Abstract
Ammonia synthesis via the high‐temperature and high‐pressure Haber‐Bosch process is one of the most important chemical processes in the world. In spite of numerous attempts over the last 100 years, continuous Haber‐Bosch type ammonia synthesis at room‐temperature had not been possible, yet. We report the development of a mechanocatalytic system operating continuously at room‐temperature and at pressures down to 1 bar. With optimized experimental conditions, a cesium‐promoted iron catalyst was shown to produce ammonia at concentrations of more than 0.2 vol. % for over 50 hours.
Keywords: ammonia synthesis, catalysis, gas-phase reactions, mechanochemistry, room-temperature
Mechanocatalytic ammonia synthesis over Fe catalysts promoted by alkali metals, especially Rb or Cs, is possible under ball milling. The reaction proceeds at appreciable rates at room‐temperature and down to atmospheric pressure in a continuously operating system under gas flow.
Ammonia production via the Haber‐Bosch process is arguably one of the most important chemical processes and certainly one of the most influential inventions of the twentieth century. [1] Its significance was acknowledged by awarding three Nobel prizes up to now: in 1918 to Fritz Haber for the development of the synthesis from hydrogen and nitrogen, in 1931 to Carl Bosch for the invention of the high‐pressure process, and in 2007 to Gerhard Ertl for the understanding of the surface processes. [2] At an annual production of about 150 Mt, ammonia and derived compounds as fertilizers are decisive for the production of food for the world's population. [3] It is also discussed as a potential carbon free energy carrier. [4] Although optimized for more than 100 years, the Haber‐Bosch process still requires essentially the conditions applied at its invention: high temperatures of up to 500 °C and reaction pressures of up to 200 bar to achieve reasonable ammonia concentrations at sufficiently high rates. [5] The most significant innovation was the introduction of ruthenium‐based catalysts, showing high activity also at somewhat lower pressures. [6] Nonetheless, ammonia production still is dominated by the iron‐catalyzed Haber‐Bosch process, with the ruthenium catalyst used in some plants in a last reactor downstream of the main, iron‐catalyzed reaction. [5b]
The extreme importance and the challenging nature of ammonia synthesis have led to the study of alternative approaches for the synthesis under milder conditions over decades. Electrochemical synthesis attempts have become popular over the last years, but even with highly sophisticated systems only very small amounts of ammonia are produced, where it is sometime not even clear that the ammonia is indeed electrocatalytically formed. [7] Systems with very high reported ammonia yields were even shown to be irreproducible. [8] Photocatalysis suffers from the drawback of only producing trace amounts of ammonia. [9] Efforts in the area of molecular catalysts for nitrogen activation—as interesting as they are—can rather be considered as model studies for the natural enzymatic way of nitrogen fixation, but not as a competitive alternative method for ammonia synthesis, due to their prevalent use of special proton donating as well as reducing agents. [10] Promising results were achieved in the catalytic conversion of nitrogen in non‐thermal plasma processes, but plasma‐catalytic processes do not meet the required targets of ammonia output and energy input. [11]
In recent years, mechanochemistry has evolved as a promising alternative strategy for the activation and transformation of molecules. [12] Also heterogeneously catalyzed gas‐phase reactions can be mechanically activated, resulting in significant enhancements of activity. [13] Due to the high importance, mechanocatalytic ammonia synthesis has also been attempted, albeit in less than a handful of reports with partly unclear validity of the claims. The first claimed mechanochemical ammonia syntheses date back to 1961 and 1974. [14] However, details are only sketchily described, ammonia concentrations were very low in the ppm range, and formation of ammonia by alternative pathways (activation of impurities) could not be excluded, so that these reports are at least questionable. In 2020, Tricker et al. claimed a mechanocatalytic synthesis of ammonia over TiN in a common laboratory shaker mill. [15] However, during milling no ammonia was observed in the gas phase, and ammonia was determined by washing the milled solid with an aqueous solution, so that hydrolytic ammonia formation from a nitride seems possible, even probable. A true step towards mechanochemical ammonia formation was taken recently in a chemical looping process. [16] Here, ammonia was cyclically produced at low temperature by nitridation of iron and subsequent hydrogenation of the formed iron nitrides. While this is certainly a highly interesting finding, this approach does not realize a Haber‐Bosch‐like continuous catalytic ammonia synthesis at room‐temperature. In the following, a room‐temperature mechanocatalytic ammonia synthesis is described, although it should be noted that during collision of balls higher local temperatures could be possible.
