Abstract
Topical metronidazole is not currently approved in Japan as a treatment for the indication of rosacea, although 0.75% metronidazole gel was authorized in 2014 for the management of cancerous skin ulcers. We conducted a randomized, double‐blind, vehicle‐controlled study to evaluate the efficacy and safety of 0.75% metronidazole gel in Japanese patients with inflammatory lesions (papules/pustules) and erythema associated with moderate to severe rosacea. Overall, 130 patients were randomly assigned to receive 0.75% metronidazole gel (n = 65) or vehicle (n = 65), and 120 patients completed 12 weeks of treatment. The primary efficacy outcome was the proportion of patients who achieved both of the following at week 12: an improvement of >50% in the number of inflammatory lesions (papules/pustules) and a positive change of at least one degree in erythema severity. This composite outcome was achieved by 72.3% of metronidazole‐treated patients versus 36.9% of vehicle‐treated patients, with the between‐group difference demonstrating significant improvement with 0.75% metronidazole gel (p < 0.0001). All secondary efficacy endpoints (patients achieving a score of ≥3 for percent change in the number of inflammatory lesions at week 12; patients achieving a score of ≥3 for change in erythema severity at week 12; patients achieving an Investigator’s Global Assessment score of 0 or 1 at week 12; percent change over time in the number of inflammatory lesions; change over time in erythema severity) also showed improvement in the 0.75% metronidazole gel group. The incidence of adverse events was higher with metronidazole (40.0%) than with vehicle (29.2%). Of these, treatment‐related, treatment‐emergent adverse events occurred in 9.2% and 6.2% in the metronidazole and the vehicle group, respectively, but there were no new safety concerns. Overall, the results of this study have confirmed the efficacy and safety of 0.75% metronidazole gel in Japanese patients with rosacea.
Keywords: clinical trial, phase III; Japanese; metronidazole; rosacea
1. INTRODUCTION
Rosacea is a common skin disease, primarily affecting the face, with an estimated global prevalence of 5.5%. 1 Prevalence rates in Asian communities are poorly characterized, 2 with estimates ranging 0.97–10.6% in China and South‐East Asian countries. 2 , 3 A longitudinal study of 67 448 Japanese dermatology patients published in 2011 reported that 0.22% were diagnosed with rosacea. 4 A chronic inflammatory condition, the four primary features of rosacea include transient erythema (flushing), non‐transient erythema, domed red papules with/without pustules, and telangiectasia. 5 , 6 , 7 Secondary features can include sensations of burning and stinging, elevated red plaques, rough and scaling skin giving the appearance of dryness, soft or solid facial edema, ocular manifestations, and phymatous changes. 7 Rosacea may also occur in other bodily locations, and this can occur with or without facial symptoms. 7 Diagnosis is based on the physical symptoms and medial history of the patient, and exclusion of disorders with overlapping signs (such as acne or lupus erythematosus); 8 for many patients, a diagnosis of rosacea is made if any one of the four primary symptoms plus one or more of the secondary symptoms are confirmed during clinical examination. Historically, rosacea has been classified into four subtypes, according to the primary clinical symptoms, 7 although a new phenotypic classification was a proposed in 2017 in which persistent, centrofacial erythema and phymatous changes were independently considered diagnostic for rosacea. 9 , 10
Although the precise etiology of rosacea remains unclear, suggested pathophysiological mechanisms include both genetic and environmental factors, such as epidermal barrier disruption, increased sensitivity to external stimuli (such as temperature and levels of ultraviolet radiation), heavy exercise, psychological stress, consumption of alcohol or spicy food, and irritants (including chemicals and possibly Demodex mite colonization), leading to inflammation and vascular proliferation. 5 , 11 , 12 , 13 , 14 , 15 , 16 , 17 Importantly, symptoms and changes in appearance due to rosacea may significantly impair patients’ quality of life (QOL), with patients’ perception of their physical appearance negatively influencing self‐esteem and elevating rates of mood disorders in affected individuals. 5 , 18 , 19
In Japan, few products are indicated for the treatment of rosacea. Available treatments include topical sulfur and vitamin B2 preparations; however, approvals were granted many years ago and there is little contemporary evidence for their efficacy. Current guideline recommendations for rosacea treatments have low levels of evidence for Japanese patients, 20 and none are approved in Japan to date. Thus, for Japanese patients with rosacea, there is no approved topical pharmacological treatment, and management is currently restricted to basic skin care.
Metronidazole gel (0.75%) is a topical product containing imidazole antibiotics, and has been in clinical use for rosacea outside Japan for more than 40 years. 21 , 22 It is also used to treat cancerous skin ulcers. 23 Its safety profile is well established, 21 and it is considered to have strong evidence for use in treating inflammatory rosacea. 24 Metronidazole is considered to exert its efficacy against the inflammatory lesions (papules/pustules) associated with rosacea via anti‐inflammatory and immunomodulatory pathways. 25 , 26 , 27 Reactive oxygen species (ROS) and oxidative stress are known to be strongly associated with a range of skin conditions, 28 and it is thought that topical metronidazole can both decrease the production of ROS and also act as a scavenger. 29
Although metronidazole has been evaluated in multiple studies in rosacea patients outside of Japan, there is no clinical evidence from randomized controlled trials conducted within Japan.
Therefore, we designed this first randomized, double‐blind, clinical study in Japanese patients with rosacea to evaluate the efficacy and safety of 0.75% metronidazole gel, using vehicle as a control.
2. METHODS
2.1. Patients
Patients with inflammatory lesions (papules/pustules) and erythema associated with rosacea were potentially eligible for study enrolment. The key inclusion criteria were age ≥18 years at the time of informed consent, an Investigator’s Global Assessment (IGA) score of ≥3 (moderate) on the day of randomization (baseline), between ≥11 and ≤40 inflammatory lesions (papules/pustules) on the whole face at baseline, and erythema severity score of ≥2 (mild) at baseline. The full list of inclusion and exclusion criteria are provided in Table S1.
