Abstract
The Lactococcus lactis temperate bacteriophage BK5-T is one of twelve type phages that define L. lactis phage species. This paper describes the nucleotide sequence and analysis of a 21-kbp region of the BK5-T genome and completes the nucleotide sequence of the genome of this phage. The 40,003-nucleotide linear genome encodes 63 open reading frames. Sequence runoff experiments showed that the cohesive ends of the BK5-T genome contained a 12-bp 3′ single-stranded overhang with the sequence 5′-CACACACATAGG-3′. Two major BK5-T structural proteins, of approximately 30 and 20 kDa, were identified, and N-terminal sequence analysis determined that they were encoded by orf7 and orf12, respectively. A 169-bp fragment containing a 37-bp direct repeat and several smaller repeat sequences conferred resistance to BK5-T infection when introduced in trans to the host cell and is likely a part of the BK5-T origin of replication (ori).
Lactic acid bacteria are used extensively as starter cultures in the dairy industry. The bacteria ferment lactose to lactic acid, a crucial process in cheese manufacture. One of the major microbiological problems faced by the dairy fermentation industry is the susceptibility of the starter bacteria to bacteriophage infection. Such infections result in lysis of starter cultures and failure of the fermentation. Since reports of bacteriophage infection of starter strains as early as 1935 (64), an increasing number of bacteriophages have been identified and considerable research has been conducted to improve our understanding of these viruses and their interaction with their hosts.
BK5-T is a temperate bacteriophage with a small isometric head and a noncontractile tail of 232 nm in length (24) first isolated from Lactococcus lactis subsp. cremoris BK5 (23). Boyce et al. (8) determined the nucleotide sequence of approximately 19 kbp of the BK5-T genome, which defined the EcoRI restriction fragments EcoRI-a and EcoRI-b (32). Thirty-two open reading frames (ORFs) were identified, and the predicted amino acid sequences encoded by several of these ORFs demonstrated significant homology with sequences available in protein databases. Analysis of the partial genome sequence also identified a putative BK5-T immunity region containing regulatory elements involved in determination of the phage lysis-or-lysogeny decision (7). This report describes the nucleotide sequence of the remaining 21 kbp of the BK5-T genome and characterizes the phage termini, major structural proteins, and the putative origin of replication.
MATERIALS AND METHODS
Bacterial strains and media.
The strains and plasmids used in this investigation are listed in Table 1. Escherichia coli strain JM107 was incubated at 37°C with shaking in 2TY medium (42). The BK5-T indicator strain L. lactis subsp. cremoris H2 was grown at 30°C for 16 h in M17 medium supplemented with 0.5% (wt/vol) glucose (M17G) (57). When necessary, the antibiotic erythromycin (2.5 μg/ml for L. lactis or 200 μg/ml for E. coli) or ampicillin (150 μg/ml for E. coli) was included in the media.
TABLE 1.
Strains and plasmids used in this investigation
| Strain or plasmid | Relevant characteristicsa | Reference |
|---|---|---|
| Strains | ||
| JM107 | endA1 gyrA96 thi hsdR17 supE44 relA1 λ− Δ(lac-proAB) (F′ traD36 proAB lacIqZΔM15) | 65 |
| H2 | Indicator strain for BK5-T phage | CSIRO Dairy Research Laboratory |
| Plasmids | ||
| pTRKH2 | Emr; E. coli lactococcal shuttle vector, high-copy-number vector | 46 |
| pJDC9 | Emr; E. coli vector, one functional transcription terminator, contains pUC19 polylinker and lacZ′ region | 12 |
| pGEM-T | Apr; E. coli cloning vector | Promega, Madison, Wis. |
| pMU1252 | Tcr Cms; EcoRI-d from BK5-T cloned in pACYC184 | 32 |
| pMU1253 | Tcr Cms; EcoRI-f from BK5-T cloned in pACYC184 | 32 |
| pMU1254 | Tcr Cms; EcoRI-g from BK5-T cloned in pACYC184 | 32 |
| pCM25 | Emr; BK5-T 2.7-kbp PstI fragment cloned in pJDC9 | This study |
| pCM29 | Emr; 921-bp PCR product containing BK5-T orf49 cloned into the SmaI site of pTRKH2 | This study |
| pCM30 | Emr; 306-bp PCR product containing the repeat (DR1 to DR3 and IR4) sequences in orf49 cloned into the SmaI site of pTR KH2 | This study |
| pCM30.2 | Emr; 250-bp exonuclease deletion version of pCM30 | This study |
| pCM30.4 | Emr; 275-bp exonuclease deletion version of pCM30 | This study |
| pCM31 | Emr; 306-bp PCR product containing the repeat (DR1 to DR3 and IR4) sequences in orf49 cloned into the SmaI site of pTR KH2 in the opposite orientation to pCM30 | This study |
| pCM31.8 | Emr; 111-bp exonuclease deletion version of pCM31 | This study |
| pCM31.31 | Emr; 177-bp exonuclease deletion version of pCM31 | This study |
| pCM31.35 | Emr; 205-bp exonuclease deletion version of pCM31 | This study |
Em, Ap, Cm, and Tc refer to antibiotic resistance markers erythromycin, ampicillin, chloramphenicol, and tetracycline, respectively.
Phage preparation.
Bacteriophage BK5-T was propagated by lytic infection of L. lactis H2 in M17G medium with 10 mM CaCl2. The bacteriophage were precipitated with 1 M NaCl and 10% (wt/vol) polyethylene glycol (PEG), and when necessary, further purified and concentrated by CsCl density gradient centrifugation (49). Phage DNA was isolated from the PEG-precipitated phage by the procedure described for coliphage λ by Qiagen (Qiagen, GmbH, Hilden, Germany).
N-terminal amino acid sequencing of phage proteins.
BK5-T phage purified by CsCl density gradient centrifugation (49) were heated at 95°C for 15 min in cracking buffer (50 mM Tris-HCl [pH 6.8], 1% [wt/vol] sodium dodocyl sulfate [SDS], 2 mM EDTA, 10% [vol/vol] glycerol, 1% [vol/vol] β-mercaptoethanol) and separated by SDS-polyacrylamide gel electrophoresis (PAGE) on a precast Novex (Amrad Biotech, Victoria, Australia) 4 to 20% acrylamide Tris-glycine gel. The denatured proteins were transferred by electroblotting to a Bio-Rad polyvinylidene difluoride membrane in a buffer containing 10 mM 3-[cyclohexylamino]-1-propanesulfonic acid (CAPS) and 20% (vol/vol) methanol. The membrane was stained with 0.1% (wt/vol) Ponceau S containing 1% (vol/vol) glacial acetic acid, the phage protein bands were excised, and their N-terminal amino acid sequences were determined by the Australian Proteome Analysis Facility (Macquarie University, Sydney, Australia).
Nucleotide sequencing.
The BK5-T EcoRI restriction fragments EcoRI-f, EcoRI-d, and EcoRI-g, which had previously been cloned in pACYC184 by Lakshmidevi et al. (33), were subcloned into the E. coli vector pGEM-3Zf (+) (Table 1). EcoRI-f and EcoRI-d were subcloned directly in both orientations and EcoRI-g was digested with HindIII and the five fragments generated were individually cloned in both orientations into pGEM-3Zf (+). All the clones were subjected to exonuclease III treatment and a series of subclones containing deleted fragments was used for sequencing templates. Plasmid DNA for sequencing was purified by the Qiagen miniprep procedure (Qiagen), followed by PEG precipitation as described by the Applied Biosystems (ABI) Taq DyeDeoxy terminator cycle sequencing kit protocol. Determination of the nucleotide sequence was conducted as outlined in the ABI sequencing manual. The sequencing reaction products were analyzed on an ABI model 373A automated sequencer.
The nucleotide sequence across junction points between clones was determined by chromosome walking, using synthesized oligonucleotides and purified BK5-T phage DNA as template. Assembly of the nucleotide sequence was conducted using Sequencher software (Sequencher 3.0, Gene Codes Corporation, Ann Arbor, Mich.). Computer analyses and database searches were conducted using programs available at the Australian National Genomic Information Service.
Sequence runoff experiments.
Two oligonucleotides, COS1 (5′-GACCATCATGGATAACTTGGC-3′) and COS2 (5′-CGCCAACAAGCACTTGCGAG-3′), were designed approximately 200 bp from either end of the linear BK5-T genome. These oligonucleotides were used for linear amplification of DNA using three different preparations of purified unligated BK5-T phage DNA as sequencing template, and the nucleotide sequences of the amplicons were determined as described above.
Nucleotide sequence accession number.
The sequence reported here is available in GenBank under accession no. AF176025.
RESULTS
Complete nucleotide sequence of BK5-T.
Boyce et al. (8) determined the nucleotide sequence of 18,935 bp of the BK5-T genome, and the remaining 21 kbp is reported here. The total circular length of the BK5-T genome was determined to be 40,003 bp. The codon usage of the BK5-T ORFs was determined and found to correlate with the codon usage of the host lactococcal genome.
Analysis of BK5-T ORFs.
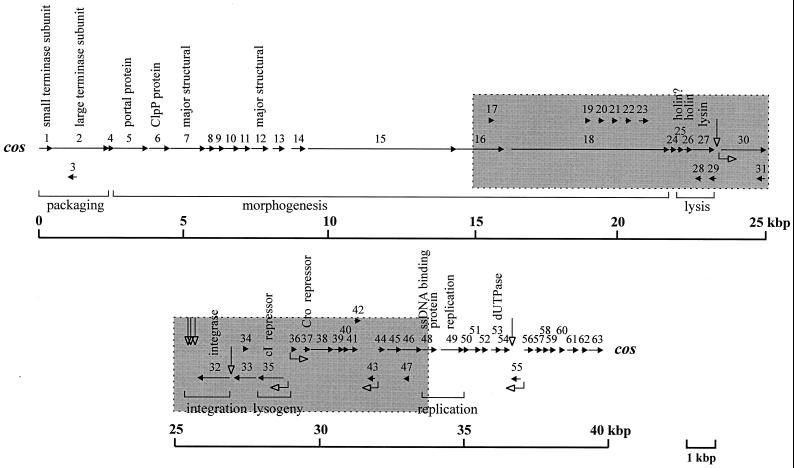
A schematic diagram of the BK5-T ORFs is shown in Fig. 1. The BK5-T ORFs were numbered sequentially along the genome, as described for other phages (10, 26, 27, 55), in contrast to the previous nomenclature of Boyce et al. (8), which was based on the number of codons in the ORF (Table 2). A total of 63 ORFs which met the following criteria were identified: (i) the ORF contained greater than 40 codons; (ii) the ORF was preceded by an identifiable ribosomal binding site (RBS) (4 to 12 nucleotides from the putative start codon) or was likely to be translationally coupled to the preceding ORF; and (iii) the ORF began with either an AUG, GUG, AUA, or UUG codon. In addition, several ORFs that had previously been identified by Boyce et al. (8) that did not meet all of the above criteria were included for consistency.