Potential catalytic systems were initially screened in a batch approach, offering advantages for screening: different catalyst systems can be tested in a very short time and at room‐temperature/high pressure (>100 bar possible) conditions, which ideally meet the thermodynamic requirements for ammonia synthesis. In a typical experiment, the milling jar (see Figure S3 and S4) was loaded inside a glovebox with a certain amount of a potential catalyst material (typically 1.0 g or 2.0 g) and three 10 mm and three 15 mm steel balls, giving a ball‐to‐powder ratio of 54:1 or 27:1 respectively. The jar was subsequently pressurized with a mixture of H2:N2 (3:1) (the resulting pressure is given as the sum of the partial pressures of H2 and N2, since a background partial pressure of 1 bar of Ar from the glovebox atmosphere was always present) and mounted in a Fritsch Pulverisette 6 planetary ball mill. After each experiment, the gas phase was analyzed by infrared (IR) spectroscopy, and the powder was transferred into the glovebox for further analyses (for more detailed descriptions see S1–S5).
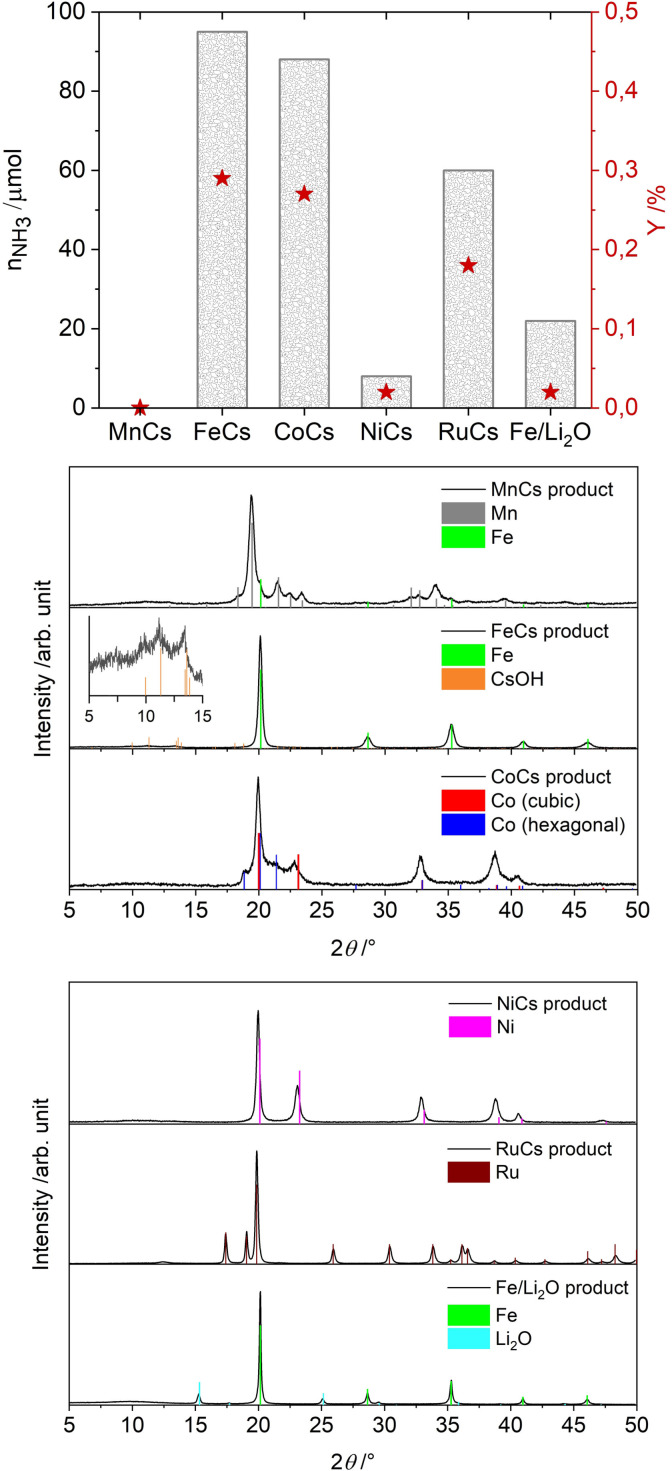
Initially, most of the attempts of a mechanochemical ammonia synthesis were not successful; not even trace amounts of ammonia were formed with any of the tested systems (selected examples, including metallic iron, and further description see Table S1). Ammonia was first unambiguously detected in the gas phase after milling mixtures of alkali metal ferrites AFeO2 (A=Li, K, Cs) with elemental iron. However, powder X‐ray diffraction (PXRD) patterns of the product from the Fe/CsFeO2 reaction very clearly showed the presence of CsOH⋅H2O, indicating an in situ reduction of the CsFeO2 under formation of water (see Figure S8–S12 for PXRDs of the products of the milling reactions and Table S2 for all details and results of the reactions with the Fe/AFeO2 system). A similar product pattern was also found for the reaction using KFeO2. In this system ammonia could be formed by hydrolysis of a nitride and reaction with the reaction water, that is, non‐catalytically. This, incidentally, also shows that great care needs to be taken in interpreting data on ammonia formation in complex systems. Nevertheless, these experiments proved the possibility of mechanochemically activating molecular nitrogen also in a mixture with hydrogen by the use of an iron‐based system. Further tests therefore focused on mixtures of iron with non‐reducible additives. In classical Haber‐Bosch a potassium promotor is present on the catalytically active ammonia iron, in the form of a [K+O] ad‐layer. [17] Also other alkali‐oxides were shown to be suitable as promotors. [18] Thus, commercially available lithium oxide was used as an additive. This indeed led to the formation of small amounts of ammonia (see Figure 1 and Table S3 for experimental conditions), however, only at high pressures and with preferentially high amounts of lithium oxide (170 bar, 1.0 g of Li2O and 1.0 g of Fe). For iron based catalysts the presence of oxygen reduces the promoting effect of the zero‐valent alkali (decreasing nitrogen sticking coefficient). [17b] However, in thermochemical ammonia synthesis operating at high temperatures, metallic