Abstract
OBJECTIVES:
The tuberculin skin test (TST) has been preferred for screening young children for latent tuberculosis infection (LTBI) because of concerns that interferon-γ release assays (IGRAs) may be less sensitive in this high-risk population. In this study, we compared the predictive value of IGRAs to the TST for progression to tuberculosis disease in children, including those <5 years old.
METHODS:
Children <15 years old at risk for LTBI or progression to disease were tested with TST, QuantiFERON-TB Gold In-Tube test (QFT-GIT), and T-SPOT.TB test (T-SPOT) and followed actively for 2 years, then with registry matches, to identify incident disease.
RESULTS:
Of 3593 children enrolled September 2012 to April 2016, 92% were born outside the United States; 25% were <5 years old. Four children developed tuberculosis over a median 4.3 years of follow-up. Sensitivities for progression to disease for TST and IGRAs were low (50%–75%), with wide confidence intervals (CIs). Specificities for TST, QFT-GIT, and T-SPOT were 73.4% (95% CI: 71.9–74.8), 90.1% (95% CI: 89.1–91.1), and 92.9% (95% CI: 92.0–93.7), respectively. Positive and negative predictive values for TST, QFT-GIT, and T-SPOT were 0.2 (95% CI: 0.1–0.8), 0.9 (95% CI: 0.3–2.5), and 0.8 (95% CI: 0.2–2.9) and 99.9 (95% CI: 99.7–100), 100 (95% CI: 99.8–100), and 99.9 (95% CI: 99.8–100), respectively. Of 533 children with TST-positive, IGRA-negative results not treated for LTBI, including 54 children <2 years old, none developed disease.
CONCLUSIONS:
Although both types of tests poorly predict disease progression, IGRAs are no less predictive than the TST and offer high specificity and negative predictive values. Results from this study support the use of IGRAs for children, especially those who are not born in the United States.
Reactivation of latent tuberculosis infection (LTBI) accounts for >80% of tuberculosis cases in the United States.1 Because treatment of LTBI can prevent progression to disease, detection and treatment of LTBI is essential to tuberculosis elimination in the United States.2 Children are a priority for LTBI screening because once infected (1) they are at high risk of progression to disease in the absence of treatment, (2) they are more likely to develop severe disease (eg, meningeal and miliary tuberculosis), and (3) those who do not develop disease in childhood are a reservoir for future tuberculosis cases.3,4
The optimal testing strategy for LTBI in children is unclear. The tuberculin skin test (TST) has been in use for more than a century, but its specificity is limited by cross-reactivity of TST antigens with those of nontuberculous mycobacteria5,6 and the bacillus Calmette-Guerin (BCG) vaccine, given to newborns in >80% of countries worldwide.7 Interferon-γ release assays (IGRAs), available since 2000, measure in vitro IFN-γ production by T lymphocytes after stimulation with Mycobacterium tuberculosis–specific antigens not found in any BCG strain or in most nontuberculous mycobacteria. The 2 commercially available IGRAs, QuantiFERON test8 and T-SPOT.TB test9 (T-SPOT), offer additional advantages of a single visit for phlebotomy and results that avoid variability because of subjectivity in interpretation observed with TSTs.10
Current US guidelines recommend IGRAs for children ≥5 years old, for whom the risk of progression to disease is the same as for adults.11 TST is recommended for younger children, in whom the risk of progression to tuberculosis is 4 to 5 times higher, primarily on the basis of studies that suggest diminished IGRA sensitivity in this age group.10,12,13 On the basis of more recent data, the American Academy of Pediatrics amended the age for the preferred use of TST to those <2 years old.14,15 Questions remain, however, about which test is best for screening children, especially children not born in the United States in whom use of TST may result in overdiagnosis and overtreatment of LTBI.15 The only reference standard for LTBI is eventual development of culture-proven disease. The positive predictive value (PPV) of TST for progression to disease has been established by multiple longitudinal studies.16–18 The predictive value of IGRAs in children is unclear because longitudinal studies involving IGRAs are scarce. There have been numerous requests for a prospective cohort study comparing IGRAs to TST in regard to their ability to predict progression to disease in this population.19–21 For clinicians, an important outcome from such studies would be the negative predictive value (NPV) of an IGRA result for subsequent development of tuberculosis disease.
We analyzed data from children enrolled in a large prospective longitudinal study to compare the predictive value of commercially available tests. Our objective was to identify optimal testing strategies for the detection of LTBI in children overall, children <5 years old, and children born outside of the United States.
METHODS
Study Population
The Tuberculosis Epidemiologic Studies Consortium (TBESC), funded by the Centers for Disease Control and Prevention (CDC), is a partnership of academic and public health programs in 11 states. TBESC-affiliated clinics recruited individuals at risk for LTBI or progression to tuberculosis to assess the ability of TST and IGRAs to predict tuberculosis disease. Children who were <15 years old and enrolled from September 2012 to April 2016 constituted the study population.
A child was eligible for enrollment if she or he was (1) a close contact (≥8 hours in a week) with an individual with infectious tuberculosis who (a) was part of an ongoing contact investigation and (b) had either a positive culture result or a negative culture result but positive smear result and positive nucleic acid amplification test result, (2) born in a country whose population in the United States had a high (≥100 cases per 100 000 population) rate of tuberculosis22 (Supplemental Table 6), (3) a recent arrival (≤5 years) from a country whose population in the United States had a moderate (10–99 cases per 100 000 population) rate of tuberculosis22 (Supplemental Table 7), (4) a member of a local population with documented LTBI prevalence ≥25%, or (5) a person with HIV infection. Participants enrolled as members of local populations with LTBI prevalence ≥25% were homeless or born in Mexico. Results for homeless persons were reported as a separate category; those for Mexican-born persons were included in results for participants not born in the United States.
