Abstract
The psychedelic psilocybin is being investigated for the treatment of depression and anxiety. Unclear is whether antidepressant treatments interact with psilocybin. The present study used a double‐blind, placebo‐controlled, crossover design with two experimental test sessions to investigate the response to psilocybin (25 mg) in healthy subjects after pretreatment with escitalopram or placebo. The treatment order was random and counterbalanced. Pretreatment consisted of 10 mg escitalopram daily for 7 days, followed by 20 mg daily for 7 days, including the day of psilocybin administration, or 14 days of placebo pretreatment before psilocybin administration. Psilocybin treatments were separated by at least 16 days. The outcome measures included self‐rating scales that evaluated subjective effects, autonomic effects, adverse effects, plasma brain‐derived neurotrophic factor (BDNF) levels, electrocardiogram QTc time, whole‐blood HTR2A and SCL6A4 gene expression, and pharmacokinetics. Escitalopram pretreatment had no relevant effect on positive mood effects of psilocybin but significantly reduced bad drug effects, anxiety, adverse cardiovascular effects, and other adverse effects of psilocybin compared with placebo pretreatment. Escitalopram did not alter the pharmacokinetics of psilocin. The half‐life of psychoactive free (unconjugated) psilocin was 1.8 hours (range 1.1–2.2 hours), consistent with the short duration of action of psilocybin. Escitalopram did not alter HTR2A or SCL6A4 gene expression before psilocybin administration, QTc intervals, or circulating BDNF levels before or after psilocybin administration. Further studies are needed with a longer antidepressant pretreatment time and patients with psychiatric disorders to further define interactions between antidepressants and psilocybin.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Case reports indicate that antidepressants generally reduce the response to psychedelics. Moreover, antidepressants are thought to increase the risks for adverse events when taken together with psychedelics.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Does an escitalopram pretreatment (10 mg for 7 days, followed by 20 mg for 7 days) interact with the acute response to psilocybin (25 mg), compared with a placebo pretreatment?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ This study gives a first indication that psilocybin is not only safe to take during escitalopram treatment, moreover, it has no relevant effects on the positive drug effects of psilocybin.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ If the results are confirmed in subsequent studies, escitalopram pretreatment would no longer need to be stopped for psilocybin treatment in phase II trials and compassionate use. Thus, the risk for adverse effects due to escitalopram treatment interruption would be eliminated.
Psilocybin is a classic psychedelic substance that is being investigated in psilocybin‐assisted psychotherapy for major depression 1 , 2 , 3 , 4 , 5 , 6 and anxiety. 4 , 5 Psilocybin has been shown to produce lasting reductions of depression and anxiety after the administration of only a few doses. 1 , 4 , 5 , 6
Similar to other classic psychedelics like lysergic acid diethylamide (LSD), 7 psilocin (the active metabolite of the prodrug psilocybin 8 ) acutely produces an altered state of mind through serotonin 5‐hydroxytryptamine‐2A (5‐HT2A) receptor activation. 9 , 10 Positive acute mood effects, including mystical‐type experiences and feelings of bliss, that are induced by psilocybin have been shown to be associated with positive long‐term effects on depression, anxiety, and addiction. 2 , 4 , 11 These findings suggest that the acute effects of a serotonergic psychedelic in humans can be used to predict, at least partially, therapeutic outcome in patients. Additionally, psychedelics, including psilocybin, may induce neuroplastogenic effects and increase markers of neuroregeneration, including brain‐derived neurotrophic factor (BDNF), which may be a possible mediator or marker of the therapeutic effects of psychedelics. 7 , 12 , 13 , 14 Negative acute effects of psilocybin include anxiety, headache, insomnia, and nausea and may in part represent serotonin toxicity. 15 It is not known whether serotonin‐associated adverse effects are increased when psilocybin is used in combination with other serotonergic agents. 15
Psychedelics are typically investigated and intended to be used in patients with psychiatric disorders who may already be treated with antidepressant medications, typically serotonin transporter inhibitors. Case reports indicated that chronic administration of the serotonin transporter inhibitors fluoxetine and sertraline reduced effects of LSD in recreational users. 16 , 17 Therefore, one assumption was that antidepressants generally reduce the response to psychedelics. Patients in clinical studies who used psilocybin typically had to be free of concomitant antidepressant treatment for 2 weeks before randomization and then for the duration of the study, 5 , 18 or medications that affect the serotonin system were stopped at least 5 half‐lives before psilocybin administration 4 or study inclusion. 6 Similarly, patients who receive LSD pause antidepressant treatment typically for 5 days before LSD treatment. 19 , 20 Adverse effects could also occur if antidepressants and psychedelics are used together, including QT‐time prolongation and serotonin toxicity. 15 However, the general practice of stopping antidepressant treatment can also be problematic in some patients who require antidepressant treatment or suffer from withdrawal when stopping it. The interruption or discontinuation of antidepressant treatment often leads to withdrawal symptoms, including dizziness, nausea/vomiting, headache, and lethargy, 21 and can also trigger the relapse of depression. Negative mood states before psilocybin administration may also result in a less positive response to psilocybin. 22 , 23
Unclear is whether an antidepressant must be stopped before psychedelic administration. Therefore, we investigated whether escitalopram pretreatment alters the acute subjective and adverse effects of a full dose of psilocybin (25 mg) in a randomized, double‐blind, placebo‐controlled, crossover study in healthy subjects. Escitalopram was administered for 14 days (10 mg daily for 7 days, followed by 20 mg daily for 7 days) to produce plasma escitalopram concentrations that are similar to patients who are on chronic antidepressant treatment. Outcome measures included subjective effects, alterations of mind, mystical‐type experiences, cardiostimulant and adverse effects, QT time, and plasma concentrations of psilocin, psilocin metabolites, escitalopram, and BDNF. Expression of the HTR2A and SCL6A4 genes (which encode the 5‐HT2A receptor and serotonin transporter, respectively) was also determined after pretreatment to explore the mechanisms that contribute to a potentially lower response to 5‐HT2A receptor stimulation after serotonin transporter inhibition. The primary study end point was the 5 Dimensions of Altered States of Consciousness (5D‐ASC) scale. 24 , 25 We hypothesized that escitalopram pretreatment would significantly reduce 5D‐ASC total scores compared with placebo pretreatment.
