Abstract
As of May 2022, there have been more than 527 million infections with severe acute respiratory disease coronavirus type 2 (SARS-CoV-2) and over 6.2 million deaths from Coronavirus Disease 2019 (COVID-19) worldwide. COVID-19 is a multisystem illness with important neurologic consequences that impact long-term morbidity and mortality. In the acutely ill, the neurologic manifestations of COVID-19 can include distressing but relatively benign symptoms such as headache, myalgias, and anosmia; however, entities such as encephalopathy, stroke, seizures, encephalitis, and Guillain–Barre Syndrome can cause neurologic injury and resulting disability that persists long after the acute pulmonary illness. Furthermore, as many as one-third of patients may experience persistent neurologic symptoms as part of a Post-Acute Sequelae of SARS-CoV-2 infection (Neuro-PASC) syndrome. This Neuro-PASC syndrome can affect patients who required hospitalization for COVID-19 or patients who did not require hospitalization and who may have had minor or no pulmonary symptoms. Given the large number of individuals affected and the ability of neurologic complications to impair quality of life and productivity, the neurologic manifestations of COVID-19 are likely to have major and long-lasting personal, public health, and economic consequences. While knowledge of disease mechanisms and therapies acquired prior to the pandemic can inform us on how to manage patients with the neurologic manifestations of COVID-19, there is a critical need for improved understanding of specific COVID-19 disease mechanisms and development of therapies that target the neurologic morbidities of COVID-19. This current perspective reviews evidence for proposed disease mechanisms as they inform the neurologic management of COVID-19 in adult patients while also identifying areas in need of further research.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01267-y.
Keywords: COVID-19, PASC, Long-COVID, Encephalopathy, SARS-CoV-2
Between the beginning of the Coronavirus Disease 2019 (COVID-19) pandemic and May 2022, there have been more than 527 million confirmed severe acute respiratory disease coronavirus type 2 (SARS-CoV-2) infections with over 6.2 million deaths worldwide, and in the USA, the cumulative rate of COVID-19 hospitalization now exceeds 974 hospitalizations per 100,000 overall population and 2722 hospitalizations per 100,000 among those 65 years and older [1, 2]. While COVID-19 was initially viewed as a pulmonary disease, it is now appreciated as a multisystem illness in which neurologic symptoms and syndromes may be prominent manifestations. In the acutely ill, COVID-19 can include neurologic complications with implications for disease recovery and long-term morbidity, and in the outpatient setting, persistent neurologic symptoms can manifest as part of Post-Acute Sequelae of SARS-CoV-2 infection (Neuro-PASC). As a result of the large number of SARS-CoV-2 infections, neurologic complications of COVID-19 are likely to create a demand for both acute and long-term neurologic evaluation and management.
In this current perspective, we review the leading neurologic manifestations and complications of COVID-19 as they occur in patients requiring acute hospitalization and in patients presenting in the outpatient setting. In addition, we review the leading neurologic complications that have been reported with vaccination against SARS-CoV-2. Given that the pathogenic mechanisms of acute COVID-19 and Neuro-PASC represent a rapidly evolving and unresolved area of research, we discuss hypothesized disease mechanisms as they might inform clinical evaluation and management. The reader should appreciate that many of these hypothesized mechanisms are a matter of ongoing debate. Furthermore, we draw from our neurocritical care service and Neuro-COVID-19 clinic experience to discuss diagnostic and therapeutic approaches for the acute and post-acute neurologic management of adult COVID-19 patients and future directions for investigation.
Neurologic Manifestations and Complications in Hospitalized COVID-19 Patients
Early in the pandemic, neurologic symptoms and syndromes were recognized as occurring frequently along with the acute pulmonary illness of COVID-19 [3–5]. In a study from our health system, we identified that 42% of hospitalized COVID-19 patients experienced some neurologic manifestation at COVID-19 symptom onset, and 63% of patients had experienced a neurologic manifestation by hospital admission [5]. By the end of acute illness, 419 out of 509 (82%) hospitalized patients had experienced some neurologic manifestation as a component of acute COVID-19 [5]. Myalgias, headache, encephalopathy, dizziness, dysgeusia, and anosmia combined accounted for 95.8% of neurologic manifestations at COVID-19 onset and 91.4% of manifestations during the course of acute COVID-19 [5]. While myalgias, headache, and anosmia/dysgeusia may be distressful to the patient, they did not impact the course of acute illness, mortality, nor occurrence of severe disability, in our experience. Therefore, our discussion of the neurologic complications of COVID-19 in hospitalized patients will focus on those syndromes that impact mortality or substantial morbidity.
Table 1 summarizes the major acute neurologic syndromes that have been associated with COVID-19 in the hospitalized population, and Fig. 1 provides examples of neuroimaging from select neurologic syndromes seen at our institution. Fortunately, with the exception of encephalopathy, most of these syndromes are infrequent and managed similarly to patients who do not have COVID-19. Furthermore, some of the neurologic syndromes seen in COVID-19 may contribute to a multifactorial encephalopathy. As such, we provide additional focus on encephalopathy as the principal acute neurologic syndrome of COVID-19. Several additional neurologic syndromes occurring more rarely in acute COVID-19 will not be discussed in this review, including: peripheral nerve and plexus syndromes [6], cranial neuropathies [7], compression peripheral nerve injuries after prone positioning [8], movement disorders [9], myositis/myopathy [5, 10] and rhino-orbital-cerebral mucormycosis [11, 12].
Table 1.
Acute neurologic syndromes in hospitalized COVID-19 patients
| Neurologic complication | Presentation | Frequency | Neurodiagnostic testing | Pathogenesis | Therapies |
|---|---|---|---|---|---|
| Encephalopathy | Subsyndromal Delirium, Delirium, and Coma. Positive CAM/CAM-ICU or ICDSC for delirium. GCS < 8 or RASS -4 or -5 for coma | 7.5–49% of all patients; 55–84% of critically ill patients |
MRI: non-specific abnormalities in over 50%, infarct/hemorrhage < 10% EEG: most often background slowing, seizures < 1% of all patients and 5.5–9.6% of critically ill CSF: protein > 45 mg/dL in 59%, WBC > 5 cells/µL in 43%, SARS-CoV-2 RT-PCR negative > 95% |
Hypoxia, sepsis, metabolic derangement, medications, systemic inflammation and organ failure, microvascular thrombosis and endothelial dysfunction, comorbid neurologic complications (eg, stroke) | Exclude neurologic emergencies, optimize systemic derangements, minimize contributory medications, address nutritional deficiencies (eg, thiamine), treat comorbid neurologic complications, early mobilization and reorientation, family/friend virtual visits, rarely immunomodulatory therapies for neurologic indications |
| Stroke | Focal neurologic deficits within first few weeks of COVID-19 onset, may be a contributor to encephalopathy |
Ischemic: 1%, up to 3.7% in critically ill Hemorrhagic: < 1%, 7% if on ECMO Venous thrombosis in COVID-19: < 0.1% Venous thrombosis after adenovirus-vector vaccination: 1.9 per million vaccine doses, 7 per million in women 18–49 years |
MRI: ischemic or hemorrhagic lesions of variable size from punctate to large with brain compression, arterial or venous occlusions |
Coagulopathy, endothelial dysfunction, cardiac dysfunction, traditional vascular risk factors Venous thrombosis after adenovirus-vector vaccination: Platelet factor 4 antibodies |
Guideline driven therapies and evaluation of stroke mechanism as for patients without COVID-19. Close attention to greater risk for treatment delay in COVID-19 Venous thrombosis after adenovirus-vector vaccination: non-heparin anticoagulation and IVIG |
| Seizures | Encephalopathy is most common reason to evaluate for seizures | < 1% of all patients; 5.5 to 9.6% of patients who receive EEG | Focal slowing and seizures may disproportionately involve frontal regions | 74% of those with seizures have either a prior brain disorder or acute or chronic structural lesion | Treated similarly to non-COVID patients |
| Non-viral encephalitis, including autoimmune encephalitis and ADEM/AHLE | Encephalopathy most commonly. Infrequently focal deficits and seizures resembling limbic encephalitis or ADEM | Potentially frequent contributor to encephalopathy in the critically ill (6.7%). Autoimmune cases are likely rare |
MRI: white matter hyperintensities, variable enhancement, variable ischemic or hemorrhagic lesions; rarely limbic encephalitis appearance EEG: generalized or focal slowing, seizures CSF: elevated protein (average 65 mg/dL), pleocytosis (average 15 cells/µL), SARS-CoV-2 RT-PCR negative, normal profile in 30% Auto-antibodies are rare |
Most frequently systemic inflammation with blood brain barrier disruption and astroglial activation; more rarely autoimmune | Supportive care and immunotherapies directed at COVID-19 pulmonary indications if due to systemic inflammation. Corticosteroids, IVIG, plasma exchange, and/or rituximab when autoimmune |
| Viral encephalitis or meningitis | Encephalopathy, seizures | Rare, case reports |
MRI: hippocampal, mesial temporal diffusion restriction or T2 hyperintensity EEG: generalized or focal slowing, seizures CSF: elevated protein (45–100 mg/dL), pleocytosis 10 s-100 s cells/µL), SARS-CoV-2 RT-PCR positive Brain Tissue positive (antigen or RNA) |
Brain parenchymal invasion by olfactory nerve, hematogenous spread, endothelial or immune cell infection | Remdesivir and immunotherapies directed at COVID-19 pulmonary indications |
| Guillain–Barre syndrome | Ascending weakness with paresthesia, frequent facial weakness (64%) and autonomic dysfunction (64%), all variants possible |
14.5 per million SARS-CoV-2 infections, < 0.5% of hospitalized patients Adenovirus-vector vaccination: 3.8 to 9.8 per million doses |
CSF: elevated protein, normal cell count, SARSCoV-2 RT-PCR negative EMG/NCS: demyelinating > motor-sensory axonal > motor axonal |
Post-infectious |
IVIG or plasma exchange Vaccine related GBS is likely less severe than COVID-19 related GBS |
CAM confusion assessment method, ICDSC intensive care delirium screening checklist, GCS Glasgow Coma Scale, RASS Richmond Agitation and Sedation Scale, MRI magnetic resonance imaging, EEG electroencephalogram, CSF cerebrospinal fluid, RT-PCR reverse transcription polymerase chain reaction, ECMO extracorporeal membrane oxygenation, IVIG intravenous immunoglobulin, ADEM acute disseminated encephalomyelitis, AHLE acute hemorrhagic leukoencephalitis, EMG/NCS electromyography/nerve conduction study
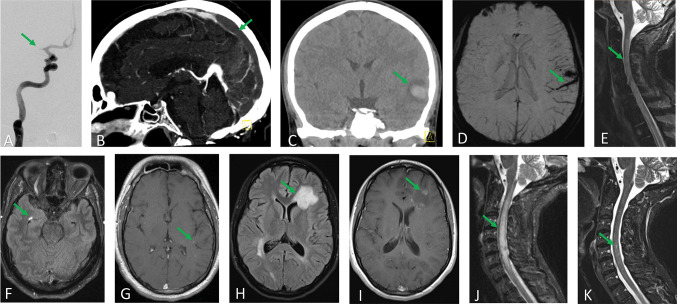
Fig. 1.
Neuroimaging case examples of select acute neurologic syndromes. A Acute ischemic stroke. A 66-year-old man presented for fevers, diarrhea, and dyspnea requiring supplemental oxygen due to COVID-19. On hospital day three, he developed new onset atrial fibrillation and later that day an acute left hemiparesis with NIH stroke scale of 15. Angiography demonstrated a proximal right MCA occlusion (arrow). Multiple passes with a stent retriever thrombectomy device with aspiration catheter plus eptifibatide infusion could not establish vessel patency. B Venous sinus thrombosis. A 64-year-old woman with no medical history presented for 3 days of diarrhea and headache. She tested positive for SARS-CoV-2. Chest imaging demonstrated opacities consistent with pneumonia and a large pulmonary embolus involving the left lower lobe, though she did not require supplemental oxygen. CT venography demonstrated acute occlusive venous thrombosis of the right transverse and sigmoid sinuses, right jugular bulb, torcula, posterior half of the straight sinus, and posterior third of the superior sagittal sinus (arrow). MRI demonstrated no infarction and her neurologic exam was unremarkable. Her headache resolved after anticoagulation. C and D Intracerebral hemorrhage secondary to venous infarction. An 18-year-old woman with no medical history presented for one week of diarrhea, mild cough without dyspnea, and two days of headache. She tested positive for SARS-CoV-2 but never required supplemental oxygen. In the emergency department, she demonstrated fluctuating aphasia and at times was mute and could not follow commands. CT demonstrated a left temporal hemorrhage with surrounding hypodensity (C, arrow) and CT angiography (not shown) suggested an overlying thrombosed cortical vein. MRI demonstrated thrombosis of the left vein of Labbe, tentorial, and cortical branch veins (D, arrow) with an associated hemorrhagic venous infarct. The aphasia improved with levetiracetam given in the emergency department, but no seizures were seen on subsequent EEG. Her aphasia gradually resolved after anticoagulation and continuation of levetiracetam. E and F Acute demyelinating encephalomyelitis (ADEM) following SARS-CoV-2 infection. A 47-year-old man with no medical history presented for lower extremity paresthesia, urinary retention, and lower extremity weakness developing over 3 days. He reported fever and cough 7 to 10 days prior and had tested positive for SARS-CoV-2. MRI spine demonstrated cervical and thoracic non-enhancing T2 hyperintensity with spinal cord expansion (E, arrow), and MRI brain demonstrated several small non-enhancing T2 hyperintense white matter lesions (F, arrow). CSF exam showed 201 white blood cells/µL, 201 mg/dL protein, and oligoclonal bands in CSF and serum; no specific pathologic antibodies were identified. He developed complete paralysis of the legs with severe left arm weakness, but he did not require intubation. He received solumedrol 1 g, 0.4 g/kg IVIG, and remdesivir daily for five days. His left arm briefly improved but again worsened when he developed hyponatremia with repeat MRI demonstrating worsened cord edema (not shown). Symptoms improved with hypertonic saline and maintenance of normal serum sodium. Surveillance MRI 12 days after admission demonstrated improved cord swelling but new patchy cervical cord enhancement. Solumedrol 1 g daily was given for three days with normalization of left arm strength and some return of leg movement. He continued to improve and was discharge to rehabilitation on a 12-week taper of prednisone. G Prolonged agitated encephalopathy. A 21-year-old man with no medical history was admitted for hypoxemic respiratory failure requiring mechanical ventilation due to COVID-19. He experienced severe agitation requiring midazolam and hydromorphone with adjunctive ketamine, dexmedetomidine, valproic acid, and scheduled quetiapine for adequate agitation control. An MRI brain was obtained when agitation returned on attempts to wean mechanical ventilation and demonstrated numerous small areas of subtle contrast enhancement (arrow). CSF exam showed 1 white blood cell/µL, 29 mg/dL protein, and was negative for SARS-CoV-2 by RT-PCR. He was diagnosed with encephalopathy and MRI findings were felt to be due to severe systemic inflammation. He was treated with supportive care and gradual reduction of adjunctive sedation agents. He was discharged to rehabilitation and, after 5 months, was living independently and exercising 45 min daily but had not returned to work. H and I Post mRNA vaccination ADEM. A 25-year-old female with no medical history presented with a generalized seizure 4 days after receiving an mRNA COVID-19 booster vaccine. Her neurologic exam was unremarkable following resolution of the seizure. MRI brain demonstrated a left frontal T2 hyperintensity (H, arrow) which enhanced after contrast injection (I, arrow). CSF exam showed 1 white blood cell/µL, elevated IgG synthesis rate of 7.1, elevated IgG index of 1.3, and 12 unique CSF oligoclonal bands. J and K Post mRNA vaccination transverse myelitis. A 65-year-old woman with rectal cancer in remission presented with back pain and saddle anesthesia 7 days after receiving an mRNA COVID-19 vaccine. MRI showed cervical to upper thoracic T2 hyperintensity (J, arrow) with a focus of enhancement at C5 (K, arrow). CSF exam showed 4 white blood cells/µL, protein 51 mg/dL, normal IgG index and synthesis rate, and oligoclonal bands present in serum and CSF. Her symptoms and imaging findings (not shown) completely resolved after two months of oral prednisone therapy. MCA middle cerebral artery, ADEM acute demyelinating encephalomyelitis, CSF cerebrospinal fluid, IVIG intravenous immunoglobulin
Neurologic complications seen in hospitalized COVID-19 patients may co-exist and might arise from common underlying mechanisms. For example, acute encephalopathy due to sepsis, regardless of the causative infection, can include contributions from cerebral ischemia (up to 30% in patients with shock), cerebral microhemorrhages, cerebral inflammation, and seizures (approximately 10% of septic patients undergoing continuous electroencephalography) [13–15]. Evaluating the patient with neurologic concerns during acute COVID-19 might result in the identification of multiple neurologic syndromes that could require diagnostic evaluation and therapy.
Systemic inflammation and its interaction with thrombosis and coagulopathy (thromboinflammation) are proposed central factors in the pathophysiology of COVID-19, including possible contributions to encephalopathy and other neurologic complications, such as stroke and encephalitis. Elevated markers of systemic inflammation (such as c-reactive protein) and coagulation dysfunction (both d-dimer and fibrinogen), as well as pro-inflammatory cytokines (including interleukin-1β, interleukin-6, tumor necrosis factor, and interferon-γ), have been widely reported in COVID-19, and increasing levels correlate with disease severity and risk of death [16–18]. COVID-19 may precipitate a profound inflammatory state akin to the cytokine storm or cytokine release syndrome described after chimeric antigen receptor (CAR) T cell therapy, hemophagocytic lymphohistiocytosis, or severe cases of sepsis [16]. A cytokine storm occurs when the immune system transitions from an adaptive response to a state of disproportionate inflammation that can result in organ dysfunction, cytokine-driven organ failure, and death. In COVID-19, a cytokine storm-like state may result either from dysregulated T lymphocytes responding to SARS-CoV-2 infection or through direct activation of macrophages and monocytes by SARS-CoV-2 infection of these cells [16, 17]. In the case of COVID-19 encephalopathy, high levels of peripheral inflammation could lead to cerebrovascular endothelial dysfunction and disruption of the blood brain barrier with astrocyte and microglial activation and central nervous system (CNS) cytokine production. A systemic hyperinflammatory state could also contribute to encephalopathy and stroke through macro- and microvascular thromboses resulting from complement activation, platelet over-excitation, endothelial cell dysfunction, and generation of neutrophil excitatory traps [18]. While thrombosis seems to predominant in COVID-19, endothelial injury and inflammation-mediated consumptive coagulopathy could result in cerebral microhemorrhages and frank hemorrhagic stroke [18].
