Abstract
Human milk oligosaccharides (HMOs) have been researched by scientists for over 100 years, driven by the substantial evidence for the nutritional and health benefits of mother's milk. Yet research has truly bloomed during the last decade, thanks to progress in biotechnology, which has allowed the production of large amounts of bona fide HMOs. The availability of HMOs has been particularly crucial for the renewed interest in HMO research because of the low abundance or even absence of HMOs in farmed animal milk. This interest is reflected in the increasing number of original research publications and reviews on HMOs. Here, we provide an overview and critical discussion on structure–function relations of HMOs that highlight why they are such interesting and important components of human milk. Clinical observations in breastfed infants backed by basic research from animal models provide guidance as to what physiological roles for HMOs are to be expected. From an evidence‐based nutrition viewpoint, we discuss the current data supporting the clinical relevance of specific HMOs based on randomised placebo‐controlled clinical intervention trials in formula‐fed infants.
Keywords: animal milk, development, growth, human milk, immunity, infections, microbiota
An illustration of the human milk oligosaccharide (HMO) mediated microbiome–host interaction, highlighting its importance for the biology of HMOs for infant health.

Key points
This review discusses different aspects of human milk oligosaccharides (HMOs): from their chemistry to biology.
It provides a comprehensive overview of our current understanding of the clinical relevance of HMOs for infant development and health.
Cumulative evidence from clinical observations and interventions backed by mechanistic basic research data indicates that HMOs are a meaningful and important component of human milk.
Increasing evidence suggests that specific HMOs help establish immune competence, both local and systemically, partly through their effect on the metabolite activity of specific microbes mainly Bifidobacterium species.
HMOs may also participate in a gut–brain connection, thereby modulating brain and cognitive development.
HMOs likely act in concert with other bioactive components and act via different mechanisms that converge to specific functions.
INTRODUCTION
Human milk is the natural, adapted and sole recommended nutrition for infants. It provides not only nutrition for the growth of an infant, but also numerous bioactive components supporting age‐appropriate development and immune protection. Consequently, pediatric societies and the World Health Organization (WHO) recommend exclusive breastfeeding to 6 months of age and breast‐feeding to be continued to at least 2 years of age. 1 , 2 Breast milk is mostly composed of water and various solid components with nutritive values and bioactive functions.
Among the breast milk bioactive components, human milk oligosaccharides (HMOs) are a highly represented category, both in terms of amounts and structural diversity. Together with their resemblance to mucous and mucosal surface glycans, and the fact that they are largely undigestible, this has triggered extensive basic and applied research. To date, more than 160 different HMO structures have been described 3 , 4 with an estimated total concentration ranging between 5 and 15 g L–1. 5 , 6 , 7
Historically, HMOs were mainly recognised as a fraction in breast milk related to the presence of beneficial bacteria such as bifidobacteria and lactobacilli in infant feces. 8 However, there is now increasing evidence that HMOs could also contribute to broader health benefits of human milk. For over a century, breast milk has been recognised as protecting infants from morbidity and mortality. Indeed, breastfed infants generally experience less gastrointestinal and respiratory infectious illnesses, and show higher cognitive development and lower risk for being overweight or obese. 9 The potential effect of breastfeeding on development of allergies later in life is less clear. Notably, in infants born preterm, breast milk reduces the risk of life‐threatening necrotising enterocolitis (NEC), as well as the risk of late onset sepsis, and supports medical treatments to prevent bronchopulmonary dysplasia. 10 , 11 , 12 These benefits of human milk for the infant are key inspirations in the quest to understand the physiological roles of breast milk components such as HMOs.
As Ajit Varki wrote; ‘Nothing in Glycobiology Makes Sense, except in the Light of Evolution’. 13 This can serve as a guiding principle when trying to understand the variety and abundance of HMOs found in breast milk and their variability between mothers. In this work, we discuss different aspects of HMOs: from their chemistry to biology, aiming to provide an overview of our current understanding on their clinical relevance for infant development and health.
WHAT ARE HMOS?
HMOs are non‐lactose oligosaccharides found in human milk. A stricter definition may be that HMOs are not only present, but also produced directly by the lactating mother's mammary glands. From a physiological angle, HMOs are not digested in the infant gut and hence are not part of the nutritive breast milk components, but, as a result of their numerous roles, are considered bioactive constituents.
From a chemical perspective, all known HMOs are elongations of the milk sugar lactose with one or several of the following monosaccharides: galactose (Gal), N‐acetyl‐glucosamine (GlcNAc), N‐acetyl‐galactosamine (GalNAc), fucose (Fuc) and sialic acid (N‐acetyl‐neuraminic acid [NeuAc]). Lactose is formed solely in the lactating mammary glands by the lactose synthase complex, starting from uridine diphosphate galactose (UDP‐Gal) and glucose (Glc). Lactose synthase is a heterodimer composed of the milk protein alpha‐lactalbumin and the enzyme beta‐1,4‐galactosyltransferase 1, which is encoded by the B4GALT1 gene. The B4GALT1 gene codes for two enzymatic forms that result from two distinct transcription initiation sites and subsequent post‐translational processing. The ubiquitously present first form, a type II membrane‐bound, trans‐Golgi resident protein, is involved in glycoconjugate biosynthesis adding Gal from UDP‐Gal to GlcNAc. The second transcription product results in the soluble lactose synthase, producing lactose by adding Gal from UDP‐Gal to free Glc.
Lactose synthase is not reported to further elongate lactose with additional Gal. Rather, the next step of lactose elongation is brought about by a series of other glycosyltransferases (GTs) with different specificities as recently reviewed. 14 Lactose can be elongated by the disaccharides lacto‐N‐biose (LNB; β‐Gal‐(1→3)‐β‐GlcNAc) and N‐acetyllactosamine (LacNAc; β‐Gal(1→4)‐β‐GlcNAc) resulting in the tetrasaccharides lacto‐N‐tetraose (LNT) and lacto‐N‐neotetraose (LNnT), respectively. The exact pathway leading towards the production of the latter two HMOs is yet to be discovered. Both LNT and LNnT are also further elongated by additional LNB and LacNAc units. Although the involved enzymes are not described, it is likely by tight sequential addition of GlcNAc and Gal units. The intermediate lacto‐N‐triose with only a GlcNAc added to lactose is only rarely reported in human milk. 15 , 16 Both LNT and LNnT as well as their further LNB‐ and LacNAc‐elongated descendants can be decorated with Fuc and NeuAc. Because many GTs are involved in the formation of several linkage types, a large variety of structures differing in composition, conformation and chain length is found in human milk. 3 , 14 , 17 Trace amounts of galactosyl‐lactoses (GLs), mainly 6'‐GL, are also found in human milk 7 , 18 , 19 and, although the enzymes involved in their formation are not known, interestingly, no further elongation with Fuc or NeuAc has been reported. This could indicate that the GL synthesis is not localised in the endomembrane system together with the GTs that are involved in the major HMO synthesis pathways. We speculate that different microbes present in milk, albeit in low concentration, could be responsible for synthesising these GLs in breast milk, similar to the GL formation during milk fermentation with starter cultures. 20
Elongation of core HMOs with Fuc is catalysed by fucosyltransferases (FUT), which add Fuc from guanosine diphosphate fucose (GDP‐Fuc) to lactose (or other acceptor oligosaccharides) through different linkages. These are encoded by FUT2 and FUT3, respectively called the secretor and Lewis gene, as well as further FUT genes (e.g., FUT5,6). This leads for example to the formation of 2'‐fucosyllactose (2'‐FL), 3‐fucosyllactose (3‐FL) and lacto‐difucosyltetraose (LDFT; 2',3‐difucosyllactose) (Figure 1). Similarly, several sialyltransferases such as St3Gal4 and St6Gal1 add NeuAc (N‐acetylneuraminic acid) to lactose (or other acceptor oligosaccharides) in different linkages, resulting in for example 3'‐sialyllactose (3'‐SL) and 6'‐sialyllactose (6'‐SL). The formation of the main HMOs that are generally analysed in most studies is depicted in Figure 1.
Figure 1.
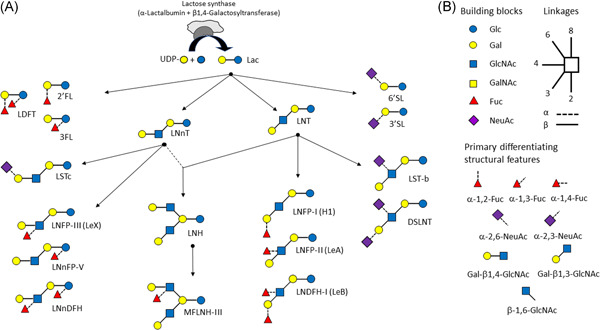
Main human milk oligosaccharides (HMOs) found in human milk. (a) Illustration of main HMOs and their synthetic route starting from lactose that is formed by lactose synthase complex from UDP‐Gal and Glc in the mammary glands. (b) Key to the monosaccharide building blocks and linkages of HMOs and illustration of the eight primary differentiating structural features represented in HMOs. 2'FL, 2'Fucosyllactose; 3FL, 3Fucosyllactose; 3'SL, 3'Sialyllactose; 6'SL, 6'Sialylactose; DSLNT, DisialyllactoNtetraose; Fuc, fucose; Gal, galactose; GalNAc, N‐acetyl‐galactosamine; GlcNAc, N‐acetyl‐glucosamine; Glc, glucose; NeuAc, N‐acetylneuraminic acid (sialic acid); LDFT, Lactodifucosyltetraose; Le (A,B,X), Lewis (A,B,X); LNnT, LactoNneotetraose; LNT, LactoNtetraose; LNFP (I,II,III), LactoNfucopentaose (I,II,III); LNnFP V, LactoNneofucopentaose V; LNnDFH, LactoNneodifucosylhexaose; LNDFH I, LactoNdifucosylhexaose I; LST (b,c), SialyllactoNtetraose (b,c); MFLNH III, MonofucosyllactoNhexaose III
Based on their chemical composition HMOs may be classified into different categories. The most apparent subclassification is the separation of acidic HMOs harboring one or more sialic acids from neutral HMOs. The latter can be further grouped into those containing one or more fucose units vs. those without a fucose moiety. The acidic sialylated HMOs may be further split to discern acidic non‐fucosylated and acidic fucosylated HMOs.
Biology is not only directed by chemical composition, but, importantly, also by the three‐dimensional structure. Hence, it is essential to consider structural features when classifying HMOs. The same sugar monomer can be attached in different ways by different enzymes to another saccharide unit. For example, adding a Fuc to Lac can result both in 2'FL or in 3‐FL depending on the catalysing GT. Investigating the structures of the major reported HMOs, eight primary differentiating structural features can be distinguished (Figure 1b). Generally, and as recently reviewed, each of these structural features is formed by a specific GT, although some can be formed by several GTs. 14 Although FUT2 specifically adds fucose in α1,2 to an acceptor galactose, FUT3 forms both α1,4 and α1,3 linkages between fucose and GlcNAc, as well as Glc. However, an additional FUT can also form the α1,3 linkages between Fuc and Glc (Figure 1). 21
The presence or absence of the FUT2 and FUT3 enzymes also allows further stratification of human milk. The FUT2 genotype is linked to secretor status (Se), with women of a FUT2 −/− genotype being unable to produce α1,2 fucosylated HMOs, such as 2'‐FL and LNFP‐I. The FUT3 enzyme determines the Lewis type (Le), resulting in strong reduction of α1,3 and absence of α1,4 fucosylated HMOs in milk. Lewis and Secretor type thus allow grouping of human milk in four main milk groups, also sometimes named ‘lactotypes’, which will be discussed later. Milk groups show significant differences in HMO profiles even beyond those HMOs directly affected by the FUT2 or FUT3 genotype. 7 Possible explanations are alterations in acceptor (e.g., LNT) or altered donor availabilities (GDP‐Fuc) if one or the other enzyme is not active: for example, absence of a downstream fucosylation enzyme results in a higher proportion of sialylated HMO structures. HMO profiles are thus not solely dependent on the direct control by specific enzymes and their expression pattern. This means that the HMO structural diversity and composition are closely tied to their biosynthesis and any maternal factors, such as genetic background, health condition, environmental factors and diet, modulating the expression and function of maternal GTs, as well as substrate availabilities. Whether or not reported differences have a physiological significance for the suckling newborns is a topic of intensive research.
Of note, GTs involved in HMO biosynthesis in the mammary glands, such as FUT2 for example, are also expressed in other body parts, where they are involved in cell and mucous glycosylation. Therefore, HMOs may be considered as soluble lactose‐bound analogues of typical mammalian cell glycocalyx and mucous glycans, which represent a dense glycan matrix at the interface with other cells and the environment, including the microbiome.
WHERE ARE HMOS FOUND?
