Abstract
Objective
Adipose tissue is a critical regulator of energy balance that must rapidly shift its metabolism between fasting and feeding to maintain homeostasis. Adenosine has been characterized as an important regulator of adipocyte metabolism primarily through its actions on A1 adenosine receptors (A1R). We sought to understand the role A1R plays specifically in adipocytes during fasting and feeding to regulate glucose and lipid metabolism.
Methods
We used Adora1 floxed mice with an inducible, adiponectin-Cre to generate FAdora1−/− mice, where F designates a fat-specific deletion of A1R. We used these FAdora1−/− mice along with specific agonists and antagonists of A1R to investigate changes in adenosine signaling within adipocytes between the fasted and fed state.
Results
We found that the adipose tissue response to adenosine is not static, but changes dynamically according to nutrient conditions through the insulin-Akt-FOXO1 axis. We show that under fasted conditions, FAdora1−/− mice had impairments in the suppression of lipolysis by insulin on normal chow and impaired glucose tolerance on high-fat diet. FAdora1−/− mice also exhibited a higher lipolytic response to isoproterenol than WT controls when fasted, however this difference was lost after a 4-hour refeeding period. We demonstrate that FOXO1 binds to the A1R promoter, and refeeding leads to a rapid downregulation of A1R transcript and desensitization of adipocytes to A1R agonism. Obesity also desensitizes adipocyte A1R, and this is accompanied by a disruption of cyclical changes in A1R transcription between fasting and refeeding.
Conclusions
We propose that FOXO1 drives high A1R expression under fasted conditions to limit excess lipolysis during stress and augment insulin action upon feeding. Subsequent downregulation of A1R under fed conditions leads to desensitization of these receptors in adipose tissue. This regulation of A1R may facilitate reentrance into the catabolic state upon fasting.
Keywords: Adora1, Adenosine receptor, Adipose, Lipolysis, FOXO1, Obesity
Graphical abstract
Highlights
-
•
A1 adenosine receptors (A1R) in adipose tissue are dynamically regulated by feeding.
-
•
Adipose A1R augments insulin action and glucose tolerance in fasted mice.
-
•
Loss of adipose A1R yields higher stimulated lipolysis in fasted but not refed mice.
-
•
Feeding downregulates and desensitizes A1R in adipose tissue via FOXO1.
-
•
Obesity disrupts feeding induced changes in adipose A1R expression.
1. Introduction
Adipose tissue is a critical regulator of energy balance, and must rapidly adapt its metabolic function to either esterify fatty acids for adequate energy storage during feeding or undergo lipolysis to provide non-esterified fatty acids (NEFA) and glycerol for energy during fasting [1]. These homeostatic set points are primarily regulated by catabolic hormones, such as catecholamines, driving lipolysis, and anabolic hormones, such as insulin, suppressing lipolysis and driving fatty acid uptake and esterification [2].
Adenosine is a nucleoside that was originally identified as an important extracellular signaling molecule by its ability to reduce heart rate through the activation of A1 adenosine receptors (A1R) present in the SA node [3,4]. Adenosine signals through four G protein-coupled receptors: A1, A2A, A2B, and A3 [5]. The A1 and A3 receptors are coupled to Gαi-proteins and act to suppress cAMP, while the A2A and A2B receptors couple to Gαs-proteins and act to increase cAMP. In white adipocytes, adenosine has been found to act primarily through the A1 adenosine receptor (A1R) to suppress lipolysis and counteract β-adrenergic signaling [6]. In brown adipocytes, adenosine appears to act primarily through A2A and A2B receptors to drive thermogenesis, with the A2A receptor being more highly expressed compared with white adipocytes [[7], [8], [9]]. The A2B receptor is also highly expressed in preadipocytes where it inhibits adipogenesis [10]. No clear role has been defined for the A3 receptor in adipose tissue, despite its expression in both white and brown adipocytes [7].
Adenosine signaling via A1R in white adipocytes has been shown to augment insulin stimulated glucose uptake, lipogenesis, and suppression of lipolysis in vitro [[11], [12], [13]]. Agonism of A1R has also been shown to reduce serum NEFA in both rats and humans, and improve insulin sensitivity in obese rats [[14], [15], [16]]. Further, reduced A1R expression in white adipocytes due to obesity, hypertension, or prolonged pharmacological stimulation are associated with insulin resistance [6,17,18]. Despite this, mice with global A1R knock out display relatively normal systemic NEFA levels, and differences in glucose and insulin tolerance were concomitant with significant confounding factors such as differences in body weight, food intake, insulin and glucagon secretion, and survival [[19], [20], [21], [22], [23]]. It therefore remains unclear whether endogenous adenosine and A1R contribute to shifts in adipocyte metabolism between the fasted and fed state in vivo.
Adipose tissue maintains basal levels of adenosine that are considered sufficient to prevent a maximal stimulation of lipolysis through tonic activation of A1R [[24], [25], [26], [27], [28]]. However, extracellular adenosine levels are increased in response to clear stressors related to damage or a metabolic imbalance, including adrenergic stress within adipose tissue [29]. This elevation is derived either from increased intracellular adenosine levels that exit adipocytes via equilibrative nucleoside transporters, or through the extracellular degradation of extracellular nucleotides such as ATP/ADP and AMP by CD39 and CD73 respectively [5,30,31]. The deletion of all four adenosine receptors in mice is non-lethal, which suggests that adenosine mainly adapts the body to stress and is not necessary for survival [19]. However, these mice displayed reduced lifespan which implies that adenosine is important for responding to normal, everyday stressors, and the loss of this functionality is maladaptive. In adipocytes, the degradation of extracellular adenosine with exogenous adenosine deaminase or inhibition of the A1R with antagonists enhances the stimulation of lipolysis by adrenergic signals such as norepinephrine or the β-adrenergic receptor agonist isoproterenol [[26], [27], [28],32]. This suggests that A1R activation may be protective since high levels of NEFA resulting from stress-induced lipolysis can have deleterious effects such as insulin resistance and lipotoxicity [33,34]. However, the effects of A1R signaling in adipocytes in response to endogenous adenosine and its subsequent impact on circulating NEFA during stress have not been investigated.
We sought to explore how the loss of A1R specifically in adipose tissue affects lipid metabolism primarily in white adipocytes in response to anabolic and catabolic signals under both fed and fasted conditions. In this study, we show that A1R acts to augment insulin action to suppress lipolysis in the transition from the fasted to the fed state, and loss of adipose A1R leads to glucose intolerance in obese mice. We also demonstrate that A1R limits the lipolytic response to adrenergic stimulation in the fasted, but not fed, state. We found that the adipose tissue response to adenosine changes dynamically according to nutrient conditions. These studies identify a mechanism of differential expression of A1R during fasted and fed states whereby insulin downregulates A1R in adipocytes and desensitizes adipose to A1R agonism in the fed state.
2. Research design and methods
2.1. Materials
All pharmacological compounds were obtained from Cayman Chemical (Ann Arbor, MI) unless otherwise stated. Capadenoson was obtained from MedChemExpress (Monmouth Junction, NJ). 3-isobutyl-1-methylxanthine (IBMX), dexamethasone, GSK3 inhibitor IX, formaldehyde, indomethacin, T3 (T6397), and peanut oil were obtained from Sigma–Aldrich (St. Louis, MO). Regular human insulin (Humulin R) was obtained from Eli Lilly (Indianapolis, IN). All antibodies were obtained from Cell Signaling (Danvers, MA) unless otherwise specified. Adora1 antibody (PA1-041a) was obtained from ThermoFisher (Waltham, MA). The FOXO1 antibody (18592-1-AP) used for ChIP-qPCR was obtained from ProteinTech (Rosemont, IL). [3H]-DPCPX was from PerkinElmer (Waltham, MA). GF/C filter plates (MAFCN0B50) were from Sigma–Aldrich (St. Louis, MO).
2.2. Animals
All animals were bred and maintained in accordance with the University of Virginia Animal Care and Use Committee regulations and the study was approved by the ACUC ethics committee. 12- to 16-week-old male and female mice were used for studies unless otherwise indicated. Adora1loxP/loxP mice were generously shared with us by Dr. Stanislav Zakharenko [35]. AdipoQ-cre-ERT2 (C57BL/6-Tg(Adipoq-cre/ERT2)1Soff/J, Jax #025124) and C57BL/6J (Jax #000664) mice were from the Jackson Laboratory. Inducible, fat-specific Adora1 knockout mice were generated by crossing Adora1loxP/loxP with AdipoQ-Cre-ERT2 mice resulting in wildtype (WT) Adora1loxP/loxP mice and knockout Adora1loxP/loxP;AdipoQ-Cre-ERT2 (FAdora1−/−) littermates on a mixed C57BL/6J and C57BL/6N background. In FAdora1−/− mice, knockout was induced at 8-weeks by daily injection of tamoxifen (50 mg/kg) dissolved in peanut oil for 10 days, followed by a 14-day washout period prior to experimentation. Cre-negative wildtype control mice were age and sex-matched littermates that received the same tamoxifen treatment protocol. For high-fat diet (HFD) studies in WT and FAdora1−/− mice, animals were placed on diet for 12 weeks starting at 8 weeks of age (BioServ, F1850). For diet studies in wildtype animals, C57BL/6J mice at Jackson Labs were put on HFD beginning at 6 weeks of age (#380050) and were shipped to the University of Virginia vivarium at 14 weeks of age. The mice were then maintained on HFD (Research Diets, D12492) for another four weeks before experiments, for a total of 12 weeks of HFD.
2.3. Animal procedures and measurements
To measure insulin and NEFA levels, blood was collected from the tail vein of mice, and serum was prepared and frozen at −80 °C until analysis. Insulin and NEFA levels were measured in serum samples or lipolysis assay buffer using the Ultra-Sensitive Mouse Insulin ELISA Kit (Crystal Chem) and the HR series NEFA-HR(2) Kit (Wako Diagnostics) respectively and according to the manufacturer's protocols. Glucose was measured in tail vein blood using a One-touch Ultra glucometer. Heart rate was measured by electrocardiogram in mice anesthetized with isoflurane. Insulin, glucose, and all pharmacological compounds were administered via i.p. injection in 0.9% NaCl, except for glucose which was administered as a 20% glucose solution. Body weight was measured once weekly at the same time of day for both normal chow and HFD mice. Fat and lean mass distribution were determined using Echo-MRI. Ambulatory activity, carbon dioxide production (VCO2), and oxygen consumption (VO2) were measured using an Oxymax metabolic chamber system (Comprehensive Laboratory Animal Monitoring System) from Columbus Instruments (Columbus, OH). One full day and night was allowed for acclimation. Mice were either fasted overnight (fasted) or then given access to chow and softened food for 4 h (refed) as indicated, with exception of the fasted condition for the isoproterenol tolerance tests where mice were fasted for 6 h. For tissue harvesting, mice were euthanized by cervical dislocation, and tissues were flash frozen in liquid nitrogen.
