Key Points
Question
Are benzodiazepines or antihistamines effective in the treatment of acute vertigo?
Findings
In this systematic review and meta-analysis of 17 trials involving 1586 participants, 7 studies comprising 802 total patients evaluated the primary outcome of change in 100-point vertigo visual analog scale scores at approximately 2 hours after treatment with an antihistamine or benzodiazepine. Antihistamines resulted in greater patient improvement than benzodiazepines (difference, 16.1) but were not superior to other active comparators, including ondansetron, droperidol, metoclopramide, and piracetam.
Meaning
The findings of this study suggest that antihistamines may be superior to benzodiazepines in the treatment of acute vertigo and that the use of the latter should be discouraged.
This systematic review and meta-analysis compares the efficacy of benzodiazepine or antihistamine treatment for patients with acute vertigo from any underlying cause.
Abstract
Importance
Acute vertigo can be disabling. Antihistamines and benzodiazepines are frequently prescribed as “vestibular suppressants,” but their efficacy is unclear.
Objective
To assess the efficacy of antihistamines and benzodiazepines in the treatment of acute vertigo from any underlying cause.
Data Sources
We searched the PubMed, CENTRAL, EMBASE, CINAHL, Scopus, and ClinicalTrials.gov databases from inception to January 14, 2019, without language restrictions. Bibliographies of the included studies and relevant reviews were also screened.
Study Selection
We included randomized clinical trials (RCTs) comparing antihistamine or benzodiazepine use with another comparator, placebo, or no intervention for patients with a duration of acute vertigo for 2 weeks or less. Studies of healthy volunteers, prophylactic treatment, or induced vertigo were excluded, as were studies that compared 2 medications from the same class.
Data Extraction and Synthesis
Following Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines, data were extracted and risk of bias was assessed by 2 authors independently for each study. Data were pooled using a random-effects model.
Main Outcomes and Measures
The predefined primary outcome was change in 10- or 100-point vertigo or dizziness visual analog scale (VAS) scores at 2 hours after treatment. Secondary outcomes included change in nausea VAS scores at 2 hours, use of rescue medication at 2 hours, and improvement or resolution of vertigo at 1 week or 1 month.
Results
Of the 27 trials identified in the systematic review, 17 contributed to the quantitative meta-analysis and involved a total of 1586 participants. Seven trials with a total of 802 participants evaluated the primary outcome of interest: single-dose antihistamines resulted in significantly more improvement on 100-point VAS scores compared with benzodiazepines (difference, 16.1 [95% CI, 7.2 to 25.0]) but not compared with other active comparators (difference, 2.7 [95% CI, –6.1 to 11.5]). At 1 week and 1 month, neither daily benzodiazepines nor antihistamines were reported to be superior to placebo. RCTs comparing the immediate effects of medications (at 2 hours) after a single dose generally had a low risk of bias, but those evaluating 1-week and 1-month outcomes had a high risk of bias.
Conclusions and Relevance
Moderately strong evidence suggests that single-dose antihistamines provide greater vertigo relief at 2 hours than single-dose benzodiazepines. Furthermore, the available evidence did not support an association of benzodiazepine use with improvement in any outcomes for acute vertigo. Other evidence suggested that daily antihistamine use may not benefit patients with acute vertigo. Larger randomized trials comparing both antihistamines and benzodiazepines with placebo could better clarify the relative efficacy of these medications.
Introduction
Vertigo is the sensation of movement (typically rotatory) and is frequently associated with nausea or imbalance. It effects up to 20% of adults, with a higher incidence in women and older individuals.1,2 Curative treatment can sometimes be tailored to the specific underlying cause,1,2,3,4,5 but medications are frequently used to suppress vertigo symptoms.2,5,6 Repositioning techniques are the preferred treatment for benign positional paroxysmal vertigo (BPPV).3,5,6 However, medications are also frequently prescribed,6 perhaps because many patients experience ongoing symptoms despite treatment with repositioning maneuvers.7 Antihistamines and benzodiazepines are frequently prescribed as “vestibular suppressants” for vertigo.2,3,4
Although treatment based on acute vertigo etiology is recommended, a specific cause is not always immediately identified.1,3,8 Symptom control for acute vertigo with vestibular suppressants may be indicated with or without a definitive diagnosis, and the efficacy of these medications remains unclear. In this systematic review and meta-analysis, we assessed the relative efficacy of benzodiazepines and antihistamines when compared with each other, other active comparators, placebo, or no intervention in the treatment of acute vertigo from any underlying cause.
Methods
Data Sources
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and is registered with PROSPERO. A medical librarian (L.M.M.) searched the PubMed, CENTRAL, EMBASE, CINAHL, and Scopus databases from inception through June 14, 2019, without language restrictions. The PubMed search was updated on December 12, 2021, to include studies published since the original search. The search was conducted using keywords and MeSH terms related to vertigo and benzodiazepines or antihistamines (eAppendix in the Supplement).
Study Selection
Randomized or quasi-randomized clinical trials (RCTs) were included if they compared any antihistamine or benzodiazepine with a comparator, placebo, or no intervention in human patients with acute vertigo (duration of ≤2 weeks). Individual study definitions of vertigo were accepted.
Studies comparing 2 drugs from the same class were excluded, as were studies of healthy volunteers, induced vertigo, or prophylactic treatment. Trials had to report on 1 or more predefined clinical outcomes. When study results lacked sufficient detail, we attempted to contact the authors via email.
Data Abstraction
After duplicates were removed, all titles and abstracts were screened by at least 2 authors independently. For articles determined by either reviewer to potentially meet the eligibility criteria, the full text was obtained and reviewed by the first author (B.R.H.) and at least 1 other author independently to determine eligibility. Disagreements were resolved through discussion. Bibliographies of the included studies and relevant reviews were screened, and the ClinicalTrials.gov database was searched for published or unpublished studies.
Demographic data, including study country, vertigo type, therapies being compared, number of patients, inclusion and exclusion criteria, and results, were abstracted from each study by the first author (B.R.H.) and at least 1 other author. Discrepancies were resolved through discussion.
Main Outcomes and Measures
The predefined primary outcome was change in 10- or 100-point vertigo or dizziness visual analog scale (VAS) score at 2 hours after treatment. Results between 30 minutes and 4 hours were allowed, but the time frame closest to 2 hours was preferentially used.
Secondary outcomes included change in nausea VAS score at 2 hours, need for rescue medication or intervention at 2 hours, resolution of vertigo at 1 week, resolution at 1 month, improvement at 1 week, improvement at 1 month, and nystagmography results. Adverse events were recorded as reported within individual studies.
We planned to report change in VAS scores at 1 week and 1 month but found essentially no studies that used VAS measures for these time frames. We decided to abstract data dichotomized as (1) complete resolution or not and (2) good or satisfactory improvement or not. Good or satisfactory improvement was defined as either patient-rated improvement as such or at least a 50% decrease in symptom severity on any numeric scale.
Quality Assessment
The risk of bias (ROB) for each study was assessed using the Cochrane Risk of Bias Tool.9 This tool assigns high, low, or unclear ROB in the following 7 domains: random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessors, incomplete outcome reporting, selective reporting, and other bias. The senior author (J.M.K.) assessed all studies for ROB. Each study was also assessed by 1 other author (A.Z.W. or A.W.B.). Discrepancies were resolved through discussion.