Although human milk is particularly rich in both amount and number of oligosaccharides with a high diversity of structural features not generally seen in other mammals, all mammals produce the milk sugar lactose in their milk and all mammals are equipped with a series of GTs such as those involved in HMO synthesis (see earlier). Milk of different mammalian species greatly varies in amount, number and diversity of structural features of their milk oligosaccharides. 22 Some are common to those found in human milk, whereas others are not. Among the HMOs more universally found in mammals, including monotremes (e.g., platypus), marsupials and eutherians (i.e., placental mammals), are the sialyllactoses, primarily 3'‐SL. 22 , 23 The milk of the egg‐laying platypus contains 3'‐SL and larger sialylated structures together with predominantly fucosylated lactose, such as LDFT, but also larger fucosylated oligosaccharides built on LNnT and LNT. 23 Similar oligosaccharides are also reported in milk of Echidna, another egg‐laying mammalian species. 24 These observations suggest that specific oligosaccharides are ancestral features of milk. As a result of the immaturity of monotremes at birth, these oligosaccharides were also suggested to be of particular importance for development and immune protection. Noteworthy, 3'‐SL and 6'‐SL are found in mouse and rat milk as highly predominant milk oligosaccharides, rendering these animals, who are born relatively immature, relevant models to study their role for growth and development. 25 , 26 , 27 , 28
Farmed animal milks also contain oligosaccharides, but at relatively low concentrations. Among them are 3'‐SL, 6'‐SL and primarily other neutral non‐fucosylated oligosaccharides such as galactosyllactoses. 29 Generally, their concentration decreases very rapidly from colostrum to mature milk. 29 In bovine milk, 3'‐SL is the most prominent oligosaccharide with a concentration reported to range between 50 and 100 mg L–1 compared to an approximately two‐fold higher amount observed in mother's milk. Notably, 3'‐SL was reported to increase in bovine milk around 2 weeks before parturition from around 100 to 700 mg L–1 and to steeply drop from around 800 mg L–1 in colostrum to around 100 mg L–1 by 3 days postpartum. 30 , 31
Several structural features characterise human as opposed to animal milk oligosaccharides. Human milk shows a predominance of type 1 structures (LNB), built on LNT cores, whereas animal milks that have larger oligosaccharides mainly show type two structures (LacNAc), built on LNnT cores. 32 , 33 Because humans cannot synthesise the N‐glycolyneuraminic acid (NeuGc) form of sialic acid, mother's milk only contains oligosaccharides with NeuAc, whereas animals do show also NeuGc on milk oligosaccharides in different proportions compared to NeuAc. 34 , 35 For example, goat milk contains approximately 70% NeuGc and 30% NeuAc, whereas, in bovine milk, over 95% of sialic acid is NeuAc. 36
ARE ALL HMOS THE SAME?
As outlined in the previous section, each HMO is structurally distinct from another. However, some commonalities exist in composition and the primary differentiating structural features. Interestingly, it should be noted that, of all theoretically possible structures, only a limited and a finite number of structures are made and present in human milk. Although HMO diversity and richness suggest many structure‐specific functions, the many different structures may also indicate a certain functional redundancy. The more universally present milk oligosaccharides, such as the sialyllactoses, are likely to contribute to similar more universal physiological needs of different mammals during the early postnatal life. On the other hand, different mammals have different postnatal nutritional and functional requirements that strongly depend on their maturity at birth, their speed of postnatal development and their environment. Hence, general milk composition differs among mammalian species and probably represents adaptation to their newborn's requirements. The question is: are milk oligosaccharides part of such an adaptation of milk?
In humans, HMO profiles strongly change by maternal polymorphisms in the Lewis blood group system (i.e. FUT2 and FUT3 polymorphisms) and by duration of lactation. 7 , 37 , 38 The distribution of the Lewis blood group polymorphisms indicates that this trait strongly depends on evolutionary pressure and selection with approximately 10%–35% FUT2 (secretor) negative genotypes in different geographies. 39 , 40 , 41 Population genetic studies confirm the presence of balancing selection acting upon FUT2, an indication of advantages linked to maintaining genetic variation. 39 , 42 The prevalence of FUT2 negative genotypes varies across different geographies, thus contributing to reported geographic differences in HMO profiles. 43 Additional environmental and maternal factors may also contribute to HMO variability, although their effect size may be rather modest. 7 , 43 Comparison of HMO profiles from mothers who gave birth to preterm vs. term infants indicated that sialyllactose is slightly higher and FUT2 dependent HMOs such as 2'‐FL and LNFP I slightly lower in early milk of mothers who gave birth to a preterm infant. 18 Whether such observations represent an adaptation of the milk for the physiological needs of the infant or rather reflect the physiological state of the mother needs further investigation. Interestingly, birth appears to trigger a program that determines how the HMO profile changes over the period of lactation. 18 The stage of lactation is a key parameter affecting HMO profiles, with most HMOs decreasing and a few increasing in concentration over the first few months and even beyond the first year of lactation. 7 , 38 3‐FL is found among the HMOs generally increasing in concentration and, although the reason for this is unknown to date, we speculate that 3‐FL may have a particular role beyond the exclusive breastfeeding period. When estimating the daily average intake, most HMOs show a relatively constant intake, whereas 3‐FL intake increases with time of lactation. 44
In basic research models, both redundancy and specificity with selected HMOs is reported. In animal models coupled with cell‐based models and modelling, both 2'‐FL and 6'‐SL were shown to affect NEC via Toll like receptor 4 (TLR‐4) mediated route. 45 Similarly, 2'‐FL and 3‐FL were shown to interact with dendritic cell‐specific intercellular adhesion molecule‐3‐grabbing non‐integrin (DC‐SIGN), whereas LNT did not bind. 46 In yet another study, 6'‐SL and LNT both activated the G‐protein coupled receptor GPR35, with both together activating stronger, whereas 2'‐FL, LNnT and 3‐'SL did not activate this receptor. 47 When it comes to stimulation of specific Bifidobacterium species, many more examples of redundancy and specificity exist. Such redundancies indicate their physiological importance and are likely to come into play to compensate FUT2 and FUT3 polymorphisms. Detailed observational studies coupled with mechanistic insight are warranted to understand such initial observations and hypothesis.
MANUFACTURED HMOS ADDED TO INFANT FORMULA: ARE THEY SAFE AND WHAT ARE THEIR BENEFITS?
The relatively large amounts of diverse HMOs found in human milk contrasts with milk from farmed animals used for human nutrition, which contain only very small amounts of oligosaccharides. To close this gap for animal milk‐derived breast milk substitutes in child nutrition, different technological strategies are possible. For example, the animal milk oligosaccharide‐rich fraction can be concentrated, an approach that can provide some oligosaccharides such as the sialyllactoses but, as a result of the low initial amounts, this is technically challenging. 48 Alternatively, individual HMOs may be produced by chemical, enzymatic or biotechnological means. Recent progress in these fields has enabled industrial production of individual HMOs that are not typically found in farmed animal milks.
Today different biotechnological processes using bacterial fermentation are the most industrially viable technologies. A handful of individual HMOs are available at industrial scale, representing some of the most abundant HMOs found in breast milk. Although they have the primary structural features of HMOs (Figure 1), they are still relatively simple compared to the some of the larger HMOs found in human milk.
These technologies involve novel processes, as defined by the regulatory authorities such as the European Food Safety Agency (EFSA) or the Federal Drug Administration (FDA). Today, a select number of HMOs (2'‐FL, 3‐FL, LDFT, LNnT, LNT, 3'‐SL, 6'‐SL) have obtained Generally Recognized As Safe (GRAS) and Novel Food status. These HMOs, produced by biotechnology, are identical in structure to those naturally present in breast milk and are therefore dubbed ‘human identical milk oligosaccharides’ abbreviated as HiMOs. These ingredients are diligently analysed, characterised and subjected to a series of safety/toxicity tests according to guidelines established by the Organisation for Economic Cooperation and Development (OECD). Generally, to best match the target application in early life nutrition, the in vivo OECD testing protocol with animals was adapted to include a juvenile period. 49 , 50
WHAT ARE THE PHYSIOLOGICAL ROLES OF HMOS?
The physical and physiological development of infants is intricately linked with their environment, including key influences such as nutrition and the developing gut microbiome. In terms of early life nutrition, breast milk feeding with its high abundance and variable amounts of different HMOs is the optimal nutrition for the developing infant and its microbiome. Yet some HMOs may have redundant roles and some may have synergistic functional effects, and hence simple associations between particular HMOs and their health effects may be difficult to establish. Ultimately, hypotheses need to be tested and causality established through mode‐of‐action research. In the following, we provide an overview of observations from different studies with breastfed infants and intervention trials with HMO formula‐fed infants. Where possible, we discuss basic research data relevant to support clinical observations. Figure 2 illustrates the main proposed functions of HMOs.
Figure 2.
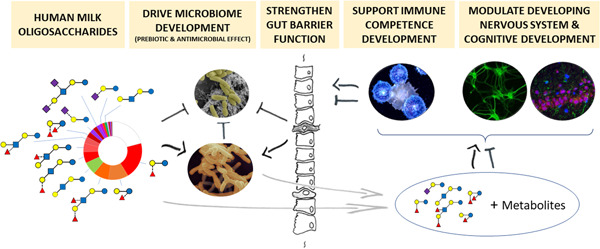
Summary illustration of main expected human milk oligosaccharide (HMO) functions reported in literature
HMOs and the development of the early life gut microbiome
A bona fide gut colonisation with microbes starts with maternal and environmental microbiota seeding, followed in part by appropriate selection through undigested dietary components. To this end, breast milk provides a multitude of diverse oligosaccharides (HMOs), together with numerous other key components such as immunoglobulins, lactoferrin and beyond. Primarily during the recommended exclusive breastfeeding period through 4–6 months of age, infants who are exclusively or partially breastfed show a different maturation trajectory of their gut microbiome compared to their non‐breastfed peers. This is first reflected in a lower microbiota diversity index and secondly in a higher microbiota age or maturity compared to the infant's chronological age in formula fed infants. 51 , 52 , 53 These measures indicate that low or no breast milk intake accelerates maturation of the gut microbiome towards that observed in adults. 52
At the microbiota taxonomic level, breastfed infants show primarily higher relative abundance of Bifidobacteriaceae family members during the early exclusive breastfeeding period. Notably, primarily strains from the classes Actinobacteria, including Bifidobacteriaceae, and Bacteroidia were found to be vertically transferred from the mother to her infant. 54 Additionally, breast milk was identified as the most important covariate explaining microbiome variance at genus and species taxonomy levels, as well as the microbiome functional capacity. 53 The importance of bifidobacteria especially during early life for gut ecology and development is well established 55 , 56 , 57 and highlights the importance of maternal seeding and feeding.
Generally, HMOs are not digested by the infant's digestive enzymes, although intestinal neuraminidase cleaving sialylated HMOs may be an exception. 58 Different studies with breastfed infant–mother dyads have investigated HMO profiles in breast milk and infant stools. Although some studies report only relatively small changes, others found that HMO profiles in some infant stools changed more dramatically. 44 , 59 , 60 , 61 In addition to important inter‐individual variability, HMO profiles are strongly associated with specific microbiota taxa, primarily Bifidobacterium, Bacteroides and Lactobacillus. 61
Bifidobacteriumspecies are genetically particularly well equipped to take advantage of the HMO diversity and abundance in breast milk. 62 , 63 , 64 Their dominance in early life is largely attributed to this unique genetic glycan‐foraging capacity, which is likely a result of coevolution with the host. 65 , 66 , 67 Several studies have investigated the complex glycan degradation capabilities of early life colonisers and all showed the unique capacity of bifidobacteria to utilise HMOs, whereas other strains favor less complex oligosaccharides and sugar monomers as carbon sources for growth. 68 , 69 , 70 In general, two large strategies are deployed by bifidobacteria to utilise HMOs: (1) the secretion of glycosidases that externally degrade HMOs followed by the uptake of sugar monomers by the bifidobacteria (e.g., Bifidobacterium bifidum and some Bifidobacterium longum strains) and (2) the expression of dedicated HMO transporters that can internalise HMOs for further internal degradation. 63 , 71 The latter strategy is found for example in Bifidobacterium breve, Bifidobacterium longum subsp. infantis and most other B. longum strains. 63 , 71 Interestingly, strain‐specific differences in HMO utilisation capacity were reported within the same B. breve and B. longum subsp. infantis species. 63 , 67 , 72 , 73 To what extent these strain specificities reflect a personalised mother–infant exchange of microbes and nutrition, or indicate a gradual gene pool loss of key microbes, needs to be established in detail.
Another group of microbes able to utilise HMOs for growth are Bacteroides species. 74 However, in a gnotobiotic mouse model associated with a Bacteroides and a Bifidobacterium species, the HMO LNnT boosted the abundance of the Bifidobacterium species over the Bacteroides, although both species were able to utilise LNnT. 75 Although the Bacteroides species can equally use mucous glycans and HMOs, the Bifidobacterium species can only use HMOs as growth substrate and HMOs appear to provide a selective advantage to Bifidobacterium species. Other early gut colonisers such as the Enterobacteriaceae, which includes several pathogens, are generally not able to grow on HMOs. 76 For Streptococcus species, 2'‐FL contrary to lactose or galactooligosaccharides (GOS) was shown not to allow for Streptococcus mutans growth and LNT was identified to interfere with a Group B Streptococcus (GBS) cell wall synthesis, which leads to cell death and higher sensitivity to antibiotics. 77 , 78 , 79 , 80 The former may have a clinical relevance for oral health, whereas the latter may be of relevance to reduce sepsis risks and antibiotic dosing especially in infants born preterm. A second reason behind the dominance of Bifidobacterium species in the gut in early life, is its social behavior. Collaboration and cross‐feeding may occur between different Bifidobacterium strains and between different species. 66 , 81 , 82 , 83 The first phenomenon relies on the presence of different HMO utilisation loci in different bifidobacteria, resulting in crossfeeding chains, further enhancing their dominance. 66 Crossfeeding of other strains relies on several HMO metabolites produced by bifidobacteria, such as the short chain fatty acid acetate supporting Anaeostipes caccae a butyrogenic species 71 , 84 or sugar oligo‐ and monomers supporting growth of other species unable to degrade HMOs, such as lactobacilli. 69 An elegant study showed that an early life gut microbiome community with a very high Bifidobacteriaceae dominance is established in presence of Bifidobacterium strains, in this case specific strains of B. breve, which have the genetic make‐up to utilise specific HMOs such as 2'‐FL. 67 Concomitantly, stool from infants with a microbiome harboring this 2'‐FL utilising capacity was also shown to have lower pH, higher acetate and lower remaining 2'‐FL from breast milk, indicating higher metabolic activity. Such gut ecology changes with higher acetate, likely combined with other metabolites, was shown in animal models to lead to improved protection against gastrointestinal and respiratory tract infections. 85 , 86 Infant gut microbiome HMO utilisation capacity was recently shown to relate to less inflammatory markers and specifically B. longum subsp. infantis derived metabolites such as indolelactate were shown to drive such pathways. 87 , 88 As expected, such bifidobacteria activity driven processes are very relevant for appropriate immune competence development.
