Abstract
COVID-19 is a rapidly spreading disease, which has caught the world by surprise. Millions of people suffer from illness, and the mortality rates are dramatically high. Currently, there is no specific and immediate treatment for this disease. Remedies are limited to supportive regiments and few antiviral and anti-inflammatory drugs. The lack of a definite cure for COVID-19 is the reason behind its high mortality and global prevalence. COVID-19 can lead to a critical illness with severe respiratory distress and cytokine release. Increased oxidative stress and excessive production of inflammatory cytokines are vital components of severe COVID-19. Micronutrients, metalloids, and vitamins such as iron, manganese, selenium, Zinc, Copper, vitamin A, B family, and C are among the essential and trace elements that play a pivotal role in human nutrition and health. They participate in metabolic processes that lead to energy production. In addition, they support immune functions and act as antioxidants. Therefore, maintaining an optimal level of micronutrients intake, particularly those with antioxidant activities, is essential to fight against oxidative stress, modulate inflammation, and boost the immune system. Therefore, these factors could play a crucial role in COVID-19 prevention and treatment. In this review, we aimed to summarize antiviral properties of different vitamins and minerals. Moreover, we will investigate the correlation between them and their effects in COVID-19 patients.
Keywords: COVID-19, Minerals, Infectious disease, SARS-CoV-2, Vitamins
Graphical Abstract
1. Introduction
Severe acute respiratory syndrome (SARS) was the first coronavirus-induced pandemic found in 2002 in Guangdong in southern China [1]. The Middle East Respiratory Syndrome (MERS) was the second pandemic caused by these viruses. MERS was first originated in Saudi Arabia in 2012 [2]. These diseases have been reported to have considerable morbidity and mortality rates [3]. The third coronavirus pandemic, which had the most destructive effects on the world, is called novel coronavirus disease-19 (COVID-19). This disease is caused by SARS coronavirus 2 (SARS-CoV-2) and was first reported in Wuhan, Hubei province, China, in December 2019 [3], [4]. Coronaviruses (CoVs) belong to the subfamily of Ortho-coronavirinaein, the family of Coronaviridaein, and the order of Nidovirales. This subfamily includes α-coronavirus, β-coronavirus, γ-coronavirus, and ƍ-coronavirus. Coronaviruses are enveloped and single-stranded RNA viruses (+ssRNA) with 26–32 kb genome size. Owing to four spike glycoproteins on their surfaces, they appear as crown-like structures. Bynoe and Tyrell first described these viruses in 1996 [5], [6], [7], [8], [9]. Coronaviruses are both zoonotic and have been shown over the last decade to infect humans [10]. SARS-CoV, MERS-CoV, and SARS-CoV-2 all belong to the β-coronavirus family, which can infect both animals and humans [6], [11]. The spike glycoprotein of SARS-CoV-2 binds to its transmembrane receptor called angiotensin-converting enzyme 2 (ACE2) on the cell surface and enters the host cell through it [12], [13]. However, SARS-CoV-2 shares more than 82% similarity to SARS-CoV, its binding affinity to ACE2 is more than SARS-CoV. This is probably why SARS-CoV-2 propagation and transmission are more intense than SARS-CoV [3], [14]. SARS-CoV-2 is transmitted from human to human and animals to humans, by respiratory droplets, cough, or contaminated hands. The virus is located in the lung by inhalation and remains in the nasopharynx for three days, if it’s dry. Then, the virus enters the lung cells through spike and ACE2 interaction [15]. There are two types of lung cells; the first type is the alveolar cells involved in the Gas exchange process; the second type is macrophage cells, which act as surfactant producers and defensive lines. The SARS-CoV-2 damages the type 2 alveolar cells, and the macrophage cells try to produce cytokines (interleukin 1, 6, and 10) and Tumor Necrosis Factors. Increased interleukins and inflammatory agents damage the alveolar cells. Vasodilation occurs near type one alveoli and increases the capillary permeability. Therefore, the fluid accumulates between alveoli and blood vessels, and consequently, gas exchange is disrupted. The latter leads to breathing shortness, hypoxia, and hypoxemia. Following the damage to type two alveoli, the amount of surfactant decreases and leads to the collapsing of the lungs, fluid enters the alveoli, which leads to edema and also causes a cough or productive cough in the COVID-19 patient [16]. Lung collapse and edema develop acute respiratory distress syndrome (ARDS). In the central nervous system, when type two cells are damaged, interleukins 1 and 6, and 10 will be released, and force the hypothalamus to liberate prostaglandin. Prostaglandin converts the energy into heat by increased metabolism. This change increases fever and can cause brain stroke, particularly among adults. Hypoxia might induce peripheral chemoreceptors and lead to tachycardia. The blood pressure would decrease, and consequently, blood volume would be reduced, perfusion would decline, and multisystem failure would occur in COVID-19 patients [11].
COVID-19 is now ubiquitous in the world as a pandemic, and up to now, there is no universally approved and available drugs against COVID-19 disease. Therefore, in the absence of a particular medication for this new virus, finding an alternative solution to control this disease is urgent. Efforts to fight against SARS-CoV-2 should not be limited to finding drugs and vaccines; a supporting managements are also beneficial in decreasing the symptoms, strengthening immunity, and accelerating the recovery process [11], [17], [18]. Supporting management efforts include wearing facial masks, public hygiene, and social distancing. Moreover, recent evidence have emphasized that nutritional supplementation could play a supportive role in COVID-19 patients [19]. The immune system can be strengthened via supplement compounds and diet. Therefore, good nutrition is required for the immune system to function correctly. Vitamins and minerals are two critical dietary factors for the proper functioning of the immune system [20], [21], [22], [23], [24]. Vitamins A, B, C, and D, as well as minerals such as Lithium, Magnesium, Selenium, and Zinc, have synergistic roles in boosting and modulating the immune system. Among them, the role of vitamin C, D, and Zinc have been strongly approved. These factors have been reported as essential factors for immune system potency [21], [25], [26], [27], [28], [29].
It is well known that proper nutrition can support optimal immune function. In this sense, it has been reported that several vitamins (vitamin A, B6, B12, folate, C, D, E) and trace metals (zinc, selenium, copper, magnesium) support the immune cells. At the same time, their deficiencies could increase the susceptibility of a host to infectious diseases. It has been shown that the micronutrients intake, particularly vitamins B12, C, D, and iron, is inversely associated with higher COVID-19 incidence and mortality. This was more evident in individuals who were genetically predisposed to a low micronutrients status. Immunomodulatory agents – including micronutrients – are among some of the current therapies applied in clinical settings for the COVID-19. This article reviews the roles of vitamins and minerals in modulating and enhancing the immune system, as well as their possible roles in the prevention and, or treatment of COVID-19. The dominant hypothesis of this study is to promote the fact that nutrition can be an essential agent in the prevention/treatment of infectious diseases like COVID-19. To this end, we reviewed the scientific literature regarding the role of these elements and even the toxic metals/metalloids, on the infectivity, development, and prognosis of COVID-19. On the other hand, the effect of various vitamins and minerals in strengthening the immune system and preventing viral diseases was surveyed.
2. Vitamin A
Vitamin A is the first fat-soluble vitamin, also known as retinol, retinal, and retinoic acid (RA). Retinol is the transported form of vitamin A stored in the liver and converted to retinyl esters. Moreover, vitamin A is considered a significant hormone and an immune modulator in structure and functionality. Retinoic acid and its cognate retinoic acid receptors (RAR and RXR) are considered the pleiotropic modulators of the adaptive and innate immune responses [30].
2.1. The immunomodulatory role of retinoid in SARS-COV-2
Vitamin A is often referred to as an "anti-infective" vitamin, as it is required for several defensive functions against infection. It has been shown that a dietary deficit of a single nutritional element could cause immune system impairment. Vitamin supplements have also been shown to protect against morbidity and mortality associated with various infectious diseases, including influenza, diarrhea, measles-related pneumonia, human immunodeficiency virus (HIV), and malaria infections. During an acute infection, the active retinol derivatives (all-trans RA, 9-Cis trans RA, and 13-Cis trans RA) facilitate the synthesis of the most potent antiviral mediator, Type-I IFN (α and β). This will enable the development of a long-term immune response against the virus [31]. Retinoids have been shown to stimulate the mRNA expression of ISGs, such as RIG-I, and IFN regulatory factor 1 (IRF-1). The effects of retinoids on IFN-I have been reported in cell and animal models of cancer, as well as clinical trials for cancer and multiple sclerosis (MS) therapy [32], [33], [34]. According to Qu et al., RA-treated peripheral blood mononuclear cells from MS patients reinforced IFN response and restored CD8 +T-suppressor cell functions [35].
2.2. COVID-19 pathogenesis and retinoic acid depletion syndrome (ARDS)
SARS-CoV-2 is the biggest enveloped single-stranded RNA virus. Although the genome size of most RNA viruses is less than 10 kB, SARS-CoV-2 has a genome of 30 kB [36]. The immune response against the ssRNA structure of the SARS-CoV-2 genome is primarily mediated by the RIG-I pathway. As an extensively reviewed and well-characterized component of the congenital immune system, this pathway is dependent on retinoic acid for its function [37]. RIG-I is a significant immune system receptor recognizing viral ssRNA as a ligand. The retinoic acid stores of the body are rapidly depleted due to the excessive stimulation of the RIG-I pathway. This activation results from factors such as acute infections, high fever, excessive catabolism, high viral load, or a large viral genome (SARS-CoV-2). Previously, it has been reported that the retinoic acid levels are severely reduced during viral infections such as measles and RVs. If the RIG-I pathway is deactivated due to retinoic acid depletion, the immune response mechanism switches to the TLR3, TLR7, TLR8, TLR9, MDA5 receptors, and UPS (ubiquitin /proteasome system) pathways. These pathways are located in neutrophils, macrophages, and dendritic cells belonging to the adaptive immune component. They collectively induce the excessive cytokine release (cytokine storm) through the NFκB arm. Such excess cytokine release triggers severe clinical manifestations that can lead to endothelial injury, hypoxia, necrosis, and multiorgan damage [38]. Regarding the respiratory disorders in COVID-19 patients, it has been shown that the development of ARDS is significantly correlated with the release of inflammatory cytokines and exudative aggregation of liquid induced by SARS-CoV-2 binding to alveolar cell receptors [38], [39]. Lack of retinol derivatives such as lecithin, Colin, and inositol in the medium, and the possible disturbance of surfactant synthesis due to retinoic acid degradation are effective in ARDS pathogenesis. Retinol derivatives play a crucial role in the surfactant structure. They are regarded as critical issues that require further investigation in terms of the growth and severity of ARDS condition ( Fig. 1) [31].
Fig. 1.
COVID-19 pathogenesis and retinoic acid depletion syndrome.
2.3. The role of retinoic acid on COVID-19ʼs ocular and nervous system
Retinitis and other vision disorders such as "pink eye," are typical in severe COVID-19 patients. They occur due to nerve cell atrophy and necrosis caused by vitamin A deficiency in the retina [40], [41]. Moreover, taste and olfactory disorders occur as a result of retinoic acid deficiency in COVID-19 patients, which is developed by retinoic acid receptors [42]. The observations from the nervous system of COVID-19 patients showed that the Retinoid receptors have a prevalent distribution. This distribution indicates that retinoid signaling could play a physiological function in the adult cortex, amygdala, hypothalamus, hippocampus, striatum, and associated areas of the brain [43], [44].
2.4. The current treatment of COVID-19 by cytochrome p450 system and RAMBAs
Cytochrome P450 is responsible for the metabolism and detoxification of toxins and drugs in the endoplasmic reticulum of liver cells. It has been demonstrated that impeding the liver cytochrome P450 could be an efficient way in COVID-19 patients by restricting retinoic acid metabolism. Serum retinoic acid levels are raised by prohibiting the excretion of retinol esters previously retained in the liver and obtained by food and by inhibiting the cytochrome oxidase mechanism [45]. The RIG-I pathway is modulated by serum retinoic acids and employs nuclear receptors to facilitate the primary immune response. RAMBAs (Retinoic acid metabolism inhibitors) could raise the endogenous retinoic acid levels and have recently been applied in dermatological indications. These effects could also be efficient against COVID-19 patients [46]. Hence, focusing on medications that have positive effects on the amount of retinol in our bodies could offer numerous advantages in terms of both public health and socio-economic experience. These advantages have previously been experienced with vitamin A treatments in measles and other viral infections.
3. Vitamin B
B vitamins are water-soluble vitamins. They serve as coenzymes to maintain physiological homeostasis. Vitamin B is essential for cell function, energy metabolism, and proper immune functions; They also decrease the pro-inflammatory cytokine levels, enhance respiratory function, retain endothelial integrity, eliminate hypercoagulability, and may diminish the hospital stays [47].
Each B vitamin serves a distinct purpose regarding the COVID-19. For instance, vitamin B1 (Thiamine) plays a significant role in immune responses. It is associated with reduced incidence of cardiovascular disease, type 2 diabetes, kidney diseases, aging-related diseases, cancer, neurological diseases, and neurodegenerative disorders. Thiamine deficiency affects the cardiovascular system, induces inflammation (including neuroinflammation), and contributes to aberrant antibody responses. Considering the fact that antibodies are required for the eradication of the SARS-CoV-2 virus, thiamine deficiency can lead to inadequate antibody responses and provoke severe symptoms [48]. Therefore, sufficient thiamine levels could assist the implementation of appropriate immune responses during SARS-CoV-2 infection.
3.1. Vitamin B2 (Riboflavin)
Vitamin B2 (riboflavin) is involved in the proper functioning of energy metabolism. Riboflavin deficiency has been reported among the elderly in the United States. Combination of Riboflavin and UV light treatment has been demonstrated to considerably reduce the MERS-CoV titer in human plasma and platelet components through irreversible damaging of nucleic acids such as DNA and RNA. Moreover, this treatment could disrupt the ability of microbial pathogens to replicate [49], [50]. These properties could decline the rate of COVID-19 transmission through transfusion and reduce the presence of other pathogens in blood products for severely ill COVID-19 patients.
3.2. Vitamin B3 (Niacin, Nicotinamide)
Vitamin B3 (Niacin) is a precursor for NAD and NADP production, required during chronic systemic inflammation. Since it serves as a coenzyme in various metabolic pathways, increased levels of NAD+ could be used for alleviation of a broad range of pathophysiological conditions. NAD+ is produced in the early stages of inflammation and has immunomodulatory properties. It could inhibit the development of pro-inflammatory cytokines, including IL-1, IL-6, and TNF-a [51], [52]. Furthermore, in patients with ventilator-induced lung damage, niacin inhibits neutrophil infiltration and has an anti-inflammatory effect. Niacin and nicotinamide have been shown to protect the lung tissue in hamsters [53]. Moreover, nicotinamide inhibits viral replication (human enteroviruses, immunodeficiency virus, vaccinia virus, and hepatitis B virus) and reinforces the immune mechanisms [54], [55]. Niacin can be used as an adjunct therapy for COVID-19 patients, owing to its lung-protective and immune-strengthening properties.
3.3. Vitamin B5 (Pantothenic acid)
Vitamin B5 (Pantothenic acid) is associated with myriads of functions promoted by Pantothenic acid. Lowering cholesterol and triglyceride levels, accelerated wound healing, decreased inflammation, and improved mental health are among the alleged functions of Vitamin B5 [48]. According to in silico analyses reported by Medha Pandya et al., vitamin B5 can bind to the spike protein of coronaviruses. In this regard, Ligplot analysis of vitamin B5-COVID-19 interactions demonstrates that several hydrogens are involved in their interaction interface [6]. Despite the fact that there have been few types of research showing the impact of pantothenic acid on the immune system and precisely coronavirus, it is a promising vitamin for further investigation.
3.4. Vitamin B6 (Pyridoxine, Pyridoxal 5`-phosphate)
Vitamin B6 is essential for protein metabolism, various reactions in tissues, and the proper functioning of our immune system. PLP (pyridoxal 5′-phosphate) is an active precursor of vitamin B6. It acts as a cofactor for a broad spectrum of inflammatory pathways, and its deficit could lead to immune dysregulation [56]. PLP supplementation ameliorates the COVID-19 symptoms, including cytokine storm and inflammation. It preserves endothelial integrity, reduces hypercoagulability, and upregulates IL-10 levels. IL10 is considered a potent anti-inflammatory and immunosuppressive cytokine capable of inhibiting antigen-presenting cells, T cells, macrophages, and monocytes [57].
3.5. Vitamin B9 (Folate, Folic acid)
Folate, also known as vitamin B9, is a type of B vitamin that has a significant role in cell development and maintenance, protein synthesis, DNA repair mechanism, adaptive immune response, and prevention of furin-associated bacterial and viral infections. Folate has been shown to inhibit the furin-bonded SARS-CoV-2 spike enzyme. Hence, it could hinder viral entry and turnover [58]. Additionally, a recent in silico study has demonstrated that folic acid and its compounds, including THF and 5-MTHF, have the highest binding affinity to Furin, spike, RdRp, and NSP3 proteins based on the molecular docking results. These observations imply that folate and its derivatives could be one of the most effective SARS-CoV-2 inhibitors [6]. Therefore, folic acid can therapeutically be used for COVID-19 regulation.
