Abstract
Overactive PI 3-kinase (PI3K) in cancer and immune-dysregulation has spurred extensive efforts to develop therapeutic PI3K inhibitors. Although progress has been hampered by issues such as poor drug tolerance and drug resistance, several PI3K inhibitors have now received regulatory approval – the PI3Kα isoform-selective inhibitor alpelisib for the treatment of breast cancer, and inhibitors mainly aimed at the leukocyte-enriched PI3Kδ in B-cell malignancies. In addition to targeting cancer-cell intrinsic PI3K activity, emerging evidence highlights the potential of PI3K inhibitors in cancer immunotherapy. This review summarises key discoveries aiding the clinical translation of PI3Kα and PI3Kδ inhibitors, highlighting lessons learned and future opportunities.
Keywords: PI3Kα, PI3Kδ, PIK3CA, PIK3CD, breast cancer, lymphoma, immunity, inflammation, cancer, immunotherapy, drug development
TOC
PI3K signalling is one of the most frequently aberrantly-activated pathways in cancer. However, the development of therapeutic PI3K pathway inhibitors has faced challenges including poor drug tolerance and drug resistance. Here, Vanhaesebroeck et al. review efforts to understand and therapeutically exploit the biology of PI3Kα and PI3Kδ — the key targets of currently approved PI3K inhibitors, highlighting lessons learned and future opportunities.
Introduction
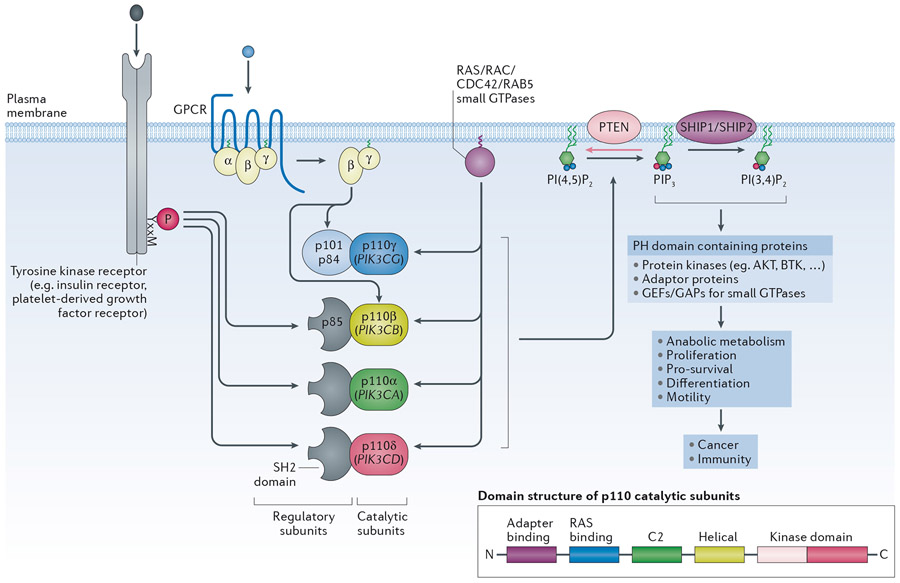
Class I PI3Ks signal downstream of tyrosine kinases, G protein-coupled receptors (GPCRs) and GTPases such as Ras, Rac and Cdc42, to regulate a range of cellular activities, including metabolism, proliferation and migration (Figure 1)1,2. PI3K signalling is one of the most frequently aberrantly-activated pathways in cancer, and early studies showed that the pan-PI3K inhibitors LY294002 and wortmannin could revert cancer cell resistance to a broad range of therapies, including chemotherapy, radiation and targeted therapies3. Some PI3K family members are also involved in inflammation and auto-immunity4-7.
Figure 1 – General overview of signalling by class I PI3K isoforms.
The class IA PI3K catalytic subunits (p110α, β and δ) bind the p85 regulatory subunits which keep the p85/p110 complex in an inactive, cytosolic form. The p85 subunits have two SH2 domains that allow the p85/p110 heterodimers to bind to phosphorylated tyrosine residues in membrane-associated proteins, such as receptors and adaptor proteins, thereby recruiting the PI3K heterodimer to its lipid substrates while simultaneously disinhibiting its enzymatic activity. Mammals have three genes for p85 regulatory subunits, namely PIK3R1 (encoding p85α, p55α and p50α), PIK3R2 (encoding p85β) and PIK3R3 (encoding p55γ). p110γ, the sole member of the class IB PI3Ks, binds p101/p84 regulatory subunits which do not have homology to p85 or other proteins, and which permit p110γ to engage with Gβγ subunits downstream of GPCRs. Class I PI3Ks can also engage with small GTPases such as members of the Ras (p110α, p110δ, p110γ) or Cdc42, Rac or Rab5 families (p110β). Unlike PI3Kα and PI3Kδ, PI3Kβ is also activated by Gβγ subunits downstream of GPCRs and appears to require more inputs to become fully activated compared to PI3Kα. (Insert): overall domain structure of the p110 catalytic subunits.
Class I PI3Ks phosphorylate the 3-position of the inositol ring of a specific phosphatidylinositol (PtdIns) lipid, namely phosphatidylinositol-(4,5)-bisphosphate (PtdIns(4,5)P2), converting it to phosphatidylinositol-(3,4,5)-trisphosphate (PtdIns(3,4,5)P3, or PIP3). PIP3 can be converted to PtdIns(3,4)P2 following dephosphorylation of the 5’-position by the 5-phosphatases SHIP1 and SHIP2. Together, PIP3 and PtdIns(3,4)P2 function as second messengers downstream of class I PI3Ks by interacting with 3-phosphoinositide-binding pleckstrin homology (PH) domains found in diverse proteins, including protein kinases (AKT, BTK), adaptor proteins and regulators of small GTPases. The tumour suppressor phosphatase and tensin homolog (PTEN) 3-phosphoinositide phosphatase dampens class I PI3K signalling, by dephosphorylating PIP3 and PtdIns(3,4)P2. PTEN is frequently somatically inactivated in cancer, through a wide range of mechanisms, including loss-of-expression and/or mutation. PTEN inactivation is also the cause of a developmental syndrome known as PTEN Hamartoma Tumour Syndrome (PHTS) in which one gene copy of PTEN has been partially or fully inactivated. Individuals with PHTS are predisposed to benign overgrowths, neurodevelopmental abnormalities as well as specific cancers in adulthood.
Class I PI3Ks consist of a regulatory subunit in complex with a p110 catalytic subunit (p110α, β, γ and δ). Below, these heterodimeric complexes will be referred to as PI3Kα, PI3Kβ, PI3Kγ and PI3Kδ, with p110α, p110β, p110γ and p110δ indicating the catalytic subunits themselves. Whereas p110α and p110β show a broad tissue distribution, p110γ and p110δ are highly enriched in all leukocyte subtypes, with emerging data of low but functionally-relevant levels of p110δ in non-leukocytes.
Class I PI3Ks generate phosphatidylinositol-(3,4,5)-trisphosphate (PtdIns(3,4,5)P3, or PIP3) which can be converted to PtdIns(3,4)P2 by 5-phosphatases such as SHIP1 and SHIP2 (Figure 1). PIP3 and PtdIns(3,4)P2 interact with 3-phosphoinositide-binding pleckstrin homology (PH) domains found in diverse proteins, including protein kinases (such as AKT/PKB, BTK), adaptor proteins and regulators of GTPases, to regulate their activities. The tumour suppressor phosphatase and tensin homolog (PTEN), which is frequently somatically inactivated in cancer, dampens class I PI3K signalling (Figure 1).
Given its key role in cancer and immunity, the PI3K pathway has been the focus of extensive drug development efforts in the past two decades. In 2014, the PI3Kδ inhibitor idelalisib (Zydelig/CAL-101/GS-1101; Gilead Sciences) became the first PI3K inhibitor to be approved, for use in specific B-cell malignancies. This was followed by the approval in 2017 of the pan-class I PI3K inhibitor copanlisib (Aliqopa/BAY 80-6946; Bayer) and in 2018 of the dual PI3Kδ/γ inhibitor duvelisib (Copiktra/IPI-145/INK1197; Verastem, now Secura Bio) for the same indications (Table 1). Umbralisib (TGR-1202; TG Therapeutics)8 has recently received fast track status in CLL in combination with the anti-CD20 antibody ublituximab, as well as conditional FDA approval in follicular lymphoma and marginal zone lymphoma9 (Table 1). In 2019, the PI3Kα inhibitor alpelisib (Piqray/NVP-BYL719; Novartis) was approved for the treatment of advanced breast cancer, in combination with the oestrogen receptor (ER) down-regulator fulvestrant10.
Table 1:
Characteristics of clinically-approved PI3K inhibitors to date (March 2020).
| Drug/Company | PI3K inhibitors class | Enzyme activities nM (selectivity fold) |
Disease indication | Monotherapy or combination | References | |||
|---|---|---|---|---|---|---|---|---|
| PI3Kα | PI3Kβ | PI3Kδ | PI3Kγ | |||||
| Alpelisib/NVP-BYL719/Piqray (Novartis) | PI3Kα inhibitor | 4.6 | 1200 (260) | 290 (63) | 250 (54) | PIK3CA-mutated, hormone receptor-positive (HR+), human epidermal growth factor receptor-2-negative (HER2−) advanced breast cancer | Combination with the oestrogen receptor (ER) down-regulator fulvestrant | 24,44 |
| Idelalisib/CAL-101/GS-1101/Zydelig (Gilead) | PI3Kδ inhibitor | 820 (330) | 570 (230) | 2.5 | 89 (36) | Chronic lymphocytic leukaemia (CLL), relapsed | Combination with the anti-CD20 antibody rituximab, in patients in whom rituximab alone would be considered appropriate therapy due to other comorbidities | 30 |
| Follicular lymphoma (FL) after at least 2 prior systemic therapies | Monotherapy | |||||||
| Small lymphocytic lymphoma (SLL) after at least 2 prior systemic therapies | Monotherapy | |||||||
| Umbralisib/TGR-1202 (TG Therapeutics) | PI3Kδ inhibitor (also Inhibits CK1ε with IC50 180 nM) | >10000 (>1000) | >10000 (>1000) | 6.2 | 1400 (225) | CLL, FL, MZ lymphoma | 2020: fast track FDA approval status in CLL in combination with the anti-CD20 antibody ublituximab; 2021: FDA approval for follicular lymphoma and marginal zone lymphoma | 8,253 |
| Duvelisib/IPI-145/Copiktra (Secura Bio) | dual PI3Kγ/δ inhibitor | 1600 (640) | 85 (34) | 2.5 | 27 (11) | Chronic lymphocytic leukemia (CLL) after at least two prior therapies; follicular lymphoma (FL) after at least two prior systemic therapies; small lymphocytic lymphoma (SLL) after at least 2 prior systemic therapies | Monotherapy | 31 |
| Copanlisib/BAY 80-6946/Aliqopa (Bayer) | Pan-PI3K inhibitor | 0.5 | 3.7 (7) | 0.7 (1.4) | 6.6 (13) | Follicular lymphoma after at least two prior systemic therapies | Monotherapy | 23 |
Although these approvals have validated the pathway as a viable drug target, the development of PI3K pathway inhibitors has proven challenging, with progress hampered by poor drug tolerance, intrinsic and acquired drug resistance and signalling feedback loops that neutralize PI3K inhibition11-14. The lack of clinical benefit and poor tolerability of pan-class I PI3K and dual PI3Kα/δ inhibitors has halted further clinical development of these compounds. Nevertheless, the development of isoform-selective PI3K inhibitors and increased clinical experience with PI3K inhibitors are now heralding a more productive phase in PI3K drug development.
Here, we overview efforts to understand and therapeutically exploit the biology of PI3Kα and PI3Kδ — the key targets of currently approved PI3K inhibitors —and the lessons learnt in their development, to realize the potential of this drug class. Data on PI3Kβ and PI3Kγ are also mentioned where relevant. The current landscape of PI3K inhibitors and the general principles of isoform-selective PI3K inhibitor development are summarized. Reflecting the differing roles of PI3Kα and PI3Kδ, their respective biologies, the clinical experience targeting these PI3Ks and emerging opportunities will be discussed, closing with a perspective on the future of the field overall.
The PI3K inhibitor landscape
The class I PI3K p110 catalytic subunits consist of an N-terminal adaptor-binding domain, a Ras binding domain, a membrane binding C2 domain, a helical domain and a C-terminal catalytic domain which is divided into N- and C-terminal sections and separated by the hinge, where ATP is bound (Figure 1; insert)15,16. The regulatory subunits bind to and maintain the p110 subunits in an inactive form until the PI3Ks become activated by engagement of their regulatory subunits with upstream signalling inputs.
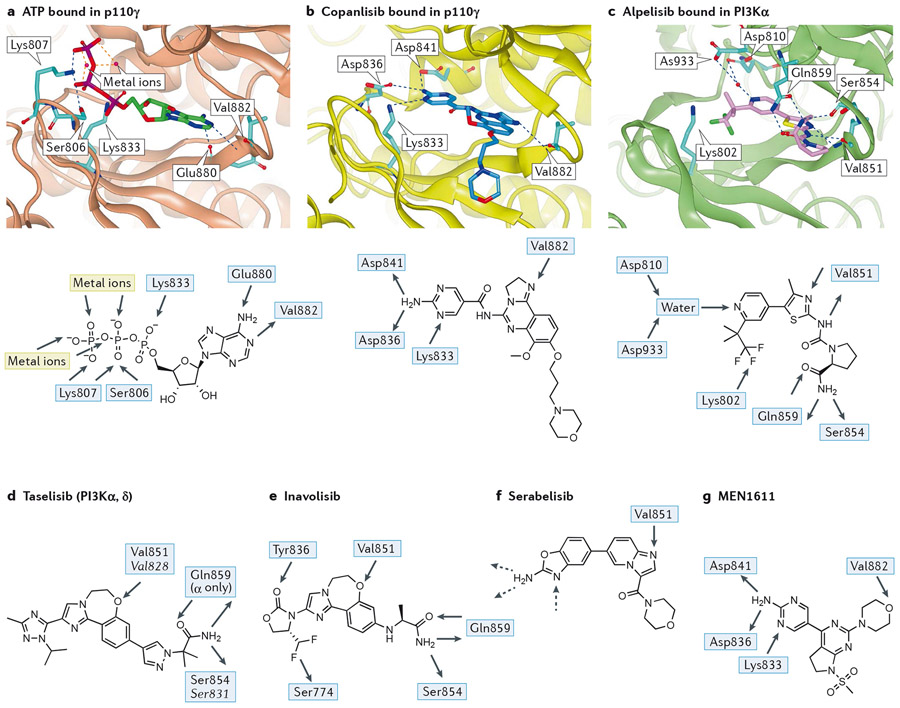
Most PI3K inhibitors are ATP-competitive. The ATP-binding pocket is in a cleft between the two lobes of the kinase domain, with a hinge valine residue at the end of the cleft (shown in p110γ in Figure 2a). This valine is conserved in all class I PI3K isoforms and forms an H-bond with the purine ring of ATP. Accordingly, all ATP-competitive PI3K inhibitors identified to date accept an H-bond from this valine residue.
Figure 2 – Key features of the interaction between PI3Ks and pan- and PI3Kα-selective inhibitors.
The native shape of PI3K enzymes is taken to be that observed by crystallography for ATP-bound p110γ (2a; PDB:1E8X)18 or the very similar apo forms observed for p110γ (PDB:1E8Y)18, p110δ (PDB:2WXR)19 and PI3Kα (in complex with a partial p85α fragment, PBD:2RD0)20. Peptides are shown as ribbons with key residues shown in stick representation. Ligands are shown in stick representation. Colour coding of atoms in stick representations - carbon: cyan, oxygen: red; nitrogen: blue; fluorine: green; phosphorus: purple; colour coding of ligands - ATP: green, copanlisib: dark blue, alpelisib: pink, idelalisib: red; hydrogen bonds are shown in blue dashed lines, metal interactions in orange dashed lines.
a) (Top panel): ATP (carbon atoms bright green) bound in p110γ (1E8X)18; p110γ shown as brown ribbon with sidechains shown in cyan for residues mentioned in text. The adenine makes an acceptor-donor pair of hydrogen bonds with the NH of hinge Val882 and carbonyl of Glu880 whilst the triphosphate is bound by two metal ions, the terminal ammonium groups of Lys807 and Lys833 and a hydrogen bond from Ser806.(Lower panel): 2D representation of the interactions of ATP with the binding pocket of p110γ.
b) (Top panel) copanlisib (dark blue) bound in p110γ (yellow ribbon, 5G2N)23. The pendant aminopyrimidine group of copanlisib fits into the affinity pocket and forms H-bonds with Asp836 and Asp841 via the amino group and receives a H-bond from Lys833 to one of the ring nitrogen atoms. The morpholinopropyl moiety extends towards solvent and does not make any significant interactions; its role in the molecule is mainly as a solubilising group. (Lower panel): 2D representation of copanlisib indicating the H-bonds made with PI3Kγ.
c) (Top panel): alpelisib (pink) bound in PI3Kα (green ribbon, 4JPS)24. Note the multiple H-bonds: to hinge Val851; involving the primary carboxamide of alpelisib with Gln859 in p110α (Asp862, Lys890, Asn836 in p110β, γ and δ, respectively) and the backbone carbonyl of Ser854; the water-mediated H-bond to the pyridine N from Asp810 and Asp933. The charged terminal amine of Lys802 is close to the CF3 group. (Lower panel): 2D representation of the major interactions of alpelisib in PI3Kα.
d) 2D representation of the PI3Kα/δ inhibitor taselisib with H-bonding interactions observed in the crystal with PI3Kα and, in italic, with PI3Kδ. The ether oxygen of taselisib makes the key hinge interaction with both PI3Kα and PI3Kδ. Taselisib has a primary amide that can make the same interactions with p110α as alpelisib25, but in p110δ a rotation of the side chain places this amide differently, where it can still interact with the backbone carbonyl of Ser831 and places the terminal carbonyl of taselisib towards solvent (PDB:5T8F)360. In the affinity pocket, taselisib appears to be capapble of accepting H-bonds from Lys779 (PI3Kδ numbering) to N2 and from a putative water molecule located between Asp787 and Tyr813 (PI3Kδ numbering) to N4.
e) 2D representation of the PI3Kα-selective inhibitor inavolisib with H-bonding interactions observed in the crystal with PI3Kα. A carbonyl group in inavolisib accepts an H-bond from Tyr836 in p110α and a difluoromethyl group interacts with the hydroxyl of Ser774 in p110α. Although both of these residues are conserved in all class I PI3K isoforms, the combination of these structural features with a primary amide interacting with the non-conserved Gln859 of p110α results in very high PI3Kα isoform selectivity25.
f) Structure of the PI3Kα-selective inhibitor serabelisib. Although a crystal structure has not been disclosed for this molecule it is probable that the binding mode mimics that of copanlisib (Figure 2b) with the nitrogen of the imidazopyridine accepting a H-bond from the hinge Val851 and the aminobenzoxazole making interactions with the residues in the affinity pocket (hashed arrows).
g) Structure of PI3Kα inhibitor MEN1611 showing the observed hydrogen bonds in PI3Kγ.
A series of non-ATP competitive PI3Kδ inhibitors have also been identified, illustrated by the PI3Kδ inhibitor IOA-244 (iOnctura; Supplementary Figure 1a)17, but the structural details of the binding mode of these molecules have not been disclosed.
Non-isoform selective inhibitors
The native shape of PI3K enzymes is taken to be that observed by crystallography for ATP-bound p110γ (PDB:1E8X)18 or the very similar apo forms observed for p110γ (PDB:1E8Y)18, p110δ (PDB: 2WXR)19 and PI3Kα (in complex with a partial p85α fragment, PBD:2RD0)20.
Early PI3K inhibitors exhibited similar activity against all class I PI3K isoforms, for example buparlisib21,22 (Supplementary Figure 1b), with copanlisib (Figure 2b) representing an optimised development of these chemotypes23. The conformation of p110γ bound to copanlisib (PDB: 5G2N)23 is almost unchanged from p110γ bound to ATP: copanlisib binds in the ATP binding site with the nitrogen atom of the imidazolidine making the obligatory H-bond with the NH group of the Val882 hinge residue, while its flat core heterocycle fits neatly between the hydrophobic faces of the cleft (Figure 2b).
Obtaining selectivity beyond the conserved ATP pocket
Although flat inhibitors are typically non-selective, it is possible to obtain selectivity from such compounds by making larger molecules whose binding extends beyond the conserved region of the ATP-binding pocket. Thus, alpelisib gains selectivity and potency for PI3Kα by addition of functionality at both termini of the molecule that make specific interactions24 (Figure 2c). Taselisib (GDC0032) (Figure 2d) makes use of a similar carboxamide to alpelisib, but is also capable of binding to PI3Kδ with high affinity25. Inavolisib (GDC-0077; Figure 2e), a further development of the taselisib structure, is significantly more selective and inhibits only PI3Kα (Table 2). Inavolisib makes more precise interactions in the affinity pocket of p110α along with the PI3Kα favouring carboxamide to give excellent PI3K isoform selectivity25.
Table 2:
PI3K inhibitors in clinical development March 2021 and other compounds discussed in the text
| Drug names | Company | Enzyme IC50(nM) (selectivity fold) |
Disease indications tested in trials |
Development phase | |||
|---|---|---|---|---|---|---|---|
| PI3Kα | PI3Kβ | PI3Kδ | PI3Kγ | ||||
| Pan PI3K inhibitors | |||||||
| Buparlisib/NVP-BKM12021,22 | Novartis → Adlai Nortye | 52 | 166 (3) | 116 (2) | 262 (5) | head and neck squamous cell carcinoma | Phase III |
| Pictilisib/GDC-0941363 | Piramed → Roche/Genentech | 3 | 33 (11) | 3 | 75 (25) | Breast cancer | Phase I (formerly in Phase II for metastatic breast cancer and non-small cell lung cancer) |
| PI3Kα inhibitors | |||||||
| inavolisib/GDC-0077/RG-611425,364-367 | Genentech/Roche | 0.034 | 100 (2900) | 12 (360) | 18 (540) | breast cancer/other solid tumours | Phase III |
| serabelisib/INK-1117/TAK-117/MLN1117/ART-001/Petra 06131 | Intellikine → Takeda → Artham (for rare diseases) and Petra Pharma (for oncology) | 15 | 4500 (300) | 1900 (130) | 14000 (930) | vascular malformations/solid tumours | Phase Ib/II |
| MEN1611/CH5132799133 | Chugai → Menarini | 14 | 120 (8) | 500 (36) | 36 (2.6) | Breast cancer/coleorectal cancer | Phase Ib/II |
| CYH-33368 | Shanghai Institute of Materia Medica → Shanghai HaiHe Biopharma | 5.9 | 600 (100) | 79 (13) | 225 (38) | Solid tumours | Phase I |
| PI3Kβ inhibitors | |||||||
| BL14043 | Xi’An Jiaotong University School of Medicine | 880 (150) | 5.7 | 4200 (145) | N/A | Not in clinical development No data reported for PI3Kγ |
|
| SAR260301369 | Sanofi | 1500 (65) | 23 | 470 (20) | >10000 (>4300) | N/A | no further clinical development |
| GSK2636771370 | GlaxoSmithKline | >5800 (>1115) | 5.2 | 58 (11) | >126000 (>24,231) | N/A | no further clinical development |
| AZD8186371 | AstraZeneca | 35 (9) | 4 | 12 (3) | 675 (170) | N/A | no further clinical development |
| PI3Kγ Inhibitors | |||||||
| eganelisib/IPI-54937 | Infinity | 3200 (200) | 3500 (220) | >8400 (>350 | 16 | immuno-oncology | Phase II |
| AZD345841 | AstraZeneca | 7900 (11000) | >31000 (>44000) | 310 (440) | 0.7 | N/A | no clinical development |
| PI3Kδ inhibitors | |||||||
| AMG319/ACP-319372 | Amgen and Cancer Research UK; Amgen → Acerta | 33000 (1800) | 2700 (150) | 18 | 850 (47) | solid tumours; haemato-oncology | Phase II235 |
| nemiralisib/GSK226955727 | GlaxoSmithKline | 5000 (39000) | 1600 (13000) | 0.13 | 6300 (50000) | airway inflammation e.g. COPD | Inhaled; on hold |
| leniolisib/CDZ173373 | Novartis → Pharming | 240 (21) | 420 (38) | 11 | 2200 (200) | activated PI3Kδ syndrome | Phase II/III |
| parsaclisib/INCB-5046533 | Incyte | >20000 (>20000) | >20000 (>20000) | 1.1 | >10000 (>10000) | haemato-oncology, solid tumours | Phase III |
| seletalisib/UCB 585732 | UCB | 3600 (300) | 2100 (177) | 12 | 280 (23) | immune-inflammation (eg. Sjögren syndrome) | on hold |
| zandelisib/PWT-143/ME-401254 | Pathway Therapeutics → MEI Pharma | 5000 (1000) | 210 (42) | 5 | 2100 (420) | haemato-oncology | Phase II |
| IOA-24417,249 | Merck AG → iOnctura | 19000 (130) | 2900 (20) | 150 | >20000 (>130) | solid tumours | ATP non-competitive; Phase I |
| linperlisib/YY-20394231 | Shanghai Yingli Pharmaceutical | 1200 (260) | 140 (30) | 4.6 | 5200 (1100) | haemato-oncology | Phase II ; Trials planned in solid tumours |
| CHF-6523251 | Chiesi | (>454) | (>454) | 2.2 | (>454) | COPD | Inhaled Phase I |
| SHC014748374 | Nanjing Sanhome Pharmaceutical | 240 (310) | 96 | 0.77 (125) | 101 (130) | Follicular lymphoma, marginal zone lymphoma | Phase II |
| dezapelisib/INCB-040093375 | Incyte | 29000 (8500) | 3800 (1100) | 3.4 | 2300 (670) | haemato-oncology | Phase II |
| dual PI3Kγ/δ inhibitors | |||||||
| tenalisib/RP6530376 | Rhizen | (>300) | (>100) | 25 | 33 (1.3) | haemato-oncology | Phase II |
| AZD8154250 | AstraZeneca | 60 (100) | 1250 (2000) | 0.6 | 0.8 | asthma | Inhaled Phase I |
| Inhibitors with undisclosed PI3K isoform-inhibitor profiles | |||||||
| KA2237377 | Karus Therapeutics | Not disclosed, referred to as a dual PI3Kβ/δ inhibitor | haemato-oncology | Phase I | |||
| TQ-B-3525378 (a) | Jiangsu Chia Tai Tianqing Pharmaceutical | Not disclosed, referred to as a dual PI3Kα/β inhibitor | haemato-oncology | Phase II | |||
| HMPL-689(a) | Hutchison China MediTech (Chi-Med) | Not disclosed | haemato-oncology | Phase I/II | |||
| Indirect PI3Kγ/δ inhibitor: SHIP1 activator | |||||||
| rosiptor/AQX-1125260-263 | Aquinox | Not applicable | bladder pain, asthma, COPD | discontinued | |||
Arrows indicate the trajectory of specific compound series through different commercial entities.
structure and data not disclosed.