HMOs and infant anthropometry
Along with environmental, genetic, epigenetic and metabolic factors, the developing gut microbiome is considered as a key factor affecting infant growth. 89 Considerable evidence suggests that the disruption of an age‐appropriate gut microbiota assembly and succession could lead to growth faltering. For example, in infants born preterm, delayed microbiota succession or maturation was related to lower weight‐for‐age z‐score (WAZ), leading to the hypothesis that the microbiome, influenced by nutrition, may play a causal role in promoting growth. 90 Similarly, studies focusing on undernourished infants found that an altered gut microbiota could be causally related to growth, with causality being established via studies in gnotobiotic animal models. 91 , 92 , 93 , 94 Similar to observations in preterm infants, the microbiota in stunted or undernourished infants is immature, as concluded from a modelling approach that used the microbiota composition to predict an infant's chronological age. 91 , 93 Based on their effect on the establishing gut microbiota, HMO are also investigated in relation to infant growth as summarised in Table 1.
Table 1.
Summary of reported associations between human milk oligosaccharides (HMOs) and anthropometric measures
| Anthropometry measure | Infant age (months) | Associated HMOs | Study type | Feeding mode | Reference | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Stunted growth | Fucosyl‐ HMOs sialyl‐ HMOs | Obs | BF | 92 | ||
| Height/length‐for‐age z‐score change | 6–12 | HMOs abundancea | Obs | BF | 96 | |
| Height/length‐for‐age z‐score | 5 | LNFP I + III, DFLNHa | Obs | BF | 107 | |
| Height/length z‐score | 3–12 | 2'‐FL | LNnT, LST‐b | Obs | BF | 100 |
| Height/length‐for‐age z‐scoreb | 5 | 3'‐SL, LDFT | LNnT, DFLNH | Obs | BF | 101 |
| Weight | 6 | LNFP I | Obs | BF | 97 | |
| Weight gain | 1–6 | LNFP II | Obs | BF | 104 | |
| Weight z‐score | 3–12 | 2'‐FL, 3‐FL | LNnT | Obs | BF | 100 |
| Weight velocityb | 0–5 | 2'‐FL | LNnT | Obs | BF | 101 |
| Weight‐for‐age z‐score | 2–6 | 3'‐SL, 6'‐SL | Obs | BF | 106 | |
| Weight‐for‐age z‐score | 5 | 3'‐SL | LST‐c | Obs | BF | 107 |
| Weight‐for‐length gain | 0–4 | 3'‐SL | Obs | BF | 103 | |
| Head circumference SDS | 3–12 | Non‐secretor milk | Obs | BF | 105 | |
| BMI‐for‐age z‐scoreb | 5 | 6'SL | Obs | BF | 101 | |
| BMI SDS | 3–6 | Non‐secretor milk | Obs | BF | 105 | |
| Lean mass | 6 | LNFP I | Obs | BF | 97 | |
| Fat mass | 6 | LNFP II, DSLNT | LNFP I | Obs | BF | 97 |
| Fat mass | 2–6 | 3'‐SL, 6'‐SL, DSLNT | Obs | BF | 106 | |
| Fat mass indexb | 5 | 2'‐FL, LDFT, | LNnT, DFLNH | Obs | BF | 101 |
| Percent fat | 6 | LNnT | Obs | BF | 97 | |
| Weight, length, head circumference and their z‐scores | 0–4 | No association seen in secretor positive vs. secretor negative milk | Obs | BF | 99 , 103 | |
| Weight, length, head circumference and their z‐scores | 0–4 | No differences observed with 2'‐FL alone or combined with LNnT or LNT, 3'‐SL, 6'‐SL, LDFT or 3‐FL | RCT | FF | 109 , 110 , 111 , 113 , 114 , 115 , 116 | |
Abbreviations: BF, breastfed; BMI, body mass index; DFLNH, DifucosyllactoNhe; FF, formula fed; HMO, human milk oligosac charides; Obs, observational; RCT, randomised placebo‐controlled trial; SDS, standard deviation score.
Abundance assessed by integration of collected ion signals.
Association seen in secretor positive milk fed infants only.
Interestingly, in two Malawian mother‐infant dyad cohorts with a total of 303 infants, lower fucosylated and sialylated HMOs (for the latter primarily LST‐b) were observed in breast milk of non‐secretor mothers whose infants were stunted compared to those showing normal growth. 92 No significant difference was seen in secretor mothers whose child was stunted or growing normally. Using gnotobiotic mice with a microbiota from stunted infants and sialylated oligosaccharides derived from bovine milk, primarily 3'‐SL, a link between the HMOs, the microbiota and infant growth was reproduced. 92 This indicates that specific milk oligosaccharides may act via the microbiota to modulate infant growth. Importantly, a mechanistic link between the sialylated bovine milk oligosaccharides, essentially sialyllactose and bone formation was shown using the gnotobiotic mouse model. 95 A recent observational study in rural Malawi (n = 647) reported a significant association of HMO absolute abundance at 6 months with length‐for‐age change from 6 to 12 months, but no relationship between sialylated HMOs and growth. 96
Several observational studies have investigated a possible link between breast milk oligosaccharides and infant growth in well‐nourished breastfed infants born at term. Although some associations were found, only a few are consistent across different studies. 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 For example, in Hispanic mother‐infant pairs (n = 157), a higher LNFP II concentration in breast milk at 1 month of lactation was associated with lower weight gain from 1 to 6 months of age, 104 whereas, in a previous analysis of a small cohort (n = 25), LNFP II at 6 months of lactation was associated with higher fat mass at 6 months of age. 97 However, major differences exist among the different studies with respect to design, geography, sampling during lactation, number of time points at which growth parameters are assessed, the specific HMOs that were analysed and the statistical methods applied to model the associations. Disialyllacto‐N‐tetraose (DSLNT) concentration at 6 months of lactation was associated with higher fat mass of infants at 6 months of age. 97 In another US cohort, DSLNT intake at 2 months was also related to subsequent fat mass through 6 months. 106 Additionally, in the same study, 3'‐SL and 6'‐SL at 2 months of lactation were associated with higher fat mass and WAZ from 2 to 6 months of age. 106 In a small cohort of mothers and infants from The Gambia, 3'‐SL was found to be associated with an increase in WAZ, whereas other sialylated HMOs such as LST‐c showed the opposite association. 107 In a recent European multicenter study of 370 mother–infant dyads, 3'‐SL was the only HMO associated with higher weight for length gain during the first 4 months of lactation. 103 Similarly, associations between fucosylated HMOs (e.g., 2'‐FL, LNFP I, LNFP II) or neutral non‐fucosylated HMOs (e.g., LNnT) and growth, show conflicting results in different observational studies. In two studies, 2'‐FL was associated with higher growth velocity and fat mass index from birth to 5 months and length and weight z‐scores at 3 months of age. 100 , 101 In the same studies, LNnT was inversely correlated with 2'‐FL, and it was proposed that the 2'‐FL/LNnT ratio at 3 months is associated with higher length and weight z‐scores. 100 , 101 To date, no other study has confirmed these observations.
Biological plausibility for some of these observations may be built on the following hypothesis. As previously mentioned, HMO intake may increase food efficiency through microbiome related processes, explaining how specific HMO–microbiota pairs could affect infant anthropometry. Another hypothesis, proposed recently, is that HMOs affect food‐responsiveness and appetite through a microbiome driven process that affects the entero‐endocrine system or central nervous system. Indeed, specific HMOs were recently shown to be both positively and negatively associated with food‐responsiveness. 108 However, a consistent picture explaining associations between HMOs and anthropometric findings has not yet emerged.
As a result of insufficient data, it is not possible to explain the above inconsistent and often contradictory associations between HMO and infant growth. However, we can speculate that factors such as gut microbiota differences (e.g., epigenetic and genetic differences) and maternal nutritional status during pregnancy may be important confounding variables. It should also be noted that, generally, the observed effect sizes of associations between breast milk HMOs and growth in healthy well‐nourished infants are modest and within normal growth trajectories. Moreover, because associations do not imply a causal relationship, causality needs to be established using randomised placebo‐controlled interventional trials (RCTs) and supporting mechanistic studies.
Under controlled and randomised conditions, growth of infants fed formula containing individual HMOs in different combinations, 2'‐FL either alone or in combination with LNnT or in combination with LNT, 3'‐SL, 6'‐SL, LDFT or 3‐FL, was similar to control formula‐fed infants, without HMOs and whenever assessed, equivalent to infants exclusively breastfed for at least 4 months. 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 These RCTs assessed infant growth in healthy infants born at term with growth as the primary study end point. All trials concluded that addition of specific HMO or HMO blends to infant formula are well tolerated and allow for age‐appropriate growth. However, whether the addition of specific HMOs could help to improve growth in specific conditions of malnutrition and growth faltering, or in infants born preterm, is unknown and needs to be established in RCTs coupled with mechanistic studies. Clinical findings on the link between HMOs and immunity and infections are summarised in Table 2.
Table 2.
Summary of observed associations between human milk oligosaccharides (HMOs) and reduced risks for infant health related outcome measures
| Measure | Infant age (months) | HMOs | study type | feeding mode | References |
|---|---|---|---|---|---|
| Necrotising enterocolitis | Preterm | DSLNT | Obs | HM | 127 , 128 |
| Necrotising enterocolitis | Preterm | HMO diversity | Obs | HM | 129 |
| Immunoglobulin E‐associated eczemaa | 48 | 2'‐FL, secretor positive milk | Obs | BF | 136 |
| Cow milk protein allergy | 18 | LNFP III, LNFP I, 6'‐SL, DSLNT | Obs | BF | 141 |
| Sensitisation | 12 | HMO profileb | Obs | BF | 145 |
| Plasma cytokine profilec, d | 1.5 | 2'‐FL | RCT | FF | 147 |
| Diarrhea | 9 | 2'‐Fucosyl‐HMOs | Obs | BF | 153 |
| Campylobacterdiarrhea | 9 | 2'‐FL | Obs | BF | 153 |
| Morbidity | 4 | 2'‐Fucosyl‐HMOs | Obs | BF | 107 |
| Diarrhea | ca 11 | 2'‐Fucosyl‐HMOs | Obs | BF | 159 |
| Morbidity | 3 | LNFP II | Obs | BF | 160 |
| Prescribed antibiotic used | 12 | 2'‐FL + LNnT | RCT | FF | 109 |
| Lower respiratory tract infectionsd | 12 | 2'‐FL + LNnT | RCT | FF | 109 |
| Overall infectionsd | 1.5 | 2'‐FL | RCT | FF | 111 |
Abbreviations: BF, breastfed; FF, formula fed; HM, human milk fed; HMO, human milk oligosaccharides; Obs, observational; RCT, randomised placebo‐controlled trial.
In C‐section born only.
Relative higher concentrations of FDSLNH, LNFPII, LNnT, LNFPI, LSTc, FLNH and lower concentarions of LNH, LNT, 2'‐FL and DSLNH.
Interleukine receptor antagonist (IL‐1ra), IL‐1a, IL‐1b, IL‐6 and tumour necrosis factor α (TNF‐αa).
Secondary exploratory outcome measures.
HMOs and immune competence development
Development of the immune system starts in utero and continues with exposure to new stimuli during postnatal development. Several stimuli important for immune development stem initially from the maternal microbiome passing through the placenta to the fetus. 117 Postnatally, although maternal microbial metabolites continue to affect the newborn (e.g., through breast milk), exposure to metabolites and components from the infant's developing intestinal microbiome is much greater and more important. HMOs are considered to affect immune system development by their major influence on the establishment of the infant gut microbiome and its metabolic activity.
Development of the immune system is particularly important in infants born preterm, who are at increased risk of numerous health problems such as NEC, sepsis and cerebral palsy, which are all at least partly linked to an inappropriate immune reaction. Human milk feeding strongly reduces the risk of developing these diseases, probably by its immune modulating effects through components with direct action and others that involve the development of the gut microbiome. 11 , 118 , 119 , 120 , 121 Several blends and individual HMOs have been investigated for their protective effects in preclinical NEC models. In rodent models, not only DSLNT, 122 but also 6'‐SL and 2'‐FL, 45 , 123 , 124 showed some protection against the severity of NEC. Although a mechanism of action has yet to be established for DSLNT, 2'‐FL appears to ameliorate NEC symptoms through the modulation of endothelial nitric oxide synthase (eNOS) leading to increased gut perfusion. 123 Interestingly, 2'‐FL was previously shown to improve vascularisation in another model system. 125 Additionally, in mouse and piglet models of NEC, both 2'‐FL and 6'‐SL reduce clinical measures of NEC and inflammation, partly through the inhibition of TLR‐4 signalling, which is implicated in the onset of NEC. 45 From in silico modelling, both 2'‐FL and 6'‐SL were predicted to dock to TLR‐4, thus inhibiting signalling. In other preterm pig models for NEC, 2'‐FL alone or in combination with other HMOs including 6'‐SL did not lead to significant reduction in NEC symptoms. 126 Of note, in one study a blend of > 20 HMOs including DSLNT was tested, but did not reduce NEC symptoms in a preterm pig model. 126 Although clinical observations have not shown associations between 2'‐FL or 6'‐SL with NEC, higher DSLNT concentrations in breast milk were found to be associated with, and to be a good predictor for, a lower risk of NEC in two independent preterm infant cohorts, 127 , 128 although this association was not confirmed in another smaller study. 129 This latter study also reported a link between low HMO diversity in breast milk and NEC. 129 Although possible explanations for the association between DSLNT and NEC remain elusive, a recent study 128 suggests that DSLNT intake may be associated with a more age‐appropriate microbiome progression. 128 Clearly, further research using an interventional design is needed to establish a causal link between DSLNT and NEC risk. It would also be worthwhile to investigate whether the same benefits of DSLNT on reduced NEC risk are observed with donor breast milk as with mothers’ own milk reported in the current studies. This will allow us to better understand whether additional maternal factors need to be considered in combination with the HMOs to understand their possible physiological role.
