ABSTRACT.
Understanding the reservoir and infectivity of Plasmodium gametocytes to vector mosquitoes is crucial to align strategies aimed at malaria transmission elimination. Yet, experimental information is scarce regarding the infectivity of Plasmodium vivax for mosquitoes in diverse epidemiological settings where the proportion of asymptomatically infected individuals varies at a microgeographic scale. We measured the transmissibility of clinical and subclinical P. vivax malaria parasite carriers to the major mosquito vector in the Amazon Basin, Nyssorhynchus darlingi (formerly Anopheles). A total of 105 participants with natural P. vivax malaria infection were recruited from a cohort study in Loreto Department, Peruvian Amazon. Four of 18 asymptomatic individuals with P. vivax positivity by blood smear infected colony-grown Ny. darlingi (22%), with 2.6% (19 of 728) mosquitoes infected. In contrast, 77% (44/57) of symptomatic participants were infectious to mosquitoes with 51% (890 of 1,753) mosquitoes infected. Infection intensity was greater in symptomatic infections (mean, 17.8 oocysts/mosquito) compared with asymptomatic infections (mean, 0.28 oocysts/mosquito), attributed to parasitemia/gametocytemia level. Paired experiments (N = 27) using direct skin-feeding assays and direct membrane mosquito-feeding assays showed that infectivity to mosquitoes was similar for both methods. Longitudinal studies with longer follow-up of symptomatic and asymptomatic parasite infections are needed to determine the natural variations of disease transmissibility.
INTRODUCTION
Malaria remains a major global health concern, with more than 228 million cases and 405,000 deaths estimated to occur annually. Plasmodium vivax is the most widespread of the malaria species, with more than one third of the world’s population—nearly 2.5 billion people—at risk.1 Unique biological characteristics of this parasite species challenge the most common strategies to reduce Plasmodium transmission, such as the ability of P. vivax to relapse from dormant liver-stage hypnozoites,2,3 and early gametocyte formation and circulation during blood-stage infection. In controlled P. vivax infections of humans, transmissible sexual stages are first detected 4 to 7 days after sporozoite infection and within 1 to 2 days after the first appearance of asexual parasites, even before clinical symptoms occur.4–6 These biological features of P. vivax distinguish this parasite’s transmission characteristics from Plasmodium falciparum, whose gametocytes are detected more than 1 week after patent blood stage infection.7 Factors that may influence a P. vivax–infected individual’s infectivity for mosquitoes may include gametocyte maturation and quantity,8 local vectorial capacity,9 and host immune factors.4,10–12
Malaria transmission is initiated when a mosquito ingests gametocytes during an infectious blood meal. The gametocytes then undergo a complex process, starting with gamete fertilization, leading to oocyst formation, then to sporozoites that travel through the mosquito hemolymph to invade the salivary glands, after which they are mature and ready to be inoculated into a human during a subsequent blood meal.4,13 Multiple factors affect successful development of oocysts midgut, including gametocyte maturation stage and gamete male-to-female ratios, especially in P. falciparum14,15; previous Plasmodium exposure16; and intrinsic permissiveness and/or vector competence of malaria vectors to Plasmodium, microbiota, and the mosquito immune system.17,18 The association between gametocyte density in blood smears and infectiousness to mosquitoes for either P. falciparum10 or P. vivax parasites10,19 is unclear and remains a knowledge gap.
Successful mosquito infection can be detected microscopically or by molecular methods in the mosquito midgut or salivary gland sporozoites. In the laboratory, direct skin-feeding assays (SFAs) reflect epidemiological reality because they mimic the natural transmission of the parasite, but some authors have ethical concerns.20 Artificial direct membrane mosquito-feeding assays (DMFA) have also been developed,20 but laboratory procedures are cumbersome and require validation to corroborate accurate measurements of mosquito infectivity in a new setting.20,21 Although comparison of DMFAs and SFAs have focused largely on P. falciparum,22–25 these methodologies are underexplored in P. vivax, primarily because of the absence of a sustainable P. vivax in vitro culture system, so that performing experimental mosquito infections depends on the availability of P. vivax carriers as parasite sources for such assays.20
In Peru, P. vivax is the most common malaria parasite; there are approximately four times as many P. vivax cases than P. falciparum cases.26 Asymptomatic P. vivax asymptomatic infections predominate, and individuals are thought to be the main reservoir of transmission in the Amazon region.27 Asymptomatic parasite carriers generally do not seek treatment, and, during this interval, these individuals may be bitten by vector mosquitoes.28 By definition, sub-microscopic parasitemia is only detectable by sensitive molecular methods, often impractical to apply during malaria surveillance.28,29 Understanding the contribution of symptomatic and asymptomatic P. vivax microscopic and sub-microscopic infections to mosquito transmissibility is critical to target malaria control resources effectively in this particular setting. Thus, this study aimed to assess malaria transmission experimentally using mosquito-feeding assays comparing symptomatic and asymptomatic subjects with parasitemia in a moderate malaria transmission region of the Peruvian Amazon. We also compared the efficiency of membrane feeding assays to direct skin feeding in P. vivax symptomatic infections with Nyssorhynchus darlingi to validate this methodology for estimating infectivity.
MATERIALS AND METHODS
Study sites and participant recruitment.