The extent and frequency with which systemic inflammation or a cytokine storm-like state may precipitate inflammation within in the brain itself during COVID-19 remains an area of debate; in fact, some COVID-19 studies have demonstrated relatively low signs of inflammation in cerebrospinal fluid (CSF) samples despite considerable systemic inflammation [19, 20]. Indeed, pro-inflammatory cytokine levels in critically ill COVID-19 patients are orders of magnitude lower than those in cytokine storm due to CAR T cell therapy, severe acute respiratory distress syndrome, or severe sepsis [21]. An alternative hypothesis argues that vascular dysfunction as a direct result of SARS-CoV-2 endothelial cell infection could produce organ system dysfunction. Studies using non-primate animal models [22, 23], non-human primate models [24], human organoid models [25], interrogation of in vivo endothelial dysfunction [26–30], human non-CNS biopsy and post-mortem samples [31–34], and some human CNS post-mortem samples [23, 33, 35, 36] provide support for the hypothesis of SARS-CoV-2 endothelial cell infection. This hypothesis holds that endothelial cell infection would lead to endothelial dysfunction, endotheliitis, thrombotic microangiopathy, and even macrothrombosis [33, 37, 38]. However, this hypothesized mechanism remains controversial, with the conclusions of some studies that claimed support of endothelial infection being directly questioned and debated [32, 39–41], and other studies of human CNS post-mortem tissue failing to find evidence of neuronal or endothelial infection [42]. Interestingly, in human studies supporting endothelial infection, the findings supporting infection did not clearly correspond with the degree of brain injury observed [23, 36]. MRI vessel wall imaging studies in COVID-19 encephalopathy patients have demonstrated instances of circumferential enhancement and thickening of basilar and vertebral arteries supporting endotheliitis as a possible contributor to encephalopathy and cerebral infarction [43].
Proposed mechanisms of encephalitis/encephalomyelitis in COVID-19 may also contribute to encephalopathy and include SARS-CoV-2 infection of the CNS by transsynaptic propagation (for example, olfactory nerve) or hematogenous invasion (vascular endothelial cell or leukocyte infection), systemic inflammation resulting in blood brain barrier disruption and astroglial activation, and autoimmune mechanisms secondary to molecular mimicry [22, 37, 39, 44–48]. The large majority of suspected encephalitis cases have not detected SARS-CoV-2 RNA in the CSF. Therefore, encephalitis as a complication of systemic hyperinflammatory pathophysiology or autoimmune mechanisms may be more frequent than encephalitis caused by direct viral infection of the CNS [44].
Acute Encephalopathy
Epidemiology and Presentation
Acute encephalopathy is a state of global brain dysfunction developing over hours to days and presenting with disturbances in cognition, attention, awareness, and responsiveness [49]. Encephalopathy severity ranges from milder “confusion” (or subsyndromal delirium) to delirium and coma. Acute encephalopathy is common in hospitalized COVID-19 patients, with studies reporting incidence from 7.5 to 49% in all hospitalized patients and exceeding 55 to 84% in critically ill patients [3–5, 50, 51]. In comparison, the incidence of delirium in hospitalized patients before the COVID-19 pandemic was estimated to be 23% [52]. Furthermore, acute encephalopathy may be the primary or only symptom leading to health care presentation for COVID-19 in nearly 15% of elderly patients [53]. Patients in the delirium portion of the encephalopathy spectrum may present either agitated (hyperactive delirium) or somnolent (hypoactive delirium). COVID-19 patients may be particularly prone to agitated encephalopathy. Studies have found that 30% to 51.8% of critically ill COVID-19 patients had at least one episode of hyperactive delirium, which is substantially higher than the pre-pandemic incidence of 12.7% in critically ill patients [50, 54, 55]. The reason behind this high rate of agitation in COVID-19 patients is unclear; this could be related to the greater severity of COVID-19 lung injury and higher levels of ventilatory support, the frequent use of prone positioning, a relatively higher incidence of young individuals needing critical care during early COVID-19 surges, crisis staffing with providers less experienced in effective sedation management, decreased family visitation, or perhaps biological mechanisms unique to SARS-CoV-2 infection [50, 55–58].
Numerous studies prior to the COVID-19 pandemic suggest increased mortality and morbidity associated with encephalopathy occurrence, and one study suggested that, even 12 months after critical illness related encephalopathy, over 30% of patients have cognitive impairments similar to moderate traumatic brain injury, with both young and old patients being affected [59]. Consistent with this literature, our group found that acute encephalopathy in COVID-19 patients was associated with a 2.9 times greater odds of 30-day mortality (22 versus 3%) and a substantially reduced odds of good functional outcome at hospital discharge (odds ratio 0.22 for a good functional outcome modified Rankin score of 0 to 2), even after adjusting for factors including age and COVID-19 disease severity [5]. We found that greater severity of COVID-19 pulmonary disease, older age, prior history of any neurologic disease, chronic kidney disease, and shorter time from COVID-19 symptom onset to hospitalization were predictive of experiencing acute encephalopathy during the COVID-19 hospitalization [5]. Given the high frequency, relative to other neurologic complications of COVID-19, combined with the morbidity of acute encephalopathy, encephalopathy related to COVID-19 may represent the greatest public health burden among the acute neurologic complications of COVID-19 [5].
Pathogenic Mechanisms
Acute encephalopathy may arise from multiple clinical entities, most commonly categorized as: hypoxia, sepsis, metabolic derangements, and medications or toxins [60]. Early in the COVID-19 pandemic, many clinicians assumed that encephalopathy in COVID-19 was due to severe hypoxia. However, observations of encephalopathy in hospitalized patients despite unremarkable hypoxia, mild encephalopathy in COVID-19 patients who never required hospitalization, and COVID-19 patients with “happy hypoxemia,” who were not encephalopathic despite remarkable hypoxemia, quickly challenged assumptions that COVID-19 encephalopathy is simply the result of hypoxia [5, 61, 62]. Since critically ill COVID-19 patients are exposed to many of the same factors as other critically ill patients, it is not surprising that multiple mechanisms may contribute to COVID-19 encephalopathy. Encephalopathy in critically ill patients without COVID-19 appears to be multifactorial more than 60% of the time, and defies easy categorization more than 10% of the time [60]. The biological mechanisms that contribute to encephalopathy as a consequence of sepsis or metabolic derangements are incompletely defined but are likely numerous and overlapping. It seems likely that COVID-19 encephalopathy shares at least some of these underlying mechanisms. Magnetic resonance imaging (MRI) in severe COVID-19 patients has identified acute abnormalities in about half of imaging studies obtained, and neuroimaging findings suggest cerebrovascular dysfunction, blood brain barrier disruption, and cerebral inflammation similar to that described in septic encephalopathy, including leukoencephalopathy, microhemorrhages, cerebral infarction, leptomeningeal enhancement (mostly subtle), and variable parenchymal enhancement [63–67]. Similar to septic encephalopathy described prior to the pandemic, in which systemic inflammatory mediators seem more responsible for brain injury than direct pathogen invasion, reverse transcription polymerase chain reaction (RT-PCR) has infrequently (< 5%) detected SARS-CoV-2 in the CSF of patients with abnormal MRI findings [14, 63, 66, 67]. In fact, the ability of SARS-CoV-2 to invade the brain and infect brain cells remains an area of debate, with studies arriving at divergent conclusions [23, 35, 36, 39, 42, 68]. While some post-mortem series reported detectable SARS-CoV-2 RNA or proteins in the majority of brain samples (for example, Matschke et al. reported 21 out of 40 [53%] patient brains positive by either SARS-CoV-2 RNA or protein and 16 out of 40 [40%] patient brains positive by nucleocapsid or spike protein immunohistochemistry), even in these studies, the presence of SARS-CoV-2 in the brain did not correlate with the severity of neuropathologic changes, suggesting brain injury and neurologic symptoms were due to systemic processes rather than direct viral injury [35, 68]. Multiple post-mortem series in COVID-19 have described hypoxic injury, ischemic lesions, microglial activation, and parenchymal and meningeal cytotoxic T lymphocyte infiltration resembling that described in pre-pandemic septic encephalopathy and acute respiratory distress syndrome [35, 68, 69].
Diagnosis and Treatment
The severity of acute encephalopathy ranges from confusion to delirium and coma. Delirium is diagnosed in the presence of acutely disturbed attention and awareness, along with an additional cognitive disturbance (for example, memory deficit, disorientation, language, visuospatial ability, or perception). The Diagnostic and Statistical Manual-5 (DSM-5) defines the criteria for delirium, with milder clinical states representing subsyndromal delirium (commonly called confusional states or altered mental status) [70]. However, delirium identification using the Confusion Assessment Method (CAM and CAM-ICU) or Intensive Care Delirium Screen Checklist (ICDSC) are likely more practical than applying the DSM-5 in clinical scenarios [71]. Coma represents a severely depressed level of responsiveness that is typically diagnosed with the Glasgow Coma Score (GCS, score < 8 representing coma) or the Full Outline of UnResponsivenss (FOUR) score [72, 73]. The Richmond Agitation-Sedation Scale (RASS) has also been used to identify coma with RASS scores of − 4 (arousable but demonstrates no attention; no response to voice, but movement or eye opening to physical stimulation) or -5 (no response to voice or physical stimulation) [60]. An important caveat when using the CAM/CAM-ICU and ICDSC is that a transition from a positive delirium evaluation to an “unassessable” evaluation due to a decline in consciousness should not be interpreted as a resolution of delirium; the clinician should evaluate if a neurologic deterioration has occurred, including a new neurologic insult or a worsening of the processes contributing to encephalopathy.
We recommend a stepwise approach to the evaluation of acute encephalopathy in COVID-19, which is generally similar to the approach in any hospitalized patient with a consultation for “altered mental status.” Conducting extensive diagnostic batteries in every encephalopathy consultation would represent an untenable expenditure of resources, especially during a pandemic, and the large majority of encephalopathy evaluations do not identify a cause that requires specific intervention [5].
Identification of neurologic emergencies. A timely initial screening history and exam should be performed. If an emergency such as acute ischemic stroke is suspected, then that possibility should be rapidly evaluated.
Clinical evaluation: When time-sensitive emergencies are excluded, a detailed history and physical and neurologic examination (with sedation held or minimized) can be performed. One should appreciate that encephalopathy is more likely to occur in those with underlying medical conditions [5]. Particularly when encephalopathy is present on hospital admission, the development of acute encephalopathy could be caused by decompensation of pre-existing conditions (for example, undiagnosed hypo- or hyperthyroidism) under the acute stress of COVID-19 [5]. In critically ill patients, the neurologic examination should be performed with the patient receiving as little sedation as medically tolerable, and ideally after a prolonged sedation hold. One prospective study of delirium in non-COVID critically ill patients receiving nurse-protocolized light sedation (RASS goal 0 to −2) with short-acting sedatives found that 12% of patients who screened positive for delirium by the CAM-ICU while on sedation subsequently screened negative for delirium after a sedation hold of up to 2 h [74]. A pooled analysis of 12,699 delirium assessments in non-COVID patients found that critically ill patients were significantly more likely to screen positive for delirium if sedated to a RASS of −2 than to a lighter RASS of -1 to 0 (77 versus 23%; p < 0.0001) [71]. Therefore, even light sedation may confound assessment in a substantial minority of patients. Coordinating with nursing staff to minimize sedation for the neurologic evaluation may be beneficial. Corticospinal tract signs including hyperreflexia and extensor plantar responses are common during encephalopathy. In severe encephalopathy, motor responses to noxious stimulation may be absent, and even brain stem reflexes can become affected in extreme cases of systemic insult. Focal findings on neurologic examination should lead to the acquisition of neuroimaging with computed tomography or magnetic resonance imaging. In our anecdotal experience, careful examination for abnormal tendon reflexes, tone, and myoclonus or ocular clonus may be of higher yield in COVID-19 patients than most other critically ill patients. Early in the pandemic, we identified several critically ill COVID-19 patients with agitated encephalopathy and signs of serotonergic toxicity (hyperreflexia, myoclonus, ocular clonus, rigidity). These cases occurred in the context of high dose fentanyl infusion for analgesia and sedation (for example, 300 µg/h) or lower doses after recent linezolid exposure. Both fentanyl and linezolid have serotonergic properties, and the serotonergic signs and encephalopathy resolved when fentanyl was converted to alternative opioids (morphine or hydromorphone) without serotonergic properties [9]. In reviewing a patient’s medication list, most clinicians will recognize sedatives and anticholinergics as possible contributors to encephalopathy. However, antibiotic associated encephalopathy from agents including penicillins, cephalosporins (especially cefepime), quinolones, macrolides, sulfonamides, metronidazole, and isoniazid is probably under-appreciated [75]. Toxicity from immunosuppressants like tacrolimus, with our without hypertension, resulting in posterior reversible encephalopathy syndrome and valproic acid resulting in hyperammonemia may also represent causes of encephalopathy secondary to easily overlooked but commonly used medications.
Laboratory studies: Screening serum laboratory studies that identify high yield toxic and metabolic contributors to encephalopathy should be obtained. These should include serum chemistry panel, magnesium, calcium (ionized calcium if critically ill), phosphorus (patients with irregular nutritional intake can experience refeeding syndrome), bilirubin, total protein, albumin, and liver function tests. Oxygen saturation should be noted and may be combined with a blood gas to assess for hypoxemia and hypercapnia. Serum ammonia level should be sent, especially in patients on medications that can contribute to hyperammonemia (valproic acid, carbamazepine, topiramate) and in patients with liver or renal failure. Blood cultures should be sent if bacteremia is a clinical concern since multifocal septic emboli can present as encephalopathy and COVID-19 patients are at risk for superimposed bacterial infections. Thyroid function tests and serum cortisol are reasonable studies; however, one should recognize that mild thyroid abnormalities may only represent the euthyroid sick syndrome. We also routinely check folate and vitamin B12 levels since critically ill patients are at risk for nutritional deficiencies; however, we more often expect these nutritional deficiencies to represent aggravating factors rather than a primary cause of encephalopathy. Additional laboratory studies, like drug levels, could be sent as guided by the history and examination and medical record review. Many patients are likely to have additional laboratory studies available, such as d-dimer and c-reactive protein. While these assays are frequently trended in COVID-19 patients and provide a sense of inflammation severity, their utility in the encephalopathy evaluation is unclear.
Neuro-imaging studies: We obtain a head CT in most COVID-19 encephalopathy patients who are able to transport for the study. Focal findings on the neurologic examination necessitate neurologic imaging for further evaluation. However, structural lesions in brain regions such as the frontal lobes or cerebellum may be more difficult to detect by physical examination in the encephalopathic patient who has reduced ability to participate in the exam. A study prior to the COVID pandemic found that out of 102 medical intensive care unit patients who received a head CT scan for “altered mental status,” 20 (19.6%) had an acute finding on CT and half of these were ischemic strokes [76]. However, it is unclear if this same degree of diagnostic yield is expected in acute COVID-19 patients who undergo neuroimaging for encephalopathy evaluation. In a study of 242 patients who presented to hospital and had a head CT or brain MRI within 14 days of COVID-19 diagnosis, the most common finding was nonspecific white matter microangiopathy (55.4%) followed by chronic infarct (19.4%); only 5.4% were found to have acute or subacute infarcts and 4.5% were found to have intracranial hemorrhages [77]. Moreover, all infarcts and hemorrhages were identified in patients with focal findings on exam, and none of the 102 patients who had “altered mental status” as the sole indication for imaging were found to have an acute or subacute infarct or hemorrhage [77]. Therefore, it may be reasonable to elect to defer neuroimaging in the select COVID-19 patient who has a non-focal neurologic exam when a cause for encephalopathy is apparent without imaging.
Additional investigations: At this point in the COVID-19 encephalopathy evaluation, most patients will be identified as having a likely multifactorial encephalopathy with components explained by septic, hypoxic, metabolic, or sedation or medication-related encephalopathy. The pre-pandemic literature on encephalopathy suggests that the next most frequent group of patients will be those in which one of these four causes seems to be the predominant or isolated cause of encephalopathy [60]. In most cases, a short period of clinical and neurologic monitoring with renewed efforts to optimize systemic insults and minimize iatrogenic contributors to encephalopathy will suggest whether additional evaluation for refractory encephalopathy is indicated.
Advanced neuroimaging including MRI brain and vascular imaging with MR or CT angiography could be considered in select cases. However, as noted previously, the yield of these studies is likely low in non-critically ill patients with a non-focal neurologic examination while the yield may be modest in the critically ill. Even acute findings on MRI in the critically ill COVID-19 patient may only represent what the pre-pandemic literature suggests are expected findings in patients with septic shock or severe hypoxemia.
Electroencephalography (EEG) should be considered in patients whose encephalopathy continues to be unexplained, especially since most seizures in critically ill patients are non-convulsive. Some non-COVID data suggests that seizures occur in up to 10% of critically ill patients with sepsis [15]. Among hospitalized patients with COVID-19, seizures appear to be detected at a similar rate of 5.5 to 9.6% of patients selected for EEG monitoring, and nonconvulsive seizures without preceding suspicious clinical events occur primarily in critically ill patients [78–80]. However, several cohort studies suggest that, overall, seizures may be rarely detected (<1%) in hospitalized COVID-19 patients [3–5, 51]. Level of consciousness may guide the duration of EEG to be acquired. In patients able to follow commands, 95% of seizures will be captured within 24 h of EEG monitoring; meanwhile, 80% of seizures in comatose patients are captured in the first 24 h of EEG monitoring, suggesting a longer duration may be needed in comatose patients [81]. The somewhat infrequent occurrence of seizures in COVID-19 may guide the allocation of limited EEG resources or inform whether a patient requires transfer to a center with continuous EEG monitoring.
Sampling CSF may be considered in encephalopathy cases with unusual presentations on history or exam or in whom neuroimaging or EEG monitoring reveal unexpected findings. Since systemic inflammation may disrupt the blood brain barrier or activate glial cells, a modest CSF protein elevation may be expected in COVID-19 encephalopathy without a specific neurologic disease mechanism. Data on CSF findings in acute COVID-19 is rather limited, though modest CSF protein elevation and leukocytosis can occur in individuals who are ultimately diagnosed with an encephalopathy from a systemic etiology. A systemic review of 113 acute COVID-19 patients with CSF analysis demonstrated protein elevation >45 mg/dL in 59% of patients and leukocyte elevation > 5 cells/µL in 43.2% of patients with encephalopathic presentation [82]. Substantial leukocytosis or presence of oligoclonal bands unique to the CSF would not be expected without a specific neurologic disease mechanism requiring further evaluation. Most case series have found SARS-CoV-2 RT-PCR to be unrevealing [82], which is consistent with our own experience of 11 lumbar punctures performed for refractory encephalopathy evaluation that were all negative for SARS-CoV-2 RT-PCR. As will be discussed later, rare cases of encephalitis or necrotizing encephalopathy have been identified in COVID-19.
Unless a specific neurologic disease process like seizures or encephalitis is found, our experience suggests that the therapeutic approach for COVID-19 encephalopathy is quite similar to the approach in non-COVID acute encephalopathy. The treatment of encephalopathy is primarily directed at supportive care and optimizing the systemic derangements and iatrogenic exposures contributing to the encephalopathy. However, we feel that there are management points to emphasize in the COVID-19 patient.
