Summary
The mechanisms underlying plant tolerance to boron (B) excess are far from fully understood. Here we characterized the role of the miR397‐CsiLAC4/CsiLAC17 (from Citrus sinensis) module in regulation of B flow.
Live‐cell imaging techniques were used in localization studies. A tobacco transient expression system tested modulations of CsiLAC4 and CsiLAC17 by miR397. Transgenic Arabidopsis were generated to analyze the biological functions of CsiLAC4 and CsiLAC17. CsiLAC4’s role in xylem lignification was determined by mRNA hybridization and cytochemistry. In situ B distribution was analyzed by laser ablation inductively coupled plasma mass spectrometry.
CsiLAC4 and CsiLAC17 are predominantly localized in the apoplast of tobacco epidermal cells. Overexpression of CsiLAC4 in Arabidopsis improves the plants’ tolerance to boric acid excess by triggering high‐B‐dependent lignification of the vascular system’s cell wall and reducing free B content in roots and shoots. In Citrus, CsiLAC4 is expressed explicitly in the xylem parenchyma and is modulated by B‐responsive miR397. Upregulation of CsiLAC4 in Citrus results in lignification of the xylem cell walls, restricting B flow from xylem vessels to the phloem.
CsiLAC4 contributes to plant tolerance to boric acid excess via high‐B‐dependent lignification of cell walls, which set up a ‘physical barrier’ preventing B flow.
Keywords: boron toxicity, cell walls, Citrus, laccase‐like protein, nutrient transport, nutrient uptake
Introduction
Boron (B) is a metalloid element belonging to group 13 in the periodic table (Tu et al., 2010). In aqueous solutions or soils with pH < 7, B occurs as undissociated boric acid (H3BO3), which can equilibrate across the phospholipid bilayers of biological membranes by permeation and penetrate cells through passive channel proteins or by secondary active efflux transporters under deficiency conditions (Raven, 1980; Takano et al., 2002; Kato et al., 2009; Reid, 2014). Boric acid in the cytoplasmic compartment of plant and animal cells can quickly react with a variety of hydroxyl‐rich compounds (Loomis & Durst, 1992; Bolaños et al., 2004).
As a micronutrient, B is required for the growth, development and reproduction of all higher vascular plants (Saleem et al., 2011; Chatterjee et al., 2014; Durbak et al., 2014). The B concentration needed for different crops varies significantly with species and cultivars; observed B concentrations in monocots are generally lower than in dicots (Princi et al., 2016). Nevertheless, the optimal B range for plant growth and development is very narrow in particular species (Bingham et al., 1987). Both deficient and excessive B levels can easily be noticed in several crops (Nable et al., 1997; Shorrocks, 1997; Chen et al., 2012; Landi et al., 2019). B excess in soils generally results in overaccumulation of B in the aboveground plant parts due to its passive transport within the transpiration stream, thereby generating toxicity to plants (Nable et al., 1990; Brdar‐Jokanovic, 2020).
Plant tolerance to excessive B varies within and between species and is primarily determined by B’s reallocation and/or transportation ability (Nable et al., 1997; Brdar‐Jokanovic, 2020). In plant species where B forms stable borates with primary photosynthetic polyols, B can be reallocated by the phloem, and the symptomology of B excess is seldom observed in mature organs. By contrast, typical symptoms of B toxicity in phloem immobile plants are expressed in older leaf margins due to enrichment of B at the end of the transpiration stream (Brown & Shelp, 1997). The mechanism underlying tolerance to B excess in B‐immobile plants can be attributed to the efflux of B by transporters. For instance, the expression of efflux transporter BOR1 in barley (Hordeum vulgare) can reduce B uptake, thereby decreasing B concentration in shoots. At the same time, the expression of BOR1 homologs in Arabidopsis (Arabidopsis thaliana) reduces B uptake by the roots and redistributes toxic B within the shoots. With this strategy, plants can reduce intracellular B levels (Hayes & Reid, 2004; Miwa et al., 2007, 2014; Sutton et al., 2007).
However, the efflux‐based theory could not explain the fact that B tolerance was not reliably correlated with lower tissue B concentrations in many plant species (Nable et al., 1990; Torun et al., 2006; Ochiai et al., 2011; Huang et al., 2014). Within‐species genotypes with similar tissue B concentrations can show remarkably different toxicity symptoms (Reid & Fitzpatrick, 2009). To explain these observations, a detoxifying mechanism exerted by B‐chelating compounds such as polyols and phenolics was proposed (Princi et al., 2016). Supporting evidence was found in a recent study on loquat (Eriobotrya japonica), which revealed an orchestrated defensive mechanism to deal with B toxicity by shifting carbohydrate metabolites from sucrose to sorbitol and fructose (Papadakis et al., 2018). Yet, such a mechanism is uncommon for those species that do not use polyols as translocating agents (Landi et al., 2019). Some authors speculated that B might be compartmentalized in the vacuoles or the cell walls via transporters (Reid & Fitzpatrick, 2009; Martinez‐Cuenca et al., 2015). However, maintenance of B distribution across membranes within cells by active transport of boric acid away from thermodynamic equilibrium is likely to be energetically expensive and inefficient, as 99.95% of B in the plant cytoplasm exists in the form of boric acid, equilibration of which occurs within minutes at high concentrations (Raven, 1980; Reid, 2014; Princi et al., 2016). To date, limited information is available on vacuolar B concentrations and molecular characterization of B‐complexes within vacuoles (Wimmer et al., 2020). The tolerance mechanisms of plants to B excess remain to be elucidated.
Citrus (Citrus sp.) is one of the most important fruit crops cultivated around the globe. Citrus plants are sensitive to B excess, which generates toxicity to old mature leaves (Han et al., 2009; Chen et al., 2012). B toxicity to Citrus mainly occurs in B‐rich soils or in soils where B fertilizers are applied inappropriately and has been reported worldwide, resulting in an overall reduction in tree vigor and yield (Nable et al., 1997; Papadakis et al., 2004; Huang et al., 2014; Li et al., 2015; Martinez‐Cuenca et al., 2015). As reported for many other species, variation in high B tolerance appeared in different genotypes of Citrus. Studies have shown that the tolerance mechanism of Citrus to B excess is independent of B efflux (Sheng et al., 2010) and that B‐chelating organic compounds made almost no contribution to detoxification under boric acid‐toxic stress (Martinez‐Cuenca et al., 2015). These studies implied a novel B tolerance mechanism in plants.
Citrus sinensis (B‐tolerant) and Citrus grandis (B‐intolerant) are two Citrus plants showing different tolerance to B excess. However, they maintain a similar total B content in the leaves under excessive B conditions (Guo et al., 2014). Previously, we found that excessive boric acid treatment triggers explicitly programmed cell death of the phloem tissue in intolerant C. grandis, and that miR397 plays a role in this process via targeting LAC4 and LAC17, both belonging to the laccase family (Huang et al., 2014, 2016). Laccases are enzymes that can oxidize quinol. They are involved in protoplast regeneration, and lignification and delignification of plant cell walls (Mayer & Staples, 2002; Schuetz et al., 2014). In Arabidopsis, LAC4 and LAC17 contribute to the constitutive lignification of stems, while LAC17 is involved in the deposition of guaiacyl lignin units in fibers (Berthet et al., 2011). In the present study, through detailed functional analysis of Csi‐miR397 and its targets, CsiLAC4 and CsiLAC17 in Arabidopsis and Citrus, we reveal a distinct B tolerance mechanism in Citrus. We show that CsiLAC4, targeted by miR397, modulates secondary lignification of the xylem parenchyma cell walls, which limits B inflow to the phloem to alleviate toxicity in B‐tolerant C. sinensis. Interestingly, the functioning of CsiLAC4 in cell wall lignification is high‐B dependent.
Materials and Methods
Plant materials and growth conditions
Five‐week‐old seedlings of ‘Xuegan’ sweet orange (C. sinensis, tolerant to B excess) and sour pummelo (C. grandis, intolerant to B excess) were cultured and treated with sufficient (10 µM, control) and excessive boric acid (400 µM) for 15 wk as previously reported by Huang et al. (2014) in a glasshouse under natural photoperiod. Mature leaves were sampled at one‐third height (c. 30 cm above ground) of the seedlings and used for total RNA extraction, cell wall extraction, histochemical analysis, in vitro hybridization and in situ B distribution analysis.
The transgenic and wild‐type Arabidopsis (A. thaliana, Col‐0) seeds were sterilized for 15 min in 1.5% bleach and then rinsed five times with double‐distilled water. Sterilized seeds were kept at 4°C for 3 d before being grown on ½ Murashige & Skoog (½MS) agar‐medium (pH 5.8). Plants were cultivated in vertical chambers with a light intensity of 222 µmol m−2 s−1 and 16 h : 8 h (day : night) photoperiod. Tobacco (Nicotiana benthamiana) and Arabidopsis seedlings were transplanted in 10 × 10 × 10 cm plastic pots containing peat moss and vermiculite (2 : 1, v/v) and grown in chambers under the same light conditions with a constant relative humidity of 45%. B in peat moss and vermiculite was leached away with 0.1 M HCl. The HCl‐treated medium was thoroughly washed with distilled water and dried before use.
Total RNA extraction and cDNA synthesis
Total RNAs were extracted from boric acid‐treated Citrus and Arabidopsis samples or tobacco leaves with TRIzol reagent (Invitrogen). The quantity and purity of the total RNAs were determined with a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA) and a 2100 bioanalyzer (Agilent, Santa Clara, CA, USA). Total RNAs with RNA integrity number > 8.0 were converted into cDNA using a Maxima First Strand cDNA Synthesis Kit (Thermo Scientific) when indicated.
Cloning of pre‐miR397, CsiLAC4 and CsiLAC17, and vector construction
For cloning of pre‐miR397, the predicted precursor of miR397 was aligned to the Orange Genomic Database (http://citrus.hzau.edu.cn/orange) (Huang et al., 2016). A genomic DNA sequence containing the precursor and ±500 bp down‐ and upstream was subjected to primer search. Primers were designed c. 300 bp up‐ and downstream from the precursor, and the expected PCR product should not change the secondary structure of the contained precursor (ViennaRNA Web Services; http://rna.tbi.univie.ac.at). The pre‐miR397 fragments were amplified from C. sinensis DNA with the primers listed in Supporting Information Table S1.
The sequences of CsiLAC4 (Cs6g07800.1) and CsiLAC17 (Cs8g17630.1) were obtained from the C. sinensis genome (http://citrus.hzau.edu.cn/orange/index.php). The open reading frames (ORFs) of CsiLAC4 and CsiLAC17 were cloned by high‐fidelity PCR amplification from C. sinensis cDNA. The primers are listed in Table S1.
