Abstract
Background:
Allergic diseases often occur in combination (multimorbidity). Human blood transcriptome studies have not addressed multimorbidity. Large-scale gene expression data were combined to retrieve biomarkers and signaling pathways to disentangle allergic multimorbidity phenotypes.
Methods:
Integrated transcriptomic analysis was conducted in 1233 participants with a discovery phase using gene expression data (Human Transcriptome Array 2.0) from whole blood of 786 children from three European birth cohorts (MeDALL), and a replication phase using RNA Sequencing data from an independent cohort (EVA-PR, n = 447). Allergic diseases (asthma, atopic dermatitis, rhinitis) were considered as single disease or multimorbidity (at least two diseases), and compared with no disease.
Results:
Fifty genes were differentially expressed in allergic diseases. Thirty-two were not previously described in allergy. Eight genes were consistently overexpressed in all types of multimorbidity for asthma, dermatitis, and rhinitis (CLC, EMR4P, IL5RA, FRRS1, HRH4, SLC29A1, SIGLEC8, IL1RL1). All genes were replicated the in EVA-PR cohort. RT-qPCR validated the overexpression of selected genes. In MeDALL, 27 genes were differentially expressed in rhinitis alone, but none was significant for asthma or dermatitis alone. The multimorbidity signature was enriched in eosinophil-associated immune response and signal transduction. Protein-protein interaction network analysis identified IL5/JAK/STAT and IL33/ST2/IRAK/TRAF as key signaling pathways in multimorbid diseases. Synergistic effect of multimorbidity on gene expression levels was found.
Conclusion:
A signature of eight genes identifies multimorbidity for asthma, rhinitis, and dermatitis. Our results have clinical and mechanistic implications, and suggest that multimorbidity should be considered differently than allergic diseases occurring alone.
Keywords: asthma, atopic dermatitis, multimorbidity, rhinitis, transcriptomics
Graphical Abstract
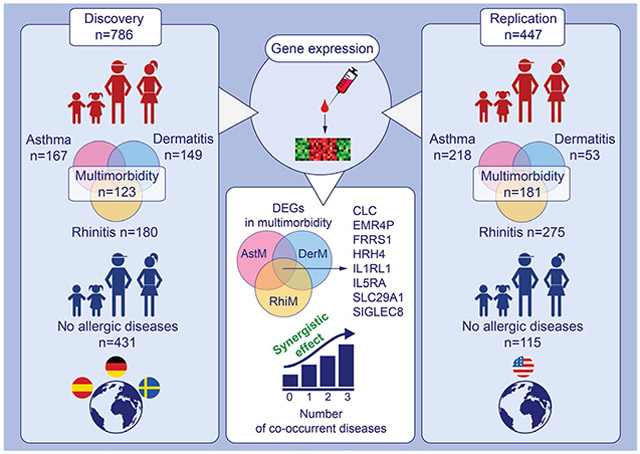
This study compares gene expression from whole blood of European children (4-16 years) with asthma and/or dermatitis and/or rhinitis to controls without allergy. Eight genes are overlapping among DEGs found in multimorbidity for asthma, dermatitis and rhinitis, which had synergistic effects along the number of co-occurrent diseases. Results were replicated in North American cohort with similar features. Abbreviations: AstM, asthma multimorbidity; CLC, charcot-leyden crystal galectin; DEGs, differentially expressed genes; DerM, dermatitis multimorbidity; EMR4P, adhesion G protein-coupled receptor E4; FRRS1, ferric chelate reductase 1; HRH4, histamine receptor H4; IL1RL1, interleukin 1 receptor like 1; IL5RA, interleukin 5 receptor subunit alpha; RhiM, rhinitis multimorbidity; SIGLEC8, sialic acid binding Ig like lectin 8;SLC29A1, solute carrier family 29 member 1
1 ∣. INTRODUCTION
Allergic diseases (asthma: A, dermatitis: D, rhinitis: R) are complex and often coexist in the same patient (multimorbidity).1 In children and adolescents, over 85% of asthmatic patients have rhinitis, suggesting common gene pathways, while only 20%-30% of rhinitis patients have asthma suggesting rhinitis-specific genes.2 Atopic dermatitis is characterized by a complex clinical phenotype varying during the life cycle.3
Mechanisms of the Development of ALLergy (MeDALL) found that multimorbidity was more common than expected by chance, suggesting genes underlying multimorbidity.1,4 MeDALL proposed that type 2 patterns are associated with multimorbidity,5 as suggested through an in silico model.6 Risk variants shared between asthma, dermatitis, and rhinitis were identified in genome-wide association studies, without considering the impact of multimorbidity.7,8 No study assessed multimorbidity using transcriptomics. Genes and pathways for atopy and atopic asthma in children and adolescents were found using transcriptome-wide data from peripheral blood.9
We used a subset of MeDALL samples as a discovery population to compare gene expression patterns between participants with and without asthma, dermatitis, or rhinitis. We examined gene expression of single diseases and multimorbidity. We replicated the results in the Epigenetic Variation and Childhood Asthma in Puerto Ricans (EVA-PR) cohort.9 To our knowledge, the present study constitutes to date the largest comprehensive whole blood gene expression dataset on allergy and the first assessment of multimorbidity molecular signatures.
2 ∣. METHODS
2.1 ∣. Study design
A cross-sectional analysis was carried out in participants from four cohorts using whole blood: three MeDALL cohorts (discovery study) and EVA-PR cohort (replication study). We compared participants with seven phenotypes: A, D, R, A + D, A + R, D + R, or A + D + R to those without asthma, dermatitis, or rhinitis (nonallergic participants, Table 1, Figure S1). We analyzed samples from the discovery study using Affymetrix Human Transcriptome Array 2.0 (HTA) and detected differentially expressed genes (DEGs). Relative expression of a randomly selected subset of eight genes was assessed by reverse transcription quantitative polymerase chain reaction (RT-qPCR). The replication arm was performed for DEGs in any multimorbidity, rhinitis alone and asthma alone in the EVA-PR cohort,9 using RNA Sequencing. We retrieved co-expressed genes with weighted gene co-expression network analysis (WGCNA). Biological interpretation involved gene, disease ontology and pathway analyses, protein-protein interaction network (PPIN), and literature review (Figure 1).
TABLE 1.
