Abstract
BACKGROUND
A comprehensive literature search shows that Sanqi and Huangjing (SQHJ) can improve diabetes treatment in vivo and in vitro, respectively. However, the combined effects of SQHJ on diabetes mellitus (DM) are still unclear.
AIM
To explore the potential mechanism of Panax notoginseng (Sanqi in Chinese) and Polygonati Rhizoma (Huangjing in Chinese) for the treatment of DM using network pharmacology.
METHODS
The active components of SQHJ and targets were predicted and screened by network pharmacology through oral bioavailability and drug-likeness filtration using the Traditional Chinese Medicine Systems Pharmacology Analysis Platform database. The potential targets for the treatment of DM were identified according to the DisGeNET database. A comparative analysis was performed to investigate the overlapping genes between active component targets and DM treatment-related targets. We constructed networks of the active component-target and target pathways of SQHJ using Cytoscape software and then analyzed the gene functions. Using the STRING database to perform an interaction analysis among overlapping genes and a topological analysis, the interactions between potential targets were identified. Gene Ontology (GO) function analyses and Kyoto Encyclopedia of Genes and Genomes enrichment analyses were conducted in DAVID.
RESULTS
We screened 18 active components from 157 SQHJ components, 187 potential targets for active components and 115 overlapping genes for active components and DM. The network pharmacology analysis revealed that quercetin, beta-sitosterol, baicalein, etc. were the major active components. The mechanism underlying the SQHJ intervention effects in DM may involve nine core targets (TP53, AKT1, CASP3, TNF, interleukin-6, PTGS2, MMP9, JUN, and MAPK1). The screening and enrichment analysis revealed that the treatment of DM using SQHJ primarily involved 16 GO enriched terms and 13 related pathways.
CONCLUSION
SQHJ treatment for DM targets TP53, AKT1, CASP3, and TNF and participates in pathways in leishmaniasis and cancer.
Keywords: Panax notoginseng (Sanqi in Chinese), Polygonati Rhizoma (Huangjing in Chinese), Diabetes mellitus, Active compounds, Network pharmacology, Hub genes
Core Tip: Due to the unsatisfactory therapeutic effects of monotherapy, combination therapy has become a preferred choice for diabetic patients. Two specific herbs are often used together for the treatment of some diseases, which is called a herb pair. Hence, we investigated the combined effects of Sanqi and Huangjing (SQHJ) on diabetes and the underlying molecular mechanisms. Network pharmacology is a new discipline based on the theory of systems biology, which analyzes the network of biological systems and selects specific signal nodes for multitarget drug molecular design 16. Therefore, we used network pharmacology to reveal the pharmacological mechanism of SQHJ in the treatment of diabetes from the following three aspects: active components, potential targets, and synthetic pathways.
INTRODUCTION
Diabetes mellitus (DM) is a metabolic disorder caused by genetic and environmental factors, and it has become a critical health problem worldwide due to its high prevalence and related disability and mortality[1]. The global prevalence of DM among adults aged 20–79 years was 8.8% in 2015, and its incidence is increasing rapidly[2]. Therefore, research on the pathogenesis and drugs of diabetes should be strengthened.
Herbal medicines present advantages over synthetic drugs that include fewer side effects and positive patient perceptions; thus, these medicines or their active natural ingredients have become increasingly popular around the world, especially for chronic metabolic diseases[3]. Traditional Chinese medicine has played an important role in improving patient quality of life and curing disease[4]. Panax notoginseng (Burk.) Chen FH (Sanqi in Chinese) has a long history of use as an herbal medicine[5] and has been well investigated in the treatment of diabetes and diabetic complications[6,7]. Polygonati Rhizoma (Huangjing in Chinese) has pharmacological activity, including antifatigue, immune promotion, antiaging, blood glucose regulation, and lipid regulation effects[8-12]. A comprehensive literature search shows that Sanqi and Huangjing (SQHJ) can improve the treatment of diabetes in vivo and in vitro, respectively.
Due to the unsatisfactory therapeutic effects of monotherapy, combination therapy has become the preferred choice for diabetic patients[13]. Two specific herbs are often used together for the treatment of certain diseases, and they are called herb pairs[14]. However, the combined effects of SQHJ on DM are still unclear.
Network pharmacology is a new discipline based on the theory of systems biology, which analyzes the network of biological systems and selects specific signal nodes for multitarget drug molecular design[15]. Therefore, we used network pharmacology to reveal the pharmacological mechanism of SQHJ in the treatment of DM according to the active components, potential targets, and pathways.
MATERIALS AND METHODS
Screening of active compounds and target proteins
We used the Traditional Chinese Medicine System Pharmacology (TCMSP) database (http://Lsp.nwu.edu.cn/tcmsp.php) to identify the active ingredients of SQHJ. As a special database that contains many herbs, active constituents, and their targets, TCMSP is a traditional Chinese medicine database and analysis platform. It also includes the pharmacokinetic properties of active compounds, such as oral bioavailability (OB), drug-likeness (DL), and other properties. OB, as one of the most important pharmacokinetic parameters, indicates the ratio of the drug taken to the blood circulation[16]. OB ≥ 30% and DL ≥ 0.18 are considered indicators for screening a clinical drug[15]. In our study, we also used those indicators as our screening criteria. The UniProt database (https://www.uniprot.org/) was employed to convert the target name to the official name.
Acquisition of gene targets for DM
DisGeNET is a discovery platform that contains one of the largest publicly available collections of genes and variants associated with human diseases[17]. The target genes for DM in this study were collected from DisGeNet databases (http://www.disgenet.org/, update 2021-8-8) by entering the key word “diabetes”.
Intersection of drug targets and disease genes
Furthermore, we matched SQHJ targets with DM targets to obtain overlapping targets. The Venn Diagram Platform (http://bioinformatics.psb.ugent.be/webtools/Venn/) was used to draw a Venn diagram.
Network construction
We constructed two networks: (1) A compound-target network; and (2) A target-pathway network. The Cytoscape 3.8.2 plug-in cytoHubba was used to construct the networks[18].
Protein-protein interaction network construction
Protein-protein interaction (PPI) data were attained from the STRING database (https://stringdb.org/), which is a database that can predict known protein interactions[19]. Initially, the abovementioned overlapping therapeutic targets were subjected to PPI analysis using the STRING database. In the platform, “Multiple proteins” was selected and the species was set to “Homo sapiens”. Target interaction information was obtained according to a confidence score of 0.4. The results were imported into Cytoscape 3.8.2 software, where the interaction network was drawn and analyzed.
Identification of Hub genes
After establishing the PPI network, hub gene networks were constructed using Cytoscape 3.8.2. The Cytoscape plug-in cytoHubba was used to filter out hub genes. Important hub genes could be selected through calculation and analysis of the network structure and the weighted reconnection between nodes. The calculations were performed by the “Degree” method.
To further screen out key targets that play an important role in the pathogenesis of DM, the nodes with topological importance in the PPI network were filtered through a series of parameters using the Cytoscape plugin CytoNCA[20]. We chose three parameters: Degree centrality (DC), betweenness centrality (BC), and closeness centrality (CC). After a series of screenings, we finally obtained a PPI network containing core genes, and it also showed the interaction between the important proteins. Furthermore, we matched the results of the plugin cytoHubba and CytoNCA through the Venn Diagram Platform (https://bioinfogp.cnb.csic.es/tools/venny/) to obtain hub genes.
Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were obtained by DAVID (https://david.ncifcrf.gov/). The GO enrichment analysis includes the following three categories: Biological process (BP), molecular function (MF), and cellular component[21]. For the GO and KEGG enrichment analyses, the evaluation criteria included a P value < 0.001 and count > 5.