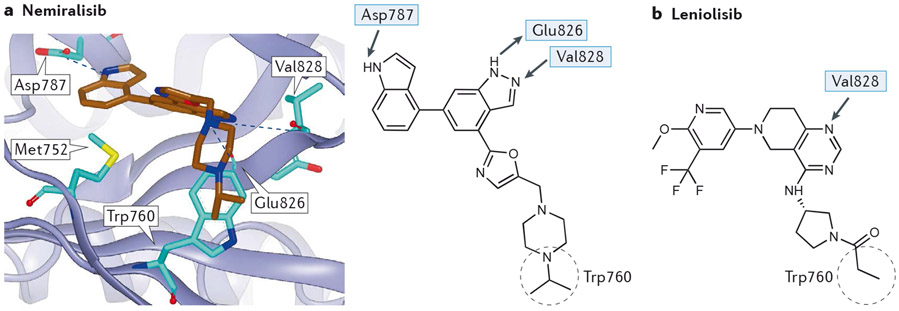
Other differences at the edge of the ATP binding pocket of the PI3K isoforms have also been exploited to identify isoform-selective PI3K inhibitors. In the case of p110δ, differences in the residue corresponding to Thr750 in p110δ (Arg770, Lys777, Lys802 in p110α, β and γ, respectively) mean that p110δ is able to accommodate large groups that can occupy the exposed face of Trp760 (the so-called Trp shelf) in p110δ26. Most notably, the exquisitely PI3Kδ-selective inhibitor nemiralisib (GSK2269557, 5AE8)27 (Figure 3a) puts an isopropyl group in this position whilst the less selective leniolisib/CDZ173 (Figure 3b) has a propionamide over Trp 760.
Figure 3 – Interactions of flat PI3Kδ-selective inhibitors with PI3Kδ.
a) (Upper panel): nemiralisib (brown) bound in p110δ (purple ribbon, 5AE8) showing H bonds with the hinge Val828 and adjacent Glu826 plus Asp787. Note that the isopropyl group, though not making any specific interactions, occupies the space above Trp760 in p110δ that is occluded in the other isoforms where the residues corresponding to Thr750 (coloured in green) are larger (Arg770, Lys777, Lys802 in p110α, β and γ, respectively) (Lower panel): 2D representation of nemiralisib, with H-bonding interactions and the isopropyl group occupying the tryptophan shelf over Trp760 as observed in the crystal with p110δ.
b) 2D representation of the PI3Kδ-selective inhibitor leniolisib, whose quinazoline 1-N accepts an H-bond from Val828 of the hinge. The substituted pyridine occupies the affintiy pocket while the propanoyl pyrrolidine occludes Trp760 giving isoform selectivity in a similar manner to nemiralisib.
In p110γ, Ala885 corresponds to serine in the other class I PI3K isoforms, whilst Gly829 corresponds to glutamine in p110α; these differences were exploited in the design of moderately PI3Kγ-selective compounds28. Further modifications retained PI3Kγ-selectivity making use only of the difference at Ala88529.
Inhibitors forming a specificity pocket
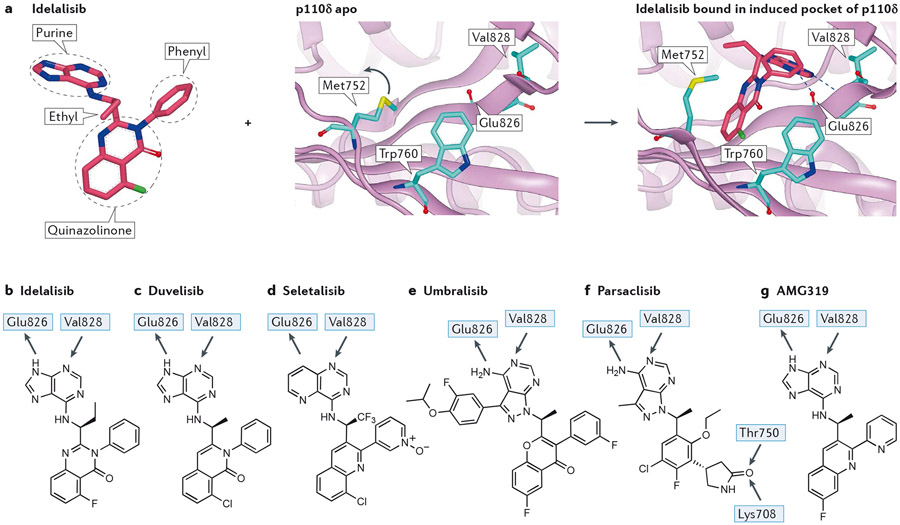
The most widely used selectivity driver in PI3Kδ is the formation of a pocket (“specificity pocket”) by inhibitors inducing the movement of a methionine (Met752 in p110δ) relative to a tryptophan (Trp760 in p110δ). Idelalisib30, duvelisib31, seletalisb32 and umbralisib8 all use this pocket (Figure 4a-e).
Figure 4 – Interactions of selected propeller-shaped PI3Kδ-selective inhibitors with PI3Kδ.
a) Inhibitor-induced specificity pocket in PI3K, illustrated by idelalisib binding to p110δ. Left panel: structure of idelalisib from 4XEO drawn to emphasise the propeller shape, thus the three ring systems of the hinge-binding purine, the quinazolinone amd the phenyl are approximately mutually orthogonal in an orientation organised by a combination of the chiral ethyl group and the phenyl ring. Middle panel: apo structure of p110δ (2WXR) with Met752 packing against Trp760. The blue arrow indicates the relative motion of Met752 in the flexing of the enzyme in solution that can open up the selectivity pocket. Right panel: crystal structure of idelalisib bound in p110δ (4XEO) with the purine making the hinge interaction with the NH of Val828 and the carbonyl of Glu826. The electron deficient quinazolinone ring system fits into the induced selectivity pocket between Met752 and Trp760 and makes a face to edge interaction with the electron rich indole of Trp760.
b) 2D representation of idelalisib showing the major interactions with p110δ.
c) 2D representation of the PI3Kγ/δ inhibitor duvelisib, with H-bonding interactions observed in the crystal with p110δ. Note the similarity to idelalisib.
d) 2D representation of the PI3Kδ-selective inhibitor seletalisib. This is another propeller-shaped PI3Kδ inhibitor, in this case it is probable that the 1 N atom accepts an H-bond from the hinge Val828, with a non-classical H-bond being formed from the CH of the adjacent pyridine ring.
e) Structure of PI3Kδ/CK 1ε inhibitor umbralisib. A crystal structure of this has not been published; however, based on the similarity with other propeller inhibitors the structural features can be identified with confidence. The 3-fluoro-4-isopropoxyphenyl ring is similar to substituents in SW13 and SW14 for which crystal structures are known19; this occupies the affinity pocket and may be responsible for the high isoform selectivity observed.
f) Structure of the PI3Kδ-selective inhibitor parsaclisib with proposed H-bonding interactions based on molecular docking. Note the additional interactions made by the pendant lactam that accepts two H-bonds from both the hydroxyl of Thr750 (p110δ, Arg770, Lys777, Lys802 in p110α, β and γ, respectively) and the terminal ammonium of Lys708 (p110δ, Gln728, Arg735, Ser760 in p110α, β and γ), respectively; other propeller inhibitors do not have an equivalent group. Despite the multiple structural differences with other PI3Kδ inhibitors, parsaclisib still forms a propeller shape.
g) Structure of PI3Kδ-selective inhibitor AMG319 showing the hinge interactions with PI3Kδ based on a crystal structure in PI3Kγ.
The highly selective PI3Kδ inhibitor parsaclisib (INCB50465; Incyte; Figure 4f)33 appears to be an optimised so-called ‘propeller structure’. Although a crystal structure of parsaclisib bound to PI3K has not been published, docking studies suggest that the carbonyl group of a pendant lactam accepts two H-bonds Thr750 and Lys708 that serve to anchor the molecule in the enzyme. Thus, as with later generation PI3Kα inhibitors, building in additional H-bonds with non-conserved residues confers increased PI3K isoform selectivity.
Other drivers for PI3K isoform selectivity with this PI3K pocket are, however, extremely subtle, since not only the original PI3Kδ-selective inhibitors make use of this pocket19 but also PI3Kγ/δ inhibitors34, PI3Kβ/δ35 and PI3Kβ-favouring36 inhibitors. Although no structural information has been disclosed, it is likely that, based on the chemical core, even the highly PI3Kγ-selective propeller-shaped inhibitor eganelisib/IPI-549 (Supplementary Figure 1c) makes use of the same pocket37.
Thus, optimisation of the shape and functionality of inhibitor structures enables multiple different PI3K isoform selectivity patterns to be obtained from a single PI3K pocket.
Other PI3K inhibitors
Other notable inhibitors include a PI3Kα inhibitor chemotype that was identifed through DNA-encoded library screening, which is very different to any previously identified PI3K inhibitor and makes key interactions through a carboxylate group with a non-conserved Arg770 in the P-loop and the non-conserved Gln859 in the C-terminal lobe (4YKN)38. Extending even further from the ATP binding site, careful design of an acrylamide substituted inhibitor (3ZIM) generated compounds that form a covalent bond with Cys86239. Whether such covalent inhibitors have additional advantages or liabilities remains to be determined.
In addition to eganelisib/IPI-549, an alternative means of obtaining selectivity for PI3Kγ has been discovered in a series of inhibitors that bind to the inactive form of the kinase but then induce a conformational change in p110γ, leading to a rearrangement of the enzyme to an active-like conformation40. This rearrangement is due to a substituent of the inhibitor extending deep into the affinity pocket and occurs in two stages, the first causing a movement of the conserved ATP-binding DFG motif in the kinase activation loop and the second a larger reorganisation of the kα12 helix and the kα4-kα5 loop. This process is thought to be easiest in p110γ and accounts for the very high PI3K isoform selectivity observed41.
PI3Kβ-selective inhibitors have been harder to find42, but BL140 represents one of the most selective tool compounds reported, with 150-fold and 430-fold selectivity against PI3Kα and PI3Kδ, respectively (no data given for PI3Kγ)43 (Supplementary Figure 1d). For PI3Kβ inhibitors that have entered clinical development, for example SAR260301, GSK2636771 and AZD8186 (Supplementary Figure 1e-g), the selectivity, where reported, has been lower.
In vivo PI3K isoform-selectivity
Whilst PI3K isoform selectivity in cells and tissues is difficult to predict based on in vitro biochemical data, it unlikely that most approved compounds inhibit only a single PI3K isoform in the clinical setting.
Thus, idelalisib has only 36-fold selectivity for PI3Kδ over PI3Kγ30. Duvelisib is closely related to idelalisib (differing only by 4 atoms) and is more potent, but less isoform-selective and is described as a dual PI3Kγδ inhibitor, with 11- and 34-fold selectivity for PI3Kδ over PI3Kγ and PI3Kβ, respectively31. It is therefore likely that idelalisib as well as duvelisib inhibit both PI3Kγ and δ in patients, at least for significant periods during drug dosing. In contrast, the second-generation PI3Kδ inhibitor umbralisib has much greater isoform selectivity (PI3Kα, PI3Kβ: >1000 fold, PI3Kγ: 225 fold) and this may contribute to a better safety profile (discussed further below).
Copanlisib is closer to a pan-class I PI3K inhibitor, with similar levels of activity against PI3Kα and PI3Kδ, and about 7- or 13-fold more selective for PI3Kα over PI3Kβ or PI3Kγ, respectively23.
While alpelisib exhibits the highest selectivity for PI3Kα, with 50-, 63- and 260-fold selectivity over PI3Kγ, PI3Kδ and PI3Kβ, respectively24,44, it is likely that some inhibition of PI3Kγ and PI3Kδ will occur, at least for some patients, during dosing of this drug.
PI3Kα: from biology to approved drugs
Physiological roles of PI3Kα
At the cellular level, a key function of PI3Kα is to convert growth factor stimulation into activation of anabolic processes (glucose uptake, glycolysis, nucleotide production, protein and lipid synthesis) and concomitant inhibition of catabolic processes (including autophagy). A key effector of PI3Kα in this response is AKT/PKB, a serine/threonine kinase with a myriad of substrates and pleiotropic functions. AKT is critical for transduction of growth factor stimulation through activation of the master regulator for cell growth, the mTORC1 protein kinase complex (which also receives class I PI3K-independent input from amino acids and glucose). Combined, AKT and mTORC1 set the stage for enhanced energy generation and biosynthetic activity, key requisites for cell proliferation and survival. The ensuing metabolic shift is associated with increased levels of several metabolites, including acetyl-CoA, that serve as substrates of chromatin-modifying enzymes45,46. This endows the PI3K pathway with the ability to elicit widespread transcriptional changes beyond those attributed to the action of individual signalling effectors.
The role of PI3Kα in the regulation of the cell cytoskeleton, for example through the regulation of GEFs and GAPs for small GTPases47 or actin-binding proteins such as gelsolin48, remains to be fully explored. Such an effect of PI3Kα has been implicated in glycolysis whereby PI3K activates Rac, resulting in actin cytoskeleton remodelling, allowing the release into the cytosol of actin-bound aldolase, a rate-limiting enzyme of glycolysis49.
The generation of mice in which endogenous PI3Kα was rendered inactive50 and the use of isoform-selective PI3K inhibitors51, positioned PI3Kα as the main insulin signalling PI3K isoform. Partial PI3Kα inactivation in mice leads to blunted insulin signalling, hyperinsulinaemia and glucose intolerance50, later found to be the main on-target adverse clinical effects of any inhibitor with activity against PI3Kα52.
PIK3CA in cancer
Genetic PI3Kα activation in cancer
PIK3CA is the most frequently mutated kinase in solid tumours (14% mutated across all cancers, but rarely in haematological malignancies53). Interestingly, normal endometrial epithelium also frequently carries oncogenic PIK3CA and PIK3R1 mutations, the burden of which increases with age and decreases with parity54.
Oncogenic mutations are present across PIK3CA,apart from the Ras-binding domain, but highly enriched for ‘hotspot’ mutations in the helical (E542K, E545K) and kinase (H1047R) domains55, which also have the strongest biological impact in experimental cell model systems compared to other PIK3CA mutations56. It is likely that different PIK3CA mutations have distinct biological outputs, as reported in a glioblastoma mouse model57, but this remains to be investigated in detail. Two activating mutations frequently co-occur in cis on the same PIK3CA allele58-60, the expression of which may render such cells more sensitive to PI3Kα inhibitors compared with cells with single-hotspot PIK3CA mutations58.
Oncogenic mutations in PIK3CA mimic and enhance dynamic events in the natural activation process of the auto-inhibited p85-p110 heterodimer15,61,62. Such processes can also be achieved by mutations in the p85 genes, most commonly in PIK3R163-65. PIK3R1 mutations, common in cancers such as endometrial carcinoma66, can also activate p110β and p110δ in addition to p110α65.
In mouse models, heterozygous PIK3CA mutation alone is a poor inducer of cancer, but is effective in combination with other oncogenic lesions, including mutated BRaf or KRas or loss of tumour suppressor genes such as Pten, Tp53 or Apc67. By contrast, transgenic over-expression of oncogenic PIK3CA can induce cancer on its own, correlating with the emerging evidence for the dose-dependency of genetic PI3K pathway activation in cancer68.
In some cancers (including breast69 and colon70), PIK3CA mutation can be an early, clonal event and thus present in all cells. In other cancers, PIK3CA mutation occurs at later stages of tumour evolution and is thus subclonal and not present in all tumour cells71. The latter has obvious therapeutic implications if PI3K inhibitors would only effectively target PIK3CA mutant cells. A substantial subset of human cancers has multiple copies of mutant PIK3CA68, in line with findings that cancers often acquire multiple oncogenic hits within the PI3K pathway72,73. Positive selection for oncogenic mutant allele imbalances is frequent in cancer and has also been documented for Ras and other oncogenes74,75. Evidence for a sharp, dose-dependent biological impact of the PIK3CAH1047R hot-spot mutation was documented in human induced pluripotent stem cell models, where PIK3CAH1047R heterozygosity led to negligible biological impact compared to homozygous expression68.
Wild-type PIK3CA is frequently amplified in some cancers53 such as endometrial76 and lung squamous carcinoma77, as part of an amplification of the 3q genomic locus. That PIK3CA amplification may have functional relevance is indicated by its ability to predict in vitro sensitivity to alpelisib in a cancer cell line panel44. However, in contrast to expression of mutant PIK3CA78,79, overexpression of wild-type p110α appears to have minor, if any, effects on PI3K pathway stimulation as assessed by phosphorylation of AKT/PKB, both under basal and growth-factor-stimulated conditions80,81. Overexpression of wild-type human PIK3CA does also not show transforming capacity in a chicken fibroblast assay, in contrast to oncogenically-mutated or membrane-targeted versions of human wild-type PIK3CA79. It is challenging, however, to overexpress p110α protein in cells, most likely because the limiting availability of p85 that is needed to stabilize the labile p110α protein.
Pleiotropic impact of PIK3CA mutation
In isogenic cancer cell lines, derived by disruption of the wild-type or mutant allele of PIK3CA78,82, PIK3CA mutation has multiple impacts: reduced growth factor dependence yet little effect on cell proliferation under nutrient-rich conditions78, increased in vitro cell migration and invasion78,83 and reduced sensitivity to starvation-induced apoptosis78 (Figure 5a). At the cellular level, the impact of activating PIK3CA mutations is context-dependent, and can range from no effect to enhancement of cell proliferation to cell senescence82,84-86 or even cell death87 (reviewed in Ref.88). Interestingly, in cells with functional p53, PIK3CA mutation leads to activation of p53-dependent growth suppression82.
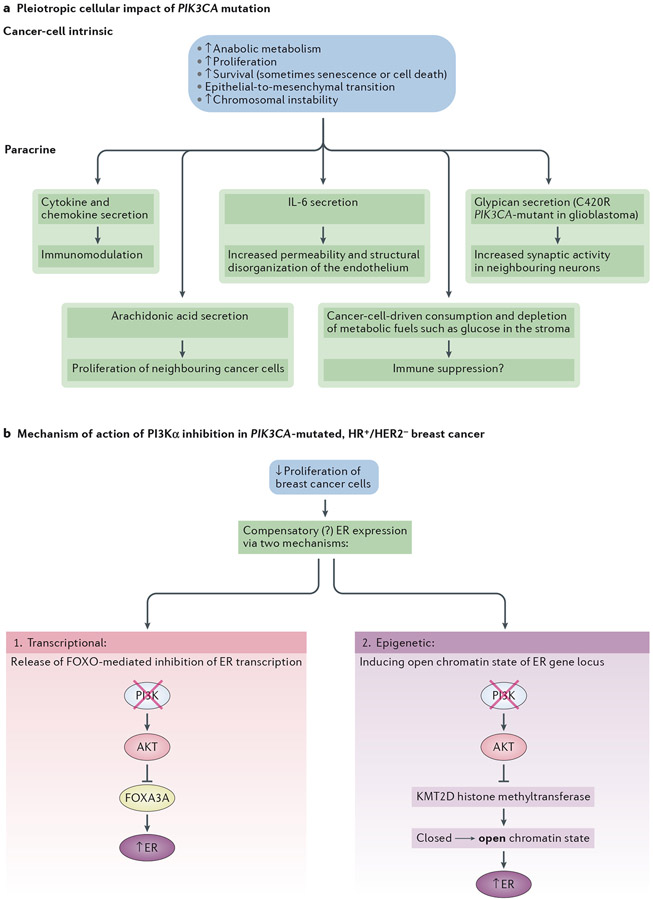
Figure 5 – Multi-pronged anti-cancer activity of PI3Kα inhibition in solid tumours.
a. Pleiotropic effect of PIK3CA mutation in solid tumours, inducing both cancer-cell intrinsic and paracrine effects.
b. Proposed mechanisms for the combinatorial anti-tumour effect of anti-PI3Kα and anti-oestrogen therapy in HR+/HER2− breast cancer. Anti-proliferation induced by PI3K inhibitionleads to a compensatory expression of the estrogen receptor (ER) and increased dependency on estrogen. The increase in ER transcription can occur via enhanced FOXO3A activity (which is no longer inhibited by active PI3K/Akt)121 and an epigenetic mechanism through the histone methyltransferase KMT2D which is inhibited upon phosphorylation by AKT361,362. Blockade of AKT by PI3Kα inhibition enhances KMT2D activity, leading to a more open chromatin state that facilitates ER-dependent transcription361. This epigenetic mechanism can also be transcriptional as it is proposed that KMT2D affects the occupancy of the transcription factor FOXA1, a key regulator of ER binding in breast cancer.
Accumulating evidence suggests that oncogenic PI3Kα activation supports the emergence of stem cell-like properties89. Activating PIK3CA mutations also promote invasive properties and epithelial-to-mesenchymal transition (EMT)80,83,90, which is strongly associated with induction of stemness, phenotypic plasticity and, ultimately, resistance to targeted therapy91-93. EMT may also facilitate tumour invasion and metastasis78,83. The mechanism(s) driving mutant PIK3CA-dependent cellular plasticity and EMT remain poorly understood, with a possible involvement of reciprocal dependency between the PI3K and TGFβ signalling pathways86,94-97.
Genetic PIK3CA activation may also induce and/or allow cells to tolerate chromosomal instability98, potentially facilitating and/or driving tumour evolution99.
There is also increasing evidence for paracrine effects of oncogenic PI3Kα activation. Transcriptional profiling of a PIK3CA-mutant derivative of the MCF10A breast cell line indicated the expression of PI3K-driven, NF-κB–dependent target-genes enriched in cytokines, chemokines or secreted proteins81. Upon overexpression of the Human Epidermal growth factor Receptor 2 (HER2) in these cells, PIK3CA mutation induced a complex secretome that promotes stem cell enrichment, angiogenesis, EMT, altered immune surveillance and vulnerability towards HSP90 inhibition86. PIK3CA mutation in breast cancer cell lines is associated with a lipogenic subtype that depends predominantly on mTORC2 activation, with intracellular and secreted arachidonic acid and its metabolites fueling cancer-cell intrinsic proliferation but also that of surrounding PIK3CA WT cells100.
PIK3CA mutation in cancer cells might also create an immunosuppressive stromal environment by induction of high glycolysis in cancer cells, leading to a high demand for glucose101,102 and subsequent depletion of metabolic fuels in the stroma,thus contributing to immune suppression103.
In a mouse model of glioblastoma, PIK3CAC420R-mutant glioblastoma cells affect neighbouring neurons through the secretion of glypican family proteins that can increase synaptic activity in neurons57, a phenomenon possibly related to the seizures often observed in glioblastoma. Likewise, the secretion of interleukin-6 by PIK3CAH1047R breast epithelial cells has been implicated in increasing permeability and structural disorganization of the neighbouring endothelium104.
Activating mutations in the PI3K/AKT/MTOR pathway commonly cause paediatric intractable epilepsy, which in mouse models has been shown to be driven by non-synaptic, neuron-intrinsic properties, involving calcium and potassium channels, and to be suppressible by acute treatment with PI3K pathway inhibitors105. PI3K pathway inhibitors therefore potentially represent novel anti-seizure therapeutics in this condition.
In conclusion, PIK3CA mutation has a diverse biological impact on cells beyond stimulation of cell proliferation.
PI3K inhibition in cancer
In vitro cytostatic effects
A common misconception is that PI3K inhibition leads to cancer cell death. However, this is not typically the case, at least upon continuous drug exposure of cancer cell lines in vitro where PI3K inhibitors in sensitive cell lines most often lead to inhibition of cell proliferation rather than cell death106-109. This arrest may be akin to a ‘dormant’ state, as observed upon inactivation of the AGE-1 class I PI3K catalytic subunit in C. elegans110. Cell-based studies with PI3K inhibitors have mainly used assays that measure protein content or metabolic activity of cells (for example, sulforhodamine B, MTT/MTS or CellTitre-Glo assays) which are not bona fide readouts of cell death. Published evidence for the induction of cell death by PI3K inhibitors mostly derives from PARP or caspase cleavage as measured by western blotting, however the levels induced are most often low and may only represent a small fraction of the total cell population (see for example Ref.111). However, significant cancer-cell cytotoxicity has been reported with some PI3K inhibitors, such as upon treatment with the pan-PI3K inhibitor copanlisib112 or upon intermittent dosing with the dual PI3Kα/δ inhibitor AZD8835113.
It should also be noted that PI3K inhibitor studies have mainly used conditions that do not reflect those in a tumour, with cells seeded at low density in 2D in nutrient-replete conditions under normoxia. Tumours in humans also have far longer doubling times than those used in xenograft models114,115.
Sensitivity to PI3Ka inhibitors
The presence of activating PIK3CA mutations is the clearest positive predictor of in vitro sensitivity of cancer cell lines to the anti-proliferative effect of alpelisib44 or the dual PI3Kα/δ inhibitors AZD8835113 and taselisib116. This correlation is not absolute but has held up well in breast cancer patients treated with alpelisib10. PIK3CA amplification is also an independent predictor of in vitro sensitivity to alpelisib44.
At the cellular level, intrinsic and acquired resistance to PI3K inhibitors is very common14 (BOX1). At the organismal level, the anti-proliferative effects of PI3K inhibitors are neutralised by compensation for the metabolic impact of PI3Kα inhibition. Indeed, PI3Kα inhibition in preclinical mouse models leads to reduced glucose uptake in insulin-responsive tissues such as adipose tissue and muscle, which results in hyperglycaemia and a compensatory insulin release from the pancreas, thereby dampening the effect of PI3K inhibition12.
BOX1 -. Cellular mechanisms of resistance to PI3K inhibitors.
PI3Kα inhibitors:
Resistance to PI3K inhibitors is often mediated by feedback loops, a non-genetic acute rebalancing of existing signalling pathways in the cell to neutralise the inhibitory effects, for example through compensatory upregulation of expression of tyrosine kinase receptors13,14. In vitro treatment of cell lines with alpelisib often leads to compensatory PI3Kβ activation314,315. Similarly, treatment with alpelisib of a patient with a PIK3CA mutant breast cancer led to recurrent PTEN loss in different metastases316. In order to overcome such compensatory mechanisms to isoform-selective PI3K inhibitors, so-called ‘balanced’ pan-PI3K/mTOR inhibitors that block all class I PI3K isoforms and mTOR pathway equally well, continue to be developed317. However, given the poor tolerance of such compounds when given systemically, these will most likely have to be administered topically, such as for skin diseases.
PI3Kα inhibitors often have a limited antiproliferative effect in cell lines with inactive PTEN44,318. Some of these cell lines have been shown to be instead sensitive to the antiproliferative effect of PI3Kβ inhibitors318-320, although this correlation is not universal321 (reviewed in Refs.322,323).