Characteristics of the MeDALL discovery population
| BAMSE | GINIplus | INMA | Total (Discovery) |
EVA-PR (Replication) |
|
|---|---|---|---|---|---|
| included in the analysis | 256 | 329 | 201 | 786 | 447 |
| male (n, %a) | 143 (55.9) | 160 (48.6) | 99 (49.2) | 402 (51.1) | 177 (53.3) |
| female (n, %a | 113 (44.1) | 169 (51.4) | 102 (50.8) | 384 (48.9) | 155 (46.7) |
| controls (n, %a | 100 (39.1) | 222 (67.5) | 109 (54.2) | 431 (54.8) | 115 (25.7) |
| A alone (n) | 32 | 15 | 18 | 65 | 47 |
| D alone (n) | 22 | 6 | 48 | 76 | 7 |
| R alone (n) | 23 | 64 | 4 | 91 | 97 |
| A + D (n) | 17 | 1 | 16 | 34 | 3 |
| A + R (n) | 33 | 15 | 2 | 50 | 135 |
| D + R (n) | 13 | 4 | 4 | 21 | 10 |
| A + D + R (n) | 16 | 2 | 0 | 18 | 33 |
| any multimorbidity (n,%b) | 79 (50.6) | 22 (20.6) | 22 (23.9) | 123 (34.6) | 181 (54.5) |
| age (Mean years ± SD) | 16.7 ± 0.4 | 15.0 ± 0.2 | 4.2 ± 0.3 | 12.8 ± 5.1 | 15.4 (10-20) |
Note: Characteristics of discovery and replication population. A: asthma, D: dermatitis, R: rhinitis.
% of total.
% of cases.
FIGURE 1.
Data analysis workflow. Gene expression levels of allergic participants were compared to nonallergic controls. Differentially expressed genes (DEGs) with FDR < 5% were detected. Expression levels of an arbitrary subset of 8 genes were validated with RT-qPCR. Subset of genes was replicated in EVA-PR cohort. Co-expression was assessed with Weighted Gene Co-expression Network Analysis (WGCNA). Biological role of genes was interpreted with gene and disease ontology functional enrichment, protein-protein network analysis (PPIN), and review of literature. *Allergy was defined as the presence of at least one disease among asthma, dermatitis, or rhinitis; allergic participants were grouped based on allergic disease co-occurrence: 1 disease, or 2 or more diseases (multimorbidity), and/or based on the nature of the disease: asthma, dermatitis or rhinitis, and compared to nonallergic participants
Methods and references are available as online Supplement.
2.2 ∣. Discovery study
2.2.1 ∣. Setting and population
Whole blood PAXgene tubes were available for 824 participants; 786 were included after quality control and outlier detection (Table 1): 256 from BAMSE,10 Sweden (GSE141623, mean age: 16.7 ± 0.4 years), 329 from GINIplus,11 Germany (15.2 ± 0.3 years), and 201 from INMA,12 Spain (GSE141631, 4.2 ± 0.3 years). Over 95% of children were of European ancestry (Figure S1). Samples were enriched for allergic cases.
2.2.2 ∣. Disease definition
Definition of current asthma, dermatitis, or rhinitis was agreed by a panel of experts and used in all MeDALL studies.1 Allergic diseases were defined by questionnaire-based parent- or self-reported doctor diagnosis and related symptoms.
2.2.3 ∣. Data production
RNA was extracted, quality-checked (RNA Integrity Number > 6), transformed into labeled cDNA and hybridized to HTA (Affymetrix, UK).
2.2.4 ∣. Statistical methods—data cleaning and differential expression
Expression levels were normalized with the robust multiarray average algorithm,13 using oligo in R v3.5.3. Multivariable models were adjusted for covariables (sex, cohort, batch, age) and fit with limma to identify DEGs (NetAffx v36 annotation). Surrogate variable analysis was used to capture cell blood heterogeneity (Figure S2).14 P-values were corrected for multiple testing (Benjamini-Hochberg false discovery rate, FDR),15 with threshold < 5%. No threshold was set for fold change (log2FC). Heatmap clustering was computed with ward method on Euclidean distance. We used G*Power v3.1.9.3 to assess power (post hoc mode with log2FC = 0.2 as the effect size, achieved power for DEGs with their actual log2FC). Sensitivity analysis was performed with adjustment for blood cell counts (neutrophils, eosinophils, lymphocytes, monocytes), or inhaled corticosteroids (BAMSE), or grouping cohorts by age. Synergistic effect of multimorbidity on gene expression was assessed from results of linear models (based on phenotype or disease number) including all samples, by computing the coefficient of linearity σ based on the difference in normalized FC between 1 and 2 diseases, and 2 and 3 diseases.
2.2.5 ∣. Biological interpretation
Functional enrichment included gene ontology, pathways (KEGG, REACTOME) with g:Profiler and disease ontology (DOSE). Treemap visualization was performed with REVIGO. PPIN was retrieved from BIOGRID v3.5 and visualized with Cytoscape v3.7.1 (network metrics from NetworkAnalyzer).
2.2.6 ∣. Validation
Expression levels were assessed with RT-qPCR (LightCycler 480 II, Roche, QuantiFAST SyBR kit, Qiagen) on a random subset of 8 genes among the significant DEGs in multimorbidity, using RNA from 45 participants.
2.2.7 ∣. Weighted gene co-expression network analysis
Modules of co-expressed genes were retrieved on discovery cohort expression values with WGCNA, discarding probesets with low variance, and using soft power thresholds of 3 and module eigengene dissimilarity threshold of 0.1 to 0.9. Genes within the same modules are likely to behave similarly and process within the same biological processes. Modules were correlated to clinical traits: presence of any multimorbidity, asthma multimorbidity, dermatitis multimorbidity, rhinitis multimorbidity, sex, age, and cohort. Gene significance to multimorbidity and gene-module membership were calculated.
2.3 ∣. Replication study
2.3.1 ∣. Setting and population
RNA sequencing data were available from white blood cells of 447 participants (15.5 years, range 10-20, San Juan, Puerto Rico) from EVA-PR cohort, without missing data on variables and outcomes of interest, including 218 participants with asthma, 275 with rhinitis, 53 with dermatitis, and 115 without allergic disease (Table 1).
2.3.2 ∣. Disease definition
Asthma: doctor's diagnosis of asthma and wheeze in the previous year. Rhinitis: physician-diagnosed allergic rhinitis and symptoms (at a time when the child did not have a cold or flu) in the previous 12 months. Dermatitis: symptoms and at least one positive allergen-specific IgE.
