the United States Department of Veterans Affairs Clinical Sciences Research and Development Service. EKS has received unrestricted research support from Dexcom (to Baltimore VA Medical Center and to University of Maryland) for the conduction of clinical trials, which (Dexcom) did not support the work monetarily or in kind. AW Grant: Novo Nordisk and Grant Salary Support: Eli Lilly, UnitedHealth. DCK is a consultant for EOFlow, Fractyl, Lifecare, Novo Nordisk, Roche Diagnostics, Samsung, and Thirdwayv.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Diabetes Technology Society received an educational grant from Abbott Diabetes Care to support the research and publication of this article.
ORCID iDs: Kevin T. Nguyen  https://orcid.org/0000-0001-9102-6537
https://orcid.org/0000-0001-9102-6537
Nicole Y. Xu  https://orcid.org/0000-0001-9353-8819
https://orcid.org/0000-0001-9353-8819
Jennifer Y. Zhang  https://orcid.org/0000-0002-3374-9777
https://orcid.org/0000-0002-3374-9777
Trisha Shang  https://orcid.org/0000-0001-9687-9336
https://orcid.org/0000-0001-9687-9336
Richard M. Bergenstal  https://orcid.org/0000-0002-9050-5584
https://orcid.org/0000-0002-9050-5584
Kristin Castorino  https://orcid.org/0000-0001-6386-9203
https://orcid.org/0000-0001-6386-9203
Kong Y. Chen  https://orcid.org/0000-0002-0306-1904
https://orcid.org/0000-0002-0306-1904
David Kerr  https://orcid.org/0000-0003-1335-1857
https://orcid.org/0000-0003-1335-1857
Suneil K. Koliwad  https://orcid.org/0000-0002-7367-1054
https://orcid.org/0000-0002-7367-1054
Lori M. Laffel  https://orcid.org/0000-0002-9675-3001
https://orcid.org/0000-0002-9675-3001
Nestoras Mathioudakis  https://orcid.org/0000-0002-0210-655X
https://orcid.org/0000-0002-0210-655X
L. Kurt Midyett  https://orcid.org/0000-0001-8486-7370
https://orcid.org/0000-0001-8486-7370
Joshua D. Miller  https://orcid.org/0000-0001-9512-461X
https://orcid.org/0000-0001-9512-461X
James H. Nichols  https://orcid.org/0000-0002-3652-1612
https://orcid.org/0000-0002-3652-1612
Francisco J. Pasquel  https://orcid.org/0000-0002-3845-6703
https://orcid.org/0000-0002-3845-6703
Mark R. Prausnitz  https://orcid.org/0000-0002-9076-8448
https://orcid.org/0000-0002-9076-8448
Jane Jeffrie Seley  https://orcid.org/0000-0003-1582-4320
https://orcid.org/0000-0003-1582-4320
Jennifer L. Sherr  https://orcid.org/0000-0001-9301-3043
https://orcid.org/0000-0001-9301-3043
Elias K. Spanakis  https://orcid.org/0000-0002-9352-7172
https://orcid.org/0000-0002-9352-7172
Guillermo E. Umpierrez  https://orcid.org/0000-0002-3252-5026
https://orcid.org/0000-0002-3252-5026
Amisha Wallia  https://orcid.org/0000-0002-3183-4062
https://orcid.org/0000-0002-3183-4062
David C. Klonoff  https://orcid.org/0000-0001-6394-6862
https://orcid.org/0000-0001-6394-6862
References
- 1. Alva S, Castorino K, Cho H, Ou J. Feasibility of continuous ketone monitoring in subcutaneous tissue using a ketone sensor. J Diabetes Sci Technol. 2021;15:768-774. doi: 10.1177/19322968211008185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15(6):412-426. doi: [DOI] [PubMed] [Google Scholar]
- 3. Dhillon KK, Gupta S. Biochemistry, Ketogenesis. StatPearls Publishing; 2021. Accessed July 2, 2021. http://www.ncbi.nlm.nih.gov/books/NBK493179/ [PubMed] [Google Scholar]
- 4. Grabacka M, Pierzchalska M, Dean M, Reiss K. Regulation of ketone body metabolism and the role of PPARα. Int J Mol Sci. 2016;17(12):2093. doi: 10.3390/ijms17122093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Selvaraj S, Kelly DP, Margulies KB. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation. 2020;141(22):1800-1812. doi: 10.1161/CIRCULATIONAHA.119.045033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gershuni VM, Yan SL, Medici V. Nutritional ketosis for weight management and reversal of metabolic syndrome. Curr Nutr Rep. 2018;7(3):97-106. doi: 10.1007/s13668-018-0235-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitchell GA, Kassovska-Bratinova S, Boukaftane Y, et al. Medical aspects of ketone body metabolism. Clin Invest Med. 1995;18(3):193-216. [PubMed] [Google Scholar]
