Abstract
Background
Work disability has serious consequences for individuals as well as society. It is possible to facilitate resumption of work by reducing barriers to return to work (RTW) and promoting collaboration with key stakeholders. This review was first published in 2009 and has now been updated to include studies published up to February 2015.
Objectives
To determine the effectiveness of workplace interventions in preventing work disability among sick‐listed workers, when compared to usual care or clinical interventions.
Search methods
We searched the Cochrane Work Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and PsycINFO databases on 2 February 2015.
Selection criteria
We included randomised controlled trials (RCTs) of workplace interventions that aimed to improve RTW for disabled workers. We only included studies where RTW or conversely sickness absence was reported as a continuous outcome.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias of the studies. We performed meta‐analysis where possible, and we assessed the quality of evidence according to GRADE criteria. We used standard methodological procedures expected by Cochrane.
Main results
We included 14 RCTs with 1897 workers. Eight studies included workers with musculoskeletal disorders, five workers with mental health problems, and one workers with cancer. We judged six studies to have low risk of bias for the outcome sickness absence.
Workplace interventions significantly improved time until first RTW compared to usual care, moderate‐quality evidence (hazard ratio (HR) 1.55, 95% confidence interval (CI) 1.20 to 2.01). Workplace interventions did not considerably reduce time to lasting RTW compared to usual care, very low‐quality evidence (HR 1.07, 95% CI 0.72 to 1.57). The effect on cumulative duration of sickness absence showed a mean difference of ‐33.33 (95% CI ‐49.54 to ‐17.12), favouring the workplace intervention, high‐quality evidence. One study assessed recurrences of sick leave, and favoured usual care, moderate‐quality evidence (HR 0.42, 95% CI 0.21 to 0.82). Overall, the effectiveness of workplace interventions on work disability showed varying results.
In subgroup analyses, we found that workplace interventions reduced time to first and lasting RTW among workers with musculoskeletal disorders more than usual care (HR 1.44, 95% CI 1.15 to 1.82 and HR 1.77, 95% CI 1.37 to 2.29, respectively; both moderate‐quality evidence). In studies of workers with musculoskeletal disorders, pain also improved (standardised mean difference (SMD) ‐0.26, 95% CI ‐0.47 to ‐0.06), as well as functional status (SMD ‐0.33, 95% CI ‐0.58 to ‐0.08). In studies of workers with mental health problems, there was a significant improvement in time until first RTW (HR 2.64, 95% CI 1.41 to 4.95), but no considerable reduction in lasting RTW (HR 0.79, 95% CI 0.54 to 1.17). One study of workers with cancer did not find a considerable reduction in lasting RTW (HR 0.88, 95% CI 0.53 to 1.47).
In another subgroup analysis, we did not find evidence that offering a workplace intervention in combination with a cognitive behavioural intervention (HR 1.93, 95% CI 1.27 to 2.93) is considerably more effective than offering a workplace intervention alone (HR 1.35, 95% CI 1.01 to 1.82, test for subgroup differences P = 0.17).
Workplace interventions did not considerably reduce time until first RTW compared with a clinical intervention in workers with mental health problems in one study (HR 2.65, 95% CI 1.42 to 4.95, very low‐quality evidence).
Authors' conclusions
We found moderate‐quality evidence that workplace interventions reduce time to first RTW, high‐quality evidence that workplace interventions reduce cumulative duration of sickness absence, very low‐quality evidence that workplace interventions reduce time to lasting RTW, and moderate‐quality evidence that workplace interventions increase recurrences of sick leave. Overall, the effectiveness of workplace interventions on work disability showed varying results. Workplace interventions reduce time to RTW and improve pain and functional status in workers with musculoskeletal disorders. We found no evidence of a considerable effect of workplace interventions on time to RTW in workers with mental health problems or cancer.
We found moderate‐quality evidence to support workplace interventions for workers with musculoskeletal disorders. The quality of the evidence on the effectiveness of workplace interventions for workers with mental health problems and cancer is low, and results do not show an effect of workplace interventions for these workers. Future research should expand the range of health conditions evaluated with high‐quality studies.
Plain language summary
Changes at the workplace for preventing disability in workers on sick leave
Background
Changes at the workplace such as working less hours or lifting less can help workers who are on sick leave get back to work earlier. Helping workers on sick leave get back to work earlier prevents long‐term disability. Because there is still uncertainty about the effectiveness of workplace changes, we evaluated whether workplace interventions decrease time to return to work more than usual care or clinical interventions.
Studies
We searched the literature until 2 February 2015. We included 14 randomised controlled trials involving 1897 workers with a follow‐up time of one year or more. In eight studies the workers had musculoskeletal disorders, in five studies they had mental health problems, and in one study they had cancer.
Key results
Considering all causes of work disability together, results showed that workplace interventions are effective in helping workers get back to work and in reducing duration of sickness absence. The effectiveness of workplace interventions is questionable regarding lasting return to work and recurrences of sick leave. The effectiveness of workplace interventions differs based on cause of work disability. We found moderate‐quality evidence to support the use of workplace interventions in reducing sickness absence among workers with musculoskeletal disorders when compared to usual care. Workplace interventions were also effective in improving pain and functional status among workers with musculoskeletal disorders. The effectiveness of workplace interventions on sickness absence was not evident for workers with mental health problems or cancer. Furthermore, it was not clear whether a workplace intervention should be offered alone or in combination with a cognitive behavioural intervention.
Quality of the evidence
We found moderate‐quality evidence that workplace interventions help workers get back to work and reduce duration of sickness absence. However, we also found very low‐quality evidence of the effectiveness of workplace interventions on lasting return to work, because the results differed based on whether the workers suffered from musculoskeletal disorders, mental health problems, or cancer.
Summary of findings
Summary of findings for the main comparison. Workplace interventions compared to Usual care for Workers on sick leave.
| Workplace interventions compared to Usual care for Workers on sick leave | ||||||
| Patient or population: Workers on sick leave Settings: Workplace Intervention: Workplace interventions Comparison: Usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Workplace interventions | |||||
| Time until first RTW Risk at return to work Follow‐up: 12 months | Study population1 | HR 1.55 (1.2 to 2.01) | 608 (5 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 799 per 10002 | 917 per 1000 (854 to 960)2 | |||||
| Low | ||||||
| 500 per 10002 | 658 per 1000 (565 to 752)2 | |||||
| High | ||||||
| 870 per 10002 | 958 per 1000 (914 to 983)2 | |||||
| Time until lasting RTW Risk at return to work Follow‐up: 12 months | Study population4 | HR 1.07 (0.72 to 1.57) | 635 (6 studies) | ⊕⊝⊝⊝ very low5,6,7 | ||
| 834 per 10002 | 853 per 1000 (725 to 940)2 | |||||
| Low | ||||||
| 560 per 10002 | 585 per 1000 (446 to 724)2 | |||||
| High | ||||||
| 920 per 10002 | 933 per 1000 (838 to 981)2 | |||||
| Cumulative duration of sickness absence Days. Scale from: 0 to 365. Scale from: 0 to 365. Follow‐up: median 12 months | The mean cumulative duration of sickness absence in the control groups was 165.7 Days8 | The mean cumulative duration of sickness absence in the intervention groups was 33.33 lower (49.54 to 17.12 lower) | 1164 (8 studies) | ⊕⊕⊕⊕ high | ||
| Recurrences Risk at recurrences of sick leave Follow‐up: 12 months | Study population | HR 0.42 (0.21 to 0.82) | 99 (1 study) | ⊕⊕⊕⊝ moderate10,11 | ||
| 250 per 10009 | 114 per 1000 (59 to 210)9 | |||||
| Functional status Roland disability questionnaire. outcome was measured on different scales in different studies. Scale from: 0 to 24. Follow‐up: median 12 months | The mean functional status ranged across control groups from 5.76 ‐ 81.79 | The mean functional status in the intervention groups was 0.33 standard deviations lower (0.58 to 0.08 lower) | 628 (6 studies) | ⊕⊕⊕⊝ moderate6 | ||
| Depression Depression Anxiety Stress scale, and PHQ‐9 depression scale Follow‐up: 12 months | The mean depression ranged across control groups from 5.9‐24.6 | The mean depression in the intervention groups was 0.12 standard deviations lower (0.35 lower to 0.11 higher) | 133 (4 studies) | ⊕⊝⊝⊝ very low7,12 | ||
| Pain Visual Analogue Scale Follow‐up: 12 months | The mean pain ranged across control groups from 3.4‐30 | The mean pain in the intervention groups was 0.26 standard deviations lower (0.47 to 0.06 lower) | 531 (5 studies) | ⊕⊕⊕⊕ high | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: Hazard ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risks ranged from 0.50 to 0.87, therefore lowest and highest risks are presented. 2 Risks are presented as the number of people who returned to work. 3 60% of studies was assigned high or unclear risk of bias 4 Risks ranged from 0.56 to 0.92, therefore lowest and highest risks are presented. 5 Three of the six studies were assessed as high risk of bias. 6 I2 > 50% 7 The CI of the pooled effect size is sufficiently wide that the estimate could either support or refute the effectiveness of the intervention. 8 Presented as the number of days of sickness absence. 9 Risks are presented as the number of people who had a recurrent period of sickness absence. 10 Not applicable 11 Sparse data, only one study for this outcome. 12 Three of the four studies were assessed as high risk of bias.
Background
Description of the condition
In recent years, the participation of individuals with chronic health problems in the workforce has become increasingly essential in order to address the decline in labour supply associated with an ageing population (OECD 2010). Many workers with health problems leave the labour market temporarily or permanently, and too few people with reduced work capacity manage to continue working. Work disability may be due to 1) musculoskeletal disorders, such as back pain and upper‐extremity disorders, 2) mental health problems, such as depression and adjustment disorders, and 3) other health conditions, such as cardiovascular diseases or cancer. Work disability is a major public health problem in Western industrialised countries. It has a considerable economic burden for society (Dall 2013; OECD 2010; Veronese 2012; Wilkie 2012). Around 6% of the working‐age population on average relies on disability benefits, leading to public spending on disability benefits of on average of 2% of the gross domestic product across Organisation for Economic Co‐operation and Development countries, rising to 4% to 5% in countries such as Norway, the Netherlands, and Sweden (OECD 2010). Employment rates of people with disability are 40% below the overall level, highlighting the need for efforts to support people with disability to return to work and stay in their jobs. Besides the high costs for society, work disability may have serious consequences for workers (Schandelmaier 2012). Being employed is a valuable societal role and is an important source of income; work disability might therefore lead to poorer quality of life and loss of social identity (Stigmar 2013). Furthermore, work disability may result in permanent exclusion from work (Waddell 2006).
Description of the intervention
Studies indicate that return‐to‐work (RTW) interventions should be carried out at the workplace instead of a rehabilitation centre, for example. It is furthermore important to involve key stakeholders in the RTW process. It has been shown that RTW interventions involving workplace adaptations and stakeholder involvement are more effective on RTW than workplace‐linked interventions such as exercise (Carroll 2010; Franche 2005a; Haugli 2011). In this review, we defined workplace interventions by either changes to the workplace or equipment, changes in work design and organisation, changes in working conditions or work environment, and involvement of (at least) the worker and the supervisor (Anema 2004).
How the intervention might work
Studies indicate that medical interventions solely do not show a positive effect on work‐related outcomes (Anema 2009; Arends 2012; Loisel 2001). If the cause of work disability is associated with the workplace, then a return to an unchanged workplace (with or without appropriate treatment for the disorder) may lead to recurrences in the longer term (Adler 2006; Pichora 2010; Sanderson 2006). By incorporating workplace adaptations, workplace interventions aim to reduce barriers for RTW. Supervisors influence health outcomes of employees, and supervisor support is associated with lower sickness absence (Munir 2012; Skakon 2010). Symptoms are generally not addressed by workplace interventions. However, we do hypothesise that earlier RTW is not associated with more severe symptoms.
Why it is important to do this review
Recent economic developments have led to policy changes. Disability benefit systems now focus more on assessing the remaining work capacity of a person applying for a benefit instead of assessing disability (OECD 2010). Timely RTW is of great benefit for both the sick‐listed workers and their employers, since there is a strong association between increased length of sickness absence and increased risk of future disability pension (Lund 2008). RTW is influenced by various psychosocial factors (Baldwin 1996; Clay 2012; Steenstra 2005; Sullivan 2005; Turner 2007; WHO 2001). It is therefore important to report on the research on individual interventions aimed at reducing workplace barriers to RTW (Nordqvist 2003; Schultz 2007; Young 2005).
This review provides insight into the effectiveness of workplace interventions on improving RTW. There is still uncertainty about the effectiveness of these interventions, especially for workers on sick leave due to mental health problems and other health conditions like cancer and cardiovascular disease.
Objectives
To determine the effectiveness of workplace interventions in preventing work disability among sick‐listed workers, when compared to usual care or clinical interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) only.
Types of participants
We incorporated all studies concerning working‐age adults (18 to 65 years) who were on sick leave. We included studies conducted with full‐ and part‐time workers.
Types of interventions
The Cochrane Work review group has classified workplace interventions as appropriate for disability management (Schonstein 2006). For this review, we used the term 'workplace intervention' for interventions focusing on changes in the workplace or equipment, work design and organisation (including working relationships), working conditions or work environment, and occupational (case) management with active stakeholder involvement of (at least) the worker and the employer (Anema 2004; Franche 2005b). We defined active involvement as face‐to‐face conversations about RTW between (at least) the worker and the supervisor. In this review, a workplace intervention must contain work changes and stakeholder involvement, specifically the employer/supervisor. This definition is a synthesis of the International Ergonomics Association definition of ergonomic interventions and the Waddell et al definition of occupational interventions (Stapleton 2000; Waddell 2001). Changes in the workplace and equipment include changes in the furniture or the materials needed to perform the work. Changes in the work design and organisation include changes in schedules or tasks, training in task performance, and altered working relationships with supervisors and coworkers. Changes in working conditions refer to the financial and contractual arrangements and changes in the work environment concerning noise, lighting, vibration, etc.
We based the definition of a workplace intervention on definitions from studies on musculoskeletal disorders. However, the definition seems suitable for interventions for RTW on workers with mental health problems as well. These interventions comprise at least advice about changes in work processes to facilitate RTW or the preparation of a RTW plan that includes the worker and the supervisor (Blonk 2006; van Oostrom 2008; Vlasveld 2008). We compared identified workplace interventions with either usual care (the care usually offered to a sick‐listed worker, for example guideline‐based care) or clinical interventions (for example graded activity, problem‐solving therapy).
As long as the workplace intervention was a structural part of the intervention (with the intention to offer the workplace intervention to all participants in the intervention group), we did not exclude studies with interventions that included more components than described in the definition of a workplace intervention. Our definition allowed us to include only interventions that were linked closely to the workplace and that focused on work adaptations or the involvement of stakeholders from the work environment. We excluded interventions that were intended to simulate the demands of work in a laboratory setting, without changes to or involvement of the workplace itself in the RTW process.
We also excluded studies if the intervention was:
focused on primary prevention of sickness absence, that is, targeted to healthy workers as opposed to those on sick leave;
not focused on RTW as the main goal;
group‐based (focused on an organisation) rather than individual‐based;
focused on education about ergonomics only, and did not result in work adaptations;
aimed at posture modifications only without RTW as the goal.
Types of outcome measures
Primary outcomes
Sickness absence is operationalised in many ways (Steenstra 2003). However, when studies used different ways of operationalisation, we only analysed the data collected in the following manners.
Time until first RTW: a period of absence from work because of sickness both preceded and followed by a period of at least one day at work (consensus definition, de Vet 2002).
Time until lasting RTW: a period of absence from the first day of sick leave to full RTW in previous or equal work for at least four weeks without dropping out.
Cumulative duration of sickness absence: total days of sick leave during the follow‐up period (resulting from one or more periods of sickness absence).
Recurrences of sickness absence: the number of days until a recurrence; or the frequency and duration of recurrent episodes of sick leave.
Our primary outcome was operationalised in different ways, and we chose to prioritise the outcome time until first RTW, as it is the most commonly used RTW outcome internationally, and hence has greater relevance. The aim of modifying work tasks or processes is to guide sick‐listed workers back to work faster than by applying only care as usual. From a socioeconomic perspective, every day of earlier RTW is beneficial. Since the outcome time until first RTW does not take recurrences into account, we also analysed the outcomes time until lasting RTW (first episode of sick leave), cumulative duration of sickness absence, and recurrences (after the first episode of sick leave). By using these outcomes besides time until first RTW we include the follow‐up of (recurrent) sick leave after first RTW.
The operationalisation for time until lasting RTW is based on the Dutch social security system. There are large differences between countries in social security systems and the way sickness absence is registered (de Vet 2002), therefore the cut‐off point for lasting RTW at four weeks is just one example. If studies reported a definition of prespecified time periods for lasting RTW, we included the data from these studies in the time until lasting RTW outcome.
A differentiation between short‐ and long‐term sickness absence is needed (Uegaki 2007). In the past, dichotomous outcomes (returned to work versus not returned to work) were often used for absence caused by sickness. However, use of these measures results in a loss of information about the exact duration of work disability, and the episodic nature of work disability is neglected. Continuous sickness absence outcomes are now more frequently used. This is especially important when an intervention is focused on RTW and when sickness absence is the primary outcome of the study, as in this review. We therefore excluded studies that only reported a dichotomous measure of sickness absence.
Secondary outcomes
Secondary outcomes were:
functional status;
quality of life;
general health;
depression;
pain levels; and
direct and indirect costs of work disability.
These outcomes are likely to be meaningful for workers who are on sick leave, their employers, their care providers (such as treating and occupational physicians), insurers, and the policymakers who are involved in decision‐making.
Search methods for identification of studies
Electronic searches
For this review, we identified studies by searching the following databases:
Cochrane Work Trials Register (31 October 2013)
The Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library) Issue 2, 2015
MEDLINE (PubMed); 2 February 2015
Embase; 2 February 2015
PsycINFO (EBSCO); 2 February 2015
We have presented the search strategies used in Appendix 1. We did not apply any language restrictions.
Searching other resources
We handsearched the reference lists of relevant review articles and eligible studies. We also made use of personal contacts with experts in occupational health to identify studies for inclusion in the review.
Data collection and analysis
The methods of this review followed the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0) (Higgins 2011).
Selection of studies
We stored titles and abstracts (if available) of all identified studies in a new database in Reference Manager. We generated a bibliography that included the title, keywords, and abstract of each reference found after removing duplicate references. We completed study selection in two steps. In the first step, two review authors (MvV and SvO) screened the titles, keywords, and abstracts of all references retrieved by the literature search to determine if articles met the inclusion criteria. The inclusion criteria were: study design was an RCT, participants were sick‐listed workers, the intervention studied met the definition of a workplace intervention, and sickness absence was measured continuously. In the second step, we retrieved the full‐text article for studies where we were could not decide upon inclusion or exclusion in the first step. We read these full‐text articles and assessed them for inclusion. We used a consensus procedure to resolve disagreements about the inclusion of RCTs, and consulted a third review author (CB) if the disagreement persisted. We documented the criteria for exclusion (design, intervention, population, and outcome) for each study we excluded.
Data extraction and management
Two review authors (MvV and SvO) independently extracted the data onto a predesigned data extraction form. This form included essential study information about participants, interventions, outcome measures, and results. We initially used a small sample of the articles to test whether the form was feasible. We resolved disagreements about the data extraction by consensus between the two review authors, or if the disagreements persisted we consulted a third review author (CB). We contacted the authors of articles that contained insufficient information. We reminded authors who did not respond. We eventually received all the information that we needed that the authors of studies had not reported.
Assessment of risk of bias in included studies
Two review authors (MvV and SvO) independently assessed the risk of bias of the included RCTs. We assessed risk of bias using the Cochrane tool for risk of bias (Higgins 2011). We assessed the blinding of outcome assessment both for sickness‐absence and health outcomes.
We assessed the following criteria:
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias): sickness‐absence outcomes
Blinding of outcome assessment (detection bias): health‐related outcomes
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other bias
We judged all studies as having high, low, or unclear risk of bias in each of the above domains. We used a consensus method to resolve disagreements, consulting a third review author (CB or HdV) if the disagreements persisted. We identified four key 'Risk of bias' domains for this review:
Random sequence generation
Allocation concealment
Blinding of outcome assessment (sickness‐absence outcomes)
Incomplete outcome data
We considered studies to be at low risk of bias overall when we judged them to be at low risk of bias in all four key domains. We did not consider blinding of participants and personnel to the intervention as a key domain because the context of the workplace does not allow blinding (Schonstein 2003; Tveito 2004). We considered studies to be at high risk of bias overall when we judged them to be at high risk of bias in one or more of the four key domains. We considered studies to be at unclear risk of bias overall when we judged them to be at unclear risk of bias in one of the four key domains and at low risk of bias in the other three key domains.
Measures of treatment effect
We entered the outcome data for each study into the data tables in Review Manager (RevMan) to calculate the treatment effects (RevMan 2014). We used hazard ratios for time‐to event outcomes (time until lasting RTW and time until first RTW). We used mean differences for the continuous outcome cumulative duration of sickness absence, since all studies measured this outcome in days. For the outcomes functional status, pain, and depression, we used standardised mean differences, because studies measured these outcomes with different instruments.
Unit of analysis issues
We included three cluster‐RCTs in this review, and these studies did not account for the design effect (Anema/Steenstra 2007; Loisel 1997a; Noordik 2013a). The Anema/Steenstra 2007 study was a replication of the Loisel 1997a study, and all three studies randomised on the level of the occupational physician. The intracluster correlation coefficient (ICC) is generally low on the level of the treatment provider. Only Anema/Steenstra 2007 reported the ICC, and it was less than 0.01. Since the Anema/Steenstra 2007 study was a replication of the Loisel 1997a study, and all three studies randomised on the level of the occupational physician, we assumed that the ICCs in the Loisel 1997a and Noordik 2013a studies were also considerably small. Because the ICCs were low and did not have a large impact on outcome data (as shown in the Anema/Steenstra 2007 study), we chose not to adjust our analyses for unit of analysis errors.
Dealing with missing data
When data was reported in a format we could not extract from the publication we contacted the primary authors.
Assessment of heterogeneity
We investigated heterogeneity due to differences in populations by performing analyses for specific causes of work disability: musculoskeletal disorders, mental health problems, or other health conditions. We investigated heterogeneity due to difference in applied interventions by performing analyses for workplace interventions offered alone, or in combination with a cognitive behavioural intervention. To determine the presence or absence of heterogeneity, we analysed I². When I² was more than 50%, we considered studies to be heterogenous. We conducted sensitivity analyses by analysing only RCTs we judged to have a low risk of bias.
Assessment of reporting biases
We used a funnel plot to check for publication bias where more than five studies were available for inclusion in the analysis.
We excluded no papers on the basis of language.
Data synthesis
We pooled the data with Review Manager 5.3 software (RevMan 2014). We plotted the results of each RCT as point estimates with corresponding 95% confidence intervals.
Most outcomes regarding sickness absence were time‐to‐event data (time until lasting RTW, time until first RTW, time until recurrence). The Cox proportional hazard model is used to analyse time‐to‐event data. In this approach, workers who do not return to work during the entire follow‐up period are censored to be sure that the total follow‐up period is analysed as sick leave. Cox proportional hazard regression models are used to determine a hazard ratio. We performed log transformations of the hazard ratios. We then combined the study results using the generic inverse‐variance method with the estimates of log hazard ratios and standard errors from the results of Cox proportional hazards regression models. For one study the results of Cox proportional hazard regression models were not available (Feuerstein 2003). We therefore estimated a hazard ratio based on the log rank test (Parmar 1998). For all analyses, we used the random‐effects model because of the heterogeneity in the type of work disability, duration of sickness absence, and the variation in interventions among studies.
Cumulative duration of sickness absence was usually presented as a continuous outcome and, we therefore calculated the mean difference. Functional status, quality of life, pain, and symptoms were continuous outcomes. For these continuous data, we determined standardised mean differences (with 95% confidence interval) to summarise the effect depending on whether or not these outcomes were measured with different scales. We could not combine changes from baseline scores and final post‐intervention scores in the forest plots when using standardised mean differences. We therefore calculated or requested the final post‐intervention scores for all self‐reported outcomes. In an RCT, mean differences based on changes from baseline can usually be assumed to address exactly the same underlying intervention effects as analyses based on final measurements (Higgins 2011). We could not pool quality‐of‐life outcomes due to large conceptual differences in measurement instruments and subscales used. Some studies only measured a couple of subscales of a quality‐of‐life measurement instrument that were related to workers' specific disabilities, while other studies measured an overall quality‐of‐life score.
We have summarised the results on the costs data in Table 2.
1. Cost outcomes.
| Study | Cost outcomes | Notes |
| Anema/Steenstra 2007 | Total costs:
Ratio of 1 day: EUR 19 |
No major difference in costs between work intervention and usual care, but work intervention is associated with larger effects |
| Arnetz 2003 | Total reimbursement from the health insurance system:
Direct cost of WI was SEK 550,000 Total savings SEK 972,900 Benefit‐to‐cost ratio: 6,8 |
Total reimbursement from the health insurance system significantly lower in work intervention group |
| Bültmann 2009a | Direct costs:
Indirect costs:
Costs per averted absence day (WI vs. UC): DKK 183 |
In terms of productivity loss, the work intervention seems to be cost saving for society |
| Busch 2011 | Total costs (10‐year follow‐up):
|
There was a decrease in costs per individual in the behavioural medicine rehabilitation group compared to usual care |
| Lambeek 2010a | Direct costs:
Indirect costs:
|
The work intervention for workers sick listed because of low back pain had substantial economic benefits over usual care |
| Loisel 1997a | 1‐year follow‐up. Saved consequence of disease costs against standard care: CAD 604 Cost‐benefit: CAD 220 6‐, 4‐year follow‐up. Saved consequence of disease costs against standard care: CAD 10,697 Cost‐benefit: CAD 16,827 Lower costs in the workplace intervention than in the control group. Significance was not calculated |
There was a small number of very costly cases |
| Tamminga 2013 | Total intervention costs per worker in the intervention group: EUR 119 Productivity loss Human Capital Approach:
Productivity loss Friction Costs Approach:
Costs work adjustments:
|
Costs did not differ statistically between groups |
| van Oostrom 2010a | Direct costs:
Indirect costs:
|
The workplace intervention had no economic benefit compared with usual care |
| Vlasveld 2012a | Direct costs:
Indirect costs:
|
Comparable findings between both groups |
BM: behavioural medicine rehabilitation CBT: cognitive behavioural therapy PT: physical therapy UC: usual care WI: workplace intervention
Quality of the evidence
We assessed the overall quality of the evidence for each outcome using the GRADE approach (Boluyt 2012; GRADE working group, Guyatt 2008a, Guyatt 2008b; Schünemann 2006). Two review authors (MvV and SvO) independently assessed the quality of the evidence. We used GRADEprofiler software (version 3.6). The GRADE approach specifies four levels of quality: high, moderate, low, and very low. The highest quality rating is for randomised trial evidence. The quality rating can be downgraded depending on the presence of the five factors specified below. Every limitation assigned downgrades the quality of the evidence by one or two levels.
