Significance
International concern about the consequences of human-induced global environmental changes has prompted a renewed focus on reducing ecological effects of biological invasions, climate change, and nutrient pollution. Our results show that the combined effects of nonnative species invasions and abiotic global environmental changes are often negative but no worse than invasion impacts alone. Invasion impacts are also more strongly detrimental than warming temperatures or nitrogen deposition, two common stressors. Thus, reducing the spread of invasive species is critical for mitigating harms from anthropogenic changes to global ecosystems.
Keywords: invasive species, climate change, nitrogen pollution, synergism, antagonism
Abstract
Human-induced abiotic global environmental changes (GECs) and the spread of nonnative invasive species are rapidly altering ecosystems. Understanding the relative and interactive effects of invasion and GECs is critical for informing ecosystem adaptation and management, but this information has not been synthesized. We conducted a meta-analysis to investigate effects of invasions, GECs, and their combined influences on native ecosystems. We found 458 cases from 95 published studies that reported individual and combined effects of invasions and a GEC stressor, which was most commonly warming, drought, or nitrogen addition. We calculated standardized effect sizes (Hedges’ d) for individual and combined treatments and classified interactions as additive (sum of individual treatment effects), antagonistic (smaller than expected), or synergistic (outside the expected range). The ecological effects of GECs varied, with detrimental effects more likely with drought than the other GECs. Invasions were more strongly detrimental, on average, than GECs. Invasion and GEC interactions were mostly antagonistic, but synergistic interactions occurred in >25% of cases and mostly led to more detrimental outcomes for ecosystems. While interactive effects were most often smaller than expected from individual invasion and GEC effects, synergisms were not rare and occurred across ecological responses from the individual to the ecosystem scale. Overall, interactions between invasions and GECs were typically no worse than the effects of invasions alone, highlighting the importance of managing invasions locally as a crucial step toward reducing harm from multiple global changes.
Humans are contributing to multiple cooccurring ecological stressors, including climate change, nitrogen deposition, and biological invasions (hereafter “invasions”), creating a challenge for practitioners who must prioritize and address threats to native species and ecosystems. Natural resource managers commonly identify invasions as a top concern for mitigation and adaptation to climate change (1, 2). However, the relative and interactive effects of abiotic global environmental changes (GECs) and invasions remain unclear. Understanding such interactions is critical for predicting impacts to ecosystems and human societies and for implementing effective policy and management (3, 4).
Invasions and GECs are major causes of biodiversity redistribution and loss (3, 4) and have impacts at all levels of biological organization, from the performance of individual organisms to ecosystem functioning (e.g., 5, 6). For example, invasions are associated with an average 25% decline in native species diversity, and increasing abundances of nonnative predators are linked to native species population declines of 44% (7). At the same time, GECs, including climate change and nitrogen deposition, are altering nutrient cycling (8), causing population declines, and increasing extinction risk (9, 10). While previous studies have compared impacts across different invasive species (7) or types of GECs [e.g., warming and drought (6)], few have compared invasive species to GECs (but see reference 11) or the combined effect of invasions with other GECs. In a recent meta-analysis of the effects of agricultural weeds and climate change on crops, Vilà et al. (12) showed that the effect of crop weeds was significantly more negative than warming and elevated carbon dioxide but comparable to the effect of drought. However, the relative ecological impacts of invasion vs. GECs remain unknown for other ecosystems.
Knowing whether and how invasions interact with GECs would also help to inform conservation and management practices (4, 13, 14). Invasions and GECs can have summed (additive) effects, such as decreasing native species abundance, that add up to more negative impacts than either stressor alone (e.g., 13, 15). Invasions and GECs can also amplify each other (a synergistic interaction), leading to more extreme outcomes than their summed effects. For example, invasive earthworms amplify the effects of warming on seedling establishment by drying soil, leading to larger than expected shifts in plant species composition (14). Alternatively, invasions and GECs could interact to lessen their ecological effects (an antagonistic interaction). For example, stressful conditions caused by drought can lessen the impacts of invasive plants (16) and pathogens (17). The broad range of potential interactions highlights the need to synthesize existing information to understand the likely outcomes of coincident stressors.
In recent years, there have been growing concerns that anthropogenic stressors will interact synergistically, leading to outsized ecological impacts (13, 15, 18, 19) and even more detrimental effects on ecosystems (19, 20). Yet, there have been no comprehensive syntheses of the individual and combined effects of biological invasions and abiotic GECs. Here, we present a meta-analysis of 95 experimental studies measuring the individual and combined ecological effects of invasions and one of six GECs, as follows: warming, nitrogen deposition, oxygen depletion, drought, carbon dioxide addition, and altered pH. We ask the following questions: 1) How do invasions, GECs, and their combination affect native species and ecosystems? 2) How often do synergistic interactions occur and are they likely to be detrimental for ecosystems? and 3) How do direct effects and interactions vary across GEC stressors, mechanisms of invasion impact (i.e., competition, predation, or chemical/physical impacts), and broad ecosystem context (marine, freshwater, and terrestrial systems)? We build on existing frameworks to classify interactions, considering both their magnitude (additive, antagonistic, or synergistic) and direction (whether the interaction has better or worse ecological effects than expected) relative to the individual stressor effects (Fig. 1). Our findings have implications for prioritizing research, policy, and management in the face of multifaceted, ongoing global change.