FIG. 1.
Genetic map of the BK5-T genome. The map is linearized at the cohesive ends and arbitrarily split at the phage attP site located between orf31 and orf32. The grey-boxed areas indicate ORFs previously determined by Boyce et al. (8). The horizontal arrows indicate ORFs and their orientation while the numbers above the arrows indicate ORF designation. Open-headed right-angled arrows indicate positions of putative promoter sequences and their direction of transcription initiation and open-headed vertical arrows indicate the positions of palindromic sequences that may be involved in termination of transcription. Bracketed regions below the map indicate the ORFs associated with the functional modules as described in the text. Putative functions assigned to the BK5-T ORFs are described above the relevant ORFs.
TABLE 2.
Position, predicted molecular sizes, RBSs, and database sequence homologies for the BK5-T ORFs
| BK5-T ORF | Previous namea | Molecular size (kDa) | Predicted start position | Predicted stop positionb | Putative RBSc | ORF homologyd | Organism matched | % Identity (over no. of amino acids) | Accession no. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 18.2 | 77 | 550 | acuaaaaGAAAGGAGaaaaaaug | ORF21 | S. thermophilus φ7201 | 46 (151) | AAF43514 | |
| ORF2 | S. thermophilus DT1 | 43 (151) | AAD21878 | ||||||
| ORF152 | S. thermophilus φSfi21 | 44 (151) | AAD41028 | ||||||
| ORF161 | S. thermophilus φSfi19 | 43 (151) | AAD44055 | ||||||
| ORF149 | L. gasseri φadh | 38 (144) | |||||||
| ORF4 | L. lactis subsp. cremories | 35 (139) | CAA11696 | ||||||
| ORF4 | L. casei A2 | 24 (146) | CAA66177 | ||||||
| 2 | 75.6 | 537 | 2,510 | ggcauucuuugauGGAGGugaug | ORF623 | S. thermophilus φSfi19 | 64 (623) | AAD44056 | |
| ORF623 | S. thermophilus φSfi21 | 63 (623) | AAD41029 | ||||||
| ORF624 | L. gasseri φadh | 59 (625) | CAB52518 | ||||||
| ORF22 | S. thermophilus φ7201 | 57 (624) | AAF43515 | ||||||
| ORF3 | S. thermophilus DT1 | 64 (365) | AAD21880 | ||||||
| ORF4 | S. thermophilus DT1 | 62 (231) | AAD21879 | ||||||
| 3 | 12.8 | 1,385 | 1,065 | ucugGAuAGGAaGUugaaauuug | |||||
| 4 | 7.7 | 2,479 | 2,688 | ccuugaugacgaaauagaugaug | ORF5 (head-tail joining) | S. thermophilus DT1 | 45 (55) | AD21881 | |
| ORF23 | S. thermophilus φ7201 | 42 (55) | AAF43516 | ||||||
| ORF59c | S. thermophilus φSfi21 | 44 (55) | AAD41030 | ||||||
| ORF59 | S. thermophilus φSfi19 | 43 (53) | AAD44057 | ||||||
| 5 | 43.5 | 2,685 | 3,863 | auagaaaaGAAgGGAGGugauug | ORF387 | S. thermophilus φSfi19 | 56 (389) | AAD44058 | |
| ORF384 | S. thermophilus φSfi21 | 56 (388) | AAD41031 | ||||||
| ORF6 | S. thermophilus DT1 | 57 (381) | AAD21882 | ||||||
| ORF24 | S. thermophilus φ7201 | 57 (383) | AAF43517 | ||||||
| ORF397 | L. gasseri φadh | 51 (356) | CAB52519 | ||||||
| ORFH | L. casei A2 | 24 (291) | CAB63683 | ||||||
| ORF4 (portal) | S. aureus φPVL | 24 (306) | BAA31877 | ||||||
| GP34 | S. actinophage φC31 | 21 (331) | CAA07104 | ||||||
| ORF1305 | P. aeruginosa D3 | 22 (368) | AAD38955 | ||||||
| 6 | 2.6 | 3,906 | 4,619 | uauucuuaGAAAGGAGGuaaaug | ORF7 | S. thermophilus DT1 | 53 (238) | AAD21883 | |
| ORF25 | S. thermophilus φ7201 | 54 (237) | AAF43518 | ||||||
| ORF221 | S. thermophilus φSfi21 | 52 (237) | AAD41032 | ||||||
| ORF242 | L. gasseri φadh | 41 (239) | CAB52520 | ||||||
| ORF891 | P. aeruginosa D3 | 38 (167) | AAD38956 | ||||||
| ClpP | Listeria monocytogenes | 29 (139) | AAF04744 | ||||||
| ClpP | Streptomyces coelicolor | 25 (146) | AAC70947 | ||||||
| 7 | 44.7 | 4,631 | 5,845 | gaaaaauAAAGGAGacucaaaug | ORF397—major structural | S. thermophilus φSfi19 | 60 (395) | AAD44059 | |
| ORF397—major structural | S. thermophilus φSfi21 | 59 (399) | AAD41033 | ||||||
| MPL—major structural | S. thermophilus φ7201 | 59 (395) | AAD43519 | ||||||
| ORF8—major structural | S. thermophilus DT1 | 65 (289) | AAD21884 | ||||||
| ORF395—major structural | L. gasseri φadh | 47 (409) | CAB52521 | ||||||
| ORF27 | B. subtilis φ105 | 34 (396) | AB016282 | ||||||
| Major structural protein | L. casei A2 | 22 (357) | CAB63685 | ||||||
| ORF1188—major structural | P. aeruginosa D3 | 20 (400) | AAD38957 | ||||||
| ORF7—major structural | S. aureus φPVL | 25 (359) | BAA31880 | ||||||
| 8 | 12.1 | 5,853 | 6,176 | ugcagGAAAGuAGGuaauuuaug | ORF27 | S. thermophilus φ7201 | 32 (103) | AAF43520 | |
| ORF106 | S. thermophilus φSfi21 | 29 (103) | AAC39275 | ||||||
| ORF104 | S. thermophilus φSfi19 | 29 (103) | AAC39289 | ||||||
| ORF9 | S. thermophilus DT1 | 27 (103) | AAD21885 | ||||||
| ORFK | Leuconostoc oenos L10 | 28 (86) | AAA66331 | ||||||
| ORF126b | L. gasseri φadh | 29 (105) | CAB52522 | ||||||
| 9 | 1.3 | 6,151 | 6,504 | uucguGgAAGGAGGcgcaagaug | ORF28 | S. thermophilus φ7201 | 31 (113) | AAF43521 | |
| ORF10 | S. thermophilus DT1 | 30 (113) | AAD21886 | ||||||
| ORF116 | S. thermophilus φSfi19 | 30 (113) | AAC39290 | ||||||
| ORF116 | S. thermophilus φSfi21 | 27 (113) | AAC39276 | ||||||
| ORF126c | L. gasseri φadh | 22 (120) | CAB52523 | ||||||
| 10 | 19.1 | 6,506 | 7,012 | gaagaaGAAgGGAGcuuaauaug | ORFA | L. oenos L10 | 39 (147) | AAA66332 | |
| ORF141b | S. thermophilus φSfi21 | 26 (163) | AAC39277 | ||||||
| ORF29 | S. thermophilus φ7201 | 29 (165) | AAC43522 | ||||||
| ORF11 | S. thermophilus DT1 | 30 (165) | AAC21887 | ||||||
| ORF140 | S. thermophilus φSfi19 | 30 (162) | AAC44060 | ||||||
| ORF159b | L. gasseri φadh | 28 (178) | CAB52524 | ||||||
| 11 | 14.7 | 7,009 | 7,404 | gaaaAAAGaAGGaauaacuuaug | ORFI | L. oenos L10 | 37 (110) | AAA66333 | |
| ORF123 | S. thermophilus φSfi19 | 32 (107) | T09268 | ||||||
| ORF30 | S. thermophilus φ7201 | 33 (107) | AAF43523 | ||||||
| ORF12 | S. thermophilus DT1 | 33 (107) | AAD21888 | ||||||
| ORF123 | S. thermophilus φSfi21 | 32 (107) | AAC39278 | ||||||
| 12 | 20.6 | 7,435 | 8,028 | auuuaaGAAAGGAauuuaaaaug | ORF203—major structural | S. thermophilus φSfi19 | 46 (198) | AAC39293 | |
| ORF13—major structural | S. thermophilus DT1 | 46 (195) | AAD21889 | ||||||
| ORF202—major structural | S. thermophilus φSfi21 | 45 (199) | AAC39279 | ||||||
| MPS—major structural | S. thermophilus φ7201 | 45 (198) | AAB71820 | ||||||
| ORF E | L. oenos L10 | 43 (193) | AAA66334 | ||||||
| ORF237—major tail | L. gasseri φadh | 28 (134) | CAB52526 | ||||||
| 13 | 15.9 | 8,173 | 8,592 | uaaaauagAGGAGauauacaaug | ORF14 | S. thermophilus DT1 | 31 (121) | AAD21890 | |
| ORF117 | S. thermophilus φSfi19 | 28 (121) | AAC39294 | ||||||
| ORF117 | S. thermophilus φSfi21 | 29 (121) | AAC39280 | ||||||
| ORF32 | S. thermophilus φ7201 | 26 (121) | AAF43524 | ||||||
| 14 | 18.2 | 8,821 | 9,312 | uuaaaaaucAGGAGaaauucaug | |||||
| 15 | 183.5 | 9,394 | 14,535 | aaaauuAGAAAGGAGuaaaaaug | ORF48 (minor capsid) | Lactobacillus plantarum φgle | 30 (1,369) | CAA66745 | |
| ORF465 | L. casei A2 | 30 (450) | CAB63691 | ||||||
| ORF16 | S. thermophilus DT1 | 37 (320) | AAD21892 | ||||||
| ORF360 | Lactobacillus delbrueckii LL-H | 35 (306) | AAC00548 | ||||||
| ORF15 | S. thermophilus DT1 | 22 (1,291) | AAD21891 | ||||||
| ORF33 | S. thermophilus φ7201 | 24 (552) | AAF43525 | ||||||
| ORF1560 (minor tail) | S. thermophilus φSfi21 | 23 (1,753) | AAC39281 | ||||||
| ORF1626 (minor tail) | S. thermophilus φSfi19 | 23 (1,789) | AAC39295 | ||||||
| 16 | ′410 | 60 | 14,532 | 16,178 | agauuAGGAGGuauuaugauuug | ORF34 | S. thermophilus φ7201 | 25 (564) | AAF43526 |
