Abstract
Eukaryotic elongation factor 2 kinase (eEF2K) has been shown to be an important molecular driver of tumorigenesis and validated as a potential novel molecular target in various solid cancers including triple negative breast cancer (TNBC). Therefore, there has been significant interest in identifying novel inhibitors of eEF2K for the development of targeted therapeutics and clinical translation. Herein, we investigated the effects of indole ring containing derivatives of etodolac, a nonsteroidal anti-inflammatory (NSAID) drug, as potential eEF2K inhibitors and we designed and synthesized seven novel compounds with a pyrano[3,4-b] indole core structure. We evaluated the eEF2K inhibitory activity of seven of these novel compounds using in silico molecular modeling and in vitro studies in TNBC cell lines. We identified two novel compounds (EC1 and EC7) with significant in vitro activity in inhibiting eEF2K in TNBC cells. In conclusion, our studies indicate that pyrano[3,4-b] indole scaffold containing compounds demonstrate marked eEF2K inhibitory activity and they may be used as eEF2K inhibitors for the development of eEF2K-targeted therapeutics.
Eukaryotic elongation factor 2 kinase (eEF2K) has been shown to be an important molecular driver of tumorigenesis and validated as a potential novel molecular target in various solid cancers including triple negative breast cancer (TNBC).
1. Introduction
Eukaryotic elongation factor 2 kinase (eEF2K), also known as calmodulin-dependent protein kinase III (CAMKIII), is a member of the atypical alpha kinase family and has been initially shown to have a role in the regulation of protein synthesis via phosphorylation of eukaryotic elongation factor 2 (eEF2).1–3 Previous reports by our group using genetic inhibition strategies demonstrated that eEF2K promotes cell proliferation, survival, invasion, migration, and tumor growth in various highly aggressive solid tumors, including triple negative breast cancer (TNBC),4–6 and pancreatic,7,8 lung9 and ovarian cancers.10 We have shown that eEF2K is highly upregulated in TNBC cells and this is associated with metastatic disease and shorter patient survival in TNBC,6,11 lung cancer9 and ovarian cancer.10 Using various genetic silencing technologies, we have demonstrated that in vivo inhibition of eEF2K suppresses tumor growth in TNBC tumor models in mice.4,6,12,13In vitro and in vivo studies suggest that eEF2K is involved in the increased activity of PI3K/Akt, mTOR, Src/FAK, and IGFR and expression of pathways, cyclin D1 and c-myc as well as modulating the tumor microenvironment by promoting accumulation of tumor associated macrophages (TAMs),4,6,12 suggesting that eEF2K not only induces oncogenic signaling but also generates a tumor-promoting immune suppressive microenvironment. Overall, recent studies suggest that eEF2K is a critical driver of tumorigenesis and progression of highly aggressive solid cancers and may serve as an excellent therapeutic target.14
Compared with traditional chemotherapy drugs small molecule kinase inhibitors have become mainstream cancer treatments as targeted therapeutics because of their efficacy and safety.15 Since the approval of the first tyrosine kinase inhibitor imatinib (STI-571) by the US Food and Drug Administration (FDA) in 2001 many small-molecule inhibitors have been developed for the treatment of cancer.15 eEF2K cannot be inhibited by the known kinase inhibitors.16 Because of the clinical significance of eEF2K and its potential as a molecular target for precision medicine, recently there has been significant interest in developing highly effective inhibitors for eEF2K for clinical translation.17–24 However, development of effective inhibitors has been challenging due to the lack of data regarding the 3D crystal structure of eEF2K. eEF2K is not inhibited by the well-known pan kinase inhibitors such as staurosporine.16 To meet this challenge, recently we have developed and utilized the homology modeling17–19 based on similarity of the kinase domain of eEF2K to its close relatives such as myosin heavy chain kinase 2 (MHCK-2) in the alpha-kinase family and focused on identifying eEF2K inhibitors with various core structures such as indoles, which are widely used in drug discovery studies because of their effective pharmacological properties.25 Indole containing drugs such as indole alkaloids vincristine and vinblastine are used for the treatment of cancers like breast cancer and other cancers.26,27 Furthermore, indole alkaloids, including vallesiachotamine and iso-vallesiachotamine isolated from natural sources and other indoles have been reported to have anticancer activity.26,28–31 Studies have shown that some indole derivatives inhibit NFκB and mTOR/PI3K/Akt pathways, and these compounds have various biological activities such as anti-HIV and anti-mycobacterial as well as anticancer activities.29–32
Pyrano indole ring containing etodolac (R,S)-2-[1,8-diethyl-1,3,4-tetrahydropyrano[3,4-b]indole-1-yl]acetic acid is an FDA-approved nonsteroidal anti-inflammatory drug (NSAID). The S-enantiomer of the drug that exists in a racemic mixture displays selective COX-2 inhibition and is responsible for the biochemical and pharmacological activities.33,34 The antineoplastic effects of the etodolac R-enantiomer have been shown in various diseases including chronic lymphocytic leukemia (CLL), multiple myeloma, colon, and prostate cancer cells.35–38 The structures of some indole ring containing drugs are shown in Fig. 1.
Fig. 1. Some indole ring containing anticancer drugs.
Considering the effective anticancer potentials of indole-containing compounds, in the current study we focused on pyrano[3,4-b] indole scaffold containing compounds as potential inhibitors for eEF2K and designed novel aliphatic amide substituted tetrahydropyrano[3,4-b]indole compounds as etodolac derivatives and evaluated their potential as eEF2K inhibitors using in silico molecular modeling and in vitro studies in TNBC cells. The molecular modeling and docking studies utilizing the eEF2K homology modeling revealed significant interactions between the target kinase domain and synthesized compounds that led to structure–activity relationships (SAR). Our in vitro study showed that compounds EC1 and EC7 demonstrated eEF2K inhibitory activity in TNBC cells. Furthermore, analyzing prediction of toxicity profiles indicates that these two compounds have no serious toxicity problems. Overall, our results suggest that pyranoindole-based compounds may be used as eEF2K inhibitors for targeting eEF2K in TNBC or other tumor models.