alkali promotors cannot be used, due to the volatility of the metals, but the low temperature mechanochemical approach opens this possibility. Addition of lithium to iron resulted in no more than traces of ammonia (see Table S2, entries 33 and 34). However, cesium is a suitable promotor for ammonia synthesis. This might be attributed to its higher electropositivity, potentially also to thermodynamic instability of its bulk nitride, which, in the case of lithium with its very stable nitride, might serve as a sink. [19] The addition of cesium to iron (2.2 mol % Cs, a total of 2.0 g catalyst) enabled the formation of ammonia not only at 150 bar (24 h of milling, n =0.378 mmol, Y=0.38 %, with n as the total amount of ammonia produced and the yield Y based on nitrogen), but also at 50 bar (same conditions, n =0.097 mmol, Y=0.29 %). As can be seen in the exemplary IR‐spectrum of the gas phase from the 150 bar reaction (see Figure S13), also methane can be detected, which results from mechanochemical hydrogenation of carbon‐containing materials (the jar itself, steel balls, rubber sealing). Comparative experiments with sodium and potassium yielded rather low amounts of ammonia (see Table S2, entries 35–37), whereas rubidium acted as a potent promotor as well, however, with somewhat reduced activity compared to cesium (see Table S2, entry 38). No promoting effect was observed for the alkaline‐earth metals calcium or barium under the conditions tested herein (see Table S3, entry 55 and 56). We tentatively attribute the promoting effect to the high electron donating capability of the heavy alkali metals, similar to electron‐donating effects observed in alternative systems. [20] Promotion of other metals than iron by cesium was also studied, but the iron‐based system was shown to be the most active one, as can be seen in Figure 1. Incidentally the most active metals are similar to those found in classical thermochemical systems. [21] The PXRD pattern of the cesium promoted iron catalyst after reaction shows very weak reflections of CsOH. This suggests the presence of water at some stage during the experiment, but it will be shown, that the formation of ammonia is not caused by hydrolysis.
Figure 1.
Results for selected batch systems. (top) Molar amount of ammonia in the gas phase and yields based on nitrogen for metals promoted by cesium or lithium oxide. Reaction conditions were: 48 repetitions of 30 min each with a pause of 5 min in between each run, 500 revolutions‐per‐minute (rpm), three 10 mm and three 15 mm steel balls were used, Fritsch Pulverisette 6. The jar was loaded with 2.0 g of material. For the systems promoted by cesium, 1.9 g of d‐metal and 0.1 g of cesium were used and the reactions were conducted at 50 bar (H2:N2, 3:1). For the reaction with iron and lithium oxide, 1.0 g of each compound was used and the milling was performed at 170 bar (H2:N2, 3:1). (middle and bottom) PXRDs of the resulting products after milling. For the MnCs reaction, additional Fe reflections from abrasion are visible and for the FeCs reaction, extremely weak reflections of CsOH were detected. For the NiCs product, the reflections of Ni are slightly shifted, possibly due to abrasion of iron and subsequent alloying.