We excluded from this analysis results for participants who (1) were diagnosed with tuberculosis disease during screening, (2) were enrolled as contacts of source cases with negative culture, smear, and nucleic acid amplification test results for M tuberculosis, (3) lacked valid TST results, or (4) did not have at least 1 valid IGRA result. The study was approved by each site’s institutional review board (IRB), or the site relied on the CDC IRB. All participants had parental consent; written assent was obtained as required by individual IRBs.
Study Procedures
Study personnel collected demographic and LTBI-related risk information using a standardized questionnaire. Participants had blood drawn for QuantiFERON-TB Gold In-Tube test (QFT-GIT) and T-SPOT followed by TST placement. TST induration was measured at 48 to 72 hours (±4 hours); an induration ≥5 mm was interpreted as positive for contacts or those with HIV; ≥10 mm was positive for all others. The QFT-GIT and T-SPOT were processed and interpreted according to manufacturers’ instructions.8,9 A positive T-SPOT result was defined by US standards as ≥8 spots. Participants with indeterminate or invalid IGRA results were retested; repeat indeterminate or invalid results were excluded from analysis. All tests were completed before LTBI treatment initiation.
Sites made their own decisions about how to define an LTBI diagnosis, whether to treat, and which LTBI regimens to use. Treated participants were defined as those who completed or were still on LTBI treatment when diagnosed with tuberculosis disease; untreated participants never started or did not complete treatment of LTBI.
Sites diagnosed tuberculosis disease as laboratory confirmed or clinically verified, on the basis of CDC definitions.23 An incident case was 1 diagnosed after enrollment in a participant who had a radiograph before diagnosis that excluded tuberculosis.
Participants with at least 1 positive test result of the 3 tests performed were contacted every 6 months for 2 years, regardless of LTBI treatment history. Study personnel interviewed participants’ parents by using standardized questions to determine if tuberculosis disease had developed since enrollment. For this cohort, the last follow-up was in June 2018. For all participants, regardless of test results, sites conducted semiannual matches between their study databases and their state tuberculosis disease registries; the last registry match for this analysis was in June 2018. In addition, sites contacted the state health departments for all children who moved out of state during the study to identify any cases who would not have been entered into the site’s state tuberculosis registry.
Statistical Analysis
The proportion positive by each test was defined as the number of participants with positive results divided by the total number of participants with test results, for TST, QFT-GIT, and T-SPOT separately. We used the Cochran-Armitage trend test to assess the association between single test positivity and age (age groups: <2, 2–4, 5–9, 10–14 years). For test agreement, IGRA was considered a positive result if either QFT-GIT or T-SPOT was positive; otherwise, IGRA was negative. TST-IGRA combinations with 1 positive and 1 negative result were considered discordant. Test agreement was evaluated with percent concordance between TST and IGRA results and with Cohen’s κ. Person-years were based on the number of years between enrollment and the date of tuberculosis disease diagnosis or the substudy stop date of June 28, 2018, whichever was earlier. The incidence rate per 100 000 person-years was computed for each test result and TST-IGRA combination; 95% confidence intervals (CIs) were calculated on the basis of a Poisson distribution. Sensitivity, specificity, PPV, and NPV for progression to tuberculosis disease were calculated for each test, along with their 95% CIs (Wilson). All statistics were calculated with SAS Version 9.4 (SAS Institute, Inc, Cary, NC). Right censoring was not taken into account for the statistical analyses.
RESULTS
Demographics
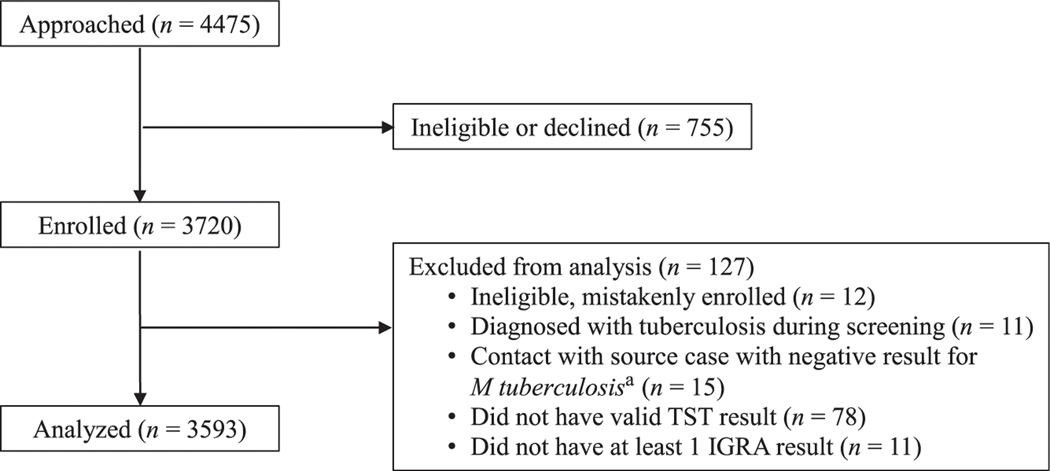
Of 3720 children enrolled, data for 3593 were eligible for analysis (Fig 1). The median age at enrollment was 8.6 years (interquartile range: 5.0–11.8). Children <5 years old and <2 years old accounted for 25.0% and 6.1% of the population, respectively. Almost all (92.0%) participants were born outside of the United States (Table 1). Most US-born children (95.1%) and 3% of non–US-born children were contacts. BCG vaccination was self-reported for 2340 (70.8%) non–US-born and 18 (6.2%) US-born children.