Prior to the start of the study, the study was explained to each patient by the investigators using the informed consent form, and all patients and their legal guardians were required to provide written informed consent for study participation.
2.2. Study design, treatments, and blinding
This was a phase 3, randomized, vehicle‐controlled, double‐blind, multicenter, parallel‐group study of 12 weeks’ duration in patients with rosacea (see Figure S1). The study was conducted at 26 study sites in Japan (see Table S2 for the full list of study sites and investigators) from April 2019 to May 2020. Study procedures were conducted in compliance with the Declaration of Helsinki, good clinical practice, and all other relevant legal and regulatory requirements. The study protocol was registered with the Japan Pharmaceutical Information Center Clinical Trials Information registry (JapicCTI‐194688; https://www.clinicaltrials.jp/cti‐user/trial/Show.jsp?clinicalTrialId=28247), and the protocol and all related documentation were reviewed and approved by the institutional review board at each participating site (see Table S3).
At baseline, after confirming their eligibility, patients were randomly assigned at 1:1 ratio to receive either 0.75% metronidazole gel or vehicle (gel product without metronidazole), using a web‐based enrolment system. The investigators, site staff, patients, and sponsor remained blinded to treatment allocation throughout the duration of the study. After washing the face or bathing, an appropriate amount of study treatment (0.75% metronidazole gel or vehicle) was applied to all inflammatory lesions (papules/pustules) and erythema areas on the face twice daily (morning and evening) for 12 weeks. Treatment continued for the full 12 weeks even if the inflammatory lesions and/or erythema disappeared.
During the study, the following medications and therapies for rosacea were prohibited: any treatments for rosacea excluding moisturizer and emollient, any topical treatments for inflammatory lesions and erythematous areas of rosacea, systemic therapies (ivermectin, retinoids, antimicrobials, metronidazole, corticosteroids, Chinese herbal medicine for rosacea, warfarin, lithium, cyclosporine, phenobarbital), topical therapies (antimicrobial medication, sulfur and camphor lotion, azelaic acid, immunosuppressants, retinoids, corticosteroids), or physical therapies (laser, intense pulsed light, photodynamic therapy, phototherapy, electrocoagulation, dermabrasion, chemical peeling, or other facial skin surgeries).
2.3. Efficacy outcomes
The primary endpoint for the study was a composite outcome comprising both improvement in the number of inflammatory lesions and change in erythema severity at week 12. The criteria for scoring changes in these measures are reported in Table 1. Both the number of inflammatory lesions and erythema severity are widely used as outcome measures in rosacea clinical studies; thus, the primary endpoint in this study was a modified outcome based on prior studies conducted outside Japan. 30 , 31 , 32
TABLE 1.
Primary outcome measures: scoring criteria
| Score | Percent change in the number of inflammatory lesions | Change in erythema severity |
|---|---|---|
| 1 | ≥−25% | Worsened |
| 2 | −26% to −50% | Unchanged |
| 3 | −51% to −75% | Improvement by 1 grade |
| 4 | −76% to −100% | Improvement by ≥2 grades |
Specifically, the investigator counted the number of inflammatory lesions (papules/pustules) on each study visit, where papules were defined as localized elevated changes ≤10 mm in diameter, and pustules were defined as skin prominences with a purulent (predominantly neutrophilic) capsule on the tegmentum with white to yellow coloration. The percent change at each visit was calculated and converted to a score from 1 to 4 (Table 1) by the study sponsor. Erythema was evaluated with reference to a standard photograph showing the criterion for each severity, and was investigator‐assessed excluding transient flushing, telangiectasia, rhinophyma, and ocular symptoms of rosacea. Erythema severity was scored as: 0 = no erythema, 1 = extremely slight erythema, 2 = slight erythema, 3 = definite erythema, or 4 = severe erythema. The change in erythema severity at each visit was converted to a score of 1 to 4 (Table 1) by the study sponsor. The primary study endpoint was set as the proportion of patients who achieved a score of ≥3 for both percent change in the number of inflammatory lesions and change in erythema severity at week 12.
Secondary endpoints were the proportion of patients who achieved a score of ≥3 for percent change in the number of inflammatory lesions at week 12; the proportion of patients who achieved score of ≥3 for change in erythema severity at week 12; the proportion of patients who achieved an IGA score of 0 or 1 at week 12; the change over time in percent change in the number of inflammatory lesions; and change over time in erythema severity. The IGA was scored by the investigator on a scale of 0 to 4: 0 = clear (no inflammatory lesion and no erythema), 1 = almost clear (almost no lesions or very slight erythema), 2 = mild (a few small papules and small pustules or slight erythema), 3 = moderate (multiple small to large papules and pustules or well‐defined erythema), and 4 = severe (many small to large papules and pustules or severe erythema).
Additional study outcomes included the proportion of patients with an improvement of ≥4 points in the Dermatology Life Quality Index (DLQI) 33 total score at week 12 and the proportion of patients with an improvement of ≥10 points in the Skindex‐16 34 overall score at week 12. The DLQI is a 10‐item questionnaire, producing a score ranging 0–30, with higher scores indicating a greater bothersome effect on daily life. The Skindex‐16 is a 16‐item list of skin symptoms experienced over the past week, with each item scored from 0 (never bothered) to 6 (always bothered), with higher scores indicating a greater bothersome effect. Improvements of ≥4 points in the DLQI and ≥10 points in the Skindex‐16 have previously been reported to be the minimum clinically important differences. 35 , 36 The DLQI and Skindex‐16 were completed by each patient on the day of randomization (baseline) and at week 12.
2.4. Safety outcomes
Safety was evaluated by adverse events (AE) and laboratory test values. Treatment‐emergent AE (TEAE) were coded using the Medical Dictionary for Regulatory Activities (MedDRA)/Japanese Edition, version 21.1. Based on the known safety profile and potential risks of metronidazole, we also defined several AE of special interest (AESI); these were peripheral neuropathy, hypersensitivity, urticaria, and angioedema.
