Abstract
Close interactions between cancer cells and cancer-associated fibroblasts (CAF) have repeatedly been reported to support tumor progression. Yet, targeting CAFs has so far failed to show a real benefit in cancer treatment, as preclinical studies have shown that such a strategy can enhance tumor growth. Accordingly, recent paradigm-shifting data suggest that certain CAF subpopulations could also show tumor-inhibitory capabilities. The present review aims to provide an in-depth description of the cellular heterogeneity of the CAF compartment in tumors. Through combining information from different cancer types, here we define 4 main CAF subpopulations that might cohabitate in any tumor microenvironment (TME). In addition, a model for the evolution of CAFs during tumor development is introduced. Moreover, the presence of tumor-inhibitory CAFs in the TME as well as their molecular characteristics are extensively discussed. Finally, the potential cellular origins of these distinct CAF subpopulations are reviewed. To our knowledge, this is the first attempt at establishing a broad but comprehensive classification of CAF subpopulations. Altogether, the present manuscript aims to provide the latest developments and innovative insights that could help refine future therapeutic targeting of CAFs for cancer treatment.
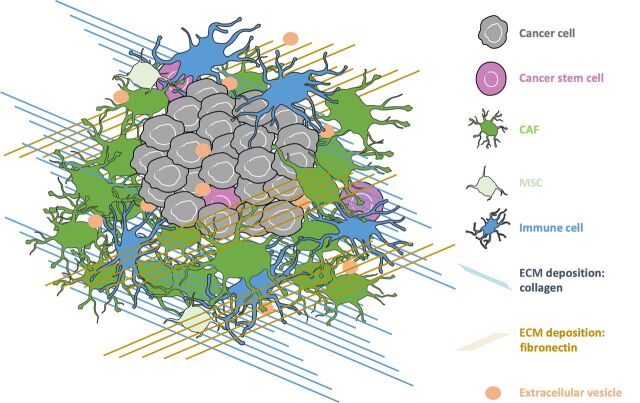
Favorable interactions between tumor cells and their surrounding tumor microenvironment (TME) are essential to cancer formation, progression and dissemination (1–4). Cancer cells are surrounded by a rich stroma, consisting of a complex meshwork of extracellular matrix (ECM) proteins, containing collagen and fibronectin among others, as well as a large number of cellular elements, such as endothelial cells, pericytes, immune cells, and cancer-associated fibroblasts (CAF; Fig. 1; refs. 2–4). Data are emerging that these stromal components, originating from myriad cell types (either resident or external to the organ system), have differing functions that are dynamic in nature.
Figure 1.
Overview of the TME. In the bulk tumor, cancer cells interact with a complex meshwork of ECM proteins, including collagen and fibronectin. Cancer cells also interact with a milieu of stromal cells, including endothelial cells, pericytes, immune cells, and CAFs. As the tumor develops, cancer cells communicate with the TME through cytokines, growth factors, and extracellular vesicles.
Figure 2.
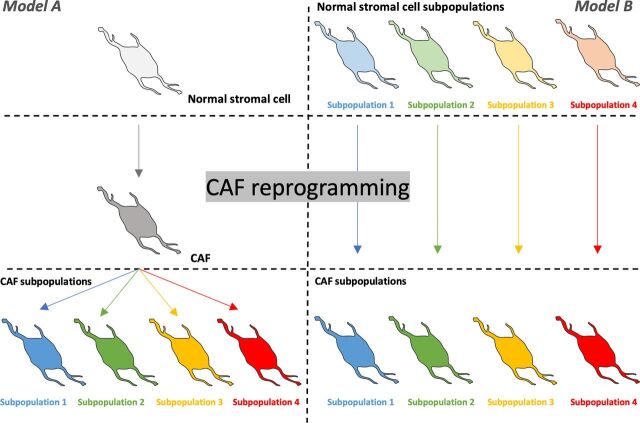
Potential cellular origins of CAFs. Multiple stromal cell types have been proposed as potential origins of CAFs, including resident-tissue normal fibroblasts, adipocytes, pericytes, MSCs, hematopoietic stem cells, epithelial cells, and endothelial cells. How different subpopulations of CAFs are formed in the growing tumor is yet to be fully understood but two main models of CAF transformation and lineage are recognized in the literature. The first of these purports that CAF subpopulations are derived from one type of normal stromal cell that undergoes CAF reprogramming followed by further differentiation that would lead to different CAF varieties and subpopulations (Model A). The second model proposes that CAF subpopulations are derived from different precursor stromal cells in the host tissue (Model B). In both models, CAFs subpopulations can further differentiate into more specialized subpopulations.
Figure 4.
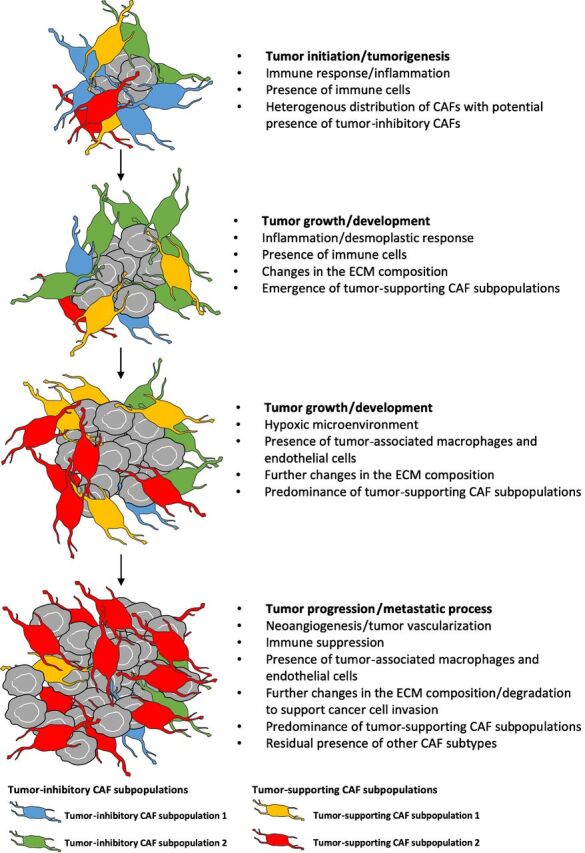
Model for the evolution of CAFs with tumor progression. CAFs are heterogenous and dynamic in nature. Tumor progression could be associated with a decrease of the tumor-inhibitory CAF/tumor-supporting CAF ratio, with highest levels seen at earlier tumor stages as a reaction to tumor emergence followed by a progressive conversion towards a tumor-supporting CAF that overtakes the CAF compartment.
In this review we discuss the heterogeneity of the CAF compartment in the TME by describing not only the well documented tumor-supporting role of CAFs but also by illuminating the existence of tumor-inhibitory CAF subpopulations (5, 6). We first summarize the cellular and molecular mechanisms underlying tumor-supporting roles of CAFs and the consequence of targeting them for cancer treatment (3). Then we introduce reports demonstrating tumor inhibitory properties of this still poorly understood subpopulation of CAFs. In addition, based on recent transcriptomics studies, we propose a broad classification of known CAF subpopulations detectable in the TME of many cancer types (7). Finally, we introduce a model for the evolution of the CAF compartment throughout tumor progression, which sheds light on the interactions of tumor-supporting CAFs with tumor inhibitory ones.