2. Results and discussion
2.1. Chemistry
The pyranoindole analogues were synthesized by using etodolac as a starting compound to obtain various substituted secondary and tertiary aliphatic amides as shown in Fig. 2. EC1, EC2, and EC3 were synthesized by an amidation reaction using a coupling reagent EDCI (N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride) between the starting compound and related heterocyclic amines including 1-(2-aminoethyl)piperidine, 4-(2-aminoethyl)morpholine, and 1-(2-aminoethyl)pyrrolidine at room temperature for 48 h. Meanwhile tertiary amide compounds (EC4, EC5, EC6 and EC7) were synthesized by using T3P (propane phosphonic acid anhydride (50% solution in ethyl acetate)) as a coupling reagent at room temperature for 24 h (Fig. 2).17,19 Thin layer chromatography (TLC) was used to observe the progress of the reaction. The reactions were performed with high yields. The purification was carried out using silica column chromatography for compounds EC6 and EC7 and it was performed by washing with various solvents such as diethyl ether and methanol to remove impurity for ECs. According to our spectroscopic analysis, Fourier-transform infrared spectroscopy (FT-IR) showed the characteristic peaks of functional groups. The characterization was performed by 1H NMR and 13C NMR analysis. The molecular weight of the compounds was determined by LC-MS/MS analysis. NMR and MS spectra are given in the ESI.† In FT-IR analysis, amide N–H stretching and C O amide peaks were approximately obtained at 3300 and 1640 cm−1. The peak for carboxylic acid was observed at 1740 cm−1. Conversion from ester to amide compounds was determined by NMR analysis. In 1H NMR spectra of EC1–EC3, characteristic peaks for aromatic protons were observed between 7.34 and 7.00 ppm.
Fig. 2. Synthesis pathway of novel etodolac derivatives as eEF2K inhibitors. Reagents and conditions: (i) EDCI, HOBt, DIPEA, rt, 48 h for EC1–EC3; (ii) T3P, DCM, rt, 24 h for EC4–EC7.
Synthesis of amide substituted pyranoindole analogues as etodolac derivatives has been reported in various studies.39–41 For instance, in one of the studies, EDCI and DIPEA were used to obtain amide derivatives to be used for hepatitis C virus (HCV) NS5B polymerase inhibitors.42 Also, hydrazide, 1,2,4-triazole and thiosemicarbazide derivatives were synthesized using etodolac to evaluate anticancer and anti HCV properties.34,43 In another study, etodolac was used as a starting material to synthesize various hydrazide derivatives in a few steps under reflux conditions using methanol or ethanol.38 We preferred T3P and EDCI reagents in our present study, Yu and Xu used HBTU (O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate) as a coupling reagent to synthesize aliphatic amide substituted etodolac derivatives in acetonitrile at room temperature.40 Furthermore, literature findings show that 1,2 diethylcyanophosphonate (DEPC) could be successfully used to obtain taurine derived amides that have a pyrano[3,4-b] indole core structure.41
2.2. Investigation of the interactions between EC1–EC7 ligands and the eEF2K target
To better understand the molecular mechanisms of the synthesized compounds at the binding pocket of eEF2K, three different docking algorithms (i.e., Glide/SP (standard precision), Glide/XP (extra precision), and quantum-polarized ligand docking (QPLD)) were used. So far the crystal structure of eEF2K has not been solved yet. However, its 3D structure prediction using alphafold (v.2) is available at the UniProt (AF-000418-F1-model_v2). When we superimpose our 3D model17 with the alphafold model, both models aligned well to each other for the ligand binding domain (i.e. regions of residue numbers 107 to 326). The RMSD between the two models was found to be 1.5 Å (Fig. S1†). Since there are unrealistic sections especially at the loop regions of the target protein at the 3D model obtained using alphafold and very good alignment at the region of residue numbers 107 to 326 between the alphafold model and the 3D model developed previously by our group, we decided to focus on the ligand binding domain (i.e., the region of 107 to 326). The domain of residue numbers 107 to 326 is truncated from the alphafold model, and this model is used as an input structure at the Rosetta relaxation. (https://www.rosettacommons.org/) “Relax” is the main protocol of the all-atom refinement of structures in the Rosetta force field which searches the local conformational space around the initial structure. To evaluate the obtained different conformers, all-atom total energy scores of Rosetta are used. 200 models were constructed, and their total energies were calculated using Rosetta all-atom total energy scores. Then, the model that has the lowest total energy (i.e., model #41) was validated with Verify3D (https://www.doe-mbi.ucla.edu/verify3d/) and PROCHECK (https://www.ebi.ac.uk/thornton-srv/software/PROCHECK/) servers (Fig. S2†). The results showed that 88.60% of the residues have averaged 3D–1D scores ≥0.2.
After protein preparation, this model was used in different docking simulations (i.e., Glide/SP, Glide/XP, and QPLD). Top-docking poses were used in all-atom MD simulations. We observed that when the MD simulations were initiated with Glide/SP and Glide/XP, docking poses were not so stable at the binding pocket and during the simulations and some of the compounds are diffused from the active site. However, when we initiated the simulations with the top-docking poses of QPLD, ligand interactions were stable throughout the MD simulations except for the simulations initiated with EC4.
Table 1 shows the docking scores and average molecular mechanics/generalized Born surface area calculation (MM/GBSA) scores of the studied compounds.
Docking scores and average MM/GBSA scores of the studied compounds.
| Synthesized compounds | Glide/SP | Glide/XP | QPLD | |||
|---|---|---|---|---|---|---|
| Docking score (kcal mol−1) | Average MM/GBSA (kcal mol−1) | Docking score (kcal mol−1) | Average MM/GBSA (kcal mol−1) | Docking score (kcal mol−1) | Average MM/GBSA (kcal mol−1) | |
| EC1 | −4.0 | −29.7 | −4.7 | NA | −4.8 | −40.9 |
| EC2 | −3.7 | NA | −4.3 | −48.6 | −4.5 | −42.1 |
| EC3 | −4.0 | −52.2 | −3.4 | −52.5 | −3.7 | −49.7 |
| EC4 | −3.7 | NA | −3.5 | −40.6 | −3.9 | NA |
| EC5 | −4.0 | −41.1 | −3.6 | NA | −4.1 | −37.8 |
| EC6 | −4.0 | −45.9 | −3.4 | −54.0 | −4.1 | −41.6 |
| EC7 | −3.8 | NA | −2.6 | NA | −3.6 | −36.8 |
Glide/SP and Glide/XP docking scores ranged from −3.7 to −4.0 kcal mol−1 and −2.6 to −4.7 kcal mol−1, respectively, and QPLD scores ranged between −3.6 and −4.8 kcal mol−1. Etodolac's docking score (Glide/SP) was measured as −5.1 kcal mol−1. MD simulations for the synthesized compounds were carried out using the Desmond MD simulations program and average binding free energy calculations were carried out using the MM/GBSA approach for all complexes.
