Abstract
Ankle osteoarthritis (OA) is much less frequent than knee or hip OA, but it can be equally disabling, greatly affecting the quality of life of the patients.
Approximately 80% of ankle OA is post-traumatic, mainly secondary to malleolar fractures, being another of the main causes untreated in chronic instability. The average age of the patient affected by ankle OA is around 50 years, being therefore active patients and in working age who seek to maintain mobility and remain active.
The authors conducted a comprehensive review of the conservative, medical, and surgical treatment of ankle OA.
Initial conservative treatment is effective and should be attempted in any stage of OA. From a pharmacological point of view, non-steroidal anti-inflammatory drugs (NSAIDs) and intra-articular infiltrations can produce temporary relief of symptoms.
After the failure of conservative-medical treatment, two large groups of surgical treatment have been described: joint-preserving and joint-sacrificing procedures.
In the early stages, only periarticular osteotomies have enough evidence to recommend in ankle OA with malalignment. Both ankle arthrodesis and ankle replacement can produce satisfactory functional results if correctly indicated in the final stages of the disease.
Finally, the authors propose a global treatment algorithm that can aid in the decision-making process.
Keywords: ankle osteoarthritis, conservative treatment, surgical treatment
Introduction
Ankle osteoarthritis (OA) is a chronic disease affecting approximately 1% of the world population, with an estimated incidence of 30 cases per 100 000 inhabitants, and corresponds to between 2 and 4% of all patients with general OA (1, 2). Cadaveric, radiologic, and clinical studies have indicated that ankle OA is far less common than knee and hip OA (1, 2, 3), what reflects in clinical practice, with symptomatic knee OA being 8 to 9 times more prevalent than ankle OA, and approximately 24 times more total knee replacements being performed than arthrodesis and arthroplasty of the ankle joint combined (3, 4). While traditionally the clinical impact and functional limitation of early ankle OA on patients have not been considered particularly relevant, it can be extremely debilitating in advanced stages and can have similar repercussions on the quality of life as severe hip OA, advanced kidney failure, and congestive heart failure (4, 5).
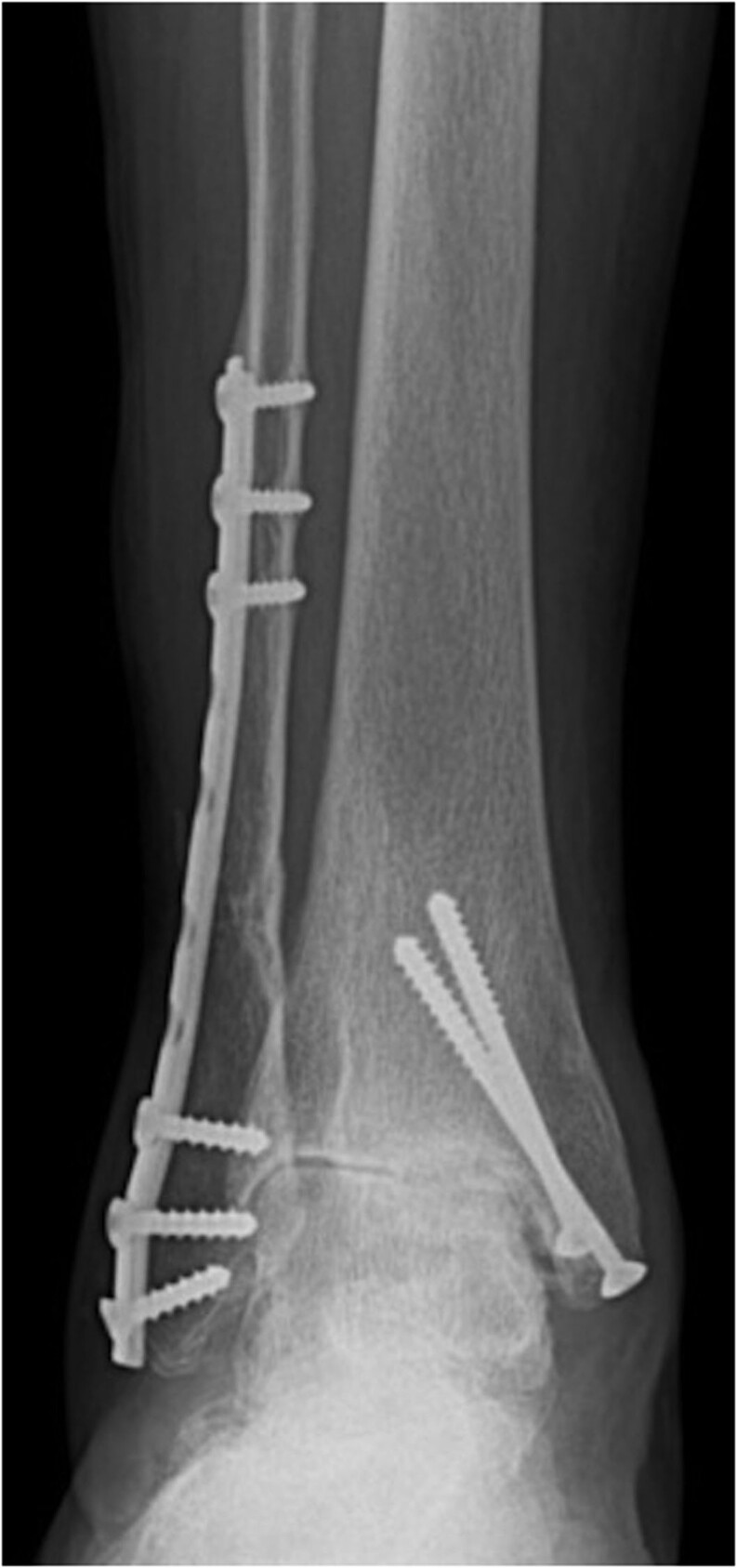
Unlike hip and knee OA, primary ankle OA is not the most common etiology (1, 6, 7, 8), with only 7–9% of the cases being idiopathic OA and 13% secondary to other causes (rheumatoid arthritis, hemochromatosis, hemophilia, or osteonecrosis). Therefore, the main etiology, representing 75–80% of all cases, is a traumatic event (post-traumatic ankle OA), with fractures in the ankle region (malleolus, distal tibia, talus, etc.) (Fig. 1) being responsible for 62% of cases and the remaining 16% due to chronic ligament instability, particularly those affecting the lateral collateral ankle ligament (which some authors call ligamentous ankle OA) (Fig. 2) (8, 9). Ankle instability increases the peak joint contact stresses of the ankle joint, resulting in cartilage deterioration that ultimately leads to ankle OA (9).
Figure 1.
Post-traumatic osteoarthritis after 12 years of bimalleolar ankle fracture.
Figure 2.
Ligamentous ankle osteoarthritis: osteoarthritis with varus deformity due to chronic lateral instability.
Another point of controversy is the relation between osteochondral lesions of the talus and the development of ankle OA. Weigelt et al. (10) reported in a 14-year-old follow-up study that osteochondral lesions of the talus that successfully undergo an initial nonoperative treatment period have minimal symptoms in the long term, a low failure rate, and no relevant ankle OA progression. On the contrary, Stufkens et al. (5) stated that anterolateral talar, posteromedial tibial, and medial malleolar osteochondral lesions are more likely to develop ankle OA.
Given its predominantly post-traumatic etiology, patients with ankle OA tend to be younger (18–44 years) than those with other degenerative joint diseases in the lower limbs (2). They also suffer a more rapid loss of function, with progression to advanced stages of ankle OA occurring 10–20 years after the onset, although clinical studies show that the initial degenerative changes secondary to an ankle fracture develop within 12–18 months of the traumatic injury (7, 11).
