ABSTRACT
Photosynthesis started to evolve some 3.5 billion years ago CO2 is the substrate for photosynthesis and in the past 200–250 years, atmospheric levels have approximately doubled due to human industrial activities. However, this time span is not sufficient for adaptation mechanisms of photosynthesis to be evolutionarily manifested. Steep increases in human population, shortage of arable land and food, and climate change call for actions, now. Thanks to substantial research efforts and advances in the last century, basic knowledge of photosynthetic and primary metabolic processes can now be translated into strategies to optimize photosynthesis to its full potential in order to improve crop yields and food supply for the future. Many different approaches have been proposed in recent years, some of which have already proven successful in different crop species. Here, we summarize recent advances on modifications of the complex network of photosynthetic light reactions. These are the starting point of all biomass production and supply the energy equivalents necessary for downstream processes as well as the oxygen we breathe.
Keywords: bioengineering, crop improvement, electron transfer, light reactions, photosynthesis, photosystem, stress tolerance
Improving photosynthesis is important for increasing future crop production. This review examines strategies to optimize protein complexes involved in photosynthetic light reactions in a range of plant/microalgal species; several of these strategies have been successfully transferred into crops, boosting yields and stress tolerance.

INTRODUCTION—WHY DO WE NEED CROPS WITH INCREASED YIELDS?
Crops and farming have sustained human existence for more than 11,000 years (Murphy, 2007). The growing world population is currently projected to reach 10.87 billion people by the end of this century in 2100 (Figure 1A, data from the Food and Agriculture Organization [FAO, https://www.fao.org/faostat/]) and requires considerably increased food production. This is a major challenge as agricultural land becomes more and more limited. In many northern latitude countries, agricultural areas have not further expanded in the past 30 years or have even declined somewhat (Figure 1B, data from FAO, see also Ramankutty et al., 2018). However, in many tropical areas, agricultural land use has increased by up to 20% in the past 30 years, with concomitant decreases in forest area. Up to 40 hectares of forest are being cleared every minute to generate more arable land in order to produce more food and feed for animals. This has led to a more than 50% loss of rainforests to date (Figure 1C, data from FAO; FAO and UNEP, 2020).
Figure 1.
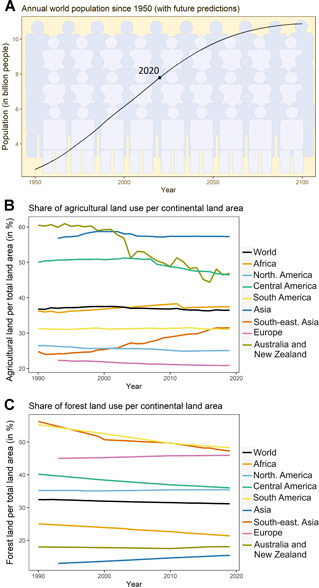
Global developments calling for solutions for improved crop yields
(A) Annual world population (in billion people) recorded from 1950 to 2020 with future predictions from 2021 to 2100. (B) Agricultural land use development and (C) forest land development per continent over the past 30 years. The shares of annual agricultural land use and forested land (in %) per total land area was determined for each area. All data were obtained from the Food and Agriculture Organization (FAO) in October–December 2021. Agricultural land use includes both crop and pasture land. Timeseries data for Asia and Europe were started at 1993 to avoid the discontinuity in 1992 due to the end of the USSR. Groupings by continent or sub‐continent follow the FAO country groupings. The data for group “South America” also includes the Caribbean countries. The data for group “Asia” combines FAO country groups for central, eastern, southern, and western Asia.
Trees absorb and fix enormous amounts of solar radiation and carbon dioxide, making a major contribution to mitigate against global warming and climate change, and serve as reservoirs for the freshwater we drink. To tackle deforestation and preserve nature, alternative approaches need to be developed to improve productivity of the farmland that is currently available. One promising target is the chemical process that sustains all life on Earth, called oxygenic photosynthesis. Plants and photosynthetic micro‐organisms fix carbon dioxide from the atmosphere and convert it into sugars and organic biomass, using the absorbed light energy from the sun and water from the soil, and releasing molecular oxygen as a by‐product (Figure 2).
Figure 2.
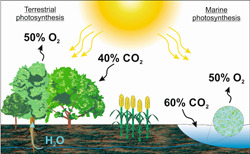
Schemes of terrestrial and marine photosynthesis
Carbon dioxide (CO2) from the air and water (H2O) from the soil are taken up by land plants and converted into sugars and biomass using the light energy of the sun (according to the equation 6 CO2 + 6 H2O↔C6H12O6 + 6 O2). As a photosynthetic by‐product, molecular oxygen (O2) is released into the air. Percentages indicate the proportions of total sequestered CO2 and released O2 by terrestrial and marine photosynthesis (source: Food and Agriculture Organization https://www.fao.org/3/y0900e/y0900e06.htm, World Ocean Review).
The predominant view up to two decades ago was that photosynthetic efficiency had reached its maximum capacity and could not be further improved in crops due to sink limitations, and the subject still sparks scientific debate (discussed in Long et al., 2006; Sinclair et al., 2019; Araus et al., 2021; Paul, 2021). However, studies simulating possible future increases in atmospheric carbon dioxide (CO2) concentrations have clearly indicated that photosynthesis can indeed be enhanced and result in improved crop yields (Ainsworth and Long, 2021), which has been further established over the years (Zhu et al., 2010; Parry et al., 2011; Gu et al., 2014; Long et al., 2015; Ort et al., 2015; Yin et al., 2015, 2021a, 2021b; Kromdijk and Long, 2016; Simkin et al., 2019; Wu et al., 2019).
In recent years, different strategies to improve crop yields have been proposed and reviewed, such as: (i) introducing photorespiratory bypasses (Betti et al., 2016; Hagemann and Bauwe, 2016; South et al., 2018; Eisenhut et al., 2019; López‐Calcagno et al., 2019; Maurino, 2019; Shen et al., 2019; Khurshid et al., 2020; Wang et al., 2020; Abbasi et al., 2021); (ii) introducing algal/cyanobacterial carbon concentrating mechanisms (McGrath and Long, 2014; Rae et al., 2017; Long et al., 2018; Atkinson et al., 2020; Hennacy and Jonikas, 2020; Chen et al., 2021; Rottet et al., 2021); (iii) introducing the C4 photosynthesis pathway into C3 plants (Ermakova et al., 2020, 2021a); (iv) improving mesophyll conductance (Hanba et al., 2004; Xu et al., 2019; Lundgren and Fleming, 2020; Ermakova et al., 2021c); (v) modifying metabolic processes (Rossi et al., 2015; South et al., 2019); and (vi) modifying circadian rhythms and introducing chronocultures (Steed et al., 2021). During the second half of the 20th century, the Green Revolution led to improved grain yields through conventional breeding techniques and improved pest/disease control. Nevertheless, photosynthesis still typically performs at a four‐ to five‐fold lower efficiency than its theoretical maximum (Long et al., 2015; Ort et al., 2015). Photosynthetic light use efficiency is a major determinant of the conversion efficiency of absorbed light energy into biomass. Only 50% of incident solar radiation (wavelengths between 400 and 740 nm) can be actively used to drive photosynthesis. Further energy losses occur due to light reflectance from the leaf, light absorption by nonphotosynthetic pigments, dissipation of excess light energy as heat, thermodynamic limits, carbohydrate biosynthesis, photorespiration, and respiration. This leaves a theoretical maximum of about 5% of total irradiance that is converted into biomass. However, in the field, photosynthetic efficiencies normally only reach 1%–2% on the individual plant level because of light saturation of the photosynthetic machinery at about 25%–50% of full sunlight and activity of energy‐dissipating photoprotective mechanisms at higher light intensities (Long et al., 2006; Zhu et al., 2008, 2010). At the canopy level this results in photosynthetic efficiencies of about 2.2% under well‐managed conditions (Yin and Struik, 2015). Calculating these theoretical efficiencies highlights the importance of crop modeling to consider further routes for crop improvement (Wu et al., 2019; Yin et al., 2021a, 2021b). Strategies to overcome these limitations include optimization of the canopy and leaf architecture (Tholen et al., 2012; Drewry et al., 2014; Mathan et al., 2016; Song et al., 2013, 2016; Xiao et al., 2016; Slattery and Ort, 2021) as well as the photosynthetic light‐dependent and light‐independent reactions. While the photochemical light‐dependent reactions involve harvesting of excitation energy from sunlight to produce the energy carriers nicotinamide adenine dinucleotide phosphate (reduced form) (NADPH) and adenosine triphosphate (ATP), in the light‐independent reactions these energy carriers are then used to fix carbon dioxide via the enzyme ribulose‐1,5‐bisphosphate carboxylase/oxygenase (Rubisco) into C3 sugars and regenerate the Rubisco substrate ribulose‐1,5‐bisphosphate (RuBP; Calvin–Benson–Bassham cycle). Both Rubisco activity and the rates of regeneration reactions have been targeted for improvement, which have proven very successful in recent years (Lefebvre et al., 2005; Kurek et al., 2007; Kumar et al., 2009; Rosenthal et al., 2011; Parry et al., 2013; Whitney et al., 2015; Driever et al., 2017; Salesse‐Smith et al., 2018; Simkin et al., 2015, 2017a, 2019; Scafaro et al., 2019a; López‐Calcagno et al., 2020; Iñiguez et al., 2021), with more promising research under way (Scafaro et al., 2019b; Degen et al., 2020).
This review focuses on the photosynthetic light‐dependent reactions, the optimization of which is relevant to improving both C3 and C4 photosynthesis in plants and photosynthetic micro‐organisms (Leister, 2012; Ruban, 2015; von Caemmerer and Furbank, 2016; Cardona et al., 2018; Simkin et al., 2019; Batista‐Silva et al., 2020; Ermakova et al., 2021b; Sales et al., 2021; Santos‐Merino et al., 2021). Modeling studies have suggested that improving the quantum yield and electron transport capacity have a greater potential for increasing the productivity of crops than other photosynthetic mechanisms, such as improving Rubisco activity (Gu et al., 2014; Yin and Struik, 2015, 2021a; Wu et al., 2019). In the past couple of years, numerous novel approaches to improve photosynthetic light reactions have been reported. This review presents a synthesis and highlights the potential of these approaches to boost crop yields.
THE “SOLAR PANELS” OF THE PLANT CELL—THE LIGHT REACTIONS OF PHOTOSYNTHESIS
Solar panels are an increasingly popular choice for generating “home‐made” sustainable energy and circumvent the use of fossil fuels (International Energy Agency, 2021). The process of light energy conversion into electricity, called photovoltaic effect, has been translated into solar cells with light conversion efficiencies of around 20%–50% (Geisz et al., 2020). A similar effect can be observed in nature in photosynthesizing organisms. Here, the light‐harvesting complexes together with the photosystems act in series to absorb energy from sunlight and fuel electron transfer and subsequent redox reactions in the thylakoid membranes, resulting in the generation of chemical energy which fuels the production of biomass. However, the photosynthetic light‐to‐biomass conversion efficiency is more difficult to estimate than the light‐to‐electricity conversion efficiency in solar cells. Nevertheless, efforts have been made to determine the theoretical photosynthetic efficiency in plants on the individual plant level as 4.6% and 6.0% (Zhu et al., 2010) for C3 and C4 plants, respectively. However, actual photosynthetic efficiencies can be as little as 1%–3.5%/4.3% in C3/C4 plants (Zhu et al., 2010; Blankenship et al., 2011). But how exactly does the light‐harvesting “solar panel” of the plant cell operate when a photon hits the leaf and initiates photosynthesis? And how has it developed during the course of evolution?
As the name suggests, light‐harvesting complex (LHC) proteins absorb light energy from the sun. The LHC proteins are located in the thylakoid membranes in close proximity to the photosystems PSII and PSI and act as a funnel, channeling the absorbed light energy, also called excitation energy, toward the photosystems' reaction center chlorophylls P680 (PSII) and P700 (PSI). There, electrons become excited by reaching a higher energy level (termed “charge separation”) and move toward electron acceptors, thus initiating a series of electron transfers in the thylakoid membrane between the two photosystems. Charge separation in PSII leads to oxidized P680+ and reduction of the stable electron acceptors plastoquinone (PQ) A and B (QA and QB, PQ pool) via the unstable intermediate pheophytin. P680+ is a very strong oxidant and extracts an electron from water, which is split into protons (H+) and molecular oxygen (O2) at the Oxygen‐Evolving Complex in PSII. Further down the line, doubly reduced QB accepts two H+ from the stroma, forming the mobile electron carrier plastoquinol (PQH2), which passes on the electron to the membrane‐embedded cytochrome b6f complex. From there, the electron either travels back to the PQ pool via the Q‐cycle or further toward the electron gap in PSI (P700+) via a mobile carrier in the thylakoid lumen, which is called plastocyanin. Within PSI then, excited electrons released from P700 using harvested light energy, are accepted by phylloquinone followed by electron transfer via three iron‐sulfur (Fe‐S) clusters to ferredoxin (Fd) and the protein Fd‐NADP+‐reductase (FNR) which regenerates NADP+ to the reducing agent NADPH. At the same time, the ultimate energy carrier ATP is produced upon acidification of the thylakoid lumen. During linear electron transfer (LET), a H+ gradient across the thylakoid membrane is established. This electrochemical force (proton motive force—pmf) drives ATP synthesis via the membrane‐spanning ATP synthase complex, which generates ATP from adenosine diphosphate (ADP) and inorganic phosphate (Pi) on the stromal side. The NADPH and the ATP produced by photosynthetic electron transport are essential to drive the Calvin–Benson–Bassham cycle for atmospheric CO2 fixation and the production of sugars used for biomass biosynthesis, and for other metabolic processes in the chloroplast (for a review see Stirbet et al., 2019; Figure 3).
Figure 3.
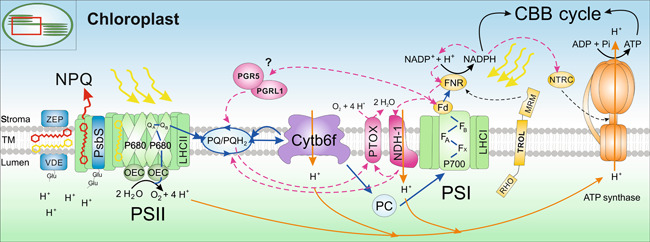
Overview of light reactions in the thylakoid membrane
In the thylakoid membrane system inside the chloroplast, two pigment–protein photosystems (PSII and PSI) operate in series in order to generate the energy equivalents nicotinamide adenine dinucleotide phosphate (reduced form) (NADPH) and adenosine triphosphate (ATP). Absorbed excitation energy is channeled by light‐harvesting complexes LHCII and LHCI toward the reaction centers of both photosystems (P680 in PSII and P700 in PSI), where an electron is liberated and passed along several electron acceptors down the linear electron transfer (LET) chain (blue arrows). Downstream of PSII, the plastoquinone/plastoquinol (PQ) pool transfers electrons to the cytochrome b6f complex (cyt b6f) and further to PSI via plastocyanin (PC). In PSI, several iron‐sulfur clusters (Fx, FA, FB) are the primary electron acceptors which then reduce ferredoxin (Fd). Lastly in LET, the Fd‐NADP+ reductase (FNR) is released from its possible anchor thylakoid rhodanase‐like (TROL) protein, which contains a rhodanese‐like motif (RHO) in the lumen and a membrane recruiting motif (MRM) in the stroma, and regenerates NADPH by oxidation of Fd. Simultaneously in PSII, oxidized P680+ is reduced by an electron deriving from the splitting of water (H2O) at the oxygen‐evolving complex, which also releases molecular oxygen (O2) and protons (H+). Protons are also transported across the thylakoid membrane by cyt b6f and the NADH dehydrogenase‐like 1 (NDH‐1) complex (orange arrows) in order to fuel ATP production at the ATP synthase complex. Both NADPH and ATP are then metabolized in the Calvin–Benson–Bassham (CBB) cycle for carbon fixation. In case of overexcitation of the LET chain, AET routes are activated downstream of PSI (magenta dashed arrows), including cyclic electron transfer via the Proton Gradient Regulation 5 (PGR5)/PGR5‐like photosynthetic phenotype 1 (PGRL1) or the NDH‐1 complexes. NDH‐1 also diverts excess electrons to the Plastid Terminal Oxidase PTOX, which reduces O2 to H2O. Photoprotection via qE‐type nonphotochemical quenching (NPQ) involves the PSII subunit S protein PsbS, which senses the acidification of the lumen upon high light exposure via protonatable residues and initiates the rearrangement of the LHCII complexes, thus inducing the dissipation of excess excitation energy or NPQ. In parallel, the xanthophyll cycle is also activated by the lumen pH, inducing the reversible conversion of violaxanthin (yellow pigment) into antheraxanthin and zeaxanthin (red pigment) in light via the violaxanthin de‐epoxidase (VDE). In dark, the xanthophyll cycle is then reversed by zeaxanthin epoxidase (ZEP).
The more sunlight, the better? How photoprotective mechanisms safeguard the light reactions from excessive sunlight
In nature, sunlight is a very variable resource. On a sunny day, plants can experience fluctuations in light exposure and spectral features when clouds cover the sun, for instance, or other plants/leaves move in the wind and shade the canopy below. Thereby, blue and red wavelengths are absorbed by the upper canopy, depleting the light of wavelengths that can be captured by the LHC proteins and drive photosynthesis in the lower canopy. Dynamic light conditions occur on a seasonal level, a daily level, and as cloud‐ and sunflecks, which can fluctuate rapidly and last seconds to minutes (Morales and Kaiser, 2020).
Harvested light energy does not always lead to electron transfer but can also be dissipated via other routes. If the sunlight is too strong (termed “high light”), it can be harmful to the plant. Photosynthesis has several rate‐limiting steps, such as the regeneration of the Rubisco substrate RuBP as electron sink in the Calvin–Benson–Bassham cycle as well as the activity of the cytochrome b6f complex and the ATP synthase in the light reactions (Farquhar et al., 1980; Price et al., 1998; Hajirezaei et al., 2002; Sage et al., 2008; Rott et al., 2011; Yamori et al., 2011; Hasan and Cramer, 2012; Magyar et al., 2018). These bottlenecks can result in a jam of electrons in the thylakoid membrane when the capacity of light‐harvesting exceeds the capacity of CO2 fixation. Under these conditions, the formation of chlorophyll triplet states from singlet excited chlorophylls increases. Triplet chlorophyll can readily react with molecular oxygen and form the harmful reactive oxygen species (ROS) singlet oxygen. This highly reactive state of oxygen can specifically damage the photosynthetic protein complexes in the thylakoid membrane, oxidize plasma membrane lipids and react with nucleic acids (Krieger‐Liszkay, 2005; Di Mascio et al., 2019; Khorobrykh et al., 2020). Particularly, the PSII core protein D1 is prone to oxidative photodamage upon long‐term high light exposure, causing a transient downregulation of PSII efficiency, termed photoinhibition of PSII (Aro et al., 1993; Long et al., 1994; Murata et al., 2007; Guidi et al., 2019). Since plants are sessile organisms, they are not able to change location to avoid unfavorable conditions and have developed numerous photoprotective mechanisms during the course of evolution. One such mechanism is via nonphotochemical dissipation of excess absorbed energy as heat (nonphotochemical quenching [NPQ]).
In addition to photochemistry and NPQ, excited chlorophylls can also return to the ground state via fluorescence, that is, emission of a red‐shifted photon. While fluorescence emission is not an appreciable energy flux, analysis of fluorescence quenching allows estimation of energy dissipation via photochemistry and NPQ. NPQ measured by fluorescence quenching analysis is a collective term that includes several different components for the avoidance responses of photodamage. These actually do not dissipate excess energy as heat but aim at decreasing light absorption and optimizing electron distribution, such as chloroplast photorelocation, redistribution of LHCII between the photosystems (state transitions) and photoinhibitory break‐down of D1 in PSII (for a comprehensive overview and depiction see Malnoë, 2018; Messant et al., 2021). Energy dissipation in the LHCs via NPQ consists of different components with contrasting response times. The fastest component of actual heat dissipation mechanisms is called energy‐dependent quenching (qE) (Wraight and Crofts, 1970, reviewed in Ruban, 2016, depicted on the left‐hand side in Figure 3). qE is triggered by lumen acidification, with the sensitivity of the response highly dependent on the PSII subunit S protein (PsbS) in higher plants. This protein is considered the main player in the induction of photoprotective mechanisms and acts as a pH sensor in the thylakoid membrane. Upon illumination and thus acidification of the thylakoid lumen, PsbS undergoes a conformational change and activates quenching of excess absorbed light energy. A very likely location of the active quenching site in land plants are the LHC antenna proteins, which are involved in light‐harvesting as well as photoprotection of the photosystems' reaction centers. LhcA1‐4 proteins form heterodimeric antennae around PSI, whereas LhcB1‐3 assemble either into strongly PSII‐bound S‐homotrimers of LhcB1 or moderately and loosely PSII‐bound M‐ and L‐heterotrimers of LhcB1‐3. These major antennae of LHCII trimers are connected to the PSII core proteins via the monomeric minor antennae LhcB4‐6. While LhcB4 (CP29) and LhcB6 (CP24) form heterodimers, connecting the M‐LHCII trimers to the PSII core via the CP47 protein, LhcB5 (CP26) connects the S‐LHCII trimers to the PSII core via the CP43 protein, thus channeling the absorbed sunlight energy from the LHCII trimers to the PSII reaction center via the monomeric antenna proteins (Figure 4A,B). Although the exact NPQ mechanisms are not known yet, it seems clear that PsbS dimers sense the change in lumenal pH upon light exposure with two protonatable lumen‐exposed glutamate residues per monomer (Li et al., 2004). Subsequently, the rearrangement of the LHCII antennae is initiated, potentially via PsbS monomerization and interactions with the CP29 and LhcB1 proteins. In a putative model for qE, conformational changes are also induced at the supercomplex level, where M‐LHCII trimers are released from the PSII‐LHCII supercomplexes for the formation of LHCII aggregates in the thylakoid membrane, forming the putative quenching site Q1 (Bergantino et al., 2003; Teardo et al., 2007; Betterle et al., 2009; Miloslavina et al., 2011; Dall'Osto et al., 2017; Kress and Jahns, 2017). Besides the acidification of the lumen pH, another prerequisite of qE is the activation of the reversible xanthophyll cycle and binding of the xanthophyll zeaxanthin to major and minor LHCII proteins (Niyogi et al., 1997; Bassi and Caffarri, 2000; Ballottari et al., 2012). Under dark and low light conditions, LHCII proteins predominantly bind violaxanthin, whereas upon exposure to high light, violaxanthin is released into the thylakoid membrane, converted into zeaxanthin via the intermediate xanthophyll antheraxanthin by the enzyme violaxanthin de‐epoxidase (VDE), and reinserted to induce NPQ and protect the cell components from oxidative stress (Havaux et al., 2007; Johnson et al., 2007; Dall'Osto et al., 2010). Zeaxanthin is reconverted into violaxanthin by the stromal enzyme zeaxanthin epoxidase (ZEP) upon light‐to‐dark transitions. Zeaxanthin is also involved in a PsbS‐independent NPQ mechanism, called qZ (zeaxanthin‐dependent quenching) for which induction and relaxation are correlated with zeaxanthin formation and depletion on a time scale of several minutes (Dall'Osto et al., 2005; Nilkens et al., 2010). Whereas further long‐term quenching forms were previously collectively termed qI (inhibitory quenching), this parameter is currently subject to further molecular dissection based on the factors involved. For example, a long‐term form of NPQ (in the range of hours), called qH, has recently been discovered to occur in the LHCII trimers, involving the plastid lipocalin LCNP and its regulators SOQ1 and ROQH1 (Malnoë et al., 2018; Amstutz et al., 2020; Bru et al., 2020, 2021; Yu et al., 2021).
