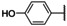
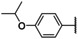
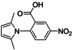
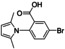
Abstract
The enzymes of the 2‐C‐methylerythritol‐d‐erythritol 4‐phosphate (MEP) pathway (MEP pathway or non‐mevalonate pathway) are responsible for the synthesis of universal precursors of the large and structurally diverse family of isoprenoids. This pathway is absent in humans, but present in many pathogenic organisms and plants, making it an attractive source of drug targets. Here, we present a high‐throughput screening approach that led to the discovery of a novel fragment hit active against the third enzyme of the MEP pathway, PfIspD. A systematic SAR investigation afforded a novel chemical structure with a balanced activity–stability profile (16). Using a homology model of PfIspD, we proposed a putative binding mode for our newly identified inhibitors that sets the stage for structure‐guided optimization.
Keywords: MEP pathway, IspD, fragment, Plasmodium falciparum, drug discovery
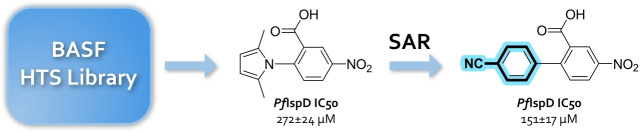
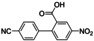
Screening of a part of the BASF library against the PfIspD enzyme led to the identification of a pyrrole as an underexplored chemical scaffold that is able to target the third enzyme in the MEP pathway. A detailed structure–activity relationship (SAR) study around the fragment hit compound helped us to successfully improve the potency as well as the physicochemical properties.
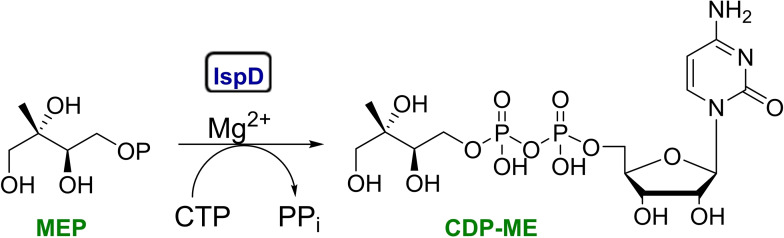
The 2‐C‐methylerythritol‐d‐erythritol 4‐phosphate (MEP) pathway, consists of seven enzymes, and is an essential biosynthetic pathway for the production of isopentenyl diphosphate (IDP) and its isomer dimethylallyl diphosphate (DMADP) both of which are universal building blocks of isoprenoids, a large and structurally diverse group of natural products with crucial physiological functions. [1] As the MEP pathway is absent in humans, but essential in most Gram‐negative bacteria, Mycobacterium tuberculosis and Plasmodium falciparum, the parasite responsible for malaria, it is an attractive source of anti‐infective drug targets. [2] Inhibitors able to target this pathway have the advantage to exhibit a novel mechanism of action without target‐based side effects. Nevertheless, despite the important functions served by the MEP pathway few inhibitors have been reported so far. [3] Importantly, fosmidomycin, [4] a potent inhibitor of the second enzyme of the MEP pathway, IspC or DXR, has undergone phase II clinical trials as antimalarial chemotherapeutic agent in combination with clindamycin and piperaquine, validating the enzymes of the MEP pathway as drug targets. [5] In the present study, we focused our attention on IspD, alternatively known as MEP cytidyltransferase or ygbP protein, that is the third enzyme in the MEP pathway. [6] IspD catalyzes the formation of 4‐diphosphocytidyl‐2‐C‐methylerythritol (CDP‐ME) from MEP and cytidine triphosphate CTP in the presence of Mg2+, with the release of inorganic diphosphate (PPi) (Figure 1). [7]
Figure 1.
Reaction catalyzed by the IspD protein. MEP: 2‐C‐methylerythritol‐d‐erythritol 4‐phosphate; CDP‐ME: 4‐diphosphocytidyl‐2‐C‐methylerythritol.
Looking at the IspD inhibitors, to date only a few compounds have been reported. [8] Aiming to enlarge the portfolio of IspD inhibitors and particularly of Plasmodium IspD (PfIspD), we performed a high‐throughput screening (HTS), using the proprietary BASF library of about 100,000 diverse selected compounds. The search for novel antimalarial compounds endowed with a novel mechanism of action has a continuous and long history of research. Although novel approved treatments and preventions helped to save many lives, malaria is still responsible for more than 400,000 deaths worldwide, mostly young children. [9]
Herein we describe the identification of a fragment‐like compound (16) able to inhibit PfIspD in vitro in the micromolar range and with a suitable physicochemical profile (Figure 2).
Figure 2.
Chemical structures of compounds reported in the present study.
