Abstract
Adipose tissue is widely recognized as an abundant and accessible human tissue that serves as a source of cells and extracellular matrix scaffolds for regenerative surgical applications. Increasingly, orthopedic surgeons are turning to adipose tissue as a resource in their treatment of osteoarthritis and related conditions. In the U.S., the regulatory landscape governing the orthopedic surgical utilization of autologous and allogeneic adipose tissue remains complex. This manuscript reviews the Food and Drug Administration's nomenclature and guidance regarding adipose tissue products. Additionally, it surveys recent pre-clinical and clinical trial literature relating to the application of adipose-derived cells and tissues in the treatment of osteoarthritis.
Keywords: Adipose, Microfragmented fat, Osteoarthritis, Regulatory agency, Regenerative medicine, Stromal/stem cells
Abbreviations: CBER, Center for Biologics Evaluation and Research; ASC, Adipose Stromal/Stem Cells; SVF, Stromal Vascular Fraction Cells; MFAT, MicroFragmented Adipose Tissue; HCT/P, Human Cells, Tissues, and Cell/Tissue related Products; OA, Osteoarthritis
Highlights
-
•
Adipose tissue is a source for stromal/stem cells, extracellular matrix, and intact tissue for regenerative surgeries
-
•
Regulatory evaluation of adipose tissue for orthopedic applications is complex
-
•
Pre-clinical studies support the safety and efficacy of adipose cells and scaffolds for orthopedic and plastic surgeries
-
•
Randomized controlled clinical trials have assessed the safety and efficacy of adipose cell therapy for osteoarthritis
1. Introduction
The Food and Drug Administration (FDA) regulates both biological products and devices developed by the emerging regenerative medicine community in the US. Within the FDA, the Center for Biologics Evaluation and Research (CBER) has the requisite expertise to evaluate the science, safety, and efficacy of potential cell-, genetic-, or tissue-based therapies. Rapid advancements in tissue engineering and regenerative medicine and their clinical translation have made this regulatory landscape complex and costly to navigate for biotech companies, physicians/surgeons, academic researchers and patients, all united by a desire to attain improved outcomes for historically unmet medical needs. This situation contrasts with the better-defined pathway for traditional drug development where the pharmaceutical industry and the FDA have established a standardized set of assays, toxicology testing, and therapeutic metrics to assess and validate small molecules as future drugs. As the audience of Bone Reports is well aware, orthopedic practitioners worldwide have embraced the infusion of adipose-derived stromal vascular fraction (SVF) cells, culture-expanded adipose stromal/stem cells (ASC), or micronized fat as a potential therapy for osteoarthritis. Nevertheless, these treatments' scientific basis, safety, and efficacy remain to be demonstrated via trials providing evidence adequate to support FDA approval. Several rulings from Congress and the FDA are of particular relevance to these matters. In 2016, the U.S. Congress passed the 21st Century Cures Act specifying that companies could request a Regenerative Medicine Advanced Therapy (RMAT) designation for their cell or tissue engineered therapy if it addressed a serious or life-threatening disease or illness and displayed preliminary clinical evidence (21st Century Cures Act, 2020). In November 2017, the FDA issued a Guidance to Industry and the Food and Drug Administration Staff entitled “Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use” which was further updated in May 2021 (Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-based Products: Minimal Manipulation and Homologous Use, 2020). The original document called for clinical practitioners currently administering cell based therapies to comply with FDA regulations and provided a 3 year moratorium to do so. Shortly thereafter, the FDA in August 2018 issued a related Guidance to Industry entitled “Osteoarthritis: Structural Endpoints for the Development of Drugs, Devices, and Biological Products for Treatment” which acknowledged that there was a need for better metrics correlating structural features with meaningful patient oriented beneficial outcomes in OA and invited industry stakeholders to help address this issue (Osteoarthritis: Structural Endpoints for the Development of Drugs, Devices, and Biological Products for Treatment Guidance for Industry, 2018). Additionally, this document identified OA as “serious disease with an unmet medical need for therapies that modify the underlying pathophysiology of the disease and potentially change its natural course to prevent long-term disability”, thereby making cell-based OA therapies eligible for RMAT designation (Osteoarthritis: Structural Endpoints for the Development of Drugs, Devices, and Biological Products for Treatment Guidance for Industry, 2018). After their three year moratorium, the FDA began taking legal actions to curtail clinical application of non-approved adipose-derived cell therapies to OA patients (Bruder, 2021). While the verdict on some of these cases has favored the authority of the FDA, others remain pending in court (Circuit U.S.C.O.A.F.T.E., 2021; Hiltzick, 2022). In part due to these ongoing controversies, the current review article has focused on adipose-derived biologic products as a potential therapy for osteoarthritis, using it as a case study for understanding the current status of FDA regulation in the regenerative medicine arena. The authors have focused particular attention to the English language literature published since 2018 using the following keywords in Pubmed: adipose, stromal/stem cell, osteoarthritis.
2. FDA nomenclature, definitions, and regulations
The following Centers within the FDA have the greatest relevance to regenerative medical (Table 1):
Center for Biologics Evaluation and Research (CBER)
Center for Devices and Radiological Health (CDRH)
Center for Drug Evaluation and Research (CDER)
Center for Veterinary Medicine (CVM)
Table 1.
Relevant centers and offices within the FDA.
| Center | Offices |
|---|---|
| CBER | Biostatistics & Epidemiology; Blood Research & Review; Communication; Compliance & Biologics Quality; Management; Outreach & Development; Tissues & Advanced Therapies; Vaccine Research & Review; |
| CDRH | Communication & Education; Management; Policy; Product Evaluation & Quality; Science & Engineering Laboratories; Strategic Partnership & Technology Innovation; |
| CDER | Communication; Compliance; Executive Programs; Generic Drugs; Management; Medical Policy; New Drugs; Pharmaceutical Quality; Regulatory Policy; Surveillance & Epidemiology; Strategic Programs; Translational Sciences |
| CVM | Management; Minor Use and Minor Species Animal Drug Development; New Animal Drug Evaluation; Research; Surveillance and Compliance |
Abbreviations: Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH), Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM).
Each Center holds primary control over a specific therapeutic or diagnostic domain as their name implies. Within each Center, specific Offices have expertise focused on targeted subjects. Of note, the CBER Offices of Compliance and Biologics Quality and Tissues & Advanced Therapies hold particular relevance to the regenerative medical arena with respect to cell and decellularized tissue products (Table 1). Nevertheless, it is essential to be aware of the related Offices as they may play a co-regulatory role if there is a “combination product” that adds drug or other non-biological elements to a cell construct. In such cases, the resulting combination product must fulfill the regulatory safety and efficacy requirements of both its biologic and drug-related elements.
When investigators are ready to begin clinical studies using a novel biologic or drug therapy, the culmination of their initial approach to the FDA comes in the form of an Investigational New Drug (IND) application (Table 2) or an Investigational Device Exemption (IDE) application. The approval of an IND or IDE permits the principal investigator and sponsor to administer the therapy to patients in a defined and mutually agreed upon manner that, first and foremost, protects the safety and authority of the participants. The IND or IDE must be registered in a national clinical trial database as part of the approval process. Detailed information relating to each trial, including the existence of the IND or IDE authorization, is kept confidential by the FDA unless disclosed to the public directly by the company. Eventually, the FDA's final approval of a biologic therapy will result in the approval of a Biologic License Application (BLA), permitting the sponsoring company or entity to use the product in interstate commerce. Clinical trials initiate as safety studies under Phase I and advance to safety and efficacy analyses in larger cohorts of test subjects in Phase II and III. In the realm of biologics, Phase I or pilot trials may focus on a single concentration or dose, or may provide for an escalating dose in small cohorts of the test article to focus on safety in subjects with the disease of interest. Phase II and III or pivotal trials typically will evaluate single or multiple concentrations of the test article in a randomized, controlled, and, often, blinded clinical trial format. Institutions conducting clinical trials will frequently register them online with www.clinicaltrials.gov, an online data repository maintained by the National Library of Medicine. In addition to a registration number, this searchable database includes the site(s) of the clinical trial, the sponsor, the number of subjects to be enrolled, a brief description of the study design, its primary and secondary endpoints, and current status with respect to enrollment. Studies registered on www.clinicaltrials.gov are just as likely to be ongoing anywhere in the world and are not exclusively those underway in the U.S. and North America. Thus, it does not accurately or effectively identify studies conducted with full authorization by the US FDA.
Table 2.
