Abstract
Drug-metabolizing enzymes (DMEs) and membrane transporters play important roles in the absorption, distribution, metabolism, and excretion processes that determine the pharmacokinetics of drugs. Inflammation has been shown to regulate the expression and function of these drug-processing proteins. Given that inflammation is a common feature of many diseases, in this review, the general mechanisms for inflammation-mediated regulation of DMEs and transporters are described. Also, evidences regarding the aberrant expression of these drug-processing proteins in several inflammatory diseases and age-related disorders are provided.
Keywords: Drug-metabolizing enzyme, Inflammation, Membrane transporter, Pharmacokinetics
1. Introduction
Pharmacokinetics describes the time course of drug levels in the body as a result of absorption, distribution, metabolism, and excretion (ADME) processes following administration. Absorption is a process in which the drugs transfer from the sites of administration to systemic blood circulation. Distribution is related to protein/tissue binding and the exchange of the drugs among various spaces in the body. Metabolism is the biotransformation process that generally converts the drugs to more water-soluble molecules, facilitating their excretion from the body. Excretion is the removal of drugs and/or their metabolites from the body, normally through the urinary or biliary pathways. It is known that drug-metabolizing enzymes (DMEs) and membrane transporters play important roles in the ADME processes.
The roles of DMEs in pharmacokinetics have been extensively investigated for years (for review, refer to Refs. [1,2]). The metabolism of drugs in the body can be mediated by enzymes responsible for phase I (oxidation, reduction, and hydrolysis) and/or phase II (conjugation) biotransformation. Among these, the cytochrome P450 (CYP) enzymes are well known for their roles in phase I oxidative metabolism; enzymes known for phase II metabolism include N-acetyl transferase, glutathione S-transferase (GST), uridine 5′-diphospho-glucuronosyltransferases (UGT), and sulfotransferase (SULT). On the other hand, membrane transporters are integral proteins that mediate the translocation of both endogenous and exogenous molecules across plasma or intracellular membranes. Transporters can be categorized into two superfamilies, namely ATP-binding cassette (ABC) transporters and solute carrier (SLC) transporters. The roles of transporters in drug absorption and disposition have been well characterized (for review, refer to Ref. [3]). Examples for the roles of DMEs and transporters involved in ADME processes are summarized in Table 1.
Table 1.
Drug-metabolizing enzymes (DMEs) and transporters that are involved in ADME processes of pharmacokinetics.
| Organs or tissues | Cell types | Subcellular localization | DMEs/transporters for drugs |
|---|---|---|---|
| Intestine | Intestinal epithelial cells (enterocytes) | Apical (luminal) membrane | OATPs, PEPT1, ASBT, MCT1, MDR1, BCRP, MRP2 |
| Inside the cell | Phase I DMEs: mainly CYPs | ||
| Phase II DMEs: UGT, SULT, and GST | |||
| Basolateral (abluminal) membrane | OCT1, OSTα/γ, MRP1, MRP3 | ||
| Liver | Hepatocytes | Basolateral (sinusoidal) membrane | OATPs, NTCP, OCT1, OAT2/7, OSTα/γ, MRP3, MRP4, MRP6 |
| Inside the cell | Phase I DMEs: mainly CYPs | ||
| Phase II DMEs: UGT, SULT, and GST | |||
| Apical (canalicular) membrane | MDR1, BCRP, MRP2, BSEP, MATE1 | ||
| Kidney | Renal tubular epithelial cells | Basolateral membrane | OCT2, OAT1-3, OATP4C1, OSTα/γ, MRP3 |
| Apical membrane | OAT4, PEPT1/2, OCTN1/2, MDR1, BCRP, MRP2/4, MATE1, MATE2-K |
ASBT: apical sodium dependent bile acid transporter; CYPs: cytochrome P450s; BCRP: breast cancer resistance protein; BSEP: bile salt export pump; DMEs: drug-metabolizing enzymes; GST: glutathione-S-transferase; MATE: multidrug and toxin extrusion protein; MCT: monocarboxylic acid transporter; MDR1: multidrug resistance protein 1 (P-glycoprotein; P-gp); MRP: multidrug resistance-associated protein; NTCP: sodium dependent cotransporting polypeptide; OAT: organic anion transporter; OATPs: organic anion transporting polypeptides; OCT: organic cation transporter; OSTα/γ: heteromeric organic solute transporter; OCTN: organic cation/carnitine transporter; PEPT: peptide transporter; SULT: sulfotransferase; UGT: uridine 5′-diphosphate-glucuronosyltransferase.
Under certain circumstances, the expression and activities of enzymes and transporters can be regulated, thereby leading to an alteration of the pharmacokinetic properties of substrate drugs. Intrinsic factors (e.g., disease, age, and gender) and extrinsic factors (e.g., drugs, smoking, alcohol use, and diet) can affect the pharmacokinetics of drugs, which may then result in insufficient efficacy or unwanted side effects. In recent years, disease–drug interaction is a rapidly growing field in drug discovery and has received much attention from drug development units and regulatory agencies. Given that inflammatory responses occurred in various diseases are known to be important for gene regulation, in this article, general mechanisms for inflammation-mediated regulation of DMEs and transporters are addressed. Also, examples regarding aberrant expression of these drug-processing proteins in inflammatory diseases (including type 1 diabetes, rheumatoid arthritis, and inflammatory bowel disease) and age-related disorders (including normal aging, metabolic disorders, and neurodegenerative diseases) are provided.
2. Factors contributing to gene regulation in inflammation
2.1. The roles of cytokines in the regulation of DMEs and transporters
Under inflammatory conditions, proinflammatory cytokines are not only produced locally around the pathological areas but may also be transferred through blood stream to activate inflammatory responses in distal tissues. In cultured human hepatocytes, direct treatments of tumor necrosis factor-α (TNF-α), interleukin-1γ (IL-1γ), interleukin-6 (IL-6), interferon-γ (IFN-γ), and transforming growth factor-γ (TGF-γ) can reduce the expression of CYP1A2, CYP2C8 and CYP3A4 [4,5]. Inhibition of IL-6 signaling using a specific antibody can abolish IL-6-induced inhibition of CYP1A2 and CYP3A4 activities in human hepatocytes [5]. Also, the knockout of IL-6 can attenuate the reduction of CYP enzymes (including Cyp1a2, Cyp2a5, Cyp2e1, and Cyp3a11) caused by turpentine treatment in mice [6]. Given that CYP enzymes and drug transporters share certain common regulatory pathways, the effects of cytokines on the regulation of CYP enzymes may be applicable to transporters. In mice, intraperitoneal administrations of TNF-α and IL-1γ/IL-6 can reduce mRNA levels of Mrp2/Mrp3/Oatp2 and Mrp2/Oatp1/Oatp2/Bsep, respectively [7]. The regulation of CYP enzymes and transporters by proinflammatory cytokines is summarized in Table 2. While the underlying mechanisms for the cytokine-induced gene regulation are not fully understood, it involves a number of transcriptional factors. Cytokines can alter the activities of various transcription factors, including nuclear factor-κB (NF-κB) and nuclear receptors. Details regarding the regulatory roles of NF-κB and nuclear receptors on DMEs and transporters are described in Sections 2.2 and 2.3, respectively.
Table 2.
The regulation of CYP enzymes and transporters by lipopolysaccharide (LPS) treatment and proinflammatory cytokines.
| Inflammatory stimulus and cytokines | Species | Target | References |
|---|---|---|---|
| LPS | Human | Decreased expression of CYP2C8 and CYP3A4 | [4] |
| Rat | Decreased expression of Cyp3a1, Cyp3a2, Mrp2, Mrp6, Mdr1a, Oatp1, Oatp2, Ntcp, Bsep, Oct1, and Oct3 | [49,50] | |
| Mouse | Decreased expression of Cyp1a2, Cyp2a5, Cyp2c29, Cyp2e1, Cyp3a11, Cyp4a10, and Cyp4a14 Increased expression of Cyp3a13 |
[51] | |
| TNF-α | Human | Decreased expression of CYP2C8 and CYP3A4 | [4] |
| Mouse | Decreased expression of Mrp2, Mrp3, and Oatp2 | [7] | |
| IL-1γ | Human | Decreased expression of CYP2C8 and CYP3A4 | [4] |
| Mouse | Decreased expression of Mrp2, Oatp1, Oatp2, and Bsep | [7] | |
| IL-6 | Human | Decreased expression of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP3A4 | [4,5] |
| Mouse | Decreased expression of Cyp1a2, Cyp2a5, Cyp2e1, Cyp3a11, Mrp2, Oatp1, Oatp2, and Bsep | [6,7] | |
| IFN-γ | Human | Decreased expression of CYP1A2, CYP2B6, CYP2C8, CYP2C9, and CYP3A4 | [4] |
| TGF-γ | Human | Decreased expression of CYP1A2, CYP2C8, CYP2C9, CYP2C19, and CYP3A4 | [4] |
Bsep: bile salt export pump; CYP: cytochrome P450; IFN-γ: interferon-γ; IL-1γ: interleukin-1γ; IL-6: interleukin-6, LPS: lipopolysaccharide; Mdr1: multidrug resistance protein 1 (P-glycoprotein; P-gp); Mrp: multidrug resistance-associated protein; Ntcp: sodium dependent cotransporting polypeptide; Oatp: organic anion transporting polypeptide; Oct: organic cation transporter; TGF-γ: transforming growth factor-γ; TNF-α: tumor necrosis factor-α.