3.6. Vitamin B12 (Cobalamin)
Vitamin B12 (cobalamin) is required for RBC synthesis, nervous system health, myelin synthesis, production and functioning of innate and adaptive immune systems, cellular growth, and accelerated DNA synthesis. Active forms of vitamin B12, including adenosyl-hydroxy and methyl-cobalamin, are essential for gut microbiome modulation. B12 deficiency could lead to increased methylmalonic acid and homocysteine. This increase could induce intensified inflammation, reactive oxygen species, and oxidative stress, contributing to endothelial dysfunction, platelet activation, and stimulating the coagulation cascade [59], [60], [61]. In some instances, megaloblastic anemia, disruption of neuron myelin sheath integrity, impaired protective immune response, neurological complications, and spinal degeneration are some of the impacts of poor cobalamin levels [61], [62], [63]. Given these circumstances, vitamin B12 deficiency symptoms, including elevated oxidative stress and lactate dehydrogenase (LDH), intravascular coagulation thrombosis, homocysteine accumulation, coagulation cascade initiation, reduced reticulocyte count, vasoconstriction, and renal dysfunction maybe shared with COVID-19 infection [60], [64]. It has been suggested that high doses of methylcobalamin can serve as potential inhibitors of the RNA-dependent RNA polymerase activity of the SARS-CoV-2 nsp12 enzyme. Inhibition of the nsp12 enzyme could decrease the viral infection and intensity of the COVID-19 disease [65]. Briefly, methylcobalamin medication will help alleviate the severity of COVID-19 illness.
4. Vitamin C
COVID-19 disease is spreading across the world, and people are looking for supportive treatments like supplementation of micronutrients such as vitamin C to protect themselves against the virus or to decrease its effects [66]. Ascorbic acid or ascorbate (vitamin C) is the primary non-enzymatic and water-soluble molecule present in plasma. Since it is not synthesized (due to a gene mutation in the gluconolactone oxidase (GULO) gene over 40 million years ago) and stored within the body, humans are dependent on exogenously supplied dietary vitamin C [67]. The Recommended Dietary Allowance (RDA) for adults for vitamin C is set at 75 mg/day for females and 90 mg/day for males [68]. Vitamin C is a practical, harmless, and inexpensive therapeutic alternative and is used to treat infections of the respiratory system. This vitamin is also a cofactor for several enzymes that catalyze the conversion of violaxanthin to zeaxanthin, synthesis of abscisic acid, biosynthesis of carnitine, and modification of the dopamine to norepinephrine. Vitamin C plays a crucial role in synthesizing collagen and neurotransmitters, wound healing, energy metabolism, and nervous system function. However, due to its anti-oxidant effects, immunomodulatory effects, and antiviral properties, it is accepted as the supportive treatment against the COVID-19 [69], [70]. The results of previous studies showed the lowering effect of vitamin C on the expression of ACE2 in alveolar epithelial cells and microvascular endothelial cells. These cells are the two kinds of cells affected by the SARS-CoV-2 [71].
4.1. Anti-oxidant effect of vitamin C
Free radicals are atoms that contain an unpaired electron that the body can handle, but the excessive level can lead to diseases like COVID-19. Oxidative stress is an imbalance between the production of free radicals and the ability of the body to counteract their harmful effects through antioxidants. Antioxidants are molecules that donate an electron to the free radicals to prevent oxidative stress [72], [73]. Previous studies have demonstrated that vitamin C has a potent antioxidant effect by scavenging the oxygen-free radicals and restoring other cellular anti-oxidants [74], [75]. COVID-19 infection can result in oxidative damage in several different organs and tissues. However, vitamin C maintains the cell redox integrity to protect the lungs against oxidative stress [76]. Recent studies spotted deficient levels of vitamin C in COVID-19 patients. Thus, improving the levels of this vitamin could be an amenable strategy in managing COVID-19 patients.
4.2. Immunomodulatory effect of vitamin C
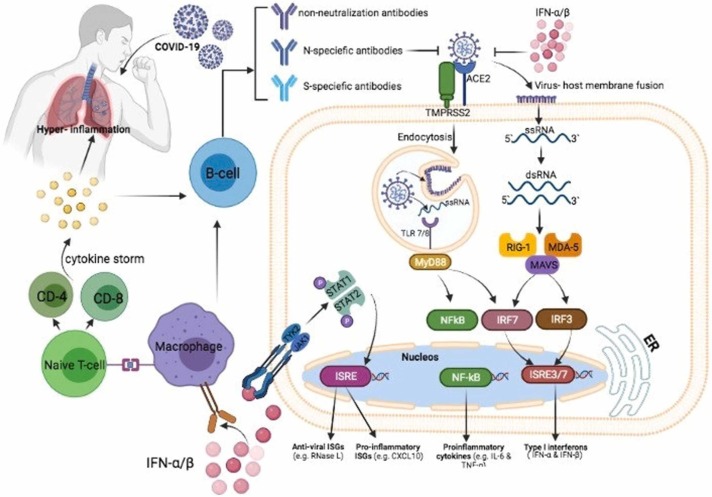
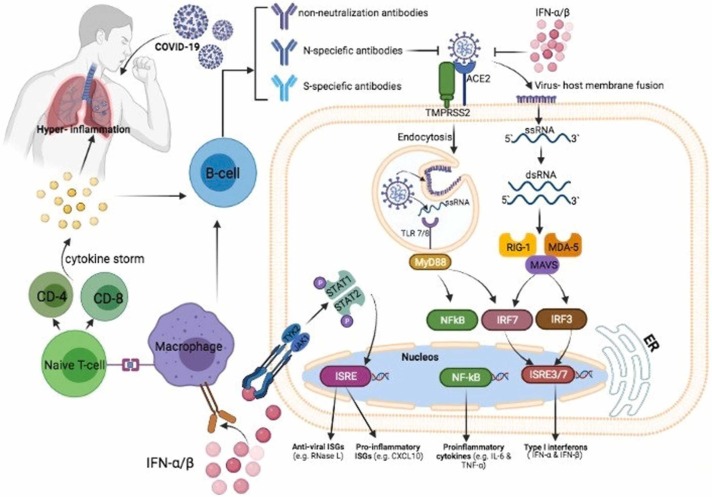
It has been approved that SARS-CoV-2 infection has a significant adverse impact on the immune system. Following the COVID-19, decreased numbers of natural killer cells and excessive release of inflammatory mediators could lead to cytokine storm and tissue damage. It has been approved that vitamin C has a significant effect on both innate (non-specific) and adaptive (specific) immune system improvement [77]. Following the release of inflammatory signals, neutrophils swarm to the site of infection randomly (chemokinesis) or in response to chemical stimuli (chemotaxis) [78]. Findings exhibit that vitamin C deficiency may affect the migration ability of phagocytes to the sites of infection. Vitamin C plays a vital role in the innate immunity against viral infection [79]. The epithelial barrier is the first line of defense against external pathogens, and vitamin C can enhance its integrity by increasing the expression of tight junction proteins and inhibiting cytoskeletal rearrangements [80]. SARS-CoV-2 infection can induce the cytokine storm and hyper inflammation syndrome, determined by the increased levels of proinflammatory mediators such as interferon-gamma, IL-1β, IL-6, IL-12, CXCL10, CCL2, and TNFα [81], [82]. This cytokine storm leads to multiple organ dysfunction and death in severe cases. Following the tissue injury, immune cells including macrophages, monocytes, and dendritic cells can secret the high mobility group box 1 (HMGB1) as a proinflammatory cytokine. However, vitamin C can inhibit the release of HMGB1 by activating Nrf2/HO-1 signals[83]. Dehydroascorbic acid (DHA) is an oxidized form of ascorbic acid, reduced to ascorbic acid in the cell. Due to structural similarity to glucose, the intracellular transport of DHA occurs via glucose transporters (GLUT), and the intracellular transport of ascorbic acid takes place through sodium-dependent vitamin C transporters (SVCT)−1 and −2 [84], [85]. The activity of natural killer cells, the suitable function of leukocytes, anti-apoptotic effects on peripheral blood neutrophils, improved proliferation, differentiation, and maturation of T lymphocytes, increased release of type I interferons, and modulation of inflammatory mediators are connected to the presence of vitamin C. These processes have the prominent role in improving the cellular immune response against viral infections [63], [86]. Neutrophil extracellular trap (NETosis) is a novel pathway different from apoptosis and necrosis, capturing and inactivating pathogens. Excessive NETosis could injure the host tissues. Based on pre-clinical findings, Vitamin C could play a critical role in the regulation of NETosis [81]. Several studies have reported that the dosage of vitamin C could affect the phagocytic functions of neutrophils. Administration of vitamin C in high doses exhibits positive results and decreases the severity of respiratory viral infections. However, mega doses greater than 2,000 mg show adverse effects like kidney stones, dry mouth, and weakness [66], [87]. The results of another study have demonstrated that a vitamin C supplement of 200 mg/day for 1–3 months raised the levels of IgG and IgM in serum and enhanced the humoral immune response in the elderly. In line with the aforementioned observations, previous studies have demonstrated that inadequate intake of vitamin C could reduce the resistance against infection and increases the complications of the disease. However, a regular supplement of vitamin C could help the immune system, and it has the potential to be deemed as a feasible treatment option in COVID-19 [88], [89].
4.3. Antiviral properties of vitamin C
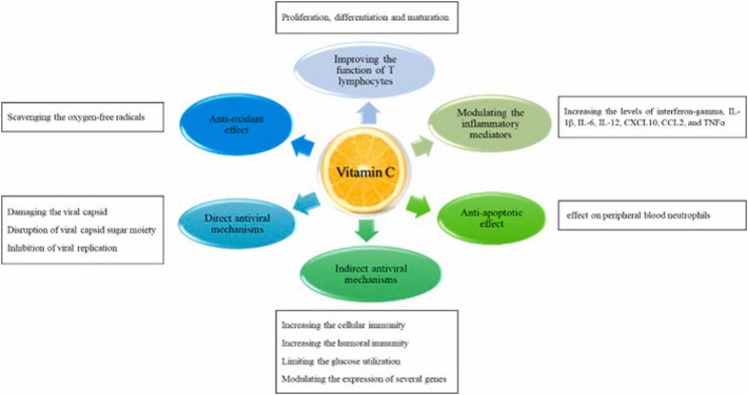
In vitro studies, animal experiments, and clinical trials exhibited that vitamin C has an antiviral effect. Due to in vitro inactivation of the viral multiplication, it is suggested that vitamin C might have a veridical impact. Vitamin C could exhibit antiviral immune responses by enhancing the production of interferon-alpha and beta [88], [90]. There are two direct and indirect antiviral mechanisms of Ascorbic Acid [91]. Damaging the viral capsid because of the redox capacity, disruption of the viral capsid sugar moiety, and inhibition of viral replication by degradation the genomes of the RNA and DNA viruses are the direct antiviral mechanisms of vitamin C [92], [93], [94]. Ascorbic Acid can manifest the indirect antiviral agents by increasing cellular and humoral immunity, limiting glucose utilization, and antioxidant action [95], [96], [97], [98]. Vitamin C also can modulate the expression of several genes, such as reducing the mitochondrial antiviral signaling, interferon regulatory factor 3, and increasing the nuclear factor κB (NF-κB) expression. These changes can lead to antiviral effects. NF-κB is a member of transcription factors that participate in inflammasome regulation, differentiation of innate immune cells, and induces the expression of different pro-inflammatory genes [99]. Based on the evidence from the mentioned clinical studies, it can be concluded that vitamin C can be helpful in the treatment of respiratory and other viral infections. Recently, it has been reported that exposure of chick embryo tracheal organ to vitamin C raised the resistance of the disease to coronavirus [100]. It has also been reported that vitamin C can reduce the risk of paralysis and improve survival in monkeys infected with poliomyelitis [88]. In light of these observations, the antiviral effects of vitamin C against both DNA and RNA viruses could introduce vitamin C as an alternative strategy in the management of COVID-19 patients ( Fig. 2).
Fig. 2.
Antiviral properties of vitamin C.
5. Vitamin D
5.1. Immunity and Vitamin D
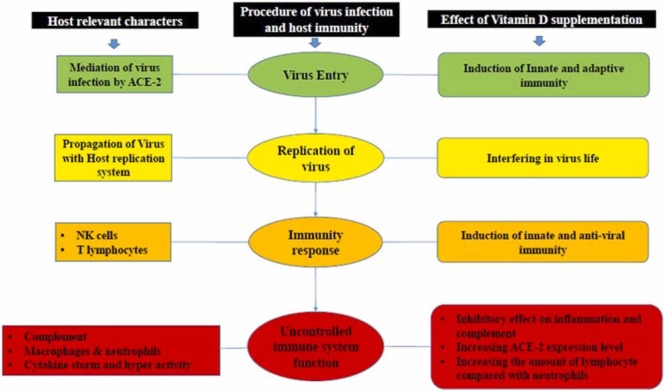
A significant correlation between vitamin D (calciferol) and health has already been established through health issues such as adult osteomalacia and children's rickets. Vitamin D can be obtained from ultraviolet (UV) radiation, supplementary components, and dietary regimens. Vitamin D can generally be found in two primary forms. UV irradiation on ergosterol from vegetable groups leads to the formation of vitamin D2 (ergocalciferol), and UV irradiation on 7-dehydrocholesterol leads to the formation of vitamin D3 (cholecalciferol). Some steps should be taken to achieve the active form of vitamin D. 1, 25-dihydroxy vitamin D3 (calcitriol) is the active form of the Vitamin D. Liver has a critical role in the production of 25-hydroxylation using several kinds of enzymes (CYP27A1, CYP2D25, and CYP2R1, etc.). The following essential enzyme for this process is the 1α-hydroxylation (CYP27B1). This enzyme is found in various cells, specifically in kidney tubules cells. The activation of this enzyme is handled by fibroblast growth factor 23 [101]. Moreover, CYP24A1 is another enzyme responsible for the breakdown of calcitriol. Vitamin D binding protein (DBP) is a multifunctional protein, which plays a crucial role in the transport of vitamin D and its metabolites. DBP facilitates the transportation of vitamin D and is considered as a modulator of inflammation and immune response [102]. Vitamin D can decrease the potential of viral infection and its mortality through three well-known immune pathways, including a physical barrier, innate immunity, and adaptive immunity. We will discuss the correlation of vitamin D with the innate and adaptive immune systems.
5.2. Regulatory effect of vitamin D in the innate immune system
The innate immune system is the first defense line in the body against several kinds of pathogens, and it generally acts as a non-specific mechanism. The response of this immune system to infectious pathogens includes different components such as neutrophils, macrophages, and complementary systems. Besides, it may cooperate in presenting antigens to the lymphocytes of other famous immune systems called adaptive immunity [103]. An early link between vitamin D and the immune system has been found through research on how this vitamin is metabolized and how its activating enzymes are induced [104]. It has been reported that expression of the vitamin D-1–hydroxylase genes and the vitamin D receptor could be upregulated due to TLR activation of human macrophages. Increased expression of these genes could lead to induction of the cathelicidin antimicrobial peptide, and cathelicidin, in turn, could trigger the killing of intracellular Mycobacterium tuberculosis [105]. Additionally, this protein can induce different types of inflammatory cytokines and plays a significant role in the stimulation of the defensive functions of neutrophils, monocytes, and macrophages against infections agents. Moreover, the induction of cathelicidin expression by 1,25(OH)2D, which has been identified in various primates, could confirm the association of vitamin D with enhanced production of this peptide [106], [107]. Due to its antimicrobial and anti-endotoxin properties, Vitamin D can improve the expression of antimicrobial peptides such as cathelicidin and β-defensin. β-Defensin2 is highly essential in the induction of chemokines and cytokines. It could create a signal for various immunogenic agents such as neutrophils, monocytes, macrophages, natural killer cells, and some epithelial cells located in the respiratory system [108], [109], [110], [111]. Production of these anti-bacterial peptides relies on CYP27B1, and vitamin D receptors (VDR), and the rate of their expression is directly correlated with the number of pathogens that bind to the membrane pattern recognition (PRR) such as toll-like receptor 2 (TLR-2) and toll-like receptor 4 (TLR-4). Another recognition receptor is nucleotide-binding oligomerization domain-containing protein 2 (NOD2). This protein can be stimulated by 1,25(OH)2D and can increase the expression of β-defensin [112], [113].
