TO THE EDITOR:
The hypomethylating agents azacitidine (AZA) and decitabine (DEC) have been backbone therapies for older or unfit patients with newly diagnosed acute myeloid leukemia (AML) as single agents and, more recently, in combination with venetoclax.1-5 Despite being widely used for >15 years, AZA and DEC have not been directly compared in a large randomized trial. Here, we sought to indirectly compare the clinical outcomes with DEC or AZA in this patient population in the context of a large prospective trial. All patients provided signed informed consent.
The ASTRAL-1 trial (registered at www.clinicaltrials.gov as #NCT02348489) was among the largest randomized phase 3 trials ever conducted for unfit or older patients with newly diagnosed AML. Patients were deemed ineligible for intensive chemotherapy by their physicians based on age ≥75 years, major organ comorbidities, or Eastern Cooperative Oncology Group performance status (ECOG PS) of 2 to 3.6 Physicians preselected a standard-of-care therapy (treatment choice [TC]) of either AZA, DEC, or low-dose cytarabine before subsequent 1:1 random assignment of the patients to guadecitabine or the preselected TC.6 Prior treatment with DEC or AZA (including treatment for myelodysplastic syndrome) was not permitted. AZA was given IV or subcutaneously at 75 mg/m2 per day on days 1 to 7, and DEC was given IV at 20 mg/m2 per day on days 1 to 5. Standard supportive care, including granulocyte colony-stimulating factor, was allowed in both groups according to investigator practice. Treatment was administered in 28-day cycles and continued until disease progression or unacceptable toxicity. This post hoc analysis includes those patients treated with AZA or DEC in the TC arm of the study.7 Other results of the ASTRAL-1 trial have been presented and will be published separately.6
Rates of complete response (CR) and overall survival (OS) were coprimary end points in the ASTRAL-1 trial and in this analysis. The composite CR (CRc), defined as a composite of CR, CR with incomplete platelet count recovery (CRp), and CR with incomplete cell count recovery (CRi), was also analyzed and reported. CR, CRp, and CRi were defined in the study protocol according to the International Working Group response criteria8 and were centrally determined by a blinded expert reviewer. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (version 4.03). Rates of CR and CRc were compared using Fisher’s exact test. OS end points were measured from randomization and were analyzed using the Kaplan-Meier method and log-rank test.
Between 19 March 2015 and 25 November 2016, 815 patients were enrolled in the ASTRAL-1 trial across 144 sites in 24 countries, of whom 171 and 167 patients were treated with AZA and DEC, respectively. Baseline patient and disease characteristics were well balanced between AZA- and DEC-treated patients; however, the use of AZA and DEC treatment was significantly different among different geographic locations (Table 1). The median age was 76 years in both groups (range, 59-94 in AZA arm and 60-87 in DEC arm), with 47% and 54% of patients having an ECOG PS of 2 to 3 in the AZA and DEC arms, respectively. Secondary AML, poor risk cytogenetics, and TP53 mutations were present in a substantial subset of patients in both groups.
Table 1.
Baseline patient characteristics
| Characteristic | Azacitidine (n = 171) |
Decitabine (n = 167) |
P |
|---|---|---|---|
| Age, years | .95 | ||
| Median | 76 | 76 | |
| Range | 59-94 | 60-87 | |
| Sex | .38 | ||
| Male | 61 | 56 | |
| Female | 39 | 44 | |
| ECOG PS | .23 | ||
| 0-1 | 53 | 46 | |
| 2-3 | 47 | 54 | |
| Secondary AML | 38 | 37 | .82 |
| Poor-risk cytogenetics | 38 | 34 | .43 |
| Total WBCs ≥20 000/μL | 15 | 13 | .64 |
| BM blasts >30% | 64 | 71 | .16 |
| TP53 mutation | 11.7 | 11.4 | >.99 |
| Geographic location * | .049 | ||
| North America (n = 75) | 45 | 55 | |
| Europe (n = 213) | 55 | 45 | |
| Rest of the world (n = 50) | 38 | 62 |
Values are given as percentages, unless otherwise indicated.
WBC, white blood cell.
Percentages under Geographic Location are based on the corresponding total n in each geographic location.
The median number of treatment cycles was 6 (range, 1-31) in the AZA arm and 5 (range, 1-34) in the DEC arm. There was no statistically significant difference in the coprimary end point of CR rate, achieved in 30 patients (17.5%) in the AZA arm and 32 patients (19.2%) in the DEC arm (P = .78). The rates of CRc (CR + CRp + CRi) were also comparable among AZA- and DEC-treated patients, at 22.2% (38 of 171 patients) and 25.1% (42 of 167 patients), respectively (P = .61).