Figure 4.
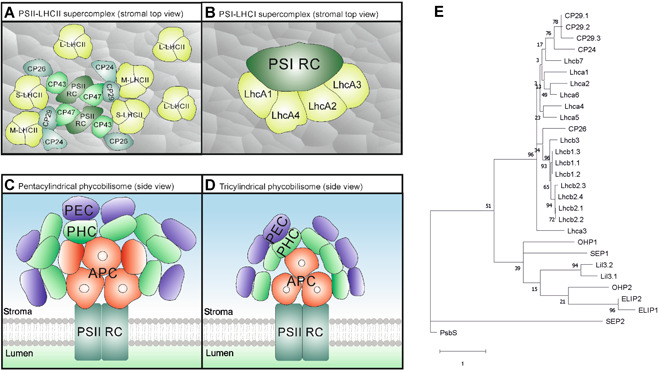
Arrangements of photosystems with light‐harvesting complexes (LHC) in higher plants and cyanobacteria in the thylakoid membranes
Top views of the pigment–protein photosystem II (PSII)‐LHCII (A) and PSI‐LHCI (B) supercomplex core components in higher plants (recreated from Protein Data Bank (PDB) entries 5MDX and 2WSC). The dimeric PSII core consists of the reaction center (RC) proteins D1 and D2 and the core proteins CP43 and CP47. The minor antennae CP29/CP24 (heterodimer) and CP26 connect moderately and strongly bound M‐ and S‐LHCII trimers to the PSII RC via CP47 and CP43, respectively. Loosely bound L‐LHCII trimers are often detached from the supercomplex. In monomeric PSI, the RC is surrounded by LhcA1‐4 in a fan‐like fashion, with LhcA1 and A4 and LhcA2 and A3 forming heterodimers. In contrast, cyanobacterial light‐harvesting antennae are not embedded within the thylakoid membrane but are attached to the core proteins on the stromal surface. These large pigment‐protein complexes are called phycobilisomes (PBS) and come in different shapes. Pentacylindrical PBS are predominantly present in filamentous cyanobacteria and consist of five allophycocyanin (APC) core cylinders (red) from which eight phycoerythrocyanin (PEC; blue)/phycocyanin (PHC; green) rods radiate (C). The colors represent the wavelengths the different PBS discs absorb upon binding phycocyanobilin pigments, inducing energy transfer from PEC→PHC→APC→PSII RC. Unicellular cyanobacteria mostly contain tricylindrical PBS with three APC core cylinders and up to six rods (D). The structures were generated from PDB entries 7EYD (Anabaena sp. PCC 7120) and 7EXT (Synechococcus sp. PCC 7002), respectively. (E) Phylogenetic tree of Arabidopsis thaliana LHC family proteins generated in MEGAX64. Protein sequences were aligned by MUSCLE with the UPGMA cluster method. The tree was built using Maximum Likelihood as statistical method, in conjunction with the bootstrap method (500 replications), the rtREV with Freqs. (+F) model, Gamma Distributed rates with Invariant Sites (G + I; five discrete gamma categories), partial deletion of gaps (95% cutoff) and the Nearest‐Neighbor‐Interchange heuristic model.
In addition to NPQ, several alternative electron transfer (AET) routes become active in response to stress in order to prevent the LET chain from overreduction. To maintain an optimal ATP/NADPH ratio for metabolic processes under such conditions, electrons are rerouted from Fd back to the PQ pool potentially via the Proton Gradient Regulation 5 (PGR5)/PGR5‐like photosynthetic phenotype 1 (PGRL1) complex or the NADH dehydrogenase‐like (NDH) complex, thus driving cyclic electron transfer (CET) around PSI and sustaining ATP synthesis (Ma et al., 2021). The membrane‐embedded NDH‐1 complex not only reduces the PQ pool for CET with electrons deriving from NADPH via FNR and Fd (Mulo and Medina, 2017) but also transfers protons into the thylakoid lumen for activation of the ATP synthase (Peltier et al., 2016). NDH‐1 is also proposed to be involved in a respiratory pathway inside the chloroplasts, called chlororespiration, which includes reoxidation of the PQ pool and reduction of oxygen to water by the membrane protein Plastid Terminal Oxidase (PTOX) as an alternative electron sink. PTOX was hypothesized to have a dual function, depending on the prevalent light intensity and thus the redox state of the PQ pool/lumen pH (Yu et al., 2014; Feilke et al., 2016; Krieger‐Liszkay and Feilke, 2016). Current models suggest that under high light conditions, PTOX associates with the thylakoid membrane and is accessible for its substrate plastoquinol (PQH2), hence oxidizing the over‐reduced PQ pool, using oxygen as an electron acceptor. However, in a side reaction, superoxide and hydroxyl radicals (ROS) are produced which could lead to photoinhibition of the photosystems if not scavenged properly by other antioxidant systems (Sun et al., 2017). Under nonsaturating light, on the other hand, it was demonstrated that PTOX can theoretically act as an extra electron sink and keep the PQ pool more oxidized and protected from photoinhibition, although its expression levels are extremely low under this condition, avoiding competition with LET (Feilke et al., 2016). However, this “antioxidant” feature is important under dynamic light conditions (Nawrocki et al., 2019b). In case superoxide is still produced at PSI, it is rapidly reduced to water by the enzymes superoxide dismutase and ascorbate peroxidase (Mehler reaction; Mehler, 1951). This reaction is part of the water–water cycle, referring to electron flow from the splitting of water at PSII to the generation of water at PSI, and acts as an alternative electron sink to increase the ATP:NADPH ratio under stress conditions when more ATP is required (Asada, 2000). Instead of stimulating ATP biosynthesis, the ATP:NADPH ratio can also be adjusted by removing NADPH either through enhanced uptake by metabolic processes, such as fatty acid production, or through transport into mitochondria via the malate shuttle (for a recent review see Dao et al., 2021).
Evolution and structure of the LHC protein family
Throughout evolution, the main components of the photosynthetic machinery have remained well‐conserved in the plant tree of life, ranging from single cell blue–green algae (called cyanobacteria) to higher plants (Nelson and Yocum, 2006). In Archean times probably more than 3.5 billion years ago, a homodimeric photosystem reaction center diverged into Type II and Type I reaction centers. Type II reaction centers then further diversified into ancestral heterodimeric water‐splitting and nonwater‐splitting systems, giving rise to oxygenic and anoxygenic photosynthesis, respectively. Cyanobacteria are the first known organisms to employ oxygenic photosynthesis, having emerged about three billion years ago, releasing molecular oxygen into the atmosphere as a by‐product of splitting water molecules at PSII. Consequently, during the “Great Oxidation Event” 2.4 billion years ago, the reduced, high CO2 atmosphere was converted into an oxidized, low CO2 atmosphere—as we know it today—allowing the development of all oxygen‐breathing organisms (Sánchez‐Baracaldo and Cardona, 2020). Cyanobacteria are deemed the evolutionary predecessors of the photosynthetic chloroplast organelles in eukaryotes. Through an endosymbiotic event in which a heterotrophic eukaryotic α‐proteobacterium engulfed an autotrophic cyanobacterium hundreds of million years ago, the cyanobacterium integrated into the host cell and slowly developed into the chloroplast over time, leading to the generation of green algae and plant cells (Gould et al., 2008; McFadden, 2014).
However, one major difference in the physiology of the photosynthetic machinery between cyanobacteria and plants is the evolution of the light‐harvesting antenna complexes. While in green algae and plants, the pigment–protein antennae are embedded in the thylakoid membrane adjacent to the photosystems, in cyanobacteria, red algae and glaucophytes, they sit in a fan‐like fashion on top of the photosystems on the stromal side of the thylakoid membrane (Figure 4C,D). These phycobilisomes (PBS) are large complexes and comprise phycobiliproteins that can bind several types of linear phycobilin pigments. The standard cyanobacterial PBS structure consists of allophycocyanin core cylinders, from which rods composed of phycocyanin and phycoerythrocyanin/phycoerythrin radiate. Depending on which phycobiliprotein the pigments are bound to, they can absorb light of different wavelengths. The distal part of the rod absorbs short wavelengths in the blue–green range with a maximum at 570 nm. The captured energy is then transferred through the rods toward the PBS core, which absorbs longer wavelengths of red light (maximum at 650 nm) and passes on all energy to the PSII reaction center. Composition and length of the rods can be adjusted according to the prevalent light conditions (Bryant, 1982; Chang et al., 2015; Stadnichuk et al., 2015; Tang et al., 2015; Green, 2019; Bag, 2021). But “what happened to the phycobilisome?” (Green, 2019) and how did the LHC protein family eventually evolve?
With the evolution of the chloroplast, many photosynthetic genes from the ancestral cyanobacterium were transferred and incorporated into the nucleus of the eukaryotic host cell. The genes for the assembly of the PBS and the associated orange carotenoid protein (OCP) for photoprotection (Muzzopappa and Kirilovsky, 2020) were likely lost, possibly due to their large size and high nitrogen requirement for protein assembly. Cyanobacteria also contain high light‐inducible proteins (HLIPs), which are considered the evolutionary progenitors of the LHC protein family. These HLIPs are small single‐helix transmembrane proteins that bind chlorophyll a and the carotenoid β‐carotene and function in assembly and photoprotection of PSII and chlorophyll metabolism in cyanobacteria (Komenda and Sobotka, 2016; Tibiletti et al., 2018). Upon endosymbiosis of cyanobacterium and eukaryote, HLIP genes were transferred into the nucleus of the eukaryote and are still present in the plant genome as one‐helix proteins (OHPs; Figure 4E). Through acquisition of additional transmembrane domains, internal gene duplications and loss of helices, two‐helix stress‐enhanced proteins (SEPs) and light‐harvesting‐like (Lil) proteins, the nonpigmented four‐helix protein PsbS, as well as the big group of three‐helix LHC proteins (including early light‐inducible proteins [ELIPs]), respectively, developed especially in the green lineage of chlorophyta, ranging from green algae to higher plants (Green, 2019; Bag, 2021). A special case is the evolution of the presumably first LHC‐like protein group of LHCSR proteins and the “younger” PsbS protein. In the case of PsbS, pH sensing and the active quenching site are located on different proteins, whereas in LHCSR both mechanisms are combined in one protein. While photoprotection in green algae mainly relies on LHCSR proteins, in mosses, both LHCSR and PsbS proteins are active. PsbS development and relocation of the active quenching site to other protein complexes was likely an adaptation process to more challenging light conditions when plants started to conquer terrestrial land. This would also explain why LHCSR proteins are completely absent in land plants (Pinnola, 2019).
Furthermore, it is believed that CP29 was the first LHCII protein to evolve due to its presence in taxonomically diverse classes of green algae. This points to the existence of an ancestral CP29 gene before the diversification of the green lineage, followed by the rise of CP26 and ancestral major antenna proteins LhcBM in green algae through gene duplications and DNA crossovers of SEP genes. In contrast, CP24 seems to be the latest addition to the LHC protein family, being present in land plants only (Koziol et al., 2007). Even though the protein sequence similarities are only 20%–40%, the overall protein structures with three membrane‐spanning domains and conserved chlorophyll a/b‐binding sites are mostly uniform across the major and minor LHCII proteins (Jansson, 1999; Ballottari et al., 2012). In addition to chlorophylls, they also bind three to four carotenoids (xanthophyll pigments: lutein, violaxanthin, zeaxanthin, neoxanthin; and β‐carotene) to designated binding sites (Jahns and Holzwarth, 2012).
IMPROVING CROP YIELDS THROUGH OPTIMIZATION OF PHOTOSYNTHETIC LIGHT REACTIONS
Decreasing antenna size to increase biomass yield
In the previous sections, we described the functions of the light‐harvesting antenna complexes in the photosynthetic thylakoid membrane on the level of an individual leaf. However, what might be beneficial for an individual plant/leaf might not necessarily be in favor for the entire population/organism, respectively. As mentioned above, photosynthesis has several rate‐limiting steps, which inhibit electron transfer even when the energy input is maximized, causing photodamage of the photosynthetic machinery. In a typical crop canopy, leaves at the top would catch most of the actinic sunlight for the activation of photosynthesis, while light incident on the leaves below will be largely composed of wavelengths that are poorly absorbed by the LHC antennae and will be too low in intensity to drive high rates of photosynthesis (Figure 5). Thus, in an optimal case, leaves in the upper canopy would sacrifice maximum light absorption to increase light penetration to leaves in the lower canopy to sustain efficient photosynthesis throughout the plant. However, this is a difficult undertaking, both in traditional plant breeding as well as with genetic engineering. Plant breeders would normally select for traits that are beneficial for the individual plant, rather than for the entire population. For that reason, plants with a light green color or upright leaves were selected against in the past, signifying lower photosynthesis rates and light absorption, respectively, on an individual plant level, even though these traits could be favorable in a plant community to allow better light distribution across the entire canopy. On the other hand, plant populations with traits beneficial for the community are often invaded by individual plants with more competitive features. In terms of light interception, examples could be taller plant height, more horizontal leaf angles or bigger leaves (Anten and Vermeulen, 2016). These invaders then have an advantage in obtaining resources over the plant community, resulting in poorer crop yields at the stand level. With regard to genetic engineering, it is possible to independently modify traits in different plant tissues, but it is more challenging to independently optimize traits in individual leaves, for instance. Nevertheless, scientists have developed a range of novel approaches to tackle this problem and reduce the LHC antenna systems for better transmittance of sunlight through plant canopies (Ort et al., 2011). Similar approaches have also been generated for microalgal/cyanobacterial liquid mass cultures in photobioreactors, which are easier and faster to manipulate. Some successful strategies could have the potential of being translated from photosynthetic micro‐organisms into crop species in the future, and are therefore briefly described hereafter.
Figure 5.
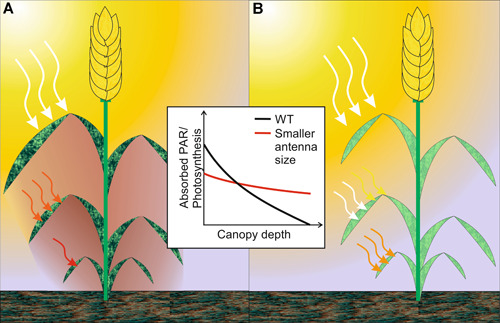
Light distribution across the plant canopy between wild type (A) and mutants with decreased light‐harvesting complex (LHC) antenna sizes (B)
(A) In a dense crop canopy, light distribution across the leaves of the plant is very uneven because of shading effects from other leaves. Leaves of the upper canopy closest to the light source absorb most of the photosynthetic active radiation (PAR) and deplete the light in the lower canopy of its PAR, resulting in a strong decrease of photosynthesis with increasing canopy depth (see insert). (B) Introduction of mutants with a decreased cross‐section of the LHC antennae allows PAR to travel deeper into the crop canopy, as the fractional PAR absorption of leaves closest to the light source has decreased. This approach may marginally decrease photosynthesis of the light‐exposed layers but improves light distribution across the entire canopy, thus improving overall photosynthesis in the otherwise shaded layers and the entire plant (see insert).
Microalgae and cyanobacteria have gained more and more attention as potential production platforms for biotechnological, pharmaceutical, and nutraceutical compounds over the past decades due to their photosynthetic ability and have many advantages over plant‐based bioengineering and production (reviewed in the past 3 years by Benedetti et al., 2018; Khan et al., 2018; Rizwan et al., 2018; Metsoviti et al., 2019; Naruka et al., 2019; Mutanda et al., 2020; Zahra et al., 2020; Dhandayuthapani et al., 2021; Khalifa et al., 2021). High production costs are currently the major drawback owing to a low light‐to‐biomass conversion rate inside the water column. Hence, several attempts have been made in different algal and cyanobacterial species to reduce the optical cross‐section of LHCII antennae to allow better light penetration into the water column of mass production bioreactors (Melis et al., 1999; Mussgnug et al., 2007; Kosourov et al., 2011; Ort et al., 2011; Cazzaniga et al., 2014; Kirst and Melis, 2014; De Mooij et al., 2015, 2016; Shin et al., 2016; Dall'Osto et al., 2019; Hu et al., 2020; Vecchi et al., 2020). In 2002, Polle and coworkers targeted the unicellular green alga species Chlamydomonas reinhardtii and Dunaliella salina for proof of this concept through DNA insertional and chemical‐induced mutations (Polle et al., 2002). Screening for mutants with reduced antenna size but increased photosynthetic performance revealed several genes that could be of interest for manipulation in order to maximize algal biomass or hydrogen production. This list of candidate genes predominantly included genes involved in the biosynthesis of chlorophylls and carotenoids and mutants had about 20%–50% smaller light‐harvesting antennae, mainly affecting PSII‐associated complexes. In a chl b‐less mutant, photosynthetic productivity could be increased two‐fold, although lower maximum PSII quantum efficiencies were observed, indicating photodamage to the photosystems. This can also be detrimental when mass cultures are grown under bright sunlight, when photoinhibition is even more pronounced and NPQ mechanisms are induced and waste absorbed light energy. Therefore, Perrine and coworkers constructed chlorophyllide a oxygenase (CAO) RNA interference (RNAi) mutants in Chlamydomonas to obtain transgenic strains with intermediate‐sized antenna complexes (20%–30% reduction in LHCII) instead of chl b‐less strains with total loss of the peripheral PSII antenna system (Perrine et al., 2012). The CAO RNAi mutants showed wild type‐like growth behavior under low light conditions but produced significantly more biomass (15%–35% compared to the wild type) when exposed to high light, without being impaired in photoprotective mechanisms, such as state transitions and xanthophyll‐dependent NPQ. However, one drawback is the reduced flexibility of these mutants to adjust antenna size to the prevailing conditions, especially outdoors, where light can show sharp dynamic fluctuations. To target this issue, the same authors improved their previous system by fusing the CAO gene to a light‐responsive transcription factor‐binding site of the LHCMB6 gene and transferring this construct into a chl b‐less mutant background (Negi et al., 2020). With this system, the translational repressor NAB1 will bind to the CAO gene upon illumination and inhibit its expression, thus downregulating the LHCII antenna size in a light‐dependent fashion. These transgenic Chlamydomonas strains indeed showed highly dynamic adjustments of the antenna systems under fluctuating light conditions, with wild type‐like PSII quantum efficiencies, slightly lower NPQ and a three‐fold increase in biomass compared to control strains under dynamic light. It seems that this successful proof of concept may also have potential to improve productivity of crop species.
In crops, modifying light‐harvesting cross‐sections has led to a range of outcomes over the past 10 years. While soybean mutants with reduced chlorophyll levels did not show any increase in biomass accumulation when grown in the field (Slattery et al., 2017), a rice mutant expressing a maize GOLDEN2‐LIKE (GLK) transcription factor demonstrated enhanced chlorophyll and antenna complex biosynthesis, surprisingly resulting in 30%–40% more biomass and grain yields compared to wild type plants (Li et al., 2020). GLK proteins induce chloroplast development through regulation of plastid and nucleus‐encoded genes. Overexpression of GLK can even lead to the development of chloroplasts in nongreen tissues, such as roots. The resulting root photosynthesis of transgenic lines of the model plant Arabidopsis thaliana contributed to a small extent to overall CO2 assimilation in addition to leaf photosynthesis (Kobayashi et al., 2013). Nevertheless, the majority of studies focused on decreasing chlorophyll contents and antenna sizes (Song et al., 2017; Bielczynski et al., 2020) with positive outcomes for improving crop yields (Jin et al., 2016; Kirst et al., 2017; Friedland et al., 2019). While Jin and colleagues knocked out a chloroplast protein that regulates translation of chlorophyll biogenesis genes (HIGH PHOTOSYNTHETIC EFFICIENCY1, HPE1), Kirst and coworkers made use of an established yellow–green line with truncated light‐harvesting antennae in the model plant Nicotiana tabacum (tobacco) and observed higher biomass accumulation per unit absorbed light. Consequently, they proposed a shift in plant agronomy by reducing the space left between sowed plants in the field, as a strategy to increase the biomass outcome per field. Nevertheless, there is a fine balance between reducing the optical cross‐section of the LHC antennae for improved yields and preserving their functions in photoprotective mechanisms (Wu et al., 2020). It is also important to note that manipulation of the light‐harvesting antennae is easier to accomplish in unicellular/multicellular microalgae/cyanobacteria compared to highly differentiated, multiorgan systems, such as plants.
Improving NPQ features increases biomass accumulation in dynamic light conditions
A complex component of photosynthesis is photoprotection of the electron transport chain from overexcitation under high light conditions. Photoprotective mechanisms are activated upon high light exposure to prevent overexcitation or dissipate excess absorbed light energy as heat (NPQ). Thus, in a quenched state, photosynthetic efficiency is downregulated until more favorable light conditions occur. However, upon shift from high light to low light conditions, NPQ mechanisms relax relatively slowly compared to their rate of induction, still inhibiting efficient photosynthesis for several minutes upon transition to more favorable light conditions (Zhu et al., 2004). During these minutes, valuable energy for photosynthetic biomass production is lost, which is particularly problematic under dynamic light conditions with short fluctuations in light intensities, as is the case in natural sunlight on a cloudy day for instance (Figure 6). Several studies have shown that plant growth is significantly reduced under fluctuating light conditions (Leakey et al., 2002; Kubásek et al., 2013; Vialet‐Chabrand et al., 2017; Kaiser et al., 2020). Scientists therefore aim to accelerate NPQ relaxation kinetics under fluctuating light through modification of the molecular players involved, in order to improve crop yields.
Figure 6.
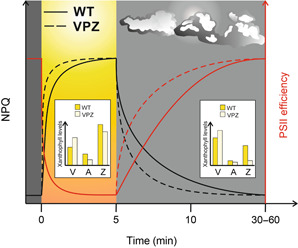
Activity of nonphotochemical quenching (NPQ) mechanisms and xanthophylls under changing light conditions and their potential for improving photosynthetic efficiency
Plants often encounter sudden changes in light intensities (sunflecks or shading), which they quickly need to respond to and adjust their metabolic setup in order to avoid photodamage or maintain their photosynthetic capacity. Upon exposure to high light intensities, NPQ (black lines) components are swiftly initiated to dissipate excess excitation energy as heat and prevent photoinhibition of the photosynthetic machinery. Acidification of the thylakoid lumen initiates the fastest NPQ component qE (energy‐dependent quenching) within seconds to minutes, which is subsequently further enhanced through activation of the xanthophyll cycle, that is, the conversion of violaxanthin (V) into photoprotective zeaxanthin (Z) via antheraxanthin (A). Simultaneously, NPQ inhibits photosynthetic efficiency (red lines), which drops to very low levels under high light conditions. Upon the shift to low light or dark conditions, NPQ relaxes and pigment–protein photosystem II (PSII) efficiency recovers, observable through reconversion of zeaxanthin to violaxanthin. However, full relaxation of NPQ after high light stress is a rather slow process (30–60 min or longer), during which photosynthetic capacity is still inhibited to some extent under otherwise optimal conditions, thereby possibly losing time for biomass production. By overexpressing the lumenal pH sensor protein PsbS and the xanthophyll‐converting enzymes in tobacco (dashed lines), transgenic VPZ plants displayed faster NPQ relaxation under changing light conditions and thus faster recovery of photosynthesis, which resulted in higher biomass accumulation compared to control plants (Kromdijk et al., 2016). Graphs displayed here are schematic representations.