To measure the suppression of lipolysis by insulin, mice were fasted overnight and administered insulin i.p. (0.75 IU/kg). Serum was collected at 0 and 15 min after insulin administration. Mice were monitored for an additional 1 h for hypoglycemia and administered glucose as needed.
2.4. Adipocyte isolation
Adipocytes were isolated as previously described [36,37]. Gonadal or inguinal fat pads were minced in low phosphate buffer (145 mmol/L NaCl, 5.4 mmol/L KCl, 1.4 mmol/L CaCl2, 1.4 mmol/L MgSO4, 0.2 mmol/L NaH2PO4, 5 mmol/L glucose, 10 mmol/L HEPES, pH 7.4) containing 2.5% bovine serum albumin (BSA, Sigma–Aldrich, St. Louis, MO), 3 mg/mL collagenase type I (Worthington, Lakewood, NJ), and 100 nmol/L adenosine, and digested for 45 min in a 37 °C water bath with mild agitation. For protein and RNA extraction, adipocytes were washed twice with buffer containing 0.1% BSA, and once with buffer without BSA. For lipolysis assays, adipocytes were washed three times with buffer containing 2.5% BSA.
2.5. Lipolysis assays
Isolated adipocytes were treated with vehicle or 1 μM AS-1842856 for 4 h in lipolysis assay buffer (145 mmol/L NaCl, 5.4 mmol/L KCl, 1.4 mmol/L CaCl2, 1.4 mmol/L MgSO4, 0.2 mmol/L NaH2PO4, 5 mmol/L glucose, 2.5% BSA, 100 nmol/L adenosine, 10 mmol/L HEPES, pH 7.4) in a 37 °C water bath with mild agitation. Adipocytes were washed twice with assay buffer without adenosine and separated into assay tubes with ∼100,000 cells per assay in a total assay volume of 200 μL. Adipocytes were treated with vehicle or 100 nmol/L CCPA followed by the addition of 1 U/mL adenosine deaminase to all lipolysis assays and incubation at 37 °C with mild agitation for 15 min. Adipocytes were then treated with vehicle or 30 nmol/L isoproterenol and incubated for an additional 30 min. Assays were centrifuged for 1 min at 200×g, adipocytes were aspirated off, and assay buffer was collected and assessed for NEFA concentration. All assays were performed in triplicate, and each N represents independently isolated adipocytes.
2.6. RNA extraction and real-time qPCR
Total RNA was isolated using the PureLink RNA Mini Kit (Invitrogen) according to the manufacturer's protocol. For tissues, RNA was first extracted using Trizol Reagent (Invitrogen). cDNA was synthesized from 500 ng RNA with the High-Capacity RNA-to-cDNA Synthesis Kit (Applied Biosystems). Real-time qPCR was performed with the iQ SYBR Green Supermix (Bio-Rad) in duplicate and analyzed with a CFX96 Real-Time PCR Detection System (Bio-Rad). Data were normalized to Ppia cDNA using the delta-delta-Ct method. Primers for Adora1, Adora2A, Adora2B, and Adora3 were sourced from the PrimerBank of the Center for Computational and Integrative Biology of Massachusetts General Hospital and Harvard University, and were used to determine changes in mRNA expression for all experiments with wildtype mice and 3T3-L1 adipocytes [[38], [39], [40]]. Primers targeting the 3′ terminal exon for Adora1 (exon 3) were designed using Primer3, and were used to determine the efficiency of the tamoxifen-induced knockout in FAdora1−/− mice [41,42].
| Target | Forward Primer | Reverse Primer |
|---|---|---|
| Ppia | CGATGACGAGCCCTTGG | TCTGCTGTCTTTGGAACTTTGTC |
| Adora1 | TGTGCCCGGAAATGTACTGG | TCTGTGGCCCAATGTTGATAAG |
| Adora2A | GCCATCCCATTCGCCATCA | GCAATAGCCAAGAGGCTGAAGA |
| Adora2B | AGCTAGAGACGCAAGACGC | GTGGGGGTCTGTAATGCACT |
| Adora3 | AAGGTGAAATCAGGTGTTGAGC | AGGCAATAATGTTGCACGAGT |
| Adora1 (exon 3) | ACTTCTTCGTCTGGGTGCTG | TCCCGTAGTACTTCTGGGGG |
2.7. Culture and treatment of 3T3-L1 adipocytes and iBACs
3T3-L1 fibroblasts (ATCC) were cultured and differentiated into adipocytes as previously reported [36]. Briefly, 3T3-L1 fibroblasts were maintained and grown to confluency in DMEM (Gibco, 11965-092) with 10% newborn calf serum (Gibco, 16010-159), 1% fetal bovine serum (FBS, Gemini Bio Products, Lot #A15H74K), and 1% antibiotic-antimycotic (AA, Gibco). Adipocyte differentiation was initiated 3 days after cells become confluent in DMEM with 10% FBS, 1% AA, and containing 0.25 IU/mL insulin, 0.5 mmol/L IBMX, 0.25 μmol/L dexamethasone, and 10 μmol/L pioglitazone. After 4 days, adipocytes were maintained in DMEM with 10% FBS.
Experiments were performed with 3T3-L1 adipocytes 10–12 days after differentiation. 3T3-L1 adipocytes were serum starved overnight in DMEM with 0.25% FBS and 0.25% BSA and treated with the indicated inhibitors at the concentrations described in figure legends 30 min prior to the addition of insulin. For qPCR analysis and FOXO1 translocation western blots, cells were treated with 1 nmol/L insulin for 4 h unless otherwise indicated. For western blot analysis of phospho-proteins, cells were treated with 1 nmol/L insulin for 15 min.
Immortalized brown preadipocytes (iBACs) were differentiated as previously described [43,44]. Briefly, iBACs were maintained and grown to confluency in DMEM containing 20% FBS. Cells were differentiated in DMEM containing 10% FBS with 20 nM insulin, 1 nM T3, 500 nM dexamethasone, 125 μM indomethacin, and 500 μM isobutylmethylxanthine for 2 days, followed by maintenance in DMEM containing 10% FBS, 20 nM insulin, and 1 nM T3 replenished every 2 days. Experiments were performed with iBACs 8 days after differentiation. iBACs were serum starved overnight and treated with insulin similar to 3T3-L1 adipocytes and as described in figure legends.
2.8. Nuclear isolation
3T3-L1 adipocytes were washed twice with cold PBS and lysed in sucrose buffer (250 mmol/L sucrose, 50 mmol/L NaF, 1 mmol/L EDTA, 50 mmol/L Tris, pH 7.4) with Dounce homogenization. Lysates were incubated on ice for 5 min, and then centrifuged at 17,000 x G for 10 min at 4 °C. Supernatant was removed and frozen at −80 °C as the cytosolic fraction. Pellets were resuspended with cell lysis buffer, briefly sonicated on ice with a probe tip sonicator, and frozen at −80 °C as the crude nuclear fraction.
2.9. Sample preparation and antibodies for western blots
Adipocytes were lysed with cell lysis buffer (10 mmol/L Na2HPO4, 50 mmol/L β-glycerophosphate disodium salt hydrate, 50 mmol/L NaF, 1 mmol/L EDTA, 1 mmol/L EGTA, pH 7.4) supplemented with protease inhibitors and 0.5 mmol/L dithiothreitol, homogenized by shearing through a 22G needle, and cleared by centrifugation at 17,000×g for 10 min at 4 °C. Tissues were homogenized by Potter-Elvehjem tissue grinder in Tissue Protein Extraction Reagent (Thermo) supplemented with protease inhibitors and 0.5 mmol/L dithiothreitol and cleared by centrifugation at 17,000×g for 10 min at 4 °C. Protein concentrations were determined using BCA assay (Pierce). Western blots were probed as indicated with β-actin (#A228, Sigma), α-tubulin (T9026, Sigma), GAPDH (#5174), TBP (#8515), A1R (#PA1-041a, ThermoFisher), phospho-Akt (S473, #9271), phospho-MAPK (T202/Y204, #9101), phospho-GSK3α/β (S21/S9, #9331), phospho-P70 S6K (T389, #9205), phospho-GYS (S641, #3891), total Akt (#2920), total P42/P44 MAPK (#4696), total GSK3β (#9315), total GYS (#3893), and FOXO1 (#2880).
2.10. Western blot and quantitative analysis
Equal amounts of proteins were separated by SDS-PAGE and transferred to PVDF. Membranes were blocked with 10% dry milk in TBST, and western blotting was carried out with appropriate primary and secondary antibodies. Blots were imaged by detecting horseradish peroxidase conjugated chemiluminescence on an Amersham ImageQuant 800. For assessing differences in protein or phospho-protein signal, membranes were stripped and reprobed with β-tubulin, α-actin, or TBP loading controls as appropriate and as indicated, and phospho-protein blots were additionally reprobed with the corresponding total antibody. Blots were quantified using densitometry with ImageQuant software.
2.11. Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed as previously described with modifications [45]. Briefly, 3T3-L1 adipocytes were serum starved overnight in DMEM with 0.25% FBS and 0.25% BSA and treated with vehicle or 1 μmol/L AS-1842856 for 4 h. Cells were fixed in 1% formaldehyde for 10 min, and fixation was quenched with 125 mmol/L glycine for 5 min. Cells were washed twice with cold PBS, lysed in hypotonic lysis buffer (10 mmol/L Tris–HCl, pH 8.0, 10 mmol/L NaCl, 3 mmol/L MgCl2, 0.5% NP-40) supplemented with protease inhibitors by Dounce homogenization, and incubated on ice for 5 min. Nuclei were pelleted by centrifugation at 17,000×g for 5 min at 4 °C. Pellets were resuspended with SDS lysis buffer (50 mmol/L Tris–HCl, pH 8.0, 10 mmol/L EDTA, 1% SDS) and chromatin was sheared by sonication using a Diagenode BioRuptor Pico with 30 s on and 30 s off for 12 cycles. ChIP was performed using 3 μg of FOXO1 rabbit-antibody (ProteinTech) or normal rabbit IgG (Cell Signaling), and antibodies were pulled down with protein A agarose (Roche). Chromatin pulldown of the Adora1 promoter region or 28S rDNA control region was assessed by qPCR using the following primers: Adora1 promoter: 5′-TGACCCTTGAAACCATGTGA-3′ and 5′-CCCAGAGTACCCAACACACA-3′, 28S rDNA: 5′-CTGGGTATAGGGGCGAAAGAC-3′ and 5′-GGCCCCAAGACCTCTAATCAT-3’. FOXO1 binding was measured as enrichment over normal rabbit IgG ChIP.