The Tumor-Supporting Roles of the CAF Compartment
Mechanisms underlying the tumor-supporting capabilities of CAFs
Normal stromal-cell reprogramming into tumor-supportive CAFs
Tumor-supportive capabilities of CAFs have been well recognized during the last two decades and has been the dogma surrounding the purpose of them in the TME (3, 8). CAFs have been shown to secrete chemokines, cytokines, growth factors, and to degrade their surrounding ECM in order to support and maintain tumor growth (3). Such tumor-supportive CAFs are known to overexpress markers such as α–smooth muscle actin (α-SMA), fibroblast activation protein (FAP) and/or platelet-derived growth factor receptor α and β (PDGF-R α and β; ref. 3). They rise from stromal cells in the TME through the direct influence of cytokines, growth factors, and other compounds secreted by tumor cells, including PDGF α/β, VEGF, stromal derived factor-1 (SDF-1), TGF-β1, or matrix metalloproteinases (MMP; ref. 9). Among the main drivers reprogramming normal stromal cells into CAFs are the molecular pathways usually associated with epithelial-to-mesenchymal transition (EMT), including the TGF-β associated signaling pathway (10). Further reports additionally showed that CAF activation might be, at least partly, redox-dependent. Indeed, evidence has repeatedly associated oxidative stress and reactive oxygen species (ROS) to CAF differentiation and tumor-supporting capabilities (11). Furthermore, constant cross-talk with the other stromal cells and cancer cells of the TME as well as autocrine signaling, involving TGF-β1 and SDF-1 pathways, help maintain the specific CAF phenotype in an integrated way (9).
CAF involvement in creating a tumor-permissive microenvironment
CAFs have been shown to directly impact tumor cell proliferation, motility, invasion, metabolism, stemness, and therapeutic resistance, which all contribute to cancer expansion. Accordingly, CAF-produced chemokines, such as SDF-1, have been reported to stimulate tumor invasiveness and metastasis through binding to specific receptors, such as C-X-C chemokine receptor type 4 (CXCR4), in various types of cancer including breast, gastric, and lung cancers (3). Tan and colleagues extensively described such communication between colorectal cancer cells and hepatic CAFs where SDF-1 secreted by CAFs activates CXCR4 in tumor cells, which subsequently stimulates the expression of TGF-β (12). It was also demonstrated that TGF-β produced by tumor cells would be directly involved in the differentiation of hepatic stellate cells into CAFs, which altogether, could promote colorectal liver metastases (12).
Other mechanisms underlying the tumor-supportive roles of CAFs include their potential to maintain tumor-cell–specific metabolism, regardless of the oxygen level in the surrounding microenvironment (13). Indeed, CAFs have been reported to help tumor-cell metabolism via transfer of intermediate metabolites such as lactate via the reverse Warburg effect, then providing them with ATP. Such metabolic coupling has recently linked lactate produced by hypoxic CAFs to enhance invasive capacity of breast cancer cells via fueling of their mitochondrial activity and activation of the TGF-β1/p38 MAPK/matrix MMP–2 and -9 signaling (14). This tight metabolic interplay between tumor cells and CAFs allows tumors to rapidly adapt and switch between glycolysis and oxidative phosphorylation, depending on oxygen levels, thus maintaining a high ATP supply that is essential to cancer cell proliferation and invasion (15). CAFs also interact closely with other stromal cells, including immune cells, to create the most tumor-permissive microenvironment to support progression and metastasis (16).
CAFs as prognostic biomarkers
Expression of specific CAF markers has been associated with poor clinical prognosis in various cancer types (3). Using in vitro coculture of primary CAFs with colorectal cancer cells, Herrera and colleagues established a gene expression ‘CAF signature’ that was able to discriminate patients into high- or low-risk groups, with high expression of ‘CAF signature’ genes linked to poor prognosis (17). In the same way, breast cancer subtype-specific gene expression profiles were described in CAFs by Tchou and colleagues, in which CAF-Integrin Alpha 3 (ITGA3) and ITGA5 were upregulated in the most aggressive HER2+ breast cancers (18). Moreover, recent extensive genomic analysis of CAFs obtained from chemosensitive versus chemoresistant breast cancer samples revealed that CAF-CXCL8, CXCL10, or CXCL11 were among genes associated with chemoresistance and poor prognosis in breast cancer (19).
Targeting CAFs for cancer treatment
Considering the important tumor-supportive capabilities of CAFs, multiple studies have shown the potential of targeting them to improve cancer treatments (9, 20–22). Specific targeting of CAFs has resulted in reduced tumor growth and reduced therapeutic resistance in several in vitro and in vivo studies (22–25). Li and colleagues demonstrated in a mouse xenograft model of colorectal cancer that targeting the CAF-activating protein FAP through the dipeptidyl peptidase inhibitor PT-100 significantly suppresses tumor growth and increases animal survival when combined with chemotherapy. In addition, authors also reported a decrease in the abundance of cancer-associated macrophages and endothelial cells in treated tumors, suggesting a central role of CAFs in organizing and integrating the TME (23). In a similar way, depletion of FAP+ CAFs resulted in improving the antitumor effect of immunotherapies in an in vivo pancreatic ductal adenocarcinoma (PDAC) model (22). Accordingly, anti-FAP vaccination has been reported to inhibit tumor growth in melanoma, breast cancer, and lymphoma mouse models (25). In another study, inhibition of the mTOR/4E-BP1 pathway was shown to selectively inhibit synthesis of proteins such as IL6 in CAFs, consecutively limiting PDAC tumor growth and chemoresistance in vivo (24). Similarly, in an elegant study by Xu and colleagues combining evidence from 3D cell culture and animal models, inhibition of CAF-derived osteopontin has been observed to prevent lung metastasis in breast cancer. Interestingly, authors also reported a correlation between osteopontin expression and tumor invasiveness in human samples, indicating a tumor-supporting role of CAFs via osteopontin in patients with breast cancer (26). In addition, as studies have indicated that CAF activation might be, at least partly, under a redox control, antioxidant treatments have been shown to inhibit CAF activation, consequently limiting their tumor-supporting roles (27). The lipid peroxidation inhibitors α-tocopherol and butylated hydroxytoluene have thus been reported to decrease the myofibroblast population in an in vitro model of skin carcinogenesis, inhibiting the expression of hepatocyte growth factor (HGF), VEGF, and IL6, then reducing cancer cell invasion (27).
Tumor Inhibitory CAFs Are Present in the TME
Based on evidence from the aforementioned reports, targeting CAFs as an antitumor strategy is, in theory, clinically promising. Nevertheless, the survival benefit of targeting CAFs as part of an anticancer treatment is yet to be proven as several recent studies have reported controversial data. For instance, talabostat, an inhibitor of FAP enzymatic activity, has showed minimal clinical activity in patients with metastatic colorectal cancer (28). By looking at the prognostic significance of CAFs in the microenvironment of PDAC, Park and colleagues revealed that low intratumoral FAP+ CAF counts significantly correlated with reduced overall survival as compared with high counts. Authors concluded that the presence of CAFs in the TME might not always be an indicator of bad prognosis, and suggested a potential tumor restraining role of the tumor stroma (29). In the same way, Özdemir and colleagues observed that depleting the myofibroblast population in PDAC led to cancer progression with reduced animal survival, via induced immunosuppression (30). Also, Rhim and colleagues surprisingly observed that reduced stromal content in a sonic hedgehog (Shh)–deficient mice model of PDAC caused tumors to be more aggressive. Authors thus suggested that at least a portion of the stromal compartment, including CAFs, can act to restrain tumor growth (31, 32).