2.5. Statistical methods
The sample size was calculated using the assumption that the proportions of patients achieving the primary endpoint would be 50% in the 0.75% metronidazole gel group and 20% in the vehicle group. Based on this, and using the χ2‐test with a two‐sided significance level of 5% and power of 95%, the required number of study patients was calculated to be 63 per treatment group.
The modified intention‐to‐treat (mITT) population was used to evaluate both efficacy and safety, and included all patients who were randomly assigned and treated with 0.75% metronidazole gel or vehicle.
The mITT was used to calculate the primary endpoint, with treatment groups compared using Pearson’s χ2‐test, with a two‐sided significance level of 5%. Missing data were imputed as non‐responders. As a pre‐specified sensitivity analysis, calculations were also conducted whereby missing data were imputed using last observation carried forward methodology.
For secondary endpoints, intergroup comparisons of binary data were performed in the same way as for the primary endpoint, using Pearson’s χ2‐test with a two‐sided significance level of 5%. For binary endpoints, missing values were imputed as a non‐responder (as per the primary endpoint). Change over time in percent change in the number of inflammatory lesions was analyzed using a mixed model for repeated measures, with visit, treatment, and the interaction between visit and treatment as explanatory variables; unstructured correlation structured between visits were assumed. Change over time in erythema severity was analyzed by conditional longitudinal data analysis, which assumed that the mean and standard deviation for the erythema severity score at baseline were the same for both treatment groups. Missing values were not imputed for continuous outcomes. Analyses were not adjusted for multiplicity of secondary endpoints or assessment time points. All analyses were performed using SAS version 9.4 (SAS Institute).
3. RESULTS
3.1. Patients
A total of 134 patients were assessed for study eligibility. Of these, three patients did not meet the inclusion criteria and one requested to withdraw and 130 were randomly assigned to treatment (n = 65 in each group). Patient disposition is shown in Figure 1. All 130 patients were included in the mITT population. Overall, 120 patients completed the study to week 12; three patients in the 0.75% metronidazole gel group discontinued early (two at the request of the patient and one due to an AE of contact dermatitis), and seven patients in the vehicle group (four at the request of the patient, two due to pregnancy, and one due to an AE of contact dermatitis).
FIGURE 1.
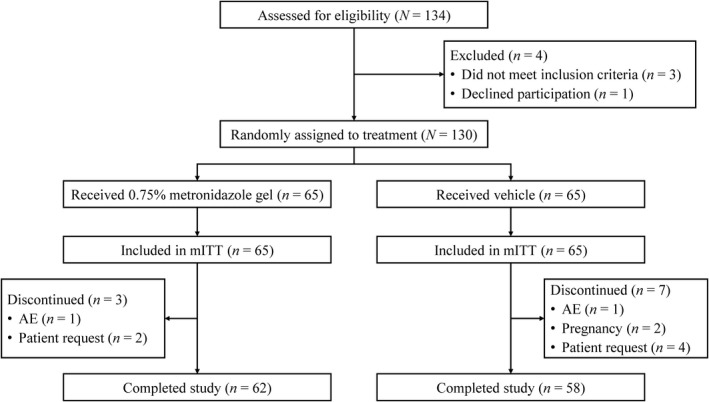
Patient disposition (Consolidated Standards of Reporting Trials). AE, adverse event; mITT, modified intention‐to‐treat
Baseline characteristics are shown in Table 2. In general, demographic and medical characteristics were similarly distributed between groups. In total, 82.3% (107/130) of patients were female, and the mean age was 47.8 years. The majority of patients had IGA and erythema severity scores of 3 (IGA 87.7%, 114/130 patients; erythema severity 60.8%, 79/130 patients), and the mean number of inflammatory lesions was 23.7. The mean age at onset and duration of disease were 43.8 and 4.7 years, respectively. The most commonly reported factor associated with worsening rosacea was temperature changes (53.8%, 70/130 patients), followed by exposure to sunlight, hot weather, seasonal changes, and heavy exercise. A further 7.7% (10/130 patients) did not report any factor associated with worsening rosacea.
TABLE 2.
Patient baseline demographics and clinical characteristics (modified intention‐to‐treat population)
| 0.75% metronidazole gel (n = 65) | Vehicle (n = 65) | Total (n = 130) | |
|---|---|---|---|
| Sex, female, n (%) | 54 (83.1) | 53 (81.5) | 107 (82.3) |
| Age, years a | |||
| Mean (SD) | 45.9 (10.6) | 49.8 (14.9) | 47.8 (13.0) |
| Range | 30–84 | 20–83 | 20–84 |
| IGA score, n (%) | |||
| 3 (moderate) | 56 (86.2) | 58 (89.2) | 114 (87.7) |
| 4 (severe) | 9 (13.8) | 7 (10.8) | 16 (12.3) |
| Erythema severity, n (%) | |||
| 2 (mild) | 14 (21.5) | 14 (21.5) | 28 (21.5) |
| 3 (moderate) | 35 (53.8) | 44 (67.7) | 79 (60.8) |
| 4 (severe) | 16 (24.6) | 7 (10.8) | 23 (17.7) |
| Inflammatory lesion counts b | |||
| Mean (SD) | 23.5 (9.3) | 23.9 (9.5) | 23.7 (9.3) |
| Range | 11–40 | 11–40 | 11–40 |
| Age of onset of rosacea, years | |||
| n | 61 | 60 | 121 |
| Mean (SD) | 41.6 (11.8) | 46.0 (16.2) | 43.8 (14.3) |
| Range | 15.6–82.2 | 12.3–82.9 | 12.3–82.9 |
| Duration of rosacea, years | |||
| n | 61 | 60 | 121 |
| Mean (SD) | 4.9 (6.4) | 4.5 (7.1) | 4.7 (6.8) |
| Range | 0.1–33.4 | 0–30.3 | 0–33.4 |
| Most commonly reported factors associated with worsening rosacea, n (%) c | |||
| Temperature changes | 38 (58.5) | 32 (49.2) | 70 (53.8) |
| Sun exposure | 30 (46.2) | 21 (32.3) | 51 (39.2) |
| Hot weather | 23 (35.4) | 23 (35.4) | 46 (35.4) |
| Seasonal variation | 24 (36.9) | 15 (23.1) | 39 (30.0) |
| Heavy exercise | 16 (24.6) | 20 (30.8) | 36 (27.7) |
Abbreviations: IGA, Investigator’s Global Assessment; SD, standard deviation.
