Abstract
In human demyelinating diseases such as multiple sclerosis (MS), an imbalance between demyelination and remyelination can trigger progressive degenerative processes. The clearance of myelin debris (phagocytosis) from the site of demyelination by microglia is critically important to achieve adequate remyelination and to slow the progression of the disease. However, how microglia phagocytose the myelin debris, and why clearance is impaired in MS, is not fully known; likewise, the role of the microglia in remyelination remains unclear. Recent studies using cuprizone (CPZ) as an animal model of central nervous system demyelination revealed that the up‐regulation of signaling proteins in microglia facilitates effective phagocytosis of myelin debris. Moreover, during demyelination, protective mediators are released from activated microglia, resulting in the acceleration of remyelination in the CPZ model. In contrast, inadequate microglial activation or recruitment to the site of demyelination, and the production of toxic mediators, impairs remyelination resulting in progressive demyelination. In addition to the microglia‐mediated phagocytosis, astrocytes play an important role in the phagocytic process by recruiting microglia to the site of demyelination and producing regenerative mediators. The current review is an update of these emerging findings from the CPZ animal model, discussing the roles of microglia and astrocytes in phagocytosis and myelination.
Keywords: aging, behavioral deficits, cuprizone, demyelination, gliosis, myelin debris, oligodendrocytes, polarization, remyelination, synaptic degeneration
Main Points
Inadequate remyelination results in longer‐lasting disability in people with multiple sclerosis. This review explores the role of microglia and astrocytes in myelin repair by modulating phagocytosis and the release of protective or detrimental substances.
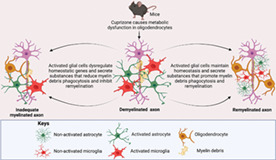
1. INTRODUCTION
Oligodendrocytes myelinate axons of the central nervous system (CNS) whereas Schwann cells myelinate axons in the peripheral nervous system, and this process facilitates the rapid propagation of action potentials. Demyelination leads to the disruption of action potential propagation and the generation of myelin fragments as debris. New myelin formation at the site of CNS demyelination occurs through the maturation of oligodendrocyte progenitor cells (OPCs) followed by remyelination (reviewed in Nave & Werner, 2014; Sen & Hossain, 2021; Sen et al., 2019b; Snaidero & Simons, 2014). Remyelination is necessary to restore myelin structure, provide metabolic support to axons, and restore rapid action potential propagation along axons. Remyelination is a tightly controlled process (regulated by the expression of several transcription factors) that depends upon the activation, proliferation, migration and maturation of OPCs (Nave & Werner, 2014; Snaidero & Simons, 2014). Where the processes of OPC recruitment and maturation are impeded, progressive demyelination and chronic neuroinflammation can prevail (Domingues et al., 2016; Nave & Werner, 2014; Sen et al., 2019b; Snaidero & Simons, 2014).
In multiple sclerosis (MS), clinically defined as a demyelinating neuroinflammatory disease of the human CNS, a progressive decline in the extent of remyelination and increased neurodegeneration is observed (Sen et al., 2020b; Stys et al., 2012; Trapp et al., 1999). Notably, currently approved immunomodulatory treatments for MS can reduce the severity of the disease and improve quality of life, but recovery from progressive myelin degeneration and disease progression has been, as yet, unattainable (Robertson & Moreo, 2016). Although the pathoetiology of remyelination failure in MS remains elusive, inadequate phagocytosis (Hochreiter‐Hufford & Ravichandran, 2013; Janda et al., 2018; Town et al., 2005) of myelin debris and secretion of toxic mediators from activated microglia and astrocytes are believed to contribute to inadequate remyelination (Franklin, 2002; Lubetzki et al., 2020; Rawji et al., 2020a). Phagocytosis is considered one of the prerequisites for remyelination in MS (Lampron et al., 2015), but the cellular mechanistic pathways of microglia and astrocyte‐driven phagocytosis and remyelination in MS remain poorly defined.
2. MICROGLIAL ACTIVATION FOLLOWING CUPRIZONE (CPZ)‐FEEDING
Microglia are the resident macrophage population in the CNS, derived from the same mesodermal origin as other peripheral immune system cells, including macrophages and monocytes (Graeber & Streit, 2010). In the CNS, microglia constitute 5%–20% of the total glial cell population, with total numbers ranging from 100 to 200 billion, depending on health status (Ginhoux et al., 2013; Harry & Kraft, 2012; Soulet & Rivest, 2008). In response to injury (e.g., traumatic brain injury) or CPZ‐feeding, microglia were traditionally described as changing from a normal (resting) branched‐shaped to an active “amoeboid” (or rod) sausage‐shaped morphology (Gudi et al., 2014; Praet et al., 2014; Sen et al., 2020a; Taylor et al., 2014; Ziebell et al., 2012). These classical morphological descriptions are now less accepted due to recent discoveries and classifications based on molecular analyses (see below). However, many publications still describe the morphological changes of microglia (and astrocytes) alone as an index of activity since it provides a quick and cost‐effective way to quantify data. Immunohistochemistry for ionized calcium‐binding adapter molecule 1 (Iba1) is the most commonly used marker to detect microglia in the CPZ model. Iba1 is a calcium‐binding actin‐cross‐linking protein specifically expressed in microglia/macrophages, although it does not differentiate between active and resting microglia (Graeber & Streit, 2010; Ohsawa et al., 2004; Sasaki et al., 2001). Other markers to detect microglia, immunohistochemistry for translocator protein (Tspo) and lysosomal‐associated membrane protein 2 marker for microglia and macrophage (Mac‐3) can be used (Gudi et al., 2009; Nack et al., 2019; Nutma et al., 2021; Oveland et al., 2021; Rubino et al., 2018; Skripuletz et al., 2008; Yao et al., 2020). To detect peripheral macrophages, antibodies to CD68 (Krauthausen et al., 2014) or CD45 antigens (Remington et al., 2007) can be used. These markers are given in Table 1.
TABLE 1.
Summary of important antibody markers/genes commonly used in microglia and astrocytes in CPZ studies
| Categories | Gene ID (marker) | Cells/expression | Functions/activities | References |
|---|---|---|---|---|
| Commonly used markers |
Iba1 |
General microglia/ macrophage |
Iba1 is a calcium‐binding cytoskeleton protein, up‐regulated in microglia/macrophages following CNS injury. | (Clarner et al., 2015; Graeber & Streit, 2010; Sen et al., 2020a) |
| Tspo |
Microglia/ macrophage |
Translocator protein (Tspo) is expressed in the mitochondrial outer membrane of microglia. Its expression is seen in both pro‐inflammatory and homeostatic microglia. Loss of Tspo (Tspo−/− mice) inhibits microglial activation. | (Nack et al., 2019; Nutma et al., 2021; Yao et al., 2020) | |
| Mac‐3 |
Microglia/ macrophage |
Activation marker of microglia/macrophages. | (Gudi et al., 2009; Rubino et al., 2018; Skripuletz et al., 2008) | |
| CD68/ED1 |
Microglia/ macrophage |
Commonly used as a pan marker for macrophages and other mononuclear phagocytes like microglia, osteoclasts and monocytes following activation/injury. | (Barros et al., 2013; Chistiakov et al., 2017; Krauthausen et al., 2014) | |
| Gfap | All astrocytes | Glial fibrillary acidic protein (Gfap) and vimentin (Vim) are intermediate filament proteins associated with the structural integrity of all astrocytes. While Gfap is expressed in all astrocytes (activated and non‐activated), Vim is expressed in reactive astrocytes. Up‐regulation of Gfap is used as an early indicator of CNS injury. | (Escartin et al., 2021; Fuller et al., 2007; Hibbits et al., 2012; Sen et al., 2019a; Sen et al., 2020a) | |
| Vim | Activated astrocytes | |||
| CD45 |
Leukocytes/ macrophage |
CD45 is a transmembrane protein tyrosine phosphatase. It is found in most hematopoietic cells. The expression of CD45 is used as a pan leukocyte marker. CD45 is also associated with hematopoietic cell activation and differentiation. | (Altin & Sloan, 1997; Ngo et al., 2007; Remington et al., 2007) | |
| Microglial polarization | CD86 and iNOS |
M1 microglia/ macrophage |
Expressed by pro‐inflammatory microglia/macrophage phenotypes. CD86 leads to Il‐2 production and regulates immune cell proliferation. Inducible nitric oxide synthase (iNOS) is associated with inflammatory responses. | (Aryanpour et al., 2021; Collmann et al., 2019; Jurga et al., 2020; Sonar & Lal, 2019; Zhou et al., 2017) |
| Arg1 and CD206 |
M2 microglia/ macrophage |
Used in assessing microglia/macrophage phenotypes and functions. Arg1+ microglia reduce Aβ plaque deposition. CD206 is a mannose receptor and is associated with various functions including phagocytosis and pinocytosis. | (Aryanpour et al., 2021; Cherry et al., 2015; Jablonski et al., 2015; Régnier‐Vigouroux, 2003) | |
| Astroglial polarization | C3 | A1 astrocytes | Expressed in reactive astrocytes and used to detect beneficial (A2) and detrimental (A1) astrocytes. This has not been tested in the CPZ model. | (Clarke et al., 2018; Li et al., 2020; Liddelow et al., 2017) |
| S100a10 and Emp1 | A2 astrocytes |
Using recent cutting‐edge techniques such as single‐cell transcriptomics, proteomics, and fluorescence‐activated cell sorting, microglia and astrocytes have been grouped into different structural and functional categories (Table 2). For example, during lipopolysaccharide (LPS)‐induced inflammation in C57Bl/6N mice, distinct clusters of microglia were identified as associated with homeostasis (e.g., Olfml3 and Tmem119), phagocytosis (Tyrobp and Trem2) and anti‐inflammatory genes (e.g., Mrc1 and Arg1), and these clusters were significantly decreased in LPS‐injected mice, while pro‐inflammatory genes (e.g., Il1b and Ccl2) were markedly increased. Microglial homeostatic genes (e.g., Tmem119 and Siglech) were down‐regulated, whereas pro‐inflammatory genes (e.g., Ccl2 and Nfkbia) were up‐regulated at the single‐cell level (Sousa et al., 2018). Similarly, using single‐cell RNA sequencing, a distinct microglial population (using C57/Bl and HIV gp120 transgenic mouse models) in the cortex and the spinal cord was found (Zheng et al., 2021). Notably, these microglia showed differential signatures based on the expression of genes involved in homeostasis (e.g., Cx3cr1 and Tmem119), regulation of immune responses (e.g., Il1a and Ccl4), and cytokine and chemokine genes (e.g., Il1b and Ccl2; Zheng et al., 2021). Another recent study by Hammond et al. (2019) revealed at least nine transcriptionally distinct microglial populations (in C57Bl/6J mice) that express unique sets of genes. Similar to the differential morphological and functional phenotypes of microglia (Hammond et al., 2019; Sousa et al., 2018; Taylor et al., 2014; Zheng et al., 2021; Ziebell et al., 2012), transcriptomic and proteomic studies revealed distinct forms, and different mRNA and protein changes involved in neurodevelopment and neurodegeneration in response to injury or aging (Ajami et al., 2018; Flowers et al., 2017; Grabert et al., 2016; Mrdjen et al., 2018). Additionally, using the CPZ model, Masuda et al. (2019) showed the differential expression of microglial genes (e.g., Apoe, Axl and Tmem119) during de‐ and remyelination, suggesting the heterogeneity of microglial populations following CPZ‐feeding. However, the way in which the products of these genes regulate phagocytosis and myelination remains untested in the CPZ model.
TABLE 2.
Summary of the heterogeneity of microglial and astrocytic structure/function using advanced technologies
| Mouse age and line used | Model system | Techniques | CNS areas and cells analyzed | Key results | References |
|---|---|---|---|---|---|
| 4‐, 12‐ and 22‐month old C57Bl/6J and Csf1r‐EGFP mice | LPS model | RNA‐seq (genome‐wide analysis) and computational analysis | CNS (e.g., cerebellum, hippocampus) microglia |
|
(Grabert et al., 2016) |
| 3–5‐ and 20‐24‐month old C57Bl/6N mice | — | Proteomics and computational analysis |
Brain Microglia |
|
(Flowers et al., 2017) |
| 4‐14‐week old C57Bl/6J, R6/2 and mSOD mice | EAE, Huntington's Disease and Amyotrophic Lateral Sclerosis models |
Mass cytometry and computational analysis |
Brain, spinal cord and blood myeloid cells |
|
(Ajami et al., 2018) |
| 3‐4‐month old C57Bl/6N mice | LPS model | RNA‐seq and computational analysis | Brain (myelin) microglia |
|
(Sousa et al., 2018) |
| Embryonic (E14.5), postnatal (4/5), 30‐, 100‐ and 540‐day old C57Bl/6J mice | Lysolecithin model and MS patients | RNA‐seq and computational analysis |
Brain Microglia |
|
(Hammond et al., 2019) |
| Embryonic (16.5), postnatal (3) and 16‐week old CD‐1 mice |
Facial nerve axotomy and CPZ models Normal and MS brain post‐mortem samples |
RNA‐seq and computational analysis | Brain and spinal cord microglia |
|
(Masuda et al., 2019) |
| 4‐month old APP/PS1 and 8‐week old C57Bl/6 and Cx3cr1CreER Rosa26‐RFP mice | EAE model | Single‐cell mass and fluorescence cytometry and computational analysis | Brain (hippocampus) leukocytes and microglia |
|
(Mrdjen et al., 2018) |
| 2‐, 4‐ and 8‐month old C57/Bl and HIVgp120 mice | — | RNA‐seq and computational analysis |
Brain (cortex) and spinal cord Microglia |
|
(Zheng et al., 2021) |
| 8‐ to 12‐week old RiboTag and C57Bl/6 mice | EAE model and MS patients | RNA‐seq and computational analysis |
CNS (e.g., spinal cord, cerebellum, optic chiasm) astrocytes |
|
(Itoh et al., 2018) |
| 8‐week old C57Bl/6J mice | — | RNA seq and computational analysis | Brain (cortex and hippocampus) astrocytes |
|
(Batiuk et al., 2020) |
| 2‐ and 8‐week old Swiss Webster, Aldh1l1‐GFP, Emx1cre, Satb2flox mice | — | RNA‐seq and computational analysis | Brain (cerebral cortex) astrocytes |
|
(Bayraktar et al., 2020) |
| 8‐ to 12‐week old C57Bl/6, RibotagGfap, TdTomatoGfap mice | EAE model and MS patients | RNA‐seq and computational analysis |
Spinal cord and human brain (cerebellum) astrocytes |
|
(Wheeler et al., 2020) |
| 4‐5‐week old Aldh1l1eGFP mice | LPS model | RNA‐seq and computational analysis | Brain astrocytes |
|
(Hasel et al., 2021) |
Abbreviations: —, not found or investigated or not relevant; CNS, central nervous system; CPZ, cuprizone; EAE, experimental autoimmune encephalomyelitis; LPS, lipopolysaccharide; MS, multiple sclerosis; RNA‐seq, RNA‐sequencing.
Based on the M1 (detrimental) and M2 (beneficial) polarization states of macrophages (Orihuela et al., 2016), in response to CNS injury, a similar classification scheme for microglia polarization (M1 and M2) has been proposed. Microglia polarize in two ways in response to injury or infection (Aguilera et al., 2018; Gudi et al., 2014; Praet et al., 2014; Wang et al., 2019; Zheng & Wong, 2019). The M1 phenotype refers to the classical activation (induced by pro‐inflammatory cytokines such as interferon [Ifn]‐γ), which mediates pro‐inflammatory responses. M1 microglia release pro‐inflammatory mediators including tumor necrosis factor‐α (Tnf‐α), interleukin (Il)‐1β, Il‐6, nitric oxide (NO), and Ifn‐γ which degenerate tissue and neurons (Lassmann & van Horssen, 2016; Rossi et al., 2014; Zheng & Wong, 2019). M1 microglia can be detected and quantified by measuring the expression of cell surface markers such as CD11b, CD16, CD32, CD86, and iNOS (Zheng & Wong, 2019). In contrast, M2‐activated microglia/macrophages are responsible for the resolution of inflammation and repair of injured tissue by phagocytosis and secretion of anti‐inflammatory mediators (e.g., Il‐10 and Il‐13), thus restoring homeostasis (Aguilera et al., 2018; Gudi et al., 2014; Praet et al., 2014; Wang et al., 2019; Zheng & Wong, 2019). M2 microglia can be detected and quantified using the expression of cell surface markers such as CD206, CD163, and Arg1 (Zheng & Wong, 2019). Despite the continued use of microglial M1 and M2 phenotype designations in the literature (Tang & Le, 2016; Zheng & Wong, 2019), this classification is still controversial because it is deemed an over‐simplification of the heterogeneous complexity of microglial structures and functions, as they can in fact exist in more than two distinct polarized states (Bachiller et al., 2018; Martinez & Gordon, 2014; Mosser & Edwards, 2008; Ransohoff, 2016). Considering the recent discoveries of the heterogeneous functions (Table 2) of microglia both in resting and activated states (Ajami et al., 2018; Flowers et al., 2017; Grabert et al., 2016; Mrdjen et al., 2018), further investigation is required to characterize the microglial phenotypes in the CPZ model.
3. ASTROCYTIC ACTIVATION FOLLOWING CPZ‐FEEDING
Routinely, astrocytes are morphologically characterized by non‐overlapping star‐shaped extensions from the cell body and comprise ~30% of the glial cells in the CNS (Liddelow & Barres, 2017; Ponath et al., 2018). Astrocytes can be identified immunohistochemically by the expression of intermediate filament proteins such as glial fibrillary acidic protein (Gfap), or vimentin (Vim; Lyck et al., 2008; Sofroniew & Vinters, 2010). In the CPZ model, the activation of astrocytes (e.g., when 0.2% CPZ is fed for 4–5 weeks) is often detected using increased expression of Gfap as a marker (Hibbits et al., 2012; Sen et al., 2019a; Sen et al., 2020a; Skripuletz et al., 2013). Likewise, Vim expression is also increased in reactive astrocytes during CPZ‐feeding to a similar level to that of Gfap (Hibbits et al., 2012). These markers are summarized in Table 1.
Recent investigations of astrocytes using single‐cell resolution methodologies (given in Table 2) reveals a differential distribution of astrocytes each with differing functions (Batiuk et al., 2020; Bayraktar et al., 2020; Hasel et al., 2021; Itoh et al., 2018; Wheeler et al., 2020). For example, Batiuk et al. (2020) showed that astrocytes can be categorized into five transcriptomically distinct subtypes (AST) in C57Bl/6J mouse cortex and hippocampus: AST1 and AST4 are predominantly found in the hippocampus, AST2 mainly in the cortex, whereas AST3 and AST5 are uniformly distributed between hippocampus and cortex. Gene enrichment (using DAVID bioinformatics; https://david.ncifcrf.gov/) analysis revealed the differential functionality of these astrocyte populations. AST3 are more consistent with mature astrocytic functions (e.g., high expression of Gfap). AST4 is associated with neurogenesis (mitosis and cell cycle control). AST5 constitute an overlapping group of astrocytes whose functions may be intermediate between neurogenesis and mature astrocytes (Batiuk et al., 2020). Similarly, following LPS‐induced acute inflammation, different clusters of astrocytes are found, at the single‐cell transcriptome level (Hasel et al., 2021). For example, while one cluster is associated with the elevated expression of complement component 3 (C3) and the C3‐like gene encoding Cd109, another cluster is enriched with Igtp, Tap1 and Stat1 genes (Hasel et al., 2021). These studies are indicative of the heterogenous characteristics of astrocytes. How these heterogeneities in gene expression and biochemical processes temporally align with morphological changes remains unclear. Moreover, while these technological advancements reveal the multifactorial characteristics of microglia and astrocytes, it is not clear how many of these genes are translated to functional proteins, nor specifically which proteoforms (Sen et al., 2021) are involved, since no parallel studies were found using transcriptomic or any (top‐down) proteomic analyses. Do microglia and astrocytes show differential transcriptional/proteome profiles in different animal models of MS? Is there any animal model that shows greater similarity to MS than any other model at either single‐cell transcriptome or proteome level? This kind of multi‐animal model analysis may provide the optimal method/model for examining the roles of microglia and astrocytes in MS.
Upon CPZ‐feeding, astrocytes become activated and hypertrophic (Gudi et al., 2014; Hibbits et al., 2012; Praet et al., 2014; Sen et al., 2020a; Sen et al., 2019b). Investigations using the CPZ model reveal that reactive astrocytes can be beneficial when they are involved in remyelination and myelin debris clearance whereas astrocytes are deemed harmful when associated with demyelination (Brück et al., 2012; Fulmer et al., 2014; Gudi et al., 2014; Houben et al., 2020; Mason et al., 2000). Studies from the Barres Lab (Clarke et al., 2018; Liddelow et al., 2017; Liddelow & Barres, 2017; Zamanian et al., 2012) showed that CNS neuroinflammation or ischemia caused astrocytes to polarize into two “reactive” types: harmful (A1) and helpful (A2). Single‐cell resolution analysis showed that A1 astrocytes up‐regulate several genes (e.g., complement component 3, C3) linked to synapse and neuronal degeneration. The prevalence of A1 reactive astrocytes is observed in different brain regions of neuroinflammatory and neurodegenerative diseases including MS, Alzheimer's disease, and Parkinson's disease (Liddelow et al., 2017). In contrast to A1 reactive astrocytes, A2 astrocytes up‐regulate many neurotrophic factors, aiding the repair of damaged synapses and neurons (Clarke et al., 2018; Liddelow et al., 2017; Liddelow & Barres, 2017; Zamanian et al., 2012). Notably, crosstalk between astrocytes and microglia has been documented. Activation of microglia leads to the activation of astrocytes, indicating that astrocytic polarization depends upon the microglial state (i.e., activated or resting; Clarke et al., 2018; Joshi et al., 2019; Liddelow et al., 2017; Rothhammer et al., 2018). However, it is not clear whether reactive astrocytes and associated mediators' do impact microglial polarization. LPS‐activated microglial secretion of Il‐1α, Tnf, and C1q cytokines induces an A1 phenotype, whereas inhibition of microglial inflammation (using neutralizing antibodies to Il‐1α, Tnf, and C1q) blocks A1 phenotype development (Liddelow et al., 2017), and blocking of microglia‐mediated reactive A1 conversion prevents neurodegeneration (Yun et al., 2018). Likewise, another study revealed the differential expression of genes associated with A1 (e.g., C3) and A2 (e.g., Emp1) reactive astrocytes (Clarke et al., 2018). Following these studies (Clarke et al., 2018; Liddelow et al., 2017; Liddelow & Barres, 2017; Zamanian et al., 2012), other studies (e.g., rat models of pain and human Creutzfeldt‐Jakob disease) also observed the existence and functionality of different types of reactive astrocytes (Guttenplan et al., 2020; Li et al., 2019; Li et al., 2020; Miller, 2018; Ugalde et al., 2020). For example, a rat model of surgical pain showed an elevation of the A1 astrocytic marker C3 and a reduction of the A2 astrocytic marker S100a10 using Western blotting (Li et al., 2020). The upregulation of these reactive astrocytic markers was also observed in a LPS‐induced inflammatory mouse model at the single‐cell level (Hasel et al., 2021). However, this classification into A1/A2 groups may be an over‐simplification (akin to that for M1/M2 microglia/macrophage phenotypes), as it has been argued that other astrocytic states may exists (e.g., An, A[n + 1]), although further studies are required to define these additional phenotypes (Liddelow & Barres, 2017). Moreover, a recent study also warned against over‐simplification of the complexity of astrocytic functions, recommending that both morphological and functional readouts be considered (Escartin et al., 2021). Thus, the interpretation of “reactive astrocytes”, “activated astrocytes” or “homogenous astrocytes” should likely be used considering the available experimental evidence and to align with suggested guidelines (Escartin et al., 2021). That said, no studies have investigated the differential polarization states of astrocytes in the CPZ model. However, to follow the consistency of described microglial polarization (M1 and M2), this review designates astrocytes as A1 or A2 to describe the detrimental and beneficial aspects of reactive astrocytes, respectively, in the CPZ model.
