Abstract
Objectives
Dementia at a young age differs from late onset dementia in pathology and care needs. This requires further research to improve the understanding of this group, support and service provision. Aim of current study is to reach consensus on the terminology and operational definition (i.e., age‐related criteria and possible causes) of dementia at a young age, to aid further research.
Methods
A classical Delphi technique was used to transform opinions into group consensus by using an online survey. In three rounds statements regarding (1) terminology, (2) age‐related criteria, and (3) aetiologies that can be considered as causes of dementia at a young age were sent to international experts in the field to give their opinions and additional comments on the statements.
Results
Forty‐four experts responded and full consensus was reached on 22 out of 35 statements. Young‐onset dementia emerged as the term of preference. Provisional consensus was found for the use of age 65 at symptom onset as preferred cut‐off age. Consensus was reached on the inclusion of 15 out of 22 aetiologies and categories of aetiologies as potential cause for dementia at a young age.
Conclusions
A clear term and operational definition have been reached. Although beneficial for conducting future research to gain more insight in pathology and care needs of young people living with dementia, still consensus about some details is lacking. To reach consensus about these details and implications for use in research and clinical practice, the organisation of an in person consensus meeting is advised.
Keywords: Delphi, operational definition, terminology, young‐onset dementia
Key points
Young‐onset dementia is the term of preference, when the first symptoms of dementia occur before the age of 65 years.
Using the age of symptom onset as cut‐off age criterion to distinguish from late‐onset dementia is important because of the existing prolonged time to diagnosis.
Consensus was reached to include the most frequently diagnosed aetiologies as potential cause of dementia at a young age.
To meet the need for different operational definitions, due to a variation in aims between research and clinical practise, the organisation of an in‐person consensus meeting is recommended.
1. INTRODUCTION
Despite the estimated prevalence of dementia in the ages 30–64 of 119/100,000 worldwide 1 and the growing interest in research considering people who develop dementia at a younger age, there is little agreement on the terminology and definition of this particular group. A clear definition is important, because people living with dementia at a young age are considered a different group due to significant differences in disease characteristics, disease mechanisms, course, and care needs compared to dementia in old age. First, in a recent literature review concerning the operational definition of dementia at a young age, a broad range of different aetiologies was identified, 2 ranging from Alzheimer's disease and frontotemporal dementia to rare aetiologies such as metabolic disorders and prion disease.3, 4 In this review various aetiologies were included as potential cause for dementia at a young age by some authors, but mentioned as exclusion criteria by others. 2 Second, differences exist in the clinical presentation, as for instance non amnestic presentations of Alzheimer's disease are more prevalent in younger individuals and include logopenic variant primary progressive aphasia, posterior cortical atrophy, frontal or behavioural/executive variants, and various parietal syndromes.5, 6 Third, there is evidence suggesting that underlying differences in pathology cause differences in the course and progress of the disease compared to dementia in old age.7, 8 Also, care needs differ partially because of the abovementioned differences and young people living active lives have various roles to fulfil in society such as being spouse, parent, and earn an income. 9 A study investigating the costs related to dementia at a young age revealed these almost doubled compared to late onset dementia. 10 Often these young people do not receive the required care timely as health care professionals struggle to recognise early symptoms of dementia at a young age. Inappropriate initial diagnoses and treatments are common, resulting in a delayed diagnosis of 4.4–4.7 years on average.11, 12 Given the differences in causes of dementia as well as the consequences of dementia at a young age a better understanding of this young group is necessary. This will aid to improve the current variations in both diagnostic and post‐diagnostic care and support, causing diverse experiences for young people living with dementia and their care takers. 13
To be able to compare and conduct research, a clear understanding of this younger group is needed, yet currently absent. In our integrative review we found that various terms, cut‐off ages and criteria are used to define dementia at a young age. Furthermore, many aetiologies that might cause dementia at a young age were found. 2 Therefore, the aim of this study was to reach consensus on the terminology, age related criteria for cut‐off, and aetiologies that might be considered as causes of dementia at a young age.
2. METHODOLOGY
2.1. Delphi study
This consensus study was part of the Prevalence REcognition and Care pathways in young Onset DEmentia (PRECODE) project on the prevalence, incidence, definition, and care pathways of dementia at a young age. The study was conducted between October 2018 and March 2019. A classical Delphi technique was used, which is an iterative multi‐stage process aimed to transform opinion into group consensus on an important topic.14, 15 Input for the surveys was generated by performing an integrative review. 2 The study was conducted in three survey rounds.
2.2. Recruitment
International experts in the field of dementia at a young age were recruited in three ways, by addressing principal investigators of research groups publishing on this topic, through networks such as Early detection and timely INTERvention in DEMentia and the Shared Interest Forum of the International Psychogeriatric Association, and through other networks in which PRECODE study group members were involved. Both researchers as well as health care professionals in the field of dementia at a young age were recruited, to ensure both clinical and scientific perspectives were captured. The experts were invited by email covering the scope, relevance, aim and method of the study.
2.3. Ethical considerations
The study protocol was submitted to the Medical Ethics Committee region Arnhem/Nijmegen (file number 2019‐5092) and appraised by the committee as not falling within the remit of the Medical Research Involving Human Subjects act. The study was organised in line with the General Data Protection Regulation and Good Clinical Practice quality standard. The individual responses of respondents were encoded, guaranteeing anonymity.