The role of breastfeeding in relation to risks of developing allergic diseases is ambiguous 9 , 130 , 131 , 132 possibly partly because of the large variability in breast milk composition. As iterated before, HMO composition in breast milk is highly variable and has profound effects on the neonatal microbiome development, which itself is related to sensitisation and development of allergic manifestations. 133 , 134 , 135 Hence, several studies have investigated possible associations between the HMO composition of human milk and allergic manifestations in breastfed infants.
In a Finnish cohort (n = 266), infants with a hereditary risk of developing allergies and born by Caesarian section had an earlier onset of immunogloulin E‐associated eczema when breastfed by secretor‐negative mothers compared to those fed by secretor‐positive mothers. 136 The infants fed secretor‐negative breastmilk also showed a more pronounced delay in establishing a bifidobacterial‐dominated microbiome at 3 months of age compared to fed with secretor‐positive breast milk. 137 Among the affected bifidobacteria, specifically B. breve has been associated in independent studies to reduce the risk of pediatric eczema. 138 , 139 As summarised in a recent systematic review, 139 observational data indicate that lower Bifidobacteriaceae abundance in infancy is associated with a higher risk of eczema, especially in infants with family history of atopy. However, the underlying mechanisms and the individual Bifidobacterium species involved remain unknown.
A small case–control study of 20 mother‐infant pairs from a larger birth cohort in Sweden found no association between the concentrations of nine neutral HMOs and risk of developing allergic disease up to 18 months of age. 140 In another case–control study (n = 39 and 41), several individual HMOs (LNFP III, LNFP I, 6'‐SL, DSLNT) were observed to be lower in breast milk fed to infants with cow milk protein allergy, with LNFP III showing the strongest signal 141 compared to non‐allergic infants. Of these, not only 6'‐SL, but also 2'‐FL provided some protection against development of a food allergy compared to lactose in an ovalbumin food allergy animal model. 142 The mechanism for this effect was probably partly related to mast cell stabilisation leading to less histamine release. For LNFP III, further mechanistic insight may be gained based on its immune modulatory functions. 143 , 144
Another large clinical observation study (n = 421 mother–infant dyads) did not observe any association between individual HMOs and food sensitisation. 145 Instead, out of the 19 measured HMOs, a specific profile classified using projection on latent structures‐discriminant analysis was found to be related to lower risk for food sensitisation. This profile could be characterised by relative higher concentrations of FDSLNH, LNFPII, LNnT, LNFPI, LSTc and FLNH, and relatively lower concentrations of LNH, LNT, 2'‐FL and DSLNH. Similarly, in another birth cohort (n = 285) of infants at risk of allergies, specific HMO profiles classified by latent class analysis (LCA) were reported to be associated with allergies up to 18 years of age. 146 Although the approach to classify HMOs in profiles is promising and deserves to be extended to other breast milk components, its interpretability can be challenging. For example, Lodge et al. 146 used LCA, a method that works with binary ‘yes/no’ data. To transform the HMO concentrations into a ‘yes/no’ signal, HMO was considered as ‘yes’ when above the median and as ‘no’ when below it.
To understand if and how HMOs modulate sensitisation and allergy risk in breastfed infants, large well‐controlled cohort studies with nested case‐control analysis are needed. To help interpretability, it may be useful to include HMO classifications with large differences between HMO concentrations. For example, profiles determined by maternal FUT2 and FUT3 genotypes or classifications considering highest and lowest quartile comparison. In addition to HMOs, other known immune active breast milk components such as transforming growth factor β should also be considered. The developing gut microbiome may also strongly affect expected functions of HMOs and should therefore be part of such investigations. Information may be gained by machine learning approaches to better understand whether symptoms or sensitisation can be explained by a combination of input features such as infant and maternal parameters, environmental factors, HMOs and infant microbiome data.
To date, no randomised controlled intervention trial has investigated the role of HMOs in the prevention of sensitisation and allergic diseases. Only one intervention trial assessed plasma cytokine profiles as a proxy for immune maturation in a subgroup analysis of infants fed formula supplemented with 2'‐FL at two concentrations and in combination with GOS, against only GOS in the control formula. 147 Infants who received 2'‐FL in the formula showed similar basal and stimulated plasma cytokine profiles compared to the profiles in breastfed infants, but not those who received formula with GOS alone.
HMOs and infectious illnesses
HMOs are largely undigested by the infant digestive enzymes. This observation, together with the recognition that the HMOs resemble mucous and cell surface glycans, triggered the hypothesis and concept that HMOs may serve as soluble ligands for pathogens and their toxins, as these often first attach via glycan ligands to epithelial cells. 148 , 149 Today, many different gastrointestinal and respiratory tract viral and bacterial pathogens have been shown to either bind to specific HMOs, or specific HMOs were shown to block adhesion of specific pathogens. 150 Interestingly, specific HMOs, including LNT for example, were reported to have antibacterial activity by interfering with biofilm formation, cell wall synthesis and function of opportunistic pathogens such as Streptococcus agalactiae (Group B Streptococcus, GBS), Staphylococcus aureus and Acinetobacter baumannii. 77 , 151 Such HMO fragilised bacteria were shown to be more sensitive to antibiotic treatment. 77 , 80 , 152 Interestingly, Chambers et al. 80 reported increased 12,13‐DiHOME production in GBS treated with HMOs. Possibly, in vivo this may trigger an increased effector immune response for pathogen clearance.
In clinical observation studies, primarily the alpha 1,2‐linked fucosylated‐HMOs were associated with protection from infectious illnesses. In a pioneering study with 93 breastfed infants and their mothers from Mexico, Morrow et al. 153 observed fewer cases of enteropathogenic induced diarrhea in infants of mothers expressing higher amounts of alpha 1,2‐linked fucosylated‐HMOs. Notably, this was observed for diarrhea caused by calicivirus, including norovirus, and for Campylobacter, against which 2'‐FL was specifically suggested to be protective. In two different mouse models, 2'‐FL was shown to reduce Campylobacter jejuni load and clinical symptoms. Moreover, in vitro adhesion to model cells was strongly reduced by 2'‐FL. 154 , 155 For specific norovirus strains, binding of 2'‐FL and also 3‐FL was shown to lead to reduced adhesion to their blood group antigen ligands in vitro. 156 , 157 Earlier work showed inhibition of norovirus particles by secretor‐positive milk, but not secretor‐negative milk indicating alpha 1,2‐linked fucosylated‐HMOs might be involved. 158 However, HMOs seem not to have been involved. Rather, the alpha 1,2‐linked fucosylated‐glycans on milk mucins and lipase were found to inhibit norovirus adhesion. 158 Alpha 1,2‐linked fucosylated‐HMOs were associated with reduced diarrhea and morbidity in independent cohorts in Africa. 107 , 159 Additionally, in another small study, the HMO LNFP II that is FUT3 dependent was associated with reduced gastrointestinal and respiratory illnesses in early infancy. 160
From a molecular point of view, fragilising, blocking and deviating pathogens from adhering to their cognate cell surface ligands comprise plausible mechanisms of action for HMOs. These add a line of innate protective functions to the colonisation resistance brought about by an appropriately developing gut microbiome. In relation to respiratory pathogens, an additional effect is expected through gut microbial metabolites as elegantly demonstrated in basic research models that show protection from respiratory viral infections through immune active microbial metabolites. 86 , 161 Together, an intricate interplay between different breast milk glycan structures, including free HMOs, the gut microbiome and specific pathogens, is expected.
Not only infants, but also adults, who are genetic non‐secretors, are often at a lower risk of diarrheal and respiratory infections caused by specific pathogens. 162 , 163 This is an important confounding factor especially when investigating associations between HMOs that strongly depend on maternal secretor status and infectious illnesses in breastfed infants. Although breastfeeding reduces the risk of diarrhea, the exact HMO composition of breast milk as determined by maternal secretor status might not have a large impact on this protective effect. This indicates that a certain functional redundancy may exist within the diversity of HMOs in mother's milk. Mechanistically, this can be exemplified by the similar effects of 2'‐FL and 3‐FL on B. longum subsp infantis, 164 and of 2'‐FL and 6'‐SL on models of NEC and allergic disease. 45 , 142
To date, only few individual manufactured HMOs, 2'‐FL alone or in combination with LNnT, have been tested in randomised controlled intervention trials in formula fed infants 109 , 110 , 111 , 112 , 113 , 114 and children. 164 All trials in infants investigated growth as the primary safety objective and also investigated infectious morbidity, not only as part of the reporting of adverse events, but also with an a priori hypothesis to investigate whether HMOs reduce infectious illnesses. For 2'‐FL alone at 0.25 g L–1, Storm et al. 111 reported a trend for a lower number of the overall infection related adverse events compared to controls. On the other hand, Marriage et al. 110 , 147 observed a higher incidence rate for reported adverse events related to overall infections in the control group of infants (GOS alone) and infants fed formula with 2'‐FL at 1 g L–1 with GOS compared to infants fed the lower dose of 2'‐FL at 0.2 g L–1 with GOS. In a cohort of healthy children aged 1–2.5 years, 2'‐FL at 3 g L–1 provided in two portions of 200 mL per day over a 6‐month period did not change the incidence of upper respiratory tract, nor gastrointestinal tract infections. 165 Rather, and somewhat surprisingly, a slight increase in duration of upper respiratory tract infections was observed. Infants fed formula with the two HMOs 2'‐FL and LNnT experienced significantly fewer reported lower respiratory tract infections up to 1 year of age. 109 In the same trial, infants fed the formula with 2'‐FL and LNnT also required significantly less antipyretics and prescribed antibiotics compared to control formula fed infants. Although these observations were based on an a priori hypothesis, they were part of the analysis of exploratory outcome measures in the trial. The observation of a lower risk of respiratory infections and lower need for antibiotics with 2'‐FL and LNnT supplementation is further supported by recent studies linking a microbiome community structure highly dominated by Bifidobacterium species at 3 months of age with a reduced requirement for antibiotics. 47 Additionally, metabolites such as acetate, derived from HMO stimulated Bifidobacterium metabolic activity, could also contribute to a lower risk of respiratory tract infections. 166 For example, in basic research models, acetate was shown to be protective against gastrointestinal pathogenic Escherichia coli 85 and respiratory syncytial virus through a type of interferon mediated pathway. 86 Similarly, in cow milk protein allergic infants fed extensively hydrolysed formula feeding with the same 2'‐FL and LNnT, there was a trend for lower respiratory tract infections and antibiotic use in supplemented vs. control fed infants although this difference did not reach statistical significance because of the limited sample size. 113
HMOs and cognitive development
The brain is highly sialylated and many developmental and functional processes in the brain depend on sialic acid bound to proteins and glycolipids (i.e., gangliosides). As a result of the high sialic acid demand during early development and the high sialic acid content in breast milk, primarily in the form of HMOs, sialic acid is considered an important conditional nutrient in early life. 167 , 168 , 169 Studies in animal models suggest that most dietary sialic acid is largely catabolised to pyruvate and GlcNAc and is not used directly as sialic acid, 26 , 170 , 171 whereas some is reused directly through a salvage pathway as shown by the uptake and incorporation of the non‐human sialic acid NeuGc. 172 Although the mechanisms are not fully established, these studies have led to the hypothesis that sialylated HMOs play a role in brain and cognitive development.