Subgroup and Sensitivity Analyses
We planned to compare antihistamines with all comparators, benzodiazepines with all comparators, and antihistamines vs benzodiazepines. We also planned sensitivity analyses of (1) each medication class vs placebo or no active comparator and (2) studies with low ROB. Planned subgroups were patients diagnosed with BPPV, those diagnosed with central vertigo, and those with peripheral vertigo not consistent with BPPV.
Statistical Analysis
All vertigo VAS results were converted to 100-point scores by multiplying the 10-point scores by 10. The mean change in VAS was compared as a continuous variable for the intervention and control groups using inverse variance and a random-effects model. Results of the meta-analysis are reported as the difference in mean VAS change with 95% CIs. Change in nausea VAS scores was calculated the same way. Dichotomous outcomes were calculated with the Mantel-Haenszel method (random effects) and are reported as relative risks (RRs) with 95% CIs. Review Manager version 5.4 (Cochrane Collaboration) was used to perform the meta-analyses and produce the corresponding forest plots.10
Results
Search Results
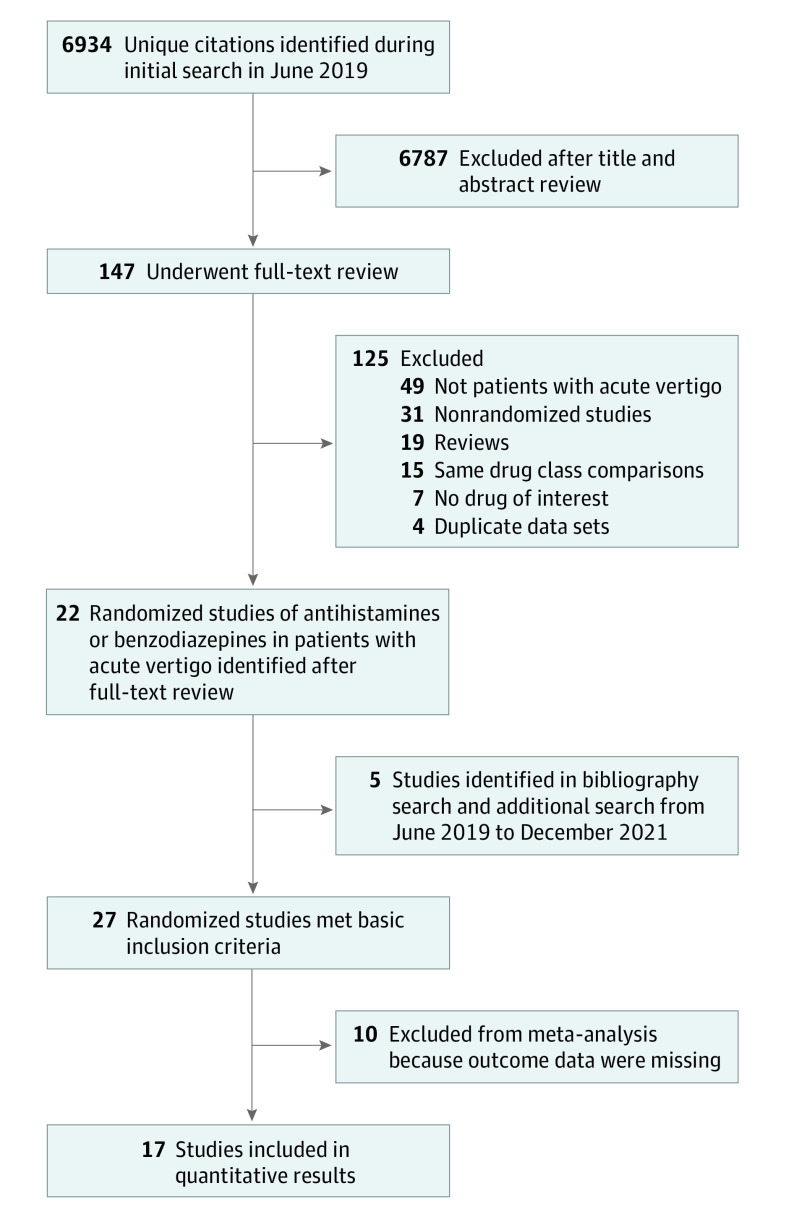
Figure 1 presents a flow diagram of the study identification. The initial search (through June 14, 2019) yielded 6934 unique citations. After title and abstract review, 6787 studies were excluded and full-text review was performed for the remaining 147 studies. Of these, 22 were RCTs of antihistamine or benzodiazepine treatment for human participants with acute vertigo. Seven RCTs lacked appropriate outcomes or data to be included in quantitative analyses, and we were unable to obtain further information from the study authors. Bibliography and ClinicalTrials.gov searches identified 2 additional RCTs, but neither had sufficient information for inclusion in the meta-analysis. Finally, 3 RCTs published since June 14, 2019, were identified as a result of the PubMed search updated on December 12, 2021; 2 were included in the quantitative analysis. In all, we identified 27 RCTs, 17 of which contributed to the quantitative results.
Figure 1. Flow Diagram of Trial Identification and Selection.
Characteristics of Excluded Studies
Table 1 summarizes the methods and results for the 10 RCTs11,12,13,14,15,16,17,18,19,20 with 918 participants that were not included in the quantitative analysis. Most of these studies were excluded because the results lacked sufficient detail (eg, only provided P values, no raw numbers). Diazepam (4 trials12,16,17,19) was the only benzodiazepine studied among these 10 RCTs. In 1 trial,12 diazepam resulted in lower “severity of vertigo” at 2 hours compared with methylprednisolone. The other 3 trials compared diazepam with antihistamines: 2 reported a greater reduction in vertigo symptoms or nystagmus with antihistamines,17,19 and 1 reported no difference between diazepam and promethazine with regard to the duration of vertigo.16 Nine studies included antihistamines against other comparators. Two studies compared antihistamines against repositioning maneuvers: 1 study15 reported no difference at 1 month and the other18 reported that the repositioning maneuver was superior. Five studies11,13,14,17,18 compared antihistamines with placebo or no treatment, and all reported benefit with antihistamines across several different primary outcomes.