2.4. Procedure
Based on an integrative review, exploring all operational definitions used to define dementia at a young age, a total of 35 statements were formulated to cover the three main topics of interest, that is, terminology (five statements), age‐related criteria (8), and aetiologies (22). 2 These statements were verified and revised by members of the PRECODE project group. All statements were checked for clarity and grammar by an English native speaker, with extensive experience in the field of dementia at a young age. Aetiologies were clustered in overarching categories based on the review, 2 and checked by an experienced neurologist not involved in the project (Table 1). An invitation letter and the survey were composed with members of the project group and sent to respondents using LimeSurvey (https://www.limesurvey.com). The experts were asked to give their opinion on each statement on a 5‐point Likert scale, ranging from ‘strongly disagree’ up to ‘strongly agree’. Furthermore, the experts were asked to comment on their opinions, to explain their answers and clarify when needed. Per round, respondents were given 4 weeks to complete the survey. Two reminders were sent to all experts per round to motivate them to participate; one after 2 weeks and one a half week before the deadline. After each round, descriptive statistics were calculated for each statement and compared to a set of consensus rules (Table 2) to establish whether full consensus was reached. Statements for which no consensus was reached were included in the next round. In the next round, experts were given insight into the group response as well as their own scores from the previous round and were asked to reconsider their original response based on the response of the group. Any additional comments given by respondents in the survey were used to gain a better understanding of their underlying considerations. The comments were also used to decide whether to add new statements that reflected shared insights among respondents. New rounds were added until no further consensus compared to the round before was reached.
TABLE 1.
Categories of clustered aetiologies
| Primary neurodegenerative dementias |
| Alzheimer's disease, frontotemporal dementia, dementia with Lewy bodies, Parkinson's disease dementia, progressive supranuclear palsy, corticobasal degeneration, multiple system atrophy, Huntington's disease, pantothenate kinase‐associated degeneration or neurodegeneration with iron accumulation (PKAN, NBIA or Hallervorden‐Spatz syndrome), aceruloplasminemia, neuroacanthocytosis, familial encephalopathy with neuroserpin inclusion bodies (FENIB), neuronal intermediate filament inclusion disease (NIFID), Fahr's syndrome, hereditary haemochromatosis, spinocerebellar ataxia, dentatorubral‐pallidoluysian atrophy (DRPLA), Lafora body disease, Mohr‐Tranebjaerg syndrome, myotonic dystrophy type 3, dentatorubral‐pallidoluysian atrophy, neuroferritinopathy, giant axonal neuropathy (GAN), progressive myoclonic epilepsy syndromes (PME), Perry syndrome. |
| Cerebrovascular dementias |
| Vascular dementia, CADASIL, multi‐infarct dementia, strategic infarct dementia, vascular cognitive impairment, cerebral amyloid angiopathy (CAA; including: familial British dementia, familial Danish dementia, Dutch variant of hereditary cerebral haemorrhage with amyloidosis, hereditary cerebral haemorrhage with amyloidosis of Icelandic type, meningovascular amyloidosis, familial amyloidosis of Finnish type), Sneddon's syndrome, Binswanger's syndrome, antiphospholipid syndrome, Susac syndrome. |
| Inflammatory diseases |
| Multiple sclerosis, chronic meningitis, paraneoplastic syndromes (e.g., limbic encephalitis), neuro‐Behçet, systemic lupus erythematosus (SLE), hepatic encephalopathy, primary central nervous system (CNS) angiitis, systemic vasculitides, Hashimoto's encephalopathy, nonvasculitic autoimmune inflammatory meningoencephalitis, celiac disease, pancreatic encephalopathy, antibasal ganglia antibodies (ABGA), autoimmune connective tissue disorders, Sjögren syndrome. |
| Infectious diseases |
| Human immunodeficiency virus (HIV), transmissible spongiform encephalopathy (TSE, e.g., Creutzfeldt‐Jakob disease. Gerstmann‐Sträussler‐Scheinker syndrome), neurosyphilis (e.g., general paresis of the insane, luetic cerebrovascular disease), herpes simplex encephalitis, Lyme disease, Whipple's disease, subacute sclerosing panencephalitis (SSPE), neurocysticercosis, progressive multi‐focal leukoencephalopathy. |
| Toxic/metabolic diseases |
| Alcohol‐related dementias (including Wernicke syndrome, Korsakoff syndrome, pellagra, vitamin B12/thiamine deficiency), vitamin E deficiency, drug‐related dementias (e.g., hashish, barbiturates, lithium), heavy metal poisoning (e.g., lead, Mercury, arsenic), uraemia, renal failure and dialysis dementia, obstructive sleep apnoea‐hypnoea syndrome, Wilson's disease, porphyria, electrolyte abnormalities, Bismuth toxicity, homocystinuria, superficial siderosis, mucopolysaccharidosis III, ornithine transcobalamine deficiency, ceftazidime toxicity, abetalipoproteinemia, galactosialidosis, mannosidosis, phenylketonuria (PKU), hereditary spastic paraparesis (HSP), Lesch‐Nyhan syndrome. |
| Mitochondrial disorders |
| Mitochondrial myopathy encephalopathy lactic acidosis and stroke (MELAS), Myoclonic epilepsy with ragged‐red fibres (MERRF), Kearns‐Sayre syndrome. |
| Lysosomal storage disorders |
| Tay‐Sachs disease, Gaucher's disease type 2 and 3, Niemann‐Pick disease type C, Fabry's disease, Kuf's disease (neuronal ceroid lipofuscinosis), adult GM2 gangliosidosis, alpha mannosidosis. |
| Leukodystrophies |
| (X‐linked) adrenoleukodystrophy, metachromatic leukodystrophy, Alexander's disease, leukoencephalopathy with vanishing white matter, Pelizaeus‐Merzbacher disease, adult polyglucosan body disease, cerebrotendineous xanthomatosis, pigmentary orthochromatic leukodystrophy (POLD), hereditary diffuse leukoencephalopathy with spheroids (HDLS), Krabbe disease, polycystic lipomembranous leukodystrophy with sclerosing leukoencephalopathy (PLOSL or Nasu‐Hakola disease). |
| Structural disorders |
| Cerebral tumours and abscess, traumatic brain injury, normal pressure hydrocephalus, subdural haematoma, sequelae of cerebral laceration, dural arteriovenous fistula, brain metastatic disease, primary CNS lymphoma, intravascular lymphoma, lymphomatoid granulomatosis, gliomatosis cerebri, malignant melanoma, chronic traumatic encephalopathy. |
| Reversible disorders |
| Epileptic dementias, iatrogenic disorders. |
| Endocrine disorders |
| Diabetes mellitus, thyroid disease, parathyroid disease, hypothyroidism, adrenal disease, nonketotic hyperosmolar hyperglycaemia, Cushing disease, Addison disease, pseudohypoparathyroidism. |
| Other (e.g. developmental disorders, psychiatric disorders) |
| Down's syndrome, depression. |
Note: Reprinted from Journal of Alzheimer's Disease, Vol. 83, Van de Veen et al., An Integrative Literature Review on the Nomenclature and Definition of Dementia at a Young Age, 1909–1910, Copyright (2021), with permission from IOS Press. The publication is available at IOS Press trough http://dx.doi.org/10.3233/JAD‐210458.