- 8. Choi A, Hallett M, Ehrlich D. Correction to: nutritional ketosis in Parkinson’s disease – a review of remaining questions and insights. Neurother J Am Soc Exp Neurother. Published online July 7, 2021. doi: 10.1007/s13311-021-01091-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu L, Li B. A case of severe diabetic ketoacidosis associated with pembrolizumab therapy in a patient with metastatic melanoma. Diabetes Metab Syndr Obes Targets Ther. 2021;14:753-757. doi: 10.2147/dmso.s297709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Primers. 2020;6(1):40-20. doi: 10.1038/s41572-020-0165-1 [DOI] [PubMed] [Google Scholar]
- 11. Diabetic ketoacidosis – NHS.nhs.uk. Published October 18, 2017. Accessed July 11, 2021. https://www.nhs.uk/conditions/diabetic-ketoacidosis/
- 12. Misra S, Oliver NS. Utility of ketone measurement in the prevention, diagnosis and management of diabetic ketoacidosis. Diabet Med. 2015;32(1):14-23. doi: 10.1111/dme.12604 [DOI] [PubMed] [Google Scholar]
- 13. Laffel LM, Wentzell K, Loughlin C, Tovar A, Moltz K, Brink S. Sick day management using blood 3-hydroxybutyrate (3-OHB) compared with urine ketone monitoring reduces hospital visits in young people with T1DM: a randomized clinical trial. Diabet Med. 2006;23(3):278-284. doi: 10.1111/j.1464-5491.2005.01771.x [DOI] [PubMed] [Google Scholar]
- 14. Ketones, urine. ucsfhealth.org. Accessed July 11, 2021. https://www.ucsfhealth.org/MedicalTests/003585
- 15. Urine diagnostics – products & solutions. Accessed July 11, 2021. https://www.analyticon-diagnostics.com/en/products_and_solutions/urine_diagnostic/urine_test_strips/portfolio_urine_stripes/Ketones.html
- 16. MacGillivray MH, Li PK, Lee JT, et al. Elevated plasma beta-hydroxybutyrate concentrations without ketonuria in healthy insulin-dependent diabetic patients. J Clin Endocrinol Metab. 1982;54(3):665-668. doi: 10.1210/jcem-54-3-665 [DOI] [PubMed] [Google Scholar]
- 17. Taboulet P, Haas L, Porcher R, et al. Urinary acetoacetate or capillary beta-hydroxybutyrate for the diagnosis of ketoacidosis in the emergency department setting. Eur J Emerg Med. 2004;11(5):251-258. [DOI] [PubMed] [Google Scholar]
- 18. Qiao Y, Gao Z, Liu Y, et al. Breath ketone testing: a new biomarker for diagnosis and therapeutic monitoring of diabetic ketosis. Biomed Res Int. 2014;2014:869186. doi: 10.1155/2014/869186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang JY, Shang T, Koliwad SK, Klonoff DC. Continuous ketone monitoring: a new paradigm for physiologic monitoring. J Diabetes Sci Technol. 2021;15:775-780. doi: 10.1177/19322968211009860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. 510(k) premarket notification. Accessed July 19, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm
- 21. Amazon.com: blood ketone & glucose monitoring system | track your ketones & ketogenic diet progress | ketosis test kit with lancing device, 10 blood glucose test strips, 30 keto strips + 50 lancets by BrunoPharma: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Glucose-Monitoring-Ketogenic-Progress-BrunoPharma/dp/B075F5VW7C/ref=as_li_ss_tl?ie=UTF8&linkCode=sl1&tag=ketovale-20&linkId=67ee1d8bf6405ca65f25355d83a66749&language=en_US
- 22. Amazon.com: CareSens N plus bluetooth blood diabetes monitoring kit (auto coding) – free APP, 1 glucose meter with 100 glucose test strips, 1 control solution, 1 lancing device, 100 lancets, 1 case, 2 batteries: health & personal care. Accessed July 17, 2021. https://www.amazon.com/CareSens-Bluetooth-Diabetes-Monitoring-Coding/dp/B08B86TM6R/ref=sr_1_87?crid=VGRLM6RB6GM6&dchild=1&keywords=blood+ketone+monitor&qid=1614023633&sprefix=blood+ketone+monitors%2Caps%2C262&sr=8-87
- 23. 510(k) premarket notification. Accessed July 17, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K083468
- 24. Care Touch Blood Glucose Meter. Direct FSA. Accessed July 17, 2021. https://directfsa.com/products/care-touch-diabetes-testing-kit
- 25. Amazon.com: femometer blood ketone monitoring kit, advanced blood Keto meter with 10 ketone test strips, 10 lancets, lancing device & case – ideal for Keto diet: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Femometet-Monitoring-Advanced-Lancets-Lancing/dp/B08MTNXJ3P/ref=sr_1_52?crid=VGRLM6RB6GM6&dchild=1&keywords=blood+ketone+monitor&qid=1614023633&sprefix=blood+ketone+monitors%2Caps%2C262&sr=8-52
- 26. Blood glucose/ketone testing/GKI & Dr. Boz Ratio. Accessed July 17, 2021. https://www.fora-shop.com/collections/fora6connect-multi-parameter-testing