At informed consent.
Papules plus pustules.
Patients could select more than one factor.
3.2. Primary efficacy outcome
The proportion of patients who achieved a score of ≥3 for both percent change in the number of inflammatory lesions and change in erythema severity at week 12 was 72.3% (47/65 patients) in the 0.75% metronidazole gel group and 36.9% (24/65 patients) in the vehicle group (Table 3). The estimated treatment difference (two‐sided exact 95% confidence interval) was 35.4% (17.9–51.3%), demonstrating significant improvement with 0.75% metronidazole gel compared with vehicle (p < 0.0001). Notably, early onset of efficacy was observed in the 0.75% metronidazole gel group, with significant improvement observed from week 2 compared with vehicle (Figure 2a). The result of the last observation carried forward sensitivity analysis was in agreement with the primary analysis with a non‐responder imputation (data not shown).
TABLE 3.
Summary of efficacy outcomes (modified intention‐to‐treat population)
| Endpoint | 0.75% metronidazole gel (n = 65) | Vehicle (n = 65) | Difference (0.75% metronidazole gel vs vehicle) | |||
|---|---|---|---|---|---|---|
| n | n (%) [95% CI] e | n | n (%) [95% CI] e | % [95% CI] e | p | |
| Primary a | ||||||
| Proportion of patients who achieved a score of ≥3 for both percent change in inflammatory lesions and change in erythema severity at week 12 | 65 | 47 (72.3) [59.8–82.7] | 65 | 24 (36.9) [25.3–49.8] | 35.4 [17.9–51.3] | <0.0001 |
| Secondary a , b | ||||||
| Proportion of patients who achieved a score of ≥3 for percent change in the number of inflammatory lesions at week 12 | 65 | 52 (80.0) [68.2–88.9] | 65 | 29 (44.6) [32.3–57.5] | 35.4 [17.9–51.3] | <0.0001 |
| Proportion of patients who achieved score of ≥3 for change in erythema severity at week 12 | 65 | 51 (78.5) [66.5–87.7] | 65 | 37 (56.9) [44.0–69.2] | 21.5 [3.6–38.4] | 0.0086 |
| Proportion of patients who achieved an IGA score of 0 or ≤1 at week 12 | 65 | 25 (38.5) [26.7–51.4] | 65 | 12 (18.5) [9.9–30.0] | 20.0 [2.1–37.0] | 0.0115 |
| n | LS mean [95% CI] | n | LS mean [95% CI] | LS mean [95% CI] | p | |
|---|---|---|---|---|---|---|
| Change over time in percent change in the number of inflammatory lesions (week 12) | 65 | ‒76.4 [‒90.5 to ‒62.4] | 65 | ‒27.5 [‒41.7 to ‒13.3] | ‒48.9 [‒68.9 to ‒28.9] | <0.0001 |
| Change over time in erythema severity (week 12) | 65 | 1.6 [1.4–1.8] | 65 | 2.2 [1.9–2.4] | ‒0.6 [‒0.9 to ‒0.3] | 0.0004 |
| n | n (%) [95% CI] e | n | n (%) [95% CI] e | % [95% CI] e | p | |
|---|---|---|---|---|---|---|
| Other | ||||||
| Proportion of patients with an improvement of ≥4 points in the DLQI total score at week 12 c | 23 | 16 (69.6) [47.1–86.8] | 28 | 18 (64.3) [44.1–81.4] | 5.3 [‒22.1 to 32.1] | 0.6906 |
| Proportion of patients with an improvement of ≥10 points in the Skindex‐16 overall score at week 12 d | 52 | 43 (82.7) [69.7–91.8] | 58 | 32 (55.2) [41.5–68.3] | 27.5 [9.0–44.8] | 0.0020 |
p‐values for binary variables were calculated using the Pearson χ2‐test. p‐values for continuous variables were calculated using the mixed model for repeated measure.
Abbreviations: CI, confidence interval; DLQI, dermatology life quality index; IGA, Investigator’s Global Assessment; LS, least squares.
For binary variables, missing values were imputed as non‐responders; no imputations were made for continuous variables.
Analyses of secondary endpoints were not adjusted for multiplicity.
Among patients with a DLQI score of ≥4 at baseline.
Among patients with a Skindex‐16 score of ≥10 at baseline.
Exact 95% CI are shown.
FIGURE 2.
