Abstract
Aims
Whereas the combination of anaemia and chronic kidney disease (CKD) has been extensively studied in patients with heart failure (HF), the contribution of iron deficiency (ID) to this dysfunctional interplay is unknown. We aimed to assess clinical associates and pathophysiological pathways related to ID in this multimorbid syndrome.
Methods and results
We studied 2151 patients with HF from the BIOSTAT‐CHF cohort. Patients were stratified based on ID (transferrin saturation <20%), anaemia (World Health Organization definition) and/or CKD (estimated glomerular filtration rate <60 ml/min/1.73 m2). Patients were mainly men (73.3%), with a median age of 70.5 (interquartile range 61.4–78.1). ID was more prevalent than CKD and anaemia (63.3%, 47.2% and 35.6% respectively), with highest prevalence in those with concomitant CKD and anaemia (77.5% vs. 59.3%; p < 0.001). There was a considerable overlap in biomarkers and pathways between patients with isolated ID, anaemia or CKD, or in combination, with processes related to immunity, inflammation, cell survival and cancer amongst the common pathways. Key biomarkers shared between syndromes with ID included transferrin receptor, interleukin‐6, fibroblast growth factor‐23, and bone morphogenetic protein 6. Having ID, either alone or on top of anaemia and/or CKD, was associated with a lower overall summary Kansas City Cardiomyopathy Questionnaire score, an impaired 6‐min walk test and increased incidence of hospitalizations and/or mortality in multivariable analyses (all p < 0.05).
Conclusion
Iron deficiency, CKD and/or anaemia in patients with HF have great overlap in biomarker profiles, suggesting common pathways associated with these syndromes. ID either alone or on top of CKD and anaemia is associated with worse quality of life, exercise capacity and prognosis of patients with worsening HF.
Keywords: Iron deficiency, Heart failure, Anaemia, Chronic kidney disease, Cardiorenal, Biomarkers
Iron deficiency (ID) in patients with heart failure (HF) retains its adverse association with clinical outcomes regardless of concomitant anaemia and/or chronic kidney disease (CKD). In a comprehensive biomarker and pathway analysis of HF patients with ID, CKD and/or anaemia, we found an extensive overlap in biomarker profiles, suggesting similar underlying mechanisms of these syndromes despite different clinical definitions. BMP6, bone morphogenetic protein 6; CRAIDS, cardio‐renal anaemia iron deficiency syndrome; CRAS, cardio‐renal anaemia syndrome; CRIDS, cardio‐renal iron deficiency syndrome; FGF23, fibroblast growth factor 23; IDA, iron deficiency anaemia; IL‐6, interleukin‐6; TFRC, transferrin receptor. Several graphical elements were adapted from www.flaticon.com.
Introduction
Heart failure (HF) is a complex syndrome further complicated by the coexistence of other comorbidities. 1 Anaemia and chronic kidney disease (CKD) are both common and associated with worse quality of life and outcomes in patients with HF, 2 , 3 especially when all three co‐exist. 4 This triad constitutes a pathophysiological entity commonly referred to as the cardio‐renal anaemia syndrome (CRAS). 5
Although pathophysiological mechanisms causing anaemia are multifactorial, iron deficiency (ID) is the most common underlying cause of anaemia in HF. 6 However, in contrast to the traditional view where ID is equated with anaemia, the majority of patients with ID do not have concomitant anaemia (IDA), indicating that the two conditions do not necessarily coexist. 7 , 8 , 9 Iron is an essential component in numerous biological processes including cellular bioenergetics, oxygen transport and storage (i.e. haemoglobin and myoglobin) as well as the synthesis of lipids, proteins, ribonucleic acid and DNA. 10 Multiple randomized controlled trials have shown that correcting ID improves symptoms, quality of life, exercise capacity and could reduce hospitalizations in patients with HF. 11 , 12 , 13 , 14 Accordingly, targeting ID using intravenous iron administration has emerged as a novel therapeutic target in HF. 15
Whereas ID, anaemia and CKD individually have been extensively studied in HF, only few studies examined the interaction between these comorbidities, particularly with regard to the additive impact of ID. 8 , 16 Although many studies have investigated CRAS in HF, 5 , 17 , 18 most of these studies failed to assess the importance of ID. It has been suggested that expanding the concept of CRAS by introducing cardio‐renal iron deficiency syndrome (CRIDS) and/or cardio‐renal anaemia iron deficiency syndrome (CRAIDS) might help to identify patients at high risk who potentially have more to gain from treatment with intravenous iron. 19 , 20
We accordingly aimed to disentangle the additive burden of ID on morbidity and mortality as well as to identify common and unique pathophysiological pathways and biomarker profiles related to isolated ID, anaemia or CKD, and their combinations.
Methods
Study population
This was a post‐hoc analysis of the BIOSTAT‐CHF (BIOlogy Study to TAilored Treatment in Chronic Heart Failure) index cohort, the details of which have been described elsewhere. 21 , 22 Briefly, BIOSTAT‐CHF was a European multicentre, prospective, observational study that was conducted from 2010–2014 across 69 centres in 11 European countries. The included 2516 participants were patients with worsening HF that were either enrolled in the outpatient setting or as inpatients if hospitalized. Included patients had to be 18 years old or older, with symptoms of either new‐onset or decompensated HF, and sub‐optimally treated for HF at inclusion. Diagnosis of HF was made either by left ventricular ejection fraction (LVEF) ≤40% or B‐type natriuretic peptide >400 pg/ml and/or N‐terminal pro‐B‐type natriuretic peptide >2000 pg/ml. The primary endpoints of interest were the time to all‐cause mortality, first unscheduled HF hospitalization and the combined endpoint of all‐cause mortality and HF hospitalization. Outcomes were independently adjudicated. The study complied with the Declaration of Helsinki, was approved by the medical ethics committees of the participating centres, and informed consent was obtained from all patients.
Study definitions
Iron deficiency was defined according to the DEFINE‐HF study as transferrin saturation (TSAT) <20%. 23 Anaemia was defined as haemoglobin level <13 g/dl in men and <12 g/dl in women according to the World Health Organization (WHO) criteria. 24 Renal function was evaluated using the estimated glomerular filtration rate (eGFR), which was calculated using the Modification of Diet in Renal Disease (MDRD) equation. CKD was defined as an eGFR of <60 ml/min/1.73 m2. 25 All measurements were determined at study inclusion.
Results of baseline clinical characteristics, determinants as well as biomarker expression profiles of ID compared to no ID in general using only the Cardiovascular III panel in this cohort have been described previously. 26 To study the additional burden of ID beyond the cardiorenal anaemia syndrome, in the present analysis, eight different groups of HF patients were formed and compared accordingly based on the presence/absence of ID, CKD and/or anaemia. Definitions of these syndromes are summarized in Table S1 .
Of all 2516 patients in the BIOSTAT‐CHF index cohort, variables needed for defining iron status, anaemia and CKD were available in 2151 (85.4%) patients.
Biomarker measurements
Four biomarker panels (the Immune response, Immuno‐oncology, Cardiovascular II and III panels) each containing 92 proteins related to different pathophysiological domains were measured. These biomarkers were measured using the Olink Proseek® Multiplex kits, which make use of proximity extension assay (PEA) to quantify plasma proteins. 27 Measurements are normalized and provided in log2 scale. Although 368 biomarkers in total were measured, only 356 biomarkers were included in the analysis because (i) eight biomarkers were excluded for being below the assay's lowest limit of detection (10%); and (ii) means of three duplicate biomarkers were used as they overlap between the different panels. The complete list of all biomarkers measured is listed in online supplementary Table S2 .
Identification of differentially expressed biomarkers
Heart failure patients with no comorbidities (ID, CKD and/or anaemia) were used as the reference group in differential biomarker expression analysis. Differential biomarker expression analysis between all of the remaining seven syndromes (online supplementary Table S1 ) and the reference group was conducted using the LIMMA package. 28 , 29 Multiple testing correction across all groups instead of only between each group was performed. Biomarkers with an absolute log fold change of 0.25 or higher and a false discovery rate (FDR) ≤0.01 were deemed statistically significant. The analysis was adjusted for potential confounders including age, sex and diabetes.
Translating biomarker lists into biological pathways
Pathway over‐representation analysis is a widely used approach to interpret gene/protein lists into biologically meaningful pathways. 30 We used the differentially expressed biomarkers to characterize the underlying pathophysiological processes for each syndrome. Pathway analysis was conducted using the online tool WebGestalt with the default parameters. 31 The over‐representation analysis was based on multiple knowledgebases including Gene Ontology (which was confined to only non‐redundant biological processes), Reactome and Kyoto Encyclopedia of Genes and Genomes (KEGG). To address the inherent redundancy across these different databases, the affinity propagation algorithm was used in order to output the most representative (exemplar) and informative pathways per syndrome. The Benjamini–Hochberg method was used for multiple testing adjustment. Over‐represented pathways with FDR <0.01 were deemed significant.