Since the docking poses showed a better stability at the binding pocket when we initiated simulations with QPLD, we investigated the trajectories of complexes using these MD simulations. Correct handling of partial charges is crucial for the success of any docking algorithm. Although commonly used force fields are capable of modeling partial atomic charges on ligands with reasonable accuracy, they are generally incapable of considering charge polarization induced by the binding pocket residues of the target protein. The protonation state of the ligand may also affect the predicted binding pose accuracy. Thus, accurate protonation state calculation of the ligand taking into account the charge polarization induced by the binding pocket residues is crucial in the docking. QPLD which uses the ab initio charge calculations overcomes this limitation. The complexes obtained by the QPLD were used as the initial structures and subjected to 200 ns MD simulation. Among the seven compounds, only ligand EC4 could not maintain its stability at the binding pocket of the receptor during 200 ns MD simulation. When we checked the average binding free energies of the compounds, all compounds have similar interaction energies.
When we checked the ligand RMSDs aligned to the protein (LigFitProt) and aligned to the docking pose conformation of the ligand (LigFitLig), we observed that in particular compound EC5 has the lowest RMSDs (Fig. 3). This shows that EC5 has a better structural stability (i.e., lowest rotational and translational motions during the simulations). Since we found that EC1 and EC7 among the seven compounds have significant activity in inhibiting eEF2K in TNBC cells, we further analyzed these two compounds. Both compounds have small LigFitLig and LigFitProt RMSD values, however, the LigFitLig RMSD of EC7 is smaller than that of EC1 which is expected since it has a small number of rotatable bonds.
Fig. 3. Normal distributions of LigFitProt (left) and LigFitLig (right) RMSDs of the studied compounds.
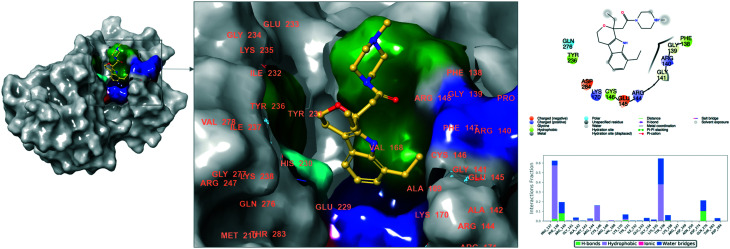
Fig. 4 and 5 show 2D and 3D ligand interaction diagrams of EC1 and EC7. Meanwhile EC1 constructs main interactions with Pro137, Tyr231 and Tyr236, and corresponding interactions were constructed by Pro137 and Lys235 with EC7.
Fig. 4. 3D and 2D ligand interaction diagram of EC1 at the binding pocket of eEF2K.
Fig. 5. 3D and 2D ligand interaction diagram of EC7 at the binding pocket of eEF2K.
We also checked the toxicity profiles of these compounds using Clarivate Analytic's MetaCore/MetaDrug program. The results showed that compounds have no serious toxicity problems (Table S1†).
2.3. Biological evaluation
To determine the effects of the synthesized compounds in inhibiting eEF2K activity in TNBC MDA-MB-231 cells, we performed western blot analysis for all compounds at concentrations ranging from 2.5 μM to 20 μM in a dose-dependent manner. eEF2K inhibition was detected by the reduction of phosphorylation levels of EF2 (p-EF2-Thr-56), which is a direct downstream substrate of the enzyme. Although compared to the original drug etodolac (EC), the other newly synthesized compounds did not display any inhibition against eEF2K at the concentrations between 2.5 to 20 μM, and a marked eEF2K inhibition was observed with compounds EC1 and EC7 at 2.5 μM as indicated by the reduced p-EF2 levels. Although these two compounds (EC1 and EC7) have heterocyclic amines containing piperidine and pyrrolidine, there was no inhibition by EC4 up to 20 μM (Fig. 6). EC1 that possesses an ethylene linker between a heterocyclic amine ring and pyrano[3,4-b] indole displayed the highest inhibition potential. Overall, these compounds exhibited highly potent activity in TNBC cells (Fig. 6).
Fig. 6. Western blot analysis of the synthesized compounds in TNBC cells. MDA-MB-231 cells were treated with the compounds for 2 h in the range of 2.5–20 μM to determine the eEF2K inhibitory activity as indicated by reduction of its downstream target pEF2 (Thr56).
Based on the literature, previously reported eEF2K inhibitors have not shown marked eEF2K inhibition. For instance, A484954 and TX1918 have been reported to inhibit eEF2K at high concentrations such as 100 μM and 10 μM, respectively. TX1918 has been shown to exert nonspecific effects and inhibit other kinases.44–46 Recently, we demonstrated that several compounds containing a coumarin scaffold were effective as eEF2K inhibitors for targeting eEF2K in cancer models.17–21 Therefore, we focused on identifying highly potent eEF2K inhibitors at low concentrations using a new core structure in cancer cells. Our findings showed that amide substituted pyranoindole derivatives are highly effective in inhibiting eEF2K and they could be used as potent eEF2K targeted anticancer candidates in the tumor models.
2.4. Structure activity relationship (SAR)
According to our combined in silico and in vitro studies, the results were evaluated with the structure–activity relationship (SAR), and it is given in Fig. 7. Among the tested compounds, EC1 and EC7 showed the highest inhibition of phospho-eEF2 (Thr56) in MDA-MB-231 cells at low concentrations of 2.5 and 5 μM for 2 h treatment (Fig. 6). To inhibit TNBC cells, it is seen that the ethylene linker between the pyrano[3,4-b]indole scaffold and heterocyclic amine contributes to the activity. This was especially observed in the presence of piperidine and pyrrolidine for the structures of secondary amide compounds EC1 and EC3, respectively. Although EC6 and EC7 are tertiary amides, EC7 has displayed eEF2K inhibition at low concentrations similar to EC1 (Fig. 6). Meanwhile EC did not display any inhibition potential in MDA-MB-231 cells (Fig. 6). Besides, EC4 that is a tertiary amide including a piperidine group linked to a pyranoindole core structure inhibits eEF2K at 20 μM following 2 h treatment in MDA-MB-231 cells. In conclusion, we observed the contribution to inhibition of the effect of structural differences between ECs and the drug etodolac.
Fig. 7. An overview of novel eEF2K inhibitors.
