Abstract
Background
Female patients with chronic cough are more likely to suffer from urinary incontinence (UI). However, there are few data in regard of risks related with UI in female adults with chronic cough.
Method
We recruited female adult patients with chronic cough from the cough specialist clinic. Demographic information and clinical characteristics including age, BMI, duration of cough, severity of cough, nature and timing of cough, cough triggers, concomitant symptoms, comorbidities and UI condition were collected. The demographics and clinical features of patients with UI and those without UI were compared.
Result
A total of 700 female patients with the main symptom of chronic cough were included, of whom 351 (50.1%) presented with UI. As compared with patients without UI, patients with UI showed a longer mean age (years) (49.5 vs. 42.4, p < 0.001), a more severe cough symptom (median of cough Visual Analogue Scale: 65 vs. 50, p < 0.001), a higher prevalence of chronic sinusitis (17.6% vs. 8.6%, p = 0.002), and combined with a higher incidence of abdominal muscle pain due to cough (39.6% vs. 18.7%, p < 0.001).In addition, patients in UI group whose cough were more easily triggered by exercise (28.2% vs. 17.2%, p = 0.048). Multivariate logistic regression analysis indicated the above five variables were risk factors for UI in female adult patients with chronic cough.
Conclusion
Urinary incontinence is a common complication in female patients with chronic cough. Older age, severe cough, combing with a higher proportion of chronic sinusitis and abdominal muscle pain, a cough easily triggered by exercise are identified as risk factors for urinary incontinence. We should pay more attention to female chronic coughers with these risk factors in clinics.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-022-02069-w.
Keywords: Urinary incontinence, Female, Chronic cough, Risk factors
Introduction
Chronic cough is defined as cough presenting as the sole or predominant symptom and lasting for more than 8 weeks, with no obvious abnormalities in chest imaging. Chronic cough accounts for up to 10 to 38% patients [1] in respiratory clinics, and cough variant asthma (CVA), eosinophilic bronchitis (EB), gastroesophageal reflux cough (GERC), upper airway cough syndrome (UACS), and atopic cough (AC) are the common causes of chronic cough. In western special cough clinics, patients with chronic cough were middle-aged and elderly women predominant [2–4]. However, there was no significant difference on the prevalence of chronic cough between males and females in China both in the cough clinics and the respiratory clinics [5–7]. Female patients usually complain about more severe cough [5] and are more likely to combine with comorbidities such as abdominal muscle pain and urinary incontinence (UI), which affect patients’ quality of life and increase their psychological burden greatly. Urinary incontinence refers to the involuntary flow of urine from the urethra.
The incidence of UI in female patients with chronic cough is much higher than that in males, a shorter urethra and weaker sphincter tension presented in females should be the main results. Meanwhile, the presence of UI is also related to the pelvic floor dysfunction provoked by pregnancy, vaginal delivery and obstetrical and gynecological surgery, all of which are female-specific [8–10]. The prevalence of UI in females with chronic cough was inconsistent among previous reports, but it is usually more than 30% [11–16], and can even be up to be 50% [12, 16]. Previous studies showed that older age, high BMI (Body mass index, kg/m2), history of vaginal delivery, obstetrical and gynecological surgery and chronic cough are common risk factors of UI in adult women [13, 17–20, 25–29]. However, it is unknown which clinical characteristics are risk factors for UI in patients with chronic cough. Therefore, the aim of this study is to investigate the risk factors of UI in adult female patients with chronic cough by comparing the demographic and clinical characteristics of patients with UI and those without UI.
Method
Subjects
Patients with cough who visited the specialist cough clinics of the First Affiliated Hospital of Guangzhou Medical University from January 2006 to December 2018 were asked to complete a standard case report form (CRF). An additional file shows the CRF in more detail (see Additional file 1). Coughers met the inclusion criterion as following were enrolled in this study: (1) Female gender, (2) aged more than 18 years, (3) cough as the only or predominant symptom lasting more than 8 weeks, (4) chest imaging showed no obvious abnormalities. Exclusion criterion: (1) information about urinary incontinence was not recorded, (2) age or duration of cough was not recorded. Basing on the above criterion, patients with UI were regarded as cases, while those without UI were deemed as controls.
Study design
This was a retrospective observational study. Investigators assisted patients in filling in a CRF the first time when patients came to the clinics. Demographics and cough-related clinical characteristics were included in the CRF, which detailly contained age, BMI, duration of cough, timing of cough, dry cough or productive cough, severity of cough, cough triggers, concomitant symptoms, comorbidities and previous treatment for cough.
Daytime/Night-time Cough symptom score (CSS) and cough Visual Analogue Scale (VAS) were used to evaluate the frequency and the severity of cough. Daytime/Night-time CSS are ranging from 0 to 5, 0 for no cough, 5 for uncontrollable cough, and inability to perform daily activities or fall asleep [21]. The cough VAS is a scale ranging from 0 to 100. Zero represents no cough and 100 represents the most severe cough.
Etiological diagnostic workflow and criteria are based on cough guidelines by the American College of Chest Physicians and the Chinese Medical Association [22–24]. We classified patients according to etiology into asthmatic cough (including cough variant asthma and classic asthma with cough as the main symptom), EB, GERC, UACS, AC, as well as rare and uncommon etiologies. In addition to the categories mentioned above, we grouped patients together with unexplained chronic cough (UC) and chronic refractory cough (CRC) with diagnosable etiology and failed in repeatedly treatment. The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (Medical Research 2,020,150). Neither this study involved any therapeutic interventions, nor did it impose any additional burden.