4. USING THE CPZ MODEL TO INVESTIGATE MICROGLIAL AND ASTROCYTIC FUNCTIONS
Animal models are the backbone of MS research, not only for long‐standing efforts to define the pathoetiology of the disease, but also for the development of therapeutics, despite the fact that no single animal model fully reflects the complex heterogeneity of MS (Ransohoff, 2012; Sen et al., 2021; Sen et al., 2020b; Stys et al., 2012). Of the commonly used animal models such as experimental autoimmune encephalomyelitis (EAE), CPZ, ethidium bromide, lysolecithin, LPS, diphtheria toxin (DPT), and Theiler's murine encephalomyelitis virus (Denic et al., 2011; Procaccini et al., 2015; Ransohoff, 2012; Sen et al., 2019b; Sousa et al., 2018; Traka et al., 2010), CPZ is mostly used to study de‐ and remyelination. Due to the progressive deterioration of motor function and limited recovery in EAE, and because of the technically demanding stereotactic injection and localized demyelination (at the site of injection) using ethidium bromide or lysolecithin, these models are chosen far less frequently to study remyelination (Hooijmans et al., 2019; Ransohoff, 2012). Moreover, CPZ‐fed C57Bl/6 mice show subtle cognitive and locomotor deficits, but these animals do not undergo severe motor deficits such as paralysis, due to limited pathology of the spinal cord, and so is quite similar to (early) human MS motor symptomology. Therefore, studies of OPC proliferation and recovery from deficits during demyelination (with CPZ‐feeding) and remyelination (without CPZ‐feeding) is possible (Gudi et al., 2014; Praet et al., 2014; Sen et al., 2020a; Sen et al., 2020b; Sen et al., 2019b).
In order to identify the early events of remyelination during which oligodendrocyte function becomes compromised by either inadequate myelin debris clearance or the production of toxic mediators, the CPZ model has proven useful. In this model, rodents are fed with CPZ, which causes metabolic disturbances in the mitochondria and endoplasmic reticulum of oligodendrocytes (Praet et al., 2014; Sen et al., 2019a; Sen et al., 2019b). Oligodendrocytes are reliant upon mitochondria for energy; CPZ interrupts mitochondria‐mediated energy production, resulting in their degeneration (termed oligodendrocytosis), leading to subsequent neuronal demyelination and glial activation in the CNS structures such as the corpus callosum, hippocampus, and cerebellar nuclei (Almuslehi et al., 2020; Goldberg et al., 2015; Sen et al., 2019a; Sen et al., 2020a; Sen et al., 2019b).
Activated microglia and astrocytes secrete both beneficial/protective (e.g., insulin‐like growth factor; Igf‐1) and detrimental/toxic cytokines (Il‐1β/6, and Ifn‐γ) that affect oligodendrocytes and thus myelination status (Figure 1). Increased production of these pro‐inflammatory mediators and prolonged glial activation enhances oligodendrocyte degeneration (Gudi et al., 2014; Olah et al., 2012; Praet et al., 2014). Moreover, within 2–3 weeks of feeding 0.2% CPZ, activation of microglia (evidenced by increased numbers and morphological changes) and astrocytes (as evidenced by morphological changes) occurs in the corpus callosum of C57Bl/6 mice, before the appearance of obvious demyelination (Hiremath et al., 1998), indicating a highly sensitive response. However, the direct effects of CPZ on microglia or astrocytes remain unclear. Analysis of primary glial cell cultures from neonatal rat brains revealed that CPZ selectively causes the degeneration of mature oligodendrocytes, whereas astrocytes and microglia remain unaffected (Benardais et al., 2013). However, no study has specifically investigated the effect of CPZ on mature microglia or astrocytes in vitro. In addition, the blood–brain barrier (BBB) apparently remains intact in the CPZ model, which is evident in both histological and proteomic investigations (Almuslehi et al., 2020; Sen et al., 2021; Tejedor et al., 2017). This is advantageous since it enables investigation of the roles of brain‐resident microglia in myelination without the interference of peripheral macrophages. However, the intact BBB limits study of the role of peripheral lymphoid and myeloid cells (and associated cytokines and chemokines). Some researchers interpret this as a limitation of the CPZ model, since BBB disruption and a prevalence of adaptive immune cells is found in the CNS of MS patients, particularly at well‐defined clinical stages of the disease (Hemmer et al., 2015; Sen et al., 2021). This, of course, also raises the issue of which studies are focussing on the earliest (pre‐clinical) models of disease versus the currently irreversible clinical condition(s). Nonetheless, when the BBB of CPZ‐fed mice is breached using pertussis toxin, and/or the immune system is primed using complete Freund's adjuvant, an infiltration by the adaptive immune system and subsequent immune‐mediated demyelination is observed (Almuslehi et al., 2020; Caprariello et al., 2018). However, the involvement of myeloid cells, including macrophages, was not investigated in these studies. Finally, the cessation of CPZ‐feeding leads to spontaneous remyelination via OPC proliferation and resolution of glial activation (reviewed in Gudi et al., 2014; Sen et al., 2019b). These features make the CPZ model an ideal tool for investigating microglia and astrocyte‐mediated myelin debris clearance and remyelination. This model has been in use since 1966 (Carlton, 1966), with over 1000 papers cited in PubMed (as of November 2021). Although the participation of microglia in myelin debris phagocytosis and remyelination was first reported in 2000 (Mason et al., 2000), no review has discussed the roles of microglia and astrocyte‐mediated phagocytosis and remyelination in the CPZ model. This is the primary focus of this review, although relevant references from other animal models of MS (e.g., EAE, LPS, ethidium bromide) are also included. This review will thus be of primary interest to the neuroscience community studying the impact of glial activation in demyelination and remyelination. Additionally, since activated microglia and astrocytes are observed in almost every neurodegenerative disease including MS, Alzheimer's, and Parkinson's diseases, this review will also be of broader interest.
FIGURE 1.
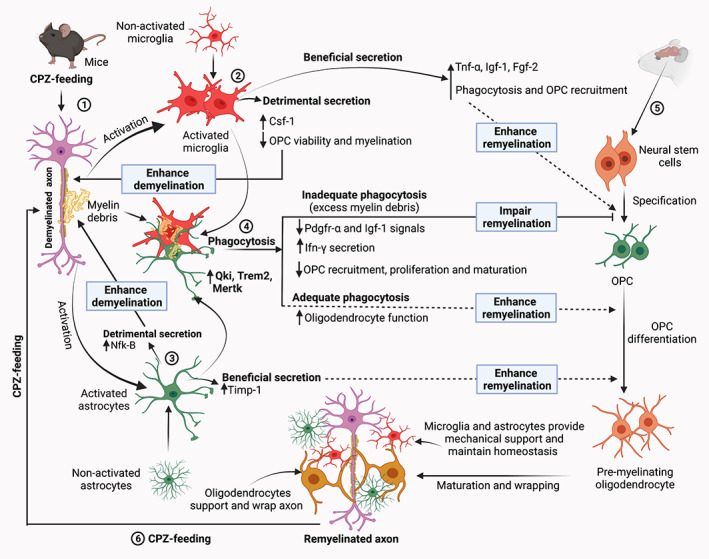
Schematic of the microglia and astrocyte‐mediated phagocytosis and myelination in the CPZ model. Step 1: CPZ‐feeding to young (7–8‐week old) rodents (mainly C57Bl/6 mice) leads to the oligodendrocytes degeneration, demyelination and generation of myelin debris and direct and indirect microglial and astrocytic activation in the CNS structures (Gudi et al., 2014; Praet et al., 2014; Sen et al., 2020a; Sen et al., 2019b). Step 2: Local proliferation and astrocyte‐mediated microglia recruit at the site of demyelination (through astrocyte regulating chemokine Cxcl10 ‐ Ifn‐γ‐induced protein signaling, not shown this figure) (Remington et al., 2007; Skripuletz et al., 2013). Activated microglia secrete substances that are both beneficial (e.g., Igf‐1) which accelerate remyelination by increasing myelin debris clearance and OPC recruitment, and detrimental (e.g., Csf‐1) which enhance demyelination (see Table 4). Step 3: Likewise, astrocytes release substances that contribute to both remyelination and demyelination (see Table 4). Step 4: One of the prerequisites of effective remyelination is the phagocytosis of myelin debris (Step 1). Recent evidence shows that this process is facilitated by the upregulation of various transcripts such as Qki, Trem2 and Mertk, expressed by microglia and astrocytes (Cignarella et al., 2020; Ren et al., 2021; Shen et al., 2021). In addition, increased expression of other microglial genes (e.g., Lrp and Calr) facilitates phagocytosis and the removal of myelin debris (Olah et al., 2012). Efficient phagocytosis of myelin debris promotes proliferation of OPCs, as well as the migration of OPC to the site of demyelination that then results in remyelination of denuded axons. However, if phagocytosis is hampered, excess myelin debris leads to the reduction of Pdgfr‐α and Igf‐1 signals and stimulates Ifn‐γ secretion. Both of these impair OPC recruitment, proliferation, and maturation, resulting in impaired remyelination (Lampron et al., 2015; Robinson & Miller, 1999; Shen et al., 2021; Skripuletz et al., 2013). Step 5: Remyelination starts with the specification of neural stem cells to OPCs and maturation of OPCs to the mature oligodendrocytes. Mature oligodendrocytes wrap and support axons (Nave & Werner, 2014; Snaidero & Simons, 2014). However, due to the detrimental secretions from microglia and astrocytes following CPZ‐feeding, inadequate phagocytosis is observed. Step 6: The myelinated axons can be demyelinated again if the rodents are again fed with CPZ. Consequently, the dynamics of de‐ and remyelination mechanisms and the role of glial activation can be investigated using CPZ‐model. ↑, increase; ↓, decrease; Csf‐1, colony‐stimulating factor‐1; CPZ, cuprizone; Fgf‐2, fibroblast growth factor‐2; Ifn‐γ, interferon‐γ; Igf‐1, insulin‐like growth factor‐1; Mertk, Mer proto‐oncogene tyrosine kinase; NfK‐B, nuclear factor kappa‐B; OPC, oligodendrocyte progenitor cell; Pdgfr‐α, platelet‐derived growth factor receptors‐α; Qki, Quaking protein; Timp‐1, tissue inhibitor of metalloproteinases‐1; Trem2, triggering receptor expressed on myeloid cells‐2; Tnf‐α, tumor necrosis factor‐α
5. MICROGLIA‐MEDIATED MYELIN DEBRIS CLEARANCE
Phagocytosis of myelin debris from damage at the lesion site(s) is crucial to the process of remyelination and reorganization of neuronal circuits. Inadequate or delayed debris clearance hampers remyelination, thus hampering the regenerative process (Neumann et al., 2009). For example, studies using mice deficient in the fractalkine receptor (Cx3cr1) showed that the clearance of myelin debris by microglia was reduced, or slowed, in CPZ‐fed mice, concomitant with slower remyelination (Lampron et al., 2015). Moreover, the disruption of myelin clearance by microglia resulted in disorganization (e.g., separation of myelin layers, hypermyelination, vacuolization of axons and large spheroid‐like structures) of the surviving myelin in the corpus callosum following CPZ‐feeding in Cx3cr1−/− mice (Lampron et al., 2015). In addition, the presence of myelin debris in microglia of wild‐type (Control), but not in Cx3cr1−/− mice, indicated the presence of an active phagocytic process in normal microglia. Furthermore, lower levels of Pdgfr‐α and Olig2+ expression (markers of OPCs) were found in the corpus callosum of Cx3cr1−/− mice. Overall, mechanistically, this study supported the concept that insufficient debris clearance is associated with low Pdgfr‐α and Igf‐1 signals, leading to impaired OPC recruitment and proliferation, which compromised the process of remyelination (Lampron et al., 2015). Interestingly, studies from another lab (Baer et al., 2009) showed that remyelination is regulated by the Fyn‐RhoA and protein kinase‐C signaling pathways. Inhibition of these pathways resulted in the induction of OPC differentiation in the presence of myelin debris (Baer et al., 2009). In the EAE model, activated microglia secrete Ifn‐β in the spinal cord at the peak of dysfunction (Kocur et al., 2015). Ifn‐β‐expressing microglia showed an enhanced capacity to phagocytose myelin in vitro and increased the expression of phagocytosis‐associated genes, which resulted in a faster removal of myelin debris (Kocur et al., 2015). Nonetheless, considering the Ifn‐β expression, the implication is that inadequate expression of Ifn‐β led to the slower removal of debris (Kocur et al., 2015). Similar to the inefficient clearance of myelin debris and inadequate remyelination in Cx3cr1−/− or Ifn‐β over‐expressing mice (Kocur et al., 2015; Lampron et al., 2015), impaired remyelination was indicated in another study by a ~60% reduction in the number of immature and mature oligodendrocyte markers (Plemel et al., 2013). Myelin debris makes contact with OPCs to inhibit their maturation (Robinson & Miller, 1999). Likewise, aggregation of myelin debris stimulates Ifn‐γ secretion, which inhibits OPC maturation resulting in the impairment of myelination (Shen et al., 2021). The inhibitory role of myelin debris load on OPC differentiation and remyelination at sites of demyelination was seen in another study using ethidium bromide administration into the cerebellar peduncle of adult‐female Sprague Dawley rats, to cause oligodendrocyte degeneration. This resulted in reduction of the oligodendrocyte differentiation transcription factor (Nkx2.2) during the pre‐myelinating stage (i.e., when oligodendrocytes are in close contact with myelin but unable to myelinate), resulting in reduced remyelination (Kotter et al., 2006). Microglia‐mediated phagocytosis of myelin debris is also supported by another study of genome‐wide gene expression analysis of microglia from the corpus callosum of CPZ‐fed C57Bl/6 mice during demyelination and remyelination (Olah et al., 2012). This analysis revealed that a subset of genes (e.g., Lrp‐1 and Calr), are involved in enhanced phagocytosis, as well as the removal of myelin debris (Olah et al., 2012). While it is clear that microglia are involved in myelin debris removal, the mechanism(s) underlying microglia‐driven phagocytosis remain unclear.
A recent study by Ren et al. (2021) has provided a new piece of evidence, showing that microglial phagocytosis is regulated by expression of the Quaking signaling protein (Qki). Quaking is an RNA‐binding signaling protein that controls alternative splicing in vascular cell differentiation (Caines et al., 2019). The study by Ren et al. (2021) rigorously analyzed the involvement of Qki in myelin debris clearance using multiple experimental approaches, including immunohistochemistry, Western blotting, transcriptomics, and transmission electron microscopy, demonstrating that deletion of the Qki gene (using an inducible conditional knockout; iCKO) preferentially impaired microglia‐mediated myelin debris clearance by affecting their phagocytic activity. Using the CPZ model, Ren et al. (2021) first observed the activation of microglia by immunofluorescence staining of enhanced yellow fluorescent protein (Eyfp) expression in mice bearing Cx3cr1CreER‐Eyfp allele (Eyfp labeled all Iba1+ microglia) in the corpus callosum. Demyelination of the same area was quantified by assessing myelin basic protein. Whether this microglial activation up‐regulated phagocytic activity in the CPZ‐fed mice was then investigated. It was found that microglial cells showed a ~6‐fold increase of Qki protein after 4 weeks of CPZ‐feeding compared to Qki expression in microglia of naïve mice (Ren et al., 2021). Importantly, there was a greater than 10‐fold increase of phago‐lysosomal components (e.g., lysosomal‐associated membrane protein‐1 and 2; assessed by Western blotting) from isolated microglia of CPZ‐fed (using Qki inducible conditional knockout mice, Qki‐iCKO) compared to control mice. The lysosomal activity of microglia (a phagocytic process) was confirmed using LysoTracker™, revealing a significant increase of lysosomal activity and suggesting an activation of phagocytic machinery in microglia following CPZ‐feeding (Ren et al., 2021). Notably, transcriptomic profiling of microglia isolated from the corpus callosum of CPZ‐fed Qk‐iCKO mice revealed a significant down‐regulation of signaling pathways other than Qki, such as peroxisome proliferator‐activated receptor (Ppar), related to phagosome formation, suggesting that Qki may selectively modulate the phagocytic function of microglia in response to (CPZ‐induced) demyelination (Ren et al., 2021). Using CNS samples from human MS patients, Ren et al. (2021) also observed a ~3‐fold increase of Qki protein in microglia from demyelinating white matter lesions. Notably, deletion of the Qki gene impaired microglia‐mediated myelin debris clearance and was associated with down‐regulation of phagosome‐related genes (e.g., CD36 and C1ra by transcriptomic analysis). Isolated microglia from Qki‐deficient mice showed a ~80% reduction of phagocytic activity in vitro. Apparently, due to impaired myelin debris clearance, less remyelination was observed (~45% remyelination in Qki deficit mice vs. ~76% in controls, Ren et al., 2021), suggesting that this was the main reason for the inhibition of OPC differentiation into mature oligodendrocytes. In addition to the involvement of Qki in the CPZ model, using a top‐down proteomic approach, an increased abundance of Qki was observed in the spinal cord of EAE mice, the primary site of demyelination in this model (Farias et al., 2012), indicating the involvement of Qki in EAE model. How Qki upregulation regulates phagocytosis and myelination in EAE or other animal models of MS (as shown in CPZ model using Qk‐iCKO mice) remains untested.
Importantly, myelin structure, and how glia support axons, changes with aging (see below). The study from Ren et al. (2021) used young mice (8‐week old) to induce demyelination; but whether Qki plays an equal role in myelin debris clearance in aged (e.g., ~2‐year old) CPZ‐fed mice remains untested. Similarly, Ren et al. (2021) fed CPZ for 6 weeks (to induce acute demyelination), but whether the up‐regulation of Qki and its functional effects persist during progressive and prolonged demyelination (e.g., ~12 weeks is considered chronic demyelination, [reviewed in Sen et al., 2019b]) remains unknown. Moreover, the study concentrated on investigation of Qki function in microglia only in the corpus callosum; whether a similar microglial response is seen in other parts of the CNS in the CPZ model also remains unreported. Additionally, this study did not concentrate on any potential behavioral changes associated with knockout of Qki in these mice.
Is only Qki associated with phagocytosis and myelination in CPZ model? Other CPZ studies indicated that the innate immune lipid‐sensing and scavenging receptor (triggering receptor expressed on myeloid cells‐2, Trem2), regulates the microglia‐mediated myelin debris clearance (Cignarella et al., 2020; Dong et al., 2021). Transgenic over‐expression of Trem2(Trem2+/+ mice) in microglia resulted in a significant amount of myelin debris clearance, whereas Trem2 deficiency (Trem2−/− mice) led to inadequate phagocytosis (Cignarella et al., 2020). These observations were further supported by experiments in which a Trem2‐agonist (AL002a antibody) treatment enhanced the amount of myelin debris clearance by microglia in the CPZ‐model in vitro, and bone marrow‐derived macrophages in BWZ cells in vivo (Cignarella et al., 2020). Antibody treatment also enhanced OPC density and maturation at the site of demyelination, suggesting an accelerated remyelination in the corpus callosum in CPZ‐fed mice (Cignarella et al., 2020). However, it is not clear how Trem2 contributes to the pathological function of microglia. In CPZ‐fed mice, microglia lacking Trem2 showed smaller cell bodies and reduced ramifications compared to wild‐type mice, suggesting dysfunctional activation of microglia (Poliani et al., 2015). Likewise, using CPZ‐feeding, Cantoni et al. (2015) showed that Trem2−/− mice had dysregulated clearance of myelin debris, in addition to reduced microglial proliferation and recruitment at the site of demyelination, and reduced expression of activation markers (e.g., major histocompatibility complex‐II and iNOS). Notably, ultrastructural and gene expression analyses revealed a dysregulation in myelin degradation and phagocytosis in the microglia of Trem2−/− mice following CPZ‐feeding (Cantoni et al., 2015). Another study of 0.2% CPZ‐fed mice showed that corpus callosum and cortex microglia up‐regulate Trem2 during both demyelination and remyelination periods (Voss et al., 2012), further emphasizing the involvement of Trem2 in microglia‐mediated phagocytosis and myelination in CPZ model.
Increased microglial expression of Trem2 to facilitate debris clearance is also supported by another recent experiment showing the phagocytosis of oxidized phosphatidylcholines (oxidized myelin debris which causes cell death and inflammation) in Trem2+/+ mice, whereas Trem2 −/− mice had reduced clearance in the spinal cord (Dong et al., 2021). Further evidence for the role of microglia in debris clearance is shown in a study analyzing inflammatory chemokine levels in the corpus callosum and cerebral cortex, identifying a reduction of key microglial‐attracting chemokines (Cxcl10, Cxcl1, and Ccl4) in Gfap‐Il6 over‐expressing mice (Gfap‐Il6) compared to CPZ‐fed wild‐type littermates (Petković et al., 2016). Reduced microglial accumulation was associated with the slower removal of degraded myelin in CPZ‐fed Gfap‐Il6 mice compared with wild‐type mice, resulting in impaired early OPC differentiation (assessed by the quantification of apoptotic cells using hematoxylin–eosin and active caspase‐3 staining). Further investigation showed that levels of microglial Trem2, as well as the phago‐lysosomal protein (CD68), were lower in CPZ‐fed Gfap‐Il6 transgenic mice compared with wild‐type mice (Petković et al., 2016). Similar to changes of Trem2 expression in the brains of CPZ or LPS models, an up‐regulation of the expression of Trem2 in microglia was observed in the spinal cord of EAE C57Bl/6 mice. Trem2 is rarely seen in naïve mouse spinal cord, while blockade of Trem2 (using an anti‐Trem2 antibody) resulted in the exacerbation of EAE symptoms, and greater demyelination and inflammation (Piccio et al., 2007). In accordance with elevated expression of Trem2 in the brain of CPZ and spinal cord of EAE models (Cignarella et al., 2020; Piccio et al., 2007), an increased abundance of Trem2 was found in the cerebrospinal fluid of MS patients (Piccio et al., 2008). The phagocytic activity in CPZ and EAE models is further supported by detection of the phagocytosis‐related protein Dynamin (Hochreiter‐Hufford & Ravichandran, 2013) via proteomic analyses of brain and spinal cord (Hasan et al., 2019; Partridge et al., 2016; Sen et al., 2019a; Werner et al., 2010) following demyelination. This collective evidence thus suggests that multiple transcripts (e.g., Qki and Trem2) are involved in microglia‐mediated myelin debris removal and maintenance of axonal health. These events are summarized in Figure 1.