The first phase of this study was conducted in the vicinity of Iquitos, Loreto Department, Amazonian Peru. Plasmodium vivax infections were identified from a cohort study in the villages of Lupuna (∼8 km from Iquitos) and Cahuide (∼60 km from Iquitos) from April 2013 to October 2015 during the dry and rainy seasons. For the direct SFAs, subjects harboring P. vivax parasites were recruited from passive surveillance at health posts around Iquitos city between June 2016 and March 2017. Symptomatic individuals presented malaria-like symptoms at the time of the survey (axillary temperature ≥ 37.5°C, headache, chills). Asymptomatic carriers did not present with malaria-compatible symptoms at the time of recruitment and did not report fever during the previous 2 weeks or the subsequent 2 weeks. Additional follow-up was done to assess malaria and symptoms at 4 weeks and at 2, 4, 8, and 12 months.
Written informed consent from all participants was obtained prior to enrollment (age, ≥ 18 years old). Thick and thin blood smears from finger pricks were prepared, stained with 10% Giemsa, and read using light microscopy to calculate sexual and asexual parasitemias and to exclude obviously mixed Plasmodium infections. Parasite and gametocyte densities (per microliter) were calculated for each participant by counting the number of parasites for 200 white blood cells (WBC), assuming a concentration of 8,000 WBC/μL. Two independent technicians read all slides.30
Sub-microscopic P. vivax–infected individuals, defined as infections detectable by molecular methods but not patent by microscopy, were enrolled from cross-sectional surveys in Cahuide and Lupuna villages and screened for malaria parasites by real-time–polymerase chain reaction RT-PCR on whole blood samples within 1 week of the survey. Parasite DNA was extracted using a QIAGEN QIAamp DNA Mini Kit and an E.Z.N.A Blood DNA kit (OMEGA Bio-tek Norcross, GA), and total parasite quantification of Plasmodium species was done using an RT quantitative PCR-adapted protocol targeting 18S ribosomal genes,31 with a limit of detection of 2.45 parasite/μL.
The second phase of the study was carried out during the dry and rainy seasons in the villages of Lupuna (∼8 km from Iquitos) and Mazan (∼35 km from Iquitos) from May to November 2019. Symptomatic individuals presented malarial symptoms at the time of the survey (axillary temperature ≥ 37.5°C, headache, chills). Asymptomatic carriers did not present with malaria-compatible symptoms during a 21-day follow-up. At the end of the follow-up, a blood sample was drawn and direct membrane mosquito-feeding assays (DMFAs) were performed either in the laboratory in Iquitos or using mosquitoes (Ny. darlingi) transported to the villages. Sub-microscopic P. vivax–infected individuals were screened for malaria by COX-18s ribosomal RNA probe RT-PCR32 on whole blood samples within 2 days of the survey, which has a limit of detection of 2.88 parasite/μL.
After obtaining blood by phlebotomy for mosquito-feeding experiments, subjects with clinical symptoms (patients) were treated with 3 days of chloroquine (10 mg/kg for 2 days and 5 mg/kg for 1 day) plus 7 days of primaquine (0.5 mg/kg), according to the regimen recommended by the Peruvian Ministry of Health.33 Asymptomatic individuals positive for P. vivax by PCR only did not receive treatment; however, this policy changed after the release in 2016 of the Norma Técnica de Malaria (Malaria Technical Regulation) by the Ministry of Health, which mandated treatment for all asymptomatic individuals diagnosed as positive by microscopy or PCR. Thus, individuals from the second phase of our study were treated according to this policy.33
Mosquito-feeding assays.
Venous blood was obtained by phlebotomy on the day of enrollment for symptomatic participants, before any treatment with medication, and 24 to 48 hours after parasitemia detection for blood smear–positive asymptomatic parasite carriers. For sub-microscopic parasitemias in asymptomatic individuals, venous blood was obtained after parasitemia detection by PCR. For DMFAs, between 4 and 6 mL of venous blood was obtained and placed in lithium heparin vacutainers tubes; 0.5 to 1 mL was added to a 3.5-cm-diameter glass membrane feeder fitted with a Parafilm® membrane and was offered immediately to mosquitoes. Mosquito feeds were performed for each volunteer using two mosquito batch replicates with 40 to 50 mosquitoes per container. Mosquito-feeding experiments were conducted using 3- to 5-day old Ny. darlingi colony females after an overnight starvation period, and all feeding assays were completed between 9 am and 12 noon. Mosquitoes were allowed to feed up to 30 minutes in the dark, and then fully fed females were sorted and maintained with glucose 10% ad libitum within the Iquitos insectary (27 ± 1°C, 70%–80% relative humidity) until dissection. Midguts were dissected on days 7 and 8 post-feeding and were inspected by light microscopy after staining with mercurochrome 0.5%. A mosquito was considered infected when at least one oocyst was detected within the midgut.34 Outcomes of the assays were infection prevalence, representing the number of subjects infecting at least one mosquito; infection intensity, or the number of dissected mosquitoes with ≥ 1 midgut oocyst divided by the total number of mosquitoes dissected (the difference in the number of mosquitoes with oocysts); and oocyst infection intensity, calculated as the geometric mean of the number of oocysts per mosquito per feeding (number of oocysts within the mosquito).