- 27. 510(k) premarket notification. Accessed July 17, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K161738
- 28. FreeStyle precision pro: next-level glucose testing. Accessed July 17, 2021. https://www.myfreestyle.com/fspp/
- 29. 510(k) premarket notification. Accessed July 17, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K132511
- 30. GlucoMen LX PLUS. Glucomen. Accessed July 17, 2021. https://www.glucomen.co.uk/glucomen-lx-plus/
- 31. GlucoRx HCT blood glucose & ketone meter. GlucoRx. Accessed July 17, 2021. https://www.glucorx.co.uk/shop/glucorx-hct-meter/
- 32. Amazon.com: KetoBM blood ketone meter kit for keto diet testing – complete ketone test kit with ketone monitor, keto strips, lancing device & lancets – easy, accurate way to check ketosis on the Ketogenic diet: health & personal care. Accessed July 17, 2021. https://www.amazon.com/KetoBM-Ketone-Blood-Meter-Kit/dp/B07QWMM4M6/ref=as_li_ss_tl?ie=UTF8&linkCode=sl1&tag=ketovale-20&linkId=5ffdd2fff6281a4584a9de7cae6db8cd&language=en_US
- 33. Ketocoach ketone meter. KetoCoach. Accessed July 17, 2021. https://ketocoachx.com/products/blood-ketone-meter
- 34. Ketodoc Advanced Ketone Blood Meter Kit. Ketodoc – Ketone blood monitoring system. Accessed July 17, 2021. https://ketodoc.com/products/ketodoc-ketone-blood-meter-kit
- 35. Amazon.com: KETO-MOJO GK+ Glucose & Ketone Bluetooth Monitor + Free APP. 20 test strips (10 each), meter, 20 lancets, lancing device, control solutions. Dual blood monitoring system for ketosis & ketogenic diets: health & personal care. Accessed July 17, 2021. https://www.amazon.com/dp/B08G5BZQVL/ref=as_li_ss_tl?ie=UTF8&linkCode=sl1&tag=ketovale-20&linkId=979b0c30d88a651438d4da4f7a4176bd&language=en_US
- 36. Amazon.com: KetoSens blood ketone monitoring starter kit + FREE APP: ideal for keto diet. Includes meter, 10 ketone test strips, 10 lancets, lancing device & case: health & personal care. Accessed July 17, 2021. https://www.amazon.com/KetoSens-Meter-Kit/dp/B07MPCX4DZ
- 37. 510(k) premarket notification. Accessed July 17, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K201551
- 38. Amazon.com: KetoTrak blood ketone testing kit, 1 Meter, 10 ketone strips, 1 lancing device, 10 lancets, optimize your result for keto diet by KetoTrak: health & personal care. Accessed July 17, 2021. https://www.amazon.com/KetoTrak-Testing-Lancing-Lancets-Optimize/dp/B07ZZM63GZ/ref=sr_1_1?keywords=KetoTrak&qid=1574634122&sr=8-1
- 39. Keto KM. Ketone Blood Meter Kit. Kiss My Keto. Accessed July 17, 2021. https://www.kissmyketo.com/products/ketone-blood-meter-kit
- 40. Nova Max plus starter kit glucose and ketone on the same meter. Accessed July 17, 2021. https://novacares.store/novamax/
- 41. 510(k) premarket notification. Accessed July 17, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K091547
- 42. Precision xceed pro glucose monitoring system by Abbott. Accessed July 17, 2021. https://www.medline.com:443/product/Precision-Xceed-Pro-Glucose-Monitoring-System-by-Abbott/Glucose-Meters-And-Accessories/Z05-PF72935
- 43. 510(k) premarket notification. Accessed July 17, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K080960
- 44. Amazon.com: precision xtra blood glucose and ketone monitoring meter kit bundle+10 precision xtra ketone test strips+one month Abbott freestyle 28 gauge Lancets+dynarex alcohol Wipes+I love keto sticker: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Precision-Glucose-Monitoring-Freestyle-Lancets/dp/B01N9NRDVI/ref=as_li_ss_tl?ie=UTF8&linkCode=sl1&tag=ketovale-20&linkId=b5e82da984255e77e8583a0a2fe1e32f&language=en_US
- 45. 510(k) premarket notification. Accessed July 17, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K060768
- 46. Amazon.com: ketone testing strips by 310 nutrition (100 strips) – test ketosis levels during low carb keto diet – accurate urine test for ketogenic measurement: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Ketone-Testing-Strips-310-Nutrition/dp/B08B1C75Q1/ref=sr_1_86?dchild=1&keywords=urine+ketone+monitor&qid=1625893098&sr=8-86
- 47. Amazon.com: Ketostix reagent strips for urinalysis, measure ketone levels, 100-count box: Carle, Frankie: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Ketostix-Reagent-Strips-100-Count-Box/dp/B0000532GH/ref=sr_1_25?dchild=1&keywords=urine+ketone+monitor&qid=1625865698&rdc=1&sr=8-25
