Abstract
Introduction
Severe burn injury involves widespread skin and tissue damage leading to systemic inflammation, hypermetabolism and multi‐organ failure. The hypermetabolic phase of burn injury has been associated with increased systemic antibiotic clearance; however, critical illness in the absence of burn may also induce similar physiologic changes. Continuous renal replacement therapy (CRRT) is often implemented in critically ill patients and may also affect antibiotic clearance. Although the pharmacokinetics (PK) of meropenem has been described in both the burn and non‐burn critically ill populations, direct comparative data is lacking.
Methods
For this study, we evaluated PK parameters of meropenem from 23 critically ill patients, burn or non‐burn, treated with or without continuous veno‐venous haemofiltration (CVVH) to determine the contribution of burn and CVVH to the variability of therapeutic meropenem levels.
Results
A two‐compartment model best described the data and revealed creatinine clearance (CrCl) and total burn surface area (TBSA) as significant covariates on clearance (CL) and peripheral volume of distribution (Vp), respectively. Of interest, non‐burn patients on CVVH displayed an overall lower inherent CL as compared to burn patients on CVVH (6.43 vs. 12.85 L/h). Probability of target attainment (PTA) simulations revealed augmented renal clearance (ARC) may necessitate dose adjustments, but TBSA and CVVH would not.
Conclusions
We recommend a standard dose of 1000 mg every 8 hours; however, if ARC is suspected, or the severity of illness requires a more stringent therapeutic target, we recommend a loading dose of 1000–2000 mg infused over 30 minutes to 1 hour followed by continuous infusion (3000–6000 mg over 24 hours), or intermittent infusion of 2000 mg every 8 hours.
Keywords: burns, continuous renal replacement therapy, critical illness, meropenem, pharmacokinetics
What is Already Known About this Subject
Pharmacokinetics (PK) of meropenem and dosing regimens are well described in various critically ill subpopulations; however, comparative data is lacking.
Traditionally, burn injury has been thought to necessitate meropenem dose increases secondary to severe physiologic derangements that impact meropenem PK.
Similarly, use of continuous renal replacement therapy (CRRT) has been thought to necessitate more aggressive meropenem dosing regimens.
What this Study Adds
We provide a comparative analysis of meropenem PK in critically ill burn and surgical patient populations treated with or without continuous veno‐venous haemofiltration (CVVH).
We found, after accounting for differences in prescribing practices of CVVH amongst critically ill subpopulations, that neither burn injury nor use of CVVH would require meropenem dose increases on account of PK considerations alone.
Our findings suggest meropenem dose increases or alternative dosing regimens should be considered on the basis of individual risk–benefit assessments given the severity of illness and entirety of clinical data available.
However, our study was limited by small sample size, large heterogeneity and the challenge of acquiring optimal PK sampling in a critical care setting. Therefore, these results should be confirmed with larger comparative PK studies.
1. INTRODUCTION
Critically ill patients are at high risk for developing life‐threatening infections, leading to sepsis and multiple organ failure, thus requiring immediate and adequate antimicrobial therapy to improve chances of survival. 1 Severe pathophysiological changes attributed to injuries such as burn alter antibiotic pharmacokinetics (PK) and complicate the prediction of appropriate therapeutic drug levels. Acute kidney injury (AKI) is relatively common among hospitalized critically ill and burn patients and is associated with an exceedingly high mortality of 80–100%, 2 , 3 increased length of mechanical ventilation and ICU hospitalization. 4 Moreover, critical injuries associated with augmented renal clearance (ARC), altered volume of distribution (Vd), and/or abnormal fluid balance can also contribute to variable drug PK, necessitating extended or continuous infusions and/or higher doses or increased dose frequency to achieve therapeutic levels. 5
Renal replacement therapy (RRT) has shown tremendous utility in the management of critically ill patients with AKI to mitigate against metabolic disturbances and achieve fluid balance. 4 Continuous RRT (CRRT) is extensively utilized in the treatment of intensive care unit (ICU) patients and is often preferred over intermittent RRT as it allows for gradual metabolic control and restoration of fluid balance without significant haemodynamic shifts. 6 , 7 There are several modalities of CRRT, although the optimal modality is not well established. 8 , 9 Continuous veno‐venous hemofiltration is a modality that relies on convection to remove solute and is the preferred modality at the US Army Burn Center. Extracorporeal antibiotic clearance due to CRRT depends on several factors including blood flow rate, ultrafiltration flow rate, dialysate flow rate and mechanism of solute removal. 7 Recent evidence has revealed that 25% of ICU patients on CRRT failed to achieve therapeutic concentrations of antibiotics regardless of the dialysis dose. 10 Thus, although RRT is vital for efficient metabolic clearance and ultrafiltration of fluids in critically ill patients, careful consideration of its impact on antibiotic PK is necessary to ensure appropriate dosing and antibiotic coverage.
Due to its high level of activity against Gram‐positive and Gram‐negative pathogens, the broad‐spectrum antimicrobial meropenem is frequently used to empirically treat critically ill patients. Meropenem is a small hydrophilic molecule in the carbapenem class with a low Vd and low protein binding (<2%), that displays time‐dependent antimicrobial activity. Meropenem is renally eliminated via glomerular filtration and tubular secretion, with a typical half‐life of 1 hour, though in the setting of renal failure the half‐life can increase up to 10‐fold. 11 Extensive literature has demonstrated that hydrophilic antimicrobials, including meropenem, display substantial PK and pharmacodynamic (PD) variability in critically ill patients due to capillary leakage and pathophysiology, 5 , 12 , 13 , 14 , 15 directly influencing drug disposition. Consequently, patients with altered pathophysiology placed on CRRT can demonstrate greater variability in drug PK, with meropenem CL affected by ultrafiltrate volume, dialysate flow rate, filter membrane surface area and duration of therapy. 16 , 17
PK/PD analyses are fundamental in determining in vivo efficacy of drugs 18 and optimal drug exposure required for maximal bacterial killing 19 in critically ill patient populations. Pathophysiological alterations associated with disease as well as adjunctive treatments can distort the PK of drugs, directly affecting the effectiveness of an administered therapeutic. For antimicrobials that exhibit time‐dependent killing activity, continuous infusion and/or increasing the dosing frequency are utilized to increase ƒ %T > MIC. While typical dosing recommendations are to maintain meropenem concentrations above the MIC for 40% of the dosing interval (ƒ 40%T > MIC), emerging evidence suggests a clinical advantage for maintaining drug concentrations longer and up to five times the MIC (ƒ 100%T > 5 × MIC). 20 , 21 Of particular relevance, administration of meropenem by continuous infusion results in similar mortality and clinical cure rates as compared with intermittent infusion; however, microbiologic success rates were higher, and ICU stays and duration of therapy were shorter with continuous infusion. 22
The objective of this study was to elucidate the effects of burn and CVVH on meropenem PK using population PK modelling methodology. Monte Carlo simulations were performed to assess different dosing regimens in the setting of normal and augmented renal function and evaluate the impact on meropenem probability of target attainment (PTA). Our findings herein provide direct comparative analyses of these distinct, critically ill patient populations and suggest dosing recommendations to optimize therapeutic levels and improve clinical outcomes.