| ORF17 | S. thermophilus DT1 | 26 (564) | AAD21893 | ||||||
| ORF515 | S. thermophilus φSfi19 | 27 (567) | AAC39296 | ||||||
| ORF515 | S. thermophilus φSfi21 | 27 (567) | AAC39282 | ||||||
| ORF I—structural tail | L. casei A2 | 26 (236) | CAB63694 | ||||||
| 17 | 64#1 | 7.7 | 15,646 | 15,840 | aggaggacaagaauuaaauaaug | ||||
| 18 | 1904 | 205.8 | 16,178 | 21,892 | uggaaAGAAAGGuaucuauaaug | ORF35 | S. thermophilus φ7201 | 36 (515) | AAF43527 |
| ORF18 | S. thermophilus DT1 | 32 (738) | AAF21894 | ||||||
| ORF45 | S. thermophilus O1205 | 38 (509) | AAC79560 | ||||||
| ORF38 | S. thermophilus φ7201 | 39 (560) | AAF43530 | ||||||
| ORF1276 | S. thermophilus φSfi21 | 33 (1,058) | AAC39283 | ||||||
| ORF1291 | S. thermophilus φSfi19 | 34 (1,058) | AAC39297 | ||||||
| 19 | 66 | 7.6 | 18,981 | 19,181 | aaaagacGGAaauaacgguaaug | ||||
| 20 | 71#1 | 8.3 | 19,434 | 19,649 | uguagccuauaugGGUaccaaug | ||||
| 21 | 71#2 | 8.3 | 19,902 | 20,117 | uguagccuauaugGGUaccaaug | ||||
| 22 | 71#3 | 8.3 | 20,370 | 20,585 | uguagccuauaugGGUaccaaug | ||||
| 23 | 69#1 | 8.1 | 20,838 | 21,044 | uguagccuauaugGGUaccaaug | ||||
| 24 | 78 | 8.7 | 21,907 | 22,143 | aacuagaaacAAGGAGaaaaaug | ||||
| 25 | 75 | 8.9 | 22,156 | 22,383 | aaaaauAGAAAGcAGGgguuaug | ||||
| 26 | 95 | 10.5 | 22,396 | 22,683 | gauucugAAGGAGaaagaacaug | ORF24 | S. aureus φPVL | 37 (70) | BAA31897 |
| BH0967 (holin) | Bacillus halodurans | 36 (72) | BAB04686 | ||||||
| GP26 | B. subtilis SPP1 | 39 (82) | CAA66519 | ||||||
| XPAF2—holin | B. subtilis | 40 (81) | P36549 | ||||||
| XPAG2—holin | B. licheniformis | 35 (81) | BAA08562 | ||||||
| 27 | 259 | 27.9 | 22,683 | 23,462 | uucacagAAGGAGGcgaauaaug | Lysin | L. lactis φ31 | 9 (173) | AC04153 |
| N-acetylmuramoyl-l- alanine amidase | Pneumococcus sp. Dp-1 | 43 (148) | CAB07986 | ||||||
| ORF288 | S. thermophilus φSfi21 | 30 (128) | AAC39288 | ||||||
| ORF288 | S. thermophilus φSfi19 | 30 (128) | AAD44061 | ||||||
| ORF288 | S. thermophilus φSfi11 | 30 (128) | AAC34421 | ||||||
| 28 | 74 | 8.1 | 22,996 | 22,772 | ugugaguguaaGAGcagugaaug | ||||
| 29 | 86 | 9.5 | 23,512 | 23,252 | aguaaucuaaaaauaguuucaug | ||||
| 30 | 536 | 61.9 | 23,679 | 25,289 | cugauucGGAGugugugagaaug | ||||
| 31 | 101 | 11.3 | 25,210 | 24,905 | uuauuccuuuaccuucucccaug | ||||
| 32 | 374 | 43.3 | 27,035 | 25,911 | aaauGGAGuaaaaaucaaauaug | —Integrase | L. lactis Tuc2009 | 99 (374) | AAA32608 |
| —Integrase | L. lactis φLC3 | 99 (374) | AAA32254 | ||||||
| ORF1—Integrase | L. lactis rlt | 98 (374) | AAB18676 | ||||||
| BH3551 | B. halodurans | 36 (376) | BAB07270 | ||||||
| ORF359 | S. thermophilus TPJ34 | 33 (376) | AAC03454 | ||||||
| ORF1 | S. thermophilus O1205 | 33 (373) | AAC79517 | ||||||
| ORF8 (Integrase) | L. plantarum φgle | 31 (381) | CAA66758 | ||||||
| —Integrase | L. lactis TPW22 | 30 (379) | AAF12706 | ||||||
| ORFB—integrase | L. casei A2 | 26 (391) | CAA73344 | ||||||
| —Integrase | S. aureus φ13 | 30 (372) | CAA57755 | ||||||
| ORF2—integrase | S. aureus φ42 | 30 (372) | AAA91615 | ||||||
| 33 | 258 | 28.7 | 27,949 | 27,173 | agcuagauaGGAGuauuuauaug | Exp3 | L. lactis MG1363 | 43 (128) | AAC14595 |
| 34 | 64#2 | 7.6 | 27,496 | 27,690 | uuuccauccauuauuuagcaaug | ||||
| 35 | 297 | 33.3 | 28,898 | 28,005 | aauauuaaggGGAGaugggaaug | cI repressor | L. lactis Tuc2009 | 73 (285) | AAA21825 |
| Rro—cI repressor | L. lactis rlt | 74 (280) | AAB18678 | ||||||
| Repressor | L. lactis φLC3 | 99 (74) | AAB53017 | ||||||
| 36 | 63 | 7.5 | 29,155 | 29,346 | gaaggcaaauGGAGGuucuaaug | ORF9 | L. lactis TPW22 | 93 (55) | AAF12713 |
| ORF61b | L. lactis u136.1 | 56 (61) | AAF74094 | ||||||
| ORF61a | L. lactis ul36.2 | 56 (61) | AAF74109 | ||||||
| ORF57 | L. lactis φ31.1 | 52 (54) | AAF43117 | ||||||
| ORF9 | L. lactis TP901-1 | 52 (54) | CAA74618 | ||||||
| ORF8 | L. lactis rlt | 52 (54) | AAB18683 | ||||||
| 37 | 79 | 9 | 29,574 | 29,813 | aagcaaAGAAAGGAGccaguaug | ORF29 | S. thermophilus DT1 | 37 (46) | AAD21905 |
| ORF67 (Cro) | S. thermophilus TPJ34 | 32 (47) | AAC03458 | ||||||
| 38 | 266 | 30.6 | 29,826 | 30,626 | cuaguuAGAAAGGcgaaaacaug | ORF5 | L. lactis rlt | 97 (266) | AAB18680 |
| ORFA | L. casei A2 | 50 (147) | CAA73339 | ||||||
| ORF291 | L. delbrueckii LL-H | 39 (280) | AAB06224 | ||||||
| ORF169a | L. delbrueckii mv4 | 44 (154) | AAG31333 | ||||||
| 39 | 111 | 12.9 | 30,639 | 30,974 | cuagcuAGAAAGGgaagcacaug | ORF6 | L. lactis rlt | 97 (109) | AAB18681 |
| ORF13 | L. plantarum φgle | 26 (107) | CAA66763 | ||||||
| ORF96 | L. casei A2 | 27 (96) | CAB63664 | ||||||
| 40 | 42 | 4.8 | 30,971 | 31,099 | uuuucAGGAGGgaguggucuaug | L. lactis phage ul36.2 | 95 (42) | AF212847 | |
| L. lactis strain SMQ-86 | 95 (42) | AF212844 | |||||||
| 41 | 113 | 13.7 | 31,112 | 31,453 | uccgcuAGAAAGGAGagauuuug | L. lactis ul36.2 | 91 (68) | AF212847 | |
| L. lactis phage ul36.1 | 91 (68) | AF212846 | |||||||
| L. lactis phage ul36 | 91 (68) | AF212845 | |||||||
| L. lactis strain SMQ-86 | 91 (68) | AF212844 | |||||||
| 42 | 72 | 8.5 | 31,333 | 31,551 | acgcuagaaaaGAGaaauuuaug | ||||
| 43 | 88 | 10.3 | 32,039 | 31,773 | augGAAAGGAGGaaugaagcaug | ||||
| 44 | 80 | 9.6 | 32,146 | 32,385 | acauAGAAAGGAaauuaaaaaug | ||||
| 45 | 169 | 20 | 32,457 | 32,966 | auaacaaaucGGAGGAaauaaug | ORF169 | L. lactis φ31 | 98 (169) | AAC48870 |
| ORF163 | L. casei A2 | 31 (160) | CAB63666 | ||||||
| ORF157 | S. thermophilus φSfi18 | 35 (165) | AAF63083 | ||||||
| ORF157 | S. thermophilus φSfi11 | 35 (165) | AAF63054 | ||||||
| ORF157 | S. thermophilus φSfi19 | 35 (165) | AAF63083 | ||||||
| ORF157 | S. thermophilus φSfi21 | 35 (165) | AAC72433 | ||||||
| ORF8 | S. thermophilus O1205 | 33 (165) | AAC79524 | ||||||
| 46 | 234 | 26.5 | 32,966 | 33,670 | gaaaguuuGAGGuuuagauaaug | ORF9 | S. thermophilus O1205 | 61 (230) | AAC79525 |
| ORF233 | S. thermophilus φSfi21 | 61 (230) | AAC72434 | ||||||
| ORF233 | S. thermophilus φSfi19 | 60 (230) | AAC97920 | ||||||
| ORF233 | S. thermophilus φSfi11 | 60 (230) | AAC63055 | ||||||
| ORF32 | S. thermophilus DT1 | 59 (230) | AAD21908 | ||||||
| ORF240 | L. casei A2 | 56 (232) | CAB63668 | ||||||
| 47 | 69 | 7.5 | 33,231 | 33,021 | ccaaguuaucguaaucaccuaug | ||||
| 48 | 70′ | 19.4 | 33,670 | 34,191 | aaauuugAAGGAGaaaaauaaug | SSB protein | B. subtilis | 28 (103) | P37455 |
| ORFE13 | L. lactis bIL170 | 31 (77) | AAC27225 | ||||||
| ORF34 | L. lactis sk1 | 30 (76) | AAB70074 | ||||||
| 49 | 31 | 34,325 | 35,134 | uauagaAGAAAGGAGaaaguuug | ORF269 | L. lactis φ31.1 | 82 (269) | AAF43120 | |
| 50 | 8.4 | 35,115 | 35,336 | agaaguGGAGGcuuuaauaaaug | ORF73 | L. lactis φ31.1 | 97 (73) | AAF43121 | |
| ORF73 | L. lactis ul36.1 | 97 (73) | AAF74098 | ||||||
| 51 | 16.2 | 35,314 | 35,715 | agcaaccgaauuaaugauauaug | ORF14 | L. lactis r1t | 96 (130) | AAB18689 | |
| ORF129 | L. lactis ul36 | 97 (129) | AAF74082 | ||||||
| ORF19 | L. lactis Tuc2009 | 96 (129) | AAD37104 | ||||||
| 52 | 9.4 | 35,719 | 35,958 | aaauuacaGAGGuauaaaccaug | ORF20 | L. lactis Tuc2009 | 99 (79) | AAD37105 | |
| ORF16 | L. lactis r1t | 92 (79) | AAB18691 | ||||||
| ORF79a | L. lactis ul36 | 91 (79) | AAF74083 | ||||||
| ORF79b | L. lactis ul36.1 | 43 (46) | AAF74100 | ||||||
| ORF79 | L. lactis φ31.1 | 43 (46) | AAF43123 | ||||||
| 53 | 13 | 36,069 | 36,416 | cuaaaauauGAGGuaguaauaug | ORF139b (dUPTase) | L. lactis φ31.1 | 82 (92) | AAF43128 | |
| ORF139a (dUPTase) | L. lactis ul36 | 82 (92) | AAF74088 | ||||||
