Abstract
Objective
To provide an estimate of the effectiveness of basivertebral nerve (BVN) radiofrequency ablation (RFA) to treat vertebrogenic low back pain (LBP).
Design
Systematic review with single-arm meta-analysis.
Population
Persons ≥18 years of age with chronic LBP associated with type 1 or 2 Modic changes.
Intervention
Intraosseous BVN RFA.
Comparison
Sham, placebo procedure, active standard care treatment, or none.
Outcomes
The proportion of patients treated with BVN RFA who reported ≥50% pain score improvement on a visual analog scale or numeric rating scale. The main secondary outcome was ≥15-point improvement in Oswestry Disability Index score.
Methods
Three reviewers independently assessed articles published before December 6, 2021, in MEDLINE and Embase. The Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) framework was used to evaluate the overall quality of evidence.
Results
Of the 856 unique records screened, 12 publications met the inclusion criteria, representing six unique study populations, with 414 participants allocated to receive BVN RFA. Single-arm meta-analysis showed a success rate of 65% (95% confidence interval [CI] 51–78%) and 64% (95% CI 43–82%) for ≥50% pain relief at 6 and 12 months, respectively. Rates of ≥15-point Oswestry Disability Index score improvement were 75% (95% CI 63–86%) and 75% (95% CI 63–85%) at 6 and 12 months, respectively.
Conclusion
According to GRADE, there is moderate-quality evidence that BVN RFA effectively reduces pain and disability in most patients with vertebrogenic LBP. Further high-quality studies will likely improve our understanding of the effectiveness of this procedure.
Keywords: Endplate, Vertebrogenic, Discogenic, Modic, Ablation
Introduction
Intraosseous basivertebral nerve (BVN) radiofrequency ablation (RFA) has gained attention as a target-specific treatment for pain arising from pathological degeneration of the vertebral endplates (VEPs) of the lumbosacral spine. At lumbar levels, the BVN is a paired branch of the bilateral sinuvertebral nerves that passes through the basivertebral foramen at the posterior aspect of the vertebral body to provide sensory innervation to the vertebral body and VEPs. Effective neurotomy of the BVN can be accomplished through careful transpedicular access under either fluoroscopy or computed tomography (CT) guidance, followed by ablation at a location 30–50% of the sagittal plane distance from the posterior cortex of the vertebral body [1, 2]. Since 2017, this treatment has been studied exclusively in populations believed to have pain arising from pathologically degenerated VEPs as evidenced by Modic 1 (MC1) and 2 changes (MC2) on magnetic resonance imaging (MRI) [1, 3–12].
Although BVN RFA is a relatively new treatment, studies investigating the pathological degeneration of the discovertebral complex began in the early 1990s. Initially, scientists postulated that the BVN might be implicated in nociception in some cases of chronic low back pain (LBP) after histological studies demonstrated that the BVN contained numerous neuropeptide Y– and PGP 9.5–positive nerve fibers [13, 14]. The case for VEP-driven pain was further strengthened when immunohistochemical analysis of the BVN demonstrated an abundance of nerve fibers in the BVN that stained positive for substance P, CGRP, and PGP 9.5 [15, 16]. Compared with controls, surgical specimens from patients with a history of chronic LBP and advanced disc degeneration were noted to have increased nociceptor density in the endplate region and adjacent vertebral body. This same pattern was observed in surgical specimens taken from patients with a history of “discogenic” LBP, where greater concentrations of PGP 9.5– and tumor necrosis factor (TNF)–immunoreactive cells near the VEPs were found compared with controls [17]. These histological findings were strongly associated with MC1 and MC2 findings on MRI.
Modic type 1 and 2 marrow changes are a radiographic manifestation of the underlying inflammatory response and fatty infiltration, respectively. These occur in the presence of prolonged mechanical stress and endplate failure, coupled with the ensuing chemical sensitization from leakage of proinflammatory cytokines from the intervertebral disc [25]. The epidemiology and clinical significance of MC1 and MC2 lesions have been discussed and debated ad nauseum [2, 18–24]. Modic changes are observed in the context of advanced disc degeneration or disc herniation [18, 19], but even stronger associations exist with endplate defects in large population-based cohort studies [20, 21]. Endplate injuries can be classified according to their pathoanatomic features: 1) avulsion of annulus fibrosus (“annulus”) fibers from their insertion at the cartilage endplate (tidemark avulsion), 2) separation of endplate from the bone (cartilage endplate avulsion), 3) degeneration of the bone–annulus interface (rim degeneration), and 4) traumatic or erosive ingrowth of nucleus pulposis material through the endplate (nodal) [22]. Although of unclear clinical significance, these descriptors help paint a more detailed picture of the disc–endplate relationship. Endplate injuries are likely the predisposing event that, in a subset of individuals, results in chronic inflammation, high bone turnover, and fatty infiltrative changes that can be observed with conventional T1- and T2-weighted MRI sequences [23]. This cascade of events can culminate in what is often referred to as “vertebrogenic” LBP, with nociception transmitted predominantly via the BVN.
Objectives and Rationale
Given the recent increase in studies investigating the treatment of vertebrogenic LBP, the present systematic review was performed to provide an updated estimate of the effectiveness of BVN RFA for the treatment of this condition. We also calculate the aggregate rates of treatment success defined by clinically important pain and functional improvement observed thus far in published clinical trials and cohort studies.
Methods
Protocol and Registration
This systematic review is an update of a prior systematic review for which the protocol was registered with PROSPERO (ID: CRD42020192001) on July 14, 2020. No changes were made to the review methodology, but to avoid redundancy, a ≥15-point Oswestry Disability Index (ODI) threshold was chosen to measure functional improvement [24]. A 15-point ODI improvement is a robust threshold that exceeds the known minimum clinically important difference for chronic LBP [25, 26]. The methods and results are reported in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [27].
Eligibility Criteria
Population
The population of interest was adults 18 years of age or older with chronic LBP associated with MC1 and MC2 changes on MRI.
Intervention
The intervention considered was intraosseous BVN RFA.
Comparison
Sham, placebo procedure, active standard care treatment, or none.
Outcome
The primary outcome considered for this review was the proportion of individuals with ≥50% pain improvement on the visual analog scale (VAS) or numeric rating scale (NRS). Secondary outcomes included ≥15-point improvement in ODI score and ≥2-point improvement in NRS score. Outcomes reported at any time point were included.
Studies
Both randomized controlled trials (RCTs) and observational study designs (nonrandomized comparative studies and single-group observational studies) were included. Non–English language articles and case reports were excluded. No restrictions were placed on the publication date.