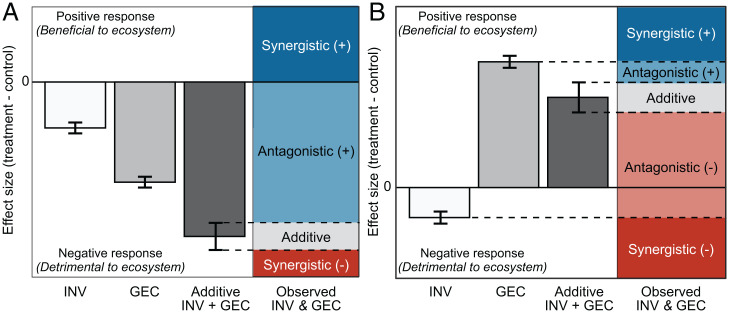
Fig. 1.
Classification of interaction types based on the relationship between individual stressor effects (INV and GEC, shown as light- and medium-gray bars, respectively) and the predicted additive effect (the sum of the individual stressor effects, dark gray), for example, cases where both INV and GEC effects are in the same direction (A) and where INV and GEC effects are in different directions (B). Observed combined stressor (INV&GEC) effects falling within the 95% confidence interval around the predicted additive effect are classified as additive. Effects that differ from the predicted additive (INV+GEC) effect but fall within the range of the individual stressor effects and the control are classified as antagonistic, and those falling outside of this range are classified as synergistic. Antagonistic and synergistic effects are further classified as “+” if the effect is more positive (beneficial) or “−“ if the observed is more negative (detrimental) than the predicted additive effect. All measured responses in the meta-analysis were coded such that negative effects indicate detrimental outcomes.
Results
Our literature search resulted in a dataset of 467 cases from 95 published studies that reported both individual and combined ecological effects of invasions with one of six abiotic GECs (SI Appendix, Part 1: Supplementary Methods for a list of studies). Eight cases had incalculable Hedges’ d values due to a measured variance of zero for multiple treatments, and one case was a clear outlier (SI Appendix, Fig. S1.2); therefore, we analyzed data on 458 cases. Most studies focused on the impacts of warming (n = 30), drought (n = 21), or nitrogen addition (n = 43), with few studies on elevated carbon dioxide (n = 3), oxygen depletion (n = 2), or altered pH (n = 3). Thus, we focused our results on the three most common GEC manipulations. Most studies were performed in the United States (n = 31), China (n = 16), and across Europe (n = 32; SI Appendix, Fig. S2.1). Studies were biased toward terrestrial systems (n = 50) and plants (n = 66) with nearly half of studies and cases (42% and 46%, respectively) focused on terrestrial invasive plants affecting native plant species via competition (SI Appendix, Figs. S2.1 and S2.2). There was some evidence of publication bias in the data, with more negative GEC effects in cases with larger sample sizes and greater precision (SI Appendix, Fig. S2.3).
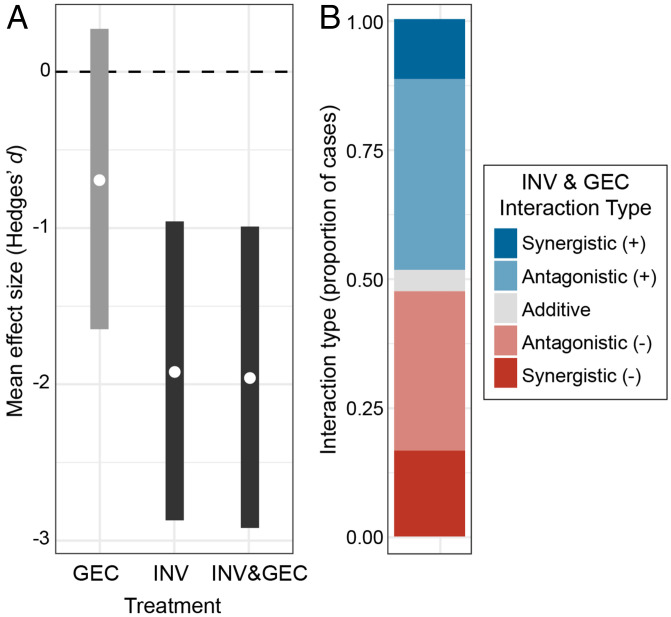
Across all cases, both invasion (INV) and the combined invasion and GEC (INV&GEC) treatments showed significantly negative (detrimental) ecological impacts (Fig. 2A). INV and INV&GEC effects were also significantly more negative than mean treatment effects for all GECs (SI Appendix, Fig. S3.4), which were not different from zero according to the 95% credible interval (Fig. 2A). Only 4% of INV&GEC interactions were classified as strictly additive, with the other 96% of interactions approximately equally likely to be more positive or more negative than the predicted additive effect of individual stressors (Fig. 2B). Antagonistic (within the range of expected values) effects were the most common, but over 25% of INV&GEC interactions were synergistic (larger than expected). Synergistic effects were most often more detrimental to the ecosystem than the predicted additive effect (17% negative vs. 12% positive synergistic interactions; Fig. 2B). These results were similar when considering only cases for which we were confident in our interpretation of the response as detrimental vs. beneficial (SI Appendix, Fig. S2.5), when we used a more conservative cutoff for removing outliers (SI Appendix, Fig. S2.5), and in cases of plant and animal invasions, respectively (SI Appendix, Fig. S2.6). All regression models converged (Gelman–Rubin statistics < 1.01) and fit the data (Bayesian P values between 0.49 and 0.52).
