Abstract
Background and Aim
Portal vein thrombosis (PVT) is a common complication of cirrhosis. The exact pathophysiology remains largely unknown, and treatment with anticoagulants does not lead to recanalization of the portal vein in all patients. A better insight into the structure and composition of portal vein thrombi may assist in developing strategies for the prevention and treatment of PVT.
Approach and Results
Sixteen prospectively and 63 retrospectively collected nonmalignant portal vein thrombi from patients with cirrhosis who underwent liver transplantation were included. Histology, immunohistochemistry, and scanning electron microscopy were used to assess structure and composition of the thrombi. Most recent CT scans were reanalyzed for thrombus characteristics. Clinical characteristics were related to histological and radiological findings. All samples showed a thickened, fibrotic tunica intima. Fibrin‐rich thrombi were present on top of the fibrotic intima in 9/16 prospective cases and in 21/63 retrospective cases. A minority of the fibrotic areas stained focally positive for fibrin/fibrinogen (16% of cases), von Willebrand factor (VWF; 10%), and CD61 (platelets, 21%), while most of the fibrin‐rich areas stained positive for those markers (fibrin/fibrinogen, 100%; VWF, 77%; CD61, 100%). No associations were found between clinical characteristics including estimated thrombus age and use of anticoagulants and presence of fibrin‐rich thrombi.
Conclusion
We demonstrate that PVT in patients with cirrhosis consists of intimal fibrosis with an additional fibrin‐rich thrombus in only one‐third of cases. We hypothesize that our observations may explain why not all portal vein thrombi in patients with cirrhosis recanalize by anticoagulant therapy.
Abbreviations
- DVT
deep vein thrombosis
- EVG
elastic van Gieson
- H&E
hematoxylin and eosin
- IQR
interquartile range
- KCH
King’s College Hospital
- LMWH
low–molecular weight heparin
- MELD
Model for End‐Stage Liver Disease
- MSB
Martius scarlet blue
- PVT
portal vein thrombosis
- SEM
scanning electron microscopy
- UMCG
University Medical Center Groningen
- VKA
vitamin K antagonist
- VWF
von Willebrand factor
INTRODUCTION
Portal vein thrombosis (PVT) is a rare condition in the general population but is common in patients with cirrhosis. The prevalence of PVT in patients with cirrhosis increases with disease severity and varies from 5% to 26% in patients with advanced disease.[ 1 ] The exact pathophysiology of PVT is still unknown, but decreased portal flow velocity and severity of liver disease are important risk factors for PVT in patients with cirrhosis.[ 2 , 3 , 4 ] Although it has been suggested that hypercoagulability may also be a risk factor for PVT development,[ 5 , 6 ] multiple recent studies did not find congenital thrombophilia to be related to PVT risk.[ 2 , 7 , 8 ] Notably, it has been debated whether PVT contributes to liver disease progression or whether it is merely a marker of severity of disease.[ 4 , 7 , 9 ] For example, a systematic review showed an increased risk of mortality and hepatic decompensation in patients with cirrhosis and PVT,[ 10 ] while other studies showed no independent association between the presence of PVT and progression of cirrhosis.[ 4 , 7 ] It is, however, generally accepted that PVT can cause technical challenges during liver transplantation that may result in decreased graft survival rates and increased morbidity.[ 11 , 12 ]
PVT is often asymptomatic and incidentally found as part of routine imaging.[ 2 ] Only a minority of patients present with symptoms such as acute abdominal pain or gastrointestinal bleeding, which is presumably a result of a portal vein thrombus that has recently been formed. For the majority of thrombi, however, the exact timing of thrombus formation is unknown, although it may be estimated by its appearance on radiological imaging or examination of sequential images over time. The age of the thrombus might be of importance for the decision whether or not to treat PVT as more recently formed portal vein thrombi seem to respond better to anticoagulant therapy than older thrombi.[ 13 , 14 ] Given the lack of precision in determination of thrombus age in the majority of patients, the distinction between acute (or recent) and chronic PVT may be considered arbitrary. In addition, whether anticoagulant therapy is indicated for both recent and chronic PVT remains a matter of debate because (1) some of the thrombi resolve spontaneously,[ 7 , 15 ] (2) anticoagulant therapy does not always result in recanalization of the portal vein,[ 13 , 16 , 17 , 18 ] (3) bleeding may complicate treatment with anticoagulants,[ 19 , 20 ] and (4) therapy may not benefit the patient, particularly if the patient is not a liver transplant candidate.[ 18 , 20 , 21 ] Therefore, some experts suggest that anticoagulant therapy is not always indicated, and observation with serial imaging every 3 months, for example, to monitor thrombus extension, is a reasonable alternative.[ 2 , 5 , 17 , 20 , 22 ]
Once the decision is made to start anticoagulants, the pharmacological treatment for PVT resembles treatment strategies used in patients with deep vein thrombosis (DVT). Given the difficulties with using vitamin K antagonists (VKAs) in patients with cirrhosis, low–molecular weight heparin (LMWH) is the most commonly used anticoagulant; but direct oral anticoagulants are gaining popularity for this indication despite the lack of randomized studies of these agents in patients with cirrhosis.[ 20 , 23 ] Whether these therapeutic strategies are optimal for treatment or (secondary) prevention of PVT remains uncertain. Importantly, the portal venous system is not directly comparable to deep venous systems: (1) the portal vein drains blood not to the heart but to hepatic sinusoids in the liver[ 24 ] and (2) the portal vein does not have venous valves, which are important in the development of DVT.[ 25 ] These features suggest that portal thrombi could be different in composition and structure compared with deep venous thrombi and might therefore require different treatment strategies.
Although the composition of venous and arterial thrombi has been studied extensively,[ 26 , 27 , 28 , 29 ] to the best of our knowledge the composition of portal vein thrombi has not been studied in detail. Better understanding of the composition and structure of portal vein thrombi will be crucial to improve treatment and preventive strategies for PVT in patients with cirrhosis and can reveal important information on the pathophysiology of PVT. Therefore, in this study, we used histology, immunohistochemistry, and electron microscopy to define the structure and composition of portal vein thrombi in patients with cirrhosis at the time of liver transplantation.
MATERIALS AND METHODS
Study population
Sixteen portal vein thrombi were prospectively collected from adult (≥18 years old) patients with cirrhosis who underwent liver transplantation at King’s College Hospital (KCH; n = 5; London, UK), University Medical Center Groningen (UMCG; n = 5; the Netherlands), or Hospital Clinic Barcelona (n = 6; Spain) between October 2018 and October 2020. All patients gave informed written consent for participation in this study. Ethical approval from the Health Research Authority and Health Care and Research Wales (study no. 19/LO/0920), from the UMCG local institutional review board (study registry no 201800967), and from the Hospital Clinic local institutional review board (HCB/2018/0546) was obtained. The study protocol was approved by the Health Research Authority and Health Care and Research Wales and the Research and Innovation Department at KCH; good clinical practice guidelines were followed. The thrombi were excised by the surgeon with preservation of the entire vessel in the specimen. Only samples with vessel wall components attached to the thrombus material were included. Eight of the thrombi were cut into two even, representative samples, of which one half was fixed in formalin and embedded in paraffin for histological assessment, and the other half was washed in a cacodylate buffer (50 mM sodium cacodylate, pH 7.4, 150 mM NaCl) and then fixed in 2% glutaraldehyde in cacodylate buffer for scanning electron microscopy (SEM). The remaining eight thrombi were fixed in formalin and embedded in paraffin only.
For the retrospective part of this study, adult (≥18 years old) patients with cirrhosis from KCH (n = 30) and UMCG (n = 33) who underwent liver transplantation between 2009 and 2018 were included. PVT was diagnosed prior to transplantation by routine imaging procedures, during liver transplantation by the liver transplant surgeon, or by the pathologist during examination of the explant. Patients with tumorous infiltration in the portal vein were excluded. Formalin‐fixed, paraffin‐embedded liver tissue with portal vein thrombus in sections taken at the liver hilum of the explant were collected from local pathology archives. Medical history and relevant clinical data were collected from patients’ medical records in an anonymized electronic database. This study was approved by the local institutional review boards (KCH liver biobank approval no. A19WBYZ11; UMCG registry no. 201900748). We also included hilar explant liver tissue from 15 patients with cirrhosis without PVT and from 5 patients with acute liver failure and no PVT (KCH liver biobank approval no. A19WBYZ11) and samples from 5 donor livers that were offered to and transplanted at the UMCG (METC no. M14.152454).
