Abstract
Background
Cancer‐related fatigue is a debilitating late effect after treatment for childhood cancer. The prevalence of fatigue in childhood cancer survivors (CCSs) and associated factors for fatigue has varied widely in previous studies. Two important aspects of cancer‐related fatigue, its severity and chronicity, are often not assessed. This study investigated the prevalence of, and risk factors for, severe chronic fatigue (CF) in a national cohort of Dutch CCSs.
Methods
In this study, 2810 CCSs (5‐year survivors of all childhood malignancies diagnosed between 1963 and 2001 with a current age of 12‐65 years) and 1040 sibling controls were included. CF was assessed with the Short Fatigue Questionnaire and was defined as a score ≥ 18 and persistence of fatigue for ≥6 months. Cancer‐ and treatment‐related characteristics, current health problems, and demographic and lifestyle variables were assessed as potential risk factors for CF via multivariable logistic regression analyses.
Results
In adult CCSs and sibling controls (≥18 years old), the prevalence of CF was 26.1% and 14.1%, respectively (P < .001). In adolescent CCSs and sibling controls (<18 years old), the prevalence of CF was 10.9% and 3.2%, respectively. Female gender (odds ratio [OR], 2.13; 95% confidence interval [CI], 1.73‐2.62), unemployment (OR, 2.18; 95% CI, 1.67‐2.85), having 1 or more health problems (OR for 1‐2, 1.48; 95% CI, 1.18‐1.87; OR for >2, 2.20; 95% CI, 1.50‐3.21), and a central nervous system diagnosis (OR, 1.74; 95% CI, 1.17‐2.60) were significantly associated with CF in adult CCSs.
Conclusions
This study shows that CCSs, regardless of their cancer diagnosis, report CF more often than sibling controls. This study provides new evidence for the prevalence of fatigue in CCSs.
Keywords: cancer‐related fatigue, childhood cancer survivors, late effects, survivorship
Short abstract
One in 4 childhood cancer survivors reports chronic fatigue. Current health problems increase the risk of reporting chronic fatigue.
Introduction
Cancer‐related fatigue is a debilitating late effect in childhood cancer survivors (CCSs) that negatively affects their quality of life. 1 , 2 It is defined as a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning. 3 According to this definition, the severity and persistence of fatigue are elements to include for the assessment of fatigue.
In the literature, widely varying prevalence rates of fatigue, ranging from 0.0% to 61.7%, have been found. 4 The variation in prevalence rates is probably due to differences in study methodology. Previous studies differed in the studied populations, sample sizes, and instruments used to assess fatigue, and they did not assess both the severity and persistence of fatigue. 4 Also, most studies did not include a control group. 4 , 5 , 6 , 7 It is likely that the differences in methodology contributed to the variation in reported prevalence rates. Conflicting results have also been reported on risk and associated factors for fatigue. 4
In the Dutch Childhood Cancer Survivor Late Effect Study (DCCSS LATER), a nationwide cohort study including survivors of all childhood malignancies, we investigated the prevalence of chronic fatigue (CF), which was defined as severe and persistent fatigue, among CCSs and a sibling control group. By combining the strengths of methodologies used in previously mentioned studies (the use of a validated fatigue questionnaire with a validated cutoff score for severe fatigue and the inclusion of a control group) and adding the duration of fatigue symptoms to the outcome measure, we aimed to investigate the prevalence of clinically relevant CF. By doing so in a nationwide cohort including all childhood malignancies, we believe the outcomes to be more broadly generalizable then findings of previous studies. Also, we studied the relationship between CF and cancer‐ and treatment‐related factors, demographic characteristics, health problems, and lifestyle variables.
Materials and Methods
Design and Participants
This study had a cross‐sectional design and included data for participants from the DCCSS LATER cohort. This cohort included survivors of all childhood malignancies who were treated before the age of 18 years at one of the pediatric oncology centers in the Netherlands between January 1, 1963, and December 31, 2001, and who survived at least 5 years after their diagnosis (n = 6165; Fig. 1). For all survivors, details on the prior cancer diagnosis and treatment were collected from medical files. 8 More details of the DCCSS LATER cohort have been described elsewhere. 8
Figure 1.
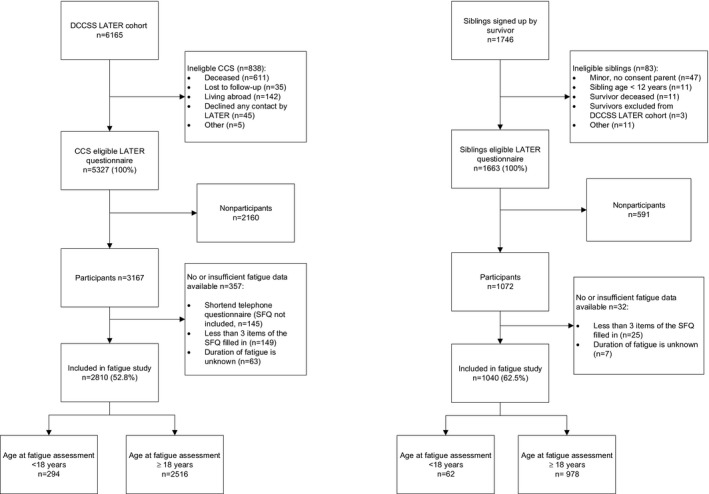
Flowchart inclusion of CCSs and siblings of CCSs. CCS indicates childhood cancer survivor; DCCSS‐LATER, Dutch Childhood Cancer Survivor Late Effect Study; SFQ, Short Fatigue Questionnaire.
All CCSs who were alive, traceable, and living in the Netherlands were eligible to participate in a questionnaire survey called the DCCSS LATER general health questionnaire. Eligible survivors received an information package in the period of September 2012 to April 2014 that included an information form and an invitation to complete the questionnaire. Survivors were included in the current study if 1) the survivor or a parent (if the survivor was younger than 16 years) signed an informed consent form and 2) sufficient data were available to determine the fatigue status. In all, 5327 survivors of the total cohort were eligible for the questionnaire survey, 3167 survivors participated, and 2810 survivors were included in the current study (response rate, 52.8%; Fig. 1).
Siblings of survivors were included as a control group. Eligible siblings were at least 12 years old, alive, and traceable and received in 2015 an information package similar to that sent to the survivors. In all, 1663 siblings were eligible, 1072 siblings participated, and 1040 siblings were included in the current study (response rate, 62.5%; Fig. 1).