Fig. 2.
On average, INV and INV&GEC had more detrimental ecological effects than single GEC stressors, and most INV&GEC interactions were classified as antagonistic (smaller than additive). (A) Hedges’ d effect sizes of GEC, INV, and INV&GEC treatments were estimated from a mixed-effects model, with white circles showing the mean and gray bars showing the 95% credible interval of the posterior distributions. Credible intervals that do not cross zero (dark-gray bars) are considered significantly different from zero. (B) INV&GEC effects were almost always different from the predicted additive effect and were equally likely to be more positive or more negative than expected from the individual stressor effects.
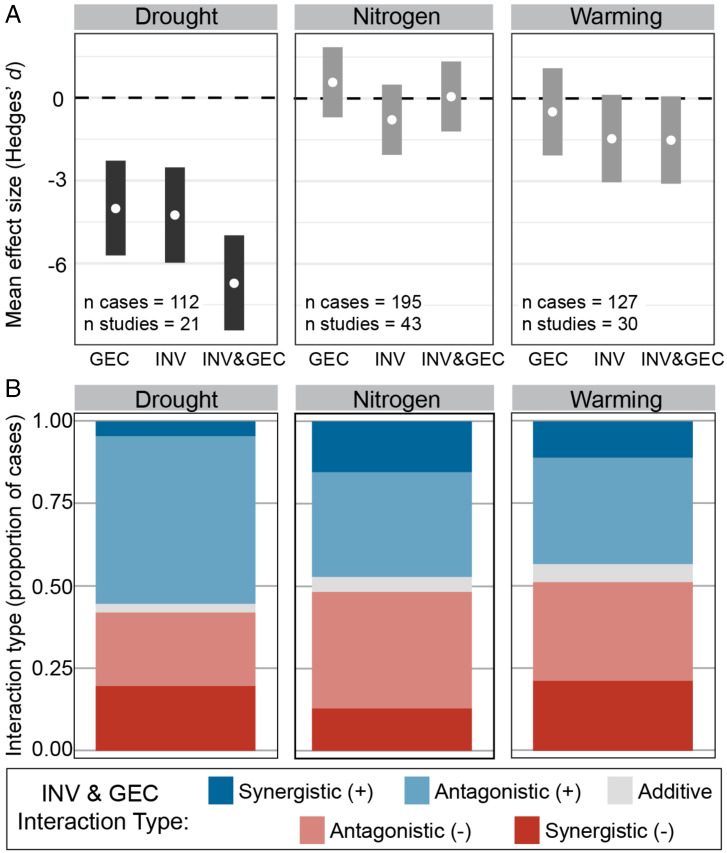
GEC type and invasion mechanism (e.g., competition, predation) both explained some of the variation in individual stressor effects across cases, but only GEC type influenced the combined INV&GEC effects. Drought, but not warming or nitrogen deposition, had a mean negative effect (Fig. 3A) that was significantly more detrimental than other individual GEC effects (SI Appendix, Fig. S2.7). Invasions acting via competition and predation also had a significant negative effect (SI Appendix, Fig. S2.8). Combined INV&GEC effects were negative with drought (Fig. 3A), as well as with invasions acting via competition (SI Appendix, Fig. S2.8). The distribution of INV&GEC interaction types (additive, negative or positive antagonistic, and negative or positive synergistic) varied across GECs (Fisher’s exact test simulated P = 0.017; simulated P = 0.002 when comparing only warming, drought, and nitrogen deposition), with more positive synergistic effects in nitrogen cases and more negative synergistic effects in drought and warming cases (Fig. 3B). However, there were no significant differences in interaction types when the dataset was reduced to one case per study (SI Appendix, Fig. S2.9). Interaction types did not vary across invasion mechanisms (Fisher’s exact test simulated P = 0.676).
Fig. 3.
INV&GEC effects were more strongly negative in cases of drought and warming, with larger proportions of interactions showing negative synergistic effects than nitrogen deposition. (A) Effects of all treatments were more detrimental in cases that manipulated drought than other GECs. (B) Bar plots show distributions of the INV&GEC interaction types across GECs. Only GECs with at least at least 10 cases from at least 5 studies (excluding CO2, O2, and pH) are shown (see SI Appendix, Fig. S3.8 for full results).