2.11.1. Membrane isolation and radioligand binding
Adipose depots were harvested from mice either fasted overnight or refed for 4 h and immediately processed to isolate membranes as previously described with minor modifications [46,47]. Briefly, adipose tissues were homogenized in sucrose buffer (250 mmol/L sucrose, 50 mmol/L NaF, 1 mmol/L EDTA, 50 mmol/L Tris–HCl, pH 7.5) by Potter-Elvehjam and placed on ice for 15 min. Homogenates were then centrifuged at 17,000 x G for 15 min at 4 °C. The membrane pellet was resuspended in 1 mL of sucrose buffer and centrifuged at 1000 x G for 10 min at 4 °C to clear nuclei. The supernatant was then centrifuged at 17,000 x G for 20 min at 4 °C to pellet the membranes. Membranes were resuspended in sucrose buffer to a protein concentration of ∼1 μg/μL and frozen at −80 °C. Upon thawing, membranes were further disrupted by homogenization through a 29G needle prior to proceeding with radioligand binding.
Radioligand binding assays were performed as previously described with minor modifications [46]. Briefly, 10 μg of membranes were combined with 2U/mL adenosine deaminase, the indicated concentration of [3H]-DPCPX, 5 nmol/L ZM 241385 (A2A antagonist), 100 nmol/L ATL-801 (A2B antagonist), and assay buffer (50 mmol/L Tris–HCl, pH 7.5) to a final volume of 200 μL. To assess non-specific binding, 1 μmol/L of unlabeled DPCPX was additionally added. Membrane binding was allowed to proceed for 2 h at room temperature before being filtered through a 96-well GF/C plate. Filters were washed 3 times with ice cold assay buffer, dried, and punched out for counting with a liquid scintillation counter. Each assay condition was run with 3 technical replicates. Total radioligand was determined by directly counting 200 μL of each assay condition in duplicate.
3. Results
3.1. Loss of A1R inhibition of lipolysis in adipocyte-specific A1R knockout mice
Several groups have previously evaluated the role of A1R in adipose tissue and fatty acid metabolism using mice with a global knockout of A1R, however these mice exhibit increased body weight and food intake [21,22], increased insulin and glucagon secretion [20,23], and decreased survival [19,48] which make it difficult to interpret the role of the A1R specifically in adipose. To avoid these complications, we crossed mice with a tamoxifen-inducible Cre driven by the adiponectin promoter (Jax #025124) with an Adora1loxP/loxP strain [35] in which the terminal 3’ exon of the Adora1 gene, which encodes A1R, is flanked by loxP sites. Both Cre negative Adora1loxP/loxP control mice (WT) and Cre positive Adora1loxP/loxP;AdipoQ-Cre-ERT2 knockout mice (FAdora1−/−), where F indicates a fat-specific deletion, were treated with tamoxifen for 10 days starting at 8-weeks of age to induce knockout, followed by a 2-week washout period. Following tamoxifen treatment, male and female WT mice displayed similar levels of A1R expression and FAdora1−/− mice exhibited similar levels of knockdown (Figure 1A–C, Fig. S1A-C). Male FAdora1−/− mice exhibited an ∼83% knockdown in A1R mRNA in gonadal white adipose tissue (gWAT), a ∼97% knockdown in inguinal white adipose tissue (iWAT), and a ∼95% knockdown in brown adipose tissue (BAT) (Figure 1A–C). This resulted in an ∼80% reduction in protein levels within isolated gonadal white adipocytes in male mice (Figure 1D). The FAdora1−/− mice did not display any compensatory changes in the mRNA expression of other adenosine receptors A2A, A2B, or A3 in gWAT, iWAT, or BAT (Fig. S2). There was no reduction of A1R protein in cardiac tissue of Fadora1−/− mice (Figure 1E) [49].
Figure 1.
Tamoxifen induces a functional knockout of adipose A1R in FAdora1−/−mice. (A–C) mRNA levels of A1R in Cre negative WT (blue) and Cre positive FAdora1−/− (red) male mice in gWAT (A), iWAT (B), and BAT (C). (D) Protein levels of A1R in isolated gonadal white adipocytes from WT and FAdora1−/− male mice assessed and quantified by western blot normalized to β-actin loading control. (E) Protein levels of A1R in whole heart lysates from WT and FAdora1−/− male mice assessed and quantified by western blot normalized to GAPDH loading control. (F and G) Serum NEFA levels (F) and heart rate (G) in WT and FAdora1−/− male mice fasted overnight and treated for 2 h with vehicle or 0.5 mg/kg CCPA. Error bars represent SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, t-test or two-way ANOVA.
Agonism of A1R in adipocytes results in lower levels of non-esterified fatty acids (NEFA) within the blood stream, and agonism of A1R in the SA node of the heart reduces heart rate [15,49,50]. We subjected WT and FAdora1−/− mice to an overnight fast and treated with either vehicle or the A1R agonist 2-chloro-N-6-cyclopentyladenosine (CCPA) for 2 h. CCPA significantly reduced serum NEFA in WT mice, but not FAdora1−/− mice (Figure 1F). Consistent with adipocyte-specific loss of A1R in our system, CCPA treatment reduced heart rate in both WT and FAdora1−/− mice (Figure 1G).
3.2. Impaired insulin suppression of lipolysis in male FAdora1−/− mice
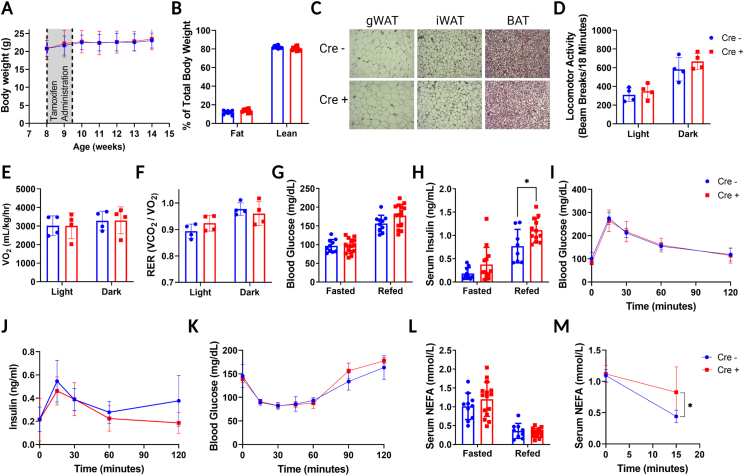
In contrast to global A1R knockout mice, FAdora1−/− mice did not develop changes in body weight or fat and lean mass distribution following tamoxifen administration, even in a long-term follow-up more than 30 weeks after A1R knockout (Figure 2A,B, Fig. S3). There were also no apparent histological differences in gWAT iWAT or BAT between WT and FAdora1−/− male mice (Figure 2C). The FAdora1−/− mice displayed normal locomotor activity, oxygen consumption, and respiratory exchange ratio (Figure 2D–F). FAdora1−/− mice also had normal blood glucose levels when fasted overnight and following a 4-hour refeeding period (Figure 2G). However, refed FAdora1−/− mice displayed significantly increased (∼45%) serum insulin levels suggestive of impaired insulin action (Figure 2H). This impairment was not apparent during a glucose tolerance test in either blood glucose levels (Figure 2I) or insulin secretion (Figure 2J), nor was it apparent in blood glucose levels during an insulin tolerance test (Figure 2K). There were also no differences in fasted or refed NEFA levels (Figure 2L), however, when we treated fasted WT and FAdora1−/− male mice with insulin, serum NEFA was significantly reduced in WT but not FAdora1−/− mice within 15 min (Figure 2M). This suggests that the loss of A1R in adipocytes impairs insulin action to suppress lipolysis. Throughout our characterization of the FAdora1−/− mice, female mice consistently lacked a distinct phenotype as compared with male mice. Female FAdora1−/− mice did not display significant differences in body weight, body mass distribution, overall histological appearance, locomotor activity, oxygen consumption, respiratory exchange ratio, fasting and refed values for glucose, insulin and NEFA, glucose tolerance, insulin secretion, or insulin tolerance (Fig. S4A-L). The ability of insulin to suppress lipolysis in female FAdora1−/− mice was impaired to an extent which reached statistical significance, however the difference was minor and likely does not represent a biologically significant change (Fig. S4M).
Figure 2.
Induced loss of A1R in adipose impairs insulin suppression of lipolysis. (A) Body weight of male Cre negative WT (blue) and Cre positive FAdora1−/− mice (red) starting at the time of tamoxifen administration at 8 weeks. Gray box indicates timing of tamoxifen. N = 6–9. (B) Fat and lean mass distribution in male WT and FAdora1−/− mice 6 weeks after the start of tamoxifen administration measured by EchoMRI. (C) Representative images for H&E staining of gWAT, iWAT, and BAT for WT and FAdora1−/− mice at ∼16 weeks of age. (D–F) Ambulatory activity (D), oxygen consumption (E), and respiratory exchange ratio (F) for one full day/night cycle measured in metabolic cages. (G and H) Blood glucose (G) and serum insulin (H) levels in male WT and FAdora1−/− mice after an overnight fast (fasted) and 4-hour refeeding period (refed). (I and J) Blood glucose (I) and serum insulin (J) in male WT and FAdora1−/− mice during an i.p. glucose tolerance test with 1 g/kg glucose. N = 6–8. (K) Blood glucose in male WT and FAdora1−/− mice during an i.p. insulin tolerance test with 0.75 IU/kg insulin. N = 3. (L) Serum NEFA levels in fasted and refed WT and FAdora1−/− mice. (M) Serum NEFA in overnight fasted male WT and FAdora1−/− mice at 0 and 15 min after administration of 0.75 IU/mL insulin. N = 5–6. Error bars represent SD. ∗p < 0.05, t-test or two-way ANOVA.