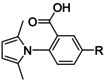
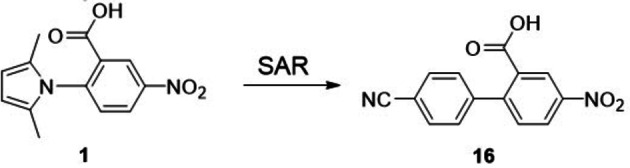
The screening performed against PfIspD led to the identification of compound 1. Despite its moderate activity it attracted our attention due to its fragment‐like size and modular structure that lends itself to chemical modification. In order to identify structural features that are critical for PfIspD inhibition, we followed a classical structure–activity relationship (SAR) study, as we could not rely on any structural information about the protein. High‐resolution structures are only available for Escherichia coli IspD, [10] besides additional structures from non‐pathogenic organisms. [11] We therefore, conducted a focused SAR study with two inter‐related objectives: i) find a replacer for the pyrrole ring as it is known to be a structural alert, [12] ii) validate the fragment hit by improving the potency and the physicochemical profile for further optimization. With these goals in mind, we initially started our exploration by keeping the core molecule constant and varying only the terminal nitro group (1–8); while, the second subset of molecules (9–18) includes modifications around the pyrrole ring.
Half‐maximal inhibitory concentration (IC50) values against purified PfIspD are reported in Table 1, while Table 2 summarizes the second set. Details about the assay are reported in the Supporting Information, section 3.
Table 1.
Inhibition values by 5‐substituted 2‐pyrrol‐1‐benzoic acid derivatives (1–8) determined using the coupled photometric assay with purified PfIspD.
|
| ||
|---|---|---|
|
Compd |
R |
IC50 [μm]* |
|
1 |
NO2 |
271±24 |
|
2 |
H |
>500 |
|
3 |
Cl |
263±35 |
|
4 |
Br |
117±20 |
|
5 |
I |
208±37 |
|
6 |
CH3 |
>500 |
|
7 |
−OCH3 |
>500 |
|
8 |
−NHCOCH3 |
>500 |
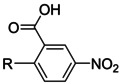
Table 2.
[*] IC50 values were obtained from two independent experiments.Table 2 Inhibition values by 2‐substituted‐5‐nitrobenzoic acid derivatives (9–18) determined with the coupled photometric assay using purified PfIspD.
|
| ||
|---|---|---|
|
Compd |
R |
IC50 [μm]* |
|
9 |
|
264±30 |
|
10 |
|
225±27 |
|
11 |
|
>500 |
|
12 |
|
>500 |
|
13 |
|
>500 |
|
14 |
|
>500 |
|
15 |
|
277±56 |
|
16 |
|
151±17 |
|
17 |
|
>500 |
|
18 |
|
280±55 |
Compounds 1–8 were synthesized following the classical Paal–Knorr pyrrole condensation by refluxing 5‐substitued anthranilic acid in toluene with 1.5 eq of 2,5‐hexanedione in the presence of molecular sieves (Scheme S1). [13]
Based on our previously reported discovery of azolopyrimidines [8a] and pseudilins [14] as halogenated and allosteric modulators of the enzyme IspD, we were intrigued to also evaluate the influence of halogens in our new scaffold, leading to derivatives 3–5. In fact, while for the azolopyrimidine scaffold only activity against the plant Arabidopsis thaliana (AtIspD) is reported, the pseudilin‐type inhibitors showed potency against the malaria parasite too, in vitro and in cell‐based assays. Specifically, in our series of compounds, the best halogen turned out to be bromine (4) that is slightly more potent than hit 1 and about two‐fold more potent than its iodo derivative 5. By contrast, activity is completely lost upon introduction of an electron‐donating group (6–8) and also a short elongation with an acetamide group 8 is not tolerated. The drop in activity observed for compound 2 suggests the influence played by a substituent in position 5 of the phenyl ring within this subset of derivatives.
Next, we moved our attention to the pyrrole core. For consistency, we maintained the initial 5‐nitrobenzoic acid scaffold, having the 2‐position occupied by a small but diverse library of aliphatic and substituted aromatic rings (Table 2). For the synthesis of this series of compounds, we relied on the classical Suzuki cross‐coupling reaction between 2‐bromo‐5‐nitrobenzoic acid and the respective boronic acid derivatives (Scheme S2).
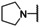
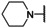
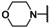
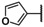
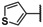
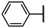
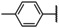
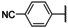
Interestingly, the pyrrolidine 9 and the piperidine 10 could replace the dimethyl pyrrole ring without a significant loss in activity. Introducing another heteroatom in the piperidine ring to give the morpholine 11 led to a further decrease in activity. Other 5‐membered heterocyclic rings such as the furan 12 and the thiophene 13 were not beneficial for the activity. Although, the unsubstituted phenyl 14 did not show significant activity, further substitution on the ring seemed to be beneficial. Substituents of variable nature such as the methyl 15 and the nitrile 16 restored the activity of the unsubstituted phenyl, suggesting there is room for further modification on this side of the molecule. The nitrile derivative 16 showed almost two‐fold higher potency than its methyl analogue 15. Exploring other polar groups such as the hydroxy 17 was not useful, hinting that the electronic effects exerted by the substituents may affect the activity. Remarkably, further growing on the hydroxyl with an isopropyl 18 regained the activity, indicating some space that could be available to modulate the activity.