Today, numerous basic research models indeed support that sialyllactoses affect brain and cognitive development. In preterm pigs, a bovine milk preparation with sialyllactoses improved cognitive performance and upregulated hippocampal genes of sialic acid metabolism, ganglioside biosynthesis and myelination, whereas the concentration of hippocampal sialic acid was not affected. 173 In neonatal pig studies, sialyllactose supplementation increased ganglioside bound sialic acid in the corpus callosum and cerebellum, 174 affected sialic acid profiles in additional brain regions such as the prefrontal cortex as well as the hippocampus, 175 and affected metabolic signatures including neurotransmitters. 176 However, such changes did not translate into improved recognition memory or sleeping patterns. 177 In different rodent models, 3'‐SL and 6'‐SL were found to improve learning and memory using different testing paradigms and models. 28 , 178 , 179 Using a cross‐feeding model with dams genetically unable to synthesise 6'‐SL in their milks, wild‐type animals fed 6'‐SL deficient milk showed long lasting deficits in prefrontal cortex mediated executive functions. 28 Analysis of early life brain, plasma and gut microbiota hinted to affected serotoninergic pathways, linking the gut and brain, as well as neurochemical and neuroanatomical adjustments in the brain. In another rodent experiment, using social disruption as a stressor, both 3'‐SL and 6'‐SL feeding prevented stress‐induced dysbiosis and anxiety such as behavior indicating that, at least in part, these HMOs may act via the microbiome involving the gut–brain axis pathways. 180
Several studies have investigated the role of sialyllactoses in breastfed infants. In a cohort of 99 infant–mother dyads, a higher breast milk 3'‐SL concentration was associated with higher scores for expressive and receptive language development. However, this association was seen only in infants who were fed breast milk that contained the HMO A‐tetrasaccharide (only produced by mothers with blood group A) but not in infants fed breast milk without this HMO. 181 In Malawian breastfed infants receiving FUT2 positive milk (n = 485), total sialylated HMOs, especially the concentration of total fucosylated HMOs, was positively associated with language development, whereas the non‐sialylated and non‐fucosylated HMOs structures showed an inverse relation. 96 In a pilot study, breast milk 6'‐SL amounts at 1 month of age correlated positively with the composite cognitive score at 18 months of age (n = 76). 182
Similar to sialyllactoses, 2'‐FL is also reported in numerous basic research models to help improve cognitive development. The first studies found that brain exposure to 2'‐FL, but not fucose or 3‐FL improved hippocampal long‐term potentiation. 183 , 184 Interestingly, direct effects of 2'‐FL and 3‐FL on enteric neuronal functions were also postulated from findings with an ex vivo model on gut contractility. Both 2'‐FL and 3‐FL, but not sialyllactoses, LNnT or GOS, had an immediate effect on colonic motor contractions, indicating that this effect is probably not driven by the gut microbiome. 185 Furthermore, additional ex vivo tests with animals that were subjected to restraint stress showed that 2'‐FL alleviated stress‐induced gut dysmotility. 186 Several recent studies tested 2'‐FL feeding in rodent models to assess cognitive abilities and its possible mode of action. 187 , 188 The long lasting and improved learning and memory outcomes with 2'‐FL feeding were related to effects on hippocampal memory related gene expression and long‐term potentiation. In additional studies, the effect of 2'‐FL was shown to be mediated via the vagus nerve 188 and not by direct uptake of 2'‐FL or derived fucose into the brain. 189 , 190 Rather, for any uptake, microbial cleavage of 2'‐FL is necessary. There are relatively few data in humans but, in one study of breastfed infants, greater 2'‐FL intake at 1 month of age predicted better infant cognitive development at 24 months of age. 191
Although infant cognitive development is affected by multiple environmental and nutrition factors, emerging data raise the possibility that HMOs make an important contribution and could partly help to explain some of the cognitive advantages of breastfeeding compared to formula feeding. Although the exact nature and mechanisms are not fully established, gut–brain communication processes involving gut microbiome metabolites are likely to be important. Well controlled and designed intervention trials with specific HMOs will be required to establish a causal link in this emerging field.
CONCLUSIONS AND OUTLOOK
From a structural perspective, HMOs represent numerous structural features that are generally present on mucosal and cell surface glycans and play important modulatory roles in cell–cell and host–microbe interactions. From a physiological perspective, HMOs show many structure function‐specific activities, only observed with specific HMO species and not generally seen with unrelated glycans that are often used as prebiotics. However, there is redundancy of some functions between different HMO species, possibly acting as safeguard mechanisms for some of their important roles.
Although human milk is particularly rich in amounts and structural diversity of HMOs, some oligosaccharides are common across the animal milks. For example, 3'‐SL appears to be quite universally present in animal and human milks, suggesting universal and important functions across mammals.
Recent progress in manufacturing of individual HMOs has triggered a revival of research and great interest in the application of HMOs as seen by the exponential increase in published studies. Together, the cumulative evidence indicates that HMOs are a meaningful and important component of human milk, the optimal nutrition for early life. Increasing evidence also suggests that specific HMOs help establish immune competence, both local and systemically, partly through their effect on the metabolite activity of specific microbes such as specific Bifidobacterium species. HMOs may also participate in a gut–brain connection, thereby modulating brain and cognitive development. As expected from many biological processes, HMOs work in concert with other bioactive components and additionally act via different mechanisms that converge to specific functions.
Although we have started to accumulate clear evidence on the benefits of specific individual HMOs and blends thereof from randomised controlled trials, observational studies in breastfed infants have added to our knowledge and evidence supporting the importance of HMOs in early life. However, why human milk contains such diverse HMOs and what are the key drivers besides genetic polymorphism and time of lactation that explain the high variability in amounts of some HMOs remain key questions. To what extent the microbes and milk composition provided by mothers to their infants is personalised also remains an intriguing question for future research.
CONFLICT OF INTERESTS
Norbert Sprenger, Hanne L. P. Tytgat, Aristea Binia and Sean Austin are employees of Société des Produits Nestlé, Switzerland. Atul Singhal has no conflict of interst regarding this work, but previously received research funding from Nestlé and Abbott Plc, as well as honoraria to give lectures and attend advisory boards for Nestlé Nutrition Institute, Danone, Wyeth Nutrition, Reckitt, Phillips, Abbott Nutrition and several academic institutions.
ETHICS STATEMENT
Not applicable.
AUTHOR CONTRIBUTIONS
Norbert Sprenger conceptualised and drafted the manuscript. Atul Singhal, Sean Austin and Atul Singhal completed the writing of the manuscript. Norbert Sprenger and Hanne L. P. Tytgat prepared the figures. Norbert Sprenger and Sean Austin prepared the tables. All authors reviewed and approved the final version of the manuscript submitted for publication.
TRANSPARENCY DECLARATION
The peer review history for this article is available at https://publons.com/publon/10.1111/jhn.12990.
ACKNOWLEDGEMENTS
We thank Dr Elizabeth Forbes Blom and Dr Eline van der Beek for their critical review and support.
Biographies
Norbert Sprenger is a Senior Research Scientist leading a team of scientists investigating microbiome–host interactions at the Nestlé Institute of Health Sciences. His main research interests are focused on understanding how dietary glycans and early life nutrition affect development and health through their effects on microbe–host interactions. After earning a PhD at the University of Basel (CH), he continued academic research related to functional complex glycans at the Universities of Zurich (CH) and Stanford (USA). He has dedicated the last 20 years to applied industrial research at Nestlé Research (Switzerland), which led to major insight and innovations related to milk oligosaccharides for infant nutrition.
Hanne L. P. Tytgat is a Microbial Glycobiologist studying microbiome–host interactions at the Nestlé Institute of Health Sciences. Her research mainly focuses on understanding how dietary glycans affect development and health in early life for nutritional applications. Hanne obtained her PhD in Bioscience Engineering from University of Leuven (BE). She continued academic research as a Marie Sklodowska‐Curie postdoctoral fellow at ETH Zurich (CH) and as a senior postdoctoral researcher at University of Wageningen (NL). Her work centered around the elucidation of the role of glycans in microbiome–host interactions. In her current role at Nestlé Research, Hanne leverages her expertise to understand how dietary glycans affect development and health for nutritional applications.
Aristea Binia is currently a Group Leader and Senior Scientist at the Nestlé Institute of Health Sciences focusing on early programming, genetics and human milk research. Aristea holds an MSc and PhD in Human Genetics and Genomics from Imperial College London, UK (2009). She joined Nestlé Research in 2012 as a Research Scientist and, subsequently, she has conducted research focusing on nutrigenetics and complex disease, early life nutrition and human milk oligosaccharides (HMO), linking genetics and glycobiology. In her current role, she manages resources, projects and leading research targeted for infant and maternal nutrition with the outlook of lifelong cardiometabolic health.
Sean Austin is a Research Scientist leading the Carbohydrates group in the Nestle Institute of Food Safety and Analytical Sciences. His research interests include the development of analytical tools for the determination of carbohydrates in food and biological samples, and the impact of carbohydrates on health. He obtained his BSc in Applied Chemistry from the Robert Gordon University in Aberdeen (UK) and his PhD on the Structural Characterization of Wheat Cell Wall Polysaccharides from the University of Nottingham in collaboration with the Rowett Research Institute in Aberdeen. Milk oligosaccharides have been a major research topic in his laboratory for more than 10 years.
Atul Singhal is Professor of Paediatric Nutrition at University College London, Institute of Child Health, and Honorary Consultant Paediatrician at Great Ormond Street Hospital. He graduated in Medicine from the Royal Free Hospital, London in 1986 and has been a Consultant in Paediatrics since 1998. Previously, he was the Director and Deputy Director of the Childhood Nutrition Research Centre, UCL Institute of Child Health. He has broad interests in paediatric nutrition, but his current research focuses on the influence of early nutrition for long‐term health, the effects of nutritional interventions to reduce long‐term cardiovascular risk and nutritional interventions for obesity.
Sprenger N, Tytgat HLP, Binia A, Austin S, Singhal A. Biology of human milk oligosaccharides: From basic science to clinical evidence. J Hum Nutr Diet. 2022;35:280–299. 10.1111/jhn.12990
DATA AVAILABILITY
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Fewtrell M, Bronsky J, Campoy C, Domellöf M, Embleton N, Fidler Mis N, et al. Complementary feeding: a position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) committee on nutrition. J Pediatr Gastroenterol Nutr. 2017;64:119–32. [DOI] [PubMed] [Google Scholar]
- 2.WHO. https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding
- 3. Urashima T, Hirabayashi J, Sato S, Kobata A. Human milk oligosaccharides as essential tools for basic and application studies on galectins. Trends Glycosci Glycotechnol. 2018;30:SE51–65. [Google Scholar]
- 4. Urashima TKT, Fukuda K, Hirabayashi J. Human milk oligosaccharides and innate immunity. In: Joseph B, Jr., ed. Comprehensive glycoscience. 2nd ed, vol. 5. Elsevier; 2021:389–439.
- 5. Kunz C, Meyer C, Collado MC, Geiger L, García‐Mantrana I, Bertua‐Ríos B, et al. Influence of gestational age, secretor, and Lewis blood group status on the oligosaccharide content of human milk. J Pediatr Gastroenterol Nutr. 2017;64:789–98. [DOI] [PubMed] [Google Scholar]
- 6. Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699–722. [DOI] [PubMed] [Google Scholar]
- 7. Samuel TM, Binia A, de Castro CA, Thakkar SK, Billeaud C, Agosti M, et al. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci Rep. 2019;9:11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kunz C. Historical aspects of human milk oligosaccharides. Adv Nutr. 2012;3:430S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–90. [DOI] [PubMed] [Google Scholar]
- 10. Altobelli E, Angeletti PM, Verrotti A, Petrocelli R. The impact of human milk on necrotizing enterocolitis: a systematic review and meta‐analysis. Nutrients. 2020;12:1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El Manouni El Hassani S, Berkhout DJC, Niemarkt HJ, Mann S, de Boode WP, Cossey V, et al. Risk factors for late‐onset sepsis in preterm infants: a multicenter case‐control study. Neonatology. 2019;116:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poets CF, Lorenz L. Prevention of bronchopulmonary dysplasia in extremely low gestational age neonates: current evidence. Arch Dis Child Fetal Neonatal Ed. 2018;103:F285–91. [DOI] [PubMed] [Google Scholar]