PCR products were gel‐purified. For overexpression, recovered fragments were double‐digested with BamHI and SacI (for overexpression) and ligated overnight with T4 DNA ligase (New England Biolabs (NEB), Ipswich, MA, USA) to the pretreated pBI121 vector (Fig. S1). For DsRed‐fused expressions, recovered fragments were directly ligated to the XbaI/BamHI‐predigested pBI121‐DsRed vector using an In‐Fusion® HD Cloning Kit (Takara Bio, Shiga, Japan). Confirmed constructs overexpressing DsRed‐fused CsiLAC4 or CsiLAC17 were mutated with a Hieff Mut™ Site‐directed Mutagenesis Kit (Yeasen, Shangai, China) according to the manufacturer’s instructions. Primers used for mutagenesis are available in Table S2. All constructs were determined by sequencing and then introduced into Agrobacterium GV3101 (pSoup) using the freeze–thaw method.
Bioinformatics, sequence alignment and phylogenetic analysis
For bioinformatics analysis of CsiLAC4 and CsiLAC17, signal peptides and transmembrane topology were predicted using the online software Pobius (http://phobius.sbc.su.se/index.html) and Sosui (http://harrier.nagahama‐i‐bio.ac.jp/sosui), and multiple sequence alignments were performed in Mega7 (Kumar et al., 2016).
Subcellular localization of CsiLAC4 and CsiLAC17
For subcellular localization assays, C‐terminally tagged CsiLAC4‐DsRed or CsiLAC17‐DsRed was transiently coexpressed with the green fluorescent protein (GFP) in tobacco epidermal cells through Agrobacterium‐mediated transfection (Agro‐inoculation) (Sparkes et al., 2006). Coexpression of untagged DsRed and GFP was set as a control. Seventy‐two hours posttransfection, leaf blades were injected with 0.8 M sorbitol before being subjected to microscopy. Fluorescence signals were examined using a DMi8 confocal laser scanning microscope (Leica, Wetzlar, Germany).
In situ mRNA hybridization
Boric acid‐treated C. sinensis and C. grandis leaf samples were fixed overnight in a 1 : 1 : 18 solution of formaldehyde, acetic acid and 50% ethanol (FAA) and applied for serial paraffin sections (5 µm) as reported by Huang et al. (2019b).
Gene‐specific primers for miR397, CsiLAC4 and CsiLAC17 (Table S1) were used to amplify the probe templates from C. sinensis cDNA. PCR products were gel‐purified and sequenced. Sense and complementary digoxigenin‐labeled RNA probes were synthesized with a DIG RNA Labeling Kit (Roche) according to the manufacturer’s instructions. In situ hybridization was performed on the paraffin sections as described by Braissant & Wahli (1998). Signals were visualized with a DIG Nucleic Acid Detection Kit (Roche) and photographed under a BX‐41 light microscope (Olympus, Tokyo, Japan).
MicroRNA blotting
Pre‐miR397 was transiently expressed in tobacco leaves via Agro‐inoculation (Sparkes et al., 2006). Inoculation of Agrobacterium without any construct was set as a mock, and those with other constructs as controls. Total RNA was extracted 48 h postinoculation, and miR397 abundance was determined by microRNA blotting.
Thirty micrograms of total RNA from each sample was used for gel blot hybridization, according to Fei et al. (2018). DNA oligo probes (Table S3) were end‐labeled with γ‐32P‐ATP using T4 Polynucleotide Kinase (NEB). U6 small nuclear RNA was used as a loading control in Citrus and tobacco (Zhao et al., 2013; Eamens et al., 2014). After overnight exposure with the hybridized membrane, phosphor screens were scanned using a Typhoon scanner (GE Healthcare Bio‐Sciences, Chicago, IL, USA).
Modulation of CsiLAC4 and CsiLAC17 by miR397
We tested the hypothesis that miR397 directly cleaves CsiLAC4 and CsiLAC17 mRNAs with the Agrobacterium‐mediated delivery system in tobacco leaves as described by Llave et al. (2002), and the 5′‐end mRNA cleavage products were detected by nested 5′‐RACE (rapid amplification of cDNA ends) PCR using a Smart Race Kit (Takara Bio) according to the manufacturer’s instructions. Gene‐specific primers are listed in Table S4. For visualization research, the empty GFP construct was coexpressed and used as an indicator of successful transfection; coexpressions of pre‐miR397 with the mutated CsiLAC4‐DsRed or CsiLAC17‐DsRed construct were set as controls. Fluorescence signals were detected 48 h after inoculation under a DMi8 confocal laser scanning microscope (Leica). For each assay, the transformation was performed in three leaves from different tobacco plants.
CsiLAC4 and CsiLAC17 functions in Arabidopsis
Transgenic Arabidopsis plants overexpressing CsiLAC4, CsiLAC17 and pre‐miR397 were obtained by Agrobacterium‐mediated transformation using the floral dip method (Zhang et al., 2006). For each gene, two transgenic lines showing a segregation ratio of 3 : 1 (resistance : susceptibility to kanamycin) were selected and grown to the homozygous T4 generation for further studies. For phenotypic analysis, vernalized Col‐0 and T4 seeds were grown on ½MS agar‐medium supplemented with 0, 0.5, 1, 2, 5 and 10 mM boric acid, or with 0, 10, 50, 100 and 200 mM NaCl, respectively. The shoot fresh weight and the hypocotyl and total root length were measured 14 d after sowing using WinRHIZO 2009b (Regent, Montreal, QC, Canada) as reported by Huang et al. (2019a). For continuous assessment of CsiLAC4 functioning in B tolerance, uniform 3‐wk‐old T4 seedlings grown in 1 l plastic pots (four plants per pot) were irrigated with 100 ml ½ liquid MS medium (supplemented with 0, 1, 2 and 5 mM boric acid) every other day for 2 wk. Aboveground plant parts were then dried and weighed.
Lignin content measurement
One gram of inflorescence stem or rosette leaf tissues from 5‐wk‐old Col‐0 and T3 seedling was used for extraction of cell wall material as described by Zhong & Lauchli (1993), and then the Klason lignin content was determined according to Hatfield et al. (1994).
Determination of B and other mineral nutrients
Citrus leaves and Arabidopsis roots and shoots were used for B determination. All the samples were ground into fine powder in liquid N2, and B fractions were extracted (soluble in water, soluble in organic solvents and insoluble) (Martinez‐Cuenca et al., 2015). Briefly, the powders were extracted twice with ice‐cold water (10 volumes) and centrifuged at 9400 g for 10 min. Combined supernatants were used for water‐soluble B (free B) determination. The residues were washed three times with 80% ethanol (10 volumes), once with a methanol : chloroform mixture (1 : 1, v/v; 10 volumes) and once with acetone (10 volumes). The combined organic extracts in which monolignols were available were used for organic‐bound B determination and the pellets for cell wall‐bound B. All the B fractions were freeze‐dried and ashed in a muffle furnace at 550°C for 12 h.
Total B and Zn, Cu, Mn, Mg and Ca concentrations from dried rosette leaves and roots extracted from mature plants were determined by inductively coupled plasma MS (ICP‐MS).
Anatomy and histochemistry
Main veins from boric acid‐treated Citrus leaves and hypocotyl and root segments from 14‐d‐old boric acid‐treated Arabidopsis were fixed in FAA for 24 h. Tissue samples were then subjected to routine serial paraffin sectioning (5 µm). Transverse sections were deparaffinized, stained with 5% phloroglucinol in 1 M HCl for 5 min according to the Wiesner’s reaction (Žárský & Cvrčková, 2019) and visualized under an Axio Imager 2 light microscope (Zeiss).
Fresh inflorescence stems from 5‐wk‐old boric acid‐treated plants were hand‐sectioned. Thin sections were stained with 5% phloroglucinol in 1 M HCl and observed under a light microscope.
Measurement of gene expression and micro‐RNA assay
To monitor the expression of laccase‐like family genes in response to boric acid excess, Citrus leaves and Arabidopsis shoots and roots were treated with boric acid. Real‐time (RT) PCRs were set up with 2× SYBR Green qPCR Master Mix (Takara Bio). Amplification levels were detected with a CFX96 Touch RT‐PCR Detection System (Bio‐Rad). Quantification was performed using three independent biological replicates. β‐actin and tubulin were set as internal controls for Citrus and GAPDH and UBQ5 (Joseph et al., 2018) for Arabidopsis. To monitor the relative abundance of miR397 in Arabidopsis treated with different levels of boric acid, stem‐loop RT‐PCR was conducted with a TaqMan® MicroRNA Assay Kit (Takara Bio) as previously described (Huang et al., 2016). All the primers employed for RT‐PCR are listed in Table S5. Relative levels were expressed as . Heatmaps were generated using excel 2016 (Microsoft, Redmond, WA, USA).
Laser ablation (LA) ICP‐MS analysis
For in situ B distribution, fresh main‐veins of boric acid‐treated Citrus leaves were transversely sectioned (24 µm) with an MEV freezing microtome (Slee Medical, Mainz, Germany). Cryosections were mounted on poly‐l‐lysine‐coated glass slides and then freeze‐dried at −40°C immediately to minimize B diffusion. Prepared slides were photographed with a light microscope before being applied to GeoLasPro LA‐ICP‐MS (Coherent, Santa Clara, CA, USA). Laser ablation was performed in line scanning mode with carrier helium gas flow of 600 ml min−1, laser power set to 20% and laser spot diameter of 20 µm. The 7500a ICP‐MS (Agilent) was set up in time‐resolved analysis mode, and the resulting amounts of B were reported in counts per second. Finally, a B distribution contour was generated from the ICP‐MS data that represents the total count of the B signal at each time point using OriginPro 9.0 (Origin Lab, Northampton, MA, USA).
Quantification and statistical analysis
Boron content, shoot fresh weight, total root length and hypocotyl length of transgenic Arabidopsis treated with different B levels were analyzed using two‐way between‐group ANOVA followed by Sidak’s post‐hoc test in Spss 22.0 (IBM, Armonk, NY, USA). An independent t‐test was used to determine the statistical significance between samples in assays related to lignin or B contents. Bivariate correlation analysis was performed to examine the correlation between relative expressions of genes in transgenic lines and the external B levels. Statistic results were presented by SigmaPlot 10.0 (Systat, Palo Alto, CA, USA) and excel 2016 (Microsoft).
