Summary
Background
Neutrophilic dermatoses (ND) are a heterogeneous group of diseases, but can often have a relatively similar histological appearance.
Aim
To identify a combination of biomarkers allowing a better differentiation of ND types.
Methods
Biopsies were obtained from normal human skin (NS; n = 4), chronic plaque‐type psoriasis (PsO; n = 7), paradoxical psoriasis (PP; n = 8), generalized pustular psoriasis (GPP; n = 9), subcorneal pustular dermatosis of Sneddon–Wilkinson (SPD; n = 3), acute generalized exanthematous pustulosis (AGEP; n = 3), hidradenitis suppurativa (HS; n = 7), Sweet syndrome (SS; n = 8) and pyoderma gangrenosum (PG; n = 8). Samples were analysed by immunofluorescence using three biomarkers, interleukin (IL)‐17E, inducible nitric oxide synthase (iNOS) and arginase1, each one in combination with two cell markers, myeloperoxidase (MPO) and CD68, which allow the identification of neutrophils and macrophages, respectively.
Results
We found that SS is characterized by high expression of IL‐17E and iNOS in the epidermis, while PG exhibits low expression. The density of the neutrophil infiltrate helps to differentiate PP (high‐density infiltrate) from PsO (low‐density infiltrate). High expression of arginase1 in the granular layer of the epidermis is a hallmark of SPD. Finally, mature neutrophils and proinflammatory macrophages are readily detectable in PP, SPD and PG, whereas immature neutrophils and anti‐inflammatory macrophages are more frequent in GPP, AGEP, HS and SS.
Conclusions
The analysis of ND by immunofluorescence using IL‐17E, iNOS and arginase1 in combination with MPO and CD68 allows for characterization of differential expression patterns in the epidermis as well as the determination of the polarization status of the dermal neutrophils and macrophages. The appropriate markers may help in the differentiation of ND in clinical practice.
Introduction
Neutrophilic dermatoses (ND) are characterized by a dense dermal infiltrate composed mostly of neutrophils and macrophages, with distinct but interconnected roles. 1 , 2 , 3 In this study we analysed several ND: paradoxical psoriasis (PP), generalized pustular psoriasis (GPP), subcorneal pustular dermatosis of Sneddon–Wilkinson (SPD), acute generalized exanthematous pustulosis (AGEP), hidradenitis suppurativa (HS), Sweet syndrome (SS) and pyoderma gangrenosum (PG).
Histologically, neutrophils and macrophages can be identified by the expression of myeloperoxidase (MPO) and CD68, respectively. 4 , 5 Both are highly plastic, with their phenotypes depending on the changing environmental milieu. Macrophages are thus classified as M1 (proinflammatory, tissue damage) and M2 (anti‐inflammatory, tissue repair) phenotypes with different subclasses. 6 , 7 When an injury occurs, neutrophils release granules that induce M1 polarization, 3 while during the resolution of inflammation, M1 induce neutrophil apoptosis, 8 which are in turn engulfed by macrophages in a process called efferocytosis. 9 This activates the switch of an M1 phenotype into an M2 phenotype, leading to restoration of homeostasis. 6 , 10
M1 and M2 macrophage phenotypes can be histologically distinguished by using two specific markers: inducible nitric oxide synthase (iNOS) is preferentially produced by M1, while arginase (arg)1 is produced by M2. 11 , 12 Based on the M1/M2 model, neutrophils were also classified 13 , 14 as N1 or N2 phenotypes using iNOS and arg1, respectively, in tumours, and also in an in vitro polarization study. 15 We hypothesized that neutrophil polarization into N1 (iNOS+) and N2 (arg1+) subtypes could also exist in inflamed ND tissues.
A third marker, interleukin (IL)‐17E (an IL‐17 family cytokine) was found to be overexpressed in skin diseases, and is critical for the recruitment of innate immune cells such as macrophages and neutrophils during skin inflammation. 16 In chronic plaque‐type psoriasis (PsO), IL‐17E epidermal expression positively correlated with the number of infiltrating neutrophils and a mixed population of dermal M1/M2 macrophages, suggesting that epidermal IL‐17E may be an important common denominator of chronic skin inflammation. 16
In this ex vivo study, we investigated if the use of three biomarkers, i.e. IL‐17E, iNOS and arg1, combined with two cell markers, MPO and CD68, which are specific markers for neutrophils and macrophages, respectively could be a tool to better characterize ND and to help with their histological differentiation.