Pathophysiology peculiarities of tibiotalar articular cartilage
Although the ankle joint is frequently injured, clinically relevant ankle OA is much less common than in other weight-bearing joints; this is probably due to specific anatomical, biochemical, and biomolecular peculiarities of the cartilage of the ankle joint (12). Ankle cartilage receives the greatest force per unit area of all hyaline cartilage in the human body (500 N/350 mm2 compared to the same force per 1100 mm2 or 1120 mm2 in the hip or knee, respectively). Furthermore, the load distribution in the ankle differs from other joints, such as the knee, which means the compressive forces are distributed in a greater area. Ankle cartilage (1–1.62 mm) is therefore thinner than knee cartilage (1.69–2.55 mm) (12, 13, 14). Biologically, ankle cartilage is believed to have a greater capacity for self-repair than knee cartilage (14). It has more stiffness and less permeability due to a higher degree of water and proteoglycan content (11). In addition, the extracellular matrix is denser which improves its load-bearing capacity and reduces its susceptibility to mechanical damage (12). It has also been shown that chondrocytes in the ankle are metabolically more active than in the knee and exhibit a greater response to anabolic factors such as osteogenic protein-1 and C-propeptide of type II collagen, upregulating cartilage synthesis. Ankle joint cartilage is also less sensitive to catabolic mediators such as fibronectin and interleukin-1 beta, which are factors that inhibit collagen synthesis (12, 15). Last but not least, for several years a biochemical alteration has been demonstrated in the synovial fluid of arthritic ankles, specifically in the concentration of certain cytokines such as matrix metalloproteinases (15) and a number of key markers have been identified in chronic OA (aggrecan and BMP-7, both increasing with OA progress). In contrast, high levels of BMP-2 have been related to a good clinical function and low signs of OA-related radiographic changes (16).
Given all these reasons, the articular cartilage in the ankle joint is less prone to degeneration than the cartilage of the knee or hip, but it is highly susceptible to lesions when there is an asymmetric distribution of stresses and forces, as in the case of joint fractures, injuries accompanied by impacts, or malalignment of the weight-bearing axis (11, 16). Such factors may explain the high correlation between ankle OA and a history of a traumatic event (17).
Clinical evaluation
Clinical diagnosis is based on the presence of joint pain with mechanical characteristics with or without joint effusion and/or deformity, as well as a loss of mobility, particularly ankle dorsiflexion (2, 11). Scarring is common due to a history of prior surgery (osteosynthesis, hardware removal, cheilectomy, etc.). Other clinical signs include leg muscle atrophy and gait disturbances (2, 3). Imaging diagnosis is based on weight-bearing anteroposterior and lateral conventional radiographs (CR), as well as a hindfoot alignment view such as the Saltzman view (2). OA may be hard to diagnose in early stages using CR as the technique often underestimates the severity of the disease, so advanced three-dimensional (3D) imaging such as CT and particularly MRI can be utilized to provide a more accurate picture of the number, location, and size of any points of cartilage damage. Newer MRI modalities, such as T2 mapping techniques, have also been tested for early diagnosis of ankle OA, although these techniques have proven more useful in assessing the quality of repair tissue after surgery (18). In recent years, the use of single-photon emission CT (SPECT-CT) has increased in popularity, adding details on the activity of the OA or chondral lesions to the anatomical information (19). The new generation of SPECT-CT scans permits exact localization of focal degenerative lesions. The SPECT-CT sensitivity can be attributed to their ability to detect increased subchondral metabolic osseous activity, which can detect early degenerative changes before appearance of clinical symptoms, for this reason it is particularly helpful in early stages of ankle OA. In addition, it can be indicated for defining OA of multiple joints with different stages and inconsistent clinical assessment of pathologies in closely adjacent structures such as talonavicular and subtalar joints (19, 20, 21). In more recent years, the appearance and development of the weight-bearing CT scan has allowed a better 3D understanding of ankle OA and associated supra- and infra-malleolar deformities of the foot and ankle, in a physiological, standing, and loaded condition (22, 23, 24, 25, 26, 27).
Classification
Perhaps the best-known system is the Tanaka Classification (28) (Table 1) which defines four stages and is clinically important because the authors established limits for joint-preserving surgery according to the grade of joint degeneration. An interesting study in 2016 by Claessen et al. assessed three different radiological classification systems (Van Dijk, Kellgren–Lawrence, and Tanaka) and concluded that none of them were reliable as decision-making tools or for establishing a prognosis for post-traumatic ankle OA (29).
Table 1.
Tanaka classification for ankle osteoarthritis.
| Stage | Radiographic finding |
|---|---|
| 1 | No narrowing of the joint space, but early sclerosis and formation of osteophytes |
| 2 | Narrowing of the medial joint space |
| 3A | Obliteration of this space with subcondral bone contact (medial gutter only) |
| 3B | Extension of the obliterarion to the roof of the dome of the talus |
| 4 | Obliteration of the whole joint space with complete bone contact |
Ankle osteoarthritis treatment
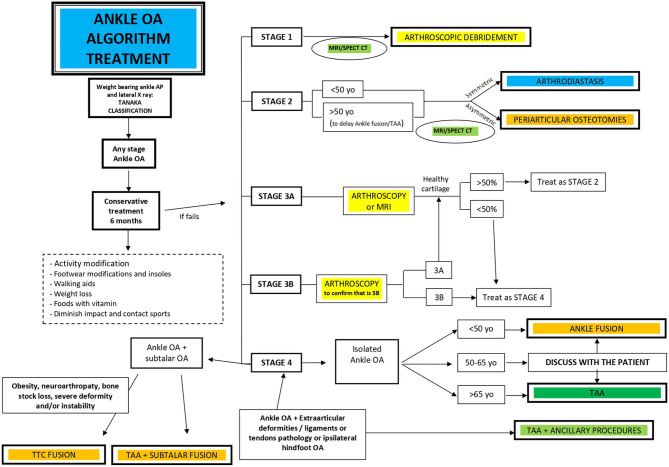
Treatment relies on three main pillars (30) (Fig. 3):
Figure 3.
Global treatment algorithm for ankle osteoarthritis.
Conservative treatment
Joint-preserving surgical procedures
Joint-sacrificing surgical procedures
Conservative treatment
Regardless of the degree of ankle OA, conservative treatment should be tried first for at least 6 months to assess its effectiveness. There are various treatment options that can be combined to alleviate the signs and symptoms of ankle OA, although there is only weak scientific evidence to support it, with mainly level IV and level V studies (30, 31, 32).
Patient education
It is important to inform patients about the modifiable risk factors related to the development/progression of OA. Obesity (BMI ≥30.0 kg/m2) is associated with an increased risk of functional impairment and is considered the most modifiable risk factor for knee OA (33, 34), being a factor associated with the onset and progression of musculoskeletal disorders as well. Additionally, it has been shown that weight loss can reduce the pain derived from OA (35). Patients should avoid lifestyle habits that provoke or aggravate OA such as high-impact sports or ascending and descending stairs and be advised to use a cane or walking stick if necessary.
Diet
Although it has not been specifically studied for the ankle, metabolic syndrome and type 2 diabetes have been associated with the risk of onset or progression of OA (36, 37). Hence, the modification of lipids in the diet of OA patients is recommended as it has been shown that a greater consumption of long-chain omega-3 fatty acids from fatty fish or fish oil supplements can improve pain and function in patients with OA. Reducing levels of blood cholesterol and increasing the intake of foods rich in vitamin K, which plays an important role in the mineralization of bones and cartilage, are also beneficial for OA. On the other hand, the influence and efficacy of vitamin D supplements are currently controversial in the treatment of OA (36). Regarding the diet–OA relationship, a systematic review recently highlighted the importance of the so-called Mediterranean diet for improved general health but could not demonstrate the long-term efficacy of this diet in preventing or improving the symptoms of OA (37).
Physical measures
Strengthening of the musculature that stabilizes the ankle joint, regular practice of stretching exercises, and locally applied cold therapy are some of the most important measures (32). As ankle OA progresses, it can cause an early decline in daily living activities, so recommending patients to seek support from occupational therapy is also essential (31, 32).
Footwear modifications
Gait pattern can be improved by wearing single rocker sole shoes which have been shown to unload pressure not only from the heel but also from the ankle joint during the push-off phase of gait (38).