FDA Definitions of relevant terminology as quoted from Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-based Products: Minimal Manipulation and Homologous Use (2020).
| BLA | Biologic License Application |
|---|---|
| IND | Investigational New Drug Application |
| IDE | Investigational Device Exemption |
| Class I, II, or III Device | Devices are categorized based on risk to patient or user: Class I (low to moderate risk); Class II (moderate to high risk); Class III (high risk) |
| HCT/P | Human cells, tissues, and cellular and tissue-based product, i.e., bone, ligament, skin, dura mater, heart valve, cornea, hematopoietic stem/progenitor cells derived from peripheral and cord blood, manipulated autologous chondrocytes, epithelial cells on a synthetic matrix, and semen or other reproductive tissue |
| Non-HCT/P | (1) Vascularized human organs for transplantation; (2) Whole Blood or blood components or blood derivative products; (3) Secreted or extracted human products, such as milk, collagen, and cell factors, except semen, are considered an HCT/P; (4) Minimally manipulated bone marrow for homologous use and not combined with another article (except for water, crystalloids, or a sterilizing, preserving, or storage agent, if the addition of the agent does not raise new clinical safety concerns with respect to the bone marrow); (5) Ancillary products used in the manufacture of HCT/P; (6) Cells, tissues, and organs derived from animals other than humans; (7) In vitro diagnostic products; and (8) Blood vessels recovered with an organ, as defined in 42 CFR 121.2 that are intended for use in organ transplantation and labeled “For use in organ transplantation only” (21 CFR 1271.3(d)) |
| Processing of HCT/P | Any activity performed on an HCT/P, other than recovery, donor screening, donor testing, storage, labeling, packaging, or distribution, such as testing for microorganisms, preparation, sterilization, steps to inactivate or remove adventitious agents, preservation for storage, and removal from storage (21 CFR 1271.3(ff)). Processing also includes cutting, grinding, shaping, culturing, enzymatic digestion, and decellularization. |
| Homologous use | The repair, reconstruction, replacement, or supplementation of a recipient's cells or tissues with an HCT/P that performs the same basic function or functions in the recipient as in the donor:
|
| Structural tissue | Tissues that physically support or serve as a barrier or conduit, or connect, cover, or cushion in the donor, i.e., adipose tissue, amniotic membrane and umbilical cord, articular cartilage, blood vessel, bone, non-articular cartilage, skin, tendon or ligament |
| Non-structural tissue | Tissues that serve predominantly metabolic or other biochemical roles in the body such as hematopoietic, immune, and endocrine functions, i.e., hematopoietic stem/progenitor cells (e.g., cord blood), lymph nodes and thymus, reproductive cells or tissues (e.g., oocytes) . |
| Minimal manipulation (structural tissue) | The processing of the HCT/P does not alter the original relevant characteristics of the tissue relating to the tissue's utility for reconstruction, repair, or replacement |
| Minimal manipulation (non-structural tissue) | The processing of the HCT/P does not alter the relevant biological characteristics of cells or tissues |
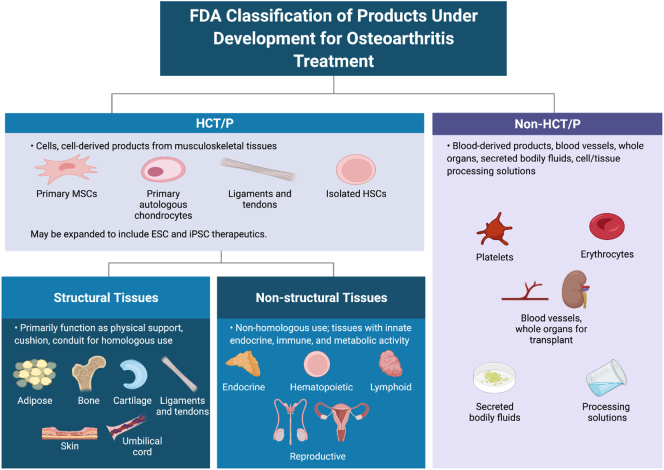
Many devices are handled distinctly from drugs under the CDRH, while selected devices that primarily function to obtain cellular material are regulated via IDE submissions handled through CBER. A subset of devices is eligible for classification as “Preamendment”. These are devices manufactured and marketed prior to 1976 and are “grandfathered” as exempt from further FDA approval; however, such devices are an exception and are not relevant to this discussion. Instead, most devices are categorized into three distinct Classes based on the degree of risk they present to the patient or user (Fig. 1 and Table 2). Forty-seven percent (47 %) of devices fall into Class I with low to moderate risk to patients; an example of a representative Class I device is an elastic bandage. Indeed, the vast majority of Class I devices are exempt from regulatory restrictions. Manufacturers can file a 510 K premarket document indicating that the device is substantially equivalent to an existing device actively being marketed with an FDA allowance. Substantial equivalence indicates that the device serves the same purpose as a currently approved device (known as a predicate) using the same or different technologies where these claims are supported by satisfactory evidence of safety and efficacy. Forty-three percent (43 %) of devices are classified as Class II with moderate to high risk; a Class II device example is a diagnostic test kit for pregnancy. The remaining 10 % of devices are classified as Class III with high risk. These are generally implanted into patients, necessary to sustain or maintain life, or present risk of disease or illness. Examples include breast implants, pacemakers, and blood collection apparatus. Class III devices are subjected to a Premarket Approval (PMA) process prior to marketing to ensure safety and efficacy by the FDA. Part of this process may include an Investigational Device Exemption (IDE) from the FDA, authorizing the manufacturer to perform interstate transportation and use of the device in clinical trials designed to assess safety and efficacy in a clinical trial.
Fig. 1.
FDA classification of products (graphical representation created with Biorender).
3. Human cells, tissues, and cell/tissue related products
Many of the products under development for osteoarthritis (OA) treatment are classified by the FDA as Human Cells, Tissues, and Cell/Tissue related Products (HCT/P) (Fig. 1). These include cells or cell-derived products from musculoskeletal tissues such as primary mesenchymal stromal/stem cells (MSC) and primary autologous chondrocytes as well as ligaments, tendons, and isolated hematopoietic stem cells. While not yet in clinical trials, this category may eventually include embryonic stem cell (ESC) and induced pluripotent stem cell (iPSC) therapeutics. In contrast, non-HCT/P products are categorized as blood-derived products such as erythrocytes and platelets, blood vessels or whole organs designed for transplant, secreted bodily fluids (except for semen), or solutions used in the processing or sterilization of cells or tissues.
From a regulatory perspective, the FDA subdivides body tissues into two categories: Structural and Non-Structural. Structural tissues are considered primarily those that serve as physical support, cushion or conduit. Examples include adipose tissue, amnion, bone, cartilage, ligament, skin, and umbilical cord. For structural tissues, the definition of homologous use is restricted to the use of the tissue or cells exclusively for supportive or cushioning functions; any activity relating to the inherent endocrine, immune or metabolic activity of the cells or structures would be characterized as non-homologous use, thereby subjecting them to greater regulatory scrutiny and evaluation. In contrast, Non-Structural tissues are those that the FDA views primarily as serving a metabolic role and examples include endocrine, hematopoietic, lymphoid, and reproductive tissues. In these cases, homologous use relates directly to the cells' endocrine, immune, and metabolic functionality. These distinctions are independent of the autologous or allogeneic origin of the tissue and have critical implications for regulation based on the meaning of minimal manipulation. For Non-Structural tissues, minimal manipulation is defined as any process that does not alter the biological properties of the cells or tissue. In contrast, for Structural tissues, minimal manipulation is defined as any process that does not alter the properties of the tissue for reconstruction, repair, or replacement purposes. While these may appear to be semantic distinctions, they have a profound impact at the level of manufacture and production. Without taking this into account, newcomers to the field may be puzzled by apparent incongruities in the FDA's rationale for their differential categorization of cells or tissues from, for example, adipose tissue vs. pancreas where the use of collagenase is treated with different levels of regulatory scrutiny depending on the tissue classification. When a pancreas is digested with type I collagenase to isolate beta islets for transplantation to an allogeneic diabetic patient, this is classified as “minimal manipulation”. In contrast, when autologous adipose tissue is digested with type I collagenase for cosmetic fat grafting, this is considered “more than minimal manipulation”. Distinctions based on the definition of what constitutes a structural vs. metabolic tissue and, by extension, minimal manipulation have served as the basis of ongoing discussions in the literature regarding the categorization of adipose tissue exclusively as a structural tissue (Rodriguez et al., 2020; Marks, 2020). Thus, the research and manufacturing communities need to share a full appreciation of these FDA distinctions based on tissue of origin and their impact on the regulatory control of subsequent cell and tissue products.
4. Case study – use of adipose-derived cells and tissues for treatment of osteoarthritis
Due to its relative abundance and accessibility, adipose tissue has attracted considerable attention in the past decade as a source of cells, tissues, and scaffolds for OA treatment. Adipose tissue can be processed by enzyme digestion with collagenase and/or dispase to yield a heterogeneous Stromal Vascular Fraction (SVF) cell population including fibroblasts, adipose-derived stromal/stem cells (ASC), pericytes, as well as lymphoid and myeloid cells (Bourin et al., 2013). The SVF cells can be adhered to plastic cell culture surfaces and expanded ex vivo as relatively homogenous ASC. Alternatively, adipose tissue can be mechanically processed to create tissue fragments known in the literature as micronized or nanofat. This can be accomplished by repeatedly injecting lipoaspirate tissue through a Luer lock syringe multiple times (30×) or with a commercially regulated device using shear forces or hand-shaken metallic beads to disrupt the tissue (Tremolada et al., 2016a; Tremolada et al., 2016b). Additionally, intact adipose tissue can be decellularized using a combination of detergents, solvents, and mechanical processing steps to yield an extracellular matrix (ECM)-rich hydrogel (Mohiuddin et al., 2020; Flynn, 2010; Kokai et al., 2019). Finally, the adipose cell-derived secretome contains soluble paracrine factors, as well as exosomal and other particulates, known collectively as extracellular microvesicles. This secretome (ASC-S) harvested from adipose cells or tissues holds promise as a biologically derived therapeutic in multiple indications, including osteoarthritis (Wei et al., 2009; Ellis et al., 2021; Niada et al., 2019; Amodeo et al., 2021).
The exosomes serve as delivery vehicles for anti-inflammatory growth factors and signal transductive microRNAs (Ni et al., 2020). Currently, pre-clinical and/or clinical level evaluations are underway for each biologic reagent as injectable therapy for osteoarthritis. Indeed, since 2019, at least 19 reviews from authors in 17 countries have been published on this topic; nearly half of these articles self-identify as meta-analyses or systematic reviews (Table 3). To avoid making the current article more repetitive than necessary, readers are referred directly to this previously published collection of eloquent reviews.