Although psilocybin has been clinically investigated for many years, pharmacokinetic data are still limited. 8 , 26 , 27 In particular, no data are available on actual psychoactive free (unconjugated) psilocin concentrations from controlled studies. 28 Therefore, we also determined the pharmacokinetics of a clinically representative dose of 25 mg of psilocybin. 29
MATERIALS AND METHODS
Study design
The study used a double‐blind, placebo‐controlled, crossover design with two experimental test sessions to investigate the response to psilocybin (25 mg) after pretreatment with escitalopram or placebo. The two treatment conditions pertinent to the double‐blind component were: (group A) 7 days of 10 mg escitalopram daily, followed by 7 days of 20 mg daily; and (group B) 14 days of placebo daily. A single 25‐mg dose of psilocybin was administered at the end of each 14‐day period. Following a washout period of at least 2 days, subjects in group A crossed over to group B, and those in group B crossed over to group A. The 14 day process was repeated with administration of the same 25 mg dose of psilocybin at the end. The treatment order was random and counterbalanced. On the day of psilocybin administration, the last daily dose of escitalopram or placebo was administered 2 hours before psilocybin. To enhance compliance of daily pretreatment administration, an electronic reminder was sent to the participant at the start of each pretreatment, blisters were checked to be empty at the end of the pretreatment, and escitalopram concentrations were measured before the final dose of escitalopram was administered under supervision at the study site. The study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization Guidelines in Good Clinical Practice and approved by the Ethics Committee of Northwest Switzerland (EKNZ) and Swiss Federal Office for Public Health (FOPH). The study was registered at ClinicalTrials.gov (NCT03912974).
Participants
Twenty‐seven participants were recruited by word of mouth or an advertisement that was posted on the web market platform of the University of Basel. There were three dropouts before psilocybin administration and one dropout after the first administration. Twenty‐three healthy subjects completed the study (12 men and 11 women; 34 ± 10 years old (mean ± SD); range: 25–55 years). Mean body weight was 70 kg. All of the subjects provided written informed consent and were paid for their participation. Drug administration timing did not consider the menstrual cycle in women for practical reasons. Six women used a hormonal contraceptive. The exclusion criteria were age < 25 years or > 65 years, pregnancy (urine pregnancy test at screening and before each test session), personal history of major psychiatric disorders (assessed by the Semi‐structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Axis I disorders), family history (first‐degree relative) of psychotic disorders, the use of medications that may interfere with the study medications (e.g., antidepressants, antipsychotics, and sedatives), chronic or acute physical illness (e.g., abnormal physical exam, electrocardiogram, or hematological and chemical blood analyses), tobacco smoking > 10 cigarettes/day, lifetime prevalence of illicit drug use > 10 times (except Δ9‐tetrahydrocannabinol (THC)), illicit drug use within the last 2 months, and illicit drug use during the study period (determined by urine drug tests). The participants were asked to consume no more than 10 standard alcoholic drinks/week and have no more than one drink on the day before the test sessions. Six participants had previously used psilocybin‐containing mushrooms (1–4 times), eight participants had used methylenedioxymethamphetamine (MDMA; 1–5 times), and three participants had previously used cocaine once, one of whom had once also used amphetamine and methylphenidate. Four participants had used nitrous oxide once previously, one of whom also used ketamine. Drug of abuse tests that were performed once during screening and once during the study in each subject were negative, with the exception of positive tests for THC in three participants (one with regular cannabis use).
Study drugs
Psilocybin was customer synthesized for this study with FOPH approval (99.7% high‐performance liquid chromatography purity; ReseaChem GmbH, Burgdorf, Switzerland) and was administered as opaque capsules that contained a 5 mg dose of psilocybin dihydrate and an exact analytically confirmed actual psilocybin content of 4.61 ± 0.09 mg (mean ± SD, n = 10 samples). Placebo consisted of identical opaque capsules that were filled with mannitol. Escitalopram was obtained as the marketed drug Escitalopram‐Mepha Lactab (10 mg; Mepha Pharma AG, Basel, Switzerland) and encapsulated to ensure blinding. Placebo consisted of identical capsules that were filled with mannitol. All drug products were produced according to good manufacturing practice (GMP) by a licensed GMP facility (Apotheke Dr. Hysek, Biel, Switzerland). The formulation of psilocybin and its use in humans was authorized by the FOPH. At the end of the study, the participants were asked to retrospectively guess their pretreatment assignment.
Study procedures
The study included a screening visit, two 10‐hour test sessions, and an end‐of‐study visit. The sessions were conducted in a calm hospital room. Only one research subject and one or two investigators were present during each test session. The test sessions began at 7:30 am. A urine sample was taken to verify abstinence from drugs of abuse, and a urine pregnancy test was performed in women. The subjects then underwent baseline measurements. The last dose of escitalopram (20 mg) or placebo was administered at 8:00 am. Psilocybin was administered at 10:00 am. Outcome measures were assessed for 7 hours after psilocybin administration. Standardized lunches were served at 1:30 pm. The subjects were never alone during the test sessions. The subjects were sent home at 5:30 pm with a partner or friend.
Subjective drug effects
To assess alterations of consciousness, the 5D‐ASC scale 30 was administered 7 hours after psilocybin administration to retrospectively rate peak drug effects. The questionnaire contains 94 items that are rated on visual analog scales (VASs) and grouped into 5 dimensions (Supplementary Methods). Three main dimension (3D‐OAV) total scores were used as the primary study end point. The three main dimensions are “Oceanic Boundlessness,” “Anxious Ego‐Dissolution,” and “Visionary Restructuralization,” which can be used as a measure of the overall intensity of psychedelic‐specific alterations of mind, in addition to five dimension (5D‐ASC) total scores. 24 Mystical experiences were assessed 7 hours after psilocybin administration using the States of Consciousness Questionnaire (SOCQ) 24 , 31 that includes the 43‐item Mystical Effects Questionnaire (MEQ43), 31 30‐item Mystical Effects Questionnaire (MEQ30), 32 and subscales for “aesthetic experience” and negative “nadir” effects. Subjective effects over time were assessed repeatedly using VASs 33 , 34 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6, and 7 hours after psilocybin administration. The Adjective Mood Rating Scale (AMRS) 35 was used before (0) and 2, 3, 5, and 7 hours after psilocybin administration. Subjective effect measurements are described in detail in the Supplementary Methods.
Autonomic and adverse effects
Blood pressure, heart rate, and tympanic body temperature were repeatedly measured. 36 Adverse effects were assessed 1 hour before and 7 hours after psilocybin administration using the List of Complaints. 37 Adverse events during pretreatment were recorded at the beginning of each test session. Adverse events that were related to the psilocybin test session and occurred after the session were recorded at the beginning of the next test session and at the end of study visit. Electrocardiograms were recorded 1 hour before and 2.5 hours after psilocybin administration using a Schiller CARDIOVIT AT‐10 Plus recorder (Patec Medical GmbH, Ebernhahn, Germany). QT times were measured, and QTc times were calculated using the device’s program or manually if no value was available.
Plasma BDNF levels
Plasma BDNF levels were measured at baseline and 4 and 7 hours after psilocybin administration using the Biosensis Mature BDNF Rapid ELISA Kit (Thebarton, Australia). 38 The lower and upper limits of sensitivity are 2 and 500 pg/mL and intra‐ and inter‐assay variability (percent coefficient of variation (CV%)) values are 3.3 and 2.3, respectively.