Histology and immunohistochemistry
The paraffin‐embedded portal vein thrombi were cut into sections 3–3.5 μm thick using a microtome and fixed on adhesive glass slides. Sections from each patient were stained with hematoxylin and eosin (H&E), elastic van Gieson (EVG), and Martius scarlet blue (MSB). In addition, portal vein thrombi sections were stained with antibodies to fibrin/fibrinogen (Abcam; Ab58207), von Willebrand factor (VWF Dako; A0082), and CD61 (Leica; PA0308) on an automated staining machine (Bond Max autostainer; Leica). Images were acquired using a light microscope (Olympus; BX51) and a digital camera (Olympus; DP26) and analyzed by an experienced liver pathologist (Y.Z.). Intimal thickness was quantified using imaging software (CellSens; Olympus). The intimal thickness in prospectively collected portal vein thrombi samples was measured in an area where the outline of the blood vessel, with a clear distinction between intima and media, was recognized (Supporting Figure S1). In samples in which only intimal tissue was recognized, we recorded the maximal thickness of the intima present in the sample.
SEM
Small pieces of prospectively collected portal vein thrombi were rinsed with saline and fixed in 2% glutaraldehyde in 50 mM cacodylate buffer containing 150 mM NaCl (pH 7.4). The fixed thrombi were washed in 50 mM sodium cacodylate and 150 mM NaCl (pH 7.4), dehydrated in ascending concentrations of ethanol (30–100 v/v%), dried using hexamethyldisilazane, and sputter‐coated with gold‐palladium. High‐definition images were obtained from randomly chosen areas of each thrombus to eliminate selection bias using a FEI Quanta 250FEG SEM (FEI, Hillsboro, OR).
Radiology analysis
From each patient, most recent CT scans before liver transplantation were analyzed by radiologists from KCH (S. G., P. K.) or UMCG (R. d. H.) to specifically estimate portal vein thrombus age and the degree of portal vein occlusion. Assignment as being acute or chronic thrombus considers the time interval between imaging where no thrombus was apparent and that when first detected. Specific morphological features also suggest an acute “fresh” thrombus, for example, specific vessel location, central thrombus position, smooth surface and vessel occlusion, while other features, such as a laminar appearance and the presence of chords or calcification, suggest a more chronic thrombosis. It is, however, evident that in practice this is not an absolute binary categorization, and both acute and chronic radiologic features may be present simultaneously.
Statistical analysis
Descriptive statistics were used to summarize the demographics and clinical characteristics of the study population. Continuous variables were reported as means and standard deviations. Categorical variables were reported as numbers and percentages, and variables of the retrospective study population were compared with a chi‐squared test, Cramer’s V test, or Fisher’s exact test as appropriate. All analyses were done using IBM SPSS Statistics 23, with a two‐sided significance level of 0.05.
RESULTS
Cirrhotic PVT consists of intimal fibrosis with or without a fibrin‐rich thrombus
First, we assessed the structure and composition of 16 prospectively collected portal vein thrombus samples of patients with cirrhosis (37.5% female, mean age 53 ± 12 years) that were obtained by the surgeon during liver transplantation. All samples showed a thickened, fibrotic tunica intima of the portal vein on H&E and MSB staining. The median thickness of this intimal fibrosis was 2325 µm (interquartile range [IQR], 1728–3695 µm). This thickened vessel wall occluded the lumen for > 50% in 5 of the 16 cases. Nine of the 16 samples also contained a fibrin thrombus as evidenced by (orange to red) MSB staining. Figure 1 shows two representative light microscopic images of the portal vein thrombi samples. Figure 1A demonstrates a focally thickened fibrotic tunica intima of the vessel wall with some hemorrhage but without a fibrin‐rich thrombus. Figure 1B shows a circumferential thickened fibrotic tunica intima of the portal vein, with a fibrin thrombus within the lumen of the vessel. Images of all 16 prospectively collected thrombi are shown in Supporting Figure S2, demonstrating heterogeneity of the thrombi in terms of structure and fibrin/fibrinogen content. To study the components of the portal vein thrombi in more detail, the samples were analyzed using immunohistochemistry for fibrin/fibrinogen, VWF, and CD61 (platelets), as shown in Supporting Figure S3. The fibrotic intima stained focally positive for fibrin/fibrinogen in 6 of the 16 cases and for VWF in 4 cases. None of the samples stained positive for CD61 in the fibrotic intima of the portal vein. The nine intravascular fibrin‐rich thrombi all stained positive for fibrin/fibrinogen, four stained positive for VWF, and four for CD61.
FIGURE 1.
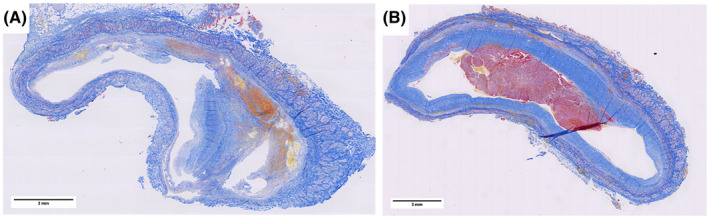
MSB‐stained sections of extrahepatic portal vein samples removed during liver transplant surgery. These are representative images of the 16 prospectively collected thrombi. (A) A portal vein thrombus consisting of a focally thickened intimal layer of the vessel wall with some hemorrhage but without a fibrin‐rich thrombus. (B) A portal vein thrombus consisting of a circumferential thickened intimal layer of the vessel wall with a fibrin‐rich thrombus on top [Color figure can be viewed at wileyonlinelibrary.com]
The prospectively collected portal vein thrombus samples were also analyzed using SEM. In the samples only containing a fibrotic intima, bundles of collagen with red blood cells in the typical biconcave shape (Figure 2A,B) were observed. In the nine samples that also contained a fibrin thrombus on MSB staining, branched fibrin networks with entrapped red blood cells, platelet remnants, and cell debris were observed (Figure 2C,D). Notably, polyhedral erythrocytes, which are a hallmark of contracted clots commonly seen in patients with venous and arterial thrombosis,[ 30 , 31 , 32 ] were only rarely observed.
FIGURE 2.
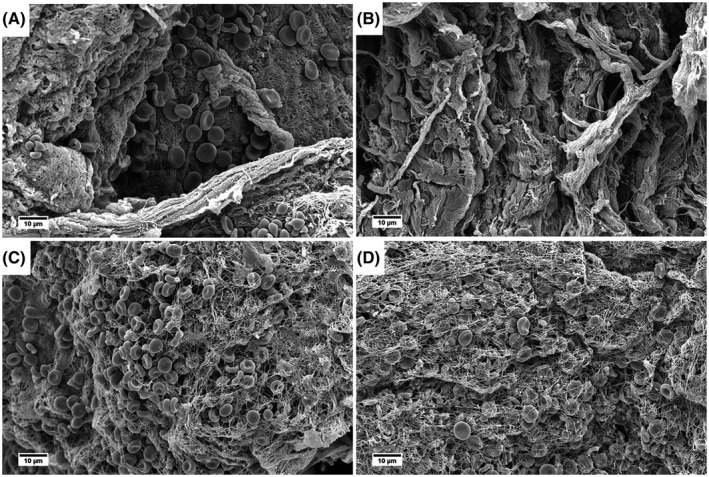
Representative SEM images from eight prospectively collected portal vein thrombus samples. (A,B) Collagen bundles and some biconcave‐shaped red blood cells. Fibrin is focally present. (C,D) Fibrin networks with mostly biconcave‐shaped red blood cells
Next, we assessed the structure and composition of retrospectively collected portal vein thrombus samples of 63 patients with cirrhosis (27% female, mean age 53 ± 13 years). Alcohol‐associated steatohepatitis (32%) was the most prevalent etiology of disease. The majority of patients had moderate to severe liver disease according to the Child‐Pugh score (i.e., Child‐Pugh B, 51%; Child‐Pugh C, 41%) at the time of liver transplantation. Median duration between diagnosis of cirrhosis and liver transplantation was 6 years (range 0–28 years), and median duration between diagnosis of PVT and liver transplantation was 5 months (range 0–125 months). In 21 patients (33%), PVT had not been diagnosed on radiological imaging prior to liver transplantation. Anticoagulant therapy was used by 19 of the 63 patients (30%) at the time of liver transplantation. Two patients had been on anticoagulant therapy that was stopped 21 months and 9 months prior to liver transplantation. The reason for stopping the therapy was chronic minor oral bleeding in the first case and unknown in the second case. At the time of liver transplantation, LMWH was used by 8 patients (12.7%), VKA by 11 patients (17.5%), and acetylsalicylic acid by 2 patients (3.2%). Patient characteristics are summarized in Table 1.