TABLE 2.
Consensus rules
| Level of agreement | Median | Interquartile range | Agree or disagree (score 4/5 or 1/2) | Consensus |
|---|---|---|---|---|
| Very high | 5 | 0 | ≥80% | Full consensus in favour |
| High | 5 | ≤1 | ≥80% | Full consensus in favour |
| Moderate | 4–5 | ≤2 | ≥60% | No consensus |
| None | 2–4 | >1 | No consensus | |
| High (against) | 1 | ≤1 | ≥80% | Full consensus against |
| Very high (against) | 1 | 0 | ≥80% | Full consensus against |
2.5. Analyses
The results were collected and analysed using SPSS version 25 for Windows (Chicago, IL). Whether or not full consensus was reached, was determined by the level of agreement between participants. Three statistics were calculated, the specific ranges of the median and interquartile range, and the percentage to which the experts disagreed (scored 1 or 2) or agreed (scored 4 or 5; Table 2). These consensus rules were defined based on Delphi research guidelines. 16 Although no common guidelines exist to determine an appropriate level of consensus in Delphi studies, a minimum of 80% of agreement between participants is often used in similar studies in the field. 17
3. RESULTS
3.1. General results
Of the 86 invited experts, approximately half (51%) completed the online survey (Table 3), and 86% of these contributed until the end of the Delphi procedure. Respondents were from 17 different countries, representing four continents, that is, Europe, North America, Asia, and Oceania. The group of experts had an average experience of 18 years in the field of dementia at a young age and consisted of a variety of health care professionals and researchers. Approximately half of all experts (55%) combined their work as a researcher with clinical care. Most common profession of the experts was neurologist (39%).
TABLE 3.
Descriptive statistics of the respondents
| Respondents | Round 1 | Round 2 | Round 3 |
|---|---|---|---|
| N = 44 (51.2%) a | N = 40 (46.5%) a | N = 38 (44.2%) a | |
| Professional background | |||
| Neurology | 17 (38.6) | 15 (37.5) | 15 (39.5) |
| Psychiatry | 7 (15.9) | 7 (17.5) | 6 (15.8) |
| Medicine | 5 (11.4) | 5 (12.5) | 5 (13.2) |
| Psychology | 5 (11.4) | 4 (10.0) | 3 (7.9) |
| Social sciences | 4 (9.1) | 3 (7.5) | 3 (7.9) |
| Other b | 4 (9.1) | 4 (10.0) | 4 (10.5) |
| Nursing | 2 (4.5) | 2 (5.0) | 2 (5.3) |
| Occupation | |||
| Health care professional and researcher | 24 (54.5) | 22 (55.0) | 21 (55.3) |
| Researcher | 12 (27.3) | 10 (25.0) | 10 (26.3) |
| Health care professional | 7 (15.9) | 7 (17.5) | 6 (15.8) |
| Other | 1 (2.3) | 1 (2.5) | 1 (2.6) |
| Country | |||
| United Kingdom | 10 (22.7) | 9 (22.5) | 9 (23.7) |
| Germany | 5 (11.4) | 4 (10.0) | 4 (10.5) |
| Belgium | 5 (11.4) | 4 (10.0) | 3 (7.9) |
| France | 5 (11.4) | 5 (12.5) | 5 (13.2) |
| Australia | 4 (9.1) | 3 (7.5) | 3 (7.9) |
| Norway | 4 (9.1) | 4 (10.0) | 4 (10.5) |
| Portugal | 2 (4.5) | 2 (5.0) | 2 (5.3) |
| Canada | 2 (4.5) | 2 (5.0) | 2 (5.3) |
| Spain | 2 (4.5) | 2 (5.0) | 2 (5.3) |
| Switzerland | 1 (2.3) | 1 (2.5) | 1 (2.6) |
| Netherlands | 1 (2.3) | 1 (2.5) | 1 (2.6) |
| Taiwan | 1 (2.3) | 1 (2.5) | 0 (0.0) |
| United States | 1 (2.3) | 1 (2.5) | 1 (2.6) |
| Sweden | 1 (2.3) | 1 (2.5) | 1 (2.6) |
| Number of years of experience with younger people with dementia (SD) | 17.3 (8.2) | 17.9 (7.9) | 18.0 (8.1) |
Abbreviation: SD, standard deviation.