RESULTS
Screening of active compounds
A total of 157 bioactive ingredients were retrieved from the TCMSP database, with 119 types in Sanqi and 38 types in Huangjing. According to OB ≥ 30% and DL ≥ 0.18, 20 active ingredients were screened, and they included 8 types in Sanqi and 12 types in Huangjing. MOL001792 (DFV, 5-deoxyflavanone) and MOL000358 (beta-sitosterol) were common compounds in SQHJ. After the removal of duplicates, 18 ingredients were stored for further study (Table 1).
Table 1.
Eighteen active compounds in Sanqi and Huangjing and their corresponding predicted oral bioavailability and drug-likeness
|
Herb
|
MOL ID
|
Molecule name
|
OB (%)
|
DL
|
| Sanqi | MOL001494 | Mandenol | 42.00 | 0.19 |
| Sanqi/Huangjing | MOL001792 | DFV | 32.76 | 0.18 |
| Sanqi | MOL002879 | Diop | 43.59 | 0.39 |
| Sanqi/Huangjing | MOL000358 | Beta-sitosterol | 36.91 | 0.75 |
| Sanqi | MOL000449 | Stigmasterol | 43.83 | 0.76 |
| Sanqi | MOL005344 | Ginsenoside rh2 | 36.32 | 0.56 |
| Sanqi | MOL007475 | Ginsenoside f2 | 36.43 | 0.25 |
| Sanqi | MOL000098 | Quercetin | 46.43 | 0.28 |
| Huangjing | MOL002714 | Baicalein | 33.52 | 0.21 |
| Huangjing | MOL002959 | 3’-Methoxydaidzein | 48.57 | 0.24 |
| Huangjing | MOL000359 | Sitosterol | 36.91 | 0.75 |
| Huangjing | MOL003889 | Methylprotodioscin_qt | 35.12 | 0.86 |
| Huangjing | MOL004941 | (2R)-7-hydroxy-2-(4-hydroxyphenyl) chroman-4-one | 71.12 | 0.18 |
| Huangjing | MOL000546 | Diosgenin | 80.88 | 0.81 |
| Huangjing | MOL006331 | 4’,5-Dihydroxyflavone | 48.55 | 0.19 |
| Huangjing | MOL009760 | Sibiricoside A_qt | 35.26 | 0.86 |
| Huangjing | MOL009763 | (+)-Syringaresinol-O-beta-D-glucoside | 43.35 | 0.77 |
| Huangjing | MOL009766 | Zhonghualiaoine 1 | 34.72 | 0.78 |
OB: Oral bioavailability; DL: Drug-likeness. Both OB ≥ 30% and DL ≥ 0.18 were obtained from TCMSP.
Target prediction and analysis.
Sanqi has 169 potential targets, and Huangjing has 79 potential targets. A total of 187 SQHJ potential targets were obtained after removing duplicates. In addition, 2359 DM-related genes were obtained from the DisGeNET database. Based on the intersection of drug and disease targets, we obtained 115 genes (Figure 1), which means that these genes could play a major role in SQHJ treatment for DM.
Figure 1.
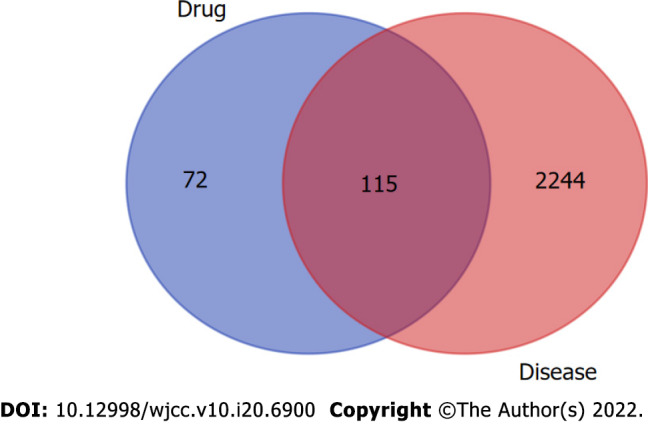
Based on the intersection of drug and disease targets, we obtained 115 genes, which means that these genes could play a major role in Sanqi and Huangjing treatment for diabetes mellitus.
Network construction and analysis
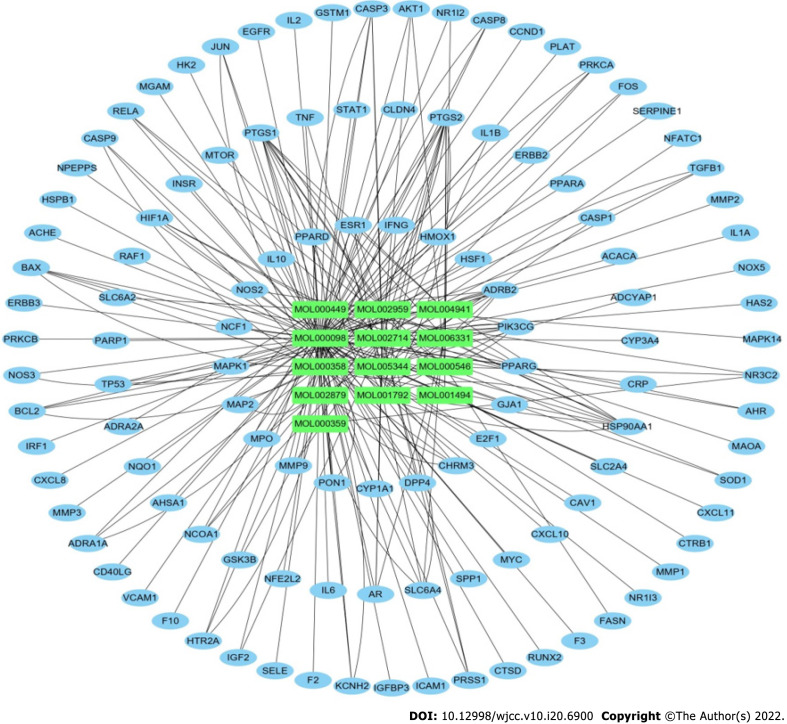
The active ingredients of SQHJ and the 115 targets were obtained using Cytoscape 3.8.2 (Figure 2). A total of 128 nodes (13 ingredients and 115 common targets) and 232 edges are depicted in Figure 2. The top 10 compounds and 9 genes are displayed based on degree and ranked in descending order (Tables 2 and 3). As depicted, MOL000098 (quercetin) displayed the greatest number of target interactions (degree = 97), followed by MOL000358 (beta-sitosterol, degree = 42), MOL002714 (baicalein, degree = 23), MOL000449 (DFV, degree = 16) and MOL174 (stigmasterol, degree = 13). The results showed that MOL000098 (quercetin), MOL000358 (beta-sitosterol), and MOL002714 (baicalein) have more target proteins and play key roles in the interaction network; thus, they may be important core compounds.
Figure 2.
The active ingredients of Sanqi and Huangjing and the 115 targets were obtained using Cytoscape 3.8.2.
Table 2.