Evidence for tumor inhibitory capabilities of CAF subpopulations
Detection of tumor-inhibitory CAFs in the TME
Preclinical studies have now provided evidence for significant, yet still controversial, tumor inhibiting effects of certain CAF subpopulations. Our recent study for instance describes how patient-derived central nervous system (CNS) metastasis-associated stromal cells (cMASC) strongly expressing known CAF markers, such as α-SMA, myosin9 (MYH9), and collagen type IV alpha 1 (COL4A1), could restrict tumor growth in vivo, hence suggesting the presence of subpopulation(s) of stromal cells/CAFs with tumor inhibitory potential in the TME of CNS metastasis (5, 33).
Recent data also suggested that Meflin-positive fibroblasts could form a specific tumor-restraining CAF subpopulation in colorectal cancer and PDAC (6, 34, 35). In CRC, conditioned medium from meflin+ CAF culture has been shown to reduce growth of cancer organoids while facilitating their differentiation in vitro (34). Detection of Meflin+ CAFs also correlated to favorable PDAC patient outcomes (6). Xenograft models of PDAC grown with immortalized human pancreatic stellate cells transduced with Meflin showed tumor regression and lower infiltration of protumoral α-SMA+ CAFs in mice when compared with controls (6). In agreement with our recent study by Tew et al., Mizutani and colleagues observed that Meflin-related tumor inhibitory effects were linked to collagen deposition, as tumors growing in Meflin-knockout (KO) mice showed straighter and wider collagen structures as compared with tumors in wild-type mice (5, 6, 28).
Tumor-inhibitory CAFs are present during early tumor development
Interestingly, tumor-supportive α-SMA+ CAFs have been reported to arise from tumor-inhibitory Meflin+ cells upon PDAC progression, suggesting that tumor-inhibitory CAFs are present at early stages of tumor development (28). Using an engineered CAF cell line expressing a suicide gene construct, iCasp9-ΔCD19, coding for an inducible caspase 9, Shen and colleagues showed the selective apoptosis of CAFs at early stages of tumor development could promote the metastatic progression of breast cancer to lungs and bone (33, 36). In addition, when eliminating CAFs at day 3 and day 10 post tumor implantation, authors observed a significant increase in the recruitment of tumor-associated macrophages to the tumor bulk, similar to data reported by Özdemir and colleagues in a mouse model of PDAC where depletion of myofibroblasts led to a decreased overall immune surveillance in tumors (30).
Altogether, it seems likely that tumor-inhibitory CAFs are present in the TME as a host defense mechanism to restrain cancer development during its early stages (5, 31, 33, 37). For the same reasons, normal fibroblasts present in the TME at the early stages of cancer growth have also been associated with tumor inhibitory properties (37–39). Nevertheless, tumor-inhibitory CAFs seem distinct from normal fibroblasts in terms of molecular background and degree of tumor inhibition even though they share common characteristics (39–41). Consequently, as for their tumor-supporting counterparts, evidence shows that interactions with tumor cells are needed for tumor-inhibitory CAFs to achieve their full inhibitory capabilities (39).
Along this line, Avagliano and colleagues recently generated a specific subpopulation of CAFs showing an intermediate proto-myofibroblast phenotype able to inhibit melanoma tumor progression. Authors observed that conditioned medium of such proto-myofibroblasts could reduce migratory capabilities of melanoma cells, while also being cytotoxic. As opposed to tumor-supporting CAFs exhibiting a phenotype close to myofibroblasts, the reported proto-myofibroblasts showed low levels of α-SMA and COX-2 expression, suggesting that such a phenotype could be an intermediate cell type in the process of fibroblast differentiation into myofibroblasts (42).
Molecular features of tumor inhibitory CAFs are context dependent
As stroma evolves along with cancer progression, it is highly possible that different tumor-inhibitory CAF subtypes are under the influence of other tumor-associated stromal cells (32, 43). Similarly, a given CAF subtype might act differently depending on the type of cancer it is associated with (44). Interestingly, we observed that a patient-derived stromal cell line, CM08, which we established from a lung adenocarcinoma to brain metastasis, could significantly inhibit the growth of a patient-derived tumor cell line CM04 in vivo, while it showed a more limited impact on the progression of a brain seeker clone derived from MDA-MB-231 cells (5). Consistent with this notion, podoplanin (PDPN) expression in CAFs has been associated with good prognosis in patients with small cell lung cancer with evidence that PDPN+ CAFs can inhibit tumor-cell proliferation. On the other hand, presence of PDPN+ CAFs has been shown to predict poor outcome in patients with lung adenocarcinoma and lung squamous cell carcinoma (SCC; ref. 45). Consistent with this complexity is the dual role of membrane type 1–MMP (MT1-MMP) in an in vitro model of breast carcinoma which acts as a tumor inhibitor when produced by normal fibroblasts but is tumor stimulating when secreted by CAFs (43). The variability of MT1-MMP substrates produced by either normal fibroblasts or CAFs could explain the observed dichotomy, meaning that the tumor-supporting capabilities of MT1-MMP are regulated by the TME context (43). Similarly, reports have indicated that both periostin (POSTN) and hyaluronic acid (HA) secreted by CAFs can contribute to either tumor-supportive or -inhibitory functions depending on their concentration (POSTN) or molecular mass (HA; refs. 46, 47).
The role of CAF-mediated desmoplasia
Tumor-supportive roles of desmoplasia
In addition to direct intercellular communication, CAFs have also been observed to affect tumor development via modification of the ECM in the TME, with overexpression and accumulation of matrix components like type I and III collagens or fibronectin, along with increased degradation of type IV collagen, overall making the TME more fibrotic. This growth of fibrous connective tissue surrounding a tumor is called desmoplasia. It is also known as the desmoplastic response or desmoplastic stroma (Fig. 1). As a ‘wound that never heals’, tumor growth recapitulates key steps of chronic inflammation such as production of proinflammatory stimuli, modulation of the immune response, neoangiogenesis, and hypoxia, in turn creating a fibrotic TME (5). In the context of cancer development, the associated desmoplastic reaction orchestrated by CAFs has mostly been reported as tumor supportive and linked with poor prognosis (48). In addition, due to its exacerbated density and stiffness, the desmoplastic ECM tends to limit blood supply, drug delivery, or immune-cell infiltration, consequently increasing hypoxia/neoangiogenesis and therapeutic resistance (48). Furthermore, related changes in the collagen composition of the ECM have been shown to support tumor cell migration, EMT, and chemoresistance, leading to poor prognosis (49). In breast cancer, Li and colleagues demonstrated that high degree of fibrosis is positively associated with cancer invasion (8). Moreover, treatments targeting CAFs have been observed to inhibit the cancer-associated desmoplastic response, consecutively limiting tumor growth (50, 51). Through adoptive transfer of FAP-targeted chimeric antigen receptor T cells, Lo and colleagues have been able to deplete FAP+ stromal cells in pancreatic and lung cancer mouse models, altering the integrity of the peritumoral ECM and vasculature and leading to a decrease in tumor growth (51).