Methods
This study was approved by the ethics committee of the University Hospitals of Geneva, Switzerland and was conducted according to the principles of the Declaration of Helsinki. Written informed consent was obtained from each participant.
Patients and biopsies
Paraffin‐embedded skin biopsies of patients with PP (n = 8; 5 women, 3 men; mean age 50 years), GPP (n = 9; 6 women, 3 men; 60 years), SPD (n = 3; all men; 80 years), AGEP (n = 3; 1 women, 2 men; 54.5 years), HS (n = 7; 3 women, 4 men; 52 years), SS (n = 8; 4 women, 4 men; 57 years) and PG (n = 8; 4 women, 4 men; 60 years) were obtained from different Swiss Hospital laboratories. Control samples were normal skin (NS) samples (n = 4; all women; 42 years) obtained from abdominoplasty surgeries, and PsO samples (n = 7; all men; 53.5 years). The diagnosis in all cases was confirmed by an experienced dermatopathologist based on haematoxylin and eosin staining. Whether PP belongs to the ND group remains a debate. 1 , 17 However, we chose to include it because of the numerous neutrophils accumulating that can mimic NDs.
Immunofluorescence
Dewaxing and rehydration
Skin sections were dewaxed in four successive washes of clearing reagent (UltraClear; Avantor/JT Baker, Deventer, The Netherlands), washed in 2‐propanol and rehydrated in four successive washes of 100% ethanol, followed by a wash in each of ethanol 95%, 70% and 50%, and a final wash in H2O.
Antigen retrieval buffers
Sections were then placed in 10 mmol/L citrate buffer pH 6 (for IL‐17E and iNOS) or 10 mmol/L Tris with 1 mmol/L EDTA pH 9 (for arg1); MPO and CD68 work in both buffers. The sections were incubated in a microwave for 8 min at 900 W, then 6 min at 160 W, and then washed and permeabilized with 1 x PBS with 0.1% Tween at room temperature for 5 min. Nonspecific binding was blocked with 1 x PBS with 0.1% Tween and 4% bovine serum albumin.
Antibodies
A combination of three primary antibodies was added to the sections for 1–2 h at room temperature and washed. Antibodies were mouse anti‐human (h)IL‐17E (cat. no. MAB1258), goat anti‐hMPO (AF3667) (both R&D Systems, Minneapolis, MN, USA) and rabbit anti‐hCD68 (ab213363; Abcam, Cambridge, MA, USA), for IL‐17E analyses, and rabbit anti‐hiNOS (ab3523) or rabbit anti‐harg1 (ab183333), mouse anti‐hCD68 (ab955) (all Abcam) and goat anti‐hMPO (R&D Systems) for iNOS or arg1 analyses.
A combination of three secondary antibodies was added for 30 min, followed by washing. These antibodies were donkey anti‐rabbit Alexa‐488 (A21206), donkey anti‐mouse Alexa‐555 (A31570) and donkey anti‐goat Alexa‐633 (A21082) (all Invitrogen/Thermo Fisher Scientific, Waltham, MA, USA). Sections were mounted with DAPI (4′,6‐diamidino‐2‐phenylindole).
Image analyses
Images were acquired with a confocal microscope (LSM 800; Zeiss, Jena, Germany). A minimum of 16 fields (0.8–1.2 mm2) with a × 40 objective lens were taken per section and analysed using ZEN software (Zeiss). Negative controls stained only with secondary antibodies and did not result in significant fluorescence. The relative evaluation of cell abundance was performed manually by two operators.
Results
Interleukin‐17E expression
We first analysed the expression of IL‐17E in combination with MPO and CD68. Skin sections from NS and PsO were used as controls.