The water could result from reduction of surface oxide species present on the iron powder (as found by X‐ray photoelectron spectroscopy (XPS), see Figure S14, whereas the bulk is pure metallic iron, see Figure S15 for PXRD), or present on the jar and the milling balls. Results proved to be well reproducible. In ten repeated runs of 3 h and 6 h in a long‐term experiment, in which just the gas phase was exchanged for fresh educts, deviations were typically below 10 % (see Table S4).
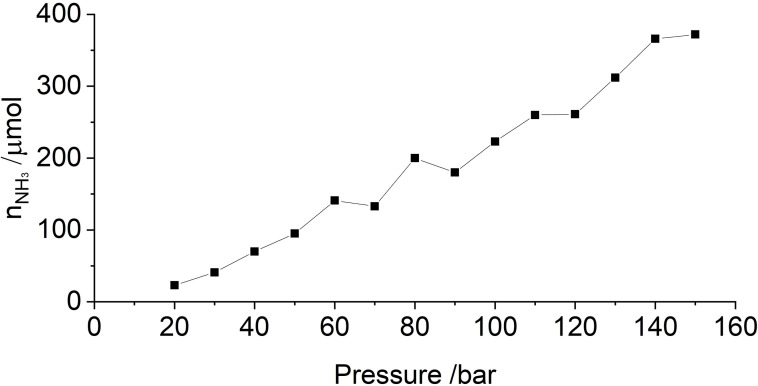
Ammonia formation in the Haber‐Bosch process is known to be favored by increased partial pressure of the reagents. Thus, reactions in the batch reactor were carried out at different pressure (see Table S2, entries 39–52). As expected, the ammonia amount increases with increased pressure, which, however, is not due to thermodynamic preference of product formation at elevated pressure (at room‐temperature, the system is far from equilibrium), but a result of positive reaction orders in hydrogen and/or nitrogen (see Figure 2).
Figure 2.
Pressure‐dependency of the mechanocatalytic ammonia formation in batch. In each experiment, a fresh FeCs mixture was used, same milling conditions like in Figure 1 (top), total milling time for each experiment was 24 h.
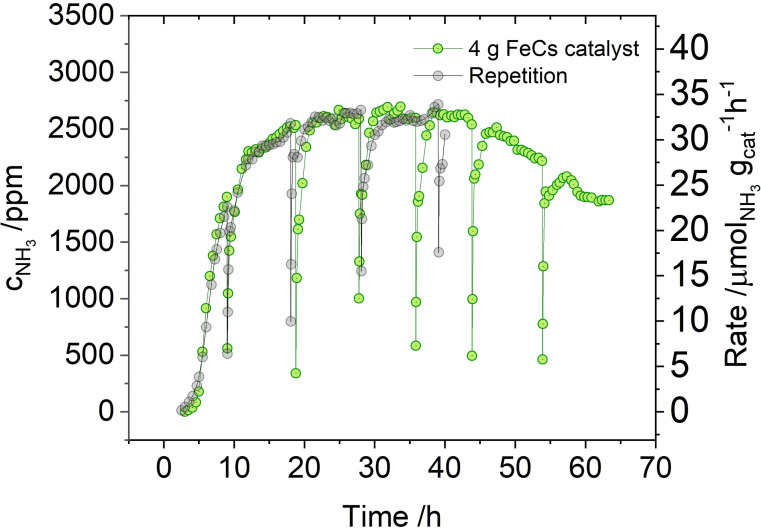
While the batch process is well suited for screening of potential catalysts, industrial ammonia synthesis, like most large‐scale processes, is continuous. Also for studying the influence of reaction parameters, a flow‐process is much better suited: temperature, pressure and residence time can be adjusted more easily, but especially the formation of the product can be monitored in a temporally resolved manner. So far, mechanocatalytic gas‐phase reactions under flow conditions at elevated pressure have not been reported. For this purpose, special milling jars were designed, that resemble small autoclaves (see Figure S6 and S7). For these experiments, a Retsch MM400 shaker mill was used. Figure 3 shows the result of a long‐term catalytic run with 4.0 g of the catalyst (details on setup, experimental procedure and results for other runs can be found in the SI in Figure S5, supplementary text S2 and S5 and Table S5). At 20 bar pressure, ammonia is produced for over 60 h with a regime of around 20 h at a stable value in the range of 0.26 vol. %, before the concentration slightly decreases. It was possible to increase the maximum concentration by a factor of 1.6 to around 0.42 vol. % by doubling the average residence time of the gas mixture inside the milling jar (see Figure S16). Ammonia is also formed at atmospheric pressure, albeit with significantly lower product concentration (around 1000 ppm, see Figure S17). Thus, mechanocatalytic ammonia synthesis is possible at room‐temperature (global vessel temperature was kept at 20 °C by a cooling system) and atmospheric pressure in a continuous fashion. Ammonia formation in this and other experiments starts after a short induction period of 2–3 h. Figure S18 shows an exemplary infrared‐spectrum of the product gas after 18 h, showing also trace amounts of methane (explanation see above). A total of 6.5 mmol ammonia was produced in this experiment (for comparison n Fe=68.04 mmol, n Cs=1.50 mmol).