Limitations of the study refer to the 'Risk of bias' assessment of studies.
Consistency refers to the similarity of estimates of treatment effects for the outcome across studies.
Directness refers to the extent to which the participants, interventions, and outcomes in the studies were comparable to those defined in the inclusion criteria of the review.
Precision of the evidence refers the degree of certainty surrounding an effect estimate.
Publication bias refers to the probability of selective publication of studies and outcomes.
The overall quality of the evidence for each outcome was the result of the combination of the assessments in all domains, leading to the following four levels of evidence (Guyatt 2008b):
High‐quality evidence: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate‐quality evidence: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low‐quality evidence: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low‐quality evidence: Any estimate of effect is very uncertain.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses in which we determined the effectiveness of workplace interventions for workers with musculoskeletal disorders, mental health problems, and other health conditions separately. Furthermore, we performed subgroup analyses in which we analysed the effectiveness of workplace interventions only, and the effectiveness of workplace interventions offered in combination with a cognitive behavioural or problem‐solving intervention.
Sensitivity analysis
We performed sensitivity analyses by including only studies we judged to have a low risk of bias.
Results
Description of studies
Results of the search
Literature search and study selection
We ran the searches in CENTRAL, the Cochrane Work Trials Register, EMBASE, and PsycINFO. We have presented the detailed search strategy in Appendix 1. We identified 1350 references from the initial electronic literature search (run up to November 2007), retrieving 30 references for full‐text scrutiny. After further examination we excluded 21 articles. We have presented reasons for exclusion in the Characteristics of excluded studies table. We included 10 articles from six studies in the initial review (Anema/Steenstra 2007; Arnetz 2003; Blonk 2006; Feuerstein 2003; Loisel 1997a; Verbeek 2002a) (see the Characteristics of included studies table).
We ran the updated database search up to 2 February 2015, which yielded at total of 1736 references. After removing double references, the electronic search resulted in 856 hits, from which we selected 21 potentially eligible studies. After further examination of the full articles, we excluded 13 articles (see the Characteristics of excluded studies table). Eight studies were eligible for inclusion, in addition to the six studies already included in the original review (Bültmann 2009a; Busch 2011; Hees 2012a; Lambeek 2010a; Noordik 2013a; Tamminga 2013; van Oostrom 2010aVlasveld 2012a) (see the Characteristics of included studies table). Handsearching of the reference lists of relevant review articles and eligible studies and personal contact with experts in the field of occupational health generated four additional potentially eligible studies (Farzanfar 2011a; Rebergen 2009b; Reme 2011b; Vermeulen 2011). However, we excluded these four studies after further examination (see the Characteristics of excluded studies table). We present a PRISMA study flow diagram of the selection process in Figure 1.
1.
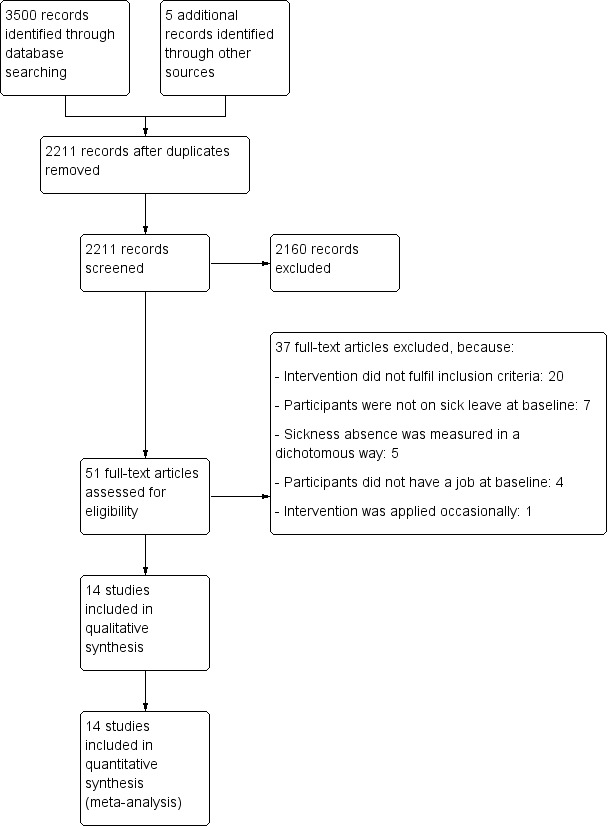
Study flow diagram.
Included studies
Participants: types of disorders and duration of work disability
All studies specified a particular condition targeted. Eight studies included workers with musculoskeletal disorders. Of these, four studies included workers with back pain (Anema/Steenstra 2007; Lambeek 2010a; Loisel 1997a; Verbeek 2002a), three studies included workers with a variety of musculoskeletal disorders (Arnetz 2003; Bültmann 2009a; Busch 2011), and one study included workers with upper‐extremity disorders (Feuerstein 2003). Five studies included workers with mental health problems (Blonk 2006; Hees 2012a; Noordik 2013a; van Oostrom 2010a; Vlasveld 2012a), and one study concerned workers with cancer (Tamminga 2013). The duration of work disability before randomisation extended from immediate inclusion of a sick‐listed worker (Blonk 2006; Tamminga 2013), a minimum of 10 days (Verbeek 2002a), two to six weeks (Anema/Steenstra 2007), two to eight weeks (Noordik 2013a; van Oostrom 2010a), at least eight weeks (Hees 2012a), four to 12 weeks (Bültmann 2009a; Loisel 1997a; Vlasveld 2012a), one to six months (Busch 2011), and three to 24 months (Lambeek 2010a). The exact duration of work disability before randomisation was not described in the Arnetz 2003 study. The duration of work disability prior to randomisation was less clear in the Feuerstein 2003 study: inclusion was possible when the work disability claim was accepted within 90 days of filing. In three studies the disability was work related; none of the other studies reported whether the condition for work disability was work related or not (Feuerstein 2003; Hees 2012a; Loisel 1997a). The only reported restrictions with regard to age, sex, and ethnicity of the participants were working age and sufficient understanding of the national language to fill in the questionnaires. Two studies reported inclusion of workers when assigned to light or modified duties (Feuerstein 2003; Loisel 1997a).
Time and setting characteristics
Of the 14 included studies, 12 were conducted in Europe (Anema/Steenstra 2007; Arnetz 2003; Blonk 2006; Bültmann 2009a; Busch 2011; Hees 2012a; Lambeek 2010a; Noordik 2013a; Tamminga 2013; van Oostrom 2010a; Verbeek 2002a; Vlasveld 2012a), one in the US (Feuerstein 2003), and one in Canada (Loisel 1997a). Of the 12 Northwestern European studies, nine were conducted in the Netherlands (Anema/Steenstra 2007; Blonk 2006; Hees 2012a; Lambeek 2010a; Noordik 2013a; Tamminga 2013; van Oostrom 2010a; Verbeek 2002a; Vlasveld 2012a), two in Sweden (Arnetz 2003; Busch 2011), and one in Denmark (Bültmann 2009a). Participants were working in several economic sectors (manufacturing, health care, office administration, and agriculture) in most studies, except for the Verbeek 2002a study, where all participants worked in hospitals. The duration of the recruitment period ranged from 12 to 34 months and was reported by 12 studies (Anema/Steenstra 2007; Blonk 2006; Bültmann 2009a; Busch 2011; Feuerstein 2003; Hees 2012a; Lambeek 2010a; Loisel 1997a; Noordik 2013a; Tamminga 2013; van Oostrom 2010a; Vlasveld 2012a).
Work interventions
We have described the content of the interventions (based on published reports and personal contact with authors) in Table 3 and Table 4. Regarding the content of the interventions, 11 studies reported changes to the workplace and equipment, 13 reported changes of work design and organisations, and six and nine studies reported changes to working conditions and work environment, respectively. According to the authors, case management with the worker and employer (supervisor) occurred in 12 studies. The worker, the supervisor or employer, and a professional in occupational health were always involved in the interventions, except for the study concerning adjustment disorders, where no supervisor was involved due to self employment (Blonk 2006). Insurer representatives were involved in three studies (Arnetz 2003; Blonk 2006; Busch 2011a), and union representatives were involved in one study (Loisel 1997a). In general, the number of contacts in the workplace intervention was not described in detail, but ranged from one to 29 contacts. Face‐to‐face contact took place in all studies: often at the workplace, and otherwise at a rehabilitation centre, hospital, psychiatry department, or at home. The Busch 2011 study includes three interventions: all three were compared to usual care. All three interventions met our inclusion criteria, therefore we included all three interventions in this review.
2. Content of the interventions ‐ 1.
| General characteristics of interventions | Specific characteristics interventions | Anema/Steenstra | Arnetz | Blonk | Bültmann | Busch | Feuerstein | Hees |
| Applied components definition workplace intervention | Changes workplace or equipment | x | x | x | x | x | x | ‐ |
| Changes work design and organisation including working relationships | x | x | x | x | x | ‐ | x | |
| Changes in working conditions | ‐ | ‐ | x | ‐ | x | ‐ | ‐ | |
| Changes to the work environment | x | ‐ | ‐ | x | x | x | x | |
| Case management with worker and employer | x | x | ‐ | x | x | x | x | |
| Contacts | Number of meetings | 3 | 1 | 5 to 6 | 2 | ? | 4 to 5 | 18 |
| Duration contact | 1 h | ? | ? | 2.5 h | 1 h | 1 to 2 h | 1 to 2 h | |
| Stakeholders involved | Worker | x | x | x | x | x | x | x |
| Employer/supervisor | x | x | Self employed | x | x | x | x | |
| Occupational physician | ‐ | ‐ | ‐ | x | ‐ | ‐ | x | |
| Occupational nurse | x | ‐ | ‐ | x | ‐ | x | ‐ | |
| Ergonomist | x | x | x | ‐ | ‐ | |||
| Representative of a union | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Representative of an insurer | ‐ | x | x | ‐ | x | ‐ | ‐ | |
| Type of contact | Face‐to‐face | x | x | x | x | x | x | x |
| By phone | ‐ | ‐ | ‐ | ‐ | x | ‐ | x | |
| Place of contact | At workplace | x | x | x | x | x | x | x |
| Other | ‐ | ‐ | ‐ | ‐ | Rehabilitation centre | Home and provider office | Psychiatry department | |
| Main treatment provider, work intervention | Ergonomist, occupational nurse | Insurance agency case manager | Labour expert | Social worker | ? | Nurse case manager | Occupational therapist | |
| Training treatment provider, work intervention | Yes | ? | Yes | ? | ? | Yes | Yes |
A 'x' mark indicates that the study fits the specific intervention characteristic. A '?' mark indicates that it is unclear whether the study fits the specific intervention characteristic.
3. Content of the interventions ‐ 2.
| General characteristics of interventions | Specific characteristics interventions | Lambeek | Loisel | Noordik | Tamminga | van Oostrom | Verbeek | Vlasveld |
| Applied components definition workplace intervention | Changes workplace or equipment | x | x | ‐ | ‐ | x | x | x |
| Changes work design and organisation including working relationships | x | x | x | x | x | x | x | |
| Changes in working conditions | x | x | ‐ | ‐ | x | ‐ | x | |
| Changes to the work environment | x | x | ‐ | ‐ | x | ‐ | x | |
| Case management with worker and employer | x | x | x | x | x | ‐ | x | |
| Contacts | Number of meetings | 3 to 29 | ? | Not prespecified | 5 | 3 | ? ˜ 3 | 6 to 12 |
| Duration contact | ? | 1 to 3 h | ? | 25 min | 1 h | 20 min | ? | |
| Stakeholders involved | Worker | x | x | x | x | x | x | x |
| Employer/supervisor | x | x | x | x | x | x | x | |
| Occupational physician | x | x | x | x | ‐ | x | x | |
| Occupational nurse | ‐ | ‐ | ‐ | ‐ | x | ‐ | ‐ | |
| Ergonomist | x | x | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Representative of a union | ‐ | x | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Representative of an insurer | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Type of contact | Face‐to‐face | x | x | x | x | x | x | x |
| By phone | ‐ | x | ‐ | x | ‐ | ‐ | ‐ | |
| Place of contact | At workplace | x | x | ‐ | x | x | ‐ | ‐ |
| Other | ‐ | ‐ | Occupational Health Service | Hospital | ‐ | Occupational Health Service | Occupational Health Service | |
| Main treatment provider, work intervention | Clinical occupational physician | Ergonomist | Occupational physician | Specialised nurse/ social worker | Return‐to‐work coordinator | Occupational physician | Occupational physician care manager | |
| Training treatment provider, work intervention | Yes | ? | Yes | Yes | Yes | Yes | Yes |
A 'x' mark indicates that the study fits the specific intervention characteristic. A '?' mark indicates that it is unclear whether the study fits the specific intervention characteristic.
Usual care
The usual‐care conditions were less extensively described in most studies. Despite the fact that studies explored the effectiveness of workplace interventions, only nine studies had a usual‐care condition in an occupational setting: guideline‐based care by the occupational physician (Anema/Steenstra 2007; Lambeek 2007; Noordik 2013a; Tamminga 2013; van Oostrom 2008; Vlasveld 2012a); an eight‐week RTW plan (Arnetz 2003); and usual case management comprising monitoring of the claims process and surveillance of medical treatment (Feuerstein 2003; Bültmann 2009b). In the other studies, usual care consisted of treatment by the attending physician (Busch 2011; Loisel 1997a), the general practitioner (Blonk 2006; Verbeek 2002a), or the psychiatrist (treatment consistent with American Psychiatric Association guidelines) (Hees 2012a).
In two studies, the workplace intervention was followed by a clinical intervention when RTW was not achieved within a predefined period of eight weeks (Anema/Steenstra 2007; Loisel 1997a). This meant that workplace and clinical interventions did not start concurrently. The Blonk 2006 study was a three‐armed trial: a workplace intervention was compared with a clinical intervention (cognitive behavioural therapy) and with usual care.
Outcomes
We selected studies if they reported on the exact duration of work disability. Four studies used self‐reported outcomes with regard to sickness absence (Hees 2012a; Loisel 1997a; Noordik 2013a; Tamminga 2013): the other 10 studies used administrative outcomes. Six studies reported time until lasting RTW, which they defined as duration of sick leave in calendar days until full RTW for at least four weeks without recurrence (Anema/Steenstra 2007; Hees 2012a; Lambeek 2010a; Noordik 2013a; Tamminga 2013; van Oostrom 2010a). Four studies reported time until first RTW (Blonk 2006; Feuerstein 2003; Loisel 1997a; Verbeek 2002a). Two studies distinguished between time until partial RTW and time until full RTW (Blonk 2006; Noordik 2013a); we decided to focus on time until first full RTW only to avoid introducing any difference from the outcomes of the other studies. One study described the outcome time until first RTW in their published study protocol (Steenstra 2003), but did not include results for this outcome in the published paper about the results (Anema 2007). We therefore requested and obtained the Cox regression output regarding this outcome from the authors. Another study did not publish results on the time until first RTW (Feuerstein 2003). It was clear from papers from the Feuerstein study that they had collected these data, and so we requested the unpublished data to avoid obvious publication bias. Eight studies reported the outcome cumulative duration of sickness absence (Anema/Steenstra 2007; Arnetz 2003; Bültmann 2009a; Busch 2011; Lambeek 2010a; van Oostrom 2010c; Verbeek 2002a; Vlasveld 2012a). Busch et al reported the cumulative duration of sickness absence for the three interventions evaluated. One study reported recurrences of sickness absence in percentages and a hazard ratio (Verbeek 2002a). Six of the included studies used the definition of lasting RTW that corrected for recurrences within four weeks of RTW (Anema/Steenstra 2007; Hees 2012a; Lambeek 2010a; Noordik 2013a; Tamminga 2013; van Oostrom 2010a). The other studies did not take the sustainability of sickness absence without recurrences into account.
Functional status was measured in the four back pain studies (Anema/Steenstra 2007; Lambeek 2010a; Loisel 1997a; Verbeek 2002a), the study on musculoskeletal disorders (Bültmann 2009b), and the upper‐extremity disorder study (Feuerstein 2003). Of these six studies, three used the Roland‐Morris Disability Questionnaire (Anema/Steenstra 2007; Lambeek 2010a; Verbeek 2002a), two used the Oswestry questionnaire (Bültmann 2009a; Loisel 1997a), and one used the upper‐extremity functional limitations scale (Feuerstein 2003). The studies on mental health problems and cancer reported no outcome on functioning.
Four of the 14 studies reported quality of life and general health as separate outcomes (Busch 2011; Hees 2012a; Tamminga 2013; Verbeek 2002a). To assess these outcomes, the studies used the Nottingham health profile, in Verbeek 2002a, and the 36‐Item Short Form Health Survey (SF‐36) (Busch 2011a; Hees 2012a; Tamminga 2013).
Six studies reported a follow‐up of symptoms. For adjustment disorders, the Blonk 2006 study used the depression anxiety stress scales, and for work‐related upper‐extremity disorders, the Feuerstein 2003 study used a modified version of the carpal tunnel symptom severity scale. The Vlasveld 2012d study reported severity of depressive symptoms (Patient Health Questionnaire), and the van Oostrom 2010c study used the Four‐Dimensional Symptom Questionnaire (4DSQ) to measure stress‐related symptoms. The Hees 2012a study assessed severity of depression with a clinician‐reported measure (Hamilton Rating Scale for Depression), as well as a self‐reported measure (Inventory of Depressive Symptomatology‐Self‐Report (IDS‐SR)). We included the self‐reported measure (IDS‐SR) in the meta‐analysis, as the depression scales the other studies used were also self‐reported. The Noordik 2013a study used the 4DSQ to measure symptoms of depression. The Arnetz 2003 study reported baseline measurements of musculoskeletal symptoms, but conducted no follow‐up of symptoms.
Five studies assessed level of pain or pain intensity (Anema/Steenstra 2007; Bültmann 2009a; Lambeek 2010a; Loisel 1997a; Verbeek 2002a). Of these studies, three used a 10‐point visual analogue scale (Anema/Steenstra 2007; Lambeek 2010a; Verbeek 2002a), one used the McGill Pain Questionnaire (Loisel 1997a), and one used the Örebro Musculoskeletal Pain Screening Questionnaire (Bültmann 2009b).
Nine studies measured direct and indirect costs of work disability, but used different perspectives (Anema/Steenstra 2007; Arnetz 2003; Bültmann 2009a; Busch 2011; Lambeek 2010a; Loisel 1997a; Tamminga 2013; van Oostrom 2010a; Vlasveld 2012d). Seven studies applied the societal perspective for cost analysis. The Loisel 1997a study applied the insurer perspective, and the Arnetz 2003 study did not report the perspective applied. All studies measured the direct intervention costs and indirect costs of sick leave. One study did not measure costs of other treatments (direct medical costs) (Arnetz 2003), while the other studies measured use of other healthcare resources and calculated the accompanying costs. The Hees 2012a study measured direct and indirect costs but has not reported them yet.
Follow‐up
Thirteen studies reported a follow‐up period of 12 months for the sickness absence outcome (Anema/Steenstra 2007; Arnetz 2003; Blonk 2006; Bültmann 2009a; Feuerstein 2003; Lambeek 2010a; Loisel 1997a; Noordik 2013a; Tamminga 2013; van Oostrom 2010a; Verbeek 2002a; Vlasveld 2012d), and the Busch 2011a study reported follow‐up of three and 10 years. We therefore did not include the Busch 2011a study in the meta‐analysis. For the other outcomes, which were all self‐reported, follow‐up was at: 10 months (Blonk 2006), 16 months (Feuerstein 2003), three years (Busch 2011a), and 12 months (Anema/Steenstra 2007; Bültmann 2009a; Hees 2012a; Lambeek 2010a; Loisel 1997a; Noordik 2013a; Tamminga 2013; van Oostrom 2010a; Verbeek 2002a; Vlasveld 2012d). One study collected cost data for a mean of 6.4 years (range 5.1 to 7.5 years) (Loisel 1997a).
Short‐term results (less than three months) for the review's outcomes were not reported. Only two studies reported either three‐month, in Verbeek 2002a, or four‐month, in Blonk 2006, results on the self‐reported outcomes (see the Characteristics of included studies table).
Excluded studies
We excluded 37 full‐text articles in total. We excluded 20 studies because the intervention did not fulfil our inclusion criteria. We excluded seven studies because participants were not on sick leave at baseline. We excluded five studies that measured sickness absence in a dichotomous way. We excluded four studies because participants did not have a job at baseline, and we excluded one study that applied the intervention only occasionally.
Risk of bias in included studies
For each study, we assessed the risk of bias by evaluating every study publication we could find. Figure 2 shows the full 'Risk of bias' assessment for each item in every study. Two review authors (MvV and SvO) independently assessed the risk of bias of the RCTs, except for the van Oostrom 2010a study, which MvV and CB assessed.
2.
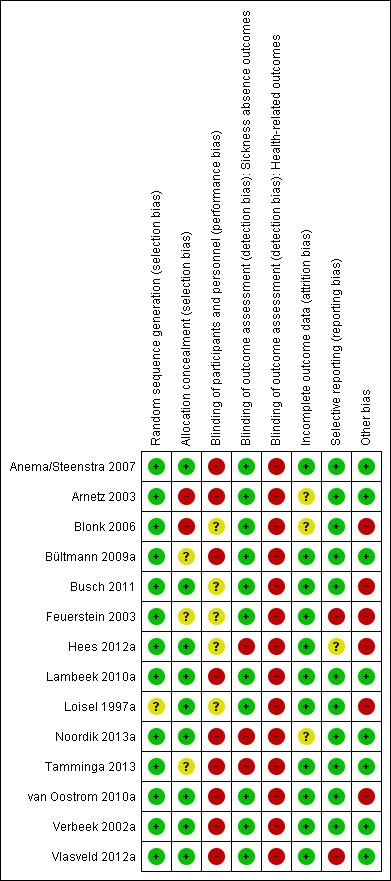
Risk of bias: review authors' judgements about each methodological quality item for each included study.
Most studies used administrative sickness absence data that was collected without knowledge of group allocation; therefore outcome assessors were blinded for these studies. We classified six studies to have a low risk of bias (Anema/Steenstra 2007; Busch 2011a; Lambeek 2010b; van Oostrom 2010c; Verbeek 2002a; Vlasveld 2012d). We classified three studies to have an unclear risk of bias (Bültmann 2009a; Feuerstein 2003; Loisel 1997a), and five studies to have a high risk of bias (Arnetz 2003; Blonk 2006; Hees 2012a; Noordik 2013a; Tamminga 2013) (see the Characteristics of included studies table).
Risk of bias was different for the secondary outcomes due to their self‐reported nature. The scores on the domain 'blinding of the outcome assessor for health‐related outcomes' changed in all studies, resulting in lower total scores for most studies (Figure 2). As expected, the most prevalent shortcomings were found in the domain 'blinding of participants and personnel', as none of the studies were able to blind participants.
Allocation
Of the 14 studies, 35.7% failed to describe or use appropriate concealment of allocation.
Blinding
Not all studies performed blinding of participants and personnel. Eleven of 14 studies performed blinding of outcome assessment of sickness absence outcomes. Not all studies performed blinding of outcome assessment of health‐related outcomes.
Incomplete outcome data
Of the 14 studies, 21.4% provided incomplete outcome data.
Selective reporting
There was selective reporting bias with 21.4% of the trials.
Other potential sources of bias
Three out of 14 studies did not perform their analyses according to the intention‐to‐treat principle. In four out of 14 studies, the groups were not similar at baseline regarding the most important prognostic factors.
Effects of interventions
See: Table 1
1. Workplace interventions compared to usual care
Primary outcomes: Sickness absence
Time until first RTW
The pooled analysis of the outcome time until first RTW showed that workplace interventions were more effective than usual care, with a pooled hazard ratio (HR) of 1.55 (95% confidence interval (CI) 1.20 to 2.01) (Figure 3; Analysis 1.1) (Anema/Steenstra 2007; Blonk 2006; Feuerstein 2003; Loisel 1997a; Verbeek 2002a). This hazard ratio means that after a workplace intervention, people return to work in a more timely fashion and more frequently when compared to the usual‐care group. The quality of this evidence was moderate (Summary of findings table 1; Table 5). We downgraded the quality of the evidence based on the inclusion of studies with high or unclear risk of bias. All studies presented the exact duration of time until first RTW with a median (see Characteristics of included studies table). The difference in median duration of time until first RTW between the workplace intervention group and the usual‐care group ranged from 14 days, in Feuerstein 2003, to 198 days, in Blonk 2006.
3.
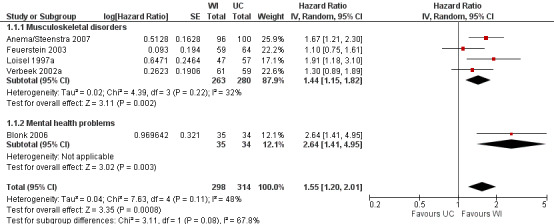
Forest plot of comparison: 1 Workplace intervention versus usual care, outcome: 1.1 Time until first RTW.
1.1. Analysis.
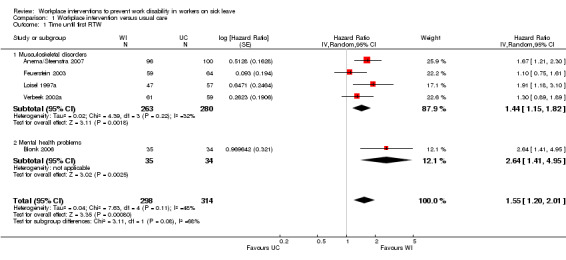
Comparison 1 Workplace intervention versus usual care, Outcome 1 Time until first RTW.