We also checked the structural similarities between our hit compounds EC1 and EC7 with FDA approved compounds. We used the Canvas module of the Maestro molecular modeling package for this aim. The Tversky similarity metric was used.47 The results showed that except for etodolac, the following drugs are structurally more similar to EC1: flavoxate, dyclonine, cinchocaine, guanethidine, and raloxifene. Corresponding drugs were diethylcarbamazine, propiverine, meperidine, agomelatine, and ethoheptazine for EC7 (Fig. S3 and S4†). Especially these highly similar structures (i.e., flavoxate and diethylcarbamazine) can be also considered at the eEF2K. Their docking scores (Glide/SP) were found as −3.6 and −3.8 kcal mol−1.
3. Conclusion
In this study, we demonstrated for the first time that two novel etodolac derivatives bearing a substituted amide moiety with heterocyclic amine groups including piperidine, morpholine, pyrrolidine and N-methylpiperazine can be used as novel eEF2K inhibitors. The novel lead candidates with pyrano[3,4-b] indole may be used for further evaluation of in vivo targeting of eEF2K for targeted cancer therapy. Besides the lead compounds did not have any predicted toxicity based on our in silico studies and they may be used for the design and development of novel drug candidates and for further testing in in vivo studies and clinical applications.
4. Experimental section
4.1. Materials
Thin layer chromatography (TLC) on silica gel plates (0.25 mm, 60G F254) was used to monitor the reactions. Silica gel (70–230 mesh, Merck) was used for column chromatography. An X-4 melting-point apparatus was used to determine the melting points. CAMAG UV light (254 and 365 nm) was used to determine the spots. FT-IR spectra and nuclear magnetic resonance spectra (1H NMR and 13C NMR, Agilent 600 MHz and JEOL 400 MHz) were recorded as given in our previous study.17,19 A Shimadzu Scientific Instruments LC-MS/MS 8040 liquid chromatograph (LC)-tandem mass spectrometer was used. Chemical shifts as δ values in parts per million (ppm) and coupling constants (J) in Hz and the peaks (br (broad), s (singlet), d (doublet), t (triplet), m (multiplet), dd (doublet of doublets)) were indicated. HPLC analysis was performed using a Shimadzu Prominence LC-20A Semi-Preparative HPLC system with a PDA detector on a Shim-pack ODS(H) 250 × 4.6 mm, 5 μm C18 column. HPLC grade MeOH (100%) was used for compounds EC2, EC4, EC5, EC6, and EC7 and MeOH : distilled water (%) (70 : 30) was used for the compounds EC1 and EC3, the flow rate was adjusted as 1 mL min−1 for HPLC analysis.17 Triple-negative breast cancer (TNBC) cell lines (ER−, PR−, and HER2−) MDA-MB-231 were purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA). MDA-MB-231 cells were cultured in Dulbecco's modified Eagle's medium (DMEM)/F12, supplemented with 10% FBS and 100 U ml−1 penicillin and streptomycin.18 Specific antibodies (p-EF2 (Thr56) (2331S), eEF2 (2332S), eEF2K (3692S), and GAPDH (5174S)) were purchased from Cell Signaling Technology (CST).
4.2. General procedure for the synthesis of novel etodolac derivatives
The compounds were synthesized by using a general procedure.17,19 For this purpose, different coupling reagents and solvents (from Sigma and Merck) were used in the reactions. EDCI and T3P were used for the synthesis of EC1–EC3 and EC4–EC7 to obtain exchanging products from carboxylic acid to amide, respectively. The reactions were performed in dimethylformamide (DMF) and dichloromethane (DCM) for EDCI and T3P reagents, respectively. N-Ethyl-di-isopropyl amine (DIPEA) was chosen as a base for all compounds. The reactions were successfully carried out at room temperature with high yields at the changing time points.
2-(1,8-Diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)-N-(2-(piperidin-1 yl)ethyl)acetamide (EC1)
White solid. Yield: 73%; m.p. 179–181 °C. FT-IR (vmax, cm−1): 3308 (amide and indole –NH), 2965–2850 (C–H), 1643 (C O), 1523. 1H NMR (400 MHz, CDCl3): δH 9.51 (s, 1H, NH), 7.34 (d, 1H, J = 7.6 Hz, ArH), 7.04 (t, 1H, J = 7.5 Hz, ArH), 6.98 (d, 1H, J = 6.8 Hz, ArH), 6.64 (br, 1H, NH), 3.91–4.12 (m, 2H), 3.19–3.41 (m, 2H), 2.71–2.93 (m, 6H), 2.36 (m, 6H), 1.99–2.12 (m, 2H), 1.54 (m, 4H), 1.43 (d, 3H, J = 5.1 Hz), 1.35 (t, 3H, J = 7.6 Hz, CH3), 0.85 (t, 3H, J = 7.4 Hz, CH3). 13C NMR (100 MHz, CDCl3) 171.10, 136.66, 134.74, 126.94, 126.34, 120.19, 119.50, 115.83, 107.76, 75.44, 60.72, 56.91, 54.29, 45.06, 36.16, 30.73, 30.14, 29.78, 27.20, 26.03, 24.41, 22.25, 13.88, 7.85. LC-MS/MS (ESI) [M + H]+ = 398. MW. 397 g mol−1. HPLC purity 99.641%. Formula C24H35N3O2.
2-(1,8-Diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)-N-(2-morpholinoethyl)acetamide (EC2)
White bright crystals. Yield: 71%; m.p. 197–199 °C. FT-IR (vmax, cm−1): 3317 (amide and indole –NH), 2967–2856 (C–H), 1645 (C O), 1525. 1H NMR (400 MHz, CDCl3): δH 9.43 (s, 1H, NH), 7.33 (d, 1H, J = 7.6 Hz, ArH), 7.04 (t, 1H, J = 7.4 Hz, ArH), 6.98 (d, 1H, J = 6.9 Hz, ArH), 6.69 (br, 1H, NH), 3.94–4.12 (m, 2H), 3.67 (t, 4H, J = 4.6 Hz), 3.21–3.45 (m, 2H), 2.82–2.93 (m, 6H), 2.40 (m, 6H), 1.93–2.17 (m, 2H), 1.33 (t, 3H, J = 7.6 Hz, CH3), 0.85 (t, 3H, J = 7.4 Hz, CH3). 13C NMR (100 MHz, CDCl3): 171.12, 136.29, 134.78, 126.92, 126.30, 120.31, 119.59, 115.82, 107.84, 75.41, 67.00, 60.70, 56.87, 53.35, 44.88, 35.73, 30.86, 29.78, 24.21, 22.45, 13.91, 7.86. LC-MS/MS (ESI) [M + H]+ = 400. MW. 399 g mol−1. HPLC purity 100%. Formula C23H33N3O3.