Orthotics and insoles
Treatment with orthotics aims to reduce pain by maintaining a correct alignment and limiting ankle mobility while walking in order to reduce the mechanical load on the ankle. Ankle–foot orthoses are effective in patients with instability or misalignment (38). Regarding the use of insoles with a lateral or medial wedge for the treatment of medial or lateral tibial OA, most studies in the literature studied the effect of wedges on knee OA, and only one clinical trial assessed the effect of using a lateral wedge insole in the ankle joint space width and found no clinical repercussion (39).
Pharmacological treatment
Although there are no clinical guidelines for the specific management of ankle OA, it seems reasonable to follow the accepted recommendations for OA in other weight-bearing joints, such as the knee (40).
Acetaminophen (paracetamol) has been classically used as a first-line analgesic; however, the efficacy of this medication in treating OA is controversial and possibly not superior to placebo (40). The American College of Rheumatology/Arthritis Foundation questions the effect of acetaminophen monotherapy for OA and only recommends it for short-term use in patients who have a contraindication to other pain reliever drugs (41).
Topical NSAIDs (diclofenac patches, ibuprofen creams, etc.) are safe options and should be considered as a complement to non-pharmacological measures. There are several systematic reviews that support the use of topical NSAIDs for pain relief and improvement of physical function related to OA, with results superior to placebo (40, 41).
Topical capsaicin has been shown to be effective in treating knee OA and could be considered as an alternative treatment to ankle OA, replacing topical NSAIDs (41).
The use of oral NSAIDs, such as the cyclooxygenase (COX)-2 inhibitors, should be considered, particularly when acetaminophen, topical NSAIDs, or capsaicin is ineffective in controlling ankle OA symptoms. Although the clinical improvement in OA-related symptoms is limited, pain control is usually greater than acetaminophen for most patients (41). This group of drugs helps relieve pain and inflammation, but its effectiveness decreases as patients develop tolerance. They should be used with caution, as their long-term use has been shown to cause gastrointestinal, renal, and cardiovascular side effects (40, 41).
The support for the use of opioids (tramadol, morphine, oxycodone, etc.) in the setting of OA is scarce in the literature and its use has been related to side effects and toxicity particularly in the elderly, as well as the increased risk of dependence. Currently, its use in both oral and transdermal forms is either contraindicated or accepted only as a third-line of treatment (41).
Symptomatic slow-acting drugs for OA such as glucosamine, chondroitin sulfate, and diacerein can also be used. OA reduces the concentration of intra-articular hyaluronic acid, which has anti-inflammatory, analgesic, lubricating, and shock-absorbing properties (42). Glucosamine can alleviate symptoms because it acts as a substrate for chondroitin sulfate formation, which in turn stimulates synovial production of hyaluronic acid (42). Although there are studies that show that both glucosamine and chondroitin are superior to placebo in reducing pain in knee OA, the evidence of these positive effects is still a matter of debate and the different international organizations do not recommend its widespread use (41).
Intra-articular therapies
Hyaluronic acid
Intra-articular injections in OA of the ankle are tolerable and effective, producing a quick clinical improvement in terms of pain, stiffness, functionality, and satisfaction, while also reducing the need for analgesics. In most studies, a significant improvement was observed for up to 6 months after the injection (30, 31, 32), while in others the effects were seen to last for up to 18 months (43).
Corticosteroids
They provide short-term symptomatic relief lasting between 4 and 8 weeks. According to the available body of evidence, they should be reserved for persistent pain in higher-grade OA with a maximum of three or four injections a year, because of the damaging effect to the joint cartilage (44).
Platelet-rich plasma
Recent studies seem to indicate that platelet-rich plasma (PRP) therapy is more effective at reducing pain than hyaluronic acid. Mei-Dan et al. studied the efficacy of intra-articular hyaluronic acid vs PRP injections over a 28- week follow-up period in 30 patients with osteochondral lesions of the talus (45). They found that patients in the PRP group reported less pain and better functionality. Angthong et al. observed an improvement in pain, with a mean follow-up of 16 months following the PRP injection; however, MRI studies did not show any radiological improvements (46). Repetto et al. reported that PRP injections once a week for a month were effective in delaying the indication for surgery in patients with ankle OA (47).
Mesenchymal stem cells
Most studies are based on their use in the knee and the evidence in the ankle is scarce, the main problem with this therapy continues to be the bias produced by the dosage, the site where the cells were obtained, the number of cells obtained, and characterization of the delivered populations, since there is no standard procedure that can answer these questions (48). Although they represent a promising field within the biological treatment options, their use in OA is still controversial.
More recently, Boffa et al. (49) have published a meta-analysis to evaluate the evidence supporting safety and effectiveness of intra-articular injective treatments for ankle lesions ranging from osteochondral lesions of the talus (OLT) to OA. Twenty-four studies (21 for OA and 3 for OLT) were included on hyaluronic acid (HA), platelet-rich plasma (PRP), saline, methylprednisolone, botulinum toxin type A (BoNT-A), mesenchymal stem cells, and prolotherapy. The authors support the safety of intra-articular treatment for ankle OA and OLT, being the HA the unique intra-articular therapy with some evidence in terms of better results vs placebo for the treatment of ankle OA.
Surgical treatment joint-preserving procedures
Arthroscopic joint debridement
It plays a relatively limited role in the treatment of ankle OA, as it is mainly confined to cases with early signs of OA (30). It reduces pain and improves function in patients with clinical signs of anterior impingement, both bony and soft tissue impingement, and is very effective in the excision of intra-articular loose bodies (50). It can also prove useful when performing cartilage repair techniques if there are localized osteochondral lesions. So, in carefully selected cases, arthroscopy can retard the disease progression and delay the need for subsequent surgical interventions. Diagnostic arthroscopy recently assumed an important place with respect to the decision-making of whether to perform joint-preserving or joint-sacrificing surgery (30, 50).
Joint distraction arthroplasty or arthrodiastasis
It involves implanting a fixed or hinged external fixator between the tibia and talus while applying a distraction force to the ankle joint, either alone or in combination with other joint techniques (chondroplasty, osteochondral holes, microfractures, hyaluronic acid or PRP injections, etc.) (51). It is mainly indicated for young patients (under 45 years) with advanced post-traumatic ankle OA, with no malalignment, and those who still have mobility. The external fixator should remain in place for at least 3 months to obtain a beneficial effect on the cartilage. This technique has proven to be valuable in reducing pain in up to 70% of cases throughout the first 5 years; however, the results decline over time and the improvement is only hypothetical, as studies have not found any evidence that joint distraction leads to marked chondral regeneration (52).