4. Grading of the quality of evidence.
| Comparison | Outcome | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | GRADE quality |
| Workplace intervention vs usual care | Time until first RTW | Yes: 60% of studies were assigned high or unclear risk of bias | No: I² < 50% | No | No | Undetected | Moderate |
| Time until lasting RTW | Yes: 50% of studies were assigned high risk of bias | Yes: I² > 50% | No | Yes: wide CI | Undetected | Very low | |
| Cumulative duration of sickness absence | No: majority low risk of bias | No: I² < 50% | No | No | Undetected | High | |
| Recurrences of sickness absence | No: study with low risk of bias | NA | No | Yes: single study | Undetected | Moderate | |
| Functional status | No: majority low risk of bias | Yes: I² > 50% | No | No | Undetected | Moderate | |
| Pain | No: majority low risk of bias | No: I² < 50% | No | No | Undetected | High | |
| Depression | Yes: > 50% of studies were assigned high risk of bias | No: I² < 50% | No | Yes: wide CI | Undetected | Very low | |
| Workplace intervention vs clinical intervention | Time until first RTW | Yes: study with high risk of bias | NA | No | Yes: single study | Undetected | Very low |
| Subgroup analyses: | |||||||
| Workplace intervention vs usual care: musculoskeletal disorders | Time until first RTW | No: Majority low risk of bias | No: I² < 50% | Yes: PICO deviant (subgroup analysis) | No | Undetected | Moderate |
| Time until lasting RTW | No: Majority low risk of bias | No: I² < 50% | Yes: PICO deviant (subgroup analysis) | No | Undetected | Moderate | |
| Cumulative duration of sickness absence | No: Majority low risk of bias | No: I² < 50% | Yes: PICO deviant (subgroup analysis) | No | Undetected | Moderate | |
| Workplace intervention vs usual care: mental health problems | Time until first RTW | Yes: Study with high risk of bias | NA | Yes: PICO deviant (subgroup analysis) | Yes: < 300 workers | Undetected | Very low |
| Time until lasting RTW | Yes: >50% of studies was assigned high risk of bias | Yes: I² > 50% | Yes: PICO deviant (subgroup analysis) | Yes: wide CI | Undetected | Very low | |
| Cumulative duration of sickness absence | No: Majority low risk of bias | No: I² < 50% | Yes: PICO deviant (subgroup analysis) | Yes: wide CI | Undetected | Low | |
| Workplace intervention only vs usual care | Time until first RTW | Yes: >50% of studies was assigned unclear risk of bias | No: I² < 50% | Yes: PICO deviant (subgroup analysis) | No | Undetected | Low |
| Time until lasting RTW | Yes: >50% of studies was assigned high risk of bias | No: I² < 50% | Yes: PICO deviant (subgroup analysis) | Yes: wide CI | Undetected | Very low | |
| Cumulative duration of sickness absence | No: Majority low risk of bias | No: I² < 50% | Yes: PICO deviant (subgroup analysis) | No | Undetected | Moderate | |
| Workplace intervention + cognitive behavioural intervention vs usual care | Time until first RTW | Yes: >25% of studies was assigned high risk of bias | No: I² < 50% | Yes: PICO deviant (subgroup analysis) | Yes: < 300 workers | Undetected | Very low |
| Time until lasting RTW | Yes: >25% of studies was assigned high risk of bias | Yes: I² > 50% | Yes: PICO deviant (subgroup analysis) | Yes: wide CI | Undetected | Very low | |
| Cumulative duration of sickness absence | No: Majority low risk of bias | No: I² < 50% | Yes: PICO deviant (subgroup analysis) | No | Undetected | Moderate | |
CI: confidence interval NA: not applicable
PICO: patients, intervention, control, outcome RTW: return to work
Subgroup analysis
The subgroups based on diagnosis were not statistically significantly different (P = 0.08). For musculoskeletal disorders, the analysis showed a pooled HR of 1.44 (95% CI 1.15 to 1.82). The quality of this evidence was moderate.
The subgroups based on inclusion of cognitive behavioural elements were not significantly different (P = 0.17). When only a workplace intervention was offered, analysis revealed a HR of 1.35 (95% CI 1.01 to 1.82), with low‐quality evidence. We downgraded the quality of the evidence based on the inclusion of studies with a high risk of bias and indirectness. When a workplace intervention was offered in combination with a cognitive behavioural intervention, the HR was 1.93 (95% CI 1.27 to 2.93). The quality of this evidence was very low, based on the inclusion of studies with a high risk of bias, indirectness, and imprecision.
Sensitivity analysis
An analysis including only studies with a low risk of bias revealed a HR of 1.50 (95% CI 1.18 to 1.92).
Time until lasting RTW
Workplace interventions did not considerably reduce time to lasting RTW compared to usual care (HR 1.07, 95% CI 0.72 to 1.57) (Figure 4; Analysis 1.2), based on six studies (Anema/Steenstra 2007; Hees 2012a; Lambeek 2010a; Noordik 2013a; Tamminga 2013; van Oostrom 2010a). The quality of this evidence was very low, downgraded based on the inclusion of studies with a high risk of bias, heterogeneity between studies, and uncertainty surrounding the effect estimate (Table 5).
4.
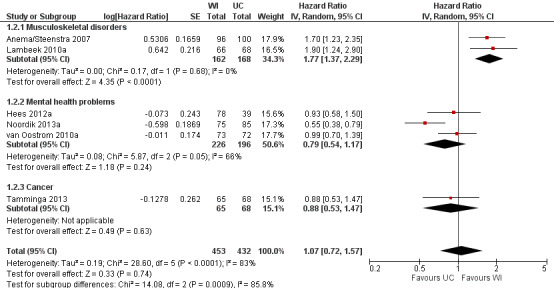
Forest plot of comparison: 1 Workplace intervention versus usual care, outcome: 1.2 Time until lasting RTW.
1.2. Analysis.
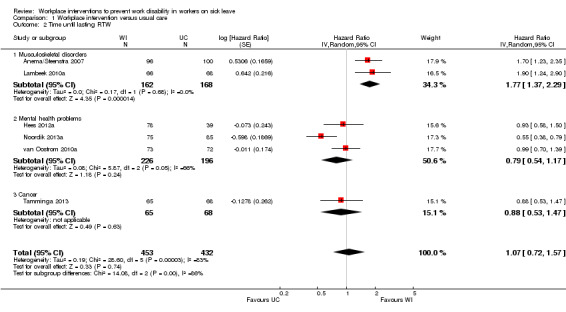
Comparison 1 Workplace intervention versus usual care, Outcome 2 Time until lasting RTW.
Subgroup analysis
The effects in subgroups based on diagnosis were significantly different (P = 0.0009). An analysis of two studies concerning musculoskeletal disorders significantly favoured the workplace intervention with a HR of 1.77 (95% CI 1.37 to 2.29). An analysis of three studies concerning mental health problems showed no difference between treatment conditions (HR 0.79, 95% CI 0.54 to 1.17). We assessed the quality of the analysis of musculoskeletal disorders as moderate, and the quality of the analysis of mental health problems as very low. We downgraded the quality of the evidence for mental health problems based on the inclusion of studies with a high risk of bias and uncertainty surrounding the effect estimate.
The effects in subgroups based on the inclusion of cognitive behavioural elements in the interventions did not differ statistically significantly (P = 0.056). An analysis of three studies offering a workplace intervention only did not show a considerable effect of the intervention on this outcome (HR 0.95, 95% CI 0.74 to 1.21). The quality of this evidence was very low, based on the inclusion of studies with a high risk of bias and uncertainty surrounding the effect estimate. An analysis of three studies offering a workplace intervention in combination with a cognitive behavioural intervention also did not show a considerable effect, with a HR of 1.21 (95% CI 0.56 to 2.62). The quality of this evidence was very low, based on the inclusion of studies with a high risk of bias, heterogeneity between studies, and uncertainty surrounding the effect estimate.
Sensitivity analysis
An analysis including only studies with a low risk of bias revealed a HR of 1.46 (95% CI 0.98 to 2.17).
Cumulative duration of sickness absence, follow‐up 12 months
Eight studies reported the cumulative duration of sickness absence (Anema/Steenstra 2007; Arnetz 2003; Bültmann 2009b; Busch 2011; Lambeek 2010b; van Oostrom 2010c; Verbeek 2002a; Vlasveld 2012c). The pooled analysis showed a non‐significant mean difference (MD) of ‐33.33 days (95% CI ‐49.54 to ‐17.12) between the workplace interventions and usual care (Figure 5; Analysis 1.3), meaning that the workplace intervention group had fewer sickness absence days when compared to the usual‐care group. The quality of this evidence was high (Table 1).
5.
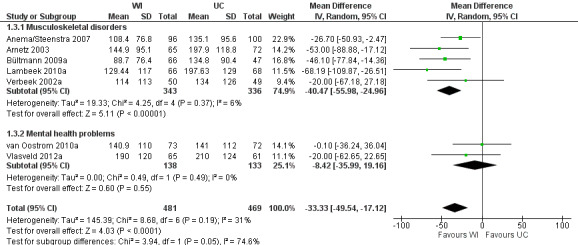
Forest plot of comparison: 1 Workplace intervention versus usual care, outcome: 1.3 Cumulative duration of sickness absence.
1.3. Analysis.
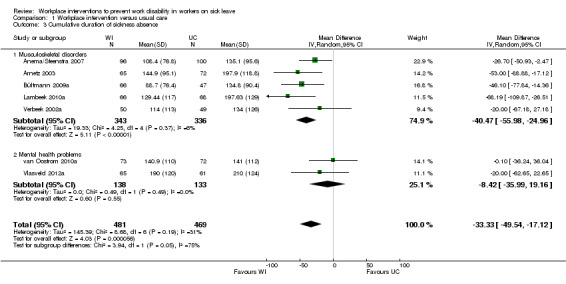
Comparison 1 Workplace intervention versus usual care, Outcome 3 Cumulative duration of sickness absence.
Subgroup analysis
Subgroups based on diagnosis were not statistically significantly different (P = 0.05). An analysis of studies concerning musculoskeletal disorders showed a significant mean difference in cumulative duration of sickness absence of ‐40.47 days (95% CI ‐55.98 to ‐24.96), favouring the workplace intervention. The quality of this evidence was moderate, due to indirectness. An analysis of studies concerning mental health problems showed a non‐significant MD of ‐8.42 days (95% CI ‐35.99 to 19.16), with low‐quality evidence for this outcome based on indirectness and imprecision.
Subgroups based on inclusion of cognitive behavioural elements in the intervention were not statistically significantly different (P = 0.79). An analysis of studies applying a workplace intervention only showed a MD of ‐31.16 days (95% CI ‐55.87 to ‐6.45). The quality of this evidence was moderate, due to indirectness. We found a significant mean difference for studies applying a workplace intervention in combination with a cognitive behavioural intervention (MD of ‐35.99 days, 95% CI ‐62.21 to ‐9.77), with moderate‐quality evidence, due to indirectness.
Sensitivity analysis
An analysis including only studies with a low risk of bias revealed a MD of ‐27.60 (95% CI ‐69.90 to 14.70).
Cumulative duration of sickness absence, follow‐up 3 to 10 years
Due to a longer follow‐up we did not include the Busch 2011 study in the meta‐analysis. After 10 years of follow‐up, workers in the behavioural medicine rehabilitation intervention had 42.98 fewer sickness absence days per year (P = 0.03). Workers in the physiotherapy or cognitive behavioural therapy groups had comparable sickness absence days with workers receiving usual care.
Recurrences of sick leave
One study reported recurrences of sick leave (Verbeek 2002a), with a recurrence rate of 25% in the usual‐care group and 51% in the workplace intervention group over 12 months, with a corresponding HR of 0.42 (95% CI 0.21 to 0.82). The evidence for this outcome was of moderate quality, since only one study measured this outcome.
Secondary outcomes
Functional status
We found a statistically significant difference in functional status at 12 months' follow‐up. The SMD was ‐0.33 (95% CI ‐0.58 to ‐0.08) between workers on sick leave who received the workplace intervention and those who received usual care (Anema/Steenstra 2007; Bültmann 2009a; Feuerstein 2003; Lambeek 2010a; Loisel 1997a; Verbeek 2002a) (Analysis 1.4). This means that workers in the workplace intervention group had better functional status. The quality assessment showed moderate‐quality evidence from six studies (628 participants) (Table 5), due to heterogeneity between studies.
1.4. Analysis.
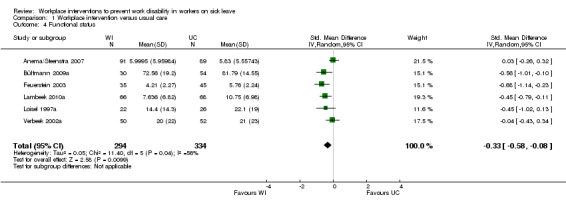
Comparison 1 Workplace intervention versus usual care, Outcome 4 Functional status.
Sensitivity analysis
An analysis including only studies with a low risk of bias revealed a MD of ‐0.15 (95% CI ‐0.45 to 0.15).
Quality of life and general health
We could not pool the data from four studies on quality of life because the studies used different measurement instruments and different subscales to measure the data (Busch 2011; Hees 2012a; Tamminga 2013; Verbeek 2002a). Hees 2012a measured three subscales of the SF‐36: mental health, role limitations due to emotional problems, and role limitations due to physical problems. The authors found a statistically significant effect between the intervention and usual‐care group on the mental health subscale. Busch 2011 calculated a global score of health‐related quality of life, based on the SF‐36. Their analysis showed that women in the intervention group reported significantly better health than women in the control group. Verbeek 2002a assessed general health perception with six separate subscales of the Nottingham Health Profile. They found no significant differences between the intervention and control group on any subscales. Tamminga 2013 measured quality of life with eight separate subscales of the SF‐36 (no overall score), and a visual analogue scale. Effects measured on quality‐of‐life scales did not differ between groups.
Symptoms
We could not pool symptom‐related data to provide one figure because one study reported the scores for each of the three scales of the Depression Anxiety Stress Scales separately (Blonk 2006). The authors of this study reported scores for depression, anxiety, and stress whilst they found no effect on anxiety and stress. We pooled depression data from the Hees 2012a, Noordik 2013a and Vlasveld 2012a studies. The pooled MD on depression was ‐0.12 (95% CI ‐0.35 to 0.11) (Analysis 1.5). The quality of this evidence was very low, based on the inclusion of studies with a high risk of bias and imprecision. The study on upper‐extremity disorders showed a MD of ‐0.30 (95% CI ‐0.63 to 0.03) for upper‐extremity symptom severity (Feuerstein 2003).
1.5. Analysis.
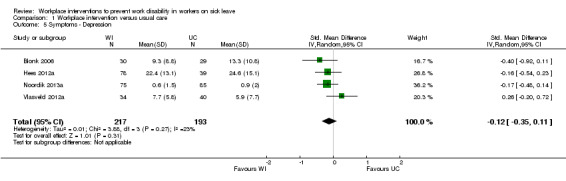
Comparison 1 Workplace intervention versus usual care, Outcome 5 Symptoms ‐ Depression.
Sensitivity analysis
An analysis including only studies with a low risk of bias for the outcome depression revealed a MD of 0.26 (95% CI ‐0.20 to 0.72).
Pain
We included five studies in the meta‐analysis regarding pain. All of these studies were on musculoskeletal disorders (Anema/Steenstra 2007; Bültmann 2009a; Lambeek 2010a; Loisel 1997a; Verbeek 2002a). Meta‐analysis resulted in a pooled SMD of ‐0.26 (95% CI ‐0.47 to ‐0.06), with workers in the workplace intervention group experiencing less pain (Analysis 1.6). We considered the quality of evidence to be high (Table 5).
1.6. Analysis.
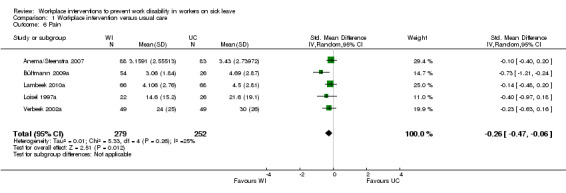
Comparison 1 Workplace intervention versus usual care, Outcome 6 Pain.
Sensitivity analysis
An analysis including only studies with a low risk of bias revealed a MD of ‐0.15 (95% CI ‐0.34 to 0.05).
Direct and indirect costs of work disability
We did not pool cost outcomes as we did not consider them comparable across studies (Table 2). Five studies reported no differences in costs (Anema/Steenstra 2007; Loisel 1997a; Tamminga 2013; van Oostrom 2010b; Vlasveld 2012d). The economic evaluation in the Steenstra 2006 study showed that the workplace intervention was more effective than usual care in RTW at slightly higher costs. The study by Loisel 1997b showed a decrease in medical costs in the intervention group compared to usual care (CAD 604 saved), but the intervention was not cost‐effective. Cost‐effectiveness analyses and cost‐utility analyses revealed no differences between the intervention and control group in quality‐adjusted life years(QALYs) or costs in the van Oostrom 2010b study. The cost‐utility analysis in the Vlasveld 2012d study showed that the intervention generated a reduction in both costs and effects compared to usual care, and was therefore not a cost‐effective intervention (Goorden). The Tamminga 2013 study compared mean direct and indirect costs between the intervention and control group. These costs did not differ between groups.
Three studies reported lower costs in the workplace intervention group (Arnetz 2003; Bültmann 2009b; Lambeek 2010c). The economic evaluation of the Arnetz 2003 study showed that the direct cost savings in the intervention group were USD 195 per case, yielding a direct benefit‐to‐cost ratio of 6.8 (Arnetz 2003). The Bültmann 2009b study estimated that the total costs saved in the intervention workers compared to controls were USD 1366 per worker at six months' follow‐up and USD 10,666 per person at 12 months' follow‐up. Lambeek 2010c carried out a cost‐effectiveness analysis from the societal perspective. Total costs in the intervention group were lower than in the usual‐care group. Cost‐effectiveness planes and acceptability curves showed that the intervention was cost effective compared with usual care for RTW and QALYs gained. The cost‐benefit analyses showed that every GBP 1 invested in the intervention would return an estimated GBP 26. Furthermore, the net societal benefit of the intervention compared with usual care was GBP 5744.
Busch 2011a compared three different interventions to a group receiving care as usual. One of these interventions, full‐time behavioural‐medicine rehabilitation, reduced the societal costs of sick leave and disability pension. The total costs in the behavioural‐medicine rehabilitation group after 10 years' follow‐up were SEK 969,077 compared to SEK 1,502,898 in the usual‐care group. The other two interventions, behaviour‐oriented physiotherapy and cognitive behavioural therapy, did not differ in costs when compared to the usual‐care group.
2. Workplace interventions compared to clinical interventions
We could perform comparisons between interventions only when interventions started at the same time. In two studies, the workplace intervention was followed by a clinical intervention if RTW was not achieved within eight weeks (Anema/Steenstra 2007; Loisel 1997a). We could not compare the workplace and clinical interventions because the clinical intervention followed the workplace intervention, and some workers received both. This meant that just one study included a valid comparison of a workplace intervention with a clinical intervention (Blonk 2006).
Sickness absence
We found a HR of 2.65 (95% CI 1.42 to 4.95) (Analysis 2.1) (Blonk 2006), with very low‐quality evidence due to imprecision and high risk of bias of this single study.
2.1. Analysis.
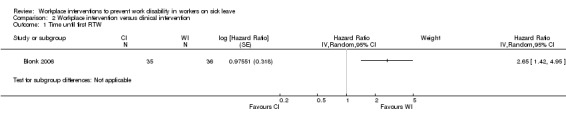
Comparison 2 Workplace intervention versus clinical intervention, Outcome 1 Time until first RTW.
Symptoms
The mean differences in anxiety, depression, and stress symptoms were not significantly different at 10 months' follow‐up (Blonk 2006). We regarded this evidence as very low quality.
Publication bias
In the funnel plots for the comparisons for the outcomes time until first RTW, time until lasting RTW, cumulative duration of sickness absence, functional status, and pain, we did not see any indication of publication bias.
Discussion
Summary of main results
We included in this review 14 RCTs that evaluated the effects of workplace interventions on work disability. Eight studies included workers with musculoskeletal disorders, five studies included workers with mental health problems, and one study included workers with cancer. The results of this review show that there is moderate‐quality evidence to support the effect of workplace interventions in reducing time until first RTW and high‐quality evidence to support the effect of workplace interventions in reducing the duration of sickness absence when compared with usual care. Furthermore, we found very low‐quality evidence that workplace interventions lead to lasting RTW, and moderate‐quality evidence that workplace interventions lead to more and faster recurrences of sick leave. Overall, the effectiveness of workplace interventions on work disability showed varying results. Our results show that the effectiveness of workplace interventions differs among workers with specific causes of work disability. Indeed, we found moderate‐quality evidence that workplace interventions reduce sickness absence (sooner first RTW, sooner lasting RTW, and shorter cumulative duration of sickness absence) among workers with musculoskeletal disorders when compared to usual care. We found that workplace interventions reduce pain and improve functional status in workers with musculoskeletal disorders. Taken together, the five studies on mental health problems did not show a considerable effect of the workplace interventions. The study on workers with cancer also did not show a considerable effect of the workplace intervention. We found no considerable evidence that a workplace intervention offered in combination with a cognitive behavioural intervention is more effective than a workplace intervention alone.
Overall completeness and applicability of evidence
There are large cross‐country differences regarding disability policies. For example, it has been shown that eligibility criteria for long‐term work disability compensation explain cross‐country differences in RTW (Anema 2009). Furthermore, the interventions applied to disability claimants in a specific country influence the success of RTW. Work interventions explained cross‐country differences more than purely medical interventions in promoting sustainable RTW (Anema 2009). Given the influence of disability policies on RTW, and the fact that most of the studies included in this review were conducted in the Netherlands, we are cautious about generalising our results to other countries.
We found moderate‐quality evidence to support the effect of workplace interventions for workers with musculoskeletal disorders on the outcomes time until first RTW, time until lasting RTW, and cumulative duration of sickness absence. A minimally clinically important change has not been defined for sickness absence. A consensus paper about definitions for episodes of low back pain proposed that an episode of work absence due to low back pain is defined as a period of work absence due to low back pain, preceded and followed by at least one day at work (de Vet 2002). Furthermore, from a socioeconomic point of view, each day of earlier RTW is important (Ostelo 2005). We found high‐quality evidence to support the effect of workplace interventions on the outcome pain, and moderate‐quality evidence on the outcome functional status. It was not possible to pool cost outcomes (Driessen 2012; Hamberg‐van Reenen 2012). However, the six studies on musculoskeletal disorders that measured costs showed beneficial results from workplace interventions: four studies were cost effective, and two other studies showed more timely RTW at equal cost.
The number of recipients of disability benefit on grounds of mental health conditions is increasing in most Organisation for Economic Co‐operation and Development countries, according to the OECD 2010. This highlights the need for interventions to support workers with mental health problems to prevent work disability. In this review, we found that workplace interventions for workers with mental health problems did not have a considerable effect on sickness absence outcomes. One might argue that the working mechanisms of workplace interventions may be less applicable for workers with mental health problems. For example, the stigma associated with mental health problems can negatively influence involvement of the workplace. This is why other intervention components may be needed to support workers with mental health problems to prevent work disability. A review of previous research on workplace interventions for workers with mental health problems indicated three intervention components that are effective on work outcomes. These components are: facilitation of access to clinical treatment; provision of workplace‐based high‐intensity psychological interventions; and facilitation of navigating through the disability management system (Pomaki 2012). Factor one and three do not involve the workplace, implying that intervention components outside the workplace are effective on work outcomes. Another Cochrane review on interventions to improve occupational health in depressed people showed moderate‐quality evidence that a work‐directed intervention in combination with a clinical intervention reduced sickness absence (Nieuwenhuijsen 2014). This finding was based on three studies, one of which was included in this review (Hees 2012a). The authors of the Nieuwenhuijsen 2014 review used less strict inclusion criteria for a workplace intervention, also including studies in which a workplace intervention was offered in combination with a clinical intervention, while we focused on workplace interventions only. This difference in inclusion criteria may account for the differing conclusions between our review and Nieuwenhuijsen 2014. Furthermore, the reviews of Nieuwenhuijsen 2014 and Pomaki 2012 imply that a combination of a workplace‐based intervention and a clinical psychological intervention might be more effective than a workplace‐based intervention only or a clinical intervention only to improve work outcomes in people with mental health problems such as depression. More research is needed to identify effective interventions or intervention components for this population. The studies by Hees 2012a and Vlasveld 2012a also combined a workplace intervention and a clinical psychological intervention, but failed to find an effect. This shows that it is not clear which intervention components are necessary to support workers with mental health problems to reduce their sickness absence.
The number of studies included in this review has increased from six studies in the original review published in 2009 to 14 studies in this updated review. This suggests that the focus of research on RTW interventions is increasingly on adapting the workplace or formulating a RTW plan instead of employing a clinical intervention in order to reduce symptoms. In this review, we included eight studies that concerned musculoskeletal disorders. RCTs concerning mental health problems or other health problems were conducted less frequently. We added four studies on mental health problems and one study on cancer to this update (Hees 2012a; Noordik 2013a; Tamminga 2013; van Oostrom 2010a; Vlasveld 2012a). It is possible that the smaller number of studies on workplace interventions for mental health problems is due to the lack of recognition of mental health problems by some compensation systems (that is, no compensation is granted in case of sickness absence caused by a mental health problem), difficulties in establishing whether a mental health problem is work related (a prerequisite for receiving compensation in several countries), and stigma concerning mental health problems. Having identified four additional studies on mental health problems since publication of the original review in 2009 highlights the increasing interest in addressing mental health problems to prevent work disability. One possible explanation for this interest is that mental health problems increasingly contribute to sickness absence and long‐term disability. Mental health problems account for about one‐third of all new disability claims, rising to between 40% to 50% in some countries (Cornelius 2011; OECD 2009). For other health conditions, such as cancer or cardiovascular disease, we included only one study in this review (Tamminga 2013). However, the importance of taking workplace factors into account in order to prevent work disability, has been recognized for both mental health problems and other health conditions (Berry 1992; Nachreiner 2007; Sanderson 2006; Tamminga 2010). More RCTs are needed to provide directions for RTW interventions for workers with mental health problems and other health conditions.
Quality of the evidence
In this updated review, we adapted the risk of bias assessment to Cochrane's current tool for risk of bias (Higgins 2011). To determine whether an included study had a high or low overall risk of bias, we set a minimum of four criteria that a study needed to meet in order to be classified as having a low risk of bias. We did not include blinding of participants and personnel in these four key domains. Blinding of participants and personnel is less applicable to the workplace setting, because the workplace often does not allow blinding of participants or personnel, especially if high degrees of worker participation and workplace changes are part of the intervention. Although we did not include blinding of participants in our key domains for the 'Risk of bias' assessment, not blinding participants and care providers could still be a source of information bias in occupational health research.