| ORF139b (dUPTase) | L. lactis ul36.1 | 82 (92) | AAF74104 | ||||||
| ORF3 (dUPTase) | L. lactis S114 | 80 (92) | CAA72644 | ||||||
| ORF20 (dUPTase) | L. lactis r1t | 80 (92) | AAB18695 | ||||||
| 54 | 11.1 | 36,413 | 36,712 | ggcucaacuggGGAGGugugaug | ORF120 | L. lactis φ31.1 | 39 (103) | AAF43131 | |
| ORF120a | L. lactis ul36 | 39 (103) | AAF7409 | ||||||
| 55 | 12.9 | 37,084 | 36,746 | aaaGAGGAAuggauauuuacaug | |||||
| 56 | 12.7 | 37,207 | 37,530 | ucaaugAAaGAGGUucaggaaug | ORF29 | L. lactis sk1 | 94 (107) | AAB70069 | |
| ORFE22 | L. lactis bIL170 | 93 (107) | AAF009630 | ||||||
| 57 | 9.3 | 37,604 | 37,834 | gacugguggGGAGGgauugaaug | |||||
| 58 | 6.7 | 37,831 | 38,004 | uuugauAAGGAGGacaaccaaug | ORF66 | L. lactis φ31.1 | 55 (60) | AAF43134 | |
| 59 | 6.9 | 38,001 | 38,180 | aacguguucggGGAGGaaugaug | |||||
| 60 | 5.5 | 38,444 | 38,587 | uaaacucaacuGGAGGaaaaaug | |||||
| 61 | 15.5 | 38,664 | 39,065 | uacucGGAGGgacuuuuuaauug | 16.9-kDa protein | L. lactis S114 | 35 (81) | CAA72647 | |
| 62 | 4.8 | 39,288 | 39,416 | caaguaguGAGugaaaguccuug | |||||
| 63 | 20.6 | 39,434 | 39,949 | ugauGAAAGGAGaugcuaucuug | ORF170 | L. gasseri φadh | 46 (162) | CAB52516 | |
| ORF170 | S. thermophilus φSfi19 | 44 (154) | AAD44054 | ||||||
| ORF20 | S. thermophilus φ7201 | 44 (154) | AAF43513 | ||||||
| ORF175 | S. thermophilus φSfi21 | 41 (155) | AAD41027 | ||||||
| ORF46 | S. thermophilus DT1 | 42 (154) | AAD21922 |
Boyce et al. (8) designation for previously identified ORFs.
The stop position includes the stop codon.
The sequence shown includes the putative start codon and the immediate upstream 20 nucleotides. The putative RBS is shown in uppercase letters.
The protein sequences in the databases which show homology with the respective BK5-T ORFs are shown. Empty cells indicate that no significant homologies were observed; experimentally determined functions are indicated after a dash.
The deduced amino acid sequences of all the ORFs were compared with protein sequences in a nonredundant peptide sequence database encompassing GenPept, TREMBL, SWISSPROT, and PIR and using the TFASTA, FASTA, or BLASTP comparison programs (2). Significant homologies are shown in Table 2 and are discussed below.
(i) ORF1 and ORF2.
The predicted size of the gene products encoded by orf1 and orf2 and the location of these genes immediately downstream from the cos site are indicative of the terminase subunits of other phages, including coliphage λ (4), Streptococcus thermophilus phages φ7201, DT1, φSfi19, and φSfi21 (16), and L. lactis phage sk1 (10). Terminase subunits form a hetero-oligomeric complex which functions in concert to bind and nick DNA at the cos site prior to packaging of the DNA concatamers into phage heads. Terminase activity is an ATP-dependent process, and an A-type Walker nucleoside triphosphate-binding motif (62) was identified between residues 49 and 53 in the putative BK5-T large terminase subunit ORF2. No such motif was detected in ORF1. In addition to homology with streptococcal phages (Table 2), BK5-T ORF1 exhibited homology to putative packaging protein GP3 found in three Salmonella enterica serovar Typhimurium bacteriophages belonging to the Podoviridae family. Although the homology between the two proteins is low (23% over 163 amino acids), it suggests an evolutionary link between two quite distantly related phage species.
(ii) ORF5.
The amino acid sequence of ORF5 showed 56 to 57% identity to four uncharacterized streptococcal phage proteins (16, 39, 58), 24% identity to ORF4 of Staphylococcus aureus φPVL (26), and 21% identity with GP34 of Streptomyces actinophage phage φC31 (54). The last two proteins showed sequence homology to the experimentally determined portal protein of coliphage HK97 (19). Portal proteins create a multisubunit ring structure that serves as an entry point for the translocation of DNA into the phage head (3).
(iii) ORF6.
ORF6 shares homology with phage proteins of unknown function from S. thermophilus, Lactobacillus gasseri, and Pseudomonas aeruginosa (Table 2) and exhibits homology to a series of endopeptidase ClpP proteins encoded in the genome of E. coli, plants, and higher eukaryotes, but not to the ClpP protein from L. lactis (22). The highly conserved Ser85 and His108 and surrounding residues, which form the proteolytic cleavage site in ClpP protein sequences, were conserved in both BK5-T ORF6 and the L. lactis ClpP protein.
(iv) ORF7.
The deduced amino acid sequence of ORF7 exhibited significant homology to major structural proteins from four S. thermophilus phages, Bacillus subtilis phage φ105 (ORF27), Lactobacillus casei A2, P. aeruginosa D3, and S. aureus phage φPVL (ORF7) (Table 2). The BK5-T orf7 encodes a major structural protein (see below).
(v) ORF12.
The predicted amino acid sequence of ORF12 showed approximately 45% identity with ORFs of unknown function from S. thermophilus phages φSfi19 (ORF203) and φSfi21 (ORF202) (15) and with an experimentally determined small major structural protein from S. thermophilus phage φ7201 (34). The gene product of BK5-T orf12 is a major structural protein (see below).
(vi) ORF25, ORF26, and ORF27.
The topological positions of orf25, orf26, and orf27 are similar to the arrangement of the holin and lysin cassette observed in prophage-like elements found in the chromosome of many Bacillus strains (29) and in bacteriophages infecting S. thermophilus (15, 40, 52). The predicted amino acid sequence of ORF25 demonstrates 28% identity to the Clostridium acetobutylicum ORF2 of unknown function, which is found upstream from the lyc gene, whose gene product exhibits sequence homology to a number of autolytic lysozymes (13). The amino acid sequence of ORF26 shows homology to the putative holin proteins from S. aureus phage φPVL (26), B. subtilis phage SPP1, and experimentally determined holin proteins from Bacillus licheniformis (30, 44) (Table 2). The BK5-T ORF25 and ORF26 are therefore likely to encode a two-component holin system similar to that suggested for phages infecting S. thermophilus (15, 40, 52). Holin proteins function to disrupt the cell membrane to allow the lysin protein access to the cell wall (67).
The amino acid sequence of ORF27 showed 99% homology to the putative lysin of L. lactis φ31, 73% homology to the N-acetylmuramoyl-l-alanine amidase (Pal) of pneumococcal phage Dp-1 (51), and 30% identity to putative lysin proteins of S. thermophilus phages (Table 2), suggesting that ORF27 encodes the BK5-T lysin.
(vii) ORF32.
Boyce et al. (9) showed that the gene product encoded by orf32 exhibited significant homology to a number of lactococcal temperate phage integrase proteins and that it was essential for the establishment and/or maintenance of lysogeny in BK5-T.
(viii) ORF35.
Boyce et al. (7) identified a helix-turn-helix DNA binding motif (amino acid positions 33 to 54) as predicted by the Dodd and Egan algorithm (18) and a putative RecA Ala-Gly cleavage site in ORF35. ORF35 showed homology to two putative cI repressor proteins from the lactococcal phages Tuc2009 and φLC3 and to an experimentally determined cI homologue from phage rlt (Table 2), suggesting that orf35 encoded the BK5-T cI repressor homologue. It was predicted that ORF35 facilitated lysogenic development by repressing a putative BK5-T promoter, P2, found in the putative BK5-T immunity region (7).