3.3. High fat diet results in overt glucose intolerance within male FAdora1−/− mice
To further explore the impacts of A1R knockout on adipose insulin sensitivity, we placed WT and FAdora1−/− mice on high fat diet (HFD) for 12 weeks. Tamoxifen was administered beginning after 7 weeks of HFD feeding to allow sufficient time for tamoxifen washout and recovery. While tamoxifen had a clear secondary impact on weight gain, there were no differences between WT and FAdora1−/− male mice in body weight, body mass distribution, or food intake (Figure 3A–C). In addition, FAdora1−/− male mice did not have any differences in activity, oxygen consumption, or respiratory exchange ratio (Figure 3D–F). There also continued to be no differences in fasting or refed glucose, insulin, and NEFA levels (Figure 3G–I). However, while there were no differences in insulin tolerance (Figure 3J), HFD fed FAdora1−/− mice displayed clear glucose intolerance during an i.p. GTT with no change in insulin secretion (Figure 3K and L). There were no significant differences in metabolic parameters between WT and FAdora1−/− among HFD fed female mice (Fig. S5).
Figure 3.
FAdora1−/−mice are more susceptible to high-fat diet-induced glucose intolerance. (A) Body weight of male Cre negative WT (blue) and Cre positive FAdora1−/− mice (red) starting at the beginning of high-fat diet feeding at 8 weeks. Gray box indicates timing of tamoxifen administration. N = 9–10. (B) Fat and lean mass distribution in male WT and FAdora1−/− mice before and after 10 weeks of high-fat diet feeding measured by EchoMRI. (C) Total food consumed per mouse during 12-weeks of HFD feeding. (D–L) Data were collected for male WT and FAdora1−/− mice after 12–15 weeks of high-fat diet feeding. (D–F) Ambulatory activity (D), oxygen consumption (E), and respiratory exchange ratio (F) for one full day/night cycle measured in metabolic cages. (G–I) Blood glucose (G), serum insulin (H), and serum NEFA (I) following an overnight fast (fasted) and 4-hour feeding period (refed). (J) Blood glucose during an i.p. insulin tolerance test with 0.75 IU/kg insulin. N = 9–10. (K and L) Blood glucose and area under the curve (K), and serum insulin (L) during an i.p. glucose tolerance test with 1 g/kg glucose. N = 9–10. Error bars represent SD. ∗∗∗∗p < 0.0001, t-test.
3.4. A1R limits the lipolytic response to adrenergic stress in fasted but not refed mice
Adenosine levels are known to rise during stress, and adenosine signaling provides numerous protective benefits during stress responses [29,49,51]. Stress-induced adrenergic stimulation activates lipolysis within adipose tissue, and exposure to high levels of NEFA during stress is associated with insulin resistance and lipotoxicity [33,34]. We hypothesized that adenosine provides a protective limit on lipolysis in adipose tissue and that the loss of adipose A1R would result in a stronger lipolytic response to adrenergic stimulation and higher serum NEFA levels. To test this, we subjected WT and FAdora1−/− mice to an isoproterenol tolerance test. After a short, 6-hour fast to normalize serum NEFA levels, we administered 10 mg/kg isoproterenol by i.p. injection to stimulate adipocyte lipolysis. In WT mice, this dose of isoproterenol induces a modest and rapid increase in serum NEFA levels that returns to baseline levels within 2 h (Figure 4A). As we expected, treatment of FAdora1−/− mice with isoproterenol resulted in a stronger stimulation of lipolysis achieving significantly higher NEFA levels, but also returning to baseline within 2 h (Figure 4A). The lipolytic response peaked 15 min after isoproterenol stimulation, which is not surprising considering the rapid induction of lipolysis and short half-life (∼2 min) of isoproterenol (Figure 4B).
Figure 4.
FAdora1−/−mice are more lipolytically responsive in the fasted but not refed state due to A1R downregulation and desensitization after feeding. (A,B) Serum NEFA in 6-hour fasted male Cre negative WT (blue) and Cre positive FAdora1−/− (red) mice at the indicated time points after i.p. injection of 10 mg/kg isoproterenol (A) or at 15 min after injection with isoproterenol (B). (C,D) Same as in (A,B) for mice that were fasted overnight and refed for 4 h. (E) Serum NEFA in 6-hour fasted wildtype male C57BL/6J mice treated with vehicle (black) or 0.1 mg/kg DPCPX (purple) for 30 min both before (control) and 15 min after (iso) i.p. injection with 10 mg/kg isoproterenol. (F) Same as in (E) for mice that were fasted overnight and refed for 4 h. (G,H) Same as in (E) and (F) except mice were treated with vehicle (black) or 0.5 mg/kg CCPA (light blue) for 30 min before isoproterenol injection. (I–N) Adipocytes and tissues from wildtype male C57BL/6J mice were harvested after either an overnight fast (white) or following a 4-hour refeeding period (shaded), and mRNA expression of A1R was measured in gonadal white adipocytes (I), inguinal white adipocytes (J), BAT (K), liver (L), heart (M), and soleus muscle (N). (O and P) Radioligand binding was performed on membranes isolated from gWAT (O) and BAT (P) from fasted or refed C57BL/6J mice. N = 4 (O) and N = 2 (P). Error bars represent SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, t-test or two-way ANOVA.
We also conducted these experiments under refed conditions by fasting mice overnight followed by a 4-hour refeeding period prior to stimulation with isoproterenol. As expected, both WT and FAdora1−/− mice had significantly lower levels of serum NEFA following refeeding. However, there was no longer any difference in the elevation of NEFA following isoproterenol stimulation (Figure 4C). Both WT and FAdora1−/− mice reached a similar peak in serum NEFA at 15 min (Figure 4D) and returned to baseline within 2 h. Once again, no differences were seen in either the fasted or refed state with female FAdora1−/− mice (Fig. S6A-D).
3.5. Feeding desensitizes adipocytes to A1R signaling in vivo
The discrepancy between the fasted and refed state in FAdora1−/− male mice suggested that refeeding may desensitize WT mice to signaling through A1R. To investigate this possibility in WT C57BL/6J mice, we used the same experimental setup as was used with the FAdora1−/− mice in Figure 4A–D; lipolysis was stimulated using isoproterenol in C57BL/6J mice that were either fasted for 6 h or fasted overnight and refed for 4-hours. We first administered a 30-minute pre-treatment with the A1R antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) and collected serum to assess NEFA levels under control conditions before subsequent induction of lipolysis with isoproterenol for 15 min. Treatment with DPCPX alone did not alter NEFA levels, however DPCPX-treated mice exhibited a stronger lipolytic response to isoproterenol compared with vehicle-treated mice under fasted conditions (Figure 4E). This difference in lipolytic response was not present in refed mice, consistent with the results seen in FAdora1−/− mice (Figure 4F).
While these data alongside the results obtained with FAdora1−/− mice suggested a loss of sensitivity to A1R signaling in response to feeding, it is possible the discrepancy in these phenotypes between the fasted and refed conditions could be explained by differences in endogenous adenosine levels. A substantial reduction in extracellular adenosine following refeeding would also produce a lack of any phenotype with both genetic knockout and antagonism of A1R. Therefore, we subjected fasted and refed C57BL/6J mice to pre-treatment with the A1R agonist CCPA and isoproterenol stimulation. Under fasted conditions, agonism of A1R with CCPA produced a significant decrease in serum NEFA in both the control and isoproterenol stimulated states (Figure 4G). Strikingly, all effects of A1R agonism on serum NEFA were eliminated under refed conditions, even with isoproterenol stimulation (Figure 4H). These data confirmed that adipocytes are indeed desensitized to A1R signaling in response to feeding, and that changes in extracellular adenosine are unlikely to be responsible for reduced signaling through A1R during the fed state.
3.6. A1R is acutely downregulated in response to feeding specifically in adipocytes
To better understand the mechanism driving A1R desensitization, we subjected wildtype C57BL/6J mice to either an overnight fast alone or an overnight fast followed by a 4-hour refeeding period and measured A1R mRNA expression levels. We found that in isolated gonadal white adipocytes there was a significant reduction in A1R mRNA levels in refed mice (Figure 4I). We saw a similar suppression of A1R expression in brown adipose tissue (Figure 4K), but intriguingly this difference was not present in inguinal white adipocytes (Figure 4J). This downregulation of A1R mRNA was also not present in liver, heart, or skeletal muscle (Figure 4L–N). Thus, feeding induces a rapid downregulation of A1R mRNA expression specifically within gWAT and BAT which may, at least partially, explain the desensitization to A1R signaling in adipocytes.
We next wanted to determine if feeding induced downregulation of A1R mRNA also correlated with a reduction in the membrane receptor density of A1R in gWAT and BAT. In order to test this, we isolated gWAT and BAT membranes from mice either fasted overnight or refed for 4 h and performed radioligand binding with [3H]-DPCPX. We found that the Bmax of gWAT was reduced from 238.8 fmol/mg tissue under fasted conditions to 95.3 fmol/mg tissue under refed conditions (Figure 4O). Similarly, the Bmax for BAT was reduced from 92.4 fmol/mg tissue under fasted conditions to 37.8 fmol/mg tissue under refed conditions (Figure 4P). Therefore, feeding not only results in reduced expression of A1R at the mRNA level, but also reduced membrane receptor density.
3.7. Insulin regulates A1R transcription in adipocytes via the PI3K-Akt pathway
To further investigate the signaling mechanisms which regulate A1R transcription in response to feeding, we used 3T3-L1 adipocytes with an overnight serum starve to approximate adipocytes under fasted conditions. A 4-hour treatment with 10% fetal bovine serum (FBS) to simulate feeding had little effect on A1R transcription (Figure 5A). However, in adipocytes insulin is perhaps the most important anabolic hormone and the amount of insulin present in media containing 10% FBS is less than 10 pmol/L according to the certificate of analysis provided for the lot number used. The introduction of additional insulin into the media in the presence or absence of serum produced a significant decrease in A1R mRNA (Figure 5A). This effect was dose-dependent, with an EC50 well within the physiological range at ∼0.15 nmol/L (∼25 μU/mL). This effect was also rapid, with near maximal reduction in mRNA within 2 h and sustained for at least 8 h in the presence of insulin (Figure 5B). We further investigated the effects of insulin on A1R mRNA within brown adipocytes using an immortalized brown preadipocyte cell line (iBACs) differentiated into brown adipocytes. We observed a similar dose-responsive effect of insulin on A1R mRNA in brown adipocytes (Fig. S7A). Importantly, iBACs are maintained in media containing 20 nM insulin. When we compared overnight serum starved iBACs with cells in maintenance media, we observed a trend towards increased A1R mRNA in the serum starved cells (Fig. S7B).
Figure 5.