Epidermis
Interleukin‐17E expression allowed differentiation of Sweet syndrome from pyoderma gangrenosum. There was high expression of IL‐17E in keratinocytes of the granular layer in NS and in all layers in PsO samples, consistent with previous work 16 (Fig. 1, Table 1). In ND, we identified some main patterns: (i) there was an overall increase in expression of IL‐17E in PP, GPP, SPD, AGEP and SS, similar to PsO; (ii) in HS, IL‐17E was distributed mainly in the granular layer, similarly to NS samples, although its expression in HS was much lower; and (iii) PG had an overall reduction in IL‐17E expression, unlike either NS or PsO. Of note, SS and PG, which may be difficult to differentiate histologically when the epidermis of PG is still preserved, consistently contained distinct levels of IL‐17E, i.e. high in SS and low in PG.
Figure 1.
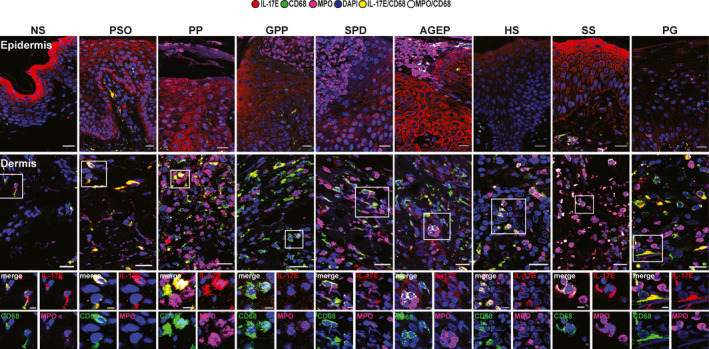
Interleukin (IL)‐17E immunofluorescence staining (mouse anti‐IL‐17E, red) in macrophages (rabbit anti‐CD68, green) and neutrophils [goat anti‐myeloperoxidase (MPO), pink] in the epidermis (first row) and dermis (second row and higher magnification). One representative cropped image is shown. Original magnification × 40; scale bar 20 µm (higher magnification scale bar 5 µm). AGEP, acute generalized exanthematous pustulosis; GPP, generalized pustular psoriasis; HS, hidradenitis suppurativa; NS, normal skin; PG, pyoderma gangrenosum; PP, paradoxical psoriasis; PsO, chronic plaque‐type psoriasis; SPD, subcorneal pustular dermatosis of Sneddon–Wilkinson; SS, Sweet syndrome.
Table 1.
Relative quantification of cells. a
| Cells | Sample | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| NS | PsO | PP | GPP | SPD | AGEP | HS | SS | PG | |
| Epidermis | |||||||||
| Keratinocytes | |||||||||
| IL‐17E | ++ | +++ | +++ | +++ | +++ | +++ | + | +++ | + |
| iNOS | ++ | +++ | +++ | +++ | +++ | +++ | + | +++ | + |
| arg1 | + | + | + | + | +++ | + | + | + | + |
| Neutrophils | |||||||||
| MPO/IL‐17Elow/iNOS | − | −/+ | ++ | + | +/++ | − | −/+ | −/+ | ++ |
| iMCs | |||||||||
| MPO/CD68/iNOS/arg1 | − | − | − | + | + | + | − | + | − |
| Pustules, Munro, b iMCs | |||||||||
| MPO/CD68/iNOS/arg1 | − | − | − | ++ | + | + | − | − | − |
| MPO/iNOS | − | + | ++ | + | ++ | + | − | − | − |
| Dermis | |||||||||
| Neutrophils | |||||||||
| MPO/IL‐17Elow | −/+ | + | +++ | ++ | ++ | ++ | ++ | ++ | +++ |
| N1 | |||||||||
| MPO/iNOS | −/+ | + | +++ | + | ++ | + | + | + | +++ |
| N2 | |||||||||
| MPO/arg1 | − | −/+ | − | + | + | + | ++ | ++ | −/+ |
| iMCs | |||||||||
| MPO/CD68/iNOS/arg1 | − | −/+ | − | + | + | + | ++ | ++ | −/+ |
| Macrophages | |||||||||
| CD68/IL‐17E | + | +++ | +++ | ++ | ++ | ++ | ++ | ++ | +++ |
| M1 | |||||||||
| CD68/iNOS | + | ++ | +++ | + | ++ | + | + | + | +++ |
| M2 | |||||||||
| CD68/arg1 | + | ++ | −/+ | ++ | + | ++ | ++ | ++ | −/+ |
AGEP, acute generalized exanthematous pustulosis; GPP, generalized pustular psoriasis; HS, hidradenitis suppurativa; iMCs, immature myeloid cells; M1, proinflammatory macrophages; M2, anti‐inflammatory macrophages; N1, inflammatory neutrophils; N2, unknown function in skin; NS, normal skin; PG, pyoderma gangrenosum; PP, paradoxical psoriasis, PsO, chronic plaque‐type psoriasis, SPD, subcorneal pustular dermatosis of Sneddon–Wilkinson; SS, Sweet syndrome.