Table 3.
Recent reviews focused on biologic therapy for osteoarthritis.
| Author (country) | Journal (ref) | Title |
|---|---|---|
| Agarwal N et al. (UK) | Cells 2021, 10:1365 (Agarwal et al., 2021) | Meta-Analysis of Adipose Tissue-Derived Cell-Based Therapy for the Treatment of Knee Osteoarthritis |
| Biazzo A et al. (Italy) | The Physician and Sportsmedicine 2020, 48: 392–399 (Biazzo et al., 2020) | Autologous adipose stem cell therapy for knee osteoarthritis: where are we now? |
| Buzaboon N & Alshammary S (Bahrain) | Stem Cells and Cloning: Advances and Applications 2020, 13: 117–136 (Buzaboon and Alshammary, 2020) | Clinical Applicability of Adult Human Mesenchymal Stem Cell Therapy in the Treatment of Knee Osteoarthritis |
| De Francesco F et al. (Italy) | International Journal of Molecular Sciences 2021, 22:10197 (De Francesco et al., 2021) | Stem Cells in Autologous Microfragmented Adipose Tissue: Current Perspectives in Osteoarthritis Disease |
| Delanois RE et al. (US) | Journal of Arthroplasty 2019, 34:801–813 (Delanois et al., 2019) | Biologic Therapies for the Treatment of Knee Osteoarthritis |
| Gentile P et al. (Italy & Greece) | International Journal of Molecular Sciences 2020, 21:4982 (Gentile et al., 2020) | Systematic Review: Allogenic Use of Stromal Vascular Fraction (SVF) and Decellularized Extracellular Matrices (ECM) as Advanced Therapy Medicinal Products (ATMP) in Tissue Regeneration |
| Ghiasloo M et al. (Belgium) | Aesthetic Surgery Journal 2020, 40: NP546–NP560 (Ghiasloo et al., 2020) | Expanding Clinical Indications of Mechanically Isolated Stromal Vascular Fraction: A Systematic Review |
| Han SB et al. (Republic of Korea) | Arthroscopy 2021, 37:292–306 (Han et al., 2021) | Intra-Articular Injections of Hyaluronic Acid or Steroids Associated With Better Outcomes Than Platelet-Rich Plasma, Adipose Mesenchymal Stromal Cells, or Placebo in Knee Osteoarthritis: A Network Meta-analysis |
| Keeling LE et al. (USA) | Am J Sports Med In Press (Keeling et al., 2021) | Bone Marrow Aspirate Concentrate for the Treatment of Knee Osteoarthritis |
| Mehranfar S et al. (Iran) | Artificial Cells, Nanomedicine, and Biotechnology 2019, 47:882–890 (Mehranfar et al., 2019) | The use of stromal vascular fraction (SVF), platelet rich plasma (PRP) and stem cells in the treatment of osteoarthritis: an overview of clinical trials |
| Migliorini F et al. (Germany) | Archives of Orthopaedic and Trauma Surgery 2020, 140:853–868 (Migliorini et al., 2020) | Improved outcomes after mesenchymal stem cells injections for knee osteoarthritis: results at 12-months follow-up: a systematic review of the literature |
| Primorac D et al. (Croatia) | Genes 2020, 11:854 (Primorac et al., 2020) | Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations |
| Shanmugasundaram S et al. (India, Oman, UAE, US) | International Orthopaedics 2021, 45:615–625 (Shanmugasundaram et al., 2021) | Assessment of safety and efficacy of intra-articular injection of stromal vascular fraction for the treatment of knee osteoarthritis—a systematic review |
| Shariatzadeh M et al. (UK) | Cell and Tissue Research 2019, 378:399–410 (Shariatzadeh et al., 2019) | The efficacy of different sources of mesenchymal stem cells for the treatment of knee osteoarthritis |
| Tan SHS et al. (Singapore) | Am J Sports Med 2021, 49: 3113–3124 (Tan et al., 2021) | Intra-articular Injections of Mesenchymal Stem Cells Without Adjuvant Therapies for Knee Osteoarthritis A Systematic Review and Meta-analysis |
| Vahedi P et al. (Iran, Turkey) | International Journal of Molecular Sciences 2021, 22:9215 (Vahedi et al., 2021) | The Use of Infrapatellar Fat Pad-Derived Mesenchymal Stem Cells in Articular Cartilage Regeneration: A Review |
| Olsson DC et al. (Brazil) | Research in Veterinary Science 2020, 135:495–503 (Olsson et al., 2021) | Administration of mesenchymal stem cells from adipose tissue at the hip joint of dogs with osteoarthritis: A systematic review |
| Zhao D et al. (China) | Journal of Arthroscopic and Related Surgery 2021, 37:2298–2314 (Zhao et al., 2021) | Intra-Articular Injections of Platelet-Rich Plasma, Adipose Mesenchymal Stem Cells, and Bone Marrow Mesenchymal Stem Cells Associated With Better Outcomes Than Hyaluronic Acid and Saline in Knee Osteoarthritis: A Systematic Review and Network Meta-analysis |
| Xiang XN et al. (China) | Stem Cell Research & Therapy 2022, 13:14 (Xiang et al., 2022) | Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis |
Likewise, since 2019, there has been an abundance of peer-reviewed published literature reporting clinical trial results using adipose-derived biological therapeutics for OA (Table 4, Table 5, Table 6, Table 7). The vast majority of these studies have monitored the quantitative response of OA patients based on measurements made with the Western Ontario and McMasters Universities Osteoarthritis Index (WOMAC), Visual Analogue Scale (VAS) metrics for pain, stiffness and activity, or the Knee Injury Osteoarthritis Outcome Score (KOOS) (Table 4, Table 5, Table 6). Additionally, a subset of studies employed non-invasive imaging analytical methods such as MRI to monitor cartilage repair, with or without enhancement agents (Zhao et al., 2019). In a single trial comparing cohorts of ten subjects each treated with microfracture alone or in combination with hyaluronic acid injection with or without autologous ASC, outcomes were further monitored using a histological evaluation of a cartilage biopsy obtained under arthroscopic examination; however, this level of post-operative invasive assessment was the exception, and only four of the sixty enrolled subjects consented to the biopsy procedure (Qiao et al., 2020). Only a single study reported a serious adverse event involving infection of an injected joint; however, this event occurred in a patient enrolled in the control arm (hyaluronate injection) study cohort and did not involve adipose-derived cells (Lu et al., 2019). Multiple studies reported adverse events, and these were frequently bruising or pain at the adipose harvest site or swelling following the joint injection.
Table 4.
Case reports, case series and retrospective studies.
| Authors/reference/nation Clinical trials or national registration # |
Study type/therapy/device | Subject # | Metrics, outcomes & serious adverse events (adverse events) |
|---|---|---|---|
| Freitag et al. (Freitag et al., 2020a) (Australia) | Ankle OA CR/Auto ASC (20 to 50 × 106 ASC at 0, 6, 12 mo) | 1 | FADI, MRI; Improvement vs baseline up to 24 mo; No SAE |
| Freitag et al. (Freitag et al., 2020b) (Australia) ACTRN12617000638336 |
OA Knee CS NR Pro/Auto ASC (50 × 106 per knee at 0 and 6 mo) | 8 | KOOS, MRI, WOMAC; Improvements with 24 mo follow up; No SAE |
| Gobbi et al. (Gobbi et al., 2021) (Italy, United Arab Emirates, USA) | Knee OA NR Retro/Auto MFAT (Lipogems) | 75 | KOOS; Significant improvement vs baseline up to 24 mo follow up; No SAE (49 % pain at lipo site, 37 % bruising at lipo site, 13 % knee swelling) |
| Lapuente et al. (Lapuente et al., 2020) (Spain) | Knee OA NR Retro/Auto SVF | 50 | Serum Cytokines, Ultra, VAS, WOMAC; Significant improvement vs baseline at 12 mo follow up; No SAE |
| Mautner et al. (Mautner et al., 2019) (USA) | OA Knee NR Retro/Auto BMAC or MFAT/Lipogems | 76 | Emory QLF, KOOS, VAS; Significant improvement pre vs post with >1 yr follow up; No SAE |
| Vinet-Jones & Darr (Vinet-Jones and Darr, 2020) (USA) | OA Shoulder CS NR Pro/Auto MFAT (Lipogems) | 25 | DASH, Rad, VAS; Significant improvement vs baseline at 12 mo follow up; No SAE |
Abbreviations: Allo, Allogeneic; ASC, Adipose-derived Stromal/stem Cells; Auto, Autologous; B, Blinded; BMAC, Bone Marrow Aspirate Concentrate; CR, Case Report; CS, Case Series; CT, Controlled Trial; DASH, Disabilities of the Arm, Shoulder and Hand; Emory QLF, Emory Quality of Life; FADI, Foot and Ankle Disability Index; HA, Hyaluronate; KOOS, Knee Injury and Osteoarthritis Outcome Score; MFAT, Microfragmented Adipose Tissue; MRI, Magnetic Resonance Imaging; NR, Not Randomized; Pro, Prospective; PRP, Platelet Rich Plasma; R, Randomized; Rad, Radiography; Retro, Retrospective; SAE, Serious Adverse Event; SVF, Stromal Vascular Fraction Cells; TBCR, To Be Conducted/Reported; Ultra, Ultrasound; VAS, Visual Analog Scale; WOMAC, Western Ontario and McMasters Universities Osteoarthritis Index.
Table 5.