Gene expression
Blood samples were collected before psilocybin administration using the PAXgene Blood RNA system (Becton Dickinson, Heidelberg, Germany). Samples were incubated for 2 hours at room temperature, followed by freezing at −80°C until further processing. Total RNA was prepared using the PAXgene Blood RNA Kit 50 (PreAnalytiX; Qiagen, Hilden, Germany). Total RNA samples were spectrophotometrically scanned (260 and 280 nm; NanoDrop One; Thermo Fisher Scientific, Reinach, Switzerland). Absorbance 260 (A260) was used for RNA quantification. The A260/A280 ratio was > 1.9, excluding relevant protein contamination. Quantitative real‐time polymerase chain reaction (PCR) was performed for the HTR2A and SCL6A4 genes and four additional reference genes (ACTB, ALAS1, RPL13A, and RRN18S) using qbasePLUS software, as described previously. 39 Total RNA (500 ng) from each sample was reverse‐transcribed using the iScript cDNA synthesis kit (Bio‐Rad, Hercules, CA, USA). Each amplification was performed in a total volume of 10 μL that contained 5 μL of the QuantiFast SYBR Green PCR kit (Qiagen) and the specific PrimerAssay (Qiagen). The PCR conditions were run on a CFX384/CFXOpus 384 device (Bio‐Rad) according to the manufacturer’s instructions (Qiagen). Melting‐point analysis was conducted for each assay to confirm specificity of the PCR products. All the PCR reactions were run in triplicate.
Plasma psilocin concentrations
Blood was collected into lithium heparin tubes. The blood samples were immediately centrifuged, and plasma was subsequently stored at −80°C until analysis. Plasma psilocin concentrations were analyzed using a validated ultra‐high‐performance liquid chromatography tandem mass spectrometry method, as described previously. 8 All samples were re‐analyzed after deglucuronidation with Escherichia coli β‐glucuronidase, 8 thereby allowing determination of the concentrations of unconjugated psilocin and psilocin glucuronide, which corresponds to the difference between samples that were incubated with and without β‐glucuronidase. 8 , 28 Plasma escitalopram concentrations were also determined using liquid chromatography tandem mass spectrometry. Briefly, the mass transitions of escitalopram (m/z 325.1/261.9, 109.1) and its internal standard escitalopram‐d6 (m/z 331.1/262.3, 109.1) were included in the psilocin method. 8 Plasma sample extraction and chromatography were unchanged. The processed samples were reanalyzed for escitalopram measurements using a larger injection volume of 20 µL. The lower limit of quantification for escitalopram in human plasma was set to 0.5 ng/mL, and the upper limit of quantification was 100 ng/mL. Pharmacokinetic parameters were estimated using noncompartmental methods in Phoenix WinNonlin 8.3 (Certara, Princeton, NJ, USA), as described previously. 40
Data analysis
Peak (maximum effect (Emax) and/or minimum effect (Emin)) or peak change from baseline (ΔEmax) values were determined for repeated measures. The values were then analyzed using paired two‐sided t‐tests. The data were analyzed using R Studio. The criterion for significance was P < 0.05. No correction for multiple testing was applied. Order effects were excluded by comparing first and second treatment sequence.
RESULTS
Subjective drug effects
Pretreatment with escitalopram had no effect on 3D‐OAV total scores, indicating no overall effect on alterations of mind compared with placebo treatment (Figure 1 , Table S1 ). Escitalopram significantly reduced psilocybin‐induced “Anxious Ego‐Dissolution” and anxiety (both P < 0.05) but not “Oceanic Boundlessness” or any other positively experienced alterations of mind that were induced by psilocybin (Figure 1 , Table S1 ).
Figure 1.
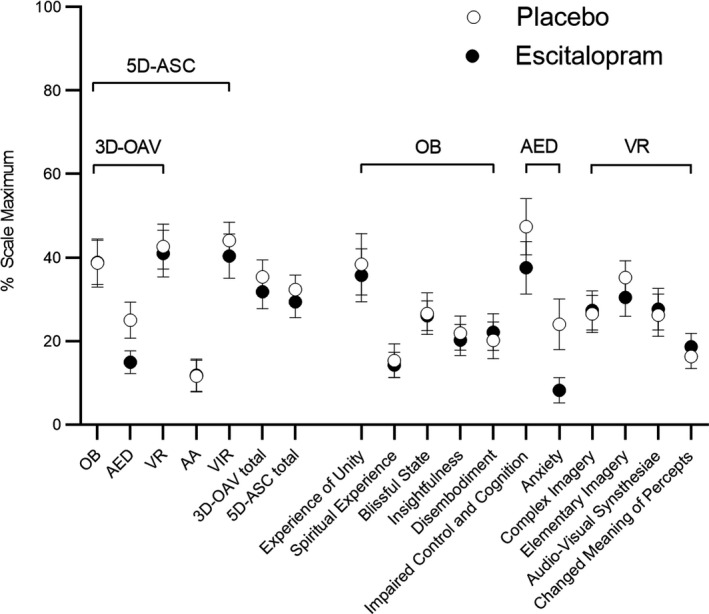
Acute alterations of mind on the 5 Dimensions of Altered States of Consciousness (5D‐ASC) Scale. Psilocybin produced similar overall alterations of mind after escitalopram pretreatment compared with placebo, indicated by similar 3D‐OAV and 5D‐ASC total scores. Psilocybin similarly increased “Oceanic Boundlessness” (OB), “Visionary Restructuralization” (VR), “Auditory Alterations” (AA), and “Vigilance Reductions” (VIR) after escitalopram compared with placebo. Escitalopram significantly reduced psilocybin‐induced increases in “Anxious Ego‐Dissolution” (AED; P = 0.03) and “Anxiety” (P = 0.03) compared with placebo. The data are expressed as the mean ± SEM percentage of maximally possible scale scores in 23 subjects. Statistics are shown in Table S1 .
Subjective effects over time on the VAS and AMRS are shown in Figure 2 and Figures S1 and S2 , respectively. The corresponding peak responses and statistics are presented in Table S2 . Statistics are summarized in Tables S2 and S3 . Escitalopram reduced psilocybin‐induced increases in VAS ratings of “any drug effects” (P = 0.02), “bad drug effects” (P = 0.004), “fear” (P = 0.004), “talkative” (P = 0.03), and “open” (P = 0.03) and attenuated reductions of ratings of “happy” (P = 0.04) and “concentration” (P = 0.01; Figure 2 , Figure S2 , Table S2 ). No effects were seen on psilocybin‐induced increases in ratings of “good drug effects” or “drug linking” (Figure 2 ). Escitalopram also reduced AMRS “anxiety” ratings in response to psilocybin (P = 0.007; Figure S2 , Table S3 ). Escitalopram did not significantly alter overall mystical experiences that were induced by psilocybin, measured by MEQ30 total score (Table 1 ). However, escitalopram significantly reduced ratings on the MEQ subscales “nadir effects” (P = 0.001) on the MEQ43 and “ineffability” (P = 0.02) on the MEQ30 but had no effect on “positive mood” that was induced by psilocybin (Table 1 ).
Figure 2.