Case series have reported rare COVID-19 patients with persistent encephalopathy from possible encephalitis or cerebrovascular endotheliitis who may have improved following high dose methylprednisolone or other immunomodulatory therapy [83–85]. We believe these cases represent exceptional instances and that, at this time, glucocorticoids or other immunotherapy should not be regarded as routine therapies in COVID-19 encephalopathy. However, many patients with COVID-19 encephalopathy will meet criteria for immunotherapy based on the severity of their pulmonary disease. Immunotherapy for COVID-19 pulmonary disease is based on the premise of mitigating organ injury by preventing excessive inflammation. However, it can be challenging to determine when an inflammatory response turns from an appropriate reaction to SARS-CoV-2 infection to one that produces more injury than benefit. While it may be tempting to institute immunotherapy in hopes of addressing cytokine-driven mechanisms, unintentionally suppressing an appropriate immune response could be detrimental. Consistent with the risks of instituting immunotherapy too early, the RECOVERY trial of dexamethasone versus standard of care in COVID-19 suggested a tendency towards worse 28-day survival in patients who received dexamethasone before pulmonary disease was severe enough to require supplemental oxygen [86]. In addition to the anti-viral agent remdesivir, immunotherapies for COVID-19 pulmonary disease supported by clinical trials include dexamethasone, baricitinib (Janus Kinase inhibitor, tofacitinib is an alternative), and tocilizumab (anti-IL-6 receptor monoclonal antibody, sarilumab is an alternative) [87]. A detailed review of the evidence-based treatments for COVID-19 pulmonary disease is beyond the scope of this current perspective, and we refer the reader to the “COVID-19 Treatment Guidelines,” which have been regularly updated by the National Institutes of Health [87]. Clinical trials of immunotherapy agents in COVID-19 focused on mortality and time to clinical recovery, rather than any specific neurologic or encephalopathy end-point. Currently, data directly comparing tocilizumab versus baricitinib in COVID-19 is limited [88], and the selection between these agents is driven by patient risk factors (for example, avoiding baricitinib in patients with known venous thrombosis given the association of baricitinib with increased thrombosis risk), though a randomized non-inferiority trial is underway (ClinicalTrials.gov Identifier: NCT05082714). One might assume an encephalopathy benefit from these agents, secondary to their ability to address the underlying systemic disease process. However, the neurologist should be aware of the theoretical possibility of encephalopathy worsening after initiation of tocilizumab. Tocilizumab is a monoclonal antibody that cannot cross the blood brain barrier to block interleukin-6 receptors in the brain, even as it blocks peripheral interleukin-6 receptor sites. Blocking peripheral interleukin-6 binding could result in an effectively greater exposure of the brain to interleukin-6 and worsened cytokine-driven toxicity, even without a change in measured cytokine levels [89]. In the absence of more data directly comparing tocilizumab and baricitinib, this theoretical possibility could be considered when weighing the initiation of tocilizumab versus baricitinib.
The effects of sedation and other medications as iatrogenic contributors to encephalopathy should not be overlooked, especially given the large doses of sedatives often provided to control agitated encephalopathy in COVID-19 patients. Evidence strongly suggests that benzodiazepine exposure is a potent risk factor for delirium and that the burden of delirium, as a clinical manifestation of encephalopathy, is associated with greater mortality and worse long-term cognitive outcomes [50, 59, 71]. A large prospective study of critically ill patients performed prior to the COVID-19 pandemic demonstrated that the burden of encephalopathy attributed to sedatives was associated with worse long term cognitive outcome to a similar degree as the encephalopathy burden due to sepsis and hypoxia; in fact, the detrimental effect of sedation was greater than the effect due to metabolic contributors to encephalopathy [60]. Furthermore, that study suggested sedation was the most frequent contributor to encephalopathy in critically ill patients [60]. The collective literature favors minimizing the depth of sedation and suggests that shorter acting sedatives, such as propofol or dexmedetomidine, and ketamine have a role in reducing benzodiazepine and opioid consumption, respectively, and may shorten mechanical ventilation time in critically ill patients [71]. The consulting neurologist can help to recognize when critically ill patients are over sedated and can provide assistance in tailoring sedation and agitation management. To minimize the lingering sedative effects of prolonged benzodiazepine infusions, we favor propofol over midazolam infusions, and when propofol is not tolerated, we use adjunctive ketamine (2.5–5 mcg/kg/min) to reduce the necessary dose of midazolam. While antipsychotics have been shown to be ineffective in treating established delirium [71], we utilize scheduled doses of antipsychotics as an adjunct to specifically target agitation and avoid a self-propagating cycle of escalating sedative infusions in response to agitation. We also utilize dexmedetomidine and ketamine to facilitate sedation weaning and speed time to ventilator liberation [90, 91]. We have recommended clonidine, guanfacine, valproic acid, gabapentin, and trazodone in select situations to further facilitate sedation weaning and to ease the transition off of dexmedetomidine and ketamine infusions.
Nutritional deficiencies should be considered a potentially treatable contributor to encephalopathy, particularly in patients with prolonged hospitalization. Thiamine deficiency is likely chief among these deficiencies for the COVID-19 patient since 20–70% of septic shock patients are biochemically thiamine deficient [92]. Patients with a history of alcoholism, poor nutrition, malabsorption, enhanced thiamine loss (as with dialysis), systemic malignancy, organ transplant, pregnancy, and high levels of metabolic stress are at risk for thiamine deficiency [92]. Thiamine deficiency can result in Wernicke encephalopathy with a classic triad of encephalopathy, gait ataxia, and ocular motor dysfunction. However, one series of 97 autopsy-proven Wernicke encephalopathy cases demonstrated the complete classic triad in only 16% of patients and one-third of patients presented only with encephalopathy [93]. Given the high risk of occult deficiency and the relative safety of thiamine supplementation, our practice is to recommend 100 mg intravenous thiamine supplementation daily for encephalopathic COVID-19 patients while critically ill, and at least one time supplementation in non-critically ill encephalopathic COVID-19 patients. When thiamine deficiency is specifically suspected, thiamine 500 mg intravenously three times daily is given for several days followed by prolonged daily supplementation.
Lastly, the role of non-pharmacologic management of encephalopathy is particularly important. Avoiding restraints when possible, optimizing mobilization, ensuring patients have their glasses and hearing aids to promote orientation, and promoting normal sleep–wake cycles are therapies beneficial for delirium reduction, both within and outside the critical care environment [71]. A unique feature of the COVID-19 pandemic has been the social isolation of patients due to visitation restrictions. A large, multicenter observational study of hospitalized COVID-19 patients found that family visitation, even if done as a “virtual” video visit, was associated with a 27% lower risk of delirium [50]. Since only 17% of patient-hospital days included any type of visitation, this may represent a modifiable target to treat COVID-19 encephalopathy [50].
Encephalitis and Encephalomyelitis
Epidemiology and Presentation
Both viral and autoimmune causes of encephalitis/encephalomyelitis have rarely been reported in COVID-19 [44, 94]. Viral encephalomyelitis with confirmed SARS-CoV-2 RNA in the CSF is quite rare [95]. A study from Mayo Clinic reported 5 patients out of 10,384 (0.05%) patients with COVID-19 who met clinical diagnostic criteria for post-infectious autoimmune encephalitis [96]. Although these five patients were negative for neuronal and glial immunoglobulin G in CSF, there have been rare reports of anti-NMDA receptor and myelin oligodendrocyte glycoprotein antibody associated cases of COVID-19 related encephalitis [96–98]. In addition, cases of acute multifocal demyelinating disease of the CNS consistent with the acute disseminated encephalomyelitis (ADEM), and the more severe variant of acute hemorrhagic leukoencephalitis (AHLE), have been reported in both children and adults following COVID-19 [94]. A systematic review of encephalitis (defined as diagnostic studies suggesting CNS inflammation) associated with COVID-19 that included 138 cases, suggested 0.2% of hospitalized patients experience encephalitis as a complication of COVID-19 [44]. However, the incidence could be as high as 6.7% in critically ill patients, and in 84% of cases, patients developed severe pulmonary COVID-19 requiring critical care before encephalitis developed [44]. The systemic review authors acknowledged that publication bias may inflate the apparent incidence of encephalitis. An epidemiological study from England that included over 2 million patients with SARS-CoV-2 infection estimated 123 events of encephalitis, meningitis, and myelitis per 10 million people infected with SARS-CoV-2 [99]. On average, patients develop encephalitis 14 days after the onset of COVID-19 symptoms [44, 94]. In the case of ADEM and AHLE, neurologic symptoms could progress over a period of time ranging from <24 h to more than 10 days and occurred most frequently 15 to 30 days after COVID-19 symptom onset [94]. The mean age of encephalitis patients was 59 years (range 43 to 80 years) and males and females were equally affected; however, in ADEM, there may be a male predominance (61%) [44]. Encephalitis did occur in a minority of patients with no other COVID-19 symptoms (24%), but fever and dyspnea were present in most [44]. Decreased level of consciousness was the most frequent neurologic symptom among all cases of encephalitis (77%), followed by altered mental status (72%), seizures (38%), headaches (27%), and weakness (15%) [44]. Among cases of ADEM, encephalopathy was also the most frequent symptom (78%) but focal motor deficits (43%) may be more frequent than in encephalitis in general [94]. The pooled mortality of patients with encephalitis was 13.4%, compared to 3.4% in the general hospitalized COVID-19 population [44]. In a systemic review of ADEM cases, functional outcome data was available in 28 cases of whom 18 (64%) were severely disabled (modified Rankin Score 4 or more) and 9 (32%) died by the time of last follow-up [94].
Diagnosis and Treatment
A diagnosis of encephalitis/encephalomyelitis is primarily suspected based on neuroimaging findings and supportive CSF studies with the exclusion of alternative diagnoses. Common MRI findings include diffuse white matter hyperintensities with variable contrast enhancement as well as possible hemorrhagic lesions varying in size from microhemorrhages to frank parenchymal hemorrhages; of note, some cases of encephalitis with normal neuroimaging have been reported [44, 94]. A systemic review of 13 studies with CSF results reported protein (average 64.8 mg/dL, range 38 to 115 mg/dL), red blood cell count (average 329 cells/µL, range 12 to 1154 cells/µL), and white blood cell count (average 15 cells/µL, range 6 to 39 cells/µL) were typically elevated [44]. CSF IgG levels were elevated (83.2 mg/L, range 5 to 112.5 mg/L) but oligoclonal bands were absent in the majority of cases. A systemic review of ADEM cases reported a normal CSF profile in 30% [94]. Multiple studies suggest that specific causative autoimmune antibodies are detected in only a small minority of cases [44, 94, 96]. Since encephalitis seems more frequent in severe COVID-19 cases, and these patients may have prolonged hospital courses characterized by persistent systemic inflammation, it may be challenging to distinguish encephalitis secondary to intense systemic inflammation versus autoimmune processes. At this time, there is no specific proven therapeutic regimen for COVID-19 encephalitis, regardless of underlying mechanism. Reported options include monotherapy or combinations of: corticosteroids (for example, methylprednisolone 1 g daily for 5–10 days), intravenous immunoglobulin, plasma exchange, and rituximab [44]. We have used methylprednisolone 1 g daily for 5 days combined with intravenous immunoglobulin or plasma exchange as the initial approach at our institution, and we preference intravenous immunoglobulin if active viral infection is felt to be present or the patient is at high risk for superimposed infections. If active systemic SARS-CoV-2 infection is a consideration, then we recommend at least a five-day course of remdesivir, and one could consider extending that course to 10 days if active viral infection remains a concern. A complete review of autoimmune encephalitis is beyond the scope of this manuscript, and we refer the reader to a recent review [100].
Stroke
Epidemiology and Presentation
Arterial ischemic stroke, cerebral infarction due to venous thrombosis, and intracranial hemorrhage have been described in acute COVID-19 [101]. Acute ischemic stroke appears to be uncommon, with many large cohort studies reporting incidence rates of approximately 1% and most cohorts reporting rates less than 3% in hospitalized patients [5, 51, 102–106]. There appears to be a higher risk of ischemic stroke in more severe COVID-19; one study reported a 3.7% incidence in patients requiring mechanical ventilation versus 0.5% in other hospitalized patients [5]. Cohort studies consistently report hemorrhagic stroke in less than 1% of hospitalized COVID-19 patients and cerebral venous thrombus likely occurs in less than 0.1% [5, 105, 107, 108]. Overall, the ratio of ischemic to hemorrhagic stroke approximates the 80:20 ratio seen in non-COVID-19 related stroke [101]. Stroke may rarely be the initial symptom leading to hospitalization in COVID-19 (0.4% in one study) [104]. However, among COVID-19 patients with a stroke, stroke was the reason for hospital presentation in about 40% and younger patients appear more likely to have stroke as their initial presentation [101, 104, 106]. Stroke in COVID-19 seems most likely within the first few weeks after COVID-19 symptom onset [104, 105]. The stroke presentation may range in severity from acutely symptomatic large vessel occlusions to punctate infarcts found during evaluation of other neurologic manifestations, like headache or encephalopathy, where the symptomatic contribution of the infarct is unclear [109, 110]. Stroke as a COVID-19 complication seems to uncommonly occur in patients younger than 50 years old (17.4% of COVID-19 stroke patients in a pooled analysis) with a median age of approximately 65 years old [103, 105, 106]. Studies are in disagreement regarding whether patients with COVID-19 and stroke are younger than contemporaneous stroke patients without COVID-19 [103, 104]. However, an analysis using “Get With the Guidelines” stroke hospital data from 41,971 acute ischemic stroke patients at 458 centers suggests that patients with COVID-19 were slightly younger (median 68 [57 to 79] versus median 71 [60 to 81] years), more likely to be Hispanic, Black, or Asian, and more likely to be on Medicaid or have no insurance than patients without COVID-19 [111]. In hospital stroke onset was also more frequent in COVID-19 than non-COVID-19 stroke patients (14 versus 3%) [111].
Pathogenic Mechanisms
Cryptogenic, followed by cardioembolic, appears to be the most common mechanism of ischemic stroke in COVID-19, with reported rates between 35 to 66% and 22 to 40%, respectively [101, 104, 105]. A meta-analysis of 67,845 patients suggested that cryptogenic stroke was significantly more likely in COVID-19 than non-COVID stroke patients (OR 3.98, 95% CI 1.62–9.77) [112]. Early case series warned of acute large vessel occlusion in young patients [110], and a pooled analysis of 126 COVID-19 ischemic stroke patients demonstrated 11 of 46 (24%) patients younger than 50 years had a large vessel occlusion [106, 110]. In general, patients with ischemic stroke and COVID-19 are more likely to have stroke due to large vessel occlusion than patients without COVID-19 (30 versus 24%), and therefore, COVID-19 patients tend to have more severe stroke symptoms (median NIH stroke scale 8 versus 4) [111]. Multiple studies of COVID-19 patients with hemorrhagic stroke demonstrate that the site of hemorrhage is lobar in more than 60% of cases [101, 105], which seems greater than the frequency of lobar location in pre-COVID-19 intracerebral hemorrhage cohorts (high-end estimates approximate 40%) [113]. This decreased frequency of hemorrhagic stroke in areas typically associated with hypertension may suggest that hypertension plays a smaller role in COVID-19 related hemorrhagic stroke.
The majority of COVID-19 patients who experience acute stroke have underlying traditional vascular risk factors such as hypertension (56 to 95%) or diabetes mellitus (34 to 60%) [101, 103–106]. Potential contributions from hypercoagulable states or endothelial dysfunction may explain why cryptogenic stroke etiology appears more likely in COVID-19 related ischemic stroke [38]. Studies predating the COVID-19 pandemic suggest that infections requiring hospitalization are associated with short-term increased stroke risk, supporting an association between systemic inflammation and stroke [103–105, 110, 114, 115]. As another potential mechanism, some studies have reported newly positive antiphospholipid antibodies in 50 to 75% of tested patients, though the significance of these antibodies in the acute setting is unclear [101, 105].
Cardiac dysfunction related to COVID-19 infection could also represent a stroke mechanism. Cardiac complications of COVID-19 that increase the risk for cardioembolic stroke include: myocarditis, stress cardiomyopathy, myocardial ischemia or infarction, and new arrhythmia [116, 117]. Acute heart failure may be present in 23% of hospitalized COVID-19 patients with evidence of cardiomyopathy in 33% [117]. A study reviewing echocardiography findings in 901 COVID-19 patients without prior cardiac disease found abnormalities in 46% and severe ventricular dysfunction in 13% [118]. A meta-analysis estimated an 11% prevalence of atrial fibrillation in hospitalized COVID-19 patients and up to 10% of patients with new-onset atrial fibrillation; atrial fibrillation was sixfold more prevalent in those with severe compared to non-severe COVID-19 [119, 120].
Data on hemorrhagic stroke in COVID-19 are more limited than for ischemic stroke. Severe levels of inflammation and coagulopathy leading to a consumptive coagulopathy, as well as endothelial injury, could contribute to hemorrhagic stroke mechanisms. A study using the American Heart Association COVID-19 Cardiovascular Disease registry reported that patients with intracranial hemorrhage had higher interleukin-6 levels, were more often on anticoagulation (75 versus 57%), and more frequently received extracorporeal membrane oxygenation (4 versus 0%, ECMO) than COVID-19 patients without ICH, but a very low number of intracranial hemorrhage patients (0.2% of the registry) precluded statistical analysis of intracranial hemorrhage risk factors [107]. The Extracorporeal Life Support Organization “ECMO in COVID-19” registry reports a 7% rate of intracranial hemorrhage and 1% rate of ischemic stroke in COVID-19 patients receiving ECMO [121]. The anticoagulation used for ECMO combined with ECMO-induced thrombocytopenia, factor XIII deficiency, fibrinogen deficiency, and platelet dysfunction may represent underlying mechanisms [122–124].
Despite these plausible mechanisms, there is some disagreement regarding whether COVID-19 actually represents a risk factor for in-hospital stroke. A large cross-sectional study of 24,808 hospital discharges in New York reported only 0.9% of SARS-CoV-2 positive patients presented with acute ischemic stroke compared to 2.4% of SARS-CoV-2 negative patients in January through April 2020 [102]. On the other hand, a case–control study comparing 86 COVID-19 patients with neuroimaging confirmed stroke to 499 matched controls suggested that COVID-19 was independently associated with a nearly 21-fold increased odds of in-hospital stroke [125].