5.3. Regulatory effect of vitamin D in the adaptive immune system
B cell and T lymphocytes have a crucial role in detecting specific antigens. B cells are a part of humoral immunity, which is responsible for the secretion of antibodies against various microorganisms. T cells are a part of cellular immunity, which has a helping role for B-lymphocytes to release antibodies and eradication of pathogens. It has been reported that resting B cells can minimally express the VDR, but signal activation and 1,25(OH)2D3 could regulate the expression of VDR in B cells [114]. Besides, B-lymphocytes have an effective role in modifying the expression of proteins involved in vitamin D metabolisms, such as 24-hydroxylase and 1-α-hydroxylase. Consequently, this kind of cells can give an autocrine response to the 1, 25(OH)2D3. It can confine the differentiation of plasma cells and prevent the production of memory B cells. However, it remains to be a controversial issue among researchers. Some researchers believe that 1,25(OH)2D3 may limit B cell function mainly due to the repression of cytokine production by macrophage and monocytes or regulation of CD4 + T Cell response [115]. Some others are convinced that 1,25(OH)2D3 has an inhibitory effect on IgE production of B cells [116]. Moreover, 1, 25(OH)2D3 can suppress immunity through T helper 1 (Th1), which can produce a higher amount of cytokines such as Interferon- gamma (IFNγ) and interleukin 2 (IL-2). Moreover, the mediating role of 1,25(OH)2D3 in T helper type 2 (Th2) response has been proven to cause the repression of Th1 function indirectly [117]. 1, 25(OH)2D3 can cooperate to induce regulatory T cells (T reg), which are essential for inhibition of inflammation. The function and development of T cells are possible with calcitriol and mediation of one of the most important transcription factors called Foxp3 [113], [118], [119]. IL-17 producing T cells (Th17) are another inhibitory target cells for calcitriol. Th17 is involved in the pathogenesis of many autoimmune diseases. The induction of Foxp3 (binding to the IL-17 promoter) and blockage of NFAT and Runx1 elements on the promoter of IL-17 have been accompanied by calcitriol [120]. Although the preventive role of calcitriol has been approved, some studies refer to the inhibitory function of calcitriol on Th17. It suppresses one of the most significant differentiation transcription factors, known as RORγt. Another task of calcitriol is the prevention of differentiation in dendritic cells, blockade of interleukin 12 (IL-12), and increased amount of secreted interleukin-10 (IL-10) by Th2 [121], [122]. The relationship between vitamin D and the innate and adaptive immune system is shown in Fig. 3.
Fig. 3.
The relationship between vitamin D and the innate and adaptive immune system.
5.4. The relation between lung and vitamin D
The main target of SARS-COV-2 is mainly the lung tissue. As the respiratory tract have a large surface, it is very suitable for pathogens. Epithelial cells, are specific cells, located in the respiration system[123]. These cells have a high potential for synthesizing 1, 25(OH)2D by expression of CYP27B1. Although CYP27B1 is consistently produced in epithelial cells of the respiratory tract, this factor must be induced in dendritic cells and alveolar macrophages via TLR and TNFα, respectively [124]. Due to the lack of diverse animal models to study antibacterial responses mediated by vitamin D and their restriction to specific species such as primates, the focus of studies was on the association of vitamin D deficiency with Mycobacterium disease [107], [125]. Several diseases are known to be correlated with insufficient vitamin D, such as HIV, HCV, HBV, and HPV [126]. It has been shown that 25-OHD is one of the most prominent factors of the antibacterial response of vitamin D, but it is also bound to the vitamin binding proteins. This fact can decrease 25-OHD for immune cells, including the dendritic cells, epithelial cells, and macrophages. Therefore, the concentration of vitamin D binding proteins and the amount of 25-OHD have an effective role in inducing antibacterial responses [127].
5.5. The relation between vitamin D, COVID-19, and cytokine storm
The role of vitamin D in COVID-19 can be analyzed by its epidemiological process, cellular process, risk factors, and genetics. Lack of vitamin D is a global issue that has a high impact on various clinical conditions. Some random clinical trials have referred to the inverse relation of vitamin D and the rate of mortality in patients who are infected with the virus [126], [128], [129]. According to the epidemiological findings, vitamin D is essential for reducing acute respiratory diseases. Besides, vitamin D can be prescribed to have antiviral effects as a preventive or therapeutic approach as an adjuvant in COVID-19 infections [130], [131], [132]. Vitamin D is involved in a wide range of cellular processes, including dipeptidyl peptidase-4 receptor (DPP-4/CD26) binding, recognition of RIG-I host, and Papain-like protease (PLpro). Among all processes, the dipeptidyl peptidase-4 receptor is associated with the S1 domain of the spike glycoprotein in COVID-19. The expression of this virulence factor causes misconception in the influence of vitamin D deficiency in animal models [133], [134]. Elderly and patients with immunological deficiencies, which have the minimum amount of vitamin D, are more prone to get COVID-19. Low vitamin D levels are also correlated with several types of diseases such as cardiovascular diseases, diabetes, and obesity. According to a survey conducted on African and American patients, more than 50% of all COVID-19 cases and about 70% of patients who died from COVID-19 suffered from the lower amount of vitamin D [135], [136], [137], [138]. It has been demonstrated that the amount of mortality among black patients is much more than in white COVID-19 patients. In other words, the rate of mortality for black males and black females is respectively 4.2 and 4.3 times more than the white group. Based on the previous studies, the amount of serum 25-OHD was lower in the black population than white people [139], [140]. The role of vitamin D in managing cytokine storms was investigated during the pandemic of flu in 1918–1919. This vitamin can block cytokine storm through the strengthening of innate immunity to decrease the load of viruses and reduction of adaptive hyper activation at the same time [141], [142]. The correlation of host immunity, virus infection, and vitamin D supplementation is depicted in Fig. 4. Extensive research on viral infection, and clinical diagnosis indicates that males have a greater rate of COVID-19 infections than females [143]. COVID-19 patients admitted to intensive care units reported elevated levels of pro-inflammatory IL-6, and it has been mentioned that coronavirus infection in humans causes cytokine/chemokine release, a process known as "cytokine storm". The initiation of cytokine storm has been suggested as a critical determinant of disease occurrence and a poor prognostic parameter for multiple organ failure and death [144].
Fig. 4.
Relation between vitamin D, COVID-19, and cytokine storm.
6. Vitamin K
Protein S (PS) has been identified in 1970 as a vitamin K-dependent natural anticoagulant protein at the intersection of multiple biological processes. Following the binding to a unique family of protein tyrosine kinase receptors, PS can regulate coagulation, activation of innate immunity, phagocytosis of apoptotic cells, vessel integrity, cellular survival, and nearby invasion, metastasis and angiogenesis. So The anti-inflammatory activity of Vitamin K, which is regulated by reducing PGE2, COX2, and IL-6, has received a lot of attention in recent years [145]. Increased levels of inflammatory cytokines such as IL-6 and C-reactive protein have been found, when vitamin K deficiency occurs due to intestinal malabsorption or drug administration. The latest findings showed that a high vitamin K intake is linked to less coronary calcification and a lower risk of cardiovascular disease (CVD) [146].
Intriguingly, the severity of COVID-19 infection in patients admitted to intensive care units is considered worse in men than in women. This may be due to the higher IL-6 and lower vitamin K levels of the males. It has been shown that vitamin K deficiency is common in COVID-19 patients. Vitamin K deficiency is more significant in males than females. Vitamin K deficiency is linked to a higher level of IL-6 in the general circulation. Furthermore, vitamin K deficiency has been indicated to help Th2 and Th1 cytokine storm by increasing pro-inflammatory cytokines such as IL-6, which is involved in the recruitment of cellular and humoral components to the inflammatory response. Vitamin K is a potentially modifiable risk factor for a more severe COVID-19 evolution in affected patients with clinical symptoms. It may also play a role in vascular calcification and contribute to thrombosis and disseminated intravascular coagulation (DIC) [147]. According to another report, vitamin K is essential for activating both pro and anticlotting factors in the liver and activation of extra-hepatically synthesized protein S, which seems to be important in the prevention of local thrombosis [148]. As vitamin deficiency occurs, the vitamin is exported to the liver to activate the procoagulant processes as mentioned above [149]. Extrahepatic vitamin K status is determined by measuring desphospho-uncarboxylated (DP- UC; i.e., inactive) MGP amounts and the ratio of uncarboxylated to carboxylated osteocalcin [150] and Matrix Gla protein (MGP) is a vitamin K-dependent regulator of calcification and elastic fiber degradation in soft tissues. Severe extrahepatic vitamin K deficiency have recently been reported In COVID-19 patients. Elevated inactive MGP levels was correlated with elastic fiber degradation rates. In other words, if vitamin K-dependent MGP activation is inadequate, elastic fibers are left vulnerable to SARS-CoV-2-induced proteolysis. Activated factor II levels in COVID-19 patients are standard, and vitamin K is preferentially transferred to the liver to activate procoagulant factors. As a result, vitamin K-dependent endothelial protein S activation is likely to be hampered, consistent with increased thrombogenicity. As a continuation of the pneumonia-induced vitamin K depletion, there is a reduction in activated MGP and protein S, which decreases pulmonary damage and coagulopathy [148].
7. Vitamin E
Vitamin E is fat-soluble compound, and it is found predominantly in sources, such as nuts like almonds and hazelnuts, legumes such as peanuts, and avocados and sunflower seeds [151], [152], [153].
7.1. Action mechanism of vitamin E in disease
Vitamin E is considered a potent antioxidant capable of neutralizing free radicals and ROS by donating a hydrogen ion from its chromanol ring. Free radicals generated from metabolic processes react with polyunsaturated fats within the cell membrane, causing peroxidative decomposition [154], [155], [156]. Vitamin E deficiency results in greater levels of lipid peroxidation in both in vivo and in vitro models. This notion is supported clinically by an inverse relationship between plasma lipoperoxidase and vitamin E in ARDS patients [157], [158], [159].
In addition to the antioxidant and anti-inflammatory properties, vitamin E also has a function in immunity. This vitamin enhances the immune response both in animal and human models through the following mechanisms: (1) Decreased production of nitrogen oxide resulting in prostaglandin E2 down regulation and inhibition of cyclooxygenase-2, (2) initiation of T-lymphocyte signals, and (3) modulation of the Th1/Th2 balance [160]. Moreover, a study reported that increased intake of vitamin E is more beneficial in maintaining immunity function in elderly as compared to younger individuals. Alphatocopherol is an inhibitor of protein kinase C, cell proliferation, and differentiation in smooth muscle cells, monocytes and platelets. Vitamin E increases the level of prostacyclin with inhibition of the metabolism of arachidonic acid. This property results in the inhibition of platelet aggregation and dilation of blood vessels [161], [162].
7.2. Vitamin E and COVID-19
COVID-19, as with most viral respiratory infections, has a predilection for those that are immunosuppressed, elderly, and those with chronic ailments. Vitamin E enhances the T lymphocyte-mediated immune function in response to mitogens and IL-2 and its ingestion could improve several immune functions in elderly [163], [164], [165], [166]. Oxidative stress is one of the driving pathological mechanisms that underpin the biology of ARDS as a result of COVID-19. The oxidant-antioxidant balance is severely shifted in COVID-19 patients, resulting in failure of biological membranes and excessive lipid peroxidation. The diffuse alveolar damage, hyaline membrane formation, and pulmonary edema are the pathological outcomes seen in the most severely affected COVID-19 patients [167], [168]. Vitamin E ingestion is known to lower the production of superoxides and tilt the balance back in favor of antioxidants. Deficiency in animal models has also been shown to cause increased genetic mutations that promote the virulence of coxsackievirus, and influenza virus, and two RNA viruses, such as COVID-19 [169], [170].
The vitamin E supplementation may enhance vaccine efficacy in those most susceptible within our society [164]. Highly complex mechanisms biological effects for vitamin E underpin the necessity of further research to unravel the potential benefits of this vitamin. Despite these beneficial roles in immunity, there is limited information on the effects of vitamin E or selenium supplementation in humans with COVID-19 infection, and patients are encouraged to have adequate intakes of different antioxidant [171].
8. Copper
Thousands of people have developed lung infections due to the coronavirus-2 (SARS-CoV-2) pandemic, and some have died. As a result, early detection and treatment of this virus-caused infection are critical. COVID-19 can be regulated through various pathways, including strengthening the immune system, inhibiting viral entry and replication, destroying the virus, and administrating drug therapies. Due to its long-term efficacy and defensive role against bacteria, improving the immune system is of acceptable significance. Copper has antibacterial, antifungal, antiviral, and anti-inflammatory effects. These properties make it an essential component in enhancing and strengthening the immune system against pathogens [172], [173], [174]. In 2020, Andriandreou et al. looked into the consequences of copper virulence in Table II (viral mechanisms vulnerable to copper virulence), Their results have shown that many Cu+2-induced biochemical processes are inactivated [175]. Various studies have been performed to elucidate the effective concentration, length, and mechanism of action for copper in COVID-19 [172], [173], [176].
The following executive processes are linked to copper performance: 1. DNA and RNA degradation and membrane loss. 2. Production of reactive oxygen species (ROS). 3. Interfering with active protein structure [172], [177]. The reaction of the immune system against inflammatory conditions demonstrates the benefits of copper. High copper serum concentrations, elevated levels of ceruloplasmin (a protein that transports copper in the blood), and IL-6, have been identified in studies [178], [179]. Copper anti-inflammatory activity has been found in acute inflammation and viral infections. Ceruloplasmin is responsible for the oxidation of iron II to iron III, which results in iron III being loaded onto transferrin and systemic distribution to other sites [180], [181]. In response to infection, autophagy is a form of immune reaction that results in an oxidative response. The induction of autophagy is synonymous with the development of autophagy vacuoles. This process contributes to the destruction of invading viruses, limitation of infection, and formation of autophagy vacuoles, which leads to the destruction of invading viruses and limitation of infection caused by the virus. Copper has been identified as an autophagy and apoptosis stimulant, resulting in the development of autophagy vacuoles and protection against infectious agents [182], [183]. Some studies have credited the inhibitory function of copper in the form of masks impregnated with copper oxide, nanoparticles (copper oxide powder), and other types of copper (Copper alloy dry surface, sodium copper, ionic copper oxide, copper iodide, Cu2+ and lay copper) in the control and irreversible degradation of viral infections. These inhibitory impacts of copper surfaces have also been studied on the survival rate of COVID-19 [184], [185], [186]. COVID-19 has a 4–8-hour survival period on copper surfaces. Another research looked at the durability of SARS-CoV-1 and SARS-CoV-2 on cardboard, stainless steel, and plastic surfaces, with shelf lives of 3, 48, and 72 h, respectively. There were no live viral particles on copper-impregnated surfaces. Data on the action mechanism of copper on COVID-19 and copper nanoparticles in the presence of oxygen showed that these formulations are the most powerful against the inhibitory effect of SARS-CoV-2 [187]. No research has been done into the virus propagation through copper-impregnated materials, and the most successful method of transmission is through respiratory droplets. Copper appears to be an efficient and low-cost technique for reducing infectious disease transmission, especially in medical centers [186]. Due to the influential role of copper in the disease process, several groups of hypothetically valuable drugs have been suggested in combination with copper, which demonstrates the role of copper as an antiseptic agent.
9. Selenium (Se)
9.1. Selenium status and viral infection
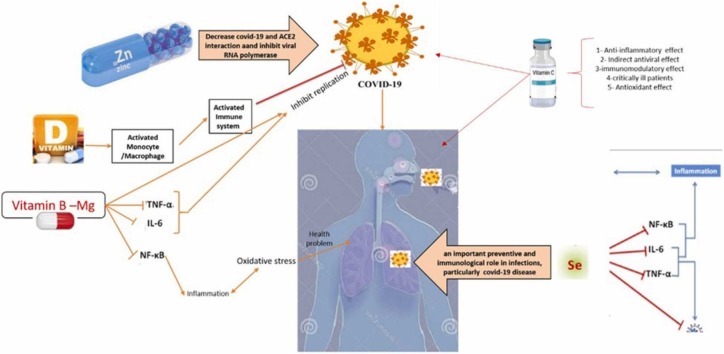
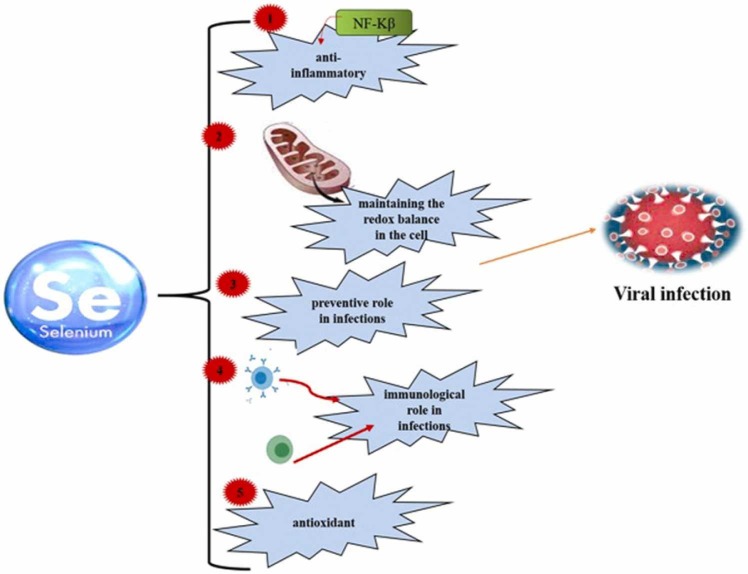
Selenium is a trace element required for the proper functioning of all organisms [188], [189] and is found in the various mineral and organic forms in the earthʼs crust and diet. This trace element has absolute protection in sodium selenite [190] and plays a vital role in maintaining the redox balance in the cell. Due to its antioxidant and anti-inflammatory properties, Se plays a preventive and immunological role in infections, particularly respiratory viral infections ( Fig. 5) [191], [192], [193].
