Abstract
Background
The incidence of tularemia has recently increased throughout Europe. Pediatric tularemia typically presents with ulceroglandular or glandular disease and requires antimicrobial therapy not used in the empirical management of childhood acute lymphadenitis. We describe the clinical presentation and course in a case series comprising 20 patients.
Methods
This is a retrospective analysis of a single-center case series of microbiologically confirmed tularemia in patients <16 years of age diagnosed between 2010 and 2021.
Results
Nineteen patients (95%) presented with ulceroglandular (n = 14) or glandular disease (n = 5), respectively. A characteristic entry site lesion (eschar) was present in 14 (74%). Fever was present at illness onset in 15 patients (75%) and disappeared in all patients before targeted therapy was initiated. The diagnosis was confirmed by serology in 18 patients (90%). While immunochromatography was positive as early as on day 7, a microagglutination test titer 1:≥160 was found no earlier than on day 13. Sixteen patients (80%) were initially treated with an antimicrobial agent ineffective against F. tularensis. The median delay (range) from illness onset to initiation of targeted therapy was 12 (6–40) days. Surgical incision and drainage were ultimately performed in 12 patients (60%).
Conclusions
Pediatric tularemia in Switzerland usually presents with early, self-limiting fever and a characteristic entry site lesion with regional lymphadenopathy draining the scalp or legs. Particularly in association with a tick exposure history, this presentation may allow early first-line therapy with an agent specifically targeting F. tularensis, potentially obviating the need for surgical therapy.
Keywords: tularemia, Francisella tularensis, child, pediatrics, ulceroglandular, glandular, case series
Human tularemia, an emerging infection in Europe [1–6] caused by Francisella tularensis, is a highly virulent zoonotic disease with sources of human infection in both wild and domestic animals, such as small rodents, lagomorphs, ticks and mosquitos, and in bodies of water [7–12]. In Central and Northern Europe, the organism is transmitted to humans predominantly through a hematophagous arthropod bite (ticks in Central Europe, mosquitos in Northern Europe), whereas reports from Eastern Europe, including Turkey, describe numerous outbreaks linked to the ingestion of contaminated water [13–17]. Inoculation may also occur through the skin, ingestion of undercooked meat, or inhalation of aerosols [18].
Two subspecies of F. tularensis are responsible for human disease [19]. F. tularensis subspecies tularensis (type A) is mainly reported in North America and often causes an invasive and aggressive clinical course. F. tularensis subspecies holarctica (type B) causes tularemia in Europe and throughout the Northern hemisphere [20].
Clinical disease in humans varies according to the mode of acquisition of F. tularensis and is described as ulceroglandular, glandular, oropharyngeal, oculoglandular, typhoidal, or pneumonic tularemia [18, 20, 21]. In Switzerland, ulceroglandular and glandular tularemia account for the majority of reported cases in all age groups, with pulmonary and abdominal disease being reported only in 20% and 5%, respectively [22]. Early systemic signs of the disease are unrelated to the inoculation site and consist of influenza-like symptoms (eg, fever, fatigue, headache, and rash) [18, 23].
Reports on pediatric tularemia in the English language literature originate mainly from North America, where type A is prevalent, from Sweden and Finland, where type B causes mosquito-borne infections [7, 24–28], and from Eastern Europe and Norway, where oropharyngeal disease from contaminated water of food is prevalent [13–16, 29]. Reports on pediatric disease in Western-Central Europe, however, are scant and limited to case reports [30–38]. No case series are available. Based on case reports and expert opinion [18, 39], Western-Central European tularemia is primarily considered a tick-borne disease, but may also result from contact with an infected animal, with the ulceroglandular or glandular form being by far the most common clinical presentation.
The purpose of this report is to provide a precise clinical description of a case series of pediatric tularemia from a single center in Switzerland.
METHODS
This is a retrospective single-center case series of patients <16 years of age diagnosed with tularemia at the University Children’s Hospital, Bern University Hospital, Switzerland, between January 1, 2010, and December 31, 2021. Case finding was performed by searching the electronic clinical microbiology database (Institute of Infectious Diseases, University of Bern, Switzerland) for cases testing positive for F. tularensis by serology, by culture, or by a positive polymerase chain reaction (PCR) test amplifying a 270-bp fopA gene fragment from a biological specimen (ie, swab from ulcer surface or surgically obtained lymph node material) [40].
Serology was defined as positive with a single microagglutination test (MAT) titer of ≥160 according to World Health Organization guidelines [41, 42] or with a >4-fold MAT titer increase in paired sera. In addition, an immunochromatographic rapid antibody test (ICT) using the Virapid Tularemia assay (Vircell, Granada, Spain) was performed in all cases occurring from 2016 onwards. The results were determined visually and considered positive with semiquantitative readings of 0.5 (“weakly positive”) or 1.0–3.0 (“positive”), respectively, according to the manufacturer’s instructions.