Statistical analysis
Data were presented as percentages(frequencies), means (standard deviations, SD), or medians (interquartile range, IQR). Correlation tests were performed between variables to identify those with collinearity (r > 0.7). Pearson correlation coefficients were used to reflect correlations between continuous variables, Spearman and Kendall's tau-b correlation coefficients were used to reflect correlations between categorical variables. Two independent samples t-test was used for normally distributed data, Mann–Whitney U test for non-normally distributed data, χ2 test or Fisher exact test for categorical data, and Bonferroni correction test for multiple comparisons. BMI (kg/m2) was classified into four grades: underweight, < 18.5; normal, 18.5–23.9; overweight, 24–27.9; obesity, ≥ 28. Age was categorized into five grades: I, 18–30 years; II, 31–40 years; III, 41–50 years; IV, 51–60 years; V, > 60 years. Variables associated with UI from univariate analysis (P < 0.1) were included in multivariate analysis. The final independent risk factor of UI was determined by the stepwise logistic regression analysis. Based on the age grade, we set the dummy variable for "age" in the logistic regression analysis. P < 0.05 was considered as a statistically significant difference. The software used for data analysis was SPSS 25.0 (IBM Corporation, Armonk, NY) and GraphPad Prism 8.
Results
Demographics and clinical characteristics
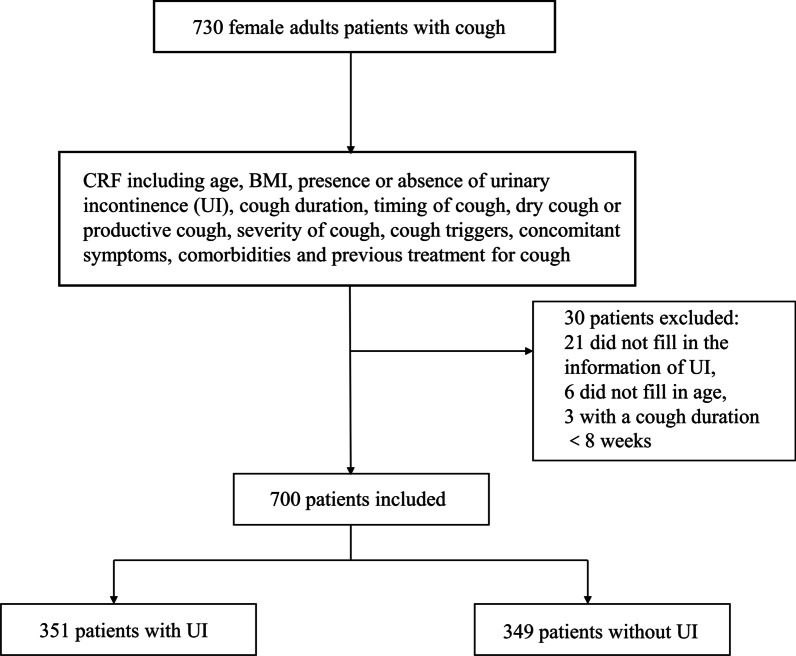
A total of 730 adult female patients were recruited from January 2006 to December 2018. Among whom, 30 cases were excluded, including 21 cases who did not fill in the presence or absence of UI, 6 cases who did not fill in their age, and 3 cases with a cough duration less than 8 weeks. The remaining 700 patients with a mean age of 45.9 years old and a median cough duration of 24 months were finally enrolled into the analysis. Among these 700 patients, 351 patients had reported UI (50.1%). The flow chart was shown in Fig. 1.
Fig. 1.
Flow chart. CRF, Case report form; UI, Urinary incontinence
The mean age and the median cough duration of patients with UI were significantly higher than that of patients without UI (49.5 ± 13.33 vs. 42.4 ± 13.85, p < 0.01; 36(12–108) vs. 18(6–48), p < 0.01 respectively). The patients with UI showed higher daytime CSS, night-time CSS, cough VAS score and lower proportion of single cough as compared with patients without UI (Table 1). In addition, patients with UI were more likely to be sensitive to “dust”, “cooking fume”, “cold air”, “supine position”, “cigarette smoke”, “exercise” as cough triggers and had significantly higher proportion of chest tightness, upper abdominal pain, chronic sinusitis and hypertension than those without UI (Table 1).
Table 1.