The insulin-Akt-FOXO1 pathway acutely regulates A1R expression to modulate adenosine signaling in adipocytes. (A–F) 3T3-L1 adipocytes 10–12 days after differentiation were serum starved overnight and treated with insulin as indicated for either 4 h (mRNA analysis) or 15 min (phospho-protein western blot analysis). (A) Dose response of insulin effects on A1R expression in the presence or absence of 10% FBS. N = 5–6. (B) Time course for the suppression of A1R expression by 10 nmol/L insulin. N = 3–4. (C) Effect of vehicle (white) or 1 nmol/L insulin (red) on A1R mRNA expression, phospho-MAPK (T202/Y204), and phospho-Akt (S473) after 15-minute pre-treatment with vehicle (Veh), 50 μmol/L LY294002 (LY), or 25 μmol/L PD98059 (PD). (D) Effect of 1 nmol/L insulin on A1R mRNA expression, phospho-GSK3α/β (S21/S9), and phospho-P70 S6K (T389) after 15-minute pre-treatment with vehicle (Veh), 25 μmol/L MK-2206 (MK), or 250 nmol/L rapamycin (Rapa). (E) Effect of 1 nmol/L insulin on A1R mRNA expression and phospho-GYS (S641) after 15-minute pre-treatment with vehicle (Veh), 1.4 μmol/L GSK3 inhibitor IX (GSK3i), or 30 mmol/L LiCl. (F) Effect of 1 nmol/L insulin on A1R mRNA expression and nuclear localization of FOXO1 after 15-minute pre-treatment with vehicle (Veh) or 1 μmol/L AS-1842856 (AS). (G) Fold-enrichment by ChIP with FOXO1 over normal rabbit IgG of A1R promoter region (A1R) or 28S rDNA control region (28S) after 4-hour treatment of 3T3-L1 adipocytes with vehicle (white) or 1 μmol/L AS1842856 (blue). (H) NEFA released into the media by isolated adipocytes that were treated with vehicle or 1 μmol/L AS-1842856 for 4 h, followed by vehicle or 100 nmol/L CCPA for 15 min in the presence of adenosine deaminase and a subsequent 30-minute treatment with vehicle or 30 nmol/L isoproterenol. Error bars represent SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, one-way or two-way ANOVA.
We next sought to determine the signaling mechanisms downstream of insulin that are responsible for A1R downregulation. The insulin receptor triggers several signaling pathways, but can be simplified to two immediate primary effectors: phosphoinositide 3-kinase (PI3K) and mitogen activated protein kinase kinase (MAP2K). To interrogate these pathways, we utilized the pharmacological inhibitors LY294002 and PD98059 to inhibit PI3K and MAP2K respectively. While a 4-hour treatment with insulin alone suppressed A1R transcription and strongly activated the PI3K pathway as seen with phosphorylation of Akt at S473, a 30-minute pretreatment with LY294002 prevented both effects (Figure 5C). Meanwhile, inhibition of MAP2K with PD98059 significantly blunted insulin-induced phosphorylation of P42/44 MAPK at T202/Y204, but did not prevent the suppression of A1R transcription.
Downstream of PI3K, the protein kinase Akt is an important intermediary effector that phosphorylates proteins important for insulin signaling in adipocytes, such as mTORC1 and GSK3β. MK-2206 is a specific inhibitor of Akt1/2/3 which was able to prevent both insulin-induced phosphorylation of GSK3α/β at S21/9 and suppression of A1R (Figure 5D). Treatment with rapamycin, a specific inhibitor of mTORC1, blocked phosphorylation of the downstream target P70 S6 kinase (S6K) at T389, however there was no impact on the suppression of A1R by insulin (Figure 5D). We also inhibited the action of GSK3 with the GSK3 inhibitor IX, and with LiCl. Both treatments significantly impaired the phosphorylation of glycogen synthase (GYS) at S641, but neither had any impact on the suppression of A1R (Figure 5E). Thus, A1R transcription is suppressed by insulin via the PI3K-Akt pathway but is not dependent on mTORC1 or GSK3β.
3.8. A1R transcription is regulated by FOXO1
The forkhead box proteins within the FOXO family are transcription factors with known sensitivity to nutrient status. FOXO1 is highly expressed in insulin sensitive tissues such as adipose and liver, and contains 3 known phosphorylation sites that are targets of Akt [52]. Phosphorylation of FOXO1 by Akt inhibits its transcriptional activity by promoting the interaction of FOXO1 with 14-3-3 proteins and retention within the cytoplasm [53]. Further, both FOXO1 and A1R are involved in adipocyte differentiation and adipogenesis and show a similar pattern of upregulation as adipocyte differentiation progresses [10,54]. We therefore hypothesized that FOXO1 may be directly regulating A1R transcription in adipocytes. To explore this hypothesis, we utilized the pharmacological inhibitor AS-1842856 which competitively inhibits the DNA binding of FOXO1 [55]. Treatment of 3T3-L1 adipocytes with insulin both suppressed A1R transcription and induced the translocation of FOXO1 out of the nucleus as determined by western blot of isolated nuclei (Figure 5F). Treatment with AS-1842856 alone resulted in the suppression of A1R transcription to a similar level as insulin treatment but did not affect FOXO1 localization. Finally, treatment with both insulin and AS-1842856 did not result in any further suppression of A1R, suggesting that insulin and AS-1842856 suppress A1R transcription through a similar mechanism.
We next wanted to confirm that FOXO1 directly binds to the promoter of A1R to regulate its transcription. We used the LASAGNA-search 2.0 tool to identify sites containing the consensus motif for FOXO1 binding within the A1R promoter [56]. We identified a region within the first intron of the A1R gene locus containing 4 consensus motifs within a span of 50 bp. We next used chromatin immunoprecipitation (ChIP) to enrich for FOXO1 binding sites, followed by qPCR and found this region within the A1R locus was significantly enriched compared with a normal IgG control ChIP under vehicle treated conditions but not in the presence of AS-1842856 (Figure 5G). There was no enrichment in the control region within 28S rDNA under either condition.
3.9. Inhibition of FOXO1 in adipocytes induces A1R insensitivity
Thus far, we have shown that feeding suppresses A1R transcription in adipocytes, and that insulin is capable of suppressing A1R transcription through the phosphorylation and inactivation of FOXO1. We therefore wondered what the effects of direct FOXO1 inhibition would be on adipocyte lipolysis and A1R sensitivity. We isolated adipocytes from the gonadal fat pads of overnight fasted male C57BL/6J mice and treated them with either vehicle or the FOXO1 inhibitor AS-1842856 for 4 h. We then treated the cells with vehicle or the A1R agonist CCPA in the presence of adenosine deaminase for 15 min followed by a 30-minute treatment with vehicle or isoproterenol and measured NEFA release into the media. In the absence of FOXO1 inhibition, isoproterenol strongly increased the concentration of NEFA within the media and the addition of CCPA suppressed NEFA back to control levels as expected (Figure 5H). While the inhibition of FOXO1 alone had no effect on NEFA release (not shown), the addition of isoproterenol in these adipocytes resulted in a significantly higher induction in lipolysis which CCPA was not able to suppress (Figure 5H). Taken together, these data demonstrate that A1R transcription is acutely regulated via the insulin-Akt-FOXO1 signaling axis within adipocytes, and that insulin stimulation results in a rapid downregulation of A1R transcription and reduced sensitivity to A1R signaling.
3.10. Obesity disrupts A1R regulation of lipolysis in both the fasted and refed state
Changes in adenosine signaling have been previously implicated in the development of insulin resistance in obesity. Multiple groups have demonstrated that obesity correlates with increased adenosine levels in human adipose tissue, yet reduced expression of A1R in adipose tissue in humans [17,57]. We wanted to know how obesity impacts not only A1R signaling in adipocytes, but also the regulation of A1R expression by feeding. We first placed C57BL/6J mice on either a normal chow or high fat diet (HFD) for 12 weeks, after which normal chow mice weighed on average 29.4 g ± 1.7 g and HFD mice weighed 46.5 g ± 3.2 g. We then compared A1R mRNA expression in isolated adipocytes from overnight fasted mice and confirmed that HFD also results in significantly reduced expression of A1R mRNA in mice within gWAT and trended towards decreased in BAT but did not reach statistical significance. (Figure 6A,B). When A1R mRNA expression between fasted and refed HFD mice is compared, we found that feeding no longer suppressed A1R transcription in gWAT. The suppression of A1R within BAT was retained, albeit with a reduced effect size (Figure 6C,D).
Figure 6.
High-fat diet downregulates and desensitizes A1R in mouse adipocytes and disrupts regulation of A1R expression by feeding. (A and B) mRNA expression of A1R in male wildtype C57BL/6J mice fed either normal chow control diet (Chow) or high-fat diet (HFD) for 12 weeks following an overnight fast in gonadal white adipocytes (A) and BAT (B). (C and D) mRNA expression of A1R in male wildtype C57BL/6J mice fed high-fat diet for 12 weeks following either an overnight fast or a 4-hour refeeding period in gonadal white adipocytes (C) and BAT (D). (E–G) Male wildtype C57BL/6J mice were fed high-fat diet for 12 weeks before experimentation. (E) Serum NEFA in 6-hour fasted mice treated with 0.1 mg/kg DPCPX for 30 min both before (control) and 15 min after (iso) i.p. injection with 10 mg/kg isoproterenol. (F) Same as in (E) for mice that were fasted overnight and refed for 4 h. (G and H) Same as in (E) and (F) except mice were treated with 0.5 mg/kg CCPA for 30 min before isoproterenol injection. Error bars represent SD. ∗∗∗p < 0.001, t-test.
We next repeated the experiments using the A1R antagonist DPCPX alongside isoproterenol stimulation of lipolysis with mice on HFD. We found that DPCPX did not result in an increased lipolytic response to isoproterenol under either fasted or refed conditions in these mice (Figure 6C,D). Further, administration of the A1R agonist CCPA was also unable to suppress lipolysis under either fasted or refed conditions in HFD mice (Figure 6E,F). These results demonstrate that, in addition to suppressing A1R transcription, obesity disrupts the regulation of A1R transcription by fasting and feeding and the regulation of lipolysis by A1R signaling.
