Abstract
Human activities have drastically increased nitrogen (N) deposition onto forests globally. This may have alleviated N limitation and thus stimulated productivity and carbon (C) sequestration in aboveground woody biomass (AGWB), a stable C pool with long turnover times. This ‘carbon bonus’ of human N use partly offsets the climate impact of human‐induced N2O emissions, but its magnitude and spatial variation are uncertain. Here we used a meta‐regression approach to identify sources of heterogeneity in tree biomass C‐N response (additional C stored per unit of N) based on data from fertilization experiments in global forests. We identified important drivers of spatial variation in forest biomass C‐N response related to climate (potential evapotranspiration), soil fertility (N content) and tree characteristics (stand age), and used these relationships to quantify global spatial variation in N‐induced forest biomass C sequestration. Results show that N deposition enhances biomass C sequestration in only one‐third of global forests, mainly in the boreal region, while N reduces C sequestration in 5% of forests, mainly in the tropics. In the remaining 59% of global forests, N addition has no impact on biomass C sequestration. Average C‐N responses were 11 (4–21) kg C per kg N for boreal forests, 4 (0–8) kg C per kg N for temperate forests and 0 (−4 to 5) kg C per kg N for tropical forests. Our global estimate of the N‐induced forest biomass C sink of 41 (−53 to 159) Tg C yr−1 is substantially lower than previous estimates, mainly due to the absence of any response in most tropical forests (accounting for 58% of the global forest area). Overall, the N‐induced C sink in AGWB only offsets ~5% of the climate impact of N2O emissions (in terms of 100‐year global warming potential), and contributes ~1% to the gross forest C sink.
Keywords: aboveground woody biomass, climate footprint, C‐N response, forest carbon sink, global warming potential, meta‐regression, N2O emissions, nitrogen deposition, spatial variation
Nitrogen deposition onto forests may enhance carbon sequestration in aboveground woody biomass (AGWB), a stable carbon pool with long turnover times. Using a meta‐regression approach, we estimated that nitrogen deposition enhances the AGWB carbon sink by 41 Tg C yr−1, explaining only 1% of the gross forest C sink. More importantly, we illustrate spatial variation in both nitrogen‐induced carbon sequestration and the balance between this ‘cooling effect’ and the warming effect of N2O emissions. Results show that nitrogen deposition enhances carbon sequestration in some, but not all forests, and that the warming effect of N2O is dominant almost everywhere.
1. INTRODUCTION
Human acceleration and disturbance of the nitrogen (N) cycle have drastically increased emissions of reactive N to the environment, resulting in adverse impacts on water, air and soil quality, ecosystems and biodiversity (Fowler et al., 2013; Galloway et al., 2008; Sutton et al., 2011; Vitousek et al., 1997). Reactive N emissions also alter radiative forcing by affecting emissions or removal of several greenhouse gases (N2O, CH4, CO2 and O3) as well as formation of aerosols (Butterbach‐Bahl et al., 2011; De Vries et al., 2011, 2017; Erisman et al., 2011). The two strongest influences of reactive N on radiative forcing are (i) warming caused by N‐induced nitrous oxide (N2O) emissions (Reay et al., 2012; Tian et al., 2020; UNEP, 2013) and (ii) cooling caused by N‐induced increases in carbon (C) sequestration in terrestrial and marine ecosystems, which occurs when N deposition stimulates productivity under conditions of N‐limitation (De Vries et al., 2011, 2017; LeBauer & Treseder, 2008; Vitousek & Howarth, 1991; Xia & Wan, 2008). This ‘carbon bonus’ can be seen as an unintended benefit of human disturbance of the N cycle (Janssens & Luyssaert, 2009) and should be considered when balancing both the threats and the benefits of N for sustainable development (Zhang et al., 2015).
The largest N‐induced C sink occurs in forests (De Vries et al., 2017; Liu & Greaver, 2009). Despite rapid deforestation and adverse effects of multiple global change drivers on forest growth, forests represented a net C sink of 2100 Tg C per year over the period 2001–2019 (Harris et al., 2021), absorbing about 18% of global fossil fuel CO2 emissions (Friedlingstein et al., 2020) and thereby slowing the increase in atmospheric CO2 concentrations (O’Sullivan et al., 2019). Regional N deposition has increased by a factor 1.5–7 since pre‐industrial times, particularly in Europe and Asia (Wang et al., 2017). Globally, about 20–30 Tg N emitted by human activities end up in forests after atmospheric transport and re‐deposition each year (Lamarque et al., 2013; Schwede et al., 2018). Many forests in Western Europe and Southeast Asia currently receive more N via atmospheric deposition (Wang et al., 2017) than the average arable field in Sub‐Saharan Africa receives via fertilizer, manure and biological N fixation combined (Liu et al., 2010). Although the amount of N supplied via deposition is small compared to the rate of internal N cycling (Cleveland et al., 2013; Du & De Vries, 2018; Högberg, 2012), it can increase forest C sequestration if this N is retained and allocated to stable C pools with long turnover times. Nitrogen deposition can increase forest C sequestration by increasing net primary productivity (NPP, C assimilation through photosynthesis), by increasing the share of C allocated to wood (with higher C:N ratios than other compartments), or by increasing litter inputs and/or reducing soil respiration and thereby increasing C storage in soils (Janssens et al., 2010; Janssens & Luyssaert, 2009). The size of the global N‐induced C sink is determined by total N deposition onto forests and their C sequestration efficiency, that is, the amount of C sequestered per unit of N deposition (‘C‐N response’ from hereon).
The magnitude and spatial variation of the N‐induced forest C sink and its contribution to ‘offsetting’ global N‐induced N2O emissions is highly uncertain. At continental or global scales, nitrogen‐induced C sequestration has been estimated with different approaches including (i) dynamic global vegetation models (DGVMs) (Fleischer et al., 2015, 2019; Jain et al., 2009; Lu & Tian, 2013; O’Sullivan et al., 2019; Zaehle et al., 2011), (ii) stoichiometric scaling (Du & De Vries, 2018; Nadelhoffer et al., 1999; Wang et al., 2017; Zhu et al., 2017), (iii) observational studies along deposition gradients (Flechard et al., 2020; Magnani et al., 2007; Solberg et al., 2009; Thomas et al., 2010) and (iv) meta‐analyses of data from forest fertilization experiments (Liu & Greaver, 2010; Schulte‐Uebbing & De Vries, 2018; Vadeboncoeur, 2010). While early studies estimated the upper limit of global N‐induced C sequestration at 2500–3000 Tg C yr−1 (Field et al., 1992; Holland et al., 1997; Hudson et al., 1994; Schindler & Bayley, 1993), more recent estimates have been constrained to a relatively narrow range of 250–560 Tg C yr−1 for both forest biomass and soils and 130–345 Tg C yr−1 for forest biomass only (estimated from De Vries et al., 2014; Du & De Vries, 2018; Fleischer et al., 2015, 2019; Jain et al., 2009; Liu & Greaver, 2009; Nadelhoffer et al., 1999; Schulte‐Uebbing & De Vries, 2018; Thomas et al., 2010; Thornton et al., 2007; Wang et al., 2017; Zaehle et al., 2011). However, the representation of site factors affecting variation in C‐N response in approaches to estimate the global N‐induced C sink is currently limited. Most empirical studies simply multiply a global average C‐N response with total global N deposition to forests (e.g. Nadelhoffer et al., 1999; Thomas et al., 2010). Some studies distinguish average C‐N responses for major biomes (e.g. Du & De Vries, 2018; Schulte‐Uebbing & De Vries, 2018); however, forest fertilization experiments show that C‐N responses vary strongly even within biomes. For example, reported responses to N addition in temperate forests range from a strong stimulation (e.g. Gentilesca et al., 2013) to no response (e.g. Finzi, 2009) or even a reduction (e.g. Lovett et al., 2013) of the forest biomass C sink. For tropical forests, most experiments find no biomass response to N addition (e.g. Fisher et al., 2013), though some find that N stimulates biomass production, especially in combination with P addition (Siddique et al., 2010). Most experiments in boreal forests find that N addition stimulates the forest biomass C sink (e.g. Gundale et al., 2014), though the strength of the response varies across sites (e.g. Helmisaari et al., 2011). Site factors potentially affecting variation in C‐N response include biome, tree and stand characteristics, nutrient and water availability, and N saturation, as further discussed below.
First, the forest C sink response to N addition decreases from boreal to tropical regions, linked to an increase in N availability with decreasing latitude. In boreal regions, low temperatures constrain N mineralization, whereas tropical forests are characterized by high N inputs from both mineralization and biological N2 fixation (BNF) as well as high N losses through leaching and denitrification (Bai et al., 2012; Brookshire et al., 2012; Cleveland et al., 1999; Vitousek et al., 2013). Both symbiotic and asymbiotic BNF strongly decline with latitude (Menge et al., 2014; Wang & Houlton, 2009), and average rates of BNF across tropical forests are a factor 2–20 higher than in temperate and boreal forests (Cleveland et al., 1999; Du & De Vries, 2018; Vitousek et al., 2013). Due to the high availability of N, NPP in tropical forests is often not constrained by N availability (Cusack et al., 2011; Lu et al., 2021).
Second, findings from forest fertilization experiments and growth inventories reveal that C‐N response is affected by stand characteristics and tree species (Allen et al., 2010; Lovett et al., 2013; Solberg et al., 2009), potentially moderated by preferential associations with either arbuscular (AM) or ectomycorrhizal (EM) fungi (Thomas et al., 2010). While both fungi provide trees with N in exchange for C from root exudates, AM fungi obtain this N mainly from inorganic N, while EM fungi can access N from soil organic matter (Bradford, 2014). Hence, trees associated with AM fungi may benefit more from N addition (Averill et al., 2018; Thomas et al., 2010). Association with EM fungi might also increase soil C storage by limiting available N to free‐living decomposers (Averill et al., 2014; Gadgil & Gadgil, 1971). Young stands have been found to respond more strongly to N addition than old stands (Schulte‐Uebbing & De Vries, 2018; Vadeboncoeur, 2010), possibly because N increases NPP through an increase in canopy leaf area index, which has less benefits in older forests where the canopy is already closed (De Vries & Posch, 2011; McCarthy et al., 2006). In addition, younger forests have a higher nutrient demand and their growth is less limited by light availability (Sun et al., 2016), and soil pH declines as forest stands mature even under natural conditions (Binkley & Högberg, 2016; Prietzel et al., 2020), which reduces availability of micro‐nutrients and thus constrains response to increased N availability.
Third, according to the Liebig's law of the minimum (Liebig, 1840), N only stimulates forest growth if growth is not limited by the availability of other nutrients or water. Phosphorus (P) limitation in tropical forests might constrain the C sink response to N addition (Cusack et al., 2011; Tanner et al., 1998), a hypothesis supported by observed increases in forest biomass production after P addition (Jiang et al., 2020; Li et al., 2016). The absence of a growth response to N in forest fertilization experiments has often been explained by (micro)nutrient deficiency such as calcium (Ca), as evidenced by low foliar Ca:N ratios (Baribault et al., 2010; Lovett et al., 2013; Mainwaring et al., 2014). Forests on fertile soils also display a higher ratio of net ecosystem productivity (NEP) to gross primary productivity (GPP) (Fernández‐Martínez et al., 2014) and allocate more C to wood production (Vicca et al., 2012). This suggests that these forests are more efficient in sequestering C assimilated through photosynthesis compared to forests on less fertile soils, ultimately resulting in larger C‐N responses (Janssens & Luyssaert, 2009). Finally, NPP response to N has been shown to increase with precipitation rates in arid and semi‐arid regions (Yahdjian et al., 2011), showing that water availability may also constrain growth response to N addition.
Lastly, at sustained high levels of N addition, N leaching from forest soils increases and N retention decreases (Aber et al., 1989; Forstner et al., 2019; Templer et al., 2012), evidenced by a decreasing response to N with increasing levels of N application in fertilization experiments (Högberg et al., 2006; Liu et al., 2021; Schulte‐Uebbing & De Vries, 2018; Tian et al., 2016). Forest C‐N response may thus be smaller at sites with high current or historical (cumulative) N deposition rates. Threshold for N saturation is likely to vary across biomes, with higher thresholds in (sub)tropical forests than in boreal and temperate regions (Yu et al., 2018).
A better understanding of site factors determining variation in C‐N response is key to improving estimates of the global N‐induced forest C sink and its spatial variation under current and future reactive N deposition. The main aim of this study is to derive spatially explicit estimates of C‐N responses and total N‐induced C sequestration in forest aboveground woody biomass (AGWB). This study focussed on N‐induced C sequestration in AGWB only, neglecting N‐induced C sequestration in belowground woody biomass (BGWB) and soil, because very few long‐term fertilization experiments have measured changes in these forest C pools simultaneously. We collected data on environmental variables and C‐N responses from forest fertilization experiments around the globe, which can isolate the effect of N addition by comparing growth responses in fertilized and unfertilized plots. We then used multiple regression to disentangle the drivers of variation in C‐N response and to quantify spatial variation in C‐N response and N‐induced forest C sequestration. Finally, we quantified spatial variation in the impact of human N use on net greenhouse gas emissions by comparing N‐induced C sequestration in AGWB to anthropogenic N2O emissions in terms of their 100‐year Global Warming Potential (GWP).
2. METHODS
Our analysis consists of four steps, which are described in more detail in the following sections:
Select the model that best explains spatial variation in forest biomass C‐N response (meta‐regression);
Use the regression model from step (1) to predict global spatial variation in forest biomass C‐N response at 0.5° × 0.5° resolution;
Estimate global spatial variation in the N‐induced forest biomass C sink by multiplying C‐N responses (kg C kg N−1) predicted under (2) with N deposition rates (kg N ha−1 yr−1) and forest area (ha);
Compare the spatial variation in 100‐year GWP of the global N‐induced forest biomass C sink estimated under (3) to that of anthropogenic N2O emissions (both expressed in CO2‐equivalents).