Desmoplasia and tumor inhibition
Desmoplasia has also been reported to show tumor-inhibitory properties. Our group has reported that CNS metastasis-associated desmoplasia could impede tumor growth in vivo, most likely through abundant collagen deposition in the vicinity of the tumor, which purportedly creates a physical barrier of growth, limiting tumor progression (5). A trial with the antifibrotic drug nintedanib in non–small cell lung cancer reported clinical benefits in adenocarcinoma albeit not in SCC, even though the stroma is fibrotic in both histotypes (44). When observing that their antidesmoplastic therapeutic strategy would actually increase pancreatic tumor progression in vivo, Özdemir and colleagues suggested that desmoplasia is most likely dynamic during tumor progression, implying that its cellular and noncellular composition is impacted by the constantly evolving molecular background of the cancer cells upon tumor progression, either through direct contact or indirect interactions with the TME (30).
Recent work by Wang and colleagues on PDAC-associated desmoplasia revealed that loose tumors (i.e., weakly desmoplastic as opposed to ‘dense’ or highly desmoplastic tumors) contained a subtype of CAFs with high metabolic activity (meCAF) that was not observed in dense tumors (48). The concentration of immune cells was also reported to be higher in loose tumors as compared with dense ones. Patients with PDAC with high levels of meCAFs were then shown to have a high risk of metastasis and a poor prognosis, while being good responders to immunotherapy. Because they observed positive correlation between the presence of meCAFs and vascular invasion in patients, the authors suggested that a loose stroma containing meCAFs could support tumor invasiveness through producing metabolic intermediates for cancer and immune cells (48). In an elegant study using 3D biomaterials, Cao and colleagues showed that CAFs can adopt a tumor inhibitory phenotype when grown in a stiff/dense microenvironment whereas the same cells would become more tumor supportive in a softer matrix (52).
During the desmoplastic response associated with cancer development, both the ECM composition and CAF compartment appear to evolve in a complex interdependent way as the tumor progresses. CAFs and their desmoplastic-induced reaction might also support both a tumor-inhibitory or -supportive function and switch between different phenotypes in a context-dependent manner (5). Therefore, it is becoming clear that cancer-associated desmoplasia is fine-tuned by the dynamic nature of the TME as it evolves during tumor progression and can be either tumor promoting or tumor inhibitory (48). These observations highlight the need for improved and in-depth analyses of the different CAF subpopulations and their respective roles in the TME.
The Heterogeneity of CAFs in the TME
Acknowledging the heterogeneity and the dynamic nature of the CAF compartment, which consists of both tumor-supporting and tumor-inhibitory CAFs, Zeltz and colleagues described CAF subtypes as ‘states’ rather than fixed cell types (20). Growing evidence is also pointing to the notion that CAF subpopulations harbor unique molecular backgrounds, cellular origins, and functions (20). Since traditional CAF markers such as α-SMA are not uniformly expressed by all CAFs, there remains a need to identify additional markers that may also be able to identify CAF subpopulations and discriminate tumor-inhibitory CAFs or states from tumor promoting ones (5, 6, 53). Such an effort would require the enumeration and molecular characterization of the distinct subpopulations present in a given tumor, along with understanding the role of CAF cellular origin (5, 32).
Influence of the cellular origin on the phenotype of CAFs
Cellular origins of CAFs
Multiple cell types have been proposed as potential origins of CAFs. These include resident-tissue normal fibroblasts, adipocytes, pericytes, mesenchymal stem cells (MSC), hematopoietic stem cells, epithelial cells, and endothelial cells (Fig. 2; ref. 3). CAFs can arise from local fibroblasts or pericytes through mesenchymal-to-mesenchymal transition (MMT). In addition, bone marrow–derived MSCs recruited to the tumor site for tissue repair or epithelial cells undergoing EMT can also differentiate into CAFs (9). In our study, we reported the isolation of a population of tumor-inhibitory CAFs termed cMASCs. We reported that cMASCs expressed many MSC markers such as annexin A5 (ANXA5), CD44, COL1A1, endoglin (ENG), or ITGB1, but also CD248, a known pericyte marker, which represents a local source of MSCs in the brain (5).
CAFs in the metastatic niche
The metastatic niche is known to contain CAFs that have circulated along with or ahead of metastatic cells. Studies recently demonstrated the association of CAFs to individual circulating tumor cells (CTC) or clusters of CTCs that can be found in the blood of patients with metastatic cancers (54). Their detection has thus been suggested as a potential biomarker for metastatic spreading (55). CTC clusters have correlated with poor prognosis in patients with metastatic cancer (56). Nevertheless, it's still unclear the extent to which circulating CAFs contribute to the CAF compartment at the metastatic site (54, 57). In addition, as highlighted by Hurtado and colleagues, the cellular origin of these circulating CAFs is yet to be identified. It is also not yet known which CAFs within the primary cancer can enter circulation to potentiate metastasis (54).
Distinct CAF subpopulations originate from different cell types
Neuzillet and colleagues observed that several different CAF subpopulations could emerge from a single cellular source (58). Alternatively, others suggested that distinct CAF subpopulations or phenotypes in a bulk tumor might originate from different cell types (Fig. 2). Su and colleagues suggested that CAF diversity they observed in the breast carcinoma TME is the result of recruiting different fibroblast precursors and paracrine signaling (Fig. 2; ref. 43).
As the cellular reprogramming of normal cells to CAFs is likely variable between different cancer types and tissues, the cellular origin for CAF subtypes might also differ in a context-dependent manner (32, 39, 59). Coffman and colleagues developed a predictive gene expression–based algorithm to classify carcinoma‐associated MSCs (CA‐MSC), the progenitor cells from which the authors proposed CAFs originate. The study reported that while bone marrow–derived MSCs could become tumor supportive when cocultured in vivo with breast cancer cells, they would display tumor-inhibitory capabilities when cocultured with ovarian cancer cells (60). In contrast, local abdominal MSCs were shown to have a tumor-supporting role when cocultured with ovarian cancer cells. Koh and colleagues recently observed that fibroblasts derived from various tissues (colon, lung, and skin) differentially impact colon cancer cell proliferation in an in vitro coculture experiment (Fig. 2; ref. 41). Bone marrow–derived MSCs have been proposed by our group and others to also be the source of tumor-inhibitory CAFs (9, 61). In addition, both tumor-inhibitory CAF and MSCs share Meflin as a common marker (62). These data collectively suggest that CAF reprogramming and subsequent function is tissue and cancer-type dependent (3, 5, 60).
The identification of CAF subpopulations
Transcriptomic analyses decipher the CAF compartment in the TME
Historically, IHC was the mainstay of CAF functional analyses. However, more recently studies have used transcriptomic analysis to elucidate the heterogeneity of the CAF TME compartment (10, 48, 53, 58, 63–69). Lambrechts and colleagues used single cell RNA (scRNA) sequencing (scRNA-seq) to characterize the stromal compartment in the lung cancer TME. The authors described 5 distinct types of fibroblasts, with a unique collagen and ECM profile for each subclass. Interestingly, they observed that fibroblast cluster 6, described as ‘normal fibroblasts’ and characterized by high elastin levels and low levels of collagens I, III, V, and VI, was significantly correlated to favorable outcome in patients with lung adenocarcinoma (63). Furthermore, gene enrichment analysis revealed that cluster 6 fibroblasts showed upregulation of inflammatory response pathways, as opposed to other clusters such as cluster 7, a subpopulation shown to be substantially associated with poor outcome. Yet, presence of cluster 6 fibroblasts was also significantly associated with poor outcome in patients with lung SCC (63). As a result, the authors suggested that the abundance and unique functions of a given CAF cluster could differ between tumor types, with consequently different associated prognosis and therapeutic response (20, 63). In another study by Neuzillet and colleagues, patient-derived PDAC CAFs were profiled for 770 genes of the PanCancer Progression panel, which revealed 4 distinct CAF clusters that overlapped with some of the CAF clusters defined by Lambrechts and colleagues (Fig. 3). A POSTN-positive subpopulation, overlapping with clusters 1 and 5 described by Lambrechts and colleagues, had the least proliferative effect on cancer cells and showed lower chemoprotection to cancer cells as compared with other CAF subpopulations in an in vitro coculture set up. However, the same POSTN-positive subpopulation was associated with poor prognosis in patients (58). In addition, as observed by the authors, POSTN is strongly expressed at the invasive front of the tumors while being involved in tumor capsule formation and metastatic niche preparation (70). Considering the low protumoral effect and association with poor prognosis, it is likely that POSTN+ CAFs arise as a host response mechanism to restrain tumor growth (5, 71).