We assessed selective outcome reporting in our risk of bias assessment, and it is a topic of concern (Chan 2004; Hróbjartsson 2013). In 2008, a review was published about empirical evidence of outcome reporting bias. Results of this review showed that, when comparing trial publications to protocols, 40% to 62% of studies had at least one primary outcome that was changed, introduced, or omitted (Dwan 2008). In our review, we had study protocols available for seven of the 14 studies included in total. For these seven studies, we were able to compare the protocol to the published outcome measures, thereby assessing the risk of selective outcome reporting. No protocols were available for the other seven studies; we therefore tried to assess selective outcome reporting based on the published reports. As we had no study protocols with which to compare the published reports, we cannot be totally sure that selective outcome reporting has not occurred.
In our review we assessed the quality of the evidence as moderate for time until first RTW and high for cumulative duration of sickness absence, but very low for time until lasting RTW. We downgraded the evidence for the outcome time until lasting RTW because the effectiveness of workplace interventions differed greatly between workers with musculoskeletal disorders and workers with mental health problems and other health conditions. Most included studies on mental health and other health problems reported on the outcome time until lasting RTW, and therefore the analysis of this outcome showed a heterogeneous result. Workplace interventions were effective for workers with musculoskeletal disorders, but not effective or even negative for workers with mental health or other health problems. Furthermore, we assessed 50% of the studies included in the analysis for the outcome time until lasting RTW as having a high risk of bias.
Potential biases in the review process
It is possible that we may have missed relevant unpublished studies. We nevertheless tried to minimise selection bias in our search by screening references of identified trials and systematic reviews, contacting experts in the research field, and using no language restrictions. Careful screening of the identified papers resulted in identification of one study that measured RTW outcomes but which had not published the results (Feuerstein 2003). We contacted the authors and obtained data about the study and the outcomes RTW, symptoms, and functioning. Publishing study protocols is an important step in reducing publication bias (for example not publishing non‐significant results). Trial registers are furthermore a tool to reduce publication bias. We will also screen trial registers in future updates of this review.
The strict inclusion criteria used are likely to have reduced the number of studies included in this review. We included RCTs only since methodologically weaker designs can easily lead to bias. Randomisation is sometimes difficult to perform in the field of occupational health. However randomisation did not seem to be a problem in the individual RTW interventions that we included in this review. It should be recognised that high‐quality prospective studies in workplaces could add relevant information to this review.
This review did not include studies that only reported a dichotomous measure of sickness absence. We excluded five studies that assessed RTW in a dichotomous way only. Of these, two workplace interventions were effective in improving RTW outcomes (Cheng 2007; Netterstrøm 2013); the study by Lindh 1997 showed the workplace intervention to be effective in maintaining work stability after RTW; and two studies did not show a considerable effect of the workplace intervention on RTW outcomes (Haldorsen 2002; Nilsson 1996). We chose not to include studies that only reported dichotomous outcomes for sickness absence, because information may be lost about the exact duration of work disability. Using continuous sickness absence outcomes is especially important when an intervention is targeted on RTW and when sickness absence is the primary outcome of the study.
Workplace interventions aim to hasten RTW and preferably lasting RTW. However, only the Dutch studies reported time until lasting RTW, while almost all studies reported time until first RTW. To use all information in the review optimally, we chose to prioritize time until first RTW.
The results on our secondary outcomes might not completely represent all the available evidence on these outcomes since we based the primary criteria for inclusion of studies on whether a study reported continuous sickness absence as an outcome. Incorporating more studies might have changed the evidence for our secondary outcomes, as we found only moderate‐quality evidence for the outcome functional status. The original version of our review also found limited effects of RTW interventions on health outcomes, as did another review on workplace‐based RTW interventions (Franche 2005a), and the Steenstra 2012 study reported a limited association between functional status and RTW.
Most studies used administrative databases for data on sickness absence, except for three studies that used self‐reported data. Current literature suggests that administrative data and self‐report data reflect different aspects of the RTW trajectory. While administrative data is closely tied to benefit provision and termination, self‐report data is more closely linked to workers' perception of their RTW trajectory and work status (Ferrie 2005; Fleten 2004; Linton 2011; Pole 2006).
Agreements and disagreements with other studies or reviews
Previous studies have investigated the effects of workplace interventions, but the way these studies operationalised interventions and made comparisons differed from our review. Schandelmaier 2012 studied the effectiveness of RTW coordination programs (defined as a direct assessment leading to an individually tailored RTW plan implemented by a RTW coordinator or team that coordinates services and communication among involved stakeholders). This review showed moderate‐quality evidence for the benefit of RTW coordination programs. Other studies compared interventions involving workplace adaptations and stakeholder involvement to workplace‐linked interventions such as ergonomic exercises, and showed a benefit of interventions conducted at the workplace with the involvement of stakeholders (Carroll 2010; Franche 2005a; Haugli 2011). Furthermore it has been shown that purely medical interventions do not have a beneficial effect on work‐related outcomes (Loisel 2001; Nieuwenhuijsen 2014).
Authors' conclusions
Implications for practice.
The effectiveness of workplace interventions differed among workers depending on the cause of work disability. For workers with musculoskeletal disorders, workplace interventions might reduce sickness absence, and we found evidence to support their effectiveness on health outcomes. The meta‐analysis of the five studies on mental health problems and one study on cancer did not show a considerable effect of workplace interventions on sickness absence outcomes.
We found no clear evidence about offering a workplace intervention alone or in combination with a cognitive behavioural intervention.
Implications for research.
The quality of evidence in this review update for workplace RTW interventions for workers on sick leave was higher than in the original review. We found moderate‐quality evidence for the effectiveness of workplace interventions for workers with musculoskeletal disorders. We added four studies on workers with mental health problems in this update. However there still remains a need for more research on effective interventions on work outcomes for workers with mental health problems and other health conditions, as it is not clear which interventions help workers with mental health problems return to work. Future research should focus on identifying effective types of workplace interventions, such as a combination of a workplace intervention with a clinical intervention. Studies evaluating cost outcomes should report on confidence intervals or P values, to enable claims about statistical significance of these results. Economic evaluations are generally underpowered because they are often carried out alongside an intervention study. We recommend that future research address the development of methods to pool cost outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 2 February 2015 | New search has been performed | The authors for this review have changed. |
| 2 February 2015 | New search has been performed | The search had been updated to 2 February 2015. Eight new studies are included in this updated review. |
| 2 February 2015 | New citation required and conclusions have changed | Eight new studies are included in this updated review. The results and conclusions have changed. |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 27 March 2008 | Amended | Converted to new review format. |
Acknowledgements
We wish to thank Ingrid Riphagen and René Otten for the contributions they made regarding the development of the search strategy; Jos Verbeek and Jean‐Baptiste Fassier for their comments on the review protocol; and Maurits van Tulder, Raymond Ostelo, and Dirk Knol for their support for the quantitative and qualitative analyses.
We furthermore wish to thank Maurice Driessen, Eva Schonstein, Patrick Loisel, and Willem van Mechelen for their contribution to the publication of the original Cochrane review published in 2009.
We thank Emma Sydenham and the Cochrane Injuries Group for hosting our review up to 2015, for organising peer review and providing advice. We thank Jos Verbeek and Jani Ruotsalainen from Cochrane Work for handling the transfer from the Injuries Group to theirs. We also thank them for their comments on the updated review. Finally we thank Lisa Winer and Jani Ruotsalainen for copy editing the text.
Appendices
Appendix 1. Detailed search strategies
Date restrictions were not applied for any of the databases. The updated search was conducted on 2 February 2015. The methodological filter we used is a best sensitive methodological filter for EMBASE.com, to identify a set of relevant RCTs that is as complete as possible (Wong 2006). Outcome terms were important for this review. However, Emtree terms such as work‐disability and disease‐duration were very broad, and were combined with population terms. On the other hand, specific terms for outcome were suitable to be incorporated without the population terms. Therefore we searched with two combinations. All references from the EMBASE.com search were combined in a Reference Manager database and further checked for double references by means of a duplicate search. Each double reference we found was deleted.
We searched for studies in EMBASE.com by combining the following: 1. RTW interventions (vocational‐rehabilitation/exp OR occupational‐intervention OR disability‐prevention OR disability‐management OR 'disability'/de/dm_dm,dm_pc,dm_th OR 'work disability'/de/dm_dm,dm_pc,dm_th OR occupational‐rehabilitation/exp OR workplace‐intervention OR modified‐duty OR modified‐duties OR vocational‐guidance OR case‐manager OR case‐management OR ergonomics OR 'ergonomic *3 approach' OR 'ergonomic *3 training' OR 'ergonomic *3 education' OR 'ergonomic *3 counselling' OR job‐accommodation OR on‐the‐job‐program OR workplace‐accommodation OR modified‐work OR supported‐employment OR work‐reintegration‐plan OR light‐duty OR work‐site‐visit OR work‐visit OR work‐adjustment OR solution‐focused‐intervention OR 'vocational *3 counselling' OR 'vocational *3 placement' OR 'vocational *3 training' OR 'occupational disease'/exp/dm_dm,dm_th)
2. Methodological filter and exclusion of chemicals and drugs (random*:ti,ab OR clinical‐trial OR clinical‐trials OR health‐care‐quality/exp) NOT ('chemicals and drugs'/exp/mj)
3. #1 AND #2
4. Specific terms for outcomes (absenteeism/exp OR (((worktime OR work‐time) OR workday*) AND (loss OR lost)) OR return‐to‐work OR returns‐to‐work OR sick‐leave OR work‐resumption/de OR sick‐absence OR sickness‐absence OR lost‐workdays OR sick‐listed OR work‐resumption OR duration‐of‐absence OR work‐reentry‐rate OR time‐loss‐from‐work OR time‐lost‐from‐work)
5. More general terms for outcomes, if used singly, were too broad, and therefore we used them in combination with terms for population. (absenteeism/exp OR (((worktime OR work‐time) OR workday*) AND (loss OR lost)) OR return‐to‐work OR returns‐to‐work OR sick‐leave OR (work AND limitation*) OR job‐performance/de OR work‐resumption/de OR sick‐absence OR sickness‐absence OR 'disease duration'/exp OR work‐disability/de OR work‐disability OR disability‐prevention OR disability/de OR disability‐management OR employment‐after‐rehabilitation OR (regain AND (employment OR work)) OR lost‐workdays OR (compensation AND cost*) OR work‐resumption OR duration‐of‐absence OR work‐reentry‐rate OR time‐loss‐from‐work OR time‐lost‐from‐work) AND (employee/exp OR employee* OR employer/exp OR employer* OR worker/exp OR worker* OR workman* OR work‐site OR worksite OR workman‐compensation/de OR workers‐compensation OR benefit‐duration OR time‐on‐benefits OR workplace/de OR workplace OR work‐environment/de OR supervisor*)
EMBASE.com #3 AND #4 #3 AND #5
We searched for studies in CENTRAL by combining the following areas: 1. Terms for population/place of application of intervention (employee* OR employer* OR worker* OR manpower OR "work site" OR worksite OR "workman compensation" OR "workers' compensation" OR workplace OR "work environment" OR "work capacity" OR supervisor*)
2. Terms for outcome (absenteeism OR ((worktime OR workday*) AND (loss OR lost)) OR "return to work" OR "returns to work" OR "sick leave" OR "job performance" OR "work resumption" OR "sick absence" OR "sickness absence" OR "disease duration" OR "work disability" OR "disability prevention" OR disability OR "disability management" OR "employment after rehabilitation" OR "regain employment" OR "regain work" OR "lost workdays" OR "duration of absence" OR "work reentry rate" OR "time loss from work" OR "time lost from work")
CENTRAL #1 AND #2 This search was restricted to the Cochrane Central Register of Controlled Trials (Clinical Trials).
PsycINFO We searched for studies in PsycINFO by combining the following areas: 1. Terms for intervention (DE=("vocational rehabilitation" or "supported employment" or "vocational evaluation" or "work adjustment training" or "occupational adjustment" or "disability management" or "case management") or KW=("workplace intervention*" or "job accommodation*" or "workplace accommodation*" or "modified work" or "work site visit" or "ergonomic*" or "occupational intervention" or "disability prevention" or "occupational rehabilitation" or "workplace intervention" or "modified duty" or "light duty" or "modified duties" or "vocational guidance" or "case manager" or "on the job program" or "work reintegration plan" or "solution focused intervention" or "vocational counselling"))
2. Terms for outcome ((DE=("employee absenteeism" or "reemployment" or "employee leave benefits")) or KW=("return to work" or "returns to work" or "work disability" or "employment after rehabilitation" or "time loss from work" or "time lost from work" or "work rehabilitation" or "absenteeism" or "work resumption" or "sick leave" or "sick listed" or "sick absence*" or "sickness absence*" or "absenteeism" or "worktime loss" or "work time loss" or "workday loss" or "work resumption" or "lost workdays" or "duration of absence" or "work reentry rate" or "time loss from work" or "time lost from work"))
PsycINFO #1 AND #2.
The database of the Cochrane Occupational Safety and Health Field was searched by combining: 1. a code for research design: RCT‐study (all non‐indexed fields) 2. a code for outcome: disability‐outcome (all non‐indexed fields)
Data and analyses
Comparison 1. Workplace intervention versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time until first RTW | 5 | 612 | Hazard Ratio (Random, 95% CI) | 1.55 [1.20, 2.01] |
| 1.1 Musculoskeletal disorders | 4 | 543 | Hazard Ratio (Random, 95% CI) | 1.44 [1.15, 1.82] |
| 1.2 Mental health problems | 1 | 69 | Hazard Ratio (Random, 95% CI) | 2.64 [1.41, 4.95] |
| 2 Time until lasting RTW | 6 | 885 | Hazard Ratio (Random, 95% CI) | 1.07 [0.72, 1.57] |
| 2.1 Musculoskeletal disorders | 2 | 330 | Hazard Ratio (Random, 95% CI) | 1.77 [1.37, 2.29] |
| 2.2 Mental health problems | 3 | 422 | Hazard Ratio (Random, 95% CI) | 0.79 [0.54, 1.17] |
| 2.3 Cancer | 1 | 133 | Hazard Ratio (Random, 95% CI) | 0.88 [0.53, 1.47] |
| 3 Cumulative duration of sickness absence | 7 | 950 | Mean Difference (IV, Random, 95% CI) | ‐33.33 [‐49.54, ‐17.12] |
| 3.1 Musculoskeletal disorders | 5 | 679 | Mean Difference (IV, Random, 95% CI) | ‐40.47 [‐55.98, ‐24.96] |
| 3.2 Mental health problems | 2 | 271 | Mean Difference (IV, Random, 95% CI) | ‐8.42 [‐35.99, 19.16] |
| 4 Functional status | 6 | 628 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.58, ‐0.08] |
| 5 Symptoms ‐ Depression | 4 | 410 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.35, 0.11] |
| 6 Pain | 5 | 531 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.47, ‐0.06] |
| 7 Time until first RTW | 5 | 612 | Hazard Ratio (Random, 95% CI) | 1.55 [1.20, 2.01] |
| 7.1 WI only | 3 | 347 | Hazard Ratio (Random, 95% CI) | 1.35 [1.01, 1.82] |
| 7.2 WI and cognitive behavioral intervention | 2 | 265 | Hazard Ratio (Random, 95% CI) | 1.93 [1.27, 2.93] |
| 8 Time until lasting RTW | 6 | 885 | Hazard Ratio (Random, 95% CI) | 1.07 [0.72, 1.57] |
| 8.1 WI only | 3 | 395 | Hazard Ratio (Random, 95% CI) | 0.95 [0.74, 1.21] |
| 8.2 WI and cognitive behavioral intervention | 3 | 490 | Hazard Ratio (Random, 95% CI) | 1.21 [0.56, 2.62] |
| 9 Cumulative duration of sickness absence | 7 | 950 | Mean Difference (IV, Random, 95% CI) | ‐33.33 [‐49.54, ‐17.12] |
| 9.1 WI only | 4 | 494 | Mean Difference (IV, Random, 95% CI) | ‐31.16 [‐55.87, ‐6.45] |
| 9.2 WI and cognitive behavioral intervention | 3 | 456 | Mean Difference (IV, Random, 95% CI) | ‐35.99 [‐62.21, ‐9.77] |
1.7. Analysis.
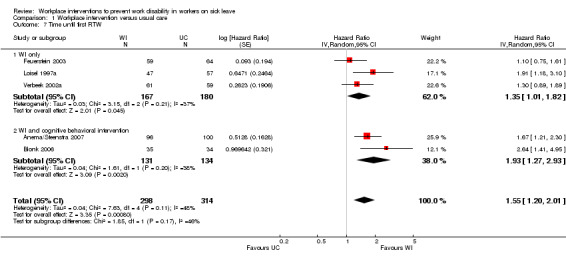
Comparison 1 Workplace intervention versus usual care, Outcome 7 Time until first RTW.
1.8. Analysis.
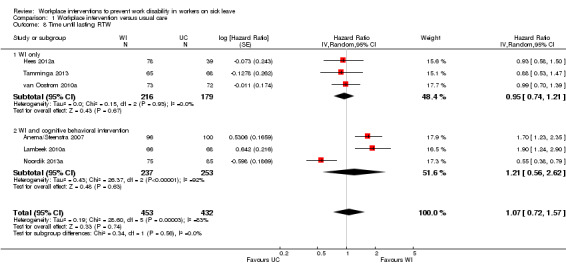
Comparison 1 Workplace intervention versus usual care, Outcome 8 Time until lasting RTW.
1.9. Analysis.
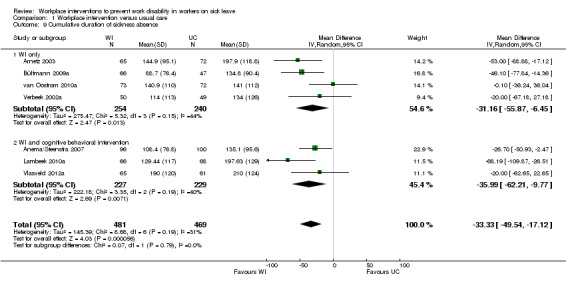
Comparison 1 Workplace intervention versus usual care, Outcome 9 Cumulative duration of sickness absence.
Comparison 2. Workplace intervention versus clinical intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time until first RTW | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anema/Steenstra 2007.
| Methods | RCT, multicentre, the Netherlands. Randomisation: cluster randomisation, level of occupational physician. A member of the research team randomised occupational physicians using a series of random numbers. Recruitment: an occupational physician informed the researchers on whether inclusion in the study was justified on medical grounds. Duration recruitment: October 2000 to October 2002. Follow‐up: 12 months. | |
| Participants | 196 were randomised (work intervention: 96; usual care: 100). Inclusion criteria: low back pain defined as pain localised in the lower back without a specific underlying cause between the lower angle of the scapulae and above the buttocks, sick leave from regular work for 2 to 6 weeks, age 18 to 65 years, understand Dutch language. Exclusion criteria: specific causes of low back pain such as herniated discs with pareses, paralysis, spinal tumour, spinal fracture, ankylosing spondylitis, spinal stenosis, spondylolisthesis, specific rheumatological diseases, pregnancy, serious psychiatric disorders (ICD‐10 code: M51, M51.2, M51.4, M51.3, M51.8, M40–M54, M45, M46.0, M46.1, M46.8, M49, and M46.9), a legal conflict at work, sick‐listed due to low back pain less than 1 month prior to the current episode of sick leave. Type of disability: musculoskeletal (diagnosis by occupational physician). Duration of absence prior to randomisation: 2 to 6 weeks. | |
| Interventions | WI Stakeholders involved: ergonomist, injured worker, supervisor, possible other stakeholders, occupational physician, general practitioner. Standardised treatment: a work site assessment and work adjustments based on methods used in participatory ergonomics, observation of the worker’s tasks by the ergonomist, ranking obstacles for RTW independently by the worker and the supervisor, meeting of the group of stakeholders to brainstorm and discuss about all possible solutions, achieving consensus regarding feasible solutions, communication between the occupational physician and the general practitioner to prevent conflicting advice, and occupational physician arranged RTW date with the worker. UC Stakeholders involved: injured worker, occupational physician, general practitioner. Standardised treatment: Dutch occupational guideline on low back pain advice for non‐specific low back pain. Education about the good prognosis, coping with low back pain, fear of movement, planning for resumption of normal activities, advice to return to work within 2 weeks in the absence of further problems, if necessary temporary work adjustments regarding working hours or job content, optional workplace visit, optional consult general practitioner or other medical specialist. | |
| Outcomes | Sickness absence: administrative data used.
Time until lasting RTW: duration of sick leave in calendar days from the first day of sick leave to full RTW in own or equal work, for at least 4 weeks without (partial or full) dropping out. Median work intervention = 77 days, usual care = 104 days, HR 1.7 (1.2 to 2.3). Time until first RTW: duration of sick leave in calendar days from the first day of sick leave to full RTW in own or equal work. Median work intervention = 69.5 days, usual care = 98.5 days, HR 1.67 (1.22 to 2.31). Cumulative duration of sickness absence: total duration of sick leave due to low back pain (including all recurrences of sick leave episodes) was calculated for the entire 12‐month follow‐up. Mean (SD) work intervention = 108.4 (76.8) days, usual care = 135.1 (95.6) days. Functional status: Roland‐Morris Disability Questionnaire. Mean at 12 months (SD) work intervention = 6.0 (5.9) days, usual care = 5.8 (5.6) days. Pain: VAS. Mean at 12 months (SD) work intervention = 3.2 (2.6), usual care = 3.4 (2.7). Costs: direct medical costs (use of pain medication, medical and alternative medical resources), direct intervention costs and indirect costs (production losses due to sick leave). Societal and employers perspective. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A series of random numbers" (p. 3, Steenstra 2003) |
| Allocation concealment (selection bias) | Low risk | Adequate (information gathered from personal contact with authors.) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Treatment allocation was made known by the OP to the worker after informed consent and completion of the first questionnaire." (p. 292, Anema 2007 |
| Blinding of outcome assessment (detection bias) Sickness absence outcomes | Low risk | "In this study records on sick leave were obtained from the occupational health services from the various co‐operating companies. Registration of sick leave is a continuous process in occupational health services." (p. 4, Steenstra 2003) |
| Blinding of outcome assessment (detection bias) Health‐related outcomes | High risk | Secondary outcomes are self‐reported, therefore secondary outcomes were assigned high risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out: "Ten of 96 (10%) workers did not receive intervention: 5 workers returned to work before an appointment for the workplace intervention was made. Five workers did not participate in the workplace intervention due to a work scheduling problem (n = 3), a medical reason (n = 1), or a work conflict (n = 1). None of the workers stopped during this intervention." (p. 294, Anema 2007) Primary outcome: none because data retrieved from administrative database. Secondary outcomes: "For 24 workers (12%), no follow‐up data regarding the secondary outcome measures could be collected." (p. 294, Anema 2007) |
| Selective reporting (reporting bias) | Low risk | The results from all important prespecified outcomes (in published study protocol) have been adequately reported in the published report of the trial (judged on basis of Steenstra 2003 and Anema 2007) |
| Other bias | Low risk | "All statistical analyses will be performed according to the intention‐to‐treat principle." (p. 4, Steenstra 2003). Groups were similar at baseline regarding important prognostic factors (p. 294, Anema 2007). No other concerns about bias were identified for this study |
Arnetz 2003.
| Methods | RCT, multicentre, Sweden. Randomisation: a worker. Participants were selected at random from the total pool of eligible workers. Every participant was allocated to either the intervention or reference group, based on the scheduled time of their visit to the local insurance branch office. In an interview with the insurance agency case manager and occupational therapist/ergonomist, potential participants were asked about their interest to participate in the project both in writing and verbally. Recruitment: potential study participants were selected from the roster of all sick leave cases at the 2 local branches of the National Insurance Agency. Duration of recruitment: not reported. Follow‐up: 12 months. | |
| Participants | 137 were randomised (work intervention: 65; usual care: 72).
Inclusion criteria: diagnosed first or recurrent musculoskeletal disorders. Prior history of musculoskeletal disorders did not disqualify a person from inclusion as long as they had recovered sufficiently to return to work during the interim period.
Type of disability: musculoskeletal (diagnostic classification based on sick leave certificate). Duration of absence prior to randomisation: not described. |
|
| Interventions | WI Stakeholders involved: insurance agency case manager, occupational therapist/ergonomist, employer, worker. Standardised treatment: early workplace‐based intervention consisting of an interview focused on the social and occupational situation, possible adaptation at work, possibility of vocational training, meeting of all stakeholders at the worker’s workplace, ergonomic assessment of workplace, introduction of appropriate ergonomic improvements, optional vocational training and instruction at work by the ergonomist, employer was encouraged to complete a rehabilitation investigation. UC Stakeholders involved: not reported. Non‐standardised treatment: 8‐week RTW plan (but only a minority actually implemented it). | |
| Outcomes | Sickness absence: administrative data used.
Cumulative duration of sickness absence: mean number of sick days. Mean at 12 months (SD) work intervention = 144.9 (11.8) days, usual care = 197.9 (14) days. Other definition: mean days of paid rehabilitation. Costs: direct intervention costs and indirect costs (total reimbursement paid). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computerized randomisation table" (Information gathered from personal contact with authors.) |
| Allocation concealment (selection bias) | High risk | Not adequate "The allocation to the control or reference group, respectively, was concealed for the employees at the local insurance branch offices as well as to the scientist responsible for data analysis. It was not possible to conceal group allocations to participating employees with musculoskeletal disorders, nor the insurance branch managers or the ergonomist that were part of the team visiting the employers together with the employee with musculoskeletal disorders‐related sickness absenteeism." (p. 500, Arnetz 2003) "All consecutive cases fulfilling the inclusion criteria were written into the randomisation table." (Information gathered from personal contact with authors.) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "It was not possible to conceal group allocations to participating employees with MSDs, nor the insurance managers or the ergonomist that were part of the team visiting the employers together with the employee with MSD‐related sickness absenteeism." (p. 500, Arnetz 2003) |
| Blinding of outcome assessment (detection bias) Sickness absence outcomes | Low risk | Primary outcomes: Table 2, cumulative number of sick leave days generated from the local branch of the National Health Insurance Agency. (p. 502, Arnetz 2003) |
| Blinding of outcome assessment (detection bias) Health‐related outcomes | High risk | Secondary outcomes are self‐reported, therefore secondary outcomes were assigned high risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐out rate not reported. Primary outcome: none because data retrieved from administrative database. Secondary outcomes not reported. |
| Selective reporting (reporting bias) | Low risk | The results from all important prespecified outcomes (in methods) have been adequately reported in the published report of the trial (judged on basis of Arnetz 2003) |
| Other bias | Low risk | Information on data on compensated sick days were tracked for all employees using the National Insurance official. (Information gathered from personal contact with authors.) Groups were similar at baseline regarding important prognostic factors (p. 501, Arnetz 2003). No other concerns about bias were identified for this study |
Blonk 2006.
| Methods | RCT, the Netherlands. Randomisation: level worker. "Randomly assigned". Recruitment: self employed individuals who called their insurance company for disability benefits were briefly informed about the study and asked whether they wished to receive additional information before deciding whether to participate. Duration recruitment: 20 months Follow‐up: 10 months, RTW 360 days. | |
| Participants | 122 were randomised (workplace intervention: 40; cognitive behavioural therapy: 40; control group: 42).