(ix) ORF36.
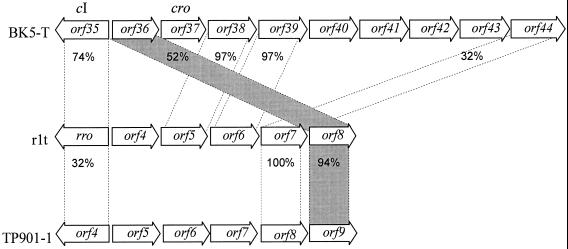
orf36 was previously proposed to encode the BK5-T Cro protein homologue, based on its size and position relative to the putative BK5-T immunity region (7). However, no helix-turn-helix DNA binding motif was identified in ORF36, and preliminary data (Mahanivong, unpublished data) suggested that ORF36 did not bind to the putative BK5-T immunity region. ORF36 shares strong identity (93% over 55 amino acids) with lactococcal phage TPW22 ORF9 (47) and 52% identity over 54 amino acids with the uncharacterized ORF8 from lactococcal phage rlt (60), ORF57 from φ31.1 (20), and ORF9 from TP901-1. It is interesting that the relative position of BK5-T ORF36 is dissimilar to the homologues from rlt and TP901-1 (Fig. 2), suggesting a horizontal introduction of the ORF into the genome or a genetic rearrangement event.
FIG. 2.
Comparison of the gene location near the immunity region of L. lactis phages. The relative locations of the ORFs in BK5-T (7), rlt (43), and TP901-1 (25) are indicated. Horizontal arrows indicate individual ORFs, with the ORF name shown within the arrows and the direction of the arrowhead indicating its orientation. The global amino acid percentage of identity between ORFs is shown between the broken lines. The horizontal hatched area illustrates the discordant genetic arrangement of BK5-T orf36 with the rlt and TP901-1 homologues orf8 and orf9, respectively.
(x) ORF37.
orf37 is located 231 bp downstream of orf36. Analysis of the predicted amino acid sequence of ORF37 identified a putative helix-turn-helix DNA binding motif at amino acid positions 23 to 42. The predicted amino acid sequence of ORF37 demonstrates 30% identity to the Cro homologue from temperate streptococcal phage TPJ34 (ORF67) and ORF39 from lytic streptococcal phage DT1 (Table 2). Preliminary DNA binding studies (Mahanivong, unpublished data) suggest that ORF37 binds to the putative BK5-T immunity region and that it is likely to be the BK5-T Cro homologue.
(xi) ORF38.
The identification of a putative helix-turn-helix DNA binding motif (18) at amino acid positions 174 to 193 suggests that orf38 encodes a DNA binding protein. The predicted amino acid sequence of ORF38 shows 97% identity to ORF5 of L. lactis phage rlt (60) and both genes are located in the same relative position immediately downstream of their Cro protein homologues (Fig. 2). BK5-T ORF38 also shows 50% identity to L. casei phage A2 ORFA, which is located immediately downstream of its cI homologue (31).
(xii) ORF48.
The predicted amino acid sequence of ORF48 exhibits limited homology with the putative single-stranded DNA binding (SSB) proteins encoded by B. subtilis, S. aureus phage φPVL, and B. subtilis phage SPP1 and with ORFs of unknown function from L. lactis phages sk1 and bIL170 (Table 2). SSB proteins are a ubiquitous class of proteins identified in prokaryotic and eukaryotic organisms. They function primarily to bind single-stranded DNA and play important roles in DNA replication, recombination, and repair. A number of SSB proteins have been characterized and some functionally relevant structural features have been identified. There are aromatic amino acid residues in the N terminus of E. coli and mitochondrial SSB proteins (36) that are important for binding single-stranded DNA, but these residues are not observed in BK5-T ORF48. The greatest region of homology between BK5-T ORF48 and the SSB protein sequences occurs at the acidic C-terminal end of the proteins. The C-terminal six amino acids (DEDLPF) of BK5-T ORF48 were compared with protein databases and showed matches to other putative SSB proteins of bacterial (B. subtilis, E. coli, L. lactis, and Thermus aquaticus) or phage (including Tuc2009, φadh, SPP1, and φ105) origin (data not shown). The functional relevance of this acidic domain is unknown; however, the loss of this domain from the E. coli SSB protein (14) results in a nonfunctional protein.
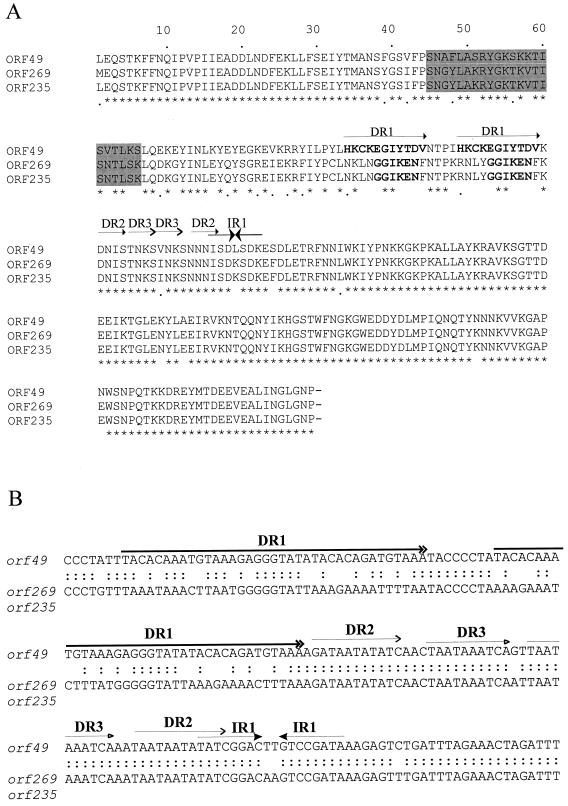
(xiii) ORF49.
ORF49 contains a helix-turn-helix region (Fig. 3A), suggesting that it is a DNA binding protein, and its nucleotide sequence contains a series of direct and indirect repeats, which are often indicative of a phage origin of replication (10, 21). The amino acid sequence of BK5-T ORF49 shows 82% identity to ORF269 in the recombinant lytic lactococcal phage φ31.1 (20) and 80% identity to ORF235 of lactococcal phage ul36.1 (6) (Table 2). Mutant phage φ31.1 was isolated after φ31 infection of cells expressing a phage resistance phenotype (Per) due to a plasmid-encoded φ31 ori. Phage φ31.1 overcame the resistance by acquiring 7.8 kbp of DNA from the host chromosome, which contained an alternative phage ori. The ori was postulated to be contained within ORF269. Mutant phage ul36.1 arose as a variant resistant to the phage resistance mechanism AbiK and had incorporated noncontiguous sections of the host chromosome DNA, one of which contained ORF235, also postulated to contain a phage ori.
FIG. 3.
(A) Comparison of the ORF49, ORF269, and ORF235 amino acid sequences from L. lactis phages BK5-T, φ31.1, and ul36.1, respectively. The asterisks indicate identical amino acid residues and the periods indicate residues conserved across all three sequences. Amino acid residues within the putative helix-turn-helix DNA binding motif and the longest direct repeat sequence are indicated by the shaded regions and in bold, respectively. The positions of the four repeat sequences (and their designations) found in all three ORFs are indicated by the horizontal lines with matching arrowheads. (B) The nucleotide sequences of the repeat sequences (DR1 to DR3 and IR1) in BK5-T orf49 are shown in the top strand. The sequence below represents the homologous region from orf235 and orf269 from L. lactis phages ul36.1 and φ31.1, respectively, which are identical. The colons indicate identical nucleotides between BK5-T orf49 and the other L. lactis phage sequences. The nucleotide positions of the four repeat sequences (DR1 to DR3 and IR1) in BK5-T are indicated by the corresponding arrows underneath the repeat label.
(xiv) ORF53.
The deduced amino acid sequence of ORF53 demonstrates significant homology with ORF20 and ORF139 of L. lactis phages rlt (60) and φ31.1 (20), respectively. Furthermore, it shows homology to dUTPase enzymes of bacterial and eukaryotic origin. The function of this enzyme, which hydrolyzes dUTP to dUMP and pyrophosphate, is to regulate intracellular levels of dUTP and to prevent incorporation of dUTP into DNA (53). The absence of dUTPase in E. coli leads to an increased recombination and mutation rate during DNA replication (59).
(xv) ORF55.
ORF55 did not show any significant homology with other protein sequences in the databases and its function is unknown. Interestingly, orf55 is located in the opposite orientation to the surrounding ORFs and is preceded by a putative RBS (AGAGGAA) and promoter (−10 [TATAAT] and −35 [TTCAAT]) located 11 and 25 nucleotides upstream from the ATG start codon, respectively. The codon usage of ORF55 is similar to that of the rest of the phage. A region of dyad symmetry is located 9 bp downstream of the stop codon of orf55 but is surrounded by a string of A nucleotides rather than the T nucleotides indicative of a rho-independent transcription terminator. The location and orientation of this ORF have not been reported in the genomes of other characterized lactococcal temperate phages (26, 27, 60). Located within ORF55, but oriented in the opposite direction, is another putative promoter (−10 [TATAAT] and −35 [ATGTTC]), which could direct transcription through orf56 and downstream genes. The arrangement of oppositely oriented putative promoters within and upstream of orf55 would generate transcripts with overlapping and complementary 5′ ends. Similar overlapping transcripts are observed in the λ oop region (28), and this region may represent a control point in the BK5-T gene expression.
Determination of the sequence of the BK5-T cos termini.
BK5-T had previously been demonstrated to contain cohesive termini (8). Sequence runoff experiments were conducted on purified phage DNA to determine the DNA sequence of cosN, which defines the single-stranded overhang. COS1 and COS2 were used as primers to determine the sequence of ligated and unligated cos ends. The nucleotide sequences obtained from unligated phage DNA and ligated DNA were compared, and the absence of a 12-bp sequence in the unligated preparations indicated that BK5-T contained a 3′ overhang of 12 bp with the sequence 5′-CACACACATAGG-3′. Thus, the BK5-T cos site, like those of all other phages infecting gram-positive organisms studied to date, possesses a single-stranded 3′ overhang (11, 26, 35, 60).