- 13. Varki A. Nothing in glycobiology makes sense, except in the light of evolution. Cell. 2006;126:841–5. [DOI] [PubMed] [Google Scholar]
- 14. Walsh C, Lane JA, Van Sinderen D, Hickey RM. From lab bench to formulated ingredient: characterization, production, and commercialization of human milk oligosaccharides. J Funct Foods. 2020;72:104074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dabrowski U, Egge H, Dabrowski J. Proton‐nuclear magnetic resonance study of peracetylated derivatives of ten oligosaccharides isolated from human milk. Arch Biochem Biophys. 1983;224:254–60. [DOI] [PubMed] [Google Scholar]
- 16. Sarkozy D, Borza B, Domokos A, Varadi E, Szigeti M, Meszaros‐Matwiejuk A, et al. Ultrafast high‐resolution analysis of human milk oligosaccharides by multicapillary gel electrophoresis. Food Chem. 2021;341:128200. [DOI] [PubMed] [Google Scholar]
- 17. Urashima T, Asakuma S, Kitaoka M et al. Indigenous oligosaccharides in milk. In: Fuquay JW, Fox PF and McSweeney PLH, editors. Encylopedia of dairy sciences, 2nd ed., 3. San Diego: Academic Press; 2011. p. 241– 73. [Google Scholar]
- 18. Austin S, De Castro CA, Sprenger N, Binia A, Affolter M, Garcia‐Rodenas CL, et al. Human milk oligosaccharides in the milk of mothers delivering term versus preterm infants. Nutrients. 2019;11:1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Donald AS, Feeney J. Separation of human milk oligosaccharides by recycling chromatography. First isolation of lacto‐N‐neo‐difucohexaose II and 3'‐galactosyllactose from this source. Carbohydr Res. 1988;178:79–91. [DOI] [PubMed] [Google Scholar]
- 20. Martínez‐Villaluenga C, Cardelle‐Cobas A, Corzo N, Olano A. Study of galactooligosaccharide composition in commercial fermented milks. J Food Comp Anal. 2008;21:540–4. [Google Scholar]
- 21. van Leeuwen SS, Stoutjesdijk E, Ten Kate GA, Schaafsma A, Dijck‐Brouwer J, Muskiet F, et al. Regional variations in human milk oligosaccharides in Vietnam suggest FucTx activity besides FucT2 and FucT3. Sci Rep. 2018;8:16790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Urashima T, Saito T, Nakamura T, Messer M. Oligosaccharides of milk and colostrum in non‐human mammals. Glycoconj J. 2001;18:357–71. [DOI] [PubMed] [Google Scholar]
- 23. Urashima T, Inamori H, Fukuda K, Saito T, Messer M, Oftedal OT. 4‐O‐Acetyl‐sialic acid (Neu4,5Ac2) in acidic milk oligosaccharides of the platypus (Ornithorhynchus anatinus) and its evolutionary significance. Glycobiology. 2015;25:683–97. [DOI] [PubMed] [Google Scholar]
- 24. Jenkins GA, Bradbury JH, Messer M, Trifonoff E. Determination of the structures of fucosyl‐lactose and difucosyl‐lactose from the milk of monotremes, using 13C‐n.m.r. spectroscopy. Carbohydr Res. 1984;126:157–61. [DOI] [PubMed] [Google Scholar]
- 25. Fuhrer A, Sprenger N, Kurakevich E, Borsig L, Chassard C, Hennet T. Milk sialyllactose influences colitis in mice through selective intestinal bacterial colonization. J Exp Med. 2010;207:2843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duncan PI, Raymond F, Fuerholz A, Sprenger N. Sialic acid utilisation and synthesis in the neonatal rat revisited. PLoS One. 2009;4:8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harris JE, Pinckard KM, Wright KR, Baer LA, Arts PJ, Abay E, et al. Exercise‐induced 3'‐sialyllactose in breast milk is a critical mediator to improve metabolic health and cardiac function in mouse offspring. Nat Metab. 2020;2:678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hauser J, Pisa E, Arias Vásquez A, Tomasi F, Traversa A, Chiodi V, et al. Sialylated human milk oligosaccharides program cognitive development through a non‐genomic transmission mode. Mol Psychiatry. 2021;26:2854–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Urashima T, Taufik E, Fukuda K, Asakuma S. Recent advances in studies on milk oligosaccharides of cows and other domestic farm animals. Biosci Biotechnol Biochem. 2013;77:455–66. [DOI] [PubMed] [Google Scholar]
- 30. Nakamura T, Kawase H, Kimura K, Watanabe Y, Ohtani M, Arai I, et al. Concentrations of sialyloligosaccharides in bovine colostrum and milk during the prepartum and early lactation. J Dairy Sci. 2003;86:1315–20. [DOI] [PubMed] [Google Scholar]
- 31. Kelly V, Davis S, Berry S, Melis J, Spelman R, Snell R, et al. Rapid, quantitative analysis of 3'‐ and 6'‐sialyllactose in milk by flow‐injection analysis‐mass spectrometry: screening of milks for naturally elevated sialyllactose concentration. J Dairy Sci. 2013;96:7684–91. [DOI] [PubMed] [Google Scholar]
- 32. Urashima T, Fukuda K, Messer M. Evolution of milk oligosaccharides and lactose: a hypothesis. Animal. 2012;6:369–74. [DOI] [PubMed] [Google Scholar]
- 33. Urashima T, Asakuma S, Leo F, Fukuda K, Messer M, Oftedal OT. The predominance of type I oligosaccharides is a feature specific to human breast milk. Adv Nutr. 2012;3:473S–82S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH, et al. A strategy for annotating the human milk glycome. J Agric Food Chem. 2006;54:7471–80. [DOI] [PubMed] [Google Scholar]
- 35. Asakuma S, Akahori M, Kimura K, Watanabe Y, Nakamura T, Tsunemi M, et al. Sialyl oligosaccharides of human colostrum: changes in concentration during the first three days of lactation. Biosci Biotechnol Biochem. 2007;71:1447–51. [DOI] [PubMed] [Google Scholar]
- 36. Spichtig V, Michaud J, Austin S. Determination of sialic acids in milks and milk‐based products. Anal Biochem. 2010;405:28–40. [DOI] [PubMed] [Google Scholar]
- 37. Lefebvre G, Shevlyakova M, Charpagne A, Marquis J, Vogel M, Kirsten T, et al. Time of lactation and maternal fucosyltransferase genetic polymorphisms determine the variability in human milk oligosaccharides. Front Nutr. 2020;7:574459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Plows JF, Berger PK, Jones RB, Alderete TL, Yonemitsu C, Najera JA, et al. Longitudinal changes in human milk oligosaccharides (HMOs) over the course of 24 months of lactation. J Nutr. 2021;151:876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferrer‐Admetlla A, Sikora M, Laayouni H, Esteve A, Roubinet F, Blancher A, et al. A natural history of FUT2 polymorphism in humans. Mol Biol Evol. 2009;26:1993–2003. [DOI] [PubMed] [Google Scholar]
- 40. Castanys‐Munoz E, Martin MJ, Prieto PA. 2'‐Fucosyllactose: an abundant, genetically determined soluble glycan present in human milk. Nutr Rev. 2013;71:773–89. [DOI] [PubMed] [Google Scholar]
- 41. Wiegering V, Kaiser J, Tappe D, Weißbrich B, Morbach H, Girschick HJ. Gastroenteritis in childhood: a retrospective study of 650 hospitalized pediatric patients. Int J Infect Dis. 2011;15:e401–7. [DOI] [PubMed] [Google Scholar]
- 42. Williams JE, McGuire MK, Meehan CL, McGuire MA, Brooker SL, Kamau‐Mbuthia EW, et al. Key genetic variants associated with variation of milk oligosaccharides from diverse human populations. Genomics. 2021;113:1867–75. [DOI] [PubMed] [Google Scholar]
- 43. McGuire MK, Meehan CL, McGuire MA, Williams JE, Foster J, Sellen DW, et al. What's normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am J Clin Nutr. 2017;105:1086–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Borewicz K, Gu F, Saccenti E, Hechler C, Beijers R, de Weerth C, et al. The association between breastmilk oligosaccharides and faecal microbiota in healthy breastfed infants at two, six, and twelve weeks of age. Sci Rep. 2020;10:4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sodhi CP, Wipf P, Yamaguchi Y, Fulton WB, Kovler M, Niño DF, et al. The human milk oligosaccharides 2'‐Fucosyllactose and 6'‐Sialyllactose protect against the development of necrotizing enterocolitis by inhibiting toll‐like receptor 4 signaling. Pediatr Res. 2021;89:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Noll AJ, Yu Y, Lasanajak Y, Duska‐McEwen G, Buck RH, Smith DF, et al. Human DC‐SIGN binds specific human milk glycans. Biochem J. 2016;473:1343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Berger B, Porta N, Foata F, Grathwohl D, Delley M, Moine D, et al. Linking human milk oligosaccharides, infant fecal community types, and later risk to require antibiotics. mBio. 2020;11:e03196‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zivkovic AM, Barile D. Bovine milk as a source of functional oligosaccharides for improving human health. Adv Nutr. 2011;2:284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Coulet M, Phothirath P, Allais L, Schilter B. Pre‐clinical safety evaluation of the synthetic human milk, nature‐identical, oligosaccharide 2'‐O‐Fucosyllactose (2'FL). Regul Toxicol Pharmacol. 2014;68:59–69. [DOI] [PubMed] [Google Scholar]
- 50. Coulet M, Phothirath P, Constable A, Marsden E, Schilter B. Pre‐clinical safety assessment of the synthetic human milk, nature‐identical, oligosaccharide Lacto‐N‐neotetraose (LNnT). Food Chem Toxicol. 2013;62:528–37. [DOI] [PubMed] [Google Scholar]
- 51. Baumann‐Dudenhoeffer AM, D'Souza AW, Tarr PI, Warner BB, Dantas G. Infant diet and maternal gestational weight gain predict early metabolic maturation of gut microbiomes. Nat Med. 2018;24:1822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ho NT, Li F, Lee‐Sarwar KA, Tun HM, Brown BP, Pannaraj PS, et al. Meta‐analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat Commun. 2018;9:4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stewart CJ, Ajami NJ, O'Brien JL, Hutchinson DS, Smith DP, Wong MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562:583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Korpela K, Costea P, Coelho LP, Kandels‐Lewis S, Willemsen G, Boomsma DI, et al. Selective maternal seeding and environment shape the human gut microbiome. Genome Res. 2018;28:561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Turroni F, Milani C, Duranti S, Ferrario C, Lugli GA, Mancabelli L, et al. Bifidobacteria and the infant gut: an example of co‐evolution and natural selection. Cell Mol Life Sci. 2018;75:103–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kumar H, Collado MC, Wopereis H, Salminen S, Knol J, Roeselers G. The Bifidogenic effect revisited ‐ ecology and health perspectives of bifidobacterial colonization in early life. Microorganisms. 2020;8:1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wong CB, Sugahara H, Odamaki T, Xiao JZ. Different physiological properties of human‐residential and non‐human‐residential bifidobacteria in human health. Benef Microbes. 2018;9:111–22. [DOI] [PubMed] [Google Scholar]
- 58. Den Tandt WR, Adriaenssens K, Scharpe S. Characteristics of human intestinal acid sialidase. Enzyme. 1987;37:155–8. [DOI] [PubMed] [Google Scholar]
- 59. Albrecht S, Schols HA, van den Heuvel EG, Voragen AG, Gruppen H. Occurrence of oligosaccharides in feces of breast‐fed babies in their first six months of life and the corresponding breast milk. Carbohydr Res. 2011;346:2540–50. [DOI] [PubMed] [Google Scholar]
- 60. Albrecht S, Schols HA, van Zoeren D, van Lingen RA, Groot Jebbink LJM, van den Heuvel EGHM, et al. Oligosaccharides in feces of breast‐ and formula‐fed babies. Carbohydr Res. 2011;346:2173–81. [DOI] [PubMed] [Google Scholar]
- 61. Borewicz K, Gu F, Saccenti E, Arts I, Penders J, Thijs C, et al. Correlating infant faecal microbiota composition and human milk oligosaccharide consumption by microbiota of one‐month old breastfed infants. Mol Nutr Food Res. 2019;e1801214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Katayama T. Host‐derived glycans serve as selected nutrients for the gut microbe: human milk oligosaccharides and bifidobacteria. Biosci Biotechnol Biochem. 2016;80:621–32. [DOI] [PubMed] [Google Scholar]
- 63. Sakanaka M, Hansen ME, Gotoh A, Katoh T, Yoshida K, Odamaki T, et al. Evolutionary adaptation in fucosyllactose uptake systems supports bifidobacteria‐infant symbiosis. Sci Adv. 2019;5:eaaw7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wong CB, Odamaki T, Xiao JZ. Insights into the reason of Human‐Residential Bifidobacteria (HRB) being the natural inhabitants of the human gut and their potential health‐promoting benefits. FEMS Microbiol Rev. 2020;44:369–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Milani C, Turroni F, Duranti S, Lugli GA, Mancabelli L, Ferrario C, et al. Genomics of the genus Bifidobacterium reveals species‐specific adaptation to the glycan‐rich gut environment. Appl Environ Microbiol. 2016;82:980–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lawson MAE, O'Neill IJ, Kujawska M, Gowrinadh Javvadi S, Wijeyesekera A, Flegg Z, et al. Breast milk‐derived human milk oligosaccharides promote Bifidobacterium interactions within a single ecosystem. ISME J. 2020;14:635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Matsuki T, Yahagi K, Mori H, Matsumoto H, Hara T, Tajima S, et al. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat Commun. 2016;7:11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kujawska M, La Rosa SL, Roger LC, Pope PB, Hoyles L, McCartney AL, et al. Succession of Bifidobacterium longum strains in response to a changing early life nutritional environment reveals dietary substrate adaptations. iScience. 2020;23:101368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Thongaram T, Hoeflinger JL, Chow J, Miller MJ. Human milk oligosaccharide consumption by probiotic and human‐associated bifidobacteria and lactobacilli. J Dairy Sci. 2017;100:7825–33. [DOI] [PubMed] [Google Scholar]
- 70. Salli K, Hirvonen J, Siitonen J, Ahonen I, Anglenius H, Maukonen J. Selective utilization of the human milk oligosaccharides 2'‐fucosyllactose, 3‐fucosyllactose, and difucosyllactose by various probiotic and pathogenic bacteria. J Agric Food Chem. 2021;69:170–82. [DOI] [PubMed] [Google Scholar]
- 71. Thomson P, Medina DA, Garrido D. Human milk oligosaccharides and infant gut bifidobacteria: molecular strategies for their utilization. Food Microbiol. 2018;75:37–46. [DOI] [PubMed] [Google Scholar]