Baseline demographic and clinical characteristics
| Total (n = 700) |
Urinary incontinence (n = 351) |
Without urinary incontinence (n = 349) | p-value | |
|---|---|---|---|---|
| Age(years) | 45.94 ± 14.01 | 49.46 ± 13.33 | 42.39 ± 13.85 | < 0.001* |
| Cough duration (months) | 24(8–84) | 36(12–108) | 18(6–48) | < 0.001* |
| BMI (kg/m2) | 22.6 ± 3.3(n = 370) | 23.2 ± 3.5(n = 193) | 21.9 ± 3.0(n = 177) | < 0.001* |
| Daytime CSS | 3(2–4) | 3(2–4) | 3(2–3) | 0.001* |
| Nighttime CSS | 2(1–2) | 2(1–3) | 2(1–2) | 0.001* |
| Cough VAS | 60(50–80) | 65(50–80) | 50(40–70) | < 0.001* |
| Dry cough, % | 52.2(360/690) | 51.7(180/349) | 52.6(180/342) | 0.811 |
| A single cough, % | 25.6(156/609) | 21.0(66/315) | 30.6(90/294) | 0.006* |
| Continuous cough, % | 69.7(435/624) | 70.4(226/321) | 69(209/303) | 0.698 |
| Seasonal, % | 29.8(200/671) | 31.6(108/342) | 28.0(92/329) | 0.306 |
| Timing of cough | ||||
| Daytime, % | 85.0(590/694) | 87.6(305/348) | 82.4(285/346) | 0.052 |
| Before sleep, % | 60.8(421/692) | 65.7(228/347) | 55.9(193/345) | 0.009* |
| Night, % | 52.6(364/692) | 57.6(200/347) | 47.5(164/345) | 0.008* |
| Morning, % | 46.4(321/692) | 49.3(171/347) | 43.5(150/345) | 0.126 |
| Triggers | ||||
| Dust, % | 51.2(354/691) | 57.3(199/347) | 45.1(155/344) | 0.001* |
| Cooking fume, % | 56.4(390/692) | 62.8(218/347) | 49.9(172/345) | 0.001* |
| Cold air, % | 54.3(375/691) | 58.7(203/346) | 49.9(172/345) | 0.020* |
| Common cold, % | 59.3(412/693) | 62.2(216/347) | 56.6(196/346) | 0.133 |
| Supine position, % | 23.6(163/690) | 29.4(102/347) | 17.8(61/343) | < 0.001* |
| Cigarette smoke, % | 55.3(383/693) | 63.2(220/348) | 47.2(163/345) | < 0.001* |
| Exercise, % | 20.2(139/689) | 23.2(80/345) | 17.2(59/344) | 0.048* |
| Talking, % | 37.6(260/692) | 39.9(138/346) | 35.3(122/346) | 0.209 |
| Alcohol, % | 2.9(17/594) | 2.9(9/306) | 2.8(8/288) | 0.905 |
| History of medication | ||||
| Oral corticosteroids, % | 19.9(112/563) | 22.8(66/289) | 16.8(46/274) | 0.072 |
| Inhaled corticosteroids, % | 34.6(197/570) | 37.5(110/293) | 31.4(87/277) | 0.124 |
| Antitussive drugs, % | 74.6(447/599) | 75.8(229/302) | 73.4(218/297) | 0.495 |
| Concomitant symptoms | ||||
| Sneezing, % | 36.7(256/697) | 38.6(135/350) | 34.9(121/347) | 0.311 |
| Rhinocnesmus, % | 25.8(179/694) | 28.0(91/347) | 23.6(82/347) | 0.193 |
| Nasal congestion, % | 28.1(195/693) | 28.7(100/348) | 27.5(95/345) | 0.726 |
| Postnasal dripping, % | 23.6(164/694) | 25.3(88/348) | 22.0(76/346) | 0.303 |
| Chest tightness, % | 31.2(216/693) | 35.0(122/349) | 27.3(94/344) | 0.030* |
| Shortness of breath, % | 22.8(158/693) | 25.9(90/348) | 19.7(68/345) | 0.054 |
| Acid regurgitation, % | 19.3(134/696) | 20.9(73/350) | 17.6(61/346) | 0.280 |
| Nausea, % | 20.4(142/696) | 22.0(77/350) | 18.8(65/346) | 0.293 |
| Belching, % | 21.4(149/695) | 22.6(79/349) | 20.2(70/346) | 0.440 |
| Heartburn, % | 8.2(57/694) | 10.0(35/349) | 6.4(22/345) | 0.080 |
| Upper abdominal pain, % | 8.8(61/696) | 11.4(40/350) | 6.1(21/346) | 0.012* |
| Throat clearing, % | 34.9(242/693) | 38.2(133/348) | 31.6(109/345) | 0.067 |
| Itchy throat, % | 60.4(420/695) | 64.3(225/350) | 56.5(195/345) | 0.036* |
| Itching below the throat, % | 29.9(209/698) | 28.3(99/350) | 31.6(110/348) | 0.338 |
|
Pharyngeal foreign body sensation, % |
34.1(221/649) | 38.2(128/335) | 29.6(93/314) | 0.021* |
| Abdominal muscle pain due to cough, % | 29.2(204/699) | 39.6(139/351) | 18.7(65/348) | < 0.001* |
| Comorbidities | ||||
| Gastrointestinal disorders, % | 24.4(164/672) | 24.7(83/336) | 24.2(81/336) | 0.857 |
| Hypertension, % | 14.9(100/670) | 19.5(65/334) | 10.4(35/336) | 0.001* |
| Allergic rhinitis, % | 25.3(170/672) | 22.6(76/336) | 28.0(94/336) | 0.110 |
| Chronic sinusitis, % | 12.6(85/672) | 17.6(59/336) | 8.6(29/336) | 0.002* |
Data presented as mean ± SD or median (IQR) or percentage (positive cases/ total cases in groups except for missing value). Patients with UI vs patients without UI
*p < 0.05
UI Urinary incontinence, CSS Cough symptom score, VAS Visual analogue scale, OCS Oral corticosteroids, ICS Inhaled corticosteroids
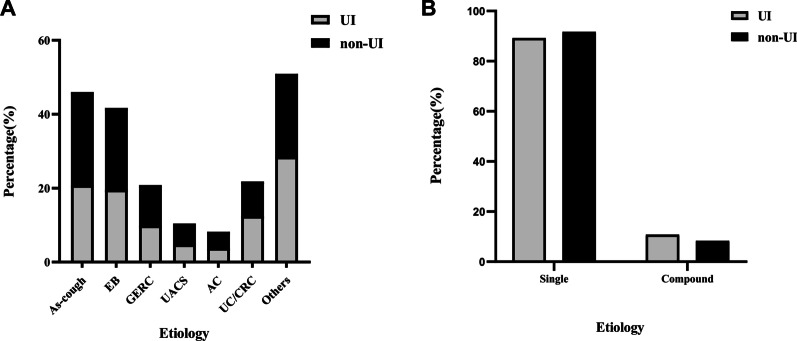
Of the 700 enrolled patients, 633 with a single cause, including asthmatic cough (146, 23.1%), EB (132, 20.9%), GERC (66, 10.4%), UACS (33, 5.2%), AC (26, 4.1%), other rare causes (161, 25.4%) and UC/CRC (69, 10.9%). There was no significant difference in etiological distribution between patients with and without UI (Fig. 2A), and no significant difference in the proportion of single etiology and compound etiology between the two groups (Fig. 2B).