Table 1. Randomized Clinical Trials Not Included in the Quantitative Results of the Systematic Review.
| Source | No. of patients | Comparators | Main outcome | Reason for study exclusion | Main results |
|---|---|---|---|---|---|
| Babin et al,11 1984 | 77 | Scopolamine vs meclizine vs placebo | Global vertigo, frequency of attacks | No result details, only P values; authors unavailable or could not provide additional detail | Both antihistamines better than placebo |
| Barzegari et al,12 2016 | 113 | Diazepam vs methylprednisolone | Dizziness severity at 30, 60, and 120 min | Data not presented in useable form; authors unavailable or could not provide additional detail | Less severity of vertigo at 2 h with diazepam compared with methylprednisolone |
| Herndon et al,13 1975 | 17 | Combination pill of pheniramine, nicotine acid, and pentylenetetrazol vs placebo | Nystagmus and vertigo scores | No results details; describes “lower” scores, but no raw numbers or data; authors unavailable or could not provide additional detail | Combination pill resulted in lower vertigo and nystagmus scores at 1 wk |
| Jalali et al,14 2020 | 117 | Betahistine vs dimenhydrinate vs placebo | Scores on Dizziness Handicap Inventory and modified Berg Balance Scale | No outcomes of interest; results not presented in useable manner; authors unavailable or could not provide additional detail | Betahistine treatment resulted in greater improvement in dizziness scores |
| Kulcu et al,15 2008 | 38 | Betahistine vs head and eye (modified Cawthorne-Cooksey) exercises | Multiple vertigo scales | Data presented as P values and graphs, without raw numbers or data; authors unavailable or could not provide additional detail | No difference in vertigo scores at 1 mo, but scores favored exercises at 2 mo |
| Massaeli et al,16 2017 | 100 | Promethazine vs diazepam | Rate of complete resolution 15 min after injection; “duration of vertigo disappearance” | Results at unclear acute time frames; no VAS or vertigo rating; no outcomes of interest | No significant difference found regarding incidence of primary and secondary outcomes |
| Mulch et al,17 1976 | 50 | High-dose vs low-dose sulpiride vs diazepam vs dimenhydrinate vs placebo | Nystagmus scores | No clinical outcomes reported, only nystagmus | High-dose sulpiride and dimenhydrinate suppressed nystagmus; diazepam, placebo, and low-dose sulpiride did not |
| Salvinelli et al,18 2004 | 156 | Semont liberatory maneuver vs flunarizine vs no treatment | “Cure” and recurrence at 6 mo | Results only provided for 6-mo outcomes; authors unavailable or could not provide additional detail | Cure rate of 94% (49 of 52 patients) with Semont, 58% (30 of 52) with flunarizine, and 36% (18 of 53) with no treatment; all differences were statistically significant |
| Shafipour et al,19 2017 | 150 | Diazepam vs promethazine | Vertigo VAS scores at 30, 120, and 240 min | VAS outcome was not provided with SD so could not be included in primary meta-analysis; authors unavailable or could not provide additional detail | Change in VAS at 2 h: 31.1 with diazepam vs 44.3 with promethazine; difference statistically significant |
| Singh et al,20 1998 | 100 | Cinnarizine vs prochlorperazine | Scale or score not defined, but reports percentage of subjective improvement | Not enough detail in results to use in meta-analysis; author contact information unavailable | At 5 wk, the average improvement in patients treated with cinnarizine plus exercises was 97%; with prochlorperazine plus exercises, the average improvement was 100% |
Abbreviation: VAS, visual analog scale.
Characteristics of Included Studies
Seventeen studies21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 with a total of 1586 participants contributed to the quantitative results (Table 2). These studies spanned 11 countries and 6 languages and each enrolled between 18 and 200 patients. Most enrolled patients with generalized or nonspecific “peripheral” vertigo. The following antihistamines were used: 2 studies administered betahistine, 4 cinnarizine, 7 dimenhydrinate, 2 flunarizine, 1 meclizine, and 2 promethazine. Antihistamines were compared with benzodiazepines in 3 studies,21,29,35 with placebo or no treatment in 7 studies,22,23,26,28,33,36,37 and with other active controls in 6 studies. The only benzodiazepines studied were lorazepam and diazepam, which were compared with placebo in 1 trial30 and with antihistamines in 3 trials.21,29,35 Four studies26,28,36,37 enrolled patients with BPPV and compared Epley repositioning maneuvers alone vs Epley maneuvers in combination with antihistamines. In 8 studies,21,24,25,27,29,31,34 medications were given as a single dose, and results were provided on immediate outcomes at 30 minutes to 2 hours. In the remaining 9 studies,22,23,26,28,30,32,33,35,36,37 medications were given enterally for 1 week to 2 months.
Table 2. Randomized Clinical Trials Included in the Systematic Review.
| Source | Country | Blind or open label | No. of patients | Vertigo type | Medications, controls, and comparators | Main outcomes reported | Inclusion criteria | Main exclusion criteria | Main results |
|---|---|---|---|---|---|---|---|---|---|
| Amini et al,21 2014 | Iran | Double blind | 184 | Peripheral | Promethazine (25 mg) vs lorazepam (2 mg) (IV) | Change in 100-point vertigo VAS score; change in 100-point VAS score nausea at 2 h (reported as average with SD); need for repeat dosing | Patients in ED aged 18-65 y with signs and symptoms consistent with peripheral vertigo | Brain injury, central vertigo, pregnancy, contraindication to medications, prior treatment, or drug-induced or orthostatic dizziness | More improvement in both vertigo and nausea VAS scores with promethazine; less need for rescue doses with promethazine |
| Boniver et al,22 1978 | Belgium | Double blind | 18 | Central (vascular) | Flunarizine taper from 40 to 10 mg/d vs placebo for 3 mo (orally) | Subjective report of resolved, improved, no change, or worse at 1 wk and 1, 2, and 3 mo; also nystagmometric data | “Definite vertigo” defined by otolaryngology examination, electronystagmography, and audiometric testing | None specified | Flunarizine better at 2 mo but not at 1 or 3 mo |
| Castellini et al,23 1969 | Italy | Double blind | 44 | Mixed, including traumatic, Meniere disease, and central nervous system associated | Cinnarizine-containing gel tabs (15 mg) or suppositories (25 mg) vs placebo for 7-60 d | Resolved enough to be satisfactory to the patient or did not; time frame only given in tables; also nystagmometric data | Unselected vertiginous patients with varying final diagnoses, including Meniere disease, traumatic vertigo, and labyrinthitis | None specified | Cinnarizine better than placebo at improving vertigo |