2.3.3 ∣. Data production
RNA Sequencing was performed using 350 ng of RNA extracted from white blood cells after removing hemoglobin (RNA Integrity Number > 7). Library preparation was done using TruSeq Stranded Total RNA Library Prep Kit with Ribo-Zero Gold High-Throughput Kit to remove cytoplasmic and mitochondrial ribosomal RNA (Illumina Inc, San Diego, CA). Libraries were run on the NextSeq 500 using NextSeq® 500/550 High Output Kit v2, using paired-end reads at 75 cycles and with 80 million reads per sample. Quality control for raw RNA Sequencing fastq files was performed using FastQC. Low quality reads and 3’ adapters were trimmed with Trim Galore! and Cutadapt. Trimmed reads were aligned to reference human genome (hg19) with STAR. Subsequently, the count of uniquely mapped read fragments was quantified for each sample as proxy for the expression level of each of the 26 335 human genes annotated in the Illumina iGenomes database using RSEM. Samples with low alignment percentage were removed from downstream analyses. Independent filtering of low-expressed genes (mean count < 2) was performed. After preprocessing, 16,880 genes and 447 samples were retained in the final analysis.
2.3.4 ∣. Statistical methods—Differential expression
DEGs were analyzed based on raw counts using DESeq2.16 Multivariable models were adjusted for age, sex, batch, and cell type proportions of eosinophils, neutrophils, monocytes, and lymphocytes in white blood cells. Subjects without any allergic disease were used as controls. A zero-mean normal prior was put on the nonintercept coefficients, to ensure that fold changes are independent of the choice of reference level. The Benjamini-Hochberg procedure was applied to adjust for multiple testing.15
2.3.5 ∣. Genes selected for replication
All the DEGs significant in any multimorbidity versus controls and in rhinitis versus controls were tested in the replication study. Moreover, a selection of genes significant in multimorbidity was tested in asthma alone.
3 ∣. RESULTS
3.1 ∣. Clinical characteristics of discovery and replication population
In MeDALL, 46% of allergic participants had asthma (61% with multimorbidity), 42% dermatitis (48%), and 51% rhinitis (49%). Asthma was more common in BAMSE (63%), and rhinitis in GINIplus (79%). Dermatitis in preschool INMA participants was predominant (74%). Fifty-five percent of the participants had no allergic disease (BAMSE 39%, GINIplus 67%, INMA 54%) (Table 1). In EVA-PR, 66% of allergic participants had asthma (78% with multimorbidity), 16% dermatitis (87%), and 83% rhinitis (65%) (Table 1). Inhaled corticosteroids in the past year were administered to 25.5% of asthmatic participants in BAMSE, 21.2% in GINIplus, 25.0% in INMA, and 25.2% in EVA-PR.
3.2 ∣. Identification and validation of differentially expressed genes in discovery study
Overall, in whole blood, FDR was reached for 50 genes comparing participants with either any multimorbidity, or asthma, dermatitis, or rhinitis multimorbidity, or rhinitis alone, to controls with no allergic disease (Table 2). Their chromosome localization is shown in Table S2 and Figure S3. Thirty-two genes were not previously reported in allergic diseases, of which 17 genes were linked to immune processes (Table 2, online Supplement).
TABLE 2.
Differentially expressed genes in MeDALL and replication in EVA-PR
| Gene identifier | Known in allergy |
Known in immune process |
MeDALL |
EVA-PR |
||||
|---|---|---|---|---|---|---|---|---|
| Any multimorbidity (62.1%) |
Any multimorbidity (51.1%) |
|||||||
| log2FC | P-value | FDR | log2FC | P-value | FDR | |||
| A. Multimorbidity | ||||||||
| CLC | Yes | Yes | 0.44 | 1.3E–08 | 8.8E–04 | 0.14 | 1.4E–04 | 4.4E–04 |
| SLC29A1 | No | No | 0.09 | 3.1E–07 | 7.1E–03 | 0.17 | 2.6E–06 | 1.9E–05 |
| HRH4 | Yes | Yes | 0.20 | 4.7E–07 | 7.1E–03 | 0.12 | 1.1E–03 | 2.7E–03 |
| FRRS1 | No | No | 0.13 | 5.1E–07 | 7.1E–03 | 0.14 | 7.2E–05 | 2.6E–04 |
| LOC101929979a | N/A | N/A | 0.32 | 5.7E–07 | 7.1E–03 | Nullb | Nullb | Nullb |
| IL5RA | Yes | Yes | 0.28 | 6.3E–07 | 7.1E–03 | 0.15 | 4.2E–05 | 1.8E–04 |
| CACNA1D | No | No | 0.04 | 3.3E–06 | 2.5E–02 | 0.07 | 3.9E–02 | >5E–02 |
| IL1RL1 | Yes | Yes | 0.20 | 3.3E–06 | 2.5E–02 | 0.09 | 1.2E–02 | 2.2E–02 |
| TTC7B | No | No | 0.08 | 4.6E–06 | 2.8E–02 | 0.03 | 3.3E–01 | >5E–02 |
| CYSLTR2 | Yes | Yes | 0.16 | 4.6E–06 | 2.8E–02 | 0.07 | 3.1E–02 | >5E–02 |
| EMR4P | No | Yes | 0.23 | 5.7E–06 | 3.0E–02 | 0.13 | 5.0E–04 | 1.4E–03 |
| PIK3R6 | No | Yes | 0.10 | 7.4E–06 | 3.6E–02 | 0.09 | 5.4E–03 | 1.1E–02 |
| ZSCAN29 | No | No | 0.06 | 9.6E–06 | 4.0E–02 | 0.03 | >5E–02 | >5E–02 |
| Gene Identifier | Known in allergy |
Known in immune process |
MeDALL |
EVA-PR |
||||
| Rhinitis alone (53.4%) |
Rhinitis alone (42.1%) |
|||||||
| log2FC | P-value | FDR | log2FC | P-value | FDR | |||
| B. Rhinitis alone | ||||||||
| SENP1 | No | No | 0.06 | 7.4E–07 | 1.4E–03 | 0.00 | >5E–02 | >5E–02 |
| BCAP29 | No | Yes | 0.07 | 3.3E–06 | 5.0E–03 | −0.01 | >5E–02 | >5E–02 |
| CEBPE | No | Yes | 0.11 | 3.6E–06 | 5.3E–03 | 0.11 | 1.6E–02 | >5E–02 |
| FAM66C | No | Yes | −0.03 | 6.5E–06 | 8.6E–03 | 0.07 | >5E–02 | >5E–02 |
| TNFSF14 | Yes | No | 0.15 | 7.9E–06 | 1.0E-02 | −0.08 | >5E–02 | >5E–02 |
| RNA5SP378a | N/A | N/A | −0.08 | 1.3E–05 | 1.6E–02 | Nullb | Nullb | Nullb |
| MAGEA9B | No | No | −0.05 | 1.5E–05 | 1.7E–02 | Nullb | Nullb | Nullb |
| RP1-149A16.12a | N/A | N/A | −0.07 | 1.6E–05 | 1.8E–02 | Nullb | Nullb | Nullb |