- 48. Keto Test Strips – CVS health reagent strips for urinanalysis. Accessed July 17, 2021. https://www.cvs.com/shop/cvs-health-reagent-strips-for-urinanalysis-ketone-test-strips-50ct-prodid-306412
- 49. Amazon.com: Easy@Home keto tests strips, 10 urine ketone tester sticks, monitor low carb ketogenic diet, individual wrapped pouches, perfect for weightloss and FSA eligible – ketone urinalysis test 10 tests/box: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Easy-Home-Ketogenic-Individual-Weightloss/dp/B08LZXV137/ref=sr_1_16?dchild=1&keywords=urine+ketone+monitor&qid=1625891110&sr=8-16
- 50. Amazon.com: EZ Keto ketone testing strips for urinalysis with free app and EZ Keto start guide – 150 test sticks measure ketones. Test if you are in ketosis. Perfect for ketogenic low carb paleo & Atkins diets: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Easy-Keto-Ketone-Testing-Strips/dp/B07YDYQ7B8/ref=sr_1_8?dchild=1&keywords=urine+ketone+monitor&qid=1625865698&sr=8-8
- 51. Amazon.com: 10 parameters urine test strips for urinalysis. Made in USA. 100 Count. Includes: pH test, ketones, protein. Reagent test paper. For whole family and pets: health & personal care. Accessed July 17, 2021. https://www.amazon.com/dp/B00XIKQCJC/ref=cm_sw_em_r_mt_dp_4HN8NTTSY6YYMKNE3C7P
- 52. Amazon.com: INVBIO pH+keto 2-in-1, urinalysis urine test strips 2 parameter, 100ct – pH and ketone urine test strips to test and monitor ketosis for low carb diet, alkaline and acid levels in your body: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Analyzer-Professional-Accuracy-Dietitian-Mouthpieces/dp/B0916ZP21J/ref=sr_1_52?crid=389087S4DOP0V&dchild=1&keywords=urine+ketone+monitor&qid=1624403203&sprefix=urine+ketone+mo%2Caps%2C206&sr=8-52
- 53. Amazon.com: JNW direct ketone test strips, 150 urinalysis keto test strips for testing body urine ketosis levels, perfect kit for ketogenic and Paleo diets: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Urinalysis-Testing-Ketosis-Diabetics-Ketogenic/dp/B07C3XHJ81/ref=sr_1_6?dchild=1&keywords=urine+ketone+monitor&qid=1625891110&sr=8-6
- 54. Amazon.com: Ketone keto urine 150 test strips. 3 resealable foil packs of 50 strips each. Look & feel fabulous on a low carb ketogenic or HCG diet. Accurately measure your fat burning ketosis levels: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Strips-Resealable-Fabulous-Ketogenic-Accurately/dp/B07DLNF4XC/ref=sr_1_15?dchild=1&keywords=urine+ketone+monitor&qid=1625891110&sr=8-15
- 55. Amazon.com: 150 ketone test strips with free keto guide eBook & free APP. Urine test for ketosis on ketogenic & low-carb diets. (extra-long strips, made in USA): health & personal care. Accessed July 17, 2021. https://www.amazon.com/KETO-MOJO-Ketosis-Ketogenic-Low-Carb-Extra-Long/dp/B088C3GF9T/ref=sr_1_22?dchild=1&keywords=urine+ketone+monitor&qid=1625865698&sr=8-22
- 56. Amazon.com: kiss my keto strips – 200 ketosis test strips for low carb diets | extra long, medical grade keto urine testing strips | keto sticks for Ketone urinalysis – accurately measure & monitor Ketone levels: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Kiss-My-Keto-Test-Strips/dp/B07C47G13X/ref=sr_1_10?dchild=1&keywords=urine+ketone+monitor&qid=1625865698&sr=8-10
- 57. Amazon.com: ketone testing kit strips for Keto urine BHB test. Made in USA. No need for keto blood monitor. First urine BHB ketosis test kit for Keto adapted. Exogenous ketones testing: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Testing-Monitor-Adapted-Exogenous-Ketones/dp/B085Q3FHJB/ref=sr_1_21?dchild=1&keywords=urine+ketone+monitor&qid=1625891110&sr=8-21
- 58. Amazon.com: nurse Hatty 150 keto test strips with free 300+ pages of eBooks & free APP (track your Ketone progress) – USA-Made – Ketone urine test for ketosis on low carb Ketogenic diets – extra-long strips: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Nurse-Hatty%C2%AE-Ketone-Strips-Proudly/dp/B016Z5XZP0/ref=sr_1_2?crid=4M7WZSXBBNAP&dchild=1&keywords=nurse+hatty+keto+strips&qid=1625866257&sprefix=nurse+hatty+%2Caps%2C248&sr=8-2
- 59. Amazon.com: ketone strips (USA made, 150 Count): accurate ketosis urine test strips for keto diet and ketogenic measurement. Lose weight with confidence: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Ketone-Strips-USA-Made-Count/dp/B01MCU8NJ6/ref=sr_1_7?dchild=1&keywords=urine+ketone+monitor&qid=1625891110&sr=8-7