We used the term “ulceroglandular” tularemia when acute lymphadenopathy was accompanied by an entry site lesion (ulcer, eschar) in the drainage area of the affected lymph nodes. In the absence of such skin lesions, we used the term “glandular” tularemia.
The clinical data of each patient were extracted from the electronic medical record system and included the variables listed in Table 1. For the presentation of the clinical course and the timing of both diagnostic and therapeutic interventions, day 1 of symptoms, that is, fever and/or lymph node swelling, was used as anchor point, and the time elapsed since day 1 was calculated for each subsequent event of interest (median, range). Narrative chart information about time intervals was converted, if needed, to numeric data; that is, the terms “several days,” “week,” and “month” were converted to 3, 7, and 30 days, respectively.
Table 1.
Demographic and Clinical Characteristics of 20 Pediatric Patients With Ulceroglandular or Glandular Tularemia
| Characteristic | Finding |
|---|---|
| Female gender, No. (%) | 8 (40) |
| Median age [range], y | 9.0 [1.1–13.4] |
| Fever at illness onset | |
| No. (%) | 15 (75) |
| Median duration [range], d | 5 [1–14] |
| Rash at illness onset, No. (%) | 2 (10) |
| Tick exposure reported, No. (%) | 10 (50) |
| Ulcer/eschar at entry site, No. (%) | 14 (70)a |
| Lymph node region involved, No. (%) | |
| Cervical | 10 (50)b |
| Axillary | 2 (10)c |
| Inguinal | 8 (40) |
| Lymph node ultrasonography performed, No. (%) | 19 (95) |
| Median C-reactive protein [range], mg/L (n = 18) | 15 [1–100] |
| Median erythrocyte sedimentation rate [range], mm/h (n = 9) | 25 [11–66] |
| Microbiology—tularemia confirmed by, No. (%) | |
| Serology (n = 20) | 18 (90) |
| First ICT performed was positive (n = 18) | 13 (72)d |
| First MAT performed was positive (n = 20) | 8 (58)d |
| Culture (n = 8) | 3 (38) |
| PCR (n = 7) | 7 (100) |
| Therapy | |
| Empiric initial therapy with amoxicillin-clavulanate,e No. (%) | 16 (80) |
| Targeted antimicrobial therapy, No. (%) | |
| Ciprofloxacin | 15 (79) |
| Doxycycline | 5 (20) |
| Median duration of targeted therapy [range], d | 16 [10–28] |
| Surgical incision and drainage, No. (%) | 12 [60] |
| Hospitalization required, No. (%) | 16 [80] |
| Median duration of hospital stay [range], d | 2 [1–7] |
| Duration of follow-up [range], d | 39 [12–167] |
Abbreviations: ICT, immunochromatography; MAT, microagglutination test; PCR, polymerase chain reaction.
Includes 13 patients with tick-borne disease and 1 patient with a mouse bite.
Includes 9 patients with tick-borne disease and 1 patient with oropharyngeal disease.
Includes 1 patient each with tick-borne disease and with a mouse bite.
P = .328 (Fisher exact test, 2-sided).
One patient was treated with amoxicillin only.
For a standardized description of involved neck lymph nodes, we used the updated neck dissection classification of the American Head and Neck Society and the American Academy of Otolaryngology [43]. The narrative description of involved lymph node levels in the medical records, photographs, and ultrasound findings were used to anatomically locate the affected lymph node levels in each patient.
For targeted antimicrobial therapy, oral or intravenous ciprofloxacin (10 mg/kg/dose every 12 hours or twice daily [BID], top dose 750 mg BID) or oral or intravenous doxycycline (2 mg/kg/dose every 12 hours or BID, top dose 100 mg BID) was prescribed.
For descriptive statistics and nonparametric statistical tests of ordinal data, we used GraphPad Prism, version 9.0.0 for Windows (GraphPad Software, San Diego, CA, USA).