FIGURE 1.
Flow diagram of selection of participants for analysis.
aNegative results for culture, smear, and nucleic acid amplification tests.
TABLE 1.
Demographic Characteristics and Medical and Social Risk Factors of Study Participants, by Age Group
| Characteristic | All Ages (N = 3593) | <2 y (n = 219) | 2–4 y (n = 681) | 5–9 y (n = 1273) | 10–14 y (n = 1420) |
|---|---|---|---|---|---|
|
| |||||
| Male, n (%) | 1813 (50.5) | 122 (55.7) | 332 (48.8) | 645 (50.7) | 714 (50.3) |
| Born outside of the United Statesa, n (%) | 3304 (92.0) | 197 (90.0) | 631 (92.7) | 1166 (91.6) | 1310 (92.3) |
| Region of birth, n (%) | |||||
| Africa | 741 (20.6) | 30 (13.7) | 147 (21.6) | 301 (23.6) | 263 (18.5) |
| Americas | 509 (14.2) | 23 (10.5) | 65 (9.5) | 180 (14.1) | 250 (17.6) |
| Eastern Mediterranean | 611 (17.0) | 23 (10.5) | 103 (15.1) | 236 (18.5) | 249 (17.5) |
| Europe | 42 (1.2) | 7 (3.2) | 8 (1.2) | 18 (1.4) | 10 (0.7) |
| Southeast Asia | 1058 (29.4) | 55 (25.1) | 165 (24.2) | 385 (30.2) | 453 (31.9) |
| Western Pacific | 547 (15.2) | 79 (36.1) | 189 (27.8) | 149 (11.7) | 190 (13.4) |
| Unknown | 15 (0.4) | 2 (0.9) | 4 (0.6) | 4 (0.3) | 5 (0.4) |
| Race or ethnicityb, n (%) | |||||
| Asian | 1222 (34.0) | 96 (43.8) | 270 (39.6) | 378 (29.7) | 478 (33.7) |
| Black or African American | 777 (21.6) | 32 (14.6) | 131 (19.2) | 294 (23.1) | 320 (22.5) |
| White or Caucasian | 190 (5.3) | 6 (2.7) | 33 (4.8) | 82 (6.4) | 69 (4.9) |
| Native Hawaiian or Pacific Islander | 72 (2.0) | 2 (0.9) | 5 (0.7) | 32 (2.5) | 33 (2.3) |
| Hispanic or Latino | 284 (7.9) | 10 (4.6) | 34 (5.0) | 108 (8.5) | 132 (9.3) |
| Other | 951 (26.5) | 61 (27.9) | 187 (27.5) | 354 (27.8) | 349 (24.6) |
| Unknown or refused | 132 (3.7) | 11 (4.6) | 23 (3.4) | 37 (2.9) | 61 (4.3) |
| Reason for enrollmentc, n (%) | |||||
| Contact | 378 (10.5) | 24 (11.0) | 67 (9.8) | 142 (11.2) | 145 (10.2) |
| Born outside of the United Statesd | 3198 (89.0) | 195 (89.0) | 612 (89.9) | 1126 (88.5) | 1265 (88.1) |
| Homelesse | 3 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 2 (0.1) |
| ≥30 d in a high-risk country in the last 5 y | 11 (0.3) | 0 (0.0) | 1 (0.1) | 4 (0.3) | 6 (0.4) |
| HIV infected | 3 (0.1) | 0 (0.0) | 1 (0.1) | 0 (0.0) | 2 (0.1) |
| BCG vaccinationf | 2358 (65.6) | 146 (66.7) | 450 (66.1) | 820 (64.4) | 942 (66.3) |
Country of birth is unknown for 2 children.
Not mutually exclusive.
Listed in hierarchical order.
Includes Mexican-born participants who were enrolled as members of a local population with documented LTBI prevalence ≥25%.
Member of a local population with documented LTBI prevalence ≥25%.
Based on self-report.
Test Results
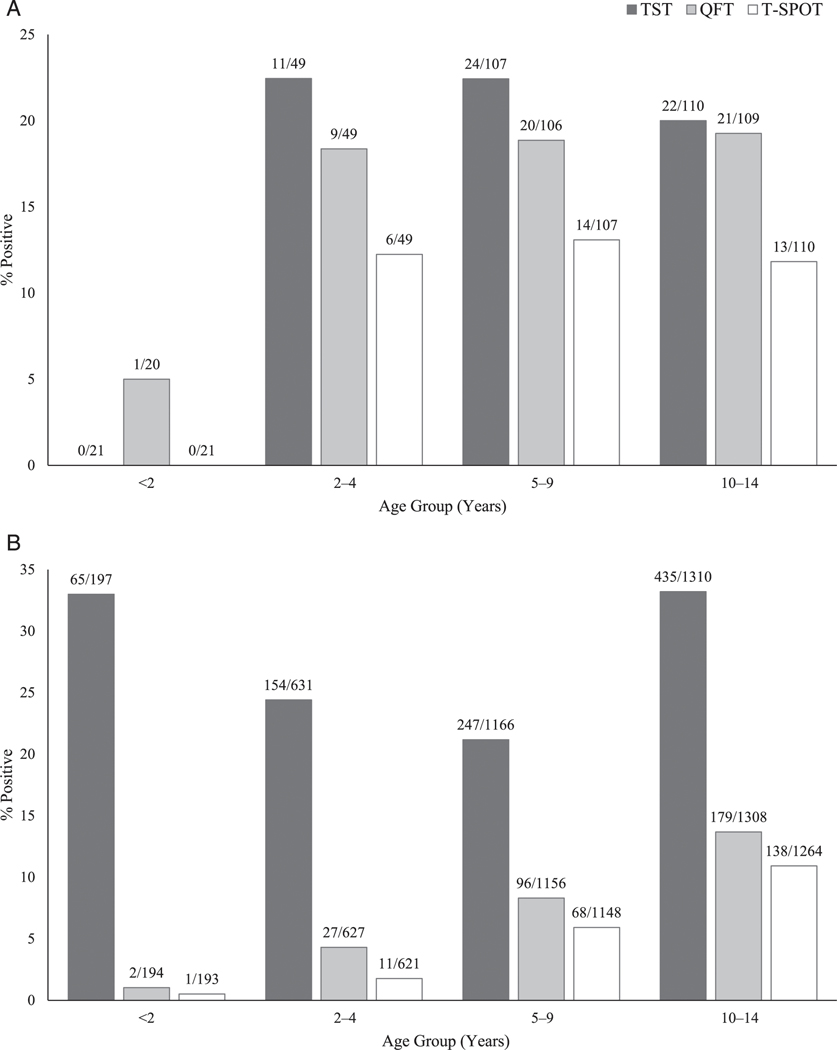
Three test results were available for all but 100 of 3593 children. The majority (71.3%) of children had negative results for all 3 tests; 6.5% were positive on all 3 tests, including 10.9% of US-born and 6.1% of non–US-born children. Thirty-three children (0.9%) had indeterminate QFT-GIT results and 15 (0.4%) invalid T-SPOT results before retesting (Supplemental Table 8). Among non–US-born children, there were approximately one-third as many with IGRA-positive results (317 out of 3304) compared with TST-positive results (901 out of 3304) (Table 