Abstract
Background and aims:
Cardiovascular Disease (CVD) poses significant health risks for seniors, especially among low-income and minority communities. Senior centers offer multiple services. We tested whether implementing two evidence-based interventions- DASH-aligned meals provided through an existing congregate meal program, and support for home Self-Measured Blood Pressure (SMBP) monitoring-lowers blood pressure among participants at two senior centers serving low-income, racially diverse communities.
Methods and results:
Open-label study, enrolling clients aged ≥60, eating ≥4 meals/week at two NYC senior centers. Participants received DASH-aligned congregate meals, and training in nutrition, BP management education, and personal SMBP device. Co-Primary outcomes: a) change in systolic BP measured by independent health professionals, and b) change in percent with “controlled BP” (Eighth Joint National Committee (JNC-8) Guidelines), at Month 1 compared to Baseline. Secondary outcomes: Changes in BP at Months 3 and 5/6 (last measure).
We enrolled 94 participants; COVID closures interrupted implementation mid-study. Mean systolic BP at Month-1 changed by −4.41 mmHg (n = 61 p = 0.07) compared to Baseline. Participants with controlled BP increased (15.7%) at Month 1. Change in mean BP at Month 1 was significantly correlated with BMI (p = 0.02), age (p = 0.04), and baseline BP (p < 0.001). Mean systolic SMBP changed by −6.9 mmHg (p = 0.004) at Months 5/6.
Conclusions:
Implementing an evidence-based multi-component BP-lowering intervention within existing congregate meal programs at senior centers serving minority and low-income communities is feasible, and early findings show promising evidence of effectiveness. This approach to cardiovascular risk reduction should be further tested for widespread adoption and impact.
Keywords: Cardiovascular risk, Hypertension, Self-efficacy, Community-based, Seniors, DASH diet, Nutritional intervention, Implementation research
1. Background
Cardiovascular Disease (CVD) poses significant health risks for older individuals, including stroke, renal failure, myocardial infarction, heart failure, premature mortality, and increases costs and suffering related to mortality, morbidity, and healthcare utilization [1]. CVD is highly prevalent, with over two thirds of adults aged 60 to 79 having one or more form of CVD [1]. The impact of CVD will increase as the current US population continues to age [2]. In the US, racial and ethnic minorities and people of lower socioeconomic status experience higher rates of CVD and higher mortality [3]. Social determinants of health further compound cardiovascular risk and worsen outcomes and increase health disparities for older individuals.
High blood pressure (BP) is one of the major modifiable risk factors for CVD-related morbidity and mortality and for stroke, and older adults are at increased risk for developing high or uncontrolled BP [4]. Reductions in systolic BP (SBP) of 10 mmHg are associated with significant reduction of CVD risks (13% for microvascular complications, 11% for myocardial infarction, 15% for deaths related to diabetes) [5]. At the population level, BP reductions of as little as 1 mmHg could have a significant impact on preventing cardiovascular events [6]. National practice guidelines have long recommended lifestyle modifications proven to reduce elevated BP [7] including specific nutritional interventions such as the Dietary-Approaches to-Stop-Hypertension (DASH), and Self-Measured Blood Pressure (SMBP) monitoring [8].
DASH is a nutritional approach with demonstrated efficacy to lower SBP in as little as 14 days in research settings [9]. The DASH diet is rich in whole grains, fruits and vegetables, nuts, healthy fats, and low-fat dairy products, and recommends limiting sweets, sodium, and red and processed meats [9,10]. DASH can decrease blood pressure in all participants, including those without hypertension, though the blood pressure reduction is greater among hypertensive individuals [9]. A 2020 systematic review found that even modest adherence to the DASH diet is associated with lower risk of all-cause and cause-specific mortality [10]. Recent studies suggest a promising role for the DASH diet in reducing measures of cardiometabolic risk among seniors [11,12]. Studies also suggest that DASH is more effective at lowering BP among Black participants than Whites [13–15] and that DASH improves hypertension and hyperlipidemia among Hispanic [16] and African American adults [17]. Additionally, studies have found that minority and lower socio-economic communities adopting DASH eating plans face adherence challenges due to food availability, cost, and cultural dietary preferences, and highlight the need for culturally responsive dietary programs [18,19]. Despite a strong evidence base for DASH efficacy in controlled settings, there are no studies testing the implementation of a DASH diet intervention among older adults in a community setting that includes congregate meals.
Seniors affected by financial need, food insecurity, immobility, or social isolation often turn to community nutrition services for resources and support. In New York City (NYC), the NYC Department for the Aging (DFTA) funds meal services at 249 senior centers throughout the five boroughs serving adults over the age of 60. In the 2019 fiscal year, DFTA provided 7.18 million congregate meals at senior centers, at no cost to 131,000 older adults [20]. The Carter Burden Network (CBN) is one such organization offering DFTA-subsidized congregate meals and other services. In 2016, The Rockefeller University Center for Clinical and Translational Science (RU) and Clinical Directors Network (CDN), a primary care practice-based research network (PBRN), engaged in a community-academic research partnership with CBN to address unmet health needs among their clients. Incorporating principles of community engagement [21] and community-based participatory research [22], a pilot study conducted at two CBN centers found a high prevalence of poorly controlled high blood pressure accompanied by high levels of food insecurity [23].
Self-Measured Blood Pressure (SMBP) monitoring is an evidence-based strategy demonstrated to improve blood pressure control [24–28] and is included in several Clinical Practice Guidelines for the management of hypertension [28]. A few studies that have focused on the impact of SMBP on blood pressure management among ethnically diverse populations in the US have produced mixed results [29–31]. Some had promising findings [29], including among older adult participants with substantial minority representation [30]. Others highlighted that additional behavioral support may be needed for SMBP to be effective in these communities [31].
Given the CBN seniors’ underlying vulnerability to excess CVD risk, high levels of food insecurity, and the popularity of the CBN congregate meals program, we saw an opportunity to build upon the strength of our established partnership to address high BP in this population. RU, CDN and CBN collaborated to develop and conduct an intervention study to test whether combining two evidence-based approaches to increasing BP self-management - (1) aligning CBN congregate meals with the DASH diet, and (2) providing high quality automated BP monitors and education to promote SMBP at home–could be implemented with senior centers to improve self-management and reduce high BP among seniors attending CBN congregate meals program.
2. Methods
Research Ethics:
The Rockefeller University Institutional Review Board (IRB) approved the project for all sites under a Single IRB mechanism. Informed consent was obtained before initiating study activities.
Study Design:
We designed an open-label study to test whether providing 1) DASH-aligned menus in an ongoing congregate meal program, and 2) educational and behavioral support for home SMBP monitoring, lowers blood pressure among community-living seniors attending two senior centers in New York City.