Figure 3.
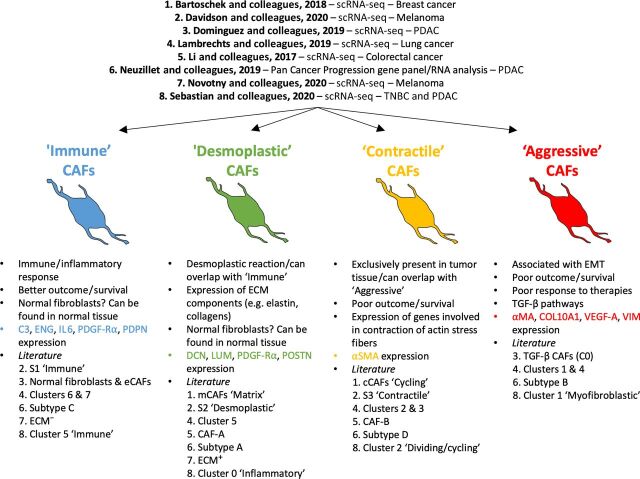
Proposed subpopulations of CAFs. The figure displays 4 broad subpopulations of CAFs in the TME as extracted from the literature: Immune, Desmoplastic, Contractile, and Aggressive. The immune and desmoplastic populations tend to be tumor inhibitory while the contractile and aggressive are more tumor supportive. The ‘immune’ subpopulation is associated with C3, ENG, IL6, PDGF-Rα, and PDPN expression. The ‘desmoplastic’ subpopulation is specifically characterized by a high expression of DCN, LUM, and POSTN as well as ECM components, such as elastin and collagen. The ‘contractile’ and ‘aggressive’ subpopulations are respectively defined by a high expression of factors involved in contraction of actin stress fibers or cell cycle (‘contractile’) and high expression of markers associated with EMT, such as vimentin or VEGF-A, or the TGF-β pathways (‘aggressive’). Both show the highest expression of α-SMA and are linked to poor patient survival/outcome. The studies used to highlight the existence of these broad CAF types are listed on top.
Using a melanoma mouse model, Davidson and colleagues also performed scRNA-seq and identified three subpopulations, namely S1 or ‘immune’, S2 or ‘desmoplastic’, and S3 or ‘contractile’ stromal cells (Fig. 3). The S1 subpopulation was shown to overexpress PDPN, PDGF-Rα, and CD34 while S3 showed high levels of α-SMA. S2 was identified as having intermediate expression of PDPN and PDGF-Rα but low expression of α-SMA and CD34. Further analysis revealed that each subpopulation had its specific functional purpose with S1 ‘immune’ subpopulation involved in the recruitment of immune cells through proinflammatory cytokines like SDF-1 or CSF-1 and receptors such as IL6ra and IL6st. S2 ‘desmoplastic’ subpopulation was thought to promote the desmoplastic reaction via overexpression of genes coding for ECM components like collagens (Col1a1, Col1a2, Col6a2), Postn, and Tnc. The S3 ‘contractile’ subpopulation overexpressed genes involved in the regulation and rearrangement of the actin cytoskeleton. S3 stromal cells were reported to be the most proliferative among all subpopulations (59). Similarly, through scRNA-seq, Sebastian and colleagues described three main subpopulations of CAFs in both breast and pancreatic cancers, namely ‘myofibroblastic’ CAFs enriched for α-SMA and other contractile proteins; ‘inflammatory’ CAFs showing overexpression of cytokines involved in inflammation and an MHC class II–expressing CAF subpopulation (7).
scRNA-seq by Bartoschek and colleagues in a mouse model of breast cancer identified ‘matrix CAF’ and ‘cycling CAF’ populations (66). Analysis of single cell transcriptomes in human colorectal tumors by Li and colleagues identified two CAF subpopulations, ‘CAF A’ cells expressing genes related to ECM remodeling while ‘CAF B’ showed ‘myofibroblastic’ features (65). As an attempt to decipher the stromal microenvironment in cutaneous melanoma spheroids through scRNA-seq, Novotny and colleagues recently described an ‘ECM-’ CAF cluster with a proinflammatory profile, an ‘ECM+’ cluster, enriched with ECM markers such as COL1A1, and a ‘ID+’ cluster, which was characterized by overexpression of factors involved in the TGF-β pathway (72).
Despite the expected differences between the different transcriptomic analyses, a common pattern has emerged from the data published describing the presence of different CAF subpopulations (Fig. 3). For instance, both the ‘inflammatory’ and the ‘Cd74-high’ subpopulations characterized by Sebastian and colleagues respectively resemble, at the molecular level, the ‘desmoplastic’ and ‘immune’ subpopulations reported by Davidson and colleagues. In the same way, the ‘CAF A’ subpopulation described by Bartoschek and colleagues and the ‘ECM+’ subpopulation reported by Novotny and colleagues also showed ‘desmoplastic’ features. Also characterized by Novotny and colleagues, the ‘ECM−’ subpopulation showed ‘immune’ characteristics. In addition, a CAF cluster (Cluster 2) defined as ‘dividing/cycling’ in triple-negative breast cancer (TNBC) seems to mirror the ‘contractile’ subpopulation of CAFs linked to melanoma (7, 59).
Accordingly, we suggest that there are 4 broad categories of CAFs described in the literature: ‘immune’, ‘desmoplastic’, ‘contractile’, and ‘aggressive’ (Fig. 3). We believe this nomenclature can help clarify and simplify comparisons between studies until a more comprehensive meta-analysis report is available, which will allow the clustering of CAFs across different cancer types to identify subpopulations and components within each subpopulation. Potential markers for the ‘immune’ subpopulation are C3, PDPN, and ENG. The ‘desmoplastic’ subpopulation can be discriminated from other CAFs through expression of markers like POSTN as well as ECM components, such as elastin and collagen (type 1 and 4). The ‘contractile’ subpopulation is defined by expression of factors involved in actin cytoskeleton rearrangement and/or cell cycle regulation. High expression of markers associated with EMT, such as vimentin or VEGF-A, or the TGF-β pathways characterizes ‘aggressive’ CAFs. Both ‘contractile’ and ‘aggressive’ subpopulations show the highest expression of α-SMA and are most often associated with poor patient survival/outcome.