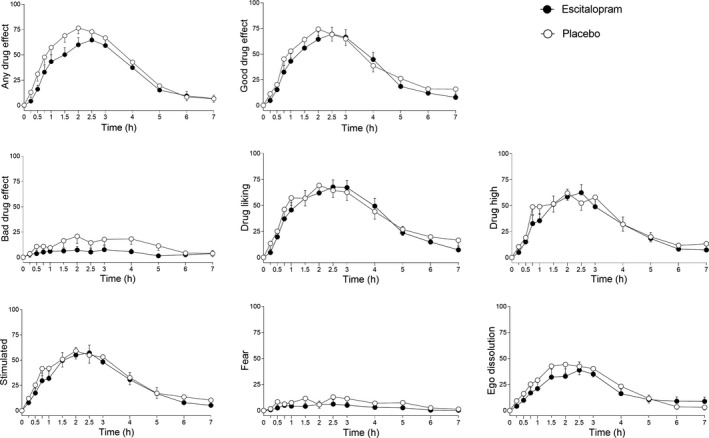
Acute subjective effects of psilocybin over time on the visual analog scale (VAS). Escitalopram slightly but significantly reduced psilocybin‐induced increases in “any drug effect” (P = 0.02), “bad drug effect” (P = 0.004), and “fear” (P = 0.004) but had no effect on “good drug effect,” “drug liking,” “drug high,” or “feeling stimulated.” Psilocybin was administered at t = 0 hour. The data are expressed as the mean ± SEM in 23 subjects. Additional subjective effects are shown in Figure S1 . The corresponding maximal responses and statistics are shown in Table S2 .
Table 1.
Acute effects of psilocybin on the Mystical Experiences Questionnaire
| Escitalopram mean ± SEM | Placebo mean ± SEM | t 22 | P value | |
|---|---|---|---|---|
| Mystical Experiences Questionnaire (MEQ43) (% score) | ||||
| Internal unity | 29 ± 5 | 28 ± 6 | −0.1 | 0.958 |
| External unity | 28 ± 5 | 32 ± 6 | 0.7 | 0.471 |
| Sacredness | 31 ± 5 | 31 ± 6 | 0.1 | 0.931 |
| Noetic quality | 27 ± 5 | 32 ± 5 | 1.0 | 0.349 |
| Deeply felt positive mood | 46 ± 6 | 42 ± 7 | −0.6 | 0.537 |
| Transcendence of time/space | 38 ± 5 | 44 ± 5 | 1.2 | 0.240 |
| Ineffability | 35 ± 4 | 42 ± 5 | 1.7 | 0.113 |
| Nadir effects | 9 ± 2 | 22 ± 4 | 3.7 | 0.001** |
| Aesthetic Experience | 42 ± 6 | 40 ± 5 | −0.4 | 0.706 |
| Mystical Experiences Questionnaire (MEQ30) (% score) | ||||
| Mystical | 28 ± 5 | 30 ± 6 | 0.4 | 0.718 |
| Positive mood | 47 ± 5 | 44 ± 6 | −0.4 | 0.701 |
| Transcendence of time/space | 38 ± 5 | 46 ± 6 | 1.5 | 0.157 |
| Ineffability | 46 ± 5 | 57 ± 5 | 2.6 | 0.017* |
| MEQ30 total score | 36 ± 4 | 39 ± 5 | 0.7 | 0.471 |
N = 23.
*P < 0.05, **P < 0.01
Autonomic and adverse effects
Autonomic effects over time and respective peak effects are shown in Figures S3 and S44 and Table S4 , respectively. Escitalopram significantly reduced psilocybin‐induced elevations of peak systolic blood pressure (P < 0.001), peak diastolic blood pressure (P = 0.02), rate pressure product (P = 0.001), and pupil dilation (P = 0.002; Figures S3, S4 ). Escitalopram tended to reduce heart rate compared with placebo before and after psilocybin administration (P = 0.09).
Escitalopram also reduced acute adverse effects, assessed by the List of Complaints, that were associated with psilocybin compared with placebo (P = 0.03; Table S4 ). Psilocybin did not increase QTc times 2.5 hours after administration compared with times measured 1 hour before administration (Table S4 ). Escitalopram did not alter QTc time before or after psilocybin administration compared with placebo (Table S4 ). However, the longest QTc intervals observed in any subject (max in Table S4 ) were 489 and 422 ms after escitalopram and placebo, respectively, 1 hour before and 509 and 481 ms 2.5 hours after psilocybin administration, respectively. Other adverse events were reported after psilocybin administration in the evening of the treatment day and on the day after treatment. These adverse effects after psilocybin administration included headaches (6 subjects after escitalopram and 6 subjects after placebo), flashbacks (one subject after escitalopram and one subject after placebo), nausea (one subject after escitalopram and one subject after placebo), abdominal bloating (one subject after escitalopram), vasovagal syncope (one subject after placebo), and lack of energy (one subject after escitalopram). Altogether, the type and amount of adverse events after psilocybin administration was comparable after escitalopram and placebo pretreatment.
Effects on BDNF
Psilocybin significantly increased plasma BDNF peak levels in the escitalopram and placebo condition compared to baseline (t 22 = 5.4, P < 0.001 and 3.6, P = 0.002, respectively). Escitalopram did not significantly alter the psilocybin‐induced moderate increase in BDNF levels (Figure S5 , Table S5 ).
Pharmacokinetics of psilocin and its metabolites
Plasma‐time curves are shown in Figure 3 , and pharmacokinetic parameters are listed in Table 2 . Plasma concentrations of psilocin and 4‐hydroxyindole‐3‐acetic acid (4‐HIAA) were quantified before and up to 7 hours posttreatment. All samples were re‐analyzed after deglucuronidation. Plasma levels of psilocin glucuronide were determined based on the difference between samples that were incubated with and without glucuronidase, corresponding to the total amount of conjugated metabolites. A large proportion of psilocin underwent glucuronidation. The 4‐HIAA concentrations were similar when they were analyzed before and after deglucuronidation. Thus, 4‐HIAA was not conjugated. Psilocin, the active metabolite of psilocybin, was metabolized to an approximately similar extent to inactive 4‐HIAA and psilocin glucuronide during the first 7 hours after drug administration (Figure 3 , Table 2 ). The increase in metabolite concentrations over time was faster for 4‐HIAA compared with the glucuronide (Figure 3 ), whereas glucuronide concentrations remained higher beyond 7 hours. Maximal plasma concentrations of unconjugated and psychoactive active psilocin were reached after a mean time of 2 hours (Figure 3 ). The elimination half‐life of unconjugated psilocin was 2 hours (Figure 3 , Table 2 ). Peak concentrations of psilocin glucuronide were reached after a mean time of 3.8 and 3.7 hours after administration, and the elimination half‐life was 4.5 and 5.2 hours after placebo and escitalopram, respectively (Figure 3 , Table 2 ). Escitalopram did not significantly alter the pharmacokinetics of psilocin, psilocin glucuronide, or 4‐HIAA (Figure 3 , Table 2 ).