For direct SFAs one container with 50 mosquitoes was applied to the right or left calf of a symptomatic P. vivax participant, and mosquitoes were allowed to feed for 30 minutes. Concomitantly, venous blood extracted from the same subject was offered through a glass membrane feeder according to the previous protocol described DMFA. Immediately after the feeding experiment, antihistamine cream was applied to the limb of the participant, and malaria treatment was begun according to Ministry of Health policy. Mosquito midguts were dissected on days 7 and 8 post-feeding, and salivary glands were dissected to detect sporozoites 14 to 25 days post-experiment.
Statistical analysis.
Prevalence and intensity of infection in feeding experiments in symptomatic and asymptomatic participants were compared using the nonparametric Kruskal-Wallis test. A nonparametric Wilcoxon test was used for paired experiments between SFAs and DMFAs. Correlation between gametocytemia and the proportion of infected mosquitoes and intensity of infection was determined with nonparametric Spearman’s rank statistics. All statistical analyses were conducted in R (version 4.0.4; R Foundation for Statistical Computing, Vienna, Austria).
Ethics statement.
This protocol was reviewed and approved by the ethics committee of the University of California-San Diego (120652), Universidad Peruana Cayetano Heredia (SIDISI 0059751 in 2015 and 102305 in 2019), Asociacion Benéfica Prisma (CE0801), and Dirección Regional de Salud de Loreto (0010-CIEI-DRSL-2015).
RESULTS
Mosquito infectivity of symptomatic and asymptomatic Plasmodium vivax carriers.
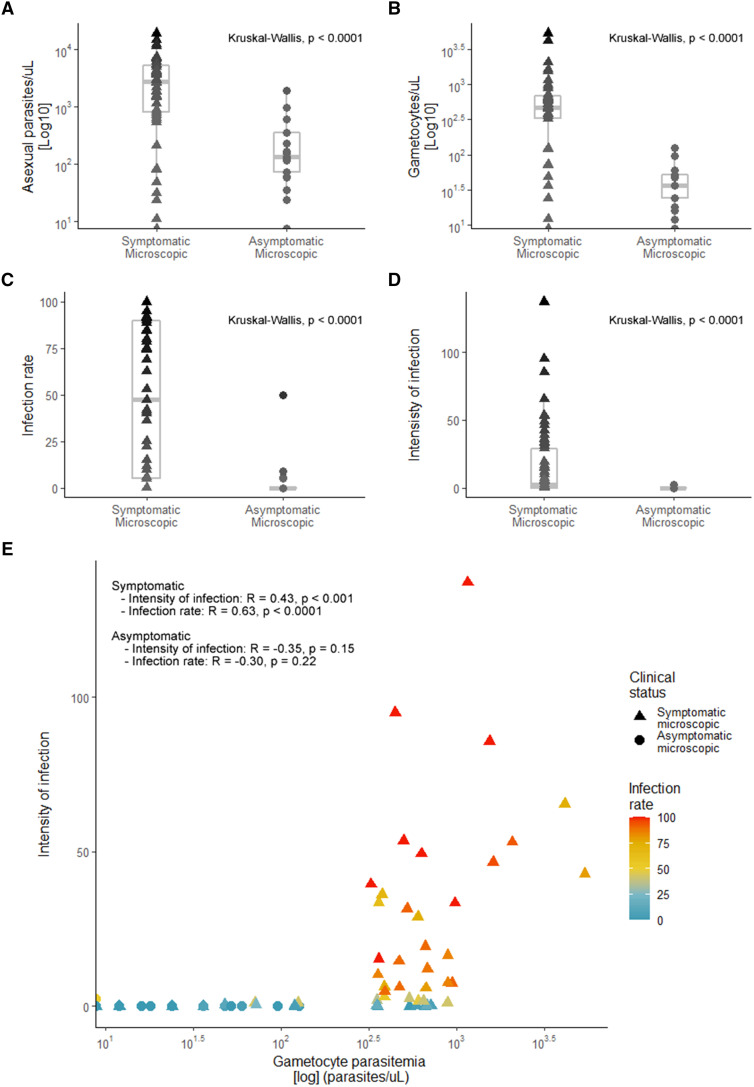
A total of 105 participants infected with P. vivax were enrolled in the study. Fifty-seven participants had malaria-compatible symptoms, and 18 subjects did not have symptoms at the time of the survey but were positive for P. vivax by thick blood smear. Thirty participants did not have malaria symptoms or detectable parasites by light microscopy, but PCR confirmed parasitemia (henceforth termed asymptomatic sub-microscopic) (Table 1). Overall, the proportion of males (57%, 60 of 105) and females (43%, 45 of 105) in the study was similar, with no significant differences between groups (Fisher’s exact test, P = 0.9289; Table 1). Parasite densities were greater in clinical malaria infections than in asymptomatic parasite carriers (Kruskal-Wallis, P < 0.0001; Table 1, Figure 1A). In the sub-microscopic infections, the median parasite density based on PCR was 7.76 parasites/µL, less than the microscopic infections (Table 1). Roughly 93% of symptomatic subjects (53 of 57 subjects) had patent gametocytemia at the time of the feeding assay, whereas only 72% of asymptomatic patients had visible sexual parasite forms (13 of 18 patients). Gametocytemia was significantly greater in symptomatic patients than in asymptomatic carriers (Kruskal-Wallis, P < 0.0001; Table 1, Figure 1B). Fluctuations in parasitemia were observed in a few asymptomatic individuals during the 21 days of follow-up, demonstrating that the asexual parasite stages can persist over time or decline without treatment, as the first sample was taken in different cases (Supplemental Figure S1).