In an attempt to speed up NPQ relaxation kinetics in dynamic light conditions, Kromdijk and coworkers simultaneously overexpressed the PsbS protein and both xanthophyll cycle enzymes VDE and ZEP in tobacco (Kromdijk et al., 2016). Transgenic tobacco VPZ overexpression lines exhibited no significant differences from wild type tobacco plants when grown under steady state light conditions. However, under fluctuating light, VPZ lines outperformed control plants by 15%–20% increase in biomass under greenhouse as well as field‐grown conditions, owing to faster NPQ relaxation kinetics and improved net CO2 assimilation. Since these NPQ components are highly conserved in higher plants, this approach has great potential of increasing future crop yields. Nevertheless, expression of a VPZ construct in Arabidopsis did not result in the previously observed growth advantage in tobacco lines (Garcia‐Molina and Leister, 2020), demonstrating that different mechanisms might be at work and that cloning vector units might need optimization in different plant species. Furthermore, an overexpression line of PsbS in rice did also show about 20% increase in canopy radiation use efficiency into biomass and grain yield under fluctuating light (Hubbart et al., 2018). Modeling and 3D reconstruction of the rice canopy revealed that leaves in the lower canopy have higher capacities for photoprotective NPQ than leaves from the upper canopy (Foo et al., 2020), which may reflect the greater occurrence of fluctuations in light due to shading from the upper canopy and sunflecks. These results show the importance of considering the canopy context when trying to design strategies for photosynthetic improvement. In contrast to overexpressing PsbS in rice, tobacco plants overexpressing PsbS did not reveal any gain in biomass compared to control plants when grown under controlled and field conditions (Głowacka et al., 2018). However, these plants did display an increased water use efficiency of 25%–33% due to dampening of the light‐induced increase in stomatal conductance. Nevertheless, photosynthesis in general was not affected by PsbS overexpression. The authors proposed that PsbS abundance might modulate the redox state of the plastoquinone pool in the thylakoid membrane, which had been put forward as an early signal for stomatal opening in response to light (Busch, 2014). Whereas these studies exemplify the importance of the PsbS protein, there might be other genetic factors that could be utilized to adjust NPQ traits for improved crop performance. Quantitative genetics and genome‐wide association studies targeting natural variation in NPQ traits have already been done in soybean, rice, and Arabidopsis, revealing 15–33 putative new targets for manipulation of NPQ (Jung and Niyogi, 2009; Herritt et al., 2016; Wang et al., 2017; Rungrat et al., 2019).
A different avenue to explore could be the introduction of a different xanthophyll cycle into future crop plants. While the violaxanthin cycle is the predominant xanthophyll cycle in most tested plant species, a lutein epoxide cycle works in parallel to the violaxanthin cycle in some nonmodel plant species (Bungard et al., 1999). Epoxidation of lutein confers higher light‐harvesting efficiency and has been successfully engineered into Arabidopsis lines through expression of a ZEP gene from Nannochloropsis oceanica (Leonelli et al., 2017). Transgenic plants only expressing the lutein epoxide cycle displayed significantly higher PSII maximum quantum efficiencies and similar NPQ kinetics compared to plants employing the violaxanthin cycle, pointing at the potential of this alternative xanthophyll cycle for future studies.
All these different approaches have one thing in common—they require acidification of the thylakoid lumen, that is, a low pH through accumulation of protons. Lumen acidification is also accompanied by fluxes of counter‐ions over the thylakoid membrane, which help to maintain the balance between the ΔpH and the electric field (ΔѰ) component of the pmf. Influx of potassium ions (K+) into the lumen upon the shift from light to dark for instance increases ΔѰ, thereby allowing a decrease in ΔpH and the relaxation of the qE component of NPQ without compromising overall pmf to maintain ATP synthase activity (simulated by Davis et al., 2017). Therefore, counter‐ion fluxes could possibly be a novel target for modification of NPQ mechanisms (Davis et al., 2017). Indeed, Armbruster and coworkers demonstrated the significance of ion fluxes in adaptation to fluctuating light conditions by the action of the K+/H+ exchange antiporter KEA3, the protein family of which is important for pH and osmoregulation inside the chloroplast (Armbruster et al., 2014, 2016; Kunz et al., 2014; Correa Galvis et al., 2020; Li et al., 2021). In higher plants, there are three splice forms of KEA3, each of which has a distinct function, but generally they export protons from the lumen with concurrent import of potassium ions. Knockout of the KEA3 gene in Arabidopsis resulted in a specific NPQ phenotype with higher NPQ levels upon the shift from dark to low light and slower NPQ relaxation from high to low light, which was attributed to the qE component of NPQ (Armbruster et al., 2014). Independent overexpression of the three KEA3 splice forms in the KEA3 knockout background both in Arabidopsis and transiently in Nicotiana benthamiana showed the specific contribution of each isoform to this NPQ phenotype. KEA3.2 is the most abundant isoform and its overexpression led to lower NPQ levels than the wild type in the initial induction response in the transition from dark to low light, but not at high light, reflecting the opposite trends in NPQ kinetics of the KEA3 knockout mutant. Thus, the oeKEA3.2 line also displayed significantly faster NPQ relaxation under fluctuating light through enhanced export of protons from the lumen. Overexpression of KEA3.3 had a similar low NPQ response to oeKEA3.2 in the shift from dark to low light. In contrast to oeKEA3.2 though, oeKEA3.3 also had a significantly lower NPQ amplitude in the transition to high light conditions, whereas oeKEA3.2 showed almost wild type levels during NPQ induction in high light despite its overexpression. These results indicate that KEA3.3 is more active than KEA3.2 in exporting protons from the lumen under high light stress, thus reducing and regulating overall NPQ levels. KEA3.2 and KEA3.3 splice forms distinguish by the presence of a KTN domain in KEA3.2, which is responsive to changes in NADH/NAD+ or ATP/ADP ratios or could possibly be regulated by the redox state of the plastoquinone pool (Armbruster et al., 2016). Overexpression of KEA3.1 did not show any special NPQ phenotype compared to the other isoforms and only slightly ameliorated the low/slow NPQ response of the KEA3 knockout mutant, which exhibited a significantly slower growth rate when grown under fluctuating light. Surprisingly, no significant growth advantage of the overexpression lines could be detected, although oeKEA3.2 showed trends of enhanced growth. Further analyses revealed that KEA3 activity requires more fine‐tuning in order to optimize photosynthesis (Wang et al., 2017) and that combinations with other players involved in the generation of a ΔpH gradient over the thylakoid lumen (NDH complex in cyclic electron flow) can amplify the KEA3 phenotype (Basso et al., 2020).
Many of the fore‐mentioned studies have not investigated the effects of ROS accumulation in the transgenic lines, but the manipulations are likely to have an impact on photo‐oxidative stress alleviation in addition to improved photosynthetic efficiencies (as suggested by Davis et al., 2017). Photo‐oxidative stress is established through the generation of the ROS singlet oxygen and superoxide, predominantly arising from PSII and PSI, respectively, through overreduction of the electron transfer chain. ROS mainly damage the core protein D1 of PSII, thereby downregulating photosynthesis under long‐term unfavorable conditions (photoinhibition). PSII employs an effective D1 repair cycle with de novo protein biosynthesis; however, this is a rather energy cost‐intensive process (Baena‐González and Aro, 2002). In attempts to engineer tobacco plants with enhanced resistance to drought stress, Almoguera and colleagues overexpressed the heat‐shock transcription factor A9 (HSFA9). This transcription factor was presumed to activate the expression of small heat‐shock proteins in chloroplasts and indeed conferred improved drought and oxidative stress tolerance as visible from sustained D1 protein levels after withholding of water (Almoguera et al., 2012). Similar results were achieved with an alternative approach in tobacco overexpressing a plastid‐encoded full‐length PsbA gene from maize under the control of the 35S promoter. These mutant lines had increased levels of the D1 protein and showed enhanced drought tolerance under stress conditions but wild type‐like growth phenotypes under optimal conditions (Huo et al., 2016). Following up on these studies, Chen and coworkers then combined and optimized both approaches and engineered a heat responsive PsbA construct expressed in the nucleus (Chen et al., 2020). The native PsbA gene is encoded in the chloroplast genome which is closer to the site of the PSII/D1 repair cycle for de novo synthesis upon recovery from photoinhibition. However, ROS production inside the chloroplast can strongly inhibit the translation of the PsbA messenger RNA (mRNA) into the D1 protein (Nishiyama et al., 2001, 2004). By transferring PsbA expression to the cell nucleus, D1 de novo synthesis could take place in the cytosol instead of the chloroplast. The mature protein was targeted to the chloroplast with the help of a chloroplast transit peptide (from RbcS) and remarkably, was able to replace degraded D1 protein in the PSII complex. Placing PsbA gene expression under the control of a heat responsive promoter from the HSFA2 (heat‐shock transcription factor A2) gene, enhanced gene expression upon exposure to increased temperatures. This presumably explained the enhanced heat stress tolerance in transgenic lines of Arabidopsis, tobacco, and rice, all of which also had significantly enhanced growth, biomass and grain yield both under nonstressed conditions and in field trials. Altogether, these results prove that photosynthesis can efficiently be upregulated through mitigation of photo‐oxidative stress and photoinhibition, leading to yield benefits in monocot as well as dicot plant species.
Increasing electron transport capacity
Photosynthesis is composed of a series of electron transfer steps in the light reactions and enzymatic reactions in the Calvin–Benson–Bassham cycle. One of the major protein complexes within the thylakoid membrane besides the two photosystems is the cyt b6f complex, which is reduced and oxidized by the mobile electron carriers plastoquinol and plastocyanin, respectively, in LET between PSII and PSI. Via the Q‐cycle, the cyt b6f complex also transfers protons across the thylakoid membrane into the lumen in addition to its electron transfer function, thus contributing to the generation of a lumenal ΔpH gradient that fuels ATP synthase activity and the initiation of NPQ upon illumination. Electron flow through cyt b6f has long been proposed to be the key rate‐limiting step of the photosynthetic light reactions, since mutants of several plant species with inhibited cyt b6f expression showed downregulated photosynthetic capacity (Holloway et al., 1983; Price et al., 1995, 1998; Anderson et al., 1997; Ruuska et al., 2000; Yamori et al., 2011, 2016; Tikhonov, 2014). One of the eight cyt b6f subunits is the Rieske‐FeS protein, which is encoded in the nucleus and the expression of which determines the accumulation of the entire cyt b6f complex (Anderson et al., 1997; Price et al., 1998). Overexpressing this subunit both in a C3 (Arabidopsis, Simkin et al., 2017b) and a C4 (Setaria viridis, Ermakova et al., 2019) plant species gave rise to increased abundance of the cyt b6f complex and enhanced photosynthetic performance. In both studies, transgenic overexpression plants had higher PSII quantum efficiencies, lower NPQ values and higher CO2 assimilation rates than control plants. In contrast to Arabidopsis transgenic plants, Setaria overexpression lines were not reported to display any growth advantage over wild type plants, despite the 10% increase in CO2 assimilation. Arabidopsis overexpression lines, on the other hand, had 30%–70% more biomass and up to 50% greater seed yield compared to control plants. In both species, nevertheless, the abundance of the cyt b6f complex and the rate of CO2 fixation seem to be correlated through control of the electron transfer rate by cyt b6f. It will be interesting to see whether this crop improvement strategy could have similar outcomes in other C3 and C4 crop species in the future.
The next step within photosynthetic light reactions downstream of the cyt b6f complex involves electron transfer through the thylakoid lumen toward PSI via the soluble protein plastocyanin, which is encoded by two genes (PetE) in higher plant genomes. This electron carrier has been shown to be essential for light‐dependent electron transfer as a double knockout mutant of both PetE genes is only viable when grown heterotrophically on media containing sucrose, but not when grown on soil only (Weigel et al., 2003). Therefore, plastocyanin‐mediated electron transfer might be a rate‐limiting step in higher plant photosynthesis as well (Burkey, 1994; Burkey et al., 1996; Schöttler et al., 2004; Finazzi et al., 2005; Höhner et al., 2020), although even low levels of this protein seem capable of efficiently sustaining photosynthetic light reactions (Abdel‐Ghany, 2009; Pesaresi et al., 2009). Attempts have been made to overexpress plastocyanin genes in Arabidopsis, which indeed resulted in enhanced biomass production of up to 1.6‐fold compared to the wild type, even though photosynthetic parameters were similar to wild type levels and no improvement of photosynthetic capacity could be observed (Pesaresi et al., 2009). This phenomenon could possibly be explained with the newly discovered role of plant plastocyanins in oxidative stress tolerance (Zhou et al., 2018). Plastocyanins are copper‐binding proteins and expression of the PetE genes is highly dependent on copper availability. While PetE2 is the predominant plastocyanin expressed under nonstress and copper‐enriched conditions and acts as a buffer in copper homeostasis, PetE1 expression increases upon copper starvation and functionally replaces PetE2 (Abdel‐Ghany, 2009). Under stress conditions, copper is released into the chloroplast via two P‐type ATPases (Abdel‐Ghany et al., 2005) and reacts with H2O2 in a Fenton reaction, generating the highly reactive ROS hydroxyl radical (Sutton and Winterbourn, 1989). Through introduction of the PetE2 gene from the halophytic plant Suaeda salsa (Song and Wang, 2014) into Arabidopsis, a plastocyanin with a greater copper‐binding capacity was able to confer a greater tolerance of transgenic Arabidopsis lines to oxidative stress than the original Arabidopsis plastocyanins (Zhou et al., 2018). This resulted in stress‐tolerant Arabidopsis plants with a fresh weight three to four times higher than that of wild type plants grown under ROS producing stress conditions. It is, therefore, of high importance to explore natural variation of protein features, especially in plant species living in extreme environments and to exploit their potential of being translated into agricultural crop species. In addition, it may also be important to look further than just proteins for the regulation of photosynthetic processes. Other regulatory elements, such as microRNAs, for post‐transcriptional gene expression regulation have gained more and more interest in the past 20 years (Meyers and Axtell, 2019; Wang et al., 2019). So far, no microRNA has been discovered that directly controls plastocyanin gene expression. However, through an indirect mechanism, one of the microRNAs with high conservation in plants, miR408, was found to impact copper levels inside the chloroplast through gene regulation of two copper transporters in the chloroplast envelope and thylakoid membranes. Overexpression of miR408, hence, led to increased expression of plastocyanin as well as other photosynthetic genes, resulting in enhanced biomass accumulation of 10%–20% in Arabidopsis, tobacco, and rice plants (Pan et al., 2018). Interestingly, this approach not only yielded improved vegetative plant growth but also boosted seed and grain size and weight even under field‐grown conditions. Moreover, miR408 seems to be highly conserved in eudicot and monocot plant species, making it a promising target for improved crop growth.
The last step of LET encompasses the protein FNR, which accepts electrons from Fd and subsequently reduces NADP+ to NADPH. Efforts have been made to overexpress FNR in tobacco, but no growth phenotype could be observed despite an increase in oxidative stress tolerance (Rodriguez et al., 2007). Knockdown of FNR, on the other hand, drastically decreased the mutants' photosynthetic capacity and made it highly susceptible to oxidative stress (Lintala et al., 2012). However, recently it has been proposed that the interaction between FNR and the Thylakoid RhOdanase‐Like protein (TROL), the protein that putatively binds FNR to the thylakoid membrane, could have potential for crop improvement (Fulgosi and Vojta, 2020). TROL is a membrane‐spanning protein close to PSI in the grana margins, with a rhodanase‐like domain (RHO) exposed to the thylakoid lumen. Here, the RHO domain may be involved in sensing redox signals, upon which FNR‐binding on the stromal side to the membrane recruiting motif (MRM) of TROL is adjusted, possibly in a light‐ and pH‐dependent manner. It is postulated that FNR is membrane‐bound in the dark, reversing its function and providing reduced Fd to a number of metabolic pathways, including oxidative stress tolerance. Upon light exposure, FNR is released into the stroma, only then being active in LET toward NADPH regeneration. Hence, TROL could potentially be a target for the switch in FNR action mode, either through modifications of the redox sensor domain RHO or the FNR‐binding domain MRM.
Instead of forwarding electrons to FNR, Fd can also reduce components of AET routes to stimulate ATP synthesis under stress conditions. However, the exact mechanisms are not yet clear and contrasting views on the function of these AET complexes as alternative electron acceptors downstream of PSI have recently been brought forward (Nawrocki et al., 2019a; Buchert et al., 2020; Rantala et al., 2020; Zhou et al., 2020; Rühle et al., 2021; Wu et al., 2021). Nevertheless, it is well‐accepted that the energy balance is of high importance when it comes to altering metabolic processes (Kramer and Evans, 2010). Reducing the levels of PGR5 protein seems to negatively impact plant growth to some extent under fluctuating light conditions (Munekage et al., 2008; Nishikawa et al., 2012), whereas in Chlamydomonas, deletion of this protein promotes biotechnological hydrogen production due to rerouting of electrons toward the hydrogenase HydA upon stress induction (Steinbeck et al., 2015; Nagy et al., 2021). Relative to C3 species, C4 plant species rely more strongly on CET around PSI in bundle sheath cells in order to provide extra ATP to fuel the carbon concentrating mechanism. However, although overexpression of PGR5 in Flaveria bidentis led to alleviation of PSI acceptor side limitation, it did not result in enhanced CO2 fixation (Munekage et al., 2010; Tazoe et al., 2020). In contrast, overexpression of PGR5 resulted in increased growth rates in diatoms under fluctuating light (Zhou et al., 2021) and enhanced high light and drought stress in Arabidopsis (Long et al., 2008). In addition, PTOX is also important as an alternative electron sink and for chloroplast biogenesis and carotenoid biosynthesis during early leaf development, and induction of its expression under stress conditions (summarized in Sun and Wen, 2011 and Johnson and Stepien, 2016; Ivanov et al., 2012; Li et al., 2016a; Ghotbi‐Ravandi et al., 2019) led to the suggestion that it could be a suitable target for enhancing stress tolerance in plants (Johnson and Stepien, 2016). However, initial experiments overexpressing PTOX in Arabidopsis and tobacco did not result in enhanced tolerance to high light stress but instead made plants more photosensitive (Joët et al., 2002; Rosso et al., 2006; Heyno et al., 2009; Ahmad et al., 2012, 2020). Nevertheless, overexpression of PTOX did provide an advantage when exposed to salt stress. This effect seemed to rely on a translocation of PTOX from the stroma lamellae to the appressed grana stacks in salt‐tolerant plants (Stepien and Johnson, 2018; Ahmad et al., 2020). This correlation between PTOX expression and salt stress tolerance was also recently confirmed in a halophyte C4 plant species (Essemine et al., 2020).
Instead of indirectly upregulating the production of ATP through upregulation of CET routes, it is conceivable to directly enhance chloroplast ATP synthase activity (Cardona et al., 2018; Davis and Kramer, 2020). The ATP synthase is composed of two rotary motor complexes (CF0/CF1) which are connected by two flexible stalks (Kühlbrandt, 2019). The CF0 complex is embedded within the thylakoid membrane and consists of a ring of several c‐subunits, the numbers of which are organism/species‐dependent but remain constant under different conditions (between eight and 17 c‐subunits; Davis and Kramer, 2020). Each c‐subunit binds one proton from the thylakoid lumen, fueling the CF0 motor and subsequently the CF1 motor on the stromal side of the membrane, thus promoting the production of three ATP molecules per 360° rotation. Davis and Kramer (2020) recently proposed a theoretical model from kinetic simulations of photosynthetic reactions that considers the size of the c‐ring/number of c‐subunits per ring and therefore the ratio of required protons per generated ATP. A lower ratio (a smaller c‐ring size) would theoretically result in higher photosynthetic energy conversion rates. However, the simulations predicted that a smaller ring size would also lead to a higher ΔpH across the membrane, thus activating photoprotective NPQ mechanisms and limiting photosynthetic efficiencies even under dark conditions. Evolution has therefore favored a bigger ring size instead, in order to avoid photodamage at the cost of conversion efficiencies. Nevertheless, the concept of optimizing the H+/ATP ratio (Pogoryelov et al., 2012) could provide a novel avenue for improving photosynthesis, considering further adjustments to the photosynthetic machinery. In a different approach, mutant lines with point mutations in ATP synthase genes were analyzed in different organisms. In the cyanobacterium Synechococcus elongatus sp. PCC 7942, a single nucleotide polymorphism (SNP) was identified in the atpA gene, which enhanced ATP synthase contents and activity, conferring improved photosynthetic efficiency as well as carbon fixation rate, especially under stress conditions (Lou et al., 2018). Certain point mutations in the AtpB gene of the CF1 complex, on the other hand, appear to be deleterious for the assembly of the entire complex and normal plant growth, so that mutants spontaneously reverted to the wild type gene sequence (Robertson et al., 1989; Malinova et al., 2021). Additionally, a SNP of a threonine residue in the β‐subunit of CF1 (T86A in AtpB) was identified in cold‐tolerant cucumber species when the gene sequences were compared to cold‐susceptible species. Threonine residues are often subject to post‐translational modification with regulatory phospho groups. Lack of a phosphorylation site in AtpB due to this SNP could possibly change its mode of action and therefore confer improved tolerance to cold stress (Oravec and Havey, 2021). Furthermore, an Arabidopsis mutant line with altered ATP synthase regulation was isolated from an ethyl methanesulfonate library, revealing a point mutation in the γ1‐subunit of the central stalk (Wu et al., 2007; Kanazawa et al., 2017). This mutation resulted in about 50% loss of ATP synthase protein content without compromising its overall activity under low light conditions when compared to the wild type, indicating a higher activity of the remaining complexes in the mutant. However, when exposed to stressful conditions, such as low CO2 and fluctuating light, the mutant was much more susceptible to photoinhibition. The authors concluded that this particular protein residue is involved in the stress‐related downregulation of the ATP synthase activity, rendering a fraction of the ATP synthase pool inactive to prevent overreduction of the electron chain (Kanazawa et al., 2017). These results show that it is not sufficient to simply aim for constantly enhanced ATP synthase activity, but that it is necessary to consider its regulatory mechanisms under suboptimal conditions as well to maintain the plant's capacity for the induction of NPQ and a healthy ATP to NADPH ratio. Such control mechanisms do not only act on the membrane‐bound CF0 complex through the pmf but are also administered through thiol‐based redox regulation of cysteine residues on the γ‐subunit of the CF1 motor (Yang et al., 2020; Buchert et al., 2021).
Thioredoxins are proteins that reduce disulphide bonds of cysteines in a light‐dependent manner, thus regulating protein activity (Nikkanen and Rintamäki, 2019). A chloroplastic NADPH‐dependent thioredoxin reductase C (NTRC) is known to interact with the ATP synthase γ‐subunit (Nikkanen et al., 2016). Overexpression of NTRC in Arabidopsis resulted in significantly enhanced biomass accumulation, starch production, photosynthetic efficiency, NDH‐dependent CET and photo‐oxidative, drought and heat stress tolerance compared to the wild type, especially under low light conditions, which was mainly attributed to lower acceptor side limitation of PSI (Chae et al., 2013; Toivola et al., 2013; Nikkanen et al., 2016, 2018; Kim et al., 2017). Interestingly, the overexpression mutant also had lower NPQ under light‐limiting conditions up to about 500 µmol photons/m2/sec and displayed much faster NPQ relaxation upon high to low light transitions under fluctuating light conditions (Guinea Diaz et al., 2020). However, NTRC overexpression in tobacco resulted in a slight growth retardation during early plant development when compared to the wild type despite higher leaf starch content (Ancín et al., 2019). The increased starch content was suggested to derive from decreased starch turnover during the night rather than enhanced starch biosynthesis during the day. A connection between redox regulation and starch metabolism is present in a range of plant species and organs (Sanz‐Barrio et al., 2013; Hou et al., 2019) and might have potential for future bioengineering of starchy crops (Nikkanen et al., 2017).