Figure 3.
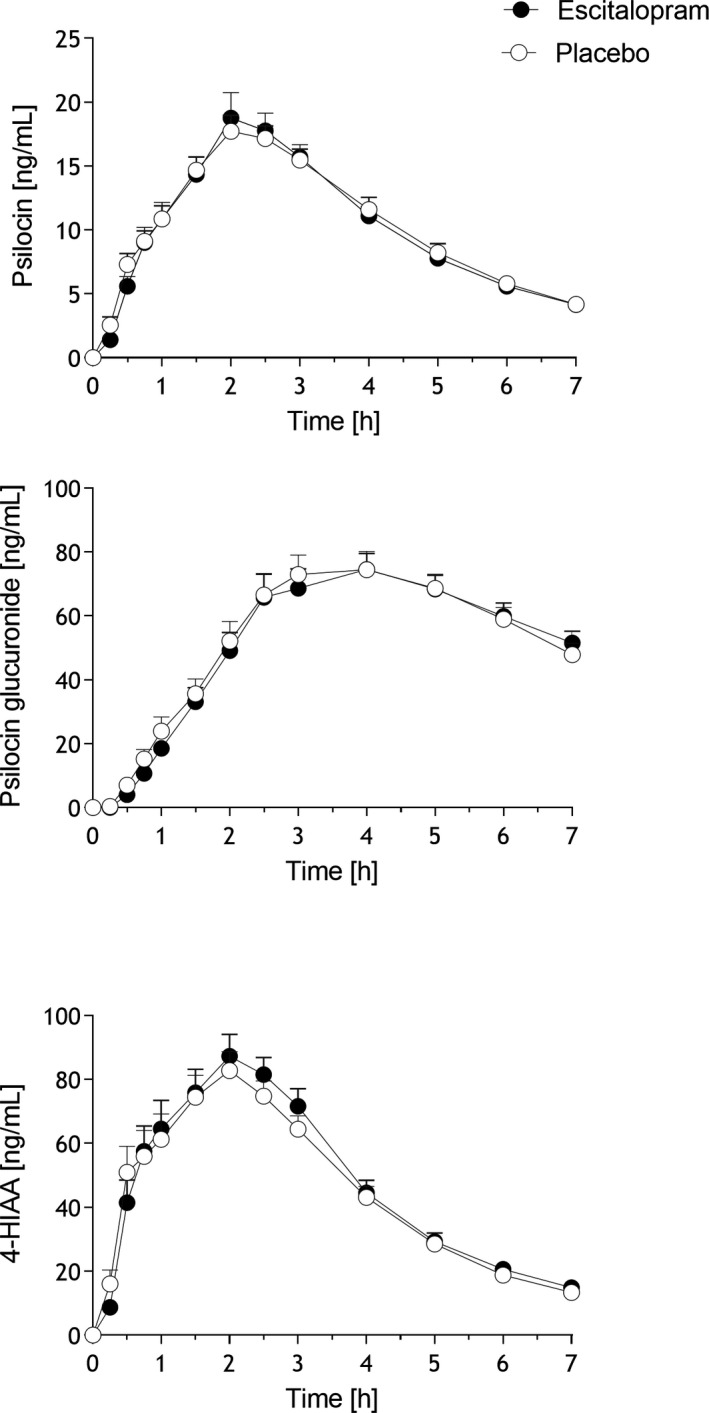
Pharmacokinetics of free psilocin (unconjugated), psilocin glucuronide, and 4‐hydroxyindole‐3‐acetic acid (4‐HIAA). Escitalopram had no effect on the pharmacokinetics of psilocin or its metabolites compared with placebo. The data are expressed as the mean ± SEM in 23 subjects. Psilocybin was administered at t = 0 hour. Pharmacokinetic parameters are listed in Table 2 .
Table 2.
Pharmacokinetic parameters [mean ± SD, range]
| Cmax, ng/mL | T max, hour | t 1/2, hour | AUC7, ng·hour/mL | AUC∞, ng·hour/mL | |
|---|---|---|---|---|---|
| Escitalopram | |||||
| Psilocin unconj. | 22 ± 8.5 | 2 | 2.0 ± 0.5 | 72 ± 18 | 84 ± 21 |
| 12–50 | 1–3 | 1.5–3.7 | 46–110 | 55–127 | |
| Psilocin glucuronide | 82 ± 30 | 4 | 5.7 ± 2.4 | 364 ± 116 | 822 ± 364 |
| 46–183 | 2.5–6 | 2.8–10.7 | 204–727 | 370–1819 | |
| Psilocin total | 97 ± 33 | 3 | 4.8 ± 1.8 | 436 ± 119 | 851 ± 322 |
| 53–207 | 2–5 | 2.7–8.8 | 252–813 | 442–1691 | |
| 4‐HIAA | 106 ± 37 | 2 | 1.7 ± 0.5 | 328 ± 80 | 367 ± 84 |
| 57–199 | 0.75–3 | 1.3–3.6 | 214–458 | 231–523 | |
| Escitalopram | 44 ± 20 | 2.75 | |||
| 25–116 | 1.9–7 | ||||
| Placebo | |||||
| Psilocin unconj. | 20 ± 5.4 | 2 | 1.8 ± 0.3 | 73 ± 17 | 83 ± 21 |
| 11–36 | 1–4 | 1.1–2.2 | 46–102 | 50–118 | |
| Psilocin glucuronide | 82 ± 28 | 4 | 4.7 ± 1.6 | 373 ± 126 | 712 ± 243 |
| 40–165 | 3–7 | 2.5–8.2 | 203–728 | 343–1262 | |
| Psilocin total | 96 ± 28 | 3 | 4.3 ± 1.3 | 446 ± 124 | 798 ± 259 |
| 50–181 | 2–7 | 2.4–6.8 | 280–802 | 414–1382 | |
| 4‐HIAA | 105 ± 30 | 2 | 1.6 ± 0.3 | 317 ± 66 | 347 ± 72 |
| 52–154 | 0.5–4 | 1.0–2.3 | 205–409 | 226–447 | |
Data are mean ± SD except for T max (median and range).
AUC, area under the plasma concentration‐time curve; AUC∞, AUC from time zero to infinity; AUC7, from time 0–7 hours; Cmax, maximum observed plasma concentration; total, after deglucuronidation (unconjugated + glucuronide); unconj., unconjugated; t 1/2, plasma half‐life; Tmax, time to reach Cmax; 4‐HIAA, 4‐hydroxyindole‐3‐acetic acid.