Fig. 2.
A Etiological distribution in patients with UI and patients without UI. B Proportion of single etiology and compound etiology between patients with and without UI. UI, Urinary incontinence; EB, Eosinophilic bronchitis; GERC, Gastroesophageal reflux cough; UACS, Upper airway cough syndrome; AC, Atopic cough; UC, Unexplained chronic cough; CRC, Chronic refractory cough
Two demographic factors "age" and "BMI" were closely related to the occurrence of UI, thus we conducted a sub-group analysis for these two variables (Table 2). The incidence of UI increased with the increase of age and BMI. In addition, most of the patients with UI were middle-aged and elderly people (> 40 years old), while the patients without UI were younger (< 40 years old). The proportion of overweight and obesity in UI patients was 1.8 and 3.1 times higher than that in those with normal BMI.
Table 2.
Relationship between urinary incontinence and age or BMI of different grades
| Occurrence rate% (95%CI) | Cases in group UI (%) | Cases in group without UI (%) | Univariate analysis | ||
|---|---|---|---|---|---|
| OR(95%CI) | p value | ||||
| Age(years) | 50.1 (46.4–53.9) | 351 (50.1%) | 349 (49.9%) | < 0.001 | |
| 18–30 | 26.2 (17.7–34.6) | 28 (8.0%) | 79 (22.6%) | 1 | |
| 31–40 | 42.0 (34.5–49.4) | 73 (20.8%) | 101 (28.9%) | 2.039 (1.205–3.450) | 0.008 |
| 41–50 | 59.1 (51.1–67.0) | 88 (25.1%) | 61 (17.5%) | 4.070 (2.370–6.990) | < 0.001 |
| 51–60 | 57.5 (49.6–65.4) | 88 (25.1%) | 65 (18.6%) | 3.820 (2.232–6.536) | < 0.001 |
| > 60 | 63.2 (54.4–72.1) | 74 (21.1%) | 43 (12.3) | 4.855 (2.741–8.602) | < 0.001 |
| BMI (kg/m2) | 52.2 (47.0–57.3) | 193 (52.2%) | 177 (47.8%) | 0.008 | |
| Underweight (< 18.5) | 39.4 (21.8–57.0) | 13 (6.7%) | 20 (11.3%) | 0.710 (0.337–1.495) | 0.367 |
| Normal (18.5–23.9) | 47.8 (41.3–54.3) | 109 (56.5%) | 119 (67.2%) | 1 | |
| Overweight (24–27.9) | 62.8 (52.4–73.2) | 54 (28.0%) | 32 (18.1%) | 1.842(1.108–3.064) | 0.019 |
| Obesity (≥ 28) | 73.9 (54.5–93.3) | 17 (8.8%) | 6 (3.4%) | 3.093 (1.177–8.130) | 0.022 |
CI, Confidence interval; BMI, Body mass index; UI, Urinary incontinence
The multivariate analysis of risk factors
The univariate analysis showed that there were obvious differences on 27 variables between groups (all p < 0.1), including age, duration of cough, BMI, daytime and night-time CSS, cough VAS, a single cough, cough at daytime, cough before sleep, cough at night, oral corticosteroids use, accompanied by chest tightness, shortness of breath, heartburn, throat clearing, upper abdominal pain (stomachache), itchy throat, foreign body sensation in the pharynx, combining with chronic sinusitis, hypertension, abdominal pain due to cough and cough triggers (e. g. dust, cooking fumes, cold air, supine position, cigarette smoke, and exercise) All the variables mentioned above were not collinear and could be entered in the logistic regression analysis. However, the variable of BMI was finally excluded considering there was so much missing data, which may cause a great impact on the results.
In multivariate analysis, older age, higher cough VAS (OR = 1.027), combined with chronic sinusitis (OR = 1.918), cough easily triggered by exercise (OR = 1.736), and abdominal muscle pain due to cough (OR = 3.167) were independent risk factors for UI in female patients with chronic cough. Compared with younger patients, patients over 40 years old are more likely to be complicated with UI, especially in patients with an age over 60 years old, whose risk of UI is about 5.5 times higher than that of younger people aged 18–30 years (OR = 5.471) (Table 3).