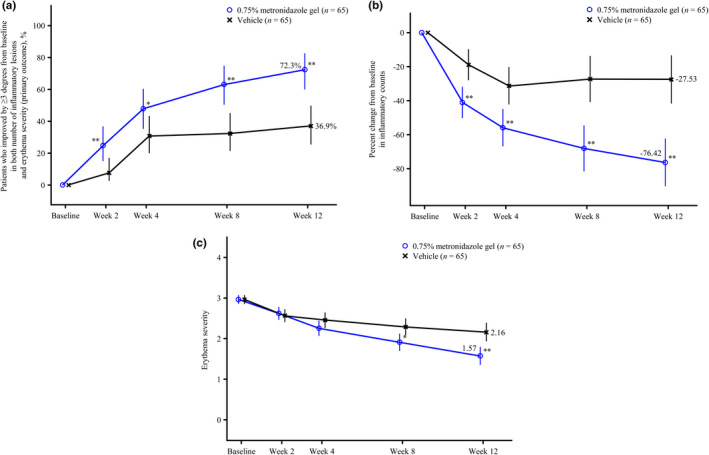
Time course of efficacy outcome measures. (a) Change over time in the proportion of patients who achieved score 3 or better for both percent change in the number of inflammatory lesions and change in erythema severity (primary outcome)†; (b) change over time in percent change in the number of inflammatory lesions‡; (c) change over time in erythema severity§ (modified intention‐to‐treat group). Missing data were imputed as non‐responders for (a), and were not imputed for (b) and (c). Data were not adjusted for multiplicity of secondary endpoints or assessment time points. *p < 0.05 vs vehicle; **p < 0.01 vs vehicle. †Data are shown as % (exact 95% confidence intervals). ‡Data are shown as least squares mean (95% confidence intervals). Mixed model for repeated measures, with visit, treatment, and the interaction between visit and treatment as explanatory variables; unstructured correlation structured between visits were assumed. §Data are shown as least squares mean (95% confidence intervals). Conditional longitudinal data analysis, which assumed that the mean and standard deviation for the erythema severity score at baseline were the same for both treatment groups
3.3. Secondary outcomes
The results of the secondary endpoints are shown in Table 3. The proportions of patients who achieved a score of ≥3 for percent change in the number of inflammatory lesions at week 12, a score of ≥3 for change in erythema severity at week 12, and an IGA score of 0 or 1 at week 12, were all significantly higher in the 0.75% metronidazole gel group than in the vehicle group (Figure S2). Both percent change in the number of inflammatory lesions and erythema severity improved over time in the 0.75% metronidazole gel group (Figure 2b,c). An example of response to treatment is shown photographically in Figure 3.
FIGURE 3.
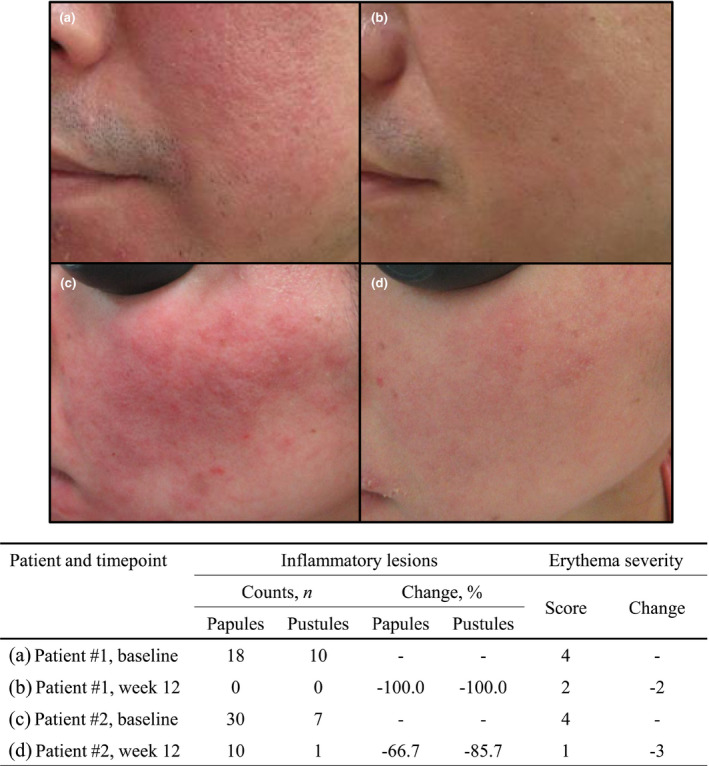
Example of response to treatment. (a) Patient #1 at baseline; (b) patient #1 at week 12; (c) patient #2 at baseline; (d) patient #2 at week 12
3.4. Other endpoints
Outcome data relating to the impact of treatment on QOL are shown in Table 3. At baseline, <50% of patients in each group had a DLQI total score of ≥4 (23/65 patients in the 0.75% metronidazole group and 28/65 patients in the vehicle group). There was no statistically significant difference between treatment groups in the proportion of patients with an improvement of ≥4 points in the DLQI total score at week 12.
There was a significant difference between the proportion of patients with an improvement of ≥10 points in the Skindex‐16 overall score at week 12 in the 0.75% metronidazole gel compared with vehicle (p = 0.0020).
3.5. Safety
Table 4 summarizes the key safety data, and a full list of all AE is provided in Table S4. The overall incidence of TEAE was 40.0% (26/65 patients) in the 0.75% metronidazole gel group and 29.2% (19/65 patients) in the vehicle group. The majority of TEAE were mild, and there were no deaths or serious TEAE. The incidence of TEAE at the application site was 21.5% (14/65 patients) in the 0.75% metronidazole gel group and 7.7% (5/65) in the vehicle group. Treatment‐related TEAE occurred in 9.2% (6/65) and 6.2% (4/65) in the metronidazole and the vehicle groups, respectively.
TABLE 4.