Osteotomies around the ankle
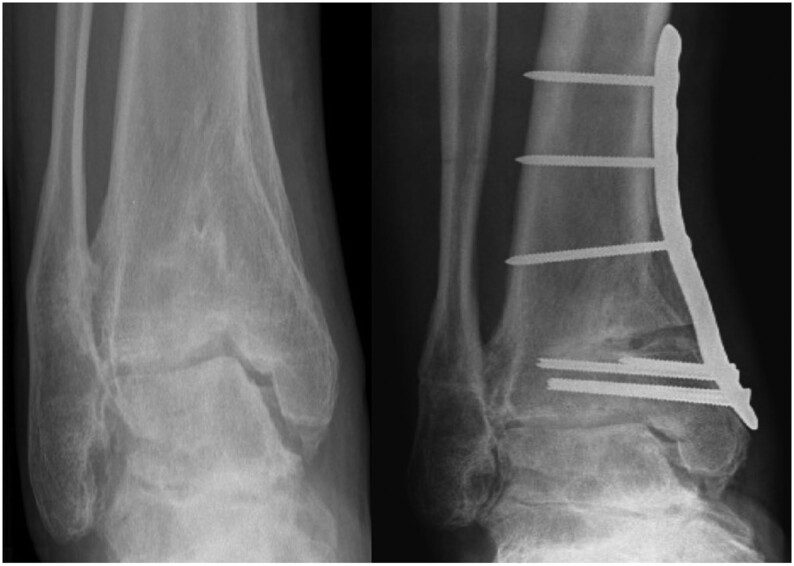
This is the only universally accepted joint-preserving procedure. Osteotomies are indicated in cases of ankle malalignment (up to 70% of post-traumatic ankle OA manifests a varus ankle) (52); the rationale is to transfer the axis of force transmission from the most to the least damaged portion of the joint and osteotomies are performed above the ankle joint (supra-malleolar), below this joint line (inframalleolar) or both. It has been shown to reduce pain in between 70 and 75% of cases and even defers the need for definitive procedures such as arthrodesis or total ankle arthroplasty (TAA) (52, 53, 54). The main indication for an osteotomy is asymmetric OA with at least 50% healthy cartilage in the tibiotalar joint, which should be confirmed by means of a preoperative MRI or intraoperative arthroscopy (52). The procedure has both general and specific contraindications. Among others, the general contraindications include acute or chronic infection (with or without osteomyelitis), severe circulatory insufficiency, and neuropathies (52). The specific contraindications are severe OA of the entire joint and the patient’s objection to the postoperative unloading protocol and rehabilitation. There is some controversy about the limits for supra-malleolar osteotomies. Tanaka et al. suggest that stage 3B ankle OA is no longer a candidate for joint-preserving surgery due to the poor results obtained, preferring to opt for ankle arthrodesis or replacement arthroplasty (28). A varus deformity can be treated by means of a medial opening wedge osteotomy (Fig. 4) or lateral closing wedge osteotomy, the former being the most used option (54). Valgus ankle deformities are more commonly corrected by performing a medial closing wedge osteotomy combined with a fibular osteotomy (53). In cases with intra-articular varus or valgus asymmetric OA, extraarticular techniques have poorer outcomes and higher rates of recurrence. In such cases, an oblique intra-articular osteotomy (known as plafondplasty) of the tibia has been described, showing low rates of recurrence, substantial postoperative pain relief, functional improvement, and a possible slowing of the degenerative process (55). Another relevant point is the treatment of malunited malleolar fractures. Reidsma et al. (56) report good or excellent results in the majority of patients indicating that reconstructive surgery is effective in most and that the beneficial effects can last for up to 27 years after the procedure.
Figure 4.
Varus osteoarthritis treated by medial opening supra-malleolar ankle osteotomy.
Joint-sacrificing surgical treatments: total ankle arthroplasty and ankle arthrodesis
This is indicated in cases of advanced, end-stage OA (stages 3B and 4 in the Takakura-Tanaka Classification) or after failure of joint-preserving techniques.
Total ankle arthroplasty
Over the past decade, TAA surgery has evolved as an alternative to ankle arthrodesis in select patients with ankle arthritis (57, 58). These include adult patients with primary, post-traumatic, and inflammatory arthritis who have moderate or severe pain, loss of mobility, and loss of function of the involved ankle. Patients with previous hindfoot fusion or significant arthritic change in neighboring joints are also considered good candidates for replacement (58). The indications for TAA were very strict up to a few years ago, but they have become more flexible with time and thanks to the incorporation of the newest designs. Indications currently include patients with end-stage OA (post-traumatic, inflammatory, etc.), sedentary lifestyles, the elderly (above 55 years at present), low functional requirements, and preserved joint mobility (Fig. 5). Indication for TAA is also well accepted when there is a contralateral ankle arthrodesis or ipsilateral major OA or previous fusion of the neighboring joints (subtalar and talonavicular joint in particular), because the preserved motion of TAA may be protective against further degeneration while providing equivalent pain relief (57, 58). The complications associated with TAA vary from 13.5 to 54.5%. Some examples include superficial and deep infections, wound dehiscence, intra- and postoperative fractures (mainly of the malleoli), aseptic loosening, polyethylene fracture, postoperative pain, stiffness, heterotopic calcifications, bone cysts, soft tissue impingement, neurovascular lesions, and deep vein thrombosis, many of which may require reoperation (4, 59). On the other hand, there are some absolute contraindications for TAA such as an active infection, major peripheral arterial disease, Charcot neuroarthropathy, or unhealthy/low-quality soft tissue coverage. Relative contraindications include tobacco consumption, morbid obesity, ankle ankylosis, younger age (under 50 years, controversial), severe lower limb malalignment, avascular necrosis of the talus (above 50%), and osteoporosis (59, 60). Unlike total knee and total hip arthroplasty, in severe malalignment deformity cases, bony and soft tissue realignment procedures might be needed to restore a more normal mechanical axis (57, 61). Although the age itself is an important factor to be considered, it has been published that at medium-term, ankle replacement is at least as effective in patients under the age of 50 as in those aged 50 or older (62).
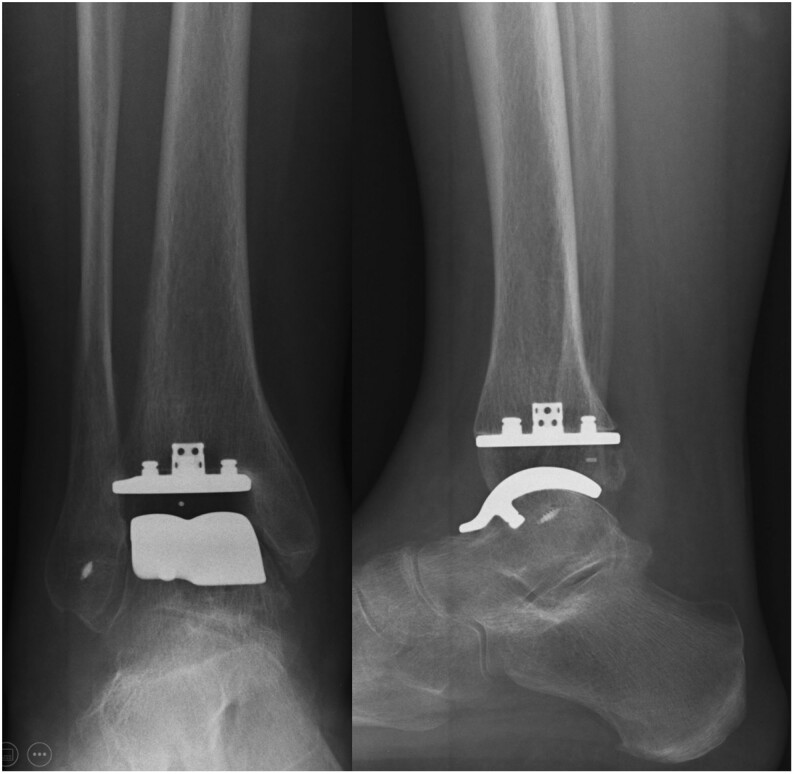
Figure 5.
Total ankle replacement and lateral ligament repair in a patient with advanced ankle osteoarthritis and lateral instability.
Ankle arthrodesis
Traditionally, arthrodesis was the favored treatment for end-stage ankle OA. The aims are to achieve a stable, pain-free, and plantigrade foot, and it is the preferred technique in physically active patients with high functional requirements (63, 64). It can be performed through a conventional open technique using different approaches (lateral transfibular with a fibular autograft, anteromedial and anterolateral mini-open, or the classic anterior approach preserving the malleoli) and different types of osteosynthesis (anterior plate, lateral plate, double plate, crossed screws, etc.) (Fig. 6). The main indications, contraindications, and ideal position for ankle arthrodesis (AA) are described in Tables 2, 3, and 4 (64). Although it is well-known classic technique, the main concerns are the overload of adjacent joints and nonunion (Fig. 7). Adjacent-joint overload is normal since these joints are specifically responsible for compensating for the absence of ankle movement. Therefore, subtalar and talonavicular OA commonly develops following an ankle arthrodesis, even though the latter is not always clinically significant (63). Arthroscopic AA has imposed itself as the technique of choice or gold standard treatment in patients with soft tissues in a poor condition due to previous surgeries (which is very common in ankle OA), although a series of contraindications have been described (Table 5) (65). Various studies have compared open and arthroscopic fusion; while they offer similar rates of nonunion and fusion times, the hospitalization period is shorter (and it can even be performed on an outpatient basis) and the functional improvement is clearly superior with arthroscopic techniques, although these differences seem to even out after 1 year of follow-up (64, 65).