Evolution of CAFs during tumor progression
Interestingly, through coculturing fibroblasts with cancer cells, Neuzillet and colleagues noticed that pancreatic stellate cells could evolve from the least protumoral POSTN+ CAF subtype (subtype A) to fully supportive CAF phenotypes [subtypes B and C, characterized by myosin heavy chain 11 (MYH11) and PDPN expression, respectively; ref. 58]. Similarly, by performing scRNA-seq at various points during tumor progression in a mouse model of PDAC, Dominguez and colleagues identified how two ‘normal fibroblast’ clusters gave rise to two distinct CAF lineages in a mouse model of PDAC (Fig. 3; ref. 10). Most importantly, IL1 and TGF-β–dependent pathways were identified to underlie the reprogramming of each lineage which eventually were observed to take over the PDAC stromal compartment. Similar IL1– and TGF-β–driven CAF subpopulations were described in patients with PDAC, although compared with the mouse model both of these lineages appeared to arise from a single early CAF subpopulation (10).
Early ‘CAF states’ in different cancer types appear to share immune/inflammatory features. These characteristics could be linked with tumor restraining properties of some CAF subpopulations during the early stages of cancer development. In a recent study, Chen and colleagues reported that a CAF subpopulation defined as ‘complement-secreting CAFs’ (csCAF) showed high expression of complement system components, such as C3 or C7, involved in regulating immune/inflammatory response. These csCAFs, which could be described as ‘immune’ CAFs, were present exclusively in early-stage PDAC and were described as potential tumor-inhibitory CAFs (73). In pancreatic tumors, transcriptional profiling of serum amyloid a3 (Saa3)-KO CAFs with tumor-inhibitory capabilities revealed an overall downregulation of cytokine expression compared with Saa3+ CAFs. These (Saa3)-KO CAFs did, however, express proinflammatory markers such as TNFα and genes in the IL6 pathway (40). Similarly, prostate cancer–associated CAFs with tumor-inhibitory capabilities have been shown to upregulate proinflammatory genes such as IL6 and CXCL2 (39).
Similar to the data reported by Dominguez and colleagues in PDAC, the distribution of stromal cell subpopulations in the murine melanoma TME described by Davidson and colleagues was observed to change upon tumor progression. This evolution progressively leads to the prevalence of the highly tumor-supportive ‘contractile’ CAF subpopulation at late stages of tumor development, as opposed to the ‘immune’ and ‘desmoplastic’ subpopulations present at earlier stages (Fig. 3; refs. 7, 59). Sebastian and colleagues also suggested that the ‘desmoplastic’ CAFs they described in murine models of triple-negative breast and pancreatic cancers may evolve and differentiate into ‘aggressive’ CAFs as the tumor progresses (Fig. 3; ref. 7). Taken together, these data suggest that CAF evolution, towards an increasingly tumor-supportive phenotype, is a common feature of many cancers during tumor progression and warrants further investigation (Fig. 4).
While the CAF compartment becomes more supportive as the tumor progresses, CAF heterogeneity appears to decrease. Support for this comes from Venning and colleagues who recently followed the evolution of CAF subpopulations in murine models of TNBC (74). Tumors were collected at day 7, 14, and 21 post injection, from which CAFs were isolated. Based on the expression of 6 markers (α-SMA, FAP, PDGF-Rα and β, CD26, and PDPN), authors defined up to 63 CAF subpopulations present in the TME of both TNBC models at day 7. Of these, 5 became more prevalent by day 21 while other CAF subpopulations decreased in abundance. Eventually, the 5 main subpopulations contributed to more than 60% of the CAF compartment in the TME of both models (74). These data suggest that the CAF compartment becomes more homogenous in its cellular composition over time, albeit the time frame studied was fairly short (74). Similar observations were made by Chen and colleagues in PDAC patient samples where late-stage tumors show only one subpopulation of CAFs, lacking other subpopulations described in early-stage tumors, including ‘immune’ CAFs (Fig. 3; ref. 73).
Overall, among the 4 subpopulations of CAFs we defined, we believe that the ‘immune’ and ‘desmoplastic’ subpopulations are the first ones to emerge as a host response to tumor growth and seem to be the most likely to have tumor inhibitory functions (73). On the other hand, the ‘contractile’ and ‘aggressive’ subpopulations show stronger tumor supporting capabilities (7, 59). In addition, the ‘immune’ and ‘desmoplastic’ subpopulations slowly disappear over time while the ‘contractile’ and ‘aggressive’ subtypes become more prevalent (7, 59, 66, 73, 75). Taken together, it can be stipulated that the tumor-inhibitory CAF/tumor-supporting CAF ratio decreases over time as the CAF compartment eventually evolves into a more homogenous and tumor-supportive environment (Fig. 4; refs. 10, 43, 58, 65, 66, 68, 74).
Conclusions
The heterogeneity of the CAF compartment is now widely acknowledged and well described. In the present review, we propose a nomenclature for 4 main CAF subpopulations that can be found in multiple cancer types and includes the ‘immune’, ‘desmoplastic’, ‘contractile’, and ‘aggressive’ CAF subtypes. In addition, as we move away from the simplistic view describing CAFs as being essentially tumor supportive, recent evidence shows that the ‘immune’ and ‘desmoplastic’ subpopulations have tumor inhibitory properties and their presence likely represents a host response to tumor growth (5).
The recognition of the existence of tumor-inhibitory CAFs in the TME challenges long-lasting paradigms in the field that CAFs are solely tumor promoting entities in the TME. Undoubtedly, this provides new opportunities to improve patient care. For these reasons, this review is also a call for a better comprehension of the mechanisms behind the emergence, roles, and evolution of the various CAF subtypes in the TME. Further work is still needed to characterize the subtype-specific functions of CAFs as well as their cross-talk with other cells in the TME. Such knowledge could eventually help future therapeutic strategies to specifically target only those tumor-supportive CAFs, while bolstering tumor inhibitory ones. Altogether, we believe that the present review provides novel insights on the complexities of CAFs by identifying major subtypes and also highlights differences in the way these subtypes emerge, evolve, and function.
Authors' Disclosures
No disclosures were reported.