Table 1.
Summary of direct membrane mosquito-feeding assays grouped by clinical and diagnosis status
| Characteristic | Symptomatic, microscopic (n = 57) | Asymptomatic, microscopic (n = 18) | Asymptomatic, sub-microscopic (n = 30) | Overall (N = 105) |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Female | 25 (44) | 8 (44) | 12 (40) | 45 (43) |
| Male | 32 (56) | 10 (56) | 18 (60) | 60 (57) |
| Parasite density, parasites/μL; median (IQR) | 2,700 (772–5,320)* | 131.5 (63–326.5)* | 7.76 (3.23–14.43)† | 1,570 (143–4,054) |
| Gametocyte density, gametocytes/μL; median (IQR) | 448 (120–669) | 24 (3–48) | – | 352 (36– 618) |
| No. of infectious individuals | 44 | 4 | 0 | 48 |
| Total oocysts/infected mosquito, median (IQR) | 211 (32.25–1,098.25) | 5 (4.25–15.50) | 0 (0–0) | 167.5 (22.25–988) |
| Mosquito infection prevalence,‡ %; median (IQR) | 76 (40–91.19) | 7.22 (5.4–19.16) | 0 (0–0) | 74.75 (33.52–90.28) |
| Intensity of infection, mean ± SD | 22.14 ± 29.96 | 0.65 ± 1.13 | 0 | 20.35 ± 29.28 |
Parasite density in symptomatic and asymptomatic microscopic groups was calculated with microscopy only.
Parasite density in the symptomatic sub-microscopic group was quantified using quantitative polymerase chain reaction methods, but we could not differentiate sexual stage from asexual stage.
Mosquito infection prevalence calculated for positive infections only.
Figure 1.
Parasitemia, the proportion of infected mosquitoes, and intensity of infection after feeding on symptomatic and asymptomatic Plasmodium vivax carrier samples. (A) Asexual parasite density (log10 parasites/μL) per clinical group. (B) Gametocyte density (log10 gametocytes/μL) per clinical group. (C) Proportion of mosquitoes (percent infection) infected by asymptomatic and symptomatic carriers. (D) Intensity of infection (mean oocyst burden/total dissected mosquitoes in one feeding experiment) by asymptomatic and symptomatic carriers. (E) Correlation between sexual parasite density with the intensity of infection and infection rate in both symptomatic and asymptomatic carriers. The parasite density for both groups was calculated using microscopy. This figure appears in color at www.ajtmh.org.
The proportion of infection of mosquitoes was significantly less in asymptomatic parasite carriers confirmed by microscopy (22%, 4 of 18), compared with symptomatic carriers (77%, 44 of 57) (Kruskal-Wallis, P < 0.0001; Table 1, Figure 1C). The median mosquito infection prevalence among asymptomatic individuals was 7.22% (interquartile range [IQR], 5.4–19.16), and 2.6% of mosquitoes (19 of 728) became infected. For symptomatic individuals, the prevalence reached 76% (IQR, 40–91.19), with 51% of mosquitoes (890 of 1,753) infected. Similarly, the intensity of infection in mosquitoes was greater in the symptomatic group (mean ± SD, 22.14 ± 29.96) compared with asymptomatic carriers (mean ± SD, 0.65 ± 1.13) (Kruskal-Wallis, P < 0.0001; Table 1, Figure 1D). Furthermore, gametocytemias had a positive correlation with the intensity of infection in the symptomatic group (R = 0.43; 95% CI, 0.19–0.62; P < 0.0001), but not in the asymptomatic one (R = –0.35; 95% CI, –0.70 to 0.14; P = 0.15). A similar association was detected for the proportion of infected mosquitoes and gametocytemia, with a strong correlation with symptomatic (R = 0.63; 95% CI, 0.45–0.77; P < 0.0001), but not asymptomatic (R = –0.30; 95% CI, –0.67 to 0.19; P = 0.22) carriers (Figure 1E). Moreover, none of the sub-microscopic infection participants infected mosquitoes (30 feeding experiments, 1,161 dissected mosquitoes).
Mosquito infectivity using direct skin feeding assays versus direct membrane mosquito-feeding assays.