Statistical analyses
Statistical analyses were performed using R version 4.0.3. Data are presented as means (standard deviation, SD) when continuous variables are normally distributed, as median (interquartile range, IQR) when continuous variables are non‐normally distributed and as number (percentage) for binary/categorical variables. Skewness of continuous variables was determined using histograms and/or Q–Q plots. Intergroup differences of baseline variables were tested using the Student's t‐test (two groups) or one‐way analysis of variance test (three groups) if normally distributed, Wilcoxon rank sum test (two groups) and Kruskal–Wallis test (three groups) if non‐normally distributed, while qualitative variables were compared using Chi‐square test. Results with a two‐tailed p‐value of <0.05 were considered significant.
Linear regression analyses were performed to test the association of ID in patients with HF, CKD and/or anaemia with quality of life and functional capacity using results from the 6‐min walk test (6MWT) and the overall summary score on the Kansas City Cardiomyopathy Questionnaire (KCCQ).
Kaplan–Meier curves stratified by increasing number of comorbidities (ID, CKD and/or anaemia) as well as each syndrome separately (as summarized in online supplementary Table S1 ) are shown, with differences between them tested using the log‐rank test for survival. To adjust for potential confounding variables and to quantify these effects, univariable and multivariable Cox proportional hazards models were constructed on all‐cause mortality and the composite endpoint of all‐cause mortality and HF rehospitalization. HF patients with no comorbidities (i.e. no ID, CKD and/or anaemia) were used as the reference group to estimate the adjusted risk associated with having ID, anaemia and/or CKD. Also, we report the additional impact of having ID on prognosis by comparing ID‐containing syndromes to syndromes without ID (i.e. CRAIDS vs. CRAS, CRIDS vs. isolated CKD and IDA vs. isolated anaemia). The proportional hazards assumption was evaluated using Schoenfeld residuals. Multivariable models were adjusted to the previously published BIOSTAT‐CHF risk models, 22 except for haemoglobin as anaemia was included as explanatory variable.
Results
Baseline characteristics
Baseline characteristics are stratified by renal dysfunction, anaemia and iron deficiency (Table 1 ). Median age of the 2151 included patients was 70.5 (IQR 61.4–78.1), median TSAT 16.7% (IQR 10.9–24.2), median ferritin 102 mg/L (IQR 50.0–192) and 26.7% were women. In general, iron‐deficient patients had higher inflammatory markers and worse quality of life compared to those with or without anaemia and/or CKD (FDR <0.05). Additionally, compared to patients with no comorbidities (i.e. without ID, CKD and/or anaemia), patients with isolated ID were less likely to be treated with β‐blockers and angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers at baseline (FDR <0.05). Levels of renal markers were comparable between patients with and without ID, except for fibroblast growth factor‐23 (FGF23) which was consistently higher in those with ID (FDR <0.05). The characteristics of iron‐deficient HF patients compared to those without ID irrespective of CKD and/or anaemia are presented in online supplementary Table S3 .
Table 1.
Baseline characteristics stratified by renal dysfunction, anaemia and iron deficiency
| Variables | Total cohort (n = 2151) | No renal dysfunction | Renal dysfunction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anaemic | Not anaemic | Anaemic | Not anaemic | ||||||||||
| No ID | ID | p‐value | No ID | ID | p‐value | No ID | ID | p‐value | No ID | ID | p‐value | ||
| (n = 76) | (n = 231) | (n = 398) | (n = 430) | (n = 106) | (n = 365) | (n = 209) | (n = 336) | ||||||
| Anaemia alone | IDA | No comorbidities | ID alone | CRAS | CRAIDS | CKD alone | CRIDS | ||||||
| Clinical parameters | |||||||||||||
| Age (years) | 70.5 [61.4–78.1] | 68.9 [58.2–73.9] | 72.0 [63.8–77.7] | 0.060 | 64.6 [56.3–73.6] | 63.6 [55.3–73.9] | 0.658 | 76.2 [67.6–81.0] | 75.4 [68.7–80.6] | 0.689 | 73.0 [65.3–80.2] | 73.3 [65.1–79.4] | 0.625 |
| Women | 575 (26.7%) | 9 (11.8%) | 56 (24.2%) | 0.033 | 69 (17.3%) | 124 (28.8%) | <0.001 * | 16 (15.1%) | 104 (28.5%) | 0.008 * | 64 (30.6%) | 133 (39.6%) | 0.043 |
| BMI (kg/m2) | 27.9 (5.58) | 27.1 (4.24) | 27.7 (5.42) | 0.380 | 28.2 (5.84) | 28.0 (5.94) | 0.707 | 26.7 (5.56) | 27.6 (5.28) | 0.147 | 27.9 (4.86) | 28.3 (5.87) | 0.397 |
| NYHA class | 0.035 | <0.001 * | 0.017 | <0.001 * | |||||||||
| I | 46 (2.20%) | 3 (3.95%) | 3 (1.35%) | 14 (3.64%) | 10 (2.43%) | 3 (2.88%) | 4 (1.13%) | 5 (2.45%) | 4 (1.21%) | ||||
| II | 734 (35.2%) | 37 (48.7%) | 75 (33.6%) | 191 (49.6%) | 137 (33.3%) | 31 (29.8%) | 87 (24.6%) | 84 (41.2%) | 92 (27.8%) | ||||
| III | 1053 (50.4%) | 29 (38.2%) | 115 (51.6%) | 153 (39.7%) | 209 (50.9%) | 64 (61.5%) | 206 (58.2%) | 101 (49.5%) | 176 (53.2%) | ||||
| IV | 255 (12.2%) | 7 (9.21%) | 30 (13.5%) | 27 (7.01%) | 55 (13.4%) | 6 (5.77%) | 57 (16.1%) | 14 (6.86%) | 59 (17.8%) | ||||
| Heart rate (bpm) | 80.2 (19.6) | 75.0 (17.3) | 81.7 (19.9) | 0.005 * | 79.5 (18.8) | 85.7 (22.4) | <0.001 * | 74.4 (16.2) | 78.3 (17.7) | 0.033 | 75.3 (17.4) | 81.3 (19.7) | <0.001 * |
| Estimated protein intake (g/day) | 54.9 (11.2) | 56.2 (13.4) | 54.9 (11.0) | 0.472 | 58.5 (12.7) | 55.5 (11.1) | <0.001 * | 51.4 (9.46) | 52.0 (8.96) | 0.576 | 55.9 (11.0) | 53.0 (10.1) | 0.004 * |
| Ischaemic aetiology | 957 (45.2%) | 28 (37.3%) | 108 (48.2%) | 0.133 | 147 (37.8%) | 171 (40.2%) | 0.520 | 61 (58.1%) | 193 (53.8%) | 0.501 | 104 (51.0%) | 145 (43.3%) | 0.099 |
| Systolic blood pressure (mmHg) | 125 (21.9) | 123 (22.1) | 123 (20.5) | 0.811 | 125 (19.2) | 128 (24.7) | 0.027 | 118 (20.6) | 124 (21.9) | 0.004 * | 125 (20.2) | 124 (23.1) | 0.628 |