Measures
The DCCSS LATER general health questionnaire evaluates self‐reported medical conditions of participants since their cancer treatment, medication use, demographic characteristics, lifestyle factors, fertility, and fatigue. An overview of relevant questions that were extracted for this study is presented in Supporting Table 1. Information about predefined, clinically relevant, self‐reported health problems 9 was validated on the basis of self‐reported medication use and, if there was still uncertainty, on the basis of medical files for survivors and general practitioner data for siblings. Clinically relevant health problems were defined as outcomes associated with clinically relevant levels of symptoms or requiring medical treatment. 9
Fatigue was assessed with the Short Fatigue Questionnaire (SFQ). The SFQ consists of 4 items rated on a 7‐point Likert scale, with the total score ranging from 4 to 28. 10 The validated cutoff score of 18 or higher indicates the presence of severe fatigue. 11 , 12 In a study including 2 cancer survivor populations (breast cancer survivors and survivors treated with stem cell transplantation), Penson et al 11 showed that a cutoff score of 18 had excellent positive and negative predictive values. The SFQ is used in the Netherlands to screen for fatigue in routine clinical care, including the survivorship care clinics for survivors of childhood cancer. Preliminary results showed the psychometric properties of the SFQ (including a cutoff score of 18 to indicate severe fatigue) to be satisfactory (good construct validity, structural validity, internal consistency, and reliability) in CCSs (A. Penson, I. Walraven, E. Bronkhorst et al, unpublished data, 2021). In the current study, Cronbach's α of the SFQ was 0.89, which indicated high internal consistency. To assess the persistence of fatigue, participants were asked to indicate the duration of fatigue in weeks, months, or years. Participants who filled in fewer than 3 items of the SFQ or did not report the duration of fatigue were excluded. CF was defined as severe fatigue (SFQ score ≥ 18) and a duration of fatigue of at least 6 months.
For survivors, the following cancer‐related variables were included: type of cancer diagnosis, type of treatment for the primary tumor and all recurrences (ie, surgery [yes/no], chemotherapy [yes/no], and radiotherapy [yes/no]), occurrence of recurrence, time since diagnosis, and age at diagnosis.
Statistical Analyses
A comparison of included and nonincluded survivors was made with respect to gender, birth decade, cancer diagnosis, cancer treatment, treatment period, and age at diagnosis to determine a possible selection bias (χ2 test).
For the included participants, descriptive statistics and missing values were examined. Eleven survivors (0.4%) and 3 siblings (0.3%) had 1 missing value on the SFQ. This missing value was imputed with the mean score of the remaining 3 items.
The prevalence of CF in survivors and siblings, including the overall prevalence, the prevalence per cancer diagnosis, and the prevalence separated by age at assessment, was calculated and compared with the χ2 test. To correct for differences between survivors and siblings, a multivariable logistic regression analysis was performed with participant characteristics as covariates. Survivors of acute lymphoblastic leukemia (ALL) were used as the reference group in the comparison between cancer diagnostic groups because it was the largest group and was previously identified to have a low prevalence of fatigue. 4 , 5
Because adolescent survivors (age at assessment < 18 years) and adult survivors (age at assessment ≥ 18 years) were not comparable concerning demographic characteristics of interest (ie, employment status, educational level, and marital status), adults and adolescents were analyzed and reported separately with respect to risk and associated factors for CF. In addition, in the Netherlands, the transition from the childhood‐care system to the adult‐care system occurs at the age of 18 years.
To examine risk factors for and factors associated with CF in adult CCSs, a multivariable logistic regression analysis was performed with CF as the dependent variable. Survivors without data on cancer treatment were excluded from the analysis. Independent variables were entered into the model in 4 blocks: demographic and general disease characteristics (gender, employment status, educational level, marital status, time since diagnosis, and age at diagnosis), current health‐related and lifestyle factors (alcohol use, smoking, drug use in the past year, body mass index, and number of health problems), cancer diagnosis, and cancer treatment. Cancer diagnosis, cancer treatment, age at diagnosis, time since diagnosis, and gender were considered potential risk factors, and all others were considered associated factors because they may or may not have preceded the onset of fatigue. Variance inflation factors were evaluated to determine multicollinearity between factors. A variance inflation factor score higher than 10 indicates serious issues with multicollinearity. 13 When this was the case, correlating factors were visually inspected to decide which factor should be deleted.
For adolescent CCSs, risk and associated factors were analyzed in 2 smaller multivariable models because of the small sample size. The following factors were examined: gender, age at diagnosis, time since diagnosis, health problems (yes/no), treatment, and cancer diagnosis.
For all analyses, a P value less than .05 was considered statistically significant. All analyses were conducted with IBM SPSS version 25. 14
Results
Survivors who were included in the study (n = 2810) were more likely to be female and had received chemotherapy more often than nonincluded survivors (Supporting Table 2). The 2 groups did not differ in age at diagnosis, period of treatment, birth decade, or cancer diagnosis.
Table 1 presents characteristics of the participating survivors and siblings. The most frequent cancer diagnosis was ALL (29.8%), and the majority of CCSs were treated with chemotherapy with or without surgery (53.0%). The median time since diagnosis was 22.4 years (range, 11.0‐50.1 years). Of the 2810 survivors, 2516 were ≥18 years old at the time of assessment, and 294 were <18 years at the time of assessment (see Supporting Table 3 for characteristics per age group).
TABLE 1.