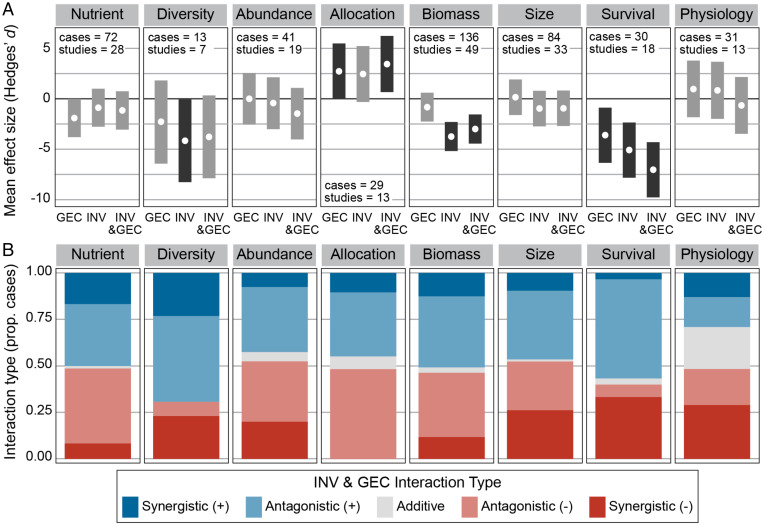
INV, GEC, and INV&GEC treatment effects and INV&GEC interaction types all differed depending on the class of ecological response. INV&GEC effects were more negative than GEC effects across almost all response classes (except for nutrients and tissue allocation) but were not always more negative than invasion effects (Fig. 4A). INVs had significant negative effects on native species biomass and community diversity; INV&GEC also had negative effects on biomass. GECs, alone and in combination with invasions had significant positive effects on tissue allocation. The most negative effects of all treatments were on native species survival (Fig. 4A), and GECs had significantly more detrimental effects on survival than on other response classes (SI Appendix, Fig. S2.7). Different response classes showed different distributions of INV&GEC interaction types (Fisher’s exact test simulated P = 0.001 in the full dataset; no differences were found in the reduced dataset; SI Appendix, Fig. S2.10). Importantly, cases measuring native species survival, body size, and physiology all had a greater than 25% likelihood of negative synergistic interactions (Fig. 4B and SI Appendix, Fig. S2.10).
Fig. 4.
INV, GEC, and INV&GEC effects varied across measured response classes, as did the interaction types. (A) On average, GEC effects were only negative for native species survival. INV and INV&GEC were both negative for biomass and survival, and INV effects were negative for native species diversity. GEC and INV&GEC effects were positive for tissure allocation. (B) Bar plots show distributions of INV&GEC interaction types across response classes. Response classes are arranged roughly from ecosystem to individual scale. Only response classes with at least at least 10 cases from at least 5 studies (excluding behavior and reproduction) are shown (SI Appendix, Fig. S3.9 for full results).
Discussion
Our meta-analysis provides a comprehensive examination of the ecological effects of interactions between invasive species and abiotic GECs across taxa. On average, the combined effects of invasions and GECs are more detrimental than individual GEC effects but no worse than invasions alone (Fig. 2A). This is consistent with Vilà and colleagues’ (12) findings in crop systems and points to an outsized role of invasions in causing ecological harm. Although combined stressor effects tend to be detrimental, antagonistic interactions predominate, leading to outcomes that are usually less extreme than expected from the individual stressor effects. Nevertheless, synergistic interactions occur in a significant minority (>25%) of cases and most often create more detrimental ecological outcomes (Fig. 2B). These results suggest that in many cases, addressing one of the stressors will ameliorate some of the impacts of both. Thus, regardless of whether interactions were antagonistic or synergistic, prioritizing the management of invasive species is most likely to lead to improved ecological outcomes.
Invasive species, which are often managed at a local scale, were more detrimental on average than GECs in our study. While GECs such as climate change and nutrient deposition are clearly linked to ecological harm (8–11), our results highlight the importance of continuing to consider local stressors when evaluating ecosystem vulnerability (21). However, detrimental invasion effects were not evident in marine systems (SI Appendix, Fig. S2.8), likely due to a mixture of impacts across trophic levels, which are often negative, and between-guild impacts in the dataset that can be positive or negative (22, 23). Our results are consistent with and add generality to a recent review of local vs. global stressors in coastal ecosystems that found the amelioration of local stressors (e.g., coastal development) to be a preferred strategy in cases where global impacts were not expected to be severe (21). Local management of invasive species includes tangible actions within the purview of many conservation organizations, governmental agencies, and land stewards and therefore may provide a more immediate benefit to native species, communities, and ecosystems than the mitigation of GECs.