The Primary Aim was to test whether the combined dietary and behavioral interventions lowered BP at Month 1. Co-Primary Outcome Measures were: 1a) the change in mean systolic BP (mmHg), and 1b) the change in proportion (%) of individuals whose BP is “controlled” according to Eighth Joint National Committee (JNC 8) guidelines (for age>60 years, SBP/DBP <150/90) [32] at Month 1 after initiation of the interventions, compared to Baseline. BP for the co-Primary Outcome measures was assessed by an independent healthcare contractor Vital Care Services (VC) (https://www.myvics.com/), using the Fora P20b BP Monitoring System according to their Community Telehealth Standard Operating Procedures, incorporating support from a Telehealth Technician Associate (TTA) on-site, and from a registered nurse remotely. Secondary Aims addressed the sustainability of changes to blood pressure and blood pressure control. Secondary outcome measures included: the change in mean systolic BP, and the change in the percentage of individuals with “controlled” BP, at Month 3 and Month 6 after initiation of the study interventions, compared to Baseline, as measured by VC. Additional secondary outcome measures assessed SMBP frequency (mean # days/week with ≥1 SMBP measure), and the change in mean systolic SMBP (average of all self-measured systolic BP in the last week of the time period), at Months 1, 3, and 6, compared to the mean systolic SMBP in the week at Baseline.
Exploratory aims related to cognitive, behavioral, nutritional, and psychosocial aspects of the intervention were assessed through the validated surveys completed at Baseline, and Months 1, 3, and 6.
Study Participants and settings:
The study was designed to enroll 200 adults, 100 at each of two sites. Eligible participants were CBN clients age 60 or older, consumed at least 4 meals/week at the study sites, and provided informed consent. There was no BP threshold for entry. Site 1, located in East Harlem, serves breakfast and lunch seven days a week; 125–150 clients attend one or more congregate meals each week. Social determinants of health among the neighborhood population include poverty, food insecurity and low rates of higher education [33]; many clients at this site are racial and ethnic minorities, and live alone; 13% do not speak English. Site 2, located on the Upper East Side of New York City, serves lunch five days a week to about 120 clients. The majority in the neighborhood are White and hold a high school diploma; many clients at Site 2 are economically distressed, live alone in rent-stabilized apartments, and some report homelessness (CBN data, unpublished).
Study procedures:
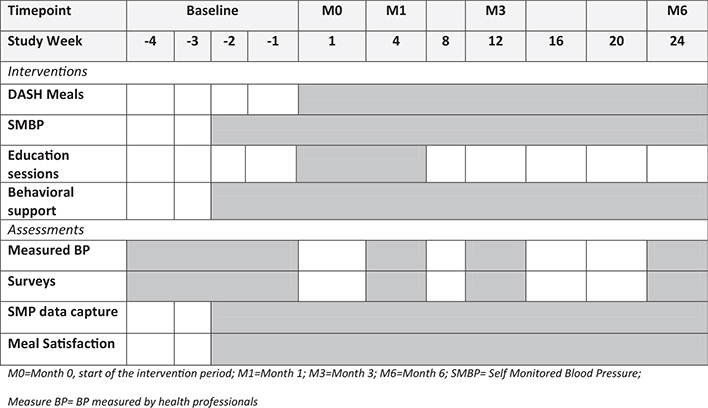
An overview of the study procedures timeline is provided in Fig. 1.
Figure 1.
Study Procedures Timeline. Schedule of planned intervention components and study assessments for this 6-month study. Weeks are relative to start of interventions at each Site.
Physiologic Measures:
At Baseline, and Months 1, 3, and 6 after start of the interventions, participants completed assessments of comorbidities, and physiologic measures including BP, pulse, BMI. Health assessments were conducted by trained technicians and nurses from healthcare contractor Vital Care Services (www.myvics.com/). BP was measured in a quiet setting, sitting with legs uncrossed, after a period of at least 5 min of rest, according to written standard operating procedures.
Cognitive, behavioral, nutritional and psychosocial measures were assessed through self-administration of 12 validated surveys [34–45] at Baseline, and Months 1, 3, and 6. Where possible, we used survey instruments validated for use in low-literacy populations in both English and Spanish. Initially surveys were completed on-site using electronic tablets. After COVID-related closures, some Month 3 and Month 6 surveys were collected remotely via weblink or returned as a paper copy, by participant preference. We used the published scales or developed measures to analyze surveys (described in Supplemental Appendix A).
Behavioral Intervention:
All participants were given a personal Bluetooth-enabled BP monitor (Omron 10, Model BP7450). Study staff trained participants to use the monitor with video and one-on-one instruction. Participants were asked to take their blood pressure twice a day (morning and evening) at the same time each day. They were to sit still with feet flat on the floor and rest 5 min before taking their blood pressure and place their left arm on a table to ensure cuff was level with their heart. After training, participants were observed putting the cuffs on correctly and using the device. Participants were asked to remain still and relax while the machines automatically took three blood pressure measurements, with 1 min of rest between measurements, and displayed the average. Participants were asked to measure their BP twice daily, at the same times each day, through Month 1, and then daily thereafter through Month 6. Participants also recorded readings manually and were encouraged to share results with their primary care clinician. Research staff met with participants every two to three weeks to download SMBP data, support adherence, and collect barriers and facilitators to SMBP. COVID-related closures shifted these activities from in-person to remote support.
The Dietary Intervention replaced the usual congregate meals served at each site with a six-week cycle of DASH-aligned meals, providing 50% of DASH components at lunch (both sites), and 70% of DASH components from breakfast and lunch combined (Site 1). Townhall meetings conducted in English and Spanish engaged CBN clients in taste-testing during menu design to incorporate seniors’ preferences. Nutrition education is described below. The New York City Department for the Aging (DFTA) provided input to ensure compliance with that agency’s nutritional guidelines, distinct from DASH requirements. A typical menu change is illustrated in Supplemental Appendix B. The timeline for implementation of DASH-aligned meals is provided in Fig. 1. COVID-related site closures interrupted the on-site meal service at Month 5 for Site 1, and Month 1 outcome data collection for Site 2. The CBN food service pivoted promptly to provide DASH-aligned “grab-and-go” packed meals for participants to pick up while social distancing. Shortly thereafter, New York City instituted a home-delivered meal service for seniors isolating under COVID and the project meals ceased. Thereafter, participants were encouraged to incorporate DASH eating principles (nutrition education sessions described below) into at-home meals, and outcome data were collected remotely.
Meal Satisfaction was measured ≥2 times weekly before and throughout the nutritional intervention. Colorful graphic Smiley Likert Food Satisfaction rating cards (in low-literacy English and Spanish) were distributed during meals to collect anonymous ratings and open-text comments.