6. ROLES OF ASTROCYTES IN MYELIN DEBRIS CLEARANCE
Since microglia are the primary cells of phagocytosis, a key question is what happens if microglia are impaired during the early phagocytosis of myelin debris; do astrocytes play a compensatory role? A previous study showed that myelin debris phagocytosis was hampered if astrocyte‐mediated microglial recruitment was compromised in CPZ‐fed mice (Skripuletz et al., 2013). Using a Gfap‐thymidine kinase transgenic mouse line 7.1 (Gfap‐TK) to induce conditional depletion of astrocytes, the role of astrocytes in myelin debris clearance in CPZ‐fed mice was investigated. In this study, CPZ‐feeding to transgenic mice produced a significant reduction of demyelination in the corpus callosum and cerebral cortex compared to the wild‐type mice. Additional experiments by Skripuletz et al. (2013) revealed that astrocytes recruited microglia to the site of demyelination, and if this process is disrupted, microglia‐mediated phagocytosis is delayed. Consequently, OPC proliferation, maturation, and recruitment to the site of demyelination was inhibited, which delayed remyelination (Skripuletz et al., 2013). This indicates that astrocytes participate in myelin debris clearance by recruiting microglia to the site of demyelination, most likely via astrocyte‐microglia crosstalk (Clarke et al., 2018; Matejuk & Ransohoff, 2020). However, while both glial cell types are activated following CPZ‐feeding, no study has yet investigated the temporal sequence (i.e., which glial cells are activated first to initiate the phagocytic process in the CPZ model). Although the involvement of both microglia and astrocytes in demyelination is evident (reviewed in Traiffort et al., 2020), the role of astrocytes in myelin debris clearance is also supported by other work. Transient ablation of Gfap+ astrocytes (using Gfap‐iCP9 transgenic mice) from the spinal cord during the first postnatal week either reduced or delayed the number of mature oligodendrocytes and inhibited myelin formation, followed by local loss of myelin integrity and regional demyelination during adulthood (Tognatta et al., 2020). Another recent study found that astrocytes are involved in debris clearance when microglia are ablated in transgenic mice (Siglechdtr and Irf8−) (Konishi et al., 2020). This suggests that astrocytes are also capable of phagocytosis and can provide a compensatory mechanism if microglia are unable to execute phagocytosis. However, this compensatory phagocytosis by astrocytes has not been studied in the CPZ model. In addition, single cell analysis of astrocytes from the brains of adult C57Bl/6 mice showed that astrocytic gene (e.g., Mertk) expression is linked with phagocytosis (Batiuk et al., 2020). The role of the Mertk gene product in phagocytosis is also supported by another recent study (Shen et al., 2021) which showed that the deletion of the Mertk gene (using Mertk‐KO mice) reduced microglial recruitment and activation following CPZ‐feeding. This reduction of microglial presence leads to the impairment of myelin debris phagocytosis. However, no difference in astrocyte numbers was found, indicating that astrocytes are not associated with phagocytic response in Mertk‐KO mice (Shen et al., 2021). Similar to Shen et al. (2021), Ren et al. (2021) and Cignarella et al. (2020) demonstrated that the roles of Qki and Trem2 in debris clearance are independent of an astrocytic response. This suggests that the sole expression of Qki or Trem2 on microglia can mediate the phagocytic response in CPZ mice, akin to that seen in Cx3cr1−/− mice (Lampron et al., 2015). An additional study showed that CD36 expression regulates microglia‐mediated phagocytosis (using niacin receptor‐lacking Hcar2−/− mice) in the lysolecithin model of demyelination (Rawji et al., 2020b). Specifically, the reduction of CD36 in microglia reduces phagocytosis of myelin debris, whereas over‐expression of CD36 using the niacin receptor (hydroxycarboxylic acid receptor 2/Gpr109a receptor) prevented the deficit in phagocytic activity (Rawji et al., 2020b). However, the role of Gpr109a in microglial function and phagocytosis remains untested in the CPZ model. Interestingly, a study using CPZ‐fed mice indicated that Cxcl10 chemokine (using Cxcl10−/− mice) regulates early microglial activation, chemotaxis, and induction of a pro‐inflammatory (iNOS and Tnf‐α) phenotype but does not play a role in phagocytosis (Clarner et al., 2015). This suggests that activated microglia can be non‐phagocytic despite the presence of myelin debris. The relevant mouse lines and functions are summarized in Table 3. Other relevant mouse lines (e.g., S100B‐Cre) and viral vectors (e.g., AAV5) are available (Eme‐Scolan & Dando, 2020; Yu et al., 2020) but have yet to be implemented using the CPZ model in order to analyze astrocytes (and microglia) from tissue to synaptic level using advanced techniques.
TABLE 3.
Summary of transgenic mouse lines used in CPZ studies investigating glial activation and phagocytosis
| Transgenic mouse lines | Expression | Functions/activities | References |
|---|---|---|---|
| Cx3cr1−/− | Microglia | Fractalkine (transmembrane chemokine) receptor signals through Cx3c chemokine receptor 1 (Cx3cr1). Cx3cr1 maintains microglial homeostasis and phagocytosis. | (Cardona et al., 2018; Lampron et al., 2015; Zheng et al., 2021) |
| Qki−/− | Microglia | Quaking (Qki) is a signal transduction and RNA‐binding protein. Qki regulates microglial phagocytosis. | (Caines et al., 2019; Ren et al., 2021) |
| Trem2−/− | Microglia/macrophage | Triggering receptor expressed on myeloid cells‐2 (Trem2) is an innate immune receptor expressed in multiple myeloid cells including CNS microglia and macrophage. The Trem2 signaling pathway regulates synaptic engulfment, microglial activation, microglial number, phagocytosis and lipid metabolism. | (Cantoni et al., 2015; Cignarella et al., 2020; Dong et al., 2021; Jay et al., 2019; Nugent et al., 2020; Poliani et al., 2015) |
| Mertk−/− | Microglia and astrocytes | Lack of tyrosine kinase phagocytic receptor (Mertk) expression impairs microglial activation. Mertk also plays a key role in phagocytosis and synapse elimination (homeostasis). | (Batiuk et al., 2020; Chung et al., 2013; Shen et al., 2021) |
| Cxcl10−/− | Microglia and astrocytes | C‐X‐C motif chemokine ligand (Cxcl10) regulates microglial chemotaxis and inflammation but not microglial proliferation or phagocytosis. | (Clarner et al., 2015) |
| Gfap‐TK | Astrocytes | Gfap up‐regulated following CPZ‐feeding. Loss‐of‐function of astrocytes using Gfap‐thymidine kinase (TK) and treatment with ganciclovir ablates astrocytes. Lack of astrocytes impairs microglial recruitment and phagocytosis which is regulated by chemokine (Cxcl10) signaling. | (Skripuletz et al., 2013) |
Studies indicate that multiple transcripts are involved in regulating phagocytosis (Cignarella et al., 2020; Ren et al., 2021; Shen et al., 2021), although no study of these genes (e.g., Qki, Trem2 and Mertk) was found using double (Qki−/− and Trem2−/−) or triple (Qki−/−, Trem2−/− and Mertk−/−) knockouts in relation to whether these gene products synergistically regulate microglial or astroglial functions and phagocytosis. However, studies using triple knockout mice lacking microglial‐secreted cytokines (Il1α, Tnf, and C1qa; an inducer of A1 astrocytes) showed a reduction of astrocytic reactivity (transcriptomic analysis), suggesting that microglia influence astrocytic function (Clarke et al., 2018). Unfortunately, no studies have investigated astrocytic secretion of cytokines with regard to microglia activation. Using the STRING Bioinformatic database (https://string-db.org/; version 11.5, accessed in November 2021), protein–protein interaction (PPI) analysis of common microglial and astrocytic transcripts (Qki, Trem2, Mertk, Cxcl10, Hcar2 and Cx3cr1) associated with phagocytosis was performed. This revealed strong interactions among Trem2, Mertk, Cxcl10, Hcar2 and Cx3cr1 indicating possible regulatory roles in phagocytosis and myelination (Figure 2). This remains to be tested experimentally. In contrast, no interactions of Qki with other transcripts were indicated, suggesting either limitations in the database or that Qki may work independently in regulating microglial function, which also remains untested. However, this inter‐connectedness is nonetheless consistent with previous observations, indicating that ~80% of the proteins do not work alone but in a complex (Berggard et al., 2007; Sen et al., 2019a; Turvey et al., 2014).
FIGURE 2.
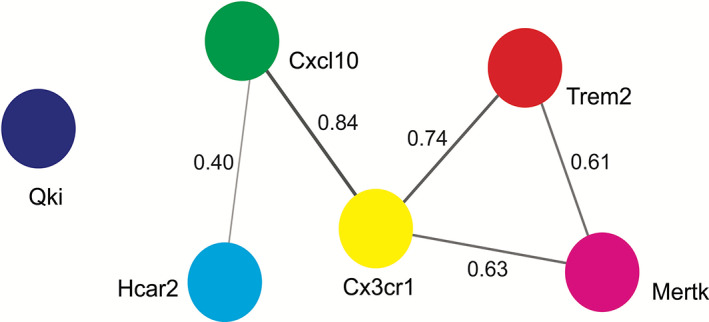
Protein–protein interaction (PPI) analysis. Using a search tool for the retrieval of interacting genes/proteins (STRING), PPI analysis (with confidence level of 0.40) shows the association of Hcar2, Cxcl10, Cx3cr1, Trem2 and Mertk, suggesting that these transcripts work in a complex way in regulating microglial and astrocytic functions. Here, each node represents a transcript and connecting lines show the interaction with each other. The thickness of the lines indicates the strength of interactions, the greater the thickness, the stronger the connection. The strength of connections is based on multiple parameters, including text‐mining, experiments, gene fusion, co‐occurrence, co‐expression, neighborhood, and databases collected in the STRING database. The interaction value is based on the combined score (highest score 0.9) found in the STRING database. Cx3cr1, CX3C chemokine receptor 1; Cxcl10, C‐X‐C motif chemokine ligand 10; Hcar2, hydroxycarboxylic acid receptor 2; Mertk, Mer proto‐oncogene tyrosine kinase; Qki, Quaking protein; Trem2, triggering receptor expressed on myeloid cells‐2
Another study showed that application of low dose irradiation strengthens immunity and improves neurodegeneration in the animal models by altering the microglial phenotypes (reviewed by Boyd et al., 2021). Administration of low dose irradiation in microglia reduces microglial activation and enhances anti‐inflammatory cytokine and anti‐oxidant properties, thus enhancing neuroprotection (Boyd et al., 2021). This irradiation strategy to examine the mechanisms underlying the regulation of myelination by microglia remains untested in the CPZ model. Moreover, microglial depletion (e.g., using clodronate liposomes) can be used to investigate the role of microglia in homeostasis and myelination (Eme‐Scolan & Dando, 2020). Studies showed microglial depletion is associated with the disruption of microglial homeostasis, behavioral deficits (e.g., motor), synaptic formation impairment and neurodegeneration (Parkhurst et al., 2013; Rubino et al., 2018). This kind of microglial depletion study has not been performed in the CPZ model. Other studies have also shown that progressive microglial activation results in phagocytosis of astrocytic end‐feet, resulting in BBB disruption (Haruwaka et al., 2019). This is of great significance for MS as BBB disruption and infiltration of adaptive immune cells into the CNS is a clinical hallmark of the disease (Daneman & Prat, 2015; Małkiewicz et al., 2019), the pathoetiology of which remains unclear. Since in the CPZ model, the BBB remains intact, the CPZ model may be ideal to investigate how microglia and astrocytes regulate BBB integrity without peripheral immune interference.
7. MICROGLIAL ROLE IN MYELINATION FOLLOWING CNS INJURY
In addition to the phagocytosis of damaged myelin fragments following demyelination, activated microglia (M2 polarization) secrete protective mediators (e.g., Tnf‐α, Igf‐1, and Pdgf‐α) that are associated with the elevation of oligodendrocyte function and myelination (Figure 1 and Table 4). For example, an increased abundance of Tnf‐α ligand is found in the cerebellum of CPZ‐fed mice (Raposo et al., 2013), and microglia have been shown to secrete Tnf‐α (Stoll et al., 1993). Another study investigated the effects of microglial Tnf‐α in oligodendrocyte function in CPZ‐fed Tnf‐α−/− mice and found it significantly delayed oligodendrocyte degeneration (presumably by Tnf‐α acting as an anti‐inflammatory molecule [Arnett et al., 2001]). Further investigation revealed that during the standard two‐week recovery phase after cessation of CPZ‐feeding, ~80% of axons remained demyelinated in Tnf‐α−/− mice compared to ~10% in wild‐type C57Bl/6J mice (Arnett et al., 2001). Notably, of the two receptors, Tnfr‐1 and Tnfr‐2, Tnfr‐2 showed greater expression in the corpus callosum during both demyelination and remyelination (Arnett et al., 2001). Also, Tnf‐α−/− or Tnfr‐2−/− mice had a greater reduction of neuron‐glial antigen‐2+ (Ng2, a chondroitin sulphate proteoglycan marker) OPC number in the corpus callosum. These observations suggest that Tnf‐α signaling may regulate oligodendrocyte proliferation and myelination in CPZ‐fed mice through Tnfr‐2 receptors (Arnett et al., 2001), possibly by protecting oligodendrocytes from cytolytic degradation (Raine et al., 1998). Likewise, the up‐regulation of Tnf‐α, Igf‐1 and fibroblast growth factor, Fgf‐2 (a potential tissue regenerating and repairing factor, [Maddaluno et al., 2017]) were found in microglia isolated from the corpus callosum and cerebral cortex of CPZ‐fed C57Bl/6 mice (Voss et al., 2012). Collectively, these studies suggest that microglia create an environment for myelination by the production of growth factors. Conversely, the toxic (pro‐inflammatory) effects of Tnf‐α have also been described in the literature (Chung & Benveniste, 1990; Li et al., 2008; Selmaj & Raine, 1988; Stoll et al., 1993; Su et al., 2011). For example, microglia‐mediated secretion of Tnf‐α has been shown to cause immune‐mediated demyelination in the peripheral nervous system in an animal model of experimental autoimmune neuritis (Stoll et al., 1993); however, the degeneration of oligodendrocytes was not investigated. Similar to the detrimental role of Tnf‐α from reactive microglia (Chung & Benveniste, 1990; Li et al., 2008; Selmaj & Raine, 1988; Stoll et al., 1993; Su et al., 2011), reactive astrocytic secretion of Tnf‐α augments immune responses and inhibits OPC differentiation into mature oligodendrocytes, thus suppressing remyelination (Chung & Benveniste, 1990; Li et al., 2008; Su et al., 2011). Likewise, elevation of Tnf‐α activates astrocytic Tnfr‐1 in the hippocampus, resulting in a disruption of hippocampal excitatory synapses, leading to the cognitive impairment (assessed using contextual fear conditioning, contextual learning, and memory) in EAE mice (Habbas et al., 2015). Similarly, elevation of Tnf‐α was observed in the cerebrospinal fluid of patients with progressive MS, and Tnf‐α has been shown to associate with excitotoxic neuronal degeneration (Rossi et al., 2014). These observations suggest a dual role of Tnf‐α. Therefore, the use of Tnf‐α as a therapeutic agent for enhancing remyelination merits further investigation.
TABLE 4.
Summary of the important beneficial or detrimental mediators secreted by microglia and astrocytes
| Mediators | |||||
|---|---|---|---|---|---|
| Beneficial | Cells | Outcome | References | Observations/validation | References |
| Tumor necrosis factor‐α (Tnf‐α) | Microglia | Tnf‐α up‐regulates following CPZ‐feeding in the corpus callosum and co‐localized with microglia. Tnf‐α−/− mice show delayed oligodendrocyte degeneration and demyelination. | (Arnett et al., 2001) | Contradictory outcomes.
|
(Hemmer et al., 2001; Stoll et al., 1993) |
| Insulin‐like growth factor‐1 (Igf‐1) | Microglia | Up‐regulation of Igf‐1 reduces CPZ‐induced demyelination. | (Mason et al., 2000) |
|
(Mason et al., 2003) |
| Sphingosine‐1 phosphate (S1P) | Microglia | Microglial secretion of S1P enhances OPC recruitment in lysolecithin‐injected mice. | (Lombardi et al., 2019) |
|
(Kim et al., 2011) |
| Activin‐A | Microglia | Microglial polarization shifts from M1 to M2 phenotype. Blocking of M2‐derived Activin secretion prevents oligodendrocyte differentiation in lysolecithin and ethidium bromide‐injected rats. | (Miron et al., 2013) |
|
(Dillenburg et al., 2018) |
| Interferon‐β (Ifn‐β) | Microglia | Microglial secretion of Ifn‐β during the peak stage of EAE removes myelin debris following autoimmune‐mediated demyelination in the spinal cord. | (Kocur et al., 2015) |
|
(Njenga et al., 2000) |
| Tissue inhibitor of metalloproteinases‐1 (Timp‐1) | Astrocytes | Astrocytic production of Timp‐1 causes OPC proliferation. | (Houben et al., 2020) |
|
(Moore et al., 2011) |
| Detrimental | |||||
| Colony‐stimulating factor‐1 (Csf‐1) | Microglia | Injection of Csf‐1 into the CNS induces microglial activation and demyelination. Csf‐1 inhibitor (PLX3397)‐mediated microglial depletion reduces the oligodendrocyte loss, astrocyte activation and demyelination. | (Marzan et al., 2021) |
|
(Wylot et al., 2019) |
| Interleukin‐3 (Il‐3) | Microglia | Il‐3 is a pro‐inflammatory cytokine. Microglial secretion of Il‐3 evokes demyelination in Gfap‐Il‐3 transgenic mice. | (Chiang et al., 1996) |
|
(Renner et al., 2016) |
| Heat shock protein‐60 (Hsp‐60) | Microglia | Production of Hsp‐60 by activated microglia (in the LPS model) causes OPC apoptosis. | (Li et al., 2017) |
|
(Rosenberger et al., 2015) |
| Nuclear factor kappa‐B (Nfk‐B) |
Astrocytes |
Astrocytic secretion of Nfk‐B causes oligodendrocyte degeneration. | (Brück et al., 2012) |
|
(Gupta et al., 2019) |
Elevation of Igf‐1, a growth hormone and associated with tissue regeneration and repair (Slavin et al., 2021), in CPZ‐fed C57Bl/6 mice coincides with the appearance of OPC, suggesting a protective role for Igf‐1 (Mason et al., 2000). While the source of Igf‐1 was not investigated in that study (Mason et al., 2000), another study revealed that the astrocytic expression of Igf‐1 was associated with remyelination (Schulz et al., 2012). Genome‐wide gene expression analysis of microglia from the corpus callosum during demyelination and remyelination in CPZ‐fed C57Bl/6 mice identified changes in 7500, 9000, and 9000 genes, respectively, for Control (without CPZ), 5 weeks CPZ‐feeding, and 2 weeks remyelination (no CPZ‐feeding), with 6200 genes shared among all groups (Olah et al., 2012). Gene ontology analysis showed the involvement of these genes in the cholesterol metabolic process, acute inflammatory response, cell cycle, immune response, and antigen processing and presentation (Olah et al., 2012). Interestingly, recruitment of OPC and oligodendrocyte‐supporting genes including Cxcl10 and Cxcl13, Igf‐1, Tfg‐β1, Pdgf‐α and ‐β was also observed (Olah et al., 2012). In particular, Pdgf‐α assisted recovery from chronic CPZ‐induced demyelination by promoting the proliferation and recruitment of OPC (Vana et al., 2007). In this work, corpus callosum demyelination was induced by CPZ‐feeding of hPdgf‐α−/− mice and following cessation of CPZ‐feeding, an increased oligodendrocyte density was observed in the transgenic mice (Vana et al., 2007). This suggests that activation of the Pdgf‐α receptor promotes remyelination in demyelinated lesions (12 weeks of 0.2% CPZ‐feeding) in the corpus callosum. These studies collectively suggest that microglia, upon activation, release protective mediators that enhance OPC proliferation and remyelination.
8. THERAPEUTIC INTERVENTIONS MODULATE MICROGLIAL POLARIZATION
The beneficial effects of myelin debris removal by microglia to facilitate remyelination is supported by various therapeutic approaches that modulate microglial functions towards a protective (M2) polarization (Aryanpour et al., 2021; Laflamme et al., 2018; Tian et al., 2021; Wang et al., 2020; Zhu et al., 2016). For example, electroacupuncture (a derivative of traditional Chinese acupuncture) improves motor behavioral deficits (measured by beam walking and pole tests) and enhances remyelination by reducing myelin debris in CPZ‐fed C57Bl/6 mice (Zhu et al., 2016). Further investigation revealed a greater number of microglial aggregations and an elevated expression of phagocytosis‐related genes in the demyelinated corpus callosum. Likewise, administering CZ‐7, a derivative of Claulansine F (a traditional Chinese medicine believed to be protective), to CPZ‐fed C57Bl/6 mice, improved motor behavioral deficits (measured by pole and grip strength tests, [Wang et al., 2020]). In addition, CZ‐7 improved remyelination via the clearance of degraded myelin debris by mediating a change of microglia from M1 to M2 polarization (measured using Q‐PCR). Moreover, higher numbers of Ng2 and O4 positive OPC were observed at the site of demyelination following CZ‐7 treatment (Wang et al., 2020). The results of these studies (Wang et al., 2020; Zhu et al., 2016) are supported by other recent investigations. Treatment with 17β‐estradiol (an estrogen steroid hormone) alleviated cognitive (learning) behavior deficits and a reduction of M1 microglial markers (CD86, iNOS and MHC‐II) in CPZ‐fed C57Bl/6 mice (Aryanpour et al., 2021). However, an up‐regulation of M2 markers (Arg1, CD206 and Trem2) was observed in the corpus callosum of CPZ‐fed mice (Aryanpour et al., 2021). Likewise, 18β‐glycyrrhetinic acid (a natural active component of Glycyrrhiza glabra root) treatment improved locomotor and balance deficits and enhanced the expression of the M2 microglial marker CD206, while reducing the M1 microglial marker CD16 in the corpus callosum of CPZ‐fed Kunming mice (Tian et al., 2021). Similarly, administration of macrophage colony‐stimulating factor, which promotes the differentiation of myeloid cells and modulates microglia towards the anti‐inflammatory M2 phenotype, thus regulating phagocytic activity to CPZ‐fed C57Bl/6J mice, resulted in reduced myelin loss and enhanced proliferation of OPC at lesion sites (Laflamme et al., 2018). Enhanced phagocytosis by microglia is also supported by a study showing that administration of Fasudil (a Rho kinase inhibitor) promoted phagocytosis in CPZ‐fed mice through the up‐regulation of the Trem2/DAP12 pathway, a key regulator of microglial function (Konishi & Kiyama, 2018), resulting in the acceleration of remyelination (Ding et al., 2021). These observations collectively suggest that CZ‐7, 17β‐estradiol or 18β‐glycyrrhetinic acid can be used as agents to promote remyelination by modulating microglial polarization. However, further investigations using different animal models of MS (e.g., EAE) and clinical validation are required.
