Abstract
Background
Transfusion is very common in the intensive care unit (ICU), but practice is highly variable, as has recently been shown in non‐bleeding critically ill patients practices survey. Bleeding patients in ICU require different blood products across a range of specific patient categories. We hypothesize that a large variety in transfusion practice exists in bleeding patients.
Study design and methods
An international online survey was performed among physicians working in the ICU. Transfusion practice in massively and non‐massively bleeding patients was examined, including transfusion ratios, thresholds, and the presence of transfusion guidelines.
Results
Six hundred eleven respondents filled in the survey of which 401 could be analyzed, representing 64 countries. Among the respondents, 52% had a massive transfusion protocol (MTP) available at their ICU. In massively bleeding patients, 46% of the respondents used fixed transfusion component ratios. Of those who used fixed blood ratios, the 1:1:1 ratio (red blood cell [RBC] concentrates: plasma: platelet concentrates) was most commonly used (33%). The presence of an MTP was associated with a more frequent use of fixed ratios (p < .001). For RBC transfusion in the general non‐massively bleeding ICU population, a hemoglobin (Hb) threshold of 7.0[7.0–7.3] g/dl was reported. In the general ICU population, a platelet count threshold of 50[26–50] × 109/L was applied.
Discussion
Half of the centers had no massive transfusion protocol available. Transfusion practice in massively bleeding critically ill patients is highly variable and driven by the presence of an MTP. In the general non‐massively bleeding ICU population restrictive transfusion triggers were chosen.
Keywords: bleeding, coagulation, critically ill, massive, transfusion, transfusion anemia
Abbreviations
- ANOVA
analysis of variance
- DOAC
direct oral anticoagulant
- ECMO
extracorporeal membrane oxygenation
- GI
gastrointestinal
- Hb
hemoglobin
- ICU
intensive care unit
- MTP
massive transfusion protocol
- PCC
prothrombin complex concentrate
- RBC(s)
red blood cell(s)
- tbi
traumatic brain injury
- TXA
tranexamic acid
- VKA
vitamin K antagonist
1. BACKGROUND
Transfusion is common practice in the intensive care unit (ICU), with about 40%–50% of the critically ill being transfused during ICU admission. 1 While the transfusion of blood products can enhance the life expectancy of critically ill patients, 2 there has been growing awareness about the possible side effects of transfusion. 2 , 3 Blood products contain inflammatory components including reactive oxygen species, foreign antigens, and various pro‐inflammatory microparticles. 4 , 5 , 6 , 7 These inflammatory components may induce harmful transfusion reactions, such as allergic reactions, hemolysis, and acute lung injury, especially in the critically ill. 8 , 9 This explains why restrictive transfusion strategies in the non‐bleeding critically ill are safe and decrease exposure to RBC transfusion as compared with liberal transfusion practices. 10 , 11 , 12 , 13 , 14
There are no data available on transfusion practices specifically for bleeding critically ill patients. The majority of transfusion studies in bleeding patients were conducted in trauma patients. In general, trauma patients are a relatively healthy population with limited comorbidities. Therefore, this evidence might not be directly generalizable to bleeding, non‐trauma, critically ill patients. Transfusion practice in bleeding patients is challenging, with multiple causes including coagulopathy, thrombocytopenia, and can occur as a consequence of surgery. Coagulopathy can also be a consequence of bleeding. To control bleeding, patients often receive different types of blood products, many of which are delivered simultaneously.
This survey aims to assess the practice of caregivers toward transfusion practices in the bleeding critically ill patient, including transfusion thresholds, choices of blood products, and diagnostic tests. We hypothesized that in this patient population a large heterogeneity exists between and within different subpopulations.
2. METHODS
2.1. Survey
A questionnaire was distributed to physicians working in adult ICUs worldwide using an online platform (SurveyMonkey, Portland, OR). This questionnaire was a follow‐up of the first TRACE survey, which focused on non‐bleeding critically ill patients. 15 This study was endorsed by the European Society of Intensive Care Medicine (ESICM) and by several national intensive care societies (Data S2).
2.2. Study design
During two focus group meetings with clinical experts on transfusion practices, themes were identified and used to compile the questionnaire. The questionnaire was piloted with physicians working in different countries within Europe and Northern America.
In this survey, the use of different blood products including red blood cells (RBCs), platelet concentrates, and plasma products in different subpopulations (e.g., trauma, obstetric, etc.) was explored. The survey included a maximum of 50 questions divided into three subsections: respondents' demographics (7 questions), transfusion practice in the massively bleeding patient (7–10 questions), and transfusion practices in the non‐massively bleeding patient (33 questions, see Data S1 for static version). Massive bleeding was defined as having one or more of the following conditions: (1) a systolic blood pressure < 90 mmHg with bleeding + non‐responsiveness to resuscitation therapy; (2) any case where a massive transfusion protocol (MTP) was initiated; or (3) the administration of ≥4 blood products within 2 h.
In non‐massively bleeding patients, hemoglobin (Hb), platelet count, and fibrinogen level thresholds were investigated for RBC transfusion, platelet transfusion, and fibrinogen administration, respectively. The use of tranexamic acid (TXA) was examined in different subpopulations (i.e., trauma patients, obstetrics, gastroenterology).