2). The proportion positive for each test was 27.3% for TST, 9.3% for QFT-GIT, and 6.8% for T-SPOT for non–US-born children and 19.9%, 18.0%, and 11.5%, respectively, for US–born. The proportion with a positive IGRA increased significantly with age among non–US-born children (P < .001) but not among US-born children (Fig 2, Supplemental Table 9). Compared with US-born children, non–US-born children had a significantly higher proportion of TST-positive, IGRA-negative results (19.3% vs 4.9%, P < .001) (Table 2).
TABLE 2.
Combination Test Results by Age Group and Nativity
| Test Result Combination | All Ages | <2 y | 2–4 y | 5–9 y | 10–14 y |
|---|---|---|---|---|---|
|
| |||||
| Born in the United States | |||||
| Two test combinations | |||||
| TST+ and IGRA+a, n (%) | 43 (15.0)b | 0 (0.0) | 9 (18.4) | 18 (16.8) | 16 (14.6) |
| TST+ and IGRA−c, n (%) | 14 (4.9)b | 0 (0.0) | 2 (4.1) | 6 (5.6) | 6 (5.5) |
| TST− and IGRA+, n (%) | 9 (3.1) | 1 (4.8) | 0 (0.0) | 2 (1.9) | 6 (5.5) |
| TST−and IGRA−, n (%) | 221 (77.0) | 20 (95.2) | 38 (77.6) | 81 (75.7) | 82 (74.6) |
| Three test combinations | |||||
| Triple +d, n (%) | 31 (10.9) | 0 (0.0) | 6 (12.2) | 14 (13.2) | 11 (10.1) |
| Triple −e, n (%) | 218 (76.8) | 19 (95.2) | 38 (77.6) | 80 (75.5) | 81 (74.3) |
| Born outside of the United States | |||||
| Two test combinations | |||||
| TST+ and IGRA+, n (%) | 263 (8.0)b | 1 (0.5) | 22 (3.5) | 79 (6.8) | 161 (12.3) |
| TST+ and IGRA−, n (%) | 638 (19.3)b | 64 (32.5) | 132 (20.9) | 168 (14.4) | 274 (20.9) |
| TST−and IGRA+, n (%) | 54 (1.6) | 1 (0.5) | 5 (0.8) | 22 (1.9) | 26 (2.0) |
| TST− and IGRA−, n (%) | 2349 (71.1) | 131 (66.5) | 472 (74.8) | 897 (76.9) | 849 (64.8) |
| Three test combinations | |||||
| Triple +, n (%) | 195 (6.1) | 1 (0.5) | 11 (1.8) | 61 (5.4) | 122 (9.7) |
| Triple −, n (%) | 2272 (70.9) | 126 (66.3) | 458 (74.2) | 872 (76.6) | 816 (64.7) |
−, negative; +, positive.
QFT-GIT–positive or T-SPOT–positive result.
P < .001 for comparison of born in the Unites States to not born in the United States.
QFT-GIT–negative and T-SPOT–negative results.
TST-positive, QFT-GIT–positive, and T-SPOT–positive results.
TST-negative, QFT-GIT–negative, and T-SPOT–negative results.
FIGURE 2.
Percentage of positive tests, by age group, for (A) US-born childrena and (B) non–US-born children. aAll TST and T-SPOT results were negative for US-born children < 2 years.
Test Agreement
Overall agreement between TST and IGRAs was 80.1% (κ = 0.37), influenced primarily by TST-negative, IGRA-negative results (2572, 71.6%). Concordance was 92.0% (κ = 0.74) among US-born children and 79.1% (κ = 0.34) among non–US-born children. More than 90% of the discordant results were TST positive and IGRA negative, including 60.9% among US-born and 92.2% among non–US-born participants Among non–US-born children, those <2 years old had the lowest concordance and the highest proportion of TST-positive, IGRA-negative results (32.5%, Supplemental Table 10). Agreement between QFT-GIT and T-SPOT was 93.0% for US-born and 96.8% for non–US-born children (data not shown). Among those with at least 1 positive IGRA result, 61.5% of US-born and 65.1% of non–US-born children had positive results for both (Supplemental Table 11).