Inclusion criteria: self employed individuals insured at a private insurance company with adjustment disorders such as burnout and job stress.
Exclusion criteria: psychiatric disorders (based on a shortened version of the CIDI), not willing to postpone current (psychotherapeutic) treatment.
Type of disability: mental health (self‐reported and CIDI interview conducted by psychologist). Duration of absence prior to randomisation: immediate inclusion in the study when reporting being disabled. |
|
| Interventions | WI Stakeholders involved: the self employed individual, a labour expert. Standardised treatment: combined intervention consisting of a brief cognitive behavioural therapy‐derived intervention focusing on work stress, relaxation, and time management including homework assignments, combined with both individual‐focused and workplace interventions. A labour expert gave advice on work processes and provided suggestions on how to lower the workload and job demands and increase the decision latitude. Partial RTW was discussed. CBT Stakeholders involved: self employed individual, psychologist. Standardised treatment: commonly used protocol in the Netherlands consisting of cognitive restructuring, and on registration of symptoms and situations, later sessions focused on work resumption, time management, workplace interventions, conflict handling, and fatigue; the assignments were related to the work situation. UC Stakeholders involved: self employed individual, general practitioner. Standardised treatment: 2 brief sessions with general practitioner to check the legitimacy of the work disability benefit (no treatment group). | |
| Outcomes | Sickness absence: administrative data used.
Time until first RTW: length of time until full RTW. Median WI = 122 days, UC = 320 days, HR = 2.6 Other definition: length of time until partial RTW. Symptoms: depression anxiety stress scale, Maslach Burnout inventory. Depression at 4 months: Mean (SD) WI = 10.6 (9.0), UC = 14.4 (10.3). Depression at 10 months: Mean (SD) WI = 9.3 (8.8), UC = 13.3 (10.8). Anxiety at 4 months: Mean (SD) WI = 7.8 (6.6), UC = 8.9 (6.9). Anxiety at 10 months: Mean (SD) WI = 6.6 (6.6), UC = 7.1 (7.2). Stress at 4 months: Mean (SD) WI =14.2 (8.3), UC = 16.6 (8.2). Stress at 10 months: Mean (SD) WI = 13.3 (7.4), UC = 14.1 (9.2). Exhaustion at 4 months: Mean (SD) WI = 3.0 (1.7), UC = 3.4 (1.7). Exhaustion at 10 months: Mean (SD) WI = 2.9 (1.5), UC = 3.0 (1.8). Depersonalisation at 4 months: Mean (SD) WI = 2.2 (1.5), UC = 2.3 (1.6). Depersonalisation at 10 months: Mean (SD) WI = 1.9 (1.4), UC = 2.3 (1.6). Professional efficacy at 4 months: Mean (SD) WI = 4.1 (1.0), UC = 3.9 (1.3). Professional efficacy at 10 months: Mean (SD) WI = 4.3 (1.0), UC = 4.0 (1.4). |
|
| Notes | Pre‐test questionnaire was received after randomisation. No numbers given of post‐hoc tests for differences between the 3 groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation done by use of a dice. (Information gathered from personal contact with authors.) |
| Allocation concealment (selection bias) | High risk | Unclear Use of a dice is not regarded as concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) Sickness absence outcomes | Low risk | Primary outcomes: "These data were extracted from the database system of the insurance company". (p. 136, Blonk 2006) |
| Blinding of outcome assessment (detection bias) Health‐related outcomes | High risk | Secondary outcomes are self‐reported, therefore secondary outcomes were assigned high risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐out not reported, 8 participants did not receive the assigned treatment, but not reported how many dropped out. Primary outcome: "At the follow‐up, the number of participants was 30, 30 and 29, respectively." (p. 134, Blonk 2006) Loss to follow‐up is 10% for CBT group, 12.5% for WI group, and 15% for UC group. Secondary outcomes: "The number of participants who returned all questionnaires was 30, 28, and 28." (p. 134, Blonk 2006) Loss to follow‐up is 25% for CBT group, 30% for WI group, and 33% for UC group. |
| Selective reporting (reporting bias) | Low risk | The results from all important prespecified outcomes (in methods) have been adequately reported in the published report of the trial (judged on basis of Blonk 2006) |
| Other bias | High risk | "Furthermore, eight participants did not receive the assigned treatment because of miscommunication at the insurance company. As a result, the number of participants who filled in the pre‐test questionnaire and received the assigned treatment was 36 in each condition." (p. 133, Blonk 2006). It was not reported whether the groups were similar at baseline regarding important prognostic factors (p. 134, Blonk 2006). |
Busch 2011.
| Methods | RCT, multicentre, Sweden. Randomisation: block randomisation, level worker. Recruitment: workers were recruited from an health insurance register which offers economic compensation in cases of sickness absence or disability pension. Duration recruitment: May 1995 to March 1998 Follow‐up: 10 years |
|
| Participants | 214 were randomised (behavioural‐oriented physical therapy: 54; cognitive behavioural therapy: 49; behavioural medicine rehabilitation: 63; usual care: 48) Inclusion criteria: being on continuous sickness absence for 1 to 6 months due to non‐specific spinal pain, being 18 to 60 years of age, and fluency in Swedish. Exclusion criteria: serious spinal pathology, exposure to physical trauma within 6 months before examination, objective neurological signs indicating a need for surgery, serious comorbidities, ongoing rehabilitation, and verified pregnancy. Type of disability: musculoskeletal (diagnosis by physician). Duration of sickness absence prior to randomisation:1 to 6 months. |
|
| Interventions | Workers were randomised to 1 of 3 active treatment conditions or to a control group receiving treatment as usual. All active treatment conditions included scheduled time for visits at the workplace. Work managers and rehabilitation officers were invited to participate in the discharge session, at which a rehabilitation plan was agreed upon. All treatment groups are specified below: PT Stakeholders involved: physiotherapist, psychologist, physician, worker, employer, insurer, ergonomist, rehabilitation official. Standardised treatment: the aim was to enhance physical functioning and promote durable behavioural change. The program included individual goal setting, gradually increased exercises to improve muscular endurance, aerobic training, pool training, relaxation techniques, and body awareness therapy. CBT Stakeholders involved: physiotherapist, psychologist, physician, worker, employer, insurer, ergonomist, rehabilitation official. Standardised treatment: the goal was to improve the worker's ability to manage pain and to resume a normal level of activity. Basic elements of the CBT were activity planning, goal setting, problem solving, applied relaxation, cognitive coping techniques, activity pacing, how to break vicious circles, assertiveness training, and the role of significant others. BM Stakeholders involved: physiotherapist, psychologist, physician, worker, employer, insurer, ergonomist, rehabilitation official. Standardised treatment: BM was a multidisciplinary program in which all parts of the PT and CBT programs were included. UC Stakeholders involved: not reported. Standardised treatment: the control group was not offered any treatment within the research project. Consequently, they underwent the normal routines in health care (treatment as usual). Research indicates that only a minority of individuals with persistent spinal pain were offered more comprehensive rehabilitation programs in Sweden at that time. |
|
| Outcomes | Sickness absence: administrative data used. Absence from work: days absent from work. Mean at 36 months (SD) females, PT intervention = 522 (386), CBT intervention = 542 (446), BM intervention = 439 (329), UC group = 572 (424). Mean at 36 months (SD) males, PT intervention = 541 (446), CBT intervention = 629 (379), BM intervention = 494 (375), UC group = 479 (408). Days on sick leave 10 years after rehabilitation. Mean at 10 years (SD), PT intervention = 873 (930), CBT intervention = 1047 (897), BM intervention = 900 (805), UC group = 972 (858). Days on disability pension 10 years after rehabilitation. Mean at 10 years (SD), PT intervention = 3108 (593), CBT intervention = 3070 (584), BM intervention = 2459 (736), UC group = 2925 (849). Costs: direct intervention costs and indirect costs (production losses due to sick leave and disability pension). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study was based on a randomised controlled multi centre trial, in which a block randomisation procedure and the sealed envelope technique were used." (p. 1728, Busch 2011a) |
| Allocation concealment (selection bias) | Low risk | "All the screening personnel were blinded to the results of the randomisation." (p. 1728, Busch 2011a) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) Sickness absence outcomes | Low risk | Primary outcome: "Sickness absence was studied using official records obtained from the Social Insurance Agency (SSIA), the Swedish authority that maintains records of all cases of sickness absence in excess of 14 consecutive days." (p. 1729, Busch 2011a) |
| Blinding of outcome assessment (detection bias) Health‐related outcomes | High risk | Secondary outcomes are self‐reported, therefore secondary outcomes were assigned high risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Sick leave: available for 208 of 214 workers (97%). self‐reported outcomes: available for 159 of 214 workers (74%). (p. 277, Jensen 2005) |
| Selective reporting (reporting bias) | Low risk | The results from all important pre specified outcomes (in methods) have been adequately reported in the published report of the trial (judged on basis of Busch 2011a and Jensen 2005) |
| Other bias | High risk | Differences between groups in sick days prior to participation, and by sex, are large. (Table 1, p. 275, Jensen 2005) |
Bültmann 2009a.
| Methods | RCT, multicentre, Denmark. Randomisation: a randomisation protocol without stratification was computer generated prior to the start of the study and was undertaken by an independent IT assistant. After informed consent, workers were randomly allocated to either the intervention group or usual‐care group. Recruitment: workers on sick leave for at least 4 weeks were invited to an information meeting at 1 of the 4 participating municipalities. Duration recruitment: April 2004 to April 2005 Follow‐up: 12 months. |
|
| Participants | 119 were randomised (work intervention: 68; usual care: 51). Inclusion criteria: absent from work for 4 to 12 weeks, have a reimbursement request indicating low back pain or MSD as the main cause of sick leave, between 18 and 65 years of age, understanding and speaking Danish. Exclusion criteria: mental health disorders, alcohol or drug addiction, pregnancy, had quit their job or had been fired before randomisation. Type of disability: musculoskeletal. Duration of absence prior to randomisation: 4 to 12 weeks. |
|
| Interventions | WI Stakeholders involved: occupational physician, chiropractor, psychologist, social worker, municipal case manager, worker, the workplace. Standardised treatment: the workplace intervention consists of 2 main components: (1) a work disability screening: a systematic, multidisciplinary assessment of disability and functioning as well as the identification of barriers for RTW; and (2) the formulation and implementation of a coordinated, tailored, and action‐oriented work rehabilitation plan collaboratively developed by an interdisciplinary team using a feedback‐guided approach. UC Stakeholders involved: employer, medical experts, vocational rehabilitation institutions, unions. Standardised treatment: municipalities are obliged to make a follow‐up assessment of all sickness benefit cases within 8 weeks after the first day of work incapacity and thereafter every 8th week. The follow‐up assessment should be based on updated medical, social, and vocational information. The sick‐listed individual can be called in for a personal interview if the case manager considers this necessary. At the interview, the case manager may advise the sick‐listed person about contacting the employer, possibilities for partial work resumption, modification of job demands, job counselling, and possibilities for vocational rehabilitation. |
|
| Outcomes | Sickness absence: administrative data used Cumulative duration of sickness absence: cumulative sickness absence hours during the 12‐month follow‐up period. Mean (SD) WI = 656.6 (565.2), UC = 997.3 (668.8). Pain: pain intensity during the last month, measured with the ÖMPSQ. Mean improvement at 12 months' follow‐up (SD) WI = ‐3.59 (2.2), UC = ‐2.46 (3.3). Functional disability: OLBPDQ. Mean improvement during 12 months' follow‐up (SD) WI = 16.23 (15.0), UC = 8.96 (20.4). Costs: direct intervention costs, direct medical costs (average outpatient treatment costs), and indirect costs (average productivity loss). Societal perspective. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation protocol without stratification was computer generated prior to the start of the study and was undertaken by an independent IT assistant (p. 83, Bültmann 2009a) |
| Allocation concealment (selection bias) | Unclear risk | Unclear. Not reported clearly |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "It was not possible to blind participants and interdisciplinary team members for the allocated intervention." (p. 91, Bültmann 2009a) |
| Blinding of outcome assessment (detection bias) Sickness absence outcomes | Low risk | Primary outcome: "administrative data on cumulative sickness absence hours was the primary outcome in this study" (p. 84, Bültmann 2009a). Secondary outcomes: "information on work status was obtained from the Danish National Health Insurance Service Registry. Information on pain intensity and functional disability was obtained by self‐report questionnaires." (p. 84, Bültmann 2009a). |
| Blinding of outcome assessment (detection bias) Health‐related outcomes | High risk | Secondary outcomes are self‐reported, therefore secondary outcomes were assigned high risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome: none because data retrieved from administrative database. Secondary outcomes: at 12 months' follow‐up, 70.77% of all workers had completed the questionnaire. |
| Selective reporting (reporting bias) | Low risk | The results from all important prespecified outcomes (in methods) have been adequately reported in the published report of the trial (judged on basis of Bültmann 2009a) |
| Other bias | Low risk | "The analysis was conducted on an intention‐to‐treat basis" (p. 85, Bültmann 2009a). "Only minor differences were observed between the CTWR group (work intervention) and the CCM group (usual care)." (p. 86, Bültmann 2009a). No other concerns about bias were identified for this study |
Feuerstein 2003.
| Methods | RCT, multicentre, in 10 metropolitan areas in the United States. Randomisation: level worker. "Randomly assigned". Recruitment: WRUED claimants were sent a letter from the Medical Director of the US Department of Labor's Office of Workers' Compensation Programs inviting them to participate in the study. Duration recruitment: March 1999 to December 2000. Follow‐up: 12‐month sickness absence, 16‐month self‐reported outcomes. | |
| Participants | 205 were randomised (work intervention: 96; usual care: 100). Inclusion criteria: age 18 to 65 years, accepted single WRUED‐related workers' compensation claim, no past claims/cases in the previous 2 years, claim accepted and adjudicated as work related within 90 days of filing, and still out of work or on modified duty at the time of claim adjudication, and at least 1 WRUED from the following ICD‐9 categories: mononeuritis, enthesopathies, tendon disorder, soft tissue, nerve root and plexus, cervical disorders, osteoarthrosis, and muscle/ligament/fascia disorders. Type of disability: musculoskeletal (diagnosis ICD‐9). Duration of absence prior to randomisation: a minimum of 90 days. | |
| Interventions | WI Stakeholders involved: worker, case manager, supervisor, injury company specialist, medical providers, claims examiner. Standardised treatment: quality medical case management and initial interview focused on domains that may affect injury recovery and RTW. Development of case management plan and active problem‐solving to overcome potential RTW barriers. Work site ergonomic assessment to identify ergonomic factors that may contribute to risk of re‐injury or delay of RTW and consequently providing ergonomic accommodations. Preventing re‐injury and follow‐up by increasing the claimants self efficacy for monitoring and preventing future symptoms. UC Stakeholders involved: worker, case manager. Standardised treatment: Usual case management in which services often are limited to monitoring of the claims process and surveillance of medical treatment. Traditional case management usually fails to properly address ergonomic and psychosocial factors shown to be risk factors for WRUEDs and their associated disability. | |
| Outcomes | Sickness absence: administrative data used.
Time until first RTW: number of days from the cleaned‐up initial evaluation date and the truncated cleaned‐up RTW date. Median WI = 21 weeks, UC = 23.1 weeks, HR = 1.09.
Functional status: UEFL. Mean at 16 months (SD) WI = 4.82 (2.6), UC = 5.31 (2.5). General health status: SF‐12. General distress at 16 months: Mean (SD) WI = 49.1 (12.8), UC = 43.0 (11.8). Physical health at 16 months: Mean (SD) WI = 36.5 (9.2), UC = 34.7 (9.1). Symptoms: carpal tunnel symptom severity scale. Mean at 16 months (SD) WI = 2.6 (0.7), UC = 2.9 (0.8). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Creation of a spreadsheet using a random‐number generator. (Information gathered from personal contact with authors.) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) Sickness absence outcomes | Low risk | Primary outcomes: "RTW was determined using administrative data extracted from computerized operational management systems used by the US Department of Labor’s Office of Workers’ Compensation Programs to administer workers’ compensation benefits to federal employees." (p. 806. Feuerstein 2003) |
| Blinding of outcome assessment (detection bias) Health‐related outcomes | High risk | Secondary outcomes are self‐reported, therefore secondary outcomes were assigned high risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome: none because data retrieved from administrative databases. Secondary outcomes: 20% at 4 months and 37% at 16 months. (Information gathered from personal contact with authors.) Therefore for the secondary outcomes 'no' is assigned. |
| Selective reporting (reporting bias) | High risk | Data about the RTW outcome are not published yet |
| Other bias | High risk | In the analysis we received from the authors, less participants were presented than were included in the study, probably due to a lack of complete baseline self‐report data of several workers. We obtained this information from personal contact with the authors. "After excluding those persons with unavailable RTW data, who were on limited/restricted duty, or RTW dates that preceded the postintervention assessment (i.e., before the 4‐month standardized time allotted for case management services), 61 individuals were examined in regression analyses for time until RTW." (p. 808, Feuerstein 2003) |
Hees 2012a.
| Methods | RCT, multicentre, the Netherlands. Randomisation: level worker. The randomisation was conducted by an independent research assistant, who used a computerized program based on the minimisation‐randomisation procedure. Recruitment: occupational physicians present potential study participants for a telephone screening, where the inclusion and exclusion criteria are globally assessed by an independent psychiatrist. Next, potentially eligible participants receive a standard 3‐hour psychiatric intake; in addition, a structured clinical interview for DSM‐IV disorders is administered. After the psychiatric intake, eligible participants are asked to sign an informed consent form. Duration recruitment: December 2007 to October 2009 Follow‐up: 18 months |
|
| Participants | 117 were randomised (WI 78; UC 39). Inclusion criteria: aged 18 to 65 years, diagnosed with a major depressive disorder according to DSM‐IV criteria, absent from work for at least 25% of their contract hours due to their depression, the duration of their depressive disorder had to be at least 3 months, or the duration of their sickness absence had to be at least 8 weeks, there had to be a relationship between the depressive disorder and the work situation. Exclusion criteria: severe alcohol or drug dependence, bipolar disorder, psychotic disorder, depression with psychotic characteristics, an indication of inpatient treatment. Type of disability: mental health (diagnosis by psychiatrist). Duration of absence prior to randomisation: at least 25% absenteeism due to the depressive disorder |
|
| Interventions | WI Stakeholders involved: worker, employer, occupational therapist, occupational physician Standardised treatment: occupational therapy consisted of 18 sessions (9 individual sessions, 8 group sessions, and a meeting with the employer), and was conducted by 2 experienced occupational therapists. During the intervention, the occupational therapist frequently communicated with the occupational physician and the treating psychiatric resident. UC Stakeholders involved: worker, psychiatrist Standardised treatment: care as usual consists of treatment by psychiatric residents in the outpatient clinic of the Mood Disorders department at the Academic Medical Center according to a treatment protocol consistent with the APA guidelines. Visits consist of clinical management, including psycho‐education, supportive therapy, and cognitive behavioral interventions. Therapies are supervised by an experienced senior psychiatrist on a weekly basis. Pharmacotherapy is started. |
|
| Outcomes | Sickness absence: self‐reported data used. Time until lasting RTW: working the full number of contract hours in own or other work for at least 4 weeks, without partial or full recurrence. Median WI = 361 days, UC = 405 days, HR 0.93 (0.57 to 1.53) Time until partial RTW: working an increment of at least 5 hours (compared with hours worked at baseline), for at least 4 weeks without partial or full recurrence. Median WI = 80 days, UC = 166 days, HR 0.72 (0.44 to 1.11) Depression: HRSD. Mean at 12 months (SD) WI = 7.1 (6.7), UC = 9.6 (7.8) Depression: IDS‐SR. Mean at 12 months (SD) WI = 22.4 (13.1), UC = 24.6 (15.1) Health‐related functioning: MOS‐SF36: subscale Mental health Mean at 12 months (SD) WI = 61.7 (18.6), UC = 57.0 (22.5) Health‐related functioning: MOS‐SF36: subscale Role limitations due to emotional problems Mean at 12 months (SD) WI = 62.1 (34.5), UC = 54.4 (37.4) Health‐related functioning: MOS‐SF36: subscale Role limitations due to physical problems Mean at 12 months (SD) WI = 63.9 (34.1), UC = 62.8 (36.1) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was conducted by an independent research assistant, who uses a computerised program based on the minimisation‐randomisation procedure" (p. 5, Hees 2010) |
| Allocation concealment (selection bias) | Low risk | "Randomisation was conducted by an independent research assistant" (p. 5, Hees 2010) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) Sickness absence outcomes | High risk | "Data are derived from diaries that patients keep on a weekly basis during the 18‐month study period" (p. 5, Hees 2010) |
| Blinding of outcome assessment (detection bias) Health‐related outcomes | High risk | "Data are derived from diaries that patients keep on a weekly basis during the 18‐month study period" (p. 5, Hees 2010) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 out of 117 workers were lost to follow‐up (p. 4, Hees 2012b) |
| Selective reporting (reporting bias) | Unclear risk | Data about neurocognitive functioning and costs are not published yet |
| Other bias | High risk | "The treatment groups were comparable at baseline, except for the number of contract hours, WLQ output scale, and HRSD scores" (p. 3, Hees 2012b) |
Lambeek 2010a.
| Methods | RCT, multicentre, the Netherlands. Randomisation: level worker. An independent statistician performs randomisation, using a computer‐generated random‐sequence table. Recruitment: workers with low back pain who had visited one of the participating hospitals received a letter from their medical specialist informing them about the trial. Duration recruitment: November 2005 to April 2007. Follow‐up: 12 months. |
|
| Participants | 134 were randomised (work intervention: 66; usual care: 68). Inclusion criteria: adults aged 18 to 65 with low back pain who had visited an outpatient clinic in 1 of the participating hospitals, had low back pain for more than 12 weeks, were in paid work for at least 8 hours a week, and were absent or partially absent from work. Exclusion criteria: people who had been absent from work for more than 2 years; had worked temporarily for an employment agency without detachment; had specific low back pain due to infection, tumour, osteoporosis, rheumatoid arthritis, fracture, or inflammatory process; had undergone lumbar spine surgery in the past 6 weeks or had to undergo surgery or invasive examinations within 3 months; had a serious psychiatric or cardiovascular illness; were pregnant; or were engaged in a lawsuit against their employer. Type of disability: musculoskeletal (diagnosis by medical specialist). Duration of absence prior to randomisation: 3 to 24 months. |
|
| Interventions | WI Stakeholders involved: clinical occupational physician, medical specialist, occupational therapist, physiotherapist, injured worker, supervisor. Standardised treatment: the overall aim of the integrated care was to restore occupational functioning and achieve lasting RTW for workers in their own job or similar work, and not to reduce pain. The integrated care was coordinated by a clinical occupational physician and consisted of a workplace intervention based on participatory ergonomics and a graded activity program, which is a time‐contingent program based on cognitive behavioural principles. UC Stakeholders involved: occupational physician, medical specialist, general practitioner, injured worker. Standardised treatment: workers allocated to the usual‐care group received the usual treatment from their medical specialist, occupational physician, general practitioner, and/or allied health professionals. |
|
| Outcomes | Sickness absence: administrative data used. Time until lasting RTW: duration of sick leave due to low back pain in calendar days from the day of randomisation until full RTW in own or other work with equal earnings for at least 4 weeks without recurrence, partial or full. Median duration WI = 88 days, UC = 208 days, HR 1.9 (1.18 to 2.76). Cumulative duration of sickness absence: median number of days of sick leave (SD) WI = 129.44 (14.52), UC = 197.63 (15.68). Functional status: Roland‐Morris Disability Questionnaire. Mean improvement (SE) WI = 7.16 (0.71), UC = 4.43 (0.72). Pain: VAS. Mean improvement (SE) WI = 1.64 (0.35), UC = 1.85 (0.36). Costs: direct medical costs, direct intervention costs, indirect costs (productivity losses and absenteeism from work). Societal perspective. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An independent statistician performs randomisation, using a computer‐generated random‐sequence table." (p. 3, Lambeek 2007) |
| Allocation concealment (selection bias) | Low risk | "The researcher will prepare for each stratum opaque, sequentially numbered, and sealed coded envelopes, with either a note for the work intervention or a note for the usual care group." (p. 3, Lambeek 2007) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "It was not possible to blind the patients for treatment allocation." (p. 3, Lambeek 2010b) |
| Blinding of outcome assessment (detection bias) Sickness absence outcomes | Low risk | Primary outcomes: "data on sick leave were collected from the database of the occupational health services." (p. 2, Lambeek 2010b) |
| Blinding of outcome assessment (detection bias) Health‐related outcomes | High risk | Secondary outcomes are self‐reported, therefore secondary outcomes were assigned high risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Data on sick leave were completed for all patients at baseline, and for 93% of the patients during the 12 months follow‐up. Follow‐up data on secondary outcomes after 12 months were incomplete for 17 patients (13%)." (p. 3, Lambeek 2010b) |
| Selective reporting (reporting bias) | Low risk | The results from all prespecified outcomes (in published study protocol) have been adequately reported in the published report of the trial (judged on basis of Lambeek 2007, Lambeek 2010c, and Lambeek 2010b) |
| Other bias | Low risk | "All analyses were done according to the intention to treat principle." (p. 3, Lambeek 2010b). "Differences in baseline characteristics between the groups were non‐significant." (p. 4, Lambeek 2010b). No other concerns about bias were identified for this study |
Loisel 1997a.
| Methods | RCT, multicentre, Canada, Quebec. Randomisation: cluster randomisation, level workplace. Randomisation was carried out during a meeting of the oversight committee. Recruitment: the management of the participating workplaces identified workers filing claims for back pain and were compared with those in the workers' compensation board master files. After 4 weeks of absence from work (or assignment to light duties) had been accumulated during 1 year, the worker and attending physician were offered the opportunity to participate in the study. Duration recruitment: 1 September 1991 to 31 December 1993. Follow‐up: 12 months for RTW; 5, 1 to 7 years for costs. | |
| Participants | 130 were randomised (after randomisation, 14 workers were excluded because they did not meet the inclusion criteria, and 12 did not respond to any follow‐up visit; work intervention: 47; usual care: 57).