2. METHODS
2.1. Data
For the study, protocol and associated documents, including informed consent forms, were reviewed and approved by the Institutional Review Board (IRB) at the United States Army Medical Research and Development Command (MRDC; Fort Detrick, MD). De‐identified patient data was obtained from an IRB‐approved protocol at the USAISR Burn Center and Brooke Army Medical Center (BAMC) Surgical Trauma Intensive Care Unit (STICU). There was a total of 23 patients, 12 with no burn injury and 11 with burn injury. Of the 12 patients with no burn injury, four received CVVH, while six of 11 patients with burn injury received CVVH. The most commonly prescribed dose was 1000 mg meropenem every 8 hours (n = 13 patients). Three patients received 2000 mg meropenem every 8 hours, three patients received 1000 mg meropenem every 12 hours, and one patient received 500 mg meropenem every 12 hours. All doses were infused over 30 minutes to 1 hour. For each patient, pre‐filter plasma, post‐filter plasma and effluent samples were collected at steady state. There was one set of samples for each patient drawn prior to the dose. The pre‐dose plasma sample was a steady‐state trough draw; however, the time in relation with the previous dose was not recorded in our dataset. The remaining samples were drawn from 0.5 hours to 12 hours post‐dose (after the infusion began). There was a total of 95 pre‐filter plasma concentration observations post‐dose. The mean number of pre‐filter post‐dose plasma samples per patient was 4.13 (range of 3–5). There were no missing post‐filter concentration data in those receiving CVVH. Three patients had either missing albumin, weight on sampling day or urine output data. Mean values were imputed in these cases. Figure S1 in the Supporting Information demonstrates all time–concentration data after the end of infusion stratified by burn and CVVH.
2.2. High‐performance liquid chromatography (HPLC)
Meropenem concentrations from patient samples were determined by HPLC using a method previously validated in our laboratory. A Dionex 3000 HPLC system (Dionex, Thermo‐Fisher Inc., Sunnyvale, CA) with UV detection at 298 nm was used for analysis. Briefly, the mobile phases consisted of 0.2 borate buffer at pH 7.2 (mobile phase A), and 100% MeOH (mobile phase B). These were run at 0.6 mL/min isocratically (97:3) for 10 minutes, followed by a ramp of mobile phase B from 3% to 26% until 20 minutes. The stationary phase was a 150 mm octadecyl column (Luna 5u C18 100A 150 × 4.6 mm; Phenomenex, Torrance, CA). This resulted in retention times for imipenem (internal standard, IS) and meropenem of approximately 7.5 minutes and 20 minutes, respectively. Standard curves were constructed for meropenem by solubilizing standards in water (for unbound meropenem concentration) or by spiking human plasma (for total meropenem concentration) and injecting reference solutions of known concentrations of analyte and IS. Peak areas of the eluted drugs were integrated, and concentrations were quantified using peak area ratios of analyte to IS. Linearity was confirmed from 0.50 μg/mL to 25.0 μg/mL, with the mean (±SD) between‐day calibration curve regression r 2 = 0.9992 ± 0.0008. Between‐day coefficients of variation at 0.5 μg/mL and 25.0 μg/mL were 0.58% and 0.48%, respectively. The limit of quantitation for the assay was 250 ng/mL (0.025 mg/L).
Some 200 μL aliquots of each plasma sample were prepared for analysis by adding 10 μL of 500 μg/mL IS and 400 μL acetonitrile (MeCN). Following centrifugation (10 000g; 10 min), 600 μL of supernatant organic phase was decanted and evaporated to dryness using N2. The remaining residue was reconstituted in 100 μL methanol (MeOH), and 50 μL aliquots were injected into the HPLC for analysis. The concentration of drug in each sample was determined by regression analysis of the peak area ratios.
2.3. Population pharmacokinetic modelling and simulations
Population pharmacokinetic modelling and simulations were performed in Pumas (version 1.05). 23 The first order conditional estimation method with interaction (FOCEI) was used to estimate population parameters. Data preparation, exploratory analysis and graphs were performed in either Pumas or R (version 3.6.1). Data from all patients, both those with or without burn and with or without CVVH were modelled simultaneously. The CL due to CVVH for each individual patient (CLCVVH) was calculated as the product of the delivered ultrafiltrate flow rate ( ), the sieving coefficient ( and correction factor for pre‐filter fluid administration ( as follows:
| (1) |
where
| (2) |
and
| (3) |
where , , , denote the observed pre‐filter, post‐filter and effluent concentrations, denotes the blood flow rate and denotes the rate of pre‐filter replacement fluid. 24 , 25
2.4. Base model
One, two, and three compartment models were explored for this study. Between‐subject variability was modelled using an exponential error model under the assumption that pharmacokinetic parameters are distributed log normally. Parameters generally took the form
| (4) |
where is the post hoc estimated parameter value for individual , is the population mean parameter and is the between‐subject random effects for individual . Renal clearance estimates in the CVVH population may be inaccurate given creatinine is cleared via CVVH. In addition, patterns of CVVH prescription are likely to differ significantly in the critically ill burn population compared to other critically ill populations. Therefore, the base model categorized patients into subgroups to understand the relative inherent meropenem CL in the CVVH patients in both the burn and non‐burn populations, compared to those without CVVH. Base model equations were, for the burn population:
| (5) |
and for the non‐burn population:
| (6) |
Selection of the base model was based on the likelihood ratio test (LRT) with , plausibility and precision of parameter estimates, and diagnostic plots.
2.5. Covariate model
Covariates were initially evaluated by plotting random effects of PK parameters from the base model against each covariate and observing the trends. Covariates evaluated were total body weight (WT), lean body mass (LBM), creatinine clearance (CrCl), age, total burn surface area (TBSA), total second degree burn surface area (SDB), total third degree burn surface area (TDB), serum albumin (ALBUM) and urine output (UOP). CrCl was calculated by the Cockcroft‐Gault (C‐G) equation 26 and LBM was calculated using Janmahasatian's formula. 27 Continuous covariates modelled as
| (7) |
or
| (8) |
where is the PK parameter in individual , is the typical value of the PK parameter at the median value of the covariate ( ), is the covariate observed in individual , and is the power or slope estimate for the covariate. The only exception was the CrCl model, where a value of 125 mg/dL was used in place of .
Categorical covariates were modelled as
| (9) |
where is binary (coded as 0 or 1), represents the typical value of the PK parameter when and represents the proportional change in when . Covariate modelling was performed with a forward addition process. A decrease of at least 3.84 units ( ) in the objective function value (OFV) was considered statistically significant.
2.6. Final model qualification
Final model qualification was based on both internal and external evaluations. The internal evaluation included examination of standard goodness‐of‐fit plots, precision of parameter estimates based on inference and bootstrap methods (n = 1000 runs), visual predictive checks (200 replicates, overall and stratified by burn and CVVH). External model evaluation was performed comparing typical value of PK parameter estimates of our final model to those of existing literature.
2.7. Monte Carlo simulations
Monte Carlo simulations were performed with the final population PK model to evaluate efficacy in various critically ill sub‐populations. Probability of target attainment (PTA) was considered a surrogate for efficacy and was defined as achieving free meropenem concentrations above minimum inhibitory concentrations (MIC) greater than 40% of the time at steady state within the dosing interval ( ). A more stringent target of achieving meropenem concentrations above the MIC for greater than 99% of the dosing interval ( ) was also explored. Specific dosing regimens tested were 1000 mg Q8 hours infused over 1 hour or 3 hours or 1500 mg infused continuously Q12 hours. In the normal renal function (NRF) and ARC populations, double of these doses were also tested. Simulated dosing regimens for renal impairment populations and respective renal function categorization were obtained from the meropenem FDA label 28 (Figure S6 in the Supporting Information). ARC was simulated with CrCl = 150–250 mL/min, where NRF was simulated with CrCl = 100–130 mL/min. Patients were simulated with an LBM of 56 kg, corresponding to 70 kg with 20% body fat. For each scenario, 1000 patient concentration–time profiles were simulated. The percentage of simulated patients that achieved and were calculated at MICs ranging from 0.5 mg/L to 16 mg/L, with PTA > 90% considered acceptable.
3. RESULTS
3.1. Patient demographics
Patient demographics by CVVH status are summarized in Table 1. There were notable imbalances in gender, weight, creatinine and CrCl between the groups. The serum creatinine (Scr) and C‐G estimates of CrCl are to be interpreted with caution in the CVVH patients as Scr is cleared by the machine. There was a wide range of total body weight (median 84.5 kg, range 59.2–173.8 kg), reflected in part by the difference in fluid resuscitation in burn compared to non‐burn critically ill patients. LBM was still imbalanced amongst the subgroups, but to a lesser degree with a tighter range (median 61.7 kg, range 33.54–70.62 kg).