TABLE 1.
Demographics and characteristics of patients with cirrhosis and PVT at the time of liver transplantation, the control cohort of patients with cirrhosis without PVT, and patients with acute liver failure without PVT
| Prospective cohort (n = 16) | Retrospective cohort (n = 63) | Control cohort, patients with cirrhosis without PVT (n = 15) | Control cohort, patients with acute liver failure without PVT (n = 5) | |
|---|---|---|---|---|
| Gender (female) | 6 (37.5) | 17 (27.0) | 9 (45.0) | 3 (60.0) |
| Age (years) at time of LT | 53 ± 12 | 53 ± 13 | 48 ± 13 | 40 ± 10 |
| BMI | 25.2 ± 4.0 | 26.4 ± 5.1 | 28.5 ± 5.0 | No data |
| MELD score | 17.8 ± 4.5 | 18.5 ± 6.0 | 12.8 ± 6.8 | N/A |
| Child‐Pugh score | ||||
| A | 1 (6.3) | 5 (7.9) | 2 (13.3) | N/A |
| B | 9 (56.3) | 32 (50.8) | 8 (53.3) | N/A |
| C | 6 (37.5) | 26 (41.3) | 5 (33.3) | N/A |
| Etiology of liver disease | ||||
| ASH | 2 (12.5) | 20 (31.7) | 5 (33.3) | 0 (0.0) |
| NASH | 2 (12.5) | 9 (14.3) | 2 (13.3) | 0 (0.0) |
| PBC | 1 (6.3) | 5 (7.9) | 1 (6.7) | 0 (0.0) |
| PSC | 2 (12.5) | 9 (14.3) | 5 (33.3) | 0 (0.0) |
| Viral | 3 (18.8) | 2 (3.2) | 1 (6.7) | 0 (0.0) |
| Autoimmune | 2 (12.5) | 9 (14.3) | 1 (6.7) | 1 (20.0) |
| Cryptogenic | 1 (6.3) | 2 (3.2) | 0 (0.0) | 0 (0.0) |
| Drug‐induced | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (60.0) |
| Other | 3 (18.8) | 7 (11.1) | 0 (0.0) | 1 (20.0) |
| HE | ||||
| No | 11 (68.8) | 32 (52.5) | 15 (100.0) | 0 (0.0) |
| Grade 1–2 | 5 (31.3) | 26 (42.6) | 0 (0.0) | 0 (0.0) |
| Grade 3–4 | 0 (0.0) | 3 (4.9) | 0 (0.0) | 5 (100.0) |
| Ascites | ||||
| No | 5 (31.3) | 13 (20.6) | 9 (60.0) | 4 (80.0) |
| Slight | 6 (37.7) | 8 (12.7) | 1 (6.7) | 1 (20.0) |
| Moderate | 4 (25.0) | 16 (25.4) | 2 (13.3) | 0 (0.0) |
| Severe | 1 (6.3) | 26 (41.3) | 3 (20.0) | 0 (0.0) |
| Smoker, currently or stopped (yes) | 6 (37.5) | 28 (50.4) | 1 (6.7) | 0 (0.0) |
| Diabetes (yes) | 5 (31.3) | 25 (42.4) | 4 (26.8) | 0 (0.0) |
| Previous abdominal surgery | 4 (25.0) | 24 (42.9) | No data | 0 (0.0) |
| HCC | 5 (31.3) | 11 (17.5) | 1 (6.7) | 0 (0.0) |
| Thrombophilic disease | 0 (0.0) | 0 (0.0) | N/A | N/A |
| Medication at time of LT | ||||
| Use of LMWH | 6 (37.5) | 8 (12.7) | 0 (0.0) | 0 (0.0) |
| Use of VKA | 6 (37.5) | 11 (17.5) | 0 (0.0) | 0 (0.0) |
| Use of acetylsalicylic acid | 1 (6.3) | 2 (3.2) | 0 (0.0) | 0 (0.0) |
| Use of beta‐blockers | 9 (56.3) | 42 (66.7) | 4 (26.8) | 0 (0.0) |
| Hormonal influence (female) | 1/6 (16.7) | 2/17 (11.8) | 0/9 (0.0) | 0/3 (0.0) |
The results are presented as mean ± SD for continuous variables, and number (percentage) for categorical variables of available data.
Abbreviations: ASH, alcohol‐associated steatohepatitis; BMI, body mass index; LT, liver transplantation; N/A, not applicable; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis.
All 63 paraffin‐embedded samples showed a thickened, fibrotic tunica intima of the portal vein on H&E, EVG, and MSB staining. The median thickness of the tunica intima was 2406 µm (IQR, 1349–3346 µm). The intimal thickening occupied > 50% of the vessel lumen in 48 patients (76%). To ascertain that a fibrotic tunica intima is specific for patients with PVT and not a general feature of a portal vein in patients with liver disease, we studied retrospectively collected tissue from patients with cirrhosis who did not have PVT. Clinical characteristics of these patients are outlined in Table 1. In hilar liver tissue from 15 patients with cirrhosis, five samples showed very mild intimal fibrosis, which was almost an order of magnitude thinner compared to the intimal thickness in patients with PVT (median thickness 358 µm [IQR, 294–378 µm]). In five samples from patients with acute liver failure, no intimal fibrosis was found. Figure 3 shows typical examples of the thickness of the tunica intima in liver donors, patients with acute liver failure, and patients with cirrhosis without PVT. From the 63 samples of patients with PVT, 21 (33%) contained a fibrin‐rich thrombus. Of these 21 samples, the fibrin structures occupied > 50% of the vessel lumen in 7 patients (33%). The median time between diagnosis of PVT and liver transplantation was 5 months (range 0–125 months) for patients who had a fibrin‐rich thrombus based on histology results and 3.5 months (range 0–81 months) for patients who did not have a fibrin‐rich thrombus based on histology results. Representative images of H&E, EVG, MSB, and immunostained hilar liver tissue samples are shown in Figures 4 and 5.
FIGURE 3.