Information Sources and Search Strategy
The databases MEDLINE and Embase were searched from inception up through December 6, 2021. MEDLINE was queried with the following terms:
("Basivertebral nerve ablation"[tiab] OR "BVN ablation"[tiab] OR "catheter ablation"[MeSH Terms] OR verteblation[tiab] OR "radiofrequency neurotomy"[tiab] OR "radiofrequency neurotomies" [tiab] OR "radiofrequency ablation"[tiab] OR "radiofrequency ablations" [tiab]) AND ("low back pain"[mesh] OR "low back pain"[tiab] OR CLBP[tiab] OR vertebrogenic[tiab] OR "modic change*"[tw] OR "disc degeneration"[All Fields] OR "endplate degeneration"[tiab] OR "endplate inflammation"[tiab] OR "disc inflammation"[tiab] OR "fatty bone marrow"[tiab] OR "fibrous bone marrow"[tiab] OR "endplate disruption"[tiab]).
Embase was queried with the following terms:
('radiofrequency ablation'/exp OR ('basivertebral nerve ablation':ab,ti,kw OR 'bvn ablation':ab,ti,kw OR 'catheter ablation':ab,ti,kw OR verteblation:ab,ti,kw OR 'radiofrequency neurotomy':ab,ti,kw OR 'radiofrequency neurotomies':ab,ti,kw OR 'radiofrequency ablation':ab,ti,kw OR 'radiofrequency ablations':ab,ti,kw OR 'radio frequency ablation':ab,ti,kw OR 'rfa therapy':ab,ti,kw OR 'rfa therapies':ab,ti,kw)) AND ('low back pain'/exp OR ('low back pain':ab,ti,kw OR clbp:ab,ti,kw OR vertebrogenic:ab,ti,kw OR 'modic change*':ab,ti,kw OR 'disc degeneration':ab,ti,kw OR 'endplate degeneration':ab,ti,kw OR 'endplate inflammation':ab,ti,kw OR 'disc inflammation':ab,ti,kw OR 'fatty bone marrow':ab,ti,kw OR 'fibrous bone marrow':ab,ti,kw OR 'endplate disruption':ab,ti,kw)).
An experienced librarian developed the search strategy. One author (AC) performed the search, and the search was confirmed for accuracy and reproducibility by a second author (TC). Additional eligible records were sought from the cited references of retrieved publications.
Study Selection
Two authors (AC and TC) independently assessed each abstract for eligibility. Any disagreements about inclusion were resolved by a third reviewer (ZM). Publications selected for full-text review were further assessed for inclusion by two authors (AC and TC), with disagreements resolved by a third reviewer (ZM).
Data Items and Collection
The following information was extracted from each study: 1) outcome measures for VAS/NRS and ODI, as well as any information related to pain medication usage or healthcare utilization; 2) bibliographic details; 3) study design; 4) selection criteria; 5) technical details of the procedure; and 6) funding and author disclosures. If data considered critical to the research questions were missing from included studies, attempts were made to contact author groups to obtain this information.
Risk of Bias and Methodological Assessment
The quality of evidence across outcomes was evaluated with the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system [32]. Accordingly, risk of bias, imprecision, inconsistency, indirectness, and publication bias were all assessed. Disagreements about determinations of evidence quality according to GRADE were resolved by consensus decision. Our previous systematic review included a GRADE evidence profile based on calculations of between-group success measurements for the included RCTs, and this was again planned for the present review.
Summary Measures and Synthesis of Results
The primary outcome of interest was the percentage of participants reporting ≥50% pain improvement, and the main secondary outcome of interest was ≥15-point ODI improvement after BVN RFA. A between-group comparison of categorical success rates with calculated relative risk and risk difference was planned. Conventional pairwise and single-arm meta-analysis was also planned for this review if the collected data were found to be sufficient for such analysis.
Meta-analyses were planned to examine the following binary outcome variables (yes/no) over time points of 6 months, 12 months, 24 months, and 60 months: 1) proportions of patients reporting ≥15-point ODI improvement and 2) proportions of patients reporting ≥50% NRS/VAS improvement. Proportions and their standard errors of the included studies were used to calculate a pooled effect size (ES) and its 95% confidence interval (CI) in each meta-analysis. A random-effects model was used for all meta-analyses, as heterogeneity was expected in observational studies [28], which was also verified by heterogeneity and I2 statistics [29]. Specifically, the calculations were performed with (inverse-variance) Freeman-Tukey double arcsine transformation [30, 31] and the exact CIs for the ESs of individual studies [32]. Forest plots were produced for the data at 6 months and 12 months to illustrate the ESs of individual studies, as well as the pooled ES from those studies [28]. Furthermore, line graphs were constructed to visualize ESs over the time points. Publication bias was assessed with Egger’s test [33, 34]. Funnel plots were not used, as the number of included studies for each meta-analysis was fewer than 10, which is the recommended minimum number of studies for the assessment of publication bias with a funnel plot [35]. Lastly, sensitivity analysis was conducted to assess the between-study heterogeneity and impact of an individual study on the pooled ES; this was done through the leave-one-out approach, which recalculated the pooled ES after a study was omitted, one by one [28, 29]. All of the analyses were performed in Stata 17.0 (StataCorp LLC, College Station, TX, USA).
Results
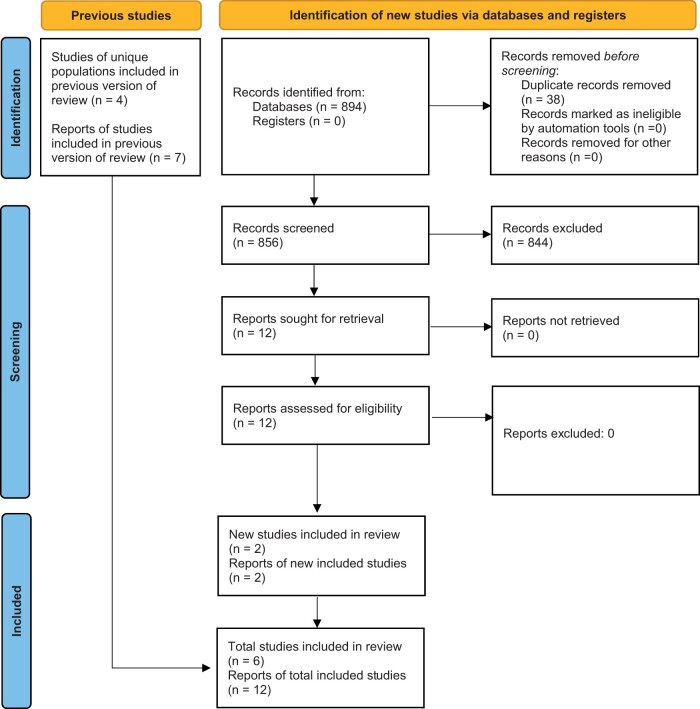
A total of 856 unique records were identified from the search (Figure 1). After abstract screening for relevant publications, 12 full-text articles were assessed and deemed eligible for inclusion. There were no disagreements among reviewers about study inclusion. These included one RCT comparing BVN RFA with sham, with outcomes reported at up to 1, 2, and 5 years [4, 7, 9, 10]; one RCT comparing BVN RFA with standard care treatment, with outcomes reported at up to 3, 6, 12, and 24 months [8, 11, 36]; and four single-group cohort studies, with outcomes reported between 3 and 12 months [1, 3, 5, 6, 12].