Non-randomized prospective clinical trials.
| Bakowski et al. (Bakowski et al., 2021) (Poland) | Knee OA NR Pro/auto lipoaspirate | 37 | IDKC2000, KOOS, NPRS, WOMAC; Satisfaction in Stage II but not Stage IV; 27 mo follow up; No SAE |
| Barfod & Blond (Barfod and Blond, 2019) (Denmark) NCT02697682 | Knee OA NR Pro/Auto MFAT (Lipogems) | 20 | KOOS, VAS; Significant improvement vs baseline up to 12 mo; No SAE |
| Bistolfi et al. (Bistolfi et al., 2021) (Italy) | Knee OA NR Retro/Auto MFAT (Lipogems or Lipocells) | 78 | FJS, KOOS, KSS, LS, NRS; Significant improvement vs baseline av. 23.5 mo follow up; No SAE (knee swelling, minor venous thrombosis) |
| Boric et al. (Boric et al., 2019) (Croatia) ISRCTN13337022 |
Knee OA NR Pro/Auto MFAT (Lipogems) | 10 | dGEMRIC, VAS; Significant improvement vs baseline up to 24 mo |
| Chen et al. (Chen et al., 2021) (Taiwan) NCT03007576 | Knee OA NR Pro/Auto IPF ASC (50 × 106 single injection) | 12 | IKDC 2000, KOOS, MOCART, VAS, WOMAC; Significant improvement vs baseline up to 11 mo follow up; No SAE |
| Dall'Oca et al. (Dall'Oca et al., 2019) (Italy) | Hip OA NR Pro/Auto MFAT (Lipogems) | 6 | HHS, WOMAC, VAS; Improvement vs baseline; 6 mo follow up; No SAE (one hematoma @ lipo site) |
| Freitag et al. (Freitag et al., 2020c) (Australia) ACTRN12617000638336 | OA Knee CT NR Pro/Auto ASC (50 × 106 per knee at 0 and 6 mo) | 27 | KOOS, MRI, MOCART, NPRS, PGIC, WOMAC; Significant improvement vs baseline with 36 mo follow up; No SAE |
| Haas et al. (Haas et al., 2020) (Germany) | Thumb Carpometacarpal OA NR Pro/Auto MFAT (Luer Lock) | 89 | MHQ; Significant improvement vs baseline at 12 mo follow up; No SAE |
| Hudetz et al. (Hudetz et al., 2019) (Croatia) | Knee OA NR Pro/Auto MFAT (Lipogems) | 20 | KOOS, VAS, WOMAC; Significant improvement vs baseline up to 12 mo; No SAE |
| Malanga et al. (Malanga et al., 2021) (USA) NCT03714659 | Knee OA NR Pro/Auto MFAT (Lipogems) | 20 | KOOS, NPRS; Significant improvement vs baseline up to 12 mo follow up; No SAE (swelling/bruising at lipo site (Niada et al., 2019) or injection site (Osteoarthritis: Structural Endpoints for the Development of Drugs, Devices, and Biological Products for Treatment Guidance for Industry, 2018)) |
| Mayoly et al. (Mayoly et al., 2019) (France) NCT03164122/EudraCT #2016-002648-18 | Wrist OA NR Pro/Auto MFAT (Hapifat) + PRP | 3 | DASH, PRWE, VAS; Significant improvement vs baseline up to 12 mo; No SAE (pain reported at adipose harvest site) |
| Natali et al. (Natali et al., 2021) (Italy) | Ankle OA NR Pro/Auto MFAT (Lipogems) | 31 | AOFAS, FADI, VAS; Significant improvement vs baseline up to 24 mo follow up; No SAE |
| Tsubosaka et al. (Tsubosaka et al., 2020) (Japan) | Knee OA NR Pro/Auto SVF (Cytori) (25 × 106 at 0 mo) | 57 | KOOS, MRI, VAS, WOMAC; Improvement vs baseline av. 13.4 mo; No SAE |
Abbreviations: Allo, Allogeneic; AOFAS, American Orthopaedic Foot and Ankle Society (AOFAS) scale; ASC, Adipose-derived Stromal/stem Cells; Auto, Autologous; B, Blinded; BMAC, Bone Marrow Aspirate Concentrate; CR, Case Report; CS, Case Series; CT, Controlled Trial; DASH, Disabilities of the Arm and Shoulders; dGEMRIC, Delayed Gadolinium Enhanced MRI of Cartilage; FJS, Forgotten Joint Scale; HA, Hyaluronate; HHS, Harris Hip Score; IKDC 2000, International Knee Documentation Committee 2000; KOOS, Knee Injury and Osteoarthritis Outcome Score; LS, Lysholm Score; MFAT, Microfragmented Adipose Tissue; MHQ, Michigan Hand Outcomes Questionnaire; MOCART, MRI Observation of Cartilage Repair Tissue; NPRS, Numeric Pain Rating Scale; NR, Not Randomized; NRS, Noise Reporting Scale; PGIC, Patient Global Impression of Change; Pro, Prospective; PRP, Platelet Rich Plasma; PRWE, Patient-Related Wrist Evaluation; R, Randomized; Retro, Retrospective; SAE, Serious Adverse Event; SVF, Stromal Vascular Fraction Cells; TBCR, To Be Conducted/Reported, VAS, Visual Analog Scale; WOMAC, Western Ontario and McMasters Universities Osteoarthritis Index.
Table 6.
Randomized controlled prospective clinical trials.
| Freitag et al. (Freitag et al., 2019) (Australia) ACTRN12614000814673 |
OA Knee RCT Pro/Auto ASC (100 × 106 per knee at 0 mo ± 6 mo) | 30 | KOOS, MOAKS, NPRS, WOMAC; Significant improvement with 1 or 2 injections vs saline at 12 mo follow up; No SAE (mild to moderate injection site pain) |
| Garza et al. (Garza et al., 2020) (USA) NCT02726945 | Knee OA B RCT Pro Phase II/Auto SVF (GID) (0, 15, or 30 × 106) | 39 | MRI, WOMAC; Dose dependent significant improvement vs placebo control; 12 mo follow up; No SAE |
| Lee et al. (Lee et al., 2019) (Republic of Korea) | OA Knee B RCT Pro Phase IIB Efficacy/Auto ASC (108 per knee) | 24 | KOOS, MRI, VAS, WOMAC; Significant improvement ASC vs placebo 6 mo follow up; No SAE (83 % treatment and 58 % control with mild to mod AE reported) |
| Lu et al. (Lu et al., 2019) (China) NCT02162693. Registered 13 June 2014 |
OA Knee B RCT Pro Phase IIB Efficacy/Auto ASC (50 × 106 per knee) | 53 | MRI, SF-36, VAS, WOMAC; Significant improvement vs hyaluronic acid injection with 12 mo follow up; SAE 1.9 % with joint infection in single HA control patient withdrawn from study |
| Pers et al. (Pers et al., 2018) (France/Germany) TC301; EudraCT N°: 2011–000183-10 |
OA Knee R Pro Phase I Safety/Auto ASC (2, 10 or 50 × 106 per knee) | 18 | Immunophenotype, WOMAC, VAS; Increased circulating Treg cells following ASC injection; No SAE |
| Qiao et al. (Qiao et al., 2020) (China) NCT02855073 |
OA Knee RCT Pro Phase IIA/Auto ASC (50 × 106 per knee with hyaluronic acid) | 60 | Histology, MRI, WOMAC; Improvement vs HA or saline alone with microfracture at 24 mo follow up; No SAE (joint swelling) |
| Sembronio et al. (Sembronio et al., 2021) (Italy) | Temporomandibular OA RCT Pro/Auto MFAT (Lipogems) vs Hyaluronate | 40 | VAS; Significant improvement vs HA control at 6 mo follow up; No SAE |
| Simunec et al. (Germany) (Simunec et al., 2020) | Knee OA R Pro/Auto SVF (Q Graft) ± PRP | 12 | KOOS, MRI; Improvement vs baseline up to 12 mo; No SAE |
| Zhao et al. (Zhao et al., 2019) (China) NCT02641860. Registered 3 December 2015 | OA Knee B RCT Pro Phase I/IIB Safety Efficacy/Allo ASC (10, 20 or 50 × 106 per knee with injections at 0 and 3 wk) | 18 | MOCART, SF-36, WOMAC; Significant improvement vs baseline at all ASC concentrations with 11 mo follow up; No SAE |
Abbreviations: Allo, Allogeneic; ASC, Adipose-derived Stromal/stem Cells; Auto, Autologous; B, Blinded; BMAC, Bone Marrow Aspirate Concentrate; CR, Case Report; CS, Case Series; CT, Controlled Trial; HA, Hyaluronate; KOOS, Knee Injury and Osteoarthritis Outcome Score; MFAT, Microfragmented Adipose Tissue; MOAKS, MRI Osteoarthritis Knee Score; NR, Not Randomized; Pro, Prospective; PRP, Platelet Rich Plasma; R, Randomized; Retro, Retrospective; SAE, Serious Adverse Event; SF-36, Quality of Life Questionnaire; SVF, Stromal Vascular Fraction Cells; TBCR, To Be Conducted/Reported; WOMAC, Western Ontario and McMasters Universities Osteoarthritis Index.
Table 7.