| LVEF (%) | 30.0 [25.0–37.0] | 30.0 [25.0–36.0] | 30.0 [25.0–39.0] | 0.730 | 30.0 [25.0–35.0] | 28.0 [21.5–35.0] | 0.460 | 33.0 [25.0–40.0] | 31.0 [25.0–40.0] | 0.634 | 30.0 [25.0–37.0] | 30.0 [24.0–35.0] | 0.020 |
| HF subgroup | 0.283 | 0.238 | 0.262 | 0.257 | |||||||||
| HFrEF | 1544 (80.3%) | 58 (84.1%) | 152 (75.2%) | 318 (85.9%) | 323 (82.6%) | 67 (72.0%) | 220 (70.7%) | 156 (80.0%) | 250 (85.6%) | ||||
| HFmrEF | 248 (12.9%) | 8 (11.6%) | 32 (15.8%) | 38 (10.3%) | 43 (11.0%) | 20 (21.5%) | 54 (17.4%) | 25 (12.8%) | 28 (9.59%) | ||||
| HFpEF | 131 (6.81%) | 3 (4.35%) | 18 (8.91%) | 14 (3.78%) | 25 (6.39%) | 6 (6.45%) | 37 (11.9%) | 14 (7.18%) | 14 (4.79%) | ||||
| Successful completion of 6MWT | 1318 (63.4%) | 56 (74.7%) | 126 (57.0%) | 0.010 * | 309 (80.1%) | 274 (65.7%) | <0.001 * | 57 (55.3%) | 179 (51.1%) | 0.524 | 129 (63.5%) | 188 (58.0%) | 0.243 |
| 6MWT (m) | 203 (172) | 253 (177) | 173 (164) | 0.001 * | 280 (173) | 225 (178) | <0.001 * | 159 (159) | 140 (139) | 0.273 | 207 (173) | 168 (162) | 0.011 * |
| KCCQ overall summary score | 48.5 (22.3) | 56.8 (20.5) | 46.9 (22.1) | 0.001 * | 56.9 (22.5) | 49.2 (21.9) | <0.001 * | 47.3 (22.6) | 40.0 (19.7) | 0.004 * | 54.2 (21.2) | 42.6 (21.1) | <0.001 * |
| Previous hospitalization for HF | 670 (31.1%) | 25 (32.9%) | 65 (28.1%) | 0.519 | 104 (26.1%) | 101 (23.5%) | 0.424 | 44 (41.5%) | 159 (43.6%) | 0.792 | 66 (31.6%) | 106 (31.5%) | 1.000 |
| Hepatomegaly | 310 (14.5%) | 10 (13.2%) | 30 (13.0%) | 1.000 | 52 (13.1%) | 57 (13.3%) | 1.000 | 27 (25.7%) | 69 (19.0%) | 0.169 | 20 (9.57%) | 45 (13.5%) | 0.215 |
| Pulmonary congestion | 1135 (54.0%) | 36 (48.6%) | 130 (57.3%) | 0.246 | 150 (39.1%) | 239 (57.2%) | <0.001 * | 49 (48.0%) | 236 (65.9%) | 0.002 * | 91 (44.2%) | 204 (61.6%) | <0.001 * |
| Comorbidities | |||||||||||||
| Atrial fibrillation | 984 (45.7%) | 33 (43.4%) | 101 (43.7%) | 1.000 | 159 (39.9%) | 156 (36.3%) | 0.310 | 55 (51.9%) | 198 (54.2%) | 0.750 | 103 (49.3%) | 179 (53.3%) | 0.413 |
| COPD | 370 (17.2%) | 11 (14.5%) | 39 (16.9%) | 0.753 | 50 (12.6%) | 72 (16.7%) | 0.110 | 18 (17.0%) | 94 (25.8%) | 0.082 | 33 (15.8%) | 53 (15.8%) | 1.000 |
| Renal disease | 616 (28.6%) | 7 (9.21%) | 22 (9.52%) | 1.000 | 14 (3.52%) | 29 (6.74%) | 0.053 | 62 (58.5%) | 244 (66.8%) | 0.141 | 78 (37.3%) | 160 (47.6%) | 0.023 |
| Peripheral arterial disease | 236 (11.0%) | 5 (6.58%) | 24 (10.4%) | 0.448 | 24 (6.03%) | 44 (10.2%) | 0.038 | 7 (6.60%) | 73 (20.0%) | 0.002 * | 22 (10.5%) | 37 (11.0%) | 0.972 |
| Hypertension | 1339 (62.3%) | 41 (53.9%) | 133 (57.6%) | 0.674 | 228 (57.3%) | 253 (58.8%) | 0.703 | 64 (60.4%) | 257 (70.4%) | 0.067 | 135 (64.6%) | 228 (67.9%) | 0.489 |
| Diabetes mellitus | 692 (32.2%) | 21 (27.6%) | 91 (39.4%) | 0.087 | 97 (24.4%) | 104 (24.2%) | 1.000 | 40 (37.7%) | 163 (44.7%) | 0.248 | 51 (24.4%) | 125 (37.2%) | 0.003 * |
| Laboratory indices | |||||||||||||
| Transferrin saturation (%) | 16.7 [10.9–24.2] | 26.5 [22.6–33.3] | 9.95 [6.63–14.3] | <0.001 * | 27.7 [23.5–34.8] | 13.3 [9.95–16.4] | <0.001 * | 26.5 [22.7–33.9] | 11.4 [8.29–15.3] | <0.001 * | 27.1 [23.2–31.0] | 13.3 [9.53–16.8] | <0.001 * |
| Ferritin (mg/L) | 102 [50.0–192] | 150 [88.8–242] | 65.0 [27.5–142] | <0.001 * | 144 [83.5–255] | 94.0 [50.2–175] | <0.001 * | 127 [72.2–316] | 69.0 [31.0–150] | <0.001 * | 130 [74.0–209] | 83.0 [46.0–171] | <0.001 * |
| Iron (mg/dl) | 44.7 [27.9–72.6] | 67.0 [54.5–90.8] | 22.3 [16.8–39.1] | <0.001 * | 83.8 [67.0–106] | 39.1 [27.9–50.3] | <0.001 * | 61.5 [50.3–83.8] | 27.9 [16.8–39.1] | <0.001 * | 78.2 [61.5–101] | 39.1 [27.9–50.3] | <0.001 * |
| sTfR (mg/L) | 1.53 [1.17–2.11] | 1.24 [0.92–1.78] | 1.81 [1.35–2.84] | <0.001 * | 1.30 [1.04–1.63] | 1.55 [1.21–2.05] | <0.001 * | 1.36 [1.00–1.92] | 1.84 [1.30–2.46] | <0.001 * | 1.37 [1.06–1.70] | 1.73 [1.35–2.29] | <0.001 * |
| Transferrin (mg/dl) | 200 [160–250] | 185 [138–240] | 190 [140–240] | 0.550 | 210 [180–240] | 220 [180–270] | 0.014 * | 160 [130–210] | 180 [130–240] | 0.051 | 200 [160–250] | 210 [170–260] | 0.090 |
| Mean corpuscular volume (fL) | 90.8 [86.5–95.2] | 92.0 [87.0–97.9] | 88.5 [82.1–93.4] | 0.005 * | 92.2 [88.6–96.4] | 90.1 [86.2–93.5] | <0.001 * | 93.9 [88.7–99.3] | 89.8 [84.7–94.7] | <0.001 * | 92.9 [89.3–96.3] | 89.9 [86.6–94.5] | <0.001 * |
| Haemoglobin (g/dl) | 13.2 (1.90) | 11.8 (1.09) | 11.4 (1.05) | 0.011 * | 14.7 (1.18) | 14.2 (1.18) | <0.001 * | 11.2 (1.25) | 11.1 (1.15) | 0.239 | 14.3 (1.34) | 14.0 (1.16) | 0.011 * |
| Haematocrit (%) | 40.0 (5.38) | 35.6 (3.33) | 35.2 (3.15) | 0.322 | 44.0 (3.62) | 42.7 (3.59) | <0.001 * | 34.4 (3.92) | 34.4 (3.56) | 0.977 | 42.9 (3.95) | 42.4 (3.47) | 0.124 |
| IL‐6 (pg/ml) | 5.40 [2.90–10.6] | 3.45 [1.87–8.10] | 7.00 [3.92–13.2] | <0.001 * | 2.95 [1.80–5.30] | 5.80 [3.10–11.6] | <0.001 * | 5.30 [3.30–11.1] | 7.85 [4.80–15.0 | <0.001 * | 3.90 [2.42–7.27] | 6.40 [3.90–13.9] | <0.001 * |
| CRP (mg/L) | 19.8 (19.1) | 15.5 (17.5) | 25.9 (20.9) | <0.001 * | 12.3 (14.5) | 21.6 (19.3) | <0.001 * | 17.8 (19.0) | 24.6 (20.1) | 0.002 * | 14.3 (16.0) | 22.3 (19.6) | <0.001 * |
| NT‐proBNP (ng/ml) | 2838 [1252–5990] | 1692 [716–4038] | 3037 [1470–5898] | 0.001 * | 1448 [641–2613] | 2408 [1099–4742] | <0.001 * | 3914 [1899–9754] | 5227 [2274–10 721] | 0.158 | 2470 [1163–4654] | 4134 [2260–8448] | <0.001 * |
| Hepcidin (nmol/L) | 6.50 [2.20–16.9] | 7.85 [4.35–20.0] | 4.00 [0.90–12.1] | <0.001 * | 9.00 [4.50–20.5] | 5.60 [2.10–15.6] | <0.001 * | 8.90 [3.90–25.2] | 4.50 [1.00–14.8] | <0.001 * | 8.80 [4.10–21.4] | 4.50 [1.40–11.9] | <0.001 * |
| Albumin (g/L) | 32.0 (8.80) | 31.4 (9.05) | 28.4 (8.76) | 0.014 * | 35.6 (7.47) | 33.3 (7.83) | <0.001 * | 29.2 (10.0) | 28.1 (9.21) | 0.320 | 34.2 (8.14) | 32.2 (8.22) | 0.006 * |