Inclusion criteria: thoracic or lumbar back pain incurred at work that had caused an absence from work (or an assignment to light duties) for more than 4 weeks and less than 3 months, age 18 to 65 years, and back pain accepted for compensation by the Quebec workers' compensation board.
Exclusion criteria: pregnant workers, workers with spinal fracture, significant degenerative spinal disease (spondylolisthesis, grade 2 or more), a non‐mechanical spinal disease (tumour or infection), major comorbid condition that might limit participation.
Type of disability: musculoskeletal (accepted for compensation by workers' compensation board). Duration of absence prior to randomisation: 4 weeks to 3 months. |
|
| Interventions | WI Stakeholders involved: worker, occupational physician, ergonomist, representatives union, representatives management, supervisor/employer, medical specialist (general practitioner, coworkers). Standardised treatment: visit to occupational physician and participatory ergonomics evaluation conducted by an ergonomist. Occupational physician could recommend investigation or treatment or set up light duties to facilitate a return to usual tasks. Ergonomic intervention consists of work site evaluation to determine the need for job modifications, observation of worker's tasks, meeting for specific ergonomic "diagnosis", submission of precise solutions to employer. UC Stakeholders involved: worker, physician (other treatment providers). Treatment: treatment from attending physician, who was at liberty to prescribe any test, treatment, or referral to a specialist for care. | |
| Outcomes | Sickness absence: administrative data used.
Time until first RTW: duration of absence from regular work. Median WI = 67 days, UC = 131 days, HR = 1.91 (1.18 to 3.1).
Other definition: duration of absence from any work (regular work or light duties).
Functional status: Oswestry questionnaire. Mean at 12 months (SD) WI = 14.4 (14.3), UC = 22.1 (19). Pain: VAS. Mean at 12 months (SD) WI = 14.6 (15.2), UC = 21.6 (19.1). Costs: direct medical costs (usual healthcare costs), direct intervention costs, and indirect costs (income replacement costs). Insurance provider perspective. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported for workplace intervention |
| Allocation concealment (selection bias) | Low risk | Adequate. "Randomization was carried out during a meeting of the oversight committee (see Organization of the Study, later in the article)." (Loisel 1997b) "The conduct of the study was overseen by a committee including investigators from the University of Sherbrooke, responsible for developing and implementing the study; investigators from McGill University, responsible for its evaluation; and representatives from the employers, the unions, and the workers compensation board, responsible for ensuring participation. The McGill evaluation team had no contact with the study site, the work sites, or the participants. It organized the randomisation process and was responsible for data analysis." (Loisel 1997b) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) Sickness absence outcomes | Low risk | Primary outcomes: return to regular work or not was collected with a question to the worker by the physician making the assessment (3, 6, 9, 12 months after work cessation) and blinded to the randomisation status. (Information gathered from personal contact with authors.) |
| Blinding of outcome assessment (detection bias) Health‐related outcomes | High risk | Secondary outcomes are self‐reported, therefore secondary outcomes were assigned high risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary and secondary outcomes: 26 workers (not specified for each group). (Loisel 1997b) |
| Selective reporting (reporting bias) | Low risk | The results from all important prespecified outcomes (in methods) have been adequately reported in the published report of the trial (judged on basis of Loisel 1997) |
| Other bias | High risk | "Fourteen out of the 130 randomised workers (11%) failed to meet the inclusion criteria (non cases). This retrospective ineligibility was because of premature inclusion (less than 28 days of absence: 2 cases, a clerical error) or late inclusion (more than 90 days of absence: 12 cases, late declaration by the employer) of the patients in the study. These cases were distributed in the four randomisation groups." (Loisel 1997b) |
Noordik 2013a.
| Methods | RCT, multicentre, the Netherlands. Randomisation: level occupational physician. A restricted randomisation was performed with blocks of 4 occupational physicians. Recruitment: workers eligible according to the occupational physician were invited to participate. Duration recruitment: November 2006 to December 2007. Follow‐up: 12 months. |
|
| Participants | 160 were randomised (work intervention: 75; usual care: 85). Inclusion criteria: workers on sick leave due to common mental disorders for > 2 and < 8 weeks. Exclusion criteria: workers with a current psychiatric disorder at the moment of inclusion, workers with a primary somatic disorder, non‐Dutch speaking workers. Type of disability: mental health (common mental disorder, stress‐related, adjustment, anxiety, or depressive disorders). Duration of absence prior to randomisation: > 2 and < 8 weeks. |
|
| Interventions | WI Stakeholders involved: occupational physician, worker, supervisor. Standardised treatment: In the RTW‐E program, workers received care as usual and were gradually exposed in vivo to more demanding work situations structured by a hierarchy of tasks evoking increasing levels of anxiety, stress to anger. The RTW‐E program provided workers with several homework assignments aimed at preparing, executing, and evaluating an exposure‐based RTW plan. UC Stakeholders involved: occupational physician, worker. Standardised treatment: Care as usual aims to help workers regain control and rebuild social and occupational contacts and activities, according to the guidelines for common mental disorders. |
|
| Outcomes | Sickness absence: self‐reported. Time until lasting RTW: the number of calendar days from the first day of sick leave to the first day of full RTW. Full RTW was defined as the total number of contracted working hours per week lasting > 28 calendar days without a recurrence of sick leave. HR 0.55 (0.33 to 0.89). Symptoms: severity of symptoms of common mental disorders: measured by the 4DSQ. Distress at 12 months: Mean (SD) WI = 6.3 (6.0), UC = 7.3 (7.7) Depression at 12 months: Mean (SD) WI = 0.6 (1.5), UC = 0.9 (2.0) Anxiety at 12 months: Mean (SD) WI = 1.5 (2.4), UC = 1.6 (3.5) Somatisation at 12 months: Mean (SD) WI = 5.2 (5.0), UC = 6.2 (5.9) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We performed a restricted randomisation with blocks of four occupational physicians". (p. 4, Noordik 2009) |
| Allocation concealment (selection bias) | Low risk | "Every time four OPs presented themselves to researcher 1, these were randomised by researcher 2, concealed from researcher 1. After randomisation, researcher 2 informed researcher 1 about the allocation of every OP and saved the randomisation file." (p. 4, Noordik 2009) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Patients were blinded to the intervention, as OPs were instructed not to inform patients about the content of their counselling. Blinding OPs to the intervention was not feasible." (p. 3, Noordik 2009) |
| Blinding of outcome assessment (detection bias) Sickness absence outcomes | High risk | "The calculated time‐to‐full RTW was based on workers' diaries and OP medical records." (p. 146, Noordik 2013b) |
| Blinding of outcome assessment (detection bias) Health‐related outcomes | High risk | "All participants were asked to complete a baseline survey and follow‐up surveys at 3, 6, 9, and 12 months". (p. 146, Noordik 2013b) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "The proportion of workers lost during follow‐up was 34% and 28% in the RTW‐E and CAU groups. Analyses of the primary outcome were based on worker’s diaries and medical records and could be performed for 63 (18% lost to FU) and 80 (11% lost to FU) workers in the RTW‐E and CAU groups." (p. 147, Noordik 2013b) |
| Selective reporting (reporting bias) | Low risk | The results from all prespecified outcomes (in published study protocol) have been adequately reported in the published report of the trial (judged on basis of Noordik 2009 and Noordik 2013b) |
| Other bias | Low risk | We performed an intention‐to‐treat analysis. (p. 147, Noordik 2013b) All participants were asked to complete a baseline survey and follow‐up surveys at 3, 6, 9, and 12 months after the first day of sick leave. (p. 146, Noordik 2013b) The characteristics of the workers and OP did not differ significantly between both groups. (p. 147, Noordik 2013b) |
Tamminga 2013.
| Methods | RCT, multicentre, the Netherlands. Randomisation: the researcher will carry out randomisation using the computerised randomisation program ALEA. Recruitment: the treating physician or nurse will inform the cancer patients about the study when they visit the hospital to discuss their treatment plans. Duration recruitment: May 2009 to December 2010. Follow‐up: 24 months |
|
| Participants | 133 were randomised (work intervention: 65; usual care: 68). Inclusion criteria: primary diagnosis of cancer, treated with curative intent, employed at the time of diagnosis, sick listed, between 18 and 60 years old. Exclusion criteria: not able to speak, read, or write Dutch; severe mental disorders or other severe comorbidity; primary diagnosis of cancer was made more than 2 months ago; primary treatment at another hospital; primary diagnosis of testicular cancer, non‐melanoma skin cancer, or melanoma skin cancer. Type of disability: cancer Duration of absence prior to randomisation: not specified. |
|
| Interventions | WI Stakeholders involved: treating physician, occupational physician, patient, supervisor, oncology nurse/medical social worker. Standardised treatment: the intervention involves patient education and support at the hospital and the improvement of the communication between the treating physician and the occupational physician. In addition, a meeting with the patient and the supervisor to make a concrete gradual RTW plan was organised by the occupational physician. UC Stakeholders involved: treating physician, occupational physician, patient. Standardised treatment: usual care according to the guidelines of the Dutch Association of Occupational Physicians. |
|
| Outcomes | Sickness absence data: self‐reported. Time until lasting RTW: the number of calendar days between the first day of sick leave and the first day at work (either part time or full time) that was sustained for at least 4 weeks. Median time (range) until partial RTW WI = 194 days (14 to 435), UC = 192 days (82 to 465). Median time (range) until full RTW WI = 283 days (25 to 394), UC = 239 (77 to 454). Overall work productivity: measured by the WLQ; mean (SD) WI = 29 (15), UC = 27 (16). Overall work ability: measured by the WAI; mean (SD) WI = 6 (2), UC = 7 (2). Quality of life: measured with the SF‐36: Physical functioning: mean (SD) WI = 81 (16), UC = 79 (20) Role‐physical: mean (SD) WI = 47 (40), UC = 61 (41) Vitality: mean (SD) WI = 59 (19), UC = 56 (16) General health: mean (SD) WI = 64 (17), UC = 70 (19) Social functioning: mean (SD) WI = 75 (20), UC = 78 (20) Role‐emotional: mean (SD) WI = 64 (42), UC = 71 (40) Mental health: mean (SD) WI = 77 (15), UC = 72 (15) Pain: mean (SD) WI = 75 (21), UC = 76 (17) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The researcher will carry out randomisation using the computerised randomisation program ALEA." (p. 4, Tamminga 2010) |
| Allocation concealment (selection bias) | Unclear risk | Not reported clearly. "Allocation of each patient is definite in such a way that allocation concealment is not possible." (p. 4, Tamminga 2010) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Patients and providers were immediately informed of the allocation as it was impossible to conceal allocation for this intervention." (p. 2, Tamminga 2012) |
| Blinding of outcome assessment (detection bias) Sickness absence outcomes | High risk | "The primary and secondary outcomes will be assessed at baseline and at 6, 12, 18 and 24 months after baseline. Participants will fill out the baseline questionnaires directly after signing the informed consent forms." (p. 6, Tamminga 2010) |
| Blinding of outcome assessment (detection bias) Health‐related outcomes | High risk | "The primary and secondary outcomes will be assessed at baseline and at 6, 12, 18 and 24 months after baseline. Participants will fill out the baseline questionnaires directly after signing the informed consent forms." (p. 6, Tamminga 2010) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The response rate at 12 months of follow‐up was 96% for the outcome of return to work and was 81% for the outcome of quality of life and secondary outcomes." (p. 181, Tamminga 2012) |
| Selective reporting (reporting bias) | Low risk | The results from all prespecified outcomes (in published study protocol) have been adequately reported in the published report of the trial (judged on basis of Tamminga 2010 and Tamminga 2012). |
| Other bias | Low risk | "All analyses will be performed according to the intention‐to‐treat principle." (p. 7, Tamminga 2010). "No statistically significant differences between the intervention group and the control group on any of the socio‐demographic or prognostic characteristics measured at baseline or any medical characteristics measured at follow‐up were identified." (p. 182‐183, Tamminga 2012). No other concerns about bias were identified for this study |
van Oostrom 2010a.
| Methods | RCT, multicentre, the Netherlands Randomisation: an independent statistician prepared the randomisation scheme by using computer‐generated randomisation. Recruitment: all employees sick listed for more than 1 week received a letter from their occupational physician together with a screening questionnaire. Duration recruitment: April 2006 to May 2008 Follow‐up: 12 months |
|
| Participants | 145 were randomised (work intervention: 73; usual care: 72). Inclusion criteria: employees on sick leave from regular work for 2 to 8 weeks, distress based on 4DSQ. Exclusion criteria: a conflict between the employee and the employer with legal involvement, working less than 12 hours a week, pregnancy, any other episode of sick leave within 1 month before the current episode, inability to complete questionnaires written in the Dutch language. Type of disability: mental health (distress, based on 4DSQ) Duration of absence prior to randomisation: 2 to 8 weeks. |
|
| Interventions | WI Stakeholders involved: worker, supervisor, RTW coordinator, occupational physician Standardised treatment: the participatory workplace intervention is a stepwise process involving the sick‐listed employee and their supervisor, aimed at reducing obstacles for RTW by reaching consensus about an action plan for RTW. UC Stakeholders involved: occupational physician, worker, supervisor. Standardised treatment: usual care from the occupational physician according to the evidence‐based guideline of the Dutch Association of Occupational Physicians published in 2000 and updated in 2007. This guideline aims to facilitate optimal functioning of employees with mental health problems and to prevent long‐term sick leave and frequent recurrences. |
|
| Outcomes | Sickness absence: administrative data used. Time until lasting RTW: duration of sick leave with distress in calendar days from the day of randomisation until full RTW to the employee's previous or another position with equal earnings, for at least 4 weeks of full RTW without (partial or full) recurrence. Median time WI = 96 days, UC = 104 days, HR 0.99 (0.70 to 1.39). Cumulative duration of sickness absence: total number of days of sick leave in the 12‐month follow‐up (SD) WI = 140.9 (13.2), UC = 141.0 (12.9). Symptoms: severity of symptoms of common mental disorders; measured by the 4DSQ. Distress at 12 months: mean (SD) WI = 9.00 (8.26), UC = 8.37 (8.07) Depression at 12 months: mean (SD) WI = 1.30 (2.40), UC = 1.04 (1.97) Anxiety at 12 months: mean (SD) WI = 2.55 (4.44), UC = 1.50 (3.05) Somatisation at 12 months: mean (SD) WI = 6.81 (6.21), UC = 7.10 (6.14) Costs: direct medical costs, direct intervention costs, indirect costs (productivity loss). Societal and employer perspective. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An independent statistician prepared the randomisation by using a computer‐generated randomisation." (p. 5, van Oostrom 2008) |
| Allocation concealment (selection bias) | Low risk | "The researchers prepared sealed envelopes before the start of the study containing either a referral to the workplace intervention or the usual care group." (p. 5, van Oostrom 2008) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The participants and occupational health professionals were not blinded for group assignment." (p. 597, van Oostrom 2010c) |
| Blinding of outcome assessment (detection bias) Sickness absence outcomes | Low risk | Primary outcome: "sick leave data were gathered from the continuous registration systems of the occupational health services after the 12‐month follow‐up." (p. 597, van Oostrom 2010c) |
| Blinding of outcome assessment (detection bias) Health‐related outcomes | High risk | Secondary outcomes are self‐reported, therefore secondary outcomes were assigned high risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Administrative sick leave data were available for all employees for the entire 12‐month follow‐up period. However, three employees left their company during the follow‐up period but registered their sick leave manually on a monthly calendar and returned the calendar to the researcher. Two employees in the usual care group withdrew from the study and so no follow‐up data regarding self‐reported outcomes could be collected for them." (p. 598, van Oostrom 2010c) |
| Selective reporting (reporting bias) | Low risk | The results from all prespecified outcomes (in published study protocol) have been adequately reported in the published report of the trial (judged on basis of van Oostrom 2008, van Oostrom 2010b, and van Oostrom 2010c) |
| Other bias | High risk | Intention to return to work despite symptoms differed between the workplace intervention group and usual‐care group. (p. 600, van Oostrom 2010c) |
Verbeek 2002a.
| Methods | RCT, multicentre, the Netherlands. Randomisation: randomisation, level worker. A sealed, opaque envelope containing a note including the allocation of the worker was opened. The assignment was based on block randomisation using a random‐numbers table. Recruitment: The administrative worker/occupational health nurse informed eligible participants about the project. Follow‐up: 12 months. | |
| Participants | 120 were randomised (work intervention: 61; usual care: 59).
Inclusion criteria: sick leave with low back pain for at least 10 days, working in department that approved participation, specified pain located below the scapula and above the gluteal fold, no consultation with occupational physician for low back pain in the past 3 months, no pregnancy, understanding of Dutch language.
Type of disability: musculoskeletal (based on diagnosis or self‐report is not described). Duration of absence prior to randomisation: a minimum of 10 days. |
|
| Interventions | WI Stakeholders involved: worker, occupational physician, supervisor (general practitioner, physiotherapist). Treatment: by an occupational physician who treated by a management guideline for low back pain consisting of early diagnostics and interventions aimed at removing barriers for return to normal work, advice about exercise and education, advice about modifying the work demands, evaluation. Optional interventions are conferring with general practitioner or physiotherapist and advising or consulting the employer. UC Stakeholders involved: worker, supervisor, general practitioner (occupational physician later). Standardised treatment: medical treatment by general practitioner, workers did not visit the occupational physician during the first 3 months of sick leave. | |
| Outcomes | Sickness absence: administrative data used.
Time until first RTW: time until RTW (working as many hours as before absence). Median WI = 51 days, UC = 62 days, HR = 1.3 Cumulative duration of sickness absence: number of days lost over a 1‐year period for all reasons and for low back pain. For low back pain mean (SD) WI = 114 (113) days, UC = 134 (126) days. For all reasons mean (SD) WI = 125 (110) days, UC = 145 (124) days. Recurrences: time until recurrence. Median WI = 262 days, UC = ?, HR = 2.4 (1.2 to 2.7). (In the original paper by Verbeek, there is a question mark for the recurrence value for usual care. See Table 2 of Verbeek 2002a.) Functional status: Roland‐Morris Disability Questionnaire. Mean at 3 months (SD) WI = 26 (24), UC = 32 (28). Mean at 12 months (SD) WI = 20 (22), UC = 21 (23). General health perception: Nottingham health profile. Pain at 3 months: mean (SD) WI = 26 (29), UC = 33 (32). Pain at 12 months: mean (SD) WI = 18 (26), UC = 22 (30). Physical mobility at 3 months: mean (SD) WI = 17 (17), UC = 23 (21). Physical mobility at 12 months: mean (SD) WI = 15 (20), UC = 19 (21). Lack of energy at 3 months: mean (SD) WI = 18 (28), UC = 22 (35). Lack of energy at 12 months: mean (SD) WI = 20 (34), UC = 10 (26). Emotional reactions at 3 months: mean (SD) WI = 11 (20), UC = 14 (24). Emotional reactions at 12 months: mean (SD) WI = 12 (23), UC = 8.7 (17). Social isolation at 3 months: mean (SD) WI = 5.5 (15), UC = 6.1 (17). Social isolation at 12 months: mean (SD) WI = 4.5 (15), UC = 3.4 (11). Sleep problems at 3 months: mean (SD) WI = 11 (20), UC = 15 (24). Sleep problems at 12 months: mean (SD) WI = 8.5 (19), UC = 8.5 (21). Pain: VAS Mean at 3 months (SD) WI = 24 (25), UC = 30 (26). Mean at 12 months (SD) WI = 31 (25), UC = 38 (26). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The assignment was based on block randomisation using a random numbers table." (p. 1844, Verbeek 2002b) |
| Allocation concealment (selection bias) | Low risk | Adequate. "The administrative worker or the occupational health nurse of the specific occupational health service informed eligible subjects about the project. After informed consent, a sealed opaque envelope containing a note was opened. This note stated whether the patient was assigned to the occupational physician (i.e., the intervention group) or to the reference group." (p. 1844, Verbeek 2002b) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "After informed consent, a sealed opaque envelope containing a note was opened. This note stated whether the patient was assigned to the occupational physician (i.e., the intervention group) or to the reference group." (p. 1844, Verbeek 2002a) |
| Blinding of outcome assessment (detection bias) Sickness absence outcomes | Low risk | Primary outcomes: "Sick leave data also were determined from computerized record systems of the occupational health services." (p. 1845, Verbeek 2002b) |
| Blinding of outcome assessment (detection bias) Health‐related outcomes | High risk | Secondary outcomes are self‐reported, therefore secondary outcomes were assigned high risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out: "In the intervention group, two patients did not visit the occupational physician" (p. 1844, Verbeek 2002b) Primary outcome: none because data retrieved from administrative database. Secondary outcomes: "The baseline questionnaire was returned by 117 patients (98%). After 3 months, 110 questionnaires were returned (92%), and 108 questionnaires were completed after 12 months (90%). The monthly questionnaires on health care utilization and sick leave during the first 3 months were returned by 110 patients (92%)." (p. 1846, Verbeek 2002b) |
| Selective reporting (reporting bias) | Low risk | The results from all important prespecified outcomes (in methods) have been adequately reported in the published report of the trial (judged on the basis of Verbeek 2002b) |
| Other bias | Low risk | "The analysis was performed on an intention‐to‐treat basis." (p. 1846, Verbeek 2002b). Groups were comparable at baseline regarding important prognostic factors (p. 1846, Verbeek 2002b). No other concerns about bias were identified for this study |
Vlasveld 2012a.