Results
miR397 mediates cleavage of CsiLAC4 and CsiLAC17 mRNAs
For validation of miR397 targeting CsiLAC4 and CsiLAC17, we used the tobacco transient expression system. We inoculated tobacco leaves with different combinations of Agrobacterium strains, which respectively carry the pre‐miR397, DsRed‐fused CsiLAC4 and CsiLAC17 constructs. Based on micro‐RNA (miRNA) blotting, we showed that heterologously expressing pre‐miR397 in tobacco efficiently generated mature miR397 (Fig. 1a). We then detected miRNA‐mediated decay of CsiLAC4 and CsiLAC17 mRNA using 5′‐RACE PCR. We only obtained a full‐length 5′‐end fragment from the nested PCR when the CsiLAC4 or CsiLAC17 construct was inoculated alone. Besides the corresponding full‐length 5′‐ends, we obtained the expected cleavage product for each target (718 bp for CsiLAC4 and 217 bp for CsiLAC17) when cotransfection was performed. Unexpectedly, we also acquired another 670 bp fragment from tobacco leaves coinoculated with the CsiLAC17 and pre‐miR397 constructs (Fig. 1b). Under the confocal microscope, the DsRed‐fused CsiLAC4 and CsiLAC17 fluorescent signals that should have appeared in the epidermal cells were suppressed when pre‐miR397 was coinoculated. Interestingly, an introduction of several site‐directed mutations in the miR397 complementary sites of CsiLAC4‐DsRed or CsiLAC17‐DsRed mRNA led to the recovery of the DsRed fluorescent signals (Fig. 1c,d). These results provided solid evidence that CsiLAC4 and CsiLAC17 are posttranscriptionally regulated through miR397‐mediated cleavage of the corresponding mRNAs.
Fig. 1.
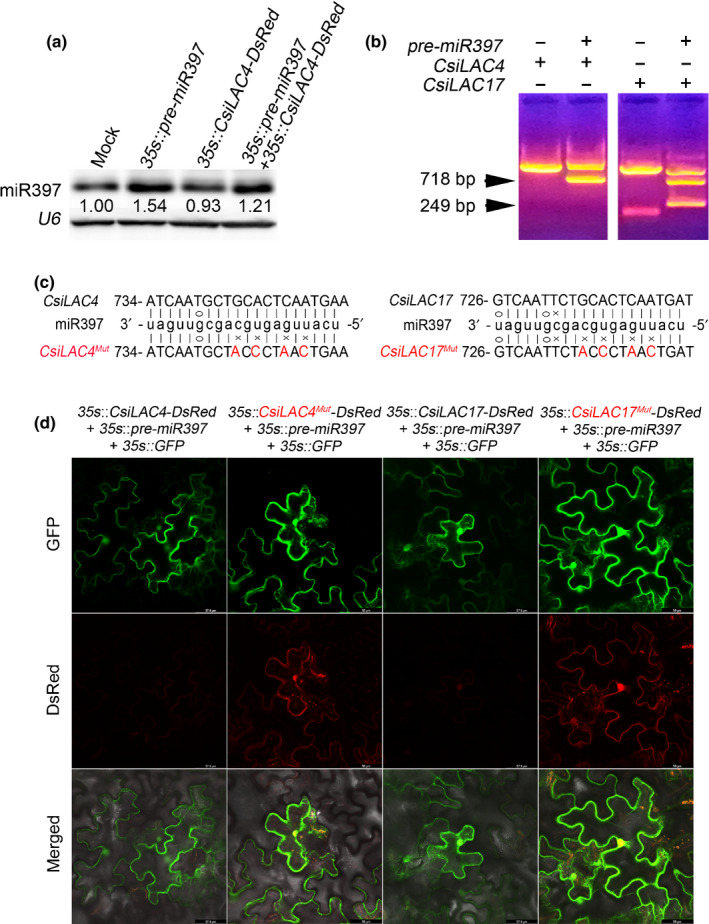
miR397 modulates expression of fluorescently tagged CsiLAC4 and CsiLAC17 in tobacco leaf via miRNA‐mediated cleavage of mRNA. (a) miRNA blot showing generation of mature miR397 by transient expression of pre‐miR397 in tobacco leaves. Total RNAs were extracted 48 h postinoculation. (b) 5′‐RACE PCR of CsiLAC4 and CsiLAC17 from tobacco leaves coexpressing miR397 and CsiLAC4‐DsRed/CsiLAC17‐DsRed. Arrowheads indicate expected fragments supporting the miRNA‐mediated cleavage. (c) Site‐directed mutations of CsiLAC4 and CsiLAC17 in complementary sites of miR397. Mutated nucleotides are indicated in red. (d) miR397 represses expression of DsRed‐tagged CsiLAC4 and CsiLAC17 rather than their corresponding mutants in the tobacco epidermis. Confocal micrographs were obtained 48 h posttransfection. GFP was used as an indicator of successful transfection.
Bioinformatics and subcellular localization of CsiLAC4 and CsiLAC17
CsiLAC4 and CsiLAC17 share 77.10% and 78.56% identity with Arabidopsis laccase‐4 and laccase‐17, respectively (Fig. S2). Both proteins were predicted in the cell membrane (plant‐mploc; http://www.csbio.sjtu.edu.cn/bioinf/plant‐multi). However, topology analysis (phobius; http://phobius.sbc.su.se/index.html) revealed that neither CsiLAC4 nor CsiLAC17 contains the transmembrane domain and that the majority of their peptides were noncytoplasmic (Fig. S3). For subcellular localization, we fused CsiLAC4 and CsiLAC17 to the N‐termini of the DsRed reporter and coexpressed them with GFP in tobacco epidermal cells. Plasmolysis of epidermal cells showed that CsiLAC4 was predominantly localized in the apoplast and CsiLAC17 in the cell walls and protoplast (Fig. S4).
A gene search of the NCBI database showed several homologs of CsiLAC4 and CsiLAC17 from other plant and animal species. According to the phylogenetic analysis, CsiLAC4 homologs, but not CsiLAC17, clustered into the dicot group, which markedly could be clustered into five subgroups by herbaceous and woody types (Figs 2, S5). This implied that the laccase‐4 homologs in woody species might function differently from those in herbaceous species.
Fig. 2.
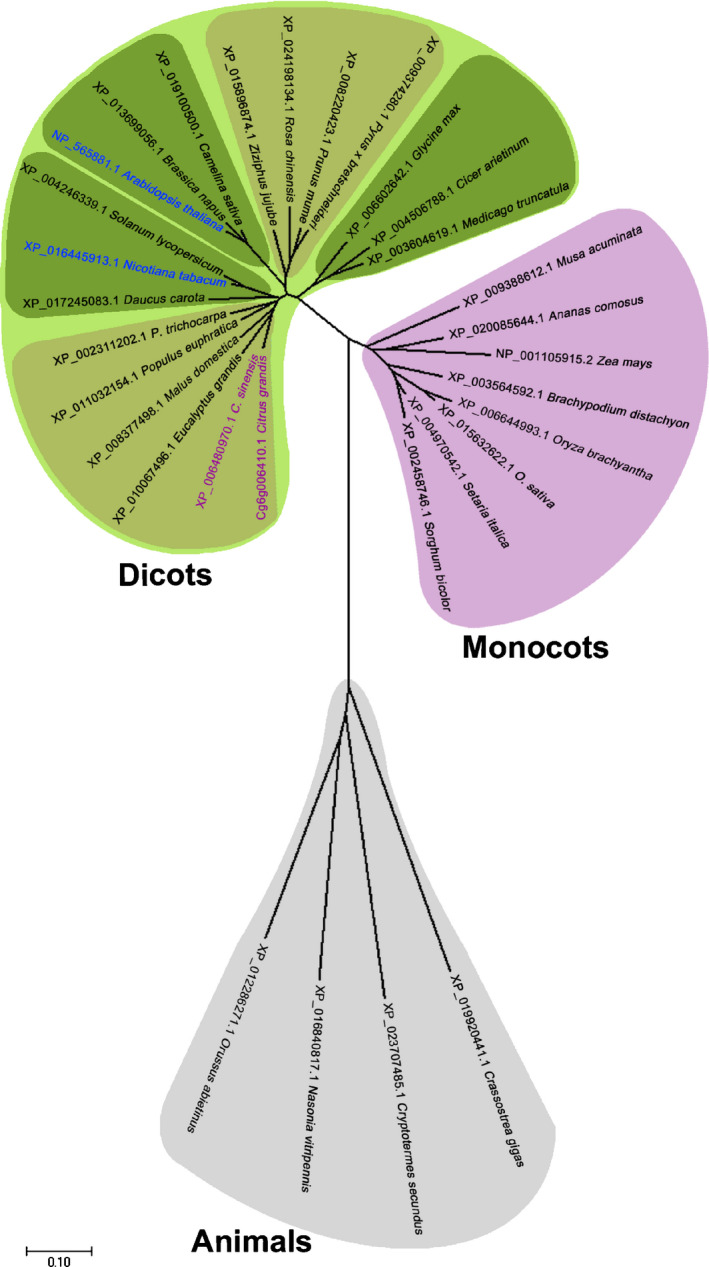
Unrooted phylogenetic tree of Citrus LACCASE‐4 (CsiLAC4) homologs by Mega7 software (neighbor‐joining method with 1000 bootstrap test). Subclustered herbaceous plant species are shown in deep green and woody plant species in brown. Bar, 0.10 substitution distance.
Overexpression of CsiLAC4 in Arabidopsis increases plant tolerance to B excess
Overexpression of AtLAC4 or AtLAC17 in Arabidopsis causes ectopic lignification of cotyledon epidermal cell walls when exogenous monolignols are supplied (Schuetz et al., 2014). To evaluate ectopic functions of Csi‐miR397, CsiLAC4 and CsiLAC17 in Arabidopsis cell wall lignification, we created several transgenic lines overexpressing pre‐miR397 (miR397OE), CsiLAC4 (CsiLAC4OE) and CsiLAC17 (CsiLAC17OE) from Col‐0 Arabidopsis. Surprisingly, under moderate B conditions, no significant difference was found for Klason lignin contents of rosette leaves and inflorescence stems between the transgenic lines and Col‐0. However, the lignin content of miR397OE inflorescence stems was lower than in those of CsiLAC4OE and CsiLAC17OE lines (Fig. S6).
Given that miR397, CsiLAC4 and CsiLAC17 are involved in the response of Citrus to B toxicity (Huang et al., 2016), we treated the T4 transgenic seedlings with excessive boric acid for 14 d. CsiLAC4OE lines showed better growth performances than Col‐0 plants after the boric acid treatment (Figs 3a, S7a). We determined the shoot fresh weight, total root length and hypocotyl length of each seedling, and the results showed significant reductions of shoot fresh weight and total root length in all plant lines as external boric acid increased (Fig. 3b,c). Nonetheless, the shoot fresh weight of CsiLAC4OE lines was higher than the remaining lines under excessive B conditions (Fig. 3b), while total root length of CsiLAC4OE lines was higher than the others when external boric acid concentrations were < 2 mM (Fig. 3c). Moreover, external boric acid for a concentration of < 5 mM increased the hypocotyl length in CsiLAC4OE lines (Fig. S7a,b).
