Abstract
Herein we report the first total synthesis of RvD2n‐3 DPA, an endogenously formed mediator biosynthesized from the omega‐3 fatty acid n‐3 docosapentaenoic acid. The key steps are the Midland Alpine borane reduction, Sonogashira cross‐coupling reactions, and a Z‐selective alkyne reduction protocol, yielding RvD2n‐3 DPA methyl ester in 13 % yield over 12 steps (longest linear sequence). The physical property data (UV chromophore, chromatography and MS/MS fragmentation) of the synthetic lipid mediator matched those obtained from biologically produced material. Moreover, synthetic RvD2n‐3 DPA also carried the potent biological activities of enhancing macrophage uptake of Staphylococcus aureus and zymosan A bioparticles.
Keywords: n-3 docosapentaenoic acid, resolution of bacterial infection and inflammation, resolvins, RvD2n-3 DPA , specialized pro-resolving mediators
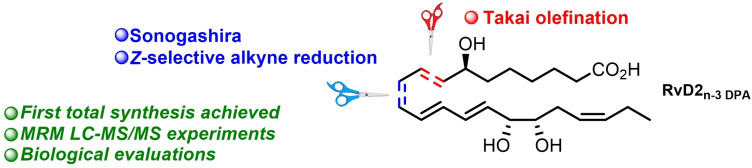
RvD2n‐3 DPA, a specialized pro‐resolving mediator, is synthesized for the first time based on the retrosynthetic outline depicted, enabling its structural elucidation and biological evaluations against S. aureus and zymosan A induced inflammation.
Introduction
The inflammatory process is an essential part of the normal protective response to tissue injury and infection by invading pathogens and is divided into acute and chronic inflammation. [1] Although the primary goal of the inflammation phase is to regain homeostasis, [2] if kept uncontrolled, it may result in the development of a highly unappreciated outcome, namely a chronic state of inflammation. Chronic inflammation is associated with diseases such as rheumatoid arthritis, cardiovascular disorders, Parkinson and Alzheimer's diseases. [3] However, the acute inflammatory response is normally self‐limited and resolves smoothly. This self‐contained process is divided into two distinct phases: the initiation and the resolution phase. The resolution of the inflammatory process is now considered to be a dynamic, programmed response, and not just a means of passive dilution of chemoattractants, as previously thought. [4] Further, evidence for this emerged with the discovery of oxygenated lipid mediators possessing pro‐resolving abilities biosynthesized from the ω‐3 polyunsaturated fatty acids (PUFAs) docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), and n‐3 docosapentaenoic acid (n‐3 DPA).[ 5 , 6 , 7 ]
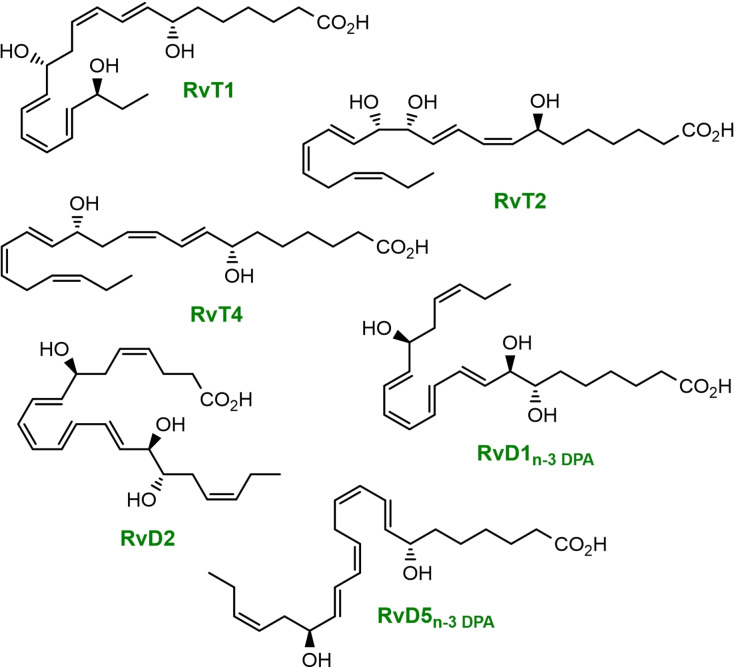
New drugs that can resolve inflammation without undesirable side effects are of great interest in drug discovery programs, [8] and are therefore attractive targets for total synthesis. [9] The pro‐resolving endogenously formed resolvins are excellent candidates displaying these actions in vivo [10] and belong to a superfamily called specialized pro‐resolving mediators (SPMs). SPMs are derived from ω‐3 PUFAs and interact with G‐protein coupled receptors (GPCRs) on the cell surface, hence limiting the infiltration of polymorph nuclear neutrophils (PMNs) and enhance the clearance of apoptotic cells by phagocytosis [11] – two important features in controlling inflammation. More in‐depth insight into the importance of SPMs in the resolution phase of an inflammatory process may lead to the development of new treatments of inflammatory driven diseases. [4] Thus, the resolution of inflammation controlled by SPMs is considered a biomedical paradigm shift. [12] The chemical structures of some resolvins are depicted in Figure 1.