Microglia‐mediated remyelination, as seen in the CPZ model, is supported by studies in other animal models (Fan et al., 2018; Miron et al., 2013). For example, in the ethidium bromide model (in C57Bl/6 mice and Sprague Dawley rats), M2 microglial cell‐derived activin‐A (a member of the transforming growth factor‐β family secreted by activated microglia/macrophages) promotes oligodendrocyte differentiation during remyelination in cerebellar slice cultures. This suggests activin‐A as a potential therapeutic target for CNS regeneration (Miron et al., 2013). Likewise, demyelination was decreased when microglia were manipulated from a M1 to M2 polarization in the EAE model (Fan et al., 2018). Furthermore, anti‐Kv 1.3 antibodies (recognizing a voltage‐gated potassium channel enriched in T‐cells and microglia/macrophages) from an anti‐Kv1.3 vaccine (PADRE‐Kv1.3) against Kv1.3 channels reduce the EAE clinical score, decreased inflammatory reactions, and reduced demyelination. Significantly, an elevated number of protective T‐cells (CD4+ Il‐10+ T‐cells and T‐regulatory cells) were observed in the vaccinated group. Most importantly, in the vaccinated group, M1 microglia (iNOS+/CD68+ double positive microglia) shifted towards the M2 phenotype (CD68+/Arg1+ double positive) (Fan et al., 2018).
Collectively, these studies suggest that administration of traditional pharmacological or complementary medicines in preclinical studies reduce demyelination and glial activation but enhance remyelination by altering the balance between the detrimental and protective effects of microglia. Thus, it can be argued that the manipulation of microglia from a toxic (M1) to a protective (M2) phenotype within appropriate time windows may be used to identify remyelinating therapeutics for demyelinating diseases.
9. ASTROCYTIC CONTRIBUTIONS TO REMYELINATION FOLLOWING CNS INJURY
In addition to the astrocyte‐mediated phagocytosis, astrocytic secretions are associated with accelerating remyelination. For example, astrocytic secretion of ciliary neurotrophic factor (Cntf) during CPZ‐induced demyelination promotes OPC proliferation, resulting in remyelination (A2 polarization) (Gudi et al., 2014; Houben et al., 2020). A recent study revealed that astrocyte‐derived tissue inhibitor of metalloproteinase‐1 (Timp‐1) drives OPC into mature oligodendrocytes and promotes remyelination in CPZ‐fed mice (Houben et al., 2020). Likewise, astrocytic secretion of Fgf‐2, a potent mitogen for OPC, has been shown to facilitate remyelination in mouse hepatitis virus (MHV‐A59) injected mice (Albrecht et al., 2003). Also, the astrocyte‐mediated release of brain‐derived neurotrophic factor (Bdnf), a neurotransmitter modulator (Bathina & Das, 2015), in CPZ‐fed mice increases the levels of metabotropic receptors in OPCs in the corpus callosum, resulting in the elevated expression of myelin proteins (e.g., myelin‐associated glycoprotein) (Fulmer et al., 2014). This astrocyte‐mediated remyelination is elevated when the secretion of Bdnf is enhanced in the CPZ model (Saitta et al., 2021). This suggests that astrocyte derived Bdnf might be a potential therapeutic for remyelination in demyelinating diseases. However, Bdnf can be produced by microglia and contributes to neuropathic pain (Trang et al., 2011). Therefore, before using Bdnf as a therapeutic for MS, further studies are required. However, no therapeutics have been found that reduce A1 (reactive and detrimental roles) but increase the number of A2 astrocytes (supportive) in the CPZ or any other animal models of MS.
To the best of our knowledge, there are no approved remyelination therapies for MS. Likewise, there are no approved therapeutics that can modulate microglia or astrocytes for efficient phagocytosis or promote production of growth factors that can accelerate remyelination. Similarly, there are no approved therapeutics that can effectively reduce/block detrimental secretions (Table 4). However, protective mediators from microglia and astrocytes (Table 4) can be used as a remyelinating therapies if they can be further validated by additional animal experiments (ideally with different animal models of MS) and approved following clinical trials with human MS patients.
10. DETRIMENTAL ROLES OF MICROGLIA AND ASTROCYTES IN MYELINATION FOLLOWING CNS INJURY
Are inadequate remyelination and progressive demyelination associated with the deleterious activity by microglia and astrocytes? Studies revealed that activated microglia (M1) and astrocytes (A1) produce a number of noxious, pro‐inflammatory molecules (summarized in Table 4), which negatively affect OPC proliferation, resulting in impaired remyelination and thus prolonged demyelination (Figure 1). Animal studies have shown that if microglia are depleted using the colony‐stimulating factor‐1 (Csf‐1) cytokine receptor inhibitor (PLX3397), the myelin sheath remains protected from demyelination in CPZ‐fed transgenic (Cx3cr1 CreER‐iresGFP/+ ; Rosa26 stop‐DsRed ) mice (Marzan et al., 2021). However, the administration of Csf‐1 to healthy transgenic mice activates microglia, causing demyelination in the corpus callosum, suggesting that microglial activation can also trigger demyelination, while blocking the Csf‐1 receptor (using PLX3397) abrogates the demyelination in CPZ‐fed mice (Marzan et al., 2021). Whether Csf‐1 injection activates astrocytes and triggers demyelination remains unclear. The involvement of Csf‐1 in myelination has been described by other investigators. One study using the Csf‐1 receptor (Csf‐1r) inhibitor (BLZ945) in the CPZ model shows the acceleration of remyelination (Beckmann et al., 2018) while another found the reduction of demyelination and immune activation using the EAE (using PLX5622 Csf‐1r inhibitor) model (Nissen et al., 2018). In contrast, administration of Csf‐1r inhibitor (PLX5622) in mice infected with a neurotropic coronavirus (a mouse model of hepatitis virus) showed an exacerbation of demyelination and an impairment of remyelination (Sariol et al., 2020). This latter result suggests that additional unknown mechanisms may be involved in regulating myelination, which requires further investigation.
Microglia‐mediated demyelination was observed in a study using Gfap‐Il3 transgenic mice, in which the expression of a microglia/macrophage activation cytokine, Il‐3, targeted astrocytes using a Gfap fusion gene (Chiang et al., 1996). This study revealed that transgenic mice with Il‐3 expression experienced progressive motor impairment (measured by the rota‐rod test), and multi‐focal, plaque‐like white matter lesions appeared in the cerebellum and brainstem (Chiang et al., 1996). A study by Li et al. (2017) revealed that LPS‐activated microglia secrete heat shock protein‐60 (Hsp‐60), which causes OPC apoptosis. While it has been argued that upon oligodendrocyte degeneration and demyelination, microglia are recruited to the injured site (Gudi et al., 2014; Sen et al., 2019b), interestingly, in stressed conditions oligodendrocytes produce factors that can modulate microglial activation. For example, in proteolipid protein/suppressor of cytokine signaling‐1 transgenic EAE mice (Plp/Socs1 mouse line), oxidative stress in oligodendrocytes resulted in the up‐regulation of many chemokines including Cxcl10, Ccl‐2 (monocyte chemoattractant protein‐1) and Ccl‐5. This suggests that mediators secreted by the interaction of Ifn‐γ (a cytokine involved in mediating responses in both the innate and adaptive immune system, [Schoenborn & Wilson, 2007]), with oligodendrocytes facilitates infiltration of microglial cells at the demyelinating site (Balabanov et al., 2007). Similarly, we have reported significant microglia (mainly in the gray matter) and astrocyte (predominately in the white matter) activation in specific regions of the spinal cord, despite the lack of demyelination and oligodendrocyte loss in CPZ‐fed mice (Sen et al., 2020a). However, it is not known which signaling process is associated with early glial activation prior to CNS injury. Moreover, whether or not stressed oligodendrocytes caused glial activation as demonstrated by Balabanov et al. (2007) has not been investigated in any CPZ study, and so merits further investigation. Is microglial and astrocytic activation the first line of response following CPZ‐feeding? As illustrated in Figure 1, gliosis can lead to oligodendrocyte degeneration. Whether CPZ differentially affects different sub‐types of oligodendrocytes (Ferrer, 2018; Simons & Nave, 2015) remains unknown. Single‐cell transcriptomics/proteomics of microglia, astrocytes or oligodendrocytes isolated from different parts of the CNS (brain, cerebellum, spinal cord) can help to identify differential signaling pathways regulating oligodendrocytosis and gliosis in the CPZ model. This kind of comprehensive and systematic CNS study is critical in helping to define the pathoetiology of localized demyelination in MS.
Astrocytic secretion of nuclear factor kappa‐B (Nfk‐B), involved in inflammatory response (Liu et al., 2017), during CPZ‐feeding also leads to the degeneration of oligodendrocytes and, thus, demyelination (Brück et al., 2012). Similarly, work by Colombo et al. (2021) revealed that mice lacking the astrocyte neurotrophin receptor tyrosine kinase B (Gfap‐TrkB KO mice) were prone to demyelination (by alteration of copper distribution), in both CPZ and EAE models of MS. Likewise, an inhibitory interaction between reactive astrocytes and OPCs resulted in inhibition of remyelination (Hammond et al., 2014; Hammond et al., 2015). Following lysolecithin‐induced demyelination, astrocyte activation and up‐regulation of endothelin‐1 resulted in reduced OPC differentiation and thus decreased remyelination. Inhibition of endothelin‐1 signaling using an endothelin‐receptor antagonist (PD142,893) blocks Notch activation, resulting in acceleration of remyelination (Hammond et al., 2014).
These studies collectively indicate that upon activation, both microglia and astrocytes are associated with inhibition of remyelination and acceleration of demyelination. However, it is not clear how the dynamics of astrocytes or microglia are altered when the status of myelination (i.e., demyelination or remyelination) changes and this merits future research. Additionally, while both astrocytes and microglia are associated with myelination, the question remains: what signaling process determines which types of glial cell(s) will be involved in either demyelination, or the remyelination of a demyelinated lesion. Moreover, while astrocytes and microglia are associated with the secretion of both protective and destructive substances, the temporal profiles and thresholds of glial activation remain to be determined. Likewise, what are the signaling pathways that determine what substances are secreted and are they all secreted in parallel upon activation of glia? Which signaling pathways then dysregulate the balance of beneficial versus detrimental molecules? Future studies are thus required to find the answers to these research questions to better understand the role of glial activation in myelination.
11. BEHAVIORAL DEFICITS FOLLOWING CNS INJURY IN CPZ MODEL
Is there any mechanistic link between glial activation and behavioral changes in the CPZ model? CPZ‐fed mice show subtle behavioral (e.g., motor and cognitive) deficits during the demyelination period which do not fully recover following remyelination (Franco‐Pons et al., 2007; Manrique‐Hoyos et al., 2012; Sen et al., 2020a; Sen et al., 2019b). These studies showed demyelination, gliosis and behavioral deficits but no study showed the correlation or causal relation of behavioral deficits with glial activation/myelin loss, particularly at the single‐cell level, in CPZ model. This raises the following questions for future studies. How much glial activation or myelin loss is required to initiate behavioral deficits? Which signaling pathways (e.g., sensory‐motor) are impacted by glial activation? Why do behavioral deficits persist after remyelination? Studies using other animal models suggest an association between changes in transcriptome profiles and behavioral changes. For example, microglial transcriptomic analysis of EAE brain and spinal cord tissue, prior to the onset of motor deficits (pre‐onset) and during the period of motor deficits, increased the gene expression in the brain and spinal cord (Acharjee et al., 2021). The magnitude of expression is greater in the spinal cord (the main site of demyelination and gliosis, and associated with motor control) than the brain during the symptomatic stage (Acharjee et al., 2021). Similar studies with other animal models (e.g., CReCOM mouse model of systemic lupus erythematosus) (Gonzalez‐Pena et al., 2016; Makinde et al., 2020) showed a correlation between microglial transcriptome change and behavioral deficits. Likewise, a positive correlation of proteoform (e.g., peroxiredoxin‐6) changes and motor score was recently observed in the Gfap‐Il‐6 mouse model of neuroinflammation (Asgarov et al., 2021). Such transcriptomic/proteomic and behavioral analyses thus hold promise for future investigations using the CPZ animal model.
12. SYNAPTIC DEGENERATION FOLLOWING ACTIVATION OF MICROGLIA AND ASTROCYTES
In addition to microglia‐mediated demyelination in the CNS, activated microglia inhibit axonal growth and engulf synapses, collectively called synaptopathy (Mandolesi et al., 2015). For example, LPS‐mediated microglial activation inhibits neurite outgrowth and induces growth cone collapse of cortical neurons in vitro (Kitayama et al., 2011). In addition, microglial activation enhances the expression of repulsive guidance molecule‐a (RGMa) and reduction of RGMa expression upon the treatment with RGMa‐neutralizing antibodies or transfection of RGMa siRNA, resulting in the regeneration of axonal structure (Kitayama et al., 2011). Likewise, activated microglia in MS patients and multiple MS animal models (EAE: C57Bl/6J mice and DPT:Plp1‐CreErt; Rosa26‐Egfp‐DTA mice) have been associated with the engulfment of synapses (Werneburg et al., 2020). Importantly, glial activation and microglia‐mediated synaptic degeneration are seen prior to marked demyelination. Furthermore, vesicular glutamate transporter‐2 terminals were engulfed by Iba1+ microglial cells (Werneburg et al., 2020). Similarly, studies with post‐mortem brain samples from MS patients showed the loss of dendritic spines and synapses in the hippocampus and cortex prior to the usual marked demyelination and neuronal injury (Dutta et al., 2011; Jürgens et al., 2015). Synaptic changes in the hippocampus and visual pathway were also observed in the EAE and CPZ animal models prior to marked changes in myelin structure and neuronal injury (Araújo et al., 2017; Bellizzi et al., 2016). Microglia‐mediated synaptic phagocytosis is further supported by other studies showing that microglia predominantly engulf presynaptic structures, which is evident in the mouse models of several human diseases (Azevedo et al., 2013; Hong et al., 2016; Schafer et al., 2012; Weinhard et al., 2018). These studies collectively suggest that activated microglia are associated with synaptic destruction. In addition to synaptic engulfment, microglia/macrophages are associated with engulfing axonal bulbs, which indicate the association of microglia in axonal injury (Huizinga et al., 2012).
Do astrocytes engulf synapses, as is seen with microglia? While one study indicated that astrocytes are not associated with synaptic engulfment (Werneburg et al., 2020), other studies showed that astrocytes are actively involved in synaptic phagocytosis (Chung et al., 2013; Jay et al., 2019; Lee et al., 2021; Vainchtein et al., 2018). For example, astrocytes can engulf both CNS excitatory and inhibitory synapses (both in vitro and in vivo), through Megf10 and Mertk phagocytic pathways in adult (Megf10tm1[Komp]Vlcg and B6;129‐Mertktm1Grl/J) mice (Chung et al., 2013). Likewise, Lee et al. (2021) showed that astrocytes eliminate excitatory synaptic connections through the Megf10 phagocytic pathway, and that Megf10‐knockout mice showed a reduction in synapse elimination (Lee et al., 2021). Another mechanism by which astrocytes engulf synapses involves Trem2 and Il‐33 signaling pathways (Jay et al., 2019; Vainchtein et al., 2018). These studies showed that reduction of astrocyte‐mediated synaptic engulfment is observed in microglia from Trem2−/− mice (Jay et al., 2019) which was also found in another study (Filipello et al., 2018). Further research revealed that astrocytes promote synaptic engulfment (through the production of Il‐33) and neuronal circuit development (Vainchtein et al., 2018). This suggests multifactorial roles for Mertk and Trem2 in maintaining both glial function and synaptic health (Chung et al., 2013; Jay et al., 2019; Poliani et al., 2015; Shen et al., 2021). Is synapse elimination age‐related, as seen in the age‐related dysregulation of myelination? Recent studies (summarized in Table 5 and illustrated in Figure 3) have shown that aged astrocytes are associated with the up‐regulation of genes that are associated with synaptic elimination (e.g., Tgf‐b2 and C3) (Boisvert et al., 2018) and assembly of excitatory synapses (e.g., Sparcl1 and Sparc) (Clarke et al., 2018). Moreover, synaptic plasticity is impaired in aged astrocytes (Popov et al., 2021). Taken together, these studies suggest an age‐related degeneration of synapses, although this phenomenon remains untested in the CPZ animal model.
TABLE 5.
Age‐related changes in microglial and astrocytic functions and myelination
| Key animal strains and cell lines | Model systems | Age of animals and cell lines | Cell analyzed | Key results | References |
|---|---|---|---|---|---|
| Sprague Dawley rats | Lysolecithin model | 8‐10‐week and 10‐13‐month old | Microglia |
|
(Zhao et al., 2006) |
| p7.2fms‐EGFP mice | LPS model | 2‐ and 18‐month old | Microglia |
|
(Sierra et al., 2007) |
| Trem2−/− mice | CPZ model | 6‐month and 1‐2‐year old | Microglia |
|
(Poliani et al., 2015) |
| C57Bl/6, LysMCre+ RXRαfl/fl mice | Lysolecithin model | 2‐ and 15‐20‐month old | Microglia |
|
(Natrajan et al., 2015) |
| Rab7∆MG and PMD mice | CPZ model | 2‐, 6‐, 7‐, 9‐, 12‐, 18‐, 24‐month old | Microglia |
|
(Safaiyan et al., 2016) |
| Cx3cr1GFP/+:Thy1YFP+, Cx3cr1GFP/+, Ccr2RFP/− and Cx3cr1GFP/− mice | Lysolecithin model | 2–3 and 9‐12‐month old | Microglia |
|
(Rawji et al., 2018) |
| C57Bl/6J mice | Lysolecithin model | Embryonic (E14.5), postnatal (P4/P5), P30‐, P100‐ and P540‐day old | Microglia |
|
(Hammond et al., 2019) |
| C57Bl/6, CX3CR1CreER:Rosa26TdT and Hcar2−/− mice | Lysolecithin model | 2–3 and 9–12‐ month old | Microglia |
|
(Rawji, Young, et al., 2020b) |
| C57Bl/6J, Trem2−/−, ApoE KO mice | — | 2‐, 6‐, 12‐, 18‐, 20‐, 24‐month old | Microglia |
|
(Safaiyan et al., 2021) |
| Rhesus macaque and Japanese macaques | — | 1–4, 10–15 and 22‐30‐year old | Astrocytes |
|
(Cargill et al., 2012) |
| Flox‐Rpl22‐HA and Gfap‐cre mice | — |
4‐ and 24‐month old |
Astrocytes |
|
(Boisvert et al., 2018) |
| LXRa KO and APOE KO mice | Lysolecithin model | 3‐ and 12‐month old | Astrocytes |
|
(Cantuti‐Castelvetri et al., 2018) |
| Aldh1l1‐eGFP‐ L10a, IL1α−/−;Tnf−/−;C1qa−/− triple KO mice | LPS model | 1‐week, 4.5‐week, 10‐week, 9.5‐month and 2‐years old | Astrocytes |
|
(Clarke et al., 2018) |
| In vitro (from C57Bl/6 mice) | Rapamycin treatment | ≤ 4‐week and ≥ 16‐weeks old astrocytes in culture | Astrocytes |
|
(Willis et al., 2020) |
| C57Bl/6 mice | — | 3–4, 9–12, and 20‐24‐month old | Astrocytes |
|
(Popov et al., 2021) |
Abbreviations: —, not found or investigated or not relevant; CPZ, cuprizone and LPS, lipopolysaccharide.
FIGURE 3.
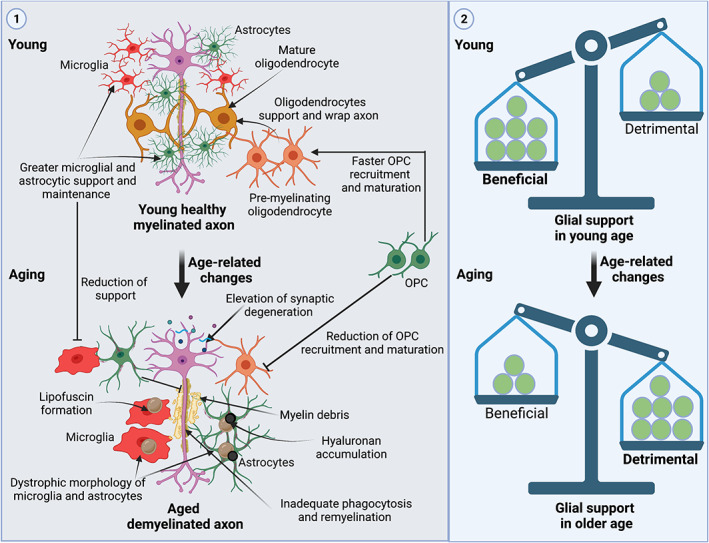
Age‐related changes of glial response in the CNS. Step 1: At younger ages (e.g., ~7–8‐week old adult mice), faster oligodendrocyte progenitor cell (OPC) migration and maturation into the site of demyelination results in rapid remyelination (e.g., CPZ‐induced demyelination and remyelination). Likewise, microglia and astrocytes also recruit and activate faster. In addition, microglia and astrocytes from a young age are associated with faster phagocytosis. Moreover, both microglia and astrocytes show more supportive and homeostatic roles. However, by ~24 months, a marked age‐related change in glial support and myelination is observed (given in Table 5). This reduction of glial support is observed in the CPZ model as well as other animal models (e.g., LPS and lysolecithin). In aged mice, microglia/macrophages show altered morphology (e.g., reduced numbers of processes), are recruited less to the site of demyelination, and express reduced levels of the scavenger receptor (CD36). They become more pro‐inflammatory and have increased aggregates of lipofuscin in microglia (Hammond et al., 2019; Natrajan et al., 2015; Poliani et al., 2015; Rawji et al., 2018; Rawji et al., 2020b; Safaiyan et al., 2016; Safaiyan et al., 2021; Sierra et al., 2007; Zhao et al., 2006). Similarly, aged astrocytes are prone to a pro‐inflammatory phenotype, secrete less cholesterol for myelin synthesis, and are enriched in lipofuscin and hyaluronan (Boisvert et al., 2018; Cantuti‐Castelvetri et al., 2018; Cargill et al., 2012; Clarke et al., 2018; Popov et al., 2021; Willis et al., 2020). In addition, OPC recruitment and maturation is delayed with aging (Sim et al., 2002). Step 2: This elevation of detrimental factors in aged glia shifts the dynamics of the hypothetical balance. At younger ages, there is marked support by glial cells (shown in the greater weight of beneficial aspects in balance) whereas the reduction of this support (as shown in the greater weight of detrimental aspects in balance) is seen with aging. These series of events impair phagocytosis and myelination with aging, resulting in progressive demyelination and neurodegeneration. OPC, Oligodendrocyte progenitor cell
Collectively, these studies suggest that both microglia and astrocytes are associated with synaptic engulfment, although the magnitude of glial reactivity required to engulf synapses remains unclear. Nonetheless, this results in the disruption of synaptic transmission and thus synaptic dysfunction (e.g., dysregulation of glutamatergic and GABAergic systems) that may lead to the early decline of motor and cognitive functions, a hallmark of neurodegenerative diseases including Alzheimer's and Parkinson's diseases, and MS (Henstridge et al., 2019; Mandolesi et al., 2015). Importantly, the destruction of synapses is not the consequence of neuronal or axonal damage; rather, synaptic changes can occur independently and prior to the marked changes in myelin structure or neuronal injury. Since synapses can be restored if disruption is detected and treated early (Mandolesi et al., 2015), functional deficits as a result of synaptic destruction can be reversed. However, while both microglia and astrocytic synaptic engulfment have been described in many studies (Azevedo et al., 2013; Chung et al., 2013; Hong et al., 2016; Jay et al., 2019; Lee et al., 2021; Schafer et al., 2012; Vainchtein et al., 2018; Weinhard et al., 2018), none were found that investigated these processes following CPZ‐feeding. Again, this clearly warrants future investigation.
