CONFLICT OF INTEREST
J.B.W. provides medical advisory consulting for Allakos, Inc. regarding the use of lirentelimab with eosinophilic gastrointestinal diseases. J.B.W. is not employed by Allakos, Inc. and does not hold stock or stock options, and did not receive consulting fees from Allakos, Inc. for this manuscript; M.B., A.W., A.P.K., and B.A.Y. are employees of and hold stock or stock options in Allakos, Inc.
To the Editor,
As of July 2021, over 30‐million Americans have recovered from documented COVID‐19 infection, and prevalence studies suggest up to twice as many may be undocumented. 1 For the survivors of COVID‐19, chronic morbidity is common, with reports of fatigue, brain fog, body aches, and loss of smell lasting for months following acute symptoms. 2 These persistent symptoms have been referred to as post‐acute sequelae of COVID‐19 (PASC), post‐acute COVID‐19 syndrome, long‐COVID, and “long‐hauler syndrome.” The etiopathogenesis of PASC remains unclear, but inflammation may play a role 3 , 4 along with metabolic disturbances 5 and autoantibodies. 6
Recently, our group and others identified evidence of mast cell (MC) activation in sera and lung tissue in patients with acute COVID‐19 infection. 7 Whether this activation is persistent and contributes to the morbidity associated with PASC is unknown. To evaluate systemic inflammation and MC activation in PASC, we obtained serum from 13 adults with symptomatic PASC and a history of positive RT‐PCR for SARS‐CoV‐2 between 39 and 305 days prior to collection (Table 1). The most reported symptoms among PASC patients were fatigue (84%), body aches (46%), loss of taste/smell (46%), anxiety (31%), shortness of breath (31%), brain fog (23%), and headaches (23%). To control for inflammatory mediators attributable to acute SARS‐CoV‐2 infection, we obtained serum from age‐matched post‐acute asymptomatic COVID‐19 (PAAC) patients with a history of positive RT‐PCR for SARS‐CoV‐2 between 22 and 322 days prior to collection, as well as healthy controls (Table 1). We evaluated 11 mediators previously implicated in COVID‐19 systemic inflammation. 8 Among the screened mediators, IL‐6 and CXCL1 were significantly elevated in PASC sera compared to controls, whereas no significant difference was detected in PAAC sera relative to controls (Figure 1A). Notably, IL‐6 levels were also significantly higher in sera from the PASC cohort compared to PAAC (Figure 1A). The following mediators showed no difference between PASC, PAAC, and control: IL‐8, TNF, CCL2, CCL3, IL‐17A, IL‐33, and VEGF (Figure S1A–B). Next, we assessed levels of MC‐derived proteases to evaluate MC activation. Active tryptase levels were significantly elevated in PASC sera compared to PAAC and heathy controls, highly suggestive of systemic MC activation (Figure 1B). Carboxypeptidase A3 (CPA3) levels were significantly elevated in PASC, but not PAAC sera compared to healthy controls (Figure 1B). In contrast, chymase levels were not significantly different across these populations (Figure S1C).
TABLE 1.
Patient Characteristics
| Patient characteristics |
PASC a n = 13 |
PAAC b n = 13 |
Healthy controls n = 20 |
P‐value c |
|---|---|---|---|---|
| Age, median (range) | 49 (24–73) | 48 (22–59) | 36 (18–51) | 0.5670 |
| Female sex, n (%) | 12 (92%) | 11 (85%) | 6 (30%) | 1.0000 |
| Race | ||||
| Black, n (%) | 1 (8%) | 0 (0%) | 0 (0%) | 1.0000 |
| Caucasian/non‐Hispanic, n (%) | 12 (92%) | 11 (85%) | 20 (100%) | 1.0000 |
| Hispanic, n (%) | 0 (0%) | 2 (15%) | 0 (0%) | 1.0000 |
| SARS‐CoV‐2 ELISA IgG, median (range) | 4.3 (0.3–11.7) | 3.8 (1.2–6.7) | N/A d | 0.2434 |
| Days between positive PCR test and serum sample collection, median (range) | 62 (39–305) | 34 (22–322) | N/A d | 0.1851 |
| Self‐reported long‐term symptom, n (%) | 13 (100%) | 0 (0%) | N/A d | N/A |
| Fatigue | 11 (85%) | 0 (0%) | ||
| Body aches | 6 (46%) | 0 (0%) | ||
| Change or loss in taste/smell | 6 (46%) | 0 (0%) | ||
| Anxiety | 4 (31%) | 0 (0%) | ||
| Shortness of breath | 4 (31%) | 0 (0%) | ||
| Brain fog | 3 (23%) | 0 (0%) | ||
| Headaches | 3 (23%) | 0 (0%) | ||
| Sore throat | 2 (15%) | 0 (0%) | ||
| Tachycardia | 2 (15%) | 0 (0%) | ||
| Anemic | 1 (8%) | 0 (0%) | ||
| Dyspnea | 1 (8%) | 0 (0%) | ||
| Joint pain | 1 (8%) | 0 (0%) | ||
| Insomnia | 1 (8%) | 0 (0%) | ||
Abbreviations: PAAC, post‐acute asymptomatic of COVID‐19; PASC, post‐acute sequelae of COVID‐19.