2.3. Statistical analysis
Only completed surveys were included for analysis. A questionnaire was defined as complete when the respondents went through all questions. Since not all questions were applicable for all respondents, some questions were allowed to leave open.
Continuous data were assessed for distribution: normally distributed variables were described by mean (standard deviations) and nonparametric data by median (first quartiles–third quartile). Exactly 10th and 90th percentiles were estimated by the largest observation less than or equal to Q3 + 1.5 × the interquartile range and the lowest observation or higher than Q1 − 1.5 × interquartile range, respectively.
Normal distributed variables were analyzed using Student's t test and analysis of variance (ANOVA). Nonparametric data were analyzed with Mann–Whitney U test or Kruskal–Wallis. The Dunn test with Bonferroni correction was used to assess the differences in applied transfusion thresholds between different subpopulations. Categorical variables were tested using the Chi‐squared test with Yates correction for continuity and were described by frequencies and percentages. Data were analyzed using R statistics (version 3.5.2) with the R Studio interface (The R Foundation, Lucent Technologies, Inc., Murray Hill, NJ, www.r-project.org).
3. RESULTS
3.1. Demographics
A total of 611 respondents participated in the survey, of which 401 finished the complete survey and were thus included for analysis (Figure 1A). These respondents represented 64 countries, of which the majority were high‐income countries (72%, Figure 1B). The majority of the respondents were board‐certified intensivists (84%) with a primary medical specialty in anesthesiology (61%) or internal medicine (19%). Participants worked in mixed ICUs (73%), surgical (16%), or medical ICUs (8%). Most participants worked at university hospitals (44%) or university‐affiliated hospitals (26%). An MTP was available in 52% of the respondents' hospitals. The availability of a hospital‐wide transfusion protocol and ICU‐specific transfusion protocol was less common—45% and 40%, respectively. The demographics of survey respondents are displayed in Table 1.
FIGURE 1.
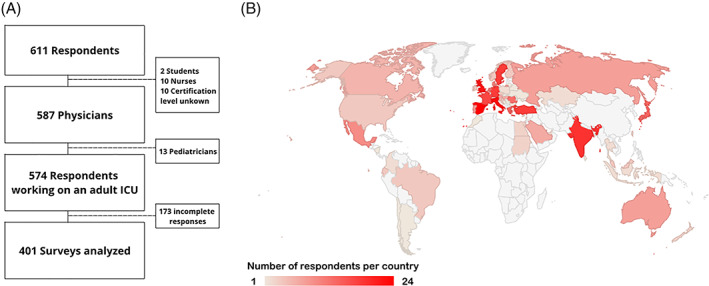
Six hundred eleven respondents filled in the survey of which 401 were analyzed (Panel A), representing 64 countries (Panel B) [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Characteristics of the survey respondents
| Demographic | No. of respondents (%) |
|---|---|
| Certification level | |
| Intensivist | 337 (84%) |
| Specialist non‐intensivist practicing ICU | 33 (8%) |
| Resident, specialist in training | 26 (6%) |
| Other | 5 (1%) |
| Primary medical specialty | 3 (1%) |
| Anesthesiology | 243 (61%) |
| Internal medicine | 78 (19%) |
| Pulmonology | 13 (3%) |
| Surgery | 9 (2%) |
| Cardiology | 7 (2%) |
| Neurology | 1 (0%) |
| Other (please specify) | 47 (12%) |
| Type of ICU | |
| Medical ICU | 33 (8%) |
| Surgical ICU | 64 (16%) |
| Mixed ICU | 294 (73%) |
| Other | 10 (4%) |
| Number of ICU beds | |
| <10 | 95 (24%) |
| 10–15 | 124 (31%) |
| 16–20 | 64 (16%) |
| >20 | 116 (29%) |
| Type of institution | |
| University hospital | 178 (44%) |
| University‐affiliated hospital | 104 (26%) |
| Non‐university public hospital | 82 (20%) |
| Private hospital | 36 (9%) |
| Other | 1 (0%) |
| Availability of transfusion guideline | |
| Hospital‐wide transfusion protocol | 180 (45%) |
| ICU‐specific transfusion protocol | 159 (40%) |
| Massive transfusion protocol | 209 (52%) |
| Unit used to measure hemoglobin | |
| g/dl | 282 (70%) |
| g/L (=mg/ml) | 94 (23%) |
| mmol/L | 25 (6%) |
| Economy | |
| High income | 287 (72%) |
| Lower middle income | 33 (8%) |
| Upper middle income | 80 (20%) |
3.2. Massive bleeding
3.2.1. Product choice
Approximately half of the respondents (46%) used fixed blood product ratios (RBC: Plasma: PLT). Among these respondents, the 1:1:1 ratio was most often reported (33%), followed by 3:3:1 ratio (24%). During massive bleeding, the use of blood products was most often guided by viscoelastic testing (73%) and conventional laboratory‐based testing (67%).
The use of fibrinogen and prothrombin complex concentrate (PCC) during massive bleeding was highly variable: fibrinogen was most often (36%) administered based on conventional laboratory‐based tests or empirically followed by laboratory test guided additional fibrinogen administration (30%). Viscoelastic testing was used by 19% of the respondents, and 11% administered fibrinogen only empirically.