References
- 1. Simon T, Coquerel B, Petit A, Kassim Y, Demange E, Cerf D, et al. Direct effect of bevacizumab on glioblastoma cell lines in vitro. NeuroMol Med 2014;16:752–71. [DOI] [PubMed] [Google Scholar]
- 2. Simon T, Gagliano T, Giamas G. Direct effects of anti-angiogenic therapies on tumor cells: VEGF signaling. Trends Mol Med 2017;23:282–92. [DOI] [PubMed] [Google Scholar]
- 3. Liao Z, Tan ZW, Zhu P, Tan NS. Cancer-associated fibroblasts in tumor microenvironment - accomplices in tumor malignancy. Cell Immunol 2019;343:103729. [DOI] [PubMed] [Google Scholar]
- 4. Brandao M, Simon T, Critchley G, Giamas G. Astrocytes, the rising stars of the glioblastoma microenvironment. Glia 2019;67:779–90. [DOI] [PubMed] [Google Scholar]
- 5. Tew BY, Legendre C, Gooden GC, Johnson KN, Martinez RA, Kiefer J, et al. Isolation and characterization of patient-derived CNS metastasis-associated stromal cell lines. Oncogene 2019;38:4002–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mizutani Y, Kobayashi H, Iida T, Asai N, Masamune A, Hara A, et al. Meflin-positive cancer-associated fibroblasts inhibit pancreatic carcinogenesis. Cancer Res 2019;79:5367. [DOI] [PubMed] [Google Scholar]
- 7. Sebastian A, Hum NR, Martin KA, Gilmore SF, Peran I, Byers SW, et al. Single-cell transcriptomic analysis of tumor-derived fibroblasts and normal tissue-resident fibroblasts reveals fibroblast heterogeneity in breast cancer. Cancers (Basel) 2020;12:1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Y, Wei Y, Tang W, Luo J, Wang M, Lin H, et al. Association between the degree of fibrosis in fibrotic focus and the unfavorable clinicopathological prognostic features of breast cancer. PeerJ 2019;7:e8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res 2011;1:482–97. [PMC free article] [PubMed] [Google Scholar]
- 10. Dominguez CX, Müller S, Keerthivasan S, Koeppen H, Hung J, Gierke S, et al. Single-cell RNA sequencing reveals stromal evolution into LRRC15(+) myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov 2020;10:232–53. [DOI] [PubMed] [Google Scholar]
- 11. Giannoni E, Bianchini F, Calorini L, Chiarugi P. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid Redox Signal 2011;14:2361–71. [DOI] [PubMed] [Google Scholar]
- 12. Tan HX, Gong WZ, Zhou K, Xiao ZG, Hou FT, Huang T, et al. CXCR4/TGF-β1 mediated hepatic stellate cells differentiation into carcinoma-associated fibroblasts and promoted liver metastasis of colon cancer. Cancer Biol Ther 2020;21:258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liberti MV, Locasale JW. The warburg effect: how does it benefit cancer cells? Trends Biochem Sci 2016;41:211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun K, Tang S, Hou Y, Xi L, Chen Y, Yin J, et al. Oxidized ATM-mediated glycolysis enhancement in breast cancer-associated fibroblasts contributes to tumor invasion through lactate as metabolic coupling. EBioMedicine 2019;41:370–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wanandi SI, Ningsih SS, Asikin H, Hosea R, Neolaka GMG. Metabolic interplay between tumour cells and cancer-associated fibroblasts (CAFs) under hypoxia versus normoxia. Malays J Med Sci 2018;25:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 2011;470:548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herrera M, Islam AB, Herrera A, Martín P, García V, Silva J, et al. Functional heterogeneity of cancer-associated fibroblasts from human colon tumors shows specific prognostic gene expression signature. Clin Cancer Res 2013;19:5914–26. [DOI] [PubMed] [Google Scholar]
- 18. Tchou J, Kossenkov AV, Chang L, Satija C, Herlyn M, Showe LC, et al. Human breast cancer associated fibroblasts exhibit subtype specific gene expression profiles. BMC Med Genomics 2012;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu Y, Zhang Z, Zhang L, Zhang C. Novel module and hub genes of distinctive breast cancer associated fibroblasts identified by weighted gene co-expression network analysis. Breast Cancer 2020;27:1017–28. [DOI] [PubMed] [Google Scholar]
- 20. Zeltz C, Primac I, Erusappan P, Alam J, Noel A, Gullberg D. Cancer-associated fibroblasts in desmoplastic tumors: emerging role of integrins. Semin Cancer Biol 2020;62:166–81. [DOI] [PubMed] [Google Scholar]
- 21. LeBleu VS, Neilson EG. Origin and functional heterogeneity of fibroblasts. Faseb J 2020;34:3519–36. [DOI] [PubMed] [Google Scholar]
- 22. Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 2013;110:20212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li M, Li M, Yin T, Shi H, Wen Y, Zhang B, et al. Targeting of cancer-associated fibroblasts enhances the efficacy of cancer chemotherapy by regulating the tumor microenvironment. Mol Med Rep 2016;13:2476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duluc C, Moatassim-Billah S, Chalabi-Dchar M, Perraud A, Samain R, Breibach F, et al. Pharmacological targeting of the protein synthesis mTOR/4E-BP1 pathway in cancer-associated fibroblasts abrogates pancreatic tumour chemoresistance. EMBO Mol Med 2015;7:735–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee J, Fassnacht M, Nair S, Boczkowski D, Gilboa E. Tumor immunotherapy targeting fibroblast activation protein, a product expressed in tumor-associated fibroblasts. Cancer Res 2005;65:11156–63. [DOI] [PubMed] [Google Scholar]
- 26. Xu K, Tian X, Oh SY, Movassaghi M, Naber SP, Kuperwasser C, et al. The fibroblast Tiam1-osteopontin pathway modulates breast cancer invasion and metastasis. Breast Cancer Res 2016;18:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cat B, Stuhlmann D, Steinbrenner H, Alili L, Holtkötter O, Sies H, et al. Enhancement of tumor invasion depends on transdifferentiation of skin fibroblasts mediated by reactive oxygen species. J Cell Sci 2006;119:2727–38. [DOI] [PubMed] [Google Scholar]
- 28. Narra K, Mullins SR, Lee HO, Strzemkowski-Brun B, Magalong K, Christiansen VJ, et al. Phase II trial of single agent Val-boroPro (talabostat) inhibiting fibroblast activation protein in patients with metastatic colorectal cancer. Cancer Biol Ther 2007;6:1691–9. [DOI] [PubMed] [Google Scholar]
- 29. Park H, Lee Y, Lee H, Kim JW, Hwang JH, Kim J, et al. The prognostic significance of cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. Tumor Biology 2017;39:1010428317718403. [DOI] [PubMed] [Google Scholar]
- 30. Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014;25:719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rhim Andrew D, Oberstein Paul E, Thomas Dafydd H, Mirek Emily T, Palermo Carmine F, Sastra Stephen A, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014;25:735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kadel D, Zhang Y, Sun HR, Zhao Y, Dong QZ, Qin LX. Current perspectives of cancer-associated fibroblast in therapeutic resistance: potential mechanism and future strategy. Cell Biol Toxicol 2019;35:407–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Z, Yang Q, Tan Y, Tang Y, Ye J, Yuan B, et al. Cancer-associated fibroblasts suppress cancer development: the other side of the coin. Front Cell Dev Biol 2021;9:613534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kobayashi H, Gieniec KA, Wright JA, Wang T, Asai N, Mizutani Y, et al. The balance of stromal BMP signaling mediated by GREM1 and ISLR drives colorectal carcinogenesis. Gastroenterology 2021;160:1224–39. [DOI] [PubMed] [Google Scholar]