Percentage of experts invited participating in each round.
Other professions mentioned by participants: researcher (2), academia and professor.
Full consensus was reached for 22 out of 35 statements after three rounds (Figure 1); for 16 statements consensus in favour and for six statements consensus against the statement was reached. In the first round, 33 statements were presented to the respondents and full consensus was reached upon 14 statements (Table 4). In round 2, full consensus was reached on another eight statements. For the third round, two statements were added, based on the comments of respondents in the previous rounds, the category ‘Other’ was broken into two separate statements on the possible inclusion of Down's syndrome and a separate statement about depression. No consensus on the remaining 11 statements and the additional two statements was reached in the final round.
FIGURE 1.
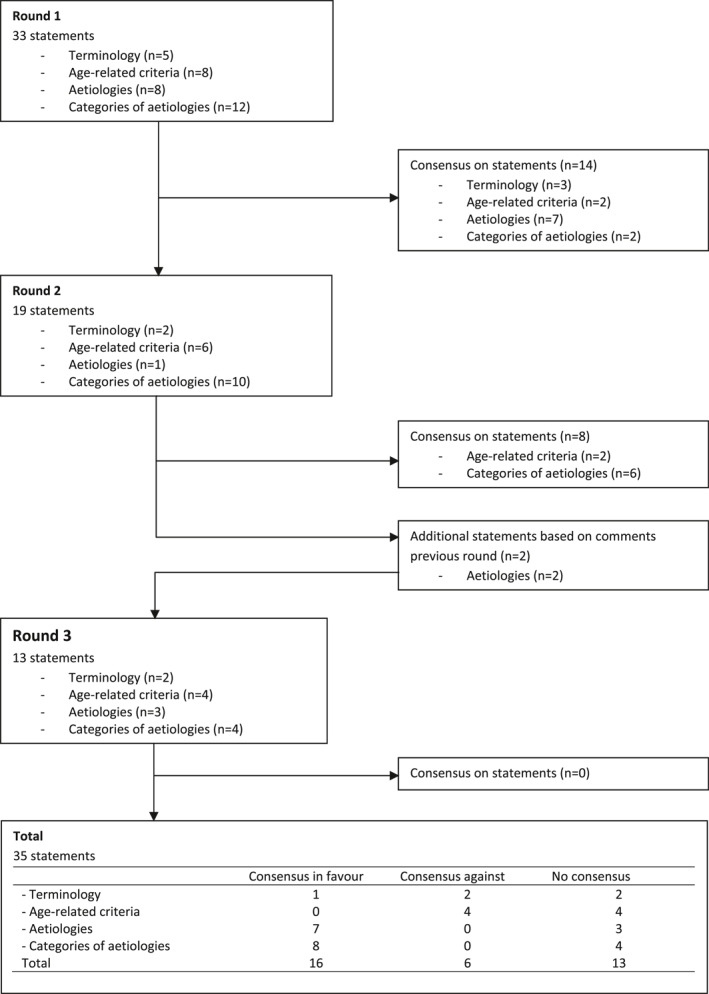
Flow chart consensus
TABLE 4.
Statements and responses in the surveys
| Statement (n, survey round) | Agreement (%) | Median | IQR a | Conclusion | ||
|---|---|---|---|---|---|---|
| Score 1 or 2 (disagree) | Score 3 | Score 4 or 5 (agree) | ||||
| Terminology | ||||||
| The term ‘Presenile dementia’ should be preferred (43, 1) | 90.7 | 4.7 | 4.7 | 1.0 | 0 | Full consensus against |
| The term ‘Young‐onset dementia’ should be preferred (44, 1) | 9.1 | 6.8 | 84.1 | 5.0 | 1 | Full consensus in favour |
| The term ‘Younger‐onset dementia’ should be preferred (37, 3) | 62.2 | 13.5 | 24.3 | 2.0 | 3 | No consensus |
| The term ‘Early‐onset dementia’ should be preferred (37, 3) | 35.1 | 10.8 | 54.1 | 4.0 | 3 | No consensus |
| The term ‘Adult‐onset dementia’ should be preferred (43, 1) | 88.4 | 4.7 | 7.0 | 1.0 | 1 | Full consensus against |
| Age | ||||||
| The age of 60 years at symptom onset should be the preferred cut‐off age (37, 3) | 83.8 | 10.8 | 5.4 | 2.0 | 1 | No consensus |
| The age of 60 years at diagnosis should be the preferred cut‐off age (40, 2) | 90.0 | 7.5 | 2.5 | 1.0 | 1 | Full consensus against |
| The age of 65 years at symptom onset should be the preferred cut‐off age (37, 3) | 16.2 | 5.4 | 78.4 | 4.0 | 1 | No consensus |
| The age of 65 years at diagnosis should be the preferred cut‐off age (36, 3) | 72.2 | 8.3 | 19.4 | 2.0 | 2 | No consensus |
| The age of 70 years at symptom onset should be the preferred cut‐off age (43, 1) | 81.4 | 9.3 | 9.3 | 1.0 | 1 | Full consensus against |
| The age of 70 years at diagnosis should be the preferred cut‐off age (42, 1) | 83.3 | 7.1 | 9.5 | 1.0 | 1 | Full consensus against |
| The age of cut‐off should be subdivided according to age range (36, 3) | 36.1 | 36.1 | 27.8 | 3.0 | 2 | No consensus |
| A lowest age limit should be used (40, 2) | 85.0 | 7.5 | 7.5 | 1.0 | 1 | Full consensus against |
| Aetiologies | ||||||
| Alzheimer's disease (44, 1) | 0.0 | 0.0 | 100 | 5.0 | 0 | Full consensus in favour |
| Frontotemporal dementia (44, 1) | 0.0 | 0.0 | 100 | 5.0 | 0 | Full consensus in favour |
| Dementia with Lewy bodies (43, 1) | 0.0 | 4.7 | 95.3 | 5.0 | 0 | Full consensus in favour |
| Parkinson's disease dementia (44, 1) | 9.1 | 6.8 | 84.1 | 5.0 | 1 | Full consensus in favour |
| Progressive supranuclear palsy (42, 1) | 0.0 | 9.5 | 90.5 | 5.0 | 0 | Full consensus in favour |
| Corticobasal degeneration (42, 1) | 0.0 | 7.1 | 92.9 | 5.0 | 0 | Full consensus in favour |
| Multiple system atrophy (38, 3) | 15.8 | 15.8 | 68.4 | 4.0 | 1 | No consensus |
| Huntington's disease (41, 1) | 9.8 | 7.3 | 82.9 | 5.0 | 1 | Full consensus in favour |
| Down's syndrome b (37,3) | 18.9 | 10.8 | 70.3 | 4.0 | 2 | No consensus |
| Depression b (37,3) | 75.7 | 5.4 | 18.9 | 1.0 | 2 | No consensus |
| Categories of aetiologies c | ||||||
| Primary neurodegenerative dementias (44, 1) | 2.3 | 2.3 | 95.5 | 5.0 | 0 | Full consensus in favour |