2-(1,8-Diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)-N-(2-(pyrrolidin-1-yl)ethyl)acetamide (EC3)
White solid. Yield: 68%; m.p. 165–167 °C. FT-IR (vmax, cm−1): 3303 (amide and indole –NH), 2962–2876 (C–H), 1647 (C O), 1527. 1H NMR (400 MHz, CDCl3): δH 9.57 (s, 1H, NH), 7.33 (d, 1H, J = 7.6 Hz, ArH), 7.03 (t, 1H, J = 11.7 Hz, ArH), 6.98 (d, 1H, J = 6.8 Hz, ArH), 6.64 (br, 1H, NH), 3.96–4.04 (m, 2H), 3.31–3.35 (m, 2H), 2.75–2.90 (m, 6H), 2.53 (t, 2H, J = 6.1 Hz), 2.43–2.49 (m, 4H), 1.94–2.15 (m, 2H), 1.75 (m, 4H), 1.34 (t, 3H, J = 7.6 Hz, CH3), 0.85 (t, 3H, J = 7.4 Hz, CH3). 13C NMR (100 MHz, CDCl3): 171.21, 136.60, 134.76, 126.95, 126.33, 120.18, 119.48, 115.80, 107.74, 75.50, 60.70, 54.41, 53.86, 44.93, 38.17, 37.19, 30.81, 24.23, 23.57, 13.89, 7.83. LC-MS/MS (ESI) [M + H]+ = 384. MW. 383 g mol−1. HPLC purity 99.544%. Formula C23H33N3O2.
2-(1,8-Diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)-1-(piperidin-1-yl)ethan-1-one (EC4)
White crystals. Yield: 64%; m.p. 109–111 °C. FT-IR (vmax, cm−1): 3276 (indole –NH), 3051, 2929–2860, 1603. 1H NMR (600 MHz, CDCl3) δH 10.01 (s, 1H, NH), 7.37 (d, 1H, J = 7.7 Hz, ArH), 7.05 (t, 1H, J = 7.4 Hz, ArH), 7.00 (d, 1H, J = 6.9 Hz, ArH), 3.97–4.07 (m, 2H), 3.57–3.63 (m, 2H), 3.40–3.45 (m, 2H), 2.81–2.99 (m, 6H), 2.18–2.29 (m, 2H), 1.52–1.65 (m, 6H), 1.38 (t, 3H, J = 7.5 Hz, CH3), 0.86 (t, 3H, J = 7.3 Hz, CH3). 13C NMR (150 MHz, CDCl3) δ 169.41, 136.90, 134.22, 126.80, 126.16, 119.98, 119.19, 115.71, 107.40, 60.50, 4.03, 42.79, 41.43, 30.36, 26.29, 25.53, 24.41, 22.67, 13.79, 7.80. LC-MS/MS (ESI) [M + H]+ = 355. MW. 354 g mol−1. HPLC purity 100%. Formula C22H30N2O2.
2-(1,8-Diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)-1-morpholinoethan-1-one (EC5)
White crystals. Yield: 60%; m.p. 108–110 °C. FT-IR (vmax, cm−1): 3334 (indole –NH), 3052, 2963–2856, 1631 (C O), 1609. 1H NMR (600 MHz, CDCl3) δH 9.75 (s, 1H, NH), 7.36 (d, 1H, J = 7.7 Hz, ArH), 7.05 (t, 1H, J = 7.4 Hz, ArH), 7.00 (d, 1H, J = 7.0 Hz, ArH), 3.96–4.05 (m, 2H), 3.61–3.69 (m, 4H), 3.47–3.53 (m, 2H), 2.77–2.95 (m, 6H), 2.13–2.28 (m, 2H), 1.37 (t, 3H, J = 7.5 Hz, CH3), 0.85 (t, 3H, J = 7.3 Hz, CH3). 13C NMR (150 MHz, CDCl3) 169.99, 136.38, 134.26, 126.72, 126.12, 120.16, 119.34, 115.78, 107.73, 76.08, 66.77, 66.40, 60.54, 60.38, 46.29, 41.90, 41.42, 30.49, 24.41, 22.51, 13.88, 7.36. LC-MS/MS (ESI) [M + H]+ = 357. MW. 356 g mol−1. HPLC purity 96.55%. Formula C21H28N2O3.
2-(1,8-Diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)-1-(pyrrolidin-1-yl)ethan-1-one (EC6)
Purification by silica column chromatography (DCM). White solid. Yield: 52%; m.p. 103–105 °C. FT-IR (vmax, cm−1): 3310 (indole –NH), 3051, 2964–2874, 1628 (C O), 1606. 1H NMR (600 MHz, CDCl3) δH 10.10 (s, 1H, NH), 7.36 (d, 1H, J = 7.7 Hz, ArH), 7.04 (t, 1H, J = 7.3 Hz, ArH), 6.99 (d, 1H, J = 7.0 Hz, ArH), 3.97–4.07 (m, 2H), 3.35–3.52 (m, 4H), 2.89–2.92 (m, 4H), 2.80–2.83 (m, 2H), 2.15–2.32 (m, 2H), 1.85–1.98 (m, 4H), 1.37 (t, 3H, J = 7.5 Hz, CH3), 0.86 (t, 3H, J = 7.1 Hz, CH3). 13C NMR (150 MHz, CDCl3) 169.88, 136.95, 134.27, 126.85, 126.15, 119.93, 119.18, 115.68, 107.30, 60.56, 47.13, 45.91, 43.14, 30.25, 26.11, 24.32, 24.27, 22.55, 13.82, 7.73. LC-MS/MS (ESI) [M + H]+ = 341. MW. 340 g mol−1. HPLC purity 97.81%. Formula C21H28N2O2.