Figure 1.
PRISMA 2020 flow diagram for updated systematic reviews.
Study Characteristics
The main characteristics of the included studies are summarized in Table 1. From 2017 to 2021, a total of 414 participants were allocated to receive BVN RFA in two RCTs and four single-group cohort studies [1, 3, 5, 7, 11, 12, 36]. Participants in all reviewed studies were adults with chronic LBP for ≥6 months with MC1 and MC2 changes within at least one of the L3–S1 vertebral bodies. In addition to MC1 and MC2 changes on MRI, participants in the cohort study by De Vivo et al. underwent SPECT/CT and CT-guided medial branch blocks to exclude lumbar facet joint pain [1]. Some studies specifically excluded individuals with disc protrusions >5 mm, spondylolisthesis >2 mm, and significant depression (Beck Depression Inventory >24) [3, 5, 7, 11]. All studies excluded individuals with clinical evidence of symptomatic spinal stenosis or radicular pain. The majority of study participants were Caucasian, nonobese, college educated, and employed [3, 7, 11] and were in their mid-40s to early 50s [1, 3, 5, 7, 11, 12]. Most study participants reported having experienced pain for ≥5 years (62–72%) [3, 7, 11], but in one study, 74% of participants reported a duration of pain between 1 and 2 years [5]. Prior treatment, where described, included opioid use (32–24%), spinal injections (61–70%), lumbosacral or sacroiliac joint RFA (16%), chiropractic care (42%), and physical therapy (70%) [3, 7, 11]. Bipolar RFA was performed in all studies with either fluoroscopic [3, 5, 7, 11, 12] or CT guidance [1] targeting the intraosseous BVN at a point 40–60% from the posterior wall [7] or 30–50% from the posterior wall [1, 3, 5, 11]. Patient-reported outcome measures were reported between 3 and 60 months and included VAS/NRS, ODI, Short Form 36 (SF-36), EuroQuol 5 Dimensions (EQ5D-5L), healthcare utilization, and opioid use.
Table 1.
Study characteristics
| Author, Year | Design | Study Size | Inclusion Criteria | Mean/Median Ages of Participants (Range) | Participant Duration of Pain | Intervention | Targeting Success (Based on Post-Ablation MRI) | Adverse Events | Author Disclosures |
|---|---|---|---|---|---|---|---|---|---|
| Fischgrund 2018* | RCT | 225 randomized, 147 received BVN RFA. 128 analyzed in PP population (excluded n = 19 for targeting/access failure or protocol noncompliance). | CLBP ≥6 months despite conservative treatment, Modic 1 or 2 changes from L3–S1, minimum ODI 30, minimum VAS 4 mm | Mean 46.9 (26–69) | 6–12 months 4.1%, 1–2 years 10.2%, 2–3 years 6.8%, 3–5 years 12.2%, ≥5 years 66.7% | Fluoroscopically guided BVN RFA, 85°C bipolar ablation for 15 minutes 40–60% from posterior wall | 95% | n = 1 nerve root injury (sham group), n = 1 vertebral compression fracture (sham group), n = 7 lumbar radicullitis, retroperitoneal hemorrhage (n = 1), and transient motor or sensory deficits, all resolved with supportive care. | Industry funded |
| Fischgrund 2019* | SGOS | 128 at 18 months and 106 at 24 months | " | " | " | " | " | No delayed adverse events | Industry funded |
| Fischgrund 2020* | SGOS | 100 | " | " | " | " | " | No delayed adverse events | Industry funded |
| Markman 2019* | PSA | 224 | " | " | " | " | " | No delayed adverse events | Industry funded |
| Khalil 2019† | RCT | 140 randomized, 51 received BVN RFA | CLBP ≥6 months despite conservative treatment, Modic 1 or 2 changes from L3–S1, minimum ODI 30, minimum VAS 4 cm | Mean 50.0 (26–70) | 6–12 months 5.8%, 1–2 years 2.9%, 2–3 years 11.5%, 3–5 years 12.5%, ≥5 years 62.7% | Fluoroscopically guided BVN RFA, 85°C bipolar ablation for 15 minutes 30–50% from posterior wall | 96% | n = 15, incisional pain, leg pain/paresthesia, back pain in a new location, urinary retention, and lateral femoral cutaneous neurapraxia. | Industry funded |
| Smuck 2021† | RCT | 66 original RFA arm, 61 crossover from standard-care arm treated with BVN RFA at average 6.3 months | " | " | " | " | 97% | No delayed adverse events | Industry funded |
| Koreckij 2021† | SGOS | 58 original BVN RFA arm | " | " | " | " | " | No delayed adverse events | Industry funded |
| Becker 2017 | SGOS | 16 | CLBP ≥6 months, Modic 1 or 2 changes from L3–S1 or positive provocation discography | Mean 48.0 (34–66) | Not reported | Fluoroscopically guided BVN RFA (n = 16), 85°C bipolar ablation for 15 minutes “at least 10 mm anterior to the posterior wall” | 91% | n = 4, lumbar pain, buttock pain, dysesthesia, and transient numbness, resolved with pain medications. | Industry funded |
| Trumees 2019 | SGOS | 28 | CLBP ≥6 months, Modic 1 or 2 changes from L3–S1, minimum ODI 30, minimum VAS 4 cm | Mean 45.2 (SD 8.89) | 1–2 years 10.7%, 2–3 years 14.3%, 3–5 years 0%, ≥5 years 75.0% | Fluoroscopically guided BVN RFA (n = 47), 85°C bipolar ablation for 15 minutes 30–50% from posterior wall | 97% | n = 3, 1 aborted procedure due to inability to access, 2 leg pain events due to pedicle breach | Industry funded |
| Macadaeg 2020‡ | SGOS | 47 | " | Median 45.0 (25–66) | 1–2 years 14.9%, 2–3 years 10.6%, 3–5 years 2.1%, ≥5 years 72.3% | " | 96% | n = 2, potential pedicle breach and associated radiculitis, resolved with oral medications | Industry funded |
| De Vivo 2021 | SGOS | 56 | CLBP ≥6 months despite conservative treatment ≥6 weeks, Modic 1 or 2 changes from L3–S1 | Median 43.0 (38–52) | Not reported | CT-guided BVN RFA (n = 56), core temperature 77°C, automatically stopped when the proximal thermocouple reached 50°C, targeted 50% distance from the posterior wall. | 100% | None | None |
| Fishchenko 2021 | SGOS | 19 | CLBP ≥6 months despite conservative treatment, Modic 1 or 2 changes from L3–S1, minimum ODI 30, minimum VAS 4 mm | Mean 52.6 (SD 6.9) | 1–2 years 73.7% , ≥5 years 26.3% | Fluoroscopically guided BVN RFA (n = 19), 85°C ablation for 15 minutes 40–50% distance from posterior wall | Not reported | n = 1, arterial injury of the “lumbalis sinistra” causing a hematoma within the iliospoas with associated plexitis, treated with endovascular embolization | None |
SGOS= single-group observational study; PP= per protocol; PSA= post-hoc secondary analysis; SD= standard deviation; CLBP= chronic low back pain.