Prothrombin complex concentrate administration was most often guided by conventional laboratory‐based testing (39%) followed by viscoelastic testing (23%) and 21% stated they initially administered PCC empirically but titrated the following doses based on conventional laboratory results.
The majority (93%) of the respondents used TXA during massive bleeding. Among those respondents, it was usually administered empirically (89%), but 9% used viscoelastic tests to guide the administration of TXA. Large differences were observed between different subpopulations (Figure 2A). The subpopulations where most respondents would always administer TXA were trauma patients (59%), followed by massively bleeding obstetric patients (40%). Few respondents would always administer TXA to septic patients (11%). In patients on extracorporeal membrane oxygenation (ECMO), respondents most often stated they would never use TXA for this patient population (35%). More data regarding massive bleeding are displayed in Table 2.
FIGURE 2.
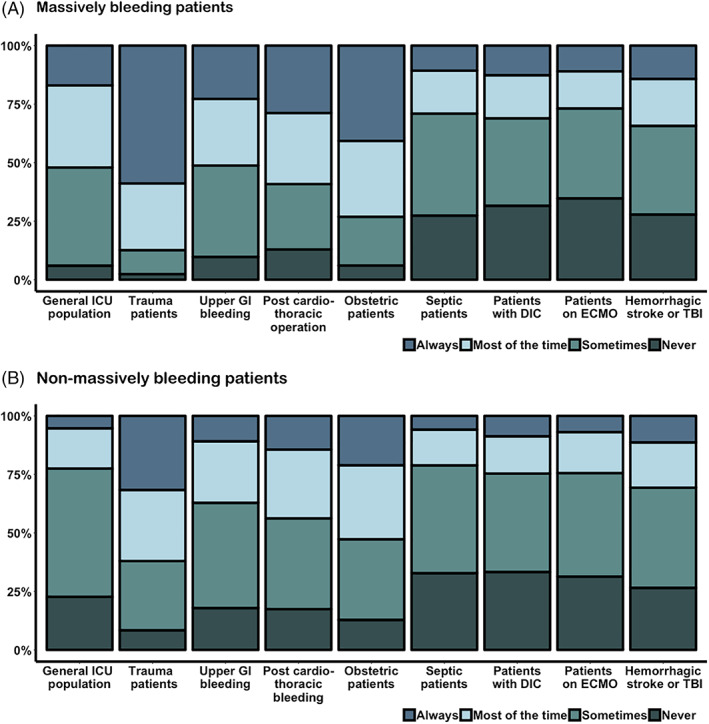
The use of tranexamic acid (TXA) in massively (Panel A) and non‐massively (Panel B) bleeding patients in the ICU [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 2.
Transfusion practice during massive bleeding
| No. of respondents (%) | |
|---|---|
| What kind of plasma do you use during massive transfusion? | |
| Pooled plasma (e.g., Omniplasma) | 29 (7%) |
| Fresh frozen plasma | 370 (92%) |
| Lyophilized plasma | 12 (3%) |
| What guides the choice of type of blood products prescribed to patients requiring massive transfusion? | |
| I use fixed ratios of blood products | 184 (46%) |
| Conventional lab based testing (e.g., International Normalized Ratio [INR], platelet count, fibrinogen, hemoglobin) | 268 (67%) |
| Point of care viscoelastic testing (Thromboelastography [TEG] or Thromboelastometry [ROTEM]) | 163 (41%) |
| What ratio of blood products do you use during massive transfusion (one platelet concentrate = pooled product from 5 donors) | |
| 1:1:1 (red blood cells:plasma:platelets concentrate) | 60 (15%) |
| 3:3:1 (red blood cells:plasma:platelets concentrate) | 45 (11%) |
| 6:6:1 (red blood cells:plasma:platelets concentrate) | 19 (5%) |
| 6:3:1 (red blood cells:plasma:platelets concentrate) | 23 (6%) |
| Whole blood | 2 (0%) |
| Other | 38 (9%) |
| How do you correct a plasmatic coagulopathy (INRx1.5 reference value or prolonged clotting time with TEG or ROTEM) in critically ill patients with massive blood loss who used vitamin K antagonists? | |
| Vitamin K | 274 (68%) |
| Prothrombin complex (Cofact/Octoplex/Beriplex) | 314 (78%) |
| Plasma | 246 (61%) |
| Other | 7 (2%) |
| Nothing | 3 (1%) |
| How do you correct a plasmatic coagulopathy in critically ill patients with massive blood loss who used direct oral anticoagulants(DOACs)? | |
| Vitamin K | 92 (23%) |
| Prothrombin complex (Cofact/Octoplex/Beriplex) | 273 (68%) |
| Plasma | 256 (64%) |
| Recombinant factor VIIa (Novoseven/Eptacog alfa) | 68 (17%) |
| Idarucizumab (for dabigatran) | 194 (48%) |
| Andexanet (for rivaroxaban or apixaban) | 84 (21%) |
| Nothing | 6 (1%) |
| Other | 28 (7%) |
| What guides your use of fibrinogen in critically ill patients with massive bleeding? | |
| I administer fibrinogen after lab testing (fibrinogen level) | 146 (36%) |
| I administer fibrinogen after viscoelastic testing (TEG/ROTEM) | 78 (19%) |
| I empirically administer fibrinogen | 43 (11%) |
| I empirically administer fibrinogen, but start titrating when first lab results are available | 121 (30%) |
| Other | 12 (3%) |
| What guides your use of prothrombin complex (Cofact,Octoplex,Beriplex) in critically ill patients with massive bleeding. | |
| I administer prothrombin complex after lab testing (PT/INR) | 157 (39%) |
| I administer prothrombin complex after viscoelastic testing (TEG/ROTEM) | 91 (23%) |