Progression to Disease
Four children developed culture-confirmed pulmonary tuberculosis during a median follow-up of 4.3 years (interquartile range: 3.2–5.0). Two were 7-month-old US-born twins exposed to a common source. Both twins developed tuberculosis disease 2 months after enrollment. The twin with all negative results developed disease while on isoniazid prophylaxis pending retesting 8 to 10 weeks after the first round of tests. The other twin had a positive QFT-GIT result but a negative T-SPOT result and developed disease while on LTBI treatment with isoniazid. The other 2 children, both born outside of the United States, had positive results for all 3 tests; 1 developed disease 19 months after enrollment and completion of 6 months of isoniazid; the other developed disease 11 months after enrollment and was not treated for LTBI because the participant had previously received 6 months of treatment of tuberculosis disease (Table 3).
TABLE 3.
Characteristics of Four Children With Incident Tuberculosis Disease
| Age at Enrollment | Sex | Country of Birth | Contact | Test Results |
LTBI Treatment at Diagnosis | Age at Diagnosis | Chest Radiograph Result | Microbiologic Confirmation for M tuberculosis | ||
|---|---|---|---|---|---|---|---|---|---|---|
| TST (mm Induration) | QFT-GIT (IU/mL IFN-γ) | T-SPOT (No. Spots) | ||||||||
|
| ||||||||||
| 7 moa | Male | United States | Yes | Negative (0) | Negative (0.26) | Negative (2) | Isoniazid, directly observed | 9 mo | Abnormal, no cavitary disease | Gastric aspirate culture positive result |
| 7 moa | Male | United States | Yes | Negative (0) | Positive (8.27) | Negative (3) | Isoniazid, directly observed | 9 mo | Abnormal, no cavitary disease | Gastric aspirate culture positive result |
| 12 y | Female | Myanmar | No | Positive (29) | Positive (7.33) | Positive (47) | Not offered | 13 y | Normal | Sputum culture positive result |
| 14 y | Female | Philippines | No | Positive (13) | Positive (7.47) | Positive (50) | Isoniazid, SAT | 16 y | Abnormal, cavitary disease | Sputum culture positive result |
SAT, self-administered therapy.
Twins.
Among untreated children, 0.16% (1 out of 630) with positive TST results developed disease compared with 0.81% (1 out of 124) with positive QFT-GIT results and 1.3% (1 out of 78) with positive T-SPOT results. None of the 533 untreated children who had positive TST results and negative IGRA results, including 54 children <2 years old, developed disease (Table 4).
TABLE 4.
Incidence Rates per 100 000 Person-Years for Single Test and Combination Test Results in Participants Treated and Untreated for LTBI
| Test Result | All Participants |
Untreated |
Treateda |
|||||
|---|---|---|---|---|---|---|---|---|
| No. Cases | IRb (95% CI) | No. Cases | No. Participants | No. PY | No. Cases | No. Participants | No. PY | |
|
| ||||||||
| Single test results | ||||||||
| TST+ | 2 | 49 (12–197) | 1 | 630 | 2639 | 1 | 328 | 1414 |
| TST− | 2 | 19 (5–75) | 0 | 2604 | 10 545 | 2 | 31 | 122 |
| QFT-GIT+ | 3 | 200 (65–620) | 1 | 124 | 524 | 2 | 231 | 976 |
| QFT-GIT− | 1 | 8 (1–54) | 0 | 3088 | 12 563 | 1 | 128 | 560 |
| T-SPOT+ | 2 | 189 (47–755) | 1 | 78 | 333 | 1 | 173 | 726 |
| T-SPOT− | 2 | 15 (4–60) | 0 | 3082 | 12 514 | 2 | 182 | 792 |
| Combination test results | ||||||||
| TST+ and IGRA+ | 2 | 154 (39–616) | 1 | 97 | 413 | 1 | 209 | 885 |
| TST+ and IGRA− | 0 | 0 | 0 | 533 | 2226 | 0 | 119 | 528 |
| TST− and IGRA+ | 1 | 389 (55–2762) | 0 | 33 | 135 | 1 | 30 | 121 |
| TST− and IGRA− | 1 | 10 (1–68) | 0 | 2571 | 10 410 | 1 | NA | NA |
IR, incidence rate; NA, not available; PY, person-years; −, negative; +, positive.
Completed LTBI treatment or was on LTBI treatment when diagnosed with tuberculosis.
Number of tuberculosis cases per 100 000 person-years.
The sensitivities of TST, QFT-GIT, and T-SPOT for progression to tuberculosis disease were 50%, 75%, and 50%, respectively, with wide CIs. The NPVs were high and the PPVs low for all 3 tests (Table 5).
TABLE 5.
Sensitivity, Specificity, PPV, and NPV of TST, QFT-GIT, and T-SPOT for Predicting Progression to Tuberculosis, Based on Incident Disease
| Test | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|
|
| ||||
| TST | 50.0 (15.0–85.0) | 73.4 (71.9–74.8) | 0.2 (0.1–0.8) | 99.9 (99.7–100.0) |
| QFT-GIT | 75.0 (30.1–95.4) | 90.1 (89.1–91.1) | 0.9 (0.3–2.5) | 100.0 (99.8–100.0) |
| T-SPOT | 50.0 (15.0–85.0) | 92.9 (92.0–93.7) | 0.8 (0.2–2.9) | 99.9 (99.8–100.0) |
DISCUSSION
This is the largest prospective longitudinal investigation to compare predictive values of IGRAs and TST in children. More than 90% of participants were individuals born outside of the United States, a priority group for tuberculosis elimination efforts, and 25% were <5 years old. In this cohort, both IGRAs and TST were poor predictors of progression to disease, with PPVs of <1%, although IGRAs performed no worse than the TST. These low values are expected given known low rates of progression to tuberculosis disease and modification of progression by LTBI treatment. More important to the clinician, IGRAs had high NPV. None of the 533 children untreated for LTBI who had positive TST results and negative IGRA results, including 54 children <2 years of age, developed disease after a median 4.3 years of follow-up. IGRAs also demonstrated high specificity, with ~70% fewer positive results compared with TST in non–US-born children. The results support the use of IGRAs to screen for LTBI in children of any age, especially those who are born ourside of the United States.