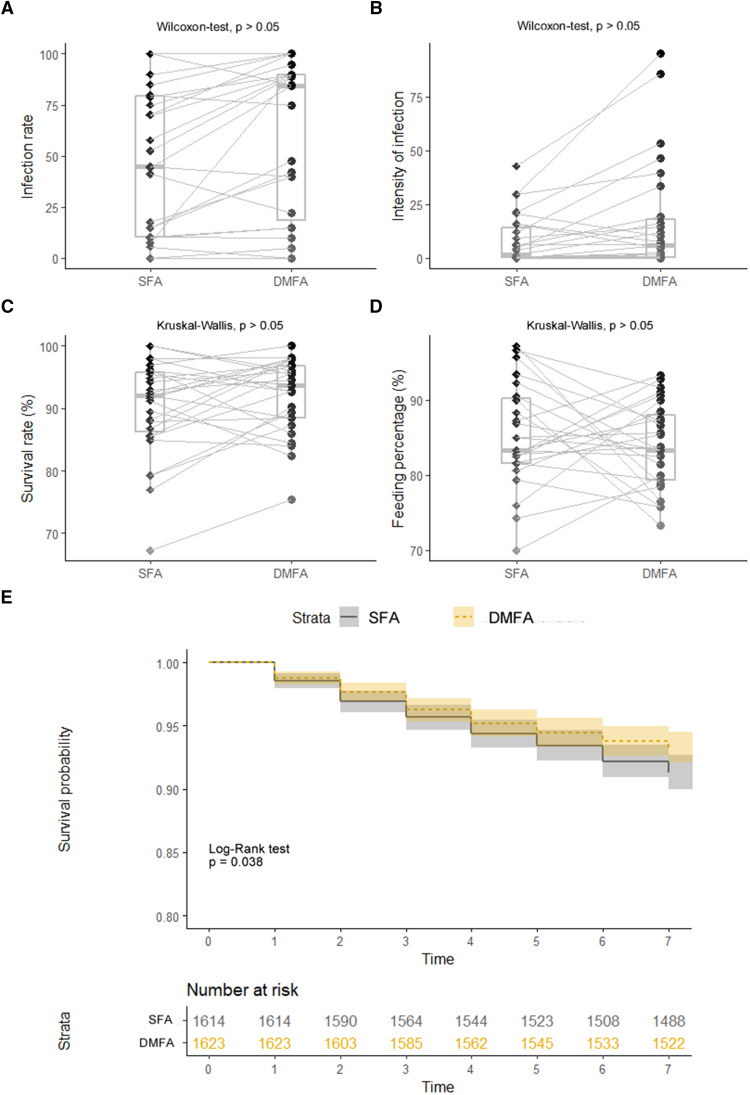
Twenty-seven paired experiments from symptomatic individuals were performed simultaneously using SFA and DMFA methodologies. The same 24 symptomatic participants were infectious to mosquitoes by both DMFA and SFA (89%, 24 of 27). The median prevalence of infection in mosquitoes after SFA was 55 (IQR, 15–81.25), whereas it was 85 (IQR, 40–91.25) for DMFA (Wilcoxon test, P > 0.05; Table 2, Figure 2A). The mean intensity of infection after DMFA was six times greater than for SFA, although this was not significant (Wilcoxon test, P > 0.05; Table 2, Figure 2B). In addition, gametocytemia correlated significantly and positively with the infection rate (R = 0.53; 95% CI, 0.19–0.76; P < 0.01) for DMFAs, but not for SFAs (R = 0.38; 95% CI, –0.001 to 0.66; P > 0.05; Supplemental Figure S2). Furthermore, the median number of sporozoites detected was not significantly different between SFA (median, 2,405; IQR, 909–13,706) and DMFA (median, 2,750; IQR, 400–20,000) (Wilcoxon test, P > 0.05; Table 2).
Table 2.
Results of paired direct membrane mosquito-feeding assays and skin-feeding assays of symptomatic Plasmodium vivax participants (N = 27)
| Characteristic | Skin-feeding assay | Direct membrane mosquito-feeding assay |
|---|---|---|
| Gender, n (%) | ||
| Female | 8 (29.6) | |
| Male | 19 (70.4) | |
| Parasite density, parasites/μL; median (IQR) | 3,000 (853–5,452) | |
| Gametocyte density, gametocytes/μL; median (IQR) | 498 (356–679.5) | |
| Proportion of infected feeds, % (n/N) | 88.8 (24/27) | 88.8 (24/27) |
| Fed mosquitoes, %; median (IQR) | 85 (70–97.2) | 83.3 (73.3–93.3) |
| Infected mosquitoes, % (n/N) | 48.5 (248/511) | 58.9 (308/523) |
| Total oocysts/infected mosquito, median (IQR) | 73 (4.75–276.75) | 140 (27.25–464.25) |
| Sporozoites/mosquito, median (IQR) | 2,405 (909–13,706) | 2,750 (400–20,000) |
| Mosquito infection rate, %; median (IQR) | 55.26 (15–81.25) | 85 (40–91.25) |
| Intensity of infection, mean ± SD | 3.32 ± 11.94 | 19.12 ± 26.84 |
IQR = interquartile range. Parasite and gametocyte densities were calculated by microscopy. Dissections were performed in paired direct membrane mosquito-feeding assays and skin-feeding assays (N = 27), and sporozoite calculations were performed with pools of mosquitoes (range, 9–35; N = 848 mosquitoes dissected). The median sporozoite number per mosquito per participant was calculated in 14 paired experiments. The percentage of infected mosquitoes was calculated only with positive infections.
Figure 2.
The proportion of infected mosquitoes, infection intensity, and mosquito survival after feeding assays on Plasmodium vivax carriers. (A) Proportion of mosquitoes (percent infection) infected by skin-feeding assay (SFA) and direct membrane mosquito-feeding assay (DMFA) experiments. (B) Intensity of infection by SFA and DMFA experiments. (C) Survival rate of mosquitoes by SFA and DMFA experiments. (D) Feeding percentage of mosquitoes by SFA and DMFA experiments. (E) Kaplan-Meier curve to test mosquito survival probability by SFA and DMFA experiments. This figure appears in color at www.ajtmh.org.