- 60. Amazon.com: perfect keto test strips – best for testing ketones in urine on low carb ketogenic diet, Ketosis home urinalysis tester kit, 100 CT: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Perfect-Keto-Ketone-Testing-Strips/dp/B01MUB7BUV/ref=sr_1_5?dchild=1&keywords=urine+ketone+monitor&qid=1625865644&sr=8-5
- 61. Amazon.com: real KetonesTM keto urine testing strips – 100 Count ketone urinalysis test sticks for measuring Ketosis levels quickly and accurately: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Real-KetonesTM-Urine-Testing-Strips/dp/B083NKH2RK/ref=sr_1_78?dchild=1&keywords=urine+ketone+monitor&qid=1625893098&sr=8-78
- 62. ReliOn ketone test strips, 50 Count. Walmart.com. Accessed July 17, 2021. https://www.walmart.com/ip/ReliOn-Ketone-Test-Strips-50-Count/33574014
- 63. Amazon.com: rock the fork keto – ketone test strips –ketosis testing for low-carb ketogenic, adkins, or PSMF diet. easy-to-use urinalysis kit. Includes: 100 sticks: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Rock-Fork-Keto-Easy-Use/dp/B08KTW9JXD/ref=sr_1_72?dchild=1&keywords=urine+ketone+monitor&qid=1625893098&sr=8-72
- 64. Amazon.com: Smackfat keto strips – keto strips urine test – high quality 100 strips: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Ketone-Strips-Smackfat-High-Quality/dp/B00SODYZQK
- 65. Amazon.com: urine test strips – stript health 10 parameter complete urinalysis testing 100ct, urinary tract infection strips (UTI) Ketones – protein – pH – great for easy testing kidney, liver, ketosis & paleo: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Urine-Test-Strips-Parameter-Urinalysis/dp/B07859M8YH/ref=sr_1_71?dchild=1&keywords=urine+ketone+monitor&qid=1625893098&sr=8-71
- 66. Ketone test strips released by top notch nutrition on Amazon.com. Accessed July 17, 2021. https://marketersmedia.com/ketone-test-strips-released-by-top-notch-nutrition-on-amazon-com/293194
- 67. TRUEplus ketone test strips. Trividia Health. Accessed July 17, 2021. https://www.trividiahealth.com/products/other-products/true-plus-ketone-test-strips/
- 68. Amazon.com: ketone test strips for ketones testing – 125 Pack keto urinalysis tester strips kit for ketogenic, paleo, Atkins & low carb diets. Premium ketosis testing, accurate measurement of ketone urine levels: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Ketone-Test-Strips-Ketones-Testing/dp/B07JHP2HVT/ref=sr_1_14?dchild=1&keywords=urine+ketone+monitor&qid=1625891110&sr=8-14
- 69. Amazon.com: ketone strips – perfect ketogenic supplement to measure ketones in urine & monitor ketosis for keto diet, 125 urinalysis test strips: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Ketone-Strips-Ketogenic-Supplement-Urinalysis/dp/B07GZ375SQ/ref=sr_1_11?dchild=1&keywords=urine+ketone+monitor&qid=1625891110&sr=8-11
- 70. Walgreens ketone test strips for urinalysis. Walgreens. Accessed July 17, 2021. https://www.walgreens.com/store/c/walgreens-ketone-test-strips-for-urinalysis/ID=prod6000723-product
- 71. Amazon.com: Wondview ketone test strips: testing ketosis based on your urine, 100 ketone urinalysis tester strips (made in USA): health & personal care. Accessed July 17, 2021. https://www.amazon.com/Wondview-Ketone-Strips-Urine-Tester/dp/B07NP2ZDHP/ref=sr_1_31?dchild=1&keywords=urine+ketone+monitor&qid=1625891110&sr=8-31
- 72. Amazon.com: Zenda naturals ketone test strips – keto strips for keto diet – measures ketones in urine & monitors ketosis, 125 urinalysis test strips: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Ketone-Strips-Ketogenic-Supplement-Urinalysis/dp/B07CF7KTCF/ref=sr_1_9?dchild=1&keywords=urine+ketone+monitor&qid=1625891110&sr=8-9
- 73. Amazon.com: Zhou Keto test strips | read ketone level with ease during Keto, paleo, low-carb diets | quick & easy | 125 test strips: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Reagent-Urinalysis-Testing-Ketones-Low-Carb/dp/B0785WXGL8/ref=sr_1_61?dchild=1&keywords=urine+ketone+monitor&qid=1625893098&rdc=1&sr=8-61
- 74. Biosense breath ketone monitoring system. Accessed July 17, 2021. https://thefeed.com/products/biosense-breath-ketone-monitoring-system?variant=31795535183935