Fig. 3.
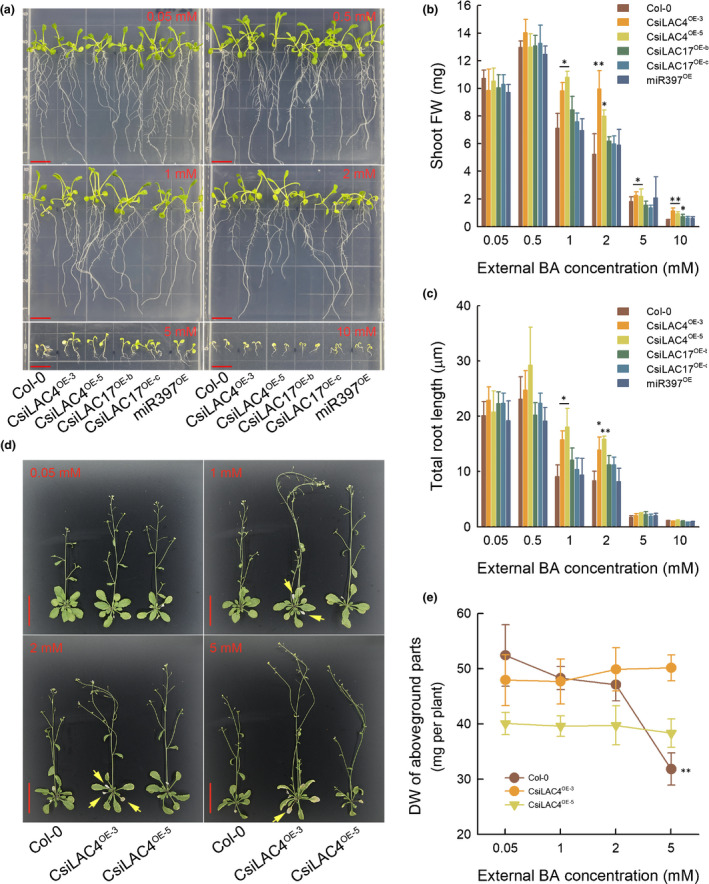
Overexpression of CsiLAC4 (CsiLAC4OE) in Arabidopsis alleviates boric acid‐toxic effects on plant growth and development at both young (a–c) and more mature (d, e) stages. (a) Sterilized seeds were sown on ½ Murashige & Skoog (½MS) solid medium containing different levels of boric acid and were vertically grown in a chamber under standard culture conditions (16 h photoperiod) for 14 d. Bar, 1 cm. (b) Shoot fresh weight (FW). (c) Total root length determined by the ImageJ software. (d) Three‐week‐old seedlings transplanted in 1 l plastic pots were irrigated with 100 ml of ½MS liquid medium supplemented with boric acid every other day for 14 d. Yellow arrowheads indicate early leaf senescence. Bar, 5 cm. (e) Dry weight (DW) of aboveground parts. Results are mean ± SD. Asterisks indicate significant differences at: *, P < 0.05; and **, P < 0.01 by one‐way ANOVA (Student‐Newman‐Keuls method, n = 6).
To further assess CsiLAC4 functions in B tolerance, we also treated mature plants of Col‐0 and the CsiLAC4OE lines with excessive boric acid. Surprisingly, boric acid excess stimulated early blooming and triggered precocious leaf senescence in the CsiLAC4OE lines (Figs 3d, S8). At the end of boric acid treatment, the dry weight of aboveground plant parts was decreased in Col‐0 treated with 5 mM boric acid. However, this phenomenon was not observed in the two CsiLAC4OE lines (Fig. 3e).
CsiLAC4 but not CsiLAC17 contributes to cell wall lignification under boric acid excess
Boron excess had a profound phenotypic effect on hypocotyl elongation in the CsiLAC4OE lines, leading us to slice and stain the hypocotyl sections with phloroglucinol‐HCl. We found that only lignified cell walls (including xylem cell walls and the Casparian strips in the endodermal cell walls) stained purple and that higher levels of external boric acid tended to decrease the size of xylem vessels in all the transgenic lines. Metaxylem vessels in the hypocotyls of CsiLAC4OE plants were enlarged after exposure to excessive boric acid and were higher in number when external boric acid reached 5 mM (Fig. S9). Since anatomical structures of the roots and hypocotyls are highly similar in Arabidopsis, we also subjected the boric acid‐treated primary root segments to paraffin sections. Excessive boric acid treatments increased the stele diameter in CsiLAC4OE primary roots (Fig. 4a), resulting in not only enlarged metaxylem vessels (Fig. 4b,c) but also B‐dose‐dependent lignification of the outer endodermal cell walls (Fig. 4a). By contrast, in Col‐0 and miR397 OE lines, a 5 mM boric acid supplement in the growing medium suppressed stele development by decreasing the number and diameter of metaxylem vessels (Fig. 4a–d).
Fig. 4.
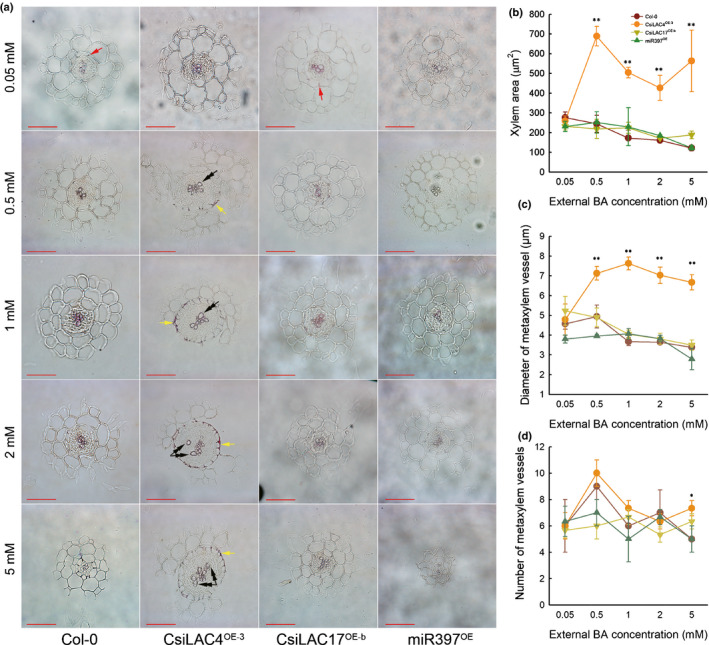
Boric acid excess results in secondary xylem development and lignin deposition in the outer endodermal cell walls (yellow arrows) in the CsiLAC4OE line, but not in the wild‐type (Col‐0), nor in CsiLAC17OE and miR397OE lines. (a) Primary roots from 14‐d‐old seedlings were transversely sectioned at the mature zone and stained with phloroglucinol‐HCl. Red arrows indicate the Casparian strips. Double arrowheads indicate the metaxylem. Xylem area (b) and diameter of metaxylem vessels (c) were determined by ImageJ software, and the number of metaxylem vessels was hand‐calculated. Results are mean ± SD. Asterisks indicate significant differences at: *, P < 0.05; and **, P < 0.01 by one‐way ANOVA (Student‐Newman‐Keuls method, n = 4). Bar, 50 µm.
By monitoring their relative abundance, we found that mRNA levels of CsiLAC4 and CsiLAC17 did not correlate with external B levels in the roots or shoots of the corresponding transgenic lines (Figs S10a,b, S11). Given that Ath‐miR397a shares 100% identity with Csi‐miR397 (Fig. S10c), and that Ath‐miR397a targets AthLAC4 and AthLAC17 in wild‐type Arabidopsis (Wang et al., 2014), we also monitored the expression of endogenous miR397a, AthLAC4 and AthLAC17 in the wild‐type and transgenic Arabidopsis treated with excessive B. We found similar expression patterns of AtLAC4 and AtLAC17 for Col‐0, CsiLAC4OE and CsiLAC17OE lines (Figs S10a,b, S11). In the roots, boric acid promoted expression of AtLAC4 and AtLAC17 for the plant lines, although both AtLAC4 and AtLAC17 were suppressed as boric acid increased. Nonetheless, lignification was only observed in the CsiLAC4OE line, suggesting a minor contribution of AthLAC4 and AthLAC17 to the high‐B‐triggered lignification for the CsiLAC4OE plants.
In mature inflorescent stems of CsiLAC4OE lines, boric acid excess resulted in lignin deposition in the metaxylem, increasing the thickness of interfascicular fibers and the metaxylem cell walls (Figs 5, S12).
Fig. 5.
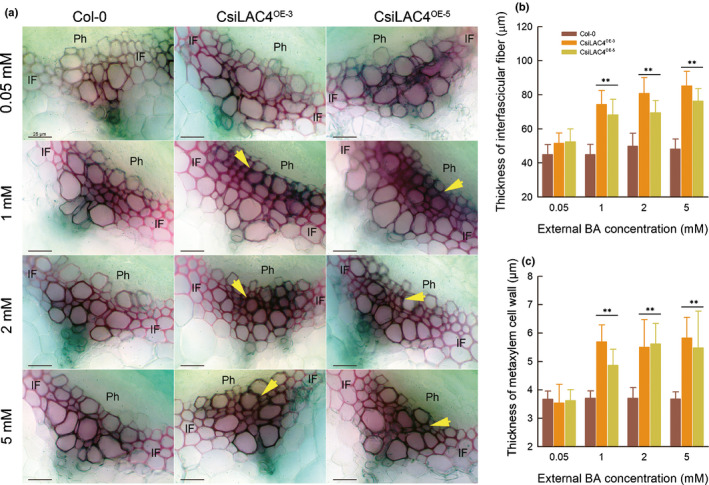
Boric acid excess results in lignin deposition in metaxylem cell walls (yellow arrows) and increase in thickness of interfascicular fiber in inflorescence stems of CsiLAC4OE lines. (a) Hand sections were cut from a mature inflorescence stem (5 cm above the rosette) 14 d after the boric acid treatment and were stained with phloroglucinol‐HCl. (b, c) Thickness of interfascicular fiber (b) and xylem cell wall (c) were measured with the ImageJ software. Results are mean ± SD. Asterisks indicate significant differences at: **, P < 0.01 level by one‐way ANOVA (Student‐Newman‐Keuls method, n = 4). Ph, phloem; IF, interfascicular fiber. Bar, 25 µm.
