Abstract
Background and Objectives
To evaluate whether plasma biomarkers of amyloid (Aβ42/Aβ40), tau (p-tau181 and p-tau231), and neuroaxonal injury (neurofilament light chain [NfL]) detect brain amyloidosis consistently across racial groups.
Methods
Individuals enrolled in studies of memory and aging who self-identified as African American (AA) were matched 1:1 to self-identified non-Hispanic White (NHW) individuals by age, APOE ε4 carrier status, and cognitive status. Each participant underwent blood and CSF collection, and amyloid PET was performed in 103 participants (68%). Plasma Aβ42/Aβ40 was measured by a high-performance immunoprecipitation–mass spectrometry assay. Plasma p-tau181, p-tau231, and NfL were measured by Simoa immunoassays. CSF Aβ42/Aβ40 and amyloid PET status were used as primary and secondary reference standards of brain amyloidosis, respectively.
Results
There were 76 matched pairs of AA and NHW participants (n = 152 total). For both AA and NHW groups, the median age was 68.4 years, 42% were APOE ε4 carriers, and 91% were cognitively normal. AA were less likely than NHW participants to have brain amyloidosis by CSF Aβ42/Aβ40 (22% vs 43% positive; p = 0.003). The receiver operating characteristic area under the curve of CSF Aβ42/Aβ40 status with the plasma biomarkers was as follows: Aβ42/Aβ40, 0.86 (95% CI 0.79–0.92); p-tau181, 0.76 (0.68–0.84); p-tau231, 0.69 (0.60–0.78); and NfL, 0.64 (0.55–0.73). In models predicting CSF Aβ42/Aβ40 status with plasma Aβ42/Aβ40 that included covariates (age, sex, APOE ε4 carrier status, race, and cognitive status), race did not affect the probability of CSF Aβ42/Aβ40 positivity. In similar models based on plasma p-tau181, p-tau231, or NfL, AA participants had a lower probability of CSF Aβ42/Aβ40 positivity (odds ratio 0.31 [95% CI 0.13–0.73], 0.30 [0.13–0.71], and 0.27 [0.12–0.64], respectively). Models of amyloid PET status yielded similar findings.
Discussion
Models predicting brain amyloidosis using a high-performance plasma Aβ42/Aβ40 assay may provide an accurate and consistent measure of brain amyloidosis across AA and NHW groups, but models based on plasma p-tau181, p-tau231, and NfL may perform inconsistently and could result in disproportionate misdiagnosis of AA individuals.
Biomarkers of Alzheimer disease (AD) brain pathology are used by research studies, clinical trials, and memory clinics for a variety of indications, including to determine whether the etiology of cognitive impairment is likely to be related to AD or another cause. Amyloid PET is a well-established technique to determine whether an individual has significant brain amyloidosis that could be causing or contributing to cognitive impairment; however, amyloid PET is expensive and has limited availability.1 CSF biomarkers are also highly accurate predictors of brain amyloidosis and are less expensive, but skilled clinicians are required to perform lumbar puncture (LP) procedures, and some individuals perceive LPs as invasive.2 Several commercial assays can be used to measure concentrations of CSF β-amyloid (Aβ) peptide 42 (Aβ42), Aβ40, total tau (t-tau), and tau phosphorylated at position 181 (p-tau181), and cutoffs consistent with brain amyloidosis have been established.3-5
Biomarker cutoffs for brain amyloidosis have been defined in cohorts largely comprised of non-Hispanic White (NHW) individuals and then applied to all individuals. However, several studies have found lower levels of CSF t-tau and p-tau181 in African American (AA) individuals as compared with NHW individuals, even after adjusting for factors such as age, sex, APOE ε4 carrier status, and cognitive impairment.6-9 Why AA individuals have lower levels of CSF t-tau and p-tau181 is unknown and could be due to differences in medical comorbidities, biological factors, or social determinants of health.8,10,11 Regardless of the underlying reasons, these differences have important implications for the utility of CSF biomarkers. Applying biomarker cutoffs defined in NHW to groups in which the biomarker has not been studied could potentially subject the other groups to additional testing, incorrect medical management, missed opportunities for treatment with AD-specific therapies, and lower enrollment in AD clinical trials.9,12 However, it is also highly problematic to adjust the interpretation of medical tests based on race, especially given the heterogeneity represented within racial groups and the dynamic nature of race because it is a social rather than a biological construct.9,13,14 It would be preferable to use AD biomarkers that perform accurately and consistently across racial and ethnic groups. Alternatively, adjusting for the factors that underlie racial differences in AD biomarkers (e.g., medical comorbidities) may be more valid and generalizable across groups.
Over the past 3 years, there has been rapid development of blood-based biomarkers for AD.15 The PrecivityAD test offered by C2N Diagnostics, which includes highly precise measurement of plasma Aβ42/Aβ40 and APOE proteotype by mass spectrometry, is now available for clinical use.16,17 Multiple plasma p-tau isoforms can also be used as biomarkers of brain amyloidosis, including p-tau181,18,19 p-tau217,20-22 and p-tau231.23 Plasma neurofilament light chain (NfL) may also be useful as a nonspecific marker of neuroaxonal injury.24 It is critical to evaluate whether these assays accurately and consistently predict brain amyloidosis across various racial and ethnic groups. In this study, one of the largest cohorts of AA individuals with CSF biomarker and amyloid PET information was used to examine the relationship of these reference measures of brain amyloidosis with the C2N Diagnostics PrecivityAD assay for plasma Aβ42/Aβ40 as well as Simoa immunoassays for p-tau181, p-tau231, and NfL.
Methods
Participants
This study analyzed samples and data from the Charles F. and Joanne Knight Alzheimer Disease Research Center (ADRC), which includes one of the largest groups of AA individuals in AD research who have undergone CSF collection or amyloid PET. The cohort consists of community-dwelling older adults recruited from the St. Louis area, including participants with and without cognitive impairment, who enrolled in research studies of memory and aging at Washington University in St. Louis. Participants underwent clinical and cognitive assessments using the Uniform Data Set (UDS)25 that includes the Clinical Dementia Rating (CDR)26 and Mini-Mental State Examination.27 The UDS includes the Hollingshead 2 factor index of social position,28 which assigns a social class based on the participant's educational level and the occupation of the head of the participant's household. Presence or absence of hypertension or diabetes was noted by the clinician. Race and sex were self-identified.
Participants with CSF biomarker information and adequate aliquots of plasma available for analysis were considered for inclusion. Each self-identified AA participant was matched 1:1 to a self-identified NHW participant by a computer algorithm. Participants were matched by age at the time of plasma collection (within 2 years), APOE ε4 status (carrier or noncarrier), and cognitive status at the time of plasma collection (cognitively normal [CDR 0] or cognitively impaired [CDR >0]). If >1 NHW participant matched an AA participant, the participant with the closest age was selected.
Standard Protocol Approvals, Registrations, and Patient Consents
Written informed consent was obtained from all participants and their study partners. All procedures were approved by Washington University's Human Research Protection Office.
Genotyping
The APOE genotype was determined by genotyping rs7412 and rs429358 with TaqMan genotyping technology.29 Genetic sex determined by sex chromosome–specific analysis was concordant with gender in all individuals in this cohort.