2.2. The roles of NF-κB in the regulation of DMEs and transporters
NF-κB is a primary transcription factor that responds to diverse stimuli, including bacterial products and proinflammatory cytokines. NF-κB has been shown to regulate the gene expression of many hepatic CYP enzymes, including CYP2E1, CYP3A7, and CYP27B1 in humans, Cyp1a1, Cyp2b1/2, Cyp2c11, and Cyp2d5 in rats, and Cyp1a1 and Cyp3a11 in mice [8–10]. NF-κB is also shown to regulate the expression of numerous ABC and SLC transporters, including MDR1 in humans and Mdr1, Mrp2, Mrp3, Bcrp, Oatp1a4, Oatp2b1, and Ntcp in rats and mice [10–12].
2.3. The roles of nuclear receptors in the regulation of DMEs and transporters
Nuclear receptors are ligand-activated transcription factors. Typical nuclear receptors contain two common structural features, namely the N-terminal DNA-binding domain and the C-terminal ligand-binding domain. These two domains specifically recognize the targeted DNA sequences and ligands, respectively. Based on their dimerization and DNA binding properties, nuclear receptors can be categorized into four groups (for review, refer to Ref. [13]). Class I nuclear receptors include steroid hormone receptors, such as glucocorticoid receptor (GR) and progesterone receptor (PR); class II include pregnane X receptor (PXR), constitutive androstane receptor (CAR), liver X receptor (LXR), peroxisome proliferator-activated receptors (PPARs) and farnesoid X receptor (FXR); class III include hepatic nuclear factors (HNFs), germ cell nuclear factor I (GCNF) and retinoid X receptors (RXRs); class IV include estrogen receptor-related receptor-α (ERR) and nuclear receptor 5A1 (SF-1). Class I and II nuclear receptors have been extensively investigated, while studies regarding class III and IV nuclear receptors are relatively limited.
Among nuclear receptors, class II nuclear receptors, especially PXR and CAR, are probably most noteworthy because many of them are considered as xenobiotic sensors. PXR is highly expressed in the liver and intestine and is considered to regulate genes whose encoded proteins protect the body against xenobiotics. Many drugs such as dexamethasone, mifepristone (RU486) and rifampicin are activators of PXR [14]. The transcriptional activity of PXR is also dependent on various endogenous steroids including progesterone and its metabolites [15]. The most notable target gene activated by PXR may be CYP3A4, in which CYP3A4 protein metabolizes more than 50% of the clinical therapeutic agents [16]. PXR also mediates the expression of other phase I DMEs (e.g., CYP2B6 [17]; and CYP2Cs; [18–20]) and phase II DMEs (e.g., GST, UGT, and SULT [21]) in humans. In addition to its effect on enzymes, the expression of mouse hepatic basolateral Oatp2 and canalicular Mrp2 has been shown to be upregulated in response to the treatment of PXR ligands [22,23]. In the intestinal epithelial cells and brain microvascular endothelial cells, PXR was reported to upregulate the expression of Mdr1 at the luminal membrane to limit the absorption and distribution of molecules into the enterocytes and the brain, respectively [24,25]. In terms of CAR, it was originally explored for the induction of CYP2B genes in response to phenobarbital treatment [26] and was later demonstrated to have a role in the regulation of a wide range of genes involved in phase I and phase II metabolism [21]. The activation of CAR has also been reported to induce phase III drug elimination pathways (i.e., transporter-mediated clearance), including MDR1 in humans [27] and Oatp2 and Mrp2 in rats [28,29]. CAR is primarily expressed in the liver with lower levels in the heart, kidney, brain, and lung [30]. PXR and CAR overlap in many aspects (e.g., ligands, action mechanisms, and target genes) and both are important regulatory factors for gene expression of enzymes/transporters [31].
Although PPARα, LXR, and FXR also belong to class II nuclear receptors, they are known for their roles in lipid metabolism [32]. Nevertheless, these nuclear receptors also contribute to the gene transcription of CYP2B6, CYP2C29, CYP3A4, CYP3A11, and MRP2 in humans [29,33–35] and Oatps and Mrp2 in rodents [34,36,37]. As for class I nuclear receptors, GR can increase gene transcription of various DMEs either by direct binding to the promoter of target genes (e.g., CYP3A5, CYP2C9, and CYP2C19) or through the interaction with other nuclear receptors (e.g., HNF-4α and PXR) to induce gene transcription (e.g., CYP2A6, CYP2B6 and CYP3A4) in humans [38–41]. For class III nuclear receptors, HNF-1α and HNF-4α are critical for regulating gene expression in the liver and kidney [42–46]. It is also noted that HNF-4α activates CYP3A4 expression not only through direct binding to CYP3A4 promoter but also through PXR- and CAR-mediated pathways [47]. These findings suggest that the interplay among nuclear receptors shapes the regulation of drug-processing proteins and thereby potentially affects the ADME profiles of their substrate drugs. The regulation of CYP enzymes and transporters via NF-κB and nuclear receptors is summarized in Table 3.
Table 3.
The regulation of CYP enzymes and transporters by ligand-activated nuclear receptors and NF-κB.
| Nuclear receptors/transcription factors | Species | Target | References |
|---|---|---|---|
| PXR | Human | CYP3A4, CYP2B6, CYP2C8, CYP2C9, CYP2C19, MDR1, MRP2 | [16–20,24,29] |
| Rat | Mrp2 | [23] | |
| Mouse | Cyp3a11, Bsep, Mdr1a, Mrp2, Mrp3, Oatp2 | [22,25] | |
| CAR | Human | CYP2C19, CYP3A4, MDR1 | [18,27] |
| Rat | Cyp3a23, Mrp2, Oatp2, Bsep | [28,29] | |
| Mouse | Cyp2b10 | [26] | |
| GR | Human | CYP2A6, CYP3A4, CYP3A5, CYP2B6, CYP2C8, CYP2C9, CYP2C19, MDR1 | [38–41] |
| HNF-1α | Human | CYP1A2 | [42] |
| Rat | Cyp2e1 | [43] | |
| HNF-4α | Human | CYP2A6, CYP3A4, CYP2B6, CYP2C8, CYP2C9, CYP2C18, CYP2D6, MDR1, ABCB11, MRP2, OATP1B1, OCT1 | [40,44–47] |
| PPARα | Human | CYP3A4 | [35] |
| Mouse | Cyp2a1, Cyp2a4, Cyp2a5, Cyp2c29, Cyp3a11 | [37] | |
| LXR | Human | CYP3A4, CYP2B6, MRP1, MRP2 | [33,34] |
| Rat | Mrp1, Mrp2 | [34] | |
| FXR | Human | MRP2 | [29] |
| Rat | Mrp2 | [29] | |
| Mouse | Oat3, Oatps, Oct2, Octn1 | [36] | |
| NF-κB | Human | CYP1A1, CYP2B1/2, CYP2C11, CYP2C11, CYP2D5, CYP2E1, CYP3A7, CYP27B1, MDR1 | [9,12] |
| Rat | Cyp1a1, Cyp2b1/2, Cyp2c11, Cyp2d5, Mdr1 | [9,11] | |
| Mouse | Cyp1a1, Cyp3a11, Mdr1a, Mrp2, Mrp3, Abcb11, Bcrp, Oatp1a4, Oatp2b1, Ntcp | [8–10] |
ABCB: ATP-binding cassette transporter B; Bcrp: breast cancer resistance protein; Bsep: bile salt export pump; CAR: constitutive androstane receptor; CYP (Cyp): cytochrome P450; GR: glucocorticoid receptor; HNF: hepatocyte nuclear factor; MDR1 (Mdr1): multidrug resistance protein 1 (P-glycoprotein; P-gp); MRP (Mrp): multidrug resistance-associated protein; NF-κB: nuclear factor-κB; Ntcp: sodium dependent cotransporting polypeptide; Oat: organic anion transporter; OATP (Oatp): organic anion transporting polypeptide; OCT (Oct): organic cation transporter; Octn: organic cation/carnitine transporter; PPAR: peroxisome proliferator-activated receptor; PXR: pregnane X receptor.