| Methods | RCT, the Netherlands. Randomisation: Computer‐generated randomisation took place at participant level by the research assistant, who informed the worker about the allocation. Recruitment: workers on sickness absence between 4 and 12 weeks whose absence was diagnosed by the occupational physician as due to mental disorders, were screened for depressive symptoms. Further screening by PHQ‐9, and MINI telephone interview by research assistant. Duration recruitment: 22 months in total. Follow‐up: 12 months. |
|
| Participants | 126 were randomised (work intervention: 65; usual care: 61) Inclusion criteria: employees who have been on sick leave for between 4 and 12 weeks. Workers who reach the cut‐off score of 10 for moderate to severe MDD on the PHQ‐9 will be contacted by the research assistant. If a worker meets the DSM‐IV criteria for MDD according to the MINI, the worker was included in the study. Exclusion criteria: suicidal, psychotic, or with a primary diagnosis of substance abuse or dependence, as assessed by the MINI interview; insufficient command of the Dutch language to fill in the questionnaires; pregnancy; workers with a legal involvement against their employer, e.g. due to a conflict at work. Type of disability: mental health (major depressive disorder) Duration of absence prior to randomisation: 4 to 12 weeks |
|
| Interventions | WI Stakeholders involved: worker, OP‐CM, employer, psychiatrist. Standardised treatment: collaborative care was applied by the OP‐CM, supported by a web‐based tracking system and a consultant psychiatrist. Treatment included problem‐solving treatment, manual guided self help, a workplace intervention, and antidepressant medication. UC Stakeholders involved: occupational physician, worker. Standardised treatment: usual care is protocolled according to the OP guidelines of the Dutch Board for Occupational Medicine. As there is considerable variation in the usual care that is provided for people with MDD, the actual care that is provided in the usual care group (e.g. medication and number of contacts with physicians) will be assessed by questionnaires. |
|
| Outcomes | Sickness absence: administrative data used. Time until lasting RTW: duration of sickness absence due to MDD in calendar days, from the day of randomisation until full RTW for at least 4 weeks without partial or full recurrence. The median duration until lasting, full RTW was 180 days for the workplace intervention group, and 199 days in the usual‐care group. Cumulative duration of sickness absence: median number of sickness absence days in the entire follow‐up period WI = 196 days, UC = 199 days. Depressive symptoms: mean (SD) at 12 months' follow‐up, WI = 7.7 (5.8), UC = 5.9 (7.7). Costs: direct medical costs, direct intervention costs, and indirect costs (absence). Societal perspective. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation took place at participant level." (p. 79, Vlasveld 2012b) |
| Allocation concealment (selection bias) | Low risk | "While assessing eligibility for the study, both the research assistant and the participant were blinded for the allocation." (p. 79, Vlasveld 2012b) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Then, the participant was informed about the computer generated allocation status by the research assistant." (p. 224, Vlasveld 2012c) |
| Blinding of outcome assessment (detection bias) Sickness absence outcomes | Low risk | Primary outcome: "sickness absence data were derived from the register of the occupational health service one year after randomisation." (p. 80, Vlasveld 2012b) |
| Blinding of outcome assessment (detection bias) Health‐related outcomes | High risk | Secondary outcomes are self‐reported, therefore secondary outcomes were assigned high risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome: none, retrieved from administrative database. Secondary outcomes: "With regard to the self‐report questionnaires, the loss to follow‐up rates at 3, 6, 9 and 12 months were respectively 22.2%, 28.6%, 33.3% and 41.3%." (p. 81, Vlasveld 2012b). Due to the loss to follow‐up for the questionnaires, 'no' was assigned to the secondary outcomes |
| Selective reporting (reporting bias) | High risk | Data about the IDS‐SR, SF‐36, and pain are not published yet |
| Other bias | Low risk | "Analyses were performed according to the intention‐to‐treat principle." (p. 80, Vlasveld 2012b). Only slight differences between work intervention group and usual‐care grouping important prognostic factors (p. 83, Vlasveld 2012b). No other concerns about bias were identified for this study |
4DSQ: Four‐Dimensional Symptom Questionnaire APA guidelines: American Psychiatric Association guidelines BM: behavioural medicine rehabilitation CAU: care as usual CBT: cognitive behavioural therapy CIDI: Composite International Diagnostic Interview DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition FU: follow‐up HR: hazard ratio HRSD: Hamilton Rating Scale for Depression ICD: International Statistical Classification of Diseases IDS‐SR: Inventory of Depressive Symptomatology‐self‐report IT assistant: information technology assistant MDD: major depressive disorder MINI: Mini‐International Neuropsychiatric Interview MOS‐SF36: Medical Outcomes Study‐36 Item Short Form Survey Instrument MSD: musculoskeletal disorder OLBPDQ: Oswestry Low Back Pain Disability Questionnaire ÖMPSQ: Örebro Musculoskeletal Pain Screening Questionnaire OP: occupational physician OP‐CM: occupational physician care manager PHQ‐9: 9‐item depression scale of the Patient Health Questionnaire PT: behaviour‐oriented physical therapy RCT: randomised controlled trial RTW: return to work SD: standard deviation SE: standard error SF‐12: 12‐Item Short Form Health Survey SF‐36: 36‐Item Short Form Health Survey UC: usual care UEFL: Upper‐Extremity Functional Limitations Scale VAS: visual analogue scale WAI: Work Ability Index WI: work intervention WLQ: Work Limitations Questionnaire WRUED: work‐related upper‐extremity disorder
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bendix 1998 | Intervention did not fulfil inclusion criteria, no supervisor involved or work adaptations planned |
| Bethge 2010 | Not all workers were on sick leave at baseline, and intervention did not fulfil inclusion criteria |
| Beutel 2005 | Not all participants had a job at baseline |
| Bonde 2005 | Not all participants had a job at baseline |
| Brouwers 2006 | Intervention did not fulfil inclusion criteria, no supervisor involved or work adaptations planned |
| Cheng 2007 | Sickness absence outcome was measured as a dichotomous outcome only: RTW rate at 4‐week follow‐up |
| Eshoj 2001 | Intervention did not fulfil inclusion criteria, no supervisor involved or work adaptations planned. The sickness absence outcome was measured as dichotomous outcome only: vocational status (inactive employment as opposed to not inactive employment) at 12‐month follow‐up |
| Farzanfar 2011 | Intervention did not fulfil inclusion criteria, no supervisor involved or work adaptations planned |
| Haldorsen 1998 | Sickness absence outcome was measured as dichotomous outcome only: RTW rate at 12 months |
| Haldorsen 2002 | Workplace intervention was occasional |
| Hubbard 2013 | Intervention did not fulfil inclusion criteria, no supervisor involved or work adaptations planned |
| Jensen 2011 | Intervention did not fulfil inclusion criteria, no supervisor involved or work adaptations planned |
| Jensen 2013 | Intervention did not fulfil inclusion criteria, no supervisor involved or work adaptations planned |
| Jousset 2004 | Not all workers were on sick leave at baseline, and intervention did not fulfil inclusion criteria |
| Karjalainen 2003 | Not all workers were on sick leave at baseline |
| Lindh 1997 | Sickness absence outcome was measured as dichotomous outcome only: work status at 9‐month, 1‐, 3‐, and 5‐year follow‐up |
| Magnussen 2007 | Intervention did not fulfil inclusion criteria, group‐based and individual follow‐up focused on medical examination and assessment of work ability only |
| Martin 2013 | Intervention did not fulfil inclusion criteria, no supervisor involved |
| Meijer 2006 | Intervention did not fulfil inclusion criteria, no supervisor involved or work adaptations planned |
| Netterstrøm 2013 | Sickness absence outcome measured as dichotomous outcome only |
| Nilsson 1996 | Sickness absence outcome measured as dichotomous outcome only: rate of RTW each 6 months to 36 months' follow‐up |
| Nystuen 2003 | Intervention did not fulfil inclusion criteria, could be group‐ or individual‐based, and no supervisor was involved or work adaptations planned |
| Nystuen 2006 | Intervention did not fulfil inclusion criteria, could be group‐ or individual‐based, and no supervisor involved or work adaptations planned |
| Poulsen 2014 | Intervention did not fulfil inclusion criteria, no work adaptations planned |
| Rebergen 2009a | Intervention did not fulfil inclusion criteria, no supervisor involved or work adaptations planned |
| Reme 2011a | Not all workers were on sick leave at baseline |
| Rupp 1994 | Intervention did not fulfil inclusion criteria, no supervisor involved or work adaptations planned. Sickness absence outcome was measured as dichotomous outcome only: employment status |
| Scheel 2002 | Intervention did not fulfil inclusion criteria, no supervisor involved or work adaptations planned |
| Schene 2007 | The primary author of this paper notified us that the intervention did not comply with the definition of a workplace intervention. The standardised intervention protocol confirmed this |
| Stapelfeldt 2011 | Intervention did not fulfil inclusion criteria, no supervisor involved or work adaptations planned |
| Streibelt 2014 | Not all participants had a job at baseline |
| Tompa 2009 | Design was not a randomised controlled trial, not all workers were on sick leave at baseline |
| Van den Hout 2003 | The workplace intervention was part of both interventions in this study |
| Vermeulen 2011 | Not all participants had a job at baseline |
| Viikari‐Juntura 2012 | Not all workers were on sick leave at baseline |
| Volker 2013 | Intervention did not fulfil inclusion criteria, no supervisor involved or work adaptations planned |
| Vonk Noordegraaf 2012 | Not all workers were on sick leave at baseline. Workplace intervention was not offered structurally |
Differences between protocol and review
The differences between the protocol and the review are as follows.
We updated the 'Risk of bias' assessment to the current Cochrane tool.
We reported on pain and symptoms as additional secondary outcomes, since these are important outcomes for workers and care providers, and most studies presented results on these outcomes.
We provided a definition of a person of working age being between 18 and 65 years old.
The primary outcome of included studies no longer needs to be return‐to‐work, as we also included studies measuring cumulative duration of sickness absence.
We conducted an additional subgroup analysis not mentioned in the protocol in which we analysed the effectiveness of workplace interventions only, and the effectiveness of workplace interventions offered in combination with a cognitive behavioural or problem‐solving intervention.
In this update we prioritised time until first RTW as the most important outcome.
Contributions of authors
MvV conducted the study selection, quality assessment, data extraction, data analysis, and drafted the text.
SvO conducted the study selection, quality assessment, data extraction, and assisted in writing the review.
HdV was the third person for the quality assessment, provided advice for the data analysis, and assisted in writing the review.
CB was the third person for the study selection, quality assessment, and data extraction, and assisted in writing the review.
RLF and JA assisted in writing the review.
JA is the guarantor of this review.
Declarations of interest
Myrthe van Vlisteren: None known.
Sandra van Oostrom: I am an author on van Oostrom 2010a. I did not participate in deciding whether to include or exclude this study nor in extracting data from it or in assessing its risk of bias.
Henrica de Vet: I am an author on Anema/Steenstra 2007. I did not participate in deciding whether to include or exclude this study nor in extracting data from it or in assessing its risk of bias.
Renée‐Louise Franche: I am a private consultant in work disability prevention. I received CAD 800 for giving a keynote address on work disability prevention in a psychology conference in Banff in March 2013.
Cécile Boot: None known.
Johannes Anema: I am an author on Anema/Steenstra 2007, Lambeek 2010a, van Oostrom 2010a and Vlasveld 2012a. I did not participate in deciding whether to include or exclude these studies nor in extracting data or assessing their risk of bias. I am a consultant for Evalua Netherlands LtD that is selling a health checklist to large employers and insurance companies to improve work ability and reduce future work disability. I also own Evalua Netherlands LtD stocks. I regularly receive travel and accommodation costs and sometimes speaker fees to present research results, including those of this review. I supervise a PhD student funded by a grant from Instituut GAK. I have also received a grant and travel support from Instituut GAK. I am an editor of the Handbook of Work Disability that includes part of the review results. I hold a chair (0.4 FTE) endowed by the Dutch Workers Compensation board.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Anema/Steenstra 2007 {published and unpublished data}
- Anema JR, Steenstra IA, Bongers PM, Vet HCW, Knol DL, Loisel P, et al. Multidisciplinary rehabilitation for subacute low back pain: Graded activity or workplace intervention or both? A randomized controlled trial. Spine 2007;32(3):291‐8. [DOI] [PubMed] [Google Scholar]
- Anema JR, Steenstra IA, Urlings IJ, Bongers PM, Vroome EM, Mechelen W. Participatory ergonomics as a return‐to‐work intervention: a future challenge?. American Journal of Industrial Medicine 2003;44(3):273‐81. [DOI] [PubMed] [Google Scholar]
- Steenstra IA, Anema JR, Bongers PM, Vet HCW, Mechelen M. Cost effectiveness of a multi‐stage return to work program for workers on sick leave due to low back pain, design of a population based controlled trial. BMC Musculoskeletal Disorders 2003;21(4):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenstra IA, Anema JR, Tulder MW, Bongers PM, Vet HCW, Mechelen W. Economic evaluation of a multi‐stage return to work program for workers on sick‐leave due to low back pain. Journal of Occupational Rehabilitation 2006;16(4):557‐78. [DOI] [PubMed] [Google Scholar]
Arnetz 2003 {published data only}
- Arnetz BB, Sjögren B, Rydéhn B, Meisel R. Early workplace intervention for employees with musculoskeletal‐related absenteeism: a prospective controlled intervention study. Journal of Occupational and Environmental Medicine/American College of Occupational and Environmental Medicine 2003;45(5):499‐506. [DOI] [PubMed] [Google Scholar]
Blonk 2006 {published and unpublished data}
- Blonk RWB, Brenninkmeijer V, Lagerveld SE, Houtman ILD. Return to work: A comparison of two cognitive behavioural interventions in cases of work‐related psychological complaints among the self‐employed. Work & Stress 2006;20(2):129‐44. [Google Scholar]
Bültmann 2009a {published data only}
- Bültmann U, Sherson D, Olsen J, Hansen CL, Lund T, Kilsgaard J. Coordinated and tailored work rehabilitation: A randomized controlled trial with economic evaluation undertaken with workers on sick leave due to musculoskeletal disorders. Journal of Occupational Rehabilitation 2009;19:81‐93. [DOI] [PubMed] [Google Scholar]
Busch 2011 {published data only}
- Busch H, Bodin L, Bergström G, Jensen IB. Patterns of sickness absence a decade after pain‐related multidisciplinary rehabilitation. Pain 2011;152:1727‐33. [DOI] [PubMed] [Google Scholar]
- Jensen IB, Bergström G, Ljungquist T, Bodin L. A 3‐year follow‐up of a multidisciplinary rehabilitation programme for back and neck pain. Pain 2005;115:273‐83. [DOI] [PubMed] [Google Scholar]
Feuerstein 2003 {published and unpublished data}
- Feuerstein M, Huang GD, Ortiz JM, Shaw WS, Miller VI, Wood PM. Integrated case management for work‐related upper‐extremity disorders: impact of patient satisfaction on health and work status. Journal of Occupational and Environmental Health 2003;45(8):803‐12. [DOI] [PubMed] [Google Scholar]
- Shaw WS, Feuerstein M, Lincoln AE, Miller VI, Wood PM. Case management services for work related upper extremity disorders. American Association of Occupational Health Nurses Journal 2001;49(8):378‐89. [PubMed] [Google Scholar]
Hees 2012a {published data only}
- Hees HL, Koeter MWJ, Vries G, Ooteman W, Schene AH. Effectiveness of adjuvant occupational therapy in employees with depression: design of a randomized controlled trial. BMC Public Health 2010;10(558):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hees HL, Vries G, Koeter MWJ, Schene AH. Adjuvant occupational therapy improves long‐term depression recovery and return‐to‐work in good health in sick‐listed employees with major depression: results of a randomised controlled trial. Occupational and Environmental Medicine 2012;70(4):252‐60. [DOI] [PubMed] [Google Scholar]
Lambeek 2010a {published data only}
- Lambeek LC, Anema JR, Royen BJ, Buijs PC, Wuisman PI, Tulder MW, et al. Multidisciplinary outpatient care program for patients with chronic low back pain: design of a randomized controlled trial and cost‐effectiveness study. BMC Public Health 2007;7(254):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeek LC, Bosmans JE, Royen BJ, Tulder MW, Mechelen W, Anema JR. Effect of integrated care for sick listed patients with chronic low back pain: economic evaluation alongside a randomised controlled trial. BMJ 2010;341:c6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeek LC, Mechelen W, Knol DL, Loisel P, Anema JR. Randomised controlled trial of integrated care to reduce disability from chronic low back pain in working and private life. BMJ 2010;340:c1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loisel 1997a {published data only}
- Loisel P, Abenhaim L, Durand P, Esdaile JM, Suissa S, Gosselin L, et al. A population‐based, randomized clinical trial on back pain management. Spine 1997;22(24):2911‐8. [DOI] [PubMed] [Google Scholar]
- Loisel P, Durand P, Abenhaim L, Gosselin L, Simard R, Turcotte J, et al. Management of occupational back pain: The Sherbrooke model. Results of a pilot and feasibility study. Occupational and Environmental Medicine 1994;51(9):597‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loisel P, Durand P, Gosselin L, Simard R, Turcotte J, Abenhaim L, et al. La Clinique des Maux de Dos; une Modèle de Prise en Charge, en Prévention de la Chronicité. Montreal: L’Institut de Recherche en Santé et en Sécurité du Travail du Québec (IRSST), May 1996. [Google Scholar]
- Loisel P, Gosselin L, Durand P, Lemaire J, Poitras S, Abenhaim L. Implementation of a participatory ergonomics program in the rehabilitation of workers suffering from subacute back pain. Applied Ergonomics 2001;32(1):53‐60. [DOI] [PubMed] [Google Scholar]
- Loisel P, Lemaire J, Poitras S, Durand MJ, Champagne F, Stock S, et al. Cost‐benefit and cost‐effectiveness analysis of a disability prevention model for back pain management: a six year follow up study. Occupational and Environmental Medicine 2002;59(12):807‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noordik 2013a {published and unpublished data}
- Noordik E, Dijk FJ, Nieuwenhuijsen K, Klink JJL. Effectiveness and cost‐effectiveness of an exposure‐based return‐to‐work programme for patients on sick leave due to common mental disorders: design of a cluster‐randomized controlled trial. BMC Public Health 2009;9(140):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordik E, Klink JJ, Geskus RB, Boer MR, Dijk FJH, Nieuwenhuijsen K. Effectiveness of an exposure‐based return‐to‐work program for workers on sick leave due to common mental disorders: a cluster‐randomized controlled trial. Scandinavian Journal of Work, Environment & Health 2013;39(2):144‐54. [DOI] [PubMed] [Google Scholar]
Tamminga 2013 {published data only}
- Tamminga SJ. Enhancing Return to Work of Cancer Patients [PhD thesis]. Amsterdam, The Netherlands: Universiteit van Amsterdam, 2012. [Google Scholar]
- Tamminga SJ, Verbeek JH, Bos MM, Fons G, Kitzen JJ, Plaisier PW, et al. Effectiveness of a hospital‐based work support intervention for female cancer patients ‐ a multi‐centre randomised controlled trial. PLoS One 2013;8(5):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga SJ, Boer AG, Verbeek JH, Taskila T, Frings‐Dresen MH. Enhancing return‐to‐work in cancer patients, development of an intervention and design of a randomized controlled trial. BMC Cancer 2010;10:345‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Oostrom 2010a {published data only}
- Oostrom SH, Anema JR, Terluin B, Vet HCW, Knol DL, Mechelen W. Cost‐effectiveness of a workplace intervention for sick‐listed employees with common mental disorders: design of a randomized controlled trial. BMC Public Health 2008;8(12):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostrom SH, Heymans MW, Vet HCW, Tulder MW, Mechelen W, Anema JR. Economic evaluation of a workplace intervention for sick‐listed employees with distress. Occupational and Environmental Medicine 2010;67:603‐10. [DOI] [PubMed] [Google Scholar]
- Oostrom SH, Mechelen W, Terluin B, Vet HCW, Knol DL, Anema JR. A workplace intervention for sick‐listed employees with distress: results of a randomised controlled trial. Occupational and Environmental Medicine 2010;67:596‐602. [DOI] [PubMed] [Google Scholar]
Verbeek 2002a {published data only}
- Verbeek JH, Weide WE, Dijk FJ. Early occupational health management of patients with back pain: a randomized controlled trial. Spine 2002;27(17):1844‐51. [DOI] [PubMed] [Google Scholar]
- Weide WE, Verbeek JH, Dijk FJ. Relation between indicators for quality of occupational rehabilitation of employees with low back pain. Occupational and Environmental Medicine 1999;56:488‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vlasveld 2012a {published data only}
- Goorden M, Vlasveld MC, Anema JR, Mechelen W, Beekman ATF, Hoedeman R, et al. Cost‐utility analysis of a collaborative care intervention for major depressive disorder in an occupational healthcare setting. Submitted In press. [DOI] [PubMed]
- Vlasveld MC. Sickness and Return to Work in Workers With Major Depressive Disorder: The Netherlands Depression Initiative in the Occupational Healthcare Setting [PhD thesis]. Amsterdam, The Netherlands: Vrije Universiteit, 2012. [Google Scholar]
- Vlasveld MC, Anema JR, Beekman ATF, Mechelen W, Hoedeman R, Marwijk HWJ, et al. Multidisciplinary collaborative care for depressive disorder in the occupational health setting: design of a randomised controlled trial and cost‐effectiveness study. BMC Health Services Research 2008;8(99):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasveld MC, Feltz‐Cornelis CM, Ader HJ, Anema JR, Hoedeman R, Mechelen W, et al. Collaborative care for major depressive disorder in an occupational healthcare setting. British Journal of Psychiatry 2012;200:510‐1. [DOI] [PubMed] [Google Scholar]
- Vlasveld MC, Feltz‐Cornelis CM, Adre HJ, Anema JR, Hoedeman R, Mechelen W, et al. Collaborative care for sick‐listed workers with major depressive disorder: a randomised controlled trial from the Netherlands Depression Initiative aimed at return to work and depressive symptoms. Occupational and Environmental Medicine 2012;70(4):223‐30. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bendix 1998 {published data only}
- Bendix AF, Bendix T, Haestrup C, Busch E. A prospective, randomized 5‐year follow‐up study of functional restoration in chronic low back pain patients. European Spine Journal 1998;7(2):111‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendix AF, Bendix T, Ostenfeld S, Bush E, Andersen A. Active treatment programs for patients with chronic low back pain: A prospective, randomized, observer‐blinded study. European Spine Journal 1995;4(3):148‐52. [DOI] [PubMed] [Google Scholar]
Bethge 2010 {published data only}
- Bethge M, Herbold D, Trowitzsch L, Jacobi C. Return to work following work‐related orthopaedic rehabilitation: A cluster randomized trial. Rehabilitation 2010;49(1):2‐12. [DOI] [PubMed] [Google Scholar]
Beutel 2005 {published data only}
- Beutel ME, Zwerenz R, Bleichner F, Vorndran A, Gustson D, Knickenberg RJ. Vocational training integrated into inpatient psychosomatic rehabilitation ‐ Short and long‐term results from a controlled study. Disability and Rehabilitation 2005;27(15):891‐900. [DOI] [PubMed] [Google Scholar]
Bonde 2005 {published data only}
- Bonde JP, Rasmussen MS, Hjollund H, Svendsen SW, Kolstad HA, Jensen LD, et al. Occupational disorders and return to work: A randomized controlled study. Journal of Rehabilitation Medicine 2005;37(4):230‐5. [DOI] [PubMed] [Google Scholar]
Brouwers 2006 {published data only}
- Brouwers EP, Tiemens BG, Terluin B, Verhaak PF. Effectiveness of an intervention to reduce sickness absence in patients with emotional distress or minor mental disorders: a randomized controlled effectiveness trial. General Hospital Psychiatry 2006;28(3):223‐9. [DOI] [PubMed] [Google Scholar]
Cheng 2007 {published data only}
- Cheng AS, Hung LK. Randomized controlled trial of workplace‐based rehabilitation for work‐related rotator cuff disorder. Journal of Occupational Rehabilitation 2007;17(3):487‐503. [DOI] [PubMed] [Google Scholar]
Eshoj 2001 {published data only}
- Eshoj P, Tarp U, Nielsen CV. Effect of early vocational intervention in a rheumatological outpatient clinic ‐ A randomized study. International Journal of Rehabilitation Research 2001;24(4):291‐7. [DOI] [PubMed] [Google Scholar]
Farzanfar 2011 {published data only}
- Farzanfar R, Locke SE, Heeren TC, Stevens A, Vachon L, Nguyen MKT, et al. Workplace telecommunications technology to identify mental health disorders and facilitate self‐help or professional referrals. American Journal of Health Promotion 2011;25(3):207‐16. [DOI] [PubMed] [Google Scholar]
Haldorsen 1998 {published data only}
- Haldorsen EM, Kronholm K, Skouen JS, Ursin H. Multimodal cognitive behavioral treatment of patients sicklisted for musculoskeletal pain: a randomized controlled study. Scandinavian Journal of Rheumatology 1998;27(1):16‐25. [DOI] [PubMed] [Google Scholar]
Haldorsen 2002 {published data only}
- Haldorsen EM, Grasdal AL, Skouen JS, Risa AE, Kronholm K, Ursin H. Is there a right treatment for a particular patient group? Comparison of ordinary treatment, light multidisciplinary treatment, and extensive multidisciplinary treatment for long‐term sick‐listed employees with musculoskeletal pain. Pain 2002;95(1‐2):49‐63. [DOI] [PubMed] [Google Scholar]
Hubbard 2013 {published data only}
- Hubbard G, Gray NM, Ayansina D, Evans JMM, Kyle RG. Case management vocational rehabilitation for women with breast cancer after surgery: A feasibility study incorporating a pilot randomised controlled trial. Trials 2013;14(1):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2011 {published data only}
- Jensen C, Jensen OK, Christiansen DH, Nielsen CV. One‐year follow‐up in employees sick‐listed because of low back pain: randomized clinical trial comparing multidisciplinary and brief intervention. Spine 2011;36(15):1180‐9. [DOI] [PubMed] [Google Scholar]
Jensen 2013 {published data only}
- Jensen C, Nielsen CV, Jensen OK, Petersen KD. Cost‐effectiveness and cost‐benefit analyses of a multidisciplinary intervention compared with a brief intervention to facilitate return to work in sick‐listed patients with low back pain. Spine 2013;38(13):1059‐67. [DOI] [PubMed] [Google Scholar]
Jousset 2004 {published data only}
- Jousset N, Fanello S, Bontoux L, Dubus V, Billabert C, Vielle B, et al. Effects of functional restoration versus 3 hours per week physical therapy: a randomized controlled study. Spine 2004;29(5):487‐93. [DOI] [PubMed] [Google Scholar]
Karjalainen 2003 {published data only}
- Karjalainen K, Malmivaara A, Mutanen P, Roine R, Hurri H, Pohjolainen T. Mini‐intervention for subacute low back pain: two‐year follow‐up and modifiers of effectiveness. Spine 2004;29(10):1069‐76. [DOI] [PubMed] [Google Scholar]
- Karjalainen K, Malmivaara A, Pohjolainen T, Hurri H, Mutanen P, Rissanen P, et al. Mini‐intervention for subacute low back pain: A randomized controlled trial. Spine 2003;28(6):533‐40. [DOI] [PubMed] [Google Scholar]
Lindh 1997 {published data only}
- Lindh M, Lurie M, Sanne H. A randomized prospective study of vocational outcome in rehabilitation of patients with non‐specific musculoskeletal pain: A multidisciplinary approach to patients identified after 90 days of sick‐leave. Scandinavian Journal of Rehabilitation Medicine 1997;29(2):103‐12. [PubMed] [Google Scholar]
Magnussen 2007 {published data only}
- Magnussen L, Strand LI, Skouen JS, Eriksen HR. Motivating disability pensioners with back pain to return to work‐‐a randomized controlled trial. Journal of Rehabilitation Medicine 2007;39(1):81‐7. [DOI] [PubMed] [Google Scholar]