4. Discussion
In this study, we investigated the role of the A1 adenosine receptor in adipose tissue in the regulation of lipid and glucose metabolism. Our findings agree with previous studies of isolated white adipocytes and show that A1R augments insulin suppression of lipolysis and limits the lipolytic response to adrenergic stress in vivo. Further, this study agrees with findings from mice with A1R overexpression using the aP2 (FABP4) promoter [58], and demonstrates that A1R action in adipose tissue serves to protect mice from obesity-induced glucose intolerance. Moreover, we found that loss of adipose A1R only resulted in a higher lipolytic response to stress under fasted conditions. This led to the key finding of this study that elevated insulin in the fed state rapidly downregulates and desensitizes A1R signaling specifically in adipocytes, and this reduction is mediated by the transcription factor FOXO1.
The regulation of A1R by FOXO1 initially seemed counterintuitive since FOXO1 drives high expression of A1R in the fasted state when conditions are primarily catabolic, while signaling through A1R is primarily anabolic. However, this view of A1R in steady state misses a critical component of this regulation, which is the lag between stimulation by insulin and an observed decrease in A1R mRNA. Considering the data presented in this study, we propose two distinct roles for A1R signaling in adipocytes which are not mutually exclusive. First, we propose that active FOXO1 drives high A1R expression in the fasted state to limit deleterious effects such as lipotoxicity resulting from the overstimulation of lipolysis by catabolic signals (Figure 7, top left). Second, we propose that during the anabolic state A1R promotes insulin action to suppress lipolysis (Figure 7, top right), but a postprandial decline in A1R levels mediated through reduced FOXO1 transcriptional activity leads to desensitization of adipocytes due to reduced signaling via A1R (Figure 7, bottom right). This desensitization to A1R may facilitate the reentrance of adipocytes into the catabolic state by removing an inhibitor of lipolysis (Figure 7, bottom left). Homeostasis posits that an organism responds to changing conditions to maintain the internal milieu constant, while allostasis suggests that organisms alter the internal milieu in order to meet anticipated demands through neuronal mechanisms [59,60]. It has been suggested that adenosine plays a more significant role in allostasis than in homeostasis, and the decline in A1R expression appears to be an allostatic adaptation to the predicted eventual shift from anabolism to catabolism [61].
Figure 7.
Proposed mechanism for FOXO1 modulation of adenosine signaling in adipocytes during fasting and feeding. Under fasted conditions, active FOXO1 drives high expression of A1R which serves to limit lipolysis stimulated by catecholamines during stress. Upon feeding, catecholamine levels decrease while insulin levels increase. Insulin acts to suppress lipolysis and induces the phosphorylation and inactivation of FOXO1, decreasing transcription of A1R. Initially, A1R expression persists at high levels and signaling through A1R augments the suppression of lipolysis by insulin. High insulin levels and inactive FOXO1 under fed conditions eventually lead to reduced A1R expression and the removal of the inhibitory effect of A1R on lipolysis. Reduced nutrient levels brought on by fasting lead to decreased insulin and increased catecholamines. Lower expression of A1R facilitates the initial induction of lipolysis by catecholamines upon fasting. Decreased insulin levels lead to active FOXO1 which begins to increase A1R to reengage its limitation on lipolysis in the fasted state.
The role of A1R in white adipocytes in vitro has been well characterized by many groups who have demonstrated that adenosine enhances insulin-stimulated glucose uptake and lipogenesis and suppresses basal and stimulated lipolysis within adipocytes [[11], [12], [13],25,28,62]. Investigating the role of adipose A1R in vivo, however, has been challenging as A1R is also involved in the regulation of numerous other tissues such as the brain, heart, skeletal muscle, and pancreatic islet cells. This has led to conflicting effects on glucose metabolism in whole-body knockouts of A1R, that have been shown to display impaired glucose tolerance [22], improved glucose tolerance [20], and no change to glucose tolerance [23]. Because all three groups saw changes in body weight and the secretion of glucagon and insulin, the role of adipose tissue A1R in these contexts has remained unclear. Dong and colleagues provided good evidence that adipose A1R activation improves glucose tolerance and insulin sensitivity by overexpressing A1R using the aP2 promoter [58]. These mice did not display the previously seen changes in body weight or insulin, however overexpression clearly enhanced the tonic activity of A1R resulting in suppression of basal lipolysis and reduced serum NEFA levels.
We now show that endogenous A1R in adipose also promotes glucose tolerance and insulin sensitivity. Our studies show that mice with an inducible, adipocyte-specific knockout of A1R are similarly devoid of changes in body weight and insulin and glucagon secretion, while also lacking any changes to basal fasted and fed serum NEFA levels. Instead, we saw that loss of adipose A1R impairs the kinetics of insulin-mediated suppression of lipolysis rather than the final homeostatic set points. FAdora1−/− mice also developed glucose intolerance when placed on HFD, without changes to insulin secretion or sensitivity. The suppression of hepatic glucose production by insulin has been shown to be dependent on the appropriate suppression of adipocyte lipolysis [63,64]. Therefore, it is likely this glucose intolerance is driven by increased endogenous glucose production within FAdora1−/− mice, consistent with their impaired ability to reduce serum NEFA in response to insulin. It is worth noting that while we did not see increased insulin secretion during an i.p. glucose tolerance test, chow fed FAdora1−/− male mice did display significantly increased insulin levels under refed conditions. It is possible that this increase in insulin can account for reduced suppression of NEFA and maintained glucose tolerance under chow conditions. Following HFD feeding however, refed insulin levels are higher in both WT and FAdora1−/− male mice relative to chow conditions, but not different from each other. After HFD, it is likely that FAdora1−/− mice cannot further increase insulin secretion to compensate for the loss of adipose A1R resulting in glucose intolerance.
Adenosine levels are increased in response to adrenergic stress, and adenosine action on A1R suppresses adrenergic stimulation of lipolysis in adipocytes [26,29,62]. A1R agonists have been clearly demonstrated to suppress basal and ß-adrenergic-stimulated lipolysis in vivo, however these experiments were performed in fasted subjects, even in human patients, as is typical for most metabolic studies [[14], [15], [16],65]. While we found that both genetic loss of adipose A1R and general antagonism of A1R resulted in a stronger lipolytic response to adrenergic stimuli in fasted conditions, this effect was not present in refed mice. We did not measure the effects of feeding on interstitial or local plasma adenosine levels, however the desensitization of adipose A1R under refed conditions was independent of any potential reduction in adenosine as lipolysis in WT refed mice was also unaffected by a high dose (0.5 mg/kg) of the full A1R agonist CCPA. It is also unlikely that alternative pathways influencing cAMP levels, such as insulin activation of phosphodiesterases, are confounding our results, as isoproterenol still produced a clear induction in lipolysis within refed mice which was not suppressed by A1R agonism. We did not, however, probe the effects of feeding on Gαi proteins, and it may also be interesting to investigate the sensitivity of adipocytes to other antilipolytic agents, such as nicotinic acid or prostaglandin E2, under refed conditions.
We found that the loss in adipose A1R sensitivity following refeeding can at least partially be explained by acute downregulation of A1R mRNA. We would like to note, however, that the reductions in mRNA measured in vivo may not be large enough to account for the full loss of A1R sensitivity observed, and other mechanisms such as post-translational modifications or changes in Gαi proteins may be involved, particularly within subcutaneous white adipose depots. Still, a significant reduction in A1R mRNA within gonadal and brown adipocytes was mediated by the insulin-Akt-FOXO1 signaling axis which correlated with a substantial decrease in A1R density, and this relationship between FOXO1 and A1R may explain a central shift in adenosine receptors that occurs during adipogenesis. Gharibi et al. demonstrated that A1R promotes adipogenesis, and that A1R expression is dramatically increased several days into the adipocyte differentiation process before falling to a level that is still significantly elevated over preadipocytes [10]. Interestingly, Nakae and colleagues demonstrated that FOXO1 also promotes adipogenesis, and FOXO1 expression follows a strikingly similar pattern and timing during adipocyte differentiation [54]. It follows that increased expression of A1R during the differentiation process is driven by FOXO1, although further work is needed to demonstrate this mechanism.
The regulation of A1R by insulin was apparent in both gonadal white and in brown adipocytes, but not inguinal white adipocytes. While there are many known differences between the gWAT and iWAT depots, it remains unclear what would cause the FOXO1 regulation of A1R to be present within gWAT but not iWAT and further investigation is needed. Regardless, the control of A1R expression by FOXO1 opens several interesting questions about the role of A1R in metabolism within both gWAT and BAT depots. In white adipocytes, hypoxia has been shown to induce adipocyte lipolysis through the production of reactive oxygen species and elevate serum NEFA [66]. At the same time, hypoxia also elevates extracellular adenosine levels through multiple mechanisms, including the upregulation of CD39 and CD73, as well as transcriptional repression of equilibrative nucleoside transporters via HIF-1α [67] that may elevate extracellular adenosine by impeding its reuptake. Intriguingly, FOXO1 is also a transcriptional target of HIF-1α which becomes upregulated during hypoxia and may serve as an indirect mechanism to upregulate adipose A1R as a protective mechanism to limit lipolysis [68].
In brown adipocytes, Gnad et al. demonstrated that adenosine acted primarily through A2A adenosine receptors to activate thermogenesis, and that brown adipocytes were significantly less responsive to A1R compared with white adipocytes [7,8]. In a more recent paper, they demonstrated that A2B adenosine receptors were also involved in activating brown adipocyte thermogenesis [8]. It is worth noting, however, that brown adipocytes are typically cultured in high levels of insulin (20 nM–750 nM), and we found that an overnight serum starve of brown adipocytes significantly increases the expression of A1R. Postprandial thermogenesis is a well-documented phenomenon in mice and humans where body temperature is elevated following feeding dependent on changes in both skeletal muscle and adipose tissues. Activation of A1R, either centrally or with global administration of A1R agonists, is known to cause hypothermia [69,70]. While a principal component of A1R signaling-induced hypothermia is mediated centrally, it is possible that additional hypothermia may occur through the activation of A1R in brown adipocytes. The downregulation of A1R by insulin in brown adipocytes may therefore be one mechanism by which postprandial thermogenesis is mediated, though a more thorough analysis of thermogenesis within FAdora1−/− mice or a more specific UCP1-induced deletion of A1R is needed to assess this hypothesis.
Obesity has been shown to reduce responsiveness to inhibitors of cyclic AMP accumulation such as adenosine [57]. This has been associated with reduced expression of A1R in adipocytes isolated from obese human patients as compared to lean patients [17,57]. We observed that A1R expression is also reduced in adipocytes isolated from obese mice and confirmed that this leads to a lack of A1R sensitivity in obese mice under both fasted and refed conditions. It is possible that both reduced A1R expression and the loss of cyclical fluctuations in A1R expression between fasting and feeding may contribute to insulin resistance and glucose intolerance.