| Renal function | |||||||||||||
| eGFR (MDRD) (ml/min/1.73 m2) | 61.7 [46.7–78.0] | 77.5 [67.1–91.4] | 75.4 [66.3–87.4] | 0.258 | 78.1 [69.2–92.7] | 77.9 [68.4–91.2] | 0.622 | 41.0 [31.9–48.6] | 42.0 [30.6–50.6] | 0.420 | 48.8 [40.4–55.2] | 48.6 [38.6–54.6] | 0.527 |
| Creatinine (µmol/ml) | 103 [84.0–130] | 88.0 [75.8–97.4] | 87.0 [74.0–98.5] | 0.853 | 87.0 [74.0–97.2] | 83.0 [72.5–97.2] | 0.167 | 152 [130–184] | 142 [120–181] | 0.049 | 124 [114–147] | 125 [112–147] | 0.677 |
| Urea (mmol/L) | 11.4 [7.60–18.4] | 9.70 [7.10–15.4] | 10.1 [6.20–15.4] | 0.734 | 8.40 [6.10–13.0] | 8.10 [6.07–12.9] | 0.519 | 19.5 [12.8–28.8] | 16.5 [11.4–25.7] | 0.121 | 11.7 [8.55–19.0] | 13.6 [9.43–22.1] | 0.078 |
| Plasma NGAL (ng/ml) | 60.3 [37.7–97.2] | 54.2 [34.6–78.9] | 52.3 [34.2–80.2] | 0.777 | 44.8 [27.8–69.6] | 45.8 [31.8–64.3] | 0.977 | 96.1 [58.1–167] | 95.2 [60.7–154] | 0.772 | 71.9 [45.6–116] | 73.1 [44.6–109] | 0.461 |
| Cystatin C (ng/ml) | 15 012 [10 183–21 902] | 14 695 [9544–21 694] | 12 446 [8390–18 762] | 0.042 | 14 068 [9881–21 132] | 13 441 [8948–18 863] | 0.012 * | 19 606 [13 464–29 116] | 17 311 [12 446–25 280] | 0.123 | 16 690 [11 865–23 494] | 15 187 [10 507–22 247] | 0.049 |
| Aldosterone (pg/ml) | 93.5 [43.0–194] | 87.0 [44.0–198] | 61.0 [26.2–146] | 0.018 | 109 [55.2–201] | 81.0 [44.0–170] | 0.001 * | 71.0 [32.0–238] | 91.0 [37.0–190] | 0.582 | 125 [65.5–235] | 100 [50.0–223] | 0.032 |
| Renin (µIU/ml) | 88.7 [28.4–255] | 81.7 [26.4–270] | 83.2 [27.4–270] | 0.986 | 71.4 [27.3–209] | 59.2 [19.5–191] | 0.068 | 112 [33.1–460] | 116 [40.8–294] | 0.574 | 125 [39.8–293] | 101 [28.8–290] | 0.210 |
| PENK (pmol/L) | 85.9 [63.7–120] | 67.9 [55.3–91.7] | 76.6 [61.4–94.7] | 0.145 | 66.5 [54.4–82.4] | 66.3 [53.5–85.3] | 0.932 | 143 [105–200] | 136 [103–192] | 0.503 | 100 [79.6–127] | 102 [80.2–133] | 0.660 |
| FGF23 (RU/ml) | 224 [120–602] | 138 [89.1–220] | 283 [154–822] | <0.001 * | 118 [87.3–179] | 189 [108–432] | <0.001 * | 348 [168–964] | 523 [255–1456] | 0.012 * | 195 [117–374] | 387 [177–1162] | <0.001 * |
| KIM‐1(NPX) | 8.47 (1.05) | 8.27 (0.92) | 8.38 (0.92) | 0.391 | 8.11 (0.88) | 8.18 (0.94) | 0.287 | 8.98 (1.10) | 8.96 (1.07) | 0.904 | 8.58 (0.92) | 8.62 (1.24) | 0.698 |
| Medications | |||||||||||||
| β‐blocker | 1773 (82.4%) | 65 (85.5%) | 181 (78.4%) | 0.233 | 352 (88.4%) | 349 (81.2%) | 0.005 * | 93 (87.7%) | 287 (78.6%) | 0.051 | 179 (85.6%) | 267 (79.5%) | 0.088 |
| ACE/ARB baseline | 1544 (71.8%) | 58 (76.3%) | 168 (72.7%) | 0.641 | 324 (81.4%) | 318 (74.0%) | 0.013 * | 74 (69.8%) | 232 (63.6%) | 0.284 | 149 (71.3%) | 221 (65.8%) | 0.212 |
| Aldosterone antagonist | 1134 (52.7%) | 41 (53.9%) | 127 (55.0%) | 0.981 | 246 (61.8%) | 232 (54.0%) | 0.027 | 52 (49.1%) | 167 (45.8%) | 0.624 | 100 (47.8%) | 169 (50.3%) | 0.640 |
| Loop diuretic | 2141 (99.5%) | 76 (100%) | 229 (99.1%) | 1.000 | 397 (99.7%) | 428 (99.5%) | 1.000 | 106 (100%) | 363 (99.5%) | 1.000 | 208 (99.5%) | 334 (99.4%) | 1.000 |
| Digoxin | 406 (18.9%) | 9 (11.8%) | 38 (16.5%) | 0.433 | 84 (21.1%) | 96 (22.3%) | 0.733 | 12 (11.3%) | 63 (17.3%) | 0.187 | 34 (16.3%) | 70 (20.8%) | 0.228 |
| Acenocoumarol | 817 (38.4%) | 30 (40.0%) | 82 (35.8%) | 0.606 | 143 (36.7%) | 152 (35.7%) | 0.826 | 39 (37.5%) | 146 (40.1%) | 0.714 | 82 (39.6%) | 143 (42.9%) | 0.501 |
| Antiplatelets | 1094 (51.4%) | 38 (50.7%) | 119 (52.0%) | 0.950 | 198 (50.8%) | 229 (53.8%) | 0.434 | 54 (51.9%) | 187 (51.4%) | 1.000 | 95 (45.9%) | 174 (52.3%) | 0.178 |
| Insulin | 285 (41.2%) | 3 (14.3%) | 33 (36.3%) | 0.092 | 35 (36.1%) | 42 (40.4%) | 0.630 | 12 (30.0%) | 92 (56.4%) | 0.005 * | 24 (47.1%) | 44 (35.2%) | 0.195 |
| Oral anti‐diabetics | 432 (62.4%) | 16 (76.2%) | 70 (76.9%) | 1.000 | 65 (67.0%) | 68 (65.4%) | 0.925 | 28 (70.0%) | 83 (50.9%) | 0.046 | 28 (54.9%) | 74 (59.2%) | 0.722 |
6MWT, 6‐min walk test; ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRAIDS, cardio‐renal anaemia iron deficiency syndrome; CRAS, cardio‐renal anaemia syndrome; CRIDS, cardio‐renal iron deficiency syndrome; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; FGF23, fibroblast growth factor‐23; HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ID, iron deficiency; IDA, iron deficiency anaemia; IL‐6, interleukin 6; KCCQ, Kansas City Cardiomyopathy Questionnaire; KIM‐1, kidney injury molecule‐1; LVEF, left ventricular ejection fraction; MDRD, Modification of Diet in Renal Disease; NGAL, neutrophil gelatinase‐associated lipocalin; NPX, Normalized Protein eXpression (Olink's arbitrary unit which is in log2 scale); NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PENK, proenkephalin; sTfR, soluble transferrin receptor.
False discovery rate ≤0.05
Prevalence of iron deficiency in relation to chronic kidney disease and anaemia
The prevalence of each condition in our study population is shown in Figure 1A . Generally, ID was more prevalent than CKD and anaemia (63.3%, 47.2% and 35.6%, respectively). Using the ‘conventional’ definition of CRAS (i.e. not considering ID when defining CRAS and, thus, with no distinction made between CRAS and CRAIDS), ID was more prevalent in patients with CRAS versus those without (77.5% vs. 59.3%, p < 0.001). The patients with CRAS and additionally ID were considered as CRAIDS from here onwards, which constituted 17% of the total population, while isolated ID was present in 20% of patients. The global prevalence of having at least one comorbidity (ID, CKD, and/or anaemia) was 81.4%. The co‐occurrence of ID, anaemia and/or CKD increases with New York Heart Association (NYHA) functional class as depicted in Figure 1B . Only syndromes containing ID show a positive correlation with NYHA class, whereas the rest of comorbidities showed a negative trend with increasing NYHA class, highlighting the additive burden of ID.