Multiple reports from the same population at various time points. Results of the per protocol analysis shown.
Multiple reports from the same population at various time points up to 12 months for the original BVN RFA arm and up to 6 months for the crossover cohort. Results of the per protocol analysis shown.
Multiple reports from the same population. Truumees et al. reported on the first 28 patients.
Synthesis of Results
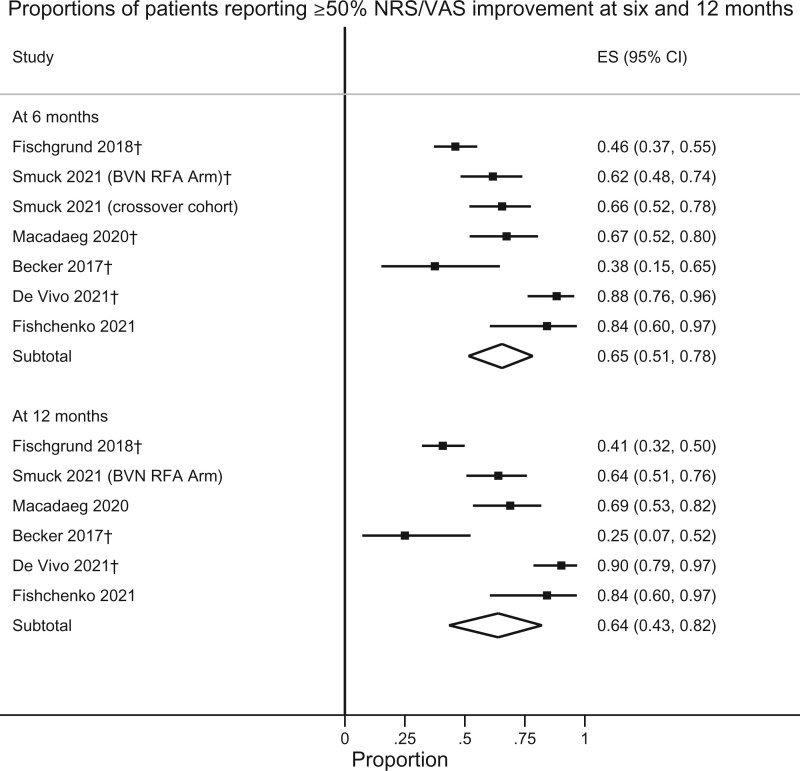
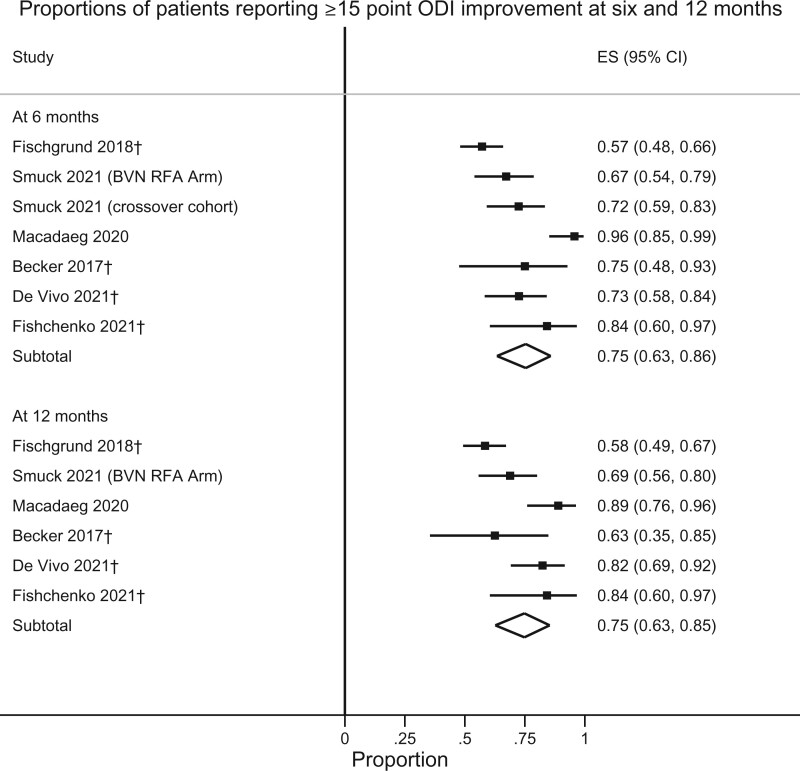
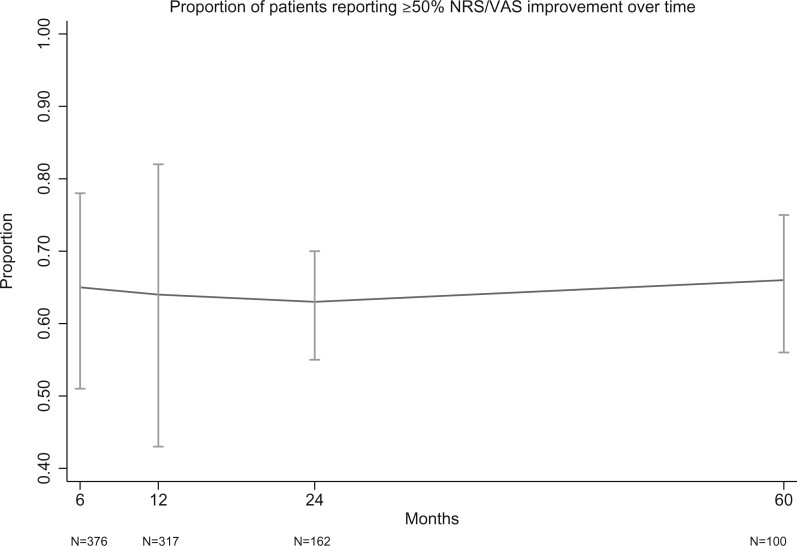
Responder rates for VAS and ODI at various thresholds are presented in Table 2. As only two RCTs (one sham-controlled trial and one active treatment–controlled trial) have been performed, conventional pairwise meta-analysis was not performed. Instead, a single-arm meta-analysis of outcomes after treatment with BVN RFA was performed to examine the percentage of responders, defined by the ≥50% VAS/NRS and ≥15-point ODI improvement thresholds at 6 and 12 months (Figures 2 and 3). For ≥50% pain improvement at 6 and 12 months, the calculated success rates were 65% (95% CI 51–78%) and 64% (95% CI 43–82%), respectively. Rates of ≥15-point ODI improvement were 75% (95% CI 63–86%) and 75% (95% CI 63–85%) at 6 and 12 months, respectively. Figures 4 and 5 illustrate these same proportions at 6, 12, 24, and 60 months longitudinally. Meta-analysis was also performed to calculate the success rates based on an intention-to-treat analysis (including lost to follow-up, protocol deviations, targeting failure, etc.) for the RCTs and a “worst-case” scenario (unreported patients were categorical failures) for cohort studies, which demonstrated slightly lower success rates for pain and functional improvement: At 6, 12, 24, and 60 months 61% (95% CI 48–74%), 59% (95% CI 40–77%), 49% (95% CI 43–56%), and 50% (95% CI 41–58%) of participants reported ≥50% pain improvement. Rates of ≥15-point ODI improvement at these same time points were 71% (95% CI 59–82%), 70% (95% CI 57–81%), 57% (95% CI 50–64%), and 57% (95% CI 49–65%).