Summary of adverse events (modified intention‐to‐treat population)
| All TEAE | Treatment‐related TEAE | |||
|---|---|---|---|---|
| 0.75% metronidazole gel (n = 65) | Vehicle (n = 65) | 0.75% metronidazole gel (n = 65) | Vehicle (n = 65) | |
| Any TEAE, n (%) | 26 (40.0) | 19 (29.2) | 6 (9.2) | 4 (6.2) |
| TEAE leading to death, n (%) | 0 | 0 | 0 | 0 |
| Serious TEAE, n (%) | 0 | 0 | 0 | 0 |
| TEAE severity, n (%) | ||||
| Severe | 0 | 0 | 0 | 0 |
| Moderate | 2 (3.1) | 5 (7.7) | 2 (3.1) | 2 (3.1) |
| Mild | 24 (36.9) | 16 (24.6) | 4 (6.2) | 2 (3.1) |
| Treatment modification, n (%) | ||||
| TEAE leading to discontinuation of treatment | 1 (1.5) | 1 (1.5) | 1 (1.5) | 1 (1.5) |
| TEAE leading to interruption of treatment | 4 (6.2) | 1 (1.5) | 3 (4.6) | 1 (1.5) |
| TEAE of special interest, n (%) | ||||
| Peripheral neuropathy | 0 | 0 | 0 | 0 |
| Hypersensitivity | 7 (10.8) | 4 (6.2) | 2 (3.1) | 1 (1.5) |
| Urticaria | 0 | 0 | 0 | 0 |
| Angioedema | 1 (1.5) | 0 | 0 | 0 |
| TEAE occurring in ≥2% of patients in any treatment group, n (%) | ||||
| Infections and infestations | ||||
| Nasopharyngitis | 3 (4.6) | 1 (1.5) | 0 | 0 |
| Otitis externa | 0 | 2 (3.1) | 0 | 0 |
| Sinusitis | 2 (3.1) | 1 (1.5) | 0 | 0 |
| Skin and subcutaneous tissue disorders | ||||
| Acne | 1 (1.5) | 2 (3.1) | 0 | 0 |
| Dermatitis contact | 6 (9.2) | 3 (4.6) | 2 (3.1) | 1 (1.5) |
| Rosacea a | 1 (1.5) | 2 (3.1) | 0 | 2 (3.1) |
| TEAE occurring at the application site, n (%) | ||||
| Any | 14 (21.5) | 5 (7.7) | 6 (9.2) | 4 (6.2) |
| General disorders and administration site conditions | 3 (4.6) | 1 (1.5) | 1 (1.5) | 1 (1.5) |
| Application site pruritus | 1 (1.5) | 0 | 0 | 0 |
| Application site dryness | 1 (1.5) | 0 | 1 (1.5) | 0 |
| Application site eczema | 1 (1.5) | 0 | 0 | 0 |
| Application site discomfort | 0 | 1 (1.5) | 0 | 1 (1.5) |
| Infections and infestations | 4 (6.2) | 0 | 0 | 0 |
| Furuncle | 1 (1.5) | 0 | 0 | 0 |
| Herpes simplex | 1 (1.5) | 0 | 0 | 0 |
| Oral herpes | 1 (1.5) | 0 | 0 | 0 |
| Demodicidosis | 1 (1.5) | 0 | 0 | 0 |
| Skin and subcutaneous tissue disorders | 9 (13.8) | 4 (6.2) | 5 (7.7) | 3 (4.6) |
| Acne | 1 (1.5) | 1 (1.5) | 0 | 0 |
| Dermatitis contact | 3 (4.6) | 1 (1.5) | 2 (3.1) | 1 (1.5) |
| Pruritus | 1 (1.5) | 0 | 1 (1.5) | 0 |
| Rosacea a | 1 (1.5) | 2 (3.1) | 0 | 2 (3.1) |
| Seborrheic dermatitis | 1 (1.5) | 0 | 0 | 0 |
| Skin tightness | 1 (1.5) | 0 | 1 (1.5) | 0 |
| Asteatosis | 1 (1.5) | 0 | 1 (1.5) | 0 |
TEAE were coded using the Medical Dictionary for Regulatory Activities/Japanese edition, version 21.1.
Abbreviation: TEAE, treatment‐emergent adverse event.
Although improvement in rosacea was assessed as an efficacy endpoint for treatment groups, worsening of rosacea could be reported as a protocol‐defined TEAE for individual patients in the safety assessment.
Treatment‐emergent adverse events that occurred in ≥2% of patients in either group were dermatitis contact (MedDRA preferred term; 9.2%, 6/65 patients), nasopharyngitis (4.6%, 3/65), and sinusitis (3.1%, 2/65) in the 0.75% metronidazole gel group, and dermatitis contact (4.6%, 3/65), otitis externa, acne, and rosacea which was reported as worsening of rosacea (3.1%, 2/65 patients each) in the vehicle group. Of these, dermatitis contact in 3.1% (2/65 patients) in the 0.75% metronidazole gel group and rosacea in 3.1% (2/65 patients) in the vehicle group were assessed as being related to study treatment. The two patients with treatment‐related dermatitis contact developed symptoms at the site of study treatment application; both recovered following treatment discontinuation or interruption.
An AE leading to treatment discontinuation was reported in one patient in the 0.75% metronidazole gel group (dermatitis contact). AE leading to treatment interruption were reported in four patients in the 0.75% metronidazole gel group (dermatitis contact, n = 2; dermatitis contact and application site dryness, n = 1; rosacea [which was reported as worsening of rosacea], n = 1).
Regarding TEAE of special interest, hypersensitivity events occurred in seven patients in the 0.75% metronidazole gel group (dermatitis contact, n = 5; eyelid edema and dermatitis contact, n = 1; application site eczema, n = 1). Vascular edema events included mild eyelid edema in the 0.75% metronidazole gel group, which was assessed as not related to treatment and subsequently resolved. No peripheral neuropathy or urticaria events were observed.
No clinically relevant changes in laboratory values were observed in the 0.75% metronidazole gel group.
4. DISCUSSION
Although 0.75% metronidazole gel has been used for the treatment of rosacea for many years in countries outside of Japan, with strong evidence to support its efficacy, 24 clinical trial data from Japanese patients with rosacea have been lacking. In this first randomized, double‐blind clinical study in Japan, 0.75% metronidazole gel twice daily for 12 weeks demonstrated superiority over vehicle in terms of the primary endpoint, namely the proportion of patients who achieved a score of ≥3 for both percent change in the number of inflammatory lesions and change in erythema severity at week 12. The early onset of efficacy observed in patients using 0.75% metronidazole gel in improving both inflammatory lesions (papules/pustules) and erythema, which are major symptoms of rosacea, underlines the clinical importance of this product to improve the treatment of rosacea in Japanese patients. Secondary endpoints were also consistently improved in the 0.75% metronidazole gel group compared with the vehicle group. The impact of 0.75% metronidazole gel on QOL was evaluated with the DLQI and Skindex‐16; our findings demonstrated that the proportion of patients with an improvement of ≥10 points in the Skindex‐16 at week 12 was significantly higher in the 0.75% metronidazole gel group, compared with the vehicle group.