Translating strategies from lower plants/microalgae/cyanobacteria into higher plants
Throughout evolution, oxygenic photosynthesis has developed in cyanobacteria first and subsequently in microalgae and plants upon endosymbiotic events with heterotrophic eukaryotes. Different photosynthetic organisms had to adjust to different environmental conditions, depending on the prevalent light and CO2 levels. Many years of basic research have revealed different traits and mechanisms and variations in protein complexes between aquatic and terrestrial species that have been optimized to adjust to certain stress conditions. It is, therefore, of high interest to translate this basic knowledge of possibly more effective ancestral proteins and potentially advantageous mechanisms from lower organisms into higher plants to ultimately improve photosynthetic performance in crops. Successful examples of this concept include the expression of cyanobacterial/algal Calvin cycle enzymes in several plant and crop species (recently reviewed in Simkin et al., 2019).
One success story has been the introduction of the algal cytochrome c6 (cyt c6) protein into Arabidopsis and tobacco, which improved photosynthetic electron transfer and biomass accumulation even under field conditions (Chida et al., 2007; Yadav et al., 2018; López‐Calcagno et al., 2020). This soluble protein is present in many cyanobacteria and green algae species and is located in the thylakoid lumen where it shuttles electrons between the cyt b6f complex and PSI for light‐dependent LET of photosynthesis, in the same fashion plastocyanin operates, as well as between cyt b6f and terminal oxidases for respiration in cyanobacteria (Torrado et al., 2019; Viola et al., 2021). Cyt c6 and plastocyanin expression levels are dependent on iron and copper availability, respectively, (García‐Cañas et al., 2021) as plastocyanin binds copper, whereas cyt c6 is an iron‐binding protein that seems to have been lost in green plants after the Great Oxidation Event (recently reviewed in Castell et al., 2021b and Slater et al., 2021). When the atmosphere became enriched in oxygen, which readily reacts with iron, the level of available iron ions as co‐factors for cyt c6 was limited, thus promoting the activity of plastocyanin in green plants instead. In cyanobacteria, there are two more cyt c6 gene homologs present, which were proposed to have arisen from cyt c6 gene duplications and were annotated as cyt c6B. Interestingly, green plants lost the cyt c6 and cyt c6B genes but instead contain a gene homolog, cyt c6A, which likely evolved from cyt c6B through insertion of a loop insertion peptide (Slater et al., 2021). However, both cyt c6A and c6B proteins show much lower redox midpoint potentials compared to cyt c6 and are, therefore, not likely to contribute to electron transfer in the thylakoid lumen, in contrast to cyt c6 (Molina‐Heredia et al., 2003; Bialek et al., 2014). It has been demonstrated that cyt c6 proteins from different algae/seaweed species are suitable for introduction into plant model species to effectively perform electron transfer, having been selected based on a similar redox midpoint potential to plant plastocyanins. While Chida and coworkers inserted a cyt c6 gene from the red alga Porphyra yezoensis into Arabidopsis (Chida et al., 2007), Yadav and coworkers utilized a cyt c6 gene from the green macroalga Ulva fasciata (sea lettuce) and introduced it into tobacco (Yadav et al., 2018). In both cases, the algal genes were under the control of the constitutive cauliflower mosaic virus 35S (CaMV35S) promoter and were fused to a plant species‐specific PetE transit peptide for correct localization of the cyt c6 protein into the chloroplast thylakoid lumen. Both studies reported enhanced growth phenotypes during the first 8 weeks of plant growth, in accordance with increased chlorophyll and photosynthetic metabolite contents, although other photosynthetic parameters were only slightly improved. An even more advanced approach was recently undertaken by López‐Calcagno and coworkers by combining enhancements of LET and carbon fixation in two tobacco cultivars through addition of an algal cyt c6 gene from Porphyra umbilicalis and the bifunctional cyanobacterial FBP/SBPase from Synechocystis sp. PCC 7942 or SBPase gene from higher plants, respectively (López‐Calcagno et al., 2020). While the single mutants had slightly increased photosynthetic rates, the double mutants showed significantly enhanced CO2 assimilation rates (up to 15% more than control plants) and PSII operational efficiencies. Consequently, single mutants had 9%–44% and double mutants had 32%–52% more biomass than control plants when grown in a controlled environment in a glasshouse. Field experiments with these mutants, on the other hand, showed much more variation in terms of biomass gains. During a small field trial in 2016, leaf material from single mutants was harvested before the flowering stage and again displayed a 20%–44% increase in biomass accumulation. However, in the following field season only a small growth advantage of the double mutants compared to control plants was visible when plant material was harvested after the onset of flowering. Surprisingly, photosynthetic parameters were not significantly different from control plants, although an improved intrinsic water use efficiency could be measured. Nevertheless, these mutant constructs targeting both the light reactions as well as carbon fixation seem to have great potential for improving crop yields, especially for plant species with short generation times. In addition, it was also demonstrated that introduction of a plastocyanin gene from Chlamydomonas into the diatom Phaeodactylum tricornutum, which normally only contains cyt c6, can enhance biomass production under iron‐deficient conditions by 60% when compared to the wild type (Castell et al., 2021a). All these studies show that it would be indeed beneficial for photosynthetic organisms to employ both soluble electron carriers plastocyanin and cyt c6 since either protein can functionally replace the other one when nutrients are short of either iron or copper ions, respectively, leaving room for biotechnological improvements of crop yields.
A similar story to plastocyanin and cyt c6 can be told about the Fd and flavodoxin proteins in plants and cyanobacteria, respectively. Fd is a conserved FeS electron carrier in cyanobacteria and plants, whereas flavodoxin binds flavin mononucleotide as cofactor and was lost from plants and green algae during the course of evolution due to its functional redundancy. In contrast to plastocyanin/cyt c6, no definite answer has been found yet as to why the iron‐containing Fd has survived the Great Oxidation Event and has been maintained in plants, where flavodoxin has not (Pierella Karlusich et al., 2014). In the past 15 years, many studies on the expression of a cyanobacterial flavodoxin in plants have been published. Most of these studies reported on enhanced tolerance of transgenic plants to different environmental stresses, particularly iron deficiency and oxidative stress, under which Fd levels would normally decrease but can now be compensated for by flavodoxin expression (Tognetti et al., 2006, 2007a, 2007b; Zurbriggen et al., 2008; Shvaleva et al., 2009; Coba de la Peña et al., 2010; Ceccoli et al., 2012; Lodeyro et al., 2012; Gharechahi et al., 2015; Mayta et al., 2018; Gómez et al., 2020; Niazian et al., 2021). It was also shown that flavodoxin could functionally replace Fd in plants (Blanco et al., 2011) and additional expression of a cyanobacterial FNR could increase oxidative stress tolerance even more (Giró et al., 2011). Flavodoxin expression is not always beneficial and may lead to stunted plant growth (Li et al., 2016b; Mayta et al., 2019) although detrimental effects on yield were offset by a higher harvest index compared to wild type plants (Mayta et al., 2019). Overall, flavodoxin expression in agricultural crops seems to have great potential to enhance abiotic stress tolerance.
Another class of photosynthetic flavoproteins that disappeared in flowering plants (angiosperms) throughout evolution are flavodiiron proteins (FDPs). FDPs serve as photoprotective excess electron valves in the so‐called “Mehler‐like reaction” or water–water cycle of photosynthesis (Allahverdiyeva et al., 2015; Ilík et al., 2017; Alboresi et al., 2019) across a large part of the green lineages from cyanobacteria up to gymnosperms. In these organisms, electron transfer on the acceptor side of PSI, initiated by the splitting of water at PSII, can switch from FNR and the regeneration of NADPH to FDPs in an alternative pathway under stress conditions. When the electron transfer chain becomes over‐reduced, FDPs help to release excess electron pressure downstream of PSI by reducing molecular oxygen to water, thereby closing the water–water cycle and preventing the generation of the highly reactive ROS superoxide for photoprotection of PSI. In cyanobacteria, FDPs are divided into two clusters and either work as homodimers (Mustila et al., 2016) or heterodimers composed of one FDP of each cluster (Santana‐Sanchez et al., 2019). The heterodimer Flv1/3 is conserved in all cyanobacteria and is responsible for oxygen photoreduction downstream of PSI in a Mehler‐like reaction that does not produce ROS (Allahverdiyeva et al., 2013), whereas the heterodimer Flv2/4 is only present in β‐cyanobacteria and was reported to be involved in photoprotection of both PSII and PSI (Zhang et al., 2009, 2012; Bersanini et al., 2014, 2017; Chukhutsina et al., 2015; Santana‐Sanchez et al., 2019). In angiosperms, in which FDPs are absent, introduction of two FDPs could therefore possibly replace several ROS scavenging enzymes and reactions, thus saving energy and nitrogen sources or adding extra protection. Indeed, transgenic lines of tobacco, Arabidopsis and barley expressing cyanobacterial Flv1/3 or Flv2/4 proteins in chloroplasts showed that FDPs are able to act as additional electron sinks in plants as well, particularly under stress conditions, such as drought and fluctuating light stress, thereby improving photosynthetic performance (Gómez et al., 2018; Tula et al., 2020; Shahinnia et al., 2021; Vicino et al., 2021). In other instances, two FDPs from the moss Physcomitrella patens were introduced into Arabidopsis and rice, revealing similar effects of FDPs and CET in maintaining the pmf under unfavorable growth conditions (Yamamoto et al., 2016; Wada et al., 2018). In summary, both sets of FDPs seem to fulfill similar roles when expressed in angiosperms, even though they were proposed to have different photoprotection targets (PSI vs. PSII) in cyanobacteria. In one case, transgenic plants even displayed improved biomass accumulation under nonstress conditions (Tula et al., 2020), suggesting that FDPs are promising tools for bioengineering of future crops (Mullineaux, 2016).
Further cyanobacterial systems with potential for improving photosynthetic efficiencies in plants are currently under investigation and include the OCP and novel chlorophylls with absorption wavelengths in the far‐red spectrum. These red‐shifted chlorophylls d and f with absorption maxima at 740 and 760 nm, respectively, have been discovered in certain cyanobacterial species (Li and Chen, 2015). The novel chlorophyll d is predicted to be able to bind to LHC proteins, expanding the range of light absorption of chlorophyll a beyond 700 nm into the far‐red region, which is often found in the lower canopy of plants and could therefore enhance light harvesting and boost crop yields (Elias et al., 2021). Cyanobacterial OCP, on the other hand, absorbs wavelengths in the blue–green region and is attached to the light‐harvesting antennae (phycobilisomes), where it is responsible for photoprotective dissipation of excess light energy as heat (NPQ) and scavenging of ROS (Muzzopappa and Kirilovsky, 2020). This photoreceptor protein consists of an effector N‐terminal domain (NTD), a sensor C‐terminal domain (CTD), and one ketocarotenoid (3‐hydroxyechinenone, echinenone, canthaxanthin) or the xanthophyll zeaxanthin. Upon absorption of strong blue–green light, the noncovalently bound ketocarotenoid transfers from the CTD to the NTD, thereby undergoing a conformational change and a shift in color from orange to red, thus activating the quenching state. OCP has not only been expressed in ketocarotenoid‐producing microalgae for better solubilization of the ketocarotenoids canthaxanthin and astaxanthin for their use as nutraceuticals (Pivato et al., 2021), but is also being exploited as a possible photoswitchable protein in plants with implementations for plastid optogenetics, artificial photosynthesis and synthetic biology due to its light‐dependent conformational changes and uniqueness in cyanobacteria (Andreoni et al., 2017; Lechno‐Yossef et al., 2017; Dominguez‐Martin and Kerfeld, 2019; Piccinini et al., 2021).
CONCLUSIONS
“Photosynthesis: Ancient, essential, complex, diverse… and in need of improvement in a changing world” (Niinemets et al., 2016). This title of a conference summary article neatly describes the need for a shift in understanding photosynthesis and its potential applications. Photosynthetic organisms have optimized photosynthesis according to their needs, which includes survival and reproduction rather than enhanced yields. Modern techniques of bioengineering, through synthetic biology and gene editing, have provided useful means to targeting specific features of metabolic pathways in a timelier manner than conventional breeding does and are rapidly gaining traction in re‐engineering of photosynthesis (Zhu et al., 2020). Our review has highlighted research demonstrating that it is possible to improve photosynthetic electron transport (Figure 7; Table 1). Targeting the light‐harvesting antenna size is a high potential approach in both plants as well as photosynthetic micro‐organisms, with a three‐fold increase in microalgae biomass (Negi et al., 2020) and 25%–40% more plant biomass in transgenic tobacco and Camelina, respectively (Kirst et al., 2017; Friedland et al., 2019). Furthermore, improving photoprotective traits, such as NPQ relaxation and D1 photoprotection, resulted in 15%–20% enhanced growth in field‐grown plants (Kromdijk et al., 2016; Hubbart et al., 2018; Chen et al., 2020). LET reactions are very well studied and gene expression manipulation of the Rieske‐FeS protein of the Cyt b6f complex and the mobile electron carrier plastocyanin were revealed as the most promising targets for boosting plant yields (Simkin et al., 2017b; Pan et al., 2018). AET pathways in higher plants, on the other hand, are less well understood, and manipulation of the putative components seems to affect stress tolerance rather than enhance photosynthetic efficiency. In contrast, introduction of alternative electron carriers and acceptors from lower plants, microalgae and cyanobacteria, which often show higher efficiencies than their equivalents in higher plants, could restore electron transfer pathways that were lost in higher plants during the course of evolution and improve plant growth (cyt c6: Chida et al., 2007; Yadav et al., 2018; López‐Calcagno et al., 2020; FDPs: Tula et al., 2020). In addition to the strategies discussed, combinations of different approaches may be strongly synergistic and when translated into staple crop species may allow an even greater boost in photosynthetic efficiency and crop productivity.
Figure 7.
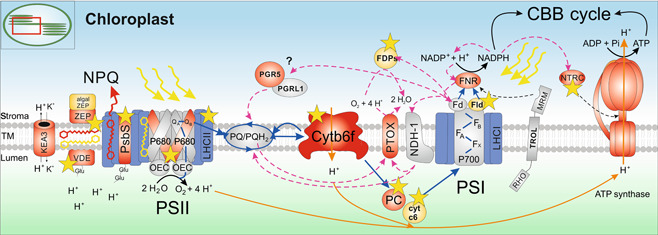
Schematic overview of light reaction components that have been targeted for bioengineering improved crops
Strategies for boosting plant photosynthesis through manipulations of light reaction components are highlighted in either red or blue in this depiction of Figure 3. Components that have not been investigated further yet are depicted in gray. Proteins and protein complexes that are highlighted in red have been directly overexpressed or their expression has been indirectly induced and resulted in a measurably improved phenotype compared to control plants. Blue highlighting of protein complexes indicates downregulation of these components. Yellow–orange proteins symbolize the introduction of alternative pathways deriving from lower plants, microalgae and cyanobacteria, including an algal zeaxanthin epoxidase (ZEP), cytochrome c6 (cyt c6), flavodoxin (FLD), and flavodiiron proteins (FDPs). Phenotypes were either associated with higher biomass accumulation (labeled with yellow asterisks) or enhanced abiotic stress tolerance (see also Table 1). For further explanation of the light reactions see the original Figure 3.
Table 1.
Overview of different approaches used to improve light reactions
| Strategy and gene of interest | Targeted process or protein complex | Species | Phenotype associated with increased biomass accumulation | Phenotype associated with enhanced abiotic stress tolerance | References |
|---|---|---|---|---|---|
| Introduction of a maize GOLDEN2‐LIKE (GLK) transcription factor for overexpression of photosynthetic genes | LHCs | Rice | 30%–40% | — | Li et al., 2020 |
| Knockout of HIGH PHOTOSYNTHETIC EFFICIENCY1 (HPE1) for impaired chlorophyll biogenesis | LHCs | Arabidopsis | Yes | — | Jin et al., 2016 |
| Truncated light‐harvesting antennae mutant | LHCs | Tobacco | Yes | — | Kirst et al., 2017 |
| RNAi of CAO for reduced chlorophyll b expression | LHCs | Camelina sativa | 40% | — | Friedland et al., 2019 |
| Overexpression of PsbS, VDE and ZEP | NPQ | Tobacco | 15%–20% | — | Kromdijk et al., 2016 |
| Overexpression of KEA3 | NPQ | Arabidopsis and tobacco | Slightly | — | Armbruster et al., 2016 |
| Overexpression of HSFA9 for D1 protection | Photoprotection | Tobacco | — | Drought stress | Almoguera et al. 2012 |
| Overexpression of maize PsbA | Photoprotection | Tobacco | — | Drought stress | Huo et al., 2016 |
| Engineering a heat responsive PsbA construct expressed in the nucleus | Photoprotection | Arabidopsis, tobacco, rice | Yes (also under nonstress conditions) | Heat stress | Chen et al., 2020 |
| Overexpression of the Rieske‐FeS protein | cyt b6f | Arabidopsis, Setaria viridis | 30%–70% (Arabidopsis) | — | Simkin et al., 2017b |
| 10 increased CO2 assimilation rate in Setaria | Ermakova et al., 2019 | ||||
| Overexpression of plastocyanin | Electron transfer | Arabidopsis | 1.6‐fold | — | Pesaresi et al., 2009 |
| Overexpression of PetE2 from Suaeda salsa | Electron transfer by plastocyanin | Arabidopsis | 3–4‐fold | Oxidative stress | Zhou et al., 2018 |
| Overexpression of miR408 for enhanced copper uptake into the chloroplast | Electron transfer by plastocyanin | Arabidopsis, tobacco, rice | 10%–20% | Pan et al., 2018 | |
| Overexpression of FNR | Electron transfer | Tobacco | — | Oxidative stress | Rodriguez et al., 2007 |
| Overexpression of (algal) PTOX | AET | Arabidopsis, Eutrema salsugineum, tobacco | — | Salt stress | Stepien and Johnson 2018; Ahmad et al., 2020 |
| SNPs in atpA for enhanced ATP synthase levels | ATP synthesis | Synechococcus elongatus sp. PCC 7942 | — | Heat stress | Lou et al., 2018 |
| SNPs in the β‐subunit of CF1 | ATP synthesis | Cucumber | — | Cold stress | Oravec and Havey 2021 |
| Overexpression of NTRC | Redox regulation | Arabidopsis, tobacco | Yes | Oxidative/drought/heat stress | Chae et al., 2013; Toivola et al., 2013; Nikkanen et al., (2016, 2018; Kim et al., 2017; Ancín et al. 2019 |
| Overexpression of algal cyt c6 | Electron transfer | Arabidopsis, tobacco | Up to 50% | — | Chida et al., 2007; Yadav et al., 2018; López‐Calcagno et al., 2020 |
| Overexpression of cyanobacterial flavodoxin | Electron transfer | Tobacco | — | Iron deficiency, oxidative stress | Tognetti et al., 2006, 2007a, 2007b; Zurbriggen et al., 2008; Shvaleva et al., 2009; Coba de la Peña et al. 2010; Giró et al., 2011; Ceccoli et al., 2012; Lodeyro et al., 2012; Gharechahi et al., 2015; Mayta et al., 2018; Gómez et al., 2020; Niazian et al., 2021 |
| Overexpression of cyanobacterial and moss FDPs | AET | Arabidopsis, tobacco, barley, rice | Yes | Drought/fluctuating light stress | Yamamoto et al., 2016; Gómez et al., 2018; Wada et al., 2018; Tula et al., 2020; Shahinnia et al., 2021; Vicino et al., 2021 |
Abbreviations: AET, alternative electron transfer; ATP, adenosine triphosphate; LHC, light‐harvesting complex; NPQ, nonphotochemical quenching.
CONFLICTS OF INTEREST
The authors declare there are no conflicts of interest.
ACKNOWLEDGEMENTS
This work was supported by the Realizing Increased Photosynthetic Efficiency (RIPE) project at the University of Illinois via a subaward to Johannes Kromdijk. RIPE is possible through support from the Bill & Melinda Gates Foundation, Foreign, Commonwealth & Development Office, and the Foundation for Food and Agriculture Research Grant No. OPP1172157.
Biographies


Walter, J., and Kromdijk, J. (2022). Here comes the sun: How optimization of photosynthetic light reactions can boost crop yields. J. Integr. Plant Biol. 64: 564–591.