| Doğan et al,24 2015 | Turkey | Double blind | 94 | Peripheral, but not specified | Dimenhydrinate (100 mg) vs piracetam (2000 mg) (IV) | Change in 10-point VAS vertigo score at 30 min (still and ambulatory); need for rescue benzodiazepines | Adults in ED with vertigo defined as the illusory sense of movement or orientation | Pregnant, contraindication to medication, already taking drugs (last 24 h), or diagnosed stroke | No statistically significant difference in outcomes |
| Ercin et al,25 2021 | Turkey | Double blind | 200 | None specified | Dimenhydrinate (50 mg) vs metoclopramide (10 mg) (IV) | Change in vertigo and nausea VAS score at 30 min | Adults in ED with vertigo defined as illusory sense of movement of orientation and rated at least 4 of 10 on VAS for associated nausea | Pregnancy, psychiatric or neurologic disorder, hemorrhage, or contraindication to study medications | No statistically significant differences in improvement in vertigo or nausea or changes in vital signs |
| İnan et al,26 2019 | Turkey | Open label | 64 | BPPV | Epley repositioning maneuvers alone vs with betahistine (24 mg) (orally) twice daily or dimenhydrinate (50 mg) daily | Change in Dizziness Handicap Inventory at 10 d | Adults diagnosed with BPPV with the Dix-Hallpike maneuver | Previous ear surgery, cervical spine disease, Meniere disease, central vertigo, or carotid stenosis | Similar decrease in Dizziness Handicap Inventory scores in all 3 groups |
| Irving et al,27 2002 | US | Double blind | 40 | Peripheral | Droperidol (2.5 mg) vs dimenhydrinate (50 mg) (IM) | Change in VAS scores at 30 min, “well enough to go home” | Patients in the ED aged 18-65 y, consistent with peripheral vertigo defined as the sensation of spinning, worse with movement and sudden in onset | Syncope, pregnancy, contraindication to study medications, taking similar medications, or concern for central or cardiac cause | No difference in any outcomes |
| Kim et al,28 2014 | South Korea | Both | 138 | Idiopathic BPPV; all received Epley repositioning | Epley alone vs with dimenhydrinate (25 mg) (orally) twice daily for a week | Residual symptoms (yes or no) at 1 wk; presence of nystagmus | Diagnosis of BPPV after bedside examination and video nystagmography; resolution with Epley (or other) repositioning; no current medications | History of inner-ear issue or surgery, psychiatric issues, failure to resolve with Epley repositioning, or >2 canals involved | Control groups had significantly more residual symptoms at 1 wk |
| Marill et al,29 2000 | US | Double blind | 74 | None specified; probably all-comers | Dimenhydrinate (50 mg) vs lorazepam (2 mg) (IV) | Decrease in average 10-point VAS score for vertigo at 1 and 2 h; measured multiple positions | Adults in the ED with vertigo defined as “hallucination of motion of self or surroundings” | Pregnancy or contraindications to study medications | Dimenhydrinate “more effective and less sedating” |
| McClure et al,30 1980 | Canada | Double blind | 20 | BPPV | Lorazepam (1 mg) vs diazepam (5 mg) vs placebo (orally) 3 times/d | 10-point VAS score for dizziness, with 10 being starting point and 0 being complete resolution at 1, 2, 3, and 4 wk | “Classical” BPPV with nystagmus on Dix-Hallpike testing | Not BPPV | No difference in improvement |
| Ozdemir et al,31 2013 | Turkey | Double blind | 200 | Peripheral | Dimenhydrinate (50 mg) vs piracetam (1000 mg) (IV) | 100-point VAS scores initially and after treatment; need for additional dose (of same medication); dizziness, drowsiness, or weakness | Aged 18-70 y, with chief complaint of vertigo and “diagnosed with peripheral vertigo” | Age >70 y, pregnancy, contraindication to study medications, or any dangerous cause of vertigo (cardiac, anemia, poisoning, etc) | No difference in efficacy; fewer minor adverse events with piracetam |
| Perelló et al,32 1998 | Spain | Double blind | 110 | Generic | Dotarizine (50 mg) vs cinnarizine (75 mg) (orally) twice daily | Complete resolution (no episodes) at 15, 30, 45, and 60 d; improvement at 60 d; normal daily activity after 60 d; rated as “very satisfactory” by investigator and by patient; side effects | Vertigo with nystagmus or abnormal vestibular tests | Pregnant, treated with medications for vertigo in the last 15 d, contraindication to study medications, ear surgery or trauma/acoustic neuroma, neurologic deficits, nonhorizontal nystagmus, alcohol abuse, or “severe metabolic diseases” | Dotarizine better than cinnarizine in multiple vertigo measures |
| Philipszoon et al,33 1961 | Netherlands | Double blind | 55 | Multiple types | Cinnarizine (30 mg) vs placebo (orally) daily | “Benefit” at 1 week (yes or no); presence of inducible nystagmus (yes or no); side effects | “Complained of vertigo”; no other criteria listed | Not stated | Cinnarizine better than placebo at improving vertigo |
| Saberi et al,34 2019 | Iran | Double blind | 170 | Acute peripheral, not BPPV | Promethazine (25 mg) (IM) vs ondansetron (4 mg) (IV) | Vertigo VAS score at 30 and 120 min as mean (SD); nausea VAS as well; side effects; need for rescue (readministration); mean relief score ranging from −6 to 9 | Aged 20-60 y, with vertigo defined as a true sense of rotation or movement | New neurologic deficits, BPPV, contraindication to medications, recent head trauma, or use of any CNS depressants | Promethazine improved vertigo more; ondansetron improved nausea more |
| Shih et al,35 2017 | US | Double blind | 40 | Peripheral | Diazepam (5 mg) vs meclizine (25 mg) (orally) | Change in 100-point VAS at 30 and 60 min, reported as mean change | Age 18-65 y; peripheral vertigo as diagnosed by an emergency physician | Mild vertigo (<40 on VAS), required parenteral therapy, pregnant, taking medications or sedatives, focal neurologic findings, or central or cardiovascular cause of vertigo | No significant differences in improvement |
| Sundararajan et al,36 2011 | India | Open label | 51 | BPPV | Epley alone vs with cinnarizine (25 mg) (orally) 3 times daily | Cured, 50% cured, no improvement, or worse at 1 and 4 wk | Diagnosed with BPPV per diagnostic criteria including positive Dix-Hallpike in ED or ENT clinic | “Severe neck problems,” recent stroke, retinal detachment, or uncontrolled hypertension | Control group did better than treatment group |
| Zhang et al,37 2012 | China | Open label | 84 | BPPV | Epley alone vs with flunarizine (10 mg) daily, betahistine (12 mg) twice daily, and Ginkgo biloba (Ginaton) drops twice daily | Cure, effective, or ineffective at 7 and 28 d; recurrence rate at 1.5 y | Diagnosed with BPPV per criteria put forth by the Chinese Society of Otolaryngology; positive Dix-Hallpike test result; seen at ENT clinic | None specified | Average cure time with 18-d treatment: 39 d for control; more cure and more “effective” at 7 and 28 d with treatment |
Abbreviations: BPPV, benign paroxysmal positional vertigo; CNS, central nervous system; ED, emergency department; ENT, ear, nose, and throat; IM, intramuscular; IV, intravenous; VAS, visual analog scale.