Importantly, there were no notable safety concerns associated with the use of 0.75% metronidazole gel in Japanese patients with rosacea, and no deaths or serious TEAE occurred. Topical formulations of metronidazole have been reported to be generally well tolerated in rosacea populations outside Japan, with local TEAE of stinging, dryness, burning, and itching sensations reported in fewer than 2% of patients. 37 In the current study, the majority of TEAE were mild in severity. All events assessed as being related to 0.75% metronidazole gel, and which led to treatment discontinuation or interruption, subsequently resolved. TEAE of special interest, including hypersensitivity and eyelid edema, also resolved. During the study, nine events of dermatitis contact were observed, of which five (0.75% metronidazole gel, n = 3; vehicle, n = 2) occurred outside of the application site, and were presumed to be due to contact with other allergens (i.e. not related to study treatment) and one (in the 0.75% metronidazole gel group) was judged to be due to the application of cosmetics at the application site and also not related to study treatment. Of note, although rosacea was reported as an AE, rosacea in patients using 0.75% metronidazole gel was considered the investigator to be unrelated to the study treatment, so this AE was likely due to a natural worsening of symptoms.
Overall, these results confirmed the efficacy and safety of 0.75% metronidazole gel in Japanese patients with rosacea. The study findings were comparable with those previously reported from clinical studies undertaken outside of Japan, 32 indicating the general utility of this treatment across different racial populations.
One potential limitation of our study is the 12‐week intervention period used to evaluate the efficacy and safety of 0.75% metronidazole gel. Rosacea is a chronic condition, and if the symptoms of rosacea remain or recur after 12 weeks, real‐world clinicians may wish to continue treatment with 0.75% metronidazole gel. Although there is no published data indicating that topical metronidazole treatment can increase microbial antibiotic resistance, it is generally known that long‐term use of antibiotics can increase the risk of developing bacterial resistance. 38 , 39 Therefore, long‐term use of 0.75% metronidazole gel to treat rosacea would require careful discussion, and demonstration of a clear clinical benefit for individual patients. Additional limitations of our study include the exclusion of patients aged <18 years, and those with erythematotelangiectatic rosacea without papules and pustules. While we cannot confirm the efficacy and safety of 0.75% metronidazole gel in children or adolescents, we consider that the potential number of affected individuals in these age groups are likely to be very small. For erythematotelangiectatic rosacea without papules and pustules, physicians should carefully discuss the choice of treatment with affected patients.
In conclusion, the use of 0.75% metronidazole gel for rosacea for 12 weeks resulted in a clinically meaningful and statistically significant improvement in inflammatory lesions and erythema, the main symptoms of rosacea. These results suggest that twice daily application of 0.75% metronidazole gel can provide benefit as a first‐line treatment for rosacea in Japan.
CONFLICT OF INTEREST
This study was funded by Maruho. Y.M. and K.Y. have received research funding, consultancy fees, speaker fees, fees for arranging education, and personal fees from Maruho. T.F. and C.F. are employees of Maruho.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to express their gratitude to the patients and their families, the investigators (Table S2), and the project team members at Maruho, especially Kanako Ito. We also thank Sally‐Anne Mitchell, PhD, of McCann Health CMC, Japan, for providing medical writing support, which was funded by Maruho.
Miyachi Y, Yamasaki K, Fujita T, Fujii C. Metronidazole gel (0.75%) in Japanese patients with rosacea: A randomized, vehicle‐controlled, phase 3 study. J Dermatol. 2022;49:330–340. 10.1111/1346-8138.16254
REFERENCES
- 1. Gether L, Overgaard LK, Egeberg A, Thyssen JP. Incidence and prevalence of rosacea: a systematic review and meta‐analysis. Br J Dermatol. 2018;179:282–9. [DOI] [PubMed] [Google Scholar]
- 2. Alexis AF, Callender VD, Baldwin HE, Desai SR, Rendon MI, Taylor SC. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: Review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722–9. [DOI] [PubMed] [Google Scholar]
- 3. Li J, Wang B, Deng Y, Shi W, Jian D, Liu F, et al. Epidemiological features of rosacea in Changsha, China: a population‐based, cross‐sectional study. J Dermatol. 2020;47:497–502. [DOI] [PubMed] [Google Scholar]
- 4. Furue M, Yamazaki S, Jimbow K, Tsuchida T, Amagai M, Tanaka T, et al. Prevalence of dermatological disorders in Japan: a nationwide, cross‐sectional, seasonal, multicenter, hospital‐based study. J Dermatol. 2011;38:310–20. [DOI] [PubMed] [Google Scholar]
- 5. Huynh TT. Burden of disease: the psychosocial impact of rosacea on a patient’s quality of life. Am Health Drug Benefits. 2013;6:348–54. [PMC free article] [PubMed] [Google Scholar]
- 6. van Zuuren EJ, Arents BWM, van der Linden MMD, Vermeulen S, Fedorowicz Z, Tan J. Rosacea: new concepts in classification and treatment. Am J Clin Dermatol. 2021;22:457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilkin J, Dahl M, Detmar M, Drake L, Feinstein A, Odom R, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584–7. [DOI] [PubMed] [Google Scholar]
- 8. Johnson SM, Berg A, Barr C. Recognizing rosacea: tips on differential diagnosis. J Drugs Dermatol. 2019;18:888–94. [PubMed] [Google Scholar]