Edited by: Zhizhong Gong, China Agricultural University, China
REFERENCES
- Abbasi, A.Z. , Bilal, M. , Khurshid, G. , Yiotis, C. , Zeb, I. , Hussain, J. , Baig, A. , Shah, M.M. , Chaudhary, S.U. , Osborne, B. , and Ahmad, R. (2021). Expression of cyanobacterial genes enhanced CO2 assimilation and biomass production in transgenic Arabidopsis thaliana . PeerJ 9: e11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel‐Ghany, S.E. (2009). Contribution of plastocyanin isoforms to photosynthesis and homeostasis in Arabidopsis thaliana grown at different copper regimes. Planta 229: 767–779. [DOI] [PubMed] [Google Scholar]
- Abdel‐Ghany, S.E. , Müller‐Moulé, P. , Niyogi, K.K. , Pilon, M. , and Shikanai, T. (2005). Two P‐type ATPases are required for copper delivery in Arabidopsis thaliana chloroplasts. Plant Cell 17: 1233–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad, N. , Michoux, F. , and Nixon, P.J. (2012). Investigating the production of foreign membrane proteins in tobacco chloroplasts: Expression of an algal plastid terminal oxidase. PLoS ONE 7: e41722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad, N. , Khan, M.O. , Islam, E. , Wei, Z.Y. , McAusland, L. , Lawson, T. , Johnson, G.N. , and Nixon, P.J. (2020). Contrasting responses to stress displayed by tobacco overexpressing an algal plastid terminal oxidase in the chloroplast. Front. Plant Sci. 11: 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth, E.A. , and Long, S.P. (2021). 30 years of free‐air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Glob. Chang. Biol. 27: 27–49. [DOI] [PubMed] [Google Scholar]
- Alboresi, A. , Storti, M. , Cendron, L. , and Morosinotto, T. (2019). Role and regulation of class‐C flavodiiron proteins in photosynthetic organisms. Biochem. J. 476: 2487–2498. [DOI] [PubMed] [Google Scholar]
- Allahverdiyeva, Y. , Isojärvi, J. , Zhang, P. , and Aro, E.‐M. (2015). Cyanobacterial oxygenic photosynthesis is protected by flavodiiron proteins. Life (Basel) 5: 716–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahverdiyeva, Y. , Mustila, H. , Ermakova, M. , Bersanini, L. , Richaud, P. , Ajlanie, G. , Battchikova, N. , Cournac, L. , and Aro, E.‐M. (2013). Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc. Natl. Acad. Sci. USA. 110: 4111–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almoguera, C. , Prieto‐Dapena, P. , Personat, J.‐M. , Tejedor‐Cano, J. , Lindahl, M. , Diaz‐Espejo, A. , and Jordano, J. (2012). Protection of the photosynthetic apparatus from extreme dehydration and oxidative stress in seedlings of transgenic tobacco. PLoS ONE 7: e51443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstutz, C.L. , Fristedt, R. , Schultink, A. , Merchant, S.S. , Niyogi, K.K. , and Malnoë, A. (2020). An atypical short‐chain dehydrogenase–reductase functions in the relaxation of photoprotective qH in Arabidopsis . Nat. Plants 6: 154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancín, M. , Larraya, L. , Fernández‐San Millán, A. , Veramendi, J. , Burch‐Smith, T. , Farran, I. (2019). NTRC and thioredoxin f overexpression differentially induces starch accumulation in tobacco leaves. Plants (Basel). 8: 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J.M. , Price, G.D. , Chow, W.S. , Hope, A.B. , and Badger, M.R. (1997). Reduced levels of cytochrome bf complex in transgenic tobacco leads to marked photochemical reduction of the plastoquinone pool, without significant change in acclimation to irradiance. Photosynth. Res. 53: 215–227. [Google Scholar]
- Andreoni, A. , Lin, S. , Liu, H. , Blankenship, R.E. , Yan, H. , and Woodbury, N.W. (2017). Orange carotenoid protein as a control element in an antenna system based on a DNA nanostructure. Nano Lett. 17: 1174–1180. [DOI] [PubMed] [Google Scholar]
- Anten, N.P.R. , and Vermeulen, P.J. (2016). Tragedies and crops: Understanding natural selection to improve cropping systems. Trends. Ecol. Evol. 31: 429–439. [DOI] [PubMed] [Google Scholar]
- Araus, J.L. , Sanchez‐Bragado, R. , and Vicente, R. (2021). Improving crop yield and resilience through optimization of photosynthesis: Panacea or pipe dream? J. Exp. Bot. 72: 3936–3955. [DOI] [PubMed] [Google Scholar]
- Armbruster, U. , Carrillo, L.R. , Venema, K. , Pavlovic, L. , Schmidtmann, E. , Kornfeld, A. , Jahns, P. , Berry, J.A. , Kramer, D.M. , and Jonikas, M.C. (2014). Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nat. Commun. 5: 5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster, U. , Leonelli, L. , Correa Galvis, V. , Strand, D. , Quinn, E.H. , Jonikas, M.C. , and Niyogi, K.K. (2016). Regulation and levels of the thylakoid K+/H+ antiporter KEA3 shape the dynamic response of photosynthesis in fluctuating light. Plant Cell Physiol. 57: 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro, E.‐M. , Virgin, I. , and Andersson, B. (1993). Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta: Bioenerg. 1143: 113–134. [DOI] [PubMed] [Google Scholar]
- Asada, K. (2000). The water‐water cycle as alternative photon and electron sinks. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 355: 1419–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson, N. , Mao, Y. , Chan, K.X. , and McCormick, A.J. (2020). Condensation of Rubisco into a proto‐pyrenoid in higher plant chloroplasts. Nat. Commun. 11: 6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena‐González, E. , and Aro, E.‐M. (2002). Biogenesis, assembly and turnover of photosystem II units. Phil. Trans. R. Soc. B 357: 1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bag, P. (2021). Light harvesting in fluctuating environments: Evolution and function of antenna proteins across photosynthetic lineage. Plants 10: 1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballottari, M. , Girardon, J. , Dall'Osto, L. , and Bassi, R. (2012). Evolution and functional properties of Photosystem II light harvesting complexes in eukaryotes. Biochim. Biophys. Acta: Bioenerg. 1817: 143–157. [DOI] [PubMed] [Google Scholar]
- Bassi, R. , and Caffarri, S. (2000). Lhc proteins and the regulation of photosynthetic light harvesting function by xanthophylls. Photosynth. Res. 64: 243–256. [DOI] [PubMed] [Google Scholar]
- Basso, L. , Yamori, W. , Szabo, I. , and Shikanai, T. (2020). Collaboration between NDH and KEA3 allows maximally efficient photosynthesis after a long dark adaptation. Plant Physiol. 184: 2078–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista‐Silva, W. , da Fonseca‐Pereira, P. , Martins, A.O. , Zsögön, A. , Nunes‐Nesi, A. , and Araújo, W.L. (2020). Engineering improved photosynthesis in the era of synthetic biology. Plant Commun. 1: 100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti, M. , Vecchi, V. , Barera, S. , and Dall'Osto, L. (2018). Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Fact. 17: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergantino, E. , Segalla, A. , Brunetta, A. , Teardo, E. , Rigoni, F. , Giacometti, G.M. , and Szabò, I. (2003). Light‐ and pH‐dependent structural changes in the PsbS subunit of photosystem II. Proc. Natl. Acad. Sci. U.S.A. 100: 15265–15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersanini, L. , Allahverdiyeva, Y. , Battchikova, N. , Heinz, S. , Lespinasse, M. , Ruohisto, E. , Mustila, H. , Nickelsen, J. , Vass, I. , and Aro, E.‐M. (2017). Dissecting the photoprotective mechanism encoded by the flv4‐2 operon: A distinct contribution of Sll0218 in photosystem II stabilization. Plant Cell Environ. 40: 378–389. [DOI] [PubMed] [Google Scholar]
- Bersanini, L. , Battchikova, N. , Jokel, M. , Rehman, A. , Vass, I. , Allahverdiyeva, Y. , and Aro, E.‐M. (2014). Flavodiiron protein Flv2/Flv4‐related photoprotective mechanism dissipates excitation pressure of PSII in cooperation with phycobilisomes in cyanobacteria. Plant Physiol. 164: 805–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betterle, N. , Ballottari, M. , Zorzan, S. , de Bianchi, S. , Cazzaniga, S. , Dall'Osto, L. , Morosinotto, T. , and Bassi, R. (2009). Light‐induced dissociation of an antenna hetero‐oligomer is needed for non‐photochemical quenching induction. J. Biol. Chem. 284: 15255–15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betti, M. , Bauwe, H. , Busch, F.A. , Fernie, A.R. , Keech, O. , Levey, M. , Ort, D.R. , Parry, M.A. , Sage, R. , Timm, S. , Walker, B. , and Weber, A.P. (2016). Manipulating photorespiration to increase plant productivity: Recent advances and perspectives for crop improvement. J. Exp. Bot. 67: 2977–2988. [DOI] [PubMed] [Google Scholar]
- Bialek, W. , Krzywda, S. , Zatwarnicki, P. , Jaskolski, M. , Kolesinski, P. , and Szczepaniak, A. (2014). Insights into the relationship between the haem‐binding pocket and the redox potential of c6 cytochromes: Four atomic resolution structures of c6 and c6‐like proteins from Synechococcus sp. PCC 7002. Acta Crystallogr. D. Biol. Crystallogr. 70: 2823–2832. [DOI] [PubMed] [Google Scholar]
- Bielczynski, L.W. , Schansker, G. , and Croce, R. (2020). Consequences of the reduction of the photosystem II antenna size on the light acclimation capacity of Arabidopsis thaliana . Plant Cell Environ. 43: 866–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship, R.E. , Tiede, D.M. , Barber, J. , Brudvig, G.W. , Fleming, G. , Ghirardi, M. , Gunner, M.R. , Junge, W. , Kramer, D.M. , Melis, A. , Moore, T.A. , Moser, C.C. , Nocera, D.G. , Nozik, A.J. , Ort, D.R. , Parson, W.W. , Prince, R.C. , and Sayre, R.T. (2011). Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 332: 805–809. [DOI] [PubMed] [Google Scholar]
- Blanco, N.E. , Ceccoli, R.D. , Segretin, M.E. , Poli, H.O. , Voss, I. , Melzer, M. , Bravo‐Almonacid, F.F. , Scheibe, R. , Hajirezaei, M.‐R. , and Carrillo, N. (2011). Cyanobacterial flavodoxin complements ferredoxin deficiency in knocked‐down transgenic tobacco plants. Plant J. 65: 922–935. [DOI] [PubMed] [Google Scholar]
- Bru, P. , Nanda, S. , and Malnoë, A. (2020). A genetic screen to identify new molecular players involved in photoprotection qH in Arabidopsis thaliana . Plants 9: 1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bru, P. , Steen, C.J. , Park, S. , Amstutz, C.L. , Sylak‐Glassman, E.J. , Leuenberger, M. , Lam, L. , Longoni, F. , Fleming, G.R. , Niyogi, K.K. , and Malnoë, A. (2021). Photoprotective qH occurs in the light‐harvesting complex II trimer. bioRxiv. 10.1101/2021.07.09.450705 [DOI]
- Buchert, F. , Bailleul, B. , and Joliot, P. (2021). Disentangling chloroplast ATP synthase regulation by proton motive force and thiol modulation in Arabidopsis leaves. Biochim. Biophys. Acta: Bioenerg. 1862: 148434. [DOI] [PubMed] [Google Scholar]
- Buchert, F. , Mosebach, L. , Gäbelein, P. , and Hippler, M. (2020). PGR5 is required for efficient Q cycle in the cytochrome b6f complex during cyclic electron flow. Biochem. J. 477: 1631–1650. [DOI] [PubMed] [Google Scholar]
- Bungard, R.A. , Ruban, A.V. , Hibberd, J.M. , Press, M.C. , Horton, P. , and Scholes, J.D. (1999). Unusual carotenoid composition and a new type of xanthophyll cycle in plants. Proc. Natl. Acad. Sci. U.S.A. 96: 1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkey, K.O. (1994). Genetic variation of photosynthetic electron transport in barley: Identification of plastocyanin as a potential limiting factor. Plant Sci. 97: 177–187. [Google Scholar]
- Burkey, K.O. , Gizlice, Z. , and Carter, T.E. (1996). Genetic variation in soybean photosynthetic electron transport capacity is related to plastocyanin concentration in the chloroplast. Photosynth. Res. 49: 141–149. [DOI] [PubMed] [Google Scholar]
- Busch, F.A. (2014). Opinion: The red‐light response of stomatal movement is sensed by the redox state of the photosynthetic electron transport chain. Photosynth. Res. 119: 131–140. [DOI] [PubMed] [Google Scholar]
- Bryant, D.A. (1982). Phycoerythrocyanin and phycoerythrin: Properties and occurrence in cyanobacteria. J. Gen. Microbiol. 128: 835–844. [Google Scholar]
- von Caemmerer, S. , and Furbank, R.T. (2016). Strategies for improving C4 photosynthesis. Curr. Opin. Plant Biol. 31: 125–134. [DOI] [PubMed] [Google Scholar]
- Cardona, T. , Shao, S. , and Nixon, P.J. (2018). Enhancing photosynthesis in plants: The light reactions. Essays Biochem. 62: 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castell, C. , Bernal‐Bayard, P. , Ortega, J.M. , Roncel, M. , Hervás, M. , and Navarro, J.A. (2021a). The heterologous expression of a plastocyanin in the diatom Phaeodactylum tricornutum improves cell growth under iron‐deficient conditions. Physiol. Plant. 171: 277–290. [DOI] [PubMed] [Google Scholar]
- Castell, C. , Rodríguez‐Lumbreras, L.A. , Hervás, M. , Fernández‐Recio, J. , and Navarro, J.A. (2021b). New insights into the evolution of the electron transfer from cytochrome f to photosystem I in the green and red branches of photosynthetic eukaryotes. Plant Cell Physiol. 29: 1082–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzaniga, S. , Dall'Osto, L. , Szaub, J. , Scibilia, L. , Ballottari, M. , Purton, S. , and Bassi, R. (2014). Domestication of the green alga Chlorella sorokiniana: Reduction of antenna size improves light‐use efficiency in a photobioreactor. Biotechnol. Biofuels 7: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccoli, R.D. , Blanco, N.E. , Segretin, M.E. , Melzer, M. , Hanke, G.T. , Scheibe, R. , Hajirezaei, M.‐R. , Bravo‐Almonacid, F.F. , and Carrillo, N. (2012). Flavodoxin displays dose‐dependent effects on photosynthesis and stress tolerance when expressed in transgenic tobacco plants. Planta 236: 1447–1458. [DOI] [PubMed] [Google Scholar]
- Chae, H.B. , Moon, J.C. , Shin, M.R. , Chi, Y.H. , Jung, Y.J. , Lee, S.Y. , Nawkar, G.M. , Jung, H.S. , Hyun, J.K. , Kim, W.Y. , Kang, C.H. , Yun, D.‐J. , Lee, K.O. , and Lee, S.Y. (2013). Thioredoxin reductase type C (NTRC) orchestrates enhanced thermotolerance to Arabidopsis by its redox‐dependent holdase chaperone function. Mol. Plant 6: 323–336. [DOI] [PubMed] [Google Scholar]
- Chang, L. , Liu, X. , Li, Y. , Liu, C.C. , Yang, F. , Zhao, J. , and Sui, S.F. (2015). Structural organization of an intact phycobilisome and its association with photosystem II. Cell Res. 25: 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J.H. , Chen, S.T. , He, N.Y. , Wang, Q.L. , Zhao, Y. , Gao, W. , and Guo, F.Q. (2020). Nuclear‐encoded synthesis of the D1 subunit of photosystem II increases photosynthetic efficiency and crop yield. Nat. Plants 6: 570–580. [DOI] [PubMed] [Google Scholar]
- Chen, T. , Fang, Y. , Jiang, Q. , Dykes, G.F. , Lin, Y. , Price, G.D. , Long, B.M. , and Liu, L.N. (2021). Incorporation of functional Rubisco activases into engineered carboxysomes to enhance carbon fixation. ACS Synth. Biol. 10.1021/acssynbio.1c00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida, H. , Nakazawa, A. , Akazaki, H. , Hirano, T. , Suruga, K. , Ogawa, M. , Satoh, T. , Kadokura, K. , Yamada, S. , Hakamata, W. , Isobe, K. , Ito, T. , Ishii, R. , Nishio, T. , Sonoike, K. , and Oku, T. (2007). Expression of the algal cytochrome c6 gene in Arabidopsis enhances photosynthesis and growth. Plant Cell Physiol. 48: 948–957. [DOI] [PubMed] [Google Scholar]
- Chukhutsina, V. , Bersanini, L. , Aro, E.‐M. , and van Amerongen, H. (2015). Cyanobacterial flv4‐2 operon‐encoded proteins optimize light harvesting and charge separation in photosystem II. Mol. Plant 8: 747–761. [DOI] [PubMed] [Google Scholar]
- Coba de la Peña, T. , Redondo, F.J. , Manrique, E. , Lucas, M.M. , and Pueyo, J.J. (2010). Nitrogen fixation persists under conditions of salt stress in transgenic Medicago truncatula plants expressing a cyanobacterial flavodoxin. Plant Biotechnol. J. 8: 954–965. [DOI] [PubMed] [Google Scholar]
- Correa Galvis, L. , Strand, D.D. , Messer, M. , Thiele, W. , Bethmann, S. , Hübner, D. , Uflewski, M. , Kaiser, E. , Siemiatkowska, B. , Morris, B.A. , Tóth, S.Z. , Watanabe, M. , Brückner, F. , Höfgen, R. , Jahns, P. , Schöttler, M.A. , and Armbruster, U. (2020). H+ transport by K+ EXCHANGE ANTIPORTER3 promotes photosynthesis and growth in chloroplast ATP synthase mutants. Plant Physiol. 182: 2126–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Osto, L. , Caffarri, S. , and Bassi, R. (2005). A mechanism of nonphotochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26. Plant Cell 17: 1217–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Osto, L. , Cazzaniga, S. , Bressan, M. , Paleček, D. , Židek, K. , Niyogi, K.K. , Fleming, G.R. , Zigmantas, D. , and Bassi, R. (2017). Two mechanisms for dissipation of excess light in monomeric and trimeric light‐harvesting complexes. Nat. Plants 3: 17033. [DOI] [PubMed] [Google Scholar]
- Dall'Osto, L. , Cazzaniga, S. , Guardini, Z. , Barera, S. , Benedetti, M. , Mannino, G. , Maffei, M.E. , and Bassi, R. (2019). Combined resistance to oxidative stress and reduced antenna size enhance light‐to‐biomass conversion efficiency in Chlorella vulgaris cultures. Biotechnol. Biofuels 12: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Osto, L. , Cazzaniga, S. , Havaux, M. , and Bassi, R. (2010). Enhanced photoprotection by protein‐bound vs free xanthophyll pools: A comparative analysis of chlorophyll b and xanthophyll biosynthesis mutants. Mol. Plant 3: 576–593. [DOI] [PubMed] [Google Scholar]
- Dao, O. , Kuhnert, F. , Weber, A.P.M. , Peltier, G. , and Li‐Beisson, Y. (2021). Physiological functions of malate shuttles in plants and algae. Trends Plant Sci. 27: S1360–S1385. [DOI] [PubMed] [Google Scholar]
- Davis, G.A. , and Kramer, D.M. (2020). Optimization of ATP synthase c‐rings for oxygenic photosynthesis. Front. Plant Sci. 10: 1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, G.A. , Rutherford, A.W. , and Kramer, D.M. (2017). Hacking the thylakoid proton motive force for improved photosynthesis: Modulating ion flux rates that control proton motive force partitioning into Δψ and ΔpH. Phil. Trans. R. Soc. B. 372: 20160381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen, G.E. , Worrall, D. , and Carmo‐Silva, E. (2020). An isoleucine residue acts as a thermal and regulatory switch in wheat Rubisco activase. Plant J. 103: 742–751. [DOI] [PubMed] [Google Scholar]
- De Mooij, T. , Janssen, M. , Cerezo‐Chinarro, O. , Mussgnug, J.H. , Kruse, O. , Ballottari, M. , Bassi, R. , Bujaldon, S. , Wollman, F.‐A. , and Wijffels, R.H. (2015). Antenna size reduction as a strategy to increase biomass productivity: A great potential not yet realized. J. Appl. Phycol. 27: 1063–1077. [Google Scholar]
- De Mooij, T. , Schediwy, K. , Wijffels, R.H. , and Janssen, M. (2016). Modeling the competition between antenna size mutant and wild type microalgae in outdoor mass culture. J. Biotechnol. 240: 1–13. [DOI] [PubMed] [Google Scholar]
- Dhandayuthapani, K. , Malathy, S. , Mulla, S.I. , and Gupta, S.K. (2021). An insight into potential application of microalgae in pharmaceutical and nutraceutical production. In: Mandotra, S.K. , Upadhyay A.K., Ahluwalia A.S. eds. Algae. Springer, Singapore. [Google Scholar]
- Di Mascio, P. , Martinez, G.R. , Miyamoto, S. , Ronsein, G.E. , Medeiros, M.H.G. , and Cadet, J. (2019). Singlet molecular oxygen reactions with nucleic acids, lipids, and proteins. Chem. Rev. 119: 2043–2086. [DOI] [PubMed] [Google Scholar]
- Dominguez‐Martin, M.A. , and Kerfeld, C.A. (2019). Engineering the orange carotenoid protein for applications in synthetic biology. Curr. Opin. Struct. Biol. 57: 110–117. [DOI] [PubMed] [Google Scholar]
- Drewry, D.T. , Kumar, P. , and Long, S.P. (2014). Simultaneous improvement in productivity, water use, and albedo through crop structural modification. Glob. Chang. Biol. 20: 1955–1967. [DOI] [PubMed] [Google Scholar]
- Driever, S.M. , Simkin, A.J. , Alotaibi, S. , Fisk, S.J. , Madgwick, P.J. , Sparks, C.A. , Jones, H.D. , Lawson, T. , Parry, M.A.J. , and Raines, C.A. (2017). Increased SBPase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 372: 20160384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhut, M. , Roell, M.S. , and Weber, A.P.M. (2019). Mechanistic understanding of photorespiration paves the way to a new green revolution. New Phytol. 223: 1762–1769. [DOI] [PubMed] [Google Scholar]
- Elias, E. , Liguori, N. , Saga, Y. , Schäfers, J. , and Croce, R. (2021). Harvesting far‐red light with plant antenna complexes incorporating chlorophyll d. Biomacromolecules 22: 3313–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermakova, M. , Arrivault, S. , Giuliani, R. , Danila, F. , Alonso‐Cantabrana, H. , Vlad, D. , Ishihara, H. , Feil, R. , Guenther, M. , Borghi, G.L. , Covshoff, S. , Ludwig, M. , Cousins, A.B. , Langdale, J.A. , Kelly, S. , Lunn, J.E. , Stitt, M. , von Caemmerer, S. , and Furbank, R.T. (2021a). Installation of C4 photosynthetic pathway enzymes in rice using a single construct. Plant Biotechnol. J. 19: 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermakova, M. , Bellasio, C. , Fitzpatrick, D. , Furbank, R.T. , Mamedov, F. , and von Caemmerer, S. (2021b). Upregulation of bundle sheath electron transport capacity under limiting light in C4 Setaria viridis . Plant J. 106: 1443–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermakova, M. , Danila, F.R. , Furbank, R.T. , and von Caemmerer, S. (2020). On the road to C4 rice: Advances and perspectives. Plant J. 101: 940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermakova, M. , López‐Calcagno, P.E. , Raines, C.A. , Furbank, R.T. , and von Caemmerer, S. (2019). Overexpression of the Rieske FeS protein of the cytochrome b6f complex increases C4 photosynthesis in Setaria viridis . Commun. Biol. 2: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermakova, M. , Osborn, H. , Groszmann, M. , Bala, S. , Bowerman, A. , McGaughey, S. , Byrt, C. , Alonso‐Cantabrana, H. , Tyerman, S. , Furbank, R.T. , Sharwood, R.E. , and von Caemmerer, S. (2021c). Expression of a CO2‐permeable aquaporin enhances mesophyll conductance in the C4 species Setaria viridis . eLife 10: e70095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essemine, J. , Lyu, M.A. , Qu, M. , Perveen, S. , Khan, N. , Song, Q. , Chen, G. , and Zhu, X.G. (2020). Contrasting responses of plastid terminal oxidase activity under salt stress in two C4 species with different salt tolerance. Front. Plant Sci. 11: 1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO and UNEP . (2020). The state of the world's forests 2020. Forests, biodiversity and people. Rome. 10.4060/ca8642en [DOI]