| Cerebrovascular dementias (44, 1) | 4.5 | 0.0 | 95.5 | 5.0 | 1 | Full consensus in favour |
| Inflammatory diseases (40, 2) | 7.5 | 10.0 | 82.5 | 5.0 | 1 | Full consensus in favour |
| Infectious diseases (40, 2) | 10.0 | 7.5 | 82.5 | 5.0 | 1 | Full consensus in favour |
| Toxic/metabolic diseases (40, 2) | 5.0 | 5.0 | 90.0 | 5.0 | 1 | Full consensus in favour |
| Mitochondrial disorders (40, 2) | 5.0 | 7.5 | 87.5 | 5.0 | 1 | Full consensus in favour |
| Lysosomal storage disorders (40, 2) | 5.0 | 10.0 | 85.0 | 5.0 | 1 | Full consensus in favour |
| Leukodystrophies (40, 2) | 5.0 | 7.5 | 87.5 | 5.0 | 1 | Full consensus in favour |
| Structural disorders (37, 3) | 8.1 | 13.5 | 78.4 | 4.0 | 1 | No consensus |
| Reversible disorders (38, 3) | 47.4 | 23.7 | 28.9 | 3.0 | 2 | No consensus |
| Endocrine disorders (38, 3) | 7.9 | 13.2 | 78.9 | 4.0 | 1 | No consensus |
| Other (e.g., developmental disorders, psychiatric disorders) (38, 3) | 18.4 | 13.2 | 68.4 | 4.0 | 1 | No consensus |
3.2. Terminology
Over 80% of the experts agreed upon using young‐onset dementia as the term of preference (Table 4). Experts also agreed that presenile dementia (90.7%) and adult‐onset dementia (88.4%) should be dismissed as terms. No consensus was reached on the terms younger‐onset dementia and early‐onset dementia, as opinions varied largely, although a tendency was found to dismiss younger‐onset dementia as a term of preference.
Regarding the term young‐onset dementia, four respondents commented that this particular term should be preferred because it ‘has gained momentum internationally’ (Box 1). Although no consensus was reached on early‐onset dementia and younger‐onset dementia several respondents expressed their concerns that these terms might cause ambiguity. Regarding the term early‐onset dementia, five experts mentioned a possible confusion with ‘early stage dementia’. According to six respondents also the term younger‐onset dementia might add ambiguity, because with this term a comparison is implied (‘younger than?’). According to two experts the term young‐onset dementia should be reserved to refer to people living with dementia aged below 45 years, while in their opinion early‐onset dementia refers to people between 45 and 65 years living with dementia.
BOX 1. Selection of additional comments.
Terminology:
‘Presenile is pejorative and old‐fashioned’.
‘Early‐onset dementia could get confused with early stage dementia’.
‘Younger is a comparative; younger than what?’
‘“Dementia at working age” or “Pre‐retirement dementia”’.
‘Young‐onset dementia and early‐onset dementia are different concepts. Young‐onset dementia begins before 45, rare causes must be considered. Early‐onset dementia starts before 65, usual neurodegenerative causes’.
‘Consensus is needed whatever the term’.
Age‐related aspects:
‘We use the age of 65 years at symptom onset based on the legal age for retirement’.
‘Below the age of 60 years ApoE‐ε4 plays less of a role’.
‘Those aged 70 and under are distinguished by having less ‘frailty’ than those who are older’.
‘Biologically it should be symptom onset (with all its caveats) rather than diagnosis age which may be very delayed’.
‘It is important to have a cut off for a diagnosis and not for symptom onset since symptoms can be caused by other illnesses than dementia’.
‘People younger than 45 years of age with dementia is a totally different group compared to those between 60 to 65 years of age’.
‘If anything, perhaps 18 to distinguish between learning disabilities and acquired dementia’.
Aetiologies:
‘The most decisive factor whether or not to name a disease as a cause of dementia is the irreversible and degenerative nature of this disease’.
‘Aetiologies should not represent nor include risk factors and co‐morbidities’.