Cell wall lignification reduces water‐soluble B in CsiLAC4OE plants under boric acid excess
Previous reports showed that cell wall hydrophobicity correlates linearly with lignin content (Hyoe & Tatsuko, 2010; Heiss‐Blanquet et al., 2011). Lignin deposition in the outer endodermal cell walls of boric acid‐treated CsiLAC4OE roots might set up an extracellular diffusion barrier for boric acid into the stele, thereby reducing B content in shoots. We determined the concentrations of water‐soluble B, organic‐bound B and cell wall‐bound B in the boric acid‐treated Arabidopsis samples (Martinez‐Cuenca et al., 2015). Our results indicated that the concentrations of all B fractions increased with the external boric acid concentrations in all tissue samples, except for cell wall‐bound B in mature roots. Most B in the roots and shoots of young seedlings and rosette leaves and roots from mature plants appeared in the water‐soluble fractions. Cell wall‐bound B accounted for a relatively small proportion in the boric acid‐treated samples. Organic‐bound B increased in seedling roots, rosette leaves and roots from mature plants under boric acid excess. There was no significant difference for organic‐bound B and cell wall‐bound B between Col‐0 and the transgenic plants under the same external boric acid levels; however, water‐soluble B was significantly lower in the CsiLAC4OE lines under the same conditions (Fig. 6).
Fig. 6.
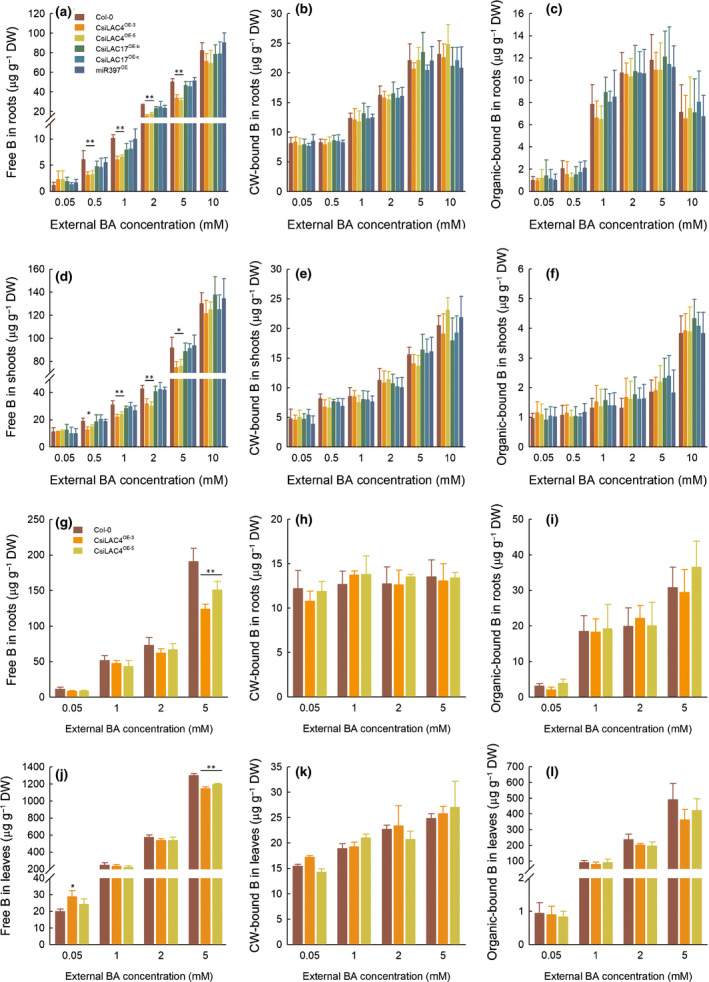
Overexpression of CsiLAC4 (CsiLAC4OE), rather than of CsiLAC17 (CsiLAC17OE) and pre‐miR397 (miR397OE) in Arabidopsis, decreases free boron (B) concentrations under excess boric acid treatments. B fractions in the root (a–c) and shoots (d–f) of 14‐d‐old seedlings, roots (g–i) and leaves (j–l) from mature plants were extracted as described by Martinez‐Cuenca et al. (2015). All fractions were finally determined by inductively coupled plasma‐MS. Free B in roots (a, d, g, j). Cell wall (CW)‐bound B (b, e, h, k). Organic‐bound B (c, f, i, l). Results are mean ± SD. Asterisks indicate significant differences at: *, P < 0.05; and **, P < 0.01 by one‐way ANOVA (Student‐Newman‐Keuls method, n = 4). FW, fresh weight; DW, dry weight.
CsiLAC4 triggers xylem cell wall lignification in Citrus under boric acid excess
We used miRNA blotting to show that excessive boric acid downregulated miR397 in C. sinensis but upregulated it in C. grandis (Fig. 7a), which negatively regulated expression of CsiLAC4 and CsiLAC17 (Fig. 7b). To examine the tissue specificity of CsiLAC4 and CsiLAC17 expressions, we conducted mRNA in situ hybridization on transverse leaf sections. The results showed that CsiLAC4 and CsiLAC17 were specifically expressed in the xylem parenchyma cells (Fig. 7c). Boric acid excess resulted in a significant increase of lignin content in C. sinensis (Fig. 7b). To verify that such lignification was caused by differential expression of CsiLAC4, we profiled expressions of laccase‐like family genes in the boric acid‐treated Citrus leaves. We obtained 34 laccase‐like family members from the Citrus genomic database and clustered them into six groups by bioinformatics analysis (Fig. S13a). CsiLAC4 was the only gene upregulated in boric acid‐treated C. sinensis leaves, accompanied by downregulation of Cs1g01800 and Cs2g29090, which are predicted to be involved in the oxidation–reduction process. Although most of the laccase‐like members in group 6 were upregulated in C. grandis leaves in response to the boric acid treatment, they did not affect lignin content in the leaves (Figs 7b, S11, S13b,c). These results suggested a contribution of CsiLAC4 to lignification in C. sinensis leaves. The effects of CsiLAC4 on lignification were evaluated by the histochemical method. Wiesner’s staining revealed a substantial lignin deposition in the metaxylem of boric acid‐treated C. sinensis leaves but not in C. grandis (Fig. 7d).
Fig. 7.
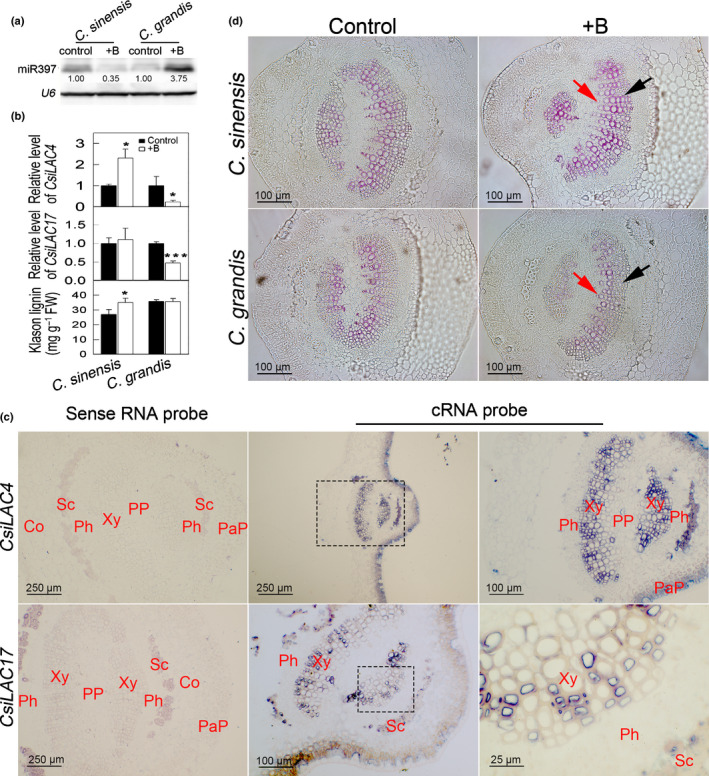
Boric acid (BA)‐responsive miR397 in Citrus leaves negatively modulates CsiLAC4, which contributes to lignification of the metaxylem parenchyma. (a) miRNA blotting. miR397 levels in BA‐treated Citrus sinensis and Citrus grandis leaves. (b) Relative levels of CsiLAC4 and CsiLAC17 and Klason lignin content in responding to excessive BA treatments. (c) In situ mRNA hybridization showed that CsiLAC4 and CsiLAC17 are specifically expressed in the metaxylem. (d) BA treatment results in lignin deposition in the metaxylem according to phloroglucinol‐HCl staining. Co, cortex; PaP, palisade parenchyma; Ph, phloem; PP, pith parenchyma; Sc, sclerenchyma; Xy, xylem. Black arrows indicate the metaxylem, while red arrows indicate the protoxylem. Results are mean ± SD. Asterisks indicate significant differences at: *, P < 0.05; and ***, P < 0.001 by independent sample t‐tests (n = 3).
Secondary lignification of xylem changes B distribution in Citrus leaves
To test our hypothesis that cell wall lignification modulates B flow, we monitored the in situ B distribution in Citrus leaves by LA‐ICP‐MS. Rebuilt images from mass spectra data showed a similar B distribution pattern in control leaf veins in C. grandis and C. sinensis. The lignified tissues (xylem and sclerenchyma) maintained relatively lower levels of B when compared to thin‐walled tissue (such as phloem and storage parenchyma) (Fig. 8a,c). Unlike this pattern, lignification in boric acid‐treated C. sinensis leaves resulted in relatively higher B levels in the xylem (particularly the metaxylem) compared to the surrounding tissues. The highest B levels in treated C. sinensis leaves were detected in the palisade parenchyma tissue (Fig. 8b). However, in boric acid‐treated C. grandis leaves, the discrepancy in B distribution was clear – most B was detected in palisade parenchyma, phloem and pith (Fig. 8d).
Fig. 8.
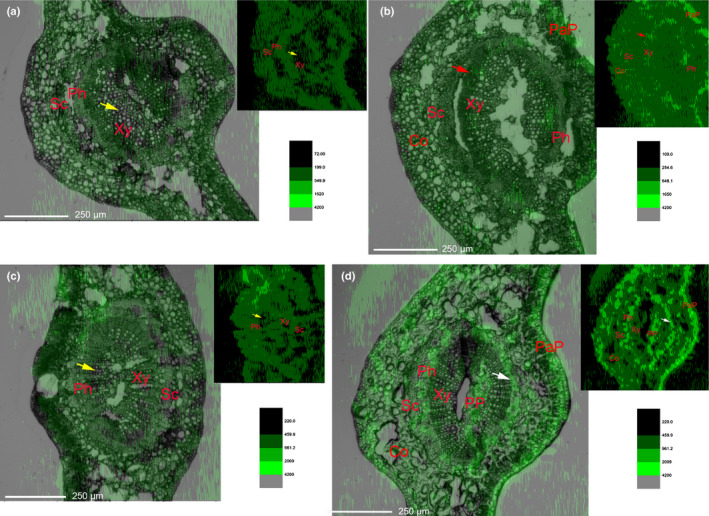
In situ boron (B) distribution in transverse Citrus leaf sections. Bright‐field micrographs of leaf sections were overlaid with corresponding B distribution contours (top right), generated from laser ablation‐inductively coupled plasma‐MS data by OriginPro software (v.9.0.0). Relatively week B signals (yellow arrows) were detected in the control xylem of both Citrus sinensis (a) and Citrus grandis (c). Excessive boric acid treatment results in stronger B signals in the metaxylem of C. sinensis (red arrows) (b), and much stronger B signals in the phloem of C. grandis (white arrows) (d). PaP, palisade parenchyma; Ph, phloem; PP, pith parenchyma; Sc, sclerenchyma; Xy, xylem. Similar results were obtained from three biological repeats.