RESULTS
Epidemiology and Demography
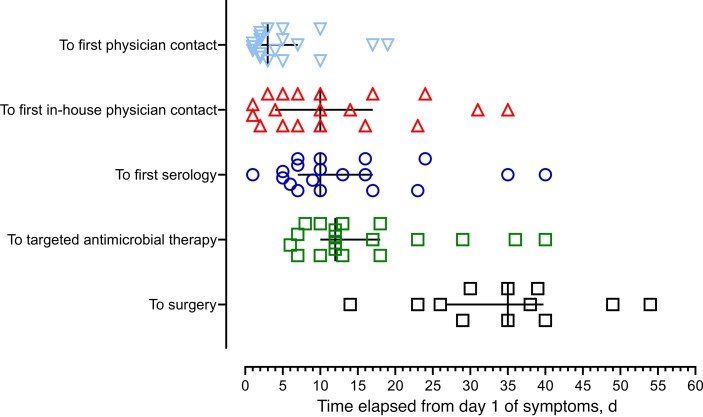
The temporal occurrence of study cases between 2010 and 2021 in relation to the total number of human tularemia cases reported to the Swiss Federal Office of Public Health [1] is shown in Supplementary Figure 1. It illustrates that the temporal occurrence of cases in this series parallels the increase of cases reported throughout the country in all age groups. The seasonal distribution of cases ranged from February to October, with 11 cases (55%) occurring between May 1 and July 31 (Supplementary Figure 2). There was no local clustering of cases. Clinical and demographic data describing the 20 patients are listed in Table 1. The time that elapsed from day 1 of symptoms attributed to tularemia to both diagnostic and therapeutic events of interest is shown in Figure 1.
Figure 1.
Intervals from day 1 of symptoms to events described on the vertical axis in 19 patients with tularemia. One patient of the case series was omitted because of late presentation and oropharyngeal disease acquired abroad. The black lines mark the median time delay and interquartile range of each event.
Clinical Manifestations
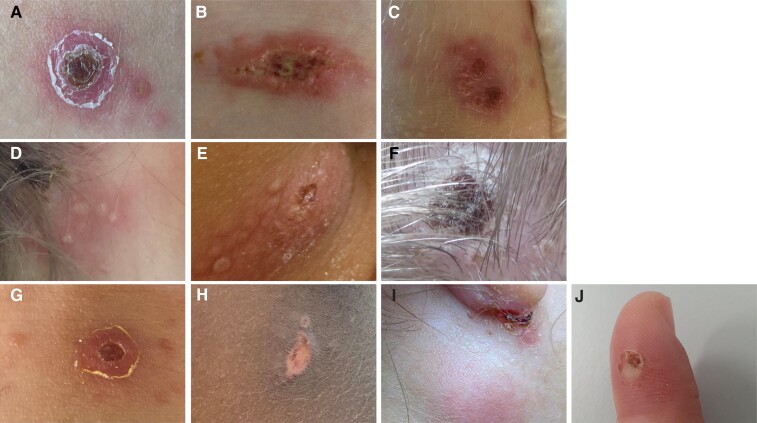
Nineteen patients, all residing in Switzerland, presented with unilateral ulceroglandular (n = 14) or glandular disease (n = 5). One additional patient, who acquired tularemia together with his father during a stay in Eastern Europe in 2010, was considered to have oropharyngeal disease. This case was excluded for the analyses in Figure 1 and other time-related analyses because of his delayed presentation 3 months after illness onset. Among the remainder, 1 patient with axillary lymphadenopathy reported a wood mouse bite to the ipsilateral index finger (Figure 2J). All other patients (n = 18) presumably had tick-borne disease, although another means of transmission, for example, direct contact with an infected mammal, cannot be excluded. Of these, 10 patients (56%) or their proxy reported a tick bite whose location was compatible with the entry site lesion or the affected lymph nodes, respectively. A cutaneous entry site lesion in the drainage area of the affected lymph nodes was clinically evident in 13 patients (72%). Figure 2 depicts the lesions of 9 patients, of whom photographs were available. With the possible exception of 2 cases (Figure 2H, I), all entry site lesions showed multiple distinct vesicles, ulcers, or eschars. Affected lymph nodes were located on the head and neck (9 patients, 6 with an entry site lesion), inguinal (8, 6), and axillary areas (1, 1), respectively. Supplementary Figure 3 illustrates the head and neck distribution of affected nodes in 9 patients. Except for 1 case (Supplementary Figure 2, dark red square), all affected lymph node locations were compatible with an entry site on the scalp. The involved areas were uniformly tender to touch, with or without erythema of the overlying skin (eg, Figure 2I), and firm to palpation. In all but 1 case, clinical and sonographic findings revealed multiple partially conglomerated lymph node enlargements. Systemic symptoms included fever during the first days of illness in 15 of 20 patients (75%), which resolved in all patients before the initiation of targeted antimicrobial therapy against F. tularensis. A generalized scarlatiniform rash occurred in 2 patients. Conjunctival manifestations were not observed. Blood markers of inflammation determined at the time of the first in-house presentation are listed in Table 1 and Supplementary Figure 4.
Figure 2.
Photographs of the inoculation sites of 10 patients with ulceroglandular tularemia. Panels (C), (D), and (E) show clustered vesicles, pustules, and ulcers; in panels (A), (B), (F), (G), and (H), multiple distinct lesions surrounding the major ulcer or eschar can be seen. Panel (J) depicts the site of a wood mouse bite, from which F. tularensis was cultivated.