Figure 1.
Chemical structures of some resolvins derived from n‐3 DPA and the DHA‐derived RvD2.
Results and Discussion
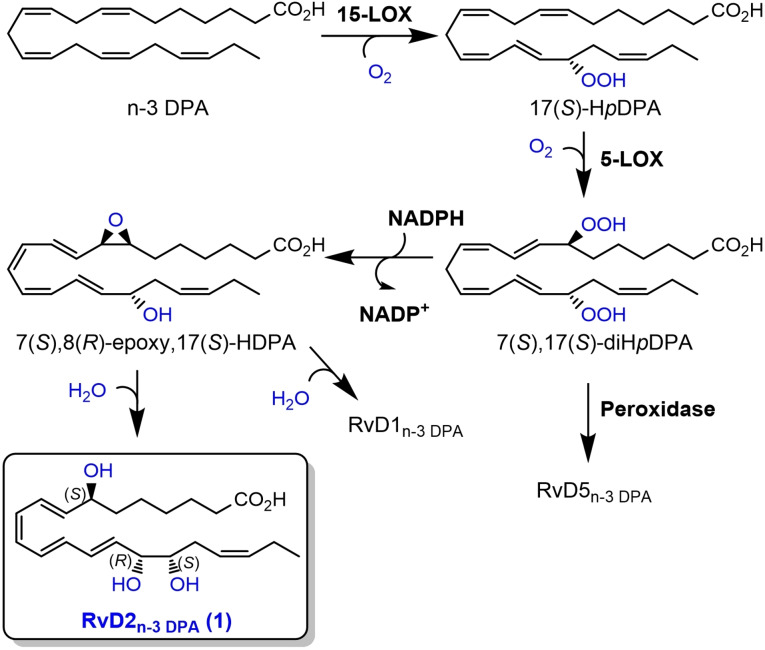
The DHA‐derived SPM resolvin D2 (RvD2, see Figure 1) was isolated from inflammatory exudates and its structure was later established by LC‐MS/MS experiments [13] and total synthesis.[ 14 , 15 ] RvD2 reduces excessive neutrophilic trafficking to inflammatory loci and has been reported to decrease leukocyte‐endothelial interactions in vivo by endothelial‐dependent nitric oxide production and by direct modulations of leukocyte adhesion receptor expression[ 10 , 11 ] Additionally, RvD2 decrease both local and systemic bacterial burden, excessive cytokine production and neutrophil recruitment, [16a] while increasing peritoneal mononuclear cells and macrophage phagocytosis. [16b] These initial reports spurred an interest in evaluating other biological properties of this SPM.[ 16b , 16c , 16d ] The congener of RvD2, RvD2n‐3 DPA (1), was reported in 2013 and its structure elucidated based on UV and LC‐MS/MS data. [5] This SPM is derived from n‐3 DPA and formed after two consecutive lipoxygenation reactions and epoxide formation, followed by ring opening of the epoxide by a hydrolytic enzyme (Scheme 1). In the anticipated biogenetic formation of 1, the first biosynthetic step in the presence of 15‐LOX forms 17(S)‐HpDPA, while the second lipoxygenation step is catalyzed by 5‐LOX. The product herein, 7(S),17(S)‐diHpDPA, is then subjected to enzymatic conversion to an epoxide intermediate that is next enzymatically hydrolyzed to either RvD1n‐3 DPA (Figure 1) or RvD2n‐3 DPA (1), see Scheme 1. Alternatively, direct reduction of the hydroperoxide intermediate 7(S),17(S)‐diHpDPA, catalyzed by peroxidase enzymes, produces RvD5n‐3 DPA (see Figure 1 and Scheme 1).
Scheme 1.
Proposed biosynthesis of RvD2n‐3 DPA (1), RvD1n‐3 DPA, and RvD5n‐3 DPA from n‐3 DPA.