3. Aberrant expression of DMEs and transporters in inflammatory diseases and age-related disorders
While the influence of inflammation on the expression of enzymes/transporters is an important issue, most of the published articles focused on inflammation models induced by lipopolysaccharide (LPS) treatment or bacterial/viral/parasitic infection (for review, refer to Ref. [48]). LPS treatment increases the levels of proinflammatory cytokines along with decreased expression of numerous DMEs and transporters [49–51] (Table 2), showing that bacterial component-induced inflammation is notable for its impacts on pharmacokinetics of drug. This LPS-mediated gene regulation is also associated with the changes in the expression and activity of nuclear receptors and transcription factors [52,53].
In addition to LPS-induced inflammation, inflammation is involved in the pathogenesis of many diseases, including inflammatory diseases (e.g., type 1 diabetes, rheumatoid arthritis, and inflammatory bowel disease) and age-related disorders (e.g., normal aging, metabolic disorders, and neurodegenerative diseases) [54,55]. Compared to LPS-induced models, inflammatory human diseases are more complex and their impacts on the expression and activity of enzymes/transporters should be evaluated individually. As summarized in Table 4, in this article, the regulation of DMEs and transporters is reviewed for type 1 diabetes, rheumatoid arthritis, and inflammatory bowel disease. In addition, as aging is recognized as a universal and multisystemic disease [56] and age-related disorders are characterized by different degrees of inflammation, the regulation of enzymes/transporters is also reviewed in normal aging, metabolic disorders (e.g., type 2 diabetes and obesity), and neurodegenerative diseases.
Table 4.
The regulation of CYP enzymes and transporters in animal models of human inflammatory diseases.
| Diseases | Models | Organs or tissues | Drug-processing proteins |
|---|---|---|---|
| Type 1 diabetes | Streptozotocin-induced diabetic rat model | Liver | Increased expression of Cyp1a2, Cyp1b1, Cyp2b1, Cyp2e1, and Mdr2 [61,67] |
| Intestine | Decreased expression of Mdr1 [68] | ||
| Kidney | Increased expression of Mdr1 [69] | ||
| Blood–brain barrier | Increased or decreased expression of Mdr1 [70,71] | ||
| Streptozotocin-induced diabetic mouse model | Liver | Increased expression of Cyp1a1, Cyp2b9, Cyp3a11, Cyp4a10, Cyp4a11, and Mdr2 [62] | |
| Rheumatoid arthritis | Adjuvant-induced arthritis rat model | Liver | Decreased expression of Cyp 1a1/2, Cyp2b1, Cyp2b2, Cyp3a1, Cyp3a2, Mdr1a, Mrp2, Oatp1a1, Oatp1a4, Oatp1a5, Oatp1b2, and Oatp2b1 [73,74,79] |
| Intestine | Decreased expression of Mrp2, Bcrp, Lat2, and Oatp1a5 [79] | ||
| Collagen-induced arthritis rat model | Liver | Decreased expression of Cyp2c6, Cyp2c7, and Cyp3a1 [75] | |
| Intestine | Decreased expression of Cyp3a1, Oatp1a1, Oatp1a4, Oatp1b2, and Mrp2 [75] | ||
| Inflammatory bowel disease | Trinitrobenzene sulfonic acid-induced colitis rat model | Liver | Decreased expression of Cyp1a2, Cyp2c11, Cyp2e1, and Cyp3a2 [82] |
| Dextran sulfate sodium-induced colitis mouse model | Liver | Decreased expression of Cyp1a2, Cyp2a5, Cyp2b9, Cyp2c29, Cyp2d9, Cyp3a25, Cyp4a10, and Cyp4a14 Increased expression of Cyp3a11, and Cyp3a13 [83] |
|
| Aging | Normal aging rats | Liver | Decreased expression of Cyp1a1, Cyp1a2, Cyp2b1, Cyp2c11, Cyp2e1, and Cyp3a2 [92] |
| Normal aging mice | Liver | Decreased expression of Cyp1a2, Cyp2b10, Cyp3a11, Oatp1a1, Ent1, and Mrp6 Increased expression of Oat2, Oatp1a4, and Mrp4 [93,94] |
|
| Metabolic disorders and type 2 diabetes | High fat diet and streptozotocin-induced type 2 diabetic rat model | Liver | Decreased expression of Mrp5 [104] |
| Kidney | Decreased expression of Oct2 Increased expression of Mrp2, Mrp4, Bcrp, and Oat2 [104] |
||
| High fat diet-fed rats | Liver | Decreased expression of Cyp3a and Mdr1 [98] | |
| High fat diet-fed mice | Liver | Decreased expression of Cyp2a4, Cyp2b10, and Cyp3a11 [97] | |
| ob/ob mice | Liver | Decreased expression of Oatp1a1 and Ntcp [103] Increased expression of Cyp2a, Cyp2b, Mrp2, and Mrp4 [99,100] |
|
| Kidney | Decreased expression of Mrp3, Oatp1a1, and Oat2 [103] | ||
| db/db mice | Liver | Decreased expression of Cyp1a2 Increased expression of Cyp2b and Cyp4a [101] |
|
| TSOD mice | Liver | Decreased expression of Cyp1a and Cyp2e Increased expression of Cyp2c and Cyp3a [102] |
|
| Monosodium glutamate-induced obese mouse model | Intestinal duodenum | Decreased expression of Mdr1 [106] | |
| Intestinal jejunum | Increased expression of Mdr1 [106] | ||
| NZO mice | Renal tubule | Decreased expression of Mdr1 [107] | |
| Blood–brain barrier | Increased expression of Mdr1 [105] | ||
| Alzheimer’s disease | Tg2576 mice | Blood–brain barrier | Decreased expression of Mdr1 [112] |
| Huntington’s disease | R6/2 mice | Blood–brain barrier | Increased expression of Mdr1 [113] |
| Epilepsy | Pilocarpine-induced acute and chronic epileptic rat model | Blood–brain barrier | Increased expression of Mdr1 [114] |
Bcrp: breast cancer resistance protein; Cyp: cytochrome P450; Ent1: equilibrative nucleoside transporter 1; Lat2: L-type amino acid transporter 2; Mdr1: multidrug resistance protein 1 (P-glycoprotein; P-gp); Mdr2: multidrug resistance protein 2; Mrp: multidrug resistance-associated protein; Ntcp: sodium dependent cotransporting polypeptide; NZO: New Zealand obese; Oat: organic anion transporter; Oatp: organic anion transporting polypeptide; Oct: organic cation transporter; TSOD: Tsumura, Suzuki, obese, diabetes.
3.1. Type 1 diabetes
Type 1 diabetes (T1D) is an autoimmune disease with selective death of γ-cells. Insulin therapy is the preferred treatment for T1D management. During the disease progression, aberrant inflammatory signalings (including cytokines and NF-κB activation) are associated with the development of multiple complications (e.g., cardiovascular, retinal, and renal complications) in T1D [57]. In the liver biopsies of patients with T1D, both total CYP content and the metabolizing capacity (using antipyrine as a probe drug for the activities of CYP1A2, CYP2C, and CYP3A) were reported to be increased, compared with those of non-diabetic subjects [58,59]. Also, the pharmacokinetic properties of various drugs are changed in patients with T1D. For example, higher oral clearance of theophylline (mainly metabolized by CYP1A2, CYP2E1, and CYP3A4 in humans) and lower steady-state plasma concentrations of phenytoin (mainly metabolized by CYP2C9 in humans) were shown in T1D patients. In contrast, a reduced clearance of lidocaine (mainly metabolized by CYP3A in humans) was reported in T1D patients (review by Refs. [59,60]). Likewise, the expression of many CYP enzymes has been demonstrated to be altered in T1D animal models [60]. In rats and mice with streptozotocin (STZ)-induced T1D, hepatic expression of numerous CYP isozymes (Cyp1a2, Cyp1b1, Cyp2b1, and Cyp2e1 in rats; Cyp1a1, Cyp2b9, Cyp2b10, Cyp3a11, Cyp4a10, and Cyp4a14 in mice) are increased [61,62]. Corroboratively, higher systemic exposure of mono-ethylglycinexylidide, the metabolite of lidocaine (converted by Cyp1a2 and Cyp3a2), was observed in STZ-treated rats receiving single intravenous administration of lidocaine [63]. Also, higher clearance of fluorouracil (metabolized by Cyp1a1/2) was reported in STZ-induced T1D rats [64]; apparent total clearance of triazolam (metabolized by Cyp3a11) was increased in STZ-treated mice than in controls [65]. The induction of CYP enzymes in STZ models may be related to NF-κB activation in the liver [66]. In addition to CYP enzymes, drug transporters are also regulated in T1D. For examples, hepatic expression of Mdr2 and renal expression of Mdr1 are significantly increased, whereas intestinal expression of Mdr1 is decreased in STZ-induced T1D rats [67–69]. On the other hand, the regulation of Mdr1 expression at the blood–brain barrier remains controversial in STZ-treated rats [70,71]. Despite that the expression of CYP enzymes and transporters are changed in T1D, pharmacokinetic data obtained from T1D animal models are not quite consistent with those from human T1D patients [60]. Thus, extrapolating data from T1D animals to humans needs to be careful.