One of the primary obstacles to the use of IGRAs in young children has been the paucity of longitudinal data on their ability to predict tuberculosis disease in this age group. Previous investigations demonstrating equivalence of IGRAs and TSTs were focused mainly on adult contacts and adolescents.24–26 Among limited pediatric studies, 1 identified 6 incident cases among 104 German children ≤15 years old who were followed for 4 years; the 28.6% of untreated children who had positive results for QFT-GIT who developed tuberculosis disease was not significantly different from the 15% of children who had positive TST results who developed disease.27 For 908 Turkish household contacts ≤15 years old, 15 of whom developed tuberculosis within 2 years, tuberculosis incidence was similar for children with positive ELISpot and TST results.28 Only a minority of cases in both studies were culture confirmed. Our study’s inclusion of 900 children <5 years old and 219 children <2 years old fills an important knowledge gap.
An important consideration for clinicians is whether potential for progression to tuberculosis will be missed by reliance on IGRAs instead of TSTs. Lack of agreement between TST and IGRA results, particularly among young and/or BCG-vaccinated children, is commonly reported in the literature and observed in clinical practice.15,20,29–31 Among California children followed for 5.7 years, none of 146 untreated children with TST-positive, IGRA-negative results, including 54 children <5 years old, developed disease.29 We found discordance, represented primarily by TST-positive, IGRA-negative results, to be more common in non–US-born children, particularly those <2 years old. More than 80% of 652 participants in our study with TST-positive, IGRA-negative results were not treated for LTBI; no cases of tuberculosis disease were identified among these children during follow-up. On the basis of this evidence, clinicians can be reassured that they can rely on negative IGRA results.
Our findings also underscore that use of TST likely results in overdiagnosis, which could lead to overtreatment of LTBI in non–US-born children. In several earlier studies, researchers have suggested that the use of TST results in the overdiagnosis of LTBI in children emigrating from areas of high tuberculosis endemicity.6,15,20,29–32 In an analysis of children diagnosed with LTBI preimmigration by TST, only 23% were positive postarrival by IGRA.30 In another study, investigators found similar discrepancies in children 2 to 14 years old tested with TST and QFT-GIT in preimmigration clinics: 26% were positive by TST and 5.6% by QFT-GIT.15 In the larger study of which this analysis is a part, TBESC investigators used latent class analysis to predict test characteristics on the basis of initial test results from 10 740 adults and children. They concluded that IGRAs had greater specificity among the 464 non–US-born children <5 years old.6 Our results support and expand on these earlier findings. Of note, we found a significant association between older age and positive IGRA results in non–US-born children, as would be expected with cumulative exposure to tuberculosis disease in high-incidence countries. No such trend was apparent for positive TSTs, which suggests that TST results do not correspond with presumed tuberculosis exposure in non–US-born children. Because most US-born children were close contacts, their test results did not reflect cumulative exposure and therefore were not associated with age. On the basis of our findings, replacing TSTs with IGRAs for non–US-born children could reduce the number of children diagnosed with LTBI by approximately two-thirds. This would translate to fewer radiographs and fewer children considered for LTBI treatment and improve use of scarce tuberculosis control resources.
An additional concern about routine use of IGRAs in young children has been the higher proportion of indeterminate QFT results reported in children <5 years old compared with older children.10,33 We found a low proportion of indeterminate results (0.9%); although the proportion was higher in the youngest children, >97% had interpretable results. Although indeterminate results require repeat testing, the observed percent of indeterminate results is lower than the proportion of persons who fail to return to have the TST read (6% to 11%), which also requires retesting.34,35
Strengths of this study include (1) a large cohort of children <5 years old; (2) data on outcomes for >500 untreated children with TST-positive, IGRA-negative results; (3) inclusion of all commercially available tests for LTBI; and (4) culture confirmation of all cases. The study’s main limitation was the small number of incident cases. This was not unexpected because half the children with single positive test results were treated. Other studies have shown that only 1% to 13% of IGRA-positive contacts develop tuberculosis disease over 2 years of follow-up, even in high-burden countries.36 Because of the limited number of tuberculosis cases, we could not evaluate possible predictors of progression to tuberculosis disease and compare incidence rates between subpopulations because the mathematical models needed would not converge. Another limitation is inherent to the tests and the population tested: IGRAs and TSTs measure immune response, which is likely to vary in young children. For example, 1 infant with negative results on all 3 tests developed disease during treatment of LTBI; the infant’s twin, who also developed disease, had a positive QFT-GIT result but negative T-SPOT and TST results. This emphasizes the need for clinical judgment in the application of these useful but imperfect tests, particularly in high-risk patients, and the need for better diagnostic tools.
This study’s findings have important implications for tuberculosis elimination efforts in the United States. Our findings support the use of IGRAs in children of all ages and especially non–US-born children, a priority group for LTBI detection and treatment. As rates of tuberculosis disease in the United States continue to decline, diminished competency with administration and interpretation of the TST will increasingly compromise this test’s utility. The value of IGRAs in tuberculosis moderate- and high-burden settings remains unclear. In the United States, where LTBI detection and treatment are paramount to tuberculosis elimination, preferential use of IGRAs may provide substantial benefits.