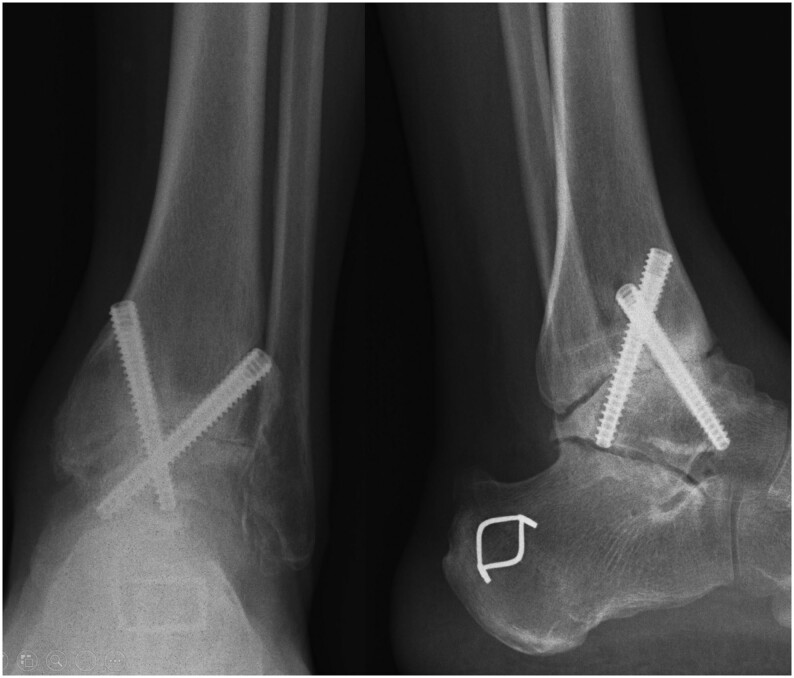
Figure 6.
Open ankle arthrodesis by lateral approach using the fibula itself as a local bone graft.
Table 2.
Indications for ankle arthrodesis.
|
*Classic indication currently under debate: there are good results of TAA in patients under 55 years of age. ^In severe necrosis (>75% of the total talus) tibiotalocalcaneal (TTC) arthrodesis may be necessary to provide stability. ^^In cases of severe bone stock loss during TAA revision surgery, TTC arthrodesis with allograft is the technique of choice.
Table 3.
Contraindications for ankle arthrodesis.
| Contraindications for ankle arthrodesis |
|---|
| Absolute contraindications |
|
| Relative contraindications |
|
Table 4.
Ideal position of ankle arthrodesis.
|
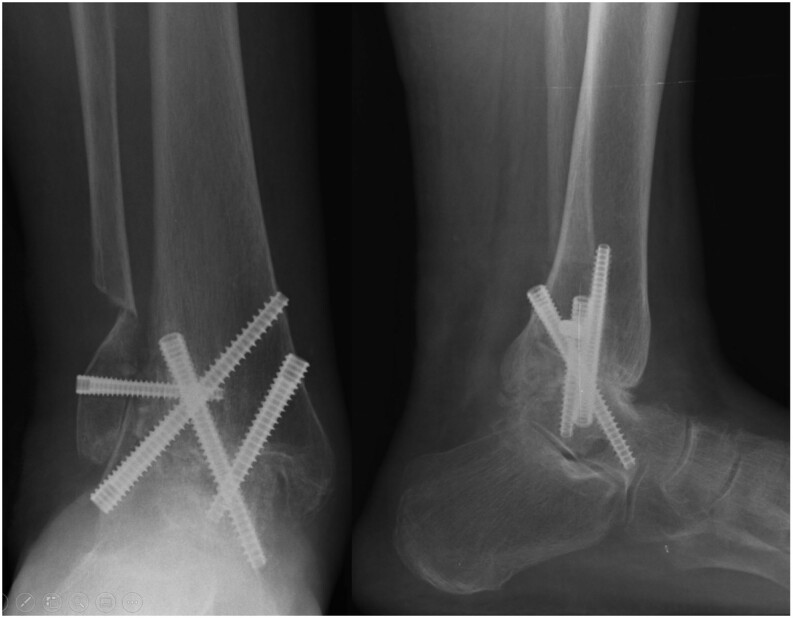
Figure 7.
Ankle arthrodesis nonunion.
Table 5.
Contraindications for ARTHROSCOPIC ankle arthrodesis.
| Contraindications for ARTHROSCOPIC ankle arthrodesis |
|---|
| Absolute contraindications |
|
| Relative contraindications |
- *Severe varus/valgus deformities >15°.
|
*Currently, arthroscopic techniques can overcome severe deformities. **Could produce a higher rate of nonunion.
Tibiotalocalcaneal arthrodesis
Tibiotalocalcaneal (TTC) arthrodesis is another procedure available for the treatment of degenerative disease in the ankle and hindfoot. The goal is to fuse the subtalar and tibiotalar joints, usually performed with a retrograde nail or plate and screws (66). TTC is indicated in attempts to salvage a failed total ankle replacement, in case of the loss of talar or distal tibial bone stock, severe hindfoot instability, morbid obesity, severe progressive collapsing foot deformity, and Charcot neuroarthropathy, among others (Fig. 8 and Table 6) (66, 67). The procedure can be performed via minimally invasive techniques; however, complications have been reported to occur in up to 25% of the cases, particularly with early nail designs, the most noteworthy being proximal fractures around the nail, nonunion (often asymptomatic), infections, neurovascular lesions, and rotational alterations. The contraindications for TTC arthrodesis are a healthy subtalar joint, poor bone stock, active infection, and severe distal tibia deformities (67).
Figure 8.
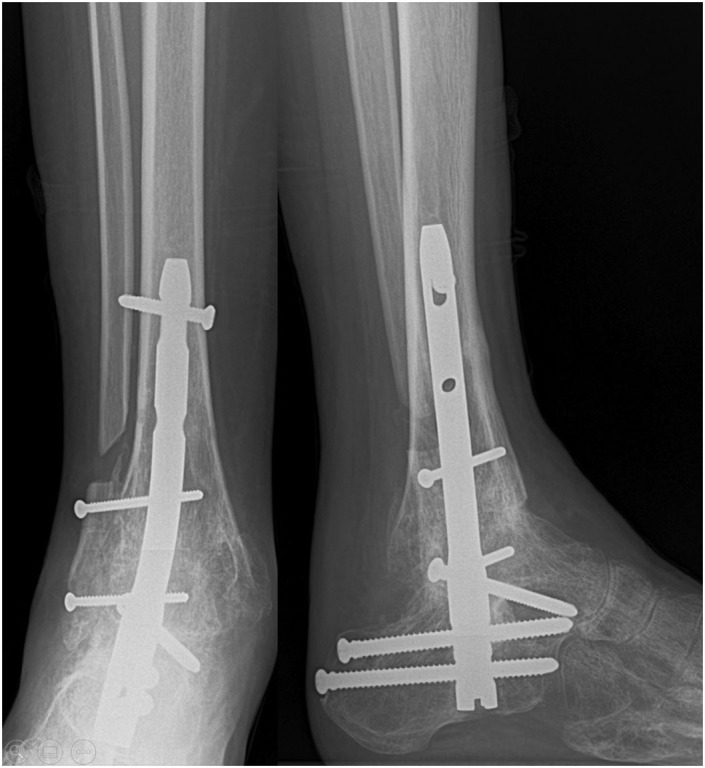
Tibiotalocalcaneal arthrodesis with retrograde nail using the fibula itself as an autograft after post-traumatic osteoarthritis secondary to pilon tibial fracture.
Table 6.