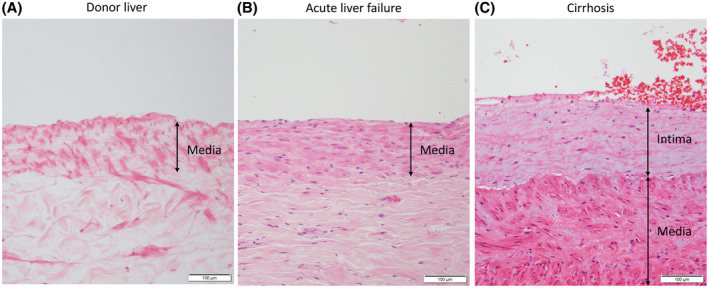
Representative images of the portal vein wall in H&E‐stained sections from hilar liver tissue samples from human donor livers, patients with acute liver failure, and patients with cirrhosis without PVT. The tunica intima at the liver hilum in human donor liver and patients with acute liver failure consists of a flat lining of endothelial cells and is almost unrecognizable, and therefore not measurable (A,B). The portal vein vessel wall at the liver hilum in patients with cirrhosis without PVT shows a thickened tunica intima (C) [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
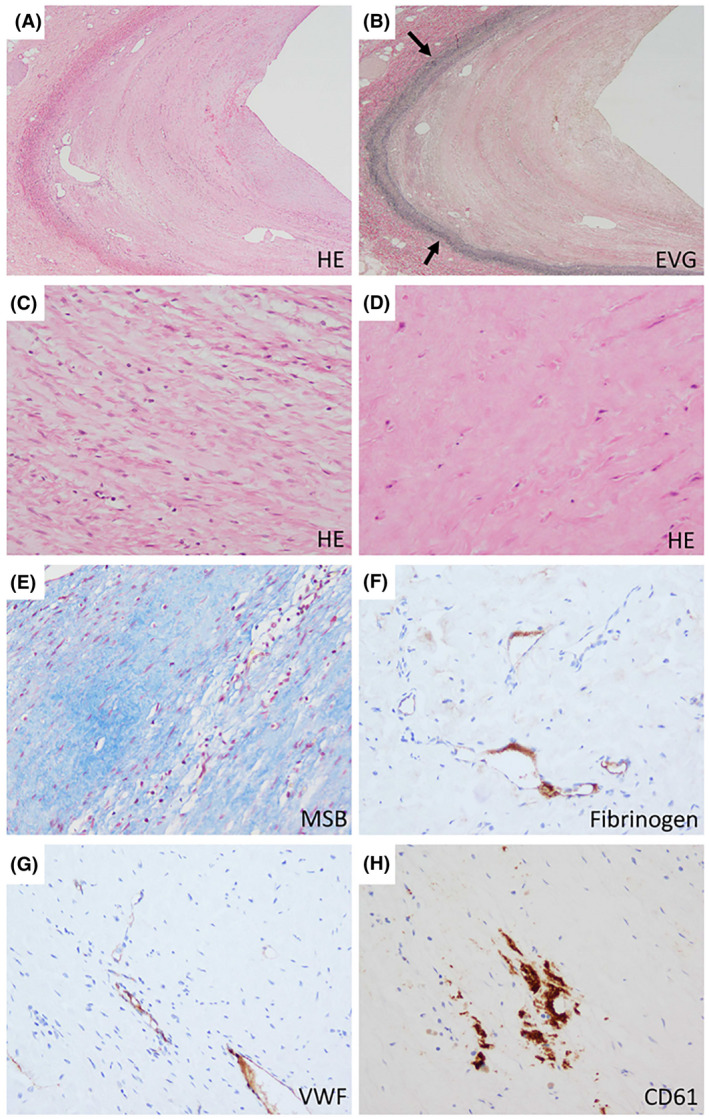
Histopathology of PVT at the hilar region of explanted livers. Representative examples of the thickened tunica intima of the portal vein. A crescent‐shaped lamellar fibrosis of the intima is observed (A; ×20). EVG staining highlights intimal thickening (B; arrows indicating the tunica media; ×20). In the thickened intima, some parts show spindle‐shaped cells arranged in a lamellar fashion (C; ×200), while others consist of hypocellular densely collagenized fibrosis (D,E; both ×200). It is only focally immunoreactive to fibrin/fibrinogen (F), VWF (G), and CD61 (H; all ×200) [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 5.
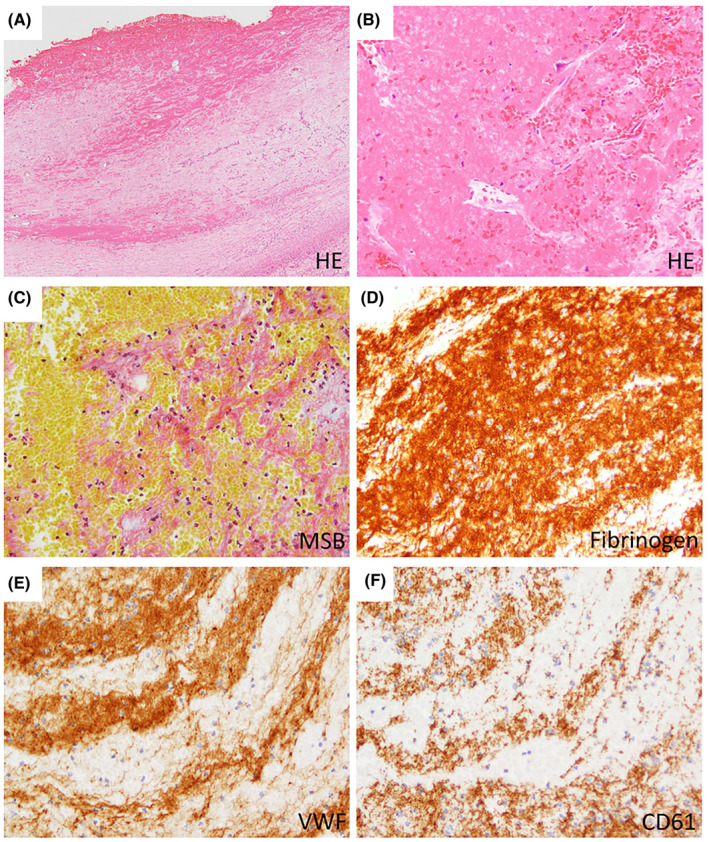
Histopathology of PVT at the hilar region of explanted livers. Representative examples of intimal fibrosis with fibrin thrombus of the portal vein. A fibrin thrombus overlying the thickened intima is observed (A; ×20). The fibrin thrombus consists of aggregated eosinophilic materials and blood contents (B; ×200), and these parts are stained orange to red by MSB, indicating fibrin (C; ×200). The fibrin thrombus is immunoreactive to fibrin/fibrinogen, VWF, and CD61; and fibrin/fibrinogen is most widely positive (D–F; ×200) [Color figure can be viewed at wileyonlinelibrary.com]
Immunohistochemical analysis of the fibrotic tunica intima of the portal vein vessel wall showed focal positive staining for fibrin/fibrinogen in 10 samples (16%), VWF in six samples (10%), and CD61 (platelets) in 13 samples (21%). The immunoreactivity to those markers was mostly restricted to small vessels or hemorrhagic foci of the thickened intima, while the sclerotic stroma was negative (Figure 4). From the 21 samples that contained a fibrin‐rich thrombus, all (100%) stained positive for fibrin/fibrinogen and CD61 (platelets), and 16 (76%) stained positive for VWF. Fibrin/fibrinogen was abundantly present in the fibrin‐rich thrombi, while platelet and VWF staining was less abundant (Figure 5).
Clinical characteristics and thrombus age do not determine whether the portal vein thrombus contains fibrin
Next to histological assessment, PVT was assessed by radiological imaging. Experienced hepatopancreatobiliary radiologists reanalyzed the most recent CT scans prior to liver transplantation from each retrospectively included patient. The median time between the most recent CT scan and liver transplantation was 4 months (range 0–39 months). The time between the most recent CT scan and liver transplantation was ≤6 months in 40 cases (64%). Although PVT was not diagnosed prior to liver transplantation in 21 patients (33%), reanalysis of their CT scans, with a specific focus on PVT, showed a thrombus in 12 of these 21 patients (57%). Thus, after close radiological inspection, only 9 of the 63 (14%) thrombi were not recognized on radiological imaging. Of these 9 patients, 5 had a fresh fibrin thrombus based on histological analyses. The most recent CT scan prior to liver transplantation of these 9 patients was older than 6 months in 7/9 (78%) patients. Of the 54 portal vein thrombi that were recognized on the most recent CT scan, the location of the thrombus was both intrahepatic and extrahepatic in 34 (62%) cases, only intrahepatic in 9 cases (16%), and only extrahepatic in 12 cases (22%). Occlusion of the portal vein was > 50% in 18 (37%) cases based on radiological examination. Of the 49 PVT cases that were analyzed by radiologists, 34 thrombi were classified as chronic, whereas 10 were classified as acute. No classification could be given for the remaining five thrombi.