Fig. 5.
The roles of selenium in viral infections.
Se reduces the entry of the virus into the respiratory cells by maintaining the integrity of the respiratory epithelial barrier. Previous studies have shown that selenium nanoparticles labeled with amantadine (an antiviral agent) suppress neuraminidase activity and inhibit virus binding to the hostʼs cell [194]. Thus, selenium can reduce the rate of infection [195]. By induction of selenium-deficient conditions, viruses are hindered due to higher mutations and reduced infectivity potential [196], [197], [198]. Se supplementation accelerates the cellular antiviral responses [199]. The effect of Se content on increasing the hostʼs ability to respond to viral infection is reflected in the cellular and molecular mechanisms involved in the control of redox homeostasis, stress response, immune response, and inflammation. These changes indicate a reduction in virus pathogenicity and infection rate [200]. In addition, various studies have shown that selenium deficiency is associated with an increased incidence of cancer and heart disease [190].
9.2. Link between Selenium and COVID-19
A healthy diet and the presence of elements such as selenium, which play a crucial role in boosting immunity, reduction of oxidative stress, and preventing of viral infections, determine the risk and outcome of SARS-CoV-2 disease [193]. Individuals with COVID-19, especially those admitted to ICU, are more vulnerable to nutritional deficiencies [201]. Therefore, deficiency of Se reduces immunity and predisposes the individuals to COVID-19 infection [202]. On the other hand, it induces viral mutations, enhanced replication, and the emergence of more pathogenic variants of RNA viruses. Some of the main functions of selenium in COVID-19 include prevention of viral infection, reduction of virus pathogenicity, strengthening of immunity, reduction of oxidative stress, prevention of inflammation, and inhibition of disease pathogenesis [196]. In a study in South Korea, a high rate of selenium deficiency was reported in COVID-19 patients [203], and in another study, selenium deficiency was associated with higher mortality in patients. Inadequate selenium intake is observed in a significant population of the world, which has a significant impact on infection and disease outcomes [204].
Sodium selenite (Na2O3Se) can oxidize thiol groups in the disulfide isomerase protein of the virus, preventing the virus from penetrating healthy cell membranes and killing the infection. On the other hand, selenite can react with the sulfhydryl group of the viral protein disulfide isomerase and convert it to a non-sulfide form, thus preventing the virus from entering the cell [190]. Therefore, this simple chemical compound could be used in appropriate doses to fight against the coronavirus epidemic as supportive therapy [190], [193]. Selenium supplementation also enhances the immune response, reduces oxidative stress, and diminishes the inflammation and the severity of viral behavior [196], [205], [206]. In addition, there is a hypothesis that selenium supplements may reduce the ability of the SARS-CoV-2 virus to infect human cells [207]. This supplement therapy can increase T cells, especially CD4 + T cells, and the percentage of NK cells, followed by NK cell cytotoxicity [208]. Moreover, mitochondrial function and inflammatory signaling are affected in this infection, which can affect the immune response and lead to severe outcomes in the host [7], [209].
10. Zinc
Zinc (Zn2+) is the second rare metal in the human body; it is essential for various cellular functions, including the maintaining a healthy immune system [210]. This trace element plays a vital role in different biological processes because it functions as a cofactor, signaling molecule, and structural element. It also regulates the metabolism of carbohydrates and lipids, and is involved in the reproductive, cardiovascular, and nervous systems [211]. On the other hand, Zn2 + plays an essential role in the differentiation of immune cells and restores the function of depleted immune cells or improves the function of normal immune cells, especially in immunocompromised or elderly patients [192], [212], [213]. In addition, it regulates the proliferation, differentiation, maturation, and functioning of leukocytes and lymphocytes in the human body [213], [214]. Various studies have shown that zinc plays an essential role in antiviral immunity [212]. People with lower levels of Zn2+ have an increased risk of infectious bacterial, fungal, and viral diseases [215], [216], [217], [218]. It can also be noted that Zn2+is probably necessary for stabilizing protein structures and altering cell affinity in various metalloproteins [219], [220]. Zn2+ may synergize with standard antiviral therapy in patients with Hepatitis C virus (HCV), Human immunodeficiency virus (HIV), and SARS-CoV-2 infections. Zn2+ is also effective against several viral species by interfering with virus attachment and uncoating [212], [218]. Zn2+ can protect or stabilize the cell membrane, which helps to prevent virus entry. It has also been shown to inhibit virus replication by altering the proteolytic processes of replicas and RNA-dependent RNA polymerase (RdRp) enzymes in rhinoviruses, influenza virus, and HCV, which reduces viral RNA synthesis [212]. Zinc is considered necessary, for proper folding and various cellular enzymes and transcription factors in multiple viruses. It may also interfere with the proteolytic activity of viral polyproteins by directly affecting viral protease (picornavirus, and poliovirus) or altering the tertiary structure (as in encephalomyocarditis virus) [221]. This element effectively prevents the fusion of respiratory virus membranes and HSV, which can be detected by binding to the histidine residue of a viral protein at a low pH of the endosome [222] and can even inactivate the VZV in vitro [223].
10.1. Potential role of zinc supplementation against SARS-CoV-2
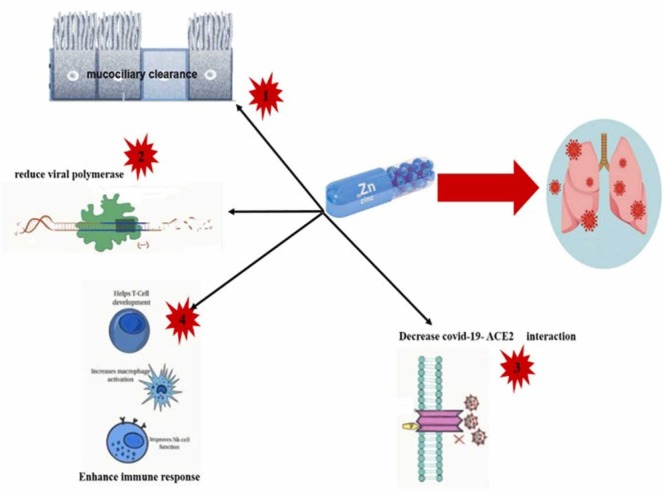
Zn2+ is a crucial element in maintaining the body's immunity and its deficiency increases the risk of infectious diseases. The role of zinc in the new coronavirus has not been proven. Prior evidence suggest that zinc may have an inhibitory effect on a type of coronavirus (SARS-CoV) and viruses involved in the common cold (Rhinovirus). In addition, zinc may help relieve the symptoms of diarrhea and lower respiratory tract infection caused by the coronavirus [224]. Zinc deficiency goes unnoticed by most people, and the World Health Organization assumes that at least one-third of the world's population is affected by its deficiency. Zinc deficiency is responsible for 16% of all deep-seated respiratory infections worldwide [225]. The first strong indication of zinc deficiency and its association with the risk of infection and the severe progression of coronary heart disease was obtained from the benefits of using it as a complementary therapy in this disease ( Fig. 6) [226]. The function of SARS-CoV2virus is highly dependent on host cell metabolism [227]. For this reason, zinc can seriously prevent the early stages of the virus from entering the late stages of the disease. For example, zinc can prevent fusion with the host membrane, reduce viral polymerase function, disrupt protein translation and processing, block the spread of viral particles, and destabilize virus envelopes [228], [229], [230]. In coronavirus infections, damage to the ciliated epithelium and ciliary dyskinesia leads to abnormal mucosal secretions [231]. On the other hand, the physiological concentrations of the Zn2+ increase ciliary beat frequency [232], which leads to the cleansing of the lash space. This function removes viral particles and increases the risk of bacterial infections [233]. Moreover, Zinc supplementation can regenerate the expression of human interferon α by leukocytes and enhance its antiviral effect through JAK/STAT1 signaling [234], [235]. As a result, this supplement can be used to treat COVID-19 patients in whom insufficient secretion of type I and II interferons is observed [236]. In various studies, the effect of zinc ions has been studied on the RdRp of SARS-CoV-2. It has been shown that this element directly inhibits the activity of RdRp in vitro. In particular, it blocks the SARS-CoV-2 RdRp elongation step by reducing the binding of the template strand. In another study, it was found that the Zn2+ mediated RdRp inhibition of SARS-CoV-2 is done by adding a Zn2+chelator [237]. It has recently been reported that administering high dosages of zinc salt to four individuals decreased their symptoms within 24 h of the initial dose [238]. As a result, zinc as an adjunct therapy reduces lung inflammation, increases mucosal clearance, inhibits ventilator-induced lung damage, and balances COVID-19 patients [239]. Zinc can modulate the coronavirus infection by targeting the structure of viral proteins. In particular, disulfiram-induced zinc release from papain protease-like has been shown to cause viral protein instability in COVID-19. Due to essential sites containing zinc, drugs such as disulfiram may be considered a potent antiviral agent and may be necessary for treatment [240], [241], [242]. There is evidence that zinc can increase the effect of chloroquine (zinc ionophore) as a therapeutic drug, and another zinc ionophore, such as epigallocatechin gallate aur quercetin, are being tested [243]. Various studies have also shown that low concentrations of zinc supplementation along with ionophore pyrithione or inositol reduce the synthesis of SARS-CoV-2 RNA by direct inhibition of RNA-dependent RNA polymerase [244], [245].
Fig. 6.
The protective mechanism of zinc in COVID-19 patients.
11. Lithium
Lithium is a monovalent cation alkali metal (Li+) and is one of the most widely used chemical elements for the treatment of Bipolar Disorder (BD) and several mental illnesses. One of these mental disorders is manic depression, known for periods of abnormally elevated mood. Treatment by lithium could reduce depressive morbidity (antidepressant drug) [26]. Inhibition of cell death and neuron growth promotion are the neuroprotective properties of lithium, which occurs due to membrane depolarization. Are a result of membrane depolarization, lithium is very effective in BD treatment [246]. The effects of lithium on the body of patients are beyond mental problems and depression. Significantly, lithium contributes to treating Alzheimer's disease (AD) [247]. However, it should also be noted that lithium harms the kidney and thyroid as its side effects [248]. In several studies, it has been suggested as a potential antiviral drug for the Coronaviridae family. The initial studies on the anti- RNA virus effects of lithium showed a reduction in the development of splenomegaly and lymphadenopathy. This report demonstrated that it could be used to treat retroviral infections [249]. In addition, further investigations have shown the efficacy of lithium in SARS-CoV-2 blockade and inhibition of its survival. It is effective in the virus replication and production, senescence and apoptosis, and immunomodulatory functions.
11.1. The virus replication and production
The study on the antiviral effect of lithium indicated that this chemical element could inhibit the entry and replication of the virus [250], so much so that in the fourth week of lithium treatment, a 40% reduction in viral transcription levels was observed [251], [252]. The lithium therapy suppresses the expression of viral proteins, leading to a significantly decreased production of virus particles and virus titer [253], [254]. Regarding, the release of the virus from infected cells, membrane hyperpolarization is a critical step. Lithium could change the hyperpolarization procedure by depolarizing and inhibiting of the virus pathogenesis [246]. On the other hand, it is observed that lithium could suppress the Inositol Polyphosphate 1-Phosphatase (IPPase) and Inositol Monophosphate Phosphatase (IMPase) in the phosphatidylinositol signaling pathway. The suppression of the IMPase leads to inhibition of IP6. It should be noted that IP6 is an essential element for viral stability and encapsulation of the newly synthesized viral genome in the capsid. Therefore, decreased IP6 could lead to the reduction of the virus replication [26], [255]. Another effect of lithium has been identified on the glycogen synthase kinase-3, isoform β (GSK-3β). The inhibition of the GSK-3β reduces the viral particle production [256]. It has been observed that the GSK-3 has a crucial role in the phosphorylation of the SARS-CoV-2 N protein. Finally, GSK-3 inhibitors barricade the phosphorylation process of N protein and ultimately destroy the replication of the virus [257]. Lithium mimetic agent, ebselen, appears to exert an inhibitory action on protease Mopar activity. The Mopar or 3 C-like protease (3CLpro) is a critical protein in the coronavirus synthesis. The protease Mopar, like other essential virus proteases, has a crucial role in the protein maturation and activation of other essential proteins of the virus. The virus infects the host cells, and the pro-proteins would be translocated to the cytosol space. The pro-proteins are subsequently cleaved by protease Mopar. These cleaved proteins would be accumulated to make the replicas transcriptase complex (RTC). As the name implies, this complex plays a crucial role in the viral life cycle, followed by the replication (full-length viral genome) and transcription (sub-genomic RNAs) [258]. The Mopar has been targeted using various newly designed drugs as an antiviral infection strategy. The assembly of mature viral particles would be subsequently disrupted by the protease inhibitors such as lopinavir, amprenavir, and tipranavir [259]. Particularly the N3 as the Mopar inhibitor substantiated the antiviral effect on COVID-19 virus infection [260].
11.2. The senescence and apoptosis process
The senescence-associated secreted phenotypes (SASP) is an aging phenotype in which these senescent cells secrete high levels of pro-inflammatory cytokines (IL-6, IL-8, and IL-1α), β-galactosidase, and cyclin-dependent kinase (CDK) inhibitors p16 and p21. It has been shown that low-dose lithium can reduce the SASP phenotype of human stem cells, suggesting that a micro dose of lithium could protect cells from senescence and the development of aging-related conditions [261]. The viral infection triggered the apoptosis induction in the infected host cells. Lithium seems to encompass a protective effect against apoptosis [262], [263], [264]. Autophagy is another regulated process of the cell that cleans out the unnecessary or dysfunctional components. It could destroy any cytoplasmic components in the lysosome. This mechanism verifies the viral infection by promoting the inflammatory responses by modulation of mediator molecules such as GSK-3β and IMPase [265]. Of course, it should be noted that viruses have acquired the ability to inhibit or so-called escape the mechanism of autophagy. Fortunately, it has been seen that lithium can also induce autophagy [26], [266].
11.3. Immunomodulatory
It is specified that the most significant mortality of SARS-CoV-2 belongs to the acute respiratory distress syndrome (ARDS), leading to lung damage. It suggests that this mortality rate is induced by the cytokine storm or inflammatory storm phenomenon. Lithium has anti-inflammatory and immunomodulatory effects to promote the body's stability conditions [26]. The “immunomodulatory” property means balancing all the contradictory (anti-inflammatory and pro-inflammatory) factors into the normal levels. The systemization of pro-inflammatory cytokines such as IL-4, and IL-6, or anti-inflammatory cytokines such as TNF-α, IL-2, and IL-10 are very well known and routine for the normalization of cytokine levels [267]. Numerous studies indicated the relationship between IL-6 and COVID-19 in the induction of cytokine release syndrome (CRS) [268]. This correlation is to the extent that it can be used as a biomarker for COVID-19 progression [269]. Furthermore, the use of lithium showed the suppression in IL-6 level and NF-κB (nuclear factor kappa B) as a cytokine storm initiator [261], [270]. Lithium appears to exert suppressive effects by inhibition of GSK-3β [271]. In fact, the GSK-3 inhibitors can inhibit viral replication (protein replication) and raise T-cell and natural killer (NK) cell responses [272]. In another mechanism, the immunomodulatory functions affect the actions and the numbers of immune cells. For instance, the plasma reactive C-Protein levels were remarkably decreased by the lithium carbonate treated in six patients with severe COVID-19 infection. Finally, this process is followed by increased lymphocyte numbers and thereby reduces the neutrophil-lymphocyte ratio (NLR) [273]. Another direct effect of lithium on immune cells is the elevation of antibody production from B-lymphocytes due to membrane depolarization. The membrane depolarization has an essential and provoking role in activating B-lymphocytes [246]. The benefits of lithium are substantial, but there are several, downsides to consider, as mentioned earlier. Lithium high consumption (more significant than 1.5 mmol/L) and abrupt change in dosage could lead to cardiotoxicity [274]. Such limitations preclude the quick recommendation of lithium application as a drug for COVID-19 patients. However, this monovalent cation alkali metal is easily consumed orally and excreted through the renal system. Accordingly, both cell-based and animal models of SARS-CoV-2 infection indicated the reduction of cytokine storm by lithium consumption. Consequently, it is possible to consider lithium as a drug for COVID-19. By reviewing previous studies, the suggested dose of lithium should be 0.5–1 mM for SARS-CoV-2 infection [275].