Top 10 ingredients based on the network of ingredients
|
Rank
|
Name
|
Molecule name
|
Degree
|
| 1 | MOL000098 | Quercetin | 97 |
| 2 | MOL000358 | Beta-sitosterol | 42 |
| 3 | MOL002714 | Baicalein | 23 |
| 4 | MOL001792 | DFV (5-deoxyflavanone) | 16 |
| 5 | MOL000449 | Stigmasterol | 13 |
| 6 | MOL002959 | 3’-Methoxydaidzein | 12 |
| 7 | MOL000546 | Diosgenin | 11 |
| 8 | MOL005344 | Ginsenoside rh2 | 10 |
| 9 | MOL004941 | (2R)-7-hydroxy-2-(4-hydroxyphenyl) chroman-4-one | 8 |
| 10 | MOL006331 | 4’,5-Dihydroxyflavone | 7 |
Table 3.
Top 9 genes based on the network of ingredients and overlapping targets
|
Rank
|
Name
|
Degree
|
| 1 | PTGS2 | 13 |
| 2 | PTGS1 | 11 |
| 3 | HSP90AA1 | 9 |
| 4 | PIK3CG | 8 |
| 5 | ADRB2 | 8 |
| 6 | SQ | 7 |
| 7 | CASP3 | 5 |
| 8 | BAX | 5 |
| 9 | SLC6A4 | 5 |
In this network, certain targets, such as PTGS2, PTGS1, HSP90AA1, PIK3CG and ADRB2, corresponded to multiple compounds. These results suggested that SQHJ compounds acted synergistically on their targets based on the multicompound and multitarget features of the herbal formula (Table 3).
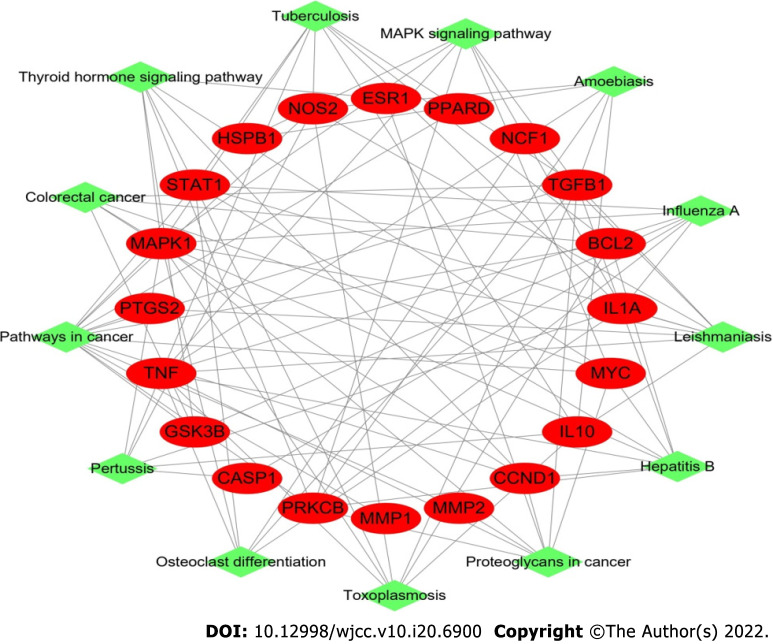
Our target-pathway network demonstrated interactions in multiple pathways, including crosstalk of the pathway terms and the transitive relation. The target-pathway network contained 33 nodes (20 DM-related target nodes and 13 pathway nodes) and 98 edges (Figure 3).
Figure 3.
Target-pathway network in Sanqi and Huangjing-diabetes mellitus. This network shows the relationship between the enriched 13 pathways and 20 genes. The green nodes represent the pathway of Sanqi and Huangjing in the treatment of diabetes mellitus. The red nodes represent the target genes. The edges represent the interaction between the red and green nodes.
Construction and analysis of the target protein PPI network
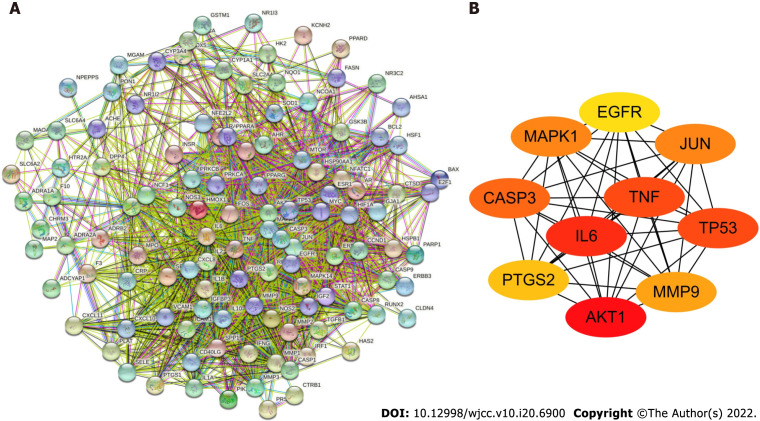
To determine how overlapping genes interact, we uploaded their information to the STRING database and then constructed a PPI network, which contained 115 nodes and 1817 edges according to the high confidence (0.4) at the protein level (Figure 4A). The top ten target proteins [AKT1, interleukin (IL)-6, TNF, TP53, CASP3, JUN, MAPK1, MMP9, PTGS2, and EGFR] were visualized by Cytoscape according to the degree (Figure 4B). Rank differences based on degree were not observed among the ten genes; thus, these genes are considered to play an important role in treating DM.
Figure 4.
Protein-protein interaction network. A: The protein-protein interaction (PPI) network of 115 overlapping genes of Sanqi and Huangjing and diabetes mellitus; B: Top 10 hub genes in the PPI network. AKT1: RAC-alpha serine/threonine-protein kinase; IL6: Interleukin 6; TNF: Tumor necrosis factor; TP53: Cellular tumor antigen p53; CASP3: Caspase-3; JUN: Transcription factor AP-1; MAPK1: Mitogen-activated protein kinase 1; MMP9: Matrix metallopeptidase-9; PTGS2: Prostaglandin G/H synthase 2; EGFR: Epidermal growth factor receptor.
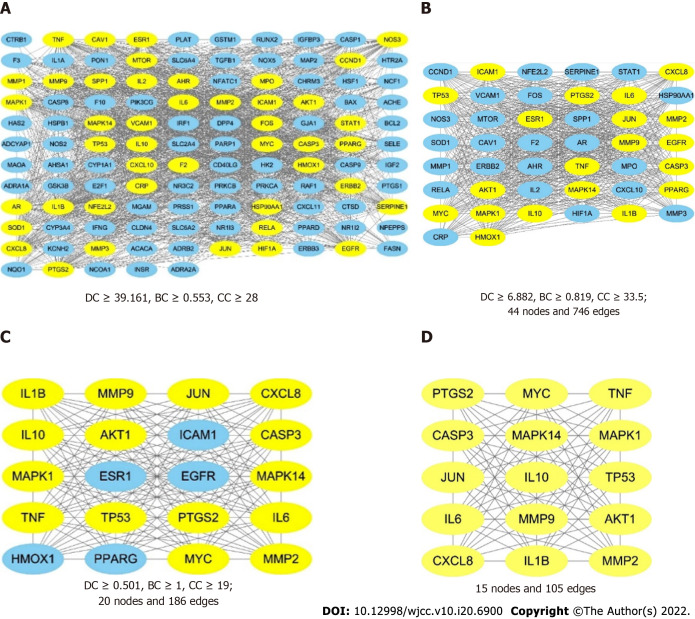
The results showed that the protein interactions involved 115 nodes and 1817 edges (Figure 5A). We then used the degree, betweenness, and closeness factors as the main parameters for critical target screening[6]. In the first topological analysis, which was based on the criteria of DC ≥ 39.161, BC ≥ 0.553, and CC ≥ 28, 44 nodes and 746 edges (Figure 5B) were obtained, and among the 44 nodes, 20 yellow nodes represent the core targets obtained in the second topology analysis while 24 blue nodes represent the noncore targets. After the third topological analysis, which was based on the criteria of DC ≥ 0.501, BC ≥ 1, and CC ≥ 19, 20 nodes (15 yellow nodes represent the core targets and 5 blue nodes represent the noncore targets) and 186 edges were obtained (Figure 5C). A PPI network (Figure 5D) was finally obtained, and it contained 15 nodes marked in yellow and 105 edges, indicating that there were 15 core targets obtained after the topological analysis, namely, MAPK14, IL-1B, JUN, TNF, TP53, MMP9, PTGS2, IL-6, CASP3, MYC, IL-10, CXCL8, MAPK1, MMP2, and AKT1.