We then compared the radiological classification as chronic or acute PVT with the histological analyses. Of the 54 portal vein thrombi identified on radiological imaging, 16 patients (30%) had an intravascular fibrin‐rich thrombus based on histology results. Of the 34 patients in whom the location of the thrombus was both intrahepatic and extrahepatic on the most recent CT scan prior to liver transplantation, 12 had a fibrin‐rich thrombus (35%); of the 9 patients who only had intrahepatic PVT on the most recent CT scan, 2 had a fibrin‐rich thrombus (22%); and of the 12 patients who only had extrahepatic PVT on the most recent CT scan, 4 had a fibrin‐rich thrombus based on histology results (33%). Of the 34 patients in whom the portal vein thrombus was classified as chronic by the radiologist during reanalysis of the images, 9 (26%) contained a fibrin thrombus within the lumen of the vessel. Of the 10 patients in whom the portal vein thrombus was classified as acute, 5 (50%) contained a fibrin thrombus within the lumen of the vessel. Supporting Figures S4–S7 show typical examples of radiological images from patients whose thrombus was classified as chronic or acute compared with representative H&E‐stained sections from these patients. Of note, the median time between the most recent CT scan and transplantation was 3 months (range 0–14) in the thrombi classified as acute by the radiologists. Of the 5 patients in whom the thrombus could not be classified as chronic or acute by radiologists, 2 (40%) contained a fibrin thrombus within the lumen of the vessel. Of the 9 patients in whom PVT was not recognized on radiological imaging, 5 (55%) had fibrin‐rich thrombi within the lumen of the vessel based on histology results. Importantly, the most recent CT scan from these patients was older than 6 months in 4/5 (80%) cases with a fibrin thrombus and in 5/9 (56%) cases without a fibrin thrombus (median time between most recent CT scan and liver transplantation was 10 months, range 2–29 months).
Lastly, we related our histological and radiological findings to clinical characteristics. There was no association between the presence of a fibrin‐rich thrombus and etiology of disease, use of anticoagulants, degree of occlusion of the portal vein, severity of disease based on the Model for End‐Stage Liver Disease (MELD) score, and the presence of collaterals and varices (Table 2). We did find that patients with a smaller portal vein diameter more often had no fibrin thrombus within the lumen of the portal vein (p < 0.05). No associations were found between patient characteristics and the time between PVT diagnosis and liver transplantation (Table 2). Nineteen patients used anticoagulant therapy at the time of liver transplantation. There were no significant differences in age, body mass index, etiology of disease, MELD score, presence of ascites, HE, HCC, smoker/nonsmoker, diabetes mellitus, time between diagnosis of PVT and liver transplantation, diameter of the portal vein, or degree of occlusion of the portal vein between patients who used anticoagulants or did not use anticoagulants (data not shown). In 5 of the 19 patients who were treated with anticoagulants, the size of the thrombus reduced after starting anticoagulant therapy, based on radiological imaging. Of these 5 patients, 0 had a fibrin‐rich thrombus at the time of liver transplantation. In the other 14 patients who used anticoagulants, the size of the thrombus increased, did not change, or is unknown because PVT was not diagnosed or no imaging was available between start of the treatment and liver transplantation. Of these 14 patients, 6 had a fibrin‐rich thrombus at the time of liver transplantation. The use of anticoagulants was not associated with a decrease in the proportion of patients with a fibrin‐rich thrombus (Table 2). In addition, the intimal thickness was similar in patients who did or did not use anticoagulants at the time of liver transplantation (2371 versus 2465 µm, p = 0.80). Seventeen of the 42 (40.5%) patients who used a beta‐blocker at the time of transplantation had a fibrin‐rich thrombus. No significant differences were observed between patients who used beta‐blockers and patients who did not use beta‐blockers on presence of a fibrin‐rich thrombus, degree of portal vein occlusion, disease severity, portal vein diameter, and presence of collaterals (data not shown).
TABLE 2.
Associations between the presence of a fibrin‐rich thrombus based on histological analysis and estimated age of thrombus based on radiological imaging and patient parameters
| Presence of a fibrin‐rich thrombus based on histology (n = 63) | Radiological diagnosis of PVT prior to LT (n = 42) | ||||||
|---|---|---|---|---|---|---|---|
| Yes (n = 21) | No (n = 42) | p | ≤6 months (n = 14) | >6 months (n = 28) | p | ||
| Presence of a fibrin‐rich thrombus | Yes | — | — | — | 7 (50.0) | 9 (32.1) | 0.26 |
| No | — | — | 7 (50.0) | 19 (67.9) | |||
| Etiology of disease | Cholestatic | 6 (28.6) | 8 (19.0) | 0.43 a | 2 (14.3) | 6 (21.4) | 0.17 a |
| NASH | 4 (19.0) | 5 (11.9) | 4 (28.6) | 2 (7.1) | |||
| Other | 11 (52.4) | 29 (69.0) | 8 (57.1) | 20 (71.4) | |||
| Use of anticoagulants (at time of LT) | Yes | 6 (28.6) | 13 (31.0) | 0.85 | 6 (42.9) | 13 (46.4) | 0.83 |
| No | 15 (71.4) | 29 (69.0) | 8 (57.1) | 15 (53.6) | |||
| Occlusion of portal vein (based on radiology) | ≤50% | 9 (56.3) | 22 (66.7) | 0.48 | 8 (66.7) | 13 (52.0) | 0.40 |
| >50% | 7 (43.8) | 11 (33.3) | 4 (33.3) | 12 (48.0) | |||
| Disease severity (based on MELD score) | ≤15 | 5 (23.8) | 14 (33.3) | 0.44 | 5 (35.7) | 6 (21.4) | 0.46 b |
| >15 | 16 (77.2) | 28 (66.7) | 9 (64.3) | 22 (78.6) | |||
| Portal vein diameter (based on radiology) | ≤15 mm | 6 (31.6) | 25 (65.8) | 0.015 | 7 (58.3) | 14 (51.9) | 0.71 |
| >15 mm | 13 (68.4) | 13 (34.2) | 5 (41.7) | 13 (48.1) | |||
| Presence of collaterals | Yes | 20 (95.2) | 36 (85.7) | 0.41 b | 12 (85.7) | 26 (92.9) | 0.59 b |
| No | 1 (4.8) | 6 (14.3) | 2 (14.3) | 2 (7.1) | |||
| Use of beta‐blockers (at time of LT) | Yes | 17 (81.0) | 25 (59.5) | 0.16 | 12 (85.7) | 20 (71.4) | 0.45 b |
| No | 4 (19.0) | 17 (40.5) | 2 (14.3) | 8 (28.6) | |||
| Presence of HCC | Yes | 3 (14.3) | 8 (19.5) | 0.74 b | 2 (15.4) | 5 (17.9) | 1.00 b |
| No | 18 (85.7) | 33 (80.5) | 11 (84.6) | 23 (82.1) | |||
Data are presented as frequency (percentage); p values are based on chi‐squared test, unless stated otherwise. Bold values denote statistical significance of p < 0.05.
Abbreviation: LT, liver transplantation.
Cramer’s V.
Fisher’s exact test.
DISCUSSION
Here, we have described the composition and structure of nonmalignant cirrhotic portal vein thrombi that were collected during liver transplantation. We demonstrated that all portal vein thrombi consist, at least in part, of tunica intima thickening of the portal vein vessel wall, specifically in an appearance resembling intimal fibrosis. Only one‐third of the thrombi examined contained a fibrin‐rich thrombus in addition to a fibrotic intima, and the presence or absence of such material appeared unrelated to thrombus age as estimated by radiological imaging, clinical characteristics, or previous anticoagulant therapy. The apparent absence of polyhedral erythrocytes, which define contracted venous and arterial thrombi,[ 30 , 31 , 32 ] reinforces the notion that the portal vein thrombus is a unique entity. As two thirds of portal vein thrombi at the time of transplantation are devoid of fibrin, our results may redefine the biological basis of PVT. We propose that the absence of fibrin in part of the thrombi may explain why not all patients with PVT show recanalization by anticoagulant therapy.