In summary, adipose A1R is acutely regulated by feeding to modulate the adenosine response and allow adipose tissue to adequately adapt to changing nutrient conditions. High expression of adipose A1R under fasted conditions is necessary to limit adrenergic-driven lipolysis and enhance insulin-mediated suppression of lipolysis. It is possible that the downregulation of A1R under fed conditions is also necessary to enable the appropriate transition of adipose back into the catabolic state once nutrient levels drop.
Funding
M.E.G. was funded by the NIH (5T32 GM008715) and by the American Heart Association (20PRE35210847). M.C.L. was funded by the NIH (5T32 GM007055). I.M.B. was funded by the NIH (R01 DK121059). Work in the lab of T.E.H. was funded by the NIH (R01 GM136900 and R01 DK101946).
Author contributions
M.E.G., S.R.H., and D.S.L. assessed knockout of A1R. M.E.G. and S.R.H. performed metabolic and lipolytic characterization of mice and assessed differences in fasting and refed conditions. S.R.H. performed metabolic caging studies. M.A.L. performed all imaging and histological assessment of adipose tissues. M.E.G., M.C.L., and D.S.L. performed 3T3-L1 studies. D.S.L. performed studies with iBACs. M.E.G., S.R.H. and T.E.H. performed isolated adipocyte studies. I.M.B. provided significant conceptual and technical input on ChIP experiments. T.E.H., J.L., and M.E.G. conceived the study and designed the experiments. M.E.G. and T.E.H. wrote the manuscript.
Acknowledgments
The authors would like to thank Dr. Stanislav Zakharenko for sharing the floxed Adora1 mice with us. We also thank Dr. Ira G. Schulman and Dr. Mohan Manjegowda for their input and resources in chromatin immunoprecipitation experiments. We would like to acknowledge the University of Virginia Research Histology Core for embedding, sectioning, and staining of all adipose tissues. Immortalized brown preadipocytes were kindly shared with us by the lab of Dr. Norbert Leitinger, and with permission of the originating lab of Dr. Bruce M Spiegelman. This data has been previously presented at the Mid-Atlantic Diabetes and Obesity Research Symposium and published on BioRxiv. Figure 7 was made with Biorender.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2022.101543.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Petersen M.C., Shulman G.I. Mechanisms of insulin action and insulin resistance. Physiological Reviews. 2018;98(4):2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duncan R.E., Ahmadian M., Jaworski K., Sarkadi-Nagy E., Sul H.S. Regulation of lipolysis in adipocytes. Annual Review of Nutrition. 2007;27(1):79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eltzschig H.K. Extracellular adenosine signaling in molecular medicine. Journal of Molecular Medicine. 2013;91(2):141–146. doi: 10.1007/s00109-013-0999-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koeppen M., Eckle T., Eltzschig H.K. Selective deletion of the A1 adenosine receptor abolishes heart-rate slowing effects of intravascular adenosine in vivo. PLoS One. 2009;4(8) doi: 10.1371/journal.pone.0006784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredholm B.B., IJzerman A.P., Jacobson K.A., Linden J., Müller C.E. International union of basic and clinical pharmacology. LXXXI. Nomenclature and classification of adenosine receptors - an update. Pharmacological Reviews. 2011;63(1):1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green A. Adenosine receptor down-regulation and insulin resistance following prolonged incubation of adipocytes with an A1 adenosine receptor agonist. Journal of Biological Chemistry. 1987;262(32):15702–15707. doi: 10.1016/s0021-9258(18)47784-8. [DOI] [PubMed] [Google Scholar]
- 7.Gnad T., Scheibler S., Kugelgen I. Von., Scheele C., Kilic A., Glode A., et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature. 2014;516(7531):395–399. doi: 10.1038/nature13816. [DOI] [PubMed] [Google Scholar]
- 8.Gnad T., Navarro G., Lahesmaa M., Reverte-Salisa L., Copperi F., Cordomi A., et al. Adenosine/A2B receptor signaling ameliorates the effects of aging and counteracts obesity. Cell Metabolism. 2020;32(1):56–70. doi: 10.1016/j.cmet.2020.06.006. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lahesmaa M., Oikonen V., Helin S., Luoto P., U Din M., Pfeifer A., et al. Regulation of human brown adipose tissue by adenosine and A2A receptors – studies with [15-O]H2O and [11C]TMSX PET/CT. European Journal of Nuclear Medicine and Molecular Imaging. 2019;46(3):743–750. doi: 10.1007/s00259-018-4120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gharibi B., Abraham A.A., Ham J., Evans B.A.J. Contrasting effects of A1 and A2b adenosine receptors on adipogenesis. International Journal of Obesity. 2012;36(3):397–406. doi: 10.1038/ijo.2011.129. [DOI] [PubMed] [Google Scholar]
- 11.Joost H.-G., Steinfelder H.-J. Modulation of insulin sensitivity by adenosine. Effects on glucose transport, lipid synthesis, and insulin receptors of the adipocyte. Molecular Pharmacology. 1982;22:614–618. [PubMed] [Google Scholar]
- 12.Heseltine L., Webster J.M., Taylor R. Adenosine effects upon insulin action on lipolysis and glucose transport in human adipocytes. Molecular and Cellular Biochemistry. 1995;144(2):147–151. doi: 10.1007/BF00944394. [DOI] [PubMed] [Google Scholar]
- 13.Vannucci S.J., Nishimura H., Satoh S., Cushman S.W., Holman G.D., Simpson I.A. Cell surface accessibility of GLUT4 glucose transporters in insulin-stimulated rat adipose cells. Modulation by isoprenaline and adenosine. Biochemical Journal. 1992;288(1):325–330. doi: 10.1042/bj2880325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoelch C., Kuhlmann J., Gossel M., Mueller G., Neumann-Haefelin C., Belz U., et al. Characterization of adenosine-A1 receptor-mediated antilipolysis in rats by tissue microdialysis, 1H-spectroscopy, and glucose clamp studies. Diabetes. 2004;53(7):1920–1926. doi: 10.2337/diabetes.53.7.1920. [DOI] [PubMed] [Google Scholar]
- 15.Dhalla A.K., Santikul M., Chisholm J.M., Belardinelli L., Reaven G.M. Comparison of the antilipolytic effects of an A1 adenosine receptor partial agonist in normal and diabetic rats. Diabetes, Obesity and Metabolism. 2009;11(2):95–101. doi: 10.1111/j.1463-1326.2008.00902.x. [DOI] [PubMed] [Google Scholar]
- 16.Staehr P., Dhalla A., Zack J., Wang X., Ho Y., Bingham J., et al. Reduction of free fatty acids, safety, and pharmacokinetics of oral GS-9667, an A(1) adenosine receptor partial agonist. Journal of Clinical Investigation. 2013;53(4):385–392. doi: 10.1002/jcph.9. [DOI] [PubMed] [Google Scholar]
- 17.Kaartinen J.M., Hreniuk S.P., Martin L.F., Ranta S., LaNoue K.F., Ohisalo J.J. Attenuated adenosine-sensitivity and decreased adenosine-receptor number in adipocyte plasma membranes in human obesity. Biochemical Journal. 1991;279(1):17–22. doi: 10.1042/bj2790017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green, A., Johnson, J.L., DiPette, D.J., 1990. Decrease in A1 adenosine receptors in adipocytes from spontaneously hypertensive rats. Metabolism 39(12): 1334–1338, Doi: 10.1016/0026-0495(90)90193-G. [DOI] [PubMed]
- 19.Xiao C., Liu N., Jacobson K.A., Gavrilova O., Reitman M.L. Physiology and effects of nucleosides in mice lacking all four adenosine receptors. PLoS Biology. 2019;17(3):1–26. doi: 10.1371/journal.pbio.3000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang G.K., Fredholm B.B., Kieffer T.J., Kwok Y.N. Improved blood glucose disposal and altered insulin secretion patterns in adenosine A 1 receptor knockout mice. American Journal of Physiology - Endocrinology and Metabolism. 2012;303(2):180–190. doi: 10.1152/ajpendo.00050.2012. [DOI] [PubMed] [Google Scholar]
- 21.Johansson S.M., Lindgren E., Yang J.N., Herling A.W., Fredholm B.B. Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue - interactions with insulin. European Journal of Pharmacology. 2008;597:92–101. doi: 10.1016/j.ejphar.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Faulhaber-Walter R., Jou W., Mizel D., Li L., Zhang J., Kim S.M., et al. Impaired glucose tolerance in the absence of adenosine A1 receptor signaling. Diabetes. 2011;60(10):2578–2587. doi: 10.2337/db11-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson S.M., Salehi A., Sandström M.E., Westerblad H., Lundquist I., Carlsson P.O., et al. A1 receptor deficiency causes increased insulin and glucagon secretion in mice. Biochemical Pharmacology. 2007;74(11):1628–1635. doi: 10.1016/j.bcp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Lonnroth P., Jansson P.A., Fredholm B.B., Smith U. Microdialysis of intercellular adenosine concentration in subcutaneous tissue in humans. American Journal of Physiology - Endocrinology and Metabolism. 1989;256(2) doi: 10.1152/ajpendo.1989.256.2.e250. [DOI] [PubMed] [Google Scholar]
- 25.Liang H.X., Belardinelli L., Ozeck M.J., Shryock J.C. Tonic activity of the rat adipocyte A1-adenosine receptor. British Journal of Pharmacology. 2002;135(6):1457–1466. doi: 10.1038/sj.bjp.0704586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szkudelski T., Szkudelska K., Nogowski L. Effects of adenosine A1 receptor antagonism on lipogenesis and lipolysis in isolated rat adipocytes. Physiological Research. 2009;58(6):863–871. doi: 10.33549/physiolres.931467. [DOI] [PubMed] [Google Scholar]
- 27.Schwabe U., Ebert R., Erbler H. Adenosine release from isolated fat cells and its significance for the effects of hormones on cyclic 3’,5’-AMP levels and lipolysis. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1973;276:133–148. doi: 10.1007/BF00501186. [DOI] [PubMed] [Google Scholar]
- 28.Fain J.N., Wieser P.B. Effects of adenosine deaminase on cyclic adenosine monophosphate accumulation, lipolysis, and glucose metabolism of fat cells. Journal of Biological Chemistry. 1975;250(3):1027–1034. doi: 10.1016/s0021-9258(19)41887-5. [DOI] [PubMed] [Google Scholar]
- 29.Fredholm B.B., Sollevi A. The release of adenosine and inosine from canine subcutaneous adipose tissue by nerve stimulation and noradrenaline. The Journal of Physiology. 1981;313(1):351–367. doi: 10.1113/jphysiol.1981.sp013670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newby A.C. Adenosine and the concept of “retaliatory metabolites. Trends in Biochemical Sciences. 1984;9(2):42–44. doi: 10.1016/0968-0004(84)90176-2. [DOI] [Google Scholar]