Supplementary Material
WHAT’S KNOWN ON THIS SUBJECT:
The tuberculin skin test (TST) has been the preferred test for screening young children for latent tuberculosis infection because of concerns that interferon-γ release assays (IGRAs) could miss infections in this high-risk population.
WHAT THIS STUDY ADDS:
In this cohort of 3593 children followed for a median 4.3 years, IGRAs had higher specificities than TST and high negative predictive value. None of 533 untreated children who had positive TST results and negative IGRA results, including 54 children <2 years old, developed tuberculosis disease.
ACKNOWLEDGMENTS
We thank the members of the TBESC. The TBESC participating sites and site principal investigators are as follows. The California Department of Public Health (Richmond): Jennifer Flood and Lisa Pascopella; San Francisco Department of Public Health: Julie Higashi; County of San Diego (CA) Health and Human Services Agency: Marisa Moore (CDC); University of California San Diego Antiviral Research Center: Richard Garfein and Constance Benson; Denver (CO) Health and Hospital Authority: Robert Belknap; Duke University (Durham, NC) and Wake County Human Services (Raleigh, NC): Jason Stout; Vanderbilt University Medical Center (Nashville, TN): Timothy Sterling and April Pettit; Emory University (Atlanta, GA): Henry M. Blumberg; DeKalb County (GA) Board of Health: Alawode Oladele; University of Florida (Gainesville): Michael Lauzardo and Marie Nancy Séraphin; Hawaii Department of Health (Honolulu): Richard Brostrom; Maryland Department of Health (Baltimore): Wendy Cronin and Susan Dorman; Public Health-Seattle and King County (WA): Masahiro Narita and David Horne; and University of North Texas Health Science Center (Fort Worth): Thaddeus Miller. The TBESC site project coordinators are as follows: Laura Romo, San Francisco; Christine Kozik and Carlos Vera, San Diego, CA; Juanita Lovato, Denver, CO; Laura Farrow and Colleen Traverse, Durham, NC; Kristian Atchley and Fernanda Maruri, Nashville, TN; Kursten Lyon and Debra Turner, Raleigh, NC; Nubia Flores, Charlotte, NC; Jane Tapia, Atlanta, GA; Livia Sura and Joanne C. Li, Gainesville, FL; Marie McMillan, Fort Lauderdale, FL; Stephanie Reynolds-Bigby, Miami and Fort Lauderdale, FL; Angela Largen and Thara Venkatappa, Honolulu, HI; Aurimar Ayala, Phoenix, AZ; Elizabeth Munk and Gina Maltas, Baltimore, MD; Yoseph Sorri and Kenji Matsumoto, Seattle, WA; and Amy Board, James Akkidas, and Jaquelyn Sanchez, Fort Worth, TX.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the authors’ affiliated institutions.
FUNDING:
Funded by the Division of Tuberculosis Elimination, Centers for Disease Control and Prevention.
ABBREVIATIONS
- BCG
bacillus Calmette-Guerin
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- IGRA
interferon-γ release assay
- IRB
institutional review board
- LTBI
latent tuberculosis infection
- NPV
negative predictive value
- PPV
positive predictive value
- QFT-GIT
QuantiFERON-TB Gold In-Tube test
- TBESC
Tuberculosis Epidemiologic Studies Consortium
- T-SPOT
T-SPOT.TB test
- TST
tuberculin skin test
Footnotes
This trial has been registered at www.clinicaltrials.gov (identifier NCT01622140).
PEDIATRICS (ISSN Numbers: Print, 0031–4005; Online, 1098–4275).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2019-3021.
REFERENCES
- 1.France AM, Grant J, Kammerer JS, Navin TR. A field-validated approach using surveillance and genotyping data to estimate tuberculosis attributable to recent transmission in the United States. Am J Epidemiol. 2015; 182(9):799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LoBue PA, Mermin JH. Latent tuberculosis infection: the final frontier of tuberculosis elimination in the USA. Lancet Infect Dis. 2017; 17(10):e327–e333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horsburgh CR Jr.. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med. 2004; 350(20):2060–2067 [DOI] [PubMed] [Google Scholar]
- 4.Pang J, Teeter LD, Katz DJ, et al. ; Tuberculosis Epidemiologic Studies Consortium. Epidemiology of tuberculosis in young children in the United States. Pediatrics. 2014;133(3). Available at: www.pediatrics.org/cgi/content/full/133/3/e494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10(11): 1192–1204 [PubMed] [Google Scholar]
- 6.Stout JE, Wu Y, Ho CS, et al. ; Tuberculosis Epidemiologic Studies Consortium. Evaluating latent tuberculosis infection diagnostics using latent class analysis. Thorax. 2018;73(11):1062–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8(3): e1001012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.QIAGEN. QuantiFERON-T B Gold ELISA [package insert]. Germantown, MD: Qiagen; 2016 [Google Scholar]
- 9.T-SPOT. TB [package insert]. Marlborough, MA: Oxford Immunotec, Inc.; 2012 [Google Scholar]
- 10.Starke JR; Committee On Infectious Diseases. Interferon-g release assays for diagnosis of tuberculosis infection and disease in children. Pediatrics. 2014;134(6). Available at: www.pediatrics.org/cgi/content/full/134/6/e1763 [DOI] [PubMed] [Google Scholar]
- 11.Lewinsohn DM, Leonard MK, LoBue PA,et al. Official American thoracic society/ infectious diseases society of America/ Centers for disease control and prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64(2): e1–e33 [DOI] [PubMed] [Google Scholar]