Indications for tibiotalocalcaneal arthrodesis.
|
Total ankle arthroplasty vs ankle arthrodesis
Veljkovic et al. compared 238 patients (88 TAA, 100 open fusions, and 50 arthroscopic fusions) and reported similar clinical and functional outcomes (62). Shih et al. published a meta-analysis in which they did not find any significant differences in terms of pain, functionality, alignment, and satisfaction among patients treated by means of TAA and AA (68). Despite the undeniable design improvements and growth in the use and indication for TAA, AA remains the most frequent surgery all over the world for end-stage ankle OA, as there is still concern about implant survival and the learning curve in TAA (61, 69). Additional studies of outcomes provide support for the performance of ankle replacement surgery in patients with OA, but most authors agree that a careful evaluation of the patient is important when selecting patients for a TAA procedure (69, 70). With this regard, Krause et al. published a review outlining the decision-making process for TAA in terms of major and minor criteria (71). Age, cause of arthritis, deformity, instability, ankle motion, and adjacent joint arthritis were all considered major considerations when selecting the appropriate procedure for a patient (71).
In general, TAA and AA are both associated with pain reduction and similar functional outcome in the mid term (68). The main problem with TAA is the greater rate of reoperations or revision surgeries in comparison with AA, whereas the main issue with AA is adjacent-joint overload and gait pattern alterations. Putting aside these concerns, TAA seems to be superior to AA with respect to gait pattern normalization, less overload of adjacent joints, and show marked improvement in the quality of life and function (70).
Talar body replacement
In the last 5 years, there has been a special interest in the development of total talar prostheses (72), indicated in avascular necrosis of the talus, failed fixation of talar fractures, and advanced ankle OA. Although many studies have shown better function than partial talar prostheses, there is a need for long-term studies to determine its long-term efficacy (73).
Bipolar allograft
Designed with the idea of replacing the ankle joint with a fresh total allograft (fixed to the distal tibia and talus with screws), it is a novel and complex procedure. Clinical outcomes are highly variable, and a 2015 systematic review proved that although the results appear promising (73), the lack of statistical power and inconsistent documentation made it difficult to determine the superiority of any one intervention compared with another for the treatment of ankle arthritis (73).
ICMJE Conflict of Interest Statement
C C N has received consultancy, lecture fees and/or royalties from Zimmer Biomet, Paragon 28, Nextremity, Artelon and Medartis; stock options and travel expenses from CurveBeam. The other authors have nothing to disclose.
Funding Statement
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Goldberg AJ, MacGregor A, Dawson J, Singh D, Cullen N, Sharp RJ, Cooke PH. The demand incidence of symptomatic ankle osteoarthritis presenting to foot and ankle surgeons in the United Kingdom. Foot 201222163–166. ( 10.1016/j.foot.2012.02.005) [DOI] [PubMed] [Google Scholar]
- 2.Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clinical Orthopaedics and Related Research 20094671800–1806. ( 10.1007/s11999-008-0543-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas RH, Daniels TR. Ankle arthritis. Journal of Bone and Joint Surgery: American Volume 200385923–936. ( 10.2106/00004623-200305000-00026) [DOI] [PubMed] [Google Scholar]
- 4.Glazebrook M, Daniels T, Younger A, Foote CJ, Penner M, Wing K, Lau J, Leighton R, Dunbar M. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. Journal of Bone and Joint Surgery: American Volume 200890499–505. ( 10.2106/JBJS.F.01299) [DOI] [PubMed] [Google Scholar]
- 5.Stufkens SA, Knupp M, Horisberger M, Lampert C, Hintermann B. Cartilage lesions and the development of osteoarthritis after internal fixation of ankle fractures: a prospective study. Journal of Bone and Joint Surgery: American Volume 201092279–286. ( 10.2106/JBJS.H.01635) [DOI] [PubMed] [Google Scholar]
- 6.Horisberger M, Valderrabano V, Hintermann B. Posttraumatic ankle osteoarthritis after ankle-related fractures. Journal of Orthopaedic Trauma 20092360–67. ( 10.1097/BOT.0b013e31818915d9) [DOI] [PubMed] [Google Scholar]
- 7.Saltzman CL, Salamon ML, Blanchard GM, Huff T, Hayes A, Buckwalter JA, Amendola A. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthopaedic Journal 20052544–46. [PMC free article] [PubMed] [Google Scholar]
- 8.Herrera-Pérez M, González-Martín D, Vallejo-Márquez M, Godoy-Santos AL, Valderrabano V, Tejero S. Ankle osteoarthritis aetiology. Journal of Clinical Medicine 202110 4489. ( 10.3390/jcm10194489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrington KD.Degenerative arthritis of the ankle secondary to long-standing lateral ligament instability. Journal of Bone and Joint Surgery: American Volume 197961354–361. ( 10.2106/00004623-197961030-00006) [DOI] [PubMed] [Google Scholar]
- 10.Weigelt L, Laux CJ, Urbanschitz L, Espinosa N, Klammer G, Götschi T, Wirth SH. Long-term prognosis after successful nonoperative treatment of osteochondral lesions of the talus: an observational 14-year follow-up study. Orthopaedic Journal of Sports Medicine 202082325967120924183. ( 10.1177/2325967120924183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindsjo J.Operative treatment of ankle fractures. Acta Orthopaedica Scandinavica, Supplement 19811891–131. ( 10.3109/ort.1981.52.suppl-189.01) [DOI] [PubMed] [Google Scholar]
- 12.Kraeutler MJ, Kaenkumchorn T, Pascual-Garrido C, Wimmer MA, Chubiskaya S. Peculiarities in ankle cartilage. Cartilage 2017812–18. ( 10.1177/1947603516642572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuettner KE, Cole AA. Cartilage degeneration in different human joints. Osteoarthritis and Cartilage 20051393–103. ( 10.1016/j.joca.2004.11.006) [DOI] [PubMed] [Google Scholar]
- 14.Treppo S, Koepp H, Quan EC, Cole AA, Kuettner KE, Grodzinsky AJ. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. Journal of Orthopaedic Research 200018739–748. ( 10.1002/jor.1100180510) [DOI] [PubMed] [Google Scholar]
- 15.Adams SB, Setton LA, Bell RD, Easley ME, Huebner JL, Stabler T, Kraus VB, Leimer EM, Olson SA, Nettles DL. Inflammatory cytokines and matrix metalloproteinases in the synovial fluid after intra-articular ankle fracture. Foot and Ankle International 2015361264–1271. ( 10.1177/1071100715611176) [DOI] [PubMed] [Google Scholar]
- 16.Schmal H, Salzmann GM, Langenmair ER, Henkelmann R, Südkamp NP, Niemeyer P. Biochemical characterization of early osteoarthritis in the ankle. Scientific World Journal 20142014 434802. ( 10.1155/2014/434802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tochigi Y, Rudert MJ, McKinley TO, Pedersen DR, Brown TD. Correlation of dynamic cartilage contact stress aberrations with severity of instability in ankle incongruity. Journal of Orthopaedic Research 2008261186–1193. ( 10.1002/jor.20589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreiner MM, Mlynarik V, Zbýň Š, Szomolanyi P, Apprich S, Windhager R, Trattnig S. New technology in imaging cartilage of the ankle. Cartilage 2017831–41. ( 10.1177/1947603516632848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul J, Barg A, Kretzchmar M, Pagenstert G, Studler U, Hügle T, Wegner NJ, Valderrabano V, Geurts J. Increased osseous (99m)Tc-DPD uptake in end-stage ankle osteoarthritis: correlation between SPECT-CT imaging and histologic findings. Foot and Ankle International 2015361438–1447. ( 10.1177/1071100715596745) [DOI] [PubMed] [Google Scholar]