Table 3.
Multivariate logistic regression analysis on risk factors of UI in female patients with chronic cough
| Multivariate analysis | |||
|---|---|---|---|
| OR | 95% CI | P value | |
| Age(years) | < 0.001 | ||
| I 18–30 | 1 | ||
| II 31–40 | 2.087 | 1.022–4.264 | 0.043 |
| III 41–50 | 3.801 | 1.798–8.036 | < 0.001 |
| IV 51–60 | 2.981 | 1.440–6.171 | 0.003 |
| V > 60 | 5.471 | 2.455–11.954 | < 0.001 |
| Cough VAS | 1.027 | 1.016–1.038 | < 0.001 |
| Combining with chronic sinusitis | 1.918 | 1.026–3.588 | 0.041 |
| Cough triggered by exercise | 1.736 | 1.036–2.907 | 0.036 |
|
Abdominal muscle pain due to cough |
3.167 | 1.931–5.194 | < 0.001 |
VAS, Visual analogue scale; CI, Confidence interval
Subsequently, 10 variables associated with UI including “age, duration of cough, cough VAS, Daytime CSS, Nighttime CSS, a single cough, combining with chronic sinusitis, concomitant with sneeze, cough triggered by exercise, abdominal muscle pain due to cough” were enrolled in the multivariate analysis to create another multivariate model. The result (See in additional file 2) was extremely similar to what shown in Table 3. Even though BMI was not included in our formal data analysis, two attempts were made about this variable: (1) enrolled “BMI” and all others variables with p < 0.1 in the univariate analyses into logistic regression; (2) enrolled “BMI” and the 10 variables mentioned above in the logistic regression. In neither attempt, “BMI” entered the regression equation (data was not shown).
Discussion
This is the first study to investigate the risk factors of UI in female adult patients with chronic cough. In this study, we found that the incidence of UI in adult female chronic cough patients was 50.1%. In addition, older age, more severe cough, combining with chronic sinusitis, cough easily triggered by exercise, and abdominal muscle pain due to cough were risk factors for UI in adult female patients.
Previous studies have proven that older age was an important risk factor for UI [13, 17, 18, 25–29], which was consistent with our observation. It might be resulted in the decline of urethral sphincter tension as the increase of age [30]. Univariate analysis showed daytime CSS, night-time CSS, and cough VAS scores in patients with UI were significantly higher than that in patients without UI. However, when the above three values were enrolled in multivariate logistic regression analysis, it was found that only the cough VAS score could be an independent risk factor. Hence, the cough VAS score might reflect the cough severity generally, and might be possible to correct for the daytime and nighttime CSS.
There are two possible reasons for “cough easily triggered by exercise” (defined as intentional exercise beyond daily activities in this study) being a risk factor for UI [31–36]. Firstly, muscle tightness could increase abdominal pressure during daily exercise, especially during moderate-to-high intensity exercise. Meanwhile, cough triggered by exercise could continuously increase abdominal pressure then increase the risk of UI. Secondly, mild-to-moderate physical exercise can train the function of pelvic floor muscles. A normal pelvic floor muscle tension is the key to offset the excessive increase in abdominal pressure after exercise, which is helpful to prevent UI. If the cough is easily induced by exercise, patients will be resistant to do exercises, which makes it difficult to train the pelvic floor function adequately. Additionally, with the increased abdominal pressure due to prolonged coughing, the risk of UI will be certainly increased.
This study also showed that “chronic sinusitis” was an independent risk factor for UI. That chronic sinusitis is often characterized by nasal congestion, runny nose, dysosmia and facial pain, is common in patients who have been diagnosed with allergic rhinitis, asthma, chronic obstructive pulmonary disease, and other respiratory diseases [37]. But up to now, there is no direct evidence that chronic sinusitis was a risk factor of UI. Bekele, et al. reported that the incidence of UI in pregnant women with respiratory diseases such as asthma, allergic rhinitis and rhinosinusitis is higher than those without respiratory diseases [38]. A few studies have shown that severe nasal symptoms such as sneezing and runny nose in women could induce UI [19, 39, 40]. However, in our study, we found that there was no significant difference in the incidence of sneezing and nasal congestion between the patients with UI and patients without UI, we speculated that this is because of the high prevalence of nasal symptoms in Chinese population and the serious air pollution in China, yet there is still lacking of epidemiological researches in this area [41]. We also compared the prevalence of UACS between the patients with UI and patients without UI and found that there was no significant difference in it (4.8% vs. 5.6%, p = 0.651). A large-scale epidemiological survey of chronic sinusitis covering 7 cities in China shows that age is a risk factor for chronic sinusitis [37]. Therefore, a large proportion of elderly patients in UI group might increase the incidence of chronic sinusitis, which may also be the reason why chronic sinusitis was a risk factor for UI in this study. However, "chronic sinusitis" was not corrected by "age" in the multivariate analysis. Xie, et al. also found that "rhinitis" was a risk factor for UI in females, but there was still no sufficient evidence to explain the result [13]. Thus, whether there was any overlapping pathogenesis between chronic sinusitis and UI needed further study.