Characteristics of Participating Survivors and Siblings
| Survivors, No. (%) | Siblings, No. (%) | P a | |
|---|---|---|---|
| Total | 2810 (100) | 1040 (100) | |
| Demographic characteristics | |||
| Age at assessment, median (range), y | 29.67 (11.75‐65.00) | 31.88 (12.42‐73.00) | <.001 |
| <18 y | 294 (10.5) | 62 (6.0) | |
| ≥18 y | 2516 (89.5) | 978 (94.0) | |
| Gender | <.001 | ||
| Male | 1464 (52.1) | 437 (42.0) | |
| Female | 1346 (47.9) | 603 (58.0) | |
| Educational level b | <.001 | ||
| Low | 512 (18.2) | 79 (7.6) | |
| Middle | 1464 (52.1) | 496 (47.7) | |
| High | 801 (28.5) | 458 (44.0) | |
| Employment status c | <.001 | ||
| Employed | 1713 (61.0) | 772 (74.2) | |
| Unemployed | 401 (14.3) | 66 (6.3) | |
| Student | 646 (23.0) | 196 (18.8) | |
| Marital status d | <.001 | ||
| Married or living as married | 1271 (45.2) | 614 (59.0) | |
| Not married | 1457 (51.9) | 339 (32.6) | |
| Divorced or widowed | 52 (1.9) | 21 (2.0) | |
| Health‐related and lifestyle variables | |||
| BMI, median (IQR), kg/m2 e | 23.37 (20.98‐26.04) | 23.51 (21.55‐25.97) | .141 |
| Current smoker f | <.001 | ||
| Yes | 366 (13.0) | 395 (38.0) | |
| No | 2432 (86.5) | 644 (61.9) | |
| Current alcohol use g | <.001 | ||
| Yes | 1183 (42.1) | 518 (49.8) | |
| No | 1623 (57.8) | 521 (50.1) | |
| Drug use in past year h | .002 | ||
| Yes | 262 (9.3) | 118 (11.3) | |
| No | 2506 (89.2) | 919 (88.4) | |
| No. of health problems i | <.001 | ||
| 0 | 1497 (53.3) | 756 (72.7) | |
| 1‐2 | 1069 (38.0) | 228 (21.9) | |
| >2 | 212 (7.5) | 10 (1.0) | |
| Cancer‐ and treatment‐related variables | |||
| Age at diagnosis, median (range), y | 5.42 (0.00‐17.92) | ||
| 0‐4 y | 1069 (38.0) | ||
| 5‐9 y | 992 (35.3) | ||
| 10‐14 y | 574 (20.4) | ||
| 15‐17 y | 175 (6.2) | ||
| Time since diagnosis, median (range), y | 22.42 (11.00‐50.08) | ||
| 10‐19 y | 1150 (40.9) | ||
| 20‐29 y | 956 (34.0) | ||
| ≥30 y | 704 (25.1) | ||
| Diagnosis | |||
| ALL | 837 (29.8) | ||
| Leukemia, not ALL | 125 (4.4) | ||
| Non‐Hodgkin lymphoma | 278 (9.9) | ||
| Hodgkin lymphoma | 186 (6.6) | ||
| CNS tumor | 329 (11.7) | ||
| Neuroblastoma | 158 (5.6) | ||
| Retinoblastoma | 13 (0.5) | ||
| Renal tumor | 314 (11.2) | ||
| Hepatic tumor | 29 (1.0) | ||
| Bone tumor | 163 (5.8) | ||
| Soft tissue and other sarcoma | 198 (7.0) | ||
| Germ cell tumor | 102 (3.6) | ||
| Other and unspecified malignant neoplasm | 78 (2.8) | ||
| Treatment combinations | |||
| Surgery only | 242 (8.6) | ||
| Chemotherapy (± surgery) | 1488 (53.0) | ||
| Radiotherapy (± surgery) | 195 (6.9) | ||
| Chemotherapy and radiotherapy (± surgery) | 876 (31.2) | ||
| No surgery, radiotherapy, or chemotherapy or unknown | 9 (0.3) | ||
| Recurrence | |||
| Yes | 372 (13.2) | ||
| No | 2438 (86.8) | ||
Abbreviations: ALL, acute lymphoblastic leukemia; BMI, body mass index; CNS, central nervous system; IQR, interquartile range.
A t test for continuous variables and a Pearson χ2 test for categorical variables were used.
Missing: 33 survivors (1.2%) and 7 siblings (0.7%). The educational levels were low (up to and including lower technical and vocational education), middle (up to and including secondary technical and vocational education), and high (up to and including higher professional education and university).
Missing: 50 survivors (1.8%) and 6 siblings (0.6%).
Missing: 8 survivors (0.3%) and 5 siblings (0.5%).
Missing: 76 survivors (2.7%) and 17 siblings (1.6%).
Missing: 12 survivors (0.4%) and 1 sibling (0.1%).
Missing: 4 survivors (0.1%) and 1 sibling (0.1%).
Missing: 42 survivors (1.5%) and 3 siblings (0.3%).
Missing: 32 survivors (1.1%) and 46 siblings (4.4%). A description of the assessment of health problems is provided in Supporting Table 1.
Compared with siblings, CCSs were slightly younger (29.7 vs 31.9 years) and were significantly more often male, unemployed, less educated, and unmarried. CCSs reported more health problems and were less likely to smoke and drink alcohol.
Prevalence of CF
The prevalence of CF was 24.4% in CCSs and 13.5% in siblings (P < .001; Fig. 2). Also, after adjustments for covariates in a multivariable model, CCSs had a significantly higher risk for reporting CF than siblings (odds ratio [OR], 2.19; 95% confidence interval [CI], 1.75‐2.76; Table 2). The prevalence of CF separated by the age at assessment was 26.1% (656 of 2516) in adult CCSs and 10.9% (32 of 294) in adolescent CCSs (P < .001; Fig. 2). Both adult and adolescent CCSs reported a higher prevalence of CF than siblings (adolescent siblings, 3.2%; adult siblings, 14.1%; Fig. 2), but this difference was only significant in adults (Supporting Table 4). Supporting Figure 1 shows the prevalence of CF in CCSs and siblings stratified by the age at assessment. The prevalence of CF followed an upside‐down U parabola shape for both CCSs and sibling controls, with higher prevalence rates for CCSs throughout all age categories.
Figure 2.
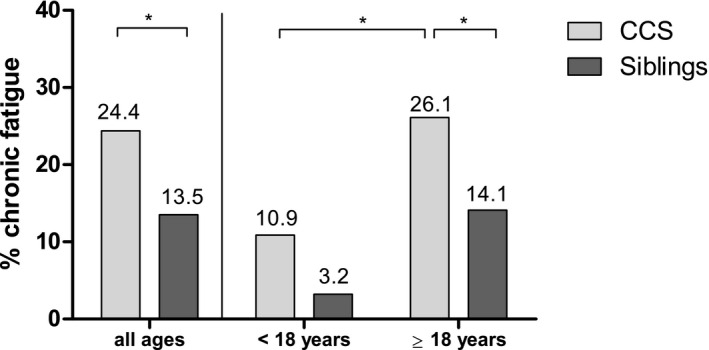
Prevalence of chronic fatigue in CCSs (n = 2810) and siblings (n = 1040) for (Left) all participants and (Right) participants separated by age at assessment (age at assessment < 18 years = adolescents; age at assessment ≥ 18 years = adults). Chronic fatigue was defined as severe (SFQ ≥ 18) and persistent fatigue (duration of complaints > 6 months). *P < .001 (χ2 test). CCS indicates childhood cancer survivor; SFQ, Short Fatigue Questionnaire.
TABLE 2.
Multivariable Analysis Assessing Differences in the Prevalence of Chronic Fatigue Between Survivors and Siblings
| OR (95% CI) | P | |
|---|---|---|
| Cohort | ||
| Siblings (reference) | 1.0 | |
| Survivors | 2.194 (1.75‐2.76) | <.001 |
| Gender | ||
| Male (reference) | 1.0 | |
| Female | 2.000 (1.68‐2.38) | <.001 |
| Age at assessment (years) | ||
| <18 y (reference) | 1.0 | |
| 18‐29 y | 1.788 (1.10‐2.90) | .018 |
| 30‐39 y | 1.905 (1.13‐3.21) | .015 |
| ≥40 y | 1.666 (0.98‐2.84) | .060 |
| No. of health problems | ||
| 0 (reference) | 1.0 | |
| 1‐2 | 1.481 (1.23‐1.78) | <.001 |
| >2 | 2.246 (1.62‐3.11) | <.001 |
| Educational level | ||
| Low (reference) | 1.0 | |
| Middle | 1.178 (0.89‐1.56) | .250 |
| High | 1.293 (0.95‐1.76) | .105 |
| Employment status | ||
| Employed (reference) | 1.0 | |
| Student | 0.768 (0.57‐1.04) | .086 |
| Unemployed | 2.061 (1.63‐2.61) | <.001 |
| Current smoker | ||
| No (reference) | 1.0 | |
| Yes | 1.304 (1.04‐1.63) | .020 |
| Current alcohol use | ||
| No (reference) | 1.0 | |
| Yes | 0.697 (0.58‐0.83) | <.001 |
| Drug use in past year | ||
| No (reference) | 1.0 | |
| Yes | 1.114 (0.82‐1.51) | .488 |
| Marital status | ||
| Married or living as married (reference) | 1.0 | |
| Not married | 0.959 (0.78‐1.17) | .681 |
| Divorced/widowed | 1.144 (0.66‐1.98) | .631 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Multivariable logistic regression analyses were used to compare the prevalence of chronic fatigue between childhood cancer survivors and sibling controls; adjustments were made for gender, age at assessment, number of health problems, educational level, employment status, smoking, alcohol consumption, drug use, and marital status. Chronic fatigue was the dependent variable.