TABLE 1.
Patient demographics
| Demographic | No burn, no CVVH | No burn, CVVH | Burn, no CVVH | Burn, CVVH |
|---|---|---|---|---|
| Age (years) | 39.75 (17.72) | 52.5 (16.46) | 57.2 (23.02) | 40 (23.05) |
| Gender | 4 M 4F | 4 M 0F | 3 M 2F | 5 M 1F |
| Admission weight (kg) | 89.1 (22.05) | 90.6 (10.53) | 65.34 (7.30) | 103.23 (19.57) |
| Weight on sampling day (kg) | 96.29 (26.75) | 89.5 (17.66) | 68.26 (8.26) | 103.57 (36.69) |
| Lean body mass on day of sampling (kg) | 54.9 (12.37) | 64.28 (3.56) | 50.15 (9.17) | 66.35 (4.71) |
| Height (cm) | 159.28 (22.91) | 180.97 (18.25) | 168.66 (8.54) | 178.65 (6.95) |
| Total burn surface area (%) | 0 | 0 | 29.1 (21.93) | 36.54 (27.08) |
| Second degree burn (%) | 0 | 0 | 14.05 (10.08) | 15.88 (13.72) |
| Third degree burn (%) | 0 | 0 | 15.05 (15.41) | 20.67 (27.32) |
| Creatinine clearance (mL/min) a | 251.24 (219.08) | 105.69 (69.55) | 116.84 (64.18) | 179.32 (109.62) |
| Creatinine (mg/dL) b | 1.32 (1.6) | 1.57 (0.78) | 0.72 (0.19) | 1.23 (0.51) |
| Blood urea nitrogen (mg/dL) | 27.84 (23.27) | 29.6 (6.52) | 35.58 (12.37) | 34.62 (5.49) |
| Albumin (g/dL) | 2.43 (0.56) | 2.52 (0.25) | 2.8 (0.58) | 2.78 (0.99) |
| Urine output (mL) | 3439.5 (1633.84) | 387.25 (706.18) | 2377.2 (1021.84) | 851.72 (841.35) |
| Ultrafiltrate flow rate (mL/kg/h) | 32.7 (11.84) | 28.25(4.75) | ||
| CLCVVH (L/h) | 2.39 (0.49) | 1.97 (0.33) | ||
| Sieving coefficient | 0.99 (0.02) | 0.85 (0.18) | ||
| Correction factor | 0.87 (0.02) | 0.89 (0.05) |
CVVH filters serum creatinine, therefore serum creatinine observations and creatinine clearance estimates in patients treated with CVVH are not reflective of their true kidney function. These values must be interpreted with caution and are included only to elucidate large trends and for completeness.
A substantial discrepancy between mean (1.32 mg/dL) and median (0.54 mg/dL) serum creatinine was observed in this patient subgroup, largely driven by a single outlier with a serum creatinine of 4.71 mg/dL.
CVVH, continuous veno‐venous haemofiltration.
3.2. Population pharmacokinetic models
3.2.1. Base model
The model‐building process is summarized in Table S1 in the Supporting Information. The data was best described by a two‐compartment model with combined additive/proportional error model. The two‐compartment model as compared to a one‐compartment model led to a decrease of 25.1 units of the OFV. The combined additive/proportional error model led to a further decrease of 16.13 units of the OFV compared to the same model with only proportional error. The two‐compartment model was parameterized with terminal clearance , intra‐compartmental clearance , volume of the central compartment and volume of the peripheral compartment . Between‐subject variability was not estimated for .
3.2.2. Covariate model
Parameter estimates of the final model are summarized in Table 2. Covariate exploratory plots with random effects generated from the base model demonstrated trends between and CrCl, and LBM, and and TBSA. For patients without CVVH, CrCl was the preferred covariate for the CL model. In contrast, weight or LBM were preferred covariates for the CL model in patients with CVVH, as Scr is filtered by CVVH. Neither weight nor LBM was found to be a significant covariate on CL. However, LBM was included as a continuous covariate for CVVH patients with exponent fixed to 0.75 on CL to standardize the comparison of estimates between burn and non‐burn subgroups and allow for extrapolation to obtain better comparison to published estimates in the literature, where patients may differ in baseline demographics. For the non‐CVVH patients, CrCl was statistically significant (P = .001) and explained 16.93% of variability on CL. There was no significant difference in the inherent meropenem CL in the burn and non‐burn groups regardless of covariate parameterization. Of note, compared to other critically ill CVVH populations, burn patients are more likely to be prescribed CVVH with preserved kidney function. Reflective of this, compared to the typical value of CL, burn CVVH patients had an average 16% decrease inherent in CL, where non‐burn CVVH patients had an average 58% decrease in inherent CL.
TABLE 2.
Pharmacokinetic parameters for final model
| Parameter | FOCEI estimate (%RSE) | FOCEI 95% CI | Bootstrap estimate (95% CI) |
|---|---|---|---|
| CL (L/h) | 15.3 (18.3) | 9.81–20.79 | 15.3 (10.66–25.13) |
| Vc (L) | 33.21 (16.07) | 22.75–43.67 | 33.21 (20.65–44.7) |
| Q (L/h) | 14.1 (38.12) | 3.76–24.43 | 14.1 (4–35.35) |
| Vp (L) | 13.45 (32.74) | 4.82–22.07 | 13.45 (3.32–27.75) |
| Covariates on CL | |||
| Weight (power) | 0.75 fixed | ‐ | ‐ |
| CrCL (power) | 0.74 (20.49) | 0.44–1.03 | 0.74 (0.26–1.27) |
| CVVH & Burn (categorical 1+) | −0.16 (150) | −0.63 to 0.31 | −0.16 (−0.61 to 0.51) |
| CVVH & no Burn (categorical 1+) | −0.58 (16.74) | −0.77 to −0.39 | −0.58 (−0.77 to −0.34) |
| Covariates on Vc | |||
| Weight (power) | 1 fixed | ‐ | ‐ |
| Covariates on Vp | |||
| TBSA (linear) | 0.71 (69.52) | 0–1.68 | 0.71 (0–4.5) |
| Random effects | |||
| ω2 CL | 0.31 (29.51) | 0.13–0.49 | 0.31 (0.11–0.46) |
| ω2 Vc | 0.35 (34.31) | 0.11–0.58 | 0.35 (0.028–0.65) |
| ω2 VP | 0.61 (46.94) | 0.049–1.17 | 0.61 (0–2.89) |
|
η‐Shrinkage CL: 0.32%, η‐shrinkage Vc: 20.99%, η‐shrinkage Vp: 32.55% Pearson's correlation coefficients: η‐Vc & η‐CL: 0.125, η‐Vp & η‐CL: 0.047, η‐Vc & η‐Vp: 0.023 | |||
| Residual unexplained variability | |||
| Proportional error | 0.22 (18.25) | 0.14–0.3 | 0.22 (0.13–0.31) |
| Additive error (mg/L) | 0.0056 (40.65) | 0.0011–0.01 | 0.0056 (0–0.12) |
| ɛ‐Shrinkage: 25.5% | |||
CI, confidence interval; CVVH, continuous veno‐venous haemofiltration; FOCEI, first order conditional estimation method with interaction; TBSA, total burn surface area.
Regarding volume, both weight and LBM were statistically significant covariates on (P = .009 and .045, respectively). LBM was chosen over body weight as it provided a more uniform comparison of weight between the different critically ill subgroups. The estimated exponent for LBM on was implausibly high at 2.47, and was fixed to 1 without a significant change in the OFV (1.2‐unit increase). This was incorporated into the model with similar rationale as for CL, to allow for extrapolation and improved external comparison to previously published estimates in the literature. TBSA was found to be statistically significant on when parameterized as a continuous linear model (P = .03). However, burn was not statistically significant on when parameterized as a categorical covariate. Despite this mixed statistical evidence, TBSA on was retained in the final model as we sought to explore the effect of burn on PTA.