- 72. Zabel BE, Gerdes S, Evans KC, Nedveck D, Singles SK, Volk B, et al. Strain‐specific strategies of 2'‐fucosyllactose, 3‐fucosyllactose, and difucosyllactose assimilation by Bifidobacterium longum subsp. infantis Bi‐26 and ATCC 15697. Sci Rep. 2020;10:15919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Duar RM, Casaburi G, Mitchell RD, Scofield L, Ortega Ramirez CA, Barile D, et al. Comparative genome analysis of Bifidobacterium longum subsp. infantis strains reveals variation in human milk oligosaccharide utilization genes among commercial probiotics. Nutrients. 2020;12:3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Marcobal A, Sonnenburg JL. Human milk oligosaccharide consumption by intestinal microbiota. Clin Microbiol Infect. 2012;18(Suppl 4):12–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P, et al. Bacteroides in the infant gut consume milk oligosaccharides via mucus‐utilization pathways. Cell Host Microbe. 2011;10:507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hoeflinger JL, Davis SR, Chow J, Miller MJ. In vitro impact of human milk oligosaccharides on Enterobacteriaceae growth. J Agric Food Chem. 2015;63:3295–302. [DOI] [PubMed] [Google Scholar]
- 77. Lin AE, Autran CA, Szyszka A, Escajadillo T, Huang M, Godula K, et al. Human milk oligosaccharides inhibit growth of Group B Streptococcus . J Biol Chem. 2017;292:11243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ackerman DL, Doster RS, Weitkamp JH, Aronoff DM, Gaddy JA, Townsend SD. Human milk oligosaccharides exhibit antimicrobial and antibiofilm properties against Group B Streptococcus . ACS Infect Dis. 2017;3:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Salli K, Söderling E, Hirvonen J, Gürsoy UK, Ouwehand AC. Influence of 2'‐fucosyllactose and galacto‐oligosaccharides on the growth and adhesion of Streptococcus mutans . Br J Nutr. 2020;124:824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chambers SA, Moore RE, Craft KM, Thomas HC, Das R, Manning SD, et al. A solution to antifolate resistance in Group B Streptococcus: untargeted metabolomics identifies human milk oligosaccharide‐induced perturbations that result in potentiation of trimethoprim. mBio. 2020;1:e00076-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Duranti S, Lugli GA, Milani C, James K, Mancabelli L, Turroni F, et al. Bifidobacterium bifidum and the infant gut microbiota: an intriguing case of microbe‐host co‐evolution. Environ Microbiol. 2019;21:3683–95. [DOI] [PubMed] [Google Scholar]
- 82. Turroni F, Milani C, Duranti S, Mahony J, van Sinderen D, Ventura M. Glycan utilization and cross‐feeding activities by bifidobacteria. Trends Microbiol. 2018;26:339–50. [DOI] [PubMed] [Google Scholar]
- 83. Gotoh A, Katoh T, Sakanaka M, Ling Y, Yamada C, Asakuma S, et al. Sharing of human milk oligosaccharides degradants within bifidobacterial communities in faecal cultures supplemented with Bifidobacterium bifidum . Sci Rep. 2018;8:13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chia LW, Mank M, Blijenberg B, Bongers RS, van Limpt K, Wopereis H, et al. Cross‐feeding between Bifidobacterium infantis and Anaerostipes caccae on lactose and human milk oligosaccharides. Benef Microbes. 2021;12:69–83. [DOI] [PubMed] [Google Scholar]
- 85. Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–7. [DOI] [PubMed] [Google Scholar]
- 86. Antunes KH, Fachi JL, de Paula R, da Silva EF, Pral LP, Dos Santos AÁ, et al. Microbiota‐derived acetate protects against respiratory syncytial virus infection through a GPR43‐type 1 interferon response. Nat Commun. 2019;10:3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Henrick BM, Rodriguez L, Lakshmikanth T, Pou C, Henckel E, Arzoomand A, et al. Bifidobacteria‐mediated immune system imprinting early in life. Cell. 2021;184:3884–98. [DOI] [PubMed] [Google Scholar]
- 88. Laursen MF, Sakanaka M , von Burg N, Mörbe U, Andersen D, Moll JM, et al. Bifidobacterium species associated with breastfeeding produce aromatic amino acids in the infant gut. Nat Microbiol. 2021;6:1367–82. [DOI] [PMC free article] [PubMed]
- 89. Robertson RC, Manges AR, Finlay BB, Prendergast AJ. The Human microbiome and child growth ‐ first 1000 days and beyond. Trends Microbiol. 2019;27:131–47. [DOI] [PubMed] [Google Scholar]
- 90. Grier A, Qiu X, Bandyopadhyay S, Holden‐Wiltse J, Kessler HA, Gill AL, et al. Impact of prematurity and nutrition on the developing gut microbiome and preterm infant growth. Microbiome. 2017;5:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510:417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Charbonneau MR, O'Donnell D, Blanton LV, Totten SM, Davis JC, Barratt MJ, et al. Sialylated milk oligosaccharides promote microbiota‐dependent growth in models of infant undernutrition. Cell. 2016;164:859–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016;351:10.1126/science.aad3311 aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339:548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cowardin CA, Ahern PP, Kung VL, Hibberd MC, Cheng J, Guruge JL, et al. Mechanisms by which sialylated milk oligosaccharides impact bone biology in a gnotobiotic mouse model of infant undernutrition. Proc Natl Acad Sci U S A. 2019;116:11988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jorgensen JM, Young R, Ashorn P, Ashorn U, Chaima D, Davis J, et al. Associations of human milk oligosaccharides and bioactive proteins with infant growth and development among Malawian mother‐infant dyads. Am J Clin Nutr. 2020;113:209 –20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Alderete TL, Autran C, Brekke BE, Knight R, Bode L, Goran MI, et al. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am J Clin Nutr. 2015;102:1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Davis EC, Wang M, Donovan SM. The role of early life nutrition in the establishment of gastrointestinal microbial composition and function. Gut Microbes. 2017;8:143–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sprenger N, Lee LY, De Castro CA, Steenhout P, Thakkar SK. Longitudinal change of selected human milk oligosaccharides and association to infants' growth, an observatory, single center, longitudinal cohort study. PLoS One. 2017;12:e0171814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lagström H, Rautava S, Ollila H, Kaljonen A, Turta O, Mäkelä J, et al. Associations between human milk oligosaccharides and growth in infancy and early childhood. Am J Clin Nutr. 2020;111:769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Larsson MW, Lind MV, Laursen RP, Yonemitsu C, Larnkjær A, Mølgaard C, et al. Human milk oligosaccharide composition is associated with excessive weight gain during exclusive breastfeeding ‐ an explorative study. Front Pediatr. 2019;7:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tonon KM, Miranda A, Abrão A, de Morais MB, Morais TB. Validation and application of a method for the simultaneous absolute quantification of 16 neutral and acidic human milk oligosaccharides by graphitized carbon liquid chromatography ‐ electrospray ionization ‐ mass spectrometry. Food Chem. 2019;274:691–7. [DOI] [PubMed] [Google Scholar]
- 103. Binia A, Lavalle L, Chen C, Austin S, Agosti M, Al‐Jashi I, et al. Human milk oligosaccharides, infant growth, and adiposity over the first 4 months of lactation. Pediatr Res. 2021;90:684–93. [DOI] [PubMed] [Google Scholar]
- 104. Berger PK, Plows JF, Jones RB, Alderete TL, Yonemitsu C, Ryoo JH, et al. Human milk oligosaccharides and Hispanic infant weight gain in the first 6 months. Obesity (Silver Spring). 2020;28:1519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Menzel P, Vogel M, Austin S, Sprenger N, Graf N, Hilbert C, et al. Concentrations of oligosaccharides in human milk and child growth. BMC Pediatrics. 2021;21:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Saben JL, Sims CR, Abraham A, Bode L, Andres A. Human milk oligosaccharide concentrations and infant intakes are associated with maternal overweight and obesity and predict infant growth. Nutrients. 2021;13:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Davis JC, Lewis ZT, Krishnan S, Bernstein RM, Moore SE, Prentice AM, et al. Growth and morbidity of gambian infants are influenced by maternal milk oligosaccharides and infant gut microbiota. Sci Rep. 2017;7:40466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Plows JF, Berger PK, Jones RB, Yonemitsu C, Ryoo JH, Alderete TL, et al. Associations between human milk oligosaccharides (HMOs) and eating behaviour in Hispanic infants at 1 and 6 months of age. Pediatr Obes. 2020;15:e12686. [DOI] [PubMed] [Google Scholar]
- 109. Puccio G, Alliet P, Cajozzo C, Janssens E, Corsello G, Sprenger N, et al. Effects of infant formula with human milk oligosaccharides on growth and morbidity: a randomized multicenter trial. J Pediatr Gastroenterol Nutr. 2017;64:624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Marriage BJ, Buck RH, Goehring KC, Oliver JS, Williams JA. Infants fed a lower calorie formula with 2'FL show growth and 2'FL uptake like breast‐fed infants. J Pediatr Gastroenterol Nutr. 2015;61:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Storm HM, Shepard J, Czerkies LM, Kineman B, Cohen SS, Reichert H, et al. 2'‐Fucosyllactose is well tolerated in a 100% whey, partially hydrolyzed infant formula with Bifidobacterium lactis: a randomized controlled trial. Glob Pediatr Health. 2019;6:2333794X19833995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nowak‐Wegrzyn A, Czerkies L, Reyes K, Collins B, Heine RG. Confirmed hypoallergenicity of a novel whey‐based extensively hydrolyzed infant formula containing two human milk oligosaccharides. Nutrients. 2019;11:1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Vandenplas Y, Zolnowska M, Berni Canani R, Ludman S, Tengelyi Zs, Moreno Álvarez A, et al. Effects of an extensively hydrolyzed formula supplemented with two human milk oligosaccharides on growth, tolerability, safety and infection risk in infants with cow's milk protein allergy: A randomized, multicenter trial Nutrients. 2022; 14:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ramirez‐Farias C, Baggs GE, Marriage BJ. Growth, tolerance, and compliance of infants fed an extensively hydrolyzed infant formula with added 2'‐FL fucosyllactose (2'‐FL) human milk oligosaccharide. Nutrients. 2021;13:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bauer V, Arciszewska M, Tarneva M, Dosev S, Dimitrova S, Nikolova O, et al. Term infant formula supplemented with a unique blend of five human milk oligosaccharides supports age‐appropriate growth, is safe and well‐tolerated: a double‐blind, randomized controlled trial. Pediatric Academic Societies Conference 2021 – abstract. https://virtual2021.pas-meeting.org/. Accessed January 2022.
- 116. Parschat K, Melsaether C, Rasch Jäpelt K, Jennewein S. Clinical evaluation of 16‐week supplementation with 5HMO‐mix in healthy‐term human infants to determine tolerability, safety, and effect on growth. Nutrients. 2021;13:2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ganal‐Vonarburg SC, Hornef MW, Macpherson AJ. Microbial‐host molecular exchange and its functional consequences in early mammalian life. Science. 2020;368:604–7. [DOI] [PubMed] [Google Scholar]
- 118. Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawöger R, Kiechl‐Kohlendorfer U, et al. An exclusively human milk‐based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk‐based products. J Pediatr. 2010;156:562–7. [DOI] [PubMed] [Google Scholar]
- 119. Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl‐Kohlendorfer U, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013;163:1592–5. [DOI] [PubMed] [Google Scholar]
- 120. Lapidaire W, Lucas A, Clayden JD, Clark C, Fewtrell MS. Human milk feeding and cognitive outcome in preterm infants: the role of infection and NEC reduction. Pediatr Res. 2021; June 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gregory KE, Samuel BS, Houghteling P, Shan G, Ausubel FM, Sadreyev RI, et al. Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome. 2016;4:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jantscher‐Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, et al. The human milk oligosaccharide disialyllacto‐N‐tetraose prevents necrotising enterocolitis in neonatal rats. Gut. 2012;61:1417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Good M, Sodhi CP, Yamaguchi Y, Jia H, Lu P, Fulton WB, et al. The human milk oligosaccharide 2'‐fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br J Nutr. 2016;116:1175–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Autran CA, Schoterman MH, Jantscher‐Krenn E, Kamerling JP, Bode L. Sialylated galacto‐oligosaccharides and 2'‐fucosyllactose reduce necrotising enterocolitis in neonatal rats. Br J Nutr. 2016;116:294–9. [DOI] [PubMed] [Google Scholar]
- 125. Zhu K, Amin MA, Zha Y, Harlow LA, Koch AE. Mechanism by which H‐2g, a glucose analog of blood group H antigen, mediates angiogenesis. Blood. 2005;105:2343–9. [DOI] [PubMed] [Google Scholar]
- 126. Rasmussen SO, Martin L, Østergaard MV, Rudloff S, Roggenbuck M, Nguyen DN, et al. Human milk oligosaccharide effects on intestinal function and inflammation after preterm birth in pigs. J Nutr Biochem. 2017;40:141–54. [DOI] [PubMed] [Google Scholar]
- 127. Autran CA, Kellman BP, Kim JH, Asztalos E, Blood AB, Spence E, et al. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut. 2018;67:1064–70. [DOI] [PubMed] [Google Scholar]
- 128. Masi AC, Embleton ND, Lamb CA, et al Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut. 2020; 70:2273–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wejryd E, Martí M, Marchini G, Werme A, Jonsson B, Landberg E, et al. Low diversity of human milk oligosaccharides is associated with necrotising Enterocolitis in extremely low birth weight infants. Nutrients. 2018;10:1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lodge CJ, Tan DJ, Lau MX, Dai X, Tham R, Lowe AJ, et al. Breastfeeding and asthma and allergies: a systematic review and meta‐analysis. Acta Paediatr. 2015;104:38–53. [DOI] [PubMed] [Google Scholar]
- 131. Oddy WH. Breastfeeding, childhood asthma, and allergic disease. Ann Nutr Metab. 2017;70(Suppl 2):26–36. [DOI] [PubMed] [Google Scholar]
- 132. Jarvinen KM, Martin H, Oyoshi MK. Immunomodulatory effects of breast milk on food allergy. Ann Allergy Asthma Immunol. 2019;123:133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Aitoro R, Paparo L, Amoroso A, Di Costanzo M, Cosenza L, Granata V, et al. Gut microbiota as a target for preventive and therapeutic intervention against food allergy. Nutrients. 2017;9:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tanaka M, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int. 2017;66:515–22. [DOI] [PubMed] [Google Scholar]