Figure 5.
The process of topological screening for the protein-protein interaction network. The yellow nodes represent the core targets and the blue nodes represent the noncore targets. A: The combined protein-protein interaction (PPI) network of the overlapping targets; B: PPI network with important targets extracted from (A); C: PPI network with important targets extracted from (B); D: PPI network with core targets extracted from (C).
According to the intersection of the cytoHubba and CytoNCA results, 9 genes were identified that could play a major role in SQHJ treatment for DM: TP53, AKT1, CASP3, TNF, IL-6, PTGS2, MMP9, JUN, and MAPK1 (Figure 6).
Figure 6.
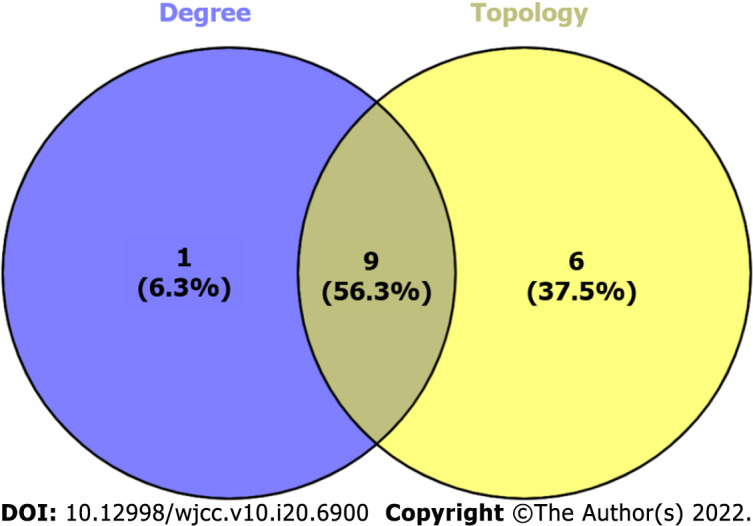
The Venn diagram of cytoHubba and CytoNCA-related targets. 1 cytoHubba non-intersection targets (left), 9 cytoHubba and CytoNCA intersection targets (middle) and 6 CytoNCA non-intersection targets (right).
GO enrichment analysis
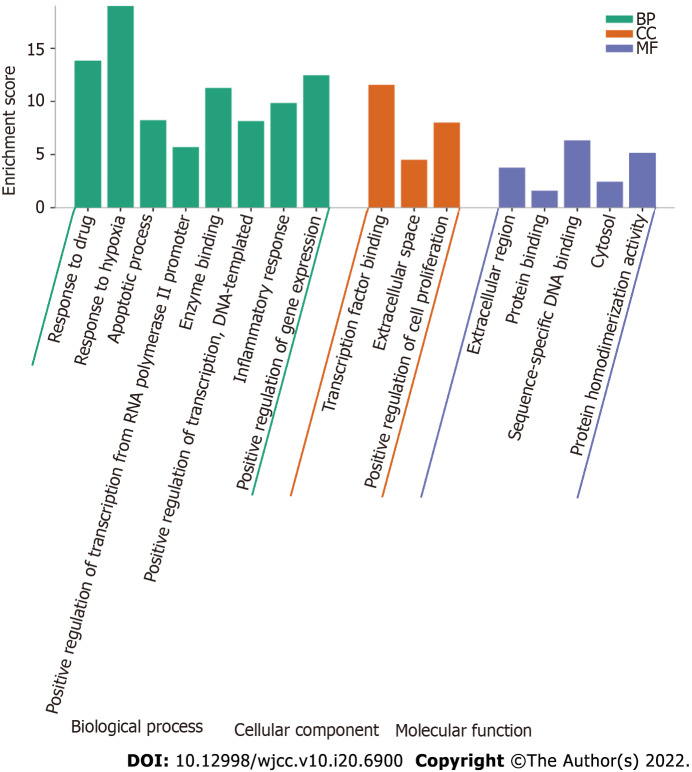
GO enrichment analysis includes 3 different categories: BP, MF, and CC. We selected the top 16 terms according to the P value and count values, as shown in Figure 7. The results indicated that SQHJ impacts DM through various GO terms in the BP category, including response to drug, response to hypoxia, apoptotic process, positive regulation of transcription from the RNA polymerase II promoter, enzyme binding, positive regulation of transcription, DNA-templated, inflammatory response, and positive regulation of gene expression. GO terms in the CC category included transcription factor binding, extracellular space, and positive regulation of cell proliferation. GO terms in the MF category were the extracellular region, protein binding, sequence-specific DNA binding, cytosol, and protein homodimerization activity.
Figure 7.
Gene Ontology enrichment analysis of 115 overlapping genes. The top Gene Ontology enriched terms with P < 0.001 and count > 5 were screened. The X-axis is the enrichment gene ratio, and the Y-axis is the molecular function or biological process. CC: Cellular component; BP: Biological process; MF: Molecular function.
KEGG enrichment analysis
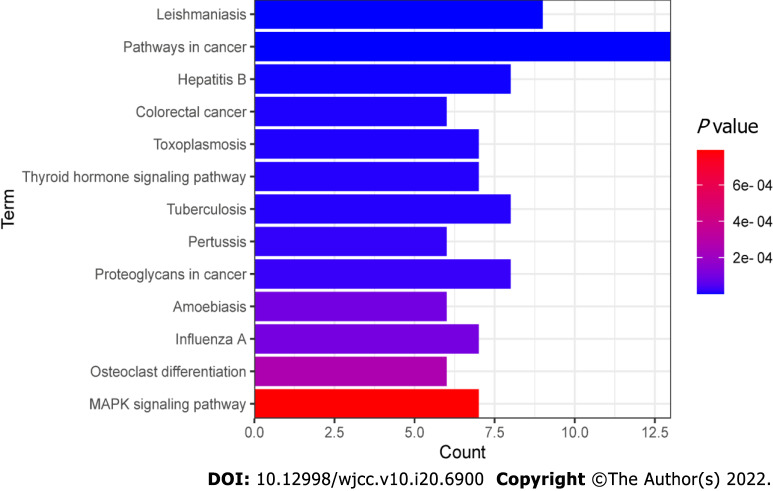
We uploaded these 115 genes to the DAVID database and performed a KEGG enrichment analysis. A total of 13 significant treatment pathways (Figure 8) were screened (P < 0.001, count > 5), including pathways in leishmaniasis, cancer, hepatitis B, colorectal cancer, toxoplasmosis, thyroid hormone signaling, tuberculosis, pertussis, proteoglycans in cancer, amoebiasis, influenza A, osteoclast differentiation and MAPK signaling. The top pathways were determined based on statistical significance and then ranked in descending order (Table 4). We found that a pathway includes multiple proteins, and one protein may also be involved in multiple pathways.
Figure 8.