Quality Assessment
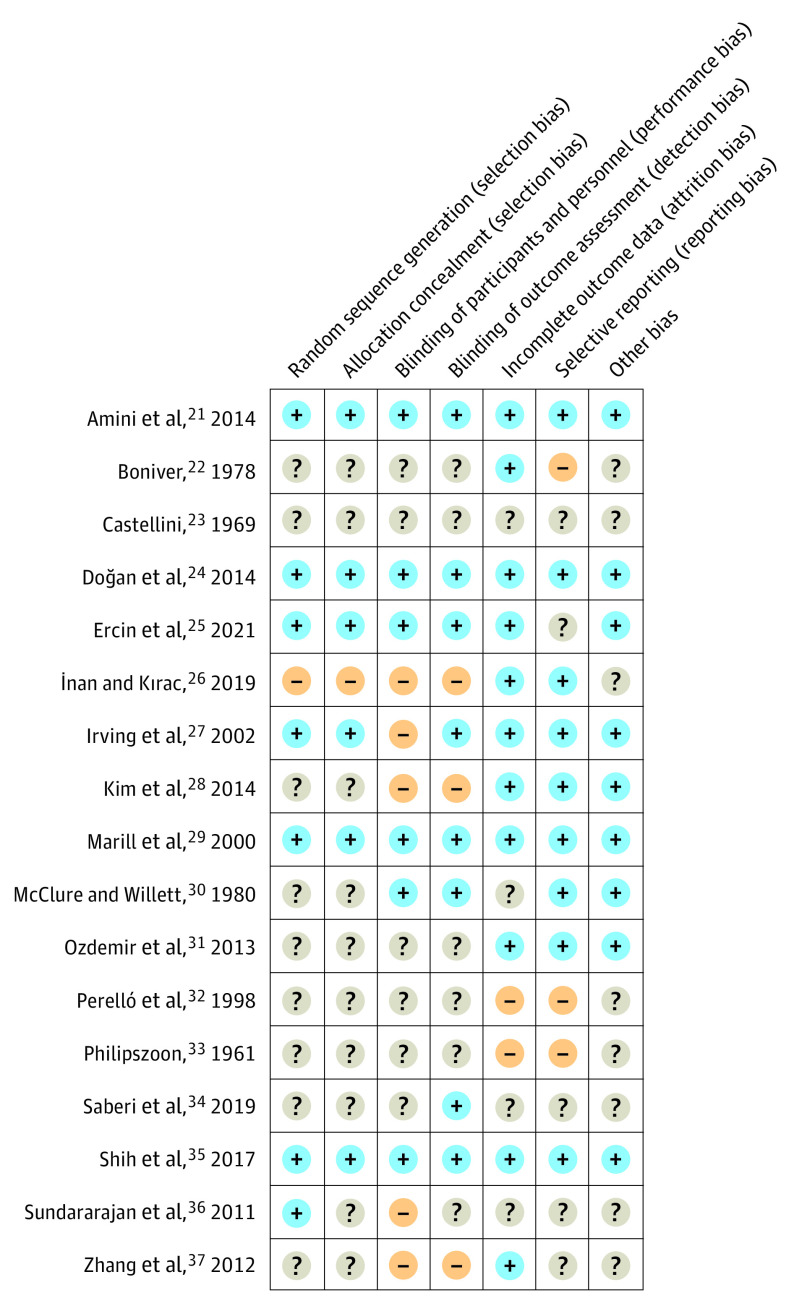
Figure 2 illustrates the ROB for individual studies, which ranged from low to high. Among the 8 trials that evaluated 2-hour outcomes, 5 trials21,24,25,29,35 had low ROB, including all 3 trials21,29,35 that compared benzodiazepines with antihistamines. One trial27 had a high ROB for blinding but low risk in all other domains, and the other34 had unclear ROB in all domains except blinding. Of the 9 trials22,23,26,28,30,32,33,36,37 that reported 1-week or 1-month outcomes, all had a high ROB. A high ROB was found for blinding in 4 studies,26,28,36,37 for selective outcomes reporting in 3 studies,22,32,33 and for attrition bias in 2 studies.32,33
Figure 2. Summary of Risk of Bias for 17 Randomized Clinical Trials Included in Meta-analyses.
Main Results
For the primary outcome, 7 trials21,24,25,27,29,34,35 encompassing 802 patients contributed data for meta-analysis (Figure 3). All studies compared antihistamines with other medications. In 3 trials with a low ROB,21,29,35 antihistamines were associated with a 16.1-point greater decrease in vertigo (95% CI, 7.2 to 25.0; I2 = 47%) than benzodiazepines. Antihistamines performed similarly to other active comparators (mean difference, 2.7 [95% CI, –6.1 to 11.5]; I2 = 72%). When all 7 studies were combined, antihistamines were not associated with a statistically significant improvement over other comparators (difference, 7.4 [95% CI, –1.1 to 15.8]; I2 = 84%). No studies compared benzodiazepines with comparators other than antihistamines.
Figure 3. Change in Vertigo Visual Analog Scale Scores at 2 Hours.
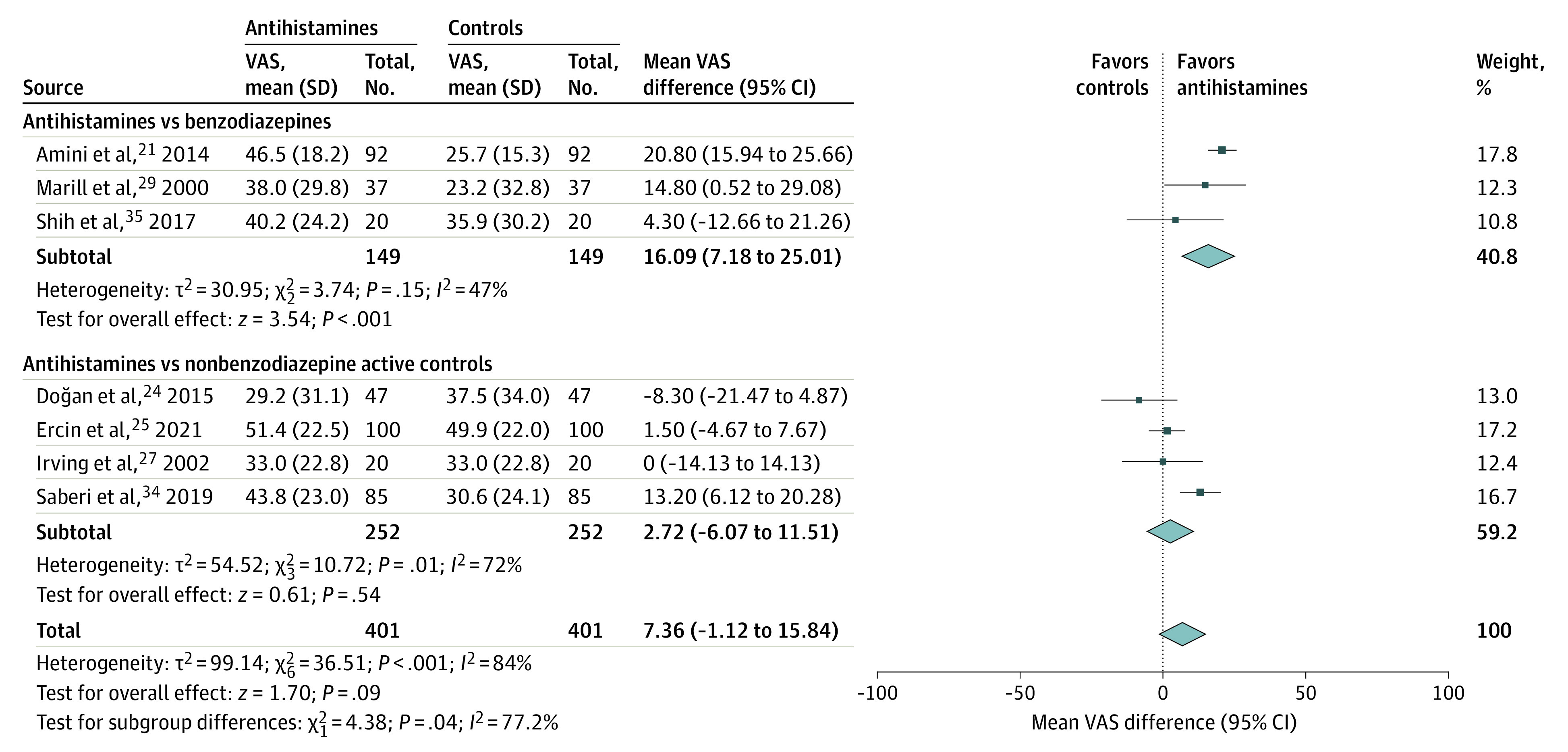
VAS indicates visual analog scale.