12. Magnesium
Magnesium (Mg), as the fourth most abundant cation in the body and the second most abundant intracellular cation after potassium, is a chemical element and a mineral part of more than 300 enzymes in the body mainly concentrated in the mitochondria [276], [277]. Noticeably except for the enzymatic cofactor role, magnesium also plays other roles in the body. These roles include the secondary messenger and cellular ion roles. These roles could guide various functions like the metabolic pathways (ATP energy complex in energy metabolism, DNA replication, apoptosis, nucleic acid synthesis, parathyroid hormone, and other protein syntheses) and the physiological functions (essential ions transporting, cytoskeletal dynamics, cell polarization, regulating the blood pressure, supporting the immune system, and better function of nerve and muscle) [276], [278]. This electrolyte is a crucial element for balancing the homeostasis maintenance of the cells. Recent studies indicated that each magnesium deficiency and administration approaches could change every symptom of COVID-19 patients. These alterations in the body are broad and generally related to some categories as summarized below; In patients with COVID-19, signs have been observed that evoke the deficiency of this element. For example, thromboembolic events, blood micro-coagulation, and blood-associated inflammatory factors. It is noteworthy that magnesium deficiency was observed in COVID-19 patients with hyperglycemia (the amount of glucose in the blood is high) and hyperinsulinemia (the higher insulin level in the blood) backgrounds [279]. Indeed, to the extent specified, the patients with diabetes, obesity, and cardiovascular diseases related to hypomagnesemia, are more susceptible to COVID-19 [280]. The mitochondria organelles regulate intracellular Mg2+ ions and balance their concentrations inside (mitochondrial compartments) and outside (the cytosol). The renal excretion of Mg was increased by the hyperinsulinemia and stress hormones (catecholamines and corticosteroids), which guide the intracellular Mg to the extracellular space and increase the renal excretion of Mg [280], [281]. Interestingly, more than 3 mg/dL of magnesium level prevented the QT (electrocardiogram from the Q wave to the T wave) prolongation of patients diagnosed with COVID-19 infection [261]. Increased vitamin D function, anti-hypertensive effect, anti-thrombotic (the blood clots formation reducer) effects, bronchodilator role, inhibition of arteriosclerosis, and reduction of the osteoporosis effects are the other consequences of magnesium consumption [282]. The association of magnesium and the immune system is not surprising; its deficiency is considered associated with respiratory problems. Nevertheless, administration of magnesium supplements (such as magnesium sulfate, 100 mg/kg, intravenously) as the anti-inflammation factor was utilized to inhibit the nuclear factor-κB (NF-κB), tumor necrosis factor-α (TNF-α), IL-6 and IL-1β cytokines, soluble vascular cell adhesion molecule 1, soluble intracellular adhesion molecule 1, and CRP [281], [283], [284], [285]. A reverse effect was observed by the Mg deficiency. It means that an enhanced intensification of immune response and a low-grade chronic inflammation have occurred [276], [286]. Magnesium is essential for synthesizing different vitamins, which are critical for immune system functions [65]. The magnesium intake changes the manifestations of COVID-19 from progression to regression [277]. Oral receiving of magnesium (as 340 mg twice a day) has led to improved lung condition [287]. It has anti-cholinergic and anti-histaminic systemic effects on the lung bronchus [286]. Daily receiving of 150 mg magnesium and other supplements for 14-days has reduced the need for oxygen therapy and intensive care support in COVID-19 patients [27]. Indeed, chronic obstructive pulmonary disease (COPD) has been associated with a low concentration of Mg in serum [286]. The inadequate intake and status of this nutrient are significantly related to the hypokalemia (low level of potassium (K+) in the serum), which makes more hospitalization period and severe level of respiratory symptoms in COVID-19 patients [288]. These data suggest that insufficient magnesium intake, as a beneficial nutrient, is so broad. The Recommended Dietary Allowance (RDA) of magnesium as a low-cost treatment could protect the immune system from optimal functions of infections.
13. Conclusion
In the present study, the effect of different vitamins, including the vitamins A, B, C, and D, was discussed in the pathogenesis of COVID-19. The deficiency of these vitamins could play a critical role in the poor prognosis of the COVID-19. A significant share of COVID-19 patients showed deficiencies of the vitamins. The experience from the COVID-19 pandemic showed that a supplement regimen of these vitamins could significantly improve the clinical outcomes of the disease. Moreover, we reviewed the scientific literature regarding the role of zinc, selenium, copper, iron, and some other metals, as well as that of the toxic metalloids, on the infectivity of SARS-CoV-2, and the prognosis of COVID-19 in infected subjects. The deficits of zinc and selenium seem to play a negative role in COVID-19 patients. Hence, their supplementation is recommended in many cases. On the contrary, the supplementation of copper and iron to COVID-19 patients is not apparent. Anyhow, the role of these trace elements is linked to the immune system of the COVID-19 patients, which is crucial in this disease. On the other hand, the role of toxic metals in COVID-19 patients is well defined. Chronic exposure of human and tissue accumulation of toxic elements such as arsenic, lead, cadmium, chromium, or vanadium, among others, means that at certain levels and times of exposure, they can cause immunotoxicity, as well as adverse effects on the respiratory system. Therefore, those individuals whose immune and respiratory systems can be negatively affected by chronic exposure to toxic elements and other environmental pollutants probably suffer from COVID-19 with greater severity. In summary, in COVID-19 patients, special attention must be paid to their load/levels of essential trace elements, mainly zinc and selenium. On the other hand, exposure to air pollutants in general, and toxic metal/metalloids in particular, should be avoided as much as possible to reduce the possibilities of viral infections, including SARS-CoV-2.
CRediT authorship contribution statement
Mohsen Karami Fath: Conceptualization, Formal analysis, Investigation, Writing – original draft. Malihe Naderi: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Hosna Hamzavi: Formal analysis, Investigation, Writing – original draft. Mahmoud Ganji: Formal analysis, Investigation, Writing – original draft. Shima Shabani: Formal analysis, Investigation, Writing – original draft. Faezeh Noorabad ghahroodi: Formal analysis, Investigation, Writing – original draft. Bahman Khalesi: Writing – original draft, Writing – review & editing. Navid Pourzardosht: Writing – original draft, Writing – review & editing. Zahra Sadat Hashemi: Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Supervision. Saeed Khalili: Conceptualization, Writing - review and editing, Supervision.
Author Declarations
The authors declare that they have no competing interests. Not specific funding was obtained for this work.
Acknowledgments
The authors wish to thank Shahid Rajaee Teacher Training University for supporting the conduct of this research.
Conflict of Interest
There is no conflict of interest in the article titled Molecular Mechanisms and therapeutic effects of different vitamins and minerals in Covid-19 patients.
Sources of support
No support was provided.
References
- 1.Cherry J.D. The chronology of the 2002–2003 SARS mini pandemic. Paediatr. Respir. Rev. 2004;5:262–269. doi: 10.1016/j.prrv.2004.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assiri A., McGeer A., Perl T.M., Price C.S., Rabeeah A.A. Al, Cummings D.A., et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N. Engl. J. Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conforti C., Cannavò S.P., Jafferany M., Dianzani C., Di Meo N., Lotti T., et al. Coronavirus disease 2019: Facts and controversies. Dermatol. Ther. 2020;33:13366. doi: 10.1111/dth.13366. [DOI] [PubMed] [Google Scholar]
- 5.Chan J.F.W., Kok K.H., Zhu Z., Chu H., Kai Wang To k, Yuan S., et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. https://doi.org/10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandya M., Shah S., Menamadathil D., Gadnayak A., Juneja T., Patel A., et al. Unravelling vitamins as wonder molecules for COVID-19 management via structure-based virtual screening. Res Squ. 2021 doi: 10.21203/rs.3.rs-144177/v1. [DOI] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyrrell D., Bynoe M. Cultivation of viruses from a high proportion of patients with colds. Lancet. 1968;1:606–610. doi: 10.1136/bmj.1.5592.606. [DOI] [PubMed] [Google Scholar]
- 9.Khan S., Faisal S., Jan H., Usman H., Zainab R., Taj F., et al. COVID-19: A brief overview on the role of vitamins specifically Vitamin C as immune modulators and in prevention and treatment of SARS-Cov-2 infections’. Biomed. J. Sci. Tech. Res. 2020;28:21580–21586. [Google Scholar]
- 10.Zhang N., Wang L., Deng X., Liang R., Su M., He C., et al. Recent advances in the detection of respiratory virus infection in humans. J. Med Virol. 2020;92:408–417. doi: 10.1002/jmv.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasmalbari E., Elobeid A. and Abbadi O. The use of Traditional Medicines, Vitamins, and minerals against COVID-19; a Review. IJRRLS 2020;7:15–24.
- 12.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsaadi E.A., Jones I.M. Membrane binding proteins of coronaviruses. Future Virol. 2019;14:275–286. doi: 10.2217/fvl-2018-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen J., Normile D. New SARS-like virus in China triggers alarm. AAAS. 2020;367:234–235. doi: 10.1126/science.367.6475.234. [DOI] [PubMed] [Google Scholar]
- 15.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:1–22. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain A., Kaler J., Tabrez E., Tabrez S., Tabrez S.S. Novel COVID-19: A comprehensive review of transmission, manifestation, and pathogenesis. Cureus. 2020;12:8184. doi: 10.7759/cureus.8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch. Pathol. Lab. 2020;144:799–805. doi: 10.5858/arpa.2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- 19.Shakoor H., Feehan J., Al Dhaheri A.S., Ali H.I., Platat C., Ismail L.C., et al. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas. 2020;143:1–9. doi: 10.1016/j.maturitas.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhok A., Butola L.K., Anjankar A., Shinde A.D.R., Kute P.K., Jha R.K. Role of vitamins and minerals in improving immunity during COVID-19 pandemic-a review. J. Evol. Med Dent. Sci. 2020;9:2296–2301. doi: 10.14260/jemds/2020/497. [DOI] [Google Scholar]
- 21.Gombart A.F., Pierre A., Maggini S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients. 2020;12:236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gleeson M., Nieman D.C., Pedersen B.K. Exercise, nutrition and immune function. J. Sports Sci. 2004;22:115–125. doi: 10.1080/0264041031000140590. [DOI] [PubMed] [Google Scholar]
- 23.Calder P.C., Field C.J., Gill H.S. CABI; 2002. Nutrition and Immune Function. [DOI] [Google Scholar]
- 24.Nieman D.C., Mitmesser S.H. Potential impact of nutrition on immune system recovery from heavy exertion: A metabolomics perspective. Nutrients. 2017;9:513. doi: 10.3390/nu9050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dehghani-Samani A., Kamali M., Hoseinzadeh-Chahkandak F. The role of vitamins on the prevention and/or treatment of COVID-19 infection; a systematic review. Mod. Care J. 2020;17 doi: 10.5812/modernc.104740. [DOI] [Google Scholar]
- 26.Murru A., Manchia M., Hajek T., Nielsen R.E., Rybakowski J.K., Sani G., et al. Lithium’s antiviral effects: a potential drug for COVID-19 disease? Int. J. Bipolar Disord. 2020:1–9. doi: 10.1186/s40345-020-00191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace T.C. Combating COVID-19 and building immune resilience: a potential role for magnesium nutrition? J. Am. Coll. Nutr. 2020;39:685–693. doi: 10.1080/07315724.2020.1785971. [DOI] [PubMed] [Google Scholar]
- 28.Bae M., Kim H. Mini-Review on the Roles of Vitamin C, Vitamin D, and selenium in the immune system against COVID-19. Molecules. 2020;25:5346. doi: 10.3390/molecules25225346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter J., Arentz S., Goldenberg J., Yang G., Beardsley J., Mertz D., et al. Rapid review protocol: zinc for the prevention or treatment of COVID-19 and other coronavirus-related respiratory tract infections. IMR. 2020;9 doi: 10.1016/j.imr.2020.100457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allenby G., Bocquel M.T., Saunders M., Kazmer S., Speck J., Rosenberger M., et al. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc. Natl. Acad. Sci. USA. 1993;90:30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarohan A.R. COVID-19: endogenous retinoic acid theory and retinoic acid depletion syndrome. Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross A.C., Stephensen C.B. Vitamin A and retinoids in antiviral responses. FASEB J. 1996;10:979–985. doi: 10.1096/fasebj.10.9.8801180. [DOI] [PubMed] [Google Scholar]
- 33.Lippman S.M., Glisson B.S., Kavanagh J.J., Lotan R., Hong W.K., Paredes-Espinoza M., et al. Retinoic acid and interferon combination studies in human cancer. Eur. J. Cancer. 1993;29:9–13. doi: 10.1016/0959-8049(93)90618-P. [DOI] [PubMed] [Google Scholar]
- 34.Lindner D.J., Borden E.C., Kalvakolanu D.V. Synergistic antitumor effects of a combination of interferons and retinoic acid on human tumor cells in vitro and in vivo. Clin. Cancer Res. 1997;3:931–937. doi: 10.1089/jir.1997.17.681. [DOI] [PubMed] [Google Scholar]
- 35.Qu Z.X., Dayal A., Jensen M.A., Arnason B.G. All-trans retinoic acid potentiates the ability of interferon beta-1b to augment suppressor cell function in multiple sclerosis. Arch. Neurol. 1998;55:315–321. doi: 10.1001/archneur.55.3.315. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kell A.M., Gale M., Jr. RIG-I in RNA virus recognition. Virology. 2015;479:110–121. doi: 10.1016/j.virol.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batah S.S., Fabro A.T. Pulmonary pathology of ARDS in COVID-19: a pathological review for clinicians. Respir. Med. 2020;176 doi: 10.1016/j.rmed.2020.106239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colavita F., Lapa D., Carletti F., Lalle E., Bordi L., Marsella P., et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann. Intern. Med. 2020;173:242–243. doi: 10.7326/M20-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiser P.D., Golczak M., Palczewski K. Chemistry of the retinoid (visual) cycle. Chem. Rev. 2014;114:194–232. doi: 10.1021/cr400107q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 43.Lane M.A., Bailey S.J. Role of retinoid signalling in the adult brain. Prog. Neurobiol. 2005;75:275–293. doi: 10.1016/j.pneurobio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Morris D.R., Levenson C.W. Zinc regulation of transcriptional activity during retinoic acid-induced neuronal differentiation. J. Nutr. Biochem. 2013;24:1940–1944. doi: 10.1016/j.jnutbio.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danielson P. á. The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans. Curr. Drug Metab. 2002;3:561–597. doi: 10.2174/1389200023337054. [DOI] [PubMed] [Google Scholar]
- 46.Vahlquist A., Blockhuys S., Steijlen P., Van Rossem K., Didona B., Blanco D., et al. Oral liarozole in the treatment of patients with moderate/severe lamellar ichthyosis: results of a randomized, double‐blind, multinational, placebo‐controlled phase II/III trial. Br. J. Dermatol. 2014;170:173–181. doi: 10.1111/bjd.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shakoor H., Feehan J., Mikkelsen K., Al Dhaheri A.S., Ali H.I., Platat C., et al. Be well: A potential role for vitamin B in COVID-19. Maturitas. 2021;144:108–111. doi: 10.1016/j.maturitas.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikkelsen K., Apostolopoulos V. Nutrition and Immunity. Springer,; 2019. Vitamin B1, B2, B3, B5, and B6 and the Immune System; pp. 115–125. [DOI] [Google Scholar]
- 49.Keil S.D., Ragan I., Yonemura S., Hartson L., Dart N.K., Bowen R. Inactivation of severe acute respiratory syndrome coronavirus 2 in plasma and platelet products using a riboflavin and ultraviolet light‐based photochemical treatment. Vox Sang. 2020;115:495–501. doi: 10.1111/vox.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ragan I., Hartson L., Pidcoke H., Bowen R., Goodrich R. Pathogen reduction of SARS-CoV-2 virus in plasma and whole blood using riboflavin and UV light. Plos One. 2020;15 doi: 10.1371/journal.pone.0233947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boergeling Y., Ludwig S. Targeting a metabolic pathway to fight the flu. FEBS J. 2017;284:218–221. doi: 10.1111/febs.13997. [DOI] [PubMed] [Google Scholar]
- 52.Mikkelsen K., Stojanovska L., Prakash M., Apostolopoulos V. The effects of vitamin B on the immune/cytokine network and their involvement in depression. Maturitas. 2017:58–71. doi: 10.1016/j.maturitas.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 53.Nagai A., Matsumiya H., Hayashi M., Yasui S., Okamoto H., Konno K. Effects of nicotinamide and niacin on bleomycin-induced acute injury and subsequent fibrosis in hamster lungs. Exp. Lung Res. 1994;20:263–281. doi: 10.3109/01902149409064387. [DOI] [PubMed] [Google Scholar]
- 54.Mehmel M., Jovanović N., Spitz U. Nicotinamide riboside-the current state of research and therapeutic uses. Nutrients. 2020;12:1616. doi: 10.3390/nu12061616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Desbarats J. Pyridoxal 5'-phosphate to mitigate immune dysregulation and coagulopathy in COVID-19. Med Pharma. 2020 doi: 10.20944/preprints202005.0144.v1. [DOI] [Google Scholar]
- 57.Mikkelsen K., Prakash M.D., Kuol N., Nurgali K., Stojanovska L., Apostolopoulos V. Anti-tumor effects of vitamin B2, B6 and B9 in promonocytic lymphoma cells. Int J. Mol. Sci. 2019;20:3763. doi: 10.3390/ijms20153763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheybani Z., Dokoohaki M.H., Negahdaripour M., Dehdashti M., Zolghadr H., Moghadami M., et al. The role of folic acid in the management of respiratory disease caused by COVID-19. 2020. https://doi.org/10.26434/chemrxiv.12034980.v1.