The study also found that abdominal muscle pain due to cough was a risk factor for UI in female patients with chronic cough. The median VAS score of patients with abdominal muscle pain due to cough was significantly higher than that in those without this complication (60 vs. 50, p = 0.002), which indicated that patients who had abdominal muscle pain often presented more severe cough symptom, However, "abdominal muscle pain due to cough" was not corrected by "cough severity (VAS)" and remained as an independent risk factor in multivariate analysis. We wondered if it was attributed to the weakness and the lack of tension of the abdominal wall muscles and the pelvic floor muscles in these patients so that persistent cough was more likely to cause more severe damage to the muscles, ultimately lead to UI. Nevertheless, the specific mechanisms and interconnections are needed to be confirmed by further studies.
We analyzed the relationship between the distribution of cause of chronic cough and the occurrence of UI, and found that neither single nor compound causes could significantly increase the risk of UI. Chronic coughers with any cause showed similar susceptible to developing UI, and if the risk factors mentioned above were considered together, the occurrence of UI would increase. With univariate analysis, it has been found that the patients with UI were more sensitive to environmental allergens such as dust, oil fume, cigarette smoke, cold air, but all of these factors were wiped off the list after using multivariate analysis. Thus, no significant correlation between allergen-induced cough and UI was found in our study.
In this study, the reasons why BMI failed to enter the multivariate model might be related to the subjects, the numerical distribution, and, most importantly, the large number of missing data. Yet, previous studies have shown that high BMI was a strong predictor of the presence of UI in female [13, 17, 18, 25–29]. Noblett et al. considered that overweight and obesity could cause the pelvic floor in a chronic state of increased pressure, thereby increase the risk of UI, by illustrating the relationship between BMI and intra-abdominal and intravesical pressure [42]. Even if BMI was not included in the multivariate analysis at the end, we can still believe that overweight and obesity were very important for UI developing by combining the results of the univariate analysis.
There were also some limitations in this study. First of all, some potential factors which could affect female pelvic floor dysfunction including surgical history (e.g. delivery and gynecological surgery), and the number of deliveries were not recorded, it might make an influence on the comprehensiveness of the result of the study. Additionally, the severity and duration of UI were not evaluated, resulting in nonsignificant effect of the risk factors. It has been reported that stress UI was more commonly presented [43, 44], thus it would be worth analyzing whether there were differences in the risk factors of different types of UI, and future study could focus on this question. Thirdly, the details of the treatment history were not recorded, which made it difficult to analyze the impact of treatment on UI. Meanwhile, this study was a single-center retrospective study, and some missing variables could not be verified, the relationship of the demographic, clinical factors and UI might not be comprehensive and accurate. However, considering the large sample size in this study, our findings could provide a helpful and referenced guiding for the management of chronic coughers. Lastly, as our sample consists of patients visiting a specialist cough clinic, it might not be representative to chronic cough patients in the community. But in our study, most of the patients were presented with the common causes of chronic cough, and the proportion of unexplained cough or chronic refractory cough was similar as previous reports [7]. Furthermore, we found that the CSS and cough VAS were similar among different causes in this study. The etiological distribution did not play an predominent role in UI. This study still provided an important implication in clinical, but further study aimed at patients from the community should be conducted to confirm it in the future.
Conclusion
In conclusion, older age, more severe cough, chronic sinusitis, cough easily triggered by exercise and abdominal muscle pain due to cough are the risk factors for urinary incontinence in female patients with chronic cough. We should pay more attention to the female patients who showed a possibility of developing urinary incontinence in clinical practice. It’s necessary for female patients to strengthen their pelvic floor muscle function in daily life to reduce the incidence of urinary incontinence. A prospective epidemiological investigation with larger sample on urinary incontinence was needed to conduct in the future, while the physiological structure and functional indicators should be taken into consideration as important risk factors.
Supplementary Information
Additional file 1: Case Report Form for adult female patients with chronic cough.
Additional file 2: Multivariate logistic regression analysis on risk factors of UI in female patients with chroniccough (after variables reduction).
Acknowledgements
Not applicable.
Abbreviations
- UI
Urinary incontinence
- BMI
Body mass index
- CVA
Cough variant asthma
- EB
Eosinophilic bronchitis
- GERC
Gastroesophageal reflux cough
- UACS
Upper airway cough syndrome
- AC
Atopic cough
- UC
Unexplained chronic cough
- CRC
Chronic refractory cough
- VAS
Visual analogue scale
- CSS
Cough symptom score
Author contributions
LK, YF: conception and design of the study. YC, FZ: data collection, data analysis, interpretation, drafting, and revising of the submitted manuscript. CZ, XD, LY: data collection, data analysis, and revising of the submitted manuscript. LK and YF: a critical review of the manuscript. All authors provided a review of the manuscript and approved its submission.
Funding
This study was funded by Science and Technology Project for Health and Family Planning of Guangzhou (20161A011063) and Incubative Project for Innovation Team of Guangzhou Medical University (grant no. 2017-159).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (Medical Research 2020150). All methods were performed in accordance with the relevant guidelines and regulations. All subjects gave informed consent for their data to be analyzed.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cunzhen Yang and Zien Feng authors contributed equally to this work.
Kefang Lai and Fang Yi authors contributed equally to this work.
Contributor Information
Kefang Lai, Email: klai@163.com.
Fang Yi, Email: upyifang@163.com.