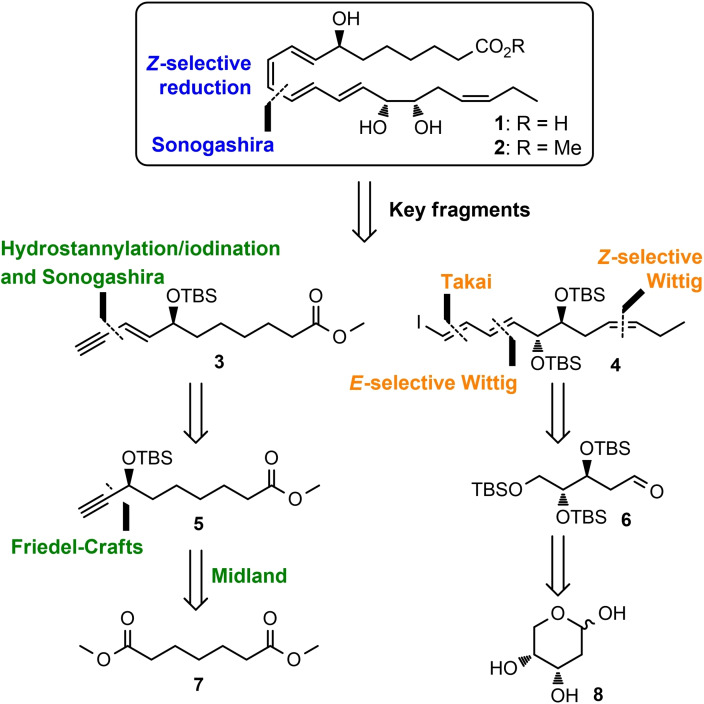
Biosynthetic considerations, LC–MS/MS data, and physical properties (MS‐ and UV‐Vis data) of the isolated endogenously produced material gave evidence for the proposed structure of 1 (Figure 2) with a highly sensitive E,Z,E,E‐tetraene embedded by two chiral allylic alcohols. An overview of the retrosynthetic proposal applied to the tentatively assigned structure of RvD2n‐3 DPA (1) suggest a disconnection based on the Sonogashira cross‐coupling reaction [17] followed by Z‐selective reduction. The analysis identified two key fragments, 3 and 4, to be convergently assembled in the synthesis.
Figure 2.
Overview of the retrosynthetic analysis of RvD2n‐3 DPA (1).
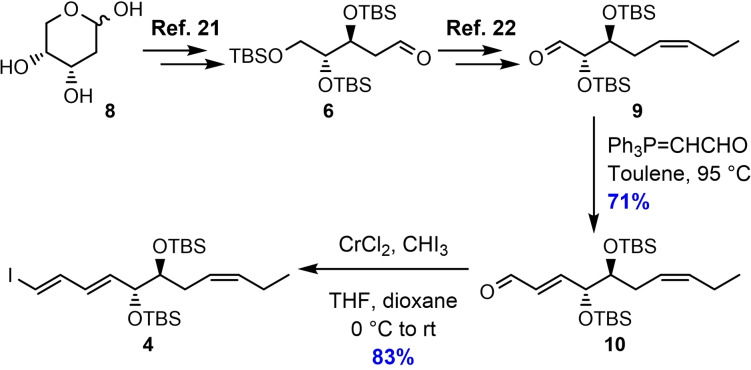
Fragment 3 was disconnected back to alkyne 5, to be prepared from commercially available dimethyl pimelate (7) with an aliphatic Friedel‐Crafts acylation, [18] Midland Alpine borane reduction, [19] and Sonogashira cross‐coupling. Two subsequent Wittig reactions and a Takai olefination [20] were chosen as the key transformations for finishing fragment 4 from 2‐deoxy‐β‐d‐ribopyranose (8).
The project commenced with the construction of compound 4, starting from commercially available and affordable 8, as shown in Scheme 2. Aldehyde 9 was prepared according to the literature,[ 21 , 22 ] and was further reacted in an E‐selective Wittig reaction with the stabilized ylide (triphenylphosphoranylidene)‐acetaldehyde at elevated temperature, to give the α,β‐unsaturated aldehyde 10. A Takai olefination protocol was then performed to complete the synthesis of vinyl iodide 4 in 59 % yield over two steps as one geometrical isomer after purification using column flash chromatography (Supporting Information).
Scheme 2.
Synthesis of vinylic iodide 4.