The final equation for in patients without CVVH, regardless of burn or not was
| (10) |
the final equation for in patients with burn and CVVH was
| (11) |
and the final equation for in patients without burn and CVVH was
| (12) |
The final equations for central and peripheral volume of distribution in all populations were
| (13) |
| (14) |
respectively.
3.2.3. Validation of final model
Goodness‐of‐fit plots revealed model predictions to be randomly scattered around the line of unity. There were no significant trend plots of conditional weighted residuals versus time or conditional weighted residuals versus predicted concentrations (Figure 1). Individual fit plots demonstrated that both the population and individual predicted concentrations fit reasonably well to the observed data (Figure S2 in the Supporting Information). Histograms of conditional weighted residuals and between‐subject variability random effects were consistent with normally distributed data centred at 0 (Figure S3 in the Supporting Information). Visual predictive checks demonstrated that the observed data and quantiles fell within the simulated 95% CIs (Figure S4, in the Supporting Information). Plots of random effects vs covariates appropriately demonstrate eliminated or diminished trends upon inclusion of the covariate in the final model (Figure S5 in the Supporting Information). External validation demonstrated that mean parameters are consistent with previously published results in all subgroups explored by our model (Table 3).
FIGURE 1.
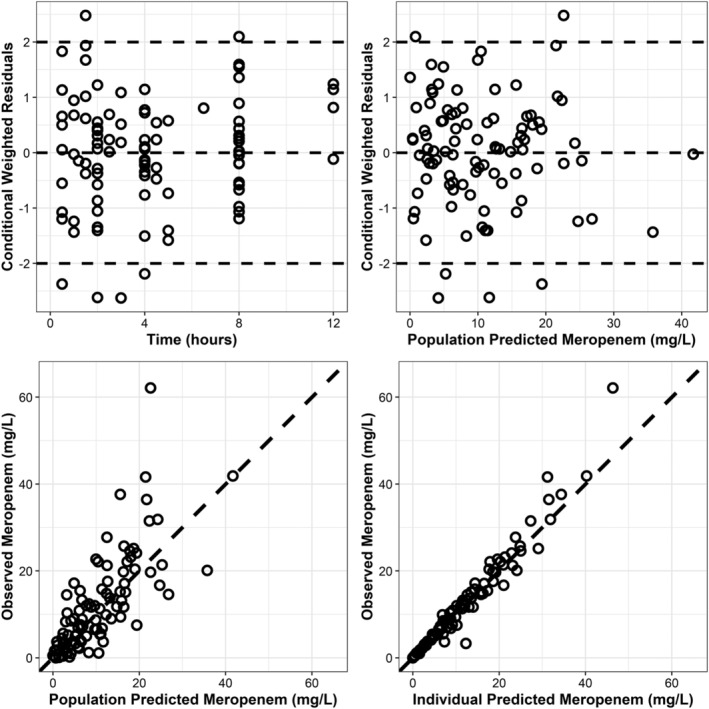
Goodness‐of‐fit plots
TABLE 3.
Comparison of final model predicted meropenem mean PK parameters to literature reported mean PK parameters in burn and critically ill patients treated with or without CRRT
| Population | Conditions a | Final model predicted mean parameters b | Literature reported mean parameters | References |
|---|---|---|---|---|
| Burn (no CRRT) |
Albumin = 2.10 g/dL LBW = 58.11 kg CrCl = 136.17 mL/min %TBSA = 41.43 |
Vc = 31.27 L Vp = 42.56 L Cl = 16.31 L/h |
Vc = 22.8–44.4 L Vp = 10.1–15.0 L Cl = 14.95–19.0 L/h |
Corcione S, et al., 2021 (LBW = 60.03 kg; %TBSA = 34; CrCl = 134 mL/min) Ramon‐Lopez A, et al., 2014 (LBW = 61.44 kg; %TBSA = 41; CrCl = 136.5 mL/min) Doh K, et al., 2010 (LBW = 52.82; %TBSA = 49.3; CrCl = 138 mL/min) |
| Critically ill (no CRRT) |
Albumin = 2.15 g/dL LBW = 56.90 kg CrCl = 75.90 mL/min |
Vc = 31.27 L Vp = 13.45 L Cl = 10.58 L/h |
Vc = 7.9–45.8 L Vp = 9.18–14.8 L Cl = 7.34–13.6 L/h |
Eisert A, et al., 2021 (LBW = 60.92 kg; CrCl = 64.5 mL/min) Sjovall F, et al., 2018 (LBW = 63.79 kg; CrCl = 67 mL/min) Muro T, et al., 2011 (LBW = 45.81 kg; CrCl = 65.5 mL/min) Roberts JA, et al., 2009 (LBW = 58.38 kg; CrCl = 99.5 mL/min) Li C, et al., 2006 (LBW = 55.61 kg; CrCl = 83 mL/min) |
| Critically ill (CRRT) |
Albumin = 2.32 g/dL LBW = 58.22 kg CrCl = 43.85 mL/min c |
Vc = 31.34 L Vp = 13.45 L Cl = 6.15 L/h |
Vc = 14–69.5 L Vp = 14–33.7 L Cl = 2.4–8.04 L/h |
Onichimowski D, et al., 2020 (LBW = 61.33 kg; CrCl = 50.3 mL/min) d Burger R, et al., 2018 (LBW = 58.14 kg; CrCl = not reported) Ulldemolins M, et al., 2015 (LBW = 55.68 kg; CrCl = not reported) Bilgrami I, et al., 2010 (LBW = 56.98 kg; CrCl = not reported) Isla A, et al., 2008 (LBW = 55.8 kg; CrCl = 37.4 mL/min) Ververs TFT, et al., 2000 (LBW = 62.68 kg; CrCl = not reported) |
Conditions used to predict mean parameters were calculated as the mean of demographics listed in the reference for each respective subgroup. When LBW was not available from an individual reference, Janmahasatian's formula was used to calculate LBW based off of mean demographics in the respective reference.
In all subgroups, Cl refers to inherent or residual meropenem Cl (not including Cl due to CRRT).
CrCl is only included here for completeness. Model‐based estimates in this subgroup were calculated using Equation 12 (the weight‐based Cl model).
Onichimowski D, et al. reported a mean meropenem Cl of 15.1 mL/h in septic patients with CRRT (mean ultrafiltrate flow 2753 mL/h, corresponding to approximately 2.7 L/hr meropenem Cl). After accounting for CRRT, this meropenem Cl is still significantly greater than other literature reported estimates for this patient subgroup and therefore was not included in the main text of the table.
CrCl, creatinine clearance; CRRT, continuous renal replacement therapy; LBW, lean body weight; PK, pharmacokinetic; TBSA, total burn surface area.
3.2.4. Simulations and probability of target attainment
Mean simulations in a patient with LBM 56 kg and CrCl 124.91 mL/min, dosed 1000 mg Q8 hours at steady state, demonstrated that increasing infusion time generally improves meropenem fT > MIC up to an MIC of 8 mg/L (Figure 2a). Mean simulations also showed a loading dose would be required prior to a continuous infusion to achieve adequate meropenem concentrations and steady state in a timely manner (Figure 2b). When considering as the ideal target, PTA demonstrated 1000 mg Q8 hours infused over 1 hour or 3 hours would be an adequate regimen to treat a patient with NRF and infected with a pathogen having MIC of 2–4 mg/L, regardless of %TBSA (Figure 3) or CVVH (Figure 4). However, patients with ARC would only be able to achieve of 1–2 mg/L at this dose. To achieve a PTA target of 2 mg/L in ARC patients, either doses of 2000 mg Q8 hours or a continuous infusion with 3000 mg or more per day are required (Figure S6 in the Supporting Information). Of note, a continuous infusion with 3000 mg total per day would be sufficient to achieve PTA targets of 4 mg/L in patients with NRF and 2 mg/L in patients with ARC. Further, a continuous infusion of 6000 mg total per day would be sufficient to achieve PTA targets of 8 mg/L in patients with NRF and 4 mg/L in patients with ARC.