Microbiology
Of 20 patients, 18 were found to have a positive MAT (reciprocal titer range, 160–5120). In 2 patients, tularemia was exclusively diagnosed by culturing F. tularensis from a skin entry site. Serologic findings are detailed in Table 1 and Supplementary Figure 4. The median time elapsed from day 1 of symptoms to first serology (range) was 10 (1–40) days (Figure 1). The first patient contact at our institution generally resulted in swift serologic testing (median [range], 0 [0–11] days). Whereas a significant MAT titer was found no earlier than on day 13, the ICT was positive as early as on day 7 (Table 1; Supplementary Figure 4). PCR of surgically removed lymph node material was positive for F. tularensis DNA in all 7 cases investigated, while the attempt to cultivate a causative agent was positive in none of 5 lymph node samples. However, F. tularensis was cultivated from all 3 entry site lesions swabbed. One isolate from a patient, who clinically failed ciprofloxacin therapy despite early initiation on day 6, was characterized further, identified as F. tularensis subspecies holarctica, and found to be susceptible to all antimicrobials tested (Supplementary Table 1).
Pathology
Histopathologic examination was performed on excised lymph node material in 3 cases 15%). It revealed areas of necrosis, acute suppurative abscess formation, and granulomatous inflammation with epitheloid cell reaction in 2 patients and nonspecific necrosis in 1 patient.
Treatment
Sixteen patients (80%) received a beta-lactam agent (amoxicillin-clavulanate in 15, amoxicillin in 1 patient) as empiric first-line treatment for acute lymphadenitis. Antibiotic therapy specifically targeting F. tularensis with ciprofloxacin (n = 15) or doxycycline (n = 5), respectively, was used exclusively as a secondary option because of clinical failure. The median duration of therapy (range) was 16 (10–28) days, and therapy was initiated with a median delay of 12 days after onset of symptoms (figure 1). The median delay to targeted therapy (range) was not significantly longer in patients ultimately requiring surgical incision and drainage (15 [6–40] vs 12 [8–18] days; P = .472). Surgery was performed at a median (range) of 13 (−1 to 43) days after starting targeted therapy.
Outcome
Sixteen patients (80%) required an in-patient stay at some time during their illness, with a median duration (range) of 2 (1–7) days. The last outpatient follow-up was recorded at a median (range) of 39 (12–167) days after day 1. All 12 patients (60%) who received surgical incision and drainage of mostly purulent material (n = 10) from affected lymph nodes had uneventful wound healing without evidence of prolonged drainage site fistulation. In 1 patient, surgery resulted in hypertrophic scar tissue on the neck. No patient required additional antimicrobial therapy after the first targeted course with ciprofloxacin or doxycycline. No secondary surgical intervention was necessary.
DISCUSSION
To our knowledge, this is the first report describing a clinical case series of pediatric tularemia from a Western-Central European country [1, 6, 44]. All cases presented with ulceroglandular or glandular disease, which is in line with previous case descriptions [30–38]. The fact that we found no other organs involved may reflect either a lack of clinical awareness of pulmonary or abdominal disease in children or truly infrequent transmission of F. tularensis via the respiratory or intestinal routes in Switzerland. Pulmonary and abdominal disease in Swiss adults does occur but is uncommon [22]. Causes for the rapidly increasing case counts in recent years may include enhanced awareness, improved performance of diagnostic techniques, changes in leisure behavior, and a truly expanding animal or environmental reservoir [39]. The proportion of all cases of pediatric acute lymphadenitis that are caused by F. tularensis is therefore likely to continue to increase in the near future, which calls for a reappraisal of the empirical management of this condition. It currently consists—as exemplified in this study with 80% of patients (Table 1)—of antibiotic first-line therapy with a beta-lactam agent or clindamycin [45, 46], which are clinically ineffective against F. tularensis [47] and contribute to therapeutic delays. In our series, we found a high rate of surgical incision and drainage needed for controlling lymphadenitis in the purulent stage (60%). For comparison, in case series of pediatric oropharyngeal disease, rates of suppuration requiring surgical intervention were 47%–58% [11, 13, 48]. This generally unsatisfactory outcome is likely related to delayed diagnosis and therapy [41, 48–50] and may be improved by accelerating early management. Currently, the diagnostic standard in cases of suspected tularemia consists of the demonstration of a serum antibody titer determined by an MAT of 1:≥160 or a 4-fold titer increase in paired sera [42, 51], or by a positive PCR or culture of an entry site drainage (eschar) or tissue biopsy specimen. Serology of acute and convalescent blood samples remains the diagnostic mainstay because culture is rarely positive and PCR is often not routinely available [18]. Because serologic confirmation is often delayed to the second or third week of illness, clinical suspicion of tularemia should lead to early antimicrobial therapy with ciprofloxacin or, alternatively, doxycycline. Beta-lactams and macrolides are ineffective [18]. Intravenous therapy with gentamicin is highly effective, but not commonly prescribed for the generally milder infections with subspecies holarctica.