A range of resistance mechanisms linked to alterations in PI3K activities or PTEN themselves have also been reported, including amplification of Myc or eIF4E324, activation of the SGK Ser/Thr kinases (which are highly related to AKT)325,326, activation of cyclin-dependent kinases CDK4/6144, persistent expression of FOXM1141 and loss of negative regulators of mTORC1327. One study identified 63 putative alpelisib resistance genes, including activation of the PIM Ser/Thr kinases328. A genome-wide shRNA-based screen identified several genes whose suppression could convert the cytostatic effect of PI3K inhibition into a cytotoxic one109. Amongst these were the PIM2 and ZAK kinases, small molecule inhibitors of which were found to synergize with PI3K inhibition109. Remarkably, no drug-induced resistance mutations in the PIK3CA gene itself have been reported.
PI3Kδ inhibitors:
In CLL, primary resistance , i.e. failure to respond at all, may be associated with mutations in the RAS/RAF/MAP2K1 pathway that result in constitutive ERK activation329. Unlike for BTK inhibitors, cell lines or CLL tumours with acquired idelalisib resistance do not display unifying recurrent mutations that could be implicated in drug resistance330,331. Similar observations were made in a mouse model of PI3Kδ inhibitor-resistant CLL, which showed a very modest increase in acquired mutations (relative to drug-sensitive tumours), with little or no overlap between independently-derived tumours, and no mutations in PIK3CD itself332. This study suggested that IGF1R overexpression may be associated with PI3Kδ inhibitor-resistant CLL, and demonstrated constitutive ERK activation associated with that overexpression. Other than the likelihood that cancer-cell-intrinsic resistance to PI3Kδ inhibition can be achieved through multiple mechanisms possibly converging on alternate signalling pathway activation (e.g. ERK), these observations also indicate that the cancer-cell-intrinsic role of PI3Kδ may not be as critical in the observed anti-leukaemic effects of PI3Kδ inhibitors as is the case for BTK inhibitors.
Based on an ex vivo co-culture system of FL patient leukaemic cells mixed with FDCs from normal tonsils treated with idelalisib, Serrat et al.215 reported a gene signature that discriminates idelalisib-sensitive from idelalsisib-non-responsive cultures. It will be of interest to test the predictive value of this idelasib-score in clinical trials. This study also reported that idelalisib treatment renders the FL cells sensitive to BCL2 inhibitors, providing a mechanistic rationale for investigating the combination of PI3Kδ and BCL2 inhibition in FL215.
Clinical development of PI3Kα inhibitors
The approved PI3Ka inhibitor alpelisib
The main rationale for the use of PI3K inhibitors in oncology has been to target cancer-cell-intrinsic PI3K activity. Given the cytostatic effect of PI3K inhibitors on tumour cells106-109, their cell-intrinsic impact as single agents may primarily result in tumour stabilisation, rather than regression. Drug combination approaches have therefore been explored.
The most compelling of these approaches is in hormone-responsive breast cancer, a prime example of context/tissue-specific effects of PI3K inhibition88. Indeed, preclinical data have shown that PI3K pathway inhibition often mediates resistance to anti-oestrogen therapies117,118, observations in line with clinical data that demonstrated improved progression-free survival (PFS) in ER-positive breast cancer by combination treatment with the mTORC1 inhibitor everolimus and an aromatase inhibitor119 (reviewed in Ref.120). Likewise, PI3Kα-selective inhibitors enhance oestrogen pathway activity in breast cancer and increase their dependence on this hormone, through modulation of ER transcription in conjunction with epigenetic effects (Figure 5b)121.
Other targeted therapies also enhance the efficacy of endocrine therapy, including inhibitors of AKT122, mTOR or CDK4/6 (reviewed in Ref.123), some of which are likely to be contenders of PI3Kα inhibitors in this clinical setting.
The PI3Kα selectivity of alpelisib (Table 1)24 and its pharmacokinetics enabled successful trials in breast cancer, leading to the drug’s FDA approval in 2019124. The SOLAR-1 trial (NCT02437318)10 compared the effect of the ER antagonist fulvestrant with or without alpelisib, finding that combination treatment prolongs PFS among patients with PIK3CA-mutated, ER-positive, HER2 receptor-negative (HR+/HER2−) advanced breast cancer who had received prior endocrine therapy. Importantly, alpelisib did not affect PFS in patients without PIK3CA mutation.
At the mature analysis of the SOLAR-1 trial, the median overall survival was 39.3 months with alpelisib plus fulvestrant compared with 31.4 months with placebo plus fulvestrant125. The most frequent side-effects with alpelisib are hyperglycaemia, rash and diarrhoea126,127, which are manageable and reversible.
The SOLAR-1 trial did not have a PI3Kα inhibitor-only arm and a key question is how much of the observed clinical response is due to direct anti-cancer effects of PI3Kα inhibition and how much derives from the restoration of cellular sensitivity to anti-oestrogen therapy by PI3Kα inhibition.
Other PI3Ka-selective inhibitors in clinical trials
Taselisib (Genentech), a highly-potent dual PI3Kα/δ inhibitor25,128 progressed to Phase III studies in breast cancer (Table 2). However, development was discontinued due to modest clinical benefit and considerable adverse side effects, with 51.4% of patients stopping treatment due to gastrointestinal toxicities129, possibly due to PI3Kδ inhibition.
Using the same core and key amide as taselisib, inavolisib (Genentech; Figure 2e) was generated, with enhanced PI3Kα isoform selectivity, and is currently being trialled in breast cancer in combination with a range of endocrine and targeted therapies (NCT03006172; NCT04802759) and other solid tumours (NCT04632992). A study currently under peer review has reported taselisib and inavolisib to lead to degradation of the E545K and H1047R mutant p110α proteins in cells, an effect not seen with other PI3Kα-selective inhibitors such as alpelisib130. This degradation of mutant p110α appears to block feedback-induced PI3K pathway reactivation in cells, resulting in enhanced potency of taselisib and inavolisib, compared to other PI3K inhibitors in cancer cell and xenograft studies130. This inhibitor-induced p110α-mutant proteasome-mediated degradation is dependent on receptor tyrosine kinase activity in PIK3CA-mutant cells, and is especially prominent in cells with HER2 amplification130. HER2-targeted therapy is the standard of care for HER2-amplified breast tumors, with HER2-positive, PIK3CA mutant breast cancer known to be less responsive to HER2-targeted therapy. Based on these data, Genentech is now planning a clinical trial to test inavolisib in combination with HER2 antibodies.
Serabelisib (INK-1117/MLN-1117/TAK-117/ART-001; Figure 2f) is another selective PI3Kα inhibitor. Though not particularly potent against PI3Kα, requiring substantial doses, serabelisib has excellent isoform selectivity131 and favourable pharmacokinetics132. The structural basis for the PI3K isoform selectivity of serabelisib has not been described. This compound has been licenced to Artham for development for vascular malformations and to Petra Pharma for oncology. Artham plans a phase II trial in 2021 while Petra Pharma plans a phase Ib/II trial in solid tumours with PIK3CA or KRAS mutations in combination with a sodium-glucose transport protein 2 (SGLT2) inhibitor (NCT04073680), based on a concept published by the Cantley group12 (see below).
MEN1611 (CH5132799) (Figure 2g) is a less selective PI3Kα inhibitor with acceptable human pharmacokinetics 133 that is in Phase 1b/2 clinical trials for breast and colorectal cancers134.
Specific inhibition of one of the PIK3CA hot-spot mutants in a manner that spares the unmutated PI3Kα in non-cancerous cells is a tantalising prospect, however, this has not yet been achieved in practice.
Insights and opportunities
PI3Kα inhibitors in breast cancer
Future efforts in this area will focus on better patient selection, expansion into other breast cancer types and novel combinations. Given that PIK3CA mutations are common across the different types of breast cancer, including triple-negative breast cancer (TNBC)135, there is an interest in clinically exploring PI3K inhibition beyond HR+/HER2− breast cancer (reviewed in Refs.136,137).
It may also be possible to refine PIK3CA-based patient stratification strategies, for example by assessing the presence of composite PIK3CA mutations (which have been shown to render cells more sensitive to PI3Kα inhibition58) or mutant PIK3CA gene copy number68. Indeed, in a recent clinical study with the AZD5363 AKT inhibitor, homozygosity of the AKT1E17K mutation was associated with an improved therapeutic response138. Similar data have been shown for Ras, where cells with multiple copies of mutant Ras are more sensitive to MAP kinase inhibition139. Predictive biomarkers beyond PIK3CA status could include a transcriptional PI3K pathway activity score140 or FOXM1 expression141. The latter has been reported as a biomarker of both response and resistance to PI3Kα inhibition in ER-positive PIK3CA-mutant breast cancer, with FOXM1-driven expression of lactate dehydrogenase allowing a targeted metabolic tissue imaging approach141.
Following the approval of alpelisib, multiple trials testing additional combinations with hormone therapy and other agents in breast cancer are now in progress or have been opened. PIK3CA mutation has been implicated in resistance to fulvestrant-CDK4/6 inhibitor combination therapy142,143. This is the basis for the BYLieve trial (NCT03056755) to test alpelisib in combination with hormone therapy in this population of previously-treated breast cancer patients. Conversely, the finding that the CDK4 pathway also mediates resistance to PI3K inhibitors in PIK3CA-mutant preclinical models144, has lead to trials evaluating the combination of inavolisib with endocrine therapy and palbociclib (CDK4/6 inhibitor) in breast cancer (NCT04191499). Another combination of alpelisib is with chemotherapy. Around 10% of TNBC are PIK3CA-mutant, further enriched in patients with apocrine or luminal tumors145. In patients with metastatic TNBC, the EPIK-B2 trial (NCT04251533) is comparing paclitaxel to paclitaxel plus alpelisib in patients with PIK3CA mutation.
PIK3CA mutations also occur in around 30% of breast cancer with amplification of the ERBB2-gene146, the target of the trastuzumab/herceptin anti-HER2 antibody, and PI3K pathway alterations have been associated with resistance to trastuzumab147. Based on these data, a phase III randomized trial has started to compare maintenance anti-HER2 therapy with or without alpelisib in patients with PIK3CA-mutant ERBB2-amplified breast cancer (NCT04208178). Based on the observation that involisib preferentially degrades mutant p110α protein in HER2-amplified cells130, this PI3K is now being tested in HER2+ breast cancer in combination with a range of endocrine therapies or targeted agents such as a CDK4/6 inhibitor, metformin and HER2 antibodies (NCT03006172; NCT04802759).
PI3Kα inhibitors in cancer beyond breast
Additional therapeutic opportunities for PI3Kα inhibitors beyond cancer include PROS, obesity and metabolic syndrome (BOX 2; Table 3). However, given that PI3Kα inhibition is mainly utilised in cancer, opportunities in this setting are described in more detail below (Table 3).
BOX 2 -. PI3Kα inhibitors in non-oncology indications.
PROS
Activating mutations in PIK3CA, similar to those in cancer, have been found in benign skin lesions (epidermal nevi and seborrheic keratoses)333 and in disorders belonging to the PIK3CA-related overgrowth spectrum (PROS; reviewed in Ref.334). The lack of cancer predisposition in these conditions illustrates the context-dependent impact of genetic PI3Kα activation88.
In most cases of PROS, PIK3CA mutations are acquired postzygotically and thus exhibit tissue mosaicism (i.e. the mutations are not present in all cells). The resulting overgrowth is asymmetric and highly variable, reflecting differences in the timing and location of mutation acquisition during development. Commonly affected tissues include adipose tissue and blood vessels, but also muscle, brain, bone and peripheral nerves334. The overgrown tissues often represent a mix of cells with wild-type and mutant PIK3CA expression, suggesting potential paracrine effects of PIK3CA-mutant cells towards their wild-type counterparts334 (Figure 5a). Evidence for the capacity of PI3K pathway mutant cells to induce lesion formation in a non-cell-autonomous manner has been reported in an AKT1-mutant-driven mouse model of the human Proteus overgrowth syndrome335. As mentioned above, PIK3CA mutation in cancer cells can result in the secretion of protein and lipid mediators that modulate the biology of surrounding neurons57, endothelial cells104 and wild-type cancer cells100. Paracrine effects upon loss of PTEN expression have also been reported336.
Treatment of PROS patients with low doses of alpelisib as part of a compassionate use program has shown a promising clinical impact (Novartis; NCT04085653) with patients experiencing negligible side effects337. This contrasts with the observations of a clinical trial of low-dose rapamycin (sirolimus) in PROS which reported only modest clinical benefit, and was associated with a considerable number of adverse effects that led to frequent treatment discontinuation338.
It remains to be seen if alpelisib treatment will be tolerated in a wider population of PROS patients and alleviate the different tissue overgrowths in PROS to the same extent. A clinical trial with well-defined endpoints has now opened to address these questions (NCT04589650/NCT04085653).
Obesity and metabolic syndrome
While heterozygous genetic PI3Kα inactivation in mice leads to adverse metabolic effects at young age50, such chronic partial PI3Kα inactivation protects older mice from age-related reduction in insulin sensitivity, glucose tolerance and fat accumulation339. Chronic partial pharmacological PI3Kα inactivation also did not lead to major toxicities or side effects in mice340. PI3K inhibitors also reduce obesity in mice and monkeys341,342, attributed to increased energy expenditure as a consequence of activation of thermogenesis in brown adipocytes343 and increased oxidative phosphorylation together with reduced anaerobic glycolysis344. Upregulation of mitochondrial activity in mouse adipocytes (as well as in Drosophila fat bodies)345 and potentiation of β-adrenergic/cAMP signalling in these cells that leads to increased catecholamine-induced energy expenditure346, have also been implicated in the beneficial metabolic effects of partial PI3Kα inactivation.
These data indicate that moderate pharmacological inhibition of PI3Kα could be a therapeutic strategy for obesity and metabolic syndrome in humans. While not clear whether this will be tested in a formal clinical trial, it is possible that supportive data will be borne out by clinical trials in other disease settings, especially if these treatments (such as in PROS) would involve long-term administration of low doses of PI3Kα inhibitors (as compared to the maximum-tolerated doses used in a cancer setting).
Table 3:
Emerging clinical opportunities for PI3K inhibitors
| Therapeutic strategy |
Therapeutic area |
Disease indication | Expected effect of drug |
|---|---|---|---|
| PI3Kα inhibitors | Cancer | Solid tumours, most effective in PIK3CA-mutant cancers? (key indications in breast cancer, head and neck cancer and ovarian cancer)s | Direct anti-proliferative effects on cancer cells |
| Potentiation of hormone therapy (breast cancer) | |||
| Overcoming anti-HER2 resistance (breast cancer) | |||
| Sensitization to PARP inhibitors or paclitaxel (ovarian and breast cancer) | |||
| Anti-angiogenesis? | |||
| immunomodulation? | |||
| Non-cancer | PROS | Reduction of tissue overgrowth | |
| Anti-seizure effects | |||
| Obesity and metabolic syndrome | Decrease in adiposity | ||
| PI3Kδ inhibitors | Cancer | B-cell malignancies | Direct anti-tumour effects (anti-proliferative / non-cytotoxic) |
| Interference with B-cell/stroma interaction | |||
| Solid tumours (most effective in ‘immune hot’ tumours) | Activation of host anti-tumour immune response | ||
| Direct anti-tumour effects in PI3Kδ-expressing cancers? | |||
| Non-cancer | APDS | Normalisation of deregulated immune signalling as consequence of PI3K activation | |
| Auto-immunity/inflammation? | Normalisation of overactive immune signalling | ||
| Diabetic retinopathy? | Dampening of angiogenesis and immunomodulation in endothelial cells | ||
| Infectious diseases such as Leishmania | Enhanced innate myeloid cell responses | ||
| Dampened regulatory T and B lymphocyte responses | |||
| Pan-PI3K inhibitors | Cancer | B-cell malignancies | Direct anti-tumour effects (non-cytotoxic) |
| Interference with B-cell/stroma interactions | |||
| Activation of a host anti-tumour immune response? | |||
| Solid tumours | Direct anti-tumour effects | ||
| Interference with how tumour cells modulate their stroma? |
The mechanism underpinning PI3K inhibitor and hormone combination therapy in breast cancer is compelling (Figure 5b). The mechanistic rationale for other combination approaches with PI3K inhibitors such as with chemotherapy, radiation and targeted therapy is not always entirely clear, other than the obviously clinically important observation that resistance against these therapies can be overcome by PI3K inhibitors in preclinical studies3.
A combination approach with a clear mechanistic rationale is provided by the finding that PI3K inhibition can inhibit homologous recombination through downregulation of BRCA1/2 expression, leading to increased DNA damage and enhanced poly ADP-ribosylation, resulting in sensitization to PARP inhibitors148,149. Evidence has been presented that this downregulation of BRCA1/2 gene expression is due to ERK-dependent activation of the ETS transcription factor148,149. In addition, the PI3K pathway is key in the production of nucleotides for DNA synthesis, the synthesis of which could be blocked by PI3K inhibitors, which could be problematic for cells under conditions that require DNA repair such as when BRCA1/2 levels are low. Based on this rationale, alpelisib has been combined with the PARP inhibitor olaparib in a phase I trial (NTC01623349). This combination was found to be feasible and led to 34% objective responses in BRCA1 wild-type ovarian cancer patients150.
Around 15% of gastric cancers present a PIK3CA mutation, with PIK3CA mutations being enriched in EBV-positive subtypes151. A phase I/II trial is currently testing the combination of alpelisib with paclitaxel in this molecular subgroup (NCT04526470). In addition, PIK3CA mutation/amplification is found in 21% of head and neck cancers, and in 56% of the HPV+ subset of head and neck cancers152. The combination of paclitaxel and alpelisib in patients with head and neck cancer is currently being evaluated (NCT02051751). Combination of radiation with alpelisib153 or GDC-0032154 has also shown promising results in preclinical head and neck cancer studies.
An upcoming trial (NCT04073680) will test the combination of PI3Kα and SGLT inhibitors in solid tumours. Indeed, PI3K inhibitors reduce glucose uptake in insulin-responsive tissues such as muscle and adipose, leading to an excess in circulating glucose. This results in a compensatory insulin release from the pancreas which partially negates the anti-tumour effects of PI3K inhibition in cancer cells12. SGLT inhibition, which helps to reduce systemic glucose levels by blocking re-uptake of glucose by the kidneys from the urine into the blood, enhances the anti-cancer effect of PI3K inhibitors in preclinical models12.
Anti-angiogenic and immunogenic effects of PI3Kα inhibition
Given the ubiquitous expression of PI3Kα, its inhibition is expected to affect the tumour stroma, including fibroblasts and endothelial cells155. PI3Kα blockade can dampen or normalize tumour angiogenesis in preclinical models156,157, which might be achieved at PI3Kα inhibitor doses which do not affect the tumour cells themselves157, similar as observed with low doses of the RAD001/everolimus mTORC1 inhibitor158. PI3Kα inhibitors would also dampen the paracrine, potentially tumour-promoting effects of PIK3CA-mutant cells discussed above.
Isoform-selective PI3Kα-inhibitors have little or no effect on lymphocytes131 and other leukocyte types, and are therefore expected to not directly affect the immune response. However, evidence is emerging that PI3Kα inhibitors can indirectly modulate the immune response, in line with emerging evidence that drugs, initially developed to target the cancer cells themselves, also have immunomodulatory effects that can be exploited in cancer therapy (reviewed in Ref.159). PI3Kα inhibition by BYL719, especially in combination with CDK4/6 inhibitors, altered the cytokine and increased expression of MHCI/II proteins on the cancer cell surface, which may increase antigen presentation of tumour antigens160. These data are in line with emerging evidence that PI3K inhibition enhances the induction of cell surface MHC I and II molecules by IFNγ161,162. The triple combination of BYL719, CDK4/6 inhibition and immune checkpoint inhibitors induced complete and durable tumor regression of a syngeneic TNBC cancer model in mice, correlating with increased activation of both adaptive and innate immunity and a decreased frequency of immune-suppressive MDSCs within the tumour environment160.
Additional indirect ways of immunomodulation by PI3Kα inhibition might result from a reduction in glucose utilisation by the cancer cells101,102, leading to enhanced availability of metabolic fuels in the stroma for immune cells103, and thus remove tumour-induced metabolic constraints on immune cells (Figure 6a). PI3Kα inhibitors could also modulate the immunomodulatory secretome induced by PIK3CA expression81.
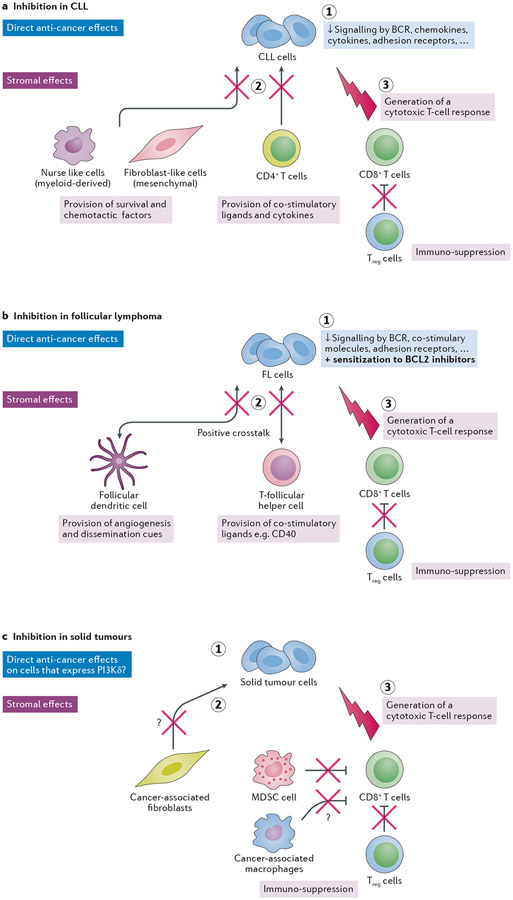
Figure 6 – Multi-pronged anti-cancer activity of PI3Kδ inhibition in cancer.
a. Proposed triple mode-of-action of PI3Kδ inhibition in CLL: (1) a cancer-cell intrinsic impact, with PI3Kδ dampening signalling by the BCR and a range of cytokines, chemokines, co-stimulatory molecules and adhesion receptors; (2) inhibition of stromal cells that support the leukaemic cells, such as myeloid-derived nurse-like cells, mesenchymal fibroblast-like cells and leukaemia-associated T-cells, and (3) a host anti-leukaemia adaptive immune response, as a consequence of dampening of Treg function upon PI3Kδ inhibition.
b. Documented effects of PI3Kδ inhibition in FL: (1) a cancer-cell intrinsic impact, with PI3Kδ dampening signalling by the BCR, the CD40/CD40L pathway as well as restoration of FL cell dependence on the BCL2 anti-apoptotic protein, resulting in a predisposition to FL cell death and sensitivity to BLC2 inhibitors; (2) dampening of recruitment of T-follicular helper cells and Treg cells through downmodulation of the CCL22 chemokine; and downregulation of proteins involved in B–T-cell synapses, leading to an inefficient crosstalk between FL cells and T-follicular helper cells; (3) dampening of follicular dendritic cell-FL interactions related to angiogenesis, cell adhesion and transendothelial migration in FL patients that show a clinical response to PI3Kδ inhibition. (4) a host anti-leukaemia adaptive immune response, as a consequence of dampening of Treg function upon PI3Kδ inhibition. Such a PI3Kδ-inhibition induced immune response has to be formally documented in FL.
c. Effect of PI3Kδ inhibition in solid tumours: (1) a cancer cell-intrinsic impact: some solid tumours (such as breast and melanoma) express high levels of PI3Kδ which may provide sensitivity to PI3Kδ inhibition. (2) dampening of the immuno-suppressive effects of MDSCs and macrophages, and dampening of cancer-stimulating fibroblasts and macrophages, and (3) preferential inhibition of Treg cells, allowing a CD8+ T-cell immune response to develop. The question marks in the figure indicate that the role of PI3Kδ in the indicated responses requires further validation, with inhibition of PI3Kγ likely to have a stronger suppressive impact on macrophages than inhibition of PI3Kδ, and blockade of PI3Kα and/or PI3Kβ having a stronger impact than PI3Kδ inhibition on fibroblasts.
Overall, these data are in line with observations mentioned above, that pulsatile pan-PI3K inhibition with copanlisib or BAY1082439 in a range of preclinical syngeneic cancer models induces favourable anti-tumour immunomodulatory effects163. Similar data have been reported with the PI3Kα/β/δ inhibitor BAY1082439 in PTEN-null tumour models164. Evidence for induction of favourable immune profile changes by AKT inhibitors in breast cancer has also been reported165.
PI3Kα inhibitor dosing regimens
Thus far, PI3K-targeted therapies in cancer have been mainly based on the principle of continuous drug dosing at the maximum-tolerated dose defined in phase I trials. Alternative dosing regimens are being explored to increase the tolerability of PI3K inhibitors, while at the same time achieving sufficient PI3K pathway inhibition.
In a preclinical study in mice, encapsulation of alpelisib/BYL719 into P-selectin-targeted nanoparticles led to drug accumulation in the tumour milieu, resulting in tumour growth inhibition and radiosensitization at lower doses of BYL719 compared with oral administration, and without inducing the metabolic side effects normally observed after BYL719 treatment153.
Intermittent dosing is another approach to improve PI3K drug tolerance. This is illustrated by the PI3Kα inhibitor serabelisib: in phase I studies, only intermittent dosing led to an acceptable safety profile and also enabled higher doses and total weekly exposures as compared to once-daily dosing132. An intermittent dosing schedule is also being used for the pan-PI3K inhibitor copanlisib166,167 which may be feasible due to the long half-life of copanlisib as a result of high volume of distribution and low clearance. In animal studies, copanlisib has shown marked accumulation in tumours over plasma167. This accumulation has been ascribed to the sequestration of the basic copanlisib molecule in acidic tumour tissue. If true clinically, this would mean that all four class I PI3K isoforms were inhibited in tumour tissue over the entire dosing interval.
Interestingly, intermittent dosing can, at least in part, convert the cytostatic effect of PI3K inhibitors into a cytotoxic one, with pulsatile dosing of the PI3Kα/δ inhibitors GDC-0941168 or AZD8835113 or the pan-PI3K inhibitor copanlisib112 inducing some level of tumour cell apoptosis in xenograft studies. While the therapeutic impact of such single-agent PI3K inhibitor dosing remained modest112,113,168, this approach may be better tolerated and allow drug combination therapies.
PI3K-based therapy assumes that all cancer-promoting effects of PI3K are reversible. However, while PIK3CA mutation might be critical at certain stages during cancer evolution, for example to tolerate the negative impact of ongoing chromosomal instability98, it may no longer be required once the cancer cell has adapted to its new genomic configuration. Such a role of genetic PIK3CA activation could be exploited by using PI3K pathway inhibitors to dampen cancer progression and evolution at any stage, and would be expected to be most effective in tumours with clonal PI3K activation such as breast cancer. Importantly, this might be achievable at lower drug doses than the maximum-tolerated doses of PI3K inhibitors currently used in the clinic99.
Interestingly, pulsatile pan-PI3K inhibition with copanlisib or BAY1082439 in a range of preclinical syngeneic cancer models induces favourable anti-tumour immunomodulatory effects163,164. A key question is whether such effects could also be achieved by PI3Kα-selective inhibitors.
PI3Kδ: from biology to approved drugs
PI3Kδ in health and disease
The highly-enriched expression of PI3Kδ in all leukocyte types has endowed this PI3K with roles in immunity and haematological malignancies. These functions are summarized below, and have turned out to be highly intertwined in the clinic.