Discussion
Plants have two essential strategies to deal with B excess: decrease B content in vivo by efflux transporters and chelate the excessive free B by organic compounds (Hayes & Reid, 2004; Papadakis et al., 2018). Speculations regarding B compartmentalization in the extracellular regions or the vacuoles are ultimately efflux‐based (Reid & Fitzpatrick, 2009; Martinez‐Cuenca et al., 2015). Here we provided strong evidence supporting a novel strategy for plants to cope with B excess, in which CsiLAC4 modulates free B flow via lignifying cell walls.
Lignins are cell wall phenolic heteropolymers resulting from the oxidative coupling of three monolignols (Barceló et al., 2004). These phenolic heteropolymers contain various oxygen groups such as hydroxyl, carboxyl, methoxyl and aldehyde that can couple to heavy metal ions to form stable chemical bonds (Sarkanen & Ludwig, 1971; Memon & Schroder, 2009). Lignin biosynthesis in plants plays an important role in detoxifying excess heavy metal ions (e.g. copper, zinc and manganese) through binding the ions in the extracellular regions (van de Mortel et al., 2006; Gao et al., 2012; Liu et al., 2014). Notably, lignin’s affinity with heavy metal ions is strongly dependent on pH and ionic strength (Guo et al., 2008). In dicots, boric acid forms stable borates with important lignin precursors such as caffeic and hydroxiferulic acids (Bolaños et al., 2004). Consequently, boric acid in plants might be bound to lignin in the extracellular regions. However, a similar detoxifying mechanism of lignin for excessive boric acid is still unknown due to the absence of evidence for binding of B to lignin (Ghanati et al., 2005; Cervilla et al., 2009).
We confirmed that CsiLAC4 and CsiLAC17 are two sterling targets of miR397 (Fig. 1). Although these two genes belong to the laccase‐like family, their coding proteins are different from their homologs in Arabidopsis (Fig. S2). Similar to the overexpressions of AtLAC4 and AtLAC17 in Col‐0 background Arabidopsis, overexpressing either CsiLAC4 or CsiLAC17 in Arabidopsis did not produce an observable phenotype (Benske, 2014). Nonetheless, overexpression of CsiLAC4 (rather than CsiLAC17) in Arabidopsis contributed to the tolerance of the transgenic plants to B excess (Fig. 3). This could be attributed to their low similarity in protein sequence (CsiLAC4 shares 58.7% identity with CsiLAC17; Figs S2, S13a). CsiLAC4 in Arabidopsis lignified the outer endodermal cell walls, metaxylem and interfascicular fibers (Figs 4, 5, S9, S10), possibly by binding itself to the lignifying cell wall and polymerizing lignin precursors (O'Malley et al., 1993). This process, however, seemed not to be able to bind B to the extracellular regions because cell wall‐bound B concentrations in the roots and shoots of CsiLAC4OE did not differ from other lines. Nevertheless, a significant decrease of water‐soluble B in the roots and shoots of CsiLAC4OE seedlings suggests that high B‐triggered lignification in the outer endodermal cell walls efficiently suppressed boric acid penetration across the endodermis into stele as well as upward transport of boric acid into shoots (Fig. 6).
Our results support the detoxifying mechanism exerted by B‐chelating compounds in Arabidopsis. As shown in Fig. 6(i,l), organically bound B in the roots and rosette leaves was dramatically increased after the boric acid treatments. In young seedlings, organically bound B in the shoots increased when the external boric acid level reached 10 mM (Fig. 6f), possibly due to its relatively small participation in secondary metabolism and the fact that metabolites might be transported into roots to chelate B (Fig. 6c). The lower water‐soluble and organically bound B in rosette leaves of the CsiLAC4OE lines suggested that lignification in roots might function as an increased ‘physical barrier’ preventing B from flowing into the plant by upward transport. By measuring concentrations of other mineral nutrients, we found that total B, Mg, Mn and Zn in rosette leaves decreased in response to the boric acid treatment (Fig. S14a,c,e,p). In the roots, boric acid reduces only total B and Mg content, although it binds more Ca, Mn, Cu and Zn into the cell walls for the CsiLAC4OE lines (Fig. S14f,l–o,q). The results suggest that lignification in the roots modulates upward transport of mineral nutrients by binding metal ions and absorbing nutrients by setting up physical barriers to ionic (Mg2+) and nonionic (B) flow.
It was found that boric acid toxicity induces ethylene‐related gene expression in plants and might trigger oxidative stress‐adaptive responses in Arabidopsis by miRNA‐based modulation of ethylene biosynthesis (Öz et al., 2009; Kayıhan et al., 2017; Kayihan et al., 2019). In CsiLAC4OE lines, we noted that boric acid excess resulted in hypocotyl and root elongation of the young seedlings, early flowering, and precocious leaf senescence of mature plants (Fig. 3). Such developmental phenomena are related to the stress hormone ethylene (Ogawara et al., 2003; Vandenbussche et al., 2012; Koyama, 2014). The salt stress treatment, which could induce ethylene biosynthesis, showed that the elongation of hypocotyls in the CsiLAC4OE lines was much more prominent, implying that CsiLAC4 might be involved in the responses of CsiLAC4OE plants to ethylene (Fig. S7) (Tao et al., 2015). Recently, Pandey et al. (2021) reported that the redistribution of volatile ethylene in compacted soil can influence plant growth and development. Similarly, cell wall lignification might alter ethylene diffusion within the boric acid‐treated CsiLAC4OE plants, thereby triggering hormone responses. Given that ethylene is involved in modulating the onset of leaf senescence and may play antagonistic roles in regulating plant tolerance to stresses, the altered ethylene distribution may further explain why, under excessive boric acid concentrations, CsiLAC4OE plants still showed severe leaf chlorosis and senescence, even though they maintained lowered water‐soluble and organic‐bound B concentrations (Fig. 3d,e,g) (Koyama, 2014; Tao et al., 2015).
Given that the majority of B in xylem existed in a water‐soluble form, our findings in Citrus provided evidence that lignification of the xylem might restrict outward transport of B (Figs 7d, 8b), which seemed to be independent from B fixation by the lignin in cell walls, since the cell wall‐bound B content in boric acid‐treated C. sinensis leaves was similar to that in the control (Table S6).
For B distribution within Citrus, our findings have several implications summarized in the model shown in Fig. 9. Given that boric acid‐induced secondary lignin in C. sinensis leaves was deposited specifically in the xylem parenchyma cell wall toward the vessel pits (the xylem/symplast interface) (Huang et al., 2016), lignification would alter the B flow between the apoplast (xylem vessel) and symplast (xylem parenchyma cells) by changing the permeability, thereby restricting further transfer of B from the symplast of xylem parenchyma into the phloem by either the symplastic or apoplastic pathway (Figs 7d, 8b,d). For radial B transport, the phloem, palisade tissue and pith might act as buffering pools under excessive boric acid conditions (Fig. 8d). In B‐phloem‐mobile species, the phloem can reallocate B from matured organs to developing young ones (Brown & Shelp, 1997). However, the phloem of Citrus seemed incapable of delivering B to other organs under boric acid excess, possibly due to its inability to chelate B to organic compounds (Table S6).
Fig. 9.
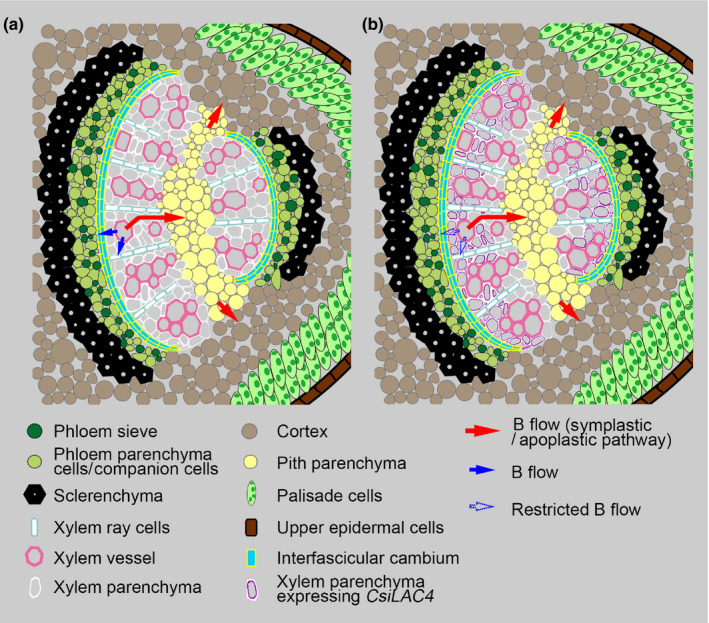
Suggested model for the role of CsiLAC4 in the preferential distribution of boron (B) in Citrus leaves. (a) Schematic diagram of B distribution in B‐intolerant Citrus grandis main veins. B in the xylem vessel is first unloaded into the xylem parenchyma by either equilibration or active transport, followed by further transfer to the phloem and pith parenchyma within the symplast. (b) Schematic diagram of B distribution in B‐tolerant Citrus sinensis main veins. Boric acid treatment triggers cell wall lignification of metaxylem parenchyma expressing CsiLAC4, which restricts B flow between the xylem/apoplast interface and further transfer to the phloem.
In conclusion, our results showed that CsiLAC4 contributes to plant tolerance to B excess by lignifying cell walls, which might set up a ‘physical barrier’ preventing the free B flow out of the xylem.
Author contributions
J‐HH, G‐CF and L‐SC designed the research; J‐HH and L‐YZ conducted the experiments; X‐JL, YG and DZ participated in the phenotypic analysis, anatomical studies and RT‐PCR; JZ participated in lignin determination; W‐LH performed B determination; J‐HH wrote the manuscript; L‐SC and RSF revised and edited the manuscript. All authors reviewed and accepted the final version of the manuscript.
Supporting information
Fig. S1 Information of the pBI121 vector.
Fig. S2 Alignment of CsiLAC4 and CsiLAC17.
Fig. S3 Topology of CsiLAC4 and CsiLAC17.
Fig. S4 Subcellular localization of CsiLAC4 and CsiLAC17.