N-terminal sequence of phage structural proteins.
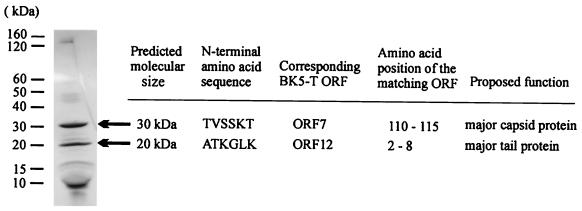
BK5-T phage was purified by CsCl density gradient centrifugation, and the proteins were analyzed by SDS-PAGE. Two major bands, with molecular masses of 20 and 30 kDa, and several bands of lower molecular mass were observed (Fig. 4). The proteins were transferred to a polyvinylidene difluoride membrane and the N-terminal sequence of the 20- and 30-kDa proteins was determined. The first six amino acids of the 30-kDa protein were TVSSKT. This sequence was identical to residues 110 to 115 of the BK5-T ORF7. The absence of the first 109 amino acids suggests that ORF7 is cleaved prior to the formation of the mature protein. The estimated molecular mass of the full length ORF7 is 45 kDa, whereas a 32-kDa protein is predicted for the mature ORF7 protein, which is consistent with observed experimental results (Fig. 4).
FIG. 4.
SDS-PAGE analysis of the BK5-T structural proteins. The N-terminal amino acid sequence of the marked proteins is indicated, together with the position of the N-terminal sequence within the corresponding BK5-T ORFs and their proposed functions. The size and location of the molecular mass markers used (Benchmark ladder; Life Technologies Inc., Rockville, Md.) are given in the left margin.
N-terminal sequence analysis of the 20-kDa BK5-T structural protein revealed the sequence ATKGLKM, which is identical to amino acids 2 to 8 of BK5-T ORF12. It appears that the N-terminal methionine residue was removed in the mature protein. The amino acid sequence of ORF12 showed homology to major structural proteins from a number of S. thermophilus phages, a leuconostoc phage protein, and the experimentally determined major tail protein from L. gasseri φadh (Table 2).
Identification of the putative BK5-T origin of replication.
Comparison of the genome organization of BK5-T with that of other lactococcal bacteriophages (41) suggested that the BK5-T ori was likely to be located in orf49. ORF49 contains a helix-turn-helix DNA binding motif (amino acid positions 47 to 66) (Fig. 3A) and may bind to the various repeat sequences within orf49 as has been observed in coliphage λ (48) and phage Tuc2009 (41). The genetic arrangement of the BK5-T orf48 and orf49, encoding a putative SSB protein and a replication protein, respectively, is similar to that of phage Tuc2009 (41), but there is no sequence similarity between the analogous proteins.
It has been shown previously that cloned lactococcal phage ori can maintain plasmid replication (63), and similar experiments were conducted to analyze the BK5-T putative ori. A BK5-T-derived 2.7-kbp PstI restriction fragment containing orf46 to orf51 was ligated into pJDC9 and the plasmid (pCM25) was cloned into E. coli. pCM25 does not contain a gram-positive replicon and therefore is unable to replicate in L. lactis unless the cloned fragment contains a functional ori. All attempts to introduce pCM25 into L. lactis were unsuccessful. This suggested that either the fragment did not contain sufficient elements to sustain autonomous replication or expression of phage-encoded proteins was necessary for DNA replication.
Attempts to introduce the same PstI fragment downstream from the constitutive L. lactis promoter P32 in a modified pJDC9 plasmid were unsuccessful in both E. coli and L. lactis.
Consequently, an alternative approach was used to investigate the BK5-T putative origin of replication. It has been previously shown that when a phage ori is introduced to host cells in trans, it confers a resistance phenotype to the host strain (21, 45). It is hypothesized that the cloned ori acts as a false target for phage replication and causes a decrease in phage DNA replication. This can be monitored by a reduction in both plaque numbers and quantity of phage genomic DNA (21, 41). A 921-bp PCR product containing BK5-T orf49 was cloned into the high-copy-number vector pTRKH2 to form pCM29, and cells containing this plasmid showed increased phage resistance as evidenced by reduced plaque size and number (efficiency of plating [EOP] = 1 × 10−5). Control strains containing pTRKH2 with no insert or an insert of BK5-T DNA from the structural region were sensitive to phage infection (EOP = 1).
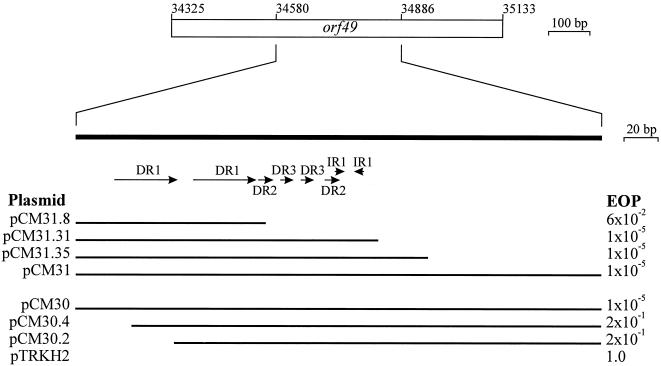
A number of direct and inverted repeat sequences were identified within orf49 (Fig. 3B) and these sequences are likely to define the BK5-T ori. A 306-bp PCR product containing this region was cloned into pTRKH2 in both orientations (plasmids pCM30 and pCM31) and shown to confer a similar level of phage sensitivity to the clone containing the complete orf49 sequence (Fig. 5). Exonuclease III deletion constructs were generated to further define the region required to confer resistance to BK5-T infection. Removal of the first 10 bp of the 5′ end of the DR1 sequence in clone pCM30.4 caused a 4-log decrease in phage resistance, indicating that the DR1 sequence was necessary to effect phage resistance.
FIG. 5.
Delineation of the putative BK5-T origin of replication. The thick solid bar represents the internal orf49 sequence generated by PCR that contained the putative ori. Horizontal arrows shown underneath the bar indicate the positions of the four repeat sequences (DR1 to DR3 and IR1). The plasmid designations of the clones containing deletion fragments are listed on the left and the EOP values (averaged from at least four different experiments) are given on the right. The horizontal thin lines indicate the DNA fragments cloned in the plasmid constructs. The plasmids were introduced into lactococcal host strain H2 (Table 1).
Similarly, deletions from the right-hand end (Fig. 5) indicated that the smaller direct and/or inverted repeat sequences were also important, as removal of these sequences (pCM31.8) caused a 3-log decrease in phage resistance.
DISCUSSION
The nucleotide sequence of the temperate lactococcal bacteriophage BK5-T was completely determined and enabled comparative genomic sequence analysis. Molecular characterization of the phage structural proteins, cos termini, and replication functions were conducted.
The BK5-T 12-nucleotide single-stranded cosN sequence was determined. In numerous other phages, the region surrounding cosN contains recognition sequences for the binding of terminase (cosB), integration host factor, and other host- or phage-encoded proteins. In phage λ, there are three direct repeat sequences in cosB, referred to as R sites, which have been shown to be important for terminase binding (4). Similar repeat sequences were identified in phage sk1 (11) and P. aeruginosa phage D3 (50). Although no such repeat sequences were found in the BK5-T genome, it does contain the sequence C3TC5 located 20 nucleotides 5′ of cosN and a string of 7 consecutive G nucleotides occurs 43 nucleotides 3′ of cosN (Fig. 6). S. thermophilus phage φ7201 contains the sequence C5GC5 16 bases 3′ of cosN, and φSfi19 and φSfi21 both contain the sequence C5GC4 21 nucleotides 5′ of cosN (39). L. lactis phage sk1 also contains a string of 8 consecutive C nucleotides found 30 bases 5′ of cosN (11). The occurrence of such sequences in these low-GC organisms is unusual and these sequences may constitute recognition sites for terminase binding and activity, although this remains to be determined. A 130-bp region surrounding BK5-T cosN contains a number of runs of four to seven identical bases, primarily A or T (Fig. 6). Similarly, the cos site of coliphage λ contains runs of adenines and thymines that are important to DNA bending which occurs upon integration host factor binding (66).
FIG. 6.
The 130-bp intergenic cosB sequence. The cosN sequence is shown in bold. Underlined residues indicate runs of at least four identical bases. The C3TC5 and G7 sequences are indicated in bold italics.
BK5-T orf7 and orf12, which encode the putative major head and tail proteins, were identified. N-terminal amino acid sequence analysis indicated that the BK5-T putative major capsid protein was processed to generate the mature protein. Similar proteolytic cleavage was observed in the mature capsid proteins of a number of phages, including φPVL (26) and L. gasseri φadh (1). BK5-T orf5, orf6, and orf7 encode gene products that are involved with phage head structure and assembly and are likely to have been acquired as a functional unit from a common ancestral phage (54). They encode the putative portal, ClpP protease, and major capsid proteins, respectively, and show identical arrangement to three functionally equivalent genes found in four phages infecting P. aeruginosa (50), S. aureus (26), S. actinophage (54), and E. coli (19). It is tempting to speculate that the putative BK5-T ClpP protease (ORF6) may be involved in the cleavage of ORF7, as observed in coliphage HK97, where the phage-encoded GP4 is a protease that cleaves the N-terminal 102 amino acids from the major capsid protein encoded by the adjacent downstream gene (19).
Investigations of the BK5-T replication functions identified a repeat-rich region within orf49 which conferred phage resistance in a manner suggestive of a phage replication origin. However, the specific function of the repeat sequences in the replication process remains to be determined. ORF49 showed significant homology with ORF269 and ORF235 from lactococcal phages φ31.1 (20) and ul36.1 (6). Cloned DNA fragments containing these latter ORFs also conferred resistance to their respective phages (6). These ORF homologues are likely to encode replication proteins which bind to the repeat regions in their coding sequences to initiate phage DNA replication in a similar manner to the coliphage λ O replication protein (56). The greatest region of sequence diversity between BK5-T and its homologues occurs at amino acid positions 40 to 120 (Fig. 3A). This region contains the putative helix-turn-helix DNA binding motif (amino acid positions 45 to 66) and DR1, a possible binding site on the DNA. Interestingly, the nucleotide sequences of the other repeat structures downstream from DR1 were identical (Fig. 3B). The variation in amino acid sequence in the helix-turn-helix motif could indicate different DNA targets for the homologues, as represented by the differences in nucleotide sequence in the region of DR1.