Expression: − (absent), + (low expression), ++ (medium expression), +++ (high expression).
Munro microabscesses.
Neutrophil infiltration inside the epidermis (MPO positive, with low‐positive levels of IL‐17E staining; MPO+IL‐17Elow) were detected mainly in PP and PG and to a lesser extent in SPD (Table 1). SS, GPP and AGEP had lower numbers of neutrophils (MPO+IL‐17low) but higher numbers of smaller cells that were MPO+CD68+IL‐17Elow.
Intraepidermal pustules contained cells resembling immature neutrophils. In GPP, SPD and AGEP pustules, there was a mixed population of cells, some resembling mature neutrophils (MPO+IL‐17Elow), with fully segmented nuclei, and others that looked like immature neutrophils (MPO+CD68+IL‐17Elow), whose nuclei were not fully segmented (Fig. 2). These pustules also contained some larger macrophages (CD68+IL‐17Elow). In PP, extraepidermal Munro microabscesses contained mainly neutrophils (MPO+IL‐17Elow) having the aspect of mature cells.
Figure 2.
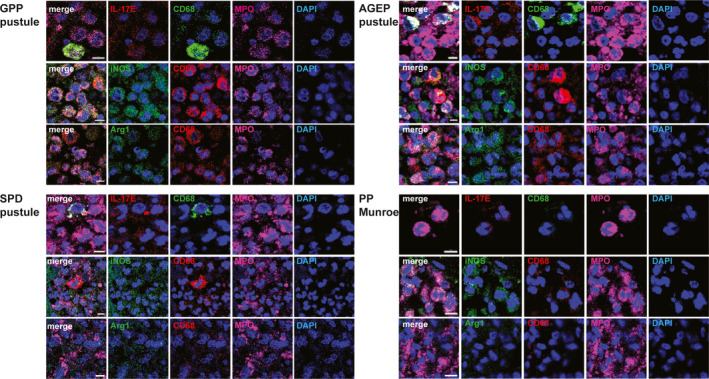
Pustules of generalized pustular psoriasis (GPP) (top left), subcorneal pustular dermatosis of Sneddon–Wilkinson (SPD) (bottom left), acute generalized exanthematous pustulosis (AGEP) (top right) and Munro microabscesses in paradoxical psoriasis (PP) (bottom right) stained with interleukin (IL)‐17E (red), inducible nitric oxide synthase (iNOS) (green) or arginase (arg)1 (green), each one in combination with markers of macrophages rabbit anti‐CD68 (green) or mouse anti‐CD68 (red) and neutrophils [goat anti‐myeloperoxidase (MPO), pink]. One representative cropped image is shown. Original magnification × 40; scale bar 5 µm.
Dermis
The frequency of particular neutrophils and macrophages allowed differentiation between different conditions. IL‐17E was mainly expressed by macrophages (CD68+) in NS and ND samples (Fig. 1, Table 1). However, larger macrophages (CD68+IL‐17E+) that were also positive for MPO were observed in PP and PG especially. These larger cells might represent macrophages that had engulfed dying neutrophils. Moreover, PP and PG contained a marked increase of neutrophils (MPO+IL‐17Elow) not observed in PsO (Table 1). Therefore, the frequency of MPO+IL‐17Elow neutrophils and of macrophages that had engulfed neutrophils is a characteristic of PG and PP, and it allows discrimination between PP and PsO.