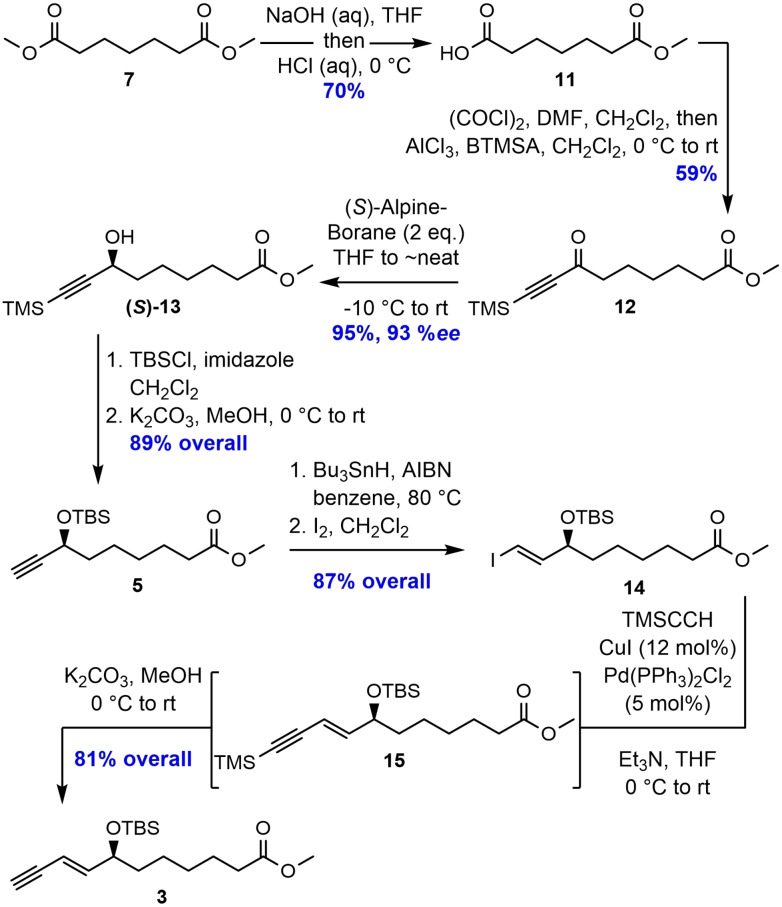
For the synthesis of alkyne ester 3, a selective monohydrolysis of dimethyl pimelate (7) using aqueous NaOH in THF, followed by acidic work‐up, was performed to give carboxylic acid 11 in 70 % yield (see Scheme 3). [23] Next, the corresponding acid chloride of 11 was prepared in situ and submitted to an aliphatic Friedel‐Crafts acylation, giving ketone 12 in 59 % yield. The asymmetric reduction of the alkynyl ketone was achieved by the addition of the Midland (S)‐Alpine borane reagent in THF at −10 °C, followed by swift removal of the solvent to essentially neat conditions, yielding the desired propargylic alcohol (S)‐13 in 93 % enantiomeric excess (ee) and 95 % yield after workup and purification (Supporting Information). The ee‐value was determined by HPLC‐analyses of the 2‐naphthoate derivative of 13 (Supporting Information).
Scheme 3.
Preparation of the alkyne ester 3.
The secondary alcohol (S)‐13 was then treated with TBS chloride and imidazole in dichloromethane, followed by TMS‐deprotection using potassium carbonate in methanol to yield 5 in 89 % over the two steps. At this point, it was necessary to convert the terminal acetylene 5 into the corresponding E‐vinyl iodide 14. This was achieved by first treating the compound with a catalytic amount of azobisisobutyronitrile (AIBN) and excess tributyltin hydride at elevated temperature, followed by iododestannylation, to give 14 in 87 % yield over the two steps. The first of the two planned palladium catalyzed reactions was accomplished using catalytic amounts of Pd(PPh3)2Cl2/CuI in THF under basic conditions, which united vinyl iodide 14 with commercially available trimethylsilylacetylene. Direct protodesilylation of the crude compound 15 was achieved after treatment with potassium carbonate in methanol, affording the terminal alkyne 3 in 81 % yield.