The mosquito survival rate, recorded daily up to 7 days post-feeding, showed no difference between the feeding assay methods (Figure 2C), with a median survival rate for SFAs of 92.0% (IQR, 86.25–95.76) and 93.65% (IQR, 88.46–96.85) for DMFAs. Furthermore, we found that a large proportion of mosquitoes had a high survival probability, with 92% surviving up to 7 days, with small differences between assays (log-rank test, P < 0.05; Figure 2E). Nonsignificant differences were detected in mosquito palatability as the proportion of engorged females between the experiments (SFA range, 70–97; DMFA range, 73–93; Wilcoxon-test, P > 0.05; Figure 2D).
DISCUSSION
Understanding infectiousness of P. vivax parasite carriers for vector mosquitoes—an important human reservoir of malaria transmission and maintenance of endemic malaria—is critical for designing control and elimination strategies. In this study, we assessed the infectiousness of natural P. vivax infections in symptomatic and asymptomatic human parasite carriers to Ny. darlingi mosquitoes in endemic settings in the Peruvian Amazon. Our results corroborated the high infectivity of symptomatic P. vivax carriers to a range of mosquito species observed elsewhere, including Southeast Asia,35 Ethiopia,36 Brazil,37 and Colombia.38 The proportion of patent asymptomatic infections leading to infected mosquitoes was at least 20% less in our study, similar to that found in other studies in Asia.39 The proportion of asymptomatic subjects with parasitemia who infected mosquitoes in our study (4 of 18) gains importance because asymptomatic and sub-microscopic infections predominate in this transmission scenario.27 An important question is whether sub-microscopic infections are important for continuing transmission.28 In our study, none of the asymptomatic, low-density PCR-detected infections infected a single mosquito, in contrast with reports in Africa36,40 and the Americas,5 where an important proportion of infected mosquitoes were from asymptomatic PCR-positive P. vivax carriers. These data suggest that low-density asymptomatic P. vivax infections may be less relevant to transmission, although it is possible that parasitemias fluctuate over time on both an individual and population basis, thus perhaps underestimating the force of infection from this potentially important human reservoir of malaria transmission.39 Further work to quantify the contribution of asymptomatic parasitemia to malaria incidence and resilience in the Americas is needed.10,41
Our results are consistent with studies showing that P. vivax gametocyte and parasite densities in symptomatic individuals are closely related to mosquito infectivity. This is likely a result of the rapid development of gametocytes in P. vivax, wherein transmission can occur at the first development of symptoms,4,42,43 as well as a result of the circulating time of P. vivax gametocytes in the bloodstream after asexual forms are cleared.44 However, other factors are involved in infection efficiency, such as adequate numbers of male and female gametocytes for successful fertilization in the mosquito midgut45 and host immune responses,12 which may also contribute to understanding of lack of close correlation and mosquito infectivity in asymptomatic carriers.
Our results show that roughly 89% of the participants were infectious to mosquitoes, and these infections were successful regardless of feeding methodology. As the proportion of infection was equal for SFAs and DMFAs, the mechanistic reasons underlying the difference in transmission efficiency of these methods in other experiments could be the result of technical issues,46–48 biological changes, or a combination of the two. Some studies have shown a greater proportion of infected mosquitoes using SFAs compared with DMFAs.21,23–25 These studies corroborate that artificial feeding on venous blood reflects natural biting and provides a reliable estimate of the infectious reservoir.
This study has some limitations. For instance, the first results found that low-density asymptomatic infections in adults were not an important source of mosquito infections, but the relatively small number of experiments cannot lead to firm conclusions about the importance of low-density parasitemia in the Peruvian Amazon. Furthermore, infectiousness was investigated exclusively from adult parasite carriers (age ≥ 18 years), potentially underestimating the fraction of the malaria reservoir in other age groups. Although in the Peruvian Amazon the greatest gametocytemia is detected most commonly in young adults,27 mosquito exposure varies depending on age group and occupation,47,48 influencing relative contributions to transmission. Our results reflect a single time point in the infectivity of the participants, and it was not possible to establish precisely when the parasite infection began or when the participant started and stopped being infective. Longitudinal studies with serialized estimations of the infectiousness to mosquitoes in parasite carriers, both with and without symptoms, would shed light on the duration of the infection period, including after antimalarial treatment. This is of vital importance in the Peruvian context, as treatment of asymptomatic P. vivax carriers is recommended only in patent parasite carriers. Molecular gametocyte quantification (quantitative RT-PCR) would improve our estimates of asymptomatic carriage, and the contribution of these carriers to the infectious reservoir in this setting.
In pre-elimination and elimination settings, accurate detection and diagnostics of the asymptomatic parasitemic fraction of the population is of paramount importance to deter the infection of natural mosquitoes and, hence, malaria propagation. Our results are critical in representing the infectiousness to P. vivax of Ny. darlingi, the major malaria vector in the Amazon Basin. Moreover, the paired feeding assays confirmed that DMFA and SFA yield comparable results and validate this indirect methodology to assess field P. vivax infectiousness to natural mosquitoes. These results have important implications for the malaria control program in Peru, which largely focuses on the treatment of symptomatic patients. Longitudinal studies with longer follow-up of symptomatic and asymptomatic parasite infections are needed to determine the natural variations of disease transmissibility.