2-(1,8-Diethyl-1,3,4,9-tetrahydropyrano[3,4-b]indol-1-yl)-1-(4-methylpiperazin-1-yl)ethan-1-one (EC7)
Purification by silica column chromatography (DCM). White. Yield: 55%; m.p. 115–118 °C. FT-IR (vmax, cm−1): 3332 (indole –NH), 3051, 2964–2875, 1629 (C O), 1608. 1H NMR (600 MHz, CDCl3) δH 9.81 (s, 1H, NH), 7.35 (d, 1H, J = 7.7 Hz, ArH), 7.03 (t, 1H, J = 7.4 Hz, ArH), 6.98 (d, 1H, J = 6.9 Hz, ArH), 3.95–4.04 (m, 2H), 3.66 (s, 2H), 3.48–3.54 (m, 2H), 2.75–2.96 (m, 4H), 2.39 (br, 2H), 2.24–2.32 (m, 4H), 2.11–2.15 (m, 2H), 1.77 (s, 3H, CH3), 1.36 (t, 3H, J = 7.6 Hz, CH3), 0.83 (t, 3H, J = 7.3 Hz, CH3). 13C NMR (150 MHz, CDCl3) 169.69, 136.51, 134.24, 126.75, 126.10, 120.07, 119.27, 115.73, 107.61, 76.08, 60.51, 54.83, 54.59, 45.86, 45.78, 41.51, 41.46, 30.47, 24.24, 22.50, 13.75, 7.75. LC-MS/MS (ESI) [M + H]+ = 370. MW. 369 g mol−1. HPLC purity 100%. Formula C22H31N3O2.
4.3. eEF2K activity
Western blot analysis was carried out according to our previously reported studies to evaluate the eEF2K inhibition activity for the synthesized compounds.17–19,47 The synthesized compounds were applied to MDA-MB-231 cells in the range of various concentrations from 2.5 to 20 μM for 2 h treatment. The expression levels of p-EF2 (Thr56) inhibition and eEF2K activity were determined by using related antibodies such as p-EF2 (Thr56), eEF2 and eEF2K. GAPDH was used as a loading control.
4.4. Preparation of the protein and ligand structure
The 2-dimensional (2D) structures of the synthesized molecules (EC1–EC7) were sketched and prepared with the Maestro LigPrep module at neutral pH using default settings and 3D structures were obtained.48 Since the crystal structure of eEF2K is not solved yet we used the model structure of alphafold (v.2) UniProt (AF-000418-F1-model_v2). However, there are unrealistic sections especially at the loop regions of the target protein at the 3D model obtained using alphafold and very good alignment at the ligand binding domain (i.e., region of residue numbers 107 to 326) between the alphafold model and the 3D model developed previously by our group. The domain of residue numbers 107 to 326 is truncated from the alphafold model, and this model is used as an input structure at the Rosetta relaxation. 200 models were constructed, and their total energies were calculated using Rosetta. Then, the model that has the lowest total energy was validated with Verify3D49 and PROCHECK50 servers. The results showed that 88.60% of the residues have averaged 3D–1D scores ≥0.2.
4.5. Docking of the EC1–EC7 ligands to the eEF2K target
Seven compounds were docked against the eEF2K target. Docking processes were conducted in three different docking algorithms: Glide extra precision (XP) docking, Glide standard precision (SP) docking and quantum polarized ligand docking (QPLD).51–53
4.6. MD simulations and MM/GBSA
To observe the conformational stability of the complexes, MD simulations were carried out using Desmond.54 Top poses of the complexes were solvated in the orthorhombic simple point charge (SPC) water model.55 The systems were neutralized with 0.15 M Na and Cl ions. To maintain the temperature at 310 K, a Nose Hoover thermostat56 was used. MD simulations were set up using 1.01 bar of pressure (Martyna–Tobias–Klein barostat)57 conditions. Throughout the simulations, 4000 trajectory frames were created, and 200 frames were recorded. Top scored complexes were further investigated in terms of free binding energy by MM/GBSA analysis in the Schrodinger Prime module.58
4.7. Binary QSAR models
Toxicity prediction analysis of the compounds was carried out in binary QSAR models of MetaCore/MetaDrug which is a comprehensive systems biology analysis suite from Clarivate Analytics.
Conflicts of interest
The authors declare that they have no known competing financial interests.
Supplementary Material
Acknowledgments
The authors thank the Çanakkale Onsekiz Mart University-COBILTUM Center Laboratory and Çankırı Karatekin University Research Center Laboratory for spectral analysis.
Electronic supplementary information (ESI) available: 1H NMR, 13C NMR and LC-MS/MS spectra and HPLC chromatograms of the synthesized compounds. See DOI: https://doi.org/10.1039/d2md00105e
References
- Ryazanov A. G. Shestakova E. A. Natapov P. G. Nature. 1988;334(6178):170–173. doi: 10.1038/334170a0. [DOI] [PubMed] [Google Scholar]
- Ryazanov A. G. Davydova E. K. FEBS Lett. 1989;251:187–190. doi: 10.1016/0014-5793(89)81452-8. [DOI] [PubMed] [Google Scholar]
- Kenney J. W. Moore C. E. Wang X. Proud C. G. Adv. Biol. Regul. 2014;55:15–27. doi: 10.1016/j.jbior.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Tekedereli I. Alpay S. N. Tavares C. D. Cobanoglu Z. E. Kaoud T. S. Sahin I. Sood A. K. Lopez-Berestein G. Dalby K. N. Ozpolat B. PLoS One. 2012;7:e41171. doi: 10.1371/journal.pone.0041171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi R. Asik E. Kahraman N. Turk M. Ozpolat B. Ulubayram K. Nanomedicine. 2017;12(16):1961–1973. doi: 10.2217/nnm-2017-0081. [DOI] [PubMed] [Google Scholar]