Kyoto encyclopedia of genes and genomes pathway enrichment analysis of 115 overlapping genes. P < 0.001 and count > 5. The X-axis is the enrichment gene count, the Y-axis is the Kyoto Encyclopedia of Genes and Genomes pathway, and the color of bar chart represents the adjusted P value.
Table 4.
Kyoto encyclopedia of genes and genomes pathway analysis based on target-pathway network
|
Pathway ID
|
Pathway name
|
Count
|
P
value
|
Gene name
|
| 05140 | Leishmaniasis | 9 | 5.61E-10 | IL-10, IL-1A, TGFB1, NCF1, NOS2, STAT1, MAPK1, PTGS2, TNF |
| 05200 | Pathways in cancer | 13 | 5.36E-08 | GSK3B, TGFB1, NOS2, PRKCB, STAT1, MMP1, MMP2, PTGS2, CCND1, MYC, BCL2, MAPK1, PPARD |
| 05161 | Hepatitis B | 8 | 2.75E-06 | TGFB1, CCND1, STAT1, PRKCB, MYC, BCL2, MAPK1, TNF |
| 05210 | Colorectal cancer | 6 | 7.16E-06 | GSK3B, TGFB1, CCND1, MYC, BCL2, MAPK1 |
| 05145 | Toxoplasmosis | 7 | 7.75E-06 | IL-10, TGFB1, NOS2, STAT1, BCL2, MAPK1, TNF |
| 04919 | Thyroid hormone signaling pathway | 7 | 1.00E-05 | GSK3B, CCND1, STAT1, PRKCB, MYC, MAPK1, ESR1 |
| 05152 | Tuberculosis | 8 | 1.03E-05 | IL-10, IL-1A, TGFB1, NOS2, STAT1, BCL2, MAPK1, TNF |
| 05133 | Pertussis | 6 | 1.83E-05 | IL-10, IL-1A, NOS2, CASP1, MAPK1, TNF |
| 05205 | Proteoglycans in cancer | 8 | 2.30E-05 | TGFB1, CCND1, PRKCB, MYC, MMP2, MAPK1, ESR1, TNF |
| 05146 | Amoebiasis | 6 | 9.76E-05 | IL-10, TGFB1, NOS2, PRKCB, HSPB1, TNF |
| 05164 | Influenza A | 7 | 1.05E-04 | IL-1A, GSK3B, STAT1, PRKCB, CASP1, MAPK1, TNF |
| 04380 | Osteoclast differentiation | 6 | 2.65E-04 | IL-1A, TGFB1, NCF1, STAT1, MAPK1, TNF |
| 04010 | MAPK signaling pathway | 7 | 7.91E-04 | IL-1A, TGFB1, PRKCB, MYC, HSPB1, MAPK1, TNF |
DISCUSSION
In the current work, we predicted the possible molecular mechanisms of SQHJ in DM using network pharmacology. Eighteen potential active ingredients and 9 corresponding potential targets were retained. According to the degree ranking in the ingredient-target network, the top 5 active ingredients are quercetin, beta-sitosterol, baicalein, DFV, and stigmasterol. Previous studies showed that quercetin alleviates ferroptosis of pancreatic β cells in type 2 diabetes[22]; beta-sitosterol attenuates insulin resistance in adipose tissue via IRS-1/Akt-mediated insulin signaling in high-fat diet- and sucrose-induced type 2 diabetic rats[23]; baicalein alleviates the erectile dysfunction associated with streptozotocin-induced diabetes[24]; and stigmasterol prevents glucolipotoxicity-induced defects in glucose-stimulated insulin secretion[25] and has potential beneficial effects on the treatment of type 2 DM[26]. The above studies show that quercetin, beta-sitosterol, baicalein, and stigmasterol are related to DM. However, few studies have been performed on DFV. We speculate that the above components are the main ingredients of SQHJ underlying its anti-DM effects. Compound DFV still needs further experimental study.
According to the PPI network and degree ranking of the main targets, cellular TP53, AKT1, CASP3, TNF, IL-6, PTGS2, MMP9, JUN, and MAPK1 are the hub genes involved in the activity of SQHJ against DM. Many studies have shown that these hub genes are related to DM. TP53, is a stress response gene in cell biology[27] that is upregulated in high glucose-treated podocytes and mesangial cells[28]. Akt1 is related to diabetes[29,30]. Streptozotocin diabetic rats exhibit increased expression of CASP3[31], and high glucose increases the expression of cleaved CASP3[32]. Inflammation is widely accepted to be responsible for the pathogenesis of hyperglycemia and its complications[33]. TNF-α and IL-6 protein levels are increased in renal glomeruli and tubules[34]. Moreover, phosphorylation of p38, JNK, and ERK1/2 is increased in diabetic control rats compared with control rats, and associated increases in the levels of TNF-α and IL-6 are also observed[35]. IL-6 plays a crucial role in insulin resistance and obesity and is increased in obese persons, thus revealing that IL-6 is the major contributor to insulin resistance in diabetic conditions[36]. PTGS is a key rate-limiting enzyme for the synthesis of prostaglandins from arachidonic acid[37]. PTGS2 (COX-2) is increased by proinflammatory factors[38]. The expression of IL-6 and COX-2 is markedly upregulated in the peripheral blood of DM cerebral infarction patients[39]. High COX-2 and iNOS levels are associated with type 2 DM[40]. MMP-9 is associated with diabetic retinopathy[41,42] and β-cell loss[43]. MAPK1 is associated with the regulation of miR-483-5p effects under high glucose conditions[44]. In total, the above targets are related to diabetes, through which SQHJ may prevent DM. JUN directly interacts with specific target DNA sequences to regulate gene expression[45]. Downregulated MAPK1 expression plays a protective role in lipopolysaccharide-induced podocyte damage in diabetic nephropathy[46]. Huangjing downregulates the expression of CASP3[47] and has potential anti-inflammatory effects in mice, and the anti-inflammatory effect may be mediated, at least in part, by inhibiting the mRNA expression of a panel of inflammatory mediators, including COX-2, TNF-α, and IL-6[48]. In summary, TP53, AKT1, CASP3, TNF, IL-6, PTGS2, MMP9, JUN, and MAPK1 are important targets for the treatment of DM.
The GO enrichment analysis showed that the protective effect of SQHJ against DM mainly included response to drugs, response to hypoxia, etc. The enrichment of GO BP involves more pathways. Therefore, we speculate that BP may be the main mechanism underlying the effects of SQHJ treatment in DM.
The KEGG pathway analysis showed enrichment in the following pathways: Leishmaniasis, cancer, hepatitis B, colorectal cancer, toxoplasmosis, thyroid hormone signaling pathway, tuberculosis, pertussis, proteoglycans in cancer, amoebiasis, influenza A, osteoclast differentiation and MAPK signaling. Previous studies have shown that the MAPK signaling pathway plays a critical role during the process of inflammation, insulin resistance and neuroinflammation combined with DM[49,50]. Osteoclast differentiation and bone absorption are inhibited by high blood glucose[51]. Hyperglycemia plays a role in aberrant osteoclast differentiation that leads to an increased capacity for bone resorption[52]. Male patients and patients with chronic pulmonary disease or DM have a higher risk of developing severe pneumonia after influenza A infection[53]. Diabetic patients are at higher risk for severe complications after amoebic infection[54]. Some pertussis infections may be a risk factor for type 1 DM[55]. Evidence for a positive association between DM and tuberculosis has been found in many studies regardless of the study design and population, and the two diseases may complicate each other to different extents[56]. Although a statistically significant relationship was not observed between diabetes and toxoplasmosis, further investigations are needed[57,58]. Diabetes has been associated with an increased risk of cancer, including breast cancer and colorectal cancer[59]. Individuals with chronic HBV infection or anti-HBc positivity may have an increased risk of diabetes, and the association may be modified by the different statuses of metabolism-related variables and age[60]. DM modifies the clinical presentation of cutaneous leishmaniasis, enhances proinflammatory cytokine production, and impairs the response to antimony therapy[61]. To date, few studies have focused on the effects of cancer pathways, the thyroid hormone signaling pathway, pertussis, and proteoglycans in cancer on DM. Therefore, the leishmaniasis, hepatitis B, colorectal cancer, toxoplasmosis, tuberculosis, amoebiasis, influenza A, osteoclast differentiation and MAPK signaling pathways may be potential pathways for the treatment of DM.