Diagnosis and Treatment
While acute stroke therapies have not been specifically tested in the COVID-19 population, there are no data to suggest the risk–benefit ratio of these interventions differs for patients with COVID-19. Therefore, the management of ischemic and hemorrhagic stroke in COVID-19 should follow the same standards of care as for patients without COVID-19. This includes a timely evaluation of candidacy for acute medical and interventional stroke therapies, such as thrombolysis or thrombectomy in acute ischemic stroke. Case series of thrombectomy in COVID-19 suggest that these patients may be more prone to clot fragmentation or re-occlusion, but at this time, no strategy to thrombectomy in COVID-19 has been proven superior to conventional approaches [126, 127]. An analysis of the multicenter “Get With the Guidelines” ischemic stroke registry data from February to June 2020 demonstrated that ischemic stroke patients with COVID-19 experience about the same rates of thrombolysis (18%) or endovascular therapy (11%) as patients without COVID-19 [111]. However, the time from arrival to initiation of thrombolysis (median 58 versus 46 min) and from arrival to endovascular therapy (median 114 versus 90 min) was significantly longer in patients with COVID-19, and the main source of greater delay in COVID-19 patients was the need to acquire appropriate personal protective equipment [111]. Developing local protocols that speed detection of SARS-CoV-2 infection and facilitate infection control measures may be a means to improve delays in acute stroke therapies. For example, our institution dedicated a specific negative airflow room adjacent to the angiography suite to allow for more rapid preparation of COVID-19 patients to undergo mechanical thrombectomy.
Given the strong association between stroke and typical vascular risk factors and stroke mechanisms in COVID-19, the approach to stroke in COVID-19 is similar to the approach in non-COVID patients. Brain and cerebrovascular imaging combined with cardiac imaging and serum laboratory studies that assess vascular risk factors are used to identify the mechanism of stroke, which then guides the appropriate therapeutic approach for secondary stroke prevention. Antithrombic therapy for long-term secondary stroke prevention (antiplatelet versus anticoagulation) is selected based on indications similar to non-COVID patients. Indications to use therapeutic dose anticoagulation for secondary stroke prevention include atrial fibrillation, severe heart failure, and also the presence of concurrent venous thromboses (extremity deep venous thromboses, pulmonary embolism, cerebral venous thromboses). The clinical course of the patient’s COVID-19 disease could influence selection of antithrombotic therapy during the acute time period. There have been several clinical trials to inform the use of anticoagulation in COVID-19. In critically ill COVID-19 patients, therapeutic dose anticoagulation (in the absence of a specific indication) did not improve survival to hospital discharge, days free of organ support, or the combined end-point of major thrombotic event or death compared to thromboprophylaxis dose heparin [128]. There was a reduced incidence of major thrombotic events (6.4 versus 10.4%) but also an increase in the rate of major bleeding (3.8 versus 2.3%) in therapeutic dose versus thromboprophylaxis dose heparin [128]. Therefore, in critically ill COVID-19 patients with stroke, we recommend therapeutic dose anticoagulation over antiplatelet therapy plus thromboprophylaxis only when there is a specific indication for therapeutic anticoagulation. In contrast, therapeutic dose anticoagulation for up to 14 days is currently recommended over thromboprophylaxis dose heparin in hospitalized COVID-19 patients not in the intensive care unit, on low flow oxygen, with a D-dimer above the upper limit of normal, and with no increased risk of bleeding because multiple clinical trials suggest mortality, organ function, and thromboembolism benefits [129–131]. Of note, patients with an indication for dual antiplatelet therapy (for example, stroke due to symptomatic intracranial atherosclerosis) are excluded from this 14-day anticoagulation recommendation. After the 14 days of anticoagulation for COVID-19 indication, we recommend continuing antithrombotic therapy as dictated by the patient’s secondary stroke prevention indications.
We do not recommend routinely starting antiplatelet therapy for primary stroke prevention in patients with COVID-19. The RECOVERY trial demonstrated that adding 150 mg of aspirin daily to standard care had no mortality benefit and only a small reduction in thrombosis (4.6 versus 5.3%) that was offset by an increase in major bleeding (1.6 versus 1.0%) [132]. However, we do routinely continue antiplatelet and anticoagulant therapies for indications preceding COVID-19, provided bleeding risks are not prohibitive. Similarly, patients may have been on angiotensin-converting enzyme inhibitors or angiotensin receptor blockers prior to COVID-19, and we do not routinely discontinue these medications. Early concerns about angiotensin pathway agents and adverse outcomes from COVID-19 have not been realized in observational studies.
Seizures and Status Epilepticus
Epidemiology and Presentation
Multiple cohort studies suggest seizures occur in fewer than 1% of hospitalized COVID-19 patients, but seizures can infrequently be a presenting symptom of COVID-19 [4, 5]. The reported incidence of seizures or status epilepticus is substantially greater in patients who undergo electroencephalography (EEG, 5.5 to 9.6%); however, clinician referral for EEG monitoring likely enriches this incidence compared to the non-monitored population [78–80]. EEG abnormalities, including seizures, may also be more frequently identified in patients who undergo at least 24 h of continuous EEG monitoring. One study reported a higher incidence of EEG abnormalities in patients who underwent continuous rather than single or intermittent routine EEG monitoring (97 versus 85%) [78]. Altered mental status is the single most frequent reason to evaluate for seizures during COVID-19 (42 to 61%), and the majority of patients who undergo EEG are mechanically ventilated (about 80%) [78–80]. One study of those who had EEG demonstrated 13.7% of patients had a clinical seizure leading to hospitalization and 5.6% had a clinical seizure during hospitalization but prior to EEG monitoring. The most frequent abnormality detected on EEG was diffuse background slowing (57 to 88%), followed by epileptiform discharges (13 to 48.7%), and then focal slowing (17 to 26%) [78–80]. One study found that seizures detected on EEG were associated with a fourfold increased risk of mortality; however, a seizure as the presenting symptom of COVID-19 was not associated with increased mortality [79].
Pathogenic Mechanisms
One study of 197 hospitalized COVID-19 patients who underwent EEG monitoring found that 74% of patients who had seizures detected had either a prior history of a CNS disorder or an acute or chronic intracranial structural lesion; the remaining patients with seizures had an acute metabolic risk factor for seizure development (sepsis, renal failure, severe hypoxia/anoxia) [79]. In fact, a chronic structural brain injury was a statistically significant risk factor for detection of seizures by EEG [133]. These authors interpreted their findings as suggesting a low likelihood of a direct epileptogenic process resulting from SARS-CoV-2 and that seizures were more likely due to predisposing structural injury or the systemic effects of severe illness [79]. However, it should be noted that several authors have suggested that focal slowing and seizures in COVID-19 disproportionately involve the frontal regions, which they argue could be related to viral entry in to the CNS along the olfactory nerve [78].
Diagnosis and Treatment
In most cases of COVID-19, seizures were detected either when evaluating a patient for encephalopathy, when a clinical episode concerning for seizure had been reported, or when a patient had known epilepsy or a predisposing structural lesion [78–80]. There is little evidence that the diagnostic evaluation or therapeutic approach for seizures detected in COVID-19 should differ from the approach taken in other patients. When seizures are detected, a patient should undergo a diagnostic evaluation to identify the likely cause of seizures since the therapeutic approach may be affected by the underlying etiology.
Guillain–Barre Syndrome
Epidemiology and Presentation
Multiple cases of Guillain–Barre syndrome (GBS) following SARS-CoV-2 infection have been reported [134, 135]. However, there is debate regarding whether SARS-CoV-2 is a meaningful cause of GBS. An epidemiological study using the United Kingdom National Immunoglobulin Database found that, compared to the prior four years (1.65–1.88 cases per 100,000 individuals per year), the incidence of GBS decreased during March through May 2020 (1.6 cases per 100,000 COVID-19 infections) when the UK was in “lock-down,” despite escalating cases of COVID-19 [136]. Furthermore, there was no correlation between regional cases of COVID-19 and regional cases of GBS in the UK [136]. The authors argued that either SARS-CoV-2 does not cause GBS and cases of GBS following SARS-CoV-2 infection were coincidental or the risk of GBS after SARS-CoV-2 is substantially less than the risk related to more established causal infectious agents (such as Campylobacter jejuni) to which exposure was reduced by social distancing and isolation. On the other hand, a large study using national health data from England, with over 2 million patients with SARS-CoV-2 infection, suggested an excess number of GBS cases related to SARS-CoV-2 infection (estimated 145 excess cases per 10 million SARS-CoV-2 infections) [99]. Given the high incidence of SARS-CoV-2 infection at any point during the pandemic, combined with the rarity of GBS, it is difficult to completely excluded the possibility of chance occurrences. Nevertheless, these epidemiological data are consistent with multiple large cohort studies of hospitalized COVID-19 patients in which GBS was a neurologic complication in 0 to < 0.5% [4, 5, 134].
Reports of GBS following SARS-CoV-2 infection suggest the entire spectrum of GBS may be observed, including axonal and demyelinating variants as well as the Miller-Fischer variant [135]. Similar to pre-pandemic GBS, cases of GBS associated with COVID-19 appear to principally develop ascending limb weakness over a few days, starting about one to two weeks after onset of viral symptoms [135]. One prospective observational study suggested a predominance of classical sensorimotor GBS variant with paresthesia (73%) but with frequent facial weakness (64%) and autonomic dysfunction (64%) [137]. Respiratory failure has been reported with COVID-19 associated GBS; however, this is difficult to distinguish from respiratory failure due to pulmonary COVID-19 disease itself.
Pathogenic Mechanisms
There is little evidence to suggest GBS related to SARS-CoV-2 infection represents a different pathogenic mechanism than postinfectious GBS from other causes. A systematic review of 77 COVID-19 associated GBS cases with electromyography demonstrated the most frequent GBS variant was acute inflammatory demyelinating polyneuropathy (77%), followed by acute motor-sensory axonal neuropathy (13%), and acute motor axonal neuropathy (10%). That review found most cases (86%) were negative for antiganglioside antibodies [135]. A complete review of GBS pathophysiology, diagnostic approaches, and therapeutic strategy is outside of the scope of this manuscript, and we refer the reader to a review of GBS [138].
Diagnosis and Treatment
GBS should be considered in the patient presenting with progressive extremity weakness and is in the differential of new onset or progressive bulbar dysfunction, suggesting a Miller Fisher GBS variant. GBS associated with COVID-19 should be considered if respiratory insufficiency seems disproportionate to pulmonary findings. Case series of GBS associated with COVID-19 suggest a CSF profile of absent or low white cell count with elevated protein, similar to other causes of GBS; a systemic review found that the CSF was negative for SARS-CoV-2 RNA in all samples tested [135]. As with other causes of GBS, both intravenous immunoglobulin and plasma exchange are reported therapies [135]. Given the limited available data, it is difficult to comment on whether the clinical severity or prognosis of GBS related to COVID-19 differs from GBS due to other causes. Cases of functional recovery from GBS after COVID-19 have been reported [134].
Neurologic Manifestations of Post-Acute Sequelae of SARS-CoV-2 Infection (Neuro-PASC)
Long lasting COVID-19 symptoms, or so-called long-COVID, have been recognized since May 2020, and became initially known through patient-reported symptom trackers and online patient groups [139]. Based on the definition from the Center for Diseases Control (CDC), symptoms persisting > 4 weeks after COVID-19 onset are now called Post-Acute Sequelae of SARS-CoV-2 infection (PASC) [140]. Long COVID, or PASC, remains a debilitating multi-system syndrome affecting a heterogenous population. It is likely that approximately one-third of COVID-19 survivors will develop PASC. Estimates of PASC range from 13 to 57% of patients [141–145]. The Government Accountability Office (GAO) report estimated that as of February 2022, there were up to 23 million people in the USA with Long Covid (up to 30% of all cases), pushing about 1 million people out of work [146]. Since Neuro-PASC symptoms, including cognitive dysfunction, are likely a significant factor in the ability to return to work, PASC is expected to have a significant impact on the US workforce and economy. Neuro-PASC may affect patients who have been hospitalized for severe COVID-19 pneumonia (post-hospitalization Neuro-PASC (PNP)) as well as those with mild or no initial respiratory presentation of COVID-19 (non-hospitalized Neuro-PASC (NNP)). PASC may still occur after breakthrough cases of COVID-19 in vaccinated individuals, although vaccinated patients are at slightly lower risk of PASC as compared to un-vaccinated individuals (HR = 0.85, 95% CI 0.82–0.89) [147].
A study of 273,618 COVID survivors found patients who developed PASC were more likely to be younger, female, and have had severe illness [143]. A survey of 4182 people who tested positive for SARS-CoV-2 also found that patients with PASC were more likely to be female and have required hospital assessment [141], but reported PASC was more common in an older population. Expanded results of the same survey found that persons who are vaccinated are half as likely to get long-COVID as compared to unvaccinated persons [148]. Other risk factors for PASC may include high levels of SARS-CoV-2 RNA at diagnosis, a variety of autoantibodies, and Epstein-Barr virus (EBV) reactivation [149]. Moreover, one study suggested EBV reactivation is seen in 66% of long Covid subjects vs. 10% of control subjects [150]. Incidentally, there are currently no pharmaceuticals licensed to specifically treat EBV reactivation.
Many patients with long COVID suffer from a wide array of neurologic symptoms affecting their cognition and quality of life, as well as their ability to work (Fig. 2 and Table 2). Our study of the first 100 non-hospitalized “long-haulers” seen in a Neuro-COVID-19 clinic showed that they are younger and predominantly female [61]. The most frequent comorbidities were depression/anxiety (42%) and autoimmune disease (16%). The main neurologic manifestations were “brain fog” (81%), headache (68%), numbness/tingling (60%), dysgeusia (59%), and anosmia (55%). In a follow-up study, there was no significant change in the frequency of most neurologic symptoms at a median of 14.8 months from disease onset [151].
Fig. 2.
Symptoms of post-acute sequelae of SARS-CoV-2 (PASC). PASC can lead to symptoms involving multiple organ systems and varies by individual patient. Common symptoms include cognitive dysfunction (often called "brain fog" by patients), dizziness, and anosmia. Treatment depends on the systems involved and often involves a multidisciplinary approach
Table 2.
Neuro-PASC symptoms
| Neuro PASC symptom | Presentation | Frequency | Neurodiagnostic testing | Pathogenesis | Therapies |
|---|---|---|---|---|---|
| Cognitive dysfunction | “Brain fog”, decreased attention, concentration, multitasking abilities, forgetfulness | 8% of all patients; 50% of critically ill patients |
NIH Toolbox, PROMIS inventory, MoCA and neuropsychological testing may be abnormal MRI, MRA, EEG, CSF may show elevated protein or non-CNS specific oligoclonal bands Screen for B12, folate deficiency, HIV infection, thyroid dysfunction, sleep apnea |
Fatigue, sleep disruption, aberrant immune response, and potential persistent infection and auto-immune reaction | Cognitive rehabilitation, adjusted work schedule, list-making, vitamin supplementation |
| Fatigue | Early fatiguing with normal activities, exercise intolerance, sleepiness | 13% of all Covid-19 survivors; 39% of hospitalized patients | Sleep study in those with daytime sleepiness, snoring | Dysfunction in sleep and wakefulness centers in the brainstem | Amantadine 100 mg upon awakening and 100 mg at noon; modafinil 100–200 mg daily |
| Anosmia/ageusia | Impaired or absent smell and/or taste | 77% of patients during initial infection; 31% prolonged | Patient self-reporting; hypometabolism of the olfactory/rectus gyrus on 18F-FDG brain PET | Injury to the nasal epithelium/supporting cells of olfactory bulb; damage to areas of the brain involved in olfaction and taste (parahippocampal gyrus, orbitofrontal cortex and insula) | Smell therapy (repeatedly smelling various strongly-scented odors) twice daily for 3–6 months. Intranasal fluticasone and/or oral triamcinolone paste for 5 days may have mild benefit |
| Dysautonomia | Heart rate and/or blood pressure variability, orthostasis, bladder and/or bowel dysfunction and fatigue | Overall rates unknown; 30% of patients seen in a neuro-COVID clinic | POTS is diagnosed by an increase in heart rate of 30 bpm, or over 120 bpm, within 10 min of standing, in the absence of orthostatic hypotension. Orthostatic hypotension is diagnosed if the systolic blood pressure drops 20 mmHg or diastolic pressure drops 10 mmHg after 3 min of standing | Damage to autonomic ganglia, vagus nerve, autoantibodies have been postulated | Maintain proper hydration, compression stockings, abdominal binders, and participate in graded exercise programs on the patient’s back. Propranolol or ivabradine for tachycardia. Midodrine or fludrocortisone may be helpful for orthostasis |
| Headache | Bilateral throbbing, persistent headaches | 47% of COVID-19 patients at onset and 10% at 30 days | MRI brain with and without contrast and vessel imaging is indicated in patients with confusion, headaches and focal neurologic deficit | Cytokine release irritating meninges; binding of ACE2 increases angiotension II and CGRP leading to trigemino-vascular activation | Indomethacin 50 mg twice a day for 5 days. Nortriptyline 25 mg nightly or propranolol 20 mg TID may be helpful |
| Neuropathy | Small fiber neuropathy (persistent tingling, burning); numbness in peripheral distribution | Overall incidence unknown; up to 60–90% of patients seen in neuro-Covid clinic | 17% had abnormal electrodiagnostic tests, 63% had abnormal skin biopsies confirming small fiber neuropathy and 50% had abnormal autonomic testing | Small fiber neuropathy may be due to autoimmune neuritis; critical illness neuropathy and compression neuropathy in hospitalized patients | Gabapentin or other neuropathic agents. IVIG has led to improvement in a small number of patients |
| Audio-vestibular symptoms | Vertigo, hearing loss, and tinnitus | Vertigo (7.2%), hearing loss (7.6%), tinnitus (4.8%) | MRI brain with internal auditory canal and VNG | Post-viral vestibular neuronitis, BPPV, PPPD. Direct viral invasion, immune dysregulation or microthrombi have been postulated | Anti-vertiginous drugs (meclizine, benzodiazepines). SSRI or SNRI for PPPD. Neuro-otology or ENT referral |
NIH National Institutes of Health, PROMIS Patient Reported Outcome Measurement Information System, MoCA Montreal Cognitive Assessment, MRI magnetic resonance imaging, MRA magnetic resonance angiography, EEG electroencephalogram, CSF cerebrospinal fluid, POTS postural orthostatic tachycardia syndrome, VNG videonystagmography, BPPV benign paroxysmal positional vertigo, PPPD persistent postural perceptual dizziness, SSRI selective serotonin reuptake inhibitor, SNRI serotonin and norepinephrine reuptake inhibitor
Cognitive Dysfunction
Epidemiology and Presentation
Cognitive dysfunction is one of the most debilitating symptoms of Neuro-PASC and a main reason for consultation to our Neuro-COVID-19 clinic. Many patients use the colloquial term of “brain fog” to describe their difficulties in carrying out usual functions at work, multitasking, or following conversations. They also describe phenomenon such as forgetting why they are in a room, repeating oneself, and word finding difficulties. At 12 months post hospitalization for severe COVID-19 pneumonia, 50% of patients without prior history of dementia or cognitive abnormality had cognitive impairment [152]. Another study showed that 8% of COVID-19 survivors had persistent cognitive dysfunction [143]. Being hospitalized for severe COVID may age the brain by 20 years, but even patients with asymptomatic Covid-19 may have prolonged cognitive dysfunction [153]. One study showed lower Montreal Cognitive Assessment (MoCA) scores in patients with asymptomatic COVID-19 than healthy controls [154]. A meta-analysis of predominantly hospitalized patients reported 18% of patients report persistent concentration difficulties and 19% reported memory loss at one year post Covid [155].