3.2. Rheumatoid arthritis
Rheumatoid arthritis (RA) is an autoimmune disease characterized by severe systemic inflammation. The production of proinflammatory cytokines, including TNF-α, IL-1γ, and IL-6, are elevated in blood circulation and synovial fluid throughout the disease stages [55]. Adjuvant- and collagen-induced arthritis rat models are widely used to mimic human RA. Studies conducted using these animal models have demonstrated that chronic inflammation in RA is not only crucial for disease progression but also influential on drug pharmacokinetics. Pharmacokinetic studies have shown that the plasma concentrations of verapamil and propranolol are significantly increased, due to the decreased clearance, in rats with adjuvant-induced arthritis [72,73]. This was suggested to be mediated by the downregulation of hepatic Cyp2b1, Cyp2b2, Cyp3a1, and Cyp3a2 in RA [73,74]. Likewise, in collagen-induced arthritis (CIA) rats, the mRNA levels of intestinal Cyp3a1 and hepatic Cyp2c6/7 and Cyp3a1 are markedly decreased, leading to differential changes in the pharmacokinetics of statins [75]. The expression changes in Cyp2b and Cyp3a enzymes are strongly correlated with the increased levels of TNF-α, IL-1γ, and IL-6 in the liver [74]. Inhibition of cytokines production by non-steroidal anti-inflammatory drugs (NSAIDs) or treatment with antibodies against specific cytokines can reverse the reduction of CYP enzymes and the changes in the clearance of propranolol in RA animal models [76,77], confirming that cytokines have an important role in the regulation of CYP enzymes in RA. In humans, it was recently reported that the levels of inflammatory cytokines are negatively associated with CYP3A4 phenotype in RA patients [78]. On the other hand, in terms of membrane transporters, the mRNA levels of numerous ABC and SLC transporters (e.g., Oatps, Mdr1a, Mrp2) are decreased in adjuvant- or collagen-induced arthritis rats [75,79]. The downregulation of Oatp1a1, Oatp1b2 and Oatp1a4 can reduce hepatic uptake of fluvastatin and atorvastatin in CIA rats [75]. These changes in transporter expression in RA are considered to be mediated by the cytokine-induced downregulation of PXR, but not of CAR [79].
3.3. Inflammatory bowel disease
Inflammatory bowel disease (IBD) is a type of disorder that is characterized by chronic and progressive inflammation in the intestine and colon. Ulcerative colitis (UC) and Crohn’s disease (CD) are two common forms of IBD. A study using DNA microarray showed a broad downregulation of genes involved in drug metabolism in the colon biopsy specimens obtained from both UC and CD patients. The expression of MDR1, but not of MRP2/3, is significantly decreased in colon samples of UC patients. This dysregulation is accompanied by a severe reduction of PXR expression [80]. To investigate the regulation of enzymes/transporters in IBD and the underlying mechanisms, several studies have been conducted using both infectious and chemical-induced animal models of IBD. Similar to the findings observed in human IBD, the gene and protein expression of many CYP enzymes are generally downregulated in the liver of these IBD animals. The changes in CYP enzymes are in parallel with increased levels of cytokines [81–83], suggesting inflammation in the colon can affect hepatic drug metabolism. Treatments of anti-inflammatory agents and antibiotics are both sufficient to attenuate the downregulation of hepatic CYP enzymes in IBD models [81–83], suggesting that the disturbance of CYP enzymes in IBD is, at least in part, caused by the combination effect of colonic inflammation and bacteria-derived molecules (e.g., endotoxin or LPS). Given that PXR expression is almost absent in colonic samples from human with IBD [80], the role of PXR dysregulation in inflammatory signaling of IBD has been extensively investigated. PXR activation has been proposed as a novel target for IBD therapy [84]. Nevertheless, the expression and activity of PXRin hepatic and renal samples from either human IBD or animal IBD model need to be further evaluated.
3.4. Aging
Human aging has been described as a chronic and low-grade inflammatory condition [85]. Under this condition, NF-κB and nuclear receptor signalings are found to be regulated during aging [86,87]. As described above, the transcription factors contribute to the regulation of enzymes/transporters, suggesting that aging can be a regulatory factor for drug metabolism/transport. However, the effects of aging on the expression and activity of CYP enzymes are controversial. The expression of many, but not all, CYP enzymes (e.g., CYP1A2, CYP2C9, CYP2C10, CYP2C18. and CYP2C19) was shown to be reduced in human aging population (reviewed by Ref. [88]). Using in-vivo metabolic probes (cocktail substrates for CYP1A2, CYP2C19, CYP2D6, CYP2E1, and CYP3A4), the metabolic activities of CYP2C19, CYP2E1, and CYP3A4, but not of CYP1A2 and CYP2D6, seem to be dependent on age [89]. However, the regulation of CYP3A4 expression during aging remains controversial [90,91] and the conditions may vary among aged human individuals due to their personal medical history. In this regard, using normal aging animals may be a promising strategy to clarify the impact of aging on the expression of enzymes/transporters [92,93]. In a study that analyzed mRNA expression of hundreds of detoxification enzymes in aged mice, the results showed that about 40–45% of these genes were downregulated during aging process [94]. Still, age-related disorders that are commonly developed during aging, rather than aging itself, may also dominate gene regulation in these animal models.
3.5. Metabolic disorders
Aging is a critical risk factor for the development of metabolic disorders. It is generally believed that chronic inflammation during aging is the major cause of metabolic abnormalities (including hyperglycemia, hyperlipidemia, insulin resistance, and obesity) that are characterized in type 2 diabetes (T2D). Total CYP content in liver biopsies of patients with T2D was reported to be decreased [58]. Consistently, patients with T2D were found to have lower CYP3A4 activity [95]. In contrast, the activity of CYP2E1 was increased and the activities of CYP1A1, CYP2C9 and CYP3As remain unchanged in patients with T2D [59,96]. In animal models with metabolic disorders, the mRNA levels of Cyp3a11, Cyp2b10, and Cyp2a4 have been demonstrated to be significantly reduced in the liver of high-fat diet-fed mice when compared with those in low-fat diet-fed mice [97]. Downregulation of hepatic Cyp3a and Mdr1 was shown to be related to the increased plasma levels of nelfinavir in high-fat diet-fed rats after an intravenous injection [98]. In addition to diet-manipulated mouse models, the regulation of DMEs has been examined in genetic diabetic mouse models. The ob/ob mice, which carry a mutation in the gene responsible for the production of leptin, can develop obesity and mild hyperglycemia similar to many cases of human T2D. In this mouse model, total levels of CYP enzymes are significantly lower, compared with lean mice. Yet, while Cyp2e1 activity is lower, the activities of Cyp2a and Cyp2b are higher in ob/ob mice than in controls. Leptin administration can correct the alterations, suggesting that the regulation of CYP enzymes may be due to direct effects of leptin or via indirect changes in insulin or endogenous hormones [99,100]. On the other hand, in db/db mice, another spontaneous obesity-induced diabetic mouse model carrying a mutation gene for leptin receptor, protein levels of Cyp2b and Cyp4a enzymes are increased, while that of Cyp1a2 is decreased. The protein levels of Cyp2c, Cyp2e1, and Cyp3a are not different between db/db and wild-type mice [101]. In TSOD (Tsumura, Suzuki, obese, diabetes)mice, higher levels of Cyp2c and Cyp3a, but lower levels of Cyp1a and Cyp2e enzymes have been reported. Following an intraperitoneal injection of triazolam, a substrate for Cyp3a, its clearance was significantly higher in TSOD mice than in controls [102]. Overall, these findings suggest that the regulation of CYP enzymes in T2D may depend on the disease models and CYP isoforms.
The expression of drug transporters is also altered in obesity and T2D [103–107]. In the liver of ob/ob mice, the expression levels of several uptake transporters (e.g., Oatps and Ntcp) are decreased, whereas the levels of efflux transporters such as Mrp2 and Mrp4 are increased [103]. In the kidney of ob/ob mice, the expression of Mrp3, Oatp1a1, and Oat2 is decreased [103]. In a polygenic T2D mouse model, New Zealand obese mice, the protein expression of Mdr1 is significantly increased in the brain and the blood–brain barrier [105], but decreased in renal tubules [107].
In addition to inflammation, several other mechanisms have been proposed to account for the regulation of enzymes/transporters in the models of metabolic disorders described above. Higher serum levels of glucose and insulin are observed in early stage of T2D. Therefore, hyperglycemia and hyperinsulinemia are considered to induce the changes in the expression of enzymes/transporters in T2D. This idea has been further confirmed by both in-vitro and in-vivo experiments [105,108,109]. Although it remains unclear how glucose and insulin influence gene transcription, it is noted that the expression of several transcription factors, including CAR, RXRα, PXR, HNF-4α, and NF-κB, is also modulated in these models [101,102,105].