- 135. Metzler S, Frei R, Schmaußer‐Hechfellner E, von Mutius E, Pekkanen J, Karvonen AM, et al. Association between antibiotic treatment during pregnancy and infancy and the development of allergic diseases. Pediatr Allergy Immunol. 2019;30:423–33. [DOI] [PubMed] [Google Scholar]
- 136. Sprenger N, Odenwald H, Kukkonen AK, Kuitunen M, Savilahti E, Kunz C. FUT2‐dependent breast milk oligosaccharides and allergy at 2 and 5 years of age in infants with high hereditary allergy risk. Eur J Nutr. 2017;56:1293–301. [DOI] [PubMed] [Google Scholar]
- 137. Korpela K, Salonen A, Hickman B, Kunz C, Sprenger N, Kukkonen K, et al. Fucosylated oligosaccharides in mother's milk alleviate the effects of caesarean birth on infant gut microbiota. Sci Rep. 2018;8:13757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ismail IH, Boyle RJ, Licciardi PV, Oppedisano F, Lahtinen S, Robins‐Browne RM, et al. Early gut colonization by Bifidobacterium breve and B. catenulatum differentially modulates eczema risk in children at high risk of developing allergic disease. Pediatr Allergy Immunol. 2016;27:838–46. [DOI] [PubMed] [Google Scholar]
- 139. Zimmermann P, Messina N, Mohn WW, Finlay BB, Curtis N. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: a systematic review. J Allergy Clin Immunol. 2019;143:467–85. [DOI] [PubMed] [Google Scholar]
- 140. Sjögren YM, Duchén K, Lindh F, Björkstén B, Sverremark‐Ekström E. Neutral oligosaccharides in colostrum in relation to maternal allergy and allergy development in children up to 18 months of age. Pediatr Allergy Immunol. 2007;18:20–6. [DOI] [PubMed] [Google Scholar]
- 141. Seppo AE, Autran CA, Bode L, Järvinen KM. Human milk oligosaccharides and development of cow's milk allergy in infants. J Allergy Clin Immunol. 2016;139:708–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Castillo‐Courtade L, Han S, Lee S, Mian FM, Buck R, Forsythe P. Attenuation of food allergy symptoms following treatment with human milk oligosaccharides in a mouse model. Allergy. 2015;70:1091–102. [DOI] [PubMed] [Google Scholar]
- 143. Velupillai P, Harn DA. Oligosaccharide‐specific induction of interleukin 10 production by B220+ cells from schistosome‐infected mice: a mechanism for regulation of CD4+ T‐cell subsets. Proc Natl Acad Sci U S A. 1994;91:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Atochina O, Da'dara AA, Walker M, Harn DA. The immunomodulatory glycan LNFPIII initiates alternative activation of murine macrophages in vivo. Immunology. 2008;125:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Miliku K, Robertson B, Sharma AK, Subbarao P, Becker AB, Mandhane PJ, et al. Human milk oligosaccharide profiles and food sensitization among infants in the CHILD Study. Eur J Allergy Clin Immunol. 2018;73:2070–3. [DOI] [PubMed] [Google Scholar]
- 146. Lodge CJ, Lowe AJ, Milanzi E, Bowatte G, Abramson MJ, Tsimiklis H, et al. Human milk oligosaccharide profiles and allergic disease up to 18 years. J Allergy Clin Immunol. 2021;147:1041–8. [DOI] [PubMed] [Google Scholar]
- 147. Goehring KC, Marriage BJ, Oliver JS, Wilder JA, Barrett EG, Buck RH. Similar to those who are breastfed, infants fed a formula containing 2'‐fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. J Nutr. 2016;146:2559–66. [DOI] [PubMed] [Google Scholar]
- 148. Sharon N, Ofek I. Safe as mother's milk: carbohydrates as future anti‐adhesion drugs for bacterial diseases. Glycoconj J. 2000;17:659–64. [DOI] [PubMed] [Google Scholar]
- 149. Andersson B, Svanborg‐Eden C. Attachment of Streptococcus pneumoniae to human pharyngeal epithelial cells. Respiration. 1989;55(Suppl 1):49–52. [DOI] [PubMed] [Google Scholar]
- 150. Morozov V, Hansman G, Hanisch FG, Schroten H, Kunz C. Human milk oligosaccharides as promising antivirals. Mol Nutr Food Res. 2018;62:e1700679. [DOI] [PubMed] [Google Scholar]
- 151. Craft KM, Thomas HC, Townsend SD. Interrogation of human milk oligosaccharide fucosylation patterns for antimicrobial and antibiofilm trends in Group b Streptococcus . ACS Infect Dis. 2018;4:1755–65. [DOI] [PubMed] [Google Scholar]
- 152. Craft KM, Gaddy JA, Townsend SD. Human milk oligosaccharides (HMOs) sensitize Group B Streptococcus to clindamycin, erythromycin, gentamicin, and minocycline on a strain specific basis. ACS Chem Biol. 2018;13:2020–6. [DOI] [PubMed] [Google Scholar]
- 153. Morrow AL, Ruiz‐Palacios GM, Altaye M, Jiang X, Guerrero ML, Meinzen‐Derr JK, et al. Human milk oligosaccharide blood group epitopes and innate immune protection against campylobacter and calicivirus diarrhea in breastfed infants. Adv Exp Med Biol. 2004;554:443–6. [DOI] [PubMed] [Google Scholar]
- 154. Ruiz‐Palacios GM, Cervantes LE, Ramos P, Chavez‐Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem. 2003;278:14112–20. [DOI] [PubMed] [Google Scholar]
- 155. Yu ZT, Nanthakumar NN, Newburg DS. The human milk oligosaccharide 2'‐fucosyllactose quenches Campylobacter jejuni‐induced inflammation in human epithelial cells HEp‐2 and HT‐29 and in mouse intestinal mucosa. J Nutr. 2016;146:1980–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Weichert S, Koromyslova A, Singh BK, Hansman S, Jennewein S, Schroten H, et al. Structural basis for norovirus inhibition by human milk oligosaccharides. J Virol. 2016;90:4843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Koromyslova A, Tripathi S, Morozov V, Schroten H, Hansman GS. Human norovirus inhibition by a human milk oligosaccharide. Virology. 2017;508:81–9. [DOI] [PubMed] [Google Scholar]
- 158. Ruvoën‐Clouet N, Mas E, Marionneau S, Guillon P, Lombardo D, Le Pendu J. Bile‐salt‐stimulated lipase and mucins from milk of ‘secretor’ mothers inhibit the binding of Norwalk virus capsids to their carbohydrate ligands. Biochem J. 2006;393:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Paganini D, Uyoga MA, Kortman GAM, Boekhorst J, Schneeberger S, Karanja S, et al. Maternal human milk oligosaccharide profile modulates the impact of an intervention with iron and galacto‐oligosaccharides in Kenyan infants. Nutrients. 2019;11:2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Stepans MBF, Wilhelm SL, Hertzog M, Rodehorst TKC, Blaney S, Clemens B, et al. Early consumption of human milk oligosaccharides is inversely related to subsequent risk of respiratory and enteric disease in infants. Breastfeed Med. 2006;1:207–15. [DOI] [PubMed] [Google Scholar]
- 161. Steed AL, Christophi GP, Kaiko GE, Sun L, Goodwin VM, Jain U, et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science. 2017;357:498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Barton SJ, Murray R, Lillycrop KA, Inskip HM, Harvey NC, Cooper C, et al. FUT2 Genetic variants and reported respiratory and gastrointestinal illnesses during infancy. J Infect Dis. 2019;219:836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Muthumuni D, Miliku K, Wade KH, Timpson NJ, Azad MB. Enhanced protection against diarrhea among breastfed infants of nonsecretor mothers. Pediatr Infect Dis J. 2021;40:260–3. [DOI] [PubMed] [Google Scholar]
- 164. Yu ZT, Chen C, Kling DE, Liu B, McCoy JM, Merighi M, et al. The principal fucosylated oligosaccharides of human milk exhibit prebiotic properties on cultured infant microbiota. Glycobiology. 2013;23:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Leung TF, Ulfman LH, Chong MKC, Hon KL, Khouw I, Chan P, et al. A randomized controlled trial of different young child formulas on upper respiratory and gastrointestinal tract infections in Chinese toddlers. Pediatr Allergy Immunol. 2020;31:745–54. [DOI] [PubMed] [Google Scholar]
- 166. Dogra SK, Martin F‐P, Donnicola D, Julita M, Berger B, Sprenger N. Human milk oligosaccharide‐stimulated Bifidobacterium species contribute to prevent later respiratory tract infections. Microorganisms. 2021;9:1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Nakano T, Sugawara M, Kawakami H. Sialic acid in human milk: composition and functions. Acta Paediatr Taiwan. 2001;42:11–7. [PubMed] [Google Scholar]
- 168. Wang B. Sialic acid is an essential nutrient for brain development and cognition. Annu Rev Nutr. 2009;29:177–222. [DOI] [PubMed] [Google Scholar]
- 169. Sprenger N, Duncan PI. Sialic acid utilization. Adv Nutr. 2012;3:392S–7S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Nohle U, Schauer R. Uptake, metabolism and excretion of orally and intravenously administered, 14C‐ and 3H‐labeled N‐acetylneuraminic acid mixture in the mouse and rat. Hoppe Seylers Z Physiol Chem. 1981;362:1495–506. [DOI] [PubMed] [Google Scholar]
- 171. Christina E, Galuska SR, Kuntz S, Borsch C, Reutzel M, Eckert G, et al. Metabolic fate and organ distribution of 13C‐3′‐sialyllactose and 13C‐N‐acetylneuraminic acid in wild‐type mice – no evidence for direct incorporation into the brain. J Funct Foods. 2020;75:104268. [Google Scholar]
- 172. Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci U S A. 2003;100:12045–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Obelitz‐Ryom K, Bering SB, Overgaard SH, Eskildsen SF, Ringgaard S, Olesen JL, et al. Bovine milk oligosaccharides with sialyllactose improves cognition in preterm pigs. Nutrients. 2019;11:1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Jacobi SK, Yatsunenko T, Li D, Dasgupta S, Yu RK, Berg BM, et al. Dietary isomers of sialyllactose increase ganglioside sialic acid concentrations in the corpus callosum and cerebellum and modulate the colonic microbiota of formula‐fed piglets. J Nutr. 2016;146:200–8. [DOI] [PubMed] [Google Scholar]
- 175. Mudd AT, Fleming SA, Labhart B, Chichlowski M, Berg BM, Donovan SM, et al. Dietary sialyllactose influences sialic acid concentrations in the prefrontal cortex and magnetic resonance imaging measures in corpus callosum of young pigs. Nutrients. 2017;9:1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Wang HX, Chen Y, Haque Z, de Veer M, Egan G, Wang B. Sialylated milk oligosaccharides alter neurotransmitters and brain metabolites in piglets: an in vivo magnetic resonance spectroscopic (MRS) study. Nutr Neurosci. 2019;24:1–11. [DOI] [PubMed] [Google Scholar]
- 177. Fleming S, Chichlowski M, Berg B, Donovan S, Dilger R. Dietary sialyllactose does not influence measures of recognition memory or diurnal activity in the young pig. Nutrients. 2018;10:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Sakai F, Ikeuchi Y, Urashima T, Fujihara M, Ohtsuki K, Yanahira S. Effects of feeding sialyllactose and galactosylated nacetylneuraminic acid on swimming learning ability and brain lipid composition in adult rats. J Appl Glycosci. 2006;53:249–54. [Google Scholar]
- 179. Oliveros E, Vázquez E, Barranco A, Ramírez M, Gruart A, Delgado‐García JM, et al. Sialic acid and sialylated oligosaccharide supplementation during lactation improves learning and memory in rats. Nutrients. 2018;10:1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Tarr AJ, Galley JD, Fisher SE, Chichlowski M, Berg BM, Bailey MT. The prebiotics 3'sialyllactose and 6'sialyllactose diminish stressor‐induced anxiety‐like behavior and colonic microbiota alterations: evidence for effects on the gut‐brain axis. Brain Behav Immun. 2015;50:166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Cho S, Zhu Z, Li T, Baluyot K, Howell BR, Hazlett HC, et al. Human milk 3'‐sialyllactose is positively associated with language development during infancy. Am J Clin Nutr. 2021;114:588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Elena Oliveros MJM, Francisco JT‐E, Teresa Segura‐Moreno M, Ramírez María, Santos A, Buck R, et al. Human milk levels of 2'‐fucosyllactose and 6'‐sialyllactose are positively associated with infant neurodevelopment and are not impacted by maternal BMI or diabetic status. J Nutr Food Sci. 2021;4:1–11. [Google Scholar]
- 183. Krug M, Wagner M, Staak S, Smalla KH. Fucose and fucose‐containing sugar epitopes enhance hippocampal long‐term potentiation in the freely moving rat. Brain Res. 1994;643:130–5. [DOI] [PubMed] [Google Scholar]
- 184. Matthies H, Staak S, Krug M. Fucose and fucosyllactose enhance in‐vitro hippocampal long‐term potentiation. Brain Res. 1996;725:276–80. [DOI] [PubMed] [Google Scholar]
- 185. Bienenstock J, Buck RH, Linke H, Forsythe P, Stanisz AM, Kunze WA. Fucosylated but not sialylated milk oligosaccharides diminish colon motor contractions. PLoS One. 2013;8:e76236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Farhin S, Wong A, Delungahawatta T, Amin JY, Bienenstock J, Buck R, et al. Restraint stress induced gut dysmotility is diminished by a milk oligosaccharide (2'‐fucosyllactose) in vitro. PLoS One. 2019;14:e0215151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Vázquez E, Barranco A, Ramírez M, Gruart A, Delgado‐García JM, Martínez‐Lara E, et al. Effects of a human milk oligosaccharide, 2'‐fucosyllactose, on hippocampal long‐term potentiation and learning capabilities in rodents. J Nutr Biochem. 2015;26:455–65. [DOI] [PubMed] [Google Scholar]
- 188. Oliveros E, Ramirez M, Vazquez E, Barranco A, Gruart A, Delgado‐Garcia JM, et al. Oral supplementation of 2'‐fucosyllactose during lactation improves memory and learning in rats. J Nutr Biochem. 2016;31:20–7. [DOI] [PubMed] [Google Scholar]
- 189. Kuntz S, Kunz C, Borsch C, Vazquez E, Buck R, Reutzel M, et al. Metabolic fate and distribution of 2‐fucosyllactose: direct influence on gut microbial activity but not on brain. Mol Nutr Food Res. 2019;e1900035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Rudloff S, Kuntz S, Borsch C, Vazquez E, Buck R, Reutzel M, et al. Fucose as a cleavage product of 2'fucosyllactose does not cross the blood‐brain barrier in mice. Mol Nutr Food Res. 2021;65:e2100045. [DOI] [PubMed] [Google Scholar]
- 191. Berger PK, Plows JF, Jones RB, Alderete TL, Yonemitsu C, Poulsen M, et al. Human milk oligosaccharide 2'‐fucosyllactose links feedings at 1 month to cognitive development at 24 months in infants of normal and overweight mothers. PLoS One. 2020;15:e0228323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