- 31.Eltzschig H.K., Weissmüller T., Mager A., Eckle T. In: Methods in molecular Biology. 341st ed. Colgan S.P., editor. Humana Press; Totowa, NJ: 2006. Cell-cell interactions; pp. 73–87. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman B.B., Chang H., Farahbakhsh Z., Reaven G. Inhibition of lipolysis by adenosine is potentiated with age. Journal of Clinical Investigation. 1984;74(5):1750–1755. doi: 10.1172/JCI111593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raje V., Ahern K.W., Martinez B.A., Howell N.L., Oenarto V., Granade M.E., et al. Adipocyte lipolysis drives acute stress-induced insulin resistance. Scientific Reports. 2020;10(1):18166. doi: 10.1038/s41598-020-75321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeFronzo R.A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53(7):1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blundon J.A., Roy N.C., Teubner B.J.W., Yu J., Eom T.Y., Sample K.J., et al. Restoring auditory cortex plasticity in adult mice by restricting thalamic adenosine signaling. Science. 2017;356(6345):1352–1356. doi: 10.1126/science.aaf4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullins G.R., Wang L., Raje V., Sherwood S.G., Grande R.C., Boroda S., et al. Catecholamine-induced lipolysis causes mTOR complex dissociation and inhibits glucose uptake in adipocytes. Proceedings of the National Academy of Sciences. 2014;111(49):17450–17455. doi: 10.1073/pnas.1410530111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar A., Lawrence J.C., Jung D.Y., Ko H.J., Keller S.R., Kim J.K., et al. Fat Cell – specific Ablation of Rictor in Mice Impairs insulin-regulated fat cell and whole-body glucose and lipid metabolism. Diabetes. 2010;59(6):1397–1406. doi: 10.2337/db09-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X., Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Research. 2003;31(24) doi: 10.1093/nar/gng154. e154–e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spandidos A., Wang X., Wang H., Dragnev S., Thurber T., Seed B. A comprehensive collection of experimentally validated primers for Polymerase Chain Reaction quantitation of murine transcript abundance. BMC Genomics. 2008;9(1):1–17. doi: 10.1186/1471-2164-9-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spandidos A., Wang X., Wang H., Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Research. 2009;38(SUPPL.1):D792–D799. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B., Remm M., et al. Primer3--new capabilities and interfaces. Nucleic Acids Research. 2012;40(15):e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koressaar T., Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics. 2007;23(10):1289–1291. doi: 10.1093/bioinformatics/btm091. [DOI] [PubMed] [Google Scholar]
- 43.Cooper M.P., Uldry M., Kajimura S., Arany Z., Spiegelman B.M. Modulation of PGC-1 coactivator pathways in brown fat differentiation through LRP130. Journal of Biological Chemistry. 2008;283(46):31960–31967. doi: 10.1074/jbc.M805431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Senthivinayagam S., Serbulea V., Upchurch C.M., Polanowska-Grabowska R., Mendu S.K., Sahu S., et al. Adaptive thermogenesis in brown adipose tissue involves activation of pannexin-1 channels. Molecular Metabolism. 2021;44(November 2020):101130. doi: 10.1016/j.molmet.2020.101130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitton H., Singh L.N., Patrick M.A., Price A.J., Osorio F.G., López-Otín C., et al. Changes at the nuclear lamina alter binding of pioneer factor Foxa2 in aged liver. Aging Cell. 2018;17(3) doi: 10.1111/acel.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lohse M.J., Klotz K., Lindenborn-fotinos J., Reddington M., Schwabe U., Olsson R.A. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) - a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1987;336:204–210. doi: 10.1007/BF00165806. [DOI] [PubMed] [Google Scholar]
- 47.McKeel D., Jarett L. Preparation and characterization of a plasma membrane fraction from isolated fat cells. Journal of Cell Biology. 1970;44:417–432. doi: 10.1083/jcb.44.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johansson B., Halldner L., Dunwiddie T.V., Masino S.A., Poelchen W., Giménez-Llort L., et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(16):9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albrecht-Küpper B.E., Leineweber K., Nell P.G. Partial adenosine A1 receptor agonists for cardiovascular therapies. Purinergic Signalling. 2012;8(SUPPL. 1):91–99. doi: 10.1007/s11302-011-9274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vannucci S.J., Klim C.M., Martin L.F., LaNoue F. A1-adenosine receptor-mediated inhibition of adipocyte adenylate cyclase and lipolysis in Zucker rats. American Journal of Physiology - Endocrinology and Metabolism. 1989;257(20):E871–E878. doi: 10.1152/ajpendo.1989.257.6.E871. [DOI] [PubMed] [Google Scholar]
- 51.Antonioli L., Pacher P., Haskó G. Adenosine and inflammation: it's time to (re)solve the problem. Trends in Pharmacological Sciences. 2022;43(1):43–55. doi: 10.1016/j.tips.2021.10.010. [DOI] [PubMed] [Google Scholar]
- 52.Cheng Z., White M.F. Targeting forkhead Box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxidants and Redox Signaling. 2011;14(4):649–661. doi: 10.1089/ars.2010.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X., Gan L., Pan H., Guo S., He X., Olson S.T., et al. Phosphorylation of serine 256 suppresses transactivation by FKHR (FOXO1) by multiple mechanisms: direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. Journal of Biological Chemistry. 2002;277(47):45276–45284. doi: 10.1074/jbc.M208063200. [DOI] [PubMed] [Google Scholar]
- 54.Nakae J., Kitamura T., Kitamura Y., Biggs W.H., Arden K.C., Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Developmental Cell. 2003;4(1):119–129. doi: 10.1016/S1534-5807(02)00401-X. [DOI] [PubMed] [Google Scholar]
- 55.Nagashima T., Shigematsu N., Maruki R., Urano Y., Tanaka H., Shimaya A., et al. Discovery of novel forkhead box O1 inhibitors for treating type 2 diabetes: improvement of fasting glycemia in diabetic db/db mice. Molecular Pharmacology. 2010;78(5):961–970. doi: 10.1124/mol.110.065714. [DOI] [PubMed] [Google Scholar]
- 56.Lee C., Huang C.H. LASAGNA-search: an integrated web tool for transcription factor binding site search and visualization. Biotechniques. 2013;54(3):141–153. doi: 10.2144/000113999. [DOI] [PubMed] [Google Scholar]
- 57.Ohisalo J.J., Ranta S., Huhtaniemi I.T. Attenuated adenosine R-site effect in adipocytes in obesity. Metabolism. 1986;35(2):143–146. doi: 10.1016/0026-0495(86)90115-0. [DOI] [PubMed] [Google Scholar]
- 58.Dong Q., Ginsberg H.N., Erlanger B.F. Overexpression of the A1 adenosine receptor in adipose tissue protects mice from obesity-related insulin resistance. Diabetes, Obesity and Metabolism. 2001;3(5):360–366. doi: 10.1046/j.1463-1326.2001.00158.x. [DOI] [PubMed] [Google Scholar]
- 59.Ramsay D.S., Woods S.C. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychological Review. 2014;121(2):225–247. doi: 10.1037/a0035942.Clarifying. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sterling P., Eyer J. In: Handbook of life stress, cognition and health. Fisher S., Reason J., editors. John Wiley & Sons; New York, NY: 1988. Allostasis: a new paradigm to explain arousal pathology; pp. 629–649. [Google Scholar]
- 61.Cunha R.A. Signaling by adenosine receptors-Homeostatic or allostatic control? PLoS Biology. 2019;17(4):5–8. doi: 10.1371/journal.pbio.3000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elks M.L., Jackson M., Manganiello V.C., Vaughan M. Effect of N6-(L-2-phenylisopropyl)adenosine and insulin on cAMP metabolism in 3T3-L1 adipocytes. American Journal of Physiology - Cell Physiology. 1987;252(21):C342–C348. doi: 10.1152/ajpcell.1987.252.3.c342. [DOI] [PubMed] [Google Scholar]
- 63.Rebrin K., Steil G.M., Mittelman S.D., Bergman R.N. Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. Journal of Clinical Investigation. 1996;98(3):741–749. doi: 10.1172/JCI118846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bergman R.N., Iyer M.S. Indirect regulation of endogenous glucose production by Insulin: the single gateway hypothesis revisited. Diabetes. 2017;66(7):1742–1747. doi: 10.2337/db16-1320. [DOI] [PubMed] [Google Scholar]
- 65.Van der Graaf P.H., Van Schaick E.A., Visser S.A., De Greef H.J., Ijzerman A.P., Danhof M. Mechanism-based pharmacokinetic-pharmacodynamic modeling of antilipolytic effects of adenosine A(1) receptor agonists in rats: prediction of tissue-dependent efficacy in vivo. Journal of Pharmacology and Experimental Therapeutics. 1999;290(2):702–709. [PubMed] [Google Scholar]
- 66.Wu G., Liu Y., Feng W., An X., Lin W., Tang C. Hypoxia-induced adipose lipolysis requires fibroblast growth factor 21. Frontiers in Pharmacology. 2020;11(August):1–11. doi: 10.3389/fphar.2020.01279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poth J.M., Brodsky K., Ehrentraut H., Grenz A., Eltzschig H.K. Transcriptional control of adenosine signaling by hypoxia-inducible transcription factors during ischemic or inflammatory disease. Journal of Molecular Medicine. 2013;91(2):183–193. doi: 10.1007/s00109-012-0988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liang R., Liu N., Cao J., Liu T., Sun P., Cai X., et al. HIF-1α/FOXO1 axis regulated autophagy is protective for β cell survival under hypoxia in human islets. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2022;1868(5):166356. doi: 10.1016/j.bbadis.2022.166356. [DOI] [PubMed] [Google Scholar]
- 69.Tupone D., Madden C.J., Morrison S.F. Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. Journal of Neuroscience. 2013;33(36):14512–14525. doi: 10.1523/JNEUROSCI.1980-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bailey I.R., Laughlin B., Moore L.A., Bogren L.K., Barati Z., Drew K.L. Optimization of thermolytic response to a1 adenosine receptor agonists in rats. Journal of Pharmacology and Experimental Therapeutics. 2017;362(3):424–430. doi: 10.1124/jpet.117.241315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.