The frequency of dermal immature neutrophils is characteristic of specific conditions. In GPP, AGEP, HS and SS, we observed smaller histiocytes (CD68+/IL‐17Elow) that were also positive for MPO (Fig. 1; Table 1). These latter cells might resemble immature myeloid cells (iMCs), as previously described in SS. 18 SPD presented a mixed population, with the majority of cells being neutrophils and macrophages, but also with a fraction of cells resembling iMCs (MPO+CD68+IL‐17Elow).
Expression of inducible nitric oxide synthase and arginase1
We next analysed the expression of iNOS and arg1 in combination with MPO and CD68, in order to define the subtypes of neutrophils and macrophages.
Epidermis
Inducible nitric oxide synthase expression allows differentiation of Sweet syndrome from pyoderma gangrenosum. iNOS was expressed by keratinocytes in all layers in both controls and ND (Fig. 3, Table 1). However, as observed with IL‐17E, iNOS expression was consistently higher in SS and lower in PG.
Figure 3.
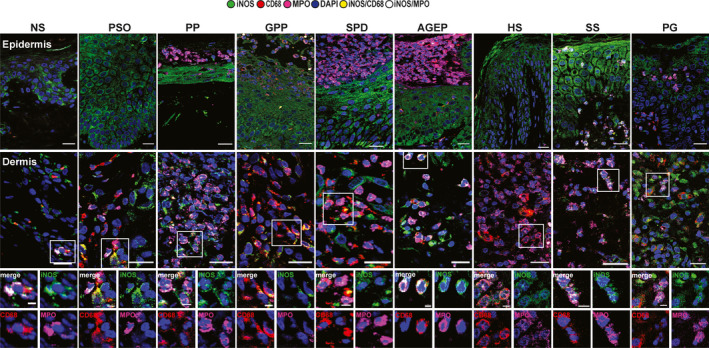
Inducible nitric oxide synthase (iNOS) immunofluorescence staining (rabbit anti‐iNOS, green) in macrophages (mouse anti‐CD68, red) and neutrophils [goat anti‐myeloperoxidase (MPO), pink] in the epidermis (first row) and dermis (second row and higher magnification). One representative cropped image is shown. Original magnification × 40; scale bar 20 µm (higher magnification scale bar 5 µm). AGEP, acute generalized exanthematous pustulosis; GPP, generalized pustular psoriasis; HS, hidradenitis suppurativa; NS, normal skin; PG, pyoderma gangrenosum; PP, paradoxical psoriasis, PsO, chronic plaque‐type psoriasis, SPD, subcorneal pustular dermatosis of Sneddon–Wilkinson; SS, Sweet syndrome.
In pustules of GPP, SPD and AGEP, and in Munro microabscesses of PP, all the MPO+ cells expressed iNOS, despite resembling iMCs (CD68+MPO+iNOS+) or mature N1 cells (MPO+iNOS+) (Fig. 2, Table 1).
Arginase1 expression is characteristic of subcorneal pustular dermatosis of Sneddon–Wilkinson. In contrast to iNOS, arg1 was markedly expressed only by the keratinocytes of the granular layer of SPD (Fig. 4, Table 1). iMC observed in the pustules were also weakly arg1+ (Fig. 2). 14 , 19
Figure 4.
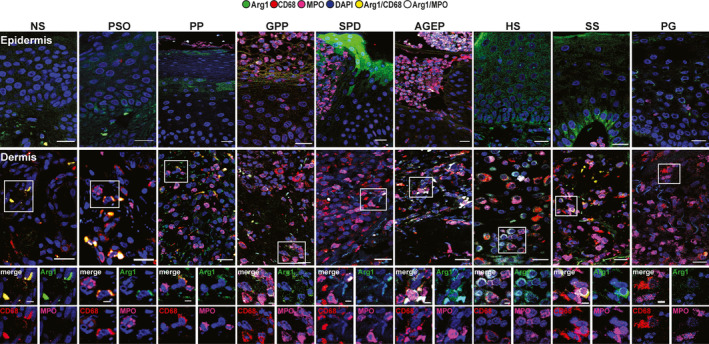
Arginase (arg) 1 immunofluorescence staining (rabbit anti‐arg1, green) in macrophages (mouse anti‐CD68, red) and neutrophils [goat anti‐myeloperoxidase (MPO), pink] in the epidermis (first row) and dermis (second row and higher magnification). One representative cropped image is shown. Original magnification × 40. Scale bar 20 µm, higher magnification scale bar 5 µm. AGEP, acute generalized exanthematous pustulosis; GPP, generalized pustular psoriasis; HS, hidradenitis suppurativa; NS, normal skin; PG, pyoderma gangrenosum; PP, paradoxical psoriasis, PsO, chronic plaque‐type psoriasis, SPD, subcorneal pustular dermatosis of Sneddon–Wilkinson; SS, Sweet syndrome.