2.1. Meta‐regression
2.1.1. Estimation of effect sizes and variances
We used meta‐regression based on data from forest N fertilization experiments to test hypotheses on drivers affecting C‐N response and to predict global variation in C‐N response. Data on forest AGWB production in response to N fertilization were collected from original studies. The steps for the literature search, criteria for the inclusion of experiments, data extraction, calculation of effect sizes and their variances, and characteristics of the study plots have been described in detail in Schulte‐Uebbing and De Vries (2018). In summary, the effect size (C‐N response) for each experiment was calculated by subtracting total AGWB increment (expressed in kg C) in unfertilized plots from total AGWB increment in fertilized plots, and dividing this by the total amount of N added during the experiment. Both mean responses of fertilized and unfertilized plots and their variances were recorded, and the variance of the effect size was calculated as the sum of the squared standard errors of the mean response in the fertilized and unfertilized plot (Borenstein et al., 2009; Hedges et al., 1999).
For the analyses in this paper, we slightly modified the original dataset described in Schulte‐Uebbing and De Vries (2018). C‐N responses were log‐transformed to better fit a normal distribution. In addition, five observations with unusually high C‐N responses (between 60 and 160 kg C per kg N) from a single study in Scotland (Gentilesca et al., 2013) were adapted. Due to high leverage of these observations, we constrained these to a maximum of 60 kg C per kg N, in line with previous studies that indicate that C‐N responses mostly range between 5 and 35 kg C per kg N with a maximum near 50 (30–70) kg C per kg N (De Vries et al., 2009, 2014; Sutton et al., 2008).
2.1.2. Deriving data on site factors
We hypothesized that C‐N response increases with water availability (precipitation and precipitation excess) and with soil fertility (cation exchange capacity, clay content, organic C, N content, pH), and decreases with tree age, N saturation ([cumulative] N deposition or N addition rate, soil C:N ratio), and from boreal to tropical regions (temperature, potential evapotranspiration, latitude), and is higher for AM‐associated tree species than for EM‐associated species. Data on site factors were obtained from the original publications (for tree age, tree species, N addition rate and latitude) or derived from global datasets (for climate, soil parameters and N deposition; see Table 1).
TABLE 1.
Global datasets used to derive data on site factors for experimental plots used in the meta‐regression and for estimating global spatial variation in C‐N responses
| Variable (abbreviation) | Data source and explanation | Spatial resolution | Reference |
|---|---|---|---|
| Mean annual temperature (MAT) | WorldClim 2.1 (1970–2000) | 0.16° × 0.16° | (Fick & Hijmans, 2017) |
| Precipitation (PREC) | Idem | Idem | Idem |
| Potential evapotranspiration (PET) | CRU TS 4.04 (2011–2019) | 0.5° × 0.5° | (Harris et al., 2020) |
| Precipitation excess (PE) | Calculated as precipitation (PREC) minus actual evapotranspiration (AET) | 0.5° × 0.5° | Own calculations |
| Soil N content (Soil N) | ISRIC soil grids (weighted average 0–30 cm) | 250 m × 250 m | (Hengl et al., 2017) |
| Soil clay content (CLAY) | Idem | Idem | Idem |
| Soil organic C content (OC) | Idem | Idem | Idem |
| Soil pH (pH) | Idem | Idem | Idem |
| Ambient N deposition (DEP) | Forest‐specific N deposition rates based on the EMEP model for the year 2010 | 1° × 1° | (Schwede et al., 2018) |
| Cumulative N deposition (DEPcum) | N deposition data for 1900, 1950 and 2000, and projections for 2050 based on SSP scenarios; interpolation based on decadal emission estimates from Galloway et al. (2004) | 1° × 1° | (Galloway et al., 2004; Rao et al., 2017) |
| Tree age (age) | For regression: extracted from original studies | ||
| Tree age | For global upscaling: Global Tree age database (GFAD V1.1); tree age was calculated as weighted mean based on forest cover fraction per plant functional type and age class | 0.5° × 0.5° | (Poulter et al., 2019) |
Climate data were obtained from WorldClim 2.1 (Fick & Hijmans, 2017) and CRU TS4.04 (Harris et al., 2020). As an indicator for water availability, precipitation excess was calculated as the difference between potential evapotranspiration (PET) and actual evapotranspiration (AET), which was calculated from precipitation and PET following Zhang et al. (2001). Soil properties (soil N content, organic C content, cation exchange capacity, clay content and soil pH) were retrieved at depth intervals of 0–5 cm, 5–15 cm and 15–30 cm from ISRIC soil grids (Hengl et al., 2017). The weighted mean for the 0–30 cm soil layer was calculated given the relevance for trees’ growing conditions. Soil C:N ratio was derived by dividing soil organic C by soil N. Ambient N deposition rates to forests were obtained from Schwede et al. (2018) who estimated forest‐specific N deposition rates for the year 2010 based on results from the EMEP model (Simpson et al., 2012). Cumulative N deposition was estimated for each experimental location by summing annual deposition values for the 50 years prior to start of the fertilization experiment. Annual N deposition for the years 1950–2010 was estimated based on data for the years 1900–2050 from Rao et al. (2017). As the temporal resolution of this dataset is coarse, we interpolated between available years based on decadal deposition trends from Galloway et al. (2004), and finally derived annual values by linear interpolation. In addition, information on tree species was extracted from the original studies, and all species were classified according to preferential association with either AM or EM fungi based on Brundrett (2009). However, as many study plots contained both trees with AM and EM associations (or with unclear associations), plots could not be unambiguously classified as either ‘AM’ or ‘EM’, and therefore mycorrhizal association was not included in the analysis. A complete overview of tested predictors is shown in Table S2.
2.1.3. Model development
To explore the drivers of forest biomass C‐N response, we built linear mixed‐effects regression models based on hypotheses regarding factors driving C‐N response (Viechtbauer et al., 2015) as follows:
| (1) |
where xi denotes the value of the jth explanatory variable in the ith study, βj represents the corresponding model coefficient indicating how the size of the effect changes as xi increases by one unit, β0 stands for the model intercept, ui denotes a random effect and εi the within‐study error.
Meta‐analytical regression models assume that observed effects among studies are independent. However, in practice dependencies exist, for example when a study reports results from multiple treatments (which are compared to the same control plot) or when a study reports observations from several locations (Gleser & Olkin, 2009). We accounted for this non‐independence using multivariate meta‐modelling with restricted maximum‐likelihood estimation, as implemented in metafor (Viechtbauer, 2010, 2017), and used “Paper ID” to specify the random‐effects structure of the model.
We first tested our hypotheses by regressing individual predictors and combinations of predictors against the log‐transformed C‐N response (kg C kg N−1). We investigated co‐linearity among explanatory variables to ensure independence. Most explanatory variables followed a log‐normal distribution and were thus log‐transformed. McFadden's pseudo‐R2 values and Akaike's Information Criterion (AIC) were used to compare regression models, where the best model is characterized by high R2 and low AIC values.
2.2. Spatial variation in nitrogen‐induced forest carbon sink and climate footprint of human nitrogen use
2.2.1. Spatial variation in nitrogen‐induced forest carbon sink
Global spatial variation in forest C‐N response was predicted from variation in site factors based on the regression model (Section 2.1.3). Global datasets for site factors best explaining variation in C‐N response (PET, soil N and tree age) were re‐projected to a common resolution of 0.5° × 0.5°. A global tree age map was obtained from the Global Forest Age Dataset (GFAD) V1.1 (Poulter et al., 2019), which provides fractions of tree cover for 15 age classes and four plant functional types (Table 1). Uncertainty in predicted C‐N responses was assessed by calculating the 95% confidence interval.
Total N‐induced C sequestration in forest AGWB was calculated by multiplying C‐N responses with forest‐specific N deposition rates from the EMEP model (Table 1). Total N deposition was calculated by multiplying forest‐specific N deposition rates (in mg N m−2) with forest area from Hansen et al. (2013) (Table 2). To summarize average effects per biome, we delineated global biomes based on a combination of WWF ecoregions and FAO Global ecological zones (Table 2, Table S1 and Figure S1a).
TABLE 2.
Global datasets used for estimating N‐induced forest C sequestration and climate footprint of human N use
| Variable | Data source and explanation | Spatial resolution | Reference |
|---|---|---|---|
| Fraction forest cover | Global Forest Monitoring Project. Forest area was calculated by multiplying forest cover fractions with total grid cell area. | 20 km × 20 km | (Hansen et al., 2013) |
| Forest biome | WWF ecoregions aggregated to 4 classes (see Table S1) combined with FAO Global Ecological Zones (GEZ) (see Figure S1b) | — (vector) | (FAO/IIASA, 2012; Olson et al., 2001) |
| N2O from cropland soils | Multi‐model mean from six dynamic global vegetation models for the years 2007–2016 | 0.5° × 0.5° | (Tian et al., 2020; Tian, Yang, et al., 2019) |
| N2O due to deposition | Idem | Idem | Idem |
| Regional N2O emissions | Average emission estimates from several models and datasets for the years 2007–2016 | 10 world regions | (Tian et al., 2020) |
2.2.2. Climate footprint of human nitrogen use
To estimate the net climate footprint of human N use, we first estimated the contribution of anthropogenic vs. natural sources to total N deposition. Anthropogenic emissions have been estimated to contribute 78% to total N deposition onto oceans (Duce et al., 2008) and 84% to terrestrial N deposition in the United States (Zhang et al., 2012). The relative contribution of natural emission sources to total N deposition declines with the total N deposition rate and rarely exceeds 2 kg N ha−1 yr−1 (Zhang et al., 2012). We thus estimated the anthropogenic share in total N deposition onto forests (Ndep) as Ndep*0.8 for regions where Ndep < 10 kg N ha−1 yr−1, and as Ndep‐2 for regions where Ndep > 10 kg N ha−1 yr−1. This resulted in a global average contribution of anthropogenic sources to total N deposition of 85%. Nitrogen‐induced C sequestration in forest AGWB attributable to human N use was calculated by multiplying anthropogenic deposition with mean C‐N responses.
Human N use leads to emissions of N2O, which absorbs ~300 times as much energy as CO2 over a 100‐year time span (Stocker et al., 2013). As greenhouse gasses are well mixed in the atmosphere, their climate impact is global, independent of the location of the emission source. However, as N‐induced forest C sequestration varies in space, the net climate impact of N use (i.e. the net result of the warming effect of N2O emissions and the cooling effect of N‐induced forest C sequestration) does depend on its spatial pattern (as the same activities generally cause both N2O emissions and N deposition). The spatial variation in the net greenhouse gas footprint of human N use was calculated by estimating the proportion of anthropogenic N2O emissions that is ‘offset’ by forest C sequestration induced by anthropogenic N deposition. For that purpose, N2O‐N emissions were converted to C‐equivalents by (i) multiplying by 44/28 (conversion factor from N to N2O), (ii) multiplying by 298 (100‐year GWP of N2O in CO2 equivalents) and (iii) multiplying by 12/44 (conversion factor from CO2 to C).
Spatially explicit direct and indirect anthropogenic N2O emissions were estimated based on data presented in Tian et al. (2020), who distinguish three sources of anthropogenic N2O: (i) agriculture (including the sub‐categories cropland soils, pastures, manure management and aquaculture); (ii) other anthropogenic sources (fossil fuels, industry, wastewater and biomass burning); and (iii) indirect emissions from anthropogenic N additions (emissions from inland waters and due to N deposition on land and oceans). Emissions of N2O from ‘perturbed fluxes’ (due to changes in climate, CO2 and land cover) were not included. Spatially explicit anthropogenic N2O emissions were estimated by combining estimates for N2O emissions from cropland soils and due to deposition on land from the multi‐model mean of six terrestrial biosphere models (NMIP; Tian, Yang, et al., 2019) with regional estimates for the other sub‐categories (Table 2). Regional emission estimates were allocated to a grid assuming that emissions from pastures, manure management and aquaculture follow the same spatial pattern as cropland N2O emissions while the remaining anthropogenic emissions were distributed homogenously over a region.
3. RESULTS
3.1. Predictors of C‐N response and model selection
Across all studies, N addition enhanced forest AGWB C sequestration (p < .01), but the direction and strength of the response was affected by site factors (Figure S2 and Table S2). Forest C‐N response increased with increasing absolute latitude (from tropical to boreal regions, p < .01), with decreasing mean annual temperature (p = .06) and with decreasing PET (p < .01). As expected, PET, temperature and latitude were strongly correlated (R2 > 0.7; Figure S3). Furthermore, C‐N response decreased with tree age (p < .01; Table S2). Neither N deposition rate nor cumulative N deposition explained variation in C‐N response, but C‐N response tended to decrease with N addition rate (p = .06; Table S2). The C‐N response strongly increased with soil N content (p < .01) and was only weakly correlated with soil organic C content (p = .15) and soil pH (p = .16). The variability in C‐N response could best be described by soil N content, PET and tree age (Table 3) where these site factors explained 68% of the observed variance in C‐N response.
TABLE 3.
Summary of selected model for predicting (log‐transformed) forest aboveground woody biomass C‐N response
| Coefficient | Lower bound CI95 | Upper bound CI95 | p value | |
|---|---|---|---|---|
| Intercept | 6.10 | 3.25 | 8.95 | <.0001 |
| log(Soil N) | 0.20 | 0.03 | 0.37 | .03 |
| log(PET) | −0.51 | −0.87 | −0.14 | .01 |
| log(Age) | −0.15 | −0.27 | −0.03 | .01 |
3.2. Forest C‐N response
Spatial variation in mean C‐N response for forest AGWB is shown in Figure 1. Overall, C‐N response decreased from the poles to the equator, from around 8–16 kg C per kg N in boreal regions to small negative responses from −4 to 0 kg C per kg N in the tropics (Figure 1a and Figure S4). The global (forest‐area weighted) average C‐N response was 2 kg C per kg N (Table 4). This low average is largely driven by the negligible response in most tropical forests (average response of 0 kg C per kg N; Table 4), which account for 58% of the global forest area. Average responses for temperate and boreal forests were 4 and 11 kg C per kg N, respectively (Table 4).
FIGURE 1.