References
- 1.Song WJ, Chang YS, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015;45(5):1479–1481. doi: 10.1183/09031936.00218714. [DOI] [PubMed] [Google Scholar]
- 2.Morice AH, Jakes AD, Faruqi S, et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J. 2014;44(5):1149–1155. doi: 10.1183/09031936.00217813. [DOI] [PubMed] [Google Scholar]
- 3.Morice AH, Kastelik JA. Cough 1: chronic cough in adults. Thorax. 2003;58:901–7. doi: 10.1136/thorax.58.10.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kavalcikova-Bogdanova N, Buday T, Plevkova J, Song WJ. Chronic cough as a female gender issue. Adv Exp Med Biol. 2016;905:69–78. doi: 10.1007/5584_2015_182. [DOI] [PubMed] [Google Scholar]
- 5.Lai K, Long L, Yi F, et al. Age and sex distribution of chinese chronic cough patients and their relationship with capsaicin cough sensitivity. Allergy Asthma Immunol Res. 2019;11(6):871–884. doi: 10.4168/aair.2019.11.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long L, Lai K. Characteristics of Chinese chronic cough patients. Pulm Pharmacol Ther. 2019;57:101811. doi: 10.1016/j.pupt.2019.101811. [DOI] [PubMed] [Google Scholar]
- 7.Lai K, Chen R, Lin J, et al. A prospective, multicenter survey on causes of chronic cough in China. Chest. 2013;143(3):613–620. doi: 10.1378/chest.12-0441. [DOI] [PubMed] [Google Scholar]
- 8.Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165(2):408–412. doi: 10.1097/00005392-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Suhr R, Lahmann NA. Urinary incontinence in home care: a representative multicenter study on prevalence, severity, impact on quality of life, and risk factors. Aging Clin Exp Res. 2018;30(6):589–594. doi: 10.1007/s40520-017-0816-6. [DOI] [PubMed] [Google Scholar]
- 10.Yang C, Chen R, Li B, et al. Survey on quality of life and urinary incontinence in female patients with chronic cough. Int J Respir. 2010;30(7):391–394. [Google Scholar]
- 11.Perera J, Kirthinanda DS, Wijeratne S, et al. Descriptive cross sectional study on prevalence, perceptions, predisposing factors and health seeking behaviour of women with stress urinary incontinence. BMC Womens Health. 2014;14:78. doi: 10.1186/1472-6874-14-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmadi B, Alimohammadian M, Golestan B, et al. The hidden epidemic of urinary incontinence in women: a population-based study with emphasis on preventive strategies. Int Urogynecol J. 2010;21(4):453–459. doi: 10.1007/s00192-009-1031-6. [DOI] [PubMed] [Google Scholar]
- 13.Xie X, Chen Y, Khan A, et al. Risk factors for urinary incontinence in Chinese women: a cross-sectional survey. Female Pelvic Med Reconstr Surg. 2021;27(6):377–381. doi: 10.1097/SPV.0000000000000871. [DOI] [PubMed] [Google Scholar]
- 14.French CL, Crawford SL, Bova C, et al. Change in psychological, physiological, and situational factors in adults after treatment of chronic cough. Chest. 2017;152(3):547–562. doi: 10.1016/j.chest.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 15.Bradaia F, Lazor R, Khouatra C, et al. Urinary incontinence due to chronic cough in interstitial lung disease. Rev Mal Respir. 2009;26(5):499–504. doi: 10.1016/S0761-8425(09)74668-6. [DOI] [PubMed] [Google Scholar]
- 16.Verett CF, Ojoo JC, Thompson RH, et al. A questionnaire survey of individuals complaining of chronic cough (abstract) Am J Respir Crit Care Med. 2003;167:A316. [Google Scholar]
- 17.Peyrat L, Haillot O, Bruyere F, et al. Prevalence and risk factors of urinary incontinence in young and middle-aged women. BJU Int. 2002;89(1):61–66. doi: 10.1046/j.1464-410X.2002.02546.x. [DOI] [PubMed] [Google Scholar]
- 18.Lamerton TJ, Torquati L, Brown WJ. Overweight and obesity as major, modifiable risk factors for urinary incontinence in young to mid-aged women: a systematic review and meta-analysis. Obes Rev. 2018;19(12):1735–1745. doi: 10.1111/obr.12756. [DOI] [PubMed] [Google Scholar]
- 19.Tähtinen RM, Cartwright R, Vernooij RWM, et al. Long-term risks of stress and urgency urinary incontinence after different vaginal delivery modes. Am J Obstet Gynecol. 2019;220(2):181.e1–181.e8. doi: 10.1016/j.ajog.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 20.Hage-Fransen MAH, Wiezer M, Otto A, et al. Pregnancy- and obstetric-related risk factors for urinary incontinence, fecal incontinence, or pelvic organ prolapse later in life: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2021;100(3):373–382. doi: 10.1111/aogs.14027. [DOI] [PubMed] [Google Scholar]
- 21.Hsu JY, Stone RA, Logan-Sinclair RB, et al. Coughing frequency in patients with persistent cough: assessment using a 24 hour ambulatory recorder. Eur Respir J. 1994;7(7):1246–53. doi: 10.1183/09031936.94.07071246. [DOI] [PubMed] [Google Scholar]
- 22.Lai K, Chen R, Liu C, et al. Etiology and a diagnostic protocol for patients with chronic cough. Zhonghua Jie He He Hu Xi Za Zhi. 2006;29:96–99. [PubMed] [Google Scholar]
- 23.Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):1s–23s. doi: 10.1378/chest.129.1_suppl.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asthma Workgroup of Chinese Society of Respiratory Diseases (CSRD) Chinese Medical Association Guidelines for diagnosis and management of cough. Zhonghua Jie He He Hu Xi Za Zhi. 2016;39(5):323–354. [Google Scholar]