3.6. Neurodegenerative diseases
Neurodegenerative diseases are a group of chronic brain disorders that are characterized by a progressive loss of neurons. While diverse neurodegenerative diseases are identified in humans, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and multiple sclerosis (MS), neuroinflammation is a common characteristic of these diseases (for review, refer to Refs. [54,55]). Neuroinflammation describes the activation of glial cells, especially microglia and astrocytes within the brain, which release a wide range of proinflammatory cytokines and chemokines that cause neurotoxicity. Although neuroinflammation is a hallmark for neurodegenerative diseases, little is known for the impacts of neuroinflammation on the expression of enzymes/transporters or on the pharmacokinetics of administered drugs. Instead, current findings in literature mainly focus on the roles of enzymes/transporters in the development of neurodegenerative diseases. For examples, the expression and function of CYP2D6 are involved in the formation of neurotoxin that induces parkinsonism [110]; reduced function of Mdr1 at the blood–brain barrier in AD and PD-related disorders may impair brain clearance of γ-amyloid [111,112]. Until recently, a study demonstrated that the mRNA and protein expression levels of Mdr1 at the blood–brain barrier are increased in R6/2 HD mouse model through NF-κB pathway, which then affects brain availability of antipsychotic drugs risperidone and paliperidone [113]. Likewise, the protein expression and activity of Mdr1 are increased in brain capillaries in pilocarpine-induced acute and chronic epileptic rats [114]. These observations suggest that the regulation of drug-processing proteins in either neurodegenerative or neurological diseases is worth an attention.
4. Conclusions and future directions
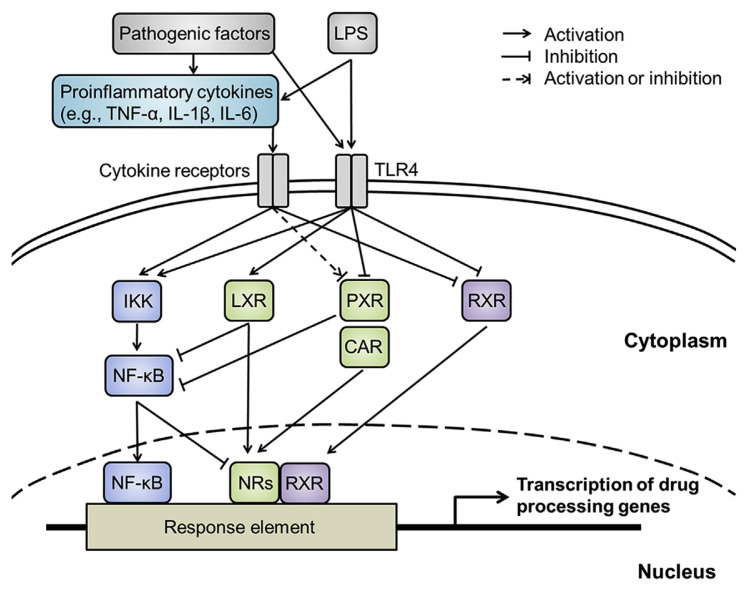
Accumulating evidences have demonstrated that the expression of DMEs and transporters can be regulated under inflammation (Fig. 1). In agreement with this notion, aberrant expression of these drug-processing proteins is observed in several animal models of human inflammatory diseases. The examples include, but are not limited to, type 1 diabetes, rheumatoid arthritis, inflammatory bowel disease, normal aging, metabolic disorders, and several neurodegenerative diseases. Thus, following the same drug administrations, patients with these diseases may be subject to different pharmacokinetic profiles from those without the same disease states. Although experimental animal models of human diseases seem to offer a feasible opportunity to explore this issue, the gap between experimental disease models and clinical observations needs to be considered. Unraveling the underlying molecular mechanisms of these regulations can enable us to see the whole picture and provide better prediction for the therapeutic outcome.
Fig. 1.
The illustration of inflammation-mediated gene regulation through NF-κB and nuclear receptor pathways under disease conditions. CAR: constitutive androstane receptor; IKK: IκB kinase; IL-1β: interleukin-1β; IL-6: interleukin-6; LPS: lipopolysaccharide; LXR: liver X receptor; NF-κB: nuclear factor-κB; NRs: nuclear receptors; PXR: pregnane X receptor; RXR: retinoid X receptor; TLR4: toll-like receptor 4; TNF-α: tumor necrosis factor-α.
Acknowledgment
This study was in part supported by Grant MOST105-2320-B-002-019 from Ministry of Science and Technology of Taiwan.
Funding Statement
This study was in part supported by Grant MOST105-2320-B-002-019 from Ministry of Science and Technology of Taiwan.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Lin JH, Lu AY. Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol Rev. 1997;49:403–49. [PubMed] [Google Scholar]
- 2. Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–41. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 3. König J, Müller F, Fromm MF. Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol Rev. 2013;65:944–66. doi: 10.1124/pr.113.007518. [DOI] [PubMed] [Google Scholar]
- 4. Aitken AE, Morgan ET. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos. 2007;35:1687–93. doi: 10.1124/dmd.107.015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dickmann LJ, Patel SK, Rock DA, Wienkers LC, Slatter JG. Effects of interleukin-6 (IL-6) and an anti-IL-6 monoclonal antibody on drug-metabolizing enzymes in human hepatocyte culture. Drug Metab Dispos. 2011;39:1415–22. doi: 10.1124/dmd.111.038679. [DOI] [PubMed] [Google Scholar]
- 6. Siewert E, Bort R, Kluge R, Heinrich PC, Castell J, Jover R. Hepatic cytochrome P450 down-regulation during aseptic inflammation in the mouse is interleukin 6 dependent. Hepatology. 2000;32:49–55. doi: 10.1053/jhep.2000.8532. [DOI] [PubMed] [Google Scholar]
- 7. Hartmann G, Cheung AK, Piquette-Miller M. Inflammatory cytokines, but not bile acids, regulate expression of murine hepatic anion transporters in endotoxemia. J Pharmacol Exp Ther. 2002;303:273–81. doi: 10.1124/jpet.102.039404. [DOI] [PubMed] [Google Scholar]
- 8. Ke S, Rabson AB, Germino JF, Gallo MA, Tian Y. Mechanism of suppression of cytochrome P-450 1A1 expression by tumor necrosis factor-alpha and lipopolysaccharide. J Biol Chem. 2001;276:39638–44. doi: 10.1074/jbc.M106286200. [DOI] [PubMed] [Google Scholar]
- 9. Zordoky BN, El-Kadi AO. Role of NF-kappaB in the regulation of cytochrome P450 enzymes. Curr Drug Metab. 2009;10:164–78. doi: 10.2174/138920009787522151. [DOI] [PubMed] [Google Scholar]
- 10. Abualsunun WA, Piquette-Miller M. Involvement of nuclear factor κB, not pregnane X receptor, in inflammation-mediated regulation of hepatic transporters. Drug Metab Dispos. 2017;45:1077–83. doi: 10.1124/dmd.117.076927. [DOI] [PubMed] [Google Scholar]
- 11. Thévenod F, Friedmann JM, Katsen AD, Hauser IA. Upregulation of multidrug resistance P-glycoprotein via nuclear factor-kappaB activation protects kidney proximal tubule cells from cadmium- and reactive oxygen species-induced apoptosis. J Biol Chem. 2000;275:1887–96. doi: 10.1074/jbc.275.3.1887. [DOI] [PubMed] [Google Scholar]
- 12. Kuo MT, Liu Z, Wei Y, Lin-Lee YC, Tatebe S, Mills GB, et al. Induction of human MDR1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate NF-kappaB signaling. Oncogene. 2002;21:1945–54. doi: 10.1038/sj.onc.1205117. [DOI] [PubMed] [Google Scholar]
- 13. Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 15. Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 16. Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102:1016–23. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goodwin B, Moore LB, Stoltz CM, McKee DD, Kliewer SA. Regulation of the human CYP2B6 gene by the nuclear pregnane X receptor. Mol Pharmacol. 2001;60:427–31. [PubMed] [Google Scholar]
- 18. Chen Y, Ferguson SS, Negishi M, Goldstein JA. Identification of constitutive androstane receptor and glucocorticoid receptor binding sites in the CYP2C19 promoter. Mol Pharmacol. 2003;64:316–24. doi: 10.1124/mol.64.2.316. [DOI] [PubMed] [Google Scholar]
- 19. Chen Y, Ferguson SS, Negishi M, Goldstein JA. Induction of human CYP2C9 by rifampicin, hyperforin, and phenobarbital is mediated by the pregnane X receptor. J Pharmacol Exp Ther. 2004;308:495–501. doi: 10.1124/jpet.103.058818. [DOI] [PubMed] [Google Scholar]
- 20. Ferguson SS, Chen Y, LeCluyse EL, Negishi M, Goldstein JA. Human CYP2C8 is transcriptionally regulated by the nuclear receptors constitutive androstane receptor, pregnane X receptor, glucocorticoid receptor, and hepatic nuclear factor 4alpha. Mol Pharmacol. 2005;68:747–57. doi: 10.1124/mol.105.013169. [DOI] [PubMed] [Google Scholar]