| RNA5SP335a | N/A | N/A | 0.10 | 1.7E–05 | 1.8E–02 | Nullb | Nullb | Nullb |
| LOC101928812a | N/A | N/A | 0.09 | 2.0E–05 | 1.9E–02 | −0.05 | >5E–02 | >5E–02 |
| LEPREL4 | No | No | −0.03 | 2.5E–05 | 2.2E–02 | 0.01 | >5E–02 | >5E–02 |
| CLINT1 | No | No | 0.05 | 2.7E–05 | 2.3E–02 | 0.00 | >5E–02 | >5E–02 |
| NDUFB5 | No | No | 0.05 | 2.7E–05 | 2.3E–02 | 0.00 | >5E–02 | >5E–02 |
| NPIPB5 | No | No | −0.08 | 3.2E–05 | 2.6E–02 | 0.05 | >5E–02 | >5E–02 |
| LA16c-395F10.1a | N/A | N/A | 0.09 | 3.4E–05 | 2.7E–02 | Nullb | Nullb | Nullb |
| AK2 | No | Yes | 0.04 | 3.9E–05 | 3.1E–02 | 0.01 | >5E–02 | >5E–02 |
| GLT6D1 | No | No | −0.08 | 3.9E–05 | 3.1E–02 | Nullb | Nullb | Nullb |
| MIR539 | No | No | −0.11 | 4.2E–05 | 3.2E–02 | Nullb | Nullb | Nullb |
| LOC101927780a | N/A | N/A | 0.08 | 4.2E–05 | 3.2E–02 | 0.18 | 1.3E–04 | 2.3E–03 |
| RNA5SP440a | N/A | N/A | −0.09 | 4.4E–05 | 3.3E–02 | Nullb | Nullb | Nullb |
| DUSP16 | No | No | 0.10 | 5.0E–05 | 3.5E–02 | −0.01 | >5E–02 | >5E–02 |
| GPR65 | No | Yes | 0.09 | 5.1E–05 | 3.5E–02 | 0.03 | >5E–02 | >5E–02 |
| RNF149 | No | No | −0.05 | 5.2E–05 | 3.5E–02 | −0.03 | >5E–02 | >5E–02 |
| SLC25A40 | No | No | 0.06 | 5.2E–05 | 3.5E–02 | −0.02 | >5E–02 | >5E–02 |
| EXOC5 | No | No | 0.04 | 5.8E–05 | 3.8E-02 | 0.01 | >5E–02 | >5E–02 |
| HMGA1P4 | No | No | 0.10 | 7.3E–05 | 4.4E–02 | Nullb | Nullb | Nullb |
| LOC101929526a | N/A | N/A | 0.09 | 8.2E–05 | 4.8E–02 | Nullb | Nullb | Nullb |
Note: Differentially expressed genes with log2FC and FDR p-values in A. any multimorbidity sorted by increasing FDR, or B. rhinitis alone for MeDALL and EVA-PR participants. Power of each group in parenthesis (%). P-values < .05 are in bold.
Long intergenic nonprotein coding RNA, pseudogenes or ribosomal pseudogenes, their role in allergy or immune processes could not be determined (N/A: not applicable)
null: not tested due to QC and RNA Sequencing issues. Genes that were specific for asthma, dermatitis, and rhinitis multimorbidity are not shown (ACSM3, SIGLEC8, ALOX15, ALOX15P1, BACE2-IT1, LGALS12, PNPLA6, SEMA7A, SMPD3, and SORD).
No DEG was associated with asthma alone or dermatitis alone but 27 DEGs were found in rhinitis alone (Figure S4.2), of which 18 were not previously described in relation to rhinitis.
FDR was reached for 13 genes when pooling participants with any multimorbidity (Figure S4.1). A multimorbidity signature of 8 genes was present in all types of multimorbidity for asthma, dermatitis, and rhinitis (Table 3, Figure 2A-C): CLC, SLC29A1, FRRS1, IL5RA, HRH4, SIGLEC8, EMR4P, IL1RL1. None of these multimorbidity genes was found in rhinitis alone.
TABLE 3.
DEGs shared between multimorbidity for asthma, dermatitis, or rhinitis
| Gene identifier | Chromosome | Name | Description | Previous identificationa |
|---|---|---|---|---|
| CLC | 19q13.2 | Charcot-Leyden crystal galectin | Eosinophil | Asthma, rhinitis |
| SLC29A1 | 6p21.1 | Solute carrier family 29 member 1 | Nucleoside transporter | No |
| FRRS1 | 1p21.2 | Ferric chelate reductase 1 | Cytochrome b561 | No |
| IL5RA | 3p26.2 | Interleukin 5 receptor subunit alpha | Eosinophil | Asthma, dermatitis, rhinitis |
| HRH4 | 18q11.2 | Histamine receptor H4 | Histamine | Asthma, dermatitis, rhinitis |
| SIGLEC8 | 19q13.41 | Sialic acid binding Ig like lectin 8 | Eosinophil | Asthma, rhinitis |
| EMR4P | 19p13.2 | Adhesion G protein-coupled receptor E4 | Leukocyte adhesion | No |
| IL1RL1 | 2q12.1 | Interleukin 1 receptor like 1 | Eosinophil | Asthma, rhinitis |
Note: Name, description and previous identification of genes shared between asthma, dermatitis, and rhinitis multimorbidity.
See online Supplement.
FIGURE 2.
Shared genes in multimorbidity. (A) Number of DEGs shared between any multimorbidity, asthma, dermatitis, or rhinitis multimorbidity. The text box lists the 7 genes shared by all groups. (B) Heatmap of −log10(FDR P-values) for the 50 genes and each group, with blue-white color scale on top right panel. Clustering on genes and groups was done with ward method on euclidean distance. EMR4P and IL1RL1 are targeted by two probesets. multi.: multimorbidity. (C) Heatmap of −log10(FDR p-values) for each gene that is shared between at least two of asthma multimorbidity, dermatitis multimorbidity, or rhinitis multimorbidity, with blue-white color scale on top right panel. The 8 genes shared between asthma, dermatitis, or rhinitis multimorbidity are in bold. Clustering on genes and groups was done with ward method on euclidean distance. (D) Normalized fold change variation across increasing number of co-occurrent disease, for the 12 genes in C, using a linear multivariate model including all allergic phenotypes. (E) KEGG and REACTOME pathways significant (FDR < 5%) with DEGs in asthma, dermatitis, or rhinitis multimorbidity versus controls (Table S7.1). Terms are sorted by decreasing significance. (F) BIOGRID PPIN from DEGs shared between asthma, dermatitis, and rhinitis multimorbidity. Octogons: proteins encoded by our candidate genes; rounded rectangle: interactors, alphabetically sorted in grid layout on the bottom right. All types of interactions were considered
These 8 genes are overexpressed in participants with multimorbidity (Figure S5), with relatively limited fold change magnitude. CLC has the highest log2FC value (0.44-0.48), followed by IL5RA (0.28-0.32), EMR4P (0.23-0.29), IL1RL1 (0.20-0.24), HRH4 (0.20-0.24).