Table 2.
Pain reduction and functional improvement
| Author, Year | NRS/VAS Responder Percentage (95% CI) | ODI Responder Percentage (95% CI) |
|---|---|---|
| Fischgrund 2018* | ||
| Fischgrund 2019* | 24 months: ≥50% VAS reduction 46% (37–55%)§, ≥2.0-cm VAS reduction 67% (58–76%)§ | 24 months: ≥15-point ODI reduction 55% (47–64%)§ |
| Fischgrund 2020* | 60 months: ≥50% VAS reduction 66% (57–75%), ≥2.0-cm VAS reduction 88% (82–94%) | 60 months: ≥15-point ODI reduction 77/100, 77% (69–85%) |
| Markman 2019* | Not reported | Not reported |
| Khalil 2019† | 3 months: ≥50% VAS reduction 63% (49–76%)§, ≥2.0-cm VAS reduction 73% (60–85%) | 3 months: ≥20-point ODI reduction 63% (49–76%) |
| Smuck 2021† |
|
|
| Koreckij 2021† | 24 months: ≥50% VAS reduction 72% (61–84%)§, ≥2.0-cm VAS reduction 79% (69–90%) | 24 months: ≥15-point ODI reduction 77% (66–88%) |
| Becker 2017 | 12 months: ≥50% VAS reduction 38% (15–65%)§, ≥2.0-cm VAS reduction 50% (26–75%)§ | 12 months: ≥15-point ODI reduction 63% (35–85%)§ |
| Macadaeg 2020‡ | 12 months: ≥50% VAS reduction 67% (52–80%)§, ≥2.0-cm VAS reduction 80% (63–89%) | 12 months: ≥15-point ODI reduction 89% (76–96%) |
| De Vivo 2021 | 12 months: ≥50% VAS reduction 90% (79–97%)§, ≥2.0-cm VAS reduction 96% (92–100%) | 12 months: ≥15-point ODI reduction 82% (69–92%)§ |
| Fishchenko 2021 | 12 months: ≥50% VAS reduction 84% (60–97%) | 12 months: ≥15-point ODI reduction 84% (63–97%)§ |
SGOS= single group observational study, PSA = post-hoc secondary analysis.
Multiple reports from the same population at various time points. Results of the per protocol analysis shown.
Multiple reports from the same population at various time points up to 12 months for the original BVN RFA arm and up to 6 months for the crossover cohort. Results of the per protocol analysis shown.
Truumees et al. reported on the first 28 patients from this study in 2019.
Exact threshold unpublished. Data requested and obtained from the study investigators.
Figure 2.
Proportions of patients reporting ≥50% NRS/VAS improvement at 6 and 12 months. †Exact threshold unpublished, data requested and obtained from the study investigators.
Figure 3.
Proportions of patients reporting ≥15-point ODI improvement at 6 and 12 months. †Exact threshold unpublished, data requested and obtained from the study investigators.
Figure 4.
Proportion of patients reporting ≥50% NRS/VAS improvement over time.
Figure 5.
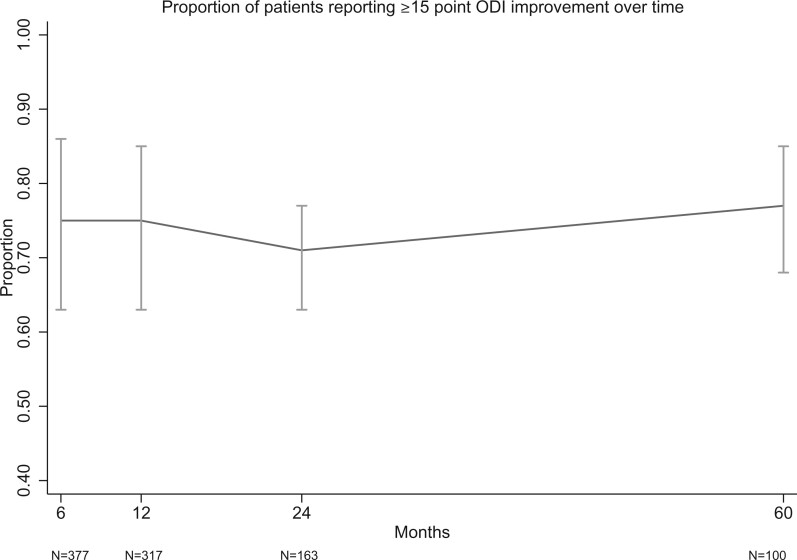
Proportion of patients reporting ≥15-point ODI improvement over time.
GRADE Quality Assessment
As no new RCTs are included in this updated systematic review, an updated GRADE evidence profile is not presented, but GRADE judgments are described narratively. The evidence from these two RCTs was downgraded from “high quality” because of the risk of bias in the form of selective outcome reporting and the inability to blind participants effectively. The possibility of publication bias was also considered, given that the majority of studies have been industry funded [3, 7, 11, 12]; however, two recently performed independent studies have shown similar results [1, 5]. According to GRADE, there is moderate-quality evidence that intraosseous BVN RFA effectively reduces LBP and related disability in those with vertebrogenic LBP, compared with sham RFA [7] and continued standard care treatment [11].
Publication Bias and Sensitivity Analysis
According to Egger’s tests, there was no evidence of serious publication bias in the meta-analysis on the proportions of patients reporting ≥15-point ODI improvement at 6 months or 12 months (P = 0.802 and 0.756, respectively) or on the proportions of patients reporting ≥50% NRS/VAS improvement at 6 months or 12 months (P = 0.409 and 0.369, respectively). The sensitivity analysis showed that the point estimate and CI of the pooled ES from all meta-analyses did not change substantially after exclusion of any individual study. Omitting the study by Fischgrund (2018) for the analysis on ≥15-point ODI improvement at 6 months and 12 months would have resulted in the pooled ESs of 0.78 (original ES = 0.75) and 0.79 (original ES = 0.75), respectively. Likewise, omitting the study by Macadaeg (2020) at 6 months and 12 months would have resulted in the pooled ESs of 0.70 (original ES = 0.75) and 0.71 (original ES = 0.75), respectively. However, all of the recalculated 95% CIs for the pooled ES largely overlapped with each other. In terms of the analysis for ≥50% NRS/VAS improvement, all of the recalculated point estimates of the ESs (after omission of any individual study) were very close to the original ESs of 0.65 and 0.64 at 6 months and 12 months, respectively, with substantial overlap of CIs.