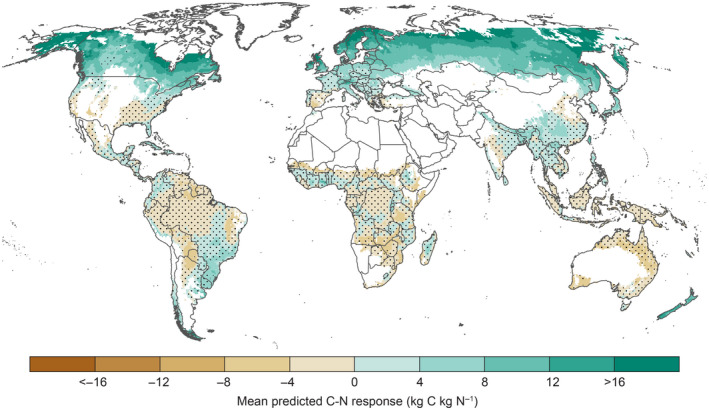
Spatial variation in mean C‐N response in forest aboveground woody biomass predicted by the regression model based on spatial variation in soil N content, mean tree age and potential evapotranspiration (PET). Dots indicate regions where the predicted response was not significant (p > .05). Maps showing spatial variation in the upper and lower confidence limits for the C‐N response are shown in Figure S5. Grid cells with <5% forest cover are masked
TABLE 4.
Total forest area, total and mean N deposition rates, total and mean N‐induced C sequestration and mean C‐N response predicted by the regression model for each biome
| Forest area (ha × 108) a | N deposition (Tg N yr−1) b | N deposition (kg N ha−1 yr−1) c | N‐induced C seq. (Tg C yr−1) d | N‐induced C seq. (kg C ha−1 yr−1) e | C‐N response (kg C kg N−1) f | |
|---|---|---|---|---|---|---|
| Boreal | 6.8 | 1.1 | 1.7 | 13 (5 to 23) | 19 (7 to 35) | 11 (4 to 21) |
| Temperate | 6.8 | 6.7 | 9.8 | 25 (0 to 55) | 37 (0 to 82) | 4 (0 to 8) |
| Tropical | 18.3 | 15.3 | 8.4 | 3 (−58 to 81) | 2 (−33 to 46) | 0 (−4 to 5) |
| World | 31.9 | 23.1 | 7.3 | 41 (−53 to 159) | 13 (−17 to 51) | 2 (−2 to 7) |
Brackets show upper and lower 95% confidence limits.
Derived from an overlay of fraction forest cover at 20 × 20 km2 from Hansen et al. (2013) with global biomes (Figure S1a).
Derived by multiplying forest‐specific N deposition rates for 2010 from Schwede et al. (2018) with total forest area in each grid cell.
Total N deposition divided by total forest area.
Derived by multiplying mean predicted C‐N response in each grid cell with total N deposition to forests. Lower/upper limits of the range are derived by multiplying the lower/upper 95% confidence limits of predicted C‐N responses with N deposition to forests.
Total N‐induced C sequestration divided by total forest area.
Derived by dividing total N‐induced C sequestration by total N deposition. Lower/upper limits of the range are derived by dividing the lower/upper confidence limit for total C sequestration by total N deposition.
For 59% of the global forest area, we found no response of forest AGWB C sequestration to N addition (Figure 1, Table 5 and Figure S5). This includes almost 90% of tropical forests (Table S3), but also temperate regions in the United States and Southern Europe (Figure 1). About one‐third of global forests responded to N addition by increasing AGWB C sequestration (p < .05; Table 5), of which the majority is situated in the boreal region (Figure 1 and Table S3). Only 5% of global forests responded to N addition by decreasing AGWB C sequestration (p < .05). These forests are mostly situated in the tropics (Figure 1 and Table S3) and on average receive the lowest rates of N deposition (4.9 kg N ha−1 yr−1, Table 5).
TABLE 5.
Share of global forests in regions where the regression model predicted a positive C‐N response (CI95,lb > 0, p < .05), negative C‐N response (CI95,ub < 0, p < .05) or no significant C‐N response (dotted areas in Figure 1)
| Share of global forest area | Mean N deposition rate (kg N ha−1 yr−1) |
Total N‐induced C sequestration (Tg C yr−1) |
Mean C‐N response (kg C kg N−1) | |
|---|---|---|---|---|
| Positive C‐N response (p < .05) | 36% | 5.4 | 45 (16 to 81) | 7 (3 to 13) |
| Negative C‐N response (p < .05) | 5% | 4.9 | −4 (−7 to −1) | −5 (−8 to −1) |
| Insignificant C‐N response | 59% | 8.5 | 3 (−65 to 85) | 0 (−4 to 5) |
3.3. Global nitrogen‐induced forest carbon sink
Globally, we estimated that N deposition onto forest increases the forest AGWB C sink by 41 Tg C yr−1 (Table 4). This additional C sequestration is realized in only one‐third of the global forest area where N significantly increased C sequestration (Table 5). The largest N‐induced C sink occurred in temperate forests of Central Europe, Southern China, Southern Korea, Japan, New Zealand and the Northeast of North America (Figure 2a). Although C‐N response was largest in boreal forests, N‐induced stimulation of the forest C sink was about twice as large in temperate forests (Table 4 and Figure 2b), due to higher N deposition in temperate regions (Table 4 and Figure S6).
FIGURE 2.
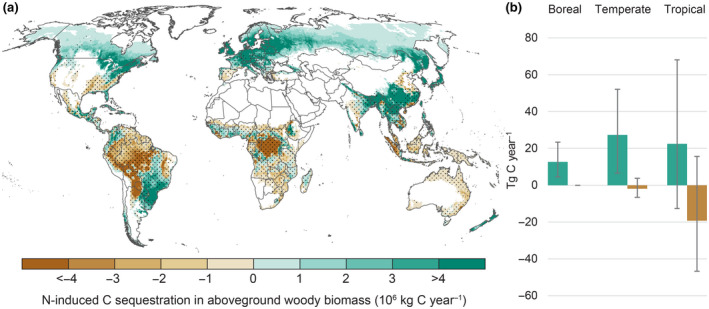
(a) Spatial variation in the N‐induced C sink in forest aboveground woody biomass estimated by multiplying mean C‐N responses (Figure 1) with N deposition to forests. Dots indicate regions where the predicted response was not significant (p > .05). Grid cells with <5% forest cover are masked. Maps showing estimated N‐induced forest C sink using the upper and lower prediction limits for C‐N response are shown in Figure S7. (b) Total N‐induced C sink in forest aboveground woody biomass in boreal, temperate and tropical forests, shown separately for regions where the mean predicted C‐N response was positive (green bars) or negative (brown bars). Error bars show 95% CI
The largest negative N‐induced C sink (i.e. N‐induced reduction in C sink) occurred in tropical rain forests (Amazon and the Congolian rainforest; Figure 2a). However, in 95% of forests where the mean predicted C‐N response was negative, the direction of this response was uncertain (p > .05, Figure 1), leading to a mean N‐induced C sink reduction of 20 Tg C yr−1 in tropical forests that might vary from a reduction of 40 Tg C yr−1 up to an increase of 18 Tg C yr−1 (Figure 2b). Overall, the uncertainty associated with the estimated N‐induced forest C sequestration increases from boreal to temperate to tropical areas (Figure 2b).
3.4. Climate footprint of human nitrogen use
Comparing spatial variation in the GWP of anthropogenic N2O emissions to that of anthropogenic N deposition‐induced forest biomass C sequestration showed that the cooling effect of C sequestration only compensated a small fraction of the warming effect of N2O almost everywhere (Figure 3). In most regions where N deposition increased forest AGWB C sequestration (blue areas in Figure 3), this cooling effect compensated less than 25% of the warming effect of N2O, implying that the net climate footprint of human N use is a warming effect almost everywhere. The only notable exception were regions across northern Russia, where the warming effect of N2O was fully offset by N‐induced C sequestration (dark blue regions in Figure 3), mainly owing to the relatively low N2O emissions in this region. For the whole boreal region, the share of N2O warming offset by N‐induced AGWB C sequestration was 22%, while this was only 6% in the temperate region and 5% in the tropics (column B in Table 6). In regions where N deposition reduced forest AGWB C sequestration (red areas in Figure 3), the associated warming effect was generally negligible compared to that of N2O. The only regions where the warming impact of an N‐induced reduction in C sequestration was of similar magnitude than that of anthropogenic N2O emissions were situated in the Amazon and Congolian rainforests (note that natural N2O emissions, which are generally high in the tropics, were not considered here). For the entire tropical region, the GWP of N‐induced reduction in C sequestration (16 Tg C yr−1) was only 4% of the GWP of anthropogenic N2O emissions (423 Tg C‐eq yr−1; Table 6).
FIGURE 3.
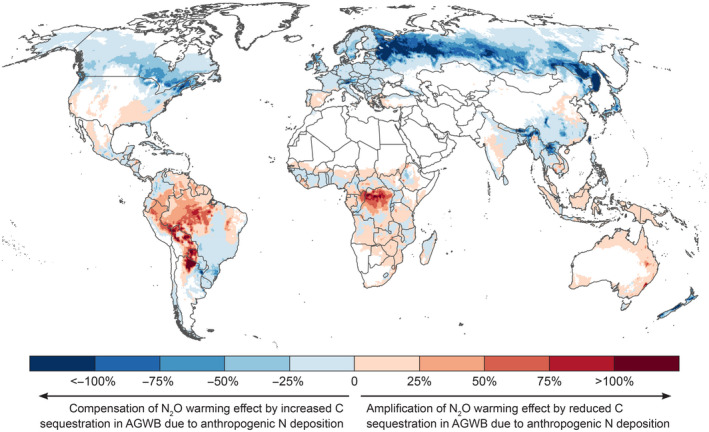
Spatial variation in the net climate footprint of human N use, expressed as the ratio between the 100‐year Global Warming Potential of N‐induced C sequestration in forest aboveground woody biomass (considering anthropogenic N deposition only) and anthropogenic N2O emissions (expressed in C‐equivalents). Blue areas indicate a reduction of the climate impact of N2O by an N‐induced increase in C sequestration, red areas an amplification the climate impact of N2O by an N‐induced reduction in C sequestration. Data on anthropogenic N2O emissions were derived from Tian et al. (2020); see Table 2. Grid cells with <5% forest cover are masked, though note that N2O emissions also occur in grid cells without forest
TABLE 6.
Anthropogenic N2O emissions, N‐induced C sequestration in forest aboveground woody biomass (considering anthropogenic N deposition only) and net climate impact of both fluxes (expressed as 100‐year Global Warming Potential in Tg C‐equivalents per year)
| Anthropogenic N2O emissions (Tg C‐eq. yr−1) a | Increased C sequestration due to anthropogenic N deposition (Tg C yr−1) b | Reduced C sequestration due to anthropogenic N deposition (Tg C yr−1) c | Net climate impact of anthropogenic N2O and net N‐induced C sink (Tg C‐eq. yr−1) | |
|---|---|---|---|---|
| A | B | C | A + B + C | |
| Boreal | 45 | −10 (−22%) | 0 (+0%) | 35 |
| Temperate | 404 | −23 (−6%) | 2 (+0%) | 383 |
| Tropical | 432 | −20 (−5%) | 16 (+4%) | 427 |
| World | 882 | −53 (−6%) | 17 (+2%) | 846 |
Positive numbers represent a flux leading to an increase in atmospheric greenhouse gas concentrations, negative numbers represent a removal from the atmosphere. Percentages in columns (B) and (C) show ratio of N‐induced forest C sequestration to anthropogenic N2O emissions in column (A) (analogue to percentages shown in Figure 3).
Obtained from an overlay of biomes with spatially explicit anthropogenic N2O emissions for the years 2007–2016 estimated based on data presented in Tian et al. (2020), see Section 2.2.2.
Obtained by multiplying predicted C‐N responses with anthropogenic N deposition to forests only for grid cells where mean predicted C‐N response >0 (i.e. where N was estimated to increase C sequestration).
Obtained by multiplying predicted C‐N responses with anthropogenic N deposition to forests only for grid cells where mean predicted C‐N response <0 (i.e. where N was estimated to reduce C sequestration).
4. DISCUSSION AND CONCLUSIONS
4.1. Site factors explaining variation in C‐N response
4.1.1. Biome
In line with previous studies, we found a decrease in C‐N response from boreal to tropical regions, with PET explaining slightly more variation in C‐N response than other climate‐related variables. This might be because PET (in contrast to latitude) captures differences between tropical lowland and highland forests, supporting the hypothesis that N limitation increases with elevation due to temperature and moisture effects on mineralization rates (Dalling et al., 2016), whereas P limitation decreases with elevation (Fisher et al., 2013), and thus high‐elevation sites (with lower PET) respond more strongly to N addition.
4.1.2. Tree and stand characteristics
Stand age emerged as an important predictor from our analyses, explaining 33% of the variance in C‐N response. The influence of mycorrhizal interaction could not be tested as classifying stands as either AM/EM was not always possible (see Section 2.1.2).
4.1.3. Nutrient and water availability
We hypothesized that forests on fertile soils respond more strongly to N addition because they convert a larger share of GPP to woody biomass (Fernández‐Martínez et al., 2014; Vicca et al., 2012) and because response to N addition is not constrained by availability of other nutrients (such as P, Ca and Mg). From all included soil fertility indicators (organic C content, pH, CEC, clay content and N content), only soil N content explained a substantial share of variation in C‐N response. This may seem unexpected as one would expect forests with high N availability to be less sensitive to N addition. However, while soil N content is considered a good proxy for N availability at the regional scale (Binkley & Hart, 1989), this is likely not the case at the global scale. Compared to tropical forests, boreal forests have higher soil N contents but lower N availability, due to lower decomposition and mineralization and thus enhanced N immobilization (Schimel & Weintraub, 2003). The soil C:N ratio is generally considered a better indicator for N availability, and N immobilization in forests has been shown to decrease at lower C:N ratios, implying that more N is available for uptake and leaching (e.g. Dise et al., 2009; van der Salm et al., 2007). Our data indeed showed a negative correlation between C‐N response and soil C:N (see Figure S2), though the relation was not significant.
Water availability (precipitation and precipitation excess) had no effect on C‐N response, possibly because of the low number of experimental fields located in semi‐arid regions where tree growth is water limited, or because annual PET or PE are poor indicators for (seasonal) water stress on local sites. Using soil moisture in the rootzone as a proxy for water availability, Baribault et al. (2010) found that NPP in northern hardwood forests was equally limited by N and water availability. This suggests that more site‐specific indicators for water availability (possibly derived from remote sensing) might help to unravel its impact on a global scale. Similarly, accurate information on soil properties across forests soils might lead to additional spatial variation in soil‐induced variation in C‐N responses.