PASC patients were defined as SARS‐CoV‐2 negative with persistent symptoms for at least ~1 month after confirmed positive infection.
PAAC patients were defined as SARS‐CoV‐2 negative with no symptoms after confirmed positive infection.
Unpaired two‐tailed t test between PASC vs PAAC performed on numerical values; Fisher's exact test performed on proportions.
Data are not collected as part of study.
FIGURE 1.
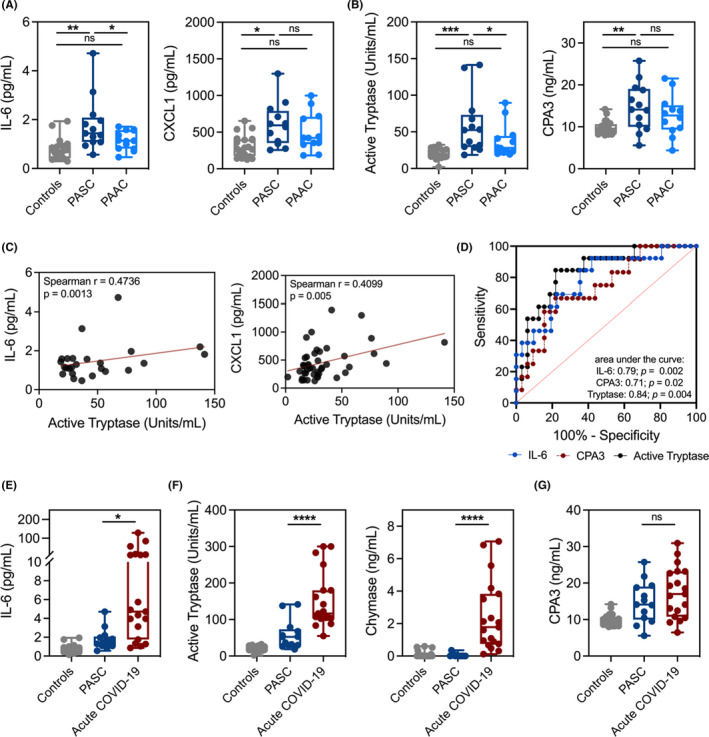
PASC patient sera display a distinct profile of elevated inflammatory cytokines and mast cell‐derived proteases. (A–B) Cytokines, chemokines, or mast cell‐derived proteases in sera from symptomatic PASC (dark blue; n = 13), PAAC (light blue; n = 13), or control (gray; n = 19–20) groups. (C) Spearman correlations for levels of active tryptase, IL‐6 and CXCL1 from panels A, B. (D) ROC curves for active tryptase, CPA3, and IL‐6 using PASC versus PAAC and controls. (E–G) Levels of cytokines and mast cell‐derived proteases in sera from PASC patients (light blue; n = 13), acute COVID‐19 patients (red; n = 19), or controls (gray; n = 19–20). *P < 0.05;**P < 0.01;***P < 0.001;****P < 0.0001; ns=not significant; PAAC, post‐acute asymptomatic COVID‐19; PASC, post‐acute sequelae of COVID‐19
To determine whether the MC activation was associated with elevated IL‐6 and CXCL1, we performed correlation analyses using sera levels of MC proteases from PASC, PAAC, and controls. Active tryptase levels showed a weak but significant correlation with both IL‐6 and CXCL1 levels, whereas CPA3 levels demonstrated modest association (Figure 1C, Figure S1D). To assess the utility of serum IL‐6 along with MC proteases as a diagnostic test for PASC, we assessed receiver operating characteristics for the outcome of PASC vs. PAAC + control. Notably, the active tryptase level was superior with an area under the curve of 0.84 (Figure 1D).