Power calculation and list of significant DEGs are reported in Table S1. Power in any single disease vs no disease was estimated to 79%, reached 62.1% in any multimorbidity, and was comparable between asthma multimorbidity, dermatitis multimorbidity, or rhinitis multimorbidity (47.3%-56.7%), as compared to asthma alone, dermatitis alone, or rhinitis alone (44.3%-53.5%).
We randomly selected 8 genes for validation with RT-qPCR, which had a higher relative gene expression in multimorbidity (Figure S6, Table S3).
Sensitivity analysis with adjustment for blood cell counts in BAMSE revealed, at most, a 13% decrease of the fold change estimate for CLC (Table S4.1). Inhaled corticosteroids did not impact the fold change magnitude (Table S4.2).
3.3 ∣. Replication of multimorbidity results in EVA-PR cohorts
Twelve of the 13 DEGs in any multimorbidity had expression values available, and 10 were replicated (83%) in white blood cells from EVA-PR. Among the multimorbidity signature of 8 genes, all were confirmed. The genes with the highest fold changes were SLC29A1, IL5RA, CLC, FRRS1, EMR4P, and HRH4 (log2FC = 0.12-0.17). Moreover, among the genes tested, none was found significant in participants with asthma alone. One gene (CEBPE) and one locus (LOC101927780) in rhinitis alone were replicated. Exhaustive results are in Table S5 and Figure S7.
3.4 ∣. Synergistic effects of multimorbidity on the significant genes
Using a linear model including all samples (Table S6), all genes had synergistic effects of multimorbidity (σ = 1.5-58.4), except LOC101929979 (σ = 1.1). Figure 2D shows the figures for the 12 genes present in at least two of asthma, dermatitis, or rhinitis multimorbidity. A linear model based on the number of diseases produced the same results (Table S6.3).
3.5 ∣. Biological interpretation of differentially expressed genes: Functional enrichment analysis
We interrogated ontology databases with the DEGs in any multimorbidity (Figure S8, Table S7). We identified biological processes and molecular functions such as “transmembrane signaling receptor activity,” “immune response,” “cell communication,” or “interleukin-5 production” (FDR P = 1.2e–03 to P = 3.0e–03), as well as REACTOME pathways such as “Signal Transduction,” “Signaling by Interleukins” and “Interleukin-33 signaling” (FDR P = 1.2e–03 to P = 1.5e–02). Leukocyte activation was involved (FDR P = 3.5e–03). Similar ontology was identified with the DEGs found in adolescents with any multimorbidity (Table S7.7). Disease ontology retrieved clusters of respiratory and skin diseases (Figure S9, Table S8).
3.6 ∣. Biological interpretation of differentially expressed genes: Protein-protein interaction network
We retrieved 37 proteins interacting with the proteins encoded by the 8 DEGs shared in multimorbidity. CLC, SLC29A1, IL5RA, SIGLEC8, and IL1RL1, but not HRH4, EMR4P, and FRRS1, were retrieved in BIOGRID (Figure 2F). The PPIN had an average clustering coefficient of 20.7%, and centralization of 49.8%. Nine of the 37 interactors were involved in IL5/JAK/STAT and IL33/ST2/IRAK/TRAF pathways, and 24 in allergy or inflammatory processes (online Supplement). IL5RA and IL1RL1 (ST2) belong to IL5/JAK/STAT and IL33/ST2/IRAK/TRAF pathways, respectively. Pathway analysis on the interactors (Table S9) retrieved 18 members of the REACTOME pathway “Immune System” (R-HSA-168256, P = 6.6e–14), and 13 members of “Signaling by Interleukins” (R-HSA-449147, P = 2.1e–15).
3.7 ∣. Co-expression modules
A module of 60 co-expressed genes (“paleturquoise”) was detected with stringent clustering height (h = 0.9, Figure S10.1). Lower height cutoff did not split this module (h = 0.6, Figure S10.2). It includes the 8 genes shared in multimorbidity. CLC scored the highest gene significance for multimorbidity (GS.multimorbidity = 0.16, P = 4.8e–06), and IL5RA scored the highest module membership (MM.paleturquoise = 0.94, P < 1.8e–311). The module was positively correlated to any multimorbidity (r2 = .14, P = 5e–05), asthma multimorbidity, dermatitis multimorbidity, and rhinitis multimorbidity (r2 = 0.13, P-value from 3e–04 to 2e–04). Gene ontology for the 60 co-expressed genes in “paleturquoise” (Table S10) retrieved an immune system and signal transduction signature (FDR from P = 4.5e–11). The implication of leukocyte (GO:0045321) was detected with 10 genes (P = 4e–06). Disease ontology retrieved clusters of respiratory and skin diseases.
4 ∣. DISCUSSION
For the first time, this study shows a notable difference between multimorbid and single allergic diseases. With a multimorbid approach, we identified important genomic features of allergic diseases that would have been missed using a single disease approach. No gene was associated to asthma or dermatitis alone but 27 were found in rhinitis alone in MeDALL. Thirteen DEGs were found in participants with any multimorbidity as compared to controls. Eight genes were common to asthma, dermatitis, and rhinitis multimorbidity. Our key results were highly replicated in the EVA-PR cohort, with 10 of the 12 tested multimorbidity genes being significant. RT-qPCR in MeDALL validated overexpression of selected genes in multimorbidity, which were found associated to immune and signaling pathway. Unsupervised analysis retrieved our candidate genes co-expressed in a same module, validating their potential involvement in related biological processes. Moreover, fold changes of our candidate genes increased along with the number of co-occurrent diseases. This synergistic effect launches new insights on the mechanisms of multimorbidity for allergic diseases. Overall, eosinophil- and T2-associated genes were identified, confirming the MeDALL hypothesis that type 2 immunity has an important role in the origin of multimorbidity.5
4.1 ∣. Strengths and limitations
We used HTA in the discovery study because of its genome-wide coverage and high number of probesets. At the time of study, the reduced costs compared to RNA sequencing allowed the analysis of a greater number of samples, thus an increased power to dig into gene expression specificities of multimorbidity. The HTA results were highly replicated and comparable with the RNA Sequencing results in EVA-PR.