13. THE EFFECT OF AGING ON MICROGLIA AND ASTROCYTES, AND MYELINATION
The efficiency of phagocytosis and CNS remyelination declines with age in MS patients (Koellhoffer et al., 2017; Neumann et al., 2019); however, the mechanism(s) by which this occurs remains unclear. Upon aging, a similar reduction of glial support to myelin, neurons and axons has been shown in experimental models (see Table 5 and Figure 3). Therefore, it is important to uncover the mechanism of age‐related reduction of debris clearance and remyelination so that remyelination therapeutics can be developed. Microglia derived from aged mice exhibit an altered inflammatory profile (Table 5). For example, using the lysolecithin model, there is delayed recruitment and activation of microglial scavenger receptors (a combination of conserved proteins expressed on the surface of microglia) in older rats (10–13‐month old) compared to young Sprague Dawley rats (8–10‐week; Zhao et al., 2006). Following the LPS challenge, microglia from 18‐month old mice (p7.2fms‐Egfp mouse line) are characterized by lipofuscin granules, a decreased complexity of processes, altered granularity, and greater expression of Il‐1β, −6, and − 10 when compared to microglia from 2‐month old mice, suggesting that older microglia may become more pro‐inflammatory (Sierra et al., 2007). Similarly, in situ hybridization analysis revealed age‐related differences in Igf‐1, Tgf‐β1, and Pdgf‐α mRNA expression during remyelination following lysolecithin‐induced demyelination in Sprague Dawley rats (Hinks & Franklin, 2000). Notably, Igf‐1 and Tgf‐β1 mRNA in old rats had a delayed and lower peak expression compared to young rats. Moreover, an age‐related difference in the expression pattern of the macrophage marker Sr‐b, a transcript unique to macrophages was found (Hinks & Franklin, 2000). This indicates a delayed expression of macrophage markers in old compared to young animals (Hinks & Franklin, 2000). Another recent study identified age‐related changes in the proteomic profiles of OPC from Sprague Dawley rats; notably, proteins associated with inflammatory responses were found to increase with aging (de la Fuente et al., 2020). However, none of these studies sought to analyze proteoforms (i.e., the active biological entities; Bogaert et al., 2020; Carbonara et al., 2021; Coorssen & Yergey, 2015; Sen et al., 2021; Zhan et al., 2019). Thus, an appropriately designed (top‐down) proteomic analysis may identify age‐dependent changes in specific proteoforms related to microglial (and astrocytic) function. However, such detailed proteomic analyses have not been performed in microglia or astrocytes. Also, young C57Bl/6J mice (7–8‐week old) after 0.2% CPZ‐feeding for 5–6 weeks, displayed marked oligodendrocyte degeneration, demyelination, and gliosis in the corpus callosum, while a higher CPZ dose (~0.4%) was needed to achieve a comparable change in older mice (~6‐month old) (Gingele et al., 2020). Furthermore, in young mice, spontaneous and complete remyelination was observed upon cessation of CPZ‐feeding, whereas incomplete remyelination was noted in older mice (Gingele et al., 2020). Again, in the ethidium bromide model, a delay in the migration of OPC in the old compared with the young Sprague Dawley rats was also observed (Sim et al., 2002). Moreover, axonal injury (assessed by electron microscopy and quantification of the axonal marker SMI‐32) in CPZ‐fed mice was significantly greater in adult (6–7‐month old) mice compared to young (8–10‐week old) C57Bl/6 mice (Irvine & Blakemore, 2006). Similarly, CPZ‐feeding to both 3‐week old juvenile and 8‐week old adult C57Bl/6 mice revealed an early onset oligodendrocyte loss, microglial activation, and acute axonal damage and demyelination in juvenile mice compared to adult mice; however in juvenile mice, rapid remyelination was also evident compared to adult mice (Pfeifenbring et al., 2015).
In addition to the secretion of toxic products by microglia, and impaired remyelination with aging, microglial phagocytosis also declines with age (Table 5 and Figure 3). For example, in wild‐type mice, a normal expansion of microglia in the corpus callosum is observed with age; however, in aged Trem2−/− mice, a reduction of microglia is observed, suggesting that Trem2 is important for maintenance of microglia number (Poliani et al., 2015). In addition, following a short term (4 weeks) phase of demyelination and remyelination in the CPZ model, both wild‐type and Trem2−/− mice showed similar pathological changes; however, when demyelination was extended (12 weeks), a greater demyelination and accumulation of myelin fragments was observed (Poliani et al., 2015). This suggests that remyelination is incomplete during prolonged progressive demyelination and that microglial expression of Trem2 plays a vital role in remyelination by promoting phagocytosis of myelin debris. Similarly, single‐cell RNA‐sequencing of microglia showed age‐dependent changes in white matter‐associated (corpus callosum) microglia (Safaiyan et al., 2021). Notably, this microglial phagocytosis relies on Trem2 signaling (Safaiyan et al., 2021). Likewise, single‐cell transcriptomics of microglia from aged mice revealed that microglia tend to be inflammatory compared to the microglia from younger mice (Hammond et al., 2019). Similarly, compared with young mice (8‐week old), aged mice (up to 24‐month old) show a greater deposition of myelin fragments resulting in the formation of insoluble, lipofuscin‐like lysosomal inclusions in microglia (Safaiyan et al., 2016). In addition, multi‐lamellar myelin fragments are observed in aged mice when examined ultra‐structurally. The sarkosyl‐insoluble lipofuscin granules (a biomarker of aged post‐mitotic cells) accumulate in microglia with age, and this is more predominant in white than in gray matter. Lipofuscin increased as early as 9 weeks after CPZ‐feeding and continued to increase throughout the period of study (Safaiyan et al., 2016). Comparable deposition of lipofuscin (in 18‐month old mice) was also observed in an earlier study (Sierra et al., 2007). Age‐dependent accumulation of intracellular lipofuscin (mostly a combination of lipids and misfolded proteins) leads to neurodegeneration and is observed in age‐related neurodegenerative disorders (Moreno‐García et al., 2018). A recent study revealed that reduction of the expression of the scavenger receptor CD36 in microglia resulting reduction of microglia‐mediated phagocytosis in both aged C57Bl/6 mice (9‐12‐month old vs. 23‐month old mice) and human microglia in culture (Rawji et al., 2020b). The age‐dependent reduction of myelin debris clearance and a decrease in microglial/macrophage motility and surveillance was also observed in other independent studies (Natrajan et al., 2015; Rawji et al., 2016; Rawji et al., 2018).
Similar to microglia, aged astrocytes or astrocytes from aging rodents disrupt the myelination process (Table 5 and Figure 3). For example, an in vitro study with aged murine astrocytes (≤ 4‐week vs. ≥ 16‐week old primary astrocytes) identified a reduced ability to support oligodendrocyte differentiation (Willis et al., 2020). Likewise, increased astrocytic expression of CNS hyaluronan (a polysaccharide that inhibits OPC maturation) in aged (~25‐year old) rhesus macaques has also been observed (Cargill et al., 2012). Similarly, hyaluronan accumulates in the demyelinating lesions of MS and the EAE animal model, and this prevents remyelination by disrupting OPC maturation in the lysolecithin model (Back et al., 2005). Age‐related changes (e.g., A1 reactivity) in astrocytes (postnatal day 7, 4.5‐week young adult, 10‐week mature, 9.5‐month middle aged and 2‐year aged astrocytes from Aldh1l1‐eGFP‐L10a transgenic mouse line) have been documented at single‐cell resolution (Clarke et al., 2018). Importantly, gene expression profiles of astrocytes from the cortical region remain unchanged whereas reactive astrocytes are more prominent in the hippocampus, suggesting a potential link with age‐related cognitive decline (Clarke et al., 2018). In addition, aging causes an upregulation of genes involved in synaptic destruction (e.g., Sparc and Tgf‐b2) and reduction of cholesterol synthesis (Boisvert et al., 2018). Astrocytic secretion of cholesterol (as well as cholesterol transporters like apolipoprotein E) is a major source of cholesterol in myelin formation during remyelination (Camargo et al., 2017; Cantuti‐Castelvetri et al., 2018) and a reduction of cholesterol during aging reflects the negative effect on the remyelination process (Cantuti‐Castelvetri et al., 2018). This is supported by a study showing CPZ‐feeding reduces the concentration of cholesterol in the demyelinating phase (Berghoff et al., 2017) whereas during remyelination an up‐regulation of cholesterol pathways (cholesterol biosynthesis I, II, and III) is observed (Voskuhl et al., 2019) and dietary cholesterol accelerates remyelination (Berghoff et al., 2017). Similar to previous observations (Boisvert et al., 2018; Cantuti‐Castelvetri et al., 2018; Clarke et al., 2018), Popov et al. (2021) showed reduced cell numbers and process length in aged astrocytes (20‐24‐month old C57Bl/6 mice), and reduced phagocytosis/pinocytosis (K+ and glutamate clearance), indicating the disruption of synaptic plasticity following aging.
These studies collectively suggest that while both microglia and astrocytes modulate different physiological pathways in de‐ and remyelination, these capacities decline with age. However, no study has investigated microglia or astrocyte‐mediated myelin debris clearance and remyelination following prolonged (e.g., 20 weeks) CPZ‐feeding. Such a study could prove quite informative since prolonged CPZ‐feeding leads to marked demyelination, gliosis and inadequate remyelination, as seen during late‐stage progressive demyelination in MS (Fancy et al., 2010; Gudi et al., 2014; Koellhoffer et al., 2017; Neumann et al., 2019; Sen et al., 2019b). While studies investigated the reduction of microglial and astrocytic activities in aging mice, rats or humans, the impact of aged microglia or astrocytes on myelination is not clear from these studies. Additionally, it is not clear from any of these exactly when (i.e., at what stage of life) these age‐related changes start. Transcriptomic or proteomic sampling of microglia or astrocytes in a longitudinal study (e.g., over the lifespan of mice models of MS, e.g., CPZ) may reveal the age when these cells start to become pro‐inflammatory/destructive. This information will be crucial for regular monitoring of disease progression, and to inform clinical trials about early therapies for demyelinating diseases.
14. CONCLUSIONS AND FUTURE PERSPECTIVES
This review has highlighted the crucial role of activated glial cells in myelination, while disruption of this process leads to progressive demyelination. Importantly, both microglia and astrocytes contribute to phagocytosis of myelin debris and are thus associated with remyelination. In addition, activated glial cells secrete several growth factors that are important for OPC proliferation and differentiation, resulting in faster remyelination. Despite these beneficial effects, secretion of detrimental mediators and progressive microglial (and astrocytic) activation is associated with disruption of the myelination and protection dynamics. This affirms the double‐edged nature of activated microglia and astrocytes in producing both beneficial and detrimental effects, the balance of which is critically important in the maintenance of myelin health. Additional studies are required to discover whether glial activation is friend or foe to animal models of MS, or to MS itself, and to translate the beneficial aspects of gliosis from preclinical studies to clinical practice. The data to date also indicates that no single glial cell (microglia or astrocyte) or its associated mediators control the complex myelination process. Importantly, different signaling molecules and pathways tightly regulate demyelination, phagocytosis and remyelination. Disruption of these regulatory pathways may trigger switching of glial phenotypes from helpful to harmful, thereby changing the balance of glial support and homeostasis. This leads to progressive demyelination and neurodegeneration akin to that observed in microglia or astrocytes from aged subjects or persons with demyelinating diseases. Additional studies are needed to investigate the temporal effects of glial activation in order to define the magnitude, duration and extent of glial activation that underlies beneficial versus detrimental roles in the CNS. Unfortunately, as yet, there is no clear, detailed molecular signature for the various glial phenotypes. Further studies are thus required to better understand the complex interaction between the roles of microglia and astrocytes to identify new potential therapeutic strategies for demyelinating diseases such as MS.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
Monokesh K. Sen designed the scope and drafted the manuscript. David A. Mahns, Jens R. Coorssen, and Peter J. Shortland critically reviewed the manuscript. All authors approved the final version.
ACKNOWLEDGMENTS
Graphical abstract, Figures 1 and 3 were constructed using BioRender (https://biorender.com/) and Figure 2 was assembled using CorelDraw‐version 2018 (www.coreldraw.com) image processing software. We acknowledge The Rotary Club of Narellan for supporting Western Sydney University multiple sclerosis research. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Sen, M. K. , Mahns, D. A. , Coorssen, J. R. , & Shortland, P. J. (2022). The roles of microglia and astrocytes in phagocytosis and myelination: Insights from the cuprizone model of multiple sclerosis. Glia, 70(7), 1215–1250. 10.1002/glia.24148
Funding information The Rotary Club of Narellan
DATA AVAILABILITY STATEMENT
N/A
REFERENCES
- Acharjee, S. , Gordon, P. M. K. , Lee, B. H. , Read, J. , Workentine, M. L. , Sharkey, K. A. , & Pittman, Q. J. (2021). Characterization of microglial transcriptomes in the brain and spinal cord of mice in early and late experimental autoimmune encephalomyelitis using a RiboTag strategy. Scientific Reports, 11(1), 14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera, G. , Colín‐González, A. L. , Rangel‐López, E. , Chavarría, A. , & Santamaría, A. (2018). Redox signaling, neuroinflammation, and neurodegeneration. Antioxidants and Redox Signaling, 28(18), 1626–1651. 10.1089/ars.2017.709 [DOI] [PubMed] [Google Scholar]
- Ajami, B. , Samusik, N. , Wieghofer, P. , Ho, P. P. , Crotti, A. , Bjornson, Z. , Prinz, M. , Fantl, W. J. , Nolan, G. P. , & Steinman, L. (2018). Single‐cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nature Neuroscience, 21(4), 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht, P. J. , Murtie, J. C. , Ness, J. K. , Redwine, J. M. , Enterline, J. R. , Armstrong, R. C. , & Levison, S. W. (2003). Astrocytes produce CNTF during the remyelination phase of viral‐induced spinal cord demyelination to stimulate FGF‐2 production. Neurobiology of Disease, 13(2), 89–101. [DOI] [PubMed] [Google Scholar]
- Almuslehi, M. S. M. , Sen, M. K. , Shortland, P. J. , Mahns, D. A. , & Coorssen, J. R. (2020). CD8 T‐cell recruitment into the central nervous system of cuprizone‐fed mice: Relevance to modeling the etiology of multiple sclerosis. Frontiers in Cellular Neuroscience, 14(43). 10.3389/fncel.2020.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altin, J. G. , & Sloan, E. K. (1997). The role of CD45 and CD45‐associated molecules in T cell activation. Immunology and Cell Biology, 75(5), 430–445. [DOI] [PubMed] [Google Scholar]
- Araújo, S. E. S. , Mendonça, H. R. , Wheeler, N. A. , Campello‐Costa, P. , Jacobs, K. M. , Gomes, F. C. A. , Fox, M. A. , & Fuss, B. (2017). Inflammatory demyelination alters subcortical visual circuits. Journal of Neuroinflammation, 14(1), 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett, H. A. , Mason, J. , Marino, M. , Suzuki, K. , Matsushima, G. K. , & Ting, J. P. (2001). TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nature Neuroscience, 4(11), 1116–1122. [DOI] [PubMed] [Google Scholar]
- Aryanpour, R. , Zibara, K. , Pasbakhsh, P. , Behnamodin Jame'ei, S. , Namjoo, Z. , Ghanbari, A. , Mahmoudi, R. , Amani, S. , & Kashani, I. R. (2021). 17β‐estradiol reduces demyelination in cuprizone‐fed mice by promoting M2 microglia polarity and regulating NLRP3 inflammasome. Neuroscience, 463, 116–127. [DOI] [PubMed] [Google Scholar]
- Asgarov, R. , Sen, M. K. , Mikhael, M. , Karl, T. , Gyengesi, E. , Mahns, D. A. , Malladi, C. S. , & Münch, G. W. (2021). Characterisation of the mouse cerebellar proteome in the GFAP‐IL6 model of chronic neuroinflammation. Cerebellum. 10.1007/s12311-021-01 [DOI] [PubMed] [Google Scholar]
- Azevedo, E. P. , Ledo, J. H. , Barbosa, G. , Sobrinho, M. , Diniz, L. , Fonseca, A. C. , Gomes, F ,, Romão, L. , Lima, F. R. S. , Palhano, F. L. , Ferreira, S. T. , & Foguel, D. (2013). Activated microglia mediate synapse loss and short‐term memory deficits in a mouse model of transthyretin‐related oculoleptomeningeal amyloidosis. Cell Death and Disease, 4(9), e789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachiller, S. , Jiménez‐Ferrer, I. , Paulus, A. , Yang, Y. , Swanberg, M. , Deierborg, T. , & Boza‐Serrano, A. (2018). Microglia in neurological diseases: A road map to brain‐disease dependent‐inflammatory response. Frontiers in Cellular Neuroscience, 12(488). https://pubmed.ncbi.nlm.nih.gov/30618635/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back, S. A. , Tuohy, T. M. , Chen, H. , Wallingford, N. , Craig, A. , Struve, J. , Luo, N. L. , Banine, F ,, Liu, Y. , Chang, A. , Trapp, B. D. , Bebo Jr, B. F. , Rao, M. S. , & Sherman, L. S. (2005). Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nature Medicine, 11(9), 966–972. [DOI] [PubMed] [Google Scholar]
- Baer, A. S. , Syed, Y. A. , Kang, S. U. , Mitteregger, D. , Vig, R. , Ffrench‐Constant, C. , Franklin, R. J. M. , Altmann, F. , Lubec, G. , & Kotter, M. R. (2009). Myelin‐mediated inhibition of oligodendrocyte precursor differentiation can be overcome by pharmacological modulation of Fyn‐RhoA and protein kinase C signalling. Brain, 132(2), 465–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanov, R. , Strand, K. , Goswami, R. , McMahon, E. , Begolka, W. , Miller, S. D. , & Popko, B. (2007). Interferon‐γ‐oligodendrocyte interactions in the regulation of experimental autoimmune encephalomyelitis. Journal of Neuroscience, 27(8), 2013–2024. 10.1523/jneurosci.4689-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros, M. H. , Hauck, F. , Dreyer, J. H. , Kempkes, B. , & Niedobitek, G. (2013). Macrophage polarisation: An immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One, 8(11), e80908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathina, S. , & Das, U. N. (2015). Brain‐derived neurotrophic factor and its clinical implications. Archives of Medical Science, 11(6), 1164–1178. 10.5114/aoms.2015.56342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batiuk, M. Y. , Martirosyan, A. , Wahis, J. , de Vin, F. , Marneffe, C. , Kusserow, C. , Koeppen, J. , Viana, J. F. , Oliveira, J. F. , Voet, T. , Ponting, C. P. , Belgard, T. G. , & Holt, M. G. (2020). Identification of region‐specific astrocyte subtypes at single cell resolution. Nature Communications, 11(1), 1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar, O. A. , Bartels, T. , Holmqvist, S. , Kleshchevnikov, V. , Martirosyan, A. , Polioudakis, D. , Ben Haim, L. , Young, A. M. H. , Batiuk, M. Y. , Prakash, K. , Brown, A. , Roberts, K. , Paredes, M. F. , Kawaguchi, R. , Stockley, J. H. , Sabeur, K. , Chang, S. M. , Huang, E. , Hutchinson, P. , … Rowitch, D. H. (2020). Astrocyte layers in the mammalian cerebral cortex revealed by a single‐cell in situ transcriptomic map. Nature Neuroscience, 23(4), 500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann, N. , Giorgetti, E. , Neuhaus, A. , Zurbruegg, S. , Accart, N. , Smith, P. , Perdoux, J. , Perrot, L. , Nash, M. , Desrayaud, S. , Wipfli, P. , Frieauff, W. , & Shimshek, D. R. (2018). Brain region‐specific enhancement of remyelination and prevention of demyelination by the CSF1R kinase inhibitor BLZ945. Acta Neuropathologica Communication, 6(1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellizzi, M. J. , Geathers, J. S. , Allan, K. C. , & Gelbard, H. A. (2016). Platelet‐activating factor receptors mediate excitatory postsynaptic hippocampal injury in experimental autoimmune encephalomyelitis. Journal of Neuroscience, 36(4), 1336–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benardais, K. , Kotsiari, A. , Skuljec, J. , Koutsoudaki, P. N. , Gudi, V. , Singh, V. , Vulinović, F. , Skripuletz, T. , & Stangel, M. (2013). Cuprizone [bis(cyclohexylidenehydrazide)] is selectively toxic for mature oligodendrocytes. Neurotoxicity Research, 24(2), 244–250. [DOI] [PubMed] [Google Scholar]
- Berggard, T. , Linse, S. , & James, P. (2007). Methods for the detection and analysis of protein‐protein interactions. Proteomics, 7(16), 2833–2842. 10.1002/pmic.200700131 [DOI] [PubMed] [Google Scholar]
- Berghoff, S. A. , Gerndt, N. , Winchenbach, J. , Stumpf, S. K. , Hosang, L. , Odoardi, F. , Ruhwedel, T. , Böhler, C. , Barrette, B. , Stassart, R. , Liebetanz, D. , Dibaj, P. , Möbius, W. , Edgar, J. M. , & Saher, G. (2017). Dietary cholesterol promotes repair of demyelinated lesions in the adult brain. Nature Communications, 8(1), 14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaert, A. , Fernandez, E. , & Gevaert, K. (2020). N‐terminal proteoforms in human disease. Trends in Biochemical Sciences, 45(4), 308–320. [DOI] [PubMed] [Google Scholar]