Figure 1.
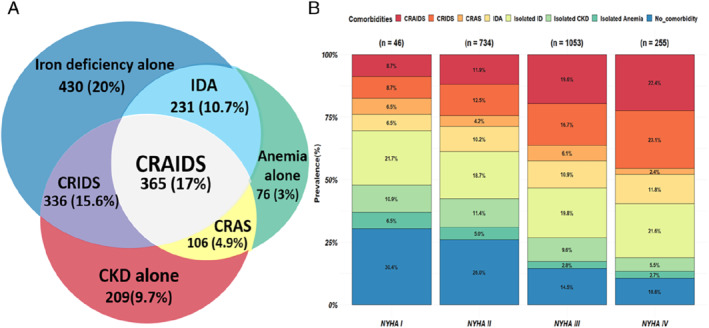
(A) Venn diagram showing the prevalence of individual or combined syndromes of the BIOSTAT‐CHF index cohort. (B) Prevalence of the studied syndromes stratified by New York Heart Association (NYHA) functional classification. CKD, chronic kidney disease; CRAIDS, cardio‐renal anaemia iron deficiency syndrome; CRAS, cardio‐renal anaemia syndrome; CRIDS, cardio‐renal iron deficiency syndrome; ID, iron deficiency; IDA, iron deficiency anaemia.
Association between iron deficiency and exercise capacity and quality of life
Having ID was associated with an average of 37 m less walked [95% confidence interval (CI) 51.2–23.4] during the 6MWT. Especially ID on top of anaemia or CKD was significantly associated with worse performance [IDA: −41 (−78.2 to −4.8), p‐value <0.05; CRIDS: −34.1 (−62 to −6.3), p‐value <0.05; Figure 2A ]. Also, patients with ID compared to patients without another comorbidity, or with anaemia alone, were less likely to complete the 6MWT (p < 0.001, online supplementary Figure S1 ). Moreover, having ID either alone or on top of CKD and/or anaemia was consistently associated with a reduced quality of life as evaluated using the KCCQ overall summary score (p‐value <0.05, Figure 2B ). On average, patients with ID score 10 points (95% CI 8.14–12.1) less compared to those without ID.
Figure 2.
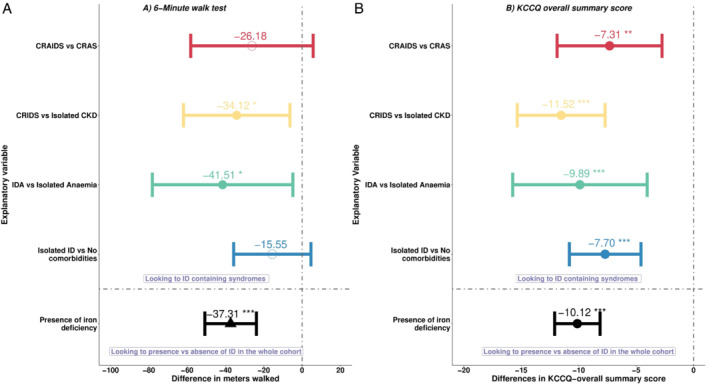
Forest plots displaying the associated burden of having iron deficiency (ID) in a head‐to‐head comparison to syndromes without ID on (A) 6‐min walk test, and (B) Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score. CKD, chronic kidney disease; CRAIDS, cardio‐renal anaemia iron deficiency syndrome; CRAS, cardio‐renal anaemia syndrome; CRIDS, cardio‐renal iron deficiency syndrome; IDA, iron deficiency anaemia. *p < 0.05; **p < 0.01; ***p < 0.001.
Results of differential protein expression
Generally, the number of differentially expressed biomarkers increased with co‐occurrence of ID, anaemia and/or CKD, with CRAIDS and CRAS having the highest number of differentially expressed biomarkers compared to patients without these comorbidities (171 and 154, respectively, online supplementary Table S4 ). There was a considerable overlap of differentially expressed biomarkers across the studied syndromes as shown in Figure 3A , with the highest number of overlapping biomarkers (61) being between CKD containing syndromes (isolated CKD, CRIDS, CRAIDS, CRAS). Key biomarkers of these include spondin 1, kidney injury molecule‐1, C‐C motif chemokine ligand 16, matrix extracellular phosphoglycoprotein, Interleukin‐18‐binding protein, insulin‐like growth factor‐binding protein 7, indicating that these markers may be associated with kidney disease in HF. Likewise, biomarkers shared between iron containing syndromes (ID alone, IDA, CRIDS and CRAIDS) include transferrin receptor, interleukin‐6, FGF23, mucin‐16 (cancer antigen‐125), carbonic anhydrase 9 and bone morphogenetic protein 6, suggesting that these biomarkers are likely to be related to pathways perturbed in iron‐deficient patients. On the other hand, hematopoietic cell‐specific lyn substrate 1, cyclin‐dependent kinase inhibitor 1A, methionine aminopeptidase 2, tyrosine‐protein kinase Lyn, were only differentially expressed in isolated anaemia.
Figure 3.
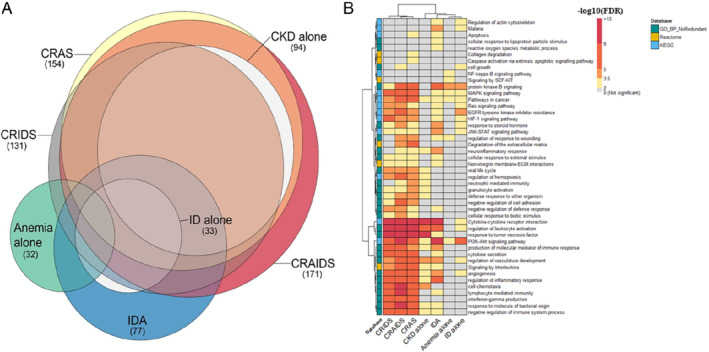
(A) Euler diagram showing overlap of the found biomarkers across the different syndromes. The size of each circle is proportional to the number of differentially expressed biomarkers found (numbers between brackets). (B) Heatmap showing enriched pathways and biological processes. The rows correspond to the pathways resulted from the affinity propagation algorithm for redundancy reduction, and the columns correspond to the studied syndromes. The colour gradient of each cell refers to the −log 10 (FDR) value, indicating the over‐representation significance of each pathway (see colour key). The full list of over‐represented pathways per syndrome can be found in online supplementary Appendix S2 . CKD, chronic kidney disease; CRAIDS, cardio‐renal anaemia iron deficiency syndrome; CRAS, cardio‐renal anaemia syndrome; CRIDS, cardio‐renal iron deficiency syndrome; ECM, extracellular matrix; EGFR, epidermal growth factor receptor; FDR, false discovery rate; HIF‐1, hypoxia‐inducible factor 1; ID, iron deficiency; IDA, iron deficiency anaemia; JAK‐STAT, Janus kinase (JAK)‐signal transducer and activator of transcription; KEGG, Kyoto Encyclopedia of Genes and Genomes; MAPK, mitogen‐activated protein kinase; NF, nuclear factor; PI3K, phosphatidylinositol‐3‐kinase; SCF, stem cell factor.
Despite the huge overlapping biomarker profiles, there was no single shared biomarker differentially expressed across all syndromes. Furthermore, there was no significant biomarker with divergent expression (i.e. upregulated in one syndrome but downregulated in another) (online supplementary Figure S2 ). Additionally, hierarchal clustering based on the patterns of differentially expressed biomarkers showed a biological clustering similar to their clinical definitions as evidenced by the dendrogram. The pattern of the found biomarkers of IDA is similar to ID and anaemia alone, while CRAIDS, CRAS and CRIDS cluster together, with the highest homogeneity between CRAS and CRAIDS. Results of differential biomarker expression analysis are presented in online supplementary Appendix S2 .
Results of pathway overrepresentation analysis
Subsequent pathway analysis per syndrome resulted in total into 46 pathways, with processes related to cancer, cell proliferation and survival [mitogen‐activated protein kinase (MAPK) and phosphatidylinositol‐3‐kinase (PI3K)‐Akt signalling] were amongst the most overlapping pathways across the studied syndromes (Figure 3B ). Immune and inflammatory related processes (including cytokine–cytokine receptor interaction and regulation of leucocyte activation) as well as angiogenesis associated pathways were enriched in all syndromes except for isolated anaemia. Pathway‐based hierarchical clustering formed two major clusters showing similar grouping as those of the found biomarkers, with CRAIDS, CRAS and CRIDS being most similar to each other and dissimilar to the other syndromes.