PI3Kδ in immunity
Preclinical studies using p110δ KO/KI mice and early-generation PI3Kδ inhibitors169 revealed roles for PI3Kδ in diverse immune functions, suggesting the potential for PI3Kδ inhibitors in autoimmune and inflammatory disorders5,6,170-173, and allowing the development of cell-based assays for PI3Kδ drug development programmes. These include B-cell activation assays174-176 and anti-IgE-mediated basophil degranulation tests177, which have also been used to monitor the impact of PI3Kδ inhibition in whole blood assays from patients (as exemplified in Ref.32).
PI3Kδ is functionally-dominant in lymphocytes whereas PI3Kγ plays a more important role in myeloid cells, although this distinction is not absolute5. In the context of an in vivo immune response, leukocytes are confronted with a range of concurrent stimuli acting through different receptor mechanisms, with PI3Kδ and PI3Kγ often working together to generate a functional output, first documented in neutrophils178. Such partnership of PI3Kδ extends to PI3Kβ, as illustrated by the cooperation of these PI3Ks in neutrophil activation by immune complexes179.
Although PI3Kα plays a minor role in lymphocyte signalling131,180, it compensates for PI3Kδ inhibition in B-cell development in mice131,181 and in human B-cell malignancies182,183. Interestingly, PI3Kβ is expressed at very low levels in B-cells181.
PI3Kδ mutation in immune disorders
Patients with homozygous bi-allelic deletion or loss-of-function mutations in PIK3CD demonstrate various forms of immunodeficiency, characterised by a profound block in B-cell development and a range of immune dysregulatory diseases including sinopulmonary infections, opportunistic pneumonias, inflammatory bowel disease, autoimmune hepatitis and juvenile idiopathic arthritis184-188. Bi-allelic loss of PIK3R1 (p85α) has also been reported and leads to a block in B-cell development189.
The first report of a PIK3CD mutation in humans with immunodeficiencies was published in 2006, but the functional impact of this E1021K mutation on PI3Kδ was not assessed at the time190. Heterozygously-expressed, activating germline mutations in PIK3CD are now known to cause the Activated PI3Kδ Syndrome (APDS)191 primary immunodeficiency, also known as PI3Kδ-activating mutation causing senescent T-cells, lymphadenopathy and immunodeficiency (PASLI)192 193-195.
Splice site mutations in PIK3R1 that lead to skipping of exon 11 resulting in a small in-frame deletion (amino acids 434–475) of the p85α inter-SH2 domain, result in a clinical phenocopy of APDS/PASLI, referred to as APDS2. This deletion ablates some of the structural inhibitory activities of p85 on the p110 subunits, leading to their de-inhibition196,197. These mutant p85α proteins preferentially activate p110δ and not p110α or p110β196,197, and therefore mainly act in the immune system. p85α proteins are ubiquitously expressed, and the selective immune impact of APDS2 mutations may relate to the observation that p110δ, compared to p110α and p110β, preferentially associates with p85α over p85β198.
Immune-related defects in APDS patients frequently include lymphadenopathy and sinopulmonary infections, with an increased predisposition to autoimmune and inflammatory complications, and lymphoma193,195. APDS patients present symptoms of both immune deficiency and autoimmunity, indicating the need for a careful balancing of organismal PI3Kδ signalling, with too little or too much PI3Kδ activity having a deleterious immune impact193,199,200.
Several mouse models with APDS mutations have been generated199-205, providing further insight into how unbalanced PI3Kδ activity leads to immune dysregulation.
PI3Kδ in B-cell lymphoma
PI3Kδ was positioned as a potential drug target in haematological malignancies, particularly B-cell malignancies, based on high PI3Kδ expression in B-cells and defects in B-cell development and function being the most apparent phenotype in mice with inactive PI3Kδ174-176.
Mutational activation of PI3Kδ is a rare event in haematological malignancies. However, the E1021K mutation in PIK3CD, which is functionally equivalent to the H1047R mutation in PIK3CA191, is present at low frequency in diffuse large B-cell lymphoma206 and T-cell acute lymphoblastic leukemia207.
Although PIK3CD is not mutated in CLL and FL, these cells show constitutive PI3K pathway activation as a consequence of chronic B-cell antigen receptor (BCR) activation and microenvironmental stimuli208-210. This is likely the basis for the superior clinical impact of PI3Kδ inhibition in this setting, compared to other haematological malignancies, as detailed below.
PI3Kδ inhibition in cancer
3-pronged action of PI3Kδ inhibition
In B-cell lymphoma, the therapeutic impact of PI3Kδ inhibition derives from a dual, most likely triple, mode-of-action (Figure 6a,b).
The first is a cancer-cell intrinsic impact, a key factor being that some B-cell malignancies (such as CLL and FL) remain highly dependent on PI3Kδ, similar to non-transformed B-cells. Such reliance of cancer cells on a single signalling pathway, and in this case on a single PI3K isoform is rare, and not observed in other cancer contexts211. This creates a unique vulnerability specifically in the B-cell malignancies in which the BCR is required for maintenance and survival. In the B-cell malignancies for which PI3Kδ inhibitors have been approved, there is no correlation between clinical drug efficacy and previously defined high-risk genetic groups. Similar to PI3Kα inhibitors, multiple cellular resistance mechanisms have been described (BOX 1).
Other than depending on PI3Kδ, BCR signalling is also regulated by the BTK, LYN and SYK tyrosine kinases212. Although blockade of BCR signalling is considered to be key to the therapeutic impact of inhibitors of these kinases, evidence is emerging that each of these inhibitors shows a distinct, pleiotropic mode-of-action in cancer therapy that does not fully overlap with that of PI3Kδ inhibitors213 212. PI3Kδ inhibition also interferes with the response of malignant B-cells to a range of cytokines, chemokines, co-stimulatory molecules and adhesion receptors, which support leukaemic cell maintenance and homing. Several of these stimulatory factors are provided by the surrounding stroma.
A likely second element of the anti-cancer activity of PI3Kδ inhibition is a direct negative impact on leukaemia-supporting stromal cells, and counteraction of microenvironment-derived proliferation and survival signalling pathways. This is best documented in CLL214 and FL215 , which both exist in complex niches containing a range of cancer-supporting cell types.
In CLL, these stromal cells include myeloid-derived nurse-like cells and mesenchymal fibroblast-like cells214,216,217 (Figure 6a). PI3Kδ inhibition in these PI3Kδ-expressing cells217 dampens their capacity to provide leukaemia-supporting signals. Leukaemia-associated T-cells can also be leukaemia-promoting by providing CD40 ligand (CD40L), IFNγ, and other stimulatory agonists for CLL cells (Figure 6a). Treatment of CLL cells in vitro with idelalisib abrogates signalling and survival induced by CD40L or TNFα208. Treatment of normal T-cells in vitro with idelalisib reduces production of IL-6, IL-10 and TNFα208. In patients, decreases in circulating cytokines and chemokines produced by both the CLL and stromal cells are observed following initiation of PI3Kδ inhibitors209,218 (Figure 6a). Disruption of the stroma-tumour interactions most likely underlies the characteristic lymphocytosis observed using PI3Kδ inhibition in CLL whereby both normal lymphocytes and leukaemic cells leave their lymph node and bone marrow niches to enter the circulation, resulting in an increase in the circulating white blood cell count upon initiation of therapy. The release of leukaemic cells from their protective niches into the blood is expected to increase cell death from loss of survival-promoting stimuli and render these cells more vulnerable to combination therapy, such as with anti-CD20 antibodies or bendamustine chemotherapy.
FL is also characterized by a strong dependence on micro-environmental cues provided by proliferating B-cells and a broad range of supportive cells, including several types of follicular T-cells: T follicular helper (TFH) cells with strong pro-survival activity through the CD40L/IL4 axis, immunosuppressive T follicular regulatory (TFR) cells, follicular reticular cells, tumor-associated macrophages (TAMs) and follicular dendritic cells (FDCs) displaying antigen presentation (Figure 6b). Notably, both TAMs and FDCs express lectins that activate stereotypic mannosylated residues in the BCR of FL cells, leading to autonomous signalling219. PI3Kδ inhibition interferes with this tumour-promoting FL micro-environmental crosstalk, including disruption of FDC-induced angiogenesis and dissemination cues, TFH-induced proliferation and recruitment of Treg cells via downmodulation of CCL22. The overall result of PI3Kδ inhibition is a less supportive and tolerogenic immune microenvironment (Figure 6b)215.
A third facet of the anti-cancer action of PI3Kδ inhibition is a potential host anti-leukaemia immune response (Figure 6a,b). Indeed, an unexpected observation was that systemic PI3Kδ inhibition in preclinical cancer models in mice, including in leukaemia, leads to enhanced anti-tumour immune responses220-228. Pharmacological inactivation of PI3Kδ showed similar effects, also on tumour cell lines resistant to the in vitro anti-proliferative effect of PI3Kδ inhibitors and/or which do not express PI3Kδ224. The contribution of a host immune response to the anti-tumour effect of PI3Kδ inhibition in leukaemic patients remains to be determined. This potential of PI3Kδ inhibition is being explored in cancer immunotherapy in solid tumours (see below).
Mechanistic studies revealed that PI3Kδ inactivation allowed mice to raise an adaptive anti-tumour immune response, through preferential inhibition of the immunosuppressive regulatory T-cell (Treg) population over the CD4+ T-helper cells and CD8+ T-cells224 (reviewed in Ref.229), leading to a ‘rebalancing’ of the adaptive immune system towards a CD8+ T-cell response (Figure 6c). This preferential inhibition of Treg cells upon PI3Kδ blockade was subsequently confirmed in other studies in mice220,222,223,226,227,230,231, in ex vivo human T-cell subpopulations from healthy donors232, in idelalisib-treated CLL patients232,233 (with patients who experience toxicity displaying a trend towards a lower Treg percentage and a lower Treg:CD4 ratio compared to patients without toxicity233,234) and most recently in a phase II trial in head and neck cancer (AMG-319; NCT02540928)235. At present, it is not clear why Tregs are more sensitive to PI3Kδ inhibition compared to other T-cell populations. This differential impact on T-cell populations is most likely complex, similar to the impact of PI3Kδ inhibition on CD4+ T-cell differentiation in mouse models which leads to both immunodeficiency and immune activation, in a context-dependent manner236.
PI3Kδ inhibition also dampens the activity of cancer-promoting myeloid-derived suppressor cells (MDSCs)224 and cancer-associated macrophages237, which also reduces the capacity of the latter cells to produce reactive oxygen species that result in the death of Natural Killer cells238. It is therefore likely that regulation of both the adaptive and innate immune system underlies the host anti-cancer immune response induced by PI3Kδ inhibition.
Clinical development of PI3Kδ inhibitors
Approved PI3Kδ inhibitors
IC87114, the first PI3Kδ-selective ATP-competitive small molecule inhibitor, was reported in 2003 (Ref.169). Studies with IC87114 and the first clinical candidate CAL-101 (Calistoga) provided some evidence for an in vitro anti-proliferative impact of PI3Kδ inhibition in leukaemic cell lines and patient-derived leukaemic cells30,208,209,239-243. The initial phase 1 trial of CAL-101 included B-cell malignancies and AML. In line with an important role of PI3Kδ in B-cells, early signs of therapeutic efficacy were mainly observed in B-cell malignancies, particularly in CLL and indolent Non-Hodgkin’s lymphoma218,244. Response rates were high and responses were durable in heavily-pretreated patients with these diseases.
In 2011, Gilead Sciences acquired Calistoga and continued clinical development of CAL-101 (renamed GS-1101/idelalisib/Zydelig), culminating in its approval in 2014 for the treatment of CLL, relapsed follicular B-cell lymphoma (FL) and relapsed small lymphocytic lymphoma (SLL), following impressive results for idelalisib monotherapy245 and combination trials with rituximab, an antibody directed against the CD20 B-cell surface marker246 (Table 1).
In 2017, the intravenously-administered pan-class I PI3K inhibitor copanlisib (Bayer) was approved for adult patients with relapsed FL, followed by approval of the orally-available dual PI3Kγ/δ inhibitor duvelisib (Verastem) in 2018 for adult patients with relapsed or refractory CLL or SLL as well as those with relapsed FL.
In 2020, the FDA granted a fast track designation to umbralisib in combination with the investigational CD20-directed monoclonal antibody ublituximab for CLL, and in 2021, the FDA granted accelerated approval to umbralisib for marginal zone (MZ) lymphoma and FL.
PI3Kδ inhibitors in development
Inhibitors with improved PI3Kδ-selectivity have now been developed (Table 2), with multiple candidates progressing to clinical studies7. The principal focus has been for haematological malignancies, though there has also been considerable interest in treating immunological/inflammatory conditions including rheumatoid arthritis, COPD, allergic asthma, psoriasis, Sjögren's syndrome, allergic rhinitis247 and airway inflammation248 (Table 2). The most selective PI3Kδ inhibitors to reach clinical trials are parsaclisib (Figure 4f) and nemiralisib (Figure 3a) and most likely also IOA-244 (iOnctura; Supplementary Figure 1a). A key question is whether drug chemistries other than idelalisib will result in similar toxicities.
Unlike all other PI3K inhibitors, which bind to the ATP site, and can be classified as type I kinase inhibitors, the PI3Kδ inhibitor IOA-244 (iOnctura; Supplementary Figure 1a) is non-ATP competitive. Though not particularly potent, IOA-244 must have a different binding mode representing the first so-called type 4 PI3K inhibitor. The expected consequences of this unique binding mode are the potential for very high PI3K isoform and overall kinase selectivity, with fewer off-target side-effects17,249. However, its in vitro selectivity is only modest against PI3Kβ (20 fold) and not exceptionally high against PI3Kα (130-fold) and it will be of interest to see the PI3K isoform inhibitor profile of this unique allosteric PI3Kδ inhibitor in patients. This compound started phase I studies as monotherapy or in combination with pemetrexed/cisplatin, focused on solid tumours with high expression of PI3Kδ protein, namely metastatic melanoma, mesothelioma or ocular/uveal melanoma (NCT04328844).
The orally available parsaclisib (Incyte) (Figure 4f) is in multiple trials for B-cell malignancies, and in combination with anti-PD1 antibodies in a range of advanced solid tumour indications (NCT02646748/NCT03589651). Although completed, results of a study of parsaclisib in Sjögren’s syndrome (NCT03627065) have not been reported. Incyte is also exploring the use of parsaclisib in myelofibrosis (NCT02718300/NCT04551053/NCT04551066).
Nemiralisib (GSK) (Figure 3a) has been developed for inhaled administration and has pharmacokinetic properties unsuitable for oral or IV use. Nemiralisib showed lack of clinical efficacy in COPD, and its further development is currently on hold. Other inhaled agents under development include the dual PI3Kγδ inhibitor AZD8154 (AstraZeneca; Supplementary Figure 1h)250 and the PI3K inhibitor CHF6523 of undisclosed profile (Chiesi; Supplementary Figure 1i)251; which are being investigated in asthma (NCT04187508) and COPD (NCT04032535), respectively.
Umbralisib (Figure 4e) (TGR-1202; TG Therapeutics)8 has is progressing in CLL, FL and MZ lymphoma9. Notably, MZ B-cell development and residence in the spleen was one of the clearest phenotypes upon genetic or pharmacological inactivation of PI3Kδ in mice175,176,252. Umbralisib is highly selective for PI3Kδ and also inhibits casein kinase 1ε253, both of which are likely to contribute to a different safety profile to that of other PI3Kδ inhibitors, as explained below.
Zandelisib (ME-401; MEI Pharma; Supplementary Figure 5j) exhibits high selectivity for PI3Kδ254, and is in a phase II registration trial, also for FL.
Another advanced agent is leniolisib (CDZ173; Novartis; now licenced to Pharming) (Figure 3b), which is in phase III for APDS255 (NCT02435173/NCT02859727). A dose-finding trial with leniolisib found evidence for immune normalisation and overall patient benefit with no discernible adverse effects255. The latter might be related to the specific condition of pre-existing overactive PI3Kδ that is being normalised by this targeted drug treatment. Recruitment has started with a larger cohort of up to 30 APDS patients, not insignificant for a rare disease. However, leniolisib did not show clear efficacy in Sjögren’s syndrome (NCT02775916)256. Seletalisib (UCB-5857) (Figure 4d), another PI3Kδ-selective inhibitor which exhibits good selectivity against PI3Kα and PI3Kβ32 has also been investigated in Sjögren’s syndrome (NCT02610543, Phase II), but development in this indication appears to have ceased257. In addition, seletalisib entered a proof-of concept study in APDS1 and APDS2 patients, in which it demonstrated modest activity and significant side effects (liver injury, colitis, infections)258. Such adverse effects were not observed in the APDS patients in the trials with leniolisib mentioned above255, for reasons that are currently unclear.
Other notable agents in development include the PI3Kδ inhibitor YY-20394/linperlisib (Shanghai Yingli Pharmaceutical; structure not disclosed), which is being investigated in both haematological and solid tumours and AMG319 (Figure 4g; Amgen) which has been tested in a window-trial in Head and Neck cancer (NCT02540928)235.
Several other companies are also exploring the utility of PI3Kδ inhibitors or dual PI3Kγ/δ or PI3Kβ/δ inhibitors in haematological malignancies, as monotherapy or in combination with other targeted agents or chemotherapy (reviewed in Ref.259; Table 2).
Another approach to reduce PI3K activity, is by activation of SHIP1. This phosphatase is mainly found in leukocytes and hydrolyses PIP3 to PI(3,4)P2 (Figure 1). Activation of SHIP1 functionally acts to reduce PI3K signalling which, in leukocytes, is principally mediated by PI3Kγ and PI3Kδ. Activation of SHIP can thus reduce the activity of these two PI3K isoforms. AQX-1125 (rosiptor; Supplementary Figure 1k)260,261 is an allosteric activator of SHIP1 that was investigated in phase II clinical trials for asthma262 and other inflammatory conditions, however all development was stopped after a phase III trial for interstitial cystitis and bladder pain failed263.
Challenges and opportunities
Below, we discuss the future of PI3Kδ inhibitors, highlighting approaches to address the key challenges of drug toxicity and tolerability, and present clinically-advanced opportunities for PI3Kδ inhibitors. Preclinical data suggests additional potential therapeutic opportunities that have yet to be tested in the clinic (BOX 3).
BOX 3 – Emerging therapeutic indications for PI3Kδ inhibition.
There is preclinical evidence to suggest that PI3Kδ-targeted therapy during the early, acute phase of some infectious diseases (such as Leishmania) could be therapeutically useful, exploiting the immunostimulatory impact induced by acute PI3Kδ inhibition through enhanced innate myeloid cell responses and dampened regulatory T and B lymphocyte responses347. This fits with the emerging concept of using kinase inhibitors as a ‘third arm’ in infectious disease, i.e. when antibiotics or vaccines are unavailable or not an option – which is often the case in an acute setting of infection.
Early studies showed that although highly enriched in all types of white blood cells, PI3Kδ can also be present in non-leukocytes289, mostly at lower levels than in white blood cells and possibly expressed from an alternative promotor which can be activated by inflammatory stimuli such as TNF348,349. These include neurons, endothelial cells and fibroblasts, with potential new therapeutic opportunities for PI3Kδ inhibitors. Diverse biological functions of PI3Kδ in these cells have been reported, such as intracellular vesicle trafficking and cytokine production, with possible functional roles in neuronal regeneration350-352 and schizophrenia349,353, angiogenesis and immunomodulation in endothelial cells (including in pathological retinal angiogenesis354,355) and inflammatory/immunomodulatory functions in fibroblast-like cells such as synoviocytes in arthritic joints348,356-359. PI3Kδ inhibition could also have an antitumour effect by suppressing tumour-promoting PI3Kδ-expressing fibroblast-like cells, namely mesenchymal fibroblast-like cells in CLL216,217 and cancer-associated fibroblasts in breast cancer359 (Figure 6).
Managing PI3Kδ inhibitor toxicities
Immunomodulation by early generation PI3Kδ inhibitors has resulted in adverse effects which hampered clinical progress in this area. These toxicities may relate to the drug characteristics and doses used (compounds not being fully selective for PI3Kδ over other PI3K isoforms, degradation of drugs resulting in metabolites with liver toxicity, use of maximum-tolerated doses which may not be required for interference with tumour:stroma interactions or immunodulation) as well as the patient population tested, with a tendency for toxicities to be lower in heavily-pretreated and/or elderly cancer patients, i.e. people with reduced immune competency.
The incomplete PI3K isoform selectivity of first generation inhibitors for PI3Kδ over PI3Kγ and/or p110β may very well have contributed to the observed toxities of these compounds. In this context, it is of interest to mention that genetic inactivation of both PI3Kγ and PI3Kδ in mice leads to severe autoimmunity and inflammation and was very poorly tolerated264. Inactivation of PI3Kδ in mice does not lead to obvious immune-related adverse effects, including auto-immunity, other than the predisposition to colitis176, which is exaggerated by non-SPF mouse housing conditions265,266.
Early trials with idelalisib in haematological malignancies enrolled mostly heavily-pretreated patients having had more than one prior treatment with chemotherapy. In these populations, the adverse effects were mostly manageable, and included infections at typical rates seen in those patients, as well as relatively rare and likely immune-related colitis, hepatotoxicity and pneumonitis 233,267,268 which were responsive to steroids. In follow-up trials in treatment-naïve and often younger patients, idelalisib was associated with more severe autoimmune adverse effects that were treatment limiting233, 269. Adverse effects observed upon PI3Kδ inhibition, mainly observed using idelalisib, included bacterial infections (possibly in part secondary to drug-induced neutropenia), opportunistic infections (fungal infections and reactivation of cytomegalovirus (CMV) and inflammatory/autoimmune toxicities including colitis, hepatotoxicity/transaminitis and pneumonitis233,267,268, which may result from an overactive immune response in tissue locations exposed to external immunogens (skin, lung, bowel and liver). Given the complex immune impact of PI3Kδ inhibition, with elements of both immune suppression and activation236, explaining the adverse effects observed is challenging. At the time of these trials, the immuno-modulatory activities of PI3Kδ inhibition were under-appreciated, as were the potential toxicities of combination therapies using novel agents270. Retrospectively, it would have been advisable to mandate antimicrobial prophylaxis in idelalisib trials271. This is standard procedure when testing immunomodulatory agents in potentially immune-compromised patients but was not mandatory in these trials.
There has also been speculation that the primary liver metabolite of idelalisib, GS-563117272, an inhibitor of the CYP3A cytochrome P450 isoform, could be a possible cause of toxicity in this organ. This was also suggested for another idelalisib-glutathione (GSH) adduct273. Evidence has been presented for a differentiated safety profile of parsaclisib relative to idelalisib and duvelisib, with a near absence of grade ≥2 transaminitis/hepatotoxicity230,274. On the other hand, an individual who had congenital loss of both PIK3CD alleles was reported to have autoimmune hepatitis187, arguing that clinical toxicity of PI3Kδ inhibitors may be at least partially due to an on-target effect of PI3Kδ inhibition, potentially exacerbated by activated CD8+ T-cells (Figure 6). Additionally, in the clinical study of idelalisib with ofatumumab in which early fulminant hepatotoxicity was seen, liver biopsies were performed in two patients, both of whom showed a liver infiltrate with activated CD8+ T-cells268. Increased transaminitis levels were also correlated with reduced Treg233. Furthermore, recurrence of transaminitis with resumption of idelalisib was blocked by concomitant corticosteroids, consistent with an immune-mediated mechanism.
An interesting observation is that the PI3Kδ inhibitor umbralisib (TG Therapeutics) appears to lead to fewer adverse events such as colitis compared to idelalisib and duvelisib8,275. This may relate to the fact that (1) umbralisib is very selective for PI3Kδ over PI3Kα and PI3Kβ and has an approximately 225-times greater selectivity over PI3Kγ (Table 1); (2) umbralisib, unlike most other kinase inhibitors, is not metabolized through the classical cytochrome P450 3A4 (CYP3A4) metabolic pathway276, and that (3) umbralisib also inhibits casein kinase 1ε (CKε)253. Inhibition of CKε on its own improves CLL Treg number and function277. CKε inhibition by umbralisib may therefore protect Tregs from the inhibitory effects of PI3Kδ blockade277. A better preservation of the number and function of Treg cells in CLL patients may translate to reduced immune-mediated side effects of umbralisib compared to other PI3Kδ inhibitors.
PI3Kδ inhibitor treatment is now considered a safer treatment option than at the time of the early clinical trials259. Antimicrobial prophylaxis as well as CMV monitoring has now been included in the guidelines for clinical use of PI3Kδ inhibitors271. Frequent monitoring for early treatment-emergent neutropenia, with the option to employ growth factors to correct it, is also indicated. Colitis, diarrhoea and transaminase elevations are now often manageable with dose interruption/reduction or drug discontinuation, particularly if identified early, and by the use of corticosteroids, including either systemic or non-absorbable budesonide for colitis278.
Recent analysis indicated idelalisib monotherapy to be suitable for heavily-pretreated relapsed/refractory FL patients, given the unmet need in these patients279. This conclusion is based on a post hoc subgroup analysis of such patients enrolled in an idelalisib monotherapy trial245 in which the benefits in increased PFS were considered to outweigh the safety concerns in this setting279.
Another reported potential side-effect of PI3Kδ inhibition is induction of genomic instability in B-cells, through activation of AID (activation-induced cytidine deaminase) which promotes DNA recombination280. These observations raised concerns about a potential mutagenic risk in patients upon long-term PI3Kδ inhibitor therapy. The extent of this biological effect in the clinic is unclear – particularly as many patients discontinue due to toxicity after short treatment times - and it remains to be determined if these biological activities are found in clinical CLL samples. Indeed, similar effects on AID of the BTK inhibitor ibrutinib, first documented in normal and neoplastic B-cell lines, are not mirrored in primary CLL samples281. It is also possible that the anti-tumour effects of PI3Kδ inhibition will outweigh this potential negative side effect.
Improving PI3Kδ inhibitor dosing regimens
Finding tolerable drug dosing regimens is a key challenge to the further development of PI3Kδ inhibitors, together with the generation of inhibitors with improved PI3Kδ isoform-selectivity.
Auto-immune and inflammatory diseases likely require lower PI3Kδ inhibitor doses than in cancer. Lower drug doses may still enable patients to mount immune responses to exogenous immunogens, as illustrated in mice where low doses of PI3Kδ inhibitor were shown to be effective in genetic models of auto-immunity, including the NOD model of type 1 diabetes, without fully inhibiting T-cell responses282. Such dosing could be intermittent, whereas for APDS which is associated with permanent genetic PI3Kδ activation, continuous dosing appears the most sensible approach.