- 35. Miyai Y, Esaki N, Takahashi M, Enomoto A. Cancer-associated fibroblasts that restrain cancer progression: hypotheses and perspectives. Cancer Sci 2020;111:1047–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen K, Luk S, Elman J, Murray R, Mukundan S, Parekkadan B. Suicide gene-engineered stromal cells reveal a dynamic regulation of cancer metastasis. Sci Rep 2016;6:21239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou L, Yang K, Randall Wickett R, Zhang Y. Dermal fibroblasts induce cell cycle arrest and block epithelial–mesenchymal transition to inhibit the early stage melanoma development. Cancer Med 2016;5:1566–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alkasalias T, Alexeyenko A, Hennig K, Danielsson F, Lebbink RJ, Fielden M, et al. RhoA knockout fibroblasts lose tumor-inhibitory capacity in vitro and promote tumor growth in vivo. Proc Natl Acad Sci 2017;114:E1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alkasalias T, Flaberg E, Kashuba V, Alexeyenko A, Pavlova T, Savchenko A, et al. Inhibition of tumor cell proliferation and motility by fibroblasts is both contact and soluble factor dependent. Proc Natl Acad Sci 2014;111:17188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Djurec M, Graña O, Lee A, Troulé K, Espinet E, Cabras L, et al. Saa3 is a key mediator of the protumorigenic properties of cancer-associated fibroblasts in pancreatic tumors. Proc Natl Acad Sci 2018;115:E1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koh B, Jeon H, Kim D, Kang D, Kim KR. Effect of fibroblast co-culture on the proliferation, viability and drug response of colon cancer cells. Oncol Lett 2019;17:2409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Avagliano A, Ruocco MR, Nasso R, Aliotta F, Sanità G, Iaccarino A, et al. Development of a stromal microenvironment experimental model containing proto-myofibroblast like cells and analysis of its crosstalk with melanoma cells: a new tool to potentiate and stabilize tumor suppressor phenotype of dermal myofibroblasts. Cells 2019;8:1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Su G, Sung KE, Beebe DJ, Friedl A. Functional screen of paracrine signals in breast carcinoma fibroblasts. PLoS One 2012;7:e46685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ikemori R, Gabasa M, Duch P, Vizoso M, Bragado P, Arshakyan M, et al. Epigenetic SMAD3 repression in tumor-associated fibroblasts impairs fibrosis and response to the antifibrotic drug nintedanib in lung squamous cell carcinoma. Cancer Res 2019;80:276–90. [DOI] [PubMed] [Google Scholar]
- 45. Takahashi A, Ishii G, Neri S, Yoshida T, Hashimoto H, Suzuki S, et al. Podoplanin-expressing cancer-associated fibroblasts inhibit small cell lung cancer growth. Oncotarget 2015;6:9531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 2013;499:346–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kanno A, Satoh K, Masamune A, Hirota M, Kimura K, Umino J, et al. Periostin, secreted from stromal cells, has biphasic effect on cell migration and correlates with the epithelial to mesenchymal transition of human pancreatic cancer cells. Int J Cancer 2008;122:2707–18. [DOI] [PubMed] [Google Scholar]
- 48. Wang Y, Liang Y, Xu H, Zhang X, Mao T, Cui J, et al. Single-cell analysis of pancreatic ductal adenocarcinoma identifies a novel fibroblast subtype associated with poor prognosis but better immunotherapy response. Cell Discov 2021;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Han W, Chen S, Yuan W, Fan Q, Tian J, Wang X, et al. Oriented collagen fibers direct tumor cell intravasation. Proc Natl Acad Sci 2016;113:11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen IX, Chauhan VP, Posada J, Ng MR, Wu MW, Adstamongkonkul P, et al. Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc Natl Acad Sci U S A 2019;116:4558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lo A, Wang LS, Scholler J, Monslow J, Avery D, Newick K, et al. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res 2015;75:2800–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cao H, Cheng HS, Wang JK, Tan NS, Tay CY. A 3D physio-mimetic interpenetrating network-based platform to decode the pro and anti-tumorigenic properties of cancer-associated fibroblasts. Acta Biomater 2021;132:448–60. [DOI] [PubMed] [Google Scholar]
- 53. Zhou Y, Yang D, Yang Q, Lv X, Huang W, Zhou Z, et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat Commun 2020;11:6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hurtado P, Martínez-Pena I, Piñeiro R. Dangerous liaisons: circulating tumor cells (CTCs) and cancer-associated fibroblasts (CAFs). Cancers (Basel) 2020;12:2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Akolkar D, Patil D, Crook T, Limaye S, Page R, Datta V, et al. Circulating ensembles of tumor-associated cells: a redoubtable new systemic hallmark of cancer. Int J Cancer 2020;146:3485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ortiz-Otero N, Marshall JR, Lash B, King MR. Chemotherapy-induced release of circulating-tumor cells into the bloodstream in collective migration units with cancer-associated fibroblasts in metastatic cancer patients. BMC Cancer 2020;20:873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Matsumura Y, Ito Y, Mezawa Y, Sulidan K, Daigo Y, Hiraga T, et al. Stromal fibroblasts induce metastatic tumor cell clusters via epithelial-mesenchymal plasticity. Life Sci Alliance 2019;2:e201900425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Neuzillet C, Tijeras-Raballand A, Ragulan C, Cros J, Patil Y, Martinet M, et al. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J Pathol 2019;248:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Davidson S, Efremova M, Riedel A, Mahata B, Pramanik J, Huuhtanen J, et al. Single-cell RNA sequencing reveals a dynamic stromal niche that supports tumor growth. Cell Rep 2020;31:107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Coffman LG, Pearson AT, Frisbie LG, Freeman Z, Christie E, Bowtell DD, et al. Ovarian carcinoma-associated mesenchymal stem cells arise from tissue-specific normal stroma. Stem Cells 2019;37:257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ryan D, Paul BT, Koziol J, ElShamy WM. The pro- and anti-tumor roles of mesenchymal stem cells toward BRCA1-IRIS-overexpressing TNBC cells. Breast Cancer Res 2019;21:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Isfoss BL, Holmqvist B, Sand E, Forsell J, Jernström H, Olsson H, et al. Stellate cells and mesenchymal stem cells in benign mammary stroma are associated with risk factors for breast cancer – an observational study. BMC Cancer 2018;18:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lambrechts D, Wauters E, Boeckx B, Aibar S, Nittner D, Burton O, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med 2018;24:1277–89. [DOI] [PubMed] [Google Scholar]
- 64. Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 2018;33:463–79.e10. [DOI] [PubMed] [Google Scholar]
- 65. Li H, Courtois ET, Sengupta D, Tan Y, Chen KH, Goh JJL, et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat Genet 2017;49:708–18. [DOI] [PubMed] [Google Scholar]
- 66. Bartoschek M, Oskolkov N, Bocci M, Lövrot J, Larsson C, Sommarin M, et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat Commun 2018;9:5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Puram SV, Tirosh I, Parikh AS, Patel AP, Yizhak K, Gillespie S, et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 2017;171:1611–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Friedman G, Levi-Galibov O, David E, Bornstein C, Giladi A, Dadiani M, et al. Cancer-associated fibroblast compositions change with breast cancer progression linking the ratio of S100A4+ and PDPN+ CAFs to clinical outcome. Nature Cancer 2020;1:692–708. [DOI] [PubMed] [Google Scholar]
- 69. Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017;214:579–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr H-A, Delaloye J-F, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 2012;481:85–9. [DOI] [PubMed] [Google Scholar]
- 71. DeClerck YA. Desmoplasia: a response or a niche? Cancer Discov 2012;2:772. [DOI] [PubMed] [Google Scholar]
- 72. Novotný J, Strnadová K, Dvořánková B, Kocourková Š, Jakša R, Dundr P, et al. Single-cell RNA sequencing unravels heterogeneity of the stromal niche in cutaneous melanoma heterogeneous spheroids. Cancers (Basel) 2020;12:3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen K, Wang Q, Li M, Guo H, Liu W, Wang F, et al. Single-cell RNA-seq reveals dynamic change in tumor microenvironment during pancreatic ductal adenocarcinoma malignant progression. EBioMedicine 2021;66:103315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Venning FA, Zornhagen KW, Wullkopf L, Sjölund J, Rodriguez-Cupello C, Kjellman P, et al. Deciphering the temporal heterogeneity of cancer-associated fibroblast subpopulations in breast cancer. J Exp Clin Cancer Res 2021;40:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang Q, Chai S, Wang W, Wan C, Zhang F, Li Y, et al. Macrophages activate mesenchymal stem cells to acquire cancer-associated fibroblast-like features resulting in gastric epithelial cell lesions and malignant transformation in vitro. Oncol Lett 2019;17:747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]