- Asik E. Akpinar Y. Caner A. Kahraman N. Guray T. Volkan M. Albarracin C. Pataer A. Arun B. Ozpolat B. Nanomedicine. 2019;14(17):2315–2338. doi: 10.2217/nnm-2019-0132. [DOI] [PubMed] [Google Scholar]
- Ashour A. A. Gurbuz N. Alpay S. N. Abdel-Aziz A. A. Mansour A. M. Huo L. Ozpolat B. J. Cell. Mol. Med. 2014;18:2235–2251. doi: 10.1111/jcmm.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour A. A. Abdel-Aziz A. A. Mansour A. M. Alpay S. N. Huo L. Ozpolat B. Apoptosis. 2014;19:241–258. doi: 10.1007/s10495-013-0927-2. [DOI] [PubMed] [Google Scholar]
- Bircan H. A. Gurbuz N. Pataer A. Caner A. Kahraman N. Bayraktar E. Bayraktar R. Erdogan M. A. Kabil N. Ozpolat B. Lung Cancer. 2018;124:31–39. doi: 10.1016/j.lungcan.2018.07.027. [DOI] [PubMed] [Google Scholar]
- Erdogan M. A. Ashour A. Yuca E. Gorgulu K. Ozpolat B. Cell. Signalling. 2021;81:109938. doi: 10.1016/j.cellsig.2021.109938. [DOI] [PubMed] [Google Scholar]
- Bayraktar R. Pichler M. Kanlikilicer P. Ivan C. Bayraktar E. Kahraman N. Aslan B. Oguztuzun S. Ulasli M. Arslan A. Calin G. Lopez-Berestein G. Ozpolat B. Oncotarget. 2017;8(7):11641–11658. doi: 10.18632/oncotarget.14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar R. Ivan C. Bayraktar E. Kanlikilicer P. Kabil N. Kahraman N. Mokhlis H. A. Karakas D. Rodriguez-Aguayo C. Arslan A. Sheng J. Wong S. T. Lopez-Berestein G. Calin G. A. Ozpolat B. Clin. Cancer Res. 2018;24(17):4225–4241. doi: 10.1158/1078-0432.CCR-17-1959. [DOI] [PubMed] [Google Scholar]
- Gorur A. Bayraktar R. Ivan C. Mokhlis H. A. Bayraktar E. Kahraman N. Karakas D. Karamil S. Kabil N. N. Kanlikilicer P. Aslan B. Tamer L. Wang Z. Cristini V. Lopez-Berestein G. Calin G. Ozpolat B. Mol. Ther.--Nucleic Acids. 2021;23:930–943. doi: 10.1016/j.omtn.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Xie J. Proud C. G. Cancers. 2017;9(12):162. doi: 10.3390/cancers9120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L. Li Y. Xiong L. Wang W. Wu M. Yuan T. Yang W. Tian C. Miao Z. Wang T. Yang S. Signal Transduction Targeted Ther. 2021;6(1):201. doi: 10.1038/s41392-021-00572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R. Proud C. G. Acta Pharmacol. Sin. 2016;37:285–294. doi: 10.1038/aps.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comert Onder F. Durdagi S. Kahraman N. Uslu T. N. Kandemir H. Atici E. B. Ozpolat B. Ay M. Bioorg. Chem. 2021;116:105296. doi: 10.1016/j.bioorg.2021.105296. [DOI] [PubMed] [Google Scholar]
- Comert Onder F. Kahraman N. Bellur Atici E. Cagir A. Kandemir H. Tatar G. Taskin Tok T. Kara G. Karliga B. Durdagi S. Ay M. Ozpolat B. ACS Pharmacol. Transl. Sci. 2021;4(2):926–940. doi: 10.1021/acsptsci.1c00030. [DOI] [PMC free article] [PubMed] [Google Scholar]; , Correction. ACS Pharmacol. Transl. Sci., 2021, 4(3), 1247
- Comert Onder F. Durdagi S. Sahin K. Ozpolat B. Ay M. J. Chem. Inf. Model. 2020;60(3):1766–1778. doi: 10.1021/acs.jcim.9b01083. [DOI] [PubMed] [Google Scholar]
- Comert Onder F. Sagbas Suner S. Sahiner N. Ay M. Ozpolat B. Pharm. Res. 2020;37(3):63. doi: 10.1007/s11095-020-2774-5. [DOI] [PubMed] [Google Scholar]
- Ay M., Ozpolat B., Comert Onder F., Taşkın Tok T., Bellur Atici E., Karlıga B., Kandemir H., Cagır A., Sahiner N. and Tatar G., WO2019/240701A1, 2019
- Zhu S. Liao M. Tan H. Zhu L. Chen Y. He G. Liu B. J. Med. Chem. 2021;64(13):8870–8883. doi: 10.1021/acs.jmedchem.0c02218. [DOI] [PubMed] [Google Scholar]
- Xiao T. Liu R. Proud C. G. Wang M. W. Acta Pharm. Sin. B. 2016;6(6):557–563. doi: 10.1016/j.apsb.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B. Zou J. Zhang Q. Wang Z. Wang N. He S. Zhao Y. Naman C. B. Int. J. Mol. Sci. 2021;22(5):2408. doi: 10.3390/ijms22052408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharief A. M. S. Ammar Y. A. Belal A. El-Sharief M. A. M. S. Mohamed Y. A. Mehany A. B. M. Elhag Ali G. A. M. Ragab A. Bioorg. Chem. 2019;85:399–412. doi: 10.1016/j.bioorg.2019.01.016. [DOI] [PubMed] [Google Scholar]
- Sachdeva H. Mathur J. Guleria A. J. Chil. Chem. Soc. 2020;65(3):4900–4907. doi: 10.4067/s0717-97072020000204900. [DOI] [Google Scholar]
- Martino E. Casamassima G. Castiglione S. Cellupica E. Pantalone S. Papagni F. Rui M. Siciliano A. M. Collina S. ACS Med. Chem. Lett. 2018;28(17):2816–2826. doi: 10.1016/j.bmcl.2018.06.044. [DOI] [PubMed] [Google Scholar]
- Mishra D. P. Khan M. A. Yadav D. K. Rawat A. K. Kumar Singh R. Ahamad T. Hussain M. K. Saquib M. Khan M. F. ChemistrySelect. 2018;3:8468–8472. doi: 10.1002/slct.201801475. [DOI] [Google Scholar]
- Devi N. Kaur K. Biharee A. Jaitak V. Anti-Cancer Agents Med. Chem. 2021;21(14):1802–1824. doi: 10.2174/1871520621999210104192644. [DOI] [PubMed] [Google Scholar]
- Kaur K. Jaitak V. Anti-Cancer Agents Med. Chem. 2019;19(8):962–983. doi: 10.2174/1871520619666190312125602. [DOI] [PubMed] [Google Scholar]
- Sidhu J. S. Singla R. Mayank V. Anti-Cancer Agents Med. Chem. 2015;16(2):160–173. doi: 10.2174/1871520615666150520144217. [DOI] [PubMed] [Google Scholar]