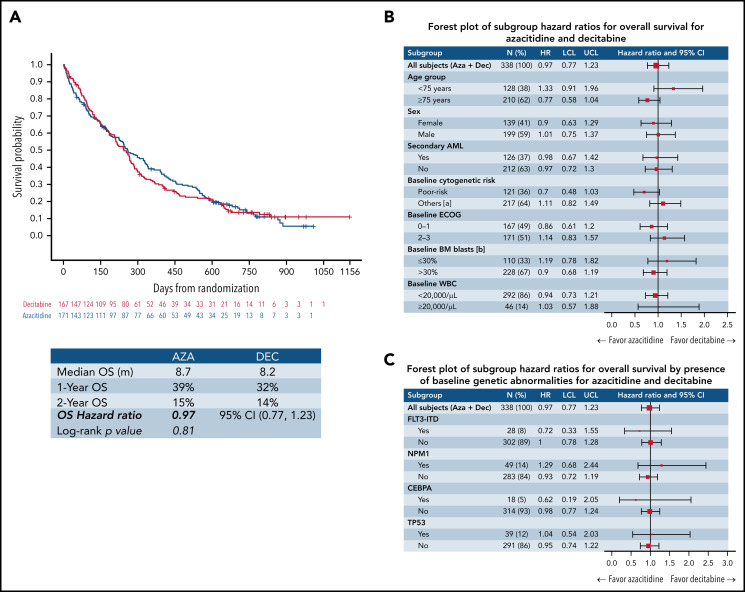
There was no statistically significant difference in OS (P = .81); the median OS times for AZA- and DEC-treated patients were 8.7 and 8.2 months, respectively; (hazard ratio for death, 0.97; 95% confidence interval, 0.77-1.23). One- and 2-year OS were also comparable, at 39% and 15% in the AZA arm and 32% and 14% in the DEC arm, respectively. Figure 1 shows the Kaplan-Meier survival estimates for both groups as well as forest plots for clinically and genetically defined subgroups.
Figure 1.
Survival outcomes for patients treated with AZA and DEC. (A) Treatment with AZA and DEC did not result in a statistically significant difference in terms of median, 1-year, or 2-year OS from the date of randomization. (B-C) OS by clinical (B) and major genetic (C) subgroups; hazard ratios (HRs) with 95% confidence intervals (CIs) for various clinical (B) and major genetic (C) subgroups are shown. All CIs included the null effect, suggesting that survival outcomes were similar in all analyzed clinical (B) and major genetic (C) subgroups. BM, bone marrow; LCL, lower confidence limit; UCL, upper confidence limit; WBC, white blood cell.
In the subgroup of patients with TP53 mutations treated with AZA (n = 20) or DEC (n = 19), there was no statistically significant difference in the CRc rate (AZA, 30.0% vs DEC, 31.6%; P > .99; supplemental Table 1). Similarly, there were no statistically significant differences in OS (P = .90) between AZA- and DEC-treated patients (median OS, 8.7 vs 11.1 months; 1-year OS, 40.0% vs 45.0%; 2-year OS, 8.0% vs 11.0%; supplemental Figure 1).
The safety profiles of AZA and DEC in the ASTRAL-1 trial were in line with prior studies. Hematologic and infectious adverse events were the most common grade ≥3 adverse events, with no statistically significant differences between AZA and DEC (supplemental Table 2). However, serious adverse events leading to death seemed to be more frequent in AZA-treated patients compared with those treated with DEC (38% vs 26%, respectively; P = .02). All-cause mortality rates at 30 and 60 days were 12% and 21% in AZA-treated patients and 8% and 13% in DEC-treated patients, respectively.
To our knowledge, this is the largest data set comparing outcomes of patients with AML treated with AZA versus DEC prospectively in the same clinical trial. No statistically significant differences in CR, CRc, or survival outcomes were observed for the overall patient population or in clinically and genetically defined subgroups, including those with TP53 mutations.
The median OS times for AZA- and DEC-treated patients of 8.7 and 8.2 months, respectively, are comparable to those of previous reports from phase 3 trials and contemporary real-world analyses.3-5 Although patients were not prospectively randomly assigned to receive AZA or DEC (the choice between the two agents was made by the treating physician), the two groups were similar in baseline characteristics and were enrolled in the same trial. Our findings have important implications for both clinicians and patients and support the use of AZA and DEC as equivalent options, potentially including in combinations with novel therapies.9,10
The safety profiles of AZA and DEC in our study were consistent with previous reports in elderly treatment-naïve patients with AML, with hematologic and infectious complications being the most common adverse events.3,4,11 Although the safety profiles were overall comparable between AZA and DEC, we observed higher rates of early mortality compared with prior trials, which had more stringent selection criteria, suggesting these outcomes might be more representative of real-life unfit patients with AML. For example, half of the enrolled patients had an ECOG PS of ≥2, and many had adverse clinical and genetic features.