- 59.Nemazannikova N., Mikkelsen K., Stojanovska L., Blatch G.L., Apostolopoulos V. Is there a link between vitamin B and multiple sclerosis? Med Chem. 2018;14:170–180. doi: 10.2174/1573406413666170906123857. [DOI] [PubMed] [Google Scholar]
- 60.Sabry W., Elemary M., Burnouf T., Seghatchian J., Goubran H. Vitamin B12 deficiency and metabolism-mediated thrombotic microangiopathy (MM-TMA) Transfus. Apher. Sci. 2020;59 doi: 10.1016/j.transci.2019.102717. [DOI] [PubMed] [Google Scholar]
- 61.Wolffenbuttel B.H., Wouters H.J., Heiner-Fokkema M.R. and van der Klauw M.M. The many faces of cobalamin (vitamin B12) deficiency. Mayo clinic proceedings: Mayo Clin Proc Innov Qual Outcomes 2019; 3: 200–14. https://doi.org/ 10.1016/j.mayocpiqo.2019.03.002. [DOI] [PMC free article] [PubMed]
- 62.Allen L.H. Efficacy and Safety of Vitamin B12 Fortification. in: Venkatesh Mannar M G, Hurrell R. Food Fortification in a Globalized World. Obes &Metab Res; 2018: 255–61. https://doi.org/10.1016/ B978–0-12–802861-2.00026–2.
- 63.Stipp M.M. SARS-CoV-2: micronutrient optimization in supporting host immunocompetence. Int J. Clin. Case Rep. Rev. 2020;2:1–10. doi: 10.31579/2690-4861/024. [DOI] [Google Scholar]
- 64.Grangé S., Bekri S., Artaud-Macari E., Francois A., Girault C., Poitou A.L., et al. Adult-onset renal thrombotic microangiopathy and pulmonary arterial hypertension in cobalamin C deficiency. Lancet. 2015;286:1011–1012. doi: 10.1016/S0140-6736(15)00076-8. [DOI] [PubMed] [Google Scholar]
- 65.Tan C.W., Ho L.P., Kalimuddin S., Cherng B.P.Z., Teh Y.E., Thien S.Y., et al. Cohort study to evaluate effect of vitamin D, magnesium, and vitamin b12 in combination on severe outcome progression in older patients with coronavirus (COVID-19) Nutrition. 2020;79–80 doi: 10.1016/j.nut.2020.111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossetti C.A., Real J.P., Palma S.D. High dose of ascorbic acid used in sars COVID-19 treatment: scientific and clinical support for its therapeutic implementation. Ars Pharm. 2020;61:145–148. doi: 10.30827/ars.v61i2.15164. [DOI] [Google Scholar]
- 67.Tóth S.Z., Lőrincz T., Szarka A. Concentration does matter: the beneficial and potentially harmful effects of ascorbate in humans and plants. Antioxid. Redox Signal. 2018;29:1516–1533. doi: 10.1089/ars.2017.7125. [DOI] [PubMed] [Google Scholar]
- 68.Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids, Washington (DC): National Academies Press (US); 2000. https://doi.org/ 10.17226/9810.
- 69.Nemet I., Monnier V.M. Monnier. Vitamin C degradation products and pathways in the human lens. J. Biol. Chem. 2011;286:37128–37136. doi: 10.1074/jbc.M111.245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hemilä H., Chalker E. Vitamin C as a possible therapy for COVID-19. Infect. Chemother. 2020;52:222–223. doi: 10.3947/ic.2020.52.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Waqas Khan H.M., Parikh N., Megala S.M., Predeteanu G.S. Unusual early recovery of a critical COVID-19 patient after administration of intravenous vitamin C. Am. J. Case Rep. 2020;21 doi: 10.12659/AJCR.925521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bafadam S., Beheshti F., Khodabakhshi T., Asghari A., Ebrahimi B., Sadeghnia H.R., et al. Trigonella foenum-graceum seed (Fenugreek) hydroalcoholic extract improved the oxidative stress status in a rat model of diabetes-induced memory impairment. Horm. Mol. Biol. Clin. Invest. 2019:39. doi: 10.1515/hmbci-2018-0074. [DOI] [PubMed] [Google Scholar]
- 73.Ebrahimi B., Forouzanfar F., Azizi H., Khoshdel-Sarkarizi H., Sadeghnia H., et al. Effect of electroacupuncture and glibenclamide on blood glucose level and oxidative stress parameters in streptozotocin-induced diabetic rats and possible human implications. Acupunct. Electro-Ther. Res. 2020;44:213–228. doi: 10.3727/036012920X15779969212955. [DOI] [Google Scholar]
- 74.Colunga Biancatelli R.M., Berrill M., Catravas J.D., Marik P.E. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19) Front Immunol. 2020;11:1451. doi: 10.3389/fimmu.2020.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horowitz R.I., Freeman P. Three novel prevention, diagnostic, and treatment options for COVID-19 urgently necessitating controlled randomized trials. Med Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.109851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wintergerst E.S., Maggini S., Hornig D.H. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann. Nutr. Metab. 2006;50:85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- 77.Hossain M.S., Koshio S., Ishikawa M., Yokoyama S., Sony N.M., Dossou S., et al. Influence of dietary inosine and vitamin C supplementation on growth, blood chemistry, oxidative stress, innate and adaptive immune responses of red sea bream, Pagrus major juvenile. Fish. Shellfish Immunol. 2018;82:92–100. doi: 10.1016/j.fsi.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 78.Ye Q., Wang B., Mao J. Cytokine storm in COVID-19 and treatment. J. Infect. 2020:80–607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calder P.C., Carr A.C., Gombart A.F., Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12:1181. doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9:1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holford P., Carr A.C., Jovic T.H., Ali S.R., Whitaker I.S., Marik P.E., et al. Vitamin C-an adjunctive therapy for respiratory infection, sepsis and COVID-19. Nutrients. 2020;12:3760. doi: 10.3390/nu12123760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lingappan K., Karmouty-Quintana H., Davies J., Akkanti B., Harting M.T. Harting. Understanding the age divide in COVID-19: why are children overwhelmingly spared? Am. J. Physiol. Lung Cell Mol. Physiol. 2020;319:39–44. doi: 10.1152/ajplung.00183.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ye F., Li X., Li L., Yuan J., Chen J. t-BHQ provides protection against lead neurotoxicity via Nrf2/HO-1 pathway. Oxid. Med Cell Longev. 2016;2016 doi: 10.1155/2016/2075915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bohndiek S.E., Kettunen M.I., Hu D.E., Kennedy B.W., Boren J.J., Gallagher F.A., et al. Hyperpolarized [1–13 C]-ascorbic and dehydroascorbic acid: vitamin C as a probe for imaging redox status in vivo. J. Am. Chem. Soc. 2011;133:11795–11801. doi: 10.1021/ja2045925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsukaguchi H., Tokui T., Mackenzie B., Berger U.V., Chen X.Z., Wang Y., et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 86.Siegel B.V., Morton J.I. Vitamin C and the immune response. Experientia. 1977;33:393–395. doi: 10.1007/BF02002847. [DOI] [PubMed] [Google Scholar]
- 87.Hoang B.X., Shaw D.G., Fang W., Han B. A Possible application of high dose vitamin C in the prevention and therapy for Coronavirus Infections. J. Glob. Antimicrob. Resist. 2020;23:256–262. doi: 10.1016/j.jgar.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abobaker A., Alzwi A., Alraied A.H. Overview of the possible role of vitamin C in management of COVID-19. Pharm. Rep. 2020;72:1517–1528. doi: 10.1007/s43440-020-00176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang L., Wang Y., Ye D., Liu Q. Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105948. https://doi.org/10.1016/j.ijantimicag.2020. 105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.White L.A., Freeman C.Y., Forrester B.D., Chappell W.A. In vitro effect of ascorbic acid on infectivity of herpesviruses and paramyxoviruses. J. Clin. Microbiol. 1986;24:527–531. doi: 10.1128/JCM.24.4.527-531.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gonzalez M.J., Miranda–Massari J.R., Rodriguez J.R. Antiviral mechanisms of vitamin C: a short communication consensus report. J. Orthomol. Med. 2020;35:1–19. [Google Scholar]
- 92.Furuya A., Uozaki M., Yamasaki H., Arakawa T., Arita M., Koyama A.H. Antiviral effects of ascorbic and dehydroascorbic acids in vitro. Int J. Mol. Med. 2008;22:541–545. doi: 10.3892/ijmm_00000053. [DOI] [PubMed] [Google Scholar]
- 93.Cheng L.L., Liu Y.Y., Li B., Li SYand Ran P.X. An in vitro study on the pharmacological ascorbate treatment of influenza virus. Zhonghua Jie He He Hu Xi Za Zhi. 2012;35:520–523. doi: 10.12659/MSM.890423. [DOI] [PubMed] [Google Scholar]
- 94.Colunga Biancatelli R.M., Berrill M., Marik P.E. The antiviral properties of vitamin C. Expert Rev. Anti-Infect. Ther. 2020;18:99–101. doi: 10.1080/14787210.2020.1706483. [DOI] [PubMed] [Google Scholar]
- 95.Frei B., Lawson S. Vitamin C and cancer revisited. Proc. Natl. Acad. Sci. 2008;105:11037–11038. doi: 10.1073/pnas.0806433105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sorice A., Guerriero E., Capone F., Colonna G., Castello G., Costantini S. Ascorbic acid: its role in immune system and chronic inflammation diseases. Mini Rev. Med Chem. 2014;14:444–452. doi: 10.2174/1389557514666140428112602. [DOI] [PubMed] [Google Scholar]
- 97.Marik P.E. Vitamin C for the treatment of sepsis: the scientific rationale. Pharm. Ther. 2018;189:63–70. doi: 10.1016/j.pharmthera.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 98.Chen Y., Luo G., Yuan J., Wang Y., Yang X., Wang X., et al. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediat. Inflamm. 2014;2014 doi: 10.1155/2014/426740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cai Y., Li Y.F., Tang L.P., Tsoi B., Chen M., Chen H., et al. A new mechanism of vitamin C effects on A/FM/1/47 (H1N1) virus-induced pneumonia in restraint-stressed mice. Biomed. Res Int. 2015;2015 doi: 10.1155/2015/675149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Atherton J.G., Kratzing C.C., Fisher A. The effect of ascorbic acid on infection of chick-embryo ciliated tracheal organ cultures by coronavirus. Arch. Virol. 1978;56:195–199. doi: 10.1007/BF01317848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Siddiqui M., Manansala J.S., Abdulrahman H.A., Nasrallah G.K., Smatti M.K., Younes N., et al. Immune modulatory effects of vitamin D on viral infections. Nutrients. 2020;12:2879. doi: 10.3390/nu12092879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martens P.J., Gysemans C., Verstuyf A., Mathieu C. Vitamin D’s effect on immune function. Nutrients. 2020;12:1248. doi: 10.3390/nu12051248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hewison M. Vitamin D and the intracrinology of innate immunity. Mol. Cell Endocrinol. 2010;321:103–111. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quarles L.D. Endocrine functions of bone in mineral metabolism regulation. J. Clin. Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu P.T., Stenger S., Li H., Wenzel L., Tan B.H., Krutzik S.R., et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 106.Yuk J.M., Shin D.M., Lee H.M., Yang C.S., Jin H.S., Kim K.K., et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 107.Gombart A.F., Borregaard N., Koeffler H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up‐regulated in myeloid cells by 1, 25–dihydroxyvitamin D3. FASEB J. 2005;19:1067–D77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 108.Wang T.T., Nestel F.P., Bourdeau V., Nagai Y., Wang Q., Liao J., et al. Cutting edge: 1, 25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. Cell Host Microbe. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 109.Kim J., Yang Y.L., Jang S.H., Jang Y.S. Human β-defensin 2 plays a regulatory role in innate antiviral immunity and is capable of potentiating the induction of antigen-specific immunity. Virol. J. 2018;15:1–12. doi: 10.1186/s12985-018-1035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bartley J. Vitamin D: emerging roles in infection and immunity. Expert Rev. Anti Infect. Ther. 2010;8:1359–1369. doi: 10.1586/eri.10.102. [DOI] [PubMed] [Google Scholar]
- 111.Ginde A.A., Mansbach J.M., Camargo C.A. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the third national health and nutrition examination survey. Arch. Intern Med. 2009;169:384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schwalfenberg G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol. Nutr. Food Res. 2011;55:96–108. doi: 10.1002/mnfr.201000174. [DOI] [PubMed] [Google Scholar]
- 113.Wang T.T., Dabbas B., Laperriere D., Bitton A.J., Soualhine H., Tavera-Mendoza L.E., et al. Direct and indirect induction by 1, 25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin β2 innate immune pathway defective in Crohn disease. J. Biol. Chem. 2010;285:2227–2231. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen S., Sims G.P., Chen X.X., Gu Y.Y., Chen S., Lipsky P.E. Modulatory effects of 1, 25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 115.Müller K., Haahr P.M., Diamant M., Rieneck K., Kharazmi A., Bendtzen K. 1, 25-Dihydroxyvitamin D3 inhibits cytokine production by human blood monocytes at the post-transcriptional level. Cytokine. 1992;4:506–512. doi: 10.1016/1043-4666(92)90012-G. [DOI] [PubMed] [Google Scholar]
- 116.Heine G., Anton K., Henz B.M., Worm M. 1α, 25–dihydroxyvitamin D3 inhibits anti‐CD40 plus IL‐4–mediated IgE production in vitro. Eur. J. Immunol. 2002;32:3395–3404. doi: 10.1002/1521-4141(200212)32:12<3395::AID-IMMU3395>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 117.Vasiliou J.E., Lui S., Walker S.A., Chohan V., Xystrakis E., Bush A., et al. Vitamin D deficiency induces T h2 skewing and eosinophilia in neonatal allergic airways disease. Allergy. 2014;69:1380–1389. doi: 10.1111/all.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Unger W.W., Laban S., Kleijwegt F.S., van der Slik A.R., Roep B.O. Induction of Treg by monocyte‐derived DC modulated by vitamin D3 or dexamethasone: differential role for PD‐L1. Eur. J. Immunol. 2009;39:3147–3159. doi: 10.1002/eji.200839103. [DOI] [PubMed] [Google Scholar]
- 119.Jeffery L.E., Burke F., Mura M., Zheng Y., Qureshi O.S., Hewison M., et al. 1, 25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Joshi S., Pantalena L.C., Liu X.K., Gaffen S.L., Liu H., Rohowsky-Kochan C., et al. 1, 25-Dihydroxyvitamin D3 ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol. Cell Biol. 2011;31:3653–3669. doi: 10.1128/MCB.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Griffin M.D., Lutz W., Phan V.A., Bachman L.A., McKean D.J., Kumar R. Dendritic cell modulation by 1α, 25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc. Natl. Acad. Sci. 2001;98:6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Penna G., Adorini L. 1α, 25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 123.Iwasaki A., Medzhitov R. Regulation of adaptive immunity by the innate immune system. science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hansdottir S., Monick M.M., Hinde S.L., Lovan N., Look D.C., Hunninghake G.W. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J. Immunol. 2008;181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aibana O., Huang C.C., Aboud S., Arnedo-Pena A., Becerra M.C., Bellido-Blasco J.B., et al. Vitamin D status and risk of incident tuberculosis disease: A nested case-control study, systematic review, and individual-participant data meta-analysis. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002907. https://doi.org/10.1371/journal. pmed.1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Teymoori‐Rad M., Shokri F., Salimi V., Marashi S.M. The interplay between vitamin D and viral infections. Rev. Med Virol. 2019;29:2032. doi: 10.1002/rmv.2032. [DOI] [PubMed] [Google Scholar]
- 127.Chun R.F., Lauridsen A.L., Suon L., Zella L.A., Pike J.W., Modlin R.L., et al. Vitamin D-binding protein directs monocyte responses to 25-hydroxy-and 1, 25-dihydroxyvitamin D. J. Clin. Endocrinol. Metab. 2010;95:3368–3376. doi: 10.1210/jc.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Inamo Y., Hasegawa M., Saito K., Hayashi R., Ishikawa T., Yoshino Y., et al. Serum vitamin D concentrations and associated severity of acute lower respiratory tract infections in Japanese hospitalized children. Pedia Int. 2011;53:199–201. doi: 10.1111/j.1442-200X.2010.03224.x. [DOI] [PubMed] [Google Scholar]
- 129.Belderbos M.E., Houben M.L., Wilbrink B., Lentjes E., Bloemen E.M., Kimpen J.L., et al. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127:1513–1520. doi: 10.1542/peds.2010-3054. [DOI] [PubMed] [Google Scholar]
- 130.Charan J., Goyal J.P., Saxena D., Yadav P. Vitamin D for prevention of respiratory tract infections: a systematic review and meta-analysis. J. Pharm. Pharm. 2012;4:300. doi: 10.4103/0976-500X.103685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. bmj. 2017;356:6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Speakman L.L., Michienzi S.M., Badowski M.E. Vitamins, supplements and COVID-19: a review of currently available evidence. Drugs Context. 2021;10:2021–6-2. doi: 10.7573/dic.2021-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McCartney D.M., Byrne D.G. Optimisation of vitamin D status for enhanced immuno-protection against COVID-19. Ir. Med J. 2020;113:58. [PubMed] [Google Scholar]