- 21. Tien ES, Negishi M. Nuclear receptors CAR and PXR in the regulation of hepatic metabolism. Xenobiotica. 2006;36:1152–63. doi: 10.1080/00498250600861827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA. 2001;98:3369–74. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Courtois A, Payen L, Guillouzo A, Fardel O. Up-regulation of multidrug resistance-associated protein 2 (MRP2) expression in rat hepatocytes by dexamethasone. FEBS Lett. 1999;459:381–5. doi: 10.1016/s0014-5793(99)01295-8. [DOI] [PubMed] [Google Scholar]
- 24. Geick A, Eichelbaum M, Burk O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem. 2001;276:14581–7. doi: 10.1074/jbc.M010173200. [DOI] [PubMed] [Google Scholar]
- 25. Bauer B, Hartz AM, Fricker G, Miller DS. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol. 2004;66:413–9. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 26. Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998;18:5652–8. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cerveny L, Svecova L, Anzenbacherova E, Vrzal R, Staud F, Dvorak Z, et al. Valproic acid induces CYP3A4 and MDR1 gene expression by activation of constitutive androstane receptor and pregnane X receptor pathways. Drug Metab Dispos. 2007;35:1032–41. doi: 10.1124/dmd.106.014456. [DOI] [PubMed] [Google Scholar]
- 28. Guo GL, Choudhuri S, Klaassen CD. Induction profile of rat organic anion transporting polypeptide 2 (oatp2) by prototypical drug-metabolizing enzyme inducers that activate gene expression through ligand-activated transcription factor pathways. J Pharmacol Exp Ther. 2002;300:206–12. doi: 10.1124/jpet.300.1.206. [DOI] [PubMed] [Google Scholar]
- 29. Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, et al. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–15. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- 30. Baes M, Gulick T, Choi HS, Martinoli MG, Simha D, Moore DD. A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol. 1994;14:1544–52. doi: 10.1128/mcb.14.3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–46. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- 32. Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol. 2006;68:159–91. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- 33. Duniec-Dmuchowski Z, Ellis E, Strom SC, Kocarek TA. Regulation of CYP3A4 and CYP2B6 expression by liver X receptor agonists. Biochem Pharmacol. 2007;74:1535–40. doi: 10.1016/j.bcp.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chisaki I, Kobayashi M, Itagaki S, Hirano T, Iseki K. Liver X receptor regulates expression of MRP2 but not that of MDR1 and BCRP in the liver. Biochim Biophys Acta. 2009;1788:2396–403. doi: 10.1016/j.bbamem.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 35. Thomas M, Burk O, Klumpp B, Kandel BA, Damm G, Weiss TS, et al. Direct transcriptional regulation of human hepatic cytochrome P450 3A4 (CYP3A4) by peroxisome proliferator-activated receptor alpha (PPARα) Mol Pharmacol. 2013;83:709–18. doi: 10.1124/mol.112.082503. [DOI] [PubMed] [Google Scholar]
- 36. Maeda T, Miyata M, Yotsumoto T, Kobayashi D, Nozawa T, Toyama K, et al. Regulation of drug transporters by the farnesoid X receptor in mice. Mol Pharm. 2004;1:281–9. doi: 10.1021/mp0499656. [DOI] [PubMed] [Google Scholar]
- 37. Rakhshandehroo M, Knoch B, Müller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 20102010 doi: 10.1155/2010/612089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pascussi JM, Drocourt L, Fabre JM, Maurel P, Vilarem MJ. Dexamethasone induces pregnane X receptor and retinoid X receptor-alpha expression in human hepatocytes: synergistic increase of CYP3A4 induction by pregnane X receptor activators. Mol Pharmacol. 2000;58:361–72. doi: 10.1124/mol.58.2.361. [DOI] [PubMed] [Google Scholar]
- 39. Wang H, Faucette SR, Gilbert D, Jolley SL, Sueyoshi T, Negishi M, et al. Glucocorticoid receptor enhancement of pregnane X receptor-mediated CYP2B6 regulation in primary human hepatocytes. Drug Metab Dispos. 2003;31:620–30. doi: 10.1124/dmd.31.5.620. [DOI] [PubMed] [Google Scholar]
- 40. Onica T, Nichols K, Larin M, Ng L, Maslen A, Dvorak Z, et al. Dexamethasone-mediated up-regulation of human CYP2A6 involves the glucocorticoid receptor and increased binding of hepatic nuclear factor 4 alpha to the proximal promoter. Mol Pharmacol. 2008;73:451–60. doi: 10.1124/mol.107.039354. [DOI] [PubMed] [Google Scholar]
- 41. Hukkanen J, Väisänen T, Lassila A, Piipari R, Anttila S, Pelkonen O, et al. Regulation of CYP3A5 by glucocorticoids and cigarette smoke in human lung-derived cells. J Pharmacol Exp Ther. 2003;304:745–52. doi: 10.1124/jpet.102.038208. [DOI] [PubMed] [Google Scholar]
- 42. Chung I, Bresnick E. Regulation of the constitutive expression of the human CYP1A2 gene: cis elements and their interactions with proteins. Mol Pharmacol. 1995;47:677–85. [PubMed] [Google Scholar]
- 43. Liu SY, Gonzalez FJ. Role of the liver-enriched transcription factor HNF-1 alpha in expression of the CYP2E1 gene. DNA Cell Biol. 1995;14:285–93. doi: 10.1089/dna.1995.14.285. [DOI] [PubMed] [Google Scholar]
- 44. Martovetsky G, Tee JB, Nigam SK. Hepatocyte nuclear factors 4α and 1α regulate kidney developmental expression of drug-metabolizing enzymes and drug transporters. Mol Pharmacol. 2013;84:808–23. doi: 10.1124/mol.113.088229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kamiyama Y, Matsubara T, Yoshinari K, Nagata K, Kamimura H, Yamazoe Y. Role of human hepatocyte nuclear factor 4alpha in the expression of drug-metabolizing enzymes and transporters in human hepatocytes assessed by use of small interfering RNA. Drug Metab Pharmacokinet. 2007;22:287–98. doi: 10.2133/dmpk.22.287. [DOI] [PubMed] [Google Scholar]
- 46. Lu H, Gonzalez FJ, Klaassen C. Alterations in hepatic mRNA expression of phase II enzymes and xenobiotic transporters after targeted disruption of hepatocyte nuclear factor 4 alpha. Toxicol Sci. 2010;118:380–90. doi: 10.1093/toxsci/kfq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tirona RG, Lee W, Leake BF, Lan LB, Cline CB, Lamba V, et al. The orphan nuclear receptor HNF4alpha determines PXR-and CAR-mediated xenobiotic induction of CYP3A4. Nat Med. 2003;9:220–4. doi: 10.1038/nm815. [DOI] [PubMed] [Google Scholar]
- 48. Renton KW. Regulation of drug metabolism and disposition during inflammation and infection. Expert Opin Drug Metab Toxicol. 2005;1:629–40. doi: 10.1517/17425255.1.4.629. [DOI] [PubMed] [Google Scholar]
- 49. Cherrington NJ, Slitt AL, Li N, Klaassen CD. Lipopolysaccharide-mediated regulation of hepatic transporter mRNA levels in rats. Drug Metab Dispos. 2004;32:734–41. doi: 10.1124/dmd.32.7.734. [DOI] [PubMed] [Google Scholar]
- 50. Kalitsky-Szirtes J, Shayeganpour A, Brocks DR, Piquette-Miller M. Suppression of drug-metabolizing enzymes and efflux transporters in the intestine of endotoxin-treated rats. Drug Metab Dispos. 2004;32:20–7. doi: 10.1124/dmd.32.1.20. [DOI] [PubMed] [Google Scholar]
- 51. Richardson TA, Morgan ET. Hepatic cytochrome P450 gene regulation during endotoxin-induced inflammation in nuclear receptor knockout mice. J Pharmacol Exp Ther. 2005;314:703–9. doi: 10.1124/jpet.105.085456. [DOI] [PubMed] [Google Scholar]
- 52. Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, et al. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006;281:17882–9. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]