‘We have to continue to think of dementia as including potentially reversible disorders so as not to miss treatments’.
‘The boundaries between inflammatory and infectious diseases may be too ambiguous and requires more elaboration and discussion’. And ‘Is Creutzfeldt‐Jakob an infectious disease or neurodegenerative?’
3.3. Age‐related criteria
Almost full consensus in favour was obtained for using the age of 65 at symptom onset as preferred cut‐off age (78.4%). Full consensus was reached to dismiss the age of 70 years at symptom onset and at diagnosis as preferred cut‐off age. Near full consensus between the experts existed to dismiss the age of 60 years at symptom onset and at diagnosis as preferred cut‐off age. After two rounds full consensus was reached against the use of a lower age limit in dementia at a young age to distinguish from developmental disorders (85.0%).
Regarding the age related aspects in general, seven respondents commented that the cut‐off age is often linked to retirement age and they emphasised using the psychosocial differences between young and old people living with dementia as a discriminator. Some of these experts explicitly mentioned the age of 65 years, which is the legal retirement age in most countries. Next to a specific age, a cut‐off criterion can be distinguished as well, that is, age at symptom onset or at diagnosis. Six experts emphasised using the age at symptom onset as cut‐off criterion, as this criterion approaches the biological onset of the disease. Some explicitly refer to a frequently seen delay in diagnosis in younger individuals. The use of the age at diagnosis provides an objective and precise date, according to three experts. According to three experts, an important reason for adopting the age of 60 years as cut‐off age is related to the changes in the role of the ApoE‐ε4 genotype around this age. The low prevalence rate of frailty in people under age 70 years should be a reason to use the age of 70 as the cut‐off age, according to two experts.
Finally, a total of five experts suggested to consider subdividing the group of people living with dementia at a young age into two groups; one group aged below 45 years and the second between 45 and 65 years. Two important reasons were the larger role of heredity, for example, the changes in the role of the ApoE‐ε4 genotype, and differences in aetiologies, such as a higher prevalence of metabolic disorders in the youngest group.
3.4. Aetiologies
After the first round, full consensus was reached to include seven aetiologies as possible causes of dementia at a young age, that is, Alzheimer's disease (100%), frontotemporal dementia (100%), dementia with Lewy bodies (95.3%), Parkinson's disease dementia (84.1%), progressive supranuclear palsy (90.5%), corticobasal degeneration (92.9%), and Huntington's disease (82.9%). A tendency towards the inclusion of multiple system atrophy (68.4%) was found. In round 3, no consensus was found in two newly added aetiologies, that is, Down's syndrome (70.3%), and depression (18.9%). A tendency towards including the first and excluding the latter aetiology was found, respectively.
Full consensus was reached to include the overarching category primary neurodegenerative dementias (95.5%) together with the category cerebrovascular dementias (95.5%), in round 1. In the second round, full consensus was reached to include six other categories of aetiologies, that is, inflammatory diseases (82.5%), infectious diseases (82.5%), toxic and metabolic diseases (90.0%), mitochondrial disorders (87.5%), lysosomal storage disorders (85.0%), and leukodystrophies (87.5%).
Nearly full consensus was found concerning structural (78.4%) and endocrine disorders (78.9%). Almost 70% of the experts agreed upon including the category ‘Other’ into the concept dementia at a young age. No consensus was reached in favour (28.9%) nor against (47.4%) the category reversible disorders.
In the comments, five experts emphasised using dementia defining features such as irreversibility and progressiveness as prerequisite and stated that conditions without these characteristics could not be aetiologies but should rather be seen as co‐morbidities and risk‐factors for developing dementia. Two experts added that in practise aetiologies lacking these dementia defining features could be considered an aetiology nonetheless, when that is in the interest of the patient, for example, to be able to refer to post‐diagnostic facilities. Furthermore, some experts questioned whether other types of categories should be used, allowing the possibility to include hereditary disorders. Other than Down's syndrome and depression, additional aetiologies were only suggested once—therefore not added into the survey—and included forms of schizophrenia, bipolar disorder, brain radiotherapy toxicity, fatal familial insomnia, and auto‐immune dementias such as anti‐NMDA encephalitis.
4. DISCUSSION
4.1. Main findings and reflection
This Delphi study resulted in consensus on 22 out of 35 statements, concerning the terminology and operational definition of dementia at a young age. Young‐onset dementia is the preferred term to refer to young people living with dementia. Also, the age of 65 years of first symptom onset is the preferred cut‐off age. A lower age limit to distinguish from for instance developmental disorders was unnecessary according to most participants. Consensus on what aetiologies can cause dementia at a young age was reached in seven out of 10 diseases and disorders: Alzheimer's disease, frontotemporal dementia, dementia with Lewy bodies, Parkinson's disease dementia, progressive supranuclear palsy, corticobasal degeneration, and Huntington's disease. Additional consensus in favour was reached on eight out of 10 overarching categories that can cause dementia at a young age: primary neurodegenerative dementias, cerebrovascular dementias, inflammatory diseases, infectious diseases, toxic/metabolic diseases, mitochondrial disorders, lysosomal storage disorders, and leukodystrophies. Near consensus was found to include structural, endocrine and other disorders. Especially the category reversible disorders proved to be difficult to reach consensus upon.