Adverse effects of escitalopram and gene expression
There was one nontreatment‐related severe adverse event in the study. One participant who started escitalopram pretreatment had a symptomatic vertebral disc hernia that required the participant to undergo surgery; hence, the participant dropped out of the study after 5 days of pretreatment and before psilocybin administration. Aside from this one subject, escitalopram pretreatment resulted in several treatment‐related adverse events. Adverse events any time during escitalopram pretreatment were nonsignificantly more frequent compared with placebo and included nausea (8 subjects), headache (6 subjects), tiredness (6 subjects), lower libido (5 subjects), feeling depressed (3 subjects), loss of appetite (3 subjects), diarrhea (2 subjects), restless legs (2 subjects), increased appetite (2 subjects), bruxism (2 subjects), insomnia (2 subjects), anorgasmia (2 subjects), dizziness (2 subjects), difficulty concentrating (one subject), and visual disturbance (one subject). Adverse events during placebo pretreatment included nausea (3 subjects), tiredness (3 subjects), nightmares (3 subjects), headaches (2 subjects), insomnia (2 subjects), diarrhea (one subject), feeling depressed (one subject), bruxism (one subject), and difficulty concentrating (one subject).
Fourteen participants correctly identified the pretreatment sequence, and nine incorrectly identified the sequence during the end‐of‐study visit (Z = 0.83, P = 0.41).
Mean peak plasma escitalopram concentrations were 41 ng/mL (range: 35–53 ng/mL; Table 2 ). Concentrations were high (< 35 ng/mL) in all subjects and remained elevated throughout the time of the psilocybin effect (Figure S6 ).
Escitalopram did not alter the expression of the HRT2A or SLC6A4 gene that was measured at the end of the 2‐week pretreatment period and compared with placebo pretreatment (Table S6 ).
DISCUSSION
We did not confirm our primary hypothesis that treatment with a serotonin transporter inhibitor would attenuate the mind‐altering effects of psilocybin. Daily escitalopram treatment for 2 weeks did not reduce the positive mood or mind‐altering effects of a full dose of psilocybin in healthy subjects. Additionally, escitalopram pretreatment reduced untoward acute effects of psilocybin, including subjective bad drug effects, anxious‐ego dissolution, anxiety, and nadir effects, compared with placebo pretreatment. QTc intervals were similar after escitalopram and placebo administration, with no evidence of an increase in serotonergic toxicity. In fact, escitalopram reduced psilocybin‐induced increases in blood pressure, pupil size, and acute adverse effects. Escitalopram did not alter the pharmacokinetics of psilocin. Higher Oceanic Boundlessness and lower anxiety ratings that were acutely induced by psychedelics were previously shown to predict better treatment efficacy in patients with depression, anxiety, and tobacco dependence. 2 , 4 , 5 , 11
There are no previous studies on the interaction of antidepressants with psilocybin comparable to the present work. The combination is currently avoided in clinical trials in patients. With regard to other psychedelics, chronic administration of the serotonin transporter inhibitors fluoxetine or sertraline mostly reduced LSD effects according to retrospective case reports. 16 , 17 In contrast, single case observations also showed that tricyclic antidepressants, including imipramine, desipramine, and clomipramine, increased physical, hallucinatory, and psychological responses to LSD. 41 Recent treatment with a serotonin transporter inhibitor reduced therapeutic responses to MDMA in a small preliminary study, 42 but this finding was not confirmed in a more conclusive larger trial. 43 Ketamine is marketed as an add‐on to an established treatment with another antidepressant.
Psilocybin itself weakly inhibits the serotonin transporter whereas LSD does not interact with this target. 9 Such pharmacological differences may theoretically result in differential interactions of LSD and psilocybin with antidepressants. Overall, the effects of escitalopram on the acute response to psilocybin in the present study could be considered positive with no evidence of increased toxicity, indicating that stopping escitalopram treatment before psilocybin administration may not be warranted.
However, several aspects of the present study may limit the above conclusion. We only tested escitalopram and the findings may not necessarily apply to other antidepressants. Escitalopram pretreatment lasted only 14 days, which may have been too short to produce more chronic neuroadaptations and changes in receptor expression that can alter the response to psilocybin. Previous studies suggested reductions of serotonin transporter expression, 44 tendency toward increases in HTR2A expression, 45 and decreases in 5‐HT2A receptor binding 46 after antidepressant treatment. We did not see changes in HTR2A or SCL6A4 gene expression (i.e., the genes that encode the 5‐HT2A receptor and serotonin transporter, respectively). We did not test HTR1A gene expression or other markers that could be altered by antidepressant treatment. 47 Escitalopram pretreatment resulted in relatively high plasma escitalopram concentrations in all participants and in the upper range of concentrations that are considered therapeutic in patients. 48 We did not measure levels of active escitalopram metabolites. Adverse events tended to be more frequent after escitalopram pretreatment compared with placebo, although complaints on the List of Complaints did not differ. Furthermore, participants could not correctly identify their pretreatment assignment at the end of the study, indicating adequate treatment blinding. Finally, the present study included healthy subjects and no therapeutic setting. Thus, subjects in different environments and patients with psychiatric disorders with longer treatment with antidepressants and in a more therapeutic setting may respond differently to psilocybin.
The present study has several strengths. The study used a powerful within‐subject design and made comparisons with placebo under double‐blind conditions in a controlled laboratory setting. We also included similar numbers of male and female participants and used internationally established standardized and validated psychometric outcome measures. The dose of psilocybin was pharmaceutically well‐characterized, and plasma psilocin and psilocin metabolite concentrations and pharmacokinetic parameters were determined. Although the present findings cannot necessarily be generalized to the therapeutic effects in patients, the results indicate no relevant acute drug‐drug interaction and no increase in adverse effects or serotonin toxicity when escitalopram is used in combination with psilocybin compared with psilocybin alone.
Psilocybin slightly elevated plasma BDNF levels, although there was no placebo control for this effect. Other psychedelics, including LSD 7 , 14 and dimethyltryptamine, 49 have similarly been reported to increase BDNF levels. BDNF could serve as a marker of potential neuroplastic and therapeutic effects of psychedelics. 49 , 50 Escitalopram had no significant effect on plasma BDNF concentrations after psilocybin administration.
Psilocybin did not significantly alter the QTc time at 2.5 hours compared with baseline, consistent with the absence of effects on QTc time at a 25 mg dose based on modeled data from a previous multidose level study. 51 However, relevant prolongation may occur in susceptible persons during the peak effects of psilocybin as also indicated by the longest QTc times of 509 ms after escitalopram and psilocybin and 481 ms after psilocybin alone among the present study participants.