Supplemental Material
ACKNOWLEDGMENTS
We appreciate the enthusiastic support of the communities of Cahuide, Lupuna, and Mazan, and the volunteers who agreed to participate in this study. We are grateful to Dirección Regional de Salud (DIRESA, Iquitos, Loreto, Peru) for collaboration and logistic support in Loreto Department and our colleague Lidia Llacsahuanga for her support with some analysis and database management.
Note: Supplemental figures and appendix appear at www.ajtmh.org.
REFERENCES
- 1. World Health Organization , 2020. World Malaria Report. Geneva, Switzerland: WHO. [Google Scholar]
- 2. Krotoski WA, 1989. The hypnozoite and malarial relapse. Prog Clin Parasitol 1: 1–19. [PubMed] [Google Scholar]
- 3. Adams JH, Mueller I, 2017. The biology of Plasmodium vivax. Cold Spring Harb Perspect Med 7: a025585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bousema T, Drakeley C, 2011. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 24: 377–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vallejo AF, García J, Amado-Garavito AB, Arévalo-Herrera M, Herrera S, 2016. Plasmodium vivax gametocyte infectivity in sub-microscopic infections. Malar J 15: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collins KA. et al. , 2020. A Plasmodium vivax experimental human infection model for evaluating efficacy of interventions. J Clin Invest 130: 2920–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smalley ME, Brown J, Bassett NM, 1981. The rate of production of Plasmodium falciparum gametocytes during natural infections. Trans R Soc Trop Med Hyg 75: 318–319. [DOI] [PubMed] [Google Scholar]
- 8. Schneider P, Greischar MA, Birget PLG, Repton C, Mideo N, Reece SE, 2018. Adaptive plasticity in the gametocyte conversion rate of malaria parasites. PLoS Pathog 14: e1007371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lefèvre T, Vantaux A, Dabiré KR, Mouline K, Cohuet A, 2013. Non-genetic determinants of mosquito competence for malaria parasites. PLoS Pathog 9: e1003365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tadesse FG, Meerstein-Kessel L, Gonçalves BP, Drakeley C, Ranford-Cartwright L, Bousema T, 2019. Gametocyte sex ratio: the key to understanding Plasmodium falciparum transmission? Trends Parasitol 35: 226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boyd MF, Stratman-Thomas WK, Kitchen SF, 1935. On the relative susceptibility of Anopheles quadrimaculatus to Plasmodium vivax and Plasmodium falciparum . Am J Trop Med Hyg 15: 485–493. [Google Scholar]
- 12. Abeles SR, Chuquiyauri R, Tong C, Vinetz JM, 2013. Human host-derived cytokines associated with Plasmodium vivax transmission from acute malaria patients to Anopheles darlingi mosquitoes in the Peruvian Amazon. Am J Trop Med Hyg 88: 1130–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meibalan E, Marti M, 2017. Biology of malaria transmission. Cold Spring Harb Perspect Med 7: a025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bennink S, Kiesow MJ, Pradel G, 2016. The development of malaria parasites in the mosquito midgut. Cell Microbiol 18: 905–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bradley J. et al. , 2018. Predicting the likelihood and intensity of mosquito infection from sex specific Plasmodium falciparum gametocyte density. eLife 7: e34463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bansal GP, Weinstein CS, Kumar N, 2016. Insight into phagocytosis of mature sexual (gametocyte) stages of Plasmodium falciparum using a human monocyte cell line. Acta Trop 157: 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clayton AM, Dong Y, Dimopoulos G, 2014. The Anopheles innate immune system in the defense against malaria infection. J Innate Immunol 6: 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Molina-Cruz A, Zilversmit MM, Neafsey DE, Hartl DL, Barillas-Mury C, 2016. Mosquito vectors and the globalization of Plasmodium falciparum malaria. Annu Rev Genet 50: 447–465. [DOI] [PubMed] [Google Scholar]
- 19. Kiattibutr K. et al. , 2017. Infectivity of symptomatic and asymptomatic Plasmodium vivax infections to a southeast Asian vector, Anopheles dirus. Int J Parasitol 47: 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bousema T. et al. , 2012. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS One 7: e42821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andolina C. et al. , 2021. Sources of persistent malaria transmission in a setting with effective malaria control in eastern Uganda: a longitudinal, observational cohort study. Lancet Infect Dis 21: 1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Muirhead-Thomson RC, 1957. The malarial infectivity of an African village population to mosquitoes (Anopheles gambiae): a random xenodiagnostic survey. Am J Trop Med Hyg 6: 971–979. [DOI] [PubMed] [Google Scholar]
- 23. Bonnet S, Gouagna C, Safeukui I, Meunier JY, Boudin C, 2000. Comparison of artificial membrane feeding with direct skin feeding to estimate infectiousness of Plasmodium falciparum gametocyte carriers to mosquitoes. Trans R Soc Trop Med Hyg 94: 103–106. [DOI] [PubMed] [Google Scholar]