The results of this study warrant the use of the term young‐onset dementia. The term early‐onset dementia often refers to the dementia stage rather than the age at onset, that is early stage of dementia. Some authors suggested the use of different terms for people living with dementia aged below 45 years and those aged between 45 and 65 years. 18
Provisional consensus was reached regarding the cut‐off criteria to distinguish from late‐onset dementia by using the age of 65 years at symptom onset as cut‐off. Only provisional consensus could be reached because full consensus in favour was not reached for any of the statements concerning this topic, nevertheless a large majority of the respondents were in favour of using the age of 65 years at symptom onset. The clear cut‐off age of 65 years at symptom onset to distinguish from late‐onset dementia will aid conducting further research concerning young people living with dementia, for example, to international prevalence and incidence studies. Using the age of 65 years as cut‐off age is frequently seen in studies2, 3 and as retirement age in many countries comprehensible from a societal perspective. From a biological perspective, the use of a specific age at symptom onset is the best approach of the disease's onset. International consensus about the cut‐off age creates an opportunity for post‐diagnostic services to universally focus on creating adequate age‐appropriate post‐diagnostic services. 19 It is recommended for clinicians diagnosing young people with dementia to explicitly investigate and report the age at symptom onset.
Consensus was reached against the use of a lower age limit to distinguish dementia from developmental disorders. The youngest group, that is, below the age of 45 years, is characterised by differences in aetiologies—more reversible disorders, metabolic diseases and lysosomal storage disorders are seen—that are more often from genetic origin.18, 20, 21 Especially the so‐called late onset forms of childhood neurodegenerative conditions, such as lysosomal storage disorders, occur in young children as well. A lower age limit would split this particular group, and this may hinder research in this area.
Although in literature some authors argue for a subdivision according to age range, because below age 45 other aetiologies are more present, 22 in our study no agreement was reached whether such a subdivision would be beneficial. Main arguments given in the Delphi against such a sub‐division were that it would reduce the visibility of the group. This may be understandable from a societal perspective, because organising care for small patient groups is more difficult. However, from a research perspective, one might argue that differences in aetiologies between very young and older young people living with dementia need to be studied.
Consensus was found to include most of the aetiologies and overarching categories of aetiologies as possible causes for dementia at a young age. In fact, only for multiple system atrophy and the category reversible disorders (i.e., epileptic dementias and iatrogenic disorders) no consensus or tendency towards inclusion was observed. The respondents included various controversial aetiologies, because these are considered risk factors or co‐morbidities, for example, toxic diseases. An unequivocal understanding of the potential causes of dementia at a young age helps health care professionals recognise early symptoms of dementia and thereby might shorten the delay in diagnosis. 11 The presumption that a large heterogeneity of disorders and diseases might cause dementia at a young age3, 4 was confirmed in this Delphi study. This rather inclusive approach of many of the experts, resulting in including a large number of aetiologies, will formally increase the prevalence of dementia at a young age when local authorities adopt these criteria. On the one hand this might help to create more post‐diagnostic services—as the consequences will be larger for society including the costs 10 —on the other hand, it would cause an increase in heterogeneity. Because in many countries care and finance of care for specific patient groups are organised differently, these systems might need to change as well.
4.2. Strengths and limitations
To ensure a rigorous discussion, experts maintained anonymity throughout the whole procedure. A second strength is the global participation of experts, spread over four continents with the majority residing in Europe. Although this distribution is not in line with the global population distribution, it corresponds with the distribution of the expertise in the field of dementia at a young age. Some countries, such as the United States, were underrepresented although several experts were invited to participate. Still, the amount of 44 experts is considered appropriate as a minimum size of 10–15 experts is advocated often. 14 Furthermore, although the inclusion of a large variety of specialisms is a strength, a minority of non‐medical experts, such as social workers, could have had less expertise regarding aetiologies that might cause dementia at a young age. Although not all experts in the field participated in this Delphi study, we believe the strict consensus rules allow us to draw firm conclusions. Maximum effect was sorted with this methodology because no new consensus was reached in round 3.
A limitation of an online survey is the absence of a real face‐to‐face conversation or discussion between experts although showing the groups responses from the previous round can be seen as peer pressure. Had this been possible, perhaps consensus on additional statements would have been reached. Moreover, it would have allowed for finding a balanced definition that works for research purposes as well as for clinical practice. However, the preferred terminology, the provisional consensus regarding the criteria for age cut‐offs, and large amount of agreement to include most of the definitions found in literature indicate it is possible to reach a shared understanding of dementia at a young age.
5. CONCLUSION AND RECOMMENDATIONS
When the first symptoms of dementia occur before the age of 65 years young‐onset dementia is the term of preference. The fact that the majority of the respondents believe symptom onset should be used as cut‐off criterion, leads to a preference of using the age of 65 years at symptom onset to distinguish from late onset dementia. Agreement for a clear operational definition was reached and beneficial for research purposes. While recent research shows a prolonged time to diagnosis still exists, 11 the use of symptom onset as criterion is important for clinical practise as well. Furthermore, some details and implications for both research and clinical practice might still lack, such as the possible inclusion of several controversial aetiologies as possible cause for dementia at a young age. To define an operational definition appeared to be a complex endeavour. Various aims in research and clinical practise should be established first and could require different operational definitions. A next step to gain more insight in the need for variations in operational definitions and investigate whether further agreement can be found could be to organise an in person consensus meeting. In such a meeting the last aetiologies and categories of aetiologies could be discussed as well as the possible use of variations in cut‐off age criteria.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
The study was set up by Dennis van de Veen, Christian Bakker and Raymond Koopmans. Dennis van de Veen draughted the manuscript. Christian Bakker, Kirsten Peetoom, Yolande Pijnenburg, Janne Papma, Marjolein de Vugt and Raymond Koopmans critically revised the manuscript. Christian Bakker and Raymond Koopmans supervised the study. The PRECODE study group critically revised the manuscript for important intellectual content.