The present study characterized the pharmacokinetics of psilocybin at a fixed dose of 25 mg psilocybin that is typically used in academic and commercial clinical trials, 1 , 18 and we included a validated analytical method to determine analyte concentrations of the relevant psychoactive free unconjugated psilocin. 8 Mean maximal plasma concentrations of unconjugated psilocin of 20 ng/mL were reached on average 2 hours after drug administration, consistent with modeled estimates for a 25 mg dose from previous studies. 26 , 51 The terminal half‐life of unconjugated psilocin was 1.8 hours (range: 1.1–2.2 hours), consistent with the short duration of action of psilocybin. This half‐life is shorter than the 3–5 hours that was previously reported. 26 , 27 , 51 However, previous studies were smaller, and unclear as to whether unconjugated or total concentrations of psilocin were measured. The effect‐time curve of acute subjective effects reflected the unconjugated psilocin plasma concentration‐time curve, consistent with the view that unconjugated psilocin is the psychoactive and pharmacologically active analyte that best reflects effects after psilocybin administration. More research is needed to determine whether unconjugated psilocin may be useful for therapeutic drug monitoring or as a marker of the response to psilocybin in addition to the acute subjective effect profile. 2 The total psilocin concentration is determined after deglucuronidation and mainly represents psilocin glucuronide. 28 The total psilocybin concentration and related pharmacokinetic parameters 26 poorly reflect the acute effects profile of psilocybin. Psilocybin glucuronide is the analyte in blood plasma best suited to document recent exposure to psilocybin for toxicological and forensic purposes 28 because of the longer presence of the psilocybin glucuronide in the body. Previous studies of the pharmacokinetics of psilocybin were small 8 , 27 and measured not clearly defined psilocin concentrations 26 , 27 or unconjugated psilocin concentrations in recreational users only. 28 Previous pharmacokinetic studies used weight‐adjusted doses (0.2, 0.3, 0.45, and 0.6 mg/kg). 26 , 27 The weight‐adjusted dosing of psilocybin has also often been used in previous clinical studies. 4 , 5 , 52 However, subjective effects have not been associated with body weight or sex, and fixed doses have thus been recommended 29 and are increasingly used. 1 , 3
CONCLUSION
In conclusion, escitalopram pretreatment for 2 weeks had no relevant effect on the acute positive mood effects of psilocybin but significantly reduced bad drug effects, anxiety, adverse autonomic effects, and other adverse effects of psilocybin compared with placebo pretreatment. Escitalopram and psilocybin can be safely administered together. However, further studies are needed with a longer antidepressant pretreatment time and in patients to clarify the interactive effects on therapeutic outcomes and whether antidepressant treatment should be maintained or stopped before psilocybin administration.
FUNDING
This work was supported by the University Hospital Basel and Mind Medicine, Inc. Knowhow and data associated with this work and owned by the University Hospital Basel were licensed by Mind Medicine, Inc. Mind Medicine, Inc., had no role in planning or conducting the present study or the present publication.
CONFLICT OF INTEREST
M.E.L. is a consultant for Mind Medicine, Inc. All other authors declare no competing interests for this work.
AUTHOR CONTRIBUTIONS
A.M.B. and M.E.L. wrote the manuscript. M.E.L. designed the research. A.M.B., K.E.K., F.H., T.G., A.K., V.E.T., U.D., N.V., A.E., and E.G. performed the research. A.M.B., K.E.K., F.H., U.D., and M.E.L. analyzed the data.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Tim Bühler for helping with study planning. The authors thank Michael Arends for proofreading the manuscript. Open Access Funding provided by Universitat Basel.
Trial registry: ClinicalTrials.gov (NCT03912974).
- 1. Carhart‐Harris, R. et al. Trial of psilocybin versus escitalopram for depression. N. Engl. J. Med. 384, 1402–1411 (2021). [DOI] [PubMed] [Google Scholar]
- 2. Roseman, L. , Nutt, D.J. & Carhart‐Harris, R.L. Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment‐resistant depression. Front. Pharmacol. 8, 974 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carhart‐Harris, R.L. et al. Psilocybin with psychological support for treatment‐resistant depression: an open‐label feasibility study. Lancet Psychiatry 3, 619–627 (2016). [DOI] [PubMed] [Google Scholar]
- 4. Griffiths, R.R. et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life‐threatening cancer: a randomized double‐blind trial. J. Psychopharmacol. 30, 1181–1197 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ross, S. et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life‐threatening cancer: a randomized controlled trial. J. Psychopharmacol. 30, 1165–1180 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis, A.K. et al. Effects of psilocybin‐assisted therapy on major depressive disorder: a randomized clinical trial. JAMA Psychiatry 78, 481–489 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holze, F. et al. Acute dose‐dependent effects of lysergic acid diethylamide in a double‐blind placebo‐controlled study in healthy subjects. Neuropsychopharmacology 46, 537–544 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kolaczynska, K.E. , Liechti, M.E. & Duthaler, U. Development and validation of an LC‐MS/MS method for the bioanalysis of psilocybin's main metabolites, psilocin and 4‐hydroxyindole‐3‐acetic acid, in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1164, 122486 (2021). [DOI] [PubMed] [Google Scholar]
- 9. Rickli, A. , Moning, O.D. , Hoener, M.C. & Liechti, M.E. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur. Neuropsychopharmacol. 26, 1327–1337 (2016). [DOI] [PubMed] [Google Scholar]
- 10. Madsen, M.K. et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology 44, 1328‐1334 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia‐Romeu, A. , Griffiths, R.R. & Johnson, M.W. Psilocybin‐occasioned mystical experiences in the treatment of tobacco addiction. Curr. Drug Abuse Rev. 7, 157–164 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ly, C. et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 23, 3170–3182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dong, C. et al. Psychedelic‐inspired drug discovery using an engineered biosensor. Cell 184, 2779–2792 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hutten, N. et al. Low doses of LSD acutely increases BDNF blood plasma levels in healthy volunteers. ACS Pharmacol. Transl. Sci. 4, 461–466 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malcolm, B. & Thomas, K. Serotonin toxicity of serotonergic psychedelics. Psychopharmacology. 10.1007/s00213-021-05876-x. [e‐pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16. Strassman, R.J. Human hallucinogen interactions with drugs affecting serotonergic neurotransmission. Neuropsychopharmacology 7, 241–243 (1992). [PubMed] [Google Scholar]
- 17. Bonson, K.R. , Buckholtz, J.W. & Murphy, D.L. Chronic administration of serotonergic antidepressants attenuates the subjective effects of LSD in humans. Neuropsychopharmacology 14, 425–436 (1996). [DOI] [PubMed] [Google Scholar]