Assuming that early diagnosis and therapy with an agent active against F. tularensis hastens the resolution of symptoms and signs [28] and potentially obviates the need for surgery, including anesthesia and subsequent wound treatment, the analysis of this case series provides several insights into how the diagnostic process of tick-borne tularemia could be expedited in pediatric primary care.
First, we found that influenza-like early systemic symptoms invariably resolved during the presumably ineffective therapy with amoxicillin-clavulanate. This may have been mistaken for effectiveness, while in fact representing the spontaneous evolution of pediatric tularemia, and thereby contributed to diagnostic delays. The immunologic events leading to the spontaneous resolution of systemic manifestations are likely linked to the adaptive T-cell cellular immune response [52, 53] confining the inflammatory process to the regional lymph nodes and preventing lymphohematogenous dissemination, which appears to be exquisitely rare in children infected with F. tularensis subspecies holarctica. Second, as with other childhood infections transmitted by Ixodes ricinus, the entry site is often located in the hairy scalp [54] and is thus easily overlooked in a cursory physical examination. We found involvement of the lymph node levels 2b, 3, 4, and 5 [43], as well as the posterior–auricular and nuchal nodes, in 8 of 9 patients with tick-borne disease and head and neck involvement (Supplementary Figure 3). These lymph nodes drain the scalp and call for a particularly diligent search for a scalp lesion. Only 1 patient had exclusively level 2a involvement, which does not allow for differentiation between the scalp and oropharynx as the primary infection site. Third, the morphology of the entry site lesion may be suggestive of tularemia. Our findings (Figure 2) concur with Byington et al. [55], who described the inoculation site as “herpes-like” with clustered vesicles or crusts. This may be particularly true in early stages, when lesions have not yet coalesced to larger ulcers or eschars. To our knowledge, however, the specificity of this finding in predicting tularemia as opposed to other etiologies has not been studied to date. Fourth, blood inflammatory markers, in particular C-reactive protein (CRP), were mostly low (Supplementary Figure 4D). This finding differs from what has been reported in adult patients with oropharyngeal disease [56]. Other investigators found that CRP values in pulmonary disease were higher than in ulceroglandular disease in adults and peaked in the first week of illness [57]. Our findings suggest that the low CRP values, which we mostly observed, are related to the advanced stage of disease when determined and may not reflect a specific characteristic of pediatric tularemia (Supplementary Figure 4E). Fifth, we found that serologic testing using an ICT yielded positive results earlier than a significant MAT titer ≥160 (Supplementary Figure 4C), which is in line with what Kilic et al. reported in a large sample of patients with oropharyngeal disease from Turkey [58]. Although the specificity of ICT (84%–94%) may be lower than that of MAT (>98%) [51], it appears to be a useful screening test and offers a very short turnaround time.
Based off of in vitro activity and clinical experience, ciprofloxacin and doxycycline are considered the antibiotics of choice for oral therapy and are generally preferred over intravenous gentamicin [47]. Johansson et al. reported that early initiation of oral ciprofloxacin within a few days after disease onset resulted in rapid cure without lymph node suppuration in 12 children below 10 years of age in Sweden [28]. In a case series of 100 children with oropharyngeal tularemia in Turkey, Tezer et al. [48] found that doxycycline was associated with more frequent need for an eventual surgical procedure than ciprofloxacin or aminoglycosides, but the finding was not statistically significant. In contrast, Oz et al., who studied 55 children with oropharyngeal tularemia, failed to identify antibiotic-related differences in outcome [50]. In vitro resistance to ciprofloxacin or doxycycline has not been reported to date, which is in line with the test results of 1 isolate investigated in this study. We did not find a differential treatment response (data not shown), but the numbers were too small and treatment delays were too long to allow for a meaningful analysis. In general, the available data emphasize the lack of high-quality, controlled treatment studies in pediatric tularemia caused by F. tularensis subspecies holarctica. Considering the rapid emergence of this disease, such trials are needed and will likely require a multicenter design. Further, studies investigating the prevalence of F. tularensis in vectors in various geographic areas are needed for an up-to-date risk assessment of outdoor leisure activities.
CONCLUSIONS
Early diagnosis of pediatric tularemia requires a high index of suspicion and should include the active search for a “herpes-like” entry site lesion on clinical examination including a careful scan of the hairy scalp. Detailed knowledge of head and neck lymph node drainage areas facilitates entry site identification. The presence of an entry site lesion and anatomically corresponding lymph node disease may justify first-line therapy with ciprofloxacin or doxycycline, with or without a history of recent tick exposure. A low threshold for serologic testing, for example, using a serum rapid antibody screening test, yields presumptive diagnostic confirmation within a few hours if positive and, if negative, requires retesting in the second week of illness. This approach is likely to shorten the diagnostic delay and may obviate hospitalization and surgery in a substantial proportion of patients.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We thank the physician staff of the Division of Pediatric Radiology at our institution for their diagnostic contributions for each patient.