Histological assessment of both prospectively and retrospectively collected portal vein thrombus samples showed that a portal vein thrombus in part or in its entirety is a thickened, fibrotic tunica intima of the portal vein. This was observed as circumferential and as focal thickening of the vessel wall (Figure 1). These observations suggest that the term “portal vein thrombosis” may be a misnomer and that “portal vein stenosis” or “nonmalignant portal vein occlusion” may be more appropriate. The origin of the intimal fibrosis of the portal vein wall cannot be derived from our study with certainty, but we propose two possible mechanisms by which portal vein intimal fibrosis develops.
First, it may be that the intimal fibrosis develops as a consequence of initial fibrin‐rich thrombus formation, which organizes into a fibrotic structure that reendothelializes over time. In this scenario, a portal vein thrombus starts as a fibrin‐rich thrombus that matures and in which fibrin is replaced by a collagen and cell‐rich structure over time. This process has been shown to occur in other vascular beds after profound injury to the vascular wall leading to thrombus formation with subsequent development of intimal fibrosis.[ 33 ] In patients with cirrhosis, damage to the portal vein wall by, for example, altered shear stress related to portal hypertension and inflammation may initiate thrombus formation, which is further facilitated by the hypercoagulable features of patients with cirrhosis.[ 5 , 34 , 35 , 36 ]
A second, intriguing hypothesis is that the intimal fibrosis develops in the absence of overt initial fibrin formation. This intimal hyperplasia can occur following all types of vascular reconstructive procedures including coronary artery bypass surgery (particularly using veins or synthetic grafts), angioplasty, vascular stenting, endarterectomy, and vascular access grafting.[ 37 ] These procedures may lead to vascular endothelial cell stress,[ 37 , 38 ] platelet aggregation, leukocyte chemotaxis, and endothelial proliferation.[ 39 ] This initial response to injury ultimately leads to proliferation of mesenchymal cells (e.g., myofibroblasts, vascular smooth muscle cells) and deposition of extracellular matrix components.[ 33 , 37 ] Interestingly, activation of coagulation and in particular the generation of thrombin is a well‐recognized contributor to intimal hyperplasia after vascular injury, which is in part mediated by activation of smooth muscle cells by thrombin.[ 40 , 41 , 42 ] In addition, tissue factor is an important driver of intimal hyperplasia,[ 40 , 43 ] and tissue factor expression on smooth muscle cells or fibrocytes may drive local thrombin generation. Indeed, local or systemic inhibition of thrombin or tissue factor decreased intimal hyperplasia in animal models.[ 40 , 41 , 44 ] This mechanism could also apply to the development of PVT. For example, one study has demonstrated that prophylactic anticoagulation profoundly reduces development of PVT,[ 9 ] and it may be that the effects of anticoagulation in this study are not primarily due to prevention of clot formation but to inhibition of local thrombin generation that drives intimal hyperplasia.
In patients with DVT and pulmonary embolism, the thrombus consists of fibrin, platelets, and blood cells.[ 27 ] However, in animal models of venous thrombosis,[ 33 ] a thickening of the vessel wall is observed over time, a phenomenon referred to as “vein wall fibrosis.” This postthrombosis vein wall remodeling has been proposed to contribute to postthrombotic syndrome, and therapeutic strategies to reduce postthrombosis vein wall fibrosis have been proposed.[ 45 ] One study in humans has shown thickening of the vein wall by high‐resolution ultrasound both in patients with DVT in the acute phase and in patients with postthrombotic syndrome.[ 46 ] Whether these post‐DVT vein wall changes have a similar pathogenesis compared to the fibrotic lesion in PVT requires additional study.
Intravascular fibrin‐rich thrombi were present in one‐third of the cases within the retrospective cohort in this study. In contrast to the PVT consisting solely of intimal fibrosis, these thrombi may be susceptible to anticoagulant therapy. Fibrin‐specific MRI contrast agents have been developed and tested in humans, and such agents may be helpful in determining which patients may benefit from anticoagulant therapy.[ 47 ] The notion that PVT can be recurrent, with spontaneously resolving and returning thrombi,[ 7 , 15 ] may also explain why patients did not have detectable fibrin‐rich thrombi at the time of transplantation. Anticoagulants may be helpful in preventing the “reappearance” of fibrin‐rich structures. Such cyclic behavior may explain why there was no difference in the proportion of fibrin‐containing thrombi between thrombi that were radiologically classified as acute or as chronic. In addition, calcification and reendothelialization of thrombi can already start a few days after initiation of thrombosis.[ 48 , 49 ] Thrombi that were not classified as fibrin‐rich on histology within this study could thus have been formed recently but could have developed into an organized thrombus at the time of transplantation. We tried to define the chronicity of thrombosis in the retrospective study population based on the most recent CT scan, but no recent imaging (≤6 months) was available from 36% of the patients within the retrospective cohort, making comparisons between radiological imaging and the possibly fast‐changing thrombus unreliable.
Our results thus suggest that part of the PVT might not always develop from a fibrin thrombus but could instead be a vascular disease resulting from cirrhosis and its complications in the portal vein. The observation that a much milder intimal thickening of the portal vein is present in part of the patients with cirrhosis without PVT supports this proposed mechanism, and it is tempting to speculate that an alteration in portal flow as a consequence of portal hypertension is the trigger of intimal fibrosis development. Indeed, alterations in shear stress have been shown to promote intimal hyperplasia in other settings.[ 50 , 51 ] It is conceivable that the composition of a portal vein thrombus has implications for treatment strategies and the effect of therapeutic interventions. Although anticoagulant therapy may be beneficial to prevent development of de novo fibrin‐rich thrombi, it will likely not be effective in recanalizing PVT that only consists of fibrotic tissue. Anticoagulants may prevent the development of fibrin‐rich thrombi on top of a fibrotic portal vein wall structure and may thereby prevent thrombus extension. Strategies to prevent portal venous intimal fibrosis in patients with cirrhosis should be the focus of future studies.
We acknowledge some limitations to our study. First, this study is largely based on a retrospective study population. We had access to samples taken at the liver hilum from pathology archives. We only included samples in which thrombus was detected in the hilum. These sections of the thrombus may be the center of the thrombus, for example, in those patients with intrahepatic thrombus only but may also have been in the periphery of the thrombus in those patients who also had thrombus in the extrahepatic portal vein. We thus cannot ascertain whether these samples were representative for the entire thrombus. However, the thrombus composition in extrahepatic portal vein thrombi samples from the prospective cohort of this study was remarkably similar to the composition of the thrombi samples from the retrospective cohort of this study, which suggests that the hilar thrombus samples were representative of the entire thrombus. Future studies with prospective analysis of portal vein thrombi sampled at different sites of the thrombus are required to confirm our present findings. Second, the most recent CT scans were older than 6 months in 36% of the patients in the retrospective study population, which complicates comparison of the radiological imaging results with histology results. In addition, we have compared the histological appearance of the thrombus at the liver hilum with the radiological appearance of the entire thrombus, which frequently was present both intrahepatically and extrahepatically.
In conclusion, this study describes the composition and structure of nonmalignant portal vein thrombi in patients with cirrhosis. All thrombi analyzed showed intimal fibrosis of the portal vein wall, and only one‐third of the patients had a fibrin‐rich thrombus. We propose that, as it is unlikely that nonfibrin portal vein occlusions recanalize by anticoagulant therapy, our findings may have direct consequences for the management of cirrhotic PVT. Also, our findings may lead to strategies for the prevention of PVT.
CONFLICT OF INTEREST
Dr. Heaton advises Sirtex.