- 75. Amazon.com: Coolker ketone breath analyzer professional grade accuracy digital ketone breath meter tracing ketosis status with 10 mouthpieces(black): health & personal care. Accessed July 17, 2021. https://www.amazon.com/dp/B07RT9FXSL/ref=cm_sw_em_r_mt_dp_WY2N5ZNYKQS3FXEX3Q9B?_encoding=UTF8&psc=1
- 76. Amazon.com: GREENWON ketone meter, digital LCD display breath ketone meter, Ketosis monitor with mouthpieces, ketone breath analyzer, keychain ketone tester, portable Ketosis monitor (G/Black): health & personal care. Accessed July 17, 2021. https://www.amazon.com/GREENWON-Digital-Display-Breathing-Monitor/dp/B07J5J8WTS/ref=sr_1_48?crid=389087S4DOP0V&dchild=1&keywords=urine+ketone+monitor&qid=1624403203&sprefix=urine+ketone+mo%2Caps%2C206&sr=8-48
- 77. House of Keto MonitorTM – Measure ketones the easy way! House of Keto. Accessed July 17, 2021. https://www.houseofketo.com/products/house-of-keto-monitor-1-1
- 78. Amazon.com: breath ketone analyzer with new technology semi-conductor sensor for keto testing: health & personal care. Accessed July 17, 2021. https://www.amazon.com/dp/B084ZJ1RDQ/ref=cm_sw_em_r_mt_dp_EVZCAMQ89PX4T96TQP26?_encoding=UTF8&psc=1
- 79. KETONIX breath ketone analyzer. Accessed July 17, 2021. https://www.ketonix.com/
- 80. Amazon.com: KETOSCAN lite breath ketone meter, diet & fitness tracker | monitor your fat metabolism or level of ketosis on low carb, ketogenic or any nutrition & fitness program: health & personal care. Accessed July 17, 2021. https://www.amazon.com/KETOSCAN-Fitness-Metabolism-Ketogenic-Nutrition/dp/B08NT1RXQ1/ref=sr_1_36?crid=389087S4DOP0V&dchild=1&keywords=urine+ketone+monitor&qid=1624403203&sprefix=urine+ketone+mo%2Caps%2C206&sr=8-36
- 81. Amazon.com: V2 KETOSCAN mini breath ketone meter, diet & fitness tracker | monitor your fat metabolism or level of ketosis on low carb, ketogenic or any nutrition & fitness program: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Upgraded-KETOSCAN-Metabolism-Ketogenic-Nutrition/dp/B07R1TW6H3/ref=sr_1_29?crid=389087S4DOP0V&dchild=1&keywords=urine+ketone+monitor&qid=1624401499&sprefix=urine+ketone+mo%2Caps%2C206&sr=8-29
- 82. Amazon.com: KetoStat – breath ketone monitoring system for ketogenic diet: health & personal care. Accessed July 17, 2021. https://www.amazon.com/KetoStat-Breath-Ketone-Monitoring-Ketogenic/dp/B07G1Z3FLZ/ref=cm_cr_arp_d_product_top?ie=UTF8
- 83. Keyto breath sensor. Keyto. Accessed July 17, 2021. https://shop.getkeyto.com/products/keyto-breath-sensor
- 84. Amazon.com: ketone breath meter, professional digital ketone breath analyzer testing ketosis with 10 mouthpieces(black): health & personal care. Accessed July 17, 2021. https://www.amazon.com/Professional-Digital-Analyzer-Testing-Mouthpieces/dp/B07SD9TSLG/ref=sr_1_50?crid=389087S4DOP0V&dchild=1&keywords=urine+ketone+monitor&qid=1624403203&sprefix=urine+ketone+mo%2Caps%2C206&sr=8-50
- 85. Amazon.com: Ketone meter, Ketone breath analyzer, professional grade accuracy digital Ketone breath meter for dietitian testing with 10 mouthpieces: health & personal care. Accessed July 17, 2021. https://www.amazon.com/Analyzer-Professional-Accuracy-Dietitian-Mouthpieces/dp/B0916ZP21J/ref=sr_1_52?crid=389087S4DOP0V&dchild=1&keywords=urine+ketone+monitor&qid=1624403203&sprefix=urine+ketone+mo%2Caps%2C206&sr=8-52
- 86. A guide to ketosis and B-Hydroxybutyrate | EKF diagnostics. Accessed July 9, 2021. https://www.ekfdiagnostics.com/ketosis-B-hydroxybutyrate.html
- 87. Levine M, Conry-Cantilena C, Wang Y, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996;93(8):3704-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pasqua M-R, Tsoukas MA, Haidar A. Strategically playing with fire: SGLT inhibitors as possible adjunct to closed-loop insulin therapy. J Diabetes Sci Technol. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Benoit SR, Zhang Y, Geiss LS, Gregg EW, Albright A. Trends in diabetic ketoacidosis hospitalizations and In-hospital mortality – United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018;67(12):362-365. doi: 10.15585/mmwr.mm6712a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Desai D, Mehta D, Mathias P, Menon G, Schubart UK. Health care utilization and burden of diabetic ketoacidosis in the U.S. Over the past decade: a nationwide analysis. Diabetes Care. 2018;41(8):1631-1638. doi: 10.2337/dc17-1379 [DOI] [PubMed] [Google Scholar]