Figure 3 shows the prevalence of CF per cancer diagnosis in CCSs. The prevalence rates ranged from 20.8% in survivors of ALL to 31.3% in survivors of central nervous system (CNS) tumors. All diagnostic groups reported a significantly higher prevalence of CF in comparison with siblings (P < .05; Fig. 3). When we compared diagnostic groups, survivors of CNS tumors, bone tumors, and other and unspecified malignancies significantly more often reported CF than survivors of ALL (P < .05; Fig. 3). For all other diagnostic groups, the prevalence of CF did not differ significantly from that of ALL survivors.
Figure 3.
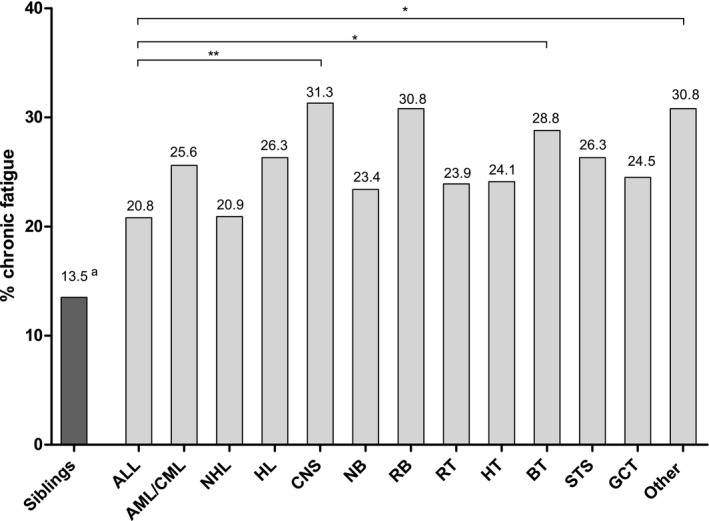
Prevalence of chronic fatigue per diagnosis in CCSs. Diagnostic groups were classified according to the third edition of the International Classification of Childhood Cancer. For the comparison between diagnostic groups, survivors of ALL served as the reference group. *P < .05 (χ2 test); **P < .001 (χ2 test). aCompared with siblings, all diagnostic groups except for RB were also found to have a significantly higher risk for chronic fatigue (P values < .05; multivariable logistic regression corrected for gender, age at assessment, employment status, educational level, marital status, number of health problems, current alcohol use, current smoker, and drug use in the past year). ALL indicates acute lymphoblastic leukemia; AML/CML, acute myeloid leukemia and chronic myeloid leukemia (leukemia, not acute lymphoblastic leukemia); BT, bone tumor; CCS, childhood cancer survivor; CNS, central nervous system tumor; GCT, germ cell tumor; HL, Hodgkin lymphoma; HT, hepatic tumor; NB, neuroblastoma; NHL, non‐Hodgkin lymphoma; Other, other and unspecified malignant neoplasm, including severe Langerhans cell histiocytosis; RB, retinoblastoma; RT, renal tumor; STS, soft tissue sarcoma.
Risk and Associated Factors With CF
The results of the multivariable regression analysis in adult CCSs are shown in Table 3. In model V, female gender (OR, 2.11; 95% CI, 1.72‐2.60), being unemployed (OR, 2.20; 95% CI, 1.69‐2.88), having 1 or 2 health problems (OR, 1.49; 95% CI, 1.18‐1.88), having more than 2 health problems (OR, 2.18; 95% CI, 1.49‐3.19), and a CNS cancer diagnosis (OR, 1.73; 95% CI, 1.16‐2.58) were all significantly associated with an increased risk of reporting CF. Alcohol use was significantly associated with a lower risk of reporting CF (OR, 0.64; 95% CI, 0.52‐0.79).
TABLE 3.
Multivariable Analyses of Risk and Associated Factors for Chronic Fatigue in Adult Childhood Cancer Survivors
| Model I: Block 1 | Model II: Blocks 1 + 2 | Model III: Blocks 1 + 2 + 3 | Model IV: Blocks 1 + 2 + 4 | Model V: Blocks 1 + 2 + 3 + 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Block 1: Demographic and general disease characteristics | ||||||||||
| Gender | ||||||||||
| Male (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Female | 2.29 (1.89‐2.77) | <.001 | 2.09 (1.71‐2.56) | <.001 | 2.12 (1.73‐2.61) | <.001 | 2.08 (1.70‐2.55) | <.001 | 2.11 (1.72‐2.60) | <.001 |
| Employment status | ||||||||||
| Employed (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Student | 0.78 (0.56‐1.11) | .16 | 0.85 (0.60‐1.21) | .36 | 0.85 (0.60‐1.21) | .36 | 0.85 (0.60‐1.21) | .36 | 0.85 (0.60‐1.22) | .38 |
| Unemployed | 2.67 (2.07‐3.44) | <.001 | 2.20 (1.70‐2.86) | <.001 | 2.17 (1.66‐2.83) | <.001 | 2.23 (1.72‐2.91) | <.001 | 2.20 (1.69‐2.88) | <.001 |
| Educational level | ||||||||||
| Low (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Middle | 1.11 (0.81‐1.51) | .52 | 1.23 (0.90‐1.69) | .20 | 1.24 (0.91‐1.71) | .18 | 1.22 (0.89‐1.68) | .21 | 1.24 (0.90‐1.71) | .18 |
| High | 1.14 (0.81‐1.60) | .46 | 1.41 (0.99‐2.00) | .06 | 1.42 (0.99‐2.03) | .05 | 1.38 (0.97‐1.97) | .07 | 1.40 (0.98‐2.01) | .06 |
| Marital status | ||||||||||
| Married/living as married (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Not married | 1.00 (0.80‐1.25) | .99 | 0.95 (0.76‐1.19) | .65 | 0.93 (0.74‐1.18) | .56 | 0.96 (0.76‐1.20) | .70 | 0.94 (0.74‐1.18) | .57 |
| Divorced or widowed | 1.25 (0.68‐2.29) | .47 | 1.18 (0.63‐2.12) | .60 | 1.20 (0.64‐2.26) | .57 | 1.18 (0.63‐2.21) | .61 | 1.17 (0.62‐2.21) | .62 |