Climate change stressors interact with invasions to produce detrimental ecological effects (Fig. 3A) and a greater likelihood of negative than positive synergistic effects (Fig. 3B). Moderate warming can benefit invasive species, leading to more detrimental effects of invasions under warmer conditions (18, 24). Drought increases stress for many organisms, including invasive species (25), causing negative ecological effects but potentially mitigating invasion impacts (e.g., 16, 26). Drought can also create negative synergistic effects when invasive plants further reduce water availability (27). The prevalence of negative synergistic interactions with climate change stressors suggests that invasive species management will benefit many systems experiencing warming and/or drought.
Nitrogen deposition had more variable ecological effects than either warming or drought, both alone and in combination with invasions (Fig. 3A). Effects of nitrogen deposition on ecosystems and on invasive species can be either positive or negative, depending on environmental conditions (28, 29) and species’ traits (28–30). Most nitrogen-focused studies in our dataset measured effects on nutrient cycling (n = 17) or biomass (n = 28), responses whose interpretation as beneficial or detrimental is highly system specific. Nitrogen studies rarely measured native species diversity (n = 4) or survival (n = 2), which tend to show the most pronounced negative response to stressor interactions. Furthermore, large differences across studies in their methods of application, concentrations, and forms of nitrogen may further explain the high variability (28).
Native species and ecosystems responded differently to the combined effects of invasions and GECs depending on what response was measured, with significant negative effects on some attributes that are important for conservation, including biodiversity (Fig. 4A). Our results are consistent with other studies showing that invasions have stronger detrimental effects on species’ survival and diversity than on ecosystem function (e.g., nutrient cycling; 5, 31) and that invasive species are detrimental to diversity at small spatial scales (7, 32). Of particular concern is that GECs, invasions, and combined stressors all have negative effects on native species survival and that a third of survival responses exhibit negative synergistic interactions (Fig. 4B). Actions to mitigate invasions and/or GECs may thus be most critical when maintaining populations of native species is a top priority, such as when management goals include protecting rare or vulnerable species.
While the frequency of negative synergistic interactions between invasions and GECs is concerning, these “worst-case scenarios” are far less common than antagonistic interactions (Fig. 2). Our results suggest that combined invasion and GEC effects are typically less extreme than the sum of the two stressors (Fig. 2) and not significantly different from invasion effects alone (SI Appendix, Fig. S2.4). Although the definitions of interaction types can influence which interactions are deemed most common (8, 33) and the method we used was conservative for assigning synergistic effects (34), several studies have found that antagonistic interactions are more common than synergistic interactions and strictly additive effects are relatively rare (33, 35, 36). While synergisms occur, especially when the measured response relates to body size, survival, or physiology or when environmental conditions are warmer and drier, in most cases one stressor dominates or mitigates the effects of the other. Thus, managing the stressor that causes the most ecological harm (often invasions) may be a wise approach when resources are limited.
Our analysis also exposes key gaps that highlight the need for more research to elucidate invasion and its interaction with GECs. Some gaps were common to invasion research and ecology in general, including significant geographic biases (particularly when meta-analyses are limited to English-language studies; 37, 38), and relatively few studies of animal invasions in terrestrial systems or plant invasion studies in aquatic systems (39). The majority of studies (n = 74) were conducted in controlled settings (i.e., laboratory/greenhouse, mesocosm) and may have failed to capture important aspects of GEC and invasive effects (although the major trends persist across experiment types; SI Appendix, Fig. S2.8). Most notable was the lack of sufficient studies to evaluate the combined impacts of acidification, carbon dioxide addition, or oxygen depletion and invasions (SI Appendix, Fig. S2.1). Oxygen depletion can have severe effects on aquatic systems, even more so than the more commonly studied effects of climate change (e.g., direct effects of warming; 40, 41), so their interactions with invasions may be particularly important to study. Carbon dioxide addition can increase the growth of invasive plants in terrestrial systems (16), potentially leading to synergistic interactions, but the generality of this trend remains unknown. Future research is needed to address these gaps to further test the generality of our findings.
Materials and Methods
Literature Search.
We searched the Web of Science Core Collection for articles and reviews that were available in English through September 30, 2020. Search terms (SI Appendix, Part 1: Supplementary Methods) were chosen to identify papers reporting impacts of invasions with one of six abiotic GECs (warming, nitrogen deposition, oxygen depletion, drought, carbon dioxide addition, and altered pH). We assessed the titles and abstracts of the 6,192 returned papers and retained those that 1) reported the ecological effects of one or more invasive species, 2) one or more GECs, and 3) both invasive species and a GEC together and 4) also reported data for a control treatment (no invasion and at current or ambient environmental conditions).
Data Extraction.
For each study meeting our design inclusion criteria (SI Appendix, Fig. S1.1, PRISMA diagram), we extracted the mean value and a measure of variability around the mean (e.g., SE) for each response variable and the number of replicates of each treatment either from the text, tables, or figures using Web Plot Digitizer (https://automeris.io/WebPlotDigitizer). If data were presented as a time series, we extracted data from the final time step only. When more than two treatment levels were examined in a study (e.g., invasion density, dose treatments), we included only the putative largest contrast (e.g., largest difference in dose treatments). For most studies, we extracted data on multiple “cases,” including multiple focal species, study locations, and/or measured responses.