Contributor Information
Nina Schöbi, Division of Pediatric Infectious Disease, Department of Pediatrics, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland.
Philipp K A Agyeman, Division of Pediatric Infectious Disease, Department of Pediatrics, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland.
Andrea Duppenthaler, Division of Pediatric Infectious Disease, Department of Pediatrics, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland.
Andreas Bartenstein, Department of Pediatric Surgery, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland.
Peter M Keller, Institute for Infectious Diseases, University of Bern, Bern, Switzerland.
Franziska Suter-Riniker, Institute for Infectious Diseases, University of Bern, Bern, Switzerland.
Kristina M Schmidt, Spiez Laboratory, Federal Office for Civil Protection and Swiss National Reference Center for Francisella tularensis (NANT), Spiez, Switzerland.
Matthias V Kopp, Division of Pediatric Infectious Disease, Department of Pediatrics, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland; 17 Center North (ARCN), Member of the German Lung Research Center (DZL), 18 University of Luebeck, Luebeck, Germany.
Christoph Aebi, Division of Pediatric Infectious Disease, Department of Pediatrics, Bern University Hospital, Inselspital, University of Bern, Bern, Switzerland.
Financial support. None.
Potential conflicts of interest. All authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. N.S. and C.A. drafted the manuscript. N.S., A.D., P.K.A.A., A.B., M.V.K., and C.A. were responsible for patient care. P.M.K., F.S., and K.M.S. were responsible for microbiological analyses. All authors made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Patient consent. The study has been approved by the Cantonal Ethics Committee (project No. 2022–00042). General or project-specific individual written informed consent was given by all patients and/or their parents.
References
- 1. Federal Office of Public Health Switzerland . Tularämie. Available at: https://www.bag.admin.ch/bag/de/home/krankheiten/krankheiten-im-ueberblick/tularaemie.html. Accessed 15 March 2022.
- 2. Appelt S, Faber M, Koppen K, Jacob D, Grunow R, Heuner K. Francisella tularensis subspecies holarctica and tularemia in Germany. Microorganisms 2020; 8:1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seiwald S, Simeon A, Hofer E, Weiss G, Bellmann-Weiler R. Tularemia goes West: epidemiology of an emerging infection in Austria. Microorganisms 2020; 8:1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mailles A, Vaillant V. Bilan de 10 années de surveillance de la tularémie chez l'Homme en France. Available at: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-transmissibles-de-l-animal-a-l-homme/tularemie/documents/rapport-synthese/bilan-de-10-annees-de-surveillance-de-la-tularemie-chez-l-homme-en-france. Accessed 15 March 2022.
- 5. Janse I, van der Plaats RQJ, de Roda Husman AM, van Passel MWJ. Environmental surveillance of zoonotic Francisella tularensis in the Netherlands. Front Cell Infect Microbiol 2018; 8:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ECDC . Tularaemia - annual epidemiological report for 2019. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/AER-tularaemia-2019.pdf. Accessed 15 March 2022.
- 7. Uhari M, Syrjala H, Salminen A. Tularemia in children caused by Francisella tularensis biovar palaearctica. Pediatr Infect Dis J 1990; 9:80–3. [DOI] [PubMed] [Google Scholar]
- 8. Lyko C, Chuard C. Tularemia, an emerging disease in Switzerland [in French]. Rev Med Suisse 2013; 9:1816–8. [PubMed] [Google Scholar]
- 9. Ellis J, Oyston PC, Green M, Titball RW. Tularemia. Clin Microbiol Rev 2002; 15:631–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oyston PCF. Francisella tularensis: unravelling the secrets of an intracellular pathogen. J Med Microbiol 2008; 57:921–30. [DOI] [PubMed] [Google Scholar]
- 11. Berdal BP, Mehl R, Meidell NK, Lorentzen-Styr AM, Scheel O. Field investigations of tularemia in Norway. FEMS Immunol Med Microbiol 1996; 13:191–5. [DOI] [PubMed] [Google Scholar]
- 12. Jenzora A, Jansen A, Ranisch H, Lierz M, Wichmann O, Grunow R. Seroprevalence study of Francisella tularensis among hunters in Germany. FEMS Immunol Med Microbiol 2008; 53:183–9. [DOI] [PubMed] [Google Scholar]