Study designs for to be conducted-randomized controlled prospective clinical trials.
| Bakowski et al. (Bakowski et al., 2020) (Poland) | Knee OA RCT Pro/Auto MFAT (Lipogems) vs PRP | Not reported | KOOS, IKDC 2000, WOMAC; TBC; 12 mo follow up |
| Krzesniak et al. (Krzesniak et al., 2021) (Poland) NCT04675359 (06 Jan 2021) |
Knee OA RCT Pro/Auto SVF vs MFAT (Lipogems) | 100 | KOOS, MRI; TBC; 12 mo follow up |
| Mikkelson et al. (Mikkelsen et al., 2021) (Denmark) NCT03771989 Registered on Dec. 13th 2018. |
OA Knee B RCT Pro Phase II Efficacy/Auto MFAT (5 ml per knee)/Lipogems | 120 | KOOS, Tegner Activity Scale; TBC; 24 mo follow up |
| Nasb et al. (Nasb et al., 2020) (China) ChiCTR1900025907 | Knee OA B RCT Pro/± Auto ASC ± Ultrasound Rx | 96 | MRI, WOMAC; TBC; 6 mo follow up |
Abbreviations: Allo, Allogeneic; ASC, Adipose-derived Stromal/stem Cells; Auto, Autologous; B, Blinded; BMAC, Bone Marrow Aspirate Concentrate; CR, Case Report; CS, Case Series; CT, Controlled Trial; HA, Hyaluronate; MFAT, Microfragmented Adipose Tissue; NR, Not Randomized; Pro, Prospective; PRP, Platelet Rich Plasma; R, Randomized; Retro, Retrospective; SVF, Stromal Vascular Fraction Cells; TBCR, To Be Conducted.
Overall, these clinical trials enrolled a total of n = 939 subjects. The studies can be sub-divided based on their level of evidence. The least rigorous were case reports, case series and retrospective reviews that primarily benchmarked treatment outcomes using the pre-operative self-reported patient metrics as baseline control (Table 4). These appeared in a total of six publications with authors enrolling subjects in five separate countries; three of the studies included at least one US-based author. One of the studies evaluated SVF cell therapy, two evaluated ASC therapy, and three examined microfragmented adipose tissue (MFAT). Only 16 % (1/6) of these publications reported a national clinical trial registration. Altogether, these trials represent findings from a total of n = 235 patients.
The next level of rigor included prospective, non-randomized clinical trials (Table 5). These appeared in a total of thirteen publications, with authors enrolling subjects in ten separate countries; one of the studies was conducted in the US. One of the trials evaluated SVF cell therapy, two examined ASC treatment, and nine focused on MFAT. Nearly half (46 %) of these publications reported a national clinical trial registration. Altogether, these trials represent findings from a total of n = 410 patients.
The most rigorous studies reported were prospective, randomized controlled clinical trials (Table 6). These appeared in a total of nine publications with authors enrolling subjects in seven countries; one of the studies was conducted in the US. Two trials evaluated SVF cell therapy, six examined ASC treatments, and one employed MFAT. Two-thirds (66 %) of these publications reported a national clinical trial registration, either with the FDA or another national regulatory agency. Altogether, these trials represent findings from a total of n = 294 patients.
While FDA clinical trials often include international patients, the agency frequently requires that a sizable percentage of patients be recruited from US to represent the nation's unique demographics. There is a single FDA registered Phase IIB clinical trial that was performed exclusively in the US using SVF cells to treat OA which merits further evaluation (Garza et al., 2020). This double-blinded, randomized controlled study was conducted at three sites (Camden NJ, Philadelphia PA, San Antonio TX) and enrolled 39 subjects. All patients, regardless of randomization, underwent lipoaspiration and both patients and healthcare providers were blinded to the therapy. The adipose tissue was processed with a single closed system device (GID-SVF2 tissue processing device, GID Group, Louisville CO) to recover isolated autologous SVF cells. Subjects were randomized to receive knee joint injections under ultrasound guidance. Under the dose escalation protocol, the first 15 consecutive participants were randomized to either the low dose (1.5 × 107) of autologous SVF cells or the placebo group (Lactated Ringer's Solution) and followed for 6 weeks with a safety and adverse events analysis. The remaining 24 patients were randomly assigned to the high dose (3 × 107), the low dose or the placebo group. Patients were monitored by WOMAC questionnaire prior to surgery and 6 weeks, 3, 6, and 12 months after surgery and by MRI exam prior to surgery and 12 months afterward. At the 6-month time point, the control or placebo group showed a mean change of 25.0 % relative to baseline in outcome scores, while the low dose and high dose SVF cell treatment groups showed clinically significant improvements of 51.5 % and 83.9 % relative to baseline. Despite a rigorous clinical design, the study faces several limitations. First, the study was unblinded after 6 months, so that the persistence of the therapeutic outcomes beyond that timepoint is not known. Second, the enrolled population was 82 % Caucasian, 15.4 % Hispanic, and 2.6 % African-American which does not adequately reflect the US demographic. Third, patients with body mass indices >35 and/or co-morbidities were excluded from the study resulting in a mean subject BMI of 27.8 (range 19–34.9). Thus, extensions of this work will need to address these potential shortcomings by enrolling a more representative cohort with respect to racial and ethnic minorities in the OA population, displaying BMIs of morbid obesity, and with co-morbidities. Despite these limitations, the report by Garza et al. represents the most comprehensive FDA-approved randomized controlled clinical evaluation of an adipose cell-derived therapy for OA completed in the US to date (Garza et al., 2020). Indeed, the GID Group is pursuing its promising findings by recruiting subjects at twelve sites within the US for a Phase III clinical trial. While clinical trial designs with comparable levels of rigor are being proposed in the literature (Table 7), all of these will be conducted exclusively outside the US in either the EU or China.
5. Clinical translation of adipose-derived products in the treatment of osteoarthritis: future directions and needs
While the literature review indicates substantial international interest in applying adipose-derived products in OA therapy, it also highlights a series of questions that must be addressed.
5.1. Do we need more US-based clinical trials?
Clinical trials remain an absolute and necessary requirement for a transparent, evidence-based assessment of new biological therapies. While a single-arm, unblinded study is sufficient for a Phase I (IND) or pilot (IDE) safety trial, an FDA-approved Phase II/III (IND) or pivotal (IDE) efficacy trial is likely to be required to include at least one “standard of care” arm to serve as a baseline control to compare against any experimental modality. From a regulatory perspective, the “gold standard” remains the randomized controlled trial (RCT) and future studies must incorporate this design. Recently, alternative approaches have been suggested, such as Practice Based Evidence or “pragmatic” clinical trials (https://www.nejm.org/doi/full/10.1056/NEJMra1510059), where large community-based populations are enrolled with minimal exclusion criteria into studies using detailed clinical monitoring metrics over extended periods. While PBE trials have proven helpful in discovering new therapies in broad patient populations, this methodology is at best likely to be viewed as only a complement to RCT by regulatory authorities in the U.S. (Horn et al., 2012; Henry et al., 2017). Therefore, it can be expected that an approved RCT study designed to enroll US-based patients, with positive outcomes and acceptable cellular product characterization, will be necessary before the FDA will approve adipose-derived biologic therapies for OA.
5.2. Can there be mechanisms for industry to establish and certify standards for adipose-derived cell and tissue therapeutics in concert with FDA?
The biomanufacturing and regulatory landscapes are evolving as basic research is translated into clinical practice. This offers an opportunity to coordinate and standardize product safety, efficacy, potency, and performance metrics. The industry developing adipose-derived therapies for OA includes partners from academia, biotech ventures, device companies, and the pharmaceutical industry. Additionally, a number of public and private organizations, institutions, and partnerships are stakeholders. These include the Advanced Regenerative Manufacturing Institute (ARMI/BioFabUSA), American Association of Orthopedic Surgeons (AAOS), Foundation for Accreditation of Cellular Therapy (FACT), International Federation of Adipose Therapeutics and Science (IFATS), International Society for Cell and Gene Therapy (ISCT), and National Institute for Standards and Technology (NIST), to name just a few. If there can be industry-wide coordination between these entities, it will be feasible to standardize and harmonize international metrics for critical elements in the biomanufacturing process with respect to product safety and efficacy. These would include definitions based on quantifiable assays associated with each cell type (SVF, ASC), biologic product (hydrogels and scaffolds from decellularized adipose tissue) and devices (point of care adipose cell isolation). Industry-wide acceptance of a standard set of assays related to functionality (differentiation, immunomodulation), composition, and/or performance could accelerate product development if appropriately coordinated with regulatory authorities (Bourin et al., 2013; Galipeau et al., 2016; Dominici et al., 2006). Similarly, the identification of a common set of questionnaires and clinical evaluation tools could benefit the field. As shown in Table 3, Table 4, Table 5, Table 6, Table 7, clinicians have used a wide range of questionnaires to evaluate their OA patients with respect to mobility, pain, and quality of life. Standardizing these protocols may set the stage for future investigators to combine data from multiple individual studies. With the sophisticated statistical tools now available, this approach may allow regulators to evaluate the safety and efficacy of cell or biologic OA therapies in larger populations more accurately and quantifiably without enrollment of an entirely new patient cohort.