| I empirically administer prothrombin complex | 24 (6%) |
| I empirically administer prothrombin complex, but start titrating when first lab results are available | 85 (21%) |
| Other (please specify) | 40 (10%) |
| Do you use tranexamic acid in critically ill patients with massive bleeding? | |
| Yes | 374 (93%) |
| No | 26 (6%) |
| What guides your use of tranexamic acid in critically ill patients with massive bleeding? | |
| I administer tranexamic acid after viscoelastic testing (TEG/ROTEM) | 33 (8%) |
| I empirically administer tranexamic acid | 332 (83%) |
| Other | 9 (2%) |
3.2.2. Correcting iatrogenic coagulopathy during massive bleeding
The strategy to correct iatrogenic coagulopathy was dependent on the class of anticoagulant medication that was used. In patients with a vitamin K antagonist (VKA) induced coagulopathy (defined as an INR >1.5× reference value), most respondents would treat this by administering vitamin K (68%), PCC (78%), and plasma (61%). When the coagulopathy was direct oral anticoagulant (DOAC)‐induced, respondents would use PCC (68%), plasma (64%), Idarucizumab for dabigatran (48%), vitamin K (23%), or andexanet alpha for rivaroxaban or apixaban (21%).
3.2.3. The effect of an MTP on transfusion practice
Several differences were observed between respondents with and without an MTP available in their ICU (Table S1 in Data S3). The respondents with an MTP available were more often working in high‐income countries (162 [80%] versus 119 [62%]; p < .001). When an MTP was available, more often fixed ratios were used (120[57%]) than when no MTP was available (64 [33%]; p < .001). In addition, when fixed ratios were used, most often the 1:1:1 ratio was used, while in the absence of the MTP, a wide range of ratios employed were reported. Tranexamic acid was more often used if an MTP was available (96% vs. 90%; p = .019).
3.3. Non‐massively bleeding patients
3.3.1. Red cell transfusion
Respondents used different thresholds in different non‐massively bleeding subpopulations (Figure 3). For the general ICU population, a Hb threshold of 7.0[7–7.3] g/dl was used. This was significantly lower than for all other specified subpopulations (Figure 3A). For patients admitted with upper gastrointestinal (GI) bleeding, obstetric complications, and sepsis, the reported RBC threshold was 7 [7–8] g/dl. The highest RBC thresholds were reported for post‐cardiothoracic surgery patients 8[7.9–9] g/dl. The highest variability was observed for patients on ECMO and patients with stroke and/or TBI: 7 [7–9] g/dl. In patients with TBI and those post‐cardiothoracic surgery, 32% of the respondents would transfuse at a Hb level of 9 g/dl or higher. In the general population, 3.5% would transfuse at a Hb level of 9 g/dl or higher. No consistent differences were observed between world regions (Figures S2–S7 in Data S1).
FIGURE 3.
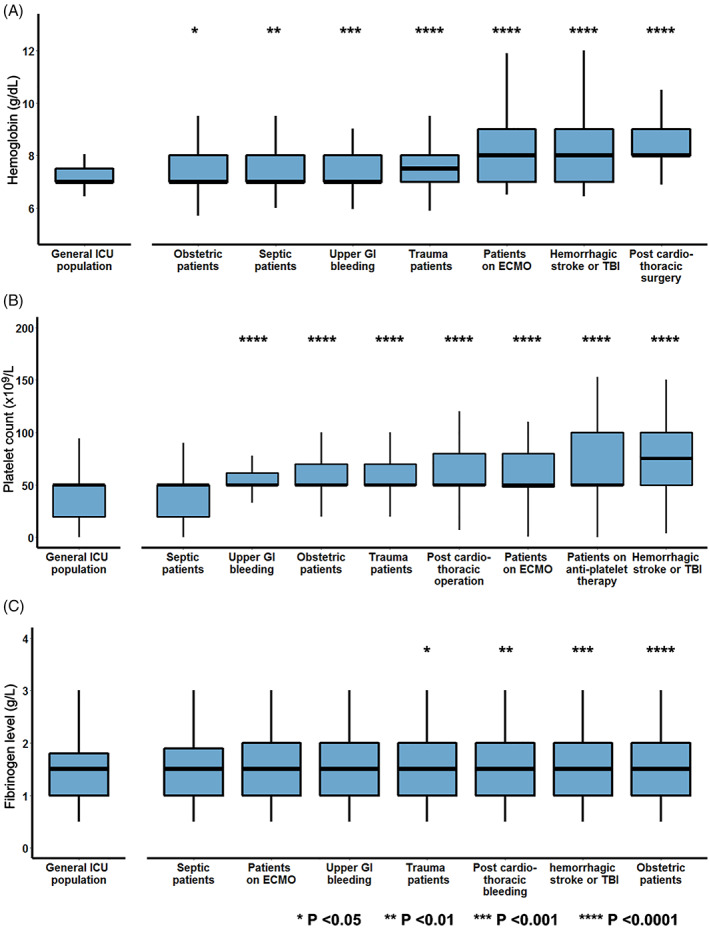
Respondents were asked to report for the general bleeding ICU population and several subpopulations their Hb threshold (Panel A), platelet count threshold (Panel B), and fibrin threshold (Panel C) for RBC transfusion, platelet transfusion, and fibrin administration, respectively. Subpopulations were compared with the general ICU population using the Dunn test with Bonferroni correction. Each boxplot represents the medians with first and third quartile. The upper and lower whiskers are estimates of the 10th and 90th percentile, respectively [Color figure can be viewed at wileyonlinelibrary.com]
Exactly 34% and 40% of respondents respectively reported always or most of the time checking the Hb level before administering additional RBC units. This was never checked by 8% of the respondents and sometimes by 18%. Whether the respondents would check the Hb in between transfusions did not correlate with the transfusion thresholds in any of the subpopulations (Figure S1 in Data S4).