Discussion
This updated systematic review identified several new publications that reported on the long-term effectiveness of BVN RFA. No new randomized trials were identified; however, substantially more data are now available to describe the short-, medium-, and long-term success rates of treatment with BVN RFA. Single-arm meta-analysis of these studies demonstrated that approximately 65% and 75% of patients report clinically significant pain and functional improvement at 6 and 12 months after BVN RFA (Figures 2 and 3). The calculated proportions of responders remained remarkably stable at 24 and 60 months, with less than 5% variance in the estimated VAS/ODI responder proportions (see Figures 4 and 5). According to GRADE, the evidence quality was determined to be “moderate.” The designation of “moderate quality” suggests that future research will likely improve our understanding of the effectiveness of BVN RFA for vertebrogenic pain [37].
In addition to safety, careful transpedicular access is required to achieve targeting success with BVN RFA. In studies that analyzed post-ablation lesion geometry on MRI, targeting success appeared slightly higher when the BVN was targeted at a point 30–50% from the posterior wall [3, 8, 11] compared with earlier targeting in SMART (40–60% from the posterior wall) [7], though the targeting success rate remained above 90% in all studies (Table 1). DeVivo et al. reported 100% targeting success with the use of CT guidance and an alternative device to that used in other studies [1]. Given that studies after SMART have reported targeting success rates greater than 95%, it appears that successful neurotomy of the BVN is achieved frequently with established techniques.
Because the BVN contains many unmyelinated fibers [14, 15, 38, 39], appropriately targeted thermal ablation can produce a long-lasting neurotomy and durable symptom improvement in those with significant pain and disability due to vertebrogenic LBP. This appears to be supported by the existing literature, in which the vast majority of study participants observed to initially benefit from BVN RFA continued to report significant pain and functional improvement at 1, 2, and 5 years (Figures 2–5). This pattern appears to be the same in industry-funded [3, 7–10, 36] and independently performed studies [1, 5]. Along with robust improvements in pain and function, healthcare utilization appears to decrease substantially after BVN RFA. In published studies, individuals treated with BVN RFA have seldom required further interventional or surgical treatment. Of the participants who were followed in the SMART trial, only 3% had received a lumbosacral facet joint RFA treatment or spinal injection in the year preceding the 5-year data collection time point [9]. Similarly, 5% of participants from INTRACEPT received an epidural steroid injection at a treated level by the 2-year data collection time point [36]. In both of these studies, a minority of participants progressed to a fusion surgery (8% and 4.5%, respectively). Opioid utilization decreased in participants over time after BVN RFA in most studies but did not differ significantly from the sham or standard-of-care groups at 3–12 months [4, 8, 10]. Despite the lack of significant difference between control arms in the RCTs at 3 months, the 5-year observational data from SMART (which included crossover of sham patients to active treatment) suggested that only 8% of participants were taking opioids at long-term follow-up (compared with 30% at baseline) [9].
No delayed complications were noted in any study report. The most common reported adverse event remains transient leg pain, which is thought to be secondary to pedicle breach (see Table 1). Investigators reported an 11% rate (n = 14) of pedicle breach resulting in “non-serious” leg pain in the 127 participants treated with BVN RFA from the INTRACEPT study [36]. The median time to resolution for these symptoms was 48.5 days, and most were successfully treated with a single course of oral steroids. Similar transient leg symptoms were observed in the SMART population [7]. Two cases of retroperitoneal hemorrhage have been reported, which may be due to excessive lateral positioning resulting in violation of the lumbar segmental artery [5, 7].
Over the years, many interventional treatments have been used for those with chronic “discogenic” LBP, with varying success. Notable examples have included biaculoplasty, intradiscal RFA, methylene blue, and intradiscal steroid injection [40–45]. More recently, case series of patients treated with extraosseous, epiduroscopic BVN/sinuvertebral nerve (SVN) laser ablation and bipolar RFA have reported positive results [46–48]. Orthobiological treatments are also being adapted to treat vertebrogenic LBP; a technique for intraosseous delivery of plasma-rich growth factor (PRGF-Endoret) has been proposed [49]. Additionally, two recent RCTs investigating the effectiveness of intradiscal steroid in patients with MC1 have been published, but they found only short-term benefit at 1 month compared with intradiscal saline or anesthetic [41, 42]. Although there are no head-to-head trials comparing BVN RFA with any of these interventions in an appropriate population, there are now multiple studies and 5-year outcomes to support the use of BVN RFA for the treatment of vertebrogenic pain. This is not the case for any of these other treatments.
Although the outcomes of BVN RFA reported in the published literature are robust, there could be opportunities to further refine patient selection. Until recently, the characteristic pain patterns for those with vertebrogenic LBP were not directly described; however, analysis of responder characteristics from a large population of patients treated with BVN RFA has been reported [50]. Those with vertebrogenic pain experience predominantly midline LBP without lower leg symptoms, but they also indicated pain in the paramidline and buttock region. Notably, these descriptions are based on a case definition of significant pain relief or functional improvement after BVN RFA (for lack of a superior gold standard of “true” vertebrogenic pain). This is perhaps not surprising given the substantial overlap in the known pain referral patterns from other spinal structures, including the disc, sacroiliac joint, and zygapophyseal facet joint [51–53].
Given the widespread use of diagnostic/prognostic blocks in interventional pain medicine, clinicians might hope for a similar tool to help select patients for BVN RFA. However, although directly anesthetizing the intraosseous BVN seems attractive as a diagnostic test at face value, there are several major issues with this approach. Anesthetic placed at the periosteum of the pedicle before access would almost certainly anesthetize the lumbar medial branch, potentially introducing confounding from relief of lumbar zygapophyseal joint pain. Because of the vascular nature of trabecular bone, local anesthetic injection within the vertebral body might not produce a consistent blockade of the BVN, leading to false-negative tests. Significant procedural pain after transpedicular access might also be a source of false-negative results. Given these challenges, clinicians are encouraged to carefully select patients for BVN RFA on the basis of the clinical and imaging paradigm described in studies published to date.
There are important limitations to this review and for the existing literature related to BVN RFA. RCTs, although not without their own limitations [54, 55], continue to represent the gold-standard study design in medical research [56]. Despite the growing interest in the treatment of vertebrogenic LBP, the present updated review found no new RCTs examining BVN RFA compared with sham or any other treatment. The majority of studies that met the inclusion criteria were supported by industry funding. When the evidence for treatment comes entirely from industry-funded studies, there is an increased risk for bias given the inherent conflict of interest, limiting the publication of negative results [57, 58]. However, it is notable that results from two independently performed studies show similarly high proportions of patients reporting clinically significant pain relief and functional improvement up to 12 months after BVN RFA [1, 5]. The present review was supported by an investigator-initiated research grant from Relievant Medsystems, which produces a device frequently used for BVN RFA. However, the sponsor had no role in the design or conduct of the review or approval of the final manuscript. The protocol, search, data extraction, and statistical analysis were all developed and performed independently without input or oversight from the sponsor.