- 91. Everett EM, Copeland TP, Moin T, Wisk LE. National trends in pediatric admissions for diabetic ketoacidosis, 2006-2016. J Clin Endocrinol Metab. 2021;106:2343-2354. doi: 10.1210/clinem/dgab287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Vellanki P, Umpierrez GE. Increasing hospitalizations for DKA: a need for prevention programs. Diabetes Care. 2018;41(9):1839-1841. doi: 10.2337/dci18-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhong VW, Juhaeri J, Mayer-Davis EJ. Trends in hospital admission for diabetic ketoacidosis in adults with type 1 and type 2 diabetes in England, 1998–2013: a retrospective cohort study. Diabetes Care. 2018;41(9):1870-1877. doi: 10.2337/dc17-1583 [DOI] [PubMed] [Google Scholar]
- 94. Fazeli Farsani S, Brodovicz K, Soleymanlou N, Marquard J, Wissinger E, Maiese BA. Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): a systematic literature review. BMJ Open. 2017;7(7):e016587. doi: 10.1136/bmjopen-2017-016587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Neu A, Hofer SE, Karges B, Oeverink R, Rosenbauer J, Holl RW. Ketoacidosis at diabetes onset is still frequent in children and adolescents: a multicenter analysis of 14,664 patients from 106 institutions. Diabetes Care. 2009;32(9):1647-1648. doi: 10.2337/dc09-0553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Limenis E, Shulman R, Daneman D. Is the frequency of ketoacidosis at onset of type 1 diabetes a child health indicator that is related to income inequality? Diabetes Care. 2012;35(2):e5. doi: 10.2337/dc11-1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dabelea D, Rewers A, Stafford JM, et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics. 2014;133(4):e938-e945. doi: 10.1542/peds.2013-2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pinkney JH, Bingley PJ, Sawtell PA, Dunger DB, Gale EAM. Presentation and progress of childhood diabetes mellitus: a prospective population-based study. Diabetologia. 1994;37(1):70-74. doi: 10.1007/bf00428780 [DOI] [PubMed] [Google Scholar]
- 99. Rewers A, Klingensmith G, Davis C, et al. Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the search for diabetes in youth study. Pediatrics. 2008;121(5):e1258-e1266. doi: 10.1542/peds.2007-1105 [DOI] [PubMed] [Google Scholar]
- 100. Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD clinical practice consensus guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(S27):155-177. doi: 10.1111/pedi.12701 [DOI] [PubMed] [Google Scholar]
- 101. Wolfsdorf JI, Allgrove J, Craig ME, et al. ISPAD clinical practice consensus guidelines 2014. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014;15(S20):154-179. doi: 10.1111/pedi.12165 [DOI] [PubMed] [Google Scholar]
- 102. Ho J, Rosolowsky E, Pacaud D, et al. Diabetic ketoacidosis at type 1 diabetes diagnosis in children during the COVID-19 pandemic. Pediatr Diabetes. 2021;22(4):552-557. doi: 10.1111/pedi.13205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Poovazhagi V. Risk factors for mortality in children with diabetic keto acidosis from developing countries. World J Diabetes. 2014;5(6):932-938. doi: 10.4239/wjd.v5.i6.932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gibb FW, Teoh WL, Graham J, Lockman KA. Risk of death following admission to a UK hospital with diabetic ketoacidosis. Diabetologia. 2016;59(10):2082-2087. doi: 10.1007/s00125-016-4034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Umpierrez GE, DiGirolamo M, Tuvlin JA, Isaacs SD, Bhoola SM, Kokko JP. Differences in metabolic and hormonal milieu in diabetic- and alcohol-induced ketoacidosis. J Crit Care. 2000;15(2):52-59. doi: 10.1053/jcrc.2000.7900 [DOI] [PubMed] [Google Scholar]
- 106. FDA Drug Safety Communication. FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. Published 2015. Accessed May 7, 2021. https://www.fda.gov/files/drugs/published/Drug-Safety-Communication–FDA-warns-that-SGLT2-inhibitors-for-diabetes-may-result-in-a-serious-condition-of-too-much-acid-in-the-blood.pdf
- 107. Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care. 2015;38(12):2258-2265. doi: 10.2337/dc15-1730 [DOI] [PubMed] [Google Scholar]