AUTHOR CONTRIBUTIONS
Ellen G. Driever collected biomaterial, performed experiments, analyzed data, and wrote the manuscript. Fien A. von Meijenfeldt collected biomaterial, performed experiments, analyzed data, and revised the manuscript. Jelle Adelmeijer performed experiments, analyzed data, and revised the manuscript. Robbert J. de Haas, Stephen Gregory, and Pauline Kane reviewed and interpreted CT scans, interpreted data, and reviewed the manuscript. Marius C. van den Heuvel, Constantino Fondevila, Robert J. Porte, Anabel Blasi, and Nigel Heaton collected biomaterial, interpreted data, and reviewed the manuscript. Chandrasekaran Nagasami performed experiments, interpreted data, and reviewed the manuscript. John W. Weisel and Yoh Zen supervised experiments, interpreted data, and reviewed the manuscript. William Bernal conceived the project, supervised experiments, interpreted data, and reviewed the manuscript. Ton Lisman conceived the project, supervised experiments, interpreted data, and wrote the manuscript.
Supporting information
Driever EG, von Meijenfeldt FA, Adelmeijer J, de Haas RJ, van den Heuvel MC, Nagasami C, et al. Nonmalignant portal vein thrombi in patients with cirrhosis consist of intimal fibrosis with or without a fibrin‐rich thrombus. Hepatology. 2022;75:898–911. 10.1002/hep.32169
Ellen G. Driever and Fien A. von Meijenfeldt are joint first authors.
William Bernal, Yoh Zen, and Ton Lisman are joint senior authors.
Funding information
Supported in part by the Dutch Thrombosis Foundation (Trombosestichting Nederland; 2018‐02).
REFERENCES
- 1. Francoz C, Valla D, Durand F. Portal vein thrombosis, cirrhosis, and liver transplantation. J Hepatol. 2012;57(1):203–12. 10.1016/j.jhep.2011.12.034 [DOI] [PubMed] [Google Scholar]
- 2. Nicoară‐Farcău O, Soy G, Magaz M, Baiges A, Turon F, Garcia‐Criado A, et al. New insights into the pathogenesis, risk factors, and treatment of portal vein thrombosis in patients with cirrhosis. Semin Thromb Hemost. 2020;46(6):673–81. 10.1055/s-0040-1715473 [DOI] [PubMed] [Google Scholar]
- 3. Gaballa D, Bezinover D, Kadry Z, Eyster E, Wang M, Northup PG, et al. Development of a model to predict portal vein thrombosis in liver transplant candidates: the Portal Vein Thrombosis Risk Index. Liver Transplant. 2019;25(12):1747–55. 10.1002/lt.25630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Noronha Ferreira C, Marinho RT, Cortez‐Pinto H, Ferreira P, Dias MS, Vasconcelos M, et al. Incidence, predictive factors and clinical significance of development of portal vein thrombosis in cirrhosis: a prospective study. Liver Int. 2019;39(8):1459–67. 10.1111/liv.14121 [DOI] [PubMed] [Google Scholar]
- 5. Intagliata NM, Caldwell SH, Tripodi A. Diagnosis, development, and treatment of portal vein thrombosis in patients with and without cirrhosis. Gastroenterology. 2019;156(6):1582–99.e1. 10.1053/j.gastro.2019.01.265 [DOI] [PubMed] [Google Scholar]
- 6. Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther. 2010;31(3):366–74. 10.1111/j.1365-2036.2009.04182.x [DOI] [PubMed] [Google Scholar]
- 7. Nery F, Chevret S, Condat B, de Raucourt E, Boudaoud L, Rautou P‐E, et al. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology. 2015;61(2):660–7. 10.1002/hep.27546 [DOI] [PubMed] [Google Scholar]
- 8. Saugel B, Lee M, Feichtinger S, Hapfelmeier A, Schmid RM, Siveke JT. Thrombophilic factor analysis in cirrhotic patients with portal vein thrombosis. J Thromb Thrombolysis. 2015;40(1):54–60. 10.1007/s11239-014-1124-z [DOI] [PubMed] [Google Scholar]
- 9. Villa E, Cammà C, Marietta M, Luongo M, Critelli R, Colopi S, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143(5):1253–60.e4. 10.1053/j.gastro.2012.07.018 [DOI] [PubMed] [Google Scholar]
- 10. Stine JG, Shah PM, Cornella SL, Rudnick SR, Ghabril MS, Stukenborg GJ, et al. Portal vein thrombosis, mortality and hepatic decompensation in patients with cirrhosis: a meta‐analysis. World J Hepatol. 2015;7(27):2774–80. 10.4254/wjh.v7.i27.2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ponziani FR, Zocco MA, Senzolo M, Pompili M, Gasbarrini A, Avolio AW. Portal vein thrombosis and liver transplantation: implications for waiting list period, surgical approach, early and late follow‐up. Transplant Rev. 2014;28(2):92–101. 10.1016/j.trre.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 12. Ghabril M, Agarwal S, Lacerda M, Chalasani N, Kwo P, Tector AJ. Portal vein thrombosis is a risk factor for poor early outcomes after liver transplantation: analysis of risk factors and outcomes for portal vein thrombosis in waitlisted patients. Transplantation. 2016;100(1):126–33. 10.1097/TP.0000000000000785 [DOI] [PubMed] [Google Scholar]
- 13. Delgado MG, Seijo S, Yepes I, Achécar L, Catalina MV, García–Criado Á, et al. Efficacy and safety of anticoagulation on patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol. 2012;10(7):776–83. 10.1016/j.cgh.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 14. Rodriguez‐Castro KI, Vitale A, Fadin M, Shalaby S, Zerbinati P, Sartori MT, et al. A prediction model for successful anticoagulation in cirrhotic portal vein thrombosis. Eur J Gastroenterol Hepatol. 2019;31(1):34–42. 10.1097/MEG.0000000000001237 [DOI] [PubMed] [Google Scholar]
- 15. Qi X, Guo X, Yoshida EM, Méndez‐Sánchez N, De Stefano V, Tacke F, et al. Transient portal vein thrombosis in liver cirrhosis. BMC Med. 2018;16(1):83. 10.1186/s12916-018-1069-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loffredo L, Pastori D, Farcomeni A, Violi F. Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: a systematic review and meta‐analysis. Gastroenterology. 2017;153(2):480–7.e1. https://doi.org/10.1053/j.gastro.2017.04. 042 [DOI] [PubMed] [Google Scholar]
- 17. To UK, Garcia‐Tsao G. PRO: patients with advanced cirrhosis and portal vein thrombosis should receive anticoagulation. Clin Liver Dis. 2018;12(3):74–9. 10.1002/cld.717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rössle MDM, Schultheiss M. Timing of the treatment of portal vein thrombosis in patients with cirrhosis: a German hepatologist’s perspective. J Transl Intern Med. 2018;6(1):11–5. 10.2478/jtim-2018-0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodríguez‐Castro KI, Antonello A, Ferrarese A. Spontaneous bleeding or thrombosis in cirrhosis: what should be feared the most? World J Hepatol. 2015;7(14):1818–27. 10.4254/wjh.v7.i14.1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Northup MDPG, Davis JPE. Timing of anticoagulation for portal vein thrombosis in liver cirrhosis: a US hepatologist’s perspective. J Transl Intern Med. 2018;6(1):1–5. 10.2478/jtim-2018-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rank KM, Lake J. CON: anticoagulation for portal vein thrombosis in advanced cirrhosis. Clin Liver Dis. 2018;12(3):80–2. 10.1002/cld.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riva N, Ageno W. Timing of anticoagulation for portal vein thrombosis in liver cirrhosis: an Italian internist’s perspective. J Transl Intern Med. 2018;6(1):6–10. 10.2478/jtim-2018-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lisman T, Kamphuisen PW, Northup PG, Porte RJ. Established and new‐generation antithrombotic drugs in patients with cirrhosis—possibilities and caveats. J Hepatol. 2013;59(2):358–66. https://doi.org/10.1016/j.jhep.2013.03. 027 [DOI] [PubMed] [Google Scholar]