Although this is the largest comparative data set of AZA- versus DEC-treated patients with AML ineligible for intensive chemotherapy, our study was an indirect comparison, because patients were not randomly assigned to receive either AZA or DEC, and the trial was not specifically designed to detect statistically significant differences between AZA and DEC. Additionally, the confidence intervals for various comparisons were wide, and therefore, we cannot completely exclude the existence of potentially relevant differences between AZA and DEC. Despite this caveat, in the absence of a trend in favor of one agent over the other, it seems unlikely that meaningful differences in activity exist between AZA and DEC in treatment-naïve patients with AML ineligible for intensive chemotherapy. Other potentially distinct clinically relevant end points, such as measurable residual disease–negative CR or CR with partial hematologic recovery, were not prospectively collected for this cohort. Although we found statistically significant differences in the use of AZA and DEC by geographic region, this was an expected finding resulting from differences in approval and use of the two hypomethylating agents between different countries. For example, DEC is not approved in certain regions like Japan and Canada, whereas it is widely used in the United States. The effect of geographic confounding cannot be accurately quantified. Finally, we cannot comment on the comparative efficacy of various administration schedules of AZA and DEC other than those used in the ASTRAL-1 trial.
In conclusion, clinical outcomes with AZA and DEC for treatment-naïve patients with AML deemed ineligible for intensive chemotherapy were comparable. No patient, disease, or molecular characteristics predicted differential outcomes with either agent. These data suggest AZA and DEC can be used interchangeably among older or unfit patients with AML.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
A.M.Z. is a Leukemia and Lymphoma Society Scholar in Clinical Research and was also supported by a National Cancer Institute (NCI) Cancer Clinical Investigator Team Leadership Award. Research reported in this publication was supported in part by the NCI, National Institutes of Health (award P30 CA016359). This study was supported by research funding from ASTEX Pharmaceuticals, Inc.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Presented in part as an oral presentation at the 25th Annual Congress of the European Hematology Association, 12 June 2020 (abstract #S142), and as a poster presentation at the 62nd Annual Meeting of the American Society of Hematology, 5-8 December 2020 (abstract #1037).
Contact the corresponding author for original data.
Authorship
Contribution: A.M.Z. and J.P.B. wrote the initial draft of the manuscript. Y.H. performed statistical analyses. All authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: A.M.Z. reports research funding (institutional) from Celgene/Bristol-Myers Squibb, AbbVie, Astex, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene/Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, and ADC Therapeutics; advisory board participation and/or consultancy for and honoraria from AbbVie, Otsuka, Pfizer, Celgene/Bristol-Myers Squibb, Jazz Pharmaceuticals, Incyte, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Cardiff Oncology, Takeda, Ionis, Amgen, Janssen, Epizyme, Syndax, Gilead, Kura, Chiesi, ALX Oncology, BioCryst, and Tyme; clinical trial committee participation for Novartis, AbbVie, Geron, and Celgene/Bristol-Myers Squibb; and travel support for meetings from Pfizer, Novartis, and Cardiff Oncology. P.F. reports honoraria and research funding from AbbVie, Bristol-Myers Squibb, Jazz Pharmaceuticals, and Novartis. G.J.R. reports consultancy for AbbVie, Agios, Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, Blueprint Medicines, Bluebird Bio, Celgene, GlaxoSmithKline, Janssen, Jasper Therapeutics, Jazz Pharmaceuticals, MEI Pharma (independent data monitoring committee chair), Mesoblast, Novartis, Pfizer, Syndax, Takeda (independent review committee chair) and research funding from Janssen. T.R. reports research funding from Acerta, F. Hoffmann-La Roche, Janssen, AbbVie, Pfizer, MorphoSys, BeiGene, UTX-TGR, AstraZeneca, GlaxoSmithKline, Bristol-Myers Squibb, and UCB; consultancy for