- 12.Machingaidze S, Wiysonge CS, Gonzalez-Angulo Y, et al. The utility of an interferon gamma release assay for diagnosis of latent tuberculosis infection and disease in children: a systematic review and meta-analysis. Pediatr Infect Dis J. 2011;30(8):694–700 [DOI] [PubMed] [Google Scholar]
- 13.Mandalakas AM, Detjen AK, Hesseling AC, Benedetti A, Menzies D. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2011;15(8):1018–1032 [DOI] [PubMed] [Google Scholar]
- 14.Kimberlin DW, Brady MT, Long SS, Jackson MA, eds. Red Book 2018: Report of the Committee on Infectious Diseases, 31st ed. Itasca, IL: American Academy of Pediatrics; 2018 [Google Scholar]
- 15.Howley MM, Painter JA, Katz DJ, et al. ; Tuberculosis Epidemiologic Studies Consortium. Evaluation of QuantiFERON-TB gold in-tube and tuberculin skin tests among immigrant children being screened for latent tuberculosis infection. Pediatr Infect Dis J. 2015; 34(1):35–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comstock GW, Livesay VT, Woolpert SF.The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. 1974;99(2):131–138 [DOI] [PubMed] [Google Scholar]
- 17.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc. 1970;26:28–106 [PubMed] [Google Scholar]
- 18.Watkins RE, Brennan R, Plant AJ. Tuberculin reactivity and the risk of tuberculosis: a review. Int J Tuberc Lung Dis. 2000;4(10):895–903 [PubMed] [Google Scholar]
- 19.Auguste P, Tsertsvadze A, Pink J, et al. Comparing interferon-gamma release assays with tuberculin skin test for identifying latent tuberculosis infection that progresses to active tuberculosis: systematic review and meta-analysis. BMC Infect Dis. 2017;17(1):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell JR, Krot J, Elwood K, Cook V, Marra F. A systematic review on TST and IGRA tests used for diagnosis of LTBI in immigrants. Mol Diagn Ther. 2015;19(1): 9–24 [DOI] [PubMed] [Google Scholar]
- 21.Song SE, Yang J, Lee KS, et al. Comparison of the tuberculin skin test and interferon gamma release assay for the screening of tuberculosis in adolescents in close contact with tuberculosis TB patients. PLoS One. 2014;9(7):e100267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cain KP, Benoit SR, Winston CA, MacKenzie WR. Tuberculosis among foreign-born persons in the United States. JAMA. 2008;300(4):405–412 [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC). Reported Tuberculosis in the United States, 2017. Atlanta, GA: US Department of Health and Human Services, CDC; 2017 [Google Scholar]
- 24.Machingaidze S, Verver S, Mulenga H,et al. Predictive value of recent QuantiFERON conversion for tuberculosis disease in adolescents. Am J Respir Crit Care Med. 2012;186(10): 1051–1056 [DOI] [PubMed] [Google Scholar]
- 25.Mahomed H, Hawkridge T, Verver S,et al. The tuberculin skin test versus QuantiFERON TB Gold® in predicting tuberculosis disease in an adolescent cohort study in South Africa. PLoS One. 2011;6(3):e17984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-g release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2012; 12(1):45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diel R, Loddenkemper R, Niemann S, Meywald-Walter K, Nienhaus A. Negative and positive predictive value of a whole-blood interferon-g release assay for developing active tuberculosis: an update. Am J Respir Crit Care Med. 2011;183(1):88–95 [DOI] [PubMed] [Google Scholar]
- 28.Bakir M, Millington KA, Soysal A, et al.Prognostic value of a T-cell-based, interferon-gamma biomarker in children with tuberculosis contact. Ann Intern Med. 2008;149(11):777–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grinsdale JA, Islam S, Tran OC, Ho CS, Kawamura LM, Higashi JM. Interferon-gamma release assays and pediatric public health tuberculosis screening: the San Francisco program experience 2005 to 2008. J Pediatr Infect Dis Soc. 2016;5(2):122–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowenthal P, Barry PM, Flood J. High-discordance between pre-US and post-US entry tuberculosis test results among immigrant children: is it time to adopt interferon gamma release assay for preentry tuberculosis screening? Pediatr Infect Dis J. 2016; 35(3):231–236 [DOI] [PubMed] [Google Scholar]
- 31.Islam S The uncertain impact of screening immigrant children with the tuberculin skin test. Pediatr Infect Dis J. 2016;35(9):1052–1053 [DOI] [PubMed] [Google Scholar]
- 32.Stout JE, Menzies D. Predicting tuberculosis: does the IGRA tell the tale? Am J Respir Crit Care Med. 2008; 177(10):1055–1057 [DOI] [PubMed] [Google Scholar]
- 33.Haustein T, Ridout DA, Hartley JC, et al.The likelihood of an indeterminate test result from a whole-blood interferon-gamma release assay for the diagnosis of Mycobacterium tuberculosis infection in children correlates with age and immune status. Pediatr Infect Dis J. 2009;28(8):669–673 [DOI] [PubMed] [Google Scholar]
- 34.Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(11):1269–1278 [DOI] [PubMed] [Google Scholar]
- 35.Lucas M, Nicol P, McKinnon E, et al. Aprospective large-scale study of methods for the detection of latent Mycobacterium tuberculosis infection in refugee children. Thorax. 2010;65(5): 442–448 [DOI] [PubMed] [Google Scholar]
- 36.Denkinger CM, Dheda K, Pai M. Guidelines on interferon-γ release assays for tuberculosis infection: concordance, discordance or confusion? Clin Microbiol Infect. 2011; 17(6):806–814 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.