4.1.4. N saturation
Decreasing C‐N responses with increasing rates of N addition have been reported by both long‐term fertilization experiments (Binkley & Högberg, 2016; Liu et al., 2021) and meta‐analyses (Schulte‐Uebbing & De Vries, 2018; Tian et al., 2016). At high rates of N addition, other nutrients (P, Ca, Mg) become increasingly limiting, and N saturation may lead to negative impacts on growth (Aber et al., 1989). However, we found no negative interaction between C‐N response and either ambient or cumulative N deposition, likely because ambient N deposition rates were lower than rates at which C‐N response decline (>40 kg N ha−1 yr−1) (Schulte‐Uebbing & De Vries, 2018; Tian et al., 2016). The C‐N response was indeed lower in experiments with high N addition rates, but spatial correlation between N addition and other site properties confounded the impact of N addition rate on the variation in observed C‐N responses. For example, tropical forests received significantly higher N addition rates than boreal forests (see Table S1 in Schulte‐Uebbing & De Vries, 2018), showing that additional experimental data are needed to disentangle this effect.
4.2. Comparison with previous studies
4.2.1. Strengths and weaknesses of approaches to estimate forest C‐N response
Each approach to estimate C‐N responses and N‐induced C sequestration has its own strengths and limitations, generally balancing between two opposing goals: observing N effects on forest growth under realistic conditions and isolating the effect of N from other drivers of forest growth. Process‐based C cycle models and growth observations along N deposition gradients are at two opposite ends of this spectrum: Models can clearly isolate the effect of N by simulating forest C sink changes under varying N deposition levels while accounting for interactions with changing CO2, temperature or land use. However, current models might overlook the impact of site properties because they do not include all relevant biochemical processes related to N cycling (Thomas et al., 2015). For example, many coupled C‐N models do not account for co‐limitation by P (Esser et al., 2011; Zaehle, 2013) or the upregulation of BNF during periods of rapid growth and biomass accumulation (Batterman et al., 2013; O’Sullivan et al., 2019), both processes that may strongly affect N‐induced C sequestration, especially in tropical forests. In contrast, growth inventories rely on actual observations at sites with different N deposition rates, but disentangling drivers forest growth is challenging due to its collinearity with undetermined site properties as well as climatic variables (De Vries et al., 2008; Magnani et al., 2007; Sutton et al., 2008).
Fertilization experiments fall between these two extremes: they observe responses in real forests and can isolate the effect of N addition by comparing growth responses in fertilized and unfertilized plots. However, both treated and control plots also receive ambient N deposition, and thus any fertilizer‐induced response is additional to the forest's response to deposition. More importantly, experimental N rates are often a factor 5–10 higher than ambient N deposition levels, which leads to an underestimation of C‐N response if C‐N response declines with N input rates (Binkley & Högberg, 2016). Also, fertilization experiments are concentrated in a few regions only, which limits extrapolation of results to the global scale (see Section 4.2.2). Finally, fertilization experiments conducted for several years only cannot provide information in changes in C‐N response over a forests’ lifetime (see also Section 4.4). These limitations of fertilization experiments also apply to stoichiometric scaling approaches, which rely on experimentally derived N retention and allocation fractions (e.g. Nadelhoffer et al., 1999). Stoichiometric scaling approaches also assume constant C:N rations even under elevated N, while N concentrations in stemwood have been shown to increase substantially in stands exposed to elevated N deposition due to luxury consumption (De Vries et al., 2021).
4.2.2. C‐N responses in temperate and boreal forests
Our estimates for average C‐N responses for boreal and temperate are lower than results from forest growth inventories along deposition gradients, which range from 20 to 30 (De Vries et al., 2008; Fleischer et al., 2013; Solberg et al., 2009) or even up to 50 kg C per kg N (Flechard et al., 2020). However, they are similar to results from stoichiometric scaling (Du & De Vries, 2018) for temperate forests (4 vs. 5 kg C per kg N) and only slightly lower for boreal forests (11 vs. 14 kg C per kg N; Table S5). Our previous estimate for temperate forests based on the same dataset was substantially higher (13 kg C per kg N, Schulte‐Uebbing & De Vries, 2018) due to differences in statistical approach (see Section 2.1.1), whereas the earlier meta‐analysis estimated mean C‐N responses as weighted averages across all experimental plots, the upscaling procedure we applied here accounts for the effect of global variation in forest site properties that drive C‐N response: compared to the experimental plots in the database, global temperate forests on average have a higher PET, lower soil N content and higher stand age (Figure 4), all leading to lower predicted C‐N responses. This highlights the relevance of robust and sound upscaling procedures from experimental fields to the global scale.
FIGURE 4.
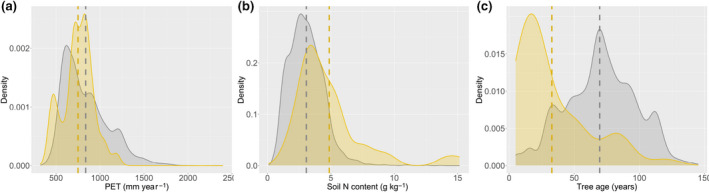
Density plots showing the distribution of the three variables included in the regression model used to predict C‐N response (a) in temperate forest experimental plots in the database (yellow) and (b) in global temperate forests (grey). For the density plots for global forests, frequency of occurrence was weighted by forest area (i.e. can be read as “frequency for km2 forest”). Dashed lines show average values across experimental plots (yellow) and global forests (grey). Density plots for other biomes are shown in Figure S8
Our results of no significant C response to N addition in one‐third of temperate forests (Table S3) are consistent with multiple forest fertilization experiments (e.g. Lovett & Goodale, 2011; McNulty et al., 2005). Possible causes of a lack of N‐induced C sequestration include (i) foliar nutrient imbalances following N addition leading to increased susceptibility to pests and pathogens; (ii) N‐induced soil pH changes lowering P availability due to enhanced sorption to iron oxides, (iii) increased soil acidity leading to a depletion of base cations and (iv) increased solubility of potentially toxic elements. For example, Mainwaring et al. (2014) found that Douglas fir stands only responded to N fertilization at soil Ca:N ratios above 0.06, indicating that Ca availability limits tree growth below this threshold. Lovett et al. (2013) also found no significant response to N addition for six tree species, possible caused by low cation availability (Templer et al., 2005) and secondary effects of long‐term excess inputs of N such as soil acidification and base cation leaching. Analyses of long‐term trends in foliar nutrients in Europe also show shifts towards P and S deficiency in forest sites subject to long‐term elevated N deposition (Jonard et al., 2015; Prietzel et al., 2020).
4.2.3. C‐N responses in tropical forests
Tropical forests account for almost two‐thirds of the global forest area and experience similar N average deposition rates as temperature forests (8.4 vs. 9.8 kg N ha−1 yr−1; Table 4). Therefore, even small differences in estimated C‐N response for tropical forests strongly affect the size of the global N‐induced forest C sink. At least two recent studies predicted an N‐induced C sink in the (sub)tropics of 123 Tg C yr−1 (Fleischer et al., 2019, based on the average for 1901–2010 using a DGVM that includes P limitation) and 80 Tg C yr−1 (Du & De Vries, 2018, based on stoichiometric scaling), which is much higher than our estimate (3 Tg C yr−1, Table 4). Most N addition experiments in the tropics find no effect of N addition on productivity (e.g. Cusack et al., 2011; Homeier et al., 2012; Lu et al., 2021), confirming the hypothesis that tree growth is mainly P‐limited on highly weathered tropical soils with high levels of N availability. Nitrogen addition has even been shown to reduce stimulating effects of P addition on biomass in tropical forests (Li et al., 2016), potentially due to detrimental effects on growth through soil acidification (Tian & Niu, 2015). Nitrogen deposition also may reduce BNF and thus not lead to more N availability at the tree level (Esser et al., 2011). A negligible effect of N deposition on C sequestration in the tropical region has also been found by another DGVM (Zaehle et al., 2011).
4.3. Uncertainties in data used in upscaling of C‐N responses to estimate nitrogen‐induced forest carbon sink
Estimates of the global N‐induced forest C sink based on upscaling approaches are sensitive not only to uncertainties in C‐N responses, but also uncertainty in input data used for upscaling, including forest cover, biome delineation and N deposition. For example, differences in estimated forest area alone explain 34% of the difference between our estimate for global N‐induced forest biomass C sequestration (41 Tg C yr−1) and the estimate by Du and De Vries (144 Tg C yr−1; see Table S5). Estimates for global forest area vary widely, due to differences in technologies, approaches and definitions of what constitutes a ‘forest’. Our estimate for global forest area is based on fraction forest cover data derived from high‐resolution Landsat imagery (Hansen et al., 2013) and is 25% lower than the value reported in the Global Forest Resources Assessment (FAO, 2020) as used by Du and De Vries (2018). While the FAO estimate is based on self‐reported values by participating countries and uses a definition based on land use (according to which a recently cut‐down forest plantation is still considered a ‘forest’, while a tree patch on agricultural land is not), satellite images detect actual land cover and are therefore considered more relevant for studying forest C dynamics (Sexton et al., 2016). However, even satellite‐based estimates of tree and forest cover show discrepancies, especially in regions with low tree density (Sexton et al., 2015, 2016).
Spatial delineation of biomes also varies between studies, with some studies including ‘sub‐tropical’ as an additional category (De Vries et al., 2014; Du & De Vries, 2018; Esser et al., 2011; Fleischer et al., 2015, 2019; Schwede et al., 2018). Not all studies clearly report criteria used to delineate biomes, while these definitions can strongly affect results of upscaling based on average response rates. As our calculations are performed at grid level, biome delineation does not affect estimates of global N‐induced C sequestration, but we show that a different biome delineation (based on FAO Global Ecological Zones) affects estimated average N deposition rates and C‐N responses per biome (Table S4).
Finally, estimates for N deposition vary substantially between studies estimating global N‐induced C sequestration. Forest‐specific deposition rates used in this study are generally higher than using grid‐cell averages, as factors such as surface roughness and canopy height exert a great influence on N deposition rates (Schwede et al., 2018). Globally, using forest‐specific deposition rates increased estimated total N deposition to forest by 12% (from 20.6 to 23.0 Tg N yr−1, with the largest relative increase in temperate forest), however, regionally the difference can be up to a factor two. As long as modelled N deposition rates strongly vary and often deviate from measured deposition rates (Tan et al., 2018), accurate quantification of the N‐induced C sink in forests is still challenging.
4.4. Nitrogen deposition as driver of the terrestrial carbon sink
The terrestrial biosphere acts as a C sink, absorbing between 18 and 25% of global CO2 emissions (Friedlingstein et al., 2020). Forests account for a substantial part of this C sink, and drivers that have been proposed to account for increased forest growth include CO2 fertilization, increasing temperatures (leading to longer growing seasons in high‐latitude forests), forest management and N deposition, as well as interactions between these drivers (De Vries & Posch, 2011; Hyvönen et al., 2007; O’Sullivan et al., 2019). The contribution of N to forest C sequestration has been disputed, with some studies claiming that N deposition controls a large part of the forest C sink (Magnani et al., 2007), while others find a minor role (Du & De Vries, 2018). We find that globally, N‐induced C sequestration accounts for ~1% of the gross forest C sink (~4100 Tg C yr−1) or 2% of the net forest C sink (~1900 Tg C yr−1), with the largest relative contribution in boreal forest where N‐induced C sequestration accounted for 2 and 3% of the gross and net C sink, respectively (Figure 5).
FIGURE 5.
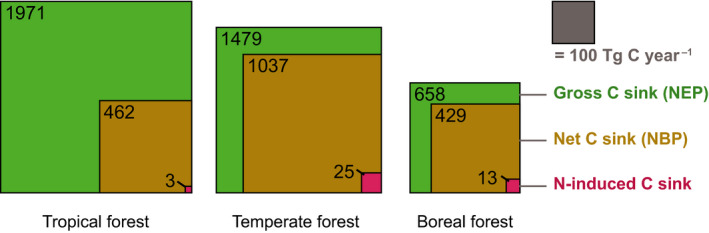
Illustration of the relative size of the N‐induced forest biomass C sink estimated by this study and the gross and net forest C sinks, per forest biome (all values are in Tg C yr−1). Estimates for gross C sink (total C removal in aboveground and belowground biomass) and net C sink (gross C sink minus C emissions due to deforestation, forestry, urbanization and wildfires) were obtained from high‐resolution maps of the global gross and net forest C sink for the years 2001–2020 estimated by combining ground‐ and earth observation data (Harris et al., 2021)
In this study, we focussed on aboveground woody biomass and ignored the impact of N deposition on C sequestration in belowground woody biomass and soils. Belowground woody biomass production is typically around 20% of AGWB (e.g. Cleveland et al., 2013), and thus assuming a similar response to N addition as for AGWB, the effect of neglecting this sink is likely small. Several experiments and meta‐analyses showed that N addition increased soil C storage due to increased litter inputs and/or reduced soil respiration through interactions with SOM stabilization and microbial community (e.g. Janssens et al., 2010; Lu et al., 2021; Maaroufi et al., 2019; Nave et al., 2009; Tian, Dungait, et al., 2019; Zhou et al., 2014). Most global studies thus concluded that the N‐induced forest soil C sink is likely in the same order of magnitude or smaller than the N‐induced forest biomass C sink (see e.g. Table 4 in Schulte‐Uebbing & De Vries, 2018). However, the notion that N deposition leads to an increase in soil C sequestration has also been challenged. In two long‐term N addition experiments, for example, N led to an increase in SOC pool in the organic horizon but a decrease in the mineral topsoil horizons, that is, a vertical redistribution of SOC pools rather than an increase in overall SOC storage (Forstner et al., 2019). Moreover, in many experiments, N effects on increased soil SOC content or reduced soil respiration are only visible at high N addition rates (>50 kg N ha−1 yr−1; e.g. Forsmark et al., 2020; Janssens et al., 2010; Liu et al., 2021; Lu et al., 2021; Maaroufi et al., 2015; Tian, Dungait, et al., 2019). Inversely, respiration has been found to increase at lower N additions near 20 kg N ha−1 yr−1 (De Vries et al., 2014), being rates that more realistically mimic atmospheric N deposition levels in most of the world. Even if experiments apply N at annual rates similar to atmospheric deposition, N is added at a much higher dose during one or several applications. Resulting high N concentrations may overwhelm microbial demand and thus induce soil acidification, resulting in reduced respiration that would not be observed under continuous ambient deposition (Averill et al., 2018). The notion that N does not increase soil C sequestration is supported by a forest inventory study that reports a negative correlation between ambient N deposition rates and soil C content in US forests, even when accounting for other variables that may affect variation in soil C content (Averill et al., 2018). An N‐induced decrease in soil C might be driven by shifts from EM‐ to AM‐associated tree species under high N deposition (Averill et al., 2018).