The organization and orientation of the ORFs in BK5-T were similar to those observed in other temperate phages infecting lactic acid bacteria (1, 27, 39, 55, 60). The majority of the ORFs are oriented in one direction, while ORFs involved in lysogeny (ORFs 32, 33, and 35) and possibly regulation (ORF43 and ORF55) are oriented in the opposite direction. Moreover, organization of functional modules within BK5-T revealed a striking correlation with the pattern of functional modules observed in the genomes of many Siphoviridae phages (26, 38, 39, 58, 61), viz. packaging, structure and morphogenesis, lysis, integration, lysogeny (in temperate phages), and replication. A detailed comparison of the genetic organization of BK5-T compared with other phages is presented elsewhere (17).
Examination of the BK5-T genome allowed comparative analyses of genomic exchange at three levels: functional modules, individual genes, and gene segments. The strong conservation of the genetic organization, ORF sequence, and gene sequence of the packaging and morphogenesis modules (ORF63 to ORF18) observed between BK5-T and the streptococcal phages (15, 17) suggests recent divergence of these modules from a common ancestor. In contrast, ORF30 to ORF61 showed greater identity with phages infecting lactococci. This latter region encompasses the integration, lysogeny, and replication modules. This structure suggests that BK5-T is a recently evolved chimeric phage containing modules that are derived from two distinct ancestral phages. Within modules, differences were also observed in the relative location of homologous ORFs. The BK5-T orf36 was located upstream of the putative BK5-T orf37 cro homologue (Fig. 2). In contrast, the ORF36 homologues from rlt and TP901-1 were both located four ORFs downstream from their corresponding cro gene homologues. Also the BK5-T orf63 was located 5′ of the cos site, whereas the homologous ORFs from the skI and bIL170 were located 3′ of their respective large terminase subunits.
The acquisition or deletion of entire ORFs was also evident. The BK5-T ORF14 homologue was absent in the streptococcal phages, indicating that it was either introduced into the BK5-T genome via a nonhomologous event or lost from the streptococcal phage genomes since their divergence. The BK5-T orf56 is surrounded by a 30-bp direct repeat sequence, which could facilitate its acquisition or deletion by a specific recombination event. ORF56 showed significant homology to ORFs from lactococcal phages sk1 and bIL170 (Table 2); however, no repeat sequences were found surrounding the ORFs in these phages. A single copy of the 30-bp direct repeat in rlt may be the remnant of an ORF deletion by recombination occurring between the repeat sequences. Examples of ORF deletions within flanking direct repeat sequences were also observed between phage bIL67 and c2 (37).
Evolution within ORF sequences was evident, as indicated by the conservation of functional domains linked to regions that show no apparent homology. Comparison of the amino acid sequence of the BK5-T cI homologue ORF35 indicates that the C-terminal domain had greater than 94% identity with the homologous proteins from lactococcal phages Tuc2009 and rlt. In contrast, there was negligible homology in the N-terminal domain of these proteins. The N-terminal domain contains the putative DNA binding motif and this divergence presumably accommodates phage-specific DNA recognition sequences.
As more sequence data on a variety of bacteriophage genomes becomes available, there is much focus on the genomic evolution and relationships between phages. These data suggest that phages have evolved by exchange of functional modules, individual genes, or gene segments by various genetic recombination events (5, 38).
ACKNOWLEDGMENTS
We acknowledge Sean Moore for his technical assistance and Scott Chandry for his helpful advice and discussions.
C.M. gratefully acknowledges receipt of a Melbourne Research Scholarship.
Footnotes
This report is dedicated to the memory of Barrie E. Davidson, who passed away in July 2000.
REFERENCES
- 1.Altermann E, Klein J R, Henrich B. Primary structure and features of the genome of the Lactobacillus gasseritemperate bacteriophage Φadh. Gene. 1999;236:333–346. doi: 10.1016/s0378-1119(99)00236-x. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Millier W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Bazinet C, King J. The DNA translocating vertex of dsDNA bacteriophage. Annu Rev Microbiol. 1985;39:109–129. doi: 10.1146/annurev.mi.39.100185.000545. [DOI] [PubMed] [Google Scholar]
- 4.Becker A, Murialdo H. Bacteriophage lambda DNA: the beginning of the end. J Bacteriol. 1990;172:2819–2824. doi: 10.1128/jb.172.6.2819-2824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botstein D. A theory of modular evolution for bacteriophages. Ann N Y Acad Sci. 1980;354:484–491. doi: 10.1111/j.1749-6632.1980.tb27987.x. [DOI] [PubMed] [Google Scholar]
- 6.Bouchard J D, Moineau S. Homologous recombination between a lactococcal bacteriophage and the chromosome of its host strain. Virology. 2000;270:65–75. doi: 10.1006/viro.2000.0226. [DOI] [PubMed] [Google Scholar]
- 7.Boyce J D, Davidson B E, Hillier A J. Identification of prophage genes expressed in lysogens of the Lactococcus lactistemperate bacteriophage BK5-T. Appl Environ Microbiol. 1995;61:4099–4104. doi: 10.1128/aem.61.11.4099-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyce J D, Davidson B E, Hillier A J. Sequence analysis of the Lactococcus lactistemperate bacteriophage BK5-T and demonstration that the phage DNA has cohesive ends. Appl Environ Microbiol. 1995;61:4089–4098. doi: 10.1128/aem.61.11.4089-4098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyce J D, Davidson B E, Hillier A J. Spontaneous deletion mutants of the Lactococcus lactis temperate bacteriophage BK5-T and localization of the BK5-T attPsite. Appl Environ Microbiol. 1995;61:4105–4109. doi: 10.1128/aem.61.11.4105-4109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandry P S, Moore S C, Boyce J D, Davidson B E, Hillier A J. Analysis of the DNA sequence, gene expression, origin of replication and modular structure of the Lactococcus lactislytic bacteriophage sk1. Mol Microbiol. 1997;26:49–64. doi: 10.1046/j.1365-2958.1997.5491926.x. [DOI] [PubMed] [Google Scholar]
- 11.Chandry P S, Moore S C, Davidson B E, Hillier A J. Analysis of the cos region of the Lactococcus lactisbacteriophage sk1. Gene. 1994;138:123–126. doi: 10.1016/0378-1119(94)90793-5. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Morrison D A. Construction and properties of a new insertion vector, pJDC9, that is protected by transcriptional terminators and useful for cloning of DNA from Streptococcus pneumoniae. Gene. 1988;64:155–164. doi: 10.1016/0378-1119(88)90489-1. [DOI] [PubMed] [Google Scholar]
- 13.Croux C, García J L. Sequence of the lyc gene encoding the autolytic lysozyme of Clostridium acetobutylicumATCC824: comparison with other lytic enzymes. Gene. 1991;104:25–31. doi: 10.1016/0378-1119(91)90460-s. [DOI] [PubMed] [Google Scholar]
- 14.Curth U, Benschel J, Urbanke C, Greipel J. In vitro and in vivo function of the C-terminus of Escherichia colisingle-stranded DNA binding protein. Nucleic Acids Res. 1996;24:2706–2711. doi: 10.1093/nar/24.14.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desiere F, Lucchini S, Brüssow H. Evolution of Streptococcus thermophilusbacteriophage genomes by modular exchanges followed by point mutations and small deletions and insertions. Virology. 1998;241:345–356. doi: 10.1006/viro.1997.8959. [DOI] [PubMed] [Google Scholar]
- 16.Desiere F, Lucchini S, Brüssow H. Comparative sequence analysis of the DNA packaging, head and tail morphogenesis modules in the temperate cos-site Streptococcus thermophilusbacteriophage Sfi21. Virology. 1999;260:244–253. doi: 10.1006/viro.1999.9830. [DOI] [PubMed] [Google Scholar]
- 17.Desiere F, Mahanivong C, Hillier A J, Chandry P S, Davidson B E, Brussow H. Comparative genomics of lactococcal phages: insight from the complete genome sequence of Lactococcus lactisphage BK5-T. Virology. 2001;283:240–252. doi: 10.1006/viro.2001.0857. [DOI] [PubMed] [Google Scholar]
- 18.Dodd I B, Egan J B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990;18:5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duda R L, Martincic K, Hendrix R W. Genetic basis of bacteriophage HK97 prohead assembly. J Mol Biol. 1995;247:636–647. doi: 10.1006/jmbi.1994.0169. [DOI] [PubMed] [Google Scholar]
- 20.Durrnaz E, Klaenhammer T R. Genetic analysis of chromosomal regions of Lactococcus lactisacquired by recombinant lytic phages. Appl Environ Microbiol. 2000;66:895–903. doi: 10.1128/aem.66.3.895-903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foley S, Lucchini S, Zwahlen M-C, Brussow H. A short noncoding viral DNA element showing characteristics of a replication origin confers bacteriophage resistance to Streptococcus thermophilus. Virology. 1998;250:377–387. doi: 10.1006/viro.1998.9387. [DOI] [PubMed] [Google Scholar]
- 22.Frees D, Ingmer H. ClpP participates in the degradation of misfolded protein in Lactococcus lactis. Mol Microbiol. 1999;31:79–87. doi: 10.1046/j.1365-2958.1999.01149.x. [DOI] [PubMed] [Google Scholar]
- 23.Huggins A R, Sandine W E. Incidence and properties of temperate bacteriophages induced from lactic streptococci. Appl Environ Microbiol. 1977;33:184–191. doi: 10.1128/aem.33.1.184-191.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvis A W. DNA-DNA homology between lactic streptococci and their temperate and lytic phages. Appl Environ Microbiol. 1984;47:1031–1038. doi: 10.1128/aem.47.5.1031-1038.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnsen M G, Appel K F, Madsen P L, Vogensen F K, Hammer K, Arnau J. A genomic region of lactococcal temperate bacteriophage TP901–1 encoding major virion proteins. Virology. 1996;218:306–315. doi: 10.1006/viro.1996.0199. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko J, Kimura T, Narita S, Tomita T, Kamio Y. Complete nucleotide sequence and molecular characterization of the temperate staphylococcal bacteriophage φPVL carrying Panton-Valentine leukocidin genes. Gene. 1998;215:57–67. doi: 10.1016/s0378-1119(98)00278-9. [DOI] [PubMed] [Google Scholar]