Dermis
N1 and M1 are predominant in pustular psoriasis, pyoderma gangrenosum and subcorneal pustular dermatosis of Sneddon–Wilkinson. Only PP and PG, and to a lesser extent SPD, contained the majority of inflammatory N1 (MPO+iNOS+) and M1 (CD68+iNOS+) cells (Fig. 3, Fig. 4, Table 1). Moreover, in PP and PG, very few cells expressed arg1 (M2 or N2). However, SPD not only presented many M1 and N1, but also contained M2 (CD68+arg1+), N2 (MPO+arg1+) and iMCs (MPO+CD68+iNOS+arg1+).
By contrast, GPP, AGEP, HS and SS contained a majority of iMCs, M2 and N2. Interestingly, N2 had more circular nuclei compared with N1 20 (Fig. 4; see HS higher magnification).
Discussion
The differential diagnosis of some types of ND can be challenging because of their very similar clinical and histological features. This can be the case for SS and PG, particularly when the epidermis in PG is still preserved. In the current study, we found that IL‐17E and iNOS are systematically expressed in the epidermis of SS but not of PG. The reasons behind this remain to be determined; we hypothesize that apoptotic keratinocytes in PG might release their contents and thus appear negative for IL‐17E and iNOS. For practical purposes, this differential expression is clear‐cut, does not require any quantification software and can be evaluated manually.
We also found that SS and PG have different dermal patterns, with infiltration of small iMCs into the dermis seen in SS but not in PG. This early infiltration of iMCs into lesions of histiocytoid SS was also reported previously. 18 As only one of our SS histological samples was histiocytoid in appearance, these results show that even in histologically ‘classic’ SS, iMCs are present in the dermis and their presence can be used to differentiate SS from PG.
We also observed the additional presence of M2 and, to a lesser extent, N2 cells in the deeper dermis in SS. More studies are needed to address the role of N2 in skin. 21 In the current study, the presence of iMCs and M2 was a shared feature of both SS and HS. In other studies, 22 , 23 persistence of M2 in HS tissues was considered responsible for excessive collagen resulting in scarring and fibrotic tissues. Similarly, we found that some lesions of SS showed fibrotic abnormalities, whereas these were typically not observed in PG. In contrast to SS and HS, PG dermis contained a predominance of N1, M1 and M1 that have engulfed neutrophils. Their persistence can be a sign of chronic inflammation, as M1 failing to clear N1 are prevented from switching to M2. 10
It can be challenging to distinguish between the different types of psoriasis (PsO, PP, GPP), as well as SPD and AGEP. SPD and AGEP can be differentiated by their epidermal content of arg1, with SPD showing a stronger accumulation in the granular layer. Of the three forms of psoriasis, only PP contained a majority of inflammatory N1, M1 and neutrophil‐engulfing M1. PsO showed a mixed population of M1 and M2 with few N1, while GPP were enriched in iMCs. Of note, iMC rather than mature neutrophils have been shown to migrate in chronic inflammation, 19 , 24 and defective neutrophils in GPP were found to impair the efferocytosis process. 25
Analysis of pustules can also help in histological discrimination. GPP and AGEP pustules contained a majority of iMCs, whereas SPD pustules presented more cells resembling N1, as seen in Munro microabscesses in PP.
Finally, our analysis highlights the possibility of classifying ND into two groups (Fig. 5): (i) ND characterized by proinflammatory M1 and N1, such as PP, PG, and to a lesser extent SPD; and (ii) ND enriched in immature neutrophils, such as GPP, AGEP, HS and SS. Further studies should be performed to confirm this hypothesis.
Figure 5.