5.3. Can there be mechanisms for patients and caregivers to identify FDA authorized clinical trials moving forward?
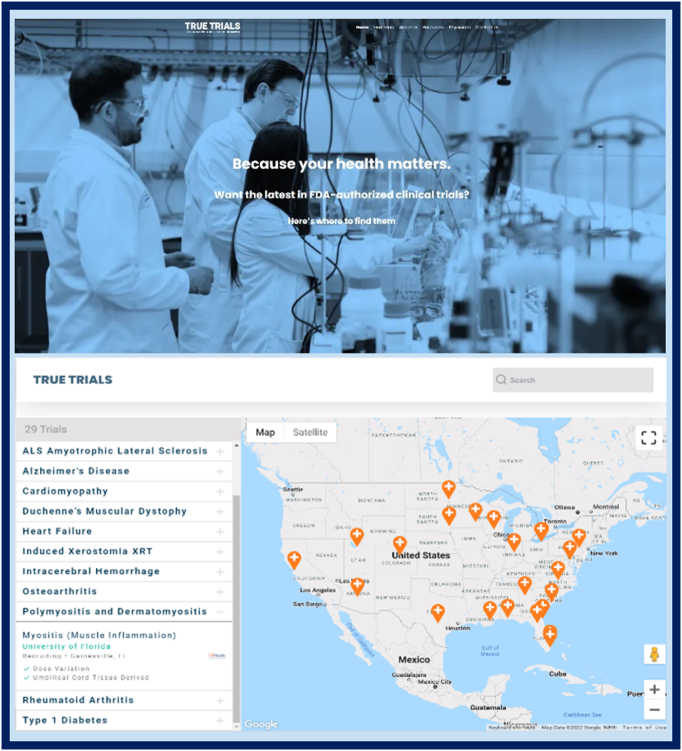
Currently, patients and their caregivers rely heavily on word of mouth and the www.Clinicaltrials.gov website to access ongoing adipose-derived therapeutic trials enrolling OA patients. Importantly, this mechanism is flawed, since FDA approval is not included as a criterion for determining whether or not a trial can be registered on the website. Steps are underway to address this challenge: a number of private sector partners are working together to develop an initiative known as TrueTrials, a 501c3 not-for-profit organization, with the website www.truetrials.org. This website will only list clinical trials authorized by the FDA to enroll patients, generally via IND or IDE; it provides a patient-directed interface with a searchable table of trials along with a linked map of trial sites (see Fig. 2). By allowing reliable access to this clinical trial website, patients and caregivers will have greater confidence in enrolling in clinical trials which have received FDA authorization, and with an appropriately reduced risk of safety, ethical or economic concerns. It will also assist in accelerating the rate of trial recruitment.
Fig. 2.
Excerpt from the development phase of the website for TrueTrials, a 501c3 organization, with the link www.truetrials.org. This website will only list clinical trials authorized by FDA to enroll patients, and provides a patient-directed interface with a searchable table of trials along with a linked map of trial sites, as shown.
5.4. Is there a continued need for basic science research into the mechanism of action of adipose-derived products in OA treatment?
Basic scientists are actively pursuing the fundamental mechanisms of the action exerted by adipose-derived biological products relevant to OA. While this body of work extends beyond the scope of the present review, one study merits comment since the majority of clinical trials have reported improvement in OA pain scores based on blinded patient-generated questionnaires (Table 4, Table 5, Table 6). Pre-clinical studies in a murine subcutaneous adipose tissue regenerative model have demonstrated that opioid receptors and resident macrophages contribute to the wound healing and scarring processes (Rabiller et al., 2021; Berthezene et al., 2021; Labit et al., 2018). By blocking nociceptive pain receptors with naloxone, genetic knockout, or supplementation with the neuropeptide Calcitonin-Gene Related Peptide (CRGF), the authors were able to reduce scarring and enhance wound healing in the subcutaneous adipose resection model (Berthezene et al., 2021; Labit et al., 2018). These novel findings indicate that cells within adipose tissue can actively direct regeneration by modulating the pain response. They suggest a possible mechanism(s) of action for adipose-derived products that merit additional investigation in the context of OA treatment. Clearly, this is just one example of how basic research in pre-clinical animal models has identified novel cellular mechanisms for OA therapies. There remains a need for further investigations into adipose cell paracrine- and secretome-based mechanisms can be utilized to modulate the underlying etiology of OA and ultimately to benefit patient care, quality of life, and overall outcomes.
CRediT authorship contribution statement
Conceptualization; TF, JRG, JMG.
Project administration; JMG.
Software; KH.
Visualization; KH, KM.
Roles/Writing - original draft; TF, JMG.
Writing - review & editing BAB, JRG, KFD, KM, ER.
Declaration of competing interest
T. Frazier and J.M. Gimble are co-founders, co-owners, and employees of Obatala Sciences, a for-profit biotechnology company focused on the development of adipose-derived cell and biomaterial products for research and clinical translational applications. They are co-inventors on patents relating to such products. Likewise, K. Hamel and E. Rogers are employees of Obatala Sciences. K. Darr is a co-owner and employee of Covington Orthopedics Sports Medicine Institute. K. March is actively involved as a developer and founder of TrueTrials.org, a 501c3 non-profit entity. J.R. Garza has received funding from the GID Group and InGeneron for the conduct of clinical trials and funding as a Medical Monitor/DSMB member for an FDA trial with 3D Bio. B.A. Bunnell, J.M. Gimble and K. March are former presidents and active board members of the International Federation of Adipose Therapeutics and Sciences (IFATS), a non-profit organization promoting research and clinical translation of adipose tissue products.
Acknowledgements
The authors thank their colleagues at Obatala Sciences for editorial support and comments.
References
- 21st Century Cures Act. 2020. [Google Scholar]
- Agarwal N., Mak C., Bojanic C., To K., Khan, W. Meta-analysis of adipose tissue derived cell-based therapy for the treatment of knee osteoarthritis. Cells. 2021;10 doi: 10.3390/cells10061365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo G., Niada S., Moschetti G., Franchi S., Savadori P., Brini A.T., Sacerdote P. Secretome of human adipose-derived mesenchymal stem cell relieves pain and neuroinflammation independently of the route of administration in experimental osteoarthritis. Brain Behav. Immunol. 2021;94:29. doi: 10.1016/j.bbi.2021.03.011. [DOI] [PubMed] [Google Scholar]
- Bakowski P., Kaszynski J., Walecka J., Ciemniewska-Gorzela K., Bakowska-Zywicka K., Piontek T. Autologous adipose tissue injection versus platelet-rich plasma (PRP) injection in the treatment of knee osteoarthritis: a randomized, controlled study - study protocol. BMC Musculoskelet Disord. 2020;21:314. doi: 10.1186/s12891-020-03345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakowski P., Kaszynski J., Baka C., Kaczmarek T., Ciemniewska-Gorzela K., Bakowska-Zywicka K., Piontek T. Patients with stage II of the knee osteoarthritis most likely benefit from the intra-articular injections of autologous adipose tissue-from 2 years of follow-up studies. Arch Orthop Trauma Surg. 2021 doi: 10.1007/s00402-021-03979-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barfod K.W., Blond L. Treatment of osteoarthritis with autologous and microfragmented adipose tissue. Dan Med J. 2019;66 [PubMed] [Google Scholar]
- Berthezene C.D., Rabiller L., Jourdan G., Cousin B., Penicaud L., Casteilla L., Lorsignol, A. Tissue regeneration: the dark side of opioids. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22147336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biazzo A., D'Ambrosi R., Masia F., Izzo V., Verde F. Autologous adipose stem cell therapy for knee osteoarthritis: where are we now? Phys. Sports Med. 2020;48:392. doi: 10.1080/00913847.2020.1758001. [DOI] [PubMed] [Google Scholar]
- Bistolfi A., Roato I., Fornelli G., Sabatini L., Masse A., Ferracini R. Treatment of knee osteoarthritis by intra-articular injection of concentrated autologous adipose tissue: a twenty four month follow-up study. Int Orthop. 2021;45:627. doi: 10.1007/s00264-020-04923-0. [DOI] [PubMed] [Google Scholar]
- Boric I., Hudetz D., Rod E., Jelec Z., Vrdoljak T., Skelin A., Polasek O., Plecko M., Trbojevic-Akmacic I., Lauc G., Primorac D. A 24-month follow-up study of the effect of intra-articular injection of autologous microfragmented fat tissue on proteoglycan synthesis in patients with knee osteoarthritis. Genes (Basel) 2019;10 doi: 10.3390/genes10121051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin P., Bunnell B.A., Casteilla L., Dominici M., Katz A.J., March K.L., Redl H., Rubin J.P., Yoshimura K., Gimble J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder S. New Warning from FDA: sales of unapproved orthobiologic products. BoneZone. 2021 [Google Scholar]
- Buzaboon N., Alshammary S. Clinical applicability of adult human mesenchymal stem cell therapy in the treatment of knee osteoarthritis. Stem Cells Cloning. 2020;13:117. doi: 10.2147/SCCAA.S268940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.H., Chen Y.C., Yu S.N., Lai W.L., Shen Y.S., Shen P.C., Lin S.H., Chang C.H., Lee S.M. Infrapatellar fat pad-derived mesenchymal stromal cell product for treatment of knee osteoarthritis: a first-in-human study with evaluation of the potency marker. Cytotherapy. 2021;1377 doi: 10.1016/j.jcyt.2021.08.006. [DOI] [PubMed] [Google Scholar]
- Circuit U.S.C.O.A.F.T.E., editor. United States v. US Stem Cell Clinic, LLC. 2021. [Google Scholar]