To gain further insights into this class of compounds, we built a homology model for PfIspD using EcIspD (PDB 1I52) as a template and docked our compounds into the substrate binding pocket (Figure 3). The docked pose of hit compound 1 (Figure 3a) shows that the carboxylic group forms two H bonds with Lys207 and Ile205 in the binding pocket. Both H bonds were also seen in the case of 4 and 16 with a substituted phenyl instead of the dimethyl pyrrole. Another H bond is formed between the compounds having the terminal NO2 group and Arg429.
Figure 3.
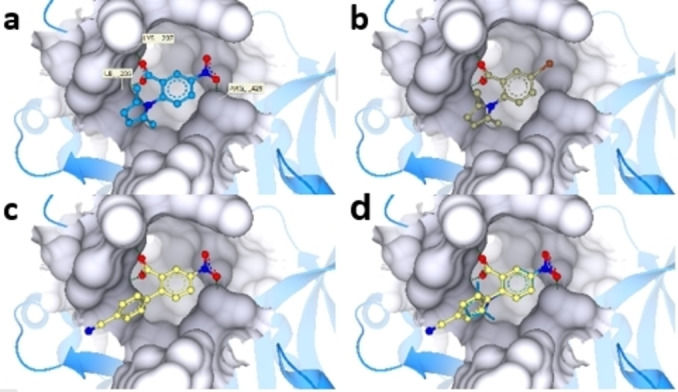
Docked poses of compounds 1, 4, and 16 in the homology model of PfIspD using SeeSAR 11.0. a) The carboxylic group of 1 (blue) forms two H bonds (green dotted lines) with Lys207 and Ile205 and the NO2 group is engaged in a H bonding interaction with Arg429. b) The carboxylic group in 4 (beige) forms the same H bonds as for compound 1. c) Compound 16 (yellow) forms the same H bonds with Lys207, Ile205 and Arg429 as the hit compound 1. d) Overlay of the docked poses of compounds 1 and 16.
Finally, as the affinity for the target is not the only aspect to be considered during fragment‐based drug discovery, we also focused our attention on the physicochemical properties of our compounds (see Table 3). [15] Despite compound 4 showing the best potency in our series of compounds, we do not consider it a suitable candidate for further fragment growing as the lipophilic ligand‐efficiency (LLE) parameter is not ideal. Most probably, the better IC50 value is due to the higher cLogP value. Conversely, with compound 16 we have a good balance in all the ligand efficiency scores evaluated. Of note, having an aromatic core with a nitrile substituent as in 16, has several advantages compared to the pyrrole liability. [16]
Table 3.
[*] IC50 values were obtained from two independent experiments.Table 3 Summary of ligand‐efficiency scores calculated on StarDrop version: 7.0.1.29911.
|
|
|
|
|
|---|---|---|---|
|
Compd |
1 |
4 |
16 |
|
PfIspD IC50 [μm] |
271±24 |
117±20 |
151±17 |
|
cLogP |
1.79 |
3.84 |
1.75 |
|
MW[a] |
262.3 |
294.1 |
270.2 |
|
HA[b] |
19 |
17 |
20 |
|
LE[c] |
0.26 |
0.32 |
0.26 |
|
LLE[d] |
1.77 |
0.085 |
2.072 |
[a] Molecular weight. [b] Non‐hydrogen atom. [c] Ligand efficiency. [d] Lipophilic ligand efficiency.
In conclusion, the present report describes the identification of compound 16 as an optimized fragment hit, targeting PfIspD with high potential for further fragment growing and optimization.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
This project received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska Curie grant agreement No 860816. A. K. H. H. acknowledges funding from the European Research Council (ERC starting grant 757913) and the Helmholtz‐Association's Initiative and Networking Fund. Open Access funding enabled and organized by Projekt DEAL.
Dedicated to the memory of Prof. Dr François Diederich (1952–2020), who initiated our consortium.
E. Diamanti, M. M. Hamed, A. Lacour, P. Bravo, B. Illarionov, M. Fischer, M. Rottmann, M. Witschel, A. K. H. Hirsch, ChemMedChem 2022, 17, e202100679.
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
References
- 1.