CSF and Plasma Collection and Analysis
CSF and blood samples from each participant were collected at a single session at approximately 8 am following overnight fasting as previously described.5,30 Concentrations of CSF Aβ40, Aβ42, t-tau, and p-tau181 were measured by chemiluminescent enzyme immunoassay using a fully automated platform (LUMIPULSE G1200; Fujirebio). CSF NfL was measured via commercial ELISA kit (UMAN Diagnostics). Plasma Aβ42 and Aβ40 were measured in the C2N Diagnostics commercial laboratory with the PrecivityAD immunoprecipitation–mass spectrometry assay.16 Plasma p-tau181 and p-tau231 were measured in the Clinical Neurochemistry Laboratory, University of Gothenburg, using in-house Single molecule array (Simoa) assays on an HD-X analyzer (Quanterix), as previously described.19,23 Plasma NfL was measured with Quanterix Nf-Light assay kits at Washington University on a HD-X analyzer. All assays were performed by personnel who were blind to participant information.
Amyloid PET
Participants underwent a dynamic scan with either florbetapir (n = 48) or Pittsburgh compound B (PiB; n = 55) in coordination with a structural MRI scan. Regional data from the 30–60 minutes postinjection window for PiB and the 50–70 minutes window for florbetapir were converted to standardized uptake value ratios (SUVRs) using cerebellar gray as a reference and partial volume corrected using a geometric transfer matrix approach based upon the Freesurfer parcellation.31 Values from regions where amyloid deposition occurs early in AD were averaged together to represent mean cortical SUVR, which was converted to Centiloid using previously published equations.32,33
Statistical Analysis
The significance of differences by self-identified race were evaluated with Wilcoxon rank sum tests for continuous variables and χ2 or Fisher exact tests for categorical variables. The covariate-adjusted significance of racial differences were evaluated using analysis of covariance models with biomarker concentrations as the outcome measure, self-identified race as the predictor variable, and including the covariates of age, sex, APOE ε4 carrier status, and cognitive status (cognitively normal [CDR = 0] or cognitively impaired [CDR > 0]). Models used natural logarithm transformed values for CSF and plasma p-tau181 and NfL, which were positively skewed. Models including the interaction between race and APOE ε4 carrier status were also evaluated.
CSF Aβ42/Aβ40 status was chosen as the primary reference standard for brain amyloidosis because all individuals in the study had both CSF and blood collected at the same session, whereas only a subcohort had an amyloid PET scan performed within 2 years of CSF/blood collection. Positive CSF Aβ42/Aβ40 was defined by a CSF Aβ42/Aβ40 <0.0673, a cutoff that maximally distinguished amyloid PET status in an overlapping cohort with a receiver operating characteristic area under the curve (ROC AUC) of 0.97.34 Amyloid PET positivity was previously defined as a mean cortical SUVR >1.42 for PiB and >1.19 for florbetapir.32,35 Logistic regression models were implemented with CSF Aβ42/Aβ40 or amyloid PET status as the outcome measure and each plasma biomarker as the predictor variable. Covariate adjusted models included self-identified race, sex, age, APOE ε4 carrier status, and cognitive status. Models that in addition included either the interaction between race and APOE ε4 carrier status or race and plasma biomarker levels were evaluated. Differences between ROC AUCs were evaluated using the DeLong test.36
Statistical analyses were implemented using SAS 9.4 (SAS Institute Inc.). Plots were created with GraphPad Prism version 9.2.0 (GraphPad Software). All p values were from 2-sided tests, and results were deemed statistically significant at p < 0.05.
Data Availability
Data are available to qualified investigators upon request to the Knight ADRC (knightadrc.wustl.edu/Research/ResourceRequest.htm).
Results
Participant Characteristics
Based on the inclusion criteria of CSF biomarker information and adequate aliquots of plasma available for analysis, 79 AA and 775 NHW participants were potentially eligible for the study. Each AA participant was matched 1:1 to a NHW participant by age, APOE ε4 carrier status and cognitive status. Three AA participants who could not be matched to a NHW participant were not included in the study. The final study cohort included a total of 152 participants (76 AA and 76 matching NHW) who contributed samples that underwent measurement of plasma biomarkers (see Table 1 for cohort characteristics). An amyloid PET scan was performed within 2 years of plasma collection in 49 AA (64%) and 54 NHW (71%) participants (eTable 1, links.lww.com/WNL/B978). All AA participants identified their ethnicity as non-Hispanic.
Table 1.
Characteristics of the Knight Alzheimer Disease Research Center Matched Cohort
For both the AA and NHW groups, the median age was 68.4 years, 42% carried at least 1 APOE ε4 allele (8% were ε4 homozygotes), and 9% were cognitively impaired as defined by a CDR >0. There was no difference in dementia severity by race as measured by the CDR. Both the AA and NHW groups were well-educated (median of 16 years of education), but the AA group had a slightly lower social position than the NHW group as measured by the Hollingshead 2-factor index of social position (median 2.0 [interquartile range (IQR) 2.0–3.5] vs 2.0 [IQR 1.0–3.0], respectively; p < 0.002). Because the AA and NHW participants had no significant differences in years of education, this suggests that the median occupational level of the head of household in the AA group was lower (e.g., fewer of the AA participants lived in households headed by executives/major professionals). Compared with NHW individuals, AA individuals were more likely to have hypertension (67% vs 45%; p = 0.006) or diabetes (28% vs 5%; p = 0.0003).
CSF and Plasma Biomarkers by Race
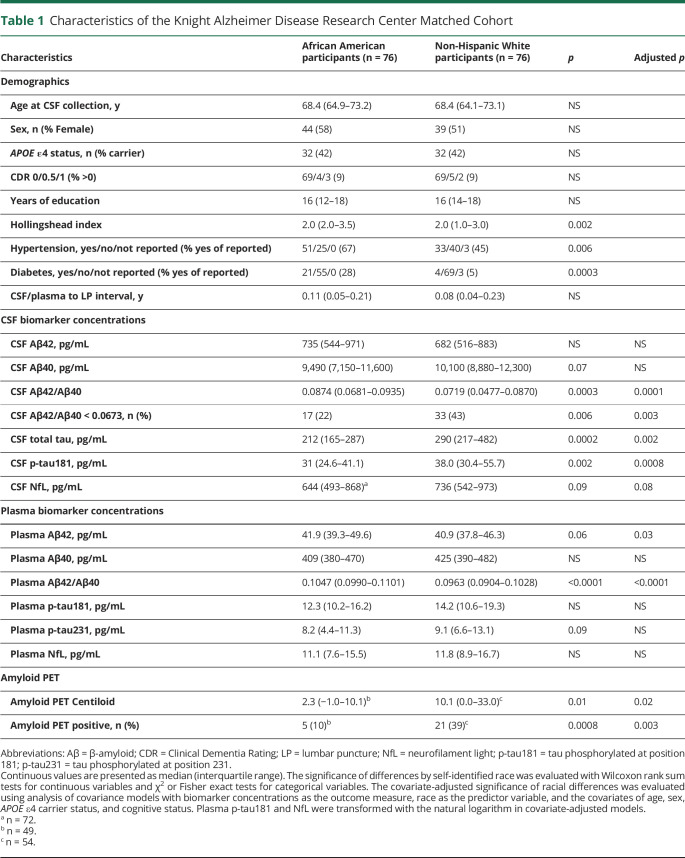
CSF Aβ42 and Aβ40 concentrations were not significantly different between the AA and NHW groups (Table 1). However, AA individuals had higher CSF Aβ42/Aβ40 (median 0.0874 [IQR 0.0681 to 0.0935] vs 0.0719 [0.0477 to 0.0870]; p < 0.0001) and lower amyloid PET Centiloid (median 2.3 [IQR –1.0 to 10.1] vs 10.1 [0.0–33.0]; p = 0.02), consistent with the AA group having lower average levels of brain amyloidosis compared with the NHW group (Figure 1). In the overall cohort, 22% of the AA and 43% of the NHW groups had brain amyloidosis by CSF Aβ42/Aβ40 status (p = 0.003); in the subcohort with amyloid PET, 10% of AA and 39% of the NHW groups had brain amyloidosis by amyloid PET status (p = 0.003). Plasma Aβ42 was only slightly higher in the AA group (p = 0.03) and plasma Aβ40 did not vary by racial group, but plasma Aβ42/Aβ40 was markedly higher in the AA group (median 0.1047 [IQR 0.0990–0.1101] vs 0.0963 [0.0904–0.1028]; p < 0.0001), again consistent with the AA group having lower average levels of brain amyloidosis compared with the NHW group. CSF total tau and p-tau181 were lower in the AA group than the NHW group (p = 0.002 and p = 0.0008, respectively), but there were no statistically significant differences in plasma p-tau181 and p-tau231 between racial groups. There was a trend towards lower CSF NfL in AA compared with NHW individuals (p = 0.08), but there was no difference in plasma NfL by racial group.