- Boisvert, M. M. , Erikson, G. A. , Shokhirev, M. N. , & Allen, N. J. (2018). The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Reports, 22(1), 269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd, A. , Byrne, S. , Middleton, R. J. , Banati, R. B. , & Liu, G. J. (2021). Control of neuroinflammation through radiation‐induced microglial changes. Cells, 10(9). 10.3390/cells10092381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brück, W. , Pförtner, R. , Pham, T. , Zhang, J. , Hayardeny, L. , Piryatinsky, V. , Hanisch, U. K. , Regen, T. , van Rossum, D. , Brakelmann, L. , Hagemeier, K. , Kuhlmann, T. , Stadelmann, C. , John, G. R. , Kramann, N. , & Wegner, C. (2012). Reduced astrocytic NF‐κB activation by laquinimod protects from cuprizone‐induced demyelination. Acta Neuropathologica, 124(3), 411–424. 10.1007/s00401-012-1009-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caines, R. , Cochrane, A. , Kelaini, S. , Vila‐Gonzalez, M. , Yang, C. , Eleftheriadou, M. , Moez, A. , Stitt, A. W. , Zeng, L. , Grieve, D. J. , & Margariti, A. (2019). The RNA‐binding protein QKI controls alternative splicing in vascular cells, producing an effective model for therapy. Journal of Cell Science, 132(16), jcs230276. [DOI] [PubMed] [Google Scholar]
- Camargo, N. , Goudriaan, A. , van Deijk, A.‐L. F. , Otte, W. M. , Brouwers, J. F. , Lodder, H. , Gutmann, D. H. , Nave, K. A. , Dijkhuizen, R. M. , Mansvelder, H. D. , Chrast, R. , Smit, A. B. , & Verheijen, M. H. G. (2017). Oligodendroglial myelination requires astrocyte‐derived lipids. PLoS Biology, 15(5), e1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantoni, C. , Bollman, B. , Licastro, D. , Xie, M. , Mikesell, R. , Schmidt, R. , Yuede, C. M. , Galimberti, D. , Olivecrona, G. , Klein, R. S. , Cross, A. H. , Otero, K. , & Piccio, L. (2015). TREM2 regulates microglial cell activation in response to demyelination in vivo. Acta Neuropathologica, 129(3), 429–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti‐Castelvetri, L. , Fitzner, D. , Bosch‐Queralt, M. , Weil, M.‐T. , Su, M. , Sen, P. , Ruhwedel, T. , Mitkovski, M. , Trendelenburg, G. , Lütjohann, D. , Möbius, W. , & Simons, M. (2018). Defective cholesterol clearance limits remyelination in the aged central nervous system. Science, 359(6376), 684–688. [DOI] [PubMed] [Google Scholar]
- Caprariello, A. V. , Rogers, J. A. , Morgan, M. L. , Hoghooghi, V. , Plemel, J. R. , Koebel, A. , Tsutsui, S. , Dunn, J. F. , Kotra, L. P. , Ousman, S. S. , Wee Yong, V. , & Stys, P. K. (2018). Biochemically altered myelin triggers autoimmune demyelination. Proceedings of the National Academy of Sciences, 115(21), 5528–5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonara, K. , Andonovski, M. , & Coorssen, J. R. (2021). Proteomes are of proteoforms: embracing the complexity. Proteomes, 9(3), 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona, S. M. , Kim, S. V. , Church, K. A. , Torres, V. O. , Cleary, I. A. , Mendiola, A. S. , Saville, S. P. , Watowich, S. S. , Parker‐Thornburg, J. , Soto‐Ospina, A. , Araque, P. , Ransohoff, R. M. , & Cardona, A. E. (2018). Role of the fractalkine receptor in CNS autoimmune inflammation: New approach utilizing a mouse model expressing the human CX3CR1I249/M280 variant. Frontiers in Cellular Neuroscience, 12(365). 10.3389/fncel.2018.0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargill, R. , Kohama, S. G. , Struve, J. , Su, W. , Banine, F. , Witkowski, E. , Back, S. A. , & Sherman, L. S. (2012). Astrocytes in aged nonhuman primate brain gray matter synthesize excess hyaluronan. Neurobiology of Aging, 33(4), 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton, W. W. (1966). Response of mice to the chelating agents sodium diethyldithiocarbamate, alpha‐benzoinoxime, and biscyclohexanone oxaldihydrazone. Toxicology and Applied Pharmacology, 8(3), 512–521. [DOI] [PubMed] [Google Scholar]
- Cherry, J. D. , Olschowka, J. A. , & O'Banion, M. K. (2015). Arginase 1+ microglia reduce Aβ plaque deposition during IL‐1β‐dependent neuroinflammation. Journal of Neuroinflammation, 12, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, C. S. , Powell, H. C. , Gold, L. H. , Samimi, A. , & Campbell, I. L. (1996). Macrophage/microglial‐mediated primary demyelination and motor disease induced by the central nervous system production of interleukin‐3 in transgenic mice. Journal of Clinical Investigation, 97(6), 1512–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov, D. A. , Killingsworth, M. C. , Myasoedova, V. A. , Orekhov, A. N. , & Bobryshev, Y. V. (2017). CD68/macrosialin: Not just a histochemical marker. Laboratory Investigation, 97(1), 4–13. [DOI] [PubMed] [Google Scholar]
- Chung, I. Y. , & Benveniste, E. N. (1990). Tumor necrosis factor‐alpha production by astrocytes. Induction by lipopolysaccharide, IFN‐gamma, and IL‐1 beta. Journal of Immunology, 144(8), 2999–3007. [PubMed] [Google Scholar]
- Chung, W.‐S. , Clarke, L. E. , Wang, G. X. , Stafford, B. K. , Sher, A. , Chakraborty, C. , Joung, J. , Foo, L. C. , Thompson, A. , Chen, C. , Smith, S. J. , & Barres, B. A. (2013). Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature, 504(7480), 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cignarella, F. , Filipello, F. , Bollman, B. , Cantoni, C. , Locca, A. , Mikesell, R. , Manis, M. , Ibrahim, A. , Deng, L. , Benitez, B. A. , Cruchaga, C. , Licastro, D. , Mihindukulasuriya, K. , Harari, O. , Buckland, M. , Holtzman, D. M. , Rosenthal, A. , Schwabe, T. , Tassi, I. , & Piccio, L. (2020). TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta Neuropathologica, 140(4), 513–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, L. E. , Liddelow, S. A. , Chakraborty, C. , Munch, A. E. , Heiman, M. , & Barres, B. A. (2018). Normal aging induces A1‐like astrocyte reactivity. Proceedings of the National Academy of Sciences, 115(8), 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarner, T. , Janssen, K. , Nellessen, L. , Stangel, M. , Skripuletz, T. , Krauspe, B. , Hess, F. M. , Denecke, B. , Beutner, C. , Linnartz‐Gerlach, B. , Neumann, H. , Vallières, L. , Amor, S. , Ohl, K. , Tenbrock, K. , Beyer, C. , & Kipp, M. (2015). CXCL10 triggers early microglial activation in the cuprizone model. Journal of Immunology, 194(7), 3400–3413. [DOI] [PubMed] [Google Scholar]
- Collmann, F. M. , Pijnenburg, R. , Hamzei‐Taj, S. , Minassian, A. , Folz‐Donahue, K. , Kukat, C. , Aswendt, M. , & Hoehn, M. (2019). Individual in vivo profiles of microglia polarization after stroke, represented by the genes iNOS and Ym1. Frontiers in Immunology, 10(1236). 10.3389/fimmu.2019.01236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo, E. , Triolo, D. , Bassani, C. , Bedogni, F. , Di Dario, M. , Dina, G. , Fredrickx, E. , Fermo, I. , Martinelli, V. , Newcombe, J. , Taveggia, C. , Quattrini, A. , Comi, G. , & Farina, C. (2021). Dysregulated copper transport in multiple sclerosis may cause demyelination via astrocytes. Proceedings of the National Academy of Sciences, 118(27). 10.1073/pnas.2025804118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coorssen, J. R. , & Yergey, A. L. (2015). Proteomics is analytical chemistry: Fitness‐for‐purpose in the application of top‐down and bottom‐up analyses. Proteomes, 3(4), 440–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman, R. , & Prat, A. (2015). The blood‐brain barrier. Cold Spring Harbor Perspectives in Biology, 7(1), a020412. 10.1101/cshperspect.a020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente, A. G. , Queiroz, R. M. L. , Ghosh, T. , McMurran, C. E. , Cubillos, J. F. , Bergles, D. E. , Fitzgerald, D. C. , Jones, C. A. , Lilley, K. S. , Glover, C. P. , & Franklin, R. J. M. (2020). Changes in the oligodendrocyte progenitor cell proteome with ageing. Molecular and Cellular Proteomics, 19(8), 1281–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denic, A. , Johnson, A. J. , Bieber, A. J. , Warrington, A. E. , Rodriguez, M. , & Pirko, I. (2011). The relevance of animal models in multiple sclerosis research. Pathophysiology, 18(1), 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillenburg, A. , Ireland, G. , Holloway, R. K. , Davies, C. L. , Evans, F. L. , Swire, M. , Bechler, M. E. , Soong, D. , Yuen, T. J. , Su, G. H. , Becher, J. C. , Smith, C. , Williams, A. , & Miron, V. E. (2018). Activin receptors regulate the oligodendrocyte lineage in health and disease. Acta Neuropathologica, 135(6), 887–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, Z.‐B. , Han, Q.‐X. , Wang, Q. , Song, L.‐J. , Chu, G.‐G. , Guo, M.‐F. , Chai, Z. , Yu, J. Z. , Xiao, B. G. , Li, X. Y. , & Ma, C.‐G. (2021). Fasudil enhances the phagocytosis of myelin debris and the expression of neurotrophic factors in cuprizone‐induced demyelinating mice. Neuroscience Letters, 753, 135880. 10.1016/j.neulet.2021.135880 [DOI] [PubMed] [Google Scholar]
- Domingues, H. S. , Portugal, C. C. , Socodato, R. , & Relvas, J. B. (2016). Oligodendrocyte, astrocyte, and microglia crosstalk in myelin development, damage, and repair. Frontiers in Cell Development Biology, 4, 71. 10.3389/fcell.2016.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Y. , D'Mello, C. , Pinsky, W. , Lozinski, B. M. , Kaushik, D. K. , Ghorbani, S. , Moezzi, D. , Brown, D. , Melo, F. C. , Zandee, S. , Vo, T. , Prat, A. , Whitehead, S. N. , & Yong, V. W. (2021). Oxidized phosphatidylcholines found in multiple sclerosis lesions mediate neurodegeneration and are neutralized by microglia. Nature Neuroscience., 24, 489–503. [DOI] [PubMed] [Google Scholar]
- Dutta, R. , Chang, A. , Doud, M. K. , Kidd, G. J. , Ribaudo, M. V. , Young, E. A. , Fox, R. J. , Staugaitis, S. M. , & Trapp, B. D. (2011). Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Annals of Neurology, 69(3), 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eme‐Scolan, E. , & Dando, S. J. (2020). Tools and approaches for studying microglia in vivo. Frontiers in Immunology, 11(2301). 10.3389/fimmu.2020.583647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin, C. , Galea, E. , Lakatos, A. , O'Callaghan, J. P. , Petzold, G. C. , Serrano‐Pozo, A. , Steinhäuser, C. , Volterra, A. , Carmignoto, G. , Agarwal, A. , Allen, N. J. , Araque, A. , Barbeito, L. , Barzilai, A. , Bergles, D. E. , Bonvento, G , Butt, A. M. , Chen, W. T. , Cohen‐Salmon, M. , … Verkhratsky, A. (2021). Reactive astrocyte nomenclature, definitions, and future directions. Nature Neuroscience, 24(3), 312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, C. , Long, R. , You, Y. , Wang, J. , Yang, X. , Huang, S. , Sheng, Y. , Peng, X. , Liu, H. , Wang, Z. , & Liu, K. (2018). A novel PADRE‐Kv1.3 vaccine effectively induces therapeutic antibodies and ameliorates experimental autoimmune encephalomyelitis in rats. Clinical Immunology, 193, 98–109. [DOI] [PubMed] [Google Scholar]
- Fancy, S. P. J. , Kotter, M. R. , Harrington, E. P. , Huang, J. K. , Zhao, C. , Rowitch, D. H. , & Franklin, R. J. M. (2010). Overcoming remyelination failure in multiple sclerosis and other myelin disorders. Experimental Neurology, 225(1), 18–23. [DOI] [PubMed] [Google Scholar]
- Farias, A. S. , Martins‐de‐Souza, D. , Guimaraes, L. , Pradella, F. , Moraes, A. S. , Facchini, G. , Novello, J. C. , & Santos, L. M. (2012). Proteome analysis of spinal cord during the clinical course of monophasic experimental autoimmune encephalomyelitis. Proteomics, 12(17), 2656–2662. [DOI] [PubMed] [Google Scholar]
- Ferrer, I. (2018). Oligodendrogliopathy in neurodegenerative diseases with abnormal protein aggregates: The forgotten partner. Progress in Neurobiology, 169, 24–54. [DOI] [PubMed] [Google Scholar]
- Filipello, F. , Morini, R. , Corradini, I. , Zerbi, V. , Canzi, A. , Michalski, B. , Erreni, M. , Markicevic, M. , Starvaggi‐Cucuzza, C. , Otero, K. , Piccio, L. , Cignarella, F. , Perrucci, F. , Tamborini, M. , Genua, M. , Rajendran, L. , Menna, E. , Vetrano, S. , Fahnestock, M. , … Matteoli, M. (2018). The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity, 48(5), 979–991. [DOI] [PubMed] [Google Scholar]
- Flowers, A. , Bell‐Temin, H. , Jalloh, A. , Stevens, S. M. , & Bickford, P. C. (2017). Proteomic analysis of aged microglia: Shifts in transcription, bioenergetics, and nutrient response. Journal of Neuroinflammation, 14(1), 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco‐Pons, N. , Torrente, M. , Colomina, M. T. , & Vilella, E. (2007). Behavioral deficits in the cuprizone‐induced murine model of demyelination/remyelination. Toxicology Letters, 169(3), 205–213. [DOI] [PubMed] [Google Scholar]
- Franklin, R. J. (2002). Why does remyelination fail in multiple sclerosis? Nature Reviews Neuroscience, 3(9), 705–714. [DOI] [PubMed] [Google Scholar]
- Fuller, M. L. , DeChant, A. K. , Rothstein, B. , Caprariello, A. , Wang, R. , Hall, A. K. , & Miller, R. H. (2007). Bone morphogenetic proteins promote gliosis in demyelinating spinal cord lesions. Annals of Neurology, 62(3), 288–300. [DOI] [PubMed] [Google Scholar]
- Fulmer, C. G. , VonDran, M. W. , Stillman, A. A. , Huang, Y. , Hempstead, B. L. , & Dreyfus, C. F. (2014). Astrocyte‐derived BDNF supports myelin protein synthesis after cuprizone‐induced demyelination. Journal of Neuroscience, 34(24), 8186–8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingele, S. , Henkel, F. , Heckers, S. , Moellenkamp, T. M. , Hümmert, M. W. , Skripuletz, T. , Stangel, M. , & Gudi, V. (2020). Delayed demyelination and impaired remyelination in aged mice in the cuprizone model. Cells, 9(4). 10.3390/cells9040945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux, F. , Lim, S. , Hoeffel, G. , Low, D. , & Huber, T. (2013). Origin and differentiation of microglia. Frontiets in Cellular Neuroscience, 7, 45. 10.3389/fncel.2013.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, J. , Clarner, T. , Beyer, C. , & Kipp, M. (2015). Anatomical distribution of cuprizone‐induced lesions in C57BL6 mice. Journal of Molecular Neuroscience, 57(2), 166–175. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Pena, D. , Nixon, S. E. , O'Connor, J. C. , Southey, B. R. , Lawson, M. A. , McCusker, R. H. , Borras, T. , Machuca, D. , Hernandez, A. G. , Dantzer, R. , Kelley, K. W. , & Rodriguez‐Zas, S. L. (2016). Microglia transcriptome changes in a model of depressive behavior after immune challenge. PLoS One, 11(3), e0150858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabert, K. , Michoel, T. , Karavolos, M. H. , Clohisey, S. , Baillie, J. K. , Stevens, M. P. , Freeman, T. C. , Summers, K. M. , & McColl, B. W. (2016). Microglial brain region‐dependent diversity and selective regional sensitivities to aging. Nature Neuroscience, 19(3), 504–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber, M. B. , & Streit, W. J. (2010). Microglia: Biology and pathology. Acta Neuropathologica, 119(1), 89–105. [DOI] [PubMed] [Google Scholar]
- Gudi, V. , Gingele, S. , Skripuletz, T. , & Stangel, M. (2014). Glial response during cuprizone‐induced de‐ and remyelination in the CNS: Lessons learned. Frontiers in Cellular Neuroscience, 8, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudi, V. , Moharregh‐Khiabani, D. , Skripuletz, T. , Koutsoudaki, P. N. , Kotsiari, A. , Skuljec, J. , Trebst, C. , & Stangel, M. (2009). Regional differences between grey and white matter in cuprizone induced demyelination. Brain Research, 1283, 127–138. [DOI] [PubMed] [Google Scholar]
- Gupta, A. S. , Biswas, D. D. , Brown, L. S. N. , Mockenhaupt, K. , Marone, M. , Hoskins, A. , Siebenlist, U. , & Kordula, T. (2019). A detrimental role of RelB in mature oligodendrocytes during experimental acute encephalomyelitis. Journal of Neuroinflammation, 16(1), 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttenplan, K. A. , Stafford, B. K. , El‐Danaf, R. N. , Adler, D. I. , Münch, A. E. , Weigel, M. K. , Huberman, A. D. , & Liddelow, S. A. (2020). Neurotoxic reactive astrocytes drive neuronal death after retinal injury. Cell Reports, 31(12), 107776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habbas, S. , Santello, M. , Becker, D. , Stubbe, H. , Zappia, G. , Liaudet, N. , Klaus, F. R. , Kollias, G. , Fontana, A. , Pryce, C. R. , Suter, T. , & Volterra, A. (2015). Neuroinflammatory TNFα impairs memory via astrocyte signaling. Cell, 163(7), 1730–1741. 10.1016/j.cell.2015.11.023 [DOI] [PubMed] [Google Scholar]
- Hammond, T. R. , Dufort, C. , Dissing‐Olesen, L. , Giera, S. , Young, A. , Wysoker, A. , Walker, A. J. , Gergits, F. , Segel, M. , Nemesh, J. , Marsh, S. E. , Saunders, A. , Macosko, E. , Ginhoux, F. , Chen, J. , Franklin, R. J. M. , Piao, X. , McCarroll, S. A. , & Stevens, B. (2019). Single‐cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell‐state changes. Immunity, 50(1), 253–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond, T. R. , Gadea, A. , Dupree, J. , Kerninon, C. , Nait‐Oumesmar, B. , Aguirre, A. , & Gallo, V. (2014). Astrocyte‐derived endothelin‐1 inhibits remyelination through notch activation. Neuron, 81(3), 588–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond, T. R. , McEllin, B. , Morton, P. D. , Raymond, M. , Dupree, J. , & Gallo, V. (2015). Endothelin‐B receptor activation in astrocytes regulates the rate of oligodendrocyte regeneration during remyelination. Cell Reports, 13(10), 2090–2097. [DOI] [PubMed] [Google Scholar]
- Harry, G. J. , & Kraft, A. D. (2012). Microglia in the developing brain: A potential target with lifetime effects. Neurotoxicology, 33(2), 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruwaka, K. , Ikegami, A. , Tachibana, Y. , Ohno, N. , Konishi, H. , Hashimoto, A. , Matsumoto, M. , Kato, D. , Ono, R. , Kiyama, H. , Moorhouse, A. J. , Nabekura, J. , & Wake, H. (2019). Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nature Communications, 10(1), 5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan, M. , Min, H. , Rahaman, K. A. , Muresan, A. R. , Kim, H. , Han, D. , & Kwon, O. S. (2019). Quantitative proteome analysis of brain subregions and spinal cord from experimental autoimmune encephalomyelitis mice by TMT‐based mass spectrometry. Proteomics, 19(5), e1800355. [DOI] [PubMed] [Google Scholar]
- Hasel, P. , Rose, I. V. L. , Sadick, J. S. , Kim, R. D. , & Liddelow, S. A. (2021). Neuroinflammatory astrocyte subtypes in the mouse brain. Nature Neuroscience, 24, 1475–1487. [DOI] [PubMed] [Google Scholar]
- Hemmer, B. , Kerschensteiner, M. , & Korn, T. (2015). Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurology, 14(4), 406–419. [DOI] [PubMed] [Google Scholar]
- Hemmer, K. , Fransen, L. , Vanderstichele, H. , Vanmechelen, E. , & Heuschling, P. (2001). An in vitro model for the study of microglia‐induced neurodegeneration: Involvement of nitric oxide and tumor necrosis factor‐alpha. Neurochemistry International, 38(7), 557–565. [DOI] [PubMed] [Google Scholar]
- Henstridge, C. M. , Tzioras, M. , & Paolicelli, R. C. (2019). Glial contribution to excitatory and inhibitory synapse loss in neurodegeneration. Frontiers in Cellular Neuroscience, 13(63). 10.3389/fncel.2019.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbits, N. , Yoshino, J. , Le, T. Q. , & Armstrong, R. C. (2012). Astrogliosis during acute and chronic cuprizone demyelination and implications for remyelination. ASN Neuro, 4(6), 393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks, G. L. , & Franklin, R. J. (2000). Delayed changes in growth factor gene expression during slow remyelination in the CNS of aged rats. Molecular and Cellular Neuroscience, 16(5), 542–556. [DOI] [PubMed] [Google Scholar]
- Hiremath, M. M. , Saito, Y. , Knapp, G. W. , Ting, J. P. , Suzuki, K. , & Matsushima, G. K. (1998). Microglial/macrophage accumulation during cuprizone‐induced demyelination in C57BL/6 mice. Journal of Neuroimmunology, 92(1–2), 38–49. [DOI] [PubMed] [Google Scholar]
- Hochreiter‐Hufford, A. , & Ravichandran, K. S. (2013). Clearing the dead: Apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harbor Perspectives in Biology, 5(1), a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, S. , Beja‐Glasser, V. F. , Nfonoyim, B. M. , Frouin, A. , Li, S. , Ramakrishnan, S. , Merry, K. M. , Shi, Q. , Rosenthal, A. , Barres, B. A. , Lemere, C. A. , Selkoe, D. J. , & Stevens, B. (2016). Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science, 352(6286), 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooijmans, C. R. , Hlavica, M. , Schuler, F. A. F. , Good, N. , Good, A. , Baumgartner, L. , Galeno, G. , Schneider, M. P. , Jung, T. , de Vries, R. , & Ineichen, B. V. (2019). Remyelination promoting therapies in multiple sclerosis animal models: A systematic review and meta‐analysis. Scientific Reports, 9(1), 822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben, E. , Janssens, K. , Hermans, D. , Vandooren, J. , Van den Haute, C. , Schepers, M. , Vanmierlo, T. , Lambrichts, I. , van Horssen, J. , Baekelandt, V. , Opdenakker, G. , Baron, W. , Broux, B. , Slaets, H. , & Hellings, N. (2020). Oncostatin M‐induced astrocytic tissue inhibitor of metalloproteinases‐1 drives remyelination. Proceedings of the National Academy of Sciences, 117(9), 5028–5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga, R. , van der Star, B. J. , Kipp, M. , Jong, R. , Gerritsen, W. , Clarner, T. , Puentes, F. , Dijkstra, C. D. , van der Valk, P. , & Amor, S. (2012). Phagocytosis of neuronal debris by microglia is associated with neuronal damage in multiple sclerosis. Glia, 60(3), 422–431. [DOI] [PubMed] [Google Scholar]
- Irvine, K. A. , & Blakemore, W. F. (2006). Age increases axon loss associated with primary demyelination in cuprizone‐induced demyelination in C57BL/6 mice. Journal of Neuroimmunology, 175(1–2), 69–76. [DOI] [PubMed] [Google Scholar]