This review has several strengths. The review was designed, executed, and reported in accordance with quality guidelines for systematic reviews [27, 59]. Although already performed in our prior work, abstract review, full-text review, and data extraction were again performed in duplicate to ensure accuracy. When data considered critical to the review's research question were missing, the authors of included studies were contacted to obtain this information. The single-arm meta-analysis presented provides clinicians with a useful estimate of the effectiveness of BVN RFA when patients are selected on the basis of the published inclusion and exclusion criteria used to date.
Conclusion
According to GRADE, there continues to be “moderate”-quality evidence that BVN RFA effectively reduces chronic LBP and associated disability in individuals with chronic vertebrogenic LBP associated with MC1 and MC2 in the L3 to S1 vertebral bodies. Between 65% and 75% of such patients report clinically significant pain and functional improvement at 6 and 12 months after BVN RFA, with similar success rates up to 5 years. Further high-quality studies will likely improve our understanding of the effectiveness of this procedure.
Contributor Information
Aaron Conger, Division of Physical Medicine and Rehabilitation, University of Utah, Salt Lake City, Utah, USA.
Taylor R Burnham, Division of Physical Medicine and Rehabilitation, University of Utah, Salt Lake City, Utah, USA.
Tyler Clark, Division of Physical Medicine and Rehabilitation, University of Utah, Salt Lake City, Utah, USA.
Masaru Teramoto, Division of Physical Medicine and Rehabilitation, University of Utah, Salt Lake City, Utah, USA.
Zachary L McCormick, Division of Physical Medicine and Rehabilitation, University of Utah, Salt Lake City, Utah, USA.
Funding sources: This investigator-initiated review was supported by a grant from Relievant MedSystems (paid directly to the University of Utah). The sponsor had no role in the design or conduct of the review or in the approval of the final manuscript. The protocol, search, data extraction, and statistical analysis were developed and performed independently.
Disclosures and Conflicts of interest: Dr. Aaron Conger and Dr. Zachary L. McCormick have received investigator-initiated research funding from Relievant MedSystems (paid directly to the University of Utah).
Supplement sponsorship: This article appears as part of the supplement entitled “Vertebrogenic Pain and Basivertebral Nerve Radiofrequency Ablation” sponsored by Relievant Medsystems Inc.
Study registration: PROSPERO (ID: CRD42020192001).
References
- 1. De Vivo AE, D'Agostino G, D'Anna G, et al. Intra-osseous basivertebral nerve radiofrequency ablation (BVA) for the treatment of vertebrogenic chronic low back pain. Neuroradiology 2021;63(5):809–15. [DOI] [PubMed] [Google Scholar]
- 2. Michalik A, Conger A, Smuck M, Maus TP, McCormick ZL.. Intraosseous basivertebral nerve radiofrequency ablation for the treatment of vertebral body endplate low back pain: Current evidence and future directions. Pain Med 2021;22(Suppl 1):S24–30. [DOI] [PubMed] [Google Scholar]
- 3. Macadaeg K, Truumees E, Boody B, et al. A prospective, single arm study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 12-month results. North Am Spine Soc J 2020;3:100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Markman JD, Rhyne AL, Sasso RC, et al. Association between opioid use and patient-reported outcomes in a randomized trial evaluating basivertebral nerve ablation for the relief of chronic low back pain. Neurosurgery 2019;86(3):343–7. [DOI] [PubMed] [Google Scholar]
- 5. Fishchenko IV, Garmish AR, Kravchuk LD, Saponenko AI, Clinic CM.. Radiofrequency ablation of the basivertebral nerve in the treatment of chronic low back pain: Analysis of a small clinical series. Hir Pozvonochnika 2021;18(3):61–7. [Google Scholar]
- 6. Truumees E, Macadaeg K, Pena E, et al. A prospective, open-label, single-arm, multi-center study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Eur Spine J 2019;28(7):1594–602. [DOI] [PubMed] [Google Scholar]
- 7. Fischgrund JS, Rhyne A, Franke J, et al. Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: A prospective randomized double-blind sham-controlled multi-center study. Eur Spine J 2018;27(5):1146–56. [DOI] [PubMed] [Google Scholar]
- 8. Smuck M, Khalil J, Barrette K, et al. Prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 12-month results. Reg Anesth Pain Med 2021;46(8):683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fischgrund JS, Rhyne A, Macadaeg K, et al. Long-term outcomes following intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 5-year treatment arm results from a prospective randomized double-blind sham-controlled multi-center study. Eur Spine J 2020;29(8):1925–34. [DOI] [PubMed] [Google Scholar]
- 10. Fischgrund JS, Rhyne A, Franke J, et al. Intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 2-year results from a prospective randomized double-blind sham-controlled multicenter study. Int J Spine Surg 2019;13(2):110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khalil JG, Smuck M, Koreckij T, et al. A prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Spine J 2019;19(10):1620–32. [DOI] [PubMed] [Google Scholar]
- 12. Becker S, Hadjipavlou A, Heggeness MH.. Ablation of the basivertebral nerve for treatment of back pain: A clinical study. Spine J 2017;17(2):218–23. [DOI] [PubMed] [Google Scholar]
- 13. Antonacci MD, Mody DR, Heggeness MH.. Innervation of the human vertebral body. J Spinal Disord 1998;11(6):526–31. [PubMed] [Google Scholar]
- 14. Brown MF, Hukkanen MVJ, McCarthy ID, et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Jt Surg 1997;79-B(1):147–53. [DOI] [PubMed] [Google Scholar]
- 15. Bailey JF, Liebenberg E, Degmetich S, Lotz JC.. Innervation patterns of PGP 9.5-positive nerve fibers within the human lumbar vertebra. J Anat 2011;218(3):263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fras C, Kravetz P, Mody DR, Heggeness MH.. Substance P–containing nerves within the human vertebral body. An immunohistochemical study of the basivertebral nerve. Spine J 2003;3(1):63–7. [DOI] [PubMed] [Google Scholar]
- 17. Ohtori S, Inoue G, Ito T, et al. Tumor necrosis factor–immunoreactive cells and PGP 9.5–immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back pain and modic type 1 or type 2 changes on MRI. Spine (Phila Pa 1976) 2006;31(9):1026–31. [DOI] [PubMed] [Google Scholar]