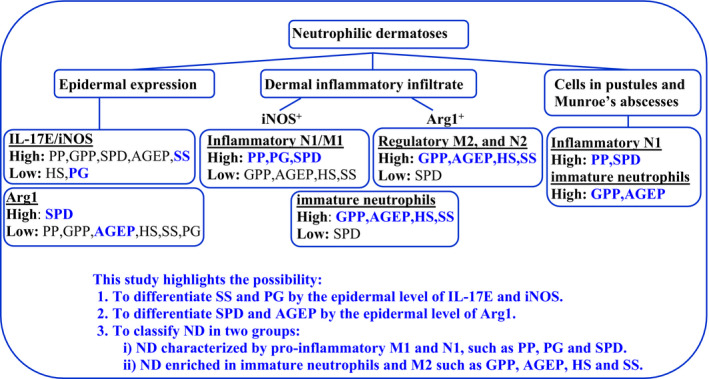
Summary of the main results obtained. AGEP, acute generalized exanthematous pustulosis; GPP, generalized pustular psoriasis; HS, hidradenitis suppurativa; M1, proinflammatory macrophages; M2, anti‐inflammatory macrophages; N1, inflammatory neutrophils; N2, unknown function in skin; NS, normal skin; PG, pyoderma gangrenosum; PP, paradoxical psoriasis, PsO, chronic plaque‐type psoriasis, SPD, subcorneal pustular dermatosis of Sneddon–Wilkinson; SS, Sweet syndrome.
Of note, the presence of M2 should be the physiological norm of homeostatic skin. However, the dermal abdominoplasties used as NS contained inflammatory M1 and a few N1. These observations were in line with the inflammatory status in obese people described by other groups. 26
This study was limited by the small number of cases. In addition, variability due to technical changes or the use of different sources of antibodies cannot be excluded.
Conclusion
The histological diagnosis of different ND can be challenging in the absence of reliable immunohistochemical markers. In this paper, we propose a set of markers (IL‐17E, iNOS and arg1) that can be used on a routine basis to help dermatopathologists with challenging cases. Our findings need to be confirmed on larger cohorts comprising additional ND not studied in this pilot project. However, these markers will help to make a more accurate ND diagnosis and contribute to a better understanding of the mechanisms of these diseases.
Acknowledgement
This study was supported by the grant no. 310030‐175470/1 from the Swiss National Science Foundation. Open Access Funding provided by Universite de Geneve. [Correction added on 12 April 2022, after first online publication: Consortium of Swiss Academic Libraries (CSAL) funding statement has been added.]
What's already known about this topic?
ND are a group of skin diseases characterized by epidermal and dermal neutrophilic infiltration.
They present several histological similarities that make the diagnosis challenging.
Misdiagnosis can lead to a delayed or inadequate treatment.
What does this study add?
This study defines three relevant skin biomarkers (IL‐17E, iNOS and arg1) that could be used to make a more accurate histological identification of ND.
This study highlights the possibility of (i) differentiating SS and PG by the epidermal level of IL‐17E and iNOS, and (ii) differentiating SPD and AGEP by the epidermal level of arg1
It also makes possible the classification of ND into two groups: (i) ND characterized by proinflammatory M1 and N1, such as PP, PG and SPD, and (ii) ND enriched in immature neutrophils and M2, such as GPP, AGEP, HS and SS.
Conflict of interest: WHB received honoraria as advisor or invited speaker from AbbVie, Almirall, BMS, Celgene, Leo, Lilly, Novartis, Sanofi, Pfizer and UCB. The other authors declare that they have no conflict of interest.