Figure 1. Biomarkers by Race.
Biomarkers of amyloid (A), tau (B), and neuroaxonal injury (C) are shown by self-identified race. The covariate-adjusted significance of racial differences was evaluated using analysis of covariance models with biomarker concentrations as the outcome measure, race as the predictor variable, and the covariates of sex, age, APOE ε4 carrier status, and cognitive status. Plasma tau phosphorylated at position 181 (p-tau181) and neurofilament light chain (NfL) were transformed with the natural logarithm for analysis. Point types denote the following: race: red, African American (AA); black, non-Hispanic White (NHW); cognitive status: open circle, Clinical Dementia Rating (CDR) 0; closed square, CDR >0. Aβ = β-amyloid.
Plasma Biomarkers, CSF Aβ42/Aβ40 or Amyloid PET Centiloid, and Race
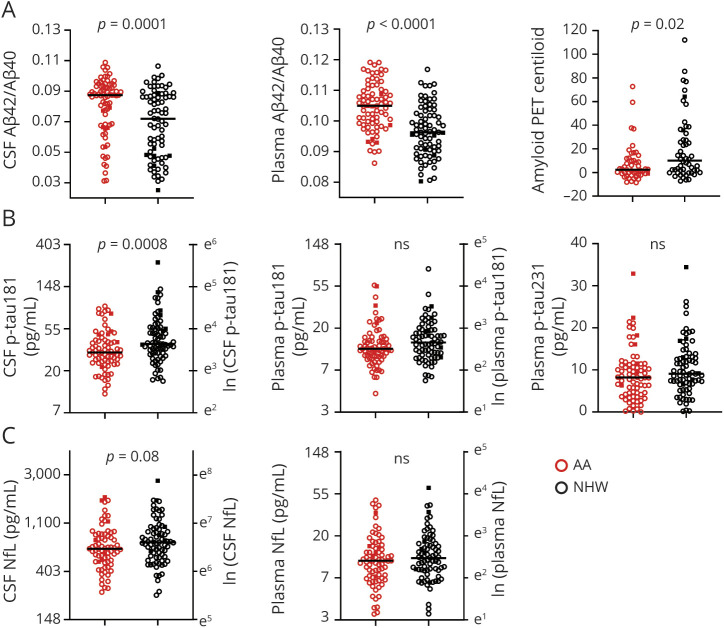
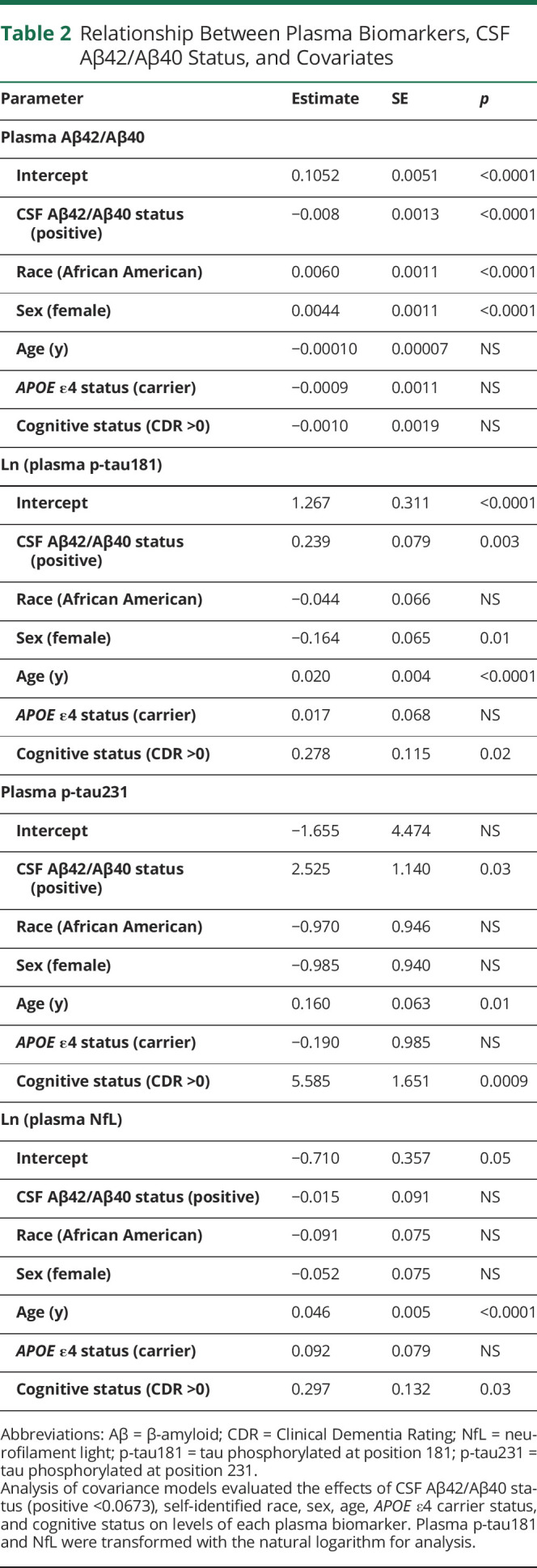
Nonlinear associations between plasma biomarkers and CSF Aβ42/Aβ40 or amyloid PET Centiloid were examined by Spearman correlations, as depicted in Figures 2 and 3 and eFigures 1–2, links.lww.com/WNL/B978 and summarized in eTable 2. Of the plasma biomarkers, Aβ42/Aβ40 had the strongest correlations with CSF Aβ42/Aβ40 (ρ = 0.52 [0.39 to 0.63]) and amyloid PET Centiloid (−0.30 [-0.10 to −0.47]) after adjustment for covariates. To examine the relationships between the plasma biomarkers, brain amyloid, and race, biomarker concentrations were modeled as a function of CSF Aβ42/Aβ40 status and included race, age, sex, APOE ε4 carrier status, and cognitive status as covariates (Table 2). More abnormal (lower) plasma Aβ42/Aβ40 was associated with NHW race (p < 0.0001), male sex (p < 0.0001), and positive CSF Aβ42/Aβ40 status (p < 0.0001). In contrast, more abnormal (higher) plasma p-tau181 levels were associated with older age (p < 0.0001), positive CSF Aβ42/Aβ40 status (p = 0.003), male sex (p = 0.01), and impaired cognitive status (p = 0.02). More abnormal (higher) p-tau231 levels were associated with impaired cognitive status (p = 0.0009), older age (p = 0.01), and positive CSF Aβ42/Aβ40 status (p = 0.03). More abnormal (higher) plasma NfL levels were associated with older age (p < 0.0001) and impaired cognitive status (p = 0.03). Similar models of plasma biomarker levels including amyloid PET status rather than CSF Aβ42/Aβ40 status yielded similar results except that cognitive status was not a significant predictor in any model (eTables 3–6); few participants with cognitive impairment had amyloid PET data (4 of 103), limiting power to detect differences by cognitive status in these models. Models that included the interaction between race and APOE ε4 carrier status were evaluated, but the interaction was not significant for any model and therefore it was not included in the final analyses.