| Time since diagnosis | ||||||||||
| 10‐19 y (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| 20‐29 y | 1.14 (0.89‐1.47) | .31 | 1.09 (0.85‐1.41) | .50 | 1.10 (0.86‐1.43) | .45 | 1.10 (0.85‐1.42) | .45 | 1.12 (0.87‐1.45) | .39 |
| ≥30 y | 1.35 (1.02‐1.78) | .04 | 1.20 (0.90‐1.61) | .21 | 1.23 (0.91‐1.66) | .17 | 1.24 (0.92‐1.67) | .16 | 1.31 (0.96‐1.79) | .08 |
| Age at diagnosis | ||||||||||
| 0‐4 y (reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| 5‐9 y | 0.91 (0.72‐1.15) | .42 | 0.87 (0.69‐1.11) | .26 | 0.84 (0.65‐1.08) | .17 | 0.88 (0.70‐1.12) | .31 | 0.86 (0.67‐1.10) | .23 |
| 10‐14 y | 1.18 (0.92‐1.53) | .20 | 1.11 (0.85‐1.44) | .44 | 1.07 (0.80‐1.42) | .66 | 1.13 (0.87‐1.47) | .37 | 1.10 (0.83‐1.48) | .51 |
| 15‐17 y | 0.95 (0.64‐1.41) | .81 | 0.88 (0.59‐1.32) | .55 | 0.83 (0.54‐1.29) | .41 | 0.91 (0.61‐1.36) | .65 | 0.87 (0.56‐1.36) | .55 |
| Block 2: Health‐related and lifestyle factors | ||||||||||
| Current alcohol use | ||||||||||
| No (reference) | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 0.64 (0.52‐0.79) | <.001 | 0.64 (0.52‐0.78) | <.001 | 0.64 (0.52‐0.79) | <.001 | 0.64 (0.52‐0.79) | <.001 | ||
| Current smoker | ||||||||||
| No (reference) | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1.24 (0.94‐1.64) | .13 | 1.26 (0.95‐1.67) | .10 | 1.23 (0.93‐1.63) | .15 | 1.26 (0.95‐1.67) | .11 | ||
| Drug use in past year | ||||||||||
| No (reference) | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| Yes | 1.00 (0.69‐1.45) | .99 | 1.02 (0.71‐1.48) | .90 | 0.99 (0.68‐1.43) | .94 | 1.02 (0.70‐1.47) | .93 | ||
| BMI | ||||||||||
| Underweight (<18.5 kg/m2) | 1.10 (0.68‐1.76) | .70 | 1.11 (0.69‐1.78) | .68 | 1.10 (0.69‐1.77) | .69 | 1.11 (0.69‐1.78) | .67 | ||
| Normal weight (18.5‐24.9 kg/m2; reference) | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| Overweight (25‐29.9 kg/m2) | 1.08 (0.86‐1.36) | .51 | 1.08 (0.86‐1.36) | .52 | 1.09 (0.87‐1.37) | .47 | 1.09 (0.86‐1.37) | .48 | ||
| Obesity (≥30 kg/m2) | 1.00 (0.72‐1.40) | .99 | 1.01 (0.72‐1.41) | .97 | 1.00 (0.72‐1.40) | 1.00 | 1.00 (0.71‐1.41) | .99 | ||
| No. of health problems | ||||||||||
| 0 (reference) | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| 1‐2 | 1.46 (1.17‐1.82) | <.01 | 1.45 (1.15‐1.82) | <.01 | 1.49 (1.19‐1.87) | <.001 | 1.49 (1.18‐1.88) | <.01 | ||
| >2 | 2.15 (1.50‐3.08) | <.001 | 2.09 (1.44‐3.03) | <.001 | 2.24 (1.55‐3.25) | <.001 | 2.18 (1.49‐3.19) | <.001 | ||
| Block 3: Cancer diagnosis | ||||||||||
| ALL (reference) | 1.0 | 1.0 | ||||||||
| Leukemia, not ALL | 1.25 (0.76‐2.06) | .39 | 1.25 (0.76‐2.06) | .38 | ||||||
| Non‐Hodgkin lymphoma | 1.31 (0.91‐1.89) | .15 | 1.29 (0.89‐1.87) | .17 | ||||||
| Hodgkin lymphoma | 1.27 (0.84‐1.94) | .26 | 1.31 (0.86‐2.00) | .21 | ||||||
| CNS tumor | 1.47 (1.06‐2.06) | .02 | 1.73 (1.16‐2.58) | <.01 | ||||||
| Neuroblastoma | 1.05 (0.65‐1.68) | .86 | 1.10 (0.67‐1.82) | .71 | ||||||
| Retinoblastoma | 1.08 (0.31‐3.75) | .91 | 1.22 (0.34‐4.39) | .76 | ||||||
| Renal tumor | 1.25 (0.88‐1.76) | .21 | 1.27 (0.90‐1.79) | .18 | ||||||
| Hepatic tumor | 1.45 (0.52‐4.05) | .48 | 1.42 (0.50‐3.98) | .51 | ||||||
| Bone tumor | 1.14 (0.73‐1.76) | .57 | 1.13 (0.73‐1.76) | .58 | ||||||
| Soft tissue tumor | 1.33 (0.89‐1.97) | .16 | 1.37 (0.92‐2.04) | .12 | ||||||
| Germ cell tumor | 0.96 (0.55‐1.67) | .87 | 1.03 (0.58‐1.82) | .93 | ||||||
| Other and unspecified | 1.49 (0.84‐2.64) | .17 | 1.61 (0.89‐2.89) | .11 | ||||||
| Block 4: Cancer treatment | ||||||||||
| Surgery only (reference) | 1.0 | 1.0 | ||||||||
| Chemotherapy (± surgery) | 0.69 (0.43‐1.10) | .12 | 0.68 (0.42‐1.10) | .11 | ||||||
| Radiotherapy (± surgery) | 0.87 (0.62‐1.23) | .43 | 1.08 (0.71‐1.64) | .72 | ||||||
| Chemotherapy and radiotherapy (± surgery) | 0.83 (0.58‐1.20) | .33 | 0.98 (0.65‐1.49) | .93 | ||||||
| Model fit statistic: AUC | 0.681 | 0.700 | 0.705 | 0.702 | 0.707 | |||||
Abbreviations: ALL, acute lymphoblastic leukemia; AUC, area under the curve; BMI, body mass index; CI, confidence interval; CNS, central nervous system; OR, odds ratio.