Educational Component:
Three bilingual educational programs were provided upon the start of the dietary and BP interventions after Day 0: 1) A classroom style review of the basics of hypertension, lifestyle management and medication adherence led by an experienced primary care physician (WP), 2) a hands-on Nutrition education class covered the DASH eating plan, portion control, shopping, utilizing food pantry items, and meal preparation, led by registered dietitians (AR, GG-A, DV), and 3) Individual and group training in use of the personal SMBP devices, using materials from the American Heart Association, the American Medical Association, the NYC Department of Health and other vetted sources (https://www.CDNetwork.org/cbn-dash).
2.1. Statistical analysis
All data were stored in REDCap files database [46] and imported into SAS Studio 9.4 for analysis. To assess changes in mean systolic BP, a paired t-test was conducted (for each site separately and for both sites combined) between Baseline and Month 1. A McNemar Chi-squared test (for paired data) examined differences in the percentage of those who had controlled blood pressure (JNC-8 criteria) between Baseline and Month 1.
From downloaded SMBP measures, we calculated the mean systolic SMBP for each individual, for each week of study. To assess the change in SMBP over the course of the study, we compared the mean change from the Baseline-SMBP to the Month 6-SMBP. We defined the Baselinemean systolic SMBP measure as the mean of the individual participant’s systolic SMBP for Week 1, and defined the Month 6 mean systolic SMBP as the mean of the individual’s systolic SMBP for the last week of measures collected in Month 6. Mean systolic SMBP differences were analyzed via a paired t-test comparing Baseline with four end points: Last Week of Month 1, Last week of Month 3, and Last week of Month 5 or 6 (last available). A sample size analysis was conducted before the study and yielded a needed sample size of 200 for 80% power, with expected dropout (defined as enrolled but no 1 month BP measurement) to be a maximum of 10%. A sample size of 200 would allow for detection of 24% of a standard deviation change in mean blood pressure, translating into a 5mm/Hg change, and an absolute proportion change of 18% in blood pressure control, holding Type I Error to 5% (two-tailed).
A linear regression analysis was conducted to identify participant characteristics associated with response to the study interventions. Change in systolic blood pressure (Vital Care Measure) from baseline to Month 1 was used as the dependent variable for all regression models. Variables that were deemed a priori to be important covariates, and variables that were statistically significant in univariate models were selected for inclusion in the final regression model presented here.
3. Results
Ninety-four older adults consented to join the study across the two sites. Interventions began at Site 1 in October 2019, and at Site 2 in February 2020. Average weekly meal attendance and meal satisfaction measured in the weeks before and after conversion to DASH-aligned menus were unchanged. Interventions were delivered as planned until March 2020 when New York City mandated COVID-19-related closure of in-person services shifted study conduct and data collection to remote platforms. The CONSORT diagram (Fig. 2) illustrates the flow of participants at each site, participation in study assessments, and study attrition. Due to COVID-mandated site closures, 19 participants at Site 2 (23% of participants overall) were unable to attend their Month 1 assessment of primary outcome. Of the 84 participants who completed the Baseline assessments, 61 (73%) completed Month 1 assessments. At study completion in July 2020, 57/84 (68%) of participants had returned a Month 6 survey.
Figure 2.
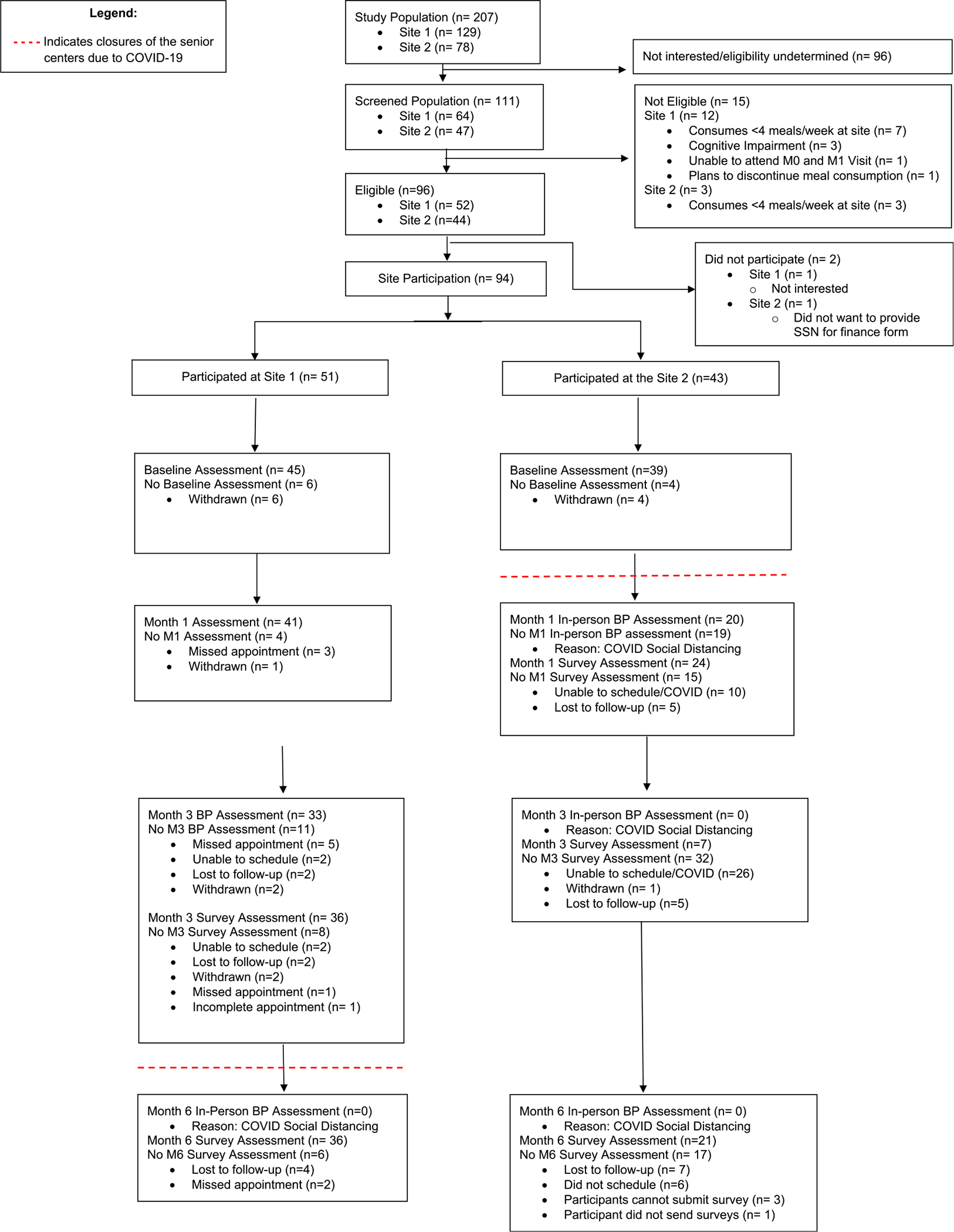
CONSORT Diagram of DASH Senior Center Intervention. Consort diagram shows the flow of participants through the study. Site closures due to COVID-19 pandemic-related public health mandates interrupted the delivery of congregate meals and in-person assessments including primary outcome measures; many assessments could be collected remotely.