Administration of PI3Kδ inhibitors in B-cell malignancies was initially based on the principle of continuous dosing at the maximum-tolerated drug dose, defined in standard dose finding phase I trials and based on the rationale to block as much cancer-cell intrinsic PI3Kδ as possible. However, these doses are not well-tolerated long-term, and guidelines for the management of adverse events associated with idelalisib treatment in B-cell malignancies are now available267,271,278, with data showing no adverse clinical impact of dose interruptions. Indeed, PFS was significantly improved in FL and CLL patients who had 1 or more treatment interruptions compared to those with none, as long as time off therapy was <8%, with overall survival also improved in CLL patients283. This finding may in part be related to duration on therapy, although it also raises the possibility that these patients exhibiting toxicity are also developing an adaptive anti-tumour response (Figure 6), as described above.
Companies are now adapting PI3Kδ inhibitor dosing and scheduling regimens, which might also facilitate combination therapies284. Non-continuous/intermittent dosing of parsaclisib and zandelisib/ME-401 (MEI Pharma) in B cell malignancies, has been reported to lower the toxicity profile of these agents274,285.
Inflammatory/autoimmune diseases
The complex immune impact of PI3Kδ inhibition, inducing elements of both immune activation and suppression, creates a challenge for chronic systemic PI3Kδ inhibition. It is possible that low doses of inhibitor are required, in order to retain overall immune responsiveness, as illustrated in a preclinical study282. Topical routes of drug administration have also been explored, for example by inhalation of the PI3Kδ inhibitor nemiralisib (GSK) for inflammatory airway diseases286. However, the development of this compound in this disease indication has now been terminated due to lack of clinical efficacy. The dual PI3Kγ/δ inhibitor duvelisib also failed to meet its primary endpoint in allergic asthma (NCT01653756) and in rheumatoid arthritis (NCT01851707)287. Further clinical development in immune dysregulation focuses on alllergic asthma (AstraZeneca, with the dual PI3Kγ/δ inhibitor AZD8154; NCT04187508) and COPD (Chiesi; with CHF-6523, a PI3K inhibitor with undisclosed PI3K isoform selectivity; NCT04032535).
B-cell malignancies
The development of PI3Kδ inhibitors for B-cell malignancies fits with the quest for non-chemotherapy-based therapies for these diseases. The efficacy of PI3Kδ inhibitors appears to be tightly linked to dependence of CLL and FL on chronic BCR signalling which is critically dependent on PI3Kδ, and is likely to be more limited in more aggressive B-cell malignancies that have activated additional cell survival pathways.
Given that most patients have partial remissions with monotherapy kinase inhibitors, interest in defining combination therapies that enable deeper remissions and discontinuation of therapy is high. While some combinations are likely safe, such as with antibodies to CD20 in relapsed CLL288, others, particularly with chemotherapy, are not without their risks270, especially in treatment-naïve patients268. PI3Kδ inhibition restores the dependence of FL cells on the anti-apoptotic protein BCL2215, providing a rationale for combined PI3Kδ and BCL2 inhibition.
It is now well-established that many CLL patients, despite showing responsive disease upon treatment with kinase inhibitors, often discontinue treatment because of adverse effects. Having access to drugs with differential toxicity profiles allows clinicians to switch these so-called kinase inhibitor-intolerant CLL patients to other drugs. An example is the use of umbralisib in CLL patients who have become intolerant to BTK inhibitors or other PI3Kδ inhibitors276. Moreover, as mentioned above, survival benefit is still observed upon treament interruption283, indicating that effective PI3Kδ inhibitor therapy might not require continued drug administation in order to be clinically effective.
Cancer immunotherapy of solid tumours
The main rationale for use of PI3Kδ inhibitors in solid tumours is the potentiation of an adaptive anticancer immune response (Figure 6c). The high expression of non-mutated PI3Kδ in some solid tumours such as melanoma or breast289 has recently also received renewed attention, with evidence emerging from xenograft studies in mice that cancer cell-intrinsic PI3Kδ may provide sensitivity to PI3Kδ inhibition237,290 (reviewed in Ref.291).
A report currently under peer-review of a window trial in head and neck cancer using AMG-319, has provided formal evidence for the cancer immunotherapy potential of PI3Kδ inhibition in human solid tumours235. This trial evaluated biomarkers suggestive of anti-tumour immune responses and, consistent with the results obtained in mice221,224,229, AMG-319 decreased tumour-infiltrating immunosuppressive Treg cells and caused heightened cytotoxic potential of tumour-infiltrating CD8+ and CD4+ T cells235. This also led to immune-mediated adverse effects, including skin rash suggesting that alternative dosing regimens will be required to effectively and safely exploit the immunomodulatory impact of PI3Kδ inhibition in human solid cancers. These adverse effects were distinct from and more severe than those experienced by AMG-319-treated lymphoma patients who had undergone prior therapies, suggesting that patients who have not previously been treated with immunosuppressive chemotherapy are more sensitive to PI3Kδ inhibitors235.
The immunomodulatory dose of PI3Kδ inhibitors is likely to differ from the maximum-tolerated dose often favoured in oncology, and intermittent dosing may also be most effective. Of interest, intermittent administration of the dual PI3Kα/δ inhibitor AZD8835 induces potent immune-mediated anti-tumour responses in syngeneic solid tumour models in mice292. Pulsatile dosing of the pan-class I PI3K inhibitors copanlisib or BAY1082439 also generates an effective anti-tumour immune response in a range of animal models163,164.
At present, there is no evidence to suggest that the break in immune tolerance induced by PI3Kδ inhibition results in sustained auto-immunity, as auto-reactive immune symptoms disappear upon termination of PI3Kδ inhibitor treatment, although this can take several months233,246. This contrasts with treatment with checkpoint inhibitors which often results in long-term auto-immunity293-296. Moreover, in contrast to checkpoint antibodies, which remain in the circulation for weeks, interruption of PI3Kδ inhibitor dosing allows rapid reversal of systemic inhibition, of critical importance upon occurrence of adverse side effects.
A handful of PI3Kδ inhibitors have been/are being studied in solid tumours but it is unlikely that PI3Kδ inhibition will be as effective as a monotherapy. One combination might be with checkpoint inhibitors, which have shown efficacy in preclinical mouse models combining pharmacological PI3Kδ inhibition with anti-CTLA4 or anti-PD1225,297, with or without radiation298. The combination with anti-PD1 is being explored in solid tumours with parsaclisib (NCT02646748/NCT03589651). Given that PI3Kδ signalling might be required for signalling reactivation in exhausted T-cells by checkpoint therapy299,300, such treatment might be most effective when used sequentially rather than concomitantly. This is also suggested by the observation that anti-PD1 antibodies do not show effective anti-cancer activity in PI3Kδ-deficient mice227. Following the observation of strong upregulation of the immune checkpoint receptor LAG-3 on Treg cells in tumours that escaped the inhibitory effects of PI3Kδ blockade, PI3Kδ inhibitor treatment followed by administration of anti-LAG3 antibodies was found to induce a superior anti-cancer effect in syngeneic mouse cancer models in which PI3Kδ inhibition induced a partial initial anti-tumour response226.
In mouse models, tumour-infiltrating CSF1-receptor (CSF1R)-positive macrophages neutralize the anti-tumour impact of PI3Kδ inhibition, with combined inhibition of CSF1R and PI3Kδ being effective in inducing an anti-tumour response228. Several inhibitors of CSF1 signalling are being tested in the clinic, and are candidates for combination with PI3Kδ inhibitors.
Other immunotherapy-based avenues that have been explored in mouse models of cancer include combination of PI3Kδ inhibition with tumour-specific vaccines222,223 or oncolytic viruses301.
Lastly, PI3Kδ inhibitors could also be used during the expansion of T-cells for adoptive cancer immunotherapy. Indeed, blockade of the PI3K/AKT/mTOR pathway, including of PI3Kδ, during the in vitro expansion of T-cells for use in adoptive transfer dampens terminal differentiation of these cells222,302-310, allowing prolonged expansion in the patient. The underlying mechanism is not entirely clear, but evidence suggests that these inhibitors do not interfere with the in vitro proliferation of these T-cells, but instead maintains them in a less differentiated state that is less prone to exhaustion, the progressive loss of effector function.
Concluding remarks
Following the approval of PI3K inhibitors for haematological malignancies, the approval of a PI3Kα inhibitor for solid tumours has heralded a new era in PI3K drug development. Encouraging clinical data are also emerging from ongoing trials with AKT/PKB inhibitors in breast and prostate cancer, in combination with hormone therapy or the anti-microtubule agent paclitaxel122,138,311,312. Indications for PI3K inhibitors beyond cancer include overgrowth conditions, obesity and metabolic syndrome, as well as diabetic retinopathy (BOX2; Table 3).
It has become more widely appreciated that PI3K inhibitors are mainly anti-proliferative rather than cytotoxic for cancer cells, at least in vitro and in xenograft studies. It is possible, however, that these experimental conditions do not adequately reflect the in vivo situation where it cannot be excluded that PI3K inhibitors might lead to the demise of cancer cells due to a combined effect on the tumour cells and the tumour stroma, angiogenesis and the immune system157.
Emerging evidence suggests that PI3K inhibitors do not need to be administered continuously, and that intermittent dosing might not only be better tolerated but even more effective as an anti-cancer approach. Tolerability of PI3K inhibitors remains an issue, with adverse events including hyperglycemia/diarrhoea for PI3Kα inhibitors10 and a range of immune-related toxicities and infections for PI3Kδ inhibitors233,268. It is clear that improved PI3K isoform selectivity will be key to further development of this class of inhibitors, not least to fully understand their mechanims of action in patients.
For PI3Kα inhibitors, a key advance will be the identification of more tolerable drug dosing regimes and rational-based combination therapies beyond sensitization to hormone therapy in breast cancer, such as combination with PARP inhibitors. Multiple trials with PI3Kα inhibitors are now ongoing or planned.
PI3Kδ biology has turned out to be more complex than anticipated, with organismal PI3Kδ inhibition inducing elements of both immune activation and suppression, creating a challenge for chronic PI3Kδ inhibition in inflammatory and auto-immune diseases. At present, it is not entirely clear if some of the adverse effects result from co-inhibition by some of these compounds of other PI3K isoforms, especially PI3Kγ. The current utility of PI3Kδ inhibitors may therefore mainly lie in cancer, both in B-cell malignancies and solid tumours. PI3Kδ inhibitors in B-cell malignancies, where there is a strong rationale for both cancer-cell-intrinsic and stromal cancer-supporting roles of PI3Kδ (Figure 6a,b), are currently positioned as a therapeutic option after the failure of other novel agents and/or chemotherapy, because of their toxicity, but can be used safely with careful monitoring and use of prophylaxis259,313. However, the development of better tolerated dosing regimens and more effective combination therapies are likely required to bring this therapeutic option back to the forefront of clinical approaches259. An exciting observation in CLL patients is that treatment interruptions upon adverse events did not negatively affect clinical impact, and in fact improved overall survival283. It is tempting to speculate that at least some of the adverse events are a hallmark of induction of a host immune response, which could be be harnessed as an anti-tumour immune effect including in solid tumours (Figure 6c)235.
Supplementary Material
Acknowledgements:
This review is dedicated to the memory of Dr. José Baselga, a champion of PI3K-related research and its clinical translation, who left us so prematurely. We thank Sabina Cosulich (AstraZeneca) for support, Eneda Toska (Johns Hopkins, Baltimore, US) for feedback on Figure 5a, Patricia Peréz-Galán and Neus Serrat (IDIBAPS, Barcelona, Spain) for help with Figure 5c, Christoph Burkhart (Novartis, Basel) and members from the B.V. group for excellent feedback on the manuscript, especially Grace Gong, Ralitsa Madsen and Alex Sullivan. Work in the B.V. laboratory is supported by PTEN Research, Cancer Research UK (C23338/A25722), the UK Biotechnology and Biological Sciences Research Council (BB/I007806/1, BB/M013278/1, BB/R017972/1), and the UK NIHR University College London Hospitals Biomedical Research Centre. J.R.B. receives research funding from NCI R01 CA 213442 (PI: J.R.B.), Gilead Sciences, Verastem and TG Therapeutics. Relevant work in the K.O. laboratory was from the Wellcome Trust (095691/Z/11/Z) and the Medical Research Council (MR/M012328/2). We apologize to all authors whose work could not be mentioned because of the limitations on the number of references that could be cited.
Glossary
- Target therapies
Therapies aimed to selectively modulate the molecules that are deregulated in disease (as opposed to non-targeted therapies, such as for example chemotherapy).
- Intrinsic drug resistance
drug resistance that exists in the cells prior to drug therapy.
- Acquired drug resistance
drug resistance that develops in cells in response to a drug therapy to which the cells were originally sensitive.
- Anabolic metabolism
The cellular process of generation and storage of energy and cell building blocks.
- Catabolic processes
The mechanisms of degradation of cellular components, in order to generate energy and recycle building blocks for macromolecules.
- Autophagy
A cellular ‘self- eating’ process that involves the sequestering of cytoplasmic material in double-membrane structures (called autophagosomes), followed by membrane traffic to the lysosome for the degradation and recycling of cellular components.
- Hotspot mutations
Commonly recurring mutations in a gene in disease, often altering key characteristics or function of the mutated protein.
- Apoptosis
A form of programmed cell death, occurring during development and tissue remodellng, in which the cell internally degrades without rupturing the cell membrane, allowing the dead cells to be taken up and degraded by the surrounding cells.
- Cell senescence
A highly stable cell cycle arrest which limits the replication of damaged or aged cells.
- Epithelial-to-mesenchymal transition (EMT)
A process whereby epithelial cells change their characteristics (such as cell-cell interactions and shape) to become more like mesenchymal cells. EMT is key to embryonic development and is re-activated in carcinoma (cancers derived from epithelial cells), allowing them to become more malignant and to metastasize.
- Reciprocal dependency
A condition in which one system influences and depends on another, an vice versa.
- Chromosomal instability
A process of ongoing acquisition of genomic alterations, most often in cancer, including the gain or loss of whole chromosomes as well as structural aberrations which range from point mutations to small-scale genomic alterations and gross chromosomal rearrangements.
- Secretome
The complement of proteins secreted by cells in the extracellular environment.
- Cytostatic
Stopping cells from proliferating, without killing them.
- Poly ADP-ribosylation
The process of covalently attaching polymers of ADP-ribose to proteins by poly(ADP-ribose) polymerases (PARPs).
- Antigen presentation
The process of displaying peptides by binding these to the major histocompatibilty complex on the surface of antigen-presenting cells, so that these antigens can be effectively recognised and bound by the antigen receptor on the surface of T lymphocytes.
- Immune checkpoint
A surface protein of immune and sometimes cancer cells, that helps to stimulate or inhibit the responsiveness of T lymphocytes to antigens.
- Micro-environmental crosstalk
Functional interactions between the tumour cells and the cells and tissues that surround around them.
- Transaminitis
The presence of high levels of liver transaminase enzymes in the blood, most often a sign of liver dysfunction such as injury or inflammation.
- Genomic instability
Increased tendency for the acquisition of alterations in the genome.
- Oncolytic viruses
A virus that infects and kill cancer cells, often engineered using molecular biology techniques to optimize these characteristics.
Footnotes
Conflicts of interest: B.V. is a consultant for iOnctura (Geneva, Switzerland), Venthera (Palo Alto, US), Olema Pharmaceuticals (San Francisco, US) and has received speaker fees from Gilead (Foster City, US). M.P. is an employee and shareholder in AstraZeneca. J.R.B. has served as a consultant for Abbvie, Acerta, Astra-Zeneca, Beigene, Catapult, Dynamo Therapeutics, Genentech/Roche, Gilead, Juno/Celgene, Kite, Loxo, MEI Pharma, Novartis, Octapharma, Pfizer, Pharmacyclics, Sunesis, TG Therapeutics, Verastem; received honoraria from Janssen and Teva; received research funding from Gilead, Loxo, Sun and Verastem; and served on data safety monitoring committees for Morphosys and Invectys. F.A. received grants from AstraZeneca, Novartis, Pfizer, Eli Lilly, Daiichi Sankyo, Roche and served as consultant/speaker with fees donated to Institute Gustave Roussy, Paris from AstraZeneca, Novartis, Pfizer, Lilly, Daiichi Sankyo, Roche and Sanofi. F.A. is a founder of Pegacsy. K.O. receives research funding from GlaxoSmithKline (Stevenage, UK) and has received consultancy and speaker fees from Gilead (Foster City, US) and Karus Therapeutics (Oxford, UK).
References
- 1.Fruman DA et al. The PI3K Pathway in Human Disease. Cell 170, 605–635, doi: 10.1016/j.cell.2017.07.029 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilanges B, Posor Y & Vanhaesebroeck B PI3K isoforms in cell signalling and vesicle trafficking. Nat Rev Mol Cell Biol 20, 515–534, doi: 10.1038/s41580-019-0129-z (2019). [DOI] [PubMed] [Google Scholar]
- 3.Hennessy BT, Smith DL, Ram PT, Lu Y & Mills GB Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 4, 988–1004, doi: 10.1038/nrd1902 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Rommel C, Camps M & Ji H PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol 7, 191–201, doi: 10.1038/nri2036 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Hawkins PT & Stephens LR PI3K signalling in inflammation. Biochim Biophys Acta 1851, 882–897, doi: 10.1016/j.bbalip.2014.12.006 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Stark AK, Sriskantharajah S, Hessel EM & Okkenhaug K PI3K inhibitors in inflammation, autoimmunity and cancer. Current opinion in pharmacology 23, 82–91, doi: 10.1016/j.coph.2015.05.017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry MWD et al. Evolution of PI3Kgamma and delta Inhibitors for Inflammatory and Autoimmune Diseases. J Med Chem 62, 4783–4814, doi: 10.1021/acs.jmedchem.8b01298 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Burris HA 3rd et al. Umbralisib, a novel PI3Kdelta and casein kinase-1epsilon inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study. The Lancet. Oncology 19, 486–496, doi: 10.1016/S1470-2045(18)30082-2 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Umbralisib: Treatment for a Rare Lymphoma? Cancer discovery 9, OF5, doi: 10.1158/2159-8290.CD-NB2019-045 (2019). [DOI] [PubMed] [Google Scholar]
- 10. Andre F et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med 380, 1929–1940, doi: 10.1056/NEJMoa1813904 (2019). This paper describes the registration trial for alpelisib (Novartis), the first approved PI3Kα inhibitor, for use in breast cancer, with evidence for a better clinical response in PIK3CA mutant cancers.
- 11.Hanker AB, Kaklamani V & Arteaga CL Challenges for the Clinical Development of PI3K Inhibitors: Strategies to Improve Their Impact in Solid Tumors. Cancer discovery 9, 482–491, doi: 10.1158/2159-8290.CD-18-1175 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hopkins BD et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560, 499–503, doi: 10.1038/s41586-018-0343-4 (2018). Preclinical study that shows that PI3K inhibition in mice leads to a compensatory insulin secretion which negates the anti-tumour effects of PI3K inhibitors, an effect which can be overcome by dietary or pharmaceutical approaches.
- 13. Chandarlapaty S et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 19, 58–71, doi: 10.1016/j.ccr.2010.10.031 (2011). One of several reports to document that inhibition of PI3K/AKT signalling results in feedback/rebound signalling in this pathway, in this study through the activation of multiple receptor tyrosine kinases.
- 14.Rozengurt E, Soares HP & Sinnet-Smith J Suppression of feedback loops mediated by PI3K/mTOR induces multiple overactivation of compensatory pathways: an unintended consequence leading to drug resistance. Mol Cancer Ther 13, 2477–2488, doi: 10.1158/1535-7163.MCT-14-0330 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke JE Structural Basis for Regulation of Phosphoinositide Kinases and Their Involvement in Human Disease. Mol Cell 71, 653–673, doi: 10.1016/j.molcel.2018.08.005 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Vadas O, Burke JE, Zhang X, Berndt A & Williams RL Structural basis for activation and inhibition of class I phosphoinositide 3-kinases. Sci Signal 4, re2, doi: 10.1126/scisignal.2002165 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Haselmayer P et al. Characterization of Novel PI3Kdelta Inhibitors as Potential Therapeutics for SLE and Lupus Nephritis in Pre-Clinical Studies. Frontiers in immunology 5, 233, doi: 10.3389/fimmu.2014.00233 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walker EH et al. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell 6, 909–919, doi: 10.1016/s1097-2765(05)00089-4(2000). The first study to reveal the binding modes of a range of inhibitors in the active site of PI3Kγ, providing a basis for the development of isoform-specific PI3K inhibitors.
- 19. Berndt A et al. The p110delta structure: mechanisms for selectivity and potency of new PI(3)K inhibitors. Nat Chem Biol 6, 244, doi: 10.1038/nchembio0310-244b (2010). This work contained the first crystal structures of p110δ with multiple ligands and provides a basis for understanding and exploiting selectivity drivers for this target.
- 20. Huang CH et al. The structure of a human p110alpha/p85alpha complex elucidates the effects of oncogenic PI3Kalpha mutations. Science (New York, N.Y 318, 1744–1748, doi: 10.1126/science.1150799.(2007). The first reported crystal structure of PI3Kα.
- 21.Maira SM et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther 11, 317–328, doi: 10.1158/1535-7163.MCT-11-0474 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Burger MT et al. Identification of NVP-BKM120 as a Potent, Selective, Orally Bioavailable Class I PI3 Kinase Inhibitor for Treating Cancer. ACS medicinal chemistry letters 2, 774–779, doi: 10.1021/ml200156t (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scott WJ et al. Discovery and SAR of Novel 2,3-Dihydroimidazo[1,2-c]quinazoline PI3K Inhibitors: Identification of Copanlisib (BAY 80-6946). ChemMedChem 11, 1517–1530, doi: 10.1002/cmdc.201600148.(2016). The discovery of the pan-PI3K inhibitor copanlisib with careful optimisation of functionality around the scaffold.
- 24. Furet P et al. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg Med Chem Lett 23, 3741–3748, doi: 10.1016/j.bmcl.2013.05.007 (2013). This work showed how the optimisation of hydrogen bonding interactions at the perimeter of the ATP-binding pocket enabled the discovery of the selective p110α inhibitor NVP-BYL719 (Novartis), which later became the first approved PI3Kα inhibitor (renamed alpelisib/Piqray) (see also Ref. 44).
- 25.Braun M-G et al. Benzoxazepin Oxazolidinone Compounds and Methods of Use. WO2017001645 (2017). [Google Scholar]
- 26. Sutherlin DP et al. Potent and selective inhibitors of PI3Kdelta: obtaining isoform selectivity from the affinity pocket and tryptophan shelf. Bioorg Med Chem Lett 22, 4296–4302,.doi: 10.1016/j.bmcl.2012.05.027.(2012). Structurally enabled work exploring means of gaining selectivity for p110δ over other isoforms.
- 27. Down K et al. Optimization of Novel Indazoles as Highly Potent and Selective Inhibitors of Phosphoinositide 3-Kinase delta for the Treatment of Respiratory Disease. J Med Chem 58, 7381–7399,.doi: 10.1021/acs.jmedchem.5b00767 (2015). The discovery and optimisation of a highly PI3Kδ-selective series without making use of the induced selectivity pocket.
- 28.Collier PN et al. Structural basis for isoform selectivity in a class of benzothiazole inhibitors of phosphoinositide 3-kinase gamma. J Med Chem 58, 517–521, doi: 10.1021/jm500362j (2015). [DOI] [PubMed] [Google Scholar]
- 29.Come JH et al. Design and Synthesis of a Novel Series of Orally Bioavailable, CNS-Penetrant, Isoform Selective Phosphoinositide 3-Kinase gamma (PI3Kgamma) Inhibitors with Potential for the Treatment of Multiple Sclerosis (MS). J Med Chem 61, 5245–5256, doi: 10.1021/acs.jmedchem.8b00085 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Lannutti BJ et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 117, 591–594, doi: 10.1182/blood-2010-03-275305 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Winkler DG et al. PI3K-delta and PI3K-gamma inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol 20, 1364–1374, doi: 10.1016/j.chembiol.2013.09.017 (2013). Exploration of the effects of dual PI3Kγδ inhibition in models of autoimmune and inflammatory disease.
- 32.Allen RA et al. Seletalisib: Characterization of a Novel, Potent, and Selective Inhibitor of PI3Kdelta. J Pharmacol Exp Ther 361, 429–440, doi: 10.1124/jpet.116.237347 (2017). [DOI] [PubMed] [Google Scholar]
- 33. Yue EW et al. INCB050465 (Parsaclisib), a Novel Next-Generation Inhibitor of Phosphoinositide 3-Kinase Delta (PI3Kdelta). ACS medicinal chemistry letters 10, 1554–1560, doi: 10.1021/acsmedchemlett.9b00334 (2019). Detailed exploration and optimisation of inhibitors for PI3Kδ with both high isoform selectivity and good pharmacokinetics.
- 34.Williams O et al. Discovery of dual inhibitors of the immune cell PI3Ks p110delta and p110gamma: a prototype for new anti-inflammatory drugs. Chem Biol 17, 123–134, doi: 10.1016/j.chembiol.2010.01.010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perreault S et al. Discovery of a Phosphoinositide 3-Kinase (PI3K) beta/delta Inhibitor for the Treatment of Phosphatase and Tensin Homolog (PTEN) Deficient Tumors: Building PI3Kbeta Potency in a PI3Kdelta-Selective Template by Targeting Nonconserved Asp856. J Med Chem 60, 1555–1567, doi: 10.1021/acs.jmedchem.6b01821 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Chandrasekhar J et al. Atropisomerism by Design: Discovery of a Selective and Stable Phosphoinositide 3-Kinase (PI3K) beta Inhibitor. J Med Chem 61, 6858–6868, doi: 10.1021/acs.jmedchem.8b00797 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Evans CA et al. Discovery of a Selective Phosphoinositide-3-Kinase (PI3K)-gamma Inhibitor (IPI-549) as an Immuno-Oncology Clinical Candidate. ACS medicinal chemistry letters 7, 862–867, doi: 10.1021/acsmedchemlett.6b00238 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang H et al. Discovery of a Potent Class of PI3Kalpha Inhibitors with Unique Binding Mode via Encoded Library Technology (ELT). ACS medicinal chemistry letters 6, 531–536, doi: 10.1021/acsmedchemlett.5b00025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nacht M et al. Discovery of a potent and isoform-selective targeted covalent inhibitor of the lipid kinase PI3Kalpha. J Med Chem 56, 712–721, doi: 10.1021/jm3008745 (2013). [DOI] [PubMed] [Google Scholar]
- 40. Gangadhara G et al. A class of highly selective inhibitors bind to an active state of PI3Kgamma. Nat Chem Biol 15, 348–357, doi: 10.1038/s41589-018-0215-0 (2019). Detailed mechanistic studies using structurally related pairs of compounds enabled deeper insights into the mechanism of conversion between inactive and active states for PI3Kγ to be identified.