FIGURE 2.
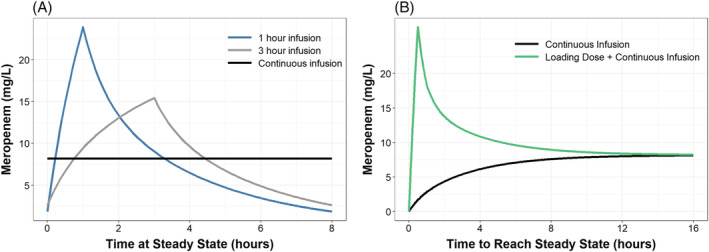
Mean simulations in a patient with LBM = 56 kg, CrCl = 125 and without CVVH. Figure 2(A) demonstrates the typical pharmacokinetic profiles when dosing meropenem 1000 mg every 8 hours at steady state with various infusion times. Figure 2(B) shows the time to reach steady state in a continuous infusion of 1500 mg every 12 hours, compared to a 1000 mg loading dose over 30 minutes followed by the same continuous infusion starting 1 hour later
FIGURE 3.
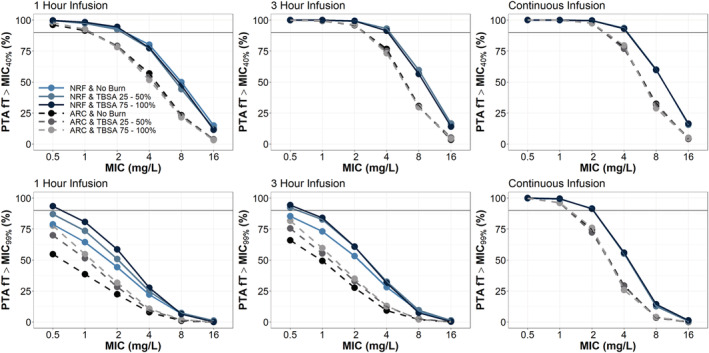
PTA results from simulations with meropenem 1000 mg Q8 hours infused over 1 hour, 3 hours or continuous infusion of 1500 mg Q12 hours. Patients (1000 per group) were simulated with varying degrees of total burn surface area (TBSA), normal renal function (NRF, CrCl = 100–130 mL/min) or augmented renal clearance (ARC, 150–250 mL/min) and fixed lean body mass (LBM) = 56 kg
FIGURE 4.
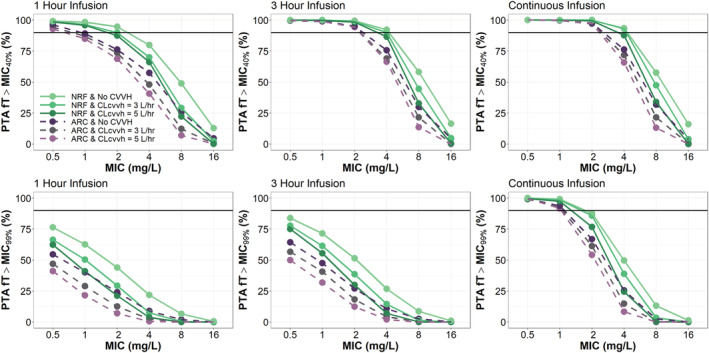
PTA results from simulations with meropenem 1000 mg Q8 hours infused over 1 hour, 3 hours or continuous infusion of 1500 mg Q12 hours. Patients (1000 per group) were simulated with varying prescriptions of CVVH (0 mL/kg/h, 42.9 mL/kg/h and 71.4 mL/kg/h), normal renal function (NRF, CrCl = 100–130 mL/min) or augmented renal clearance (ARC, 150–250 mL/min) and fixed lean body mass (LBM) = 56 kg
When considering as the ideal target, only continuous infusion dosing strategies would consistently be able to achieve adequate exposure to treat pathogens with MICs ≥ 0.5 mg/L. If a more stringent target is desired, a continuous infusion with 3000 mg total per day would be sufficient to achieve PTA targets of 2 mg/L in patients with NRF and 1 mg/L in patients with ARC (Figure 3). A continuous infusion of 6000 mg total per day would be sufficient to achieve PTA targets of 4 mg/L in patients with NRF and 2 mg/L in patients with ARC (Figure S6 in the Supporting Information). Unlike the target, where CVVH had minimal effect on PTA, patients with NRF and CVVH could only achieve targets equivalent to ARC patients as above. For the , burn severity was associated with improvement in PTA for the Q8 hour dosing strategies, but had minimal effect on the PTA for the continuous infusion dosing strategy.
4. DISCUSSION
Appropriate antibiotic dosing is essential in the care of critically ill patients, to effectively manage infection but also to reduce the development of antibiotic‐resistant bacteria. Extensive evaluation of meropenem PK has provided considerable evidence to support current clinical dosing regimens to achieve appropriate PK/PD targets. 11 , 15 , 16 , 17 , 20 Our findings from this study provide comparative data analyses of critically ill patients, burn and non‐burn, treated with or without CVVH. In accordance with existing literature, we found that a two‐compartment model best fit the data with CrCl identified as a significant covariate on meropenem Cl (Figure S1 in the Supporting Information). Importantly, PTA simulations suggest neither CVVH nor %TBSA necessitate dosing adjustments; however, continuous infusion and/or an increased dose of meropenem may be required in cases of suspected ARC or clinical scenarios that demand a more stringent PK/PD target (i.e., ƒT > MIC99%).
Of note, burn demonstrated mixed statistical evidence as a significant covariate on Vp. Burn as a categorical covariate was not statistically significant on Vp, but TBSA as a linear covariate was statistically significant (Table S1, Models 17–20). Although the comparison is indirect, prior literature shows similar Vc and Vp in both the burn and non‐burn populations, providing further evidence that burn is unlikely a significant covariate on meropenem Vc or Vp. Nevertheless, we included TBSA on Vp in the final model to investigate via simulation the effect of large changes in peripheral volume on meropenem PTA. We found that large changes in Vp have minimal effect on PTA, and dosing changes would not be required. We also found that burn is not a statistically significant covariate on meropenem Cl, which is consistent with prior literature after accounting for differences in CrCl (Table 3). Therefore, even the most severe burn injuries are unlikely to require dosing changes, on the consideration of PK alone, in the absence of renal failure or ARC.
This was consistent with our PTA simulations, which indicate that continuous infusion and/or dosing adjustments for intermittent infusion may be necessary in patients with ARC (Figure 3). These findings are also in agreement with previous literature that supports continuous or extended infusion of meropenem in critically ill patients, 22 , 29 , 30 which has been to shown to translate to higher clinical cure rates and better PK/PD target attainment. 31 Although CVVH had minimal impact on PTA in simulated patients with NRF or ARC, target attainment in the setting of ARC was more readily achieved with continuous infusion, specifically at the more stringent PK/PD target, ƒT > MIC99% (Figure 4). Thus, in the setting of ARC, or clinical scenarios requiring the most stringent PK/PD targets, increased meropenem doses (3000–6000 mg, 24 hrs) via continuous infusion may be required to adequately achieve therapeutic levels.
Although a continuous infusion may more consistently achieve desired ƒT > MIC, there is mixed evidence on clinical cure rates compared to intermittent infusion. 22 , 31 , 32 , 33 Further, continuous infusion may take several hours before achieving desired steady state concentrations, necessitating an initial loading dose and additional loading doses if the infusion is interrupted for more than 3–4 hours. A reasonable loading dose would be 1000–2000 mg infused over 30 minutes to 1 hour, based on the clinical severity and desired PKPD target corresponding to total daily doses of 3000–6000 mg. Other considerations are the constant use of an intravenous line and potential for frequent interruptions in the ICU setting, making continuous infusion less practical.