Characteristics of the study participants are shown in Table 1. Participants at Site 1 were more likely to be persons of color and to have lower educational attainment than those at Site 2. The income distribution was similar in both sites: 43% of participants reported an annual income of less than $20,000. Overall, 63% of participants were either overweight or obese. At Baseline, few participants had BP in the normal range (19%), most had stage 1 or stage 2 hypertension (71%) and some were in hypertensive crisis (4%). Overall, 42% of the 84 participants who completed Baseline measures scored as Food Insecure on the Food Insecurity survey.
Table 1.
Participant characteristics at baseline.
| Characteristics – Baseline | Site 1 (n = 45) | Site 2 (n = 39) | Total (n = 84) |
|---|---|---|---|
|
| |||
| Sex (Female) | 34 (76%) | 23 (59%) | 57 (68%) |
| Age, years (Mean ± SD) | 70.6 ± 7.7 | 76.1 ± 8 | 73.2 ± 8.3 |
| Race | |||
| American Indian/Alaskan Native | 1 (2%) | 0 (0%) | 1 (1%) |
| Asian | 0 (0%) | 2 (5%) | 2 (2%) |
| Black | 22 (48%) | 3 (8%) | 25 (30%) |
| Multiple Races | 3 (7%) | 1 (3%) | 4 (5%) |
| Other/Unknown | 9 (20%) | 5 (13%) | 14 (17%) |
| White | 10 (22%) | 28 (72%) | 38 (45%) |
| Hispanic Ethnicity | 24 (53%) | 2 (5%) | 26 (31%) |
| Survey Language (Spanish) | 10 (22%) | 4 (10%) | 14 (17%) |
| Annual Income (dollars) | |||
| Less than $20,000 | 20 (44%) | 16 (41%) | 36 (43%) |
| $20,000 to less than $35,000 | 10 (22%) | 9 (23%) | 19 (23%) |
| $35,000 or more | 8 (18%) | 7 (18%) | 15 (18%) |
| Unknown | 7 (16%) | 7 (18%) | 14 (17%) |
| Education | |||
| Less than high school graduate | 8 (18%) | 3 (8%) | 11 (13%) |
| High school graduate | 13 (29%) | 6 (15%) | 19 (23%) |
| At least some college | 12 (27%) | 3 (8%) | 15 (18%) |
| College graduate | 12 (27%) | 24 (62%) | 36 (43%) |
| Unknown | 0 (0%) | 2 (5%) | 2 (2%) |
| Retired/Not currently employed | 44 (98%) | 29 (74%) | 73 (87%) |
| Marital Status | |||
| Married/member of a couple | 7 (16%) | 6 (15%) | 13 (15%) |
| Divorced/Widowed/Separated | 30 (67%) | 19 (49%) | 49 (58%) |
| Never married | 6 (13%) | 11 (28%) | 17 (20%) |
| Unknown | 1 (2%) | 3 (8%) | 4 (5%) |
| BMIa | |||
| Normal weight (18.5–24.9) | 12 (27%) | 15 (38%) | 27 (32%) |
| Overweight (25–29.9) | 13 (29%) | 14 (36%) | 27 (32%) |
| Obese (<30) | 19 (42%) | 7 (18%) | 26 (31%) |
| Unknown | 1 (2%) | 3 (8%) | 4 (5%) |
| Blood Pressure Group (mmHg)b | |||
| Normal (<120 SBP and <80 DBP) | 9 (20%) | 7 (18%) | 16 (19%) |
| Elevated (120–129 SBP and <80 DBP) | 6 (13%) | 1 (3%) | 7 (8%) |
| Hypertension Stage 1 (130–139 SBP or 80–89 DBP) | 13 (29%) | 13 (33%) | 26 (31%) |
| Hypertension Stage 2 (≥140 SBP or ≥90 DBP) | 17 (38%) | 17 (44%) | 34 (40%) |
| Hypertensive Crisis (>180 SBP and/or >120 DBP) | 3 (7%) | 0 (0%) | 3 (4%) |
| Unknown | 3 (7%) | 3 (8%) | 6 (7%) |
For the 61 participants who completed assessment at both timepoints, the change in mean systolic BP was −4.41 mmHg (p = 0.07) at Month-1 compared to Baseline (Table 2). The percentage of participants whose BP was “controlled” (according to JNC 8 guidelines) increased by 15.7% for the same time period. Neither primary outcome for the combined cohort was statistically significant. Among Site 1 participants, there was minimal BP decline (−2.66 mmHg p = 0.39) at Month 1, whereas among the Site 2 participants who completed Month 1 visits before COVID site-closure, the change in mean BP was −8.0 mmHg, and statistically significant (p < 0.05). At Month 3, the change in mean BP, was −3.51 mmHg, slightly greater than at Month 1, and not statistically significant (p = 0.25). The corresponding measures at Months 6 for Site 1, and Months 3 and 6 for Site 2 were not collected due to COVID-related closures.
Table 2.
Changes to blood pressure, as measured on-site by the health contractor.
| Site 1 | Site 2 | Total | |
|---|---|---|---|
|
| |||
| Systolic BP Baseline, mmHg | |||
| Mean ± SD | 137.62 ± 20.5 | 138.15 ± 16.97 | 137.87 ± 18.8 |
| (Range), n | (98, 191) n = 45 | (101, 175) n = 39 | (98, 191) n = 84 |
| Systolic BP Month 1, mmHg | |||
| Mean ± SD | 135.29 ± 17.09 | 129.65 ± 16.24 | 133.44 ± 16.9 |
| (Range), n | (98, 191) n = 41 | (100, 156) n = 20 | (98, 191) n = 61 |
| Primary Outcome Month 1 – Baseline, mmHg | |||
| Mean Change ± SD | −2.66b ± 19.56 | −8.0 ± 16.9 | −4.41 ± 18.76 |
| t = −0.87, p = 0.39 | t = −2.12, p = 0.048 | t = −1.84, p = 0.07 | |
| n = 41 | n = 20 | n = 61 | |
| Systolic BP Month 3, mmHg | |||
| Mean ± SD | 134.48 ± 14.09 | ||
| (Range), n | (105, 165), n = 33 | ||
| Month 3-Baseline, mmHg | |||
| Mean Change ± SD | −3.51 ± 17.2 | ||
| t = −1.17, p = 0.25 | |||
| n = 33 | |||
| Controlleda BP, Baseline | 71.1% | 64.1% | 67.9% |
| Controlleda BP, Month 1 | 80.5% (χ2 = 2.67, p = 0.10) | 90% (χ2 = 0.33, p = 0.56) | 83.6% (χ2 = 2.78, p = 0.10) |
| Controlleda BP, Month 3 | 81.8% (χ2 = 1.90, p = 0.17) | ||
As defined by JNC-8 Guidelines (32).