Results from studies assessing N‐induced C sequestration alone do not allow to draw conclusions on the long‐term fate of C sequestered in forests, as they assess N impacts on Net Ecosystem Productivity (C uptake by photosynthesis minus respiration losses) rather than Net Biome Productivity (accounting for C removal from disturbances and harvest). Whether the additional C sequestered in forests will be removed from the atmosphere over the course of decades or centuries (policy‐relevant timescales for climate change mitigation) depends on its persistence in biomass and soil. It has been proposed, for example, that trees that grow faster (due to N deposition, CO2 fertilization or warming) also die younger (Büntgen et al., 2019), which would reduce the residence time of C in wood (Körner, 2017). For managed forests, increased woody biomass growth may also lead to earlier harvesting and the fate of sequestered C would then depend on how the harvested wood is used (i.e. burned or used in products with a long lifespan). For natural forests, faster growth may just imply that forests reach their steady‐state C pool faster, thus not increasing net C sequestration over the forests’ lifetime (Körner, 2017). While even old‐growth forests may continue to accumulate C (Luyssaert et al., 2008), the largest part of the global forest C sink has been attributed to demographic changes in regrowth forests (Pugh et al., 2019). Finally, actual C sequestration strongly depends on anthropogenic or natural disturbances that are not related to N deposition, such as forest fires, deforestation and forest degradation.
5. CONCLUSIONS
We conclude that the contribution of N deposition to forest biomass C sequestration likely is small. Nitrogen‐induced biomass C sequestration accounts for only ~1% of the current gross forest C sink. The climate mitigation effect of N‐induced C sequestration in forests is not sufficient to balance the warming impact of anthropogenic N2O emissions, with N‐induced C sequestration only offsetting ~5% of the global warming potential of N2O emissions, though with large regional variation. Overall, the climate footprint of N decreases from tropical to boreal regions, due to both higher N2O emissions and lower N‐induced C sequestration at low latitudes. Assuming N‐induced C sequestration in soils in the same order of magnitude as for biomass, N‐induced C sequestration may offset 10% of global N2O emissions, but the impact of N on soil C sequestration and its spatial variation is uncertain. While N may also lead to cooling by increasing CH4 consumption and by increasing formation of aerosols that reflect sunlight, available evidence shows that this effect is likely small compared to the warming effect of N2O (De Vries et al., 2017; Erisman et al., 2011). Therefore, reducing reactive N losses is likely to benefit climate mitigation.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We thank Lex Bouwman, Michael Gundale and two anonymous reviewers for valuable suggestions on improving the manuscript. L.S. acknowledges funding by the NWO (project number 022.003.009), provided by a project initiated by the SENSE Research School.
Schulte‐Uebbing, L. F. , Ros, G. H. , & de Vries, W. (2022). Experimental evidence shows minor contribution of nitrogen deposition to global forest carbon sequestration. Global Change Biology, 28, 899–917. 10.1111/gcb.15960
See also Commentary on this article by Gundale, https://doi.org/10.1111/gcb.15959
DATA AVAILABILITY STATEMENT
Original geo‐tiffs of maps shown in figures 1, 2, 3, S3 and S5 can be downloaded from the data‐repository pangea at https://doi.org/10.1594/PANGAEA.940283. Additional data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- Aber, J. D. , Nadelhoffer, K. J. , Steudler, P. , & Melillo, J. M. (1989). Nitrogen saturation in northern forest ecosystems. BioScience, 39, 378–386. 10.2307/1311067 [DOI] [Google Scholar]
- Allen, M. F. , Allen, E. B. , Lansing, J. L. , Pregitzer, K. S. , Hendrick, R. L. , Ruess, R. W. , & Collins, S. L. (2010). Responses to chronic N fertilization of ectomycorrhizal piñon but not arbuscular mycorrhizal juniper in a piñon‐juniper woodland. Journal of Arid Environments, 74, 1170–1176. 10.1016/j.jaridenv.2010.05.001 [DOI] [Google Scholar]
- Averill, C. , Dietze, M. C. , & Bhatnagar, J. M. (2018). Continental‐scale nitrogen pollution is shifting forest mycorrhizal associations and soil carbon stocks. Global Change Biology, 24, 4544–4553. 10.1111/gcb.14368 [DOI] [PubMed] [Google Scholar]
- Averill, C. , Turner, B. L. , & Finzi, A. C. (2014). Mycorrhiza‐mediated competition between plants and decomposers drives soil carbon storage. Nature, 505, 543–545. 10.1038/nature12901 [DOI] [PubMed] [Google Scholar]
- Bai, E. , Houlton, B. Z. , & Wang, Y. P. (2012). Isotopic identification of nitrogen hotspots across natural terrestrial ecosystems. Biogeosciences, 9, 3287–3304. 10.5194/bg-9-3287-2012 [DOI] [Google Scholar]
- Baribault, T. W. , Kobe, R. K. , & Rothstein, D. E. (2010). Soil calcium, nitrogen, and water are correlated with aboveground net primary production in northern hardwood forests. Forest Ecology and Management, 260, 723–733. 10.1016/j.foreco.2010.05.029 [DOI] [Google Scholar]
- Batterman, S. A. , Hedin, L. O. , van Breugel, M. , Ransijn, J. , Craven, D. J. , & Hall, J. S. (2013). Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature, 502(7470), 224–227. 10.1038/nature12525 [DOI] [PubMed] [Google Scholar]
- Binkley, D. , & Hart, S. C. (1989). The components of nitrogen availability assessments in forest soils. In Stewart B. A. (Ed.), Advances in soil sciences (pp. 57–112). Springer. [Google Scholar]
- Binkley, D. , & Högberg, P. (2016). Tamm Review: Revisiting the influence of nitrogen deposition on Swedish forests. Forest Ecology and Management, 368, 222–239. 10.1016/j.foreco.2016.02.035 [DOI] [Google Scholar]
- Borenstein, M. , Hedges, L. V. , Higgins, J. P. , & Rothstein, H. R. (2009). Introduction to meta‐analysis. John Wiley & Sons. [Google Scholar]
- Bradford, M. A. (2014). Ecology: Good dirt with good friends. Nature, 505, 486–487. 10.1038/nature12849 [DOI] [PubMed] [Google Scholar]
- Brookshire, E. N. J. , Gerber, S. , Menge, D. N. L. , & Hedin, L. O. (2012). Large losses of inorganic nitrogen from tropical rainforests suggest a lack of nitrogen limitation. Ecology Letters, 15, 9–16. 10.1111/j.1461-0248.2011.01701.x [DOI] [PubMed] [Google Scholar]
- Brundrett, M. C. (2009). Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant and Soil, 320, 37–77. 10.1007/s11104-008-9877-9 [DOI] [Google Scholar]
- Büntgen, U. , Krusic, P. J. , Piermattei, A. , Coomes, D. A. , Esper, J. , Myglan, V. S. , Kirdyanov, A. V. , Camarero, J. J. , Crivellaro, A. , & Körner, C. (2019). Limited capacity of tree growth to mitigate the global greenhouse effect under predicted warming. Nature Communications, 10, 1–6. 10.1038/s41467-019-10174-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterbach‐Bahl, K. , Nemitz, E. , Zaehle, S. , Billen, G. , Boeckx, P. , Erisman, J. W. , Garnier, J. , Upstill‐Goddard, R. , Kreuzer, M. , Oenema, O. , Reis, S. , Schaap, M. , Simpson, D. , De Vries, W. , Winiwarter, W. , & Sutton, M. A. (2011). Nitrogen as a threat to the European greenhouse balance. European Nitrogen Assessment, 434–462. 10.1017/cbo9780511976988.022 [DOI] [Google Scholar]
- Cleveland, C. C. , Houlton, B. Z. , Smith, W. K. , Marklein, A. R. , Reed, S. C. , Parton, W. , Del Grosso, S. J. , & Running, S. W. (2013). Patterns of new versus recycled primary production in the terrestrial biosphere. Proceedings of the National Academy of Sciences, 110, 12733–12737. 10.1073/pnas.1302768110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland, C. C. , Townsend, A. R. , Schimel, D. S. , Fisher, H. , Howarth, R. W. , Hedin, L. O. , Perakis, S. S. , Latty, E. F. , Von Fischer, J. C. , Elseroad, A. , & Wasson, M. F. (1999). Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Global Biogeochemical Cycles, 13(2), 623–645. 10.1029/1999gb900014 [DOI] [Google Scholar]
- Cusack, D. F. , Silver, W. L. , Torn, M. S. , & McDowell, W. H. (2011). Effects of nitrogen additions on above‐ and belowground carbon dynamics in two tropical forests. Biogeochemistry, 104, 203–225. 10.1007/s10533-010-9496-4 [DOI] [Google Scholar]
- Dalling, J. W. , Heineman, K. , Lopez, O. R. , Wright, S. J. , & Turner, B. L. (2016). Nutrient availability in tropical rain forests: The paradigm of phosphorus limitation. In: Goldstein G., & Santiago L. (Eds.), Tropical tree physiology. Springer; (pp. 261–273). 10.1007/978-3-319-27422-5_12 [DOI] [Google Scholar]
- De Vries, W. , de Jong, A. , Kros, J. , & Spijker, J. (2021). The use of soil nutrient balances in deriving forest biomass harvesting guidelines specific to region, tree species and soil type in the Netherlands. Forest Ecology and Management, 479, 118591. 10.1016/j.foreco.2020.118591 [DOI] [Google Scholar]
- De Vries, W. , Du, E. , & Butterbach‐Bahl, K. (2014). Short and long‐term impacts of nitrogen deposition on carbon sequestration by forest ecosystems. Current Opinion in Environmental Sustainability, 9–10, 90–104. 10.1016/j.cosust.2014.09.001 [DOI] [Google Scholar]
- De Vries, W. , Du, E. , Butterbach‐Bahl, K. , Dentener, F. , & Schulte‐Uebbing, L. (2017). Global‐scale impact of human nitrogen fixation on greenhouse gas emissions. Oxford Research Encyclopedias. Environmental Science. [Google Scholar]
- De Vries, W. , Kros, J. , Reinds, G. J. , & Butterbach‐Bahl, K. (2011). Quantifying impacts of nitrogen use in European agriculture on global warming potential. Current Opinion in Environmental Sustainability, 3, 291–302. 10.1016/j.cosust.2011.08.009 [DOI] [Google Scholar]
- De Vries, W. , & Posch, M. (2011). Modelling the impact of nitrogen deposition, climate change and nutrient limitations on tree carbon sequestration in Europe for the period 1900–2050. Environmental Pollution, 159, 2289–2299. 10.1016/j.envpol.2010.11.023 [DOI] [PubMed] [Google Scholar]
- De Vries, W. , Solberg, S. , Dobbertin, M. , Sterba, H. , Laubhahn, D. , Reinds, G. J. , Nabuurs, G.‐J. , Gundersen, P. , & Sutton, M. A. (2008). Ecologically implausible carbon response? Nature, 451, E1–E3. 10.1038/nature06579 [DOI] [PubMed] [Google Scholar]
- De Vries, W. , Solberg, S. , Dobbertin, M. , Sterba, H. , Laubhann, D. , van Oijen, M. , Evans, C. , Gundersen, P. , Kros, J. , Wamelink, G. W. W. , Reinds, G. J. , & Sutton, M. A. (2009). The impact of nitrogen deposition on carbon sequestration by European forests and heathlands. Forest Ecology and Management, 258, 1814–1823. 10.1016/j.foreco.2009.02.034 [DOI] [Google Scholar]
- Dise, N. B. , Rothwell, J. J. , Gauci, V. , van der Salm, C. , & De Vries, W. (2009). Predicting dissolved inorganic nitrogen leaching in European forests using two independent databases. Science of the Total Environment, 407, 1798–1808. 10.1016/j.scitotenv.2008.11.003 [DOI] [PubMed] [Google Scholar]
- Du, E. , & De Vries, W. (2018). Nitrogen‐induced new net primary production and carbon sequestration in global forests. Environmental Pollution, 242, 1476–1487. 10.1016/j.envpol.2018.08.041 [DOI] [PubMed] [Google Scholar]
- Duce, R. A. , LaRoche, J. , Altieri, K. , Arrigo, K. R. , Baker, A. R. , Capone, D. G. , Cornell, S. , Dentener, F. , Galloway, J. , Ganeshram, R. S. , Geider, R. J. , Jickells, T. , Kuypers, M. M. , Langlois, R. , Liss, P. S. , Liu, S. M. , Middelburg, J. J. , Moore, C. M. , Nickovic, S. , … Zamora, L. (2008). Impacts of atmospheric anthropogenic nitrogen on the open ocean. Science, 320, 893–897. 10.1126/science.1150369 [DOI] [PubMed] [Google Scholar]
- Erisman, J. W. , Galloway, J. , Seitzinger, S. , Bleeker, A. , & Butterbach‐Bahl, K. (2011). Reactive nitrogen in the environment and its effect on climate change. Current Opinion in Environmental Sustainability, 3, 281–290. 10.1016/j.cosust.2011.08.012 [DOI] [Google Scholar]
- Esser, G. , Kattge, J. , & Sakalli, A. (2011). Feedback of carbon and nitrogen cycles enhances carbon sequestration in the terrestrial biosphere. Global Change Biology, 17, 819–842. 10.1111/j.1365-2486.2010.02261.x [DOI] [Google Scholar]
- FAO . (2020). Global Forest Resources Assessment 2020. Global Forest Resources Assessment, 2020. 10.4060/ca9825en [DOI] [Google Scholar]
- FAO/IIASA . (2012). Global Agro‐ecological Zones (GAEZ): model documentation.