- 13. Karli A, Sensoy G, Paksu S, Korkmaz MF, Ertugrul O, Karli R. Treatment-failure tularemia in children. Korean J Pediatr 2018; 61:49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ulu-Kilic A, Gulen G, Sezen F, Kilic S, Sencan I. Tularemia in central Anatolia. Infection 2013; 41:391–9. [DOI] [PubMed] [Google Scholar]
- 15. Gozel MG, Engin A, Altuntas EE, et al. . Evaluation of clinical and laboratory findings of pediatric and adult patients with oropharyngeal tularemia in Turkey: a combination of surgical drainage and antibiotic therapy increases treatment success. Jpn J Infect Dis 2014; 67:295–9. [DOI] [PubMed] [Google Scholar]
- 16. Celebi S, Hacimustafaoglu M, Gedikoglu S. Tularemia in children. Indian J Pediatr 2008; 75:1129–32. [DOI] [PubMed] [Google Scholar]
- 17. Hennebique A, Boisset S, Maurin M. Tularemia as a waterborne disease: a review. Emerg Microbes Infect 2019; 8:1027–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maurin M, Gyuranecz M. Tularaemia: clinical aspects in Europe. Lancet Infect Dis 2016; 16:113–24. [DOI] [PubMed] [Google Scholar]
- 19. Petersen JM, Molins CR. Subpopulations of Francisella tularensis ssp. tularensis and holarctica: identification and associated epidemiology. Future Microbiol 2010; 5:649–61. [DOI] [PubMed] [Google Scholar]
- 20. Sjostedt A. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann N Y Acad Sci 2007; 1105:1–29. [DOI] [PubMed] [Google Scholar]
- 21. Frischknecht M, Meier A, Mani B, et al. . Tularemia: an experience of 13 cases including a rare myocarditis in a referral center in Eastern Switzerland (Central Europe) and a review of the literature. Infection 2019; 47:683–95. [DOI] [PubMed] [Google Scholar]
- 22. Federal Office of Public Health Switzerland . Tularämie: eine seltene zeckenübertragene krankheit breitet sich aus. Bulletin BAG 2018; 18:13–8. [Google Scholar]
- 23. Eliasson H, Broman T, Forsman M, Back E. Tularemia: current epidemiology and disease management. Infect Dis Clin North Am 2006; 20:289–311. [DOI] [PubMed] [Google Scholar]
- 24. Desvars A, Furberg M, Hjertqvist M, et al. . Epidemiology and ecology of tularemia in Sweden, 1984–2012. Emerg Infect Dis 2015; 21:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rossow H, Ollgren J, Klemets P, et al. . Risk factors for pneumonic and ulceroglandular tularaemia in Finland: a population-based case-control study. Epidemiol Infect 2014; 142:2207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rydén P, Björk R, Schäfer ML, et al. . Outbreaks of tularemia in a boreal forest region depends on mosquito prevalence. J Infect Dis 2012; 205:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jounio U, Renko M, Uhari M. An outbreak of holarctica-type tularemia in pediatric patients. Pediatr Infect Dis J 2010; 29:160–2. [DOI] [PubMed] [Google Scholar]
- 28. Johansson A, Berglund L, Gothefors L, Sjostedt A, Tarnvik A. Ciprofloxacin for treatment of tularemia in children. Pediatr Infect Dis J 2000; 19:449–53. [DOI] [PubMed] [Google Scholar]
- 29. Larssen KW, Bergh K, Heier BT, Vold L, Afset JE. All-time high tularaemia incidence in Norway in 2011: report from the national surveillance. Eur J Clin Microbiol Infect Dis 2014; 33:1919–26. [DOI] [PubMed] [Google Scholar]
- 30. Cognard J, Falque L, Zimmermann B, Pietrement C. Tularemia: a rare cause of pediatric lymph nodes adenitis. Arch Pediatr 2021; 28:580–2. [DOI] [PubMed] [Google Scholar]
- 31. Miacz K, Sledz J, Karwacki MW. Unique does not mean impossible: infant presenting with complicated course of ulceroglandular tularemia. Oxf Med Case Reports. 2021; 2021(9):omab086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rastawicki W, Chmielewski T, Łasecka-Zadrożna J. Kinetics of immune response to Francisella tularensis and Borrelia burgdorferi in a 10-year-old girl with oculoglandular form of tularemia after a tick bite: a case report. J Vector Borne Dis 2020; 57:189–92. [DOI] [PubMed] [Google Scholar]
- 33. Deak C, Relly C. Tularämie auf dem vormarch. Available at: https://www.paediatrieschweiz.ch/tularamie-auf-dem-vormarsch/. Accessed 15 March 2022.