- 108. Musso G, Sircana A, Saba F, Cassader M, Gambino R. Assessing the risk of ketoacidosis due to sodium-glucose cotransporter (SGLT)-2 inhibitors in patients with type 1 diabetes: a meta-analysis and meta-regression. PLoS Med. 2020;17(12):e1003461. doi: 10.1371/journal.pmed.1003461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Danne T, Garg S, Peters AL, et al. International consensus on risk management of diabetic ketoacidosis in patients with type 1 Diabetes treated with sodium-glucose cotransporter (SGLT) inhibitors. Diabetes Care. 2019;42(6):1147-1154. doi: 10.2337/dc18-2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Garg SK, Peters AL, Buse JB, Danne T. Strategy for mitigating DKA risk in patients with type 1 diabetes on adjunctive treatment with SGLT inhibitors: a STICH protocol. Diabetes Technol Ther. 2018;20(9):571-575. doi: 10.1089/dia.2018.0246 [DOI] [PubMed] [Google Scholar]
- 111. Sussman D, van Eede M, Wong MD, Adamson SL, Henkelman M. Effects of a ketogenic diet during pregnancy on embryonic growth in the mouse. BMC Pregnancy Childbirth. 2013;13:109. doi: 10.1186/1471-2393-13-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gosmanov AR, Gosmanova EO, Kitabchi AE. Hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. In: Feingold KR, Anawalt B, Boyce A, et al. (eds) Endotext. MDText.com, Inc; 2000. Accessed July 1, 2021. http://www.ncbi.nlm.nih.gov/books/NBK279052/. [PubMed] [Google Scholar]
- 113. Møller N. Ketone body, 3-hydroxybutyrate: minor metabolite – major medical manifestations. J Clin Endocrinol Metab. 2020;105(9):dgaa370. doi: 10.1210/clinem/dgaa370 [DOI] [PubMed] [Google Scholar]
- 114. Hettiarachchi D, Lakmal K, Dissanayake VHW. A concise review of ketogenic dietary interventions in the management of rare diseases. J Nutr Metab. 2021;2021:6685581. doi: 10.1155/2021/6685581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yuan X, Wang J, Yang S, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. 2020;10:38. doi: 10.1038/s41387-020-00142-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol. 2019;10:348. doi: 10.3389/fendo.2019.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hallberg SJ, McKenzie AL, Williams PT, et al. Author correction: effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther. 2018;9(2):613-621. doi: 10.1007/s13300-018-0386-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Flores M, Amir M, Ahmed R, et al. Causes of diabetic ketoacidosis among adults with type 1 diabetes mellitus: insulin pump users and non-users. BMJ Open Diabetes Res Care. 2020;8(2):e001329. doi: 10.1136/bmjdrc-2020-001329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Guerci B, Meyer L, Sallé A, et al. Comparison of metabolic deterioration between insulin analog and regular insulin after a 5-hour interruption of a continuous subcutaneous insulin infusion in type 1 diabetic patients 1. J Clin Endocrinol Metab. 1999;84(8):2673-2678. doi: 10.1210/jcem.84.8.5912 [DOI] [PubMed] [Google Scholar]
- 120. Orsini-Federici M, Akwi JA, Canonico V, et al. Early detection of insulin deprivation in continuous subcutaneous insulin infusion-treated patients with type 1 Diabetes. Diabetes Technol Ther. 2006;8(1):67-75. doi: 10.1089/dia.2006.8.67 [DOI] [PubMed] [Google Scholar]
- 121. Lee MH, Paldus B, Krishnamurthy B, et al. The clinical case for the integration of a ketone sensor as part of a closed loop insulin Pump system. J Diabetes Sci Technol. 2019;13(5):967-973. doi: 10.1177/1932296818822986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Shivers JP, Mackowiak L, Anhalt H, Zisser H. “Turn it off!”: diabetes device alarm fatigue considerations for the present and the future. J Diabetes Sci Technol. 2013;7(3):789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Klonoff DC. The expanding role of real-world evidence trials in health care decision making. J Diabetes Sci Technol. 2020;14(1):174-179. doi: 10.1177/1932296819832653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Klonoff DC, Freckmann G, Heinemann L. Insulin Pump occlusions: for patients who have been around the (infusion) block. J Diabetes Sci Technol. 2017;11(3):451-454. doi: 10.1177/1932296817700545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Heinemann L, Klonoff DC. Input of patients for new diabetes technology products. J Diabetes Sci Technol. 2021. Published online August 12, 2021:19322968211033612. doi: 10.1177/19322968211033611 [DOI] [PMC free article] [PubMed] [Google Scholar]