3.3.2. Platelet transfusion
The applied platelet threshold for the general non‐massively bleeding ICU population was 50 [20–50] × 109/L (Figure 3B). This was similar in septic patients and patients with disseminated intravascular coagulation (DIC, p = 1). Significantly higher thresholds (p < .001) were reported in several other bleeding subpopulations including patients with upper gastrointestinal bleeding (50 [50–62] × 109/L), obstetric complications (50 [50–70] × 109/L), after cardiothoracic surgery (50 [50–80] × 109/L), ECMO (50 [48–80] × 109/L), and with a hemorrhagic stroke or traumatic brain injury (75 [50–100] × 109/L, Figure 3B). Patients with hemorrhagic stroke or traumatic brain injury were transfused at the highest platelet count, and 31.2% of the respondents would transfuse this population to platelet levels of 100 × 109/L or higher. Also, in patients receiving antiplatelet therapy, a high variance in the platelet threshold utilized was observed (50[50–100] × 109/L). In these patients, 27% of the respondents would transfuse to platelet levels of 100 × 109/L or higher. No consistent differences were observed between world regions (Figures S2–S7 in Data S4).
Of the respondents, 67% reported that they always checked the platelet count before transfusing a second unit of platelets. Furthermore, 13% reported doing this most of the time, and 13% only sometimes. Respondents who only sometimes or never checked the platelet count transfused at higher platelet counts in patients after cardiothoracic surgery (p = .044; Figure S1 in Data S4).
3.3.3. Coagulation supportive therapy
Fibrinogen administration was triggered at a level of 1.5[1–1.8] g/L in the general ICU population for non‐massive bleeding. The differences in fibrinogen thresholds used in other subpopulations were small, but statistically significant. Trauma patients, obstetric patients, patients on ECMO, upper GI bleeding cases, post‐cardiothoracic surgery patients, and patients with traumatic brain injury would receive fibrinogen at a threshold of 1.5 [1–2] g/L, and in patients with sepsis, fibrinogen would be administered at a fibrinogen level of 1.5[1–1.9] g/L (Figure 3C).
The use of TXA differed between subpopulations. It was most often considered in trauma followed by obstetric patients (Figure 2B). TXA was mostly administered empirically in non‐massively bleeding patients (68%), whereas some respondents (24.4%) performed viscoelastic testing before administering TXA.
Most respondents reported that they use the INR or PT to decide whether a non‐massively bleeding patient could benefit from a plasma transfusion (88%), followed by activated partial thromboplastin time (aPTT, 59%), fibrinogen level (48%), and viscoelastic testing (42%). An INR of 2 (IQR: 1.6–2.5) was used as the threshold for plasma transfusion. Of the respondents, 24% and 31.9% respectively reported that they always or most of the time checked the INR, PT, or the viscoelastic test again before transfusing a second unit of plasma. Exactly 23% and 20% checked these tests sometimes or never.
3.3.4. Effect of respondents' primary specialty on transfusion practices during non‐massive bleeding
The primary specialties of anesthesiology and internal medicine were sufficiently powered to test the effect of specialty on transfusion practice. For RBC transfusion, only for patients with traumatic brain injury or a hemorrhagic stroke was a small difference seen. Anesthesiologists would transfuse at a higher Hb level of 8[7.4–9] g/dl versus internists 8 [7–9] g/dl (p = .044) (see Table S2 in Data S3). For platelet transfusion, significant differences were observed in more patient categories (Table S2 in Data S3). In cardiothoracic surgery, obstetric complications, septic patients, and those who recently used antiplatelet drugs, anesthesiologists would transfuse at higher platelet levels. For fibrinogen administration, no association was found between the primary specialty and the reported fibrinogen threshold.
3.3.5. Effect of having transfusion guidelines during non‐massive bleeding
The effect of a hospital‐wide and an ICU‐specific transfusion protocol was assessed for bleeding critically ill patients. When a hospital‐wide transfusion protocol was available, lower platelet transfusion thresholds were applied to patients with upper GI bleeding and in post‐cardiothoracic surgery patients. A hospital‐wide transfusion protocol did not affect the thresholds for RBC transfusion and fibrinogen administration (Table S3 in Data S3). The availability of an ICU‐specific transfusion protocol only showed an effect on the RBC transfusion threshold in ECMO patients (Table S4 in Data S3). When this protocol was available, ECMO patients were transfused at lower Hb levels (p = .026).