Figure 2. Relationship of Plasma Aβ42/Aβ40 With CSF Aβ42/Aβ40 and Amyloid PET.
The relationship between plasma Aβ42/Aβ40 and CSF Aβ42/Aβ40 (A) or amyloid PET Centiloid (C) was evaluated by partial Spearman correlation and was adjusted for age, sex, APOE ε4 carrier status, self-identified race, and cognitive status. Vertical dotted lines represent cutoff values for amyloid positivity. Plasma Aβ42/Aβ40 for African American (AA) and non-Hispanic White (NHW) groups were evaluated by CSF Aβ42/Aβ40 status (positive <0.0673) (B) or amyloid PET status (D). Cutoff values for plasma Aβ42/Aβ40 with the highest combined sensitivity and specificity for distinguishing amyloid status were selected and are denoted by horizontal dashed lines. The receiver operating characteristic area under the curve (ROC AUC), positive percent agreement (PPA), and negative percent agreement (NPA) are shown. Point types denote the following: race: red, AA; black, NHW; cognitive status: open circle, Clinical Dementia Rating (CDR) 0; closed square, CDR > 0.
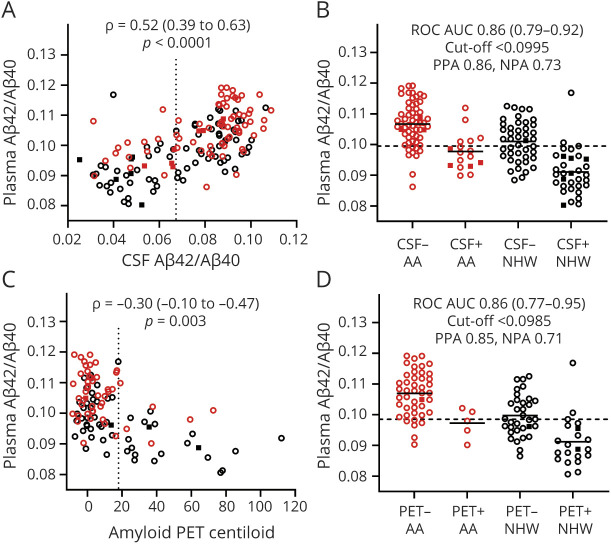
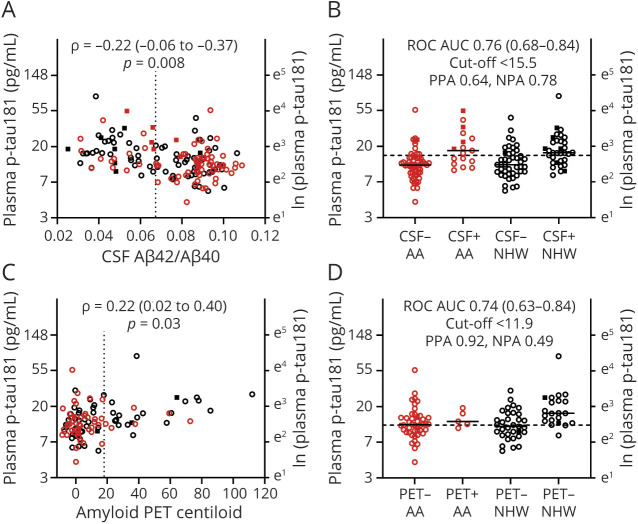
Figure 3. Relationship of Plasma p-tau181 With CSF Aβ42/Aβ40 and Amyloid PET.
Plasma p-tau181 was transformed with the natural logarithm for analysis. The relationship between plasma p-tau181 and CSF Aβ42/Aβ40 (A) or amyloid PET Centiloid (C) was evaluated by partial Spearman correlation and was adjusted for age, sex, APOE ε4 carrier status, self-identified race, and cognitive status. Vertical dotted lines represent cutoff values for amyloid positivity. Plasma p-tau181 levels for African American (AA) and non-Hispanic White (NHW) groups were evaluated by CSF Aβ42/Aβ40 status (positive <0.0673) (B) or amyloid PET status (D). Cutoff values for plasma p-tau181 with the highest combined sensitivity and specificity for distinguishing amyloid status were selected and are denoted by horizontal dashed lines. The receiver operating characteristic area under the curve (ROC AUC), positive percent agreement (PPA), and negative percent agreement (NPA) are shown. Point types denote the following: race: red, AA; black, NHW; cognitive status: open circle, Clinical Dementia Rating (CDR) 0; closed square, CDR > 0.
Table 2.
Relationship Between Plasma Biomarkers, CSF Aβ42/Aβ40 Status, and Covariates
Correspondence of Plasma Biomarkers With CSF Aβ42/Aβ40 and Amyloid PET Status
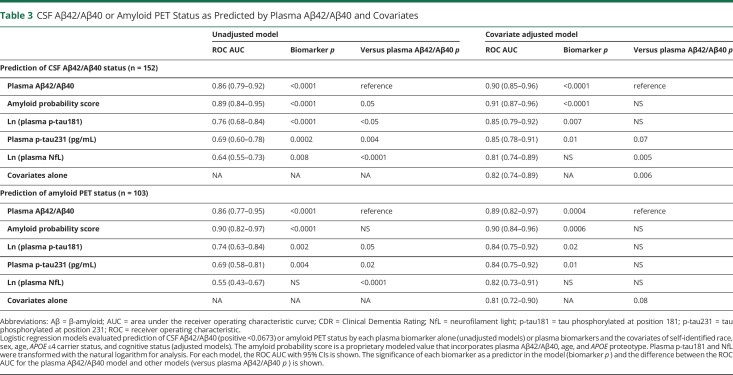
Prediction of CSF Aβ42/Aβ40 or amyloid PET status by plasma biomarkers was evaluated by logistic regression analyses, as depicted in Figures 2 and 3 and eFigures 1 and 2, links.lww.com/WNL/B978, shown in eTables 7–11, and summarized in Tables 3 and 4. Models predicting CSF Aβ42/Aβ40 status based on plasma biomarker levels had ROC AUCs as follows: Aβ42/Aβ40, 0.86 (95% CI 0.79–0.92); p-tau181, 0.76 (0.68–0.84); p-tau231, 0.69 (0.60–0.78); and NfL, 0.64 (0.55–0.73). The amyloid probability score, a proprietary modeled value provided by C2N Diagnostics that is based on plasma Aβ42/Aβ40, APOE proteotype and age,17 had an ROC AUC of 0.89 (0.84–0.95) with CSF Aβ42/Aβ40 status. Comparisons of ROC AUCs showed that plasma Aβ42/Aβ40 had significantly better prediction of CSF Aβ42/Aβ40 status compared with p-tau181, p-tau231, and NfL (p < 0.05, 0.004, and <0.0001, respectively; Table 3).