- 20.Pagenstert GI, Barg A, Leumann AG, Tasch H, Müller-Brand J, Hintermann B, Valderrabano V. SPECT-CT imaging in degenerative joint disease of the foot and ankle. Journal of Bone and Joint Surgery: British Volume 2009911191–1196. ( 10.1302/0301-620X.91B9.22570) [DOI] [PubMed] [Google Scholar]
- 21.Claassen L, Yao D, Ettinger S, Lerch M, Daniilidis K, Stukenborg-Colsman C, Plaass C. Relevance of SPECT-CT in complex cases of foot and ankle surgery: a comparison with MRI. Foot and Ankle Specialist 202013451–462. ( 10.1177/1938640019890987) [DOI] [PubMed] [Google Scholar]
- 22.Conti MS, Ellis SJ. Weight-bearing CT scans in foot and ankle surgery. Journal of the American Academy of Orthopaedic Surgeons 202028e595–e603. ( 10.5435/JAAOS-D-19-00700) [DOI] [PubMed] [Google Scholar]
- 23.Rojas EO, Barbachan Mansur NS, Dibbern K, Lalevee M, Auch E, Schmidt E, Vivtcharenko V, Li S, Phisitkul P, Femino J, et al. Weightbearing computed tomography for assessment of foot and ankle deformities: the Iowa experience. Iowa Orthopaedic Journal 202141111–119. ( 10.1177/2473011421S00419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lôbo CFT, Bordalo-Rodrigues M. & Weight-Bearing Computed Tomography International Study Group. Weight-bearing cone beam Ct scans and its uses in ankle, foot, and knee: an update article. Acta Ortopédica Brasileira 202129105–110. ( 10.1590/1413-785220212902236939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godoy-Santos AL, Cesar C. & W eight -B earing CT I nternational S tudy G roup. Weight-bearing computed tomography of the foot and ankle: an update and future directions. Acta Ortopédica Brasileira 201826135–139. ( 10.1590/1413-785220182602188482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lintz F, de Cesar Netto C, Barg A, Burssens A, Richter MWeight Bearing CT International Study Group & International Study Group. Weight-bearing cone beam CT scans in the foot and ankle. EFORT Open Reviews 20183278–286. ( 10.1302/2058-5241.3.170066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barg A, Bailey T, Richter M, de Cesar Netto C, Lintz F, Burssens A, Phisitkul P, Hanrahan CJ, Saltzman CL. Weightbearing computed tomography of the foot and ankle: emerging technology topical review. Foot and Ankle International 201839376–386. ( 10.1177/1071100717740330) [DOI] [PubMed] [Google Scholar]
- 28.Tanaka Y, Takakura Y, Hayashi K, Taniguchi A, Kumai T, Sugimoto K. Low tibial osteotomy for varus-type osteoarthritis of the ankle. Journal of Bone and Joint Surgery: British Volume 200688909–913. ( 10.1302/0301-620X.88B7.17325) [DOI] [PubMed] [Google Scholar]
- 29.Claessen FM, Meijer DT, van den Bekerom MP, Gevers Deynoot BD, Mallee WH, Doornberg JN, van Dijk CN. Reliability of classification for post-traumatic ankle osteoarthritis. Knee Surgery, Sports Traumatology, Arthroscopy 2016241332–1337. ( 10.1007/s00167-015-3871-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloch B, Srinivasan S, Manwani J. Current concepts in the management of ankle osteoarthritis: a systematic review. Journal of Foot and Ankle Surgery 201554932–939. ( 10.1053/j.jfas.2014.12.042) [DOI] [PubMed] [Google Scholar]
- 31.Grunfeld R, Aydogan U, Juliano P, Bustillo J. Ankle arthritis: diagnosis and conservative management. OA Musculoskeletal Medicine 20131 32. ( 10.13172/2052-9287-1-4-997) [DOI] [Google Scholar]
- 32.Tejero S, Prada-Chamorro E, González-Martín D, García-Guirao A, Galhoum A, Valderrabano V, Herrera-Pérez M. Conservative treatment of ankle osteoarthritis. Journal of Clinical Medicine 202110 4561. ( 10.3390/jcm10194561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koonce RC, Bravman JT. Obesity and osteoarthritis: more than just wear and tear. Journal of the American Academy of Orthopaedic Surgeons 201321161–169. ( 10.5435/JAAOS-21-03-161) [DOI] [PubMed] [Google Scholar]
- 34.Lementowski PW, Zelicof SB. Obesity and osteoarthritis. American Journal of Orthopedics 200837148–151. [PubMed] [Google Scholar]
- 35.Aaboe J, Bliddal H, Messier SP, Alkjær T, Henriksen M. Effects of an intensive weight loss program on knee joint loading in obese adults with knee osteoarthritis. Osteoarthritis and Cartilage 201119822–828. ( 10.1016/j.joca.2011.03.006) [DOI] [PubMed] [Google Scholar]
- 36.Thomas S, Browne H, Mobasheri A, Rayman MP. What is the evidence for a role for diet and nutrition in osteoarthritis? Rheumatology 201857 (Supplement4) iv61–iv74. ( 10.1093/rheumatology/key011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morales-Ivorra I, Romera-Baures M, Roman-Viñas B, Serra-Majem L. Osteoarthritis and the mediterranean diet: a systematic review. Nutrients 201810 1030. ( 10.3390/nu10081030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.John S, Bongiovanni F. Brace management for ankle arthritis. Clinics in Podiatric Medicine and Surgery 200926193–197. ( 10.1016/j.cpm.2008.12.004) [DOI] [PubMed] [Google Scholar]
- 39.Tezcan ME, Goker B, Lidtke R, Block JA. Long-term effects of lateral wedge orthotics on hip and ankle joint space widths. Gait and Posture 20175136–40. ( 10.1016/j.gaitpost.2016.09.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paterson KL, Gates L. Clinical assessment and management of foot and ankle osteoarthritis: a review of current evidence and focus on pharmacological treatment. Drugs and Aging 201936203–211. ( 10.1007/s40266-019-00639-y) [DOI] [PubMed] [Google Scholar]
- 41.Primorac D, Molnar V, Matišić V, Hudetz D, Jeleč Ž, Rod E, Čukelj F, Vidović D, Vrdoljak T, Dobričić B, et al. Comprehensive review of knee osteoarthritis pharmacological treatment and the latest professional societies’ guidelines. Pharmaceuticals 202114 205. ( 10.3390/ph14030205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCarthy MF.Enhanced synovial production of hyaluronic acid my explain rapid clinical response to high-dose glucosamine in osteoarthritis. Medical Hypotheses 199850507–510. ( 10.1016/s0306-9877(9890272-9) [DOI] [PubMed] [Google Scholar]
- 43.Bossert M, Boublil D, Parisaux JM, Bozgan AM, Richelme E, Conrozier T. Imaging guidance improves the results of viscosuplementation with HANOX-M-XL in patients with ankle osteoarthritis: results of a clinical survey in 50 patients treated in daily practice. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders 20169195–199. ( 10.4137/CMAMD.S40401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward ST, Williams PL, Purkayashta S. Intra-articular corticosteroid injections in the foot and ankle: a prospective 1-year follow-up investigation. Journal of Foot and Ankle Surgery 200847138–144. ( 10.1053/j.jfas.2007.12.007) [DOI] [PubMed] [Google Scholar]
- 45.Mei-Dan O, Carmont MR, Laver L, Mann G, Maffuli N, Nyska M. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. American Journal of Sports Medicine 201240534–541. ( 10.1177/0363546511431238) [DOI] [PubMed] [Google Scholar]