- 25.Bø K, Pauck Øglund G, Sletner L, et al. The prevalence of urinary incontinence in pregnancy among a multi-ethnic population resident in Norway. BJOG. 2012;119(11):1354–1360. doi: 10.1111/j.1471-0528.2012.03435.x. [DOI] [PubMed] [Google Scholar]
- 26.Lasserre A, Pelat C, Guéroult V, et al. Urinary incontinence in French women: prevalence, risk factors, and impact on quality of life. Eur Urol. 2009;56(1):177–183. doi: 10.1016/j.eururo.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Demir O, Sen V, Irer B, et al. Prevalence and possible risk factors for urinary incontinence: a cohort study in the city of Izmir. Urol Int. 2017;99(1):84–90. doi: 10.1159/000466705. [DOI] [PubMed] [Google Scholar]
- 28.Linde JM, Nijman RJM, Trzpis M, et al. Urinary incontinence in the Netherlands: prevalence and associated risk factors in adults. Neurourol Urodyn. 2017;36(6):1519–1528. doi: 10.1002/nau.23121. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber Pedersen L, Lose G, Høybye MT, et al. Prevalence of urinary incontinence among women and analysis of potential risk factors in Germany and Denmark. Acta Obstet Gynecol Scand. 2017;96(8):939–948. doi: 10.1111/aogs.13149. [DOI] [PubMed] [Google Scholar]
- 30.Kenton K, Mueller E, Brubaker L. Neuromuscular characterization of the urethra in continent women. Female Pelvic Med Reconstr Surg. 2011;17(5):226–30. doi: 10.1097/SPV.0b013e31822dd012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson S, Deeble M, Thompson J, et al. Should women with incontinence and prolapse do abdominal curls? Int Urogynecol J. 2016;27(10):1507–1512. doi: 10.1007/s00192-016-3005-9. [DOI] [PubMed] [Google Scholar]
- 32.Bump RC, Norton PA. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin North Am. 1998;25(4):723–746. doi: 10.1016/S0889-8545(05)70039-5. [DOI] [PubMed] [Google Scholar]
- 33.Bø K. Urinary incontinence, pelvic floor dysfunction, exercise and sport. Sports Med. 2004;34(7):451–464. doi: 10.2165/00007256-200434070-00004. [DOI] [PubMed] [Google Scholar]
- 34.Faleiro DJA, Menezes EC, Capeletto E, et al. Association of physical activity with urinary incontinence in older women: a systematic review. J Aging Phys Act. 2019;27(4):906–913. doi: 10.1123/japa.2018-0313. [DOI] [PubMed] [Google Scholar]
- 35.Bø K, Talseth T, Holme I. Single blind, randomised controlled trial of pelvic floor exercises, electrical stimulation, vaginal cones, and no treatment in management of genuine stress incontinence in women. BMJ. 1999;318(7182):487–493. doi: 10.1136/bmj.318.7182.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw JM, Hamad NM, Coleman TJ, et al. Intra-abdominal pressures during activity in women using an intra-vaginal pressure transducer. J Sports Sci. 2014;32(12):1176–1185. doi: 10.1080/02640414.2014.889845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi JB, Fu QL, Zhang H, et al. Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy. 2015;70(5):533–539. doi: 10.1111/all.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bekele A, Adefris M, Demeke S. Urinary incontinence among pregnant women, following antenatal care at University of Gondar Hospital, North West Ethiopia. BMC Pregnancy Childbirth. 2016;16(1):333. doi: 10.1186/s12884-016-1126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fritel X, Ringa V, Varnoux N, et al. Mode of delivery and severe stress incontinence. A cross-sectional study among 2,625 perimenopausal women. BJOG. 2005;112(12):1646–51. doi: 10.1111/j.1471-0528.2005.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elia G. Stress urinary incontinence in women removing the barriers to exercise. Phys Sportsmed. 1999;27(1):39–52. doi: 10.3810/psm.1999.01.644. [DOI] [PubMed] [Google Scholar]
- 41.Xian M, Wang K, Lou H, et al. Short-term haze exposure predisposes healthy volunteers to nasal inflammation. Allergy Asthma Immunol Res. 2019;11(5):632–643. doi: 10.4168/aair.2019.11.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noblett KL, Jensen JK, Ostergard DR. The relationship of body mass index to intra-abdominal pressure as measured by multichannel cystometry. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8(6):323–326. doi: 10.1007/BF02765589. [DOI] [PubMed] [Google Scholar]
- 43.Legendre G, Fritel X, Panjo H, et al. Incidence and remission of stress, urge, and mixed urinary incontinence in midlife and older women: a longitudinal cohort study. Neurourol Urodyn. 2020;39(2):650–657. doi: 10.1002/nau.24237. [DOI] [PubMed] [Google Scholar]
- 44.Moossdorff-Steinhauser HFA, Berghmans BCM, Spaanderman MEA, et al. Prevalence, incidence and bothersomeness of urinary incontinence in pregnancy: a systematic review and meta-analysis. Int Urogynecol J. 2021;32(7):1633–1652. doi: 10.1007/s00192-020-04636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Case Report Form for adult female patients with chronic cough.
Additional file 2: Multivariate logistic regression analysis on risk factors of UI in female patients with chroniccough (after variables reduction).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.