Pathogenic Mechanisms
The mechanism of persistent cognitive dysfunction in Neuro-COVID is likely a complex interplay of fatigue, sleep disruption, aberrant immune response, and potential persistent infection [156, 157]. Lack of oxygen does not seem to be responsible as impaired cognition can occur in patients with mild COVID-19 who were never hypoxic.
The role of SARS-CoV-2 as a neurotropic virus is still debated. Proposed mechanisms of the virus invading the brain through the olfactory bulb are theoretical, and, to date, the frequency and significance of direct viral invasion of the brain are debated. Moreover, olfactory neurons, which constitute a direct route to the brain by anterograde axonal transport, do not appear to express the obligatory entry proteins for SARS-CoV-2. Some studies report SARS-CoV-2 is rarely detected in brain samples, possibly due to low levels of ACE2 expression on brain cells [158]. However, brainstem dysfunction has also been posited as a possible etiology of long COVID as there are more ACE2 receptors in the pons and medulla compared to other brain regions [159].
There is evidence for local immune overactivation in the CSF even in the absence of SARS-CoV-2 in the CSF in patients with Neuro-COVID [160]. However, despite a relative overactivation of the immune response, there is also impairment in the normal function. Monocytes remain de-differentiated instead of following appropriate signals to turn into macrophages or dendritic cells as they would normally. T cells become “exhausted,” indicating they are less able to kill infected cells. These findings suggest a dysregulation of the immune response by the virus.
Vascular dysfunction and thrombosis may also play a role in cognitive dysfunction. SARS-CoV-2 infection has been shown to lead to endotheliitis, inflammation of the endothelium of the blood vessel where ACE2 receptors are present. As the vascular endothelium is present throughout the body, this may be a mechanism by which COVID-19 can cause dysfunction in all major organs. The vascular endothelium serves not only as a structural support but also plays a dynamic role in hormonal and cytokine signaling [161]. Finally, there is evidence of microthrombi in the brain on autopsy in patients who have died of COVID-19 [68]. We do not know if this may also occur in patients with mild or moderate COVID-19.
Diagnosis and Treatment
We recommended to screen for and rule-out alternate causes of cognitive dysfunction including B12 or folate deficiency, HIV infection, syphilis, thyroid dysfunction, and obstructive sleep apnea. A study analyzing MRI brains of 401 patients who tested positive for SARS-CoV2 before and after their infection found that patients who had COVID-19 had a 0.2–2% brain tissue loss in the parahippocampal gyrus, orbitofrontal cortex, and insula [162]. These are all areas that are involved in the sense of smell and could atrophy in patients who have anosmia. A study of two patients post-SARS-CoV2 infection reported hypometabolism of the olfactory/rectus gyrus on 18F-FDG brain PET, but the long-term consequence of this finding is uncertain [163]. Conventional MRI brain may not show abnormalities given these mild changes. A study in patients with cognitive symptoms after mild COVID-19 showed that cerebrospinal fluid studies were abnormal in 10/13 (77%) of post-COVID-19 patients with cognitive symptoms [164]. One patient had two well-defined oligoclonal bands in the CSF that were not present in the serum and 8 patients had matched bands in serum and CSF which are nonspecific. Two patients (15%) had mildly elevated protein. CSF Aβ42 and Aβ40 have been described as abnormal in neuro-PASC, indicating impaired amyloid processing suggesting a possible degenerative component in this population [165]. Serum neurofilament, a marker of neuronal damage, has been shown to be elevated in hospitalized COVID-19 patients, although this test is not widely available for clinical use due to need for specialized lab equipment [166].
Tests such as the NIH Toolbox and MOCA are useful screens for cognitive dysfunction in COVID-19 [167]. In particular, the NIH Toolbox tests screen for impairment in four different cognitive domains including working memory, executive function, processing speed, and attention. Scores are based on a large normative US population and a T score of 50 is the average expected result and accounts for age, education, gender, and race. We refer patients with NIH toolbox T scores below 40 (> 1 standard deviation below average) on any of the tests to a cognitive neurologist or neuropsychologist for further evaluation of their cognitive problems, followed by referral to precision cognitive rehabilitation. Medications for Alzheimer’s Disease including donepezil and memantine have not been studied in neuro-PASC cognitive dysfunction.
Fatigue
Epidemiology and Presentation
Fatigue is one of the most frequently reported symptoms in long COVID. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection [168]. At an average of 48 days post discharge, 72% of hospitalized patients reported ongoing fatigue [169]. In another study, at 12-week follow-up, 39% of hospitalized patients reported ongoing fatigue [170]. Around 13% of all COVID-19 survivors have persistent fatigue at 6 months [143]. In our Neuro-COVID-19 clinic, 85% of non-hospitalized Neuro-PASC patients complained of fatigue [61], which persisted an average of 14.8 months after COVID-19 symptoms onset in a follow-up study [151].
Pathogenic Mechanisms
PASC symptoms resemble the prominent fatigue and cognitive complaints seen after mild traumatic brain injury, and in patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) [171–173]. Yet, despite two decades of research, the etiology of chronic fatigue syndrome remains unclear. Unlike chronic fatigue syndrome, which likely has multiple different etiologies, PASC has a known single viral trigger. The mechanisms leading to post-COVID fatigue are likely similar to that of cognitive dysfunction, although the fatigue can also present as early physical tiring.
Post-COVID fatigue may also, in part, be a result of dysfunction in sleep and wakefulness centers in the brainstem leading to insomnia. In our study, the rate of insomnia in long COVID patients increased from 16% pre-COVID to 33% post-COVID [61]. Alterations in the sleep cycle may also lead to immune dysregulation and impair the body’s ability to clear SARS-CoV-2, thus extending the cycle.
Diagnosis and Management
Patients seen in our Neuro-COVID-19 clinic had significantly worse than expected quality of life due to fatigue as assessed by Patient Reported Outcome Measurement Information System (PROMIS) [174]. PROMIS inventories are validated for clinical use to assess how much fatigue interferes with a patient’s daily life. Scores are expressed as T-scores with a score of 50 representing the normative mean/median of the US reference population with a standard deviation of 10.
When evaluating a patient with PASC and fatigue in the clinic, screening for other comorbid causes for fatigue including for insomnia, sleep apnea, depression and thyroid dysfunction is important. Patients with sleep dysfunction may be referred to cognitive behavioral therapy for insomnia (CBT-I). Medications such as amantadine (100 mg upon awakening and 100 mg at noon) and modafinil 100–200 mg daily may be helpful for more severe cases of fatigue. Amantadine was originally developed as an antiviral mediation against influenza, although it is no longer used for this purpose. Amantadine has a metabolite that is similar to amphetamine and boosts brain levels of dopamine, a neurotransmitter linked to arousal. Side effects of amantadine include worsening anxiety and insomnia, and therefore should not be used after noontime, as well as extremity swelling and retiform rash. Other stimulants, modafinil and armodafinil, are weak dopamine reuptake inhibitors, and can be used clinically as awake-promoting agents.
Finally, screening for depression and anxiety is important as around 40% of patients with long-COVID endorse depression or anxiety [61, 175]. For patients with comorbid depression, bupropion XL 150–300 mg daily may be beneficial as this is also a norepinephrine–dopamine reuptake inhibitor that can improve energy. For patients with anxiety, CBT and selective serotonin reuptake inhibitors (SSRI) such as escitalopram 10 mg daily may be useful.
Anosmia/Dysgeusia
Epidemiology and Presentation
Anosmia is one of the distinguishing factors of COVID-19 compared to other viral upper respiratory tract infections. Most patients develop some degree of anosmia, though they may not subjectively identify it. In a study by Hannum et al., 77% of patients with COVID-19 had impaired smell when tested, while only 44% of patients self-reported anosmia [176]. Dysgeusia, or impaired sense of taste, often coincides with impaired sense of smell, but at times, patients may report one or the other and not both. The incidence of impairment in smell or taste may be up to three times higher in Western countries than East Asia [177]. The reason for this is unclear and may be due to polymorphisms in the ACE2 receptor or differences in ACE2 expression levels in various tissues in different populations [178].
For most patients, anosmia and dysgeusia typically resolves within a few days to 2 weeks. Although it can persist for much longer and it is yet to be determined if COVID-19 can cause permanent hyposmia or dysgeusia. In one study, 14/44 (31%) patients had impaired smell or taste at 244 days post infection [179].
Phantosmia, or detecting smells that are not really present, as well as parosmia, alteration in smell, occur frequently Neuro-PASC patients [179]. It is unclear if phantosmia and parosmia are due to initial damage or signs of early recovery, as these symptoms may be reported later in the disease course.
Pathogenic Mechanisms
Anosmia in COVID-19 is likely due to injury to the nasal epithelium/supporting cells of olfactory bulb. MRIs have shown abnormalities in olfactory bulbs in patients with anosmia; however, pathologic samples do not support direct viral invasion of the olfactory bulb [180]. This lack of evidence that SARS-CoV-2 can infect the olfactory bulbs further supports that SARS-CoV-2 is not likely a neurotropic virus. However, SARS-CoV-2 has been found in the sustentacular cells in olfactory epithelium, cells that support the olfactory sensory neurons [181, 182].
Diagnosis and Treatment
For patients with anosmia > 4 weeks, olfactory training should be encouraged. Olfactory training, or repeatedly smelling various strongly-scented odors, may speed smell recovery process [183]. This is typically done at least twice a day for 3–6 months. Patients should think of a memory of that odor while smelling the scent and take 10 s breaks between scents. They may start with odors such as rose, lemon, and clove. This may help reorganize nerve connections. Other scents can be added after 1 month. The typical timeframe of recovery is poorly known. Patients should ensure home smoke and carbon monoxide detectors are working. Patients with anosmia should be screened for concomitant mood disorders as one study found that anosmia/ageusia was an independent risk factor for depression or suicidal ideation [184].
Intranasal and oral steroids have shown mixed results. One study assessed systemic prednisone plus a nasal irrigation with betamethasone, ambroxol, and naphazoline vs. untreated controls and found improvement in olfactory scores in the treatment group, although this was not a controlled study [185]. In another study, there was improvement in smell and taste recognition after five days of intranasal fluticasone spray and oral triamcinolone paste [186]. A third study showed no difference between mometasone furoate nasal spray plus olfactory training vs. olfactory training alone, therefore questioning the impact of inhaled nasal steroids [187].
Dysautonomia
Epidemiology and Presentation
Dysautonomia involves the malfunction of the sympathetic and parasympathetic nervous system. Symptoms include heart rate and/or blood pressure variability, orthostasis, bladder and/or bowel dysfunction, and fatigue. In our study, 30% of patients reported heart rate or blood pressure variability [61]. In a study of 20 COVID-19 patient with autonomic symptoms, 60% were unable to return to work at 6–8 weeks [188]. Postural Orthostatic Tachycardia Syndrome (POTS) is the most common symptom experienced followed by neurogenic syncope and orthostatic hypotension. Orthostatic cerebral blood flow velocity (CBFv) on tilt table testing has been shown to be lower in PASC patients compared to controls, consistent with cerebrovascular dysregulation [189].
Pathogenic Mechanisms
Damage to the autonomic nervous system typically occurs at the autonomic ganglia. Damage to the vagus nerve is also possible [68]. Autoantibodies have been previously associated with orthostasis and postural orthostatic tachycardia syndrome (POTS), but no clear antibodies associated with post-Covid dysautonomia have been established.
Diagnosis and Treatment
POTS is diagnosed by an increase in heart rate of 30 beats per minute (bpm), or over 120 bpm, within 10 min of standing, in the absence of orthostatic hypotension. Orthostatic hypotension is diagnosed if the systolic blood pressure drops 20 mmHg or diastolic pressure drops 10 mmHg after 3 min of standing. If the diagnosis is unclear, patients with symptoms of tachycardia, orthostasis, and blood pressure fluctuations should undergo autonomic testing such as tilt-table test, where available. Patients with confirmed POTS may benefit from a beta-blocker such as propranolol or ivabradine, a heart failure drug which can lower heart rate without reducing blood pressure [190]. Patients with orthostasis should maintain proper hydration, use compression stockings, abdominal binders, and participate in graded exercise programs on their back [191]. Midodrine or fludrocortisone may be helpful for orthostasis [191]. There is little data on use of IVIg or steroids for post-COVID dysautonomia. Only one of three patients treated with steroids noted improvement [188].
Headaches
Epidemiology and Presentation
Headaches are common at presentation and in long-COVID. Headaches are present in 47% of COVID-19 patients at onset and 10% at 30 days [192]. The quality of post-COVID headache can be tension-type or migraine-like and is often persistent. It can occur at the time of initial infection or be delayed [193]. In a survey of 262 COVID positive patients, COVID headaches were more often bilateral, long-lasting, and resistant to analgesics. Patients with pre-existing headache disorders noted a change in the quality of their headaches post-COVID [194]. About one in five patients who present with headache during the acute phase of COVID-19 develop chronic daily headaches [195].
Pathogenic Mechanisms
The COVID-related cytokine release is one of the likely mechanisms for headaches via irritation of meninges. Additionally, SARS-CoV-2 binding of ACE2 leads to an increased production of angiotensin II which is known to increased calcium gene related peptide (CGRP) [196]. CGRP is a key neuropeptide leading to trigemino-vascular activation resulting in headache [197].
Diagnosis and Treatment
MRI brain with and without contrast and vessel imaging is indicated in patients with confusion, headaches, and focal neurologic deficit. Patients with pre-existing headache disorders are prone to have an increase in their baseline headaches.
A five-day course of indomethacin 50 mg twice a day has been shown to reduce post-COVID headaches by about 50%. Migraine specific treatments including nortriptyline, propranolol, topiramate, gepants, and CGRP antagonists have not yet been studied systematically for post-COVID headache [198]. We frequently use nortriptyline 25 mg nightly for treatment of post-COVID headache in our Neuro-COVID-19 clinic.
Numbness/Tingling
Epidemiology and Presentation
In our study, 60% of patients seen in the neuro-COVID clinic reported numbness or tingling. Another study showed 8/9 (89%) of patients with PASC had small fiber neuropathy, although no systematic studies have been done [189]. Small fiber neuropathy may be due to inflammation triggered by infection and can be seen in a range of mild to severe COVID-19. There is also evidence for autoimmune neuritis based on an autopsy study with perivascular macrophage infiltrates in the nerves [199]. Critical illness neuropathy is not necessarily unique to COVID but rather can be seen in any prolonged ICU stay.
Diagnosis and Treatment
In a review of 17 post-Covid patients without prior neuropathy referred for peripheral neuropathy evaluation, 17% had abnormal electrodiagnostic tests, 63% had abnormal skin biopsies confirming small fiber neuropathy, and 50% had abnormal autonomic testing [199].
Treatment with neuropathic pain agents including gabapentin may be helpful [200]. Five patients who received repeated intravenous immunoglobulins reported an improvement in sensory symptoms [199]. There is need for further study as patients who did not receive immunotherapy also reported improvement over time.
Audio-Vestibular Symptoms
Epidemiology and Presentation
According to a systematic review of post-COVID audio-vestibular symptoms, 4.8% of patients experience tinnitus, 7.6% have hearing loss, and 7.2% have vertigo, or a spinning sensation. This same review also concluded that these symptoms are not clearly related to COVID-19 [201]. Vestibular symptoms are less reported than other neurologic symptoms of PASC, but tend to be quite debilitating when they do occur. Post COVID-19 vestibular neuronitis and BPPV have been reported [202, 203]. In our experience, some patients may report a persistent feeling of unsteadiness with normal vestibular testing, similar to Persistent Postural Perceptual Dizziness (PPPD). This syndrome is typically provoked by an event affecting the vestibular system.
Pathogenic Mechanisms
Similar to other neuro-COVID syndromes, direct viral invasion, immune dysregulation, or microthrombi have been postulated as causes of audio-vestibular symptoms following COVID-19.
Diagnosis and Treatment
After exclusion of alternate etiology such as vestibular migraines, Meniere’s disease, stroke, or demyelinating disease, patients should be referred to vestibular therapy. MRI brain with internal auditory canal and videonystagmography (VNG) may be helpful to determine etiology. Anti-vertiginous drugs such as meclizine, antihistamines, and benzodiazepines are useful in vestibular neuritis. Benzodiazepines and vestibular suppressants such as meclizine are not helpful in PPPD. Studies have shown SSRI or serotonin and norepinephrine reuptake inhibitors (SNRI) reduce symptoms in 60–70% of patients with PPPD. Those with persistent symptoms despite vestibular therapy should be referred to neuro-otology or ENT for further testing.
Experimental Therapies
There are 26 ongoing studies for post-Covid syndromes [190]. However, due to the limited knowledge and lack of completed, successful studies for treatment in neuro-PASC, many doctors and patients have sought alternative routes for treatment. Treating a new syndrome can be daunting, but treating the individual symptoms based on known principles can bring as much or more success than any experimental treatment.
Low dose naltrexone (LDN) has gained clinical interest over the past decade as a treatment that may improve overall sense of wellbeing, fatigue, and pain. LDN has been used in chronic fatigue syndrome, fibromyalgia, multiple sclerosis, and other autoimmune diseases with success in a few studies [204, 205]. LDN is proposed to reduce levels of pro-inflammatory cytokines TNF-alpha and IL-6. However, a recent study showed no difference in quality of life in glioma patients taking LDN [206]. There is an ongoing clinical trial researching LDN 4.5 mg daily in long-COVID [207].
Fluvoxamine has been used in the acute COVID-19 inpatient setting with improvement in outcomes, but there is no current evidence for use in long-COVID [87]. Fluvoxamine is an SSRI that may lower pro-inflammatory cytokines including IL-6 and TNF-a through the sigma-1 receptor on the endoplasmic reticulum [208]. In addition, treatments including steroids, ivermectin, CCR-5 inhibitors, aspirin, and statins have been proposed. Ivermectin has been shown to be ineffective in acute COVID-19 in multiple studies. Cryotherapy, acupuncture, lymphatic drainage massages, cupping, IV glutathione, hyperbaric oxygen chambers, and infrared saunas have not yet been studied for PASC.
Acute Neurologic Complications of SARS-CoV-2 Vaccination
Rare cases of thrombotic events with thrombocytopenia, including cerebral venous thrombosis (up to 70% of thromboses), have been reported in patients vaccinated with the adenovirus-vector SARS-CoV-2 vaccines (AstraZeneca and Johnson & Johnson) but not with the mRNA-based vaccines [209]. It is believed that DNA from adenovirus vectors binds to platelet factor 4 (PF4) and triggers autoantibody production, resulting in a vaccine-associated immune thrombotic thrombocytopenia (VITT) developing most often 1 to 2 weeks after vaccination [209]. Patients with cerebral venous thrombosis present with the typical signs and symptoms of that condition (headache in 90%), and the PF4 antibody test is confirmatory of VITT [209]. Since the mechanism resembles heparin-induced thrombocytopenia, recommended treatment is anticoagulation with a non-heparin anticoagulant and intravenous immunoglobulin (1 g/kg daily for 2 days) [209]. Plasma exchange may be used for refractory cases. In the USA, the overall risk of VITT is about 1.9 cases per million Johnson & Johnson doses, but the risk is 7 cases of VITT per million doses in women aged 18 to 49 years [210]. Despite this risk, the Advisory Committee on Immunization Practices found the benefits of continued vaccination using Johnson & Johnson exceed the risks [210].