Two additional studies19,31 reported the change in VAS scores at 2 hours but did not include SDs, which are required for meta-analysis. To include these trials, we performed an unplanned sensitivity analysis in which we calculated the average SD across the 7 included studies and applied it to the results of the 2 additional studies. Point-estimate results of the sensitivity analysis (with 1152 patients) were nearly identical to the primary analysis. However, with the slight increase in statistical power in the sensitivity analysis, antihistamines were associated with greater VAS score improvement against all comparators combined (difference, 7.5 [95% CI, 0.8 to 9.2]; I2 = 81%; eFigure 1 in the Supplement).
Secondary 2-hour outcomes are displayed in eFigure 2 in the Supplement. Overall, there were no differences in any 2-hour outcomes for antihistamines and comparators, although antihistamines were reported as superior to benzodiazepines in the only study comparing both for these outcomes.21 No studies reporting 2-hour secondary outcomes had a placebo or nontreatment group, and none compared benzodiazepines with comparators other than antihistamines.
The results of antihistamines vs comparators at 1 week and 1 month are provided in eFigure 3 in the Supplement. At 1 week, the difference in improvement favoring antihistamines was not statistically significant (RR, 1.15 [95% CI, 1.00 to 1.32]; I2 = 41%) among the 269 patients in 5 trials22,26,33,36,37 of antihistamines vs placebo or no intervention. There was no suggestion of an increase in likelihood of complete resolution at 1 week with antihistamines (RR, 1.03 [95% CI, 0.56 to 1.89]; I2 = 81%). At 1 month, there was no difference in antihistamines vs other comparators for improvement or complete resolution. In subgroup analyses, antihistamines showed a trend toward greater effect in patients without vs with BPPV, with only 1 subgroup comparison (improvement at 1 week for patients without BPPV) showing a statistically significant improvement with antihistamines (RR, 1.66 [95% CI, 1.06 to 2.59]).
Only 1 benzodiazepine study30 reported 1-week or 1-month results. None of the 13 benzodiazepine-treated or 7 placebo-treated patients reported improvement at 1 week. Improvement was reported for 5 of 12 benzodiazepine-treated patients (42%) and 4 of 7 placebo-treated patients (57%) at 1 month. Complete resolution was reported for 2 of 12 benzodiazepine-treated patients (17%) and 3 of 7 placebo-treated patients (43%), respectively. These differences were not statistically significant.
Adverse events were inconsistently reported and rarely predefined. None of the comparisons of antihistamines and benzodiazepines, other controls, or placebo were statistically significant (eFigure 4 in the Supplement). Most events were not considered serious by the study authors. Two patients who received 2 mg of intravenous lorazepam developed hypoventilation requiring bag-valve-mask treatment. One patient was diagnosed with Quinke edema after taking oral cinnarizine, which was treated conservatively. This patient then resumed cinnarizine treatment without recurrence.
Six trials17,22,23,30,32,33 included nystagmometry measurements of any kind and are summarized in the eTable in the Supplement. Two studies17,30 comparing benzodiazepines with placebo reported no difference, and 1 study17 comparing diazepam with dimenhydrinate suggested that dimenhydrinate was superior. Four studies17,22,23,33 compared antihistamines with placebo, and all reported greater improvement in nystagmus with antihistamines. Finally, 1 study32 compared an antihistamine with dotarizine, reporting no difference.
Discussion
On the basis of the results of this systematic review and meta-analysis, moderately strong evidence suggests that single-dose antihistamines result in greater improvement of vertigo at 2 hours than single-dose benzodiazepines, but they are similarly effective to other studied medications. Low-quality evidence suggests that daily enteral use of antihistamines is not superior to placebo or no medication, with regard to improving or resolving vertigo at 1 week or 1 month. Only a single high-ROB study30 compared oral benzodiazepines (diazepam and lorazepam) with placebo and reported no benefit.
Treatment of acute vertigo is challenging. There is general consensus that repositioning maneuvers are the preferred first-line treatment for BPPV.3,5,6,38 However, these maneuvers are not always effective,7,38 and patients often require further intervention for vertigo symptoms. BPPV is the most common cause of vertigo.1,3,6 However, patients with alternative causes of vertigo, including vestibular dysfunction, Meniere disease, or central nervous system pathology,1 do not respond to repositioning maneuvers. Furthermore, there is often diagnostic uncertainty regarding the cause of and pathology underlying vertigo in the acute phase when the diagnostic workup remains incomplete.1,5,8 Patients with acute vertigo may need or desire treatment to alleviate their symptoms regardless of the underlying cause.
Both benzodiazepines and antihistamines are used as vestibular suppressants for patients with vertigo.2,4 For decades, antihistamines have been studied for vertigo, with mixed results. In contrast with our findings, a 2015 review of 13 studies39 reported that antihistamines taken daily for durations of 1 to 20 weeks produced higher odds of improvement than placebo. The studies in that review included patients with chronic vertigo symptoms. During this meta-analysis, we identified 5 RCTs11,13,14,17,18 investigating antihistamines against placebo or no treatment that we could not include because of insufficient results reporting or lack of data on any of the extracted outcomes. In all 5 studies, the investigators suggested that antihistamine treatment was superior. In addition, there were inconsistent trends favoring antihistamines over placebo or no treatment after 1 week of daily use. It remains unclear whether antihistamines may provide relief for patients with acute vertigo when taken for 1 week to 1 month, but the results of our review were not consistent with a robust effect. Although meclizine is a popular treatment for vertigo in the US, we did not find direct or indirect evidence that its efficacy differs from that of other antihistamines.11,35
There is less evidence available on benzodiazepine treatment for acute vertigo, but existing studies do not support their use. In this meta-analysis, benzodiazepines were statistically inferior to antihistamines, and the only RCT30 comparing benzodiazepines with placebo reported numerically better results in the placebo group. Four additional RCTs12,16,17,19 including a benzodiazepine group could not be included in this meta-analysis, but the results were generally consistent with inferiority compared with antihistamines. Interestingly, benzodiazepines did not seem to reduce or improve nystagmus compared with placebo, although antihistamines did. Despite the limited clinical importance, this observation is consistent with clinical results suggesting no benefit with benzodiazepines for acute vertigo. Given the consistent signal of better improvement with antihistamines and no evidence that benzodiazepines improve any outcomes, the results of this study suggest that benzodiazepine use for acute vertigo should be discouraged.
Limitations
There are several limitations to this review, largely based on those of the existing literature. First, the suggestion that benzodiazepines seem to be ineffective must be taken in the context of the small number of included studies and participants. One of the largest studies identified (with 150 patients)19 comparing benzodiazepines with antihistamines for our primary outcome did not report SD values around the effect size, so it could not be included in this meta-analysis. However, this study reported that the antihistamine treatment was superior, and the finding in our sensitivity analysis including those results with an assumed average SD was similar to our primary analysis, further strengthening confidence that antihistamines result in greater improvement at 2 hours than benzodiazepines.