- 18. Carhart‐Harris, R.L. et al. Psilocybin with psychological support for treatment‐resistant depression: six‐month follow‐up. Psychopharmacology 235, 399–408 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmid, Y. , Gasser, P. , Oehen, P. & Liechti, M.E. Acute subjective effects in LSD‐ and MDMA‐assisted psychotherapy. J. Psychopharmacol. 35, 362–374 (2021). [DOI] [PubMed] [Google Scholar]
- 20. Gasser, P. et al. Safety and efficacy of lysergic acid diethylamide‐assisted psychotherapy for anxiety associated with life‐threatening diseases. J. Nerv. Ment. Dis. 202, 513–520 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tamam, L. & Ozpoyraz, N. Selective serotonin reuptake inhibitor discontinuation syndrome: a review. Adv. Ther. 19, 17–26 (2002). [DOI] [PubMed] [Google Scholar]
- 22. Studerus, E. , Gamma, A. , Kometer, M. & Vollenweider, F.X. Prediction of psilocybin response in healthy volunteers. PLoS One 7, e30800 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haijen, E. et al. Predicting responses to psychedelics: a prospective study. Front. Pharmacol. 9, 897 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liechti, M.E. , Dolder, P.C. & Schmid, Y. Alterations in conciousness and mystical‐type experiences after acute LSD in humans. Psychopharmacology 234, 1499–1510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Studerus, E. , Kometer, M. , Hasler, F. & Vollenweider, F.X. Acute, subacute and long‐term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. J. Psychopharmacology 25, 1434–1452 (2011). [DOI] [PubMed] [Google Scholar]
- 26. Brown, R.T. et al. Pharmacokinetics of escalating doses of oral psilocybin in healthy adults. Clin. Pharmacokinet. 56, 1543–1554 (2017). [DOI] [PubMed] [Google Scholar]
- 27. Hasler, F. , Bourquin, D. , Brenneisen, R. , Bär, T. & Vollenweider, F.X. Determination of psilocin and 4‐hydroxyindole‐3‐acetic acid in plasma by HPLC‐ECD and pharmacokinetic profiles of oral and intravenous psilocybin in man. Pharm. Acta Helv. 72, 175–184 (1997). [DOI] [PubMed] [Google Scholar]
- 28. Kamata, T. , Nishikawa, M. , Katagi, M. & Tsuchihashi, H. Direct detection of serum psilocin glucuronide by LC/MS and LC/MS/MS: time‐courses of total and free (unconjugated) psilocin concentrations in serum specimens of a “magic mushroom” user. Forens. Toxicol. 24, 36–40 (2006). [Google Scholar]
- 29. Garcia‐Romeu, A. , Barrett, F.S. , Carbonaro, T.M. , Johnson, M.W. & Griffiths, R.R. Optimal dosing for psilocybin pharmacotherapy: considering weight‐adjusted and fixed dosing approaches. J. Psychopharmacol. 35, 353–361 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Studerus, E. , Gamma, A. & Vollenweider, F.X. Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS One 5, e12412 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Griffiths, R.R. , Richards, W.A. , McCann, U. & Jesse, R. Psilocybin can occasion mystical‐type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology 187, 268–283; discussion 284–292 (2006). [DOI] [PubMed] [Google Scholar]
- 32. Barrett, F.S. , Johnson, M.W. & Griffiths, R.R. Validation of the revised Mystical Experience Questionnaire in experimental sessions with psilocybin. J. Psychopharmacol. 29, 1182–1190 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holze, F. et al. Distinct acute effects of LSD, MDMA, and D‐amphetamine in healthy subjects. Neuropsychopharmacology 45, 462–471 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmid, Y. et al. Acute effects of lysergic acid diethylamide in healthy subjects. Biol. Psychiatry 78, 544–553 (2015). [DOI] [PubMed] [Google Scholar]
- 35. Janke, W. & Debus, G. Die Eigenschaftswörterliste. Hogrefe, Göttingen. (1978). [Google Scholar]
- 36. Hysek, C.M. , Vollenweider, F.X. & Liechti, M.E. Effects of a b‐blocker on the cardiovascular response to MDMA (ecstasy). Emerg. Med. J. 27, 586–589 (2010). [DOI] [PubMed] [Google Scholar]
- 37. Zerssen, D.V. Die Beschwerden‐Liste. Münchener Informationssystem (Psychis, München, 1976). [Google Scholar]
- 38. Akimoto, H. , Oshima, S. , Sugiyama, T. , Negishi, A. , Nemoto, T. & Kobayashi, D. Changes in brain metabolites related to stress resilience: metabolomic analysis of the hippocampus in a rat model of depression. Behav. Brain Res. 359, 342–352 (2019). [DOI] [PubMed] [Google Scholar]
- 39. Grünblatt, E. et al. Gene expression as peripheral biomarkers for sporadic Alzheimer's disease. J. Alzheimers Dis. 16, 627–634 (2009). [DOI] [PubMed] [Google Scholar]
- 40. Holze, F. , Duthaler, U. , Vizeli, P. , Müller, F. , Borgwardt, S. & Liechti, M.E. Pharmacokinetics and subjective effects of a novel oral LSD formulation in healthy subjects. Br. J. Clin. Pharmacol. 85, 1474–1483 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bonson, K.R. & Murphy, D.L. Alterations in response to LSD in humans associated with chronic administration of tricyclic antidepressants, monoamine oxidase inhibitors or lithium. Behav. Brain Res. 73, 229–233 (1996). [DOI] [PubMed] [Google Scholar]
- 42. Feduccia, A.A. , Jerome, L. , Mithoefer, M.C. & Holland, J. Discontinuation of medications classified as reuptake inhibitors affects treatment response of MDMA‐assisted psychotherapy. Psychopharmacology 238(2), 581–588 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43. Mitchell, J.M. et al. MDMA‐assisted therapy for severe PTSD: a randomized, double‐blind, placebo‐controlled phase 3 study. Nat. Med. 27, 1025–1033 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iga, J. et al. Altered HDAC5 and CREB mRNA expressions in the peripheral leukocytes of major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 31, 628–632 (2007). [DOI] [PubMed] [Google Scholar]
- 45. Belzeaux, R. et al. Clinical variations modulate patterns of gene expression and define blood biomarkers in major depression. J. Psychiatr. Res. 44, 1205–1213 (2010). [DOI] [PubMed] [Google Scholar]
- 46. Meyer, J.H. et al. The effect of paroxetine on 5‐HT(2A) receptors in depression: an [(18)F]setoperone PET imaging study. Am. J. Psychiatry 158, 78–85 (2001). [DOI] [PubMed] [Google Scholar]
- 47. Fabbri, C. et al. Consensus paper of the WFSBP Task Force on Genetics: genetics, epigenetics and gene expression markers of major depressive disorder and antidepressant response. World J. Biol. Psychiatry 18, 5–28 (2017). [DOI] [PubMed] [Google Scholar]
- 48. Florio, V. , Porcelli, S. , Saria, A. , Serretti, A. & Conca, A. Escitalopram plasma levels and antidepressant response. Eur. Neuropsychopharmacol. 27, 940–944 (2017). [DOI] [PubMed] [Google Scholar]
- 49. de Almeida, R.N. et al. Modulation of serum brain‐derived neurotrophic factor by a single dose of ayahuasca: observation from a randomized controlled trial. Front. Psychol. 10, 1234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang, T. et al. The role of BDNF on neural plasticity in depression. Front. Cell. Neurosci. 14, 82 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dahmane, E. , Hutson, P.R. & Gobburu, J.V.S. Exposure‐response analysis to assess the concentration‐QTc relationship of psilocybin/psilocin. Clin. Pharmacol. Drug Dev. 10, 78–85 (2021). [DOI] [PubMed] [Google Scholar]
- 52. Griffiths, R.R. , Johnson, M.W. , Richards, W.A. , Richards, B.D. , McCann, U. & Jesse, R. Psilocybin occasioned mystical‐type experiences: immediate and persisting dose‐related effects. Psychopharmacology 218, 649–665 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