For this study, we chose to model data from all patients simultaneously, regardless of burn or CVVH status. This approach allowed for a direct statistical comparison of PK parameters in the different ICU subgroups via covariate analysis. The main limitation to this modelling approach is significant variability in patient characteristics and provider practices among different ICU patient populations. This limitation is highlighted by a recent study that demonstrated bias and variable predictive accuracy when extrapolating results of their meropenem PK model to external ICU populations. 34 In reference to our ICU populations, burn patients may have physiologic derangements, namely severe capillary leak, that make them not comparable to surgical ICU patients. Provider practices with fluid resuscitation and thresholds to implement CVVH may also differ heavily between burn and surgical ICU populations. 35 , 36 , 37 Therefore, in order to maximize the clinical relevance of our statistical comparisons, we decided to use lean body weight (LBW) as a covariate and categorically group patients into burn with or without CVVH and non‐burn with or without CVVH as the base model.
By implementing LBW as a covariate, we minimized the effect of weight attributed to fluid resuscitation or fluid loss via capillary leak in the burn population and provide a more uniform comparison between burn and surgical ICU patients. Regarding the decision to categorize the base model, it is important to note that CVVH is associated with a mortality benefit in burn patients with shock. 38 , 39 Although the subset of burn patients that benefit the most from CVVH is not firmly established, and results have not consistently achieved statistical significance, CVVH has a large reported effect size (33% absolute reduction) in 28‐day mortality compared to historic controls. For this reason, CVVH is implemented early and aggressively in the burn population at the US Army Burn Center, including in patients with preserved renal function. CVVH in other ICU populations is typically reserved for patients with definitive renal failure, while a moderate portion of burn patients receiving CVVH have only stage 1 AKI (22%) or no AKI at all (6%). 35
Meropenem is almost exclusively renally cleared and CrCl has the largest effect size on meropenem Cl and dosing requirements. Thus, with improper categorization, it may appear that burn itself is the cause of increased meropenem Cl, rather than an artifact of preserved renal function in burn patients receiving CVVH due to aggressive prescribing patterns. This conclusion was made by Li and Xie who noted that burn imparted an 82% increase in imipenem Cl compared to non‐burn patients all receiving CRRT. 40 Importantly, this study did not specify differences in provider prescribing practices of CRRT and that their finding could be entirely explained by preserved renal function in the burn CRRT population.
By categorizing the base model, standardizing Cl and Vd by LBW and including data from all ICU subgroups, we provided clinically relevant direct statistical comparison and demonstrated that burn did not have a significant effect on meropenem Cl. This approach also allowed us to demonstrate that burn patients treated with CVVH had only a 16% reduction in inherent meropenem Cl compared to those not treated with CVVH, where the STICU patients treated with CVVH had a 58% reduction in inherent meropenem Cl compared to those not treated with CVVH. These results likely reflect the differing CVVH prescribing practices amongst these ICU subpopulations. An alternative approach would have been to model all the subgroups separately; however, this would not have allowed for direct statistical comparison and the sample size of some subgroups was small (n = 4), possibly leading to less precise parameter estimates.
We utilized the C‐G equation to estimate CrCl in this study, although it is generally known to overestimate CrCl and consequently may not accurately predict CrCl in ICU populations. We also noted a surprisingly high mean CrCl in the no burn and no CVVH group (251.24 ± 219.08 mL/min) that was discrepant from calculating the mean CrCl based off of mean demographics in that subgroup (102 mL/min). This is explained by a large difference in the mean (1.32 mg/dL) and median (0.54 mg/dL) serum creatinine observed in this patient subgroup, largely driven by a single outlier with a serum creatinine of 4.71 mg/dL. Nevertheless, our CrCl covariate model with exponent estimate of 0.74 (including 1 in the 95% confidence interval) is highly consistent with the literature, where multiple ICU papers estimate the CrCl exponent to be 1 with similar covariate parametrizations. 34 , 41 , 42 Table 3 demonstrates our model predicts the mean meropenem CL reported in the literature reasonably well when extrapolating to similar mean CrCls reported in those manuscripts. Therefore, although there are limitations to our CrCl data, our CrCl covariate model is reasonable and performs its function of accounting for CrCl prior to making conclusions about the effect of burn on meropenem CL. Using the Jelliffe equation may have led to more accurate CrCl estimates 43 ; however, it is not validated in the burn population. In addition, due to the extensive use of the C‐G equation in clinical practice, and its ability to outperform other equations in terms of predictive performance for a number of antibiotics, 29 , 44 we selected this method for CrCl estimation.
A final consideration is the limitation of our CrCl model to extrapolate to the ARC population. Doh et al. measured CrCl by 24‐hour urine collection in 44 patients and demonstrated a linear relationship of CrCl to estimated renal meropenem CL. 41 Our estimate of exponent 0.74 is consistent with the CrCl model of Doh et al. and therefore, for the purpose of an exploratory PTA analysis, our CrCl model is adequate. Further, although the use of CVVH in a patient with ARC is unlikely, commonly cited reasons for antibiotic failure are ARC and CVVH. In addition, we hypothesize there is a small, but clinically relevant proportion of critically ill patients with burn that have undiagnosed ARC and are incidentally prescribed CVVH. Therefore, we sought to investigate the theoretical impact of such conditions on antibiotic failure as an exploratory analysis. We found that a high proportion of patients would be able to achieve adequate meropenem exposure with commonly used ICU doses to target clinically relevant MICs despite a combination of ARC and CVVH (Figures 4 and S6). This finding was surprising and should alert clinicians to promptly investigate reasons for antibiotic failure. These investigations could include either therapeutic drug monitoring or more accurate assessments of CrCl. However, empiric antibiotic dose increases or alternative antibiotic selection would be appropriate alternatives given severity of illness and possibility of significant delay in adequate antibiotic therapy awaiting test results.
Emerging evidence amassed from a variety of critically ill patient populations has provided clinical insight into the impact of renal failure, sepsis and/or burn on meropenem PK. 22 , 41 , 45 , 46 , 47 Collectively, these data have demonstrated the impact of critical injuries and altered physiology on the disposition of meropenem, specifically with regard to clearance and attainment of therapeutic levels. To our knowledge, this is the first study to directly compare the effect of burn and non‐burn injury on meropenem PK in critically ill patients with and without CVVH. Parameter estimates generated from this study align with previously published literature, while PTA simulations support clinical data that suggests loading doses and continuous infusions of meropenem may be required to achieve therapeutic levels and improve patient outcomes. We recommend a standard dose of 1000 mg every 8 hours; however, if ARC is suspected, or the severity of illness requires a more stringent therapeutic target, we recommend a loading dose of 1000–2000 mg infused over 30 minutes to 1 hour followed by continuous infusion (3000–6000 mg over 24 hours), or intermittent infusion of 2000 mg every 8 hours.
COMPETING INTERESTS
The authors have no conflicts of interest to report. Material has been reviewed by the Walter Reed Army Institute of Research, the Uniformed Services University of the Health Sciences and the United States Institute of Surgical Research. There is no objection to its presentation and/or publication. The views expressed in this article are those of the authors and do not reflect the official policy or position of the US Army Medical Department, Department of the Army, DoD or the US Government.
CONTRIBUTORS
All authors contributed significantly to the work and met the criteria of authorship as defined by the ICMJE.