Post-authorization safety surveillance has identified a potential association between GBS and the adenovirus-vector SARS-CoV-2 vaccines. In the USA, the estimated incidence of GBS following the Johnson & Johnson vaccine is 9.8 cases per million doses, which corresponds to a four-fold increase above the background rate of GBS [211]. The median time from vaccination to GBS onset was 13 days and nearly all cases began within 42 days [211]. Most cases were of serious severity (93.8%), and there was a male predominance [211].
It is especially important for patient education to note that the risks of neurologic complications from SARS-CoV-2 infection itself likely exceed the risks associated with vaccination. A study using national health data from England with over 20 million doses of AstraZeneca (adenovirus-based) vaccine, 12 million doses of Pfizer (mRNA-based) vaccine, and 2 million patients with SARS-CoV-2 infection compared the excess incidence of neurologic complications following vaccination and SARS-CoV-2 infection. AstraZeneca vaccination result in an estimated 38 excess cases of GBS per 10 million doses compared to 145 excess cases of GBS per 10 million SARS-CoV-2 infections, and there was no statistically significant association between GBS and Pfizer vaccination [99]. While the Pfizer vaccine was associated with 60 excess cases of hemorrhagic stroke per 10 million doses, this was offset by 163 excess cases of myasthenic disorder and 123 excess cases of encephalitis per 10 million SARS-CoV2 infections [99]. Data from the US Vaccine Adverse Event Reporting System similarly suggests that the risks of neurologic complications from SARS-CoV-2 infection far exceed the risks associated with vaccination [212].
Directions for the Future: Clinical Care, Research, and Education on Neurologic Manifestations of COVID-19
The COVID-19 pandemic is evolving very rapidly in unpredictable ways all over the world due to low rates of vaccination and the emergence of new SARS-CoV-2 variants. Therefore, we focus on delineating the most urgent and important future directions for clinical management, research, and education.
Risk Factors for Hospitalization and Long COVID Syndrome
While some individuals remain asymptomatic after SARS-CoV-2 infection, others develop severe pneumonia requiring mechanical ventilation. Furthermore, people often fully recover from a mild case of COVID-19, but others develop lingering and debilitating neurologic manifestations of Long-COVID. Therefore, further research is needed on the risk factors of severe disease and Long-COVID syndrome in diverse populations.
Clinical Characterization of Neuro-PASC
Unfortunately, the definition of PASC is imprecise, as it encompasses all post-acute sequela lasting > 4 weeks from symptom onset and does not differentiate between post -hospitalized and non-hospitalized patients. Therefore, an 80-year-old man with multiple co-morbidities who has cognitive problems post-hospitalization for severe COVID-19 pneumonia requiring mechanical ventilation complicated by encephalopathy, and a previously healthy 20-year-old woman with persistent “brain fog” after mild initial SARS-CoV-2 infection are both considered to have Neuro-PASC using the NIH definition. Since the two populations of post-hospitalization and non-hospitalized Neuro-PASC patients are very different, this has caused significant confusion and decreased scientific rigor in the field. Indeed, most publications have lumped those two populations of patients together [142]. Clinical studies should aim at improving the scientific rigor of the Neuro-PASC field by differentiating post-hospitalization Neuro-PASC (PNP) from non-hospitalized Neuro-PASC (NNP) patients.
The Case for SARS-CoV-2-Negative “Long Haulers”
In view of the limited availability of SARS-CoV-2 nasopharyngeal testing by RT-PCR in some geographic locations during the first year of the pandemic, and the low sensitivity of the first commercially available serological test for SARS-CoV-2 Nucleocapsid antibodies (Abbott), it has been estimated that approximately 10 million people in the USA may have developed long COVID symptoms in 2020 but did not have a positive test result of SARS-CoV-2 infection [213]. Those individuals, predominantly females in their forties, have unfortunately experienced rejection and stigma from medical providers as well as from the majority of post-Covid clinics in the USA [61, 214–216]. However, we argue that those patients fall within the categories of “probable” or “suspected” SARS-CoV-2 infections according to WHO case definitions and their post-viral syndrome should be managed symptomatically similarly to the Long COVID syndrome [217].
Peripheral Biomarkers of CNS Injury
Many Neuro-PASC patients have normal brain imaging and CSF studies. However, there is emerging data on blood-based biomarkers of CNS injury in the acute phase of both severe and mild-to-moderate COVID-19 that assess the nature of CNS injury in acute infection. Plasma neurofilament light chain (pNfL) is an intra-axonal structural protein which has been validated as a biomarker for neuroaxonal damage, and plasma glial fibrillary acidic protein (pGFAP) is an astrocytic cytoskeletal protein which is upregulated in activated astrocytes [218, 219]. Both biomarkers have been shown to increase in the acute phase of severe COVID-19, indicating CNS injury associated with neuronal damage and astrocytic activation [220–223]. Furthermore, elevation of serum (s)GFAP and (s)NfL has been observed in acute mild to moderate cases of COVID-19 [166, 224–226]. However, whether SARS-CoV-2 directly infects the brain parenchyma remains unclear [68]. Collectively, these findings suggest that CNS damage may occur in COVID-19 patients presenting with a wide range of acute disease severity that is likely independent of direct viral CNS infection. The dynamic evolution of those biomarkers in Neuro-PASC and how they related to quality of life or cognitive function measures over time is not known and should be measured prospectively.
Pathogenic Mechanisms of Neuro-PASC and Interventions
Immunity against viral pathogens requires robust activation of T cells, and there are ample examples that peripheral T cell activation is critical to help clear viral infections with CNS manifestations. However, most T cell studies in COVID-19 have focused on hospitalized patients with pneumonia [227]. The rapidly waning T and B cell responses after vaccination, breakthrough infections, and need for booster shots has become painfully apparent, underscoring our persistent knowledge gaps and urgent need for further research on the immune response to SARS-CoV-2 [228]. Pilot studies on the characterization of the T cell response in Neuro-PASC patients and its impact on neuropsychiatric symptoms show an emerging pattern consistent with continuous antigenic stimulation or persistent infection with SARS-CoV-2 [157, 229]. Such persistent infection may lead to auto-immunity, driven either by auto-antibodies or auto-reactive T cells. Future research should focus on identification of potential hidden reservoirs of SARS-CoV-2 including nasopharyngeal olfactory mucosae, endothelial cells of micro-vessels in the CNS and the gut in PNP and NNP patients [157]. Identification of such reservoirs could justify therapeutic interventions with monoclonal antibodies or antiviral medications.
Sleep/Fatigue/Cognition Axis
Neuro-PASC patients suffer from intense fatigue, sleep difficulties, and cognitive dysfunction. Future research should focus on the interplay of sleep and circadian rhythms disruption and fatigue, and their impact on quality of life and cognitive measures.
Changing Spectrum of Acute and Chronic COVID-19 Presentations Caused by Viral Variants After Vaccines/Booster
SARS-CoV-2 continues to evolve. Omicron and its associated variants of SARS-CoV-2 have become the dominant strain worldwide, causing a steep increase in the number of confirmed infections [230]. While Omicron is more infectious than the initial Wuhan strain, it appears to be less pathogenic during acute infection [230]. Whether these variants causes similar or different Neuro-PASC manifestations than the initial strain and whether these will be altered when infection is contracted after vaccination require further study.
Role of Global Neurology in a Global Pandemic
Multiple factors have shaped the COVID-19 pandemic in various geographic locations, including access to intensive or specialized care and medications, rate of vaccination, socio-economic factors, age and genetic background of the populations, and local viral variants. All of those may have a profound impact on the acute and chronic neurologic manifestations of COVID-19 and may shed light on pathogenic mechanism of Neuro-PASC.
Unsanctioned Use of Experimental Medications and Role of Social Media
While research takes time and patients are often desperate for treatments that could alleviate their symptoms, a word of caution is necessary to avoid the use of unnecessary, unproven or potentially toxic medications. Such potential “cures” are often amplified on social media, and patients may fall prey to unscrupulous providers selling expensive and uninterpretable blood tests that they use to justify cocktails of drugs that are neither FDA-approved nor evidence-based for COVID-19. Instead, patients should be encouraged to participate to clinical treatment trials for COVID-19 held by academic institutions.
Need for Dedicated Research Funding and Peer Review for Neuro-PASC Research
While COVID-19 in primarily a respiratory disease, the most frequent and debilitating manifestations of PASC are neurological, as is the case for the majority of patients seen at the Comprehensive COVID-19 Center of our institution [231]. Although the NIH has organized the RECOVER study [232], media outlets have reported on frustration over the slow pace of enrollment and lack of transparency in the selection process of participating sites expressed by NIH officials, COVID-19 experts and patients alike [233–236]. A panel of physicians, scientists, public health experts, and patients advocates have recommended that the NIH earmark dedicated funding for research on neuropsychiatric manifestations of COVID-19 in a manner that facilitates investigators outside of the RECOVER study group contributing urgently needed knowledge in a nimble and timely fashion based on their own patient populations and expertise [234]. Immediate creation of a specialized NIH study section comprising subject experts who review competitive, independent COVID-19 grant applications could help address existing frustrations over the pace of research. This would be analogous to the Neuro-AIDS and end organ disease (NAED) study section created by the NIH early in the AIDS pandemic, which specifically adjudicates research grant applications on neurologic complications of HIV—an important public health concern but also a virus affecting far fewer people in the USA than SARS-CoV-2 does today.
Education of Medical Students, Residents, and Fellows
As of May 2022, over 80 million people in the USA have survived COVID-19 and approximately 23 million have developed some manifestations of PASC affecting the nervous system [146]. This makes Neuro-PASC, the third most frequent neurologic disorder in this country after tension headache and migraine, and far ahead of stroke, Alzheimer’s, spinal cord, and traumatic brain injury [237]. Patients with Neuro-PASC may present with cognitive dysfunction, headache, vestibular problems, sleep difficulties, neuromuscular and vascular complications as well as dysautonomia, which constitute the commonly considered “bread and butter” of neurology [238]. Since COVID-19 is very likely to become endemic and a persistent disease, the clinical management of Neuro-PASC patients should be taught to medical students and neurology trainees. Furthermore, since Neuro-PASC in non-hospitalized patients has many of the hallmarks of an autoimmune disease, clinicians in Neuroimmunology or Multiple Sclerosis fellowships should participate in the care of these patients in specialized outpatient clinics.
Conclusion
It is apparent that the neurologic manifestations and complications of COVID-19, both during acute illness and during the subsequent chronic phase of Neuro-PASC, are extremely diverse and likely to represent an ongoing challenge to the field of neurology as COVID-19 becomes an endemic illness. While there has been an unprecedented explosion in research productivity in response to the pandemic, our knowledge of the mechanisms underlying the neurologic features of COVID-19 is far from complete. Although we have leveraged existing knowledge from disease and symptom management that predated the pandemic, the large burden of neurologic morbidity represented by COVID-19 demands the development of adequate funding mechanisms and infrastructure to support Neuro-PASC research and clinical care, which will allow the development of specific therapeutics that can mitigate the extensive personal and public health consequences of the pandemic.
Supplementary Information
Below is the link to the electronic supplementary material.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC COVID-NET: COVID-10-Associated Hospitalization Surveillance Network [online]. 2021. Available at: https://gis.cdc.gov/grasp/covidnet/covid19_3.html. Accessed 27 Apr 2022.
- 3.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero-Sanchez CM, Diaz-Maroto I, Fernandez-Diaz E, et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95:e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liotta EM, Batra A, Clark JR, et al. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 2020. [DOI] [PMC free article] [PubMed]
- 6.Siepmann T, Kitzler HH, Lueck C, Platzek I, Reichmann H, Barlinn K. Neuralgic amyotrophy following infection with SARS-CoV-2. Muscle Nerve. 2020;62:E68–E70. doi: 10.1002/mus.27035. [DOI] [PubMed] [Google Scholar]
- 7.Goh Y, Beh DLL, Makmur A, Somani J, Chan ACY. Pearls & Oy-sters: facial nerve palsy in COVID-19 infection. Neurology. 2020;95:364–367. doi: 10.1212/WNL.0000000000009863. [DOI] [PubMed] [Google Scholar]
- 8.Malik GR, Wolfe AR, Soriano R, et al. Injury-prone: peripheral nerve injuries associated with prone positioning for COVID-19-related acute respiratory distress syndrome. Br J Anaesth. 2020;125:e478–e480. doi: 10.1016/j.bja.2020.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark JR, Liotta EM, Reish NJ, et al. Abnormal movements in hospitalized COVID-19 patients: a case series. J Neurol Sci. 2021;423:117377. doi: 10.1016/j.jns.2021.117377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aschman T, Schneider J, Greuel S, et al. Association between SARS-CoV-2 infection and immune-mediated myopathy in patients who have died. JAMA Neurol. 2021;78:948–960. doi: 10.1001/jamaneurol.2021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choksi T, Agrawal A, Date P, et al. Cumulative mortality and factors associated with outcomes of mucormycosis after COVID-19 at a multispecialty tertiary care center in India. JAMA Ophthalmol. 2022;140:66–72. doi: 10.1001/jamaophthalmol.2021.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharyya A, Sarma P, Sharma DJ, et al. Rhino-orbital-cerebral-mucormycosis in COVID-19: a systematic review. Indian J Pharmacol. 2021;53:317–327. doi: 10.4103/ijp.ijp_419_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8:557–566. doi: 10.1038/nrneurol.2012.183. [DOI] [PubMed] [Google Scholar]
- 14.Mazeraud A, Righy C, Bouchereau E, Benghanem S, Bozza FA, Sharshar T. Septic-associated encephalopathy: a comprehensive review. Neurotherapeutics. 2020;17:392–403. doi: 10.1007/s13311-020-00862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oddo M, Carrera E, Claassen J, Mayer SA, Hirsch LJ. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37:2051–2056. doi: 10.1097/CCM.0b013e3181a00604. [DOI] [PubMed] [Google Scholar]
- 16.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pensato U, Muccioli L, Cani I, et al. Brain dysfunction in COVID-19 and CAR-T therapy: cytokine storm-associated encephalopathy. Ann Clin Transl Neurol. 2021;8:968–979. doi: 10.1002/acn3.51348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu SX, Tyagi T, Jain K, et al. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. 2021;18:194–209. doi: 10.1038/s41569-020-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernard-Valnet R, Perriot S, Canales M, et al. Encephalopathies associated with severe COVID-19 present neurovascular unit alterations without evidence for strong neuroinflammation. Neurol Neuroimmunol Neuroinflamm. 2021;8. PMC8210172. [DOI] [PMC free article] [PubMed]
- 20.Garcia MA, Barreras PV, Lewis A, et al. Cerebrospinal fluid in COVID-19 neurological complications: neuroaxonal damage, anti-SARS-Cov2 antibodies but no evidence of cytokine storm. J Neurol Sci. 2021;427:117517. doi: 10.1016/j.jns.2021.117517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wenzel J, Lampe J, Muller-Fielitz H, et al. The SARS-CoV-2 main protease M(pro) causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat Neurosci. 2021;24:1522–1533. doi: 10.1038/s41593-021-00926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song E, Zhang C, Israelow B, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med 2021;218. PMC7808299. [DOI] [PMC free article] [PubMed]
- 24.Rutkai I, Mayer MG, Hellmers LM, et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat Commun. 2022;13:1745. doi: 10.1038/s41467-022-29440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabioni L, De Lorenzo A, Lamas C, et al. Systemic microvascular endothelial dysfunction and disease severity in COVID-19 patients: evaluation by laser Doppler perfusion monitoring and cytokine/chemokine analysis. Microvasc Res. 2021;134:104119. doi: 10.1016/j.mvr.2020.104119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruhl L, Pink I, Kuhne JF, et al. Endothelial dysfunction contributes to severe COVID-19 in combination with dysregulated lymphocyte responses and cytokine networks. Signal Transduct Target Ther. 2021;6:418. doi: 10.1038/s41392-021-00819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jud P, Gressenberger P, Muster V, et al. Evaluation of endothelial dysfunction and inflammatory vasculopathy after SARS-CoV-2 infection-a cross-sectional study. Front Cardiovasc Med. 2021;8:750887. doi: 10.3389/fcvm.2021.750887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mejia-Renteria H, Travieso A, Sagir A, et al. In-vivo evidence of systemic endothelial vascular dysfunction in COVID-19. Int J Cardiol. 2021;345:153–155. doi: 10.1016/j.ijcard.2021.10.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moretta P, Maniscalco M, Papa A, Lanzillo A, Trojano L, Ambrosino P. Cognitive impairment and endothelial dysfunction in convalescent COVID-19 patients undergoing rehabilitation. Eur J Clin Invest. 2022;52:e13726. doi: 10.1111/eci.13726. [DOI] [PubMed] [Google Scholar]
- 31.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bryce C, Grimes Z, Pujadas E, et al. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol. 2021;34:1456–1467. doi: 10.1038/s41379-021-00793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colmenero I, Santonja C, Alonso-Riano M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thakur KT, Miller EH, Glendinning MD, et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain. 2021;144:2696–2708. doi: 10.1093/brain/awab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 37.Hernandez-Fernandez F, Sandoval Valencia H, Barbella-Aponte RA, et al. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain. 2020;143:3089–3103. doi: 10.1093/brain/awaa239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fara MG, Stein LK, Skliut M, Morgello S, Fifi JT, Dhamoon MS. Macrothrombosis and stroke in patients with mild Covid-19 infection. J Thromb Haemost. 2020;18:2031–2033. doi: 10.1111/jth.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paniz-Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bullock HA, Goldsmith CS, Zaki SR, Martines RB, Miller SE. Difficulties in differentiating coronaviruses from subcellular structures in human tissues by electron microscopy. Emerg Infect Dis. 2021;27:1023–1031. doi: 10.3201/eid2704.204337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varga Z, Flammer AJ, Steiger P, et al. Electron microscopy of SARS-CoV-2: a challenging task - Authors' reply. Lancet. 2020;395:e100. doi: 10.1016/S0140-6736(20)31185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid-19. N Engl J Med. 2020;383:989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uginet M, Breville G, Hofmeister J, et al. Cerebrovascular complications and vessel wall imaging in COVID-19 encephalopathy-a pilot study. Clin Neuroradiol. 2021. PMC7995679. [DOI] [PMC free article] [PubMed]
- 44.Siow I, Lee KS, Zhang JJY, Saffari SE, Ng A. Encephalitis as a neurological complication of COVID-19: a systematic review and meta-analysis of incidence, outcomes, and predictors. Eur J Neurol. 2021;28:3491–3502. doi: 10.1111/ene.14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho CY, Salimian M, Hegert J, et al. Postmortem assessment of olfactory tissue degeneration and microvasculopathy in patients with COVID-19. JAMA Neurol. 2022. [DOI] [PMC free article] [PubMed]
- 46.Vasilevska V, Guest PC, Bernstein HG, Schroeter ML, Geis C, Steiner J. Molecular mimicry of NMDA receptors may contribute to neuropsychiatric symptoms in severe COVID-19 cases. J Neuroinflammation. 2021;18:245. doi: 10.1186/s12974-021-02293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Angileri F, Legare S, Gammazza AM, de Macario EC, Macario AJ, Cappello F. Molecular mimicry may explain multi-organ damage in COVID-19. Autoimmun Rev. 2020;19:102591. doi: 10.1016/j.autrev.2020.102591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marino Gammazza A, Legare S, Lo Bosco G, et al. Human molecular chaperones share with SARS-CoV-2 antigenic epitopes potentially capable of eliciting autoimmunity against endothelial cells: possible role of molecular mimicry in COVID-19. Cell Stress Chaperones. 2020;25:737–741. doi: 10.1007/s12192-020-01148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slooter AJC, Otte WM, Devlin JW, et al. Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Med. 2020;46:1020–1022. doi: 10.1007/s00134-019-05907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pun BT, Badenes R, La Calle GH, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. 2021;9:239–250. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chou SH, Beghi E, Helbok R, et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19-a report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw Open. 2021;4:e2112131. doi: 10.1001/jamanetworkopen.2021.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gibb K, Seeley A, Quinn T, et al. The consistent burden in published estimates of delirium occurrence in medical inpatients over four decades: a systematic review and meta-analysis study. Age Ageing. 2020;49:352–360. doi: 10.1093/ageing/afaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kennedy M, Helfand BKI, Gou RY, et al. Delirium in older patients with COVID-19 presenting to the emergency department. JAMA Netw Open. 2020;3:e2029540. doi: 10.1001/jamanetworkopen.2020.29540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krewulak KD, Stelfox HT, Leigh JP, Ely EW, Fiest KM. Incidence and prevalence of delirium subtypes in an adult ICU: a systematic review and meta-analysis. Crit Care Med. 2018;46:2029–2035. doi: 10.1097/CCM.0000000000003402. [DOI] [PubMed] [Google Scholar]
- 55.Batra A, Clark JR, Kang AK, Ali S, Patel TR, Shlobin NA, Hoffman SC, Lim PH, Orban ZS, Visvabharathy L, Graham EL, Sullivan DP, Muller WA, Chou SH, Ungvári Z, Koralnik IJ, Liotta EM. Persistent viral RNA shedding of SARS‑CoV‑2 is associated with delirium incidence and six‑month mortality in hospitalized COVID‑19 patients. Geroscience. 2022 Jun;44(3):1241–1254. 10.1007/s11357-022-00561-z. Epub 2022 May 11. PMID: 35538386; PMCID: PMC9090540. [DOI] [PMC free article] [PubMed]
- 56.Sher Y, Rabkin B, Maldonado JR, Mohabir P. COVID-19-associated hyperactive intensive care unit delirium with proposed pathophysiology and treatment: a case report. Psychosomatics. 2020;61:544–550. doi: 10.1016/j.psym.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim B, Cho J, Park JY, Kim HE, Oh J. Delirium and anxiety outcomes related to visiting policy changes in the intensive care unit during the COVID-19 pandemic. Front Aging Neurosci. 2022;14:845105. doi: 10.3389/fnagi.2022.845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ragheb J, McKinney A, Zierau M, et al. Delirium and neuropsychological outcomes in critically Ill patients with COVID-19: a cohort study. BMJ Open. 2021;11:e050045. doi: 10.1136/bmjopen-2021-050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Girard TD, Thompson JL, Pandharipande PP, et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med. 2018;6:213–222. doi: 10.1016/S2213-2600(18)30062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graham EL, Clark JR, Orban ZS, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 "long haulers". Ann Clin Transl Neurol. 2021. [DOI] [PMC free article] [PubMed]
- 62.Brouqui P, Amrane S, Million M, et al. Asymptomatic hypoxia in COVID-19 is associated with poor outcome. Int J Infect Dis. 2021;102:233–238. doi: 10.1016/j.ijid.2020.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stubbs DJ, Yamamoto AK, Menon DK. Imaging in sepsis-associated encephalopathy–insights and opportunities. Nat Rev Neurol. 2013;9:551–561. doi: 10.1038/nrneurol.2013.177. [DOI] [PubMed] [Google Scholar]
- 64.Kandemirli SG, Dogan L, Sarikaya ZT, et al. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. 2020;297:E232–E235. doi: 10.1148/radiol.2020201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agarwal S, Jain R, Dogra S, et al. Cerebral microbleeds and leukoencephalopathy in critically ill patients with COVID-19. Stroke. 2020;51:2649–2655. doi: 10.1161/STROKEAHA.120.030940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kremer S, Lersy F, Anheim M, et al. Neurologic and neuroimaging findings in patients with COVID-19: a retrospective multicenter study. Neurology. 2020;95:e1868–e1882. doi: 10.1212/WNL.0000000000010112. [DOI] [PubMed] [Google Scholar]
- 67.Kremer S, Lersy F, de Seze J, et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;297:E242–E251. doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matschke J, Lutgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deigendesch N, Sironi L, Kutza M, et al. Correlates of critical illness-related encephalopathy predominate postmortem COVID-19 neuropathology. Acta Neuropathol. 2020;140:583–586. doi: 10.1007/s00401-020-02213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fifth ed. Diagnostic and statistical manual of mental disorders. Am Psychiatric Assoc. 2013.