Fig. S5 Phylogenetic analysis of CsiLAC17.
Fig. S6 Lignin contents of transgenic Arabidopsis.
Fig. S7 Effects of boric acid and NaCl supplements on hypocotyl growth.
Fig. S8 Boric acid excess stimulates early flowering in the CsiLAC4OE lines.
Fig. S9 Effects of boric acid excess on hypocotyl anatomy.
Fig. S10 Effects of boric acid treatments on gene expression in Arabidopsis.
Fig. S11 Melting curves of genes tested in this study.
Fig. S12 Effects of boric acid excess on interfascicular fiber thickness in the CsiLAC4OE lines.
Fig. S13 Expression of LACCASE‐like family genes in boric acid‐treated Citrus leaves.
Fig. S14 Effects of boric acid excess on the nutrient accumulation in Arabidopsis.
Table S1 Primers for PCR cloning and hybridization.
Table S2 Primers for site‐directed mutagenesis.
Table S3 miRNA northern blotting probe.
Table S4 Specific and nested primers for 5′‐RACE PCR.
Table S5 Primers for RT‐PCR of laccase‐like family genes.
Table S6 Boron fractions in Citrus leaves treated with different boric acid treatments.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
We thank Dr Chong Zhang for providing the pBI121‐DsRed/GFP vectors; Dr Wei Wang for help with miRNA blotting; Bi‐Fei Huang for technical assistance with LA‐ICP‐MS analysis; and Dr Yuan Li and the anonymous reviewers for critical comments and suggestions on the manuscript. This work was supported by the National Natural Science Foundation of China (31701901), Collaborative Innovation Project from PGFP & CAAS (XTCXGC2021006), the Science and Technology Innovation Team of FAAS (CXTD2021003‐3), and the State Scholarship Fund by the China Scholarship Council (201909350003).
Contributor Information
Jing‐Hao Huang, Email: jhuang1982@126.com.
Guo‐Cheng Fan, Email: guochengfan@126.com.
Li‐Song Chen, Email: lisongchen2002@hotmail.com.
Data availability
Data are available in the article’s Supporting Information.
References
- Barceló AR, Ros LVG, Gabaldón C, López‐Serrano M, Pomar F, Carrión JS, Pedreño MA. 2004. Basic peroxidases: the gateway for lignin evolution? Phytochemistry Reviews 3: 61–78. [Google Scholar]
- Benske A. 2014. Laccase‐dependent lignification of secondary cell walls of protoxylem tracheary elements in Arabidopsis thaliana . MSc thesis, University of British Columbia, Vancouver, BC, Canada. [Google Scholar]
- Berthet S, Demont‐Caulet N, Pollet B, Bidzinski P, Cezard L, Le Bris P, Borrega N, Herve J, Blondet E, Balzergue S et al. 2011. Disruption of LACCASE4 and 17 results in tissue‐specific alterations to lignification of Arabidopsis thaliana stems. Plant Cell 23: 1124–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham FT, Strong JE, Rhoades JD, Keren R. 1987. Effects of salinity and varying boron concentrations on boron uptake and growth of wheat. Plant and Soil 97: 345–351. [Google Scholar]
- Bolaños L, Lukaszewski K, Bonilla I, Blevins D. 2004. Why boron? Plant Physiology and Biochemistry 42: 907–912. [DOI] [PubMed] [Google Scholar]
- Braissant O, Wahli W. 1998. A simplified in situ hybridization protocol using non‐radioactively labelled probes to detect abundant and rare mRNAs on tissue sections. Biochemica 1: 10–16. [Google Scholar]
- Brdar‐Jokanovic M. 2020. Boron toxicity and deficiency in agricultural plants. International Journal of Molecular Sciences 21: 1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PH, Shelp BJ. 1997. Boron mobility in plants. Plant and Soil 193: 85–101. [Google Scholar]
- Cervilla LM, Rosales MA, Rubio‐Wilhelmi MM, Sanchez‐Rodriguez E, Blasco B, Rios JJ, Romero L, Ruiz JM. 2009. Involvement of lignification and membrane permeability in the tomato root response to boron toxicity. Plant Science 176: 545–552. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Tabi Z, Galli M, Malcomber S, Buck A, Muszynski M, Gallavotti A. 2014. The boron efflux transporter ROTTEN EAR is required for maize inflorescence development and fertility. Plant Cell 26: 2962–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L‐S, Han S, Qi Y‐P, Yang L‐T. 2012. Boron stresses and tolerance in citrus. African Journal of Biotechnology 11: 5961–5969. [Google Scholar]
- Durbak AR, Phillips KA, Pike S, O'Neill MA, Mares J, Gallavotti A, Malcomber ST, Gassmann W, McSteen P. 2014. Transport of boron by the tassel‐less1 aquaporin is critical for vegetative and reproductive development in maize. Plant Cell 26: 2978–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eamens AL, Smith NA, Dennis ES, Wassenegger M, Wang MB. 2014. In Nicotiana species, an artificial microRNA corresponding to the virulence modulating region of Potato spindle tuber viroid directs RNA silencing of a soluble inorganic pyrophosphatase gene and the development of abnormal phenotypes. Virology 450–451: 266–277. [DOI] [PubMed] [Google Scholar]
- Fei Q, Yu Y, Liu L, Zhang Y, Baldrich P, Dai Q, Chen X, Meyers BC. 2018. Biogenesis of a 22‐nt microRNA in Phaseoleae species by precursor‐programmed uridylation. Proceedings of the National Academy of Sciences, USA 115: 8037–8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Peng K, Chen YQ, Wang G, Shen Z. 2012. Roles of apoplastic peroxidases, laccases, and lignification in the manganese tolerance of hyperaccumulator Phytolacca americana . Acta Physiologiae Plantarum 34: 151–159. [Google Scholar]
- Ghanati F, Morita A, Yokota H. 2005. Deposition of suberin in roots of soybean induced by excess boron. Plant Science 168: 397–405. [Google Scholar]
- Guo P, Qi YP, Yang LT, Ye X, Jiang HX, Huang JH, Chen LS. 2014. cDNA‐AFLP analysis reveals the adaptive responses of citrus to long‐term boron‐toxicity. BMC Plant Biology 14: 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zhang S, Shan XQ. 2008. Adsorption of metal ions on lignin. Journal of Hazardous Materials 151: 134–142. [DOI] [PubMed] [Google Scholar]
- Han S, Tang N, Jiang H‐X, Yang L‐T, Li Y, Chen L‐S. 2009. CO2 assimilation, photosystem II photochemistry, carbohydrate metabolism and antioxidant system of citrus leaves in response to boron stress. Plant Science 176: 143–153. [Google Scholar]
- Hatfield RD, Jung HG, Ralph J, Buxton DR, Weimer PJ. 1994. A comparison of the insoluble residues produced by the Klason lignin and acid detergent lignin procedures. Journal of the Science of Food and Agriculture 65: 51–58. [Google Scholar]
- Hayes JE, Reid RJ. 2004. Boron tolerance in barley is mediated by efflux of boron from the roots. Plant Physiology 136: 3376–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss‐Blanquet S, Zheng D, Lopes Ferreira N, Lapierre C, Baumberger S. 2011. Effect of pretreatment and enzymatic hydrolysis of wheat straw on cell wall composition, hydrophobicity and cellulase adsorption. Bioresource Technology 102: 5938–5946. [DOI] [PubMed] [Google Scholar]
- Huang J‐H, Cai ZJ, Wen SX, Guo P, Ye X, Lin GZ, Chen LS. 2014. Effects of boron toxicity on root and leaf anatomy in two Citrus species differing in boron tolerance. Trees‐Structure and Function 28: 1653–1666. [Google Scholar]
- Huang J‐H, Lin X‐J, Zhang L‐Y, Wang X‐D, Fan G‐C, Chen L‐S. 2019a. MicroRNA sequencing revealed Citrus adaptation to long‐term boron toxicity through modulation of root development by miR319 and miR171. International Journal of Molecular Sciences 20: 1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J‐H, Qi YP, Wen SX, Guo P, Chen XM, Chen LS. 2016. Illumina microRNA profiles reveal the involvement of miR397a in Citrus adaptation to long‐term boron toxicity via modulating secondary cell‐wall biosynthesis. Scientific Reports 6: 22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J‐H, Xu J, Ye X, Luo TY, Ren LH, Fan GC, Qi YP, Li Q, Ferrarezi RS, Chen L‐S. 2019b. Magnesium deficiency affects secondary lignification of the vascular system in Citrus sinensis seedlings. Trees – Structure and Function 33: 171–182. [Google Scholar]
- Hyoe H, Tatsuko H. 2010. Lignin structure, properties, and applications. In: Abe A, Dusek K, Kobayashi S, eds. Biopolymers: lignin, proteins, bioactive nanocomposites. Berlin/Heidelberg, Germany: Springer, 1–63. [Google Scholar]
- Joseph JT, Poolakkalody NJ, Shah JM. 2018. Plant reference genes for development and stress response studies. Journal of Biosciences 43: 173–187. [PubMed] [Google Scholar]
- Kato Y, Miwa K, Takano J, Wada M, Fujiwara T. 2009. Highly boron deficiency‐tolerant plants generated by enhanced expression of NIP5;1, a boric acid channel. Plant and Cell Physiology 50: 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayıhan C, Öz MT, Eyidoğan F, Yücel M, Öktem HA. 2017. Physiological, biochemical, and transcriptomic responses to boron toxicity in leaf and root tissues of contrasting wheat cultivars. Plant Molecular Biology Reporter 35: 97–109. [Google Scholar]
- Kayihan DS, Kayihan C, Özden Çiftçi Y. 2019. Moderate level of toxic boron causes differential regulation of microRNAs related to jasmonate and ethylene metabolisms in Arabidopsis thaliana . Turkish Journal of Botany 43: 167–172. [Google Scholar]
- Koyama T. 2014. The roles of ethylene and transcription factors in the regulation of onset of leaf senescence. Frontiers in Plant Science 5: 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. 2016. Mega7: molecular evolutionary genetics analysis v.7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi M, Margaritopoulou T, Papadakis IE, Araniti F. 2019. Boron toxicity in higher plants: an update. Planta 250: 1011–1032. [DOI] [PubMed] [Google Scholar]
- Li Y, Han M‐Q, Lin F, Ten Y, Lin J, Zhu D‐H, Guo P, Weng YB, Chen L‐S. 2015. Soil chemical properties, ‘Guanximiyou’ pummelo leaf mineral nutrient status and fruit quality in the southern region of Fujian province, China. Journal of Soil Science and Plant Nutrition 15: 615–628. [Google Scholar]