- Fernández‐Martínez, M. , Vicca, S. , Janssens, I. A. , Sardans, J. , Luyssaert, S. , Campioli, M. , Chapin, F. S. III , Ciais, P. , Malhi, Y. , Obersteiner, M. , Papale, D. , Piao, S. L. , Reichstein, M. , Rodà, F. , & Peñuelas, J. (2014). Nutrient availability as the key regulator of global forest carbon balance. Nature Climate Change, 4, 471–476. 10.1038/nclimate2177 [DOI] [Google Scholar]
- Fick, S. E. , & Hijmans, R. J. (2017). WorldClim 2: New 1‐km spatial resolution climate surfaces for global land areas. International Journal of Climatology, 37(12), 4302–4315. [Google Scholar]
- Field, C. B. , Chapin, F. S. III , Matson, P. A. , & Mooney, H. A. (1992). Responses of terrestrial ecosystems to the changing atmosphere: a resource‐based approach. Annual Review of Ecology and Systematics, 23, 201–235. 10.1146/annurev.es.23.110192.001221 [DOI] [Google Scholar]
- Finzi, A. C. (2009). Decades of atmospheric deposition have not resulted in widespread phosphorus limitation or saturation of tree demand for nitrogen in southern New England. Biogeochemistry, 92, 217–229. 10.1007/s10533-009-9286-z [DOI] [Google Scholar]
- Fisher, J. B. , Malhi, Y. , Torres, I. C. , Metcalfe, D. B. , van de Weg, M. J. , Meir, P. , Silva‐Espejo, J. E. , & Huasco, W. H. (2013). Nutrient limitation in rainforests and cloud forests along a 3,000‐m elevation gradient in the Peruvian Andes. Oecologia, 172, 889–902. 10.1007/s00442-012-2522-6 [DOI] [PubMed] [Google Scholar]
- Flechard, C. R. , Van Oijen, M. , Cameron, D. R. , De Vries, W. , Ibrom, A. , Buchmann, N. , Dise, N. B. , Janssens, I. A. , Neirynck, J. , Montagnani, L. , Varlagin, A. , Loustau, D. , Legout, A. , Ziemblińska, K. , Aubinet, M. , Aurela, M. , Chojnicki, B. H. , Drewer, J. , Eugster, W. , … Sutton, M. A. (2020). Carbon‐nitrogen interactions in European forests and semi‐natural vegetation ‐ Part 2: Untangling climatic, edaphic, management and nitrogen deposition effects on carbon sequestration potentials. Biogeosciences, 17, 1621–1654. 10.5194/bg-17-1621-2020 [DOI] [Google Scholar]
- Fleischer, K. , Dolman, A. J. , van der Molen, M. K. , Rebel, K. T. , Erisman, J. W. , Wassen, M. J. , Pak, B. , Lu, X. , Rammig, A. , & Wang, Y. P. (2019). Nitrogen deposition maintains a positive effect on terrestrial carbon sequestration in the 21st century despite growing phosphorus limitation at regional scales. Global Biogeochemical Cycles, 33, 810–824. 10.1029/2018GB005952 [DOI] [Google Scholar]
- Fleischer, K. , Rebel, K. T. , van der Molen, M. K. , Erisman, J. W. , Wassen, M. J. , van Loon, E. E. , Montagnani, L. , Gough, C. M. , Herbst, M. , Janssens, I. A. , Gianelle, D. , & Dolman, A. J. (2013). The contribution of nitrogen deposition to the photosynthetic capacity of forests. Global Biogeochemical Cycles, 27, 187–199. 10.1002/gbc.20026 [DOI] [Google Scholar]
- Fleischer, K. , Wårlind, D. , van der Molen, M. , Rebel, K. , Arneth, A. , Erisman, J. W. , Wassen, M. , Smith, B. , Gough, C. , Margolis, H. , Cescatti, A. , Montagnani, L. , Arain, A. , & Dolman, A. J. (2015). Low historical nitrogen deposition effect on carbon sequestration in the boreal zone. Journal of Geophysical Research: Biogeosciences, 120, 2542–2561. 10.1002/2015JG002988 [DOI] [Google Scholar]
- Forsmark, B. , Nordin, A. , Maaroufi, N. I. , Lundmark, T. , & Gundale, M. J. (2020). Low and high nitrogen deposition rates in Northern coniferous forests have different impacts on aboveground litter production, soil respiration, and soil carbon stocks. Ecosystems, 23(7), 1423–1436. 10.1007/s10021-020-00478-8 [DOI] [Google Scholar]
- Forstner, S. J. , Wechselberger, V. , Müller, S. , Keibinger, K. M. , Díaz‐Pinés, E. , Wanek, W. , Scheppi, P. , Hagedorn, F. , Gundersen, P. , Tatzber, M. , Gerzabek, M. H. , & Zechmeister‐Boltenstern, S. (2019). Vertical redistribution of soil organic carbon pools after twenty years of nitrogen addition in two temperate coniferous forests. Ecosystems, 22, 379–400. 10.1007/s10021-018-0275-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, D. , Coyle, M. , Skiba, U. , Sutton, M. A. , Cape, J. N. , Reis, S. , Sheppard, L. J. , Jenkins, A. , Grizzetti, B. , Galloway, J. N. , Vitousek, P. , Leach, A. , Bouwman, A. F. , Butterbach‐Bahl, K. , Dentener, F. , Stevenson, D. , Amann, M. , & Voss, M. (2013). The global nitrogen cycle in the twenty‐first century. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 368, 20130164.– 10.1098/rstb.2013.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlingstein, P. , O’Sullivan, M. , Jones, M. W. , Andrew, R. M. , Hauck, J. , Olsen, A. , Peters, G. P. , Peters, W. , Pongratz, J. , Sitch, S. , Le Quéré, C. , Canadell, J. G. , Ciais, P. , Jackson, R. B. , Alin, S. , Aragão, L. E. O. C. , Arneth, A. , Arora, V. , Bates, N. R. , … Zaehle, S. (2020). Global Carbon Budget 2020. Earth System Science Data, 12, 3269–3340. 10.5194/essd-12-3269-2020 [DOI] [Google Scholar]
- Gadgil, R. L. , & Gadgil, P. D. (1971). Mycorrhiza and litter decomposition. Nature, 233, 133. 10.1038/233133a0 [DOI] [PubMed] [Google Scholar]
- Galloway, J. N. , Dentener, F. J. , Capone, D. G. , Boyer, E. W. , Howarth, R. W. , Seitzinger, S. P. , Asner, G. P. , Cleveland, C. C. , Green, P. A. , Holland, E. A. , Karl, D. M. , Michaels, A. F. , Porter, J. H. , Townsend, A. R. , & Vörösmarty, C. J. (2004). Nitrogen cycles: Past, present, and future. Biogeochemistry, 70, 153–226. 10.1007/s10533-004-0370-0 [DOI] [Google Scholar]
- Galloway, J. N. , Townsend, A. R. , Erisman, J. W. , Bekunda, M. , Cai, Z. , Freney, J. R. , Martinelli, L. A. , Seitzinger, S. P. , & Sutton, M. A. (2008). Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science, 320, 889–892. 10.1126/science.1136674 [DOI] [PubMed] [Google Scholar]
- Gentilesca, T. , Vieno, M. , Perks, M. P. , Borghetti, M. , & Mencuccini, M. (2013). Effects of long‐term nitrogen addition and atmospheric nitrogen deposition on carbon accumulation in Picea sitchensis plantations. Ecosystems, 16, 1310–1324. 10.1007/s10021-013-9685-9 [DOI] [Google Scholar]
- Gleser, L. J. , & Olkin, I. (2009). Stochastically dependent effect sizes. In: The handbook of research synthesis and meta‐analysis (2nd ed., pp. 357–376). Russell Sage Foundation. [Google Scholar]
- Gundale, M. J. , From, F. , Bach, L. H. , & Nordin, A. (2014). Anthropogenic nitrogen deposition in boreal forests has a minor impact on the global carbon cycle. Global Change Biology, 20, 276–286. 10.1111/gcb.12422 [DOI] [PubMed] [Google Scholar]
- Hansen, M. C. , Potapov, P. V. , Moore, R. , Hancher, M. , Turubanova, S. A. , Tyukavina, A. , Thau, D. , Stehman, S. V. , Goetz, S. J. , Loveland, T. R. , Kommareddy, A. , Egorov, A. , Chini, L. , Justice, C. O. , & Townshend, J. R. G. (2013). High‐resolution global maps of 21st‐century forest cover change. Science, 342, 850–853. 10.1126/science.1244693 [DOI] [PubMed] [Google Scholar]
- Harris, I. C. , Jones, P. D. , & Osborn, T. (2020). CRU TS4.04: Climatic Research Unit (CRU) Time‐Series (TS) version 4.04 of high‐resolution gridded data of month‐by‐month variation in climate (Jan. 1901–Dec. 2019).
- Harris, N. L. , Gibbs, D. A. , Baccini, A. , Birdsey, R. A. , de Bruin, S. , Farina, M. , Fatoyinbo, L. , Hansen, M. C. , Herold, M. , Houghton, R. A. , Potapov, P. V. , Suarez, D. R. , Roman‐Cuesta, R. M. , Saatchi, S. S. , Slay, C. M. , Turubanova, S. A. , & Tyukavina, A. (2021). Global maps of twenty‐first century forest carbon fluxes. Nature Climate Change, 2021, 1–7. 10.1038/s41558-020-00976-6 [DOI] [Google Scholar]
- Hedges, L. V. , Gurevitch, J. , & Curtis, P. S. (1999). The meta‐analysis of response ratios in experimental ecology. Ecology, 80(4), 1150–1156. [Google Scholar]
- Helmisaari, H.‐S. , Hanssen, K. H. , Jacobson, S. , Kukkola, M. , Luiro, J. , Saarsalmi, A. , Tamminen, P. , & Tveite, B. (2011). Logging residue removal after thinning in Nordic boreal forests: Long‐term impact on tree growth. Forest Ecology and Management, 261, 1919–1927. 10.1016/j.foreco.2011.02.015 [DOI] [Google Scholar]
- Hengl, T. , De Jesus, J. M. , Heuvelink, G. B. M. , Gonzalez, M. R. , Kilibarda, M. , Blagotić, A. , Shangguan, W. , Wright, M. N. , Geng, X. , Bauer‐Marschallinger, B. , Guevara, M. A. , Vargas, R. , MacMillan, R. A. , Batjes, N. H. , Leenaars, J. G. B. , Ribeiro, E. , Wheeler, I. , Mantel, S. , & Kempen, B. (2017). SoilGrids250m: Global gridded soil information based on machine learning. PLoS One, 12, e0169748. 10.1371/journal.pone.0169748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högberg, P. (2012). What is the quantitative relation between nitrogen deposition and forest carbon sequestration? Global Change Biology, 18(1), 1–2. 10.1111/j.1365-2486.2011.02553.x [DOI] [Google Scholar]
- Högberg, P. , Fan, H. , Quist, M. , Binkley, D. , & Tamm, C. O. (2006). Tree growth and soil acidification in response to 30 years of experimental nitrogen loading on boreal forest. Global Change Biology, 12, 489–499. 10.1111/j.1365-2486.2006.01102.x [DOI] [Google Scholar]
- Holland, E. A. , Braswell, B. H. , Lamarque, J. F. , Townsend, A. , Sulzman, J. , Müller, J. F. , Dentener, F. , Brasseur, G. , Levy, H. , Penner, J. E. , & Roelofs, G. J. (1997). Variations in the predicted spatial distribution of atmospheric nitrogen deposition and their impact on carbon uptake by terrestrial ecosystems. Journal of Geophysical Research: Atmospheres, 102, 15849–15866. 10.1029/96JD03164 [DOI] [Google Scholar]
- Homeier, J. , Hertel, D. , Camenzind, T. , Cumbicus, N. L. , Maraun, M. , Martinson, G. O. , Poma, L. N. , Rillig, M. C. , Sandmann, D. , Scheu, S. , Veldkamp, E. , Wilcke, W. , Wullaert, H. , & Leuschner, C. (2012). Tropical Andean forests are highly susceptible to nutrient inputs–rapid effects of experimental N and P addition to an Ecuadorian montane forest. PLoS One, 7, e47128. 10.1371/journal.pone.0047128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, R. J. M. , Gherini, S. A. , & Goldstein, R. A. (1994). Modeling the global carbon cycle: nitrogen fertilization of the terrestrial biosphere and the “missing” CO2 sink. Global Biogeochemical Cycles, 8, 307–333. [Google Scholar]
- Hyvönen, R. , Ågren, G. I. , Linder, S. , Persson, T. , Cotrufo, M. F. , Ekblad, A. , Freeman, M. , Grelle, A. , Janssens, I. A. , Jarvis, P. G. , Kellomäki, S. , Lindroth, A. , Loustau, D. , Lundmark, T. , Norby, R. J. , Oren, R. , Pilegaard, K. , Ryan, M. G. , Sigurdsson, B. D. , … Wallin, G. (2007). The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: A literature review. New Phytologist, 173, 463–480. 10.1111/j.1469-8137.2007.01967.x [DOI] [PubMed] [Google Scholar]
- Jain, A. , Yang, X. , Kheshgi, H. , McGuire, A. D. , Post, W. , & Kicklighter, D. (2009). Nitrogen attenuation of terrestrial carbon cycle response to global environmental factors. Global Biogeochemical Cycles, 23, GB4028. 10.1029/2009GB003519 [DOI] [Google Scholar]
- Janssens, I. A. , Dieleman, W. , Luyssaert, S. , Subke, J.‐A. , Reichstein, M. , Ceulemans, R. , Ciais, P. , Dolman, A. J. , Grace, J. , Matteucci, G. , Papale, D. , Piao, S. L. , Schulze, E.‐D. , Tang, J. , & Law, B. E. (2010). Reduction of forest soil respiration in response to nitrogen deposition. Nature Geoscience, 3, 315–322. 10.1038/ngeo844 [DOI] [Google Scholar]
- Janssens, I. A. , & Luyssaert, S. (2009). Carbon cycle: Nitrogen’s carbon bonus. Nature Geoscience, 2(5), 318–319. 10.1038/ngeo505 [DOI] [Google Scholar]
- Jiang, M. , Caldararu, S. , Zhang, H. , Fleischer, K. , Crous, K. Y. , Yang, J. , De Kauwe, M. G. , Ellsworth, D. S. , Reich, P. B. , Tissue, D. T. , Zaehle, S. , & Medlyn, B. E. (2020). Low phosphorus supply constrains plant responses to elevated CO2: A meta‐analysis. Global Change Biology, 26, 5856–5873. 10.1111/gcb.15277 [DOI] [PubMed] [Google Scholar]