- 53. Ghose R, Zimmerman TL, Thevananther S, Karpen SJ. Endotoxin leads to rapid subcellular re-localization of hepatic RXRalpha: a novel mechanism for reduced hepatic gene expression in inflammation. Nucl Recept. 2004;2:4. doi: 10.1186/1478-1336-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001 May;8(3):131–6. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 55. Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843:2563–82. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 56. Bulterijs S, Hull RS, Björk VC, Roy AG. It is time to classify biological aging as a disease. Front Genet. 2015;6:205. doi: 10.3389/fgene.2015.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79(8 Suppl):1527–34. doi: 10.1902/jop.2008.080246. [DOI] [PubMed] [Google Scholar]
- 58. Sotaniemi EA, Pelkonen O, Arranto AJ, Tapanainen P, Rautio A, Pasanen M. Diabetes and elimination of antipyrine in man: an analysis of 298 patients classified by type of diabetes, age, sex, duration of disease and liver involvement. Pharmacol Toxicol. 2002;90:155–60. doi: 10.1034/j.1600-0773.2002.900308.x. [DOI] [PubMed] [Google Scholar]
- 59. Dostalek M, Akhlaghi F, Puzanovova M. Effect of diabetes mellitus on pharmacokinetic and pharmacodynamic properties of drugs. Clin Pharmacokinet. 2012;51:481–99. doi: 10.2165/11631900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 60. Lee JH, Yang SH, Oh JM, Lee MG. Pharmacokinetics of drugs in rats with diabetes mellitus induced by alloxan or streptozocin: comparison with those in patients with type I diabetes mellitus. J Pharm Pharmacol. 2010;62:1–23. doi: 10.1211/jpp.62.01.0001. [DOI] [PubMed] [Google Scholar]
- 61. Sindhu RK, Koo JR, Sindhu KK, Ehdaie A, Farmand F, Roberts CK. Differential regulation of hepatic cytochrome P450 monooxygenases in streptozotocin-induced diabetic rats. Free Radic Res. 2006;40:921–8. doi: 10.1080/10715760600801272. [DOI] [PubMed] [Google Scholar]
- 62. Chatuphonprasert W, Nemoto N, Sakuma T, Jarukamjorn K. Modulations of cytochrome P450 expression in diabetic mice by berberine. Chem Biol Interact. 2012;196:23–9. doi: 10.1016/j.cbi.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 63.Gawrońska-Szklarz B, Musiał DH, Pawlik A, Paprota B.Effect of experimental diabetes on pharmacokinetic parameters of lidocaine and MEGX in rats Pol J Pharmacol 2003. Jul–Aug554619–24. [PubMed] [Google Scholar]
- 64. Choi YH, Lee AK, Bae SK, Kim SO, Lee MG. Pharmacokinetics of 5-fluorouracil in rats with diabetes mellitus induced by streptozotocin. Biopharm Drug Dispos. 2005 Apr;26(3):93–8. doi: 10.1002/bdd.436. [DOI] [PubMed] [Google Scholar]
- 65. Kudo T, Toda T, Ushiki T, Ohi K, Ikarashi N, Ochiai W, et al. Differences in the pharmacokinetics of Cyp3a substrates in TSOD and streptozotocin-induced diabetic mice. Xenobiotica. 2010;40:282–90. doi: 10.3109/00498251003596809. [DOI] [PubMed] [Google Scholar]
- 66. Dias AS, Porawski M, Alonso M, Marroni N, Collado PS, González-Gallego J. Quercetin decreases oxidative stress, NF-kappaB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J Nutr. 2005;135:2299–304. doi: 10.1093/jn/135.10.2299. [DOI] [PubMed] [Google Scholar]
- 67. van Waarde WM, Verkade HJ, Wolters H, Havinga R, Baller J, Bloks V, et al. Differential effects of streptozotocin-induced diabetes on expression of hepatic ABC-transporters in rats. Gastroenterology. 2002;122:1842–52. doi: 10.1053/gast.2002.33582. [DOI] [PubMed] [Google Scholar]
- 68. Nawa A, Fujita Hamabe W, Tokuyama S. Inducible nitric oxide synthase-mediated decrease of intestinal P-glycoprotein expression under streptozotocin-induced diabetic conditions. Life Sci. 2010;86:402–9. doi: 10.1016/j.lfs.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 69. Zhang LL, Lu L, Jin S, Jing XY, Yao D, Hu N, et al. Tissue-specific alterations in expression and function of P-glycoprotein in streptozotocin-induced diabetic rats. Acta Pharmacol Sin. 2011;32:956–66. doi: 10.1038/aps.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu H, Xu X, Yang Z, Deng Y, Liu X, Xie L. Impaired function and expression of P-glycoprotein in blood-brain barrier of streptozotocin-induced diabetic rats. Brain Res. 2006;1123:245–52. doi: 10.1016/j.brainres.2006.09.061. [DOI] [PubMed] [Google Scholar]
- 71. Maeng HJ, Kim MH, Jin HE, Shin SM, Tsuruo T, Kim SG, et al. Functional induction of P-glycoprotein in the blood-brain barrier of streptozotocin-induced diabetic rats: evidence for the involvement of nuclear factor-kappaB, a nitrosative stress-sensitive transcription factor, in the regulation. Drug Metab Dispos. 2007;35:1996–2005. doi: 10.1124/dmd.107.015800. [DOI] [PubMed] [Google Scholar]
- 72. Guirguis MS, Jamali F. Disease-drug interaction: reduced response to propranolol despite increased concentration in the rat with inflammation. J Pharm Sci. 2003;92:1077–84. doi: 10.1002/jps.10381. [DOI] [PubMed] [Google Scholar]
- 73. Ling S, Jamali F. Effect of early phase adjuvant arthritis on hepatic P450 enzymes and pharmacokinetics of verapamil: an alternative approach to the use of an animal model of inflammation for pharmacokinetic studies. Drug Metab Dispos. 2005;33:579–86. doi: 10.1124/dmd.104.002360. [DOI] [PubMed] [Google Scholar]
- 74. Sanada H, Sekimoto M, Kamoshita A, Degawa M. Changes in expression of hepatic cytochrome P450 subfamily enzymes during development of adjuvant-induced arthritis in rats. J Toxicol Sci. 2011;36:181–90. doi: 10.2131/jts.36.181. [DOI] [PubMed] [Google Scholar]
- 75. Lin CH, Hsu KW, Chen CH, Uang YS, Lin CJ. Differential changes in the pharmacokinetics of statins in collagen-induced arthritis rats. Biochem Pharmacol. 2017;142:216–28. doi: 10.1016/j.bcp.2017.06.118. [DOI] [PubMed] [Google Scholar]
- 76. Piquette-Miller M, Jamali F. Influence of severity of inflammation on the disposition kinetics of propranolol enantiomers in ketoprofen-treated and untreated adjuvant arthritis. Drug Metab Dispos. 1995;23:240–5. [PubMed] [Google Scholar]
- 77. Ashino T, Arima Y, Shioda S, Iwakura Y, Numazawa S, Yoshida T. Effect of interleukin-6 neutralization on CYP3A11 and metallothionein-1/2 expressions in arthritic mouse liver. Eur J Pharmacol. 2007;558:199–207. doi: 10.1016/j.ejphar.2006.11.072. [DOI] [PubMed] [Google Scholar]
- 78. Wollmann BM, Syversen SW2, Vistnes M, Lie E, Mehus LL, Molden E. Associations between cytokine levels and CYP3A4 phenotype in patients with rheumatoid arthritis. Drug Metab Dispos. 2018;46:1384–9. doi: 10.1124/dmd.118.082065. [DOI] [PubMed] [Google Scholar]
- 79. Uno S, Uraki M, Ito A, Shinozaki Y, Yamada A, Kawase A, et al. Changes in mRNA expression of ABC and SLC transporters in liver and intestines of the adjuvant-induced arthritis rat. Biopharm Drug Dispos. 2009;30:49–54. doi: 10.1002/bdd.639. [DOI] [PubMed] [Google Scholar]
- 80. Langmann T, Moehle C, Mauerer R, Scharl M, Liebisch G, Zahn A, et al. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology. 2004;127:26–40. doi: 10.1053/j.gastro.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 81. Masubuchi Y, Horie T. Endotoxin-mediated disturbance of hepatic cytochrome P450 function and development of endotoxin tolerance in the rat model of dextran sulfate sodium-induced experimental colitis. Drug Metab Dispos. 2004;32:437–41. doi: 10.1124/dmd.32.4.437. [DOI] [PubMed] [Google Scholar]
- 82. Masubuchi Y, Enoki K, Horie T. Down-regulation of hepatic cytochrome P450 enzymes in rats with trinitrobenzene sulfonic acid-induced colitis. Drug Metab Dispos. 2008;36:597–603. doi: 10.1124/dmd.107.018754. [DOI] [PubMed] [Google Scholar]