3.4. Viscoelastic tests
The majority of the respondents reported the use of viscoelastic tests to guide the blood product choice (RBC, plasma, and platelet concentrates) during massive hemorrhage (73%). However, only 23% reported using viscoelastic tests to guide the use of PCC and 19% to guide the use of fibrinogen. In the decision‐making process for the administration of TXA during massive bleeding, 8% reported using viscoelastic tests to guide its use. This is significantly lower (p < .001) than in non‐massively bleeding patients, where 24% reported using viscoelastic tests prior to TXA administration. When deciding to transfuse non‐massively bleeding ICU patients with plasma, 42% reported using viscoelastic tests. The use of viscoelastic tests during non‐massive bleeding for administration of other blood products was not studied in this survey.
4. DISCUSSION
This is the first international survey among ICU physicians assessing transfusion practices in bleeding critically ill patients. The main findings of this study were: (1) half of the respondents did not have an ICU‐specific transfusion protocol available at their ICU; (2) the presence of an MTP was correlated with the use of fixed transfusion ratios during massive bleeding; (3) a high variation in practice in the use of diagnostic tests, transfusion ratios, fibrinogen, TXA, and PCC in the setting of hemorrhage; (4) during non‐massive bleeding, a high variability in platelet and RBC transfusion thresholds within and between different subpopulations; and (5) plasma was still often administered for VKA induced coagulopathy during massive bleeding.
In general, this survey showed that the majority of the respondents did not use fixed transfusion ratios in the ICU—only 46% would consider this during massive bleeding. The 1:1:1 ratio was most commonly reported (33%). The use of this ratio is controversial as no beneficial effect on mortality in trauma patients was observed in a large RCT. 16 In addition, the potential harm of a high FFP ratio in an ICU setting was reported in a retrospective study, where a high plasma:RBC ratio was associated with increased mortality in patients in general surgery and medicine. 17
Tranexamic acid use in the ICU differed significantly across all subpopulations. Overall, trauma and obstetric patients most often received TXA in the ICU during bleeding as compared with the general ICU population. We speculate that the rationale behind this is that both obstetric and trauma patients have relatively fewer comorbidities compared with the other subpopulations and the benefit of early TXA administration was proven in these patients in a non‐ICU setting: the CRASH‐2 Trial 18 showed reduced mortality in trauma patients in the emergency room and in the WOMAN‐trial, early TXA administration in women with post‐partum hemorrhage decreased mortality due to bleeding. 19 In contrast, a recent study showed that in patients with upper GI bleeding, the use of high dose TXA did not result in a reduction in mortality. 20 In this survey, half of the respondents reported that they would administer TXA always or most of the time during massive upper GI bleeding. However, it should be mentioned that the abovementioned study was published after closing this survey. Therefore, the results on the use of TXA in this specific patient group may already be obsolete.
In the general ICU populations and several subpopulations, including septic, obstetric, trauma, and patients with upper GI bleeding, a relatively restrictive RBC transfusion strategy was reported, with a median Hb threshold of 7–7.5 g/dl. This is in accordance with several large RCTs comparing liberal and restrictive transfusion strategies. 12 , 13 The highest Hb thresholds in this survey were reported for bleeding patients after cardiothoracic surgery 8[7.9–9] g/dl and bleeding patients supported with ECMO 8 [7–9] g/dl. This is in contrast to multiple RCTs showing that a liberal transfusion strategy was not superior to a restrictive transfusion strategy after cardiothoracic surgery. 10 , 11 , 21 In our previous survey, there were also significantly higher Hb thresholds reported in patients with acute coronary syndrome compared with the general ICU population (9[8–9.7] g/dl vs. 7[7–7.5] g/dl). 15 Physicians might associate cardiothoracic surgery with an increased risk of coronary syndrome, which is an indication to consider higher Hb thresholds in several guidelines. 22 , 23 The high variety in Hb thresholds in ECMO patients is not surprising, as this was reported earlier. 15 , 24 As long as no randomized studies are performed in patients receiving ECMO, the optimal Hb trigger in ECMO patients will remain a matter of debate, thus explaining the heterogeneity in the Hb thresholds applied to transfuse these patients.
Despite limited evidence in the ICU, a large proportion of respondents were using viscoelastic tests to guide the choice of blood products during massive bleeding (73.3%). But when deciding to administer fibrinogen or PCC, the number of respondents who use viscoelastic tests was lower: 19% and 23%, respectively. In this survey, the use of viscoelastic tests during non‐massive bleeding to guide platelet and plasma transfusion was not assessed. However, viscoelastic testing did play a role in the use of TXA during non‐massive bleeding, as 24% of respondents used viscoelastic tests to assess whether a patient would benefit from TXA administration. None of these indications have been studied yet in the ICU setting, but there may be potential to reduce the amount of transfusion and thereby the exposure to the potential harmful side effects of blood products. 25
In this survey, 78% would correct a VKA induced coagulopathy with PCC in massively bleeding patients. However, 61% of the respondents also reported that they considered using plasma for this indication, although no evidence is available to support this practice. Multiple RCTs have shown the superiority of PCC versus plasma for VKA reversal in patients with major bleeding or for patients prior to urgent surgical procedures. 26 , 27 Since plasma transfusion has several disadvantages including slower infusion rate, risk of transfusion reactions, and risk of fluid overload, 28 we expected a smaller number of respondents administering plasma for iatrogenic coagulopathy. Therefore, we conclude that the use of plasma could be safely reduced by evidence‐based transfusion guidelines.