We recorded information from studies on variables expected to explain the variability in responses. We recorded the type of GEC(s) manipulated in each study, as well as the identity of the manipulated invasive species. The effects of invasions depend on the trophic relationships between invasions and native species (7, 42) as well as other ecological roles of invasions (e.g., habitat modification; 31). We thus categorized the invasion impact mechanisms (“invasion mechanism”) identified by study authors based on those defined by the Environmental Impact Classification for Alien Taxa (43) and simplified to competition, predation (including predation, parasitism, and grazing/herbivory/browsing), or chemical/physical (including chemical, physical, or structural impact on ecosystem and poisoning/toxicity). Other meta-analyses have shown that invasions and GEC effects and their interactions vary across ecosystem settings (terrestrial, freshwater, or marine; 13, 27, 39) and experiment types (field, mesocosm, or laboratory/greenhouse; 19). Thus, we recorded these data for use as model covariates. Moreover, we classified each response variable into a response class (abundance, allocation, behavior, biomass, diversity, physiology, nutrient, reproduction, size, or survival; SI Appendix, Table S1.2 for definitions).
To make the directionality of all responses comparable and meaningful, we made an expert judgement on whether the effects on native species and ecosystems were detrimental or beneficial. We then changed the sign of responses as necessary so that negative effect sizes would indicate poorer performance and positive effect sizes would indicate higher performance, relative to the control treatment. For example, we changed the sign of measures of mortality (where lower mortality indicated better population outcomes). In some cases, we had low confidence in our assessment of whether a response was beneficial or detrimental, especially concerning behavior, resource allocation, and ecosystem properties (e.g., responses of nutrient pools and fluxes). To test the sensitivity of the results to these uncertain cases, we reran our analyses without them (nstudies = 78, ncases = 310).
Meta-analysis.
We calculated Hedges’ d effect sizes to examine the effects of invasions, GECs, and their interactions (INV&GEC) across studies. Hedges’ d is an estimate of the standardized mean difference of treatment from control and is not biased by small sample size (44). We calculated the effect size (d) as:
| [1] |
where XT and XC are the observed mean treatment and control responses, respectively; S is the pooled SD; and J is a weighting factor based on the sample size (44, 45). S is calculated as:
| [2] |
and J is calculated as:
| [3] |
where nT and nC represent the number of replicates and and are the SDs of the treatment and control, respectively (44). Prior to analysis, we removed eight cases with null or infinite Hedges’ d values NA (due to recorded SDs of zero for multiple treatments) and one outlier with a Hedges’ d value less than −200 (SI Appendix, Fig. S1.2). We examined publication bias in effect sizes using funnel plots and Spearman’s rank correlation tests (SI Appendix, Fig. S2.3; 18, 46). All analyses were performed in R (47).
We used Bayesian mixed-effects models (run with the “rjags” package in R; 48) to evaluate treatment effects across cases, with study and case included as random effects and using uninformative priors. These models estimated the true effect size for each case and treatment from the calculated Hedges’ d and associated variance. We fit separate models to 1) compare overall effects of treatments, 2) compare treatment effects across categories of individual predictor variables (GEC, invasion mechanism, measured response class, ecosystem setting, and experiment type), and 3) compare the effects of individual treatments across categories of all predictor variables (SI Appendix, Part 2: Supplementary Results). We report means and 95% credible intervals derived from parameter posterior distributions.
To identify types of INV&GEC interactions, we calculated a Hedges’ d effect size comparing the observed INV&GEC effect to a predicted additive effect, defined as the sum of the individual stressor effects, for each case (18, 34; Fig. 1; SI Appendix, Part 1: Supplementary Results for details on the calculation). Interactions were considered additive if the INV&GEC effect was not different from the predicted additive (if the 95% confidence intervals around Hedges’ d for the predicted additive effect overlapped zero; 34). Observed interactions that differed from the predicted additive effect were considered synergistic if they fell outside the range of values of the individual stressor effects and the control (if the 95% confidence intervals of the observed INV&GEC Hedges’ d did not overlap the Hedges’ d values for the individual stressors or zero); otherwise, they were categorized as antagonistic (34). We further classified interactions based on whether the INV&GEC effect was more positive or negative than the predicted additive effect. Thus, the possible interaction categories were additive, antagonistic (−), antagonistic (+), synergistic (−), and synergistic (+), where the + and − indicate whether the interaction was more beneficial or detrimental than expected, respectively (Fig. 1). We used Fisher’s exact tests to examine differences in interaction types across GECs, invasion mechanisms, measured response classes, ecosystem settings, and experiment types. To account for nonindependence in cases from the same study, we also performed Fisher’s tests on a reduced dataset with one case per study. Full details of the analysis can be found in SI Appendix, Part 1: Supplementary Results; data are archived at reference 49, and code is available at reference 50.