Multivariable logistic regression analyses were used with possible risk and associated factors added in blocks. The dependent variable was chronic fatigue. The educational levels were low (up to and including lower technical and vocational education), middle (up to and including secondary technical and vocational education), and high (up to and including higher professional education and university).
Among adolescent CCSs, only female gender was found to be significantly associated with increased CF (OR, 3.56; 95% CI, 1.36‐9.33; Supporting Table 5).
Discussion
This study reports on the prevalence of and risk and associated factors for CF in a national cohort of Dutch CCSs. By taking into account the duration of fatigue (CF is defined as severe fatigue for a duration of 6 months or longer), we build on the previous literature and ensure that the outcome measure is more in accordance with the definition of cancer‐related fatigue as stated by the National Comprehensive Cancer Network. 3 The prevalence of CF was significantly higher in CCSs than sibling controls. Other studies have also found elevated prevalence rates of CF in CCSs compared with controls. 5 , 6 , 15 Hamre et al 5 found a prevalence rate in CCSs comparable to that in the current study (28%), whereas Johannsdottir et al 6 and Puhr et al 15 found lower prevalence rates (13.6% and 14.5%, respectively). Variations in the study populations and assessment might explain these differences in prevalence rates. Our study demonstrates that the high prevalence of CF applies to all childhood malignancies, but prevalence rates vary between age and diagnostic groups. This might explain the lower prevalence rates found in the previous literature in comparison with the current study. For example, our study found the highest rates of fatigue in those aged 40 to 49 years; however, the study by Johannsdottir et al did not include participants with an age > 34 years (most participants were younger than 25 years), and the participants in the study by Puhr et al had a mean age of 23.4 years (SD, ±3.5 years).
In addition to the differences seen between stratified age groups, the adult survivors (≥18 years old) had a significantly higher prevalence of CF than adolescents (<18 years old). A previous study that compared survivors aged 13 to 18 years with survivors aged 19 to 34 years also found a significantly higher prevalence in the older group. 6 We also showed an increased risk for CF in adult survivors who suffered from health problems. The risk for having health problems in CCSs generally increases with the duration of follow‐up 16 ; thus, health problems might have contributed to the difference in the prevalence of CF between adolescents and adults and the finding that the prevalence of CF was highest in those aged 40 to 49 years. Also, adults generally have more responsibilities than adolescents, and survivors may experience a discrepancy between the demands of a successful transition to adulthood and their resources, which might lead to fatigue in adults. 17 , 18
Our study showed that survivors of CNS tumors had an elevated risk of reporting CF in comparison with survivors of ALL, and this does not correspond to 4 previous studies. 19 , 20 , 21 , 22 These studies assessed fatigue on a continuous scale 19 , 20 , 22 or assessed fatigue status on the basis of scores of controls, 21 and this might explain the different results. The current study used a validated cutoff point to indicate severe fatigue rather than a continuous measure of fatigue to most effectively capture those who are in need of services. CNS tumor survivors more often report neurocognitive problems, 15 , 23 which have also been related to fatigue. 24 , 25 , 26 , 27 In addition to the fact that neurocognitive problems themselves might trigger symptoms of fatigue, the 2 outcomes might also exacerbate each other because it is likely that symptoms of fatigue might influence a person's attention or memory skills, for example. Therefore, it is plausible that CNS survivors are at risk for both fatigue and neurocognitive problems because both outcomes are highly prevalent. It might be interesting for future studies to more extensively study this relation.
In our study population, no association was found between the type of treatment and CF. This is consistent with 7 previous studies showing the type of treatment to not be related to (chronic) fatigue in multivariable analyses 6 , 19 , 25 , 28 , 29 , 30 , 31 ; this suggests that a strong role of treatment is unlikely, and the increased prevalence rate of CF in CCSs is likely related to other factors. This suggestion is strengthened by contradictory results presented by Mulrooney et al, 21 who showed that CCSs treated with radiation therapy were more likely to be fatigued. However, only cancer‐ and treatment‐related variables were included in their multivariable analysis, whereas in the current study, we also included sociodemographic, health‐related, and lifestyle factors in the analysis to ensure a possible interaction of these variables to be adjusted for. Because the cancer treatment variables were not shown to be associated with CF in the current study, it is, therefore, likely that other factors play a more prominent role. The results of Ho et al 19 complement these thoughts because they showed the type of cancer treatment to be related to fatigue in a univariate analysis; however, this effect no longer occurred in a multivariable analysis when it was adjusted for sociodemographic factors, depressive symptoms, and physical activity level. Nonetheless, cancer treatment might have indirectly affected fatigue levels by increasing the likelihood of health conditions (eg, heart disease) that are associated with fatigue. 32 , 33 The current study showed, in accordance with other studies, 20 , 25 , 28 that CCSs having 1 or 2 health problems were associated with CF, and this association became even stronger for CCSs having 2 or more health problems. Combining these results, we hypothesize that cancer treatment in childhood can induce health problems at a later stage in life that may increase the risk for CF.
Gender (female) and employment status (unemployment) were found to be associated with CF. Five and 2 studies, respectively, also reported that female and unemployed survivors were at risk for reporting fatigue. 20 , 21 , 25 , 34 , 35 , 36 In general, females tend to more often report physical symptoms such as fatigue, 37 , 38 and this might explain the association that we found. As for employment status, CCSs are more often unemployed than healthy controls. 39 Higher fatigue levels in CCSs could contribute to this higher unemployment rate, as suggested for survivors of adult‐onset cancer, 40 but further research is needed to investigate this relationship in CCSs.
Finally, we found that alcohol use was associated with a decreased risk of CF. In our study, fatigued survivors less often consumed alcohol than nonfatigued survivors. Low alcohol intake has also been described in patients with persistent medically unexplained fatigue with the hypothesis that alcohol intake is reduced or stopped because of fatigue‐related alcohol intolerance. 41 , 42 This might explain the difference in alcohol consumption that we found in our study.
The area under the curve of the regression models indicates that additional factors need to be considered for explaining the occurrence of CF in CSS. Factors to consider are physical activity, 19 , 43 depression, 4 sleep problems, 44 fear of disease recurrence, and fatigue‐related cognitive‐behavioral factors. 45 Not including these factors in the current study is considered a limitation. Additionally, our results demonstrate a relationship between the number of clinically relevant health problems and fatigue, but the influence of fatigue severity and specific health problems on fatigue needs to be assessed in future studies. Longitudinal studies are necessary to assess causal relations between associated factors and fatigue.
Our study has limitations. One limitation is the lack of statistical power in the analysis of CF in adolescent CCSs. We limited the number of predictors in the regression analysis; subsequently, the results should be interpreted with caution. Also, the general categorization of cancer treatment does not exclude the possibility that specific agents or combination regimens do contribute directly to fatigue in specific CCS subgroups. Furthermore, no information was available to identify racial diversity in our cohort because of Dutch law and regulations on registering personal information regarding race and ethnicity. Finally, we did not assess to what extent fatigue leads to limitations of daily life, which are part of the definition of cancer‐related fatigue. Unfortunately, this information was not available.