Table 3.
CSF Aβ42/Aβ40 or Amyloid PET Status as Predicted by Plasma Aβ42/Aβ40 and Covariates
Covariate adjusted models of CSF Aβ42/Aβ40 status incorporating each plasma biomarker and covariates (age, sex, APOE ε4 carrier status, race, and cognitive status) are summarized in Table 4. The model based on plasma Aβ42/Aβ40 had an ROC AUC of 0.90 (0.85–0.96) (eTable 7, links.lww.com/WNL/B978), which was superior to a model of covariates alone (0.82 [0.74–0.89] (eTable 12); p = 0.006 for difference in ROC AUCs). In the model of CSF Aβ42/Aβ40 status incorporating plasma Aβ42/Aβ40 and covariates, a higher probability of CSF Aβ42/Aβ40 positivity was associated with APOE ε4 carriers (odds ratio [OR] 5.6 [95% CI 2.0–16]; p = 0.001), older age in years (OR 1.12 [1.03–1.21]; p = 0.007), and cognitive impairment (OR 9.2 [1.9–46]; p = 0.007). Notably, in models incorporating plasma Aβ42/Aβ40 and covariates, race did not significantly affect correspondence with CSF Aβ42/Aβ40 or amyloid PET status.
Table 4.
CSF Aβ42/Aβ40 Status as Predicted by Plasma Biomarkers and Covariates
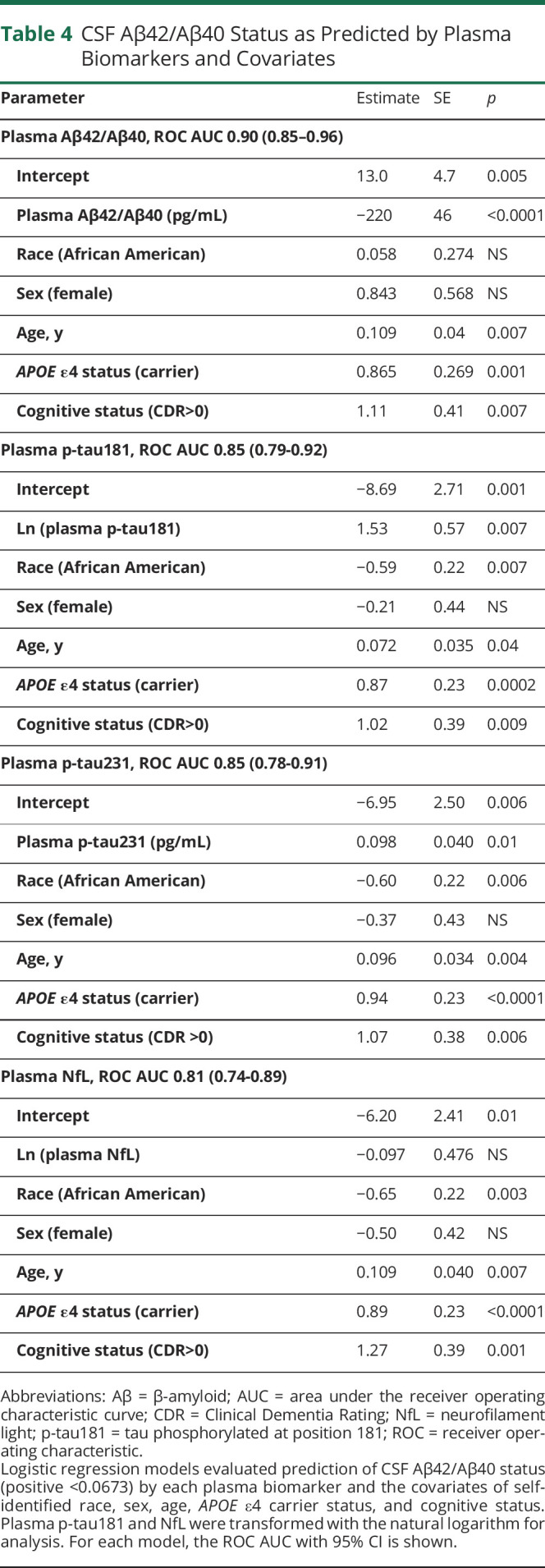
The covariate-adjusted model for CSF Aβ42/Aβ40 status based on p-tau181 had an ROC AUC of 0.85 (0.79–0.92) (Table 4 and eTable 9, links.lww.com/WNL/B978). In this model, a higher probability of CSF Aβ42/Aβ40 positivity was associated with APOE ε4 carriers (OR 5.7 [2.3–14]; p = 0.0002), cognitive impairment (OR 7.7 [1.7–36]; p = 0.009), and older age in years (OR 1.08 [1.00–1.15]; p = 0.04); AA race was associated with a lower probability of positivity (OR 0.31 [0.13–0.73]; p = 0.007). Models of CSF Aβ42/Aβ40 or amyloid PET status based on p-tau231 (eTable 10) or NfL (eTable 11) were also evaluated and are summarized in Table 4.
A model of CSF Aβ42/Aβ40 status based only on covariates demonstrated that AA race was associated with a lower probability of CSF Aβ42/Aβ40 positivity (OR 0.27 [0.12–0.64]; p = 0.003) (eTable 12, links.lww.com/WNL/B978). AA race significantly decreased the probability of CSF Aβ42/Aβ40 positivity in models based on plasma p-tau181 (OR 0.31 [0.13–0.73]; p = 0.007), p-tau231 (OR 0.30 [0.13–0.71]; p = 0.006), or NfL (OR 0.27 [0.12–0.64]; p = 0.003) levels. Consistent with these results, AA race decreased the probability of amyloid PET positivity in models including plasma p-tau181 (OR 0.19 [0.06–0.63]; p = 0.007), p-tau231 (OR 0.17 [0.05–0.59]; p = 0.005), or NfL (OR 0.17 [0.05–0.55]; p = 0.003) levels (eTables 9–11, respectively). In contrast, race did not affect the probability of CSF Aβ42/Aβ40 or amyloid PET positivity associated with plasma Aβ42/Aβ40 (eTable 7). Models of CSF Aβ42/Aβ40 status including only cognitively normal individuals (91% of cohort) showed the same major findings as models that included the entire cohort (eTable 13). Models of CSF Aβ42/Aβ40 status were also evaluated that incorporated either the interaction between race and APOE ε4 carrier status or race and plasma biomarker levels, but neither interaction was significant for any model and therefore the interactions were not included in the final analyses.