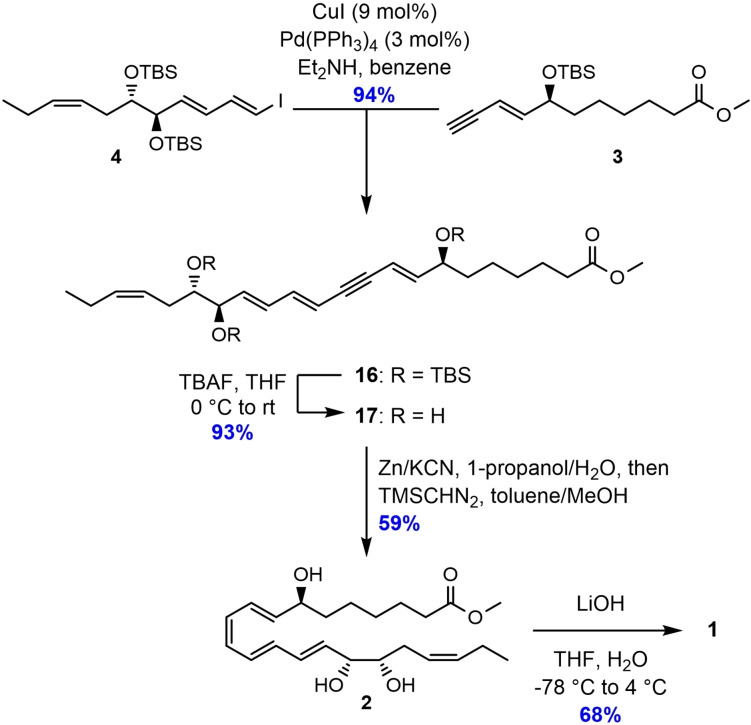
For the assembly of the two key fragments, 3 and 4, the Sonogashira cross‐coupling reaction using Pd(PPh3)4/CuI and diethylamine in benzene produced 16 in excellent yields (Scheme 4). Removal of the three TBS‐groups in 16 was performed by adding tetra‐n‐butylammonium fluoride (TBAF) in THF to give the triol 17 in 93 % yield. However, for the Z‐selective reduction of the internal alkyne in 17, several different protocols were attempted. Firstly, a Zn(Cu/Ag) mediated reduction protocol, [24] reported to be highly Z‐selective for conjugated systems, was tested, but the conversion was rather poor. Additionally, elimination of the (7S)‐alcohol moiety was observed. Next, a Lindlar hydrogenation protocol [25] using a mixture of EtOAc/pyridine/1‐octene as solvent system was attempted, but no product formation was observed. Then, a hydrosilylation protocol [26] using the Karstedt catalyst was tried, which was successfully employed in the preparation of RvD1n‐3 DPA. [21] Unfortunately, major byproduct formation and difficulties in the purification step was observed. Finally, a reaction using zinc powder and potassium cyanide in a mixture of 1‐propanol/H2O, [27] followed by a solvent switch to toluene/methanol and addition of TMS‐diazomethane, gave RvD2n‐3 DPA methyl ester (2) in 59 % yield and with chemical purity >96 % after purification by column chromatography (Supporting Information). The NMR‐ (1H, 13C, and COSY), MS‐, and UV‐data were all in accordance with the structure of 2 (Supporting Information).
Scheme 4.
Sonogashira cross‐coupling reaction and Z‐selective hydrogenation to complete the synthesis of RvD2n‐3 DPA methyl ester (2).
MRM LC‐MS/MS matching experiments were performed to assure that our synthetic material was identical to that of authentic RvD2n‐3 DPA (1). For SPMs, direct NMR analyses for structural elucidation and configurational assignments are not possible, since their biosynthetic formation yields nano‐ to picogram amounts. [9a] As previously reported, SPMs are chemically labile compounds. [28] Hence, hydrolysis of the methyl ester 2 to the free acid 1 was performed just prior to the LC‐MS/MS experiments, [5] since the E,Z,E,E‐tetraene in SPMs easily undergo conversion to other geometrical isomers. [1]
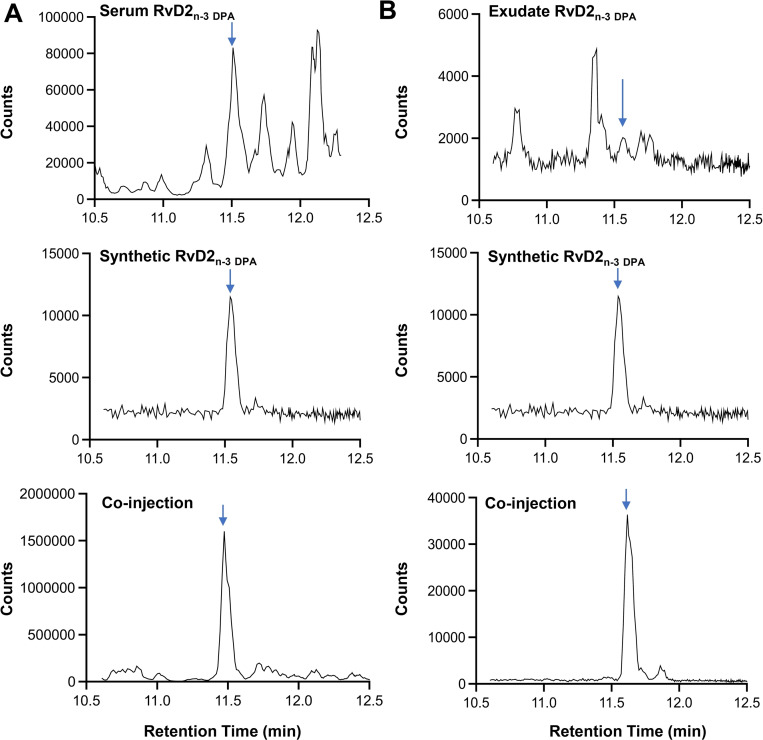
Injection of biological material obtained from human peripheral blood and mouse infectious exudate with synthetic materials gave a single sharp peak (RT=11.5 min). Co‐elution of the synthetic material with biological material was further corroborated by co‐injecting the synthetic material with biological material (see Figure 3). In addition, the UV‐spectrum of 1 (λmax (EtOH)=301 nm, shoulders at 288 and 315 nm, Figure S‐1, Supporting Information), is in agreement with earlier reported data of RvD2n‐3 DPA (1) [5] and RvD2.[ 7 , 14 ]
Figure 3.