In conclusion, the results of this study strongly suggest that CCSs, regardless of their cancer diagnosis, report CF more often than siblings. CF seems to be less directly related to cancer diagnosis and treatment (only survivors of CNS tumors showed an increased OR in the multivariable analysis) and more related to demographic and health‐related factors (experiencing health problems, being female, and being unemployed). Overall, this study provides new evidence about the occurrence of fatigue in CCSs.
Funding Support
This work was supported by the Dutch Cancer Society (KUN 2014‐6985). The work of Nina Streefkerk was also financially supported by the Dutch Cancer Society (grant UVA2014‐6805).
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
Sylvia van Deuren: Drafting of the manuscript. Adriaan Penson: Drafting of the manuscript. Eline van Dulmen‐den Broeder: Critical revision of the manuscript. Martha A. Grootenhuis: Critical revision of the manuscript. Margriet van der Heiden‐van der Loo: Critical revision of the manuscript. Ewald Bronkhorst: Critical revision of the manuscript. Nicole M. A. Blijlevens: Critical revision of the manuscript. Nina Streefkerk: Critical revision of the manuscript. Jop C. Teepen: Critical revision of the manuscript. Wim J. E. Tissing: Critical revision of the manuscript. Helena J. H. van der Pal: Critical revision of the manuscript. Marry M. van den Heuvel‐Eibrink: Critical revision of the manuscript. Birgitta A. B. Versluys: Critical revision of the manuscript. Dorine Bresters: Critical revision of the manuscript. Flora E. van Leeuwen: Critical revision of the manuscript. Cécile M. Ronckers: Critical revision of the manuscript. Leontien C. M. Kremer: Critical revision of the manuscript. Hans Knoop: Drafting of the manuscript. Jacqueline J. Loonen: Drafting of the manuscript. All authors contributed to the design and data collection of the study and to the interpretation of data, and all authors approved the final version of the manuscript.
Supporting information
Supplementary Material
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
The results of this study were presented in part at the 52nd Congress of the International Society of Paediatric Oncology (virtual congress); October 14 to 17, 2020.
We acknowledge the contributions from physicians and other medical professionals who cared for children with cancer in the Netherlands. We thank all data managers at the 7 participating centers for obtaining the data for this study.
Data Availability
The data underlying this article were provided by the DCCSS‐LATER consortium under license. Data will be shared on request to the corresponding author with permission of the DCCSS‐LATER consortium.
References
- 1. Macartney G, VanDenKerkhof E, Harrison MB, Stacey D. Symptom experience and quality of life in pediatric brain tumor survivors: a cross‐sectional study. J Pain Symptom Manage. 2014;48:957‐967. [DOI] [PubMed] [Google Scholar]
- 2. Kanellopoulos A, Hamre HM, Dahl AA, Fossa SD, Ruud E. Factors associated with poor quality of life in survivors of childhood acute lymphoblastic leukemia and lymphoma. Pediatr Blood Cancer. 2013;60:849‐855. [DOI] [PubMed] [Google Scholar]
- 3. Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32:1840‐1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Deuren S, Boonstra A, van Dulmen‐den Broeder E, Blijlevens N, Knoop H, Loonen J. Severe fatigue after treatment for childhood cancer. Cochrane Database Syst Rev. 2020;3:CD012681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamre H, Zeller B, Kanellopoulos A, et al. High prevalence of chronic fatigue in adult long‐term survivors of acute lymphoblastic leukemia and lymphoma during childhood and adolescence. J Adolesc Young Adult Oncol. 2013;2:0015. [Google Scholar]
- 6. Johannsdottir IM, Hjermstad MJ, Moum T, et al. Increased prevalence of chronic fatigue among survivors of childhood cancers: a population‐based study. Pediatr Blood Cancer. 2012;58:415‐420. [DOI] [PubMed] [Google Scholar]
- 7. Kenney LB, Nancarrow CM, Najita J, et al. Health status of the oldest adult survivors of cancer during childhood. Cancer. 2010;116:497‐505. [DOI] [PubMed] [Google Scholar]
- 8. Teepen JC, Leeuwen FEV, Tissing WJ, et al. Long‐term risk of subsequent malignant neoplasms after treatment of childhood cancer in the DCOG LATER study cohort: role of chemotherapy. J Clin Oncol. 2017;35:2288‐2298. [DOI] [PubMed] [Google Scholar]
- 9. Streefkerk N, Tissing WJE, van der Heiden‐van der Loo M, et al. The Dutch LATER physical outcomes set for self‐reported data in survivors of childhood cancer. J Cancer Surviv. 2020;14:666‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alberts M, Smets EM, Vercoulen JH, Garssen B, Bleijenberg G. Verkorte vermoeidheids vragenlijst: een praktisch hulpmiddel bij het scoren van vermoeidheid. Ned Tijdschr Geneeskd. 1997;141:1526‐1530. [PubMed] [Google Scholar]
- 11. Penson A, Van Deuren S, Worm‐Smeitink M, et al. Short Fatigue Questionnaire: screening for severe fatigue. J Psychosom Res. Published online August 28, 2020. doi: 10.1016/j.jpsychores.2020.110229 [DOI] [PubMed] [Google Scholar]
- 12. Bleijenberg G, Knoop H, Gielissen M, Bleijenberg G, Knoop H, Gielissen M. De Verkorte VermoeidheidsVragenlijst voor het vaststellen van de ernst van chronische vermoeidheid. Bijblijven. 2009;25(1):19–21. 10.1007/bf03087615. [DOI] [Google Scholar]
- 13. Hair J, Black W, Babin B, Anderson R. Multivariate Data Analysis: A Global Perspective. Pearson; 2010. [Google Scholar]
- 14. IBM Corp . IBM SPSS Statistics for Windows, Version 25.0. IBM Corp; 2017. [Google Scholar]
- 15. Puhr A, Ruud E, Anderson V, et al. Self‐reported executive dysfunction, fatigue, and psychological and emotional symptoms in physically well‐functioning long‐term survivors of pediatric brain tumor. Dev Neuropsychol. 2019;44:88‐103. [DOI] [PubMed] [Google Scholar]
- 16. Sieswerda E, Font‐Gonzalez A, Reitsma JB, et al. High hospitalization rates in survivors of childhood cancer: a longitudinal follow‐up study using medical record linkage. PLoS One. 2016;11:e0159518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stam H, Hartman EE, Deurloo JA, Groothoff J, Grootenhuis MA. Young adult patients with a history of pediatric disease: impact on course of life and transition into adulthood. J Adolesc Health. 2006;39:4‐13. [DOI] [PubMed] [Google Scholar]