Martin 2013 {published data only}
- Martin MHT, Nielsen MB, Madsen IEH, Petersen SMA, Lange T, Rugulies R. Effectiveness of a coordinated and tailored return‐to‐work intervention for sickness absence beneficiaries with mental health problems. Journal of Occupational Rehabilitation 2013;23(4):621‐30. [DOI] [PubMed] [Google Scholar]
Meijer 2006 {published data only}
- Meijer EM, Sluiter JK, Heyma A, Sadiraj K, Frings‐Dresen MHW. Cost‐effectiveness of multidisciplinary treatment in sick‐listed patients with upper extremity musculoskeletal disorders: A randomized, controlled trial with one‐year follow‐up. International Archives of Occupational and Environmental Health 2006;79(8):654‐64. [DOI] [PubMed] [Google Scholar]
Netterstrøm 2013 {published data only}
- Netterstrøm B, Friebel L, Ladegaard Y. Effects of a multidisciplinary stress treatment programme on patient return to work and symptom reduction: results from a randomised, wait‐list controlled trial. Psychotherapy and Psychosomatics 2013;82(3):177‐86. [DOI] [PubMed] [Google Scholar]
Nilsson 1996 {published data only}
- Nilsson I, Buxhoeveden L. An attempt to work rehabilitation after long sick‐leave. Work 1996;7:183‐9. [DOI] [PubMed] [Google Scholar]
Nystuen 2003 {published data only}
- Nystuen P, Hagen KB. Feasibility and effectiveness of offering a solution‐focused follow‐up to employees with psychological problems or muscle skeletal pain: a randomised controlled trial. BMC Public Health 2003;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nystuen 2006 {published data only}
- Nystuen P, Hagen KB. Solution‐focused intervention for sick listed employees with psychological problems or muscle skeletal pain: A randomised controlled trial. BMC Public Health 2006;16(6):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poulsen 2014 {published data only}
- Poulsen OM, Aust B, Bjorner JB, Rugulies R, Hansen JV, Tverborgvik T, et al. Effect of the Danish return‐to‐work program on long‐term sickness absence: Results from a randomized controlled trial in three municipalities. Scandinavian Journal of Work, Environment & Health 2014;40(1):47‐56. [DOI] [PubMed] [Google Scholar]
Rebergen 2009a {published data only}
- Rebergen DS, Bruinvels DJ, Bezemer PD, Beek AJ, Mechelen W. Guideline‐based care of common mental disorders by occupational physicians (CO‐OP study): a randomized controlled trial. Journal of Occupational and Environmental Medicine 2009;51(3):305‐12. [DOI] [PubMed] [Google Scholar]
Reme 2011a {published data only}
- Reme SE, Tveito TH, Chalder T, Bjorkkjaer T, Indahl A, Brox JI, et al. Protocol for the cognitive interventions and nutritional supplements (CINS) trial: a randomized controlled multicenter trial of a brief intervention (BI) versus a BI plus cognitive behavioural treatment (CBT) versus nutritional supplements for patients with long‐lasting muscle and back pain. BMC Musculoskeletal Disorders 2011;12(152):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rupp 1994 {published data only}
- Rupp K, Bell SH, McManus LA. Design of the Project NetWork return‐to‐work experiment for persons with disabilities. Social Security Bulletin 1994;57(2):3‐20. [PubMed] [Google Scholar]
Scheel 2002 {published data only}
- Scheel IB, Birger Hagen K, Herrin J, Carling C, Oxman AD. Blind faith? The effects of promoting active sick leave for back pain patients: A cluster‐randomized controlled trial. Spine 2002;27(23):2734‐40. [DOI] [PubMed] [Google Scholar]
Schene 2007 {published data only}
- Schene AH, Koeter MWJ, Kikkert MJ, Swinkels JA, McCrone P. Adjuvant occupational therapy for work‐related major depression works: Randomized trial including economic evaluation. Psychological Medicine 2007;37(3):351‐62. [DOI] [PubMed] [Google Scholar]
Stapelfeldt 2011 {published data only}
- Stapelfeldt CM, Christiansen DH, Jensen OK, Nielsen CV, Petersen KD, Jensen C. Subgroup analyses on return to work in sick‐listed employees with low back pain in a randomised trial comparing brief and multidisciplinary intervention. BMC Musculoskeletal Disorders 2011;12(112):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Streibelt 2014 {published data only}
- Streibelt M, Bethge M. Effects of intensified work‐related multidisciplinary rehabilitation on occupational participation: a randomized‐controlled trial in patients with chronic musculoskeletal disorders. International Journal of Rehabilitation Research 2014;37(1):61‐6. [DOI] [PubMed] [Google Scholar]
Tompa 2009 {published data only}
- Tompa E, Dolinschi R, Laing A. An economic evaluation of a participatory ergonomics process in an auto parts manufacturer. Journal of Safety Research 2009;40(1):41‐7. [DOI] [PubMed] [Google Scholar]
Van den Hout 2003 {published data only}
- Hout JHC, Vlaeyen JWS, Heuts PHTG, Zijlema JHL, Wijnen JAG. Secondary prevention of work‐related disability in nonspecific low back pain: Does problem‐solving therapy help? A randomized clinical trial. Clinical Journal of Pain 2003;19(2):87‐96. [DOI] [PubMed] [Google Scholar]
Vermeulen 2011 {published data only}
- Vermeulen SJ, Anema JR, Schellart AJM, Knol DL, Mechelen W, Beek AJ. A participatory return‐to‐work intervention for temporary agency workers and unemployed workers sick‐listed due to musculoskeletal disorders: results of a randomized controlled trial. Journal of Occupational Rehabilitation 2011;21:313‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Viikari‐Juntura 2012 {published data only}
- Viikari‐Juntura E, Kausto J, Shiri R, Kaila‐Kangas L, Takala EP, Karppinen J, et al. Return to work after early part‐time sick leave due to musculoskeletal disorders: a randomized controlled trial. Scandinavian Journal of Work, Environment & Health 2012;38(2):134‐43. [DOI] [PubMed] [Google Scholar]
Volker 2013 {published data only}
- Volker D, Vlasveld MC, Anema JR, Beekman ATF, Hakkaart‐van Roijen LH, Brouwers EPM, et al. Blended E‐health module on return to work embedded in collaborative occupational health care for common mental disorders: Design of a cluster randomized controlled trial. Neuropsychiatric Disease and Treatment 2013;9:529‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vonk Noordegraaf 2012 {published data only}
- Vonk NA, Huirne JA, Brölmann HA, Emanuel MH, Kesteren PJ, Kleiverda G, et al. Effectiveness of a multidisciplinary care program on recovery and return to work of patients after gynaecological surgery: design of a randomized controlled trial. BMC Health Services Research 2012;12(29):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Adler 2006
- Adler DA, McLaughlin TJ, Rogers WH, Chang H, Lapitsky L, Lerner D. Job performance deficits due to depression. American Journal of Psychiatry 2006;163(9):1569‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anema 2004
- Anema JR. Low Back Pain, Workplace Intervention and Return‐to‐Work [PhD thesis]. Amsterdam, The Netherlands: Vrije Universiteit, 2004. [Google Scholar]
Anema 2007
- Anema JR, Steenstra IA, Bongers PM, Vet HCW, Knol DL, Mechelen W. Multidisciplinary rehabilitation for subacute low back pain: Graded activity or workplace intervention or both? A randomized controlled trial. Spine 2007;32(3):291‐8. [DOI] [PubMed] [Google Scholar]
Anema 2009
- Anema JR, Schellart AJM, Cassidy JD, Loisel P, Veerman TJ, Beek AJ. Can cross country differences in return‐to‐work after chronic occupational back pain be explained? An exploratory analysis on disability policies in a six country cohort study. Journal of Occupational Rehabilitation 2009;19(4):419‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arends 2012
- Arends I, Bruinvels DJ, Rebergen DS, Nieuwenhuijsen K, Madan I, Neumeyer‐Gromen A, et al. Interventions to facilitate return to work in adults with adjustment disorders. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD006389.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baldwin 1996
- Baldwin ML, Johnson WG, Butler RJ. The error of using returns‐to‐work to measure the outcomes of health care. American Journal of Industrial Medicine 1996;29:632‐41. [DOI] [PubMed] [Google Scholar]
Berry 1992
- Berry DL, Catanzaro M. Persons with cancer and their return to the workplace. Cancer Nursing 1992;15(1):40‐6. [PubMed] [Google Scholar]
Boluyt 2012
- Boluyt N, Rottier BL, Langendam MW. Guidelines are made more transparent with the GRADE method: considerations for recommendations are explicit in the new method. Nederlands Tijdschrift voor Geneeskunde 2012;156:1‐7. [PubMed] [Google Scholar]
Busch 2011a
- Busch H, Bodin L, Bergström G, Jensen IB. Patterns of sickness absence a decade after pain‐related multidisciplinary rehabilitation. Pain 2011;152:1727‐33. [DOI] [PubMed] [Google Scholar]
Bültmann 2009b
- Bültmann U, Sherson D, Olsen J, Hansen CL, Lund T, Kilsgaard J. Coordinated and tailored work rehabilitation: A randomized controlled trial with economic evaluation undertaken with workers on sick leave due to musculoskeletal disorders. Journal of Occupational Rehabilitation 2009;19:81‐93. [DOI] [PubMed] [Google Scholar]
Carroll 2010
- Carroll C, Rick J, Pilgrim H, Cameron J, Hillage J. Workplace involvement improves return to work rates among employees with back pain on long‐term sick leave: a systematic review of the effectiveness and cost‐effectiveness of interventions. Disability and Rehabilitation 2010;32(8):607‐21. [DOI] [PubMed] [Google Scholar]
Chan 2004
- Chan A, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: Comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. [DOI] [PubMed] [Google Scholar]
Clay 2012
- Clay FJ, Fitzharris M, Kerr E, McClure RJ, Watson WL. The association of social functioning, social relationships and the receipt of compensation with time to return to work following unintentional injuries to Victorian workers. Journal of Occupational Rehabilitation 2012;22:363‐75. [DOI] [PubMed] [Google Scholar]
Cornelius 2011
- Cornelius LR, Klink JJL, Groothoff JW, Brouwer S. Prognostic factors of long term disability due to mental disorders: A systematic review. Journal of Occupational Rehabilitation 2011;21:259‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dall 2013
- Dall TM, Gallo P, Koenig L, Gu Q, Ruiz D. Modeling the indirect economic implications of musculoskeletal disorders and treatment. Cost‐effectiveness and resource allocation 2013;11(5):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Vet 2002
- Vet HCW, Heymans MW, Dunn KM, Pope DP, Beek AJ, Macfarlane GJ, et al. Episodes of low back pain: a proposal for uniform definitions to be used in research. Spine 2002;27(21):2409‐16. [DOI] [PubMed] [Google Scholar]
Driessen 2012
- Driessen MT, Lin CC, Tulder MW. Cost‐effectiveness of conservative treatments for neck pain: a systematic review on economic evaluations. European Spine Journal 2012;21:1441‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwan 2008
- Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan A, Cronin E, et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS One 2008;3(8):1‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farzanfar 2011a
- Farzanfar R, Locke SE, Heeren TC, Stevens A, Vachon L, Nguyen MKT, et al. Workplace telecommunications technology to identify mental health disorders and facilitate self‐help or professional referrals. American Journal of Health Promotion 2011;25(3):207‐16. [DOI] [PubMed] [Google Scholar]
Ferrie 2005
- Ferrie JE, Kivimäki M, Head J, Shipley MJ, Vahtera J, Marmot MG. A comparison of self‐reported sickness absence with absences recorded in employers' registers: evidence from the Whitehall II study. Occupational and Environmental Medicine 2005;62(2):74‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleten 2004
- Fleten N, Johnsen R, Forde OH. Length of sick leave ‐ why not ask the sick‐listed? Sick‐listed individuals predict their length of sick leave more accurately than professionals. BMC Public Health 2004;4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Franche 2005a
- Franche RL, Cullen K, Clarke J, Irvin E, Sinclair S, Frank J, et al. Workplace‐based return‐to‐work interventions: A systematic review of the quantitative literature. Journal of Occupational Rehabilitation 2005;15(4):607‐31. [DOI] [PubMed] [Google Scholar]
Franche 2005b
- Franche RL, Baril R, Shaw W, Nicholas M, Loisel P. Workplace‐based return‐to‐work interventions: optimizing the role of stakeholders in implementation and research. Journal of Occupational Rehabilitation 2005;15(4):525‐42. [DOI] [PubMed] [Google Scholar]
Goorden
GRADE working group
- GRADE working group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEprofiler [Computer program]
- GRADE Working Group. GRADEprofiler. Version 3.6. GRADE Working Group, 2004‐2007.
Guyatt 2008a
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Schünemann HJ. GRADE: what is 'quality of evidence' and why is it important to clinicians?. BMJ 2008;336:995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008b
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamberg‐van Reenen 2012
- Hamberg‐van Reenen HH, Proper KI, Berg M. Worksite mental health interventions: a systematic review of economic evaluations. Occupational and Environmental Medicine 2012;69:837‐45. [DOI] [PubMed] [Google Scholar]
Haugli 2011
- Haugli L, Maeland S, Magnussen LH. What facilitates return to work? Patients' experiences 3 years after occupational rehabilitation. Journal of Occupational Rehabilitation 2011;21:573‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hees 2010
- Hees HL, Koeter MWJ, Vries G, Ooteman W, Schene AH. Effectiveness of adjuvant occupational therapy in employees with depression: design of a randomized controlled trial. BMC Public Health 2010;10(558):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hees 2012b
- Hees HL, Vries G, Koeter MWJ, Schene AH. Adjuvant occupational therapy improves long‐term depression recovery and return‐to‐work in good health in sick‐listed employees with major depression: results of a randomised controlled trial. Occupational and Environmental Medicine 2012;70(4):252‐60. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hróbjartsson 2013
- Hróbjartsson A, Boutron I, Turner L, Altman DG, Moher D. Assessing risk of bias in randomised clinical trials included in Cochrane Reviews: the why is easy, the how is a challenge [editorial]. Cochrane Database of Systematic Reviews 2013; Vol. 4. [DOI] [PMC free article] [PubMed]
Jensen 2005
- Jensen IB, Bergström G, Ljungquist T, Bodin L. A 3‐year follow‐up of a multidisciplinary rehabilitation programme for back and neck pain. Pain 2005;115:273‐83. [DOI] [PubMed] [Google Scholar]
Lambeek 2007
- Lambeek LC, Anema JR, Royen BJ, Buijs PC, Wuisman PI, Tulder MW, et al. Multidisciplinary outpatient care program for patients with chronic low back pain: design of a randomized controlled trial and cost‐effectiveness study. BMC Public Health 2007;7:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lambeek 2010b
- Lambeek LC, Mechelen W, Knol DL, Loisel P, Anema JR. Randomised controlled trial of integrated care to reduce disability from chronic low back pain in working and private life. BMJ 2010;340:c1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lambeek 2010c
- Lambeek LC, Bosmans JE, Royen BJ, Tulder MW, Mechelen W, Anema JR. Effect of integrated care for sick listed patients with chronic low back pain: economic evaluation alongside a randomised controlled trial. BMJ 2010;341:c6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linton 2011
- Linton SJ. Correspondence of back pain patients' self‐reports of sick leave and Swedish National Insurance Authority Register. Perceptual and Motor Skills 2011;112:133‐7. [DOI] [PubMed] [Google Scholar]
Loisel 1997b
- Loisel P, Abenhaim L, Durand P, Esdaile JM, Suissa S, Gosselin L, et al. A population‐based, randomized clinical trial on back pain management. Spine 1997;22(24):2911‐8. [DOI] [PubMed] [Google Scholar]
Loisel 2001
- Loisel P, Durand MJ, Berthelette D, Vezina N, Baril R, Gagnon D, et al. Disability prevention ‐ New paradigm for the management of occupational back pain. Disease Management & Health Outcomes 2001;9(7):351‐60. [Google Scholar]
Lund 2008
- Lund T, Kivimäki M, Labriola M, Villadsen E, Christensen KB. Using administrative sickness absence data as a marker of future disability pension: the prospective DREAM study of Danish private sector employees. Occupational and Environmental Medicine 2008;65:28‐31. [DOI] [PubMed] [Google Scholar]
Munir 2012
- Munir F, Yarker J, Hicks B, Donaldson‐Feilder E. Returning employees back to work: developing a measure for Supervisors to Support Return to Work (SSRW). Journal of Occupational Rehabilitation 2012;22:196‐208. [DOI] [PubMed] [Google Scholar]
Nachreiner 2007
- Nachreiner NM, Dagher RK, McGovern PM, Baker BA, Alexander BH, Gerberich SG. Successful return to work for cancer survivors. Official Journal of the American Association of Occupational Health Nurses 2007;55(7):290‐5. [DOI] [PubMed] [Google Scholar]
Nieuwenhuijsen 2014
- Nieuwenhuijsen K, Faber B, Verbeek JH, Neumeyer‐Gromen A, Hees HL, Verhoeven AC, et al. Interventions to improve return to work in depressed people. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD006237.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noordik 2009
- Noordik E, Dijk FJ, Nieuwenhuijsen K, Klink JJL. Effectiveness and cost‐effectiveness of an exposure‐based return‐to‐work programme for patients on sick leave due to common mental disorders: design of a cluster‐randomized controlled trial. BMC Public Health 2009;9(140):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noordik 2013b
- Noordik E, Klink JJ, Geskus RB, Boer MR, Dijk FJH, Nieuwenhuijsen K. Effectiveness of an exposure‐based return‐to‐work program for workers on sick leave due to common mental disorders: a cluster‐randomized controlled trial. Scandinavian Journal of Work, Environment & Health 2013;39(2):144‐54. [DOI] [PubMed] [Google Scholar]
Nordqvist 2003
- Nordqvist C, Holmqvist C, Alexanderson K. Views of laypersons on the role employers play in return to work when sick‐listed. Journal of Occupational Rehabilitation 2003;13(1):11‐20. [DOI] [PubMed] [Google Scholar]
OECD 2009
- OECD. Sickness, disability and work: Keeping track in the economic downturn ‐ Background Paper. Organisation for Economic Co‐operation and Development Directorate for Employment, Labour and Social Affairs 2009.
OECD 2010
- OECD. Sickness, disability and work: Breaking the barriers ‐ A synthesis of findings across OECD countries. Organisation for Economic Co‐operation and Development 2010.
Ostelo 2005
- Ostelo RW, Vet HC. Clinically important outcomes in low back pain. Best Practice & Research. Clinical Rheumatology 2005;19(4):593‐607. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Pichora 2010
- Pichora D, Grant H. Upper extremity injured workers stratified by current work status: an examination of health characteristics, work limitations and work instability. International Journal of Occupational and Environmental Medicine 2010;1(3):124‐31. [PubMed] [Google Scholar]
Pole 2006
- Pole JD, Franche RL, Hogg‐Johnson S, Vidmar M, Krause N. Duration of work disability: a comparison of self‐report and administrative data. American Journal of Industrial Medicine 2006;49(5):394‐401. [DOI] [PubMed] [Google Scholar]
Pomaki 2012
- Pomaki G, Franche RL, Murray E, Khushrushahi N, Lampinen TM. Workplace‐based work disability prevention interventions for workers with common mental health conditions: A review of the literature. Journal of Occupational Rehabilitation 2012;22:182‐95. [DOI] [PubMed] [Google Scholar]
Rebergen 2009b
- Rebergen DS, Bruinvels DJ, Bezemer PD, Beek AJ, Mechelen W. Guideline‐based care of common mental disorders by occupational physicians (CO‐OP study): a randomized controlled trial. Journal of Occupational and Environmental Medicine 2009;51(3):305‐12. [DOI] [PubMed] [Google Scholar]
Reference Manager [Computer program]
- Thomson ResearchSoft.. Reference Manager 11. Thomson ResearchSoft., 2005.
Reme 2011b
- Reme SE, Tveito TH, Chalder T, Bjorkkjaer T, Indahl A, Brox JI, et al. Protocol for the cognitive interventions and nutritional supplements (CINS) trial: a randomized controlled multicenter trial of a brief intervention (BI) versus a BI plus cognitive behavioural treatment (CBT) versus nutritional supplements for patients with long‐lasting muscle and back pain. BMC Musculoskeletal Disorders 2011;12(152):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2014.
Sanderson 2006
- Sanderson K, Andrews G. Common mental disorders in the workforce: recent findings from descriptive and social epidemiology. Canadian Journal of Psychiatry 2006;51(2):63‐75. [DOI] [PubMed] [Google Scholar]
Schandelmaier 2012
- Schandelmaier S, Ebrahim S, Burkhardt SCA, Boer WEL, Zumbrunn T, Guyatt GH, et al. Return to work coordination programmes for work disability: A meta‐analysis of randomised controlled trials. PLoS One 2012;7(11):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schonstein 2003
- Schonstein E, Kenny DT, Keating J, Koes BW. Work conditioning, work hardening and functional restoration for workers with back and neck pain. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD001822] [DOI] [PubMed] [Google Scholar]
Schonstein 2006
- Schonstein E, Verbeek JH. Occupational health systematic reviews: An overview. Work 2006;26(3):255‐8. [PubMed] [Google Scholar]
Schultz 2007
- Schultz IZ, Stowell AW, Feuerstein M, Gatchel RJ. Models of return to work for musculoskeletal disorders. Journal of Occupational Rehabilitation 2007;17(2):327‐52. [DOI] [PubMed] [Google Scholar]
Schünemann 2006
- Schünemann HJ, Jaeschke R, Cook DJ, Bria WF, El‐Sohl AA, Ernst A, et al. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. American Journal of Respiratory and Critical Care Medicine 2006;174:605‐14. [DOI] [PubMed] [Google Scholar]
Skakon 2010
- Skakon J, Nielsen K, Borg V, Guzman J. Are leaders' well‐being, behaviours and style associated with the affective well‐being of their employees? A systematic review of three decades of research. Work & Stress 2010;24:107‐39. [Google Scholar]
Stapleton 2000
- Stapleton C, editor. Classification scheme. Ergnonomics Abstracts. London: Taylor & Francis Ltd, 2000; Vol. 32:1‐7.
Steenstra 2003
- Steenstra IA, Anema JR, Bongers PM, Vet HC, Mechelen W. Cost effectiveness of a multi‐stage return to work program for workers on sick leave due to low back pain, design of a population based controlled trial. BMC Musculoskeletal Disorders 2003;4(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steenstra 2005
- Steenstra IA, Verbeek JH, Heymans MW, Bongers PM. Prognostic factors for duration of sick leave in patients sick listed with acute low back pain: a systematic review of the literature. Occupational and Environmental Medicine 2005;62(12):851‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steenstra 2006
- Steenstra IA, Anema JR, Tulder MW, Bongers PM, Vet HCW, Mechelen W. Economic evaluation of a multi‐stage return to work program for workers on sick‐leave due to low back pain. Occupational and Environmental Medicine 2006;16(4):557‐78. [DOI] [PubMed] [Google Scholar]
Steenstra 2012
- Steenstra IA, Lee H, Vroome EMM, Busse JW, Hogg‐Johnson SJ. Comparing current definitions of return to work: A measurement approach. Journal of Occupational Rehabilitation 2012;22:394‐400. [DOI] [PubMed] [Google Scholar]
Stigmar 2013
- Stigmar KG, Petersson IF, Jöud A, Grahn BE. Promoting work ability in a structured national rehabilitation program in patients with musculoskeletal disorders: outcomes and predictors in an prospective cohort study. BMC Musculoskeletal Disorders 2013;14(57):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sullivan 2005
- Sullivan MJ, Feuerstein M, Gatchel R, Linton SJ, Pransky G. Integrating psychosocial and behavioral interventions to achieve optimal rehabilitation outcomes. Journal of Occupational Rehabilitation 2005;15(4):475‐89. [DOI] [PubMed] [Google Scholar]
Tamminga 2010
- Tamminga SJ, Boer AG, Verbeek JH, Taskila T, Frings‐Dresen MH. Enhancing return‐to‐work in cancer patients, development of an intervention and design of a randomized controlled trial. BMC Cancer 2010;10:345‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tamminga 2012
- Tamminga SJ. Enhancing Return to Work of Cancer Patients [PhD thesis]. Amsterdam, The Netherlands: Universiteit van Amsterdam, 2012. [Google Scholar]
Turner 2007
- Turner JA, Franklin G, Fulton‐Kehoe D, Sheppard L, Wickizer TM, Wu R, et al. Early predictors of chronic work disability associated with carpal tunnel syndrome: a longitudinal workers' compensation cohort study. American Journal of Industrial Medicine 2007;50(7):489‐500. [DOI] [PubMed] [Google Scholar]
Tveito 2004
- Tveito TH, Hysing M, Eriksen HR. Low back pain interventions at the workplace: a systematic literature review. Occupational Medicine 2004;54(1):3‐13. [DOI] [PubMed] [Google Scholar]
Uegaki 2007
- Uegaki K, Bruijne MC, Anema JR, Beek AJ, Tulder MW, Mechelen W. Consensus‐based findings and recommendations for estimating the costs of health‐related productivity loss from a company's perspective. Scandinavian Journal of Work Environment and Health 2007;33(2):122‐30. [DOI] [PubMed] [Google Scholar]
van Oostrom 2008
- Oostrom SH, Anema JR, Terluin B, Vet HC, Knol DL, Mechelen W. Cost‐effectiveness of a workplace intervention for sick‐listed employees with common mental disorders: design of a randomized controlled trial. BMC Public Health 2008;14(8):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Oostrom 2010b
- Oostrom SH, Heymans MW, Vet HCW, Tulder MW, Mechelen W, Anema JR. Economic evaluation of a workplace intervention for sick‐listed employees with distress. Occupational and Environmental Medicine 2010;67:603‐10. [DOI] [PubMed] [Google Scholar]
van Oostrom 2010c
- Oostrom SH, Mechelen W, Terluin B, Vet HCW, Knol DL, Anema JR. A workplace intervention for sick‐listed employees with distress: results of a randomised controlled trial. Occupational and Environmental Medicine 2010;67:596‐602. [DOI] [PubMed] [Google Scholar]
Verbeek 2002b
- Verbeek JH, Weide WE, Dijk FJ. Early occupational health management of patients with back pain: a randomized controlled trial. Spine 2002;27(17):1844‐51. [DOI] [PubMed] [Google Scholar]
Veronese 2012
- Veronese A, Ayuso‐Mateos JL, Cabello M, Chatterji S, Nuevo R. Work disability and depressive disorders: Impact on the European population. American Journal of Physical Medicine & Rehabilitation 2012;91(13):S62‐8. [DOI] [PubMed] [Google Scholar]
Vlasveld 2008
- Vlasveld MC, Anema JR, Beekman AT, Mechelen W, Hoedeman R, Marwijk HW, et al. Multidisciplinary collaborative care for depressive disorder in the occupational health setting: design of a randomised controlled trial and cost‐effectiveness study. BMC Health Services Research 2008;5(8):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vlasveld 2012b
- Vlasveld MC. Sickness and Return to Work in Workers With Major Depressive Disorder: The Netherlands Depression Initiative in the Occupational Healthcare Setting [PhD thesis]. Amsterdam, The Netherlands: Vrije Universiteit, 2012. [Google Scholar]
Vlasveld 2012c
- Vlasveld MC, Feltz‐Cornelis CM, Adre HJ, Anema JR, Hoedeman R, Mechelen W, et al. Collaborative care for sick‐listed workers with major depressive disorder: a randomised controlled trial from the Netherlands Depression Initiative aimed at return to work and depressive symptoms. Occupational and Environmental Medicine 2012;70(4):223‐30. [DOI] [PubMed] [Google Scholar]
Vlasveld 2012d
- Vlasveld MC, Feltz‐Cornelis CM, Ader HJ, Anema JR, Hoedeman R, Mechelen W, et al. Collaborative care for major depressive disorder in an occupational healthcare setting. British Journal of Psychiatry 2012;200:510‐1. [DOI] [PubMed] [Google Scholar]
Waddell 2001
- Waddell G, Burton AK. Occupational health guidelines for the management of low back pain at work: evidence review. Occupational Medicine (Oxford, England) 2001;51(2):124‐35. [DOI] [PubMed] [Google Scholar]
Waddell 2006
- Waddell G, Burton AK. Is Work Good for Your Health and Well‐Being?. 1st Edition. United Kingdom: The Stationery Office, 2006. [Google Scholar]
WHO 2001
- World Health Organization. International Classification of Functioning, Disability and Health. Geneva: World Health Organization, 2001. [Google Scholar]
Wilkie 2012
- Wilkie R, Pransky G. Improving work participation for adults with musculoskeletal conditions. Best Practices & Research Clinical Rheumatology 2012;26(5):733‐42. [DOI] [PubMed] [Google Scholar]
Wong 2006
- Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. Journal of the Medical Library Association 2006;94(1):41‐7. [PMC free article] [PubMed] [Google Scholar]
Young 2005
- Young AE, Roessler RT, Wasiak R, McPherson KM, Poppel MN, Anema JR. A developmental conceptualization of return to work. Journal of Occupational Rehabilitation 2005;15(4):557‐68. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
van Oostrom 2009
- Oostrom SH, Driessen MT, Vet HCW, Franche RL, Schonstein E, Loisel P, et al. Workplace interventions for preventing work disability. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006955.pub2] [DOI] [PubMed] [Google Scholar]