- 34. Wetzstein N, Karcher I, Kupper-Tetzel CP, et al. . Clinical characteristics in a sentinel case as well as in a cluster of tularemia patients associated with grape harvest. Int J Infect Dis 2019; 84:116–20. [DOI] [PubMed] [Google Scholar]
- 35. Hanke CA, Otten JE, Berner R, Serr A, Splettstoesser W, von Schnakenburg C. Ulceroglandular tularemia in a toddler in Germany after a mosquito bite. Eur J Pediatr 2009; 168:937–40. [DOI] [PubMed] [Google Scholar]
- 36. Bloch C, Friedl A, Zucol F, Widmer A, Khanna N. Fever and lymphadenopathy. report of 4 cases of tularemia [in German]. Internist (Berl) 2013; 54:491–7. [DOI] [PubMed] [Google Scholar]
- 37. Passiouk N, Heininger U. Ulceroglandular tularemia following contact with a boar. Pediatr Infect Dis J 2016; 35:453–5. [DOI] [PubMed] [Google Scholar]
- 38. Buettcher M, Imbimbo C. Ulceroglandular tularemia. N Engl J Med 2021; 384:1349. [DOI] [PubMed] [Google Scholar]
- 39. Imbimbo C, Karrer U, Wittwer M, Buettcher M. Tularemia in children and adolescents. Pediatr Infect Dis J 2020; 39:e435–8. [DOI] [PubMed] [Google Scholar]
- 40. Wittwer M, Altpeter E, Pilo P, et al. . Population genomics of Francisella tularensis subsp. holarctica and its implication on the eco-epidemiology of tularemia in Switzerland. Front Cell Infect Microbiol 2018; 8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maurin M, Pelloux I, Brion JP, Del Bano JN, Picard A. Human tularemia in France, 2006–2010. Clin Infect Dis 2011; 53:e133–41. [DOI] [PubMed] [Google Scholar]
- 42. WHO WHO Guidelines on Tularaemia. World Heath Organization; 2007. [Google Scholar]
- 43. Robbins KT, Clayman G, Levine PA, et al. . Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg 2002; 128:751–8. [DOI] [PubMed] [Google Scholar]
- 44. Federal Office of Public Health Switzerland . Tularämie: eine seltene zeckenübertragene krankheit breitet sich aus. BAG Bull 2018; 18:19–28. [Google Scholar]
- 45. Royal College of Paediatrics and Child Health . Manual of Childhood Infections: The Blue Book. 4th ed. Oxford University Press; 2016. [Google Scholar]
- 46. DGPI . DGPI Handbuch. 7th ed. Georg Thieme Verlag; 2018.
- 47. Caspar Y, Maurin M. Francisella tularensis susceptibility to antibiotics: a comprehensive review of the data obtained in vitro and in animal models. Front Cell Infect Microbiol 2017; 7:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tezer H, Ozkaya-Parlakay A, Aykan H, et al. . Tularemia in children, Turkey, September 2009-November 2012. Emerg Infect Dis 2015; 21:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kaya A, Uysal IO, Guven AS, et al. . Treatment failure of gentamicin in pediatric patients with oropharyngeal tularemia. Med Sci Monit 2011; 17:CR376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oz F, Eksioglu A, Tanir G, Bayhan G, Metin O, Teke TA. Evaluation of clinical and sonographic features in 55 children with tularemia. Vector Borne Zoonotic Dis 2014; 14:571–5. [DOI] [PubMed] [Google Scholar]
- 51. Maurin M. Francisella tularensis. Tularemia and serological diagnosis. Front Cell Infect Microbiol 2020; 10:512090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Elkins KL, Cowley SC, Bosio CM. Innate and adaptive immunity to Francisella. Ann N Y Acad Sci 2007; 1105:284–324. [DOI] [PubMed] [Google Scholar]
- 53. Pechous RD, McCarthy TR, Zahrt TC. Working toward the future: insights into Francisella tularensis pathogenesis and vaccine development. Microbiol Mol Biol Rev 2009; 73:684–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cull B, Pietzsch ME, Gillingham EL, McGinley L, Medlock JM, Hansford KM. Seasonality and anatomical location of human tick bites in the United Kingdom. Zoonoses Public Health 2020; 67:112–21. [DOI] [PubMed] [Google Scholar]
- 55. Byington CL, Bender JM, Ampofo K, et al. . Tularemia with vesicular skin lesions may be mistaken for infection with herpes viruses. Clin Infect Dis 2008; 47:e4–6. [DOI] [PubMed] [Google Scholar]
- 56. Karlidag T, Keles E, Kaygusuz I, Yuksel K, Yalcin S. Tularemia: a rare cause of neck mass. Turk Arch Otorhinolaryngol 2015; 53:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Syrjala H. Peripheral blood leukocyte counts, erythrocyte sedimentation rate and C-reactive protein in tularemia caused by the type B strain of Francisella tularensis. Infection 1986; 14:51–4. [DOI] [PubMed] [Google Scholar]
- 58. Kilic S, Celebi B, Yesilyurt M. Evaluation of a commercial immunochromatographic assay for the serologic diagnosis of tularemia. Diagn Microbiol Infect Dis 2012; 74:1–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.