- 18. Maurice‐Stam H, Grootenhuis MA, Caron HN, Last BF. Course of life of survivors of childhood cancer is related to quality of life in young adulthood. J Psychosoc Oncol. 2007;25:43‐58. [DOI] [PubMed] [Google Scholar]
- 19. Ho KY, Li WHC, Lam KWK, et al. Relationships among fatigue, physical activity, depressive symptoms, and quality of life in Chinese children and adolescents surviving cancer. Eur J Oncol Nurs. 2019;38:21‐27. [DOI] [PubMed] [Google Scholar]
- 20. Langeveld NE, Grootenhuis MA, Voute PA, de Haan RJ, van den Bos C. No excess fatigue in young adult survivors of childhood cancer. Eur J Cancer. 2003;39:204‐214. [DOI] [PubMed] [Google Scholar]
- 21. Mulrooney DA, Ness KK, Neglia JP, et al. Fatigue and sleep disturbance in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study (CCSS). Sleep. 2008;31:271‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crom DB, Chathaway DK, Tolley EA, Mulhern RK, Hudson MM. Health status and health‐related quality of life in long‐term adult survivors of pediatric solid tumors. Int J Cancer Suppl. 1999;12:25‐31. [DOI] [PubMed] [Google Scholar]
- 23. Krull KR, Hardy KK, Kahalley LS, Schuitema I, Kesler SR. Neurocognitive outcomes and interventions in long‐term survivors of childhood cancer. J Clin Oncol. 2018;36:2181‐2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clanton NR, Klosky JL, Li C, et al. Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2011;117:2559‐2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meeske KA, Siegel SE, Globe DR, Mack WJ, Bernstein L. Prevalence and correlates of fatigue in long‐term survivors of childhood leukemia. J Clin Oncol. 2005;23:5501‐5510. [DOI] [PubMed] [Google Scholar]
- 26. Rueegg CS, Gianinazzi ME, Rischewski J, et al. Health‐related quality of life in survivors of childhood cancer: the role of chronic health problems. J Cancer Surviv. 2013;7:511‐522. [DOI] [PubMed] [Google Scholar]
- 27. Nugent BD, Bender CM, Sereika SM, Tersak JM, Rosenzweig M. Cognitive and occupational function in survivors of adolescent cancer. J Adolesc Young Adult Oncol. 2018;7:79‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frederick NN, Kenney L, Vrooman L, Recklitis CJ. Fatigue in adolescent and adult survivors of non‐CNS childhood cancer: a report from project REACH. Support Care Cancer. 2016;24:3951‐3959. [DOI] [PubMed] [Google Scholar]
- 29. Zeller B, Loge JH, Kanellopoulos A, Hamre H, Wyller VB, Ruud E. Chronic fatigue in long‐term survivors of childhood lymphomas and leukemia: persistence and associated clinical factors. J Pediatr Hematol Oncol. 2014;36:438‐444. [DOI] [PubMed] [Google Scholar]
- 30. Mort S, Lahteenmaki PM, Matomaki J, Salmi TT, Salantera S. Fatigue in young survivors of extracranial childhood cancer: a Finnish nationwide survey. Oncol Nurs Forum. 2011;38:E445‐E454. [DOI] [PubMed] [Google Scholar]
- 31. Harila MJ, Salo J, Lanning M, Vilkkumaa I, Harila‐Saari AH. High health‐related quality of life among long‐term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010;55:331‐336. [DOI] [PubMed] [Google Scholar]
- 32. Geenen MM, Cardous‐Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long‐term survivors of childhood cancer. JAMA. 2007;297:2705‐2715. [DOI] [PubMed] [Google Scholar]
- 33. Schwartz CL. Long‐term survivors of childhood cancer: the late effects of therapy. Oncologist. 1999;4:45‐54. [PubMed] [Google Scholar]
- 34. Pemberger S, Jagsch R, Frey E, et al. Quality of life in long‐term childhood cancer survivors and the relation of late effects and subjective well‐being. Support Care Cancer. 2005;13:49‐56. [DOI] [PubMed] [Google Scholar]
- 35. Barrera M, Teall T, Barr R, Silva M, Greenberg M. Health related quality of life in adolescent and young adult survivors of lower extremity bone tumors. Pediatr Blood Cancer. 2012;58:265‐273. [DOI] [PubMed] [Google Scholar]
- 36. Rach AM, Crabtree VM, Brinkman TM, et al. Predictors of fatigue and poor sleep in adult survivors of childhood Hodgkin's lymphoma: a report from the Childhood Cancer Survivor Study. J Cancer Surviv. 2017;11:256‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gijsbers van Wijk CMT, Huisman H, Kolk AM. Gender differences in physical symptoms and illness behavior: a health diary study. Soc Sci Med. 1999;49:1061‐1074. [DOI] [PubMed] [Google Scholar]
- 38. Bensing JM, Hulsman RL, Schreurs KMG. Gender differences in fatigue: biopsychosocial factors relating to fatigue in men and women. Med Care. 1999;37:1078‐1083. [DOI] [PubMed] [Google Scholar]
- 39. de Boer AG, Verbeek JH, van Dijk FJ. Adult survivors of childhood cancer and unemployment: a metaanalysis. Cancer. 2006;107:1‐11. [DOI] [PubMed] [Google Scholar]
- 40. Behringer K, Goergen H, Müller H, et al. Cancer‐related fatigue in patients with and survivors of Hodgkin lymphoma: the impact on treatment outcome and social reintegration. J Clin Oncol. 2016;34:4329‐4337. [DOI] [PubMed] [Google Scholar]
- 41. Goedendorp MM, Knoop H, Schippers GM, Bleijenberg G. The lifestyle of patients with chronic fatigue syndrome and the effect on fatigue and functional impairments. J Hum Nutr Diet. 2009;22:226‐231. [DOI] [PubMed] [Google Scholar]
- 42. Woolley J, Allen R, Wessely S. Alcohol use in chronic fatigue syndrome. J Psychosom Res. 2004;56:203‐206. [DOI] [PubMed] [Google Scholar]
- 43. Van Dijk‐Lokkart EM, Steur LMH, Braam KI, et al. Longitudinal development of cancer‐related fatigue and physical activity in childhood cancer patients. Pediatr Blood Cancer. 2019;66:e27949. [DOI] [PubMed] [Google Scholar]
- 44. Steur LMH, Kaspers GJL, Van Someren EJW, et al. Sleep‐wake rhythm disruption is associated with cancer‐related fatigue in pediatric acute lymphoblastic leukemia. Sleep. 2020;43:zsz320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boonstra A, Gielissen M, van Dulmen‐den Broeder E, Blijlevens N, Knoop H, Loonen J. Cognitive behavior therapy for persistent severe fatigue in childhood cancer survivors: a pilot study. J Pediatr Hematol Oncol. 2019;41:313‐318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data underlying this article were provided by the DCCSS‐LATER consortium under license. Data will be shared on request to the corresponding author with permission of the DCCSS‐LATER consortium.