Noteworthily, the hypoxia‐inducible factor 1 (HIF‐1) signalling pathway, which is known as oxygen‐sensing pathway, was enriched even in isolated ID, suggesting that ID in HF is associated with hypoxia‐related pathways. The comprehensive list of enriched pathways per syndrome are provided in online supplementary Appendix S2 .
Differential protein expression and subsequent pathway enrichment analyses defining ID as per the FAIR‐HF study (serum ferritin <100 µg/L, or ferritin, 100 to 300 µg/L, with TSAT of <20%) was performed as sensitivity analysis, which resulted in essentially similar findings (see online supplementary Appendix S1 , Figure S4 ). Furthermore, the overall difference in biomarker and pathway analyses between iron‐deficient HF patients compared to those without ID can be seen in online supplementary Figure S5 , which shows similar observations with ID containing syndromes as in the present analysis.
Association of the studied comorbidities with outcomes
During a median follow‐up of 21.2 (IQR 16–27) months, 899 (41.7%) patients died or were hospitalized for HF. Isolated ID remained an independent predictor of all‐cause mortality [1.53 (1.03–2.28), p = 0.03] as well as combined outcomes [1.30 (1.05–1.70), p = 0.045], while isolated anaemia and CKD were only associated with all‐cause mortality after adjustments for the BIOSTAT‐CHF risk models (except for haemoglobin) (Table 2 ). Compared to HF patients with no comorbidities, higher hazards ratios (HR) were found for ID containing syndromes, but not significantly different when compared to the syndrome without ID for both all‐cause mortality [IDA: 2.58 (95% CI 1.73–3.85), p < 0.001 vs. isolated anaemia: 1.90 (95% CI 1.06–3.41), p = 0.0.02, p for difference = 0.275; and CRIDS: 1.74 (95% CI 1.18–2.58), p = 0.004 vs. isolated CKD: 1.66 (95% CI 1.08–2.55), p = 0.020, p for difference = 0.774] as well as combined endpoints [IDA: 1.71 (1.29–2.26), p < 0.001 vs. isolated anaemia: 1.4 (95% CI 0.91–2.15), p = 0.50, p for difference = 0.366; and CRIDS: 1.67 (95% CI 1.29–2.17), p < 0.001 vs. isolated CKD: 1.18 (95% CI 0.87–1.61), p = 0.270, p for difference = 0.014] (Table 2 and online supplementary Figure S3 ).
Table 2.
Results of the Cox proportional hazards models for the association between the studied syndromes with all‐cause mortality and the combined endpoint
| All‐cause mortality | Combined endpoint | |||||||
|---|---|---|---|---|---|---|---|---|
| Conditions (no. of patients) | Model A | Model B | Model A | Model B | ||||
| HR (95% CI) | p‐value | HR (95% CI) | p‐value | HR (95% CI) | p‐value | HR (95% CI) | p‐value | |
| No comorbidities (398) | 1 [Reference] | |||||||
| Isolated ID (430) | 1.66 (1.12–2.46) | 0.011 | 1.53 (1.03–2.28) | 0.03 | 1.43 (1.10–1.86) | 0.006 | 1.30 (1.05–1.70) | 0.045 |
| Isolated anaemia (76) | 2.3 (1.28–4.12) | 0.005 | 1.90 (1.06–3.41) | 0.02 | 1.63 (1.06–2.51) | 0.023 | 1.4 (0.91–2.15) | 0.11 |
| Isolated CKD (209) | 2.53 (1.66–3.86) | <0.001 | 1.66 (1.08–2.55) | 0.02 | 1.60 (1.18–2.16) | <0.002 | 1.18 (0.87–1.61) | 0.27 |
| IDA (231) | 3.33 (2.24–4.94) | <0.001 | 2.58 (1.73–3.85) | <0.001 | 2.36 (1.79–3.12) | <0.001 | 1.71 (1.29–2.26) | <0.001 |
| CRIDS (336) | 3.08 (2.12–4.48) | <0.001 | 1.74 (1.18–2.58) | 0.004 | 2.6 (2.06–3.4) | <0.001 | 1.67 (1.29–2.17) | <0.001 |
| CRAS (106) | 5.25 (3.39–8.12) | <0.001 | 2.62 (1.66–4.14) | <0.001 | 3.3 (2.39–4.56) | <0.001 | 1.87 (1.34–2.61) | <0.001 |
| CRAIDS (365) | 4.93 (3.46–7.03) | <0.001 | 2.42 (1.66–3.53) | <0.001 | 3.62 (2.84–4.6) | <0.001 | 1.94 (1.50–2.51) | <0.001 |
Model A = univariable analysis. Model B = adjusted for BIOSTAT‐CHF models, but without haemoglobin. 23
CI, confidence interval; CKD, chronic kidney disease; CRAIDS, cardio‐renal anaemia iron deficiency syndrome; CRAS, cardio‐renal anaemia syndrome; CRIDS, cardio‐renal iron deficiency syndrome; HR, hazard ratio; ID, iron deficiency; IDA, iron deficiency anaemia.
Discussion
The present study confirms that isolated ID, or on top of anaemia and/or CKD is associated with worse quality of life, exercise capacity, hospitalization and mortality. Differentially expressed biomarkers in the syndromes formed by the combination of ID, CKD and/or anaemia show considerable overlap, suggesting a pathophysiological continuum where common pathways associated with these comorbidities are involved (Graphical Abstract). Key enriched pathways in the studied syndromes were related to inflammatory and immune responses, cell survival and cancer. Our results provide supportive evidence that ID, anaemia and CKD in HF may have shared underlying pathways, and that ID is associated with clinical and functional impairments in HF even in the absence of CKD and anaemia.
Prevalence of individual or combined syndromes
The coexistence of ID, CKD and/or anaemia increases with the severity of HF, suggesting that these comorbidities tend to coexist in severe HF and as such, their coexistence may play an important role in exacerbating HF. Multiple studies have reported a global ID prevalence reaching up to 60% in HF (63% in the current study), we report that the prevalence of ID without other comorbidities (20%) is substantial, especially in patients with lower NYHA classes, potentially indicating that ID is a comorbidity which manifests itself early in disease progression caused by mechanisms not yet resulting in CKD and/or anaemia. We already have shown that decreased protein intake, fluid retention, inflammation and antiplatelet use were associated with ID in HF. 26 Additionally, the exceptionally high prevalence of ID without anaemia even in HF patients with minimal or few symptoms (NYHA class I, II), may point towards the progressive nature of theses syndromes since hematopoiesis remains unaffected until late stages of persisting ID. 32 Hence, early recognition and treatment of ID may aid in preventing HF progression.
Despite other studies reporting a prevalence of CRAS of around 20%–27% of HF patients, 5 , 17 prevalence of CRAS in the current study was found to be 4.9%. This large difference is likely attributable to the fact that ID was not considered in these studies and thus, no distinction was made between CRIDS, CRAIDS and CRAS. Accordingly, the term CRAS disregards the interaction between ID and anaemia/CKD as well as its impact beyond the complex interplay of CRAS.
The additive burden of iron deficiency on exercise capacity and quality of life
Whereas it is known that both anaemia and ID are associated with reduced quality of life and exercise capacity, 33 , 34 we herein specifically show that ID is additively and consistently associated with reduced quality of life and exercise capacity. Despite that reduced cardiac reserve is the main underlying cause of exercise intolerance and reduced quality of life in patients with HF, 35 other factors such as skeletal muscle perfusion and function contribute to the severity of these cardinal signs. Given the crucial role of iron in cellular energetics beside its role in oxygen transport and storage, cells that are metabolically active such as skeletal and heart muscles are particularly sensitive to ID. 9 , 36 Intriguingly, the recent FERRIC‐HF II trial demonstrated that administering iron isomaltoside resulted in better skeletal muscle energetics with no significant changes in haemoglobin, linking ID mechanistically with exercise intolerance in HF independently of anaemia. 37
Regarding the additive burden of ID on quality of life, we report that compared to those without, patients with ID either alone or on top of anaemia and/or CKD scored on average more than 5 points less on the KCCQ overall summary score. This suggests that ID, on top of anaemia and/or CKD, has a substantial and clinically relevant impact on the perceived health status of HF patients. 38
Pathways and biomarkers associated with the studied syndromes
In our study, a considerable overlap in the found biomarkers across the seven syndromes was observed, with immune and inflammatory related pathways being amongst the most enriched and common pathways between the studied syndromes. This may indicate that these syndromes fit in a spectrum where inflammation is a key denominator, which is extensively supported by existing evidence. 39 , 40 , 41 Many of the top enriched pathways were also related to MAPK and PI3K/AKT signalling pathways. These pathways are known to regulate a plethora of biological functions, including cell proliferation, differentiation, survival and cancer. 42 Notably, multiple cancer‐related terms were also enriched such as ‘pathways in cancer’, ‘melanoma’, and ‘glioma’. A study found that incidence of cancer is significantly higher in patients with CRAS. 43 Yet, the contribution of ID associated with the incidence of cancer in HF has not been recognized despite the known significant role of iron in cancer. 44 Whether the studied syndromes are associated with increased risk of cancer development through perturbations in these pathways remains to be elucidated.