Since allergic diseases are systemic and we assessed multimorbidity, we did not use specific tissues from the skin, nose and bronchi but whole blood. We detected genes associated with eosinophils and immune response, which partly overlap with the findings from our recent MeDALL epigenome-wide study on asthma,17 where CLC, IL5RA, SLC29A1, and SIGLEC8 still reach significance after eosinophil correction. Since blood samples were not drawn after allergen challenge, which may increase the signal, we expected to find low effect sizes (small log2FC values). The limited magnitude of fold change values, observed in the literature for asthma,18 does not prevent biological significance.19 Moreover, we validated the biological relevance of our genes with RT-qPCR, ontology and network-based analyses. We retrieved 13 DEGs comparing 123 participants with any multimorbidity to 431 participants without allergic disease. The pooling approach of MeDALL allowed us to increase FDR significance.
Power was anticipated with a low effect size (d = 0.2). In the present study, we retrieved more DEGs in 123 participants with any multimorbidity (13 DEGs with power = 62%) than in 232 participants with single disease (no DEGs, power = 79%, Table S1.11). Each discovery cohort alone had an insufficient power, validating the pooling approach of MeDALL.
Our discovery study included two cohorts of adolescents and one of children, thus limiting our capacity to assess the role of age.20 Nevertheless, FDR was reached for 9 genes grouping adolescents with any multimorbidity, including CLC, FRRS1, HRH4, IL5RA, EMR4P, and IL1RL1, shared between asthma, dermatitis, and rhinitis multimorbidity (Table S4.3).
There were some differences between the European and Puerto Rican cohorts, for example in age distribution and disease prevalences, that may be explained by the different study designs, populations, and/or lifestyle factors. A complete harmonization of disease definitions was not available between discovery and replication cohorts. The resulting dissimilarities (eg, inclusion of positive IgE sensitization, or symptom-based definition versus self-reported doctor diagnosis) could have led to bias in overselection of IgE mechanism–associated genes, or differences in disease prevalence (overselection of more severe cases), but the reproducibility of the results showed the generalizability of the findings.
4.2 ∣. Replication analyses of multimorbidity
The results for multimorbidity were consistent in particular since the replication cohort was from a different environment (Puerto Rico), and RNA Sequencing on white blood cells was used.9,21 For example, the log2FC value for CLC in discovery is 0.44 in any multimorbidity (expression increased by 36% in allergic participants compared to controls), and 0.14 in replication (10% increase). The inter-platform difference is thus 26% for CLC, and less than 10% in the other multimorbidity genes. Thus, although some differences in effect sizes were noted between the discovery and replication cohorts, our analyses identify robust multimorbidity genes such as CLC.
In children and adolescents, asthma or dermatitis alone was not associated with any DEG whereas rhinitis alone was associated with 27 DEGs, in line with the clinical knowledge that 85% of asthma patients have also rhinitis, whereas only a third of rhinitis patients have asthma.2 This study suggests that mechanistic studies in asthma could also benefit from considering the presence of rhinitis as multimorbidity. However, despite a distinct signature of rhinitis alone in the discovery cohort, the limited replication suggests that further studies need to be done.
4.3 ∣. Candidate genes function
Of the eight multimorbidity genes, five were eosinophil-specific genes. CLC (Galectin-10),22 which is involved in regulation of T-cell proliferation, and predicts oral corticosteroid response in asthma (see Supplementary Bibliography), featured the highest log2FC and was significant in any type of multimorbidity. IL5RA has been involved in dermatitis23 and asthma,24 IL1RL1 in asthma,25 and was found associated with multimorbidity in our computational study of the protein interaction network.5 SIGLEC8 is a susceptibility locus for asthma.26 Expression levels of SIGLEC8 in our RT-qPCR validation were comparable in asthma multimorbidity and asthma alone, highlighting the importance of SIGLEC8 in asthma. EMR4P may be involved in leukocyte adhesion and migration (Supplementary Bibliography). Among the three other genes, HRH4 was related to allergic diseases and immune response (Table 3).27 FRRS1 is a member of cytochrome b561, and SLC29A1 mediates the cellular uptake of nucleosides from the surrounding medium (Supplementary Bibliography). EMR4P, FRRS1 and SLC29A1 were not previously described in allergic diseases.
Moreover, among the genes found with any multimorbidity, or multimorbidity for asthma, dermatitis or rhinitis, eight were linked to allergic inflammation and immune response (Table 2 and online Supplement). In addition to genes cited above, SEMA7A was eosinophil-specific and may play a role in airway remodeling of asthma.28 CLC, IL5RA, SIGLEC8, SEMA7A correlated strongly to differentially methylated CpG sites in asthma.17 In addition to HRH4, ALOX15,29 and CYSLTR230 were related to allergic diseases and immune response.
Among genes found in rhinitis alone, SENP1 is important for the epithelial-mesenchymal trophic unit, AK2, CEBPE, GPR65, are involved in white blood cells differentiation, and BCAP29 influences the traffic of MHC class I molecules (online Supplement). TNFSF14 was known in allergy with no known role in inflammation.31
Thirty-two genes were potential novel targets, notably EMR4P, FRRS1, and SLC29A1. Nine genes had known roles in inflammatory processes, and nine uncharacterized genes corresponded to long intergenic nonprotein coding RNA, or pseudogenes.
These results enforce the role of eosinophil activation in childhood allergic diseases through DNA methylation and gene expression processes. Without exhaustive information on blood cell composition in MeDALL, we used surrogate variable analysis to detect and adjust for unknown surrogate variables, which demonstrated the best performance for cell type mixture.14 Moreover, we validated our approach with a sensitivity analysis in one discovery cohort (Table S4) and found no major overall influence on the association results. All analyses in EVA-PR were adjusted for white blood cell type. Thus, our overall findings should not be confounded by higher eosinophil counts in allergic patients per se. Inhaled corticosteroids and ß2 agonists did not impact the observed effect (Table S4.2).
4.4 ∣. Multimorbidity-related pathways and unsupervised analysis
PPIN retrieved IL5/JAK/STAT pathway, well known in IL-5 inflammation, and IL33/ST2/IRAK/TRAF pathway, involved in IL-33 and IL1RL1 inflammation (online Supplement). IL1RL1 and IL5RA were shared between asthma, dermatitis, and rhinitis multimorbidity. Fifteen other interactors were related to allergy, immune, or inflammatory processes (online Supplement).