Takeda and Momenta; honoraria from Sandoz, Novartis, and Octapharma; and travel expenses from F. Hoffmann-LaRoche, Janssen, and AbbVie. H.K. reports honoraria from AbbVie, Amgen, Daiichi Sankyo, Novartis, Pfizer, Adaptive Biotechnologies, Aptitute Health, BioAscend, Delta-Fly, Janssen, Oxford Biomedical; research funding from AbbVie, Amgen, Ascentage, Bristol-Myers Squibb, Daiichi Sankyo, Immunogen, Jazz Pharmaceuticals, Novartis, Pfizer, and Sanofi; and advisory committee participation for Actinium. J.N. reports consultancy for Novartis, Roche, Pfizer, Amgen, and Takeda and travel expenses from Amgen and Janssen. W.W.J. reports honoraria from Roche, Novartis, and Bristol-Myers Squibb and travel expenses from Celgene. Y.M. reports honoraria from Novartis, Kyowa Kirin, Chugai Pharmaceuticals, Otsuka, Astellas, Celgene, Nippon Shinyaku, and Sumitomo Dainippon Pharma. S.-P.Y. reports advisory committee participation for AbbVie, Amgen, Janssen, Astellas, and Astex. J.M.B. reports honoraria from Celgene/Bristol-Myers Squibb, AbbVie, Astellas, Taiho, Jazz Pharmaceuticals, Pfizer, and Amgen. K.W.L.Y. reports honoraria and research funding from AbbVie, Astex, Forma Therapeutics, F. Hoffmann-La Roche, Genentech, Geron, Janssen, Jazz Pharmaceuticals, MedImmune, Novartis, Onconova, and Tolero and consultancy for Astex, Celgene/Bristol-Myers Squibb, F. Hoffmann-La Roche, Novartis, Otsuka, Paladin, Pfizer, Shattuck Labs, Taiho, and Takeda. H.K., Y.H., and M.A. are current employees of Astex Pharmaceuticals, Inc. H.D. reports honoraria from AbbVie, Agios, Amgen, Astellas, AstraZeneca, Berlin-Chemie, Bristol-Myers Squibb, Celgene, GEMoaB, Gilead, Janssen, Jazz Pharmaceuticals, Novartis, and Syndax and research funding from AbbVie, Agios, Amgen, Astellas, Bristol-Myers Squibb, Jazz Pharmaceuticals, Kronos Bio, and Novartis. None of these relationships were related to the development of this manuscript. The remaining authors declare no competing financial interests.
Correspondence: Amer M. Zeidan, Section of Hematology, Department of Medicine, Yale University, 333 Cedar St, PO Box 208028, New Haven, CT 06520-8028; e-mail: amer.zeidan@yale.edu.
REFERENCES
- 1.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020; 383(7):617-629. [DOI] [PubMed] [Google Scholar]
- 2.Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for patients with untreated AML ineligible for intensive chemotherapy: phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeidan AM, Wang R, Wang X, et al. Clinical outcomes of older patients with AML receiving hypomethylating agents: a large population-based study in the United States. Blood Adv. 2020;4(10):2192-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenaux P, Gobbi M, Kropf P, et al. Results of ASTRAL-1 study, a phase 3 randomized trial of guadecitabine (G) vs treatment choice (TC) in treatment naïve acute myeloid leukemia (TN-AML) not eligible for intensive chemotherapy (IC). Presented at the 24th Congress of the European Hematology Association. 13-16 June 2019. Amsterdam, The Netherlands.
- 7.Zeidan AM, Fenaux P, Gobbi M, et al. Comparative results of azacitidine and decitabine from a large prospective phase 3 study in treatment naive patients with acute myeloid leukemia not eligible for intensive chemotherapy. Presented at the 62nd American Society of Hematology Annual Meeting. 5-8 December 2020.
- 8.Cheson BD, Bennett JM, Kopecky KJ, et al. ; International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia . Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia [published correction appears in J Clin Oncol. 2004;22(3):576]. J Clin Oncol. 2003;21(24):4642-4649. [DOI] [PubMed] [Google Scholar]
- 9.DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunner AM, Esteve J, Porkka K, et al. Efficacy and safety of sabatolimab (MBG453) in combination with hypomethylating agents (HMAs) in patients (Pts) with very high/high-risk myelodysplastic syndrome (vHR/HR-MDS) and acute myeloid leukemia (AML): final analysis from a phase Ib study [abstract]. Blood. 2021;138(suppl 1): 244. [Google Scholar]
- 11.Short NJ, Kantarjian HM, Loghavi S, et al. Treatment with a 5-day versus a 10-day schedule of decitabine in older patients with newly diagnosed acute myeloid leukaemia: a randomised phase 2 trial. Lancet Haematol. 2019;6(1):e29-e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.