- 83. Chaluvadi MR, Nyagode BA, Kinloch RD, Morgan ET. TLR4-dependent and -independent regulation of hepatic cytochrome P450 in mice with chemically induced inflammatory bowel disease. Biochem Pharmacol. 2009;77:464–71. doi: 10.1016/j.bcp.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cheng J, Shah YM, Gonzalez FJ. Pregnane X receptor as a target for treatment of inflammatory bowel disorders. Trends Pharmacol Sci. 2012;33:323–30. doi: 10.1016/j.tips.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 86. Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD. NF-κB in aging and disease. Aging Dis. 2011;2:449–65. [PMC free article] [PubMed] [Google Scholar]
- 87. Bertolotti M, Gabbi C, Anzivino C, Crestani M, Mitro N, Del Puppo M, et al. Age-related changes in bile acid synthesis and hepatic nuclear receptor expression. Eur J Clin Invest. 2007;37:501–8. doi: 10.1111/j.1365-2362.2007.01808.x. [DOI] [PubMed] [Google Scholar]
- 88. Kinirons MT, O’Mahony MS. Drug metabolism and ageing. Br J Clin Pharmacol. 2004;57:540–4. doi: 10.1111/j.1365-2125.2004.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bebia Z, Buch SC, Wilson JW, Frye RF, Romkes M, Cecchetti A, et al. Bioequivalence revisited: influence of age and sex on CYP enzymes. Clin Pharmacol Ther. 2004;76:618–27. doi: 10.1016/j.clpt.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 90. Hunt CM, Westerkam WR, Stave GM, Wilson JA. Hepatic cytochrome P-4503A (CYP3A) activity in the elderly. Mech Ageing Dev. 1992;64:189–99. doi: 10.1016/0047-6374(92)90106-n. [DOI] [PubMed] [Google Scholar]
- 91. George J, Byth K, Farrell GC. Age but not gender selectively affects expression of individual cytochrome P450 proteins in human liver. Biochem Pharmacol. 1995;50:727–30. doi: 10.1016/0006-2952(95)00192-3. [DOI] [PubMed] [Google Scholar]
- 92. Vyskočilová E, Szotáková B, Skálová L, Bártíková H, Hlaváčová J, Boušová I. Age-related changes in hepatic activity and expression of detoxification enzymes in male rats. Biomed Res Int. 2013;2013:408573. doi: 10.1155/2013/408573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kwak HC, Kim HC, Oh SJ, Kim SK. Effects of age increase on hepatic expression and activity of cytochrome P450 in male C57BL/6 mice. Arch Pharm Res. 2015;38:857–64. doi: 10.1007/s12272-014-0452-z. [DOI] [PubMed] [Google Scholar]
- 94. Fu ZD, Csanaky IL, Klaassen CD. Effects of aging on mRNA profiles for drug-metabolizing enzymes and transporters in livers of male and female mice. Drug Metab Dispos. 2012;40:1216–25. doi: 10.1124/dmd.111.044461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Marques MP, Coelho EB, Dos Santos NA, Geleilete TJ, Lanchote VL. Dynamic and kinetic disposition of nisoldipine enantiomers in hypertensive patients presenting with type-2 diabetes mellitus. Eur J Clin Pharmacol. 2002;58:607–14. doi: 10.1007/s00228-002-0528-4. [DOI] [PubMed] [Google Scholar]
- 96. Wang Z, Hall SD, Maya JF, Li L, Asghar A, Gorski JC. Diabetes mellitus increases the in vivo activity of cytochrome P450 2E1 in humans. Br J Clin Pharmacol. 2003;55:77–85. doi: 10.1046/j.1365-2125.2003.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ghose R, Omoluabi O, Gandhi A, Shah P, Strohacker K, Carpenter KC, et al. Role of high-fat diet in regulation of gene expression of drug metabolizing enzymes and transporters. Life Sci. 2011;89:57–64. doi: 10.1016/j.lfs.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sugioka N, Haraya K, Fukushima K, Ito Y, Takada K. Effects of obesity induced by high-fat diet on the pharmacokinetics of nelfinavir, a HIV protease inhibitor, in laboratory rats. Biopharm Drug Dispos. 2009;30:532–41. doi: 10.1002/bdd.689. [DOI] [PubMed] [Google Scholar]
- 99. Watson AM, Poloyac SM, Howard G, Blouin RA. Effect of leptin on cytochrome P-450, conjugation, and antioxidant enzymes in the ob/ob mouse. Drug Metab Dispos. 1999;27:695–700. [PubMed] [Google Scholar]
- 100. Leclercq IA, Field J, Enriquez A, Farrell GC, Robertson GR. Constitutive and inducible expression of hepatic CYP2E1 in leptin-deficient ob/ob mice. Biochem Biophys Res Commun. 2000;268:337–44. doi: 10.1006/bbrc.2000.2125. [DOI] [PubMed] [Google Scholar]
- 101. Yoshinari K, Takagi S, Sugatani J, Miwa M. Changes in the expression of cytochromes P450 and nuclear receptors in the liver of genetically diabetic db/db mice. Biol Pharm Bull. 2006;29:1634–8. doi: 10.1248/bpb.29.1634. [DOI] [PubMed] [Google Scholar]
- 102. Kudo T, Shimada T, Toda T, Igeta S, Suzuki W, Ikarashi N, et al. Altered expression of CYP in TSOD mice: a model of type 2 diabetes and obesity. Xenobiotica. 2009;39:889–902. doi: 10.3109/00498250903242592. [DOI] [PubMed] [Google Scholar]
- 103. Cheng Q, Aleksunes LM, Manautou JE, Cherrington NJ, Scheffer GL, Yamasaki H, et al. Drug-metabolizing enzyme and transporter expression in a mouse model of diabetes and obesity. Mol Pharm. 2008;5:77–91. doi: 10.1021/mp700114j. [DOI] [PubMed] [Google Scholar]
- 104. Nowicki MT, Aleksunes LM, Sawant SP, Dnyanmote AV, Mehendale HM, Manautou JE. Renal and hepatic transporter expression in type 2 diabetic rats. Drug Metab Lett. 2008;2:11–7. doi: 10.2174/187231208783478425. [DOI] [PubMed] [Google Scholar]
- 105. Wu KC, Pan HJ, Yin HS, Chen MR, Lu SC, Lin CJ. Change in P-glycoprotein and caveolin protein expression in brain striatum capillaries in New Zealand obese mice with type 2 diabetes. Life Sci. 2009;85:775–81. doi: 10.1016/j.lfs.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 106. Nawa A, Fujita-Hamabe W, Tokuyama S. Altered intestinal P-glycoprotein expression levels in a monosodium glutamate-induced obese mouse model. Life Sci. 2011;89:834–8. doi: 10.1016/j.lfs.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 107. Yeh SY, Pan HJ, Lin CC, Kao YH, Chen YH, Lin CJ. Hyperglycemia induced down-regulation of renal Pglycoprotein expression. Eur J Pharmacol. 2012;690:42–50. doi: 10.1016/j.ejphar.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 108. Woodcroft KJ, Novak RF. Insulin differentially affects xenobiotic-enhanced, cytochrome P-450 (CYP)2E1, CYP2B, CYP3A, and CYP4A expression in primary cultured rat hepatocytes. J Pharmacol Exp Ther. 1999;289:1121–7. [PubMed] [Google Scholar]
- 109. Davidson MD, Ballinger KR, Khetani SR. Long-term exposure to abnormal glucose levels alters drug metabolism pathways and insulin sensitivity in primary human hepatocytes. Sci Rep. 2016;6:28178. doi: 10.1038/srep28178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bajpai P, Sangar MC, Singh S, Tang W, Bansal S, Chowdhury G, et al. Metabolism of 1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine by mitochondrion-targeted cytochrome P450 2D6: implications in Parkinson disease. J Biol Chem. 2013;288:4436–51. doi: 10.1074/jbc.M112.402123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bartels AL, Willemsen AT, Kortekaas R, de Jong BM, de Vries R, de Klerk O, et al. Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. J Neural Transm (Vienna) 2008;115:1001–9. doi: 10.1007/s00702-008-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hartz AM, Miller DS, Bauer B. Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer’s disease. Mol Pharmacol. 2010;77:715–23. doi: 10.1124/mol.109.061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kao YH, Chern Y, Yang HT, Chen HM, Lin CJ. Regulation of P-glycoprotein expression in brain capillaries in Huntington’s disease and its impact on brain availability of antipsychotic agents risperidone and paliperidone. J Cereb Blood Flow Metab. 2016;36:1412–23. doi: 10.1177/0271678X15606459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hartz AM, Pekcec A, Soldner EL, Zhong Y, Schlichtiger J, Bauer B. P-gp protein expression and transport activity in rodent seizure models and human epilepsy. Mol Pharm. 2017;14:999–1011. doi: 10.1021/acs.molpharmaceut.6b00770. [DOI] [PMC free article] [PubMed] [Google Scholar]