- Farquhar, G.D. , von Caemmerer, S. , and Berry, J.A. (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90. [DOI] [PubMed] [Google Scholar]
- Feilke, K. , Streb, P. , Cornic, G. , Perreau, F. , Kruk, J. , and Krieger‐Liszkay, A. (2016). Effect of Chlamydomonas plastid terminal oxidase 1 expressed in tobacco on photosynthetic electron transfer. Plant J. 85: 219–228. [DOI] [PubMed] [Google Scholar]
- Finazzi, G. , Sommer, F. , and Hippler, M. (2005). Release of oxidized plastocyanin from photosystem I limits electron transfer between photosystem I and cytochrome b6f complex in vivo. Proc. Natl. Acad. Sci. U.S.A. 102: 7031–7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo, C.C. , Burgess, A.J. , Retkute, R. , Tree‐Intong, P. , Ruban, A.V. , and Murchie, E.H. (2020). Photoprotective energy dissipation is greater in the lower, not the upper, regions of a rice canopy: A 3D analysis. J. Exp. Bot. 71: 7382–7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland, N. , Negi, S. , Vinogradova‐Shah, T. , Wu, G. , Ma, L. , Flynn, S. , Kumssa, T. , Lee, C.‐H. , and Sayre, R.T. (2019). Fine‐tuning the photosynthetic light harvesting apparatus for improved photosynthetic efficiency and biomass yield. Sci. Rep. 9: 13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulgosi, H. , and Vojta, L. (2020). Tweaking photosynthesis: FNR‐TROL interaction as potential target for crop fortification. Front. Plant Sci. 11: 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Cañas, R. , Giner‐Lamia, J. , Florencio, F.J. , and López‐Maury, L. (2021). A protease‐mediated mechanism regulates the cytochrome c6/plastocyanin switch in Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. U.S.A. 118: e2017898118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Molina, A. , and Leister, D. (2020). Accelerated relaxation of photoprotection impairs biomass accumulation in Arabidopsis . Nat. Plants 6: 9–12. [DOI] [PubMed] [Google Scholar]
- Geisz, J.F. , France, R.M. , Schulte, K.L. , Steiner, M.A. , Norman, A.G. , Guthrey, H.L. , Young, M.R. , Song, T. , and Moriarty, T. (2020). Six‐junction III–V solar cells with 47.1% conversion efficiency under 143 Suns concentration. Nat. Energy 5: 326–335. [Google Scholar]
- Gharechahi, J. , Hajirezaei, M.‐R. , and Salekdeh, G.H. (2015). Comparative proteomic analysis of tobacco expressing cyanobacterial flavodoxin and its wild type under drought stress. J. Plant Physiol. 175: 48–58. [DOI] [PubMed] [Google Scholar]
- Ghotbi‐Ravandi, A.A. , Shariati, M. , Shobbar, Z.‐S. , and Shahbazi, M. (2019). Expression pattern and physiological roles of plastid terminal oxidase (PTOX) in wild and cultivated barley genotypes under drought stress. Environ. Exper. Bot. 169: 319–320. [Google Scholar]
- Giró, M. , Ceccoli, R.D. , Poli, H.O. , Carrillo, N. , and Lodeyro, A.F. (2011). An in vivo system involving co‐expression of cyanobacterial flavodoxin and ferredoxin‐NADP(+) reductase confers increased tolerance to oxidative stress in plants. FEBS Open Biol. 1: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Głowacka, K. , Kromdijk, J. , Kucera, K. , Xie, J. , Cavanagh, A.P. , Leonelli, L. , Leakey, A.D.B. , Ort, D.R. , Niyogi, K.K. , and Long, S.P. (2018). Photosystem II Subunit S overexpression increases the efficiency of water use in a field‐grown crop. Nat. Commun. 9: 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez, R. , Carrillo, N. , Morelli, M.P. , Tula, S. , Shahinnia, F. , Hajirezaei, M.‐R. , and Lodeyro, A.F. (2018). Faster photosynthetic induction in tobacco by expressing cyanobacterial flavodiiron proteins in chloroplasts. Photosynth. Res. 136: 129–138. [DOI] [PubMed] [Google Scholar]
- Gómez, R. , Figueroa, N. , Melzer, M. , Hajirezaei, M.‐R. , Carrillo, N. , and Lodeyro, A.F. (2020). Photosynthetic characterization of flavodoxin‐expressing tobacco plants reveals a high light acclimation‐like phenotype. Biochim. Biophys. Acta: Bioenerg. 1861: 148211. [DOI] [PubMed] [Google Scholar]
- Gould, S.B. , Waller, R.F. , and McFadden, G.I. (2008). Plastid evolution. Annu. Rev. Plant Biol. 59: 491–517. [DOI] [PubMed] [Google Scholar]
- Green, B.R. (2019). What happened to the phycobilisome? Biomolecules 9: 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, J. , Yin, X. , Stomph, T.J. , and Struik, P.C. (2014). Can exploiting natural genetic variation in leaf photosynthesis contribute to increasing rice productivity? A simulation analysis. Plant Cell Environ. 37: 22–34. [DOI] [PubMed] [Google Scholar]
- Guidi, L. , Lo Piccolo, E. , and Landi, M. (2019). Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 10: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinea Diaz, M. , Nikkanen, L. , Himanen, K. , Toivola, J. , and Rintamäki, E. (2020). Two chloroplast thioredoxin systems differentially modulate photosynthesis in Arabidopsis depending on light intensity and leaf age. Plant J. 104: 718–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann, M. , and Bauwe, H. (2016). Photorespiration and the potential to improve photosynthesis. Curr. Opin. Chem. Biol. 35: 109–116. [DOI] [PubMed] [Google Scholar]
- Hajirezaei, M.R. , Peisker, M. , Tschiersch, H. , Palatnik, J.F. , Valle, E.M. , Carrillo, N. , and Sonnewald, U. (2002). Small changes in the activity of chloroplastic NADP(+)‐dependent ferredoxin oxidoreductase lead to impaired plant growth and restrict photosynthetic activity of transgenic tobacco plants. Plant J. 29: 281–293. [DOI] [PubMed] [Google Scholar]
- Hanba, Y.T. , Shibasaka, M. , Hayashi, Y. , Hayakawa, T. , Kasamo, K. , Terashima, I. , and Katsuhara, M. (2004). Overexpression of the barley aquaporin HvPIP2;1 increases internal CO(2) conductance and CO(2) assimilation in the leaves of transgenic rice plants. Plant Cell Physiol. 45: 521–529. [DOI] [PubMed] [Google Scholar]
- Hasan, S.S. , and Cramer, W.A. (2012). On rate limitations of electron transfer in the photosynthetic cytochrome b6f complex. Phys. Chem. Chem. Phys. 14: 13853–13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux, M. , Dall'Osto, L. , and Bassi, R. (2007). Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol. 145: 1506–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennacy, J.H. , and Jonikas, M.C. (2020). Prospects for engineering biophysical CO2 concentrating mechanisms into land plants to enhance yields. Annu. Rev. Plant Biol. 71: 461–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herritt, M. , Dhanapal, A.P. , and Fritschi, F.B. (2016). Identification of genomic loci associated with the photochemical reflectance index by genome‐wide association study in soybean. Plant Genome 9: 1–12. [DOI] [PubMed] [Google Scholar]
- Heyno, E. , Gross, C.M. , Laureau, C. , Culcasi, M. , Pietri, S. , and Krieger‐Liszkay, A. (2009). Plastid alternative oxidase (PTOX) promotes oxidative stress when overexpressed in tobacco. J. Biol. Chem. 284: 31174–31180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhner, R. , Pribil, M. , Herbstová, M. , Lopez, L.S. , Kunz, H.‐H. , Li, M. , Wood, M. , Svoboda, V. , Puthiyaveetil, S. , Leister, D. , and Kirchhoff, H. (2020). Plastocyanin is the long‐range electron carrier between photosystem II and photosystem I in plants. Proc. Natl. Acad. Sci. U.S.A. 117: 15354–15362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway, P.J. , Maclean, D.J. , and Scott, K.J. (1983). Rate‐limiting steps of electron transport in chloroplasts during ontogeny and senescence of barley. Plant Physiol. 72: 795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, L.‐Y. , Ehrlich, M. , Thormählen, I. , Lehmann, M. , Krahnert, I. , Obata, T. , Cejudo, F.J. , Fernie, A.R. , and Geigenberger, P. (2019). NTRC plays a crucial role in starch metabolism, redox balance, and tomato fruit growth. Plant Physiol. 181: 976–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, G.R. , Fan, Y. , Zheng, Y.L. , Xu, F. , Zhang, L. , and Li, F.L. (2020). Photoprotection capacity of microalgae improved by regulating the antenna size of light‐harvesting complexes. J. Appl. Phycol. 32: 1027–1039. [Google Scholar]
- Hubbart, S. , Smillie, I.R.A. , Heatley, M. , Swarup, R. , Foo, C.C. , Zhao, L. , and Murchie, E.H. (2018). Enhanced thylakoid photoprotection can increase yield and canopy radiation use efficiency in rice. Commun. Biol. 1: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo, Y. , Wang, M. , Wei, Y. , and Xia, Z. (2016). Overexpression of the maize PsbA gene enhances drought tolerance through regulating antioxidant system, photosynthetic capability, and stress defense gene expression in tobacco. Front. Plant Sci. 6: 1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Energy Agency. (2021). Global energy review 2021. Paris. https://www.iea.org/reports/global‐energy‐review‐2021
- Ilík, P. , Pavlovič, A. , Kouřil, R. , Alboresi, A. , Morosinotto, T. , Allahverdiyeva, Y. , Aro, E.‐M. , Yamamoto, H. , and Shikanai, T. (2017). Alternative electron transport mediated by flavodiiron proteins is operational in organisms from cyanobacteria up to gymnosperms. New Phytol. 214: 967–972. [DOI] [PubMed] [Google Scholar]
- Iñiguez, C. , Aguiló‐Nicolau, P. , and Galmés, J. (2021). Improving photosynthesis through the enhancement of Rubisco carboxylation capacity. Biochem. Soc. Trans. 10.1042/BST20201056 [DOI] [PubMed] [Google Scholar]
- Ivanov, A.G. , Rosso, D. , Savitch, L.V. , Stachula, P. , Rosembert, M. , Oquist, G. , Hurry, V. , and Hüner, N.P.A. (2012). Implications of alternative electron sinks in increased resistance of PSII and PSI photochemistry to high light stress in cold‐acclimated Arabidopsis thaliana . Photosynth. Res. 113: 191–206. [DOI] [PubMed] [Google Scholar]
- Jahns, P. , and Holzwarth, A.R. (2012). The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta: Bioenerg. 1817: 182–193. [DOI] [PubMed] [Google Scholar]
- Jansson, S. (1999). A guide to the Lhc genes and their relatives in Arabidopsis . Trends Plant Sci. 4: 236–240. [DOI] [PubMed] [Google Scholar]
- Jin, H. , Li, M. , Duan, S. , Fu, M. , Dong, X. , Liu, B. , Feng, D. , Wang, J. , and Wang, H.B. (2016). Optimization of light‐harvesting pigment improves photosynthetic efficiency. Plant Physiol. 172: 1720–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joët, T. , Genty, B. , Josse, E.M. , Kuntz, M. , Cournac, L. , and Peltier, G. (2002). Involvement of a plastid terminal oxidase in plastoquinone oxidation as evidenced by expression of the Arabidopsis thaliana enzyme in tobacco. J. Biol. Chem. 277: 31623–31630. [DOI] [PubMed] [Google Scholar]
- Johnson, G.N. , and Stepien, P. (2016). Plastid terminal oxidase as a route to improving plant stress tolerance: Known knowns and known unknowns. Plant Cell Physiol. 57: 1387–1396. [DOI] [PubMed] [Google Scholar]
- Johnson, M.P. , Havaux, M. , Triantaphylidès, C. , Ksas, B. , Pascal, A.A. , Robert, B. , Davison, P.A. , Ruban, A.V. , and Horton, P. (2007). Elevated zeaxanthin bound to oligomeric LHCII enhances the resistance of Arabidopsis to photooxidative stress by a lipid‐protective, antioxidant mechanism. J. Biol. Chem. 282: 22605–22618. [DOI] [PubMed] [Google Scholar]
- Jung, H.‐S. , and Niyogi, K.K. (2009). Quantitative genetic analysis of thermal dissipation in Arabidopsis . Plant Physiol. 150: 977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser, E. , Walther, D. , and Armbruster, U. (2020). Growth under fluctuating light reveals large trait variation in a panel of Arabidopsis accessions. Plants (Basel) 9: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa, A. , Ostendorf, E. , Kohzuma, K. , Hoh, D. , Strand, D.D. , Sato‐Cruz, M. , Savage, L. , Cruz, J.A. , Fisher, N. , Froehlich, J.E. , and Kramer, D.M. (2017). Chloroplast ATP synthase modulation of the thylakoid proton motive force: Implications for photosystem I and photosystem II photoprotection. Front. Plant Sci. 8: 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifa, S.A.M. , Shedid, E.S. , Saied, E.M. , Jassbi, A.R. , Jamebozorgi, F.H. , Rateb, M.E. , Du, M. , Abdel‐Daim, M.M. , Kai, G.‐Y. , Al‐Hammady, M.A.M. , Xiao, J. , Guo, Z. , and El‐Seedi, H.R. (2021). Cyanobacteria—From the oceans to the potential biotechnological and biomedical applications. Mar. Drugs 19: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M.I. , Shin, J.H. , and Kim, J.D. (2018). The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 17: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorobrykh, S. , Havurinne, V. , Mattila, H. , and Tyystjärvi, E. (2020). Oxygen and ROS in photosynthesis. Plants (Basel) 9: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurshid, G. , Abbassi, A.Z. , Khalid, M.F. , Gondal, M.N. , Naqvi, T.A. , Shah, M.M. , Chaudhary, S.U. , and Ahmad, R. (2020). A cyanobacterial photorespiratory bypass model to enhance photosynthesis by rerouting photorespiratory pathway in C3 plants. Sci. Rep. 10: 20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M.R. , Khaleda, L. , Jung, I.J. , Kim, J.Y. , Lee, S.Y. , Cha, J.‐Y. , and Kim, W.‐Y. (2017). Overexpression of chloroplast‐localized NADPH‐dependent thioredoxin reductase C (NTRC) enhances tolerance to photo‐oxidative and drought stresses in Arabidopsis thaliana . J. Plant Biol. 60: 175–180. [Google Scholar]
- Kirst, H. , and Melis, A. (2014). The chloroplast signal recognition particle (CpSRP) pathway as a tool to minimize chlorophyll antenna size and maximize photosynthetic productivity. Biotechnol. Adv. 32: 66–72. [DOI] [PubMed] [Google Scholar]
- Kirst, H. , Gabilly, S.T. , Niyogi, K.K. , Lemaux, P.G. , and Melis, A. (2017). Photosynthetic antenna engineering to improve crop yields. Planta 245: 1009–1020. [DOI] [PubMed] [Google Scholar]
- Kobayashi, K. , Sasaki, D. , Noguchi, K. , Fujinuma, D. , Komatsu, H. , Kobayashi, M. , Sato, M. , Toyooka, K. , Sugimoto, K. , Niyogi, K.K. , Wada, H. , and Masuda, T. (2013). Photosynthesis of root chloroplasts developed in Arabidopsis lines overexpressing GOLDEN2‐LIKE transcription factors. Plant Cell Physiol. 54: 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komenda, J. , and Sobotka, R. (2016). Cyanobacterial high‐light‐inducible proteins—Protectors of chlorophyll–protein synthesis and assembly. Biochim. Biophys. Acta: Bioenerg. 1857: 288–295. [DOI] [PubMed] [Google Scholar]
- Kosourov, S.N. , Ghirardi, M.L. , and Seibert, M. (2011). A truncated antenna mutant of Chlamydomonas reinhardtii can produce more hydrogen than the parental strain. Int. J. Hydrog. Energy 36: 2044–2048. [Google Scholar]
- Koziol, A.G. , Borza, T. , Ishida, K.‐I. , Keeling, P. , Lee, R.W. , and Durnford, D.G. (2007). Tracing the evolution of the light‐harvesting antennae in chlorophyll a/b‐containing organisms. Plant Physiol. 143: 1802–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, D.M. , and Evans, J.R. (2010). The importance of energy balance in improving photosynthetic productivity. Plant Physiol. 155: 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress, E. , and Jahns, P. (2017). The dynamics of energy dissipation and xanthophyll conversion in Arabidopsis indicate an indirect photoprotective role of zeaxanthin in slowly inducible and relaxing components of non‐photochemical quenching of excitation energy. Front. Plant Sci. 8: 2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger‐Liszkay, A. (2005). Singlet oxygen production in photosynthesis. J. Exp. Bot. 56: 337–346. [DOI] [PubMed] [Google Scholar]
- Krieger‐Liszkay, A. , and Feilke, K. (2016). The dual role of the plastid terminal oxidase PTOX: Between a protective and a pro‐oxidant function. Front. Plant Sci. 6: 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromdijk, J. , Głowacka, K. , Leonelli, L. , Gabilly, S.T. , Iwai, M. , Niyogi, K.K. , and Long, S.P. (2016). Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354: 857–861. [DOI] [PubMed] [Google Scholar]
- Kromdijk, J. , and Long, S.P. (2016). One crop breeding cycle from starvation? How engineering crop photosynthesis for rising CO2 and temperature could be one important route to alleviation. Proc. Biol. Sci. 283: 20152578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubásek, J. , Urban, O. , and Šantrůček, J. (2013). C4 plants use fluctuating light less efficiently than do C3 plants: A study of growth, photosynthesis and carbon isotope discrimination. Physiol. Plant. 149: 528–539. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt, W. (2019). Structure and mechanisms of F‐type ATP synthases. Annu. Rev. Biochem. 88: 515–549. [DOI] [PubMed] [Google Scholar]
- Kumar, A. , Li, C. , Portis, A.R., Jr. (2009). Arabidopsis thaliana expressing a thermostable chimeric Rubisco activase exhibits enhanced growth and higher rates of photosynthesis at moderately high temperatures. Photosynth. Res. 100: 143–153. [DOI] [PubMed] [Google Scholar]
- Kunz, H.‐H. , Gierth, M. , Herdean, A. , Satoh‐Cruz, M. , Kramer, D.M. , Spetea, C. , and Schroeder, J.I. (2014). Plastidial transporters KEA1, ‐2, and ‐3 are essential for chloroplast osmoregulation, integrity, and pH regulation in Arabidopsis . Proc. Natl. Acad. Sci. U.S.A. 111: 7480–7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurek, I. , Chang, T.K. , Bertain, S.M. , Madrigal, A. , Liu, L. , Lassner, M.W. , and Zhu, G. (2007). Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell 19: 3230–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey, A.D.B. , Press, M.C. , Scholes, J.D. , and Watling, J.R. (2002). Relative enhancement of photosynthesis and growth at elevated CO2 is greater under sunflecks than uniform irradiance in a tropical rain forest tree seedling. Plant Cell Environ. 25: 1701–1714. [Google Scholar]
- Lechno‐Yossef, S. , Melnicki, M.R. , Bao, H. , Montgomery, B.L. , and Kerfeld, C.A. (2017). Synthetic OCP heterodimers are photoactive and recapitulate the fusion of two primitive carotenoproteins in the evolution of cyanobacterial photoprotection. Plant J. 91: 646–656. [DOI] [PubMed] [Google Scholar]
- Lefebvre, S. , Lawson, T. , Zakhleniuk, O.V. , Lloyd, J.C. , Raines, C.A. , and Fryer, M. (2005). Increased sedoheptulose‐1,7‐bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol. 138: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leister, D. (2012). How can the light reactions of photosynthesis be improved in plants? Front. Plant Sci. 3: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonelli, L. , Brooks, M.D. , Niyogi, K.K. (2017). Engineering the lutein epoxide cycle into Arabidopsis thaliana . Proc. Natl. Acad. Sci. U.S.A. 114: E7002–E7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. , Svoboda, V. , Davis, G. , Kramer, D. , Kunz, H.‐H. , and Kirchhoff, H. (2021). Impact of ion fluxes across thylakoid membranes on photosynthetic electron transport and photoprotection. Nat. Plants 7: 979–988. [DOI] [PubMed] [Google Scholar]
- Li, Q. , Yao, Z. J. , and Mi, H. (2016a). Alleviation of photoinhibition by co‐ordination of chlororespiration and cyclic electron flow mediated by NDH under heat stressed condition in tobacco. Front. Plant Sci. 7: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Wang, P. , Li, J. , Wei, S. , Yan, Y. , Yang, J. , Zhao, M. , Langdale, J.A. , and Zhou, W. (2020). Maize GOLDEN2‐LIKE genes enhance biomass and grain yields in rice by improving photosynthesis and reducing photoinhibition. Commun. Biol. 3: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X.‐P. , Gilmore, A.M. , Caffarri, S. , Bassi, R. , Golan, T. , Kramer, D. , and Niyogi, K.K. (2004). Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J. Biol. Chem. 279: 22866–22874. [DOI] [PubMed] [Google Scholar]
- Li, Y. , and Chen, M. (2015). Novel chlorophylls and new directions in photosynthesis research. Funct. Plant Biol. 42: 493–501. [DOI] [PubMed] [Google Scholar]
- Li, Z. , Yuan, S. , Jia, H. , Gao, F. , Zhou, M. , Yuan, N. , Wu, P. , Hu, Q. , Sun, D. , and Luo, H. (2016b). Ectopic expression of a cyanobacterial flavodoxin in creeping bentgrass impacts plant development and confers broad abiotic stress tolerance. Plant Biotechnol. J. 15: 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintala, M. , Lehtimäki, N. , Benz, J.P. , Jungfer, A. , Soll, J. , Aro, E.‐M. , Bölter, B. , and Mulo, P. (2012). Depletion of leaf‐type ferredoxin‐NADP+ oxidoreductase results in the permanent induction of photoprotective mechanisms in Arabidopsis chloroplasts. Plant J. 70: 809–817. [DOI] [PubMed] [Google Scholar]
- Lodeyro, A.F. , Ceccoli, R.D. , Pierella Karlusich, J.J. , and Carrillo, N. (2012). The importance of flavodoxin for environmental stress tolerance in photosynthetic microorganisms and transgenic plants. Mechanism, evolution and biotechnological potential. FEBS Lett. 586: 2917–2924. [DOI] [PubMed] [Google Scholar]
- Long, B.M. , Hee, W.Y. , Sharwood, R.E. , Rae, B.D. , Kaines, S. , Lim, Y.L. , Nguyen, N.D. , Massey, B. , Bala, S. , von Caemmerer, S. , Badger, M.R. , and Price, G.D. (2018). Carboxysome encapsulation of the CO2‐fixing enzyme Rubisco in tobacco chloroplasts. Nat. Commun. 9: 3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, S.P. , Humphries, S. , and Falkowski, P.G. (1994). Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45: 633–662. [Google Scholar]
- Long, S.P. , Marshall‐Colon, A. , and Zhu, X.G. (2015). Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161: 56–66. [DOI] [PubMed] [Google Scholar]
- Long, S.P. , Zhu, X.‐G. , Naidu, S.L. , and Ort, D.R. (2006). Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 29: 315–330. [DOI] [PubMed] [Google Scholar]
- Long, T.A. , Okegawa, Y. , Shikanai, T. , Schmidt, G.W. , and Covert, S.F. (2008). Conserved role of proton gradient regulation 5 in the regulation of PSI cyclic electron transport. Planta 228: 907. [DOI] [PubMed] [Google Scholar]