Synthetic RvD2n‐3 DPA (1) elutes at the same retention time as endogenous RvD2n‐3 DPA (1) in human serum and mouse inflammatory exudates. (A) Human serum was obtained from commercial sources and (B) inflammatory exudates were collected from mice 2 h after inoculation of E. coli (105 CFU) via peritoneal lavage. Results are from n=3 determinations for A and n=4 mice for B. See Supporting Information for details.
Additionally, the MS/MS spectra of biologically and synthetically produced material of 1 (Figure S‐2, Supporting Information), confirmed that the synthetically produced compound matched the data of the biogenic material. Key diagnostic fragments were identified in MS/MS spectra from biological and synthetic material, including m/z 143, 197, 233 and 249 (Figure S‐2, Supporting Information).
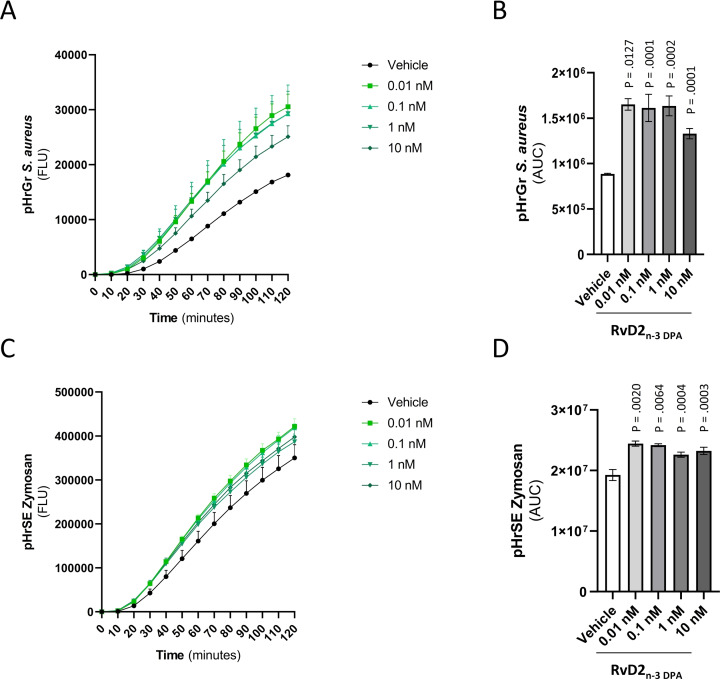
In order to investigate the biological properties of the synthetic material, murine macrophages were differentiated from bone marrows in vitro. Macrophages were incubated with either vehicle or RvD2n‐3 DPA (1) for 15 minutes before adding pHrodo‐labelled Staphylococcus aureus or zymosan A bioparticles, as previously described. [29] Here we observed that synthetic RvD2n‐3 DPA (1) potently increased the uptake of both S. aureus (Figure 4 A–B) and zymosan A bioparticles (Figure 4 C–D) in a dose dependent matter. S. aureus is a bacterium commonly located on the skin and mucosa of healthy individuals that can cause serious infections if in contact with internal tissues or by dissemination through the bloodstream. [29] Zymosan A is a macromolecule derived from the yeast wall of Saccharomyces cerevisiae, commonly used to induce sterile inflammation. Our findings demonstrate that RvD2n‐3 DPA (1) not only promotes the uptake of bacterial and fungal particles, but also their degradation since the dye employed is a pH‐sensitive dye and thus indicates that this mediator also promotes the phagolysosome acidification, a key step in microbial killing. Clearing of such pathogens and inflammatory molecules by tissue‐infiltrating macrophages is a fundamental step in the resolution of inflammation that, when impaired, can lead to increased tissue damage and systemic inflammation. [29] Notably, SPMs have been demonstrated to effectively increase the clearance of pathogens in several inflammatory settings.[ 30 , 31 ]
Figure 4.