- 41.Pemberton N et al. Discovery of Highly Isoform Selective Orally Bioavailable Phosphoinositide 3-Kinase (PI3K)-gamma Inhibitors. J Med Chem 61, 5435–5441, doi: 10.1021/acs.jmedchem.8b00447 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Miller MS, Thompson PE & Gabelli SB Structural Determinants of Isoform Selectivity in PI3K Inhibitors. Biomolecules 9, doi: 10.3390/biom9030082 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He C et al. Characterization of a novel p110beta-specific inhibitor BL140 that overcomes MDV3100-resistance in castration-resistant prostate cancer cells. Prostate 77, 1187–1198, doi: 10.1002/pros.23377 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fritsch C et al. Characterization of the novel and specific PI3Kalpha inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol Cancer Ther 13, 1117–1129, doi: 10.1158/1535-7163.MCT-13-0865 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Lee JV et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab 20, 306–319, doi: 10.1016/j.cmet.2014.06.004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinnaird A, Zhao S, Wellen KE & Michelakis ED Metabolic control of epigenetics in cancer. Nat Rev Cancer 16, 694–707, doi: 10.1038/nrc.2016.82 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Stephens L, Milne L & Hawkins P Moving towards a better understanding of chemotaxis. Curr Biol 18, R485–494, doi: 10.1016/j.cub.2008.04.048 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Patel VB et al. PI3Kalpha-regulated gelsolin activity is a critical determinant of cardiac cytoskeletal remodeling and heart disease. Nature communications 9, 5390, doi: 10.1038/s41467-018-07812-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu H et al. Phosphoinositide 3-Kinase Regulates Glycolysis through Mobilization of Aldolase from the Actin Cytoskeleton. Cell 164, 433–446, doi: 10.1016/j.cell.2015.12.042 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Foukas LC et al. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature 441, 366–370, doi: 10.1038/nature04694 (2006). Together with Ref. 51 (Knight) - first formal demonstration of the critical role of PI3Kα in insulin signalling.
- 51.Knight ZA et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 125, 733–747, doi: 10.1016/j.cell.2006.03.035 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paddock MN, Field SJ & Cantley LC Treating cancer with phosphatidylinositol-3-kinase inhibitors: increasing efficacy and overcoming resistance. J Lipid Res 60, 747–752, doi: 10.1194/jlr.S092130 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y et al. A Pan-Cancer Proteogenomic Atlas of PI3K/AKT/mTOR Pathway Alterations. Cancer Cell 31, 820–832 e823, doi: 10.1016/j.ccell.2017.04.013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Moore L et al. The mutational landscape of normal human endometrial epithelium. Nature 580, 640–646, doi: 10.1038/s41586-020-2214-z (2020). The first documentation of the presence of oncogenic PIK3CA and PIK3R1 mutations in normal endometrial epithelium, long before the occurrence of detectable cancer.
- 55.Arafeh R & Samuels Y PIK3CA in cancer: The past 30 years. Seminars in cancer biology 59, 36–49, doi: 10.1016/j.semcancer.2019.02.002 (2019). [DOI] [PubMed] [Google Scholar]
- 56. Dogruluk T et al. Identification of Variant-Specific Functions of PIK3CA by Rapid Phenotyping of Rare Mutations. Cancer Res 75, 5341–5354, doi: 10.1158/0008-5472.CAN-15-1654 (2015). Assessment of the functionality of a broad range of PIK3CA variants, one of the widests mammalian cell-based screens of its kind.
- 57.Yu K et al. PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature 578, 166–171, doi: 10.1038/s41586-020-1952-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vasan N et al. Double PIK3CA mutations in cis increase oncogenicity and sensitivity to PI3Kalpha inhibitors. Science (New York, N.Y 366, 714–723, doi: 10.1126/science.aaw9032 (2019). First report on the existence of double PIK3CA mutations in cis, and how they lead to further increases in PI3K pathway activation.
- 59.Saito Y et al. Landscape and function of multiple mutations within individual oncogenes. Nature 582, 95–99, doi: 10.1038/s41586-020-2175-2 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Gorelick AN et al. Phase and context shape the function of composite oncogenic mutations. Nature 582, 100–103, doi: 10.1038/s41586-020-2315-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burke JE, Perisic O, Masson GR, Vadas O & Williams RL Oncogenic mutations mimic and enhance dynamic events in the natural activation of phosphoinositide 3-kinase p110alpha (PIK3CA). Proc Natl Acad Sci U S A 109, 15259–15264, doi: 10.1073/pnas.1205508109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burke JE, Perisic O & Williams RL Allosteric activation of PI3Kalpha by oncogenic mutations. Oncotarget 4, 180–181, doi: 10.18632/oncotarget.913 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Philp AJ et al. The phosphatidylinositol 3'-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res 61, 7426–7429 (2001). [PubMed] [Google Scholar]
- 64.Berenjeno IM & Vanhaesebroeck B PI3K regulatory subunits lose control in cancer. Cancer Cell 16, 449–450, doi: 10.1016/j.ccr.2009.11.017 (2009). [DOI] [PubMed] [Google Scholar]
- 65.Jaiswal BS et al. Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell 16, 463–474, doi: 10.1016/j.ccr.2009.10.016 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheung LW et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer discovery 1, 170–185, doi: 10.1158/2159-8290.CD-11-0039 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mitchell CB & Phillips WA Mouse Models for Exploring the Biological Consequences and Clinical Significance of PIK3CA Mutations. Biomolecules 9, doi: 10.3390/biom9040158 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Madsen RR et al. Oncogenic PIK3CA promotes cellular stemness in an allele dose-dependent manner. Proc Natl Acad Sci U S A 116, 8380–8389, doi: 10.1073/pnas.1821093116 (2019). The first formal documentation of functional allele dose-dependent consequences of mutant PIK3CA, and documentation of the occurrence of multiple oncogenic PIK3CA copies or additional PI3K signalling pathway-activating genetic lesions in human cancer (see also Ref. 58).
- 69.Berenjeno IM et al. Oncogenic PIK3CA induces centrosome amplification and tolerance to genome doubling. Nat. Comm 8, 1773, doi: 10.1038/s41467-017-02002-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gerstung M et al. The evolutionary history of 2,658 cancers. Nature 578, 122–128, doi: 10.1038/s41586-019-1907-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McGranahan N & Swanton C Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 168, 613–628, doi: 10.1016/j.cell.2017.01.018 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Stemke-Hale K et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res 68, 6084–6091, doi: 10.1158/0008-5472.CAN-07-6854 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oda K et al. PIK3CA cooperates with other phosphatidylinositol 3'-kinase pathway mutations to effect oncogenic transformation. Cancer Res 68, 8127–8136, doi: 10.1158/0008-5472.CAN-08-0755 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Bielski CM et al. Widespread Selection for Oncogenic Mutant Allele Imbalance in Cancer. Cancer Cell 34, 852–862 e854, doi: 10.1016/j.ccell.2018.10.003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mueller S et al. Evolutionary routes and KRAS dosage define pancreatic cancer phenotypes. Nature 554, 62–68, doi: 10.1038/nature25459 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shayesteh L et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet 21, 99–102, doi: 10.1038/5042 (1999). [DOI] [PubMed] [Google Scholar]
- 77.Cancer Genome Atlas Research, N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068, doi: 10.1038/nature07385 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Samuels Y et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell 7, 561–573, doi: 10.1016/j.ccr.2005.05.014 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Kang S, Denley A, Vanhaesebroeck B & Vogt PK Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci U S A 103, 1289–1294, doi: 10.1073/pnas.0510772103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Isakoff SJ et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res 65, 10992–11000, doi: 10.1158/0008-5472.CAN-05-2612 (2005). [DOI] [PubMed] [Google Scholar]
- 81.Hutti JE et al. Oncogenic PI3K mutations lead to NF-kappaB-dependent cytokine expression following growth factor deprivation. Cancer Res 72, 3260–3269, doi: 10.1158/0008-5472.CAN-11-4141 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim JS, Lee C, Bonifant CL, Ressom H & Waldman T Activation of p53-dependent growth suppression in human cells by mutations in PTEN or PIK3CA. Molecular and cellular biology 27, 662–677, doi: 10.1128/MCB.00537-06 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wallin JJ et al. Active PI3K pathway causes an invasive phenotype which can be reversed or promoted by blocking the pathway at divergent nodes. PLoS One 7, e36402, doi: 10.1371/journal.pone.0036402 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Astle MV et al. AKT induces senescence in human cells via mTORC1 and p53 in the absence of DNA damage: implications for targeting mTOR during malignancy. Oncogene 31, 1949–1962, doi: 10.1038/onc.2011.394 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu XL et al. Membrane metallo-endopeptidase mediates cellular senescence induced by oncogenic PIK3CA(H1047R) accompanied with pro-tumorigenic secretome. Int J Cancer 145, 817–829, doi: 10.1002/ijc.32153 (2019). [DOI] [PubMed] [Google Scholar]
- 86.Chakrabarty A et al. The H1047R PIK3CA oncogene induces a senescence-like state, pleiotropy and acute HSP90 dependency in HER2+ mammary epithelial cells. Carcinogenesis 40, 1179–1190, doi: 10.1093/carcin/bgz118 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Klippel A et al. Activation of phosphatidylinositol 3-kinase is sufficient for cell cycle entry and promotes cellular changes characteristic of oncogenic transformation. Molecular and cellular biology 18, 5699–5711, doi: 10.1128/mcb.18.10.5699 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Madsen RR & Vanhaesebroeck B Cracking the context-specific PI3K signaling code. Sci Signal 13, doi: 10.1126/scisignal.aay2940 (2020). [DOI] [PubMed] [Google Scholar]
- 89.Madsen RR PI3K in stemness regulation: from development to cancer. Biochem Soc Trans 48, 301–315, doi: 10.1042/BST20190778 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bonelli MA et al. Inhibition of PI3K Pathway Reduces Invasiveness and Epithelial-to-Mesenchymal Transition in Squamous Lung Cancer Cell Lines Harboring PIK3CA Gene Alterations. Mol Cancer Ther 14, 1916–1927, doi: 10.1158/1535-7163.MCT-14-0892 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Boumahdi S & de Sauvage FJ The great escape: tumour cell plasticity in resistance to targeted therapy. Nat Rev Drug Discov 19, 39–56, doi: 10.1038/s41573-019-0044-1 (2020). [DOI] [PubMed] [Google Scholar]
- 92.Shibue T & Weinberg RA EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nature reviews. Clinical oncology 14, 611–629, doi: 10.1038/nrclinonc.2017.44 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nassar D & Blanpain C Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annual review of pathology 11, 47–76, doi: 10.1146/annurev-pathol-012615-044438 (2016). [DOI] [PubMed] [Google Scholar]
- 94.Madsen RR et al. NODAL/TGFbeta signalling mediates the self-sustained stemness induced by PIK3CA (H1047R) homozygosity in pluripotent stem cells. Disease models & mechanisms, doi: 10.1242/dmm.048298 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Katsuno Y et al. Chronic TGF-beta exposure drives stabilized EMT, tumor stemness, and cancer drug resistance with vulnerability to bitopic mTOR inhibition. Sci Signal 12, doi: 10.1126/scisignal.aau8544 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang L, Zhou F & ten Dijke P Signaling interplay between transforming growth factor-beta receptor and PI3K/AKT pathways in cancer. Trends Biochem Sci 38, 612–620, doi: 10.1016/j.tibs.2013.10.001 (2013). [DOI] [PubMed] [Google Scholar]
- 97.Du L et al. Overexpression of PIK3CA in murine head and neck epithelium drives tumor invasion and metastasis through PDK1 and enhanced TGFbeta signaling. Oncogene 35, 4641–4652, doi: 10.1038/onc.2016.1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Berenjeno IM et al. Oncogenic PIK3CA induces centrosome amplification and tolerance to genome doubling. Nature communications 8, 1773, doi: 10.1038/s41467-017-02002-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vanhaesebroeck B et al. Perspective: Potential Impact and Therapeutic Implications of Oncogenic PI3K Activation on Chromosomal Instability. Biomolecules 9, doi: 10.3390/biom9080331 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koundouros N et al. Metabolic Fingerprinting Links Oncogenic PIK3CA with Enhanced Arachidonic Acid-Derived Eicosanoids. Cell 181, 1596–1611 e1527, doi: 10.1016/j.cell.2020.05.053 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hao Y et al. Oncogenic PIK3CA mutations reprogram glutamine metabolism in colorectal cancer. Nature communications 7, 11971, doi: 10.1038/ncomms11971 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Elstrom RL et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 64, 3892–3899, doi: 10.1158/0008-5472.CAN-03-2904 (2004). [DOI] [PubMed] [Google Scholar]
- 103.Biswas SK Metabolic Reprogramming of Immune Cells in Cancer Progression. Immunity 43, 435–449, doi: 10.1016/j.immuni.2015.09.001 (2015). [DOI] [PubMed] [Google Scholar]
- 104.Kutys ML et al. Uncovering mutation-specific morphogenic phenotypes and paracrine-mediated vessel dysfunction in a biomimetic vascularized mammary duct platform. Nature communications 11, 3377, doi: 10.1038/s41467-020-17102-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roy A et al. Suppression of PIK3CA-driven epileptiform activity by acute pathway control. bioRxiv, 2021.2003.2003.433821, doi: 10.1101/2021.03.03.433821 (2021). [DOI] [Google Scholar]
- 106.Tarantelli C et al. PQR309 Is a Novel Dual PI3K/mTOR Inhibitor with Preclinical Antitumor Activity in Lymphomas as a Single Agent and in Combination Therapy. Clin Cancer Res 24, 120–129, doi: 10.1158/1078-0432.CCR-17-1041 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Hafner M et al. Quantification of sensitivity and resistance of breast cancer cell lines to anti-cancer drugs using GR metrics. Sci Data 4, 170166, doi: 10.1038/sdata.2017.166 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hafner M, Niepel M & Sorger PK Alternative drug sensitivity metrics improve preclinical cancer pharmacogenomics. Nat Biotechnol 35, 500–502, doi: 10.1038/nbt.3882 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zwang Y et al. Synergistic interactions with PI3K inhibition that induce apoptosis. eLife 6, doi: 10.7554/eLife.24523 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Morris JZ, Tissenbaum HA & Ruvkun G A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature 382, 536–539 (1996). Reports that inactivation of the PIK3CA paralog in C. elegans induces a dormant developmental state of this model organism, without inducing cell death.
- 111.Elkabets M et al. mTORC1 inhibition is required for sensitivity to PI3K p110alpha inhibitors in PIK3CA-mutant breast cancer. Science translational medicine 5, 196ra199, doi: 10.1126/scitranslmed.3005747 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Will M et al. Rapid induction of apoptosis by PI3K inhibitors is dependent upon their transient inhibition of RAS-ERK signaling. Cancer discovery 4, 334–347, doi: 10.1158/2159-8290.CD-13-0611 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hudson K et al. Intermittent High-Dose Scheduling of AZD8835, a Novel Selective Inhibitor of PI3Kalpha and PI3Kdelta, Demonstrates Treatment Strategies for PIK3CA-Dependent Breast Cancers. Mol Cancer Ther 15, 877–889, doi: 10.1158/1535-7163.MCT-15-0687 (2016). Convincingly shows in preclinical cancer models that intermittent dosing of a PI3K inhibitor has potent anti-tumour effect.
- 114.Komlodi-Pasztor E, Sackett D, Wilkerson J & Fojo T Mitosis is not a key target of microtubule agents in patient tumors. Nature reviews. Clinical oncology 8, 244–250, doi: 10.1038/nrclinonc.2010.228 (2011). [DOI] [PubMed] [Google Scholar]
- 115.Komlodi-Pasztor E, Sackett DL & Fojo AT Inhibitors targeting mitosis: tales of how great drugs against a promising target were brought down by a flawed rationale. Clin Cancer Res 18, 51–63, doi: 10.1158/1078-0432.CCR-11-0999 (2012). [DOI] [PubMed] [Google Scholar]
- 116.Moore HM et al. Predictive and Pharmacodynamic Biomarkers of Response to the Phosphatidylinositol 3-Kinase Inhibitor Taselisib in Breast Cancer Preclinical Models. Mol Cancer Ther 19, 292–303, doi: 10.1158/1535-7163.MCT-19-0284 (2020). [DOI] [PubMed] [Google Scholar]
- 117. Miller TW et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. The Journal of clinical investigation 120, 2406–2413, doi: 10.1172/JCI41680 (2010). One of the first reports to show that PI3K pathway inhibition often mediates resistance to anti-oestrogen therapies in breast cancers, which forms the basis for the use of the PI3Kα inhibitors in this clinical context.
- 118.Miller TW, Balko JM & Arteaga CL Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol 29, 4452–4461, doi: 10.1200/JCO.2010.34.4879 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baselga J et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366, 520–529, doi: 10.1056/NEJMoa1109653 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vasan N, Toska E & Scaltriti M Overview of the relevance of PI3K pathway in HR-positive breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 30, x3–x11, doi: 10.1093/annonc/mdz281 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bosch A et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci. Transl. Med 7, 283ra251, doi: 10.1126/scitranslmed.aaa4442 (2015). Provides a mechanistic understanding of how PI3Kα inhibtion potentiates hormone therapy in breast cancer.
- 122.Jones RH et al. Capivasertib (AZD5363) plus fulvestrant versus placebo plus fulvestrant after relapse or progression on an aromatase inhibitor in metastatic ER -positive breast cancer (FAKTION): A randomized, double-blind, placebo-controlled, phase II trial. Journal of Clinical Oncology 37, 1005–1005, doi:DOI 10.1200/JCO.2019.37.15_suppl.1005 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Turner NC, Neven P, Loibl S & Andre F Advances in the treatment of advanced oestrogen-receptor-positive breast cancer. Lancet 389, 2403–2414, doi: 10.1016/S0140-6736(16)32419-9 (2017). [DOI] [PubMed] [Google Scholar]
- 124.Narayan P et al. FDA Approval Summary: Alpelisib plus fulvestrant for patients with HR-positive, HER2-negative, PIK3CA-mutated, advanced or metastatic breast cancer. Clin Cancer Res, doi: 10.1158/1078-0432.CCR-20-3652 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Andre F et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 32, 208–217, doi: 10.1016/j.annonc.2020.11.011 (2021). [DOI] [PubMed] [Google Scholar]
- 126.Rugo HS et al. Time course and management of key adverse events during the randomized phase III SOLAR-1 study of PI3K inhibitor alpelisib plus fulvestrant in patients with HR-positive advanced breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 31, 1001–1010, doi: 10.1016/j.annonc.2020.05.001 (2020). [DOI] [PubMed] [Google Scholar]
- 127.Nunnery SE & Mayer IA Management of toxicity to isoform alpha-specific PI3K inhibitors. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 30, x21–x26, doi: 10.1093/annonc/mdz440 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ndubaku CO et al. Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1 ,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): a beta-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J Med Chem 56, 4597–4610, doi: 10.1021/jm4003632 (2013). [DOI] [PubMed] [Google Scholar]
- 129.Dent S et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: the SANDPIPER trial. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 32, 197–207, doi: 10.1016/j.annonc.2020.10.596 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Song KW e. a. RTK-dependent inducible degradation of mutant PI3Kα drives 1 GDC-0077 (Inavolisib) efficacy. BioRxiv (2021). Shows that GDC-0077 (Inavolisib) induces degradation of mutant PIK3CA protein in cells in an RTK-dependent manner, resulting in enhanced efficacy in preclincal cancer models compared to non PI3Kα-degraders.
- 131.So L et al. Selective inhibition of phosphoinositide 3-kinase p110alpha preserves lymphocyte function. J Biol Chem 288, 5718–5731, doi: 10.1074/jbc.M112.379446 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Juric D et al. A First-in-Human, Phase I, Dose-Escalation Study of TAK-117, a Selective PI3Kalpha Isoform Inhibitor, in Patients with Advanced Solid Malignancies. Clin Cancer Res 23, 5015–5023, doi: 10.1158/1078-0432.CCR-16-2888 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ohwada J et al. Discovery and biological activity of a novel class I PI3K inhibitor, CH5132799. Bioorg Med Chem Lett 21, 1767–1772, doi: 10.1016/j.bmcl.2011.01.065 (2011). [DOI] [PubMed] [Google Scholar]
- 134.Blagden S et al. First-in-human study of CH5132799, an oral class I PI3K inhibitor, studying toxicity, pharmacokinetics, and pharmacodynamics, in patients with metastatic cancer. Clin Cancer Res 20, 5908–5917, doi: 10.1158/1078-0432.CCR-14-1315 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pereira B et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nature communications 7, 11479, doi: 10.1038/ncomms11479 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Verret B, Cortes J, Bachelot T, Andre F & Arnedos M Efficacy of PI3K inhibitors in advanced breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 30, x12–x20, doi: 10.1093/annonc/mdz381 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pascual J & Turner NC Targeting the PI3-kinase pathway in triple-negative breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 30, 1051–1060, doi: 10.1093/annonc/mdz133 (2019). [DOI] [PubMed] [Google Scholar]
- 138.Hyman DM et al. AKT Inhibition in Solid Tumors With AKT1 Mutations. J Clin Oncol 35, 2251–2259, doi: 10.1200/JCO.2017.73.0143 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Burgess MR et al. KRAS Allelic Imbalance Enhances Fitness and Modulates MAP Kinase Dependence in Cancer. Cell 168, 817–829 e815, doi: 10.1016/j.cell.2017.01.020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Madsen RR et al. Relationship between stemness and transcriptionally-inferred PI3K activity in human breast cancer. bioRxiv, 2020.2007.2009.195974, doi: 10.1101/2020.07.09.195974 (2020). [DOI] [Google Scholar]
- 141.Ros S et al. Metabolic Imaging Detects Resistance to PI3Kalpha Inhibition Mediated by Persistent FOXM1 Expression in ER(+) Breast Cancer. Cancer Cell 38, 516–533 e519, doi: 10.1016/j.ccell.2020.08.016 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.O'Leary B et al. The Genetic Landscape and Clonal Evolution of Breast Cancer Resistance to Palbociclib plus Fulvestrant in the PALOMA-3 Trial. Cancer discovery 8, 1390–1403, doi: 10.1158/2159-8290.CD-18-0264 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Clark AS, Makhlin I & DeMichele A Setting the Pick: Can PI3K Inhibitors Circumvent CDK4/6 Inhibitor Resistance? Clin Cancer Res 27, 371–373, doi: 10.1158/1078-0432.CCR-20-3624 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vora SR et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 26, 136–149, doi: 10.1016/j.ccr.2014.05.020 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mosele F et al. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 31, 377–386, doi: 10.1016/j.annonc.2019.11.006 (2020). [DOI] [PubMed] [Google Scholar]
- 146.Andre F et al. Molecular Alterations and Everolimus Efficacy in Human Epidermal Growth Factor Receptor 2-Overexpressing Metastatic Breast Cancers: Combined Exploratory Biomarker Analysis From BOLERO-1 and BOLERO-3. J Clin Oncol 34, 2115–2124, doi: 10.1200/JCO.2015.63.9161 (2016). [DOI] [PubMed] [Google Scholar]
- 147.Berns K et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12, 395–402, doi: 10.1016/j.ccr.2007.08.030 (2007). [DOI] [PubMed] [Google Scholar]
- 148.Juvekar A et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer discovery 2, 1048–1063, doi: 10.1158/2159-8290.CD-11-0336 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ibrahim YH et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer discovery 2, 1036–1047, doi: 10.1158/2159-8290.CD-11-0348 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Konstantinopoulos PA et al. Olaparib and alpha-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: a dose-escalation and dose-expansion phase 1b trial. The Lancet. Oncology 20, 570–580, doi: 10.1016/S1470-2045(18)30905-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cancer Genome Atlas Research, N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209, doi: 10.1038/nature13480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cancer Genome Atlas, N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517, 576–582, doi: 10.1038/nature14129 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mizrachi A et al. Tumour-specific PI3K inhibition via nanoparticle-targeted delivery in head and neck squamous cell carcinoma. Nature communications 8, 14292, doi: 10.1038/ncomms14292 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zumsteg ZS et al. Taselisib (GDC-0032), a Potent beta-Sparing Small Molecule Inhibitor of PI3K, Radiosensitizes Head and Neck Squamous Carcinomas Containing Activating PIK3CA Alterations. Clin Cancer Res 22, 2009–2019, doi: 10.1158/1078-0432.CCR-15-2245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Graupera M et al. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature 453, 662–666, doi: 10.1038/nature06892 (2008). [DOI] [PubMed] [Google Scholar]
- 156.Soler A et al. Inhibition of the p110alpha isoform of PI 3-kinase stimulates nonfunctional tumor angiogenesis. J Exp Med 210, 1937–1945, doi: 10.1084/jem.20121571 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Okkenhaug K, Graupera M & Vanhaesebroeck B Targeting PI3K in Cancer: Impact on Tumor Cells, Their Protective Stroma, Angiogenesis, and Immunotherapy. Cancer discovery 6, 1090–1105, doi: 10.1158/2159-8290.CD-16-0716 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang S et al. Selective inhibition of mTORC1 in tumor vessels increases antitumor immunity. JCI insight 5, e139237, doi: 10.1172/jci.insight.139237 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Petroni G, Buque A, Zitvogel L, Kroemer G & Galluzzi L Immunomodulation by targeted anticancer agents. Cancer Cell 39, 310–345, doi: 10.1016/j.ccell.2020.11.009 (2021). [DOI] [PubMed] [Google Scholar]
- 160.Teo ZL et al. Combined CDK4/6 and PI3Kalpha Inhibition Is Synergistic and Immunogenic in Triple-Negative Breast Cancer. Cancer Res 77, 6340–6352, doi: 10.1158/0008-5472.CAN-17-2210 (2017). [DOI] [PubMed] [Google Scholar]
- 161.Chandrasekaran S et al. Phosphoinositide 3-Kinase Signaling Can Modulate MHC Class I and II Expression. Mol Cancer Res 17, 2395–2409, doi: 10.1158/1541-7786.MCR-19-0545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Marijt KA et al. Metabolic stress in cancer cells induces immune escape through a PI3K-dependent blockade of IFNgamma receptor signaling. Journal for immunotherapy of cancer 7, 152, doi: 10.1186/s40425-019-0627-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Glaeske S et al. Pulsatile inhibition of PI3K converts immune suppression by T(reg)s and M2-TAM to anti-tumor immune response in animal models insensitive or resistant to the monotherapies of PI3K and checkpoint inhibitors. Cancer Research 78, LB-123-LB-123, doi: 10.1158/1538-7445.Am2018-Lb-123 (2018). [DOI] [Google Scholar]
- 164.Qi Z et al. Overcoming resistance to immune checkpoint therapy in PTEN-null prostate cancer by sequential intermittent anti-PI3Kα/β/δ and anti-PD-1 treatment. bioRxiv, 2020.2010.2017.343608, doi: 10.1101/2020.10.17.343608 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Marks DK et al. Akt Inhibition Is Associated With Favorable Immune Profile Changes Within the Tumor Microenvironment of Hormone Receptor Positive, HER2 Negative Breast Cancer. Frontiers in oncology 10, 968, doi: 10.3389/fonc.2020.00968 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Patnaik A et al. First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin's lymphomas. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 27, 1928–1940, doi: 10.1093/annonc/mdw282 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu N et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110alpha and p110delta activities in tumor cell lines and xenograft models. Mol Cancer Ther 12, 2319–2330, doi: 10.1158/1535-7163.MCT-12-0993-T (2013). [DOI] [PubMed] [Google Scholar]
- 168.Yang W et al. Strategically Timing Inhibition of Phosphatidylinositol 3-Kinase to Maximize Therapeutic Index in Estrogen Receptor Alpha-Positive, PIK3CA-Mutant Breast Cancer. Clin Cancer Res 22, 2250–2260, doi: 10.1158/1078-0432.CCR-15-2276 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Sadhu C, Masinovsky B, Dick K, Sowell CG & Staunton DE Essential role of phosphoinositide 3-kinase delta in neutrophil directional movement. J Immunol 170, 2647–2654, doi: 10.4049/jimmunol.170.5.2647 (2003). The first report on a PI3K isoform-selective inhibitor, the PI3Kδ inhibitor IC87114 (ICOS Corporation), and its use in cell-based models.