Combining Plasma Biomarkers
A model of CSF Aβ42/Aβ40 status including levels of all plasma biomarkers and covariates had an ROC AUC of 0.92 (0.88–0.96), which was not significantly different from the ROC AUC of the model including Aβ42/Aβ40 as the only plasma biomarker (eTable 14, links.lww.com/WNL/B978). In the model with all plasma biomarkers, plasma Aβ42/Aβ40 was the only biomarker that was a significant predictor (p < 0.0001): plasma p-tau181, p-tau231, and NfL were not significant predictors of CSF Aβ42/Aβ40 after adjusting for the effects of plasma Aβ42/Aβ40 and covariates. In a similar model of amyloid PET status, plasma Aβ42/Aβ40 and plasma NfL levels were both significant predictors (p = 0.0004 and p = 0.007, respectively). In models of CSF Aβ42/Aβ40 or amyloid PET status with all plasma biomarkers and covariates (including plasma Aβ42/Aβ40), race was not a significant predictor.
Discussion
This study found that the C2N Diagnostics PrecivityAD plasma Aβ42/Aβ40 assay more accurately classified CSF Aβ42/Aβ40 or amyloid PET status compared with Simoa-based assays for plasma p-tau181, p-tau231, and NfL in a mostly cognitively normal cohort of matched AA and NHW research participants. Self-identified race did not affect prediction of CSF Aβ42/Aβ40 or amyloid PET status by plasma Aβ42/Aβ40. However, AA individuals had a significantly lower probability of CSF or amyloid PET positivity compared with NHW in models incorporating plasma p-tau181, p-tau231, or NfL levels, suggesting that predictive algorithms for these assays would perform inconsistently across racial groups and that applying cutoffs established in NHW individuals to AA individuals could lead to disproportionate misdiagnosis of AA.
Plasma biomarkers have been almost exclusively studied in non-Hispanic White cohorts, with little data available on the performance of these biomarkers in other groups. A recent study of a multiracial cohort found good performance of plasma p-tau217 in distinguishing clinical, pathologic, and amyloid PET status, but performance of the assay in predicting amyloid PET status across racial groups could not be ascertained because only 40 individuals had amyloid PET data.37 Another study found that plasma p-tau181 and plasma p-tau181/Aβ42 were associated with brain amyloidosis and hippocampal atrophy in a Singaporean AD cohort with high burden of cerebrovascular disease, but it did not investigate potential plasma biomarker differences across racial groups.38 Plasma NfL has been studied in a large Latino cohort, but amyloid PET data were only available in a relatively small subset of participants.39 To reduce racial disparities in research and clinical care, it is important to confirm that plasma biomarker assays have accurate and consistent performance in identifying amyloid status across racial and ethnic groups.
Comparing the absolute values of biomarkers corrected for covariates may be misleading in evaluating which biomarkers perform consistently across racial groups. For example, in this study AA individuals had higher average plasma Aβ42/Aβ40 compared with NHW individuals, but this reflected lower levels of brain amyloidosis in AA individuals and did not affect the probability of CSF Aβ42/Aβ40 positivity associated with a given plasma Aβ42/Aβ40 value. In contrast, plasma p-tau181 levels did not vary by race, but AA individuals were less likely to be amyloid positive at a given plasma p-tau181 value. Without a comparison to reference standards, investigators might have concluded that plasma Aβ42/Aβ40 was more variable across racial groups and that p-tau isoforms were more consistent, when in fact plasma Aβ42/Aβ40 was accurately detecting differences in brain amyloidosis by racial group. Confirming that plasma biomarker assays have accurate and consistent performance in identifying amyloid status across racial and ethnic groups requires comparison with a reference standard, and not just covariate-adjusted models of absolute levels.
Previous studies have found an inconsistent relationship between amyloid biomarkers and race. One study found that AA individuals had higher measures of amyloid PET40; another recent study found the opposite result.12 Some studies have found no differences in CSF Aβ42 levels by racial group,6-8 but the current findings demonstrate that CSF Aβ42 alone may not reveal significant racial differences that are apparent when CSF Aβ42/Aβ40 is evaluated. The inconsistent relationship between race and amyloid biomarkers could reflect variation in recruitment methods: NHW and AA individuals are often recruited differently (e.g., NHW are more often referred by health care providers and AA individuals are more often referred by community contacts).41,42 Recruitment differences could result in racial groups having significantly different comorbidities, social determinants of health, or frequencies of brain amyloidosis. Potential differences in brain amyloidosis by racial group again suggest that comparison of plasma biomarkers with a reference standard, rather than comparison of absolute values, may be more helpful in establishing which plasma biomarker assays are accurate and consistent across racial groups.
One important issue in the fluid biomarker field is that different assays for plasma analytes have widely varying performance. A recent head-to-head comparison of 8 different plasma Aβ42/Aβ40 assays found ROC AUCs with CSF Aβ42/Aβ40 status ranging from a maximum of 0.86 for the Washington University assay that is the basis for the C2N assay used in this study down to a minimum of 0.69 for some immunoassays (0.50 is chance alone).43 In another head-to-head comparison study, different p-tau assays yielded somewhat different findings, even for the same p-tau isoform.44 The differences in assay performance complicate comparisons of the relationship of different biomarker analytes to factors such as race. For example, it is unclear whether the probability of CSF Aβ42/Aβ40 or amyloid PET positivity would be affected by race in models incorporating plasma p-tau181, p-tau231, or p-tau217 measured with higher performing assays (e.g., ROC AUC of >0.85 with CSF Aβ42/Aβ40 or amyloid PET status). Performance of plasma assays may vary markedly in prediction of brain amyloidosis depending on the study cohort. For example, the p-tau181 assay used in the current study performed very well in predicting amyloid PET status in a cohort including both cognitively normal and cognitively impaired individuals (ROC AUC 0.88),19 but the performance was lower when predicting amyloid PET status in cognitively normal individuals (ROC AUC 0.82).45 Overall, use of consistently high-performing assays is needed to make accurate conclusions about comparative associations of biomarkers.
Although this study made use of one of the largest AD research cohorts with CSF and amyloid PET data, there are major limitations in the conclusions. Individuals enrolled in this study were primarily from the greater St. Louis metropolitan area and individuals from other geographic regions may vary in key characteristics such as medical comorbidities or social determinants of health. The very small number of individuals with cognitive impairment (7 of 76 in each group) was not sufficient to allow analysis of the relationships between cognitive impairment, race, and biomarker levels. This study of 76 matched pairs of individuals, in which 6 variables had significant effects, was also not sufficiently powered to evaluate the underlying reasons for the racial differences. The Hollingshead index of social position demonstrated that AA individuals had a slightly lower social position compared with NHW individuals. However, this measure does not capture the complex social factors that may underlie biomarker differences between the groups. AA individuals had a higher rate of hypertension and diabetes compared with NHW individuals, but the relatively small cohort did not permit a detailed investigation of these effects. For example, only 4 NHW individuals had diabetes, which does not permit analysis of race by diabetes interactions. Although this study is insufficiently powered or does not have the data available to answer many important questions, it does document racial differences in plasma biomarkers that could potentially lead to clinical misdiagnosis, bias clinical trials that use a biomarker cutoff for inclusion,12,46 and affect interpretation of biomarkers as a secondary end point. These findings should encourage investigators to evaluate the performance of plasma biomarker assays in diverse cohorts. This report strengthens the justification for the creation of large, diverse cohorts that are adequately powered to evaluate the underlying reasons for racial differences.