RvD2n‐3 DPA (1) increases bone marrow‐derived macrophages uptake of S. aureus and zymosan A bioparticles. Results in A and C are expressed as change in signal intensity recorded at baseline (0 min), (mean ± s.e.m., N=3). Results in B and D are expressed as AUC values (mean ± s.e.m., N=3). See Supporting Information for details.
Conclusion
In summary, the first total synthesis of the methyl ester 2 of the specialized pro‐resolving mediator RvD2n‐3 DPA (1) has been stereoselectively obtained in 13 % overall yield over 12 steps (longest linear sequence). The synthesis featured the rarely used synthetic method of Z‐selective alkyne hydrogenation using potassium cyanide and zinc. Our synthesis differs significantly to earlier reported synthesis of the congener RvD2,[ 14 , 15 ] and produced multi milligrams of 2. The stereoselective synthesis of RvD2n‐3 DPA (1) and data from LC‐MS/MS matching experiments enabled the configurational assignment of this oxygenated natural product as (7S,8E,10Z,12E,14E,16R,17S,19Z)‐7,16,17‐trihydroxydocosa‐8,10,12,14,19‐pentaenoic acid. In addition, using synthetic material of 1 in the 0.01 to 10 nM range, experiments showed that 1 potently increases bone marrow‐derived macrophage uptake of S. aureus and zymosan A bioparticles. Such bioactions are of interest towards developing new immunoresolvents[ 8 , 30 , 31 ] targeting development of new anti‐bacterial drugs. [32]
Conflict of interest
The authors declare no conflict of interest.
1.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
The Department of Pharmacy is gratefully acknowledged for a scholarship to A.F.R. This work was also supported by funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant no: 677542) and the Barts Charity (grant no: MRC&U0032) to J.D.
A. F. Reinertsen, K. G. Primdahl, R. De Matteis, J. Dalli, T. V. Hansen, Chem. Eur. J. 2022, 28, e202103857.
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
References
- 1. Serhan C. N., Petasis N. A., Chem. Rev. 2011, 111, 5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Serhan C. N., Am. J. Pathol. 2010, 177, 1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gordon S., Immunity 2016, 44, 463. [DOI] [PubMed] [Google Scholar]
- 4. Gilroy D. W., in Fundamentals of Inflammation (Eds: Serhan C. N., Ward P. A., Gilroy D. W.) Cambridge University Press, New York, 2010, p. 17. [Google Scholar]
- 5. Dalli J., Colas R. A., Serhan C. N., Sci. Rep. 2013, 3, p. 1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Serhan C. N., Clish C. B., Brannon J., Colgan S. P., Chiang N., Gronert K., J. Exp. Med. 2000, 192, 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Serhan C. N., Hong S., Gronert K., Colgan S. P., Devchand P. R., Mirick G., Moussignac R.-L., J. Exp. Med. 2002, 196, 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fullerton J., Gilroy D., Nat. Rev. Drug Discovery 2016, 15, 551. [DOI] [PubMed] [Google Scholar]
- 9.
- 9a. Vik A., Hansen T. V., From Biosynthesis to Total Synthesis: Strategies and Tactics for Natural Products (Ed: Zografos A. L.), John Wiley and Sons, Hoboken, 2016, p. 130; [Google Scholar]
- 9b. Vik A., Hansen T. V., Org. Biomol. Chem. 2021, 19, 705; [DOI] [PubMed] [Google Scholar]
- 9c. Reinertsen A. F., Primdahl K. G., Shay A. E., Serhan C. N., Hansen T. V., Aursnes M., J. Org. Chem. 2021, 86, 3535; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9d. Urbitsch F., Elbert B. L., Llaveria J., Streatfeild P. E., Anderson E. A., Org. Lett. 2020, 22, 1510; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9e. Nanba Y., Morita Y. M., Kobayashi Y. Synlett 2018, 29, 1791; [Google Scholar]
- 9f. Rodrigues A. R., Spur B. W., Tetrahedron Lett. 2020, 61, 151473; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9g. Rodrigues A. R., Spur B. W., Tetrahedron Lett. 2020, 61, 151857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Serhan C. N., Levy B. D., J. Clin. Invest. 2018, 128, 2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Serhan C. N., Nature 2014, 510, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maderna P., Godson C., Br. J. Pharmacol. 2009, 158, 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hong S., Gronert K., Devchand P. R., Moussignac R.-L., Serhan C. N., J. Biol. Chem. 2003, 278, 14677. [DOI] [PubMed] [Google Scholar]
- 14. Rodrigues A. R., Spur B. W., Tetrahedron Lett. 2004, 45, 8717. [Google Scholar]
- 15. Li J., Leong M. M., Stewart A., Rizzacasa M. A., Beilstein J. Org. Chem. 2013, 9, 2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.