Supplementary Material
Acknowledgments
We thank A. Bates and R. Early for valuable discussions and C.R. Field for advice on statistical analysis. This work was initiated at a working group led by C.J.B.S. and B.A.B. that was supported by the Albert and Elaine Borchard Foundation. B.E.L. was supported by the U.S. Geological Survey Northeast Climate Adaptation Science Center, Grant/Award Number G19AC00091; J.M.A., B.A.B., and M.W.O. by National Science Foundation award BCS1560925; M.V., B.A.B., and C.J.B.S. by the project InvasiBES through the 2017–2018 Belmont Forum and BIODIVERSA joint call for research proposals, under the BiodivScen ERANet COFUND program, with support from the National Science Foundation (ICER-1852326 to B.A.B., ICER-1852060 to C.J.B.S. and PCI2018-092939) and Ministerio de Ciencia, Innovación y Universidades to M.V.. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2117389119/-/DCSupplemental.
Data Availability
Data used for meta-analysis have been deposited in Scholarworks (https://scholarworks.umass.edu/data/140/).
References
- 1.Peters C. B., Schwartz M. W., Lubell M. N., Identifying climate risk perceptions, information needs, and barriers to information exchange among public land managers. Sci. Total Environ. 616-617, 245–254 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Ernest Johnson M., “2020 AFWA climate adaptation surveys: A review of activities at state fish & wildlife agencies” Association of Fish & Wildlife Agencies (2020). https://www.fishwildlife.org/application/files/7416/1012/0886/2020_Climate_Adaptation_Survey_Report.pdf. Accessed 22 June 2021.
- 3.Pecl G. T., et al. , Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 355, eaai9214 (2017). [DOI] [PubMed] [Google Scholar]
- 4.IPBES, Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES Secretariat, 2019). [Google Scholar]
- 5.Vilà M., et al. , Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 14, 702–708 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Wu Z., Dijkstra P., Koch G. W., Peñuelas J., Hungate B. A., Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob. Change Biol. 17, 927–942 (2011). [Google Scholar]
- 7.Bradley B. A., et al. , Disentangling the abundance-impact relationship for invasive species. Proc. Natl. Acad. Sci. U.S.A. 116, 9919–9924 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song J., et al. , A meta-analysis of 1,119 manipulative experiments on terrestrial carbon-cycling responses to global change. Nat. Ecol. Evol. 3, 1309–1320 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Maclean I. M. D., Wilson R. J., Recent ecological responses to climate change support predictions of high extinction risk. Proc. Natl. Acad. Sci. U.S.A. 108, 12337–12342 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClean C. J., Van den Berg L. J., Ashmore M. R., Preston C. D., Atmospheric nitrogen deposition explains patterns of plant species loss. Glob. Change Biol. 17, 2882–2892 (2011). [Google Scholar]
- 11.Hooper D. U., et al. , A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486, 105–108 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Vilà M., et al. , Understanding the combined impacts of weeds and climate change on crops. Environ. Res. Lett. 16, 034043 (2021). [Google Scholar]
- 13.Staudt A., et al. , The added complications of climate change: Understanding and managing biodiversity and ecosystems. Front. Ecol. Environ. 11, 494–501 (2013). [Google Scholar]
- 14.Côté I. M., Darling E. S., Brown C. J., Interactions among ecosystem stressors and their importance in conservation. Proc. Biol. Sci. 283, 20152592 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brook B. W., Sodhi N. S., Bradshaw C. J. A., Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Holway D. A., Suarez A. V., Case T. J., Role of abiotic factors in governing susceptibility to invasion: A test with Argentine ants. Ecology 83, 1610–1619 (2002). [Google Scholar]
- 17.Lyons K. G., Maldonado-Leal B. G., Owen G., Community and ecosystem effects of buffelgrass (Pennisetum ciliare) and nitrogen deposition in the Sonoran Desert. Invasive Plant Sci. Manag. 6, 65–78 (2013). [Google Scholar]
- 18.Jackson M. C., Loewen C. J., Vinebrooke R. D., Chimimba C. T., Net effects of multiple stressors in freshwater ecosystems: A meta-analysis. Glob. Change Biol. 22, 180–189 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Paine R. T., Tegner M. J., Johnson E. A., Compounded perturbations yield ecological surprises. Ecosystems (N. Y.) 1, 535–545 (1998). [Google Scholar]
- 20.Liu Y., et al. , Do invasive alien plants benefit more from global environmental change than native plants? Glob. Change Biol. 23, 3363–3370 (2017). [DOI] [PubMed] [Google Scholar]
- 21.He Q., Silliman B. R., Climate change, human impacts, and coastal ecosystems in the Anthropocene. Curr. Biol. 29, R1021–R1035 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Thomsen M. S., et al. , Impacts of marine invaders on biodiversity depend on trophic position and functional similarity. Mar. Ecol. Prog. Ser. 495, 39–47 (2014). [Google Scholar]
- 23.Maggi E., et al. , Ecological impacts of invading seaweeds: A meta-analysis of their effects at different trophic levels. Divers. Distrib. 21, 1–12 (2015). [Google Scholar]