It is critical to understand that biomarker differences associated with race likely reflect differences in medical comorbidities, social determinants of health, or the effects of systemic racism rather than inherent biological differences.10 For example, in this study cohort there were differences in the rates of hypertension and diabetes by racial group, and recent work has demonstrated that major medical comorbidities such as heart and kidney disease may affect plasma biomarker levels.47 AD research cohorts have traditionally not collected detailed information about social determinants of health such as economic stability, access to healthy foods, neighborhood safety, and quality of education that may be associated with dementia; the importance of these factors is now gaining greater recognition.48 The greater accessibility and acceptance of blood-based AD biomarkers may enable creation of larger cohorts and increased inclusion of groups, such as AA individuals, that have been underrepresented in AD biomarker studies.49 Much larger longitudinal studies of diverse cohorts are needed to evaluate the intersection of race, AD biomarkers, cognitive impairment, medical comorbidities, and social determinants of health.50 Improved understanding of these complex factors will enable more accurate AD diagnosis and improve patient care for all groups.
Acknowledgment
The authors thank the research volunteers who participated in the studies from which these data were obtained and their families and the Clinical, Fluid Biomarker and Imaging Cores at the Knight Alzheimer Disease Research Center for sample and data collection.
Glossary
- AA
African American
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADRC
Alzheimer Disease Research Center
- AUC
area under the curve
- CDR
Clinical Dementia Rating
- IQR
interquartile range
- LP
lumbar puncture
- NfL
neurofilament light chain
- NHW
non-Hispanic White
- OR
odds ratio
- p-tau181
tau phosphorylated at position 181
- PiB
Pittsburgh compound B
- ROC
receiver operating characteristic
- SUVR
standardized uptake value ratio
- t-tau
total tau
- UDS
Uniform Data Set
Appendix. Authors
Study Funding
This study was supported by National Institute on Aging grants R01AG070941 (S.E.S.), K23AG053426 (S.E.S.), P30AG066444 (J.C.M.), P01AG003991 (J.C.M.), P01AG026276 (J.C.M.), R01AG067505 (C.X.), and RF1R01AG053550 (C.X.) and the Cure Alzheimer's Fund (K.L.M.). C2N Diagnostics provided the plasma Aβ42/Aβ40 assays for this study. T.K.K. was funded by the Alzheimer's Association Research Fellowship (850325), the BrightFocus Foundation (A2020812F), the International Society for Neurochemistry's Career Development Grant, the Swedish Alzheimer Foundation (Alzheimerfonden; AF-930627), the Swedish Brain Foundation (Hjärnfonden; FO2020-0240), the Swedish Dementia Foundation (Demensförbundet), the Swedish Parkinson Foundation (Parkinsonfonden), the Gamla Tjänarinnor Foundation, the Aina (Ann) Wallströms and Mary-Ann Sjöbloms Foundation, the Agneta Prytz-Folkes & Gösta Folkes Foundation (2020-00124), the Gun and Bertil Stohnes Foundation, and the Anna Lisa and Brother Björnsson's Foundation. H.Z. is a Wallenberg Scholar supported by grants from the Swedish Research Council (2018–02532), the European Research Council (681712), Swedish State Support for Clinical Research (ALFGBG-720931), the Alzheimer Drug Discovery Foundation (ADDF) USA (201809–2016862), the AD Strategic Fund and the Alzheimer's Association (ADSF-21-831376-C, ADSF-21-831381-C, and ADSF-21-831377-C), the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (FO2019-0228), the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement 860197 (MIRIADE), and the UK Dementia Research Institute at UCL. K.B. is supported by the Swedish Research Council (2017-00915), the Swedish Alzheimer Foundation (AF-742881), Hjärnfonden, Sweden (FO2017-0243), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (ALFGBG-715986), and the Alzheimer's Association 2021 Zenith Award (ZEN-21-848,495).
Disclosure
S.E. Schindler has received data on behalf of Washington University from C2N Diagnostics at no cost. T.K. Karikari, N.J. Ashton, and R.L. Henson report no disclosures relevant to the manuscript. K.E. Yarasheski, T. West, M.R. Meyer, and K.M. Kirmess are employees of C2N Diagnostics, which offers the PrecivityAD test described in this article. Y. Li, B. Saef, K.L. Moulder, and D. Bradford report no disclosures relevant to the manuscript. A.M. Fagan has received research funding from Biogen, Centene, Fujirebio, and Roche Diagnostics; is a member of the scientific advisory boards for Roche Diagnostics, Genentech, and Diadem; and consults for DiamiR and Siemens Healthcare Diagnostics Inc. B.A. Gordon reports no disclosures relevant to the manuscript. T.L.S. Benzinger has investigator-initiated research funding from the NIH, the Alzheimer's Association, the Barnes-Jewish Hospital Foundation, and Avid Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly); participates as a site investigator in clinical trials sponsored by Avid Radiopharmaceuticals, Eli Lilly, Biogen, Eisai, Janssen, and Roche; serves as an unpaid consultant to Eisai and Siemens; and is on the speaker's bureau for Biogen. J. Balls-Berry is a member of the patient advisory board and receives financial support for the Dartmouth University project Implementation of Uterine Fibroid Option Grid Patient Decision Aids Across Five Organizational Settings (UPFRONT; NCT03985449). R.J. Bateman co-founded C2N Diagnostics, receives income from C2N Diagnostics for serving on the scientific advisory board, and consults for Roche, Genentech, AbbVie, Pfizer, Boehringer-Ingelheim, and Merck. Dr. Bateman and Washington University have equity ownership interest in C2N Diagnostics and receive royalty income based on technology (stable isotope labeling kinetics and blood plasma assay) licensed by Washington University to C2N Diagnostics. Washington University, with Dr. Bateman as coinventor, has submitted the US provisional patent application “Plasma Based Methods for Detecting CNS Amyloid Deposition.” C. Xiong consults for Diadem. H. Zetterberg has served on scientific advisory boards and/or as a consultant for Alector, Eisai, Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, Nervgen, AZTherapies, CogRx, and Red Abbey Labs; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, and Biogen; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. K. Blennow has served as a consultant, on advisory boards, or on data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Prothena, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. J.C. Morris is Chair of the Research Strategy Council of the Cure Alzheimer's Fund. Go to Neurology.org/N for full disclosures.