Figure 3.
Development of ammonia production during continuous mechanocatalysis with 4.0 g of the catalyst (see Table S5 entry 68 and 80). The experiments were performed using a Retsch MM400 at a frequency of 25 Hz and with two 15 mm steel balls. The temperature was kept at 20 °C by external cooling. The jar was fed with 20 mL min−1 (STP) of a H2:N2 (3:1) mixture at 20 bar. Due to safety reasons, the milling process had to be paused after every day; which is the reason for the sharp spikes, for further explanations see S2. For the repetition run (Table S5 entry 80) the mill stopped working after about 40 h and had to undergo maintenance. For the time of the experiment, the repetition has proven very good reproducibility of the mechanocatalytic ammonia formation.
This high amount proves, that ammonia is formed catalytically and not from hydrolysis due to water impurities formed from reduction of potential oxide surface species. The observed slight deactivation at extended milling times could so far not be suppressed completely. Potential causes for this could be slowly proceeding reaction of the active cesium species towards CsOH by impurities, as indicated by the presence of extremely weak reflections of CsOH in the PXRD of the product after milling (see Figure S19) as well as oxidized cesium species on the surface, as indicated by XPS investigation (see Figure S20 and S14 for comparison with the iron starting material). Elemental analysis also indicated incorporation of nitrogen into the catalyst (see Table S6).
Test experiments, during which the mill was stopped at certain stages of the reaction showed that ammonia formation does not proceed further and quickly declines without the influence of ball milling (see Figure S21 and S22). Furthermore, other additives besides cesium were tested (see Table S5). Lithium oxide, which showed a slight promoting effect in the batch reaction, did not yield ammonia in a continuous‐flow experiment when combined with iron, which might be explained by the low pressure compared to the experiments under batch conditions. Also both the addition of h‐BN (to prevent cold welding of pure iron) or Li3N did not result in ammonia formation. Since hydrolysis of Li3N readily results in formation of ammonia, this experiment again proves that no hydrolysis is involved in the reactions with the FeCs mechanocatalyst. The only other active compound when mixed with iron was CsH, whereas an analogous experiment with NaH did not result in ammonia production (see Figure S23 and Table S5, entries 78 and 79). CsOH⋅x H2O (obtained from thermal treatment of CsOH⋅H2O, which lead to partial dehydration, see Figure S1) in combination with iron, produced almost no ammonia during milling (see Figure S24 and Table S5, entry 77).
The low amounts of ammonia still produced are most likely caused by the presence of the crystal water and subsequent hydrolysis of nitride species, again showing that through this route at best only small amounts of ammonia can be obtained.
To finally prove that ammonia formation results from activation of nitrogen and not impurities (e.g. dissolved N in the stainless steel), two control experiments were performed: A batch reaction using 15N2 produced exclusively 15NH3 (see Table S3, entry 64 and Figure S25) and a continuous‐flow experiment under exclusion of nitrogen gas resulted only in insignificant values of ≤10 ppm of ammonia (see Table S5, entry 81 and Figure S26). Significant amounts of ammonia in this experiment could only be produced after again dosing nitrogen gas.