- Itoh, N. , Itoh, Y. , Tassoni, A. , Ren, E. , Kaito, M. , Ohno, A. , Ao, Y. , Farkhondeh, V. , Johnsonbaugh, H. , Burda, J. , Sofroniew, M. V. , & Voskuhl, R. R. (2018). Cell‐specific and region‐specific transcriptomics in the multiple sclerosis model: Focus on astrocytes. Proceedings of the National Academy of Sciences, 115(2), 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski, K. A. , Amici, S. A. , Webb, L. M. , Ruiz‐Rosado, J. , Popovich, P. G. , Partida‐Sanchez, S. , & Guerau‐de‐Arellano, M. (2015). Novel markers to delineate murine M1 and M2 macrophages. PLoS One, 10(12), e0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda, E. , Boi, L. , & Carta, A. R. (2018). Microglial phagocytosis and its regulation: A therapeutic target in Parkinson's disease? Frontiers in Molecular Neuroscience, 11(144). 10.3389/fnmol.2018.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay, T. R. , von Saucken, V. E. , Muñoz, B. , Codocedo, J. F. , Atwood, B. K. , Lamb, B. T. , & Landreth, G. E. (2019). TREM2 is required for microglial instruction of astrocytic synaptic engulfment in neurodevelopment. Glia, 67(10), 1873–1892. [DOI] [PubMed] [Google Scholar]
- Joshi, A. U. , Minhas, P. S. , Liddelow, S. A. , Haileselassie, B. , Andreasson, K. I. , Dorn, G. W. , & Mochly‐Rosen, D. (2019). Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nature Neuroscience, 22(10), 1635–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurga, A. M. , Paleczna, M. , & Kuter, K. Z. (2020). Overview of general and discriminating markers of differential microglia phenotypes. Frontiers in Cellular Neuroscience, 14(198). 10.3389/fncel.2020.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens, T. , Jafari, M. , Kreutzfeldt, M. , Bahn, E. , Brück, W. , Kerschensteiner, M. , & Merkler, D. (2015). Reconstruction of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain, 139(1), 39–46. 10.1093/brain/awv353 [DOI] [PubMed] [Google Scholar]
- Kim, H. J. , Miron, V. E. , Dukala, D. , Proia, R. L. , Ludwin, S. K. , Traka, M. , Antel, J. P. , & Soliven, B. (2011). Neurobiological effects of sphingosine 1‐phosphate receptor modulation in the cuprizone model. FASEB Journal, 25(5), 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama, M. , Ueno, M. , Itakura, T. , & Yamashita, T. (2011). Activated microglia inhibit axonal growth through RGMa. PLoS One, 6(9), e25234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocur, M. , Schneider, R. , Pulm, A. K. , Bauer, J. , Kropp, S. , Gliem, M. , Ingwersen, J. , Goebels, N. , Alferink, J. , Prozorovski, T. , Aktas, O. , & Scheu, S. (2015). IFNβ secreted by microglia mediates clearance of myelin debris in CNS autoimmunity. Acta Neuropathologica Communication, 3, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koellhoffer, E. C. , McCullough, L. D. , & Ritzel, R. M. (2017). Old maids: Aging and its impact on microglia function. International Journal of Molecular Sciences, 18(4). 10.3390/ijms18040769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi, H. , & Kiyama, H. (2018). Microglial TREM2/DAP12 signaling: A double‐edged sword in neural diseases. Frontiers in Cellular Neuroscience, 12(206). 10.3389/fncel.2018.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi, H. , Okamoto, T. , Hara, Y. , Komine, O. , Tamada, H. , Maeda, M. , Osako, F. , Kobayashi, M. , Nishiyama, A. , Kataoka, Y. , Takai, T. , Udagawa, N. , Jung, S. , Ozato, K. , Tamura, T. , Tsuda, M. , Yamanaka, K. , Ogi, T. , Sato, K. , & Kiyama, H. (2020). Astrocytic phagocytosis is a compensatory mechanism for microglial dysfunction. EMBO Journal, 39(22), e104464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter, M. R. , Li, W. W. , Zhao, C. , & Franklin, R. J. (2006). Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. Journal of Neuroscience, 26(1), 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauthausen, M. , Saxe, S. , Zimmermann, J. , Emrich, M. , Heneka, M. T. , & Muller, M. (2014). CXCR3 modulates glial accumulation and activation in cuprizone‐induced demyelination of the central nervous system. Journal of Neuroinflammation, 11, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme, N. , Cisbani, G. , Préfontaine, P. , Srour, Y. , Bernier, J. , St‐Pierre, M. K. , Tremblay, M. È. , & Rivest, S. (2018). mCSF‐induced microglial activation prevents myelin loss and promotes its repair in a mouse model of multiple sclerosis. Frontiers in Cellular Neuroscience, 12, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampron, A. , Larochelle, A. , Laflamme, N. , Prefontaine, P. , Plante, M. M. , Sanchez, M. G. , Yong, V. W. , Stys, P. K. , Tremblay, M. È. , & Rivest, S. (2015). Inefficient clearance of myelin debris by microglia impairs remyelinating processes. Journal of Experimental Medicine, 212(4), 481–495. 10.1084/jem.20141656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann, H. , & van Horssen, J. (2016). Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochimica et Biophysica Acta, 1862(3), 506–510. [DOI] [PubMed] [Google Scholar]
- Lee, J.‐H. , Noh, S. , Lee, H. , Lee, S. Y. , Mun, J. Y. , Park, H. , & Chung, W.‐S. (2021). Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature, 590(7847), 612–617. [DOI] [PubMed] [Google Scholar]
- Li, J. , Ramenaden, E. R. , Peng, J. , Koito, H. , Volpe, J. J. , & Rosenberg, P. A. (2008). Tumor necrosis factor alpha mediates lipopolysaccharide‐induced microglial toxicity to developing oligodendrocytes when astrocytes are present. Journal of Neuroscience, 28(20), 5321–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T. , Chen, X. , Zhang, C. , Zhang, Y. , & Yao, W. (2019). An update on reactive astrocytes in chronic pain. Journal of Neuroinflammation, 16(1), 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T. , Liu, T. , Chen, X. , Li, L. , Feng, M. , Zhang, Y. , Wan, L. , Zhang, C. , & Yao, W. (2020). Microglia induce the transformation of A1/A2 reactive astrocytes via the CXCR7/PI3K/Akt pathway in chronic post‐surgical pain. Journal of Neuroinflammation, 17(1), 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Zhang, R. , Hou, X. , Zhang, Y. , Ding, F. , Li, F. , Yao, Y. , & Wang, Y. (2017). Microglia activation triggers oligodendrocyte precursor cells apoptosis via HSP60. Molecular Medicine Reports, 16(1), 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow, S. A. , & Barres, B. A. (2017). Reactive astrocytes: Production, function, and therapeutic potential. Immunity, 46(6), 957–967. [DOI] [PubMed] [Google Scholar]
- Liddelow, S. A. , Guttenplan, K. A. , Clarke, L. E. , Bennett, F. C. , Bohlen, C. J. , Schirmer, L. , Bennett, M. L. , Münch, A. E. , Chung, W. S. , Peterson, T. C. , Wilton, D. K. , Frouin, A. , Napier, B. A. , Panicker, N. , Kumar, M. , Buckwalter, M. S. , Rowitch, D. H. , Dawson, V. L. , Dawson, T. M. , … Barres, B. A. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 541(7638), 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, T. , Zhang, L. , Joo, D. , & Sun, S.‐C. (2017). NF‐κB signaling in inflammation. Signal Transduction and Targeted Therapy, 2(1), 17023. 10.1038/sigtrans.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi, M. , Parolisi, R. , Scaroni, F. , Bonfanti, E. , Gualerzi, A. , Gabrielli, M. , Kerlero de Rosbo, N. , Uccelli, A. , Giussani, P. , Viani, P. , Garlanda, C. , Abbracchio, M. P. , Chaabane, L. , Buffo, A. , Fumagalli, M. , & Verderio, C. (2019). Detrimental and protective action of microglial extracellular vesicles on myelin lesions: Astrocyte involvement in remyelination failure. Acta Neuropathologica, 138(6), 987–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubetzki, C. , Zalc, B. , Williams, A. , Stadelmann, C. , & Stankoff, B. (2020). Remyelination in multiple sclerosis: From basic science to clinical translation. Lancet Neurology, 19(8), 678–688. [DOI] [PubMed] [Google Scholar]
- Lyck, L. , Dalmau, I. , Chemnitz, J. , Finsen, B. , & Schroder, H. D. (2008). Immunohistochemical markers for quantitative studies of neurons and glia in human neocortex. Journal of Histochemistry and Cytochemistry, 56(3), 201–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddaluno, L. , Urwyler, C. , & Werner, S. (2017). Fibroblast growth factors: Key players in regeneration and tissue repair. Development, 144(22), 4047–4060. [DOI] [PubMed] [Google Scholar]
- Makinde, H. M. , Winter, D. R. , Procissi, D. , Mike, E. V. , Stock, A. D. , Kando, M. J. , Gadhvi, G. T. , Droho, S. , Bloomfield, C. L. , Dominguez, S. T. , Mayr, M. G. , Lavine, J. A. , Putterman, C. , & Cuda, C. M. (2020). A novel microglia‐specific transcriptional signature correlates with behavioral deficits in neuropsychiatric lupus. Frontiers in Immunology, 11(230). 10.3389/fimmu.2020.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Małkiewicz, M. A. , Szarmach, A. , Sabisz, A. , Cubała, W. J. , Szurowska, E. , & Winklewski, P. J. (2019). Blood‐brain barrier permeability and physical exercise. Journal of Neuroinflammation, 16(1), 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandolesi, G. , Gentile, A. , Musella, A. , Fresegna, D. , De Vito, F. , Bullitta, S. , Sepman, D. , Marfia, G. A. , & Centonze, D. (2015). Synaptopathy connects inflammation and neurodegeneration in multiple sclerosis. Nature Reviews Neurology, 11(12), 711–724. [DOI] [PubMed] [Google Scholar]
- Manrique‐Hoyos, N. , Jürgens, T. , Grønborg, M. , Kreutzfeldt, M. , Schedensack, M. , Kuhlmann, T. , Schrick, C. , Brück, W. , Urlaub, H. , Simons, M. , & Merkler, D. (2012). Late motor decline after accomplished remyelination: Impact for progressive multiple sclerosis. Annals of Neurology, 71(2), 227–244. [DOI] [PubMed] [Google Scholar]
- Martinez, F. O. , & Gordon, S. (2014). The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Reports, 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzan, D. E. , Brügger‐Verdon, V. , West, B. L. , Liddelow, S. , Samanta, J. , & Salzer, J. L. (2021). Activated microglia drive demyelination via CSF1R signaling. Glia, 69(6), 1583–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, J. L. , Jones, J. J. , Taniike, M. , Morell, P. , Suzuki, K. , & Matsushima, G. K. (2000). Mature oligodendrocyte apoptosis precedes IGF‐1 production and oligodendrocyte progenitor accumulation and differentiation during demyelination/remyelination. Journal of Neuroscience Research, 61(3), 251–262. [DOI] [PubMed] [Google Scholar]
- Mason, J. L. , Xuan, S. , Dragatsis, I. , Efstratiadis, A. , & Goldman, J. E. (2003). Insulin‐like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. Journal of Neuroscience, 23(20), 7710–7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda, T. , Sankowski, R. , Staszewski, O. , Böttcher, C. , Amann, L. , Sagar, Scheiwe, C. , Nessler, S. , Kunz, P. , van Loo, G. , Coenen, V. A. , Reinacher, P. C. , Michel, A. , Sure, U. , Gold, R. , Grün, D. , Priller, J. , Stadelmann, C. , & Prinz, M. (2019). Spatial and temporal heterogeneity of mouse and human microglia at single‐cell resolution. Nature, 566(7744), 388–392. [DOI] [PubMed] [Google Scholar]
- Matejuk, A. , & Ransohoff, R. M. (2020). Crosstalk between astrocytes and microglia: An overview. Frontiers in Immunology, 11(1416). 10.3389/fimmu.2020.01416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, S. J. (2018). Astrocyte heterogeneity in the adult central nervous system. Frontiers in Cellular Neuroscience, 12(401). 10.3389/fncel.2018.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron, V. E. , Boyd, A. , Zhao, J. W. , Yuen, T. J. , Ruckh, J. M. , Shadrach, J. L. , van Wijngaarden, P. , Wagers, A. J. , Williams, A. , Franklin, R. J. M. , & Ffrench‐Constant, C. (2013). M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nature Neuroscience, 16(9), 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, C. S. , Milner, R. , Nishiyama, A. , Frausto, R. F. , Serwanski, D. R. , Pagarigan, R. R. , Whitton, J. L. , Miller, R. H. , & Crocker, S. J. (2011). Astrocytic tissue inhibitor of metalloproteinase‐1 (TIMP‐1) promotes oligodendrocyte differentiation and enhances CNS myelination. Journal of Neuroscience, 31(16), 6247–6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno‐García, A. , Kun, A. , Calero, O. , Medina, M. , & Calero, M. (2018). An overview of the role of lipofuscin in age‐related neurodegeneration. Frontiers in Neuroscience, 12(464). 10.3389/fnins.2018.00464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser, D. M. , & Edwards, J. P. (2008). Exploring the full spectrum of macrophage activation. Nature Reviews Immunology, 8(12), 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrdjen, D. , Pavlovic, A. , Hartmann, F. J. , Schreiner, B. , Utz, S. G. , Leung, B. P. , Lelios, I. , Heppner, F. L. , Kipnis, J. , Merkler, D. , Greter, M. , & Becher, B. (2018). High‐dimensional single‐cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity, 48(2), 380–395. [DOI] [PubMed] [Google Scholar]
- Nack, A. , Brendel, M. , Nedelcu, J. , Daerr, M. , Nyamoya, S. , Beyer, C. , Focke, C. , Deussing, M. , Hoornaert, C. , Ponsaerts, P. , Schmitz, C. , Bartenstein, P. , Rominger, A. , & Kipp, M. (2019). Expression of translocator protein and [18F]‐GE180 ligand uptake in multiple sclerosis animal models. Cells, 8(2), 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natrajan, M. S. , de la Fuente, A. G. , Crawford, A. H. , Linehan, E. , Nuñez, V. , Johnson, K. R. , Wu, T. , Fitzgerald, D. C. , Ricote, M. , Bielekova, B. , & Franklin, R. J. (2015). Retinoid X receptor activation reverses age‐related deficiencies in myelin debris phagocytosis and remyelination. Brain, 138(12), 3581–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave, K.‐A. , & Werner, H. B. (2014). Myelination of the nervous system: Mechanisms and functions. Annual Review of Cell and Developmental Biology, 30(1), 503–533. [DOI] [PubMed] [Google Scholar]
- Neumann, B. , Segel, M. , Chalut, K. J. , & Franklin, R. J. (2019). Remyelination and ageing: Reversing the ravages of time. Multiple Sclerosis Journal, 25(14), 1835–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann, H. , Kotter, M. R. , & Franklin, R. J. (2009). Debris clearance by microglia: An essential link between degeneration and regeneration. Brain, 132(2), 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo, N. , Patel, K. , Isaacson, P. G. , & Naresh, K. N. (2007). Leucocyte common antigen (CD45) and CD5 positivity in an "undifferentiated" carcinoma: A potential diagnostic pitfall. Journal of Clinical Pathology, 60(8), 936–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen, J. C. , Thompson, K. K. , West, B. L. , & Tsirka, S. E. (2018). Csf1R inhibition attenuates experimental autoimmune encephalomyelitis and promotes recovery. Experimental Neurology, 307, 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njenga, M. K. , Coenen, M. J. , DeCuir, N. , Yeh, H. Y. , & Rodriguez, M. (2000). Short‐term treatment with interferon‐alpha/beta promotes remyelination, whereas long‐term treatment aggravates demyelination in a murine model of multiple sclerosis. Journal of Neuroscience Research, 59(5), 661–670. [DOI] [PubMed] [Google Scholar]
- Nugent, A. A. , Lin, K. , van Lengerich, B. , Lianoglou, S. , Przybyla, L. , Davis, S. S. , Llapashtica, C. , Wang, J. , Kim, D. J. , Xia, D. , Lucas, A. , Baskaran, S. , Haddick, P. C. G. , Lenser, M. , Earr, T. K. , Shi, J. , Dugas, J. C. , Andreone, B. J. , Logan, T. , … Di Paolo, G. (2020). TREM2 regulates microglial cholesterol metabolism upon chronic phagocytic challenge. Neuron, 105(5), 837–854. [DOI] [PubMed] [Google Scholar]
- Nutma, E. , Gebro, E. , Marzin, M. C. , van der Valk, P. , Matthews, P. M. , Owen, D. R. , & Amor, S. (2021). Activated microglia do not increase 18 kDa translocator protein (TSPO) expression in the multiple sclerosis brain. Glia, 69(10), 2447–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa, K. , Imai, Y. , Sasaki, Y. , & Kohsaka, S. (2004). Microglia/macrophage‐specific protein Iba1 binds to fimbrin and enhances its actin‐bundling activity. Journal of Neurochemistry, 88(4), 844–856. [DOI] [PubMed] [Google Scholar]
- Olah, M. , Amor, S. , Brouwer, N. , Vinet, J. , Eggen, B. , Biber, K. , & Boddeke, H. W. (2012). Identification of a microglia phenotype supportive of remyelination. Glia, 60(2), 306–321. [DOI] [PubMed] [Google Scholar]
- Orihuela, R. , McPherson, C. A. , & Harry, G. J. (2016). Microglial M1/M2 polarization and metabolic states. British Journal of Pharmacology, 173(4), 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oveland, E. , Ahmad, I. , Lereim, R. R. , Kroksveen, A. C. , Barsnes, H. , Guldbrandsen, A. , Myhr, K. M. , Bø, L. , Berven, F. S. , & Wergeland, S. (2021). Cuprizone and EAE mouse frontal cortex proteomics revealed proteins altered in multiple sclerosis. Scientific Reports, 11(1), 7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst, C. N. , Yang, G. , Ninan, I. , Savas, J. N. , Yates, J. R. , Lafaille, J. J. , Hempstead, B. L. , Littman, D. R. , & Gan, Gan (2013). Microglia promote learning‐dependent synapse formation through brain‐derived neurotrophic factor. Cell, 155(7), 1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge, M. A. , Gopinath, S. , Myers, S. J. , & Coorssen, J. R. (2016). An initial top‐down proteomic analysis of the standard cuprizone mouse model of multiple sclerosis. Journal of Chemical Biology, 9(1), 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petković, F. , Campbell, I. L. , Gonzalez, B. , & Castellano, B. (2016). Astrocyte‐targeted production of interleukin‐6 reduces astroglial and microglial activation in the cuprizone demyelination model: Implications for myelin clearance and oligodendrocyte maturation. Glia, 64(12), 2104–2119. [DOI] [PubMed] [Google Scholar]
- Pfeifenbring, S. , Nessler, S. , Wegner, C. , Stadelmann, C. , & Bruck, W. (2015). Remyelination after cuprizone‐induced demyelination is accelerated in juvenile mice. Journal of Neuropathology and Experimental Neurology, 74(8), 756–766. [DOI] [PubMed] [Google Scholar]
- Piccio, L. , Buonsanti, C. , Cella, M. , Tassi, I. , Schmidt, R. E. , Fenoglio, C. , Rinker, J., II , Naismith, R. T. , Panina‐Bordignon, P. , Passini, N. , Galimberti, D. , Scarpini, E. , Colonna, M. , & Cross, A. H. (2008). Identification of soluble TREM‐2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain, 131(11), 3081–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccio, L. , Buonsanti, C. , Mariani, M. , Cella, M. , Gilfillan, S. , Cross, A. H. , Colonna, M. , & Panina‐Bordignon, P. (2007). Blockade of TREM‐2 exacerbates experimental autoimmune encephalomyelitis. European Journal of Immunology, 37(5), 1290–1301. [DOI] [PubMed] [Google Scholar]
- Plemel, J. R. , Manesh, S. B. , Sparling, J. S. , & Tetzlaff, W. (2013). Myelin inhibits oligodendroglial maturation and regulates oligodendrocytic transcription factor expression. Glia, 61(9), 1471–1487. [DOI] [PubMed] [Google Scholar]
- Poliani, P. L. , Wang, Y. , Fontana, E. , Robinette, M. L. , Yamanishi, Y. , Gilfillan, S. , & Colonna, M. (2015). TREM2 sustains microglial expansion during aging and response to demyelination. Journal of Clinical Investigation, 125(5), 2161–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponath, G. , Park, C. , & Pitt, D. (2018). The role of astrocytes in multiple sclerosis. Frontiers in Immunology, 9, 217. 10.3389/fimmu.2018.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov, A. , Brazhe, A. , Denisov, P. , Sutyagina, O. , Li, L. , Lazareva, N. , Verkhratsky, A. , & Semyanov, A. (2021). Astrocyte dystrophy in ageing brain parallels impaired synaptic plasticity. Aging Cell, 20(3), e13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praet, J. , Guglielmetti, C. , Berneman, Z. , Van der Linden, A. , & Ponsaerts, P. (2014). Cellular and molecular neuropathology of the cuprizone mouse model: Clinical relevance for multiple sclerosis. Neuroscience and Biobehavioural Reviews, 47, 485–505. [DOI] [PubMed] [Google Scholar]
- Procaccini, C. , De Rosa, V. , Pucino, V. , Formisano, L. , & Matarese, G. (2015). Animal models of multiple sclerosis. European Journal of Pharmacology, 759, 182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine, C. S. , Bonetti, B. , & Cannella, B. (1998). Multiple sclerosis: Expression of molecules of the tumor necrosis factor ligand and receptor families in relationship to the demyelinated plaque. Revue Neurologique, 154(8–9), 577–585. [PubMed] [Google Scholar]
- Ransohoff, R. M. (2012). Animal models of multiple sclerosis: The good, the bad and the bottom line. Nature Neuroscience, 15(8), 1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff, R. M. (2016). A polarizing question: do M1 and M2 microglia exist? Nature Neuroscience, 19(8), 987–991. [DOI] [PubMed] [Google Scholar]
- Raposo, C. , Nunes, A. K. , Luna, R. L. , Araujo, S. M. , da Cruz‐Hofling, M. A. , & Peixoto, C. A. (2013). Sildenafil (Viagra) protective effects on neuroinflammation: The role of iNOS/NO system in an inflammatory demyelination model. Mediators in Inflammation, 2013, 321460. 10.1155/2013/321460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawji, K. S. , Gonzalez Martinez, G. A. , Sharma, A. , & Franklin, R. J. M. (2020a). The role of astrocytes in remyelination. Trends in Neuroscience, 43(8), 596–607. [DOI] [PubMed] [Google Scholar]
- Rawji, K. S. , Kappen, J. , Tang, W. , Teo, W. , Plemel, J. R. , Stys, P. K. , & Yong, V. W. (2018). Deficient surveillance and phagocytic activity of myeloid cells within demyelinated lesions in aging mice visualized by ex vivo live multiphoton imaging. The Journal of Neuroscience, 38(8), 1973–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawji, K. S. , Mishra, M. K. , Michaels, N. J. , Rivest, S. , Stys, P. K. , & Yong, V. W. (2016). Immunosenescence of microglia and macrophages: Impact on the ageing central nervous system. Brain, 139(3), 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawji, K. S. , Young, A. M. H. , Ghosh, T. , Michaels, N. J. , Mirzaei, R. , Kappen, J. , Kolehmainen, K. L. , Alaeiilkhchi, N. , Lozinski, B. , Mishra, M. K. , Pu, A. , Tang, W. , Zein, S. , Kaushik, D. K. , Keough, M. B. , Plemel, J. R. , Calvert, F. , Knights, A. J. , Gaffney, D. J. , … Yong, V. W. (2020b). Niacin‐mediated rejuvenation of macrophage/microglia enhances remyelination of the aging central nervous system. Acta Neuropathologica, 139(5), 893–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Régnier‐Vigouroux, A. (2003). The mannose receptor in the brain. International Review of Cytology, 226, 321–342. [DOI] [PubMed] [Google Scholar]