- 27.Kodaira K-I, Oki M, Kakikawa M, Watanabe N, Hirakawa M, Yamada K, Taketo A. Genome structure of the Lactobacillustemperate phage Φgle: the whole genome sequence and the putative promoter/repressor system. Gene. 1997;187:45–53. doi: 10.1016/s0378-1119(96)00687-7. [DOI] [PubMed] [Google Scholar]
- 28.Krinke L, Mahoney M, Wulff D L. The role of the OOP antisense RNA in coliphage lambda development. Mol Microbiol. 1991;5:1265–1272. doi: 10.1111/j.1365-2958.1991.tb01900.x. [DOI] [PubMed] [Google Scholar]
- 29.Krogh S, O'Reilly M, Nolan N, Kevin D M. The phage-like element PBSX and part of the skin element, which are resident at different locations on the Bacillus subtilischromosome, are highly homologous. Microbiology. 1996;142:2031–2040. doi: 10.1099/13500872-142-8-2031. [DOI] [PubMed] [Google Scholar]
- 30.Kyogoku K, Sekiguchi J. Cloning and sequencing of a new holin-encoding gene of Bacillus licheniformis. Gene. 1996;168:61–65. doi: 10.1016/0378-1119(95)00690-7. [DOI] [PubMed] [Google Scholar]
- 31.Ladero V, García P, Bascaran V, Herrero M, Alvarez M A, Suarez J E. Identification of the repressor-encoding gene of the Lactobacillusbacteriophage A2. J Bacteriol. 1998;180:3474–3476. doi: 10.1128/jb.180.13.3474-3476.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lakshmidevi G, Davidson B E, Hillier A J. Circular permutation of the genome of a temperate bacteriophage from Streptococcus cremorisBK5. Appl Environ Microbiol. 1988;54:1039–1045. doi: 10.1128/aem.54.4.1039-1045.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lakshmidevi G, Davidson B E, Hillier A J. Molecular characterization of promoters of the Lactococcus lactis subsp. cremoristemperate bacteriophage BK5-T and identification of a phage gene implicated in the regulation of promoter activity. Appl Environ Microbiol. 1990;56:934–942. doi: 10.1128/aem.56.4.934-942.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Marrec C, van Sinderen D, Walsh L, Stanley E, Vlegels E, Moineau S, Heinze P, Fitzgerald G, Fayard B. Two groups of bacteriophages infecting Streptococcus thermophiluscan be distinguished on the basis of mode of packaging and genetic determinants for major structural proteins. Appl Environ Microbiol. 1997;63:3246–3253. doi: 10.1128/aem.63.8.3246-3253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lillehaug D, Lindqvist B H, Birkland N K. Characterization of φLC3, a Lactococcus lactis subsp. cremoristemperate bacteriophage with cohesive single-stranded DNA ends. Appl Environ Microbiol. 1993;57:3206–3211. doi: 10.1128/aem.57.11.3206-3211.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lohman T M, Ferrari M E. Escherichia colisingle-stranded DNA-binding protein: multiple DNA-binding modes and cooperativities. Annu Rev Biochem. 1994;63:527–570. doi: 10.1146/annurev.bi.63.070194.002523. [DOI] [PubMed] [Google Scholar]
- 37.Lubbers M W, Waterfield N R, Beresford T P J, LePage R W F, Jarvis A W. Sequencing and analysis of the prolate-headed lactococcal bacteriophage c2 genome and identification of the structural genes. Appl Environ Microbiol. 1995;61:4348–4356. doi: 10.1128/aem.61.12.4348-4356.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucchini S, Desiere F, Brüssow H. Comparative genomics of Streptococcus thermophilusphage species supports a modular evolution theory. J Virol. 1999;73:8647–8656. doi: 10.1128/jvi.73.10.8647-8656.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucchini S, Desiere F, Brüssow H. The genetic relationship between virulent and temperate Streptococcus thermophilus bacteriophages: whole genome comparison of cos-site phages Sfi19 and Sfi21. Virology. 1999;260:232–243. doi: 10.1006/viro.1999.9814. [DOI] [PubMed] [Google Scholar]
- 40.Lucchini S, Desiere F, Brüssow H. The structural gene module in Streptococcus thermophilusbacteriophage φSfi11 shows a hierarchy of relatedness to Siphoviridae from a wide range of bacterial hosts. Virology. 1998;246:63–73. doi: 10.1006/viro.1998.9190. [DOI] [PubMed] [Google Scholar]
- 41.McGrath S, Seegers J F M L, Fitzgerald G, van Sinderen D. Molecular characterization of a phage-encoded resistance system in Lactococcus lactis. Appl Environ Microbiol. 1999;65:1891–1899. doi: 10.1128/aem.65.5.1891-1899.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 43.Nauta A, van Sinderen D, Karsens H, Smit E, Venema G, Kok J. Inducible gene expression mediated by a repressor-operator system isolated from Lactococcus lactisbacteriophage rlt. Mol Microbiol. 1996;19:1331–1341. doi: 10.1111/j.1365-2958.1996.tb02477.x. [DOI] [PubMed] [Google Scholar]
- 44.Oda Y, Nakayama R, Kuroda A, Sekiguchi J. Molecular cloning, sequence analysis and characterization of a new cell wall hydrolase, CwIL, of Bacillus licheniformis. Mol Gen Genet. 1993;241:380–388. doi: 10.1007/BF00284691. [DOI] [PubMed] [Google Scholar]
- 45.O'Sullivan D J, Hill C, Klaenhammer T R. Effect of increasing the copy number of bacteriophage origins of replication, in trans, on incoming-phage proliferation. Appl Environ Microbiol. 1993;59:2449–2456. doi: 10.1128/aem.59.8.2449-2456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Sullivan D J, Klaenhammer T R. High- and low-copy-number Lactococcusshuttle cloning vectors with features for clone screening. Gene. 1993;137:227–231. doi: 10.1016/0378-1119(93)90011-q. [DOI] [PubMed] [Google Scholar]
- 47.Petersen A, Josephsen J, Johnsen M G. TPW22, a lactococcal temperate phage with a site-specific integrase closely related to Streptococcus thermophilusphage integrases. J Bacteriol. 1999;181:7034–7042. doi: 10.1128/jb.181.22.7034-7042.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts J D, McMacken R. The bacteriophage lambda O replication protein: isolation and characterization of the amplified initiator. Nucleic Acids Res. 1983;11:7435–7452. [PMC free article] [PubMed] [Google Scholar]
- 49.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 50.Sharp R, Jansons I S, Gertman E, Kropinski A M. Genetic and sequence analysis of the cos region of the temperate Pseudomonas aeruginosabacteriophage, D3. Gene. 1996;177:47–53. doi: 10.1016/0378-1119(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 51.Sheehan M M, García J L, Lopez R, García P. The lytic enzyme of the pneumococcal phage Dp-1: a chimeric lysin of intergeneric origin. Mol Microbiol. 1997;25:717–725. doi: 10.1046/j.1365-2958.1997.5101880.x. [DOI] [PubMed] [Google Scholar]
- 52.Sheehan M M, Stanley E, Fitzgerald G F, van Sinderen D. Identification and characterization of a lysis module present in a large proportion of bacteriophages infecting Streptococcus thermophilus. Appl Environ Microbiol. 1999;65:569–577. doi: 10.1128/aem.65.2.569-577.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shlomai J, Kornberg A. Deoxyuridine triphosphatase of Escherichia coli. J Biol Chem. 1978;253:3305–3312. [PubMed] [Google Scholar]
- 54.Smith M C M, Burns R N, Wilson S E, Gregory M A. The complete genome sequence of the Streptomycestemperate phage ΦC31: evolutionary relationships to other viruses. Nucleic Acids Res. 1999;27:2145–2155. doi: 10.1093/nar/27.10.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stanley E, Fitzgerald G F, Le Marrec C, Fayard B, van Sinderen D. Sequence analysis and characterization of φO1205, a temperate bacteriophage infecting Streptococcus thermophilusCNRZ1205. Microbiology. 1997;143:3417–3429. doi: 10.1099/00221287-143-11-3417. [DOI] [PubMed] [Google Scholar]
- 56.Taylor K, Wegrzyn G. Replication of coliphage lambda DNA. FEMS Microbiol Rev. 1995;17:109–119. doi: 10.1111/j.1574-6976.1995.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 57.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tremblay D M, Moineau S. Complete genomic sequence of the lytic bacteriophage DT1 of Streptococcus thermophilus. Virology. 1999;255:63–76. doi: 10.1006/viro.1998.9525. [DOI] [PubMed] [Google Scholar]
- 59.Tye B-K, Chien J, Lehman I R, Duncan B K, Warner J R. Uracil incorporation: a source of pulse-labeled DNA fragments in the replication of the Escherichia colichromosome. Proc Natl Acad Sci USA. 1978;75:233–237. doi: 10.1073/pnas.75.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Sinderen D, Karsens H, Kok J, Terpstra P, Ruiters M H J, Venema G, Nauta A. Sequence analysis and molecular characterization of the temperate lactococcal bacteriophage rlt. Mol Microbiol. 1996;19:1343–1355. doi: 10.1111/j.1365-2958.1996.tb02478.x. [DOI] [PubMed] [Google Scholar]
- 61.Venema G, Kok J, van Sinderen D. From DNA sequence to application: possibilities and complications. Antonie Leeuwenhoek. 1999;76:3–23. [PubMed] [Google Scholar]
- 62.Walker J E, Saraste M, Runswick J, Gay N J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waterfield N R, Lubbers M W, Polzin K M, Le Page R W F, Jarvis A W. An origin of DNA replication from Lactococcus lactisbacteriophage c2. Appl Environ Microbiol. 1996;62:1452–1453. doi: 10.1128/aem.62.4.1452-1453.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitehead H R, Cox G A. The occurrence of bacteriophage in cultures of lactic streptococci. N Z J Dairy Sci Technol. 1935;16:319–320. [Google Scholar]
- 65.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 66.Yeo A, Kosturka L D, Feiss M. Structure of the bacteriophage lambda cohesive end site: bent DNA on both sides of the site, cosN, at which terminase introduces nicks during chromosome maturation. Virology. 1990;174:329–334. doi: 10.1016/0042-6822(90)90085-6. [DOI] [PubMed] [Google Scholar]
- 67.Young R. Bacteriophage lysis: mechanism and regulation. Microbiol Rev. 1992;56:430–481. doi: 10.1128/mr.56.3.430-481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]