- 9. Gallo RL, Granstein RD, Kang S, Mannis M, Steinhoff M, Tan J, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148–55. [DOI] [PubMed] [Google Scholar]
- 10. Tan J, Almeida LM, Bewley A, Cribier B, Dlova NC, Gallo R, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br J Dermatol. 2017;176:431–8. [DOI] [PubMed] [Google Scholar]
- 11. Thiboutot D, Anderson R, Cook‐Bolden F, Draelos Z, Gallo RL, Granstein RD, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82:1501–10. [DOI] [PubMed] [Google Scholar]
- 12. Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–80. [DOI] [PubMed] [Google Scholar]
- 13. Yamasaki K, Gallo RL. Antimicrobial peptides in human skin disease. Eur J Dermatol. 2008;18:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15:12–5. [DOI] [PubMed] [Google Scholar]
- 15. Yamasaki K, Kanada K, Macleod DT, Borkowski AW, Morizane S, Nakatsuji T, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimada‐Omori R, Yamasaki K, Koike S, Yamauchi T, Aiba S. TLR3 augments glucocorticoid‐synthetic enzymes expression in epidermal keratinocytes; Implications of glucocorticoid metabolism in rosacea epidermis. J Dermatol Sci. 2020;100:58–66. [DOI] [PubMed] [Google Scholar]
- 17. Plewig G, Melnik B, Chen WC. Plewig and Kligman’s acne and rosacea. Switzerland: Springer International Publishing; 2019. [Google Scholar]
- 18. Baldwin HE, Harper J, Baradaran S, Patel V. Erythema of rosacea affects health‐related quality of life: results of a survey conducted in collaboration with the National Rosacea Society. Dermatol Ther. 2019;9:725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singam V, Rastogi S, Patel KR, Lee HH, Silverberg JI. The mental health burden in acne vulgaris and rosacea: an analysis of the US National Inpatient Sample. Clin Exp Dermatol. 2019;44:766–72. [DOI] [PubMed] [Google Scholar]
- 20. Hayashi N, Akamatsu H, Iwatsuki K, Shimada‐Omori R, Kaminaka C, Kurokawa I, et al. Japanese Dermatological Association Guidelines: guidelines for the treatment of acne vulgaris 2017. J Dermatol. 2018;45:898–935. [DOI] [PubMed] [Google Scholar]
- 21. Aronson IK, Rumsfield JA, West DP, Alexander J, Fischer JH, Paloucek FP. Evaluation of topical metronidazole gel in acne rosacea. Drug Intell Clin Pharm. 1987;21:346–51. [DOI] [PubMed] [Google Scholar]
- 22. Pye RJ, Burton JL. Treatment of rosacea by metronidazole. Lancet. 1976;1:1211–2. [DOI] [PubMed] [Google Scholar]
- 23. Watanabe K, Shimo A, Tsugawa K, Tokuda Y, Yamauchi H, Miyai E, et al. Safe and effective deodorization of malodorous fungating tumors using topical metronidazole 0.75 % gel (GK567): a multicenter, open‐label, phase III study (RDT.07.SRE.27013). Support Care Cancer. 2016;24:2583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Searle T, Al‐Niaimi F, Ali FR. Rosacea. Br J Hosp Med. 2021;82:1–8. [DOI] [PubMed] [Google Scholar]
- 25. Fararjeh M, Mohammad MK, Bustanji Y, Alkhatib H, Abdalla S. Evaluation of immunosuppression induced by metronidazole in Balb/c mice and human peripheral blood lymphocytes. Int Immunopharmacol. 2008;8:341–50. [DOI] [PubMed] [Google Scholar]
- 26. Krehmeier U, Bardenheuer M, Voggenreiter G, Obertacke U, Schade FU, Majetschak M. Effects of antimicrobial agents on spontaneous and endotoxin‐induced cytokine release of human peripheral blood mononuclear cells. J Infect Chemother. 2002;8:194–7. [DOI] [PubMed] [Google Scholar]
- 27. Miyachi Y, Imamura S, Niwa Y. Anti‐oxidant action of metronidazole: a possible mechanism of action in rosacea. Br J Dermatol. 1986;114:231–4. [DOI] [PubMed] [Google Scholar]
- 28. Trüeb RM. Oxidative stress and its impact on skin, scalp and hair. Int J Cosmet Sci. 2021;43(S1):S9–S13. [DOI] [PubMed] [Google Scholar]
- 29. Narayanan S, Hunerbein A, Getie M, Jackel A, Neubert RH. Scavenging properties of metronidazole on free oxygen radicals in a skin lipid model system. J Pharm Pharmacol. 2007;59:1125–30. [DOI] [PubMed] [Google Scholar]
- 30. Nielsen PG. Treatment of rosacea with I% metronidazole cream. A double‐blind study. Br J Dermatol. 1983;108:327–32. [DOI] [PubMed] [Google Scholar]
- 31. Dahl MV, Jarratt M, Kaplan D, Tuley MR, Baker MD. Once‐daily topical metronidazole cream formulations in the treatment of the papules and pustules of rosacea. J Am Acad Dermatol. 2001;45:723–30. [DOI] [PubMed] [Google Scholar]
- 32. Taieb A, Ortonne JP, Ruzicka T, Roszkiewicz J, Berth‐Jones J, Peirone MH, et al. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator‐blinded trial. Br J Dermatol. 2015;172:1103–10. [DOI] [PubMed] [Google Scholar]
- 33. Takahashi N, Suzukamo Y, Nakamura M, Miyachi Y, Green J, Ohya Y, et al. Japanese version of the Dermatology Life Quality Index: validity and reliability in patients with acne. Health Qual Life Outcomes. 2006;4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Higaki Y, Kawamoto K, Kamo T, Horikawa N, Kawashima M, Chren MM. The Japanese version of Skindex‐16: a brief quality‐of‐life measure for patients with skin diseases. J Dermatol. 2002;29:693–8. [DOI] [PubMed] [Google Scholar]
- 35. Basra MK, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology. 2015;230:27–33. [DOI] [PubMed] [Google Scholar]
- 36. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality‐of‐life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127:1351–7. [DOI] [PubMed] [Google Scholar]
- 37. McClellan KJ, Noble S. Topical metronidazole. A review of its use in rosacea. Am J Clin Dermatol. 2000;1:191–9. [DOI] [PubMed] [Google Scholar]
- 38. Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5:229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ventola CL. The antibiotic resistance crisis: Part 1: causes and threats. P T. 2015;40:277–83. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