- Remington, L. T. , Babcock, A. A. , Zehntner, S. P. , & Owens, T. (2007). Microglial recruitment, activation, and proliferation in response to primary demyelination. American Journal of Pathology, 170(5), 1713–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, J. , Dai, C. , Zhou, X. , Barnes, J. A. , Chen, X. , Wang, Y. , Yuan, L. , Shingu, T. , Heimberger, A. B. , Chen, Y. , & Hu, J. (2021). Qki is an essential regulator of microglial phagocytosis in demyelination. Journal of Experimental Medicine, 218(1). 10.1084/jem.20190348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner, K. , Hellerbrand, S. , Hermann, F. , Riedhammer, C. , Talke, Y. , Schiechl, G. , Gomez, M. R. , Kutzi, S. , Halbritter, D. , Goebel, N. , Brühl, H. , Weissert, R. , & Mack, M. (2016). IL‐3 promotes the development of experimental autoimmune encephalitis. JCI Insight, 1(16), e87157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, D. , & Moreo, N. (2016). Disease‐modifying therapies in multiple sclerosis: Overview and treatment considerations. Federal Practioner, 33(6), 28–34. [PMC free article] [PubMed] [Google Scholar]
- Robinson, S. , & Miller, R. H. (1999). Contact with central nervous system myelin inhibits oligodendrocyte progenitor maturation. Developmental Biology, 216(1), 359–368. [DOI] [PubMed] [Google Scholar]
- Rosenberger, K. , Dembny, P. , Derkow, K. , Engel, O. , Krüger, C. , Wolf, S. A. , Kettenmann, H. , Schott, E. , Meisel, A. , & Lehnardt, S. (2015). Intrathecal heat shock protein 60 mediates neurodegeneration and demyelination in the CNS through a TLR4‐ and MyD88‐dependent pathway. Molecular Neurodegeneration, 10(1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, S. , Motta, C. , Studer, V. , Barbieri, F. , Buttari, F. , Bergami, A. , Sancesario, G. , Bernardini, S. , de Angelis, G. , Martino, G. , Furlan, R. , & Centonze, D. (2014). Tumor necrosis factor is elevated in progressive multiple sclerosis and causes excitotoxic neurodegeneration. Multiple Sclerosis, 20(3), 304–312. [DOI] [PubMed] [Google Scholar]
- Rothhammer, V. , Borucki, D. M. , Tjon, E. C. , Takenaka, M. C. , Chao, C.‐C. , Ardura‐Fabregat, A. , de Lima, K. A. , Gutiérrez‐Vázquez, C. , Hewson, P. , Staszewski, O. , Blain, M. , Healy, L. , Neziraj, T. , Borio, M. , Wheeler, M. , Dragin, L. L. , Laplaud, D. A. , Antel, J. , Alvarez, J. I. , … Quintana, F. J. (2018). Microglial control of astrocytes in response to microbial metabolites. Nature, 557(7707), 724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino, S. J. , Mayo, L. , Wimmer, I. , Siedler, V. , Brunner, F. , Hametner, S. , Madi, A. , Lanser, A. , Moreira, T. , Donnelly, D. , Cox, L. , Rezende, R. M. , Butovsky, O. , Lassmann, H. , & Weiner, H. L. (2018). Acute microglia ablation induces neurodegeneration in the somatosensory system. Nature Communications, 9(1), 4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaiyan, S. , Besson‐Girard, S. , Kaya, T. , Cantuti‐Castelvetri, L. , Liu, L. , Ji, H. , Schifferer, M. , Gouna, G. , Usifo, F. , Kannaiyan, N. , Fitzner, D. , Xiang, X. , Rossner, M. J. , Brendel, M. , Gokce, O. , & Simons, M. (2021). White matter aging drives microglial diversity. Neuron, 109(7), 1100–1117. [DOI] [PubMed] [Google Scholar]
- Safaiyan, S. , Kannaiyan, N. , Snaidero, N. , Brioschi, S. , Biber, K. , Yona, S. , Edinger, A. L. , Jung, S. , Rossner, M. J. , & Simons, M. (2016). Age‐related myelin degradation burdens the clearance function of microglia during aging. Nature Neuroscience, 19(8), 995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitta, K. S. , Lercher, L. D. , Sainato, D. M. , Patel, A. , Huang, Y. , McAuliffe, G. , & Dreyfus, C. F. (2021). CHPG enhances BDNF and myelination in cuprizone‐treated mice through astrocytic metabotropic glutamate receptor 5. Glia, 69(8), 1950–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariol, A. , Mackin, S. , Allred, M. G. , Ma, C. , Zhou, Y. , Zhang, Q. , Zou, X. , Abrahante, J. E. , Meyerholz, D. K. , & Perlman, S. (2020). Microglia depletion exacerbates demyelination and impairs remyelination in a neurotropic coronavirus infection. Proceedings of the National Academy of Sciences, 117(39), 24464–24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, Y. , Ohsawa, K. , Kanazawa, H. , Kohsaka, S. , & Imai, Y. (2001). Iba1 Is an actin‐cross‐linking protein in macrophages/microglia. Biochemical and Biophysical Research Communications, 286(2), 292–297. [DOI] [PubMed] [Google Scholar]
- Schafer, D. P. , Lehrman, E. K. , Kautzman, A. G. , Koyama, R. , Mardinly, A. R. , Yamasaki, R. , Ransohoff, R. M. , Greenberg, M. E. , Barres, B. A. , & Stevens, B. (2012). Microglia sculpt postnatal neural circuits in an activity and complement‐dependent manner. Neuron, 74(4), 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn, J. R. , & Wilson, C. B. (2007). Regulation of interferon‐gamma during innate and adaptive immune responses. Advances in Immunology, 96, 41–101. [DOI] [PubMed] [Google Scholar]
- Schulz, K. , Kroner, A. , & David, S. (2012). Iron efflux from astrocytes plays a role in remyelination. Journal of Neuroscience, 32(14), 4841–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj, K. W. , & Raine, C. S. (1988). Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Annals of Neurology, 23(4), 339–346. [DOI] [PubMed] [Google Scholar]
- Sen, M. K. , Almuslehi, M. S. M. , Coorssen, J. R. , Mahns, D. A. , & Shortland, P. J. (2020a). Behavioural and histological changes in cuprizone‐fed mice. Brain, Behavior, and Immunity, 87, 508–523. [DOI] [PubMed] [Google Scholar]
- Sen, M. K. , Almuslehi, M. S. M. , Gyengesi, E. , Myers, S. J. , Shortland, P. J. , Mahns, D. A. , & Coorssen, J. R. (2019a). Suppression of the peripheral immune system limits the central immune response following cuprizone‐feeding: Relevance to modelling multiple sclerosis. Cells, 8(11), 1314. 10.3390/cells8111314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, M. K. , Almuslehi, M. S. M. , Shortland, P. J. , Coorssen, J. R. , & Mahns, D. A. (2020b). Revisiting the pathoetiology of multiple sclerosis: Has the tail been wagging the mouse? Frontiers in Immunology, 11, 572186. 10.3389/fimmu.2020.572186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, M. K. , Almuslehi, M. S. M. , Shortland, P. J. , Mahns, D. A. , & Coorssen, J. R. (2021). Proteomics of multiple sclerosis: Inherent issues in defining the pathoetiology and identifying (early) biomarkers. International Journal of Molecular Sciences, 22(14), 7377. 10.3390/ijms22147377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, M. K. , & Hossain, M. J. (2021). Oligodendrocyte‐specific mechanisms of myelin thinning: Implications for neurodegenerative diseases. Frontiers in Neuroscience, 15(290). 10.3389/fnins.2021.663053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, M. K. , Mahns, D. A. , Coorssen, J. R. , & Shortland, P. J. (2019b). Behavioural phenotypes in the cuprizone model of central nervous system demyelination. Neuroscience and Biobehavioral Reviews, 107, 23–46. [DOI] [PubMed] [Google Scholar]
- Shen, K. , Reichelt, M. , Kyauk, R. V. , Ngu, H. , Shen, Y. A. , Foreman, O. , Modrusan, Z. , Friedman, B. A. , Sheng, M. , & Yuen, T. J. (2021). Multiple sclerosis risk gene Mertk is required for microglial activation and subsequent remyelination. Cell Reports, 34(10), 108835. [DOI] [PubMed] [Google Scholar]
- Sierra, A. , Gottfried‐Blackmore, A. C. , McEwen, B. S. , & Bulloch, K. (2007). Microglia derived from aging mice exhibit an altered inflammatory profile. Glia, 55(4), 412–424. [DOI] [PubMed] [Google Scholar]
- Sim, F. J. , Zhao, C. , Penderis, J. , & Franklin, R. J. (2002). The age‐related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. Journal of Neuroscience, 22(7), 2451–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, M. , & Nave, K. A. (2015). Oligodendrocytes: Myelination and axonal support. Cold Spring Harb Perspectives in Biology, 8(1), a020479. 10.1101/cshperspect.a020479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skripuletz, T. , Hackstette, D. , Bauer, K. , Gudi, V. , Pul, R. , Voss, E. , Berger, K. , Kipp, M. , Baumgärtner, W. , & Stangel, M. (2013). Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone‐induced demyelination. Brain, 136(1), 147–167. [DOI] [PubMed] [Google Scholar]
- Skripuletz, T. , Lindner, M. , Kotsiari, A. , Garde, N. , Fokuhl, J. , Linsmeier, F. , Trebst, C. , & Stangel, M. (2008). Cortical demyelination is prominent in the murine cuprizone model and is strain‐dependent. American Journal of Pathology, 172(4), 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin, B. R. , Sarhane, K. A. , von Guionneau, N. , Hanwright, P. J. , Qiu, C. , Mao, H.‐Q. , Höke, A. , & Tuffaha, S. H. (2021). Insulin‐like growth factor‐1: A promising therapeutic target for peripheral nerve injury. Frontiers in Bioengineering and Biotechnology, 9(549). 10.3389/fbioe.2021.695850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaidero, N. , & Simons, M. (2014). Myelination at a glance. Jounral of Cell Science, 127(14), 2999–3004. 10.1242/jcs.151043 [DOI] [PubMed] [Google Scholar]
- Sofroniew, M. V. , & Vinters, H. V. (2010). Astrocytes: Biology and pathology. Acta Neuropathologica, 119(1), 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonar, S. A. , & Lal, G. (2019). The iNOS activity during an immune response controls the CNS pathology in experimental autoimmune encephalomyelitis. Frontiers in Immunology, 10(710). 10.3389/fimmu.2019.00710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulet, D. , & Rivest, S. (2008). Microglia. Current Biology, 18(12), 506–508. 10.1016/j.cub.2008.04.047 [DOI] [PubMed] [Google Scholar]
- Sousa, C. , Golebiewska, A. , Poovathingal, S. K. , Kaoma, T. , Pires‐Afonso, Y. , Martina, S. , Coowar, D. , Azuaje, F. , Skupin, A. , Balling, R. , Biber, K. , Niclou, S. P. , & Michelucci, A. (2018). Single‐cell transcriptomics reveals distinct inflammation‐induced microglia signatures. EMBO Reports, 19(11). 10.15252/embr.201846171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll, G. , Jung, S. , Jander, S. , van der Meide, P. , & Hartung, H. P. (1993). Tumor necrosis factor‐alpha in immune‐mediated demyelination and Wallerian degeneration of the rat peripheral nervous system. Journal of Neuroimmunology, 45(1–2), 175–182. [DOI] [PubMed] [Google Scholar]
- Stys, P. K. , Zamponi, G. W. , van Minnen, J. , & Geurts, J. J. (2012). Will the real multiple sclerosis please stand up? Nature Reviews Neuroscience, 13(7), 507–514. [DOI] [PubMed] [Google Scholar]
- Su, Z. , Yuan, Y. , Chen, J. , Zhu, Y. , Qiu, Y. , Zhu, F. , Huang, A. , & He, C. (2011). Reactive astrocytes inhibit the survival and differentiation of oligodendrocyte precursor cells by secreted TNF‐α. Journal of Neurotrauma, 28(6), 1089–1100. 10.1089/neu.2010.1597 [DOI] [PubMed] [Google Scholar]
- Tang, Y. , & Le, W. (2016). Differential roles of M1 and M2 microglia in neurodegenerative diseases. Molecular Neurobiology, 53(2), 1181–1194. [DOI] [PubMed] [Google Scholar]
- Taylor, S. E. , Morganti‐Kossmann, C. , Lifshitz, J. , & Ziebell, J. M. (2014). Rod microglia: a morphological definition. PLoS One, 9(5), e97096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor, L. S. , Wostradowski, T. , Gingele, S. , Skripuletz, T. , Gudi, V. , & Stangel, M. (2017). The effect of stereotactic injections on demyelination and remyelination: A study in the cuprizone model. Journal of Molecular Neuroscience, 61(4), 479–488. [DOI] [PubMed] [Google Scholar]
- Tian, H. , Cheng, Y. , Zhang, Y. , Bai, X. , Jiang, Y. , Li, J. , Fan, S. , & Ding, H. (2021). 18β‐Glycyrrhetinic acid alleviates demyelination by modulating the microglial M1/M2 phenotype in a mouse model of cuprizone‐induced demyelination. Neuroscience Letters, 871, 135871. [DOI] [PubMed] [Google Scholar]
- Tognatta, R. , Karl, M. T. , Fyffe‐Maricich, S. L. , Popratiloff, A. , Garrison, E. D. , Schenck, J. K. , Abu‐Rub, M. , & Miller, R. H. (2020). Astrocytes are required for oligodendrocyte survival and maintenance of myelin compaction and integrity. Frontiers in Cellular Neuroscience, 14(74). 10.3389/fncel.2020.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town, T. , Nikolic, V. , & Tan, J. (2005). The microglial "activation" continuum: From innate to adaptive responses. Journal of Neuroinflammation, 2, 24. 10.1186/1742-2094-2-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traiffort, E. , Kassoussi, A. , Zahaf, A. , & Laouarem, Y. (2020). Astrocytes and microglia as major players of myelin production in normal and pathological conditions. Frontiers in Cellular Neuroscience, 14(79). 10.3389/fncel.2020.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traka, M. , Arasi, K. , Avila, R. L. , Podojil, J. R. , Christakos, A. , Miller, S. D. , Soliven, B. , & Popko, B. (2010). A genetic mouse model of adult‐onset, pervasive central nervous system demyelination with robust remyelination. Brain, 133(10), 3017–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang, T. , Beggs, S. , & Salter, M. W. (2011). Brain‐derived neurotrophic factor from microglia: A molecular substrate for neuropathic pain. Neuron Glia Biology, 7(1), 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp, B. D. , Ransohoff, R. M. , Fisher, E. , & Rudick, R. A. (1999). Neurodegeneration in multiple sclerosis: Relationship to neurological disability. The Neuroscientist, 5(1), 48–57. [Google Scholar]
- Turvey, M. E. , Koudelka, T. , Comerford, I. , Greer, J. M. , Carroll, W. , Bernard, C. C. , Hoffmann, S. R. , & McColl, P. (2014). Quantitative proteome profiling of CNS‐infiltrating autoreactive CD4+ cells reveals selective changes during experimental autoimmune encephalomyelitis. Journal of Proteome Research, 13(8), 3655–3670. [DOI] [PubMed] [Google Scholar]
- Ugalde, C. L. , Lewis, V. , Stehmann, C. , McLean, C. A. , Lawson, V. A. , Collins, S. J. , & Hill, A. F. (2020). Markers of A1 astrocytes stratify to molecular sub‐types in sporadic Creutzfeldt‐Jakob disease brain. Brain Communications, 2(2). 10.1093/braincomms/fcaa029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchtein, I. D. , Chin, G. , Cho, F. S. , Kelley, K. W. , Miller, J. G. , Chien, E. C. , Liddelow, S. A. , Nguyen, P. T. , Nakao‐Inoue, H. , Dorman, L. C. , Akil, O. , Joshita, S. , Barres, B. A. , Paz, J. T. , Molofsky, A. B. , & Molofsky, A. V. (2018). Astrocyte‐derived interleukin‐33 promotes microglial synapse engulfment and neural circuit development. Science, 359(6381), 1269–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vana, A. C. , Flint, N. C. , Harwood, N. E. , Le, T. Q. , Fruttiger, M. , & Armstrong, R. C. (2007). Platelet‐derived growth factor promotes repair of chronically demyelinated white matter. Journal of Neuropathology and Experimental Neurology, 66(11), 975–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl, R. R. , Itoh, N. , Tassoni, A. , Matsukawa, M. A. , Ren, E. , Tse, V. , Jang, E. , Suen, T. T. , & Itoh, Y. (2019). Gene expression in oligodendrocytes during remyelination reveals cholesterol homeostasis as a therapeutic target in multiple sclerosis. Proceedings of the National Academy of Sciences, 116(20), 10130–10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss, E. V. , Skuljec, J. , Gudi, V. , Skripuletz, T. , Pul, R. , Trebst, C. , & Stangel, M. (2012). Characterisation of microglia during de‐ and remyelination: Can they create a repair promoting environment? Neurobiology of Disease, 45(1), 519–528. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Wang, J. , Wang, J. , Yang, B. , Weng, Q. , & He, Q. (2019). Targeting microglia and macrophages: A potential treatment strategy for multiple sclerosis. Frontiers in Pharmacology, 10(286). 10.3389/fphar.2019.00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. S. , Bi, H. Z. , Chu, S. F. , Dong, Y. X. , He, W. B. , Tian, Y. J. , Zang, Y. D. , Zhang, D. M. , Zhang, Z. , & Chen, N. H. (2020). CZ‐7, a new derivative of claulansine F, promotes remyelination induced by cuprizone by enhancing myelin debris clearance. Brain Research Bulletin, 159, 67–78. [DOI] [PubMed] [Google Scholar]
- Weinhard, L. , di Bartolomei, G. , Bolasco, G. , Machado, P. , Schieber, N. L. , Neniskyte, U. , Exiga, M. , Vadisiute, A. , Raggioli, A. , Schertel, A. , Schwab, Y. , & Gross, C. T. (2018). Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nature Communications, 9(1), 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneburg, S. , Jung, J. , Kunjamma, R. B. , Ha, S. K. , Luciano, N. J. , Willis, C. M. , Gao, G. , Biscola, N. P. , Havton, L. A. , Crocker, S. J. , Popko, B. , Reich, D. S. , & Schafer, D. P. (2020). Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity, 52(1), 167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner, S. R. , Saha, J. K. , Broderick, C. L. , Zhen, E. Y. , Higgs, R. E. , Duffin, K. L. , & Smith, R. C. (2010). Proteomic analysis of demyelinated and remyelinating brain tissue following dietary cuprizone administration. Journal of Molecular Neuroscience, 42(2), 210–225. [DOI] [PubMed] [Google Scholar]
- Wheeler, M. A. , Clark, I. C. , Tjon, E. C. , Li, Z. , Zandee, S. E. J. , Couturier, C. P. , Watson, B. R. , Scalisi, G. , Alkwai, S. , Rothhammer, V. , Rotem, A. , Heyman, J. A. , Thaploo, S. , Sanmarco, L. M. , Ragoussis, J. , Weitz, D. A. , Petrecca, K. , Moffitt, J. R. , Becher, B. , … Quintana, F. J. (2020). MAFG‐driven astrocytes promote CNS inflammation. Nature, 578(7796), 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis, C. M. , Nicaise, A. M. , Bongarzone, E. R. , Givogri, M. , Reiter, C. R. , Heintz, O. , Jellison, E. R. , Sutter, P. A. , TeHennepe, G. , Ananda, G. , Vella, A. T. , & Crocker, S. J. (2020). Astrocyte support for oligodendrocyte differentiation can be conveyed via extracellular vesicles but diminishes with age. Scientific Reports, 10(1), 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylot, B. , Mieczkowski, J. , Niedziolka, S. , Kaminska, B. , & Zawadzka, M. (2019). Csf1 deficiency dysregulates glial responses to demyelination and disturbs CNS white matter Remyelination. Cells, 9(1). 10.3390/cells9010099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, R. , Pan, R. , Shang, C. , Li, X. , Cheng, J. , Xu, J. , & Li, Y. (2020). Translocator protein 18 kDa (TSPO) deficiency inhibits microglial activation and impairs mitochondrial function. Frontiers in Pharmacology, 11(986). 10.3389/fphar.2020.00986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X. , Nagai, J. , & Khakh, B. S. (2020). Improved tools to study astrocytes. Nature Reviews Neuroscience, 21(3), 121–138. [DOI] [PubMed] [Google Scholar]
- Yun, S. P. , Kam, T.‐I. , Panicker, N. , Kim, S. , Oh, Y. , Park, J.‐S. , Kwon, S. H. , Park, Y. J. , Karuppagounder, S. S. , Park, H. , Kim, S. , Oh, N. , Kim, N. A. , Lee, S. , Brahmachari, S. , Mao, X. , Lee, J. H. , Kumar, M. , An, D. , … Ko, H. S. (2018). Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nature Medicine, 24(7), 931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian, J. L. , Xu, L. , Foo, L. C. , Nouri, N. , Zhou, L. , Giffard, R. G. , & Barres, B. A. (2012). Genomic analysis of reactive astrogliosis. Journal of Neuroscience, 32(18), 6391–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan, X. , Li, B. , Zhan, X. , Schlüter, H. , Jungblut, P. R. , & Coorssen, J. R. (2019). Innovating the concept and practice of two‐dimensional gel electrophoresis in the analysis of proteomes at the proteoform level. Proteomes, 7(4). 10.3390/proteomes7040036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, C. , Li, W. W. , & Franklin, R. J. (2006). Differences in the early inflammatory responses to toxin‐induced demyelination are associated with the age‐related decline in CNS remyelination. Neurobiology of Aging, 27(9), 1298–1307. [DOI] [PubMed] [Google Scholar]
- Zheng, J. , Ru, W. , Adolacion, J. R. , Spurgat, M. S. , Liu, X. , Yuan, S. , Liang, R. X. , Dong, J. , Potter, A. S. , Potter, S. S. , Chen, K. , Chen, R. , Varadarajan, N. , & Tang, S.‐J. (2021). Single‐cell RNA‐seq analysis reveals compartment‐specific heterogeneity and plasticity of microglia. iScience, 24(3), 102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Z. V. , & Wong, K. C. G. (2019). Microglial activation and polarization after subarachnoid hemorrhage. Neuroimmunology and Neuroinflammation, 6, 1. 10.20517/2347-8659.2018.52 [DOI] [Google Scholar]
- Zhou, T. , Huang, Z. , Sun, X. , Zhu, X. , Zhou, L. , Li, M. , & He, C. (2017). Microglia polarization with M1/M2 phenotype changes in rd1 mouse model of retinal degeneration. Frontiers in Neuroanatomy, 11(77). 10.3389/fnana.2017.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, K. , Sun, J. , Kang, Z. , Zou, Z. , Wu, G. , & Wang, J. (2016). Electroacupuncture promotes remyelination after cuprizone treatment by enhancing myelin debris clearance. Frontiers in Neuroscience, 10, 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebell, J. M. , Taylor, S. E. , Cao, T. , Harrison, J. L. , & Lifshitz, J. (2012). Rod microglia: Elongation, alignment, and coupling to form trains across the somatosensory cortex after experimental diffuse brain injury. Journal of Neuroinflammation, 9(1), 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A