Bold items reflect the primary outcomes and associated measures of their significance.
We assessed the change in SMBP across the duration of the study for all participants and each study site using individuals’ assessments from the first and last weeks of the observation periods (Table 3). For some participants at Site 1 who did not have Week 1 measures, we used Week 2 measures for their Baseline-SMBP. Week 1 measures for SMBP were available for all Site 2 participants. SMBP Baselines are presented as Week 1/2. Similarly, not all participants continued SMBP measures to the end of Month 6, so we used the last available measure in Month 5 and Month 6 as the last measure, called Month 5/6.
Table 3.
Change in self-measured (systolic) BP (SMBP), both sites.
| Time Periods | N | Mean Change, mmHg | T-value (p-value) |
|---|---|---|---|
|
| |||
| Week 1/2 to End of Month 1 | 43 | −0.86 ± 9.74 | −0.58 (p = 0.56) |
| Week 1/2 to End of Month 3 | 34 | 0.43 ± 2.46 | 0.86 (p = 0.86) |
| Week 1/2 to End of Month 5/6 | 25 | −6.90a ± 10.70 | −3.23 (p = 0.004) |
Statistically significant values, and measures of significance, are shown in bold text.
Participants reported facilitators to taking SMBP included leaving the device out in a highly visible location, pairing the timing of measurements with other established habits (e.g. breakfast, brushing teeth), engaging a household member for assistance, setting an alarm, and other active reminders. Participants main barriers were discomfort with the cuff, lack of confidence about the measures, and forgetting.
Overall, 43/84 (51%) participants who received a personal BP device completed SMBP measures through Month 1, and 25/84 (30%) continued taking measures through Months 5/6 (Table 3). Participants conducting SMBP took at least one measure on an average of 3 days/week (Table 4). During Week 1/2, the mean systolic SMBP measured across the cohort was 125.1 mmHg (Range: 96.3–160.9 mmHg, n = 45), and at Month 5/6, was 120.6 (Range: 96.3–151 mmHg, n = 25). The mean change in mean systolic SMBP for participants who had both Baseline and Month 5/6 data was −6.9 mmHg (p = 0.004) (Table 3).
Table 4.
Mean days/week participants self-measured BP (SMBP) at least once.
| Time period | Mean Days/Week With At Least One SMBP Measurement |
||
|---|---|---|---|
| Site 1 | Site 2 | Total | |
|
| |||
| DASH Diet Day 1 to Month 1a | 2.74 ± 1.71 n = 44 | 3.48 ± 2.26 n = 32 | 3.05 ± 1.98 n = 76 |
| Month 1 to Month 3a | 3.11 ± 1.66 n = 38 | 3.51 ± 2.23 n = 22 | 3.26 ± 1.88 n = 60 |
| Month 3 to Month 6a | 3.32 ± 1.66 n = 35 | 2.82 ± 1.88 n = 25 | 3.11 ± 1.76 n = 60 |
| DASH Diet Day 1 – Month 5/6b | 3.41 ± 1.35 n = 34 | 3.82 ± 1.67 n = 21 | 3.57 ± 1.48 n = 55 |
Participants who captured at least one HSBP measure in the specified time periods were included in the analysis.
Participants who captured at least one HSBP measure during Month 1 and Month 5 or 6 were included in the analysis.
To evaluate potential self-selection bias among those who completed the measurements over time, we compared the individuals who continued taking their home blood pressure until the end of the study with those who did not. Race and sex were the only statistically significant differences among the groups; women (n = 22 (88%), and p = 0.002) and Black participants (n = 15 (60%), and p = 0.04) were more heavily represented in the group who continued taking their home blood pressure measurements (n = 25), than among those who dropped out (n = 20). Baseline blood pressure was not associated with continuing to record home BP self-measurements.
We conducted a multiple linear regression analysis to identify characteristics associated with changes in the primary outcome (Table 5). We found statistically significant associations of BMI, age, and baseline blood pressure with change in mean BP from baseline to Month 1. Higher BMI was associated with an increase in BP, and higher baseline blood pressure was associated with a decrease in BP.
Table 5.
Final regression model.
| Observed (n = 52) | Coefficient | Standard Error (SE) | P-value |
|---|---|---|---|
|
| |||
| Modelb (F = 6.92) R2 = 52.4% | < 0.0001 | ||
| BMI (Normal Weight (Ref) vs. Overweight/Obese) | 10.56 | 4.56 | 0.02 |
| History of Hypertension1 (No History of Hypertension (Ref), History of Hypertension) | 5.07 | 4.30 | 0.24 |
| Food Insecurity Status (Not Food Insecure (Ref), vs. Food Insecure) | 3.64 | 4.16 | 0.38 |
| Age (years) | −0.56 | −0.27 | 0.04 |
| Baseline Blood Pressure (mmHg) | −0.73 | 0.13 | < 0.001 |
| Meal Attendance Baseline – Month 1 (meals/week) | 0.69 | 0.40 | 0.09 |
| SMBP Frequency Percentagea Baseline – Month 1 | −0.08 | 0.06 | 0.16 |
Percentage of days with at least one SMBP measurement.
Statistically significant model, variables, and values are shown in bold.
4. Discussion
Senior centers are an under-recognized venue to disseminate, implement and evaluate evidence-based health interventions [48] and there are few examples of centerbased blood pressure interventions [49,50]. In its first months, our study demonstrated the feasibility of implementing a multi-component intervention involving DASH-aligned congregate meals, SMBP and educational support for community-living seniors attending a senior center congregate meal program. Diet is an important modifiable risk factor for CVD [51], and our study provides the first evidence of implementation and potential effectiveness of DASH as part of a congregate meal program for seniors. In a smaller-than-planned cohort, our primary outcome (change of −4.41 mmHg (p = 0.07) at Month-1 compared to Baseline) did not reach statistical significance. However the impact of the combined interventions may accrue over time: some participants were able to sustain SMBP monitoring until the end of study and lowered their blood pressure in both a clinically meaningful and statistically significant way (Table 3). Our findings are aligned with the literature on the effectiveness of SMBP in lowering blood pressure [52]. Home self- BP monitoring in conjunction with other interventions such as counseling and education have been found to be more effective at lowering blood pressure than self-monitoring alone [24–26,53]. Further research is needed to identify the adaptations needed to implement and optimize multi-component interventions including DASH-aligned congregate meals, health education and SBPM in senior service settings and other community-based organizations.