We validated the signature with WGCNA, and retrieved, in an unsupervised manner, a module of co-expressed genes with a high clustering coefficient, which survived a stringent height cutoff, indicating biological relationship and strong expression similarities with other genes of the module. All 8 genes shared in multimorbidity were co-expressed in this module. The module correlation to multimorbidity was significant. Fourteen co-expressed genes were involved in allergy and immune processes (online Supplement).
5 ∣. CONCLUSION
Among the 50 genes differentially expressed in participants with any allergic disease, 8 were common to multimorbidity, and 4 associated with eosinophil expression. All had synergistic effect associated to multimorbidity. This genetic signature of multimorbidity can provide new diagnostic and therapeutic opportunities. Thirty-two genes previously unknown in allergic diseases were discovered. Our results support the need to assess the presence of multimorbidity in clinical and genetic investigations of allergic diseases in children and adolescents.
Supplementary Material
ACKNOWLEDGMENTS
We thank all other investigators of the MeDALL Study Group for their advices and support: Annesi-Maesano Isabella, Baiz Nour, Bedbrook Anna, Cambon-Thomsen Anne, Jacquemin Benedicte, Kauffmann Francine, Pin Isabelle, Rial-Sebbag Emmanuelle, Nadif Rachel, Basagna Xavier, Benet Mora Marta, Kogevinas Manolis, Lavi Iris, Mestre Jordi, Pinart Mariona, Colli Matthias, Hettler-Chen Chih-Mei, Hohmann Cynthia, Keller Teresa, Lau Susanne, Schietinger Andrea, van Hofmann Ingrid, Worm Margitta, Zuberbier Torsten, Pison Christophe, Kerkhof Marjan, Nawijn Martijn C., van Oosterhout Antoon J. M., Wijmenga Cisca, Bachert Claus, Coquet Jonathan, Hammad Hamida, Lambrecht Bart, Saeys Yvan, Haahtela Tari, Hanninen Sinikka, Makela Mika, Reitamo Sakari, von Hertzen Leena, Klimek Magdalena, Kowalski Marek, Carlsen Kai Hakon, Lodrup-Carlsen Karin C., Baar Alexandra, Lupinek Christian, Pahr Sandra, Valenta Rudolf, van de Veen Willem, Andersson Niklas, Ballardini Natalia, Johansson SGO, Kumar Ashish, Merid Simon Kebede, Thacher Jesse, van Hage Marianne, Westman Marit, Yazdanbakhsh Maria, Tischer Christina, Brunekreef Bert, Gehring Ulrike, Smit Henriette A, Le Naour Stéphanie, Smagghe Delphine, Albang Richard, Arno Albert, Mascaro Angels, Roda Xavier, Sanchez Cristina, Vega Mireia, Baumgartner Ursula, Neubauer Angela, Stolz Franck, McEachan Rosie, Oddie Sam, Petherick Emily, Raynor Pauline, Waiblinger Dagmar, Wright John, Martinez Fernando D., De Carlo Giuseppe, Palkonen Susanna, Salvi Roberta, Wecksell Per-Ake, Bindslev-Jensen Carsten, Eller Esben, Steensen Jens Peter, Forestiere Francesco, Narduzzi Silvia, Porta Daniela. We acknowledge IRT BIOASTER for hosting MeDALL data production team: Alain Troesch and Nathalie Garçon ; Vincent Lotteau from INSERM for kindly hosting part of MeDALL data production ; and members of the CNRS USR3010: Charles Auffray, Stéphane Ballereau and Johann Pellet, plus Bertr and De Meulderand Diane Lefaudeux from U-BIOPRED project for the active discussions on sample selection and analyses. We thank Dieter Maier for the knowledge management platform and the data sharing between partners. The authors thank all children and parents participating in the BAMSE cohort, the nurses, and other staff working with the BAMSE project. The authors thank all the families for their participation in the GINIplus study. Furthermore, we thank all members of the GINIplus Study Group for their excellent work. The GINIplus Study group consists of the following: Institute of Epidemiology I, Helmholtz Zentrum München, German Research Centre for Environmental Health, Neuherberg (Heinrich J, Brüske I, Schulz H, Flexeder C, Zeller C, Standl M, Schnappinger M, Ferland M, Thiering E, Tiesler C); Department of Pediatrics, Marien-Hospital, Wesel (Berdel D, von Berg A); Ludwig-Maximilians-University of Munich, Dr von Hauner Children's Hospital (Koletzko S); Child and Adolescent Medicine, University Hospital rechts der Isar of the Technical University Munich (Bauer CP, Hoffmann U); IUF- Environmental Health Research Institute, Düsseldorf (Schikowski T, Link E, Klümper C). The authors thank all the families for their participation in the INMA project. A full list of INMA researchers can be found at http://www.proyectoinma.org/presentacion-inma/listado-investigadores/listado-investigadores.html.