- 170.Oak JS & Fruman DA Role of phosphoinositide 3-kinase signaling in autoimmunity. Autoimmunity 40, 433–441, doi: 10.1080/08916930701464780 (2007). [DOI] [PubMed] [Google Scholar]
- 171.Banham-Hall E, Clatworthy MR & Okkenhaug K The Therapeutic Potential for PI3K Inhibitors in Autoimmune Rheumatic Diseases. The open rheumatology journal 6, 245–258, doi: 10.2174/1874312901206010245 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Puri KD & Gold MR Selective inhibitors of phosphoinositide 3-kinase delta: modulators of B-cell function with potential for treating autoimmune inflammatory diseases and B-cell malignancies. Frontiers in immunology 3, 256, doi: 10.3389/fimmu.2012.00256 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee KS, Lee HK, Hayflick JS, Lee YC & Puri KD Inhibition of phosphoinositide 3-kinase delta attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. FASEB J 20, 455–465, doi: 10.1096/fj.05-5045com (2006). [DOI] [PubMed] [Google Scholar]
- 174.Jou ST et al. Essential, nonredundant role for the phosphoinositide 3-kinase p110delta in signaling by the B-cell receptor complex. Molecular and cellular biology 22, 8580–8591, doi: 10.1128/mcb.22.24.8580-8591.2002 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Clayton E et al. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med 196, 753–763, doi: 10.1084/jem.20020805 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Okkenhaug K et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science (New York, N.Y 297, 1031–1034, doi: 10.1126/science.1073560 (2002). The first formal documentation for a functional role of PI3Kδ in T lymphocytes.
- 177.Ali K et al. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature 431, 1007–1011, doi: 10.1038/nature02991 (2004). [DOI] [PubMed] [Google Scholar]
- 178.Condliffe AM et al. Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood 106, 1432–1440, doi: 10.1182/blood-2005-03-0944 (2005). [DOI] [PubMed] [Google Scholar]
- 179.Kulkarni S et al. PI3Kbeta plays a critical role in neutrophil activation by immune complexes. Sci Signal 4, ra23, doi: 10.1126/scisignal.2001617 (2011). [DOI] [PubMed] [Google Scholar]
- 180.Aragoneses-Fenoll L et al. T-Cell-Specific Loss of the PI-3-Kinase p110alpha Catalytic Subunit Results in Enhanced Cytokine Production and Antitumor Response. Frontiers in immunology 9, 332, doi: 10.3389/fimmu.2018.00332 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ramadani F et al. The PI3K isoforms p110alpha and p110delta are essential for pre-B cell receptor signaling and B cell development. Sci Signal 3, ra60, doi: 10.1126/scisignal.2001104 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Iyengar S et al. P110alpha-mediated constitutive PI3K signaling limits the efficacy of p110delta-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood 121, 2274–2284, doi: 10.1182/blood-2012-10-460832 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pongas GN, Annunziata CM & Staudt LM PI3Kdelta inhibition causes feedback activation of PI3Kalpha in the ABC subtype of diffuse large B-cell lymphoma. Oncotarget 8, 81794–81802, doi: 10.18632/oncotarget.20864 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sogkas G et al. Primary immunodeficiency disorder caused by phosphoinositide 3-kinase delta deficiency. J Allergy Clin Immunol 142, 1650–1653 e1652, doi: 10.1016/j.jaci.2018.06.039 (2018). [DOI] [PubMed] [Google Scholar]
- 185.Sharfe N et al. Dual loss of p110delta PI3-kinase and SKAP (KNSTRN) expression leads to combined immunodeficiency and multisystem syndromic features. J Allergy Clin Immunol 142, 618–629, doi: 10.1016/j.jaci.2017.10.033 (2018). [DOI] [PubMed] [Google Scholar]
- 186.Swan DJ et al. Immunodeficiency, autoimmune thrombocytopenia and enterocolitis caused by autosomal recessive deficiency of PIK3CD-encoded phosphoinositide 3-kinase delta. Haematologica 104, e483–e486, doi: 10.3324/haematol.2018.208397 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang K, Husami A, Marsh R & Jordan MB Identification of a phosphoinositide 3-kinase (PI-3K) p110delta (PIK3CD) deficient individual. Journal of clinical immunology 33, 673–674 (2013). [Google Scholar]
- 188.Cohen SB et al. Human primary immunodeficiency caused by expression of a kinase-dead p110delta mutant. J Allergy Clin Immunol 143, 797–799 e792, doi: 10.1016/j.jaci.2018.10.005 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Conley ME et al. Agammaglobulinemia and absent B lineage cells in a patient lacking the p85alpha subunit of PI3K. J Exp Med 209, 463–470, doi: 10.1084/jem.20112533 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Jou ST et al. Identification of variations in the human phosphoinositide 3-kinase p110delta gene in children with primary B-cell immunodeficiency of unknown aetiology. International journal of immunogenetics 33, 361–369, doi: 10.1111/j.1744-313X.2006.00627.x (2006). The first documenation of the presence of PIK3CD-activating mutations in human leukemia.
- 191. Angulo I et al. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science (New York, N.Y 342, 866–871, doi: 10.1126/science.1243292 (2013). Discovery and functional characterisation of hereditary PIK3CD-activating mutations leading to immune dysregulation, the cause of a primary immune deficiency syndrome, now most commonly named Activating PI3Kδ syndrome (APDS).
- 192. Lucas CL et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nat Immunol 15, 88–97, doi: 10.1038/ni.2771 (2014). Discovery and functional characterisation of hereditary PIK3CD-activating mutations leading to immune dysregulation, the cause of a primary immune deficiency syndrome, now most commonly named Activating PI3Kδ syndrome (APDS).
- 193.Lucas CL, Chandra A, Nejentsev S, Condliffe AM & Okkenhaug K PI3Kdelta and primary immunodeficiencies. Nat Rev Immunol 16, 702–714, doi: 10.1038/nri.2016.93 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Michalovich D & Nejentsev S Activated PI3 Kinase Delta Syndrome: From Genetics to Therapy. Frontiers in immunology 9, 369, doi: 10.3389/fimmu.2018.00369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Coulter TI et al. Clinical spectrum and features of activated phosphoinositide 3-kinase delta syndrome: A large patient cohort study. J Allergy Clin Immunol 139, 597–606 e594, doi: 10.1016/j.jaci.2016.06.021 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dornan GL & Burke JE Molecular Mechanisms of Human Disease Mediated by Oncogenic and Primary Immunodeficiency Mutations in Class IA Phosphoinositide 3-Kinases. Frontiers in immunology 9, 575, doi: 10.3389/fimmu.2018.00575 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dornan GL et al. Conformational disruption of PI3Kdelta regulation by immunodeficiency mutations in PIK3CD and PIK3R1. Proc Natl Acad Sci U S A 114, 1982–1987, doi: 10.1073/pnas.1617244114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tsolakos N et al. Quantitation of class IA PI3Ks in mice reveals p110-free-p85s and isoform-selective subunit associations and recruitment to receptors. Proc Natl Acad Sci U S A 115, 12176–12181, doi: 10.1073/pnas.1803446115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tangye SG et al. Immune Dysregulation and Disease Pathogenesis due to Activating Mutations in PIK3CD-the Goldilocks' Effect. Journal of clinical immunology 39, 148–158, doi: 10.1007/s10875-019-00612-9 (2019). [DOI] [PubMed] [Google Scholar]
- 200.Preite S, Gomez-Rodriguez J, Cannons JL & Schwartzberg PLT and B-cell signaling in activated PI3K delta syndrome: From immunodeficiency to autoimmunity. Immunol Rev 291, 154–173, doi: 10.1111/imr.12790 (2019). [DOI] [PubMed] [Google Scholar]
- 201.Stark AK et al. PI3Kdelta hyper-activation promotes development of B cells that exacerbate Streptococcus pneumoniae infection in an antibody-independent manner. Nature communications 9, 3174, doi: 10.1038/s41467-018-05674-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Preite S et al. Hyperactivated PI3Kdelta promotes self and commensal reactivity at the expense of optimal humoral immunity. Nat Immunol 19, 986–1000, doi: 10.1038/s41590-018-0182-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wray-Dutra MN et al. Activated PIK3CD drives innate B cell expansion yet limits B cell-intrinsic immune responses. J Exp Med 215, 2485–2496, doi: 10.1084/jem.20180617 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bier J et al. Activating mutations in PIK3CD disrupt the differentiation and function of human and murine CD4(+) T cells. J Allergy Clin Immunol 144, 236–253, doi: 10.1016/j.jaci.2019.01.033 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Avery DT et al. Germline-activating mutations in PIK3CD compromise B cell development and function. J Exp Med 215, 2073–2095, doi: 10.1084/jem.20180010 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang J et al. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A 110, 1398–1403, doi: 10.1073/pnas.1205299110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liu Y et al. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet 49, 1211–1218, doi: 10.1038/ng.3909 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Herman SE et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood 116, 2078–2088, doi: 10.1182/blood-2010-02-271171 (2010). Together with Ref. 242 (Ikeda) - the first reports on CAL-101, the first clinically-used PI3Kδ inhibitor (ICOS Corporation), and its in vitro anti-cancer potential in B-cell malignancies such as CLL.
- 209.Hoellenriegel J et al. The phosphoinositide 3'-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood 118, 3603–3612, doi: 10.1182/blood-2011-05-352492 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Niemann CU & Wiestner A B-cell receptor signaling as a driver of lymphoma development and evolution. Seminars in cancer biology 23, 410–421, doi: 10.1016/j.semcancer.2013.09.001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Foukas LC, Berenjeno IM, Gray A, Khwaja A & Vanhaesebroeck B Activity of any class IA PI3K isoform can sustain cell proliferation and survival. Proc Natl Acad Sci U S A 107, 11381–11386, doi: 10.1073/pnas.0906461107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Nguyen PH, Niesen E & Hallek M New roles for B cell receptor associated kinases: when the B cell is not the target. Leukemia 33, 576–587, doi: 10.1038/s41375-018-0366-8 (2019). [DOI] [PubMed] [Google Scholar]
- 213.Skanland SS et al. An in vitro assay for biomarker discovery and dose prediction applied to ibrutinib plus venetoclax treatment of CLL. Leukemia 34, 478–487, doi: 10.1038/s41375-019-0569-7 (2020). [DOI] [PubMed] [Google Scholar]
- 214.Aydin E et al. Phosphoinositide 3-Kinase Signaling in the Tumor Microenvironment: What Do We Need to Consider When Treating Chronic Lymphocytic Leukemia With PI3K Inhibitors? Frontiers in immunology 11, 595818, doi: 10.3389/fimmu.2020.595818 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Serrat N et al. PI3Kdelta inhibition reshapes follicular lymphoma-immune microenvironment cross talk and unleashes the activity of venetoclax. Blood advances 4, 4217–4231, doi: 10.1182/bloodadvances.2020001584 (2020). First detailed characterisation of the impact of PI3Kδ inhibition on the immune crosstalk of follicular lymphoma with the cancer microenvironment, also showing that PI3Kδ inhibition leads to sensitivity to a BCL2 inhibitor.
- 216.Burger JA & Wiestner A Targeting B cell receptor signalling in cancer: preclinical and clinical advances. Nat Rev Cancer 18, 148–167, doi: 10.1038/nrc.2017.121 (2018). [DOI] [PubMed] [Google Scholar]
- 217.Ali AY et al. Expression and function of phosphoinositide 3-kinase delta in mesenchymal stromal cells from normal and leukaemic bone marrow. British journal of haematology 185, 883–887, doi: 10.1111/bjh.15865 (2019). [DOI] [PubMed] [Google Scholar]
- 218.Brown JR et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood 123, 3390–3397, doi: 10.1182/blood-2013-11-535047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kuppers R & Stevenson FK Critical influences on the pathogenesis of follicular lymphoma. Blood 131, 2297–2306, doi: 10.1182/blood-2017-11-764365 (2018). [DOI] [PubMed] [Google Scholar]
- 220. Dong S et al. PI3K p110delta inactivation antagonizes chronic lymphocytic leukemia and reverses T cell immune suppression. The Journal of clinical investigation 129, 122–136, doi: 10.1172/JCI99386 (2019). Shows that PI3Kδ inhibition in preclincal models of CLL leads to a host anti- tumour immune response.
- 221.Abu Eid R et al. Selective inhibition of regulatory T cells by targeting the PI3K-Akt pathway. Cancer immunology research 2, 1080–1089, doi: 10.1158/2326-6066.CIR-14-0095 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Abu Eid R et al. Enhanced Therapeutic Efficacy and Memory of Tumor-Specific CD8 T Cells by Ex Vivo PI3K-delta Inhibition. Cancer Res 77, 4135–4145, doi: 10.1158/0008-5472.CAN-16-1925 (2017). [DOI] [PubMed] [Google Scholar]
- 223.Ahmad S et al. Differential PI3Kdelta Signaling in CD4(+) T-cell Subsets Enables Selective Targeting of T Regulatory Cells to Enhance Cancer Immunotherapy. Cancer Res 77, 1892–1904, doi: 10.1158/0008-5472.CAN-16-1839 (2017). [DOI] [PubMed] [Google Scholar]
- 224. Ali K et al. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature 510, 407–411, doi: 10.1038/nature13444 (2014). This paper provides the first evidence that PI3Kδ inhibition in mice induces an anti-tumour immune response, as the result of a preferential inhibition on the immuno-suppressive Treg cells.
- 225.Lu X et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 543, 728–732, doi: 10.1038/nature21676 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Lauder SN et al. Tumour control by sequential targeting of regulatory T cells with PI3Kdelta inhibition and LAG3 Blockade. Journal for immunotherapy of cancer 8, 8 (2020). This study in mice shows that (1) that upregulation of the LAG3 checkpoint receptor on Treg cells is a bottleneck to successful PI3Kδ-targeted immunotherapy, with superior anti-cancer activity of combined PI3Kdelta/LAG3 blockade and (2) that some degree of primary anti-cancer immune response is required in order to achieve an anti-tumour effect of PI3Kδ-targeted immunotherapy.
- 227.Lim EL et al. Phosphoinositide 3-kinase delta inhibition promotes antitumor responses but antagonizes checkpoint inhibitors. JCI insight 3, e120626, doi: 10.1172/jci.insight.120626 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gyori D et al. Compensation between CSF1R+ macrophages and Foxp3+ Treg cells drives resistance to tumor immunotherapy. JCI insight 3, doi: 10.1172/jci.insight.120631 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lim EL & Okkenhaug K Phosphoinositide 3-kinase delta is a regulatory T-cell target in cancer immunotherapy. Immunology 157, 210–218, doi: 10.1111/imm.13082 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Shin N et al. Parsaclisib Is a Next-Generation Phosphoinositide 3-Kinase delta Inhibitor with Reduced Hepatotoxicity and Potent Antitumor and Immunomodulatory Activities in Models of B-Cell Malignancy. J Pharmacol Exp Ther 374, 211–222, doi: 10.1124/jpet.120.265538 (2020). [DOI] [PubMed] [Google Scholar]
- 231.Xu ZS et al. A novel PI3K inhibitor suppresses tumor progression by immune modulation. Cancer Research 77, doi: 10.1158/1538-7445.Am2017-5675 (2017). [DOI] [Google Scholar]
- 232.Chellappa S et al. The PI3K p110delta Isoform Inhibitor Idelalisib Preferentially Inhibits Human Regulatory T Cell Function. J Immunol 202, 1397–1405, doi: 10.4049/jimmunol.1701703 (2019). [DOI] [PubMed] [Google Scholar]
- 233.Lampson BL et al. Idelalisib given front-line for treatment of chronic lymphocytic leukemia causes frequent immune-mediated hepatotoxicity. Blood 128, 195–203, doi: 10.1182/blood-2016-03-707133 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gadi D et al. Imbalance in T Cell Subsets Triggers the Autoimmune Toxicity of PI3K Inhibitors in CLL. Blood 134, 1745–1745, doi: 10.1182/blood-2019-122961 (2019).31558466 [DOI] [Google Scholar]
- 235. Eschweiler S et al. Nature Portfolio under review, doi: 10.21203/rs.3.rs-337290/v1 (2021). Randomized phase II trial that provides the first formal demonstration of the cancer immunotherapy potential of PI3Kδ inhibition in human solid tumours.
- 236.Preite S, Huang B, Cannons JL, McGavern DB & Schwartzberg PL PI3K Orchestrates T Follicular Helper Cell Differentiation in a Context Dependent Manner: Implications for Autoimmunity. Frontiers in immunology 9, 3079, doi: 10.3389/fimmu.2018.03079 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Goulielmaki E et al. Pharmacological inactivation of the PI3K p110delta prevents breast tumour progression by targeting cancer cells and macrophages. Cell death & disease 9, 678, doi: 10.1038/s41419-018-0717-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Akhiani AA et al. Idelalisib Rescues Natural Killer Cells from Monocyte-Induced Immunosuppression by Inhibiting NOX2-Derived Reactive Oxygen Species. Cancer immunology research 8, 1532–1541, doi: 10.1158/2326-6066.CIR-20-0055 (2020). [DOI] [PubMed] [Google Scholar]
- 239.Sujobert P et al. Essential role for the p110delta isoform in phosphoinositide 3-kinase activation and cell proliferation in acute myeloid leukemia. Blood 106, 1063–1066, doi: 10.1182/blood-2004-08-3225 (2005). [DOI] [PubMed] [Google Scholar]
- 240.Billottet C, Banerjee L, Vanhaesebroeck B & Khwaja A Inhibition of class I phosphoinositide 3-kinase activity impairs proliferation and triggers apoptosis in acute promyelocytic leukemia without affecting atra-induced differentiation. Cancer Res 69, 1027–1036, doi: 10.1158/0008-5472.CAN-08-2608 (2009). [DOI] [PubMed] [Google Scholar]
- 241.Billottet C et al. A selective inhibitor of the p110delta isoform of PI 3-kinase inhibits AML cell proliferation and survival and increases the cytotoxic effects of VP16. Oncogene 25, 6648–6659, doi: 10.1038/sj.onc.1209670 (2006). [DOI] [PubMed] [Google Scholar]
- 242.Ikeda H et al. PI3K/p110{delta} is a novel therapeutic target in multiple myeloma. Blood 116, 1460–1468, doi: 10.1182/blood-2009-06-222943 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Meadows SA et al. PI3Kdelta inhibitor, GS-1101 (CAL-101), attenuates pathway signaling, induces apoptosis, and overcomes signals from the microenvironment in cellular models of Hodgkin lymphoma. Blood 119, 1897–1900, doi: 10.1182/blood-2011-10-386763 (2012). [DOI] [PubMed] [Google Scholar]
- 244.Flinn IW et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-delta, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood 123, 3406–3413, doi: 10.1182/blood-2013-11-538546 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Gopal AK et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 370, 1008–1018, doi: 10.1056/NEJMoa1314583 (2014). Phase III trial with idelalisib in human lymphoma, showing impressive clinical impact, resulting in the approval of this compound for clinical use.
- 246. Furman RR et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 370, 997–1007, doi: 10.1056/NEJMoa1315226 (2014). Phase III trial with idelalisib in human lymphoma, showing impressive clinical impact, resulting in the approval of this compound for clinical use.
- 247.Horak F et al. Randomized phase 1 study of the phosphatidylinositol 3-kinase delta inhibitor idelalisib in patients with allergic rhinitis. J Allergy Clin Immunol 137, 1733–1741, doi: 10.1016/j.jaci.2015.12.1313 (2016). [DOI] [PubMed] [Google Scholar]
- 248.Khindri S et al. A Multicentre, Randomized, Double-Blind, Placebo-Controlled, Crossover Study To Investigate the Efficacy, Safety, Tolerability, and Pharmacokinetics of Repeat Doses of Inhaled Nemiralisib in Adults with Persistent, Uncontrolled Asthma. J Pharmacol Exp Ther 367, 405–413, doi: 10.1124/jpet.118.249516 (2018). [DOI] [PubMed] [Google Scholar]
- 249.Ewings K et al. Abstract 2692: Preclinical development of a novel, highly selective PI3Kδ inhibitor, IOA-244, for the treatment of solid malignancies. Cancer Research 79, 2692–2692, doi: 10.1158/1538-7445.Am2019-2692 (2019). [DOI] [Google Scholar]
- 250.Perry MDW e. a. The discovery of AZD8154, a dual PI3Kγδ inhibitor for the treatment of asthma. J Med Chem under review (2021). [DOI] [PubMed] [Google Scholar]
- 251.Biagetti M, Ronchi P, Fiorelli C & Bruno P Isochromene derivatives as phosphoinositide 3-kinases inhibitors. WO2020100918 (2020). [Google Scholar]
- 252.Durand CA et al. Phosphoinositide 3-kinase p110 delta regulates natural antibody production, marginal zone and B-1 B cell function, and autoantibody responses. J Immunol 183, 5673–5684, doi: 10.4049/jimmunol.0900432 (2009). [DOI] [PubMed] [Google Scholar]
- 253.Deng C et al. Silencing c-Myc translation as a therapeutic strategy through targeting PI3Kdelta and CK1epsilon in hematological malignancies. Blood 129, 88–99, doi: 10.1182/blood-2016-08-731240 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.O'Farrell M et al. Preclinical Characterization of PWT143, a Novel Selective and Potent Phosphatidylinositol 3-kinase Delta (PI3K delta) Inhibitor with Ex-Vivo Activity in Hematologic Malignancies. Blood 120, 2907–2907, doi:DOI 10.1182/blood.V120.21.2907.2907 (2012). [DOI] [Google Scholar]
- 255. Rao VK et al. Effective “activated PI3Kdelta syndrome”-targeted therapy with the PI3Kdelta inhibitor leniolisib. Blood 130, 2307–2316, doi: 10.1182/blood-2017-08-801191 (2017). First documentation of clinical efficacy of PI3Kδ inhibition in human activated PI3Kδ syndrome.
- 256.Dörner T et al. OP0250 A randomised, double-blind study to assess the safety, tolerability and preliminary efficacy of leniolisib (CDZ173) in patients with primary Sjögren's syndrome. Annals of the Rheumatic Diseases 77, 174–174, doi: 10.1136/annrheumdis-2018-eular.3111 (2018). [DOI] [Google Scholar]
- 257.Juarez M et al. A phase 2 randomized, double-blind, placebo-controlled, proof-of-concept study of oral seletalisib in primary Sjogren's syndrome. Rheumatology (Oxford), doi: 10.1093/rheumatology/keaa410 (2020). [DOI] [PubMed] [Google Scholar]
- 258.Diaz N et al. Seletalisib for Activated PI3Kdelta Syndromes: Open-Label Phase 1b and Extension Studies. J Immunol 205, 2979–2987, doi: 10.4049/jimmunol.2000326 (2020). [DOI] [PubMed] [Google Scholar]
- 259.Kienle DL & Stilgenbauer S Approved and emerging PI3K inhibitors for the treatment of chronic lymphocytic leukemia and non-Hodgkin lymphoma. Expert Opin Pharmacother 21, 917–929, doi: 10.1080/14656566.2020.1737010 (2020). [DOI] [PubMed] [Google Scholar]
- 260.Stenton GR et al. Characterization of AQX-1125, a small-molecule SHIP1 activator: Part 1. Effects on inflammatory cell activation and chemotaxis in vitro and pharmacokinetic characterization in vivo. Br J Pharmacol 168, 1506–1518, doi: 10.1111/bph.12039 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Stenton GR et al. Characterization of AQX-1125, a small-molecule SHIP1 activator: Part 2. Efficacy studies in allergic and pulmonary inflammation models in vivo. Br J Pharmacol 168, 1519–1529, doi: 10.1111/bph.12038 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Leaker BR et al. The effects of the novel SHIP1 activator AQX-1125 on allergen-induced responses in mild-to-moderate asthma. Clin Exp Allergy 44, 1146–1153, doi: 10.1111/cea.12370 (2014). [DOI] [PubMed] [Google Scholar]
- 263.Nickel JC et al. Targeting the SHIP1 Pathway Fails to Show Treatment Benefit in Interstitial Cystitis/Bladder Pain Syndrome: Lessons Learned from Evaluating Potentially Effective Therapies in This Enigmatic Syndrome. J Urol 202, 301–308, doi: 10.1097/JU.0000000000000192 (2019). [DOI] [PubMed] [Google Scholar]
- 264.Ji H et al. Inactivation of PI3Kgamma and PI3Kdelta distorts T-cell development and causes multiple organ inflammation. Blood 110, 2940–2947, doi: 10.1182/blood-2007-04-086751 (2007). [DOI] [PubMed] [Google Scholar]
- 265.Steinbach EC et al. Innate PI3K p110delta regulates Th1/Th17 development and microbiota-dependent colitis. J Immunol 192, 3958–3968, doi: 10.4049/jimmunol.1301533 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Uno JK et al. Altered macrophage function contributes to colitis in mice defective in the phosphoinositide-3 kinase subunit p110delta. Gastroenterology 139, 1642–1653, 1653 e1641-1646, doi: 10.1053/j.gastro.2010.07.008 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Coutre SE et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma 56, 2779–2786, doi: 10.3109/10428194.2015.1022770 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Lampson BL & Brown JR PI3Kdelta-selective and PI3Kalpha/delta-combinatorial inhibitors in clinical development for B-cell non-Hodgkin lymphoma. Expert Opin Investig Drugs 26, 1267–1279, doi: 10.1080/13543784.2017.1384815 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Barr PM et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood 127, 2411–2415, doi: 10.1182/blood-2015-12-683516 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Smith SM et al. Safety and tolerability of idelalisib, lenalidomide, and rituximab in relapsed and refractory lymphoma: the Alliance for Clinical Trials in Oncology A051201 and A051202 phase 1 trials. The Lancet. Haematology 4, e176–e182, doi: 10.1016/S2352-3026(17)30028-5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Cuneo A et al. Management of adverse events associated with idelalisib treatment in chronic lymphocytic leukemia and follicular lymphoma: A multidisciplinary position paper. Hematological oncology 37, 3–14, doi: 10.1002/hon.2540 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ramanathan S, Jin F, Sharma S & Kearney BP Clinical Pharmacokinetic and Pharmacodynamic Profile of Idelalisib. Clin Pharmacokinet 55, 33–45, doi: 10.1007/s40262-015-0304-0 (2016). [DOI] [PubMed] [Google Scholar]
- 273.Zhu J et al. Identification of Novel Pathways in Idelalisib Metabolism and Bioactivation. Chem Res Toxicol 31, 548–555, doi: 10.1021/acs.chemrestox.8b00023 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Forero-Torres A et al. Parsaclisib, a potent and highly selective PI3Kdelta inhibitor, in patients with relapsed or refractory B-cell malignancies. Blood 133, 1742–1752, doi: 10.1182/blood-2018-08-867499 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Fowler NH et al. Umbralisib, a Dual PI3Kδ/CK1ε Inhibitor in Patients With Relapsed or Refractory Indolent Lymphoma. Journal of Clinical Oncology 0, JCO.20.03433, doi: 10.1200/jco.20.03433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mato AR et al. Phase 2 Study of the Safety and Efficacy of Umbralisib in Patients with CLL Who Are Intolerant to BTK or PI3Kdelta Inhibitor Therapy. Blood, doi: 10.1182/blood.2020007376 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Maharaj K et al. The dual PI3Kdelta/CK1epsilon inhibitor umbralisib exhibits unique immunomodulatory effects on CLL T cells. Blood advances 4, 3072–3084, doi: 10.1182/bloodadvances.2020001800 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hanlon A & Brander DM Managing toxicities of phosphatidylinositol-3-kinase (PI3K) inhibitors. Hematology Am Soc Hematol Educ Program 2020, 346–356, doi: 10.1182/hematology.2020000119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Salles G et al. Efficacy and safety of idelalisib in patients with relapsed, rituximab- and alkylating agent-refractory follicular lymphoma: a subgroup analysis of a phase 2 study. Haematologica 102, e156–e159, doi: 10.3324/haematol.2016.151738 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Compagno M et al. Phosphatidylinositol 3-kinase delta blockade increases genomic instability in B cells. Nature 542, 489–493, doi: 10.1038/nature21406 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Morande PE et al. Ibrutinib therapy downregulates AID enzyme and proliferative fractions in chronic lymphocytic leukemia. Blood 133, 2056–2068, doi: 10.1182/blood-2018-09-876292 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Franks SE, Getahun A & Cambier JC A Precision B Cell-Targeted Therapeutic Approach to Autoimmunity Caused by Phosphatidylinositol 3-Kinase Pathway Dysregulation. J Immunol 202, 3381–3393, doi: 10.4049/jimmunol.1801394 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Ma S et al. Retrospective Analysis of the Impact of Adverse Event-Triggered Idelalisib Interruption and Dose Reduction on Clinical Outcomes in Patients With Relapsed/Refractory B-Cell Malignancies. Clin Lymphoma Myeloma Leuk, doi: 10.1016/j.clml.2020.12.016 (2020). Documents extended survival of patients who had treatment interruptions with the PI3Kδ inhibitor idelalisib in follicular lymphoma and CLL, indicating that continuous PI3Kδ therapy might not be required to achieve clinical benefit.