Congregate meals provided by senior centers provide a sustainable setting to improve food security, nutritional status, blood pressure control and CVD risk among seniors [54]. Behavior-change theory highlights the importance of positive social influence and a supportive environment in helping individuals maintain newly adopted behavior over time [55]. By targeting a program and social space already frequented by older adults, this intervention sought to modify participants’ environments to facilitate behavior change. This approach has particular promise for communities facing health disparities. SMBP, combined with implementation of the DASH diet, can strengthen a participant’s sense of autonomy and control over their own health and can also improve collaboration with the clinician who monitors and manages their blood pressure. This might be empowering particularly for minority participants who have greater vulnerability to CVD risk, and face barriers to healthcare access. Several DASH trials that found African Americans were less likely to adhere to the DASH diet recommended additional adaptations for minority communities [17]. Our study found that Black women, who are at higher risk of adverse CVD outcomes, were more likely to continue SMBP monitoring than other participants. This gender difference warrants further exploration.
Our study has several limitations. We did not meet the planned sample size for the study due to under-enrollment. COVID curtailed delivery of the interventions as planned and interfered with collection of primary outcome data. The substitution of Week 2 SMBP measures for missing Week 1 data at Site 1 may have under-estimated the change in systolic SMBP, and many seniors conducted SMBP monitoring infrequently, reducing the number of paired observations available for analysis. Mean systolic blood pressure at Baseline for the cohort was 137 mmHg, a reflection of a broadly inclusive approach that permitted enrollment of non-hypertensive individuals.
A 2016 meta-analysis of randomized controlled trials found that healthy dietary patterns such as DASH significantly lowered systolic BP by 4.26 mm Hg [56], and our study, although under-powered, yielded a reduction of similar magnitude (−4.41 mmHg). A larger sample size would be required to detect effects of this size, especially if including non-hypertensive individuals [57]. If our findings were replicated in a larger cohort, we might observe a statistically significant difference for this clinically meaningful reduction in SBP at Month 1, and a longer follow-up time would be important to determine whether this reduction can be sustained over time.
While the observed effect sizes are lower than the prespecified outcomes of the study, given the challenges imposed by the pandemic, the signals we observed justify designing a follow-on study in a larger cohort at a time when all the planned outcome data can be collected, and other lessons learned can be attended to. Viewed through the lens of implementation science, the current study was a pilot Type I hybrid effectiveness-implementation study [58], designed to assess clinical outcomes (systolic BP reduction and improvement in BP control) while gathering preliminary information on implementation. Since the study was conducted with a single agency (CBN) there is no organizational-level variation to examine here, such as inner and outer context [59], although the participant characteristics did differ by location (see Table 2). Here, we report the frequency of home blood pressure self-monitoring as a measure of feasibility, which is one dimension to assess implementation. Table 4 demonstrates that participants were willing and able to perform home self BP monitoring, and that those who measured their BP at least once during the follow-up interval (65% of participants), self-monitored about three times per week. This number was fairly consistent over the 6-month follow-up. A future manuscript will describe the barriers and facilitators we observed and describe these within the context of the Consolidated Framework for Implementation Research (CFIR) [59].
A redesigned study could be readily streamlined by incorporating our top lessons learned: 1) Implement DASH-aligned congregate meals using the study’s NYC DFTAapproved menus; 2) Reduce the burdens to participants by omitting psychosocial surveys that added little value; 3) Support participants for SMBP while reducing burden on the research team by partnering with a peer-supported SMBP support programs or Federally Qualified Health Centers; 4) Engage more congregate meal sites to boost enrollment and cohort size; 5) In alignment with the multiple linear regression model which found initial SBP, age and BMI were significantly associated with lowering SBP, focus future efforts on the participants with higher SBP and higher BMI as those most likely to benefit from the interventions. This reinforces our continued effort to focus on older adults at a higher risk of CVD to maximize the impact of the intervention. 6) Capture setting-specific measures to understand the contribution of organization-related variables to uptake, effectiveness and sustainability.
5. Summary/significance
Our study demonstrates the potential of leveraging community-engaged research partnership models to develop and implement community-based interventions incorporating evidence-based interventions to improve health.
6. In summary
It is feasible to implement DASH aligned meals through a congregate meals program; pairing it with self-monitoring of blood pressure (SMBP) as part of multi-component evidence-based interventions.
A 4 mmHg decrease across both sites at Month 1 may be clinically meaningful; the 8 mmHg decrease at Site 2 was clinically and statistically meaningful.
Social determinants of health, including food insecurity and low-income, can drive seniors to access nutrition services from senior serving organizations. Broader adoption and dissemination of programs such as this one could meaningfully impact health outcomes among seniors experiencing health disparities.
The burdens related to research could be simplified by: using the study’s DASH-aligned menus; eliminating the surveys as they did not explain differences in outcomes, and engaging partners such as local health organizations and peer partner programs to support SMBP effectively without requiring senior centers to develop this capacity.
A community-based intervention involving congregate meals and SMBP, once streamlined, implemented and proven effective in a larger study, has potential for widespread dissemination, adoption and public health impact.
Our engagement model demonstrated the effectiveness of a multi-partner collaboration that included a government agency, nonprofit agencies and academia in developing and evaluating a community-based health intervention.
Supplementary Material
Acknowledgements
Supported by a 2018–2020 NIH/Administration for Community Living Nutritional Innovation Award to Carter Burden Network (grant #90INNU0007-01-00), and by grants from the National Institutes of Health, National Center for Advancing Translational Science, Clinical and Translational Science Award to the Rockefeller University (UL1TR0001866, UL1TR000043, and UL1TR000043-07S1) and by a grant from the Agency for Healthcare Research and Quality grant 1 P30-HS-021667 to Clinical Directors Network (CDN). The authors thank Advisory Committee Members Jacqueline Berman and Esther Maleh (New York City Department for the Aging (NYCDFTA); Margaret Casey (New York State Department of Health (NYSDOH)); David Putrino (Mount Sinai Hospital); Mia Oberlink (Visiting Nurses of New York); Alina Moran (NYC Health + Hospitals/Metropolitan Hospital); Allison Nickerson (LiveOnNY); Beth Shapiro (City Meals on Wheels); Khristel Simmons; Stanley Isaacs; Kris Allen (Leonard Covello Senior Representative); George Davis (Luncheon Club Senior Representative); David Guar, Christopher Gaur and Pramod Gaur (Vital Care Telehealth); Victor Baez (Bionutrition Department at Rockefeller University Hospital); William Dionne, Rina Desai, Debra Perez, Sharon Halliday, Sonia Diaz, Joshua Watkins, Enerdyn Almanzar, Andrew Bell, Ewart Coote, Sybil Frances, Dhurata Pjeshka, David Robinson, Alexis Saddler, Jared Tate and Mel Torres (Carter Burden Network); Cecilia Covenas, Chamanara Khalida MD MPH, Pa’Malick Mbye, Dena Moftah (Clinical Directors Network); Lara Cemo, Michale Akers and Calla Tse (Lehman University), as well as staff from the Administration for Community Living including Keri Ann Lipperini, Monique Bolton and Kathleen Otte. In Memoriam, Schuyler Antonio.