An interesting observation was the over‐representation of hypoxia‐associated pathways (HIF‐1 signaling pathway and angiogenesis) even in those with isolated ID (Figure 3B ). This pathway may represent the convergence/intersection point between iron metabolism, hypoxia, immunity, inflammation and cancer. Recently, results of multiple phase 3 trials have shown that targeting this pathway using oral HIF‐prolyl hydroxylase inhibitors such as roxadustat or vadadustat, improved iron bioavailability and increased haemoglobin levels with no major side effects compared to darbepoetin alfa in patients with CKD. 45 , 46 These findings have potentially fascinating prospects in treating HF patients with ID as these agents have advantages above the intravenous administration of iron such as being orally administered, reducing hepcidin levels, inducing physiological levels of endogenous erythropoietin production and having potentially positive effects on lipid metabolism. 47 Whether HIF stabilizers could be a safe therapeutic option for targeting CKD anaemia and/or ID in HF is currently unknown.
Lastly, the patterns of clustering syndromes based on the associated pathways (Figure 3B ) and biomarkers (online supplementary Figure S2) are quite similar to their clinical definitions, exemplifying the commonalities and differences between these syndromes. The greatest resemblance in the associated pathways and biomarkers was observed between CRAIDS, CRAS and CRIDS, potentially indicating that these three syndromes, although having different clinical definitions, may actually be different manifestations of the same entity in the same spectrum with varying degrees of impact on outcomes. FGF23 and 21, interleukin‐6, mucin 16, growth differentiation factor 15, natriuretic peptide A and B, are amongst the topmost upregulated proteins in these syndromes, which are known biomarkers with clinical significance in patients with HF. 39 , 48 , 49 Further whole proteome and/or transcriptome analysis is warranted to gain mechanistic insights on the underlying biological similarities and differences between these syndromes.
Prognostic impact
Numerous studies have shown that ID is associated with worse mortality independently of haemoglobin and eGFR. 8 , 50 Using a large cohort of HF patients, the current evaluation extends on these observations by showing that ID itself without comorbid anaemia or CKD was significantly associated with increased incidence of not only mortality but also HF rehospitalization. Additionally, comorbidity specific analysis revealed that both IDA and CRIDS were consistently associated with lower survival rates and increased hospitalization compared to having only anaemia or CKD alone, underscoring the additive contribution of ID on outcomes as well as indicating that simultaneous existence of these comorbidities may worsen each other. Sub‐analyses of the FAIR‐HF trial have highlighted the benefits of correcting ID on clinical and functional metrics even in non‐anaemic patients 51 as well as those with preserved kidney function, 52 suggesting ID as a therapeutic target in all symptomatic HF patients irrespective of anaemia or CKD. Whether its correction is also associated with improved outcomes with or without the presence of CKD and/or anaemia remains to be established by the ongoing trials. 50
Limitations
There are several limitations to be acknowledged. Firstly, the analysis included measurements obtained from blood samples only at study inclusion, making it difficult to look into longitudinal changes. Additionally, although our sample size was large, dividing our patients into comorbidity groups has diminished this advantage. The resulting imbalance in syndromes could have influenced the power of our analysis and therefore, our results should be validated with larger sample size. Secondly, although we used quite a large number of biomarkers, it remains a small part of the whole proteome. The measured panels are pre‐selected and most of them are related to inflammation and cardiovascular disease, making our analysis potentially biased towards finding overlap between the studied syndromes in these domains. Therefore, comprehensive analysis of the whole proteome is warranted to extend and validate our findings. Lastly, our cohort is mainly comprised of patients with HF with a reduced ejection fraction. As such, extrapolation of these observations to HF with preserved ejection fraction may not be applicable.
Conclusion
Our results show that ID, CKD and/or anaemia in patients with HF have great overlap in biomarker profiles, suggesting common pathways associated with these syndromes. Also, we provide supportive evidence that ID either alone or on top of CKD and anaemia is associated with adverse effects on quality of life, exercise capacity and prognosis of patients with worsening HF.
Funding
The BIOSTAT‐CHF study was supported by the European Commission (FP7‐242209‐BIOSTAT‐CHF; EudraCT 2010–020808‐29).
Conflict of interest: N.G.B.: received speaker fees from Vifor Pharma. J.G.F.C. received research support from Pharmacosmos and Vifor Pharma. S.D.A. reports receiving fees from Abbott, Bayer, Boehringer Ingelheim, Cardiac Dimension, Cordio, Impulse Dynamics, Novartis, Occlutech, Servier, and Vifor Pharma, and grant support from Abbott and Vifor Pharma. P.v.d.M. received consultancy and/or research grants from Vifor Pharma, AstraZeneca, Servier, Pharmacosmos, Novartis, Pfizer, Ionis. All other authors have nothing to disclose.
Supporting information
Appendix S1. Supporting Information.
Appendix S2. Supporting Information.
References
- 1. Chamberlain AM, Sauver LS, Gerber Y, Manemann SM. Multimorbidity in heart failure: a community perspective. Am J Med. 2015;128:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, et al. Anemia and mortality in heart failure patients. A systematic review and meta‐analysis. J Am Coll Cardiol. 2008;52:818–27. [DOI] [PubMed] [Google Scholar]
- 3. Silverberg DS, Wexler D, Palazzuoli A, Iaina A, Schwartz D. The anemia of heart failure. Acta Haematol. 2009;122:109–19. [DOI] [PubMed] [Google Scholar]
- 4. Herzog CA, Muster HA, Li S, Collins AJ. Impact of congestive heart failure, chronic kidney disease, and anemia on survival in the Medicare population. J Card Fail. 2004;10:467–72. [DOI] [PubMed] [Google Scholar]
- 5. Kim CJ, Choi IJ, Park HJ, Kim TH, Kim PJ, Chang K, et al. Impact of cardiorenal anemia syndrome on short‐ and long‐term clinical outcomes in patients hospitalized with heart failure. Cardiorenal Med. 2016;6:269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new‐onset heart failure. Circulation. 2003;107:223–5. [DOI] [PubMed] [Google Scholar]
- 7. Hoes MF, Grote Beverborg N, Kijlstra JD, Kuipers J, Swinkels DW, Giepmans BNG, et al. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail. 2018;20:910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klip IT, Jankowska EA, Enjuanes C, Voors AA, Banasiak W, Bruguera J, et al. The additive burden of iron deficiency in the cardiorenal‐anaemia axis: scope of a problem and its consequences. Eur J Heart Fail. 2014;16:655–62. [DOI] [PubMed] [Google Scholar]
- 9. Okonko DO, Mandal AKJ, Missouris CG, Poole‐Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol. 2011;58:1241–51. [DOI] [PubMed] [Google Scholar]
- 10. Ponikowski P, Kirwan BA, Anker SD, Dorobantu M, Drozdz J, Fabien V, et al. Rationale and design of the AFFIRM‐AHF trial: a randomised, double‐blind, placebo‐controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalisations and mortality in iron‐deficient patients admitted for acute heart failure. Eur J Heart Fail. 2019;21:1651–8. [DOI] [PubMed] [Google Scholar]
- 11. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al.; FAIR‐HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–48. [DOI] [PubMed] [Google Scholar]
- 12. Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, et al.; AFFIRM‐AHF Investigators. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double‐blind, randomised, controlled trial. Lancet. 2020;396:1895–904. [DOI] [PubMed] [Google Scholar]
- 13. Ponikowski P, Van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, et al.; CONFIRM‐HF Investigators. Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36:657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Veldhuisen DJ, Ponikowski P, Van Der Meer P, Metra M, Böhm M, Doletsky A, et al.; EFFECT‐HF Investigators. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. 2017;136:1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726. [DOI] [PubMed] [Google Scholar]