- Laporte M. G. Jackson R. W. Draper T. L. Gaboury J. A. Galie K. Herbertz T. Hussey A. R. Rippin S. R. Benetatos C. A. Chunduru S. K. Christensen J. S. Coburn G. A. Rizzo C. J. Rhodes G. O'Connell J. Howe A. Y. Mansour T. S. Collett M. S. Pevear D. C. Young D. C. Gao T. Tyrrell D. L. Kneteman N. M. Burns C. J. Condon S. M. ChemMedChem. 2008;3(10):1508–1515. doi: 10.1002/cmdc.200800168. [DOI] [PubMed] [Google Scholar]
- Demerson C. A. Humber L. G. Abraham N. A. Schilling G. Martel R. R. Pace-Asciak C. J. Med. Chem. 1983;26(12):1778–1780. doi: 10.1021/jm00366a025. [DOI] [PubMed] [Google Scholar]
- Çıkla-Süzgün P. Kaushik-Basu N. Basu A. Talele T. T. Durmaz I. Çetin-Atalay R. Küçükgüzel Ş. G. J. Enzyme Inhib. Med. Chem. 2015;30(5):778–785. doi: 10.3109/14756366.2014.971780. [DOI] [PubMed] [Google Scholar]
- Behari J. Zeng G. Otruba W. Thompson M. D. Muller P. Micsenyi A. Sekhon S. S. Leoni L. Monga S. P. J. Hepatol. 2007;46(5):849–857. doi: 10.1016/j.jhep.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri S. K. Corr M. James S. Y. Bernasconi M. Lu D. Liu W. Cottam H. B. Leoni L. M. Carson D. A. Zhang X. K. Proc. Natl. Acad. Sci. U. S. A. 2005;102:2525–2530. doi: 10.1073/pnas.0409721102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui H. Hideshima T. Hamasaki M. Roccaro A. M. Shiraishi N. Kumar S. Tassone P. Ishitsuka K. Raje N. Tai Y. T. Podar K. Chauhan D. Leoni L. M. Kanekal S. Elliott G. Munshi N. C. Anderson K. C. Blood. 2005;106:706–712. doi: 10.1182/blood-2005-02-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çıkla P. Özsavcı D. Bingöl-Özakpınar Ö. Şener A. Çevik Ö. Özbaş-Turan S. Akbuğa J. Şahin F. Küçükgüzel Ş. G. Arch. Pharm. 2013;346(5):367–379. doi: 10.1002/ardp.201200449. [DOI] [PubMed] [Google Scholar]
- Chongxi Yu. and Lina X., WO2008017903A1, 2008
- Chongxi Yu. and Lina X., US20090238763A1, 2009
- Vizioli E. D. O., Chin C. M., Menegon R. F., Blau L., Santos J. L. D. and Longo M. D. J., WO2009124371A3, 2009
- Gopalsamy A. Lim K. Ciszewski G. Park K. Ellingboe J. W. Bloom J. Insaf S. Upeslacis J. Mansour T. S. Krishnamurthy G. Damarla M. Pyatski Y. Ho D. Howe A. Y. Orlowski M. Feld B. O'Connell J. J. Med. Chem. 2004;47(26):6603–6608. doi: 10.1021/jm0401255. [DOI] [PubMed] [Google Scholar]
- Çıkla P. Arora P. Basu A. Talele T. T. Kaushik-Basu N. Küçükgüzel Ş. G. Marmara Pharm. J. 2013;17:138–146. doi: 10.12991/201317382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edupuganti R. Wang Q. Tavares C. D. J. Chitjian C. A. Bachman J. L. Ren P. Anslyn E. V. Dalby K. N. Bioorg. Med. Chem. 2014;22:4910–4916. doi: 10.1016/j.bmc.2014.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H. Nagasawa H. Ishibashi M. Uto Y. Hirata A. Saijo K. Ohkura K. Kirk K. L. Uehara Y. Bioorg. Med. Chem. 2002;10:3257–3265. doi: 10.1016/S0968-0896(02)00160-8. [DOI] [PubMed] [Google Scholar]
- Kabil N. Bayraktar R. Kahraman N. Mokhlis H. A. Calin G. A. Lopez-Berestein G. Ozpolat B. Breast Cancer Res. Treat. 2018;171(3):593–605. doi: 10.1007/s10549-018-4847-2. [DOI] [PubMed] [Google Scholar]
- Tversky A. Features of similarity. Psychol. Res. 1977;84:327–352. [Google Scholar]
- Madhavi Sastry G. Adzhigirey M. Day T. Annabhimoju R. Sherman W. J. Comput.-Aided Mol. Des. 2013;27(3):221–234. doi: 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- Luthy R. Bowei J. Einsenberg D. Methods Enzymol. 1997;277:396–404. doi: 10.1016/s0076-6879(97)77022-8. [DOI] [PubMed] [Google Scholar]
- Laskowski R. A. MacArthur M. W. Moss D. S. Thornton J. M. J. Appl. Crystallogr. 1993;26(2):283–291. doi: 10.1107/S0021889892009944. [DOI] [Google Scholar]
- Friesner R. A. Murphy R. B. Repasky M. P. Frye L. L. Greenwood J. R. Halgren T. A. Sanschagrin P. C. Mainz D. T. J. Med. Chem. 2006;49(21):6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- Friesner R. A. Banks J. L. Murphy R. B. Halgren T. A. Klicic J. J. Mainz D. T. Repasky M. P. Knoll E. H. Shelley M. Perry J. K. Shaw D. E. Francis P. Shenkin P. S. J. Med. Chem. 2004;47(7):1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- Cho A. E. Guallar V. Berne B. J. Friesner R. J. Comput. Chem. 2005;26(9):915–931. doi: 10.1002/jcc.20222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers K. J., Chow E., Xu H., Dror R. O., Eastwood M. P., Gregersen B. A., Klepeis J. L., Kolossvary I., Moraes M. A., Sacerdoti F. D., Salmon J. K., Shan Y. and Shaw D. E., SC'06: Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, 2006, (11), p. 43
- Hoover W. G. Phys. Rev. A: At., Mol., Opt. Phys. 1985;31(3):1695–1697. doi: 10.1103/PhysRevA.31.1695. [DOI] [PubMed] [Google Scholar]
- Evans D. J. Holian B. L. J. Chem. Phys. 1985;83(8):4069–4074. doi: 10.1063/1.449071. [DOI] [Google Scholar]
- Martyna G. J. Tobias D. J. Klein M. L. J. Chem. Phys. 1994;101(5):4177–4189. doi: 10.1063/1.467468. [DOI] [Google Scholar]
- Jacobson M. P. Pincus D. L. Rapp C. S. Day T. J. F. Honig B. Shaw D. E. Friesner R. A. A. Proteins: Struct., Funct., Genet. 2004;55(2):351–367. doi: 10.1002/prot.10613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.