- 284.Patel K & Pagel JM Exploring a Future for PI3K Inhibitors in Chronic Lymphocytic Leukemia. Curr Hematol Malig Rep 14, 292–301, doi: 10.1007/s11899-019-00525-9 (2019). [DOI] [PubMed] [Google Scholar]
- 285.Zelenetz AD et al. The Pi3kδ Inhibitor Me-401 ± Rituximab in Relapsed/Refractory (R/R) Follicular Lymphoma (Fl), Chronic Lymphocytic Leukemia (Cll), and Small Lymphocytic Lymphoma (Sll). Hematological oncology 37, 176–177, doi: 10.1002/hon.133_2629 (2019).30261551 [DOI] [Google Scholar]
- 286.Cahn A et al. Safety, pharmacokinetics and dose-response characteristics of GSK2269557, an inhaled PI3Kdelta inhibitor under development for the treatment of COPD. Pulm Pharmacol Ther 46, 69–77, doi: 10.1016/j.pupt.2017.08.008 (2017). [DOI] [PubMed] [Google Scholar]
- 287.Schmalbach T et al. Duvelisib, a PI3K-δ,γ inhibitor, in subjects with mild asthma. European Respiratory Journal 46, PA2122, doi: 10.1183/13993003.congress-2015.PA2122 (2015). [DOI] [Google Scholar]
- 288.Jones JA et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: an open-label, randomised phase 3 trial. The Lancet. Haematology 4, e114–e126, doi: 10.1016/S2352-3026(17)30019-4 (2017). [DOI] [PubMed] [Google Scholar]
- 289.Sawyer C et al. Regulation of breast cancer cell chemotaxis by the phosphoinositide 3-kinase p110delta. Cancer Res 63, 1667–1675 (2003). [PubMed] [Google Scholar]
- 290.Tzenaki N et al. High levels of p110delta PI3K expression in solid tumor cells suppress PTEN activity, generating cellular sensitivity to p110delta inhibitors through PTEN activation. FASEB J 26, 2498–2508, doi: 10.1096/fj.11-198192 (2012). [DOI] [PubMed] [Google Scholar]
- 291.Xenou L & Papakonstanti EA p110delta PI3K as a therapeutic target of solid tumours. Clin Sci (Lond) 134, 1377–1397, doi: 10.1042/CS20190772 (2020). [DOI] [PubMed] [Google Scholar]
- 292.Carnevalli LS et al. PI3Kalpha/delta inhibition promotes anti-tumor immunity through direct enhancement of effector CD8(+) T-cell activity. Journal for immunotherapy of cancer 6, 158, doi: 10.1186/s40425-018-0457-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Tan MH et al. Spectrum of immune checkpoint inhibitors-induced endocrinopathies in cancer patients: a scoping review of case reports. Clinical diabetes and endocrinology 5, 1, doi: 10.1186/s40842-018-0073-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Mitchell EL et al. Rheumatic immune-related adverse events secondary to anti-programmed death-1 antibodies and preliminary analysis on the impact of corticosteroids on anti-tumour response: A case series. Eur J Cancer 105, 88–102, doi: 10.1016/j.ejca.2018.09.027 (2018). [DOI] [PubMed] [Google Scholar]
- 295.Solinas C et al. Cancer immunotherapy-associated hypophysitis. Semin Oncol 45, 181–186, doi: 10.1053/j.seminoncol.2018.09.002 (2018). [DOI] [PubMed] [Google Scholar]
- 296.Miranda Poma J, Ostios Garcia L, Villamayor Sanchez J & D'Errico G What do we know about cancer immunotherapy? Long-term survival and immune-related adverse events. Allergologia et immunopathologia 47, 303–308, doi: 10.1016/j.aller.2018.04.005 (2019). [DOI] [PubMed] [Google Scholar]
- 297.Taylor NA et al. Treg depletion potentiates checkpoint inhibition in claudin-low breast cancer. The Journal of clinical investigation 127, 3472–3483, doi: 10.1172/JCI90499 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Chang WI et al. PI3Kalphadelta Inhibitor Combined With Radiation Enhances the Antitumor Immune Effect of Anti-PD1 in a Syngeneic Murine Triple-Negative Breast Cancer Model. Int J Radiat Oncol Biol Phys, doi: 10.1016/j.ijrobp.2021.01.025 (2021). [DOI] [PubMed] [Google Scholar]
- 299.Parry RV et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Molecular and cellular biology 25, 9543–9553, doi: 10.1128/MCB.25.21.9543-9553.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Hui E et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science (New York, N.Y 355, 1428–1433, doi: 10.1126/science.aaf1292 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ferguson MS et al. Transient Inhibition of PI3Kdelta Enhances the Therapeutic Effect of Intravenous Delivery of Oncolytic Vaccinia Virus. Molecular therapy : the journal of the American Society of Gene Therapy 28, 1263–1275, doi: 10.1016/j.ymthe.2020.02.017 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Crompton JG et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res 75, 296–305, doi: 10.1158/0008-5472.CAN-14-2277 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.van der Waart AB et al. Inhibition of Akt signaling promotes the generation of superior tumor-reactive T cells for adoptive immunotherapy. Blood 124, 3490–3500, doi: 10.1182/blood-2014-05-578583 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Abu Eid R et al. Akt1 and -2 inhibition diminishes terminal differentiation and enhances central memory CD8(+) T-cell proliferation and survival. Oncoimmunology 4, e1005448, doi: 10.1080/2162402X.2015.1005448 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Petersen CT et al. Improving T-cell expansion and function for adoptive T-cell therapy using ex vivo treatment with PI3Kdelta inhibitors and VIP antagonists. Blood advances 2, 210–223, doi: 10.1182/bloodadvances.2017011254 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Klebanoff CA et al. Inhibition of AKT signaling uncouples T cell differentiation from expansion for receptor-engineered adoptive immunotherapy. JCI insight 2, doi: 10.1172/jci.insight.95103 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Bowers JS et al. PI3Kdelta Inhibition Enhances the Antitumor Fitness of Adoptively Transferred CD8(+) T Cells. Frontiers in immunology 8, 1221, doi: 10.3389/fimmu.2017.01221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Zheng W et al. PI3K orchestration of the in vivo persistence of chimeric antigen receptor-modified T cells. Leukemia 32, 1157–1167, doi: 10.1038/s41375-017-0008-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Urak R et al. Ex vivo Akt inhibition promotes the generation of potent CD19CAR T cells for adoptive immunotherapy. Journal for immunotherapy of cancer 5, 26, doi: 10.1186/s40425-017-0227-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Dwyer CJ et al. Ex vivo blockade of PI3K gamma or delta signaling enhances the antitumor potency of adoptively transferred CD8(+) T cells. Eur J Immunol 50, 1386–1399, doi: 10.1002/eji.201948455 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.de Bono JS et al. Randomized Phase II Study Evaluating Akt Blockade with Ipatasertib, in Combination with Abiraterone, in Patients with Metastatic Prostate Cancer with and without PTEN Loss. Clin Cancer Res 25, 928–936, doi: 10.1158/1078-0432.CCR-18-0981 (2019). [DOI] [PubMed] [Google Scholar]
- 312.Kim SB et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet. Oncology 18, 1360–1372, doi: 10.1016/S1470-2045(17)30450-3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Burger JA Treatment of Chronic Lymphocytic Leukemia. N Engl J Med 383, 460–473, doi: 10.1056/NEJMra1908213 (2020). [DOI] [PubMed] [Google Scholar]
- 314.Costa C et al. Measurement of PIP3 levels reveals an unexpected role for p110beta in early adaptive responses to p110alpha-specific inhibitors in luminal breast cancer. Cancer Cell 27, 97–108, doi: 10.1016/j.ccell.2014.11.007 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Leroy C et al. Activation of IGF1R/p110beta/AKT/mTOR confers resistance to alpha-specific PI3K inhibition. Breast Cancer Res 18, 41, doi: 10.1186/s13058-016-0697-1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Juric D et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kalpha inhibitor. Nature 518, 240–244, doi: 10.1038/nature13948 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Rageot D et al. (S)-4-(Difluoromethyl)-5-(4-(3-methylmorpholino)-6-morpholino-1,3,5-triazin-2-yl) pyridin-2-amine (PQR530), a Potent, Orally Bioavailable, and Brain-Penetrable Dual Inhibitor of Class I PI3K and mTOR Kinase. J Med Chem 62, 6241–6261, doi: 10.1021/acs.jmedchem.9b00525 (2019). [DOI] [PubMed] [Google Scholar]
- 318.Hosford SR et al. Combined Inhibition of Both p110alpha and p110beta Isoforms of Phosphatidylinositol 3-Kinase Is Required for Sustained Therapeutic Effect in PTEN-Deficient, ER(+) Breast Cancer. Clin Cancer Res 23, 2795–2805, doi: 10.1158/1078-0432.CCR-15-2764 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Wee S et al. PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci U S A 105, 13057–13062, doi: 10.1073/pnas.0802655105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Torbett NE et al. A chemical screen in diverse breast cancer cell lines reveals genetic enhancers and suppressors of sensitivity to PI3K isoform-selective inhibition. Biochem J 415, 97–110, doi: 10.1042/BJ20080639 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Schmit F et al. PI3K isoform dependence of PTEN-deficient tumors can be altered by the genetic context. Proc Natl Acad Sci U S A 111, 6395–6400, doi: 10.1073/pnas.1323004111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M & Bilanges B The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 11, 329–341, doi: 10.1038/nrm2882 (2010). [DOI] [PubMed] [Google Scholar]
- 323.Weigelt B & Downward J Genomic Determinants of PI3K Pathway Inhibitor Response in Cancer. Frontiers in oncology 2, 109, doi: 10.3389/fonc.2012.00109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ilic N, Utermark T, Widlund HR & Roberts TM PI3K-targeted therapy can be evaded by gene amplification along the MYC-eukaryotic translation initiation factor 4E (eIF4E) axis. Proc Natl Acad Sci U S A 108, E699–708, doi: 10.1073/pnas.1108237108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Castel P et al. PDK1-SGK1 Signaling Sustains AKT-Independent mTORC1 Activation and Confers Resistance to PI3Kalpha Inhibition. Cancer Cell 30, 229–242, doi: 10.1016/j.ccell.2016.06.004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Bago R et al. The hVps34-SGK3 pathway alleviates sustained PI3K/Akt inhibition by stimulating mTORC1 and tumour growth. The EMBO journal 35, 2263, doi: 10.15252/embj.201670010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Cai Y et al. Genomic alterations in PIK3CA-mutated breast cancer result in mTORC1 activation and limit sensitivity to PI3Kalpha inhibitors. Cancer Res, doi: 10.1158/0008-5472.CAN-20-3232 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Le X et al. Systematic Functional Characterization of Resistance to PI3K Inhibition in Breast Cancer. Cancer discovery 6, 1134–1147, doi: 10.1158/2159-8290.CD-16-0305 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Murali I et al. Activation of the MAPK Pathway Mediates Resistance to PI3K Inhibitors in Chronic Lymphocytic Leukemia (CLL). Blood in press (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Yahiaoui A et al. PI3Kdelta inhibitor idelalisib in combination with BTK inhibitor ONO/GS-4059 in diffuse large B cell lymphoma with acquired resistance to PI3Kdelta and BTK inhibitors. PLoS One 12, e0171221, doi: 10.1371/journal.pone.0171221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Ghia P et al. Whole-Exome Sequencing Revealed No Recurrent Mutations within the PI3K Pathway in Relapsed Chronic Lymphocytic Leukemia Patients Progressing Under Idelalisib Treatment. Blood 128, doi:DOI 10.1182/blood.V128.22.2770.2770 (2016). [DOI] [Google Scholar]
- 332.Scheffold A et al. IGF1R as druggable target mediating PI3K-delta inhibitor resistance in a murine model of chronic lymphocytic leukemia. Blood 134, 534–547, doi: 10.1182/blood.2018881029 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Hafner C et al. Oncogenic PIK3CA mutations occur in epidermal nevi and seborrheic keratoses with a characteristic mutation pattern. Proc Natl Acad Sci U S A 104, 13450–13454, doi: 10.1073/pnas.0705218104 (2007). First ever demonstration of the presence of oncogenic PIK3CA mutations in human tissues (epidermal nevi and seborrheic keratoses), without predisposing to cancer.
- 334.Madsen RR, Vanhaesebroeck B & Semple RK Cancer-Associated PIK3CA Mutations in Overgrowth Disorders. Trends Mol Med 24, 856–870, doi: 10.1016/j.molmed.2018.08.003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Lindhurst MJ et al. A mouse model of Proteus syndrome. Hum Mol Genet 28, 2920–2936, doi: 10.1093/hmg/ddz116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lee YR, Chen M & Pandolfi PP The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol 19, 547–562, doi: 10.1038/s41580-018-0015-0 (2018). [DOI] [PubMed] [Google Scholar]
- 337.Venot Q et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature 558, 540–546, doi: 10.1038/s41586-018-0217-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Parker VER et al. Safety and efficacy of low-dose sirolimus in the PIK3CA-related overgrowth spectrum. Genetics in medicine : official journal of the American College of Medical Genetics 21, 1189–1198, doi: 10.1038/s41436-018-0297-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Foukas LC et al. Long-term p110alpha PI3K inactivation exerts a beneficial effect on metabolism. EMBO molecular medicine 5, 563–571, doi: 10.1002/emmm.201201953 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Hedges CP et al. Efficacy of Providing the PI3K p110alpha Inhibitor BYL719 (Alpelisib) to Middle-Aged Mice in Their Diet. Biomolecules 11, doi: 10.3390/biom11020150 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Ortega-Molina A et al. Pharmacological inhibition of PI3K reduces adiposity and metabolic syndrome in obese mice and rhesus monkeys. Cell Metab 21, 558–570, doi: 10.1016/j.cmet.2015.02.017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Lopez-Guadamillas E et al. PI3Kalpha inhibition reduces obesity in mice. Aging 8, 2747–2753, doi: 10.18632/aging.101075 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Ortega-Molina A et al. Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell Metab 15, 382–394, doi: 10.1016/j.cmet.2012.02.001 (2012). [DOI] [PubMed] [Google Scholar]
- 344.Garcia-Cao I et al. Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell 149, 49–62, doi: 10.1016/j.cell.2012.02.030 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Bettedi L, Yan A, Schuster E, Alic N & Foukas LC Increased mitochondrial and lipid metabolism is a conserved effect of Insulin/PI3K pathway downregulation in adipose tissue. Scientific reports 10, 3418, doi: 10.1038/s41598-020-60210-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Araiz C et al. Enhanced beta-adrenergic signalling underlies an age-dependent beneficial metabolic effect of PI3K p110alpha inactivation in adipose tissue. Nature communications 10, 1546, doi: 10.1038/s41467-019-09514-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Adefemi F, Fruman DA & Marshall AJ A Case for Phosphoinositide 3-Kinase-Targeted Therapy for Infectious Disease. J Immunol 205, 3237–3245, doi: 10.4049/jimmunol.2000599 (2020). [DOI] [PubMed] [Google Scholar]
- 348.Whitehead MA, Bombardieri M, Pitzalis C & Vanhaesebroeck B Isoform-selective induction of human p110delta PI3K expression by TNFalpha: identification of a new and inducible PIK3CD promoter. Biochem J 443, 857–867, doi: 10.1042/BJ20112214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Hood VL, Berger R, Freedman R & Law AJ Transcription of PIK3CD in human brain and schizophrenia: regulation by proinflammatory cytokines. Hum Mol Genet 28, 3188–3198, doi: 10.1093/hmg/ddz144 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Martinez-Marmol R et al. p110delta PI3-Kinase Inhibition Perturbs APP and TNFalpha Trafficking, Reduces Plaque Burden, Dampens Neuroinflammation, and Prevents Cognitive Decline in an Alzheimer's Disease Mouse Model. J Neurosci 39, 7976–7991, doi: 10.1523/JNEUROSCI.0674-19.2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Eickholt BJ et al. Control of axonal growth and regeneration of sensory neurons by the p110delta PI 3-kinase. PLoS One 2, e869, doi: 10.1371/journal.pone.0000869 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Nieuwenhuis B et al. PI 3-kinase delta enhances axonal PIP3 to support axon regeneration in the adult CNS. EMBO molecular medicine 12, e11674, doi: 10.15252/emmm.201911674 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Law AJ et al. Neuregulin 1-ErbB4-PI3K signaling in schizophrenia and phosphoinositide 3-kinase-p110delta inhibition as a potential therapeutic strategy. Proc Natl Acad Sci U S A 109, 12165–12170, doi: 10.1073/pnas.1206118109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Puri KD et al. Mechanisms and implications of phosphoinositide 3-kinase delta in promoting neutrophil trafficking into inflamed tissue. Blood 103, 3448–3456, doi: 10.1182/blood-2003-05-1667 (2004). [DOI] [PubMed] [Google Scholar]
- 355.Wu W et al. PI3Kdelta as a Novel Therapeutic Target in Pathological Angiogenesis. Diabetes 69, 736–748, doi: 10.2337/db19-0713 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Bartok B et al. PI3 kinase delta is a key regulator of synoviocyte function in rheumatoid arthritis. Am J Pathol 180, 1906–1916, doi: 10.1016/j.ajpath.2012.01.030 (2012). [DOI] [PubMed] [Google Scholar]
- 357.Bartok B, Hammaker D & Firestein GS Phosphoinositide 3-kinase delta regulates migration and invasion of synoviocytes in rheumatoid arthritis. J Immunol 192, 2063–2070, doi: 10.4049/jimmunol.1300950 (2014). [DOI] [PubMed] [Google Scholar]
- 358.Ko J, Kim JY, Lee EJ & Yoon JS Inhibitory Effect of Idelalisib, a Selective Phosphatidylinositol 3-Kinase delta Inhibitor, on Adipogenesis in an In Vitro Model of Graves' Orbitopathy. Invest Ophthalmol Vis Sci 59, 4477–4485, doi: 10.1167/iovs.18-24509 (2018). [DOI] [PubMed] [Google Scholar]
- 359.Gagliano T et al. PIK3Cdelta expression by fibroblasts promotes triple-negative breast cancer progression. The Journal of clinical investigation 130, 3188–3204, doi: 10.1172/JCI128313 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Castanedo GM et al. Structure-Based Design of Tricyclic NF-kappaB Inducing Kinase (NIK) Inhibitors That Have High Selectivity over Phosphoinositide-3-kinase (PI3K). J Med Chem 60, 627–640, doi: 10.1021/acs.jmedchem.6b01363 (2017). [DOI] [PubMed] [Google Scholar]
- 361. Toska E et al. PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D. Science (New York, N.Y 355, 1324–1330, doi: 10.1126/science.aah6893 (2017). Provides a mechanistic understanding of how PI3Kα inhibition potentiates hormone therapy in breast cancer.
- 362.Koren S & Bentires-Alj M Tackling Resistance to PI3K Inhibition by Targeting the Epigenome. Cancer Cell 31, 616–618, doi: 10.1016/j.ccell.2017.04.010 (2017). [DOI] [PubMed] [Google Scholar]
- 363.Folkes AJ et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-t hieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem 51, 5522–5532, doi: 10.1021/jm800295d (2008). [DOI] [PubMed] [Google Scholar]
- 364.Han C et al. Synthesis of PI3K inhibitor GDC-0077 via a stereocontrolled N-arylation of alpha-amino acids. Tetrahedron 75, 4351–4357, doi: 10.1016/j.tet.2019.04.057 (2019). [DOI] [Google Scholar]
- 365.Turner N et al. Phase III study of GDC-0077 or placebo (pbo) with palbociclib (P) plus fulvestrant (F) in patients (pts) with PIK3CA-mutant/hormone receptor-positive/HER2-negative locally advanced or metastatic breast cancer (HR+/HER2-LA/MBC). Annals of Oncology 31, S391–S392, doi: 10.1016/j.annonc.2020.08.457 (2020). [DOI] [Google Scholar]
- 366.Juric D et al. A first-in-human phase Ia dose escalation study of GDC-0077, a p110a-selective and mutant-degrading PI3K inhibitor, in patients with PIK3CA-mutant solid tumors. Cancer Research 80, doi: 10.1158/1538-7445.Sabcs19-Ot1-08-04 (2020). [DOI] [Google Scholar]
- 367.Edgar K et al. Preclinical characterization of GDC-0077, a specific PI3K alpha inhibitor in early clinical development. Cancer Research 77, doi: 10.1158/1538-7445.Am2017-156 (2017). [DOI] [Google Scholar]
- 368.Xiang HY et al. Identification of methyl (5-(6-((4-(methylsulfonyl)piperazin-1-yl)methyl)-4-morpholinopyrrolo[2,1-f][1,2,4]triazin-2-yl)-4-(trifluoromethyl)pyridin-2-yl)carbamate (CYH33) as an orally bioavailable, highly potent, PI3K alpha inhibitor for the treatment of advanced solid tumors. Eur J Med Chem 209, 112913, doi: 10.1016/j.ejmech.2020.112913 (2021). [DOI] [PubMed] [Google Scholar]
- 369.Certal V et al. Discovery and optimization of pyrimidone indoline amide PI3Kbeta inhibitors for the treatment of phosphatase and tensin homologue (PTEN)-deficient cancers. J Med Chem 57, 903–920, doi: 10.1021/jm401642q (2014). [DOI] [PubMed] [Google Scholar]
- 370.Mateo J et al. A First-Time-in-Human Study of GSK2636771, a Phosphoinositide 3 Kinase Beta-Selective Inhibitor, in Patients with Advanced Solid Tumors. Clin Cancer Res 23, 5981–5992, doi: 10.1158/1078-0432.CCR-17-0725 (2017). [DOI] [PubMed] [Google Scholar]
- 371.Barlaam B et al. Discovery of (R)-8-(1-(3,5-difluorophenylamino)ethyl)-N,N-dimethyl-2-morpholino-4-oxo-4H-chrom ene-6-carboxamide (AZD8186): a potent and selective inhibitor of PI3Kbeta and PI3Kdelta for the treatment of PTEN-deficient cancers. J Med Chem 58, 943–962, doi: 10.1021/jm501629p (2015). [DOI] [PubMed] [Google Scholar]
- 372.Cushing TD et al. Discovery and in vivo evaluation of (S)-N-(1-(7-fluoro-2-(pyridin-2-yl)quinolin-3-yl)ethyl)-9H-purin-6-amine (AMG319) and related PI3Kdelta inhibitors for inflammation and autoimmune disease. J Med Chem 58, 480–511, doi: 10.1021/jm501624r (2015). [DOI] [PubMed] [Google Scholar]
- 373.Hoegenauer K et al. Discovery of CDZ173 (Leniolisib), Representing a Structurally Novel Class of PI3K Delta-Selective Inhibitors. ACS medicinal chemistry letters 8, 975–980, doi: 10.1021/acsmedchemlett.7b00293 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Fan L et al. SHC014748M, a novel selective inhi-bitor of PI3Kdelta, demonstrates promising preclinical antitumor activity in B cell lymphomas and chronic lymphocytic leukemia. Neoplasia 22, 714–724, doi: 10.1016/j.neo.2020.10.004 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Shin N et al. INCB040093 Is a Novel PI3Kdelta Inhibitor for the Treatment of B Cell Lymphoid Malignancies. J Pharmacol Exp Ther 364, 120–130, doi: 10.1124/jpet.117.244947 (2018). [DOI] [PubMed] [Google Scholar]
- 376.Sneha S, Nivetha R, Srikant V, Swaroop V & Ganesan TS Abstract 3331: RP6530, a dual PI3K δ/γ inhibitor attenuates cancer stem cell proliferation in serous adenocarcinoma of ovary. Cancer Research 76, 3331–3331, doi: 10.1158/1538-7445.Am2016-3331 (2016). [DOI] [Google Scholar]
- 377.Nastoupil LJ et al. Results of a First in Human, Dose Ascending, Phase I Study Examining the Safety and Tolerability of KA2237, an Oral PI3K p110β/δ Inhibitor in Patients with Relapsed/Refractory (R/R) B-Cell Lymphoma. Blood 134, 4099–4099, doi: 10.1182/blood-2019-130401 (2019). [DOI] [Google Scholar]
- 378.Wang H et al. Safety and efficacy of TQ-B3525, a novel and selective oral PI3K α/δ inhibitor, in Chinese patients with advanced malignancies: A phase I dose-escalation and expansion trial. Journal of Clinical Oncology 38, 8058–8058, doi: 10.1200/JCO.2020.38.15_suppl.8058 (2020). [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.