- 16. Cleland JGF, Zhang J, Pellicori P, Dicken B, Dierckx R, Shoaib A, et al. Prevalence and outcomes of anemia and hematinic deficiencies in patients with chronic heart failure. JAMA Cardiol. 2016;1:539–47. [DOI] [PubMed] [Google Scholar]
- 17. Al‐Jarallah M, Rajan R, Al‐Zakwani I, Dashti R, Bulbanat B, Sulaiman K, et al. Incidence and impact of cardiorenal anaemia syndrome on all‐cause mortality in acute heart failure patients stratified by left ventricular ejection fraction in the Middle East. ESC Heart Fail. 2019;6:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pallangyo P, Fredrick F, Bhalia S, Nicholaus P, Kisenge P, Mtinangi B, et al. Cardiorenal anemia syndrome and survival among heart failure patients in Tanzania: a prospective cohort study. BMC Cardiovasc Disord. 2017;17:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Macdougall IC, Canaud B, De Francisco ALM, Filippatos G, Ponikowski P, Silverberg D, et al. Beyond the cardiorenal anaemia syndrome: recognizing the role of iron deficiency. Eur J Heart Fail. 2012;14:882–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silverberg DS, Wexler D, Iaina A, Schwartz D. Correction of iron deficiency in the cardiorenal syndrome. Int J Nephrol. 2011;2011:365301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, et al. A systems BIOlogy Study to TAilored Treatment in Chronic Heart Failure: rationale, design, and baseline characteristics of BIOSTAT‐CHF. Eur J Heart Fail. 2016;18:716–26. [DOI] [PubMed] [Google Scholar]
- 22. Voors AA, Ouwerkerk W, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P, et al. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur J Heart Fail. 2017;19:627–34. [DOI] [PubMed] [Google Scholar]
- 23. Beverborg NG, Klip IjT, Meijers WC, Voors AA, Vegter EL, Van Der Wal HH, et al. Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circ Heart Fail. 2018;11:e004519. [DOI] [PubMed] [Google Scholar]
- 24. Von Haehling S, Anker MS, Jankowska EA, Ponikowski P, Anker SD. Anemia in chronic heart failure: can we treat? What to treat? Heart Fail Rev. 2012;17:203–10. [DOI] [PubMed] [Google Scholar]
- 25. National Kidney Foundation . K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 26. van der Wal HH, Grote Beverborg N, Dickstein K, Anker SD, Lang CC, Ng LL, et al. Iron deficiency in worsening heart failure is associated with reduced estimated protein intake, fluid retention, inflammation, and antiplatelet use. Eur Heart J. 2019;40:3616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Precision Proteomics Solutions for biomarker discovery | Olink [Internet]. [cited 2020 Dec 29]. Available from: https://www.olink.com/products/
- 28. Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3. [DOI] [PubMed] [Google Scholar]
- 29. Kammers K, Cole RN, Tiengwe C, Ruczinski I. Detecting significant changes in protein abundance. EuPA Open Proteom. 2015;7:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khatri P, Sirota M, Butte AJ. Ten years of pathway analysis: current approaches and outstanding challenges. PLoS Comput Biol. 2012;8:e1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liao Y, Wang J, Jaehnig EJ, Shi Z, Zhang B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019;47:W199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hanif N, Anwer F. Chronic iron deficiency. StatPearls. StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 33. Ebner N, Jankowska EA, Ponikowski P, Lainscak M, Elsner S, Sliziuk V, et al. The impact of iron deficiency and anaemia on exercise capacity and outcomes in patients with chronic heart failure. Results from the Studies Investigating Co‐morbidities Aggravating Heart Failure. Int J Cardiol. 2016;205:6–12. [DOI] [PubMed] [Google Scholar]
- 34. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail. 2011;17:899–906. [DOI] [PubMed] [Google Scholar]
- 35. Del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DL, et al. Exercise intolerance in patients with heart failure: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019;73:2209–25. [DOI] [PubMed] [Google Scholar]
- 36. Stugiewicz M, Tkaczyszyn M, Kasztura M, Banasiak W, Ponikowski P, Jankowska EA. The influence of iron deficiency on the functioning of skeletal muscles: experimental evidence and clinical implications. Eur J Heart Fail. 2016;18:762–73. [DOI] [PubMed] [Google Scholar]
- 37. Charles‐Edwards G, Amaral N, Sleigh A, Ayis S, Catibog N, McDonagh T, et al. Effect of iron isomaltoside on skeletal muscle energetics in patients with chronic heart failure and iron deficiency: FERRIC‐HF II randomized mechanistic trial. Circulation. 2019;139:2386–98. [DOI] [PubMed] [Google Scholar]
- 38. Butler J, Shahzeb Khan M, Mori C, Filippatos GS, Ponikowski P, Comin‐Colet J, et al. Minimal clinically important difference in quality of life scores for patients with heart failure and reduced ejection fraction. Eur J Heart Fail. 2020;22:999–1005. [DOI] [PubMed] [Google Scholar]
- 39. Markousis‐Mavrogenis G, Tromp J, Ouwerkerk W, Devalaraja M, Anker SD, Cleland JG, et al. The clinical significance of interleukin‐6 in heart failure: results from the BIOSTAT‐CHF study. Eur J Heart Fail. 2019;21:965–73. [DOI] [PubMed] [Google Scholar]
- 40. Colombo PC, Ganda A, Lin J, Onat D, Harxhi A, Iyasere JE, et al. Inflammatory activation: cardiac, renal, and cardio‐renal interactions in patients with the cardiorenal syndrome. Heart Fail Rev. 2012;17:177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kleijn L, Belonje AMS, Voors AA, De Boer RA, Jaarsma T, Ghosh S, et al. Inflammation and anaemia in a broad spectrum of patients with heart failure. Heart. 2012;98:1237–41. [DOI] [PubMed] [Google Scholar]
- 42. Cao Z, Liao Q, Su M, Huang K, Jin J, Cao D. AKT and ERK dual inhibitors: the way forward? Cancer Lett. 2019;459:30–40. [DOI] [PubMed] [Google Scholar]
- 43. Schroten NF, Van Der Putten K, Rutten FH, Diepenbroek A, Mosterd A, Gaillard CAJM. High cumulative incidence of cancer in patients with cardio‐renal‐anaemia syndrome. Eur J Heart Fail. 2010;12:855–60. [DOI] [PubMed] [Google Scholar]
- 44. Jung M, Mertens C, Tomat E, Brüne B. Iron as a central player and promising target in cancer progression. Int J Mol Sci. 2019;20:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chertow GM, Pergola PE, Farag YMK, Agarwal R, Arnold S, Bako G, et al.; PRO2TECT Study Group . Vadadustat in patients with anemia and non‐dialysis‐dependent CKD. N Engl J Med. 2021;384:1589–600. [DOI] [PubMed] [Google Scholar]
- 46. Zheng Q, Yang H, Fu X, Huang Y, Wei R, Wang Y, et al. The efficacy and safety of roxadustat for anemia in patients with chronic kidney disease: a meta‐analysis. Nephrol Dial Transplant. 2020;36:1603–15. [DOI] [PubMed] [Google Scholar]
- 47. Sanghani NS, Haase VH. Hypoxia‐inducible factor activators in renal anemia: current clinical experience. Adv Chronic Kidney Dis. 2019;26:253–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Piek A, Du W, de Boer RA, Silljé HHW. Novel heart failure biomarkers: why do we fail to exploit their potential? Crit Rev Clin Lab Sci. 2018;55:246–63. [DOI] [PubMed] [Google Scholar]
- 49. Topf A, Mirna M, Ohnewein B, Jirak P, Kopp K, Fejzic D, et al. The diagnostic and therapeutic value of multimarker analysis in heart failure. An approach to biomarker‐targeted therapy. Front Cardiovasc Med. 2020;7:579567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron deficiency in heart failure: an overview. JACC Heart Fail. 2019;7:36–46. [DOI] [PubMed] [Google Scholar]
- 51. Filippatos G, Farmakis D, Colet JC, Dickstein K, Lüscher TF, Willenheimer R, et al. Intravenous ferric carboxymaltose in iron‐deficient chronic heart failure patients with and without anaemia: a subanalysis of the FAIR‐HF trial. Eur J Heart Fail. 2013;15:1267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ponikowski P, Filippatos G, Colet JC, Willenheimer R, Dickstein K, Lüscher T, et al.; FAIR‐HF Investigators. The impact of intravenous ferric carboxymaltose on renal function: an analysis of the FAIR‐HF study. Eur J Heart Fail. 2015;17:329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Appendix S2. Supporting Information.