This study has several limitations. First, due to the nature of the design of the study, the survey reflects the perceived practice of respondents. Actual practice may still differ from the responses given in the survey. Second, as it is unknown who the nonresponders were, we cannot estimate the effect of this participation bias. Physicians with more interest in blood transfusion might be more likely to fill in this survey, and this group of physicians is likely to be more aware of the latest literature on transfusion practices. Third, to avoid a too long survey, we did not question the use of cryoprecipitate. Since the majority of the respondents work in countries where fibrinogen is used, we believe this did not influence our results. Fourth, the majority of our respondents are working in high‐income countries, therefore are our findings mainly generalizable to high resource settings. Finally, the definition of massive bleeding is currently still under debate. We used a broad definition of massive bleeding; however, respondents may have used their own personal definitions for this term.
5. CONCLUSION
In conclusion, we observed a high variety in transfusion practice among intensive care physicians and a lack of guidelines for the management of bleeding critically ill patients. The presence of a massive transfusion protocol influenced transfusion practices. Current transfusion practice was influenced by large transfusion studies in trauma patients. However, since these studies might not be completely applicable to all critically ill patients, more research specifically into the management of bleeding critically ill patients is warranted.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors contributed to the study concept, study design. All authors and collaborators critically revised the questionnaire. Sanne de Bruin and Dorus Eggermont collected the data. Sanne de Bruin is responsible for the statistical analysis. Sanne de Bruin, Dorus Eggermont, and Alexander P.J. Vlaar drafted the manuscript. All authors and collaborators critically revised the manuscript. All authors read and approved the final manuscript.
CONSENT FOR PUBLICATION
Not applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
Supporting information
Data S1. Static version survey
Data S2. Overview endorsement national societies
Data S3. Supplemental tables
Data S4. Supplemental figures
ACKNOWLEDGMENT
This survey was endorsed by the European Society of Intensive Care Medicine (ESICM). Members of the Cardiovascular Dynamics Section and Transfusion Guideline Task Force of the ESICM. Members of the Cardiovascular Dynamics Section and/or Transfusion Guideline Task Force of the ESICM include: Riccardo G. Abbasciano, Massimo Antonelli, Cécile Aubron, Frank E.H.P. van Baarle, Maurizio Cecconi, Joanna C. Dionne, Jacques Duranteau, Gordon Gyatt, Beverley J Hunt, Nicole P. Juffermans, Marcus Lance, Jens Meier, Marcella C.A. Muller, Gavin J. Murphy, Nathan Nielsen, Simon J. Oczkowski, Anders Perner, S. Jorinde Raasveld, Herbert Schöchel, and Marije Wijnberge.
APPENDIX A. COLLABORATORS
The Guideline Task Force of the ESICM includes Riccardo G. Abbasciano, Massimo Antonelli, Cécile Aubron, Frank E.H.P. van Baarle, Maurizio Cecconi, Joanna C. Dionne, Jacques Duranteau, Gordon Gyatt, Beverley J Hunt, Nicole P. Juffermans, Marcus Lance, Jens Meier, Marcella C.A. Muller, Gavin J. Murphy, Nathan Nielsen, Simon J. Oczkowski, Anders Perner, S. Jorinde Raasveld, Herbert Schöchel, and Marije Wijnberge.
Contact person is chair of the Transfusion Guideline Task Force of the ESICM: Alexander P. J. Vlaar (a.p.vlaar@amsterdamumc.nl)
de Bruin S, Eggermont D, van Bruggen R, de Korte D, Scheeren TWL, Bakker J, et al. Transfusion practice in the bleeding critically ill: An international online survey—The TRACE‐2 survey. Transfusion. 2022;62:324–335. 10.1111/trf.16789
Collaborators are provided in Appendix A.
Funding informationNone.
Contributor Information
Alexander P. J. Vlaar, Email: a.p.vlaar@amsterdamumc.nl.