- 24.Homet P., et al. , Exploring interactive effects of climate change and exotic pathogens on Quercus suber performance: Damage caused by Phytophthora cinnamomi varies across contrasting scenarios of soil moisture. Agric. For. Meteorol. 276, 107605 (2019). [Google Scholar]
- 25.Bennett S., et al. , Climate-driven impacts of exotic species on marine ecosystems. Glob. Ecol. Biogeogr. 30, 1043–1055 (2021). [Google Scholar]
- 26.Keller J. A., Shea K., Warming and shifting phenology accelerate an invasive plant life cycle. Ecology 102, e03219 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorte C. J., et al. , Poised to prosper? A cross-system comparison of climate change effects on native and non-native species performance. Ecol. Lett. 16, 261–270 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Lu M., et al. , Responses of ecosystem nitrogen cycle to nitrogen addition: A meta-analysis. New Phytol. 189, 1040–1050 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Simkin S. M., et al. , Conditional vulnerability of plant diversity to atmospheric nitrogen deposition across the United States. Proc. Natl. Acad. Sci. U.S.A. 113, 4086–4091 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee M. R., et al. , Invasive species’ leaf traits and dissimilarity from natives shape their impact on nitrogen cycling: A meta-analysis. New Phytol. 213, 128–139 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Castro-Díez P., Godoy O., Alonso A., Gallardo A., Saldaña A., What explains variation in the impacts of exotic plant invasions on the nitrogen cycle? A meta-analysis. Ecol. Lett. 17, 1–12 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Powell K. I., Chase J. M., Knight T. M., Invasive plants have scale-dependent effects on diversity by altering species-area relationships. Science 339, 316–318 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Tekin E., et al. , Using a newly introduced framework to measure ecological stressor interactions. Ecol. Lett. 23, 1391–1403 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Piggott J. J., Townsend C. R., Matthaei C. D., Reconceptualizing synergism and antagonism among multiple stressors. Ecol. Evol. 5, 1538–1547 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darling E. S., Côté I. M., Quantifying the evidence for ecological synergies. Ecol. Lett. 11, 1278–1286 (2008). [DOI] [PubMed] [Google Scholar]
- 36.Galic N., Sullivan L. L., Grimm V., Forbes V. E., When things don’t add up: Quantifying impacts of multiple stressors from individual metabolism to ecosystem processing. Ecol. Lett. 21, 568–577 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Pyšek P., et al. , Geographical and taxonomic biases in invasion ecology. Trends Ecol. Evol. 23, 237–244 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Amano T., González-Varo J. P., Sutherland W. J., Languages are still a major barrier to global science. PLoS Biol. 14, e2000933 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephens K. L., Dantzler-Kyer M. E., Patten M. A., Souza L., Differential responses to global change of aquatic and terrestrial invasive species: Evidences from a meta-analysis. Ecosphere 10, e02680 (2019). [Google Scholar]
- 40.Sperling E. A., Frieder C. A., Levin L. A., Biodiversity response to natural gradients of multiple stressors on continental margins. Proc. Biol. Sci. 283, 20160637 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sampaio E., et al. , Impacts of hypoxic events surpass those of future ocean warming and acidification. Nat. Ecol. Evol. 5, 311–321 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Anton A., et al. , Global ecological impacts of marine exotic species. Nat. Ecol. Evol. 3, 787–800 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Hawkins C. L., et al. , Framework and guidelines for implementing the proposed IUCN Environmental Impact Classification for Alien Taxa (EICAT). Divers. Distrib. 21, 1360–1363 (2015). [Google Scholar]
- 44.Borenstein M., Cooper H., Hedges L., Valentine J., “Effect sizes for continuous data” in The Handbook of Research Synthesis and Meta-analysis, H. Cooper, L. V. Hedges, J. C. Valentine, Eds. (Russell Sage Foundation, 2009), vol. 2, pp. 221–235. [Google Scholar]
- 45.Koricheva J., Gurevitch J., Mengersen K., Handbook of Meta-analysis in Ecology and Evolution (Princeton University Press, 2013). [Google Scholar]
- 46.Nakagawa S., Santos E. S., Methodological issues and advances in biological meta-analysis. Evol. Ecol. 26, 1253–1274 (2012). [Google Scholar]
- 47.R Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2020). www.R-project.org/ [Google Scholar]
- 48.Plummer M., rjags: Bayesian Graphical Models using MCMC (2019). https://cran.r-project.org/web/packages/rjags/. Accessed 23 January 2022.
- 49.Lopez B. E., et al. , Invasion and global change meta-analysis data. 10.7275/w98a-zs36. Accessed 20 September 2021. [DOI]
- 50.Lopez B. E., et al. , Invasion-global-change-meta: First release of invasion and global environmental change meta-analysis code (2022. revision). Zenodo. 10.5281/zenodo.6111731. Accessed 16 February 2022. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used for meta-analysis have been deposited in Scholarworks (https://scholarworks.umass.edu/data/140/).