CONCLUSION
In this study, the active ingredients of SQHJ and the potential mechanisms underlying their effects on DM were explored using network pharmacology methods. We screened 5 ingredients and identified 9 hub targets in the relevant pathways. This work provides new clues on SQHJ pharmacological targets and pathways in DM. We speculated that SQHJ was effective for the treatment of DM; however, experiments must be performed to verify these results.
ARTICLE HIGHLIGHTS
Research background
Sanqi and Huangjing (SQHJ) ameliorate diabetes mellitus (DM) in vivo and in vitro respectively. However, the combined effects of SQHJ on DM are unclear.
Research motivation
To provide an objective basis for the treatment of DM with traditional Chinese medicine.
Research objectives
To explore the potential mechanism of Panax notoginseng (Sanqi in Chinese) and Polygonati Rhizoma (Huangjing in Chinese) for the treatment of DM using network pharmacology.
Research methods
Active components of SQHJ and targets were predicted and screened by network pharmacology using the Traditional Chinese Medicine Systems Pharmacology Analysis Platform, DisGeNET database, STRING database, Gene Ontology (GO) function and Kyoto Encyclopedia of Genes and Genomes enrichment analysis.
Research results
Of 18 active components from 157 SQHJ components, 187 potential targets for active components and 115 overlapping genes for active components and DM were screened. Quercetin, beta-sitosterol, baicalein and so on were the major active components. The mechanism of intervention of SQHJ in treating DM may involve nine core targets (TP53, AKT1, CASP3, TNF, interleukin-6, PTGS2, MMP9, JUN, and MAPK1). The treatment of DM using SQHJ primarily involved 16 GO enriched terms and 13 related pathways.
Research conclusions
SQHJ treats DM by targeting TP53, AKT1, CASP3, and TNF and participating pathways in leishmaniasis, pathways in cancer, and so on.
Research perspectives
SQHJ is effective for the treatment of DM. In the future, experiments will be needed to verify these results.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest, financial or otherwise.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 28, 2021
First decision: January 23, 2022
Article in press: April 15, 2022
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee KS, South Korea; Papazafiropoulou A, Greece S-Editor: Fan JR L-Editor: Webster JR P-Editor: Fan JR
Contributor Information
Xiao-Yan Cui, Hebei Institute for Drug and Medical Device Control, Shijiazhuang 050011, Hebei Province, China.
Xiao Wu, Department of Basic Medical, HE's University, Shenyang 110163, Liaoning Province, China.
Dan Lu, College of Clinical, HE's University, Shenyang 110163, Liaoning Province, China.
Dan Wang, College of Human Kinesiology, Shenyang Sport University, Shenyang 110102, Liaoning Province, China. 595680903@qq.com.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IFD Diabetes Altas Globally, 7th Edn. [cited 10 December 2021]. Available from: http://www.diabetesatlas.org .
- 3.Tian X, Liu F, Li Z, Lin Y, Liu H, Hu P, Chen M, Sun Z, Xu Z, Zhang Y, Han L, Pan G, Huang C. Enhanced Anti-diabetic Effect of Berberine Combined With Timosaponin B2 in Goto-Kakizaki Rats, Associated With Increased Variety and Exposure of Effective Substances Through Intestinal Absorption. Front Pharmacol. 2019;10:19. doi: 10.3389/fphar.2019.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo MF, Dai YJ, Gao JR, Chen PJ. Uncovering the Mechanism of Astragalus membranaceus in the Treatment of Diabetic Nephropathy Based on Network Pharmacology. J Diabetes Res. 2020;2020:5947304. doi: 10.1155/2020/5947304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu C, Wang W, Wang B, Zhang T, Cui X, Pu Y, Li N. Analytical methods and biological activities of Panax notoginseng saponins: Recent trends. J Ethnopharmacol. 2019;236:443–465. doi: 10.1016/j.jep.2019.02.035. [DOI] [PubMed] [Google Scholar]
- 6.Piao C, Sun Z, Jin , Wang H, Wu X, Zhang N, Lian F, Tong X. Network Pharmacology-based Investigation of the Underlying Mechanism of Panax notoginseng Treatment of Diabetic Retinopathy. Comb Chem High Throughput Screen. 2020;23:334–344. doi: 10.2174/1386207323666200305093709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo X, Sun W, Luo G, Wu L, Xu G, Hou D, Hou Y, Guo X, Mu X, Qin L, Liu T. Panax notoginseng saponins alleviate skeletal muscle insulin resistance by regulating the IRS1-PI3K-AKT signaling pathway and GLUT4 expression. FEBS Open Bio. 2019;9:1008–1019. doi: 10.1002/2211-5463.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang XX, Wang X, Shi TT, Dong JC, Li FJ, Zeng LX, Yang M, Gu W, Li JP, Yu J. Mitochondrial dysfunction in high-fat diet-induced nonalcoholic fatty liver disease: The alleviating effect and its mechanism of Polygonatum kingianum. Biomed Pharmacother. 2019;117:109083. doi: 10.1016/j.biopha.2019.109083. [DOI] [PubMed] [Google Scholar]
- 9.Yan H, Lu J, Wang Y, Gu W, Yang X, Yu J. Intake of total saponins and polysaccharides from Polygonatum kingianum affects the gut microbiota in diabetic rats. Phytomedicine. 2017;26:45–54. doi: 10.1016/j.phymed.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Yang XX, Wei JD, Mu JK, Liu X, Li FJ, Li YQ, Gu W, Li JP, Yu J. Mitochondrial metabolomic profiling for elucidating the alleviating potential of Polygonatum kingianum against high-fat diet-induced nonalcoholic fatty liver disease. World J Gastroenterol. 2019;25:6404–6415. doi: 10.3748/wjg.v25.i43.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato A, Miura T, Yano H, Masuda K, Ishida H, Seino Y. Suppressive effects of polygonati rhizoma on hepatic glucose output, GLUT2 mRNA expression and its protein content in rat liver. Endocr J. 1994;41:139–144. doi: 10.1507/endocrj.41.139. [DOI] [PubMed] [Google Scholar]
- 12.Miura T, Kato A, Usami M, Kadowaki S, Seino Y. Effect of polygonati rhizoma on blood glucose and facilitative glucose transporter isoform 2 (GLUT2) mRNA expression in Wistar fatty rats. Biol Pharm Bull. 1995;18:624–625. doi: 10.1248/bpb.18.624. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakar PK, Kumar A, Doble M. Combination therapy: a new strategy to manage diabetes and its complications. Phytomedicine. 2014;21:123–130. doi: 10.1016/j.phymed.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Meng Y, Du Z, Li Y, Gao P, Song J, Lu Y, Tu P, Jiang Y, Guo X. The synergistic mechanism of total saponins and flavonoids in Notoginseng-Safflower pair against myocardial ischemia uncovered by an integrated metabolomics strategy. Biomed Pharmacother. 2020;130:110574. doi: 10.1016/j.biopha.2020.110574. [DOI] [PubMed] [Google Scholar]