- López‐Calcagno, P.E. , Brown, K.L. , Simkin, A.J. , Fisk, S.J. , Vialet‐Chabrand, S. , Lawson, T. , and Raines, C.A. (2020). Stimulating photosynthetic processes increases productivity and water‐use efficiency in the field. Nat. Plants 6: 1054–1063. [DOI] [PubMed] [Google Scholar]
- López‐Calcagno, P.E. , Fisk, S. , Brown, K.L. , Bull, S.E. , South, P.F. , and Raines, C.A. (2019). Overexpressing the H‐protein of the glycine cleavage system increases biomass yield in glasshouse and field‐grown transgenic tobacco plants. Plant Biotechnol. J. 17: 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou, W. , Tan, X. , Song, K. , Zhang, S. , Luan, G. , Li, C. , and Lu, X. (2018). A specific single nucleotide polymorphism in the ATP synthase gene significantly improves environmental stress tolerance of Synechococcus elongatus PCC 7942. Appl. Environ. Microbiol. 84: e01222–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren, M.R. , and Fleming, A.J. (2020). Cellular perspectives for improving mesophyll conductance. Plant J. 101: 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, M. , Liu, Y. , Bai, C. , Yang, Y. , Sun, Z. , Liu, X. , Zhang, S. , Han, X. , and Yong, J.W.H. (2021). The physiological functionality of PGR5/PGRL1‐dependent cyclic electron transport in sustaining photosynthesis. Front. Plant Sci. 12: 702196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar, M. , Sipka, G. , Kovács, L. , Ughy, B. , Zhu, Q. , Han, G. , Špunda, V. , Lambrev, P.H. , Shen, J.R. , and Garab, G. (2018). Rate‐limiting steps in the dark‐to‐light transition of photosystem II‐revealed by chlorophyll‐a fluorescence induction. Sci. Rep. 8: 2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinova, I. , Zupok, A. , Massouh, A. , Schöttler, M.A. , Meyer, E.H. , Yaneva‐Roder, L. , Szymanski, W. , Rößner, M. , Ruf, S. , Bock, R. , and Greiner, S. (2021). Correction of frameshift mutations in the AtpB gene by translational recoding in chloroplasts of Oenothera and tobacco. Plant Cell 33: 1682–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnoë, A. (2018). Photoinhibition or photoprotection of photosynthesis? Update on the (newly termed) sustained quenching component qH. Environ. Exp. Bot. 154: 123–133. [Google Scholar]
- Malnoë, A. , Schultink, A. , Shahrasbi, S. , Rumeau, D. , Havaux, M. , and Niyogi, K.K. (2018). The plastid lipocalin LCNP is required for sustained photoprotective energy dissipation in Arabidopsis . Plant Cell 30: 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathan, J. , Bhattacharya, J. , and Ranjan, A. (2016). Enhancing crop yield by optimizing plant developmental features. Development 143: 3283–3294. [DOI] [PubMed] [Google Scholar]
- Maurino, V.G. (2019). Using energy‐efficient synthetic biochemical pathways to bypass photorespiration. Biochem. Soc. Trans. 47: 1805–1813. [DOI] [PubMed] [Google Scholar]
- Mayta, M.L. , Arce, R.C. , Zurbriggen, M.D. , Valle, E.M. , Hajirezaei, M.‐R. , Zanor, M.I. , and Carrillo, N. (2019). Expression of a chloroplast‐targeted cyanobacterial flavodoxin in tomato plants increases harvest index by altering plant size and productivity. Front. Plant Sci. 10: 1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayta, M.L. , Lodeyro, A.F. , Guiamet, J.J. , Tognetti, V.B. , Melzer, M. , Hajirezaei, M.‐R. , and Carrillo, N. (2018). Expression of a plastid‐targeted flavodoxin decreases chloroplast reactive oxygen species accumulation and delays senescence in aging tobacco leaves. Front. Plant Sci. 9: 1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden, G.I. (2014). Origin and evolution of plastids and photosynthesis in eukaryotes. Cold Spring Harb. Perspect. Biol. 6: a016105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath, J.M. , and Long, S.P. (2014). Can the cyanobacterial carbon‐concentrating mechanism increase photosynthesis in crop species? A theoretical analysis. Plant Physiol. 164: 2247–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler, A.H. (1951). Studies on reactions of illuminated chloroplasts: I. Mechanism of the reduction of oxygen and other hill reagents. Arch. Biochem. Biophys. 33: 65–77. [DOI] [PubMed] [Google Scholar]
- Melis, A. , Neidhardt, J. , and Benemann, J. (1999). Dunaliella salina (chlorophyta) with small chlorophyll antenna sizes exhibit higher photosynthetic productivities and photon use efficiencies than normally pigmented cells. J. Appl. Phycol. 10: 515–525. [Google Scholar]
- Messant, M. , Krieger‐Liszkay, A. , and Shimakawa, G. (2021). Dynamic changes in protein‐membrane association for regulating photosynthetic electron transport. Cells 10: 1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsoviti, M.N. , Katsoulas, N. , Karapanagiotidis, I.T. , and Papapolymerou, G. (2019). Current and potential applications of microalgae: A mini review. Oceanogr. Fish. Open Access J. 11: 555811. [Google Scholar]
- Meyers, B.C. , and Axtell, M.J. (2019). MicroRNAs in plants: Key findings from the early years. Plant Cell 31: 1206–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miloslavina, Y. , de Bianchi, S. , Dall'Osto, L. , Bassi, R. , and Holzwarth, A.R. (2011). Quenching in Arabidopsis thaliana mutants lacking monomeric antenna proteins of photosystem II. J. Biol. Chem. 286: 36830–36840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina‐Heredia, F.P. , Wastl, J. , Navarro, J.A. , Bendall, D.S. , Hervás, M. , Howe, C.J. , and De la Rosa, M.A. (2003). A new function for an old cytochrome? Nature 424: 33–34. [DOI] [PubMed] [Google Scholar]
- Morales, A. , and Kaiser, E. (2020). Photosynthetic acclimation to fluctuating irradiance in plants. Front. Plant Sci. 11: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux, C.W. (2016). Photosynthesis: Rewiring an angiosperm. Nat. Plants 2: 16018. [DOI] [PubMed] [Google Scholar]
- Mulo, P. , and Medina, M. (2017). Interaction and electron transfer between ferredoxin‐NADP+ oxidoreductase and its partners: Structural, functional, and physiological implications. Photosynth. Res. 134: 265–280. [DOI] [PubMed] [Google Scholar]
- Munekage, Y.N. , Genty, B. , and Peltier, G. (2008). Effect of PGR5 impairment on photosynthesis and growth in Arabidopsis thaliana . Plant Cell Physiol. 49: 1688–1698. [DOI] [PubMed] [Google Scholar]
- Munekage, Y.N. , Eymery, F. , Rumeau, D. , Cuiné, S. , Oguri, M. , Nakamura, N. , Yokota, A. , Genty, B. , and Peltier, G. (2010). Elevated expression of PGR5 and NDH‐H in bundle sheath chloroplasts in C4 Flaveria species. Plant Cell Physiol. 51: 664–668. [DOI] [PubMed] [Google Scholar]
- Murata, N. , Takahashi, S. , Nishiyama, Y. , and Allakhverdiev, S.I. (2007). Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta, Bioenerg. 1767: 414–421. [DOI] [PubMed] [Google Scholar]
- Murphy, D.J. (2007). People, plants and genes: The story of crops and humanity. 1st ed. Oxford University Press, Oxford, UK. [Google Scholar]
- Mussgnug, J.H. , Thomas‐Hall, S. , Rupprecht, J. , Foo, A. , Klassen, V. , McDowall, A. , Schenk, P.M. , Kruse, O. , and Hankamer, B. (2007). Engineering photosynthetic light capture: Impacts on improved solar energy to biomass conversion. Plant Biotechnol. J. 5: 802–814. [DOI] [PubMed] [Google Scholar]
- Mustila, H. , Paananen, P. , Battchikova, N. , Santana‐Sánchez, A. , Muth‐Pawlak, D. , Hagemann, M. , Aro, E.‐M. , and Allahverdiyeva, Y. (2016). The flavodiiron protein Flv3 functions as a homo‐oligomer during stress acclimation and is distinct from the Flv1/Flv3 hetero‐oligomer specific to the O2 photoreduction pathway. Plant Cell Physiol. 57: 1468–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutanda, T. , Naidoo, D. , Bwapwa, J.K. , and Anandraj, A. (2020). Biotechnological applications of microalgal oleaginous compounds: Current trends on microalgal bioprocessing of products. Front. Energy Res. 8: 299. [Google Scholar]
- Muzzopappa, F. , and Kirilovsky, D. (2020). Changing color for photoprotection: The orange carotenoid protein. Trends Plant Sci. 25: 92–104. [DOI] [PubMed] [Google Scholar]
- Nagy, V. , Podmaniczki, A. , Vidal‐Meireles, A. , Kuntam, S. , Herman, E. , Kovács, L. , Tóth, D. , Scoma, A. , and Tóth, S.Z. (2021). Thin cell layer cultures of Chlamydomonas reinhardtii L159I‐N230Y, pgrl1 and pgr5 mutants perform enhanced hydrogen production at sunlight intensity. Bioresour. Technol. 333: 125217. [DOI] [PubMed] [Google Scholar]
- Naruka, M. , Khadka, M. , Upadhayay, S. , and Kumar, S. (2019). Potential applications of microalgae in bioproduct production: A review. Octa J. Biosci. 7: 1–5. [Google Scholar]
- Nawrocki, W.J. , Bailleul, B. , Picot, D. , Cardol, P. , Rappaport, F. , Wollman, F.‐A. , and Joliot, P. (2019a). The mechanism of cyclic electron flow. Biochim. Biophys. Acta: Bioenerg. 1860: 433–438. [DOI] [PubMed] [Google Scholar]
- Nawrocki, W.J. , Buchert, F. , Joliot, P. , Rappaport, F. , Bailleul, B. , and Wollman, F.A. (2019b). Chlororespiration controls growth under intermittent light. Plant Physiol. 179: 630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi, S. , Perrine, Z. , Friedland, N. , Kumar, A. , Tokutsu, R. , Minagawa, J. , Berg, H. , Barry, A.N. , Govindjee, and Sayre, R. (2020). Light regulation of light‐harvesting antenna size substantially enhances photosynthetic efficiency and biomass yield in green algae. Plant J. 103: 584–603. [DOI] [PubMed] [Google Scholar]
- Nelson, N. , and Yocum, C.F. (2006). Structure and function of photosystems I and II. Annu. Rev. Plant Biol. 57: 521–565. [DOI] [PubMed] [Google Scholar]
- Niazian, M. , Sadat‐Noori, S.A. , Tohidfar, M. , Mortazavian, S.M.M. , and Sabbatini, P. (2021). Betaine aldehyde dehydrogenase (BADH) vs. flavodoxin (Fld): Two important genes for enhancing plants stress tolerance and productivity. Front. Plant Sci. 12: 650215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets, Û. , Berry, J.A. , von Caemmerer, S. , Ort, D.R. , Parry, M.A.J. , and Poorter, H. (2016). Photosynthesis: Ancient, essential, complex, diverse… and in need of improvement in a changing world. New Phytol. 213: 43–47. [DOI] [PubMed] [Google Scholar]
- Nikkanen, L. , Toivola, J. , and Rintamäki, E. (2016). Crosstalk between chloroplast thioredoxin systems in regulation of photosynthesis. Plant Cell Environ. 39: 1691–1705. [DOI] [PubMed] [Google Scholar]
- Nikkanen, L. , Toivola, J. , Guinea Diaz, M. , and Rintamäki, E. (2017). Chloroplast thioredoxin systems: Prospects for improving photosynthesis. Phil. Trans. R. Soc. B 372: 20160474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkanen, L. , Toivola, J. , Trotta, A. , Guinea Diaz, M. , Tikkanen, M. , Aro, E.‐M. , and Rintamäki, E. (2018). Regulation of cyclic electron flow by chloroplast NADPH‐dependent thioredoxin system. Plant Direct 2: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkanen, L. , and Rintamäki, E. (2019). Chloroplast thioredoxin systems dynamically regulate photosynthesis in plants. Biochem. J. 476: 1159–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilkens, M. , Kress, E. , Lambrev, P. , Miloslavina, Y. , Müller, M. , Holzwarth, A.R. , and Jahns, P. (2010). Identification of a slowly inducible zeaxanthin‐dependent component of non‐photochemical quenching of chlorophyll fluorescence generated under steady‐state conditions in Arabidopsis . Biochim. Biophys. Acta: Bioenerg. 1797: 466–475. [DOI] [PubMed] [Google Scholar]
- Nishiyama, Y. , Allakhverdiev, S.I. , Yamamoto, H. , Hayashi, H. , and Murata, N. (2004). Singlet oxygen inhibits the repair of photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp. PCC 6803. Biochemistry 43: 11321–11330. [DOI] [PubMed] [Google Scholar]
- Nishiyama, Y. , Yamamoto, H. , Allakhverdiev, S.I. , Inaba, M. , Yokota, A. , and Murata, N. (2001). Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J. 20: 5587–5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa, Y. , Yamamoto, H. , Okegawa, Y. , Wada, S. , Sato, N. , Taira, Y. , Sugimoto, K. , Makino, A. , and Shikanai, T. (2012). PGR5‐dependent cyclic electron transport around PSI contributes to the redox homeostasis in chloroplasts rather than CO2 fixation and biomass production in rice. Plant Cell Physiol. 53: 2117–2126. [DOI] [PubMed] [Google Scholar]
- Niyogi, K.K. , Björkman, O. , and Grossman, A.R. (1997). The roles of specific xanthophylls in photoprotection. Proc. Natl. Acad. Sci. U.S.A. 94: 14162–14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oravec, M.W. , and Havey, M.J. (2021). Polymorphism in the chloroplast ATP synthase beta‐subunit is associated with a maternally inherited enhanced cold recovery in cucumber. Plants (Basel) 10: 1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort, D.R. , Merchant, S.S. , Alric, J. , Barkan, A. , Blankenship, R.E. , Bock, R. , Croce, R. , Hanson, M.R. , Hibberd, J.M. , Long, S.P. , Moore, T.A. , Moroney, J. , Niyogi, K.K. , Parry, M.A.J. , Peralta‐Yahya, P.P. , Prince, R.C. , Redding, K.E. , Spalding, M.H. , van Wijk, K.J. , Vermaas, W.F.J. , von Caemmerer, S. , Weber, A.P.M. , Yeates, T.O. , Yuan, J.S. , and Zhu, X.G. (2015). Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. U.S.A. 112: 8529–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort, D.R. , Zhu, X. , and Melis, A. (2011). Optimizing antenna size to maximize photosynthetic efficiency. Plant Physiol. 155: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, J. , Huang, D. , Guo, Z. , Kuang, Z. , Zhang, H. , Xie, X. , Ma, Z. , Gao, S. , Lerdau, M.T. , Chu, C. , and Li, L. (2018). Overexpression of microRNA408 enhances photosynthesis, growth, and seed yield in diverse plants. J. Integr. Plant Biol. 60: 323–340. [DOI] [PubMed] [Google Scholar]
- Parry, M.A.J. , Andralojc, P.J. , Scales, J.C. , Salvucci, M.E. , Carmo‐Silva, A.E. , Alonso, H. , and Whitney, S.M. (2013). Rubisco activity and regulation as targets for crop improvement. J. Exp. Bot. 64: 717–730. [DOI] [PubMed] [Google Scholar]
- Parry, M.A.J. , Reynolds, M. , Salvucci, M.E. , Raines, C. , Andralojc, P.J. , Zhu, X.G. , Price, G.D. , Condon, A.G. , and Furbank, R.T. (2011). Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J. Exp. Bot. 62: 453–467. [DOI] [PubMed] [Google Scholar]
- Paul, M.J. (2021). Improving photosynthetic metabolism for crop yields: What is going to work? Front. Plant Sci. 12: 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier, G. , Aro, E.‐M. , and Shikanai, T. (2016). NDH‐1 and NDH‐2 plastoquinone reductases in oxygenic photosynthesis. Annu. Rev. Plant Biol. 67: 55–80. [DOI] [PubMed] [Google Scholar]
- Perrine, Z. , Negi, S. , and Sayre, R.T. (2012). Optimization of photosynthetic light energy utilization by microalgae. Algal Res. 1: 134–142. [Google Scholar]
- Pesaresi, P. , Scharfenberg, M. , Weigel, M. , Granlund, I. , Schröder, W.P. , Finazzi, G. , Rappaport, F. , Masiero, S. , Furini, A. , Jahns, P. , and Leister, D. (2009). Mutants, overexpressors, and interactors of Arabidopsis plastocyanin isoforms: Revised roles of plastocyanin in photosynthetic electron flow and thylakoid redox state. Mol. Plant 2: 236–248. [DOI] [PubMed] [Google Scholar]
- Piccinini, L. , Cazzaniga, S. , Iacopino, S. , Ballottari, M. , Giuntoli, B. , and Licausi, F. (2021). A synthetic switch based on orange carotenoid protein to control blue light responses in chloroplasts. bioRxiv. 10.1101/2021.01.27.428448 [DOI] [PMC free article] [PubMed]
- Pierella Karlusich, J.J. , Lodeyro, A.F. , and Carrillo, N. (2014). The long goodbye: The rise and fall of flavodoxin during plant evolution. J. Exp. Bot. 65: 5161–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnola, A. (2019). The rise and fall of light‐harvesting complex stress‐related proteins as photoprotection agents during evolution. J. Exp. Bot. 70: 5527–5535. [DOI] [PubMed] [Google Scholar]
- Pivato, M. , Perozeni, F. , Licausi, F. , Cazzaniga, S. , and Ballottari, M. (2021). Heterologous expression of cyanobacterial orange carotenoid protein (OCP2) as a soluble carrier of ketocarotenoids in Chlamydomonas reinhardtii . Algal Res. 55: 102255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogoryelov, D. , Klyszejko, A.L. , Krasnoselska, G.O. , Heller, E.‐M. , Leone, V. , Langer, J.D. , Vonck, J. , Müller, D.J. , Faraldo‐Gómez, J.D. , and Meier, T. (2012). Engineering rotor rings in ATP synthase. Proc. Natl. Acad. Sci. U.S.A. 109: E1599–E1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle, J.E.W. , Kanakagiri, S. , Jin, E.S. , Masuda, T. , and Melis, A. (2002). Truncated chlorophyll antenna size of the photosystems—A practical method to improve microalgal productivity and hydrogen production in mass culture. Int. J. Hydrogen Energy 27: 1257–1264. [Google Scholar]
- Price, G.D. , von Caemmerer, S. , Evans, J.R. , Siebke, K. , Anderson, J.M. , and Badger, M.R. (1998). Photosynthesis is strongly reduced by antisense suppression of chloroplastic cytochrome bf complex in transgenic tobacco. Aust. J. Plant Physiol. 25: 445–452. [Google Scholar]
- Price, G.D. , Yu, J.‐W. , von Caemmerer, S. , Evans, J.R. , Chow, W.S. , Anderson, J.M. , Hurry, V. , and Badger, M.R. (1995). Chloroplast cytochrome b6/f and ATP synthase complexes in tobacco: Transformation with antisense RNA against nuclear‐encoded transcripts for the Rieske FeS and ATP polypeptides. Aust. J. Plant Physiol. 22: 285–297. [Google Scholar]
- Rae, B.D. , Long, B.M. , Förster, B. , Nguyen, N.D. , Velanis, C.N. , Atkinson, N. , Hee, W.Y. , Mukherjee, B. , Price, G.D. , and McCormick, A.J. (2017). Progress and challenges of engineering a biophysical CO2‐concentrating mechanism into higher plants. J. Exp. Bot. 68: 3717–3737. [DOI] [PubMed] [Google Scholar]
- Ramankutty, N. , Mehrabi, Z. , Waha, K. , Jarvis, L. , Kremen, C. , Herrero, M. , and Rieseberg, L.H. (2018). Trends in global agricultural land use: Implications for environmental health and food security. Annu. Rev. Plant Biol. 69: 789–815. [DOI] [PubMed] [Google Scholar]
- Rantala, S. , Lempiäinen, T. , Gerotto, C. , Tiwari, A. , Aro, E.‐M. , and Tikkanen, M. (2020). PGR5 and NDH‐1 systems do not function as protective electron acceptors but mitigate the consequences of PSI inhibition. Biochim. Biophys. Acta: Bioenerg. 1861: 148154. [DOI] [PubMed] [Google Scholar]
- Rizwan, M. , Mujtaba, G. , Memon, S.A. , Lee, K. , and Rashid, N. (2018). Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 92: 394–404. [Google Scholar]
- Robertson, D. , Woessner, J.P. , Gillham, N.W. , and Boynton, J.E. (1989). Molecular characterization of two point mutants in the chloroplast atpB gene of the green alga Chlamydomonas reinhardtii defective in assembly of the ATP synthase complex. J. Biol. Chem. 264: 2331–2337. [PubMed] [Google Scholar]
- Rodriguez, R.E. , Lodeyro, A. , Poli, H.O. , Zurbriggen, M. , Peisker, M. , Palatnik, J.F. , Tognetti, V.B. , Tschiersch, H. , Hajirezaei, M.‐R. , Valle, E.M. , and Carrillo, N. (2007). Transgenic tobacco plants overexpressing chloroplastic Ferredoxin‐NADP(H) Reductase display normal rates of photosynthesis and increased tolerance to oxidative stress. Plant Physiol. 143: 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal, D.M. , Locke, A.M. , Khozaei, M. , Raines, C.A. , Long, S.P. , and Ort, D.R. (2011). Over‐expressing the C(3) photosynthesis cycle enzyme Sedoheptulose‐1‐7 Bisphosphatase improves photosynthetic carbon gain and yield under fully open air CO2 fumigation (FACE). BMC Plant Biol. 11: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, M. , Bermudez, L. , and Carrari, F. (2015). Crop yield: Challenges from a metabolic perspective. Curr. Opin. Plant Biol. 25: 79–89. [DOI] [PubMed] [Google Scholar]
- Rosso, D. , Ivanov, A.G. , Fu, A. , Geisler‐Lee, J. , Hendrickson, L. , Geisler, M. , Stewart, G. , Krol, M. , Hurry, V. , Rodermel, S.R. , Maxwell, D.P. , and Hüner, N.P.A. (2006). IMMUTANS does not act as a stress‐induced safety valve in the protection of the photosynthetic apparatus of Arabidopsis during steady‐state photosynthesis. Plant Physiol. 142: 574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott, M. , Martins, N.F. , Thiele, W. , Lein, W. , Bock, R. , Kramer, D.M. , and Schöttler, M.A. (2011). ATP synthase repression in tobacco restricts photosynthetic electron transport, CO2 assimilation, and plant growth by overacidification of the thylakoid lumen. Plant Cell 23: 304–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottet, S. , Förster, B. , Hee, W.Y. , Rourke, L.M. , Price, G.D. , and Long, B.M. (2021). Engineered accumulation of bicarbonate in plant chloroplasts: Known knowns and known unknowns. Front. Plant Sci. 12: 727118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban, A.V. (2015). Evolution under the sun: Optimizing light harvesting in photosynthesis. J. Exp. Bot. 66: 7–23. [DOI] [PubMed] [Google Scholar]
- Ruban, A.V. (2016). Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 170: 1903–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühle, T. , Dann, M. , Reiter, B. , Schünemann, D. , Naranjo, B. , Penzler, J.‐F. , Kleine, T. , and Leister, D. (2021). PGRL2 triggers degradation of PGR5 in the absence of PGRL1. Nat. Commun. 12: 3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungrat, T. , Almonte, A.A. , Cheng, R. , Gollan, P.J. , Stuart, T. , Aro, E.‐M. , Borevitz, J.O. , Pogson, B. , and Wilson, P.B. (2019). A genome‐wide association study of non‐photochemical quenching in response to local seasonal climates in Arabidopsis thaliana . Plant Direct 3: e00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuska, S.A. , Andrews, T.J. , Badger, M.R. , Price, G.D. , and von Caemmerer, S. (2000). The role of chloroplast electron transport and metabolites in modulating Rubisco activity in tobacco. Insights from transgenic plants with reduced amounts of cytochrome b/f complex or glyceraldehyde 3‐phosphate dehydrogenase. Plant Physiol. 122: 491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage, R.F. , Way, D.A. , and Kubien, D.S. (2008). Rubisco, Rubisco activase, and global climate change. J. Exp. Bot. 59: 1581–1595. [DOI] [PubMed] [Google Scholar]