ACKNOWLEDGEMENTS
We are especially thankful for the contribution the experts made to this Delphi study. Special thanks go to Dr. Janet Carter aiding in translation of the surveys from her experience and expertise in the field of dementia at a young age; the PRECODE study group members for sharing their networks and draughting the surveys, Prof. Dr. Edo Richard for editing of the categories of aetiologies, and many thanks to Anita Oude Bos for her work as research assistant and support. Funding for this article was provided by the Gieskes‐Strijbis Fund, Alzheimer Netherlands, the Dutch Young‐Onset Dementia Knowledge Center and Florence Care Group in the Netherlands. We thank the PRECODE project team for their suggestions and collaboration.
van de Veen D, Bakker C, Peetoom K, et al. Provisional consensus on the nomenclature and operational definition of dementia at a young age, a Delphi study. Int J Geriatr Psychiatry. 2022;37(3):1‐11. 10.1002/gps.5691
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Hendriks S, Peetoom K, Bakker C, et al. Global prevalence of young‐onset dementia: a systematic review and meta‐analysis. JAMA Neurol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van de Veen D, Bakker C, Peetoom K, et al. An integrative literature review on the nomenclature and definition of dementia at a young age. J Alzheimers Dis. 2021;83(4):1891‐1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rossor MN, Fox NC, Mummery CJ, Schott JM, Warren JD. The diagnosis of young‐onset dementia. Lancet Neurol. 2010;9(8):793‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sampson EL, Warren JD, Rossor MN. Young onset dementia. Postgrad Med J. 2004;80(941):125‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mendez MF. Early‐onset Alzheimer disease and its variants. Contin (Minneap Minn). 2019;25(1):34‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koedam EL, Lauffer V, van der Vlies AE, van der Flier WM, Scheltens P, Pijnenburg YA. Early‐versus late‐onset Alzheimer's disease: more than age alone. J Alzheimers Dis. 2010;19(4):1401‐1408. [DOI] [PubMed] [Google Scholar]
- 7. Gerritsen AAJ, Bakker C, Verhey FRJ, et al. The progression of dementia and cognitive decline in a Dutch 2‐year cohort study of people with young‐onset dementia. J Alzheimers Dis. 2018;63(1):343‐351. [DOI] [PubMed] [Google Scholar]
- 8. van der Vlies AE, Koedam EL, Pijnenburg YA, Twisk JW, Scheltens P, van der Flier WM. Most rapid cognitive decline in APOE epsilon4 negative Alzheimer's disease with early onset. Psychol Med. 2009;39(11):1907‐1911. [DOI] [PubMed] [Google Scholar]
- 9. Millenaar JK, Bakker C, Koopmans RT, Verhey FR, Kurz A, de Vugt ME. The care needs and experiences with the use of services of people with young‐onset dementia and their caregivers: a systematic review. Int J Geriatr Psychiatr. 2016;31(12):1261‐1276. [DOI] [PubMed] [Google Scholar]
- 10. Kandiah N, Wang V, Lin X, et al. Cost related to dementia in the young and the impact of etiological subtype on cost. J Alzheimers Dis. 2016;49(2):277‐285. [DOI] [PubMed] [Google Scholar]
- 11. Draper B, Cations M, White F, et al. Time to diagnosis in young‐onset dementia and its determinants: the INSPIRED study. Int J Geriatr Psychiatr. 2016;31(11):1217‐1224. [DOI] [PubMed] [Google Scholar]
- 12. van Vliet D, de Vugt ME, Bakker C, et al. Time to diagnosis in young‐onset dementia as compared with late‐onset dementia. Psychol Med. 2013;43(2):423‐432. [DOI] [PubMed] [Google Scholar]
- 13. Stamou V, La Fontaine J, Gage H, et al. Services for people with young onset dementia: the ‘Angela’ project national UK survey of service use and satisfaction. Int J Geriatr Psychiatr. 2020. [DOI] [PubMed] [Google Scholar]
- 14. Keeney S, Hasson F, McKenna H. The Delphi Technique in Nursing and Health Research. Wiley‐Blackwell; 2011. [Google Scholar]
- 15. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008‐1015. [PubMed] [Google Scholar]
- 16. Keeney S, Hasson F, McKenna H. Consulting the oracle: ten lessons from using the Delphi technique in nursing research. J Adv Nurs. 2006;53(2):205‐212. [DOI] [PubMed] [Google Scholar]
- 17. van der Steen JT, Radbruch L, Hertogh CM, et al. White paper defining optimal palliative care in older people with dementia: a Delphi study and recommendations from the European Association for Palliative Care. Palliat Med. 2014;28(3):197‐209. [DOI] [PubMed] [Google Scholar]
- 18. Masellis M, Sherborn K, Neto P, et al. Early‐onset dementias: diagnostic and etiological considerations. Alzheimer's Res Ther. 2013;5:S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mayrhofer A, Mathie E, McKeown J, Bunn F, Goodman C. Age‐appropriate services for people diagnosed with young onset dementia (YOD): a systematic review. Aging Ment Health. 2018;22(8):927‐935. [DOI] [PubMed] [Google Scholar]
- 20. Ridha B, Josephs KA. Young‐onset dementia: a practical approach to diagnosis. Neurologist. 2006;12(1):2‐13. [DOI] [PubMed] [Google Scholar]
- 21. Kuruppu DK, Matthews BR. Young‐onset dementia. Semin Neurol. 2013;33(4):365‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Draper B, Withall A. Young onset dementia. Intern Med J. 2016;46(7):779‐786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