- 15.Fang T, Liu L, Liu W. Network pharmacology-based strategy for predicting therapy targets of Tripterygium wilfordii on acute myeloid leukemia. Medicine (Baltimore) 2020;99:e23546. doi: 10.1097/MD.0000000000023546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg AX, Moist L, Pannu N, Tobe S, Walsh M, Weir M Curcumin AAA AKI Investigators. Bioavailability of oral curcumin. CMAJ. 2019;191:E428. doi: 10.1503/cmaj.71719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piñero J, Saüch J, Sanz F, Furlong LI. The DisGeNET cytoscape app: Exploring and visualizing disease genomics data. Comput Struct Biotechnol J. 2021;19:2960–2967. doi: 10.1016/j.csbj.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin Q, Hao XF, Xie LK, Xu J, Sun M, Yuan H, Wang SH, Wu GP, Miao ML. A Network Pharmacology to Explore the Mechanism of Astragalus Membranaceus in the Treatment of Diabetic Retinopathy. Evid Based Complement Alternat Med. 2020;2020:8878569. doi: 10.1155/2020/8878569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu M, Li Z, Yang L, Zhai W, Wei N, Zhang Q, Chao B, Huang S, Cui H. Elucidation of the Mechanisms and Molecular Targets of Sanhuang Xiexin Decoction for Type 2 Diabetes Mellitus Based on Network Pharmacology. Biomed Res Int. 2020;2020:5848497. doi: 10.1155/2020/5848497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li D, Jiang C, Mei G, Zhao Y, Chen L, Liu J, Tang Y, Gao C, Yao P. Quercetin Alleviates Ferroptosis of Pancreatic β Cells in Type 2 Diabetes. Nutrients. 2020;12 doi: 10.3390/nu12102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babu S, Krishnan M, Rajagopal P, Periyasamy V, Veeraraghavan V, Govindan R, Jayaraman S. Beta-sitosterol attenuates insulin resistance in adipose tissue via IRS-1/Akt mediated insulin signaling in high fat diet and sucrose induced type-2 diabetic rats. Eur J Pharmacol. 2020;873:173004. doi: 10.1016/j.ejphar.2020.173004. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Zhou B, Yu Z, Yuan P, Sun T, Gong J, Zhang Y, Wang T, Wang S, Liu K, Liu J. Baicalein Alleviates Erectile Dysfunction Associated With Streptozotocin-Induced Type I Diabetes by Ameliorating Endothelial Nitric Oxide Synthase Dysfunction, Inhibiting Oxidative Stress and Fibrosis. J Sex Med. 2020;17:1434–1447. doi: 10.1016/j.jsxm.2020.04.390. [DOI] [PubMed] [Google Scholar]
- 25.Ward MG, Li G, Barbosa-Lorenzi VC, Hao M. Stigmasterol prevents glucolipotoxicity induced defects in glucose-stimulated insulin secretion. Sci Rep. 2017;7:9536. doi: 10.1038/s41598-017-10209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Huang M, Yang J, Ma X, Zheng S, Deng S, Huang Y, Yang X, Zhao P. Anti-diabetic activity of stigmasterol from soybean oil by targeting the GLUT4 glucose transporter. Food Nutr Res. 2017;61:1364117. doi: 10.1080/16546628.2017.1364117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray-Zmijewski F, Slee EA, Lu X. A complex barcode underlies the heterogeneous response of p53 to stress. Nat Rev Mol Cell Biol. 2008;9:702–712. doi: 10.1038/nrm2451. [DOI] [PubMed] [Google Scholar]
- 28.Lu Q, Zhou Y, Hao M, Li C, Wang J, Shu F, Du L, Zhu X, Zhang Q, Yin X. The mTOR promotes oxidative stress-induced apoptosis of mesangial cells in diabetic nephropathy. Mol Cell Endocrinol. 2018;473:31–43. doi: 10.1016/j.mce.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Chen WS, Peng XD, Wang Y, Xu PZ, Chen ML, Luo Y, Jeon SM, Coleman K, Haschek WM, Bass J, Philipson LH, Hay N. Leptin deficiency and beta-cell dysfunction underlie type 2 diabetes in compound Akt knockout mice. Mol Cell Biol. 2009;29:3151–3162. doi: 10.1128/MCB.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin X, Xu Z, Zhang Z, Li L, Pan Q, Zheng F, Li H. Association of PI3K/AKT/mTOR pathway genetic variants with type 2 diabetes mellitus in Chinese. Diabetes Res Clin Pract. 2017;128:127–135. doi: 10.1016/j.diabres.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Al-Megrin WA, El-Khadragy MF, Hussein MH, Mahgoub S, Abdel-Mohsen DM, Taha H, Bakkar AAA, Abdel Moneim AE, Amin HK. Green Coffea arabica Extract Ameliorates Testicular Injury in High-Fat Diet/Streptozotocin-Induced Diabetes in Rats. J Diabetes Res. 2020;2020:6762709. doi: 10.1155/2020/6762709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan S, Liu X, Zhu X, Qu Z, Gong Z, Li J, Xiao L, Yang Y, Liu H, Sun L, Liu F. The Role of TLR4 on PGC-1α-Mediated Oxidative Stress in Tubular Cell in Diabetic Kidney Disease. Oxid Med Cell Longev. 2018;2018:6296802. doi: 10.1155/2018/6296802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed HH, Abd El-Maksoud MD, Abdel Moneim AE, Aglan HA. Pre-Clinical Study for the Antidiabetic Potential of Selenium Nanoparticles. Biol Trace Elem Res. 2017;177:267–280. doi: 10.1007/s12011-016-0876-z. [DOI] [PubMed] [Google Scholar]
- 34.Mora C, Navarro JF. Inflammation and pathogenesis of diabetic nephropathy. Metabolism. 2004;53:265–6; author reply 266. doi: 10.1016/j.metabol.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Malik S, Suchal K, Khan SI, Bhatia J, Kishore K, Dinda AK, Arya DS. Apigenin ameliorates streptozotocin-induced diabetic nephropathy in rats via MAPK-NF-κB-TNF-α and TGF-β1-MAPK-fibronectin pathways. Am J Physiol Renal Physiol. 2017;313:F414–F422. doi: 10.1152/ajprenal.00393.2016. [DOI] [PubMed] [Google Scholar]
- 36.Allen TL, Febbraio MA. IL6 as a mediator of insulin resistance: fat or fiction? Diabetologia. 2010;53:399–402. doi: 10.1007/s00125-009-1627-x. [DOI] [PubMed] [Google Scholar]
- 37.Cebola I, Custodio J, Muñoz M, Díez-Villanueva A, Paré L, Prieto P, Aussó S, Coll-Mulet L, Boscá L, Moreno V, Peinado MA. Epigenetics override pro-inflammatory PTGS transcriptomic signature towards selective hyperactivation of PGE2 in colorectal cancer. Clin Epigenetics. 2015;7:74. doi: 10.1186/s13148-015-0110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu W, Yang X, Zhe C, Zhang Q, Sun L, Cao K. Puerarin inhibits iNOS, COX-2 and CRP expression via suppression of NF-κB activation in LPS-induced RAW264.7 macrophage cells. Pharmacol Rep. 2011;63:781–789. doi: 10.1016/s1734-1140(11)70590-4. [DOI] [PubMed] [Google Scholar]