To further characterize the inflammatory profile identified in PASC patient sera, we compared cytokine, chemokine, and MC protease levels against sera from a previously published cohort of acute COVID‐19 patients. 7 Sera from symptomatic PASC patients displayed significantly reduced inflammatory cytokines and chemokines compared to sera from acute COVID‐19 patients (Figure 1E, Figure S2). Levels of active tryptase and chymase were also significantly lower in PASC patients compared to acute COVID‐19 patients (Figure 1F). Notably, CPA3 levels in the serum were not significantly different between PASC and acute COVID‐19 patients (Figure 1G), suggesting CPA3 levels may remain similarly elevated post‐acute infection.
Taken together, our findings support a potential role for immune dysfunction associated with MC activation in a subgroup of patients with PASC. Findings from this study also suggest that MCs are differentially activated in acute SARS‐CoV‐2 infection compared to PASC. Additional studies are needed to determine if these differences are based on distinct populations of activated MCs or local environmental cues. Interestingly, IL‐6 has been shown to increase MC proliferation and induce a more reactive phenotype 9 providing a possible link between elevated IL‐6 levels and MC activation in PASC. While it remains unclear if MC activation is causative in PASC or simply a consequence, larger longitudinal studies to validate our findings and assess the natural history are critical. Additional limitations of our study include using unmatched healthy controls and lack of medical history from PASC and PAAC patients. Importantly, our findings highlight MCs as potential therapeutic targets for patients with PASC, which could be targeted with agents that (1) reduce MC‐derived mediators, 10 (2) engage inhibitory receptors, 11 or (3) attenuate inflammation. 12
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
Professional writing and editing support was provided by Jocelyn Hybiske, PhD, an independent consultant, funded by Allakos. We thank Drs. Julia Schanin and Wouter Korver for their critical review.
REFERENCES
- 1. Demonbreun AR, McDade TW, Pesce L, et al. Patterns and persistence of SARS‐CoV‐2 IgG antibodies in Chicago to monitor COVID‐19 exposure. JCI Insight. 2021;6(9). 10.1172/jci.insight.146148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626‐631. 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doykov I, Hallqvist J, Gilmour KC, Grandjean L, Mills K, Heywood WE. ‘The long tail of Covid‐19’ ‐ The detection of a prolonged inflammatory response after a SARS‐CoV‐2 infection in asymptomatic and mildly affected patients. F1000Research. 2020;9:1349. 10.12688/f1000research.27287.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patterson BK, Guevara‐Coto J, Yogendra R, et al. Immune‐based prediction of COVID‐19 severity and chronicity decoded using machine learning. Front Immunol. 2021;12:700782. 10.3389/fimmu.2021.700782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holmes E, Wist J, Masuda R, et al. Incomplete systemic recovery and metabolic phenoreversion in post‐acute‐phase nonhospitalized COVID‐19 patients: implications for assessment of post‐acute COVID‐19 syndrome. J Proteome Res. 2021;20(6):3315‐3329. 10.1021/acs.jproteome.1c00224 [DOI] [PubMed] [Google Scholar]
- 6. Wallukat G, Hohberger B, Wenzel K, et al. Functional autoantibodies against G‐protein coupled receptors in patients with persistent Long‐COVID‐19 symptoms. J Transl Autoimmun. 2021;4:100100. 10.1016/j.jtauto.2021.100100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gebremeskel S, Schanin J, Coyle KM, et al. Mast cell and eosinophil activation are associated with COVID‐19 and TLR‐mediated viral inflammation: implications for an anti‐Siglec‐8 antibody. Front Immunol. 2021;12:650331. 10.3389/fimmu.2021.650331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang L, Xie X, Tu Z, Fu J, Xu D, Zhou Y. The signal pathways and treatment of cytokine storm in COVID‐19. Signal Transduct Target Ther. 2021;6(1):255. 10.1038/s41392-021-00679-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Desai A, Jung MY, Olivera A, et al. IL‐6 promotes an increase in human mast cell numbers and reactivity through suppression of suppressor of cytokine signaling 3. J Allergy Clin Immunol. 2016;137(6):1863–1871 e6. 10.1016/j.jaci.2015.09.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aigner L, Pietrantonio F, Bessa de Sousa DM, et al. The leukotriene receptor antagonist montelukast as a potential COVID‐19 therapeutic. Front Mol Biosci. 2020;7:610132. 10.3389/fmolb.2020.610132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schanin J, Gebremeskel S, Korver W, et al. A monoclonal antibody to Siglec‐8 suppresses non‐allergic airway inflammation and inhibits IgE‐independent mast cell activation. Mucosal Immunol. 2020;14:366–376. 10.1038/s41385-020-00336-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peter AE, Sandeep BV, Rao BG, Kalpana VL. Calming the storm: natural immunosuppressants as adjuvants to target the cytokine storm in COVID‐19. Front Pharmacol. 2020;11:583777. 10.3389/fphar.2020.583777 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