- 134.Vankadari N., Wilce J.A. Emerging COVID-19 coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microbes Infect. 2020;9:601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Meehan M., Penckofer S. The role of vitamin D in the aging adult. J. Aging Gerontol. 2014;2:60–71. doi: 10.12974/2309-6128.2014.02.02.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peterlik M., Cross H.S. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur. J. Clin. Invest. 2005;35:290–304. doi: 10.1111/j.1365-2362.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 137.Yancy C.W. COVID-19 and african americans. Jama. 2020;323:1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 138.Alzaman N.S., Dawson-Hughes B., Nelson J., D’Alessio D., Pittas A.G. Vitamin D status of black and white Americans and changes in vitamin D metabolites after varied doses of vitamin D supplementation. Am. J. Clin. Nutr. 2016;104:205–214. doi: 10.3945/ajcn.115.129478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)- China, 2020[J]. C CDC Weekly 2020; 2: 113–22. https://doi.org/10.3760/cma.j. issn. 0254–6450.2020.02.003. [PMC free article] [PubMed]
- 140.Autier P., Mullie P., Macacu A., Dragomir M., Boniol M., Coppens K., et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986–1004. doi: 10.1016/S2213-8587(17)30357-1. [DOI] [PubMed] [Google Scholar]
- 141.Aranow C. Vitamin D and the immune system. J. Invest Med. 2011;59:881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Grant W.B., Giovannucci E. The possible roles of solar ultraviolet-B radiation and vitamin D in reducing case-fatality rates from the 1918–1919 influenza pandemic in the United States. Derm. Endocrinol. 2009;1:215–219. doi: 10.4161/derm.1.4.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhao S., Cao P., Chong M.K., Gao D., Lou Y., Ran J., et al. COVID-19 and gender-specific difference: Analysis of public surveillance data in Hong Kong and Shenzhen, China, from January 10 to February 15, 2020. Infect. Control Hosp. Epidemiol. 2020;41:750–751. doi: 10.1017/ice.2020.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Suleiman L., Négrier C., Boukerche H. Protein S: A multifunctional anticoagulant vitamin K-dependent protein at the crossroads of coagulation, inflammation, angiogenesis, and cancer. Crit. Rev. Oncol. Hematol. 2013;88:637–654. doi: 10.1016/j.critrevonc.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 146.Shioi A., Morioka T., Shoji T., Emoto M. The inhibitory roles of vitamin K in progression of vascular calcification. Nutrients. 2020;12:583. doi: 10.3390/nu12020583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Anastasi E., Ialongo C., Labriola R., Ferraguti G., Lucarelli M., Angeloni A. Vitamin K deficiency and COVID-19. Scand. J. Clin. Lab Invest. 2020;80:525–527. doi: 10.1080/00365513.2020.1805122. [DOI] [PubMed] [Google Scholar]
- 148.Janssen R., Visser M.P., Dofferhoff A.S., Vermeer C., Janssens, W, Walk J. Vitamin K metabolism as the potential missing link between lung damage and thromboembolism in Coronavirus disease 2019. Br. J. Nut. 2021;126:191–198. doi: 10.1017/S0007114520003979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ames B.N. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc. Natl. Acad. Sci. 2006;103:17589–17594. doi: 10.1073/pnas.0608757103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shea M., Booth S.L. Concepts and controversies in evaluating vitamin K status in population-based studies. Nutrients. 2016;8:8. doi: 10.3390/nu8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Traber M.G., Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Traber M.G. Vitamin E regulatory mechanisms. Annu. Rev. Nutr. 2007;27:347–362. doi: 10.1146/annurev.nutr.27.061406.093819. [DOI] [PubMed] [Google Scholar]
- 153.Jovic T.H., Ali S.R., Ibrahim N., Jessop Z.M., Tarassoli S.P., Dobbs T.D., et al. Could Vitamins Help in the Fight Against COVID-19? Nutrients. 2020;12(9):2550. doi: 10.3390/nu12092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bieri J.G., Corash L., Van Hubbard S. Medical Uses of Vitamin. E. N. Engl. J. Med. 1983;308:1063–1071. doi: 10.1056/NEJM198305053081805. [DOI] [PubMed] [Google Scholar]
- 155.Jayawardena R., Sooriyaarachchi P., Chourdakis M., Jeewandara C., Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab. Syndr. Clin. Res Rev. 2020 doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Verhagen H., Buijsse B., Jansen E., Bueno-De-Mesquita B. The state of antioxidant affairs. Nutr. Today. 2006;4:244–252. [Google Scholar]
- 157.Tappel A.L., Dillard C.J. In vivo lipid peroxidation: Measurement via exhaled pentane and protection by vitamin E. Fed. Proc. 1981;40:174–178. [PubMed] [Google Scholar]
- 158.Hafeman D.G., Hoekstra W.G. Lipid Peroxidation in Vivo during Vitamin E and Selenium Deficiency in the Rat as Monitored by Ethane Evolution. J. Nutr. 1977;107:666–672. doi: 10.1093/jn/107.4.666. [DOI] [PubMed] [Google Scholar]
- 159.Richard C., Lemonnier F., Thibault M., Couturier M., Auzepy P. Vitamin E deficiency and lipoperoxidation during adult respiratory distress syndrome. Crit. Care Med. 1990;18:4–9. doi: 10.1097/00003246-199001000-00002. [DOI] [PubMed] [Google Scholar]
- 160.Hemila H. Vitamin E administration may decrease the incidence of pneumonia in elderly males. Clin. Inter. Aging. 2016;11:1379–1385. doi: 10.2147/CIA.S114515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Meydani S.N., Lewis E.D., Wu D. Perspective: Should vitamin E recommendations for older adults be increased? Adv. Nutr. 2018;9(5):533–543. doi: 10.1093/advances/nmy035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee G.Y., Han S.N. The role of vitamin E in immunity. Nutrients. 2018;10:1614. doi: 10.3390/nu10111614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Meydani S.N., Leka L.S., Fine B.C., Dallal G.E., Keusch G.T., Singh M.F., Hamer D.H. Vitamin E and respiratory tract infections in elderly nursing home residents: A randomized Controlled trial. J. Am. Med. Assoc. 2004;292:828–836. doi: 10.1001/jama.292.7.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Meydani S.N. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. JAMA. 1997;277:1380–1386. doi: 10.1001/jama.1997.03540410058031. [DOI] [PubMed] [Google Scholar]
- 165.Wu D., Meydani S. Age-associated changes in immune function: Impact of vitamin E intervention and the underlying mechanisms. Endocr. Metab. Immune Disord. Targets. 2014;14:283–289. doi: 10.2174/1871530314666140922143950. [DOI] [PubMed] [Google Scholar]
- 166.De la Fuente M., Hernanz A., Guayerbas N., Victor V.M., Arnalich F. Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic. Res. 2008;42:272–280. doi: 10.1080/10715760801898838. [DOI] [PubMed] [Google Scholar]
- 167.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Calder P.C., Carr A.C., Gombart A.F., Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12:1181. doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Beck M.A., Handy J., Levander O.A. Host nutritional status: The neglected virulence factor. Trends Microbiol. 2004;12:417–423. doi: 10.1016/j.tim.2004.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shakoor H., Feehan J., Al Dhaheri A.S., Ali H.I., Platat C., Ismail L.C., Apostolopoulos V., Stojanovska L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: could they help against COVID-19? Maturitas. 2021;143:1–9. doi: 10.1016/j.maturitas.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Grass G., Rensing C., Solioz M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011;77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fujimori Y., Sato T., Hayata T., Nagao T., Nakayama M., Nakayama T., et al. Novel antiviral characteristics of nanosized copper (I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl. Environ. Microbiol. 2012;78:951–955. doi: 10.1128/AEM.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen T.D., Rotival M., Chiu L.Y., Bagnati M., Ko J.H., Srivastava P.K., et al. Identification of ceruloplasmin as a gene that affects susceptibility to glomerulonephritis through macrophage function. Genetics. 2017;206:1139–1151. doi: 10.1534/genetics.116.197376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Andreou A., Trantza S., Filippou D., Sipsas N., Tsiodras S. COVID-19: The potential role of copper and N-acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS-CoV-2. vivo. 2020;34:1567–1588. doi: 10.21873/invivo.11946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Horie M., Ogawa H., Yoshida Y., Yamada K., Hara A., Ozawa K., et al. Inactivation and morphological changes of avian influenza virus by copper ions. Arch. Virol. 2008;153:1467–1472. doi: 10.1007/s00705-008-0154-2. [DOI] [PubMed] [Google Scholar]
- 177.Powanda M. Trace elements in the pathogenesis and treatment of inflammation. Agents Actions Suppl. 1981;8:1–617. doi: 10.1385/BTER:93:1-3:95. [DOI] [PubMed] [Google Scholar]
- 178.Neve J., Fontaine J., Peretz A., Famaey J.P. Changes in zinc, copper and selenium status during adjuvant-induced arthritis in rats. Agents Actions. 1988;25:146–155. doi: 10.1007/BF01969106. [DOI] [PubMed] [Google Scholar]
- 179.Di Bella L.M., Alampi R., Biundo F., Toscano G., Felice M.R. Copper chelation and interleukin-6 proinflammatory cytokine effects on expression of different proteins involved in iron metabolism in HepG2 cell line. BMC Biochem. 2017;18:1–11. doi: 10.1186/s12858-017-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dignass A., Farrag K., Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J. Chronic Dis. 2018;2018 doi: 10.1155/2018/9394060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cherukuri S., Potla R., Sarkar J., Nurko S., Harris Z.L., Fox P.L. Unexpected role of ceruloplasmin in intestinal iron absorption. Cell Metab. 2005;2:309–319. doi: 10.1016/j.cmet.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 182.Liao J., Yang F., Chen H., Yu W., Han Q., Li Y., et al. Effects of copper on oxidative stress and autophagy in hypothalamus of broilers. Ecotoxicol. Environ. Saf. 2019;185 doi: 10.1016/j.ecoenv.2019.109710. [DOI] [PubMed] [Google Scholar]
- 183.Zischka H., Kroemer G. Copper–a novel stimulator of autophagy. Cell Stress. 2020;4:92–94. doi: 10.15698/cst2020.05.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Borkow G., Covington C.Y., Gautam B., Anzala O., Oyugi J., Juma M., et al. Prevention of human immunodeficiency virus breastmilk transmission with copper oxide: proof-of-concept study. Breast Med. 2011;6:165–170. doi: 10.1089/bfm.2010.0090. [DOI] [PubMed] [Google Scholar]
- 185.Borkow G., Zhou S.S., Page T., Gabbay J. A novel anti-influenza copper oxide containing respiratory face mask. PloS One. 2010;5:11295. doi: 10.1371/journal.pone.0011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cortes A.A., Zuñiga J.M. The use of copper to help prevent transmission of SARS-coronavirus and influenza viruses. A general review. Diagn. Microbiol Infect. Dis. 2020;98 doi: 10.1016/j.diagmicrobio.2020.115176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kieliszek M. Selenium–fascinating microelement, properties and sources in food. Molecules. 2019;24:1298. doi: 10.3390/molecules24071298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fairweather-Tait S.J., Bao Y., Broadley M.R., Collings R., Ford D., Ford D., et al. Selenium in human health and disease. Antioxid. Redox Signal. 2011;14:1337–1383. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- 190.Kieliszek M., Lipinski B. Selenium supplementation in the prevention of coronavirus infections (COVID-19) Med Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Guillin O.M., Vindry C., Ohlmann T., Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;11:2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hoffmann P.R., Berry M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008;52:1273–1280. doi: 10.1002/mnfr.200700330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Khatiwada S., Subedi A. A mechanistic link between selenium and coronavirus disease 2019 (COVID-19) Curr. Nutr. Rep. 2021;10:125–136. doi: 10.1007/s13668-021-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li Y., Lin Z., Gong G., Guo M., Xu T., Wang C., et al. Inhibition of H1N1 influenza virus-induced apoptosis by selenium nanoparticles functionalized with arbidol through ROS-mediated signaling pathways. J. Mater. Chem. B. 2019;7:4252–4262. doi: 10.1039/C9TB00531E. [DOI] [Google Scholar]
- 195.Fang L.Q., Goeijenbier M., Zuo S.Q., Wang L.P., Liang S., Klein S.L., et al. The association between hantavirus infection and selenium deficiency in mainland China. Viruses. 2015;7:333–351. doi: 10.3390/v7010333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Steinbrenner H., Al-Quraishy S., Dkhil M.A., Wunderlich F., Sies H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv. Nutr. 2015;6:73–82. doi: 10.3945/an.114.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Beck M.A., Nelson H.K., Shi Q., Van Dael P., Schiffrin E.J., Blum S., et al. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J. 2001;15:1481–1483. doi: 10.1096/fj.00-0721fje. [DOI] [PubMed] [Google Scholar]
- 198.Beck M.A., Kolbeck P.C., Rohr L.H., Shi Q., Morris V.C., Levander O.A. Benign human enterovirus becomes virulent in selenium‐deficient mice. J. Med Virol. 1994;43:166–170. doi: 10.1002/jmv.1890430213. [DOI] [PubMed] [Google Scholar]
- 199.Bermano G., Méplan C., Mercer D.K., Hesketh J.E. Selenium and viral infection: are there lessons for COVID-19? Br. J. Nutr. 2021;125:1–10. doi: 10.1017/S0007114520003128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Marshall J.S., Warrington R., Watson W., Kim H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018;14:1–10. doi: 10.1186/s13223-018-0278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bourke C.D., Berkley J.A., Prendergast A.J. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 2016;37:386–398. doi: 10.1016/j.it.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Im J.H., Je Y.S., Baek J., Chung M.H., Kwon H.Y., Lee J.S. Nutritional status of patients with COVID-19. Int J. Infect. Dis. 2020;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Moghaddam A., Heller R.A., Sun Q., Seelig J., Cherkezov A., Seibert L., et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020;12:2098. doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mehdi Y., Hornick J.L., Istasse L., Dufrasne I. Selenium in the environment, metabolism and involvement in body functions. Molecules. 2013;18:3292–3311. doi: 10.3390/molecules18033292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fakhrolmobasheri M., Nasr-Esfahany Z., Khanahmad H., Zeinalian M. Selenium supplementation can relieve the clinical complications of COVID-19 and other similar viral infections. Int J. Vitam. Nutr. Res. 2020;91:197–199. doi: 10.1024/0300-9831/a000663. [DOI] [PubMed] [Google Scholar]
- 207.Zhang J., Saad R., Taylor E.W., Rayman M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wood S.M., Beckham C., Yosioka A., Darban H., Watson R.R. β-Carotene and selenium supplementation enhances immune response in aged humans. Integr. Mol. 2000;2:85–92. doi: 10.1016/S1096-2190(00)00009-3. [DOI] [PubMed] [Google Scholar]
- 209.Wong C.K., Lam C.W., Wu A.K., Ip W.K., Lee N.L., Chan I., et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pal A., Squitti R., Picozza M., Pawar A., Rongioletti M., Dutta A.K., et al. Zinc and COVID-19: basis of current clinical trials. Biol. Trace Elem. Res. 2020;199(8):2882–2892. doi: 10.1007/s12011-020-02437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Prasad A.S. discovery of zinc for human health and biomarkers of zinc deficiency. Mol. Genet. Nutr. Asp. Major Trace Min. 2017:241–260. doi: 10.1016/B978-0-12-802168-2.00020-8. [DOI] [Google Scholar]
- 212.Kumar A., Kubota Y., Chernov M., Kasuya H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wessels I., Maywald M., Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9:1286. doi: 10.1016/j.abb.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Maares M., Haase H. Zinc and immunity: an essential interrelation. Arch. Biochem Biophys. 2016;611:58–65. doi: 10.1016/j.abb.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 215.Roohani N., Hurrell R., Kelishadi R., Schulin R. Zinc and its importance for human health: An integrative review. J. Res Med Sci. 2013;18:144–157. [PMC free article] [PubMed] [Google Scholar]
- 216.Wessels I., Rink L. Micronutrients in autoimmune diseases: possible therapeutic benefits of zinc and vitamin D. J. Nutr. Biochem. 2020;77 doi: 10.1016/j.jnutbio.2019.108240. [DOI] [PubMed] [Google Scholar]