- Dall'Oca C., Breda S., Elena N., Valentini R., Samaila E.M., Magnan B. Mesenchymal stem cells injection in hip osteoarthritis: preliminary results. Acta Biomed. 2019;90(75) doi: 10.23750/abm.v90i1-S.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Francesco F., Gravina P., Busato A., Farinelli L., Soranzo C., Vidal L., Zingaretti N., Zavan B., Sbarbati A., Riccio M., Gigante, A. Stem cells in autologous microfragmented adipose tissue: current perspectives in osteoarthritis disease. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms221910197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanois R.E., Etcheson J.I., Sodhi N., Gwam C.U., George N.E., Henn R.F., 3rd, Mont M.A. Biologic therapies for the treatment of knee osteoarthritis. J. Arthroplasty. 2019;34:801. doi: 10.1016/j.arth.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Ellis B.W., Traktuev D.O., Merfeld-Clauss S., Can U.I., Wang M., Bergeron R., Zorlutuna P., March K.L. Adipose stem cell secretome markedly improves rodent heart and human induced pluripotent stem cell-derived cardiomyocyte recovery from cardioplegic transport solution exposure. Stem Cells. 2021;39:170. doi: 10.1002/stem.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn L.E. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials. 2010;31:4715. doi: 10.1016/j.biomaterials.2010.02.046. [DOI] [PubMed] [Google Scholar]
- Freitag J., Bates D., Wickham J., Shah K., Huguenin L., Tenen A., Paterson K., Boyd R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med. 2019;14:213. doi: 10.2217/rme-2018-0161. [DOI] [PubMed] [Google Scholar]
- Freitag J., Wickham J., Shah K., Tenen A. Effect of autologous adipose-derived mesenchymal stem cell therapy in the treatment of an osteochondral lesion of the ankle. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-234595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag J., Shah K., Wickham J., Li D., Norsworthy C., Tenen A. Evaluation of autologous adipose-derived mesenchymal stem cell therapy in focal chondral defects of the knee: a pilot case series. Regen. Med. 2020;15:1703. doi: 10.2217/rme-2020-0027. [DOI] [PubMed] [Google Scholar]
- Freitag J., Wickham J., Shah K., Li D., Norsworthy C., Tenen A. Mesenchymal stem cell therapy combined with arthroscopic abrasion arthroplasty regenerates cartilage in patients with severe knee osteoarthritis: a case series. Regen Med. 2020;15(1957) doi: 10.2217/rme-2020-0128. [DOI] [PubMed] [Google Scholar]
- Galipeau J., Krampera M., Barrett J., Dazzi F., Deans R.J., DeBruijn J., Dominici M., Fibbe W.E., Gee A.P., Gimble J.M., Hematti P., Koh M.B., LeBlanc K., Martin I., McNiece I.K., Mendicino M., Oh S., Ortiz L., Phinney D.G., Planat V., Shi Y., Stroncek D.F., Viswanathan S., Weiss D.J., Sensebe L. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18:151. doi: 10.1016/j.jcyt.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza J.R., Campbell R.E., Tjoumakaris F.P., Freedman K.B., Miller L.S., Santa Maria D., Tucker B.S. Clinical efficacy of intra-articular mesenchymal stromal cells for the treatment of knee osteoarthritis: a double-blinded prospective randomized controlled clinical trial. Am. J. Sports Med. 2020;48:588. doi: 10.1177/0363546519899923. [DOI] [PubMed] [Google Scholar]
- Gentile P., Sterodimas A., Pizzicannella J., Dionisi L., De Fazio D., Calabrese C., Garcovich S. Systematic review: allogenic use of stromal vascular fraction (SVF) and decellularized extracellular matrices (ECM) as advanced therapy medicinal products (ATMP) in tissue regeneration. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21144982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiasloo M., Lobato R.C., Diaz J.M., Singh K., Verpaele A., Tonnard P. Expanding clinical indications of mechanically isolated stromal vascular fraction: a systematic review. Aesthet Surg J. 2020;40 doi: 10.1093/asj/sjaa111. [DOI] [PubMed] [Google Scholar]
- Gobbi A., Dallo I., Rogers C., Striano R.D., Mautner K., Bowers R., Rozak M., Bilbool N., Murrell W.D. Two-year clinical outcomes of autologous microfragmented adipose tissue in elderly patients with knee osteoarthritis: a multi-centric, international study. Int Orthop. 2021;45:1179. doi: 10.1007/s00264-021-04947-0. [DOI] [PubMed] [Google Scholar]
- Haas E.M., Eisele A., Arnoldi A., Paolini M., Ehrl D., Volkmer E., Giunta R.E. One-year outcomes of intraarticular fat transplantation for thumb carpometacarpal joint osteoarthritis: case review of 99 joints. Plast Reconstr Surg. 2020;145:151. doi: 10.1097/PRS.0000000000006378. [DOI] [PubMed] [Google Scholar]
- Han S.B., Seo I.W., andShin Y.S. Intra-articular injections of hyaluronic acid or steroids associated with better outcomes than platelet-rich plasma, adipose mesenchymal stromal cells, or placebo in knee osteoarthritis: a network meta-analysis. Arthroscopy. 2021;37:292. doi: 10.1016/j.arthro.2020.03.041. [DOI] [PubMed] [Google Scholar]
- Henry D., Tolan P., Gorman-Smith D., Schoeny M. Alternatives to randomized control trial designs for community-based prevention evaluation. Prev. Sci. 2017;18:671. doi: 10.1007/s11121-016-0706-8. [DOI] [PubMed] [Google Scholar]
- Hiltzick M. Los Angeles Times; Los Angeles CA: 2022. Column: Mark Berman, Pusher of Unproven Stem Cell Therapies, Dies While Awaiting Verdict in FDA Lawsuit. [Google Scholar]
- Horn S.D., DeJong G., Deutscher D. Practice-based evidence research in rehabilitation: an alternative to randomized controlled trials and traditional observational studies. Arch. Phys. Med. Rehabil. 2012;93:S127. doi: 10.1016/j.apmr.2011.10.031. [DOI] [PubMed] [Google Scholar]
- Hudetz D., Boric I., Rod E., Jelec Z., Kunovac B., Polasek O., Vrdoljak T., Plecko M., Skelin A., Polancec D., Zenic L., Primorac D. Early results of intra-articular micro-fragmented lipoaspirate treatment in patients with late stages knee osteoarthritis: a prospective study. Croat Med J. 2019;60:227. doi: 10.3325/cmj.2019.60.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling L.E., Belk J.W., Kraeutler M.J., Kallner A.C., Lindsay A., McCarty E.C., Postma W.F. Bone marrow aspirate concentrate for the treatment of knee osteoarthritis: a systematic review. Am J Sports Med. 2021;8:2315–2323. doi: 10.1177/03635465211018837. 3635465211018837. [DOI] [PubMed] [Google Scholar]
- Kokai L.E., Schilling B.K., Chnari E., Huang Y.C., Imming E.A., Karunamurthy A., Khouri R.K., D'Amico R.A., Coleman S.R., Marra K.G., Rubin J.P. Injectable allograft adipose matrix supports adipogenic tissue remodeling in the nude mouse and human. Plast. Reconstr. Surg. 2019;143:299e. doi: 10.1097/PRS.0000000000005269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzesniak A.M., Radzimowski K., Stolarczyk A. Comparison of the treatment results of knee osteoarthritis using adipose tissue mesenchymal stromal cells derived through enzymatic digestion and mechanically fragmented adipose tissue. Medicine (Baltimore) 2021;100 doi: 10.1097/MD.0000000000024777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labit E., Rabiller L., Rampon C., Guissard C., Andre M., Barreau C., Cousin B., Carriere A., Eddine M.A., Pipy B., Penicaud L., Lorsignol A., Vriz S., Dromard C., Casteilla L. Opioids prevent regeneration in adult mammals through inhibition of ROS production. Sci. Rep. 2018;8:12170. doi: 10.1038/s41598-018-29594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapuente J.P., Dos-Anjos S., Blazquez-Martinez A. Intra-articular infiltration of adipose-derived stromal vascular fraction cells slows the clinical progression of moderate-severe knee osteoarthritis: hypothesis on the regulatory role of intra-articular adipose tissue. J Orthop Surg Res. 2020;15:137. doi: 10.1186/s13018-020-01664-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.S., Kim H.J., Kim K.I., Kim G.B., Jin W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8:504. doi: 10.1002/sctm.18-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Dai C., Zhang Z., Du H., Li S., Ye P., Fu Q., Zhang L., Wu X., Dong Y., Song Y., Zhao D., Pang Y., Bao C. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res. Ther. 2019;10:143. doi: 10.1186/s13287-019-1248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanga G.A., Chirichella P.S., Hogaboom N.S., Capella T. Clinical evaluation of micro-fragmented adipose tissue as a treatment option for patients with meniscus tears with osteoarthritis: a prospective pilot study. Int Orthop. 2021;45:473. doi: 10.1007/s00264-020-04835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P.W. Clear evidence of safety and efficacy is needed for stromal vascular fraction products: commentary on "Arguments for a different regulatory categorization and framework for stromal vascular Fraction". Stem Cells Dev. 2020;29:263. doi: 10.1089/scd.2020.0011. [DOI] [PubMed] [Google Scholar]
- Mautner K., Bowers R., Easley K., Fausel Z., Robinson R. Functional outcomes following microfragmented adipose tissue versus bone marrow aspirate concentrate injections for symptomatic knee osteoarthritis. Stem Cells Transl Med. 2019;8:1149. doi: 10.1002/sctm.18-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoly A., Iniesta A., Curvale C., Kachouh N., Jaloux C., Eraud J., Vogtensperger M., Veran J., Grimaud F., Jouve E., Casanova D., Sabatier F., Legre R., Magalon J. Development of autologous platelet-rich plasma mixed-microfat as an advanced therapy medicinal product for intra-articular injection of radio-carpal osteoarthritis: from validation data to preliminary clinical results. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehranfar S., Abdi Rad I., Mostafav E., Akbarzadeh A. The use of stromal vascular fraction (SVF), platelet-rich plasma (PRP) and stem cells in the treatment of osteoarthritis: an overview of clinical trials. Artif. Cells Nanomed. Biotechnol. 2019;47:882. doi: 10.1080/21691401.2019.1576710. [DOI] [PubMed] [Google Scholar]