- 24. Carneiro C, Brito J, Bilreiro C, Barros M, Bahia C, Santiago I, et al. All about portal vein: a pictorial display to anatomy, variants and physiopathology. Insights Imaging. 2019;10(1):38. 10.1186/s13244-019-0716-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karino T, Motomiya M. Flow through a venous valve and its implication for thrombus formation. Thromb Res. 1984;36(3):245–57. 10.1016/0049-3848(84)90224-x [DOI] [PubMed] [Google Scholar]
- 26. Khismatullin RR, Nagaswami C, Shakirova AZ, Vrtková A, Procházka V, Gumulec J, et al. Quantitative morphology of cerebral thrombi related to intravital contraction and clinical features of ischemic stroke. Stroke. 2020;51(12):3640–50. 10.1161/STROKEAHA.120.031559 [DOI] [PubMed] [Google Scholar]
- 27. Chernysh IN, Nagaswami C, Kosolapova S, Peshkova AD, Cuker A, Cines DB, et al. The distinctive structure and composition of arterial and venous thrombi and pulmonary emboli. Sci Rep. 2020;10(1):5112. 10.1038/s41598-020-59526-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riegger J, Byrne RA, Joner M, Chandraratne S, Gershlick AH, ten Berg JM, et al. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: a report of the prevention of late stent thrombosis by an interdisciplinary global European effort consortium. Eur Heart J. 2016;37(19):1538–49. 10.1093/eurheartj/ehv419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Di Meglio L, Desilles J‐P, Ollivier V, Nomenjanahary MS, Di Meglio S, Deschildre C, et al. Acute ischemic stroke thrombi have an outer shell that impairs fibrinolysis. Neurology. 2019;93(18):e1686–98. 10.1212/WNL.0000000000008395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zalewski J, Lewicki L, Krawczyk K, Zabczyk M, Targonski R, Molek P, et al. Polyhedral erythrocytes in intracoronary thrombus and their association with reperfusion in myocardial infarction. Clin Res Cardiol. 2019;108(8):950–62. 10.1007/s00392-019-01425-x [DOI] [PubMed] [Google Scholar]
- 31. Cines DB, Lebedeva T, Nagaswami C, Hayes V, Massefski W, Litvinov RI, et al. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood. 2014;123(10):1596–603. 10.1182/blood-2013-08-523860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peshkova A, Malyasyov D, Bredikhin R, Le Minh G, Andrianova I, Tutwiler V, et al. Reduced contraction of blood clots in venous thromboembolism is a potential thrombogenic and embologenic mechanism. TH Open. 2018;2(1):e104–15. 10.1055/s-0038-1635572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sigel B, Swami V, Can A, Parsons RE, Golub RM, Kolecki R, et al. Intimal hyperplasia producing thrombus organization in an experimental venous thrombosis model. J Vasc Surg. 1994;19(2):350–60. 10.1016/s0741-5214(94)70110-5 [DOI] [PubMed] [Google Scholar]
- 34. Rautou P‐E, Vion A‐C, Luyendyk JP, Mackman N. Circulating microparticle tissue factor activity is increased in patients with cirrhosis. Hepatology. 2014;60(5):1793–5. 10.1002/hep.27033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116(6): 878–85. 10.1182/blood-2010-02-261891 [DOI] [PubMed] [Google Scholar]
- 36. Bos S, van den Boom B, Kamphuisen P, Adelmeijer J, Blokzijl H, Schreuder T, et al. Haemostatic profiles are similar across all aetiologies of cirrhosis. Thromb Haemost. 2019;119(2):246–53. 10.1055/s-0038-1676954 [DOI] [PubMed] [Google Scholar]
- 37. Anwar MA, Shalhoub J, Lim CS, Gohel MS, Davies AH. The effect of pressure‐induced mechanical stretch on vascular wall differential gene expression. J Vasc Res. 2012;49(6):463–78. 10.1159/000339151 [DOI] [PubMed] [Google Scholar]
- 38. Jennette JC, Stone JR. Diseases of medium‐sized and small vessels. In: Willis MS, Homeister JW, Stone JR, editors. Cellular and molecular pathology of cardiovascular disease. San Diego: Academic Press; 2014. p. 197–219. 10.1016/B978-0-12-405206-2.00011-9 [DOI] [Google Scholar]
- 39. Ahanchi SS, Tsihlis ND, Kibbe MR. The role of nitric oxide in the pathophysiology of intimal hyperplasia. J Vasc Surg. 2007;45(6, Suppl):A64‐73. 10.1016/j.jvs.2007.02.027 [DOI] [PubMed] [Google Scholar]
- 40. Chen D, Ma L, Tham E‐L, Maresh S, Lechler RI, McVey JH, et al. Fibrocytes mediate intimal hyperplasia post‐vascular injury and are regulated by two tissue factor–dependent mechanisms. J Thromb Haemost. 2013;11(5):963–74. 10.1111/jth.12198 [DOI] [PubMed] [Google Scholar]
- 41. Rade JJ, Schulick AH, Virmani R, Dichek DA. Local adenoviral‐mediated expression of recombinant hirudin reduces neointima formation after arterial injury. Nat Med. 1996;2(3):293–8. 10.1038/nm0396-293 [DOI] [PubMed] [Google Scholar]
- 42. McNamara CA, Sarembock IJ, Gimple LW, Fenton JW 2nd, Coughlin SR, Owens GK. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J Clin Invest. 1993;91(1):94–8. 10.1172/JCI116206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pyo RT, Sato Y, Mackman N, Taubman MB. Mice deficient in tissue factor demonstrate attenuated intimal hyperplasia in response to vascular injury and decreased smooth muscle cell migration. Thromb Haemost. 2004;92(3):451–8. 10.1160/TH04-02-0122 [DOI] [PubMed] [Google Scholar]
- 44. Gerdes C, Faber‐Steinfeld V, Yalkinoglu O, Wohlfeil S. Comparison of the effects of the thrombin inhibitor r‐hirudin in four animal models of neointima formation after arterial injury. Arterioscler Thromb Vasc Biol. 1996;16(10):1306–11. 10.1161/01.atv.16.10.1306 [DOI] [PubMed] [Google Scholar]
- 45. Deroo S, Deatrick KB, Henke PK. The vessel wall: a forgotten player in post thrombotic syndrome. Thromb Haemost. 2010;104(4):681–92. 10.1160/TH10-03-0183 [DOI] [PubMed] [Google Scholar]
- 46. Chandrashekar A, Garry J, Gasparis A, Labropoulos N. Vein wall remodeling in patients with acute deep vein thrombosis and chronic postthrombotic changes. J Thromb Haemost. 2017;15(10):1989–93. 10.1111/jth.13793 [DOI] [PubMed] [Google Scholar]
- 47. Vymazal J, Spuentrup E, Cardenas‐Molina G, Wiethoff AJ, Hartmann MG, Caravan P, et al. Thrombus imaging with fibrin‐specific gadolinium‐based MR contrast agent EP‐2104R: results of a phase II clinical study of feasibility. Invest Radiol. 2009;44(11):697–704. 10.1097/RLI.0b013e3181b092a7 [DOI] [PubMed] [Google Scholar]
- 48. Almekhlafi MA, Hu WY, Hill MD, Auer RN. Calcification and endothelialization of thrombi in acute stroke. Ann Neurol. 2008;64(3):344–7. 10.1002/ana.21404 [DOI] [PubMed] [Google Scholar]
- 49. Tanaka K, Hirst AE, Smith LL. Rate of endothelialization in venous thrombi: an experimental study. Arch Surg. 1982;117(8):1045–8. 10.1001/archsurg.1982.01380320033009 [DOI] [PubMed] [Google Scholar]
- 50. Heise M, Krüger U, Rückert R, Pfitzman R, Neuhaus P, Settmacher U. Correlation of intimal hyperplasia development and shear stress distribution at the distal end‐side‐anastomosis, in vitro study using particle image velocimetry. Eur J Vasc Endovasc Surg. 2003;26(4):357–66. 10.1016/s1078-5884(02)00567-1 [DOI] [PubMed] [Google Scholar]
- 51. Gnasso A, Carallo C, Irace C, Spagnuolo V, De Novara G, Mattioli PL, et al. Association between intima‐media thickness and wall shear stress in common carotid arteries in healthy male subjects. Circulation. 1996;94(12):3257–62. 10.1161/01.CIR.94.12.3257 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