- 16a. Spite M., Norling L., Summers L., Yang R., Cooper D., Petasis N. A., Flower R., Perretti M., Serhan C.N, Nature 2009, 461, 1287; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16b. Dort J., Orfi Z., Fabre P., Molina T., Conte T. C., Greffard K., Pellerito O., Bilodeau J.-F., Dumont N. A., Nat. Commun. 2021, 12, 6264; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16c. Joan C., Dalli J., Yacoubian S., Gao F., Serhan C. N., J. Immunol. 2012, 189, 2597; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16d. Park C.-K., Xu Z.-Z., Liu T., Lü N., Serhan C. N., Ji R.-R., J. Neurosci. 2011, 31, 18433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sonogashira K., Tohda Y., Hagihara N., Sonogashira K., Tohda Y., Hagihara N., Tetrahedron Lett. 1975, 16, 4467. [Google Scholar]
- 18. Birkofer L., Ritter A., Uhlenbrauck H., Chem. Ber. 1963, 96, 3280. [Google Scholar]
- 19. Midland M. M., McDowell D. C., Hatch R. L., Tramontano A., J. Am. Chem. Soc. 1980, 102, 867. [Google Scholar]
- 20. Takai K., Nitta K., Utimoto K., J. Am. Chem. Soc. 1986, 108, 7408. [DOI] [PubMed] [Google Scholar]
- 21. Tungen J. E., Gerstmann L., Vik A., De Matteis R., Colas R. A., Dalli J., Chiang N., Serhan C. N., Kalesse M., Hansen T. V., Chem. Eur. J. 2019, 25, 1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tungen J. E., Primdahl K. G., Hansen T. V., J. Nat. Prod. 2020, 83, 2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Niwayama S., J. Org. Chem. 2000, 65, 5834. [DOI] [PubMed] [Google Scholar]
- 24. Boland W., Schroer N., Sieler C., Feigel M., Helv. Chim. Acta. 1987, 70, 1025. [Google Scholar]
- 25.
- 25a. Aursnes M., Tungen J. E., Vik A., Dalli J., Hansen T. V., Org. Biomol. Chem. 2014, 12, 432; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25b. Aursnes M., Tungen J. E., Vik A., Colas R. A., Cheng C. C., Dalli J., Serhan C.N, Hansen T. V., J. Nat. Prod. 2014, 77, 910; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25c. Tungen J. E., Aursnes M., Hansen T. V., Tetrahedron Lett. 2015, 56, 1843; [Google Scholar]
- 25d. Tungen J. E., Aursnes M., Dalli J., Arnardottir H., Serhan C. N., Hansen T. V., Chem. Eur. J. 2014, 20, 14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rooke D.A, Ferreira E. M., Angew. Chem. Int. Ed. 2012, 51, 3225. [DOI] [PubMed] [Google Scholar]
- 27. Näf F., Decorzant R., Thommen W., Willhalm B., Ohloff G., Helv. Chim. Acta. 1975, 58, 1016. [DOI] [PubMed] [Google Scholar]
- 28. Hansen T. V., Dalli J., Serhan C. N., Prostaglandins Other Lipid Mediators 2017, 133, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koenis D. S., Beegun I., Jouvene C. C., Aguirre G. A., Souza P. R., Gonzalez-Nunez M., Ly L., Pistorius K., Kocher H. M., Ricketts W., Thomas G., Perretti M., Alusi G., Pfeffer P., Dalli J., Circ. Res. 2021, 129, e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dalli J., Mol. Aspects Med. 2017, 58, 12. [DOI] [PubMed] [Google Scholar]
- 31. Serhan C. N., FASEB J. 2017, 31, 1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown E., Wright G., Nature 2016, 529, 336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.