- Sales, C.R.G. , Wang, Y. , Evers, J.B. , and Kromdijk, J. (2021). Improving C4 photosynthesis to increase productivity under optimal and suboptimal conditions. J. Exp. Bot. 72: 5942–5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salesse‐Smith, C.E. , Sharwood, R.E. , Busch, F.A. , Kromdijk, J. , Bardal, V. , and Stern, D.B. (2018). Overexpression of Rubisco subunits with RAF1 increases Rubisco content in maize. Nat. Plants 4: 802–810. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Baracaldo, P. , and Cardona, T. (2020). On the origin of oxygenic photosynthesis and cyanobacteria. New Phytol. 225: 1440–1446. [DOI] [PubMed] [Google Scholar]
- Santana‐Sanchez, A. , Solymosi, D. , Mustila, H. , Bersanini, L. , Aro, E.‐M. , and Allahverdiyeva, Y. (2019). Flavodiiron proteins 1‐to‐4 function in versatile combinations in O2 photoreduction in cyanobacteria. eLife 8: e45766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos‐Merino, M. , Torrado, A. , Davis, G.A. , Röttig, A. , Bibby, T.S. , Kramer, D.M. , and Ducat, D.C. (2021). Improved photosynthetic capacity and photosystem I oxidation via heterologous metabolism engineering in cyanobacteria. Proc. Natl. Acad. Sci. U.S.A. 118: e2021523118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz‐Barrio, R. , Corral‐Martinez, P. , Ancin, M. , Segui‐Simarro, J.M. , and Farran, I. (2013). Overexpression of plastidial thioredoxin f leads to enhanced starch accumulation in tobacco leaves. Plant Biotechnol. J. 11: 618–627. [DOI] [PubMed] [Google Scholar]
- Scafaro, A.P. , Bautsoens, N. , den Boer, B. , Van Rie, J. , and Gallé, A. (2019a). A conserved sequence from heat‐adapted species improves Rubisco activase thermostability in wheat. Plant Physiol. 181: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafaro, A.P. , De Vleesschauwer, D. , Bautsoens, N. , Hannah, M.A. , den Boer, B. , Gallé, A. , and Van Rie, J. (2019b). A single point mutation in the C‐terminal extension of wheat Rubisco activase dramatically reduces ADP inhibition via enhanced ATP binding affinity. J. Biol. Chem. 294: 17931–17940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöttler, M.A. , Kirchhoff, H. , and Weis, E. (2004). The role of plastocyanin in the adjustment of the photosynthetic electron transport to the carbon metabolism in tobacco. Plant Physiol. 136: 4265–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahinnia, F. , Tula, S. , Hensel, G. , Reiahisamani, N. , Nasr, N. , Kumlehn, J. , Gómez, R. , Lodeyro, A.F. , Carrillo, N. , and Hajirezaei, M.‐R. (2021). Plastid‐targeted cyanobacterial flavodiiron proteins maintain carbohydrate turnover and enhance drought stress tolerance in barley. Front. Plant Sci. 11: 613731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, B.R. , Wang, L.M. , Lin, X.L. , Yao, Z. , Xu, H.W. , Zhu, C.H. , Teng, H.Y. , Cui, L.L. , Liu, E.E. , Zhang, J.J. , He, Z.H. , and Peng, X.X. (2019). Engineering a new chloroplastic photorespiratory bypass to increase photosynthetic efficiency and productivity in rice. Mol. Plant 12: 199–214. [DOI] [PubMed] [Google Scholar]
- Shin, W.‐S. , Lee, B. , Jeong, B.‐R. , Chang, Y.K. , and Kwon, J.‐H. (2016). Truncated light‐harvesting chlorophyll antenna size in Chlorella vulgaris improves biomass productivity. J. Appl. Phycol. 28: 3193–3202. [Google Scholar]
- Shvaleva, A. , de la Peña, T.C. , Rincón, A. , Morcillo, C.N. , García de la Torre, V.S. , Lucas, M.M. , and Pueyo, J.J. (2009). Flavodoxin overexpression reduces cadmium‐induced damage in alfalfa root nodules. Plant Soil 326: 109–121. [Google Scholar]
- Simkin, A.J. , Lopez‐Calcagno, P.E. , Davey, P.A. , Headland, L.R. , Lawson, T. , Timm, S. , Bauwe, H. , and Raines, C.A. (2017a). Simultaneous stimulation of sedoheptulose 1,7‐bisphosphatase, fructose 1,6‐bisphophate aldolase and the photorespiratory glycine decarboxylase‐H protein increases CO2 assimilation, vegetative biomass and seed yield in Arabidopsis . Plant Biotechnol. J. 15: 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin, A.J. , López‐Calcagno, P.E. , and Raines, C.A. (2019). Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 70: 1119–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin, A.J. , McAusland, L. , Headland, L.R. , Lawson, T. , and Raines, C.A. (2015). Multigene manipulation of photosynthetic carbon assimilation increases CO2 fixation and biomass yield in tobacco. J. Exp. Bot. 66: 4075–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin, A.J. , McAusland, L. , Lawson, T. , and Raines, C.A. (2017b). Overexpression of the Rieske FeS protein increases electron transport rates and biomass yield. Plant Physiol. 175: 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair, T.R. , Rufty, T.W. , and Lewis, R.S. (2019). Increasing photosynthesis: Unlikely solution for world food problem. Trends Plant Sci. 24: 1032–1039. [DOI] [PubMed] [Google Scholar]
- Slater, B. , Kosmützky, D. , Nisbet, R.E.R. , and Howe, C.J. (2021). The evolution of the cytochrome c6 family of photosynthetic electron transfer proteins. Genome Biol. Evol. 13: evab146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery, R.A. , and Ort, D.R. (2021). Perspectives on improving light distribution and light use efficiency in crop canopies. Plant Physiol. 185: 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery, R.A. , VanLoocke, A. , Bernacchi, C.J. , Zhu, X.‐G. , and Ort, D.R. (2017). Photosynthesis, light use efficiency, and yield of reduced‐chlorophyll soybean mutants in field conditions. Front. Plant Sci. 8: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, J. , and Wang, B. (2014). Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. 115: 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Q. , Chu, C. , Parry, M.A. , and Zhu, X.‐G. (2016). Genetics‐based dynamic systems model of canopy photosynthesis: The key to improve light and resource use efficiencies for crops. Food Energy Secur. 5: 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Q. , Wang, Y. , Qu, M. , Ort, D.R. , and Zhu, X.‐G. (2017). The impact of modifying photosystem antenna size on canopy photosynthetic efficiency—Development of a new canopy photosynthesis model scaling from metabolism to canopy level processes. Plant Cell Environ. 40: 2946–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Q. , Zhang, G. , and Zhu, X.‐G. (2013). Optimal crop canopy architecture to maximize canopy photosynthetic CO2 uptake under elevated CO2—A theoretical study using a mechanistic model of canopy photosynthesis. Funct. Plant Biol. 40: 109–124. [DOI] [PubMed] [Google Scholar]
- South, P.F. , Cavanagh, A.P. , Lopez‐Calcagno, P.E. , Raines, C.A. , and Ort, D.R. (2018). Optimizing photorespiration for improved crop productivity. J. Integr. Plant Biol. 60: 1217–1230. [DOI] [PubMed] [Google Scholar]
- South, P.F. , Cavanagh, A.P. , Liu, H.W. , and Ort, D.R. (2019). Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 363: eaat9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnichuk, I.N. , Krasilnikov, P.M. , and Zlenko, D.V. (2015). Cyanobacterial phycobilisomes and phycobiliproteins. Microbiology 84: 101–111. [PubMed] [Google Scholar]
- Steed, G. , Cano Ramirez, D. , Hannah, M.A. , and Webb, A.A.R. (2021). Chronoculture, harnessing the circadian clock to improve crop yield and sustainability. Science 372: eabc9141. [DOI] [PubMed] [Google Scholar]
- Steinbeck, J. , Nikolova, D. , Weingarten, R. , Johnson, X. , Richaud, P. , Peltier, G. , Hermann, M. , Magneschi, L. , and Hippler, M. (2015). Deletion of proton gradient regulation 5 (PGR5) and PGR5‐like 1 (PGRL1) proteins promote sustainable light‐driven hydrogen production in Chlamydomonas reinhardtii due to increased PSII activity under sulfur deprivation. Front. Plant Sci. 6: 892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepien, P. , and Johnson, G.N. (2018). Plastid terminal oxidase requires translocation to the grana stacks to act as a sink for electron transport. Proc. Natl. Acad. Sci. U.S.A. 115: 9634–9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirbet, A. , Lazár, D. , Guo, Y. , and Govindjee (2019). Photosynthesis: Basics, history and modelling. Ann. Bot. 126: 511–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X. , Liu, M.J. , Yang, M.Y. , Lu, J. , Du, J.B. , Shu, K. , Wang, X.C. , and Yang, W.Y. (2017). Implications of terminal oxidases in the regulation of soybean photosynthetic performance under different light intensities. Acta Physiol. Plant. 39: 266. [Google Scholar]
- Sun, X. , and Wen, T. (2011). Physiological roles of plastid terminal oxidase in plant stress responses. J. Biosci. 36: 951–956. [DOI] [PubMed] [Google Scholar]
- Sutton, H.C. , and Winterbourn, C.C. (1989). On the participation of higher oxidation states of iron and copper in Fenton reactions. Free Rad. Biol. Med. 6: 53–60. [DOI] [PubMed] [Google Scholar]
- Tang, K. , Ding, W.L. , Höppner, A. , Zhao, C. , Zhang, L. , Hontani, Y. , Kennis, J.T. , Gärtner, W. , Scheer, H. , Zhou, M. , and Zhao, K.H. (2015). The terminal phycobilisome emitter, LCM: A light‐harvesting pigment with a phytochrome chromophore. Proc. Natl. Acad. Sci. U.S.A. 112: 15880–15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazoe, Y. , Ishikawa, N. , Shikanai, T. , Ishiyama, K. , Takagi, D. , Makino, A. , Sato, F. , and Endo, T. (2020). Overproduction of PGR5 enhances the electron sink downstream of photosystem I in a C4 plant Flaveria bidentis. Plant J. 103: 814–823. [DOI] [PubMed] [Google Scholar]
- Teardo, E. , de Laureto, P.P. , Bergantino, E. , Dalla Vecchia, F. , Rigoni, F. , Szabò, I. , and Giacometti, G.M. (2007). Evidences for interaction of PsbS with photosynthetic complexes in maize thylakoids. Biochim. Biophys. Acta: Bioenerg. 1767: 703–711. [DOI] [PubMed] [Google Scholar]
- Tholen, D. , Boom, C. , and Zhu, X.G. (2012). Opinion: Prospects for improving photosynthesis by altering leaf anatomy. Plant Sci. 197: 92–101. [DOI] [PubMed] [Google Scholar]
- Tibiletti, T. , Rehman, A.U. , Vass, I. , and Funk, C. (2018). The stress‐induced SCP/HLIP family of small light‐harvesting‐like proteins (ScpABCDE) protects photosystem II from photoinhibitory damages in the cyanobacterium Synechocystis sp. PCC 6803. Photosynth. Res. 135: 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonov, A.N. (2014). The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways. Plant Physiol. Biochem. 81: 163–183. [DOI] [PubMed] [Google Scholar]
- Tognetti, V.B. , Monti, M.R. , Valle, E.M. , Carrillo, N. , and Smania, A.M. (2007b). Detoxification of 2,4‐dinitrotoluene by transgenic tobacco plants expressing a bacterial flavodoxin. Environ. Sci. Technol. 41: 4071–4076. [DOI] [PubMed] [Google Scholar]
- Tognetti, V.B. , Palatnik, J.F. , Fillat, M.F. , Melzer, M. , Hajirezaei, M.‐R. , Valle, E.M. , and Carrillo, N. (2006). Functional replacement of ferredoxin by a cyanobacterial flavodoxin in tobacco confers broad‐range stress tolerance. Plant Cell 18: 2035–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetti, V.B. , Zurbriggen, M.D. , Morandi, E.N. , Fillat, M.F. , Valle, E.M. , Hajirezaei, M.‐R. , and Carrillo, N. (2007a). Enhanced plant tolerance to iron starvation by functional substitution of chloroplast ferredoxin with a bacterial flavodoxin. Proc. Natl. Acad. Sci. U.S.A. 104: 11495–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivola, J. , Nikkanen, L. , Dahlström, K.M. , Salminen, T.A. , Lepistö, A. , Vignols, H.F. , and Rintamäki, E. (2013). Overexpression of chloroplast NADPH‐dependent thioredoxin reductase in Arabidopsis enhances leaf growth and elucidates in vivo function of reductase and thioredoxin domains. Front. Plant Sci. 4: 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrado, A. , Ramírez‐Moncayo, C. , Navarro, J.A. , Mariscal, V. , and Molina‐Heredia, F.P. (2019). Cytochrome c6 is the main respiratory and photosynthetic soluble electron donor in heterocysts of the cyanobacterium Anabaena sp. PCC 7120. Biochim. Biophys. Acta, Bioenerg. 1860: 60–68. [DOI] [PubMed] [Google Scholar]
- Tula, S. , Shahinnia, F. , Melzer, M. , Rutten, T. , Gómez, R. , Lodeyro, A.F. , von Wirén, N. , Carrillo, N. , and Hajirezaei, M.‐R. (2020). Providing an additional electron sink by the introduction of cyanobacterial flavodiirons enhances growth of A. thaliana under various light intensities. Front. Plant Sci. 11: 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchi, V. , Barera, S. , Bassi, R. , and Dall'Osto, L. (2020). Potential and challenges of improving photosynthesis in algae. Plants 9: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialet‐Chabrand, S. , Matthews, J.S.A. , Simkin, A.J. , Raines, C.A. , and Lawson, T. (2017). Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiol. 173: 2163–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicino, P. , Carrillo, J. , Gómez, R. , Shahinnia, F. , Tula, S. , Melzer, M. , Rutten, T. , Carrillo, N. , Hajirezaei, M.‐R. , and Lodeyro, A.F. (2021). Expression of flavodiiron proteins Flv2‐Flv4 in chloroplasts of Arabidopsis and tobacco plants provides multiple stress tolerance. Int. J. Mol. Sci. 22: 1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola, S. , Sellés, J. , Bailleul, B. , Joliot, P. , and Wollman, F.‐A. (2021). In vivo electron donation from plastocyanin and cytochrome c6 to PSI in Synechocystis sp. PCC6803. Biochim. Biophys. Acta: Bioenerg. 1862: 148449. [DOI] [PubMed] [Google Scholar]
- Wada, S. , Yamamoto, H. , Suzuki, Y. , Yamori, W. , Shikanai, T. , and Makino, A. (2018). Flavodiiron protein substitutes for cyclic electron flow without competing CO2 assimilation in rice. Plant Physiol. 176: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Yamamoto, H. , Narumiya, F. , Munekage, Y.N. , Finazzi, G. , Szabo, I. , and Shikanai, T. (2017). Fine‐tuned regulation of the K+/H+ antiporter KEA3 is required to optimize photosynthesis during induction. Plant J. 89: 540–553. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Mei, J. , and Ren, G. (2019). Plant microRNAs: Biogenesis, homeostasis, and degradation. Front. Plant Sci. 10: 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L.M. , Shen, B.R. , Li, B.D. , Zhang, C.L. , Lin, M. , Tong, P.P. , Cui, L.L. , Zhang, Z.S. , and Peng, X.X. (2020). A synthetic photorespiratory shortcut enhances photosynthesis to boost biomass and grain yield in rice. Mol. Plant 13: 1802–1815. [DOI] [PubMed] [Google Scholar]
- Wang, Q. , Zhao, H. , Jiang, J. , Xu, J. , Xie, W. , Fu, X. , Liu, C. , He, Y. , and Wang, G. (2017). Genetic architecture of natural variation in rice nonphotochemical quenching capacity revealed by genome‐wide association study. Front. Plant Sci. 8: 1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel, M. , Varotto, C. , Pesaresi, P. , Finazzi, G. , Rappaport, F. , Salamini, F. , and Leister, D. (2003). Plastocyanin is indispensable for photosynthetic electron flow in Arabidopsis thaliana . J. Biol. Chem. 278: 31286–31289. [DOI] [PubMed] [Google Scholar]
- Whitney, S.M. , Birch, R. , Kelso, C. , Beck, J.L. , and Kapralov, M.V. (2015). Improving recombinant Rubisco biogenesis, plant photosynthesis and growth by coexpressing its ancillary RAF1 chaperone. Proc. Natl. Acad. Sci. U.S.A. 112: 3564–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Ocean Review . (2010). World Ocean Review 1, Living with the oceans – A report on the state of the world's oceans. Hamburg. https://worldoceanreview.com/en/wor-1/ocean-chemistry/co2-reservoir/
- Wraight, C.A. , and Crofts, A.R. (1970). Energy‐dependent quenching of chlorophyll alpha fluorescence in isolated chloroplasts. Eur. J. Biochem. 17: 319–327. [DOI] [PubMed] [Google Scholar]
- Wu, A. , Hammer, G.L. , Doherty, A. , von Caemmerer, S. , and Farquhar, G.D. (2019). Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 5: 380–388. [DOI] [PubMed] [Google Scholar]
- Wu, G. , Ma, L. , Sayre, R.T. , and Lee, C.‐H. (2020). Identification of the optimal light harvesting antenna size for high‐light stress mitigation in plants. Front. Plant Sci. 11: 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, G. , Ortiz‐Flores, G. , Ortiz‐Lopez, A. , and Ort, D.R. (2007). A point mutation in AtpC1 raises the redox potential of the Arabidopsis chloroplast ATP synthase gamma‐subunit regulatory disulfide above the range of thioredoxin modulation. J. Biol. Chem. 282: 36782–36789. [DOI] [PubMed] [Google Scholar]
- Wu, X. , Wu, J. , Wang, Y. , He, M. , He, M. , Liu, W. , Shu, S. , Sun, J. , and Guo, S. (2021). The key cyclic electron flow protein PGR5 associates with cytochrome b6f, and its function is partially influenced by the LHCII state transition. Hortic. Res. 8: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Y. , Tholen, D. , and Zhu, X.G. (2016). The influence of leaf anatomy on the internal light environment and photosynthetic electron transport rate: Exploration with a new leaf ray tracing model. J. Exp. Bot. 67: 6021–6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, F. , Wang, K. , Yuan, W. , Xu, W. , Liu, S. , Kronzucker, H.J. , Chen, G. , Miao, R. , Zhang, M. , Ding, M. , Xiao, L. , Kai, L. , Zhang, J. , and Zhu, Y. (2019). Overexpression of rice aquaporin OsPIP1;2 improves yield by enhancing mesophyll CO2 conductance and phloem sucrose transport. J. Exp. Bot. 70: 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, S.K. , Khatri, K. , Rathore, M.S. , and Jha, B. (2018). Introgression of Ufcyt c6, a thylakoid lumen protein from a green seaweed Ulva fasciata Delile enhanced photosynthesis and growth in tobacco. Mol. Biol. Rep. 45: 1745–1758. [DOI] [PubMed] [Google Scholar]
- Yamamoto, H. , Takahashi, S. , Badger, M.R. , and Shikanai, T. (2016). Artificial remodelling of alternative electron flow by flavodiiron proteins in Arabidopsis . Nat. Plants 2: 16012. [DOI] [PubMed] [Google Scholar]
- Yamori, W. , Takahashi, S. , Makino, A. , Price, G.D. , Badger, M.R. , and von Caemmerer, S. (2011). The roles of ATP synthase and the cytochrome b6/f complexes in limiting chloroplast electron transport and determining photosynthetic capacity. Plant Physiol. 155: 956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori, W. , Kondo, E. , Sugiura, D. , Terashima, I. , Suzuki, Y. , and Makino, A. (2016). Enhanced leaf photosynthesis as a target to increase grain yield: Insights from transgenic rice lines with variable Rieske FeS protein content in the cytochrome b6/f complex. Plant Cell Environ. 39: 80–87. [DOI] [PubMed] [Google Scholar]
- Yang, J.H. , Williams, D. , Kandiah, E. , Fromme, P. , and Chiu, P.L. (2020). Structural basis of redox modulation on chloroplast ATP synthase. Commun. Biol. 3: 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, X. , and Struik, P.C. (2015). Constraints to the potential efficiency of converting solar radiation into phytoenergy in annual crops: From leaf biochemistry to canopy physiology and crop ecology. J. Exp. Bot. 66: 6535–6549. [DOI] [PubMed] [Google Scholar]
- Yin, X. , and Struik, P.C. (2021a). Exploiting differences in the energy budget among C4 subtypes to improve crop productivity. New Phytol. 229: 2400–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, X. , Struik, P.C. , and Goudriaan, J. (2021b). On the needs for combining physiological principles and mathematics to improve crop models. Field Crops Res. 271: 108254. [Google Scholar]
- Yu, G. , Pan, X. , Hao, J. , Shi, L. , Zhang, Y. , Wang, J. , Xiao, Y. , Yang, F. , Lou, J. , Chang, W. , Malnoë, A. , and Li, M. (2021). Structure of SOQ1 lumenal domains identifies potential disulfide exchange for negative regulation of photoprotection, qH. bioRxiv. 10.1101/2021.03.16.435614 [DOI]
- Yu, Q. , Feilke, K. , Krieger‐Liszkay, A. , and Beyer, P. (2014). Functional and molecular characterization of plastid terminal oxidase from rice (Oryza sativa). Biochim. Biophys. Acta, Bioenerg. 1837: 1284–1292. [DOI] [PubMed] [Google Scholar]
- Zahra, Z. , Choo, D.H. , Lee, H. , and Parveen, A. (2020). Cyanobacteria: Review of current potentials and applications. Environments. 7: 13. [Google Scholar]
- Zhang, P. , Allahverdiyeva, Y. , Eisenhut, M. , and Aro, E.‐M. (2009). Flavodiiron proteins in oxygenic photosynthetic organisms: Photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS ONE 4: e5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, P. , Eisenhut, M. , Brandt, A.‐M. , Carmel, D. , Silén, H.M. , Vass, I. , Allahverdiyeva, Y. , Salminen, T.A. , and Aro, E.‐M. (2012). Operon flv4‐flv2 provides cyanobacterial photosystem II with flexibility of electron transfer. Plant Cell 24: 1952–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, L. , Gao, S. , Wu, S. , Han, D. , Wang, H. , Gu, W. , Hu, Q. , Wang, J. , and Wang, G. (2020). PGRL1 overexpression in Phaeodactylum tricornutum inhibits growth and reduces apparent PSII activity. Plant J. 103: 1850–1857. [DOI] [PubMed] [Google Scholar]
- Zhou, L. , Wu, S. , Gu, W. , Wang, L. , Wang, J. , Gao, S. , and Wang, G. (2021). Photosynthesis acclimation under severely fluctuating light conditions allows faster growth of diatoms compared with dinoflagellates. BMC Plant Biol. 21: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X.T. , Wang, F. , Ma, Y.P. , Jia, L.J. , Liu, N. , Wang, H.Y. , Zhao, P. , Xia, G.X. , and Zhong, N.Q. (2018). Ectopic expression of SsPETE2, a plastocyanin from Suaeda salsa, improves plant tolerance to oxidative stress. Plant Sci. 268: 1–10. [DOI] [PubMed] [Google Scholar]
- Zhu, X.G. , Long, S.P. , and Ort, D.R. (2008). What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotechnol. 19: 153–159. [DOI] [PubMed] [Google Scholar]
- Zhu, X.G. , Long, S.P. , and Ort, D.R. (2010). Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 61: 235–261. [DOI] [PubMed] [Google Scholar]
- Zhu, X.G. , Ort, D.R. , Parry, M.A.J. , and von Caemmerer, S. (2020). A wish list for synthetic biology in photosynthesis research. J. Exp. Bot. 71: 2219–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X.G. , Ort, D.R. , Whitmarsh, J. , and Long, S.P. (2004). The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: A theoretical analysis. J. Exp. Bot. 55: 1167–1175. [DOI] [PubMed] [Google Scholar]
- Zurbriggen, M.D. , Tognetti, V.B. , Fillat, M.F. , Hajirezaei, M.‐R. , Valle, E.M. , and Carrillo, N. (2008). Combating stress with flavodoxin: A promising route for crop improvement. Trends Biotechnol. 26: 531–537. [DOI] [PubMed] [Google Scholar]