Supporting information
TABLE S1 Model building process
FIGURE S1 Linear (left) and semi‐log (right) plots of raw data with LOESS trend lines (thick solid lines) after the end‐of‐infusion stratified by burn and CVVH. The black dashed line is the LOESS trend of all data combined
FIGURE S2 Individual goodness‐of‐fit plots. Open circles represent observed concentrations, solid black lines represent individual predicted concentrations and blue dashed lines represent population predicted concentrations. Burn and CVVH are noted next to the pseudo‐id. (Right panel log‐linear scale, left panel linear scale)
FIGURE S3 Histogram of conditional weighted residuals and BSV diagnostic plots of final model
FIGURE S4 Visual predictive checks comparing simulated and observed time–concentration data at the 5th, 50th and 95th percentiles (200 replicates). Purple circles represent observed data, red lines represent observed quantile trend lines, black lines represent simulated quantile trend lines and gray bands represent simulated 95% CI
FIGURE S5 Eta vs covariate plots. Left columns are plots from base model, right columns are plots from final model
FIGURE S6 PTA results from simulations with meropenem 2000 mg Q8 hours infused over 1 hour, 3 hours or continuous infusion of 3000 mg Q12 hours. Patients (1000 per group) were simulated with varying prescriptions of CVVH (0 mL/kg/h, 42.9 mL/kg/h and 71.4 mL/kg/h), normal renal function (NRF, CrCl = 100–130 mL/min) or augmented renal clearance (ARC, 150–250 mL/min) and fixed lean body mass (LBM) = 56 kg
ACKNOWLEDGEMENTS
We thank Ms. Zanete Wright for her support of the WRAIR/USUHS Clinical Pharmacology Fellowship. We also thank the participating subjects, clinical research coordinators and laboratory personnel, without whose assistance this research would not have been possible.
This work was supported by the WRAIR/USUHS Clinical Pharmacology Fellowship P8 funds.
Selig DJ, Akers KS, Chung KK, Pruskowski KA, Livezey JR, Por ED. Meropenem pharmacokinetics in critically ill patients with or without burn treated with or without continuous veno‐venous haemofiltration. Br J Clin Pharmacol. 2022;88(5):2156-2168. doi: 10.1111/bcp.15138
This was an observational study with no experimental interventions. Patients were cared for under the supervision of the respective intensive care unit attending physician(s) while enrolled in the study. MAJ Elaine Por was the site principal investigator at Walter Reed Army Institute of Research and LTC Kevin Akers was the site principal investigator at the United States Army Institute of Surgical Research.
Funding information WRAIR/USUHS Clinical Pharmacology Fellowship P8
DATA AVAILABILITY STATEMENT
Data presented in this article cannot be shared. For any other questions, please contact the corresponding author.
REFERENCES
- 1. Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient—concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev. 2014;77:3‐11. 10.1016/j.addr.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 2. Brusselaers N, Monstrey S, Colpaert K, Decruyenaere J, Blot SI, Hoste EA. Outcome of acute kidney injury in severe burns: a systematic review and meta‐analysis. Intensive Care Med. 2010;36(6):915‐925. 10.1007/s00134-010-1861-1 [DOI] [PubMed] [Google Scholar]
- 3. Leblanc M, Thibeault Y, Querin S. Continuous haemofiltration and haemodiafiltration for acute renal failure in severely burned patients. Burns. 1997;23(2):160‐165. 10.1016/s0305-4179(96)00085-x [DOI] [PubMed] [Google Scholar]
- 4. Merchant M, Kim C, Grossman P. Acute Kidney Injury and CRRT in Burn Patients. Proceedings of UCLA Health; 2018:22.
- 5. Roberts JA, Abdul‐Aziz MH, Lipman J, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14(6):498‐509. 10.1016/S1473-3099(14)70036-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bagshaw SM, Berthiaume LR, Delaney A, Bellomo R. Continuous versus intermittent renal replacement therapy for critically ill patients with acute kidney injury: a meta‐analysis. Crit Care Med. 2008;36(2):610‐617. 10.1097/01.CCM.0B013E3181611F552 [DOI] [PubMed] [Google Scholar]
- 7. Gordon AC, Mason AJ, Thirunavukkarasu N, et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. JAMA. 2016;316(5):509‐518. 10.1001/jama.2016.10485 [DOI] [PubMed] [Google Scholar]
- 8. Cerda J, Ronco C. Modalities of continuous renal replacement therapy: technical and clinical considerations. Semin Dial. 2009;22(2):114‐122. 10.1111/j.1525-139X.2008.00549.x [DOI] [PubMed] [Google Scholar]
- 9. Ruiz EF, Ortiz‐Soriano VM, Talbott M, et al. Development, implementation and outcomes of a quality assurance system for the provision of continuous renal replacement therapy in the intensive care unit. Sci Rep. 2020;10(1):20616. 10.1038/s41598-020-76785-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roberts DM, Liu X, Roberts JA, et al. A multicenter study on the effect of continuous hemodiafiltration intensity on antibiotic pharmacokinetics. Crit Care. 2015;19(1):84. Published 2015 Mar 13. 10.1186/s13054-015-0818-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burger R, Guidi M, Calpini V, et al. Effect of renal clearance and continuous renal replacement therapy on appropriateness of recommended meropenem dosing regimens in critically ill patients with susceptible life‐threatening infections. J Antimicrob Chemother. 2018;73(12):3413‐3422. 10.1093/jac/dky370 [DOI] [PubMed] [Google Scholar]
- 12. Craig WA. The pharmacology of meropenem, a new carbapenem antibiotic. Clin Infect Dis. 1997;24(Suppl 2):S266‐S275. 10.1093/clinids/24.supplement_2.s266 [DOI] [PubMed] [Google Scholar]
- 13. De Waele JJ, Lipman J, Akova M, et al. Risk factors for target non‐attainment during empirical treatment with beta‐lactam antibiotics in critically ill patients. Intensive Care Med. 2014;40(9):1340‐1351. 10.1007/s00134-014-3403-8 [DOI] [PubMed] [Google Scholar]
- 14. Dulhunty JM, Roberts JA, Davis JS, et al. A Multicenter Randomized Trial of Continuous versus Intermittent beta‐Lactam Infusion in Severe Sepsis. Am J Respir Crit Care Med. 2015;192(11):1298‐1305. 10.1164/rccm.201505-0857OC [DOI] [PubMed] [Google Scholar]
- 15. Goncalves‐Pereira J, Povoa P. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of beta‐lactams. Crit Care. 2011;15(5):R206. 10.1186/cc10441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Robatel C, Decosterd LA, Biollaz J, Eckert P, Schaller MD, Buclin T. Pharmacokinetics and dosage adaptation of meropenem during continuous venovenous hemodiafiltration in critically ill patients. J Clin Pharmacol. 2003;43(12):1329‐1340. 10.1177/0091270003260286 [DOI] [PubMed] [Google Scholar]
- 17. Krueger WA, Schroeder TH, Hutchison M, et al. Pharmacokinetics of meropenem in critically ill patients with acute renal failure treated by continuous hemodiafiltration. Antimicrob Agents Chemother. 1998;42(9):2421‐2424. 10.1128/AAC.42.9.2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steele AN, Grimsrud KN, Sen S, Palmieri TL, Greenhalgh DG, Tran NK. Gap analysis of pharmacokinetics and pharmacodynamics in burn patients: a review. J Burn Care Res. 2015;36(3):e194‐e211. 10.1097/BCR.0000000000000120 [DOI] [PubMed] [Google Scholar]
- 19. Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37(3):840‐851; quiz 859. 10.1097/CCM.0b013e3181961bff [DOI] [PubMed] [Google Scholar]
- 20. Ariano RE, Nyhlen A, Donnelly JP, Sitar DS, Harding GK, Zelenitsky SA. Pharmacokinetics and pharmacodynamics of meropenem in febrile neutropenic patients with bacteremia. Ann Pharmacother. 2005;39(1):32‐38. 10.1345/aph.1E271 [DOI] [PubMed] [Google Scholar]
- 21. Li C, Du X, Kuti JL, Nicolau DP. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother. 2007;51(5):1725‐1730. 10.1128/AAC.00294-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chytra I, Stepan M, Benes J, et al. Clinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: a randomized open‐label controlled trial. Crit Care. 2012;16(3):R113. 10.1186/cc11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rackauckas C, Ma Y, Noack A, et al. Accelerated predictive healthcare analytics with Pumas, a high performance pharmaceutical modeling and simulation platform. bioRxiv. 2020:1‐27. 10.1101/2020.11.28.402297 [DOI]
- 24. Choi G, Gomersall CD, Tian Q, Joynt GM, Li AM, Lipman J. Principles of antibacterial dosing in continuous renal replacement therapy. Blood Purif. 2010;30(3):195‐212. 10.1159/000321488 [DOI] [PubMed] [Google Scholar]
- 25. Hulko M, Haug U, Gauss J, Boschetti‐de‐Fierro A, Beck W, Krause B. Requirements and pitfalls of dialyzer sieving coefficients comparisons. Artif Organs. 2018;42(12):1164‐1173. 10.1111/aor.13278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. FDA . Guidance for Industry Pharmacokinetics in Patients with Impaired Renal Function – Study Design, Data Analysis, and Impact on Dosing. https://www.fda.gov/media/78573/download. Accessed May 4, 2021.