There is substantial clinical heterogeneity in antihistamine studies. Several antihistamines were studied against a variety of comparators with a wide range of outcomes reported, and statistical heterogeneity was high for most outcomes. Some studies did not report on any of our outcomes of interest or lacked sufficient detail to be included in the meta-analysis. Attempts to contact authors to obtain additional data for meta-analysis were largely unsuccessful, as many studies were performed decades ago. In addition, only a limited number of studies reported on each individual outcome, limiting power to find differences between comparators.
To date, there is no established clinically relevant difference in vertigo VASs. For a pain VAS, the minimum clinically important difference is generally considered to be at least 10.40,41,42 We found a difference of 16 between antihistamines and benzodiazepines; this may be a patient-important difference, but its clinical relevance on a vertigo VAS is unclear.
All studies that evaluated the efficacy of medication at 1 week or 1 month had a high ROB, which may have worked for or against antihistamines in particular. Bias is more likely to favor interventions over controls43,44; in some instances, studies used antihistamines as the control to assess a new or novel treatment. Conversely, other studies compared antihistamines with placebo or with no treatment, so it is impossible to say whether the ROB in this literature base would skew results in one direction or the other.
Because of the small number of studies for each of our reported outcomes, subgroup analyses were either not feasible or were underpowered. Most studies included a nonspecific population of patients with vertigo or peripheral vertigo without further defining the population. Five studies26,28,30,36,37 specifically enrolled patients with BPPV, but most analyses only included 1 to 2 studies for this subgroup.
Finally, for our primary outcome of change in VAS scores, some studies measured change at 1 hour instead of 2 hours. We pooled these results, but it is possible that the effects of medications at 1 hour could be underestimated if this time point was before the peak onset of action.
Conclusions
In this systematic review and meta-analysis, the available evidence did not support an association of benzodiazepine use with improvement in any outcomes for patients with acute vertigo. Moderately strong evidence suggested that single-dose antihistamines provide more relief for acute vertigo than benzodiazepines at 2 hours. There was weak evidence suggesting that antihistamines are comparable with other nonbenzodiazepine medications for immediate relief of vertigo symptoms. Daily antihistamine use may not be superior to placebo or no treatment for improvement at 1 week or 1 month, but this is based on very weak evidence from heterogenous trials with a high ROB. Larger randomized trials comparing both antihistamines and benzodiazepines to placebo could better clarify the relative efficacy of these medications.
eAppendix. PubMed Search
eFigure 1. Sensitivity Analysis for Primary Outcomes of 2 Additional Studies Reporting Visual Analog Score Changes at 2 Hours But Lacking SDs
eFigure 2. Two-Hour Secondary End Points
eFigure 3. Antihistamines vs Placebo or No Treatment at 1 Week and 1 Month
eFigure 4. Adverse Events
eTable. Nystagmography Studies
eReferences
References
- 1.Neuhauser HK. The epidemiology of dizziness and vertigo. Handb Clin Neurol. 2016;137:67-82. doi: 10.1016/B978-0-444-63437-5.00005-4 [DOI] [PubMed] [Google Scholar]
- 2.Casani AP, Gufoni M, Capobianco S. Current insights into treating vertigo in older adults. Drugs Aging. 2021;38(8):655-670. doi: 10.1007/s40266-021-00877-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg. 2017;156(3 suppl):S1-S47. doi: 10.1177/0194599816689667 [DOI] [PubMed] [Google Scholar]
- 4.Hain TC, Uddin M. Pharmacological treatment of vertigo. CNS Drugs. 2003;17(2):85-100. doi: 10.2165/00023210-200317020-00002 [DOI] [PubMed] [Google Scholar]
- 5.Swartz R, Longwell P. Treatment of vertigo. Am Fam Physician. 2005;71(6):1115-1122. [PubMed] [Google Scholar]
- 6.Neely P, Patel H, Wellings T. Benign paroxysmal positional vertigo in the emergency department: an observational study of an Australian regional hospital’s acute clinical practice. Emerg Med Australas. 2021;33(6):1082-1087. doi: 10.1111/1742-6723.13810 [DOI] [PubMed] [Google Scholar]
- 7.Sim E, Tan D, Hill K. Poor treatment outcomes following repositioning maneuvers in younger and older adults with benign paroxysmal positional vertigo: a systematic review and meta-analysis. J Am Med Dir Assoc. 2019;20(2):224.e1-224.e23. doi: 10.1016/j.jamda.2018.11.019 [DOI] [PubMed] [Google Scholar]
- 8.Zwergal A, Dieterich M. Vertigo and dizziness in the emergency room. Curr Opin Neurol. 2020;33(1):117-125. doi: 10.1097/WCO.0000000000000769 [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Review Manager (RevMan). Version 5.4. Cochrane Collaboration; 2020. Accessed June 9, 2022. https://training.cochrane.org/online-learning/core-software/revman [Google Scholar]
- 11.Babin RW, Balkany TJ, Fee WE. Transdermal scopolamine in the treatment of acute vertigo. Ann Otol Rhinol Laryngol. 1984;93(1 Pt 1):25-27. doi: 10.1177/000348948409300106 [DOI] [PubMed] [Google Scholar]
- 12.Barzegari HMJ, Yousefian MA, Zohrevandi B. Comparing the effectiveness of intravenous diazepam and methyl prednisolone in treatment of acute peripheral vertigo; a clinical trial. Iran J Emerg Med. 2016;3(3):92-97. [Google Scholar]
- 13.Herndon JW, Haug O, Horowitz MJ, Lynes TE. Benign paroxysmal positional vertigo: a clinical study. Ann Otol Rhinol Laryngol. 1975;84(2 Pt 1):218-222. doi: 10.1177/000348947508400214 [DOI] [PubMed] [Google Scholar]
- 14.Jalali MM, Gerami H, Saberi A, Razaghi S. The impact of betahistine versus dimenhydrinate in the resolution of residual dizziness in patients with benign paroxysmal positional vertigo: a randomized clinical trial. Ann Otol Rhinol Laryngol. 2020;129(5):434-440. doi: 10.1177/0003489419892285 [DOI] [PubMed] [Google Scholar]
- 15.Kulcu DG, Yanik B, Boynukalin S, Kurtais Y. Efficacy of a home-based exercise program on benign paroxysmal positional vertigo compared with betahistine. J Otolaryngol Head Neck Surg. 2008;37(3):373-379. [PubMed] [Google Scholar]
- 16.Massaeli MRF, Shahabian M. Comparison of the effectiveness of intravenous promethazine and intravenous diazepam in the treatment of benign paroxysmal positional vertigo in the emergency department. Asian J Med Health. 2017;8(4):1-7. doi: 10.9734/AJMAH/2017/38001 [DOI] [Google Scholar]
- 17.Mulch G. Comparison of the effectiveness of antivertiginous drugs by double blind procedure. The effect of diazepam, dimenhydrinate and sulpirid on the human vestibular spontaneous nystagmus. Laryngol Rhinol Otol (Stuttg). 1976;55(5):392-399. [PubMed] [Google Scholar]