In summary, we have developed a system for the mechanocatalytic synthesis of ammonia from its elements working at room‐temperature and down to atmospheric pressure.
While several systems were identified that led to ammonia formation, the most promising systems consists of a mixture of iron with small amounts of elemental cesium. This catalytic system operates both under batch conditions and in a continuous process for more than 60 h, leading to continuous formation of ammonia at values up to 0.26 vol. %. This study demonstrates the continuous catalytic synthesis of ammonia from the elements in a manner probably similar to the Haber‐Bosch process, but at ambient temperature and pressure conditions due to the influence of mechanical forces, something which has remained elusive in spite of more than hundred years of efforts via different approaches.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
Jan Ternieden and PD Dr. Claudia Weidenthaler are gratefully acknowledged for the measurement and analysis of powder x‐ray diffraction data. We thank Sebastian Leiting and PD Dr. Claudia Weidenthaler for the measurement and interpretation of XPS data. Dr. Alexander Bodach and Frederik Winkelmann are acknowledged for providing lithium sand. We thank Mikrolab Kolbe for the elemental analyses. We gratefully acknowledge the fine mechanics department at the Max‐Planck‐Institut für Kohlenforschung led by Wolfgang Kersten for conception and construction of the milling equipment. Max‐Planck‐Institut für Kohlenforschung is acknowledged for financial support. Open Access funding enabled and organized by Projekt DEAL.
S. Reichle, M. Felderhoff, F. Schüth, Angew. Chem. Int. Ed. 2021, 60, 26385.
References
- 1. Smil V., Nature 1999, 400, 415. [Google Scholar]
- 2. Ertl G., Angew. Chem. Int. Ed. 2008, 47, 3524–3535; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 3578–3590. [Google Scholar]
- 3.
- 3a.U. S. Geological Survey, 2021;
- 3b. Galloway J. N., Townsend A. R., Erisman J. W., Bekunda M., Cai Z. C., Freney J. R., Martinelli L. A., Seitzinger S. P., Sutton M. A., Science 2008, 320, 889–892. [DOI] [PubMed] [Google Scholar]
- 4.
- 4a. Klerke A., Christensen C. H., Norskov J. K., Vegge T., J. Mater. Chem. 2008, 18, 2304–2310; [Google Scholar]
- 4b. Schüth F., Palkovits R., Schlögl R., Su D. S., Energy Environ. Sci. 2012, 5, 6278–6289; [Google Scholar]
- 4c. Liu T. Y., Sartori S., MRS Bull. 2020, 45, 698–699. [Google Scholar]
- 5.
- 5a. Schlögl R., Angew. Chem. Int. Ed. 2003, 42, 2004–2008; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2003, 115, 2050–2055; [Google Scholar]
- 5b. Smith C., Hill A. K., Torrente-Murciano L., Energy Environ. Sci. 2020, 13, 331–344; [Google Scholar]
- 5c. Jennings J. R., Catalytic ammonia synthesis: fundamentals and practice, Springer Science+Business Media, New York, 1991. [Google Scholar]
- 6. Brown D. E., Edmonds T., Joyner R. W., McCarroll J. J., Tennison S. R., Catal. Lett. 2014, 144, 545–552. [Google Scholar]
- 7.
- 7a. Andersen S. Z., Colic V., Yang S., Schwalbe J. A., Nielander A. C., McEnaney J. M., Enemark-Rasmussen K., Baker J. G., Singh A. R., Rohr B. A., Statt M. J., Blair S. J., Mezzavilla S., Kibsgaard J., Vesborg P. C. K., Cargnello M., Bent S. F., Jaramillo T. F., Stephens I. E. L., Norskov J. K., Chorkendorff I., Nature 2019, 570, 504–508; [DOI] [PubMed] [Google Scholar]
- 7b. Kibsgaard J., Norskov J. K., Chorkendorff I., ACS Energy Lett. 2019, 4, 2986–2988. [Google Scholar]
- 8. Choi J., Du H. L., Nguyen C. K., Suryanto B. H. R., Simonov A. N., MacFarlane D. R., ACS Energy Lett. 2020, 5, 2095–2097. [Google Scholar]
- 9.
- 9a. Zhu D., Zhang L. H., Ruther R. E., Hamers R. J., Nat. Mater. 2013, 12, 836–841; [DOI] [PubMed] [Google Scholar]
- 9b. Ali M., Zhou F. L., Chen K., Kotzur C., Xiao C. L., Bourgeois L., Zhang X. Y., MacFarlane D. R., Nat. Commun. 2016, 7, 11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.