- 39.Cai F, Wu F, Cao J, Chen X. MicroRNA-146b-3p regulates the development and progression of cerebral infarction with diabetes through RAF1/P38MAPK/COX-2 signaling pathway. Am J Transl Res. 2018;10:618–628. [PMC free article] [PubMed] [Google Scholar]
- 40.Ozbayer C, Kebapci MN, Kurt H, Colak E, Gunes HV, Degirmenci I. Potential associations between variants of genes encoding regulators of inflammation, and mediators of inflammation in type 2 diabetes and insulin resistance. J Clin Pharm Ther. 2021;46:1395–1403. doi: 10.1111/jcpt.13471. [DOI] [PubMed] [Google Scholar]
- 41.Opdenakker G, Abu El-Asrar A. Metalloproteinases mediate diabetes-induced retinal neuropathy and vasculopathy. Cell Mol Life Sci. 2019;76:3157–3166. doi: 10.1007/s00018-019-03177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jayashree K, Yasir M, Senthilkumar GP, Ramesh Babu K, Mehalingam V, Mohanraj PS. Circulating matrix modulators (MMP-9 and TIMP-1) and their association with severity of diabetic retinopathy. Diabetes Metab Syndr. 2018;12:869–873. doi: 10.1016/j.dsx.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Meier DT, Tu LH, Zraika S, Hogan MF, Templin AT, Hull RL, Raleigh DP, Kahn SE. Matrix Metalloproteinase-9 Protects Islets from Amyloid-induced Toxicity. J Biol Chem. 2015;290:30475–30485. doi: 10.1074/jbc.M115.676692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu D, Liu F, Li Z, Pan S, Xie J, Zhao Z, Liu Z, Zhang J. HNRNPA1-mediated exosomal sorting of miR-483-5p out of renal tubular epithelial cells promotes the progression of diabetic nephropathy-induced renal interstitial fibrosis. Cell Death Dis. 2021;12:255. doi: 10.1038/s41419-021-03460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun Y, Yang J. A bioinformatics investigation into the pharmacological mechanisms of the effect of Fufang Danshen on pain based on methodologies of network pharmacology. Sci Rep. 2019;9:5913. doi: 10.1038/s41598-019-40694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao D, Liu Z, Zhang H. The protective effect of the TUG1/miR197/MAPK1 axis on lipopolysaccharideinduced podocyte injury. Mol Med Rep. 2019;20:49–56. doi: 10.3892/mmr.2019.10216. [DOI] [PubMed] [Google Scholar]
- 47.Cai J, Tian Y, Lin R, Chen X, Liu Z, Xie J. Protective effects of kidney-tonifying Chinese herbal preparation on substantia nigra neurons in a mouse model of Parkinson's disease. Neural Regen Res. 2012;7:413–420. doi: 10.3969/j.issn.1673-5374.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xian YF, Lin ZX, Xu XY, Su ZR, Chen JN, Lai XP, Ip SP. Effect of Rhizoma Polygonati on 12-O-tetradecanoylphorbol-acetate-induced ear edema in mice. J Ethnopharmacol. 2012;142:851–856. doi: 10.1016/j.jep.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 49.Cui X, Qian DW, Jiang S, Shang EX, Zhu ZH, Duan JA. Scutellariae Radix and Coptidis Rhizoma Improve Glucose and Lipid Metabolism in T2DM Rats via Regulation of the Metabolic Profiling and MAPK/PI3K/Akt Signaling Pathway. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19113634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang J, Liu Y, Zheng Y, Luo Y, Du Y, Zhao Y, Guan J, Zhang X, Fu J. TREM-2-p38 MAPK signaling regulates neuroinflammation during chronic cerebral hypoperfusion combined with diabetes mellitus. J Neuroinflammation. 2020;17:2. doi: 10.1186/s12974-019-1688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong W, Qi M, Wang Y, Feng X, Liu H. Zoledronate and high glucose levels influence osteoclast differentiation and bone absorption via the AMPK pathway. Biochem Biophys Res Commun. 2018;505:1195–1202. doi: 10.1016/j.bbrc.2018.10.059. [DOI] [PubMed] [Google Scholar]
- 52.Catalfamo DL, Britten TM, Storch DL, Calderon NL, Sorenson HL, Wallet SM. Hyperglycemia induced and intrinsic alterations in type 2 diabetes-derived osteoclast function. Oral Dis. 2013;19:303–312. doi: 10.1111/odi.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou Q, Zheng S, Wang X, Liu S, Bao J, Yu F, Wu W, Shen B, Zhou T, Zhao Z, Wang Y, Chen R, Wang W, Ma J, Li Y, Wu X, Shen W, Xie F, Vijaykrishna D, Chen Y. Influenza A-associated severe pneumonia in hospitalized patients: Risk factors and NAI treatments. Int J Infect Dis. 2020;92:208–213. doi: 10.1016/j.ijid.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 54.Bredin C, Margery J, Bordier L, Mayaudon H, Dupuis O, Vergeau B, Bauduceau B. [Diabetes and amoebiasis: a high-risk combination] Med Trop (Mars) 2004;64:195–198. [PubMed] [Google Scholar]
- 55.Montgomery SM, Ehlin AG, Ekbom A, Wakefield AJ. Pertussis infection in childhood and subsequent type 1 diabetes mellitus. Diabet Med. 2002;19:986–993. doi: 10.1046/j.1464-5491.2002.00841.x. [DOI] [PubMed] [Google Scholar]
- 56.Cheng J, Zhang H, Zhao YL, Wang LX, Chen MT. Mutual Impact of Diabetes Mellitus and Tuberculosis in China. Biomed Environ Sci. 2017;30:384–389. doi: 10.3967/bes2017.051. [DOI] [PubMed] [Google Scholar]
- 57.Khalili M, Mahami-Oskouei M, Shahbazi A, Safaiyan A, Mohammadzadeh-Gheshlaghi N, Mahami-Oskouei L. The Correlation between Serum Levels of Anti-Toxoplasma gondii Antibodies and the Risk of Diabetes. Iran J Parasitol. 2018;13:637–642. [PMC free article] [PubMed] [Google Scholar]
- 58.Majidiani H, Dalvand S, Daryani A, Galvan-Ramirez ML, Foroutan-Rad M. Is chronic toxoplasmosis a risk factor for diabetes mellitus? Braz J Infect Dis. 2016;20:605–609. doi: 10.1016/j.bjid.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rennert G, Rennert HS, Gronich N, Pinchev M, Gruber SB. Use of metformin and risk of breast and colorectal cancer. Diabetes Res Clin Pract. 2020;165:108232. doi: 10.1016/j.diabres.2020.108232. [DOI] [PubMed] [Google Scholar]
- 60.Lei S, Chen S, Zhao X, Zhang Y, Cheng K, Zhang X, Wang Z, Sun Y, Wu S, Wang L. Hepatitis B virus infection and diabetes mellitus: the Kailuan prospective cohort study in China. Hepatol Int. 2020;14:743–753. doi: 10.1007/s12072-020-10086-2. [DOI] [PubMed] [Google Scholar]
- 61.Lago AS, Lima FR, Carvalho AM, Sampaio C, Lago N, Guimarães LH, Lago J, Machado PRL, Carvalho LP, Arruda S, Carvalho EM. Diabetes Modifies the Clinic Presentation of Cutaneous Leishmaniasis. Open Forum Infect Dis. 2020;7:ofaa491. doi: 10.1093/ofid/ofaa491. [DOI] [PMC free article] [PubMed] [Google Scholar]