- 18.Salvinelli F, Trivelli M, Casale M, et al. Treatment of benign positional vertigo in the elderly: a randomized trial. Laryngoscope. 2004;114(5):827-831. doi: 10.1097/00005537-200405000-00007 [DOI] [PubMed] [Google Scholar]
- 19.Shafipour LKI, Shafipour V, Ahidashti HA, Charati JY. Intravenous promethazine versus diazepam for treatment of peripheral vertigo in emergency department. J Mazandaran Univ Med Sci. 2017;27(149):88-98. [Google Scholar]
- 20.Singh AK, Chaturvedi VN. Prochlorperazine versus cinnarizine in cases of vertigo. Indian J Otolaryngol Head Neck Surg. 1998;50(4):392-397. doi: 10.1007/BF03000698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amini A, Heidari K, Asadollahi S, et al. Intravenous promethazine versus lorazepam for the treatment of peripheral vertigo in the emergency department: A double blind, randomized clinical trial of efficacy and safety. J Vestib Res. 2014;24(1):39-47. doi: 10.3233/VES-130506 [DOI] [PubMed] [Google Scholar]
- 22.Boniver R. Vertigo, particularly of vascular origin, treated with flunarizine (R 14 950). Arzneimittelforschung. 1978;28(10):1800-1804. [PubMed] [Google Scholar]
- 23.Castellini V. Clinical and electronystagmographic experience with a new drug containing cinnarizine in the treatment of vertigo. Article in Italian. Boll Mal Orecch Gola Naso. 1969;87(2):107-131. [PubMed] [Google Scholar]
- 24.Doğan NÖ, Avcu N, Yaka E, Yılmaz S, Pekdemir M. Comparison of the therapeutic efficacy of intravenous dimenhydrinate and intravenous piracetam in patients with vertigo: a randomised clinical trial. Emerg Med J. 2015;32(7):520-524. doi: 10.1136/emermed-2014-204006 [DOI] [PubMed] [Google Scholar]
- 25.Ercin D, Erdur B, Turkcuer I, et al. Comparison of efficacy dimenhydrinate and metoclopramide in the treatment of nausea due to vertigo; a randomized study. Am J Emerg Med. 2021;40:77-82. doi: 10.1016/j.ajem.2020.12.010 [DOI] [PubMed] [Google Scholar]
- 26.İnan HC, Kıraç M. An evaluation of the effects of betahistine and dimenhydrinate on posterior canal benign paroxysmal positional vertigo. Turk Arch Otorhinolaryngol. 2019;57(4):191-196. doi: 10.5152/tao.2019.4185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irving C, Richman P, Kaiafas C, Eskin B, Allegra J. Intramuscular droperidol versus intramuscular dimenhydrinate for the treatment of acute peripheral vertigo in the emergency department: a randomized clinical trial. Acad Emerg Med. 2002;9(6):650-653. doi: 10.1197/aemj.9.6.650 [DOI] [PubMed] [Google Scholar]
- 28.Kim MB, Lee HS, Ban JH. Vestibular suppressants after canalith repositioning in benign paroxysmal positional vertigo. Laryngoscope. 2014;124(10):2400-2403. doi: 10.1002/lary.24741 [DOI] [PubMed] [Google Scholar]
- 29.Marill KA, Walsh MJ, Nelson BK. Intravenous Lorazepam versus dimenhydrinate for treatment of vertigo in the emergency department: a randomized clinical trial. Ann Emerg Med. 2000;36(4):310-319. doi: 10.1067/mem.2000.110580 [DOI] [PubMed] [Google Scholar]
- 30.McClure JA, Willett JM. Lorazepam and diazepam in the treatment of benign paroxysmal vertigo. J Otolaryngol. 1980;9(6):472-477. [PubMed] [Google Scholar]
- 31.Ozdemir H, Akinci E, Coskun F. Comparison of the effectiveness of intravenous piracetam and intravenous dimenhydrinate in the treatment of acute peripheral vertigo in the emergency department. Singapore Med J. 2013;54(11):649-652. doi: 10.11622/smedj.2013225 [DOI] [PubMed] [Google Scholar]
- 32.Perelló E, Esteller E, Colls A, et al. Efficacy and safety of dotarizine vs. cinnarizine in the symptomatic treatment of acute balance disorders (common vertigo). Article in Spanish. An Otorrinolaringol Ibero Am. 1998;25(3):291-310. [PubMed] [Google Scholar]
- 33.Philipszoon AJ. The effect of cinnarizine on vertigo; an electronystagmographic examination in 55 patients. Article in Dutch. Ned Tijdschr Geneeskd. 1961;105:657-660. [PubMed] [Google Scholar]
- 34.Saberi A, Pourshafie SH, Kazemnejad-Leili E, Nemati S, Sutohian S, Sayad-Fathi S. Ondansetron or promethazine: Which one is better for the treatment of acute peripheral vertigo? Am J Otolaryngol. 2019;40(1):10-15. doi: 10.1016/j.amjoto.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 35.Shih RD, Walsh B, Eskin B, et al. Diazepam and meclizine are equally effective in the treatment of vertigo: an emergency department randomized double-blind placebo-controlled trial. J Emerg Med. 2017;52(1):23-27. doi: 10.1016/j.jemermed.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 36.Sundararajan I, Rangachari V, Sumathi V, Kumar K. Epley’s manoeuvre versus Epley’s manoeuvre plus labyrinthine sedative as management of benign paroxysmal positional vertigo: prospective, randomised study. J Laryngol Otol. 2011;125(6):572-575. doi: 10.1017/S0022215110002781 [DOI] [PubMed] [Google Scholar]
- 37.Zhang H, Geng M, Yan B, Lu X. Epley’s manoeuvre versus Epley’s manoeuvre plus labyrinthine sedative in the management of benign paroxysmal positional vertigo: prospective, randomised study. Article in Chinese. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012;26(16):750-752. [PubMed] [Google Scholar]
- 38.Hilton MP, Pinder DK. The Epley (canalith repositioning) manoeuvre for benign paroxysmal positional vertigo. Cochrane Database Syst Rev. 2014;(12):CD003162. doi: 10.1002/14651858.CD003162.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amini A, Heidari K, Kariman H, et al. Histamine antagonists for treatment of peripheral vertigo: a meta-analysis. J Int Adv Otol. 2015;11(2):138-142. doi: 10.5152/iao.2015.1169 [DOI] [PubMed] [Google Scholar]
- 40.Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18(3):205-207. doi: 10.1136/emj.18.3.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly AM. Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or cause of pain? Acad Emerg Med. 1998;5(11):1086-1090. doi: 10.1111/j.1553-2712.1998.tb02667.x [DOI] [PubMed] [Google Scholar]
- 42.Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elbow Surg. 2009;18(6):927-932. doi: 10.1016/j.jse.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 43.Page MJ, Higgins JP, Clayton G, Sterne JA, Hróbjartsson A, Savović J. Empirical evidence of study design biases in randomized trials: systematic review of meta-epidemiological studies. PLoS One. 2016;11(7):e0159267. doi: 10.1371/journal.pone.0159267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood L, Egger M, Gluud LL, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336(7644):601-605. doi: 10.1136/bmj.39465.451748.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. PubMed Search
eFigure 1. Sensitivity Analysis for Primary Outcomes of 2 Additional Studies Reporting Visual Analog Score Changes at 2 Hours But Lacking SDs
eFigure 2. Two-Hour Secondary End Points
eFigure 3. Antihistamines vs Placebo or No Treatment at 1 Week and 1 Month
eFigure 4. Adverse Events
eTable. Nystagmography Studies
eReferences