This project was supported, in part by grant 90INNU0007-01-00, from the U.S. Administration for Community Living, Department of Health and Human Services, Washington, D.C. 20201. Grantees undertaking projects with government sponsorship are encouraged to express freely their findings and conclusions. Points of view or opinions do not, therefore, necessarily represent official ACL policy. This project was also supported in part by grants UL1 TR001866 and UL1 TR000043 from the National Center for Advancing Translational Sciences (NCATS, National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program) and by a 2011 CTSA Community-Engaged Research Administrative Supplement Award NIH-NCATS grant UL1 TR000043-07S1, as well as by funding from the Agency for Healthcare Research and Quality grant 1 P30-HS-021667.
Abbreviations:
- BP
Blood Pressure
- CBN
Carter Burden Network
- CDN
Clinical Directors Network
- CVD
Cardiovascular Disease
- DASH
Dietary-Approaches to-Stop-Hypertension
- DFTA
NYC Department for the Aging
- NYC
New York City
- PBRN
Practice-Based Research Network
- RU
Rockefeller University
- SBP
Systolic Blood Pressure
- SMBP
Self-Measured Blood Pressure
- VC
Vital Care
Footnotes
Declaration of competing interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.numecd.2022.05.018.
References
- [1].Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2016 update. Circulation 2016;133:e38–360. 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- [2].Vespa J, Medina L, Armstrong DM. Demographic turning points for the United States: population projections for 2020 to 2060. Current Population Reports, P25–1144. Washington, DC: U.S. Census Bureau; 2020. [Google Scholar]
- [3].Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American heart association. Circulation 2015:873–98. 10.1161/CIR.0000000000000228. [DOI] [PubMed] [Google Scholar]
- [4].Lionakis N, Mendrinos D, Sanidas E, Favatas G, Georgopoulou M. Hypertension in the elderly. World J Cardiol 2012;4:135–47. 10.4330/wjc.v4.i5.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000;321:412–9. 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hardy ST, Loehr LR, Butler KR, Chakladar S, Chang PP, Folsom AR, et al. Reducing the blood pressure-related burden of cardiovascular disease: impact of achievable improvements in blood pressure prevention and control. J Am Heart Assoc 2015;4:e002276. 10.1161/JAHA.115.002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American heart association Task Force on clinical practice guidelines. Hypertension 2018;71: e13–115. 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- [8].Writing Group of the PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 2003;289. 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- [9].Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM, et al. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 2006;47:296–308. 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- [10].Soltani S, Arablou T, Jayedi A, Salehi-Abargouei A. Adherence to the dietary approaches to stop hypertension (DASH) diet in relation to all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Nutr J 2020;19:37. 10.1186/s12937-02000554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ferguson CC, Knol LL, Ellis AC. Visceral adiposity index and its association with dietary approaches to stop hypertension (DASH) diet scores among older adults: national health and nutrition examination surveys 2011–2014. Clin Nutr 2021;40:4085–9. 10.1016/j.clnu.2021.02.008. [DOI] [PubMed] [Google Scholar]
- [12].Ortolá R, García-Esquinas E, Buño-Soto A, Sotos-Prieto M, Struijk EA, Caballero FF, et al. Healthy dietary patterns are associated with lower concentrations of growth differentiation factor 15 in older adults. Am J Clin Nutr 2021;113:1619–26. 10.1093/ajcn/nqaa444. [DOI] [PubMed] [Google Scholar]
- [13].Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, SimonsMorton DG, et al. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium trial. Ann Intern Med 2001;135:1019–28. 10.7326/0003-4819-13512-200112180-00005. [DOI] [PubMed] [Google Scholar]
- [14].Bray GA, Vollmer WM, Sacks FM, Obarzanek E, Svetkey LP, Appel LJ, et al. A further subgroup analysis of the effects of the DASH diet and three dietary sodium levels on blood pressure: results of the DASH-Sodium Trial. Am J Cardiol 2004;94:222–7. 10.1016/j.amjcard.2004.03.070. [DOI] [PubMed] [Google Scholar]
- [15].Svetkey LP. Effects of dietary patterns on blood pressure: subgroup Analysis of the dietary approaches to stop hypertension (DASH) randomized clinical trial. Arch Intern Med 1999:159:285. 10.1001/archinte.159.3.285. [DOI] [PubMed] [Google Scholar]
- [16].Joyce BT, Wu D, Hou L, Dai Q, Castaneda SF, Gallo LC, et al. DASH diet and prevalent metabolic syndrome in the hispanic community health study/study of latinos. Prev Med Rep 2019:15:100950. 10.1016/j.pmedr.2019.100950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Baker EA, Barnidge EK, Schootman M, Sawicki M, MottonKershaw FL. Adaptation of a modified DASH diet to a rural african American community setting. Am J Prev Med 2016;51:967–74. 10.1016/j.amepre.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bertoni AG, Foy CG, Hunter JC, Quandt SA, Vitolins MZ, Whitt-Glover MC. A multilevel assessment of barriers to adoption of dietary approaches to stop hypertension (DASH) among african Americans of low socioeconomic status. J Health Care for the Poor and Underserved 2011;22:1205–20. 10.1353/hpu.2011.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Epstein DE, Sherwood A, Smith PJ, Craighead L, Caccia P-C, Lin H, et al. Determinants and consequences of adherence to the dietary approaches to stop hypertension diet in African-American and white adults with high blood pressure: results from the ENCORE trial. J Acad Nutr Diet 2012;112:1763–73. 10.1016/j.jand.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].LaTrella P, Bosnick M, Chin J, Di J. NYC department of aging: services snapshot. 2020. [Google Scholar]
- [21].McCloskey DJ, Akintobi TH, Bonham A, Cook J, Coyne-Beasley T. Principles of community engagement. second ed. 2011.