References
- 1.Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. Alzheimers Dement. 2013;9:e1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw LM, Arias J, Blennow K, et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer's disease. Alzheimers Dement. 2018;14:1505-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplow J, Vandijck M, Gray J, et al. Concordance of Lumipulse cerebrospinal fluid t-tau/Abeta42 ratio with amyloid PET status. Alzheimers Dement. 2020;16:144-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer's disease concord with amyloid-beta PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 2018;14:1470-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schindler SE, Gray JD, Gordon BA, et al. Cerebrospinal fluid biomarkers measured by Elecsys assays compared with amyloid imaging. Alzheimers Dement. 2018;14:1460-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howell JC, Watts KD, Parker MW, et al. Race modifies the relationship between cognition and Alzheimer's disease cerebrospinal fluid biomarkers. Alzheimers Res Ther. 2017;9(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris JC, Schindler SE, McCue LM, et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. 2019;76(3):264-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schindler SE, Cruchaga C, Joseph A, et al. African Americans have differences in CSF soluble TREM2 and associated genetic variants. Neurol Genet. 2021;7(2):e571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrett SL, McDaniel D, Obideen M, et al. Racial disparity in cerebrospinal fluid amyloid and tau biomarkers and associated cutoffs for mild cognitive impairment. JAMA Netw Open. 2019;2(12):e1917363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on ethnic and racial disparities in Alzheimer's disease and related dementias: update and areas of immediate need. Alzheimers Dement. 2019;15:292-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meeker KL, Wisch JK, Hudson D, et al. Socioeconomic status mediates racial differences seen using the AT(N) framework. Ann Neurol. 2021;89(2):254-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deters KD, Napolioni V, Sperling RA, et al. Amyloid PET imaging in self-identified non-Hispanic black participants of the Anti-Amyloid in Asymptomatic Alzheimer's Disease (A4) study. Neurology. 2021;96(11):e1491-e1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powe NR. Black kidney function matters: use or misuse of race? JAMA. 2020;324:737-738. [DOI] [PubMed] [Google Scholar]
- 14.Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight: reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383(9):874-882. [DOI] [PubMed] [Google Scholar]
- 15.Ashton NJ, Leuzy A, Karikari TK, et al. The validation status of blood biomarkers of amyloid and phospho-tau assessed with the 5-phase development framework for AD biomarkers. Eur J Nucl Med Mol Imaging. 2021;48(7):2140-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirmess KM, Meyer MR, Holubasch MS, et al. The PrecivityAD™ test: accurate and reliable LC-MS/MS assays for quantifying plasma amyloid beta 40 and 42 and apolipoprotein E proteotype for the assessment of brain amyloidosis. Clin Chim Acta. 2021;519:267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West T, Kirmess KM, Meyer MR, et al. A blood-based diagnostic test incorporating plasma Abeta42/40 ratio, ApoE proteotype, and age accurately identifies brain amyloid status: findings from a multi cohort validity analysis. Mol Neurodegener. 2021;16:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moscoso A, Grothe MJ, Ashton NJ, et al. Time course of phosphorylated-tau181 in blood across the Alzheimer's disease spectrum. Brain. 2021;144(1):325-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19:422-433. [DOI] [PubMed] [Google Scholar]
- 20.Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324(8):772-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barthélemy NR, Horie K, Sato C, Bateman RJ. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer's disease. J Exp Med. 2020;217(11):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thijssen EH, La Joie R, Strom A, et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer's disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol. 2021;20(9):739-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashton NJ, Pascoal TA, Karikari TK, et al. Plasma p-tau231: a new biomarker for incipient Alzheimer's disease pathology. Acta Neuropathol. 2021;141(5):709-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preische O, Schultz S, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med. 2019;25:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris JC, Weintraub S, Chui HC, et al. The Uniform data Set (UDS): clinical and cognitive variables and descriptive data from alzheimer disease centers. Alzheimer Dis Assoc Disord. 2006;20:210-216. [DOI] [PubMed] [Google Scholar]
- 26.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412-2414. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198. [DOI] [PubMed] [Google Scholar]
- 28.Hollingshead Two Factor Index of Social Position, 5th ed. Sage Publications; 1991. [Google Scholar]
- 29.Cruchaga C, Kauwe JS, Mayo K, et al. SNPs associated with cerebrospinal fluid phospho-tau levels influence rate of decline in Alzheimer's disease. PLoS Genet. 2010;6(9):e1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schindler SE, Bollinger JG, Ovod V, et al. High-precision plasma beta-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93:e1647-e1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su Y, Blazey TM, Snyder AZ, et al. Partial volume correction in quantitative amyloid imaging. NeuroImage. 2015;107:55-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su Y, Flores S, Wang G, et al. Comparison of Pittsburgh compound B and florbetapir in cross-sectional and longitudinal studies. Alzheimers Dement. 2019;11:180-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su Y, Flores S, Hornbeck RC, et al. Utilizing the Centiloid scale in cross-sectional and longitudinal PiB PET studies. Neuroimage Clin. 2018;19:406-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volluz KE, Schindler SE, Henson RL, et al. Correspondence of CSF Biomarkers Measured by Lumipulse Assays with Amyloid PET. 2021 Alzheimer's Association International Conference; 2021. [Google Scholar]
- 35.Vlassenko AG, McCue L, Jasielec MS, et al. Imaging and cerebrospinal fluid biomarkers in early preclinical Alzheimer disease. Ann Neurol. 2016;80(3):379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. [PubMed] [Google Scholar]
- 37.Brickman AM, Manly JJ, Honig LS, et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer's disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 2021;17:1353-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chong JR, Ashton NJ, Karikari TK, et al. Plasma P-tau181 to Abeta42 ratio is associated with brain amyloid burden and hippocampal atrophy in an Asian cohort of Alzheimer's disease patients with concomitant cerebrovascular disease. Alzheimers Dement. 2021;17(10):1649-1662. [DOI] [PubMed] [Google Scholar]
- 39.O'Bryant S, Petersen M, Hall J, et al. Characterizing plasma NfL in a community-dwelling multi-ethnic cohort: results from the HABLE study. Alzheimers Dement. 2022;18(2):240-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gottesman RF, Schneider AL, Zhou Y, et al. The ARIC-PET amyloid imaging study: brain amyloid differences by age, race, sex, and APOE. Neurology. 2016;87(5):473-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raman R, Quiroz YT, Langford O, et al. Disparities by race and ethnicity among adults recruited for a preclinical alzheimer disease trial. JAMA Netw Open. 2021;4(7):e2114364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gleason CE, Norton D, Zuelsdorff M, et al. Association between enrollment factors and incident cognitive impairment in Blacks and Whites: data from the Alzheimer's Disease Center. Alzheimers Dement. 2019;15:1533-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janelidze S, Teunissen CE, Zetterberg H, et al. Head-to-head comparison of 8 plasma amyloid-beta 42/40 assays in Alzheimer disease. JAMA Neurol. 2021;78(11):1375-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mielke MM, Frank RD, Dage JL, et al. Comparison of plasma phosphorylated tau species with amyloid and tau positron emission tomography, neurodegeneration, vascular pathology, and cognitive outcomes. JAMA Neurol. 2021;78(9):1108-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keshavan A, Pannee J, Karikari TK, et al. Population-based blood screening for preclinical Alzheimer's disease in a British birth cohort at age 70. Brain. 2021;144(2):434-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gottesman RF, Hamilton R. Recruiting diverse populations in clinical trials: how do we overcome selection bias? Neurology. 2021;96:509-510. [DOI] [PubMed] [Google Scholar]
- 47.Syrjanen JA, Campbell MR, Algeciras-Schimnich A, et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. Epub 2021 Sep 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilkins CH, Schindler SE, Morris JC. Addressing health disparities among minority populations: why clinical trial recruitment is not enough. JAMA Neurol. 2020;77(9):1063-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howell JC, Parker MW, Watts KD, Kollhoff A, Tsvetkova DZ, Hu WT. Research lumbar punctures among African Americans and Caucasians: perception predicts experience. Front Aging Neurosci. 2016;8:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barnes LL. Alzheimer disease in African American individuals: increased incidence or not enough data? Nat Rev Neurol. 2022;18:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available to qualified investigators upon request to the Knight ADRC (knightadrc.wustl.edu/Research/ResourceRequest.htm).