- Jonard, M. , Fürst, A. , Verstraeten, A. , Thimonier, A. , Timmermann, V. , Potočić, N. , Waldner, P. , Benham, S. , Hansen, K. , Merilä, P. , Ponette, Q. , de la Cruz, A. C. , Roskams, P. , Nicolas, M. , Croisé, L. , Ingerslev, M. , Matteucci, G. , Decinti, B. , Bascietto, M. , & Rautio, P. (2015). Tree mineral nutrition is deteriorating in Europe. Global Change Biology, 21, 418–430. 10.1111/gcb.12657 [DOI] [PubMed] [Google Scholar]
- Körner, C. (2017). A matter of tree longevity. Science, 355, 130–131. 10.1126/science.aal2449 [DOI] [PubMed] [Google Scholar]
- Lamarque, J. F. , Dentener, F. , McConnell, J. , Ro, C. U. , Shaw, M. , Vet, R. , Bergmann, D. , Cameron‐Smith, P. , Dalsoren, S. , Doherty, R. , Faluvegi, G. , Ghan, S. J. , Josse, B. , Lee, Y. H. , Mackenzie, I. A. , Plummer, D. , Shindell, D. T. , Skeie, R. B. , Stevenson, D. S. , … Nolan, M. (2013). Multi‐model mean nitrogen and sulfur deposition from the atmospheric chemistry and climate model intercomparison project (ACCMIP): Evaluation of historical and projected future changes. Atmospheric Chemistry and Physics, 13, 7997–8018. 10.5194/acp-13-7997-2013 [DOI] [Google Scholar]
- LeBauer, D. S. , & Treseder, K. K. (2008). Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology, 89, 371–379. 10.1890/06-2057.1 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Niu, S. , & Yu, G. (2016). Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: A meta‐analysis. Global Change Biology, 22, 934–943. 10.1111/gcb.13125 [DOI] [PubMed] [Google Scholar]
- Liebig, J. (1840). Die organische Chemie in ihrer Anwendung auf Agricultur und Physiologie (Organic chemistry in its applications to agriculture and physiology). Friedrich Vieweg und Sohn Publ. Co. [Google Scholar]
- Liu, G. , Yan, G. , Chang, M. , Huang, B. , Sun, X. , Han, S. , Xing, Y. , & Wang, Q. (2021). Long‐term nitrogen addition further increased carbon sequestration in a boreal forest. European Journal of Forest Research, 140(5), 1113–1126. 10.1007/s10342-021-01386-9 [DOI] [Google Scholar]
- Liu, J. , You, L. , Amini, M. , Obersteiner, M. , Herrero, M. , Zehnder, A. J. B. , & Yang, H. (2010). A high‐resolution assessment on global nitrogen flows in cropland. Proceedings of the National Academy of Sciences, 107, 8035–8040. 10.1073/pnas.0913658107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , & Greaver, T. L. (2009). A review of nitrogen enrichment effects on three biogenic GHGs: the CO2 sink may be largely offset by stimulated N2O and CH4 emission. Ecology Letters, 12, 1103–1117. 10.1111/j.1461-0248.2009.01351.x [DOI] [PubMed] [Google Scholar]
- Liu, L. , & Greaver, T. L. (2010). A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecology Letters, 13, 819–828. 10.1111/j.1461-0248.2010.01482.x [DOI] [PubMed] [Google Scholar]
- Lovett, G. M. , Arthur, M. A. , Weathers, K. C. , Fitzhugh, R. D. , & Templer, P. H. (2013). Nitrogen addition increases carbon storage in soils, but not in trees, in an Eastern U.S. deciduous forest. Ecosystems, 16, 980–1001. 10.1007/s10021-013-9662-3 [DOI] [Google Scholar]
- Lovett, G. M. , & Goodale, C. L. (2011). A new conceptual model of nitrogen saturation based on experimental nitrogen addition to an oak forest. Ecosystems, 14, 615–631. 10.1007/s10021-011-9432-z [DOI] [Google Scholar]
- Lu, C. , & Tian, H. (2013). Net greenhouse gas balance in response to nitrogen enrichment: Perspectives from a coupled biogeochemical model. Global Change Biology, 19, 571–588. 10.1111/gcb.12049 [DOI] [PubMed] [Google Scholar]
- Lu, X. , Vitousek, P. M. , Mao, Q. , Gilliam, F. S. , Luo, Y. , Turner, B. L. , Zhou, G. , & Mo, J. (2021). Nitrogen deposition accelerates soil carbon sequestration in tropical forests. Proceedings of the National Academy of Sciences, 118, e2020790118. 10.1073/pnas.2020790118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyssaert, S. , Schulze, E.‐D. , Börner, A. , Knohl, A. , Hessenmöller, D. , Law, B. E. , Ciais, P. , & Grace, J. (2008). Old‐growth forests as global carbon sinks. Nature, 455, 213–215. 10.1038/nature07276 [DOI] [PubMed] [Google Scholar]
- Maaroufi, N. I. , Nordin, A. , Hasselquist, N. J. , Bach, L. H. , Palmqvist, K. , & Gundale, M. J. (2015). Anthropogenic nitrogen deposition enhances carbon sequestration in boreal soils. Global Change Biology, 21, 3169–3180. 10.1111/gcb.12904 [DOI] [PubMed] [Google Scholar]
- Maaroufi, N. I. , Nordin, A. , Palmqvist, K. , Hasselquist, N. J. , Forsmark, B. , Rosenstock, N. P. , Wallander, H. , & Gundale, M. J. (2019). Anthropogenic nitrogen enrichment enhances soil carbon accumulation by impacting saprotrophs rather than ectomycorrhizal fungal activity. Global Change Biology, 25, 2900–2914. 10.1111/gcb.14722 [DOI] [PubMed] [Google Scholar]
- Magnani, F. , Mencuccini, M. , Borghetti, M. , Berbigier, P. , Berninger, F. , Delzon, S. , Grelle, A. , Hari, P. , Jarvis, P. G. , Kolari, P. , Kowalski, A. S. , Lankreijer, H. , Law, B. E. , Lindroth, A. , Loustau, D. , Manca, G. , Moncrieff, J. B. , Rayment, M. , Tedeschi, V. , … Grace, J. (2007). The human footprint in the carbon cycle of temperate and boreal forests. Nature, 447, 848–850. 10.1038/nature05847 [DOI] [PubMed] [Google Scholar]
- Mainwaring, D. B. , Maguire, D. A. , & Perakis, S. S. (2014). Three‐year growth response of young Douglas‐fir to nitrogen, calcium, phosphorus, and blended fertilizers in Oregon and Washington. Forest Ecology and Management, 327, 178–188. 10.1016/j.foreco.2014.05.005 [DOI] [Google Scholar]
- McCarthy, H. R. , Oren, R. , Finzi, A. C. , & Johnsen, K. H. (2006). Canopy leaf area constrains [CO2]‐induced enhancement of productivity and partitioning among aboveground carbon pools. Proceedings of the National Academy of Sciences, 103, 19356–19361. 10.1073/pnas.0609448103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty, S. G. , Boggs, J. , Aber, J. D. , Rustad, L. , & Magill, A. (2005). Red spruce ecosystem level changes following 14 years of chronic N fertilization. Forest Ecology and Management, 219, 279–291. 10.1016/j.foreco.2005.09.004 [DOI] [Google Scholar]
- Menge, D. N. L. , Lichstein, J. W. , & Ángeles‐Pérez, G. (2014). Nitrogen fixation strategies can explain the latitudinal shift in nitrogen‐fixing tree abundance. Ecology, 95, 2236–2245. 10.1890/13-2124.1 [DOI] [PubMed] [Google Scholar]
- Nadelhoffer, K. J. , Emmett, B. A. , Gundersen, P. , Kjønaas, O. J. , Koopmans, C. J. , Schleppi, P. , Tietema, A. , & Wright, R. F. (1999). Nitrogen deposition makes a minor contribution to carbon sequestration in temperate forests. Nature, 398, 145–148. 10.1038/18205 [DOI] [Google Scholar]
- Nave, L. E. , Vance, E. D. , Swanston, C. W. , & Curtis, P. S. (2009). Impacts of elevated N inputs on north temperate forest soil C storage, C/N, and net N‐mineralization. Geoderma, 153, 231–240. 10.1016/j.geoderma.2009.08.012 [DOI] [Google Scholar]
- O’Sullivan, M. , Spracklen, D. V. , Batterman, S. A. , Arnold, S. R. , Gloor, M. , & Buermann, W. (2019). Have synergies between nitrogen deposition and atmospheric CO2 driven the recent enhancement of the terrestrial carbon sink? Global Biogeochemical Cycles, 33, 163–180. 10.1029/2018GB005922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, D. M. , Dinerstein, E. , Wikramanayake, E. D. , Burgess, N. D. , Powell, G. V. N. , Underwood, E. C. , D'amico, J. A. , Itoua, I. , Strand, H. E. , Morrison, J. C. , Loucks, C. J. , Allnutt, T. F. , Ricketts, T. H. , Kura, Y. , Lamoreux, J. F. , Wettengel, W. W. , Hedao, P. , & Kassem, K. R. (2001). Terrestrial ecoregions of the world: a new map of life on Earth. BioScience, 51, 933. [Google Scholar]
- Poulter, B. , Aragao, L. , Andela, N. , Bellassen, V. , Ciais, P. , Kato, T. , Lin, X. , Nachin, B. , Luyssaert, S. , Pederson, N. , Peylin, P. , Piao, S. , Pugh, T. , Saatchi, S. , Schepaschenko, D. , Schelhaas, M. , & Shivdenko, A. (2019). The global forest age dataset and its uncertainties (GFADv1.1). National Aeronautics and Space Administration. 10.1594/PANGAEA.897392 [DOI] [Google Scholar]
- Prietzel, J. , Falk, W. , Reger, B. , Uhl, E. , Pretzsch, H. , & Zimmermann, L. (2020). Half a century of Scots pine forest ecosystem monitoring reveals long‐term effects of atmospheric deposition and climate change. Global Change Biology, 26, 5796–5815. 10.1111/gcb.15265 [DOI] [PubMed] [Google Scholar]
- Pugh, T. A. M. , Lindeskog, M. , Smith, B. , Poulter, B. , Arneth, A. , Haverd, V. , & Calle, L. (2019). Role of forest regrowth in global carbon sink dynamics. Proceedings of the National Academy of Sciences, 116, 4382–4387. 10.1073/pnas.1810512116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, S. , Klimont, Z. , Smith, S. J. , Van Dingenen, R. , Dentener, F. , Bouwman, L. , Riahi, K. , Amann, M. , Bodirsky, B. L. , van Vuuren, D. P. , Aleluia Reis, L. , Calvin, K. , Drouet, L. , Fricko, O. , Fujimori, S. , Gernaat, D. , Havlik, P. , Harmsen, M. , Hasegawa, T. , … Tavoni, M. (2017). Future air pollution in the shared socio‐economic pathways. Global Environmental Change, 42, 346–358. 10.1016/j.gloenvcha.2016.05.012 [DOI] [Google Scholar]
- Reay, D. S. , Davidson, E. A. , Smith, K. A. , Smith, P. , Melillo, J. M. , Dentener, F. , & Crutzen, P. J. (2012). Global agriculture and nitrous oxide emissions. Nature Climate Change, 2, 410–416. 10.1038/nclimate1458 [DOI] [Google Scholar]
- Schimel, J. P. , & Weintraub, M. N. (2003). The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biology & Biochemistry, 35, 549–563. 10.1016/S0038-0717(03)00015-4 [DOI] [Google Scholar]
- Schindler, D. W. , & Bayley, S. E. (1993). The biosphere as an increasing sink for atmospheric carbon: Estimates from increased nitrogen depostion. Global Biogeochemical Cycles, 7, 717–733. 10.1029/93GB02562 [DOI] [Google Scholar]
- Schulte‐Uebbing, L. , & De Vries, W. (2018). Global‐scale impacts of nitrogen deposition on tree carbon sequestration in tropical, temperate, and boreal forests: A meta‐analysis. Global Change Biology, 24, e416–e431. 10.1111/gcb.13862 [DOI] [PubMed] [Google Scholar]
- Schwede, D. B. , Simpson, D. , Tan, J. , Fu, J. S. , Dentener, F. , Du, E. , & DeVries, W. (2018). Spatial variation of modelled total, dry and wet nitrogen deposition to forests at global scale. Environmental Pollution, 243, 1287–1301. 10.1016/j.envpol.2018.09.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton, J. O. , Noojipady, P. , Anand, A. , Song, X. P. , McMahon, S. , Huang, C. , Feng, M. , Channan, S. , & Townshend, J. R. (2015). A model for the propagation of uncertainty from continuous estimates of tree cover to categorical forest cover and change. Remote Sensing of Environment, 156, 418–425. 10.1016/j.rse.2014.08.038 [DOI] [Google Scholar]
- Sexton, J. O. , Noojipady, P. , Song, X. P. , Feng, M. , Song, D. X. , Kim, D. H. , Anand, A. , Huang, C. , Channan, S. , Pimm, S. L. , & Townshend, J. R. (2016). Conservation policy and the measurement of forests. Nature Climate Change, 6, 192–196. 10.1038/nclimate2816 [DOI] [Google Scholar]
- Siddique, I. , Vieira, I. C. G. , Schmidt, S. , Lamb, D. , Carvalho, C. J. R. , Figueiredo, R. D. O. , Blomberg, S. , & Davidson, E. A. (2010). Nitrogen and phosphorus additions negatively affect tree species diversity in tropical forest regrowth trajectories. Ecology, 91, 2121–2131. 10.1890/09-0636.1 [DOI] [PubMed] [Google Scholar]
- Simpson, D. , Benedictow, A. , Berge, H. , Bergström, R. , Emberson, L. D. , Fagerli, H. , Flechard, C. R. , Hayman, G. D. , Gauss, M. , Jonson, J. E. , Jenkin, M. E. , Nyúri, A. , Richter, C. , Semeena, V. S. , Tsyro, S. , Tuovinen, J. P. , Valdebenito, A. , & Wind, P. (2012). The EMEP MSC‐W chemical transport model – Technical description. Atmospheric Chemistry and Physics, 12, 7825–7865. 10.5194/acp-12-7825-2012 [DOI] [Google Scholar]