- [22].Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: assessing partnership approaches to improve public health. Annu Rev Public Health 1998;19:173–202. 10.1146/annurev.publhealth.19.1.173. [DOI] [PubMed] [Google Scholar]
- [23].Vasquez K, Guishard D, Desai R, Naji M, Jiang C, Ronning A, et al. 3310 A community-academic partnership to understand the association among health status and senior services utilization to improve nutrition and blood pressure control for low income seniors aging in place. J Clinical and Trans Sci 2019;3:79–80. 10.1017/cts.2019.185. [DOI] [Google Scholar]
- [24].McManus RJ, Mant J, Bray EP, Holder R, Jones MI, Greenfield S, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. The Lancet 2010; 376:163–72. 10.1016/S0140-6736(10)60964-6. [DOI] [PubMed] [Google Scholar]
- [25].McManus RJ, Mant J, Haque MS, Bray EP, Bryan S, Greenfield SM, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA 2014;312:799–808. 10.1001/jama.2014.10057. [DOI] [PubMed] [Google Scholar]
- [26].McManus RJ, Mant J, Franssen M, Nickless A, Schwartz C, Hodgkinson J, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet 2018;391:949–59. 10.1016/S0140-6736(18)30309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bray EP, Holder R, Mant J, McManus RJ. Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Annals of Medicine 2010;42:371–86. 10.3109/07853890.2010.489567. [DOI] [PubMed] [Google Scholar]
- [28].Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self-measured blood pressure monitoring in the management of hypertension. Ann Intern Med 2013;159:185–94. 10.7326/0003-4819-159-3-201308060-00008. [DOI] [PubMed] [Google Scholar]
- [29].Angell S, Guthartz S, Dalal M, Foster V, Pogue V, Wei A, et al. Integrating self blood pressure monitoring into the routine management of uncontrolled hypertension: translating evidence to practice. J Clin Hypertens (Greenwich) 2013;15:180–5. 10.1111/jch.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Muldoon MF, Einhorn J, Yabes JG, Burton D, Irizarry T, Basse J, et al. Randomized feasibility trial of a digital intervention for hypertension self-management. J Hum Hypertens 2021:1–8. 10.1038/s41371-021-00574-9. [DOI] [PubMed] [Google Scholar]
- [31].Yi SS, Tabaei BP, Angell SY, Rapin A, Buck MD, Pagano WG, et al. Self-blood pressure monitoring in an urban, ethnically diverse population: a randomized clinical trial utilizing the electronic health record. Circ Cardiovasc Qual Outcomes 2015;8:138–45. 10.1161/CIRCOUTCOMES.114.000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20. 10.1001/jama.2013.284427.2014. [DOI] [PubMed] [Google Scholar]
- [33].New York City’s Department for the Aging. Profile of older New yorkers. 2017.
- [34].Townsend MS, Kaiser LL, Allen LH, Joy AB, Murphy SP. Selecting items for a food behavior checklist for a limited-resource audience. J Nutr Educ Behav 2003;35:69–77. 10.1016/s1499-4046(06)60043-2. [DOI] [PubMed] [Google Scholar]
- [35].Hager ER, Quigg AM, Black MM, Coleman SM, Heeren T, Rose-Jacobs R, et al. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics 2010;126: e26–32. 10.1542/peds.2009-3146. [DOI] [PubMed] [Google Scholar]
- [36].Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient care committee & adherence working group of the outcomes committee of the adult AIDS clinical trials group (AACTG). AIDS Care 2000;12:255–66. 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- [37].National Center for Health Statistics. National health interview survey. 2017. https://www.cdc.gov/nchs/nhis/data-questionnairesdocumentation.htm. [Accessed 16 July 2021].
- [38].Hahn EA, DeWalt DA, Bode RK, Garcia SF, DeVellis RF, Correia H, et al. New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol 2014;33: 490–9. 10.1037/hea0000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Center for Disease Control and Prevention. Behavioral risk factor surveillance System survey data. Atlanta, GA: US Department of Health and Human Services; 2016. [Google Scholar]
- [40].Center for Disease Control and Prevention. National health and nutrition examination survey questionnaire. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/questionnaires.aspx?BeginYearZ2017. [Accessed 16 July 2021].
- [41].Global Adult Tobacco Survey Collaborative Group. Tobacco questions for surveys: a subset of key questions from the global adult tobacco survey (gats). second ed. Atlanta, GA: Centers for Disease Control and Prevention; 2011. [Google Scholar]
- [42].Babor TF, Higgins-Bundle JC, Saunders JB, Monteiro MG. Audit: the Alcohol Use Disorders Identification Test: guidelines for use in primary health care. WHO; 2021. [Google Scholar]
- [43].Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Diseases 1987;40:373–83. 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- [44].Kroenke K, Spitzer RL, Williams JBW. The patient health questionnaire-2: validity of a two-item depression screener. Medical Care 2003;41:1284–92. 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- [45].NYC Department of Health and Mental Hygiene Bureau of Epidemiology Services, 2017 New York City Community Health Survey (NYC CHS), https://www1.nyc.gov/assets/doh/downloads/pdf/episrv/chs2017survey.pdf, accessed June 8, 2022.
- [46].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Normal weight ranges: body mass index (BMI), https://www.cancer.org/cancer/cancer-causes/diet-physical-activity/bodyweight-and-cancer-risk/adult-bmi.html, accessed April 27, 2022.
- [48].Felix HC, Adams B, Cornell CE, Fausett JK, Krukowski RA, Love SJ, et al. Barriers and facilitators to senior centers participating in translational research. Res Aging 2014;36:22–39. 10.1177/0164027512466874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Truncali A, Dumanovsky T, Stollman H, Angell SY. Keep on track: a volunteer-run community-based intervention to lower blood pressure in older adults. J Am Geriatr Soc 2010;58:1177–83. 10.1111/j.1532-5415.2010.02874.x. [DOI] [PubMed] [Google Scholar]
- [50].Fernandez S, Scales KL, Pineiro JM, Schoenthaler AM, Ogedegbe G. A senior center–based pilot trial of the effect of lifestyle intervention on blood pressure in minority elderly people with hypertension. J Am Geriatr Soc 2008;56:1860–6. 10.1111/j.1532-5415.2008.01863.x. [DOI] [PubMed] [Google Scholar]
- [51].Arnett DK, Blumenthal RS, Albert MA, Michos ED, Buroker AB, Miedema MD, et al. ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary. Circulation 2019;140:e563–95. 10.1161/CIR.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tucker KL, Sheppard JP, Stevens R, Bosworth HB, Bove A, Bray EP, et al. Individual patient data meta-analysis of self-monitoring of blood pressure (BP-SMART): a protocol. BMJ Open 2015;5:e008532. 10.1136/bmjopen-2015-008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tucker KL, Sheppard JP, Stevens R, Bosworth HB, Bove A, Bray EP, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med 2017;14:e1002389. 10.1371/journal.pmed.1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].An R Association of home-delivered meals on daily energy and nutrient intakes: findings from the national health and nutrition examination surveys. J Nutr Gerontol Geriatr 2015;34:263–72. 10.1080/21551197.2015.1031604. [DOI] [PubMed] [Google Scholar]
- [55].Kwasnicka D, Dombrowski SU, White M, Sniehotta F. Theoretical explanations for maintenance of behaviour change: a systematic review of behaviour theories. Health Psychol Rev 2016;10: 277–96. 10.1080/17437199.2016.1151372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ndanuko RN, Tapsell LC, Charlton KE, Neale EP, Batterham MJ. Dietary patterns and blood pressure in adults: a systematic review and meta-analysis of randomized controlled Trials12. Adv Nutr 2016;7:76–89. 10.3945/an.115.009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vollmer WM, Appel LJ, Svetkey LP, Moore TJ, vogt T, Conlin PR, et al. Comparing office-based and ambulatory blood pressure monitoring in clinical trials. Journal of Human Hypertension 2005;19:77–82. [DOI] [PubMed] [Google Scholar]
- [58].Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50:217–26. 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50. 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.