References
- 1. Marzano AV, Borghi A, Wallach D, Cugno M. A comprehensive review of neutrophilic diseases. Clin Rev Allergy Immunol 2018; 54: 114–30. [DOI] [PubMed] [Google Scholar]
- 2. Marzano AV, Ortega‐Loayza AG, Heath M et al. Mechanisms of inflammation in neutrophil‐mediated skin diseases. Front Immunol 2019; 10: 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prame Kumar K, Nicholls AJ, Wong CHY. Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res 2018; 371: 551–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amanzada A, Malik IA, Nischwitz M et al. Myeloperoxidase and elastase are only expressed by neutrophils in normal and in inflamed liver. Histochem Cell Biol 2011; 135: 305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chistiakov DA, Killingsworth MC, Myasoedova VA et al. CD68/macrosialin: not just a histochemical marker. Lab Invest 2017; 97: 4–13. [DOI] [PubMed] [Google Scholar]
- 6. Yurdagul A Jr, Subramanian M, Wang X et al. Macrophage metabolism of apoptotic cell‐derived arginine promotes continual efferocytosis and resolution of injury. Cell Metab 2020; 31: 518–33.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci 2018; 19: 1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allenbach C, Zufferey C, Perez C et al. Macrophages induce neutrophil apoptosis through membrane TNF, a process amplified by Leishmania major. J Immunol 2006; 176: 6656–64. [DOI] [PubMed] [Google Scholar]
- 9. Peiseler M, Kubes P. More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest 2019; 129: 2629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro‐wound healing phenotypes. Front Physiol 2018; 9: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suwanpradid J, Shih M, Pontius L et al. Arginase1 deficiency in monocytes/macrophages upregulates inducible nitric oxide synthase to promote cutaneous contact hypersensitivity. J Immunol 2017; 199: 1827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lisi L, Ciotti GM, Braun D et al. Expression of iNOS, CD163 and ARG‐1 taken as M1 and M2 markers of microglial polarization in human glioblastoma and the surrounding normal parenchyma. Neurosci Lett 2017; 645: 106–12. [DOI] [PubMed] [Google Scholar]
- 13. Masucci MT, Minopoli M, Carriero MV. Tumor associated neutrophils. their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol 2019; 9: 1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grzywa TM, Sosnowska A, Matryba P et al. Myeloid cell‐derived arginase in cancer immune response. Front Immunol 2020; 11: 938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohms M, Möller S, Laskay T. An attempt to polarize human neutrophils toward N1 and N2 phenotypes in vitro. Front Immunol 2020; 11: 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Senra L, Stalder R, Alvarez Martinez D et al. Keratinocyte‐derived IL‐17E contributes to inflammation in psoriasis. J Invest Dermatol 2016; 136: 1970–80. [DOI] [PubMed] [Google Scholar]
- 17. Hu JZ, Billings SD, Yan D, Fernandez AP. Histologic comparison of tumor necrosis factor‐α inhibitor–induced psoriasis and psoriasis vulgaris. J Am Acad Dermatol 2020; 83: 71–7. [DOI] [PubMed] [Google Scholar]
- 18. Alegría‐Landa V, Rodríguez‐Pinilla SM, Santos‐Briz A et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol 2017; 153: 651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ren W, Zhang X, Li W et al. Circulating and tumor‐infiltrating arginase 1‐expressing cells in gastric adenocarcinoma patients were mainly immature and monocytic myeloid‐derived suppressor cells. Sci Rep 2020; 10: 8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fridlender ZG, Sun J, Kim S et al. Polarization of tumor‐associated neutrophil phenotype by TGF‐beta: "N1" versus "N2" TAN. Cancer Cell 2009; 16: 183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scalerandi MV, Peinetti N, Leimgruber C et al. Inefficient N2‐Like neutrophils are promoted by androgens during infection. Front Immunol 2018; 9: 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Byrd AS, Kerns ML, Williams DW et al. Collagen deposition in chronic hidradenitis suppurativa: potential role for CD163(+) macrophages. Br J Dermatol 2018; 179: 792–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim SY, Nair MG. Macrophages in wound healing: activation and plasticity. Immunol Cell Biol 2019; 97: 258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ilkovitch D, Ferris LK. Myeloid‐derived suppressor cells are elevated in patients with psoriasis and produce various molecules. Mol Med Rep 2016; 14: 935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haskamp S, Bruns H, Hahn M et al. Myeloperoxidase modulates inflammation in generalized pustular psoriasis and additional rare pustular skin diseases. Am J Hum Genet 2020; 107: 527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chylikova J, Dvorackova J, Tauber Z, Kamarad V. M1/M2 macrophage polarization in human obese adipose tissue. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2018; 162: 79–82. [DOI] [PubMed] [Google Scholar]