- 71.Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 72.Wijdicks EF, Bamlet WR, Maramattom BV, Manno EM, McClelland RL. Validation of a new coma scale: The FOUR score. Ann Neurol. 2005;58:585–593. doi: 10.1002/ana.20611. [DOI] [PubMed] [Google Scholar]
- 73.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 74.Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 2014;189:658–665. doi: 10.1164/rccm.201310-1815OC. [DOI] [PubMed] [Google Scholar]
- 75.Bhattacharyya S, Darby RR, Raibagkar P, Gonzalez Castro LN, Berkowitz AL. Antibiotic-associated encephalopathy. Neurology. 2016;86:963–971. doi: 10.1212/WNL.0000000000002455. [DOI] [PubMed] [Google Scholar]
- 76.Salerno D, Marik PE, Daskalakis C, Kolm P, Leone F. The role of head computer tomographic scans on the management of MICU patients with neurological dysfunction. J Intensive Care Med. 2009;24:372–375. doi: 10.1177/0885066609344940. [DOI] [PubMed] [Google Scholar]
- 77.Radmanesh A, Raz E, Zan E, Derman A, Kaminetzky M. Brain imaging use and findings in COVID-19: a single academic center experience in the epicenter of disease in the United States. AJNR Am J Neuroradiol. 2020;41:1179–1183. doi: 10.3174/ajnr.A6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Antony AR, Haneef Z. Systematic review of EEG findings in 617 patients diagnosed with COVID-19. Seizure. 2020;83:234–241. doi: 10.1016/j.seizure.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin L, Al-Faraj A, Ayub N, et al. Electroencephalographic abnormalities are common in COVID-19 and are associated with outcomes. Ann Neurol. 2021;89:872–883. doi: 10.1002/ana.26060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pellinen J, Carroll E, Friedman D, et al. Continuous EEG findings in patients with COVID-19 infection admitted to a New York academic hospital system. Epilepsia. 2020;61:2097–2105. doi: 10.1111/epi.16667. [DOI] [PubMed] [Google Scholar]
- 81.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–1748. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- 82.Tandon M, Kataria S, Patel J, et al. A comprehensive systematic review of CSF analysis that defines neurological manifestations of COVID-19. Int J Infect Dis. 2021;104:390–397. doi: 10.1016/j.ijid.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cao A, Rohaut B, Le Guennec L, et al. Severe COVID-19-related encephalitis can respond to immunotherapy. Brain. 2020;143:e102. doi: 10.1093/brain/awaa337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pugin D, Vargas MI, Thieffry C, et al. COVID-19-related encephalopathy responsive to high-dose glucocorticoids. Neurology. 2020;95:543–546. doi: 10.1212/WNL.0000000000010354. [DOI] [PubMed] [Google Scholar]
- 85.Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Group RC. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Health NIo. Therapeutic management of hospitalized adults With COVID-19 [online]. 2022. Available at: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management/. Accessed 20 May 2022.
- 88.Kojima Y, Nakakubo S, Takei N, et al. Comparative efficacy of tocilizumab and baricitinib administration in COVID-19 treatment: a retrospective cohort study. Medicina (Kaunas). 2022;58. [DOI] [PMC free article] [PubMed]
- 89.Siegler EL, Kenderian SS. Neurotoxicity and cytokine release syndrome after chimeric antigen receptor T cell therapy: insights into mechanisms and novel therapies. Front Immunol. 2020;11:1973. doi: 10.3389/fimmu.2020.01973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Buckley MS, Smithburger PL, Wong A, et al. Dexmedetomidine for facilitating mechanical ventilation extubation in difficult-to-wean ICU patients: systematic review and meta-analysis of clinical trials. J Intensive Care Med. 2021;36:925–936. doi: 10.1177/0885066620937673. [DOI] [PubMed] [Google Scholar]
- 91.Groetzinger LM, Rivosecchi RM, Bain W, et al. Ketamine infusion for adjunct sedation in mechanically ventilated adults. Pharmacotherapy. 2018;38:181–188. doi: 10.1002/phar.2065. [DOI] [PubMed] [Google Scholar]
- 92.Attaluri P, Castillo A, Edriss H, Nugent K. Thiamine deficiency: an important consideration in critically ill patients. Am J Med Sci. 2018;356:382–390. doi: 10.1016/j.amjms.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 93.Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49:341–345. doi: 10.1136/jnnp.49.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Manzano GS, McEntire CRS, Martinez-Lage M, Mateen FJ, Hutto SK. Acute disseminated encephalomyelitis and acute hemorrhagic leukoencephalitis following COVID-19: systematic review and meta-synthesis. Neurol Neuroimmunol Neuroinflamm. 2021;8. PMC8404207. [DOI] [PMC free article] [PubMed]
- 95.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Valencia Sanchez C, Theel E, Binnicker M, Toledano M, McKeon A. Autoimmune encephalitis after SARS-CoV-2 infection: case frequency, findings, and outcomes. Neurology. 2021;97:e2262–e2268. doi: 10.1212/WNL.0000000000012931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Panariello A, Bassetti R, Radice A, et al. Anti-NMDA receptor encephalitis in a psychiatric Covid-19 patient: a case report. Brain Behav Immun. 2020;87:179–181. doi: 10.1016/j.bbi.2020.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Durovic E, Bien C, Bien CG, Isenmann S. MOG antibody-associated encephalitis secondary to Covid-19: case report. BMC Neurol. 2021;21:414. doi: 10.1186/s12883-021-02449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Patone M, Handunnetthi L, Saatci D, et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021;27:2144–2153. doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Richie MB. Autoimmune meningitis and encephalitis. Neurol Clin. 2022;40:93–112. doi: 10.1016/j.ncl.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 101.Trifan G, Goldenberg FD, Caprio FZ, et al. Characteristics of a diverse cohort of stroke patients with SARS-CoV-2 and outcome by sex. J Stroke Cerebrovasc Dis. 2020;29:105314. doi: 10.1016/j.jstrokecerebrovasdis.2020.105314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bekelis K, Missios S, Ahmad J, et al. Ischemic stroke occurs less frequently in patients with COVID-19: a multicenter cross-sectional study. Stroke. 2020;51:3570–3576. doi: 10.1161/STROKEAHA.120.031217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qureshi AI, Baskett WI, Huang W, et al. Acute ischemic stroke and COVID-19: an analysis of 27 676 patients. Stroke. 2021;52:905–912. doi: 10.1161/STROKEAHA.120.031786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yaghi S, Ishida K, Torres J, et al. SARS-CoV-2 and stroke in a New York Healthcare System. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rothstein A, Oldridge O, Schwennesen H, Do D, Cucchiara BL. Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke. 2020;51:e219–e222. doi: 10.1161/STROKEAHA.120.030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fridman S, Bres Bullrich M, Jimenez-Ruiz A, et al. Stroke risk, phenotypes, and death in COVID-19: systematic review and newly reported cases. Neurology. 2020;95:e3373–e3385. doi: 10.1212/WNL.0000000000010851. [DOI] [PubMed] [Google Scholar]
- 107.Leasure AC, Khan YM, Iyer R, et al. Intracerebral hemorrhage in patients with COVID-19: an analysis from the COVID-19 Cardiovascular Disease Registry. Stroke. 2021;52:e321–e323. doi: 10.1161/STROKEAHA.121.034215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Al-Mufti F, Amuluru K, Sahni R, et al. Cerebral venous thrombosis in COVID-19: a New York Metropolitan Cohort Study. AJNR Am J Neuroradiol. 2021;42:1196–1200. doi: 10.3174/ajnr.A7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Batra A, Clark JR, LaHaye K, et al. Transcranial doppler ultrasound evidence of active cerebral embolization in COVID-19. J Stroke Cerebrovasc Dis. 2020;30:105542. doi: 10.1016/j.jstrokecerebrovasdis.2020.105542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Srivastava PK, Zhang S, Xian Y, et al. Acute ischemic stroke in patients with COVID-19: an analysis from Get With The Guidelines-Stroke. Stroke. 2021;52:1826–1829. doi: 10.1161/STROKEAHA.121.034301. [DOI] [PubMed] [Google Scholar]
- 112.Katsanos AH, Palaiodimou L, Zand R, et al. The impact of SARS-CoV-2 on stroke epidemiology and care: a meta-analysis. Ann Neurol. 2021;89:380–388. doi: 10.1002/ana.25967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liotta EM, Prabhakaran S, Sangha RS, et al. Magnesium, hemostasis, and outcomes in patients with intracerebral hemorrhage. Neurology. 2017;89:813–819. doi: 10.1212/WNL.0000000000004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Elkind MS, Carty CL, O'Meara ES, et al. Hospitalization for infection and risk of acute ischemic stroke: the Cardiovascular Health Study. Stroke. 2011;42:1851–1856. doi: 10.1161/STROKEAHA.110.608588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cowan LT, Alonso A, Pankow JS, et al. Hospitalized infection as a trigger for acute ischemic stroke: the Atherosclerosis Risk in Communities Study. Stroke. 2016;47:1612–1617. doi: 10.1161/STROKEAHA.116.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zeng JH, Liu YX, Yuan J, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48:773–777. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dweck MR, Bularga A, Hahn RT, et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21:949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li Z, Shao W, Zhang J, et al. Prevalence of atrial fibrillation and associated mortality among hospitalized patients with COVID-19: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:720129. doi: 10.3389/fcvm.2021.720129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bhatla A, Mayer MM, Adusumalli S, et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Organization ELS. ECMO in COVID-19 [online]. 2022. Available at: https://www.elso.org/Registry/FullCOVID19RegistryDashboard.aspx. Accessed 14 Jan 2022.
- 122.Fletcher-Sandersjoo A, Thelin EP, Bartek J, Jr, et al. Incidence, outcome, and predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: a systematic and narrative review. Front Neurol. 2018;9:548. doi: 10.3389/fneur.2018.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Usman AA, Han J, Acker A, et al. A case series of devastating intracranial hemorrhage during venovenous extracorporeal membrane oxygenation for COVID-19. J Cardiothorac Vasc Anesth. 2020;34:3006–3012. doi: 10.1053/j.jvca.2020.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bermea RS, Raz Y, Sertic F, et al. Increased intracranial hemorrhage amid elevated inflammatory markers in those with COVID-19 supported with extracorporeal membrane oxygenation. Shock. 2021;56:206–214. doi: 10.1097/SHK.0000000000001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Katz JM, Libman RB, Wang JJ, et al. Cerebrovascular complications of COVID-19. Stroke. 2020;51:e227–e231. doi: 10.1161/STROKEAHA.120.031265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang A, Mandigo GK, Yim PD, Meyers PM, Lavine SD. Stroke and mechanical thrombectomy in patients with COVID-19: technical observations and patient characteristics. J Neurointerv Surg. 2020;12:648–653. doi: 10.1136/neurintsurg-2020-016220. [DOI] [PubMed] [Google Scholar]
- 127.Escalard S, Maier B, Redjem H, et al. Treatment of acute ischemic stroke due to large vessel occlusion with COVID-19: experience from Paris. Stroke. 2020;51:2540–2543. doi: 10.1161/STROKEAHA.120.030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.RC Investigators. AC-a Investigators. A Investigators et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.A Investigators. AC-a Investigators. R-C Investigators et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sholzberg M, Tang GH, Rahhal H, et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400. doi: 10.1136/bmj.n2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Spyropoulos AC, Goldin M, Giannis D, et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID Randomized Clinical Trial. JAMA Intern Med. 2021;181:1612–1620. doi: 10.1001/jamainternmed.2021.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.RECOVERY Collaborative Group. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:143–151. PMC8598213. [DOI] [PMC free article] [PubMed]
- 133.Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barre syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Aladawi M, Elfil M, Abu-Esheh B, et al. Guillain Barre syndrome as a complication of COVID-19: A SYSTEMATIC REView. Can J Neurol Sci. 2022;49:38–48. doi: 10.1017/cjn.2021.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Keddie S, Pakpoor J, Mousele C, et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barre syndrome. Brain. 2021;144:682–693. doi: 10.1093/brain/awaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Luijten LWG, Leonhard SE, van der Eijk AA, et al. Guillain-Barre syndrome after SARS-CoV-2 infection in an international prospective cohort study. Brain. 2021;144:3392–3404. doi: 10.1093/brain/awab279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sheikh KA. Guillain-Barre Syndrome. Continuum (Minneap Minn) 2020;26:1184–1204. doi: 10.1212/CON.0000000000000929. [DOI] [PubMed] [Google Scholar]
- 139.Menni C, Valdes AM, Freidin MB, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 2020;26:1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Centers for Disease Control and Prevention. Long COVID or post-COVID conditions [online]. 2022. Available at: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html. Accessed 19 May 2022.
- 141.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4:e2128568. doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18:e1003773. PMC8478214. [DOI] [PMC free article] [PubMed]
- 144.Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after Covid-19: qualitative study of 114 "long Covid" patients and draft quality principles for services. BMC Health Serv Res. 2020;20:1144. doi: 10.1186/s12913-020-06001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Centers for Disease Control and Prevention. Estimated COVID-19 Burden [online]. 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html. Accessed 30 Jan 2022.
- 146.US Government Accountability Office. Science & Tech Spotlight: Long COVID [online]. 2020. Available at: https://www.gao.gov/products/gao-22-105666. Accessed 27 May 2022.