- Solberg, S. , Dobbertin, M. , Reinds, G. J. , Lange, H. , Andreassen, K. , Fernandez, P. G. , Hildingsson, A. , & De Vries, W. (2009). Analyses of the impact of changes in atmospheric deposition and climate on forest growth in European monitoring plots: A stand growth approach. Forest Ecology and Management, 258, 1735–1750. 10.1016/j.foreco.2008.09.057 [DOI] [Google Scholar]
- Stocker, T. F. , Qin, D. , Plattner, G.‐K. , Alexander, L. V. , Allen, S. K. , Bindoff, N. L. , Bréon, F.‐M. , Church, J. A. , Cubasch, U. , Emori, S. , Forster, P. , Friedlingstein, P. , Gillett, N. , Gregory, J. M. , Hartmann, D. L. , Jansen, E. , Kirtman, B. , Knutti, R. , Kumar, K. K. , Lemke, P. , Marotzke, J. , Masson‐Delmotte, V. , Meehl, G. A. , Mokhov, I. I. , Piao, S. , Ramaswamy, V. , Randall, D. , Rhein, M. , Rojas, M. , Sabine, C. , Shindell, D. , Talley, L. D. , Vaughan, D. G. , & Xie, S.‐P. (2013). Technical summary. In: Stocker T. F., Qin D., Plattner G.‐K., Tignor M., Allen S. K., Boschung J., Nauels A., Xia Y., Bex V., & Midgley P. M. (Eds.), Climate change 2013: The physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change (pp. 31–116). Cambridge University Press. 10.1017/cbo9781107415324.005 [DOI] [Google Scholar]
- Sun, Z. , Liu, L. , Peng, S. , Peñuelas, J. , Zeng, H. , & Piao, S. (2016). Age‐related modulation of the nitrogen resorption efficiency response to growth requirements and soil nitrogen availability in a temperate pine plantation. Ecosystems, 19, 698–709. 10.1007/s10021-016-9962-5 [DOI] [Google Scholar]
- Sutton, M. A. , Oenema, O. , Erisman, J. W. , Leip, A. , Van Grinsven, H. , & Winiwarter, W. (2011). Too much of a good thing. Nature, 472(7342), 159–161. 10.1038/472159a [DOI] [PubMed] [Google Scholar]
- Sutton, M. A. , Simpson, D. , Levy, P. E. , Smith, R. I. , Reis, S. , van Oijen, M. , & De Vries, W. (2008). Uncertainties in the relationship between atmospheric nitrogen deposition and forest carbon sequestration. Global Change Biology, 14, 2057–2063. 10.1111/j.1365-2486.2008.01636.x [DOI] [Google Scholar]
- Tan, J. , Fu, J. S. , Dentener, F. , Sun, J. , Emmons, L. , Tilmes, S. , Sudo, K. , Flemming, J. , Jonson, J. E. , Gravel, S. , Bian, H. , Davila, Y. , Henze, D. K. , Lund, M. T. , Kucsera, T. , Takemura, T. , & Keating, T. (2018). Multi‐model study of HTAP II on sulfur and nitrogen deposition. Atmospheric Chemistry and Physics, 18, 6847–6866. 10.5194/acp-18-6847-2018 [DOI] [Google Scholar]
- Tanner, E. V. J. , Vltousek, P. M. , & Cuevas, E. (1998). Experimental investigation of nutrient limitation of forest growth on wet tropical mountains. Ecology, 79, 10–22. [Google Scholar]
- Templer, P. H. , Lovett, G. M. , Weathers, K. C. , Findlay, S. E. , & Dawson, T. E. (2005). Influence of tree species on forest nitrogen retention in the Catskill Mountains, New York, USA. Ecosystems, 8, 1–16. 10.1007/s10021-004-0230-8 [DOI] [Google Scholar]
- Templer, P. H. , Mack, M. C. , Chapin, F. S. III , Christenson, L. M. , Compton, J. E. , Crook, H. D. , Currie, W. S. , Curtis, C. J. , Dail, D. B. , D’Antonio, C. M. , Emmett, B. A. , Epstein, H. E. , Goodale, C. L. , Gundersen, P. , Hobbie, S. E. , Holland, K. , Hooper, D. U. , Hungate, B. A. , Lamontagne, S. , … Zak, D. R. (2012). Sinks for nitrogen inputs in terrestrial ecosystems: a meta‐analysis of 15 N tracer field studies. Ecology, 93, 1816–1829. 10.1890/11-1146.1 [DOI] [PubMed] [Google Scholar]
- Thomas, R. Q. , Brookshire, E. N. J. , & Gerber, S. (2015). Nitrogen limitation on land: How can it occur in earth system models? Global Change Biology, 21, 1777–1793. 10.1111/gcb.12813 [DOI] [PubMed] [Google Scholar]
- Thomas, R. Q. , Canham, C. D. , Weathers, K. C. , & Goodale, C. L. (2010). Increased tree carbon storage in response to nitrogen deposition in the US. Nature Geoscience, 3, 13–17. 10.1038/ngeo721 [DOI] [Google Scholar]
- Thornton, P. E. , Lamarque, J.‐F. , Rosenbloom, N. A. , & Mahowald, N. M. (2007). Influence of carbon‐nitrogen cycle coupling on land model response to CO2 fertilization and climate variability. Global Biogeochemical Cycles, 21, GB4018. 10.1029/2006GB002868 [DOI] [Google Scholar]
- Tian, D. , & Niu, S. (2015). A global analysis of soil acidification caused by nitrogen addition. Environmental Research Letters, 10. 10.1088/1748-9326/10/2/024019 [DOI] [Google Scholar]
- Tian, D. , Wang, H. , Sun, J. , & Niu, S. (2016). Global evidence on nitrogen saturation of terrestrial ecosystem net primary productivity. Environmental Research Letters, 11. 10.1088/1748-9326/11/2/024012 [DOI] [Google Scholar]
- Tian, H. , Xu, R. , Canadell, J. G. , Thompson, R. L. , Winiwarter, W. , Suntharalingam, P. , Davidson, E. A. , Ciais, P. , Jackson, R. B. , Janssens‐maenhout, G. , Prather, M. J. , Regnier, P. , Pan, N. , Pan, S. , Peters, G. P. , Shi, H. , Tubiello, F. N. , Zaehle, S. , Zhou, F. , … Yao, Y. (2020). A comprehensive quantification of global nitrous oxide sources and sinks. Nature, 586, 10.1038/s41586-020-2780-0 [DOI] [PubMed] [Google Scholar]
- Tian, H. , Yang, J. , Xu, R. , Lu, C. , Canadell, J. G. , Davidson, E. A. , Jackson, R. B. , Arneth, A. , Chang, J. , Ciais, P. , Gerber, S. , Ito, A. , Joos, F. , Lienert, S. , Messina, P. , Olin, S. , Pan, S. , Peng, C. , Saikawa, E. , … Zhang, B. (2019). Global soil nitrous oxide emissions since the preindustrial era estimated by an ensemble of terrestrial biosphere models: Magnitude, attribution, and uncertainty. Global Change Biology, 25, 640–659. 10.1111/gcb.14514 [DOI] [PubMed] [Google Scholar]
- Tian, J. , Dungait, J. A. J. , Lu, X. , Yang, Y. , Hartley, I. P. , Zhang, W. , Mo, J. , Yu, G. , Zhou, J. , & Kuzyakov, Y. (2019). Long‐term nitrogen addition modifies microbial composition and functions for slow carbon cycling and increased sequestration in tropical forest soil. Global Change Biology, 25, 3267–3281. 10.1111/gcb.14750 [DOI] [PubMed] [Google Scholar]
- UNEP . (2013). Drawing down N2O to protect climate and the ozone layer. A UNEP synthesis report. [Google Scholar]
- Vadeboncoeur, M. A. (2010). Meta‐analysis of fertilization experiments indicates multiple limiting nutrients in northeastern deciduous forests. Canadian Journal of Forest Research, 40, 1766–1780. 10.1139/X10-127 [DOI] [Google Scholar]
- van der Salm, C. , de Vries, W. , Reinds, G. J. , & Dise, N. B. (2007). N leaching across European forests: Derivation and validation of empirical relationships using data from intensive monitoring plots. Forest Ecology and Management, 238, 81–91. 10.1016/j.foreco.2006.09.092 [DOI] [Google Scholar]
- Vicca, S. , Luyssaert, S. , Peñuelas, J. , Campioli, M. , Chapin, F. S. , Ciais, P. , Heinemeyer, A. , Högberg, P. , Kutsch, W. L. , Law, B. E. , Malhi, Y. , Papale, D. , Piao, S. L. , Reichstein, M. , Schulze, E. D. , & Janssens, I. A. (2012). Fertile forests produce biomass more efficiently. Ecology Letters, 15(6), 520–526. 10.1111/j.1461-0248.2012.01775.x [DOI] [PubMed] [Google Scholar]
- Viechtbauer, W. (2010). Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software, 36, 1–48. [Google Scholar]
- Viechtbauer, W. (2017). Package “metafor” Title Meta‐Analysis Package for R.
- Viechtbauer, W. , Antonio Lopez‐Lopez, J. , Sanchez‐Meca, J. , & Marin‐Martinez, F. (2015). A comparison of procedures to test for moderators in mixed‐effects meta‐regression models. Psychological Methods, 20(3), 360–374. [DOI] [PubMed] [Google Scholar]
- Vitousek, P. P. M. , Aber, J. J. D. , Howarth, R. W. , Likens, G. E. , Matson, P. A. , Schindler, D. W. , Schlesinger, W. H. , & Tilman, D. G. (1997). Human alteration of the global nitrogen cycle: Sources and consequences. Ecological Applications, 7, 737–750. [Google Scholar]
- Vitousek, P. M. , & Howarth, R. W. (1991). Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry, 13, 87–115. 10.1007/BF00002772 [DOI] [Google Scholar]
- Vitousek, P. M. , Menge, D. N. L. , Reed, S. C. , & Cleveland, C. C. (2013). Biological nitrogen fixation: Rates, patterns and ecological controls in terrestrial ecosystems. Philosophical Transactions of the Royal Society B: Biological Sciences, 368. 10.1098/rstb.2013.0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. , Goll, D. , Balkanski, Y. , Hauglustaine, D. , Boucher, O. , Ciais, P. , Janssens, I. , Penuelas, J. , Guenet, B. , Sardans, J. , Bopp, L. , Vuichard, N. , Zhou, F. , Li, B. , Piao, S. , Peng, S. , Huang, Y. , & Tao, S. (2017). Global forest carbon uptake due to nitrogen and phosphorus deposition from 1850 to 2100. Global Change Biology, 23, 4854–4872. 10.1111/gcb.13766 [DOI] [PubMed] [Google Scholar]
- Wang, Y. P. , & Houlton, B. Z. (2009). Nitrogen constraints on terrestrial carbon uptake: Implications for the global carbon‐climate feedback. Geophysical Research Letters, 36, 10.1029/2009GL041009 [DOI] [Google Scholar]
- Xia, J. , & Wan, S. (2008). Global response patterns of terrestrial plant species to nitrogen addition. New Phytologist, 179, 428–439. 10.1111/j.1469-8137.2008.02488.x [DOI] [PubMed] [Google Scholar]
- Yahdjian, L. , Gherardi, L. , & Sala, O. E. (2011). Nitrogen limitation in arid‐subhumid ecosystems: A meta‐analysis of fertilization studies. Journal of Arid Environments, 75, 675–680. 10.1016/j.jaridenv.2011.03.003 [DOI] [Google Scholar]
- Yu, Q. , Duan, L. , Yu, L. , Chen, X. , Si, G. , Ke, P. , Ye, Z. , & Mulder, J. (2018). Threshold and multiple indicators for nitrogen saturation in subtropical forests. Environmental Pollution, 241, 664–673. 10.1016/j.envpol.2018.06.001 [DOI] [PubMed] [Google Scholar]
- Zaehle, S. (2013). Terrestrial nitrogen‐carbon cycle interactions at the global scale. Philosophical Transactions of the Royal Society B: Biological Sciences, 368. 10.1098/rstb.2013.0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaehle, S. , Ciais, P. , Friend, A. D. , & Prieur, V. (2011). Carbon benefits of anthropogenic reactive nitrogen offset by nitrous oxide emissions. Nature Geoscience, 4, 601–605. 10.1038/ngeo1207 [DOI] [Google Scholar]
- Zhang, L. , Dawes, W. R. , & Walker, G. R. (2001). Response of mean annual evapotranspiration to vegetation changes at catchment scale. Water Resources Research, 37, 701–708. 10.1029/2000WR900325 [DOI] [Google Scholar]
- Zhang, L. , Jacob, D. J. , Knipping, E. M. , Kumar, N. , Munger, J. W. , Carouge, C. C. , Van Donkelaar, A. , Wang, Y. X. , & Chen, D. (2012). Nitrogen deposition to the United States: Distribution, sources, and processes. Atmospheric Chemistry and Physics, 12, 4539–4554. 10.5194/acp-12-4539-2012 [DOI] [Google Scholar]
- Zhang, X. , Davidson, E. A. , Mauzerall, D. L. , Searchinger, T. D. , Dumas, P. , & Shen, Y. (2015). Managing nitrogen for sustainable development. Nature, 528, 51–59. 10.1038/nature15743 [DOI] [PubMed] [Google Scholar]
- Zhou, L. , Zhou, X. , Zhang, B. , Lu, M. , Luo, Y. , Liu, L. , & Li, B. (2014). Different responses of soil respiration and its components to nitrogen addition among biomes: A meta‐analysis. Global Change Biology, 20, 2332–2343. 10.1111/gcb.12490 [DOI] [PubMed] [Google Scholar]
- Zhu, J. , He, N. , Zhang, J. , Wang, Q. , Zhao, N. , Jia, Y. , Ge, J. , & Yu, G. (2017). Estimation of carbon sequestration in China’s forests induced by atmospheric wet nitrogen deposition using the principles of ecological stoichiometry. Environmental Research Letters, 12, 10.1088/1748-9326/aa94a4 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Original geo‐tiffs of maps shown in figures 1, 2, 3, S3 and S5 can be downloaded from the data‐repository pangea at https://doi.org/10.1594/PANGAEA.940283. Additional data that support the findings of this study are available on request from the corresponding author.