Cardiovascular Dynamics Section and Transfusion Task Force of the ESICM:
Riccardo G. Abbasciano, Massimo Antonelli, Cécile Aubron, Frank E.H.P. van Baarle, Maurizio Cecconi, Joanna C. Dionne, Jacques Duranteau, Gordon Gyatt, Beverley J Hunt, Nicole P. Juffermans, Marcus Lance, Jens Meier, Marcella C.A. Muller, Gavin J. Murphy, Nathan Nielsen, Simon J. Oczkowski, Anders Perner, S. Jorinde Raasveld, Herbert Schöchel, and Marije Wijnberge
DATA AVAILABILITY STATEMENT
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Juffermans NP, Walsh TS. Transfusion in the intensive care unit. Cham: Springer International Publishing; 2015. [Google Scholar]
- 2. Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red‐cell transfusion. N Engl J Med. 2017;377(13):1261–72. [DOI] [PubMed] [Google Scholar]
- 3. Shander A, Goodnough LT. Can blood transfusion be not only ineffective, but also injurious? Ann Thorc Surg. 2014;97(1):11–4. [DOI] [PubMed] [Google Scholar]
- 4. Kim‐Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion. 2011;51(4):844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hess JR. Measures of stored red blood cell quality. Vox Sang. 2014;107(1):1–9. [DOI] [PubMed] [Google Scholar]
- 6. Gajic O, Dzik WH, Toy P. Fresh frozen plasma and platelet transfusion for nonbleeding patients in the intensive care unit: benefit or harm? Crit Care Med. 2006;34(5 Suppl):S170–3. [DOI] [PubMed] [Google Scholar]
- 7. Dara SI, Rana R, Afessa B, Moore SB, Gajic O. Fresh frozen plasma transfusion in critically ill medical patients with coagulopathy. Crit Care Med. 2005;33(11):2667–71. [DOI] [PubMed] [Google Scholar]
- 8. Veelo DP, Vlaar APJ, Klanderman RB, Murphy MF, Bosboom JJ, Migdady Y, et al. Transfusion associated circulatory overload; a clinical perspective. Transfus Med Rev. 2019;33(2):69–77. [DOI] [PubMed] [Google Scholar]
- 9. Vlaar APJ, Juffermans NP. Transfusion‐related acute lung injury: a clinical review. Lancet. 2013;382(9896):984–94. [DOI] [PubMed] [Google Scholar]
- 10. Mazer CD, Whitlock RP, Fergusson DA, Hall J, Belley‐Cote E, Connolly K, et al. Restrictive or liberal red‐cell transfusion for cardiac surgery. N Engl J Med. 2017;377(22):2133–44. [DOI] [PubMed] [Google Scholar]
- 11. Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, et al. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015;372(11):997–1008. [DOI] [PubMed] [Google Scholar]
- 12. Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez‐Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11–21. [DOI] [PubMed] [Google Scholar]
- 13. Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371(15):1381–91. [DOI] [PubMed] [Google Scholar]
- 14. Hebert PC, Wells G, Blajchman M, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340(13):1056–6. [DOI] [PubMed] [Google Scholar]
- 15. de Bruin S, Scheeren TWL, Bakker J, van Bruggen R, Vlaar APJ. Cardiovascular dynamics section and transfusion guideline task force of the ESICM. Transfusion practice in the non‐bleeding critically ill: an international online survey‐the TRACE survey. Crit Care. 2019;23(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mesar T, Larentzakis A, Dzik W, Chang Y, Velmahos G, Yeh DD. Association between ratio of fresh frozen plasma to red blood cells during massive transfusion and survival among patients without traumatic injury. JAMA Surg. 2017;152(6):574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. CRASH‐2 trial collaborators , Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH‐2): a randomised, placebo‐controlled trial. Lancet. 2010;376(9734):23–32. 10.1016/S0140-6736(10)60835-5 [DOI] [PubMed] [Google Scholar]
- 19. Shakur H, Roberts I, Fawole B, Chaudhri R, El‐Sheikh M, Akintan A, et al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post‐partum haemorrhage (WOMAN): an international, randomised, double‐blind, placebo‐controlled trial. Lancet. 2017;389(10084):2105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roberts I, Shakur‐Still H, Afolabi A, Akere A, Arribas M, Brenner A, et al. Effects of a high‐dose 24‐h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT‐IT): an international randomised, double‐blind, placebo‐controlled trial. Lancet. 2020;395(10241):1927–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hajjar LA, Vincent J‐L, Galas FRBG, Nakamura RE, Silva CMP, Santos MH, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304(14):1559–67. [DOI] [PubMed] [Google Scholar]
- 22. National Institute for Health and Care Excellence . Blood Transfusion. London, UK: National Institute for Health and Care Excellence; 2015. [Google Scholar]
- 23. Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. 2016;316(19):2025–35. [DOI] [PubMed] [Google Scholar]
- 24. Martucci G, Grasselli G, Tanaka K, Tuzzolino F, Panarello G, Schmidt M, et al. Hemoglobin trigger and approach to red blood cell transfusions during veno‐venous extracorporeal membrane oxygenation: the international TRAIN‐ECMO survey. Perfusion. 2019;34(1_suppl):39–48. [DOI] [PubMed] [Google Scholar]
- 25. Crochemore T, Corrêa TD, Lance MD, Solomon C, Neto AS, De Campos Guerra JC, et al. Thromboelastometry profile in critically ill patients: a single‐center, retrospective, observational study. PLoS One. 2018;13(2):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sarode R. Four‐factor prothrombin complex concentrate versus plasma for urgent vitamin K antagonist reversal: new evidence. Clin Lab Med. 2014. Sep;34(3):613–21. 10.1016/S0140-6736(14)61685-8 [DOI] [PubMed] [Google Scholar]
- 27. Sarode R, Milling TJ, Refaai MA, Mangione A, Schneider A, Durn BL, et al. Efficacy and safety of a 4‐factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma‐controlled, phase IIIb study. Circulation. 2013;128(11):1234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. MacLennan S, Williamson LM. Risks of fresh frozen plasma and platelets. J Trauma Inj Infect Crit Care. 2006;60(6 Suppl):41–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Static version survey
Data S2. Overview endorsement national societies
Data S3. Supplemental tables
Data S4. Supplemental figures
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