- 24. Diallo M. et al. , 2008. Evaluation and optimization of membrane feeding compared to direct feeding as an assay for infectivity. Malar J 7: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sattabongkot J, Maneechai N, Phunkitchar V, Eikarat N, Khuntirat B, Sirichaisinthop J, Burge R, Coleman RE, 2003. Comparison of artificial membrane feeding with direct skin feeding to estimate the infectiousness of Plasmodium vivax gametocyte carriers to mosquitoes. Am J Trop Med Hyg 69: 529–535. [PubMed] [Google Scholar]
- 26. Rosas-Aguirre A, Guzman-Guzman M, Gamboa D, Chuquiyauri R, Ramirez R, Manrique P, Carrasco-Escobar G, Puemape C, Llanos-Cuentas A, Vinetz JM, 2017. Micro-heterogeneity of malaria transmission in the Peruvian Amazon: a baseline assessment underlying a population-based cohort study. Malar J 16: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rovira-Vallbona E, Contreras-Mancilla JJ, Ramirez R, Guzmán-Guzmán M, Carrasco-Escobar G, Llanos-Cuentas A, Vinetz JM, Gamboa D, Rosanas-Urgell A, 2017. Predominance of asymptomatic and sub-microscopic infections characterizes the Plasmodium gametocyte reservoir in the Peruvian Amazon. PLoS Negl Trop Dis 11: e0005674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin JT, Saunders DL, Meshnick SR, 2014. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol 30: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Recker M, Bull PC, Buckee CO, 2018. Recent advances in the molecular epidemiology of clinical malaria. F1000Research 7: 1159. [DOI] [PMC free article] [PubMed]
- 30. Ministerio de Salud del Perú (MINSA) , 2010. Norma Técnica de Salud para el Control de Calidad del Diagnóstico Microscópico de Malaria. Lima, Peru: MINSA. [Google Scholar]
- 31. Mangold KA, Manson RU, Koay ESC, Stephens L, Regner M, Thomson RB, Peterson LR, Kaul KL, 2005. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol 43: 2435–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rougemont M, Van Saanen M, Sahli R, Hinrikson HP, Bille J, Jaton K, 2004. Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J Clin Microbiol 42: 5636–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ministerio de Salud del Perú (MINSA) , 2015. Norma Técnica de Salud para la Atención de la Malaria y Malaria Grave en el Perú. Lima, Perú: MINSA.
- 34. Kim K, Tsuda Y, Yamada A, 2009. Bloodmeal identification and detection of avian malaria parasite from mosquitoes (Diptera: Culicidae) inhabiting coastal areas of Tokyo Bay, Japan. J Med Entomol 46: 1230–1234. [DOI] [PubMed] [Google Scholar]
- 35. Vantaux A. et al. , 2018. Contribution to malaria transmission of symptomatic and asymptomatic parasite carriers in Cambodia. J Infect Dis 217: 1561–1568. [DOI] [PubMed] [Google Scholar]
- 36. Tadesse FG. et al. , 2018. The relative contribution of symptomatic and asymptomatic Plasmodium vivax and Plasmodium falciparum infections to the infectious reservoir in a low-endemic setting in Ethiopia. Clin Infect Dis Off Publ Infect Dis Soc Am 66: 1883–1891. [DOI] [PubMed] [Google Scholar]
- 37. Alves MJCP, Mayo RC, Donalisio MR, 2004. History, epidemiology and control of malaria in Campinas Region, São Paulo State, Brazil, 1980 to 2000. Rev Soc Bras Med Trop 37: 41–45. [DOI] [PubMed] [Google Scholar]
- 38. Vallejo AF, Rubiano K, Amado A, Krystosik AR, Herrera S, Arévalo-Herrera M, 2016. Optimization of a membrane feeding assay for Plasmodium vivax infection in Anopheles albimanus. PLoS Negl Trop Dis 10: e0004807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin JT. et al. , 2016. Microscopic Plasmodium falciparum gametocytemia and infectivity to mosquitoes in Cambodia. J Infect Dis 213: 1491–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ouédraogo AL. et al. , 2009. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PLoS One 4: e8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bousema T, Okell L, Felger I, Drakeley C, 2014. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 12: 833–840. [DOI] [PubMed] [Google Scholar]
- 42. Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, del Portillo HA, 2009. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis 9: 555–566. [DOI] [PubMed] [Google Scholar]
- 43. Boyd MF, Kitchen SF, 1937. On the infectiousness of patients infected with Plasmodium vivax and Plasmodium falciparum . Am J Trop Med Hyg 17: 253–262. [Google Scholar]
- 44. McCarthy JS. et al. , 2013. Experimentally induced blood-stage Plasmodium vivax infection in healthy volunteers. J Infect Dis 208: 1688–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tadesse FG. et al. , 2021. Anopheles stephensi mosquitoes as vectors of Plasmodium vivax and falciparum, Horn of Africa, 2019. Emerg Infect Dis 27: 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chali W. et al. , 2020. Comparison of infectivity of Plasmodium vivax to wild-caught and laboratory-adapted (colonized) Anopheles arabiensis mosquitoes in Ethiopia. Parasit Vectors 13: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gonçalves BP. et al. , 2017. Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat Commun 8: 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tiono AB, Guelbeogo MW, Sagnon NF, Nébié I, Sirima SB, Mukhopadhyay A, Hamed K, 2013. Dynamics of malaria transmission and susceptibility to clinical malaria episodes following treatment of Plasmodium falciparum asymptomatic carriers: results of a cluster-randomized study of community-wide screening and treatment, and a parallel entomology study. BMC Infect Dis 13: 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.