- 18. Jensen TS, Bendix T, Sorensen JS, et al. Characteristics and natural course of vertebral endplate signal (Modic) changes in the Danish general population. BMC Musculoskelet Disord 2009;10:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jensen TS, Kjaer P, Korsholm L, et al. Predictors of new vertebral endplate signal (Modic) changes in the general population. Eur Spine J 2010;19(1):129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rade M, Määttä JH, Freidin MB, et al. Vertebral endplate defect as initiating factor in intervertebral disc degeneration; strong association between endplate defect and disc degeneration in the general population. Spine (Phila Pa 1976) 2018;43(6):412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Määttä JH, Rade M, Freidin MB, et al. Strong association between vertebral endplate defect and Modic change in the general population. Sci Rep 2018;8(1):16630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berg-Johansen B, Jain D, Liebenberg EC, et al. Tidemark avulsions are a predominant form of endplate irregularity. Spine (Phila Pa 1976) 2018;43(16):1095–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dudli S, Fields AJ, Samartzis D, Karppinen J, Lotz JC.. Pathobiology of Modic changes. Eur Spine J 2016;25(11):3723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Conger A, Schuster NM, Cheng DS, et al. The effectiveness of intraosseous basivertebral nerve radiofrequency neurotomy for the treatment of chronic low back pain in patients with Modic changes: A systematic review. Pain Med 2021;22(5):1039–54. [DOI] [PubMed] [Google Scholar]
- 25. Hung M, Saltzman CL, Kendall R, et al. What are the MCIDs for PROMIS, NDI, and ODI instruments among patients with spinal conditions? Clin Orthop Relat Res 2018;476(10):2027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ostelo RWJG, de Vet HCW.. Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol 2005;19(4):593–607. [DOI] [PubMed] [Google Scholar]
- 27. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol 2021;134:178–89. [DOI] [PubMed] [Google Scholar]
- 28. Sutton AJ, Abrams KR, Jones DR, Sheldon TS.. Methods for Meta-Analysis in Medical Research. Chichester, West Sussex, UK: John Wiley & Sons, Ltd; 2000. [Google Scholar]
- 29. Higgins JPT, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miller JJ. The inverse of the Freeman–Tukey double arcsine transformation. Am Stat 1978;32(4):138. [Google Scholar]
- 31. Freeman MF, Tukey JW.. Transformations related to the angular and the square root. Ann Math Stat 1950;21(4):607–11. [Google Scholar]
- 32. Newcombe RG. Two-sided confidence intervals for the single proportion: Comparison of seven methods. Stat Med 1998;17(8):857–72. [DOI] [PubMed] [Google Scholar]
- 33. Harbord RM, Egger M, Sterne JAC.. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Stat Med 2006;25(20):3443–57. [DOI] [PubMed] [Google Scholar]
- 34. Egger M, Smith GD, Schneider M, Minder C.. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 36. Koreckij T, Kreiner S, Khalil JG, et al. Prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 24-Month treatment arm results. North Am Spine Soc J 2021;8:100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guyatt GH, Oxman AD, Vist GE, GRADE Working Group, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fields AJ, Liebenberg EC, Lotz JC.. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J 2014;14(3):513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lotz JC, Fields AJ, Liebenberg EC.. The role of the vertebral end plate in low back pain. Glob Spine J 2013;3(3):153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barendse GAM, van den Berg SGM, Kessels AHF, Weber WEJ, van Kleef M.. Randomized controlled trial of percutaneous intradiscal radiofrequency thermocoagulation for chronic discogenic back pain. Spine (Phila Pa 1976) 2001;26(3):287–92. [DOI] [PubMed] [Google Scholar]
- 41. Tavares I, Thomas E, Cyteval C, et al. Intradiscal glucocorticoids injection in chronic low back pain with active discopathy: A randomized controlled study. Ann Phys Rehabil Med 2021;64(2):101396. [DOI] [PubMed] [Google Scholar]
- 42. Nguyen C, Boutron I, Baron G, et al. Intradiscal glucocorticoid injection for patients with chronic low back pain associated with active discopathy. Ann Intern Med 2017;166(8):547. [DOI] [PubMed] [Google Scholar]
- 43. Kallewaard JW, Wintraecken VM, Geurts JW, et al. A multicenter randomized controlled trial on the efficacy of intradiscal methylene blue injection for chronic discogenic low back pain: The IMBI study. Pain 2019;160(4):945–53. [DOI] [PubMed] [Google Scholar]
- 44. Kapural L, Vrooman B, Sarwar S, et al. A randomized, placebo-controlled trial of transdiscal radiofrequency, biacuplasty for treatment of discogenic lower back pain. Pain Med 2013;14(3):362–73. [DOI] [PubMed] [Google Scholar]
- 45. Khot A, Bowditch M, Powell J, Sharp D.. The use of intradiscal steroid therapy for lumbar spinal discogenic pain: A randomized controlled trial. Spine (Phila Pa 1976) 2004;29(8):833–6. [DOI] [PubMed] [Google Scholar]
- 46. Kim HS, Adsul N, Yudoyono F, et al. Transforaminal epiduroscopic basivertebral nerve laser ablation for chronic low back pain associated with Modic changes: A preliminary open-label study. Pain Res Manag 2018;2018:6857983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim JY, Kim HS, Wu PH, Jang I-T.. Alleviating paravertebral muscle spasm after radiofrequency ablation treatment of hypersensitive basivertebral and sinuvertebral nerves for chronic discogenic back pain. Pain Physician 2021;24(6):E883–92. [PubMed] [Google Scholar]
- 48. Kim HS, Wu PH, Jang IT.. Lumbar degenerative disease part 1: Anatomy and pathophysiology of intervertebral discogenic pain and radiofrequency ablation of basivertebral and sinuvertebral nerve treatment for chronic discogenic back pain: A prospective case series and review of lite. Int J Mol Sci 2020;21(4):1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kirchner F, Pinar A, Milani I, et al. Vertebral intraosseous plasma rich in growth factor (PRGF-Endoret) infiltrations as a novel strategy for the treatment of degenerative lesions of endplate in lumbar pathology: Description of technique and case presentation. J Orthop Surg Res 2020;15(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McCormick ZL, Sperry BP, Boody BS, et al. Pain location and exacerbating activities associated with treatment success following basivertebral nerve ablation: An aggregated cohort study of multicenter prospective clinical trial data. Pain Med 2022; (doi: 10.1093/pm/pnac069). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. DePalma MJ, Ketchum JM, Saullo T.. What is the source of chronic low back pain and does age play a role? Pain Med 2011;12(2):224–33. [DOI] [PubMed] [Google Scholar]
- 52. Depalma MJ, Ketchum JM, Trussell BS, Saullo TR, Slipman CW.. Does the location of low back pain predict its source? PM R 2011;3(1):33–9. [DOI] [PubMed] [Google Scholar]
- 53. Hancock MJ, Maher CG, Latimer J, et al. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J 2007;16(10):1539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kaptchuk TJ. The double-blind, randomized, placebo-controlled trial: Gold standard or golden calf? J Clin Epidemiol 2001;54(6):541–9. [DOI] [PubMed] [Google Scholar]
- 55. Mielke D, Rohde V.. Randomized controlled trials—a critical re-appraisal. Neurosurg Rev 2021;44(4):2085–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol 2011;64(4):407–15. [DOI] [PubMed] [Google Scholar]
- 57. Canestaro WJ, Hendrix N, Bansal A, et al. Favorable and publicly funded studies are more likely to be published: A systematic review and meta-analysis. J Clin Epidemiol 2017;92:58–68. [DOI] [PubMed] [Google Scholar]
- 58. Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K.. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst Rev 2009;2009(1):MR000006. doi: 10.1002/14651858.MR000006.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]