- 46.Angthong C, Khadsongkram A, Angthong W. Outcomes and quality of life after platelet-rich plasma therapy in patients with recalcitrant hindfoot and ankle diseases: a preliminary report of 12 patients. Journal of Foot and Ankle Surgery 201352475–480. ( 10.1053/j.jfas.2013.04.005) [DOI] [PubMed] [Google Scholar]
- 47.Repetto I, Biti B, Cerruti P, Trentini R, Felli L. Conservative treatment of ankle osteoarthritis: can platelet-rich plasma effectively postpone surgery? Journal of Foot and Ankle Surgery 201756362–365. ( 10.1053/j.jfas.2016.11.015) [DOI] [PubMed] [Google Scholar]
- 48.Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, Volarevic V. Mesenchymal stem cell-based therapy of osteoarthritis: current knowledge and future perspectives. Biomedicine and Pharmacotherapy 20191092318–2326. ( 10.1016/j.biopha.2018.11.099) [DOI] [PubMed] [Google Scholar]
- 49.Boffa A, Previtali D, Di Laura Frattura G, Vannini F, Candrian C, Filardo G. Evidence on ankle injections for osteochondral lesions and osteoarthritis: a systematic review and meta-analysis. International Orthopaedics 202145509–523. ( 10.1007/s00264-020-04689-5) [DOI] [PubMed] [Google Scholar]
- 50.Osti L, Del Buono A, Maffulli N. Arthroscopic debridement of the ankle for mild to moderate osteoarthritis: a midterm follow-up study in former professional soccer players. Journal of Orthopaedic Surgery and Research 201611 37. ( 10.1186/s13018-016-0368-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith NC, Beaman D, Rozbruch SR, Glazebrook MA. Evidence-based indications for distraction ankle arthroplasty. Foot and Ankle International 201233632–636. ( 10.3113/FAI.2012.0632) [DOI] [PubMed] [Google Scholar]
- 52.Herrera-Pérez M, Alrashidi Y, Galhoum AE, Kahn TL, Valderrabano V, Barg A. Debridement and hinged motion distraction is superior to debridement alone in patients with ankle osteoarthritis: a prospective randomized controlled trial. Knee Surgery, Sports Traumatology, Arthroscopy 2019272802–2812. ( 10.1007/s00167-018-5156-3) [DOI] [PubMed] [Google Scholar]
- 53.Valderrabano V, Paul J, Monika H, Pagenstert GI, Henninger HB, Barg A. Joint-preserving surgery of valgus ankle osteoarthritis. Foot and Ankle Clinics 201318481–502. ( 10.1016/j.fcl.2013.06.008) [DOI] [PubMed] [Google Scholar]
- 54.Barg A, Saltzman CL. Joint-preserving procedures in patients with varus deformity: role of supramalleolar osteotomies. Foot and Ankle Clinics 201924239–264. ( 10.1016/j.fcl.2019.02.004) [DOI] [PubMed] [Google Scholar]
- 55.Al-Nammari SS, Myerson MS. The use of tibial osteotomy (ankle Plafondplasty) for joint preservation of ankle deformity and early arthritis. Foot and Ankle Clinics 20162115–26. ( 10.1016/j.fcl.2015.09.009) [DOI] [PubMed] [Google Scholar]
- 56.Reidsma II, Nolte PA, Marti RK, Raaymakers EL. Treatment of malunited fractures of the ankle: a long-term follow-up of reconstructive surgery. Journal of Bone and Joint Surgery 20109266–70. ( 10.1302/0301-620X.92B1.22540) [DOI] [PubMed] [Google Scholar]
- 57.Barg A, Wimmer MD, Wiewiorski M, Wirtz DC, Pagenstert GI, Valderrabano V. Total ankle replacement indications, implant designs, and results. Deutsches Arzteblatt International 2015112177–184. ( 10.3238/arztebl.2015.0177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cody EA, Scott DJ, Easley ME. Total ankle arthroplasty, a critical analysis review. JBJS Reviews 20186 e8. ( 10.2106/JBJS.RVW.17.00182) [DOI] [PubMed] [Google Scholar]
- 59.Jeyaseelan L, Si-Hyeong S, Al-Rumaih H, Veljkovic A, Penner MJ, Wing KJ, Younger A. Outcomes following total ankle arthroplasty: a review of the registry data and current literature. Orthopedic Clinics of North America 201950539–548. ( 10.1016/j.ocl.2019.06.004) [DOI] [PubMed] [Google Scholar]
- 60.Gross CE, Palanca AA, DeOrio JK. Design rationale for total ankle arthroplasty systems: an update. Journal of the American Academy of Orthopaedic Surgeons 201826353–359. ( 10.5435/JAAOS-D-16-00715) [DOI] [PubMed] [Google Scholar]
- 61.Alsayel F, Alttahir M, Mosca M, Barg A, Herrera-Pérez M, Valderrabano V. Mobile anatomical total ankle arthroplasty-improvement of talus recentralization. Journal of Clinical Medicine 202110 554. ( 10.3390/jcm10030554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodrigues-Pinto R, Muras J, Martín Oliva X, Amado P. Total ankle replacement in patients under the age of 50. Should the indications be revised? Foot and Ankle Surgery 201319229–233. ( 10.1016/j.fas.2013.05.004) [DOI] [PubMed] [Google Scholar]
- 63.Kennedy JG, Hodgkins CW, Brodsky A, Bohne WH. Outcomes after standarized screw fixation technique of ankle arthrodesis. Clinical Orthopaedics and Related Research 2006447112–118. ( 10.1097/01.blo.0000203480.04174.0e) [DOI] [PubMed] [Google Scholar]
- 64.Arias A, Dalmau A. Artrodesis de tobillo abierta. En Artrodesis vs. Artroplastia de Tobillo. Estado Actual y Fuego Cruzado de Casos Clínicos. Eds Alvarez F, Viladot R, Martín X, Asunción J. Monografía Nº 6; S; ociedad Española de Medicina y Cirugía de Pie y Tobillo, 2014. [Google Scholar]
- 65.Townshend D, Di Silvestro M, Krause F, Penner M, Younger A, Glazebrrok M, Wing K. Arthroscopic versus open ankle arthrodesis: a multicenter comparative case series. Journal of Bone and Joint Surgery: American Volume 20139598–102. ( 10.2106/JBJS.K.01240) [DOI] [PubMed] [Google Scholar]
- 66.Jehan S, Shakeel M, Bing AJF, Hill SO. The success of tibiotalocalcaneal arthrodesis with intramedullary nailing: a systematic review of the literature. Acta Orthopaedica Belgica 201177644–651. [PubMed] [Google Scholar]
- 67.Donnenwerth MP, Roukis TS. Tibio-talo-calcaneal arthrodesis with retrograde compression intramedullary nail fixation for salvage of failed total ankle replacement: a systematic review. Clinics in Podiatric Medicine and Surgery 201330199–206. ( 10.1016/j.cpm.2012.10.007) [DOI] [PubMed] [Google Scholar]
- 68.Veljkovic AN, Daniels TR, Glazebrook MA, Dryden PJ, Penner MJ, Wing KJ, Younger ASE. Outcomes of total ankle replacement, arthroscopic ankle arthrodesis, and open ankle arthrodesis for isolated non-deformed end-stage ankle arthritis. Journal of Bone and Joint Surgery: American Volume 20191011523–1529. ( 10.2106/JBJS.18.01012) [DOI] [PubMed] [Google Scholar]
- 69.Shih CL, Chen SJ, Huang PJ. Clinical outcomes of total ankle arthroplasty versus ankle arthrodesis for the treatment of end-stage ankle arthritis in the last decade: a systematic review and meta-analysis. Journal of Foot and Ankle Surgery 2020591032–1039. ( 10.1053/j.jfas.2019.10.008) [DOI] [PubMed] [Google Scholar]
- 70.Wąsik J, Stołtny T, Pasek J, Szyluk K, Pyda M, Ostałowska A, Kasperczyk S, Koczy B. Effect of total ankle arthroplasty and ankle arthrodesis for ankle osteoarthritis: a comparative study. Medical Science Monitor 2019256797–6804. ( 10.12659/MSM.915574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krause FG, Schmid T. Ankle arthrodesis versus total ankle replacement: how do I decide? Foot and Ankle Clinics 201217529–543. ( 10.1016/j.fcl.2012.08.002) [DOI] [PubMed] [Google Scholar]
- 72.Dimitrov AS, Westover L, Jomha NM. Clinical use of talar prostheses. JBJS Reviews 20219 34101700. ( 10.2106/JBJS.RVW.20.00209) [DOI] [PubMed] [Google Scholar]
- 73.Johnson P, Lee DK. Evidence-based rationale for ankle cartilage allograft replacement: a systematic review of clinical outcomes. Journal of Foot and Ankle Surgery 201554940–943. ( 10.1053/j.jfas.2014.12.008) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a