- 27. Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44(10):1051‐1065. 10.2165/00003088-200544100-00004 [DOI] [PubMed] [Google Scholar]
- 28. FDA . Label: Merrem IV (meropenem for injection). https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/050706s037lbl.pdf. Accessed May 4, 2021.
- 29. Minichmayr IK, Roberts JA, Frey OR, Roehr AC, Kloft C, Brinkmann A. Development of a dosing nomogram for continuous‐infusion meropenem in critically ill patients based on a validated population pharmacokinetic model. J Antimicrob Chemother. 2018;73(5):1330‐1339. 10.1093/jac/dkx526 [DOI] [PubMed] [Google Scholar]
- 30. Ververs TF, van Dijk A, Vinks SA, et al. Pharmacokinetics and dosing regimen of meropenem in critically ill patients receiving continuous venovenous hemofiltration. Crit Care Med. 2000;28(10):3412‐3416. 10.1097/00003246-200010000-00006 [DOI] [PubMed] [Google Scholar]
- 31. Abdul‐Aziz MH, Sulaiman H, Mat‐Nor MB, et al. Beta‐Lactam Infusion in Severe Sepsis (BLISS): a prospective, two‐centre, open‐labelled randomised controlled trial of continuous versus intermittent beta‐lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016;42(10):1535‐1545. 10.1007/s00134-015-4188-0 [DOI] [PubMed] [Google Scholar]
- 32. Taccone FS. Continuous infusion of meropenem in critically ill patients: practical considerations. Crit Care. 2012;16(4):444. Published 2012 Aug 27. 10.1186/cc11459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao HY, Gu J, Lyu J, et al. Pharmacokinetic and pharmacodynamic efficacies of continuous versus intermittent administration of meropenem in patients with severe sepsis and septic shock: a prospective randomized pilot study. Chin Med J (Engl). 2017;130(10):1139‐1145. 10.4103/0366-6999.205859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dhaese SAM, Farkas A, Colin P, et al. Population pharmacokinetics and evaluation of the predictive performance of pharmacokinetic models in critically ill patients receiving continuous infusion meropenem: a comparison of eight pharmacokinetic models. J Antimicrob Chemother. 2019;74(2):432‐441. 10.1093/jac/dky434 [DOI] [PubMed] [Google Scholar]
- 35. Chung KK, Coates EC, Hickerson WL, et al. Renal replacement therapy in severe burns: a multicenter observational study. J Burn Care Res. 2018;39(6):1017‐1021. 10.1093/jbcr/iry036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kang D, Yoo KY. Fluid management in perioperative and critically ill patients. Acute Crit Care. 2019;34(4):235‐245. 10.4266/acc.2019.00717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pham TN, Cancio LC, Gibran NS, American Burn Association . American Burn Association practice guidelines burn shock resuscitation. J Burn Care Res. 2008;29(1):257‐266. 10.1097/BCR.0b013e31815f3876 [DOI] [PubMed] [Google Scholar]
- 38. Chung KK, Lundy JB, Matson JR, et al. Continuous venovenous hemofiltration in severely burned patients with acute kidney injury: a cohort study. Crit Care. 2009;13(3):R62. 10.1186/cc7801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hill DM, Rizzo JA, Aden JK, Hickerson WL, Chung KK, RESCUE Investigators . Continuous venovenous hemofiltration is associated with improved survival in burn patients with shock: a subset analysis of a multicenter observational study. Blood Purif. 2021;50(4‐5):473‐480. 10.1159/000512101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li S, Xie F. Population pharmacokinetics and simulations of imipenem in critically ill patients undergoing continuous renal replacement therapy. Int J Antimicrob Agents. 2019;53(1):98‐105. 10.1016/j.ijantimicag.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 41. Doh K, Woo H, Hur J, et al. Population pharmacokinetics of meropenem in burn patients. J Antimicrob Chemother. 2010;65(11):2428‐2435. 10.1093/jac/dkq317 [DOI] [PubMed] [Google Scholar]
- 42. Isla A, Rodriguez‐Gascon A, Troconiz IF, et al. Population pharmacokinetics of meropenem in critically ill patients undergoing continuous renal replacement therapy. Clin Pharmacokinet. 2008;47(3):173‐180. 10.2165/00003088-200847030-00003 [DOI] [PubMed] [Google Scholar]
- 43. Bouchard J, Macedo E, Soroko S, et al. Comparison of methods for estimating glomerular filtration rate in critically ill patients with acute kidney injury. Nephrol Dial Transplant. 2010;25(1):102‐107. 10.1093/ndt/gfp392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Charhon N, Neely MN, Bourguignon L, Maire P, Jelliffe RW, Goutelle S. Comparison of four renal function estimation equations for pharmacokinetic modeling of gentamicin in geriatric patients. Antimicrob Agents Chemother. 2012;56(4):1862‐1869. 10.1128/AAC.05634-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carlier M, Carrette S, Roberts JA, et al. Meropenem and piperacillin/tazobactam prescribing in critically ill patients: does augmented renal clearance affect pharmacokinetic/pharmacodynamic target attainment when extended infusions are used? Crit Care. 2013;17(3):R84. 10.1186/cc12705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kothekar AT, Divatia JV, Myatra SN, et al. Clinical pharmacokinetics of 3‐h extended infusion of meropenem in adult patients with severe sepsis and septic shock: implications for empirical therapy against Gram‐negative bacteria. Ann Intensive Care. 2020;10(1):4. Published 2020 Jan 10. 10.1186/s13613-019-0622-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ramon‐Lopez A, Allen JM, Thomson AH, et al. Dosing regimen of meropenem for adults with severe burns: a population pharmacokinetic study with Monte Carlo simulations. J Antimicrob Chemother. 2015;70(3):882‐890. 10.1093/jac/dku429 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Model building process
FIGURE S1 Linear (left) and semi‐log (right) plots of raw data with LOESS trend lines (thick solid lines) after the end‐of‐infusion stratified by burn and CVVH. The black dashed line is the LOESS trend of all data combined
FIGURE S2 Individual goodness‐of‐fit plots. Open circles represent observed concentrations, solid black lines represent individual predicted concentrations and blue dashed lines represent population predicted concentrations. Burn and CVVH are noted next to the pseudo‐id. (Right panel log‐linear scale, left panel linear scale)
FIGURE S3 Histogram of conditional weighted residuals and BSV diagnostic plots of final model
FIGURE S4 Visual predictive checks comparing simulated and observed time–concentration data at the 5th, 50th and 95th percentiles (200 replicates). Purple circles represent observed data, red lines represent observed quantile trend lines, black lines represent simulated quantile trend lines and gray bands represent simulated 95% CI
FIGURE S5 Eta vs covariate plots. Left columns are plots from base model, right columns are plots from final model
FIGURE S6 PTA results from simulations with meropenem 2000 mg Q8 hours infused over 1 hour, 3 hours or continuous infusion of 3000 mg Q12 hours. Patients (1000 per group) were simulated with varying prescriptions of CVVH (0 mL/kg/h, 42.9 mL/kg/h and 71.4 mL/kg/h), normal renal function (NRF, CrCl = 100–130 mL/min) or augmented renal clearance (ARC, 150–250 mL/min) and fixed lean body mass (LBM) = 56 kg
Data Availability Statement
Data presented in this article cannot be shared. For any other questions, please contact the corresponding author.