- 1a. Gershenzon J., Dudareva N., Nat. Chem. Biol. 2007, 3, 408–414; [DOI] [PubMed] [Google Scholar]
- 1b. Masini T., Kroezen B. S., Hirsch A. K. H., Drug Discov. 2013, 18, 1256–1262. [DOI] [PubMed] [Google Scholar]
- 2. Heuston S., Begley M., Gahan C. G. M., Hill C., Microbiology 2012, 158, 1389–1401. [DOI] [PubMed] [Google Scholar]
- 3. Masini T., Hirsch A. K. H., J. Med. Chem. 2014, 57, 9740–9763. [DOI] [PubMed] [Google Scholar]
- 4. Lell B., Ruangweerayut R., Wiesner J., Missinou M. A., Schindler A., Baranek T., Hintz M., Hutchinson D., Jomaa H., Kremsner P. G., Antimicrob. Agents Chemother. 2003, 47, 735–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.
- 5a. Jomaa J. W. H., Sanderbrand S., Altincicek C. W. B., Hintz M., Turbachova M. E. I., Zeidler H. K. L. J., Soldati E. B. D., Science 1999, 285, 1573–1576; [DOI] [PubMed] [Google Scholar]
- 5b. Mombo-Ngoma G., Remppis J., Sievers M., Zoleko Manego R., Endamne L., Kabwende L., Veletzky L., Nguyen T. T., Groger M., Lotsch F., Mischlinger J., Flohr L., Kim J., Cattaneo C., Hutchinson D., Duparc S., Moehrle J., Velavan T. P., Lell B., Ramharter M., Adegnika A. A., Mordmuller B., Kremsner P. G., Clin. Infect. Dis. 2018, 66, 1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rohdich J. W. F., Fellermeier M., Sagner S., Herz S., Kis K., Eisenreich W., Bacher a. M. H. Z. A., PNAS 1999, 96, 11758–11763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frank A., Groll M., Chem. Rev. 2016, 117, 5675–5703. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Witschel M. C., Höffken H. W., Seet M., Parra L., Mietzner T., Thater F., Niggeweg R., Röhl F., Illarionov B., Rohdich F., Kaiser J., Fischer M., Bacher A., Diederich F., Angew. Chem. Int. Ed. 2011, 50, 7931–7935; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 8077–8081; [Google Scholar]
- 8b. Baatarkhuu Z., Chaignon P., Borel F., Ferrer J.-L., Wagner A., Seemann M., Sci. Rep. 2018, 8 17892; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8c. Imlay L. S., Armstrong C. M., Masters M. C., Li T., Price K. E., Edwards R. L., Mann K. M., Li L. X., Stallings C. L., Berry N. G., O′Neill P. M., Odom A. R., ACS Infect. Dis. 2015, 1, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. https://www.cdc.gov/malaria/features/world_malaria_day_2021.html, 2021.
- 10.
- 10a. Stéphane A. M. L., Richard B., Cane D. E., Bowman M. E., Kwiatkowski W., Noel J. P., Kang I., Chow C., Nat. Struct. Biol. 2001, 8, 641–648; [DOI] [PubMed] [Google Scholar]
- 10b. Kemp L. E., Bond C. S., Hunter W. N., Acta Crystallogr. D Biol. Crystallogr. 2003, 59, 607–610. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a. Gabrielsen M., Kaiser J., Rohdich F., Eisenreich W., Laupitz R., Bacher A., Bond C. S., Hunter W. N., FEBS J. 2006, 273, 1065–1073; [DOI] [PubMed] [Google Scholar]
- 11b. Björkelid C., Bergfors T., Henriksson L. M., Stern A. L., Unge T., Mowbray S. L., Jones T. A., Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 403–414. [DOI] [PubMed] [Google Scholar]
- 12.
- 12a. Ropponen H. K., Bader C. D., Diamanti E., Illarionov B., Rottmann M., Fischer M., Witschel M., Müller R., Hirsch A. K. H., ChemMedChem 2021, 16, 2089–2093; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12b. Zhu W., Groh M., Haupenthal J., Hartmann R. W., Chem. Eur. J. 2013, 19, 8397–8400. [DOI] [PubMed] [Google Scholar]
- 13.J. E. Bradner, M. R. Mckeown, P. B. Rahl, R. A. Young, J. J. Marineau, Int. PCT Pub. No. WO2014071247 A1, 2014.
- 14. Kunfermann A., Witschel M., Illarionov B., Martin R., Rottmann M., Hoffken H. W., Seet M., Eisenreich W., Knolker H. J., Fischer M., Bacher A., Groll M., Diederich F., Angew. Chem. Int. Ed. 2014, 53, 2235–2239; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 2267–2272. [Google Scholar]
- 15. Schultes S., de Graaf C., Haaksma E. E. J., de Esch I. J. P., Leurs R., Krämer O., Drug Discovery Today Technol. 2010, 7, e157–e162. [Google Scholar]
- 16. Fleming F. F., Yao L., Ravikumar P. C., Funk L., Shook B. C., J. Med. Chem. 2010, 53, 7902–7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.