- Liu Q, Zheng L, He F, Zhao F, Shen Z, Zheng L. 2014. Transcriptional and physiological analyses identify a regulatory role for hydrogen peroxide in the lignin biosynthesis of copper‐stressed rice roots. Plant and Soil 387: 323–336. [Google Scholar]
- Llave C, Xie Z, Kasschau KD, Carrington JC. 2002. Cleavage of Scarecrow‐like mRNA targets directed by a class of Arabidopsis miRNA. Science 297: 2053–2056. [DOI] [PubMed] [Google Scholar]
- Loomis WD, Durst RW. 1992. Chemistry and biology of boron. BioFactors 3: 229–239. [PubMed] [Google Scholar]
- Martinez‐Cuenca MR, Martinez‐Alcantara B, Quinones A, Ruiz M, Iglesias DJ, Primo‐Millo E, Forner‐Giner MA. 2015. Physiological and molecular responses to excess boron in Citrus macrophylla W. PLoS ONE 10: e0134372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AM, Staples RC. 2002. Laccase: new functions for an old enzyme. Phytochemistry 60: 551–565. [DOI] [PubMed] [Google Scholar]
- Memon AR, Schroder P. 2009. Implications of metal accumulation mechanisms to phytoremediation. Environmental Science and Pollution Research International 16: 162–175. [DOI] [PubMed] [Google Scholar]
- Miwa K, Aibara I, Fujiwara T. 2014. Arabidopsis thaliana BOR4 is upregulated under high boron conditions and confers tolerance to high boron. Soil Science and Plant Nutrition 60: 349–355. [Google Scholar]
- Miwa K, Takano J, Omori H, Seki M, Shinozaki K, Fujiwara T. 2007. Plants tolerant of high boron levels. Science 318: 1417. [DOI] [PubMed] [Google Scholar]
- van de Mortel JE, Almar Villanueva L, Schat H, Kwekkeboom J, Coughlan S, Moerland PD, Loren V, van Themaat E, Koornneef M, Aarts MG. 2006. Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens . Plant Physiology 142: 1127–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nable RO, Bañuelos GS, Paull JG. 1997. Boron toxicity. Plant and Soil 193: 181–198. [Google Scholar]
- Nable RO, Paull JG, Cartwright B. 1990. Problems associated with the use of foliar analysis for diagnosing boron toxicity in barley. Plant and Soil 128: 225–232. [Google Scholar]
- Ochiai K, Shimizu A, Okumoto Y, Fujiwara T, Matoh T. 2011. Suppression of a NAC‐like transcription factor gene improves boron‐toxicity tolerance in rice. Plant Physiology 156: 1457–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawara T, Higashi K, Kamada H, Ezura H. 2003. Ethylene advances the transition from vegetative growth to flowering in Arabidopsis thaliana . Journal of Plant Physiology 160: 1335–1340. [DOI] [PubMed] [Google Scholar]
- O'Malley DM, Whetten R, Bao W, Chen CL, Sederoff RR. 1993. The role of laccase in lignification. The Plant Journal 4: 751–757. [Google Scholar]
- Öz MT, Yilmaz R, Eyidoğan F, De Graaff L, Yücel M, Öktem HA. 2009. Microarray analysis of late response to boron toxicity in barley (Hordeum vulgare L.) leaves. Turkish Journal of Agriculture and Forestry 33: 191–202. [Google Scholar]
- Pandey BK, Huang G, Bhosale R, Hartman S, Sturrock CJ, Jose L, Martin OC, Karady M, Voesenek LACJ, Ljung K et al. 2021. Plant roots sense soil compaction through restricted ethylene diffusion. Science 371: 276–280. [DOI] [PubMed] [Google Scholar]
- Papadakis IE, Dimassi KN, Bosabalidis AM, Therios IN, Patakas A, Giannakoula A. 2004. Boron toxicity in ‘Clementine’ mandarin plants grafted on two rootstocks. Plant Science 166: 539–547. [Google Scholar]
- Papadakis IE, Tsiantas PI, Tsaniklidis G, Landi M, Psychoyou M, Fasseas C. 2018. Changes in sugar metabolism associated to stem bark thickening partially assist young tissues of Eriobotrya japonica seedlings under boron stress. Journal of Plant Physiology 231: 337–345. [DOI] [PubMed] [Google Scholar]
- Princi MP, Lupini A, Araniti F, Longo C, Mauceri A, Sunseri F, Abenavoli MR. 2016. Boron toxicity and tolerance in plants: recent advances and future perspectives. In: Ahmad P, ed. Plant metal interaction. Amsterdam, the Netherlands: Elsevier, 115–147. [Google Scholar]
- Raven JA. 1980. Short‐ and long‐distance transport of boric acid in plants. New Phytologist 84: 231–249. [Google Scholar]
- Reid R. 2014. Understanding the boron transport network in plants. Plant and Soil 385: 1–13. [Google Scholar]
- Reid RJ, Fitzpatrick KL. 2009. Redistribution of boron in leaves reduces boron toxicity. Plant Signaling & Behavior 4: 1091–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M, Khanif YM, Fauziah I, Samsuri AW, Hafeez B. 2011. Importance of boron for agriculture productivity: a review. International Research Journal of Agricultural Science and Soil Science 1: 293–300. [Google Scholar]
- Sarkanen KV, Ludwig CH. 1971. Liguins. Occurrence, formation, structure, and reactions. New York, NY, USA: Wiley‐Interscience. [Google Scholar]
- Schuetz M, Benske A, Smith RA, Watanabe Y, Tobimatsu Y, Ralph J, Demura T, Ellis B, Samuels AL. 2014. Laccases direct lignification in the discrete secondary cell wall domains of protoxylem. Plant Physiology 166: 798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng O, Zhou G, Wei Q, Peng S, Deng X. 2010. Effects of excess boron on growth, gas exchange, and boron status of four orange scion‐rootstock combinations. Journal of Plant Nutrition and Soil Science 173: 469–476. [Google Scholar]
- Shorrocks VM. 1997. The occurrence and correction of boron deficiency. Plant and Soil 193: 121–148. [Google Scholar]
- Sparkes IA, Runions J, Kearns A, Hawes C. 2006. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nature Protocols 1: 2019–2025. [DOI] [PubMed] [Google Scholar]
- Sutton T, Baumann U, Hayes J, Collins NC, Shi B‐J, Schnurbusch T, Hay A, Mayo G, Pallotta M, Tester M et al. 2007. Boron‐toxicity tolerance in barley arising from efflux transporter amplification. Science 318: 1446–1449. [DOI] [PubMed] [Google Scholar]
- Takano J, Noguchi K, Yasumori M, Kobayashi M, Gajdos Z, Miwa K, Hayashi H, Yoneyama T, Fujiwara T. 2002. Arabidopsis boron transporter for xylem loading. Nature 420: 337–340. [DOI] [PubMed] [Google Scholar]
- Tao JJ, Chen HW, Ma B, Zhang WK, Chen SY, Zhang JS. 2015. The role of ethylene in plants under salinity stress. Frontiers in Plant Science 6: 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torun AA, Yazici A, Erdem H, Çakmak İ. 2006. Genotypic variation in tolerance to boron toxicity in 70 durum wheat genotypes. Turkish Journal of Agriculture and Forestry 30: 49–58. [Google Scholar]
- Tu KL, Nghiem LD, Chivas AR. 2010. Boron removal by reverse osmosis membranes in seawater desalination applications. Separation and Purification Technology 75: 87–101. [Google Scholar]
- Vandenbussche F, Vaseva I, Vissenberg K, Van Der Straeten D. 2012. Ethylene in vegetative development: a tale with a riddle. New Phytologist 194: 895–909. [DOI] [PubMed] [Google Scholar]
- Wang CY, Zhang S, Yu Y, Luo YC, Liu Q, Ju C, Zhang YC, Qu LH, Lucas WJ, Wang X et al. 2014. MiR397b regulates both lignin content and seed number in Arabidopsis via modulating a laccase involved in lignin biosynthesis. Plant Biotechnology Journal 12: 1132–1142. [DOI] [PubMed] [Google Scholar]
- Wimmer MA, Abreu I, Bell RW, Bienert MD, Brown PH, Dell B, Fujiwara T, Goldbach HE, Lehto T, Mock HP et al. 2020. Boron: an essential element for vascular plants: a comment on Lewis (2019) ‘Boron: the essential element for vascular plants that never was’. New Phytologist 226: 1232–1237. [DOI] [PubMed] [Google Scholar]
- Žárský V, Cvrčková F. 2019. Plant cell morphogenesis: methods and Protocols. In: Walker JM, ed. Methods in molecular biology. New York, NY, USA: Humana Press, 34–35. [Google Scholar]
- Zhang X, Henriques R, Lin SS, Niu QW, Chua NH. 2006. Agrobacterium‐mediated transformation of Arabidopsis thaliana using the floral dip method. Nature Protocols 1: 641–646. [DOI] [PubMed] [Google Scholar]
- Zhao H, Sun R, Albrecht U, Padmanabhan C, Wang A, Coffey MD, Girke T, Wang Z, Close TJ, Roose M et al. 2013. Small RNA profiling reveals phosphorus deficiency as a contributing factor in symptom expression for citrus huanglongbing disease. Molecular Plant 6: 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Lauchli A. 1993. Changes of cell wall composition and polymer size in primary roots of cotton seedlings under high salinity. Journal of Experimental Botany 44: 773–778. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Information of the pBI121 vector.
Fig. S2 Alignment of CsiLAC4 and CsiLAC17.
Fig. S3 Topology of CsiLAC4 and CsiLAC17.
Fig. S4 Subcellular localization of CsiLAC4 and CsiLAC17.
Fig. S5 Phylogenetic analysis of CsiLAC17.
Fig. S6 Lignin contents of transgenic Arabidopsis.
Fig. S7 Effects of boric acid and NaCl supplements on hypocotyl growth.
Fig. S8 Boric acid excess stimulates early flowering in the CsiLAC4OE lines.
Fig. S9 Effects of boric acid excess on hypocotyl anatomy.
Fig. S10 Effects of boric acid treatments on gene expression in Arabidopsis.
Fig. S11 Melting curves of genes tested in this study.
Fig. S12 Effects of boric acid excess on interfascicular fiber thickness in the CsiLAC4OE lines.
Fig. S13 Expression of LACCASE‐like family genes in boric acid‐treated Citrus leaves.
Fig. S14 Effects of boric acid excess on the nutrient accumulation in Arabidopsis.
Table S1 Primers for PCR cloning and hybridization.
Table S2 Primers for site‐directed mutagenesis.
Table S3 miRNA northern blotting probe.
Table S4 Specific and nested primers for 5′‐RACE PCR.
Table S5 Primers for RT‐PCR of laccase‐like family genes.
Table S6 Boron fractions in Citrus leaves treated with different boric acid treatments.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data Availability Statement
Data are available in the article’s Supporting Information.