- 10a. Laplaza C. E., Cummins C. C., Science 1995, 268, 861–863; [DOI] [PubMed] [Google Scholar]
- 10b. Yandulov D. V., Schrock R. R., Science 2003, 301, 76–78; [DOI] [PubMed] [Google Scholar]
- 10c. Ashida Y., Arashiba K., Nakajima K., Nishibayashi Y., Nature 2019, 568, 536–540. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a. Iwamoto M., Akiyama M., Aihara K., Deguchi T., ACS Catal. 2017, 7, 6924–6929; [Google Scholar]
- 11b. Rouwenhorst K. H. R., Engelmann Y., van't Veer K., Postma R. S., Bogaerts A., Lefferts L., Green Chem. 2020, 22, 6258–6287. [Google Scholar]
- 12.
- 12a. James S. L., Adams C. J., Bolm C., Braga D., Collier P., Friščić T., Grepioni F., Harris K. D. M., Hyett G., Jones W., Krebs A., Mack J., Maini L., Orpen A. G., Parkin I. P., Shearouse W. C., Steed J. W., Waddell D. C., Chem. Soc. Rev. 2012, 41, 413–447; [DOI] [PubMed] [Google Scholar]
- 12b. Friščić T., Mottillo C., Titi H. M., Angew. Chem. Int. Ed. 2020, 59, 1018–1029; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 1030–1041. [Google Scholar]
- 13.
- 13a. Mori S., Xu W. C., Ishidzuki T., Ogasawara N., Imai J., Kobayashi K., Appl. Catal. A 1996, 137, 255–268; [Google Scholar]
- 13b. Eckert R., Felderhoff M., Schüth F., Angew. Chem. Int. Ed. 2017, 56, 2445–2448; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 2485–2488; [Google Scholar]
- 13c. Bilke M., Losch P., Vozniuk O., Bodach A., Schüth F., J. Am. Chem. Soc. 2019, 141, 11212–11218. [DOI] [PubMed] [Google Scholar]
- 14.
- 14a. Heinicke G., Meyer K., Senzky U., Z. Anorg. Allg. Chem. 1961, 312, 180–185; [Google Scholar]
- 14b. Thiejen P. A., Heinicke G., Bock N., Z. Chem. 1974, 14, 76. [Google Scholar]
- 15. Tricker A. W., Hebisch K. L., Buchmann M., Liu Y. H., Rose M., Stavitski E., Medford A. J., Hatzell M. C., Sievers C., ACS Energy Lett. 2020, 5, 3362–3367. [Google Scholar]
- 16. Han G. F., Li F., Chen Z. W., Coppex C., Kim S. J., Noh H. J., Fu Z. P., Lu Y. L., Singh C. V., Siahrostami S., Jiang Q., Baek J. B., Nat. Nanotechnol. 2021, 16, 325–330. [DOI] [PubMed] [Google Scholar]
- 17.
- 17a. Pirug G., Broden G., Bonzel H. P., Surf. Sci. 1980, 94, 323–338; [Google Scholar]
- 17b. Paál Z., Ertl G., Lee S. B., Appl. Surf. Sci. 1981, 8, 231–249. [Google Scholar]
- 18.
- 18a. Bosch H., Vanommen J. G., Gellings P. J., Appl. Catal. 1985, 18, 405–408; [Google Scholar]
- 18b. Arabczyk W., Jasinska I., Jedrzejewski R., Catal. Commun. 2009, 10, 1821–1823. [Google Scholar]
- 19. Gregory D. H., Coord. Chem. Rev. 2001, 215, 301–345. [Google Scholar]
- 20.
- 20a. Kitano M., Inoue Y., Yamazaki Y., Hayashi F., Kanbara S., Matsuishi S., Yokoyama T., Kim S. W., Hara M., Hosono H., Nat. Chem. 2012, 4, 934–940; [DOI] [PubMed] [Google Scholar]
- 20b. Hattori M., Iijima S., Nakao T., Hosono H., Hara M., Nat. Commun. 2020, 11, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jacobsen C. J. H., Dahl S., Clausen B. S., Bahn S., Logadottir A., Norskov J. K., J. Am. Chem. Soc. 2001, 123, 8404–8405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information