- 217.Haase H., Schomburg L. You’d better zinc-trace element homeostasis in infection and inflammation. Nutrients. 2019;11:2078. doi: 10.3390/nu11092078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Overbeck S., Rink L., Haase H. Modulating the immune response by oral zinc supplementation: a single approach for multiple diseases. Arch. Immunol. Ther. Exp. 2008;56:15–30. doi: 10.1007/s00005-008-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Christianson D.W., Alexander R.S. Carboxylate-histidine-zinc interactions in protein structure and function. J. Am. Chem. Soc. 1989;111:6412–6419. doi: 10.1021/ja00198a065. [DOI] [Google Scholar]
- 220.Cox E.H., McLendon G.L. Zinc-dependent protein folding. Curr. Opin. Chem. Biol. 2000;4:162–165. doi: 10.1016/S1367-5931(99)00070-8. [DOI] [PubMed] [Google Scholar]
- 221.Lanke K., Krenn B.M., Melchers W.J., Seipelt J., Van Kuppeveld F.J. PDTC inhibits picornavirus polyprotein processing and RNA replication by transporting zinc ions into cells. J. Gen. Virol. 2007;88:1206–1217. doi: 10.1099/vir.0.82634-0. [DOI] [PubMed] [Google Scholar]
- 222.Liu C.Y., Kielian M. Identification of a specific region in the E1 fusion protein involved in zinc inhibition of Semliki Forest virus fusion. J. Virol. 2012;86:3588–3594. doi: 10.1128/JVI.07115-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Shishkov S., Varadinova T., Bontchev P., Nachev C., Michailova E. Complexes of zinc with picolinic and aspartic acids inactivate free varicella-zoster virions. Met Based Drugs. 1996;3:11–14. doi: 10.1155/MBD.1996.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Jayawardena R., Sooriyaarachchi P., Chourdakis M., Jeewandara C., Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes Metab. Syndr. 2020;14:367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wessels I., Rolles B., Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.team E. e. Updated rapid risk assessment from ECDC on coronavirus disease (COVID-19) pandemic in the EU/EEA and the UK: resurgence of cases. Eur. Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.32.2008131. https://doi.org/10.2807/15607917.ES.2020.25.32.2008131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ishida T. Review on the role of Zn2+ ions in viral pathogenesis and the effect of Zn2+ ions for host cell-virus growth inhibition. Am. J. Biomed. Sci. Res. 2019;2:28–37. doi: 10.34297/AJBSR.2019.02.000566. [DOI] [Google Scholar]
- 228.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv. Nutr. 2019;10:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Suara R.O., Crowe J.E., Jr. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob. Agents Chemother. 2004;48:783–790. doi: 10.1128/AAC.48.3.783-790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kümel G., Schrader S., Zentgraf H., Daus H., Brendel M. The mechanism of the antiherpetic activity of zinc sulphate. J. Gen. Virol. 1990;71:2989–2997. doi: 10.1099/0022-1317-71-12-2989. [DOI] [PubMed] [Google Scholar]
- 231.Chilvers M.A., McKean M., Rutman A., Myint B.S., Silverman M., O’Callaghan C. The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur. Respir. J. 2001;18:965–970. doi: 10.1183/09031936.01.00093001. [DOI] [PubMed] [Google Scholar]
- 232.Woodworth B.A., Zhang S., Tamashiro E., Bhargave G., Palmer J.N., Cohen N.A. Zinc increases ciliary beat frequency in a calcium-dependent manner. Am. J. Rhinol. Allergy. 2010;24:6–10. doi: 10.2500/ajra.2010.24.3379. [DOI] [PubMed] [Google Scholar]
- 233.Biaggio V.S., Salvetti N.R., Chaca M.V., Valdez S.R., Ortega H.H., Gimenez M.S., et al. Alterations of the extracellular matrix of lung during zinc deficiency. Br. J. Nutr. 2012;108:62–70. doi: 10.1017/S0007114511005290. [DOI] [PubMed] [Google Scholar]
- 234.Berg K., Bolt G., Andersen H., Owen T.C. Zinc potentiates the antiviral action of human IFN-α tenfold. J. Interferon Cytokine Res. 2001;21:471–474. doi: 10.1089/10799900152434330. [DOI] [PubMed] [Google Scholar]
- 235.Cakman I., Kirchner H., Rinkl L. Zinc supplementation reconstitutes the production of interferon-α by leukocytes from elderly persons. J. Interferon Cytokine Res. 1997;17:469–472. doi: 10.1089/jir.1997.17.469. [DOI] [PubMed] [Google Scholar]
- 236.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Møller R., Panis M., Sachs D., et al. SARS-CoV-2 launches a unique transcriptional signature from in vitro, ex vivo, and in vivo systems. BioRxiv. 2020 doi: 10.1101/2020.03.24.004655. [DOI] [Google Scholar]
- 237.Te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Finzi E. Treatment of SARS-CoV-2 with high dose oral zinc salts: a report on four patients. Int J. Infect. Dis. 2020;99:307–309. doi: 10.1016/j.ijid.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S.I., et al. Zinc and respiratory tract infections: Perspectives for COVID‑19. Int J. Mol. Med. 2020;46:17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lin M.H., Moses D.C., Hsieh C.H., Cheng S.C., Chen Y.H., Sun C.Y., et al. Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antivir. Res. 2018;150:155–163. doi: 10.1016/j.antiviral.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sargsyan K., Chen T., Grauffel C., Lim C. Identifying COVID-19 drug-sites susceptible to clinically safe Zn-ejector drugs using evolutionary/physical principles. Chem. Sci. 2020;11:9904–9909. doi: 10.31219/osf.io/snuqf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Xu L., Tong J., Wu Y., Zhao S. and Lin B.L. Targeted oxidation strategy (TOS) for potential inhibition of coronaviruses by disulfiram-a 70-year old anti-alcoholism drug 2020. https://doi.org/10.26434/chem rxiv.11936292.v1.
- 243.Shittu M.O., Afolami O.I. Improving the efficacy of Chloroquine and Hydroxychloroquine against SARS-CoV-2 may require Zinc additives-A better synergy for future COVID-19 clinical trials. Infez. Med. 2020;28:192–197. [PubMed] [Google Scholar]
- 244.Cao J.W., Duan S.Y., Zhang H.X., Chen Yand Guo M. Zinc deficiency promoted fibrosis via ROS and TIMP/MMPs in the myocardium of mice. Biol. Trace Elem. Res. 2020;196:145–152. doi: 10.1007/s12011-019-01902-4. [DOI] [PubMed] [Google Scholar]
- 245.Krenn B.M., Gaudernak E., Holzer B., Lanke K., Van Kuppeveld F.J., Seipelt J. Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections. J. Virol. 2009;83:58–64. doi: 10.1128/JVI.01543-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Qaswal A.B., Suleiman A., Guzu H., Harb T., Atiyat B. The potential role of lithium as an antiviral agent against SARS-CoV-2 via membrane depolarization: review and hypothesis. Sci. Pharm. 2021;89:11. doi: 10.3390/scipharm89010011. [DOI] [Google Scholar]
- 247.Forlenza O.V., de Paula V.J., Machado-Vieira R., Diniz B.S., Gattaz W.F. Does lithium prevent Alzheimer’s disease? Drugs Aging. 2012;29:335–342. doi: 10.2165/11599180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 248.Shine B., McKnight R.F., Leaver L., Geddes J.R. Long-term effects of lithium on renal, thyroid, and parathyroid function: a retrospective analysis of laboratory data. Lancet. 2015;386:461–468. doi: 10.1016/j.rvsc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 249.Gallicchio V.S., Cibull M.L., Hughes N.K., Tse K.F. Effect of lithium in murine immunodeficiency virus infected animals. Pathobiol. 1993;61:216–221. doi: 10.1159/000163797. [DOI] [PubMed] [Google Scholar]
- 250.Li H.J., Gao D.S., Li Y.T., Wang Y.S., Liu H.Y., Zhao J. Antiviral effect of lithium chloride on porcine epidemic diarrhea virus in vitro. Res Vet. Sci. 2018;118:288–294. doi: 10.1016/j.rvsc.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Muñoz-Moreno J.A., Prats A., Moltó J., Garolera M., Pérez-Álvarez N., Díez-Quevedo C., et al. Transdermal rivastigmine for HIV-associated cognitive impairment: A randomized pilot study. PloS One. 2017;12 doi: 10.1371/journal.pone.0182547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Puertas M.C., Salgado M., Morón-López S., Ouchi D., Muñoz-Moreno J.A., Moltó J., et al. Effect of lithium on HIV-1 expression and proviral reservoir size in the CD4+ T cells of antiretroviral therapy suppressed patients. Aids. 2014;28:2157–2159. doi: 10.1097/QAD.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 253.Harrison S.M., Tarpey I., Rothwell L., Kaiser P., Hiscox J.A. Lithium chloride inhibits the coronavirus infectious bronchitis virus in cell culture. Avian Pathol. 2007;36:109–114. doi: 10.1080/03079450601156083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Li J., Yin J., Sui X., Li G., Ren X. Comparative analysis of the effect of glycyrrhizin diammonium and lithium chloride on infectious bronchitis virus infection in vitro. Avian Pathol. 2009;38:215–221. doi: 10.1080/03079450902912184. [DOI] [PubMed] [Google Scholar]
- 255.Mallery D.L., Márquez C.L., McEwan W.A., Dickson C.F., Jacques D.A., Anandapadamanaban M., et al. IP6 is an HIV pocket factor that prevents capsid collapse and promotes DNA synthesis. Elife. 2018;7:35335. doi: 10.7554/eLife.35335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Quiroz J.A., Gould T.D., Manji H.K. Molecular effects of lithium. Mol. Inter. 2004;4:259–272. doi: 10.1124/mi.4.5.6. [DOI] [PubMed] [Google Scholar]
- 257.Liu X., Verma A., Ramage H., Garcia G., Myers R.L., Lucas A., et al. Targeting the Coronavirus Nucleocapsid Protein through GSK-3 Inhibition. medRxiv 2021. https://doi.org/10.1101/20 21.02.17.21 251933. [DOI] [PMC free article] [PubMed]
- 258.Jeong G.U., Song H., Yoon G.Y., Kim D., Kwon Y.C. Therapeutic strategies against COVID-19 and structural characterization of SARS-CoV-2: a review. Front. Microbiol. 2020;11:1723. doi: 10.3389/fmicb.2020.01723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mengist H.M., Dilnessa T., Jin T. Structural basis of potential inhibitors targeting SARS-CoV-2 main protease. Front. Chem. 2021;9 doi: 10.3389/fchem.2021.622898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Jin Z., Liu F., Du X., Xu Y., Deng Y., Liu M., et al. Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1101/2020.02.26.964882. [DOI] [PubMed] [Google Scholar]
- 261.Viel T., Chinta S., Rane A., Chamoli M., Buck H., Andersen J. Microdose lithium reduces cellular senescence in human astrocytes-a potential pharmacotherapy for COVID-19? Aging (Albany NY) 2020;12:10035. doi: 10.18632/aging.103449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Rajkumar R.P. Lithium as a candidate treatment for COVID‐19: promises and pitfalls. Drug Dev. Res. 2020;81:782–785. doi: 10.1002/ddr.21701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ren X., Meng F., Yin J., Li G., Li X., Wang C., et al. Action mechanisms of lithium chloride on cell infection by transmissible gastroenteritis coronavirus. PloS One. 2011;6 doi: 10.1371/journal.pone.0018669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Gómez Bernal G. Lithium for the 2019 novel coronavirus. Med. Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Motoi Y., Shimada K., Ishiguro K., Hattori N. Lithium and autophagy. ACS Chem. Neurosci. 2014;5:434–442. doi: 10.1021/cn500056q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Khalil R.B. Lithium chloride combination with rapamycin for the treatment of COVID-19 pneumonia. Med Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Nassar A., Azab A.N. Effects of lithium on inflammation. ACS Chem. Neurosci. 2014;5:451–458. doi: 10.1021/cn500038f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020;111 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ulhaq Z.S., Soraya G.V. Interleukin-6 as a potential biomarker of COVID-19 progression. Med Mal. Infect. 2020;50:382–383. doi: 10.1016/j.medmal.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Troib A., Azab A.N. Effects of psychotropic drugs on nuclear factor kappa B. Eur. Rev. Med. Pharm. Sci. 2015;19:1198–1208. [PubMed] [Google Scholar]
- 271.Wang H.M., Zhang T., Li Q., Huang J.K., Chen R.F., Sun X.J. Inhibition of glycogen synthase kinase-3β by lithium chloride suppresses 6-hydroxydopamine-induced inflammatory response in primary cultured astrocytes. Neurochem Int. 2013;63:345–353. doi: 10.1016/j.neuint.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 272.Rudd C.E. GSK-3 inhibition as a therapeutic approach against SARs CoV2: dual benefit of inhibiting viral replication while potentiating the immune response. Front. Immunol. 2020;11:1638. doi: 10.3389/fimmu.2020.01638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Spuch C., López-García M., Rivera-Baltanás T., Rodrígues-Amorím D., Olivares J.M. Does lithium deserve a place in the treatment against COVID-19? A preliminary observational study in six patients, case report. Front Pharm. 2020;11:1347. doi: 10.3389/fphar.2020.557629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Mehta N., Vannozzi Vannozzi R. Lithium‐induced electrocardiographic changes: a complete review. Clin. Cardiol. 2017;40:1363–1367. doi: 10.1002/clc.22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Nowak J.K. and Walkowiak J. Lithium and coronaviral infections. A scoping review. F1000Res 2020;9:93. https://doi.org/10.12688/f1000research.22299.2.eCollection 2020. [DOI] [PMC free article] [PubMed]
- 276.Maier J.A., Castiglioni S., Locatelli L., Zocchi M., Mazur A. Magnesium and inflammation: advances and perspectives. Semin Cell Dev. Biol. 2020;115:37–44. doi: 10.1016/j.semcdb.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 277.Zeng H.L., Yang Q., Yuan P., Wang X., Cheng L. Associations of essential and toxic metals/metalloids in whole blood with both disease severity and mortality in patients with COVID‐19. FASEB J. 2021;35:21392. doi: 10.1096/fj.202002346R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tang C.F., Ding H., Jiao R.Q., Wu X.X., Kong L.D. Possibility of magnesium supplementation for supportive treatment in patients with COVID-19. Eur. J. Pharm. 2020;886 doi: 10.1016/j.ejphar.2020.173546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Errasfa M. Magnesium therapeutic potential against COVID-19: Could it be an“ All-in-one” therapy? Magnes. Res. 2021;34:32–34. doi: 10.1684/mrh.2020.0474. [DOI] [PubMed] [Google Scholar]
- 280.Iotti S., Wolf F., Mazur A., Maier J.A. The COVID-19 pandemic: is there a role for magnesium? Hypotheses and perspectives. Magnes. Res. 2020;33:21–27. doi: 10.1684/mrh.2020.0465. [DOI] [PubMed] [Google Scholar]
- 281.Cooper I.D., Crofts C.A., DiNicolantonio J.J., Malhotra A., Elliott B., Kyriakidou Y., et al. Relationships between hyperinsulinaemia, magnesium, vitamin D, thrombosis and COVID-19: rationale for clinical management. Open Heart. 2020;7 doi: 10.1136/openhrt-2020-001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Goddek S. Vitamin D3 and K2 and their potential contribution to reducing the COVID-19 mortality rate. Int. J. Infect. Dis. 2020;99:286–290. doi: 10.1016/j.ijid.2020.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Jie M., Wu Y., Gao M., Li X., Liu C., Ouyang Q., et al. CircMRPS35 suppresses gastric cancer progression via recruiting KAT7 to govern histone modification. Mol. Cancer. 2020;19:1–16. doi: 10.1186/s12943-020-01160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Alnafiey M.O., Alangari A.M., Alarifi A.M., Abushara A. Persistent Hypokalemia post SARS-coV-2 infection, is it a life-long complication? Case report. Ann. Med. Surg. (Lond. ) 2021;62:358–361. doi: 10.1016/j.amsu.2021.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sarvazad H., Cahngaripour S.H., Roozbahani N.E., Izadi B. Evaluation of electrolyte status of sodium, potassium and magnesium, and fasting blood sugar at the initial admission of individuals with COVID-19 without underlying disease in Golestan Hospital, Kermanshah. N. Microbes N. Infect. 2020;38 doi: 10.1016/j.nmni.2020.100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Micke O., Vormann J., Kisters K. Magnesium and COVID-19–Some further comments–a commentary on wallace TC. Combating COVID-19 and building immune resilience: a potential role for magnesium nutrition? J. Am. Coll. Nutr. 2020;1–9 doi: 10.1080/07315724.2020.1785971. [DOI] [PubMed] [Google Scholar]
- 287.Kazaks A.G., Uriu-Adams J.Y., Albertson T.E., Shenoy S.F., Stern J.S. Effect of oral magnesium supplementation on measures of airway resistance and subjective assessment of asthma control and quality of life in men and women with mild to moderate asthma: a randomized placebo controlled trial. J. Asthma. 2010;47:83–92. doi: 10.3109/02770900903331127. [DOI] [PubMed] [Google Scholar]
- 288.Alfano G., Ferrari A., Fontana F., Perrone R., Mori G., Ascione E., et al. Hypokalemia in patients with COVID-19. Clin. Exp. Nephrol. 2021;25:401–409. doi: 10.1007/s10157-020-01996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]