- Migliorini F., Rath B., Colarossi G., Driessen A., Tingart M., Niewiera M., Eschweiler J. Improved outcomes after mesenchymal stem cells injections for knee osteoarthritis: results at 12-months follow-up: a systematic review of the literature. Arch Orthop Trauma Surg. 2020;140:853. doi: 10.1007/s00402-019-03267-8. [DOI] [PubMed] [Google Scholar]
- Mikkelsen R.K., Blond L., Holmich L.R., Molgaard C., Troelsen A., Holmich P., Barfod K.W. Treatment of osteoarthritis with autologous, micro-fragmented adipose tissue: a study protocol for a randomized controlled trial. Trials. 2021;22:748. doi: 10.1186/s13063-021-05628-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohiuddin O.A., Campbell B., Poche J.N., Thomas-Porch C., Hayes D.A., Bunnell B.A., Gimble J.M. Decellularized adipose tissue: biochemical composition, in vivo analysis and potential clinical applications. Adv. Exp. Med. Biol. 2020;1212:57. doi: 10.1007/5584_2019_371. [DOI] [PubMed] [Google Scholar]
- Nasb M., Liangjiang H., Gong C., Hong C. Human adipose-derived Mesenchymal stem cells, low-intensity pulsed ultrasound, or their combination for the treatment of knee osteoarthritis: study protocol for a first-in-man randomized controlled trial. BMC Musculoskelet Disord. 2020;21:33. doi: 10.1186/s12891-020-3056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali S., Screpis D., Farinelli L., Iacono V., Vacca V., Gigante A., Zorzi C. The use of intra-articular injection of autologous micro-fragmented adipose tissue as pain treatment for ankle osteoarthritis: a prospective not randomized clinical study. Int Orthop. 2021;45:2239. doi: 10.1007/s00264-021-05093-3. [DOI] [PubMed] [Google Scholar]
- Ni Z., Zhou S., Li S., Kuang L., Chen H., Luo X., Ouyang J., He M., Du X., Chen L. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8:25. doi: 10.1038/s41413-020-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niada S., Giannasi C., Gomarasca M., Stanco D., Casati S., Brini A.T. Adipose-derived stromal cell secretome reduces TNFalpha-induced hypertrophy and catabolic markers in primary human articular chondrocytes. Stem Cell Res. 2019;38 doi: 10.1016/j.scr.2019.101463. [DOI] [PubMed] [Google Scholar]
- Olsson D.C., Teixeira B.L., Jeremias T.D.S., Reus J.C., De Luca Canto G., Porporatti A.L., Trentin A.G. Administration of mesenchymal stem cells from adipose tissue at the hip joint of dogs with osteoarthritis: A systematic review. Res Vet Sci. 2021;135:495. doi: 10.1016/j.rvsc.2020.11.014. [DOI] [PubMed] [Google Scholar]
- Osteoarthritis: Structural Endpoints for the Development of Drugs, Devices, and Biological Products for Treatment Guidance for Industry. 2018. [Google Scholar]
- Pers Y.M., Quentin J., Feirreira R., Espinoza F., Abdellaoui N., Erkilic N., Cren M., Dufourcq-Lopez E., Pullig O., Noth U., Jorgensen C., Louis-Plence P. Injection of adipose-derived stromal cells in the knee of patients with severe osteoarthritis has a systemic effect and promotes an anti-inflammatory phenotype of circulating immune cells. Theranostics. 2018;8:5519. doi: 10.7150/thno.27674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primorac D., Molnar V., Rod E., Jelec Z., Cukelj F., Matisic V., Vrdoljak T., Hudetz D., Hajsok H., Boric I. Knee osteoarthritis: a review of pathogenesis and state-of-the-art non-operative therapeutic considerations. Genes (Basel) 2020;11 doi: 10.3390/genes11080854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Z., Tang J., Yue B., Wang J., Zhang J., Xuan L., Dai C., Li S., Li M., Xu C., Dai K., Wang Y. Human adipose-derived mesenchymal progenitor cells plus microfracture and hyaluronic acid for cartilage repair: a phase IIa trial. Regen. Med. 2020;15:1193. doi: 10.2217/rme-2019-0068. [DOI] [PubMed] [Google Scholar]
- Rabiller L., Labit E., Guissard C., Gilardi S., Guiard B.P., Mouledous L., Silva M., Mithieux G., Penicaud L., Lorsignol A., Casteilla L., Dromard C. Pain sensing neurons promote tissue regeneration in adult mice. NPJ Regen. Med. 2021;6:63. doi: 10.1038/s41536-021-00175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-based Products: Minimal Manipulation and Homologous Use. US Department of Health and Human Services F.a.D.A., Center for Biologic Evaluation and Research, Center for Devices and Radiological Health, Office of Combination Products; Silver Spring MD: 2020. [Google Scholar]
- Rodriguez R.L., Frazier T., Bunnell B.A., Mouton C.A., March K.L., Katz A.J., Rubin J.P., Llull R., Sorensen J.A., Gimble J.M. Arguments for a different regulatory categorization and framework for stromal vascular fraction. Stem Cells Dev. 2020;29:257. doi: 10.1089/scd.2019.0096. [DOI] [PubMed] [Google Scholar]
- Sembronio S., Tel A., Tremolada C., Lazzarotto A., Isola M., Robiony M. Temporomandibular joint arthrocentesis and microfragmented adipose tissue injection for the treatment of internal derangement and osteoarthritis: a randomized clinical trial. J Oral Maxillofac Surg. 2021;79:1447. doi: 10.1016/j.joms.2021.01.038. [DOI] [PubMed] [Google Scholar]
- Shanmugasundaram S., Vaish A., Chavada V., Murrell W.D., Vaishya R. Assessment of safety and efficacy of intra-articular injection of stromal vascular fraction for the treatment of knee osteoarthritis-a systematic review. Int. Orthop. 2021;45:615. doi: 10.1007/s00264-020-04926-x. [DOI] [PubMed] [Google Scholar]
- Shariatzadeh M., Song J., Wilson S.L. The efficacy of different sources of mesenchymal stem cells for the treatment of knee osteoarthritis. Cell Tissue Res. 2019;378:399. doi: 10.1007/s00441-019-03069-9. [DOI] [PubMed] [Google Scholar]
- Simunec D., Salari H., Meyer J. Treatment of grade 3 and 4 osteoarthritis with intraoperatively separated adipose tissue-derived stromal vascular fraction: a comparative case series. Cells. 2020;9 doi: 10.3390/cells9092096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S.H.S., Kwan Y.T., Neo W.J., Chong J.Y., Kuek T.Y.J., See J.Z.F., Wong K.L., Toh W.S., Hui J.H.P. Intra-articular injections of mesenchymal stem cells without adjuvant therapies for knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2021;49:3113. doi: 10.1177/0363546520981704. [DOI] [PubMed] [Google Scholar]
- Tremolada C., Colombo V., Ventura C. Adipose tissue and mesenchymal stem cells: state of the art and lipogems(R) technology development. Curr. Stem Cell Rep. 2016;2:304. doi: 10.1007/s40778-016-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremolada C., Ricordi C., Caplan A.I., Ventura C. Mesenchymal stem cells in lipogems, a reverse story: from clinical practice to basic science. Methods Mol Biol. 2016;1416:109. doi: 10.1007/978-1-4939-3584-0_6. [DOI] [PubMed] [Google Scholar]
- Tsubosaka M., Matsumoto T., Sobajima S., Matsushita T., Iwaguro H., Kuroda R. The influence of adipose-derived stromal vascular fraction cells on the treatment of knee osteoarthritis. BMC Musculoskelet Disord. 2020;21:207. doi: 10.1186/s12891-020-03231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahedi P., Moghaddamshahabi R., Webster T.J., Calikoglu Koyuncu A.C., Ahmadian E., Khan W.S., Jimale Mohamed A., Eftekhari A. The use of infrapatellar fat pad-derived mesenchymal stem cells in articular cartilage regeneration: a review. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22179215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinet-Jones H., Darr K.F. Clinical use of autologous micro-fragmented fat progressively restores pain and function in shoulder osteoarthritis. Regen Med. 2020;15(2153) doi: 10.2217/rme-2020-0069. [DOI] [PubMed] [Google Scholar]
- Wei X., Du Z., Zhao L., Feng D., Wei G., He Y., Tan J., Lee W.H., Hampel H., Dodel R., Johnstone B.H., March K.L., Farlow M.R., Du Y. IFATS collection: the conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells. 2009;27:478. doi: 10.1634/stemcells.2008-0333. [DOI] [PubMed] [Google Scholar]
- Xiang X.N., Zhu S.Y., He H.C., Yu X., Xu Y., He C.Q. Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis. Stem Cell Res Ther. 2022;13(14) doi: 10.1186/s13287-021-02689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Pan J.K., Yang W.Y., Han Y.H., Zeng L.F., Liang G.H., Liu J. Intra-articular injections of platelet-rich plasma, adipose mesenchymal stem cells, and bone marrow mesenchymal stem cells associated with better outcomes than hyaluronic acid and saline in knee osteoarthritis: a systematic review and network meta-analysis. Arthroscopy. 2021;37:2298. doi: 10.1016/j.arthro.2021.02.045. [DOI] [PubMed] [Google Scholar]
- Zhao X., Ruan J., Tang H., Li J., Shi Y., Li M., Li S., Xu C., Lu Q., Dai C. Multi-compositional MRI evaluation of repair cartilage in knee osteoarthritis with treatment of allogeneic human adipose-derived mesenchymal progenitor cells. Stem Cell Res. Ther. 2019;10:308. doi: 10.1186/s13287-019-1406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]