- 147.Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022. [DOI] [PMC free article] [PubMed]
- 148.Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(881–895):e820. doi: 10.1016/j.cell.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gold JE, Okyay RA, Licht WE, Hurley DJ. Investigation of long COVID prevalence and its relationship to epstein-barr virus reactivation. Pathogens. 2021;10. PMC8233978. [DOI] [PMC free article] [PubMed]
- 151.Ali ST, Kang AK, Patel TR, et al. Evolution of neurologic symptoms in non-hospitalized COVID-19 "long haulers". Ann Clin Transl Neurol. 2022. [DOI] [PMC free article] [PubMed]
- 152.Frontera JA, Yang D, Medicherla C, et al. Trajectories of neurologic recovery 12 Months after hospitalization for COVID-19: a prospective longitudinal study. Neurology. 2022. [DOI] [PMC free article] [PubMed]
- 153.Hampshire A, Chatfield DA, MPhil AM, et al. Multivariate profile and acute-phase correlates of cognitive deficits in a COVID-19 hospitalised cohort. EClinicalMedicine. 2022;47:101417. doi: 10.1016/j.eclinm.2022.101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Amalakanti S, Arepalli KVR, Jillella JP. Cognitive assessment in asymptomatic COVID-19 subjects. Virusdisease. 2021;32:146–149. doi: 10.1007/s13337-021-00663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Han Q, Zheng B, Daines L, Sheikh A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens. 2022;11. PMC8875269. [DOI] [PMC free article] [PubMed]
- 156.Spudich S, Nath A. Nervous system consequences of COVID-19. Science. 2022;375:267–269. doi: 10.1126/science.abm2052. [DOI] [PubMed] [Google Scholar]
- 157.Batra A, Clark JR, Kang AK, et al. Persistent viral RNA shedding of SARS-CoV-2 is associated with delirium incidence and six-month mortality in hospitalized COVID-19 patients. Geroscience. 2022. PMC9090540. [DOI] [PMC free article] [PubMed]
- 158.Chen R, Wang K, Yu J, et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front Neurol. 2020;11:573095. doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yong SJ. Persistent brainstem dysfunction in long-COVID: a hypothesis. ACS Chem Neurosci. 2021;12:573–580. doi: 10.1021/acschemneuro.0c00793. [DOI] [PubMed] [Google Scholar]
- 160.Heming M, Li X, Rauber S, et al. Neurological manifestations of COVID-19 feature T cell exhaustion and dedifferentiated monocytes in cerebrospinal fluid. Immunity. 2021;54(164–175):e166. doi: 10.1016/j.immuni.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hattori Y, Hattori K, Machida T, Matsuda N. Vascular endotheliitis associated with infections: Its pathogenetic role and therapeutic implication. Biochem Pharmacol. 2022;197:114909. doi: 10.1016/j.bcp.2022.114909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Douaud G, Lee S, Alfaro-Almagro F, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Guedj E, Million M, Dudouet P, et al. (18)F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Eur J Nucl Med Mol Imaging. 2021;48:592–595. doi: 10.1007/s00259-020-04973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Apple AC, Oddi A, Peluso MJ, et al. Risk factors and abnormal cerebrospinal fluid associate with cognitive symptoms after mild COVID-19. Ann Clin Transl Neurol. 2022;9:221–226. doi: 10.1002/acn3.51498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ziff OJ, Ashton NJ, Mehta PR, et al. Amyloid processing in COVID-19-associated neurological syndromes. J Neurochem. 2022;161:146–157. doi: 10.1111/jnc.15585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ameres M, Brandstetter S, Toncheva AA, et al. Association of neuronal injury blood marker neurofilament light chain with mild-to-moderate COVID-19. J Neurol. 2020;267:3476–3478. doi: 10.1007/s00415-020-10050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Aiello EN, Fiabane E, Manera MR, et al. Screening for cognitive sequelae of SARS-CoV-2 infection: a comparison between the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) Neurol Sci. 2022;43:81–84. doi: 10.1007/s10072-021-05630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE. 2020;15:e0240784. doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93:1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 170.Arnold DT, Hamilton FW, Milne A, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2021;76:399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry. 2003;160:221–236. doi: 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- 172.Norrie J, Heitger M, Leathem J, Anderson T, Jones R, Flett R. Mild traumatic brain injury and fatigue: a prospective longitudinal study. Brain Inj. 2010;24:1528–1538. doi: 10.3109/02699052.2010.531687. [DOI] [PubMed] [Google Scholar]
- 173.Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585:339–341. doi: 10.1038/d41586-020-02598-6. [DOI] [PubMed] [Google Scholar]
- 174.Lai JS, Cella D, Choi S, et al. How item banks and their application can influence measurement practice in rehabilitation medicine: a PROMIS fatigue item bank example. Arch Phys Med Rehabil. 2011;92:S20–27. doi: 10.1016/j.apmr.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Scherlinger M, Felten R, Gallais F, et al. Refining "long-COVID" by a prospective multimodal evaluation of patients with long-term symptoms attributed to SARS-CoV-2 infection. Infect Dis Ther. 2021;10:1747–1763. doi: 10.1007/s40121-021-00484-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hannum ME, Ramirez VA, Lipson SJ, et al. Objective sensory testing methods reveal a higher prevalence of olfactory loss in COVID-19-positive patients compared to subjective methods: a systematic review and meta-analysis. Chem Senses. 2020;45:865–874. doi: 10.1093/chemse/bjaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.von Bartheld CS, Hagen MM, Butowt R. Prevalence of Chemosensory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis Reveals Significant Ethnic Differences. ACS Chem Neurosci. 2020;11:2944–2961. doi: 10.1021/acschemneuro.0c00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cao Y, Li L, Feng Z, et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schambeck SE, Crowell CS, Wagner KI, et al. Phantosmia, parosmia, and dysgeusia are prolonged and late-onset symptoms of COVID-19. J Clin Med. 2021;10. PMC8618742. [DOI] [PMC free article] [PubMed]
- 180.Aragao M, Leal MC, Cartaxo Filho OQ, Fonseca TM, Valenca MM. Anosmia in COVID-19 associated with injury to the olfactory bulbs evident on MRI. AJNR Am J Neuroradiol. 2020;41:1703–1706. doi: 10.3174/ajnr.A6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Khan M, Yoo SJ, Clijsters M, et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell. 2021;184(5932–5949):e5915. doi: 10.1016/j.cell.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Butowt R, von Bartheld CS. Anosmia in COVID-19: underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist. 2021;27:582–603. doi: 10.1177/1073858420956905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Patel ZM. Olfactory loss and olfactory training. JAMA Otolaryngol Head Neck Surg. 2021;147:840. doi: 10.1001/jamaoto.2021.1507. [DOI] [PubMed] [Google Scholar]
- 184.Yom-Tov E, Lekkas D, Jacobson NC. Association of COVID19-induced anosmia and ageusia with depression and suicidal ideation. J Affect Disord Rep. 2021;5:100156. doi: 10.1016/j.jadr.2021.100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vaira LA, Hopkins C, Petrocelli M, et al. Efficacy of corticosteroid therapy in the treatment of long- lasting olfactory disorders in COVID-19 patients. Rhinology. 2021;59:21–25. doi: 10.4193/Rhin20.515. [DOI] [PubMed] [Google Scholar]
- 186.Singh CV, Jain S, Parveen S, Deshmukh P. The outcome of fluticasone nasal spray on anosmia and triamcinolone oral paste in taste dysgeusia in COVID-19 patients. Am J Otolaryngol. 2021;42:103009. doi: 10.1016/j.amjoto.2021.103009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Abdelalim AA, Mohamady AA, Elsayed RA, Elawady MA, Ghallab AF. Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: a randomized controlled trial. Am J Otolaryngol. 2021;42:102884. doi: 10.1016/j.amjoto.2020.102884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res. 2021;69:205–211. doi: 10.1007/s12026-021-09185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Novak P, Mukerji SS, Alabsi HS, et al. Multisystem involvement in post-acute sequelae of coronavirus disease 19. Ann Neurol. 2021. [DOI] [PMC free article] [PubMed]
- 190.Carson E, Hemenway AN. A scoping review of pharmacological management of postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection in 2021. Am J Ther. 2022;29:e305–e321. doi: 10.1097/MJT.0000000000001486. [DOI] [PubMed] [Google Scholar]
- 191.Dani M, Dirksen A, Taraborrelli P, et al. Autonomic dysfunction in 'long COVID': rationale, physiology and management strategies. Clin Med (Lond) 2021;21:e63–e67. doi: 10.7861/clinmed.2020-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fernandez-de-Las-Penas C, Navarro-Santana M, Gomez-Mayordomo V, et al. Headache as an acute and post-COVID-19 symptom in COVID-19 survivors: a meta-analysis of the current literature. Eur J Neurol. 2021;28:3820–3825. doi: 10.1111/ene.15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Caronna E, Alpuente A, Torres-Ferrus M, Pozo-Rosich P. Toward a better understanding of persistent headache after mild COVID-19: three migraine-like yet distinct scenarios. Headache. 2021;61:1277–1280. doi: 10.1111/head.14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Uygun O, Ertas M, Ekizoglu E, et al. Headache characteristics in COVID-19 pandemic-a survey study. J Headache Pain. 2020;21:121. doi: 10.1186/s10194-020-01188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Garcia-Azorin D, Layos-Romero A, Porta-Etessam J, et al. Post-COVID-19 persistent headache: a multicentric 9-months follow-up study of 905 patients. Cephalalgia. 2022:3331024211068074. [DOI] [PubMed]
- 196.Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97:553–622. doi: 10.1152/physrev.00034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bolay H, Gul A, Baykan B. COVID-19 is a real headache! Headache. 2020;60:1415–1421. doi: 10.1111/head.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Krymchantowski AV, Silva-Neto RP, Jevoux C, Krymchantowski AG. Indomethacin for refractory COVID or post-COVID headache: a retrospective study. Acta Neurol Belg. 2022;122:465–469. doi: 10.1007/s13760-021-01790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Oaklander AL, Mills AJ, Kelley M, et al. Peripheral neuropathy evaluations of patients with prolonged long COVID. Neurol Neuroimmunol Neuroinflamm. 2022;9. PMC8889896. [DOI] [PMC free article] [PubMed]
- 200.Aksan F, Nelson EA, Swedish KA. A COVID-19 patient with intense burning pain. J Neurovirol. 2020;26:800–801. doi: 10.1007/s13365-020-00887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Almufarrij I, Munro KJ. One year on: an updated systematic review of SARS-CoV-2, COVID-19 and audio-vestibular symptoms. Int J Audiol. 2021;60:935–945. doi: 10.1080/14992027.2021.1896793. [DOI] [PubMed] [Google Scholar]
- 202.Motawea KR, Monib FA. New onset vertigo after COVID-19 infection. A case report. Indian J Otolaryngol Head Neck Surg. 2021:1–3. PMC8255056. [DOI] [PMC free article] [PubMed]
- 203.Picciotti PM, Passali GC, Sergi B, De Corso E. Benign paroxysmal positional vertigo (BPPV) in COVID-19. Audiol Res. 2021;11:418–422. doi: 10.3390/audiolres11030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cree BA, Kornyeyeva E, Goodin DS. Pilot trial of low-dose naltrexone and quality of life in multiple sclerosis. Ann Neurol. 2010;68:145–150. doi: 10.1002/ana.22006. [DOI] [PubMed] [Google Scholar]
- 205.Patten DK, Schultz BG, Berlau DJ. The safety and efficacy of low-dose naltrexone in the management of chronic pain and inflammation in multiple sclerosis, fibromyalgia, Crohn's disease, and other chronic pain disorders. Pharmacotherapy. 2018;38:382–389. doi: 10.1002/phar.2086. [DOI] [PubMed] [Google Scholar]
- 206.Peters KB, Affronti ML, Woodring S, et al. Effects of low-dose naltrexone on quality of life in high-grade glioma patients: a placebo-controlled, double-blind randomized trial. Support Care Cancer. 2022. [DOI] [PubMed]
- 207.World Health Organization. Pilot study into LDN and NAD+ for treatment of patients with post-COVID-19 syndrome [online]. 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04604704. Accessed 30 Jan 2022.
- 208.Hashimoto Y, Suzuki T, Hashimoto K. Mechanisms of action of fluvoxamine for COVID-19: a historical review. Mol Psychiatry. 2022. PMC8739627. [DOI] [PMC free article] [PubMed]
- 209.See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448–2456. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.MacNeil JR, Su JR, Broder KR, et al. Updated Recommendations from the Advisory Committee on Immunization Practices for Use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients - United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:651–656. PMC8084127. [DOI] [PMC free article] [PubMed]
- 211.Woo EJ, Mba-Jonas A, Dimova RB, Alimchandani M, Zinderman CE, Nair N. Association of receipt of the Ad26.COV2.S COVID-19 vaccine with presumptive Guillain-Barre syndrome, February-July 2021. JAMA. 2021;326:1606–1613. doi: 10.1001/jama.2021.16496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Frontera JA, Tamborska AA, Doheim MF, et al. Neurological events reported after COVID-19 vaccines: an analysis of VAERS. Ann Neurol. 2022. [DOI] [PMC free article] [PubMed]
- 213.Ali ST, Kang AK, Patel TR, Clark JR, Perez-Giraldo GS, Orban ZS, Lim PH, Jimenez M, Graham EL, Batra A, Liotta EM, Koralnik I. Evolution of neurologic symptoms in non-hospitalized COVID-19 “long haulers”. Ann Clin Transl Neurol. 2022 May 24. 10.1002/acn3.51570. Epub ahead of print. PMID: 35607826. [DOI] [PMC free article] [PubMed]
- 214.Rubin R. As their numbers grow, COVID-19 "long haulers" stump experts. JAMA. 2020. [DOI] [PubMed]
- 215.Asbring P, Narvanen AL. Women's experiences of stigma in relation to chronic fatigue syndrome and fibromyalgia. Qual Health Res. 2002;12:148–160. doi: 10.1177/104973230201200202. [DOI] [PubMed] [Google Scholar]
- 216.Garner P. For 7 weeks I have been through a roller coaster of ill health, extreme emotions, and utter exhaustion. BMJ Opinion. 2020.
- 217.World Health Organization. WHO COVID-19 Case definition [online]. 2020. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2. Accessed 24 May 2022.
- 218.Abdelhak A, Huss A, Kassubek J, Tumani H, Otto M. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep. 2018;8:14798. doi: 10.1038/s41598-018-33158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Watanabe M, Nakamura Y, Michalak Z, et al. Serum GFAP and neurofilament light as biomarkers of disease activity and disability in NMOSD. Neurology. 2019;93:e1299–e1311. doi: 10.1212/WNL.0000000000008160. [DOI] [PubMed] [Google Scholar]
- 220.Sutter R, Hert L, De Marchis GM, et al. Serum neurofilament light chain levels in the intensive care unit: comparison between severely ill patients with and without coronavirus disease 2019. Ann Neurol. 2021;89:610–616. doi: 10.1002/ana.26004. [DOI] [PubMed] [Google Scholar]
- 221.Chung HY, Neu C, Wickel J, Kuckertz SL, Coldewey SM. Neurofilament light chain in patients with COVID-19 and bacterial pneumonia. Ann Neurol. 2021;90:174–175. doi: 10.1002/ana.26135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cooper J, Stukas S, Hoiland RL, et al. Quantification of neurological blood-based biomarkers in critically ill patients with coronavirus disease 2019. Crit Care Explor. 2020;2:e0238. doi: 10.1097/CCE.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kanberg N, Simren J, Eden A, et al. Neurochemical signs of astrocytic and neuronal injury in acute COVID-19 normalizes during long-term follow-up. EBioMedicine. 2021;70:103512. doi: 10.1016/j.ebiom.2021.103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hanson BA, Visvabharathy L, Ali ST, et al. Plasma biomarkers of neuropathogenesis in hospitalized patients with COVID-19 and those with postacute sequelae of SARS-CoV-2 Infection. Neurol Neuroimmunol Neuroinflammation. 2022;9:e1151. doi: 10.1212/NXI.0000000000001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Geis T, Brandstetter S, Toncheva AA, et al. Serum neurofilament light chain (sNfL) values in a large cross-sectional population of children with asymptomatic to moderate COVID-19. J Neurol. 2021;268:3969–3974. doi: 10.1007/s00415-021-10554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kanberg N, Ashton NJ, Andersson LM, et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;95:e1754–e1759. doi: 10.1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- 227.Weiskopf D, Schmitz KS, Raadsen MP, et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5. PMC7319493. [DOI] [PMC free article] [PubMed]
- 228.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Visvabharathy L, Hanson B, Orban Z, et al. Neuro-COVID long-haulers exhibit broad dysfunction in T cell memory generation and responses to vaccination. Available at: https://www.medrxiv.org/content/10.1101/2021.08.08.21261763v1?rss=1. medRxiv. 2021. PMC8366804. Accesed 20 May 2022.
- 230.Khandia R, Singhal S, Alqahtani T, et al. Emergence of SARS-CoV-2 Omicron (B.1.1.529) variant, salient features, high global health concerns and strategies to counter it amid ongoing COVID-19 pandemic. Environ Res. 2022:112816. [DOI] [PMC free article] [PubMed]
- 231.Nortwestern Medicine. Northwestern Medicine Comprehensive COVID-19 Center [online]. 2020. Available at: https://www.nm.org/conditions-and-care-areas/infectious-disease/covid-19-overview/comprehensive-care-after-covid-19. Accessed 25 May 2022.
- 232.National Institutes of Health. Recover: Researching COVID to Enhance Recovery [online]. 2022. Available at: https://recovercovid.org/. Accessed 25 May /2022.
- 233.Cohrs R. ‘A slow-moving glacier’: NIH’s sluggish and often opaque efforts to study long Covid draw patient, expert ire [online]. 2022. Available at: https://www.statnews.com/2022/03/29/nih-long-covid-sluggish-study/. Accessed 25 May 2022.
- 234.Issuu.com. Getting to and sustaining the next normal: a roadmap to living with COVID [online]. 2022. Available at: https://issuu.com/covidstrategy/docs/covidnextnormal_ee2307fb01e43f/2?ff. Accessed 24 May 2022.
- 235.Patient-Led Research Collaborative. Open Letter regarding the RECOVER initiative to study Long COVID [online]. 2021. Available at: https://patientresearchcovid19.com/open-letter-regarding-the-recover-initiative-to-study-long-covid/. Accessed 24 May 2022.
- 236.Body Politic. Open letter to the NIH [online]. 2021. Available at: https://www.wearebodypolitic.com/bodytype/2021/4/22/open-letter-to-nih. Accessed 24 May 2022.
- 237.Collaborators GUND, Feigin VL, Vos T, et al. Burden of neurological disorders across the US From 1990–2017: a global burden of disease study. JAMA Neurol. 2021;78:165–176. doi: 10.1001/jamaneurol.2020.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Balcom EF, Nath A, Power C. Acute and chronic neurological disorders in COVID-19: potential mechanisms of disease. Brain. 2021;144:3576–3588. doi: 10.1093/brain/awab302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.