Funding information
NIH, Grant/Award Number: HL079966, HL117191, MD011764 and U54MD007587; ANR, Grant/Award Number: ANR-15-IDEX-02; European Research Council, Grant/Award Number: 757919; Federal Ministry for Education, Science, Research and Technology – Helmholtz Zentrum Munich – Research Institute at Marien-Hospital Wesel – LMU Munich – TU Munich – IUF - Leibniz Research-Institute for Environmental Medicine, University of Düsseldorf; CLARA – CHUGA – UGA, Grant/Award Number: Chair of Excellence in Translational Research; Instituto de Salud Carlos III, Grant/Award Number: CB06/02/0041, G03/176, PI041436 and PI081151; Federal Ministry for Environment, Grant/Award Number: FKZ 20462296; The Swedish Research Council – The Swedish Heart and Lung Foundation – The Swedish Research Council for Working Life and Social Welfare – The Swedish Asthma and Allergy Association Research Foundation – The Swedish Research Council Formas – Stockholm County Council; Generalitat de Catalunya, Grant/Award Number: CIRIT 1999SGR 00241; Mechanisms of the Development of ALLergy (MeDALL) project, European framework programme 7, Grant/Award Number: 261357; Bart
Abbreviations:
- A
Asthma
- BAMSE
Barn Allergi Miljö Stockholm Epidemiologi Projektet (Children Allergy, Milieu, Stockholm, an Epidemiological study)
- D
Atopic Dermatitis
- DEGs
Differentially Expressed Genes
- EVA-PR
Epigenetic Variation and Childhood Asthma in Puerto Ricans
- FC
Fold Change
- FDR
False Discovery Rate
- GINIplus
German Infant Study on the influence of Nutrition Intervention PLUS environmental and genetic influences on allergy development
- HTA
Affymetrix Human Transcriptome Array 2.0
- INMA
INfancia y Medio Ambiente (Environment and Childhood)
- MeDALL
Mechanisms of the Development of ALLergy
- PPIN
Protein-Protein Interaction Network
- R
allergic Rhinitis
- RT-qPCR
Reverse Transcription quantitative Polymerase Chain Reaction
- WGCNA
Weighted Gene Co-Expression Network Analysis
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Pinart M, Benet M, Annesi-Maesano I, et al. Comorbidity of eczema, rhinitis, and asthma in IgE-sensitised and non-IgE-sensitised children in MeDALL: a population-based cohort study. Lancet Respir Med. 2014;2:131–140. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl 86):8–160. [DOI] [PubMed] [Google Scholar]
- 3.Bieber T Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. [DOI] [PubMed] [Google Scholar]
- 4.Anto JM, Bousquet J, Akdis M, et al. Mechanisms of the Development of Allergy (MeDALL): Introducing novel concepts in allergy phenotypes. J Allergy Clin Immunol. 2017;139:388–399. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet J, Anto JM, Wickman M, et al. Are allergic multimorbidities and IgE polysensitization associated with the persistence or re-occurrence of foetal type 2 signalling? The MeDALL hypothesis. Allergy. 2015;70:1062–1078. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar D, Pinart M, Koppelman GH, et al. Computational analysis of multimorbidity between asthma, eczema and rhinitis. PLoS ONE. 2017;12:e0179125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demenais F, Margaritte-Jeannin P, Barnes KC, et al. Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nat Genet. 2018;50:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira MA, Vonk JM, Baurecht H, et al. Shared genetic origin of asthma, hay fever and eczema elucidates allergic disease biology. Nat Genet. 2017;49:1752–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y, Gruzieva O, Wang T, et al. Transcriptomics of atopy and atopic asthma in white blood cells from children and adolescents. Eur Respir J. 2019;53(5):1900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gref A, Merid SK, Gruzieva O, et al. Genome-wide interaction analysis of air pollution exposure and childhood asthma with functional follow-up. Am J Respir Crit Care Med. 2017;195:1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Von Berg A, Kramer U, Link E, et al. Impact of early feeding on childhood eczema: development after nutritional intervention compared with the natural course–the GINIplus study up to the age of 6 years. Clin Exp Allergy. 2010;40:627–636. [DOI] [PubMed] [Google Scholar]
- 12.Guxens M, Ballester F, Espada M, et al. Cohort profile: the INMA-INfancia y Medio Ambiente-(Environment and Childhood) project. Int J Epidemiol. 2012;41:930–940. [DOI] [PubMed] [Google Scholar]
- 13.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. [DOI] [PubMed] [Google Scholar]
- 14.McGregor K, Bernatsky S, Colmegna I, et al. An evaluation of methods correcting for cell-type heterogeneity in DNA methylation studies. Genome Biol. 2016;17:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B. 1995;57:289–300. [Google Scholar]
- 16.MI Love, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu CJ, Söderhäll C, Bustamante M, et al. DNA methylation in childhood asthma: an epigenome-wide meta-analysis. Lancet Respir Med. 2018;6:379–388. [DOI] [PubMed] [Google Scholar]
- 18.Morrow JD, Qiu W, Chhabra D, et al. Identifying a gene expression signature of frequent COPD exacerbations in peripheral blood using network methods. BMC Med Genomics. 2015;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.St Laurent G, Shtokalo D, Tackett MR, et al. On the importance of small changes in RNA expression. Methods. 2013;63(1):18–24. [DOI] [PubMed] [Google Scholar]
- 20.Belgrave CD, Granell R, Simpson A, et al. Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies. PLoS Med. 2014;11:e1001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao S, Fung-Leung WP, Bittner A, Ngo K, Liu X. Comparison of RNA-Seq and Microarray in Transcriptome Profiling of Activated T Cells. PLoS ONE. 2014;9(1):e78644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su J A Brief history of charcot-leyden crystal protein/Galectin-10 research. Molecules. 2018;23:2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semic-Jusufagic A, Gevaert P, Bachert C, Murray C, Simpson A, Custovic A. Increased serum-soluble interleukin-5 receptor alpha level precedes the development of eczema in children. Pediatr Allergy Immunol. 2010;21:1052–1058. [DOI] [PubMed] [Google Scholar]
- 24.Yasruel Z, Humbert M, Kotsimbos TC, et al. Membrane-bound and soluble alpha IL-5 receptor mRNA in the bronchial mucosa of atopic and nonatopic asthmatics. Am J Respir Crit Care Med. 1997;155:1413–1418. [DOI] [PubMed] [Google Scholar]
- 25.Dijk FN, Xu C, Melén E, et al. Genetic regulation of IL1RL1 methylation and IL1RL1-a protein levels in asthma. Eur Respir J. 2018;51:1701377. [DOI] [PubMed] [Google Scholar]
- 26.Gao PS, Shimizu K, Grant AV, et al. Polymorphisms in the sialic acid-binding immunoglobulin-like lectin-8 (Siglec-8) gene are associated with susceptibility to asthma. Eur J Hum Genet. 2010;18:713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walter M, Kottke T, Stark H. The histamine H4 receptor: Targeting inflammatory disorders. Eur J Pharmacol. 2011;668:1–5. [DOI] [PubMed] [Google Scholar]
- 28.Mizutani N, Nabe T, Yoshino S. Semaphorin 7A plays a critical role in IgE-mediated airway inflammation in mice. Eur J Pharmacol. 2015;764:149–156. [DOI] [PubMed] [Google Scholar]
- 29.Wen Y, Gu J, Chakrabarti SK, et al. The role of 12/15-lipoxygenase in the expression of interleukin-6 and tumor necrosis factor-alpha in macrophages. Endocrinol. 2007;148:1313–1322. [DOI] [PubMed] [Google Scholar]
- 30.Thompson MD, Capra V, Clunes MT, et al. Cysteinyl leukotrienes pathway genes, atopic asthma and drug response: from population isolates to large genome-wide association studies. Front Pharmacol. 2016;1(7):299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi F, Xiong Y, Zhang Y, et al. The role of TNF family molecules light in cellular interaction between airway smooth muscle cells and T cells during chronic allergic inflammation. Inflammation. 2018;41:1021–1031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




